User login
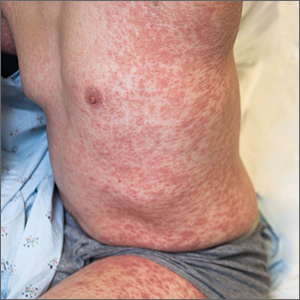
Generalized pustular eruption
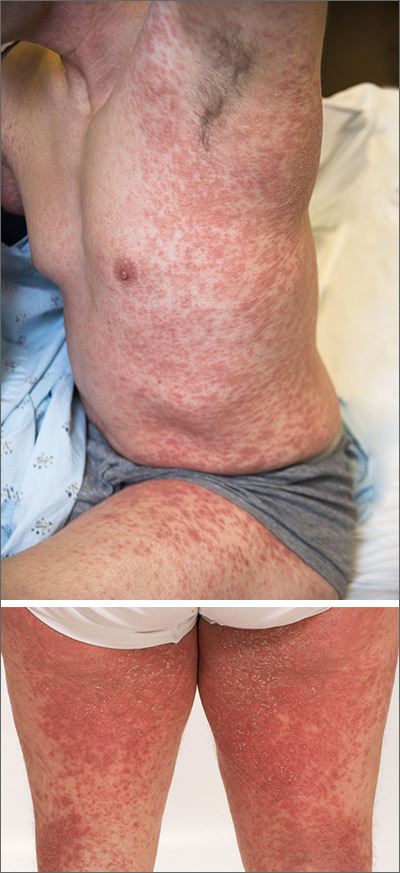
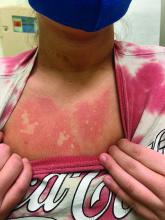
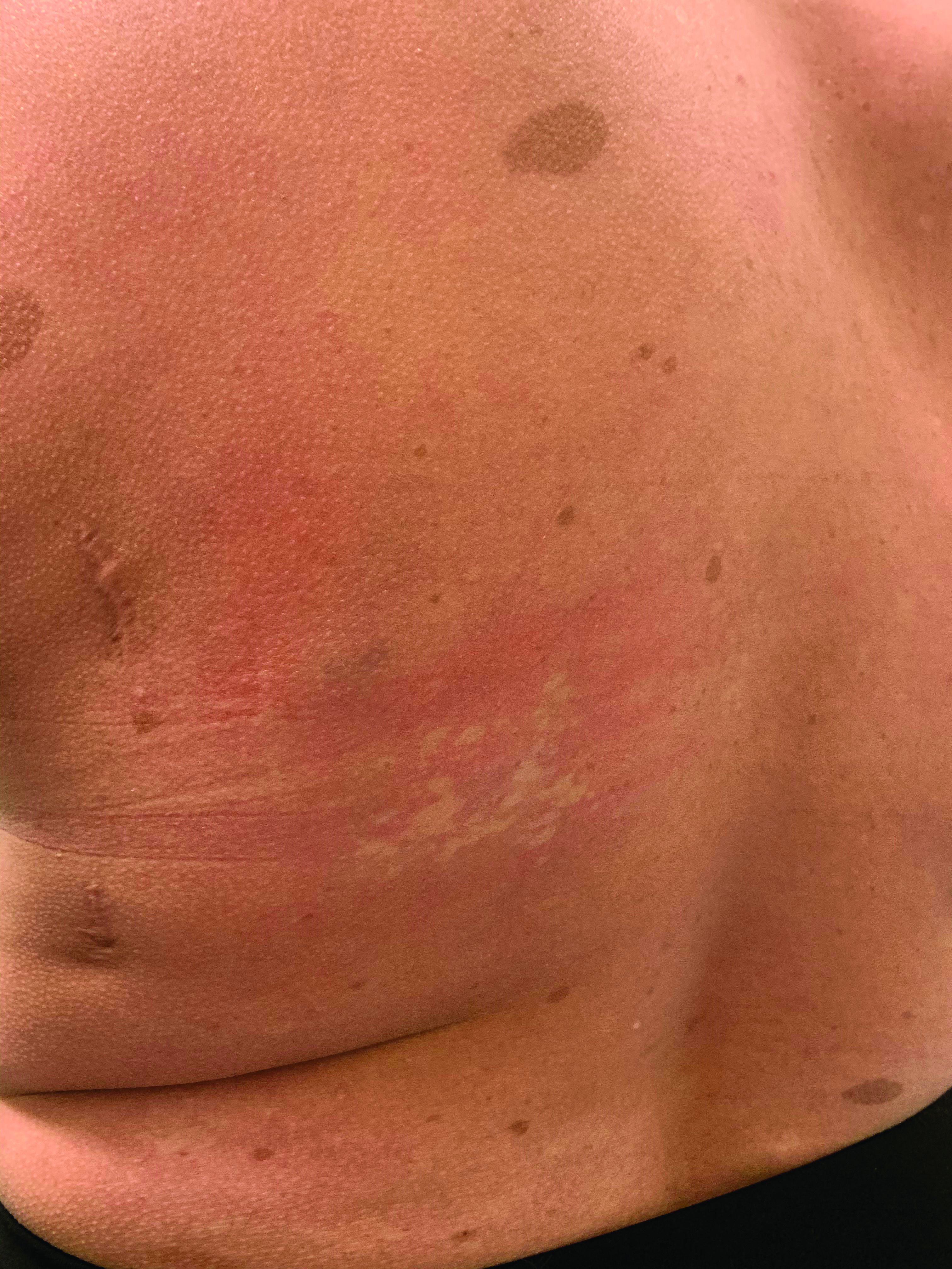
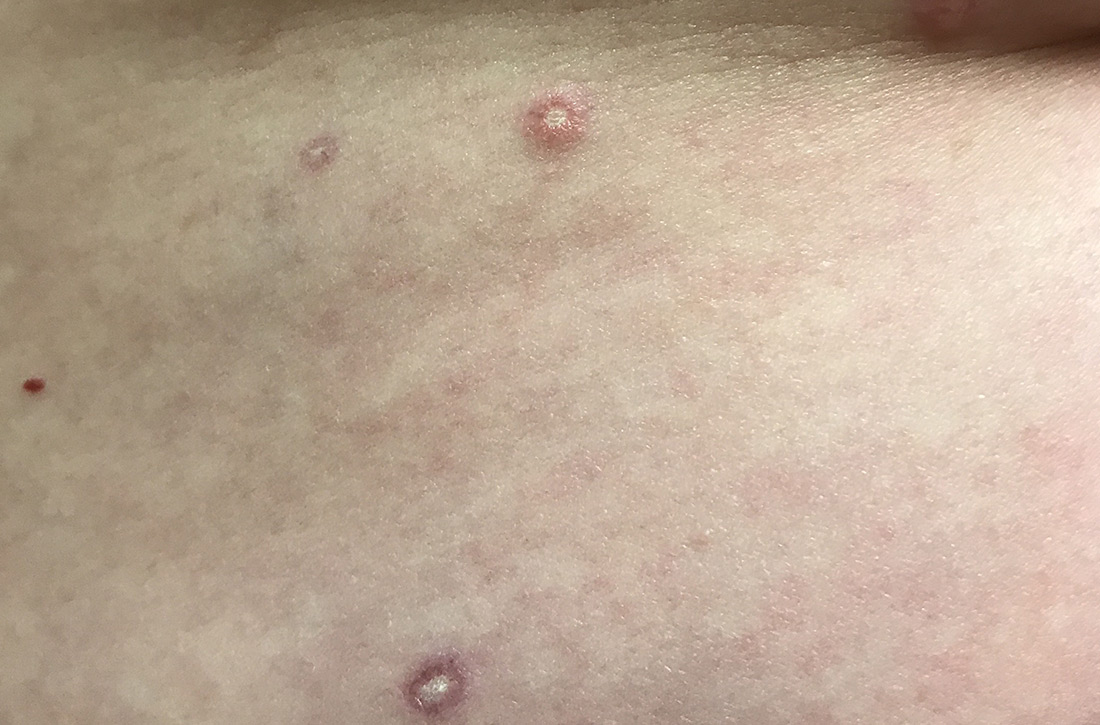
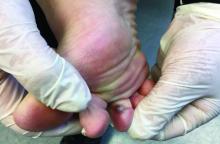
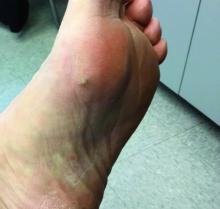
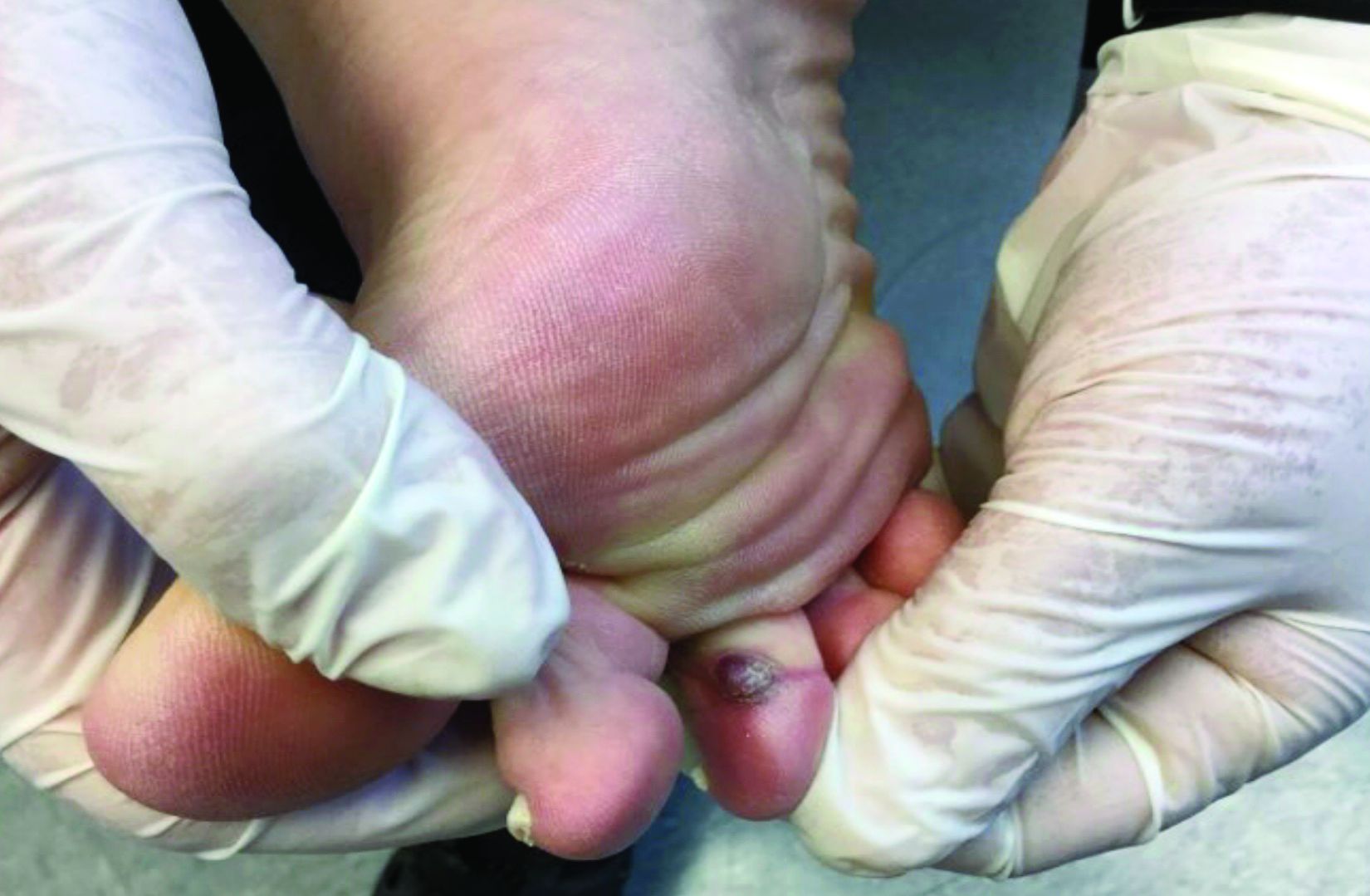
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to a diagnosis of acute generalized exanthematous pustulosis (AGEP), which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized. The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%). Leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days. Although common, fever is not always documented in patients with AGEP. (This patient was a case in point.)
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis. In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days. In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs. A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men. While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include a drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, toxic epidermal necrolysis, and pustular psoriasis. The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated. A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.
This patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared and only minimal residual erythema was noted. The patient was instructed to avoid azithromycin.
This case was adapted from: Tolkachjov SN, Wetter DA, Sandefur BJ. Generalized pustular eruption. J Fam Pract. 2018;67:309-310,312.

The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to a diagnosis of acute generalized exanthematous pustulosis (AGEP), which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized. The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%). Leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days. Although common, fever is not always documented in patients with AGEP. (This patient was a case in point.)
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis. In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days. In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs. A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men. While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include a drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, toxic epidermal necrolysis, and pustular psoriasis. The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated. A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.
This patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared and only minimal residual erythema was noted. The patient was instructed to avoid azithromycin.
This case was adapted from: Tolkachjov SN, Wetter DA, Sandefur BJ. Generalized pustular eruption. J Fam Pract. 2018;67:309-310,312.

The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to a diagnosis of acute generalized exanthematous pustulosis (AGEP), which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized. The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%). Leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days. Although common, fever is not always documented in patients with AGEP. (This patient was a case in point.)
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis. In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days. In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs. A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men. While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include a drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, toxic epidermal necrolysis, and pustular psoriasis. The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated. A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.
This patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared and only minimal residual erythema was noted. The patient was instructed to avoid azithromycin.
This case was adapted from: Tolkachjov SN, Wetter DA, Sandefur BJ. Generalized pustular eruption. J Fam Pract. 2018;67:309-310,312.
COVID-19 vaccines: Safe for immunocompromised patients?
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Preadolescent acne: Management from birth requires increasing vigilance
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
No treatment may be necessary for acne in the first few months of life, but the condition can leave scars in children as young as ages 3-6 months, said Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology, Penn State University, Hershey, Penn., said in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Neonatal acne occurs in more than 20% of newborns aged 2 weeks to 3 months. “Typically we don’t need to treat it. But if you do, you could use a topical antifungal like clotrimazole cream twice a day,” but in most babies, “this will just improve over time and resolve without any scarring or sequelae,” she said.
Infantile acne begins about 3-6 months of age typically, or a little bit older, and lasts up to 2 years of age, Dr. Zaenglein said. “You will see comedones in infantile acne, so this is actually a true form of acne. It’s due to increased adrenal production of androgens.”
She added: “The scarring can be permanent. It’s important that you recognize infantile acne and treat it, even though it seems pretty mild.”
For infantile acne, she recommends performing a full-skin exam to rule out hyperandrogenic disorders such as Cushing syndrome, congenital adrenal hyperplasia, premature adrenarche, a gonadal/adrenal tumor and precocious puberty.
Treatments are similar to those in teenagers, she said, “but make sure you use baby-friendly formulations,” with lower concentrations of active ingredients – and avoid tetracyclines and benzoyl peroxide (BPO) washes. BPO can be used in leave-on formulations/creams at lower strengths (2.5%-5%).
One possible combination option is tretinoin 0.025% cream or adapalene 0.1% gel plus BPO 2.5% cream or clindamycin/BPO gel. Another combination is adapalene/BPO 2.5% gel.
Erythromycin can be appropriate at 30-50 mg/kg per day divided in two or three doses a day, but beware of possible gastrointestinal upset. Azithromycin at 5 mg/kg per day is another option.
“Rarely do we have to go to isotretinoin,” Dr. Zaenglein said. “I think in all my years, I’ve only treated one baby with isotretinoin for infantile acne. But severe forms can occur.”
Midchildhood and preadolescent acne conditions occur in children starting at ages 1 up to 10 years, Dr. Zaenglein said. In this population, she also recommends ruling out hyperandrogenism by looking for secondary sexual characteristics with full-body skin exams. “The workup can be broad and includes looking at adrenal androgens and total and free testosterone, as well as looking at growth charts and bone age. Typically, you’ll refer these kids to pediatric endocrinology.”
Keep in mind, she said, that early adrenarche starts at ages 6-7 years in girls and 7-8 years in boys. “That’s when we expect to start seeing that very early acne. You can see it even earlier in patients with elevated BMI, and it’s more common in Hispanic and Black children as well.”
She added that it’s important to remember that early adrenarche is a risk factor for polycystic ovarian syndrome (PCOS). “So ask patients about their family history and look for other signs of PCOS as they move further into adolescence.”
Milder cases of acne in this age group can be treated with “salicylic acid wipes and things that are kind of a rite of passage. But if they have any more severe acne, you’re going to want to treat it more or less like you do adolescent acne.”
MedscapeLive and this news organization are owned by the same parent company. Dr. Zaenglein disclosed receiving consulting fees from Cassiopea, Dermata, and Regeneron and fees for contracted research support from Incyte.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Beware a pair of dermatologic emergencies in children
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
in a presentation at MedscapeLive’s virtual Women’s & Pediatric Dermatology Seminar.
Eczema herpeticum is a condition in which a herpes simplex virus (HSV-1 or HSV-2) is superimposed over preexisting eczema. “The infection may be primary and sustained from a close contact or result in some of our older patients from reactivation and spread through autoinoculation,” said Dr. Hightower, of Rady Children’s Hospital and the University of California, both in San Diego.
Signs, he said, include acute worsening of atopic dermatitis with new-onset vesicles, pustules, and “punched-out” hemorrhagic crusted erosions. “Presentation ranges from mild to transient to life threatening.”
Potential complications include meningitis, encephalitis, hepatitis, and chronic conjunctivitis. “That’s why immediate ophthalmological evaluation is needed when there’s involvement on the face near the eye,” he said.
As for management and care, “where I have concern for HSV patients, I get HSV [polymerase chain reaction] as well as a bacterial culture,” he said. But even before the results are available, empiric treatment with acyclovir can be appropriate. “It’s got to be systemic for these kids with severe involvement,” he said, and they should also be started on medication for staphylococci and streptococci.
During his presentation, Dr. Hightower also highlighted staphylococcal scalded skin syndrome. Patients with the disease commonly have concurrent skin pain (which can appear to be fussiness), fever, irritability, malaise, and poor feeding. Examination may reveal widespread erythema with accentuation at folds/peeling at hands and large sheets of superficial peeling scale with diffuse erythema.
Widespread skin involvement “results not from the presence of staph throughout the skin, but the exotoxin that it produces that becomes systemic,” he said. “Clinical diagnosis is supported by presence of S. aureus on bacterial culture, but the presence of staph is not necessary to make the diagnosis. When in doubt, histopathology is helpful. But again, it’s not necessary to make the diagnosis.”
Cases can be managed with a first- or second-generation cephalosporin, he said. Alternative therapies include antistaphylococcus penicillinase-resistant penicillins (oxacillin or nafcillin) or vancomycin.
While Dr. Hightower doesn’t use clindamycin in these patients, he said it’s an option that some dermatologists consider because of its antistaphylococcus activity. “Historically, people thought it may decrease exotoxin production. The big concern if you are going to use clindamycin is that there are high rates of community resistance,” he said. “So you want to be careful that you know your resistance patterns wherever you are. Follow up on culture to make sure that you have adequate coverage for the bug that the kiddo in front of you has.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
A girl presents with blotchy, slightly itchy spots on her chest, back
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
On close evaluation of the picture on her chest, she has pale macules and patches surrounded by erythematous ill-defined patches consistent with nevus anemicus. The findings of the picture raise the suspicion for neurofibromatosis, and it was recommended for her to be evaluated in person.
She comes several days later to the clinic. The caretaker, who is her aunt, reports she does not know much of the girl’s medical history as she recently moved from South America to live with her. The girl is a very nice and pleasant 8-year-old. She reports noticing the spots on her chest for about a year and that they seem to get a little itchier and more noticeable when she is hot or when she is running. She also reports increasing headaches for several months. She is being home schooled, and according to her aunt she is at par with her cousins who are about the same age. There is no history of seizures. She has had back surgery in the past. There is no history of hypertension. There is no family history of any genetic disorder or similar lesions.
On physical exam, her vital signs are normal, but her head circumference is over the 90th percentile. She is pleasant and interactive. On skin examination, she has slightly noticeable pale macules and patches on the chest and back that become more apparent after rubbing her skin. She has multiple light brown macules and oval patches on the chest, back, and neck. She has no axillary or inguinal freckling. She has scars on the back from her prior surgery.
As she was having worsening headaches, an MRI of the brain was ordered, which showed a left optic glioma. She was then referred to ophthalmology, neurology, and genetics.
Neurofibromatosis type 1 (NF1) is a common genetic autosomal dominant disorder cause by mutations on the NF1 gene on chromosome 17, which encodes for the protein neurofibromin. This protein works in the Ras-mitogen–activated protein kinase pathway as a negative regulator. Based on the National Institute of Health criteria, children need two or more of the following to be diagnosed with NF1: more than six café au lait macules larger than 5 mm in prepubescent children and 2.5 cm after puberty; axillary or inguinal freckling; two or more Lisch nodules; optic gliomas; two or more neurofibromas or one plexiform neurofibroma; or a first degree relative with a diagnosis of NF1. With these criteria, about 70% of the children can be diagnosed before the age of 1 year.1
Nevus anemicus is an uncommon birthmark, sometimes overlooked, that is characterized by pale, hypopigmented, well-defined macules and patches that do not turn red after trauma or changes in temperature. Nevus anemicus is usually localized on the torso but can be seen on the face, neck, and extremities. These lesions are present in 1%-2% of the general population. They are thought to occur because of increased sensitivity of the affected blood vessels to catecholamines, which causes permanent vasoconstriction, which leads to hypopigmentation on the area.2 These lesions are usually present at birth and have been described in patients with tuberous sclerosis, neurofibromatosis, and phakomatosis pigmentovascularis.
Recent studies of patients with neurofibromatosis and other RASopathies have noticed that nevus anemicus is present in about 8.8%-51% of the patients studied with a diagnosis NF1, compared with only 2% of the controls.3,4 The studies failed to report any cases of nevus anemicus in patients with other RASopathies associated with café au lait macules. Bulteel and colleagues recently reported two cases of non-NF1 RASopathies also associated with nevus anemicus in a patient with Legius syndrome and a patient with Noonan syndrome with multiple lentigines.5 The nevus anemicus was reported to occur most commonly on the anterior chest and be multiple, as seen in our patient.
The authors of the published studies advocate for the introduction of nevus anemicus as part of the diagnostic criteria for NF1, especially because it can be an early finding seen in babies, which can aid in early diagnosis of NF1.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She has no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Pediatrics. 2000 Mar. doi: 10.1542/peds.105.3.608.
2. Nevus Anemicus. StatPearls [Internet] (Treasure Island, Fla.: StatPearls Publishing; 2020 Jan).
3. J Am Acad Dermatol. 2013 Nov. doi: 10.1016/j.jaad.2013.06.039.
4. Pediatr Dermatol. 2015 May-Jun. doi: 10.1111/pde.12525.
5. JAAD Case Rep. 2018 Apr 5. doi: 10.1016/j.jdcr.2017.09.037.
Worsening skin lesions but no diagnosis
A 50-year-old woman presented to her family physician for a urinary tract infection (UTI) and an itchy rash. She said the rash had developed 2 years earlier and had gotten worse, with additional lesions emerging on her skin as time went on. She noted that other physicians had evaluated the rash but provided no clear diagnosis and had done no testing.
A physical exam revealed scattered erythematous papules with white centers on the patient’s trunk, arms, and legs (FIGURE 1). The patient’s medical history was significant for asthma, obstructive sleep apnea, obesity, gastroesophageal reflux disease, urinary incontinence, and depression. Her medications included montelukast, inhaled fluticasone, albuterol, tolterodine, omeprazole, and fluoxetine.
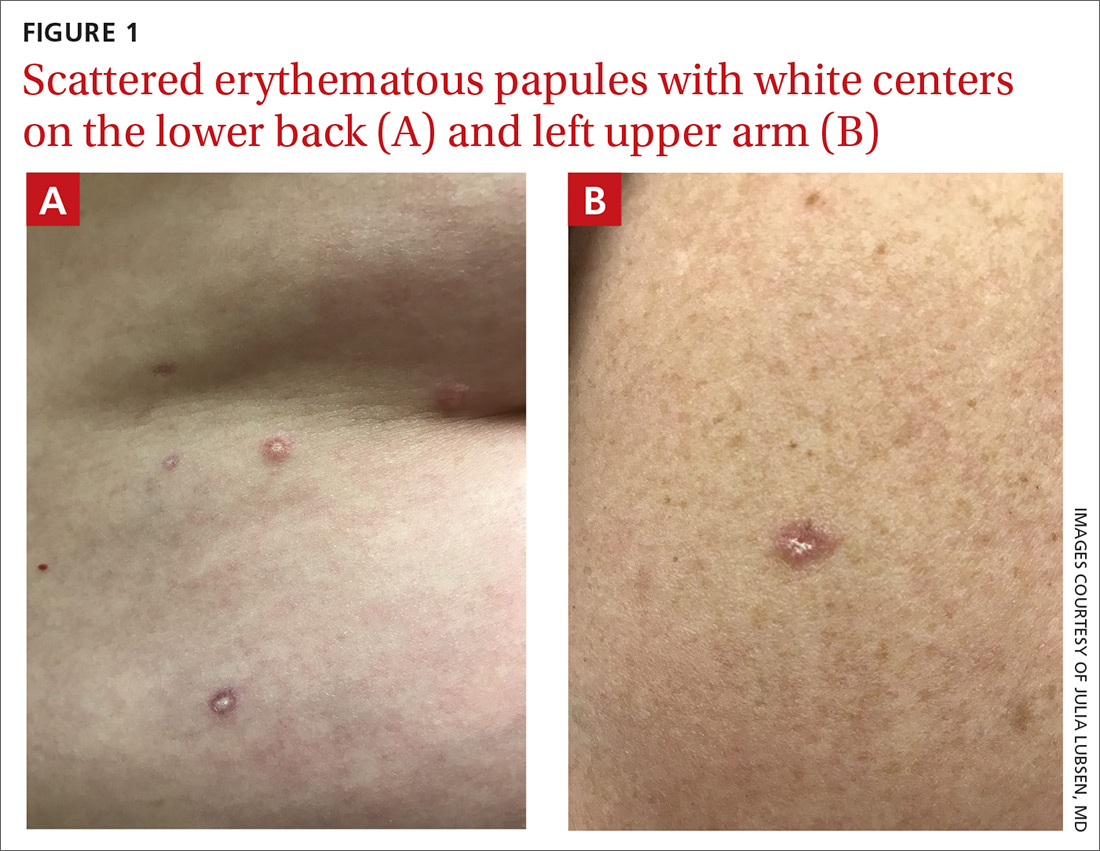
The patient was prescribed nitrofurantoin, 100 mg twice daily for 5 days, to treat her UTI, and a punch biopsy was performed on one of the patient’s lesions to determine the cause of the rash.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophic papulosis
Pathology suggested a diagnosis of atrophic papulosis. A consultation with a dermatologist and additional biopsies confirmed the diagnosis. The biopsies showed wedge-shaped areas of superficial dermal sclerosis with thinning of the overlying epidermis. The superficial dermal vessels contained scattered, small thrombi at the periphery of the areas of sclerosis.
Atrophic papulosis (also known as Degos disease or Kölmeier-Degos disease) is a vasculopathy characterized by thrombotic occlusion of small arteries.1 Although rare—with fewer than 200 published case reports in the literature—it is likely underdiagnosed.1 Atrophic papulosis can be distinguished by hallmark skin findings, including 0.5- to 1-cm papular skin lesions with central porcelain-white atrophy and an erythematous, telangiectatic rim.1 It usually manifests between ages 20 to 50 but can occur in infants and children.1 The etiology is unknown, but case evidence suggests the condition is sometimes familial.1,2
Easy to confuse with common conditions
Clinical presentation of atrophic papulosis can vary, but evaluation should rule out systemic lupus erythematosus and other connective tissue diseases.1 In addition, the lesions can easily be confused with other common conditions such as molluscum contagiosum or insect bites.
The hallmark finding of molluscum contagiosum is raised papules with central umbilication, whereas atrophic papulosis lesions are characterized by white centers. While insect bites typically disappear within weeks, atrophic papulosis lesions persist for years or are even lifelong.1
Is it benign or malignant?
Benign atrophic papulosis is limited to the skin.1 The probability of a patient having benign atrophic papulosis is about 70% at the onset of skin lesions and 97% after 7 years without other symptoms.2
Malignant atrophic papulosis—although less common—is systemic and life-threatening. About 30% of patients with atrophic papulosis develop lesions manifesting both on the skin and in internal organs.1,2 Systemic involvement can develop at any time, sometimes years after the appearance of skin lesions, but the risk declines over time.2 In a case series, systemic signs were shown to develop, on average, 1 year after skin lesions.2 Some evidence suggests a mortality rate of 50% within 2 to 3 years of the onset of systemic involvement, making regular follow-up necessary.1
Continue to: Patients with malignant atrophic papulosis...
Patients with malignant atrophic papulosis may have systemic involvement in multiple organ systems. Gastrointestinal (GI) involvement can cause bowel perforation. Central nervous system (CNS) involvement may put the patient at risk for stroke, intracranial bleeding, meningitis, and encephalitis.1,3 There can also be cardiopulmonary involvement that causes pleuritis and pericarditis.1 Ocular involvement can affect the eyelids, conjunctiva, retina, sclera, and choroid plexus.1 Renal involvement has been noted in a few cases.2
In a prospective, single-center cohort study of 39 patients with atrophic papulosis, systemic involvement (malignant atrophic papulosis) was reported in 29% (n = 11) of the patients.2 In these patients, involved organ systems included the GI tract (73%; n = 8), CNS (64%; n = 7), eye (18%; n = 2), heart (18%; n = 2), and lungs (9%; n = 1); 64% (n = 7) had multiorgan involvement. Mortality was reported in 73% of the patients with systemic disease.
Ongoing testing is required
For a patient presenting with atrophic papulosis, initial and follow-up visits should include evaluation for systemic manifestations through a full skin examination, fecal occult blood test, and ocular fundus examination.1,2 If the patient shows any symptoms that suggest systemic involvement, further testing is advised, including evaluation of renal function, colonoscopy, endoscopy, magnetic resonance imaging of the brain, an echocardiogram, and chest computed tomography.
Because internal organ involvement in malignant atrophic papulosis can develop within years of (benign) cutaneous manifestations, regular follow-up is recommended.1 Research suggests evaluation of patients with benign atrophic papulosis every 6 months for the first 7 years after disease onset and then yearly between 7 and 10 years after onset.2
Treatment options are limited
Antiplatelet agents (aspirin, pentoxifylline, dipyridamole, and ticlodipine) and anticoagulants (heparin) have led to partial regression of skin lesions in case reports.1 Some lesions seem to disappear after treatment, but due to limited evidence, it is difficult to determine whether treatment leads to a reduction of future lesions.
When it comes to malignant atrophic papulosis, there is no uniformly effective treatment. Antiplatelet agents and anticoagulants are often used as initial treatment, but efficacy has not been clearly demonstrated. In case reports, eculizumab and treprostinil have shown effectiveness in treating CNS involvement, but there are no uniform dosage recommendations.3,4
In this case, the patient had mild GI symptoms. A colonoscopy showed evidence of microscopic colitis, but there was no evidence of atrophic papulosis in the GI tract.
Additional laboratory work-up was ordered to evaluate for signs of organ involvement and to rule out any associated connective tissue disease or hypercoagulable state. Her results showed a mildly elevated erythrocyte sedimentation rate (29 mm/h) and a positive antinuclear antibodies assay (1:640, speckled pattern). She was referred to a rheumatologist, who found no evidence of a connective tissue disorder. A complete blood count, comprehensive metabolic panel, urinalysis, and hypercoagulability work-up were all within normal limits. A complete eye exam was also normal.
The patient was started on aspirin 81 mg/d. Because she continued to develop new lesions, her dermatologist added pentoxifylline extended release and gradually increased the dose to 400 mg in the morning and 800 mg in the evening. About 4 years after onset of the rash, the patient showed no signs of systemic involvement, but her skin lesions were still present.
1. Theodoridis A, Makrantonaki E, Zouboulis CC, et al. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10.
2. Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115.
3. Huang YC, Wang JD, Lee FY, et al. Pediatric malignant atrophic papulosis. Pediatrics. 2018;141(suppl 5):S481-S484.
4. Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52.
A 50-year-old woman presented to her family physician for a urinary tract infection (UTI) and an itchy rash. She said the rash had developed 2 years earlier and had gotten worse, with additional lesions emerging on her skin as time went on. She noted that other physicians had evaluated the rash but provided no clear diagnosis and had done no testing.
A physical exam revealed scattered erythematous papules with white centers on the patient’s trunk, arms, and legs (FIGURE 1). The patient’s medical history was significant for asthma, obstructive sleep apnea, obesity, gastroesophageal reflux disease, urinary incontinence, and depression. Her medications included montelukast, inhaled fluticasone, albuterol, tolterodine, omeprazole, and fluoxetine.

The patient was prescribed nitrofurantoin, 100 mg twice daily for 5 days, to treat her UTI, and a punch biopsy was performed on one of the patient’s lesions to determine the cause of the rash.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophic papulosis
Pathology suggested a diagnosis of atrophic papulosis. A consultation with a dermatologist and additional biopsies confirmed the diagnosis. The biopsies showed wedge-shaped areas of superficial dermal sclerosis with thinning of the overlying epidermis. The superficial dermal vessels contained scattered, small thrombi at the periphery of the areas of sclerosis.
Atrophic papulosis (also known as Degos disease or Kölmeier-Degos disease) is a vasculopathy characterized by thrombotic occlusion of small arteries.1 Although rare—with fewer than 200 published case reports in the literature—it is likely underdiagnosed.1 Atrophic papulosis can be distinguished by hallmark skin findings, including 0.5- to 1-cm papular skin lesions with central porcelain-white atrophy and an erythematous, telangiectatic rim.1 It usually manifests between ages 20 to 50 but can occur in infants and children.1 The etiology is unknown, but case evidence suggests the condition is sometimes familial.1,2
Easy to confuse with common conditions
Clinical presentation of atrophic papulosis can vary, but evaluation should rule out systemic lupus erythematosus and other connective tissue diseases.1 In addition, the lesions can easily be confused with other common conditions such as molluscum contagiosum or insect bites.
The hallmark finding of molluscum contagiosum is raised papules with central umbilication, whereas atrophic papulosis lesions are characterized by white centers. While insect bites typically disappear within weeks, atrophic papulosis lesions persist for years or are even lifelong.1
Is it benign or malignant?
Benign atrophic papulosis is limited to the skin.1 The probability of a patient having benign atrophic papulosis is about 70% at the onset of skin lesions and 97% after 7 years without other symptoms.2
Malignant atrophic papulosis—although less common—is systemic and life-threatening. About 30% of patients with atrophic papulosis develop lesions manifesting both on the skin and in internal organs.1,2 Systemic involvement can develop at any time, sometimes years after the appearance of skin lesions, but the risk declines over time.2 In a case series, systemic signs were shown to develop, on average, 1 year after skin lesions.2 Some evidence suggests a mortality rate of 50% within 2 to 3 years of the onset of systemic involvement, making regular follow-up necessary.1
Continue to: Patients with malignant atrophic papulosis...
Patients with malignant atrophic papulosis may have systemic involvement in multiple organ systems. Gastrointestinal (GI) involvement can cause bowel perforation. Central nervous system (CNS) involvement may put the patient at risk for stroke, intracranial bleeding, meningitis, and encephalitis.1,3 There can also be cardiopulmonary involvement that causes pleuritis and pericarditis.1 Ocular involvement can affect the eyelids, conjunctiva, retina, sclera, and choroid plexus.1 Renal involvement has been noted in a few cases.2
In a prospective, single-center cohort study of 39 patients with atrophic papulosis, systemic involvement (malignant atrophic papulosis) was reported in 29% (n = 11) of the patients.2 In these patients, involved organ systems included the GI tract (73%; n = 8), CNS (64%; n = 7), eye (18%; n = 2), heart (18%; n = 2), and lungs (9%; n = 1); 64% (n = 7) had multiorgan involvement. Mortality was reported in 73% of the patients with systemic disease.
Ongoing testing is required
For a patient presenting with atrophic papulosis, initial and follow-up visits should include evaluation for systemic manifestations through a full skin examination, fecal occult blood test, and ocular fundus examination.1,2 If the patient shows any symptoms that suggest systemic involvement, further testing is advised, including evaluation of renal function, colonoscopy, endoscopy, magnetic resonance imaging of the brain, an echocardiogram, and chest computed tomography.
Because internal organ involvement in malignant atrophic papulosis can develop within years of (benign) cutaneous manifestations, regular follow-up is recommended.1 Research suggests evaluation of patients with benign atrophic papulosis every 6 months for the first 7 years after disease onset and then yearly between 7 and 10 years after onset.2
Treatment options are limited
Antiplatelet agents (aspirin, pentoxifylline, dipyridamole, and ticlodipine) and anticoagulants (heparin) have led to partial regression of skin lesions in case reports.1 Some lesions seem to disappear after treatment, but due to limited evidence, it is difficult to determine whether treatment leads to a reduction of future lesions.
When it comes to malignant atrophic papulosis, there is no uniformly effective treatment. Antiplatelet agents and anticoagulants are often used as initial treatment, but efficacy has not been clearly demonstrated. In case reports, eculizumab and treprostinil have shown effectiveness in treating CNS involvement, but there are no uniform dosage recommendations.3,4
In this case, the patient had mild GI symptoms. A colonoscopy showed evidence of microscopic colitis, but there was no evidence of atrophic papulosis in the GI tract.
Additional laboratory work-up was ordered to evaluate for signs of organ involvement and to rule out any associated connective tissue disease or hypercoagulable state. Her results showed a mildly elevated erythrocyte sedimentation rate (29 mm/h) and a positive antinuclear antibodies assay (1:640, speckled pattern). She was referred to a rheumatologist, who found no evidence of a connective tissue disorder. A complete blood count, comprehensive metabolic panel, urinalysis, and hypercoagulability work-up were all within normal limits. A complete eye exam was also normal.
The patient was started on aspirin 81 mg/d. Because she continued to develop new lesions, her dermatologist added pentoxifylline extended release and gradually increased the dose to 400 mg in the morning and 800 mg in the evening. About 4 years after onset of the rash, the patient showed no signs of systemic involvement, but her skin lesions were still present.
A 50-year-old woman presented to her family physician for a urinary tract infection (UTI) and an itchy rash. She said the rash had developed 2 years earlier and had gotten worse, with additional lesions emerging on her skin as time went on. She noted that other physicians had evaluated the rash but provided no clear diagnosis and had done no testing.
A physical exam revealed scattered erythematous papules with white centers on the patient’s trunk, arms, and legs (FIGURE 1). The patient’s medical history was significant for asthma, obstructive sleep apnea, obesity, gastroesophageal reflux disease, urinary incontinence, and depression. Her medications included montelukast, inhaled fluticasone, albuterol, tolterodine, omeprazole, and fluoxetine.

The patient was prescribed nitrofurantoin, 100 mg twice daily for 5 days, to treat her UTI, and a punch biopsy was performed on one of the patient’s lesions to determine the cause of the rash.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophic papulosis
Pathology suggested a diagnosis of atrophic papulosis. A consultation with a dermatologist and additional biopsies confirmed the diagnosis. The biopsies showed wedge-shaped areas of superficial dermal sclerosis with thinning of the overlying epidermis. The superficial dermal vessels contained scattered, small thrombi at the periphery of the areas of sclerosis.
Atrophic papulosis (also known as Degos disease or Kölmeier-Degos disease) is a vasculopathy characterized by thrombotic occlusion of small arteries.1 Although rare—with fewer than 200 published case reports in the literature—it is likely underdiagnosed.1 Atrophic papulosis can be distinguished by hallmark skin findings, including 0.5- to 1-cm papular skin lesions with central porcelain-white atrophy and an erythematous, telangiectatic rim.1 It usually manifests between ages 20 to 50 but can occur in infants and children.1 The etiology is unknown, but case evidence suggests the condition is sometimes familial.1,2
Easy to confuse with common conditions
Clinical presentation of atrophic papulosis can vary, but evaluation should rule out systemic lupus erythematosus and other connective tissue diseases.1 In addition, the lesions can easily be confused with other common conditions such as molluscum contagiosum or insect bites.
The hallmark finding of molluscum contagiosum is raised papules with central umbilication, whereas atrophic papulosis lesions are characterized by white centers. While insect bites typically disappear within weeks, atrophic papulosis lesions persist for years or are even lifelong.1
Is it benign or malignant?
Benign atrophic papulosis is limited to the skin.1 The probability of a patient having benign atrophic papulosis is about 70% at the onset of skin lesions and 97% after 7 years without other symptoms.2
Malignant atrophic papulosis—although less common—is systemic and life-threatening. About 30% of patients with atrophic papulosis develop lesions manifesting both on the skin and in internal organs.1,2 Systemic involvement can develop at any time, sometimes years after the appearance of skin lesions, but the risk declines over time.2 In a case series, systemic signs were shown to develop, on average, 1 year after skin lesions.2 Some evidence suggests a mortality rate of 50% within 2 to 3 years of the onset of systemic involvement, making regular follow-up necessary.1
Continue to: Patients with malignant atrophic papulosis...
Patients with malignant atrophic papulosis may have systemic involvement in multiple organ systems. Gastrointestinal (GI) involvement can cause bowel perforation. Central nervous system (CNS) involvement may put the patient at risk for stroke, intracranial bleeding, meningitis, and encephalitis.1,3 There can also be cardiopulmonary involvement that causes pleuritis and pericarditis.1 Ocular involvement can affect the eyelids, conjunctiva, retina, sclera, and choroid plexus.1 Renal involvement has been noted in a few cases.2
In a prospective, single-center cohort study of 39 patients with atrophic papulosis, systemic involvement (malignant atrophic papulosis) was reported in 29% (n = 11) of the patients.2 In these patients, involved organ systems included the GI tract (73%; n = 8), CNS (64%; n = 7), eye (18%; n = 2), heart (18%; n = 2), and lungs (9%; n = 1); 64% (n = 7) had multiorgan involvement. Mortality was reported in 73% of the patients with systemic disease.
Ongoing testing is required
For a patient presenting with atrophic papulosis, initial and follow-up visits should include evaluation for systemic manifestations through a full skin examination, fecal occult blood test, and ocular fundus examination.1,2 If the patient shows any symptoms that suggest systemic involvement, further testing is advised, including evaluation of renal function, colonoscopy, endoscopy, magnetic resonance imaging of the brain, an echocardiogram, and chest computed tomography.
Because internal organ involvement in malignant atrophic papulosis can develop within years of (benign) cutaneous manifestations, regular follow-up is recommended.1 Research suggests evaluation of patients with benign atrophic papulosis every 6 months for the first 7 years after disease onset and then yearly between 7 and 10 years after onset.2
Treatment options are limited
Antiplatelet agents (aspirin, pentoxifylline, dipyridamole, and ticlodipine) and anticoagulants (heparin) have led to partial regression of skin lesions in case reports.1 Some lesions seem to disappear after treatment, but due to limited evidence, it is difficult to determine whether treatment leads to a reduction of future lesions.
When it comes to malignant atrophic papulosis, there is no uniformly effective treatment. Antiplatelet agents and anticoagulants are often used as initial treatment, but efficacy has not been clearly demonstrated. In case reports, eculizumab and treprostinil have shown effectiveness in treating CNS involvement, but there are no uniform dosage recommendations.3,4
In this case, the patient had mild GI symptoms. A colonoscopy showed evidence of microscopic colitis, but there was no evidence of atrophic papulosis in the GI tract.
Additional laboratory work-up was ordered to evaluate for signs of organ involvement and to rule out any associated connective tissue disease or hypercoagulable state. Her results showed a mildly elevated erythrocyte sedimentation rate (29 mm/h) and a positive antinuclear antibodies assay (1:640, speckled pattern). She was referred to a rheumatologist, who found no evidence of a connective tissue disorder. A complete blood count, comprehensive metabolic panel, urinalysis, and hypercoagulability work-up were all within normal limits. A complete eye exam was also normal.
The patient was started on aspirin 81 mg/d. Because she continued to develop new lesions, her dermatologist added pentoxifylline extended release and gradually increased the dose to 400 mg in the morning and 800 mg in the evening. About 4 years after onset of the rash, the patient showed no signs of systemic involvement, but her skin lesions were still present.
1. Theodoridis A, Makrantonaki E, Zouboulis CC, et al. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10.
2. Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115.
3. Huang YC, Wang JD, Lee FY, et al. Pediatric malignant atrophic papulosis. Pediatrics. 2018;141(suppl 5):S481-S484.
4. Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52.
1. Theodoridis A, Makrantonaki E, Zouboulis CC, et al. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10.
2. Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115.
3. Huang YC, Wang JD, Lee FY, et al. Pediatric malignant atrophic papulosis. Pediatrics. 2018;141(suppl 5):S481-S484.
4. Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52.
Itchy scalp with scale
An 11-year-old boy sought care at a small village’s health center in Panama for scalp itching and subtle hair loss. He was seen by a family physician (RU) and a team of medical students who were there as part of a humanitarian trip. The patient denied any hair pulling. He had a history of treatment for head lice.
Our physical examination revealed mild alopecia and scaling on the scalp (FIGURE 1), but what we saw through the dermatoscope (FIGURE 2) made the diagnosis clear.
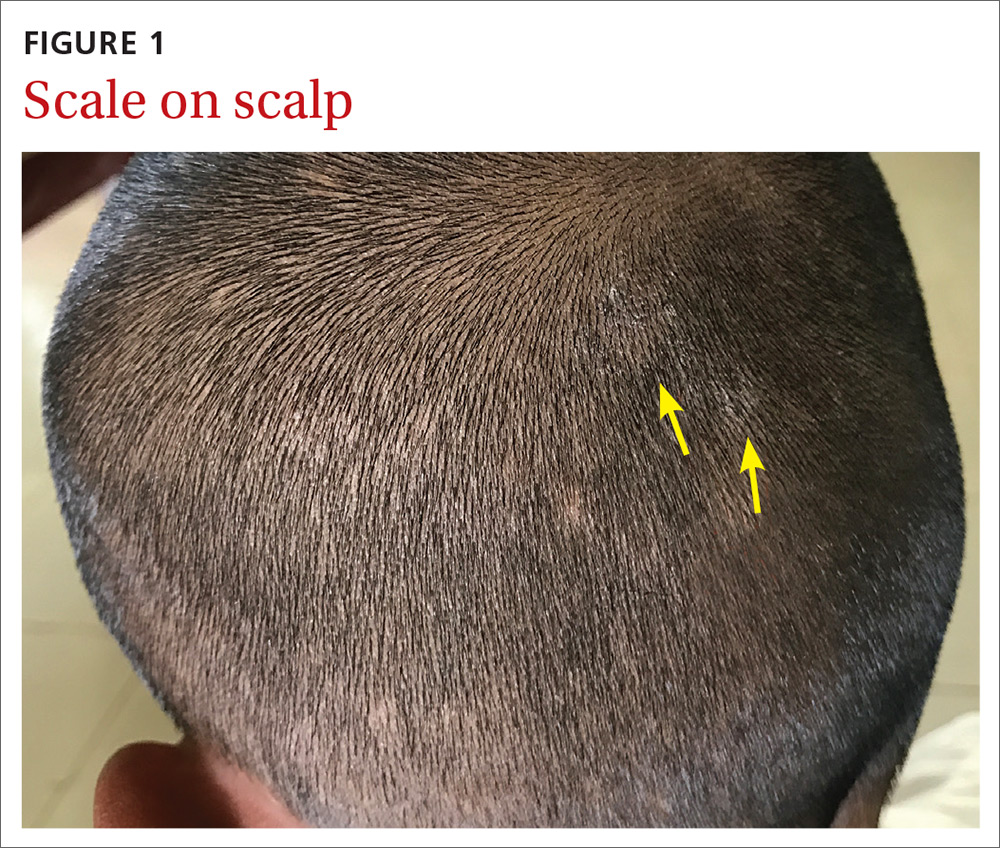
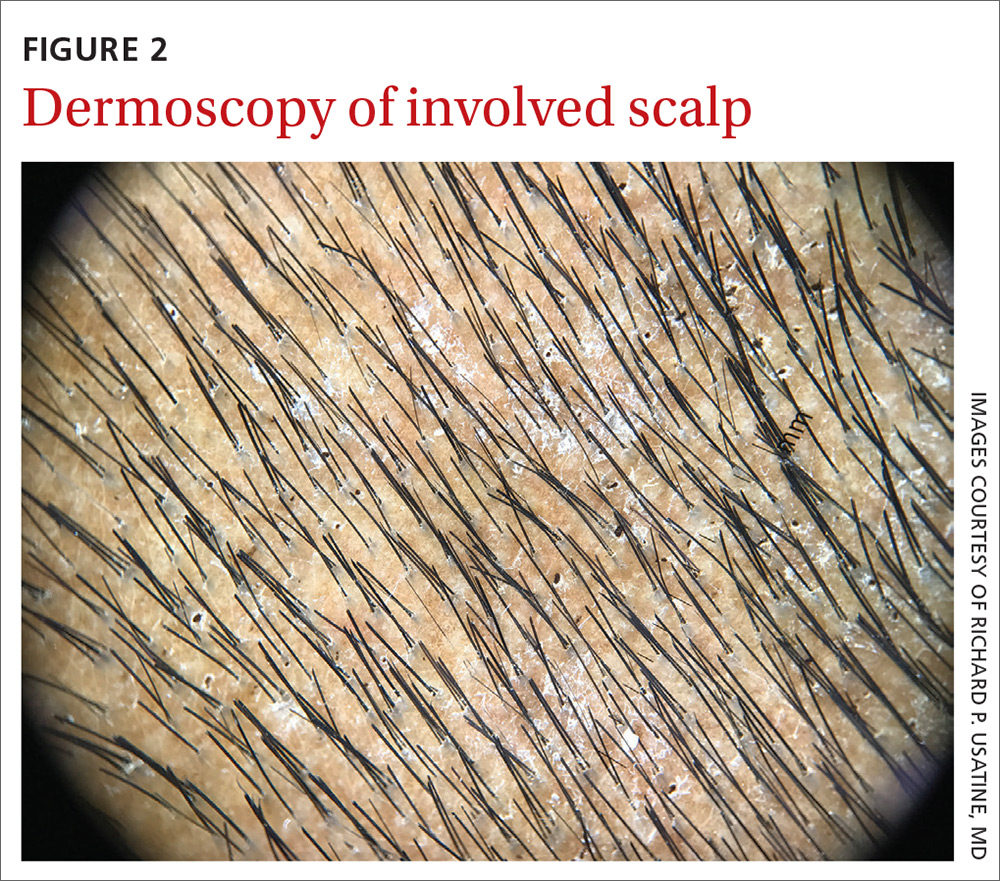
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tinea capitis
On dermatoscopic examination (10× magnification), there were numerous “black dots” or broken hair shafts within patches of hair loss (FIGURE 3), which is indicative of tinea capitis.1,2 This condition causes hair shafts to break, creating “comma hairs” and black dots. The hairs are uniform in thickness and color and bend distally, like a comma.3
Tinea capitis (commonly called ringworm of the scalp) is a fungal infection caused by Trichophyton and Microsporum dermatophytes. It is the most common pediatric dermatophyte infection in the world; the usual age of onset is 5 to 10 years.2 The incidence of tinea capitis in the United States is not known because cases are no longer registered by public health agencies. That said, a Northern California study that tracked occurrences in children younger than 15 years from 1998 to 2007 found that the incidence was on the decline and lower in girls compared to boys (111.9 vs 146.4, respectively, in 1998; 27.9 vs 39.9, respectively, in 2007).4 Incidence rates were calculated per 10,000 eligible children.4
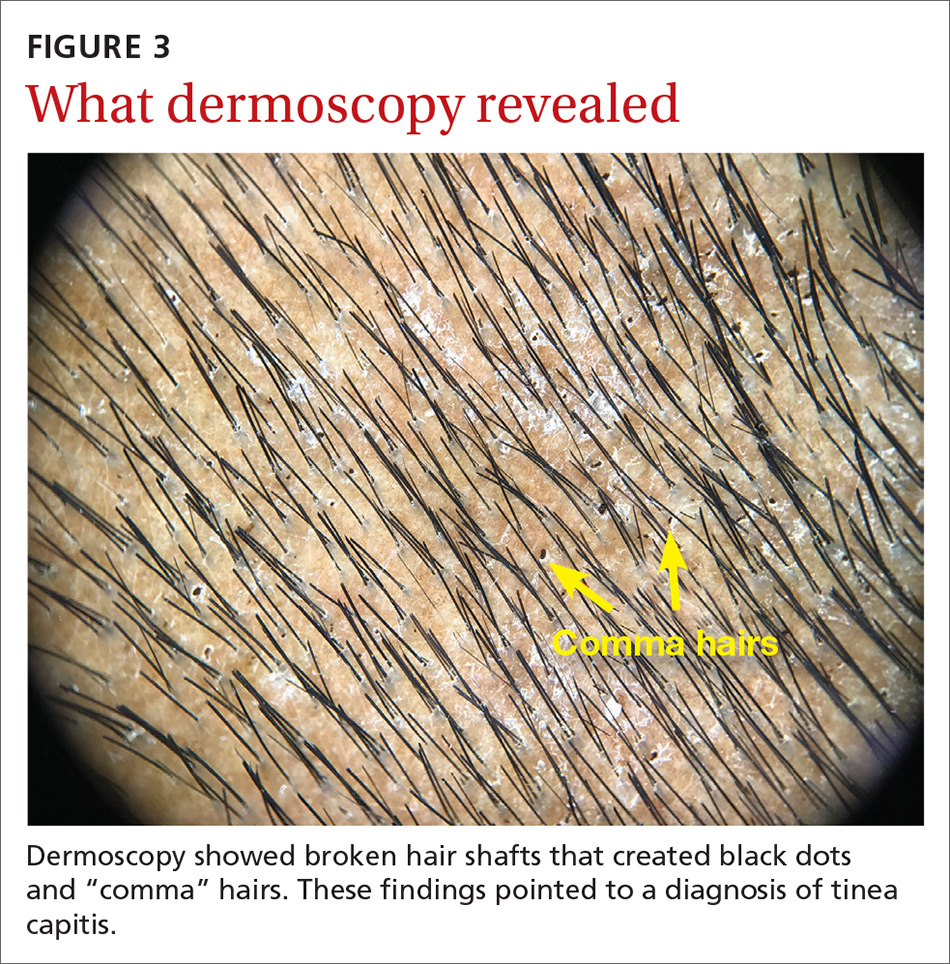
Tinea capitis can spread by contact with infected individuals and contaminated objects, including combs, towels, toys, and bedding.1 Fungal spores can remain viable on these surfaces for months.
In a study of 69 patients with tinea capitis (23 females, 46 males; mean age, 12 years), the risk factors for spreading infection included participation in sports, contact with an animal, a recent haircut, and use of a swimming pool.5
4 conditions you’ll want to rule out
The following conditions should be considered as part of the differential when a patient presents with an itchy scalp and/or hair loss.
Continue to: Psoriasis of the scalp...
Psoriasis of the scalp is characterized by scaling of the scalp along with crusted plaques. It is often accompanied by similar psoriatic plaques on the elbows, knees, and other areas of the body. Examination of our patient showed no psoriatic plaques.
Seborrhea of the scalp (also known as dandruff) is a very common diagnosis. However, it is unlikely to cause hair loss. It has widespread involvement of the scalp compared to tinea capitis, which is local and patchy. Our patient’s patches of hair loss indicated that seborrhea was unlikely.
Alopecia areata. Individuals develop this condition due to an autoimmune process affecting hair follicles. However, the resulting hair loss does not cause significant scaling, inflammation, scarring, or pain in the affected area. Further, this condition can cause the loss of the entire hair shaft.
Trichotillomania is an impulse control disorder that causes patients to pull out their own hair. There is no scaling of the scalp in this condition.
A dermatoscope can beuseful in making the Dx
Although clinical appearance and patient presentation are adequate to establish the diagnosis of tinea capitis, this case demonstrates the utility of a dermatoscope in making the diagnosis of tinea capitis. Previous studies have shown that dermoscopy allows for rapid identification of the broken hair shafts, which are a key distinction from alopecia areata.3,6
Microscopic inspection. Samples from the scaling of the scalp can be examined with potassium hydroxide (KOH) on a microscope slide. Hyphae, spores, and endo/ectothrix invasion can be seen through the microsope.
Continue to: Laboratory testing is helpful, but not needed.
Laboratory testing is helpful, but not needed. Testing for tinea capitis would require that you obtain a sample from the affected area using a swab, edge of a scalpel blade, or scalp brush.7 Because treatment can require weeks of medication, diagnosis should be confirmed with a KOH or culture when possible.
Newer antifungalsprovide a Tx advantage
Oral antifungal medications are the treatment of choice for tinea capitis. Newer antifungals, such as terbinafine and fluconazole, require a 3- to 6-week course compared to the standard 6- to 8-week course of griseofulvin.1 Also, antifungal shampoos—such as those that contain selenium sulfide—may be used for topical treatment but only as adjuvant therapy.1,2
For our patient, we dispensed a 3-week course of oral fluconazole, 3 to 6 mg/kg, to be given daily by his parents. We also recommended the use of an antidandruff shampoo, if possible. The treatment outcome was not known because our team’s humanitarian global health trip had ended.
1. Usatine R, Smith MA, Mayeaux Jr EJ, Chumley HS. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019.
2. Handler MZ. Tinea capitis. Medscape. https://emedicine.medscape.com/article/1091351-overview. Updated February 21, 2020. Accessed November 30, 2020.
3. Hernández-Bel P, Malvehy J, Crocker A, et al. Comma hairs: a new dermoscopic marker for tinea capitis [in Spanish]. Actas Dermosifiliogr. 2012;103:836-837.
4. Mirmirani P, Lue-Yen T. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California. J Am Acad Dermatol. 2013;69:916-921.
5. Mikaeili A, Kavaoussi H, Hashemian AH, et al. Clinico-mycological profile of tinea capitis and its comparative response to griseofulvin versus terbinafine. Curr Med Mycol. 2019;5:15-20.
6. Slowinska M, Rudnicka L, Schwartz RA, et al. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. Journal of the American Academy of Dermatology. 2008;59(suppl 5):S77-S79.
An 11-year-old boy sought care at a small village’s health center in Panama for scalp itching and subtle hair loss. He was seen by a family physician (RU) and a team of medical students who were there as part of a humanitarian trip. The patient denied any hair pulling. He had a history of treatment for head lice.
Our physical examination revealed mild alopecia and scaling on the scalp (FIGURE 1), but what we saw through the dermatoscope (FIGURE 2) made the diagnosis clear.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tinea capitis
On dermatoscopic examination (10× magnification), there were numerous “black dots” or broken hair shafts within patches of hair loss (FIGURE 3), which is indicative of tinea capitis.1,2 This condition causes hair shafts to break, creating “comma hairs” and black dots. The hairs are uniform in thickness and color and bend distally, like a comma.3
Tinea capitis (commonly called ringworm of the scalp) is a fungal infection caused by Trichophyton and Microsporum dermatophytes. It is the most common pediatric dermatophyte infection in the world; the usual age of onset is 5 to 10 years.2 The incidence of tinea capitis in the United States is not known because cases are no longer registered by public health agencies. That said, a Northern California study that tracked occurrences in children younger than 15 years from 1998 to 2007 found that the incidence was on the decline and lower in girls compared to boys (111.9 vs 146.4, respectively, in 1998; 27.9 vs 39.9, respectively, in 2007).4 Incidence rates were calculated per 10,000 eligible children.4

Tinea capitis can spread by contact with infected individuals and contaminated objects, including combs, towels, toys, and bedding.1 Fungal spores can remain viable on these surfaces for months.
In a study of 69 patients with tinea capitis (23 females, 46 males; mean age, 12 years), the risk factors for spreading infection included participation in sports, contact with an animal, a recent haircut, and use of a swimming pool.5
4 conditions you’ll want to rule out
The following conditions should be considered as part of the differential when a patient presents with an itchy scalp and/or hair loss.
Continue to: Psoriasis of the scalp...
Psoriasis of the scalp is characterized by scaling of the scalp along with crusted plaques. It is often accompanied by similar psoriatic plaques on the elbows, knees, and other areas of the body. Examination of our patient showed no psoriatic plaques.
Seborrhea of the scalp (also known as dandruff) is a very common diagnosis. However, it is unlikely to cause hair loss. It has widespread involvement of the scalp compared to tinea capitis, which is local and patchy. Our patient’s patches of hair loss indicated that seborrhea was unlikely.
Alopecia areata. Individuals develop this condition due to an autoimmune process affecting hair follicles. However, the resulting hair loss does not cause significant scaling, inflammation, scarring, or pain in the affected area. Further, this condition can cause the loss of the entire hair shaft.
Trichotillomania is an impulse control disorder that causes patients to pull out their own hair. There is no scaling of the scalp in this condition.
A dermatoscope can beuseful in making the Dx
Although clinical appearance and patient presentation are adequate to establish the diagnosis of tinea capitis, this case demonstrates the utility of a dermatoscope in making the diagnosis of tinea capitis. Previous studies have shown that dermoscopy allows for rapid identification of the broken hair shafts, which are a key distinction from alopecia areata.3,6
Microscopic inspection. Samples from the scaling of the scalp can be examined with potassium hydroxide (KOH) on a microscope slide. Hyphae, spores, and endo/ectothrix invasion can be seen through the microsope.
Continue to: Laboratory testing is helpful, but not needed.
Laboratory testing is helpful, but not needed. Testing for tinea capitis would require that you obtain a sample from the affected area using a swab, edge of a scalpel blade, or scalp brush.7 Because treatment can require weeks of medication, diagnosis should be confirmed with a KOH or culture when possible.
Newer antifungalsprovide a Tx advantage
Oral antifungal medications are the treatment of choice for tinea capitis. Newer antifungals, such as terbinafine and fluconazole, require a 3- to 6-week course compared to the standard 6- to 8-week course of griseofulvin.1 Also, antifungal shampoos—such as those that contain selenium sulfide—may be used for topical treatment but only as adjuvant therapy.1,2
For our patient, we dispensed a 3-week course of oral fluconazole, 3 to 6 mg/kg, to be given daily by his parents. We also recommended the use of an antidandruff shampoo, if possible. The treatment outcome was not known because our team’s humanitarian global health trip had ended.
An 11-year-old boy sought care at a small village’s health center in Panama for scalp itching and subtle hair loss. He was seen by a family physician (RU) and a team of medical students who were there as part of a humanitarian trip. The patient denied any hair pulling. He had a history of treatment for head lice.
Our physical examination revealed mild alopecia and scaling on the scalp (FIGURE 1), but what we saw through the dermatoscope (FIGURE 2) made the diagnosis clear.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tinea capitis
On dermatoscopic examination (10× magnification), there were numerous “black dots” or broken hair shafts within patches of hair loss (FIGURE 3), which is indicative of tinea capitis.1,2 This condition causes hair shafts to break, creating “comma hairs” and black dots. The hairs are uniform in thickness and color and bend distally, like a comma.3
Tinea capitis (commonly called ringworm of the scalp) is a fungal infection caused by Trichophyton and Microsporum dermatophytes. It is the most common pediatric dermatophyte infection in the world; the usual age of onset is 5 to 10 years.2 The incidence of tinea capitis in the United States is not known because cases are no longer registered by public health agencies. That said, a Northern California study that tracked occurrences in children younger than 15 years from 1998 to 2007 found that the incidence was on the decline and lower in girls compared to boys (111.9 vs 146.4, respectively, in 1998; 27.9 vs 39.9, respectively, in 2007).4 Incidence rates were calculated per 10,000 eligible children.4

Tinea capitis can spread by contact with infected individuals and contaminated objects, including combs, towels, toys, and bedding.1 Fungal spores can remain viable on these surfaces for months.
In a study of 69 patients with tinea capitis (23 females, 46 males; mean age, 12 years), the risk factors for spreading infection included participation in sports, contact with an animal, a recent haircut, and use of a swimming pool.5
4 conditions you’ll want to rule out
The following conditions should be considered as part of the differential when a patient presents with an itchy scalp and/or hair loss.
Continue to: Psoriasis of the scalp...
Psoriasis of the scalp is characterized by scaling of the scalp along with crusted plaques. It is often accompanied by similar psoriatic plaques on the elbows, knees, and other areas of the body. Examination of our patient showed no psoriatic plaques.
Seborrhea of the scalp (also known as dandruff) is a very common diagnosis. However, it is unlikely to cause hair loss. It has widespread involvement of the scalp compared to tinea capitis, which is local and patchy. Our patient’s patches of hair loss indicated that seborrhea was unlikely.
Alopecia areata. Individuals develop this condition due to an autoimmune process affecting hair follicles. However, the resulting hair loss does not cause significant scaling, inflammation, scarring, or pain in the affected area. Further, this condition can cause the loss of the entire hair shaft.
Trichotillomania is an impulse control disorder that causes patients to pull out their own hair. There is no scaling of the scalp in this condition.
A dermatoscope can beuseful in making the Dx
Although clinical appearance and patient presentation are adequate to establish the diagnosis of tinea capitis, this case demonstrates the utility of a dermatoscope in making the diagnosis of tinea capitis. Previous studies have shown that dermoscopy allows for rapid identification of the broken hair shafts, which are a key distinction from alopecia areata.3,6
Microscopic inspection. Samples from the scaling of the scalp can be examined with potassium hydroxide (KOH) on a microscope slide. Hyphae, spores, and endo/ectothrix invasion can be seen through the microsope.
Continue to: Laboratory testing is helpful, but not needed.
Laboratory testing is helpful, but not needed. Testing for tinea capitis would require that you obtain a sample from the affected area using a swab, edge of a scalpel blade, or scalp brush.7 Because treatment can require weeks of medication, diagnosis should be confirmed with a KOH or culture when possible.
Newer antifungalsprovide a Tx advantage
Oral antifungal medications are the treatment of choice for tinea capitis. Newer antifungals, such as terbinafine and fluconazole, require a 3- to 6-week course compared to the standard 6- to 8-week course of griseofulvin.1 Also, antifungal shampoos—such as those that contain selenium sulfide—may be used for topical treatment but only as adjuvant therapy.1,2
For our patient, we dispensed a 3-week course of oral fluconazole, 3 to 6 mg/kg, to be given daily by his parents. We also recommended the use of an antidandruff shampoo, if possible. The treatment outcome was not known because our team’s humanitarian global health trip had ended.
1. Usatine R, Smith MA, Mayeaux Jr EJ, Chumley HS. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019.
2. Handler MZ. Tinea capitis. Medscape. https://emedicine.medscape.com/article/1091351-overview. Updated February 21, 2020. Accessed November 30, 2020.
3. Hernández-Bel P, Malvehy J, Crocker A, et al. Comma hairs: a new dermoscopic marker for tinea capitis [in Spanish]. Actas Dermosifiliogr. 2012;103:836-837.
4. Mirmirani P, Lue-Yen T. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California. J Am Acad Dermatol. 2013;69:916-921.
5. Mikaeili A, Kavaoussi H, Hashemian AH, et al. Clinico-mycological profile of tinea capitis and its comparative response to griseofulvin versus terbinafine. Curr Med Mycol. 2019;5:15-20.
6. Slowinska M, Rudnicka L, Schwartz RA, et al. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. Journal of the American Academy of Dermatology. 2008;59(suppl 5):S77-S79.
1. Usatine R, Smith MA, Mayeaux Jr EJ, Chumley HS. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019.
2. Handler MZ. Tinea capitis. Medscape. https://emedicine.medscape.com/article/1091351-overview. Updated February 21, 2020. Accessed November 30, 2020.
3. Hernández-Bel P, Malvehy J, Crocker A, et al. Comma hairs: a new dermoscopic marker for tinea capitis [in Spanish]. Actas Dermosifiliogr. 2012;103:836-837.
4. Mirmirani P, Lue-Yen T. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California. J Am Acad Dermatol. 2013;69:916-921.
5. Mikaeili A, Kavaoussi H, Hashemian AH, et al. Clinico-mycological profile of tinea capitis and its comparative response to griseofulvin versus terbinafine. Curr Med Mycol. 2019;5:15-20.
6. Slowinska M, Rudnicka L, Schwartz RA, et al. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. Journal of the American Academy of Dermatology. 2008;59(suppl 5):S77-S79.
How to identify and treat common bites and stings
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19
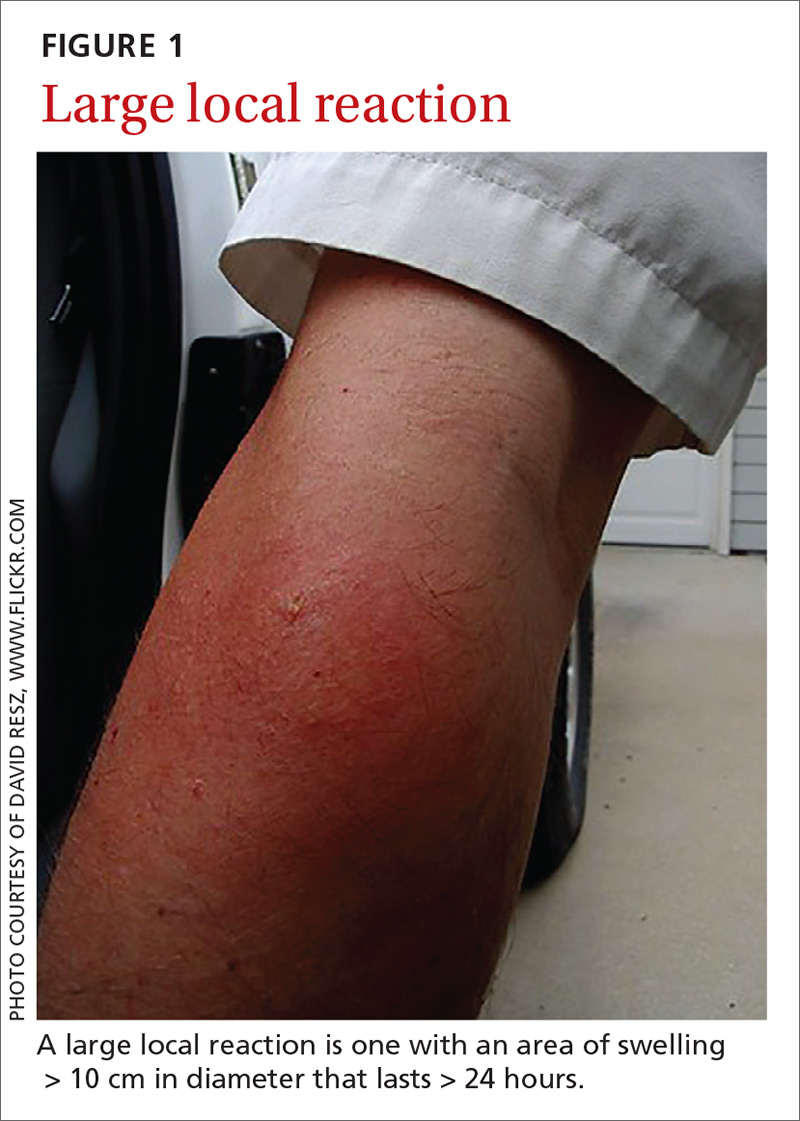
Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2
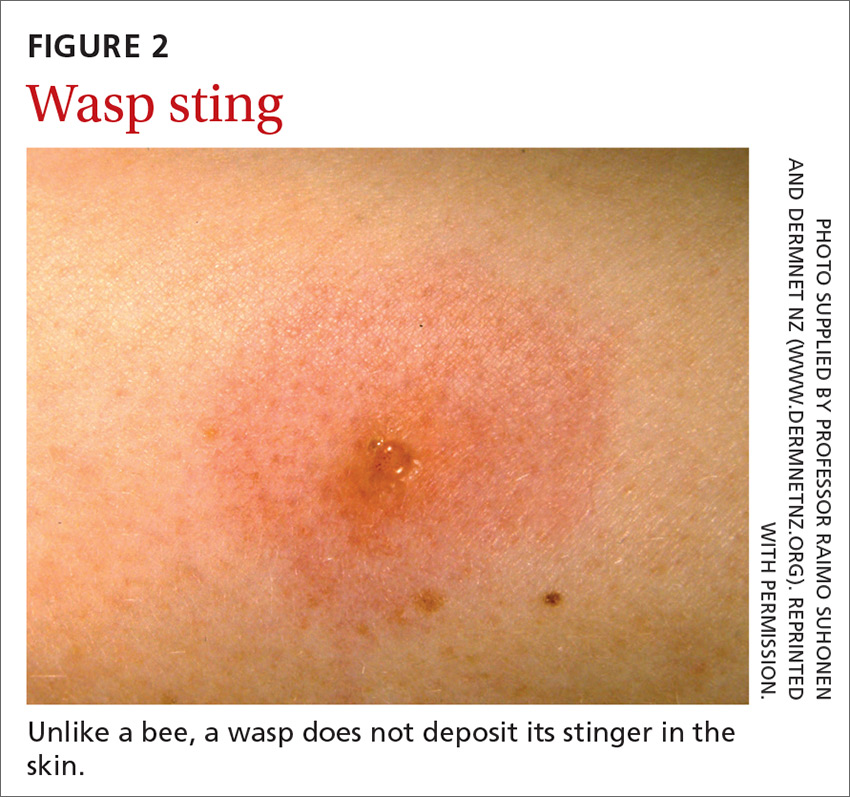
Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30
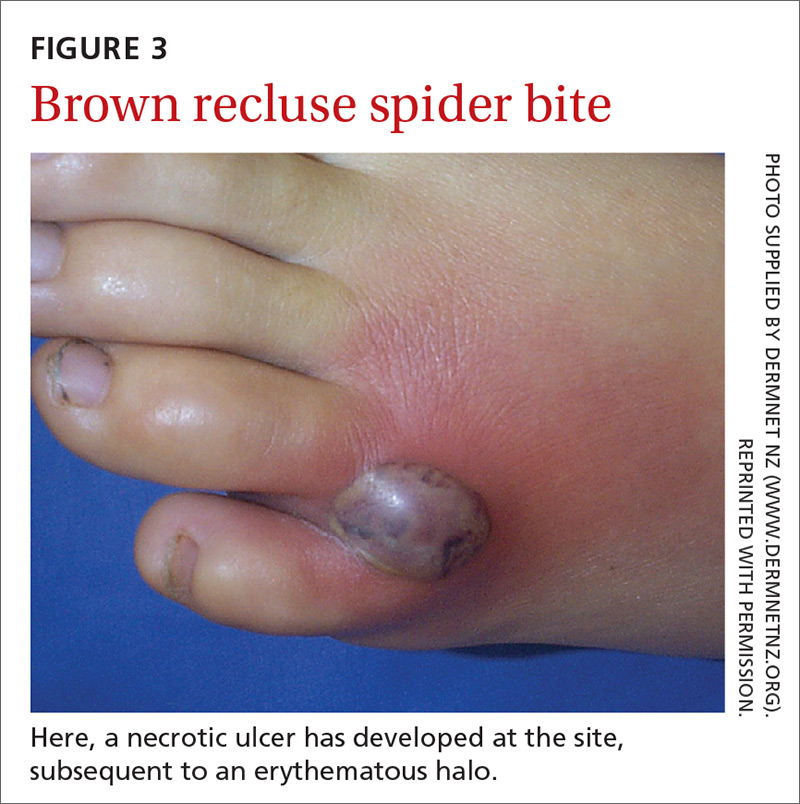
Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37
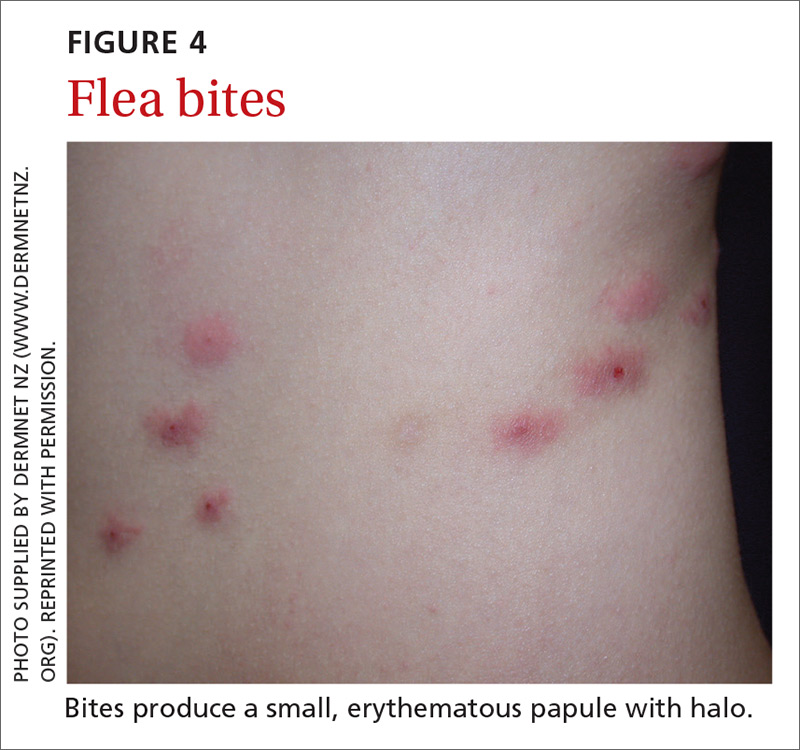
Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43
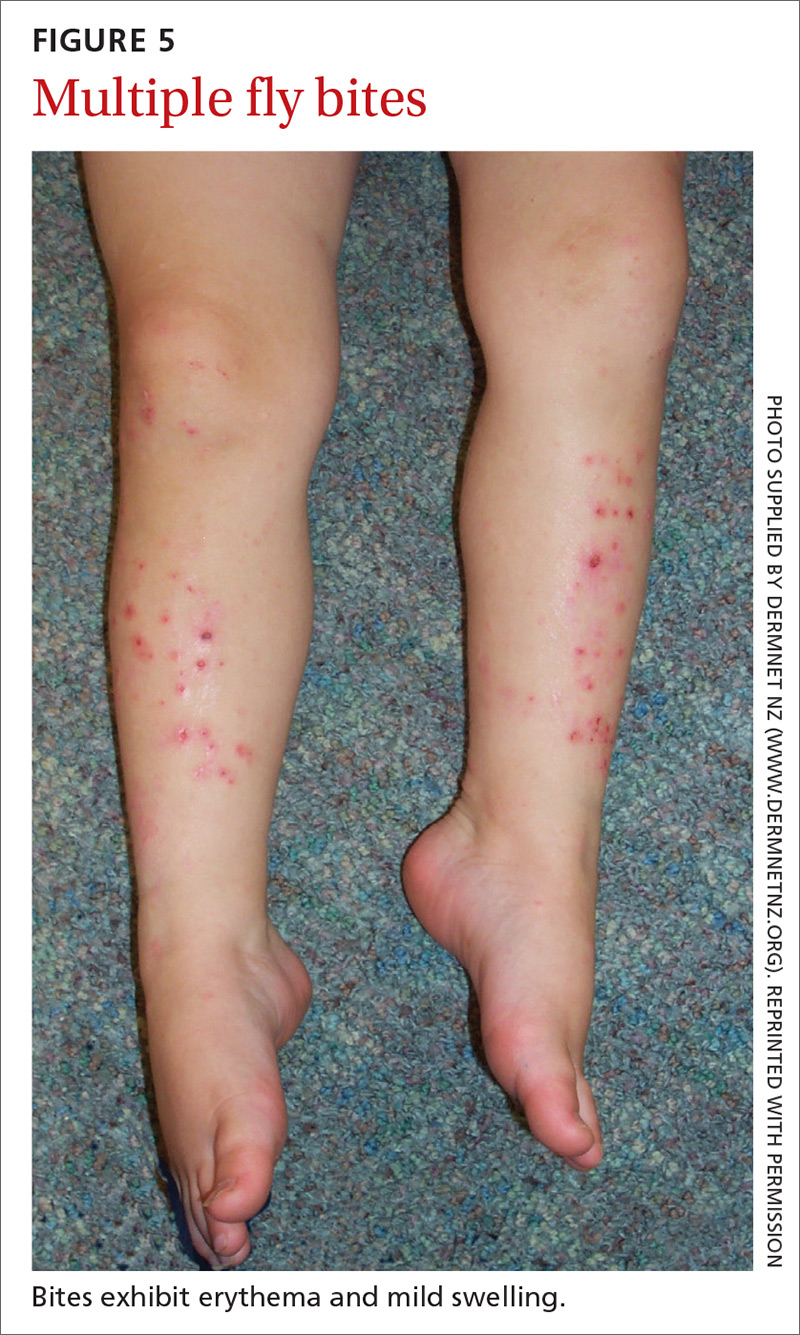
Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47
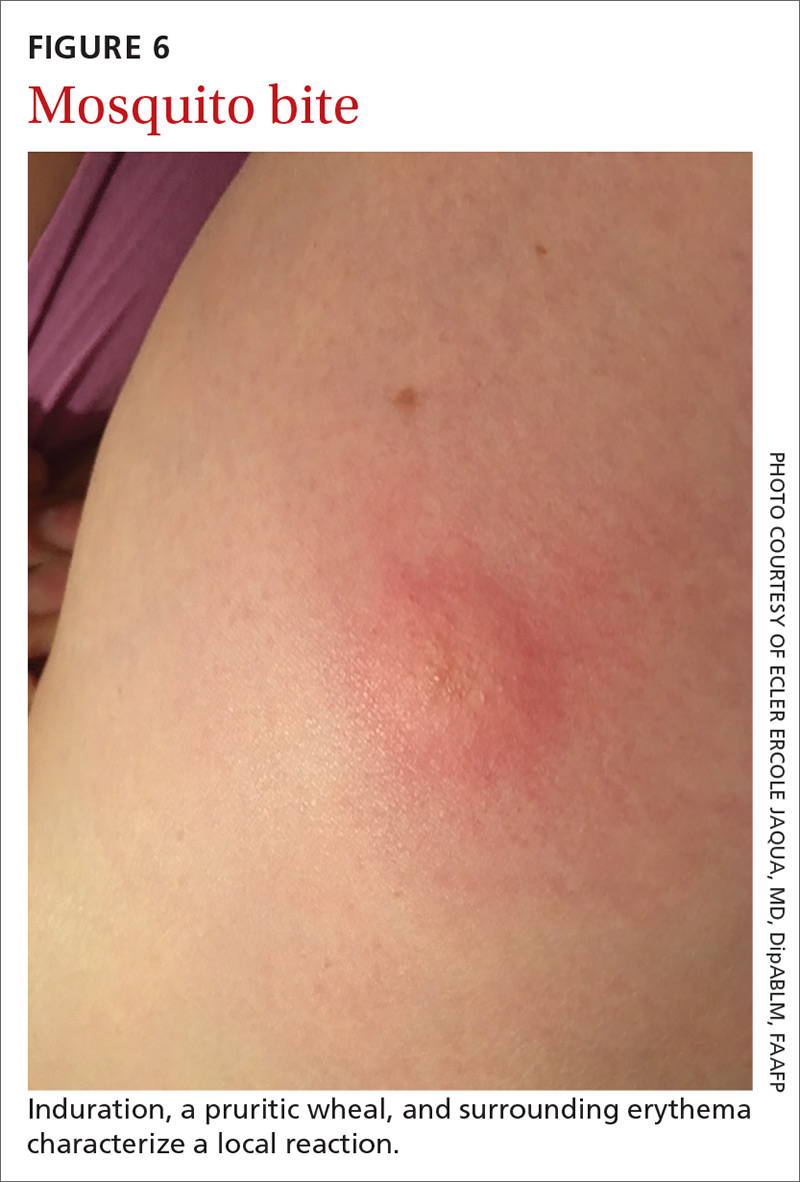
Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49
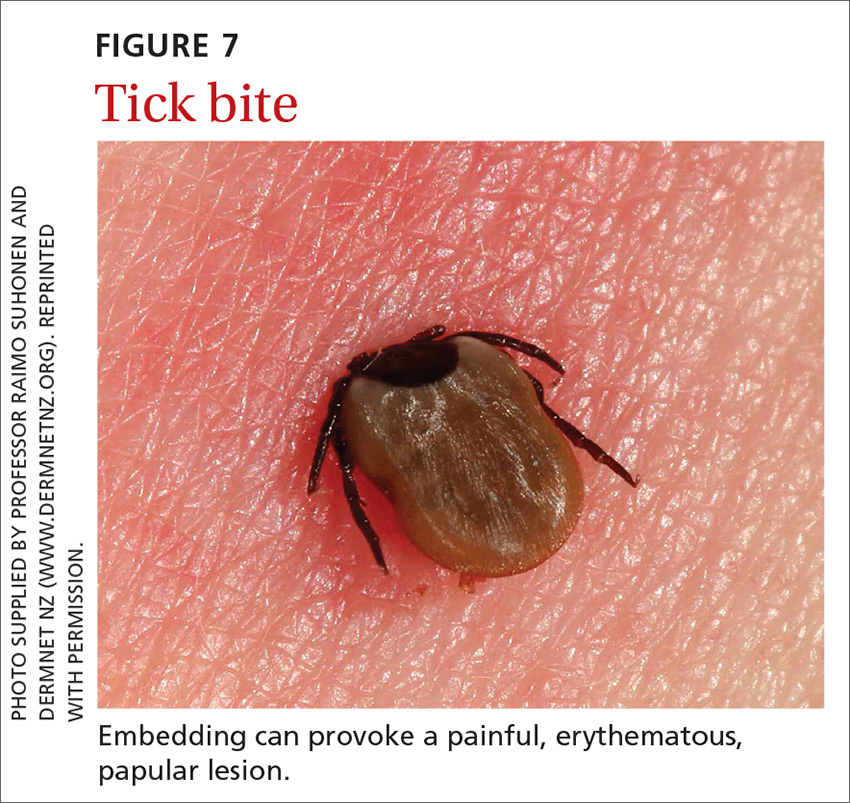
Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.
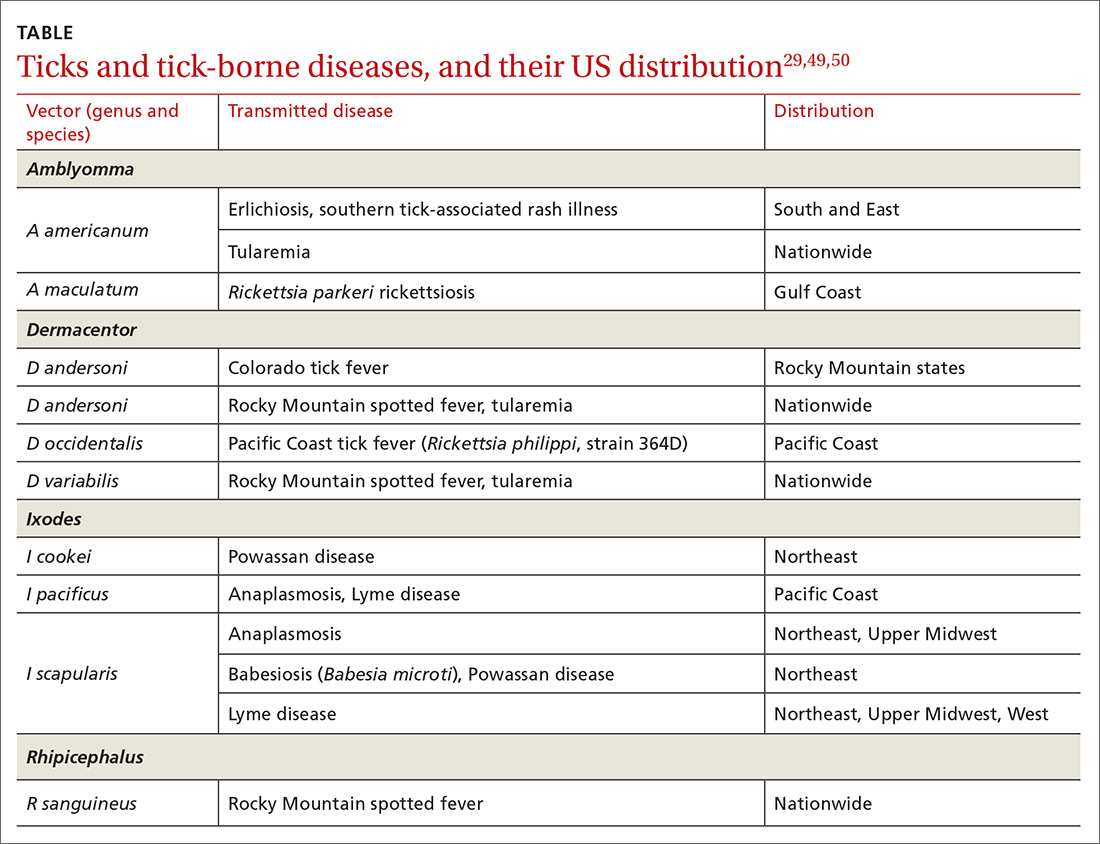
Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19

Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2

Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30

Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37

Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43

Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47

Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49

Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.

Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19

Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2

Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30

Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37

Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43

Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47

Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49

Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.

Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
PRACTICE RECOMMENDATIONS
❯ Recommend that patients use an insect repellent, such as an over-the-counter formulation that contains DEET, picaridin, or PMD (a chemical constituent of Eucalyptus citriodora oil) to prevent flea bites. C
❯ Prescribe nonsedating oral antihistamines as first-line symptomatic treatment of mild-to-moderate pruritus secondary to an insect bite. C
❯ When indicated, refer patients for venom immunotherapy, which is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis and death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antihistamine prescribing for AD varies by specialty
(AD), according to an analysis of a national database.
Dermatologists were more likely to prescribe sedating than nonsedating antihistamines (0.68 vs. 0.32) for patients with AD, but the reverse applied to nondermatologists, whose antihistamine distribution was 0.23 sedating and 0.77 nonsedating, based on 2011-2016 data from the National Ambulatory Medical Care Survey.
The numbers were similar for new antihistamine prescriptions, with sedating/nonsedating proportions of 0.60/0.40 for dermatologists and 0.24/0.76 for nondermatologists. Addition of guideline-recommended drugs such as topical corticosteroids and calcineurin inhibitors to the AD equation did not change the result, as dermatologists again showed a preference for sedating antihistamines, compared with nondermatologists, the investigators said.
The data also showed that Black patients with AD were more likely than were White patients to receive prescriptions for first-generation antihistamines and for therapies recommended by the AAD guidelines, and that patients under 21 years received more sedating antihistamines than did patients over age 21, they reported.
The age disparity “may be due to patient preference, as sedation effects may be less desirable to adult patients,” the investigators noted.
SOURCE: Garg S et al. Pediatr Dermatol. 2020 Nov 27. doi: 10.1111/pde.14445.
(AD), according to an analysis of a national database.

Dermatologists were more likely to prescribe sedating than nonsedating antihistamines (0.68 vs. 0.32) for patients with AD, but the reverse applied to nondermatologists, whose antihistamine distribution was 0.23 sedating and 0.77 nonsedating, based on 2011-2016 data from the National Ambulatory Medical Care Survey.
The numbers were similar for new antihistamine prescriptions, with sedating/nonsedating proportions of 0.60/0.40 for dermatologists and 0.24/0.76 for nondermatologists. Addition of guideline-recommended drugs such as topical corticosteroids and calcineurin inhibitors to the AD equation did not change the result, as dermatologists again showed a preference for sedating antihistamines, compared with nondermatologists, the investigators said.
The data also showed that Black patients with AD were more likely than were White patients to receive prescriptions for first-generation antihistamines and for therapies recommended by the AAD guidelines, and that patients under 21 years received more sedating antihistamines than did patients over age 21, they reported.
The age disparity “may be due to patient preference, as sedation effects may be less desirable to adult patients,” the investigators noted.
SOURCE: Garg S et al. Pediatr Dermatol. 2020 Nov 27. doi: 10.1111/pde.14445.
(AD), according to an analysis of a national database.

Dermatologists were more likely to prescribe sedating than nonsedating antihistamines (0.68 vs. 0.32) for patients with AD, but the reverse applied to nondermatologists, whose antihistamine distribution was 0.23 sedating and 0.77 nonsedating, based on 2011-2016 data from the National Ambulatory Medical Care Survey.
The numbers were similar for new antihistamine prescriptions, with sedating/nonsedating proportions of 0.60/0.40 for dermatologists and 0.24/0.76 for nondermatologists. Addition of guideline-recommended drugs such as topical corticosteroids and calcineurin inhibitors to the AD equation did not change the result, as dermatologists again showed a preference for sedating antihistamines, compared with nondermatologists, the investigators said.
The data also showed that Black patients with AD were more likely than were White patients to receive prescriptions for first-generation antihistamines and for therapies recommended by the AAD guidelines, and that patients under 21 years received more sedating antihistamines than did patients over age 21, they reported.
The age disparity “may be due to patient preference, as sedation effects may be less desirable to adult patients,” the investigators noted.
SOURCE: Garg S et al. Pediatr Dermatol. 2020 Nov 27. doi: 10.1111/pde.14445.
FROM PEDIATRIC DERMATOLOGY
A 70-year-old presented with a 3-week history of asymptomatic violaceous papules on his feet
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].