User login
International expert group agrees on redefining psoriasis severity
It’s high time to say farewell to the traditional categorization of psoriasis severity into mild, moderate, or severe disease, according to the International Psoriasis Council.
The mild/moderate/severe categorization is vague and defined differently by different organizations and in different countries. It often underestimates disease severity because it ignores psoriasis involvement in particularly tough-to-treat special areas, including the scalp, palms, soles, face, nails, and genitalia, Bruce E. Strober, MD, PhD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year. He chaired an IPC project in which prominent psoriasis experts in 32 countries employed a Delphi consensus approach aimed at achieving agreement on a more practical recategorization of psoriasis severity for use in both daily clinical practice and enrolling appropriate participants in clinical trials. What emerged was a simplified dichotomous categorization system.
“What we came up with is a very sensible approach to defining whether patients should get either topical or systemic therapy. In fact, there are only two groups of patients in psoriasis: those who should get topicals alone, and those who should get systemic therapy. It’s topicals or systemics,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., who also works in private practice in Cromwell, Conn.
Under the IPC classification, psoriasis patients are candidates for systemic therapy if they meet at least one of three criteria: body surface area of involvement greater than 10%, disease involving the previously mentioned special areas, or failure of topical therapy.
“This approach is about practically treating patients who are in need,” Dr. Strober said. “If patients meet just one of these three criteria they can move on to our current toolbox of systemic therapies, be they older systemic treatments, apremilast, phototherapy, or 1 of the 11 biologics currently approved for the treatment of psoriasis. The key point is that for patients with moderate to severe psoriasis – or should I say, systemic therapy–appropriate psoriasis – treatment should be based on individual patient characteristics. We don’t work on a stepwise approach. If a patient walks in with more than 10% body surface area involved, don’t make them fail topicals; you can go right to systemics.”
European dermatologists often use the Psoriasis Area and Severity Index (PASI) score to characterize disease severity and monitor response to therapy. In contrast, American dermatologists generally find PASI too complex and time-consuming for use in clinical practice, relying instead on the amount of body surface area involved with psoriasis. Neither of these measures incorporates disease involvement in special areas, which when present ought to automatically place a patient in the systemic therapy–appropriate category, according to Dr. Strober.
“I find this [IPC recategorization] a very practical approach. I hope you write this down and use this in your own practice,” Dr. Strober said.
The full IPC report was published in the Journal of the American Academy of Dermatology.
The IPC psoriasis severity reclassification project was unfunded. Dr. Strober reported receiving institutional research funding from and serving as a paid consultant to more than two dozen pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
It’s high time to say farewell to the traditional categorization of psoriasis severity into mild, moderate, or severe disease, according to the International Psoriasis Council.
The mild/moderate/severe categorization is vague and defined differently by different organizations and in different countries. It often underestimates disease severity because it ignores psoriasis involvement in particularly tough-to-treat special areas, including the scalp, palms, soles, face, nails, and genitalia, Bruce E. Strober, MD, PhD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year. He chaired an IPC project in which prominent psoriasis experts in 32 countries employed a Delphi consensus approach aimed at achieving agreement on a more practical recategorization of psoriasis severity for use in both daily clinical practice and enrolling appropriate participants in clinical trials. What emerged was a simplified dichotomous categorization system.
“What we came up with is a very sensible approach to defining whether patients should get either topical or systemic therapy. In fact, there are only two groups of patients in psoriasis: those who should get topicals alone, and those who should get systemic therapy. It’s topicals or systemics,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., who also works in private practice in Cromwell, Conn.
Under the IPC classification, psoriasis patients are candidates for systemic therapy if they meet at least one of three criteria: body surface area of involvement greater than 10%, disease involving the previously mentioned special areas, or failure of topical therapy.
“This approach is about practically treating patients who are in need,” Dr. Strober said. “If patients meet just one of these three criteria they can move on to our current toolbox of systemic therapies, be they older systemic treatments, apremilast, phototherapy, or 1 of the 11 biologics currently approved for the treatment of psoriasis. The key point is that for patients with moderate to severe psoriasis – or should I say, systemic therapy–appropriate psoriasis – treatment should be based on individual patient characteristics. We don’t work on a stepwise approach. If a patient walks in with more than 10% body surface area involved, don’t make them fail topicals; you can go right to systemics.”
European dermatologists often use the Psoriasis Area and Severity Index (PASI) score to characterize disease severity and monitor response to therapy. In contrast, American dermatologists generally find PASI too complex and time-consuming for use in clinical practice, relying instead on the amount of body surface area involved with psoriasis. Neither of these measures incorporates disease involvement in special areas, which when present ought to automatically place a patient in the systemic therapy–appropriate category, according to Dr. Strober.
“I find this [IPC recategorization] a very practical approach. I hope you write this down and use this in your own practice,” Dr. Strober said.
The full IPC report was published in the Journal of the American Academy of Dermatology.
The IPC psoriasis severity reclassification project was unfunded. Dr. Strober reported receiving institutional research funding from and serving as a paid consultant to more than two dozen pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
It’s high time to say farewell to the traditional categorization of psoriasis severity into mild, moderate, or severe disease, according to the International Psoriasis Council.
The mild/moderate/severe categorization is vague and defined differently by different organizations and in different countries. It often underestimates disease severity because it ignores psoriasis involvement in particularly tough-to-treat special areas, including the scalp, palms, soles, face, nails, and genitalia, Bruce E. Strober, MD, PhD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year. He chaired an IPC project in which prominent psoriasis experts in 32 countries employed a Delphi consensus approach aimed at achieving agreement on a more practical recategorization of psoriasis severity for use in both daily clinical practice and enrolling appropriate participants in clinical trials. What emerged was a simplified dichotomous categorization system.
“What we came up with is a very sensible approach to defining whether patients should get either topical or systemic therapy. In fact, there are only two groups of patients in psoriasis: those who should get topicals alone, and those who should get systemic therapy. It’s topicals or systemics,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., who also works in private practice in Cromwell, Conn.
Under the IPC classification, psoriasis patients are candidates for systemic therapy if they meet at least one of three criteria: body surface area of involvement greater than 10%, disease involving the previously mentioned special areas, or failure of topical therapy.
“This approach is about practically treating patients who are in need,” Dr. Strober said. “If patients meet just one of these three criteria they can move on to our current toolbox of systemic therapies, be they older systemic treatments, apremilast, phototherapy, or 1 of the 11 biologics currently approved for the treatment of psoriasis. The key point is that for patients with moderate to severe psoriasis – or should I say, systemic therapy–appropriate psoriasis – treatment should be based on individual patient characteristics. We don’t work on a stepwise approach. If a patient walks in with more than 10% body surface area involved, don’t make them fail topicals; you can go right to systemics.”
European dermatologists often use the Psoriasis Area and Severity Index (PASI) score to characterize disease severity and monitor response to therapy. In contrast, American dermatologists generally find PASI too complex and time-consuming for use in clinical practice, relying instead on the amount of body surface area involved with psoriasis. Neither of these measures incorporates disease involvement in special areas, which when present ought to automatically place a patient in the systemic therapy–appropriate category, according to Dr. Strober.
“I find this [IPC recategorization] a very practical approach. I hope you write this down and use this in your own practice,” Dr. Strober said.
The full IPC report was published in the Journal of the American Academy of Dermatology.
The IPC psoriasis severity reclassification project was unfunded. Dr. Strober reported receiving institutional research funding from and serving as a paid consultant to more than two dozen pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Itchy, scaly lesions with sweating

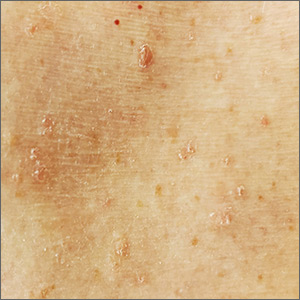
The patient’s history of a rash that worsened with sweating and clinical findings of erythematous papulosquamous lesions was consistent with Grover disease, also known as transient acantholytic dermatosis. Typically, this is a short-lived condition; however, when symptoms have manifested for years, it is deemed persistent acantholytic dermatosis. The gold standard for diagnostic confirmation is a skin biopsy, although it can also be diagnosed clinically. Since the patient had previously received this diagnosis from another clinician, and his clinical presentation was consistent, a confirmatory biopsy was not performed.
The specific pathophysiology of acantholytic dermatosis is unclear. A study to assess for an autoimmune component found that all participants had autoantibodies that are reactive to proteins involved in cell development, activation, growth, death, adhesion, and motility. Another hypothesis involves occlusion of eccrine sweat glands.
Typical triggers are sweating, heat, sunlight, and mechanical irritation, although it can also be triggered by end-stage renal disease and solid organ transplantation. It has also been linked to certain drugs (eg, ribavirin, anastrozole), other skin diseases (eg, atopic dermatitis, xerosis cutis), bacterial/viral infections, and malignancies.
As heat and perspiration are common triggers, avoidance of activities that expose patients to these conditions is recommended. Otherwise, topical corticosteroids and emollients are the recommended first-line therapy, along with antihistamines to control itching. Other therapies include systemic corticosteroids, topical vitamin D analogs (eg, calcipotriene), systemic retinoids (acitretin or isotretinoin), phototherapy and photochemotherapy (PUVA), red-light 5-aminolevulinic acid photodynamic therapy (ALA-PDT), and etanercept.
Although the patient could not remember the name of the previously prescribed medication, his description suggested that a systemic retinoid had already been tried, with no improvement. Treatment with emollients and oral antihistamines was also unsuccessful, as was topical antiperspirants to control perspiration on his affected skin. The patient agreed to try topical calcipotriene twice daily. He also agreed to switch to a topical emollient containing ceramides.
Image courtesy of Esther Walker, MD, and text courtesy of Esther Walker, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Phillips C, Kalantari-Dehaghi M, Marchenko S, et al. Is Grover's disease an autoimmune dermatosis? Exp Dermatol. 2013;22:781-784.

The patient’s history of a rash that worsened with sweating and clinical findings of erythematous papulosquamous lesions was consistent with Grover disease, also known as transient acantholytic dermatosis. Typically, this is a short-lived condition; however, when symptoms have manifested for years, it is deemed persistent acantholytic dermatosis. The gold standard for diagnostic confirmation is a skin biopsy, although it can also be diagnosed clinically. Since the patient had previously received this diagnosis from another clinician, and his clinical presentation was consistent, a confirmatory biopsy was not performed.
The specific pathophysiology of acantholytic dermatosis is unclear. A study to assess for an autoimmune component found that all participants had autoantibodies that are reactive to proteins involved in cell development, activation, growth, death, adhesion, and motility. Another hypothesis involves occlusion of eccrine sweat glands.
Typical triggers are sweating, heat, sunlight, and mechanical irritation, although it can also be triggered by end-stage renal disease and solid organ transplantation. It has also been linked to certain drugs (eg, ribavirin, anastrozole), other skin diseases (eg, atopic dermatitis, xerosis cutis), bacterial/viral infections, and malignancies.
As heat and perspiration are common triggers, avoidance of activities that expose patients to these conditions is recommended. Otherwise, topical corticosteroids and emollients are the recommended first-line therapy, along with antihistamines to control itching. Other therapies include systemic corticosteroids, topical vitamin D analogs (eg, calcipotriene), systemic retinoids (acitretin or isotretinoin), phototherapy and photochemotherapy (PUVA), red-light 5-aminolevulinic acid photodynamic therapy (ALA-PDT), and etanercept.
Although the patient could not remember the name of the previously prescribed medication, his description suggested that a systemic retinoid had already been tried, with no improvement. Treatment with emollients and oral antihistamines was also unsuccessful, as was topical antiperspirants to control perspiration on his affected skin. The patient agreed to try topical calcipotriene twice daily. He also agreed to switch to a topical emollient containing ceramides.
Image courtesy of Esther Walker, MD, and text courtesy of Esther Walker, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient’s history of a rash that worsened with sweating and clinical findings of erythematous papulosquamous lesions was consistent with Grover disease, also known as transient acantholytic dermatosis. Typically, this is a short-lived condition; however, when symptoms have manifested for years, it is deemed persistent acantholytic dermatosis. The gold standard for diagnostic confirmation is a skin biopsy, although it can also be diagnosed clinically. Since the patient had previously received this diagnosis from another clinician, and his clinical presentation was consistent, a confirmatory biopsy was not performed.
The specific pathophysiology of acantholytic dermatosis is unclear. A study to assess for an autoimmune component found that all participants had autoantibodies that are reactive to proteins involved in cell development, activation, growth, death, adhesion, and motility. Another hypothesis involves occlusion of eccrine sweat glands.
Typical triggers are sweating, heat, sunlight, and mechanical irritation, although it can also be triggered by end-stage renal disease and solid organ transplantation. It has also been linked to certain drugs (eg, ribavirin, anastrozole), other skin diseases (eg, atopic dermatitis, xerosis cutis), bacterial/viral infections, and malignancies.
As heat and perspiration are common triggers, avoidance of activities that expose patients to these conditions is recommended. Otherwise, topical corticosteroids and emollients are the recommended first-line therapy, along with antihistamines to control itching. Other therapies include systemic corticosteroids, topical vitamin D analogs (eg, calcipotriene), systemic retinoids (acitretin or isotretinoin), phototherapy and photochemotherapy (PUVA), red-light 5-aminolevulinic acid photodynamic therapy (ALA-PDT), and etanercept.
Although the patient could not remember the name of the previously prescribed medication, his description suggested that a systemic retinoid had already been tried, with no improvement. Treatment with emollients and oral antihistamines was also unsuccessful, as was topical antiperspirants to control perspiration on his affected skin. The patient agreed to try topical calcipotriene twice daily. He also agreed to switch to a topical emollient containing ceramides.
Image courtesy of Esther Walker, MD, and text courtesy of Esther Walker, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Phillips C, Kalantari-Dehaghi M, Marchenko S, et al. Is Grover's disease an autoimmune dermatosis? Exp Dermatol. 2013;22:781-784.
Phillips C, Kalantari-Dehaghi M, Marchenko S, et al. Is Grover's disease an autoimmune dermatosis? Exp Dermatol. 2013;22:781-784.
Expanded indications likely for apremilast
Big changes are coming in the use of oral apremilast, currently approved for moderate to severe psoriasis and plaque psoriasis in adults, Bruce E. Strober, MD, PhD, predicted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
“We’ll have , meaning we can use this drug in patients in whom we typically think about using only topical therapies. Keep on the lookout: I think the mild to moderate indication may be coming next year, and that’s going to really shake up the whole landscape of psoriasis therapy,” said Dr. Strober, a dermatologist at Yale University in New Haven, Conn., and Central Connecticut Dermatology in Cromwell, Conn.
Mild or moderate psoriasis
Apremilast manufacturer Amgen has announced positive topline results from the phase 3 ADVANCE trial, a multicenter, placebo-controlled, double-blind, study of 595 patients with mild or moderate psoriasis as defined by an involved body surface area of 2%-15% and a Psoriasis Area and Severity Index score of 2-15. Participants were randomized to the approved dose of apremilast (Otezla) – 30 mg twice daily – or placebo for 16 weeks, followed by 16 weeks of open-label apremilast for all. The full study findings haven’t yet been published or presented at a medical conference, but Amgen announced that the results were positive for all primary and secondary endpoints, and the company plans to file a request with the Food and Drug Administration for an expanded indication for the oral agent.
Pediatric studies
A recently published phase 2, open-label, 1-year study of apremilast in 42 children and adolescents with moderate to severe plaque psoriasis demonstrated that weight-based dosing is the best approach in the pediatric population. The study, which serves as the template for coming phase 3 trials, showed that dosing apremilast at 20 mg twice daily in youths weighing not more than 35 kg and 30 mg twice daily in those who weighed more provided pharmacokinetic exposure similar to that achieved with apremilast at the standard adult dose of 30 mg twice daily. Most participants liked the taste of the tablet.
“My prediction is apremilast will have efficacy in children and teenagers comparable to what it has in adults, with a similar safety and adverse event profile,” Dr. Strober said.
Apremilast works by blocking phosphodiesterase type 4, thereby reducing cyclic AMP metabolism, with a resultant increase in cyclic AMP levels. Cyclic AMP is a regulator of inflammation. Boosting its level has the effect of decreasing tumor necrosis factor and other proinflammatory cytokines while increasing anti-inflammatory mediators, such as interleukin-10.
Dr. Strober characterized apremilast’s efficacy as “modest” by contemporary standards in adults with moderate to severe psoriasis, with week 16 PASI 75 rates of about 30% in randomized trials, compared with 5% in placebo-treated controls. He considers it a good option in patients with moderate disease who are needle phobic and in those averse to the inconvenience of laboratory monitoring. The drug is useful in treating psoriasis in especially challenging locations. Apremilast is specifically approved for scalp psoriasis, and Dr. Strober has anecdotally found it helpful in patients with palmoplantar psoriasis or genital psoriasis.
“Apremilast has tolerability issues: first and foremost diarrhea, nausea, and headache. Probably 15%-20% of patients have nausea or diarrhea ranging from mild to severe, and 1 in 20 have headache. You have to warn patients,” he said.
Roughly 1% of patients experience depressed mood. “I’ve seen it in a few patients. I definitely believe it’s real, so query patients about mood changes while taking apremilast,” the dermatologist advised.
One in 5 patients loses 5% of body weight during the first 6 months on apremilast, but there’s no additional weight loss thereafter. It’s wrong to characterize the oral agent as a weight-loss drug, though, since 80% of patients don’t lose weight, Dr. Strober noted.
Topical PDE-4 inhibitor shows promise
Separately at the Las Vegas meeting, Linda Stein Gold, MD, provided highlights of a phase 2b randomized trial of a topical cream formulation of an extremely potent PDE-4 inhibitor, roflumilast, in patients with chronic plaque psoriasis. This molecule is a couple hundred times more effective at inhibiting the PDE-4 receptor than either oral apremilast or topical crisaborole (Eucrisa). And as a once-daily topical agent with very little systemic absorption, roflumilast cream sidesteps the tolerability issues that accompany apremilast.
“Roflumilast is currently available as an oral formulation for treatment of [chronic obstructive pulmonary disease], so it has a fairly well-established safety profile,” noted Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
The 12-week, multicenter, phase 2b study sponsored by Arcutis Biotherapeutics included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had baseline moderate disease.
A week-8 Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear skin or almost clear, plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls. On the secondary endpoint of improvement in tough-to-treat intertriginous psoriasis, at week 12 an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls. Moreover, the clinical improvements in IGA and itch kicked in quickly, with significant separation from placebo by week 2, Dr. Stein Gold noted.
The phase 3 program is now recruiting participants.
Dr. Strober and Dr. Stein Gold reported receiving research funding from and serving as consultants to Amgen and numerous other pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
Big changes are coming in the use of oral apremilast, currently approved for moderate to severe psoriasis and plaque psoriasis in adults, Bruce E. Strober, MD, PhD, predicted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
“We’ll have , meaning we can use this drug in patients in whom we typically think about using only topical therapies. Keep on the lookout: I think the mild to moderate indication may be coming next year, and that’s going to really shake up the whole landscape of psoriasis therapy,” said Dr. Strober, a dermatologist at Yale University in New Haven, Conn., and Central Connecticut Dermatology in Cromwell, Conn.
Mild or moderate psoriasis
Apremilast manufacturer Amgen has announced positive topline results from the phase 3 ADVANCE trial, a multicenter, placebo-controlled, double-blind, study of 595 patients with mild or moderate psoriasis as defined by an involved body surface area of 2%-15% and a Psoriasis Area and Severity Index score of 2-15. Participants were randomized to the approved dose of apremilast (Otezla) – 30 mg twice daily – or placebo for 16 weeks, followed by 16 weeks of open-label apremilast for all. The full study findings haven’t yet been published or presented at a medical conference, but Amgen announced that the results were positive for all primary and secondary endpoints, and the company plans to file a request with the Food and Drug Administration for an expanded indication for the oral agent.
Pediatric studies
A recently published phase 2, open-label, 1-year study of apremilast in 42 children and adolescents with moderate to severe plaque psoriasis demonstrated that weight-based dosing is the best approach in the pediatric population. The study, which serves as the template for coming phase 3 trials, showed that dosing apremilast at 20 mg twice daily in youths weighing not more than 35 kg and 30 mg twice daily in those who weighed more provided pharmacokinetic exposure similar to that achieved with apremilast at the standard adult dose of 30 mg twice daily. Most participants liked the taste of the tablet.
“My prediction is apremilast will have efficacy in children and teenagers comparable to what it has in adults, with a similar safety and adverse event profile,” Dr. Strober said.
Apremilast works by blocking phosphodiesterase type 4, thereby reducing cyclic AMP metabolism, with a resultant increase in cyclic AMP levels. Cyclic AMP is a regulator of inflammation. Boosting its level has the effect of decreasing tumor necrosis factor and other proinflammatory cytokines while increasing anti-inflammatory mediators, such as interleukin-10.
Dr. Strober characterized apremilast’s efficacy as “modest” by contemporary standards in adults with moderate to severe psoriasis, with week 16 PASI 75 rates of about 30% in randomized trials, compared with 5% in placebo-treated controls. He considers it a good option in patients with moderate disease who are needle phobic and in those averse to the inconvenience of laboratory monitoring. The drug is useful in treating psoriasis in especially challenging locations. Apremilast is specifically approved for scalp psoriasis, and Dr. Strober has anecdotally found it helpful in patients with palmoplantar psoriasis or genital psoriasis.
“Apremilast has tolerability issues: first and foremost diarrhea, nausea, and headache. Probably 15%-20% of patients have nausea or diarrhea ranging from mild to severe, and 1 in 20 have headache. You have to warn patients,” he said.
Roughly 1% of patients experience depressed mood. “I’ve seen it in a few patients. I definitely believe it’s real, so query patients about mood changes while taking apremilast,” the dermatologist advised.
One in 5 patients loses 5% of body weight during the first 6 months on apremilast, but there’s no additional weight loss thereafter. It’s wrong to characterize the oral agent as a weight-loss drug, though, since 80% of patients don’t lose weight, Dr. Strober noted.
Topical PDE-4 inhibitor shows promise
Separately at the Las Vegas meeting, Linda Stein Gold, MD, provided highlights of a phase 2b randomized trial of a topical cream formulation of an extremely potent PDE-4 inhibitor, roflumilast, in patients with chronic plaque psoriasis. This molecule is a couple hundred times more effective at inhibiting the PDE-4 receptor than either oral apremilast or topical crisaborole (Eucrisa). And as a once-daily topical agent with very little systemic absorption, roflumilast cream sidesteps the tolerability issues that accompany apremilast.
“Roflumilast is currently available as an oral formulation for treatment of [chronic obstructive pulmonary disease], so it has a fairly well-established safety profile,” noted Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
The 12-week, multicenter, phase 2b study sponsored by Arcutis Biotherapeutics included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had baseline moderate disease.
A week-8 Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear skin or almost clear, plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls. On the secondary endpoint of improvement in tough-to-treat intertriginous psoriasis, at week 12 an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls. Moreover, the clinical improvements in IGA and itch kicked in quickly, with significant separation from placebo by week 2, Dr. Stein Gold noted.
The phase 3 program is now recruiting participants.
Dr. Strober and Dr. Stein Gold reported receiving research funding from and serving as consultants to Amgen and numerous other pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
Big changes are coming in the use of oral apremilast, currently approved for moderate to severe psoriasis and plaque psoriasis in adults, Bruce E. Strober, MD, PhD, predicted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
“We’ll have , meaning we can use this drug in patients in whom we typically think about using only topical therapies. Keep on the lookout: I think the mild to moderate indication may be coming next year, and that’s going to really shake up the whole landscape of psoriasis therapy,” said Dr. Strober, a dermatologist at Yale University in New Haven, Conn., and Central Connecticut Dermatology in Cromwell, Conn.
Mild or moderate psoriasis
Apremilast manufacturer Amgen has announced positive topline results from the phase 3 ADVANCE trial, a multicenter, placebo-controlled, double-blind, study of 595 patients with mild or moderate psoriasis as defined by an involved body surface area of 2%-15% and a Psoriasis Area and Severity Index score of 2-15. Participants were randomized to the approved dose of apremilast (Otezla) – 30 mg twice daily – or placebo for 16 weeks, followed by 16 weeks of open-label apremilast for all. The full study findings haven’t yet been published or presented at a medical conference, but Amgen announced that the results were positive for all primary and secondary endpoints, and the company plans to file a request with the Food and Drug Administration for an expanded indication for the oral agent.
Pediatric studies
A recently published phase 2, open-label, 1-year study of apremilast in 42 children and adolescents with moderate to severe plaque psoriasis demonstrated that weight-based dosing is the best approach in the pediatric population. The study, which serves as the template for coming phase 3 trials, showed that dosing apremilast at 20 mg twice daily in youths weighing not more than 35 kg and 30 mg twice daily in those who weighed more provided pharmacokinetic exposure similar to that achieved with apremilast at the standard adult dose of 30 mg twice daily. Most participants liked the taste of the tablet.
“My prediction is apremilast will have efficacy in children and teenagers comparable to what it has in adults, with a similar safety and adverse event profile,” Dr. Strober said.
Apremilast works by blocking phosphodiesterase type 4, thereby reducing cyclic AMP metabolism, with a resultant increase in cyclic AMP levels. Cyclic AMP is a regulator of inflammation. Boosting its level has the effect of decreasing tumor necrosis factor and other proinflammatory cytokines while increasing anti-inflammatory mediators, such as interleukin-10.
Dr. Strober characterized apremilast’s efficacy as “modest” by contemporary standards in adults with moderate to severe psoriasis, with week 16 PASI 75 rates of about 30% in randomized trials, compared with 5% in placebo-treated controls. He considers it a good option in patients with moderate disease who are needle phobic and in those averse to the inconvenience of laboratory monitoring. The drug is useful in treating psoriasis in especially challenging locations. Apremilast is specifically approved for scalp psoriasis, and Dr. Strober has anecdotally found it helpful in patients with palmoplantar psoriasis or genital psoriasis.
“Apremilast has tolerability issues: first and foremost diarrhea, nausea, and headache. Probably 15%-20% of patients have nausea or diarrhea ranging from mild to severe, and 1 in 20 have headache. You have to warn patients,” he said.
Roughly 1% of patients experience depressed mood. “I’ve seen it in a few patients. I definitely believe it’s real, so query patients about mood changes while taking apremilast,” the dermatologist advised.
One in 5 patients loses 5% of body weight during the first 6 months on apremilast, but there’s no additional weight loss thereafter. It’s wrong to characterize the oral agent as a weight-loss drug, though, since 80% of patients don’t lose weight, Dr. Strober noted.
Topical PDE-4 inhibitor shows promise
Separately at the Las Vegas meeting, Linda Stein Gold, MD, provided highlights of a phase 2b randomized trial of a topical cream formulation of an extremely potent PDE-4 inhibitor, roflumilast, in patients with chronic plaque psoriasis. This molecule is a couple hundred times more effective at inhibiting the PDE-4 receptor than either oral apremilast or topical crisaborole (Eucrisa). And as a once-daily topical agent with very little systemic absorption, roflumilast cream sidesteps the tolerability issues that accompany apremilast.
“Roflumilast is currently available as an oral formulation for treatment of [chronic obstructive pulmonary disease], so it has a fairly well-established safety profile,” noted Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
The 12-week, multicenter, phase 2b study sponsored by Arcutis Biotherapeutics included 331 patients with chronic plaque psoriasis who were randomized to once-daily 0.3% roflumilast cream, 0.15% roflumilast cream, or vehicle. Three-quarters of participants had baseline moderate disease.
A week-8 Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear skin or almost clear, plus at least a 2-grade improvement from baseline occurred in 32% of the high-dose roflumilast group, 25% of those on the 0.15% formulation, and 10% of controls. On the secondary endpoint of improvement in tough-to-treat intertriginous psoriasis, at week 12 an intertriginous IGA score of 0 or 1 plus at least a 2-point improvement from baseline was seen in 86% of the 0.3% roflumilast cream group, 50% on low-dose therapy, and 29% of controls. Moreover, the clinical improvements in IGA and itch kicked in quickly, with significant separation from placebo by week 2, Dr. Stein Gold noted.
The phase 3 program is now recruiting participants.
Dr. Strober and Dr. Stein Gold reported receiving research funding from and serving as consultants to Amgen and numerous other pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
TNF inhibitor–induced psoriasis treatment algorithm maintains TNF inhibitor if possible
In a single-center retrospective analysis of 102 patients with psoriasis induced by tumor necrosis factor (TNF) inhibitors, most cases improved or resolved with use of topical medications or with discontinuation of the inciting TNF inhibitor, with or without other interventions. All patients were treated and diagnosed by dermatologists.
While TNF inhibitors have revolutionized management of numerous debilitating chronic inflammatory diseases, they are associated with mild and potentially serious adverse reactions, including de novo psoriasiform eruptions, noted Sean E. Mazloom, MD, and colleagues, at the Cleveland Clinic, Cleveland, Ohio, in the Journal of the American Academy of Dermatology. Despite the fact that it has been more than 15 years since the first reports of TNF inhibitor-induced psoriasis, optimal treatment strategies still remain poorly understood.
IBD and RA most common
Dr. Mazloom and colleagues identified 102 patients (median onset, 41 years; 72.5% female) with TNF inhibitor-induced psoriasis seen at a single tertiary care institution (the Cleveland Clinic) over a 10-year period. The authors proposed a treatment algorithm based on their findings.
Inciting TNF inhibitors were prescribed most commonly for inflammatory bowel disease (IBD) (52%) and rheumatoid arthritis (RA) (24.5%). The most common inciting TNF inhibitor was infliximab (52%). TNF inhibitor-induced psoriasis improved or resolved with topical medications alone in 63.5% of patients, and cyclosporine and methotrexate (10 mg weekly) were often effective (cyclosporine in five of five patients; methotrexate in 7 of 13) if topicals failed.
Noting that the success with topicals in this cohort exceeded that of earlier reports, the authors suggested that more accurate diagnoses and optimal strategies attributable to the involvement of dermatologists may be explanatory.
In 67% of refractory cases, discontinuation of the inciting TNF inhibitor with or without other interventions improved or resolved TNF inhibitor-induced psoriasis. With switching of TNF inhibitors, persistence or worsening of TNF inhibitor-induced psoriasis was reported in 16 of 25 patients (64%).
Algorithm aims at balancing control
The treatment algorithm proposed by Dr. Mazloom and colleagues aims at balancing control of the primary disease with minimization of skin symptom discomfort and continuation of the inciting TNF inhibitor if possible. Only with cyclosporine or methotrexate failure amid severe symptoms and less-than-optimal primary disease control should TNF inhibitors be discontinued and biologics and/or small-molecule inhibitors with alternative mechanisms of action be introduced. Transitioning to other TNF inhibitors may be tried before alternative strategies when the underlying disease is well-controlled but TNF inhibitor-induced psoriasis remains severe.
“Most dermatologists who see TNF-induced psoriasis often are likely already using strategies like the one proposed in the algorithm,” commented senior author Anthony Fernandez, MD, PhD, of the Cleveland (Ohio) Clinic, in an interview. “The concern is over those who may not see TNF inhibitor-induced psoriasis very often, and who may, as a knee-jerk response to TNF-induced psoriasis, stop the inciting medication. When strong side effects occur in IBD and RA, it’s critical to know how well the TNF inhibitor is controlling the underlying disease because lack of control can lead to permanent damage.”
Risk to benefit ratio favors retaining TNF inhibitors
The dermatologist’s goal, if the TNF inhibitor is working well, should be to exhaust all reasonable options to control the psoriasiform eruption and keep the patient on the TNF inhibitor rather than turn to potentially less effective alternatives, Dr. Fernandez added. “The risk:benefit ratio still usually favors adding more immune therapies to treat these reactions in order to enable patients to stay” on their TNF inhibitors.
Study authors disclosed no direct funding for the study. Dr Fernandez, the senior author, receives research funding from Pfizer, Mallinckrodt, and Novartis, consults for AbbVie and Celgene, and is a speaker for AbbVie and Mallinckrodt.
SOURCE: Mazloom SE et al. J Am Acad Dermatol. 2020 Dec;83(6):1590-8.
In a single-center retrospective analysis of 102 patients with psoriasis induced by tumor necrosis factor (TNF) inhibitors, most cases improved or resolved with use of topical medications or with discontinuation of the inciting TNF inhibitor, with or without other interventions. All patients were treated and diagnosed by dermatologists.
While TNF inhibitors have revolutionized management of numerous debilitating chronic inflammatory diseases, they are associated with mild and potentially serious adverse reactions, including de novo psoriasiform eruptions, noted Sean E. Mazloom, MD, and colleagues, at the Cleveland Clinic, Cleveland, Ohio, in the Journal of the American Academy of Dermatology. Despite the fact that it has been more than 15 years since the first reports of TNF inhibitor-induced psoriasis, optimal treatment strategies still remain poorly understood.
IBD and RA most common
Dr. Mazloom and colleagues identified 102 patients (median onset, 41 years; 72.5% female) with TNF inhibitor-induced psoriasis seen at a single tertiary care institution (the Cleveland Clinic) over a 10-year period. The authors proposed a treatment algorithm based on their findings.
Inciting TNF inhibitors were prescribed most commonly for inflammatory bowel disease (IBD) (52%) and rheumatoid arthritis (RA) (24.5%). The most common inciting TNF inhibitor was infliximab (52%). TNF inhibitor-induced psoriasis improved or resolved with topical medications alone in 63.5% of patients, and cyclosporine and methotrexate (10 mg weekly) were often effective (cyclosporine in five of five patients; methotrexate in 7 of 13) if topicals failed.
Noting that the success with topicals in this cohort exceeded that of earlier reports, the authors suggested that more accurate diagnoses and optimal strategies attributable to the involvement of dermatologists may be explanatory.
In 67% of refractory cases, discontinuation of the inciting TNF inhibitor with or without other interventions improved or resolved TNF inhibitor-induced psoriasis. With switching of TNF inhibitors, persistence or worsening of TNF inhibitor-induced psoriasis was reported in 16 of 25 patients (64%).
Algorithm aims at balancing control
The treatment algorithm proposed by Dr. Mazloom and colleagues aims at balancing control of the primary disease with minimization of skin symptom discomfort and continuation of the inciting TNF inhibitor if possible. Only with cyclosporine or methotrexate failure amid severe symptoms and less-than-optimal primary disease control should TNF inhibitors be discontinued and biologics and/or small-molecule inhibitors with alternative mechanisms of action be introduced. Transitioning to other TNF inhibitors may be tried before alternative strategies when the underlying disease is well-controlled but TNF inhibitor-induced psoriasis remains severe.
“Most dermatologists who see TNF-induced psoriasis often are likely already using strategies like the one proposed in the algorithm,” commented senior author Anthony Fernandez, MD, PhD, of the Cleveland (Ohio) Clinic, in an interview. “The concern is over those who may not see TNF inhibitor-induced psoriasis very often, and who may, as a knee-jerk response to TNF-induced psoriasis, stop the inciting medication. When strong side effects occur in IBD and RA, it’s critical to know how well the TNF inhibitor is controlling the underlying disease because lack of control can lead to permanent damage.”
Risk to benefit ratio favors retaining TNF inhibitors
The dermatologist’s goal, if the TNF inhibitor is working well, should be to exhaust all reasonable options to control the psoriasiform eruption and keep the patient on the TNF inhibitor rather than turn to potentially less effective alternatives, Dr. Fernandez added. “The risk:benefit ratio still usually favors adding more immune therapies to treat these reactions in order to enable patients to stay” on their TNF inhibitors.
Study authors disclosed no direct funding for the study. Dr Fernandez, the senior author, receives research funding from Pfizer, Mallinckrodt, and Novartis, consults for AbbVie and Celgene, and is a speaker for AbbVie and Mallinckrodt.
SOURCE: Mazloom SE et al. J Am Acad Dermatol. 2020 Dec;83(6):1590-8.
In a single-center retrospective analysis of 102 patients with psoriasis induced by tumor necrosis factor (TNF) inhibitors, most cases improved or resolved with use of topical medications or with discontinuation of the inciting TNF inhibitor, with or without other interventions. All patients were treated and diagnosed by dermatologists.
While TNF inhibitors have revolutionized management of numerous debilitating chronic inflammatory diseases, they are associated with mild and potentially serious adverse reactions, including de novo psoriasiform eruptions, noted Sean E. Mazloom, MD, and colleagues, at the Cleveland Clinic, Cleveland, Ohio, in the Journal of the American Academy of Dermatology. Despite the fact that it has been more than 15 years since the first reports of TNF inhibitor-induced psoriasis, optimal treatment strategies still remain poorly understood.
IBD and RA most common
Dr. Mazloom and colleagues identified 102 patients (median onset, 41 years; 72.5% female) with TNF inhibitor-induced psoriasis seen at a single tertiary care institution (the Cleveland Clinic) over a 10-year period. The authors proposed a treatment algorithm based on their findings.
Inciting TNF inhibitors were prescribed most commonly for inflammatory bowel disease (IBD) (52%) and rheumatoid arthritis (RA) (24.5%). The most common inciting TNF inhibitor was infliximab (52%). TNF inhibitor-induced psoriasis improved or resolved with topical medications alone in 63.5% of patients, and cyclosporine and methotrexate (10 mg weekly) were often effective (cyclosporine in five of five patients; methotrexate in 7 of 13) if topicals failed.
Noting that the success with topicals in this cohort exceeded that of earlier reports, the authors suggested that more accurate diagnoses and optimal strategies attributable to the involvement of dermatologists may be explanatory.
In 67% of refractory cases, discontinuation of the inciting TNF inhibitor with or without other interventions improved or resolved TNF inhibitor-induced psoriasis. With switching of TNF inhibitors, persistence or worsening of TNF inhibitor-induced psoriasis was reported in 16 of 25 patients (64%).
Algorithm aims at balancing control
The treatment algorithm proposed by Dr. Mazloom and colleagues aims at balancing control of the primary disease with minimization of skin symptom discomfort and continuation of the inciting TNF inhibitor if possible. Only with cyclosporine or methotrexate failure amid severe symptoms and less-than-optimal primary disease control should TNF inhibitors be discontinued and biologics and/or small-molecule inhibitors with alternative mechanisms of action be introduced. Transitioning to other TNF inhibitors may be tried before alternative strategies when the underlying disease is well-controlled but TNF inhibitor-induced psoriasis remains severe.
“Most dermatologists who see TNF-induced psoriasis often are likely already using strategies like the one proposed in the algorithm,” commented senior author Anthony Fernandez, MD, PhD, of the Cleveland (Ohio) Clinic, in an interview. “The concern is over those who may not see TNF inhibitor-induced psoriasis very often, and who may, as a knee-jerk response to TNF-induced psoriasis, stop the inciting medication. When strong side effects occur in IBD and RA, it’s critical to know how well the TNF inhibitor is controlling the underlying disease because lack of control can lead to permanent damage.”
Risk to benefit ratio favors retaining TNF inhibitors
The dermatologist’s goal, if the TNF inhibitor is working well, should be to exhaust all reasonable options to control the psoriasiform eruption and keep the patient on the TNF inhibitor rather than turn to potentially less effective alternatives, Dr. Fernandez added. “The risk:benefit ratio still usually favors adding more immune therapies to treat these reactions in order to enable patients to stay” on their TNF inhibitors.
Study authors disclosed no direct funding for the study. Dr Fernandez, the senior author, receives research funding from Pfizer, Mallinckrodt, and Novartis, consults for AbbVie and Celgene, and is a speaker for AbbVie and Mallinckrodt.
SOURCE: Mazloom SE et al. J Am Acad Dermatol. 2020 Dec;83(6):1590-8.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Embrace new and classic acne treatments
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
Recognizing the ongoing value of benzoyl peroxide, educating patients about the role of antibiotics, and embracing spironolactone are among the acne treatment pearls provided by Hilary Baldwin, MD, during a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Benzoyl peroxide celebrates its 60th birthday and is still going strong as an acne treatment, said Dr. Baldwin, of the department of dermatology, Rutgers Robert Wood Johnston Medical Center, New Brunswick, N.J. Benzoyl peroxide can be used as a stand-alone and has the added benefit of not being associated with antimicrobial resistance. In addition, “benzoyl peroxide is the heavy lifter in combinations,” she said. In fact, benzoyl peroxide can prevent the development of resistance to topical and oral antibiotics such as clindamycin, and can reverse resistance that has occurred, she noted.
However, patient compliance can be an issue. Benzoyl peroxide often is underused because of its tendency to bleach fabric, noted Dr. Baldwin, who is also medical director of The Acne Treatment and Research Center in New York. To help combat this problem and improve compliance, she advises patients to establish a dosing schedule for benzoyl peroxide, such as using it first thing in the morning, or applying in the afternoon and using a paper towel first, or a white towel, to wash their faces at bedtime, she said. When dealing with teenagers, “it sounds like a lot of work, but it makes the mothers much happier not to have their towels bleached.”
Although clinicians want to reduce unnecessary antibiotic use in acne, there is a place for antibiotics, but not as monotherapy, Dr. Baldwin said. Instead, initiate topical therapy, such as a retinoid or benzoyl peroxide, simultaneously with antibiotics and evaluate the response in 6-8 weeks, she advised. At that point, the antibiotics can be stopped, even if 100% clearing has not been achieved, and “the topicals can carry you on for months and months,” she noted.
Also, in female patients, consider oral contraceptive pills or spironolactone at the same time as oral antibiotics, then discontinue the antibiotics and continue with the hormonal therapy, she added. “Plan your exit strategy early,” she said. Explain to patients that you will stop the oral antibiotics after 2 months, so they must continue with the topicals.
“Embrace spironolactone if you haven’t already,” said Dr. Baldwin, who noted that spironolactone has been underused in recent years. Spironolactone use for acne has not been well studied, “but consensus groups and expert opinions certainly favor its use,” she added.
Spironolactone takes 3-6 months to reach its full effect, so Dr. Baldwin recommends beginning the therapy in combination with other strategies. “I begin in combination with oral antibiotics,” she said. Also, be sure to check hormone levels before initiating therapy if appropriate. Potential side effects include menstrual irregularities and breast tenderness, but they tend to decrease over time, Dr. Baldwin noted. Other side effects such as CNS symptoms (fatigue, dizziness, and headache) can be eased by paying attention to proper hydration and starting with a lower dose, she added. Studies in younger adults show no reason for concern about potassium levels, but potassium should be checked at baseline in older patients, after the first month, and after a dose increase, she said.
Dr. Baldwin was enthusiastic about the recent introduction of several new treatments for acne: Sarecycline, now approved by the Food and Drug Administration for use in patients as young as 9 years; trifarotene 0.005% cream, the first 4th generation retinoid, with truncal acne data; tazarotene 0.045% lotion, with improved tolerability; minocycline 4% foam, with high cutaneous levels and minimal systemic absorption; and clascoterone 1% cream, “the first topical antiandrogen and safe for use in males,” she said.
Relevant to her presentation, Dr. Baldwin disclosed relationships as an adviser, speaker, and/or investigator for Almirall, EPI Health, Foamix, Galderma, Johnson & Johnson, LaRoche-Posay, Menlo Therapeutics, Ortho Dermatologics, Sol-Gel, and Sun.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Treatment pipeline holds promise for rosacea
, according to Linda Stein Gold, MD, director of clinical research, in the department of dermatology, Henry Ford Hospital in Detroit.
In addition, topical minocycline has recently been approved by the Food and Drug Administration for the treatment of rosacea in a 1.5% foam formulation. “The reason it has taken so long to have a minocycline product is that it is challenging to deliver it topically,” she said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually. Studies of higher concentrations were not significantly better for rosacea, so development of the 1.5% foam was pursued, although a 4% foam is approved for the treatment of acne.
Dr. Stein Gold shared results from a pair of 12-week randomized trials in which significantly more patients treated with topical minocycline foam showed treatment success, compared with those on vehicle, based on Investigator’s Global Assessment (IGA) scores of clear or almost clear and a decrease of at least two grades from baseline: 52.1% versus 43.0% in one study and 49.1% versus 39.0% in the second, statistically significant differences. The product also was well tolerated, with most patients reporting no side effects or mild side effects.
Research on how to maximize effectiveness of available treatments such as ivermectin is ongoing, but several new treatments in the pipeline continue to show promising results, she noted.
An up-and-coming rosacea treatment is an old product used in a new way: Benzoyl peroxide in a microencapsulated form. “Benzoyl peroxide is encased in silica molecules that allow very slow release of the benzoyl peroxide into the skin and that leads to decreased irritation,” Dr. Stein Gold explained. The deposit of active ingredient on the skin appears to stay below the level of irritation.
Dr. Stein Gold and colleagues conducted two randomized, vehicle-controlled trials in which 733 adults with moderate to severe rosacea were treated with either the encapsulated benzoyl peroxide cream formulation or a vehicle applied once daily for 12 weeks.
At 12 weeks, IGA success increased over the course of the studies, and reached 43.5% in one and 50.1% in the other, compared with 16.1% and 25.9%, respectively, for the vehicle groups in those studies (P < .001 for both). Overall, she described this as “a nice improvement for a drug that we had not considered to be part of our treatment armamentarium for our rosacea patients.”
Dr. Stein Gold also shared data from a phase 2 study of low-dose oral minocycline in adults with papulopustular rosacea. A group of 200 patients used the drug or a placebo once daily for 16 weeks. The study examined 20-mg and 40-mg extended-release formulations, and found a significant improvement with the 40-mg dose over the 20-mg dose and over placebo, in terms of those who reached an IGA of 0 or 1, with a 2 grade improvement. While this is a phase 2 study, it may lead to oral minocycline as another treatment option, she said.
“It is an exciting time for the treatment of rosacea, with a variety of options and an active pipeline, so we can aim for clear skin for our patients,” she commented.
Dr. Stein Gold disclosed serving as an investigator and consultant for Galderma, Vyne, Sun, Sol Gel, and Almirall; she is a consultant and speaker for Ortho.
MedscapeLive and this news organization are owned by the same parent company.
, according to Linda Stein Gold, MD, director of clinical research, in the department of dermatology, Henry Ford Hospital in Detroit.
In addition, topical minocycline has recently been approved by the Food and Drug Administration for the treatment of rosacea in a 1.5% foam formulation. “The reason it has taken so long to have a minocycline product is that it is challenging to deliver it topically,” she said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually. Studies of higher concentrations were not significantly better for rosacea, so development of the 1.5% foam was pursued, although a 4% foam is approved for the treatment of acne.
Dr. Stein Gold shared results from a pair of 12-week randomized trials in which significantly more patients treated with topical minocycline foam showed treatment success, compared with those on vehicle, based on Investigator’s Global Assessment (IGA) scores of clear or almost clear and a decrease of at least two grades from baseline: 52.1% versus 43.0% in one study and 49.1% versus 39.0% in the second, statistically significant differences. The product also was well tolerated, with most patients reporting no side effects or mild side effects.
Research on how to maximize effectiveness of available treatments such as ivermectin is ongoing, but several new treatments in the pipeline continue to show promising results, she noted.
An up-and-coming rosacea treatment is an old product used in a new way: Benzoyl peroxide in a microencapsulated form. “Benzoyl peroxide is encased in silica molecules that allow very slow release of the benzoyl peroxide into the skin and that leads to decreased irritation,” Dr. Stein Gold explained. The deposit of active ingredient on the skin appears to stay below the level of irritation.
Dr. Stein Gold and colleagues conducted two randomized, vehicle-controlled trials in which 733 adults with moderate to severe rosacea were treated with either the encapsulated benzoyl peroxide cream formulation or a vehicle applied once daily for 12 weeks.
At 12 weeks, IGA success increased over the course of the studies, and reached 43.5% in one and 50.1% in the other, compared with 16.1% and 25.9%, respectively, for the vehicle groups in those studies (P < .001 for both). Overall, she described this as “a nice improvement for a drug that we had not considered to be part of our treatment armamentarium for our rosacea patients.”
Dr. Stein Gold also shared data from a phase 2 study of low-dose oral minocycline in adults with papulopustular rosacea. A group of 200 patients used the drug or a placebo once daily for 16 weeks. The study examined 20-mg and 40-mg extended-release formulations, and found a significant improvement with the 40-mg dose over the 20-mg dose and over placebo, in terms of those who reached an IGA of 0 or 1, with a 2 grade improvement. While this is a phase 2 study, it may lead to oral minocycline as another treatment option, she said.
“It is an exciting time for the treatment of rosacea, with a variety of options and an active pipeline, so we can aim for clear skin for our patients,” she commented.
Dr. Stein Gold disclosed serving as an investigator and consultant for Galderma, Vyne, Sun, Sol Gel, and Almirall; she is a consultant and speaker for Ortho.
MedscapeLive and this news organization are owned by the same parent company.
, according to Linda Stein Gold, MD, director of clinical research, in the department of dermatology, Henry Ford Hospital in Detroit.
In addition, topical minocycline has recently been approved by the Food and Drug Administration for the treatment of rosacea in a 1.5% foam formulation. “The reason it has taken so long to have a minocycline product is that it is challenging to deliver it topically,” she said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually. Studies of higher concentrations were not significantly better for rosacea, so development of the 1.5% foam was pursued, although a 4% foam is approved for the treatment of acne.
Dr. Stein Gold shared results from a pair of 12-week randomized trials in which significantly more patients treated with topical minocycline foam showed treatment success, compared with those on vehicle, based on Investigator’s Global Assessment (IGA) scores of clear or almost clear and a decrease of at least two grades from baseline: 52.1% versus 43.0% in one study and 49.1% versus 39.0% in the second, statistically significant differences. The product also was well tolerated, with most patients reporting no side effects or mild side effects.
Research on how to maximize effectiveness of available treatments such as ivermectin is ongoing, but several new treatments in the pipeline continue to show promising results, she noted.
An up-and-coming rosacea treatment is an old product used in a new way: Benzoyl peroxide in a microencapsulated form. “Benzoyl peroxide is encased in silica molecules that allow very slow release of the benzoyl peroxide into the skin and that leads to decreased irritation,” Dr. Stein Gold explained. The deposit of active ingredient on the skin appears to stay below the level of irritation.
Dr. Stein Gold and colleagues conducted two randomized, vehicle-controlled trials in which 733 adults with moderate to severe rosacea were treated with either the encapsulated benzoyl peroxide cream formulation or a vehicle applied once daily for 12 weeks.
At 12 weeks, IGA success increased over the course of the studies, and reached 43.5% in one and 50.1% in the other, compared with 16.1% and 25.9%, respectively, for the vehicle groups in those studies (P < .001 for both). Overall, she described this as “a nice improvement for a drug that we had not considered to be part of our treatment armamentarium for our rosacea patients.”
Dr. Stein Gold also shared data from a phase 2 study of low-dose oral minocycline in adults with papulopustular rosacea. A group of 200 patients used the drug or a placebo once daily for 16 weeks. The study examined 20-mg and 40-mg extended-release formulations, and found a significant improvement with the 40-mg dose over the 20-mg dose and over placebo, in terms of those who reached an IGA of 0 or 1, with a 2 grade improvement. While this is a phase 2 study, it may lead to oral minocycline as another treatment option, she said.
“It is an exciting time for the treatment of rosacea, with a variety of options and an active pipeline, so we can aim for clear skin for our patients,” she commented.
Dr. Stein Gold disclosed serving as an investigator and consultant for Galderma, Vyne, Sun, Sol Gel, and Almirall; she is a consultant and speaker for Ortho.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Merino wool clothing improves atopic dermatitis, studies find
Conventional wisdom holds that Joseph F. Fowler, Jr., MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
“We’ve always though that wool is bad in atopics, right? Indeed, rough wool might be. But fine wool garments can actually improve atopic dermatitis, probably because wool is the most breathable fabric and has the best temperature regulation qualities of any fabric we can wear,” said Dr. Fowler, a dermatologist at the University of Louisville (Ky).
He was first author of a randomized, 12-week, crossover, assessor-blinded clinical trial which showed precisely that. And a second, similarly designed study, this one conducted in Australia, also concluded that fine merino wool assists in the management of AD.
The study by Dr. Fowler and coinvestigators included 50 children and adults with mild or moderate AD who either wore top-and-bottom base layer merino wool ensembles for 6 weeks and then switched to their regular nonwoolen clothing, or vice versa. The mean Eczema Area and Severity Index (EASI) score in those initially randomized to merino wool improved from a mean baseline of 4.5 to 1.7 at week 6, a significantly greater improvement than in the group wearing their regular clothing. Similarly, those who switched to merino wool after 6 weeks experienced a significant decrease in EASI scores from that point on to week 12, while those who switched from merino wool to their regular clothing did not.
Mean Dermatology Life Quality Index (DLQI) scores in patients who wore merino wool first improved from 6.9 at baseline to 3.4 at week 6. Those who wore their regular clothing first went from a mean baseline DLQI of 6.7 to 6.2 at week 6 – a nonsignificant change – but then improved to a week 12 mean DLQI of 3.7 while wearing wool. There was no improvement in DLQI scores while participants were wearing their regular clothing.
Static Investigator’s Global Assessment scores showed significantly greater improvement while patients wore merino wool garments than their regular clothing.
The Australian study included 39 patients with mild to moderate AD aged between 4 weeks and 3 years. This, too, was a 12-week, randomized, crossover, assessor-blinded clinical trial. Participating children wore merino wool for 6 weeks and cotton ensembles chosen by their parents for an equal time. The primary endpoint was change in the SCORing Atopic Dermatitis (SCORAD) index after each 6-week period. The mean 7.6-point greater SCORAD reduction at 6 weeks while wearing merino wool, compared with cotton, was “a pretty impressive reduction,” Dr. Fowler observed.
Reductions in the secondary endpoints of Atopic Dermatitis Severity Index and Infants’ Dermatitis Quality of Life Index while wearing merino wool followed suit. In contrast, switching from wool to cotton resulted in an increase in both scores. Also, use of topical corticosteroids was significantly reduced while patients wore merino wool.
Wool harvested from merino sheep is characterized by fine-diameter fibers. In Dr. Fowler’s study the mean fiber diameter was 17.5 mcm. This makes for a soft fabric with outstanding moisture absorbance capacity, a quality that’s beneficial in patients with AD, since their lesional skin loses the ability to regulate moisture, the dermatologist explained.
Both randomized trials were funded by Australian Wool Innovation and the Australian government.
MedscapeLive and this news organization are owned by the same parent company.
Conventional wisdom holds that Joseph F. Fowler, Jr., MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
“We’ve always though that wool is bad in atopics, right? Indeed, rough wool might be. But fine wool garments can actually improve atopic dermatitis, probably because wool is the most breathable fabric and has the best temperature regulation qualities of any fabric we can wear,” said Dr. Fowler, a dermatologist at the University of Louisville (Ky).
He was first author of a randomized, 12-week, crossover, assessor-blinded clinical trial which showed precisely that. And a second, similarly designed study, this one conducted in Australia, also concluded that fine merino wool assists in the management of AD.
The study by Dr. Fowler and coinvestigators included 50 children and adults with mild or moderate AD who either wore top-and-bottom base layer merino wool ensembles for 6 weeks and then switched to their regular nonwoolen clothing, or vice versa. The mean Eczema Area and Severity Index (EASI) score in those initially randomized to merino wool improved from a mean baseline of 4.5 to 1.7 at week 6, a significantly greater improvement than in the group wearing their regular clothing. Similarly, those who switched to merino wool after 6 weeks experienced a significant decrease in EASI scores from that point on to week 12, while those who switched from merino wool to their regular clothing did not.
Mean Dermatology Life Quality Index (DLQI) scores in patients who wore merino wool first improved from 6.9 at baseline to 3.4 at week 6. Those who wore their regular clothing first went from a mean baseline DLQI of 6.7 to 6.2 at week 6 – a nonsignificant change – but then improved to a week 12 mean DLQI of 3.7 while wearing wool. There was no improvement in DLQI scores while participants were wearing their regular clothing.
Static Investigator’s Global Assessment scores showed significantly greater improvement while patients wore merino wool garments than their regular clothing.
The Australian study included 39 patients with mild to moderate AD aged between 4 weeks and 3 years. This, too, was a 12-week, randomized, crossover, assessor-blinded clinical trial. Participating children wore merino wool for 6 weeks and cotton ensembles chosen by their parents for an equal time. The primary endpoint was change in the SCORing Atopic Dermatitis (SCORAD) index after each 6-week period. The mean 7.6-point greater SCORAD reduction at 6 weeks while wearing merino wool, compared with cotton, was “a pretty impressive reduction,” Dr. Fowler observed.
Reductions in the secondary endpoints of Atopic Dermatitis Severity Index and Infants’ Dermatitis Quality of Life Index while wearing merino wool followed suit. In contrast, switching from wool to cotton resulted in an increase in both scores. Also, use of topical corticosteroids was significantly reduced while patients wore merino wool.
Wool harvested from merino sheep is characterized by fine-diameter fibers. In Dr. Fowler’s study the mean fiber diameter was 17.5 mcm. This makes for a soft fabric with outstanding moisture absorbance capacity, a quality that’s beneficial in patients with AD, since their lesional skin loses the ability to regulate moisture, the dermatologist explained.
Both randomized trials were funded by Australian Wool Innovation and the Australian government.
MedscapeLive and this news organization are owned by the same parent company.
Conventional wisdom holds that Joseph F. Fowler, Jr., MD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
“We’ve always though that wool is bad in atopics, right? Indeed, rough wool might be. But fine wool garments can actually improve atopic dermatitis, probably because wool is the most breathable fabric and has the best temperature regulation qualities of any fabric we can wear,” said Dr. Fowler, a dermatologist at the University of Louisville (Ky).
He was first author of a randomized, 12-week, crossover, assessor-blinded clinical trial which showed precisely that. And a second, similarly designed study, this one conducted in Australia, also concluded that fine merino wool assists in the management of AD.
The study by Dr. Fowler and coinvestigators included 50 children and adults with mild or moderate AD who either wore top-and-bottom base layer merino wool ensembles for 6 weeks and then switched to their regular nonwoolen clothing, or vice versa. The mean Eczema Area and Severity Index (EASI) score in those initially randomized to merino wool improved from a mean baseline of 4.5 to 1.7 at week 6, a significantly greater improvement than in the group wearing their regular clothing. Similarly, those who switched to merino wool after 6 weeks experienced a significant decrease in EASI scores from that point on to week 12, while those who switched from merino wool to their regular clothing did not.
Mean Dermatology Life Quality Index (DLQI) scores in patients who wore merino wool first improved from 6.9 at baseline to 3.4 at week 6. Those who wore their regular clothing first went from a mean baseline DLQI of 6.7 to 6.2 at week 6 – a nonsignificant change – but then improved to a week 12 mean DLQI of 3.7 while wearing wool. There was no improvement in DLQI scores while participants were wearing their regular clothing.
Static Investigator’s Global Assessment scores showed significantly greater improvement while patients wore merino wool garments than their regular clothing.
The Australian study included 39 patients with mild to moderate AD aged between 4 weeks and 3 years. This, too, was a 12-week, randomized, crossover, assessor-blinded clinical trial. Participating children wore merino wool for 6 weeks and cotton ensembles chosen by their parents for an equal time. The primary endpoint was change in the SCORing Atopic Dermatitis (SCORAD) index after each 6-week period. The mean 7.6-point greater SCORAD reduction at 6 weeks while wearing merino wool, compared with cotton, was “a pretty impressive reduction,” Dr. Fowler observed.
Reductions in the secondary endpoints of Atopic Dermatitis Severity Index and Infants’ Dermatitis Quality of Life Index while wearing merino wool followed suit. In contrast, switching from wool to cotton resulted in an increase in both scores. Also, use of topical corticosteroids was significantly reduced while patients wore merino wool.
Wool harvested from merino sheep is characterized by fine-diameter fibers. In Dr. Fowler’s study the mean fiber diameter was 17.5 mcm. This makes for a soft fabric with outstanding moisture absorbance capacity, a quality that’s beneficial in patients with AD, since their lesional skin loses the ability to regulate moisture, the dermatologist explained.
Both randomized trials were funded by Australian Wool Innovation and the Australian government.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Sunscreen myths, controversies continue
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Expert shares key facts about keloid therapy
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Improvements in chronic hand eczema seen with oral gusacitinib in phase 2 study
in a phase 2b, randomized trial, Howard Sofen, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The once-daily drug proved effective for this challenging condition, regardless of whether an individual’s chronic hand eczema was driven chiefly by irritant contact dermatitis, allergic contact dermatitis, or atopic dermatitis, added Dr. Sofen, medical director of Dermatology Research Associates, Los Angeles, and chief of the dermatology division at LA County/Olive View Medical Center.
Gusacitinib is a once-daily oral inhibitor of Janus kinase 1, 2, and 3, tyrosine kinase 2, and spleen tyrosine kinase (SYK). As such, it targets the Th1, Th2, Th17, and Th22 cytokine pathways, as well as SYK-mediated interleukin-17 signaling of keratinocyte proliferation and differentiation. Thus, its spectrum of activity makes it a candidate for the treatment of a variety of other inflammatory dermatologic diseases, although chronic hand eczema alone affects an estimated 7 million Americans, the dermatologist noted.
The phase 2b, double-blind, 16-week, multicenter, randomized trial included 97 patients who were randomized to oral gusacitinib as monotherapy at 40 or 80 mg once daily or placebo. All participants had chronic hand eczema of more than 6 months duration that was refractory to potent or superpotent topical and/or systemic steroids. Participants were split 60/40 between those with severe chronic hand eczema, defined by a baseline score on the 0-4 Physician’s Global Assessment scale, and moderate disease, with a PGA of 3.
The primary endpoint was the percent improvement in modified total lesion severity score (mTLSS) at week 16 from a mean baseline of 13.2. A clearcut dose response was evident: Gusacitinib at 80 mg/day achieved a 69.5% decrease, while 40 mg brought a 40% reduction, which wasn’t significantly better than the 33.5% decrease in placebo-treated controls.
The rapidity of response was noteworthy in these steroid-refractory patients. The 80-mg group showed significant separation from placebo by 2 weeks, with a mean 40.1% reduction in mTLSS versus 13.6% with placebo.
The secondary endpoint was achievement of a PGA score of 0 or 1 – that is, clear or almost clear – with a 2-grade improvement over placebo. This was achieved in 31.3% of patients assigned to the higher dose of gusacitinib at week 16, a success rate fivefold higher than the 6.3% rate in controls. The two groups separated on this endpoint at week 2, the first assessment. At week 8 there was an eightfold difference in response: 25% in patients on gusacitinib at 80 mg, 3.1% with placebo.
The other secondary endpoint was improvement in itch as measured by the mTLSS pruritus 0-3 subscore. As for the other outcomes, the improvement in itch was rapid. At week 2, patients on gusacitinib at 80 mg averaged a 43.1% reduction from their baseline pruritus score, compared with 4.6% with placebo. At week 16, the reductions were 65.7% and 29.8%, respectively.
Both doses of gusacitinib were well tolerated, according to Dr. Sofer. No thromboembolic events, major adverse cardiovascular events, or opportunistic infections occurred during the short 16-week study. The drug’s safety profile was consistent with what’s been seen in a collective gusacitinib clinical trial experience totaling more than 350 patients: mild to moderate nasopharyngitis, headache, asymptomatic elevations in creatine phosphokinase, and a slight increase in HDL cholesterol accompanied by a small reduction in LDL cholesterol.
Dr. Sofen reported receiving research funding from and serving as a consultant to Asana BioSciences, the study sponsor, as well as more than half a dozen other pharmaceutical companies.
in a phase 2b, randomized trial, Howard Sofen, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The once-daily drug proved effective for this challenging condition, regardless of whether an individual’s chronic hand eczema was driven chiefly by irritant contact dermatitis, allergic contact dermatitis, or atopic dermatitis, added Dr. Sofen, medical director of Dermatology Research Associates, Los Angeles, and chief of the dermatology division at LA County/Olive View Medical Center.
Gusacitinib is a once-daily oral inhibitor of Janus kinase 1, 2, and 3, tyrosine kinase 2, and spleen tyrosine kinase (SYK). As such, it targets the Th1, Th2, Th17, and Th22 cytokine pathways, as well as SYK-mediated interleukin-17 signaling of keratinocyte proliferation and differentiation. Thus, its spectrum of activity makes it a candidate for the treatment of a variety of other inflammatory dermatologic diseases, although chronic hand eczema alone affects an estimated 7 million Americans, the dermatologist noted.
The phase 2b, double-blind, 16-week, multicenter, randomized trial included 97 patients who were randomized to oral gusacitinib as monotherapy at 40 or 80 mg once daily or placebo. All participants had chronic hand eczema of more than 6 months duration that was refractory to potent or superpotent topical and/or systemic steroids. Participants were split 60/40 between those with severe chronic hand eczema, defined by a baseline score on the 0-4 Physician’s Global Assessment scale, and moderate disease, with a PGA of 3.
The primary endpoint was the percent improvement in modified total lesion severity score (mTLSS) at week 16 from a mean baseline of 13.2. A clearcut dose response was evident: Gusacitinib at 80 mg/day achieved a 69.5% decrease, while 40 mg brought a 40% reduction, which wasn’t significantly better than the 33.5% decrease in placebo-treated controls.
The rapidity of response was noteworthy in these steroid-refractory patients. The 80-mg group showed significant separation from placebo by 2 weeks, with a mean 40.1% reduction in mTLSS versus 13.6% with placebo.
The secondary endpoint was achievement of a PGA score of 0 or 1 – that is, clear or almost clear – with a 2-grade improvement over placebo. This was achieved in 31.3% of patients assigned to the higher dose of gusacitinib at week 16, a success rate fivefold higher than the 6.3% rate in controls. The two groups separated on this endpoint at week 2, the first assessment. At week 8 there was an eightfold difference in response: 25% in patients on gusacitinib at 80 mg, 3.1% with placebo.
The other secondary endpoint was improvement in itch as measured by the mTLSS pruritus 0-3 subscore. As for the other outcomes, the improvement in itch was rapid. At week 2, patients on gusacitinib at 80 mg averaged a 43.1% reduction from their baseline pruritus score, compared with 4.6% with placebo. At week 16, the reductions were 65.7% and 29.8%, respectively.
Both doses of gusacitinib were well tolerated, according to Dr. Sofer. No thromboembolic events, major adverse cardiovascular events, or opportunistic infections occurred during the short 16-week study. The drug’s safety profile was consistent with what’s been seen in a collective gusacitinib clinical trial experience totaling more than 350 patients: mild to moderate nasopharyngitis, headache, asymptomatic elevations in creatine phosphokinase, and a slight increase in HDL cholesterol accompanied by a small reduction in LDL cholesterol.
Dr. Sofen reported receiving research funding from and serving as a consultant to Asana BioSciences, the study sponsor, as well as more than half a dozen other pharmaceutical companies.
in a phase 2b, randomized trial, Howard Sofen, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
The once-daily drug proved effective for this challenging condition, regardless of whether an individual’s chronic hand eczema was driven chiefly by irritant contact dermatitis, allergic contact dermatitis, or atopic dermatitis, added Dr. Sofen, medical director of Dermatology Research Associates, Los Angeles, and chief of the dermatology division at LA County/Olive View Medical Center.
Gusacitinib is a once-daily oral inhibitor of Janus kinase 1, 2, and 3, tyrosine kinase 2, and spleen tyrosine kinase (SYK). As such, it targets the Th1, Th2, Th17, and Th22 cytokine pathways, as well as SYK-mediated interleukin-17 signaling of keratinocyte proliferation and differentiation. Thus, its spectrum of activity makes it a candidate for the treatment of a variety of other inflammatory dermatologic diseases, although chronic hand eczema alone affects an estimated 7 million Americans, the dermatologist noted.
The phase 2b, double-blind, 16-week, multicenter, randomized trial included 97 patients who were randomized to oral gusacitinib as monotherapy at 40 or 80 mg once daily or placebo. All participants had chronic hand eczema of more than 6 months duration that was refractory to potent or superpotent topical and/or systemic steroids. Participants were split 60/40 between those with severe chronic hand eczema, defined by a baseline score on the 0-4 Physician’s Global Assessment scale, and moderate disease, with a PGA of 3.
The primary endpoint was the percent improvement in modified total lesion severity score (mTLSS) at week 16 from a mean baseline of 13.2. A clearcut dose response was evident: Gusacitinib at 80 mg/day achieved a 69.5% decrease, while 40 mg brought a 40% reduction, which wasn’t significantly better than the 33.5% decrease in placebo-treated controls.
The rapidity of response was noteworthy in these steroid-refractory patients. The 80-mg group showed significant separation from placebo by 2 weeks, with a mean 40.1% reduction in mTLSS versus 13.6% with placebo.
The secondary endpoint was achievement of a PGA score of 0 or 1 – that is, clear or almost clear – with a 2-grade improvement over placebo. This was achieved in 31.3% of patients assigned to the higher dose of gusacitinib at week 16, a success rate fivefold higher than the 6.3% rate in controls. The two groups separated on this endpoint at week 2, the first assessment. At week 8 there was an eightfold difference in response: 25% in patients on gusacitinib at 80 mg, 3.1% with placebo.
The other secondary endpoint was improvement in itch as measured by the mTLSS pruritus 0-3 subscore. As for the other outcomes, the improvement in itch was rapid. At week 2, patients on gusacitinib at 80 mg averaged a 43.1% reduction from their baseline pruritus score, compared with 4.6% with placebo. At week 16, the reductions were 65.7% and 29.8%, respectively.
Both doses of gusacitinib were well tolerated, according to Dr. Sofer. No thromboembolic events, major adverse cardiovascular events, or opportunistic infections occurred during the short 16-week study. The drug’s safety profile was consistent with what’s been seen in a collective gusacitinib clinical trial experience totaling more than 350 patients: mild to moderate nasopharyngitis, headache, asymptomatic elevations in creatine phosphokinase, and a slight increase in HDL cholesterol accompanied by a small reduction in LDL cholesterol.
Dr. Sofen reported receiving research funding from and serving as a consultant to Asana BioSciences, the study sponsor, as well as more than half a dozen other pharmaceutical companies.
FROM THE EADV CONGRESS