User login
Subcutaneous nemolizumab eases itching for atopic dermatitis
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
of 215 patients in Japan.
Controlling the pruritus associated with atopic dermatitis (AD) can have a significant impact on patients’ quality of life, wrote Kenji Kabashima, MD, PhD, of the department of dermatology at Kyoto University, and coauthors. Frequent scratching can cause not only mechanical skin damage, but also may enhance inflammatory reactions and contribute to sleep problems.
In earlier phase studies, nemolizumab, a humanized monoclonal antibody against interleukin-31 receptor A, showed efficacy in reducing pruritus in patients with AD, but has not been well studied in patients who are also using topical agents, they wrote.
In the study published in the New England Journal of Medicine, the researchers randomized 143 patients with AD and moderate to severe pruritus to 60 mg of subcutaneous nemolizumab and 72 patients to a placebo every 4 weeks for 16 weeks. All patients were aged 13 years and older with a confirmed AD diagnosis and a history of inadequate response to or inability to use treatments, including topical glucocorticoids and oral antihistamines. Their average age was 40 years, approximately two-thirds were male, and the average disease duration was approximately 30 years. Topical treatments included a medium potency glucocorticoid in 97% of patients in both groups, and a topical calcineurin inhibitor in 41% of those on nemolizumab, and 40% of those on placebo; almost 90% of the patients in both groups were on oral antihistamines.
At 16 weeks, scores on the visual analog scale for pruritus (the primary outcome) significantly improved from baseline in the nemolizumab group, compared with the placebo group (a mean change of –42.8% and –21.4%, respectively, P < .001).
In addition, more patients in the nemolizumab group, compared with the placebo group (40% vs. 22%) achieved a score of 4 or less on the Dermatology Life Quality Index, with lower scores reflecting less impact of disease on daily life. In addition, more patients in the nemolizumab group, compared with the placebo group (55% vs. 21%) achieved a score of 7 or less on the Insomnia Severity Index.
During the study, 71% of the patients in each group reported adverse events, most were mild or moderate. The most common adverse event was worsening AD, reported by 24% of the nemolizumab patients and 21% of the placebo patients. Reactions related to the injection occurred in 8% of nemolizumab patients and 3% of placebo patients. Cytokine abnormalities, which included an increased level of thymus and activation regulated chemokine, were reported in 10 (7%) of the patients on nemolizumab, none of which occurred in those on placebo. “Most were not accompanied by a worsening of signs of or the extent of atopic dermatitis,” the authors wrote.
Severe adverse events were reported in three patients (2%) in the nemolizumab group, which were Meniere’s disease, acute pancreatitis, and AD in one patient each. No severe adverse events were reported in the placebo group. In addition, three patients in the nemolizumab group experienced four treatment-related adverse events that led them to discontinue treatment: AD, Meniere’s disease, alopecia, and peripheral edema.
The study findings were limited by several factors including the relatively short treatment period, inclusion only of Japanese patients, inclusion of patients aged as young as 13 years, and the inability to draw conclusions from the secondary endpoints such as quality of life and sleep issues, the researchers noted.
However, the results suggest that “nemolizumab plus topical agents may ameliorate both pruritus and signs of eczema and may lessen the severity of atopic dermatitis by disrupting the itch-scratch cycle,” they added.
“Novel therapies [for AD] are needed, as there are still patients who need better disease control despite current therapies, and AD is a heterogeneous disease that may need different treatment approaches,” Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, said in an interview.
Dr. Simpson, who was not an investigator in this study, said that he was somewhat surprised that the itch reduction was lower in the current study, compared with previous studies by the same group. Also surprising was the increase in cytokine abnormalities in the nemolizumab group, which “needs further study.”
Overall, the data “provide support that blockade of the IL-31 receptor improves itch in AD and appears to have some effect on inflammation,” Dr. Simpson said.
One challenge to the clinical use of nemolizumab will be identifying “where this type of drug fits into the treatment paradigm,” and determining whether specific patients whose disease is driven more by this neuroimmune pathway could benefit more than with the traditional IL-4 or IL-13 blockade, he said.
The study was supported by Maruho. Dr. Kabashima disclosed consulting fees from Maruho and two coauthors were Maruho employees. Dr. Simpson had no financial conflicts relevant to this study, but he reported receiving research grants and other financial relationships with manufacturers of AD therapies.
SOURCE: Kabashima K et al. N Engl J Med. 2020 Jul 9. doi: 10.1056/NEJMoa1917006.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Diagnosing molluscum contagiosum can be tricky
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
The way James R. Treat, MD, sees it, if there ever were a truism in the field of dermatology, it’s that everyone hates molluscum contagiosum.
“It tortures all of us,” Dr. Treat, a pediatric dermatologist at Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “It’s very distressing to parents, but often more distressing to parents than to kids.”
A viral disorder of the skin and mucous membranes characterized by discrete single or multiple, flesh-colored papules, “When you look at inflamed molluscum it can be very difficult to recognize because it looks like a more complicated infection,” said Dr. Treat, who is also associate professor of clinical pediatrics and dermatology, at the University of Pennsylvania, Philadelphia.
An epidemiologic review of 302 MC cases found that 80% of patients were aged younger than 8 years, 63% had more than 15 lesions, and 24% had concomitant atopic dermatitis (J Am Acad Dermatol. 2006; 2006;54[1]:47-54). “Children with atopic dermatitis often have their molluscum last longer,” he said. “The average time course for molluscum is 18 months, but it can certainly be longer than that. So if you say, ‘it’s probably going to go away in a few months,’ that’s probably not going to happen.”
The telltale MC lesion is glossy and contains a white core in the center that can be revealed by shining an otoscope sideways on the lesion. “Umbilication doesn’t always occur, but if the center part looks white, that will help with diagnosis,” Dr. Treat said. “If they’re inflamed and they’re red and you’re worried that there’s a bacterial infection, do a culture, pop the lesion open, and get some of the pus out. If you’re concerned, start them on antibiotics. It’s always worse to miss an infection than to overtreat molluscum. But once you’ve done it a few times and you realize that the cultures are coming back negative, then you’ll probably have your threshold a little higher.”
The most useful clinical sign of MC is the so-called “BOTE” (beginning of the end) sign, which is characterized by erythema and swelling of MC skin lesions. “When the parents come to us in pediatric dermatology, often it’s because their kids have had molluscum for a while,” he said. “It spreads and becomes inflamed and the parents ask, ‘Is it infected?’ The answer is, yes, it’s an infection, but it’s not infected with what you think it is [which is Staphylococcus or Streptococcus], it’s the virus being recognized by the body. When the virus is recognized by the body, it creates a huge inflammatory reaction. That’s usually the time at which the body has had enough of the virus, and it eradicates the rest of it. It means the inflammatory response is finding the molluscum and it’s going to take care of it.”
MC brings its own eczematous response, which can complicate efforts to confirm the diagnosis. Dr. Treat spoke of a young patient he recently saw who had an eczematous reaction on the inner parts of the arms and the upper flank – with no such clinical history. “It kind of came out of the blue,” he said. “You think about contact allergies and other types of dermatitis, but molluscum brings its own eczema. Often what the parents recognize is the eczematous eruption and not the little dots of molluscum. So if you see someone with a new eruption in typical molluscum areas – the flank and your thighs and the back of the legs – and they’ve never had eczema in the past, or they’ve only had mild eczema, think about eczema as a response to molluscum.”
MC can also result in a Gianotti-Crosti syndrome-like reactions (Arch Dermatol. 2012;148[11]:1257-64). “These are angry, inflamed red papules on the knees and on the elbows and on the buttocks and on the cheeks,” Dr. Treat said. “It typically spares the trunk, and they look like molluscum.”
He went on to note that MC can present as cysts, and that MC in the gluteal cleft is a mimicker of condyloma. MC can also cause conjunctivitis, which is increased in HIV patients and in those with atopic dermatitis. “These are patients who should probably see an ophthalmologist” to make no damage has occurred, Dr. Treat said.
He closed his remarks by noting that rarely, MC can be the presenting sign of an immunodeficiency. “The immune system dysregulation that shows up this way is called a DOCK8 mutation, which have eczema and widespread viral disease including warts and molluscum,” Dr. Treat said.
He reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY 2020
Oral difelikefalin quells severe chronic kidney disease–associated itch
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
, in a first-of-its-kind randomized clinical trial, Gil Yosipovitch, MD, said at the virtual annual meeting of the American Academy of Dermatology.
“Difelikefalin at 1.0 mg was associated with clinically meaningful improvements in pruritus. The improvement in itch was significant by week 2. And nearly 40% of patients achieved a complete response, which was more than two-and-one-half times more than with placebo,” noted Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami.
Pruritus associated with chronic kidney disease (CKD) is a common, underrecognized, and distressing condition that causes markedly impaired quality of life. It occurs in patients across all stages of CKD, not just in those on hemodialysis, as is widely but mistakenly believed. And at present there is no approved drug in any country for treatment of CKD-associated itch.
Difelikefalin, a novel selective agonist of peripheral kappa opioid receptors, is designed to have very limited CNS penetration. The drug, which is renally excreted, doesn’t bind to mu or delta opioid receptors. Its antipruritic effect arises from activation of kappa opioid receptors on peripheral sensory neurons and immune cells, the dermatologist explained.
Dr. Yosipovitch presented the results of a phase 2, randomized, double-blind, placebo-controlled, 12-week trial in which 240 patients with severe chronic pruritus and stage 3-5 CKD were assigned to once-daily oral difelikefalin at 0.25 mg, 0.5 mg, or 1.0 mg, or placebo. More than 80% of participants were not on dialysis. Indeed, this was the first-ever clinical trial targeting itch in patients across such a broad spectrum of CKD stages.
The primary study endpoint was change from baseline to week 12 in the weekly mean score on the 24-hour Worst Itching Intensity Numerical Rating Scale. The average baseline score was 7, considered severe pruritus on the 0-10 scale. Patients randomized to difelikefalin at 1.0 mg/day had a mean 4.4-point decrease, a significantly greater improvement than the 3.3-point reduction in placebo-treated controls.
“More than a 4-point decrease is considered a very meaningful itch reduction,” Dr. Yosipovitch noted.
The mean reductions in itch score in patients on 0.25 mg and 0.5 mg/day of difelikefalin were 4.0 and 3.8 points, respectively, which fell short of statistical significance versus placebo.
A key prespecified secondary endpoint was the proportion of subjects with at least a 3-point improvement in itch score over 12 weeks. This was achieved in 72% of patients on the top dose of difelikefalin, compared with 58% of controls, a significant difference. A 4-point or larger decrease in itch score occurred in 65% of patients on 1.0 mg/day of the kappa opioid recent agonist, versus 50% of controls, also a significant difference.
A complete response, defined as an itch score of 0 or 1 at least 80% of the time, was significantly more common in all three active treatment groups than in controls, with rates of 33%, 31.6%, and 38.6% at difelikefalin 0.25, 0.5, and 1.0 mg, compared with 4.4% among those on placebo.
Falls occurred in 1.5% of patients on difelikefalin. “The therapy does seem to increase the risk of dizziness, falls, fatigue, and GI complaints,” according to the investigator.
Still, most of these adverse events were mild or moderate in severity. Only about 1% of participants discontinued treatment for such reasons.
Earlier this year, a positive phase 3 trial of an intravenous formulation of difelikefalin for pruritus was reported in CKD patients on hemodialysis (N Engl J Med. 2020 Jan 16;382[3]:222-32).
In an interview, Dr. Yosipovitch said that this new phase 2 oral dose-finding study wasn’t powered to detect differences in treatment efficacy between the dialysis and nondialysis groups. However, the proportion of patients with at least a 3-point improvement in itch at week 12 was similar in the two groups.
“The oral formulation would of course be more convenient and would be preferred for patients not undergoing hemodialysis,” he said. “I would expect that the IV formulation would be the preferred route of administration for a patient undergoing hemodialysis. An IV formulation would be very convenient for such patients because it’s administered at the dialysis clinic at the end of the hemodialysis session.”
The oral difelikefalin phase 3 program is scheduled to start later in 2020.
CKD-associated itch poses a therapeutic challenge because it has so many contributory factors. These include CKD-induced peripheral neuropathy, functional and structural neuropathic changes in the brain, cutaneous mast cell activation, an imbalance between mu opioid receptor overexpression and kappa opioid receptor downregulation, secondary parathyroidism, and systemic accumulation of aluminum, beta 2 microglobulin, and other dialysis-related substances, the dermatologist observed.
Dr. Yosipovitch reported receiving research grants from a half-dozen pharmaceutical companies. He also serves as a consultant to numerous companies, including Cara Therapeutics, which sponsored the phase 2 trial.
FROM AAD 2020
Even a few days of steroids may be risky, new study suggests
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
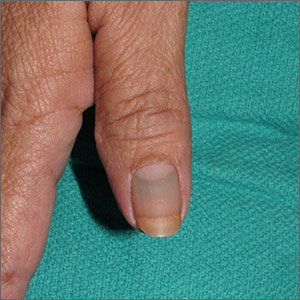
Nail discoloration
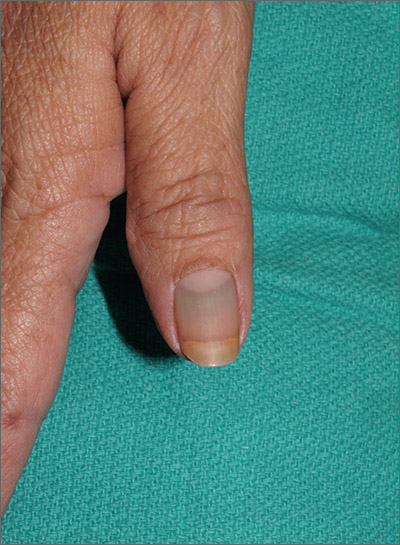
The clinical findings and medical history were consistent with drug-induced hyperpigmentation and minocycline was the likely culprit. Other medications commonly implicated in drug-induced hyperpigmentation include heavy metals (eg, gold, iron, silver), anticonvulsants, hydroxychloroquine, and amiodarone.
Common sites for minocycline pigment deposition—besides the fingernails—include the gingiva, dorsal hands, shins, and old scars. The diagnosis of drug-induced hyperpigmentation is clinical and does not require a biopsy.
While the use of antibiotics for chronic disease can lead to antimicrobial resistance (and should be avoided when possible), certain cases of rosacea may require chronic therapy, and tetracyclines most commonly are used. When minocycline is chosen as a chronic therapy, part of the treatment surveillance should include monitoring for drug-induced hyperpigmentation.
When drug-induced hyperpigmentation does occur, treatment involves discontinuing the offending agent. It can take years for pigment to develop and years for it to resolve—if it completely resolves at all.
In this case, the physician was concerned about the pigmentation worsening or spreading to other sites, so he discontinued the minocycline and prescribed a topical agent for the patient’s rosacea. Ultimately, the patient required occasional use of doxycycline for flares.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.

The clinical findings and medical history were consistent with drug-induced hyperpigmentation and minocycline was the likely culprit. Other medications commonly implicated in drug-induced hyperpigmentation include heavy metals (eg, gold, iron, silver), anticonvulsants, hydroxychloroquine, and amiodarone.
Common sites for minocycline pigment deposition—besides the fingernails—include the gingiva, dorsal hands, shins, and old scars. The diagnosis of drug-induced hyperpigmentation is clinical and does not require a biopsy.
While the use of antibiotics for chronic disease can lead to antimicrobial resistance (and should be avoided when possible), certain cases of rosacea may require chronic therapy, and tetracyclines most commonly are used. When minocycline is chosen as a chronic therapy, part of the treatment surveillance should include monitoring for drug-induced hyperpigmentation.
When drug-induced hyperpigmentation does occur, treatment involves discontinuing the offending agent. It can take years for pigment to develop and years for it to resolve—if it completely resolves at all.
In this case, the physician was concerned about the pigmentation worsening or spreading to other sites, so he discontinued the minocycline and prescribed a topical agent for the patient’s rosacea. Ultimately, the patient required occasional use of doxycycline for flares.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The clinical findings and medical history were consistent with drug-induced hyperpigmentation and minocycline was the likely culprit. Other medications commonly implicated in drug-induced hyperpigmentation include heavy metals (eg, gold, iron, silver), anticonvulsants, hydroxychloroquine, and amiodarone.
Common sites for minocycline pigment deposition—besides the fingernails—include the gingiva, dorsal hands, shins, and old scars. The diagnosis of drug-induced hyperpigmentation is clinical and does not require a biopsy.
While the use of antibiotics for chronic disease can lead to antimicrobial resistance (and should be avoided when possible), certain cases of rosacea may require chronic therapy, and tetracyclines most commonly are used. When minocycline is chosen as a chronic therapy, part of the treatment surveillance should include monitoring for drug-induced hyperpigmentation.
When drug-induced hyperpigmentation does occur, treatment involves discontinuing the offending agent. It can take years for pigment to develop and years for it to resolve—if it completely resolves at all.
In this case, the physician was concerned about the pigmentation worsening or spreading to other sites, so he discontinued the minocycline and prescribed a topical agent for the patient’s rosacea. Ultimately, the patient required occasional use of doxycycline for flares.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
Nisar MS, Iyer K, Brodell RT, et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol. 2013;6:159-162.
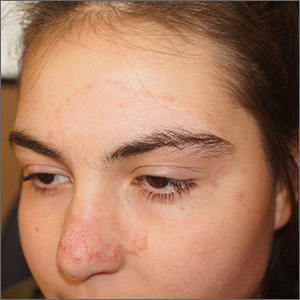
Sorting out the many mimickers of psoriasis
“It has an earlier age of onset, usually in infancy, and can occur with the atopic triad that presents with asthma and seasonal allergies as well,” Israel David “Izzy” Andrews, MD, said at the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “There is typically a very strong family history, as this is an autosomal dominant condition, and it’s far more common than psoriasis. The annual incidence is estimated to be 10%-15% of pediatric patients. It has classic areas of involvement depending on the age of the patient, and lesions are intensely pruritic at all times. There is induration and crust, but it’s important to distinguish crust from scale. Whereas crust is dried exudate, and scale is usually secondary to a hyperproliferation of the skin. Initially, treatments (especially topical) are similar and may also delay the formalized diagnosis of either of the two.”
Another psoriasis mimicker, pityriasis rosea, is thought to be secondary to human herpes virus 6 or 7 infection, said Dr. Andrews, of the department of dermatology at Phoenix Children’s Hospital. It typically appears in the teens and tweens and usually presents as a large herald patch or plaque on the trunk. As the herald patch resolves, smaller lesions will develop on the trunk following skin folds. “It’s rarely symptomatic and it’s very short lived, and clears within 6-12 weeks,” Dr. Andrews noted. “It can present with an inverse pattern involving the face, neck, and groin, but sparing the trunk. This variant, termed inverse pityriasis rosea, can be confused with inverse psoriasis, which has a similar distribution. However, the inverse pattern of pityriasis rosea will still resolve in a similar time frame to its more classic variant.”
Pityriasis lichenoides can also be mistaken for psoriasis. The acute form can present with erythematous, scaly papules and plaques, but lesions are often found in different phases of resolution or healing. “This benign lymphoproliferative skin disorder can be very difficult to distinguish from psoriasis and may require a biopsy to rule in or out,” Dr. Andrews said. “It can last months to years and there are few treatments that are effective. It is typically nonresponsive to topical steroids and other treatments that would be more effective for psoriasis, helping to distinguish the two. It is thought to exist in the spectrum with other lymphoproliferative diseases including cutaneous T-cell lymphoma [CTCL]. However, there are only a few cases in the literature that support a transformation from pityriasis lichenoides to CTCL.”
Seborrheic dermatitis is more common than atopic dermatitis and psoriasis, but it can be mistaken for psoriasis. It is caused by an inflammatory response secondary to overgrowth of Malassezia yeast and has a bimodal age distribution. “Seborrheic dermatitis affects babies, teens, and tweens, and can persist into adulthood,” he said. “Infants with cradle cap usually resolve with moisturization, gentle brushing, and occasional antifungal shampoos.” Petaloid seborrheic dermatitis can predominately involve the face with psoriatic-appearing induration, plaques, and varying degrees of scales. “In skin of color, this can be confused with discoid lupus, sarcoidosis, and psoriasis, occasionally requiring a biopsy to distinguish,” said Dr. Andrews, who is also an assistant professor of pediatrics at the Mayo Clinic College of Medicine and Science in Scottsdale, Ariz.
Another psoriasis mimicker, pityriasis amiantacea, is thought to be a more severe form of seborrheic dermatitis. It presents with concretions of scale around hair follicles that are highly adherent and are sometimes called sebopsoriasis. “It may be associated with cutaneous findings of psoriasis elsewhere, but may also be found with secondarily infected atopic dermatitis and tinea capitis; however, in my clinical experience, it is most often found in isolation,” he said. “There may be a seasonal association with exacerbation in warm temperatures, and treatment often consists of humectants like salicylic acid for loosening scale, topical steroids for inflammation, and gentle combing out of scale.”
Infections can also mimic psoriasis. For example, tinea infections are often misdiagnosed as eczema or psoriasis and treated with topical steroids. “This can lead to tinea incognito, making it harder to diagnose either condition without attention to detail,” Dr. Andrews said. “On the body, look for expanding lesions with more raised peripheral edges, and central flattening, giving a classic annular appearance. It’s also important to inquire about family history and contacts including pets, contact sports/mat sports (think yoga, gymnastics, martial arts), or other contacts with similar rashes.” Work-up typically includes a fungal culture and starting empiric oral antifungal medications. “It is important to be able to distinguish scalp psoriasis from tinea capitis to prevent the more inflammatory form of tinea capitis, kerion (a deeper more symptomatic, painful and purulent dermatitis), which can lead to permanent scarring alopecia,” he said.
Bacterial infections can also mimic psoriasis, specifically nonbullous impetigo and ecthyma, the more ulcerative form of impetigo. The most frequent associations are group A Streptococcus, methicillin-susceptible Staphylococcus aureus and methicillin-resistant S. aureus.
Dr. Andrews closed his presentation by noting that tumor necrosis factor–alpha inhibitor–induced psoriasiform drug eruptions can occur in psoriasis-naive patients or unmask a predilection for psoriasis in patients with Crohn’s disease, juvenile idiopathic arthritis, or other autoinflammatory or autoimmune conditions. “They may improve with continued treatment and resolve with switching treatments,” he said. “Early biopsy in psoriasiform drug eruptions can appear like atopic dermatitis on pathology. When suspecting psoriasis in a pediatric patient, it is important to consider the history and physical exam as well as family history and associated comorbidities. While a biopsy may aide in the work-up, diagnosis can be made clinically.”
Dr. Andrews reported having no financial disclosures.
“It has an earlier age of onset, usually in infancy, and can occur with the atopic triad that presents with asthma and seasonal allergies as well,” Israel David “Izzy” Andrews, MD, said at the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “There is typically a very strong family history, as this is an autosomal dominant condition, and it’s far more common than psoriasis. The annual incidence is estimated to be 10%-15% of pediatric patients. It has classic areas of involvement depending on the age of the patient, and lesions are intensely pruritic at all times. There is induration and crust, but it’s important to distinguish crust from scale. Whereas crust is dried exudate, and scale is usually secondary to a hyperproliferation of the skin. Initially, treatments (especially topical) are similar and may also delay the formalized diagnosis of either of the two.”
Another psoriasis mimicker, pityriasis rosea, is thought to be secondary to human herpes virus 6 or 7 infection, said Dr. Andrews, of the department of dermatology at Phoenix Children’s Hospital. It typically appears in the teens and tweens and usually presents as a large herald patch or plaque on the trunk. As the herald patch resolves, smaller lesions will develop on the trunk following skin folds. “It’s rarely symptomatic and it’s very short lived, and clears within 6-12 weeks,” Dr. Andrews noted. “It can present with an inverse pattern involving the face, neck, and groin, but sparing the trunk. This variant, termed inverse pityriasis rosea, can be confused with inverse psoriasis, which has a similar distribution. However, the inverse pattern of pityriasis rosea will still resolve in a similar time frame to its more classic variant.”
Pityriasis lichenoides can also be mistaken for psoriasis. The acute form can present with erythematous, scaly papules and plaques, but lesions are often found in different phases of resolution or healing. “This benign lymphoproliferative skin disorder can be very difficult to distinguish from psoriasis and may require a biopsy to rule in or out,” Dr. Andrews said. “It can last months to years and there are few treatments that are effective. It is typically nonresponsive to topical steroids and other treatments that would be more effective for psoriasis, helping to distinguish the two. It is thought to exist in the spectrum with other lymphoproliferative diseases including cutaneous T-cell lymphoma [CTCL]. However, there are only a few cases in the literature that support a transformation from pityriasis lichenoides to CTCL.”
Seborrheic dermatitis is more common than atopic dermatitis and psoriasis, but it can be mistaken for psoriasis. It is caused by an inflammatory response secondary to overgrowth of Malassezia yeast and has a bimodal age distribution. “Seborrheic dermatitis affects babies, teens, and tweens, and can persist into adulthood,” he said. “Infants with cradle cap usually resolve with moisturization, gentle brushing, and occasional antifungal shampoos.” Petaloid seborrheic dermatitis can predominately involve the face with psoriatic-appearing induration, plaques, and varying degrees of scales. “In skin of color, this can be confused with discoid lupus, sarcoidosis, and psoriasis, occasionally requiring a biopsy to distinguish,” said Dr. Andrews, who is also an assistant professor of pediatrics at the Mayo Clinic College of Medicine and Science in Scottsdale, Ariz.
Another psoriasis mimicker, pityriasis amiantacea, is thought to be a more severe form of seborrheic dermatitis. It presents with concretions of scale around hair follicles that are highly adherent and are sometimes called sebopsoriasis. “It may be associated with cutaneous findings of psoriasis elsewhere, but may also be found with secondarily infected atopic dermatitis and tinea capitis; however, in my clinical experience, it is most often found in isolation,” he said. “There may be a seasonal association with exacerbation in warm temperatures, and treatment often consists of humectants like salicylic acid for loosening scale, topical steroids for inflammation, and gentle combing out of scale.”
Infections can also mimic psoriasis. For example, tinea infections are often misdiagnosed as eczema or psoriasis and treated with topical steroids. “This can lead to tinea incognito, making it harder to diagnose either condition without attention to detail,” Dr. Andrews said. “On the body, look for expanding lesions with more raised peripheral edges, and central flattening, giving a classic annular appearance. It’s also important to inquire about family history and contacts including pets, contact sports/mat sports (think yoga, gymnastics, martial arts), or other contacts with similar rashes.” Work-up typically includes a fungal culture and starting empiric oral antifungal medications. “It is important to be able to distinguish scalp psoriasis from tinea capitis to prevent the more inflammatory form of tinea capitis, kerion (a deeper more symptomatic, painful and purulent dermatitis), which can lead to permanent scarring alopecia,” he said.
Bacterial infections can also mimic psoriasis, specifically nonbullous impetigo and ecthyma, the more ulcerative form of impetigo. The most frequent associations are group A Streptococcus, methicillin-susceptible Staphylococcus aureus and methicillin-resistant S. aureus.
Dr. Andrews closed his presentation by noting that tumor necrosis factor–alpha inhibitor–induced psoriasiform drug eruptions can occur in psoriasis-naive patients or unmask a predilection for psoriasis in patients with Crohn’s disease, juvenile idiopathic arthritis, or other autoinflammatory or autoimmune conditions. “They may improve with continued treatment and resolve with switching treatments,” he said. “Early biopsy in psoriasiform drug eruptions can appear like atopic dermatitis on pathology. When suspecting psoriasis in a pediatric patient, it is important to consider the history and physical exam as well as family history and associated comorbidities. While a biopsy may aide in the work-up, diagnosis can be made clinically.”
Dr. Andrews reported having no financial disclosures.
“It has an earlier age of onset, usually in infancy, and can occur with the atopic triad that presents with asthma and seasonal allergies as well,” Israel David “Izzy” Andrews, MD, said at the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “There is typically a very strong family history, as this is an autosomal dominant condition, and it’s far more common than psoriasis. The annual incidence is estimated to be 10%-15% of pediatric patients. It has classic areas of involvement depending on the age of the patient, and lesions are intensely pruritic at all times. There is induration and crust, but it’s important to distinguish crust from scale. Whereas crust is dried exudate, and scale is usually secondary to a hyperproliferation of the skin. Initially, treatments (especially topical) are similar and may also delay the formalized diagnosis of either of the two.”
Another psoriasis mimicker, pityriasis rosea, is thought to be secondary to human herpes virus 6 or 7 infection, said Dr. Andrews, of the department of dermatology at Phoenix Children’s Hospital. It typically appears in the teens and tweens and usually presents as a large herald patch or plaque on the trunk. As the herald patch resolves, smaller lesions will develop on the trunk following skin folds. “It’s rarely symptomatic and it’s very short lived, and clears within 6-12 weeks,” Dr. Andrews noted. “It can present with an inverse pattern involving the face, neck, and groin, but sparing the trunk. This variant, termed inverse pityriasis rosea, can be confused with inverse psoriasis, which has a similar distribution. However, the inverse pattern of pityriasis rosea will still resolve in a similar time frame to its more classic variant.”
Pityriasis lichenoides can also be mistaken for psoriasis. The acute form can present with erythematous, scaly papules and plaques, but lesions are often found in different phases of resolution or healing. “This benign lymphoproliferative skin disorder can be very difficult to distinguish from psoriasis and may require a biopsy to rule in or out,” Dr. Andrews said. “It can last months to years and there are few treatments that are effective. It is typically nonresponsive to topical steroids and other treatments that would be more effective for psoriasis, helping to distinguish the two. It is thought to exist in the spectrum with other lymphoproliferative diseases including cutaneous T-cell lymphoma [CTCL]. However, there are only a few cases in the literature that support a transformation from pityriasis lichenoides to CTCL.”
Seborrheic dermatitis is more common than atopic dermatitis and psoriasis, but it can be mistaken for psoriasis. It is caused by an inflammatory response secondary to overgrowth of Malassezia yeast and has a bimodal age distribution. “Seborrheic dermatitis affects babies, teens, and tweens, and can persist into adulthood,” he said. “Infants with cradle cap usually resolve with moisturization, gentle brushing, and occasional antifungal shampoos.” Petaloid seborrheic dermatitis can predominately involve the face with psoriatic-appearing induration, plaques, and varying degrees of scales. “In skin of color, this can be confused with discoid lupus, sarcoidosis, and psoriasis, occasionally requiring a biopsy to distinguish,” said Dr. Andrews, who is also an assistant professor of pediatrics at the Mayo Clinic College of Medicine and Science in Scottsdale, Ariz.
Another psoriasis mimicker, pityriasis amiantacea, is thought to be a more severe form of seborrheic dermatitis. It presents with concretions of scale around hair follicles that are highly adherent and are sometimes called sebopsoriasis. “It may be associated with cutaneous findings of psoriasis elsewhere, but may also be found with secondarily infected atopic dermatitis and tinea capitis; however, in my clinical experience, it is most often found in isolation,” he said. “There may be a seasonal association with exacerbation in warm temperatures, and treatment often consists of humectants like salicylic acid for loosening scale, topical steroids for inflammation, and gentle combing out of scale.”
Infections can also mimic psoriasis. For example, tinea infections are often misdiagnosed as eczema or psoriasis and treated with topical steroids. “This can lead to tinea incognito, making it harder to diagnose either condition without attention to detail,” Dr. Andrews said. “On the body, look for expanding lesions with more raised peripheral edges, and central flattening, giving a classic annular appearance. It’s also important to inquire about family history and contacts including pets, contact sports/mat sports (think yoga, gymnastics, martial arts), or other contacts with similar rashes.” Work-up typically includes a fungal culture and starting empiric oral antifungal medications. “It is important to be able to distinguish scalp psoriasis from tinea capitis to prevent the more inflammatory form of tinea capitis, kerion (a deeper more symptomatic, painful and purulent dermatitis), which can lead to permanent scarring alopecia,” he said.
Bacterial infections can also mimic psoriasis, specifically nonbullous impetigo and ecthyma, the more ulcerative form of impetigo. The most frequent associations are group A Streptococcus, methicillin-susceptible Staphylococcus aureus and methicillin-resistant S. aureus.
Dr. Andrews closed his presentation by noting that tumor necrosis factor–alpha inhibitor–induced psoriasiform drug eruptions can occur in psoriasis-naive patients or unmask a predilection for psoriasis in patients with Crohn’s disease, juvenile idiopathic arthritis, or other autoinflammatory or autoimmune conditions. “They may improve with continued treatment and resolve with switching treatments,” he said. “Early biopsy in psoriasiform drug eruptions can appear like atopic dermatitis on pathology. When suspecting psoriasis in a pediatric patient, it is important to consider the history and physical exam as well as family history and associated comorbidities. While a biopsy may aide in the work-up, diagnosis can be made clinically.”
Dr. Andrews reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY 2020
Expert shares his approach to treating warts in children
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
FROM PEDIATRIC DERMATOLOGY 2020
Treat acne aggressively upfront, expert advises
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
First, determine the types of lesions they have. “Do they have comedones, papules/pustules, and nodules present?” she asked during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. Second, quantify the number of lesions that they have. Is it few? Several? Many? Third, determine the extent of their acne. “Is it limited to half the face, or is it generalized to the face, back, chest, and shoulders?” added Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey.
Fourth, identify postinflammatory changes such as erythema, hyperpigmentation, and scarring “because that’s going to influence your management,” she said. “Finally, you want to give a quick investigative global assessment of the acne severity where you quantify them as being clear, almost clear, mild, moderate, or severe. You want to do this with each patient at every visit so you can determine what their initial treatment’s going to be and what their management going forward is going to be.”
According to Dr. Zaenglein, the best acne treatments are based on the pathogenesis of the skin condition and trying to target as many pathogenic factors as possible. The four main pathogenic factors in acne include hyperkeratinization, increased sebum production, cutibacterium, and inflammation. “This is not a stepwise process; there’s an interplay between all of those factors,” she said. “All acne is inflammatory, but each of the treatments we have target specific factors. Retinoids target hyperkeratinization and inflammation, whereas the hormonal therapies will address decreased sebum production. Antimicrobial agents like benzoyl peroxide and antibiotics will work to decrease cutibacterium acnes. All of these are influenced by the exposome. This includes your genetics, external factors like pollution or changes in seasons that can affect your skin and the severity of your acne.” A state of hyperandrogenism, she added, “can definitely increase acne” and is seen in patients with polycystic ovary syndrome (PCOS).
For patients with mild acne, initial treatment should consist of a topical retinoid and, almost always, benzoyl peroxide, “unless it’s a pure comedonal form of acne,” Dr. Zaenglein said. She recommended using the combination of a topical retinoid and benzoyl peroxide, noting that while it used to be difficult to find benzoyl peroxide, “nowadays there are numerous manufacturers and different formulations of benzoyl peroxide. We also have over-the-counter adapalene now, which is great. So now we have a complete routine for patients with adapalene and benzoyl peroxide that you can combine together in a cost-effective way.”
If the initial regimen fails to improve the patient’s mild acne, a second-line treatment would be to change the retinoid and continue on the existing benzoyl peroxide formulation or to add dapsone gel if the patient is experiencing skin irritation. The four retinoids currently available include adapalene, tretinoin, tazarotene, and trifarotene. “These normalize keratinocyte differentiation, reduce keratinocyte proliferation, and decrease expression of inflammatory markers,” Dr. Zaenglein noted. “They also prevent scarring. Adapalene is considered to be the most tolerable, whereas tazarotene may have an edge on efficacy. There’s a lot of overlap; head-to-head studies may not always match them up exactly, but generally this is how it’s considered. Picking the right retinoid for your patient based on efficacy and tolerability is most important.”
The newest topical retinoid, trifarotene 50 mcg/g cream, is a fourth-generation retinoid which is retinoic acid receptor gamma selective. Pivotal trials were conducted in patients aged 9 years and older with moderate facial and truncal acne. With monotherapy there was a success rate of 36% at 12 weeks and 60% at 52 weeks based on the Investigator’s Global Assessment. Another newcomer, tazarotene 0.045% lotion, is a third-generation retinoid which is retinoic acid receptor alpha beta gamma selective. It’s approved for moderate to severe facial acne in patients 9 years and older.
To optimize tolerance to retinoids, Dr. Zaenglein asks patients about their typical skin care regimen. “I ask them what they’re washing their face with,” she said. “Are they using apricot scrubs or harsh cleansers? Make sure they’re applying it to the entire face and not spot-treating. You get less irritation when it’s applied to dry skin, so you can recommend that. Make sure that they use a bland unscented moisturizer in the morning and apply it over top of their retinoid. I always warn them that irritation usually peaks at about 2 weeks. If they can power through, the irritation will improve with continued use.”
To optimize adherence to retinoids, she asks patients how many nights per week that they apply it. If they are using it all seven nights, “they’re good at using it,” she said. “If they say three nights, then they need to work on getting it on more frequently.”
Topical dapsone gel (5% and 7.5%) is mainly used for patients with papular-pustular acne. “Its mechanism of action for acne is not known, but presumptively it’s anti-inflammatory,” Dr. Zaenglein said. “It doesn’t require G6PD [glucose-6-phosphate dehydrogenase] testing. It can cause some orange discoloration of your skin or fabrics if you use it with benzoyl peroxide, so you want to apply them at different times of the day. It’s well tolerated. I tend to use it in patients who have problems tolerating any topical retinoid or any benzoyl peroxide but have mild to moderate acne.”
For patients with moderate acne, consider combination therapy to target as many pathogenic factors as possible. “Use a topical retinoid plus benzoyl peroxide with or without a systemic antibiotic,” Dr. Zaenglein advised. “I may give them an oral antibiotic if their acne is not responsive to the routine. But you wouldn’t want to combine the systemic antibiotic with a topical antibiotic, like clindamycin with doxycycline, because you don’t need two antibiotics. Make sure that you treat aggressively up front. It can take up to 3 months to see improvement. I counsel my patients that we’ll rescue with the antibiotic and then we maintain, but we’re going to stop that antibiotic after 3 months.”
Systemic antibiotic options for acne include tetracyclines, doxycycline, minocycline, and sarecycline. “Tetracycline itself we don’t use too much because you have to take it on an empty stomach, and availability is sometimes an issue,” she said. “Primarily, we use doxycycline. You can take it with food, so that helps. The main side effects are gastrointestinal upset and photosensitivity. Alternately, you can use minocycline, which is also okay to take with food. It does have more potentially worrisome side effects, including pseudotumor cerebri, blue pigmentation, autoimmune hepatitis, and DRESS [drug reaction with eosinophilia and systemic symptoms].”
Sarecycline is the first narrow spectrum tetracycline for acne, with fewer vestibular and phototoxic side effects, compared with other tetracyclines. “It also has less effect on the GI flora,” Dr. Zaenglein said. “It’s a good alternative but it can be costly, so make sure to check the pricing for your patients.” She does not use other antibiotics such as TMP/SMX, penicillins, or cephalosporins for acne patients. “The reason is, the tetracyclines are not only antibacterial, but they’re anti-inflammatory,” she explained. “They also are lipophilic, so they will penetrate into the sebaceous unit where the heart of the acne is.”
For patients who don’t want to take an oral antibiotic, consider minocycline 4% foam, which was studied in moderate to severe acne in patients aged 9 years and older. The pooled results from the three studies showed a 47% mean improvement in inflammatory acne, compared with 37% among those in the vehicle arm. “You wouldn’t use this as monotherapy; you’d use this in combination with the topical retinoid and the benzoyl peroxide,” Dr. Zaenglein said.
Most primary care providers do not prescribe isotretinoin for patients with severe acne, but they can start patients on triple therapy with a topical retinoid, benzoyl peroxide, and a systemic antibiotic at its full dose. “The efficacy of triple therapy in patients you would typically deem as isotretinoin worthy is actually pretty good,” she said. “There have been several studies looking at this, and about 70%-80% of patients will respond to triple therapy, where they are no longer deemed isotretinoin candidates. They still may need to move on to isotretinoin, but they will be improved.”
Dr. Zaenglein disclosed that she is a consultant for Cassiopea, Novartis, and Pfizer. She has also received grants or research support from AbbVie, Incyte, and Pfizer.
FROM PEDIATRIC DERMATOLOGY 2020
Lifestyle changes may explain skin lesions in pandemic-era patients
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
Scaly nose plaque
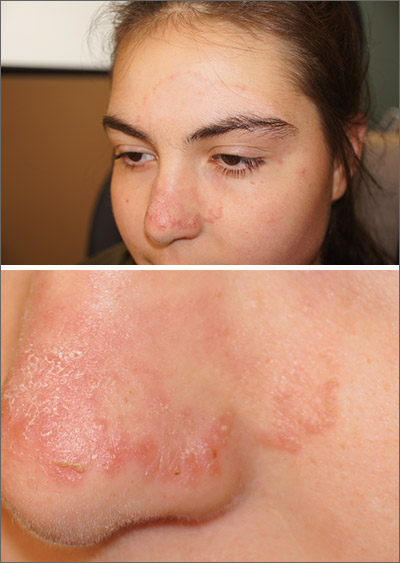
An annular morphology was appreciated on close inspection and small pustules were seen at the edges—features consistent with tinea faciei, a fungal infection of facial skin. A skin exam did not reveal any scaling or erythema on the scalp, hands, feet, trunk, or nails. The diagnosis was confirmed during the visit with a skin scraping and examination in potassium hydroxide with parker pen blue ink (Swartz-Lamkins stain) which revealed hyphae. The diagnosis was made with the knowledge that a history of eczema increases the risk of fungal, viral, and bacterial infections due to an impaired skin barrier.
Tinea faciei is an uncommon diagnosis that often is misdiagnosed as facial dermatitis, rosacea, or acne. The differential diagnosis also includes discoid lupus and psoriasis. Rarely is the annular presentation as obvious as it was here. Diagnosing tinea faciei in a patient can be made more challenging if the patient is already being treated with steroids. That’s because the steroids may decrease the clinical signs of tinea and allow subtle, slow progression of disease.
The location of fungal disease has implications for treatment. While some cases of tinea faciei may respond to topical antifungals, involvement of the eyebrows and glandular structures of the mid-face are beyond the depth of penetration of topical formulations. In these cases, systemic antifungals such as terbinafine, griseofulvin, or itraconazole are more effective.
Because of eyebrow and glandular involvement, this patient was given oral terbinafine 250 mg/d for 3 weeks and the lesion cleared completely in that time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Amigo M, Milani-Nejad N, Mosser-Goldfarb J. Periocular tinea faciei. J Pediatr. 2020;221:255-256.

An annular morphology was appreciated on close inspection and small pustules were seen at the edges—features consistent with tinea faciei, a fungal infection of facial skin. A skin exam did not reveal any scaling or erythema on the scalp, hands, feet, trunk, or nails. The diagnosis was confirmed during the visit with a skin scraping and examination in potassium hydroxide with parker pen blue ink (Swartz-Lamkins stain) which revealed hyphae. The diagnosis was made with the knowledge that a history of eczema increases the risk of fungal, viral, and bacterial infections due to an impaired skin barrier.
Tinea faciei is an uncommon diagnosis that often is misdiagnosed as facial dermatitis, rosacea, or acne. The differential diagnosis also includes discoid lupus and psoriasis. Rarely is the annular presentation as obvious as it was here. Diagnosing tinea faciei in a patient can be made more challenging if the patient is already being treated with steroids. That’s because the steroids may decrease the clinical signs of tinea and allow subtle, slow progression of disease.
The location of fungal disease has implications for treatment. While some cases of tinea faciei may respond to topical antifungals, involvement of the eyebrows and glandular structures of the mid-face are beyond the depth of penetration of topical formulations. In these cases, systemic antifungals such as terbinafine, griseofulvin, or itraconazole are more effective.
Because of eyebrow and glandular involvement, this patient was given oral terbinafine 250 mg/d for 3 weeks and the lesion cleared completely in that time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

An annular morphology was appreciated on close inspection and small pustules were seen at the edges—features consistent with tinea faciei, a fungal infection of facial skin. A skin exam did not reveal any scaling or erythema on the scalp, hands, feet, trunk, or nails. The diagnosis was confirmed during the visit with a skin scraping and examination in potassium hydroxide with parker pen blue ink (Swartz-Lamkins stain) which revealed hyphae. The diagnosis was made with the knowledge that a history of eczema increases the risk of fungal, viral, and bacterial infections due to an impaired skin barrier.
Tinea faciei is an uncommon diagnosis that often is misdiagnosed as facial dermatitis, rosacea, or acne. The differential diagnosis also includes discoid lupus and psoriasis. Rarely is the annular presentation as obvious as it was here. Diagnosing tinea faciei in a patient can be made more challenging if the patient is already being treated with steroids. That’s because the steroids may decrease the clinical signs of tinea and allow subtle, slow progression of disease.
The location of fungal disease has implications for treatment. While some cases of tinea faciei may respond to topical antifungals, involvement of the eyebrows and glandular structures of the mid-face are beyond the depth of penetration of topical formulations. In these cases, systemic antifungals such as terbinafine, griseofulvin, or itraconazole are more effective.
Because of eyebrow and glandular involvement, this patient was given oral terbinafine 250 mg/d for 3 weeks and the lesion cleared completely in that time.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Amigo M, Milani-Nejad N, Mosser-Goldfarb J. Periocular tinea faciei. J Pediatr. 2020;221:255-256.
Amigo M, Milani-Nejad N, Mosser-Goldfarb J. Periocular tinea faciei. J Pediatr. 2020;221:255-256.