User login
Skin patterns of COVID-19 vary widely
according to Christine Ko, MD.
“Things are very fluid,” Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn., said during the virtual annual meeting of the American Academy of Dermatology. “New studies are coming out daily. Due to the need for rapid dissemination, a lot of the studies are case reports, but there are some nice case series. Another caveat for the literature is that a lot of these cases were not necessarily confirmed with testing for SARS-CoV-2, but some were.”
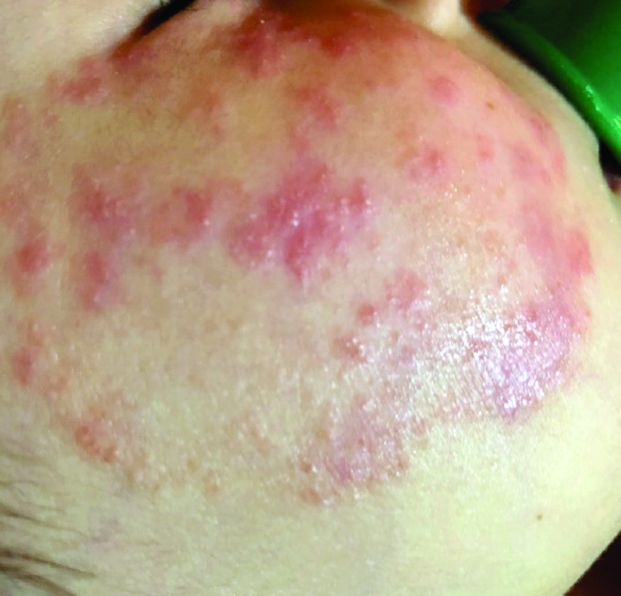
Dr. Ko framed her remarks largely on a case collection survey of images and clinical data from 375 patients in Spain with suspected or confirmed COVID-19 that was published online April 29, 2020, in the British Journal of Dermatology (doi: 10.1111/bjd.19163). Cutaneous manifestations included early vesicular eruptions mainly on the trunk or limbs (9%), maculopapular (47%) to urticarial lesions (19%) mainly on the trunk, and acral areas of erythema sometimes with vesicles or erosion (perniosis-like) (19%) that seemed to be a later manifestation of COVID-19. Retiform purpura or necrosis (6%) was most concerning in terms of skin disease, with an associated with a mortality of 10%.
On histology, the early vesicular eruptions are typically marked by dyskeratotic keratinocytes, Dr. Ko said, while urticarial lesions are characterized by a mixed dermal infiltrate; maculopapular lesions were a broad category. “There are some case reports that show spongiotic dermatitis or parakeratosis with a lymphocytic infiltrate,” she said. “A caveat to keep in mind is that, although these patients may definitely have COVID-19 and be confirmed to have it by testing, hypersensitivity reactions may be due to the multiple medications they’re on.”
Patients can develop a spectrum of lesions that are suggestive of vascular damage or occlusion, Dr. Ko continued. Livedoid lesions may remain static and not eventuate into necrosis or purpura but will self-resolve. Purpuric lesions and acral gangrene have been described, and these lesions correspond to vascular occlusion on biopsy.
A later manifestation are the so-called “COVID toes” with a superficial and deep lymphocytic infiltrate, as published June 1, 2020, in JAAD Case Reports: (doi: 10.1016/j.jdcr.2020.04.011).
“There are patients in the literature that have slightly different pathology, with lymphocytic inflammation as well as occlusion of vessels,” Dr. Ko said. A paper published June 20, 2020, in the British Journal of Dermatology used immunohistochemical staining against the SARS-CoV-2 spike protein, and biopsies of “COVID toes” had positive staining of endothelial cells, supporting the notion that “COVID toes” are a direct manifestation of viral infection (doi: 10.1111/bjd.19327).
“There’s a lot that we still don’t know, and some patterns are going to be outliers,” Dr. Ko concluded. “[As for] determining which skin manifestations are directly from coronavirus infection within the skin, more study is needed and likely time will tell.” She reported having no financial disclosures relevant to her talk.
according to Christine Ko, MD.
“Things are very fluid,” Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn., said during the virtual annual meeting of the American Academy of Dermatology. “New studies are coming out daily. Due to the need for rapid dissemination, a lot of the studies are case reports, but there are some nice case series. Another caveat for the literature is that a lot of these cases were not necessarily confirmed with testing for SARS-CoV-2, but some were.”
Dr. Ko framed her remarks largely on a case collection survey of images and clinical data from 375 patients in Spain with suspected or confirmed COVID-19 that was published online April 29, 2020, in the British Journal of Dermatology (doi: 10.1111/bjd.19163). Cutaneous manifestations included early vesicular eruptions mainly on the trunk or limbs (9%), maculopapular (47%) to urticarial lesions (19%) mainly on the trunk, and acral areas of erythema sometimes with vesicles or erosion (perniosis-like) (19%) that seemed to be a later manifestation of COVID-19. Retiform purpura or necrosis (6%) was most concerning in terms of skin disease, with an associated with a mortality of 10%.
On histology, the early vesicular eruptions are typically marked by dyskeratotic keratinocytes, Dr. Ko said, while urticarial lesions are characterized by a mixed dermal infiltrate; maculopapular lesions were a broad category. “There are some case reports that show spongiotic dermatitis or parakeratosis with a lymphocytic infiltrate,” she said. “A caveat to keep in mind is that, although these patients may definitely have COVID-19 and be confirmed to have it by testing, hypersensitivity reactions may be due to the multiple medications they’re on.”
Patients can develop a spectrum of lesions that are suggestive of vascular damage or occlusion, Dr. Ko continued. Livedoid lesions may remain static and not eventuate into necrosis or purpura but will self-resolve. Purpuric lesions and acral gangrene have been described, and these lesions correspond to vascular occlusion on biopsy.
A later manifestation are the so-called “COVID toes” with a superficial and deep lymphocytic infiltrate, as published June 1, 2020, in JAAD Case Reports: (doi: 10.1016/j.jdcr.2020.04.011).
“There are patients in the literature that have slightly different pathology, with lymphocytic inflammation as well as occlusion of vessels,” Dr. Ko said. A paper published June 20, 2020, in the British Journal of Dermatology used immunohistochemical staining against the SARS-CoV-2 spike protein, and biopsies of “COVID toes” had positive staining of endothelial cells, supporting the notion that “COVID toes” are a direct manifestation of viral infection (doi: 10.1111/bjd.19327).
“There’s a lot that we still don’t know, and some patterns are going to be outliers,” Dr. Ko concluded. “[As for] determining which skin manifestations are directly from coronavirus infection within the skin, more study is needed and likely time will tell.” She reported having no financial disclosures relevant to her talk.
according to Christine Ko, MD.
“Things are very fluid,” Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn., said during the virtual annual meeting of the American Academy of Dermatology. “New studies are coming out daily. Due to the need for rapid dissemination, a lot of the studies are case reports, but there are some nice case series. Another caveat for the literature is that a lot of these cases were not necessarily confirmed with testing for SARS-CoV-2, but some were.”
Dr. Ko framed her remarks largely on a case collection survey of images and clinical data from 375 patients in Spain with suspected or confirmed COVID-19 that was published online April 29, 2020, in the British Journal of Dermatology (doi: 10.1111/bjd.19163). Cutaneous manifestations included early vesicular eruptions mainly on the trunk or limbs (9%), maculopapular (47%) to urticarial lesions (19%) mainly on the trunk, and acral areas of erythema sometimes with vesicles or erosion (perniosis-like) (19%) that seemed to be a later manifestation of COVID-19. Retiform purpura or necrosis (6%) was most concerning in terms of skin disease, with an associated with a mortality of 10%.
On histology, the early vesicular eruptions are typically marked by dyskeratotic keratinocytes, Dr. Ko said, while urticarial lesions are characterized by a mixed dermal infiltrate; maculopapular lesions were a broad category. “There are some case reports that show spongiotic dermatitis or parakeratosis with a lymphocytic infiltrate,” she said. “A caveat to keep in mind is that, although these patients may definitely have COVID-19 and be confirmed to have it by testing, hypersensitivity reactions may be due to the multiple medications they’re on.”
Patients can develop a spectrum of lesions that are suggestive of vascular damage or occlusion, Dr. Ko continued. Livedoid lesions may remain static and not eventuate into necrosis or purpura but will self-resolve. Purpuric lesions and acral gangrene have been described, and these lesions correspond to vascular occlusion on biopsy.
A later manifestation are the so-called “COVID toes” with a superficial and deep lymphocytic infiltrate, as published June 1, 2020, in JAAD Case Reports: (doi: 10.1016/j.jdcr.2020.04.011).
“There are patients in the literature that have slightly different pathology, with lymphocytic inflammation as well as occlusion of vessels,” Dr. Ko said. A paper published June 20, 2020, in the British Journal of Dermatology used immunohistochemical staining against the SARS-CoV-2 spike protein, and biopsies of “COVID toes” had positive staining of endothelial cells, supporting the notion that “COVID toes” are a direct manifestation of viral infection (doi: 10.1111/bjd.19327).
“There’s a lot that we still don’t know, and some patterns are going to be outliers,” Dr. Ko concluded. “[As for] determining which skin manifestations are directly from coronavirus infection within the skin, more study is needed and likely time will tell.” She reported having no financial disclosures relevant to her talk.
FROM AAD 20
First validated classification criteria for discoid lupus erythematosus unveiled
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
FROM AAD 20
In scleroderma, GERD questionnaires are essential tools
Every rheumatologist ought to be comfortable in using a validated gastrointestinal symptom scale for evaluation of gastroesophageal reflux disease in patients with scleroderma, Tracy M. Frech, MD, declared at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
About 90% of scleroderma patients will develop GI tract involvement during the course of their connective tissue disease. And while any portion of the GI tract from esophagus to anus can be involved, the most common GI manifestation is gastroesophageal reflux disease (GERD), affecting up to 90% of scleroderma patients, observed Dr. Frech, a rheumatologist and director of the systemic sclerosis clinic at the University of Utah and the George E. Wahlen Department of Veterans Affairs Medical Center, both in Salt Lake City.
“It is essential to ask scleroderma patients questions in order to understand their gastrointestinal tract symptoms. The questionnaires are really critical for us to grade the severity and then properly order tests,” she explained. “The goal is symptom identification, ideally with minimal time burden and at no cost, to guide decisions that move our patients’ care forward.”
Three of the most useful validated instruments for assessment of GERD symptoms in scleroderma patients in routine clinical practice are the GerdQ, the University of California, Los Angeles, Scleroderma Clinical Trial Consortium GI Tract Questionnaire (UCLA GIT) 2.0 reflux scale, and the Patient-Reported Outcomes Measurement Information System (PROMIS) reflux scale.
The GerdQ is a six-item, self-administered questionnaire in which patients specify how many days in the past week they have experienced heartburn, regurgitation, nausea, sleep interference, upper abdominal pain, and need for medication. A free online tool is available for calculating the likelihood of having GERD based upon GerdQ score. A score of 8 or more points out of a possible 18 has the highest sensitivity and specificity for diagnosis of GERD.
The UCLA GIT 2.0 – the most commonly used instrument for GI symptom assessment in scleroderma patients – includes 34 items. It takes 6-8 minutes to complete the whole thing, but patients being assessed for GERD only need answer the eight GERD-specific questions. Six of these eight questions are the same as in the GerdQ. One of the two extra questions asks about difficulty in swallowing solid food, which if answered affirmatively warrants early referral to a gastroenterologist. The other question inquires about any food triggers for the reflux, providing an opportunity for a rheumatologist to educate the patient about the importance of avoiding acidic foods, such as tomatoes, and other food and drink generally considered healthy but which actually exacerbate GERD.
The National Institutes of Health PROMIS scale, the newest of the three instruments, is a 60-item questionnaire; however, only 20 questions relate to reflux and dysphagia and are thus germane to a focused GERD assessment in scleroderma.
When a clinical diagnosis of GERD is made in a scleroderma patient based upon symptoms elicited by questionnaire, guidelines recommend a trial of empiric proton pump inhibitor therapy and behavioral interventions, such as raising the head of the bed, in order to confirm the diagnosis. If the patient reports feeling better after these basic interventions, the diagnosis is confirmed. If not, it’s time to make a referral to a gastroenterologist for specialized care, Dr. Frech said.
Dr. Frech was a coinvestigator in an international, prospective, longitudinal study of patient-reported outcomes measures in 116 patients with scleroderma and GERD. All study participants had to complete the UCLA GIT 2.0, the PROMIS reflux scale, and a third patient-reported GERD measure both before and after the therapeutic intervention. The UCLA GIT 2.0 and PROMIS instruments demonstrated similarly robust sensitivity for identifying changes in GERD symptoms after therapeutic intervention.
“It doesn’t really matter what questionnaire we’re using,” according to the rheumatologist. “But I will point out that there is significant overlap in symptoms among GERD, gastroparesis, functional dyspepsia, and eosinophilic esophagitis, all of which cause symptoms of heartburn and regurgitation. So we don’t want to ask these questions just once, we want to make an intervention and then reask the questions to ensure that we’re continuously moving forward with the gastrointestinal tract management plan.”
Dr. Frech reported having no financial conflicts regarding her presentation.
Every rheumatologist ought to be comfortable in using a validated gastrointestinal symptom scale for evaluation of gastroesophageal reflux disease in patients with scleroderma, Tracy M. Frech, MD, declared at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
About 90% of scleroderma patients will develop GI tract involvement during the course of their connective tissue disease. And while any portion of the GI tract from esophagus to anus can be involved, the most common GI manifestation is gastroesophageal reflux disease (GERD), affecting up to 90% of scleroderma patients, observed Dr. Frech, a rheumatologist and director of the systemic sclerosis clinic at the University of Utah and the George E. Wahlen Department of Veterans Affairs Medical Center, both in Salt Lake City.
“It is essential to ask scleroderma patients questions in order to understand their gastrointestinal tract symptoms. The questionnaires are really critical for us to grade the severity and then properly order tests,” she explained. “The goal is symptom identification, ideally with minimal time burden and at no cost, to guide decisions that move our patients’ care forward.”
Three of the most useful validated instruments for assessment of GERD symptoms in scleroderma patients in routine clinical practice are the GerdQ, the University of California, Los Angeles, Scleroderma Clinical Trial Consortium GI Tract Questionnaire (UCLA GIT) 2.0 reflux scale, and the Patient-Reported Outcomes Measurement Information System (PROMIS) reflux scale.
The GerdQ is a six-item, self-administered questionnaire in which patients specify how many days in the past week they have experienced heartburn, regurgitation, nausea, sleep interference, upper abdominal pain, and need for medication. A free online tool is available for calculating the likelihood of having GERD based upon GerdQ score. A score of 8 or more points out of a possible 18 has the highest sensitivity and specificity for diagnosis of GERD.
The UCLA GIT 2.0 – the most commonly used instrument for GI symptom assessment in scleroderma patients – includes 34 items. It takes 6-8 minutes to complete the whole thing, but patients being assessed for GERD only need answer the eight GERD-specific questions. Six of these eight questions are the same as in the GerdQ. One of the two extra questions asks about difficulty in swallowing solid food, which if answered affirmatively warrants early referral to a gastroenterologist. The other question inquires about any food triggers for the reflux, providing an opportunity for a rheumatologist to educate the patient about the importance of avoiding acidic foods, such as tomatoes, and other food and drink generally considered healthy but which actually exacerbate GERD.
The National Institutes of Health PROMIS scale, the newest of the three instruments, is a 60-item questionnaire; however, only 20 questions relate to reflux and dysphagia and are thus germane to a focused GERD assessment in scleroderma.
When a clinical diagnosis of GERD is made in a scleroderma patient based upon symptoms elicited by questionnaire, guidelines recommend a trial of empiric proton pump inhibitor therapy and behavioral interventions, such as raising the head of the bed, in order to confirm the diagnosis. If the patient reports feeling better after these basic interventions, the diagnosis is confirmed. If not, it’s time to make a referral to a gastroenterologist for specialized care, Dr. Frech said.
Dr. Frech was a coinvestigator in an international, prospective, longitudinal study of patient-reported outcomes measures in 116 patients with scleroderma and GERD. All study participants had to complete the UCLA GIT 2.0, the PROMIS reflux scale, and a third patient-reported GERD measure both before and after the therapeutic intervention. The UCLA GIT 2.0 and PROMIS instruments demonstrated similarly robust sensitivity for identifying changes in GERD symptoms after therapeutic intervention.
“It doesn’t really matter what questionnaire we’re using,” according to the rheumatologist. “But I will point out that there is significant overlap in symptoms among GERD, gastroparesis, functional dyspepsia, and eosinophilic esophagitis, all of which cause symptoms of heartburn and regurgitation. So we don’t want to ask these questions just once, we want to make an intervention and then reask the questions to ensure that we’re continuously moving forward with the gastrointestinal tract management plan.”
Dr. Frech reported having no financial conflicts regarding her presentation.
Every rheumatologist ought to be comfortable in using a validated gastrointestinal symptom scale for evaluation of gastroesophageal reflux disease in patients with scleroderma, Tracy M. Frech, MD, declared at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
About 90% of scleroderma patients will develop GI tract involvement during the course of their connective tissue disease. And while any portion of the GI tract from esophagus to anus can be involved, the most common GI manifestation is gastroesophageal reflux disease (GERD), affecting up to 90% of scleroderma patients, observed Dr. Frech, a rheumatologist and director of the systemic sclerosis clinic at the University of Utah and the George E. Wahlen Department of Veterans Affairs Medical Center, both in Salt Lake City.
“It is essential to ask scleroderma patients questions in order to understand their gastrointestinal tract symptoms. The questionnaires are really critical for us to grade the severity and then properly order tests,” she explained. “The goal is symptom identification, ideally with minimal time burden and at no cost, to guide decisions that move our patients’ care forward.”
Three of the most useful validated instruments for assessment of GERD symptoms in scleroderma patients in routine clinical practice are the GerdQ, the University of California, Los Angeles, Scleroderma Clinical Trial Consortium GI Tract Questionnaire (UCLA GIT) 2.0 reflux scale, and the Patient-Reported Outcomes Measurement Information System (PROMIS) reflux scale.
The GerdQ is a six-item, self-administered questionnaire in which patients specify how many days in the past week they have experienced heartburn, regurgitation, nausea, sleep interference, upper abdominal pain, and need for medication. A free online tool is available for calculating the likelihood of having GERD based upon GerdQ score. A score of 8 or more points out of a possible 18 has the highest sensitivity and specificity for diagnosis of GERD.
The UCLA GIT 2.0 – the most commonly used instrument for GI symptom assessment in scleroderma patients – includes 34 items. It takes 6-8 minutes to complete the whole thing, but patients being assessed for GERD only need answer the eight GERD-specific questions. Six of these eight questions are the same as in the GerdQ. One of the two extra questions asks about difficulty in swallowing solid food, which if answered affirmatively warrants early referral to a gastroenterologist. The other question inquires about any food triggers for the reflux, providing an opportunity for a rheumatologist to educate the patient about the importance of avoiding acidic foods, such as tomatoes, and other food and drink generally considered healthy but which actually exacerbate GERD.
The National Institutes of Health PROMIS scale, the newest of the three instruments, is a 60-item questionnaire; however, only 20 questions relate to reflux and dysphagia and are thus germane to a focused GERD assessment in scleroderma.
When a clinical diagnosis of GERD is made in a scleroderma patient based upon symptoms elicited by questionnaire, guidelines recommend a trial of empiric proton pump inhibitor therapy and behavioral interventions, such as raising the head of the bed, in order to confirm the diagnosis. If the patient reports feeling better after these basic interventions, the diagnosis is confirmed. If not, it’s time to make a referral to a gastroenterologist for specialized care, Dr. Frech said.
Dr. Frech was a coinvestigator in an international, prospective, longitudinal study of patient-reported outcomes measures in 116 patients with scleroderma and GERD. All study participants had to complete the UCLA GIT 2.0, the PROMIS reflux scale, and a third patient-reported GERD measure both before and after the therapeutic intervention. The UCLA GIT 2.0 and PROMIS instruments demonstrated similarly robust sensitivity for identifying changes in GERD symptoms after therapeutic intervention.
“It doesn’t really matter what questionnaire we’re using,” according to the rheumatologist. “But I will point out that there is significant overlap in symptoms among GERD, gastroparesis, functional dyspepsia, and eosinophilic esophagitis, all of which cause symptoms of heartburn and regurgitation. So we don’t want to ask these questions just once, we want to make an intervention and then reask the questions to ensure that we’re continuously moving forward with the gastrointestinal tract management plan.”
Dr. Frech reported having no financial conflicts regarding her presentation.
FROM SOTA 2020
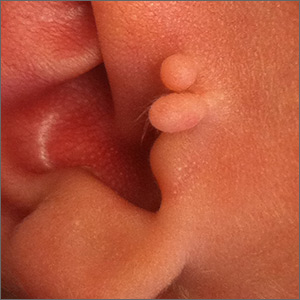
Newborn ear lesion
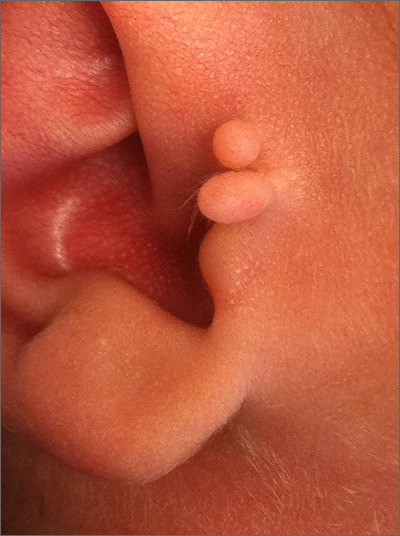
This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.

This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.
Eczema may increase lymphoma risk, cohort studies suggest
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
FROM JAMA DERMATOLOGY
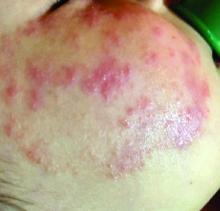
Pilot study shows apremilast effective for severe recurrent canker sores
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
FROM AAD 20
Increased hypothyroidism risk seen in young men with HS
Anna Figueiredo, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
The surprise about this finding from a large retrospective case-control study stems from the fact that the elevated risk for hypothyroidism didn’t also extend to younger women with hidradenitis suppurativa (HS) nor to patients older than 40 years of either gender, explained Dr. Figueiredo of the department of dermatology at Northwestern University, Chicago.
She presented a retrospective case-control study based on information extracted from a medical records database of more than 8 million Midwestern adults. Among nearly 141,000 dermatology patients with follow-up in the database for at least 1 year, there were 405 HS patients aged 18-40 years and 327 aged 41-89.
In an age-matched comparison with the dermatology patients without HS, the younger HS cohort was at a significant 1.52-fold increased risk for comorbid hypothyroidism. Upon further stratification by sex, only the younger men with HS were at increased risk. Those patients were at 3.95-fold greater risk for having a diagnosis of hypothyroidism than were age-matched younger male dermatology patients.
Both younger and older HS patients were at numerically increased risk for being diagnosed with hyperthyroidism; however, this difference didn’t approach statistical significance because there were so few cases: a total of just eight in the HS population across the full age spectrum.
Hidradenitis suppurativa is a chronic inflammatory disease with an estimated prevalence of up to 4% in the United States. Growing evidence suggests it is an immune-mediated disorder because the tumor necrosis factor inhibitor adalimumab (Humira) has been approved for treatment of HS.
Thyroid disease is also often autoimmune-mediated, but its relationship with HS hasn’t been extensively examined. A recent meta-analysis of five case-control studies concluded that HS was associated with a 1.36-fold increased risk of thyroid disease; however, the Nepalese investigators didn’t distinguish between hypo- and hyperthyroidism (J Am Acad Dermatol. 2020 Feb;82[2]:491-3).
Dr. Figueiredo reported having no financial conflicts regarding her study, which was without commercial support.
Anna Figueiredo, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
The surprise about this finding from a large retrospective case-control study stems from the fact that the elevated risk for hypothyroidism didn’t also extend to younger women with hidradenitis suppurativa (HS) nor to patients older than 40 years of either gender, explained Dr. Figueiredo of the department of dermatology at Northwestern University, Chicago.
She presented a retrospective case-control study based on information extracted from a medical records database of more than 8 million Midwestern adults. Among nearly 141,000 dermatology patients with follow-up in the database for at least 1 year, there were 405 HS patients aged 18-40 years and 327 aged 41-89.
In an age-matched comparison with the dermatology patients without HS, the younger HS cohort was at a significant 1.52-fold increased risk for comorbid hypothyroidism. Upon further stratification by sex, only the younger men with HS were at increased risk. Those patients were at 3.95-fold greater risk for having a diagnosis of hypothyroidism than were age-matched younger male dermatology patients.
Both younger and older HS patients were at numerically increased risk for being diagnosed with hyperthyroidism; however, this difference didn’t approach statistical significance because there were so few cases: a total of just eight in the HS population across the full age spectrum.
Hidradenitis suppurativa is a chronic inflammatory disease with an estimated prevalence of up to 4% in the United States. Growing evidence suggests it is an immune-mediated disorder because the tumor necrosis factor inhibitor adalimumab (Humira) has been approved for treatment of HS.
Thyroid disease is also often autoimmune-mediated, but its relationship with HS hasn’t been extensively examined. A recent meta-analysis of five case-control studies concluded that HS was associated with a 1.36-fold increased risk of thyroid disease; however, the Nepalese investigators didn’t distinguish between hypo- and hyperthyroidism (J Am Acad Dermatol. 2020 Feb;82[2]:491-3).
Dr. Figueiredo reported having no financial conflicts regarding her study, which was without commercial support.
Anna Figueiredo, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
The surprise about this finding from a large retrospective case-control study stems from the fact that the elevated risk for hypothyroidism didn’t also extend to younger women with hidradenitis suppurativa (HS) nor to patients older than 40 years of either gender, explained Dr. Figueiredo of the department of dermatology at Northwestern University, Chicago.
She presented a retrospective case-control study based on information extracted from a medical records database of more than 8 million Midwestern adults. Among nearly 141,000 dermatology patients with follow-up in the database for at least 1 year, there were 405 HS patients aged 18-40 years and 327 aged 41-89.
In an age-matched comparison with the dermatology patients without HS, the younger HS cohort was at a significant 1.52-fold increased risk for comorbid hypothyroidism. Upon further stratification by sex, only the younger men with HS were at increased risk. Those patients were at 3.95-fold greater risk for having a diagnosis of hypothyroidism than were age-matched younger male dermatology patients.
Both younger and older HS patients were at numerically increased risk for being diagnosed with hyperthyroidism; however, this difference didn’t approach statistical significance because there were so few cases: a total of just eight in the HS population across the full age spectrum.
Hidradenitis suppurativa is a chronic inflammatory disease with an estimated prevalence of up to 4% in the United States. Growing evidence suggests it is an immune-mediated disorder because the tumor necrosis factor inhibitor adalimumab (Humira) has been approved for treatment of HS.
Thyroid disease is also often autoimmune-mediated, but its relationship with HS hasn’t been extensively examined. A recent meta-analysis of five case-control studies concluded that HS was associated with a 1.36-fold increased risk of thyroid disease; however, the Nepalese investigators didn’t distinguish between hypo- and hyperthyroidism (J Am Acad Dermatol. 2020 Feb;82[2]:491-3).
Dr. Figueiredo reported having no financial conflicts regarding her study, which was without commercial support.
FROM AAD 20
More phase 3 data reported for abrocitinib for atopic dermatitis
Melinda Gooderham, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The positive results of this 391-patient, international, randomized, double-blind, placebo-controlled clinical trial mirror those previously reported in the identically designed JADE-MONO-1 pivotal phase 3 trial, noted Dr. Gooderham, medical director of the SKiN Centre for Dermatology and a dermatologist at Queen’s University in Kingston, Ont.
Participants in JADE-MONO-2 were randomized 2:2:1 to abrocitinib at 200 mg once daily, 100 mg once daily, or placebo for 12 weeks. The coprimary endpoint of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with an improvement of at least two grades at week 12 was achieved in 38.1% and 28.4% of patients on 200 and 100 mg of the JAK-1 inhibitor, respectively, compared with 9.1% of placebo-treated controls. The other coprimary endpoint – significant improvement in disease extent as defined by at least a 75% reduction from baseline in Eczema Area and Severity Index (EASI-75 response) at 12 weeks – was reached in 61% of patients on abrocitinib at 200 mg/day, 44.5% on 100 mg/day, and 10.4% of controls.
A key secondary endpoint was improvement in itch based on at least a 4-point improvement at week 12 on the Peak Pruritus Numerical Rating Scale from a mean baseline score of 7. This outcome was reached by 55.3% of patients on abrocitinib at 200 mg, 45.2% on 100 mg, and 11.5% on placebo. Of note, the reduction in itch was impressively fast, with significant separation from placebo occurring within the first 24 hours of the study, after just a single dose of abrocitinib. By week 2, roughly one-third of patients on high-dose and one-quarter of those on low-dose abrocitinib had already reached the itch endpoint, the dermatologist continued.
The improvement in pruritus scores in abrocitinib-treated patients was accompanied by significantly greater gains on validated measures of quality of life, another secondary endpoint. The EASI-90 response rate, yet another key secondary outcome, was 37.7% with abrocitinib at 200 mg, 23.9% with 100 mg, and 3.9% with placebo.
The safety profile of abrocitinib was essentially the same as for placebo with the exception of a 3.2% incidence of thrombocytopenia in patients on abrocitinib at 200 mg/day; no cases occurred in controls or patients on abrocitinib at 100 mg/day. Although venous thromboembolism has arisen as a potential concern in clinical trials of oral JAK inhibitors for rheumatoid arthritis, there were no cases in JADE-MONO-2. A long-term safety extension study in JADE-MONO participants is underway.
In an interview, Dr. Gooderham said that, based on the phase 2 study data that’s available for upadacitinib, another JAK-1-selective oral agent, abrocitinib and upadacitinib appear to be in the same ballpark with respect to efficacy as defined by IGA response, EASI improvement, and itch relief.
“The JAK-1 selectivity does seem to offer some advantage in levels of response over more broad JAK inhibition, such as with baritinib,” she added.
Asked how she foresees abrocitinib fitting into clinical practice, should it win regulatory approval for treatment of atopic dermatitis, Dr. Gooderham said it might be considered on a par with the injectable interleukin-4 and -13 inhibitor dupilumab (Dupixent) as next-line therapy after failure on topical therapy or as an option in patients who haven’t responded to or could not tolerate dupilumab. Abrocitinib will be an attractive option for patients who prefer oral therapy and will be an especially appealing medication in patients with a strong itch component to their atopic dermatitis, she added.
The results of JADE COMPARE, a phase 3, head-to-head randomized comparison of abrocitinib and dupilumab, are expected to be presented later this year at the virtual annual congress of the European Academy of Dermatology and Venereology. Pfizer has announced the key results, reporting that the JAK inhibitor at 200 mg/day achieved significantly greater improvements than dupilumab in the coprimary IGA and EASI-75 endpoints at 12 weeks.
JADE-MONO-2 was sponsored by Pfizer. Dr. Gooderham reported receiving research grants from that company and close to two dozen others.
The JADE-MONO-2 results have been published online (JAMA Dermatol. 2020 Jun 3;e201406. doi: 10.1001/jamadermatol.2020.1406).
Melinda Gooderham, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The positive results of this 391-patient, international, randomized, double-blind, placebo-controlled clinical trial mirror those previously reported in the identically designed JADE-MONO-1 pivotal phase 3 trial, noted Dr. Gooderham, medical director of the SKiN Centre for Dermatology and a dermatologist at Queen’s University in Kingston, Ont.
Participants in JADE-MONO-2 were randomized 2:2:1 to abrocitinib at 200 mg once daily, 100 mg once daily, or placebo for 12 weeks. The coprimary endpoint of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with an improvement of at least two grades at week 12 was achieved in 38.1% and 28.4% of patients on 200 and 100 mg of the JAK-1 inhibitor, respectively, compared with 9.1% of placebo-treated controls. The other coprimary endpoint – significant improvement in disease extent as defined by at least a 75% reduction from baseline in Eczema Area and Severity Index (EASI-75 response) at 12 weeks – was reached in 61% of patients on abrocitinib at 200 mg/day, 44.5% on 100 mg/day, and 10.4% of controls.
A key secondary endpoint was improvement in itch based on at least a 4-point improvement at week 12 on the Peak Pruritus Numerical Rating Scale from a mean baseline score of 7. This outcome was reached by 55.3% of patients on abrocitinib at 200 mg, 45.2% on 100 mg, and 11.5% on placebo. Of note, the reduction in itch was impressively fast, with significant separation from placebo occurring within the first 24 hours of the study, after just a single dose of abrocitinib. By week 2, roughly one-third of patients on high-dose and one-quarter of those on low-dose abrocitinib had already reached the itch endpoint, the dermatologist continued.
The improvement in pruritus scores in abrocitinib-treated patients was accompanied by significantly greater gains on validated measures of quality of life, another secondary endpoint. The EASI-90 response rate, yet another key secondary outcome, was 37.7% with abrocitinib at 200 mg, 23.9% with 100 mg, and 3.9% with placebo.
The safety profile of abrocitinib was essentially the same as for placebo with the exception of a 3.2% incidence of thrombocytopenia in patients on abrocitinib at 200 mg/day; no cases occurred in controls or patients on abrocitinib at 100 mg/day. Although venous thromboembolism has arisen as a potential concern in clinical trials of oral JAK inhibitors for rheumatoid arthritis, there were no cases in JADE-MONO-2. A long-term safety extension study in JADE-MONO participants is underway.
In an interview, Dr. Gooderham said that, based on the phase 2 study data that’s available for upadacitinib, another JAK-1-selective oral agent, abrocitinib and upadacitinib appear to be in the same ballpark with respect to efficacy as defined by IGA response, EASI improvement, and itch relief.
“The JAK-1 selectivity does seem to offer some advantage in levels of response over more broad JAK inhibition, such as with baritinib,” she added.
Asked how she foresees abrocitinib fitting into clinical practice, should it win regulatory approval for treatment of atopic dermatitis, Dr. Gooderham said it might be considered on a par with the injectable interleukin-4 and -13 inhibitor dupilumab (Dupixent) as next-line therapy after failure on topical therapy or as an option in patients who haven’t responded to or could not tolerate dupilumab. Abrocitinib will be an attractive option for patients who prefer oral therapy and will be an especially appealing medication in patients with a strong itch component to their atopic dermatitis, she added.
The results of JADE COMPARE, a phase 3, head-to-head randomized comparison of abrocitinib and dupilumab, are expected to be presented later this year at the virtual annual congress of the European Academy of Dermatology and Venereology. Pfizer has announced the key results, reporting that the JAK inhibitor at 200 mg/day achieved significantly greater improvements than dupilumab in the coprimary IGA and EASI-75 endpoints at 12 weeks.
JADE-MONO-2 was sponsored by Pfizer. Dr. Gooderham reported receiving research grants from that company and close to two dozen others.
The JADE-MONO-2 results have been published online (JAMA Dermatol. 2020 Jun 3;e201406. doi: 10.1001/jamadermatol.2020.1406).
Melinda Gooderham, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The positive results of this 391-patient, international, randomized, double-blind, placebo-controlled clinical trial mirror those previously reported in the identically designed JADE-MONO-1 pivotal phase 3 trial, noted Dr. Gooderham, medical director of the SKiN Centre for Dermatology and a dermatologist at Queen’s University in Kingston, Ont.
Participants in JADE-MONO-2 were randomized 2:2:1 to abrocitinib at 200 mg once daily, 100 mg once daily, or placebo for 12 weeks. The coprimary endpoint of skin clearance as reflected in an Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with an improvement of at least two grades at week 12 was achieved in 38.1% and 28.4% of patients on 200 and 100 mg of the JAK-1 inhibitor, respectively, compared with 9.1% of placebo-treated controls. The other coprimary endpoint – significant improvement in disease extent as defined by at least a 75% reduction from baseline in Eczema Area and Severity Index (EASI-75 response) at 12 weeks – was reached in 61% of patients on abrocitinib at 200 mg/day, 44.5% on 100 mg/day, and 10.4% of controls.
A key secondary endpoint was improvement in itch based on at least a 4-point improvement at week 12 on the Peak Pruritus Numerical Rating Scale from a mean baseline score of 7. This outcome was reached by 55.3% of patients on abrocitinib at 200 mg, 45.2% on 100 mg, and 11.5% on placebo. Of note, the reduction in itch was impressively fast, with significant separation from placebo occurring within the first 24 hours of the study, after just a single dose of abrocitinib. By week 2, roughly one-third of patients on high-dose and one-quarter of those on low-dose abrocitinib had already reached the itch endpoint, the dermatologist continued.
The improvement in pruritus scores in abrocitinib-treated patients was accompanied by significantly greater gains on validated measures of quality of life, another secondary endpoint. The EASI-90 response rate, yet another key secondary outcome, was 37.7% with abrocitinib at 200 mg, 23.9% with 100 mg, and 3.9% with placebo.
The safety profile of abrocitinib was essentially the same as for placebo with the exception of a 3.2% incidence of thrombocytopenia in patients on abrocitinib at 200 mg/day; no cases occurred in controls or patients on abrocitinib at 100 mg/day. Although venous thromboembolism has arisen as a potential concern in clinical trials of oral JAK inhibitors for rheumatoid arthritis, there were no cases in JADE-MONO-2. A long-term safety extension study in JADE-MONO participants is underway.
In an interview, Dr. Gooderham said that, based on the phase 2 study data that’s available for upadacitinib, another JAK-1-selective oral agent, abrocitinib and upadacitinib appear to be in the same ballpark with respect to efficacy as defined by IGA response, EASI improvement, and itch relief.
“The JAK-1 selectivity does seem to offer some advantage in levels of response over more broad JAK inhibition, such as with baritinib,” she added.
Asked how she foresees abrocitinib fitting into clinical practice, should it win regulatory approval for treatment of atopic dermatitis, Dr. Gooderham said it might be considered on a par with the injectable interleukin-4 and -13 inhibitor dupilumab (Dupixent) as next-line therapy after failure on topical therapy or as an option in patients who haven’t responded to or could not tolerate dupilumab. Abrocitinib will be an attractive option for patients who prefer oral therapy and will be an especially appealing medication in patients with a strong itch component to their atopic dermatitis, she added.
The results of JADE COMPARE, a phase 3, head-to-head randomized comparison of abrocitinib and dupilumab, are expected to be presented later this year at the virtual annual congress of the European Academy of Dermatology and Venereology. Pfizer has announced the key results, reporting that the JAK inhibitor at 200 mg/day achieved significantly greater improvements than dupilumab in the coprimary IGA and EASI-75 endpoints at 12 weeks.
JADE-MONO-2 was sponsored by Pfizer. Dr. Gooderham reported receiving research grants from that company and close to two dozen others.
The JADE-MONO-2 results have been published online (JAMA Dermatol. 2020 Jun 3;e201406. doi: 10.1001/jamadermatol.2020.1406).
FROM AAD 20
ID dermatology: Advancements, but new challenges, over 50 years
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
When Stephen Tyring, MD, PhD, an infectious disease dermatologist, started his career in the early 1980s, he said “we were diagnosing Kaposi’s sarcoma right and left. We would see a new case every day or two.”
It was the early days of the HIV/AIDS epidemic, and dermatologists were at the forefront because HIV/AIDS often presented with skin manifestations. Dr. Tyring, clinical professor in the departments of dermatology, microbiology & molecular genetics and internal medicine at the University of Texas Health Science Center, Houston, and his colleagues referred Kaposi’s patients for chemotherapy and radiation, but the outlook was often grim, especially if lesions developed in the lungs.
Dermatologist don’t see much Kaposi’s anymore because of highly effective treatments for HIV.
Members of the original editorial advisory board saw it coming. In a feature in which board members provided their prediction for the 1970s that appeared in the first issue, New York dermatologist Norman Orentreich, MD, counted the “probable introduction of virucidal agents” as one of the “significant advances or changes that I foresee in the next 10 years.” J. Lamar Callaway, MD, professor of dermatology at Duke University, Durham, N.C., predicted that “the next 10 years should develop effective anti-viral agents for warts, herpes simplex, and herpes zoster.”
To celebrate the 50th anniversary of Dermatology News, we are looking back at how the field has changed since that first issue. The focus this month is infectious disease. There’s a lot to be grateful for but there are also challenges like antibiotic resistance that weren’t on the radar screens of Dr. Orentreich, Dr. Callaway, and their peers in 1970.
All in all, “the only thing I wish we did the old way is sit at the bedside and talk to patients more. We rely so much on technology now that we sometimes lose the art of medicine, which is comforting to the patient,” said Theodore Rosen, MD, an ID dermatologist and professor of dermatology at Baylor College of Medicine, Houston, who’s been in practice for 42 years.
“A lot of advancements against herpes viruses”
One of the biggest wins for ID dermatology over the last 5 decades has been the management of herpes, both herpes simplex virus 1 and 2, as well as herpes zoster virus. It started with the approval of acyclovir in 1981. Before then, “we had no direct therapy for genital herpes, herpes zoster, or disseminated herpes in immunosuppressed or cancer patients,” Dr. Rosen said.
“I can remember doing an interview with Good Morning America when I gave the first IV dose of acyclovir in the city of Houston for really bad disseminated herpes” in an HIV patient, he said, and it worked.
Two derivatives, valacyclovir and famciclovir, became available in the mid-1990s, so today “we have three drugs and some others at the periphery that are all highly effective not only” against herpes, but also for preventing outbreaks; valacyclovir can even prevent asymptomatic shedding, therefore possibly preventing new infections. “That’s a concept we didn’t even have 40 years ago,” Dr. Rosen said.
Cidofovir has also made a difference. The IV formulation was approved for AIDS-associated cytomegalovirus retinitis in 1996 but discontinued a few years later amid concerns of severe renal toxicity. It’s found a new home in dermatology since then, explained ID dermatologist Carrie Kovarik, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
Dermatologists see acyclovir-resistant herpes “heaped up on the genitals in HIV patients,” and there weren’t many options in the past. A few years ago, “we [tried] injecting cidofovir directly into the skin lesions, and it’s been remarkably successful. It is a good way to treat these lesions” if dermatologists can get it compounded, she said.
Shingles vaccines, first the live attenuated zoster vaccine (Zostavax) approved by the Food and Drug Administration in 2006 and the more effective recombinant zoster vaccine (Shingrix) approved in 2017, have also had a significant impact.
Dr. Rosen remembers what it was like when he first started practicing over 40 years ago. Not uncommonly, “we saw horrible cases of shingles,” including one in his uncle, who was left with permanent hand pain long after the rash subsided.
Today, “I see much less shingles, and when I do see it, it’s in a much-attenuated form. [Shingrix], even if it doesn’t prevent the disease, often prevents postherpetic neuralgia,” he said.
Also, with pediatric vaccinations against chicken pox, “we’re probably going to see a whole new generation without shingles, which is huge. We’ve made a lot of advancements against herpes viruses,” Dr. Kovarik said.
“We finally found something that helps”
“We’ve [also] come a really long way with genital wart treatment,” Dr. Kovarik said.
It started with approval of topical imiquimod in 1997. “Before that, we were just killing one wart here and one wart there” but they would often come back and pop up in other areas. Injectable interferon was an option at the time, but people didn’t like all the needles.
With imiquimod, “we finally [had] a way to target HPV [human papillomavirus] and not just scrape” or freeze one wart at a time, and “we were able to generate an inflammatory response in the whole area to clear the virus.” Working with HIV patients, “I see sheets and sheets of confluent warts throughout the whole genital area; to try to freeze that is impossible. Now I have a way to get rid of [genital] warts and keep them away even if you have a big cluster,” she said.
“Sometimes, we’ll do both liquid nitrogen and imiquimod. That’s a good way to tackle people who have a high burden of warts,” Dr. Kovarik noted. Other effective treatments have come out as well, including an ointment formulation of sinecatechins, extracted from green tea, “but you have to put it on several times a day, and insurance companies don’t cover it often,” she said.
Intralesional cidofovir is also proving to be boon for potentially malignant refractory warts in HIV and transplant patients. “It’s an incredible treatment. We can inject that antiviral into warts and get rid of them. We finally found something that helps” these people, Dr. Kovarik said.
The HPV vaccine Gardasil is making a difference, as well. In addition to cervical dysplasia and anogenital cancers, it protects against two condyloma strains. Dr. Rosen said he’s seeing fewer cases of genital warts now than when he started practicing, likely because of the vaccine.
“Organisms that weren’t pathogens are now pathogens”
Antibiotic resistance probably tops the list for what’s changed in a bad way in ID dermatology since 1970. Dr. Rosen remembers at the start of his career that “we never worried about antibiotic resistance. We’d put people on antibiotics for acne, rosacea, and we’d keep them on them for 3 years, 6 years”; resistance wasn’t on the radar screen and was not mentioned once in the first issue of Dermatology News, which was packed with articles and ran 24 pages.
The situation is different now. Driven by decades of overuse in agriculture and the medical system, antibiotic resistance is a concern throughout medicine, and unfortunately, “we have not come nearly as far as fast with antibiotics,” at least the ones dermatologists use, “as we have with antivirals,” Dr. Tyring said.
For instance, methicillin-resistant Staphylococcus aureus (MRSA), first described in the United States in 1968, is “no longer the exception to the rule, but the rule” itself, he said, with carbuncles, furuncles, and abscesses not infrequently growing out MRSA. There are also new drug-resistant forms of old problems like gonorrhea and tuberculosis, among other developments, and impetigo has shifted since 1970 from mostly a Streptococcus infection easily treated with penicillin to often a Staphylococcus disease that’s resistant to it. There’s also been a steady march of new pathogens, including the latest one, SARS-CoV-2, the virus that causes COVID-19, which has been recognized as having a variety of skin manifestations.
“No matter how smart we think we are, nature has a way of putting us back in our place,” Dr. Rosen said.
The bright spot is that “we’ve become very adept at identifying and characterizing” microbes “based on techniques we didn’t even have when I started practicing,” such as polymerase chain reaction. “It has taken a lot of guess work out of treating infectious diseases,” he said.
The widespread use of immunosuppressives such as cyclophosphamide, mycophenolate, azathioprine, rituximab, and other agents used in conjunction with solid organ transplantation, has also been a challenge. “We are seeing infections with really odd organisms. Just recently, I had a patient with fusarium in the skin; it’s a fungus that lives in the dirt. I saw a patient with a species of algae” that normally lives in stagnant water, he commented. “We used to get [things like that] back on reports, and we’d throw them away. You can’t do that anymore. Organisms that weren’t pathogens in the past are now pathogens,” particularly in immunosuppressed people, Dr. Rosen said.
Venereologists no more
There’s been another big change in the field. “Back in the not too distant past, dermatologists in the U.S. were referred to as ‘dermatologist-venereologists.’ ” It goes back to the time when syphilis wasn’t diagnosed and treated early, so patients often presented with secondary skin complications and went to dermatologists for help. As a result, “dermatologists became the most experienced at treating it,” Dr. Tyring said.
That’s faded from practice. Part of the reason is that as late as 2000, syphilis seemed to be on the way out; the Centers for Disease and Control and Prevention even raised the possibility of elimination. Dermatologists turned their attention to other areas.
It might have been short-sighted, Dr. Rosen said. Syphilis has made a strong comeback, and drug-resistant gonorrhea has also emerged globally and in at least a few states. No other medical field has stepped in to take up the slack. “Ob.gyns. are busy delivering babies, ID [physicians are] concerned about HIV, and urologists are worried about kidney stones and cancer.” Other than herpes and genital warts, “we have not done well” with management of sexually transmitted diseases, he said.
“I could sense” his frustration
The first issue of Dermatology News carried an article and photospread about scabies that could run today, except that topical permethrin and oral ivermectin have largely replaced benzyl benzoate and sulfur ointments for treatment in the United States. In the article, Scottish dermatologist J. O’D. Alexander, MD, called scabies “the scourge of mankind” and blamed it’s prevalence on “an offhand attitude to the disease which makes control very difficult.”
“I could sense this man’s frustration that people were not recognizing scabies,” Dr. Kovarik said, and it’s no closer to being eradicated than it was in 1970. “It’s still around, and we see it in our clinics. It’s a horrible disease in kids we see in dermatology not infrequently,” and treatment has only advanced a bit.
The article highlights what hasn’t changed much in ID dermatology over the years. Common warts are another one. “With all the evolution in medicine, we don’t have any better treatments approved for common warts than we ever had.” Injecting cidofovir “works great,” but access is a problem, Dr. Tyring said.
Onychomycosis has also proven a tough nut to crack. Readers back in 1970 counted the introduction of the antifungal, griseofulvin, as a major advancement in the 1960s; it’s still a go-to for tinea capitis, but it didn’t work very well for toenail fungus. Terbinafine (Lamisil), approved in 1993, and subsequent developments have helped, but the field still awaits more effective options; a few potential new agents are in the pipeline.
Although there have been major advancements for serious systemic fungal infections, “we’ve mainly seen small steps forward” in ID dermatology, Dr. Tyring said.
Dr. Tyring, Dr. Kovarik, and Dr. Rosen said they had no relevant disclosures.
What presents as deep erythematous papules, pustules, and may form an annular or circular plaque?
Scrapings of the child’s rash were analyzed with potassium hydroxide (KOH) under microscopy which revealed multiple septate hyphae.
She was diagnosed with Majocchi’s granuloma. The fungal culture was positive for Trichophyton rubrum.
Majocchi’s granuloma (MG) is cutaneous mycosis in which the fungal infection goes deeper into the hair follicle causing granulomatous folliculitis and perifolliculitis.1 It was first described by Domenico Majocchi in 1883, and he named the condition “granuloma tricofitico.”2
It is commonly caused by T. rubrum but also can be caused by T. mentagrophytes, T. tonsurans, T. verrucosum, Microsporum canis, and Epidermophyton floccosum.2,3 Patients at risk for developing this infection include those previously treated with topical corticosteroids, immunosuppressed patients, patients with areas under occlusion, and those with areas traumatized by shaving. This infection is most commonly seen in the lower extremities, but can happen anywhere in the body. The lesions present as deep erythematous papules, pustules, and may form an annular or circular plaque as seen on our patient.
A KOH test of skin scrapings and hair extractions often can reveal fungal hyphae. Identification of the pathogen can be performed with culture or polymerase chain reaction of skin samples. If the diagnosis is uncertain or the KOH is negative, a skin biopsy can be performed. Histopathologic examination reveals perifollicular granulomas with associated dermal abscesses. Giant cells may be observed. MG is associated with chronic inflammation with lymphocytes, macrophages, epithelioid cells, and scattered multinucleated giant cells.2,3
The differential diagnosis for these lesions in children includes other granulomatous conditions such as granulomatous rosacea, sarcoidosis, and granuloma faciale, as well as bacterial or atypical mycobacterial infections, cutaneous leishmaniasis, and eosinophilic pustular folliculitis.
Treatment of MG requires systemic treatment with griseofulvin, itraconazole, or terbinafine for at least 4-8 weeks or until all the lesions have resolved. Our patient was treated with 6 weeks of high-dose griseofulvin with resolution of her lesions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Dermatol Online J. 2018 Dec 15;24(12):13030/qt89k4t6wj.
2. Med Mycol. 2012 Jul;50(5):449-57.
3. Clin Microbiol Rev. 2011 Apr;24(2):247-80.
Scrapings of the child’s rash were analyzed with potassium hydroxide (KOH) under microscopy which revealed multiple septate hyphae.
She was diagnosed with Majocchi’s granuloma. The fungal culture was positive for Trichophyton rubrum.
Majocchi’s granuloma (MG) is cutaneous mycosis in which the fungal infection goes deeper into the hair follicle causing granulomatous folliculitis and perifolliculitis.1 It was first described by Domenico Majocchi in 1883, and he named the condition “granuloma tricofitico.”2
It is commonly caused by T. rubrum but also can be caused by T. mentagrophytes, T. tonsurans, T. verrucosum, Microsporum canis, and Epidermophyton floccosum.2,3 Patients at risk for developing this infection include those previously treated with topical corticosteroids, immunosuppressed patients, patients with areas under occlusion, and those with areas traumatized by shaving. This infection is most commonly seen in the lower extremities, but can happen anywhere in the body. The lesions present as deep erythematous papules, pustules, and may form an annular or circular plaque as seen on our patient.
A KOH test of skin scrapings and hair extractions often can reveal fungal hyphae. Identification of the pathogen can be performed with culture or polymerase chain reaction of skin samples. If the diagnosis is uncertain or the KOH is negative, a skin biopsy can be performed. Histopathologic examination reveals perifollicular granulomas with associated dermal abscesses. Giant cells may be observed. MG is associated with chronic inflammation with lymphocytes, macrophages, epithelioid cells, and scattered multinucleated giant cells.2,3
The differential diagnosis for these lesions in children includes other granulomatous conditions such as granulomatous rosacea, sarcoidosis, and granuloma faciale, as well as bacterial or atypical mycobacterial infections, cutaneous leishmaniasis, and eosinophilic pustular folliculitis.
Treatment of MG requires systemic treatment with griseofulvin, itraconazole, or terbinafine for at least 4-8 weeks or until all the lesions have resolved. Our patient was treated with 6 weeks of high-dose griseofulvin with resolution of her lesions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Dermatol Online J. 2018 Dec 15;24(12):13030/qt89k4t6wj.
2. Med Mycol. 2012 Jul;50(5):449-57.
3. Clin Microbiol Rev. 2011 Apr;24(2):247-80.
Scrapings of the child’s rash were analyzed with potassium hydroxide (KOH) under microscopy which revealed multiple septate hyphae.
She was diagnosed with Majocchi’s granuloma. The fungal culture was positive for Trichophyton rubrum.
Majocchi’s granuloma (MG) is cutaneous mycosis in which the fungal infection goes deeper into the hair follicle causing granulomatous folliculitis and perifolliculitis.1 It was first described by Domenico Majocchi in 1883, and he named the condition “granuloma tricofitico.”2
It is commonly caused by T. rubrum but also can be caused by T. mentagrophytes, T. tonsurans, T. verrucosum, Microsporum canis, and Epidermophyton floccosum.2,3 Patients at risk for developing this infection include those previously treated with topical corticosteroids, immunosuppressed patients, patients with areas under occlusion, and those with areas traumatized by shaving. This infection is most commonly seen in the lower extremities, but can happen anywhere in the body. The lesions present as deep erythematous papules, pustules, and may form an annular or circular plaque as seen on our patient.
A KOH test of skin scrapings and hair extractions often can reveal fungal hyphae. Identification of the pathogen can be performed with culture or polymerase chain reaction of skin samples. If the diagnosis is uncertain or the KOH is negative, a skin biopsy can be performed. Histopathologic examination reveals perifollicular granulomas with associated dermal abscesses. Giant cells may be observed. MG is associated with chronic inflammation with lymphocytes, macrophages, epithelioid cells, and scattered multinucleated giant cells.2,3
The differential diagnosis for these lesions in children includes other granulomatous conditions such as granulomatous rosacea, sarcoidosis, and granuloma faciale, as well as bacterial or atypical mycobacterial infections, cutaneous leishmaniasis, and eosinophilic pustular folliculitis.
Treatment of MG requires systemic treatment with griseofulvin, itraconazole, or terbinafine for at least 4-8 weeks or until all the lesions have resolved. Our patient was treated with 6 weeks of high-dose griseofulvin with resolution of her lesions.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Dermatol Online J. 2018 Dec 15;24(12):13030/qt89k4t6wj.
2. Med Mycol. 2012 Jul;50(5):449-57.
3. Clin Microbiol Rev. 2011 Apr;24(2):247-80.
A 3-year-old girl with a known history of eczema presented to our dermatology clinic for evaluation of a persistent rash for about a year on the right cheek.
The mother reported she was treating the lesions with hydrocortisone cream 2.5% as instructed previously for her eczema. Initially the rash got partially better but then started getting worse again. The area was itchy.
The child was later seen in the emergency department, where she was recommended to treat the area with a combination cream of terbinafine 1% and betamethasone dipropionate 0.05%. The mother applied this cream as instructed for 3 weeks with some improvement of the lesions on the cheek.
A few weeks later, pimples started coming back. The mother tried the medication again but this time it was not helpful and the rash continued to expand.
The mother reported having a rash on her hand months back, which she successfully treated with the combination cream provided at the emergency department. They have no pets at home.
The child has a little sister who also has mild eczema.
She goes to day care and dances ballet.