User login
Ecchymotic patches
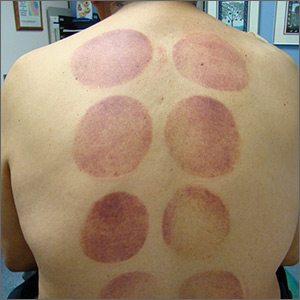
This patient’s circular ecchymotic patches were due to cupping. One of the clues that this was iatrogenic was the regular and repeated pattern on the skin.
Cupping is a centuries old treatment for pain relief (among other things) that involves applying glass globes or other hollow materials to the skin to create a vacuum. Traditionally, this vacuum is created by heating the air inside the vessel and then holding the vessel in place as the air cools. Practitioners may also use more modern instruments to induce the vacuum that are similar to those used to assist in vaginal deliveries. The mechanical devices leave these circular ecchymotic marks. The ecchymosis fades over time, and this procedure has been shown to significantly reduce myofascial neck and back pain in small trials.
It is important to recognize geometric patterns that are iatrogenic or due to abuse when evaluating skin findings. If skin findings do not follow dermatomal distributions, typical exanthem, or other classic patterns or presentations, there is the possibility that the pattern may be the result of neglect or abuse. On inspection, consider whether an odd pattern may have been caused from a belt buckle, striking instrument, furniture, medical equipment, or a hand strike.
This patient’s findings were consistent with his history of visiting a physical therapist for cupping. No treatment was required; the patient’s back pain from his car accident was improving, and the cupping marks were not troubling him.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Wang YT, Qi Y, Tang FY, et al. The effect of cupping therapy for low back pain: a meta-analysis based on existing randomized controlled trials. J Back Musculoskelet Rehabil. 2017;30:1187-1195.

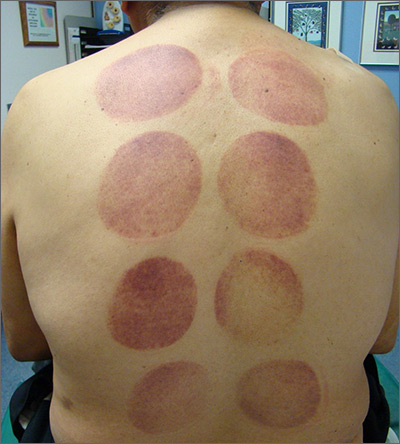
This patient’s circular ecchymotic patches were due to cupping. One of the clues that this was iatrogenic was the regular and repeated pattern on the skin.
Cupping is a centuries old treatment for pain relief (among other things) that involves applying glass globes or other hollow materials to the skin to create a vacuum. Traditionally, this vacuum is created by heating the air inside the vessel and then holding the vessel in place as the air cools. Practitioners may also use more modern instruments to induce the vacuum that are similar to those used to assist in vaginal deliveries. The mechanical devices leave these circular ecchymotic marks. The ecchymosis fades over time, and this procedure has been shown to significantly reduce myofascial neck and back pain in small trials.
It is important to recognize geometric patterns that are iatrogenic or due to abuse when evaluating skin findings. If skin findings do not follow dermatomal distributions, typical exanthem, or other classic patterns or presentations, there is the possibility that the pattern may be the result of neglect or abuse. On inspection, consider whether an odd pattern may have been caused from a belt buckle, striking instrument, furniture, medical equipment, or a hand strike.
This patient’s findings were consistent with his history of visiting a physical therapist for cupping. No treatment was required; the patient’s back pain from his car accident was improving, and the cupping marks were not troubling him.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This patient’s circular ecchymotic patches were due to cupping. One of the clues that this was iatrogenic was the regular and repeated pattern on the skin.
Cupping is a centuries old treatment for pain relief (among other things) that involves applying glass globes or other hollow materials to the skin to create a vacuum. Traditionally, this vacuum is created by heating the air inside the vessel and then holding the vessel in place as the air cools. Practitioners may also use more modern instruments to induce the vacuum that are similar to those used to assist in vaginal deliveries. The mechanical devices leave these circular ecchymotic marks. The ecchymosis fades over time, and this procedure has been shown to significantly reduce myofascial neck and back pain in small trials.
It is important to recognize geometric patterns that are iatrogenic or due to abuse when evaluating skin findings. If skin findings do not follow dermatomal distributions, typical exanthem, or other classic patterns or presentations, there is the possibility that the pattern may be the result of neglect or abuse. On inspection, consider whether an odd pattern may have been caused from a belt buckle, striking instrument, furniture, medical equipment, or a hand strike.
This patient’s findings were consistent with his history of visiting a physical therapist for cupping. No treatment was required; the patient’s back pain from his car accident was improving, and the cupping marks were not troubling him.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Wang YT, Qi Y, Tang FY, et al. The effect of cupping therapy for low back pain: a meta-analysis based on existing randomized controlled trials. J Back Musculoskelet Rehabil. 2017;30:1187-1195.
Wang YT, Qi Y, Tang FY, et al. The effect of cupping therapy for low back pain: a meta-analysis based on existing randomized controlled trials. J Back Musculoskelet Rehabil. 2017;30:1187-1195.
Daily Recap 6/17
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Comorbidities increase COVID-19 deaths by factor of 12
COVID-19 patients with an underlying condition are 6 times as likely to be hospitalized and 12 times as likely to die, compared with those who have no such condition, according to the CDC.
The most frequently reported underlying conditions were cardiovascular disease (32%), diabetes (30%), chronic lung disease (18%), and renal disease (7.6%), and there were no significant differences between males and females.
The pandemic “continues to affect all populations and result in severe outcomes including death,” noted the CDC, emphasizing “the continued need for community mitigation strategies, especially for vulnerable populations, to slow COVID-19 transmission.” Read more.
Preventive services coalition recommends routine anxiety screening for women
Women and girls aged 13 years and older with no current diagnosis of anxiety should be screened routinely for anxiety, according to a new recommendation from the Women’s Preventive Services Initiative.
The lifetime prevalence of anxiety disorders in women in the United States is 40%, approximately twice that of men, and anxiety can be a manifestation of underlying issues including posttraumatic stress, sexual harassment, and assault.
“The WPSI based its rationale for anxiety screening on several considerations,” the researchers noted. “Anxiety disorders are the most prevalent mental health disorders in women, and the problems created by untreated anxiety can impair function in all areas of a woman’s life.” Read more.
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, noted investigators.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded.
Population study supports migraine-dementia link
Preliminary results from a population-based cohort study support previous reports that migraine is a midlife risk factor for dementia later in life, but further determined that migraine with aura and frequent hospital contacts significantly increased dementia risk after age 60 years, according to results from a Danish registry presented at the virtual annual meeting of the American Headache Society.
The preliminary findings revealed that the median age at diagnosis was 49 years and about 70% of the migraine population were women. “There was a 50% higher dementia rate in individuals who had any migraine diagnosis,” Dr. Islamoska said.
“To the best of our knowledge, no previous national register–based studies have investigated the risk of dementia among individuals who suffer from migraine with aura,” Dr. Sabrina Islamoska said.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Vulvar melanoma is increasing in older women
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
FROM AAD 2020
Study compares pulse vs. continuous therapy for dermatophyte toenail onychomycosis
There appear to be results from a systematic review and network meta-analysis showed.
“Previous meta-analyses of pulse and continuous therapies have generated ambiguous results,” study authors led by Aditya K. Gupta, MD, PhD, wrote in a poster abstract presented at the virtual annual meeting of the American Academy of Dermatology. “There are few head-to-head clinical studies and no meta-analyses comparing regimens of terbinafine to regimens of itraconazole.”
In what is believed to be the first study of its kind, Dr. Gupta, professor of dermatology at the University of Toronto, and colleagues used network meta-analysis to compare pulse and continuous systemic therapies for toenail onychomycosis. They used PubMed to search for randomized, controlled trials of oral antifungal treatments for the condition in patients aged 18 years and older that included data on mycologic cure, complete cure, adverse events, and dropout rates. Treatment effects were based on intention-to-treat cure rates, and the researchers excluded studies of ketoconazole and griseofulvin since they are no longer indicated for the condition.
For their network meta-analysis, Dr. Gupta and colleagues evaluated 22 studies from 20 publications that included 4,205 randomized patients. Data on complete cure were excluded because of a lack of studies. When the researchers compared all treatments to placebo, the likelihood of mycologic cure did not differ significantly between continuous and pulse regimens for terbinafine and itraconazole. Compared with placebo, the most successful treatments were continuous terbinafine 250 mg daily for 24 weeks (risk ratio of achieving mycologic cure, 11.0) and continuous terbinafine 250 mg daily for 16 weeks (RR, 8.90). The researchers also observed no significant differences in the likelihood of adverse events between any continuous and pulse regimens of terbinafine, itraconazole, and fluconazole.
“Although continuous terbinafine 250 mg for 24 weeks was significantly more likely to produce mycologic cure than continuous itraconazole 200 mg for 12 weeks and weekly fluconazole (150-450 mg), it is not significantly different from the other included treatments,” Dr. Gupta and colleagues wrote in the abstract. “Considering the fungal life cycle, pulse therapy should theoretically be as effective as, or more effective than, continuous therapies: the sudden high concentration of an antifungal drug eliminates hyphae, sparing already-present spores. During the ‘off’ portion, these spores may germinate and be eliminated during the next pulse. Continuous therapy spares the spores, allowing them to germinate once treatment ends.”
They went on to note that, in clinical practice, “neither continuous nor pulse therapy is necessarily better. It is possible that the drug concentration in the nail is maintained during the ‘off’ period of pulse therapy. In both therapies, it may be that residual spores that have not been eliminated by the end of therapy are left to germinate, possibly contributing to the recalcitrant nature of onychomycosis.”
The study was awarded fourth place in the AAD’s 2020 poster awards. Dr. Gupta disclosed that he is a clinical trials investigator for Moberg Pharma and Bausch Health Canada and a speaker for Bausch Health Canada.
SOURCE: Gupta A et al. AAD 20, Abstract 16014.
There appear to be results from a systematic review and network meta-analysis showed.
“Previous meta-analyses of pulse and continuous therapies have generated ambiguous results,” study authors led by Aditya K. Gupta, MD, PhD, wrote in a poster abstract presented at the virtual annual meeting of the American Academy of Dermatology. “There are few head-to-head clinical studies and no meta-analyses comparing regimens of terbinafine to regimens of itraconazole.”
In what is believed to be the first study of its kind, Dr. Gupta, professor of dermatology at the University of Toronto, and colleagues used network meta-analysis to compare pulse and continuous systemic therapies for toenail onychomycosis. They used PubMed to search for randomized, controlled trials of oral antifungal treatments for the condition in patients aged 18 years and older that included data on mycologic cure, complete cure, adverse events, and dropout rates. Treatment effects were based on intention-to-treat cure rates, and the researchers excluded studies of ketoconazole and griseofulvin since they are no longer indicated for the condition.
For their network meta-analysis, Dr. Gupta and colleagues evaluated 22 studies from 20 publications that included 4,205 randomized patients. Data on complete cure were excluded because of a lack of studies. When the researchers compared all treatments to placebo, the likelihood of mycologic cure did not differ significantly between continuous and pulse regimens for terbinafine and itraconazole. Compared with placebo, the most successful treatments were continuous terbinafine 250 mg daily for 24 weeks (risk ratio of achieving mycologic cure, 11.0) and continuous terbinafine 250 mg daily for 16 weeks (RR, 8.90). The researchers also observed no significant differences in the likelihood of adverse events between any continuous and pulse regimens of terbinafine, itraconazole, and fluconazole.
“Although continuous terbinafine 250 mg for 24 weeks was significantly more likely to produce mycologic cure than continuous itraconazole 200 mg for 12 weeks and weekly fluconazole (150-450 mg), it is not significantly different from the other included treatments,” Dr. Gupta and colleagues wrote in the abstract. “Considering the fungal life cycle, pulse therapy should theoretically be as effective as, or more effective than, continuous therapies: the sudden high concentration of an antifungal drug eliminates hyphae, sparing already-present spores. During the ‘off’ portion, these spores may germinate and be eliminated during the next pulse. Continuous therapy spares the spores, allowing them to germinate once treatment ends.”
They went on to note that, in clinical practice, “neither continuous nor pulse therapy is necessarily better. It is possible that the drug concentration in the nail is maintained during the ‘off’ period of pulse therapy. In both therapies, it may be that residual spores that have not been eliminated by the end of therapy are left to germinate, possibly contributing to the recalcitrant nature of onychomycosis.”
The study was awarded fourth place in the AAD’s 2020 poster awards. Dr. Gupta disclosed that he is a clinical trials investigator for Moberg Pharma and Bausch Health Canada and a speaker for Bausch Health Canada.
SOURCE: Gupta A et al. AAD 20, Abstract 16014.
There appear to be results from a systematic review and network meta-analysis showed.
“Previous meta-analyses of pulse and continuous therapies have generated ambiguous results,” study authors led by Aditya K. Gupta, MD, PhD, wrote in a poster abstract presented at the virtual annual meeting of the American Academy of Dermatology. “There are few head-to-head clinical studies and no meta-analyses comparing regimens of terbinafine to regimens of itraconazole.”
In what is believed to be the first study of its kind, Dr. Gupta, professor of dermatology at the University of Toronto, and colleagues used network meta-analysis to compare pulse and continuous systemic therapies for toenail onychomycosis. They used PubMed to search for randomized, controlled trials of oral antifungal treatments for the condition in patients aged 18 years and older that included data on mycologic cure, complete cure, adverse events, and dropout rates. Treatment effects were based on intention-to-treat cure rates, and the researchers excluded studies of ketoconazole and griseofulvin since they are no longer indicated for the condition.
For their network meta-analysis, Dr. Gupta and colleagues evaluated 22 studies from 20 publications that included 4,205 randomized patients. Data on complete cure were excluded because of a lack of studies. When the researchers compared all treatments to placebo, the likelihood of mycologic cure did not differ significantly between continuous and pulse regimens for terbinafine and itraconazole. Compared with placebo, the most successful treatments were continuous terbinafine 250 mg daily for 24 weeks (risk ratio of achieving mycologic cure, 11.0) and continuous terbinafine 250 mg daily for 16 weeks (RR, 8.90). The researchers also observed no significant differences in the likelihood of adverse events between any continuous and pulse regimens of terbinafine, itraconazole, and fluconazole.
“Although continuous terbinafine 250 mg for 24 weeks was significantly more likely to produce mycologic cure than continuous itraconazole 200 mg for 12 weeks and weekly fluconazole (150-450 mg), it is not significantly different from the other included treatments,” Dr. Gupta and colleagues wrote in the abstract. “Considering the fungal life cycle, pulse therapy should theoretically be as effective as, or more effective than, continuous therapies: the sudden high concentration of an antifungal drug eliminates hyphae, sparing already-present spores. During the ‘off’ portion, these spores may germinate and be eliminated during the next pulse. Continuous therapy spares the spores, allowing them to germinate once treatment ends.”
They went on to note that, in clinical practice, “neither continuous nor pulse therapy is necessarily better. It is possible that the drug concentration in the nail is maintained during the ‘off’ period of pulse therapy. In both therapies, it may be that residual spores that have not been eliminated by the end of therapy are left to germinate, possibly contributing to the recalcitrant nature of onychomycosis.”
The study was awarded fourth place in the AAD’s 2020 poster awards. Dr. Gupta disclosed that he is a clinical trials investigator for Moberg Pharma and Bausch Health Canada and a speaker for Bausch Health Canada.
SOURCE: Gupta A et al. AAD 20, Abstract 16014.
FROM AAD 20
High-fat, high-sugar diet may promote adult acne
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
A diet higher in fat, sugar, and milk was associated with having acne in a cross-sectional study of approximately 24,000 adults in France.
Acne in adults has been associated with social, emotional, and psychological consequences similar to those found with chronic diseases such as asthma, arthritis, epilepsy, and diabetes, wrote Laetitia Penso, MSc, of the University of Paris in Bobigny, France, and colleagues.
Although acne patients may believe that eating certain foods exacerbates acne, data on the effects of nutrition on acne, including associations between acne and a high-glycemic diet, are limited and have produced conflicting results, they noted.
In their study, published in JAMA Dermatology, the researchers identified 24,452 adults who participated in the NutriNet-Santé study, an ongoing, web-based study in France. Approximately 75% of the participants were women, the average age was 57 years, and 46% reported past or current acne.
Participants responded to an 11-item questionnaire between November 2008 and July 2019. Questions were related to the occurrence and diagnosis of acne, as well as medical history. Based on their acne status, participants were identified as falling into the categories of never acne, past acne, or current acne, and their dietary intake was assessed at baseline and every 6 months using three nonconsecutive 24-hour dietary records for 2 weekdays and 1 weekend day.
In an analysis, after adjustment for confounders, current acne was significantly associated with consumption of fatty and sugary foods (per portion, adjusted odds ratio, 1.54; P = .01), as well as with consumption of sugary drinks (per glass, aOR, 1.18; P = .04) and milk (per glass, aOR, 1.12; P = .04). In addition, carbohydrate intake and saturated fatty acid intake were significantly associated with current acne (aOR, 1.43; P = .02; and aOR, 3.90; P = .048, respectively).
Three dietary patterns accounted for 42% of the total variability, the researchers said. A healthy pattern of higher fruit, vegetable, and fish intake accounted for 18%, a fatty and sugary pattern of higher fat and sugar intake (including chocolate) accounted for 13%, and an animal product and cereal pattern of higher intake of meat, milk, and refined cereals accounted for 11%, they explained.
“The results of our study appear to support the hypothesis that the Western diet (rich in animal products and fatty and sugary foods) is associated with the presence of acne in adulthood,” the researchers concluded. Possible explanations for the findings include the effects of a high glycemic-load diet on circulating IGF-1 and insulin, which ultimately increases both oxidative stress and inflammation that promotes the development of acne, they noted.
The study findings were limited by several factors including the use of relatively homogenous younger and female patient population and the reliance on self-reported acne, as well as the observational design, which did not allow for identification of direct, causal associations between diet and acne, the researchers noted. Larger studies are needed to examine the relationship between diet and adult acne to inform prevention and treatment, they wrote.
“Much of the previous literature on the role of diet in acne has focused on the association of milk consumption and high glycemic-load diet with acne,” John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, wrote in an accompanying editorial.
Dr. Barbieri acknowledged the inability to make causal associations given the study design and noted that dietary interventions should be implemented with caution because of the potential for other effects such as reduced calcium or vitamin D.
“Nevertheless, given the potential overall health benefits of a healthy or low glycemic-load diet, and 2 small trials supporting its effectiveness in acne, a low glycemic-load diet is a reasonable recommendation for patients looking for dietary modifications that may improve their acne,” he said.
Dr. Barbieri said that he was encouraged to see that the study findings reflected previous research identifying an association between acne and high-glycemic load foods, as well as milk consumption, but he emphasized that more research is needed before general recommendations about diet and acne can be made.
“Trials are needed to evaluate whether dietary interventions can improve or prevent acne and how the effect size of such interventions compares with other standard treatment modalities,” he emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Barbieri disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and from a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
SOURCE: Penso L et al. JAMA Dermatol. 2020 June 10. doi: 10.1001/jamadermatol.2020.1602.
FROM JAMA DERMATOLOGY
Tralokinumab found effective in phase 3 atopic dermatitis studies
presented at the virtual annual meeting of the American Academy of Dermatology.
Tralokinumab is a fully human monoclonal antibody which binds specifically to interleukin-13 and thereby prevents downstream IL-13 signaling. In contrast, dupilumab (Dupixent), at present the only approved biologic agent for AD, blocks both the IL-13 and IL-4 pathways.
Two of the pivotal phase 3 trials presented at AAD 2020 – ECZTRA 1 and ECZTRA 2 – were identically designed, randomized, double-blind, placebo-controlled, 52-week, multinational monotherapy studies including a collective 1,596 adults with moderate to severe AD. In contrast, ECZTRA 3 was a 380-patient, double-blind, randomized, 32-week study of tralokinumab in combination with a topical corticosteroid versus placebo injections plus a topical corticosteroid.
“I would say the take-home point of these trials is they are proof of principle that blocking just IL-13 can be an effective approach. The studies help us understand that IL-13 is an important driver cytokine for the disease,” Eric Simpson, MD, lead clinical investigator for ECZTRA 2, said in an interview.
In all three phase 3 trials, the primary endpoint was achievement of a clinical response as defined by an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) plus at least a 75% improvement in the Eczema Area and Severity Index score (EASI-75) at week 16. In ECZTRA 1 and 2, this was accomplished in 16% and 22% of patients on 300 mg of tralokinumab administered subcutaneously every 2 weeks, compared with 7% and 11% of placebo-treated controls.
Patients with a clinical response at week 16 were then rerandomized to tralokinumab either every other week or every 4 weeks or to placebo for an additional 36 weeks. At 52 weeks, 51% and 59% of patients in ECZTRA 1 and 2, respectively, who had a clinical response at week 16 maintained an IGA 0/1 response while on tralokinumab every 2 weeks, as did 39% and 45% of those switched to treatment every 4 weeks. Similarly, 60% and 56% of clinical responders at week 16 maintained an EASI-75 response at week 52 with tralokinumab every 2 weeks, as did 49% and 51% of those rerandomized to treatment every 4 weeks.
The safety profile of tralokinumab in the two monotherapy trials was comparable with placebo.
In the ECZTRA studies, tralokinumab achieved significant improvement at week 16 in secondary endpoints including itch, health-related quality of life, and severity and extent of skin lesions.
How does tralokinumab, with its narrower focus targeting a single cytokine, stack up against dupilumab, the dual IL-13/IL-4 inhibitor that’s transformed the treatment of patients with moderate or severe AD?
Dr. Simpson, who was also principal investigator in a pivotal phase 3 trial for dupilumab, emphasized that no firm conclusions can be drawn because there have been no head-to-head comparative trials and the tralokinumab and dupilumab trials had different patient populations, geographic locations, and washout periods. With those caveats, however, he commented that, “just on the surface, numerically, for the monotherapy studies, dupilumab hit some higher targets than tralokinumab in terms of the percentage of patients clear or almost clear.”
In terms of safety, it appears that the risk of conjunctivitis may be lower with tralokinumab than dupilumab, with rates of 7% and 3% through 52 weeks in ECZTRA 1 and 2, respectively, versus 2% with placebo, although again this is “a caveated conclusion,” said Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland.
Tralokinumab combination therapy in ECZTRA 3
At 16 weeks, 39% of patients treated with tralokinumab plus topical corticosteroids had an IGA of 0/1 and 56% had an EASI-75 response, compared with 26% and 36% of patients on topical corticosteroids plus biweekly placebo injections. More than 90% of patients with a good clinical response at week 16 maintained that response at week 32 while on tralokinumab biweekly plus topical steroids. Among good responders at week 16 who were rerandomized to 300 mg of tralokinumab every 4 weeks plus topical steroids, 78% still had an IGA of 0/1 at week 32, and 91% had an EASI-75, reported Jonathan I. Silverberg, MD, PhD, director of clinical research and contact dermatitis at George Washington University, Washington.
A randomized, placebo-controlled combination therapy study such as this provides information that’s especially useful in clinical practice, Dr. Simpson observed.
“When I’m talking to patients about any biologics or oral therapies, I usually quote the figures from the combination therapy studies because the vast majority of our patients are using topical therapy in addition to systemics,” he said in the interview.
Asked how he envisions tralokinumab’s role in clinical practice, should the drug receive regulatory approval, Dr. Simpson said that he welcomes the prospect of having an additional treatment option to discuss with patients. Tralokinumab could be considered either as first-line therapy in patients who are failing on topical therapy or for patients who don’t respond adequately to or experience limiting side effects on dupilumab.
“There isn’t any established, published treatment algorithm in atopic dermatitis, probably for good reason, since we don’t have data to tell us you should start here and then move there. Those are long, difficult studies to perform,” Dr. Simpson said.
LEO Pharma has announced that it has applied for marketing approval for tralokinumab to the European Medicines Agency and plans to do so with the Food and Drug Administration by year’s end.
Dr. Simpson reported receiving research grants from and serving as a consultant to LEO Pharma, sponsor of the ECZTRA trials. He has similar financial relationships with close to a dozen other pharmaceutical companies.
presented at the virtual annual meeting of the American Academy of Dermatology.
Tralokinumab is a fully human monoclonal antibody which binds specifically to interleukin-13 and thereby prevents downstream IL-13 signaling. In contrast, dupilumab (Dupixent), at present the only approved biologic agent for AD, blocks both the IL-13 and IL-4 pathways.
Two of the pivotal phase 3 trials presented at AAD 2020 – ECZTRA 1 and ECZTRA 2 – were identically designed, randomized, double-blind, placebo-controlled, 52-week, multinational monotherapy studies including a collective 1,596 adults with moderate to severe AD. In contrast, ECZTRA 3 was a 380-patient, double-blind, randomized, 32-week study of tralokinumab in combination with a topical corticosteroid versus placebo injections plus a topical corticosteroid.
“I would say the take-home point of these trials is they are proof of principle that blocking just IL-13 can be an effective approach. The studies help us understand that IL-13 is an important driver cytokine for the disease,” Eric Simpson, MD, lead clinical investigator for ECZTRA 2, said in an interview.
In all three phase 3 trials, the primary endpoint was achievement of a clinical response as defined by an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) plus at least a 75% improvement in the Eczema Area and Severity Index score (EASI-75) at week 16. In ECZTRA 1 and 2, this was accomplished in 16% and 22% of patients on 300 mg of tralokinumab administered subcutaneously every 2 weeks, compared with 7% and 11% of placebo-treated controls.
Patients with a clinical response at week 16 were then rerandomized to tralokinumab either every other week or every 4 weeks or to placebo for an additional 36 weeks. At 52 weeks, 51% and 59% of patients in ECZTRA 1 and 2, respectively, who had a clinical response at week 16 maintained an IGA 0/1 response while on tralokinumab every 2 weeks, as did 39% and 45% of those switched to treatment every 4 weeks. Similarly, 60% and 56% of clinical responders at week 16 maintained an EASI-75 response at week 52 with tralokinumab every 2 weeks, as did 49% and 51% of those rerandomized to treatment every 4 weeks.
The safety profile of tralokinumab in the two monotherapy trials was comparable with placebo.
In the ECZTRA studies, tralokinumab achieved significant improvement at week 16 in secondary endpoints including itch, health-related quality of life, and severity and extent of skin lesions.
How does tralokinumab, with its narrower focus targeting a single cytokine, stack up against dupilumab, the dual IL-13/IL-4 inhibitor that’s transformed the treatment of patients with moderate or severe AD?
Dr. Simpson, who was also principal investigator in a pivotal phase 3 trial for dupilumab, emphasized that no firm conclusions can be drawn because there have been no head-to-head comparative trials and the tralokinumab and dupilumab trials had different patient populations, geographic locations, and washout periods. With those caveats, however, he commented that, “just on the surface, numerically, for the monotherapy studies, dupilumab hit some higher targets than tralokinumab in terms of the percentage of patients clear or almost clear.”
In terms of safety, it appears that the risk of conjunctivitis may be lower with tralokinumab than dupilumab, with rates of 7% and 3% through 52 weeks in ECZTRA 1 and 2, respectively, versus 2% with placebo, although again this is “a caveated conclusion,” said Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland.
Tralokinumab combination therapy in ECZTRA 3
At 16 weeks, 39% of patients treated with tralokinumab plus topical corticosteroids had an IGA of 0/1 and 56% had an EASI-75 response, compared with 26% and 36% of patients on topical corticosteroids plus biweekly placebo injections. More than 90% of patients with a good clinical response at week 16 maintained that response at week 32 while on tralokinumab biweekly plus topical steroids. Among good responders at week 16 who were rerandomized to 300 mg of tralokinumab every 4 weeks plus topical steroids, 78% still had an IGA of 0/1 at week 32, and 91% had an EASI-75, reported Jonathan I. Silverberg, MD, PhD, director of clinical research and contact dermatitis at George Washington University, Washington.
A randomized, placebo-controlled combination therapy study such as this provides information that’s especially useful in clinical practice, Dr. Simpson observed.
“When I’m talking to patients about any biologics or oral therapies, I usually quote the figures from the combination therapy studies because the vast majority of our patients are using topical therapy in addition to systemics,” he said in the interview.
Asked how he envisions tralokinumab’s role in clinical practice, should the drug receive regulatory approval, Dr. Simpson said that he welcomes the prospect of having an additional treatment option to discuss with patients. Tralokinumab could be considered either as first-line therapy in patients who are failing on topical therapy or for patients who don’t respond adequately to or experience limiting side effects on dupilumab.
“There isn’t any established, published treatment algorithm in atopic dermatitis, probably for good reason, since we don’t have data to tell us you should start here and then move there. Those are long, difficult studies to perform,” Dr. Simpson said.
LEO Pharma has announced that it has applied for marketing approval for tralokinumab to the European Medicines Agency and plans to do so with the Food and Drug Administration by year’s end.
Dr. Simpson reported receiving research grants from and serving as a consultant to LEO Pharma, sponsor of the ECZTRA trials. He has similar financial relationships with close to a dozen other pharmaceutical companies.
presented at the virtual annual meeting of the American Academy of Dermatology.
Tralokinumab is a fully human monoclonal antibody which binds specifically to interleukin-13 and thereby prevents downstream IL-13 signaling. In contrast, dupilumab (Dupixent), at present the only approved biologic agent for AD, blocks both the IL-13 and IL-4 pathways.
Two of the pivotal phase 3 trials presented at AAD 2020 – ECZTRA 1 and ECZTRA 2 – were identically designed, randomized, double-blind, placebo-controlled, 52-week, multinational monotherapy studies including a collective 1,596 adults with moderate to severe AD. In contrast, ECZTRA 3 was a 380-patient, double-blind, randomized, 32-week study of tralokinumab in combination with a topical corticosteroid versus placebo injections plus a topical corticosteroid.
“I would say the take-home point of these trials is they are proof of principle that blocking just IL-13 can be an effective approach. The studies help us understand that IL-13 is an important driver cytokine for the disease,” Eric Simpson, MD, lead clinical investigator for ECZTRA 2, said in an interview.
In all three phase 3 trials, the primary endpoint was achievement of a clinical response as defined by an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) plus at least a 75% improvement in the Eczema Area and Severity Index score (EASI-75) at week 16. In ECZTRA 1 and 2, this was accomplished in 16% and 22% of patients on 300 mg of tralokinumab administered subcutaneously every 2 weeks, compared with 7% and 11% of placebo-treated controls.
Patients with a clinical response at week 16 were then rerandomized to tralokinumab either every other week or every 4 weeks or to placebo for an additional 36 weeks. At 52 weeks, 51% and 59% of patients in ECZTRA 1 and 2, respectively, who had a clinical response at week 16 maintained an IGA 0/1 response while on tralokinumab every 2 weeks, as did 39% and 45% of those switched to treatment every 4 weeks. Similarly, 60% and 56% of clinical responders at week 16 maintained an EASI-75 response at week 52 with tralokinumab every 2 weeks, as did 49% and 51% of those rerandomized to treatment every 4 weeks.
The safety profile of tralokinumab in the two monotherapy trials was comparable with placebo.
In the ECZTRA studies, tralokinumab achieved significant improvement at week 16 in secondary endpoints including itch, health-related quality of life, and severity and extent of skin lesions.
How does tralokinumab, with its narrower focus targeting a single cytokine, stack up against dupilumab, the dual IL-13/IL-4 inhibitor that’s transformed the treatment of patients with moderate or severe AD?
Dr. Simpson, who was also principal investigator in a pivotal phase 3 trial for dupilumab, emphasized that no firm conclusions can be drawn because there have been no head-to-head comparative trials and the tralokinumab and dupilumab trials had different patient populations, geographic locations, and washout periods. With those caveats, however, he commented that, “just on the surface, numerically, for the monotherapy studies, dupilumab hit some higher targets than tralokinumab in terms of the percentage of patients clear or almost clear.”
In terms of safety, it appears that the risk of conjunctivitis may be lower with tralokinumab than dupilumab, with rates of 7% and 3% through 52 weeks in ECZTRA 1 and 2, respectively, versus 2% with placebo, although again this is “a caveated conclusion,” said Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland.
Tralokinumab combination therapy in ECZTRA 3
At 16 weeks, 39% of patients treated with tralokinumab plus topical corticosteroids had an IGA of 0/1 and 56% had an EASI-75 response, compared with 26% and 36% of patients on topical corticosteroids plus biweekly placebo injections. More than 90% of patients with a good clinical response at week 16 maintained that response at week 32 while on tralokinumab biweekly plus topical steroids. Among good responders at week 16 who were rerandomized to 300 mg of tralokinumab every 4 weeks plus topical steroids, 78% still had an IGA of 0/1 at week 32, and 91% had an EASI-75, reported Jonathan I. Silverberg, MD, PhD, director of clinical research and contact dermatitis at George Washington University, Washington.
A randomized, placebo-controlled combination therapy study such as this provides information that’s especially useful in clinical practice, Dr. Simpson observed.
“When I’m talking to patients about any biologics or oral therapies, I usually quote the figures from the combination therapy studies because the vast majority of our patients are using topical therapy in addition to systemics,” he said in the interview.
Asked how he envisions tralokinumab’s role in clinical practice, should the drug receive regulatory approval, Dr. Simpson said that he welcomes the prospect of having an additional treatment option to discuss with patients. Tralokinumab could be considered either as first-line therapy in patients who are failing on topical therapy or for patients who don’t respond adequately to or experience limiting side effects on dupilumab.
“There isn’t any established, published treatment algorithm in atopic dermatitis, probably for good reason, since we don’t have data to tell us you should start here and then move there. Those are long, difficult studies to perform,” Dr. Simpson said.
LEO Pharma has announced that it has applied for marketing approval for tralokinumab to the European Medicines Agency and plans to do so with the Food and Drug Administration by year’s end.
Dr. Simpson reported receiving research grants from and serving as a consultant to LEO Pharma, sponsor of the ECZTRA trials. He has similar financial relationships with close to a dozen other pharmaceutical companies.
FROM AAD 2020
Study spotlights the skin microbiome’s evolving nature
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
FROM AAD 20
Key clinical point: The skin’s microbial diversity changes with increasing age in children while remaining stable in adult mothers.
Major finding: The skin microbiome in children becomes more diverse between the ages of 3-4 to age 10.
Study details: A longitudinal analysis of 30 mothers and their 31 children.
Disclosures: The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
Source: Capone K et al. AAD 20, Abstract F053.
Daily Recap: FDA revokes emergency use of hydroxychloroquine; Hardest hit specialties ranked in financial report
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
It’s official: COVID-19 is bad for your health care business
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
“Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology,” according to the report. Read more.
FDA revokes emergency use of hydroxychloroquine
The FDA revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
"Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19," the agency announced in a June 15 statement.
"In light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use," noted the FDA. Read more.
Secondary infections common in COVID-19, implications unclear
Secondary respiratory infections appear to be highly prevalent among patients with severe COVID-19, but at this point, most physicians aren’t sure what to make of this understudied phenomenon.
“We really do not understand the implications of secondary infections on outcomes in COVID-19 patients,” David L. Bowton, MD, FCCP, said in an interview. “In most early reports the incidence of secondary infections was much higher in patients dying from COVID-19, compared to survivors, but it isn’t clear whether this indicates that the secondary infection itself led to excess mortality or was more a marker of the severity of the COVID-19 infection."
An early retrospective cohort study including 191 COVID-19 patients in Wuhan, China found that of the 54 who died in hospital, half had secondary bacterial lung infections (Lancet. 2020 Mar 28;395[10229]:1054-62). That comes as no surprise to U.S. physicians, who learned in training that many deaths during the so-called Spanish influenza epidemic were actually caused by secondary pneumonia involving Staphylococcus aureus, commented Daniel L. Ouellette, MD, FCCP. Read more.
Automated insulin delivery system ‘getting better and better’
Medtronic’s next-generation automated insulin delivery system offers significant improvements over the currently available model, particularly in young people with type 1 diabetes, new data suggest.
Data from three trials of such systems using Medtronic’s advanced hybrid closed-loop (AHCL) algorithm (trade name SmartGuard) were presented during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
Taken together, the data from the three trials showed that the AHCL-based system improved glycemic time-in-range with no increased risk for hypoglycemia, including in children and teenagers, with high patient-reported satisfaction.
“None of these devices is perfect, but they are a substantial improvement over what we’ve had ... They might make the quality of [patient] lives better. That’s really underappreciated,” session moderator Timothy S. Bailey, MD, commented. Read more.
Access more top news from the ADA virtual meeting.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Be vigilant for scleroderma renal crisis
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
FROM SOTA 2020
Pediatric Dermatology: A Supplement to Pediatric News & Dermatology News
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness
Content includes:
- Early onset of atopic dermatitis linked to poorer control, could signify more persistent disease
- Patients with actopic dermatitis should be routinely asked about conjunctivitis
- Hope on the horizon: New cantharidin formulation alleviates molluscum contagiosum in pivotal trials
- Patch testing in atopic dermatitis: When and how
- Topical calcineurin inhibitors are an effective treatment option for periorificial dermatitis
- Psychology consults for children’s skin issues can boost adherence, wellness