User login
Coronavirus outbreak prompts cancellation of AAD annual meeting
The American Academy of Dermatology annual meeting is the latest large medical conference to be canceled because of the coronavirus disease 2019 (COVID-19) outbreak.
“After carefully weighing the emerging facts, as well as our duties to the Academy, our members, other meeting attendees, and the local communities we as dermatologists serve, the AAD has made the difficult but necessary decision to cancel the AAD 2020 Annual Meeting in Denver,” AAD President George Hruza, MD, said in an announcement posted on the AAD’s website late on March 9. “We also want to respect our physicians’ need to be available to and healthy for their own patients, communities, and countries,” he added.
Earlier in the day, the American College of Cardiology announced that its annual meeting would be canceled, as did the Society of Gynecologic Oncology.
In his statement, Dr. Hruza said that the AAD is looking into “virtual meeting options” to provide content that was scheduled to be presented at the meeting.
Updates on those plans will be posted on the AAD’s website at www.aad.org.
The American Academy of Dermatology annual meeting is the latest large medical conference to be canceled because of the coronavirus disease 2019 (COVID-19) outbreak.
“After carefully weighing the emerging facts, as well as our duties to the Academy, our members, other meeting attendees, and the local communities we as dermatologists serve, the AAD has made the difficult but necessary decision to cancel the AAD 2020 Annual Meeting in Denver,” AAD President George Hruza, MD, said in an announcement posted on the AAD’s website late on March 9. “We also want to respect our physicians’ need to be available to and healthy for their own patients, communities, and countries,” he added.
Earlier in the day, the American College of Cardiology announced that its annual meeting would be canceled, as did the Society of Gynecologic Oncology.
In his statement, Dr. Hruza said that the AAD is looking into “virtual meeting options” to provide content that was scheduled to be presented at the meeting.
Updates on those plans will be posted on the AAD’s website at www.aad.org.
The American Academy of Dermatology annual meeting is the latest large medical conference to be canceled because of the coronavirus disease 2019 (COVID-19) outbreak.
“After carefully weighing the emerging facts, as well as our duties to the Academy, our members, other meeting attendees, and the local communities we as dermatologists serve, the AAD has made the difficult but necessary decision to cancel the AAD 2020 Annual Meeting in Denver,” AAD President George Hruza, MD, said in an announcement posted on the AAD’s website late on March 9. “We also want to respect our physicians’ need to be available to and healthy for their own patients, communities, and countries,” he added.
Earlier in the day, the American College of Cardiology announced that its annual meeting would be canceled, as did the Society of Gynecologic Oncology.
In his statement, Dr. Hruza said that the AAD is looking into “virtual meeting options” to provide content that was scheduled to be presented at the meeting.
Updates on those plans will be posted on the AAD’s website at www.aad.org.
Antifungal drug appears safe for pregnancy
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
FROM JAMA DERMATOLOGY
Breaking bacterial communication may heal EB wounds
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
LONDON – Disrupting how microorganisms communicate with each other could be a way to overcome antibiotic resistance and to help heal chronic wounds in patients with epidermolysis bullosa (EB), according to presenters at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
The majority of chronic wounds in patients with EB are colonized with microorganisms, with a predominance of Staphylococcus species, said Erik Gerner, an industrial PhD student at Mölnlycke Health Care in Gothenburg, Sweden, and Gothenburg University.
Because of the growing problem of antibiotic resistance, alternative treatments are needed, and one possible alternative for treating infected wounds could be interfering with quorum sensing, the cell-to-cell communication used by bacteria, he said. He is hoping to explore this possibility as a novel treatment strategy for infected wounds.
“Quorum sensing is defined as the ability to detect and respond to population density,” Mr. Gerner said, noting that, when there is a sufficient density of bacteria, “they start to communicate with each other.” This enables them to act as a community and perform actions that they could not do as individual cells. Such actions include forming biofilms, which helps protect bacteria from their environment, such as the immune system. Other actions include collectively switching on the production of virulence factors and becoming resistant to treatments.
“Bacteria use quorum sensing to act collectively,” Mr. Gerner said. “If we could shut down this quorum sensing system, it would be very beneficial … and increase the chances to heal the wound.”
The quorum sensing system is based on the production of signaling molecules called AHL (N-acyl homoserine lactones), which are constantly produced at a low rate. This isn’t a problem until the level of bacteria increases and the level of quorum sensing breaches a threshold, he explained.
There are several benefits of inhibiting bacterial communication through disrupting quorum sensing, namely, “a low risk of resistance,” Mr. Gerner said. There is also potentially less toxin production by bacteria, and this could help the immune system in killing the invading bacteria.
One approach to disrupting quorum testing that Mr. Gerner has been investigating is the use of sodium salicylate (NaSa). So far, preclinical work shows that NaSa can reduce toxin production but not the growth rate of bacteria. The advantage of using NaSa is that it is nontoxic to human dermal fibroblasts, with similar results seen in human keratinocytes and immune cells. His work to date has shown that NaSa reduced activity of NF-kB (a proinflammatory signaling pathway) in differentiated and lipopolysaccharide-stimulated monocytes; NF-kB activated production of proinflammatory cytokines (such as interleukin-1 beta and IL-6) are elevated in EB wounds. “My studies support the bodies of evidence that bacteria use quorum sensing to coordinate … and to produce a large number of toxic factors,” Mr. Gerner concluded. Future studies will look at the potential of NaSa to disrupt this activity.
Skin microbiome of EB wounds
Liat Samuelov, MD, of the department of molecular dermatology at Tel Aviv (Israel) Sourasky Medical Center, presented data on skin microbiome characteristics in eight patients with recessive dystrophic EB (RDEB). This showed that there was reduced bacterial diversity in wounds, and a “progressive development of dysbiosis across different stages of DEB wound formation.”
The skin microbiome has been implicated in several skin diseases, Dr. Samuelov and associates observed in a poster presentation. That includes the autoimmune blistering disease bullous pemphigoid (Exp Dermatol. 2017 Dec;26[12]:1221-7). “Colonization of DEB chronic wounds may lead to systemic infections, result in delayed healing, and possibly be involved in the development of squamous cell carcinoma,” they noted in the poster, “thus accurate delineation of the dysbiotic profile … may point to corrective measures of great therapeutic potential.”
The aim was to see what microorganisms were present in the chronic wounds of the patients. To be included in the study, patients must not have had any antibiotic treatment – oral or topical – in the past 6 months. Samples were taken from an untreated wound, around the wound, and from uninvolved skin, which were compared with samples taken from similar areas in age-matched controls.
Reduced bacterial diversity was observed in RDEB wounds, compared with uninvolved or perilesional areas and the skin of control subjects, Dr. Samuelov said in an oral presentation of the study results. There was increased abundance of Staphylococcus epidermidis and decreased Cutibacterium acnes, which she noted was in contrast to other studies where S. aureus was the most common colonizer in RDEB wounds.
Bacterial composition in each group was calculated using the beta-diversity score, while control samples showed similar microbial composition, the DEB samples had no microbial similarities among different samples. These data “suggest the need to ascertain the potential therapeutic benefit of interventions aimed at restoring normal microbiome composition in DEB,” Dr. Samuelov concluded.
Wound colonization and squamous cell carcinoma
Other research on wound microbiology was presented by Laura E. Levin, MD, a dermatologist at New York–Presbyterian, and associates. “Given the potential role of bacteria-induced inflammation in the development of wound-associated SCC [squamous cell carcinoma] in a subset of patients, we sought to improve our understanding of what microbes colonize and infect the wounds of patients with epidermolysis bullosa,” they explained in their poster.
The researchers, from New York–Presbyterian Morgan Stanley Children’s Hospital and Columbia University Irvine Medical Center, New York, presented data from a retrospective analysis of 739 wound cultures taken between 2001 and 2017 from 158 patients enrolled in the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. In the analysis, just under 70% of patients had DEB, of which 90% were of the RDEB subtype; 13% had EB simplex, 14% had junctional EB, and 3% had an unknown EB subtype.
At least one organism grew in 87% of cultures, with the most common microorganism isolated being Staphylococcus aureus (84% of cultures). Other commonly isolated microbes were Pseudomonas aeruginosa in 35% of cultures, Streptococcus group A in 34% of cultures (of which 22% were Streptococcus pyogenes), Corynebacterium species in 31% of cultures, and Proteus species in 18% of cultures.
“Improved understanding of what microbes are colonizing the wounds of our patients may help improve antibiotic stewardship,” the researchers stated.
Looking at the antibiotic susceptibilities, Dr. Levin and associates found that 68% of 115 cultures were sensitive to methicillin and 60% of 15 cultures were sensitive to mupirocin. “Resistance to many systemic and topical antibiotic agents in EB patients supports surveillance cultures with routine testing for mupirocin susceptibility,” they suggested.
A total of 23 patients developed SCC of whom 10 had cultures that grew S. aureus (90%) and P. aeruginosa (50%), and Proteus species (20%). Among the patients who did not develop SCC, the respective cultures positive for each of those microorganisms were 83%, 34%, and 11%. Perhaps “gram-negative and flagellated organisms may be more common in wounds of patients at risk for SCC,” they observed, adding that further studies were needed to determine if “wound microbiome interventions inhibit the risk of development of SCC and improve outcomes.”
Mr. Gerner’s research is supported by Mölnlycke Health Care. Dr. Samuelov had no disclosures. The work by Dr. Levin and associates is supported by the Pediatric Dermatology Research Alliance, EB Research Partnership, and the Epidermolysis Bullosa Medical Research Foundation.
REPORTING FROM EB 2020
‘Natural is not always good’ when it comes to treatments for alopecia
ORLANDO – Biotin is the most popular consumer supplement for alopecia and highly popular online, but should patients be taking it?
Patients may want something “natural” to treat their hair loss, but “natural is not always good,” Amy McMichael, MD, professor and chair of the department of dermatology at Wake Forest Baptist Health, Winston-Salem, N.C., said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dosages of commercially available biotin supplements can vary significantly, with some doses as high as 10,000 mcg, making supraphysiological dosing possible. Dr. McMichael said that, not only is biotin unlikely to help with a patient’s alopecia, but a high intake of biotin can interfere with certain assays that use streptavidin-biotin capture techniques (Clin Chem Lab Med. 2017 May 1;55[6]:817-25). This could present a problem for a patient who experiences an MI or has a thyroid disorder where a high level of biotin could affect lab results, she noted.
Dr. McMichael advises patients that there is a
Questioning side effects of 5-alpha reductase inhibitors
Research in the early 2010s associated the 5-alpha reductase inhibitor finasteride with persistent sexual side effects and depression. But a later meta-analysis of the Prostate Cancer Prevention Trial and other trials did not find evidence of persistent sexual side effects or depression in men on finasteride, and the authors said that double-blind, placebo-controlled studies were needed (J Clin Aesthet Dermatol. 2014 Dec;7[12]:51-5).
However, two meta-analyses of 34 clinical trials published in 2015 found that none of the clinical trials evaluating finasteride treatment in patients with androgenic alopecia had accurate safety reporting (JAMA Dermatol. 2015 Jun;151[6]:600-6). Another study published by the same group in 2017 found that 0.8% of men aged 16-42 years to exposed to a 5-alpha reductase inhibitor developed persistent erectile dysfunction after a longer duration of exposure (median, 1,534 days). compared with a shorter duration of exposure (PeerJ. 2017 Mar 9;5:e3020).
The bottom line when considering use of 5-alpha reductase inhibitors in men is to discuss the outlier data on persistent sexual dysfunction in the studies, ask patients whether they have a history of sexual dysfunction and depression, and then only treat appropriate patients with no such history, Dr. McMichael said.
Use of 5-alpha reductase inhibitors also appears to be related to an increased risk of type 2 diabetes in men with benign prostatic hyperplasia, according to more recent data. In a study of patients in the U.K. Clinical Practice Research Datalink and Taiwanese National Health Insurance Research Database who received dutasteride, finasteride, or the alpha blocker tamsulosin, there was a slightly increased risk of type 2 diabetes among those who took the two 5-alpha reductase inhibitors, compared with those on tamsulosin (BMJ. 2019;365:l1204). In light of these results, Dr. McMichael advised clinicians to be aware of these risks and to consider screening patients for type 2 diabetes and ask them about their family history, but the results shouldn’t affect patients at risk for type 2 diabetes or metabolic disorder.
JAK inhibitors for alopecia areata
Within the past few years, promising results for Janus kinase (JAK) inhibitors like tofacitinib and ruxolitinib for alopecia areata have been reported, but they are currently not a first-line therapy, Dr. McMichael said. Consider methotrexate first in older adolescents and adults with more than 50% hair loss, followed by a JAK inhibitor if there is no improvement or methotrexate is not tolerated well.
Clinicians can consider enrolling their patients in a clinical trial to give them access to JAK inhibitors as a treatment option, she noted, and if a trial is not available, it may be worth appealing to an insurance company using an article titled “Alopecia areata is a medical disease” coauthored by Dr. McMichael and others (Am Acad Dermatol. 2018 Apr;78[4]:832-4). After two denials by insurance, the manufacturer’s patient assistance program (Xelsource) may be helpful in obtaining tofacitinib (Xeljanz) through a letter and references. There are adolescent data on JAK inhibitors for alopecia, but “absolutely no data” in very young children, so prior to adolescence, she would not recommend this treatment. In a study of 13 adolescents with alopecia areata, totalis, or universalis, those treated with tofacitinib for a mean of 6.5 months, 9 had significant hair regrowth with treatment and adverse events were mild (J Am Acad Dermatol. 2017 Jan;76[1]:29-32).
Dr. McMichael reports being an investigator for Allergan, Intendis, Proctor & Gamble, Samumed, Cassiopia, Concert, Aclaris, and Incyte; and is a consultant for Johnson & Johnson, Proctor & Gamble, Allergan, Bayer, Galderma, Incyte, Samumed, Aclaris, Anacor, Pfizer, Nutrafol, Bioniz, and Almirall.
ORLANDO – Biotin is the most popular consumer supplement for alopecia and highly popular online, but should patients be taking it?
Patients may want something “natural” to treat their hair loss, but “natural is not always good,” Amy McMichael, MD, professor and chair of the department of dermatology at Wake Forest Baptist Health, Winston-Salem, N.C., said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dosages of commercially available biotin supplements can vary significantly, with some doses as high as 10,000 mcg, making supraphysiological dosing possible. Dr. McMichael said that, not only is biotin unlikely to help with a patient’s alopecia, but a high intake of biotin can interfere with certain assays that use streptavidin-biotin capture techniques (Clin Chem Lab Med. 2017 May 1;55[6]:817-25). This could present a problem for a patient who experiences an MI or has a thyroid disorder where a high level of biotin could affect lab results, she noted.
Dr. McMichael advises patients that there is a
Questioning side effects of 5-alpha reductase inhibitors
Research in the early 2010s associated the 5-alpha reductase inhibitor finasteride with persistent sexual side effects and depression. But a later meta-analysis of the Prostate Cancer Prevention Trial and other trials did not find evidence of persistent sexual side effects or depression in men on finasteride, and the authors said that double-blind, placebo-controlled studies were needed (J Clin Aesthet Dermatol. 2014 Dec;7[12]:51-5).
However, two meta-analyses of 34 clinical trials published in 2015 found that none of the clinical trials evaluating finasteride treatment in patients with androgenic alopecia had accurate safety reporting (JAMA Dermatol. 2015 Jun;151[6]:600-6). Another study published by the same group in 2017 found that 0.8% of men aged 16-42 years to exposed to a 5-alpha reductase inhibitor developed persistent erectile dysfunction after a longer duration of exposure (median, 1,534 days). compared with a shorter duration of exposure (PeerJ. 2017 Mar 9;5:e3020).
The bottom line when considering use of 5-alpha reductase inhibitors in men is to discuss the outlier data on persistent sexual dysfunction in the studies, ask patients whether they have a history of sexual dysfunction and depression, and then only treat appropriate patients with no such history, Dr. McMichael said.
Use of 5-alpha reductase inhibitors also appears to be related to an increased risk of type 2 diabetes in men with benign prostatic hyperplasia, according to more recent data. In a study of patients in the U.K. Clinical Practice Research Datalink and Taiwanese National Health Insurance Research Database who received dutasteride, finasteride, or the alpha blocker tamsulosin, there was a slightly increased risk of type 2 diabetes among those who took the two 5-alpha reductase inhibitors, compared with those on tamsulosin (BMJ. 2019;365:l1204). In light of these results, Dr. McMichael advised clinicians to be aware of these risks and to consider screening patients for type 2 diabetes and ask them about their family history, but the results shouldn’t affect patients at risk for type 2 diabetes or metabolic disorder.
JAK inhibitors for alopecia areata
Within the past few years, promising results for Janus kinase (JAK) inhibitors like tofacitinib and ruxolitinib for alopecia areata have been reported, but they are currently not a first-line therapy, Dr. McMichael said. Consider methotrexate first in older adolescents and adults with more than 50% hair loss, followed by a JAK inhibitor if there is no improvement or methotrexate is not tolerated well.
Clinicians can consider enrolling their patients in a clinical trial to give them access to JAK inhibitors as a treatment option, she noted, and if a trial is not available, it may be worth appealing to an insurance company using an article titled “Alopecia areata is a medical disease” coauthored by Dr. McMichael and others (Am Acad Dermatol. 2018 Apr;78[4]:832-4). After two denials by insurance, the manufacturer’s patient assistance program (Xelsource) may be helpful in obtaining tofacitinib (Xeljanz) through a letter and references. There are adolescent data on JAK inhibitors for alopecia, but “absolutely no data” in very young children, so prior to adolescence, she would not recommend this treatment. In a study of 13 adolescents with alopecia areata, totalis, or universalis, those treated with tofacitinib for a mean of 6.5 months, 9 had significant hair regrowth with treatment and adverse events were mild (J Am Acad Dermatol. 2017 Jan;76[1]:29-32).
Dr. McMichael reports being an investigator for Allergan, Intendis, Proctor & Gamble, Samumed, Cassiopia, Concert, Aclaris, and Incyte; and is a consultant for Johnson & Johnson, Proctor & Gamble, Allergan, Bayer, Galderma, Incyte, Samumed, Aclaris, Anacor, Pfizer, Nutrafol, Bioniz, and Almirall.
ORLANDO – Biotin is the most popular consumer supplement for alopecia and highly popular online, but should patients be taking it?
Patients may want something “natural” to treat their hair loss, but “natural is not always good,” Amy McMichael, MD, professor and chair of the department of dermatology at Wake Forest Baptist Health, Winston-Salem, N.C., said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
Dosages of commercially available biotin supplements can vary significantly, with some doses as high as 10,000 mcg, making supraphysiological dosing possible. Dr. McMichael said that, not only is biotin unlikely to help with a patient’s alopecia, but a high intake of biotin can interfere with certain assays that use streptavidin-biotin capture techniques (Clin Chem Lab Med. 2017 May 1;55[6]:817-25). This could present a problem for a patient who experiences an MI or has a thyroid disorder where a high level of biotin could affect lab results, she noted.
Dr. McMichael advises patients that there is a
Questioning side effects of 5-alpha reductase inhibitors
Research in the early 2010s associated the 5-alpha reductase inhibitor finasteride with persistent sexual side effects and depression. But a later meta-analysis of the Prostate Cancer Prevention Trial and other trials did not find evidence of persistent sexual side effects or depression in men on finasteride, and the authors said that double-blind, placebo-controlled studies were needed (J Clin Aesthet Dermatol. 2014 Dec;7[12]:51-5).
However, two meta-analyses of 34 clinical trials published in 2015 found that none of the clinical trials evaluating finasteride treatment in patients with androgenic alopecia had accurate safety reporting (JAMA Dermatol. 2015 Jun;151[6]:600-6). Another study published by the same group in 2017 found that 0.8% of men aged 16-42 years to exposed to a 5-alpha reductase inhibitor developed persistent erectile dysfunction after a longer duration of exposure (median, 1,534 days). compared with a shorter duration of exposure (PeerJ. 2017 Mar 9;5:e3020).
The bottom line when considering use of 5-alpha reductase inhibitors in men is to discuss the outlier data on persistent sexual dysfunction in the studies, ask patients whether they have a history of sexual dysfunction and depression, and then only treat appropriate patients with no such history, Dr. McMichael said.
Use of 5-alpha reductase inhibitors also appears to be related to an increased risk of type 2 diabetes in men with benign prostatic hyperplasia, according to more recent data. In a study of patients in the U.K. Clinical Practice Research Datalink and Taiwanese National Health Insurance Research Database who received dutasteride, finasteride, or the alpha blocker tamsulosin, there was a slightly increased risk of type 2 diabetes among those who took the two 5-alpha reductase inhibitors, compared with those on tamsulosin (BMJ. 2019;365:l1204). In light of these results, Dr. McMichael advised clinicians to be aware of these risks and to consider screening patients for type 2 diabetes and ask them about their family history, but the results shouldn’t affect patients at risk for type 2 diabetes or metabolic disorder.
JAK inhibitors for alopecia areata
Within the past few years, promising results for Janus kinase (JAK) inhibitors like tofacitinib and ruxolitinib for alopecia areata have been reported, but they are currently not a first-line therapy, Dr. McMichael said. Consider methotrexate first in older adolescents and adults with more than 50% hair loss, followed by a JAK inhibitor if there is no improvement or methotrexate is not tolerated well.
Clinicians can consider enrolling their patients in a clinical trial to give them access to JAK inhibitors as a treatment option, she noted, and if a trial is not available, it may be worth appealing to an insurance company using an article titled “Alopecia areata is a medical disease” coauthored by Dr. McMichael and others (Am Acad Dermatol. 2018 Apr;78[4]:832-4). After two denials by insurance, the manufacturer’s patient assistance program (Xelsource) may be helpful in obtaining tofacitinib (Xeljanz) through a letter and references. There are adolescent data on JAK inhibitors for alopecia, but “absolutely no data” in very young children, so prior to adolescence, she would not recommend this treatment. In a study of 13 adolescents with alopecia areata, totalis, or universalis, those treated with tofacitinib for a mean of 6.5 months, 9 had significant hair regrowth with treatment and adverse events were mild (J Am Acad Dermatol. 2017 Jan;76[1]:29-32).
Dr. McMichael reports being an investigator for Allergan, Intendis, Proctor & Gamble, Samumed, Cassiopia, Concert, Aclaris, and Incyte; and is a consultant for Johnson & Johnson, Proctor & Gamble, Allergan, Bayer, Galderma, Incyte, Samumed, Aclaris, Anacor, Pfizer, Nutrafol, Bioniz, and Almirall.
EXPERT ANALYSIS FROM ODAC 2020
Emollients didn’t prevent atopic dermatitis in high-risk infants
The use of including those at high risk, in two new clinical trials.
The BEEP (Barrier Enhancement for Eczema Prevention) study compared the rates of AD among infants identified as at risk of AD because of family history who had daily applications of emollients (Diprobase cream or Doublebase gel) for the first year of life, compared with a standard skin care group. PreventADALL (Preventing Atopic Dermatitis and Allergies in Children) is a randomized, primary-prevention study conducted in Norway and Sweden that randomized infants into one of four groups: controls whose parents followed regular skin care advice and nutrition guidelines; those who received skin emollients (the addition of emulsified oil to their bath and application of facial cream on at least 4 days a week from age 2 weeks to 8 months); those who received early complementary feeding of peanut, cow’s milk, wheat, and egg introduced between aged 12 and 16 weeks; and a group that combined both the emollient and diet interventions.
Neither of the studies, published in the Lancet, found statistically significant differences in AD rates between the intervention and control groups.
The results put a damper on hopes raised by previous studies that included two small pilot studies, which found that daily use of leave-on emollients in infants considered at high risk of AD prevented the development of AD (J Allergy Clin Immunol 2014 Oct;134:824-30.e6; J Allergy Clin Immunol Oct 2014;134:818-23).
“It was maybe a little bit overly hopeful to think that we could just moisturize and prevent such a complex disorder,” Robert Sidbury, MD, chief of dermatology at Seattle Children’s Hospital, said in an interview. He emphasized that the studies only addressed emollients as a preventative, and that “there’s no question that emollients are still critical for the therapy of eczema.”
Bruce Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia, suggested that homogeneous patient populations or insufficient numbers might explain the negative findings. PreventADALL drew patients from Norway and Sweden, while BEEP recruited from the United Kingdom. “They’re important studies, but I think they still lend themselves to further studies with different patient populations and larger groups of patients,” Dr. Brod said in an interview.
BEEP was headed by Joanne Chalmers, PhD, and Hywel Williams, DSc, of the Centre of Evidence-Based Dermatology at the University of Nottingham (England). Håvard Ove Skjerven, PhD, and Karin C Lødrup Carlsen, PhD, of Oslo University Hospital led the PreventADALL study.
The BEEP study randomized 1,394 newborns at 16 sites in the United Kingdom to daily emollient treatment with standard skin care, or standard skin care alone. At one year, compliance was 74% in the intervention group. At age 2, 23% of the intervention group had AD, compared with 25% of controls (hazard ratio, 0.95; P =.61). Skin infections were also higher in the treatment arm (mean, 0.23 per year vs. 0.15 per year; adjusted incidence ratio, 1.55; 95% confidence interval, 1.15-2.09).
“Our study does not support the use of emollients for preventing eczema in high-risk infants, a finding supported by PreventADALL, another large trial using a skin barrier enhancing intervention,” they concluded. Their data “relate only to prevention of eczema and do not directly challenge the practice of using emollients as first-line treatment for eczema.”
In the PreventADALL study, 2,397 newborn infants born between 2015 and 2017 were randomized to one of the four groups. Use of facial cream and emollients during bathing began at 2 weeks, and early complementary feeding of peanut, cow’s milk, wheat, and egg at 3-4 months. The frequency of AD at aged 12 months in the control group was 8%, compared with 11% in the skin-intervention group, 9% in the food-intervention group, and 5% in the combined-intervention group.
These differences were not statistically significant, and “the primary hypothesis that either skin intervention or food intervention reduced atopic dermatitis were not confirmed,” the authors wrote. Parental atopy did not influence the effects of the interventions. Their results were in line with the BEEP results, and the authors “cannot recommend these interventions as primary prevention strategies.”
The researchers will continue to follow children until age 3 years to evaluate the food allergy rates, if the combined-treatment group experiences a long-term benefit. Adherence to the protocol was poor, with 44% compliance with the facial cream application and 27% compliance with bathing emollients; 32% fully adhered to the diet protocols.
The studies were funded by the National Institute for Health Research Health Technology Assessment (BEEP); and a range of public and private funders (PreventADALL). One author of the PreventADALL study disclosed receiving honoraria for presentations from several pharmaceutical companies, and one author received honoraria for presentations from Thermo Fisher Scientific; the rest had no disclosures. Dr. Sidbury has been an investigator for Regeneron. Dr. Brod had no relevant financial disclosures.
SOURCES: Chalmers JR et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32984-8; Skjerven HO et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32983-6.
The “null findings” of these two studies were “unexpected,” Kirsten P. Perrett, MBBS, Phd, and Rachel L. Peters, PhD, of the department of population allergy at Murdoch Children’s Research Institute, Parkville, Australia, wrote in an accompanying editorial. They noted that emollients are used regularly in the management of atopic dermatitis, where they help maintain the skin barrier and reduce the need for anti-inflammatory therapies.
These two large prevention studies were “prompted” by the results of small, proof-of-concept pilot studies, which “provided strong efficacy signals for the hypothesis that daily emollient use could prevent atopic dermatitis,” they wrote. But the two studies “found no evidence that daily emollient use in either a population-based or high-risk cohort of infants during the first year of life could delay, suppress, or prevent atopic dermatitis.” The lower incidence of atopic dermatitis among those in the dietary and emollient combination, compared with controls (5% vs. 8%) in PreventADALL, could be a chance finding.
The large, randomized Prevention of Eczema by a Barrier Lipid Equilibrium Strategy (PEBBLES) trial is ongoing to confirm results from a small study suggesting the efficacy of a ceramide-dominant emollient. But the PreventADALL study showed low compliance, suggesting that this intervention, if effective, a twice-daily emollient regimen may be tough to implement. “At this stage, emollients should not be recommended for the primary prevention of atopic dermatitis in infants,” they concluded.
Dr. Perrett and Dr. Peters declared no competing interests. Their comments appeared in the Lancet (2020 Feb 19. doi: 10.1016/S0140-6736[19]33174-5).
The “null findings” of these two studies were “unexpected,” Kirsten P. Perrett, MBBS, Phd, and Rachel L. Peters, PhD, of the department of population allergy at Murdoch Children’s Research Institute, Parkville, Australia, wrote in an accompanying editorial. They noted that emollients are used regularly in the management of atopic dermatitis, where they help maintain the skin barrier and reduce the need for anti-inflammatory therapies.
These two large prevention studies were “prompted” by the results of small, proof-of-concept pilot studies, which “provided strong efficacy signals for the hypothesis that daily emollient use could prevent atopic dermatitis,” they wrote. But the two studies “found no evidence that daily emollient use in either a population-based or high-risk cohort of infants during the first year of life could delay, suppress, or prevent atopic dermatitis.” The lower incidence of atopic dermatitis among those in the dietary and emollient combination, compared with controls (5% vs. 8%) in PreventADALL, could be a chance finding.
The large, randomized Prevention of Eczema by a Barrier Lipid Equilibrium Strategy (PEBBLES) trial is ongoing to confirm results from a small study suggesting the efficacy of a ceramide-dominant emollient. But the PreventADALL study showed low compliance, suggesting that this intervention, if effective, a twice-daily emollient regimen may be tough to implement. “At this stage, emollients should not be recommended for the primary prevention of atopic dermatitis in infants,” they concluded.
Dr. Perrett and Dr. Peters declared no competing interests. Their comments appeared in the Lancet (2020 Feb 19. doi: 10.1016/S0140-6736[19]33174-5).
The “null findings” of these two studies were “unexpected,” Kirsten P. Perrett, MBBS, Phd, and Rachel L. Peters, PhD, of the department of population allergy at Murdoch Children’s Research Institute, Parkville, Australia, wrote in an accompanying editorial. They noted that emollients are used regularly in the management of atopic dermatitis, where they help maintain the skin barrier and reduce the need for anti-inflammatory therapies.
These two large prevention studies were “prompted” by the results of small, proof-of-concept pilot studies, which “provided strong efficacy signals for the hypothesis that daily emollient use could prevent atopic dermatitis,” they wrote. But the two studies “found no evidence that daily emollient use in either a population-based or high-risk cohort of infants during the first year of life could delay, suppress, or prevent atopic dermatitis.” The lower incidence of atopic dermatitis among those in the dietary and emollient combination, compared with controls (5% vs. 8%) in PreventADALL, could be a chance finding.
The large, randomized Prevention of Eczema by a Barrier Lipid Equilibrium Strategy (PEBBLES) trial is ongoing to confirm results from a small study suggesting the efficacy of a ceramide-dominant emollient. But the PreventADALL study showed low compliance, suggesting that this intervention, if effective, a twice-daily emollient regimen may be tough to implement. “At this stage, emollients should not be recommended for the primary prevention of atopic dermatitis in infants,” they concluded.
Dr. Perrett and Dr. Peters declared no competing interests. Their comments appeared in the Lancet (2020 Feb 19. doi: 10.1016/S0140-6736[19]33174-5).
The use of including those at high risk, in two new clinical trials.
The BEEP (Barrier Enhancement for Eczema Prevention) study compared the rates of AD among infants identified as at risk of AD because of family history who had daily applications of emollients (Diprobase cream or Doublebase gel) for the first year of life, compared with a standard skin care group. PreventADALL (Preventing Atopic Dermatitis and Allergies in Children) is a randomized, primary-prevention study conducted in Norway and Sweden that randomized infants into one of four groups: controls whose parents followed regular skin care advice and nutrition guidelines; those who received skin emollients (the addition of emulsified oil to their bath and application of facial cream on at least 4 days a week from age 2 weeks to 8 months); those who received early complementary feeding of peanut, cow’s milk, wheat, and egg introduced between aged 12 and 16 weeks; and a group that combined both the emollient and diet interventions.
Neither of the studies, published in the Lancet, found statistically significant differences in AD rates between the intervention and control groups.
The results put a damper on hopes raised by previous studies that included two small pilot studies, which found that daily use of leave-on emollients in infants considered at high risk of AD prevented the development of AD (J Allergy Clin Immunol 2014 Oct;134:824-30.e6; J Allergy Clin Immunol Oct 2014;134:818-23).
“It was maybe a little bit overly hopeful to think that we could just moisturize and prevent such a complex disorder,” Robert Sidbury, MD, chief of dermatology at Seattle Children’s Hospital, said in an interview. He emphasized that the studies only addressed emollients as a preventative, and that “there’s no question that emollients are still critical for the therapy of eczema.”
Bruce Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia, suggested that homogeneous patient populations or insufficient numbers might explain the negative findings. PreventADALL drew patients from Norway and Sweden, while BEEP recruited from the United Kingdom. “They’re important studies, but I think they still lend themselves to further studies with different patient populations and larger groups of patients,” Dr. Brod said in an interview.
BEEP was headed by Joanne Chalmers, PhD, and Hywel Williams, DSc, of the Centre of Evidence-Based Dermatology at the University of Nottingham (England). Håvard Ove Skjerven, PhD, and Karin C Lødrup Carlsen, PhD, of Oslo University Hospital led the PreventADALL study.
The BEEP study randomized 1,394 newborns at 16 sites in the United Kingdom to daily emollient treatment with standard skin care, or standard skin care alone. At one year, compliance was 74% in the intervention group. At age 2, 23% of the intervention group had AD, compared with 25% of controls (hazard ratio, 0.95; P =.61). Skin infections were also higher in the treatment arm (mean, 0.23 per year vs. 0.15 per year; adjusted incidence ratio, 1.55; 95% confidence interval, 1.15-2.09).
“Our study does not support the use of emollients for preventing eczema in high-risk infants, a finding supported by PreventADALL, another large trial using a skin barrier enhancing intervention,” they concluded. Their data “relate only to prevention of eczema and do not directly challenge the practice of using emollients as first-line treatment for eczema.”
In the PreventADALL study, 2,397 newborn infants born between 2015 and 2017 were randomized to one of the four groups. Use of facial cream and emollients during bathing began at 2 weeks, and early complementary feeding of peanut, cow’s milk, wheat, and egg at 3-4 months. The frequency of AD at aged 12 months in the control group was 8%, compared with 11% in the skin-intervention group, 9% in the food-intervention group, and 5% in the combined-intervention group.
These differences were not statistically significant, and “the primary hypothesis that either skin intervention or food intervention reduced atopic dermatitis were not confirmed,” the authors wrote. Parental atopy did not influence the effects of the interventions. Their results were in line with the BEEP results, and the authors “cannot recommend these interventions as primary prevention strategies.”
The researchers will continue to follow children until age 3 years to evaluate the food allergy rates, if the combined-treatment group experiences a long-term benefit. Adherence to the protocol was poor, with 44% compliance with the facial cream application and 27% compliance with bathing emollients; 32% fully adhered to the diet protocols.
The studies were funded by the National Institute for Health Research Health Technology Assessment (BEEP); and a range of public and private funders (PreventADALL). One author of the PreventADALL study disclosed receiving honoraria for presentations from several pharmaceutical companies, and one author received honoraria for presentations from Thermo Fisher Scientific; the rest had no disclosures. Dr. Sidbury has been an investigator for Regeneron. Dr. Brod had no relevant financial disclosures.
SOURCES: Chalmers JR et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32984-8; Skjerven HO et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32983-6.
The use of including those at high risk, in two new clinical trials.
The BEEP (Barrier Enhancement for Eczema Prevention) study compared the rates of AD among infants identified as at risk of AD because of family history who had daily applications of emollients (Diprobase cream or Doublebase gel) for the first year of life, compared with a standard skin care group. PreventADALL (Preventing Atopic Dermatitis and Allergies in Children) is a randomized, primary-prevention study conducted in Norway and Sweden that randomized infants into one of four groups: controls whose parents followed regular skin care advice and nutrition guidelines; those who received skin emollients (the addition of emulsified oil to their bath and application of facial cream on at least 4 days a week from age 2 weeks to 8 months); those who received early complementary feeding of peanut, cow’s milk, wheat, and egg introduced between aged 12 and 16 weeks; and a group that combined both the emollient and diet interventions.
Neither of the studies, published in the Lancet, found statistically significant differences in AD rates between the intervention and control groups.
The results put a damper on hopes raised by previous studies that included two small pilot studies, which found that daily use of leave-on emollients in infants considered at high risk of AD prevented the development of AD (J Allergy Clin Immunol 2014 Oct;134:824-30.e6; J Allergy Clin Immunol Oct 2014;134:818-23).
“It was maybe a little bit overly hopeful to think that we could just moisturize and prevent such a complex disorder,” Robert Sidbury, MD, chief of dermatology at Seattle Children’s Hospital, said in an interview. He emphasized that the studies only addressed emollients as a preventative, and that “there’s no question that emollients are still critical for the therapy of eczema.”
Bruce Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia, suggested that homogeneous patient populations or insufficient numbers might explain the negative findings. PreventADALL drew patients from Norway and Sweden, while BEEP recruited from the United Kingdom. “They’re important studies, but I think they still lend themselves to further studies with different patient populations and larger groups of patients,” Dr. Brod said in an interview.
BEEP was headed by Joanne Chalmers, PhD, and Hywel Williams, DSc, of the Centre of Evidence-Based Dermatology at the University of Nottingham (England). Håvard Ove Skjerven, PhD, and Karin C Lødrup Carlsen, PhD, of Oslo University Hospital led the PreventADALL study.
The BEEP study randomized 1,394 newborns at 16 sites in the United Kingdom to daily emollient treatment with standard skin care, or standard skin care alone. At one year, compliance was 74% in the intervention group. At age 2, 23% of the intervention group had AD, compared with 25% of controls (hazard ratio, 0.95; P =.61). Skin infections were also higher in the treatment arm (mean, 0.23 per year vs. 0.15 per year; adjusted incidence ratio, 1.55; 95% confidence interval, 1.15-2.09).
“Our study does not support the use of emollients for preventing eczema in high-risk infants, a finding supported by PreventADALL, another large trial using a skin barrier enhancing intervention,” they concluded. Their data “relate only to prevention of eczema and do not directly challenge the practice of using emollients as first-line treatment for eczema.”
In the PreventADALL study, 2,397 newborn infants born between 2015 and 2017 were randomized to one of the four groups. Use of facial cream and emollients during bathing began at 2 weeks, and early complementary feeding of peanut, cow’s milk, wheat, and egg at 3-4 months. The frequency of AD at aged 12 months in the control group was 8%, compared with 11% in the skin-intervention group, 9% in the food-intervention group, and 5% in the combined-intervention group.
These differences were not statistically significant, and “the primary hypothesis that either skin intervention or food intervention reduced atopic dermatitis were not confirmed,” the authors wrote. Parental atopy did not influence the effects of the interventions. Their results were in line with the BEEP results, and the authors “cannot recommend these interventions as primary prevention strategies.”
The researchers will continue to follow children until age 3 years to evaluate the food allergy rates, if the combined-treatment group experiences a long-term benefit. Adherence to the protocol was poor, with 44% compliance with the facial cream application and 27% compliance with bathing emollients; 32% fully adhered to the diet protocols.
The studies were funded by the National Institute for Health Research Health Technology Assessment (BEEP); and a range of public and private funders (PreventADALL). One author of the PreventADALL study disclosed receiving honoraria for presentations from several pharmaceutical companies, and one author received honoraria for presentations from Thermo Fisher Scientific; the rest had no disclosures. Dr. Sidbury has been an investigator for Regeneron. Dr. Brod had no relevant financial disclosures.
SOURCES: Chalmers JR et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32984-8; Skjerven HO et al. Lancet. 2020 Feb 19. doi: 10.1016/S0140-6736(19)32983-6.
FROM THE LANCET
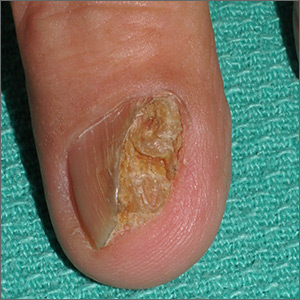
Nail growth
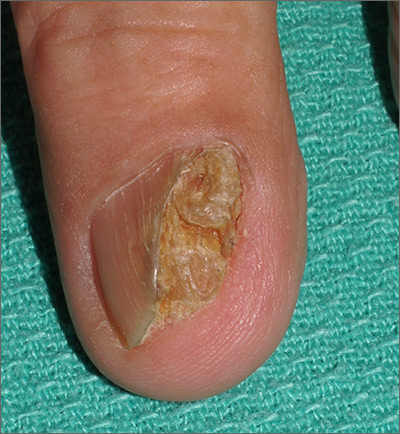
The treatment failure with the terbinafine made onychomycosis unlikely, and the appearance of the finger did not suggest that this was a wart. So, the physician opted for a 4-mm punch biopsy of the lateral nail fold, which confirmed that this was a well-differentiated squamous cell carcinoma (SCC) of the fingertip.
Periungual SCC is twice as common in men as women. Lesions tend to appear as hyperkeratotic plaques or nodules, pushing the nail plate away from the nail bed. With onychomycosis, one would expect the nail to be more thickened and discolored. A wart would not be as keratotic as this lesion was, and there would be thrombosed capillaries on closer inspection.
SCC is the second most common skin cancer in humans. It is most common on sun-exposed areas but may present in sites not exposed to the sun. It has been hypothesized that high risk human papillomavirus (HPV) types—particularly HPV 16—may contribute to diseases of the fingertips and nail unit in older adults. (There was no known history of HPV in this patient.)
A surgical approach often is curative. Achieving appropriate margins occasionally requires partial amputation. Mohs micrographic surgery (MMS) offers the highest cure rate and spares as much uninvolved tissue as possible. Radiation therapy is another tissue-sparing technique. It requires 15 to 30 sessions over 3 to 6 weeks and has a lower cure rate than MMS.
In this case, the patient underwent MMS. Follow-up skin surveillance exams revealed other small nonmelanoma skin cancers at other sites. The patient also developed a dystrophic nail spicule near the surgical site that was re-excised and deemed benign.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011 Jun;64:1147-1153.

The treatment failure with the terbinafine made onychomycosis unlikely, and the appearance of the finger did not suggest that this was a wart. So, the physician opted for a 4-mm punch biopsy of the lateral nail fold, which confirmed that this was a well-differentiated squamous cell carcinoma (SCC) of the fingertip.
Periungual SCC is twice as common in men as women. Lesions tend to appear as hyperkeratotic plaques or nodules, pushing the nail plate away from the nail bed. With onychomycosis, one would expect the nail to be more thickened and discolored. A wart would not be as keratotic as this lesion was, and there would be thrombosed capillaries on closer inspection.
SCC is the second most common skin cancer in humans. It is most common on sun-exposed areas but may present in sites not exposed to the sun. It has been hypothesized that high risk human papillomavirus (HPV) types—particularly HPV 16—may contribute to diseases of the fingertips and nail unit in older adults. (There was no known history of HPV in this patient.)
A surgical approach often is curative. Achieving appropriate margins occasionally requires partial amputation. Mohs micrographic surgery (MMS) offers the highest cure rate and spares as much uninvolved tissue as possible. Radiation therapy is another tissue-sparing technique. It requires 15 to 30 sessions over 3 to 6 weeks and has a lower cure rate than MMS.
In this case, the patient underwent MMS. Follow-up skin surveillance exams revealed other small nonmelanoma skin cancers at other sites. The patient also developed a dystrophic nail spicule near the surgical site that was re-excised and deemed benign.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The treatment failure with the terbinafine made onychomycosis unlikely, and the appearance of the finger did not suggest that this was a wart. So, the physician opted for a 4-mm punch biopsy of the lateral nail fold, which confirmed that this was a well-differentiated squamous cell carcinoma (SCC) of the fingertip.
Periungual SCC is twice as common in men as women. Lesions tend to appear as hyperkeratotic plaques or nodules, pushing the nail plate away from the nail bed. With onychomycosis, one would expect the nail to be more thickened and discolored. A wart would not be as keratotic as this lesion was, and there would be thrombosed capillaries on closer inspection.
SCC is the second most common skin cancer in humans. It is most common on sun-exposed areas but may present in sites not exposed to the sun. It has been hypothesized that high risk human papillomavirus (HPV) types—particularly HPV 16—may contribute to diseases of the fingertips and nail unit in older adults. (There was no known history of HPV in this patient.)
A surgical approach often is curative. Achieving appropriate margins occasionally requires partial amputation. Mohs micrographic surgery (MMS) offers the highest cure rate and spares as much uninvolved tissue as possible. Radiation therapy is another tissue-sparing technique. It requires 15 to 30 sessions over 3 to 6 weeks and has a lower cure rate than MMS.
In this case, the patient underwent MMS. Follow-up skin surveillance exams revealed other small nonmelanoma skin cancers at other sites. The patient also developed a dystrophic nail spicule near the surgical site that was re-excised and deemed benign.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011 Jun;64:1147-1153.
Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011 Jun;64:1147-1153.
Does screening by primary care providers effectively detect melanoma and other skin cancers?
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE-BASED ANSWER:
Possibly. No trials have directly assessed detection of melanoma and other skin cancers by primary care providers.
Training a group comprised largely of primary care physicians to perform skin cancer screening was associated with a 41% increase in skin cancer diagnoses but no change in melanoma mortality.
Visual screening for melanoma by primary care physicians is 40% sensitive and 86% specific (compared with 49% and 98%, respectively, for dermatologists and plastic surgeons).
Melanomas found by visual screening are 38% more likely to be thin (≤ 0.75 mm) than melanomas discovered without screening, which correlates with improved outcomes.
Visual skin cancer screening overall is associated with false-positive rates as follows: 28 biopsies for each melanoma detected, 9 to 10 biopsies for each basal cell carcinoma, and 28 to 56 biopsies for squamous cell carcinoma. False-positive rates are higher for women—as much as double the rate for men—and younger patients—as much as 20-fold the rate for older patients (strength of recommendations for all foregoing statements: B, cohort studies).
Esophageal stricture signals urgent treatment in kids with butterfly skin
LONDON – A quarter of urgent contacts in 20 children with generalized severe recessive dystrophic epidermolysis bullosa (GS-RDEB) were tied to esophageal narrowing, according data from a 12-month review of electronic health records.
Urgent advice was sought 102 times outside of regular or scheduled appointments by the parents of 20 children with GS-RDEB, Christine Prodinger, MD, of the University Clinic of Dermatology at Paracelsus Medical University, Salzburg, Austria, and colleagues reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). The researchers looked at the records from the EB clinic at Great Ormond Street Hospital for Children NHS Foundation Trust, London, during April 2018–April 2019.
The mean number of urgent contacts with the specialist unit was 5.1 per patient per year, the researchers reported, with 24 of the 102 contacts (23.5%) resulting in the child being admitted to a hospital. Most of the contacts were made via email or telephone to EB nurses (94%), by contacts during home visits (3%), or in an appointment with the palliative or symptom care team (3%).
“The most common reason [for the urgent contact] was acute dysphagia,” which was experienced as choking, throat pain, difficulty eating, reflux, and vomiting, the researchers observed. Dysphagia affected children in 27 of the contacts (26.5%), and resulted in esophageal dilatation in 90% of the cases. Other reasons for urgent contact were skin infection (15.7% of contacts), uncontrolled pain (15.7% of the contacts), and eye problems (11.8%).
Esophageal dilatation
Strictures are just one of the esophageal manifestations of the disease, noted Anna Bruckner, MD, associate professor of dermatology and pediatrics in the department of dermatology at the University of Colorado at Denver, Aurora, during an oral presentation. Other possible manifestations include blisters and erosions, the formation of webs – a thin extension of esophageal tissue, perforations, and rupture. “These are primarily problems with dystrophic EB” but can occur with other EB subtypes, she noted.
“We don’t have great evidence” on whether the onset of esophageal strictures can be delayed or prevented, Dr. Bruckner observed. As for management, “fluoroscopy-guided balloon dilatation is probably best” for most patients, but the best procedural approach needs to be discussed on a patient-by-patient basis.
Citing a paper that documents her own experience on the use of esophageal dilatation in 24 children who underwent 231 fluoroscopy-guided balloon dilatation procedures, Dr. Bruckner noted that strictures were most commonly located in the proximal part of the esophagus, with a median distance of 13 cm down from the lips (J Pediatr Gastroenterol Nutr. 2018;67[6]:701-5).
The retrospective chart review reported by Dr. Bruckner showed that there were a median of seven dilatation procedures per patient, and 20 patients had repeated procedures at a median interval of 164 days. About 10% of procedures resulted in adverse events – mostly vomiting, pain, and fever – but there were no perforations or other serious effects, and the rate of subsequent hospitalization was 6.9%.
Dysphagia
Dysphagia was the predominant symptom caused by esophageal stricture in another dataset reported in a poster by Elena Pope, MD, MSc, of the Hospital for Sick Children at the University of Toronto, and colleagues.
Of 125 EB patients who had experienced at least 1 esophageal stricture episode, 497 esophageal stricture events were reported, and 85.5% of patients had difficulty swallowing at presentation, with 29.8% unable to swallow solids and 7.2% unable to swallow liquids. Other symptoms at presentation were painful swallowing (11%), food being stuck in the esophagus (8%), regurgitation (5%), coughing (4.8%), and dyspepsia (2.8%).
The aim of the retrospective, multicenter cohort study was to determine the prevalence of, and predisposing factors for, restenosis of esophageal strictures and factors that may predispose to restenosis. The study population consisted of 66 men and 59 women who had experienced their esophageal stricture at around ages 12-13 years. The majority (98.4%) had dystrophic EB, of which almost half (46.5%) had GS-RDEB.
The researchers found that the location of the esophageal stricture was important for restenosis, and that strictures occurring in the lower esophagus were 67.5% less likely to result in restenosis than if they occurred in the upper esophagus (P = .057; hazard ratio, 0.675).
A higher number of strictures was associated with a higher rate of restenosis, they reported. Indeed, patients who had two esophageal strictures had a 29.4% increased risk of restenosis, compared with those who had just one stricture (P = .038; HR, 1.294), and those with three or more strictures had an increased risk of 78.5%, compared with those having one stricture (P = .005; HR, 1.785).
Strictures longer than 1 cm also were associated with a greater (34.7%) risk of restenosis, compared with shorter strictures (P = .032; HR, 1.347). Various methods of resolving the stricture were used, from fluoroscopy-guided balloon dilatation to retro- or antegrade endoscopy. “Irrespective of method, dilatations are successful,” Dr. Pope and colleagues reported. The overall success of dilatation was 99.3%, with full dilatation achieved in almost all of the patients (96%). Of note is that there was a low risk (2.6%) of complications, they observed.
Medications were used in 46.8% of the patients, with the most popular choice being corticosteroids (90.3%), but the researchers noted that the “potential benefit of periprocedural corticosteroids use in decreasing the risk of restenosis needs further exploration.”
Dr. Bruckner had noted in her presentation that her group did not favor the use of periprocedural corticosteroids, but that antifibrotic therapy “could be attractive” for preventing future strictures.
Dr. Prodinger, Dr. Pope, and their colleagues did not provide disclosure information. Dr. Bruckner is the principal investigator for the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. She disclosed the receipt of grants or research funding, honoraria, or consultation fees from a number of drug companies, as well as other support from the EB Research Partnership and the EB Medical Research Foundation.
SOURCES: Prodinger et al. EB 2020. Poster 3; Bruckner A et al. Pediatr Gastroenterol Nutr. 2018;67(6):701-5; Pope et al. EB 2020. Poster 8.
LONDON – A quarter of urgent contacts in 20 children with generalized severe recessive dystrophic epidermolysis bullosa (GS-RDEB) were tied to esophageal narrowing, according data from a 12-month review of electronic health records.
Urgent advice was sought 102 times outside of regular or scheduled appointments by the parents of 20 children with GS-RDEB, Christine Prodinger, MD, of the University Clinic of Dermatology at Paracelsus Medical University, Salzburg, Austria, and colleagues reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). The researchers looked at the records from the EB clinic at Great Ormond Street Hospital for Children NHS Foundation Trust, London, during April 2018–April 2019.
The mean number of urgent contacts with the specialist unit was 5.1 per patient per year, the researchers reported, with 24 of the 102 contacts (23.5%) resulting in the child being admitted to a hospital. Most of the contacts were made via email or telephone to EB nurses (94%), by contacts during home visits (3%), or in an appointment with the palliative or symptom care team (3%).
“The most common reason [for the urgent contact] was acute dysphagia,” which was experienced as choking, throat pain, difficulty eating, reflux, and vomiting, the researchers observed. Dysphagia affected children in 27 of the contacts (26.5%), and resulted in esophageal dilatation in 90% of the cases. Other reasons for urgent contact were skin infection (15.7% of contacts), uncontrolled pain (15.7% of the contacts), and eye problems (11.8%).
Esophageal dilatation
Strictures are just one of the esophageal manifestations of the disease, noted Anna Bruckner, MD, associate professor of dermatology and pediatrics in the department of dermatology at the University of Colorado at Denver, Aurora, during an oral presentation. Other possible manifestations include blisters and erosions, the formation of webs – a thin extension of esophageal tissue, perforations, and rupture. “These are primarily problems with dystrophic EB” but can occur with other EB subtypes, she noted.
“We don’t have great evidence” on whether the onset of esophageal strictures can be delayed or prevented, Dr. Bruckner observed. As for management, “fluoroscopy-guided balloon dilatation is probably best” for most patients, but the best procedural approach needs to be discussed on a patient-by-patient basis.
Citing a paper that documents her own experience on the use of esophageal dilatation in 24 children who underwent 231 fluoroscopy-guided balloon dilatation procedures, Dr. Bruckner noted that strictures were most commonly located in the proximal part of the esophagus, with a median distance of 13 cm down from the lips (J Pediatr Gastroenterol Nutr. 2018;67[6]:701-5).
The retrospective chart review reported by Dr. Bruckner showed that there were a median of seven dilatation procedures per patient, and 20 patients had repeated procedures at a median interval of 164 days. About 10% of procedures resulted in adverse events – mostly vomiting, pain, and fever – but there were no perforations or other serious effects, and the rate of subsequent hospitalization was 6.9%.
Dysphagia
Dysphagia was the predominant symptom caused by esophageal stricture in another dataset reported in a poster by Elena Pope, MD, MSc, of the Hospital for Sick Children at the University of Toronto, and colleagues.
Of 125 EB patients who had experienced at least 1 esophageal stricture episode, 497 esophageal stricture events were reported, and 85.5% of patients had difficulty swallowing at presentation, with 29.8% unable to swallow solids and 7.2% unable to swallow liquids. Other symptoms at presentation were painful swallowing (11%), food being stuck in the esophagus (8%), regurgitation (5%), coughing (4.8%), and dyspepsia (2.8%).
The aim of the retrospective, multicenter cohort study was to determine the prevalence of, and predisposing factors for, restenosis of esophageal strictures and factors that may predispose to restenosis. The study population consisted of 66 men and 59 women who had experienced their esophageal stricture at around ages 12-13 years. The majority (98.4%) had dystrophic EB, of which almost half (46.5%) had GS-RDEB.
The researchers found that the location of the esophageal stricture was important for restenosis, and that strictures occurring in the lower esophagus were 67.5% less likely to result in restenosis than if they occurred in the upper esophagus (P = .057; hazard ratio, 0.675).
A higher number of strictures was associated with a higher rate of restenosis, they reported. Indeed, patients who had two esophageal strictures had a 29.4% increased risk of restenosis, compared with those who had just one stricture (P = .038; HR, 1.294), and those with three or more strictures had an increased risk of 78.5%, compared with those having one stricture (P = .005; HR, 1.785).
Strictures longer than 1 cm also were associated with a greater (34.7%) risk of restenosis, compared with shorter strictures (P = .032; HR, 1.347). Various methods of resolving the stricture were used, from fluoroscopy-guided balloon dilatation to retro- or antegrade endoscopy. “Irrespective of method, dilatations are successful,” Dr. Pope and colleagues reported. The overall success of dilatation was 99.3%, with full dilatation achieved in almost all of the patients (96%). Of note is that there was a low risk (2.6%) of complications, they observed.
Medications were used in 46.8% of the patients, with the most popular choice being corticosteroids (90.3%), but the researchers noted that the “potential benefit of periprocedural corticosteroids use in decreasing the risk of restenosis needs further exploration.”
Dr. Bruckner had noted in her presentation that her group did not favor the use of periprocedural corticosteroids, but that antifibrotic therapy “could be attractive” for preventing future strictures.
Dr. Prodinger, Dr. Pope, and their colleagues did not provide disclosure information. Dr. Bruckner is the principal investigator for the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. She disclosed the receipt of grants or research funding, honoraria, or consultation fees from a number of drug companies, as well as other support from the EB Research Partnership and the EB Medical Research Foundation.
SOURCES: Prodinger et al. EB 2020. Poster 3; Bruckner A et al. Pediatr Gastroenterol Nutr. 2018;67(6):701-5; Pope et al. EB 2020. Poster 8.
LONDON – A quarter of urgent contacts in 20 children with generalized severe recessive dystrophic epidermolysis bullosa (GS-RDEB) were tied to esophageal narrowing, according data from a 12-month review of electronic health records.
Urgent advice was sought 102 times outside of regular or scheduled appointments by the parents of 20 children with GS-RDEB, Christine Prodinger, MD, of the University Clinic of Dermatology at Paracelsus Medical University, Salzburg, Austria, and colleagues reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). The researchers looked at the records from the EB clinic at Great Ormond Street Hospital for Children NHS Foundation Trust, London, during April 2018–April 2019.
The mean number of urgent contacts with the specialist unit was 5.1 per patient per year, the researchers reported, with 24 of the 102 contacts (23.5%) resulting in the child being admitted to a hospital. Most of the contacts were made via email or telephone to EB nurses (94%), by contacts during home visits (3%), or in an appointment with the palliative or symptom care team (3%).
“The most common reason [for the urgent contact] was acute dysphagia,” which was experienced as choking, throat pain, difficulty eating, reflux, and vomiting, the researchers observed. Dysphagia affected children in 27 of the contacts (26.5%), and resulted in esophageal dilatation in 90% of the cases. Other reasons for urgent contact were skin infection (15.7% of contacts), uncontrolled pain (15.7% of the contacts), and eye problems (11.8%).
Esophageal dilatation
Strictures are just one of the esophageal manifestations of the disease, noted Anna Bruckner, MD, associate professor of dermatology and pediatrics in the department of dermatology at the University of Colorado at Denver, Aurora, during an oral presentation. Other possible manifestations include blisters and erosions, the formation of webs – a thin extension of esophageal tissue, perforations, and rupture. “These are primarily problems with dystrophic EB” but can occur with other EB subtypes, she noted.
“We don’t have great evidence” on whether the onset of esophageal strictures can be delayed or prevented, Dr. Bruckner observed. As for management, “fluoroscopy-guided balloon dilatation is probably best” for most patients, but the best procedural approach needs to be discussed on a patient-by-patient basis.
Citing a paper that documents her own experience on the use of esophageal dilatation in 24 children who underwent 231 fluoroscopy-guided balloon dilatation procedures, Dr. Bruckner noted that strictures were most commonly located in the proximal part of the esophagus, with a median distance of 13 cm down from the lips (J Pediatr Gastroenterol Nutr. 2018;67[6]:701-5).
The retrospective chart review reported by Dr. Bruckner showed that there were a median of seven dilatation procedures per patient, and 20 patients had repeated procedures at a median interval of 164 days. About 10% of procedures resulted in adverse events – mostly vomiting, pain, and fever – but there were no perforations or other serious effects, and the rate of subsequent hospitalization was 6.9%.
Dysphagia
Dysphagia was the predominant symptom caused by esophageal stricture in another dataset reported in a poster by Elena Pope, MD, MSc, of the Hospital for Sick Children at the University of Toronto, and colleagues.
Of 125 EB patients who had experienced at least 1 esophageal stricture episode, 497 esophageal stricture events were reported, and 85.5% of patients had difficulty swallowing at presentation, with 29.8% unable to swallow solids and 7.2% unable to swallow liquids. Other symptoms at presentation were painful swallowing (11%), food being stuck in the esophagus (8%), regurgitation (5%), coughing (4.8%), and dyspepsia (2.8%).
The aim of the retrospective, multicenter cohort study was to determine the prevalence of, and predisposing factors for, restenosis of esophageal strictures and factors that may predispose to restenosis. The study population consisted of 66 men and 59 women who had experienced their esophageal stricture at around ages 12-13 years. The majority (98.4%) had dystrophic EB, of which almost half (46.5%) had GS-RDEB.
The researchers found that the location of the esophageal stricture was important for restenosis, and that strictures occurring in the lower esophagus were 67.5% less likely to result in restenosis than if they occurred in the upper esophagus (P = .057; hazard ratio, 0.675).
A higher number of strictures was associated with a higher rate of restenosis, they reported. Indeed, patients who had two esophageal strictures had a 29.4% increased risk of restenosis, compared with those who had just one stricture (P = .038; HR, 1.294), and those with three or more strictures had an increased risk of 78.5%, compared with those having one stricture (P = .005; HR, 1.785).
Strictures longer than 1 cm also were associated with a greater (34.7%) risk of restenosis, compared with shorter strictures (P = .032; HR, 1.347). Various methods of resolving the stricture were used, from fluoroscopy-guided balloon dilatation to retro- or antegrade endoscopy. “Irrespective of method, dilatations are successful,” Dr. Pope and colleagues reported. The overall success of dilatation was 99.3%, with full dilatation achieved in almost all of the patients (96%). Of note is that there was a low risk (2.6%) of complications, they observed.
Medications were used in 46.8% of the patients, with the most popular choice being corticosteroids (90.3%), but the researchers noted that the “potential benefit of periprocedural corticosteroids use in decreasing the risk of restenosis needs further exploration.”
Dr. Bruckner had noted in her presentation that her group did not favor the use of periprocedural corticosteroids, but that antifibrotic therapy “could be attractive” for preventing future strictures.
Dr. Prodinger, Dr. Pope, and their colleagues did not provide disclosure information. Dr. Bruckner is the principal investigator for the Epidermolysis Bullosa Clinical Characterization and Outcomes Database. She disclosed the receipt of grants or research funding, honoraria, or consultation fees from a number of drug companies, as well as other support from the EB Research Partnership and the EB Medical Research Foundation.
SOURCES: Prodinger et al. EB 2020. Poster 3; Bruckner A et al. Pediatr Gastroenterol Nutr. 2018;67(6):701-5; Pope et al. EB 2020. Poster 8.
REPORTING FROM EB 2020
Consider toys as culprits in children with contact allergies
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
A variety of according to the results of a review of 25 published articles.
“In recent years the products have become a reflection of the compounds used frequently in manufacturing, including metals and plastic compounds,” wrote Justine Fenner, MD, and coauthors, from the departments of dermatology and pediatrics at the Icahn School of Medicine at Mount Sinai, New York,
In a study published in Contact Dermatitis, the researchers identified 25 articles describing dermatitis, rash, or eczema associated with a range of toy and play product terms including Nintendo, PlayStation, putty, glue, doll, game, car, bicycle, slime, iPad, and iPhone.
Overall, nickel was the most common allergen. Cases of nickel dermatitis were associated with laptops, videogame controllers, iPads, and cell phones. Cell phones were the most common electronics associated with contact dermatitis, which was observed on the cheek, periauricular area, and hand, as well as the breast in one case of a patient who kept her phone in her bra.
Other sources of metal allergens were identified in toy cars and costume jewelry, the researchers noted.
In addition, temporary tattoos have been associated with contact dermatitis in children, as have homemade “slime” products, which often contain not only borax or other household detergents, but also glue, shaving cream, or coloring.
However, identification of true allergic contact dermatitis from toys “requires both identification of the chemical contents of toys, which are proprietary in nature, and then epicutaneous allergy testing of these ingredients,” the researchers said.
The study findings were limited by several factors including the consideration only of English-language articles and of cases in children, which thus eliminates other potential cases, the researchers noted. However, the results suggest that dermatologists consider toys as a source of contact dermatitis in children, especially if the time to diagnosis is months to years, they said. “Additionally, it may be useful, as it was in several of the above cases, to have the patient bring in his or her favorite toys for the dermatologist to examine and help further understand the etiology of patient’s rash,” they noted. Moreover, “there is an unmet need for corporations to reveal the chemical ingredients of their toys when allergic contact dermatitis is suspected in order to properly evaluate the patient,” they added.
“Contact dermatitis has been underreported in children and constitutes an ongoing concern,” senior author Nanette Silverberg, MD, chief of pediatric dermatology for the Mount Sinai Health System, said in an interview.
“In particular, toy-related allergy is concerning due to the rise in allergen inclusion in common play items,” she commented. The current analysis identified many case reports of allergens that pediatric dermatologists are frequently seeing in their offices, notably metals such as nickel, she pointed out. “The allergen that always stands out ahead of others is nickel,” Dr. Silverberg said. “Nickel allergy affects about 25% of Americans, often starting in early childhood,” she said. “In the European Union, legislation has been passed to reduce nickel release from metals, which has resulted in less sensitization to nickel. We lack such legislation in the United States,” she added.
Other trending allergens include methylchloroisothiazolinone/methylisothiazolinone, which may be components of glue or other ingredients in some “slime” products, Dr. Silverberg said.
She advised clinicians to consider patch testing when addressing localized or persistent dermatitis in children. “Furthermore, consider toys as potential relevant allergens that should be modified in order to achieve skin improvement,” she said.
“Greater reporting of pediatric allergic contact dermatitis is needed,” Dr. Silverberg emphasized. “Additionally, surveillance and monitoring for trends in allergen exposures in toys and personal care items is required to analyze this ongoing concern of childhood,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Fenner J et al. Contact Dermatitis. 2020 Feb 22. doi: 10.1111/cod.13500.
FROM CONTACT DERMATITIS
Isotretinoin data provide postmeal absorption guidance
LAHAINA, HAWAII – Recent , Hilary E. Baldwin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
It is recommended that isotretinoin, which is fat-soluble, be taken with food, preferably high-fat foods. So it has been unclear what the effect would be when taken with lower-fat food, such as low-fat cereal and raspberries, for example, Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York, pointed out.
“We’ve been trying for years to figure out how we’re going to get around this,” and there have not been any relevant data available until recently, other than in the setting of taking isotretinoin on an empty stomach or with a high-fat meal, she commented.
She referred to a open-label, single-dose, randomized crossover study that compared the bioavailability of the lidose formulation of isotretinoin (Absorica) and brand name Accutane, at a dose of 40 mg either on top of a fatty meal (the Food and Drug Administration-stipulated high-fat, high-calorie diet) or after a 10-hour fast; 60 patients did all four arms, with a 21-day washout period between them (J Am Acad Dermatol. 2013 Nov;69[5]:762-7).
In the fed state, both isotretinoin formulations were absorbed to the same extent, “but in the fasting state, there was a considerable difference,” Dr. Baldwin said. Absorption of both dropped in the fasting state, but the drop was more extreme with Accutane, “about a 50% difference between the two, in terms of how much drug was getting into the system,” she noted.
That is important because weight-based dosing is considered with isotretinoin, so at the end of treatment, a patient who has been taking it on an empty stomach may be getting a 60% lower dose than prescribed, “which could lead to a lessening of the effectiveness of the drug and also an increase in relapse over time.”
But how would a low-fat meal, like low-fat cereal and raspberries, affect the absorption, and ultimate efficacy?
This question was addressed in an open-label, single-arm study of 163 patients with acne, who were taking the lidose isotretinoin formulation without food, at the standard dose, for no longer than 20 weeks. Whether they relapsed was evaluated in a 2-year observational phase of the study, Dr. Baldwin said.
At the end of the trial, the drug was considered effective, with improvements in IGA (the 5-point Investigator’s Global Assessment scale). But the change from baseline was maintained at the 2-year posttreatment period, so the benefits of treatment lasted, which indicates that patients can take it “on top of absolutely no food whatsoever ... so if they eat anything, we are headed in the right direction,” including a low-fat meal. During the 2-year period, most patients did not need to be retreated. Of those people who needed treatment, only 4.2% needed treatment with isotretinoin, which is better than the historical relapse rates with isotretinoin, she noted.
Dr. Baldwin’s disclosures included being on the speakers’ bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent , Hilary E. Baldwin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
It is recommended that isotretinoin, which is fat-soluble, be taken with food, preferably high-fat foods. So it has been unclear what the effect would be when taken with lower-fat food, such as low-fat cereal and raspberries, for example, Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York, pointed out.
“We’ve been trying for years to figure out how we’re going to get around this,” and there have not been any relevant data available until recently, other than in the setting of taking isotretinoin on an empty stomach or with a high-fat meal, she commented.
She referred to a open-label, single-dose, randomized crossover study that compared the bioavailability of the lidose formulation of isotretinoin (Absorica) and brand name Accutane, at a dose of 40 mg either on top of a fatty meal (the Food and Drug Administration-stipulated high-fat, high-calorie diet) or after a 10-hour fast; 60 patients did all four arms, with a 21-day washout period between them (J Am Acad Dermatol. 2013 Nov;69[5]:762-7).
In the fed state, both isotretinoin formulations were absorbed to the same extent, “but in the fasting state, there was a considerable difference,” Dr. Baldwin said. Absorption of both dropped in the fasting state, but the drop was more extreme with Accutane, “about a 50% difference between the two, in terms of how much drug was getting into the system,” she noted.
That is important because weight-based dosing is considered with isotretinoin, so at the end of treatment, a patient who has been taking it on an empty stomach may be getting a 60% lower dose than prescribed, “which could lead to a lessening of the effectiveness of the drug and also an increase in relapse over time.”
But how would a low-fat meal, like low-fat cereal and raspberries, affect the absorption, and ultimate efficacy?
This question was addressed in an open-label, single-arm study of 163 patients with acne, who were taking the lidose isotretinoin formulation without food, at the standard dose, for no longer than 20 weeks. Whether they relapsed was evaluated in a 2-year observational phase of the study, Dr. Baldwin said.
At the end of the trial, the drug was considered effective, with improvements in IGA (the 5-point Investigator’s Global Assessment scale). But the change from baseline was maintained at the 2-year posttreatment period, so the benefits of treatment lasted, which indicates that patients can take it “on top of absolutely no food whatsoever ... so if they eat anything, we are headed in the right direction,” including a low-fat meal. During the 2-year period, most patients did not need to be retreated. Of those people who needed treatment, only 4.2% needed treatment with isotretinoin, which is better than the historical relapse rates with isotretinoin, she noted.
Dr. Baldwin’s disclosures included being on the speakers’ bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent , Hilary E. Baldwin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
It is recommended that isotretinoin, which is fat-soluble, be taken with food, preferably high-fat foods. So it has been unclear what the effect would be when taken with lower-fat food, such as low-fat cereal and raspberries, for example, Dr. Baldwin, medical director of the Acne Treatment and Research Center in New York, pointed out.
“We’ve been trying for years to figure out how we’re going to get around this,” and there have not been any relevant data available until recently, other than in the setting of taking isotretinoin on an empty stomach or with a high-fat meal, she commented.
She referred to a open-label, single-dose, randomized crossover study that compared the bioavailability of the lidose formulation of isotretinoin (Absorica) and brand name Accutane, at a dose of 40 mg either on top of a fatty meal (the Food and Drug Administration-stipulated high-fat, high-calorie diet) or after a 10-hour fast; 60 patients did all four arms, with a 21-day washout period between them (J Am Acad Dermatol. 2013 Nov;69[5]:762-7).
In the fed state, both isotretinoin formulations were absorbed to the same extent, “but in the fasting state, there was a considerable difference,” Dr. Baldwin said. Absorption of both dropped in the fasting state, but the drop was more extreme with Accutane, “about a 50% difference between the two, in terms of how much drug was getting into the system,” she noted.
That is important because weight-based dosing is considered with isotretinoin, so at the end of treatment, a patient who has been taking it on an empty stomach may be getting a 60% lower dose than prescribed, “which could lead to a lessening of the effectiveness of the drug and also an increase in relapse over time.”
But how would a low-fat meal, like low-fat cereal and raspberries, affect the absorption, and ultimate efficacy?
This question was addressed in an open-label, single-arm study of 163 patients with acne, who were taking the lidose isotretinoin formulation without food, at the standard dose, for no longer than 20 weeks. Whether they relapsed was evaluated in a 2-year observational phase of the study, Dr. Baldwin said.
At the end of the trial, the drug was considered effective, with improvements in IGA (the 5-point Investigator’s Global Assessment scale). But the change from baseline was maintained at the 2-year posttreatment period, so the benefits of treatment lasted, which indicates that patients can take it “on top of absolutely no food whatsoever ... so if they eat anything, we are headed in the right direction,” including a low-fat meal. During the 2-year period, most patients did not need to be retreated. Of those people who needed treatment, only 4.2% needed treatment with isotretinoin, which is better than the historical relapse rates with isotretinoin, she noted.
Dr. Baldwin’s disclosures included being on the speakers’ bureau, serving as an advisor, and/or an investigator for companies that include Almirall, BioPharmx, Foamix, Galderma, Ortho Dermatologics, Sun Pharmaceuticals, Johnson & Johnson, and La Roche–Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR