User login
Keep your eye on tapinarof, a topical antipsoriatic therapy
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Tapinarof is an investigational drug whose novel mechanism of action – and encouraging performance in phase 2 studies – are making waves for the topical treatment of both psoriasis and atopic dermatitis, Linda F. Stein Gold, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Tapinarof is a first-in-class agonist of the aryl hydrocarbon receptor.
“An aryl hydrocarbon receptor agonist – what in the world does that mean? It means that this drug actually acts at the receptor level inside the cell, and it does a lot of different things,” explained Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
For one, tapinarof down-regulates Th17 cytokines, an attribute that positions the drug very well as a potential topical treatment for psoriasis. But in addition, the drug has a skin barrier repair element through up-regulation of the filaggrin and involucrin genes in keratinocytes, and it also down-regulates Th2 cytokines, actions desirable in a treatment for atopic dermatitis.
Dr. Stein Gold focused mainly on tapinarof’s potential as a novel treatment for psoriasis, a disease that hasn’t seen approval of a new nonsteroidal topical therapy in decades. There is a huge unmet need for safe and effective new topical therapies for this disease; despite all the attention devoted to biologics and other systemic therapies, the great majority of psoriasis patients are managed via topical therapy only.
The definitive trial was initiated based upon the results of a phase 2b, double-blind, six-arm study including 141 adults with body surface involvement of 1%-15% and a baseline Physician Global Assessment (PGA) score of 2 or more who were assigned to tapinarof at 0.5% or 1% once or twice daily or placebo. The phase 2b results, she commented, were very encouraging.
“When we look at the clinical efficacy, it looks like this drug has legs. It does work even as monotherapy to get patients clear,” she said.
The phase 2b, dose-finding study showed dose-dependent treatment efficacy. At week 12, the proportion of participants with a PGA of 0-1 and at least a 2-grade improvement – that is, clear or almost clear – was 36% with tapinarof monotherapy at 0.5% once daily, 46% with 0.5% twice daily, 56% with 1% once daily, and 65% with 1% twice daily, compared with 5% in controls on once-daily application of vehicle and 11% with twice-daily vehicle. Moreover, the improvement was maintained for 4 weeks post treatment. The drug was well tolerated other than some mild to moderate folliculitis and contact dermatitis (J Am Acad Dermatol. 2019 Mar;80[3]:714-21).
“With such small numbers in phase 2, we don’t necessarily need to see statistical significance, but we want to see a trend in the right direction. But every one of the active treatment arms was statistically significantly better than with vehicle. And at higher concentrations, greater efficacy,” noted Dr. Stein Gold.
A phase 2 study of tapinarof cream has also been completed in adults and adolescents with atopic dermatitis, again with positive results. A phase 3 study in atopic dermatitis is still in the planning stages.
Dr. Stein Gold wasn’t involved in the tapinarof psoriasis phase 2b study, sponsored by GlaxoSmithKline. She reported research funding from nine other pharmaceutical companies and serves as a consultant and/or scientific to more than a dozen companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Studies add clarity to link between rosacea and Demodex, coffee
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
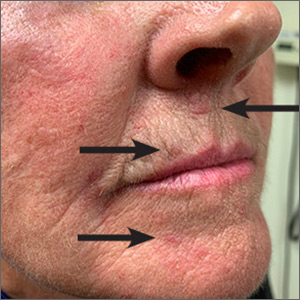
Red lesions on face

These small (1-3 mm) grouped and solitary erythematous papules distributed around the mouth and nares were classic presentations of perioral dermatitis. Although perioral dermatitis typically affects the skin around the mouth, a newer term—periorificial dermatitis—is used because the eruption can, as seen in this patient, involve the skin around the mouth, nares, and/or eyes. Pustules also may occur. There also is a granulomatous form of periorificial dermatitis that occurs in children.
Periorificial dermatitis more closely resembles a rosacea-like eruption than a true dermatitis. Patients often report that the affected areas burn or sting, although occasionally they may be pruritic. Like rosacea, the pathogenesis of periorificial dermatitis is not completely understood. A major risk factor for the development of this condition is the use of topical corticosteroids—especially high-potency products—on the face. Therefore, the most important step in treating periorificial dermatitis is the discontinuation of topical corticosteroids (if they were being used).
In adults, oral tetracycline antibiotics are the drug of choice. As in rosacea, oral antibiotics are used not for their antimicrobial effect, but for their anti-inflammatory effect. For this reason, subantimicrobial dosing of doxycycline has become increasingly common. This reduces the likelihood of antibiotic-related adverse effects and bacterial resistance. In children or adults with a contraindication to tetracyclines, erythromycin is often used. Topical alternatives include erythromycin, metronidazole, and pimecrolimus.
In this case, the patient was advised to discontinue the topical corticosteroid and was started on subantimicrobial dosing of delayed-release doxycycline 40 mg. If the delayed-release form is not available, or is prohibitively expensive, doxycycline 20 mg bid may be used. This patient was told that it could take several weeks for the condition to improve, and that tapering the medication might help reduce recurrence, which is common.
Image courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of D. Alexander Phillips, MD, and Daniel Stulberg, MD, FAAFP Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Wollina U. Subantimicrobial-dose doxycycline monohydrate in dermatology. Wien Med Wochenschr. 2015;165:499-503.

These small (1-3 mm) grouped and solitary erythematous papules distributed around the mouth and nares were classic presentations of perioral dermatitis. Although perioral dermatitis typically affects the skin around the mouth, a newer term—periorificial dermatitis—is used because the eruption can, as seen in this patient, involve the skin around the mouth, nares, and/or eyes. Pustules also may occur. There also is a granulomatous form of periorificial dermatitis that occurs in children.
Periorificial dermatitis more closely resembles a rosacea-like eruption than a true dermatitis. Patients often report that the affected areas burn or sting, although occasionally they may be pruritic. Like rosacea, the pathogenesis of periorificial dermatitis is not completely understood. A major risk factor for the development of this condition is the use of topical corticosteroids—especially high-potency products—on the face. Therefore, the most important step in treating periorificial dermatitis is the discontinuation of topical corticosteroids (if they were being used).
In adults, oral tetracycline antibiotics are the drug of choice. As in rosacea, oral antibiotics are used not for their antimicrobial effect, but for their anti-inflammatory effect. For this reason, subantimicrobial dosing of doxycycline has become increasingly common. This reduces the likelihood of antibiotic-related adverse effects and bacterial resistance. In children or adults with a contraindication to tetracyclines, erythromycin is often used. Topical alternatives include erythromycin, metronidazole, and pimecrolimus.
In this case, the patient was advised to discontinue the topical corticosteroid and was started on subantimicrobial dosing of delayed-release doxycycline 40 mg. If the delayed-release form is not available, or is prohibitively expensive, doxycycline 20 mg bid may be used. This patient was told that it could take several weeks for the condition to improve, and that tapering the medication might help reduce recurrence, which is common.
Image courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of D. Alexander Phillips, MD, and Daniel Stulberg, MD, FAAFP Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

These small (1-3 mm) grouped and solitary erythematous papules distributed around the mouth and nares were classic presentations of perioral dermatitis. Although perioral dermatitis typically affects the skin around the mouth, a newer term—periorificial dermatitis—is used because the eruption can, as seen in this patient, involve the skin around the mouth, nares, and/or eyes. Pustules also may occur. There also is a granulomatous form of periorificial dermatitis that occurs in children.
Periorificial dermatitis more closely resembles a rosacea-like eruption than a true dermatitis. Patients often report that the affected areas burn or sting, although occasionally they may be pruritic. Like rosacea, the pathogenesis of periorificial dermatitis is not completely understood. A major risk factor for the development of this condition is the use of topical corticosteroids—especially high-potency products—on the face. Therefore, the most important step in treating periorificial dermatitis is the discontinuation of topical corticosteroids (if they were being used).
In adults, oral tetracycline antibiotics are the drug of choice. As in rosacea, oral antibiotics are used not for their antimicrobial effect, but for their anti-inflammatory effect. For this reason, subantimicrobial dosing of doxycycline has become increasingly common. This reduces the likelihood of antibiotic-related adverse effects and bacterial resistance. In children or adults with a contraindication to tetracyclines, erythromycin is often used. Topical alternatives include erythromycin, metronidazole, and pimecrolimus.
In this case, the patient was advised to discontinue the topical corticosteroid and was started on subantimicrobial dosing of delayed-release doxycycline 40 mg. If the delayed-release form is not available, or is prohibitively expensive, doxycycline 20 mg bid may be used. This patient was told that it could take several weeks for the condition to improve, and that tapering the medication might help reduce recurrence, which is common.
Image courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of D. Alexander Phillips, MD, and Daniel Stulberg, MD, FAAFP Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Wollina U. Subantimicrobial-dose doxycycline monohydrate in dermatology. Wien Med Wochenschr. 2015;165:499-503.
Wollina U. Subantimicrobial-dose doxycycline monohydrate in dermatology. Wien Med Wochenschr. 2015;165:499-503.
Clinical management guidelines for hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that affects hair follicles, with predilection for intertriginous sites. The prevalence of HS ranges from 0.1% to 2%, with HS significantly affecting the quality of life for patients, with both physical and emotional consequences.
Guidelines from the U.S. and Canadian Hidradenitis Suppurativa Foundations provide a summary of management and treatment for patients.
Grading
Hurley staging is recommended to determine therapies. Stage I is classified by recurrent nodules and abscesses with minimal scars. Stage II is classified by one or a limited number of sinuses and/or scarring within a body region. Stage III is classified by multiple or extensive sinuses and/or scarring. The Dermatology Life Quality Index and pain visual analog scale scores can be used in addition to the Hurley staging for management.
Diagnostic testing/comorbidities screening
There is limited evidence for microbiological testing for HS because skin flora is the main bacteria cultured. Patients should be screened for smoking use, diabetes, metabolic syndrome, depression/anxiety, follicular occlusion tetrad, and squamous cell carcinoma. Some studies have suggested an association between the severity of HS and smoking; therefore, smoking cessation is recommended. Patients should also be counseled on weight loss.
Zinc supplementation (90 mg daily) may be helpful. However, there is insufficient evidence for recommendations to avoid diary, brewer’s yeast, friction, deodorant, depilation, or shaving. There is also insufficient data to support vitamin D supplementation.
Topical/intralesional therapies
Expert opinion supports the use of chlorhexidine, benzoyl peroxide, or zinc pyrithione. A keratolytic and antiseptic cream such as resorcinol 15% cream may be used but can cause contact dermatitis. Topical clindamycin may decrease pustules formation, but it can increase resistance to Staphylococcus aureus. Triamcinolone intravlesional injections may decrease inflamed HS lesions in the short term.
Systemic antibiotics
Systemic antibiotics have been used for decades to treat HS. Tetracyclines for a 12-week course or long-term maintenance can be used in mild to moderate HS. Clindamycin and rifampin combination can be used as second-line therapy for mild to moderate HS. Moxifloxacin, metronidazole, and rifampin combination can also be considered second-line treatment for moderate to severe disease. Dapsone can be used in patients with Hurley stage I or II for maintenance therapy. Ertapenem IV can be used as a rescue or as bridge therapy for severe disease.
The duration of antibiotics and frequency of use depends on each patient and resistance.
Hormonal agents and retinoids
Although androgens may influence HS, evidence for hormonal agents is limited. Hormonal agents, such as ethinyl estradiol and spironolactone, can be considered for females with mild to moderate HS. Retinoids may be considered as a second- or third-line agent, especially in patients with severe acne and HS.
Immunosuppressants and biologics
Immunosuppressants such as methotrexate and azathioprine provide limited benefit; therefore, they are not recommended. Colchicine with minocycline may provide slight benefit in refractory mild to moderate HS. Cyclosporine may be considered in recalcitrant, severe HS. Systemic corticosteroids can be used short term for acute flares or long term for severe HS.
Biologic therapy is becoming more common and the choice of therapy for moderate to severe HS. Adalimumab is currently the only Food and Drug Administration–approved tumor necrosis factor–inhibitor treatment for HS. Other biologics – including infliximab, anakinra, and ustekinumab – may be effective for HS, but optimal dosing needs to be determined.
Pain management
While there are no studies about pain in HS, acute pain management should include topical analgesics and oral nonsteroidal anti-inflammatory drugs. Anticonvulsants such as pregabalin or gabapentin may help with neuropathic pain, and opioids can be considered if there is no improvement with first-line agents.
Surgical management
Recurrent nodules and tunnels can be deroofed or excised. Acute abscesses may be relieved by incision and drainage. Extensive lesions may require wide local scalpel excision, carbon dioxide laser excision, or electrosurgical excision. Surgery alone does not affect the biology of HS; therefore, surgical interventions should be reserved for disease that is not managed by medical therapy.
The bottom line
HS is a chronic inflammatory condition with complex medical management and surgical treatment options. Hurley staging I-III can be used to grade severity and determine therapy. Management of pain, tobacco cessation, weight loss, and mental health are important aspects of HS. Zinc supplementation (90 mg daily) may be helpful. Experts opinion supports the use of chlorhexidine, benzoyl peroxide or zinc pyrithione.
Acute lesions may be managed with short-term oral or intralesional corticosteroids, as well as deroofing or incision and drainage. Moderate-to-severe HS may be managed with systemic antibiotics or biologics and surgical therapy. Adalimumab is the only FDA-approved biologic for treatment of HS.
Dr. Chuong is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
References
Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations. Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019 Jul;81(1):76-90.
Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication form the United States and Canadian Hidradenitis Suppurativa Foundations. Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that affects hair follicles, with predilection for intertriginous sites. The prevalence of HS ranges from 0.1% to 2%, with HS significantly affecting the quality of life for patients, with both physical and emotional consequences.
Guidelines from the U.S. and Canadian Hidradenitis Suppurativa Foundations provide a summary of management and treatment for patients.
Grading
Hurley staging is recommended to determine therapies. Stage I is classified by recurrent nodules and abscesses with minimal scars. Stage II is classified by one or a limited number of sinuses and/or scarring within a body region. Stage III is classified by multiple or extensive sinuses and/or scarring. The Dermatology Life Quality Index and pain visual analog scale scores can be used in addition to the Hurley staging for management.
Diagnostic testing/comorbidities screening
There is limited evidence for microbiological testing for HS because skin flora is the main bacteria cultured. Patients should be screened for smoking use, diabetes, metabolic syndrome, depression/anxiety, follicular occlusion tetrad, and squamous cell carcinoma. Some studies have suggested an association between the severity of HS and smoking; therefore, smoking cessation is recommended. Patients should also be counseled on weight loss.
Zinc supplementation (90 mg daily) may be helpful. However, there is insufficient evidence for recommendations to avoid diary, brewer’s yeast, friction, deodorant, depilation, or shaving. There is also insufficient data to support vitamin D supplementation.
Topical/intralesional therapies
Expert opinion supports the use of chlorhexidine, benzoyl peroxide, or zinc pyrithione. A keratolytic and antiseptic cream such as resorcinol 15% cream may be used but can cause contact dermatitis. Topical clindamycin may decrease pustules formation, but it can increase resistance to Staphylococcus aureus. Triamcinolone intravlesional injections may decrease inflamed HS lesions in the short term.
Systemic antibiotics
Systemic antibiotics have been used for decades to treat HS. Tetracyclines for a 12-week course or long-term maintenance can be used in mild to moderate HS. Clindamycin and rifampin combination can be used as second-line therapy for mild to moderate HS. Moxifloxacin, metronidazole, and rifampin combination can also be considered second-line treatment for moderate to severe disease. Dapsone can be used in patients with Hurley stage I or II for maintenance therapy. Ertapenem IV can be used as a rescue or as bridge therapy for severe disease.
The duration of antibiotics and frequency of use depends on each patient and resistance.
Hormonal agents and retinoids
Although androgens may influence HS, evidence for hormonal agents is limited. Hormonal agents, such as ethinyl estradiol and spironolactone, can be considered for females with mild to moderate HS. Retinoids may be considered as a second- or third-line agent, especially in patients with severe acne and HS.
Immunosuppressants and biologics
Immunosuppressants such as methotrexate and azathioprine provide limited benefit; therefore, they are not recommended. Colchicine with minocycline may provide slight benefit in refractory mild to moderate HS. Cyclosporine may be considered in recalcitrant, severe HS. Systemic corticosteroids can be used short term for acute flares or long term for severe HS.
Biologic therapy is becoming more common and the choice of therapy for moderate to severe HS. Adalimumab is currently the only Food and Drug Administration–approved tumor necrosis factor–inhibitor treatment for HS. Other biologics – including infliximab, anakinra, and ustekinumab – may be effective for HS, but optimal dosing needs to be determined.
Pain management
While there are no studies about pain in HS, acute pain management should include topical analgesics and oral nonsteroidal anti-inflammatory drugs. Anticonvulsants such as pregabalin or gabapentin may help with neuropathic pain, and opioids can be considered if there is no improvement with first-line agents.
Surgical management
Recurrent nodules and tunnels can be deroofed or excised. Acute abscesses may be relieved by incision and drainage. Extensive lesions may require wide local scalpel excision, carbon dioxide laser excision, or electrosurgical excision. Surgery alone does not affect the biology of HS; therefore, surgical interventions should be reserved for disease that is not managed by medical therapy.
The bottom line
HS is a chronic inflammatory condition with complex medical management and surgical treatment options. Hurley staging I-III can be used to grade severity and determine therapy. Management of pain, tobacco cessation, weight loss, and mental health are important aspects of HS. Zinc supplementation (90 mg daily) may be helpful. Experts opinion supports the use of chlorhexidine, benzoyl peroxide or zinc pyrithione.
Acute lesions may be managed with short-term oral or intralesional corticosteroids, as well as deroofing or incision and drainage. Moderate-to-severe HS may be managed with systemic antibiotics or biologics and surgical therapy. Adalimumab is the only FDA-approved biologic for treatment of HS.
Dr. Chuong is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
References
Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations. Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019 Jul;81(1):76-90.
Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication form the United States and Canadian Hidradenitis Suppurativa Foundations. Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that affects hair follicles, with predilection for intertriginous sites. The prevalence of HS ranges from 0.1% to 2%, with HS significantly affecting the quality of life for patients, with both physical and emotional consequences.
Guidelines from the U.S. and Canadian Hidradenitis Suppurativa Foundations provide a summary of management and treatment for patients.
Grading
Hurley staging is recommended to determine therapies. Stage I is classified by recurrent nodules and abscesses with minimal scars. Stage II is classified by one or a limited number of sinuses and/or scarring within a body region. Stage III is classified by multiple or extensive sinuses and/or scarring. The Dermatology Life Quality Index and pain visual analog scale scores can be used in addition to the Hurley staging for management.
Diagnostic testing/comorbidities screening
There is limited evidence for microbiological testing for HS because skin flora is the main bacteria cultured. Patients should be screened for smoking use, diabetes, metabolic syndrome, depression/anxiety, follicular occlusion tetrad, and squamous cell carcinoma. Some studies have suggested an association between the severity of HS and smoking; therefore, smoking cessation is recommended. Patients should also be counseled on weight loss.
Zinc supplementation (90 mg daily) may be helpful. However, there is insufficient evidence for recommendations to avoid diary, brewer’s yeast, friction, deodorant, depilation, or shaving. There is also insufficient data to support vitamin D supplementation.
Topical/intralesional therapies
Expert opinion supports the use of chlorhexidine, benzoyl peroxide, or zinc pyrithione. A keratolytic and antiseptic cream such as resorcinol 15% cream may be used but can cause contact dermatitis. Topical clindamycin may decrease pustules formation, but it can increase resistance to Staphylococcus aureus. Triamcinolone intravlesional injections may decrease inflamed HS lesions in the short term.
Systemic antibiotics
Systemic antibiotics have been used for decades to treat HS. Tetracyclines for a 12-week course or long-term maintenance can be used in mild to moderate HS. Clindamycin and rifampin combination can be used as second-line therapy for mild to moderate HS. Moxifloxacin, metronidazole, and rifampin combination can also be considered second-line treatment for moderate to severe disease. Dapsone can be used in patients with Hurley stage I or II for maintenance therapy. Ertapenem IV can be used as a rescue or as bridge therapy for severe disease.
The duration of antibiotics and frequency of use depends on each patient and resistance.
Hormonal agents and retinoids
Although androgens may influence HS, evidence for hormonal agents is limited. Hormonal agents, such as ethinyl estradiol and spironolactone, can be considered for females with mild to moderate HS. Retinoids may be considered as a second- or third-line agent, especially in patients with severe acne and HS.
Immunosuppressants and biologics
Immunosuppressants such as methotrexate and azathioprine provide limited benefit; therefore, they are not recommended. Colchicine with minocycline may provide slight benefit in refractory mild to moderate HS. Cyclosporine may be considered in recalcitrant, severe HS. Systemic corticosteroids can be used short term for acute flares or long term for severe HS.
Biologic therapy is becoming more common and the choice of therapy for moderate to severe HS. Adalimumab is currently the only Food and Drug Administration–approved tumor necrosis factor–inhibitor treatment for HS. Other biologics – including infliximab, anakinra, and ustekinumab – may be effective for HS, but optimal dosing needs to be determined.
Pain management
While there are no studies about pain in HS, acute pain management should include topical analgesics and oral nonsteroidal anti-inflammatory drugs. Anticonvulsants such as pregabalin or gabapentin may help with neuropathic pain, and opioids can be considered if there is no improvement with first-line agents.
Surgical management
Recurrent nodules and tunnels can be deroofed or excised. Acute abscesses may be relieved by incision and drainage. Extensive lesions may require wide local scalpel excision, carbon dioxide laser excision, or electrosurgical excision. Surgery alone does not affect the biology of HS; therefore, surgical interventions should be reserved for disease that is not managed by medical therapy.
The bottom line
HS is a chronic inflammatory condition with complex medical management and surgical treatment options. Hurley staging I-III can be used to grade severity and determine therapy. Management of pain, tobacco cessation, weight loss, and mental health are important aspects of HS. Zinc supplementation (90 mg daily) may be helpful. Experts opinion supports the use of chlorhexidine, benzoyl peroxide or zinc pyrithione.
Acute lesions may be managed with short-term oral or intralesional corticosteroids, as well as deroofing or incision and drainage. Moderate-to-severe HS may be managed with systemic antibiotics or biologics and surgical therapy. Adalimumab is the only FDA-approved biologic for treatment of HS.
Dr. Chuong is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
References
Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations. Part I: Diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019 Jul;81(1):76-90.
Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication form the United States and Canadian Hidradenitis Suppurativa Foundations. Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019 Jul;81(1):91-101.
SCC survival remains poor in epidermolysis bullosa
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
REPORTING FROM EB 2020
Prioritize oral health in children with DEB
LONDON – , pediatric dentist Susanne Krämer told attendees at the first EB World Congress.
While it may not be the first thing on the minds of families coming to terms with their children having a chronic and potentially debilitating skin disease, it is important to consider oral health early to ensure healthy dentition and mouth function, both of which will affect the ability to eat and thus nutrition.
When there are a lot of other health issues, “dentistry is not a priority,” Dr. Krämer acknowledged in an interview at the meeting, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Something as simple as brushing teeth can be very distressing for parents of a child with EB, she observed, especially if there is dysphagia and toothpaste may be getting into the airways accidentally.
Oral health was one of the topics that patients with EB and their families said would be good to have some guidance on when they were surveyed by DEBRA International. This led the charity to develop its first clinical practice guideline in 2012. Dr. Krämer was the lead author of the guidelines, which are about to be updated and republished.
The “Oral Health for Patients with Epidermolysis Bullosa – Best Clinical Practice Guidelines” (Int J Paediatr Dent. 2012;22 Suppl 1:1-35) are in the final stages of being revised, said Dr. Krämer, who is head of the department of pediatric dentistry at the University of Chile in Santiago. Although there is not much new evidence since the guidelines were first published, “we do have a lot of new technologies within dentistry that can aid the care of EB,” she said.
An important addition to the upcoming 2020 guidelines is a chapter on the patient-clinician partnership. This was added because “you can have fantastic technologies, but if you don’t have a confident relationship with the family and the patient, you won’t be able to proceed.” Dr. Krämer explained: “Patients with EB are so fragile and so afraid of being hurt that they won’t open their mouth unless there is a confidence with the clinician and they trust [him or her]; once they trust, they [will] open the mouth and you can work.”
Dr. Krämer noted that timing of the first dental appointment will depend on the referral pathway for every country and then every service. In her specialist practice the aim is to see newly diagnosed babies before the age of 3 months. “Lots of people would argue they don’t have teeth, but I need to educate the families on several aspects of oral health from early on.”
Older patients with EB may be more aware of the importance of a healthy mouth from a functional point of view and the need to eat and swallow normally, Dr. Krämer said, adding that the “social aspects of having a healthy smile are very important as well.”
Oral care in EB has come a long way since the 1970s when teeth extraction was recommended as the primary dental treatment option. “If you refer to literature in the 90s, that said we can actually restore the teeth in the patients with EB, and what we are now saying is that we have to prevent oral disease,” Dr. Krämer said.
Can oral disease be prevented completely? Yes, she said, but only in a few patients. “We still have decay in a lot of our patients, but far less than what we have had before. It will depend on the compliance of the family and the patient,” Dr. Krämer noted.
Compliance also is a factor in improving mouth function after surgery, which may be done to prevent the tongue from fusing to the bottom of the mouth and to relieve or prevent microstomia, which limits mouth opening.
“We are doing a lot of surgeries to release the fibrotic scars ... we have done it in both children and adults, but there have been better results in adults, because they are able to comply with the course of exercises” after surgery, Dr. Krämer said.
Results of an as-yet unpublished randomized controlled trial of postoperative mouth exercises demonstrate that patients who did the exercises, which involved using a device to stretch the mouth three times a day for 3 months, saw improvements in mouth opening. Once they stopped doing the exercises, however, these improvements faded. Considering the time spent on dressing changes and other exercises, this is perhaps understandable, she acknowledged.
Prevention, education, continual follow-up, and early referral are key to good oral health, Dr. Krämer emphasized. “If there is patient-clinician partnership confidence, they can have regular checkups with dental cleaning, with a fluoride varnish, different preventive strategies so they do not need to get to the point where they need general anesthesia or extractions.” Extractions still will be done, she added, but more for orthodontic reasons, because the teeth do not fit in the mouth. “That is our ideal world, that is where we want to go.”
LONDON – , pediatric dentist Susanne Krämer told attendees at the first EB World Congress.
While it may not be the first thing on the minds of families coming to terms with their children having a chronic and potentially debilitating skin disease, it is important to consider oral health early to ensure healthy dentition and mouth function, both of which will affect the ability to eat and thus nutrition.
When there are a lot of other health issues, “dentistry is not a priority,” Dr. Krämer acknowledged in an interview at the meeting, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Something as simple as brushing teeth can be very distressing for parents of a child with EB, she observed, especially if there is dysphagia and toothpaste may be getting into the airways accidentally.
Oral health was one of the topics that patients with EB and their families said would be good to have some guidance on when they were surveyed by DEBRA International. This led the charity to develop its first clinical practice guideline in 2012. Dr. Krämer was the lead author of the guidelines, which are about to be updated and republished.
The “Oral Health for Patients with Epidermolysis Bullosa – Best Clinical Practice Guidelines” (Int J Paediatr Dent. 2012;22 Suppl 1:1-35) are in the final stages of being revised, said Dr. Krämer, who is head of the department of pediatric dentistry at the University of Chile in Santiago. Although there is not much new evidence since the guidelines were first published, “we do have a lot of new technologies within dentistry that can aid the care of EB,” she said.
An important addition to the upcoming 2020 guidelines is a chapter on the patient-clinician partnership. This was added because “you can have fantastic technologies, but if you don’t have a confident relationship with the family and the patient, you won’t be able to proceed.” Dr. Krämer explained: “Patients with EB are so fragile and so afraid of being hurt that they won’t open their mouth unless there is a confidence with the clinician and they trust [him or her]; once they trust, they [will] open the mouth and you can work.”
Dr. Krämer noted that timing of the first dental appointment will depend on the referral pathway for every country and then every service. In her specialist practice the aim is to see newly diagnosed babies before the age of 3 months. “Lots of people would argue they don’t have teeth, but I need to educate the families on several aspects of oral health from early on.”
Older patients with EB may be more aware of the importance of a healthy mouth from a functional point of view and the need to eat and swallow normally, Dr. Krämer said, adding that the “social aspects of having a healthy smile are very important as well.”
Oral care in EB has come a long way since the 1970s when teeth extraction was recommended as the primary dental treatment option. “If you refer to literature in the 90s, that said we can actually restore the teeth in the patients with EB, and what we are now saying is that we have to prevent oral disease,” Dr. Krämer said.
Can oral disease be prevented completely? Yes, she said, but only in a few patients. “We still have decay in a lot of our patients, but far less than what we have had before. It will depend on the compliance of the family and the patient,” Dr. Krämer noted.
Compliance also is a factor in improving mouth function after surgery, which may be done to prevent the tongue from fusing to the bottom of the mouth and to relieve or prevent microstomia, which limits mouth opening.
“We are doing a lot of surgeries to release the fibrotic scars ... we have done it in both children and adults, but there have been better results in adults, because they are able to comply with the course of exercises” after surgery, Dr. Krämer said.
Results of an as-yet unpublished randomized controlled trial of postoperative mouth exercises demonstrate that patients who did the exercises, which involved using a device to stretch the mouth three times a day for 3 months, saw improvements in mouth opening. Once they stopped doing the exercises, however, these improvements faded. Considering the time spent on dressing changes and other exercises, this is perhaps understandable, she acknowledged.
Prevention, education, continual follow-up, and early referral are key to good oral health, Dr. Krämer emphasized. “If there is patient-clinician partnership confidence, they can have regular checkups with dental cleaning, with a fluoride varnish, different preventive strategies so they do not need to get to the point where they need general anesthesia or extractions.” Extractions still will be done, she added, but more for orthodontic reasons, because the teeth do not fit in the mouth. “That is our ideal world, that is where we want to go.”
LONDON – , pediatric dentist Susanne Krämer told attendees at the first EB World Congress.
While it may not be the first thing on the minds of families coming to terms with their children having a chronic and potentially debilitating skin disease, it is important to consider oral health early to ensure healthy dentition and mouth function, both of which will affect the ability to eat and thus nutrition.
When there are a lot of other health issues, “dentistry is not a priority,” Dr. Krämer acknowledged in an interview at the meeting, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Something as simple as brushing teeth can be very distressing for parents of a child with EB, she observed, especially if there is dysphagia and toothpaste may be getting into the airways accidentally.
Oral health was one of the topics that patients with EB and their families said would be good to have some guidance on when they were surveyed by DEBRA International. This led the charity to develop its first clinical practice guideline in 2012. Dr. Krämer was the lead author of the guidelines, which are about to be updated and republished.
The “Oral Health for Patients with Epidermolysis Bullosa – Best Clinical Practice Guidelines” (Int J Paediatr Dent. 2012;22 Suppl 1:1-35) are in the final stages of being revised, said Dr. Krämer, who is head of the department of pediatric dentistry at the University of Chile in Santiago. Although there is not much new evidence since the guidelines were first published, “we do have a lot of new technologies within dentistry that can aid the care of EB,” she said.
An important addition to the upcoming 2020 guidelines is a chapter on the patient-clinician partnership. This was added because “you can have fantastic technologies, but if you don’t have a confident relationship with the family and the patient, you won’t be able to proceed.” Dr. Krämer explained: “Patients with EB are so fragile and so afraid of being hurt that they won’t open their mouth unless there is a confidence with the clinician and they trust [him or her]; once they trust, they [will] open the mouth and you can work.”
Dr. Krämer noted that timing of the first dental appointment will depend on the referral pathway for every country and then every service. In her specialist practice the aim is to see newly diagnosed babies before the age of 3 months. “Lots of people would argue they don’t have teeth, but I need to educate the families on several aspects of oral health from early on.”
Older patients with EB may be more aware of the importance of a healthy mouth from a functional point of view and the need to eat and swallow normally, Dr. Krämer said, adding that the “social aspects of having a healthy smile are very important as well.”
Oral care in EB has come a long way since the 1970s when teeth extraction was recommended as the primary dental treatment option. “If you refer to literature in the 90s, that said we can actually restore the teeth in the patients with EB, and what we are now saying is that we have to prevent oral disease,” Dr. Krämer said.
Can oral disease be prevented completely? Yes, she said, but only in a few patients. “We still have decay in a lot of our patients, but far less than what we have had before. It will depend on the compliance of the family and the patient,” Dr. Krämer noted.
Compliance also is a factor in improving mouth function after surgery, which may be done to prevent the tongue from fusing to the bottom of the mouth and to relieve or prevent microstomia, which limits mouth opening.
“We are doing a lot of surgeries to release the fibrotic scars ... we have done it in both children and adults, but there have been better results in adults, because they are able to comply with the course of exercises” after surgery, Dr. Krämer said.
Results of an as-yet unpublished randomized controlled trial of postoperative mouth exercises demonstrate that patients who did the exercises, which involved using a device to stretch the mouth three times a day for 3 months, saw improvements in mouth opening. Once they stopped doing the exercises, however, these improvements faded. Considering the time spent on dressing changes and other exercises, this is perhaps understandable, she acknowledged.
Prevention, education, continual follow-up, and early referral are key to good oral health, Dr. Krämer emphasized. “If there is patient-clinician partnership confidence, they can have regular checkups with dental cleaning, with a fluoride varnish, different preventive strategies so they do not need to get to the point where they need general anesthesia or extractions.” Extractions still will be done, she added, but more for orthodontic reasons, because the teeth do not fit in the mouth. “That is our ideal world, that is where we want to go.”
REPORTING FROM EB 2020
Reassurance on general anesthesia in young kids
LAHAINA, HAWAII – Two recent large, well-conducted, and persuasive Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“These two studies can be cited in conversation with parents and are very reassuring for a single episode of general anesthesia,” observed Dr. Sprague, a dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
“As a take home, I think we can feel pretty confident that single exposure to short-duration general anesthesia does not have any adverse neurocognitive effects,” she added.
In 2016, the Food and Drug Administration issued a drug safety communication that general anesthesia lasting for more than 3 hours in children aged less than 3 years, or repeated shorter-duration general anesthesia, may affect the development of children’s brains. This edict caused considerable turmoil among both physicians and parents. The warning was based upon animal studies suggesting adverse effects, including abnormal axon formation and other structural changes, impaired learning and memory, and heightened emotional reactivity to threats. Preliminary human cohort studies generated conflicting results, but were tough to interpret because of potential confounding issues, most prominently the distinct possibility that the very reason the child was undergoing general anesthesia might inherently predispose to neurodevelopmental problems, the dermatologist explained.
Enter the GAS trial, a multinational, assessor-blinded study in which 722 generally healthy infants undergoing hernia repair at 28 centers in the United States and six other countries were randomized to general anesthesia for a median of 54 minutes or awake regional anesthesia. Assessment via a detailed neuropsychological test battery and parent questionnaires at age 2 and 5 years showed no between-group differences at all. Of note, the GAS trial was funded by the FDA, the National Institutes of Health, and similar national health care agencies in the other participating countries (Lancet. 2019 Feb 16;393[10172]:664-77).
The other major recent research contribution was a province-wide Ontario study led by investigators at the Hospital for Sick Children in Toronto. This retrospective study included 2,346 sibling pairs aged 4-5 years in which one child in each pair received general anesthesia as a preschooler. All participants underwent testing using the comprehensive Early Development Instrument. Reassuringly, no between-group differences were found in any of the five domains assessed by the testing: language and cognitive development, physical health and well-being, emotional health and maturity, social knowledge and competence, and communication skills and general knowledge (JAMA Pediatr. 2019 Jan 1;173[1]:29-36).
These two studies address a pressing issue, since 10% of children in the United States and other developed countries receive general anesthesia within their first 3 years of life. Common indications in dermatology include excisional surgery, laser therapy for extensive port wine birthmarks, and diagnostic MRIs.
Dr. Sprague advised that, based upon the new data, “you definitely do not want to delay necessary imaging studies or surgeries, but MRIs can often be done without general anesthesia in infants less than 2 months old. If you have an infant who needs an MRI for something like PHACE syndrome [posterior fossa brain malformations, hemangioma, arterial lesions, cardiac abnormalities, and eye abnormalities], if you can get them in before 2 months of age sometimes you can avoid the general anesthesia if you wrap them tight enough. But once they get over 2 months ,there’s too much wiggle and it’s pretty impossible.”
Her other suggestions:
- Consider delaying nonurgent surgeries and imaging until at least age 6 months and ideally 3 years. “Parents will eventually want surgery to be done for a benign-appearing congenital nevus on the cheek, but it doesn’t necessarily need to be done before 6 months. The same with a residual hemangioma. I would recommend doing it before they go to kindergarten and before they get a sort of sense of what their self looks like, but you have some time between ages 3 and 5 to do that,” Dr. Sprague said.
- Seek out an anesthesiologist who has extensive experience with infants and young children, as is common at a dedicated children’s hospital. “If you live somewhere where the anesthesiologists are primarily seeing adult patients, they’re just not as good,” according to the pediatric dermatologist.
- Definitely consider a topical anesthesia strategy in infants who require multiple procedures, because there remains some unresolved concern about the potential neurodevelopmental impact of multiple bouts of general anesthesia.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Two recent large, well-conducted, and persuasive Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“These two studies can be cited in conversation with parents and are very reassuring for a single episode of general anesthesia,” observed Dr. Sprague, a dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
“As a take home, I think we can feel pretty confident that single exposure to short-duration general anesthesia does not have any adverse neurocognitive effects,” she added.
In 2016, the Food and Drug Administration issued a drug safety communication that general anesthesia lasting for more than 3 hours in children aged less than 3 years, or repeated shorter-duration general anesthesia, may affect the development of children’s brains. This edict caused considerable turmoil among both physicians and parents. The warning was based upon animal studies suggesting adverse effects, including abnormal axon formation and other structural changes, impaired learning and memory, and heightened emotional reactivity to threats. Preliminary human cohort studies generated conflicting results, but were tough to interpret because of potential confounding issues, most prominently the distinct possibility that the very reason the child was undergoing general anesthesia might inherently predispose to neurodevelopmental problems, the dermatologist explained.
Enter the GAS trial, a multinational, assessor-blinded study in which 722 generally healthy infants undergoing hernia repair at 28 centers in the United States and six other countries were randomized to general anesthesia for a median of 54 minutes or awake regional anesthesia. Assessment via a detailed neuropsychological test battery and parent questionnaires at age 2 and 5 years showed no between-group differences at all. Of note, the GAS trial was funded by the FDA, the National Institutes of Health, and similar national health care agencies in the other participating countries (Lancet. 2019 Feb 16;393[10172]:664-77).
The other major recent research contribution was a province-wide Ontario study led by investigators at the Hospital for Sick Children in Toronto. This retrospective study included 2,346 sibling pairs aged 4-5 years in which one child in each pair received general anesthesia as a preschooler. All participants underwent testing using the comprehensive Early Development Instrument. Reassuringly, no between-group differences were found in any of the five domains assessed by the testing: language and cognitive development, physical health and well-being, emotional health and maturity, social knowledge and competence, and communication skills and general knowledge (JAMA Pediatr. 2019 Jan 1;173[1]:29-36).
These two studies address a pressing issue, since 10% of children in the United States and other developed countries receive general anesthesia within their first 3 years of life. Common indications in dermatology include excisional surgery, laser therapy for extensive port wine birthmarks, and diagnostic MRIs.
Dr. Sprague advised that, based upon the new data, “you definitely do not want to delay necessary imaging studies or surgeries, but MRIs can often be done without general anesthesia in infants less than 2 months old. If you have an infant who needs an MRI for something like PHACE syndrome [posterior fossa brain malformations, hemangioma, arterial lesions, cardiac abnormalities, and eye abnormalities], if you can get them in before 2 months of age sometimes you can avoid the general anesthesia if you wrap them tight enough. But once they get over 2 months ,there’s too much wiggle and it’s pretty impossible.”
Her other suggestions:
- Consider delaying nonurgent surgeries and imaging until at least age 6 months and ideally 3 years. “Parents will eventually want surgery to be done for a benign-appearing congenital nevus on the cheek, but it doesn’t necessarily need to be done before 6 months. The same with a residual hemangioma. I would recommend doing it before they go to kindergarten and before they get a sort of sense of what their self looks like, but you have some time between ages 3 and 5 to do that,” Dr. Sprague said.
- Seek out an anesthesiologist who has extensive experience with infants and young children, as is common at a dedicated children’s hospital. “If you live somewhere where the anesthesiologists are primarily seeing adult patients, they’re just not as good,” according to the pediatric dermatologist.
- Definitely consider a topical anesthesia strategy in infants who require multiple procedures, because there remains some unresolved concern about the potential neurodevelopmental impact of multiple bouts of general anesthesia.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Two recent large, well-conducted, and persuasive Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“These two studies can be cited in conversation with parents and are very reassuring for a single episode of general anesthesia,” observed Dr. Sprague, a dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
“As a take home, I think we can feel pretty confident that single exposure to short-duration general anesthesia does not have any adverse neurocognitive effects,” she added.
In 2016, the Food and Drug Administration issued a drug safety communication that general anesthesia lasting for more than 3 hours in children aged less than 3 years, or repeated shorter-duration general anesthesia, may affect the development of children’s brains. This edict caused considerable turmoil among both physicians and parents. The warning was based upon animal studies suggesting adverse effects, including abnormal axon formation and other structural changes, impaired learning and memory, and heightened emotional reactivity to threats. Preliminary human cohort studies generated conflicting results, but were tough to interpret because of potential confounding issues, most prominently the distinct possibility that the very reason the child was undergoing general anesthesia might inherently predispose to neurodevelopmental problems, the dermatologist explained.
Enter the GAS trial, a multinational, assessor-blinded study in which 722 generally healthy infants undergoing hernia repair at 28 centers in the United States and six other countries were randomized to general anesthesia for a median of 54 minutes or awake regional anesthesia. Assessment via a detailed neuropsychological test battery and parent questionnaires at age 2 and 5 years showed no between-group differences at all. Of note, the GAS trial was funded by the FDA, the National Institutes of Health, and similar national health care agencies in the other participating countries (Lancet. 2019 Feb 16;393[10172]:664-77).
The other major recent research contribution was a province-wide Ontario study led by investigators at the Hospital for Sick Children in Toronto. This retrospective study included 2,346 sibling pairs aged 4-5 years in which one child in each pair received general anesthesia as a preschooler. All participants underwent testing using the comprehensive Early Development Instrument. Reassuringly, no between-group differences were found in any of the five domains assessed by the testing: language and cognitive development, physical health and well-being, emotional health and maturity, social knowledge and competence, and communication skills and general knowledge (JAMA Pediatr. 2019 Jan 1;173[1]:29-36).
These two studies address a pressing issue, since 10% of children in the United States and other developed countries receive general anesthesia within their first 3 years of life. Common indications in dermatology include excisional surgery, laser therapy for extensive port wine birthmarks, and diagnostic MRIs.
Dr. Sprague advised that, based upon the new data, “you definitely do not want to delay necessary imaging studies or surgeries, but MRIs can often be done without general anesthesia in infants less than 2 months old. If you have an infant who needs an MRI for something like PHACE syndrome [posterior fossa brain malformations, hemangioma, arterial lesions, cardiac abnormalities, and eye abnormalities], if you can get them in before 2 months of age sometimes you can avoid the general anesthesia if you wrap them tight enough. But once they get over 2 months ,there’s too much wiggle and it’s pretty impossible.”
Her other suggestions:
- Consider delaying nonurgent surgeries and imaging until at least age 6 months and ideally 3 years. “Parents will eventually want surgery to be done for a benign-appearing congenital nevus on the cheek, but it doesn’t necessarily need to be done before 6 months. The same with a residual hemangioma. I would recommend doing it before they go to kindergarten and before they get a sort of sense of what their self looks like, but you have some time between ages 3 and 5 to do that,” Dr. Sprague said.
- Seek out an anesthesiologist who has extensive experience with infants and young children, as is common at a dedicated children’s hospital. “If you live somewhere where the anesthesiologists are primarily seeing adult patients, they’re just not as good,” according to the pediatric dermatologist.
- Definitely consider a topical anesthesia strategy in infants who require multiple procedures, because there remains some unresolved concern about the potential neurodevelopmental impact of multiple bouts of general anesthesia.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Nail dystrophy and nail plate thinning
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
An 8-year-old female child comes to our pediatric dermatology clinic for evaluation of onychomycosis on her fingernails. The mother stated the child started developing funny-looking nails 1 year prior to the visit. It started with only two fingernails affected and now has spread to all her fingernails. Her toenails are not involved.
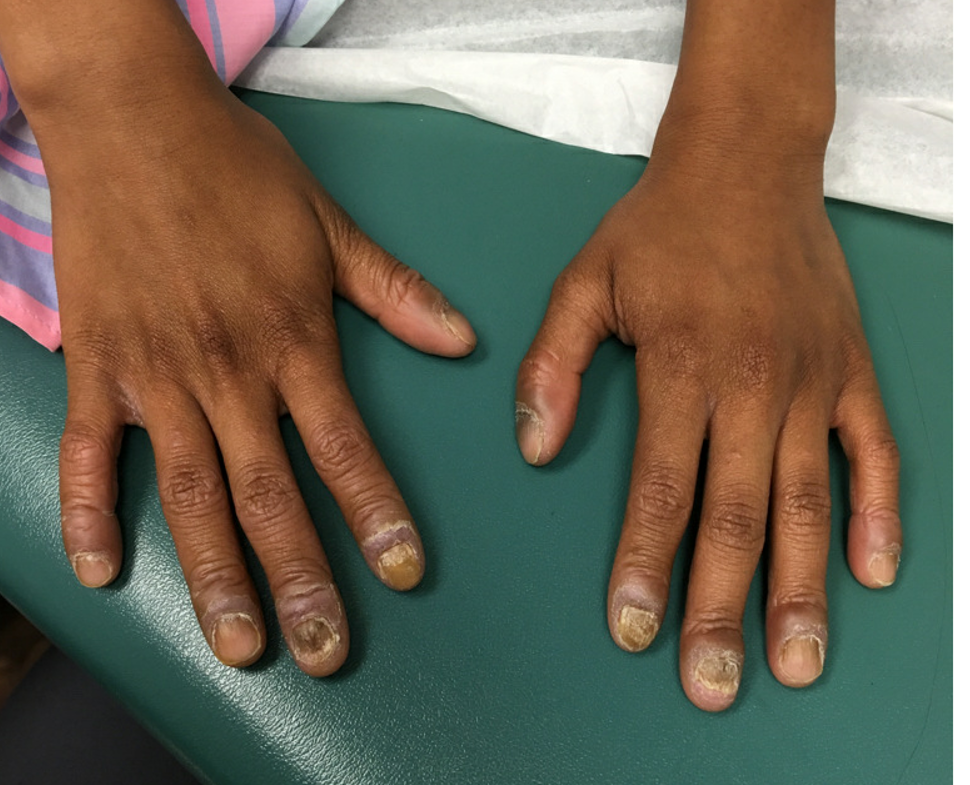
She denied any pain or itching. She initially was treated with topical antifungal medications as well as tea tree oil, apple cider vinegar, and a 6-week course of oral griseofulvin without any improvement. Her nails progressively have gotten much worse. She has no history of atopic dermatitis or any other skin conditions. She denied any joint pain, sun sensitivity, hair loss, or any other symptoms. The mother denied any family history of nail fungus, ringworm, psoriasis, or eczema.
She likes to play basketball and enjoys arts and crafts. She has a cat and a dog; neither of them have any skin problems.
On physical examination, there is nail dystrophy with nail plate thinning and longitudinal fissuring of all fingernails but not of the toenails. She also has hyperpigmented violaceous plaques on the surrounding periungual skin. There are no other skin lesions, and there are no oral or genital lesions. There is no scalp involvement or hair loss. At follow-up several months later, she had complete destruction of the nail plate with scar formation.
A fungal culture was performed, as well as microscopic analysis of the nail with periodic acid fast and giemsa stains, which showed no fungal organisms.
She initially was treated with topical betamethasone twice a day for 6 weeks and then 2 weeks on and 2 weeks off without much change.
Psoriasis elevates cancer risk
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
FROM JAMA DERMATOLOGY
Pruritic rash on trunk
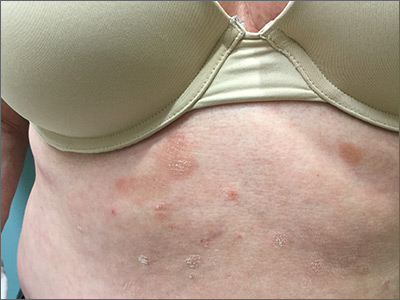
The patient underwent a skin biopsy, which was consistent with mycosis fungoides (MF) a form of cutaneous T cell lymphoma. Common MF complaints are a persistent pruritic rash that slowly progresses in size and shape. Nodules, papules, alopecia, and excoriations are often seen and can become secondarily infected. Sun spared areas are most affected. Itching and erythroderma can be quite intense, especially in Sezary Syndrome (SS)—a rare subtype of cutaneous T cell lymphoma that has a worse prognosis than localized MF.
It is a common for many patients with MF to go undiagnosed or incorrectly diagnosed for years. The differential on initial presentation can include eczema, dermatitis, psoriasis, or a drug reaction. Clues to this patient’s diagnosis were that the rash involved sun-spared areas and didn’t improve with a change to her oral medications or a course of topical steroids. Other clues that pointed to the diagnosis were that she had no history of prior scaling skin disease or psoriasis, her age (usual age of onset for MF is between 50 and 60 years), and the observation that the rash, although it looked like eczema or tinea, presented in atypical and multiple locations.
MF and SS are the most common cutaneous T cell lymphomas. Although these disorders initially involve the skin, later stages can spread to internal organs. Early MF can be difficult to diagnose on histology and can require multiple biopsies. The most accurate biopsy practice involves stopping topical medications for 4 weeks, then taking multiple biopsies of clinically different areas to confirm the diagnosis and clonality (the same cell line in more than one location). SS typically presents with a larger area of involvement and may be associated with lymphadenopathy.
The patient was seen by Dermatology. Due to the extent of the disease, which was suggestive of SS, she underwent peripheral blood flow cytometry, which revealed > 1000 Sezary cells/mcL. This confirmed the diagnosis of SS. The patient was started on photophoresis, interferon, high-potency topical steroids for the local symptoms, and bexarotene, which blocks abnormal cell growth by binding to retinoid receptors.
Photos and text courtesy of John Durkin, MD, Pigmented Lesions Clinic, University of New Mexico, and Kirill Balatsky, MS II, University of New Mexico School of Medicine.
Submitted for publication by Dr. Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Hoppe RT, Kim YH. Clinical manifestations, pathologic features, and diagnosis of mycosis fungoides. UpToDate Web site. https://www.uptodate.com/contents/clinical-manifestations-pathologic-features-and-diagnosis-of-mycosis-fungoides. Updated February 27, 2019. Accessed January 29, 2020.

The patient underwent a skin biopsy, which was consistent with mycosis fungoides (MF) a form of cutaneous T cell lymphoma. Common MF complaints are a persistent pruritic rash that slowly progresses in size and shape. Nodules, papules, alopecia, and excoriations are often seen and can become secondarily infected. Sun spared areas are most affected. Itching and erythroderma can be quite intense, especially in Sezary Syndrome (SS)—a rare subtype of cutaneous T cell lymphoma that has a worse prognosis than localized MF.
It is a common for many patients with MF to go undiagnosed or incorrectly diagnosed for years. The differential on initial presentation can include eczema, dermatitis, psoriasis, or a drug reaction. Clues to this patient’s diagnosis were that the rash involved sun-spared areas and didn’t improve with a change to her oral medications or a course of topical steroids. Other clues that pointed to the diagnosis were that she had no history of prior scaling skin disease or psoriasis, her age (usual age of onset for MF is between 50 and 60 years), and the observation that the rash, although it looked like eczema or tinea, presented in atypical and multiple locations.
MF and SS are the most common cutaneous T cell lymphomas. Although these disorders initially involve the skin, later stages can spread to internal organs. Early MF can be difficult to diagnose on histology and can require multiple biopsies. The most accurate biopsy practice involves stopping topical medications for 4 weeks, then taking multiple biopsies of clinically different areas to confirm the diagnosis and clonality (the same cell line in more than one location). SS typically presents with a larger area of involvement and may be associated with lymphadenopathy.
The patient was seen by Dermatology. Due to the extent of the disease, which was suggestive of SS, she underwent peripheral blood flow cytometry, which revealed > 1000 Sezary cells/mcL. This confirmed the diagnosis of SS. The patient was started on photophoresis, interferon, high-potency topical steroids for the local symptoms, and bexarotene, which blocks abnormal cell growth by binding to retinoid receptors.
Photos and text courtesy of John Durkin, MD, Pigmented Lesions Clinic, University of New Mexico, and Kirill Balatsky, MS II, University of New Mexico School of Medicine.
Submitted for publication by Dr. Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient underwent a skin biopsy, which was consistent with mycosis fungoides (MF) a form of cutaneous T cell lymphoma. Common MF complaints are a persistent pruritic rash that slowly progresses in size and shape. Nodules, papules, alopecia, and excoriations are often seen and can become secondarily infected. Sun spared areas are most affected. Itching and erythroderma can be quite intense, especially in Sezary Syndrome (SS)—a rare subtype of cutaneous T cell lymphoma that has a worse prognosis than localized MF.
It is a common for many patients with MF to go undiagnosed or incorrectly diagnosed for years. The differential on initial presentation can include eczema, dermatitis, psoriasis, or a drug reaction. Clues to this patient’s diagnosis were that the rash involved sun-spared areas and didn’t improve with a change to her oral medications or a course of topical steroids. Other clues that pointed to the diagnosis were that she had no history of prior scaling skin disease or psoriasis, her age (usual age of onset for MF is between 50 and 60 years), and the observation that the rash, although it looked like eczema or tinea, presented in atypical and multiple locations.
MF and SS are the most common cutaneous T cell lymphomas. Although these disorders initially involve the skin, later stages can spread to internal organs. Early MF can be difficult to diagnose on histology and can require multiple biopsies. The most accurate biopsy practice involves stopping topical medications for 4 weeks, then taking multiple biopsies of clinically different areas to confirm the diagnosis and clonality (the same cell line in more than one location). SS typically presents with a larger area of involvement and may be associated with lymphadenopathy.
The patient was seen by Dermatology. Due to the extent of the disease, which was suggestive of SS, she underwent peripheral blood flow cytometry, which revealed > 1000 Sezary cells/mcL. This confirmed the diagnosis of SS. The patient was started on photophoresis, interferon, high-potency topical steroids for the local symptoms, and bexarotene, which blocks abnormal cell growth by binding to retinoid receptors.
Photos and text courtesy of John Durkin, MD, Pigmented Lesions Clinic, University of New Mexico, and Kirill Balatsky, MS II, University of New Mexico School of Medicine.
Submitted for publication by Dr. Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Hoppe RT, Kim YH. Clinical manifestations, pathologic features, and diagnosis of mycosis fungoides. UpToDate Web site. https://www.uptodate.com/contents/clinical-manifestations-pathologic-features-and-diagnosis-of-mycosis-fungoides. Updated February 27, 2019. Accessed January 29, 2020.
Hoppe RT, Kim YH. Clinical manifestations, pathologic features, and diagnosis of mycosis fungoides. UpToDate Web site. https://www.uptodate.com/contents/clinical-manifestations-pathologic-features-and-diagnosis-of-mycosis-fungoides. Updated February 27, 2019. Accessed January 29, 2020.