User login
Don’t Just Look at the Lesion
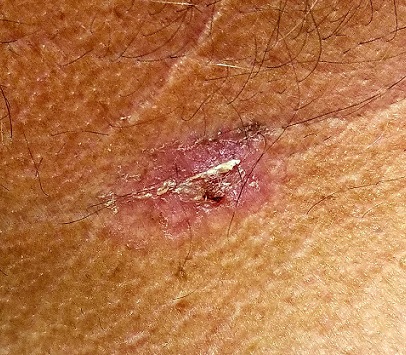
A 52-year-old man self-refers to dermatology for evaluation of an irritated lesion on the posterolateral aspect of his neck. It’s been there for years, waxing and waning but always being irritated by his shirt collar or seat belt.
He has shown the lesion to his primary care provider on several occasions. Various topical preparations, including steroid and antifungal creams, have been prescribed—to no avail.
The patient owns a landscaping business. He has worked outdoors since he was in his teens and acknowledges that in his younger years, he was not careful about sun protection. For the past 20 years or so, however, he has worn a wide-brimmed hat and long-sleeved shirt while working.
His health is good in all other respects.
EXAMINATION
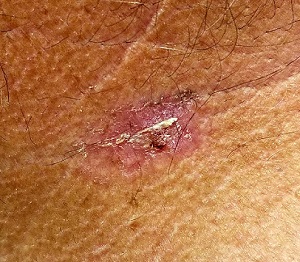
The lesion is a pink, scaly, 2-cm ovoid patch on the left side of the patient’s neck. Several areas of scabbing are seen within the bounds of the lesion, the margins of which are very well defined.
There is much evidence of sun damage on his neck and arms, with prominent pilosebaceous units and rhomboidal lining of the neck skin. Elsewhere, on sun-exposed skin, there are numerous actinic keratoses, telangiectasias, and poikilodermatous changes.
The lesion is biopsied by shave technique and the sample submitted to pathology.
What’s the diagnosis?
DISCUSSION
The biopsy results confirmed the impression of superficial squamous cell carcinoma (SSC) with focal areas of invasion.
One thinks of skin cancer as being “a thing,” a papule or nodule, and that’s usually true. But there are several types of skin cancer that manifest as a fixed rash—meaning it stays in the same place year after year, unlike other rashes (eg, psoriasis, eczema, or fungal infection). Common examples, besides superficial SCC (also known as Bowen disease), include superficial basal cell carcinoma (BCC), Paget disease (mammary and extramammary), cutaneous T-cell lymphoma, and even local recurrence of breast cancer.
This patient’s occupation and history of (abundantly evident) sun damage put him at risk for a sun-caused skin cancer. As so often happens, the problem was initially misdiagnosed by a provider who was only looking at the lesion instead of factoring in the context in which it appeared. Who has the lesion is almost as important as the lesion itself.
Without a biopsy, Bowen disease is often indistinguishable from superficial BCC; the latter can take on a variety of appearances, from scar-like (cicatricial) to pigmented (especially in patients with darker skin) to the most common, nodular/noduloulcerative. Another item in the differential is discoid lupus erythematosus, particularly when the lesion manifests in a highly sun-exposed area.
Treatment of this patient’s lesion entailed full excision with 3-mm margins. This option was chosen based on the focal areas of invasion and theoretical potential for metastasis.
TAKE-HOME LEARNING POINTS
- Intraepidermal squamous cell carcinoma (Bowen disease) presents as a red, scaly rash with a rounded, well-demarcated border, almost always on skin directly overexposed to the sun.
- These cancers grow slowly, are fixed in location, never heal, and—given enough time—often become focally invasive.
- They will often be misdiagnosed as fungal infection, psoriasis, or eczema, because of the misperception that skin cancers are always papular or nodular.
- A history of sun exposure and resulting markers of dermatoheliosis serve to corroborate the putative diagnosis.
A 52-year-old man self-refers to dermatology for evaluation of an irritated lesion on the posterolateral aspect of his neck. It’s been there for years, waxing and waning but always being irritated by his shirt collar or seat belt.
He has shown the lesion to his primary care provider on several occasions. Various topical preparations, including steroid and antifungal creams, have been prescribed—to no avail.
The patient owns a landscaping business. He has worked outdoors since he was in his teens and acknowledges that in his younger years, he was not careful about sun protection. For the past 20 years or so, however, he has worn a wide-brimmed hat and long-sleeved shirt while working.
His health is good in all other respects.

EXAMINATION
The lesion is a pink, scaly, 2-cm ovoid patch on the left side of the patient’s neck. Several areas of scabbing are seen within the bounds of the lesion, the margins of which are very well defined.
There is much evidence of sun damage on his neck and arms, with prominent pilosebaceous units and rhomboidal lining of the neck skin. Elsewhere, on sun-exposed skin, there are numerous actinic keratoses, telangiectasias, and poikilodermatous changes.
The lesion is biopsied by shave technique and the sample submitted to pathology.
What’s the diagnosis?
DISCUSSION
The biopsy results confirmed the impression of superficial squamous cell carcinoma (SSC) with focal areas of invasion.
One thinks of skin cancer as being “a thing,” a papule or nodule, and that’s usually true. But there are several types of skin cancer that manifest as a fixed rash—meaning it stays in the same place year after year, unlike other rashes (eg, psoriasis, eczema, or fungal infection). Common examples, besides superficial SCC (also known as Bowen disease), include superficial basal cell carcinoma (BCC), Paget disease (mammary and extramammary), cutaneous T-cell lymphoma, and even local recurrence of breast cancer.
This patient’s occupation and history of (abundantly evident) sun damage put him at risk for a sun-caused skin cancer. As so often happens, the problem was initially misdiagnosed by a provider who was only looking at the lesion instead of factoring in the context in which it appeared. Who has the lesion is almost as important as the lesion itself.
Without a biopsy, Bowen disease is often indistinguishable from superficial BCC; the latter can take on a variety of appearances, from scar-like (cicatricial) to pigmented (especially in patients with darker skin) to the most common, nodular/noduloulcerative. Another item in the differential is discoid lupus erythematosus, particularly when the lesion manifests in a highly sun-exposed area.
Treatment of this patient’s lesion entailed full excision with 3-mm margins. This option was chosen based on the focal areas of invasion and theoretical potential for metastasis.
TAKE-HOME LEARNING POINTS
- Intraepidermal squamous cell carcinoma (Bowen disease) presents as a red, scaly rash with a rounded, well-demarcated border, almost always on skin directly overexposed to the sun.
- These cancers grow slowly, are fixed in location, never heal, and—given enough time—often become focally invasive.
- They will often be misdiagnosed as fungal infection, psoriasis, or eczema, because of the misperception that skin cancers are always papular or nodular.
- A history of sun exposure and resulting markers of dermatoheliosis serve to corroborate the putative diagnosis.
A 52-year-old man self-refers to dermatology for evaluation of an irritated lesion on the posterolateral aspect of his neck. It’s been there for years, waxing and waning but always being irritated by his shirt collar or seat belt.
He has shown the lesion to his primary care provider on several occasions. Various topical preparations, including steroid and antifungal creams, have been prescribed—to no avail.
The patient owns a landscaping business. He has worked outdoors since he was in his teens and acknowledges that in his younger years, he was not careful about sun protection. For the past 20 years or so, however, he has worn a wide-brimmed hat and long-sleeved shirt while working.
His health is good in all other respects.

EXAMINATION
The lesion is a pink, scaly, 2-cm ovoid patch on the left side of the patient’s neck. Several areas of scabbing are seen within the bounds of the lesion, the margins of which are very well defined.
There is much evidence of sun damage on his neck and arms, with prominent pilosebaceous units and rhomboidal lining of the neck skin. Elsewhere, on sun-exposed skin, there are numerous actinic keratoses, telangiectasias, and poikilodermatous changes.
The lesion is biopsied by shave technique and the sample submitted to pathology.
What’s the diagnosis?
DISCUSSION
The biopsy results confirmed the impression of superficial squamous cell carcinoma (SSC) with focal areas of invasion.
One thinks of skin cancer as being “a thing,” a papule or nodule, and that’s usually true. But there are several types of skin cancer that manifest as a fixed rash—meaning it stays in the same place year after year, unlike other rashes (eg, psoriasis, eczema, or fungal infection). Common examples, besides superficial SCC (also known as Bowen disease), include superficial basal cell carcinoma (BCC), Paget disease (mammary and extramammary), cutaneous T-cell lymphoma, and even local recurrence of breast cancer.
This patient’s occupation and history of (abundantly evident) sun damage put him at risk for a sun-caused skin cancer. As so often happens, the problem was initially misdiagnosed by a provider who was only looking at the lesion instead of factoring in the context in which it appeared. Who has the lesion is almost as important as the lesion itself.
Without a biopsy, Bowen disease is often indistinguishable from superficial BCC; the latter can take on a variety of appearances, from scar-like (cicatricial) to pigmented (especially in patients with darker skin) to the most common, nodular/noduloulcerative. Another item in the differential is discoid lupus erythematosus, particularly when the lesion manifests in a highly sun-exposed area.
Treatment of this patient’s lesion entailed full excision with 3-mm margins. This option was chosen based on the focal areas of invasion and theoretical potential for metastasis.
TAKE-HOME LEARNING POINTS
- Intraepidermal squamous cell carcinoma (Bowen disease) presents as a red, scaly rash with a rounded, well-demarcated border, almost always on skin directly overexposed to the sun.
- These cancers grow slowly, are fixed in location, never heal, and—given enough time—often become focally invasive.
- They will often be misdiagnosed as fungal infection, psoriasis, or eczema, because of the misperception that skin cancers are always papular or nodular.
- A history of sun exposure and resulting markers of dermatoheliosis serve to corroborate the putative diagnosis.
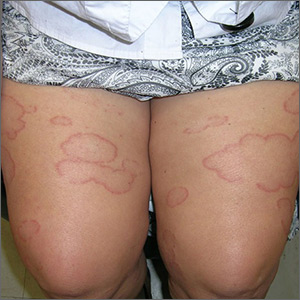
Geometric rash on arms and legs
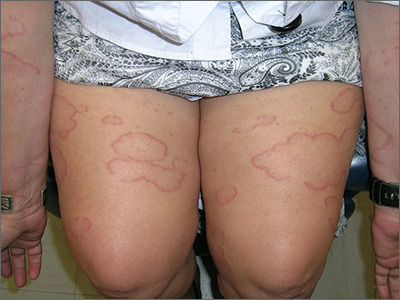
The FP thought this looked like granuloma annulare (GA) but had never seen so many lesions on a patient. Some lesions were not round (more oval or elongated), while others were not completely enclosed geometric shapes (with breaks in the multiple ring patterns). However, all of the lesions were slightly raised with erythema and no scale. (The FP also considered tinea corporis, but ruled it out because there was no scale.)
He consulted some Internet resources and was convinced that this was disseminated GA. The greatest risk factor for this somewhat mysterious disease of unknown origin is female gender. It is also often associated with diabetes, but diabetes is not the cause.
Although intralesional steroids work when treating disseminated GA, this approach is not realistic for widespread disease. A number of options have been studied and reported in small series or case reports. The FP presented various options to the patient, and together they decided to try pentoxifylline 400 mg 3 times daily for systemic treatment. Pentoxifylline is approved for claudication and has few adverse effects or risks. While this treatment is off-label for GA, there are no FDA approved treatments.
During a follow-up visit 1 month later, the number of lesions had been reduced by half. The FP continued treating the patient with pentoxifylline with the hope of achieving full resolution.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP thought this looked like granuloma annulare (GA) but had never seen so many lesions on a patient. Some lesions were not round (more oval or elongated), while others were not completely enclosed geometric shapes (with breaks in the multiple ring patterns). However, all of the lesions were slightly raised with erythema and no scale. (The FP also considered tinea corporis, but ruled it out because there was no scale.)
He consulted some Internet resources and was convinced that this was disseminated GA. The greatest risk factor for this somewhat mysterious disease of unknown origin is female gender. It is also often associated with diabetes, but diabetes is not the cause.
Although intralesional steroids work when treating disseminated GA, this approach is not realistic for widespread disease. A number of options have been studied and reported in small series or case reports. The FP presented various options to the patient, and together they decided to try pentoxifylline 400 mg 3 times daily for systemic treatment. Pentoxifylline is approved for claudication and has few adverse effects or risks. While this treatment is off-label for GA, there are no FDA approved treatments.
During a follow-up visit 1 month later, the number of lesions had been reduced by half. The FP continued treating the patient with pentoxifylline with the hope of achieving full resolution.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP thought this looked like granuloma annulare (GA) but had never seen so many lesions on a patient. Some lesions were not round (more oval or elongated), while others were not completely enclosed geometric shapes (with breaks in the multiple ring patterns). However, all of the lesions were slightly raised with erythema and no scale. (The FP also considered tinea corporis, but ruled it out because there was no scale.)
He consulted some Internet resources and was convinced that this was disseminated GA. The greatest risk factor for this somewhat mysterious disease of unknown origin is female gender. It is also often associated with diabetes, but diabetes is not the cause.
Although intralesional steroids work when treating disseminated GA, this approach is not realistic for widespread disease. A number of options have been studied and reported in small series or case reports. The FP presented various options to the patient, and together they decided to try pentoxifylline 400 mg 3 times daily for systemic treatment. Pentoxifylline is approved for claudication and has few adverse effects or risks. While this treatment is off-label for GA, there are no FDA approved treatments.
During a follow-up visit 1 month later, the number of lesions had been reduced by half. The FP continued treating the patient with pentoxifylline with the hope of achieving full resolution.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Hormone use linked to hair loss in transgender adults
Gender-affirming hormone use was significantly associated with reports of androgenetic alopecia in transgender men, based on data from a survey of 991 individuals.
Given the importance of hair in body image and gender identity, hair concerns are important to the quality of life of gender-minority individuals, wrote Dustin Marks, of Massachusetts General Hospital, Boston, and colleagues.
To explore the impact of hormone use on hair loss in gender-minority patients, the researchers conducted a web-based survey of transgender individuals aged 18 years and older, who self-identified as gender minority. Participants were invited based on profiles on Facebook, YouTube, and Instagram. The findings were published in a research letter in the British Journal of Dermatology.
The 991 survey respondents included 59% transmen, 31% transwomen, and 9% gender nonbinary or gender queer. The average age of the participants was 33 years; 79% were white, 89% had medical insurance, and 91% reported using gender-affirming hormones.
Overall, 65% of transwomen, 43% of transmen, and 35% of nonbinary individuals reported scalp hair loss or thinning. Scalp hair loss was significantly more common among transmen on masculinizing hormones compared to transmen not on hormones (45% vs. 17%). Scalp hair loss was not significantly different between transwomen on feminizing hormones and those not on hormones.
The transwomen who reported scalp hair loss and were on hormones reported significantly less severe Sinclair grades, compared with transwomen with scalp hair loss and were not on hormones. By contrast, transmen and nonbinary individuals on testosterone reported significantly more hair loss (using Hamilton-Norwood and Sinclair scores) between a baseline before hormone use and their present state of hormone use.
The findings support the impact of testosterone use on androgenic alopecia (AGA) in gender-minority patients similar to the established role of testosterone in male pattern hair loss overall, the researchers wrote.
“Some transmen, moreover, may view AGA as a wanted masculine trait, while others seek dermatologic evaluation and treatment for their hair loss,” they noted. By contrast, some transwomen may find AGA especially distressing. In this study, AGA scores were stable for transwomen, which suggests that feminizing hormones may be enough to stabilize hair loss in these patients.
The study was limited by several factors including use of a convenience sample study population without cisgender controls, lack of data on the duration of hormone use, and specific focus on AGE, the researchers noted.
“Mindful of these limitations, clinicians should appreciate the impact of gender-affirming hormones on androgenetic alopecia severity and continue to address the hair concerns of each patient individually,” they wrote.
The researchers had no financial conflicts to disclose, and no sources of study funding were reported.
SOURCE: Marks D et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18099.
Gender-affirming hormone use was significantly associated with reports of androgenetic alopecia in transgender men, based on data from a survey of 991 individuals.
Given the importance of hair in body image and gender identity, hair concerns are important to the quality of life of gender-minority individuals, wrote Dustin Marks, of Massachusetts General Hospital, Boston, and colleagues.
To explore the impact of hormone use on hair loss in gender-minority patients, the researchers conducted a web-based survey of transgender individuals aged 18 years and older, who self-identified as gender minority. Participants were invited based on profiles on Facebook, YouTube, and Instagram. The findings were published in a research letter in the British Journal of Dermatology.
The 991 survey respondents included 59% transmen, 31% transwomen, and 9% gender nonbinary or gender queer. The average age of the participants was 33 years; 79% were white, 89% had medical insurance, and 91% reported using gender-affirming hormones.
Overall, 65% of transwomen, 43% of transmen, and 35% of nonbinary individuals reported scalp hair loss or thinning. Scalp hair loss was significantly more common among transmen on masculinizing hormones compared to transmen not on hormones (45% vs. 17%). Scalp hair loss was not significantly different between transwomen on feminizing hormones and those not on hormones.
The transwomen who reported scalp hair loss and were on hormones reported significantly less severe Sinclair grades, compared with transwomen with scalp hair loss and were not on hormones. By contrast, transmen and nonbinary individuals on testosterone reported significantly more hair loss (using Hamilton-Norwood and Sinclair scores) between a baseline before hormone use and their present state of hormone use.
The findings support the impact of testosterone use on androgenic alopecia (AGA) in gender-minority patients similar to the established role of testosterone in male pattern hair loss overall, the researchers wrote.
“Some transmen, moreover, may view AGA as a wanted masculine trait, while others seek dermatologic evaluation and treatment for their hair loss,” they noted. By contrast, some transwomen may find AGA especially distressing. In this study, AGA scores were stable for transwomen, which suggests that feminizing hormones may be enough to stabilize hair loss in these patients.
The study was limited by several factors including use of a convenience sample study population without cisgender controls, lack of data on the duration of hormone use, and specific focus on AGE, the researchers noted.
“Mindful of these limitations, clinicians should appreciate the impact of gender-affirming hormones on androgenetic alopecia severity and continue to address the hair concerns of each patient individually,” they wrote.
The researchers had no financial conflicts to disclose, and no sources of study funding were reported.
SOURCE: Marks D et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18099.
Gender-affirming hormone use was significantly associated with reports of androgenetic alopecia in transgender men, based on data from a survey of 991 individuals.
Given the importance of hair in body image and gender identity, hair concerns are important to the quality of life of gender-minority individuals, wrote Dustin Marks, of Massachusetts General Hospital, Boston, and colleagues.
To explore the impact of hormone use on hair loss in gender-minority patients, the researchers conducted a web-based survey of transgender individuals aged 18 years and older, who self-identified as gender minority. Participants were invited based on profiles on Facebook, YouTube, and Instagram. The findings were published in a research letter in the British Journal of Dermatology.
The 991 survey respondents included 59% transmen, 31% transwomen, and 9% gender nonbinary or gender queer. The average age of the participants was 33 years; 79% were white, 89% had medical insurance, and 91% reported using gender-affirming hormones.
Overall, 65% of transwomen, 43% of transmen, and 35% of nonbinary individuals reported scalp hair loss or thinning. Scalp hair loss was significantly more common among transmen on masculinizing hormones compared to transmen not on hormones (45% vs. 17%). Scalp hair loss was not significantly different between transwomen on feminizing hormones and those not on hormones.
The transwomen who reported scalp hair loss and were on hormones reported significantly less severe Sinclair grades, compared with transwomen with scalp hair loss and were not on hormones. By contrast, transmen and nonbinary individuals on testosterone reported significantly more hair loss (using Hamilton-Norwood and Sinclair scores) between a baseline before hormone use and their present state of hormone use.
The findings support the impact of testosterone use on androgenic alopecia (AGA) in gender-minority patients similar to the established role of testosterone in male pattern hair loss overall, the researchers wrote.
“Some transmen, moreover, may view AGA as a wanted masculine trait, while others seek dermatologic evaluation and treatment for their hair loss,” they noted. By contrast, some transwomen may find AGA especially distressing. In this study, AGA scores were stable for transwomen, which suggests that feminizing hormones may be enough to stabilize hair loss in these patients.
The study was limited by several factors including use of a convenience sample study population without cisgender controls, lack of data on the duration of hormone use, and specific focus on AGE, the researchers noted.
“Mindful of these limitations, clinicians should appreciate the impact of gender-affirming hormones on androgenetic alopecia severity and continue to address the hair concerns of each patient individually,” they wrote.
The researchers had no financial conflicts to disclose, and no sources of study funding were reported.
SOURCE: Marks D et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18099.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Use of hormone therapy had a significant impact on scalp and hair loss in trans men who used masculinizing hormones.
Major finding: Scalp hair loss or hair thinning was reported by 65% of trans women, 43% of trans men, and 35% of nonbinary individuals.
Study details: The data come from a cross-sectional study including 991 adults self-identifying as gender minorities.
Disclosures: The researchers had no financial conflicts to disclose, and no sources of study funding were reported.
Source: Marks D et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18099.
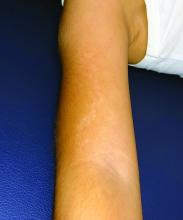
A 5-year-old boy with a papular rash on his arm
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
Atopic dermatitis in adults associated with increased risk of dementia
CHICAGO – Atopic dermatitis in adulthood was associated with a twofold increase in the risk of developing dementia late in life, based on results from a large longitudinal cohort study presented at the annual meeting of the Society for Investigative Dermatology.
“After adjusting for potential mediators such as smoking status, depression, cardiovascular disease, and asthma or rhinitis, the effect was decreased slightly but still remained strongly statistically significant,” reported Katrina Abuabara, MD, of the University of California, San Francisco.
Atopic dermatitis is the latest in growing list of chronic inflammatory conditions that have been associated with an increased risk of dementia, according to Dr. Abuabara, who cited a body of evidence suggesting that inflammation triggers or exacerbates the processes that drive risk of developing dementia late in life.
Interest in the potential association of atopic dermatitis and dementia has been triggered “by a paradigm shift in which we now think of atopic dermatitis as a systemic inflammatory condition.” Dr. Abuabara reported.
In a primary care database of more than 1 million patients, both atopic dermatitis and dementia were common in those aged 60 years or older. The two disorders were identified in 6.75% and 6.49% of patients, respectively.
Cox proportional hazard ratios were employed to determine the relationship between the presence of atopic dermatitis and subsequent development of dementia. The median follow-up was 8 years. Atopic dermatitis was classified as mild, moderate, or severe involvement based on treatment records.
Patients with dementia associated with infectious diseases such as HIV, alcoholism, and other exogenous toxins were excluded from the analysis.
For those with atopic dermatitis relative to those without, the unadjusted hazard ratios were 1.91 for dementia of any type, 2.14 for Alzheimer’s dementia, and 2.25 for vascular-related dementia. After adjustment for confounders such as age, sex, and socioeconomic status, these hazard ratios, respectively, were only somewhat lower and remained statistically significant.
There was a trend for greater dementia risk with greater atopic dermatitis severity, rising from 2.07 in those with mild atopic dermatitis to 2.72 to those with severe disease, according to Dr. Abuabara.
“The important next step is to determine if better control of atopic dermatitis results in a lower risk of dementia,” she said.
According to Dr. Abuabara, some experimental studies have supported the hypothesis that downregulation of systemic markers of inflammation may be protective.
“Even if you reduced risk by a small amount, it would translate into a large health impact because of the large and growing prevalence of dementia,” she said.
Dr. Abuabara is a consultant for the TARGET-DERM study, sponsored by Target PharmaSolutions.
CHICAGO – Atopic dermatitis in adulthood was associated with a twofold increase in the risk of developing dementia late in life, based on results from a large longitudinal cohort study presented at the annual meeting of the Society for Investigative Dermatology.
“After adjusting for potential mediators such as smoking status, depression, cardiovascular disease, and asthma or rhinitis, the effect was decreased slightly but still remained strongly statistically significant,” reported Katrina Abuabara, MD, of the University of California, San Francisco.
Atopic dermatitis is the latest in growing list of chronic inflammatory conditions that have been associated with an increased risk of dementia, according to Dr. Abuabara, who cited a body of evidence suggesting that inflammation triggers or exacerbates the processes that drive risk of developing dementia late in life.
Interest in the potential association of atopic dermatitis and dementia has been triggered “by a paradigm shift in which we now think of atopic dermatitis as a systemic inflammatory condition.” Dr. Abuabara reported.
In a primary care database of more than 1 million patients, both atopic dermatitis and dementia were common in those aged 60 years or older. The two disorders were identified in 6.75% and 6.49% of patients, respectively.
Cox proportional hazard ratios were employed to determine the relationship between the presence of atopic dermatitis and subsequent development of dementia. The median follow-up was 8 years. Atopic dermatitis was classified as mild, moderate, or severe involvement based on treatment records.
Patients with dementia associated with infectious diseases such as HIV, alcoholism, and other exogenous toxins were excluded from the analysis.
For those with atopic dermatitis relative to those without, the unadjusted hazard ratios were 1.91 for dementia of any type, 2.14 for Alzheimer’s dementia, and 2.25 for vascular-related dementia. After adjustment for confounders such as age, sex, and socioeconomic status, these hazard ratios, respectively, were only somewhat lower and remained statistically significant.
There was a trend for greater dementia risk with greater atopic dermatitis severity, rising from 2.07 in those with mild atopic dermatitis to 2.72 to those with severe disease, according to Dr. Abuabara.
“The important next step is to determine if better control of atopic dermatitis results in a lower risk of dementia,” she said.
According to Dr. Abuabara, some experimental studies have supported the hypothesis that downregulation of systemic markers of inflammation may be protective.
“Even if you reduced risk by a small amount, it would translate into a large health impact because of the large and growing prevalence of dementia,” she said.
Dr. Abuabara is a consultant for the TARGET-DERM study, sponsored by Target PharmaSolutions.
CHICAGO – Atopic dermatitis in adulthood was associated with a twofold increase in the risk of developing dementia late in life, based on results from a large longitudinal cohort study presented at the annual meeting of the Society for Investigative Dermatology.
“After adjusting for potential mediators such as smoking status, depression, cardiovascular disease, and asthma or rhinitis, the effect was decreased slightly but still remained strongly statistically significant,” reported Katrina Abuabara, MD, of the University of California, San Francisco.
Atopic dermatitis is the latest in growing list of chronic inflammatory conditions that have been associated with an increased risk of dementia, according to Dr. Abuabara, who cited a body of evidence suggesting that inflammation triggers or exacerbates the processes that drive risk of developing dementia late in life.
Interest in the potential association of atopic dermatitis and dementia has been triggered “by a paradigm shift in which we now think of atopic dermatitis as a systemic inflammatory condition.” Dr. Abuabara reported.
In a primary care database of more than 1 million patients, both atopic dermatitis and dementia were common in those aged 60 years or older. The two disorders were identified in 6.75% and 6.49% of patients, respectively.
Cox proportional hazard ratios were employed to determine the relationship between the presence of atopic dermatitis and subsequent development of dementia. The median follow-up was 8 years. Atopic dermatitis was classified as mild, moderate, or severe involvement based on treatment records.
Patients with dementia associated with infectious diseases such as HIV, alcoholism, and other exogenous toxins were excluded from the analysis.
For those with atopic dermatitis relative to those without, the unadjusted hazard ratios were 1.91 for dementia of any type, 2.14 for Alzheimer’s dementia, and 2.25 for vascular-related dementia. After adjustment for confounders such as age, sex, and socioeconomic status, these hazard ratios, respectively, were only somewhat lower and remained statistically significant.
There was a trend for greater dementia risk with greater atopic dermatitis severity, rising from 2.07 in those with mild atopic dermatitis to 2.72 to those with severe disease, according to Dr. Abuabara.
“The important next step is to determine if better control of atopic dermatitis results in a lower risk of dementia,” she said.
According to Dr. Abuabara, some experimental studies have supported the hypothesis that downregulation of systemic markers of inflammation may be protective.
“Even if you reduced risk by a small amount, it would translate into a large health impact because of the large and growing prevalence of dementia,” she said.
Dr. Abuabara is a consultant for the TARGET-DERM study, sponsored by Target PharmaSolutions.
REPORTING FROM SID 2019
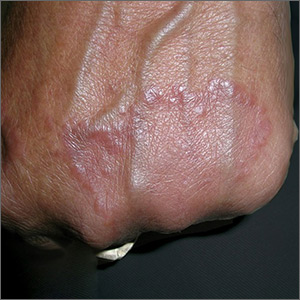
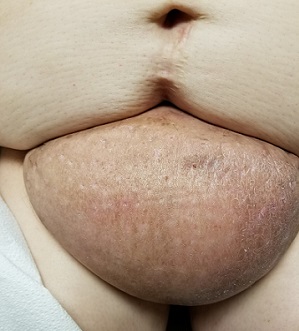
Raised lesion on hand

The FP diagnosed granuloma annulare (GA) in this patient, based on the typical clinical appearance of a raised ring on the back of the hand with no scale.
GA is a common benign cutaneous, inflammatory disorder of unknown origin. It affects twice as many women as men. It features annular lesions that have raised borders and are skin-colored to erythematous. The rings may become hyperpigmented and often feature a central depression. These lesions are typically 1 to 5 cm wide. Although the classical appearance of GA is annular, the rings may not always be complete. Most importantly, there is no scaling, which one would expect to see in tinea infections, also known as “ringworm.”
The most common form of granuloma annulare is localized, as it was in this case. It typically presents on the dorsal surfaces of extremities, especially of the hands and feet. When the presentation is classic, there is no need for a biopsy to confirm the diagnosis.
The most effective treatment for local disease is intralesional triamcinolone (5mg/mL). (See Watch & Learn: Intralesional injections.) The FP offered this treatment to the patient, and she tolerated the procedure well. The GA resolved over the weeks that followed with some faint hypopigmentation that lasted for several months. The patient was happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP diagnosed granuloma annulare (GA) in this patient, based on the typical clinical appearance of a raised ring on the back of the hand with no scale.
GA is a common benign cutaneous, inflammatory disorder of unknown origin. It affects twice as many women as men. It features annular lesions that have raised borders and are skin-colored to erythematous. The rings may become hyperpigmented and often feature a central depression. These lesions are typically 1 to 5 cm wide. Although the classical appearance of GA is annular, the rings may not always be complete. Most importantly, there is no scaling, which one would expect to see in tinea infections, also known as “ringworm.”
The most common form of granuloma annulare is localized, as it was in this case. It typically presents on the dorsal surfaces of extremities, especially of the hands and feet. When the presentation is classic, there is no need for a biopsy to confirm the diagnosis.
The most effective treatment for local disease is intralesional triamcinolone (5mg/mL). (See Watch & Learn: Intralesional injections.) The FP offered this treatment to the patient, and she tolerated the procedure well. The GA resolved over the weeks that followed with some faint hypopigmentation that lasted for several months. The patient was happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP diagnosed granuloma annulare (GA) in this patient, based on the typical clinical appearance of a raised ring on the back of the hand with no scale.
GA is a common benign cutaneous, inflammatory disorder of unknown origin. It affects twice as many women as men. It features annular lesions that have raised borders and are skin-colored to erythematous. The rings may become hyperpigmented and often feature a central depression. These lesions are typically 1 to 5 cm wide. Although the classical appearance of GA is annular, the rings may not always be complete. Most importantly, there is no scaling, which one would expect to see in tinea infections, also known as “ringworm.”
The most common form of granuloma annulare is localized, as it was in this case. It typically presents on the dorsal surfaces of extremities, especially of the hands and feet. When the presentation is classic, there is no need for a biopsy to confirm the diagnosis.
The most effective treatment for local disease is intralesional triamcinolone (5mg/mL). (See Watch & Learn: Intralesional injections.) The FP offered this treatment to the patient, and she tolerated the procedure well. The GA resolved over the weeks that followed with some faint hypopigmentation that lasted for several months. The patient was happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Is it measles? – Diagnosis and management for the pediatric provider
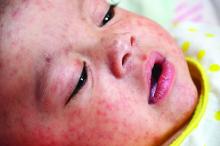
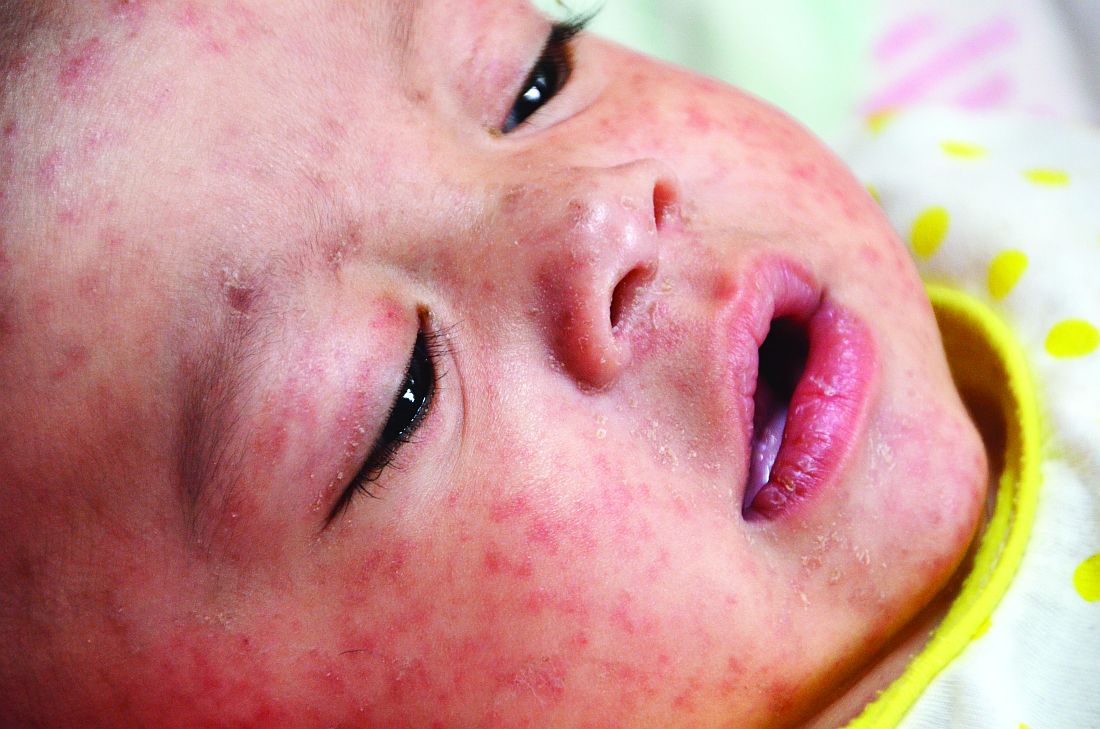
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
Gentamicin restores wound healing in hereditary epidermolysis bullosa
Rare progress seen in challenging disease
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
Rare progress seen in challenging disease
Rare progress seen in challenging disease
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
CHICAGO – Topical gentamicin counters the nonsense mutations that inhibit production of laminin 332 in infants with Herlitz junctional epidermolysis bullosa (H-JEB) to allow lesion healing, according to results of a small clinical study presented at the annual meeting of the Society for Investigative Dermatology.
“All of the children treated so far have responded,” reported Andrew Kwong, who will soon graduate from the Keck School of Medicine at the University of Southern California, Los Angeles.
H-JEB is an inherited blistering skin disease associated with nonsense mutations in the LAMA3, LAMB3, or LAMC2 genes that result in impaired production of functional laminin 332, an essential protein for epidermal-dermal adherence. At this time there are no effective therapies, and the disease is fatal.
The small clinical study was initiated after in vitro studies demonstrated that gentamicin restored functional laminin 332 in cultured keratinocytes from infants with H-JEB. The dose-dependent effect was credited to the ability of gentamicin to induce readthrough of premature stop codons that block production of laminin 332.
Data were presented on the first three infants with H-JEB treated with oral gentamicin. In each child, lesions were treated with topical 0.5% gentamicin twice daily for two weeks. Biopsies were taken prior to the initiation of treatment and at one and three months after treatment. The primary outcome was change in laminin 332, but clinical improvement was also monitored.
Although none of the infants had measurable laminin 332 prior to treatment, all lesions treated with topical gentamicin developed localized laminin 332 at the dermal-epidermal junction of the skin, Mr. Kwong reported. This expression, which was about 40% to 60% of that seen in normal skin, still persisted when evaluated three months after treatment.
The expression was associated with resolution of existing lesions and a reduced risk of developing new lesions, according to Mr. Kwong. In lesions that went untreated, there was no change.
Other molecular changes in the skin, such as increased expression and polarization of beta-4 integrin, were consistent with the ability of gentamicin to address the underlying pathophysiology of H-JEB. There were no adverse events observed.
By restoring functional laminin 332 in the skin, topical gentamicin appears to address the underlying cause of the bullae associated with H-JEB, but Mr. Kwong said that the next step is to determine whether intravenous gentamicin can address the systemic effects. If so, this treatment has the potential to improve survival. He reported that an infant with H-JEB was recently started on intravenous treatment, and initial results were encouraging.
Asked whether he would recommend topical gentamicin on the basis of these findings, Mr. Kwong cautioned that the case series remains very small, but he noted that the uniformity of the positive response is encouraging. He expects that off-label use of this novel and low-cost approach might be warranted in a population that has very limited therapeutic options.
SOURCE: Kwong A. SID 2019;S102, Abstract 594. Annual meeting of the Society for Investigative Dermatology.
REPORTING FROM SID 2019
Intradermal etanercept improves discoid lupus
BIRMINGHAM, ENGLAND – Intradermal delivery of a tumor necrosis factor inhibitor (TNFi) could offer patients with discoid lupus erythematosus (DLE) a much-needed additional treatment option, according to results of a phase 2, “proof-of-concept” study.
Overall, 14 (56%) of the 25 patients in the study achieved a 20% or greater reduction in disease activity from baseline to week 12 via intradermal injection of etanercept (Enbrel), which was assessed via the modified limited Score of Activity and Damage in DLE (ML-SADDLE). About half (48%) and one-fifth (20%) also achieved greater reductions of 50% and 70%, respectively.
“Discoid lupus is a chronic form of cutaneous lupus. Usually it occurs in visible areas like the face and scalp, causing scarring, so it’s really disabling and affects patients’ quality of life,” observed the lead study investigator Md Yuzaiful Md Yusof, MBChB, PhD, NIHR Academic Clinical Lecturer at the University of Leeds, England.
“It’s also one of the most resistant manifestations of lupus,” he said during a poster presentation at the annual conference of the British Society for Rheumatology. “Usually, when people have discoid lupus, the dermatologist gives antimalarial treatment, but only 50% of people respond to these drugs. So, what happens to the rest of them?” Basically, it is trial and error, Dr. Md Yusof said; some patients may be given disease-modifying antirheumatic drugs and in some patients this may work well, but in others there may be toxicity that contraindicates treatment.
B-cell therapy with rituximab (Rituxan) has not been successful, he said. In a previous study of 35 patients with refractory discoid lupus, none of the patients responded to rituximab and half of them actually flared after taking the drug.
There is a pathologic case for using anti-TNF therapy in DLE, but the use of TNFis is not without concern. Such treatment can increase antinuclear antibody production and make lupus worse. “In order to overcome this, as the lesion is quite small, we don’t need to use a systemic approach,” Dr. Md Yusof explained in an interview. “If you give directly, it should just be confined to the lesion and not absorbed, that’s the whole idea of thinking outside the box.” He noted that if it worked, such treatment would be for inducing remission and not for maintenance.
The study, “Targeted therapy using intradermal injection of etanercept for remission induction in discoid lupus erythematosus” (TARGET-DLE) was designed to test the validity of using intradermal rather than subcutaneous TNFi therapy in patients with discoid lupus.
Dr. Md Yusof noted that only 25 patients needed to be recruited into the single-arm, prospective trial as a “Simon’s two-stage minimized design” was used (Control Clin Trials. 1989;10[1]:1-10). This involved treating the first few patients to see if a response occurred and if it did, carrying on with treating the others, but if no response occurred in at least two patients, the trial would stop completely.
Adult patients were eligible for inclusion if they had one or more active DLE lesions and had not responded to antimalarial treatment. Stable doses of DMARDs and up to 10 mg of oral prednisolone daily was permitted if already being taken prior to entering the study.
Etanercept was injected intradermally around the most symptomatic lesion once a week for up to 12 weeks. The dosage was determined based on the radius of the selected discoid lesion. Over an 18-month period, all 25 patients were recruited, including 18 women. The median age of patients was 47 years, and six had systemic lupus erythematosus. The median number of prior DMARDs was 5 but ranged from 1 to 16, indicating a very resistant patient population.
The primary endpoint was at least 6 of the 25 patients having at least a 20% reduction in ML-SADDLE at week 12; 14 (56%) patients achieved this.
“We didn’t use CLASI [Cutaneous Lupus Area and Severity Index Activity Score] because that only includes erythema and atrophy,” Dr. Md Yusof explained. “In discoid lupus, induration is quite important as well, so that’s why we used ML-SADDLE. We called it ‘modified limited’ because the original SADDLE score is based on the whole organ score, but we only calculated the one lesion that we wanted to treat.”
In addition to meeting the primary endpoint, several secondary endpoints were met, including significant improvements in scores on visual analog scales as determined by pre- and posttreatment scoring by physicians (53.1 mm vs. 23.2 mm; P less than .001) and patients (56.9 mm vs. 29.7 mm; P = .001). Mean Dermatology Life Quality Index (DLQI) score significantly improved between pre- and post treatment, as did blood perfusion under the skin based on laser Doppler imaging and infrared thermography. However, no difference was seen with optical coherence tomography.
“There were only four grade 3/4 toxicities, and importantly, none of the SLE patients got worse, and none with DLE only converted into SLE,” Dr. Md Yusof reported. Of the four grade 3/4 adverse events, two were chest infections, one was heart failure, and one was a worsening of chilblains.
“It was a full-powered phase 2 trial, and because it was positive, now we can go to phase 3 trial,” he added.
Before conducting a phase 3 trial, however, Dr. Md Yusof wants to refine how the TNFi is delivered. Perhaps an intradermal patch with microneedles could be used. This would be left on the skin for a short amount of time to allow drug delivery and then removed. It could help ensure that all patients comply with treatment and perhaps even self-administer, he noted.
“The median compliance rate was 80%, which is not too bad, but I think when we come to run a phase 3 trial, I’m looking to improve the drug delivery,” he said. Changing the delivery method will need to be validated before a phase 3 trial can be started.
The study was not commercially funded. Dr. Md Yusof had no disclosures. Pfizer provided the study drug free of charge.
SOURCE: Md Yusof MY et al. Rheumatology. 2019;58(suppl 3): Abstract 244. doi: 10.1093/rheumatology/kez107.060.
BIRMINGHAM, ENGLAND – Intradermal delivery of a tumor necrosis factor inhibitor (TNFi) could offer patients with discoid lupus erythematosus (DLE) a much-needed additional treatment option, according to results of a phase 2, “proof-of-concept” study.
Overall, 14 (56%) of the 25 patients in the study achieved a 20% or greater reduction in disease activity from baseline to week 12 via intradermal injection of etanercept (Enbrel), which was assessed via the modified limited Score of Activity and Damage in DLE (ML-SADDLE). About half (48%) and one-fifth (20%) also achieved greater reductions of 50% and 70%, respectively.
“Discoid lupus is a chronic form of cutaneous lupus. Usually it occurs in visible areas like the face and scalp, causing scarring, so it’s really disabling and affects patients’ quality of life,” observed the lead study investigator Md Yuzaiful Md Yusof, MBChB, PhD, NIHR Academic Clinical Lecturer at the University of Leeds, England.
“It’s also one of the most resistant manifestations of lupus,” he said during a poster presentation at the annual conference of the British Society for Rheumatology. “Usually, when people have discoid lupus, the dermatologist gives antimalarial treatment, but only 50% of people respond to these drugs. So, what happens to the rest of them?” Basically, it is trial and error, Dr. Md Yusof said; some patients may be given disease-modifying antirheumatic drugs and in some patients this may work well, but in others there may be toxicity that contraindicates treatment.
B-cell therapy with rituximab (Rituxan) has not been successful, he said. In a previous study of 35 patients with refractory discoid lupus, none of the patients responded to rituximab and half of them actually flared after taking the drug.
There is a pathologic case for using anti-TNF therapy in DLE, but the use of TNFis is not without concern. Such treatment can increase antinuclear antibody production and make lupus worse. “In order to overcome this, as the lesion is quite small, we don’t need to use a systemic approach,” Dr. Md Yusof explained in an interview. “If you give directly, it should just be confined to the lesion and not absorbed, that’s the whole idea of thinking outside the box.” He noted that if it worked, such treatment would be for inducing remission and not for maintenance.
The study, “Targeted therapy using intradermal injection of etanercept for remission induction in discoid lupus erythematosus” (TARGET-DLE) was designed to test the validity of using intradermal rather than subcutaneous TNFi therapy in patients with discoid lupus.
Dr. Md Yusof noted that only 25 patients needed to be recruited into the single-arm, prospective trial as a “Simon’s two-stage minimized design” was used (Control Clin Trials. 1989;10[1]:1-10). This involved treating the first few patients to see if a response occurred and if it did, carrying on with treating the others, but if no response occurred in at least two patients, the trial would stop completely.
Adult patients were eligible for inclusion if they had one or more active DLE lesions and had not responded to antimalarial treatment. Stable doses of DMARDs and up to 10 mg of oral prednisolone daily was permitted if already being taken prior to entering the study.
Etanercept was injected intradermally around the most symptomatic lesion once a week for up to 12 weeks. The dosage was determined based on the radius of the selected discoid lesion. Over an 18-month period, all 25 patients were recruited, including 18 women. The median age of patients was 47 years, and six had systemic lupus erythematosus. The median number of prior DMARDs was 5 but ranged from 1 to 16, indicating a very resistant patient population.
The primary endpoint was at least 6 of the 25 patients having at least a 20% reduction in ML-SADDLE at week 12; 14 (56%) patients achieved this.
“We didn’t use CLASI [Cutaneous Lupus Area and Severity Index Activity Score] because that only includes erythema and atrophy,” Dr. Md Yusof explained. “In discoid lupus, induration is quite important as well, so that’s why we used ML-SADDLE. We called it ‘modified limited’ because the original SADDLE score is based on the whole organ score, but we only calculated the one lesion that we wanted to treat.”
In addition to meeting the primary endpoint, several secondary endpoints were met, including significant improvements in scores on visual analog scales as determined by pre- and posttreatment scoring by physicians (53.1 mm vs. 23.2 mm; P less than .001) and patients (56.9 mm vs. 29.7 mm; P = .001). Mean Dermatology Life Quality Index (DLQI) score significantly improved between pre- and post treatment, as did blood perfusion under the skin based on laser Doppler imaging and infrared thermography. However, no difference was seen with optical coherence tomography.
“There were only four grade 3/4 toxicities, and importantly, none of the SLE patients got worse, and none with DLE only converted into SLE,” Dr. Md Yusof reported. Of the four grade 3/4 adverse events, two were chest infections, one was heart failure, and one was a worsening of chilblains.
“It was a full-powered phase 2 trial, and because it was positive, now we can go to phase 3 trial,” he added.
Before conducting a phase 3 trial, however, Dr. Md Yusof wants to refine how the TNFi is delivered. Perhaps an intradermal patch with microneedles could be used. This would be left on the skin for a short amount of time to allow drug delivery and then removed. It could help ensure that all patients comply with treatment and perhaps even self-administer, he noted.
“The median compliance rate was 80%, which is not too bad, but I think when we come to run a phase 3 trial, I’m looking to improve the drug delivery,” he said. Changing the delivery method will need to be validated before a phase 3 trial can be started.
The study was not commercially funded. Dr. Md Yusof had no disclosures. Pfizer provided the study drug free of charge.
SOURCE: Md Yusof MY et al. Rheumatology. 2019;58(suppl 3): Abstract 244. doi: 10.1093/rheumatology/kez107.060.
BIRMINGHAM, ENGLAND – Intradermal delivery of a tumor necrosis factor inhibitor (TNFi) could offer patients with discoid lupus erythematosus (DLE) a much-needed additional treatment option, according to results of a phase 2, “proof-of-concept” study.
Overall, 14 (56%) of the 25 patients in the study achieved a 20% or greater reduction in disease activity from baseline to week 12 via intradermal injection of etanercept (Enbrel), which was assessed via the modified limited Score of Activity and Damage in DLE (ML-SADDLE). About half (48%) and one-fifth (20%) also achieved greater reductions of 50% and 70%, respectively.
“Discoid lupus is a chronic form of cutaneous lupus. Usually it occurs in visible areas like the face and scalp, causing scarring, so it’s really disabling and affects patients’ quality of life,” observed the lead study investigator Md Yuzaiful Md Yusof, MBChB, PhD, NIHR Academic Clinical Lecturer at the University of Leeds, England.
“It’s also one of the most resistant manifestations of lupus,” he said during a poster presentation at the annual conference of the British Society for Rheumatology. “Usually, when people have discoid lupus, the dermatologist gives antimalarial treatment, but only 50% of people respond to these drugs. So, what happens to the rest of them?” Basically, it is trial and error, Dr. Md Yusof said; some patients may be given disease-modifying antirheumatic drugs and in some patients this may work well, but in others there may be toxicity that contraindicates treatment.
B-cell therapy with rituximab (Rituxan) has not been successful, he said. In a previous study of 35 patients with refractory discoid lupus, none of the patients responded to rituximab and half of them actually flared after taking the drug.
There is a pathologic case for using anti-TNF therapy in DLE, but the use of TNFis is not without concern. Such treatment can increase antinuclear antibody production and make lupus worse. “In order to overcome this, as the lesion is quite small, we don’t need to use a systemic approach,” Dr. Md Yusof explained in an interview. “If you give directly, it should just be confined to the lesion and not absorbed, that’s the whole idea of thinking outside the box.” He noted that if it worked, such treatment would be for inducing remission and not for maintenance.
The study, “Targeted therapy using intradermal injection of etanercept for remission induction in discoid lupus erythematosus” (TARGET-DLE) was designed to test the validity of using intradermal rather than subcutaneous TNFi therapy in patients with discoid lupus.
Dr. Md Yusof noted that only 25 patients needed to be recruited into the single-arm, prospective trial as a “Simon’s two-stage minimized design” was used (Control Clin Trials. 1989;10[1]:1-10). This involved treating the first few patients to see if a response occurred and if it did, carrying on with treating the others, but if no response occurred in at least two patients, the trial would stop completely.
Adult patients were eligible for inclusion if they had one or more active DLE lesions and had not responded to antimalarial treatment. Stable doses of DMARDs and up to 10 mg of oral prednisolone daily was permitted if already being taken prior to entering the study.
Etanercept was injected intradermally around the most symptomatic lesion once a week for up to 12 weeks. The dosage was determined based on the radius of the selected discoid lesion. Over an 18-month period, all 25 patients were recruited, including 18 women. The median age of patients was 47 years, and six had systemic lupus erythematosus. The median number of prior DMARDs was 5 but ranged from 1 to 16, indicating a very resistant patient population.
The primary endpoint was at least 6 of the 25 patients having at least a 20% reduction in ML-SADDLE at week 12; 14 (56%) patients achieved this.
“We didn’t use CLASI [Cutaneous Lupus Area and Severity Index Activity Score] because that only includes erythema and atrophy,” Dr. Md Yusof explained. “In discoid lupus, induration is quite important as well, so that’s why we used ML-SADDLE. We called it ‘modified limited’ because the original SADDLE score is based on the whole organ score, but we only calculated the one lesion that we wanted to treat.”
In addition to meeting the primary endpoint, several secondary endpoints were met, including significant improvements in scores on visual analog scales as determined by pre- and posttreatment scoring by physicians (53.1 mm vs. 23.2 mm; P less than .001) and patients (56.9 mm vs. 29.7 mm; P = .001). Mean Dermatology Life Quality Index (DLQI) score significantly improved between pre- and post treatment, as did blood perfusion under the skin based on laser Doppler imaging and infrared thermography. However, no difference was seen with optical coherence tomography.
“There were only four grade 3/4 toxicities, and importantly, none of the SLE patients got worse, and none with DLE only converted into SLE,” Dr. Md Yusof reported. Of the four grade 3/4 adverse events, two were chest infections, one was heart failure, and one was a worsening of chilblains.
“It was a full-powered phase 2 trial, and because it was positive, now we can go to phase 3 trial,” he added.
Before conducting a phase 3 trial, however, Dr. Md Yusof wants to refine how the TNFi is delivered. Perhaps an intradermal patch with microneedles could be used. This would be left on the skin for a short amount of time to allow drug delivery and then removed. It could help ensure that all patients comply with treatment and perhaps even self-administer, he noted.
“The median compliance rate was 80%, which is not too bad, but I think when we come to run a phase 3 trial, I’m looking to improve the drug delivery,” he said. Changing the delivery method will need to be validated before a phase 3 trial can be started.
The study was not commercially funded. Dr. Md Yusof had no disclosures. Pfizer provided the study drug free of charge.
SOURCE: Md Yusof MY et al. Rheumatology. 2019;58(suppl 3): Abstract 244. doi: 10.1093/rheumatology/kez107.060.
REPORTING FROM BSR 2019
A Literally Massive Problem
A 60-year-old woman presents to dermatology with a longstanding complaint of a tender, irritated mass hanging from her lower abdomen. The patient says it started as a large fold in her lower abdomen but over the years has grown and become more pendulous. It is now large enough to interfere with normal activity, including walking.
Numerous providers, dermatologists included, have rendered diagnoses—most recently, hidradenitis suppurativa. The antibiotics prescribed for that diagnosis have not helped, however. Similarly, cultures performed on samples from draining sores on the mass’s posterior have failed to illuminate the situation, showing only mixed normal flora.
The patient’s primary care provider referred her to surgery for consideration of removal, or at least reduction, of the mass. The surgeon offered a presumptive diagnosis of elephantiasis nostras verrucosa of the pannus and agreed to perform the surgery. But the patient’s primary care provider requested a second opinion from dermatology.
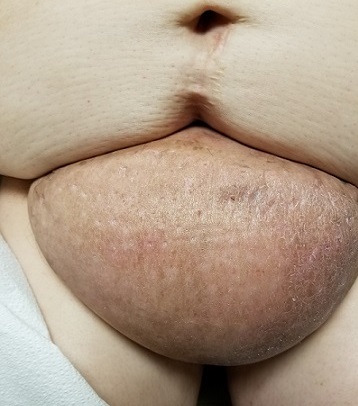
EXAMINATION
The edematous, pendulous mass is the size of a soccer ball and hangs down from its abdominal base. When the patient stands, the mass stretches almost to the level of her knees. The anterior surface is edematous but otherwise normal in appearance. The intertriginous surfaces of the lesion look entirely different, with multiple small, draining puncta and a few open comedones.
The body of the mass is quite indurated but is neither hot nor tender. There are no comedones or cysts in other intertriginous locations, as might be seen in hidradenitis.
What’s the diagnosis?
DISCUSSION
This case involves a rare entity: vast lymphedema of the pannus leading to the formation of a pendulous mass so large that it filled the space between the patient’s legs, causing pain and discomfort. These findings are analogous to those seen in advanced venous insufficiency. Both manifestations share a name: elephantiasis nostras verrucosa. (Neither has anything to do with the more notorious cases of filarial elephantiasis seen in tropical locations.)
Elephantiasis nostras verrucosa of the lower extremities involves striking skin changes: edema, along with extreme thickening, papularity, and roughness of the skin. These typically manifest downward from just below the knee. The condition represents the effects of late-stage chronic venous insufficiency, often worsened by obesity and a sedentary lifestyle. Other causes of dependent lymphedema, such as congestive heart failure, can also contribute to the problem.
This same pathophysiologic process can affect other areas as well—including the pannus, as seen in this case. Since I had only ever encountered this problem in legs, I did a literature search. I found several references, all of which indicated that surgical removal (panniculectomy) was the best treatment. I could not find any information on the success rate of this surgery, but I did refer the patient back to the surgeon, who had made the correct diagnosis.
TAKE-HOME LEARNING POINTS
- Elephantiasis nostras verrucosa (ENV) is a consequence of uncontrolled venous insufficiency that commonly manifests on lower extremities.
- ENV is a distinctly rare (though not unknown) problem when areas other than legs are affected.
- This patient’s condition is, in my opinion, beyond the reach of medical treatment. But in milder cases, approaches such as weight loss and use of diuretics have been tried with mixed success.
- The best treatment appears to be surgical removal, which is not without potential complications: risk for infection, pain, prolonged recovery time, and wound dehiscence; these issues were discussed thoroughly with the case patient.
A 60-year-old woman presents to dermatology with a longstanding complaint of a tender, irritated mass hanging from her lower abdomen. The patient says it started as a large fold in her lower abdomen but over the years has grown and become more pendulous. It is now large enough to interfere with normal activity, including walking.
Numerous providers, dermatologists included, have rendered diagnoses—most recently, hidradenitis suppurativa. The antibiotics prescribed for that diagnosis have not helped, however. Similarly, cultures performed on samples from draining sores on the mass’s posterior have failed to illuminate the situation, showing only mixed normal flora.
The patient’s primary care provider referred her to surgery for consideration of removal, or at least reduction, of the mass. The surgeon offered a presumptive diagnosis of elephantiasis nostras verrucosa of the pannus and agreed to perform the surgery. But the patient’s primary care provider requested a second opinion from dermatology.

EXAMINATION
The edematous, pendulous mass is the size of a soccer ball and hangs down from its abdominal base. When the patient stands, the mass stretches almost to the level of her knees. The anterior surface is edematous but otherwise normal in appearance. The intertriginous surfaces of the lesion look entirely different, with multiple small, draining puncta and a few open comedones.

The body of the mass is quite indurated but is neither hot nor tender. There are no comedones or cysts in other intertriginous locations, as might be seen in hidradenitis.
What’s the diagnosis?
DISCUSSION
This case involves a rare entity: vast lymphedema of the pannus leading to the formation of a pendulous mass so large that it filled the space between the patient’s legs, causing pain and discomfort. These findings are analogous to those seen in advanced venous insufficiency. Both manifestations share a name: elephantiasis nostras verrucosa. (Neither has anything to do with the more notorious cases of filarial elephantiasis seen in tropical locations.)
Elephantiasis nostras verrucosa of the lower extremities involves striking skin changes: edema, along with extreme thickening, papularity, and roughness of the skin. These typically manifest downward from just below the knee. The condition represents the effects of late-stage chronic venous insufficiency, often worsened by obesity and a sedentary lifestyle. Other causes of dependent lymphedema, such as congestive heart failure, can also contribute to the problem.
This same pathophysiologic process can affect other areas as well—including the pannus, as seen in this case. Since I had only ever encountered this problem in legs, I did a literature search. I found several references, all of which indicated that surgical removal (panniculectomy) was the best treatment. I could not find any information on the success rate of this surgery, but I did refer the patient back to the surgeon, who had made the correct diagnosis.
TAKE-HOME LEARNING POINTS
- Elephantiasis nostras verrucosa (ENV) is a consequence of uncontrolled venous insufficiency that commonly manifests on lower extremities.
- ENV is a distinctly rare (though not unknown) problem when areas other than legs are affected.
- This patient’s condition is, in my opinion, beyond the reach of medical treatment. But in milder cases, approaches such as weight loss and use of diuretics have been tried with mixed success.
- The best treatment appears to be surgical removal, which is not without potential complications: risk for infection, pain, prolonged recovery time, and wound dehiscence; these issues were discussed thoroughly with the case patient.
A 60-year-old woman presents to dermatology with a longstanding complaint of a tender, irritated mass hanging from her lower abdomen. The patient says it started as a large fold in her lower abdomen but over the years has grown and become more pendulous. It is now large enough to interfere with normal activity, including walking.
Numerous providers, dermatologists included, have rendered diagnoses—most recently, hidradenitis suppurativa. The antibiotics prescribed for that diagnosis have not helped, however. Similarly, cultures performed on samples from draining sores on the mass’s posterior have failed to illuminate the situation, showing only mixed normal flora.
The patient’s primary care provider referred her to surgery for consideration of removal, or at least reduction, of the mass. The surgeon offered a presumptive diagnosis of elephantiasis nostras verrucosa of the pannus and agreed to perform the surgery. But the patient’s primary care provider requested a second opinion from dermatology.

EXAMINATION
The edematous, pendulous mass is the size of a soccer ball and hangs down from its abdominal base. When the patient stands, the mass stretches almost to the level of her knees. The anterior surface is edematous but otherwise normal in appearance. The intertriginous surfaces of the lesion look entirely different, with multiple small, draining puncta and a few open comedones.

The body of the mass is quite indurated but is neither hot nor tender. There are no comedones or cysts in other intertriginous locations, as might be seen in hidradenitis.
What’s the diagnosis?
DISCUSSION
This case involves a rare entity: vast lymphedema of the pannus leading to the formation of a pendulous mass so large that it filled the space between the patient’s legs, causing pain and discomfort. These findings are analogous to those seen in advanced venous insufficiency. Both manifestations share a name: elephantiasis nostras verrucosa. (Neither has anything to do with the more notorious cases of filarial elephantiasis seen in tropical locations.)
Elephantiasis nostras verrucosa of the lower extremities involves striking skin changes: edema, along with extreme thickening, papularity, and roughness of the skin. These typically manifest downward from just below the knee. The condition represents the effects of late-stage chronic venous insufficiency, often worsened by obesity and a sedentary lifestyle. Other causes of dependent lymphedema, such as congestive heart failure, can also contribute to the problem.
This same pathophysiologic process can affect other areas as well—including the pannus, as seen in this case. Since I had only ever encountered this problem in legs, I did a literature search. I found several references, all of which indicated that surgical removal (panniculectomy) was the best treatment. I could not find any information on the success rate of this surgery, but I did refer the patient back to the surgeon, who had made the correct diagnosis.
TAKE-HOME LEARNING POINTS
- Elephantiasis nostras verrucosa (ENV) is a consequence of uncontrolled venous insufficiency that commonly manifests on lower extremities.
- ENV is a distinctly rare (though not unknown) problem when areas other than legs are affected.
- This patient’s condition is, in my opinion, beyond the reach of medical treatment. But in milder cases, approaches such as weight loss and use of diuretics have been tried with mixed success.
- The best treatment appears to be surgical removal, which is not without potential complications: risk for infection, pain, prolonged recovery time, and wound dehiscence; these issues were discussed thoroughly with the case patient.