User login
Persistent facial hyperpigmentation
A 59-year-old woman presented to a dermatology clinic with an asymptomatic brown facial hyperpigmentation that had developed several years earlier, and had persisted, despite regular face washing. Physicians who previously treated this patient interpreted this as melasma and advised her to wear sunscreen. The condition was not aggravated by sun exposure. The patient reported that she was otherwise healthy.
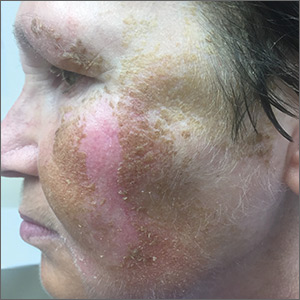
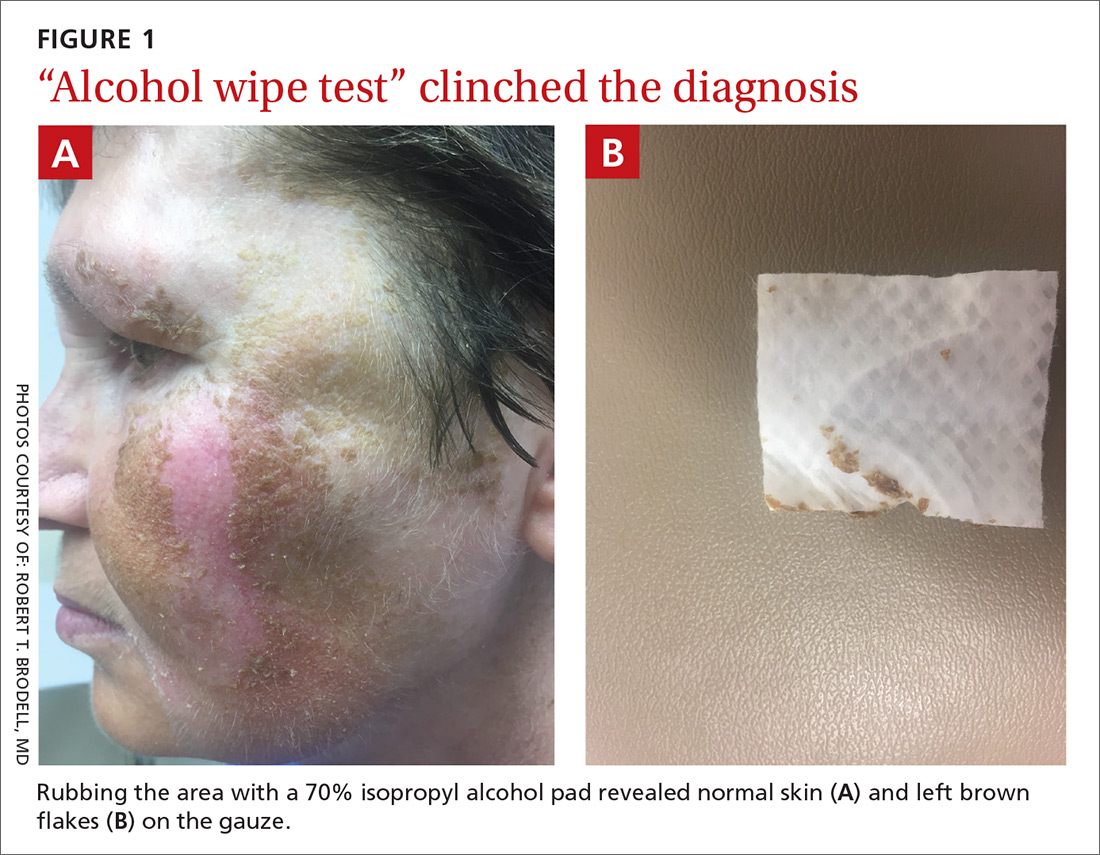
Physical examination revealed a brown discoloration with a slightly rough texture. Upon rubbing the affected area with a 70% isopropyl alcohol pad, normal skin was revealed (FIGURE 1A) and brown flakes were apparent on the gauze (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Terra firma-forme dermatosis
The physician diagnosed terra firma-forme dermatosis (TFFD) in this patient, noting the “dirty brown coloration” and distribution that did not suggest post-inflammatory hyperpigmentation or melasma. TFFD is a rare and benign form of acquired hyperpigmentation characterized by “velvety, pigmented patches or plaques.”1 A simple bedside test, known as an “alcohol wipe test,” both confirms and treats TFFD; it involves rubbing the affected area with a 70% isopropyl alcohol pad.1
TFFD typically affects the face, neck, trunk, or ankles, but the scalp, axilla, back, and pubis also can be affected.1 Histopathology will show negligible amounts of dermal inflammation, hyperkeratosis with mild acanthosis, and hyperkeratosis and papillomatosis.1 Most patients diagnosed with TFFD report that the hyperpigmentation does not improve despite washing with soap and water.2
Hygiene is not a factor
In 2015, Greywal and Cohen followed the case presentations of 10 Caucasian patients with TFFD who presented with “brown and/or black plaques or papules or both.”2 Many of the individuals followed in this case series reported “[practicing] good hygiene and showered a minimum of every other day or daily.”2 The same was reported by the patient in this case. This suggests that TFFD is not a consequence of poor hygiene but perhaps a result of “sticky” sebum that produces a buildup of keratin debris, sebum, and bacteria on the skin.3 This produces the hyperpigmentation seen clinically.
Differential includes post-inflammatory hyperpigmentation
Several other hyperpigmentation disorders were considered on the initial differential diagnosis for this case, including melasma and post-inflammatory hyperpigmentation. However, these 2 conditions are macular, whereas this hyperpigmented condition had a rough, mildly papular texture. Additionally, melasma flares up in the summer with UV exposure, and post-inflammatory hyperpigmentation presents with pruritus and/or a pre-existing rash.4 This patient reported that the condition did not itch nor change with increased sunlight, thus making melasma and post-inflammatory hyperpigmentation unlikely diagnoses.
Acanthosis nigricans also was considered because it presents with a velvety brown pigmentation similar to what was seen with this patient. Acanthosis nigricans, however, primarily affects flexural areas, not the face, making it improbable.
Continue to: Our patient
Our patient. A “wipe test” was performed on the patient. This removed the brown flaky scaling and revealed the underlying normal skin. We instructed the patient to wash daily with a soapy wash cloth and scrub with 70% isopropyl alcohol should the hyperpigmentation recur. The patient did not return.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected]
1. Lunge S, Supraja C. Terra firma-forme dermatosis—a dirty dermatosis: report of two cases. Our Dermatol Online. 2016;7:338-340.
2. Greywal T, Cohen PR. Terra firma-forme dermatosis: A report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
3. Alonso-Usero V, Gavrilova M, et al. Dermatosis neglecta or terra firma-forme dermatosis. Actas Dermosifiliogr. 2012;103:932-934.
4. Lucas J, Brodell RT, Feldman SR. Dermatosis neglecta: a series of case reports and review of other dirty-appearing dermatoses. Dermatol Online J. 2006;12:5.
A 59-year-old woman presented to a dermatology clinic with an asymptomatic brown facial hyperpigmentation that had developed several years earlier, and had persisted, despite regular face washing. Physicians who previously treated this patient interpreted this as melasma and advised her to wear sunscreen. The condition was not aggravated by sun exposure. The patient reported that she was otherwise healthy.
Physical examination revealed a brown discoloration with a slightly rough texture. Upon rubbing the affected area with a 70% isopropyl alcohol pad, normal skin was revealed (FIGURE 1A) and brown flakes were apparent on the gauze (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Terra firma-forme dermatosis
The physician diagnosed terra firma-forme dermatosis (TFFD) in this patient, noting the “dirty brown coloration” and distribution that did not suggest post-inflammatory hyperpigmentation or melasma. TFFD is a rare and benign form of acquired hyperpigmentation characterized by “velvety, pigmented patches or plaques.”1 A simple bedside test, known as an “alcohol wipe test,” both confirms and treats TFFD; it involves rubbing the affected area with a 70% isopropyl alcohol pad.1
TFFD typically affects the face, neck, trunk, or ankles, but the scalp, axilla, back, and pubis also can be affected.1 Histopathology will show negligible amounts of dermal inflammation, hyperkeratosis with mild acanthosis, and hyperkeratosis and papillomatosis.1 Most patients diagnosed with TFFD report that the hyperpigmentation does not improve despite washing with soap and water.2
Hygiene is not a factor
In 2015, Greywal and Cohen followed the case presentations of 10 Caucasian patients with TFFD who presented with “brown and/or black plaques or papules or both.”2 Many of the individuals followed in this case series reported “[practicing] good hygiene and showered a minimum of every other day or daily.”2 The same was reported by the patient in this case. This suggests that TFFD is not a consequence of poor hygiene but perhaps a result of “sticky” sebum that produces a buildup of keratin debris, sebum, and bacteria on the skin.3 This produces the hyperpigmentation seen clinically.
Differential includes post-inflammatory hyperpigmentation
Several other hyperpigmentation disorders were considered on the initial differential diagnosis for this case, including melasma and post-inflammatory hyperpigmentation. However, these 2 conditions are macular, whereas this hyperpigmented condition had a rough, mildly papular texture. Additionally, melasma flares up in the summer with UV exposure, and post-inflammatory hyperpigmentation presents with pruritus and/or a pre-existing rash.4 This patient reported that the condition did not itch nor change with increased sunlight, thus making melasma and post-inflammatory hyperpigmentation unlikely diagnoses.
Acanthosis nigricans also was considered because it presents with a velvety brown pigmentation similar to what was seen with this patient. Acanthosis nigricans, however, primarily affects flexural areas, not the face, making it improbable.
Continue to: Our patient
Our patient. A “wipe test” was performed on the patient. This removed the brown flaky scaling and revealed the underlying normal skin. We instructed the patient to wash daily with a soapy wash cloth and scrub with 70% isopropyl alcohol should the hyperpigmentation recur. The patient did not return.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected]
A 59-year-old woman presented to a dermatology clinic with an asymptomatic brown facial hyperpigmentation that had developed several years earlier, and had persisted, despite regular face washing. Physicians who previously treated this patient interpreted this as melasma and advised her to wear sunscreen. The condition was not aggravated by sun exposure. The patient reported that she was otherwise healthy.
Physical examination revealed a brown discoloration with a slightly rough texture. Upon rubbing the affected area with a 70% isopropyl alcohol pad, normal skin was revealed (FIGURE 1A) and brown flakes were apparent on the gauze (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Terra firma-forme dermatosis
The physician diagnosed terra firma-forme dermatosis (TFFD) in this patient, noting the “dirty brown coloration” and distribution that did not suggest post-inflammatory hyperpigmentation or melasma. TFFD is a rare and benign form of acquired hyperpigmentation characterized by “velvety, pigmented patches or plaques.”1 A simple bedside test, known as an “alcohol wipe test,” both confirms and treats TFFD; it involves rubbing the affected area with a 70% isopropyl alcohol pad.1
TFFD typically affects the face, neck, trunk, or ankles, but the scalp, axilla, back, and pubis also can be affected.1 Histopathology will show negligible amounts of dermal inflammation, hyperkeratosis with mild acanthosis, and hyperkeratosis and papillomatosis.1 Most patients diagnosed with TFFD report that the hyperpigmentation does not improve despite washing with soap and water.2
Hygiene is not a factor
In 2015, Greywal and Cohen followed the case presentations of 10 Caucasian patients with TFFD who presented with “brown and/or black plaques or papules or both.”2 Many of the individuals followed in this case series reported “[practicing] good hygiene and showered a minimum of every other day or daily.”2 The same was reported by the patient in this case. This suggests that TFFD is not a consequence of poor hygiene but perhaps a result of “sticky” sebum that produces a buildup of keratin debris, sebum, and bacteria on the skin.3 This produces the hyperpigmentation seen clinically.
Differential includes post-inflammatory hyperpigmentation
Several other hyperpigmentation disorders were considered on the initial differential diagnosis for this case, including melasma and post-inflammatory hyperpigmentation. However, these 2 conditions are macular, whereas this hyperpigmented condition had a rough, mildly papular texture. Additionally, melasma flares up in the summer with UV exposure, and post-inflammatory hyperpigmentation presents with pruritus and/or a pre-existing rash.4 This patient reported that the condition did not itch nor change with increased sunlight, thus making melasma and post-inflammatory hyperpigmentation unlikely diagnoses.
Acanthosis nigricans also was considered because it presents with a velvety brown pigmentation similar to what was seen with this patient. Acanthosis nigricans, however, primarily affects flexural areas, not the face, making it improbable.
Continue to: Our patient
Our patient. A “wipe test” was performed on the patient. This removed the brown flaky scaling and revealed the underlying normal skin. We instructed the patient to wash daily with a soapy wash cloth and scrub with 70% isopropyl alcohol should the hyperpigmentation recur. The patient did not return.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected]
1. Lunge S, Supraja C. Terra firma-forme dermatosis—a dirty dermatosis: report of two cases. Our Dermatol Online. 2016;7:338-340.
2. Greywal T, Cohen PR. Terra firma-forme dermatosis: A report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
3. Alonso-Usero V, Gavrilova M, et al. Dermatosis neglecta or terra firma-forme dermatosis. Actas Dermosifiliogr. 2012;103:932-934.
4. Lucas J, Brodell RT, Feldman SR. Dermatosis neglecta: a series of case reports and review of other dirty-appearing dermatoses. Dermatol Online J. 2006;12:5.
1. Lunge S, Supraja C. Terra firma-forme dermatosis—a dirty dermatosis: report of two cases. Our Dermatol Online. 2016;7:338-340.
2. Greywal T, Cohen PR. Terra firma-forme dermatosis: A report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
3. Alonso-Usero V, Gavrilova M, et al. Dermatosis neglecta or terra firma-forme dermatosis. Actas Dermosifiliogr. 2012;103:932-934.
4. Lucas J, Brodell RT, Feldman SR. Dermatosis neglecta: a series of case reports and review of other dirty-appearing dermatoses. Dermatol Online J. 2006;12:5.
Comorbidities may cut effectiveness of psoriasis biologics
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
REPORTING FROM THE EADV CONGRESS
Key clinical point: As the number of baseline comorbid conditions increases, the complete clearance rate in response to biologic agents for psoriasis falls.
Major finding: The complete clearance rate after 6 months of biologic therapy ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more.
Study details: This multinational, prospective, observational, 12-month study included 846 patients initiating biologic therapy for moderate to severe psoriasis.
Disclosures: The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma and was presented by a Leo executive.
Source: Ziegler F. EADV Congress, Abstract FC04.01.
Isotretinoin treatment reorganizes dermal microbiome in acne patients
GRAND CAYMAN, CAYMAN ISLANDS – Isotretinoin, the go-to guy for severe acne, may not be so much a local cop as a community organizer, Kenneth B. Gordon, MD, said at the meeting provided by Global Academy for Medical Education.
“It now appears that with and that the microbial community is replenished with the types associated with healthy skin,” said Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee. When these new bacteria move in, they push pathogenic species out of the neighborhood “and create a new skin microbial community. Maybe this is the real reason our patients tend to stay better, once we get them better with isotretinoin.”
Dr. Gordon discussed new data published last October in the Journal of Investigative Dermatology (J Invest Dermatol. 2018 Oct 24. doi: 10.1016/j.jid.2018.09.023). In a letter to the editor, William H. McCoy, IV, MD, PhD, of Washington University, St. Louis, and his associates suggest that isotretinoin induces a “sebaceous drought,” which shifts the skin microbiome from pathogenic to normophysiological.
Isotretinoin is the gold standard treatment for severe acne, but its method of action has never been fully elucidated, Dr. Gordon said. It clearly targets the sebaceous gland – decreasing sebocyte proliferation and suppressing sebum production – but an emerging body of research suggests that the drug also markedly affects dermal microbial colonization.
The entire concept of a skin microbiome is nearly as new as this new concept of isotretinoin’s effect upon it. Only in the last few years have researchers begun to characterize the complex microbial film that keeps skin healthy and resistant to infection. Dermal dysbiosis has now been associated with acne, psoriasis and psoriatic arthritis, and atopic dermatitis.
The 2-year pilot study compared the dermal microbiome of isotretinoin-treated acne patients with that of patients with untreated acne and normal skin. Skin samples underwent genomic analysis before isotretinoin treatment, at several periods during treatment, and about 5 months after treatment stopped. Untreated controls were evaluated at baseline and at 2, 5, and 10 months.
Not surprisingly, before treatment the microbiome was similar in both acne groups, but markedly different from that seen in normal skin. As isotretinoin’s “oil drought” dragged on, levels of Cutibacterium acnes (the new appellation for P. acnes) declined. Staphylococcus species initially increased, but then declined as well. Simultaneously, four new taxa (Rothia, Flavobacterium, Enterobacter, and Micrococcus) increased. Most patients had a restructuring of their Propionibacterium community, populated largely by the less-pathogenic strains found on normal skin.
“We suggest that isotretinoin creates a Propionibacterium ‘population bottleneck’ that selects for ‘healthy’ Propionibacterium communities and other sebaceous skin taxa that persist after treatment, resulting in long-term acne remission [i.e., normal skin],” the investigators wrote.
This is a new and very exciting finding, Dr. Gordon commented. “It appears that the reason our isotretinoin patients stay better once they get better is not from targeting the sebaceous gland itself, but by repairing the skin’s microbiome and getting it back to normal.”
Dr. Gordon reported financial relationships with numerous pharmaceutical companies. Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – Isotretinoin, the go-to guy for severe acne, may not be so much a local cop as a community organizer, Kenneth B. Gordon, MD, said at the meeting provided by Global Academy for Medical Education.
“It now appears that with and that the microbial community is replenished with the types associated with healthy skin,” said Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee. When these new bacteria move in, they push pathogenic species out of the neighborhood “and create a new skin microbial community. Maybe this is the real reason our patients tend to stay better, once we get them better with isotretinoin.”
Dr. Gordon discussed new data published last October in the Journal of Investigative Dermatology (J Invest Dermatol. 2018 Oct 24. doi: 10.1016/j.jid.2018.09.023). In a letter to the editor, William H. McCoy, IV, MD, PhD, of Washington University, St. Louis, and his associates suggest that isotretinoin induces a “sebaceous drought,” which shifts the skin microbiome from pathogenic to normophysiological.
Isotretinoin is the gold standard treatment for severe acne, but its method of action has never been fully elucidated, Dr. Gordon said. It clearly targets the sebaceous gland – decreasing sebocyte proliferation and suppressing sebum production – but an emerging body of research suggests that the drug also markedly affects dermal microbial colonization.
The entire concept of a skin microbiome is nearly as new as this new concept of isotretinoin’s effect upon it. Only in the last few years have researchers begun to characterize the complex microbial film that keeps skin healthy and resistant to infection. Dermal dysbiosis has now been associated with acne, psoriasis and psoriatic arthritis, and atopic dermatitis.
The 2-year pilot study compared the dermal microbiome of isotretinoin-treated acne patients with that of patients with untreated acne and normal skin. Skin samples underwent genomic analysis before isotretinoin treatment, at several periods during treatment, and about 5 months after treatment stopped. Untreated controls were evaluated at baseline and at 2, 5, and 10 months.
Not surprisingly, before treatment the microbiome was similar in both acne groups, but markedly different from that seen in normal skin. As isotretinoin’s “oil drought” dragged on, levels of Cutibacterium acnes (the new appellation for P. acnes) declined. Staphylococcus species initially increased, but then declined as well. Simultaneously, four new taxa (Rothia, Flavobacterium, Enterobacter, and Micrococcus) increased. Most patients had a restructuring of their Propionibacterium community, populated largely by the less-pathogenic strains found on normal skin.
“We suggest that isotretinoin creates a Propionibacterium ‘population bottleneck’ that selects for ‘healthy’ Propionibacterium communities and other sebaceous skin taxa that persist after treatment, resulting in long-term acne remission [i.e., normal skin],” the investigators wrote.
This is a new and very exciting finding, Dr. Gordon commented. “It appears that the reason our isotretinoin patients stay better once they get better is not from targeting the sebaceous gland itself, but by repairing the skin’s microbiome and getting it back to normal.”
Dr. Gordon reported financial relationships with numerous pharmaceutical companies. Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – Isotretinoin, the go-to guy for severe acne, may not be so much a local cop as a community organizer, Kenneth B. Gordon, MD, said at the meeting provided by Global Academy for Medical Education.
“It now appears that with and that the microbial community is replenished with the types associated with healthy skin,” said Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee. When these new bacteria move in, they push pathogenic species out of the neighborhood “and create a new skin microbial community. Maybe this is the real reason our patients tend to stay better, once we get them better with isotretinoin.”
Dr. Gordon discussed new data published last October in the Journal of Investigative Dermatology (J Invest Dermatol. 2018 Oct 24. doi: 10.1016/j.jid.2018.09.023). In a letter to the editor, William H. McCoy, IV, MD, PhD, of Washington University, St. Louis, and his associates suggest that isotretinoin induces a “sebaceous drought,” which shifts the skin microbiome from pathogenic to normophysiological.
Isotretinoin is the gold standard treatment for severe acne, but its method of action has never been fully elucidated, Dr. Gordon said. It clearly targets the sebaceous gland – decreasing sebocyte proliferation and suppressing sebum production – but an emerging body of research suggests that the drug also markedly affects dermal microbial colonization.
The entire concept of a skin microbiome is nearly as new as this new concept of isotretinoin’s effect upon it. Only in the last few years have researchers begun to characterize the complex microbial film that keeps skin healthy and resistant to infection. Dermal dysbiosis has now been associated with acne, psoriasis and psoriatic arthritis, and atopic dermatitis.
The 2-year pilot study compared the dermal microbiome of isotretinoin-treated acne patients with that of patients with untreated acne and normal skin. Skin samples underwent genomic analysis before isotretinoin treatment, at several periods during treatment, and about 5 months after treatment stopped. Untreated controls were evaluated at baseline and at 2, 5, and 10 months.
Not surprisingly, before treatment the microbiome was similar in both acne groups, but markedly different from that seen in normal skin. As isotretinoin’s “oil drought” dragged on, levels of Cutibacterium acnes (the new appellation for P. acnes) declined. Staphylococcus species initially increased, but then declined as well. Simultaneously, four new taxa (Rothia, Flavobacterium, Enterobacter, and Micrococcus) increased. Most patients had a restructuring of their Propionibacterium community, populated largely by the less-pathogenic strains found on normal skin.
“We suggest that isotretinoin creates a Propionibacterium ‘population bottleneck’ that selects for ‘healthy’ Propionibacterium communities and other sebaceous skin taxa that persist after treatment, resulting in long-term acne remission [i.e., normal skin],” the investigators wrote.
This is a new and very exciting finding, Dr. Gordon commented. “It appears that the reason our isotretinoin patients stay better once they get better is not from targeting the sebaceous gland itself, but by repairing the skin’s microbiome and getting it back to normal.”
Dr. Gordon reported financial relationships with numerous pharmaceutical companies. Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
REPORTING FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
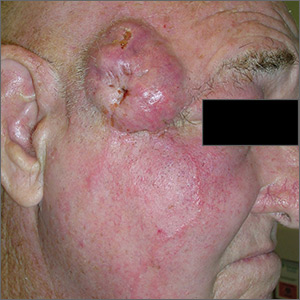
Growing cyst on face
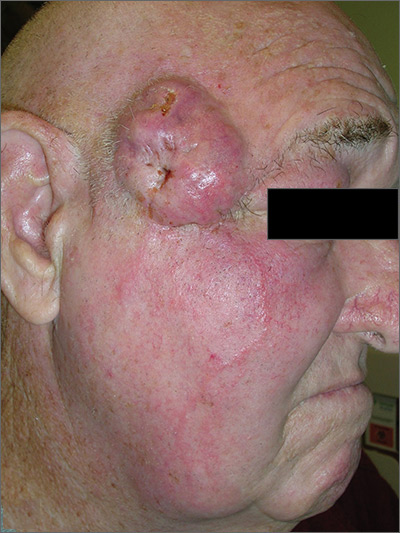
The FP suspected this was more than just a simple cyst, and his differential diagnosis included basal cell carcinoma and squamous cell carcinoma (SCC). The FP advised the patient that a punch biopsy was needed to determine the diagnosis and that it was not possible to just remove the cyst. The patient consented to the biopsy, and the FP performed a 4-mm punch biopsy. (See the Watch & Learn video on “Punch biopsy.”)
The pathology showed an invasive SCC. Note that cutaneous SCCs can appear cystic on presentation. Due to the location and size of the SCC, the patient was referred to Head and Neck Surgery for resection of the tumor and flap repair. The temporal branch of the facial nerve was spared. And, while it appeared that the red lines radiating down the cheek from the tumor were lymphangitic spread, the pathology at the time of the tumor resection did not show this. The surgery achieved clear margins, and the patient recovered well.
On a follow-up visit, the FP performed a total body skin exam to look for other skin cancers and found none. He also counseled the patient on sun avoidance, the consistent use of a hat outdoors, and the use of sunscreen when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected this was more than just a simple cyst, and his differential diagnosis included basal cell carcinoma and squamous cell carcinoma (SCC). The FP advised the patient that a punch biopsy was needed to determine the diagnosis and that it was not possible to just remove the cyst. The patient consented to the biopsy, and the FP performed a 4-mm punch biopsy. (See the Watch & Learn video on “Punch biopsy.”)
The pathology showed an invasive SCC. Note that cutaneous SCCs can appear cystic on presentation. Due to the location and size of the SCC, the patient was referred to Head and Neck Surgery for resection of the tumor and flap repair. The temporal branch of the facial nerve was spared. And, while it appeared that the red lines radiating down the cheek from the tumor were lymphangitic spread, the pathology at the time of the tumor resection did not show this. The surgery achieved clear margins, and the patient recovered well.
On a follow-up visit, the FP performed a total body skin exam to look for other skin cancers and found none. He also counseled the patient on sun avoidance, the consistent use of a hat outdoors, and the use of sunscreen when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected this was more than just a simple cyst, and his differential diagnosis included basal cell carcinoma and squamous cell carcinoma (SCC). The FP advised the patient that a punch biopsy was needed to determine the diagnosis and that it was not possible to just remove the cyst. The patient consented to the biopsy, and the FP performed a 4-mm punch biopsy. (See the Watch & Learn video on “Punch biopsy.”)
The pathology showed an invasive SCC. Note that cutaneous SCCs can appear cystic on presentation. Due to the location and size of the SCC, the patient was referred to Head and Neck Surgery for resection of the tumor and flap repair. The temporal branch of the facial nerve was spared. And, while it appeared that the red lines radiating down the cheek from the tumor were lymphangitic spread, the pathology at the time of the tumor resection did not show this. The surgery achieved clear margins, and the patient recovered well.
On a follow-up visit, the FP performed a total body skin exam to look for other skin cancers and found none. He also counseled the patient on sun avoidance, the consistent use of a hat outdoors, and the use of sunscreen when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Study shows evidence of herd immunity with HPV vaccine
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
FROM PEDIATRICS
Key clinical point:
Major finding: Infection rates for quadrivalent vaccine-covered HPV strains declined by 81% among vaccinated women.
Study details: Surveillance studies in 1,580 vaccinated and unvaccinated young women.
Disclosures: The study was funded by the National Institutes of Health. One author declared shares of Merck, but no other conflicts of interest were declared.
Source: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
Bidirectional relationship found between depression, vitiligo
Vitiligo and major depressive disorder have a bidirectional relationship, according to a new study that examined data from a cohort of more than 6 million people.
“Ultimately, this suggests that mental health appears to play a large role in the pathogenesis of autoimmune diseases like vitiligo, which in turn can increase the risk of MDD, especially in younger patients,” wrote Isabelle Vallerand, PhD, and her colleagues. The report is in the Journal of the American Academy of Dermatology.
Dr. Vallerand and her colleagues found that patients with major depressive disorder (MDD, n = 405,397) had a 64% increased risk of vitiligo, compared with a referent cohort (n = 5,739,048; 95% confidence interval, 1.43-1.87; P less than .0001). Conversely, patients who had vitiligo also were at an increased risk of MDD. Patients who were younger than 30 years old at diagnosis (n = 7,104) had a hazard ratio of 1.31 for MDD (P less than .0001), compared with 1.22 for patients aged 30 years and older (P = .001).
Individuals who took antidepressants, whether or not they also had an MDD diagnosis, had a decreased risk for vitiligo.
Though it’s known that vitiligo increases the risk of MDD, less clarity has been in the literature about whether the converse also might be true. “The question of whether vitiligo onset can be precipitated by MDD has received less attention, despite the notion that patients often ask their dermatologists if stress or depression may have contributed to their disease,” wrote Dr. Vallerand, an epidemiologist and medical student at the University of Calgary, Alberta, and her colleagues.
There is a biologic plausibility for a bidirectional relationship, said Dr. Vallerand and her colleagues, since depression can boost systemic inflammation, and the risk for autoimmune disease such as vitiligo can be increased by proinflammatory states.
Access to a large dataset gave Dr. Vallerand and her collaborators the numbers to look at the relationship between vitiligo and MDD in the context of potential confounders, and to correct for those in their statistical analysis. Using medical records from The Health Improvement Network (THIN) database in the United Kingdom, the investigators conducted two independent population-based cohort studies. Each looked at risk in one direction of the MDD-vitiligo association.
The first analysis looked at MDD as a risk factor for vitiligo, following all patients with an incident diagnostic code for MDD. Patients without the MDD diagnosis code were the referent cohort. Patients in each cohort were followed until they reached the outcome of interest – a diagnosis of vitiligo – or were censored. Patients who had a vitiligo diagnosis before receiving an MDD diagnosis were not included.
The second analysis examined whether vitiligo was a risk factor for MDD, with a similar design that used nonvitiligo patients as the referent cohort. This analysis followed all patients until a diagnosis of MDD was recorded, or patients were censored. Again, patients with MDD diagnoses that came before the vitiligo diagnosis were excluded.
For the analysis of risk of vitiligo, the investigators looked at the effects of multiple covariates, including age, sex, alcohol use and smoking status, socioeconomic status, medical comorbidities, and whether patients were taking antidepressants. The covariates included in the analysis of risk of MDD were age, sex, medical comorbidities, and type of vitiligo treatment.
After the researchers determined unadjusted hazard ratios, each covariate was removed one at a time to see where there were substantial changes to the HR. Two additional models, one unadjusted and one that fully adjusted for all covariates, also were built.
The sensitivity analyses showed “an overall protective effect of antidepressants among both cohorts,” wrote Dr. Vallerand and her colleagues. The incidence rate of vitiligo among patients with MDD using antidepressants was 19.7 per 100,000 person-years, compared with 27.5 among MDD patients not using antidepressants (P = .0053).
“Similarly, those in the referent cohort who used antidepressants had about half the risk of vitiligo,” compared with the nonusers in the referent group, the investigators said. Serotonin also is present in the skin, and neurons and melanocytes share embryonic ectodermal origins, Dr. Vallerand and her colleagues said. Though the exact mechanisms are not known, in the THIN cohorts, they noted.
Though younger patients with vitiligo were at higher risk for MDD than were those aged 30 years and older, the overall cohort of individuals with vitiligo still had an unadjusted elevated risk for MDD, compared with the referent cohort (HR 1.27; 95% confidence interval, 1.16-1.40; P less than .0001).
“Unexpectedly, the magnitude of the reciprocal association was highest with MDD being a risk factor for vitiligo,” wrote Dr. Vallerand and her colleagues. “This highlights the notion that mental health may have a greater impact on the body, specifically with dermatologic manifestations, than previously thought.”
Some misclassification of both conditions is likely in such a large dataset, the investigators acknowledged. Also, subclinical depression was not evaluated, and there was no way to track the severity of either depression or vitiligo, they noted. Still, the big data approach “renders this one of the largest studies on psychodermatology to date,” said Dr. Vallerand and her colleagues, and the independent bidirectional analyses support causality.
Dr. Vallerand is a partner in a pharmaceutical consulting firm, GlacierRX, and was funded by Alberta Innovates. The authors reported having no conflicts of interest.
SOURCE: Vallerand IA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.11.047.
Vitiligo and major depressive disorder have a bidirectional relationship, according to a new study that examined data from a cohort of more than 6 million people.
“Ultimately, this suggests that mental health appears to play a large role in the pathogenesis of autoimmune diseases like vitiligo, which in turn can increase the risk of MDD, especially in younger patients,” wrote Isabelle Vallerand, PhD, and her colleagues. The report is in the Journal of the American Academy of Dermatology.
Dr. Vallerand and her colleagues found that patients with major depressive disorder (MDD, n = 405,397) had a 64% increased risk of vitiligo, compared with a referent cohort (n = 5,739,048; 95% confidence interval, 1.43-1.87; P less than .0001). Conversely, patients who had vitiligo also were at an increased risk of MDD. Patients who were younger than 30 years old at diagnosis (n = 7,104) had a hazard ratio of 1.31 for MDD (P less than .0001), compared with 1.22 for patients aged 30 years and older (P = .001).
Individuals who took antidepressants, whether or not they also had an MDD diagnosis, had a decreased risk for vitiligo.
Though it’s known that vitiligo increases the risk of MDD, less clarity has been in the literature about whether the converse also might be true. “The question of whether vitiligo onset can be precipitated by MDD has received less attention, despite the notion that patients often ask their dermatologists if stress or depression may have contributed to their disease,” wrote Dr. Vallerand, an epidemiologist and medical student at the University of Calgary, Alberta, and her colleagues.
There is a biologic plausibility for a bidirectional relationship, said Dr. Vallerand and her colleagues, since depression can boost systemic inflammation, and the risk for autoimmune disease such as vitiligo can be increased by proinflammatory states.
Access to a large dataset gave Dr. Vallerand and her collaborators the numbers to look at the relationship between vitiligo and MDD in the context of potential confounders, and to correct for those in their statistical analysis. Using medical records from The Health Improvement Network (THIN) database in the United Kingdom, the investigators conducted two independent population-based cohort studies. Each looked at risk in one direction of the MDD-vitiligo association.
The first analysis looked at MDD as a risk factor for vitiligo, following all patients with an incident diagnostic code for MDD. Patients without the MDD diagnosis code were the referent cohort. Patients in each cohort were followed until they reached the outcome of interest – a diagnosis of vitiligo – or were censored. Patients who had a vitiligo diagnosis before receiving an MDD diagnosis were not included.
The second analysis examined whether vitiligo was a risk factor for MDD, with a similar design that used nonvitiligo patients as the referent cohort. This analysis followed all patients until a diagnosis of MDD was recorded, or patients were censored. Again, patients with MDD diagnoses that came before the vitiligo diagnosis were excluded.
For the analysis of risk of vitiligo, the investigators looked at the effects of multiple covariates, including age, sex, alcohol use and smoking status, socioeconomic status, medical comorbidities, and whether patients were taking antidepressants. The covariates included in the analysis of risk of MDD were age, sex, medical comorbidities, and type of vitiligo treatment.
After the researchers determined unadjusted hazard ratios, each covariate was removed one at a time to see where there were substantial changes to the HR. Two additional models, one unadjusted and one that fully adjusted for all covariates, also were built.
The sensitivity analyses showed “an overall protective effect of antidepressants among both cohorts,” wrote Dr. Vallerand and her colleagues. The incidence rate of vitiligo among patients with MDD using antidepressants was 19.7 per 100,000 person-years, compared with 27.5 among MDD patients not using antidepressants (P = .0053).
“Similarly, those in the referent cohort who used antidepressants had about half the risk of vitiligo,” compared with the nonusers in the referent group, the investigators said. Serotonin also is present in the skin, and neurons and melanocytes share embryonic ectodermal origins, Dr. Vallerand and her colleagues said. Though the exact mechanisms are not known, in the THIN cohorts, they noted.
Though younger patients with vitiligo were at higher risk for MDD than were those aged 30 years and older, the overall cohort of individuals with vitiligo still had an unadjusted elevated risk for MDD, compared with the referent cohort (HR 1.27; 95% confidence interval, 1.16-1.40; P less than .0001).
“Unexpectedly, the magnitude of the reciprocal association was highest with MDD being a risk factor for vitiligo,” wrote Dr. Vallerand and her colleagues. “This highlights the notion that mental health may have a greater impact on the body, specifically with dermatologic manifestations, than previously thought.”
Some misclassification of both conditions is likely in such a large dataset, the investigators acknowledged. Also, subclinical depression was not evaluated, and there was no way to track the severity of either depression or vitiligo, they noted. Still, the big data approach “renders this one of the largest studies on psychodermatology to date,” said Dr. Vallerand and her colleagues, and the independent bidirectional analyses support causality.
Dr. Vallerand is a partner in a pharmaceutical consulting firm, GlacierRX, and was funded by Alberta Innovates. The authors reported having no conflicts of interest.
SOURCE: Vallerand IA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.11.047.
Vitiligo and major depressive disorder have a bidirectional relationship, according to a new study that examined data from a cohort of more than 6 million people.
“Ultimately, this suggests that mental health appears to play a large role in the pathogenesis of autoimmune diseases like vitiligo, which in turn can increase the risk of MDD, especially in younger patients,” wrote Isabelle Vallerand, PhD, and her colleagues. The report is in the Journal of the American Academy of Dermatology.
Dr. Vallerand and her colleagues found that patients with major depressive disorder (MDD, n = 405,397) had a 64% increased risk of vitiligo, compared with a referent cohort (n = 5,739,048; 95% confidence interval, 1.43-1.87; P less than .0001). Conversely, patients who had vitiligo also were at an increased risk of MDD. Patients who were younger than 30 years old at diagnosis (n = 7,104) had a hazard ratio of 1.31 for MDD (P less than .0001), compared with 1.22 for patients aged 30 years and older (P = .001).
Individuals who took antidepressants, whether or not they also had an MDD diagnosis, had a decreased risk for vitiligo.
Though it’s known that vitiligo increases the risk of MDD, less clarity has been in the literature about whether the converse also might be true. “The question of whether vitiligo onset can be precipitated by MDD has received less attention, despite the notion that patients often ask their dermatologists if stress or depression may have contributed to their disease,” wrote Dr. Vallerand, an epidemiologist and medical student at the University of Calgary, Alberta, and her colleagues.
There is a biologic plausibility for a bidirectional relationship, said Dr. Vallerand and her colleagues, since depression can boost systemic inflammation, and the risk for autoimmune disease such as vitiligo can be increased by proinflammatory states.
Access to a large dataset gave Dr. Vallerand and her collaborators the numbers to look at the relationship between vitiligo and MDD in the context of potential confounders, and to correct for those in their statistical analysis. Using medical records from The Health Improvement Network (THIN) database in the United Kingdom, the investigators conducted two independent population-based cohort studies. Each looked at risk in one direction of the MDD-vitiligo association.
The first analysis looked at MDD as a risk factor for vitiligo, following all patients with an incident diagnostic code for MDD. Patients without the MDD diagnosis code were the referent cohort. Patients in each cohort were followed until they reached the outcome of interest – a diagnosis of vitiligo – or were censored. Patients who had a vitiligo diagnosis before receiving an MDD diagnosis were not included.
The second analysis examined whether vitiligo was a risk factor for MDD, with a similar design that used nonvitiligo patients as the referent cohort. This analysis followed all patients until a diagnosis of MDD was recorded, or patients were censored. Again, patients with MDD diagnoses that came before the vitiligo diagnosis were excluded.
For the analysis of risk of vitiligo, the investigators looked at the effects of multiple covariates, including age, sex, alcohol use and smoking status, socioeconomic status, medical comorbidities, and whether patients were taking antidepressants. The covariates included in the analysis of risk of MDD were age, sex, medical comorbidities, and type of vitiligo treatment.
After the researchers determined unadjusted hazard ratios, each covariate was removed one at a time to see where there were substantial changes to the HR. Two additional models, one unadjusted and one that fully adjusted for all covariates, also were built.
The sensitivity analyses showed “an overall protective effect of antidepressants among both cohorts,” wrote Dr. Vallerand and her colleagues. The incidence rate of vitiligo among patients with MDD using antidepressants was 19.7 per 100,000 person-years, compared with 27.5 among MDD patients not using antidepressants (P = .0053).
“Similarly, those in the referent cohort who used antidepressants had about half the risk of vitiligo,” compared with the nonusers in the referent group, the investigators said. Serotonin also is present in the skin, and neurons and melanocytes share embryonic ectodermal origins, Dr. Vallerand and her colleagues said. Though the exact mechanisms are not known, in the THIN cohorts, they noted.
Though younger patients with vitiligo were at higher risk for MDD than were those aged 30 years and older, the overall cohort of individuals with vitiligo still had an unadjusted elevated risk for MDD, compared with the referent cohort (HR 1.27; 95% confidence interval, 1.16-1.40; P less than .0001).
“Unexpectedly, the magnitude of the reciprocal association was highest with MDD being a risk factor for vitiligo,” wrote Dr. Vallerand and her colleagues. “This highlights the notion that mental health may have a greater impact on the body, specifically with dermatologic manifestations, than previously thought.”
Some misclassification of both conditions is likely in such a large dataset, the investigators acknowledged. Also, subclinical depression was not evaluated, and there was no way to track the severity of either depression or vitiligo, they noted. Still, the big data approach “renders this one of the largest studies on psychodermatology to date,” said Dr. Vallerand and her colleagues, and the independent bidirectional analyses support causality.
Dr. Vallerand is a partner in a pharmaceutical consulting firm, GlacierRX, and was funded by Alberta Innovates. The authors reported having no conflicts of interest.
SOURCE: Vallerand IA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.11.047.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: The findings suggest that “mental health appears to play a large role in the pathogenesis of autoimmune diseases like vitiligo.”
Major finding: Patients with major depressive disorder had a 64% increased risk of vitiligo.
Study details: Retrospective records review of 405,397 patients with MDD and 5,738,048 patients in a referent cohort.
Disclosures: Dr. Vallerand is a partner in a pharmaceutical consulting firm, GlacierRx, and was funded by Alberta Innovates. The authors reported having no conflicts of interest.
Source: Vallerand IA et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2018.11.047.
Biopsy? What Biopsy?
This 80-year-old man has been complaining to health care providers about the asymptomatic lesion on his right forearm for at least 5 years—“maybe more,” he says. During that time, he has been given a variety of diagnoses, mostly “infection” of some sort, along with prescriptions for oral antibiotics (cephalexin, trimethoprim/sulfa), topical antibiotics (mupirocin, triple-antibiotic cream), and germicidal washes (povidone and others). None of these treatment attempts has achieved results.
Prior to now, there has been no referral to dermatology, nor has any provider suggested biopsy of the lesion. The patient has a history of skin cancer (basal cell or squamous cell carcinomas) on his face, back, and arms. These followed a lifetime of sun exposure due to his work in construction and roofing.
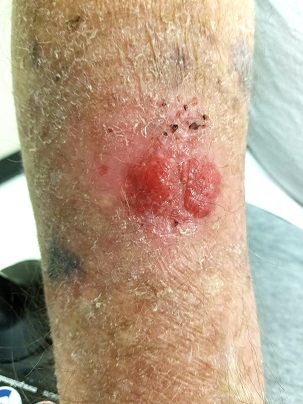
EXAMINATION
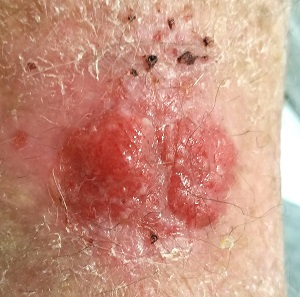
The lesion in question is located on the dorsal aspect of his mid right forearm. Measuring almost 4 cm in aggregate, the lesion is composed of 2 adjacent bright red half-moon friable nodules in a circular configuration. There is almost no erythema or edema in or around the lesion, which is neither warm nor tender to touch.
There are no palpable nodes in the adjacent epitrochlear or axillary nodal locations.
The surrounding skin of this arm, as well as that of the opposite arm, is quite thin, discolored, and scaly and is covered with stellate scars. Examination of all other sun-exposed areas reveals similar changes but no other notable lesions.
What’s the diagnosis?
DISCUSSION
It would be obvious to most readers that a biopsy is in order, given the likelihood that this lesion is either a basal cell or squamous cell carcinoma—an impression bolstered by the contextual findings of advanced sun damage and the history of skin cancer. And yet, this patient finds himself with a nonhealing friable lesion that is at least 5 years old.
Repeated misdiagnosis as infection is actually a common occurrence. One suspects that at least some of the providers making this mistake followed the patient’s lead: Many affected patients assume such lesions represent infection and therefore demand treatment appropriate for that diagnosis.
As a rule, however, bacterial infections don’t last years, especially in the absence of pain and swelling. Yes, there are nonbacterial infections (eg, those caused by acid-fast bacilli) that can be just as, if not more, indolent, but they too are apt to be painful and swollen.
Biopsy, the obvious missing step in this entire saga, would rule out any of these odd things (including melanoma, which has been known to present in this atypical morphologic manner). Other items in the differential include pyogenic granuloma and metastatic cancer (eg, breast, colon, lung).
In this case, biopsy confirmed the lesion was (as expected) a basal cell carcinoma. The lesion was excised in 2 stages over a 2.5-month period. And the patient was reminded that he will remain at extremely high risk for additional sun-caused skin cancers for the rest of his life.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be assumed to be skin cancer until proven otherwise, especially in sun-damaged individuals.
- Correct treatment is dictated by correct diagnosis; in this case, a biopsy was clearly indicated.
- Besides the more common cancers (eg, basal cell and squamous cell carcinoma), there were numerous other items in the differential, including melanoma and even metastatic cancer.
- Only rarely do skin infections smolder for years without pain or surrounding erythema.
This 80-year-old man has been complaining to health care providers about the asymptomatic lesion on his right forearm for at least 5 years—“maybe more,” he says. During that time, he has been given a variety of diagnoses, mostly “infection” of some sort, along with prescriptions for oral antibiotics (cephalexin, trimethoprim/sulfa), topical antibiotics (mupirocin, triple-antibiotic cream), and germicidal washes (povidone and others). None of these treatment attempts has achieved results.
Prior to now, there has been no referral to dermatology, nor has any provider suggested biopsy of the lesion. The patient has a history of skin cancer (basal cell or squamous cell carcinomas) on his face, back, and arms. These followed a lifetime of sun exposure due to his work in construction and roofing.

EXAMINATION
The lesion in question is located on the dorsal aspect of his mid right forearm. Measuring almost 4 cm in aggregate, the lesion is composed of 2 adjacent bright red half-moon friable nodules in a circular configuration. There is almost no erythema or edema in or around the lesion, which is neither warm nor tender to touch.
There are no palpable nodes in the adjacent epitrochlear or axillary nodal locations.
The surrounding skin of this arm, as well as that of the opposite arm, is quite thin, discolored, and scaly and is covered with stellate scars. Examination of all other sun-exposed areas reveals similar changes but no other notable lesions.
What’s the diagnosis?
DISCUSSION
It would be obvious to most readers that a biopsy is in order, given the likelihood that this lesion is either a basal cell or squamous cell carcinoma—an impression bolstered by the contextual findings of advanced sun damage and the history of skin cancer. And yet, this patient finds himself with a nonhealing friable lesion that is at least 5 years old.
Repeated misdiagnosis as infection is actually a common occurrence. One suspects that at least some of the providers making this mistake followed the patient’s lead: Many affected patients assume such lesions represent infection and therefore demand treatment appropriate for that diagnosis.
As a rule, however, bacterial infections don’t last years, especially in the absence of pain and swelling. Yes, there are nonbacterial infections (eg, those caused by acid-fast bacilli) that can be just as, if not more, indolent, but they too are apt to be painful and swollen.
Biopsy, the obvious missing step in this entire saga, would rule out any of these odd things (including melanoma, which has been known to present in this atypical morphologic manner). Other items in the differential include pyogenic granuloma and metastatic cancer (eg, breast, colon, lung).
In this case, biopsy confirmed the lesion was (as expected) a basal cell carcinoma. The lesion was excised in 2 stages over a 2.5-month period. And the patient was reminded that he will remain at extremely high risk for additional sun-caused skin cancers for the rest of his life.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be assumed to be skin cancer until proven otherwise, especially in sun-damaged individuals.
- Correct treatment is dictated by correct diagnosis; in this case, a biopsy was clearly indicated.
- Besides the more common cancers (eg, basal cell and squamous cell carcinoma), there were numerous other items in the differential, including melanoma and even metastatic cancer.
- Only rarely do skin infections smolder for years without pain or surrounding erythema.
This 80-year-old man has been complaining to health care providers about the asymptomatic lesion on his right forearm for at least 5 years—“maybe more,” he says. During that time, he has been given a variety of diagnoses, mostly “infection” of some sort, along with prescriptions for oral antibiotics (cephalexin, trimethoprim/sulfa), topical antibiotics (mupirocin, triple-antibiotic cream), and germicidal washes (povidone and others). None of these treatment attempts has achieved results.
Prior to now, there has been no referral to dermatology, nor has any provider suggested biopsy of the lesion. The patient has a history of skin cancer (basal cell or squamous cell carcinomas) on his face, back, and arms. These followed a lifetime of sun exposure due to his work in construction and roofing.

EXAMINATION
The lesion in question is located on the dorsal aspect of his mid right forearm. Measuring almost 4 cm in aggregate, the lesion is composed of 2 adjacent bright red half-moon friable nodules in a circular configuration. There is almost no erythema or edema in or around the lesion, which is neither warm nor tender to touch.
There are no palpable nodes in the adjacent epitrochlear or axillary nodal locations.
The surrounding skin of this arm, as well as that of the opposite arm, is quite thin, discolored, and scaly and is covered with stellate scars. Examination of all other sun-exposed areas reveals similar changes but no other notable lesions.
What’s the diagnosis?
DISCUSSION
It would be obvious to most readers that a biopsy is in order, given the likelihood that this lesion is either a basal cell or squamous cell carcinoma—an impression bolstered by the contextual findings of advanced sun damage and the history of skin cancer. And yet, this patient finds himself with a nonhealing friable lesion that is at least 5 years old.
Repeated misdiagnosis as infection is actually a common occurrence. One suspects that at least some of the providers making this mistake followed the patient’s lead: Many affected patients assume such lesions represent infection and therefore demand treatment appropriate for that diagnosis.
As a rule, however, bacterial infections don’t last years, especially in the absence of pain and swelling. Yes, there are nonbacterial infections (eg, those caused by acid-fast bacilli) that can be just as, if not more, indolent, but they too are apt to be painful and swollen.
Biopsy, the obvious missing step in this entire saga, would rule out any of these odd things (including melanoma, which has been known to present in this atypical morphologic manner). Other items in the differential include pyogenic granuloma and metastatic cancer (eg, breast, colon, lung).
In this case, biopsy confirmed the lesion was (as expected) a basal cell carcinoma. The lesion was excised in 2 stages over a 2.5-month period. And the patient was reminded that he will remain at extremely high risk for additional sun-caused skin cancers for the rest of his life.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be assumed to be skin cancer until proven otherwise, especially in sun-damaged individuals.
- Correct treatment is dictated by correct diagnosis; in this case, a biopsy was clearly indicated.
- Besides the more common cancers (eg, basal cell and squamous cell carcinoma), there were numerous other items in the differential, including melanoma and even metastatic cancer.
- Only rarely do skin infections smolder for years without pain or surrounding erythema.
Lesions around anus
The FP suspected invasive squamous cell carcinoma (SCC) related to human papillomavirus. He recognized that the patient was at a higher risk of this secondary to the HIV/AIDS diagnosis.
The FP recommended a shave biopsy (See the Watch & Learn video on “Shave biopsy.”) of the ulcerated lesion, and the patient consented to this procedure. The FP used a surgical marker to mark an area at the edge of the ulcer including some tissue outside of the ulcer. (This is recommended for biopsy of any ulcerated skin lesion.) He then injected 1% lidocaine with epinephrine into the edge of the ulcer for anesthesia and to minimize bleeding during the shave biopsy. The shave was performed and aluminum chloride, along with electrosurgery, was used to stop the bleeding.
The pathology showed an invasive SCC. Due to the size and location of the lesions, the patient was referred to Colorectal Surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected invasive squamous cell carcinoma (SCC) related to human papillomavirus. He recognized that the patient was at a higher risk of this secondary to the HIV/AIDS diagnosis.
The FP recommended a shave biopsy (See the Watch & Learn video on “Shave biopsy.”) of the ulcerated lesion, and the patient consented to this procedure. The FP used a surgical marker to mark an area at the edge of the ulcer including some tissue outside of the ulcer. (This is recommended for biopsy of any ulcerated skin lesion.) He then injected 1% lidocaine with epinephrine into the edge of the ulcer for anesthesia and to minimize bleeding during the shave biopsy. The shave was performed and aluminum chloride, along with electrosurgery, was used to stop the bleeding.
The pathology showed an invasive SCC. Due to the size and location of the lesions, the patient was referred to Colorectal Surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected invasive squamous cell carcinoma (SCC) related to human papillomavirus. He recognized that the patient was at a higher risk of this secondary to the HIV/AIDS diagnosis.
The FP recommended a shave biopsy (See the Watch & Learn video on “Shave biopsy.”) of the ulcerated lesion, and the patient consented to this procedure. The FP used a surgical marker to mark an area at the edge of the ulcer including some tissue outside of the ulcer. (This is recommended for biopsy of any ulcerated skin lesion.) He then injected 1% lidocaine with epinephrine into the edge of the ulcer for anesthesia and to minimize bleeding during the shave biopsy. The shave was performed and aluminum chloride, along with electrosurgery, was used to stop the bleeding.
The pathology showed an invasive SCC. Due to the size and location of the lesions, the patient was referred to Colorectal Surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Warmth and moisture help keep preterm neonates’ skin healthy
The skin of premature infants is very fragile and can take up to 4 weeks to become cornified. Until then, it’s apt to rapidly lose water and heat, putting babies at risk of hypothermia, dehydration, and electrolyte imbalances, Ayan Kusari and his colleagues wrote in Pediatric Dermatology.
The team examined evidence-based skin care in these tiny patients, extracting recommendations from a meta-analysis of 68 studies.
“There are a number of unifying features that distinguish preterm skin from term skin,” wrote Mr. Kusari, a clinical research associate at the Rady Children’s Hospital–San Diego, and his associates. “Preterm skin is thinner, making preterm neonates more susceptible to skin infections and caustic agents. The vernix caseosa is typically thicker in preterm neonates [though thinner in extremely preterm neonates]. Accordingly, there are a number of general principles that can guide skin care for most preterm neonates.”
Bathing
The team identified eight studies of bathing preterm neonates and concluded that a daily bath isn’t necessary.
“Colonization by pathogenic bacterial strains, size of the total bacterial population, and incidence of skin infection do not vary between preterm infants bathed every 2 days and preterm infants bathed every 4 days in all studies,” the authors wrote.
These less frequent baths appear to decrease the risk of temperature variability, and tub baths are preferable to sponge baths. “In sponge bathing, wet skin is more exposed to ambient air, which is typically colder than body temperature. Physiological and behavioral parameters in preterm infants are often disrupted during sponge bathing. In contrast, tub bathing results in less variability in body temperature and warmer temperatures after bathing,” Mr. Kusari and his associates found.
However, premoistened baby wipes appeared beneficial, lowering skin pH, which might help “facilitate acid mantle development, infection control, and barrier repair,” they wrote.
Emollients
Seven studies and one meta-analysis examined the use of emollients in preterm infants; there was agreement that emollients do improve skin condition. Plant-based emollients appeared superior to petrolatum-based products.
“In developing countries where oil massage of infants and children is traditional, there appears to be a clear benefit to massage with some oils. In developed countries, research has emphasized petrolatum-based creams and ointments, whose benefits are tempered by the increased risk of serious infections with some products,” Mr. Kusari and his colleagues wrote.
Sunflower seed oil was particularly beneficial in studies carried out in developing countries. A mixture of 70% lanolin and 30% olive oil proved better than olive oil alone. Coconut oil also displayed positive impact on skin condition.
“In contrast, multiple studies show an increased risk of sepsis with the application of petrolatum ointment to preterm neonates,” they noted.
In one study, following the adoption of a new skin care protocol involving regular application of petrolatum‐based ointments for extremely low-birth-weight neonates, researchers in Texas observed a significant, 200% increase in the incidence of systemic candidiasis. A study in Saudi Arabia replicated this finding. The largest study of a petrolatum-based ointment on premature babies was conducted in Vermont and found a statistically significant increase in infection with coagulase-negative staphylococcus (CoNS). “This ... study appears to be the driving force in a Cochrane Database meta-analysis, which concludes that topical emollients are associated with increased CoNS infection in preterm neonates,” the authors wrote.
Temperature regulation
It’s notoriously tough to maintain core temperature in preterm newborns. Six studies in the meta-analysis tackled this issue using impermeable plastic wraps or garments after birth and semipermeable barriers in the weeks after.
“Plastic wraps or bags can help neonates to retain their body heat, and greater skin coverage with plastic devices appears to be associated with a better outcome. In infants less than 28 weeks’ gestational age, the use of polyethylene occlusive wraps prevents heat loss after delivery and results in higher NICU admission temperatures and a lower incidence of hypothermia,” Mr Kusari and his associates wrote.
Semipermeable wraps can be used for an extended period after birth to reduce transepidermal water loss. Seven studies examined this technique, using both adhesive and nonadhesive polyurethane dressings.
“These studies show that semipermeable adhesive membranes decrease water loss, reduce skin breakdown, and decrease erythema while applied, but may strip superficial skin layers when they are removed, leading to a transient post-removal increase in transepidermal water loss. Furthermore, due to their semipermeable design, application of these adhesive membranes does not appear to decrease fluid requirement or affect electrolyte status in preterm neonates; however, skin barrier function is disrupted following removal of plastic tape, with increased transepidermal water loss at sites of tape removal,” the investigators wrote.
Pectin-based dressings and those containing hydrocolloid or acrylate can damage preterm neonatal skin by inflicting medical adhesive-related skin injury, the team wrote; this can involve epidermal stripping, tension injury, shearing, maceration, folliculitis, or contact dermatitis.
Skin sterilization
There’s little consensus when it comes to sterilization choices for preterm neonatal skin about to undergo a venipuncture or other procedure. Popular methods are povidone-iodine and chlorhexidine, with gestational age affecting choice. Iodine-based antiseptics have been associated with thyroid disruption and chlorhexidine with chemical burns.
“Some studies suggest 0.2% chlorhexidine gluconate may be an attractive alternative to povidone-iodine for the very and extremely preterm,” the authors wrote. One study they examined compared chlorhexidine gluconate 0.2% and 0.5% in extremely preterm infants, showing a significant decrease in skin irritation in the lower-concentration group.
But a randomized trial following this finding, which compared 0.2% chlorhexidine gluconate with 10% aqueous povidone-iodine, found no differences in any infection outcome or skin irritation, but there was more thyroid suppression in the povidone-iodine group.
More research is needed, the team concluded.
Cord care
Tincture of time may be the best alternative here.
The investigators examined a meta-analysis of 21 umbilical cord care studies and found that cleaning the cord with antiseptic prolonged the time to cord separation, compared with simple air drying.
“Interestingly, one study does suggest that one-time cleansing with chlorhexidine reduces neonatal mortality when compared to dry cord care; however, most of the existing evidence suggests that antiseptic treatment does not offer a benefit over dry cord care,” they wrote.
“Further studies, particularly in the very preterm and extremely preterm neonates, with an emphasis placed on subclassifying the preterm patient population based on gestational age, are needed to further examine and validate the real‐world utility of these interventions,” Mr. Kusari and his associates concluded. “In the meantime, it may be useful to establish practice guidelines based on the evidence we have presented here.”
The authors reported no relevant financial disclosures.
SOURCE: Kusari A et al. Pediatr Dermatol. 2018 Dec 12. doi: 10.1111/pde.13725.
The skin of premature infants is very fragile and can take up to 4 weeks to become cornified. Until then, it’s apt to rapidly lose water and heat, putting babies at risk of hypothermia, dehydration, and electrolyte imbalances, Ayan Kusari and his colleagues wrote in Pediatric Dermatology.
The team examined evidence-based skin care in these tiny patients, extracting recommendations from a meta-analysis of 68 studies.
“There are a number of unifying features that distinguish preterm skin from term skin,” wrote Mr. Kusari, a clinical research associate at the Rady Children’s Hospital–San Diego, and his associates. “Preterm skin is thinner, making preterm neonates more susceptible to skin infections and caustic agents. The vernix caseosa is typically thicker in preterm neonates [though thinner in extremely preterm neonates]. Accordingly, there are a number of general principles that can guide skin care for most preterm neonates.”
Bathing
The team identified eight studies of bathing preterm neonates and concluded that a daily bath isn’t necessary.
“Colonization by pathogenic bacterial strains, size of the total bacterial population, and incidence of skin infection do not vary between preterm infants bathed every 2 days and preterm infants bathed every 4 days in all studies,” the authors wrote.
These less frequent baths appear to decrease the risk of temperature variability, and tub baths are preferable to sponge baths. “In sponge bathing, wet skin is more exposed to ambient air, which is typically colder than body temperature. Physiological and behavioral parameters in preterm infants are often disrupted during sponge bathing. In contrast, tub bathing results in less variability in body temperature and warmer temperatures after bathing,” Mr. Kusari and his associates found.
However, premoistened baby wipes appeared beneficial, lowering skin pH, which might help “facilitate acid mantle development, infection control, and barrier repair,” they wrote.
Emollients
Seven studies and one meta-analysis examined the use of emollients in preterm infants; there was agreement that emollients do improve skin condition. Plant-based emollients appeared superior to petrolatum-based products.
“In developing countries where oil massage of infants and children is traditional, there appears to be a clear benefit to massage with some oils. In developed countries, research has emphasized petrolatum-based creams and ointments, whose benefits are tempered by the increased risk of serious infections with some products,” Mr. Kusari and his colleagues wrote.
Sunflower seed oil was particularly beneficial in studies carried out in developing countries. A mixture of 70% lanolin and 30% olive oil proved better than olive oil alone. Coconut oil also displayed positive impact on skin condition.
“In contrast, multiple studies show an increased risk of sepsis with the application of petrolatum ointment to preterm neonates,” they noted.
In one study, following the adoption of a new skin care protocol involving regular application of petrolatum‐based ointments for extremely low-birth-weight neonates, researchers in Texas observed a significant, 200% increase in the incidence of systemic candidiasis. A study in Saudi Arabia replicated this finding. The largest study of a petrolatum-based ointment on premature babies was conducted in Vermont and found a statistically significant increase in infection with coagulase-negative staphylococcus (CoNS). “This ... study appears to be the driving force in a Cochrane Database meta-analysis, which concludes that topical emollients are associated with increased CoNS infection in preterm neonates,” the authors wrote.
Temperature regulation
It’s notoriously tough to maintain core temperature in preterm newborns. Six studies in the meta-analysis tackled this issue using impermeable plastic wraps or garments after birth and semipermeable barriers in the weeks after.
“Plastic wraps or bags can help neonates to retain their body heat, and greater skin coverage with plastic devices appears to be associated with a better outcome. In infants less than 28 weeks’ gestational age, the use of polyethylene occlusive wraps prevents heat loss after delivery and results in higher NICU admission temperatures and a lower incidence of hypothermia,” Mr Kusari and his associates wrote.
Semipermeable wraps can be used for an extended period after birth to reduce transepidermal water loss. Seven studies examined this technique, using both adhesive and nonadhesive polyurethane dressings.
“These studies show that semipermeable adhesive membranes decrease water loss, reduce skin breakdown, and decrease erythema while applied, but may strip superficial skin layers when they are removed, leading to a transient post-removal increase in transepidermal water loss. Furthermore, due to their semipermeable design, application of these adhesive membranes does not appear to decrease fluid requirement or affect electrolyte status in preterm neonates; however, skin barrier function is disrupted following removal of plastic tape, with increased transepidermal water loss at sites of tape removal,” the investigators wrote.
Pectin-based dressings and those containing hydrocolloid or acrylate can damage preterm neonatal skin by inflicting medical adhesive-related skin injury, the team wrote; this can involve epidermal stripping, tension injury, shearing, maceration, folliculitis, or contact dermatitis.
Skin sterilization
There’s little consensus when it comes to sterilization choices for preterm neonatal skin about to undergo a venipuncture or other procedure. Popular methods are povidone-iodine and chlorhexidine, with gestational age affecting choice. Iodine-based antiseptics have been associated with thyroid disruption and chlorhexidine with chemical burns.
“Some studies suggest 0.2% chlorhexidine gluconate may be an attractive alternative to povidone-iodine for the very and extremely preterm,” the authors wrote. One study they examined compared chlorhexidine gluconate 0.2% and 0.5% in extremely preterm infants, showing a significant decrease in skin irritation in the lower-concentration group.
But a randomized trial following this finding, which compared 0.2% chlorhexidine gluconate with 10% aqueous povidone-iodine, found no differences in any infection outcome or skin irritation, but there was more thyroid suppression in the povidone-iodine group.
More research is needed, the team concluded.
Cord care
Tincture of time may be the best alternative here.
The investigators examined a meta-analysis of 21 umbilical cord care studies and found that cleaning the cord with antiseptic prolonged the time to cord separation, compared with simple air drying.
“Interestingly, one study does suggest that one-time cleansing with chlorhexidine reduces neonatal mortality when compared to dry cord care; however, most of the existing evidence suggests that antiseptic treatment does not offer a benefit over dry cord care,” they wrote.
“Further studies, particularly in the very preterm and extremely preterm neonates, with an emphasis placed on subclassifying the preterm patient population based on gestational age, are needed to further examine and validate the real‐world utility of these interventions,” Mr. Kusari and his associates concluded. “In the meantime, it may be useful to establish practice guidelines based on the evidence we have presented here.”
The authors reported no relevant financial disclosures.
SOURCE: Kusari A et al. Pediatr Dermatol. 2018 Dec 12. doi: 10.1111/pde.13725.
The skin of premature infants is very fragile and can take up to 4 weeks to become cornified. Until then, it’s apt to rapidly lose water and heat, putting babies at risk of hypothermia, dehydration, and electrolyte imbalances, Ayan Kusari and his colleagues wrote in Pediatric Dermatology.
The team examined evidence-based skin care in these tiny patients, extracting recommendations from a meta-analysis of 68 studies.
“There are a number of unifying features that distinguish preterm skin from term skin,” wrote Mr. Kusari, a clinical research associate at the Rady Children’s Hospital–San Diego, and his associates. “Preterm skin is thinner, making preterm neonates more susceptible to skin infections and caustic agents. The vernix caseosa is typically thicker in preterm neonates [though thinner in extremely preterm neonates]. Accordingly, there are a number of general principles that can guide skin care for most preterm neonates.”
Bathing
The team identified eight studies of bathing preterm neonates and concluded that a daily bath isn’t necessary.
“Colonization by pathogenic bacterial strains, size of the total bacterial population, and incidence of skin infection do not vary between preterm infants bathed every 2 days and preterm infants bathed every 4 days in all studies,” the authors wrote.
These less frequent baths appear to decrease the risk of temperature variability, and tub baths are preferable to sponge baths. “In sponge bathing, wet skin is more exposed to ambient air, which is typically colder than body temperature. Physiological and behavioral parameters in preterm infants are often disrupted during sponge bathing. In contrast, tub bathing results in less variability in body temperature and warmer temperatures after bathing,” Mr. Kusari and his associates found.
However, premoistened baby wipes appeared beneficial, lowering skin pH, which might help “facilitate acid mantle development, infection control, and barrier repair,” they wrote.
Emollients
Seven studies and one meta-analysis examined the use of emollients in preterm infants; there was agreement that emollients do improve skin condition. Plant-based emollients appeared superior to petrolatum-based products.
“In developing countries where oil massage of infants and children is traditional, there appears to be a clear benefit to massage with some oils. In developed countries, research has emphasized petrolatum-based creams and ointments, whose benefits are tempered by the increased risk of serious infections with some products,” Mr. Kusari and his colleagues wrote.
Sunflower seed oil was particularly beneficial in studies carried out in developing countries. A mixture of 70% lanolin and 30% olive oil proved better than olive oil alone. Coconut oil also displayed positive impact on skin condition.
“In contrast, multiple studies show an increased risk of sepsis with the application of petrolatum ointment to preterm neonates,” they noted.
In one study, following the adoption of a new skin care protocol involving regular application of petrolatum‐based ointments for extremely low-birth-weight neonates, researchers in Texas observed a significant, 200% increase in the incidence of systemic candidiasis. A study in Saudi Arabia replicated this finding. The largest study of a petrolatum-based ointment on premature babies was conducted in Vermont and found a statistically significant increase in infection with coagulase-negative staphylococcus (CoNS). “This ... study appears to be the driving force in a Cochrane Database meta-analysis, which concludes that topical emollients are associated with increased CoNS infection in preterm neonates,” the authors wrote.
Temperature regulation
It’s notoriously tough to maintain core temperature in preterm newborns. Six studies in the meta-analysis tackled this issue using impermeable plastic wraps or garments after birth and semipermeable barriers in the weeks after.
“Plastic wraps or bags can help neonates to retain their body heat, and greater skin coverage with plastic devices appears to be associated with a better outcome. In infants less than 28 weeks’ gestational age, the use of polyethylene occlusive wraps prevents heat loss after delivery and results in higher NICU admission temperatures and a lower incidence of hypothermia,” Mr Kusari and his associates wrote.
Semipermeable wraps can be used for an extended period after birth to reduce transepidermal water loss. Seven studies examined this technique, using both adhesive and nonadhesive polyurethane dressings.
“These studies show that semipermeable adhesive membranes decrease water loss, reduce skin breakdown, and decrease erythema while applied, but may strip superficial skin layers when they are removed, leading to a transient post-removal increase in transepidermal water loss. Furthermore, due to their semipermeable design, application of these adhesive membranes does not appear to decrease fluid requirement or affect electrolyte status in preterm neonates; however, skin barrier function is disrupted following removal of plastic tape, with increased transepidermal water loss at sites of tape removal,” the investigators wrote.
Pectin-based dressings and those containing hydrocolloid or acrylate can damage preterm neonatal skin by inflicting medical adhesive-related skin injury, the team wrote; this can involve epidermal stripping, tension injury, shearing, maceration, folliculitis, or contact dermatitis.
Skin sterilization
There’s little consensus when it comes to sterilization choices for preterm neonatal skin about to undergo a venipuncture or other procedure. Popular methods are povidone-iodine and chlorhexidine, with gestational age affecting choice. Iodine-based antiseptics have been associated with thyroid disruption and chlorhexidine with chemical burns.
“Some studies suggest 0.2% chlorhexidine gluconate may be an attractive alternative to povidone-iodine for the very and extremely preterm,” the authors wrote. One study they examined compared chlorhexidine gluconate 0.2% and 0.5% in extremely preterm infants, showing a significant decrease in skin irritation in the lower-concentration group.
But a randomized trial following this finding, which compared 0.2% chlorhexidine gluconate with 10% aqueous povidone-iodine, found no differences in any infection outcome or skin irritation, but there was more thyroid suppression in the povidone-iodine group.
More research is needed, the team concluded.
Cord care
Tincture of time may be the best alternative here.
The investigators examined a meta-analysis of 21 umbilical cord care studies and found that cleaning the cord with antiseptic prolonged the time to cord separation, compared with simple air drying.
“Interestingly, one study does suggest that one-time cleansing with chlorhexidine reduces neonatal mortality when compared to dry cord care; however, most of the existing evidence suggests that antiseptic treatment does not offer a benefit over dry cord care,” they wrote.
“Further studies, particularly in the very preterm and extremely preterm neonates, with an emphasis placed on subclassifying the preterm patient population based on gestational age, are needed to further examine and validate the real‐world utility of these interventions,” Mr. Kusari and his associates concluded. “In the meantime, it may be useful to establish practice guidelines based on the evidence we have presented here.”
The authors reported no relevant financial disclosures.
SOURCE: Kusari A et al. Pediatr Dermatol. 2018 Dec 12. doi: 10.1111/pde.13725.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: Limiting baths, using plant-based emollients, and using plastic wraps benefit preterm neonates’ skin early in life.
Major finding: The team identified eight studies of bathing preterm neonates and concluded that a daily bath isn’t necessary.
Study details: A meta-analysis of 68 studies.
Disclosures: The authors reported no relevant financial disclosures.
Source: Kusari A et al. Pediatr Dermatol. 2018 Dec 12. doi: 10.1111/pde.13725.
AAP infantile hemangioma guideline should empower primary care clinicians
given the dramatic increase in information available over the past decade. 
The aim in providing an evidence-based approach to evaluating, triaging, and managing IH cases is to arm primary care providers with the confidence needed to successfully treat high-risk cases, reported Daniel P. Krowchuk, MD, of the department of pediatrics and dermatology, Wake Forest University, Winston-Salem, N.C., and his associates who are members of the AAP subcommittee on the management of IHs.
With an occurrence rate of 4%-5%, IHs are the most common benign tumor presenting in childhood, especially occurring in girls, twins, preterm or low-birth-weight infants, and white neonates.
The AAP’s guideline “provides a framework for clinical decision-making” – it should not be considered a sole source of guidance. It also should not be used to replace clinical judgment or as a protocol for managing all patients with IHs, explained Dr. Krowchuk and his associates.
Clinicians, especially, are encouraged to consult promptly with a hemangioma specialist if they are not experienced in managing IHs.
According to one study cited by the authors, the mean age of examination by a dermatologist is 5 months, when most growth has already been completed. Lesions are first noticed, on average, at 2 weeks; 4 weeks has been recommended as the ideal time for professional consultation. It is important for clinicians to recognize the difficulty families are likely to face in obtaining an appointment, which makes caregiver and clinician advocacy on behalf of infants affected critical, urged Dr. Krowchuk and his colleagues. In cases or locations where hemangioma specialists are in short supply, telemedicine triage or photographic consultation is especially helpful.
Dr. Krowchuk and his associates noted several possible challenges in implementing this clinical practice guideline (CPG) published in Pediatrics. The growth of individual IHs is difficult to predict, especially in young infants, and there are no markers or imaging studies to correct this challenge. For this reason, they advised: “Prompt evaluation, either in-person or via photographs, is warranted for any infant reported by parents to have a changing birthmark during the first 2 months of life.”
Wide heterogeneity in terms of size, location, patterns, of distribution, and depth, when coupled with unpredictable growth, makes management of IHs unpredictable. Thus, there can be no one-size-fits-all treatment approach.
Further complicating implementation of the CPG is the long-held myth that IHs are benign and resolve spontaneously. While this may accurately describe the vast majority of outcomes, “ample evidence” demonstrates what can happen when family and/or caregivers yield to such “false reassurance.” According to Dr. Krowchuk and his associates, hemangioma specialists have seen their share of “examples of lost opportunities to intervene and prevent poor outcomes because of lack of or delayed referral.”
The paucity of data on high-risk cases in primary care and referral care settings should be the subject of future research, the authors noted. Scorings systems, such as the Hemangioma Severity Score, are growing in popularity as a triage tool, but more research is needed to demonstrate that primary care physicians are accurately interpreting findings and that high-risk cases are accurately identified to avoid over-referral to specialists.
Dr. Krowchuk and his colleagues did call attention to important evidence gaps that may be answered by research currently underway, or that may require further research in the future by asking the following questions: How safe is treatment with topical timolol in early infancy, and what proportion of patients can be observed without referral? For healthy infants 5 weeks or older, to what extent, if any, is cardiovascular monitoring for propranolol necessary? How should pediatricians be involved in beta-blocker management of infants and when should specialty reevaluation be made? What is the accuracy of primary care identification of high-risk IH cases using many of the parameters offered within this CPG? Are pediatric trainees being sufficiently trained in stratifying and managing IH risk?
One noteworthy barrier to improved management and outcomes noted by the authors is the “imprecision of current diagnostic codes.” At present, the existing coding in the International Classification of Diseases, 10th Revision does not include specific reference to IH but rather describes “hemangioma of the skin and subcutaneous tissues” and can include congenital as well as verrucous hemangioma. The codes also do not address the details characteristic of IHs or the higher risk aspects of IH, such as location or multifocality. Advocacy, in this instance, would be appropriate, advised Dr. Krowchuk and his associates.
In an interview, Dr. Krowchuk provided additional insight into what sets the AAP’s CPG apart from consensus statements published previously by European and Australasian expert groups. Although these might appear to be similar documents with analogous content at first glance, there are important differences, he said.
The consensus statements were based on expert opinion, while “the academy’s CPG was founded on an extensive review of the medical literature (1982-2017) regarding the potential benefits and harms of diagnostic modalities and pharmacologic and surgical treatments,” Dr. Krowchuk explained. The information that came out of this extensive review is what members of the subcommittee used to develop key action statements that pediatricians can use to evaluate and manage infants with IHs.
“The scope of the consensus statements was more limited, focusing primarily on the treatment of IH with propranolol. While the benefits of propranolol, its use and dosing, and potential adverse effects were addressed in depth in the academy’s CPG, the document went well beyond this,” he clarified.
The AAP also previously published a clinical report that provides a comprehensive evaluation of the pathogenesis, clinical features, and treatment of IH (Pediatrics. 2015 Oct. doi: 10.1542/peds.2015-2485).
There was no external funding for the CPG, and the authors said there were no potential conflicts of interest. Ilona J. Frieden, MD, is a member of the data monitoring safety board for Pfizer and the scientific advisory board for Venthera/Bridge Bio; Anthony J. Mancini, MD, said he has advisory board relationships with Verrica, Valeant, and Pfizer.
SOURCE: Krowchuk, DP et al. Pediatrics 2019;143(1):e20183475.
given the dramatic increase in information available over the past decade. 
The aim in providing an evidence-based approach to evaluating, triaging, and managing IH cases is to arm primary care providers with the confidence needed to successfully treat high-risk cases, reported Daniel P. Krowchuk, MD, of the department of pediatrics and dermatology, Wake Forest University, Winston-Salem, N.C., and his associates who are members of the AAP subcommittee on the management of IHs.
With an occurrence rate of 4%-5%, IHs are the most common benign tumor presenting in childhood, especially occurring in girls, twins, preterm or low-birth-weight infants, and white neonates.
The AAP’s guideline “provides a framework for clinical decision-making” – it should not be considered a sole source of guidance. It also should not be used to replace clinical judgment or as a protocol for managing all patients with IHs, explained Dr. Krowchuk and his associates.
Clinicians, especially, are encouraged to consult promptly with a hemangioma specialist if they are not experienced in managing IHs.
According to one study cited by the authors, the mean age of examination by a dermatologist is 5 months, when most growth has already been completed. Lesions are first noticed, on average, at 2 weeks; 4 weeks has been recommended as the ideal time for professional consultation. It is important for clinicians to recognize the difficulty families are likely to face in obtaining an appointment, which makes caregiver and clinician advocacy on behalf of infants affected critical, urged Dr. Krowchuk and his colleagues. In cases or locations where hemangioma specialists are in short supply, telemedicine triage or photographic consultation is especially helpful.
Dr. Krowchuk and his associates noted several possible challenges in implementing this clinical practice guideline (CPG) published in Pediatrics. The growth of individual IHs is difficult to predict, especially in young infants, and there are no markers or imaging studies to correct this challenge. For this reason, they advised: “Prompt evaluation, either in-person or via photographs, is warranted for any infant reported by parents to have a changing birthmark during the first 2 months of life.”
Wide heterogeneity in terms of size, location, patterns, of distribution, and depth, when coupled with unpredictable growth, makes management of IHs unpredictable. Thus, there can be no one-size-fits-all treatment approach.
Further complicating implementation of the CPG is the long-held myth that IHs are benign and resolve spontaneously. While this may accurately describe the vast majority of outcomes, “ample evidence” demonstrates what can happen when family and/or caregivers yield to such “false reassurance.” According to Dr. Krowchuk and his associates, hemangioma specialists have seen their share of “examples of lost opportunities to intervene and prevent poor outcomes because of lack of or delayed referral.”
The paucity of data on high-risk cases in primary care and referral care settings should be the subject of future research, the authors noted. Scorings systems, such as the Hemangioma Severity Score, are growing in popularity as a triage tool, but more research is needed to demonstrate that primary care physicians are accurately interpreting findings and that high-risk cases are accurately identified to avoid over-referral to specialists.
Dr. Krowchuk and his colleagues did call attention to important evidence gaps that may be answered by research currently underway, or that may require further research in the future by asking the following questions: How safe is treatment with topical timolol in early infancy, and what proportion of patients can be observed without referral? For healthy infants 5 weeks or older, to what extent, if any, is cardiovascular monitoring for propranolol necessary? How should pediatricians be involved in beta-blocker management of infants and when should specialty reevaluation be made? What is the accuracy of primary care identification of high-risk IH cases using many of the parameters offered within this CPG? Are pediatric trainees being sufficiently trained in stratifying and managing IH risk?
One noteworthy barrier to improved management and outcomes noted by the authors is the “imprecision of current diagnostic codes.” At present, the existing coding in the International Classification of Diseases, 10th Revision does not include specific reference to IH but rather describes “hemangioma of the skin and subcutaneous tissues” and can include congenital as well as verrucous hemangioma. The codes also do not address the details characteristic of IHs or the higher risk aspects of IH, such as location or multifocality. Advocacy, in this instance, would be appropriate, advised Dr. Krowchuk and his associates.
In an interview, Dr. Krowchuk provided additional insight into what sets the AAP’s CPG apart from consensus statements published previously by European and Australasian expert groups. Although these might appear to be similar documents with analogous content at first glance, there are important differences, he said.
The consensus statements were based on expert opinion, while “the academy’s CPG was founded on an extensive review of the medical literature (1982-2017) regarding the potential benefits and harms of diagnostic modalities and pharmacologic and surgical treatments,” Dr. Krowchuk explained. The information that came out of this extensive review is what members of the subcommittee used to develop key action statements that pediatricians can use to evaluate and manage infants with IHs.
“The scope of the consensus statements was more limited, focusing primarily on the treatment of IH with propranolol. While the benefits of propranolol, its use and dosing, and potential adverse effects were addressed in depth in the academy’s CPG, the document went well beyond this,” he clarified.
The AAP also previously published a clinical report that provides a comprehensive evaluation of the pathogenesis, clinical features, and treatment of IH (Pediatrics. 2015 Oct. doi: 10.1542/peds.2015-2485).
There was no external funding for the CPG, and the authors said there were no potential conflicts of interest. Ilona J. Frieden, MD, is a member of the data monitoring safety board for Pfizer and the scientific advisory board for Venthera/Bridge Bio; Anthony J. Mancini, MD, said he has advisory board relationships with Verrica, Valeant, and Pfizer.
SOURCE: Krowchuk, DP et al. Pediatrics 2019;143(1):e20183475.
given the dramatic increase in information available over the past decade. 
The aim in providing an evidence-based approach to evaluating, triaging, and managing IH cases is to arm primary care providers with the confidence needed to successfully treat high-risk cases, reported Daniel P. Krowchuk, MD, of the department of pediatrics and dermatology, Wake Forest University, Winston-Salem, N.C., and his associates who are members of the AAP subcommittee on the management of IHs.
With an occurrence rate of 4%-5%, IHs are the most common benign tumor presenting in childhood, especially occurring in girls, twins, preterm or low-birth-weight infants, and white neonates.
The AAP’s guideline “provides a framework for clinical decision-making” – it should not be considered a sole source of guidance. It also should not be used to replace clinical judgment or as a protocol for managing all patients with IHs, explained Dr. Krowchuk and his associates.
Clinicians, especially, are encouraged to consult promptly with a hemangioma specialist if they are not experienced in managing IHs.
According to one study cited by the authors, the mean age of examination by a dermatologist is 5 months, when most growth has already been completed. Lesions are first noticed, on average, at 2 weeks; 4 weeks has been recommended as the ideal time for professional consultation. It is important for clinicians to recognize the difficulty families are likely to face in obtaining an appointment, which makes caregiver and clinician advocacy on behalf of infants affected critical, urged Dr. Krowchuk and his colleagues. In cases or locations where hemangioma specialists are in short supply, telemedicine triage or photographic consultation is especially helpful.
Dr. Krowchuk and his associates noted several possible challenges in implementing this clinical practice guideline (CPG) published in Pediatrics. The growth of individual IHs is difficult to predict, especially in young infants, and there are no markers or imaging studies to correct this challenge. For this reason, they advised: “Prompt evaluation, either in-person or via photographs, is warranted for any infant reported by parents to have a changing birthmark during the first 2 months of life.”
Wide heterogeneity in terms of size, location, patterns, of distribution, and depth, when coupled with unpredictable growth, makes management of IHs unpredictable. Thus, there can be no one-size-fits-all treatment approach.
Further complicating implementation of the CPG is the long-held myth that IHs are benign and resolve spontaneously. While this may accurately describe the vast majority of outcomes, “ample evidence” demonstrates what can happen when family and/or caregivers yield to such “false reassurance.” According to Dr. Krowchuk and his associates, hemangioma specialists have seen their share of “examples of lost opportunities to intervene and prevent poor outcomes because of lack of or delayed referral.”
The paucity of data on high-risk cases in primary care and referral care settings should be the subject of future research, the authors noted. Scorings systems, such as the Hemangioma Severity Score, are growing in popularity as a triage tool, but more research is needed to demonstrate that primary care physicians are accurately interpreting findings and that high-risk cases are accurately identified to avoid over-referral to specialists.
Dr. Krowchuk and his colleagues did call attention to important evidence gaps that may be answered by research currently underway, or that may require further research in the future by asking the following questions: How safe is treatment with topical timolol in early infancy, and what proportion of patients can be observed without referral? For healthy infants 5 weeks or older, to what extent, if any, is cardiovascular monitoring for propranolol necessary? How should pediatricians be involved in beta-blocker management of infants and when should specialty reevaluation be made? What is the accuracy of primary care identification of high-risk IH cases using many of the parameters offered within this CPG? Are pediatric trainees being sufficiently trained in stratifying and managing IH risk?
One noteworthy barrier to improved management and outcomes noted by the authors is the “imprecision of current diagnostic codes.” At present, the existing coding in the International Classification of Diseases, 10th Revision does not include specific reference to IH but rather describes “hemangioma of the skin and subcutaneous tissues” and can include congenital as well as verrucous hemangioma. The codes also do not address the details characteristic of IHs or the higher risk aspects of IH, such as location or multifocality. Advocacy, in this instance, would be appropriate, advised Dr. Krowchuk and his associates.
In an interview, Dr. Krowchuk provided additional insight into what sets the AAP’s CPG apart from consensus statements published previously by European and Australasian expert groups. Although these might appear to be similar documents with analogous content at first glance, there are important differences, he said.
The consensus statements were based on expert opinion, while “the academy’s CPG was founded on an extensive review of the medical literature (1982-2017) regarding the potential benefits and harms of diagnostic modalities and pharmacologic and surgical treatments,” Dr. Krowchuk explained. The information that came out of this extensive review is what members of the subcommittee used to develop key action statements that pediatricians can use to evaluate and manage infants with IHs.
“The scope of the consensus statements was more limited, focusing primarily on the treatment of IH with propranolol. While the benefits of propranolol, its use and dosing, and potential adverse effects were addressed in depth in the academy’s CPG, the document went well beyond this,” he clarified.
The AAP also previously published a clinical report that provides a comprehensive evaluation of the pathogenesis, clinical features, and treatment of IH (Pediatrics. 2015 Oct. doi: 10.1542/peds.2015-2485).
There was no external funding for the CPG, and the authors said there were no potential conflicts of interest. Ilona J. Frieden, MD, is a member of the data monitoring safety board for Pfizer and the scientific advisory board for Venthera/Bridge Bio; Anthony J. Mancini, MD, said he has advisory board relationships with Verrica, Valeant, and Pfizer.
SOURCE: Krowchuk, DP et al. Pediatrics 2019;143(1):e20183475.
FROM PEDIATRICS