User login
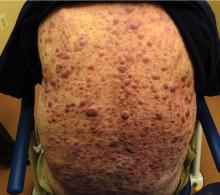
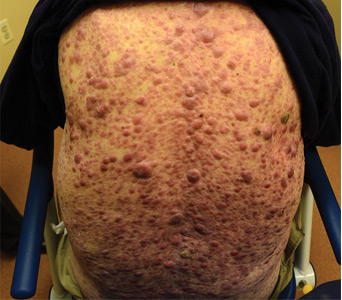
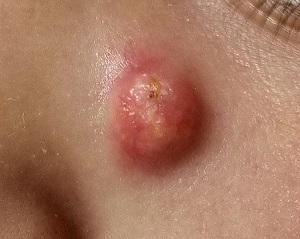
Aleukemic leukemia cutis
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
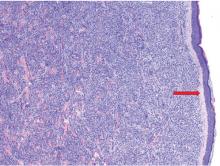
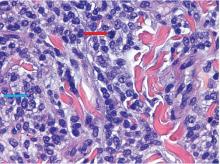
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
On examination, the numerous firm, indurated nodules ranged in size from 1 to 4 cm. There was no palpable lymphadenopathy.
Results of a peripheral blood cell count showed the following:
- Hemoglobin 12.5 g/dL (reference range 13.0–17.0)
- Platelet count 154 × 109/L (130–400)
- White blood cell count 5.0 × 109/L (4.0–11.0)
- Neutrophils 1.7 × 109/L (1.5–8.0)
- Lymphocytes 2.2 × 109/L (1.0–4.0)
- Monocytes 1.0 × 109/L (0.2–1.0)
- Eosinophils 0 (0–0.4)
- Basophils 0 (0–0.2)
- Blasts 0.
The findings were consistent with leukemic cells with monocytic differentiation. The infiltrate was unusual because leukemic infiltrates typically demonstrate a high nuclear-to-cytoplasmic ratio, but in this case the malignant cells had moderate amounts of cytoplasm due to the monocytic differentiation. Also, a grenz zone is more typically seen in B-cell lymphomas, and T cells more typically demonstrate epidermotropism.
Bone marrow aspiration was performed and revealed a hypercellular bone marrow with trilineage maturation with only 2% blasts. The fluorescence in situ hybridization testing for myelodysplastic syndrome and acute myeloid leukemia was normal. A diagnosis of aleukemic leukemia cutis was made.
After 2 months of chemotherapy with azacitidine, the nodules were less indurated. Treatment was briefly withdrawn due to the development of acute pneumonia, leading to a rapid progression of cutaneous involvement. Despite restarting chemotherapy, the patient died.
ALEUKEMIC LEUKEMIA CUTIS
The differential diagnosis of leukemia cutis is diverse and extensive. Patients often present with painless, firm, indurated nodules, papules, and plaques.1 The lesions can be small, involving a small amount of body surface area, but can also be very large and diffuse.
In our patient’s case, there were no new drugs or exposures to suggest a drug-related eruption, or pruritus or pain to suggest an inflammatory process. The rapid progression of the lesions suggested either an infectious or malignant process. The top 3 conditions in the differential diagnosis, based on his clinical presentation, were cutaneous T-cell lymphoma, cutaneous CD30+ anaplastic large-cell lymphoma, and a drug-induced cutaneous pseudolymphoma.
Skin biopsy is required to differentiate leukemia cutis from the other conditions. On skin biopsy study, leukemia cutis is characterized by infiltration of the skin by leukemic cells and is seen in 10% to 15% of patients with acute myeloid leukemia.2 In 5% of cases, leukemia cutis can present without bone marrow or peripheral signs of leukemia, hence the term aleukemic leukemia cutis.3 Cutaneous signs can occur before, after, or simultaneously with systemic leukemia.4
In the absence of systemic symptoms, the diagnosis is made when progressive cutaneous symptoms are present. The prognosis for aleukemic leukemia cutis is poor. Prompt diagnosis with skin biopsy is paramount to improve outcomes.
Acknowledgment: We would like to recognize Maanasa Devabhaktuni for her assistance in reporting this case.
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
- Yonal I, Hindilerden F, Coskun R, Dogan OI, Nalcaci M. Aleukemic leukemia cutis manifesting with disseminated nodular eruptions and a plaque preceding acute monocytic leukemia: a case report. Case Rep Oncol 2011; 4(3):547–554. doi:10.1159/000334745
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130–142. doi:10.1309/WYACYWF6NGM3WBRT
- Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci 2013; 28(4):614–619. doi:10.3346/jkms.2013.28.4.614
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J 2015; 21(8)pii:13030/qt6m18g35f. pmid:26437164
Mohs Micrographic Surgery in the VHA (FULL)
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
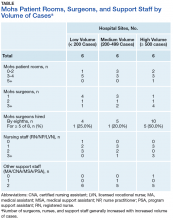
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
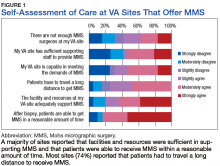
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
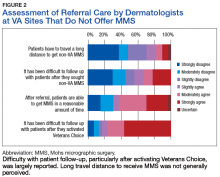
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
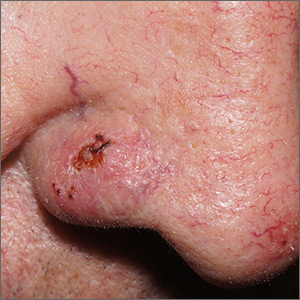
Sore on nose
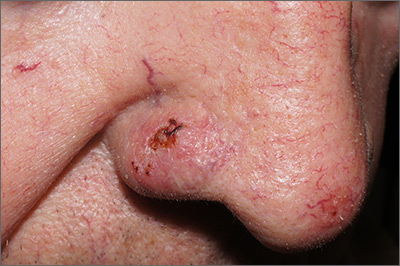
The FP suspected that it could be a skin cancer because any nonhealing lesion in sun-exposed areas could be skin cancer (especially basal cell carcinoma and squamous cell carcinoma).
He explained this to the patient and obtained written consent for a shave biopsy. He told the patient that the biopsy would likely leave an indentation in the involved area, but since treating the sore would likely require a second surgery, this indented area could be cut out and repaired with sutures. The patient indicated that he was more concerned about getting a proper diagnosis than he was about the appearance of the biopsy site.
The physician injected the area with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. (Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. See “Biopsies for skin cancer detection: Dispelling the myths.”) The shave biopsy was performed using a Dermablade. (See the Watch & Learn video on “Shave biopsy.”) The pathology report revealed squamous cell carcinoma. The patient was referred for Mohs surgery to get the best cure and cosmetic result.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that it could be a skin cancer because any nonhealing lesion in sun-exposed areas could be skin cancer (especially basal cell carcinoma and squamous cell carcinoma).
He explained this to the patient and obtained written consent for a shave biopsy. He told the patient that the biopsy would likely leave an indentation in the involved area, but since treating the sore would likely require a second surgery, this indented area could be cut out and repaired with sutures. The patient indicated that he was more concerned about getting a proper diagnosis than he was about the appearance of the biopsy site.
The physician injected the area with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. (Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. See “Biopsies for skin cancer detection: Dispelling the myths.”) The shave biopsy was performed using a Dermablade. (See the Watch & Learn video on “Shave biopsy.”) The pathology report revealed squamous cell carcinoma. The patient was referred for Mohs surgery to get the best cure and cosmetic result.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that it could be a skin cancer because any nonhealing lesion in sun-exposed areas could be skin cancer (especially basal cell carcinoma and squamous cell carcinoma).
He explained this to the patient and obtained written consent for a shave biopsy. He told the patient that the biopsy would likely leave an indentation in the involved area, but since treating the sore would likely require a second surgery, this indented area could be cut out and repaired with sutures. The patient indicated that he was more concerned about getting a proper diagnosis than he was about the appearance of the biopsy site.
The physician injected the area with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. (Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. See “Biopsies for skin cancer detection: Dispelling the myths.”) The shave biopsy was performed using a Dermablade. (See the Watch & Learn video on “Shave biopsy.”) The pathology report revealed squamous cell carcinoma. The patient was referred for Mohs surgery to get the best cure and cosmetic result.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
A Lesion Hits Its Growth Spurt
When she was 3 years old, a lesion appeared on this child’s face. It was small and caused little to no concern for several years. The child is now 9, and about a year ago, the lesion began to enlarge, ultimately reaching its present size.
First thought to be a pimple, the lesion was later deemed to be “cystic in nature” by another provider. By that point, however, the lesion was quite prominent—to the extent that it intrudes into the patient’s visual field. Perhaps more significantly for someone her age, it has prompted looks and comments that make her uncomfortable.
Fortunately, the lesion causes no pain or physical discomfort, and no other lesions have manifested. The child’s health is generally excellent.
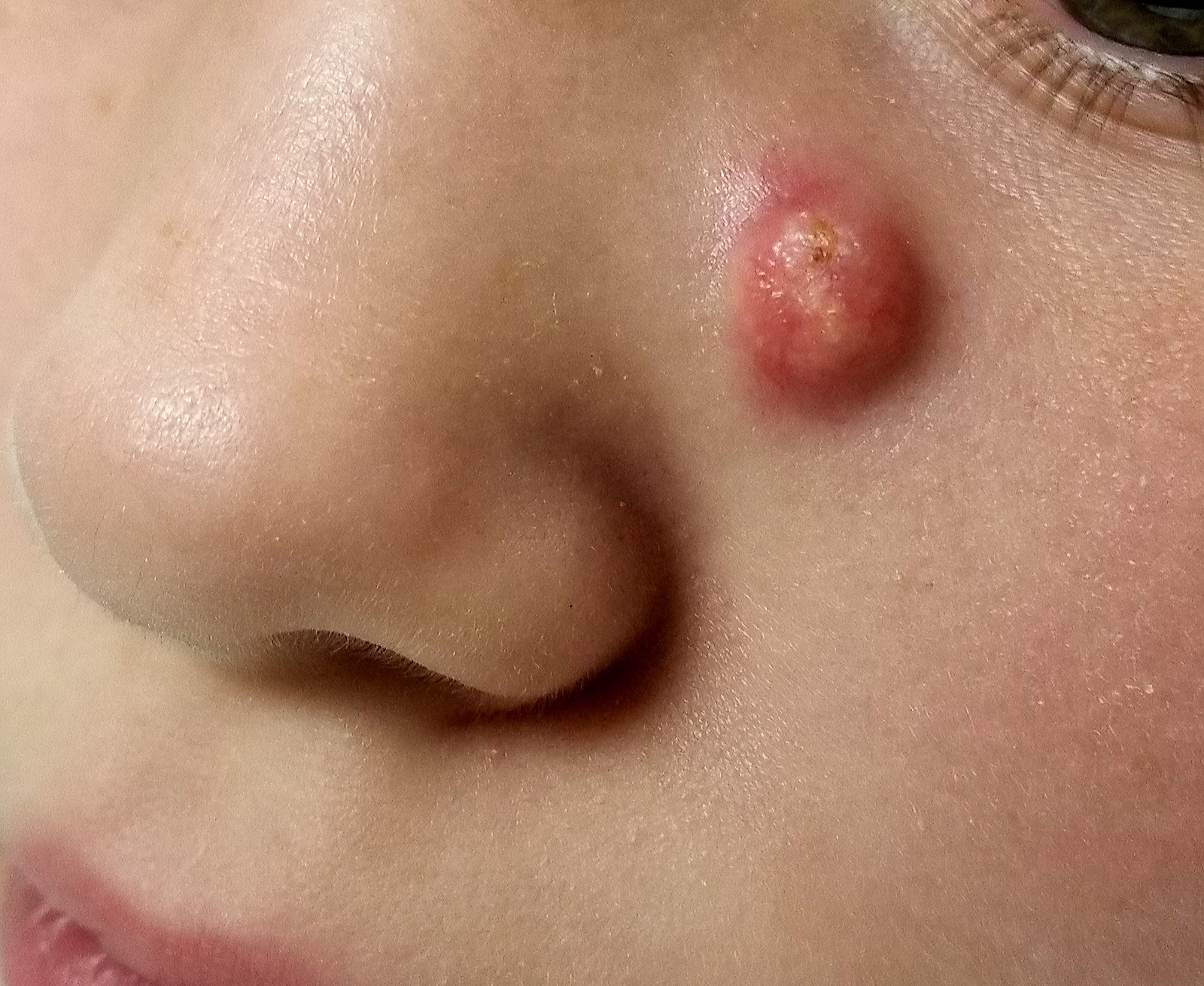
EXAMINATION
A firm nodule, measuring 1.0 by 0.8 cm, is located on the patient’s left upper nasal sidewall. It stands out on an otherwise pristine face free of other blemishes. The lesion is predominantly red, with faint epidermal disturbance in the center. No punctum is appreciated. The lesion is quite firm on palpation, with just a hint of fluctuance but no tenderness or increased warmth.
Excision is clearly indicated; however, the wait for an appointment with a plastic surgeon is currently weeks to months. So an attempt is made to reduce the prominence of the lesion through incision and drainage, which also offers an opportunity to visualize its contents and possibly confirm a diagnosis. The lesion is opened with a #11 blade, and copious amounts of whitish, grainy material is digitally extruded.
What’s the diagnosis?
DISCUSSION
The contents are consistent with those of a somewhat unusual lesion, commonly called pilomatricoma. It is also known as calcifying epithelioma of Malherbe and pilomatrixoma.
This type of cyst is derived from the hair matrix and is commonly seen on the face, neck, scalp, and arms of children and young adults. This patient’s lesion was atypical in its prominence and erythema, at odds with the firm bluish intradermal papule or nodule usually seen in these cases. But the unique contents established the diagnosis with considerable certainty.
All that remained was the excision—which, given the patient’s age and the cosmetic concerns, would require above-average surgical skills. Once removed, the sample will be sent for pathologic examination, which should show anucleate squamous cells (“ghost cells”), benign viable squamous cells with a lining consisting of basaloid cells. Calcifications with foreign body giant cells account for the pathognomic white flecks seen in the extruded material.
Pilomatricoma’s cause is debatable, but it appears to involve increased levels of beta catenin caused by mutations of the APC gene. This effectively inhibits apoptosis, leading to focal increases in cell growth.
The differential for this type of lesion includes simple acne cyst (unlikely in such a young child), carbuncle (which would have been quite painful and full of pus), or even squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts usually seen on the face, neck, scalp, and arms of children and young adults.
- The typical pilomatricoma (sometimes called calcifying epithelioma of Malherbe) is an intradermal papule or nodule, often displaying a faintly bluish color, that is relatively firm on palpation.
- The contents of a pilomatricoma usually consist of whitish curds or flecks of material that represent calcified tissue mixed with foreign body giant cells.
- Pilomatricoma has little or no malignant potential but is often cosmetically significant.
When she was 3 years old, a lesion appeared on this child’s face. It was small and caused little to no concern for several years. The child is now 9, and about a year ago, the lesion began to enlarge, ultimately reaching its present size.
First thought to be a pimple, the lesion was later deemed to be “cystic in nature” by another provider. By that point, however, the lesion was quite prominent—to the extent that it intrudes into the patient’s visual field. Perhaps more significantly for someone her age, it has prompted looks and comments that make her uncomfortable.
Fortunately, the lesion causes no pain or physical discomfort, and no other lesions have manifested. The child’s health is generally excellent.

EXAMINATION
A firm nodule, measuring 1.0 by 0.8 cm, is located on the patient’s left upper nasal sidewall. It stands out on an otherwise pristine face free of other blemishes. The lesion is predominantly red, with faint epidermal disturbance in the center. No punctum is appreciated. The lesion is quite firm on palpation, with just a hint of fluctuance but no tenderness or increased warmth.
Excision is clearly indicated; however, the wait for an appointment with a plastic surgeon is currently weeks to months. So an attempt is made to reduce the prominence of the lesion through incision and drainage, which also offers an opportunity to visualize its contents and possibly confirm a diagnosis. The lesion is opened with a #11 blade, and copious amounts of whitish, grainy material is digitally extruded.
What’s the diagnosis?
DISCUSSION
The contents are consistent with those of a somewhat unusual lesion, commonly called pilomatricoma. It is also known as calcifying epithelioma of Malherbe and pilomatrixoma.
This type of cyst is derived from the hair matrix and is commonly seen on the face, neck, scalp, and arms of children and young adults. This patient’s lesion was atypical in its prominence and erythema, at odds with the firm bluish intradermal papule or nodule usually seen in these cases. But the unique contents established the diagnosis with considerable certainty.
All that remained was the excision—which, given the patient’s age and the cosmetic concerns, would require above-average surgical skills. Once removed, the sample will be sent for pathologic examination, which should show anucleate squamous cells (“ghost cells”), benign viable squamous cells with a lining consisting of basaloid cells. Calcifications with foreign body giant cells account for the pathognomic white flecks seen in the extruded material.
Pilomatricoma’s cause is debatable, but it appears to involve increased levels of beta catenin caused by mutations of the APC gene. This effectively inhibits apoptosis, leading to focal increases in cell growth.
The differential for this type of lesion includes simple acne cyst (unlikely in such a young child), carbuncle (which would have been quite painful and full of pus), or even squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts usually seen on the face, neck, scalp, and arms of children and young adults.
- The typical pilomatricoma (sometimes called calcifying epithelioma of Malherbe) is an intradermal papule or nodule, often displaying a faintly bluish color, that is relatively firm on palpation.
- The contents of a pilomatricoma usually consist of whitish curds or flecks of material that represent calcified tissue mixed with foreign body giant cells.
- Pilomatricoma has little or no malignant potential but is often cosmetically significant.
When she was 3 years old, a lesion appeared on this child’s face. It was small and caused little to no concern for several years. The child is now 9, and about a year ago, the lesion began to enlarge, ultimately reaching its present size.
First thought to be a pimple, the lesion was later deemed to be “cystic in nature” by another provider. By that point, however, the lesion was quite prominent—to the extent that it intrudes into the patient’s visual field. Perhaps more significantly for someone her age, it has prompted looks and comments that make her uncomfortable.
Fortunately, the lesion causes no pain or physical discomfort, and no other lesions have manifested. The child’s health is generally excellent.

EXAMINATION
A firm nodule, measuring 1.0 by 0.8 cm, is located on the patient’s left upper nasal sidewall. It stands out on an otherwise pristine face free of other blemishes. The lesion is predominantly red, with faint epidermal disturbance in the center. No punctum is appreciated. The lesion is quite firm on palpation, with just a hint of fluctuance but no tenderness or increased warmth.
Excision is clearly indicated; however, the wait for an appointment with a plastic surgeon is currently weeks to months. So an attempt is made to reduce the prominence of the lesion through incision and drainage, which also offers an opportunity to visualize its contents and possibly confirm a diagnosis. The lesion is opened with a #11 blade, and copious amounts of whitish, grainy material is digitally extruded.
What’s the diagnosis?
DISCUSSION
The contents are consistent with those of a somewhat unusual lesion, commonly called pilomatricoma. It is also known as calcifying epithelioma of Malherbe and pilomatrixoma.
This type of cyst is derived from the hair matrix and is commonly seen on the face, neck, scalp, and arms of children and young adults. This patient’s lesion was atypical in its prominence and erythema, at odds with the firm bluish intradermal papule or nodule usually seen in these cases. But the unique contents established the diagnosis with considerable certainty.
All that remained was the excision—which, given the patient’s age and the cosmetic concerns, would require above-average surgical skills. Once removed, the sample will be sent for pathologic examination, which should show anucleate squamous cells (“ghost cells”), benign viable squamous cells with a lining consisting of basaloid cells. Calcifications with foreign body giant cells account for the pathognomic white flecks seen in the extruded material.
Pilomatricoma’s cause is debatable, but it appears to involve increased levels of beta catenin caused by mutations of the APC gene. This effectively inhibits apoptosis, leading to focal increases in cell growth.
The differential for this type of lesion includes simple acne cyst (unlikely in such a young child), carbuncle (which would have been quite painful and full of pus), or even squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts usually seen on the face, neck, scalp, and arms of children and young adults.
- The typical pilomatricoma (sometimes called calcifying epithelioma of Malherbe) is an intradermal papule or nodule, often displaying a faintly bluish color, that is relatively firm on palpation.
- The contents of a pilomatricoma usually consist of whitish curds or flecks of material that represent calcified tissue mixed with foreign body giant cells.
- Pilomatricoma has little or no malignant potential but is often cosmetically significant.
Antibiotic use in dermatology declining, with one exception
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Reducing antibiotic prescribing in dermatology – as in so many other areas of medical practice – is a challenge, but there are a number of strategies that can help.
The first is to take a wait-and-see approach, which has been shown to be effective for childhood otitis media. Communication training for physicians can also help them to manage patient requests for antibiotics by working out the patient’s level of understanding of their condition and treatment options, and their expectations, and getting them to agree to keep antibiotics as a contingency plan. There are clinical decision support tools available to help physicians identify high-risk surgical patients who may require postoperative antibiotics.
It will help to have alternative treatment options for conditions such as acne and rosacea, such as better topical therapies, and an increase in clinical trials for these therapies will hopefully provide more options for patients.
Joslyn S. Kirby, MD, and Jordan S. Lim, MB, are in the department of dermatology, Penn State University, Hershey. These comments are taken from an accompanying editorial (JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4877). They had no disclosures.
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Dermatologists are prescribing fewer antibiotics for acne and rosacea, but prescribing after dermatologic surgery has increased in the past decade.
In a study published online Jan. 16 in JAMA Dermatology, researchers report the results of a cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists between 2008 and 2016, using commercial claims data.
The analysis showed that, over this period of time, the overall rate of antibiotic prescribing by dermatologists decreased by 36.6%, from 3.36 courses per 100 dermatologist visits to 2.13 courses. In particular, antibiotic prescribing for acne decreased by 28.1%, from 11.76 courses per 100 visits to 8.45 courses, and for rosacea it decreased by 18.1%, from 10.89 courses per 100 visits to 8.92 courses.
John S. Barbieri, MD, of the department of dermatology, University of Pennsylvania, and his coauthors described the overall decline in antibiotic prescribing as “encouraging,” considering that in 2013 dermatologists were identified as the “most frequent prescribers of oral antibiotics per clinician.” The decline resulted in an estimated 480,000 fewer antibiotic courses a year, they noted.
“Much of the decrease in extended courses of antibiotic therapy is associated with visits for acne and rosacea,” they wrote. “Although recent guidelines suggest limiting the duration of therapy in this patient population, course duration has remained stable over time, suggesting that this decrease may be due to fewer patients being treated with antibiotics rather than patients being treated for a shorter duration.”
However, the rate of oral antibiotic prescriptions associated with surgical visits increased by 69.6%, from 3.92 courses per 100 visits to 6.65. This increase was concerning, given the risk of surgical-site infections was low, the authors pointed out. “In addition, a 2008 advisory statement on antibiotic prophylaxis recommends single-dose perioperative antibiotics for patients at increased risk of surgical-site infection,” they added.
The study also noted a 35.3% increase in antibiotic prescribing for cysts and a 3.2% increase for hidradenitis suppurativa.
Over the entire study period, nearly 1 million courses of oral antibiotics were prescribed. Doxycycline hyclate accounted for around one quarter of prescriptions, as did minocycline, while 19.9% of prescriptions were for cephalexin.
“Given the low rate of infectious complications, even for Mohs surgery, and the lack of evidence to support the use of prolonged rather than single-dose perioperative regimens, the postoperative courses of antibiotics identified in this study may increase risks to patients without substantial benefits,” they added.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. No conflicts of interest were declared.
SOURCE: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
FROM JAMA DERMATOLOGY
Key clinical point: Antibiotic prescriptions by dermatologists have decreased since 2008.
Major finding: Between 2008 and 2016, antibiotic prescriptions by dermatologists dropped by 36.6%.
Study details: Cross-sectional analysis of antibiotic prescribing by 11,986 dermatologists from 2008 to 2016.
Disclosures: The study was partly supported by the National Institute of Arthritis and Musculoskeletal Skin Diseases. The authors had no disclosures.
Source: Barbieri J et al. JAMA Dermatology. 2019 Jan 16. doi: 10.1001/jamadermatol.2018.4944.
Vulvar disease treatment tips: From lice to lichen sclerosus
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS
When sweaty palms are more than just sweaty palms
ORLANDO – When you extend your hand to a new patient, and he reflexively wipes his palm before shaking hands, be alert. It’s possible you’re seeing primary hyperhidrosis, a condition that’s both more common and more disabling than once thought.
“Looking at the biology of sweating, normally, it’s a good thing – we need it to survive. However, hyperhidrosis is too much of a good thing – it’s an excess of what is needed for normal biology,” said Adam Friedman, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Recent data, he pointed out, show that hyperhidrosis is more prevalent than previously thought – about 4.8% of individuals may have the condition, with about half having axillary hyperhidrosis. Symptoms peak in early adulthood, with adults aged 18-54 most affected. “These are the prime working years,” he said.
About 2% of teens are affected, and many adults report that symptoms began before they were 12 years old. Hand hyperhidrosis is a factor for computer and electronic device work, sports, and even handling paper and pencils, noted Dr. Friedman, professor of dermatology at George Washington University, Washington.
“Does it affect quality of life? Yes. We have data to support the impact. The adverse impact is actually greater than that of eczema and psoriasis,” he said, adding that patients won’t always bring up their concerns about sweating. “Often, it’s the patient who apologizes for having sweaty palms or who sticks to the paper on the exam table. It’s worth asking these patients if they are bothered by excessive sweating.”
Is it hyperhidrosis?
A 2016 paper defined hyperhidrosis as “a condition that involves chronic excessive sweating of the underarms, hands, feet, face, groin, or other bodily areas, which is much more than what is normal, and occurs regardless of temperature, exercise, or situation, and may have an impact on quality of life” (Arch Dermatol Res. 2016 Dec;308[10]:743-9). The amount of sweating can be four to five times that seen in healthy controls.
Other clues that excess sweating might be hyperhidrosis? General hyperhidrosis is a secondary syndrome that can be caused by a variety of conditions including endocrine and metabolic disorders and malignancies. Drugs and toxins can also cause generalized excessive sweating.
Focal hyperhidrosis can be primary idiopathic disease; some neuropathies and certain spinal diseases and spinal cord injury can also cause focal hyperhidrosis, though not usually in the axillary/palmar/plantar distribution seen in primary hyperhidrosis.
Before settling on primary hyperhidrosis, the history and exam should also account for other possibilities in the differential: social anxiety disorder, eccrine nevus, gustatory sweating, Frey syndrome, and impaired evaporation could all account for excess sweating, which is also a postsurgical phenomenon for some patients.
Diagnostic criteria call for “focal, visible, excessive sweating” persisting for at least 6 months with no apparent cause. Additionally, patients must have at least two of the additional following criteria: sweating that is bilateral and symmetric, occurs at least once weekly, impairs daily activities, and starts before age 25 years, as well as a positive family history of hyperhidrosis and cessation of sweating during sleep.
The last point is critical, Dr. Friedman said. “If you sweat a lot at night, it’s not hyperhidrosis!”
Though gravimetric evaluation is used in hyperhidrosis research, the history and exam are really where the diagnosis is made in practice, he noted. The Hyperhidrosis Disease Severity Scale is a brief, useful clinical tool that asks patients to peg the extent to which their sweating interferes with daily life.
Topical treatments to try
Topical antiperspirants and other topical agents are a logical place to start and may be required as part of step therapy by insurers. Many patients will already have tried clinical strength over-the-counter antiperspirants containing aluminum zirconium trichlorohydrex, but these products rate low in patient satisfaction among those with primary hyperhidrosis.
Prescription aluminum salts can be compounded to various strengths, with 10%-20% concentration appropriate for axillae and 30%-40% a good strength for palms and soles, according to Dr. Friedman. All of these agents work by precipitating out solids that form a shallow plug in sweat ducts, slowing the flow of perspiration.
Pearls for topical treatment include the need for the product to be on the skin for 6-8 hours overnight. “Remember hyperhidrosis patients do not sweat at night,” so this is the time when the occlusive plugs can form. Then residue can be washed off in the morning, and patients can apply a deodorant. “I remind my patients that antiperspirants are for sweating, and deodorants are for odor,” said Dr. Friedman. These products can damage fabric, and they can be irritating, a problem addressed with low-potency topical steroids.
Topical regimens don’t need to be adjusted for pediatric patients, said Dr. Friedman.
Iontophoresis has been around since the 1950s, is effective, has few side effects, and is considered first-line treatment for severe palmar and plantar hyperhidrosis. But he said there’s one big rub: time. To be effective, patients need 20-30 minutes of application of 15-20 milliamperes of current 3-4 times weekly, not a schedule that works for most patients or practitioners, Dr. Friedman noted.
A treatment recently approved by the Food and Drug Administration for primary axillary hyperhidrosis is a topical anticholinergic, glycopyrronium tosylate, applied with wipes impregnated with glycopyrronium solution. This product significantly outperformed placebo in two clinical trials, with up to 64% of users meeting the primary endpoint of improving by at least 4 points on the Axillary Sweating Daily Diary (ASDD) scale. This product significantly outperformed placebo in two clinical trials, with 53% and 66% of users meeting the primary endpoint, improvement of at least 4 points from baseline in the weekly mean ASDD Item #2. It was approved in those aged 9 years and older.
“You can use this in kids, but you need to educate the kid and the parent or adult,” he said. “This is the last thing you do before bed, after brushing your teeth and after washing your face.”
Patients should apply one swipe to the clean skin of each underarm, and then wash their hands thoroughly. Clinical trials saw a greater proportion of off-target effects such as dry eyes and mouth and mydriasis in the active arm; unilateral mydriasis was more common than bilateral, underscoring the importance of hand washing as this was probably secondary transfer from hands to face during sleep, said Dr. Friedman. Patients can expect results in 2-3 weeks, and doses can be held as needed for anticholinergic side effects.
Systemic choices are limited
There are no FDA-approved systemic agents for hyperhidrosis, and the literature holds only case reports or small series, Dr. Friedman pointed out.
Though systemic treatment may be more effective in generalized hyperhidrosis and for patients with dysautonomia-associated hyperhidrosis, glycopyrrolate is a logical choice if a systemic anticholinergic is desired. A starting dose of 1 mg twice daily can be titrated for effect to about 6 mg daily. Though off-target effects may be a dose-limiting factor, glycopyrrolate is not very lipid soluble, so it penetrates the blood-brain barrier relatively poorly, he said.
Oxybutynin is available in many forms, including a slow-release tablet that permits once-daily dosing. Starting at 5-10 mg daily is a good idea, but dosing may need to be increased to as high as 20 mg daily to be effective. However, patients will often experience “major side effects” with oxybutynin, including significant xerostomia, constipation, blurred vision, and difficulty urinating.
For children, small studies have seen improvement with glycopyrrolate at an average dose of about 2 mg/day. Oxybutynin, which has been extensively studied in the pediatric population, was also effective, but central nervous system adverse events were common.
For some, beta-adrenergic blockade can be an extremely valuable tool, said Dr. Friedman. When sweating is linked to social phobia or performance anxiety, 10-20 mg of atenolol about an hour before the performance or public appearance can make a big difference. Bradycardia, atrioventricular block, and asthma are all contraindications, and the usual precautions should be taken with a host of other comorbidities, he noted.
It’s a good idea to check resting blood pressure and heart rate and take body mass into consideration, and adjust the dose downward appropriately. A key pearl: “Have them do a test run at home, to make sure they don’t keel over on the podium!” said Dr. Friedman.
Botulinum toxin tips and tricks
Botulinum toxin can be very effective and works directly by blocking acetylcholine release at the junction of the sympathetic sudomotor neuron and the sweat gland.
Before treatment, make sure the patient prepares correctly by abstaining from over-the-counter deodorants or antiperspirants, and resting without exertion or drinking hot beverages for about 30 minutes before the procedure.
To ascertain the follicular outline of the area to be injected, the iodine starch test can be used: Paint the axilla with iodine, allow it to dry, and then dust corn starch over the area. The follicular outline is mapped by the purple-blue reaction of the starch and iodine in the presence of moisture from perspiration, Dr. Friedman said.
Applying topical analgesia 30 minutes prior to the procedure helps with patient discomfort with axillary injections. When it comes time to inject, a shallow approach with the bevel side up works well, with a goal of blanketing the field identified by the iodine starch test with small aliquots of toxin placed 1-2 centimeters apart, said Dr. Friedman. However, for patients who might have tattoos that extend to the axillary area, “Avoid the ink!”
Patients will start to see improvement within 2-4 days, and although the literature says a toxin treatment can last 6-9 months, Dr. Friedman said he sees patients coming back in 4-5 months.
Obtaining botulinum toxin can be done in one of two ways: the “buy and bill” approach has the dermatologist purchasing the medication, using CPT 64650 and J code J0585 – “Remember the units!” said Dr. Friedman, because reimbursement will be based on the volume of toxin purchased.. This route may be cheaper for the patient because it avoids a medication copay. The physician obtains preauthorization for both the medication and procedure with this strategy.
The other route is to have the provider prescribe botulinum toxin and the patient purchase it at a regular or specialty pharmacy. In this case, the pharmacist obtains precertification for the medication, but the physician still needs to be precertified – and bill – for the injection procedure itself. This scenario is less risky for the physician but may trigger two separate copays for the patient.
Botulinum toxin can be effective for up to 90% of patients, but at a cost: Without insurance reimbursement, treatments can cost in the neighborhood of $1,500.
A good resource for patients and clinicians is the International Hyperhidrosis Society’s website (sweathelp.org), said Dr. Friedman.
Dr. Friedman disclosed relationships with multiple pharmaceutical and cosmetic companies, including Dermira, which markets topical glycopyrronium tosylate as Qbrexza.
ORLANDO – When you extend your hand to a new patient, and he reflexively wipes his palm before shaking hands, be alert. It’s possible you’re seeing primary hyperhidrosis, a condition that’s both more common and more disabling than once thought.
“Looking at the biology of sweating, normally, it’s a good thing – we need it to survive. However, hyperhidrosis is too much of a good thing – it’s an excess of what is needed for normal biology,” said Adam Friedman, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Recent data, he pointed out, show that hyperhidrosis is more prevalent than previously thought – about 4.8% of individuals may have the condition, with about half having axillary hyperhidrosis. Symptoms peak in early adulthood, with adults aged 18-54 most affected. “These are the prime working years,” he said.
About 2% of teens are affected, and many adults report that symptoms began before they were 12 years old. Hand hyperhidrosis is a factor for computer and electronic device work, sports, and even handling paper and pencils, noted Dr. Friedman, professor of dermatology at George Washington University, Washington.
“Does it affect quality of life? Yes. We have data to support the impact. The adverse impact is actually greater than that of eczema and psoriasis,” he said, adding that patients won’t always bring up their concerns about sweating. “Often, it’s the patient who apologizes for having sweaty palms or who sticks to the paper on the exam table. It’s worth asking these patients if they are bothered by excessive sweating.”
Is it hyperhidrosis?
A 2016 paper defined hyperhidrosis as “a condition that involves chronic excessive sweating of the underarms, hands, feet, face, groin, or other bodily areas, which is much more than what is normal, and occurs regardless of temperature, exercise, or situation, and may have an impact on quality of life” (Arch Dermatol Res. 2016 Dec;308[10]:743-9). The amount of sweating can be four to five times that seen in healthy controls.
Other clues that excess sweating might be hyperhidrosis? General hyperhidrosis is a secondary syndrome that can be caused by a variety of conditions including endocrine and metabolic disorders and malignancies. Drugs and toxins can also cause generalized excessive sweating.
Focal hyperhidrosis can be primary idiopathic disease; some neuropathies and certain spinal diseases and spinal cord injury can also cause focal hyperhidrosis, though not usually in the axillary/palmar/plantar distribution seen in primary hyperhidrosis.
Before settling on primary hyperhidrosis, the history and exam should also account for other possibilities in the differential: social anxiety disorder, eccrine nevus, gustatory sweating, Frey syndrome, and impaired evaporation could all account for excess sweating, which is also a postsurgical phenomenon for some patients.
Diagnostic criteria call for “focal, visible, excessive sweating” persisting for at least 6 months with no apparent cause. Additionally, patients must have at least two of the additional following criteria: sweating that is bilateral and symmetric, occurs at least once weekly, impairs daily activities, and starts before age 25 years, as well as a positive family history of hyperhidrosis and cessation of sweating during sleep.
The last point is critical, Dr. Friedman said. “If you sweat a lot at night, it’s not hyperhidrosis!”
Though gravimetric evaluation is used in hyperhidrosis research, the history and exam are really where the diagnosis is made in practice, he noted. The Hyperhidrosis Disease Severity Scale is a brief, useful clinical tool that asks patients to peg the extent to which their sweating interferes with daily life.
Topical treatments to try
Topical antiperspirants and other topical agents are a logical place to start and may be required as part of step therapy by insurers. Many patients will already have tried clinical strength over-the-counter antiperspirants containing aluminum zirconium trichlorohydrex, but these products rate low in patient satisfaction among those with primary hyperhidrosis.
Prescription aluminum salts can be compounded to various strengths, with 10%-20% concentration appropriate for axillae and 30%-40% a good strength for palms and soles, according to Dr. Friedman. All of these agents work by precipitating out solids that form a shallow plug in sweat ducts, slowing the flow of perspiration.
Pearls for topical treatment include the need for the product to be on the skin for 6-8 hours overnight. “Remember hyperhidrosis patients do not sweat at night,” so this is the time when the occlusive plugs can form. Then residue can be washed off in the morning, and patients can apply a deodorant. “I remind my patients that antiperspirants are for sweating, and deodorants are for odor,” said Dr. Friedman. These products can damage fabric, and they can be irritating, a problem addressed with low-potency topical steroids.
Topical regimens don’t need to be adjusted for pediatric patients, said Dr. Friedman.
Iontophoresis has been around since the 1950s, is effective, has few side effects, and is considered first-line treatment for severe palmar and plantar hyperhidrosis. But he said there’s one big rub: time. To be effective, patients need 20-30 minutes of application of 15-20 milliamperes of current 3-4 times weekly, not a schedule that works for most patients or practitioners, Dr. Friedman noted.
A treatment recently approved by the Food and Drug Administration for primary axillary hyperhidrosis is a topical anticholinergic, glycopyrronium tosylate, applied with wipes impregnated with glycopyrronium solution. This product significantly outperformed placebo in two clinical trials, with up to 64% of users meeting the primary endpoint of improving by at least 4 points on the Axillary Sweating Daily Diary (ASDD) scale. This product significantly outperformed placebo in two clinical trials, with 53% and 66% of users meeting the primary endpoint, improvement of at least 4 points from baseline in the weekly mean ASDD Item #2. It was approved in those aged 9 years and older.
“You can use this in kids, but you need to educate the kid and the parent or adult,” he said. “This is the last thing you do before bed, after brushing your teeth and after washing your face.”
Patients should apply one swipe to the clean skin of each underarm, and then wash their hands thoroughly. Clinical trials saw a greater proportion of off-target effects such as dry eyes and mouth and mydriasis in the active arm; unilateral mydriasis was more common than bilateral, underscoring the importance of hand washing as this was probably secondary transfer from hands to face during sleep, said Dr. Friedman. Patients can expect results in 2-3 weeks, and doses can be held as needed for anticholinergic side effects.
Systemic choices are limited
There are no FDA-approved systemic agents for hyperhidrosis, and the literature holds only case reports or small series, Dr. Friedman pointed out.
Though systemic treatment may be more effective in generalized hyperhidrosis and for patients with dysautonomia-associated hyperhidrosis, glycopyrrolate is a logical choice if a systemic anticholinergic is desired. A starting dose of 1 mg twice daily can be titrated for effect to about 6 mg daily. Though off-target effects may be a dose-limiting factor, glycopyrrolate is not very lipid soluble, so it penetrates the blood-brain barrier relatively poorly, he said.
Oxybutynin is available in many forms, including a slow-release tablet that permits once-daily dosing. Starting at 5-10 mg daily is a good idea, but dosing may need to be increased to as high as 20 mg daily to be effective. However, patients will often experience “major side effects” with oxybutynin, including significant xerostomia, constipation, blurred vision, and difficulty urinating.
For children, small studies have seen improvement with glycopyrrolate at an average dose of about 2 mg/day. Oxybutynin, which has been extensively studied in the pediatric population, was also effective, but central nervous system adverse events were common.
For some, beta-adrenergic blockade can be an extremely valuable tool, said Dr. Friedman. When sweating is linked to social phobia or performance anxiety, 10-20 mg of atenolol about an hour before the performance or public appearance can make a big difference. Bradycardia, atrioventricular block, and asthma are all contraindications, and the usual precautions should be taken with a host of other comorbidities, he noted.
It’s a good idea to check resting blood pressure and heart rate and take body mass into consideration, and adjust the dose downward appropriately. A key pearl: “Have them do a test run at home, to make sure they don’t keel over on the podium!” said Dr. Friedman.
Botulinum toxin tips and tricks
Botulinum toxin can be very effective and works directly by blocking acetylcholine release at the junction of the sympathetic sudomotor neuron and the sweat gland.
Before treatment, make sure the patient prepares correctly by abstaining from over-the-counter deodorants or antiperspirants, and resting without exertion or drinking hot beverages for about 30 minutes before the procedure.
To ascertain the follicular outline of the area to be injected, the iodine starch test can be used: Paint the axilla with iodine, allow it to dry, and then dust corn starch over the area. The follicular outline is mapped by the purple-blue reaction of the starch and iodine in the presence of moisture from perspiration, Dr. Friedman said.
Applying topical analgesia 30 minutes prior to the procedure helps with patient discomfort with axillary injections. When it comes time to inject, a shallow approach with the bevel side up works well, with a goal of blanketing the field identified by the iodine starch test with small aliquots of toxin placed 1-2 centimeters apart, said Dr. Friedman. However, for patients who might have tattoos that extend to the axillary area, “Avoid the ink!”
Patients will start to see improvement within 2-4 days, and although the literature says a toxin treatment can last 6-9 months, Dr. Friedman said he sees patients coming back in 4-5 months.
Obtaining botulinum toxin can be done in one of two ways: the “buy and bill” approach has the dermatologist purchasing the medication, using CPT 64650 and J code J0585 – “Remember the units!” said Dr. Friedman, because reimbursement will be based on the volume of toxin purchased.. This route may be cheaper for the patient because it avoids a medication copay. The physician obtains preauthorization for both the medication and procedure with this strategy.
The other route is to have the provider prescribe botulinum toxin and the patient purchase it at a regular or specialty pharmacy. In this case, the pharmacist obtains precertification for the medication, but the physician still needs to be precertified – and bill – for the injection procedure itself. This scenario is less risky for the physician but may trigger two separate copays for the patient.
Botulinum toxin can be effective for up to 90% of patients, but at a cost: Without insurance reimbursement, treatments can cost in the neighborhood of $1,500.
A good resource for patients and clinicians is the International Hyperhidrosis Society’s website (sweathelp.org), said Dr. Friedman.
Dr. Friedman disclosed relationships with multiple pharmaceutical and cosmetic companies, including Dermira, which markets topical glycopyrronium tosylate as Qbrexza.
ORLANDO – When you extend your hand to a new patient, and he reflexively wipes his palm before shaking hands, be alert. It’s possible you’re seeing primary hyperhidrosis, a condition that’s both more common and more disabling than once thought.
“Looking at the biology of sweating, normally, it’s a good thing – we need it to survive. However, hyperhidrosis is too much of a good thing – it’s an excess of what is needed for normal biology,” said Adam Friedman, MD, speaking at the Orlando Dermatology Aesthetic and Clinical Conference.
Recent data, he pointed out, show that hyperhidrosis is more prevalent than previously thought – about 4.8% of individuals may have the condition, with about half having axillary hyperhidrosis. Symptoms peak in early adulthood, with adults aged 18-54 most affected. “These are the prime working years,” he said.
About 2% of teens are affected, and many adults report that symptoms began before they were 12 years old. Hand hyperhidrosis is a factor for computer and electronic device work, sports, and even handling paper and pencils, noted Dr. Friedman, professor of dermatology at George Washington University, Washington.
“Does it affect quality of life? Yes. We have data to support the impact. The adverse impact is actually greater than that of eczema and psoriasis,” he said, adding that patients won’t always bring up their concerns about sweating. “Often, it’s the patient who apologizes for having sweaty palms or who sticks to the paper on the exam table. It’s worth asking these patients if they are bothered by excessive sweating.”
Is it hyperhidrosis?
A 2016 paper defined hyperhidrosis as “a condition that involves chronic excessive sweating of the underarms, hands, feet, face, groin, or other bodily areas, which is much more than what is normal, and occurs regardless of temperature, exercise, or situation, and may have an impact on quality of life” (Arch Dermatol Res. 2016 Dec;308[10]:743-9). The amount of sweating can be four to five times that seen in healthy controls.
Other clues that excess sweating might be hyperhidrosis? General hyperhidrosis is a secondary syndrome that can be caused by a variety of conditions including endocrine and metabolic disorders and malignancies. Drugs and toxins can also cause generalized excessive sweating.
Focal hyperhidrosis can be primary idiopathic disease; some neuropathies and certain spinal diseases and spinal cord injury can also cause focal hyperhidrosis, though not usually in the axillary/palmar/plantar distribution seen in primary hyperhidrosis.
Before settling on primary hyperhidrosis, the history and exam should also account for other possibilities in the differential: social anxiety disorder, eccrine nevus, gustatory sweating, Frey syndrome, and impaired evaporation could all account for excess sweating, which is also a postsurgical phenomenon for some patients.
Diagnostic criteria call for “focal, visible, excessive sweating” persisting for at least 6 months with no apparent cause. Additionally, patients must have at least two of the additional following criteria: sweating that is bilateral and symmetric, occurs at least once weekly, impairs daily activities, and starts before age 25 years, as well as a positive family history of hyperhidrosis and cessation of sweating during sleep.
The last point is critical, Dr. Friedman said. “If you sweat a lot at night, it’s not hyperhidrosis!”
Though gravimetric evaluation is used in hyperhidrosis research, the history and exam are really where the diagnosis is made in practice, he noted. The Hyperhidrosis Disease Severity Scale is a brief, useful clinical tool that asks patients to peg the extent to which their sweating interferes with daily life.
Topical treatments to try
Topical antiperspirants and other topical agents are a logical place to start and may be required as part of step therapy by insurers. Many patients will already have tried clinical strength over-the-counter antiperspirants containing aluminum zirconium trichlorohydrex, but these products rate low in patient satisfaction among those with primary hyperhidrosis.
Prescription aluminum salts can be compounded to various strengths, with 10%-20% concentration appropriate for axillae and 30%-40% a good strength for palms and soles, according to Dr. Friedman. All of these agents work by precipitating out solids that form a shallow plug in sweat ducts, slowing the flow of perspiration.
Pearls for topical treatment include the need for the product to be on the skin for 6-8 hours overnight. “Remember hyperhidrosis patients do not sweat at night,” so this is the time when the occlusive plugs can form. Then residue can be washed off in the morning, and patients can apply a deodorant. “I remind my patients that antiperspirants are for sweating, and deodorants are for odor,” said Dr. Friedman. These products can damage fabric, and they can be irritating, a problem addressed with low-potency topical steroids.
Topical regimens don’t need to be adjusted for pediatric patients, said Dr. Friedman.
Iontophoresis has been around since the 1950s, is effective, has few side effects, and is considered first-line treatment for severe palmar and plantar hyperhidrosis. But he said there’s one big rub: time. To be effective, patients need 20-30 minutes of application of 15-20 milliamperes of current 3-4 times weekly, not a schedule that works for most patients or practitioners, Dr. Friedman noted.
A treatment recently approved by the Food and Drug Administration for primary axillary hyperhidrosis is a topical anticholinergic, glycopyrronium tosylate, applied with wipes impregnated with glycopyrronium solution. This product significantly outperformed placebo in two clinical trials, with up to 64% of users meeting the primary endpoint of improving by at least 4 points on the Axillary Sweating Daily Diary (ASDD) scale. This product significantly outperformed placebo in two clinical trials, with 53% and 66% of users meeting the primary endpoint, improvement of at least 4 points from baseline in the weekly mean ASDD Item #2. It was approved in those aged 9 years and older.
“You can use this in kids, but you need to educate the kid and the parent or adult,” he said. “This is the last thing you do before bed, after brushing your teeth and after washing your face.”
Patients should apply one swipe to the clean skin of each underarm, and then wash their hands thoroughly. Clinical trials saw a greater proportion of off-target effects such as dry eyes and mouth and mydriasis in the active arm; unilateral mydriasis was more common than bilateral, underscoring the importance of hand washing as this was probably secondary transfer from hands to face during sleep, said Dr. Friedman. Patients can expect results in 2-3 weeks, and doses can be held as needed for anticholinergic side effects.
Systemic choices are limited
There are no FDA-approved systemic agents for hyperhidrosis, and the literature holds only case reports or small series, Dr. Friedman pointed out.
Though systemic treatment may be more effective in generalized hyperhidrosis and for patients with dysautonomia-associated hyperhidrosis, glycopyrrolate is a logical choice if a systemic anticholinergic is desired. A starting dose of 1 mg twice daily can be titrated for effect to about 6 mg daily. Though off-target effects may be a dose-limiting factor, glycopyrrolate is not very lipid soluble, so it penetrates the blood-brain barrier relatively poorly, he said.
Oxybutynin is available in many forms, including a slow-release tablet that permits once-daily dosing. Starting at 5-10 mg daily is a good idea, but dosing may need to be increased to as high as 20 mg daily to be effective. However, patients will often experience “major side effects” with oxybutynin, including significant xerostomia, constipation, blurred vision, and difficulty urinating.
For children, small studies have seen improvement with glycopyrrolate at an average dose of about 2 mg/day. Oxybutynin, which has been extensively studied in the pediatric population, was also effective, but central nervous system adverse events were common.
For some, beta-adrenergic blockade can be an extremely valuable tool, said Dr. Friedman. When sweating is linked to social phobia or performance anxiety, 10-20 mg of atenolol about an hour before the performance or public appearance can make a big difference. Bradycardia, atrioventricular block, and asthma are all contraindications, and the usual precautions should be taken with a host of other comorbidities, he noted.
It’s a good idea to check resting blood pressure and heart rate and take body mass into consideration, and adjust the dose downward appropriately. A key pearl: “Have them do a test run at home, to make sure they don’t keel over on the podium!” said Dr. Friedman.
Botulinum toxin tips and tricks
Botulinum toxin can be very effective and works directly by blocking acetylcholine release at the junction of the sympathetic sudomotor neuron and the sweat gland.
Before treatment, make sure the patient prepares correctly by abstaining from over-the-counter deodorants or antiperspirants, and resting without exertion or drinking hot beverages for about 30 minutes before the procedure.
To ascertain the follicular outline of the area to be injected, the iodine starch test can be used: Paint the axilla with iodine, allow it to dry, and then dust corn starch over the area. The follicular outline is mapped by the purple-blue reaction of the starch and iodine in the presence of moisture from perspiration, Dr. Friedman said.
Applying topical analgesia 30 minutes prior to the procedure helps with patient discomfort with axillary injections. When it comes time to inject, a shallow approach with the bevel side up works well, with a goal of blanketing the field identified by the iodine starch test with small aliquots of toxin placed 1-2 centimeters apart, said Dr. Friedman. However, for patients who might have tattoos that extend to the axillary area, “Avoid the ink!”
Patients will start to see improvement within 2-4 days, and although the literature says a toxin treatment can last 6-9 months, Dr. Friedman said he sees patients coming back in 4-5 months.
Obtaining botulinum toxin can be done in one of two ways: the “buy and bill” approach has the dermatologist purchasing the medication, using CPT 64650 and J code J0585 – “Remember the units!” said Dr. Friedman, because reimbursement will be based on the volume of toxin purchased.. This route may be cheaper for the patient because it avoids a medication copay. The physician obtains preauthorization for both the medication and procedure with this strategy.
The other route is to have the provider prescribe botulinum toxin and the patient purchase it at a regular or specialty pharmacy. In this case, the pharmacist obtains precertification for the medication, but the physician still needs to be precertified – and bill – for the injection procedure itself. This scenario is less risky for the physician but may trigger two separate copays for the patient.
Botulinum toxin can be effective for up to 90% of patients, but at a cost: Without insurance reimbursement, treatments can cost in the neighborhood of $1,500.
A good resource for patients and clinicians is the International Hyperhidrosis Society’s website (sweathelp.org), said Dr. Friedman.
Dr. Friedman disclosed relationships with multiple pharmaceutical and cosmetic companies, including Dermira, which markets topical glycopyrronium tosylate as Qbrexza.
EXPERT ANALYSIS FROM ODAC 2019
A theory of relativity for rosacea patients
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.
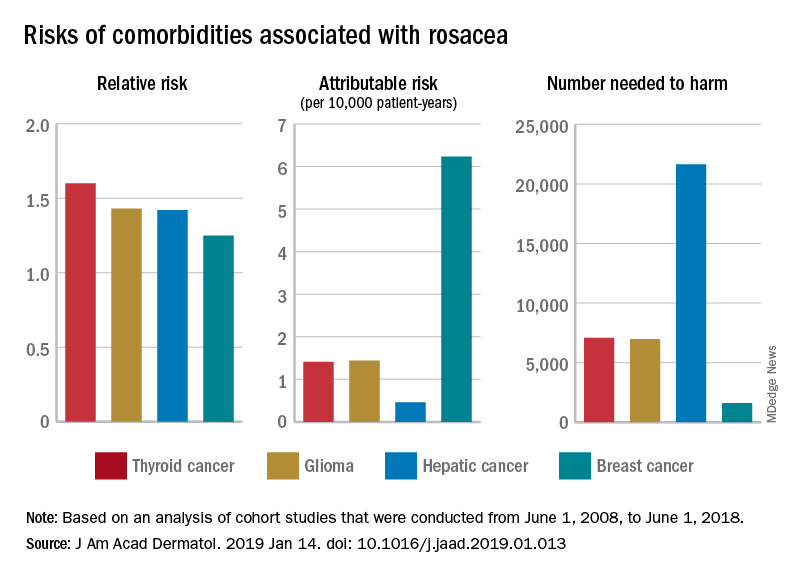
For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.

For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
Rosacea has several known comorbidities, but the widespread use of their relative, rather than absolute, risks “may result in overestimation of the clinical importance of exposures,” Leonardo A. Tjahjono and his associates wrote.

For a patient with rosacea, the relative risk of hepatic cancer is 1.42, but the attributable risk and the number needed to harm (NNH), which provide “a clearer, absolute picture regarding the association” with rosacea, are 0.46 per 10,000 patient-years and 21,645, respectively. The relative risk of comorbid breast cancer is 1.25, compared with an attributable risk of 6.23 per 10,000 patient-years and an NNH of 1,606, Mr. Tjahjono of Wake Forest University in Winston-Salem, N.C., and his associates reported in the Journal of the American Academy of Dermatology.
Physician misconceptions based on patients’ relative risks of comorbidities may lead to increased cancer screenings, which “can provide great benefit in a proper context; however, they are not without consequences. For example, 0.7 % of liver biopsies result in severe intraperitoneal hematoma,” the investigators said.
The absolute risks – calculated by the investigators using cohort studies that were conducted from June 1, 2008, to June 1, 2018 – present “a better understanding regarding rosacea’s impact on public health and clinical settings, “ they wrote.
SOURCE: Tjahjono LA et al. J Am Acad Dermatol. 2019 Jan 14. doi: 10.1016/j.jaad.2019.01.013.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
A young girl with a painful rash
A 3-year-old girl presented with a rapidly progressing rash. The rash began the previous day with redness around her lips and nose (FIGURE 1). Twelve hours later, the rash had progressed to involve her neck, trunk, and inguinal area (FIGURE 2). The child’s parents reported that she had no recent illnesses or treatment with antibiotics.
On physical examination, she was febrile (101.8° F) and irritable throughout the encounter. She had perioral and nasolabial erythema and dryness. Her lips were dry with no intraoral mucosal lesions, and her conjunctiva was clear.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Staphylococcal scalded skin syndrome
Based on the patient’s classic presentation and exam findings, the physician suspected staphylococcal scalded skin syndrome. SSSS is a rare but serious condition that progresses quickly with high fevers and diffuse painful erythema. The exact epidemiology of SSSS is unclear; some articles report incidences between 0.09 and 0.13 cases per 1 million people.1 The mortality rate is about 5% due to complications of sepsis, superinfection, and electrolyte disturbances.2
SSSS is caused by Staphylococcus aureus from a localized source that produces exfoliative toxins A and B that spread hematogenously, causing extensive epidermal damage. Exotoxins bind to desmosomes, causing skin cells to lose adherence.3 Histopathology shows intraepidermal cleavage through the stratum granulosum.
Infants and younger children appear more likely to be affected by SSSS, although it may occur in older children or adults who are immunocompromised. It may be that younger children are most susceptible due to a lack of antibodies to the toxin produced or because of a delayed clearance of the toxin-antibody complex from an immature renal system.
What you’ll see. Patients with SSSS may have a prodrome of irritability, malaise, and fever. The rash is first noticeable as erythema in the flexural areas.4 The erythematous tender patches spread and coalesce into a scarlatiniform erythema. Fragile bullae become large sheets of epidermis that slough (a positive Nikolsky’s sign).5 The desquamated areas can exhibit a scalded appearance.3
Differential diagnosis includes TEN and SJS
There is a broad differential for vesiculobullous rashes, ranging from self-limiting conditions to those that are life threatening.
Toxic epidermal necrolysis (TEN), Stevens-Johnson Syndrome (SJS), and erythema multiforme major (EMM) are immunological reactions to certain drugs or infections varying in the severity of their presentation. EMM, SJS, and TEN involve the mucosal surfaces, while SSSS does not. The histopathology of these conditions also differs from SSSS as they have keratinocyte necrosis of varying levels of the skin, whereas SSSS only involves the epidermis.
SSSS also may be confused with drug reactions, such as DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. DRESS typically is associated with anticonvulsants and sulfonamides and may have peripheral eosinophilia and a transaminitis.4
Continue to: Other more self-limited vesiculobullous rashes...
Other more self-limited vesiculobullous rashes include human enteroviruses such as coxsackie virus (hand-foot-mouth disease), echovirus, and enterovirus. However, unlike SSSS, which only affects the epidermis, these disorders may produce epidermal necrosis resulting in epidermal-dermal separation and mucocutaneous blistering.4
Making the diagnosis
When a patient has classic SSSS, the diagnosis can be made based on exam findings and the patient’s history. Families will usually report a generalized rash in neonates with desquamation of the entire skin. Fever is often present. Recent exposures to other family members with skin and soft-tissue infections is a possibility. If there is doubt, a skin biopsy can be obtained for histology. Lab work may reveal an elevated white blood cell count; blood culture is often negative.
The primary site of S aureus infection is usually the nasopharynx, causing a mild upper respiratory tract infection; therefore, nasopharyngeal cultures may be positive.4 Cultures can also be drawn from blood, wounds, nares, and ocular exudates if there is suspicion. Cultures from the actual blisters are typically negative, as the toxin—not the actual bacteria—is responsible for the blistering. Unlike adults who experience SSSS, children typically have negative blood cultures.4
Prompt treatment is essential
Swift diagnosis and management of SSSS is important due to the risk of severe disease. It is important to start antibiotics early because methicillin-sensitive S aureus is a predominant cause of SSSS.2 The epidemiology of methicillin-sensitive and methicillin-resistant S aureus (MRSA) continues to shift. A recent study suggests that empiric therapy with penicillinase-resistant penicillins, along with clindamycin, be employed until culture sensitivities are available to guide therapy.2 Local resistance patterns to S aureus should help guide initial empiric antibiotic treatment. Patients should receive intravenous (IV) fluids to compensate for insensible fluid losses similar to an extensive burn wound. Wound dressings placed over sloughed skin can help prevent secondary infection.2 Lastly, the use of anti-inflammatory drugs and opiates often depends upon the extent of pain the patient experiences.
Our patient was immediately started on IV clindamycin 10 mg/kg tid and IV fluids. She was given morphine 0.01 mg/kg for pain control. As expected, cultures of her nasopharynx, blood, and vulva did not grow S aureus. Although no organism was isolated, her rash rapidly improved, and she was discharged home to complete a 10-day oral course of clindamycin 10 mg/kg tid.
CORRESPONDENCE
Nicholas M. Potisek, MD, Wake Forest School of Medicine, Department of Pediatrics, Medical Center Blvd, Winston-Salem, NC 27157; [email protected]
1. Mockenhaupt M, Idzko M, Grosber M, et al. Epidemiology of staphylococcal scalded skin syndrome in Germany. J Invest Dermatol. 2005;124:700-703.
2. Braunstein I, Wanat K, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
3. Mishra AK, Yadav, P, Mishra A. A systemic review on Staphylococcal Scalded Skin Syndrome (SSSS): A rare and critical disease of neonates. Open Microbiol J. 2016;10: 150-159.
4. Handler MZ, Schwarz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
5. Franco L, Pereira P. Staphylococcal scalded skin syndrome. Indian Pediatr. 2016. 53:939.
A 3-year-old girl presented with a rapidly progressing rash. The rash began the previous day with redness around her lips and nose (FIGURE 1). Twelve hours later, the rash had progressed to involve her neck, trunk, and inguinal area (FIGURE 2). The child’s parents reported that she had no recent illnesses or treatment with antibiotics.

On physical examination, she was febrile (101.8° F) and irritable throughout the encounter. She had perioral and nasolabial erythema and dryness. Her lips were dry with no intraoral mucosal lesions, and her conjunctiva was clear.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Staphylococcal scalded skin syndrome
Based on the patient’s classic presentation and exam findings, the physician suspected staphylococcal scalded skin syndrome. SSSS is a rare but serious condition that progresses quickly with high fevers and diffuse painful erythema. The exact epidemiology of SSSS is unclear; some articles report incidences between 0.09 and 0.13 cases per 1 million people.1 The mortality rate is about 5% due to complications of sepsis, superinfection, and electrolyte disturbances.2
SSSS is caused by Staphylococcus aureus from a localized source that produces exfoliative toxins A and B that spread hematogenously, causing extensive epidermal damage. Exotoxins bind to desmosomes, causing skin cells to lose adherence.3 Histopathology shows intraepidermal cleavage through the stratum granulosum.
Infants and younger children appear more likely to be affected by SSSS, although it may occur in older children or adults who are immunocompromised. It may be that younger children are most susceptible due to a lack of antibodies to the toxin produced or because of a delayed clearance of the toxin-antibody complex from an immature renal system.
What you’ll see. Patients with SSSS may have a prodrome of irritability, malaise, and fever. The rash is first noticeable as erythema in the flexural areas.4 The erythematous tender patches spread and coalesce into a scarlatiniform erythema. Fragile bullae become large sheets of epidermis that slough (a positive Nikolsky’s sign).5 The desquamated areas can exhibit a scalded appearance.3
Differential diagnosis includes TEN and SJS
There is a broad differential for vesiculobullous rashes, ranging from self-limiting conditions to those that are life threatening.
Toxic epidermal necrolysis (TEN), Stevens-Johnson Syndrome (SJS), and erythema multiforme major (EMM) are immunological reactions to certain drugs or infections varying in the severity of their presentation. EMM, SJS, and TEN involve the mucosal surfaces, while SSSS does not. The histopathology of these conditions also differs from SSSS as they have keratinocyte necrosis of varying levels of the skin, whereas SSSS only involves the epidermis.
SSSS also may be confused with drug reactions, such as DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. DRESS typically is associated with anticonvulsants and sulfonamides and may have peripheral eosinophilia and a transaminitis.4
Continue to: Other more self-limited vesiculobullous rashes...
Other more self-limited vesiculobullous rashes include human enteroviruses such as coxsackie virus (hand-foot-mouth disease), echovirus, and enterovirus. However, unlike SSSS, which only affects the epidermis, these disorders may produce epidermal necrosis resulting in epidermal-dermal separation and mucocutaneous blistering.4
Making the diagnosis
When a patient has classic SSSS, the diagnosis can be made based on exam findings and the patient’s history. Families will usually report a generalized rash in neonates with desquamation of the entire skin. Fever is often present. Recent exposures to other family members with skin and soft-tissue infections is a possibility. If there is doubt, a skin biopsy can be obtained for histology. Lab work may reveal an elevated white blood cell count; blood culture is often negative.
The primary site of S aureus infection is usually the nasopharynx, causing a mild upper respiratory tract infection; therefore, nasopharyngeal cultures may be positive.4 Cultures can also be drawn from blood, wounds, nares, and ocular exudates if there is suspicion. Cultures from the actual blisters are typically negative, as the toxin—not the actual bacteria—is responsible for the blistering. Unlike adults who experience SSSS, children typically have negative blood cultures.4
Prompt treatment is essential
Swift diagnosis and management of SSSS is important due to the risk of severe disease. It is important to start antibiotics early because methicillin-sensitive S aureus is a predominant cause of SSSS.2 The epidemiology of methicillin-sensitive and methicillin-resistant S aureus (MRSA) continues to shift. A recent study suggests that empiric therapy with penicillinase-resistant penicillins, along with clindamycin, be employed until culture sensitivities are available to guide therapy.2 Local resistance patterns to S aureus should help guide initial empiric antibiotic treatment. Patients should receive intravenous (IV) fluids to compensate for insensible fluid losses similar to an extensive burn wound. Wound dressings placed over sloughed skin can help prevent secondary infection.2 Lastly, the use of anti-inflammatory drugs and opiates often depends upon the extent of pain the patient experiences.
Our patient was immediately started on IV clindamycin 10 mg/kg tid and IV fluids. She was given morphine 0.01 mg/kg for pain control. As expected, cultures of her nasopharynx, blood, and vulva did not grow S aureus. Although no organism was isolated, her rash rapidly improved, and she was discharged home to complete a 10-day oral course of clindamycin 10 mg/kg tid.
CORRESPONDENCE
Nicholas M. Potisek, MD, Wake Forest School of Medicine, Department of Pediatrics, Medical Center Blvd, Winston-Salem, NC 27157; [email protected]
A 3-year-old girl presented with a rapidly progressing rash. The rash began the previous day with redness around her lips and nose (FIGURE 1). Twelve hours later, the rash had progressed to involve her neck, trunk, and inguinal area (FIGURE 2). The child’s parents reported that she had no recent illnesses or treatment with antibiotics.

On physical examination, she was febrile (101.8° F) and irritable throughout the encounter. She had perioral and nasolabial erythema and dryness. Her lips were dry with no intraoral mucosal lesions, and her conjunctiva was clear.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Staphylococcal scalded skin syndrome
Based on the patient’s classic presentation and exam findings, the physician suspected staphylococcal scalded skin syndrome. SSSS is a rare but serious condition that progresses quickly with high fevers and diffuse painful erythema. The exact epidemiology of SSSS is unclear; some articles report incidences between 0.09 and 0.13 cases per 1 million people.1 The mortality rate is about 5% due to complications of sepsis, superinfection, and electrolyte disturbances.2
SSSS is caused by Staphylococcus aureus from a localized source that produces exfoliative toxins A and B that spread hematogenously, causing extensive epidermal damage. Exotoxins bind to desmosomes, causing skin cells to lose adherence.3 Histopathology shows intraepidermal cleavage through the stratum granulosum.
Infants and younger children appear more likely to be affected by SSSS, although it may occur in older children or adults who are immunocompromised. It may be that younger children are most susceptible due to a lack of antibodies to the toxin produced or because of a delayed clearance of the toxin-antibody complex from an immature renal system.
What you’ll see. Patients with SSSS may have a prodrome of irritability, malaise, and fever. The rash is first noticeable as erythema in the flexural areas.4 The erythematous tender patches spread and coalesce into a scarlatiniform erythema. Fragile bullae become large sheets of epidermis that slough (a positive Nikolsky’s sign).5 The desquamated areas can exhibit a scalded appearance.3
Differential diagnosis includes TEN and SJS
There is a broad differential for vesiculobullous rashes, ranging from self-limiting conditions to those that are life threatening.
Toxic epidermal necrolysis (TEN), Stevens-Johnson Syndrome (SJS), and erythema multiforme major (EMM) are immunological reactions to certain drugs or infections varying in the severity of their presentation. EMM, SJS, and TEN involve the mucosal surfaces, while SSSS does not. The histopathology of these conditions also differs from SSSS as they have keratinocyte necrosis of varying levels of the skin, whereas SSSS only involves the epidermis.
SSSS also may be confused with drug reactions, such as DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome. DRESS typically is associated with anticonvulsants and sulfonamides and may have peripheral eosinophilia and a transaminitis.4
Continue to: Other more self-limited vesiculobullous rashes...
Other more self-limited vesiculobullous rashes include human enteroviruses such as coxsackie virus (hand-foot-mouth disease), echovirus, and enterovirus. However, unlike SSSS, which only affects the epidermis, these disorders may produce epidermal necrosis resulting in epidermal-dermal separation and mucocutaneous blistering.4
Making the diagnosis
When a patient has classic SSSS, the diagnosis can be made based on exam findings and the patient’s history. Families will usually report a generalized rash in neonates with desquamation of the entire skin. Fever is often present. Recent exposures to other family members with skin and soft-tissue infections is a possibility. If there is doubt, a skin biopsy can be obtained for histology. Lab work may reveal an elevated white blood cell count; blood culture is often negative.
The primary site of S aureus infection is usually the nasopharynx, causing a mild upper respiratory tract infection; therefore, nasopharyngeal cultures may be positive.4 Cultures can also be drawn from blood, wounds, nares, and ocular exudates if there is suspicion. Cultures from the actual blisters are typically negative, as the toxin—not the actual bacteria—is responsible for the blistering. Unlike adults who experience SSSS, children typically have negative blood cultures.4
Prompt treatment is essential
Swift diagnosis and management of SSSS is important due to the risk of severe disease. It is important to start antibiotics early because methicillin-sensitive S aureus is a predominant cause of SSSS.2 The epidemiology of methicillin-sensitive and methicillin-resistant S aureus (MRSA) continues to shift. A recent study suggests that empiric therapy with penicillinase-resistant penicillins, along with clindamycin, be employed until culture sensitivities are available to guide therapy.2 Local resistance patterns to S aureus should help guide initial empiric antibiotic treatment. Patients should receive intravenous (IV) fluids to compensate for insensible fluid losses similar to an extensive burn wound. Wound dressings placed over sloughed skin can help prevent secondary infection.2 Lastly, the use of anti-inflammatory drugs and opiates often depends upon the extent of pain the patient experiences.
Our patient was immediately started on IV clindamycin 10 mg/kg tid and IV fluids. She was given morphine 0.01 mg/kg for pain control. As expected, cultures of her nasopharynx, blood, and vulva did not grow S aureus. Although no organism was isolated, her rash rapidly improved, and she was discharged home to complete a 10-day oral course of clindamycin 10 mg/kg tid.
CORRESPONDENCE
Nicholas M. Potisek, MD, Wake Forest School of Medicine, Department of Pediatrics, Medical Center Blvd, Winston-Salem, NC 27157; [email protected]
1. Mockenhaupt M, Idzko M, Grosber M, et al. Epidemiology of staphylococcal scalded skin syndrome in Germany. J Invest Dermatol. 2005;124:700-703.
2. Braunstein I, Wanat K, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
3. Mishra AK, Yadav, P, Mishra A. A systemic review on Staphylococcal Scalded Skin Syndrome (SSSS): A rare and critical disease of neonates. Open Microbiol J. 2016;10: 150-159.
4. Handler MZ, Schwarz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
5. Franco L, Pereira P. Staphylococcal scalded skin syndrome. Indian Pediatr. 2016. 53:939.
1. Mockenhaupt M, Idzko M, Grosber M, et al. Epidemiology of staphylococcal scalded skin syndrome in Germany. J Invest Dermatol. 2005;124:700-703.
2. Braunstein I, Wanat K, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
3. Mishra AK, Yadav, P, Mishra A. A systemic review on Staphylococcal Scalded Skin Syndrome (SSSS): A rare and critical disease of neonates. Open Microbiol J. 2016;10: 150-159.
4. Handler MZ, Schwarz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
5. Franco L, Pereira P. Staphylococcal scalded skin syndrome. Indian Pediatr. 2016. 53:939.
History of melanoma in situ • dyspnea • rib pain • Dx?
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.

A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.

A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).

A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.

THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.

A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).

A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.

THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.