User login
What's your diagnosis?
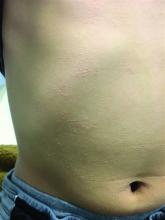
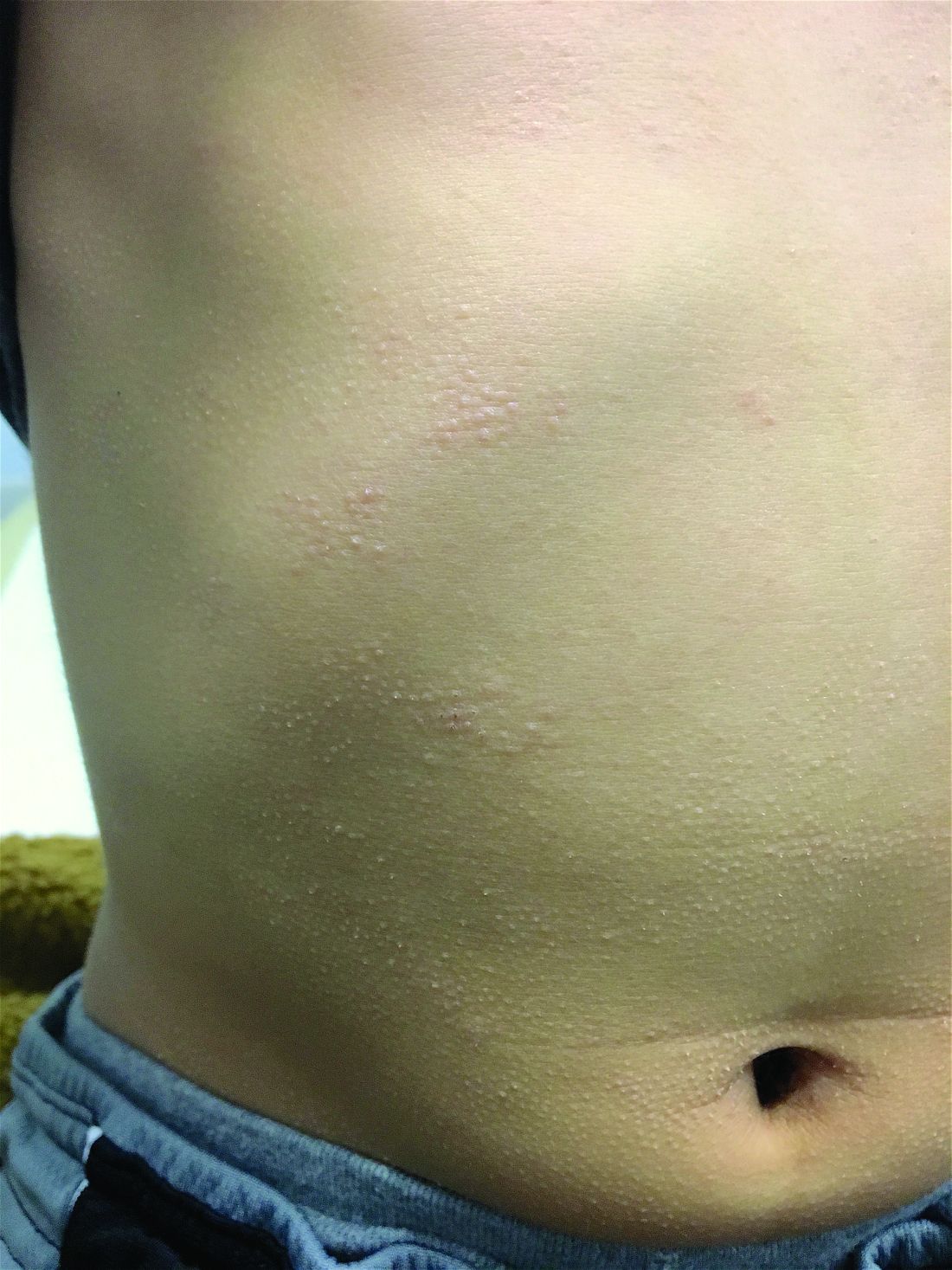
The patient was diagnosed with lichen spinulosus (LS) based on the physical appearance of the lesions (hyperkeratotic spiny papules forming plaques), the lack of pruritus, and negative personal history of atopic dermatitis.
Lichen spinulosus is an underreported entity, first described in 1908 by Adamson as superficial circumscribed chronic dermatitis in children and adolescents. The median age of presentation is age 16 years. There are several reports of possible associations with systemic infections such as HIV, fungi, and syphilis, as well as chronic diseases such as Crohn’s disease, Hodgkin disease, seborrhea, and secondary to certain medications such as omeprazole. There are no prior reports of infliximab being associated with LS, but it has been reported to cause other lichenoid reactions such as lichen planus and lichen planopilaris.
Clinically the lesions are characterized by asymptomatic, small (1 cm), skin color, hyperkeratotic, follicular papules that coalesce into plaques. The lesions usually occur on the extensor surfaces of the arms, neck, torso, and buttocks. Mild pruritus can occur in some patients.
The lesions in keratosis pilaris can be very similar to lichen spinulosus, but usually they don’t coalesce into plaques and are commonly present on the extensor surfaces of the arms, thighs, and cheeks. Histopathology of both conditions is very similar.
Another condition to consider includes papular eczema. The lesions in papular eczema tend to be pruritic and are not as circumscribed as LS lesions. Papular eczema responds well to the use of topical corticosteroids, while LS lesions usually do not. Lichen nitidus (LN) is characterized by monomorphic, skin color, 1-mm, flat-topped papules. Lesions tend to occur in crops rather than circumscribed papules forming plaques. LN most commonly presents on the extensor surface of the arms, trunk, dorsal hands, and genitalia. Koebner phenomenon is usually seen. Although uncommon in children, a more generalized type of follicular mucinosis can present very similar to lichen spinulosus. A recent study found LS-like lesions with associated hypopigmentation and hair loss should be suspicious for folliculotropic mycosis fungoides.
Keratolytics such as lactic acid, urea, and salicylic acid can help improve LS, although they do not cure it. Other reported treatments include the use of topical adapalene, tacalcitol cream, and tretinoin gel with hydroactive adhesive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
The patient was diagnosed with lichen spinulosus (LS) based on the physical appearance of the lesions (hyperkeratotic spiny papules forming plaques), the lack of pruritus, and negative personal history of atopic dermatitis.
Lichen spinulosus is an underreported entity, first described in 1908 by Adamson as superficial circumscribed chronic dermatitis in children and adolescents. The median age of presentation is age 16 years. There are several reports of possible associations with systemic infections such as HIV, fungi, and syphilis, as well as chronic diseases such as Crohn’s disease, Hodgkin disease, seborrhea, and secondary to certain medications such as omeprazole. There are no prior reports of infliximab being associated with LS, but it has been reported to cause other lichenoid reactions such as lichen planus and lichen planopilaris.
Clinically the lesions are characterized by asymptomatic, small (1 cm), skin color, hyperkeratotic, follicular papules that coalesce into plaques. The lesions usually occur on the extensor surfaces of the arms, neck, torso, and buttocks. Mild pruritus can occur in some patients.
The lesions in keratosis pilaris can be very similar to lichen spinulosus, but usually they don’t coalesce into plaques and are commonly present on the extensor surfaces of the arms, thighs, and cheeks. Histopathology of both conditions is very similar.
Another condition to consider includes papular eczema. The lesions in papular eczema tend to be pruritic and are not as circumscribed as LS lesions. Papular eczema responds well to the use of topical corticosteroids, while LS lesions usually do not. Lichen nitidus (LN) is characterized by monomorphic, skin color, 1-mm, flat-topped papules. Lesions tend to occur in crops rather than circumscribed papules forming plaques. LN most commonly presents on the extensor surface of the arms, trunk, dorsal hands, and genitalia. Koebner phenomenon is usually seen. Although uncommon in children, a more generalized type of follicular mucinosis can present very similar to lichen spinulosus. A recent study found LS-like lesions with associated hypopigmentation and hair loss should be suspicious for folliculotropic mycosis fungoides.
Keratolytics such as lactic acid, urea, and salicylic acid can help improve LS, although they do not cure it. Other reported treatments include the use of topical adapalene, tacalcitol cream, and tretinoin gel with hydroactive adhesive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
The patient was diagnosed with lichen spinulosus (LS) based on the physical appearance of the lesions (hyperkeratotic spiny papules forming plaques), the lack of pruritus, and negative personal history of atopic dermatitis.
Lichen spinulosus is an underreported entity, first described in 1908 by Adamson as superficial circumscribed chronic dermatitis in children and adolescents. The median age of presentation is age 16 years. There are several reports of possible associations with systemic infections such as HIV, fungi, and syphilis, as well as chronic diseases such as Crohn’s disease, Hodgkin disease, seborrhea, and secondary to certain medications such as omeprazole. There are no prior reports of infliximab being associated with LS, but it has been reported to cause other lichenoid reactions such as lichen planus and lichen planopilaris.
Clinically the lesions are characterized by asymptomatic, small (1 cm), skin color, hyperkeratotic, follicular papules that coalesce into plaques. The lesions usually occur on the extensor surfaces of the arms, neck, torso, and buttocks. Mild pruritus can occur in some patients.
The lesions in keratosis pilaris can be very similar to lichen spinulosus, but usually they don’t coalesce into plaques and are commonly present on the extensor surfaces of the arms, thighs, and cheeks. Histopathology of both conditions is very similar.
Another condition to consider includes papular eczema. The lesions in papular eczema tend to be pruritic and are not as circumscribed as LS lesions. Papular eczema responds well to the use of topical corticosteroids, while LS lesions usually do not. Lichen nitidus (LN) is characterized by monomorphic, skin color, 1-mm, flat-topped papules. Lesions tend to occur in crops rather than circumscribed papules forming plaques. LN most commonly presents on the extensor surface of the arms, trunk, dorsal hands, and genitalia. Koebner phenomenon is usually seen. Although uncommon in children, a more generalized type of follicular mucinosis can present very similar to lichen spinulosus. A recent study found LS-like lesions with associated hypopigmentation and hair loss should be suspicious for folliculotropic mycosis fungoides.
Keratolytics such as lactic acid, urea, and salicylic acid can help improve LS, although they do not cure it. Other reported treatments include the use of topical adapalene, tacalcitol cream, and tretinoin gel with hydroactive adhesive.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
A 7-year-old male with a history of Crohn's disease presents with 6 months of asymptomatic, bumpy lesions on the torso and extremities. He has been using over-the-counter hydrocortisone and moisturizers without it helping. His Crohn's disease has been controlled with infliximab infusions for 2 years. The mother is concerned the rash could be a side effect of the medication.
He denies any prior history of atopic dermatitis or psoriasis. The mother had eczema as a child. He has two brothers who have been diagnosed and treated for allergic rhinitis.
On physical examination, he is a thin, pleasant young boy in no distress.
His skin is somewhat dry, and there are several hyperkeratotic follicular papules forming plaques on the torso and extremities. There is no associated hair loss on the affected areas or inflammation noted.
Novel atopic dermatitis cream shows promise in phase 2 study
Adults with mild to moderate atopic dermatitis showed significant improvement after 8 weeks of treatment with a novel topical cream, compared with a placebo group, based on data from 194 patients.
“Transient receptor potential vanilloid subfamily, member 1 (TRPV1) is expressed not only on sensory nerves, but also on keratinocytes, dendritic cells and sebocytes in the skin,” wrote Y.W. Lee, MD, of Konkuk University, Seoul, South Korea, and colleagues. Previous research suggests that TRPV1 may play a role in the inflammation and itching associated with atopic dermatitis, but use of a TRPV antagonist as treatment has not been well studied, the researchers said.
In a phase 2b trial published in the British Journal of Dermatology, the researchers randomized 194 adults with atopic dermatitis to one of three concentrations of a topical cream containing the selective TRPV1 antagonist PAC‐14028, or a placebo vehicle. The patients had baseline scores of 2 or 3 (mild to moderate) on the Investigator’s Global Assessment (IGA) scale. Patients were instructed to apply the cream twice daily to AD-affected areas.
After 8 weeks, treatment success (defined as a score of 0 or 1 on the IGA) occurred in 57% of patients given 1% cream, 38% of those given 0.3% cream, 43% of those given 0.1% cream, and 15% of those given a placebo cream.
In addition, other measures of improvement including the Scoring of Atopic Dermatitis (SCORAD) index, EASI 75/90, sleep disturbance score, and pruritus visual analogue scale (VAS) trended toward improvement in patients who received the treatment cream.
The mean change in the SCORAD index was significantly greater in the 0.1% and 1.0% groups, compared with the placebo group. Also of note, patients in the 1.0% cream group showed significant improvements in both sleep disturbance and VAS scores, compared with the placebo patients, the researchers said.
The incidence of adverse events was similar among the groups, and no treatment-related serious adverse events were reported. A total of 18 patients discontinued the study, but 193 received at least one dose of treatment cream.
The study findings were limited by several factors, including the small size and lack of comparison to treatment with topical corticosteroids and topical calcineurin inhibitors, the researchers noted.
However, the results support the safety and efficacy of PAC‐14028, they added. And “based on these results, a phase III program is underway to assess the efficacy and safety of PAC-14028 topical cream 10% in adolescent and adult patients with mild to moderate AD,” they said.
AmorePacific funded the study. Dr. Lee disclosed relationships with AmorePacific, as well as LG Household & Health Care and Medytox.
SOURCE: Lee YW et al. Br J Dermatol. 2019 Jan 8. doi: 10.1111/bjd.17455.
Adults with mild to moderate atopic dermatitis showed significant improvement after 8 weeks of treatment with a novel topical cream, compared with a placebo group, based on data from 194 patients.
“Transient receptor potential vanilloid subfamily, member 1 (TRPV1) is expressed not only on sensory nerves, but also on keratinocytes, dendritic cells and sebocytes in the skin,” wrote Y.W. Lee, MD, of Konkuk University, Seoul, South Korea, and colleagues. Previous research suggests that TRPV1 may play a role in the inflammation and itching associated with atopic dermatitis, but use of a TRPV antagonist as treatment has not been well studied, the researchers said.
In a phase 2b trial published in the British Journal of Dermatology, the researchers randomized 194 adults with atopic dermatitis to one of three concentrations of a topical cream containing the selective TRPV1 antagonist PAC‐14028, or a placebo vehicle. The patients had baseline scores of 2 or 3 (mild to moderate) on the Investigator’s Global Assessment (IGA) scale. Patients were instructed to apply the cream twice daily to AD-affected areas.
After 8 weeks, treatment success (defined as a score of 0 or 1 on the IGA) occurred in 57% of patients given 1% cream, 38% of those given 0.3% cream, 43% of those given 0.1% cream, and 15% of those given a placebo cream.
In addition, other measures of improvement including the Scoring of Atopic Dermatitis (SCORAD) index, EASI 75/90, sleep disturbance score, and pruritus visual analogue scale (VAS) trended toward improvement in patients who received the treatment cream.
The mean change in the SCORAD index was significantly greater in the 0.1% and 1.0% groups, compared with the placebo group. Also of note, patients in the 1.0% cream group showed significant improvements in both sleep disturbance and VAS scores, compared with the placebo patients, the researchers said.
The incidence of adverse events was similar among the groups, and no treatment-related serious adverse events were reported. A total of 18 patients discontinued the study, but 193 received at least one dose of treatment cream.
The study findings were limited by several factors, including the small size and lack of comparison to treatment with topical corticosteroids and topical calcineurin inhibitors, the researchers noted.
However, the results support the safety and efficacy of PAC‐14028, they added. And “based on these results, a phase III program is underway to assess the efficacy and safety of PAC-14028 topical cream 10% in adolescent and adult patients with mild to moderate AD,” they said.
AmorePacific funded the study. Dr. Lee disclosed relationships with AmorePacific, as well as LG Household & Health Care and Medytox.
SOURCE: Lee YW et al. Br J Dermatol. 2019 Jan 8. doi: 10.1111/bjd.17455.
Adults with mild to moderate atopic dermatitis showed significant improvement after 8 weeks of treatment with a novel topical cream, compared with a placebo group, based on data from 194 patients.
“Transient receptor potential vanilloid subfamily, member 1 (TRPV1) is expressed not only on sensory nerves, but also on keratinocytes, dendritic cells and sebocytes in the skin,” wrote Y.W. Lee, MD, of Konkuk University, Seoul, South Korea, and colleagues. Previous research suggests that TRPV1 may play a role in the inflammation and itching associated with atopic dermatitis, but use of a TRPV antagonist as treatment has not been well studied, the researchers said.
In a phase 2b trial published in the British Journal of Dermatology, the researchers randomized 194 adults with atopic dermatitis to one of three concentrations of a topical cream containing the selective TRPV1 antagonist PAC‐14028, or a placebo vehicle. The patients had baseline scores of 2 or 3 (mild to moderate) on the Investigator’s Global Assessment (IGA) scale. Patients were instructed to apply the cream twice daily to AD-affected areas.
After 8 weeks, treatment success (defined as a score of 0 or 1 on the IGA) occurred in 57% of patients given 1% cream, 38% of those given 0.3% cream, 43% of those given 0.1% cream, and 15% of those given a placebo cream.
In addition, other measures of improvement including the Scoring of Atopic Dermatitis (SCORAD) index, EASI 75/90, sleep disturbance score, and pruritus visual analogue scale (VAS) trended toward improvement in patients who received the treatment cream.
The mean change in the SCORAD index was significantly greater in the 0.1% and 1.0% groups, compared with the placebo group. Also of note, patients in the 1.0% cream group showed significant improvements in both sleep disturbance and VAS scores, compared with the placebo patients, the researchers said.
The incidence of adverse events was similar among the groups, and no treatment-related serious adverse events were reported. A total of 18 patients discontinued the study, but 193 received at least one dose of treatment cream.
The study findings were limited by several factors, including the small size and lack of comparison to treatment with topical corticosteroids and topical calcineurin inhibitors, the researchers noted.
However, the results support the safety and efficacy of PAC‐14028, they added. And “based on these results, a phase III program is underway to assess the efficacy and safety of PAC-14028 topical cream 10% in adolescent and adult patients with mild to moderate AD,” they said.
AmorePacific funded the study. Dr. Lee disclosed relationships with AmorePacific, as well as LG Household & Health Care and Medytox.
SOURCE: Lee YW et al. Br J Dermatol. 2019 Jan 8. doi: 10.1111/bjd.17455.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Significantly more atopic dermatitis patients improved when treated with PAC-14028 cream, compared with those treated with a placebo vehicle.
Major finding: After 8 weeks, 58% of patients given the 1.0 cream achieved scores of 0 or 1 on the Investigator’s Global Assessment scale, compared with 15% for placebo patients.
Study details: The data come from a phase 2b randomized, double-blind trial including 194 atopic dermatitis patients.
Disclosures: AmorePacific funded the study. Dr. Lee disclosed relationships with AmorePacific, as well as LG Household & Health Care and Medytox.
Source: Lee YW et al. Br J Dermatol. 2019 Jan 8. doi: 10.1111/bjd.17455.
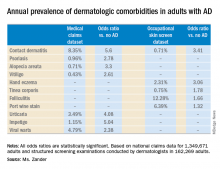
Adult atopic dermatitis is fraught with dermatologic comorbidities
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Adult atopic dermatitis patients have higher prevalence of psoriasis, alopecia areata, vitiligo, and other dermatologic disorders.
Major finding: The risk of contact dermatitis is increased 3.4- to 5.6-fold.
Study details: This German population-based study used both a medical claims database including more than 1.3 million adults and dermatologist-conducted occupational skin screening in more than 162,000 workers.
Disclosures: The study presenter reported having no financial conflicts regarding her study, conducted without commercial support.
Deep sleep decreases, Alzheimer’s increases
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
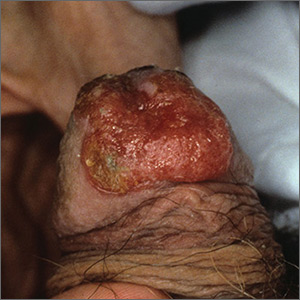
Growing lesion
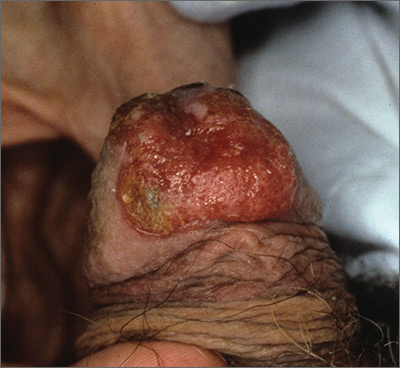
The FP recognized this as squamous cell carcinoma (SCC) of the penis.
The FP knew that a biopsy would be needed to confirm his clinical impression and obtained informed consent for a shave biopsy of a portion of the lesion. While the FP was taught in medical school to never use epinephrine on the penis, he realized that this was merely a myth (see “Biopsies for skin cancer detection: Dispelling the myths”). He injected 1% lidocaine with epinephrine into the lesion for anesthesia and to minimize bleeding during the shave biopsy. (See the Watch & Learn video on “Shave biopsy.”) The FP performed a shave biopsy of a small portion of the lesion farthest from the urethra.
Aluminum chloride was used to stop most of the bleeding, but since the penis is very vascular, some electrocoagulation was needed to stop all of the bleeding. The pathology came back as an invasive SCC. Due to the location of the lesion on the glans and around the urethra, the patient was referred to Urology.
A partial penectomy was performed and clear surgical margins were achieved. If the lesion had been on the shaft of the penis (rather than the glans penis), a Mohs surgeon would have attempted to save the whole penis with tissue sparing surgery.
Photo courtesy of Jeff Meffert, MD, and text courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP recognized this as squamous cell carcinoma (SCC) of the penis.
The FP knew that a biopsy would be needed to confirm his clinical impression and obtained informed consent for a shave biopsy of a portion of the lesion. While the FP was taught in medical school to never use epinephrine on the penis, he realized that this was merely a myth (see “Biopsies for skin cancer detection: Dispelling the myths”). He injected 1% lidocaine with epinephrine into the lesion for anesthesia and to minimize bleeding during the shave biopsy. (See the Watch & Learn video on “Shave biopsy.”) The FP performed a shave biopsy of a small portion of the lesion farthest from the urethra.
Aluminum chloride was used to stop most of the bleeding, but since the penis is very vascular, some electrocoagulation was needed to stop all of the bleeding. The pathology came back as an invasive SCC. Due to the location of the lesion on the glans and around the urethra, the patient was referred to Urology.
A partial penectomy was performed and clear surgical margins were achieved. If the lesion had been on the shaft of the penis (rather than the glans penis), a Mohs surgeon would have attempted to save the whole penis with tissue sparing surgery.
Photo courtesy of Jeff Meffert, MD, and text courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP recognized this as squamous cell carcinoma (SCC) of the penis.
The FP knew that a biopsy would be needed to confirm his clinical impression and obtained informed consent for a shave biopsy of a portion of the lesion. While the FP was taught in medical school to never use epinephrine on the penis, he realized that this was merely a myth (see “Biopsies for skin cancer detection: Dispelling the myths”). He injected 1% lidocaine with epinephrine into the lesion for anesthesia and to minimize bleeding during the shave biopsy. (See the Watch & Learn video on “Shave biopsy.”) The FP performed a shave biopsy of a small portion of the lesion farthest from the urethra.
Aluminum chloride was used to stop most of the bleeding, but since the penis is very vascular, some electrocoagulation was needed to stop all of the bleeding. The pathology came back as an invasive SCC. Due to the location of the lesion on the glans and around the urethra, the patient was referred to Urology.
A partial penectomy was performed and clear surgical margins were achieved. If the lesion had been on the shaft of the penis (rather than the glans penis), a Mohs surgeon would have attempted to save the whole penis with tissue sparing surgery.
Photo courtesy of Jeff Meffert, MD, and text courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Chronic infections such as HCV, HIV, and TB cause unique problems for psoriasis patients
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
In a review of therapeutic issues for psoriasis patients who have such chronic infections as hepatitis, HIV, or latent tuberculosis infection (LTBI) or those who fall into the category of special populations (pregnant women or children), significant concerns were directly tied to the mode of action of the drugs involved.
In particular, “Most systemic agents for psoriasis are immunosuppressive, which poses a unique treatment challenge in patients with psoriasis with chronic infections because they are already immunosuppressed,” according to Shivani B. Kaushik, MD, a resident in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and her colleague Mark G. Lebwohl, MD, professor and system chair of the department.
For example, the reviewers detailed a report of hepatitis B virus (HBV) and hepatitis C virus (HCV) reactivation in patients with psoriasis who were taking biologics. Virus reactivation was noted in 2/175 patients who were positive for anti-HBc antibody, 3/97 patients with HCV infection, and 8/40 patients who were positive for HBsAg (the surface antigen of HBV). From this, they concluded that “biologics pose minimal risk for viral reactivation in patients with anti-HCV or anti-HBc antibodies, but they are of considerable risk in HBsAg-positive patients.” (J Amer Acad Derm. 2019 Jan;80:43-53).
Giving a specific example, Dr. Kaushik and her colleague pointed out that the safety of ustekinumab in patients with psoriasis with concurrent HCV and HBV infection was not clear. Viral reactivation and hepatocellular cancer were reported in one of four patients with HCV and in two of seven HBsAg-positive patients; and yet, another study showed that the successful use of ustekinumab for psoriasis had no impact on liver function or viral load in a patient with coexisting HCV.
Overall, “Patients should not be treated with immunosuppressive therapies during the acute stage. However, biologic treatment can be initiated in patients with chronic or resolved hepatitis under close monitoring and collaboration with a gastroenterologist,” the researchers stated.
In addition, they pointed out that methotrexate, another commonly prescribed drug for psoriasis, is absolutely contraindicated, although the use of cyclosporine remains controversial for those patients who are HCV-antibody positive.
“Most systemic agents used in psoriasis are immunosuppressive and require appropriate screening, monitoring, and prophylaxis when used in [psoriasis] patients with chronic infections, such as hepatitis, HIV, and LTBI,” the authors concluded.
The authors reported receiving funding from a number of pharmaceutical companies.
SOURCE: Kaushik BS et al. J Amer Acad Derm. 2019;80:43-53.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Danish study finds reassuring data on pregnancy outcomes in atopic dermatitis patients
PARIS – over an 18-year period, which showed no increased risk of pregnancy and birth problems other than modestly increased risks of premature rupture of membranes and neonatal staphylococcal septicemia, according to Jacob P. Thyssen, MD, PhD.
At a session of the European Task Force of Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology, he presented a case control study of 10,668 births to Danish women with atopic dermatitis (AD) during 1997-2014. They were matched 1:10 by age, parity, and birth year to mothers without AD.
The risk of premature rupture of membranes was 15% higher in mothers with AD. And while the increased relative risk of neonatal staphylococcal septicemia was more substantial – a 145% increase – this was in fact a rare complication, observed Dr. Thyssen, a dermatologist at the University of Copenhagen.
There was no significant difference between women with or without AD in rates of preeclampsia, prematurity, pregnancy-induced hypertension, placenta previa, placental abruption, neonatal nonstaphylococcal septicemia, or other complications. The two groups had a similar number of visits to physicians and midwives during pregnancy.
Moreover, although the body mass index was similar in women with or without AD, the risk of gestational diabetes in women with the disease was significantly reduced by 21%; their risk of having a large-for-gestational-age baby with a birth weight of 4,500 g or more was also significantly lower than in controls.
Women received less treatment for AD during their pregnancy than they did beforehand. While pregnant, their disease was managed predominantly with topical corticosteroids and UV therapy. There was very little use of superpotent topical steroids, topical calcineurin inhibitors, or immunosuppressants, although 10% of pregnant women received systemic corticosteroids for their AD.
Dr. Thyssen reported serving as a scientific adviser and paid speaker for Leo Pharma, Roche, Eli Lilly, and Sanofi-Genzyme, although this study was conducted without commercial support.
PARIS – over an 18-year period, which showed no increased risk of pregnancy and birth problems other than modestly increased risks of premature rupture of membranes and neonatal staphylococcal septicemia, according to Jacob P. Thyssen, MD, PhD.
At a session of the European Task Force of Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology, he presented a case control study of 10,668 births to Danish women with atopic dermatitis (AD) during 1997-2014. They were matched 1:10 by age, parity, and birth year to mothers without AD.
The risk of premature rupture of membranes was 15% higher in mothers with AD. And while the increased relative risk of neonatal staphylococcal septicemia was more substantial – a 145% increase – this was in fact a rare complication, observed Dr. Thyssen, a dermatologist at the University of Copenhagen.
There was no significant difference between women with or without AD in rates of preeclampsia, prematurity, pregnancy-induced hypertension, placenta previa, placental abruption, neonatal nonstaphylococcal septicemia, or other complications. The two groups had a similar number of visits to physicians and midwives during pregnancy.
Moreover, although the body mass index was similar in women with or without AD, the risk of gestational diabetes in women with the disease was significantly reduced by 21%; their risk of having a large-for-gestational-age baby with a birth weight of 4,500 g or more was also significantly lower than in controls.
Women received less treatment for AD during their pregnancy than they did beforehand. While pregnant, their disease was managed predominantly with topical corticosteroids and UV therapy. There was very little use of superpotent topical steroids, topical calcineurin inhibitors, or immunosuppressants, although 10% of pregnant women received systemic corticosteroids for their AD.
Dr. Thyssen reported serving as a scientific adviser and paid speaker for Leo Pharma, Roche, Eli Lilly, and Sanofi-Genzyme, although this study was conducted without commercial support.
PARIS – over an 18-year period, which showed no increased risk of pregnancy and birth problems other than modestly increased risks of premature rupture of membranes and neonatal staphylococcal septicemia, according to Jacob P. Thyssen, MD, PhD.
At a session of the European Task Force of Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology, he presented a case control study of 10,668 births to Danish women with atopic dermatitis (AD) during 1997-2014. They were matched 1:10 by age, parity, and birth year to mothers without AD.
The risk of premature rupture of membranes was 15% higher in mothers with AD. And while the increased relative risk of neonatal staphylococcal septicemia was more substantial – a 145% increase – this was in fact a rare complication, observed Dr. Thyssen, a dermatologist at the University of Copenhagen.
There was no significant difference between women with or without AD in rates of preeclampsia, prematurity, pregnancy-induced hypertension, placenta previa, placental abruption, neonatal nonstaphylococcal septicemia, or other complications. The two groups had a similar number of visits to physicians and midwives during pregnancy.
Moreover, although the body mass index was similar in women with or without AD, the risk of gestational diabetes in women with the disease was significantly reduced by 21%; their risk of having a large-for-gestational-age baby with a birth weight of 4,500 g or more was also significantly lower than in controls.
Women received less treatment for AD during their pregnancy than they did beforehand. While pregnant, their disease was managed predominantly with topical corticosteroids and UV therapy. There was very little use of superpotent topical steroids, topical calcineurin inhibitors, or immunosuppressants, although 10% of pregnant women received systemic corticosteroids for their AD.
Dr. Thyssen reported serving as a scientific adviser and paid speaker for Leo Pharma, Roche, Eli Lilly, and Sanofi-Genzyme, although this study was conducted without commercial support.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Birth complications are uncommon for women with atopic dermatitis in pregnancy.
Major finding: The risk of premature rupture of membranes was increased by 15% in women with atopic dermatitis in pregnancy, but their risk of gestational diabetes was reduced by 21%.
Study details: This case control study included 10,668 births to Danish women with atopic dermatitis and 10 times as many matched controls without the disease.
Disclosures: The study presenter reported serving as a scientific adviser and paid speaker for Leo Pharma, Roche, Eli Lilly, and Sanofi-Genzyme, although this study was conducted without commercial support.
Difelikefalin shows promise for hemodialysis-associated itch
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
PARIS – while achieving across-the-board clinically meaningful improvements in quality of life measures in patients with hemodialysis-associated pruritus in a phase 2 study, Frédérique Menzaghi, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
At present there is no approved medication in the United States or Europe for the often intense itching associated with chronic kidney disease. Off-label treatments have limited efficacy.
Dr. Menzaghi is senior vice president for research and development at Cara Therapeutics, which is developing difelikefalin.
More than half – 60% to 70% – of patients on hemodialysis for end-stage renal disease experience chronic pruritus, as do a smaller proportion of individuals with chronic kidney disease (CKD) not requiring dialysis. CKD-associated pruritus is a day-and-night itch that makes life miserable for affected patients. Not only must they endure the predictable complications of skin excoriation, including impetigo, ulcerations, papules, and prurigo nodularis, but they also experience sleep disruption, depressed mood, and a 10%-20% increased mortality risk compared with CKD patients without pruritus.
Difelikefalin is a potent and selective peripheral kappa opioid receptor agonist that doesn’t activate mu or delta opioid receptors. It’s a synthetic drug that mimics endogenous dynorphin. Its key attribute is that it doesn’t cross the blood/brain barrier, so it doesn’t pose a risk for adverse events caused by activation of central opioid receptors. Difelikefalin has two mechanisms of action in CKD-associated pruritus: an antipruritic effect due to inhibition of ion channels responsible for afferent peripheral nerve activity; and an anti-inflammatory effect mediated by activation of kappa opioid receptors expressed by immune system cells, according to Dr. Menzaghi.
She reported on 174 hemodialysis patients with moderate to severe CKD-associated pruritus who were randomized to a double-blind, phase 2, dose-ranging study featuring an intravenous bolus of difelikefalin at 0.5, 1.0, or 1.5 mcg/kg or placebo given immediately after each of the thrice-weekly hemodialysis sessions for 8 weeks.
An oral formulation of difelikefalin is also under investigation for treatment of CKD-associated pruritus. The IV version is being developed for hemodialysis patients because difelikefalin is renally excreted.
“We’re taking advantage of the fact that their kidneys aren’t working. The drug stays in the system until the next dialysis because it can’t be eliminated. It’s quite convenient for these patients,” she explained.
The primary endpoint in the phase 2 study was change from baseline through week 8 in the weekly average of a patient’s daily self-rated 0-10 worst itching intensity numeric rating scale (NRS) scores. All participants had to have a baseline NRS score of at least 4, considered the lower threshold for moderate itch. In fact, the mean baseline score was 6.7-7.1 in the four study arms.
The results
Sixty-four percent of patients on difelikefalin 0.5 mcg/kg – the most effective dose – experienced at least a 3-point reduction, compared with 29% of placebo-treated controls. And a 4-point or greater reduction in NRS from baseline was documented in 51% of patients on difelikefalin at 0.5 mcg/kg, compared with 24% of controls.
Although a 4-point difference is widely considered to represent clinically meaningful improvement in atopic dermatitis studies, Dr. Menzaghi said psychometric analyses of the difelikefalin trial data indicated that a 3-point or greater improvement in NRS score was associated with clinically meaningful change.
“Our data suggest that a 4-point change may not be generalizable to all conditions,” she said.
Hemodialysis patients with severe baseline itch typically improved to moderate itch on difelikefalin, while those with baseline moderate itch – that is, an NRS of 4-6 – dropped down to mild or no itch while on the drug.
“But that’s just a number. The question is, is that really clinically meaningful?” Dr. Menzaghi noted.
The answer, she continued, is yes. A high correlation was seen between reduction in itch intensity and improvement in quality of life. Scores on the 5-D Itch Scale and Skindex-10 improved two- to threefold more in the difelikefalin 0.5-mcg group than in controls. So did scores on the 12-item Medical Outcomes Study Sleep Scale assessing sleep restlessness, awakening during sleep, and trouble falling asleep.
“We think these results suggest that peripheral kappa opioid receptors play an integral role in the modulation of itch signals and represent a primary target for the development of antipruritic agents,” said Dr. Menzaghi.
Indeed, a phase 3 randomized trial of difelikefalin 0.5 mcg/kg versus placebo in 350 hemodialysis patients with CKD-associated itch is ongoing in the United States, Europe, Australia, and Korea. Also ongoing is a phase 2 U.S. study of oral difelikefalin in patients with CKD-associated pruritus, many of whom are not on hemodialysis. In January, the company announced that enrollment in a phase 3 U.S. study of difelikefalin injection (0.5 mcg/kg) in hemodialysis patients with moderate to severe CKD-associated pruritus had been completed. The trials are funded by Cara Therapeutics.
SOURCE: Menzaghi F. EADV Congress, Abstract FC0.4.7.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Moderate to severe chronic itching associated with chronic kidney disease is a common and underrecognized problem with a huge quality of life impact.
Major finding: Sixty-four percent of hemodialysis patients on difelikefalin 0.5 mcg/kg experienced at least a 3-point reduction on a 0-10 worst daily itch numeric rating scale, compared with 29% of placebo-treated controls.
Study details: This phase 2, multicenter, 8-week, double-blind study comprised 174 patients with moderate to severe hemodialysis-related itching.
Disclosures: The study was sponsored by Cara Therapeutics and presented by a company officer.
Source: Menzaghi F. EADV Congress, Abstract FC0.4.7.
Click for Credit: STIs on the rise; psoriasis & cardiac risk; more
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
All the Little Lesions in a Row
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.
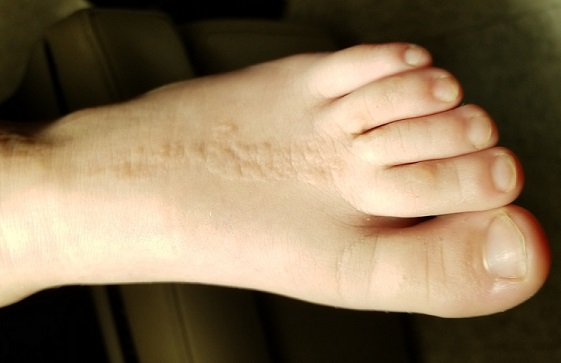
EXAMINATION
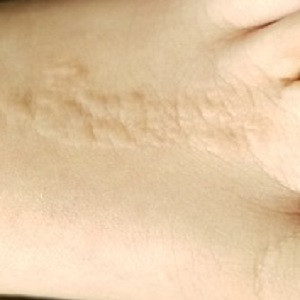
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.

EXAMINATION
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.

EXAMINATION
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.