User login
Increased cancer risk in dermatomyositis has temporal limits
The increased risk of cancer associated with anti-TIF1-Ab-positive dermatomyositis is limited almost exclusively to 3 years on either side of the onset of dermatomyositis, new research suggests.
Idiopathic inflammatory myopathy have been associated with malignancy, in particular dermatomyositis (DM) and the DM-specific antitranscriptional intermediary factor 1 antibody (anti-TIF1-Ab).
Around one-fifth of the 236 patients diagnosed with DM in the current study, published online Dec. 7 in Rheumatology, were anti-TIF1-Ab positive, and these patients had a more than threefold higher risk of developing cancer comapared with patients who were anti-TIF1-Ab negative (hazard ratio = 3.4, 95% confidence interval, 2.2-5.4; P less than .01).
Overall, 38% of patients in the anti-TIF1-Ab-positive group developed cancer during the 10-year follow-up, compared with 15% of patients with anti-TIF1-Ab-negative DM.
However, all the cancers in the anti-TIF1-Ab-positive group occurred within the 3 years before the onset of DM or within 2.5 years after onset. No anti-TIF1-Ab-positive patients developed cancers after this time, but some patients in the anti-TIF1-Ab-negative group did.
“This finding is not likely to be due to a disparity in follow-up time between anti-TIF1-Ab-positive and -negative cases, as the median follow-up times were similar for both groups: 10 years and 12 years, respectively,” wrote Alexander Oldroyd, MBChB, a clinical research fellow in the Centre for Musculoskeletal Research at the University of Manchester (England), and his coauthors. “Further, this finding is unlikely to be due to differences in cancer detection methods, as both cohorts’ cancer diagnoses were identified through HSCIC [U.K. Health and Social Care Information Centre] data, ensuring capture of all incident cancers during the follow-up period.”
Anti-TIF1-Ab-positive patients were more likely to develop cancer if they were older. None of the 15 anti-TIF1-Ab-positive patients who were aged under 39 when they developed DM went on to develop cancer. But cancer developed in around half of the anti-TIF1-Ab-positive patients who were aged 39 years or older when their DM began.
The anti-TIF1 antibody is commonly found in juvenile DM, but previous research has not found an association with an increased risk of cancer in this younger patient population.
“Our findings add strength to the hypothesis that there exists a subset of young adult anti-TIF1-Ab-positive cases who do not have a discernible increased risk of cancer, similar to that observed in TIF1-Ab-positive juvenile DM,” the authors wrote. They suggested that given the increased risk of malignancy in older patients who were anti-TIF1-Ab positive, this group should be subject to more detailed cancer screening.
Breast cancer was the most common malignancy among both anti-TIF1-Ab-positive and anti-TIF1-Ab-negative patients (33% and 25%, respectively). However, ovarian cancer was significantly more common among the anti-TIF1-Ab-positive patients than among the anti-TIF1-Ab-negative patients (19% vs. 2%; P less than .05); four of the five ovarian cancers in the entire cohort occurred in the anti-TIF1-Ab-positive group.
The authors noted that this confirmed the finding of a number of previous studies suggesting an increased risk of ovarian cancer with DM.
“However, this is the first large study to identify that ovarian cancer is overrepresented in anti-TIF1-Ab-positive individuals, suggesting that the true association between DM and ovarian cancer may be through possession of anti-TIF1-Abs,” they noted.
The authors wrote that they had aimed to inform cancer screening strategies among patients with DM.
“It may be that a focus on screening for cancer within the first 3 years after DM onset and particularly screening for ovarian cancer in anti-TIF1-Ab-positive female patients may be required,” they wrote. “Our findings also strengthen the hypothesis that inflammatory myopathies represent a paraneoplastic reaction initiated by attempted immune-mediated clearance of a cancer.”
The study was supported by Arthritis Research UK, Myositis UK, the European Science Foundation for EuMyoNet, Association Francaise Contre Les Myopathies, the Medical Research Council, and the Manchester Academic Health Science Centre. No conflicts of interest were declared.
SOURCE: Oldroyd A et al. Rheumatology. 2018 Dec 7. doi: 10.1093/rheumatology/key357.
The increased risk of cancer associated with anti-TIF1-Ab-positive dermatomyositis is limited almost exclusively to 3 years on either side of the onset of dermatomyositis, new research suggests.
Idiopathic inflammatory myopathy have been associated with malignancy, in particular dermatomyositis (DM) and the DM-specific antitranscriptional intermediary factor 1 antibody (anti-TIF1-Ab).
Around one-fifth of the 236 patients diagnosed with DM in the current study, published online Dec. 7 in Rheumatology, were anti-TIF1-Ab positive, and these patients had a more than threefold higher risk of developing cancer comapared with patients who were anti-TIF1-Ab negative (hazard ratio = 3.4, 95% confidence interval, 2.2-5.4; P less than .01).
Overall, 38% of patients in the anti-TIF1-Ab-positive group developed cancer during the 10-year follow-up, compared with 15% of patients with anti-TIF1-Ab-negative DM.
However, all the cancers in the anti-TIF1-Ab-positive group occurred within the 3 years before the onset of DM or within 2.5 years after onset. No anti-TIF1-Ab-positive patients developed cancers after this time, but some patients in the anti-TIF1-Ab-negative group did.
“This finding is not likely to be due to a disparity in follow-up time between anti-TIF1-Ab-positive and -negative cases, as the median follow-up times were similar for both groups: 10 years and 12 years, respectively,” wrote Alexander Oldroyd, MBChB, a clinical research fellow in the Centre for Musculoskeletal Research at the University of Manchester (England), and his coauthors. “Further, this finding is unlikely to be due to differences in cancer detection methods, as both cohorts’ cancer diagnoses were identified through HSCIC [U.K. Health and Social Care Information Centre] data, ensuring capture of all incident cancers during the follow-up period.”
Anti-TIF1-Ab-positive patients were more likely to develop cancer if they were older. None of the 15 anti-TIF1-Ab-positive patients who were aged under 39 when they developed DM went on to develop cancer. But cancer developed in around half of the anti-TIF1-Ab-positive patients who were aged 39 years or older when their DM began.
The anti-TIF1 antibody is commonly found in juvenile DM, but previous research has not found an association with an increased risk of cancer in this younger patient population.
“Our findings add strength to the hypothesis that there exists a subset of young adult anti-TIF1-Ab-positive cases who do not have a discernible increased risk of cancer, similar to that observed in TIF1-Ab-positive juvenile DM,” the authors wrote. They suggested that given the increased risk of malignancy in older patients who were anti-TIF1-Ab positive, this group should be subject to more detailed cancer screening.
Breast cancer was the most common malignancy among both anti-TIF1-Ab-positive and anti-TIF1-Ab-negative patients (33% and 25%, respectively). However, ovarian cancer was significantly more common among the anti-TIF1-Ab-positive patients than among the anti-TIF1-Ab-negative patients (19% vs. 2%; P less than .05); four of the five ovarian cancers in the entire cohort occurred in the anti-TIF1-Ab-positive group.
The authors noted that this confirmed the finding of a number of previous studies suggesting an increased risk of ovarian cancer with DM.
“However, this is the first large study to identify that ovarian cancer is overrepresented in anti-TIF1-Ab-positive individuals, suggesting that the true association between DM and ovarian cancer may be through possession of anti-TIF1-Abs,” they noted.
The authors wrote that they had aimed to inform cancer screening strategies among patients with DM.
“It may be that a focus on screening for cancer within the first 3 years after DM onset and particularly screening for ovarian cancer in anti-TIF1-Ab-positive female patients may be required,” they wrote. “Our findings also strengthen the hypothesis that inflammatory myopathies represent a paraneoplastic reaction initiated by attempted immune-mediated clearance of a cancer.”
The study was supported by Arthritis Research UK, Myositis UK, the European Science Foundation for EuMyoNet, Association Francaise Contre Les Myopathies, the Medical Research Council, and the Manchester Academic Health Science Centre. No conflicts of interest were declared.
SOURCE: Oldroyd A et al. Rheumatology. 2018 Dec 7. doi: 10.1093/rheumatology/key357.
The increased risk of cancer associated with anti-TIF1-Ab-positive dermatomyositis is limited almost exclusively to 3 years on either side of the onset of dermatomyositis, new research suggests.
Idiopathic inflammatory myopathy have been associated with malignancy, in particular dermatomyositis (DM) and the DM-specific antitranscriptional intermediary factor 1 antibody (anti-TIF1-Ab).
Around one-fifth of the 236 patients diagnosed with DM in the current study, published online Dec. 7 in Rheumatology, were anti-TIF1-Ab positive, and these patients had a more than threefold higher risk of developing cancer comapared with patients who were anti-TIF1-Ab negative (hazard ratio = 3.4, 95% confidence interval, 2.2-5.4; P less than .01).
Overall, 38% of patients in the anti-TIF1-Ab-positive group developed cancer during the 10-year follow-up, compared with 15% of patients with anti-TIF1-Ab-negative DM.
However, all the cancers in the anti-TIF1-Ab-positive group occurred within the 3 years before the onset of DM or within 2.5 years after onset. No anti-TIF1-Ab-positive patients developed cancers after this time, but some patients in the anti-TIF1-Ab-negative group did.
“This finding is not likely to be due to a disparity in follow-up time between anti-TIF1-Ab-positive and -negative cases, as the median follow-up times were similar for both groups: 10 years and 12 years, respectively,” wrote Alexander Oldroyd, MBChB, a clinical research fellow in the Centre for Musculoskeletal Research at the University of Manchester (England), and his coauthors. “Further, this finding is unlikely to be due to differences in cancer detection methods, as both cohorts’ cancer diagnoses were identified through HSCIC [U.K. Health and Social Care Information Centre] data, ensuring capture of all incident cancers during the follow-up period.”
Anti-TIF1-Ab-positive patients were more likely to develop cancer if they were older. None of the 15 anti-TIF1-Ab-positive patients who were aged under 39 when they developed DM went on to develop cancer. But cancer developed in around half of the anti-TIF1-Ab-positive patients who were aged 39 years or older when their DM began.
The anti-TIF1 antibody is commonly found in juvenile DM, but previous research has not found an association with an increased risk of cancer in this younger patient population.
“Our findings add strength to the hypothesis that there exists a subset of young adult anti-TIF1-Ab-positive cases who do not have a discernible increased risk of cancer, similar to that observed in TIF1-Ab-positive juvenile DM,” the authors wrote. They suggested that given the increased risk of malignancy in older patients who were anti-TIF1-Ab positive, this group should be subject to more detailed cancer screening.
Breast cancer was the most common malignancy among both anti-TIF1-Ab-positive and anti-TIF1-Ab-negative patients (33% and 25%, respectively). However, ovarian cancer was significantly more common among the anti-TIF1-Ab-positive patients than among the anti-TIF1-Ab-negative patients (19% vs. 2%; P less than .05); four of the five ovarian cancers in the entire cohort occurred in the anti-TIF1-Ab-positive group.
The authors noted that this confirmed the finding of a number of previous studies suggesting an increased risk of ovarian cancer with DM.
“However, this is the first large study to identify that ovarian cancer is overrepresented in anti-TIF1-Ab-positive individuals, suggesting that the true association between DM and ovarian cancer may be through possession of anti-TIF1-Abs,” they noted.
The authors wrote that they had aimed to inform cancer screening strategies among patients with DM.
“It may be that a focus on screening for cancer within the first 3 years after DM onset and particularly screening for ovarian cancer in anti-TIF1-Ab-positive female patients may be required,” they wrote. “Our findings also strengthen the hypothesis that inflammatory myopathies represent a paraneoplastic reaction initiated by attempted immune-mediated clearance of a cancer.”
The study was supported by Arthritis Research UK, Myositis UK, the European Science Foundation for EuMyoNet, Association Francaise Contre Les Myopathies, the Medical Research Council, and the Manchester Academic Health Science Centre. No conflicts of interest were declared.
SOURCE: Oldroyd A et al. Rheumatology. 2018 Dec 7. doi: 10.1093/rheumatology/key357.
FROM RHEUMATOLOGY
Key clinical point: Patients with dermatomyositis are at increased risk of cancer only in the 3-year periods before and after the onset of dermatomyositis.
Major finding: Overall, 38% of patients in the anti-TIF1-Ab-positive group developed cancer during the 10-year follow-up, compared with 15% of patients with anti-TIF1-Ab-negative DM.
Study details: Cohort study of 236 people with dermatomyositis.
Disclosures: The study was supported by Arthritis Research UK, Myositis UK, the European Science Foundation for EuMyoNet, Association Francaise Contre Les Myopathies, the Medical Research Council, and the Manchester Academic Health Science Centre. No conflicts of interest were declared.
Source: Oldroyd A et al. Rheumatology. 2018 Dec 7. doi: 10.1093/rheumatology/key357.
New worldwide atopic dermatitis survey brings big surprises
PARIS – A major worldwide survey of the 12-month prevalence of atopic dermatitis (AD) across the course of life provides new insights into global disease trends, Jonathan I. Silverberg, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Among the most important takeaways from this Internet-based survey of more than 273,645 infants, children, and adults in 18 countries across five continents conducted in 2017 was that “global atopic dermatitis prevalence appears to be higher in adults, at 10%, than in younger cohorts, where it’s 4%-8%, which I think is quite provocative and requires further study and confirmation,” said Dr. Silverberg, a dermatologist at Northwestern University in Chicago.
“Let’s keep in mind that there’s this accepted dogma in the literature than atopic dermatitis is somehow only a childhood disorder – it doesn’t affect adults. Well, these data tell a very different story because we’re actually seeing overall highest prevalences throughout the world occurring in adulthood,” based on the U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis (Br J Dermatol. 1994 Sep;131[3]:383-96).
This is the biggest epidemiologic survey ever to examine the 12-month prevalence and severity of AD around the world for both adults and children. Survey respondents included 172,627 adults aged 18 years and older, 34,212 adolescents aged 12-17 years, 54,806 children aged 2-11 years, and more than 12,000 infants.
Key findings from the study include the following:
- AD prevalence rates varied widely from country to country around the world, as well as by age groups (see graphic).
- The highest rate in adults was observed in China. South Korea had the highest rates in both children and adolescents. The top AD rates in infancy occurred in France and the United Kingdom.
- Rates across the age spectrum were consistently lowest in Israel and Switzerland.

“These kinds of patterns raise fascinating questions about the potential risk factors or protective factors that happen in different countries. There are some startling differences in terms of the different regions,” Dr. Silverberg observed. “Certain regions of the world really stand out as having much higher prevalences, particularly China and South Korea, and then as you get into the adult years, Brazil and Mexico, which I think are areas that, at least in the global atopic dermatitis epidemiology community, are not quite as well recognized as being hot spots for atopic dermatitis.”
Indeed, the 12-month prevalence rate of AD among adults was 14% in Mexico and 12% in Brazil, as compared with 13% in Saudi Arabia, 11% in Australia and Spain, 10% in Canada and the United Kingdom, and 9% in the United States.
The prevalence was generally lowest in infants, then jumped substantially within countries during the childhood years, declined slightly in adolescents, and then peaked in adulthood.
AD severity was assessed using PO-SCORAD, the Patient-Oriented Scoring AD measure. Most affected individuals had moderate AD as defined by a PO-SCORAD score of 25-50. Across the age spectrum, the highest proportion of infants with AD who had moderate disease was in China, with 72%. In Taiwan, 63% of children with AD had moderate disease, as did 68% of adolescents and an equal proportion of adults.
In the United Kingdom, 49% percent of infants with AD had severe disease, making that country the world leader in the youngest age group. Severe AD was most common among Turkish children, where 30% of kids with the skin disease had a PO-SCORAD score greater than 50. In Brazil, 31% of adolescents with AD had severe disease, the world’s highest rate in that age group. Among adults with AD, the world’s highest rate of severe disease was 25%, which was seen in the United States, Brazil, and Saudi Arabia.
Across the age spectrum, Japan had a consistently lower-end, overall, 12-month AD prevalence rate of 5%. Germany, Italy, and France had overall rates of 6%, 7%, and 8%, respectively. The rate was 9% in the United States and Canada, and it was 10% in Australia.
Dr. Silverberg performed validation analyses using the Patient-Oriented Eczema Measure (POEM) and diagnostic criteria similar to the earlier landmark International Study of Asthma and Allergies in Childhood, or ISAAC (Lancet. 1998 Apr 25;351[9111]:1225-32). This was a huge study that excluded the United States, leaving a hole in the epidemiologic picture of the disease that the new survey fills. The validation analyses were supportive of the main findings based on the U.K. Working Party criteria.
Dr. Silverberg reported serving as a consultant to Pfizer, which sponsored the global epidemiologic survey, as well as to roughly a dozen other pharmaceutical companies.
SOURCE: Silverberg JI. EADV Congress, Abstract FC01.01.
PARIS – A major worldwide survey of the 12-month prevalence of atopic dermatitis (AD) across the course of life provides new insights into global disease trends, Jonathan I. Silverberg, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Among the most important takeaways from this Internet-based survey of more than 273,645 infants, children, and adults in 18 countries across five continents conducted in 2017 was that “global atopic dermatitis prevalence appears to be higher in adults, at 10%, than in younger cohorts, where it’s 4%-8%, which I think is quite provocative and requires further study and confirmation,” said Dr. Silverberg, a dermatologist at Northwestern University in Chicago.
“Let’s keep in mind that there’s this accepted dogma in the literature than atopic dermatitis is somehow only a childhood disorder – it doesn’t affect adults. Well, these data tell a very different story because we’re actually seeing overall highest prevalences throughout the world occurring in adulthood,” based on the U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis (Br J Dermatol. 1994 Sep;131[3]:383-96).
This is the biggest epidemiologic survey ever to examine the 12-month prevalence and severity of AD around the world for both adults and children. Survey respondents included 172,627 adults aged 18 years and older, 34,212 adolescents aged 12-17 years, 54,806 children aged 2-11 years, and more than 12,000 infants.
Key findings from the study include the following:
- AD prevalence rates varied widely from country to country around the world, as well as by age groups (see graphic).
- The highest rate in adults was observed in China. South Korea had the highest rates in both children and adolescents. The top AD rates in infancy occurred in France and the United Kingdom.
- Rates across the age spectrum were consistently lowest in Israel and Switzerland.

“These kinds of patterns raise fascinating questions about the potential risk factors or protective factors that happen in different countries. There are some startling differences in terms of the different regions,” Dr. Silverberg observed. “Certain regions of the world really stand out as having much higher prevalences, particularly China and South Korea, and then as you get into the adult years, Brazil and Mexico, which I think are areas that, at least in the global atopic dermatitis epidemiology community, are not quite as well recognized as being hot spots for atopic dermatitis.”
Indeed, the 12-month prevalence rate of AD among adults was 14% in Mexico and 12% in Brazil, as compared with 13% in Saudi Arabia, 11% in Australia and Spain, 10% in Canada and the United Kingdom, and 9% in the United States.
The prevalence was generally lowest in infants, then jumped substantially within countries during the childhood years, declined slightly in adolescents, and then peaked in adulthood.
AD severity was assessed using PO-SCORAD, the Patient-Oriented Scoring AD measure. Most affected individuals had moderate AD as defined by a PO-SCORAD score of 25-50. Across the age spectrum, the highest proportion of infants with AD who had moderate disease was in China, with 72%. In Taiwan, 63% of children with AD had moderate disease, as did 68% of adolescents and an equal proportion of adults.
In the United Kingdom, 49% percent of infants with AD had severe disease, making that country the world leader in the youngest age group. Severe AD was most common among Turkish children, where 30% of kids with the skin disease had a PO-SCORAD score greater than 50. In Brazil, 31% of adolescents with AD had severe disease, the world’s highest rate in that age group. Among adults with AD, the world’s highest rate of severe disease was 25%, which was seen in the United States, Brazil, and Saudi Arabia.
Across the age spectrum, Japan had a consistently lower-end, overall, 12-month AD prevalence rate of 5%. Germany, Italy, and France had overall rates of 6%, 7%, and 8%, respectively. The rate was 9% in the United States and Canada, and it was 10% in Australia.
Dr. Silverberg performed validation analyses using the Patient-Oriented Eczema Measure (POEM) and diagnostic criteria similar to the earlier landmark International Study of Asthma and Allergies in Childhood, or ISAAC (Lancet. 1998 Apr 25;351[9111]:1225-32). This was a huge study that excluded the United States, leaving a hole in the epidemiologic picture of the disease that the new survey fills. The validation analyses were supportive of the main findings based on the U.K. Working Party criteria.
Dr. Silverberg reported serving as a consultant to Pfizer, which sponsored the global epidemiologic survey, as well as to roughly a dozen other pharmaceutical companies.
SOURCE: Silverberg JI. EADV Congress, Abstract FC01.01.
PARIS – A major worldwide survey of the 12-month prevalence of atopic dermatitis (AD) across the course of life provides new insights into global disease trends, Jonathan I. Silverberg, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Among the most important takeaways from this Internet-based survey of more than 273,645 infants, children, and adults in 18 countries across five continents conducted in 2017 was that “global atopic dermatitis prevalence appears to be higher in adults, at 10%, than in younger cohorts, where it’s 4%-8%, which I think is quite provocative and requires further study and confirmation,” said Dr. Silverberg, a dermatologist at Northwestern University in Chicago.
“Let’s keep in mind that there’s this accepted dogma in the literature than atopic dermatitis is somehow only a childhood disorder – it doesn’t affect adults. Well, these data tell a very different story because we’re actually seeing overall highest prevalences throughout the world occurring in adulthood,” based on the U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis (Br J Dermatol. 1994 Sep;131[3]:383-96).
This is the biggest epidemiologic survey ever to examine the 12-month prevalence and severity of AD around the world for both adults and children. Survey respondents included 172,627 adults aged 18 years and older, 34,212 adolescents aged 12-17 years, 54,806 children aged 2-11 years, and more than 12,000 infants.
Key findings from the study include the following:
- AD prevalence rates varied widely from country to country around the world, as well as by age groups (see graphic).
- The highest rate in adults was observed in China. South Korea had the highest rates in both children and adolescents. The top AD rates in infancy occurred in France and the United Kingdom.
- Rates across the age spectrum were consistently lowest in Israel and Switzerland.

“These kinds of patterns raise fascinating questions about the potential risk factors or protective factors that happen in different countries. There are some startling differences in terms of the different regions,” Dr. Silverberg observed. “Certain regions of the world really stand out as having much higher prevalences, particularly China and South Korea, and then as you get into the adult years, Brazil and Mexico, which I think are areas that, at least in the global atopic dermatitis epidemiology community, are not quite as well recognized as being hot spots for atopic dermatitis.”
Indeed, the 12-month prevalence rate of AD among adults was 14% in Mexico and 12% in Brazil, as compared with 13% in Saudi Arabia, 11% in Australia and Spain, 10% in Canada and the United Kingdom, and 9% in the United States.
The prevalence was generally lowest in infants, then jumped substantially within countries during the childhood years, declined slightly in adolescents, and then peaked in adulthood.
AD severity was assessed using PO-SCORAD, the Patient-Oriented Scoring AD measure. Most affected individuals had moderate AD as defined by a PO-SCORAD score of 25-50. Across the age spectrum, the highest proportion of infants with AD who had moderate disease was in China, with 72%. In Taiwan, 63% of children with AD had moderate disease, as did 68% of adolescents and an equal proportion of adults.
In the United Kingdom, 49% percent of infants with AD had severe disease, making that country the world leader in the youngest age group. Severe AD was most common among Turkish children, where 30% of kids with the skin disease had a PO-SCORAD score greater than 50. In Brazil, 31% of adolescents with AD had severe disease, the world’s highest rate in that age group. Among adults with AD, the world’s highest rate of severe disease was 25%, which was seen in the United States, Brazil, and Saudi Arabia.
Across the age spectrum, Japan had a consistently lower-end, overall, 12-month AD prevalence rate of 5%. Germany, Italy, and France had overall rates of 6%, 7%, and 8%, respectively. The rate was 9% in the United States and Canada, and it was 10% in Australia.
Dr. Silverberg performed validation analyses using the Patient-Oriented Eczema Measure (POEM) and diagnostic criteria similar to the earlier landmark International Study of Asthma and Allergies in Childhood, or ISAAC (Lancet. 1998 Apr 25;351[9111]:1225-32). This was a huge study that excluded the United States, leaving a hole in the epidemiologic picture of the disease that the new survey fills. The validation analyses were supportive of the main findings based on the U.K. Working Party criteria.
Dr. Silverberg reported serving as a consultant to Pfizer, which sponsored the global epidemiologic survey, as well as to roughly a dozen other pharmaceutical companies.
SOURCE: Silverberg JI. EADV Congress, Abstract FC01.01.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Worldwide, the 12-month prevalence of atopic dermatitis (AD) varies substantially but is unexpectedly highest in adults.
Major finding: The global 12-month prevalence of AD in adults is 10%, substantially higher than in infants, children, or adolescents.
Study details: This was an Internet survey of 273,654 subjects conducted in 2017 in 18 countries on five continents.
Disclosures: The presenter reported serving as a consultant to Pfizer, the study sponsor, as well as to roughly a dozen other pharmaceutical companies.
Source: Silverberg JI. EADV Congress, Abstract FC01.01.
Algorithm proposes approach for managing TNF inhibitor–induced psoriasis
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
Patients with tumor necrosis factor inhibitor–induced psoriasis could potentially be switched to a different drug class if they have moderate to severe skin eruption or mild skin eruption with an uncontrolled underlying disease such as inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis, according to a new treatment algorithm proposed by researchers from Brigham and Women’s Hospital and Harvard Medical School in Boston.
The researchers outlined the prevalence of tumor necrosis factor–alpha inhibitor (TNFi)-induced psoriasis in a literature review of inflammatory bowel disease (IBD), psoriasis, psoriatic arthritis (PsA), and rheumatoid arthritis (RA) and identified an estimated rate of between 2.3% and 5% in patients with RA and between 1.6% and 2.7% in patients with IBD. Although there have been reports of TNFi-induced psoriasis in patients with psoriasis and PsA, the prevalence is unclear, they wrote in the Journal of Psoriasis and Psoriatic Arthritis.
The authors then created an algorithm to manage and treat TNFi-induced psoriasiform skin eruptions with decisions to continue therapy and “treat through” symptoms, switch to a different anti-TNF therapy, or switch to a different drug class based on severity of symptoms, whether the underlying disease is well controlled, and how patients with those underlying diseases have fared with those specific therapies or agents.
“We’ve shifted gears over the past decade, and we’ve gone from having very few agents and trying to keep patients desperately on one or two agents because we didn’t want to have to give up on them for their other comorbid disease, whether it was Crohn’s, colitis, RA, or whatever it may be,” senior author Joseph Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston, said in an interview. “We’re now in an area where we can have an algorithm like this, and we have so many more mechanistic options to move to.”
Dr. Merola, who is board certified in dermatology and rheumatology, said the algorithm is meant to “open a dialogue” with other specialists in different areas and raise awareness of treatments in related but separate fields. For diseases not often seen by more than one specialty, with the exception of psoriasis and PsA, he said that “the idea is to start a dialogue and increase communication between specialists.”
Dr. Merola noted that while the algorithm in many respects is meant to guide a physician in a specialty in appropriate medication decisions, at the same time he hopes that “it opens a dialogue and communication with the other specialty who tends to oversee this particular disease state or class of medicine to really work together to try to find the right drug for the right person.”
For patients with a mild skin eruption and a controlled underlying disease, the algorithm recommends a “treat through” approach by continuing anti-TNF therapy and treating psoriasis symptoms with topical steroids, ultraviolet therapy, methotrexate, cyclosporine, or acitretin, and to consider dapsone in cases of pustular psoriasis. However, the researchers noted that “treat through” studies have reported complete symptom resolution in 26%-41% of patients.
For patients with recalcitrant or worsening TNFi-induced psoriasis or patients with mild skin eruptions with an uncontrolled underlying disease, the researchers proposed considering switching to a different anti-TNF therapy, although studies have shown complete resolution of symptoms in only 5%-37% of patients.
If patients worsen from there, or if they have moderate to-severe skin eruption with uncontrolled underlying disease, they could be considered for switching to a different drug class and treated based on their underlying disease, along with treatment for psoriasis symptoms. This approach has been shown to completely resolve lesions in up to 64% of cases, they said. IBD patients could benefit from ustekinumab, vedolizumab, 6-mercaptopurine, or azathioprine as an alternative to anti-TNF therapy. Those patients with psoriasis should be considered for guselkumab, while ustekinumab, ixekizumab, secukinumab, and apremilast are effective treatments for patients with psoriasis and PsA. Patients with RA could receive treatment with tocilizumab, rituximab, abatacept, and tofacitinib, the authors wrote.
Dr. Merola reported serving as a consultant and/or investigator for Merck Research Laboratories, AbbVie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Samumed, Celgene, Sanofi Regeneron, GlaxoSmithKline, Almirall, Sun Pharma, Biogen, Pfizer, Incyte, Aclaris, and Leo Pharma.
SOURCE: Li SJ et al. J Psoriasis Psoriatic Arthritis. 2018 Nov 21. doi: 10.1177/2475530318810851.
FROM JOURNAL OF PSORIASIS AND PSORIATIC ARTHRITIS
When Skin Damage Takes Sides
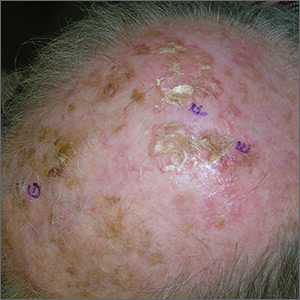
A 61-year-old man is sent to dermatology by his primary care provider for evaluation of several facial lesions. Although they have been present for years, the patient says they have never caused any symptoms.
The patient’s work history includes extensive outdoor activity, as well as more than 20 years of driving a truck. He is now retired and spends most of his days fishing.
He has a history of at least two unspecified skin cancers but denies receiving facial radiation treatment. He has been a smoker since age 12 and admits to heavy drinking.
EXAMINATION
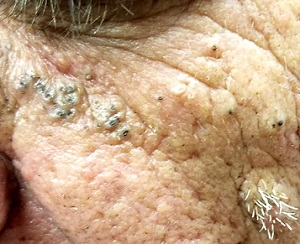
The patient’s face is uneven in appearance, with deep wrinkles and multiple areas of discoloration. His skin is type III.
Multiple open and closed comedones are seen around the left malar and brow areas, where the underlying skin has a whitish look to it. The comedones are not inflamed, and no pustules are observed. Some of the comedones extend into the infra-orbital area on the left side of his face. Almost no such changes are seen on the right side.
There is no increase in hair in the affected areas and no skin changes observed on his hands or arms, other than moderately severe sun damage.
What is the diagnosis?
DISCUSSION
In the 1930s, two French dermatologists, Favre and Racouchot, began to see several cases like this one. Following a review of available literature, they published their findings, calling the condition “nodular elastosis with cysts and comedones.” This quickly became known as Favre-Racouchot syndrome (FRS), the name it still bears today.
FRS is quite common, especially on the faces of white men older than 50. More men than woman are affected, and smoking almost certainly contributes to the problem.
The connection between FRS and sun exposure is well established: the basophilic degeneration of the dermis (solar elastosis) seen in FRS is identical to that seen from overexposure to the sun. Another clue to the cause is exemplified in cases involving truck drivers, since the side of the face nearest the window is usually affected more than the other side. In most countries, the left side of the face sustains the most damage; in countries with right-hand drive, the right side of the face is affected.
Significant focal nodular solar elastosis, along with open and closed comedones, notably worse on the more sun-exposed side, is unique to FRS. The lack of increase in facial hair in the affected area is significant, in that it effectively rules out the only other major item in the differential: porphyria cutanea tarda.
Treatment is problematic at best. Except in younger patients, sunscreens are largely a waste. The comedones can be manually extracted, but recurrence is certain. For the truly motivated patient, laser resurfacing and peels can help. To slow the progression of the disease, smoking cessation is necessary.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is most commonly seen in older men with a history of excessive chronic sun exposure. Most are smokers.
- FRS primarily affects the malar and lateral brow areas with nodular elastosis, in which are embedded multiple cysts and comedones.
- In the United States, FRS is usually more pronounced on the left side of the face.
- Other signs of chronic sun damage (actinic weathering, also known as dermatoheliosis) are almost always seen over the entire face of FRS patients.
A 61-year-old man is sent to dermatology by his primary care provider for evaluation of several facial lesions. Although they have been present for years, the patient says they have never caused any symptoms.
The patient’s work history includes extensive outdoor activity, as well as more than 20 years of driving a truck. He is now retired and spends most of his days fishing.
He has a history of at least two unspecified skin cancers but denies receiving facial radiation treatment. He has been a smoker since age 12 and admits to heavy drinking.

EXAMINATION
The patient’s face is uneven in appearance, with deep wrinkles and multiple areas of discoloration. His skin is type III.
Multiple open and closed comedones are seen around the left malar and brow areas, where the underlying skin has a whitish look to it. The comedones are not inflamed, and no pustules are observed. Some of the comedones extend into the infra-orbital area on the left side of his face. Almost no such changes are seen on the right side.
There is no increase in hair in the affected areas and no skin changes observed on his hands or arms, other than moderately severe sun damage.
What is the diagnosis?
DISCUSSION
In the 1930s, two French dermatologists, Favre and Racouchot, began to see several cases like this one. Following a review of available literature, they published their findings, calling the condition “nodular elastosis with cysts and comedones.” This quickly became known as Favre-Racouchot syndrome (FRS), the name it still bears today.
FRS is quite common, especially on the faces of white men older than 50. More men than woman are affected, and smoking almost certainly contributes to the problem.
The connection between FRS and sun exposure is well established: the basophilic degeneration of the dermis (solar elastosis) seen in FRS is identical to that seen from overexposure to the sun. Another clue to the cause is exemplified in cases involving truck drivers, since the side of the face nearest the window is usually affected more than the other side. In most countries, the left side of the face sustains the most damage; in countries with right-hand drive, the right side of the face is affected.
Significant focal nodular solar elastosis, along with open and closed comedones, notably worse on the more sun-exposed side, is unique to FRS. The lack of increase in facial hair in the affected area is significant, in that it effectively rules out the only other major item in the differential: porphyria cutanea tarda.
Treatment is problematic at best. Except in younger patients, sunscreens are largely a waste. The comedones can be manually extracted, but recurrence is certain. For the truly motivated patient, laser resurfacing and peels can help. To slow the progression of the disease, smoking cessation is necessary.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is most commonly seen in older men with a history of excessive chronic sun exposure. Most are smokers.
- FRS primarily affects the malar and lateral brow areas with nodular elastosis, in which are embedded multiple cysts and comedones.
- In the United States, FRS is usually more pronounced on the left side of the face.
- Other signs of chronic sun damage (actinic weathering, also known as dermatoheliosis) are almost always seen over the entire face of FRS patients.
A 61-year-old man is sent to dermatology by his primary care provider for evaluation of several facial lesions. Although they have been present for years, the patient says they have never caused any symptoms.
The patient’s work history includes extensive outdoor activity, as well as more than 20 years of driving a truck. He is now retired and spends most of his days fishing.
He has a history of at least two unspecified skin cancers but denies receiving facial radiation treatment. He has been a smoker since age 12 and admits to heavy drinking.

EXAMINATION
The patient’s face is uneven in appearance, with deep wrinkles and multiple areas of discoloration. His skin is type III.
Multiple open and closed comedones are seen around the left malar and brow areas, where the underlying skin has a whitish look to it. The comedones are not inflamed, and no pustules are observed. Some of the comedones extend into the infra-orbital area on the left side of his face. Almost no such changes are seen on the right side.
There is no increase in hair in the affected areas and no skin changes observed on his hands or arms, other than moderately severe sun damage.
What is the diagnosis?
DISCUSSION
In the 1930s, two French dermatologists, Favre and Racouchot, began to see several cases like this one. Following a review of available literature, they published their findings, calling the condition “nodular elastosis with cysts and comedones.” This quickly became known as Favre-Racouchot syndrome (FRS), the name it still bears today.
FRS is quite common, especially on the faces of white men older than 50. More men than woman are affected, and smoking almost certainly contributes to the problem.
The connection between FRS and sun exposure is well established: the basophilic degeneration of the dermis (solar elastosis) seen in FRS is identical to that seen from overexposure to the sun. Another clue to the cause is exemplified in cases involving truck drivers, since the side of the face nearest the window is usually affected more than the other side. In most countries, the left side of the face sustains the most damage; in countries with right-hand drive, the right side of the face is affected.
Significant focal nodular solar elastosis, along with open and closed comedones, notably worse on the more sun-exposed side, is unique to FRS. The lack of increase in facial hair in the affected area is significant, in that it effectively rules out the only other major item in the differential: porphyria cutanea tarda.
Treatment is problematic at best. Except in younger patients, sunscreens are largely a waste. The comedones can be manually extracted, but recurrence is certain. For the truly motivated patient, laser resurfacing and peels can help. To slow the progression of the disease, smoking cessation is necessary.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is most commonly seen in older men with a history of excessive chronic sun exposure. Most are smokers.
- FRS primarily affects the malar and lateral brow areas with nodular elastosis, in which are embedded multiple cysts and comedones.
- In the United States, FRS is usually more pronounced on the left side of the face.
- Other signs of chronic sun damage (actinic weathering, also known as dermatoheliosis) are almost always seen over the entire face of FRS patients.
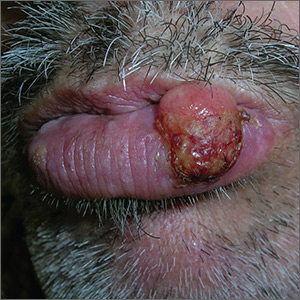
Growing lesion on lip
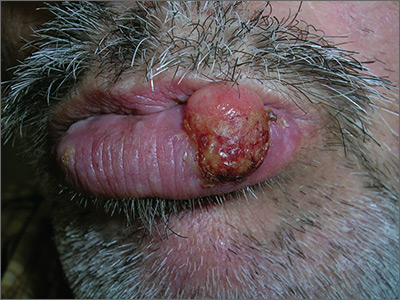
The FP recognized the lesion as a probable squamous cell carcinoma (SCC) due to its appearance and location on the lower lip. He was also aware that immunosuppressive medications increase a patient's risk for SCC.
The FP performed a shave biopsy of a portion of the lesion and the result confirmed SCC. (See the Watch & Learn video on “Shave biopsy.”) A careful head and neck exam did not reveal palpable lymph nodes. Given the location of the lesion and the risk for metastases, the FP referred the patient for Mohs surgery and provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the lesion as a probable squamous cell carcinoma (SCC) due to its appearance and location on the lower lip. He was also aware that immunosuppressive medications increase a patient's risk for SCC.
The FP performed a shave biopsy of a portion of the lesion and the result confirmed SCC. (See the Watch & Learn video on “Shave biopsy.”) A careful head and neck exam did not reveal palpable lymph nodes. Given the location of the lesion and the risk for metastases, the FP referred the patient for Mohs surgery and provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the lesion as a probable squamous cell carcinoma (SCC) due to its appearance and location on the lower lip. He was also aware that immunosuppressive medications increase a patient's risk for SCC.
The FP performed a shave biopsy of a portion of the lesion and the result confirmed SCC. (See the Watch & Learn video on “Shave biopsy.”) A careful head and neck exam did not reveal palpable lymph nodes. Given the location of the lesion and the risk for metastases, the FP referred the patient for Mohs surgery and provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
What is your diagnosis?
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
Epidermal nevi are a subset of cutaneous hamartomas resulting from somatic mutations of epidermal cells, presenting as keratinocyte or epidermal appendage overgrowths. The most common type appear in a linear distribution and are termed linear epidermal nevi or linear verrucous epidermal nevi.
There are variations of epidermal nevi (EN) that can be composed of superficial epidermal keratinocytes, sebaceous glands, apocrine or eccrine glands, hair follicles, or smooth muscle. For example, many consider a nevus sebaceous to be a type of epidermal nevus as well. The incidence of EN is approximately 1 in 1,000 newborns. Postzygotic cell mutations result in a mosaic distribution that follows embryonic migration patterns, appearing in a Blaschkoid distribution.
EN present most frequently as unilateral linear or whorled hyperpigmented coalescing papules. The lesions can be present at birth or during childhood, and after appearing, grow with the patient. Typically the lesions become more raised and verrucous around puberty. The differential diagnosis of linear EN include lichen striatus, warts, and incontinentia pigmenti. Lichen striatus can be differentiated because it presents later in life and self-resolves. Verrucae are the most commonly mistaken diagnosis for EN; warts do not usually persist in the same pattern over time with proportionate growth and typically respond to locally destructive treatments such as liquid nitrogen, unlike EN. Incontinentia pigmenti presents as vesicles initially and shows a quick evolution, differentiating it from EN. Inflammatory linear verrucous epidermal nevus (ILVEN) is a variant of linear EN that has associated chronic and intermittent erythema, scale, and pruritus. Lichen nitidus often has a pruritic presentation; however, it is flat topped and skin colored, helping differentiate it from linear EN.
There has been recent research advancing gene associations for linear EN displaying many lesions associated with mosaic mutations in oncogenes. Multiple genes have been identified with EN including RAS, FGFR3, and PIK3CA1. FGFR3 and PIK3CA mutations are associated with 50% of keratinocytic nevi. Of the RAS family, the HRAS pathway has been most closely associated with nevus sebaceous. While KRAS and NRAS genes have been associated with EN, it is to a lesser degree. However, there are multiple recent case reports demonstrating a potential association of G12D mosaicism of the KRAS gene in EN with rhabdomyosarcoma and bladder cancers2.
The diagnosis of epidermal nevus syndrome should be considered when there is a nevus with associated developmental abnormality of the central nervous system, eyes, or musculoskeletal systems. The most common systemic symptoms include delays in developmental milestones, seizure disorders, coloboma, strabismus, muscle weakness, and hemihypertrophy. To date, there are six specific epidermal nevus syndromes identified: sebaceous nevus syndrome, nevus comedonicus syndrome, Becker nevus syndrome, phakomatosis pigmentokeratotica, Proteus syndrome, congenital hemidysplasia with ichthyosiform nevus and limb defects, and cutaneous-skeletal hypophosphatemia syndrome3. In addition to the syndromes described, there are reports of associations between keratinocytic nevi and ILVEN with hypophosphatemic rickets and precocious puberty.
Linear EN are rarely associated with malignant transformation to basal cell carcinoma or squamous cell carcinoma, depending on the cell type involved. Given the low risk of malignancy, the lesions do not need to be removed routinely. For small lesions, monitoring often is the preferred management. However, lesions with functional significance, or causing strangulation or deformity, can be treated with surgical excision, curettage, or laser destruction
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Pediatr Dermatol. 2004 Jul-Aug;21(4):432-9.
2. J Med Genet. 2010 Dec;47(12):859-62.
3. Pediatr Dermatol. 2018 Jan;35(1):21-9.
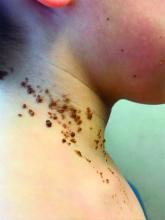
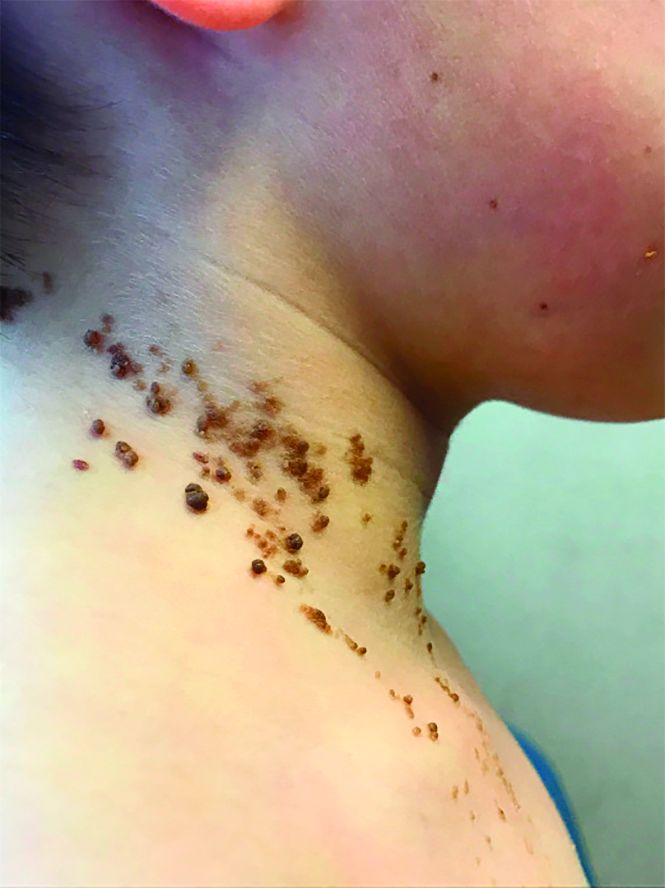
A 6-year-old, otherwise-healthy male is brought into clinic for evaluation of papules on his neck. The rash has been present since 1 year of age and has been growing in size proportionately. He claims there is occasional itching but no pain or redness. He does not seem to be disturbed by his rash. He has two siblings, aged 2 and 4 years, without lesions.
On physical exam, he is noted to have a linear plaque of hyperpigmented verrucous papules on his neck.
Debunking Psoriasis Myths: Psoriasis Is More Than Skin Deep
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
ACR, NPF unveil new psoriatic arthritis treatment guideline
The American College of Rheumatology and the National Psoriasis Foundation have released a joint treatment guideline for psoriatic arthritis that, for the first time, includes a conditional recommendation to use tumor necrosis factor–inhibitor(TNFi) biologics over methotrexate and other oral small molecules as a first-line therapy in patients with active disease.
“The available low-quality evidence is inconclusive regarding the efficacy of OSMs [oral small molecules] in management of PsA, whereas there is moderate-quality evidence of the benefits of TNFi biologics, in particular regarding their impact on the prevention of disease progression and joint damage,” wrote the panel of authors, who were led by Jasvinder A. Singh, MD, of the University of Alabama at Birmingham. “In making their recommendation, the panel recognized the cost implications, but put considerations of quality of evidence for benefit over other considerations. This guideline provides recommendations for early and aggressive therapy in patients with newly diagnosed PsA.”
The 28-page guideline, published online Nov. 30 in the Journal of Psoriasis and Psoriatic Arthritis and also in Arthritis Care & Research and Arthritis & Rheumatology, is the first set of PsA-specific recommendations to be assembled using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology that the ACR has used for RA and other conditions. GRADE uses systematic reviews of the scientific literature available to evaluate and grade the quality of evidence in a particular domain. The evidence reviews are then used to create guideline recommendations for or against particular therapy options that range from strong to conditional, depending on the quality of evidence available.
Based on the GRADE methodology and consensus building, the guideline authors crafted recommendations for eight different clinical scenarios, including the initial treatment of patients with active PsA who have not received either OSMs or other treatments; treatment of patients with active PsA despite treatment with an OSM; treatment of patients with active PsA despite treatment with a TNFi biologic either as monotherapy or in combination with methotrexate; treatment of patients with active PsA despite treatment with an interleukin (IL)-17 inhibitor or IL-12/23 inhibitor monotherapy; treatment of patients with active PsA including treat-to-target, active axial disease, enthesitis, or active inflammatory bowel disease; treatment of patients with active PsA and comorbidities, including concomitant diabetes and recurrent serious infections; vaccination in patients with active PsA; and treatment of patients with active PsA with nonpharmacologic interventions such as yoga and weight loss. Most of the treatment recommendations are conditional based on very low to moderate quality evidence. “Health care providers and patients must take into consideration all active disease domains, comorbidities, and the patient’s functional status in choosing the optimal therapy for an individual at a given point in time,” the authors emphasized.
Only five of the recommendations are listed as strong, including smoking cessation. Three of the strong recommendations concern adult patients with active PsA and concomitant active inflammatory bowel disease despite treatment with an OSM. They are “switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic,” “switch to a TNFi monoclonal antibody biologic over an IL-7i biologic,” and “switch to an IL-12/23i biologic over switching to an IL-17i biologic.”
The process of creating the guideline included input from a panel of nine adults who provided the authors with perspective on their values and preferences. “The concept of treat-to-target was challenging for patients,” the authors noted. “Although they saw value in improved outcomes, they also thought this strategy could increase costs to the patient (e.g., copayments, time traveling to more frequent appointments, etc.) and potentially increase adverse events. Therefore, a detailed conversation with the patient is needed to make decisions regarding treat-to-target.”
The authors concluded the guideline by calling for more comparative data to inform treatment selection in the future. “Several ongoing trials, including a trial to compare a TNFi biologic combination therapy with a TNFi biologic monotherapy and MTX monotherapy, will inform treatment decisions,” they wrote. “We anticipate future updates to the guideline when new evidence is available.”
Dr. Singh, who is also a staff rheumatologist at the Birmingham (Ala.) Veterans Affairs Medical Center, led development of the 2012 and 2015 ACR treatment guidelines for RA. He has received consulting fees from a variety of companies marketing rheumatologic drugs as well as research support from Takeda and Savient. The other guideline authors reported having numerous financial ties to industry.
SOURCE: Singh J et al. Arthritis Care Res. 2018 Nov 30. doi: 10.1002/acr.23789.
The American College of Rheumatology and the National Psoriasis Foundation have released a joint treatment guideline for psoriatic arthritis that, for the first time, includes a conditional recommendation to use tumor necrosis factor–inhibitor(TNFi) biologics over methotrexate and other oral small molecules as a first-line therapy in patients with active disease.
“The available low-quality evidence is inconclusive regarding the efficacy of OSMs [oral small molecules] in management of PsA, whereas there is moderate-quality evidence of the benefits of TNFi biologics, in particular regarding their impact on the prevention of disease progression and joint damage,” wrote the panel of authors, who were led by Jasvinder A. Singh, MD, of the University of Alabama at Birmingham. “In making their recommendation, the panel recognized the cost implications, but put considerations of quality of evidence for benefit over other considerations. This guideline provides recommendations for early and aggressive therapy in patients with newly diagnosed PsA.”
The 28-page guideline, published online Nov. 30 in the Journal of Psoriasis and Psoriatic Arthritis and also in Arthritis Care & Research and Arthritis & Rheumatology, is the first set of PsA-specific recommendations to be assembled using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology that the ACR has used for RA and other conditions. GRADE uses systematic reviews of the scientific literature available to evaluate and grade the quality of evidence in a particular domain. The evidence reviews are then used to create guideline recommendations for or against particular therapy options that range from strong to conditional, depending on the quality of evidence available.
Based on the GRADE methodology and consensus building, the guideline authors crafted recommendations for eight different clinical scenarios, including the initial treatment of patients with active PsA who have not received either OSMs or other treatments; treatment of patients with active PsA despite treatment with an OSM; treatment of patients with active PsA despite treatment with a TNFi biologic either as monotherapy or in combination with methotrexate; treatment of patients with active PsA despite treatment with an interleukin (IL)-17 inhibitor or IL-12/23 inhibitor monotherapy; treatment of patients with active PsA including treat-to-target, active axial disease, enthesitis, or active inflammatory bowel disease; treatment of patients with active PsA and comorbidities, including concomitant diabetes and recurrent serious infections; vaccination in patients with active PsA; and treatment of patients with active PsA with nonpharmacologic interventions such as yoga and weight loss. Most of the treatment recommendations are conditional based on very low to moderate quality evidence. “Health care providers and patients must take into consideration all active disease domains, comorbidities, and the patient’s functional status in choosing the optimal therapy for an individual at a given point in time,” the authors emphasized.
Only five of the recommendations are listed as strong, including smoking cessation. Three of the strong recommendations concern adult patients with active PsA and concomitant active inflammatory bowel disease despite treatment with an OSM. They are “switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic,” “switch to a TNFi monoclonal antibody biologic over an IL-7i biologic,” and “switch to an IL-12/23i biologic over switching to an IL-17i biologic.”
The process of creating the guideline included input from a panel of nine adults who provided the authors with perspective on their values and preferences. “The concept of treat-to-target was challenging for patients,” the authors noted. “Although they saw value in improved outcomes, they also thought this strategy could increase costs to the patient (e.g., copayments, time traveling to more frequent appointments, etc.) and potentially increase adverse events. Therefore, a detailed conversation with the patient is needed to make decisions regarding treat-to-target.”
The authors concluded the guideline by calling for more comparative data to inform treatment selection in the future. “Several ongoing trials, including a trial to compare a TNFi biologic combination therapy with a TNFi biologic monotherapy and MTX monotherapy, will inform treatment decisions,” they wrote. “We anticipate future updates to the guideline when new evidence is available.”
Dr. Singh, who is also a staff rheumatologist at the Birmingham (Ala.) Veterans Affairs Medical Center, led development of the 2012 and 2015 ACR treatment guidelines for RA. He has received consulting fees from a variety of companies marketing rheumatologic drugs as well as research support from Takeda and Savient. The other guideline authors reported having numerous financial ties to industry.
SOURCE: Singh J et al. Arthritis Care Res. 2018 Nov 30. doi: 10.1002/acr.23789.
The American College of Rheumatology and the National Psoriasis Foundation have released a joint treatment guideline for psoriatic arthritis that, for the first time, includes a conditional recommendation to use tumor necrosis factor–inhibitor(TNFi) biologics over methotrexate and other oral small molecules as a first-line therapy in patients with active disease.
“The available low-quality evidence is inconclusive regarding the efficacy of OSMs [oral small molecules] in management of PsA, whereas there is moderate-quality evidence of the benefits of TNFi biologics, in particular regarding their impact on the prevention of disease progression and joint damage,” wrote the panel of authors, who were led by Jasvinder A. Singh, MD, of the University of Alabama at Birmingham. “In making their recommendation, the panel recognized the cost implications, but put considerations of quality of evidence for benefit over other considerations. This guideline provides recommendations for early and aggressive therapy in patients with newly diagnosed PsA.”
The 28-page guideline, published online Nov. 30 in the Journal of Psoriasis and Psoriatic Arthritis and also in Arthritis Care & Research and Arthritis & Rheumatology, is the first set of PsA-specific recommendations to be assembled using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology that the ACR has used for RA and other conditions. GRADE uses systematic reviews of the scientific literature available to evaluate and grade the quality of evidence in a particular domain. The evidence reviews are then used to create guideline recommendations for or against particular therapy options that range from strong to conditional, depending on the quality of evidence available.
Based on the GRADE methodology and consensus building, the guideline authors crafted recommendations for eight different clinical scenarios, including the initial treatment of patients with active PsA who have not received either OSMs or other treatments; treatment of patients with active PsA despite treatment with an OSM; treatment of patients with active PsA despite treatment with a TNFi biologic either as monotherapy or in combination with methotrexate; treatment of patients with active PsA despite treatment with an interleukin (IL)-17 inhibitor or IL-12/23 inhibitor monotherapy; treatment of patients with active PsA including treat-to-target, active axial disease, enthesitis, or active inflammatory bowel disease; treatment of patients with active PsA and comorbidities, including concomitant diabetes and recurrent serious infections; vaccination in patients with active PsA; and treatment of patients with active PsA with nonpharmacologic interventions such as yoga and weight loss. Most of the treatment recommendations are conditional based on very low to moderate quality evidence. “Health care providers and patients must take into consideration all active disease domains, comorbidities, and the patient’s functional status in choosing the optimal therapy for an individual at a given point in time,” the authors emphasized.
Only five of the recommendations are listed as strong, including smoking cessation. Three of the strong recommendations concern adult patients with active PsA and concomitant active inflammatory bowel disease despite treatment with an OSM. They are “switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic,” “switch to a TNFi monoclonal antibody biologic over an IL-7i biologic,” and “switch to an IL-12/23i biologic over switching to an IL-17i biologic.”
The process of creating the guideline included input from a panel of nine adults who provided the authors with perspective on their values and preferences. “The concept of treat-to-target was challenging for patients,” the authors noted. “Although they saw value in improved outcomes, they also thought this strategy could increase costs to the patient (e.g., copayments, time traveling to more frequent appointments, etc.) and potentially increase adverse events. Therefore, a detailed conversation with the patient is needed to make decisions regarding treat-to-target.”
The authors concluded the guideline by calling for more comparative data to inform treatment selection in the future. “Several ongoing trials, including a trial to compare a TNFi biologic combination therapy with a TNFi biologic monotherapy and MTX monotherapy, will inform treatment decisions,” they wrote. “We anticipate future updates to the guideline when new evidence is available.”
Dr. Singh, who is also a staff rheumatologist at the Birmingham (Ala.) Veterans Affairs Medical Center, led development of the 2012 and 2015 ACR treatment guidelines for RA. He has received consulting fees from a variety of companies marketing rheumatologic drugs as well as research support from Takeda and Savient. The other guideline authors reported having numerous financial ties to industry.
SOURCE: Singh J et al. Arthritis Care Res. 2018 Nov 30. doi: 10.1002/acr.23789.
FROM ARTHRITIS CARE & RESEARCH
Growths on scalp
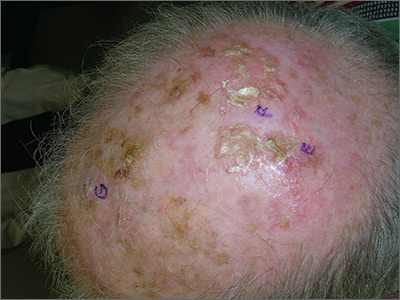
The FP recognized the severely sun damaged scalp as a major risk factor for skin cancers. He looked closely at the lesions and realized that the ulcerated areas were at particularly high risk for squamous cell carcinoma (SCC).
He performed broad shave biopsies with sufficient depth to obtain the needed diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The pathology demonstrated that 2 of the 3 biopsy sites were positive for SCC (E and G were SCC, while F was read as actinic keratosis). Cutaneous SCC is a malignant tumor of keratinocytes. Most cutaneous SCCs arise from precursor lesions, often actinic keratoses. SCC usually spreads by local extension, but it is also capable of regional lymph node metastasis and distant metastasis.
Unsure of the margins of the tumors and aware that surgery of the scalp can be challenging, the FP referred the patient for Mohs surgery. The FP also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
The Mohs surgeon recommended field treatment with 5% fluorouracil cream twice daily for 4 weeks before surgery to minimize the amount of cutting that would be needed to clear the SCC from this diffusely sun-damaged scalp. After the 5% fluorouracil cream treatment, the surgeon waited 1 month to allow the scalp to heal before performing surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the severely sun damaged scalp as a major risk factor for skin cancers. He looked closely at the lesions and realized that the ulcerated areas were at particularly high risk for squamous cell carcinoma (SCC).
He performed broad shave biopsies with sufficient depth to obtain the needed diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The pathology demonstrated that 2 of the 3 biopsy sites were positive for SCC (E and G were SCC, while F was read as actinic keratosis). Cutaneous SCC is a malignant tumor of keratinocytes. Most cutaneous SCCs arise from precursor lesions, often actinic keratoses. SCC usually spreads by local extension, but it is also capable of regional lymph node metastasis and distant metastasis.
Unsure of the margins of the tumors and aware that surgery of the scalp can be challenging, the FP referred the patient for Mohs surgery. The FP also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
The Mohs surgeon recommended field treatment with 5% fluorouracil cream twice daily for 4 weeks before surgery to minimize the amount of cutting that would be needed to clear the SCC from this diffusely sun-damaged scalp. After the 5% fluorouracil cream treatment, the surgeon waited 1 month to allow the scalp to heal before performing surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized the severely sun damaged scalp as a major risk factor for skin cancers. He looked closely at the lesions and realized that the ulcerated areas were at particularly high risk for squamous cell carcinoma (SCC).
He performed broad shave biopsies with sufficient depth to obtain the needed diagnosis. (See the Watch & Learn video on “Shave biopsy.”) The pathology demonstrated that 2 of the 3 biopsy sites were positive for SCC (E and G were SCC, while F was read as actinic keratosis). Cutaneous SCC is a malignant tumor of keratinocytes. Most cutaneous SCCs arise from precursor lesions, often actinic keratoses. SCC usually spreads by local extension, but it is also capable of regional lymph node metastasis and distant metastasis.
Unsure of the margins of the tumors and aware that surgery of the scalp can be challenging, the FP referred the patient for Mohs surgery. The FP also provided counseling about sun avoidance, the consistent use of a hat outdoors, and the use of sunscreens when exposed to the sun.
The Mohs surgeon recommended field treatment with 5% fluorouracil cream twice daily for 4 weeks before surgery to minimize the amount of cutting that would be needed to clear the SCC from this diffusely sun-damaged scalp. After the 5% fluorouracil cream treatment, the surgeon waited 1 month to allow the scalp to heal before performing surgery.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
The new third edition will be available in January 2019: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/.
You can also get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Dermoscopy in family medicine: A primer
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.
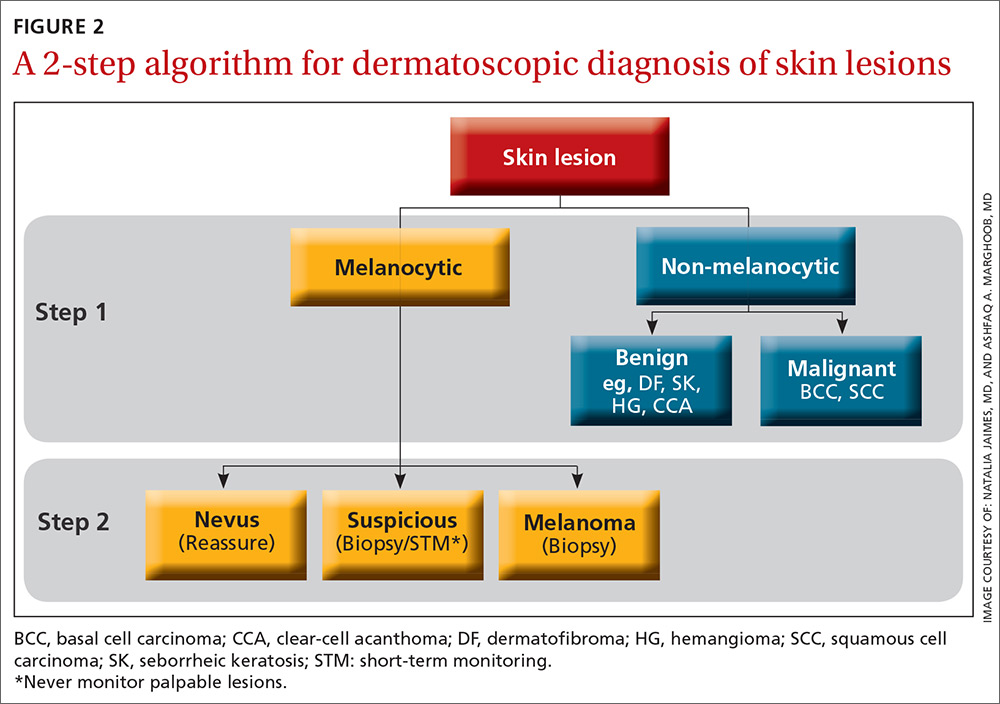
Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).
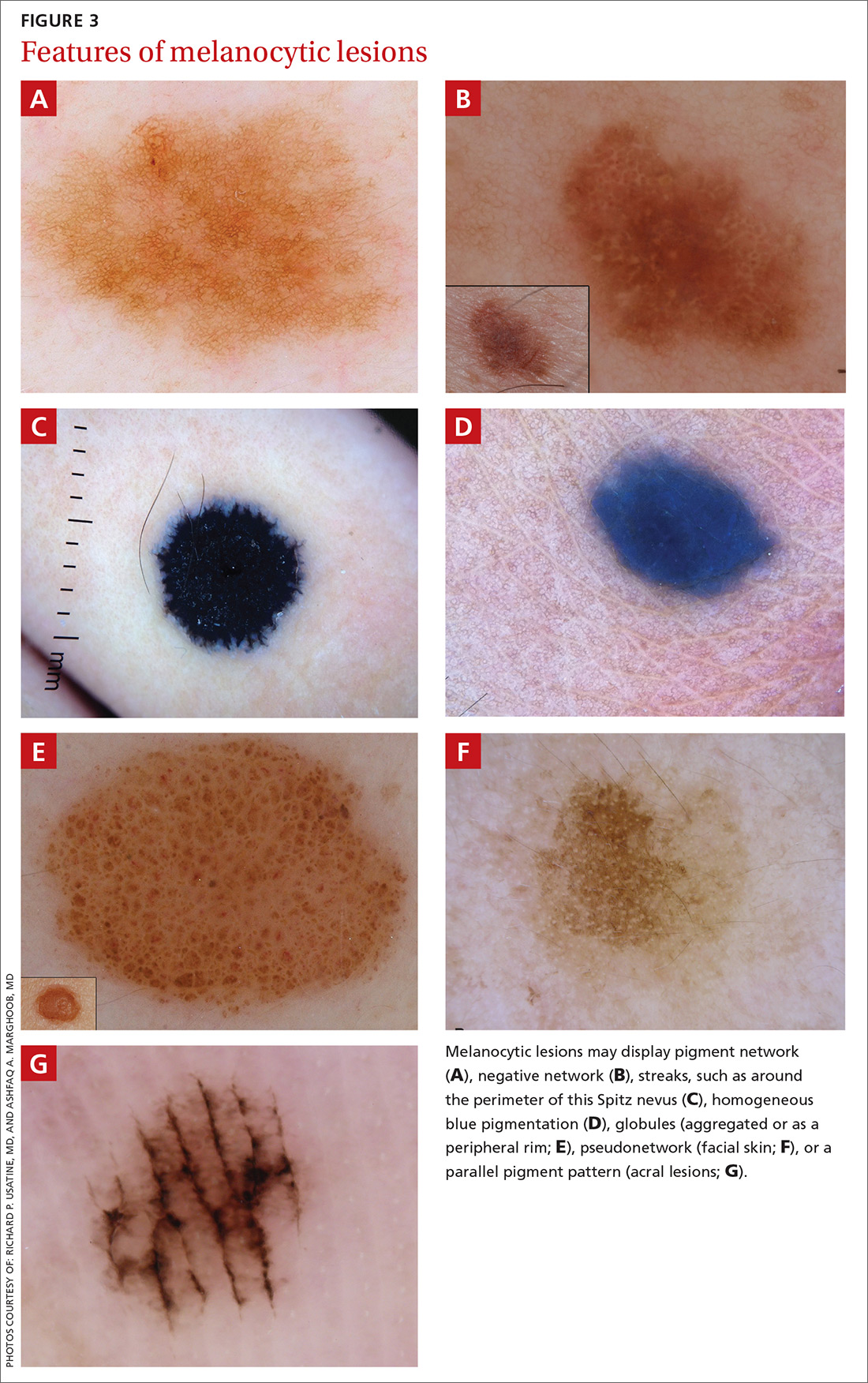
Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.
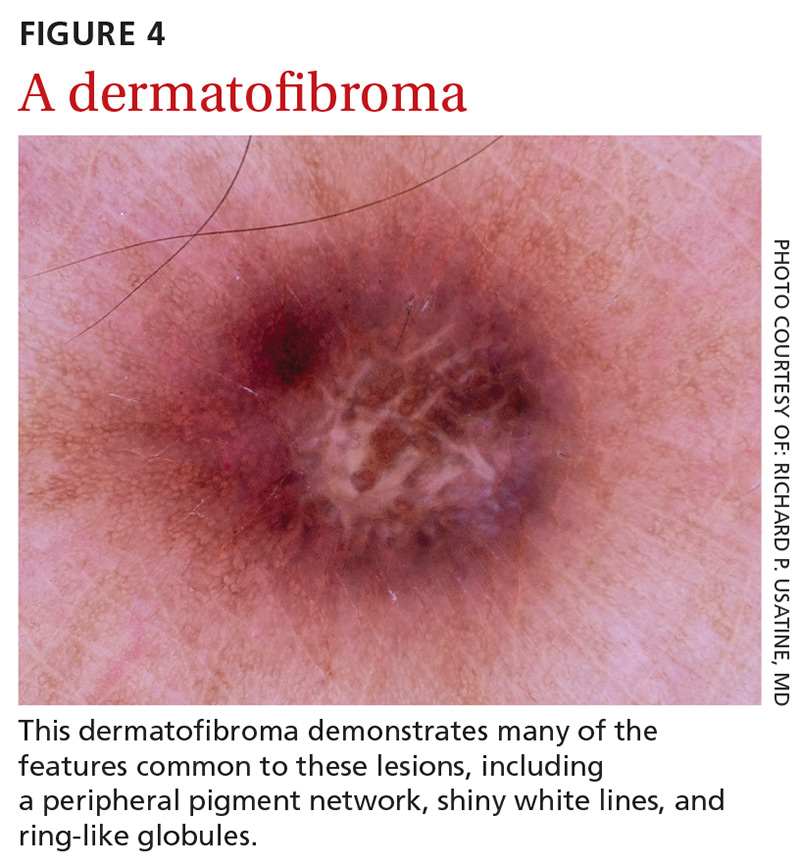
Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).
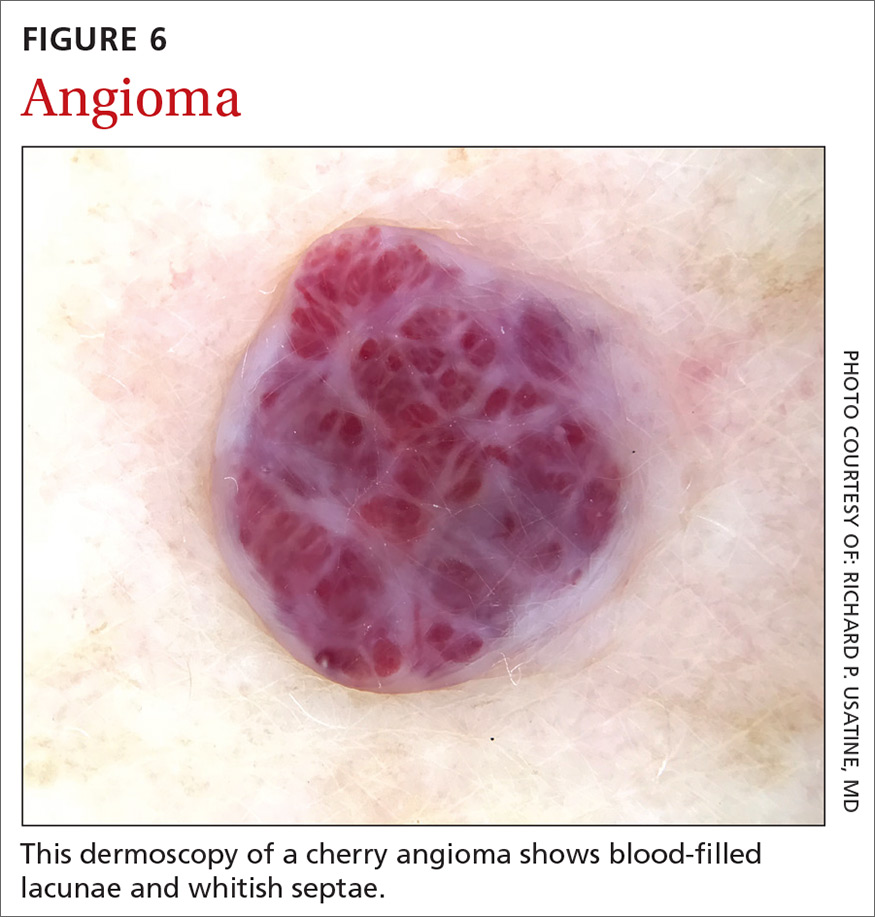
Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.
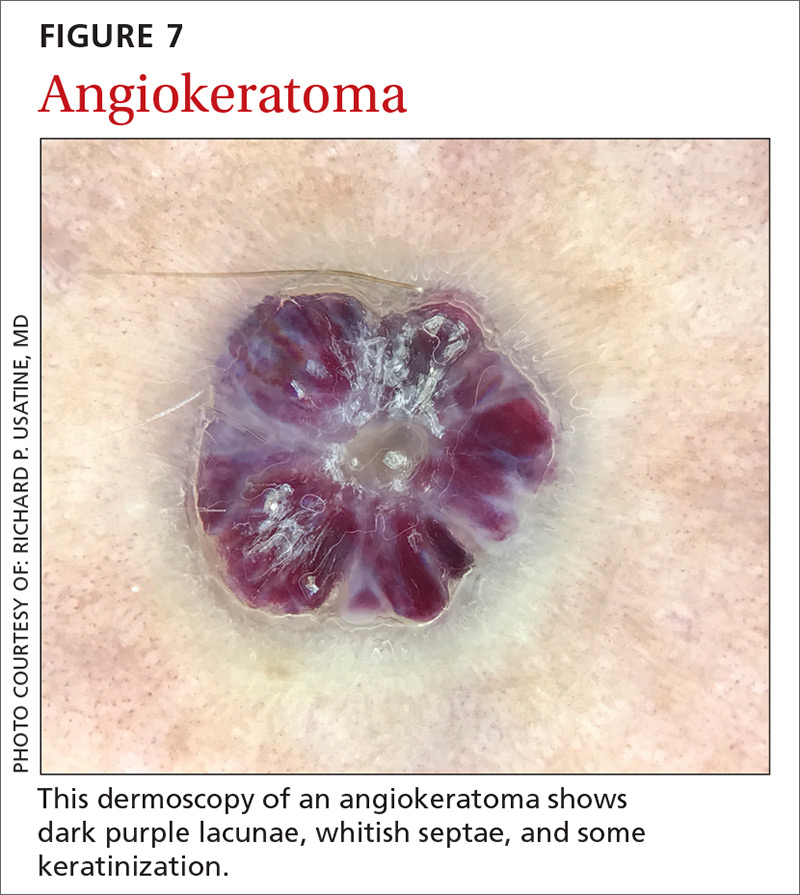
Continue to: Sebaceous hyperplasia...
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).
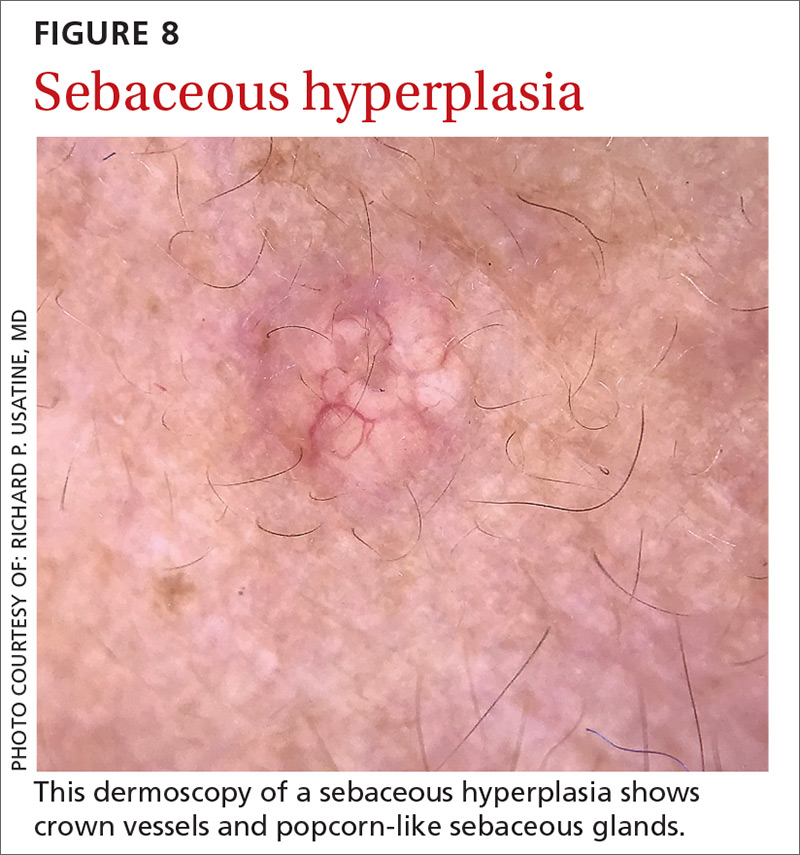
Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).
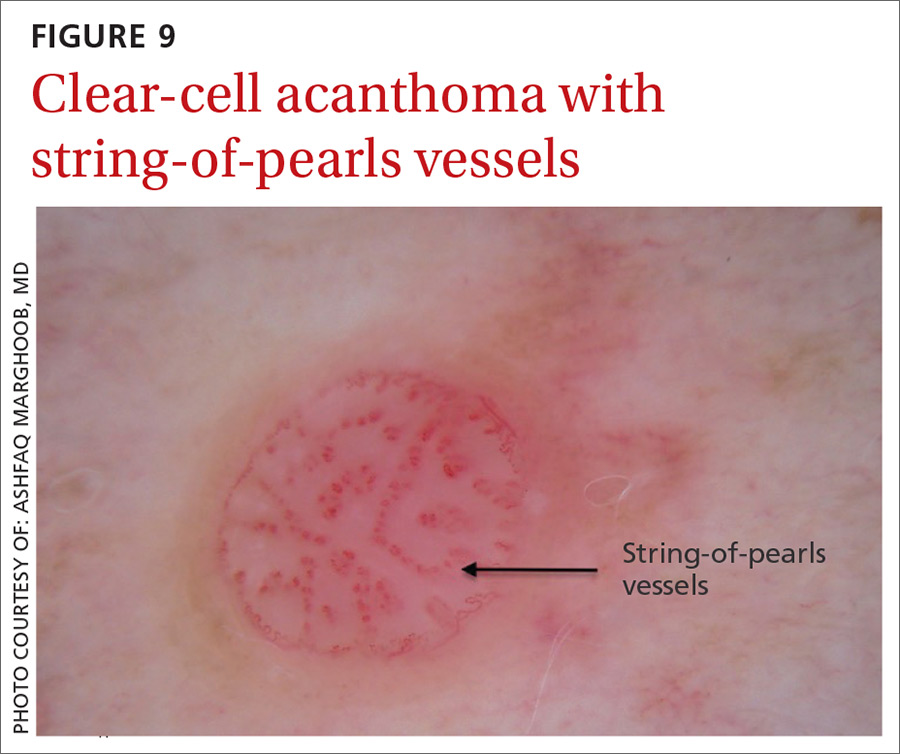
Malignant nonmelanocytic lesions
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).
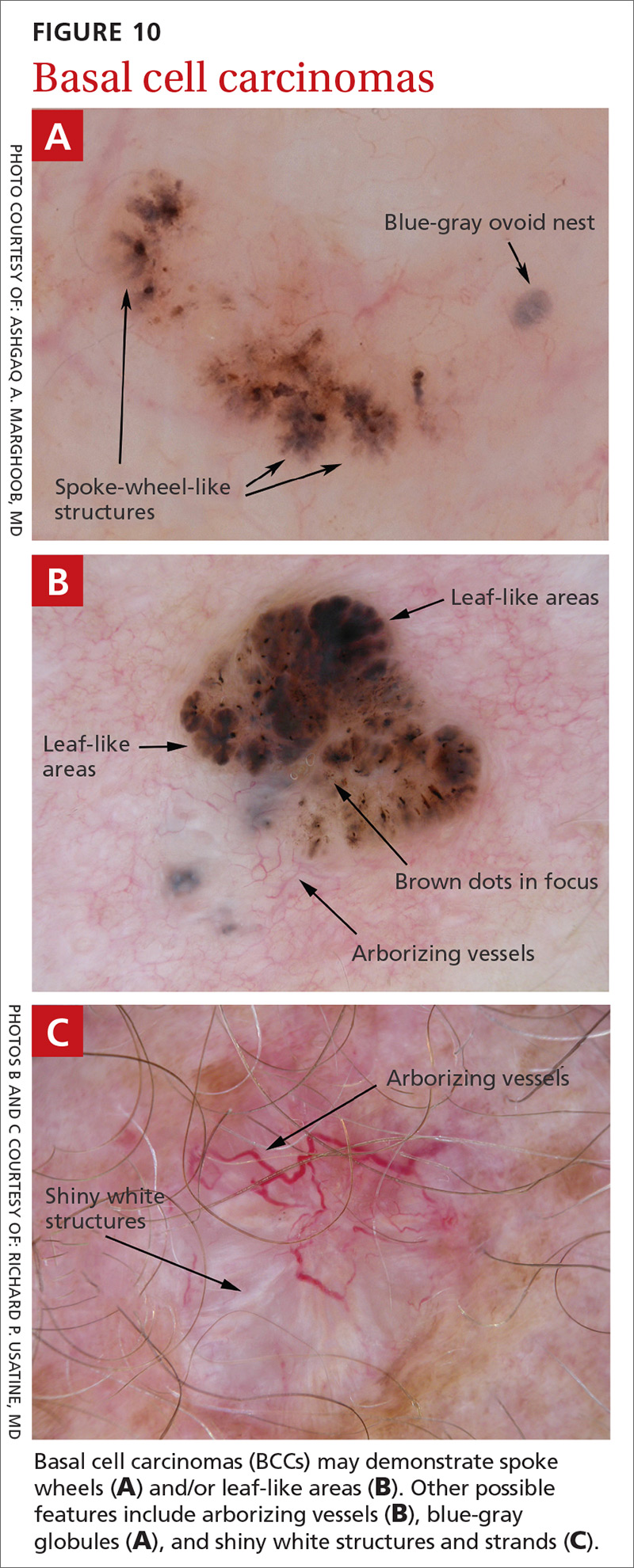
Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.
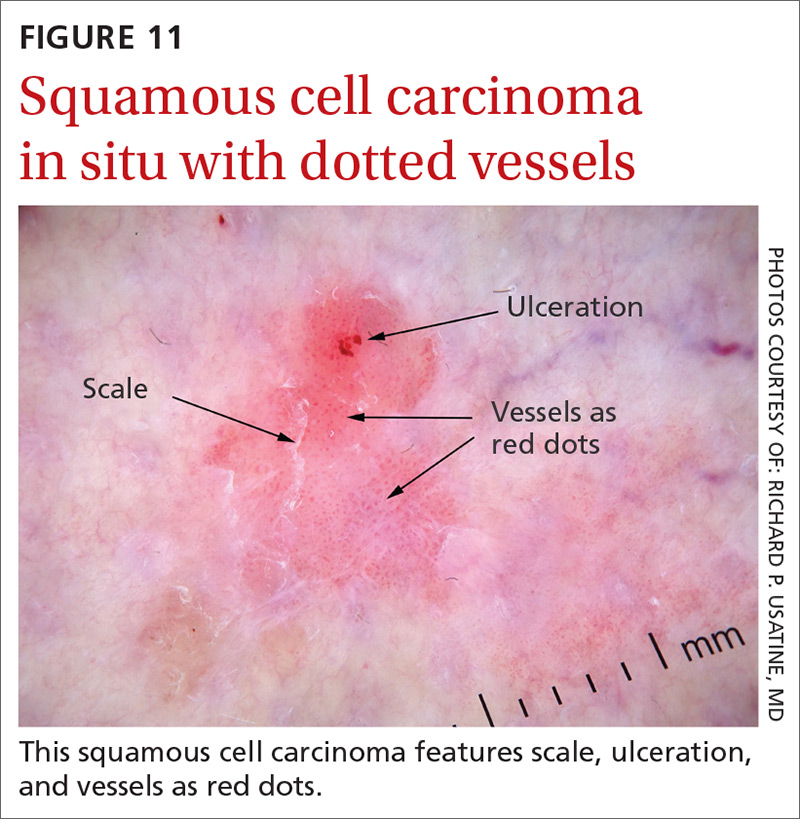
Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17
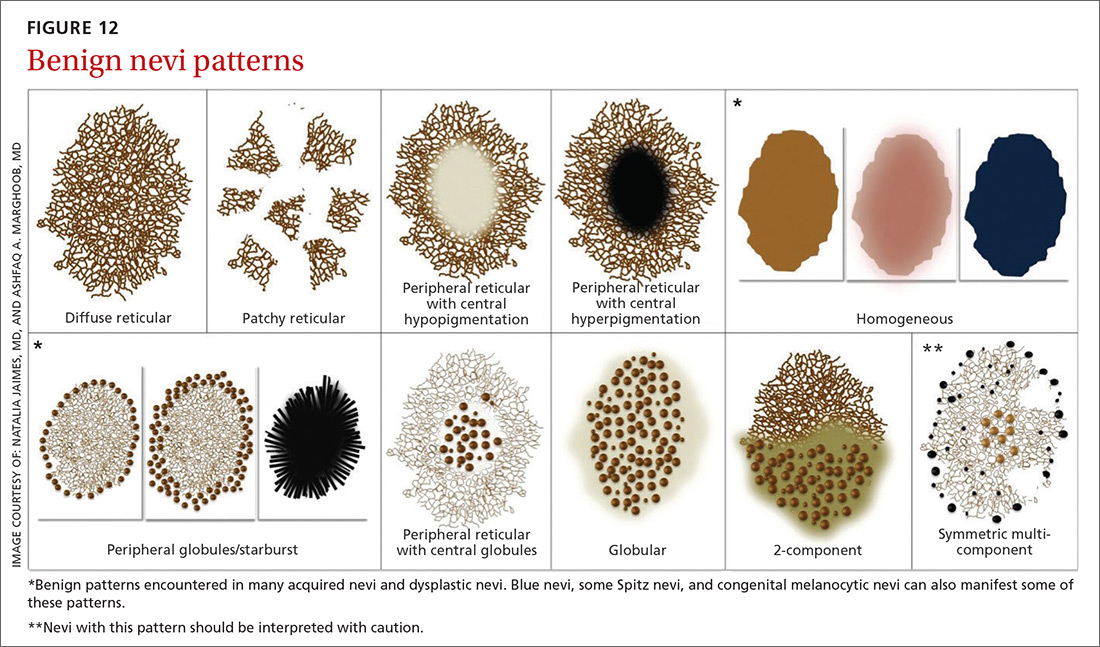
Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).
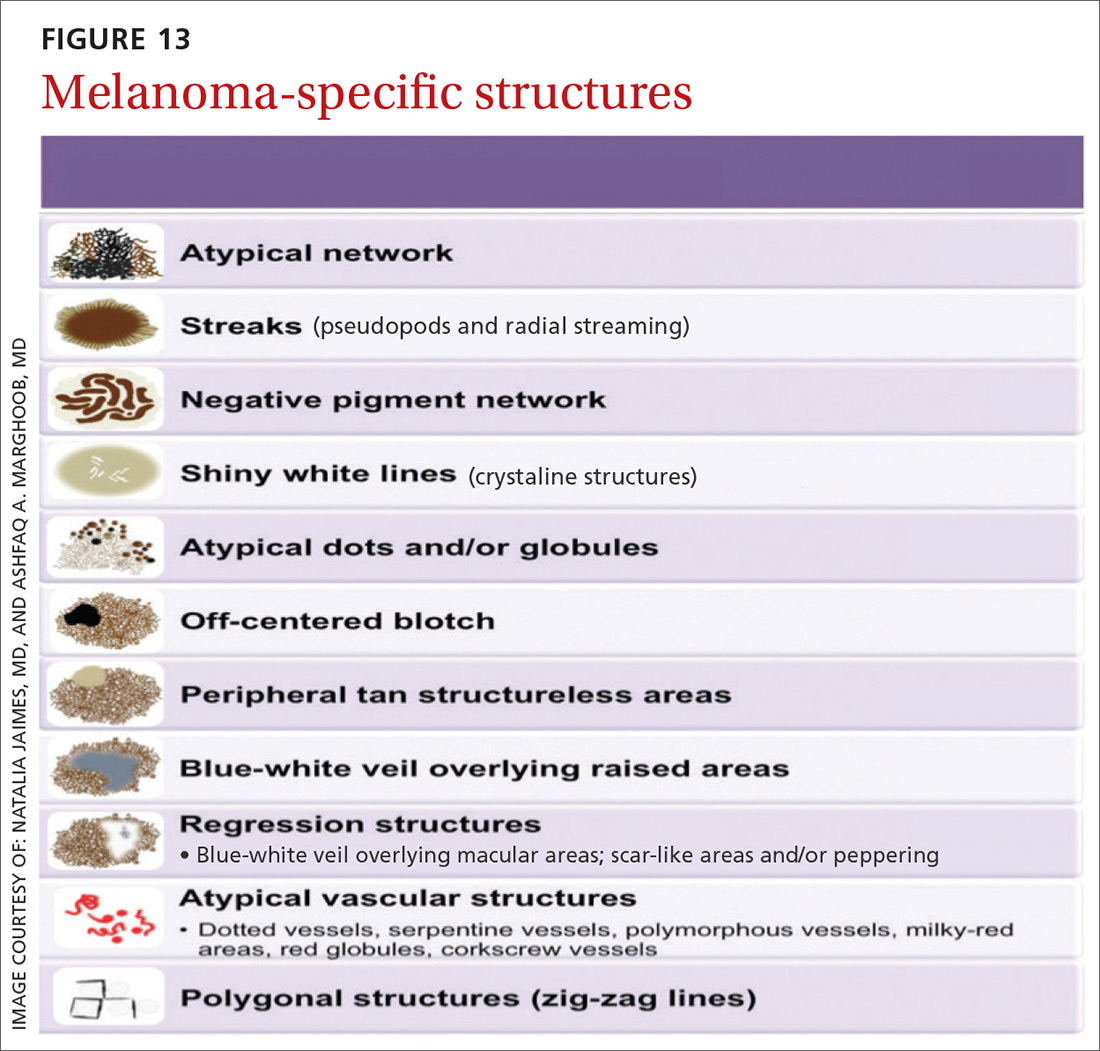
Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).
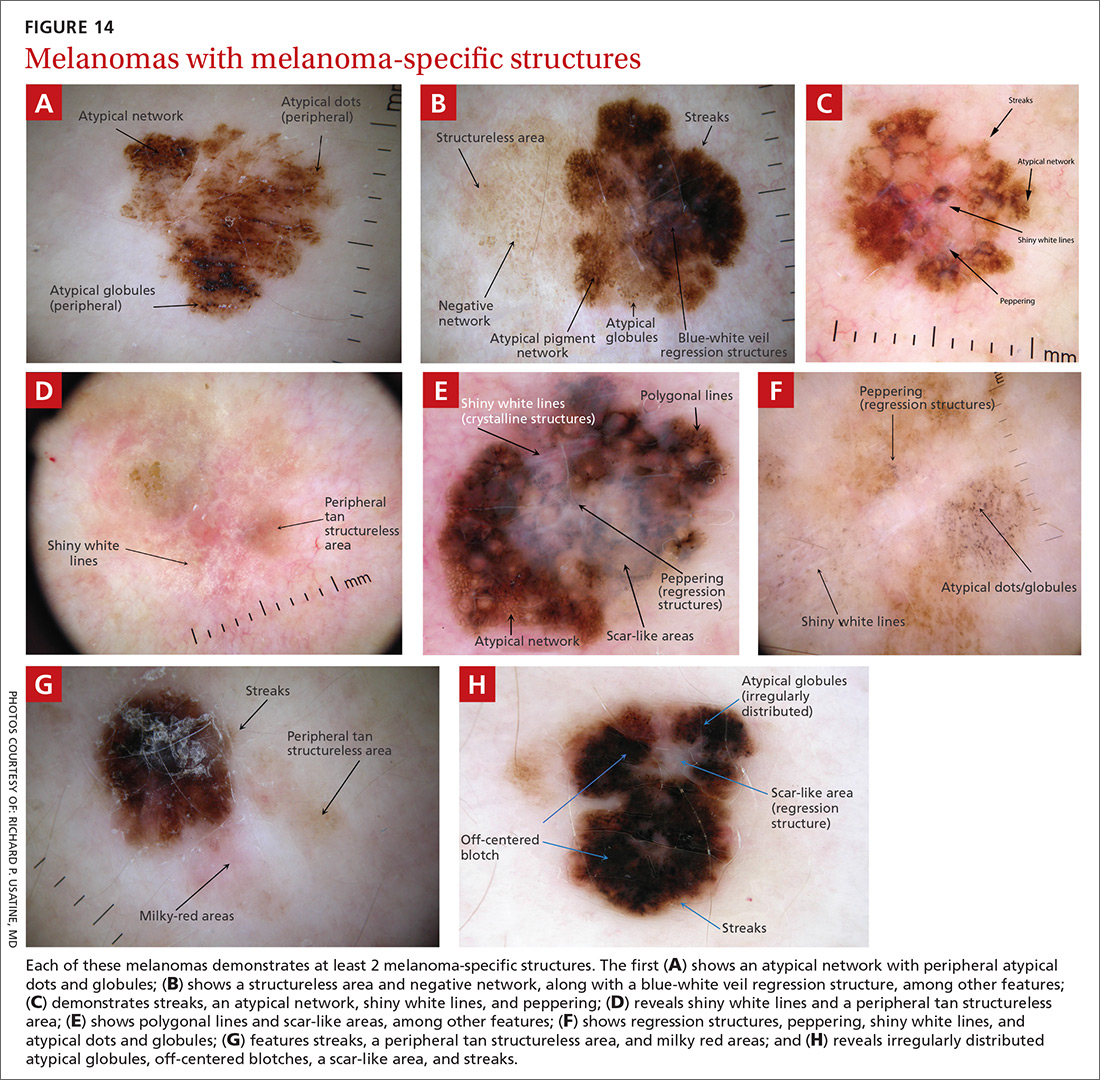
How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.
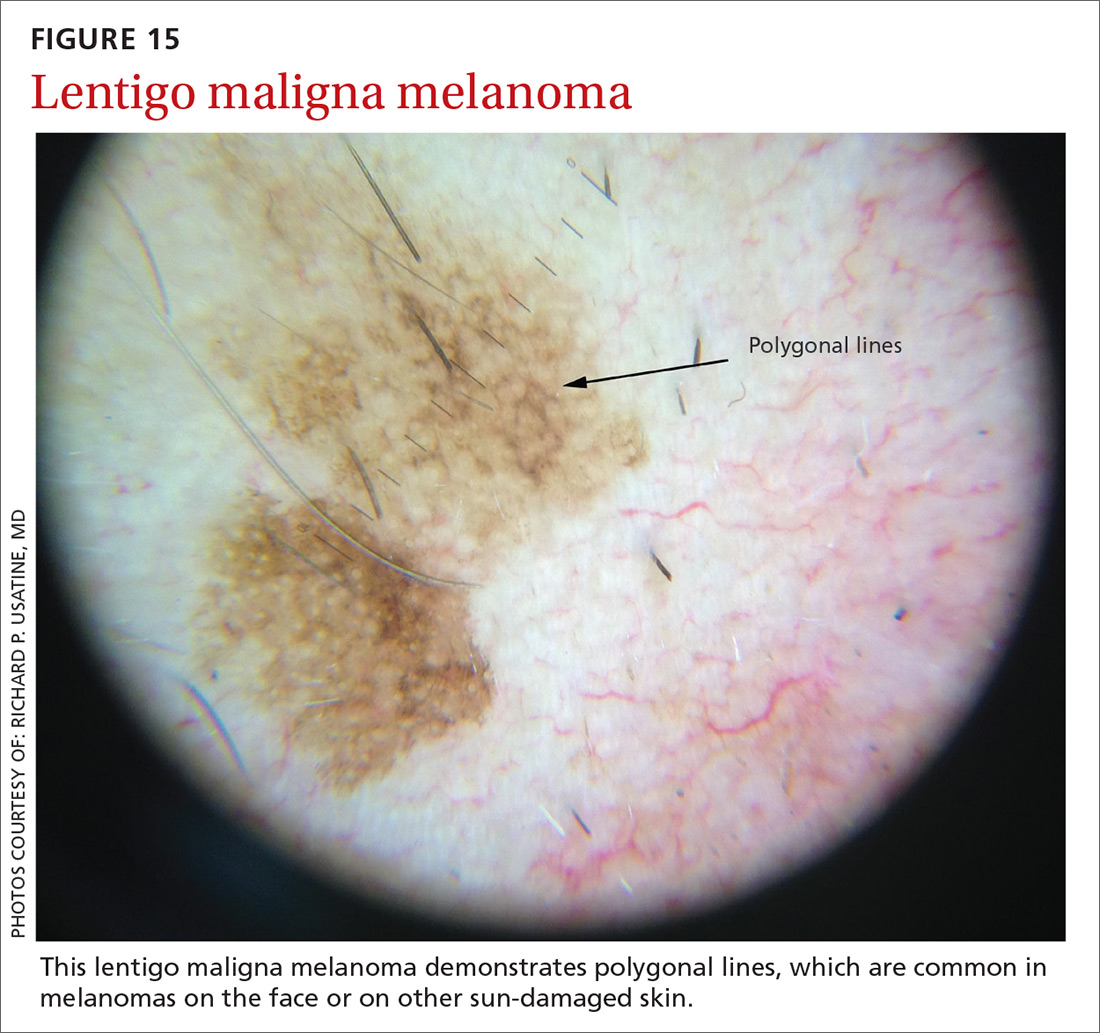
This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.
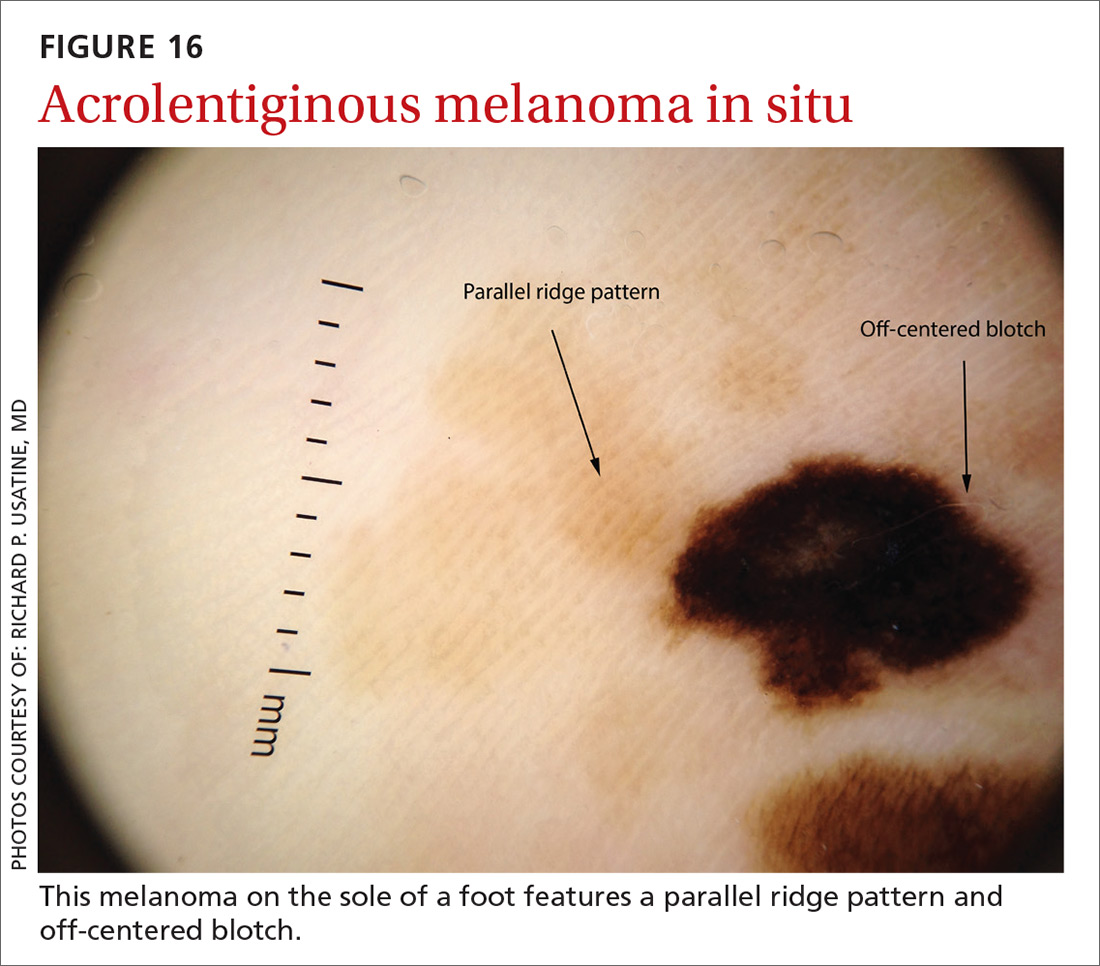
Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.

Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).

Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.

Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).

Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.

Continue to: Sebaceous hyperplasia...
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).

Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).

Malignant nonmelanocytic lesions
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).

Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.

Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17

Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).

Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).

How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.

This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.

Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
Dermoscopy, the use of a handheld instrument to magnify the skin 10-fold while providing a light source, is a quick, useful, cost-effective tool for detecting melanoma in family medicine.1-4 The device, which allows the physician to visualize structures below the stratum corneum that are not routinely discernible with the naked eye, can be attached to a smartphone so that photos can be taken and reviewed with the patient. The photo can also be reviewed after a biopsy result is obtained.
Its use among non-dermatologist US physicians appears to be relatively low, but rising. One small study of physicians working in family medicine, internal medicine, and plastic surgery found that only 15% had ever used a dermatoscope and 6% were currently using one.5
As a family physician, you can expand your diagnostic abilities in dermatology with the acquisition of a dermatoscope (FIGURE 1) and some time invested in learning to interpret visible patterns. With that in mind, this review focuses on the diagnosis of skin cancers and benign growths using dermoscopy. We begin with a brief look at the research on dermoscopy and how it is performed. From there, we’ll detail an algorithm to guide dermoscopic analysis. And to round things out, we provide guidance that will help you to get started. (See “Choosing a dermatoscope—and making the most of it,” and “To learn more about dermoscopy …”.)

SIDEBAR
Choosing a dermatoscope—and making the most of it
1. Consider acquiring a hybrid dermatoscope.
Nonpolarized dermatoscopes (NPDs) and polarized dermatoscopes (PDs) provide different but complementary information. PDs enable users to identify features such as vessels and shiny white structures that are highly indicative of skin cancer. Because PDs are highly sensitive for detecting skin cancer and do not require a liquid interface or direct skin contact, they are the ideal dermatoscopes to use for skin cancer screening.
However, maintaining the highest specificity requires the complementary use of NPDs, which are better at identifying surface structures seen in seborrheic keratoses and other benign lesions. Thus, if the aim is to maintain the highest diagnostic accuracy for all types of lesions, then the preferred dermatoscope is a hybrid that permits the user to toggle between polarized and nonpolarized features in one device.
2. Choose a dermatoscope that attaches to your smartphone and/or camera.
This helps you capture digital dermoscopic images that can be analyzed on a larger screen, which permits:
- enlarging certain areas for in-depth analysis of structures and patterns
- sharing the image with the patient to explain why a biopsy is, or isn’t, needed
- sharing the image with a colleague for the purpose of a consult or a referral, or using the images for teaching purposes
- saving the images in order to follow lesions over time when monitoring is indicated
- ongoing learning. After each biopsy result comes back, we recommend correlating the dermoscopic images with the biopsy report. If your suspected diagnosis was correct, this reinforces your knowledge. If the pathology diagnosis is unexpected, you can learn by revisiting the original images to look for structures or patterns you may have missed upon first examination. You may even question the pathology report based on the dermoscopy, prompting a call to the pathologist.
- keeping a safe distance from the patient when looking for scabies mites.
SIDEBAR
To learn more about dermoscopy…
FREE APPS:
Dermoscopy 2-Step Algorithm. Available for free on iTunes, Google Play, and at https://usatinemedia.com/app/dermoscopy-two-step-algorithm/, this free app (developed by 3 of the 4 authors) is intended to help you interpret the dermoscopic patterns seen with your dermatoscope. It asks a series of questions that lead you to the most probable diagnosis. The app also contains more than 80 photos and charts to help you with your diagnosis. No Internet connection is needed to view the full app. There are 50 interactive cases to solve.
YOUdermoscopy Training (Available for free on iTunes, Google Play, and at https://www.youdermoscopytraining.org/) offers a fun game interface to test and expand your dermoscopy skills.
OTHER INTERNET RESOURCES:
- Dermoscopedia provides state-of-the-art information on dermoscopy. It’s available at: https://dermoscopedia.org.
- A free dermoscopy tutorial is available at: http://www.dermoscopy.org/
- The International Dermoscopy Society’s Web site, which offers various tutorials and other information, can be found at: http://www.dermoscopy-ids.org/.
COURSES:
Dermoscopy courses are a great way to get started and/or to advance your skills. The following courses are taught by the authors of this article:
- The American Dermoscopy Meeting is held yearly in the summer in a national park. See http://www.americandermoscopy.com/.
- Memorial Sloan Kettering Cancer Center holds a yearly dermoscopy workshop each fall in New York City. See http://www.mskcc.org/events/.
- The yearly American Academy of Family Physicians' FMX meeting offers dermoscopy workshops. See https://www.aafp.org/events/fmx.html.
Continue to: What the research says
What the research says
Dermoscopy improves sensitivity for detecting melanoma over the naked eye alone; it also allows for the detection of melanoma at earlier stages, which improves prognosis.6
A meta-analysis of dermoscopy use in clinical settings showed that, following training, dermoscopy increases the average sensitivity of melanoma diagnosis from 71% to more than 90% without a significant decrease in specificity.7 In a study of 74 primary care physicians, there was an improvement in both clinical and dermoscopic diagnosis of melanoma among those who received training in dermoscopy, compared with a control group.8 Another study found that primary care physicians can reduce their baseline benign-to-melanoma ratio (the number of suspicious benign lesions biopsied to find 1 melanoma) from 9.5:1 with naked eye examination to 3.5:1 with dermoscopy.9
The exam begins by choosing 1 of 3 modes of dermoscopy
Dermatoscopes can have a polarized or nonpolarized light source. Some dermatoscopes combine both types of light (hybrid dermatoscopes; see “Choosing a dermatoscope—and making the most of it.”)
There are 3 modes of dermoscopy:
- nonpolarized contact dermoscopy
- polarized contact dermoscopy
- polarized non-contact dermoscopy.
Dermatoscopes with nonpolarized light require direct skin contact and a liquid interface (eg, alcohol, gel, mineral oil) between the scope’s glass plate and the skin for the visualization of subsurface structures. In contrast, dermatoscopes with polarized light do not require direct skin contact or a liquid interface; however, contacting the skin and using a liquid interface will provide a sharper image.
Continue to: Two major algorithms guide dermoscopic analysis
Two major algorithms guide dermoscopic analysis
The first of 2 major algorithms that can be used to guide dermoscopic analysis is a modified pattern analysis put forth by Kittler.10 This descriptive system based on geometric elements, patterns, colors, and clues guides the observer to a specific diagnosis without categorizing lesions as being either melanocytic or nonmelanocytic. Because this is not the preferred method of the authors, we will move on to Method 2.
The second method, a 2-step algorithm, is a qualitative system that guides the observer through differentiating melanocytic from nonmelanocytic lesions in order to differentiate nevi from melanoma (FIGURE 2). At the same time, it serves as an aid to correctly diagnose non-melanocytic lesions. The 2-step algorithm forms the foundation for the dermoscopic evaluation of skin lesions in this article.

Not all expert dermoscopists employ structured analytical systems or methods to reach a diagnosis. Because of their vast experience, many rely purely on pattern recognition. But algorithms can facilitate non-experts in dermoscopy in the differentiation of nevi from melanoma or, simply, in differentiating the benign from the malignant.
Although each algorithm has its unique criteria, all of them require training and practice and familiarity with the terms used to describe morphologic structures. The International Dermoscopy Society recently published a consensus paper designating some terms as preferred over others.11
Continue to: Step 1...
Step 1: Melanocytic vs non-melanocytic
Step 1 of the 2-step algorithm requires the observer to determine whether the lesion is melanocytic (ie, originates from melanocytes and, therefore, could be a melanoma) or nonmelanocytic in origin.
A melanocytic lesion usually will display at least 1 of the following structures:
- pigment network (FIGURE 3A) (This can include angulated lines.)
- negative network (FIGURE 3B) (hypopigmented lines connecting pigmented structures in a serpiginous fashion)
- streaks (FIGURE 3C)
- homogeneous blue pigmentation (FIGURE 3D)
- globules (aggregated or as a peripheral rim) (FIGURE 3E)
- pseudonetwork (facial skin) (FIGURE 3F)
- parallel pigment pattern (acral lesions) (FIGURE 3G).

Exceptions. Sometimes, nonmelanocytic lesions will present with pigment network. Dermatofibromas, for example, are one exception in which the pattern trumps the network. Two other exceptions are solar lentigo and supernumerary or accessory nipple.
If the lesion does not display any structure, it is considered structureless. In these cases, proceed to the second step to rule out a melanoma.
Doesn’t meet criteria for a melanocytic lesion?
If the lesion does not reveal any of the criteria for a melanocytic lesion, then look for structures seen in nonmelanocytic lesions: dermatofibromas; seborrheic keratosis; angiomas and angiokeratomas; sebaceous hyperplasia; clear-cell acanthomas; basal cell carcinomas (BCCs); and squamous cell carcinomas (SCCs).
Continue to: Benign nonmelanocytic lesions
Benign nonmelanocytic lesions
Dermatofibromas are benign symmetric lesions that feel firm and may dimple upon application of lateral pressure. They are fibrotic scar-like lesions that present with 1 or more of the following dermoscopic features (FIGURE 4):
- peripheral pigment network, due to increased melanin in keratinocytes
- homogeneous brown pigmented areas
- central scar-like area
- shiny white lines
- vascular structures (ie, dotted, polymorphous vessels), usually seen within the scar-like area
- ring-like globules, usually seen in the zone between the scar-like depigmentation and the peripheral network. They correspond to widened hyperpigmented rete ridges.

Seborrheic keratosis (SK) is a benign skin growth that often has a stuck-on appearance (FIGURE 5). Features often include:
- multiple (>2) milia-like cysts
- comedo-like openings
- a network-like structure that corresponds to gyri and sulci and which in some cases can create a cerebriform pattern
- fingerprint-like structures
- moth-eaten borders
- jelly sign. This consists of semicircular u-shaped structures that have a smudged appearance and are aligned in the same direction. The appearance resembles jelly as it is spread on a piece of bread.
- hairpin (looped or twisted-looped) vessels surrounded by a white halo.

Other clues include a sharp demarcation and a negative wobble sign (which we’ll describe in a moment). The presence or absence of a wobble sign is determined by using a dermatoscope that touches the skin. Mild vertical pressure is applied to the lesion while moving the scope back and forth horizontally. If the lesion slides across the skin surface, the diagnosis of an epidermal keratinocytic tumor (ie, SK) is favored. If, on the other hand, the lesion wobbles (rolls back and forth), then the diagnosis of a neoplasm with a dermal component (ie, intradermal or compound nevus) is more likely.
Angiomas and angiokeratomas. Angiomas demonstrate lacunae that are often separated by septae (FIGURE 6). Lacunae can vary in size and color. They can be red, red-white, red-blue, maroon, blue, blue-black, or even black (when thrombosis is present).

Angiokeratomas (FIGURE 7) can reveal lacunae of varying colors including black, red, purple, and maroon. In addition, a blue-whitish veil, erythema, and hemorrhagic crusts can be present.

Continue to: Sebaceous hyperplasia...
Sebaceous hyperplasia is the overgrowth of sebaceous glands. It can mimic BCC on the face. Sebaceous hyperplasia presents with multiple vessels in a crown-like arrangement that do not cross the center of the lesion. The sebaceous glands resemble popcorn (FIGURE 8).

Clear-cell acanthoma is a benign erythematous epidermal tumor usually found on the leg with a string-of-pearls pattern. This pattern is vascular so the pearls are red in color (FIGURE 9).

Malignant nonmelanocytic lesions
BCC is the most common type of skin cancer. Features often include:
- spoke-wheel-like structures or concentric structures (FIGURE 10A)
- leaf-like areas (FIGURE 10B)
- arborizing vessels (FIGURE 10b and 10C)large blue-gray ovoid nest (FIGURE 10A)
- multiple blue-gray non-aggregated globules
- ulceration or multiple small erosions
- shiny white structures and strands (FIGURE 10C).

Additional dermoscopic clues include short, fine, superficial telangiectasias and multiple in-focus dots in a buck-shot scatter distribution.
Squamous cell carcinomas (SCCs) of the skin are keratinizing malignant tumors. Each SCC generally has some of the following features (FIGURE 11):
- dotted and/or glomerular vessels, commonly distributed focally at the periphery. They can also be diffuse or aligned linearly within the lesion.
- scale (yellow or white)
- rosettes (seen with polarized light)
- white circles or keratin pearls
- brown circles
- ulcerations
- brown dots or globules arranged in a linear configuration.

Continue to: Step 2...
Step 2: It’s melanocytic, but is it a nevus or a melanoma?
If, by following Step 1 of the algorithm, the lesion is determined to be of melanocytic origin, then one proceeds to Step 2 to decide whether the growth is a nevus, a suspicious lesion, or a melanoma. For this purpose, several additional algorithms are available.12-17

Benign nevi tend to manifest with 1 of the following 10 patterns: (FIGURE 12)
- diffuse reticular
- patchy reticular
- peripheral reticular with central hypopigmentation
- peripheral reticular with central hyperpigmentation
- homogeneous
- peripheral globules/starburst. It has been suggested that lesions that show starburst morphology on dermoscopy require complete excision and follow-up since 13% of Spitzoid-looking symmetric lesions in patients older than 12 years were found to be melanoma in one study.18
- peripheral reticular with central globules
- globular
- 2-component
- symmetric multicomponent (this pattern should be interpreted with caution, and a biopsy is probably warranted for dermoscopic novices).

Melanomas tend to deviate from the benign patterns described earlier. Structures in melanomas are often distributed in an asymmetric fashion (which is the basis for diagnosis in many of the other algorithms), and most of them will reveal 1 or more of the melanoma-specific structures (FIGURE 13). The melanomas in FIGURES 14 A-H each show at least 2 melanoma-specific structures. On the face or sun-damaged skin, melanoma may present with grey color, a circle-in-circle pattern, and/or polygonal lines (FIGURE 15). Note that melanoma on the soles or palms may present with a parallel ridge pattern (FIGURE 16).

How to proceed after the evaluation of melanocytic lesions
After evaluating the lesion for benign patterns and melanoma-specific structures, there are 3 possible pathways:
1. The lesion adheres to one of the nevi patterns and does not display a melanoma-specific structure. You can reassure the patient that the lesion is benign.
2. The lesion:
A. Adheres to one nevus pattern, but also displays a melanoma-specific structure.
B. Does not adhere to any of the benign patterns and does not have any melanoma-specific structures.

This is considered a suspicious lesion, and the choices of action include performing a biopsy or short-term monitoring by comparing dermoscopic images over a 3-month interval. (Caveat: Never monitor raised lesions because nodular melanomas can grow quickly and develop a worsened prognosis in a short time. Instead you’ll want to biopsy the lesion that day or very soon thereafter.)
3. The lesion deviates from the benign patterns and has at least 1 melanoma-specific structure. Biopsy the lesion to rule out melanoma.

Continue to: A bonus...
A bonus: Diagnosing scabies
Increasingly, dermoscopy is being used in the diagnosis of many other skin, nail, and hair problems. In fact, one great bonus to owning a dermatoscope is the accurate diagnosis of scabies. Dermoscopy can be helpful in detecting the scabies mite without having to scrape and use the microscope. Moreover, the sensitivity and specificity of a dermoscopic diagnosis is higher than for scraping and microscopy.19
What you’ll see
The anterior legs and mouth parts of the mite resemble a triangle (arrowhead, delta-wing jet) (FIGURE 17). Look for a burrow, and the mite can be seen at the end of the burrow as a faint circle with a leading darker triangle. The burrow itself has a distinctive pattern that has more morphology than an excoriation and has been described as the contrail of a jet plane. Using a dermatoscope attached to your smartphone allows you to magnify the image even further while maintaining a safe distance from the mite.

CORRESPONDENCE
Richard P. Usatine, MD, 903 W. Martin, Skin Clinic – Historic Building, San Antonio, TX 78207; [email protected].
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.
1. Herschorn A. Dermoscopy for melanoma detection in family practice. Can Fam Physician. 2012;58:740-745.
2. Buckley D, McMonagle C. Melanoma in primary care. The role of the general practitioner. Ir J Med Sci. 2014;183:363-368.
3. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part I Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71:599.e1-599.e12.
4. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: Part II Screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10.
5. Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:2.
6. Salerni G, Terán T, Alonso C, et al. The role of dermoscopy and digital dermoscopy follow-up in the clinical diagnosis of melanoma: clinical and dermoscopic features of 99 consecutive primary melanomas. Dermatol Pract Concept. 2014;4:39-46.
7. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
8. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
9. Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270-1277.
10. Kittler H. Dermatoscopy: introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13:3.
11. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093-1106.
12. Stolz W, Riemann A, Cognetta AB, et al. ABCD rule of dermoscopy: a new practical method for early recognition of malignant melanoma. Eur J Dermatol. 1994;4:521-527.
13. Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions I Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571-583.
14. Menzies SW, Ingvar C, McCarthy WH. A sensitivity and specificity analysis of the surface microscopy features of invasive melanoma. Melanoma Res. 1996;6:55-62.
15. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
16. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
17. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
18. Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015;72:47-53.
19. Dupuy A, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007;56:53-62.