User login
Survey finds oral minoxidil shortage in Washington-area pharmacies
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
AI flagged skin cancer with near-perfect accuracy, in UK study
. AI detected more than 99% of all skin cancers.
The researchers tested the AI by integrating it into a clinical diagnosis process – anticipating a future in which AI helps doctors catch skin cancer faster and triage patients.
Skin cancer is the most common cancer in the United States; one in five 5 Americans develop skin cancer by age 70. With melanoma, the deadliest skin cancer, the 5-year survival rate is better than 99% if caught early, though only about three-quarters of melanomas are caught at this stage.
Amid rising skin cancer rates come concerns that the number of dermatologists in the workforce isn’t keeping pace. That may be why the average wait time for a dermatology appointment is trending up – in 2022, it reached 34.5 days.
The study, which was presented at the European Academy of Dermatology and Venereology Congress recently and has not yet been published, involved 6,900 patients in the United Kingdom with suspected skin cancer. The patients had been referred by their primary care physicians. The researchers took images of the suspicious areas and uploaded them to the AI software. The AI’s assessment was then shared with a dermatologist.
“Note that the diagnosis issued by the AI was not hidden from the dermatologist doing the second assessment,” said lead researcher Kashini Andrew, MBBS, a dermatologist and specialist registrar at University Hospitals Birmingham NHS Foundation Trust.
Dr. Andrew acknowledged that this may have influenced the dermatologist’s opinion. But that’s the vision of how doctors could use this tool.
The AI caught 59 of 59 melanomas and 189 of 190 total skin cancers (99.5%). (The one case that the AI missed was caught by the dermatologist.) It also flagged 541 of 585 precancerous lesions (92.5%). This represented a big improvement from a 2021 version of the model, which detected 86% of melanomas, 84% of all skin cancers, and 54% of precancerous lesions.
Over the 10-month period of the study, the system saved more than 1,000 face-to-face consultations, freeing dermatologists’ time to catch more cancers and serve more patients.
Limitations
The patients in the study were from “one hospital in a single region of the UK,” and the sample was not large enough to allow broad statements to be made about the use of AI in dermatology, Dr. Andrew said.
But it can open the conversation. Roxana Daneshjou, MD, PhD, a dermatologist at Stanford (Calif.) University who has studied the pros and cons of AI in medicine, had some concerns. For one thing, doctors can gather more in-depth information during an in-person exam than AI can glean from a photo, Dr. Daneshjou noted. They can examine skin texture, gather patient history, and take photos with special lighting and magnification.
And the AI needs to get better at ruling out malignancy, Dr. Daneshjou said. In this study, the AI identified 75% of benign lesions, a decline from the earlier version. The researchers noted in the abstract that this is a potential trade-off for increased sensitivity.
“[Unnecessary] biopsies can clog up the health care system, cost money, and cause stress and scarring,” said Dr. Daneshjou. “You don’t want to increase the burden of that.”
Still, if AI software such as the kind used in the study proves just as accurate in larger, more diverse sample sizes, then it could be a powerful tool for triage, Dr. Daneshjou said. “If AI gets particularly good at finding malignancy and also ruling it out, that would be a win.”
A version of this article appeared on Medscape.com.
. AI detected more than 99% of all skin cancers.
The researchers tested the AI by integrating it into a clinical diagnosis process – anticipating a future in which AI helps doctors catch skin cancer faster and triage patients.
Skin cancer is the most common cancer in the United States; one in five 5 Americans develop skin cancer by age 70. With melanoma, the deadliest skin cancer, the 5-year survival rate is better than 99% if caught early, though only about three-quarters of melanomas are caught at this stage.
Amid rising skin cancer rates come concerns that the number of dermatologists in the workforce isn’t keeping pace. That may be why the average wait time for a dermatology appointment is trending up – in 2022, it reached 34.5 days.
The study, which was presented at the European Academy of Dermatology and Venereology Congress recently and has not yet been published, involved 6,900 patients in the United Kingdom with suspected skin cancer. The patients had been referred by their primary care physicians. The researchers took images of the suspicious areas and uploaded them to the AI software. The AI’s assessment was then shared with a dermatologist.
“Note that the diagnosis issued by the AI was not hidden from the dermatologist doing the second assessment,” said lead researcher Kashini Andrew, MBBS, a dermatologist and specialist registrar at University Hospitals Birmingham NHS Foundation Trust.
Dr. Andrew acknowledged that this may have influenced the dermatologist’s opinion. But that’s the vision of how doctors could use this tool.
The AI caught 59 of 59 melanomas and 189 of 190 total skin cancers (99.5%). (The one case that the AI missed was caught by the dermatologist.) It also flagged 541 of 585 precancerous lesions (92.5%). This represented a big improvement from a 2021 version of the model, which detected 86% of melanomas, 84% of all skin cancers, and 54% of precancerous lesions.
Over the 10-month period of the study, the system saved more than 1,000 face-to-face consultations, freeing dermatologists’ time to catch more cancers and serve more patients.
Limitations
The patients in the study were from “one hospital in a single region of the UK,” and the sample was not large enough to allow broad statements to be made about the use of AI in dermatology, Dr. Andrew said.
But it can open the conversation. Roxana Daneshjou, MD, PhD, a dermatologist at Stanford (Calif.) University who has studied the pros and cons of AI in medicine, had some concerns. For one thing, doctors can gather more in-depth information during an in-person exam than AI can glean from a photo, Dr. Daneshjou noted. They can examine skin texture, gather patient history, and take photos with special lighting and magnification.
And the AI needs to get better at ruling out malignancy, Dr. Daneshjou said. In this study, the AI identified 75% of benign lesions, a decline from the earlier version. The researchers noted in the abstract that this is a potential trade-off for increased sensitivity.
“[Unnecessary] biopsies can clog up the health care system, cost money, and cause stress and scarring,” said Dr. Daneshjou. “You don’t want to increase the burden of that.”
Still, if AI software such as the kind used in the study proves just as accurate in larger, more diverse sample sizes, then it could be a powerful tool for triage, Dr. Daneshjou said. “If AI gets particularly good at finding malignancy and also ruling it out, that would be a win.”
A version of this article appeared on Medscape.com.
. AI detected more than 99% of all skin cancers.
The researchers tested the AI by integrating it into a clinical diagnosis process – anticipating a future in which AI helps doctors catch skin cancer faster and triage patients.
Skin cancer is the most common cancer in the United States; one in five 5 Americans develop skin cancer by age 70. With melanoma, the deadliest skin cancer, the 5-year survival rate is better than 99% if caught early, though only about three-quarters of melanomas are caught at this stage.
Amid rising skin cancer rates come concerns that the number of dermatologists in the workforce isn’t keeping pace. That may be why the average wait time for a dermatology appointment is trending up – in 2022, it reached 34.5 days.
The study, which was presented at the European Academy of Dermatology and Venereology Congress recently and has not yet been published, involved 6,900 patients in the United Kingdom with suspected skin cancer. The patients had been referred by their primary care physicians. The researchers took images of the suspicious areas and uploaded them to the AI software. The AI’s assessment was then shared with a dermatologist.
“Note that the diagnosis issued by the AI was not hidden from the dermatologist doing the second assessment,” said lead researcher Kashini Andrew, MBBS, a dermatologist and specialist registrar at University Hospitals Birmingham NHS Foundation Trust.
Dr. Andrew acknowledged that this may have influenced the dermatologist’s opinion. But that’s the vision of how doctors could use this tool.
The AI caught 59 of 59 melanomas and 189 of 190 total skin cancers (99.5%). (The one case that the AI missed was caught by the dermatologist.) It also flagged 541 of 585 precancerous lesions (92.5%). This represented a big improvement from a 2021 version of the model, which detected 86% of melanomas, 84% of all skin cancers, and 54% of precancerous lesions.
Over the 10-month period of the study, the system saved more than 1,000 face-to-face consultations, freeing dermatologists’ time to catch more cancers and serve more patients.
Limitations
The patients in the study were from “one hospital in a single region of the UK,” and the sample was not large enough to allow broad statements to be made about the use of AI in dermatology, Dr. Andrew said.
But it can open the conversation. Roxana Daneshjou, MD, PhD, a dermatologist at Stanford (Calif.) University who has studied the pros and cons of AI in medicine, had some concerns. For one thing, doctors can gather more in-depth information during an in-person exam than AI can glean from a photo, Dr. Daneshjou noted. They can examine skin texture, gather patient history, and take photos with special lighting and magnification.
And the AI needs to get better at ruling out malignancy, Dr. Daneshjou said. In this study, the AI identified 75% of benign lesions, a decline from the earlier version. The researchers noted in the abstract that this is a potential trade-off for increased sensitivity.
“[Unnecessary] biopsies can clog up the health care system, cost money, and cause stress and scarring,” said Dr. Daneshjou. “You don’t want to increase the burden of that.”
Still, if AI software such as the kind used in the study proves just as accurate in larger, more diverse sample sizes, then it could be a powerful tool for triage, Dr. Daneshjou said. “If AI gets particularly good at finding malignancy and also ruling it out, that would be a win.”
A version of this article appeared on Medscape.com.
FROM THE EADV CONGRESS
FDA OKs first ustekinumab biosimilar
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
FDA approves second treatment for adults with hidradenitis suppurativa
The U.S.
The development, which was announced Oct. 31, makes secukinumab the first and only interleukin (IL)–17A inhibitor approved by the FDA for HS, which affects an estimated 1% of the worldwide population. It joins the tumor necrosis factor blocker adalimumab as the only FDA-approved treatment options for HS.
Secukinumab (Cosentyx) was previously approved by the FDA for treatment of moderate-to-severe plaque psoriasis in adults, and several other indications including psoriatic arthritis and ankylosing spondylitis.
Approval for HS was based on the pivotal phase 3 SUNSHINE and SUNRISE trials, which had a combined enrollment of more than 1,000 patients with HS in 40 countries. The studies evaluated efficacy, safety, and tolerability of two dose regimens of the drug in adults with moderate to severe HS at 16 weeks and up to 52 weeks.
According to a press release from Novartis announcing the approval, results at week 16 showed that a significantly higher proportion of patients achieved a Hidradenitis Suppurativa Clinical Response (HiSCR) when treated with secukinumab 300 mg every 2 weeks, compared with placebo: 44.5% vs. 29.4%, respectively, in the SUNSHINE trial and 38.3.% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
Similarly, results at week 16 showed that a significantly higher proportion of patients achieved an HiSCR when treated with secukinumab 300 mg every 4 weeks, compared with placebo: 41.3% vs. 29.4% in the SUNSHINE trial and 42.5% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
In addition, in an exploratory analysis out to 52 weeks, HiSCR values observed at week 16 following either dose regimen of secukinumab were improved over time up to week 52. In SUNSHINE, the values improved by 56.4% in patients treated with secukinumab every 3 weeks and by 56.3% in those treated with secukinumab every 4 weeks. In SUNRISE, the values improved by 65% in patients who were treated with secukinumab every 2 weeks and by 62.2% in those who were treated with the drug every 4 weeks.
In an interview, Haley Naik, MD, a dermatologist who directs the hidradenitis suppurativa program at the University of California, San Francisco, characterized the approval as a win for HS patients. “Patients now not only have a second option for approved therapy for HS, but also an option that raises the bar for what we can expect from therapeutic response,” she told this news organization. “I am excited to see a novel therapy that improves HS and quality of life for patients make it through the regulatory pipeline.”
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, Union Chimique Belge, and Novartis; investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
A version of this article first appeared on Medscape.com.
The U.S.
The development, which was announced Oct. 31, makes secukinumab the first and only interleukin (IL)–17A inhibitor approved by the FDA for HS, which affects an estimated 1% of the worldwide population. It joins the tumor necrosis factor blocker adalimumab as the only FDA-approved treatment options for HS.
Secukinumab (Cosentyx) was previously approved by the FDA for treatment of moderate-to-severe plaque psoriasis in adults, and several other indications including psoriatic arthritis and ankylosing spondylitis.
Approval for HS was based on the pivotal phase 3 SUNSHINE and SUNRISE trials, which had a combined enrollment of more than 1,000 patients with HS in 40 countries. The studies evaluated efficacy, safety, and tolerability of two dose regimens of the drug in adults with moderate to severe HS at 16 weeks and up to 52 weeks.
According to a press release from Novartis announcing the approval, results at week 16 showed that a significantly higher proportion of patients achieved a Hidradenitis Suppurativa Clinical Response (HiSCR) when treated with secukinumab 300 mg every 2 weeks, compared with placebo: 44.5% vs. 29.4%, respectively, in the SUNSHINE trial and 38.3.% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
Similarly, results at week 16 showed that a significantly higher proportion of patients achieved an HiSCR when treated with secukinumab 300 mg every 4 weeks, compared with placebo: 41.3% vs. 29.4% in the SUNSHINE trial and 42.5% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
In addition, in an exploratory analysis out to 52 weeks, HiSCR values observed at week 16 following either dose regimen of secukinumab were improved over time up to week 52. In SUNSHINE, the values improved by 56.4% in patients treated with secukinumab every 3 weeks and by 56.3% in those treated with secukinumab every 4 weeks. In SUNRISE, the values improved by 65% in patients who were treated with secukinumab every 2 weeks and by 62.2% in those who were treated with the drug every 4 weeks.
In an interview, Haley Naik, MD, a dermatologist who directs the hidradenitis suppurativa program at the University of California, San Francisco, characterized the approval as a win for HS patients. “Patients now not only have a second option for approved therapy for HS, but also an option that raises the bar for what we can expect from therapeutic response,” she told this news organization. “I am excited to see a novel therapy that improves HS and quality of life for patients make it through the regulatory pipeline.”
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, Union Chimique Belge, and Novartis; investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
A version of this article first appeared on Medscape.com.
The U.S.
The development, which was announced Oct. 31, makes secukinumab the first and only interleukin (IL)–17A inhibitor approved by the FDA for HS, which affects an estimated 1% of the worldwide population. It joins the tumor necrosis factor blocker adalimumab as the only FDA-approved treatment options for HS.
Secukinumab (Cosentyx) was previously approved by the FDA for treatment of moderate-to-severe plaque psoriasis in adults, and several other indications including psoriatic arthritis and ankylosing spondylitis.
Approval for HS was based on the pivotal phase 3 SUNSHINE and SUNRISE trials, which had a combined enrollment of more than 1,000 patients with HS in 40 countries. The studies evaluated efficacy, safety, and tolerability of two dose regimens of the drug in adults with moderate to severe HS at 16 weeks and up to 52 weeks.
According to a press release from Novartis announcing the approval, results at week 16 showed that a significantly higher proportion of patients achieved a Hidradenitis Suppurativa Clinical Response (HiSCR) when treated with secukinumab 300 mg every 2 weeks, compared with placebo: 44.5% vs. 29.4%, respectively, in the SUNSHINE trial and 38.3.% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
Similarly, results at week 16 showed that a significantly higher proportion of patients achieved an HiSCR when treated with secukinumab 300 mg every 4 weeks, compared with placebo: 41.3% vs. 29.4% in the SUNSHINE trial and 42.5% vs. 26.1% in the SUNRISE trial (P < .05 for both associations).
In addition, in an exploratory analysis out to 52 weeks, HiSCR values observed at week 16 following either dose regimen of secukinumab were improved over time up to week 52. In SUNSHINE, the values improved by 56.4% in patients treated with secukinumab every 3 weeks and by 56.3% in those treated with secukinumab every 4 weeks. In SUNRISE, the values improved by 65% in patients who were treated with secukinumab every 2 weeks and by 62.2% in those who were treated with the drug every 4 weeks.
In an interview, Haley Naik, MD, a dermatologist who directs the hidradenitis suppurativa program at the University of California, San Francisco, characterized the approval as a win for HS patients. “Patients now not only have a second option for approved therapy for HS, but also an option that raises the bar for what we can expect from therapeutic response,” she told this news organization. “I am excited to see a novel therapy that improves HS and quality of life for patients make it through the regulatory pipeline.”
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, Union Chimique Belge, and Novartis; investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
A version of this article first appeared on Medscape.com.
Review finds no CV or VTE risk signal with use of JAK inhibitors for skin indications
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
FROM JAMA DERMATOLOGY
Phase 3 trial supports topical JAK inhibitor for AD in young children
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
BERLIN – as previously shown in adolescents and adults for whom it already has an approved indication.
In this study – TRUE-AD3 – systemic exposure to ruxolitinib, which is selective for JAK1 and 2, was followed closely, and the low mean plasma concentrations “suggest systemic JAK inhibition is highly unlikely,” Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the annual congress of the European Academy of Dermatology and Venereology.
For example, at a plasma concentration no greater than 27 nM in both younger and older patients at 4 weeks and again at 8 weeks, the systemic exposure was about a tenth of that (281 nM) previously associated with myelosuppression, he reported.
Given the boxed warning for oral JAK inhibitors, which was based largely on a 2022 study in adults with rheumatoid arthritis that associated tofacitinib, a nonspecific JAK inhibitor, with an increased risk of thrombotic events in adults already at risk for these events, safety was a focus of this phase 3 trial. The boxed warning is also in the labeling for topical ruxolitinib, 1.5% (Opzelura), approved for treating to mild to moderate atopic dermatitis in patients 12 years of age and older.
Dr. Eichenfield said there were no significant safety signals in the younger pediatric population. “There were no treatment-emergent adverse events suggestive of systemic JAK inhibition,” he said. This not only included the absence of serious infections, cardiac events, thromboses, or malignancies, but there was no signal of hematologic abnormalities, such as change in hemoglobin or neutrophil count.
Application site reactions
Rather, in the study of children ages 2-11, the only adverse events associated with topical ruxolitinib not observed in the control arm, which received the vehicle alone, were application site reactions, such as pain, erythema, and irritation. None of these occurred in more than 3% of those randomized to ruxolitinib regardless of dose.
Overall, in the trial, which randomized 329 patients ages from 2 to under 12 years with mild to moderate AD to ruxolitinib 1.5% cream, ruxolitinib 0.75% cream, or vehicle in a 2:2:1 fashion, there were just two (0.8%) discontinuations in the ruxolitinib groups (one in each dosing arm). There were none in the vehicle arm.
The safety supports an expansion of the AD indication for topical ruxolitinib in young children, because the rates of response were very similar to that seen in adolescents and adults in the previously published TRUE AD-1 and TRUE AD-2 trials, he said.
For the primary endpoint of Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) with at least a 2 grade improvement in IGA score from baseline, the response rates were 56.5%, 36.6%, and 10.8% for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively, at 8 weeks (P < .0001 for both doses relative to vehicle).
For the secondary efficacy endpoint of 75% or greater clearance on the Eczema Area and Severity Index, the rates were 67.2%, 51.5%, and 15.4%, for ruxolitinib 1.5%, ruxolitinib 0.75%, and vehicle respectively. Again, the advantage of both doses of ruxolitinib relative to vehicle was highly statistically significant (P < .0001).
Control of itch, evaluated with the Numerical Rating Scale was only evaluated in children 6-2 because of concern of the reliability of reporting in younger children. Control was defined as at least a 4-point improvement from baseline. It was achieved by 43.4%, 37.5%, and 29.7% by week 8 in the arms receiving the higher dose of ruxolitinib, the lower dose, and vehicle, respectively. The median time to achieving itch control was 11 days, 13 days, and 23 days, respectively. For all of these endpoints, the separation of the curves was readily apparent within the first 2 weeks.
The efficacy and tolerability of ruxolitinib appeared to be similar in younger children (ages 2-6) relative to older children.
Extension study in children near completion
Most of the patients who participated in TRUE AD-3 have been rolled over to the open-label extension trial, which is nearing completion. Those originally randomized to vehicle have been rerandomized to the lower or higher dose of ruxolitinib.
While this trial was focused on ruxolitinib as monotherapy, Thrasyvoulos Tzellos, MD, head of the department of dermatology, Nordland Hospital Trust, Bødo, Norway, questioned whether this is will be how it will be used in clinical practice. With the increasing array of therapies for AD, the “concept of combination therapy becomes more and more relevant,” he said after Dr. Eichenfield’s presentation.
Questioning whether an effective nonsteroidal anti-inflammatory agent like ruxolitinib should be considered a first-line treatment in mild disease or an adjunctive treatment for AD of any severity, he suggested that it might be best considered within a combination.
Dr. Eichenfield agreed. “Once we get the drug approved in a controlled trial, I think we then figure out how to use it in clinical practice.” Based on his own use of ruxolitinib in adults, he noted that he has not seen this drug replace other therapies so much as provide another option for control.
“We have an increasing armamentarium of drugs to use for involvement in different areas of the body in order to get more long-term control of disease,” he said. As an effective topical nonsteroidal drug, he believes its addition to clinical care in younger children, if approved, will be meaningful.
Dr. Eichenfield disclosed financial relationships with more multiple pharmaceutical companies, including Incyte, the manufacturer of ruxolitinib cream that provided funding for the True-AD trials. Dr. Tzellos reported financial relationships with AbbVie and UCB.
AT THE EADV CONGRESS
Painful axillary plaque
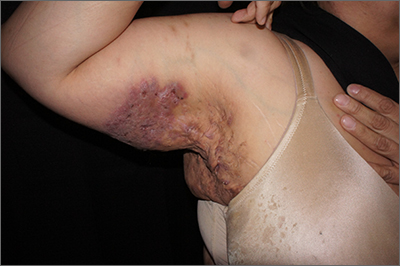
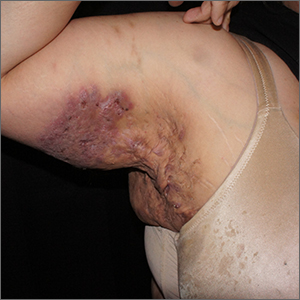
The persistent scars with recurrent abscesses and sinuses are indicative of advanced hidradenitis suppurativa. This painful and debilitating disease is characterized by the recurrent formation and inflammation of papules, cysts, sinuses, and scars in the axillae, inguinal folds, gluteal cleft, and inframammary folds. Pain, social isolation, depression, increased risk of substance abuse, and increased suicidality are all associated with hidradenitis suppurativa.
The disease may be graded based on severity, which can guide medical treatment options. The earliest stage appears similar to acne without significant sinus tract or scar formation and may be treated with topical therapies—including clindamycin 1% lotion or gel. When larger cysts associated with sinus tracts occur, systemic options with oral antibiotics (including doxycycline 100 mg bid for 3 months or combination clindamycin 300 mg and rifampin 300 mg, both bid for 3 months) are reasonable options. Intralesional triamcinolone in a concentration of 10 mg/mL injected directly into an inflamed cyst can provide acute relief. Severe disease is characterized by diffuse scars and sinus tracts. The TNF-alpha inhibitors adalimumab and infliximab are excellent options for severe disease that does not respond to antibiotics.
Surgical treatment may include either “deroofing” the sinuses or performing a wide excision of the whole area of involvement. Widely excised areas may be grafted, allowed to granulate, or closed if small enough. Although these options create significant wounds, patients experience good results; there is a 27% recurrence with deroofing and a 13% recurrence with wide excision.1
This patient underwent wide local excision of both axillae and the areas of involvement were allowed to granulate. Secondary intention healing occurred over 12 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Orenstein LAV, Nguyen TV, Damiani G, et al. Medical and surgical management of hidradenitis suppurativa: a review of international treatment guidelines and implementation in general dermatology practice. Dermatology. 2020;236:393-412. doi: 10.1159/000507323

The persistent scars with recurrent abscesses and sinuses are indicative of advanced hidradenitis suppurativa. This painful and debilitating disease is characterized by the recurrent formation and inflammation of papules, cysts, sinuses, and scars in the axillae, inguinal folds, gluteal cleft, and inframammary folds. Pain, social isolation, depression, increased risk of substance abuse, and increased suicidality are all associated with hidradenitis suppurativa.
The disease may be graded based on severity, which can guide medical treatment options. The earliest stage appears similar to acne without significant sinus tract or scar formation and may be treated with topical therapies—including clindamycin 1% lotion or gel. When larger cysts associated with sinus tracts occur, systemic options with oral antibiotics (including doxycycline 100 mg bid for 3 months or combination clindamycin 300 mg and rifampin 300 mg, both bid for 3 months) are reasonable options. Intralesional triamcinolone in a concentration of 10 mg/mL injected directly into an inflamed cyst can provide acute relief. Severe disease is characterized by diffuse scars and sinus tracts. The TNF-alpha inhibitors adalimumab and infliximab are excellent options for severe disease that does not respond to antibiotics.
Surgical treatment may include either “deroofing” the sinuses or performing a wide excision of the whole area of involvement. Widely excised areas may be grafted, allowed to granulate, or closed if small enough. Although these options create significant wounds, patients experience good results; there is a 27% recurrence with deroofing and a 13% recurrence with wide excision.1
This patient underwent wide local excision of both axillae and the areas of involvement were allowed to granulate. Secondary intention healing occurred over 12 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The persistent scars with recurrent abscesses and sinuses are indicative of advanced hidradenitis suppurativa. This painful and debilitating disease is characterized by the recurrent formation and inflammation of papules, cysts, sinuses, and scars in the axillae, inguinal folds, gluteal cleft, and inframammary folds. Pain, social isolation, depression, increased risk of substance abuse, and increased suicidality are all associated with hidradenitis suppurativa.
The disease may be graded based on severity, which can guide medical treatment options. The earliest stage appears similar to acne without significant sinus tract or scar formation and may be treated with topical therapies—including clindamycin 1% lotion or gel. When larger cysts associated with sinus tracts occur, systemic options with oral antibiotics (including doxycycline 100 mg bid for 3 months or combination clindamycin 300 mg and rifampin 300 mg, both bid for 3 months) are reasonable options. Intralesional triamcinolone in a concentration of 10 mg/mL injected directly into an inflamed cyst can provide acute relief. Severe disease is characterized by diffuse scars and sinus tracts. The TNF-alpha inhibitors adalimumab and infliximab are excellent options for severe disease that does not respond to antibiotics.
Surgical treatment may include either “deroofing” the sinuses or performing a wide excision of the whole area of involvement. Widely excised areas may be grafted, allowed to granulate, or closed if small enough. Although these options create significant wounds, patients experience good results; there is a 27% recurrence with deroofing and a 13% recurrence with wide excision.1
This patient underwent wide local excision of both axillae and the areas of involvement were allowed to granulate. Secondary intention healing occurred over 12 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Orenstein LAV, Nguyen TV, Damiani G, et al. Medical and surgical management of hidradenitis suppurativa: a review of international treatment guidelines and implementation in general dermatology practice. Dermatology. 2020;236:393-412. doi: 10.1159/000507323
1. Orenstein LAV, Nguyen TV, Damiani G, et al. Medical and surgical management of hidradenitis suppurativa: a review of international treatment guidelines and implementation in general dermatology practice. Dermatology. 2020;236:393-412. doi: 10.1159/000507323
Novel hydrogel holds promise for skin regeneration
CARLSBAD, CALIF. – For the estimated 10 million wounds that clinicians treat in the United States each year resulting from surgical procedures, trauma, burns, and other causes, the best outcome is a scar, a fibrotic dermis with a flattened epidermis that contains no sweat glands, no pilosebaceous units, and impaired nerve function.
But what if the outcome was skin regeneration instead of scar formation? At the annual symposium of the California Society of Dermatology & Dermatologic Surgery, Philip O. Scumpia, MD, PhD, described the .
“We’re preprogrammed to undergo scarring,” said Dr. Scumpia, associate professor of dermatology at the University of California, Los Angeles. “Tissue fibrosis is an evolutionary process” where a fibrotic matrix is deposited “as quickly as possible to close the gap caused by an injury,” he noted. “All of the cues in the normal wound healing process result in fibrosis, but we want to move from scarring to tissue regeneration. The goal is to make something that can shift from this evolutionary process, and it’s proven to be inherently difficult.”
Common approaches to wound treatment include simple and advanced dressings, negative pressure, and hyperbaric oxygen. For wounds that persist beyond 30 days, advanced treatment options include decellularized grafts such as placental membranes, amniotic membranes, and acellular dermal matrices. “There are also cellularized grafts such as dressings that contain neonatal dermal fibroblasts,” which are expensive, said Dr. Scumpia, director of dermatopathology at the West Los Angeles VA Medical Center. “There are also semi-synthetic grafts such as single or double layer dermal replacement templates and synthetic dermal substitutes in the form of sheets or foam. All of these can help with wound coverage and help chronic wounds close on their own.”
Meanwhile, tissue regeneration – or efforts to restore tissue to its original functionality – include growth factors, stem cells, or replacement extracellular matrix (skin substitutes), or a combination. “Bioengineered dressings and bioengineered skin substitutes have shown modest improvement in wound healing but not tissue regeneration,” Dr. Scumpia said. “At best, we can accelerate scar formation and close the wound quicker, but nothing has been shown to regenerate tissue.”
Approaches to skin regeneration
Studies from the embryology literature have helped researchers develop better approaches to skin regeneration. For example, fetal skin heals without scarring when injured. “Hairs form from placodes, then sebaceous glands form, and fibroblasts that are part of the papillary mesenchymal body expressing special factors such as engrailed or CRABP1 drive hair follicle formation,” he said. “Many studies have shown that sonic hedgehog signaling, and Wnt/beta-catenin signaling can play a role in the development of new hair follicles. Also, fibroblasts in the dermis can drive hair follicle formation.”
Researchers are also learning about tissue regeneration from mouse models. For example, African spiny mice have been shown to heal regeneratively. “If you make wounds large enough on lab mice, the center heals regeneratively,” Dr. Scumpia said. “What’s interesting is that these same signals are present in embryonic hair follicle development. Why is this important? Who wants a hairy scar? It’s an organized structure that develops in the wound. That can help us understand what we need to put in so that our body regenerates on its own. In mouse models, the immune system has been shown to play a role in regeneration.”
Expanding on initial work conducted at UCLA, Dr. Scumpia and his colleagues founded San Diego-based Tempo Therapeutics, which is commercializing the MAP hydrogel to mimic the natural porosity and stiffness of skin. They sought to develop a new biomaterial, he said, noting that “the skin is porous on a microscale level, allowing cells to infiltrate different areas.” And the problem with existing biomaterials “is that they don’t incorporate into the skin very well,” he explained. “They’re usually stiff and rubbery and can cause a foreign body reaction, which can result in fibrous encapsulation and inflammation.”
The MAP hydrogel is composed of randomly packed “microsphere building blocks,” including an amino acid that promotes an immune response. When injected into a wound, the hydrogel forms a porous matrix in the tissue. Surface annealing locks in porosity and tissue grows into porous spaces, which avoids scar formation pathways and enables critical organs to regain function.
During in vivo tests, researchers observed decreases in inflammation compared with traditional hydrogels in the first 48 hours. “In mouse models, we found that if you inject in a hydrogel that has no porosity, the body tries to spit it out, and you have an immune reaction,” Dr. Scumpia said. “But when we used the MAP hydrogel, we found that cells can migrate through it, which allows wounds to heal quicker. When we added an antigen in the hydrogel trying to allow the hydrogel to degrade slower, it actually degraded more rapidly, but we found that new hair follicles formed in the center of these wounds, a hallmark of skin regeneration. My lab has been studying why this occurs and trying to use this to our advantage in other models.”
In an unpublished mouse burn wound model study, he and his colleagues excised a wound, but it never healed with regeneration in the center. “We don’t understand why,” he said. But when the researchers used the MAP gel in wounds of hairless mice, they observed the formation of sebaceous glands and hair follicles over the wound beds. “It’s an exciting finding to see hair follicles develop in the center of a wound,” Dr. Scumpia said. He noted that to date, use of the MAP hydrogel has demonstrated tissue regeneration in some of the 27 veterinary cases that have been performed, including for wounds following traumatic injuries or following tumor resections on paws that allowed the pets to avoid amputation.
Clinical trials planned
The first clinical trials of the MAP hydrogel are planned for treating complex diabetic wounds in early 2024 but will likely expand to other difficult-to-treat wounds, including venous stasis ulcers, decubitus ulcers, and use following large surgical resections. Dr. Scumpia and colleagues will also examine the regenerative biomaterial for tissue aesthetics, including dermal and deep tissue filler applications. The next steps in his laboratory, he said, are to combine biomaterials with stem cells, immune factors, or small molecular activators/inhibitors to improve sweat gland, nerve, or hair follicle regeneration.
Dr. Scumpia disclosed that he is a cofounder and shareholder in Tempo Therapeutics. He has also received grant support from the National Institutes of Health, Department of Veteran Affairs, and the LEO Foundation.
CARLSBAD, CALIF. – For the estimated 10 million wounds that clinicians treat in the United States each year resulting from surgical procedures, trauma, burns, and other causes, the best outcome is a scar, a fibrotic dermis with a flattened epidermis that contains no sweat glands, no pilosebaceous units, and impaired nerve function.
But what if the outcome was skin regeneration instead of scar formation? At the annual symposium of the California Society of Dermatology & Dermatologic Surgery, Philip O. Scumpia, MD, PhD, described the .
“We’re preprogrammed to undergo scarring,” said Dr. Scumpia, associate professor of dermatology at the University of California, Los Angeles. “Tissue fibrosis is an evolutionary process” where a fibrotic matrix is deposited “as quickly as possible to close the gap caused by an injury,” he noted. “All of the cues in the normal wound healing process result in fibrosis, but we want to move from scarring to tissue regeneration. The goal is to make something that can shift from this evolutionary process, and it’s proven to be inherently difficult.”
Common approaches to wound treatment include simple and advanced dressings, negative pressure, and hyperbaric oxygen. For wounds that persist beyond 30 days, advanced treatment options include decellularized grafts such as placental membranes, amniotic membranes, and acellular dermal matrices. “There are also cellularized grafts such as dressings that contain neonatal dermal fibroblasts,” which are expensive, said Dr. Scumpia, director of dermatopathology at the West Los Angeles VA Medical Center. “There are also semi-synthetic grafts such as single or double layer dermal replacement templates and synthetic dermal substitutes in the form of sheets or foam. All of these can help with wound coverage and help chronic wounds close on their own.”
Meanwhile, tissue regeneration – or efforts to restore tissue to its original functionality – include growth factors, stem cells, or replacement extracellular matrix (skin substitutes), or a combination. “Bioengineered dressings and bioengineered skin substitutes have shown modest improvement in wound healing but not tissue regeneration,” Dr. Scumpia said. “At best, we can accelerate scar formation and close the wound quicker, but nothing has been shown to regenerate tissue.”
Approaches to skin regeneration
Studies from the embryology literature have helped researchers develop better approaches to skin regeneration. For example, fetal skin heals without scarring when injured. “Hairs form from placodes, then sebaceous glands form, and fibroblasts that are part of the papillary mesenchymal body expressing special factors such as engrailed or CRABP1 drive hair follicle formation,” he said. “Many studies have shown that sonic hedgehog signaling, and Wnt/beta-catenin signaling can play a role in the development of new hair follicles. Also, fibroblasts in the dermis can drive hair follicle formation.”
Researchers are also learning about tissue regeneration from mouse models. For example, African spiny mice have been shown to heal regeneratively. “If you make wounds large enough on lab mice, the center heals regeneratively,” Dr. Scumpia said. “What’s interesting is that these same signals are present in embryonic hair follicle development. Why is this important? Who wants a hairy scar? It’s an organized structure that develops in the wound. That can help us understand what we need to put in so that our body regenerates on its own. In mouse models, the immune system has been shown to play a role in regeneration.”
Expanding on initial work conducted at UCLA, Dr. Scumpia and his colleagues founded San Diego-based Tempo Therapeutics, which is commercializing the MAP hydrogel to mimic the natural porosity and stiffness of skin. They sought to develop a new biomaterial, he said, noting that “the skin is porous on a microscale level, allowing cells to infiltrate different areas.” And the problem with existing biomaterials “is that they don’t incorporate into the skin very well,” he explained. “They’re usually stiff and rubbery and can cause a foreign body reaction, which can result in fibrous encapsulation and inflammation.”
The MAP hydrogel is composed of randomly packed “microsphere building blocks,” including an amino acid that promotes an immune response. When injected into a wound, the hydrogel forms a porous matrix in the tissue. Surface annealing locks in porosity and tissue grows into porous spaces, which avoids scar formation pathways and enables critical organs to regain function.
During in vivo tests, researchers observed decreases in inflammation compared with traditional hydrogels in the first 48 hours. “In mouse models, we found that if you inject in a hydrogel that has no porosity, the body tries to spit it out, and you have an immune reaction,” Dr. Scumpia said. “But when we used the MAP hydrogel, we found that cells can migrate through it, which allows wounds to heal quicker. When we added an antigen in the hydrogel trying to allow the hydrogel to degrade slower, it actually degraded more rapidly, but we found that new hair follicles formed in the center of these wounds, a hallmark of skin regeneration. My lab has been studying why this occurs and trying to use this to our advantage in other models.”
In an unpublished mouse burn wound model study, he and his colleagues excised a wound, but it never healed with regeneration in the center. “We don’t understand why,” he said. But when the researchers used the MAP gel in wounds of hairless mice, they observed the formation of sebaceous glands and hair follicles over the wound beds. “It’s an exciting finding to see hair follicles develop in the center of a wound,” Dr. Scumpia said. He noted that to date, use of the MAP hydrogel has demonstrated tissue regeneration in some of the 27 veterinary cases that have been performed, including for wounds following traumatic injuries or following tumor resections on paws that allowed the pets to avoid amputation.
Clinical trials planned
The first clinical trials of the MAP hydrogel are planned for treating complex diabetic wounds in early 2024 but will likely expand to other difficult-to-treat wounds, including venous stasis ulcers, decubitus ulcers, and use following large surgical resections. Dr. Scumpia and colleagues will also examine the regenerative biomaterial for tissue aesthetics, including dermal and deep tissue filler applications. The next steps in his laboratory, he said, are to combine biomaterials with stem cells, immune factors, or small molecular activators/inhibitors to improve sweat gland, nerve, or hair follicle regeneration.
Dr. Scumpia disclosed that he is a cofounder and shareholder in Tempo Therapeutics. He has also received grant support from the National Institutes of Health, Department of Veteran Affairs, and the LEO Foundation.
CARLSBAD, CALIF. – For the estimated 10 million wounds that clinicians treat in the United States each year resulting from surgical procedures, trauma, burns, and other causes, the best outcome is a scar, a fibrotic dermis with a flattened epidermis that contains no sweat glands, no pilosebaceous units, and impaired nerve function.
But what if the outcome was skin regeneration instead of scar formation? At the annual symposium of the California Society of Dermatology & Dermatologic Surgery, Philip O. Scumpia, MD, PhD, described the .
“We’re preprogrammed to undergo scarring,” said Dr. Scumpia, associate professor of dermatology at the University of California, Los Angeles. “Tissue fibrosis is an evolutionary process” where a fibrotic matrix is deposited “as quickly as possible to close the gap caused by an injury,” he noted. “All of the cues in the normal wound healing process result in fibrosis, but we want to move from scarring to tissue regeneration. The goal is to make something that can shift from this evolutionary process, and it’s proven to be inherently difficult.”
Common approaches to wound treatment include simple and advanced dressings, negative pressure, and hyperbaric oxygen. For wounds that persist beyond 30 days, advanced treatment options include decellularized grafts such as placental membranes, amniotic membranes, and acellular dermal matrices. “There are also cellularized grafts such as dressings that contain neonatal dermal fibroblasts,” which are expensive, said Dr. Scumpia, director of dermatopathology at the West Los Angeles VA Medical Center. “There are also semi-synthetic grafts such as single or double layer dermal replacement templates and synthetic dermal substitutes in the form of sheets or foam. All of these can help with wound coverage and help chronic wounds close on their own.”
Meanwhile, tissue regeneration – or efforts to restore tissue to its original functionality – include growth factors, stem cells, or replacement extracellular matrix (skin substitutes), or a combination. “Bioengineered dressings and bioengineered skin substitutes have shown modest improvement in wound healing but not tissue regeneration,” Dr. Scumpia said. “At best, we can accelerate scar formation and close the wound quicker, but nothing has been shown to regenerate tissue.”
Approaches to skin regeneration
Studies from the embryology literature have helped researchers develop better approaches to skin regeneration. For example, fetal skin heals without scarring when injured. “Hairs form from placodes, then sebaceous glands form, and fibroblasts that are part of the papillary mesenchymal body expressing special factors such as engrailed or CRABP1 drive hair follicle formation,” he said. “Many studies have shown that sonic hedgehog signaling, and Wnt/beta-catenin signaling can play a role in the development of new hair follicles. Also, fibroblasts in the dermis can drive hair follicle formation.”
Researchers are also learning about tissue regeneration from mouse models. For example, African spiny mice have been shown to heal regeneratively. “If you make wounds large enough on lab mice, the center heals regeneratively,” Dr. Scumpia said. “What’s interesting is that these same signals are present in embryonic hair follicle development. Why is this important? Who wants a hairy scar? It’s an organized structure that develops in the wound. That can help us understand what we need to put in so that our body regenerates on its own. In mouse models, the immune system has been shown to play a role in regeneration.”
Expanding on initial work conducted at UCLA, Dr. Scumpia and his colleagues founded San Diego-based Tempo Therapeutics, which is commercializing the MAP hydrogel to mimic the natural porosity and stiffness of skin. They sought to develop a new biomaterial, he said, noting that “the skin is porous on a microscale level, allowing cells to infiltrate different areas.” And the problem with existing biomaterials “is that they don’t incorporate into the skin very well,” he explained. “They’re usually stiff and rubbery and can cause a foreign body reaction, which can result in fibrous encapsulation and inflammation.”
The MAP hydrogel is composed of randomly packed “microsphere building blocks,” including an amino acid that promotes an immune response. When injected into a wound, the hydrogel forms a porous matrix in the tissue. Surface annealing locks in porosity and tissue grows into porous spaces, which avoids scar formation pathways and enables critical organs to regain function.
During in vivo tests, researchers observed decreases in inflammation compared with traditional hydrogels in the first 48 hours. “In mouse models, we found that if you inject in a hydrogel that has no porosity, the body tries to spit it out, and you have an immune reaction,” Dr. Scumpia said. “But when we used the MAP hydrogel, we found that cells can migrate through it, which allows wounds to heal quicker. When we added an antigen in the hydrogel trying to allow the hydrogel to degrade slower, it actually degraded more rapidly, but we found that new hair follicles formed in the center of these wounds, a hallmark of skin regeneration. My lab has been studying why this occurs and trying to use this to our advantage in other models.”
In an unpublished mouse burn wound model study, he and his colleagues excised a wound, but it never healed with regeneration in the center. “We don’t understand why,” he said. But when the researchers used the MAP gel in wounds of hairless mice, they observed the formation of sebaceous glands and hair follicles over the wound beds. “It’s an exciting finding to see hair follicles develop in the center of a wound,” Dr. Scumpia said. He noted that to date, use of the MAP hydrogel has demonstrated tissue regeneration in some of the 27 veterinary cases that have been performed, including for wounds following traumatic injuries or following tumor resections on paws that allowed the pets to avoid amputation.
Clinical trials planned
The first clinical trials of the MAP hydrogel are planned for treating complex diabetic wounds in early 2024 but will likely expand to other difficult-to-treat wounds, including venous stasis ulcers, decubitus ulcers, and use following large surgical resections. Dr. Scumpia and colleagues will also examine the regenerative biomaterial for tissue aesthetics, including dermal and deep tissue filler applications. The next steps in his laboratory, he said, are to combine biomaterials with stem cells, immune factors, or small molecular activators/inhibitors to improve sweat gland, nerve, or hair follicle regeneration.
Dr. Scumpia disclosed that he is a cofounder and shareholder in Tempo Therapeutics. He has also received grant support from the National Institutes of Health, Department of Veteran Affairs, and the LEO Foundation.
AT CALDERM 2023
Multicenter study aims to find new treatments for hidradenitis suppurativa
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
When Haley Naik, MD, joined the University of California, San Francisco, as a dermatologist in 2015, she was struck by the dearth of data in the medical literature about hidradenitis suppurativa (HS).
“For decades there were no datasets to begin to understand HS – its clinical course, how patients respond to medications, and how quality of life improves for patients with therapy,” Dr. Naik, who directs the HS program at UCSF, said in an interview. Inspired to improve the bleak HS knowledge landscape, she began to systematically collect information from HS patient visits, “to try to better understand how treatments were helping them or not and also to better understand their quality-of-life impact,” she said. “This also facilitated research in HS, but over time it became clear that there was a growing need for a larger effort.”
But in 2020, Dr. Naik teamed up with investigative dermatologist Michelle Lowes, MBBS, PhD, to . To date, more than 500 patients are enrolled at 12 participating sites, and 4 more sites plan to join the consortium by the end of 2023. The goal is to enroll a total of 8,000 patients, which will make it the largest dataset of its kind.
“Each site investigator is a physician who specializes in taking care of HS patients,” said Dr. Naik, who is the study’s principal investigator. “These are people who are conducting active research in various aspects of HS, and they’re trusted members of the medical community.”
She highlighted the three main objectives of HS PROGRESS. The first objective is to develop a longitudinal cohort of HS patients so that investigators can understand the clinical course of HS and effectiveness of treatments. The second is to collect biospecimens from patients with HS for translational studies “that can help to drive drug development, help us identify biomarkers that can help us predict disease course and predict patient response to therapies, so we know exactly what to give them,” she explained. The third objective is to provide patients with HS with the opportunity to be recruited for clinical trials, “so they have access to cutting-edge therapies and know what’s happening in this space.”
Collecting biospecimens
The goal of collecting biospecimens is to provide them to multiple investigators to improve the understanding of HS biology and treatment. “Our thought is to apply next generation techniques to these biospecimens to get metagenomic, transcriptomic, and genomic data to better understand HS biology so that we can identify targets for novel therapy,” Dr. Naik said.
Although HS is estimated to affect 1% of Western populations, the tumor necrosis alpha (TNF)-inhibitor adalimumab remains the only Food and Drug Administration-approved therapy for the condition.
However, Dr. Naik said that there are many promising drugs on the horizon for HS, especially interleukin (IL)-17 inhibitors. “One of the most exciting things about these drugs is that they set the bar higher for what we can expect out of therapies for HS, such as reporting a HiSCR (HS Clinical Response) score 75, which is the equivalent of 75% improvement in inflammatory HS lesions without an increase in draining tunnels,” she said. “This is well beyond what adalimumab had demonstrated in landmark trials in 2015. The safety profile on IL-17 inhibitors looks great, too.”
JAK inhibitors also hold promise for HS. “It’s going to be key to see how these drugs perform in the real-world setting in our average HS patients who may have comorbidities,” Dr. Naik said. “This is where an effort like HS PROGRESS will carry weight, because in a dataset like this, we’re going to be able to ask questions like, is there a class of drugs that works better for one specific phenotype of HS, or for patients who have a younger age of onset, or who are earlier in their disease course? These are questions we can’t ask in the context of a clinical trial, but we can ask in the context of real-world data from many practices.”
In addition to USCF, the 11 study locations participating in HS PROGRESS are the University of North Carolina at Chapel Hill; Mayo Clinic; Penn State University, Hershey; University of Virginia, Charlottesville; Washington University in St. Louis; University of Southern California, Los Angeles; Henry Ford Health, Detroit; University of Minnesota; University of Pennsylvania, Philadelphia; Duke University, Durham, N.C.; and University of Miami.
Dr. Naik disclosed that she has received grant support from AbbVie; consulting fees from 23andme, AbbVie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, UCB, and Novartis; and investigator fees from Pfizer; and holds shares in Radera. She is also an associate editor for JAMA Dermatology and a board member of the Hidradenitis Suppurativa Foundation.
Systematic review spotlights the use of nutraceuticals for acne
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
.
“While many topical and systemic prescription options are available for the treatment of acne, some patients may be interested in natural and complementary therapies as either an adjunctive or an alternative to prescription medications,” researchers led by John S. Barbieri, MD, MBA, of the department of dermatology at Brigham and Women’s Hospital, Boston, wrote in their study, which was published online in JAMA Dermatology. The researchers defined nutraceuticals as products derived from food sources that provide both nutritional and medicinal benefits, such as vitamins, dietary supplements, and herbal products. “Although patients may be interested in nutraceuticals as a potential treatment option for acne, there is uncertainty regarding the efficacy and safety of these products,” they wrote.
For the systematic review, they searched the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases from inception through January 30, 2023, to identify randomized clinical trials that evaluated oral nutraceutical interventions such as vitamins and minerals, botanical extracts, prebiotics, and probiotics in individuals with acne. They extracted clinician-reported outcomes, patient-reported outcomes, and adverse events from the included studies, and used the Cochrane Risk of Bias checklist tool to assess the quality of evidence in randomized clinical trials. Based on this tool, they used Agency for Healthcare Research and Quality standards to categorize the articles as good, fair, or poor quality.
The search yielded 42 unique studies with 3,346 participants. Of these 42 studies, 27 were considered poor quality, 11 were considered fair quality, and 4 were considered good quality. The good-quality studies separately evaluated four interventions: vitamin D, green tea extract, probiotics, and cheongsangbangpoong-tang, an herbal formula approved for use in acne by the Korea Food and Drug Administration.
The 11 fair-quality studies suggested potential effectiveness for pantothenic acid (vitamin B5), the fatty acids omega-3 (eicosapentaenoic acid [EPA] and/or docosahexaenoic acid [DHA]) and omega-6 (gamma-linoleic acid), and probiotics.
Zinc was the most studied nutraceutical identified in the review, but “there was substantial heterogeneity in the results, with only slightly greater than one-half of studies finding zinc to be efficacious,” the authors noted. “Studies using higher doses more often found zinc to be efficacious,” they said, adding that zinc “had the highest rate of adverse effect reporting of any nutraceuticals assessed in this review.”
Dr. Barbieri and colleagues acknowledged limitations of their analysis, including the fact that few of the nutraceuticals considered to have good or fair evidence for their use were evaluated in more than one study. “In addition, some studies had inconsistent results depending on the outcome measure assessed,” they wrote. “For instance, although green tea extract led to statistically significant improvements in lesion counts, it did not result in statistically significant improvements in quality of life, suggesting the observed lesion count differences may not be clinically meaningful to patients.”
And while probiotics had the most studies supporting their efficacy, they were generally of very small sample size.
Asked to comment on the study, Jonette Keri, MD, PhD, a dermatologist who directs the Acne and Rosacea Treatment Center at the University of Miami, who was not involved with the study, said that while the review was exhaustive, more research is needed to better determine the efficacy and side effects of the products studied. “The real strength of this wonderful review is now we have all of this information in one place, and this will serve as a great patient care resource,” she told this news organization.
Dr. Barbieri reported personal fees from Dexcel Pharma for consulting outside the submitted work. Dr. Keri disclosed that she is a consultant for L’Oréal.
FROM JAMA DERMATOLOGY