User login
Can online mindfulness and self-compassion training improve quality of life for patients with atopic dermatitis?
, according to results of a small randomized controlled trial in Japan.
“We found that skin disease–specific QOL improved over time with a large effect size,” lead study author Sanae Kishimoto, MHS, MPH, of the School of Public Health, Graduate School of Medicine at Kyoto University and colleagues write in JAMA Dermatology. “These findings suggest that mindfulness and self-compassion training is an effective treatment option for adults with AD.”
A bothersome disease that worsens quality of life
AD, a chronic, relapsing, inflammatory, multifactorial skin disease involving intense itching, affects an estimated 15%-30% of children and 2%-10% of adults, with the incidence increasing in industrialized countries, the authors state.
Measured by disability-adjusted life years, AD has the highest disease burden among skin diseases, and people with AD commonly have anxiety, depression, and sleep problems. Treatments include medications, other skin care, and lifestyle changes. New biologics appear to be effective but are expensive and need to be studied for their long-term safety, the authors add.
“Stress can make the skin worse, but at the same time the skin disease and symptoms cause stress,” Peter A. Lio, MD, who was not involved in the study, told this news organization by email. “This vicious cycle contributes greatly to impairing quality of life.”
A program focused on wise, kind self-care
In the SMiLE study, the authors recruited adults with moderate to severe AD and Dermatology Life Quality Index (DLQI) score above 6 from dermatology clinics and through online announcements over 1 year beginning in July 2019.
Participants averaged 36.3 years of age, 80% were women, and their mean AD duration was 26.6 years. Everyone was allowed to receive usual care during the study, except for dupilumab (a newly marketed drug when the study started), psychotherapy, or other mindfulness training.
The researchers randomly assigned 56 adults to receive mindfulness training in addition to their usual care and 51 to the wait list plus usual care. Those in the training group received eight weekly 90-minute online mindfulness and self-compassion sessions. Each group-based session was conducted at the same time and day of the week and included meditation, informal psychoeducation, inquiry, and a short lecture, along with an optional 1-day silent meditation retreat at week 7 and an optional 2-hour videoconferencing booster session at week 13.
The intervention encouraged a nonjudgmental relationship with stress using mindfulness-based stress reduction (MBSR) and emphasized a compassionate relationship with oneself during suffering using mindful self-compassion (MSC). The program was developed and taught by lead author Dr. Kishimoto, a Japanese licensed clinical psychologist who has a history of AD, the paper notes.
At 13 weeks, after completing electronic assessments, patients in the training group showed greater improvement in the DLQI score than those on the wait list (between-group difference estimate, –6.34; 95% confidence interval, –8.27 to –4.41; P < .001). The standardized effect size (Cohen’s d) at 13 weeks was –1.06 (95% CI, –1.39 to –0.74).
Patients in the training group also improved more in all secondary outcomes: severity, itch- and scratch-related visual analog scales, self-compassion, mindfulness, psychological symptoms, and adherence to dermatologist-advised treatments.
They were also more likely to follow their dermatologist’s medical treatment plans, including moisturizer and topical steroid use.
One serious adverse event, endometrial cancer in one patient, was judged to be unrelated to the intervention.
Online format may give more patients access to treatment
“With relatively limited data in the literature, this particularly well-done, important study is likely to positively shape thinking around this topic,” said Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago. “This study nicely demonstrates that an online approach can be effective.
“In theory, these methods or techniques could democratize treatments like this, and open them up to many more patients,” he added. He would like to see partially or entirely automated apps (free of cost), similar to meditation “apps,” to treat patients more cost-effectively.
Dr. Lio explained that excluding participants on dupilumab (Dupixent) makes the results slightly less generalizable to patients with moderate to severe AD, who may have the most serious QOL challenges and who are often candidates for dupilumab.
“However, given that we almost never have all the known variables for a study, we are generally comfortable extrapolating that the intervention would likely be helpful for patients taking dupilumab as well, despite it not being specifically evaluated in that group,” he said.
Susan Massick, MD, of the department of dermatology at the Ohio State University Wexner Medical Center in Columbus, advises clinicians to take a multipronged approach to treating the physical and behavioral components of AD and to embrace therapies beyond prescription medications.
“Self-compassion training is another tool in our toolbox toward finding the right fix for our patients,” Dr. Massick said by email. She was not involved with this research.
“I applaud the focus of this study on behavioral health training as a means toward wellness and improved mindfulness,” she added. “I was impressed by the extent to which these simple measures helped improve the quality of life for patients who used the training.”
U.S. patients can benefit from these findings
“My sense is that AD patients the world over have many similar characteristics and concerns, so I would anticipate that the results would be comparable in a U.S. population,” Dr. Lio said. “Other studies performed in the U.S. also support this line of thinking.”
Although the study involved highly motivated patients in Japan, the suffering that patients with AD experience is universal regardless of race or ethnicity, Dr. Massick said. “Americans may be even more willing to embrace mindfulness and self-compassion training as a path toward better health and wellness.”
The study was funded by the Japan Agency for Medical Research and Development and the Mental Health Okamoto Memorial Foundation, the KDDI Foundation, the Pfizer Health Research Foundation, and the Japan Society for the Promotion of Science.
Dr. Kishimoto and several coauthors report relevant financial relationships with pharmaceutical companies. Dr. Lio reports financial relationships with Sanofi and Regeneron, the joint developers of dupilumab. Dr. Massick reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results of a small randomized controlled trial in Japan.
“We found that skin disease–specific QOL improved over time with a large effect size,” lead study author Sanae Kishimoto, MHS, MPH, of the School of Public Health, Graduate School of Medicine at Kyoto University and colleagues write in JAMA Dermatology. “These findings suggest that mindfulness and self-compassion training is an effective treatment option for adults with AD.”
A bothersome disease that worsens quality of life
AD, a chronic, relapsing, inflammatory, multifactorial skin disease involving intense itching, affects an estimated 15%-30% of children and 2%-10% of adults, with the incidence increasing in industrialized countries, the authors state.
Measured by disability-adjusted life years, AD has the highest disease burden among skin diseases, and people with AD commonly have anxiety, depression, and sleep problems. Treatments include medications, other skin care, and lifestyle changes. New biologics appear to be effective but are expensive and need to be studied for their long-term safety, the authors add.
“Stress can make the skin worse, but at the same time the skin disease and symptoms cause stress,” Peter A. Lio, MD, who was not involved in the study, told this news organization by email. “This vicious cycle contributes greatly to impairing quality of life.”
A program focused on wise, kind self-care
In the SMiLE study, the authors recruited adults with moderate to severe AD and Dermatology Life Quality Index (DLQI) score above 6 from dermatology clinics and through online announcements over 1 year beginning in July 2019.
Participants averaged 36.3 years of age, 80% were women, and their mean AD duration was 26.6 years. Everyone was allowed to receive usual care during the study, except for dupilumab (a newly marketed drug when the study started), psychotherapy, or other mindfulness training.
The researchers randomly assigned 56 adults to receive mindfulness training in addition to their usual care and 51 to the wait list plus usual care. Those in the training group received eight weekly 90-minute online mindfulness and self-compassion sessions. Each group-based session was conducted at the same time and day of the week and included meditation, informal psychoeducation, inquiry, and a short lecture, along with an optional 1-day silent meditation retreat at week 7 and an optional 2-hour videoconferencing booster session at week 13.
The intervention encouraged a nonjudgmental relationship with stress using mindfulness-based stress reduction (MBSR) and emphasized a compassionate relationship with oneself during suffering using mindful self-compassion (MSC). The program was developed and taught by lead author Dr. Kishimoto, a Japanese licensed clinical psychologist who has a history of AD, the paper notes.
At 13 weeks, after completing electronic assessments, patients in the training group showed greater improvement in the DLQI score than those on the wait list (between-group difference estimate, –6.34; 95% confidence interval, –8.27 to –4.41; P < .001). The standardized effect size (Cohen’s d) at 13 weeks was –1.06 (95% CI, –1.39 to –0.74).
Patients in the training group also improved more in all secondary outcomes: severity, itch- and scratch-related visual analog scales, self-compassion, mindfulness, psychological symptoms, and adherence to dermatologist-advised treatments.
They were also more likely to follow their dermatologist’s medical treatment plans, including moisturizer and topical steroid use.
One serious adverse event, endometrial cancer in one patient, was judged to be unrelated to the intervention.
Online format may give more patients access to treatment
“With relatively limited data in the literature, this particularly well-done, important study is likely to positively shape thinking around this topic,” said Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago. “This study nicely demonstrates that an online approach can be effective.
“In theory, these methods or techniques could democratize treatments like this, and open them up to many more patients,” he added. He would like to see partially or entirely automated apps (free of cost), similar to meditation “apps,” to treat patients more cost-effectively.
Dr. Lio explained that excluding participants on dupilumab (Dupixent) makes the results slightly less generalizable to patients with moderate to severe AD, who may have the most serious QOL challenges and who are often candidates for dupilumab.
“However, given that we almost never have all the known variables for a study, we are generally comfortable extrapolating that the intervention would likely be helpful for patients taking dupilumab as well, despite it not being specifically evaluated in that group,” he said.
Susan Massick, MD, of the department of dermatology at the Ohio State University Wexner Medical Center in Columbus, advises clinicians to take a multipronged approach to treating the physical and behavioral components of AD and to embrace therapies beyond prescription medications.
“Self-compassion training is another tool in our toolbox toward finding the right fix for our patients,” Dr. Massick said by email. She was not involved with this research.
“I applaud the focus of this study on behavioral health training as a means toward wellness and improved mindfulness,” she added. “I was impressed by the extent to which these simple measures helped improve the quality of life for patients who used the training.”
U.S. patients can benefit from these findings
“My sense is that AD patients the world over have many similar characteristics and concerns, so I would anticipate that the results would be comparable in a U.S. population,” Dr. Lio said. “Other studies performed in the U.S. also support this line of thinking.”
Although the study involved highly motivated patients in Japan, the suffering that patients with AD experience is universal regardless of race or ethnicity, Dr. Massick said. “Americans may be even more willing to embrace mindfulness and self-compassion training as a path toward better health and wellness.”
The study was funded by the Japan Agency for Medical Research and Development and the Mental Health Okamoto Memorial Foundation, the KDDI Foundation, the Pfizer Health Research Foundation, and the Japan Society for the Promotion of Science.
Dr. Kishimoto and several coauthors report relevant financial relationships with pharmaceutical companies. Dr. Lio reports financial relationships with Sanofi and Regeneron, the joint developers of dupilumab. Dr. Massick reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results of a small randomized controlled trial in Japan.
“We found that skin disease–specific QOL improved over time with a large effect size,” lead study author Sanae Kishimoto, MHS, MPH, of the School of Public Health, Graduate School of Medicine at Kyoto University and colleagues write in JAMA Dermatology. “These findings suggest that mindfulness and self-compassion training is an effective treatment option for adults with AD.”
A bothersome disease that worsens quality of life
AD, a chronic, relapsing, inflammatory, multifactorial skin disease involving intense itching, affects an estimated 15%-30% of children and 2%-10% of adults, with the incidence increasing in industrialized countries, the authors state.
Measured by disability-adjusted life years, AD has the highest disease burden among skin diseases, and people with AD commonly have anxiety, depression, and sleep problems. Treatments include medications, other skin care, and lifestyle changes. New biologics appear to be effective but are expensive and need to be studied for their long-term safety, the authors add.
“Stress can make the skin worse, but at the same time the skin disease and symptoms cause stress,” Peter A. Lio, MD, who was not involved in the study, told this news organization by email. “This vicious cycle contributes greatly to impairing quality of life.”
A program focused on wise, kind self-care
In the SMiLE study, the authors recruited adults with moderate to severe AD and Dermatology Life Quality Index (DLQI) score above 6 from dermatology clinics and through online announcements over 1 year beginning in July 2019.
Participants averaged 36.3 years of age, 80% were women, and their mean AD duration was 26.6 years. Everyone was allowed to receive usual care during the study, except for dupilumab (a newly marketed drug when the study started), psychotherapy, or other mindfulness training.
The researchers randomly assigned 56 adults to receive mindfulness training in addition to their usual care and 51 to the wait list plus usual care. Those in the training group received eight weekly 90-minute online mindfulness and self-compassion sessions. Each group-based session was conducted at the same time and day of the week and included meditation, informal psychoeducation, inquiry, and a short lecture, along with an optional 1-day silent meditation retreat at week 7 and an optional 2-hour videoconferencing booster session at week 13.
The intervention encouraged a nonjudgmental relationship with stress using mindfulness-based stress reduction (MBSR) and emphasized a compassionate relationship with oneself during suffering using mindful self-compassion (MSC). The program was developed and taught by lead author Dr. Kishimoto, a Japanese licensed clinical psychologist who has a history of AD, the paper notes.
At 13 weeks, after completing electronic assessments, patients in the training group showed greater improvement in the DLQI score than those on the wait list (between-group difference estimate, –6.34; 95% confidence interval, –8.27 to –4.41; P < .001). The standardized effect size (Cohen’s d) at 13 weeks was –1.06 (95% CI, –1.39 to –0.74).
Patients in the training group also improved more in all secondary outcomes: severity, itch- and scratch-related visual analog scales, self-compassion, mindfulness, psychological symptoms, and adherence to dermatologist-advised treatments.
They were also more likely to follow their dermatologist’s medical treatment plans, including moisturizer and topical steroid use.
One serious adverse event, endometrial cancer in one patient, was judged to be unrelated to the intervention.
Online format may give more patients access to treatment
“With relatively limited data in the literature, this particularly well-done, important study is likely to positively shape thinking around this topic,” said Dr. Lio, of the departments of dermatology and pediatrics at Northwestern University, Chicago. “This study nicely demonstrates that an online approach can be effective.
“In theory, these methods or techniques could democratize treatments like this, and open them up to many more patients,” he added. He would like to see partially or entirely automated apps (free of cost), similar to meditation “apps,” to treat patients more cost-effectively.
Dr. Lio explained that excluding participants on dupilumab (Dupixent) makes the results slightly less generalizable to patients with moderate to severe AD, who may have the most serious QOL challenges and who are often candidates for dupilumab.
“However, given that we almost never have all the known variables for a study, we are generally comfortable extrapolating that the intervention would likely be helpful for patients taking dupilumab as well, despite it not being specifically evaluated in that group,” he said.
Susan Massick, MD, of the department of dermatology at the Ohio State University Wexner Medical Center in Columbus, advises clinicians to take a multipronged approach to treating the physical and behavioral components of AD and to embrace therapies beyond prescription medications.
“Self-compassion training is another tool in our toolbox toward finding the right fix for our patients,” Dr. Massick said by email. She was not involved with this research.
“I applaud the focus of this study on behavioral health training as a means toward wellness and improved mindfulness,” she added. “I was impressed by the extent to which these simple measures helped improve the quality of life for patients who used the training.”
U.S. patients can benefit from these findings
“My sense is that AD patients the world over have many similar characteristics and concerns, so I would anticipate that the results would be comparable in a U.S. population,” Dr. Lio said. “Other studies performed in the U.S. also support this line of thinking.”
Although the study involved highly motivated patients in Japan, the suffering that patients with AD experience is universal regardless of race or ethnicity, Dr. Massick said. “Americans may be even more willing to embrace mindfulness and self-compassion training as a path toward better health and wellness.”
The study was funded by the Japan Agency for Medical Research and Development and the Mental Health Okamoto Memorial Foundation, the KDDI Foundation, the Pfizer Health Research Foundation, and the Japan Society for the Promotion of Science.
Dr. Kishimoto and several coauthors report relevant financial relationships with pharmaceutical companies. Dr. Lio reports financial relationships with Sanofi and Regeneron, the joint developers of dupilumab. Dr. Massick reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Does Ozempic cause hair loss?
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves Yuflyma as ninth adalimumab biosimilar
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the biosimilar adalimumab-aaty (Yuflyma) in a citrate-free, high-concentration formulation, the manufacturer, Celltrion USA, announced today. It is the ninth biosimilar of adalimumab (Humira) to be approved in the United States.
Yuflyma is approved for the treatment of adult patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa. It is also approved for polyarticular juvenile idiopathic arthritis for patients aged 2 years or older, as well as for Crohn’s disease in adults and in pediatric patients aged 6 years or older.
The formulation was approved on the basis of a comprehensive data package of analytic, preclinical, and clinical studies, according to Celltrion USA, “demonstrating that Yuflyma is comparable to the reference product Humira in terms of efficacy, safety, pharmacokinetics, and immunogenicity up to 24 weeks and 1 year following treatment.”
The company conducted a double-blind, randomized phase 3 trial that compared switching from reference adalimumab to Yuflyma with continuing either reference adalimumab or Yuflyma for patients with active rheumatoid arthritis. In that trial, the efficacy, pharmacokinetics, safety, and immunogenicity of Yuflyma and reference adalimumab were comparable after 1 year of treatment, including after switching from reference adalimumab to Yuflyma.
“Currently, more than 80% of patients treated with Humira in the United States rely on a high-concentration and citrate-free formulation of this medication. The availability of a high-concentration and citrate-free formulation adalimumab biosimilar provides an important treatment option for patients with inflammatory diseases who benefit from this effective therapy,” said Jonathan Kay, MD, of the University of Massachusetts, Worcester, in the press release.
The citrate-free formulation is thought to lead to less pain on injection.
Yuflyma will be available in prefilled syringe and autoinjector administration options.
Celltrion USA plans to market the drug in the United States in July 2023. Following the initial launch of 40 mg/0.4 mL, the company plans to launch dose forms of 80 mg/0.8 mL and 20 mg/0.2 mL.
Celltrion USA is also seeking an interchangeability designation from the FDA following the completion of an interchangeability trial of 366 patients with chronic plaque psoriasis. The interchangeability designation would mean that patients successfully switched from Humira to Yuflyma multiple times in the trial. The interchangeability designation would allow pharmacists to autosubstitute Humira with Yuflyma. In these cases, individual state laws control how and whether physicians will be notified of this switch.
If interchangeability is approved for Yuflyma, which the company tentatively expects in the fourth quarter of 2024, it would be just the third interchangeable biosimilar approved by the FDA overall and the second adalimumab biosimilar to be designated as such, after adalimumab-adbm (Cyltezo) in October 2021.
Yuflyma was approved in Canada in December 2021 for 10 indications: rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, adult ulcerative colitis, hidradenitis suppurativa, plaque psoriasis, adult uveitis, and pediatric uveitis.
In February 2022, the European Commission granted marketing authorization for Yuflyma across those 10 indications, as well as for nonradiographic axial spondyloarthritis, pediatric plaque psoriasis, and pediatric Crohn’s disease.
In April 2022, Celltrion USA signed a licensing agreement with AbbVie, the manufacturer of Humira. Under that agreement, Celltrion will pay royalties to AbbVie on sales of their individual biosimilars, and AbbVie agreed to drop all patent litigation.
The full prescribing information for Yuflyma is available here.
A version of this article first appeared on Medscape.com.
FDA approves autoinjector pen for Humira biosimilar, Cyltezo
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
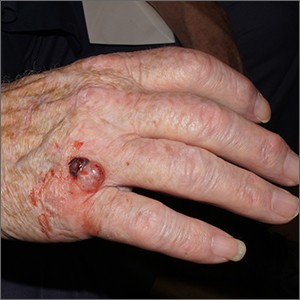
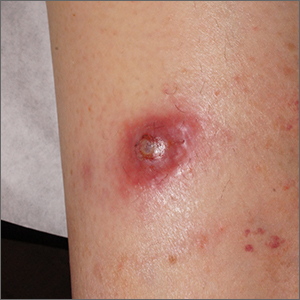
AD in infancy: Diagnostic advice and treatment tips
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
AT RAD 2023
Expunging ‘penicillin allergy’: Your questions answered
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Nodule on farmer’s hand
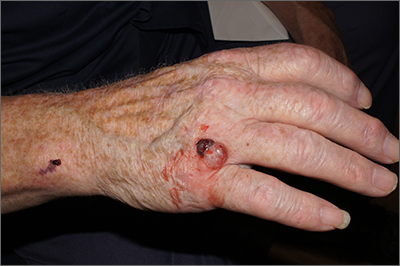
A broad shave biopsy was performed at the base of the lesion and the results were consistent with a thick nodular melanoma with a Breslow depth of 5.5 mm.
Melanoma is the deadliest skin cancer in the United States with mortality risk corresponding with the depth of the tumor.1 Nodular melanomas grow faster than all other types of melanoma. For this reason, a concerning raised lesion with a risk of melanoma should not be observed for change over time; it should be biopsied promptly. In this case, a depth of 5.5 mm was cause for quick action. Patients with tumors > 1 mm in depth (and some tumors > 0.8 mm) should be offered sentinel lymph node biopsy (SLNB) along with wide local excision to evaluate for lymphatic spread. Patients with thinner tumors may undergo wide local excision without SLNB.
In this case, National Comprehensive Cancer Network guidelines would dictate a 2-cm margin for a wide local incision; the patient underwent a modified version of this with Surgical Oncology to accommodate maintenance of hand function. This patient’s SLNB was negative, so the melanoma was classified as Stage IIC.
In the recent past, there were no additional treatments for patients with late Stage II disease (thick tumors without evidence of metastasis). However, in December 2021, the US Food and Drug Administration approved the use of immunotherapy with pembrolizumab in patients with node-negative late Stage II melanoma after demonstration of improved recurrence-free survival in the KEYNOTE trial.2 Evidence of improved long-term survival is mixed with adjuvant therapy, and studies evaluating the best role of adjuvant therapy are ongoing.
This patient was started on a regimen of pembrolizumab 200 mg IV every 3 weeks, which he will continue for as long as 1 year. He has tolerated this regimen without difficulty and has no evidence of disease.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Epstein DS, Lange JR, Gruber SB, et al. Is physician detection associated with thinner melanomas? JAMA. 1999;281:640-643. doi: 10.1001/jama.281.7.640
2. Luke JJ, Rutkowski P, Queirolo P, et al; KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718-1729. doi: 10.1016/S0140-6736(22)00562-1

A broad shave biopsy was performed at the base of the lesion and the results were consistent with a thick nodular melanoma with a Breslow depth of 5.5 mm.
Melanoma is the deadliest skin cancer in the United States with mortality risk corresponding with the depth of the tumor.1 Nodular melanomas grow faster than all other types of melanoma. For this reason, a concerning raised lesion with a risk of melanoma should not be observed for change over time; it should be biopsied promptly. In this case, a depth of 5.5 mm was cause for quick action. Patients with tumors > 1 mm in depth (and some tumors > 0.8 mm) should be offered sentinel lymph node biopsy (SLNB) along with wide local excision to evaluate for lymphatic spread. Patients with thinner tumors may undergo wide local excision without SLNB.
In this case, National Comprehensive Cancer Network guidelines would dictate a 2-cm margin for a wide local incision; the patient underwent a modified version of this with Surgical Oncology to accommodate maintenance of hand function. This patient’s SLNB was negative, so the melanoma was classified as Stage IIC.
In the recent past, there were no additional treatments for patients with late Stage II disease (thick tumors without evidence of metastasis). However, in December 2021, the US Food and Drug Administration approved the use of immunotherapy with pembrolizumab in patients with node-negative late Stage II melanoma after demonstration of improved recurrence-free survival in the KEYNOTE trial.2 Evidence of improved long-term survival is mixed with adjuvant therapy, and studies evaluating the best role of adjuvant therapy are ongoing.
This patient was started on a regimen of pembrolizumab 200 mg IV every 3 weeks, which he will continue for as long as 1 year. He has tolerated this regimen without difficulty and has no evidence of disease.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A broad shave biopsy was performed at the base of the lesion and the results were consistent with a thick nodular melanoma with a Breslow depth of 5.5 mm.
Melanoma is the deadliest skin cancer in the United States with mortality risk corresponding with the depth of the tumor.1 Nodular melanomas grow faster than all other types of melanoma. For this reason, a concerning raised lesion with a risk of melanoma should not be observed for change over time; it should be biopsied promptly. In this case, a depth of 5.5 mm was cause for quick action. Patients with tumors > 1 mm in depth (and some tumors > 0.8 mm) should be offered sentinel lymph node biopsy (SLNB) along with wide local excision to evaluate for lymphatic spread. Patients with thinner tumors may undergo wide local excision without SLNB.
In this case, National Comprehensive Cancer Network guidelines would dictate a 2-cm margin for a wide local incision; the patient underwent a modified version of this with Surgical Oncology to accommodate maintenance of hand function. This patient’s SLNB was negative, so the melanoma was classified as Stage IIC.
In the recent past, there were no additional treatments for patients with late Stage II disease (thick tumors without evidence of metastasis). However, in December 2021, the US Food and Drug Administration approved the use of immunotherapy with pembrolizumab in patients with node-negative late Stage II melanoma after demonstration of improved recurrence-free survival in the KEYNOTE trial.2 Evidence of improved long-term survival is mixed with adjuvant therapy, and studies evaluating the best role of adjuvant therapy are ongoing.
This patient was started on a regimen of pembrolizumab 200 mg IV every 3 weeks, which he will continue for as long as 1 year. He has tolerated this regimen without difficulty and has no evidence of disease.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Epstein DS, Lange JR, Gruber SB, et al. Is physician detection associated with thinner melanomas? JAMA. 1999;281:640-643. doi: 10.1001/jama.281.7.640
2. Luke JJ, Rutkowski P, Queirolo P, et al; KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718-1729. doi: 10.1016/S0140-6736(22)00562-1
1. Epstein DS, Lange JR, Gruber SB, et al. Is physician detection associated with thinner melanomas? JAMA. 1999;281:640-643. doi: 10.1001/jama.281.7.640
2. Luke JJ, Rutkowski P, Queirolo P, et al; KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718-1729. doi: 10.1016/S0140-6736(22)00562-1
Firm nodule following tick bite
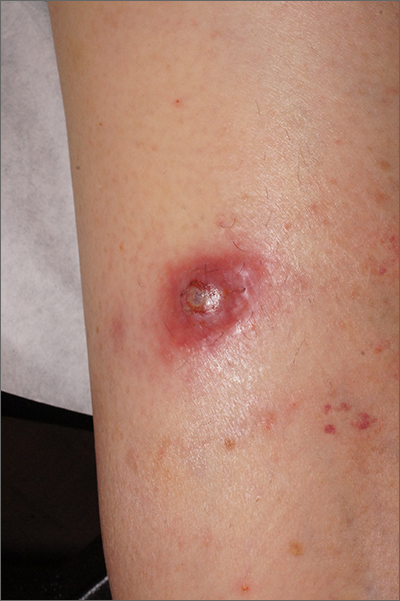
The biopsy revealed abundant lymphocytes, neutrophils, and eosinophils consistent with a diagnosis of cutaneous B cell pseudolymphoma. The pseudolymphoma was caused by an exaggerated response to a tick bite.
As the name implies, B cell pseudolymphomas clinically and histologically mimic the various patterns of cutaneous lymphoma, often appearing as a firm, pink to dark violet nodule. A biopsy is mandatory to distinguish between pseudolymphoma and true lymphoma. There is an association between pseudolymphomas and Borrelia burgdorferi, the organism responsible for Lyme disease. Thus, testing for Lyme disease is recommended for patients with pseudolymphomas who live in endemic areas. Patients who test positive should be treated with doxycycline 100 mg bid for 10 days.
In the absence of Lyme disease, a pseudolymphoma may resolve spontaneously over weeks or months. Resolution can be hastened with topical superpotent steroids, intralesional steroids, or systemic steroids. Treatment can begin with topical clobetasol 0.05% bid. If the lesion does not resolve, the next step would be intralesional triamcinolone 10 mg/mL injected directly into the nodule until it blanches slightly. Injections should be repeated every 3 to 4 weeks for a total of 2 to 3 injections.
The patient in this case had negative Lyme serology and was treated with 2 injections of triamcinolone 10 mg/mL administered 3 weeks apart. She experienced complete resolution.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi: 10.1016/j.path.2017.01.002

The biopsy revealed abundant lymphocytes, neutrophils, and eosinophils consistent with a diagnosis of cutaneous B cell pseudolymphoma. The pseudolymphoma was caused by an exaggerated response to a tick bite.
As the name implies, B cell pseudolymphomas clinically and histologically mimic the various patterns of cutaneous lymphoma, often appearing as a firm, pink to dark violet nodule. A biopsy is mandatory to distinguish between pseudolymphoma and true lymphoma. There is an association between pseudolymphomas and Borrelia burgdorferi, the organism responsible for Lyme disease. Thus, testing for Lyme disease is recommended for patients with pseudolymphomas who live in endemic areas. Patients who test positive should be treated with doxycycline 100 mg bid for 10 days.
In the absence of Lyme disease, a pseudolymphoma may resolve spontaneously over weeks or months. Resolution can be hastened with topical superpotent steroids, intralesional steroids, or systemic steroids. Treatment can begin with topical clobetasol 0.05% bid. If the lesion does not resolve, the next step would be intralesional triamcinolone 10 mg/mL injected directly into the nodule until it blanches slightly. Injections should be repeated every 3 to 4 weeks for a total of 2 to 3 injections.
The patient in this case had negative Lyme serology and was treated with 2 injections of triamcinolone 10 mg/mL administered 3 weeks apart. She experienced complete resolution.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The biopsy revealed abundant lymphocytes, neutrophils, and eosinophils consistent with a diagnosis of cutaneous B cell pseudolymphoma. The pseudolymphoma was caused by an exaggerated response to a tick bite.
As the name implies, B cell pseudolymphomas clinically and histologically mimic the various patterns of cutaneous lymphoma, often appearing as a firm, pink to dark violet nodule. A biopsy is mandatory to distinguish between pseudolymphoma and true lymphoma. There is an association between pseudolymphomas and Borrelia burgdorferi, the organism responsible for Lyme disease. Thus, testing for Lyme disease is recommended for patients with pseudolymphomas who live in endemic areas. Patients who test positive should be treated with doxycycline 100 mg bid for 10 days.
In the absence of Lyme disease, a pseudolymphoma may resolve spontaneously over weeks or months. Resolution can be hastened with topical superpotent steroids, intralesional steroids, or systemic steroids. Treatment can begin with topical clobetasol 0.05% bid. If the lesion does not resolve, the next step would be intralesional triamcinolone 10 mg/mL injected directly into the nodule until it blanches slightly. Injections should be repeated every 3 to 4 weeks for a total of 2 to 3 injections.
The patient in this case had negative Lyme serology and was treated with 2 injections of triamcinolone 10 mg/mL administered 3 weeks apart. She experienced complete resolution.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi: 10.1016/j.path.2017.01.002
1. Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi: 10.1016/j.path.2017.01.002
CDC: Drug-resistant ringworm reported in New York
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
Facial, hand, and foot dermatitis: Lebrikizumab and dupilumab show efficacy in new studies
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
AT RAD 2023