User login
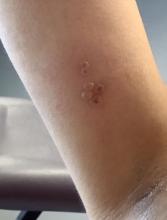
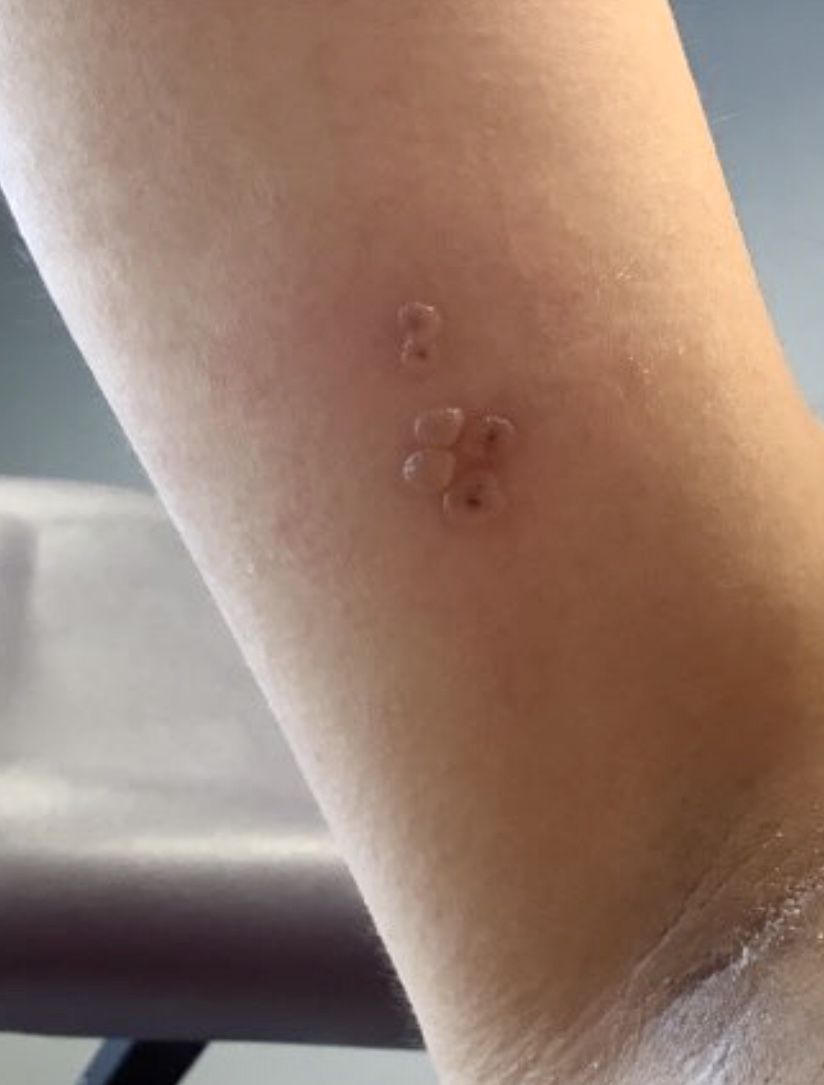
A healthy 36-year-old female presented with 4 days of itchy lesions on the right upper extremity
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
Cutaneous vasculitis curtails quality of life
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
FROM JAMA DERMATOLOGY
Can this tool forecast peanut allergies?
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PAS 2023
Two phase 3 trials show benefits of dupilumab for prurigo nodularis
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
FROM NATURE MEDICINE
Itchy pustules over hair follicles
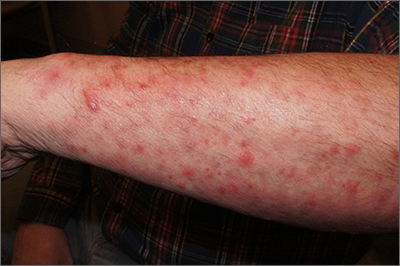
A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
Study shifts burden of IgG4-related disease to women
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.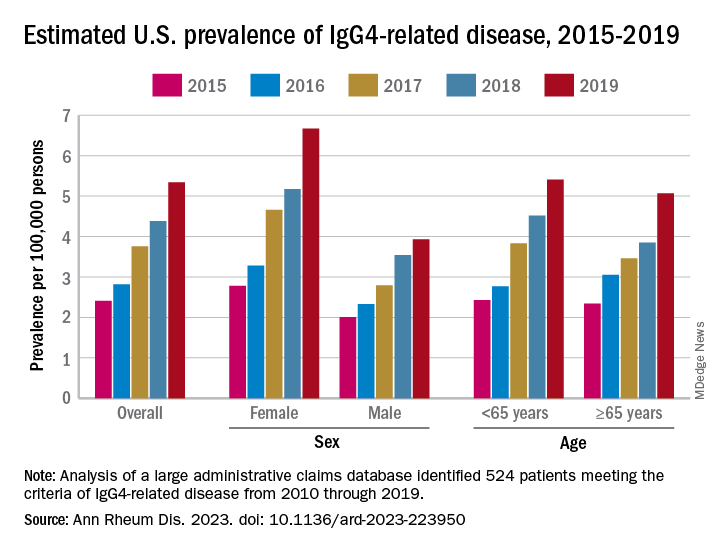
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
FROM ANNALS OF THE RHEUMATIC DISEASES
1,726-nm lasers poised to revolutionize acne treatment, expert predicts
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
AT ASLMS 2023
Contact allergens lurk in diabetes devices
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
FROM ACDS 2023
Beware the hidden allergens in nutritional supplements
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACDS 2023
Controlled hyperthermia: Novel treatment of BCCs without surgery continues to be refined
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
AT ASLMS 2023