User login
52-week data show lebrikizumab atopic dermatitis effects maintained
from the phase 3 ADvocate1 and ADvocate2 trials.
“We’re focused on the responders,” said Andrew Blauvelt, MD, MBA, as he presented the positive findings at the annual congress of the European Academy of Dermatology and Venereology.
Responders were the 291 people whose atopic dermatitis greatly improved after an initial 16 weeks’ treatment with lebrikizumab in both trials and who were then randomly allocated to receive injections every 2 weeks (Q2W, n = 113) or every 4 weeks (Q4W, n = 118), or to receive placebo injections Q2W (n = 60).
“Very interestingly, for me, the Q4W maintenance dosing was just as good as the Q2W maintenance dosing,” said Dr. Blauvelt, president of Oregon Medical Research Center, Portland.
“Another highlight of these data is that the patients who went on to placebo, about 50% of the patients maintained good responses, despite no treatment from week 16 to week 52,” he added.
Most patients did not require topical steroids, and “there were no surprises here” in terms of the safety profile. Lebrikizumab, a monoclonal antibody, binds to soluble interleukin-13 and blocks IL-13 signaling.
“So, the study really shows that specific targeting of IL-13 with lebrikizumab, either Q2W or Q4W, has high maintenance of efficacy and is reasonably tolerated and safe in adolescents and adults with atopic dermatitis,” Dr. Blauvelt concluded.
“We know now that IL-13 is a critical cytokine in AD [atopic dermatitis] pathogenesis. The unique features of this drug I want to highlight is that it has high binding affinity for IL-13,” he said.
“It has a slow dissociation off rate, meaning it binds IL-13 tightly, very potently, and stays blocking and stays hold of IL-13 in a strong manner,” he added. The drug has a half-life of 25 days.
These features could be very important for long-term dosing of the drug, he argued.
Lebrikizumab phase 3 trials
ADvocate1 and ADvocate2 are two of several phase 3 trials evaluating the efficacy and safety of lebrikizumab for the treatment of atopic dermatitis.
These include the completed ADhere study, in which lebrikizumab was used in combination with topical steroids and showed positive results in skin improvement and relief of pruritus.
The ADore study, an open-label trial in adolescents, is yet to report. The ongoing ADjoin study, a long-term extension study, is actively recruiting.
ADvocate1 and ADvocate2 are two identically designed – multicenter, randomized, double-blind, placebo-controlled, parallel-group – monotherapy trials that initially pitched two dosing regimens of lebrikizumab (250 mg) against placebo with a double loading dose at baseline and week 2 and then one dose every 2 weeks. The pair of trials enrolled a total of 869 adolescents and adults.
After the 16-week induction period, all patients in the lebrikizumab arm who had responded to treatment were rerandomly assigned to receive lebrikizumab 250 mg Q2W or Q4W, or placebo Q2W during a 36-week long-term maintenance treatment period.
This brought the total treatment time to 52 weeks for those whose atopic dermatitis had initially responded to lebrikizumab, explained Blauvelt.
Responders were those who, at 16 weeks, had an Investigator’s Global Assessment score of 0 or 1 (IGA 0/1) with a 2-point improvement or who had a 75% improvement in the Eczema Area and Severity Index score (EASI 75) without the need for rescue medication, compared with baseline values.
Induction and maintenance phase results
At the end of the 16-week induction period, a greater proportion of patients who had been treated with lebrikizumab than placebo met a primary outcome of IGA 0/1 in each trial (43.1% vs. 12.7% in ADvocate1 and 33.2% vs. 10.8% in ADvocate2).
A similar result was seen for another primary outcome, EASI 75 (58.8% vs. 16.2% and 52.1% vs. 18.1%) and for a secondary outcome, improvement in pruritus using a numerical rating scale (45.9% vs. 13.0% and 39.8% vs. 11.5%).
In the maintenance phase, with respect to responders, Dr. Blauvelt reported “very similar results” between the QW2 and Q4W maintenance dosing, “and still a quite high response in [half] the patients who were randomized to placebo at week 16.”
In the ADvocate1 and ADvocate2 trials, respectively, an IGA 0/1 with at least a 2-point improvement was maintained at week 52 in 75.8% and 64.6% of patients treated with the Q2W lebrikizumab dose, 74.2% and 80.6% of those treated with the Q4W dose, and 46.5% and 49.8% of those given placebo.
EASI 75 was maintained at week 52 in a respective 79.2% and 77.4% of patients treated with the Q2W dose, 79.2% and 84.7% with the Q4W dose, and 61.3% and 72.0% with placebo.
As for maintenance of at least a 4-point improvement in pruritus score, results at 52 weeks were 81.2% and 90.3% for the 2-week dose, 80.4% and 88.1% for the 4-week dose, and 65.4% and 67.6% for placebo.
Although topical corticosteroid treatment was allowed during the maintenance phase, only about 15% of patients needed this, Dr. Blauvelt said.
Different dosing results questioned
During the discussion period, one delegate highlighted that the twice-weekly maintenance dosing schedule seemed to “do worse a little bit” than the 4-week dosing, with both “close to placebo,” although “the long-term effect is already very impressive.”
Dr. Blauvelt noted that a pooled analysis had been done and that “it’s very clear that being on lebrikizumab works better than not being on lebrikizumab.
“Now, Q2W versus Q4W. We believe that this may be due to the long half-life of the drug possibly. It could be due to the slow disassociation rate, it’s binding tightly,” he suggested.
“We also could talk about disease modification, right. So, it opens up the concept of hit hard, hit early for 16 weeks, and then maybe you can modify disease over time,” Dr. Blauvelt said.
He added: “That’s highly speculative, of course.”
Short-term safety data
The 52-week safety profile of lebrikizumab is consistent with previously published data at 16 weeks, Dr. Blauvelt said. The most common adverse events during the studies included atopic dermatitis, nasopharyngitis, conjunctivitis, conjunctivitis allergic, headache, and COVID-19.
“This drug has comparable efficacy with dupilumab and tralokinumab,” said Jashin J. Wu, MD, from the Dermatology Research and Education Foundation in Irvine, Calif., in an interview. He was not involved in the study.
“As it does not have any significant advantages with less long-term safety data, I do not see a place for it in my practice,” Dr. Wu said.
Dupilumab (Dupixent) and tralokinumab (Adbry) are monoclonal antibodies that also block IL-13. Both are already licensed for treating atopic dermatitis. Dupilumab was approved by the Food and Drug Administration in 2017, and tralokinumab was approved in 2021.
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. Eli Lilly has exclusive rights for the development and commercialization of lebrikizumab in the United States and all countries outside Europe; European rights belong to Almirall for all dermatology indications, including atopic dermatitis. Dr. Blauvelt acts as an investigator and adviser to these companies as well as many other pharmaceutical companies that are involved in developing new dermatologic treatments. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
from the phase 3 ADvocate1 and ADvocate2 trials.
“We’re focused on the responders,” said Andrew Blauvelt, MD, MBA, as he presented the positive findings at the annual congress of the European Academy of Dermatology and Venereology.
Responders were the 291 people whose atopic dermatitis greatly improved after an initial 16 weeks’ treatment with lebrikizumab in both trials and who were then randomly allocated to receive injections every 2 weeks (Q2W, n = 113) or every 4 weeks (Q4W, n = 118), or to receive placebo injections Q2W (n = 60).
“Very interestingly, for me, the Q4W maintenance dosing was just as good as the Q2W maintenance dosing,” said Dr. Blauvelt, president of Oregon Medical Research Center, Portland.
“Another highlight of these data is that the patients who went on to placebo, about 50% of the patients maintained good responses, despite no treatment from week 16 to week 52,” he added.
Most patients did not require topical steroids, and “there were no surprises here” in terms of the safety profile. Lebrikizumab, a monoclonal antibody, binds to soluble interleukin-13 and blocks IL-13 signaling.
“So, the study really shows that specific targeting of IL-13 with lebrikizumab, either Q2W or Q4W, has high maintenance of efficacy and is reasonably tolerated and safe in adolescents and adults with atopic dermatitis,” Dr. Blauvelt concluded.
“We know now that IL-13 is a critical cytokine in AD [atopic dermatitis] pathogenesis. The unique features of this drug I want to highlight is that it has high binding affinity for IL-13,” he said.
“It has a slow dissociation off rate, meaning it binds IL-13 tightly, very potently, and stays blocking and stays hold of IL-13 in a strong manner,” he added. The drug has a half-life of 25 days.
These features could be very important for long-term dosing of the drug, he argued.
Lebrikizumab phase 3 trials
ADvocate1 and ADvocate2 are two of several phase 3 trials evaluating the efficacy and safety of lebrikizumab for the treatment of atopic dermatitis.
These include the completed ADhere study, in which lebrikizumab was used in combination with topical steroids and showed positive results in skin improvement and relief of pruritus.
The ADore study, an open-label trial in adolescents, is yet to report. The ongoing ADjoin study, a long-term extension study, is actively recruiting.
ADvocate1 and ADvocate2 are two identically designed – multicenter, randomized, double-blind, placebo-controlled, parallel-group – monotherapy trials that initially pitched two dosing regimens of lebrikizumab (250 mg) against placebo with a double loading dose at baseline and week 2 and then one dose every 2 weeks. The pair of trials enrolled a total of 869 adolescents and adults.
After the 16-week induction period, all patients in the lebrikizumab arm who had responded to treatment were rerandomly assigned to receive lebrikizumab 250 mg Q2W or Q4W, or placebo Q2W during a 36-week long-term maintenance treatment period.
This brought the total treatment time to 52 weeks for those whose atopic dermatitis had initially responded to lebrikizumab, explained Blauvelt.
Responders were those who, at 16 weeks, had an Investigator’s Global Assessment score of 0 or 1 (IGA 0/1) with a 2-point improvement or who had a 75% improvement in the Eczema Area and Severity Index score (EASI 75) without the need for rescue medication, compared with baseline values.
Induction and maintenance phase results
At the end of the 16-week induction period, a greater proportion of patients who had been treated with lebrikizumab than placebo met a primary outcome of IGA 0/1 in each trial (43.1% vs. 12.7% in ADvocate1 and 33.2% vs. 10.8% in ADvocate2).
A similar result was seen for another primary outcome, EASI 75 (58.8% vs. 16.2% and 52.1% vs. 18.1%) and for a secondary outcome, improvement in pruritus using a numerical rating scale (45.9% vs. 13.0% and 39.8% vs. 11.5%).
In the maintenance phase, with respect to responders, Dr. Blauvelt reported “very similar results” between the QW2 and Q4W maintenance dosing, “and still a quite high response in [half] the patients who were randomized to placebo at week 16.”
In the ADvocate1 and ADvocate2 trials, respectively, an IGA 0/1 with at least a 2-point improvement was maintained at week 52 in 75.8% and 64.6% of patients treated with the Q2W lebrikizumab dose, 74.2% and 80.6% of those treated with the Q4W dose, and 46.5% and 49.8% of those given placebo.
EASI 75 was maintained at week 52 in a respective 79.2% and 77.4% of patients treated with the Q2W dose, 79.2% and 84.7% with the Q4W dose, and 61.3% and 72.0% with placebo.
As for maintenance of at least a 4-point improvement in pruritus score, results at 52 weeks were 81.2% and 90.3% for the 2-week dose, 80.4% and 88.1% for the 4-week dose, and 65.4% and 67.6% for placebo.
Although topical corticosteroid treatment was allowed during the maintenance phase, only about 15% of patients needed this, Dr. Blauvelt said.
Different dosing results questioned
During the discussion period, one delegate highlighted that the twice-weekly maintenance dosing schedule seemed to “do worse a little bit” than the 4-week dosing, with both “close to placebo,” although “the long-term effect is already very impressive.”
Dr. Blauvelt noted that a pooled analysis had been done and that “it’s very clear that being on lebrikizumab works better than not being on lebrikizumab.
“Now, Q2W versus Q4W. We believe that this may be due to the long half-life of the drug possibly. It could be due to the slow disassociation rate, it’s binding tightly,” he suggested.
“We also could talk about disease modification, right. So, it opens up the concept of hit hard, hit early for 16 weeks, and then maybe you can modify disease over time,” Dr. Blauvelt said.
He added: “That’s highly speculative, of course.”
Short-term safety data
The 52-week safety profile of lebrikizumab is consistent with previously published data at 16 weeks, Dr. Blauvelt said. The most common adverse events during the studies included atopic dermatitis, nasopharyngitis, conjunctivitis, conjunctivitis allergic, headache, and COVID-19.
“This drug has comparable efficacy with dupilumab and tralokinumab,” said Jashin J. Wu, MD, from the Dermatology Research and Education Foundation in Irvine, Calif., in an interview. He was not involved in the study.
“As it does not have any significant advantages with less long-term safety data, I do not see a place for it in my practice,” Dr. Wu said.
Dupilumab (Dupixent) and tralokinumab (Adbry) are monoclonal antibodies that also block IL-13. Both are already licensed for treating atopic dermatitis. Dupilumab was approved by the Food and Drug Administration in 2017, and tralokinumab was approved in 2021.
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. Eli Lilly has exclusive rights for the development and commercialization of lebrikizumab in the United States and all countries outside Europe; European rights belong to Almirall for all dermatology indications, including atopic dermatitis. Dr. Blauvelt acts as an investigator and adviser to these companies as well as many other pharmaceutical companies that are involved in developing new dermatologic treatments. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
from the phase 3 ADvocate1 and ADvocate2 trials.
“We’re focused on the responders,” said Andrew Blauvelt, MD, MBA, as he presented the positive findings at the annual congress of the European Academy of Dermatology and Venereology.
Responders were the 291 people whose atopic dermatitis greatly improved after an initial 16 weeks’ treatment with lebrikizumab in both trials and who were then randomly allocated to receive injections every 2 weeks (Q2W, n = 113) or every 4 weeks (Q4W, n = 118), or to receive placebo injections Q2W (n = 60).
“Very interestingly, for me, the Q4W maintenance dosing was just as good as the Q2W maintenance dosing,” said Dr. Blauvelt, president of Oregon Medical Research Center, Portland.
“Another highlight of these data is that the patients who went on to placebo, about 50% of the patients maintained good responses, despite no treatment from week 16 to week 52,” he added.
Most patients did not require topical steroids, and “there were no surprises here” in terms of the safety profile. Lebrikizumab, a monoclonal antibody, binds to soluble interleukin-13 and blocks IL-13 signaling.
“So, the study really shows that specific targeting of IL-13 with lebrikizumab, either Q2W or Q4W, has high maintenance of efficacy and is reasonably tolerated and safe in adolescents and adults with atopic dermatitis,” Dr. Blauvelt concluded.
“We know now that IL-13 is a critical cytokine in AD [atopic dermatitis] pathogenesis. The unique features of this drug I want to highlight is that it has high binding affinity for IL-13,” he said.
“It has a slow dissociation off rate, meaning it binds IL-13 tightly, very potently, and stays blocking and stays hold of IL-13 in a strong manner,” he added. The drug has a half-life of 25 days.
These features could be very important for long-term dosing of the drug, he argued.
Lebrikizumab phase 3 trials
ADvocate1 and ADvocate2 are two of several phase 3 trials evaluating the efficacy and safety of lebrikizumab for the treatment of atopic dermatitis.
These include the completed ADhere study, in which lebrikizumab was used in combination with topical steroids and showed positive results in skin improvement and relief of pruritus.
The ADore study, an open-label trial in adolescents, is yet to report. The ongoing ADjoin study, a long-term extension study, is actively recruiting.
ADvocate1 and ADvocate2 are two identically designed – multicenter, randomized, double-blind, placebo-controlled, parallel-group – monotherapy trials that initially pitched two dosing regimens of lebrikizumab (250 mg) against placebo with a double loading dose at baseline and week 2 and then one dose every 2 weeks. The pair of trials enrolled a total of 869 adolescents and adults.
After the 16-week induction period, all patients in the lebrikizumab arm who had responded to treatment were rerandomly assigned to receive lebrikizumab 250 mg Q2W or Q4W, or placebo Q2W during a 36-week long-term maintenance treatment period.
This brought the total treatment time to 52 weeks for those whose atopic dermatitis had initially responded to lebrikizumab, explained Blauvelt.
Responders were those who, at 16 weeks, had an Investigator’s Global Assessment score of 0 or 1 (IGA 0/1) with a 2-point improvement or who had a 75% improvement in the Eczema Area and Severity Index score (EASI 75) without the need for rescue medication, compared with baseline values.
Induction and maintenance phase results
At the end of the 16-week induction period, a greater proportion of patients who had been treated with lebrikizumab than placebo met a primary outcome of IGA 0/1 in each trial (43.1% vs. 12.7% in ADvocate1 and 33.2% vs. 10.8% in ADvocate2).
A similar result was seen for another primary outcome, EASI 75 (58.8% vs. 16.2% and 52.1% vs. 18.1%) and for a secondary outcome, improvement in pruritus using a numerical rating scale (45.9% vs. 13.0% and 39.8% vs. 11.5%).
In the maintenance phase, with respect to responders, Dr. Blauvelt reported “very similar results” between the QW2 and Q4W maintenance dosing, “and still a quite high response in [half] the patients who were randomized to placebo at week 16.”
In the ADvocate1 and ADvocate2 trials, respectively, an IGA 0/1 with at least a 2-point improvement was maintained at week 52 in 75.8% and 64.6% of patients treated with the Q2W lebrikizumab dose, 74.2% and 80.6% of those treated with the Q4W dose, and 46.5% and 49.8% of those given placebo.
EASI 75 was maintained at week 52 in a respective 79.2% and 77.4% of patients treated with the Q2W dose, 79.2% and 84.7% with the Q4W dose, and 61.3% and 72.0% with placebo.
As for maintenance of at least a 4-point improvement in pruritus score, results at 52 weeks were 81.2% and 90.3% for the 2-week dose, 80.4% and 88.1% for the 4-week dose, and 65.4% and 67.6% for placebo.
Although topical corticosteroid treatment was allowed during the maintenance phase, only about 15% of patients needed this, Dr. Blauvelt said.
Different dosing results questioned
During the discussion period, one delegate highlighted that the twice-weekly maintenance dosing schedule seemed to “do worse a little bit” than the 4-week dosing, with both “close to placebo,” although “the long-term effect is already very impressive.”
Dr. Blauvelt noted that a pooled analysis had been done and that “it’s very clear that being on lebrikizumab works better than not being on lebrikizumab.
“Now, Q2W versus Q4W. We believe that this may be due to the long half-life of the drug possibly. It could be due to the slow disassociation rate, it’s binding tightly,” he suggested.
“We also could talk about disease modification, right. So, it opens up the concept of hit hard, hit early for 16 weeks, and then maybe you can modify disease over time,” Dr. Blauvelt said.
He added: “That’s highly speculative, of course.”
Short-term safety data
The 52-week safety profile of lebrikizumab is consistent with previously published data at 16 weeks, Dr. Blauvelt said. The most common adverse events during the studies included atopic dermatitis, nasopharyngitis, conjunctivitis, conjunctivitis allergic, headache, and COVID-19.
“This drug has comparable efficacy with dupilumab and tralokinumab,” said Jashin J. Wu, MD, from the Dermatology Research and Education Foundation in Irvine, Calif., in an interview. He was not involved in the study.
“As it does not have any significant advantages with less long-term safety data, I do not see a place for it in my practice,” Dr. Wu said.
Dupilumab (Dupixent) and tralokinumab (Adbry) are monoclonal antibodies that also block IL-13. Both are already licensed for treating atopic dermatitis. Dupilumab was approved by the Food and Drug Administration in 2017, and tralokinumab was approved in 2021.
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. Eli Lilly has exclusive rights for the development and commercialization of lebrikizumab in the United States and all countries outside Europe; European rights belong to Almirall for all dermatology indications, including atopic dermatitis. Dr. Blauvelt acts as an investigator and adviser to these companies as well as many other pharmaceutical companies that are involved in developing new dermatologic treatments. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Ruxolitinib repigments many vitiligo-affected body areas
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
.
Those difficult areas include the hands and feet, said Thierry Passeron, MD, PhD, of Université Côte d’Azur and Centre Hospitalier Universitaire de Nice (France).
Indeed, a 50% or greater improvement in the Vitiligo Area Scoring Index (VASI-50) of the hands and feet was achieved with ruxolitinib cream (Opzelura) in around one-third of patients after 52 weeks’ treatment, and more than half of patients showed improvement in the upper and lower extremities.
During one of the late-breaking news sessions, Dr. Passeron presented a pooled analysis of the Topical Ruxolitinib Evaluation in Vitiligo Study 1 (TRuE-V1) and Study 2 (TruE-V2), which assessed VASI-50 data by body regions.
Similarly positive results were seen on the head and neck and the trunk, with VASI-50 being reached in a respective 68% and 48% of patients after a full year of treatment.
“VASI-50 response rates rose steadily through 52 weeks for both the head and trunk,” said Dr. Passeron. He noted that the trials were initially double-blinded for 24 weeks and that there was a further open-label extension phase through week 52.
In the latter phase, all patients were treated with ruxolitinib; those who originally received a vehicle agent as placebo crossed over to the active treatment.
First FDA-approved treatment for adults and adolescents with vitiligo
Ruxolitinib is a Janus kinase 1/2 inhibitor that has been available for the treatment for atopic dermatitis for more than a year. It was recently approved by the U.S. Food and Drug Administration for the treatment of vitiligo in adults and pediatric patients aged 12 years and older.
This approval was based on the positive findings of the TRuE-V1 and TRuE-V2 studies, which showed that after 24 weeks, 30% of patients treated with ruxolitinib had at least 75% improvement in the facial VASI, compared with 10% of placebo-treated patients.
“These studies demonstrated very nice results, especially on the face, which is the easiest part to repigment in vitiligo,” Dr. Passeron said.
“We know that the location is very important when it comes to repigmentation of vitiligo,” he added. He noted that other body areas, “the extremities, for example, are much more difficult.”
The analysis he presented specifically assessed the effect of ruxolitinib cream on repigmentation in other areas.
Pooled analysis performed
Data from the two TRuE-V trials were pooled. The new analysis included a total of 661 individuals; of those patients, 443 had been treated with topical ruxolitinib, and 218 had received a vehicle cream as a placebo.
For the first 24 weeks, patients received twice-daily 1.5% ruxolitinib cream or vehicle cream. This was followed by a 28-week extension phase in which everyone was treated with ruxolitinib cream, after which there was a 30-day final follow-up period.
Dr. Passeron reported data by body region for weeks 12, 24, and 52, which showed an increasing percentage of patients with VASI-50.
“We didn’t look at the face; that we know well, that is a very good result,” he said.
The best results were seen for the head and neck. VASI-50 was reached by 28.3%, 45.3%, and 68.1% of patients treated with ruxolitinib cream at weeks 12, 24, and 52, respectively. Corresponding rates for the placebo-crossover group were 19.8%, 23.8%, and 51%.
Repigmentation rates of the hand, upper extremities, trunk, lower extremities, and feet were about 9%-15% for both ruxolitinib and placebo at 12 weeks, but by 24 weeks, there was a clear increase in repigmentation rates in the ruxolitinib group for all body areas.
The 24-week VASI-50 rates for hand repigmentation were 24.9% for ruxolitinib cream and 14.4% for placebo. Corresponding rates for upper extremity repigmentation were 33.2% and 8.2%; for the trunk, 26.4% and 12.2%; for the lower extremities, 29.5% and 12.2%; and for the feet, 18.5% and 12.5%.
“The results are quite poor at 12 weeks,” Dr. Passeron said. “It’s very important to keep this in mind; it takes time to repigment vitiligo, it takes to 6-24 months. We have to explain to our patients that they will have to wait to see the results.”
Steady improvements, no new safety concerns
Regarding VASI-50 over time, there was a steady increase in total body scores; 47.7% of patients who received ruxolitinib and 23.3% of placebo-treated patients hit this target at 52 weeks.
“And what is also very important to see is that we didn’t reach the plateau,” Dr. Passeron reported.
Similar patterns were seen for all the other body areas. Again there was a suggestion that rates may continue to rise with continued long-term treatment.
“About one-third of the patients reached at least 50% repigmentation after 1 year of treatment in the hands and feet,” Dr. Passeron said. He noted that certain areas, such as the back of the hand or tips of the fingers, may be unresponsive.
“So, we have to also to warn the patient that probably on these areas we have to combine it with other treatment because it remains very, very difficult to treat.”
There were no new safety concerns regarding treatment-emergent adverse events, which were reported in 52% of patients who received ruxolitinib and in 36% of placebo-treated patients.
The most common adverse reactions included COVID-19 (6.1% vs. 3.1%), acne at the application site (5.3% vs. 1.3%), and pruritus at the application site (3.9% vs. 2.7%), although cases were “mild or moderate,” said Dr. Passeron.
An expert’s take-home
“The results of TRuE-V phase 3 studies are encouraging and exciting,” Viktoria Eleftheriadou, MD, MRCP(UK), SCE(Derm), PhD, said in providing an independent comment for this news organization.
“Although ruxolitinib cream is applied on the skin, this novel treatment for vitiligo is not without risks; therefore, careful monitoring of patients who are started on this topical treatment would be prudent,” said Dr. Eleftheriadou, who is a consultant dermatologist for Walsall Healthcare NHS Trust and the Royal Wolverhampton NHS Trust, Birmingham, United Kingdom.
“I would like to see how many patients achieved VASI-75 or VASI-80 score, which from patients’ perspectives is a more meaningful outcome, as well as how long these results will last for,” she added.
The study was funded by Incyte Corporation. Dr. Passeron has received grants, honoraria, or both from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte Corporation, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, and UCB. Dr. Passeron is the cofounder of YUKIN Therapeutics and has patents on WNT agonists or GSK2b antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. Dr. Eleftheriadou is an investigator and trial development group member on the HI-Light Vitiligo Trial (specific), a lead investigator on the pilot HI-Light Vitiligo Trial, and a medical advisory panel member of the Vitiligo Society UK. Dr. Eleftheriadou also provides consultancy services to Incyte and Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
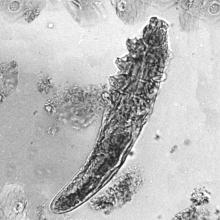
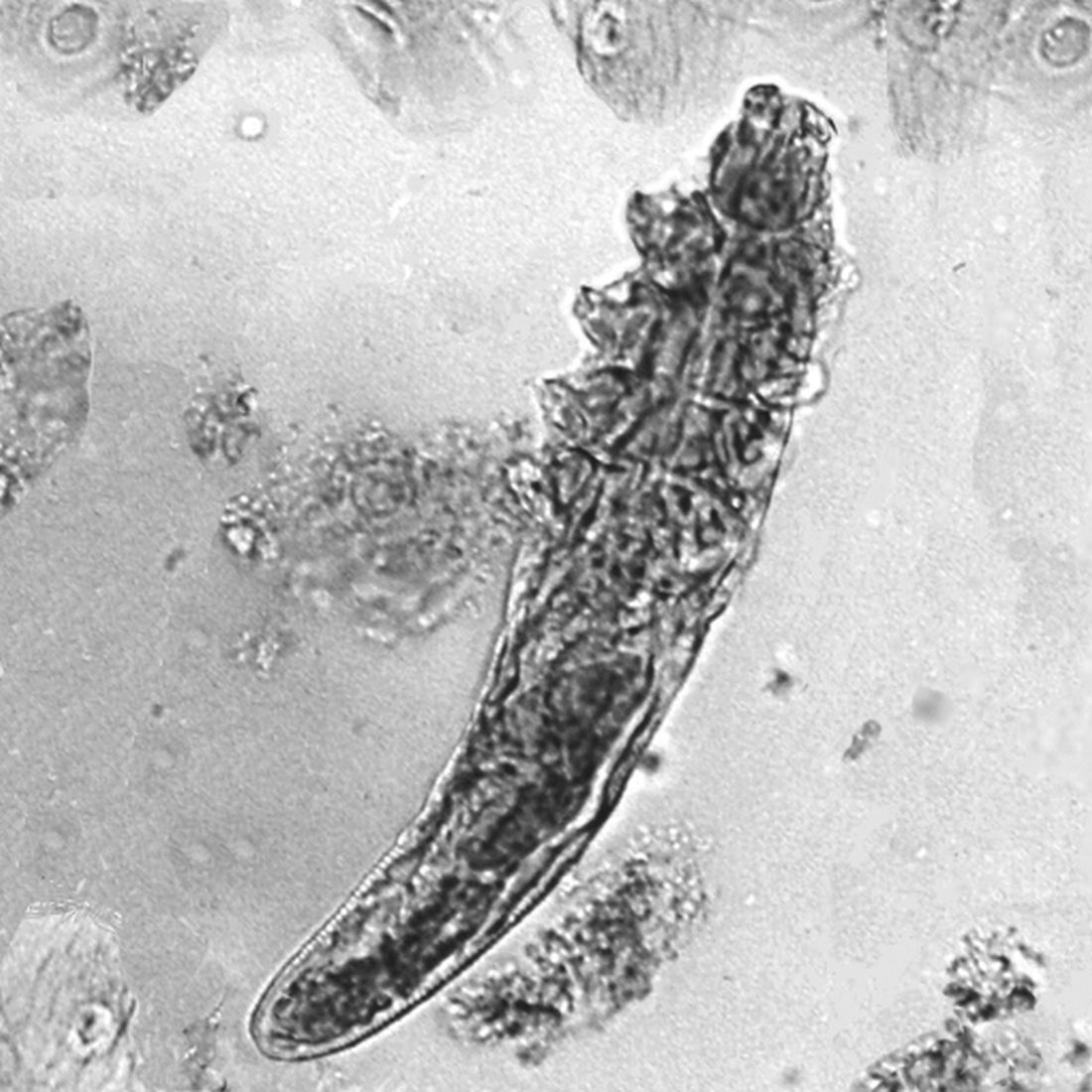
How to handle pesky molluscum contagiosum lesions
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
Breakthrough COVID studies lend support to use of new boosters in immunosuppressed patients
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
Don’t make children with head lice leave school, report says
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
The American Academy of Pediatrics says children with head lice don’t need to be sent home from school.
Head lice infestations aren’t really a health hazard because of low transmission rates, a new report from the academy says, and sending students home “may stigmatize children suspected of having head lice.” The group says schools should instead offer education programs to help families understand how to manage head lice.
“Head lice are an unpleasant part of the human experience, but they can be successfully managed and are no reason for a child to miss school,” Dawn Nolt, MD, lead author of the report on head lice, said in a news release.
The report advises schools to abandon “no-nit” policies, which call for a student to be lice-free before being allowed back in class.
“A child or adolescent should not be restricted from school attendance because of head lice, given the low contagion within classrooms. ‘No-nit’ policies that exclude children or adolescents until all nits are removed may violate a child’s or adolescent’s civil liberties and are best addressed with legal counsel for schools,” the report says.
The report notes that lice almost always spread through head-to-head contact, not by “jumping” from one person to another. It’s possible for lice to spread by touching the belongings of a person with lice, such as combs or sports helmets, but the chances of that happening are very low, the academy said.
“Lice found on combs are likely to be injured or dead, and a louse is not likely to leave a healthy head unless there is a heavy infestation,” the report says.
The report lists new medications for treatment and gives an algorithm for managing head lice cases.
“The ideal treatment of head lice should be safe, free of toxic chemicals, readily available, simple to apply, effective, and inexpensive,” the report says.
This is the first updated guidance on head lice from the American Academy of Pediatrics since 2015. The CDC also says students with head lice don’t need to be sent home.
“Students diagnosed with live head lice do not need to be sent home early from school; they can go home at the end of the day, be treated, and return to class after appropriate treatment has begun. Nits may persist after treatment, but successful treatment should kill crawling lice,” the CDC says.
A version of this article first appeared on WebMD.com.
BREEZE-AD-PEDS: First data for baricitinib in childhood eczema reported
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
The oral Janus kinase
After 16 weeks of treatment, the primary endpoint – an Investigators Global Assessment (IGA) score of 0 or 1 with at least a 2-point improvement from baseline – was met by 41.7% of patients given 2 mg (those younger than age 10) or 4 mg of baricitinib (those aged 10-17 years), the highest dose studied in each of those two age groups.
By comparison, the primary endpoint was met in 16.4% of children in the placebo group (P < .001).
Baricitinib is approved for the treatment of AD in adults in many countries, Antonio Torrelo, MD, of the Hospital Infantil Niño Jesús, Madrid, said at the annual congress of the European Academy of Dermatology and Venereology. It was approved by the U.S. Food and Drug Administration for treating adults with severe alopecia areata in June and is under FDA review for the treatment of AD.
The phase 3 BREEZE-AD-PEDS trial
BREEZE-AD-PEDS was a randomized, double-blind trial that evaluated the safety and efficacy of baricitinib in 483 children and adolescents with moderate to severe AD. Participants were aged 2-17 years. Those aged 2-5 years had been diagnosed with AD for at least 6 months; if they were older, they had been diagnosed for at least 12 months.
Three dosing levels of baricitinib were tested: 121 patients were given a low dose, which was 0.5 mg/day in children aged 2 to less than 10 years and 1 mg/day in those aged 10 to less than 18 years. A medium dose – 1 mg/day in the younger children and 2 mg/day in the older children – was given to 120 children, while a high dose – 2 mg/day and 4 mg/day, respectively – was given to another 120 children.
Topical treatments were permitted, although for entry into the trial, participants had to have had an inadequate response to steroids and an inadequate or no response to topical calcineurin inhibitors. In all groups, age, gender, race, geographic region, age at diagnosis of AD, and duration of AD “were more or less similar,” Dr. Torello said.
Good results, but only with highest dose
The primary IGA endpoint was reached by 25.8% of children in the medium-dose group and by 18.2% in the low-dose group. Neither result was statistically significant in comparison with placebo (16.4%).
When breaking down the results between different ages, “the results in the IGA scores are consistent in both age subgroups – below 10 years and over 10 years,” Dr. Torello noted. The results are also consistent across body weights (< 20 kg, 20-60 kg, and > 60 kg), he added.
Among those treated with the high dose of baricitinib, Eczema Area and Severity Index (EASI) 75% and 95% improvement scores were reached in 52.5% and 30% of patients, respectively. Corresponding figures for the medium dose were 40% and 21.7%; for the low baricitinib dose, 32.2% and 11.6%; and for placebo, 32% and 12.3%. Again, only the results for the highest baricitinib dose were significant in comparison with placebo.
A similar pattern was seen for improvement in itch, and there was a 75% improvement in Scoring Atopic Dermatitis (SCORAD75) results.
Safety of baricitinib in children
The labeling for JAK inhibitors that have been approved to date, including baricitinib, include a boxed warning regarding risks for thrombosis, major adverse cardiovascular events, and all-cause mortality. The warning is based on use by patients with rheumatoid arthritis.
Dr. Torello summarized baricitinib’s safety profile in the trial as being “consistent with the well-known safety profile for baricitinib in adults with moderate to severe atopic dermatitis.”
In the study, no severe adverse effects were noted, and no new safety signals were observed, he said. The rate of any treatment-emergent effect among patients was around 50% and was similar across all baricitinib and placebo groups. Study discontinuations because of a side effect were more frequent in the placebo arm (1.6% of patients) than in the baricitinib low-, medium-, and high-dose arms (0.8%, 0%, and 0.8%, respectively).
There were no cases of deep-vein thrombosis, pulmonary embolism, or other adverse effects of special interest, including major adverse cardiovascular events, gastrointestinal perforations, and opportunistic infections, Dr. Torrelo said.
No patient experienced elevations in liver enzyme levels, although there were some cases of elevated creatinine phosphokinase levels (16% in the placebo group and 19% in the baricitinib arms altogether) that were not from muscle injury. There was a possible increase in low-density cholesterol level (3.3% of those taking placebo vs. 10.1% of baricitinib-treated patients).
Is there a role for baricitinib?
“Baricitinib is a potential therapeutic option with a favorable benefit-to-risk profile for children between 2 and 18 years who have moderate to severe atopic dermatitis, and candidates for systemic therapy,” Dr. Torrelo said. “No single drug is capable to treat every patient with atopic dermatitis,” he added in discussing the possible place of baricitinib in pediatric practice.
“There are patients who do not respond to dupilumab, who apparently respond later to JAK inhibitors,” he noted.
“We are trying to work phenotypically, trying to learn what kind of patients – especially children who have a more heterogeneous disease than adults – can be better treated with JAK inhibitors or dupilumab.” There may be other important considerations in choosing a treatment in children, Dr. Torrelo said, including that JAK inhibitors can be given orally, while dupilumab is administered by injection.
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation in Irvine, Calif., pointed out that “only the higher dose is significantly more effective than placebo.”
In his view, “the potentially severe adverse events are not worth the risk compared to more effective agents, such as dupilumab, in this pediatric population,” added Dr. Wu, who recently authored a review of the role of JAK inhibitors in skin disease. He was not involved with the baricitinib study.
The study was funded by Eli Lilly in collaboration with Incyte. Dr. Torello has participated in advisory boards and/or has served as a principal investigator in clinical trials for AbbVie, Eli Lilly and Company, Novartis, Pfizer, Pierre Fabre, and Sanofi. Dr. Wu has been an investigator, consultant, or speaker for multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
What’s the true role of Demodex mites in the development of papulopustular rosacea?
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
FROM JEADV
Monkeypox features include mucocutaneous involvement in almost all cases
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
AT THE EADV CONGRESS
Alopecia areata: Positive results reported for two investigational JAK inhibitors
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
in separate studies reported at the annual congress of the European Academy of Dermatology and Venereology.
In the THRIVE-AA1 study, the primary endpoint of a Severity of Alopecia Tool (SALT) score of 20 or lower –which indicates that hair regrowth has occurred on at least 80% of the scalp – was achieved among patients taking deuruxolitinib, which was a significantly higher proportion than with placebo (P < .0001). Importantly, the JAK inhibitor’s effects were seen in as early as 4 weeks, and there was significant improvement in both eyelash and eyebrow hair regrowth.
In the unrelated ALLEGRO-LT study, effects from treatment with the JAK inhibitor ritlecitinib appeared to be sustained for 2 years; 69.6% of patients treated with ritlecitinib had a SALT score of 20 or lower by 24 months.
These data are “very exciting for alopecia areata because the patients selected are very severe,” observed Mahtab Samimi, MD, PhD, who cochaired the late-breaking session in which the study findings were discussed.
THRIVE-AA1 included only patients with hair loss of 50% or more. The ALLEGRO-LT study included patients with total scalp or total body hair loss (areata totalis/areata universalis) of 25%-50% at enrollment.
Moreover, “very stringent criteria” were used. SALT scores of 10 or less were evaluated in both studies, observed Dr. Samimi, professor of dermatology at the University of Tours (France).
“We can be ambitious now for our patients with alopecia areata; that’s really good news,” Dr. Samimi added.
Deuruxolitinib and the THRIVE trials
Deuruxolitinib is an oral JAK1/JAK2 inhibitor that has been tested in two similarly designed, multinational, randomized, double-blind, placebo-controlled phase 3 trials in patients with AA, THRIVE-AA1 and THRIVE-AA2.
Two doses of deuruxolitinib, 8 mg and 12 mg given twice daily, were evaluated in the trials, which altogether included just over 1,200 patients.
Results of THRIVE-AA1 have been reported by the manufacturer. Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., presented a more comprehensive review at the EADV meeting.
He reported that at 24 weeks, SALT scores of 20 or lower were achieved by 30% of adults with AA who were treated with deuruxolitinib 8 mg and by 42% of those treated with deuruxolitinib 12 mg. This primary endpoint was seen in only 1% of the placebo-treated patients.
The more stringent endpoint of having a SALT score of 10 or less, which indicates that hair regrowth has occurred over 90% of the scalp, was met by 21% of patients who received deuruxolitinib 8 mg twice a day and by 35% of those who received the 12-mg dose twice a day at 24 weeks. This endpoint was not reached by any of the placebo-treated patients.
“This is truly transformative therapy,” Dr. King said when presenting the findings. “We know that the chances of spontaneous remission when you have severe disease is next to zero,” he added.
There were reasonably high rates of patient satisfaction with the treatment, according to Dr. King. He said that 42% of those who took 8 mg twice a day and 53% of those who took 12 mg twice a day said they were “very satisfied” or “satisfied” with the degree of scalp hair regrowth achieved, compared with 5% for placebo.
Safety was as expected, and there were no signs of any blood clots, said Dr. King. Common treatment-emergent adverse events (TEAEs) that affected 5% or more of patients included acne and headache. Serious TEAEs were reported by 1.1% and 0.5% of those taking the 8-mg and 12-mg twice-daily doses, respectively, compared with 2.9% of those who received placebo.
Overall, the results look promising for deuruxolitinib, he added. He noted that almost all patients included in the trial have opted to continue in the open-label long-term safety study.
Prescribing information of the JAK inhibitors approved by the U.S. Food and Drug Administration includes a boxed warning about risk of serious infections, mortality, malignancy, major adverse cardiovascular events (MACE), and thrombosis. The warning is based on experience with another JAK inhibitor for patients with rheumatoid arthritis.
Ritlecitinib and the ALLEGRO studies
Interim results of the ongoing, open-label, phase 3 ALLEGRO-LT study with ritlecitinib were presented separately by Athanasios Tsianakas, MD, head of the department of dermatology at Fachklinik Bad Bentheim, Germany.
Ritlecitinib, which targets JAK3 and also the TEC family of tyrosine kinases, had met all of its endpoints in the prior ALLEGRO Phase 2b/3 study, Dr. Tsianakas said. Those included the benchmarks of a SALT score of 20 or less and a SALT score of 10 or less.
“Ritlecitinib showed a very good long-term efficacy and good safety profile in our adolescent and adult patients suffering from alopecia areata,” said Dr. Tsianakas.
A total of 447 patients were included in the trial. They were treated with 50 mg of ritlecitinib every day; some had already participated in the ALLEGRO trial, while others had been newly recruited. The latter group entered the trial after a 4-week run-in period, during which a 200-mg daily loading dose was given for 4 weeks.
Most (86%) patients had been exposed to ritlecitinib for at least 12 months; one-fifth had discontinued treatment at the data cutoff, generally because the patients no longer met the eligibility criteria for the trial.
Safety was paramount, Dr. Tsianakas highlighted. There were few adverse events that led to temporary or permanent discontinuation of the study drug. The most common TEAEs that affected 5% or more of patients included headache and acne. There were two cases of MACE (one nonfatal myocardial infarction and one nonfatal stroke).
The proportion of patients with a SALT score of 20 or less was 2.5% at 1 month, 27.9% at 3 months, 50.1% at 6 months, 59.8% at 9 months, and 65.5% at 12 months. Thereafter, there was little shift in the response. A sustained effect, in which a SALT score of 20 or less was seen out to 24 months, occurred in 69.9% of patients.
A similar pattern was seen for SALT scores of 10 or less, ranging from 16.5% at 3 months to 62.5% at 24 months.
Following in baricitinib’s footsteps?
This not the first time that JAK inhibitors have been shown to have beneficial effects for patients with AA. Baricitinib (Olumiant) recently became the first JAK inhibitor to be granted marketing approval for AA in the United States, largely on the basis of two pivotal phase 3 studies, BRAVE-AA1 and BRAVE-AA2.
“This is just such an incredibly exciting time,” said Dr. King. “Our discoveries in the lab are being translated into effective therapies for patients with diseases for which we’ve not previously had therapies,” he commented.
“Our concept of interferon gamma– and interleukin-15–mediated disease is probably not true for everybody,” said, Dr. King, who acknowledged that some patients with AA do not respond to JAK-inhibitor therapy or may need additional or alternative treatment.
“It’s probably not that homogeneous a disease,” he added. “It’s fascinating that the very first drugs for this disease are showing efficacy in as many patients as they are.”
The THRIVE-AAI study was funded by CONCERT Pharmaceuticals. Dr. King has served on advisory boards, has provided consulting services to, or has been a trial investigator for multiple pharmaceutical companies, including CoNCERT Pharmaceuticals. The ALLEGRO-LT study was funded by Pfizer. Dr. Tsianakas has acted as a clinical trial investigator and speaker for Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Training program linked to less hand eczema for hairdressers
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS