User login
Study identifies skin biomarkers that predict newborn eczema risk
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It might be possible to develop a simple test to identify newborn children who are at risk of later developing atopic dermatitis (AD), according to findings from a Danish prospective birth cohort study.
but also for having more severe disease.
“We are able to identify predictive immune biomarkers of atopic dermatitis using a noninvasive method that was not associated with any pain,” one of the study’s investigators, Anne-Sofie Halling, MD, said at a press briefing at the annual congress of the European Academy of Dermatology and Venereology.
“Importantly, we were able to predict atopic dermatitis occurring months after [sample] collection,” said Dr. Halling, who works at Bispebjerg Hospital and is a PhD student at the University of Copenhagen.
These findings could hopefully be used to help identify children “so that preventive strategies can target these children ... and decrease the incidence of this common disease,” she added.
AD is caused “by a complex interplay between skin barrier dysfunction and immune dysregulation,” Dr. Halling said, and it is “the first step in the so-called atopic march, where children also develop food allergy, asthma, and rhinitis.” Almost all cases of AD begin during the first years of life. Approximately 15%-20% of children can be affected, she noted, emphasizing the high burden of the disease and pointing out that strategies are shifting toward trying to prevent the disease in those at risk.
Copenhagen BABY cohort
This is where the BABY study comes in, Dr. Halling said. The study enrolled 450 children at birth and followed them until age 2 years. Gene mutation testing was performed at enrollment. All children underwent skin examination, and skin samples were taken using tape strips. Tape strips were applied to the back of the hand of children born at term and between the shoulder blades on the back of children who were premature.
Skin examinations were repeated, and skin samples were obtained again at age 2 months. They were taken again only if there were any signs of AD. For those diagnosed with AD, disease severity was assessed using the Eczema Area and Severity Index (EASI) by the treating physician. Children were excluded if they had AD at the time the tape strip testing was due to be performed.
Comparing term and preterm children
Dr. Halling noted that analyses were performed separately for the 300 children born at term and for the 150 who were preterm.
The prevalence of AD was higher among children born at term than among the preterm children (34.6% vs. 21.2%), and the median time to onset was shorter (6 months vs. 8 months). There were also differences in the EASI scores among those who developed AD; median scores were higher in the children born at term than in the preterm children (4.1 vs. 1.6).
More children born at term than preterm children had moderate to severe AD (23.3% vs. 8%), Dr. Halling reported.
TARC, IL-8, and IL-18 predictive of AD
Multiple immune biomarkers were tested, including various cytokines and filaggrin degradation products. On examination of skin samples collected at birth, no particular biomarkers were found at higher levels among children who developed AD in comparison with those who did not develop AD.
With regard to biomarkers examined in skin samples at 2 months of age, however, the results were different, Dr. Halling said. One particular cytokine, thymus and activation-regulated chemokine (TARC), was seen to double the risk of AD in the first 2 years of a child’s life.
This doubled risk was seen not only among the children born at term but also among those born preterm, although the data were only significant with regard to the children born at term.
The unadjusted hazard ratios and adjusted HRs (adjusted for parental atopy and filaggrin gene mutations) in term children were 2.11 (95% confidence interval, 1.36-3.26; P = .0008) and 1.85 (95% CI, 1.18-2.89; P = .007), respectively.
For preterm children, the HRs were 2.23 (95% CI, 0.85-5.86; P = .1) and 2.60 (95% CI, 0.98-6.85; P =.05), respectively.
These findings were in line with findings of other studies, Dr. Halling said. “It is well recognized that TARC is currently the best biomarker in patients with established atopic dermatitis.” Moreover, she reported that TARC was associated with a cumulative increase in the risk for AD and that levels were found to be higher in children in whom onset occurred at a later age than among those diagnosed before 6 months of age.
“This is important, as these findings shows that TARC levels predict atopic dermatitis that occurred many months later,” Dr. Halling said.
And, in term-born children at least, TARC upped the chances that the severity of AD would be greater than had it not been present (adjusted HR, 4.65; 95% CI, 1.91-11.31; P = .0007).
Increased levels of interleukin-8 (IL-8) and IL-18 at 2 months of age were also found to be predictive of having moderate to severe AD. The risk was more than double in comparison with those in whom levels were not increased, again only in term-born children.
‘Stimulating and interesting findings’
These data are “very stimulating and interesting,” Dedee Murrell, MD, professor and head of the department of dermatology at St. George Hospital, University of New South Wales, Sydney, observed at the press briefing.
“You found this significant association mainly in the newborn children born at term, and the association in the preterm babies wasn’t as high. Is that anything to do with how they were taken care of in the hospital?” Dr. Murrell asked.
“That’s a really good question,” Dr. Halling said. “Maybe they need to be exposed for a month or two before we are actually able to identify which children will develop atopic dermatitis.”
The study was funded by the Lundbeck Foundation. Dr. Halling has acted as a consultant for Coloplast and as a speaker for Leo Pharma. Dr. Murrell has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
FDA approves dupilumab for treatment of prurigo nodularis
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
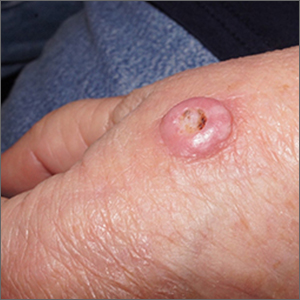
Fast growing hand lesion
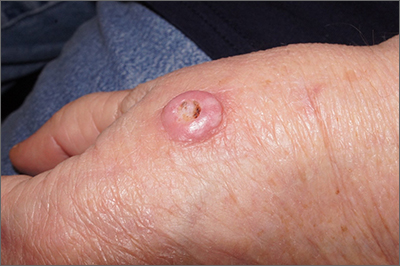
A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003

A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A scoop shave biopsy at the lower edge of the lesion revealed that this was a well-differentiated squamous cell carcinoma.
Squamous cell carcinoma is the second most common cancer in the United States and the most common skin cancer in Black people.1 A patient’s age and their accumulated UV radiation from sun exposure or artificial tanning is a major contributing factor. Lesions may manifest as precancers characterized as rough pink or brown papules with a sandpaper-like texture on sun-exposed skin. These lesions may clear spontaneously or develop into invasive disease, as occurred in this case.
Surgical treatment is often curative. Fusiform excision and Mohs micrographic surgery are 2 common options. More advanced squamous cell carcinomas that are large or found to have poorly differentiated cells or large perineural invasion carry a risk of metastasis.
In elderly patients, optimal treatment isn’t always straightforward.1 Nonsurgical options include radiation and intralesional chemotherapy. These nonsurgical choices may seem less aggressive, but total inconvenience, wound care, and discomfort can be equal to or worse than a single session of curative surgery.
This patient’s lesion was excised with a 5-mm margin. The patient tolerated an in-office procedure lasting about 45 minutes but would have struggled with a longer session with Mohs microsurgery. The postoperative period required limiting full use of his left hand for about 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003
1. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178. 2. Renzi M Jr, Schimmel J, Decker A, et al. Management of skin cancer in the elderly. Dermatol Clin. 2019;37:279-286. doi: 10.1016/j.det.2019.02.003
Consider the mnemonic ‘CLEAR’ when counseling acne patients
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
Many factors linked with higher, lower risk for hand eczema
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
Apremilast may have some cardiometabolic benefits in patients with psoriasis
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Early emollient use reduces dermatitis in at-risk infants
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY
Psoriasis, psoriatic arthritis insurance coverage remains restrictive
Insurance coverage for specialty drugs to treat psoriasis and psoriatic arthritis varies extensively among insurance companies and often restricts coverage beyond the drug labels, according to a review of data from commercial health plans in the United States.
Although specialty medications have demonstrated effectiveness for psoriasis and psoriatic arthritis, data on insurance coverage for these indications are limited and costs are often a barrier to treatment, Christine Learned, of Tufts Medical Center, Boston, and colleagues wrote.
In a study published in the Journal of Psoriasis and Psoriatic Arthritis, the researchers used the Tufts Medical Center Specialty Drug Evidence and Coverage database, which includes information on 158 specialty drugs covered by 17 U.S. commercial health plans, to review data on a total of 11 medications indicated for psoriasis (etanercept, adalimumab, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, risankizumab, and apremilast) and 11 indicated for psoriatic arthritis (etanercept, adalimumab, certolizumab pegol, golimumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tofacitinib, apremilast, and abatacept) at the time of the study.
Overall, an average of 78.6% and 66.8% of insurance plans were more restrictive than the Food and Drug Association label in coverage of specialty medications for psoriasis and psoriatic arthritis, respectively.
Disease severity affected insurance coverage for psoriasis. The percentage of plans with a body surface area requirement for specialty medications ranged from 11% for apremilast to 39% for tildrakizumab, adalimumab, and certolizumab pegol. The percentage of plans with exceptions for special body locations affected by psoriasis ranged from 6% for risankizumab and brodalumab to 39% for certolizumab pegol. In addition, 6% of plans had Psoriasis Area and Severity Index requirements for etanercept and ixekizumab, and 11% had PASI requirements for adalimumab, certolizumab pegol, and tildrakizumab.
The percentage of plans with prescriber restrictions for both psoriasis and psoriatic arthritis ranged from 33% to 50%.
All 11 medications for psoriatic arthritis were approved as first-line treatments by at least one plan, compared with 3 the 11 medications with indications for psoriasis. However, medications for both psoriasis and psoriatic arthritis were approved mainly as second-line therapies.
Study designs may impact insurance coverage, as randomized, controlled trials are often used as the basis for coverage decisions for psoriasis, while coverage for psoriatic arthritis is more often based on clinical guidelines, the researchers explained.
“Our analysis confirms that variability exists for the indications of psoriasis and psoriatic arthritis,” they wrote.
The comorbidities associated with psoriasis are not always considered in insurance coverage, and coverage complications may contribute to the persistent undertreatment of many patients with psoriasis, the researchers added.
“Insurance restrictions may blunt provider and patient autonomy in selection of specialty medications and have the potential to diminish a provider’s ability to tailor regimens so as to optimize outcomes while minimizing risks,” they emphasized.
The study findings were limited by the inclusion only of publicly available policy information; therefore, some plans’ restrictions may have been missed in the analysis, the researchers said.
The results suggest that patients should review their insurance coverage of specialty drugs when choosing a health plan, and clinicians should factor in a patient’s plan a likely drug access when considering treatment options, they concluded.
The study received no outside funding. Ms. Learned had no relevant financial conflicts to disclose, but two coauthors reported financial relationships with pharmaceutical companies that manufacturer drugs for psoriasis and psoriatic arthritis.
Insurance coverage for specialty drugs to treat psoriasis and psoriatic arthritis varies extensively among insurance companies and often restricts coverage beyond the drug labels, according to a review of data from commercial health plans in the United States.
Although specialty medications have demonstrated effectiveness for psoriasis and psoriatic arthritis, data on insurance coverage for these indications are limited and costs are often a barrier to treatment, Christine Learned, of Tufts Medical Center, Boston, and colleagues wrote.
In a study published in the Journal of Psoriasis and Psoriatic Arthritis, the researchers used the Tufts Medical Center Specialty Drug Evidence and Coverage database, which includes information on 158 specialty drugs covered by 17 U.S. commercial health plans, to review data on a total of 11 medications indicated for psoriasis (etanercept, adalimumab, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, risankizumab, and apremilast) and 11 indicated for psoriatic arthritis (etanercept, adalimumab, certolizumab pegol, golimumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tofacitinib, apremilast, and abatacept) at the time of the study.
Overall, an average of 78.6% and 66.8% of insurance plans were more restrictive than the Food and Drug Association label in coverage of specialty medications for psoriasis and psoriatic arthritis, respectively.
Disease severity affected insurance coverage for psoriasis. The percentage of plans with a body surface area requirement for specialty medications ranged from 11% for apremilast to 39% for tildrakizumab, adalimumab, and certolizumab pegol. The percentage of plans with exceptions for special body locations affected by psoriasis ranged from 6% for risankizumab and brodalumab to 39% for certolizumab pegol. In addition, 6% of plans had Psoriasis Area and Severity Index requirements for etanercept and ixekizumab, and 11% had PASI requirements for adalimumab, certolizumab pegol, and tildrakizumab.
The percentage of plans with prescriber restrictions for both psoriasis and psoriatic arthritis ranged from 33% to 50%.
All 11 medications for psoriatic arthritis were approved as first-line treatments by at least one plan, compared with 3 the 11 medications with indications for psoriasis. However, medications for both psoriasis and psoriatic arthritis were approved mainly as second-line therapies.
Study designs may impact insurance coverage, as randomized, controlled trials are often used as the basis for coverage decisions for psoriasis, while coverage for psoriatic arthritis is more often based on clinical guidelines, the researchers explained.
“Our analysis confirms that variability exists for the indications of psoriasis and psoriatic arthritis,” they wrote.
The comorbidities associated with psoriasis are not always considered in insurance coverage, and coverage complications may contribute to the persistent undertreatment of many patients with psoriasis, the researchers added.
“Insurance restrictions may blunt provider and patient autonomy in selection of specialty medications and have the potential to diminish a provider’s ability to tailor regimens so as to optimize outcomes while minimizing risks,” they emphasized.
The study findings were limited by the inclusion only of publicly available policy information; therefore, some plans’ restrictions may have been missed in the analysis, the researchers said.
The results suggest that patients should review their insurance coverage of specialty drugs when choosing a health plan, and clinicians should factor in a patient’s plan a likely drug access when considering treatment options, they concluded.
The study received no outside funding. Ms. Learned had no relevant financial conflicts to disclose, but two coauthors reported financial relationships with pharmaceutical companies that manufacturer drugs for psoriasis and psoriatic arthritis.
Insurance coverage for specialty drugs to treat psoriasis and psoriatic arthritis varies extensively among insurance companies and often restricts coverage beyond the drug labels, according to a review of data from commercial health plans in the United States.
Although specialty medications have demonstrated effectiveness for psoriasis and psoriatic arthritis, data on insurance coverage for these indications are limited and costs are often a barrier to treatment, Christine Learned, of Tufts Medical Center, Boston, and colleagues wrote.
In a study published in the Journal of Psoriasis and Psoriatic Arthritis, the researchers used the Tufts Medical Center Specialty Drug Evidence and Coverage database, which includes information on 158 specialty drugs covered by 17 U.S. commercial health plans, to review data on a total of 11 medications indicated for psoriasis (etanercept, adalimumab, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, risankizumab, and apremilast) and 11 indicated for psoriatic arthritis (etanercept, adalimumab, certolizumab pegol, golimumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tofacitinib, apremilast, and abatacept) at the time of the study.
Overall, an average of 78.6% and 66.8% of insurance plans were more restrictive than the Food and Drug Association label in coverage of specialty medications for psoriasis and psoriatic arthritis, respectively.
Disease severity affected insurance coverage for psoriasis. The percentage of plans with a body surface area requirement for specialty medications ranged from 11% for apremilast to 39% for tildrakizumab, adalimumab, and certolizumab pegol. The percentage of plans with exceptions for special body locations affected by psoriasis ranged from 6% for risankizumab and brodalumab to 39% for certolizumab pegol. In addition, 6% of plans had Psoriasis Area and Severity Index requirements for etanercept and ixekizumab, and 11% had PASI requirements for adalimumab, certolizumab pegol, and tildrakizumab.
The percentage of plans with prescriber restrictions for both psoriasis and psoriatic arthritis ranged from 33% to 50%.
All 11 medications for psoriatic arthritis were approved as first-line treatments by at least one plan, compared with 3 the 11 medications with indications for psoriasis. However, medications for both psoriasis and psoriatic arthritis were approved mainly as second-line therapies.
Study designs may impact insurance coverage, as randomized, controlled trials are often used as the basis for coverage decisions for psoriasis, while coverage for psoriatic arthritis is more often based on clinical guidelines, the researchers explained.
“Our analysis confirms that variability exists for the indications of psoriasis and psoriatic arthritis,” they wrote.
The comorbidities associated with psoriasis are not always considered in insurance coverage, and coverage complications may contribute to the persistent undertreatment of many patients with psoriasis, the researchers added.
“Insurance restrictions may blunt provider and patient autonomy in selection of specialty medications and have the potential to diminish a provider’s ability to tailor regimens so as to optimize outcomes while minimizing risks,” they emphasized.
The study findings were limited by the inclusion only of publicly available policy information; therefore, some plans’ restrictions may have been missed in the analysis, the researchers said.
The results suggest that patients should review their insurance coverage of specialty drugs when choosing a health plan, and clinicians should factor in a patient’s plan a likely drug access when considering treatment options, they concluded.
The study received no outside funding. Ms. Learned had no relevant financial conflicts to disclose, but two coauthors reported financial relationships with pharmaceutical companies that manufacturer drugs for psoriasis and psoriatic arthritis.
FROM THE JOURNAL OF PSORIASIS AND PSORIATIC ARTHRITIS
When is an allergic reaction to raw plant food due to tree pollen?
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
Scalp plaque
A punch biopsy was performed, and the results were consistent with pityriasis amiantacea arising from psoriasis. In an older patient, a keratinaceous horn would be worrisome for a squamous cell carcinoma. In a younger patient, like this one, it is more likely an atypical manifestation of a more common dermatosis.
Pityriasis amiantacea is an unusual disorder in which thick adherent scales form on the scalp; it is most common in children, adolescents, and young adults. There is no racial predilection. With this condition, patients complain of a fixed plaque that may shed scale but not as quickly as it accumulates. It can be an isolated finding, but more often it is a secondary manifestation of an underlying case of psoriasis, seborrheic dermatitis, tinea capitis, or atopic dermatitis.1
A punch biopsy performed on the scalp should include the skin underlying the compact keratin scale. However, to avoid excessive bleeding, use lidocaine with epinephrine. Allow 15 minutes for the anesthesia to take effect before beginning the procedure.
Treatment depends on the underlying cause but includes debridement of the aggregated scale with a topical keratolytic (such as salicylic acid or topical fluocinolone oil 0.01% applied) at night and washed out 7 to 10 hours later.
The patient was advised to use over-the-counter 2% salicylic acid shampoo daily and to apply topical clobetasol 0.05% solution nightly for 4 weeks and once weekly after clearance for another 3 months. At the 3-month follow-up, the patient’s scalp was clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641. doi: 10.1111/j.1365-2230.2011.04286.x

A punch biopsy was performed, and the results were consistent with pityriasis amiantacea arising from psoriasis. In an older patient, a keratinaceous horn would be worrisome for a squamous cell carcinoma. In a younger patient, like this one, it is more likely an atypical manifestation of a more common dermatosis.
Pityriasis amiantacea is an unusual disorder in which thick adherent scales form on the scalp; it is most common in children, adolescents, and young adults. There is no racial predilection. With this condition, patients complain of a fixed plaque that may shed scale but not as quickly as it accumulates. It can be an isolated finding, but more often it is a secondary manifestation of an underlying case of psoriasis, seborrheic dermatitis, tinea capitis, or atopic dermatitis.1
A punch biopsy performed on the scalp should include the skin underlying the compact keratin scale. However, to avoid excessive bleeding, use lidocaine with epinephrine. Allow 15 minutes for the anesthesia to take effect before beginning the procedure.
Treatment depends on the underlying cause but includes debridement of the aggregated scale with a topical keratolytic (such as salicylic acid or topical fluocinolone oil 0.01% applied) at night and washed out 7 to 10 hours later.
The patient was advised to use over-the-counter 2% salicylic acid shampoo daily and to apply topical clobetasol 0.05% solution nightly for 4 weeks and once weekly after clearance for another 3 months. At the 3-month follow-up, the patient’s scalp was clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A punch biopsy was performed, and the results were consistent with pityriasis amiantacea arising from psoriasis. In an older patient, a keratinaceous horn would be worrisome for a squamous cell carcinoma. In a younger patient, like this one, it is more likely an atypical manifestation of a more common dermatosis.
Pityriasis amiantacea is an unusual disorder in which thick adherent scales form on the scalp; it is most common in children, adolescents, and young adults. There is no racial predilection. With this condition, patients complain of a fixed plaque that may shed scale but not as quickly as it accumulates. It can be an isolated finding, but more often it is a secondary manifestation of an underlying case of psoriasis, seborrheic dermatitis, tinea capitis, or atopic dermatitis.1
A punch biopsy performed on the scalp should include the skin underlying the compact keratin scale. However, to avoid excessive bleeding, use lidocaine with epinephrine. Allow 15 minutes for the anesthesia to take effect before beginning the procedure.
Treatment depends on the underlying cause but includes debridement of the aggregated scale with a topical keratolytic (such as salicylic acid or topical fluocinolone oil 0.01% applied) at night and washed out 7 to 10 hours later.
The patient was advised to use over-the-counter 2% salicylic acid shampoo daily and to apply topical clobetasol 0.05% solution nightly for 4 weeks and once weekly after clearance for another 3 months. At the 3-month follow-up, the patient’s scalp was clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641. doi: 10.1111/j.1365-2230.2011.04286.x
1. Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641. doi: 10.1111/j.1365-2230.2011.04286.x