User login
Subcutaneous Panniculitislike T-Cell Lymphoma
Subcutaneous panniculitislike T-cell lymphoma (SPTL) is a cutaneous lymphoma of α and β phenotype cytotoxic T cells in which the neoplastic cells are found almost exclusively in the subcutaneous layer and resemble a panniculitis.1 It affects males and females with equal incidence and is seen in both adults and children. Clinically, this disease presents as a nonspecific panniculitis with indurated but typically nonulcerated erythematous plaques and nodules most commonly located on the extremities. Plaques and nodules may appear on other body sites and may be generalized.1 In some cases, patients present with associated systemic symptoms including fever, malaise, weight loss, and fatigue.2
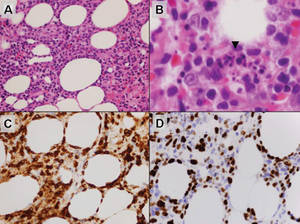
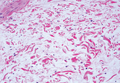
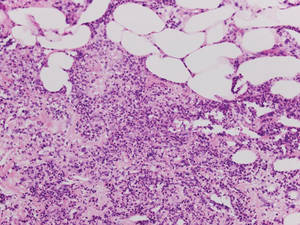
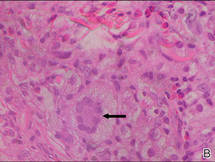
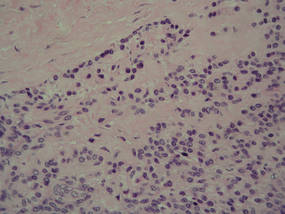
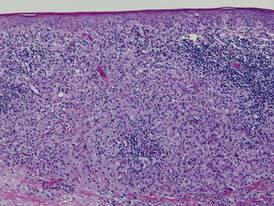
Histologically, SPTL presents as a predominantly lobular panniculitis (Figure 1) with rimming of adipocytes by neoplastic cells that appear as small and medium-sized atypical lymphocytes with hyperchromatic nuclei (Figure 2A). A less dominant septal component may be present, and neoplastic cells may encroach into the lower reticular dermis, rarely involving the papillary dermis or epidermis.2 Although rimming of adipocytes is classic, it is not specific to this entity, as rimming also can be found in other lymphomas and infectious panniculitis. Reactive lymphocytes and macrophages with ingested lipid material also are seen intermixed with neoplastic cells.2 Necrosis is a common finding, including destructive fragmentation of the nucleus, known as karyorrhexis. If necrosis is extensive, appreciation of other histologic features may be hindered.3 Histiocytes engulfing the nuclear debris known as beanbag cells also can be seen (Figure 2B). The diagnosis can be made on immunohistologic analysis demonstrating neoplastic cells with a cytotoxic α and β T-suppressor phenotype centered around and rimming the adipocytes in the subcutaneous fat.3 Immunohistochemistry reveals positive CD3, CD8 (Figure 2C), and βF1 markers, as well as T-cell intracellular antigen 1 (TIA-1), granzyme B, and perforin.1,2 The neoplastic cells often have a high proliferation index as evidenced by MIB-1 (Ki-67) labeling (Figure 2D). The neoplastic cells are negative for CD4, CD56, and CD30.1,2 Subcutaneous panniculitislike T-cell lymphoma cells are negative for Epstein-Barr virus by in situ hybridization.2
 |
| Figure 1. Subcutaneous panniculitislike T-cell lymphoma showing a predominantly lobular panniculitis (H&E, original magnification ×20). |
|
| Figure 2. Rimming of adipocytes by hyperchromatic lymphocytes (A)(H&E, original magnification ×400). Arrowhead indicates a histiocyte (ie, beanbag cell) that has undergone cytophagocytosis of nuclear debris (B)(H&E, original magnification ×600). Immunohistochemistry with CD8 highlights the cells rimming the adipocytes (C)(original magnification ×600). Immunohistochemistry with MIB-1 shows an increased proliferative rate in the lymphocytes rimming the adipocytes (D)(original magnification ×600). |
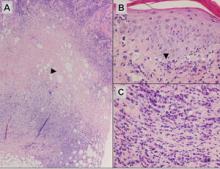
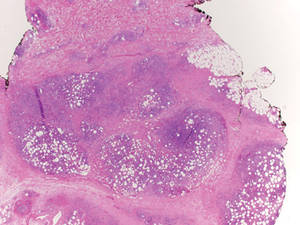
Subcutaneous panniculitislike T-cell lymphoma must be distinguished from lupus erythematosus panniculitis (LEP) and other cutaneous lymphomas. Importantly, LEP and SPTL clinically may appear similar and are not mutually exclusive diagnoses.2 On histology, they may look similar, showing T cell aggregates and necrosis; however, thickening of the basement membrane, vacuolar change at the dermoepidermal junction, plasma cells, hyaline sclerosis, mucin deposition, a lymphocytic perivascular infiltrate, and nodular aggregates of B cells are more common in LEP (Figure 3).2,4 Additionally, in LEP the T cell aggregates typically will not have a high proliferative rate as evidenced by MIB-1.3
Additionally, other lobular panniculitides can be considered in the differential diagnosis, including erythema induratum (EI), α1-antitrypsin deficiency panniculitis (A1ATDP), and infectious panniculitis. Histologically, EI (Figure 4), also known as nodular vasculitis when not associated with Mycobacterium tuberculosis, has a lobular pattern of inflammation. Early in the disease process there are discrete collections of neutrophils; later, granulomas with histiocytes, giant cells, and foamy macrophages are seen.4 The reactive infiltrate of EI is more mixed than in SPTL, with small lymphocytes, plasma cells, and eosinophils. Leukocytoclastic vasculitis and extravascular caseous or fibrinoid necrosis also may be present.4,5 Substantial caseous necrosis may extend to the dermis and epidermis with EI. Importantly, EI lacks true tuberculoid granulomas and stains negative for acid-fast bacilli, as it is a reactive rather than a local infectious process, but a history of M tuberculosis exposure is common.4 α1-Antitrypsin deficiency panniculitis results from a deficiency of proteinase activity and can be distinguished from SPTL by a neutrophil-rich panniculitis (Figure 5) as well as the classic appearance of splaying of neutrophils between collagen bundles in the deep reticular dermis. Additionally, the panniculitis is characterized by focal areas of necrotic lobules and septa with an infiltrate of neutrophils and macrophages that abut areas of normal-appearing subcutaneous fat without infiltrate.6 Clinically, the A1ATDP lesions may have ulceration and express an oily substance from fat necrosis. Panniculitis with A1ATDP may precede liver and lung disease.4 Panniculitis from bacterial or fungal infection is more common in immunocompromised patients but should be considered when subcutaneous inflammation and/or necrosis is present. Depending on the responsible organism and the status of a patient’s immune system, infectious panniculitis can have variable presentations, including suppurative granulomas with mycobacterial organisms, a dermal focus of infection if the primary source is cutaneous, or a deeper reticular and subcuticular focus in the subcutaneous fat if the infectious panniculitis occurs from hematogenous spread.4 An example of an infectious panniculitis having more of a granulomatous pattern secondary to Cryptococcus can be seen in Figure 6. Ultimately, special stains to identify infectious organisms (eg, Gram, periodic acid–Schiff, Ziehl-Neelsen) can be ordered to aid in the diagnosis if a responsible organism is not visible on hematoxylin and eosin staining.
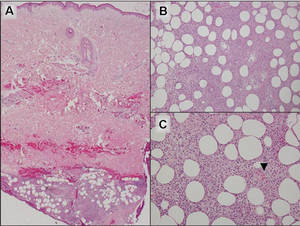 |
Figure 4. Erythema induratum is characterized by a lobular panniculitis (A and B)(both H&E, original magnifications ×40 and ×200). Vascular changes (arrowhead) are present in a majority of cases with endothelial swelling and extravasation of erythrocytes (C)(H&E, original magnification ×400). |
 |
| Figure 5. Neutrophilic panniculitis that can be seen in α1-antitrypsin deficiency panniculitis (H&E, original magnification ×400). |
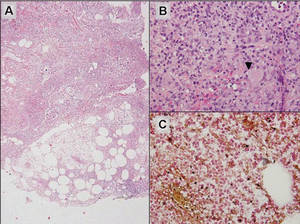 |
Figure 6. Infectious panniculitis secondary to Cryptococcus showing a granulomatous reaction in the subcutis (A)(H&E, original magnification ×40). Closer inspection shows a dense infiltrate of chronic inflammatory cells including numerous histiocytes and multinucleated giant cells. Some of the giant cells contain refractile organisms (arrowhead)(B)(H&E, original magnification ×400). Mucicarmine histochemical stain highlights the capsule of the organism (C)(original magnification ×400). |
Acknowledgment
The authors would like to thank Drake Poeschl, MD, St. Louis, Missouri, for proofreading the manuscript.
1. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3765-3785.
2. Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111:838-845.
3. Cerroni L, Gatter K, Kerl H. Subcutaneous “panniculitis-like” T-cell lymphoma. In: Cerroni L, Gatter K, Kerl H. Skin Lymphoma: The Illustrated Guide. 3rd ed. Hoboken, NJ: Wiley-Blackwell Publishing; 2011:87-96.
4. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
5. Sharon V, Goodarzi H, Chambers CJ, et al. Erythema induratum of Bazin. Dermatol Online J. 2010;16:1.
6. Rajagopal R, Malik AK, Murthy PS, et al. Alpha-1 antitrypsin deficiency panniculitis. Indian J Dermatol Venereol Leprol. 2002;68:362-364.
Subcutaneous panniculitislike T-cell lymphoma (SPTL) is a cutaneous lymphoma of α and β phenotype cytotoxic T cells in which the neoplastic cells are found almost exclusively in the subcutaneous layer and resemble a panniculitis.1 It affects males and females with equal incidence and is seen in both adults and children. Clinically, this disease presents as a nonspecific panniculitis with indurated but typically nonulcerated erythematous plaques and nodules most commonly located on the extremities. Plaques and nodules may appear on other body sites and may be generalized.1 In some cases, patients present with associated systemic symptoms including fever, malaise, weight loss, and fatigue.2
Histologically, SPTL presents as a predominantly lobular panniculitis (Figure 1) with rimming of adipocytes by neoplastic cells that appear as small and medium-sized atypical lymphocytes with hyperchromatic nuclei (Figure 2A). A less dominant septal component may be present, and neoplastic cells may encroach into the lower reticular dermis, rarely involving the papillary dermis or epidermis.2 Although rimming of adipocytes is classic, it is not specific to this entity, as rimming also can be found in other lymphomas and infectious panniculitis. Reactive lymphocytes and macrophages with ingested lipid material also are seen intermixed with neoplastic cells.2 Necrosis is a common finding, including destructive fragmentation of the nucleus, known as karyorrhexis. If necrosis is extensive, appreciation of other histologic features may be hindered.3 Histiocytes engulfing the nuclear debris known as beanbag cells also can be seen (Figure 2B). The diagnosis can be made on immunohistologic analysis demonstrating neoplastic cells with a cytotoxic α and β T-suppressor phenotype centered around and rimming the adipocytes in the subcutaneous fat.3 Immunohistochemistry reveals positive CD3, CD8 (Figure 2C), and βF1 markers, as well as T-cell intracellular antigen 1 (TIA-1), granzyme B, and perforin.1,2 The neoplastic cells often have a high proliferation index as evidenced by MIB-1 (Ki-67) labeling (Figure 2D). The neoplastic cells are negative for CD4, CD56, and CD30.1,2 Subcutaneous panniculitislike T-cell lymphoma cells are negative for Epstein-Barr virus by in situ hybridization.2
 |
| Figure 1. Subcutaneous panniculitislike T-cell lymphoma showing a predominantly lobular panniculitis (H&E, original magnification ×20). |
|
| Figure 2. Rimming of adipocytes by hyperchromatic lymphocytes (A)(H&E, original magnification ×400). Arrowhead indicates a histiocyte (ie, beanbag cell) that has undergone cytophagocytosis of nuclear debris (B)(H&E, original magnification ×600). Immunohistochemistry with CD8 highlights the cells rimming the adipocytes (C)(original magnification ×600). Immunohistochemistry with MIB-1 shows an increased proliferative rate in the lymphocytes rimming the adipocytes (D)(original magnification ×600). |
Subcutaneous panniculitislike T-cell lymphoma must be distinguished from lupus erythematosus panniculitis (LEP) and other cutaneous lymphomas. Importantly, LEP and SPTL clinically may appear similar and are not mutually exclusive diagnoses.2 On histology, they may look similar, showing T cell aggregates and necrosis; however, thickening of the basement membrane, vacuolar change at the dermoepidermal junction, plasma cells, hyaline sclerosis, mucin deposition, a lymphocytic perivascular infiltrate, and nodular aggregates of B cells are more common in LEP (Figure 3).2,4 Additionally, in LEP the T cell aggregates typically will not have a high proliferative rate as evidenced by MIB-1.3
Additionally, other lobular panniculitides can be considered in the differential diagnosis, including erythema induratum (EI), α1-antitrypsin deficiency panniculitis (A1ATDP), and infectious panniculitis. Histologically, EI (Figure 4), also known as nodular vasculitis when not associated with Mycobacterium tuberculosis, has a lobular pattern of inflammation. Early in the disease process there are discrete collections of neutrophils; later, granulomas with histiocytes, giant cells, and foamy macrophages are seen.4 The reactive infiltrate of EI is more mixed than in SPTL, with small lymphocytes, plasma cells, and eosinophils. Leukocytoclastic vasculitis and extravascular caseous or fibrinoid necrosis also may be present.4,5 Substantial caseous necrosis may extend to the dermis and epidermis with EI. Importantly, EI lacks true tuberculoid granulomas and stains negative for acid-fast bacilli, as it is a reactive rather than a local infectious process, but a history of M tuberculosis exposure is common.4 α1-Antitrypsin deficiency panniculitis results from a deficiency of proteinase activity and can be distinguished from SPTL by a neutrophil-rich panniculitis (Figure 5) as well as the classic appearance of splaying of neutrophils between collagen bundles in the deep reticular dermis. Additionally, the panniculitis is characterized by focal areas of necrotic lobules and septa with an infiltrate of neutrophils and macrophages that abut areas of normal-appearing subcutaneous fat without infiltrate.6 Clinically, the A1ATDP lesions may have ulceration and express an oily substance from fat necrosis. Panniculitis with A1ATDP may precede liver and lung disease.4 Panniculitis from bacterial or fungal infection is more common in immunocompromised patients but should be considered when subcutaneous inflammation and/or necrosis is present. Depending on the responsible organism and the status of a patient’s immune system, infectious panniculitis can have variable presentations, including suppurative granulomas with mycobacterial organisms, a dermal focus of infection if the primary source is cutaneous, or a deeper reticular and subcuticular focus in the subcutaneous fat if the infectious panniculitis occurs from hematogenous spread.4 An example of an infectious panniculitis having more of a granulomatous pattern secondary to Cryptococcus can be seen in Figure 6. Ultimately, special stains to identify infectious organisms (eg, Gram, periodic acid–Schiff, Ziehl-Neelsen) can be ordered to aid in the diagnosis if a responsible organism is not visible on hematoxylin and eosin staining.
 |
Figure 4. Erythema induratum is characterized by a lobular panniculitis (A and B)(both H&E, original magnifications ×40 and ×200). Vascular changes (arrowhead) are present in a majority of cases with endothelial swelling and extravasation of erythrocytes (C)(H&E, original magnification ×400). |
 |
| Figure 5. Neutrophilic panniculitis that can be seen in α1-antitrypsin deficiency panniculitis (H&E, original magnification ×400). |
 |
Figure 6. Infectious panniculitis secondary to Cryptococcus showing a granulomatous reaction in the subcutis (A)(H&E, original magnification ×40). Closer inspection shows a dense infiltrate of chronic inflammatory cells including numerous histiocytes and multinucleated giant cells. Some of the giant cells contain refractile organisms (arrowhead)(B)(H&E, original magnification ×400). Mucicarmine histochemical stain highlights the capsule of the organism (C)(original magnification ×400). |
Acknowledgment
The authors would like to thank Drake Poeschl, MD, St. Louis, Missouri, for proofreading the manuscript.
Subcutaneous panniculitislike T-cell lymphoma (SPTL) is a cutaneous lymphoma of α and β phenotype cytotoxic T cells in which the neoplastic cells are found almost exclusively in the subcutaneous layer and resemble a panniculitis.1 It affects males and females with equal incidence and is seen in both adults and children. Clinically, this disease presents as a nonspecific panniculitis with indurated but typically nonulcerated erythematous plaques and nodules most commonly located on the extremities. Plaques and nodules may appear on other body sites and may be generalized.1 In some cases, patients present with associated systemic symptoms including fever, malaise, weight loss, and fatigue.2
Histologically, SPTL presents as a predominantly lobular panniculitis (Figure 1) with rimming of adipocytes by neoplastic cells that appear as small and medium-sized atypical lymphocytes with hyperchromatic nuclei (Figure 2A). A less dominant septal component may be present, and neoplastic cells may encroach into the lower reticular dermis, rarely involving the papillary dermis or epidermis.2 Although rimming of adipocytes is classic, it is not specific to this entity, as rimming also can be found in other lymphomas and infectious panniculitis. Reactive lymphocytes and macrophages with ingested lipid material also are seen intermixed with neoplastic cells.2 Necrosis is a common finding, including destructive fragmentation of the nucleus, known as karyorrhexis. If necrosis is extensive, appreciation of other histologic features may be hindered.3 Histiocytes engulfing the nuclear debris known as beanbag cells also can be seen (Figure 2B). The diagnosis can be made on immunohistologic analysis demonstrating neoplastic cells with a cytotoxic α and β T-suppressor phenotype centered around and rimming the adipocytes in the subcutaneous fat.3 Immunohistochemistry reveals positive CD3, CD8 (Figure 2C), and βF1 markers, as well as T-cell intracellular antigen 1 (TIA-1), granzyme B, and perforin.1,2 The neoplastic cells often have a high proliferation index as evidenced by MIB-1 (Ki-67) labeling (Figure 2D). The neoplastic cells are negative for CD4, CD56, and CD30.1,2 Subcutaneous panniculitislike T-cell lymphoma cells are negative for Epstein-Barr virus by in situ hybridization.2
 |
| Figure 1. Subcutaneous panniculitislike T-cell lymphoma showing a predominantly lobular panniculitis (H&E, original magnification ×20). |
|
| Figure 2. Rimming of adipocytes by hyperchromatic lymphocytes (A)(H&E, original magnification ×400). Arrowhead indicates a histiocyte (ie, beanbag cell) that has undergone cytophagocytosis of nuclear debris (B)(H&E, original magnification ×600). Immunohistochemistry with CD8 highlights the cells rimming the adipocytes (C)(original magnification ×600). Immunohistochemistry with MIB-1 shows an increased proliferative rate in the lymphocytes rimming the adipocytes (D)(original magnification ×600). |
Subcutaneous panniculitislike T-cell lymphoma must be distinguished from lupus erythematosus panniculitis (LEP) and other cutaneous lymphomas. Importantly, LEP and SPTL clinically may appear similar and are not mutually exclusive diagnoses.2 On histology, they may look similar, showing T cell aggregates and necrosis; however, thickening of the basement membrane, vacuolar change at the dermoepidermal junction, plasma cells, hyaline sclerosis, mucin deposition, a lymphocytic perivascular infiltrate, and nodular aggregates of B cells are more common in LEP (Figure 3).2,4 Additionally, in LEP the T cell aggregates typically will not have a high proliferative rate as evidenced by MIB-1.3
Additionally, other lobular panniculitides can be considered in the differential diagnosis, including erythema induratum (EI), α1-antitrypsin deficiency panniculitis (A1ATDP), and infectious panniculitis. Histologically, EI (Figure 4), also known as nodular vasculitis when not associated with Mycobacterium tuberculosis, has a lobular pattern of inflammation. Early in the disease process there are discrete collections of neutrophils; later, granulomas with histiocytes, giant cells, and foamy macrophages are seen.4 The reactive infiltrate of EI is more mixed than in SPTL, with small lymphocytes, plasma cells, and eosinophils. Leukocytoclastic vasculitis and extravascular caseous or fibrinoid necrosis also may be present.4,5 Substantial caseous necrosis may extend to the dermis and epidermis with EI. Importantly, EI lacks true tuberculoid granulomas and stains negative for acid-fast bacilli, as it is a reactive rather than a local infectious process, but a history of M tuberculosis exposure is common.4 α1-Antitrypsin deficiency panniculitis results from a deficiency of proteinase activity and can be distinguished from SPTL by a neutrophil-rich panniculitis (Figure 5) as well as the classic appearance of splaying of neutrophils between collagen bundles in the deep reticular dermis. Additionally, the panniculitis is characterized by focal areas of necrotic lobules and septa with an infiltrate of neutrophils and macrophages that abut areas of normal-appearing subcutaneous fat without infiltrate.6 Clinically, the A1ATDP lesions may have ulceration and express an oily substance from fat necrosis. Panniculitis with A1ATDP may precede liver and lung disease.4 Panniculitis from bacterial or fungal infection is more common in immunocompromised patients but should be considered when subcutaneous inflammation and/or necrosis is present. Depending on the responsible organism and the status of a patient’s immune system, infectious panniculitis can have variable presentations, including suppurative granulomas with mycobacterial organisms, a dermal focus of infection if the primary source is cutaneous, or a deeper reticular and subcuticular focus in the subcutaneous fat if the infectious panniculitis occurs from hematogenous spread.4 An example of an infectious panniculitis having more of a granulomatous pattern secondary to Cryptococcus can be seen in Figure 6. Ultimately, special stains to identify infectious organisms (eg, Gram, periodic acid–Schiff, Ziehl-Neelsen) can be ordered to aid in the diagnosis if a responsible organism is not visible on hematoxylin and eosin staining.
 |
Figure 4. Erythema induratum is characterized by a lobular panniculitis (A and B)(both H&E, original magnifications ×40 and ×200). Vascular changes (arrowhead) are present in a majority of cases with endothelial swelling and extravasation of erythrocytes (C)(H&E, original magnification ×400). |
 |
| Figure 5. Neutrophilic panniculitis that can be seen in α1-antitrypsin deficiency panniculitis (H&E, original magnification ×400). |
 |
Figure 6. Infectious panniculitis secondary to Cryptococcus showing a granulomatous reaction in the subcutis (A)(H&E, original magnification ×40). Closer inspection shows a dense infiltrate of chronic inflammatory cells including numerous histiocytes and multinucleated giant cells. Some of the giant cells contain refractile organisms (arrowhead)(B)(H&E, original magnification ×400). Mucicarmine histochemical stain highlights the capsule of the organism (C)(original magnification ×400). |
Acknowledgment
The authors would like to thank Drake Poeschl, MD, St. Louis, Missouri, for proofreading the manuscript.
1. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3765-3785.
2. Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111:838-845.
3. Cerroni L, Gatter K, Kerl H. Subcutaneous “panniculitis-like” T-cell lymphoma. In: Cerroni L, Gatter K, Kerl H. Skin Lymphoma: The Illustrated Guide. 3rd ed. Hoboken, NJ: Wiley-Blackwell Publishing; 2011:87-96.
4. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
5. Sharon V, Goodarzi H, Chambers CJ, et al. Erythema induratum of Bazin. Dermatol Online J. 2010;16:1.
6. Rajagopal R, Malik AK, Murthy PS, et al. Alpha-1 antitrypsin deficiency panniculitis. Indian J Dermatol Venereol Leprol. 2002;68:362-364.
1. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3765-3785.
2. Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group study of 83 cases. Blood. 2008;111:838-845.
3. Cerroni L, Gatter K, Kerl H. Subcutaneous “panniculitis-like” T-cell lymphoma. In: Cerroni L, Gatter K, Kerl H. Skin Lymphoma: The Illustrated Guide. 3rd ed. Hoboken, NJ: Wiley-Blackwell Publishing; 2011:87-96.
4. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
5. Sharon V, Goodarzi H, Chambers CJ, et al. Erythema induratum of Bazin. Dermatol Online J. 2010;16:1.
6. Rajagopal R, Malik AK, Murthy PS, et al. Alpha-1 antitrypsin deficiency panniculitis. Indian J Dermatol Venereol Leprol. 2002;68:362-364.
Yellowish Papulonodular Periorbital Eruption
The Diagnosis: Adult-Onset Xanthogranuloma
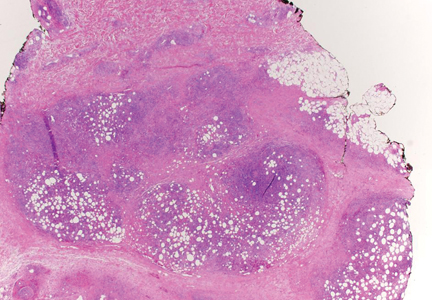
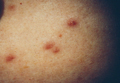
Biopsies of the lesions on the neck (Figure 1) and back were performed. Histologic analyses revealed a diffuse dermatitis consisting of foamy histiocytes admixed with a few Touton-type giant cells in the dermis (Figure 2), which was associated with an inflammatory infiltrate of eosinophils and lymphocytes. Laboratory investigations revealed mild thrombocytopenia with a platelet count of 134×109/L (reference range, 140–440×109/L). Other investigations including biochemistry, lipid, serum electrophoresis, and chest radiogram were normal. A bone marrow trephine biopsy and flow cytometry were performed and were normal. Magnetic resonance imaging revealed periorbital soft-tissue masses that did not extend into the orbits.
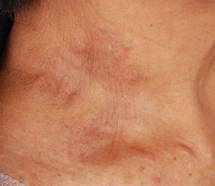 |
| Figure 1. Firm plaques with a yellowish tinge over the side of the neck |
 |
 |
| Figure 2. A dense infiltrate of foamy histiocytes in the dermis (A) associated with a Touton-type giant cell (arrow) and an inflammatory infiltrate consisting of eosinophils and lymphocytes (B)(both H&E, original magnifications ×200 and ×400). |
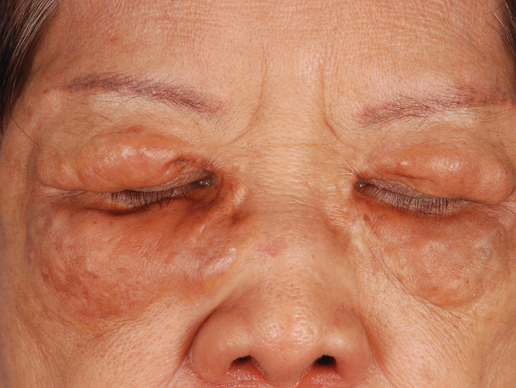
Adult-onset xanthogranuloma (AXG) is a rare disease entity, usually presenting in the third to fourth decades of life. The condition typically presents as a red to yellow-brown nodular cutaneous lesion located on the scalp, face, neck, trunk, or limbs. The presentation typically consists of a solitary lesion, occurring in 70% to 89% of cases,1 but more rarely, as in this case, lesions can be multiple or even disseminated.
Histologically, AXG presents as a dense, well-circumscribed, histiocytic infiltrate consisting of lipophages possessing foamy cytoplasm and giant cells. The presence of histiocytic giant cells differentiates AXG from xanthelasma, a clinical differential diagnosis in this case, and xanthoma. In AXG, there are 4 main types of histiocytes: xanthomatized, spindle shaped, vacuolated, and oncocytic.2 They can be seen in variable proportions, together with different types of giant cells (eg, Touton, foreign body, ground glass, nonspecific). A mixed infiltrate of eosinophils, lymphocytes, plasma cells, and neutrophils also may be seen scattered throughout the lesion.2
Correlating with the clinical and histological features of xanthogranuloma, the firm plaques and nodules represent the dense dermal infiltration of histiocytes that may extend into the subcutis. The lesions demonstrate a time-dependent progression both clinically and histologically. Early lesions are comprised of a dense monomorphous nonlipid histiocytic inflammatory infiltrate, and they clinically appear more erythematous. In mature lesions, as in our patient, the infiltrate is predominantly composed of lipid-laden histiocytes with associated Touton giant cells. They appear more yellowish on clinical presentation.
Adult-onset xanthogranuloma is part of a rare heterogenous group of disorders termed adult orbital xanthogranulomatous disease, which includes 3 other syndromes: necrobiotic xanthogranuloma, adult-onset asthma and periocular xanthogranuloma, and Erdheim-Chester disease.
Necrobiotic xanthogranuloma is clinically characterized by the presence of subcutaneous lesions that ulcerate in approximately 40% to 50% of cases and is histologically characterized by necrobiosis with palisading epithelioid histiocytes. It also is systemically associated with paraproteinemia and multiple myeloma.3
Adult-onset asthma and periocular xanthogranuloma is characterized by yellowish papules and nodules predominantly over the lower eyelids that are histologically comprised of lymphoid follicles with reactive germinal centers. It is associated with asthma, which normally is severe and often occurs almost simultaneously with the periorbital lesions.4
There are no definite diagnostic criteria for Erdheim-Chester disease and the diagnosis is usually based on radiologic findings of osteosclerosis and histopathologic evidence of foamy histiocytic infiltration. Systemic manifestations are common with lymphohistiocytic infiltration of the heart, lungs, pericardium, bones, and intestines. Prognosis is uniformly dismal.
Based on the clinical presentation of a nonulcerative papulonodular eruption and the absence of systemic involvement including asthma, we made the diagnosis of AXG. In view of the heterogeneity among these clinical entities as well as the time-based evolution of the lesions involved, we continued to monitor the patient for 2 years and there was no development of other systemic manifestations and hematologic abnormalities.
In contrast to the more common form of juvenile-onset xanthogranuloma, the adult type is not associated with widespread visceral lesions. Hence extensive screening investigations for systemic disease generally are not necessary. Another difference is that AXG has been associated with hematologic abnormalities, including essential thrombocytosis, chronic lymphocytic leukemia, large B-cell lymphoma, and monoclonal gammopathy.5,6 In our patient, the presence of thrombocytopenia and older age caused us to be concerned about an associated hematologic malignancy; therefore, a bone marrow biopsy and flow cytometry were performed.
Adult-onset xanthogranuloma typically is an asymptomatic and self-healing disease and therefore treatment usually is not required. Spontaneous regression of xanthogranuloma was observed to occur in 54% of cases with a median duration of 22 months,7 though lesions were noted to last as long as 15 years.8 When treatment is necessary, a combination of local and systemic corticosteroids, cytotoxic agents, and radiotherapy have been routinely used. In particular, intralesional corticosteroid therapy has been found to be efficacious in controlling symptoms and signs of AXG affecting the eyelids and orbits while avoiding the systemic side effects of other agents.9
Because our patient’s lesions were longstanding and disfiguring, we opted for active intervention with intralesional triamcinolone, which resulted in only a slight reduction in size of the lesions. The lesions remain largely unchanged in 2 years of follow-up.
1. Chang SE, Cho S, Choi JC, et al. Clinicohistopathologic comparison of adult type and juvenile type xanthogranulomas in Korea. J Dermatol. 2001;28:413-418.
2. Gelmetti C. Non-Langerhans cell histiocytosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
3. Mehregan DA, Winkelmann RK. Necrobiotic xanthogranuloma. Arch Dermatol. 1992;128:94-100.
4. Jakobiec FA, Mills MD, Hidayat AA, et al. Periocular xanthogranulomas associated with severe adult-onset asthma. Trans Am Ophthalmol Soc. 1993;91:99-125.
5. Shoo BA, Shinkai K, McCalmont TH, et al. Xanthogranulomas associated with hematologic malignancy in adulthood. J Am Acad Dermatol. 2008;59:488-493.
6. Chiou CC, Wang PN, Yang LC, et al. Disseminated xanthogranulomas associated with adult T-cell leukaemia/lymphoma: a case report and review the association of haematologic malignancies. J Eur Acad Dermatol Venereol. 2007;21:532-535.
7. Robinson HM, Harmon CE, Firminger HI. Multiple lipoidal histiocytomas with regression. Arch Dermatol. 1963;88:660-667.
8. Winkelmann RK. Cutaneous syndromes of non-X histiocytosis: a review of the macrophage-histiocyte diseases of the skin. Arch Dermatol. 1981;117:667-672.
9. Elner VM, Mintz R, Demirci H, et al. Local corticosteroid treatment of eyelid and orbital xanthogranuloma. Ophthal Plast Reconstr Surg. 2006;22:36-40.
The Diagnosis: Adult-Onset Xanthogranuloma
Biopsies of the lesions on the neck (Figure 1) and back were performed. Histologic analyses revealed a diffuse dermatitis consisting of foamy histiocytes admixed with a few Touton-type giant cells in the dermis (Figure 2), which was associated with an inflammatory infiltrate of eosinophils and lymphocytes. Laboratory investigations revealed mild thrombocytopenia with a platelet count of 134×109/L (reference range, 140–440×109/L). Other investigations including biochemistry, lipid, serum electrophoresis, and chest radiogram were normal. A bone marrow trephine biopsy and flow cytometry were performed and were normal. Magnetic resonance imaging revealed periorbital soft-tissue masses that did not extend into the orbits.
 |
| Figure 1. Firm plaques with a yellowish tinge over the side of the neck |
 |
 |
| Figure 2. A dense infiltrate of foamy histiocytes in the dermis (A) associated with a Touton-type giant cell (arrow) and an inflammatory infiltrate consisting of eosinophils and lymphocytes (B)(both H&E, original magnifications ×200 and ×400). |
Adult-onset xanthogranuloma (AXG) is a rare disease entity, usually presenting in the third to fourth decades of life. The condition typically presents as a red to yellow-brown nodular cutaneous lesion located on the scalp, face, neck, trunk, or limbs. The presentation typically consists of a solitary lesion, occurring in 70% to 89% of cases,1 but more rarely, as in this case, lesions can be multiple or even disseminated.
Histologically, AXG presents as a dense, well-circumscribed, histiocytic infiltrate consisting of lipophages possessing foamy cytoplasm and giant cells. The presence of histiocytic giant cells differentiates AXG from xanthelasma, a clinical differential diagnosis in this case, and xanthoma. In AXG, there are 4 main types of histiocytes: xanthomatized, spindle shaped, vacuolated, and oncocytic.2 They can be seen in variable proportions, together with different types of giant cells (eg, Touton, foreign body, ground glass, nonspecific). A mixed infiltrate of eosinophils, lymphocytes, plasma cells, and neutrophils also may be seen scattered throughout the lesion.2
Correlating with the clinical and histological features of xanthogranuloma, the firm plaques and nodules represent the dense dermal infiltration of histiocytes that may extend into the subcutis. The lesions demonstrate a time-dependent progression both clinically and histologically. Early lesions are comprised of a dense monomorphous nonlipid histiocytic inflammatory infiltrate, and they clinically appear more erythematous. In mature lesions, as in our patient, the infiltrate is predominantly composed of lipid-laden histiocytes with associated Touton giant cells. They appear more yellowish on clinical presentation.
Adult-onset xanthogranuloma is part of a rare heterogenous group of disorders termed adult orbital xanthogranulomatous disease, which includes 3 other syndromes: necrobiotic xanthogranuloma, adult-onset asthma and periocular xanthogranuloma, and Erdheim-Chester disease.
Necrobiotic xanthogranuloma is clinically characterized by the presence of subcutaneous lesions that ulcerate in approximately 40% to 50% of cases and is histologically characterized by necrobiosis with palisading epithelioid histiocytes. It also is systemically associated with paraproteinemia and multiple myeloma.3
Adult-onset asthma and periocular xanthogranuloma is characterized by yellowish papules and nodules predominantly over the lower eyelids that are histologically comprised of lymphoid follicles with reactive germinal centers. It is associated with asthma, which normally is severe and often occurs almost simultaneously with the periorbital lesions.4
There are no definite diagnostic criteria for Erdheim-Chester disease and the diagnosis is usually based on radiologic findings of osteosclerosis and histopathologic evidence of foamy histiocytic infiltration. Systemic manifestations are common with lymphohistiocytic infiltration of the heart, lungs, pericardium, bones, and intestines. Prognosis is uniformly dismal.
Based on the clinical presentation of a nonulcerative papulonodular eruption and the absence of systemic involvement including asthma, we made the diagnosis of AXG. In view of the heterogeneity among these clinical entities as well as the time-based evolution of the lesions involved, we continued to monitor the patient for 2 years and there was no development of other systemic manifestations and hematologic abnormalities.
In contrast to the more common form of juvenile-onset xanthogranuloma, the adult type is not associated with widespread visceral lesions. Hence extensive screening investigations for systemic disease generally are not necessary. Another difference is that AXG has been associated with hematologic abnormalities, including essential thrombocytosis, chronic lymphocytic leukemia, large B-cell lymphoma, and monoclonal gammopathy.5,6 In our patient, the presence of thrombocytopenia and older age caused us to be concerned about an associated hematologic malignancy; therefore, a bone marrow biopsy and flow cytometry were performed.
Adult-onset xanthogranuloma typically is an asymptomatic and self-healing disease and therefore treatment usually is not required. Spontaneous regression of xanthogranuloma was observed to occur in 54% of cases with a median duration of 22 months,7 though lesions were noted to last as long as 15 years.8 When treatment is necessary, a combination of local and systemic corticosteroids, cytotoxic agents, and radiotherapy have been routinely used. In particular, intralesional corticosteroid therapy has been found to be efficacious in controlling symptoms and signs of AXG affecting the eyelids and orbits while avoiding the systemic side effects of other agents.9
Because our patient’s lesions were longstanding and disfiguring, we opted for active intervention with intralesional triamcinolone, which resulted in only a slight reduction in size of the lesions. The lesions remain largely unchanged in 2 years of follow-up.
The Diagnosis: Adult-Onset Xanthogranuloma
Biopsies of the lesions on the neck (Figure 1) and back were performed. Histologic analyses revealed a diffuse dermatitis consisting of foamy histiocytes admixed with a few Touton-type giant cells in the dermis (Figure 2), which was associated with an inflammatory infiltrate of eosinophils and lymphocytes. Laboratory investigations revealed mild thrombocytopenia with a platelet count of 134×109/L (reference range, 140–440×109/L). Other investigations including biochemistry, lipid, serum electrophoresis, and chest radiogram were normal. A bone marrow trephine biopsy and flow cytometry were performed and were normal. Magnetic resonance imaging revealed periorbital soft-tissue masses that did not extend into the orbits.
 |
| Figure 1. Firm plaques with a yellowish tinge over the side of the neck |
 |
 |
| Figure 2. A dense infiltrate of foamy histiocytes in the dermis (A) associated with a Touton-type giant cell (arrow) and an inflammatory infiltrate consisting of eosinophils and lymphocytes (B)(both H&E, original magnifications ×200 and ×400). |
Adult-onset xanthogranuloma (AXG) is a rare disease entity, usually presenting in the third to fourth decades of life. The condition typically presents as a red to yellow-brown nodular cutaneous lesion located on the scalp, face, neck, trunk, or limbs. The presentation typically consists of a solitary lesion, occurring in 70% to 89% of cases,1 but more rarely, as in this case, lesions can be multiple or even disseminated.
Histologically, AXG presents as a dense, well-circumscribed, histiocytic infiltrate consisting of lipophages possessing foamy cytoplasm and giant cells. The presence of histiocytic giant cells differentiates AXG from xanthelasma, a clinical differential diagnosis in this case, and xanthoma. In AXG, there are 4 main types of histiocytes: xanthomatized, spindle shaped, vacuolated, and oncocytic.2 They can be seen in variable proportions, together with different types of giant cells (eg, Touton, foreign body, ground glass, nonspecific). A mixed infiltrate of eosinophils, lymphocytes, plasma cells, and neutrophils also may be seen scattered throughout the lesion.2
Correlating with the clinical and histological features of xanthogranuloma, the firm plaques and nodules represent the dense dermal infiltration of histiocytes that may extend into the subcutis. The lesions demonstrate a time-dependent progression both clinically and histologically. Early lesions are comprised of a dense monomorphous nonlipid histiocytic inflammatory infiltrate, and they clinically appear more erythematous. In mature lesions, as in our patient, the infiltrate is predominantly composed of lipid-laden histiocytes with associated Touton giant cells. They appear more yellowish on clinical presentation.
Adult-onset xanthogranuloma is part of a rare heterogenous group of disorders termed adult orbital xanthogranulomatous disease, which includes 3 other syndromes: necrobiotic xanthogranuloma, adult-onset asthma and periocular xanthogranuloma, and Erdheim-Chester disease.
Necrobiotic xanthogranuloma is clinically characterized by the presence of subcutaneous lesions that ulcerate in approximately 40% to 50% of cases and is histologically characterized by necrobiosis with palisading epithelioid histiocytes. It also is systemically associated with paraproteinemia and multiple myeloma.3
Adult-onset asthma and periocular xanthogranuloma is characterized by yellowish papules and nodules predominantly over the lower eyelids that are histologically comprised of lymphoid follicles with reactive germinal centers. It is associated with asthma, which normally is severe and often occurs almost simultaneously with the periorbital lesions.4
There are no definite diagnostic criteria for Erdheim-Chester disease and the diagnosis is usually based on radiologic findings of osteosclerosis and histopathologic evidence of foamy histiocytic infiltration. Systemic manifestations are common with lymphohistiocytic infiltration of the heart, lungs, pericardium, bones, and intestines. Prognosis is uniformly dismal.
Based on the clinical presentation of a nonulcerative papulonodular eruption and the absence of systemic involvement including asthma, we made the diagnosis of AXG. In view of the heterogeneity among these clinical entities as well as the time-based evolution of the lesions involved, we continued to monitor the patient for 2 years and there was no development of other systemic manifestations and hematologic abnormalities.
In contrast to the more common form of juvenile-onset xanthogranuloma, the adult type is not associated with widespread visceral lesions. Hence extensive screening investigations for systemic disease generally are not necessary. Another difference is that AXG has been associated with hematologic abnormalities, including essential thrombocytosis, chronic lymphocytic leukemia, large B-cell lymphoma, and monoclonal gammopathy.5,6 In our patient, the presence of thrombocytopenia and older age caused us to be concerned about an associated hematologic malignancy; therefore, a bone marrow biopsy and flow cytometry were performed.
Adult-onset xanthogranuloma typically is an asymptomatic and self-healing disease and therefore treatment usually is not required. Spontaneous regression of xanthogranuloma was observed to occur in 54% of cases with a median duration of 22 months,7 though lesions were noted to last as long as 15 years.8 When treatment is necessary, a combination of local and systemic corticosteroids, cytotoxic agents, and radiotherapy have been routinely used. In particular, intralesional corticosteroid therapy has been found to be efficacious in controlling symptoms and signs of AXG affecting the eyelids and orbits while avoiding the systemic side effects of other agents.9
Because our patient’s lesions were longstanding and disfiguring, we opted for active intervention with intralesional triamcinolone, which resulted in only a slight reduction in size of the lesions. The lesions remain largely unchanged in 2 years of follow-up.
1. Chang SE, Cho S, Choi JC, et al. Clinicohistopathologic comparison of adult type and juvenile type xanthogranulomas in Korea. J Dermatol. 2001;28:413-418.
2. Gelmetti C. Non-Langerhans cell histiocytosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
3. Mehregan DA, Winkelmann RK. Necrobiotic xanthogranuloma. Arch Dermatol. 1992;128:94-100.
4. Jakobiec FA, Mills MD, Hidayat AA, et al. Periocular xanthogranulomas associated with severe adult-onset asthma. Trans Am Ophthalmol Soc. 1993;91:99-125.
5. Shoo BA, Shinkai K, McCalmont TH, et al. Xanthogranulomas associated with hematologic malignancy in adulthood. J Am Acad Dermatol. 2008;59:488-493.
6. Chiou CC, Wang PN, Yang LC, et al. Disseminated xanthogranulomas associated with adult T-cell leukaemia/lymphoma: a case report and review the association of haematologic malignancies. J Eur Acad Dermatol Venereol. 2007;21:532-535.
7. Robinson HM, Harmon CE, Firminger HI. Multiple lipoidal histiocytomas with regression. Arch Dermatol. 1963;88:660-667.
8. Winkelmann RK. Cutaneous syndromes of non-X histiocytosis: a review of the macrophage-histiocyte diseases of the skin. Arch Dermatol. 1981;117:667-672.
9. Elner VM, Mintz R, Demirci H, et al. Local corticosteroid treatment of eyelid and orbital xanthogranuloma. Ophthal Plast Reconstr Surg. 2006;22:36-40.
1. Chang SE, Cho S, Choi JC, et al. Clinicohistopathologic comparison of adult type and juvenile type xanthogranulomas in Korea. J Dermatol. 2001;28:413-418.
2. Gelmetti C. Non-Langerhans cell histiocytosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
3. Mehregan DA, Winkelmann RK. Necrobiotic xanthogranuloma. Arch Dermatol. 1992;128:94-100.
4. Jakobiec FA, Mills MD, Hidayat AA, et al. Periocular xanthogranulomas associated with severe adult-onset asthma. Trans Am Ophthalmol Soc. 1993;91:99-125.
5. Shoo BA, Shinkai K, McCalmont TH, et al. Xanthogranulomas associated with hematologic malignancy in adulthood. J Am Acad Dermatol. 2008;59:488-493.
6. Chiou CC, Wang PN, Yang LC, et al. Disseminated xanthogranulomas associated with adult T-cell leukaemia/lymphoma: a case report and review the association of haematologic malignancies. J Eur Acad Dermatol Venereol. 2007;21:532-535.
7. Robinson HM, Harmon CE, Firminger HI. Multiple lipoidal histiocytomas with regression. Arch Dermatol. 1963;88:660-667.
8. Winkelmann RK. Cutaneous syndromes of non-X histiocytosis: a review of the macrophage-histiocyte diseases of the skin. Arch Dermatol. 1981;117:667-672.
9. Elner VM, Mintz R, Demirci H, et al. Local corticosteroid treatment of eyelid and orbital xanthogranuloma. Ophthal Plast Reconstr Surg. 2006;22:36-40.
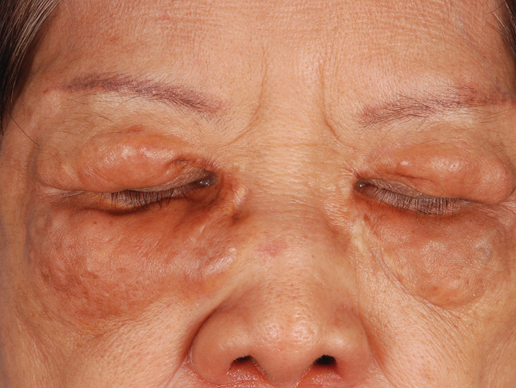
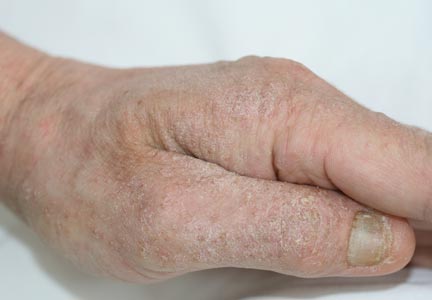
A 66-year-old woman with a history of type 2 diabetes mellitus and mild dyslipidemia presented with persistent lesions over the eyelids and cheeks of 10 years’ duration. Systemic review was unremarkable. There was no family or personal history of atopy, asthma, or other dermatologic disorders. Physical examination revealed confluent yellowish plaques and nodules over the periorbital regions as well as yellowish plaques over the neck and back. The lesions were firm to palpation and the epidermis appeared unaffected. The ophthalmic examination was normal and other mucosal surfaces were unaffected.
Glomus Tumor of Uncertain Malignant Potential on the Forehead
Glomus tumors (GTs) are uncommon benign tumors originating in the neuromyoarterial elements of the glomus body, an arteriovenous shunt specialized in thermoregulation.1 Glomus tumors usually occur in the distal extremities of young adults2 and rarely are seen in the deep soft tissue or viscera. Malignant GTs are rare and highly aggressive tumors that have been associated with both local recurrence and distant metastasis.1-11 Glomus tumors have been subdivided into 3 groups with different prognoses2: (1) malignant GT with metastatic potential (subfascial or visceral location, >2 cm in size, atypical mitotic figures, >5 mitoses per 50 high-power fields [HPFs], marked nuclear atypia); (2) symplastic GT (benign tumor with nuclear pleomorphism without mitotic activity); and (3) GT of uncertain malignant potential (GTUMP)(absence of metastatic disease, favorable prognosis, at least 1 feature of malignant GTs other than marked nuclear atypia [eg, high mitotic activity, >2 cm in size, deep location]).1,2
We report a case of GTUMP with unusual clinicopathologic features in a 74-year-old man that was treated via wide surgical excision. No local recurrence or distant metastasis was noted at 3-year follow-up.
Case Report
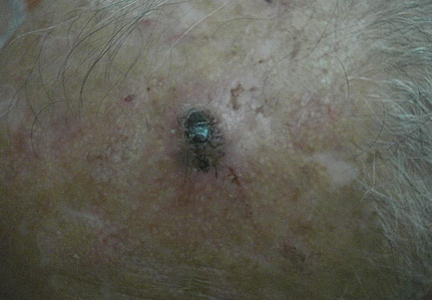
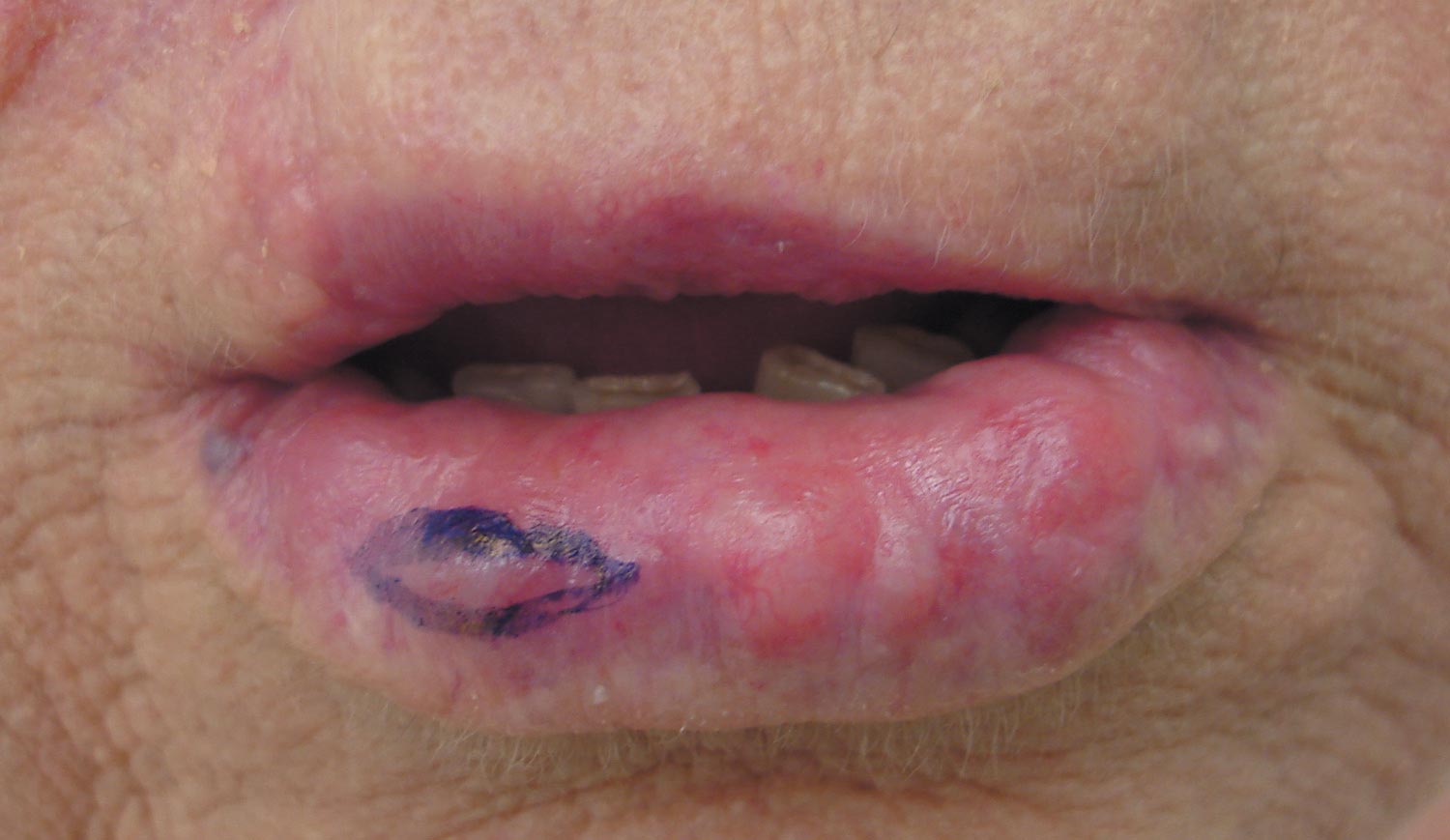
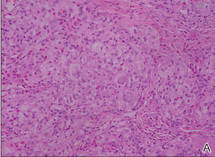
A 74-year-old man presented to the Unit of Surgery with a slowly progressing, painful, ulcerated, 2.5-cm, red-blue nodule on the forehead (Figure 1). An excisional biopsy of the nodule was performed. Histologically, the dermis and superficial subcutis were filled with a proliferation of atypical epithelioid cells to slightly spindled cells. Both cells displayed a weakly eosinophilic cytoplasm with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli. Neoplastic cells showed a disordered arrangement or were organized in short fascicles separated by slitlike spaces, vascular lumens of various sizes, or hemorrhagic stroma resembling angiosarcoma or Kaposi sarcoma (Figure 2). No areas of necrosis were noted. Pleomorphic nuclei and some mitotic figures also were identified, but they were not atypical and showed fewer than 5 mitoses per 50 HPFs. Immunohistochemically, the neoplastic cells stained positive for vimentin, caldesmon (Figure 3), and a–smooth muscle actin, and they stained negative for cytokeratins, desmin, CD34, factor VIII–related antigen, S-100 protein, and the latent nuclear antigen of Kaposi sarcoma–associated herpesvirus. The Ki-67 labeling index revealed less than 20% positive cells.
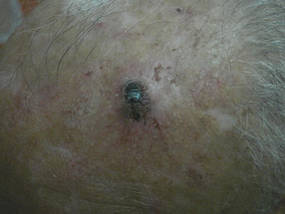 |
| Figure 1. A painful, red-blue, ulcerated nodule on the forehead of a 74-year-old man. |
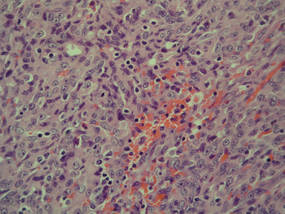 |
| Figure 2. The tumor was composed of atypical epithelioid cells to slightly spindled cells with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli in a disordered arrangement or rather in short fascicles separated in a hemorrhagic background (H&E, original magnification ×40). |
 |
| Figure 3. The neoplastic cells stained positive for caldesmon (original magnification ×20). |
 |
| Figure 4. A focus of round to polygonal tumor cells reminiscent of a preexisting benign-appearing glomus tumor was found on biopsy following reexcision (H&E, original magnification ×20). |
Because the tumor involved margins of excision, the patient successfully underwent wide reexcision with adequate margins. Histological examination of the reexcised specimen showed a focus of bland, round to polygonal tumor cells with the features of glomus cells (Figure 4). On the basis of the histologic features of both specimens, the lesion was classified as a GT. The biggest problem in our case was the classification of the lesion according to established pathologic criteria. We considered this case to be borderline because the lesion was greater than 2 cm but the location was superficial; marked atypia with sarcomatoid features also were present, but there was an absence of necrosis and fewer than 5 mitoses per 50 HPFs. For these reasons, we diagnosed this problematic lesion as a GTUMP. Following reexcision, the patient underwent strict follow-up. Wound healing was uncomplicated and the patient showed no local recurrence or distant metastasis at 3-year follow-up.
Comment
Clinically metastatic and histologically malignant GTs are exceptional.3-11 The classification system for GTs based on histologic criteria subdivided these tumors into 3 groups with different prognoses.2 Malignant GTs are highly aggressive tumors with metastatic potential, symplastic GTs are considered to be a degenerative phenomenon, and GTUMPs have a favorable clinical outcome and absence of metastatic disease.12
Diagnosis of malignant GTs and symplastic GTs is relatively easy in the presence of typical uniform, small, round epithelioid cells (glomus cells) located around blood vessels. Immunohistochemistry may be useful, as GTs express smooth muscle actin and caldesmon. Over the years the existence and diagnosis of malignant GT has been questioned because a residual component of benign GT in the surgical biopsy is useful in diagnosis but is not always present1-3 and because an unusual pattern may be present in malignant tumors with prevalent spindle cells resembling fibrosarcoma, leiomyosarcoma, spindle cell angiosarcoma, and spindle cell melanoma.1 In the absence of a preexisting GT, the differential diagnosis may be difficult; in such cases, a panel of immunohistochemical markers including smooth muscle actin, caldesmon, desmin, S-100, human melanoma black-45 (HMB-45), CD34, and CD31 is always necessary. The GTUMP category was introduced for GTs that demonstrate marked nuclear atypia but do not fulfill histologic criteria for malignancy. Along with other tumors of uncertain malignant potential, borderline cases should be considered GTUMPs to guarantee wide excision of the tumor with negative margins and an adequate follow-up due to the possibility of local recurrence or distant metastasis. In our patient, a diagnosis of GTUMP was made. Additionally, our case demonstrates some previously unreported features of GTUMPs, such as spindled cells in short fascicles separated by slitlike spaces, small vessels, and hemorrhagic stroma resembling Kaposi sarcoma. Along with these unusual sarcomatous features, the superficial location of the lesion, absence of necrosis, and a mitotic count of less than 5 per 50 HPFs were suggestive of an uncertain malignant potential for this tumor.
Distinction between malignant GTs and GTUMPs in the presence of unusual histologic features may be difficult.12 Glomus tumors that do not fulfill criteria for malignancy but have at least 1 atypical feature other than nuclear pleomorphism should be named GTUMPs. According to classification criteria, a true malignant GT is a highly aggressive tumor with metastatic potential. In a case series reported by Folpe et al,2 38% (20/52) of malignant GTs showed metastases, while metastatic disease was not observed in the tumors classified as GTUMPs. Wide surgical excision or Mohs micrographic surgery13 are the treatments of choice for malignant GTs and GTUMPs. Complete excision of the lesion with negative margins is always necessary in cases of GTUMPs. After the diagnosis of GTUMP, adequate follow-up should berecommended due to the possibility of local recurrence or distant metastasis.
Conclusion
Malignant GTs and GTUMPs are rare, and the nomenclature and classification of these tumors is controversial. These findings and the difficulty of differential diagnosis in a continuum between benignity and malignancy prompted our report.
1. Weiss SW, Goldblum JR. Perivascular tumors. In: Enzinger FM, Weiss SW, eds. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. St Louis, MO: Mosby; 2008:751-768.
2. Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12.
3. Aiba M, Hirayama A, Kuramochi S. Glomangiosarcoma in a glomus tumor. an immunohistochemical and ultrastructural study. Cancer. 1988;61:1467-1471.
4. Gould EW, Manivel JC, Albores-Saavedra J, et al. Locally infiltrative glomus tumors and glomangiosarcomas. a clinical, ultrastructural, and immunohistochemical study. Cancer. 1990;65:310-318.
5. Noer H, Krogdahl A. Glomangiosarcoma of the lower extremity. Histopathology. 1991;18:365-366.
6. Hiruta N, Kameda N, Tokudome T, et al. Malignant glomus tumor: a case report and review of the literature. Am J Surg Pathol. 1997;21:1096-1103.
7. Watanabe K, Sugino T, Saito A, et al. Glomangiosarcoma of the hip: report of a highly aggressive tumour with widespread distant metastases. Br J Dermatol. 1998;139:1097-1101.
8. Park JH, Oh SH, Yang MH, et al. Glomangiosarcoma of the hand: a case report and review of the literature. J Dermatol. 2003;30:827-833.
9. Kayal JD, Hampton RW, Sheehan DJ, et al. Malignant glomus tumor: a case report and review of the literature. Dermatol Surg. 2001;27:837-840.
10. Pérez de la Fuente T, Vega C, Gutierrez Palacios A, et al. Glomangiosarcoma of the hypothenar eminence: a case report. Chir Main. 2005;24:199-202.
11. Terada T, Fujimoto J, Shirakashi Y, et al. Malignant glomus tumor of the palm: a case report. J Cutan Pathol. 2011;38:381-384.
12. Gill J, Van Vliet C. Infiltrating glomus tumor of uncertain malignant potential arising in the kidney. Hum Pathol. 2010;41:145-149.
13. Cecchi R, Pavesi M, Apicella P. Malignant glomus tumor of the trunk treated with Mohs micrographic surgery [in English, German]. J Dtsch Dermatol Ges. 2011;9:391-392.
Glomus tumors (GTs) are uncommon benign tumors originating in the neuromyoarterial elements of the glomus body, an arteriovenous shunt specialized in thermoregulation.1 Glomus tumors usually occur in the distal extremities of young adults2 and rarely are seen in the deep soft tissue or viscera. Malignant GTs are rare and highly aggressive tumors that have been associated with both local recurrence and distant metastasis.1-11 Glomus tumors have been subdivided into 3 groups with different prognoses2: (1) malignant GT with metastatic potential (subfascial or visceral location, >2 cm in size, atypical mitotic figures, >5 mitoses per 50 high-power fields [HPFs], marked nuclear atypia); (2) symplastic GT (benign tumor with nuclear pleomorphism without mitotic activity); and (3) GT of uncertain malignant potential (GTUMP)(absence of metastatic disease, favorable prognosis, at least 1 feature of malignant GTs other than marked nuclear atypia [eg, high mitotic activity, >2 cm in size, deep location]).1,2
We report a case of GTUMP with unusual clinicopathologic features in a 74-year-old man that was treated via wide surgical excision. No local recurrence or distant metastasis was noted at 3-year follow-up.
Case Report
A 74-year-old man presented to the Unit of Surgery with a slowly progressing, painful, ulcerated, 2.5-cm, red-blue nodule on the forehead (Figure 1). An excisional biopsy of the nodule was performed. Histologically, the dermis and superficial subcutis were filled with a proliferation of atypical epithelioid cells to slightly spindled cells. Both cells displayed a weakly eosinophilic cytoplasm with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli. Neoplastic cells showed a disordered arrangement or were organized in short fascicles separated by slitlike spaces, vascular lumens of various sizes, or hemorrhagic stroma resembling angiosarcoma or Kaposi sarcoma (Figure 2). No areas of necrosis were noted. Pleomorphic nuclei and some mitotic figures also were identified, but they were not atypical and showed fewer than 5 mitoses per 50 HPFs. Immunohistochemically, the neoplastic cells stained positive for vimentin, caldesmon (Figure 3), and a–smooth muscle actin, and they stained negative for cytokeratins, desmin, CD34, factor VIII–related antigen, S-100 protein, and the latent nuclear antigen of Kaposi sarcoma–associated herpesvirus. The Ki-67 labeling index revealed less than 20% positive cells.
 |
| Figure 1. A painful, red-blue, ulcerated nodule on the forehead of a 74-year-old man. |
 |
| Figure 2. The tumor was composed of atypical epithelioid cells to slightly spindled cells with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli in a disordered arrangement or rather in short fascicles separated in a hemorrhagic background (H&E, original magnification ×40). |
 |
| Figure 3. The neoplastic cells stained positive for caldesmon (original magnification ×20). |
 |
| Figure 4. A focus of round to polygonal tumor cells reminiscent of a preexisting benign-appearing glomus tumor was found on biopsy following reexcision (H&E, original magnification ×20). |
Because the tumor involved margins of excision, the patient successfully underwent wide reexcision with adequate margins. Histological examination of the reexcised specimen showed a focus of bland, round to polygonal tumor cells with the features of glomus cells (Figure 4). On the basis of the histologic features of both specimens, the lesion was classified as a GT. The biggest problem in our case was the classification of the lesion according to established pathologic criteria. We considered this case to be borderline because the lesion was greater than 2 cm but the location was superficial; marked atypia with sarcomatoid features also were present, but there was an absence of necrosis and fewer than 5 mitoses per 50 HPFs. For these reasons, we diagnosed this problematic lesion as a GTUMP. Following reexcision, the patient underwent strict follow-up. Wound healing was uncomplicated and the patient showed no local recurrence or distant metastasis at 3-year follow-up.
Comment
Clinically metastatic and histologically malignant GTs are exceptional.3-11 The classification system for GTs based on histologic criteria subdivided these tumors into 3 groups with different prognoses.2 Malignant GTs are highly aggressive tumors with metastatic potential, symplastic GTs are considered to be a degenerative phenomenon, and GTUMPs have a favorable clinical outcome and absence of metastatic disease.12
Diagnosis of malignant GTs and symplastic GTs is relatively easy in the presence of typical uniform, small, round epithelioid cells (glomus cells) located around blood vessels. Immunohistochemistry may be useful, as GTs express smooth muscle actin and caldesmon. Over the years the existence and diagnosis of malignant GT has been questioned because a residual component of benign GT in the surgical biopsy is useful in diagnosis but is not always present1-3 and because an unusual pattern may be present in malignant tumors with prevalent spindle cells resembling fibrosarcoma, leiomyosarcoma, spindle cell angiosarcoma, and spindle cell melanoma.1 In the absence of a preexisting GT, the differential diagnosis may be difficult; in such cases, a panel of immunohistochemical markers including smooth muscle actin, caldesmon, desmin, S-100, human melanoma black-45 (HMB-45), CD34, and CD31 is always necessary. The GTUMP category was introduced for GTs that demonstrate marked nuclear atypia but do not fulfill histologic criteria for malignancy. Along with other tumors of uncertain malignant potential, borderline cases should be considered GTUMPs to guarantee wide excision of the tumor with negative margins and an adequate follow-up due to the possibility of local recurrence or distant metastasis. In our patient, a diagnosis of GTUMP was made. Additionally, our case demonstrates some previously unreported features of GTUMPs, such as spindled cells in short fascicles separated by slitlike spaces, small vessels, and hemorrhagic stroma resembling Kaposi sarcoma. Along with these unusual sarcomatous features, the superficial location of the lesion, absence of necrosis, and a mitotic count of less than 5 per 50 HPFs were suggestive of an uncertain malignant potential for this tumor.
Distinction between malignant GTs and GTUMPs in the presence of unusual histologic features may be difficult.12 Glomus tumors that do not fulfill criteria for malignancy but have at least 1 atypical feature other than nuclear pleomorphism should be named GTUMPs. According to classification criteria, a true malignant GT is a highly aggressive tumor with metastatic potential. In a case series reported by Folpe et al,2 38% (20/52) of malignant GTs showed metastases, while metastatic disease was not observed in the tumors classified as GTUMPs. Wide surgical excision or Mohs micrographic surgery13 are the treatments of choice for malignant GTs and GTUMPs. Complete excision of the lesion with negative margins is always necessary in cases of GTUMPs. After the diagnosis of GTUMP, adequate follow-up should berecommended due to the possibility of local recurrence or distant metastasis.
Conclusion
Malignant GTs and GTUMPs are rare, and the nomenclature and classification of these tumors is controversial. These findings and the difficulty of differential diagnosis in a continuum between benignity and malignancy prompted our report.
Glomus tumors (GTs) are uncommon benign tumors originating in the neuromyoarterial elements of the glomus body, an arteriovenous shunt specialized in thermoregulation.1 Glomus tumors usually occur in the distal extremities of young adults2 and rarely are seen in the deep soft tissue or viscera. Malignant GTs are rare and highly aggressive tumors that have been associated with both local recurrence and distant metastasis.1-11 Glomus tumors have been subdivided into 3 groups with different prognoses2: (1) malignant GT with metastatic potential (subfascial or visceral location, >2 cm in size, atypical mitotic figures, >5 mitoses per 50 high-power fields [HPFs], marked nuclear atypia); (2) symplastic GT (benign tumor with nuclear pleomorphism without mitotic activity); and (3) GT of uncertain malignant potential (GTUMP)(absence of metastatic disease, favorable prognosis, at least 1 feature of malignant GTs other than marked nuclear atypia [eg, high mitotic activity, >2 cm in size, deep location]).1,2
We report a case of GTUMP with unusual clinicopathologic features in a 74-year-old man that was treated via wide surgical excision. No local recurrence or distant metastasis was noted at 3-year follow-up.
Case Report
A 74-year-old man presented to the Unit of Surgery with a slowly progressing, painful, ulcerated, 2.5-cm, red-blue nodule on the forehead (Figure 1). An excisional biopsy of the nodule was performed. Histologically, the dermis and superficial subcutis were filled with a proliferation of atypical epithelioid cells to slightly spindled cells. Both cells displayed a weakly eosinophilic cytoplasm with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli. Neoplastic cells showed a disordered arrangement or were organized in short fascicles separated by slitlike spaces, vascular lumens of various sizes, or hemorrhagic stroma resembling angiosarcoma or Kaposi sarcoma (Figure 2). No areas of necrosis were noted. Pleomorphic nuclei and some mitotic figures also were identified, but they were not atypical and showed fewer than 5 mitoses per 50 HPFs. Immunohistochemically, the neoplastic cells stained positive for vimentin, caldesmon (Figure 3), and a–smooth muscle actin, and they stained negative for cytokeratins, desmin, CD34, factor VIII–related antigen, S-100 protein, and the latent nuclear antigen of Kaposi sarcoma–associated herpesvirus. The Ki-67 labeling index revealed less than 20% positive cells.
 |
| Figure 1. A painful, red-blue, ulcerated nodule on the forehead of a 74-year-old man. |
 |
| Figure 2. The tumor was composed of atypical epithelioid cells to slightly spindled cells with indistinct membranes and larger ovoid nuclei, some with prominent nucleoli in a disordered arrangement or rather in short fascicles separated in a hemorrhagic background (H&E, original magnification ×40). |
 |
| Figure 3. The neoplastic cells stained positive for caldesmon (original magnification ×20). |
 |
| Figure 4. A focus of round to polygonal tumor cells reminiscent of a preexisting benign-appearing glomus tumor was found on biopsy following reexcision (H&E, original magnification ×20). |
Because the tumor involved margins of excision, the patient successfully underwent wide reexcision with adequate margins. Histological examination of the reexcised specimen showed a focus of bland, round to polygonal tumor cells with the features of glomus cells (Figure 4). On the basis of the histologic features of both specimens, the lesion was classified as a GT. The biggest problem in our case was the classification of the lesion according to established pathologic criteria. We considered this case to be borderline because the lesion was greater than 2 cm but the location was superficial; marked atypia with sarcomatoid features also were present, but there was an absence of necrosis and fewer than 5 mitoses per 50 HPFs. For these reasons, we diagnosed this problematic lesion as a GTUMP. Following reexcision, the patient underwent strict follow-up. Wound healing was uncomplicated and the patient showed no local recurrence or distant metastasis at 3-year follow-up.
Comment
Clinically metastatic and histologically malignant GTs are exceptional.3-11 The classification system for GTs based on histologic criteria subdivided these tumors into 3 groups with different prognoses.2 Malignant GTs are highly aggressive tumors with metastatic potential, symplastic GTs are considered to be a degenerative phenomenon, and GTUMPs have a favorable clinical outcome and absence of metastatic disease.12
Diagnosis of malignant GTs and symplastic GTs is relatively easy in the presence of typical uniform, small, round epithelioid cells (glomus cells) located around blood vessels. Immunohistochemistry may be useful, as GTs express smooth muscle actin and caldesmon. Over the years the existence and diagnosis of malignant GT has been questioned because a residual component of benign GT in the surgical biopsy is useful in diagnosis but is not always present1-3 and because an unusual pattern may be present in malignant tumors with prevalent spindle cells resembling fibrosarcoma, leiomyosarcoma, spindle cell angiosarcoma, and spindle cell melanoma.1 In the absence of a preexisting GT, the differential diagnosis may be difficult; in such cases, a panel of immunohistochemical markers including smooth muscle actin, caldesmon, desmin, S-100, human melanoma black-45 (HMB-45), CD34, and CD31 is always necessary. The GTUMP category was introduced for GTs that demonstrate marked nuclear atypia but do not fulfill histologic criteria for malignancy. Along with other tumors of uncertain malignant potential, borderline cases should be considered GTUMPs to guarantee wide excision of the tumor with negative margins and an adequate follow-up due to the possibility of local recurrence or distant metastasis. In our patient, a diagnosis of GTUMP was made. Additionally, our case demonstrates some previously unreported features of GTUMPs, such as spindled cells in short fascicles separated by slitlike spaces, small vessels, and hemorrhagic stroma resembling Kaposi sarcoma. Along with these unusual sarcomatous features, the superficial location of the lesion, absence of necrosis, and a mitotic count of less than 5 per 50 HPFs were suggestive of an uncertain malignant potential for this tumor.
Distinction between malignant GTs and GTUMPs in the presence of unusual histologic features may be difficult.12 Glomus tumors that do not fulfill criteria for malignancy but have at least 1 atypical feature other than nuclear pleomorphism should be named GTUMPs. According to classification criteria, a true malignant GT is a highly aggressive tumor with metastatic potential. In a case series reported by Folpe et al,2 38% (20/52) of malignant GTs showed metastases, while metastatic disease was not observed in the tumors classified as GTUMPs. Wide surgical excision or Mohs micrographic surgery13 are the treatments of choice for malignant GTs and GTUMPs. Complete excision of the lesion with negative margins is always necessary in cases of GTUMPs. After the diagnosis of GTUMP, adequate follow-up should berecommended due to the possibility of local recurrence or distant metastasis.
Conclusion
Malignant GTs and GTUMPs are rare, and the nomenclature and classification of these tumors is controversial. These findings and the difficulty of differential diagnosis in a continuum between benignity and malignancy prompted our report.
1. Weiss SW, Goldblum JR. Perivascular tumors. In: Enzinger FM, Weiss SW, eds. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. St Louis, MO: Mosby; 2008:751-768.
2. Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12.
3. Aiba M, Hirayama A, Kuramochi S. Glomangiosarcoma in a glomus tumor. an immunohistochemical and ultrastructural study. Cancer. 1988;61:1467-1471.
4. Gould EW, Manivel JC, Albores-Saavedra J, et al. Locally infiltrative glomus tumors and glomangiosarcomas. a clinical, ultrastructural, and immunohistochemical study. Cancer. 1990;65:310-318.
5. Noer H, Krogdahl A. Glomangiosarcoma of the lower extremity. Histopathology. 1991;18:365-366.
6. Hiruta N, Kameda N, Tokudome T, et al. Malignant glomus tumor: a case report and review of the literature. Am J Surg Pathol. 1997;21:1096-1103.
7. Watanabe K, Sugino T, Saito A, et al. Glomangiosarcoma of the hip: report of a highly aggressive tumour with widespread distant metastases. Br J Dermatol. 1998;139:1097-1101.
8. Park JH, Oh SH, Yang MH, et al. Glomangiosarcoma of the hand: a case report and review of the literature. J Dermatol. 2003;30:827-833.
9. Kayal JD, Hampton RW, Sheehan DJ, et al. Malignant glomus tumor: a case report and review of the literature. Dermatol Surg. 2001;27:837-840.
10. Pérez de la Fuente T, Vega C, Gutierrez Palacios A, et al. Glomangiosarcoma of the hypothenar eminence: a case report. Chir Main. 2005;24:199-202.
11. Terada T, Fujimoto J, Shirakashi Y, et al. Malignant glomus tumor of the palm: a case report. J Cutan Pathol. 2011;38:381-384.
12. Gill J, Van Vliet C. Infiltrating glomus tumor of uncertain malignant potential arising in the kidney. Hum Pathol. 2010;41:145-149.
13. Cecchi R, Pavesi M, Apicella P. Malignant glomus tumor of the trunk treated with Mohs micrographic surgery [in English, German]. J Dtsch Dermatol Ges. 2011;9:391-392.
1. Weiss SW, Goldblum JR. Perivascular tumors. In: Enzinger FM, Weiss SW, eds. Enzinger and Weiss’s Soft Tissue Tumors. 5th ed. St Louis, MO: Mosby; 2008:751-768.
2. Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12.
3. Aiba M, Hirayama A, Kuramochi S. Glomangiosarcoma in a glomus tumor. an immunohistochemical and ultrastructural study. Cancer. 1988;61:1467-1471.
4. Gould EW, Manivel JC, Albores-Saavedra J, et al. Locally infiltrative glomus tumors and glomangiosarcomas. a clinical, ultrastructural, and immunohistochemical study. Cancer. 1990;65:310-318.
5. Noer H, Krogdahl A. Glomangiosarcoma of the lower extremity. Histopathology. 1991;18:365-366.
6. Hiruta N, Kameda N, Tokudome T, et al. Malignant glomus tumor: a case report and review of the literature. Am J Surg Pathol. 1997;21:1096-1103.
7. Watanabe K, Sugino T, Saito A, et al. Glomangiosarcoma of the hip: report of a highly aggressive tumour with widespread distant metastases. Br J Dermatol. 1998;139:1097-1101.
8. Park JH, Oh SH, Yang MH, et al. Glomangiosarcoma of the hand: a case report and review of the literature. J Dermatol. 2003;30:827-833.
9. Kayal JD, Hampton RW, Sheehan DJ, et al. Malignant glomus tumor: a case report and review of the literature. Dermatol Surg. 2001;27:837-840.
10. Pérez de la Fuente T, Vega C, Gutierrez Palacios A, et al. Glomangiosarcoma of the hypothenar eminence: a case report. Chir Main. 2005;24:199-202.
11. Terada T, Fujimoto J, Shirakashi Y, et al. Malignant glomus tumor of the palm: a case report. J Cutan Pathol. 2011;38:381-384.
12. Gill J, Van Vliet C. Infiltrating glomus tumor of uncertain malignant potential arising in the kidney. Hum Pathol. 2010;41:145-149.
13. Cecchi R, Pavesi M, Apicella P. Malignant glomus tumor of the trunk treated with Mohs micrographic surgery [in English, German]. J Dtsch Dermatol Ges. 2011;9:391-392.
Practice Points
- Glomus tumors have been subdivided into 3 groups with different prognoses.
- The term glomus tumor of uncertain malignant potential (GTUMP) was introduced to describe glomus tumors that demonstrate marked nuclear atypia but do not fulfill histologic criteria for malignancy.
- Complete excision with negative margins is always necessary in cases of GTUMPs.
Adult-Type Langerhans Cell Histiocytosis: Minimal Treatment for Maximal Results
To the Editor:
A 78-year-old man presented with erythematous circular skin papules that were widely scattered over the trunk. He denied recent contact with ill individuals and denied any systemic symptoms indicating internal involvement or malignancy leading to possible paraneoplastic presentation. Physical examination showed erythematous, circular, slightly elevated plaques of varying sizes scattered over the trunk (Figure 1) and right axilla.
 |
| Figure 1. Nontender, erythematous, brown nodules scattered over the trunk. |
 |
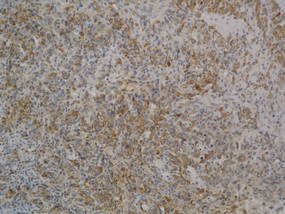
| Figure 2. Light microscopy revealed Langerhans cells filling the superficial dermis, abutting the epidermis, and extending into the deep dermis with surrounding inflammatory infiltrates (CD1a, original magnification ×100). |
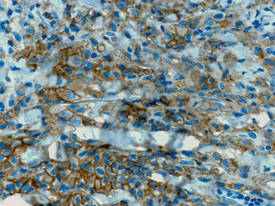 |
| Figure 3. Langerhans cells appeared strongly positive for CD1a (original magnification ×400). |
Biopsies of lesions were taken and stained with immunoperoxidase. On light microscopy there was a reticular and papillary dermal dense infiltrate of cells with indented nuclei (Figure 2). At higher magnification, cells appeared strongly positive for CD1a (Figure 3) and S-100 protein, which was histologically consistent with adult-type Langerhans cell histiocytosis (ALCH).
Computed tomography of the head, chest, abdomen, and pelvis were ordered to rule out spread of ALCH to other organ sites. Results were clear of evidence of systemic spread. Additionally, a complete blood cell count and comprehensive metabolic panel were within reference range.
He was started on topical tacrolimus; however, most of the lesions resolved on their own. As a result, tacrolimus was discontinued due to its propensity to cause skin irritation and lack of change in disease progression. At 3-month follow-up, he was prescribed triamcinolone acetonide cream 0.1% for minor outbreaks. After 2 years, he was completely clear of all skin signs of ALCH.
Adult-type Langerhans cell histiocytosis is characterized as a group of disorders associated with abnormal spread and proliferation of dendritic cells of the epidermis. The disease primarily affects children aged 1 to 4 years. It is estimated that only 1 to 2 cases of ALCH per million occur.1 The pathophysiology of ALCH is unknown; it is speculated that it may be associated with a reactive inflammatory process triggered by proliferation of Langerhans-type dendritic cells. It is possible that the release of multiple cytokines by dendritic cells and T cells in ALCH lesions leads to erythematous eruptions and can contribute to spontaneous remission of the disorder.2 Various cases of ALCH have reported high serum levels of IL-17 and IL-10 proinflammatory cytokines, supporting the theory of an inflammatory etiology of ALCH.3
Comparative genomic hybridization with loss of heterozygosity of pulmonary lesions has provided further evidence to suggest that chromosomal aberrations also may contribute to the pathophysiology of ALCH.4 One study evaluated 14 cases of pulmonary ALCH for loss of heterozygosity and found allelic loss of 1p, 1q, 3p, 5p, 17p, and 22q.5 In addition, allelic loss of 1 or more tumor suppressor genes was identified in 19 of 24 specimens, suggesting a neoplastic type of pathology through uncontrolled cellular proliferation.6
Lesions of ALCH can be broad but typically present as red-brown maculopapular lesions with petechiae that erupt over the trunk, axilla, and perivulvar or retroauricular regions.7 The papules may unify to form an erythematous, weeping, or crusted eruption that appears similar to seborrheic dermatitis. Typically the lesions remit on their own; however, lesions can recur with the same or decreased severity as the primary eruption. Complications have been noted with lesions, particularly secondary infection and ulceration.7
Systemic involvement has been noted in adults, particularly in the lungs. Patients typically present with chronic cough, dyspnea, and chest pain with evidence of a solitary nodular lesion on radiologic testing. In addition, bone involvement has been noted as eosinophilic granulomas that can produce osteolytic lesions that lead to spontaneous fractures. Use of corticosteroids and immunosuppressive agents, as opposed to just observation, is warranted in cases of systemic involvement, according to the National Cancer Institute.7
Exact treatment modalities have not yet been elucidated due to the ambiguity of pathogenesis. In addition, ALCH is known to remit and relapse in patients, which increases the difficulty in evaluating the efficacy of particular treatments. Trials conducted by the Histiocyte Society have shown that treatment regimens should be tailored to disease severity. Epidermal involvement of ALCH typically responds to corticosteroid creams, whereas patients with systemic involvement respond well to strong chemotherapeutic agents such as vincristine and prednisone with mercaptopurine.8 However, as demonstrated in our case, lesions may remit on their own and use of corticosteroids and immunosuppressive agents may lead to further detriment without treating disease progression.
Because of a low prevalence among adults, ALCH is difficult to recognize and diagnose, and the uncertainty of the pathogenesis of ALCH limits treatment alternatives. Further study into proper treatment modalities is warranted given that the remitting and relapsing course of the disease and cosmetic quandaries are detrimental to patient well-being. Our case illustrates that it is appropriate to simply monitor lesions for cases limited to cutaneous involvement. Systemic agents may be used when there are signs of organ involvement outside the skin, but providers must proceed to do so with caution.
1. Baumgartner I, von Hochstetter A, Baumert B, et al. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28:9-14.
2. Egeler RM, Favara BE, van Meurs M, et al. Differential in situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood. 1999;94:4195-4201.
3. da Costa CE, Szuhai K, van Eijk R, et al. No genomic aberrations in Langerhans cell histiocytosis as assessed by diverse molecular technologies. Genes Chromosomes Cancer. 2009;48:239-249.
4. Murakami I, Gogusev J, Fournet JC, et al. Detection of molecular cytogenetic aberrations in Langerhans cell histiocytosis of bone. Hum Pathol. 2002;33:555-560.
5. Dacic S, Trusky C, Bakker A, et al. Genotypic analysis of pulmonary Langerhans cell histiocytosis. Hum Pathol. 2003;34:1345-1349.
6. Chikwava KR, Hunt JL, Mantha GS, et al. Analysis of loss of heterozygosity in single-system and multisystem Langerhans’ cell histiocytosis. Pediatr Dev Pathol. 2007;10:18-24.
7. Langerhans cell histiocytosis treatment. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/pdq/treatment/lchistio/HealthProfessional/page5. Updated June 4, 2014. Accessed August 27, 2014.
8. Weitzman S, Wayne AS, Arceci R, et al. Nucleoside analogues in the therapy of Langerhans cell histiocytosis: a survey of members of the histiocyte society and review of the literature. Med Pediatr Oncol. 1999;33:476-481.
To the Editor:
A 78-year-old man presented with erythematous circular skin papules that were widely scattered over the trunk. He denied recent contact with ill individuals and denied any systemic symptoms indicating internal involvement or malignancy leading to possible paraneoplastic presentation. Physical examination showed erythematous, circular, slightly elevated plaques of varying sizes scattered over the trunk (Figure 1) and right axilla.
 |
| Figure 1. Nontender, erythematous, brown nodules scattered over the trunk. |
 |
| Figure 2. Light microscopy revealed Langerhans cells filling the superficial dermis, abutting the epidermis, and extending into the deep dermis with surrounding inflammatory infiltrates (CD1a, original magnification ×100). |
 |
| Figure 3. Langerhans cells appeared strongly positive for CD1a (original magnification ×400). |
Biopsies of lesions were taken and stained with immunoperoxidase. On light microscopy there was a reticular and papillary dermal dense infiltrate of cells with indented nuclei (Figure 2). At higher magnification, cells appeared strongly positive for CD1a (Figure 3) and S-100 protein, which was histologically consistent with adult-type Langerhans cell histiocytosis (ALCH).
Computed tomography of the head, chest, abdomen, and pelvis were ordered to rule out spread of ALCH to other organ sites. Results were clear of evidence of systemic spread. Additionally, a complete blood cell count and comprehensive metabolic panel were within reference range.
He was started on topical tacrolimus; however, most of the lesions resolved on their own. As a result, tacrolimus was discontinued due to its propensity to cause skin irritation and lack of change in disease progression. At 3-month follow-up, he was prescribed triamcinolone acetonide cream 0.1% for minor outbreaks. After 2 years, he was completely clear of all skin signs of ALCH.
Adult-type Langerhans cell histiocytosis is characterized as a group of disorders associated with abnormal spread and proliferation of dendritic cells of the epidermis. The disease primarily affects children aged 1 to 4 years. It is estimated that only 1 to 2 cases of ALCH per million occur.1 The pathophysiology of ALCH is unknown; it is speculated that it may be associated with a reactive inflammatory process triggered by proliferation of Langerhans-type dendritic cells. It is possible that the release of multiple cytokines by dendritic cells and T cells in ALCH lesions leads to erythematous eruptions and can contribute to spontaneous remission of the disorder.2 Various cases of ALCH have reported high serum levels of IL-17 and IL-10 proinflammatory cytokines, supporting the theory of an inflammatory etiology of ALCH.3
Comparative genomic hybridization with loss of heterozygosity of pulmonary lesions has provided further evidence to suggest that chromosomal aberrations also may contribute to the pathophysiology of ALCH.4 One study evaluated 14 cases of pulmonary ALCH for loss of heterozygosity and found allelic loss of 1p, 1q, 3p, 5p, 17p, and 22q.5 In addition, allelic loss of 1 or more tumor suppressor genes was identified in 19 of 24 specimens, suggesting a neoplastic type of pathology through uncontrolled cellular proliferation.6
Lesions of ALCH can be broad but typically present as red-brown maculopapular lesions with petechiae that erupt over the trunk, axilla, and perivulvar or retroauricular regions.7 The papules may unify to form an erythematous, weeping, or crusted eruption that appears similar to seborrheic dermatitis. Typically the lesions remit on their own; however, lesions can recur with the same or decreased severity as the primary eruption. Complications have been noted with lesions, particularly secondary infection and ulceration.7
Systemic involvement has been noted in adults, particularly in the lungs. Patients typically present with chronic cough, dyspnea, and chest pain with evidence of a solitary nodular lesion on radiologic testing. In addition, bone involvement has been noted as eosinophilic granulomas that can produce osteolytic lesions that lead to spontaneous fractures. Use of corticosteroids and immunosuppressive agents, as opposed to just observation, is warranted in cases of systemic involvement, according to the National Cancer Institute.7
Exact treatment modalities have not yet been elucidated due to the ambiguity of pathogenesis. In addition, ALCH is known to remit and relapse in patients, which increases the difficulty in evaluating the efficacy of particular treatments. Trials conducted by the Histiocyte Society have shown that treatment regimens should be tailored to disease severity. Epidermal involvement of ALCH typically responds to corticosteroid creams, whereas patients with systemic involvement respond well to strong chemotherapeutic agents such as vincristine and prednisone with mercaptopurine.8 However, as demonstrated in our case, lesions may remit on their own and use of corticosteroids and immunosuppressive agents may lead to further detriment without treating disease progression.
Because of a low prevalence among adults, ALCH is difficult to recognize and diagnose, and the uncertainty of the pathogenesis of ALCH limits treatment alternatives. Further study into proper treatment modalities is warranted given that the remitting and relapsing course of the disease and cosmetic quandaries are detrimental to patient well-being. Our case illustrates that it is appropriate to simply monitor lesions for cases limited to cutaneous involvement. Systemic agents may be used when there are signs of organ involvement outside the skin, but providers must proceed to do so with caution.
To the Editor:
A 78-year-old man presented with erythematous circular skin papules that were widely scattered over the trunk. He denied recent contact with ill individuals and denied any systemic symptoms indicating internal involvement or malignancy leading to possible paraneoplastic presentation. Physical examination showed erythematous, circular, slightly elevated plaques of varying sizes scattered over the trunk (Figure 1) and right axilla.
 |
| Figure 1. Nontender, erythematous, brown nodules scattered over the trunk. |
 |
| Figure 2. Light microscopy revealed Langerhans cells filling the superficial dermis, abutting the epidermis, and extending into the deep dermis with surrounding inflammatory infiltrates (CD1a, original magnification ×100). |
 |
| Figure 3. Langerhans cells appeared strongly positive for CD1a (original magnification ×400). |
Biopsies of lesions were taken and stained with immunoperoxidase. On light microscopy there was a reticular and papillary dermal dense infiltrate of cells with indented nuclei (Figure 2). At higher magnification, cells appeared strongly positive for CD1a (Figure 3) and S-100 protein, which was histologically consistent with adult-type Langerhans cell histiocytosis (ALCH).
Computed tomography of the head, chest, abdomen, and pelvis were ordered to rule out spread of ALCH to other organ sites. Results were clear of evidence of systemic spread. Additionally, a complete blood cell count and comprehensive metabolic panel were within reference range.
He was started on topical tacrolimus; however, most of the lesions resolved on their own. As a result, tacrolimus was discontinued due to its propensity to cause skin irritation and lack of change in disease progression. At 3-month follow-up, he was prescribed triamcinolone acetonide cream 0.1% for minor outbreaks. After 2 years, he was completely clear of all skin signs of ALCH.
Adult-type Langerhans cell histiocytosis is characterized as a group of disorders associated with abnormal spread and proliferation of dendritic cells of the epidermis. The disease primarily affects children aged 1 to 4 years. It is estimated that only 1 to 2 cases of ALCH per million occur.1 The pathophysiology of ALCH is unknown; it is speculated that it may be associated with a reactive inflammatory process triggered by proliferation of Langerhans-type dendritic cells. It is possible that the release of multiple cytokines by dendritic cells and T cells in ALCH lesions leads to erythematous eruptions and can contribute to spontaneous remission of the disorder.2 Various cases of ALCH have reported high serum levels of IL-17 and IL-10 proinflammatory cytokines, supporting the theory of an inflammatory etiology of ALCH.3
Comparative genomic hybridization with loss of heterozygosity of pulmonary lesions has provided further evidence to suggest that chromosomal aberrations also may contribute to the pathophysiology of ALCH.4 One study evaluated 14 cases of pulmonary ALCH for loss of heterozygosity and found allelic loss of 1p, 1q, 3p, 5p, 17p, and 22q.5 In addition, allelic loss of 1 or more tumor suppressor genes was identified in 19 of 24 specimens, suggesting a neoplastic type of pathology through uncontrolled cellular proliferation.6
Lesions of ALCH can be broad but typically present as red-brown maculopapular lesions with petechiae that erupt over the trunk, axilla, and perivulvar or retroauricular regions.7 The papules may unify to form an erythematous, weeping, or crusted eruption that appears similar to seborrheic dermatitis. Typically the lesions remit on their own; however, lesions can recur with the same or decreased severity as the primary eruption. Complications have been noted with lesions, particularly secondary infection and ulceration.7
Systemic involvement has been noted in adults, particularly in the lungs. Patients typically present with chronic cough, dyspnea, and chest pain with evidence of a solitary nodular lesion on radiologic testing. In addition, bone involvement has been noted as eosinophilic granulomas that can produce osteolytic lesions that lead to spontaneous fractures. Use of corticosteroids and immunosuppressive agents, as opposed to just observation, is warranted in cases of systemic involvement, according to the National Cancer Institute.7
Exact treatment modalities have not yet been elucidated due to the ambiguity of pathogenesis. In addition, ALCH is known to remit and relapse in patients, which increases the difficulty in evaluating the efficacy of particular treatments. Trials conducted by the Histiocyte Society have shown that treatment regimens should be tailored to disease severity. Epidermal involvement of ALCH typically responds to corticosteroid creams, whereas patients with systemic involvement respond well to strong chemotherapeutic agents such as vincristine and prednisone with mercaptopurine.8 However, as demonstrated in our case, lesions may remit on their own and use of corticosteroids and immunosuppressive agents may lead to further detriment without treating disease progression.
Because of a low prevalence among adults, ALCH is difficult to recognize and diagnose, and the uncertainty of the pathogenesis of ALCH limits treatment alternatives. Further study into proper treatment modalities is warranted given that the remitting and relapsing course of the disease and cosmetic quandaries are detrimental to patient well-being. Our case illustrates that it is appropriate to simply monitor lesions for cases limited to cutaneous involvement. Systemic agents may be used when there are signs of organ involvement outside the skin, but providers must proceed to do so with caution.
1. Baumgartner I, von Hochstetter A, Baumert B, et al. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28:9-14.
2. Egeler RM, Favara BE, van Meurs M, et al. Differential in situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood. 1999;94:4195-4201.
3. da Costa CE, Szuhai K, van Eijk R, et al. No genomic aberrations in Langerhans cell histiocytosis as assessed by diverse molecular technologies. Genes Chromosomes Cancer. 2009;48:239-249.
4. Murakami I, Gogusev J, Fournet JC, et al. Detection of molecular cytogenetic aberrations in Langerhans cell histiocytosis of bone. Hum Pathol. 2002;33:555-560.
5. Dacic S, Trusky C, Bakker A, et al. Genotypic analysis of pulmonary Langerhans cell histiocytosis. Hum Pathol. 2003;34:1345-1349.
6. Chikwava KR, Hunt JL, Mantha GS, et al. Analysis of loss of heterozygosity in single-system and multisystem Langerhans’ cell histiocytosis. Pediatr Dev Pathol. 2007;10:18-24.
7. Langerhans cell histiocytosis treatment. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/pdq/treatment/lchistio/HealthProfessional/page5. Updated June 4, 2014. Accessed August 27, 2014.
8. Weitzman S, Wayne AS, Arceci R, et al. Nucleoside analogues in the therapy of Langerhans cell histiocytosis: a survey of members of the histiocyte society and review of the literature. Med Pediatr Oncol. 1999;33:476-481.
1. Baumgartner I, von Hochstetter A, Baumert B, et al. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28:9-14.
2. Egeler RM, Favara BE, van Meurs M, et al. Differential in situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood. 1999;94:4195-4201.
3. da Costa CE, Szuhai K, van Eijk R, et al. No genomic aberrations in Langerhans cell histiocytosis as assessed by diverse molecular technologies. Genes Chromosomes Cancer. 2009;48:239-249.
4. Murakami I, Gogusev J, Fournet JC, et al. Detection of molecular cytogenetic aberrations in Langerhans cell histiocytosis of bone. Hum Pathol. 2002;33:555-560.
5. Dacic S, Trusky C, Bakker A, et al. Genotypic analysis of pulmonary Langerhans cell histiocytosis. Hum Pathol. 2003;34:1345-1349.
6. Chikwava KR, Hunt JL, Mantha GS, et al. Analysis of loss of heterozygosity in single-system and multisystem Langerhans’ cell histiocytosis. Pediatr Dev Pathol. 2007;10:18-24.
7. Langerhans cell histiocytosis treatment. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/pdq/treatment/lchistio/HealthProfessional/page5. Updated June 4, 2014. Accessed August 27, 2014.
8. Weitzman S, Wayne AS, Arceci R, et al. Nucleoside analogues in the therapy of Langerhans cell histiocytosis: a survey of members of the histiocyte society and review of the literature. Med Pediatr Oncol. 1999;33:476-481.
Pretibial Myxedema
Pretibial myxedema (PM) is a cutaneous mucinosis associated with thyroid dysfunction. It most commonly presents in the setting of Graves disease and is seen less often in patients with hypothyroidism and euthyroidism.1 The anterior tibia is most frequently affected, but lesions also may present on the feet, thighs, and upper extremities. Physical examination generally demonstrates skin thickening, hyperkeratosis, hyperpigmentation, yellow-red discoloration, and hyperhidrosis. Classically, the term peau d’orange has been used to characterize these clinical features.1 Histologic examination of PM typically reveals marked deposition of mucin throughout the reticular dermis with sparing of the papillary dermis (Figure 1) and may be accompanied by overlying hyperkeratosis. Collagen fibers are splayed and appear decreased in density (Figure 2). Alcian blue, periodic acid–Schiff, colloidal iron, and toluidine blue staining can be used to highlight dermal mucin.
 | 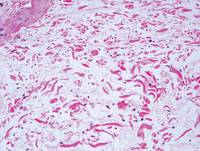 |
Figure 1. Prominent mucin deposition throughout the reticular dermis in pretibial myxedema (H&E, original magnification ×40). | Figure 2. Increased mucin deposition and collagen fiber splaying in pretibial myxedema (H&E, original magnification ×200). |
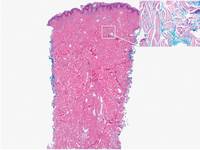 | 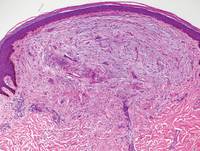 |
Figure 3. Scleredema with increased dermal thickness (H&E, original magnification ×20) and interstitial mucin on colloidal iron–stained sections (inset in upper right corner, original magnification ×400). | Figure 4. Scleromyxedema with mucin deposition primarily in the superficial dermis as well as increased cellularity and fibrosis (H&E, original magnification ×200). |
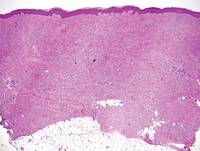 | 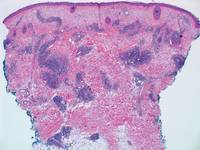 |
Figure 5. Nephrogenic systemic fibrosis with prominent mucinous fibrosis (H&E, original magnification ×40). | Figure 6. Tumid lupus erythematosus with a superficial and perivascular lymphoid infiltrate as well as increased dermal mucin (H&E, original magnification ×40). |
Similar to PM, histologic examination of scleredema and scleromyxedema usually demonstrate prominent mucin deposition. In scleredema, mucin primarily is visualized in the deep dermis between thick collagen bundles and typically is localized to the back (Figure 3). Scleromyxedema is distinguished by mucin deposition in the superficial dermis with associated fibroblast proliferation and fibrosis (Figure 4).
Nephrogenic systemic fibrosis is characterized by proliferation of CD34+ dermal spindle cells, fibroblasts, interstitial mucin, altered elastic fibers, and thickened collagen bundles that involve the dermis and subcutaneous septa (Figure 5).2 Tumid lupus erythematosus classically demonstrates perivascular and periadnexal superficial and deep lymphocytic inflammation (Figure 6). Similar to scleredema and PM, mucin deposition in tumid lupus erythematosus is interspersed between collagen bundles in the reticular dermis.3
1. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
2. Cowper SE, Lyndon DS, Bhawan J, et al. Nephro-genic fibrosing dermopathy. Am J Dermatopathol.2001;23:383-393.
3. Kuhn A, Dagmar RH, Oslislo C, et al. Lupus erythematosus tumidus: a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
Pretibial myxedema (PM) is a cutaneous mucinosis associated with thyroid dysfunction. It most commonly presents in the setting of Graves disease and is seen less often in patients with hypothyroidism and euthyroidism.1 The anterior tibia is most frequently affected, but lesions also may present on the feet, thighs, and upper extremities. Physical examination generally demonstrates skin thickening, hyperkeratosis, hyperpigmentation, yellow-red discoloration, and hyperhidrosis. Classically, the term peau d’orange has been used to characterize these clinical features.1 Histologic examination of PM typically reveals marked deposition of mucin throughout the reticular dermis with sparing of the papillary dermis (Figure 1) and may be accompanied by overlying hyperkeratosis. Collagen fibers are splayed and appear decreased in density (Figure 2). Alcian blue, periodic acid–Schiff, colloidal iron, and toluidine blue staining can be used to highlight dermal mucin.
 |  |
Figure 1. Prominent mucin deposition throughout the reticular dermis in pretibial myxedema (H&E, original magnification ×40). | Figure 2. Increased mucin deposition and collagen fiber splaying in pretibial myxedema (H&E, original magnification ×200). |
 |  |
Figure 3. Scleredema with increased dermal thickness (H&E, original magnification ×20) and interstitial mucin on colloidal iron–stained sections (inset in upper right corner, original magnification ×400). | Figure 4. Scleromyxedema with mucin deposition primarily in the superficial dermis as well as increased cellularity and fibrosis (H&E, original magnification ×200). |
 |  |
Figure 5. Nephrogenic systemic fibrosis with prominent mucinous fibrosis (H&E, original magnification ×40). | Figure 6. Tumid lupus erythematosus with a superficial and perivascular lymphoid infiltrate as well as increased dermal mucin (H&E, original magnification ×40). |
Similar to PM, histologic examination of scleredema and scleromyxedema usually demonstrate prominent mucin deposition. In scleredema, mucin primarily is visualized in the deep dermis between thick collagen bundles and typically is localized to the back (Figure 3). Scleromyxedema is distinguished by mucin deposition in the superficial dermis with associated fibroblast proliferation and fibrosis (Figure 4).
Nephrogenic systemic fibrosis is characterized by proliferation of CD34+ dermal spindle cells, fibroblasts, interstitial mucin, altered elastic fibers, and thickened collagen bundles that involve the dermis and subcutaneous septa (Figure 5).2 Tumid lupus erythematosus classically demonstrates perivascular and periadnexal superficial and deep lymphocytic inflammation (Figure 6). Similar to scleredema and PM, mucin deposition in tumid lupus erythematosus is interspersed between collagen bundles in the reticular dermis.3
Pretibial myxedema (PM) is a cutaneous mucinosis associated with thyroid dysfunction. It most commonly presents in the setting of Graves disease and is seen less often in patients with hypothyroidism and euthyroidism.1 The anterior tibia is most frequently affected, but lesions also may present on the feet, thighs, and upper extremities. Physical examination generally demonstrates skin thickening, hyperkeratosis, hyperpigmentation, yellow-red discoloration, and hyperhidrosis. Classically, the term peau d’orange has been used to characterize these clinical features.1 Histologic examination of PM typically reveals marked deposition of mucin throughout the reticular dermis with sparing of the papillary dermis (Figure 1) and may be accompanied by overlying hyperkeratosis. Collagen fibers are splayed and appear decreased in density (Figure 2). Alcian blue, periodic acid–Schiff, colloidal iron, and toluidine blue staining can be used to highlight dermal mucin.
 |  |
Figure 1. Prominent mucin deposition throughout the reticular dermis in pretibial myxedema (H&E, original magnification ×40). | Figure 2. Increased mucin deposition and collagen fiber splaying in pretibial myxedema (H&E, original magnification ×200). |
 |  |
Figure 3. Scleredema with increased dermal thickness (H&E, original magnification ×20) and interstitial mucin on colloidal iron–stained sections (inset in upper right corner, original magnification ×400). | Figure 4. Scleromyxedema with mucin deposition primarily in the superficial dermis as well as increased cellularity and fibrosis (H&E, original magnification ×200). |
 |  |
Figure 5. Nephrogenic systemic fibrosis with prominent mucinous fibrosis (H&E, original magnification ×40). | Figure 6. Tumid lupus erythematosus with a superficial and perivascular lymphoid infiltrate as well as increased dermal mucin (H&E, original magnification ×40). |
Similar to PM, histologic examination of scleredema and scleromyxedema usually demonstrate prominent mucin deposition. In scleredema, mucin primarily is visualized in the deep dermis between thick collagen bundles and typically is localized to the back (Figure 3). Scleromyxedema is distinguished by mucin deposition in the superficial dermis with associated fibroblast proliferation and fibrosis (Figure 4).
Nephrogenic systemic fibrosis is characterized by proliferation of CD34+ dermal spindle cells, fibroblasts, interstitial mucin, altered elastic fibers, and thickened collagen bundles that involve the dermis and subcutaneous septa (Figure 5).2 Tumid lupus erythematosus classically demonstrates perivascular and periadnexal superficial and deep lymphocytic inflammation (Figure 6). Similar to scleredema and PM, mucin deposition in tumid lupus erythematosus is interspersed between collagen bundles in the reticular dermis.3
1. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
2. Cowper SE, Lyndon DS, Bhawan J, et al. Nephro-genic fibrosing dermopathy. Am J Dermatopathol.2001;23:383-393.
3. Kuhn A, Dagmar RH, Oslislo C, et al. Lupus erythematosus tumidus: a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
1. Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
2. Cowper SE, Lyndon DS, Bhawan J, et al. Nephro-genic fibrosing dermopathy. Am J Dermatopathol.2001;23:383-393.
3. Kuhn A, Dagmar RH, Oslislo C, et al. Lupus erythematosus tumidus: a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
Buschke-Ollendorff Syndrome: Sparing Unnecessary Investigations
Buschke-Ollendorff syndrome (BOS) is a rare disease that is inherited in an autosomal-dominant fashion with high penetrance. It is characterized by osteopoikilosis associated with skin manifestations. The approximate incidence of the disease is 1:20,000, with few cases reported in the literature since 1928.1 Skeletal lesions known as osteopoikilosis are areas of increased bone density that can be seen on radiographic imaging and typically are located in the substantia spongiosa of the epiphyses and metaphyses of long bones and the pelvis. In BOS, cutaneous lesions consist of elastic or collagen nevi. Phenotypic expression of the disease is variable, and skeletal and cutaneous lesions may occur separately. Gene mutations of proteins involved in bone and connective tissue morphogenesis have been described in patients with BOS.2-5
Case Reports
Patient 1
A 17-year-old adolescent girl was referred to our hospital for evaluation of an incidental finding of osteopoikilosis that had been noted in the setting of a traumatic event. Clinical examination revealed cutaneous lesions characterized by asymptomatic, linear, stringlike and atrophic fibrotic plaques localized symmetrically on the trunk, right buttock, and right thigh (Figure 1). The lesions on the thigh were present at birth and had spread progressively to the other areas. There was no known history of inflammatory skin disease to explain the presence of the lesions, and no family history of similar signs or symptoms was reported. Histopathologic examination of a punch biopsy of a plaque from the trunk revealed increased collagen bundles associated with thick interlacing elastic fibers. The epidermis did not show any specific histologic alterations. These histopathologic features were diagnostic of a connective tissue nevus (Figure 2).
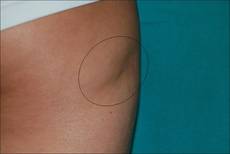 |
Figure 1. An asymptomatic atrophic fibrotic plaque localized on the right thigh. |
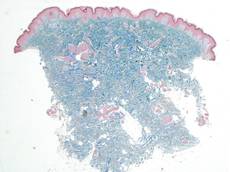 |
Figure 2. Low-power view of a punch biopsy showing a normal epidermis. Collagen bundles of the dermis were thickened and somewhat homogenized, as highlighted by the Masson stain (original magnification ×2.5). |
 |
Figure 3. Radiograph of the legs revealed numerous small, ovoid or round foci of sclerosis on the substantia spongiosa of the metaphyses and the epiphyses of the femur, tibia, and fibula on both sides of the body. |
 |
Figure 4. Multiple asymptomatic flesh-colored papules with elastic consistency on the back that were characteristic of dermatofibrosis lenticularis disseminata. |
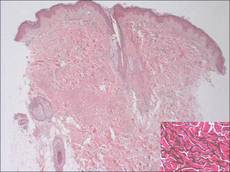 |
Figure 5. The epidermis and dermis appeared normal on histopathology; however, the elastic fibers were markedly increased in both size and number up to the deep dermis in the absence of degenerating changes (H&E, original magnification ×2.5; vascular endothelial growth factor, original magnification ×20 [inset in bottom right corner]). |
 |
| Figure 6. A radiograph of the left hand revealed small sporadic areas of sclerosis in the substantia spongiosa on the heads of some phalanges (white arrows). |
Radiographic imaging of the hands and feet revealed numerous small, ovoid or round foci of sclerosis that were a few millimeters in diameter and were occasionally confluent. This finding was prevalent on the carpal and tarsal bones but less evident on the phalanges and the epiphysis of the metatarsal and metacarpal bones. Full radiographic imaging of both arms and legs subsequently was obtained and showed similar lesions predominantly in the substantia spongiosa of the metaphyses and the epiphyses of the humerus, femur, tibia, and fibula bilaterally (Figure 3). Evaluation of the patient’s parents revealed that her mother had sporadic lenticular areas of increased bone density seen on radiography of the carpal and tarsal bones, particularly at the level of calcaneus, and the proximal and distal humeral epiphyses.
These lesions in our patient were consistent with an incomplete form of osteopoikilosis, which reflects the known variable expression of BOS. The radiologic findings in addition to the cutaneous lesions and the positive family history supported a diagnosis of BOS.
Patient 2
A 5-year-old boy presented with multiple congenital, asymptomatic, flesh-colored papules with elastic consistency on the left thigh, back, and pubic area that were characteristic of dermatofibrosis lenticularis disseminata (Figure 4). The patient’s 7-year-old sister had similar cutaneous lesions. The patient underwent a skin biopsy from the back that revealed thickening of collagen bundles in the dermis with an increase in elastic fibers (Figure 5). On radiographic imaging, small sporadic areas of sclerosis were noted in the substantia spongiosa of the lateral aspect of the humeral capitulum, the radial neck, the capitate, the head of the proximal phalanx of the fourth finger on the left hand, and the heads of the third and fifth middle phalanges on the left hand (Figure 6). Radiography revealed osteopoikilosis on both humeral heads in the patient’s mother. These findings in addition to the dermatologic and histopathologic features suggested a diagnosis of BOS.
Comment
In 1928, Buschke and Ollendorff1 first reported the association between osteopoikilosis and dermatofibrosis lenticularis disseminata, which showed autosomal-dominant inheritance. Skin and bone lesions can occur independently in different family members. Two different clinical cutaneous patterns are described in BOS.2-4 The first and most frequent form is characterized by yellowish nodules often grouped in plaques that are asymmetrically distributed, such as those seen in patient 1. The second form is known as dermatofibrosis lenticularis disseminata, which was seen in patient 2. Histologically, most lesions show normal collagen fibrils and an increased number of elastic fibers.
Osteopoikilosis, also known as spotted bone disease or osteopathia condensans, is a rare asymptomatic bone dysplasia of unknown etiology. It is characterized by an abnormality in the bone maturation process and often is found incidentally on radiologic examination, as seen in patient 1. Radiologic signs of osteopoikilosis consist of small, disseminated, well-circumscribed areas of increased radiodensity located in the epiphyses and metaphyses of long bones, as well as the pelvis, hands, and feet. These lesions, which typically are asymptomatic, could sometimes be associated with bone and/or joint pain but do not cause a predisposition to fractures. Documentation of these bone lesions by early adult life is important to avoid confusion with osteoblastic metastases on the skeleton.6
Buschke-Ollendorff syndrome is characterized by a variable expression. Loss-of-function mutations of the LEMD3 gene have been described in association with this disorder.7 This gene encodes an inner nuclear protein membrane with a C-terminal domain that binds SMAD2 and SMAD3 and antagonizes the bone morphogenetic proteins and transforming growth factor b. These proteins are involved in connective tissue morphogenesis, inducing elastin production from fibroblasts.7 Seven novel loss-of-function mutations of LEMD3 have been identified.8 The segmental manifestation of BOS could be a consequence of the mosaicism resulting from a somatic mutation.9,10 A case describing the absence of LEMD3 mutation in an affected family suggested the genetic heterogeneity of BOS.11
Conclusion
Buschke-Ollendorff syndrome is a benign condition with no repercussions on the patient’s health or quality of life. It does not require any specific treatment because the lesions generally remain asymptomatic and do not generate any substantial cosmetic burden. Mutation analysis was not performed in our patients because BOS has a good prognosis and the parents refused further investigation in both patients; therefore, a correct diagnosis was essential in our cases to rule out malignant bone disease in patient 1 whose osteopoikilosis prompted the workup and other disorders (eg, tuberous sclerosis, pseudoxanthoma elasticum) in patient 2 whose cutaneous lesions were the primary cause for presentation. A correct diagnosis of BOS is necessary to spare patients from expensive investigations and to provide reassurance about the benign nature of the disease.
1. Buschke A, Ollendorff H. Ein fall von dermatofibrosis lenticularis disseminata und osteopathia condensas disseminata. Derm Worchenschr. 1928;86:257-262.
2. Ramme K, Kolde G, Stadler R. Dermatofibrosis lenticularis disseminata with osteopoikilosis. Buschke-Ollendorff syndrome [in German]. Hautarzt. 1993;44:312-314.
3. Schena D, Germi L, Zamperetti MR, et al. Buschke-Ollendorff syndrome. Int J Dermatol. 2008;47:1159-1161.
4. Kawamura A, Ochiai T, Tan-Kinoshita M, et al. Buschke-Ollendorff syndrome: three generations in a Japanese family. Pediatr Dermatol. 2005;22:133-137.
5. Woodrow SL, Pope FM, Handfield-Jones SE. The Buschke-Ollendorff syndrome presenting as familial elastic tissue naevi. Br J Dermatol. 2001;144:890-893.
6. Whyte MP, Murphy WA, Siegel BA. 99mTc-pyrophosphate bone imaging in osteopoikilosis, osteopathia striata, and melorheostosis. Radiology. 1978;127:439-443.
7. Giro MG, Duvic M, Smith LT, et al. Buschke-Ollendorff syndrome associated with elevated elastin production by affected skin fibroblasts in culture. J Invest Dermatol. 1992;99:129-137.
8. Hellemans J, Preobrazhenska O, Willaert A, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis [published online ahead of print October 17, 2004]. Nat Genet. 2004;36:1213-1218.
9. Ehrig T, Cockerell CJ. Buschke-Ollendorff syndrome: report of a case and interpretation of the clinical phenotype as a type 2 segmental manifestation of an autosomal dominant skin disease. J Am Acad Dermatol. 2003;49:1163-1166.
10. Hellemans J, Debeer P, Wright M, et al. Germline LEMD3 mutations are rare in sporadic patients with isolated melorheostosis. Hum Mutat. 2006;27:290.
11. Yadegari M, Whyte MP, Mumm S, et al. Buschke-Ollendorff syndrome: absence of LEMD3 mutation in an affected family. Arch Dermatol. 2010;146:63-68.
Buschke-Ollendorff syndrome (BOS) is a rare disease that is inherited in an autosomal-dominant fashion with high penetrance. It is characterized by osteopoikilosis associated with skin manifestations. The approximate incidence of the disease is 1:20,000, with few cases reported in the literature since 1928.1 Skeletal lesions known as osteopoikilosis are areas of increased bone density that can be seen on radiographic imaging and typically are located in the substantia spongiosa of the epiphyses and metaphyses of long bones and the pelvis. In BOS, cutaneous lesions consist of elastic or collagen nevi. Phenotypic expression of the disease is variable, and skeletal and cutaneous lesions may occur separately. Gene mutations of proteins involved in bone and connective tissue morphogenesis have been described in patients with BOS.2-5
Case Reports
Patient 1
A 17-year-old adolescent girl was referred to our hospital for evaluation of an incidental finding of osteopoikilosis that had been noted in the setting of a traumatic event. Clinical examination revealed cutaneous lesions characterized by asymptomatic, linear, stringlike and atrophic fibrotic plaques localized symmetrically on the trunk, right buttock, and right thigh (Figure 1). The lesions on the thigh were present at birth and had spread progressively to the other areas. There was no known history of inflammatory skin disease to explain the presence of the lesions, and no family history of similar signs or symptoms was reported. Histopathologic examination of a punch biopsy of a plaque from the trunk revealed increased collagen bundles associated with thick interlacing elastic fibers. The epidermis did not show any specific histologic alterations. These histopathologic features were diagnostic of a connective tissue nevus (Figure 2).
 |
Figure 1. An asymptomatic atrophic fibrotic plaque localized on the right thigh. |
 |
Figure 2. Low-power view of a punch biopsy showing a normal epidermis. Collagen bundles of the dermis were thickened and somewhat homogenized, as highlighted by the Masson stain (original magnification ×2.5). |
 |
Figure 3. Radiograph of the legs revealed numerous small, ovoid or round foci of sclerosis on the substantia spongiosa of the metaphyses and the epiphyses of the femur, tibia, and fibula on both sides of the body. |
 |
Figure 4. Multiple asymptomatic flesh-colored papules with elastic consistency on the back that were characteristic of dermatofibrosis lenticularis disseminata. |
 |
Figure 5. The epidermis and dermis appeared normal on histopathology; however, the elastic fibers were markedly increased in both size and number up to the deep dermis in the absence of degenerating changes (H&E, original magnification ×2.5; vascular endothelial growth factor, original magnification ×20 [inset in bottom right corner]). |
 |
| Figure 6. A radiograph of the left hand revealed small sporadic areas of sclerosis in the substantia spongiosa on the heads of some phalanges (white arrows). |
Radiographic imaging of the hands and feet revealed numerous small, ovoid or round foci of sclerosis that were a few millimeters in diameter and were occasionally confluent. This finding was prevalent on the carpal and tarsal bones but less evident on the phalanges and the epiphysis of the metatarsal and metacarpal bones. Full radiographic imaging of both arms and legs subsequently was obtained and showed similar lesions predominantly in the substantia spongiosa of the metaphyses and the epiphyses of the humerus, femur, tibia, and fibula bilaterally (Figure 3). Evaluation of the patient’s parents revealed that her mother had sporadic lenticular areas of increased bone density seen on radiography of the carpal and tarsal bones, particularly at the level of calcaneus, and the proximal and distal humeral epiphyses.
These lesions in our patient were consistent with an incomplete form of osteopoikilosis, which reflects the known variable expression of BOS. The radiologic findings in addition to the cutaneous lesions and the positive family history supported a diagnosis of BOS.
Patient 2
A 5-year-old boy presented with multiple congenital, asymptomatic, flesh-colored papules with elastic consistency on the left thigh, back, and pubic area that were characteristic of dermatofibrosis lenticularis disseminata (Figure 4). The patient’s 7-year-old sister had similar cutaneous lesions. The patient underwent a skin biopsy from the back that revealed thickening of collagen bundles in the dermis with an increase in elastic fibers (Figure 5). On radiographic imaging, small sporadic areas of sclerosis were noted in the substantia spongiosa of the lateral aspect of the humeral capitulum, the radial neck, the capitate, the head of the proximal phalanx of the fourth finger on the left hand, and the heads of the third and fifth middle phalanges on the left hand (Figure 6). Radiography revealed osteopoikilosis on both humeral heads in the patient’s mother. These findings in addition to the dermatologic and histopathologic features suggested a diagnosis of BOS.
Comment
In 1928, Buschke and Ollendorff1 first reported the association between osteopoikilosis and dermatofibrosis lenticularis disseminata, which showed autosomal-dominant inheritance. Skin and bone lesions can occur independently in different family members. Two different clinical cutaneous patterns are described in BOS.2-4 The first and most frequent form is characterized by yellowish nodules often grouped in plaques that are asymmetrically distributed, such as those seen in patient 1. The second form is known as dermatofibrosis lenticularis disseminata, which was seen in patient 2. Histologically, most lesions show normal collagen fibrils and an increased number of elastic fibers.
Osteopoikilosis, also known as spotted bone disease or osteopathia condensans, is a rare asymptomatic bone dysplasia of unknown etiology. It is characterized by an abnormality in the bone maturation process and often is found incidentally on radiologic examination, as seen in patient 1. Radiologic signs of osteopoikilosis consist of small, disseminated, well-circumscribed areas of increased radiodensity located in the epiphyses and metaphyses of long bones, as well as the pelvis, hands, and feet. These lesions, which typically are asymptomatic, could sometimes be associated with bone and/or joint pain but do not cause a predisposition to fractures. Documentation of these bone lesions by early adult life is important to avoid confusion with osteoblastic metastases on the skeleton.6
Buschke-Ollendorff syndrome is characterized by a variable expression. Loss-of-function mutations of the LEMD3 gene have been described in association with this disorder.7 This gene encodes an inner nuclear protein membrane with a C-terminal domain that binds SMAD2 and SMAD3 and antagonizes the bone morphogenetic proteins and transforming growth factor b. These proteins are involved in connective tissue morphogenesis, inducing elastin production from fibroblasts.7 Seven novel loss-of-function mutations of LEMD3 have been identified.8 The segmental manifestation of BOS could be a consequence of the mosaicism resulting from a somatic mutation.9,10 A case describing the absence of LEMD3 mutation in an affected family suggested the genetic heterogeneity of BOS.11
Conclusion
Buschke-Ollendorff syndrome is a benign condition with no repercussions on the patient’s health or quality of life. It does not require any specific treatment because the lesions generally remain asymptomatic and do not generate any substantial cosmetic burden. Mutation analysis was not performed in our patients because BOS has a good prognosis and the parents refused further investigation in both patients; therefore, a correct diagnosis was essential in our cases to rule out malignant bone disease in patient 1 whose osteopoikilosis prompted the workup and other disorders (eg, tuberous sclerosis, pseudoxanthoma elasticum) in patient 2 whose cutaneous lesions were the primary cause for presentation. A correct diagnosis of BOS is necessary to spare patients from expensive investigations and to provide reassurance about the benign nature of the disease.
Buschke-Ollendorff syndrome (BOS) is a rare disease that is inherited in an autosomal-dominant fashion with high penetrance. It is characterized by osteopoikilosis associated with skin manifestations. The approximate incidence of the disease is 1:20,000, with few cases reported in the literature since 1928.1 Skeletal lesions known as osteopoikilosis are areas of increased bone density that can be seen on radiographic imaging and typically are located in the substantia spongiosa of the epiphyses and metaphyses of long bones and the pelvis. In BOS, cutaneous lesions consist of elastic or collagen nevi. Phenotypic expression of the disease is variable, and skeletal and cutaneous lesions may occur separately. Gene mutations of proteins involved in bone and connective tissue morphogenesis have been described in patients with BOS.2-5
Case Reports
Patient 1
A 17-year-old adolescent girl was referred to our hospital for evaluation of an incidental finding of osteopoikilosis that had been noted in the setting of a traumatic event. Clinical examination revealed cutaneous lesions characterized by asymptomatic, linear, stringlike and atrophic fibrotic plaques localized symmetrically on the trunk, right buttock, and right thigh (Figure 1). The lesions on the thigh were present at birth and had spread progressively to the other areas. There was no known history of inflammatory skin disease to explain the presence of the lesions, and no family history of similar signs or symptoms was reported. Histopathologic examination of a punch biopsy of a plaque from the trunk revealed increased collagen bundles associated with thick interlacing elastic fibers. The epidermis did not show any specific histologic alterations. These histopathologic features were diagnostic of a connective tissue nevus (Figure 2).
 |
Figure 1. An asymptomatic atrophic fibrotic plaque localized on the right thigh. |
 |
Figure 2. Low-power view of a punch biopsy showing a normal epidermis. Collagen bundles of the dermis were thickened and somewhat homogenized, as highlighted by the Masson stain (original magnification ×2.5). |
 |
Figure 3. Radiograph of the legs revealed numerous small, ovoid or round foci of sclerosis on the substantia spongiosa of the metaphyses and the epiphyses of the femur, tibia, and fibula on both sides of the body. |
 |
Figure 4. Multiple asymptomatic flesh-colored papules with elastic consistency on the back that were characteristic of dermatofibrosis lenticularis disseminata. |
 |
Figure 5. The epidermis and dermis appeared normal on histopathology; however, the elastic fibers were markedly increased in both size and number up to the deep dermis in the absence of degenerating changes (H&E, original magnification ×2.5; vascular endothelial growth factor, original magnification ×20 [inset in bottom right corner]). |
 |
| Figure 6. A radiograph of the left hand revealed small sporadic areas of sclerosis in the substantia spongiosa on the heads of some phalanges (white arrows). |
Radiographic imaging of the hands and feet revealed numerous small, ovoid or round foci of sclerosis that were a few millimeters in diameter and were occasionally confluent. This finding was prevalent on the carpal and tarsal bones but less evident on the phalanges and the epiphysis of the metatarsal and metacarpal bones. Full radiographic imaging of both arms and legs subsequently was obtained and showed similar lesions predominantly in the substantia spongiosa of the metaphyses and the epiphyses of the humerus, femur, tibia, and fibula bilaterally (Figure 3). Evaluation of the patient’s parents revealed that her mother had sporadic lenticular areas of increased bone density seen on radiography of the carpal and tarsal bones, particularly at the level of calcaneus, and the proximal and distal humeral epiphyses.
These lesions in our patient were consistent with an incomplete form of osteopoikilosis, which reflects the known variable expression of BOS. The radiologic findings in addition to the cutaneous lesions and the positive family history supported a diagnosis of BOS.
Patient 2
A 5-year-old boy presented with multiple congenital, asymptomatic, flesh-colored papules with elastic consistency on the left thigh, back, and pubic area that were characteristic of dermatofibrosis lenticularis disseminata (Figure 4). The patient’s 7-year-old sister had similar cutaneous lesions. The patient underwent a skin biopsy from the back that revealed thickening of collagen bundles in the dermis with an increase in elastic fibers (Figure 5). On radiographic imaging, small sporadic areas of sclerosis were noted in the substantia spongiosa of the lateral aspect of the humeral capitulum, the radial neck, the capitate, the head of the proximal phalanx of the fourth finger on the left hand, and the heads of the third and fifth middle phalanges on the left hand (Figure 6). Radiography revealed osteopoikilosis on both humeral heads in the patient’s mother. These findings in addition to the dermatologic and histopathologic features suggested a diagnosis of BOS.
Comment
In 1928, Buschke and Ollendorff1 first reported the association between osteopoikilosis and dermatofibrosis lenticularis disseminata, which showed autosomal-dominant inheritance. Skin and bone lesions can occur independently in different family members. Two different clinical cutaneous patterns are described in BOS.2-4 The first and most frequent form is characterized by yellowish nodules often grouped in plaques that are asymmetrically distributed, such as those seen in patient 1. The second form is known as dermatofibrosis lenticularis disseminata, which was seen in patient 2. Histologically, most lesions show normal collagen fibrils and an increased number of elastic fibers.
Osteopoikilosis, also known as spotted bone disease or osteopathia condensans, is a rare asymptomatic bone dysplasia of unknown etiology. It is characterized by an abnormality in the bone maturation process and often is found incidentally on radiologic examination, as seen in patient 1. Radiologic signs of osteopoikilosis consist of small, disseminated, well-circumscribed areas of increased radiodensity located in the epiphyses and metaphyses of long bones, as well as the pelvis, hands, and feet. These lesions, which typically are asymptomatic, could sometimes be associated with bone and/or joint pain but do not cause a predisposition to fractures. Documentation of these bone lesions by early adult life is important to avoid confusion with osteoblastic metastases on the skeleton.6
Buschke-Ollendorff syndrome is characterized by a variable expression. Loss-of-function mutations of the LEMD3 gene have been described in association with this disorder.7 This gene encodes an inner nuclear protein membrane with a C-terminal domain that binds SMAD2 and SMAD3 and antagonizes the bone morphogenetic proteins and transforming growth factor b. These proteins are involved in connective tissue morphogenesis, inducing elastin production from fibroblasts.7 Seven novel loss-of-function mutations of LEMD3 have been identified.8 The segmental manifestation of BOS could be a consequence of the mosaicism resulting from a somatic mutation.9,10 A case describing the absence of LEMD3 mutation in an affected family suggested the genetic heterogeneity of BOS.11
Conclusion
Buschke-Ollendorff syndrome is a benign condition with no repercussions on the patient’s health or quality of life. It does not require any specific treatment because the lesions generally remain asymptomatic and do not generate any substantial cosmetic burden. Mutation analysis was not performed in our patients because BOS has a good prognosis and the parents refused further investigation in both patients; therefore, a correct diagnosis was essential in our cases to rule out malignant bone disease in patient 1 whose osteopoikilosis prompted the workup and other disorders (eg, tuberous sclerosis, pseudoxanthoma elasticum) in patient 2 whose cutaneous lesions were the primary cause for presentation. A correct diagnosis of BOS is necessary to spare patients from expensive investigations and to provide reassurance about the benign nature of the disease.
1. Buschke A, Ollendorff H. Ein fall von dermatofibrosis lenticularis disseminata und osteopathia condensas disseminata. Derm Worchenschr. 1928;86:257-262.
2. Ramme K, Kolde G, Stadler R. Dermatofibrosis lenticularis disseminata with osteopoikilosis. Buschke-Ollendorff syndrome [in German]. Hautarzt. 1993;44:312-314.
3. Schena D, Germi L, Zamperetti MR, et al. Buschke-Ollendorff syndrome. Int J Dermatol. 2008;47:1159-1161.
4. Kawamura A, Ochiai T, Tan-Kinoshita M, et al. Buschke-Ollendorff syndrome: three generations in a Japanese family. Pediatr Dermatol. 2005;22:133-137.
5. Woodrow SL, Pope FM, Handfield-Jones SE. The Buschke-Ollendorff syndrome presenting as familial elastic tissue naevi. Br J Dermatol. 2001;144:890-893.
6. Whyte MP, Murphy WA, Siegel BA. 99mTc-pyrophosphate bone imaging in osteopoikilosis, osteopathia striata, and melorheostosis. Radiology. 1978;127:439-443.
7. Giro MG, Duvic M, Smith LT, et al. Buschke-Ollendorff syndrome associated with elevated elastin production by affected skin fibroblasts in culture. J Invest Dermatol. 1992;99:129-137.
8. Hellemans J, Preobrazhenska O, Willaert A, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis [published online ahead of print October 17, 2004]. Nat Genet. 2004;36:1213-1218.
9. Ehrig T, Cockerell CJ. Buschke-Ollendorff syndrome: report of a case and interpretation of the clinical phenotype as a type 2 segmental manifestation of an autosomal dominant skin disease. J Am Acad Dermatol. 2003;49:1163-1166.
10. Hellemans J, Debeer P, Wright M, et al. Germline LEMD3 mutations are rare in sporadic patients with isolated melorheostosis. Hum Mutat. 2006;27:290.
11. Yadegari M, Whyte MP, Mumm S, et al. Buschke-Ollendorff syndrome: absence of LEMD3 mutation in an affected family. Arch Dermatol. 2010;146:63-68.
1. Buschke A, Ollendorff H. Ein fall von dermatofibrosis lenticularis disseminata und osteopathia condensas disseminata. Derm Worchenschr. 1928;86:257-262.
2. Ramme K, Kolde G, Stadler R. Dermatofibrosis lenticularis disseminata with osteopoikilosis. Buschke-Ollendorff syndrome [in German]. Hautarzt. 1993;44:312-314.
3. Schena D, Germi L, Zamperetti MR, et al. Buschke-Ollendorff syndrome. Int J Dermatol. 2008;47:1159-1161.
4. Kawamura A, Ochiai T, Tan-Kinoshita M, et al. Buschke-Ollendorff syndrome: three generations in a Japanese family. Pediatr Dermatol. 2005;22:133-137.
5. Woodrow SL, Pope FM, Handfield-Jones SE. The Buschke-Ollendorff syndrome presenting as familial elastic tissue naevi. Br J Dermatol. 2001;144:890-893.
6. Whyte MP, Murphy WA, Siegel BA. 99mTc-pyrophosphate bone imaging in osteopoikilosis, osteopathia striata, and melorheostosis. Radiology. 1978;127:439-443.
7. Giro MG, Duvic M, Smith LT, et al. Buschke-Ollendorff syndrome associated with elevated elastin production by affected skin fibroblasts in culture. J Invest Dermatol. 1992;99:129-137.
8. Hellemans J, Preobrazhenska O, Willaert A, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis [published online ahead of print October 17, 2004]. Nat Genet. 2004;36:1213-1218.
9. Ehrig T, Cockerell CJ. Buschke-Ollendorff syndrome: report of a case and interpretation of the clinical phenotype as a type 2 segmental manifestation of an autosomal dominant skin disease. J Am Acad Dermatol. 2003;49:1163-1166.
10. Hellemans J, Debeer P, Wright M, et al. Germline LEMD3 mutations are rare in sporadic patients with isolated melorheostosis. Hum Mutat. 2006;27:290.
11. Yadegari M, Whyte MP, Mumm S, et al. Buschke-Ollendorff syndrome: absence of LEMD3 mutation in an affected family. Arch Dermatol. 2010;146:63-68.
Practice Points
- Buschke-Ollendorff syndrome (BOS) is an autosomal-dominant disease characterized by the rare association of skin lesions consisting of collagen or elastic nevi and bone lesions known as osteopoikilosis that are reported radiologically.
- Buschke-Ollendorff syndrome is characterized by elevated genetic heterogeneity and is transmitted with a variable expression.
- Although BOS is a benign condition and does not require any treatment, a correct diagnosis is important to spare patients from unnecessary investigations.
Eleven Years of Itching: A Case Report of Crusted Scabies
Case Report
A 48-year-old man presented to our dermatology clinic with pruritus of 11 years’ duration that worsened at night. He had been followed at a different clinic for several years and was unsuccessfully treated with topical permethrin and oral antihistamines on multiple occasions for scabies. He also had been intermittently treated for contact dermatitis with topical and systemic steroids, which also brought no relief. Just prior to his presentation, the patient’s wife and 8-year-old son had sought medical attention at our institution for chronic pruritus and elevated IgE levels. They had been unsuccessfully treated with topical permethrin, topical steroids, and oral antihistamines for atopic dermatitis at a different clinic. When they presented to our clinic, they were both diagnosed with and treated for scabies. At this visit the patient revealed similar concerns and subsequently underwent examination.
Physical examination revealed large erythematous, hyperkeratotic, scaly plaques on the gluteal fold, um-bilicus, glans penis, scrotum, bilateral elbows, knees, nipples, and ear helices (Figure 1). Numerous small erythematous papules and wavy threadlike gray burrows measuring 1 to 10 mm in diameter were distributed primarily around the wrists, ankles, proximal extremities, abdominal and pubic area (Figure 2), and interdigital spaces. Wide oval patches of nonscarring alopecia developed on the scalp, and atrophic glossitis and angular cheilitis were noted on the oral mucosa, along with a white pseudomembranous exudate on the palatum. The patient’s nails also were thickened and discolored (Figure 3). His medical history was remarkable for hypoparathyroidism (42 years), alopecia areata (15 years), oral candidiasis and angular cheilitis (10 years), and primary hypothyroidism (1 year) that was currently being treated with levothyroxine.
 | 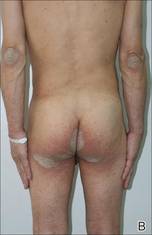 |
| Figure 1. Large erythematous, hyperkeratotic, scaly plaques on the ear helices (A), gluteal fold, and bilateral elbows (B) in a 48-year-old man with crusted scabies. | |
 | |
| Figure 2. Numerous small erythematous papules and wavy, threadlike, grayish, 1- to 10-mm burrows distributed in the abdominal and pubic area. | |
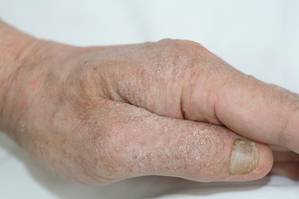 | |
| Figure 3. Thickened and discolored nails in a 48-year-old man with crusted scabies. | |
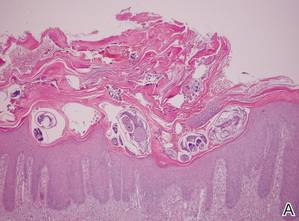 | |
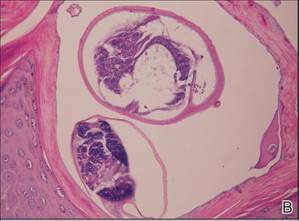 | |
| Figure 4. The psoriasiform epidermis showed massive hyperkeratosis and burrows in the subcorneal layer containing a large number of female mites and feces (A)(H&E, original magnification ×40). A substantial lymphohistiocytic infiltrate with numerous eosinophils was seen diffusely throughout the dermis, though most prominently within the upper half. High-power examination demonstrated mites in the burrow (B)(H&E, original magnification ×400). | |
Laboratory studies revealed normal hemogram with an eosinophil level of 0% (reference range, 0.9%–6%). Biochemistry and hormone profiles were consistent with hypoparathyroidism, with the following levels: serum calcium, 7.6 mg/dL (reference range, 8.4–10.2 mg/dL); phosphorus, 5.1 mg/dL (reference range, 2.4–4.4 mg/dL); and parathyroid hormone, 5.23 pg/mL (reference range, 15–65 pg/mL). The patient tested negative for human immunodeficiency virus.
Dermoscopic examination of the gray threadlike burrows revealed distinctive brown-colored triangular structures that were removed with a fine scalpel. Microscopic examination of the tissue revealed moving mites, eggs, and red-brown scybala.
A biopsy was taken from the thick, scaly, crusted white plaques in the gluteal area. The epidermis showed massive hyperkeratosis and burrows in the subcorneal layer containing a large number of female mites and feces, while the remainder of the epidermis was substantially psoriasiform. A substantial lymphohistiocytic infiltrate with numerous eosinophils was seen diffusely throughout the dermis, though it was most prominent within the upper half (Figure 4). The patient subsequently was diagnosed with crusted scabies.
Crusted scabies typically develops in patients with defective T-cell immunity or a reduced ability to mechanically debride the mites. Because our patient had a history of persistent oral candidiasis, further investigation for immunosuppression was initiated. An immunosuppression panel revealed low IgA levels (46 mg/dL [reference range, 82–453 mg/dL]), low absolute CD8 level (145 cells/µL [reference range, 300–1800 cells/µL), and CD4:CD8 ratio of 4.1. Serum IgG, IgM, IgE, complement levels, and immunoelectrophoresis were within reference range. Abdominal ultrasonography was unremarkable. Taking into account the patient’s history of autoimmune hypoparathyroidism, hypothyroidism, oral candidiasis, and alopecia areata, he was diagnosed with autoimmune polyglandular syndrome.
The patient and his family were successfully treated with modified Wilkinson ointment (goudron végétal 12.5%; sulfur 12.5% in petrolatum) for 3 consecutive days. Within 1 week, the scaly plaques had disappeared and the erythematous papules had faded. The pruritus had resolved and no new papules emerged. Treatment was reapplied once more the following week and complete cure was achieved. We have been following this family for 12 months and no recurrences have occurred.
Comment
Crusted scabies is a rare and highly contagious form of scabies that is characterized by uncontrolled proliferation of mites in the skin, extensive hyperkeratotic scaling, crusted lesions, and variable pruritus.1 The stratum corneum thickens and forms warty crusts as a reaction to the high mite burden.2 The uncontrolled proliferation of mites in the skin typically develops in patients with a defective T-cell response or decreased cutaneous sensation and reduced ability to mechanically debride the mites.3 Patients should be investigated for a predisposition to crusted scabies due to an underlying condition. Crusted scabies also has been shown to develop in Australian natives with normal immunity, though the etiology of the increased susceptibility in this patient population remains unclear. Some studies have shown an association with HLA-A11.4,5 It also has been hypothesized that these patients may have a specific immunodeficiency predisposing them to hyperinfestation.6
Unlike classic scabies, crusted scabies usually does not present acutely, and it usually is insidious at onset. The eruption typically has 2 components: localized horny plaques and a more distinct erythema.3 Crusted scabies can mimic a variety of conditions such as psoriasis, eczema, seborrheic dermatitis, Darier disease, contact dermatitis, and pityriasis rubra pilaris.7 When pruritus is resistant to permethrin therapy, as in our patient, crusted scabies often is misdiagnosed as eczema or contact dermatitis. Topical and systemic corticosteroids often are prescribed, causing progression to scabies incognito.
The diagnosis of crusted scabies is confirmed by examination of scrapings and biopsies, as in classic scabies; however, treatment can be challenging due to compromised immunity, a large mite burden, and limited penetration of topical medications into the hyperkeratotic lesions. Thus treatment should include both keratolytic and scabicidal agents to remove the crusts, reduce the mite load, and enhance the scabicidal therapy.1 Our patient and his affected family members had previously been treated with topical permethrin several times without any benefit. Oral ivermectin has been proven to be effective but is not available in Turkey. Therefore, we treated the patient and his household contacts (other extended family members treated separately) with modified Wilkinson ointment (goudron végétal 12.5%; sulfur 12.5% in petrolatum) for 3 consecutive days, which is known to have both a keratolytic and scabicidal effect.8-11
Conclusion
This case highlights the importance of obtaining a complete family history, skin examination, and thorough investigation for underlying immunodeficiencies that can lead to a predisposition for crusted scabies. It is important to note that the treatment of crusted scabies can be challenging, and effective management of the condition requires a keratolytic agent in conjunction with a scabicidal agent.
1. Douri T, Shawaf AZ. Treatment of crusted scabies with albenzdazole: a case report. Dermatol Online J. 2009;15:17.
2. Burns DA. Diseases caused by arthropods and other noxious animals. In: Champion RH, Burton JL, Burns DA, et al, eds. Textbook of Dermatology. 6th ed. Oxford, England: Wiley-Blackwell; 1998:1423-1482.
3. Karthiyekan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
4. Falk ES, Thorsby E. HLA antigens in patients with scabies. Br J Dermatol. 1981;104:317-320.
5. Morsy TA, Romia SA, al-Ganayni GA, et al. Histocompatibility (HLA) antigens in Egyptians with two parasitic skin diseases (scabies and leishmaniasis). J Egypt Soc Parasitol. 1990;20:565-572.
6. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
7. Jucowics P, Ramon ME, Don PC, et al. Norwegian scabies in an infant with acquired immunodeficiency syndrome. Arch Dermatol. 1989;125:1670-1671.
8. Goldsmith WN. Wilkinson’s ointment. Br Med J. 1945;1:347-348.
9. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
10. Gupta AK, Nikol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
11. Lin AN, Moses K. Tar revisited. Int J Dermatol. 1985;24:216-219.
Case Report
A 48-year-old man presented to our dermatology clinic with pruritus of 11 years’ duration that worsened at night. He had been followed at a different clinic for several years and was unsuccessfully treated with topical permethrin and oral antihistamines on multiple occasions for scabies. He also had been intermittently treated for contact dermatitis with topical and systemic steroids, which also brought no relief. Just prior to his presentation, the patient’s wife and 8-year-old son had sought medical attention at our institution for chronic pruritus and elevated IgE levels. They had been unsuccessfully treated with topical permethrin, topical steroids, and oral antihistamines for atopic dermatitis at a different clinic. When they presented to our clinic, they were both diagnosed with and treated for scabies. At this visit the patient revealed similar concerns and subsequently underwent examination.
Physical examination revealed large erythematous, hyperkeratotic, scaly plaques on the gluteal fold, um-bilicus, glans penis, scrotum, bilateral elbows, knees, nipples, and ear helices (Figure 1). Numerous small erythematous papules and wavy threadlike gray burrows measuring 1 to 10 mm in diameter were distributed primarily around the wrists, ankles, proximal extremities, abdominal and pubic area (Figure 2), and interdigital spaces. Wide oval patches of nonscarring alopecia developed on the scalp, and atrophic glossitis and angular cheilitis were noted on the oral mucosa, along with a white pseudomembranous exudate on the palatum. The patient’s nails also were thickened and discolored (Figure 3). His medical history was remarkable for hypoparathyroidism (42 years), alopecia areata (15 years), oral candidiasis and angular cheilitis (10 years), and primary hypothyroidism (1 year) that was currently being treated with levothyroxine.
 |  |
| Figure 1. Large erythematous, hyperkeratotic, scaly plaques on the ear helices (A), gluteal fold, and bilateral elbows (B) in a 48-year-old man with crusted scabies. | |
 | |
| Figure 2. Numerous small erythematous papules and wavy, threadlike, grayish, 1- to 10-mm burrows distributed in the abdominal and pubic area. | |
 | |
| Figure 3. Thickened and discolored nails in a 48-year-old man with crusted scabies. | |
 | |
 | |
| Figure 4. The psoriasiform epidermis showed massive hyperkeratosis and burrows in the subcorneal layer containing a large number of female mites and feces (A)(H&E, original magnification ×40). A substantial lymphohistiocytic infiltrate with numerous eosinophils was seen diffusely throughout the dermis, though most prominently within the upper half. High-power examination demonstrated mites in the burrow (B)(H&E, original magnification ×400). | |
Laboratory studies revealed normal hemogram with an eosinophil level of 0% (reference range, 0.9%–6%). Biochemistry and hormone profiles were consistent with hypoparathyroidism, with the following levels: serum calcium, 7.6 mg/dL (reference range, 8.4–10.2 mg/dL); phosphorus, 5.1 mg/dL (reference range, 2.4–4.4 mg/dL); and parathyroid hormone, 5.23 pg/mL (reference range, 15–65 pg/mL). The patient tested negative for human immunodeficiency virus.
Dermoscopic examination of the gray threadlike burrows revealed distinctive brown-colored triangular structures that were removed with a fine scalpel. Microscopic examination of the tissue revealed moving mites, eggs, and red-brown scybala.
A biopsy was taken from the thick, scaly, crusted white plaques in the gluteal area. The epidermis showed massive hyperkeratosis and burrows in the subcorneal layer containing a large number of female mites and feces, while the remainder of the epidermis was substantially psoriasiform. A substantial lymphohistiocytic infiltrate with numerous eosinophils was seen diffusely throughout the dermis, though it was most prominent within the upper half (Figure 4). The patient subsequently was diagnosed with crusted scabies.
Crusted scabies typically develops in patients with defective T-cell immunity or a reduced ability to mechanically debride the mites. Because our patient had a history of persistent oral candidiasis, further investigation for immunosuppression was initiated. An immunosuppression panel revealed low IgA levels (46 mg/dL [reference range, 82–453 mg/dL]), low absolute CD8 level (145 cells/µL [reference range, 300–1800 cells/µL), and CD4:CD8 ratio of 4.1. Serum IgG, IgM, IgE, complement levels, and immunoelectrophoresis were within reference range. Abdominal ultrasonography was unremarkable. Taking into account the patient’s history of autoimmune hypoparathyroidism, hypothyroidism, oral candidiasis, and alopecia areata, he was diagnosed with autoimmune polyglandular syndrome.
The patient and his family were successfully treated with modified Wilkinson ointment (goudron végétal 12.5%; sulfur 12.5% in petrolatum) for 3 consecutive days. Within 1 week, the scaly plaques had disappeared and the erythematous papules had faded. The pruritus had resolved and no new papules emerged. Treatment was reapplied once more the following week and complete cure was achieved. We have been following this family for 12 months and no recurrences have occurred.
Comment
Crusted scabies is a rare and highly contagious form of scabies that is characterized by uncontrolled proliferation of mites in the skin, extensive hyperkeratotic scaling, crusted lesions, and variable pruritus.1 The stratum corneum thickens and forms warty crusts as a reaction to the high mite burden.2 The uncontrolled proliferation of mites in the skin typically develops in patients with a defective T-cell response or decreased cutaneous sensation and reduced ability to mechanically debride the mites.3 Patients should be investigated for a predisposition to crusted scabies due to an underlying condition. Crusted scabies also has been shown to develop in Australian natives with normal immunity, though the etiology of the increased susceptibility in this patient population remains unclear. Some studies have shown an association with HLA-A11.4,5 It also has been hypothesized that these patients may have a specific immunodeficiency predisposing them to hyperinfestation.6
Unlike classic scabies, crusted scabies usually does not present acutely, and it usually is insidious at onset. The eruption typically has 2 components: localized horny plaques and a more distinct erythema.3 Crusted scabies can mimic a variety of conditions such as psoriasis, eczema, seborrheic dermatitis, Darier disease, contact dermatitis, and pityriasis rubra pilaris.7 When pruritus is resistant to permethrin therapy, as in our patient, crusted scabies often is misdiagnosed as eczema or contact dermatitis. Topical and systemic corticosteroids often are prescribed, causing progression to scabies incognito.
The diagnosis of crusted scabies is confirmed by examination of scrapings and biopsies, as in classic scabies; however, treatment can be challenging due to compromised immunity, a large mite burden, and limited penetration of topical medications into the hyperkeratotic lesions. Thus treatment should include both keratolytic and scabicidal agents to remove the crusts, reduce the mite load, and enhance the scabicidal therapy.1 Our patient and his affected family members had previously been treated with topical permethrin several times without any benefit. Oral ivermectin has been proven to be effective but is not available in Turkey. Therefore, we treated the patient and his household contacts (other extended family members treated separately) with modified Wilkinson ointment (goudron végétal 12.5%; sulfur 12.5% in petrolatum) for 3 consecutive days, which is known to have both a keratolytic and scabicidal effect.8-11
Conclusion
This case highlights the importance of obtaining a complete family history, skin examination, and thorough investigation for underlying immunodeficiencies that can lead to a predisposition for crusted scabies. It is important to note that the treatment of crusted scabies can be challenging, and effective management of the condition requires a keratolytic agent in conjunction with a scabicidal agent.
Case Report
A 48-year-old man presented to our dermatology clinic with pruritus of 11 years’ duration that worsened at night. He had been followed at a different clinic for several years and was unsuccessfully treated with topical permethrin and oral antihistamines on multiple occasions for scabies. He also had been intermittently treated for contact dermatitis with topical and systemic steroids, which also brought no relief. Just prior to his presentation, the patient’s wife and 8-year-old son had sought medical attention at our institution for chronic pruritus and elevated IgE levels. They had been unsuccessfully treated with topical permethrin, topical steroids, and oral antihistamines for atopic dermatitis at a different clinic. When they presented to our clinic, they were both diagnosed with and treated for scabies. At this visit the patient revealed similar concerns and subsequently underwent examination.
Physical examination revealed large erythematous, hyperkeratotic, scaly plaques on the gluteal fold, um-bilicus, glans penis, scrotum, bilateral elbows, knees, nipples, and ear helices (Figure 1). Numerous small erythematous papules and wavy threadlike gray burrows measuring 1 to 10 mm in diameter were distributed primarily around the wrists, ankles, proximal extremities, abdominal and pubic area (Figure 2), and interdigital spaces. Wide oval patches of nonscarring alopecia developed on the scalp, and atrophic glossitis and angular cheilitis were noted on the oral mucosa, along with a white pseudomembranous exudate on the palatum. The patient’s nails also were thickened and discolored (Figure 3). His medical history was remarkable for hypoparathyroidism (42 years), alopecia areata (15 years), oral candidiasis and angular cheilitis (10 years), and primary hypothyroidism (1 year) that was currently being treated with levothyroxine.
 |  |
| Figure 1. Large erythematous, hyperkeratotic, scaly plaques on the ear helices (A), gluteal fold, and bilateral elbows (B) in a 48-year-old man with crusted scabies. | |
 | |
| Figure 2. Numerous small erythematous papules and wavy, threadlike, grayish, 1- to 10-mm burrows distributed in the abdominal and pubic area. | |
 | |
| Figure 3. Thickened and discolored nails in a 48-year-old man with crusted scabies. | |
 | |
 | |
| Figure 4. The psoriasiform epidermis showed massive hyperkeratosis and burrows in the subcorneal layer containing a large number of female mites and feces (A)(H&E, original magnification ×40). A substantial lymphohistiocytic infiltrate with numerous eosinophils was seen diffusely throughout the dermis, though most prominently within the upper half. High-power examination demonstrated mites in the burrow (B)(H&E, original magnification ×400). | |
Laboratory studies revealed normal hemogram with an eosinophil level of 0% (reference range, 0.9%–6%). Biochemistry and hormone profiles were consistent with hypoparathyroidism, with the following levels: serum calcium, 7.6 mg/dL (reference range, 8.4–10.2 mg/dL); phosphorus, 5.1 mg/dL (reference range, 2.4–4.4 mg/dL); and parathyroid hormone, 5.23 pg/mL (reference range, 15–65 pg/mL). The patient tested negative for human immunodeficiency virus.
Dermoscopic examination of the gray threadlike burrows revealed distinctive brown-colored triangular structures that were removed with a fine scalpel. Microscopic examination of the tissue revealed moving mites, eggs, and red-brown scybala.
A biopsy was taken from the thick, scaly, crusted white plaques in the gluteal area. The epidermis showed massive hyperkeratosis and burrows in the subcorneal layer containing a large number of female mites and feces, while the remainder of the epidermis was substantially psoriasiform. A substantial lymphohistiocytic infiltrate with numerous eosinophils was seen diffusely throughout the dermis, though it was most prominent within the upper half (Figure 4). The patient subsequently was diagnosed with crusted scabies.
Crusted scabies typically develops in patients with defective T-cell immunity or a reduced ability to mechanically debride the mites. Because our patient had a history of persistent oral candidiasis, further investigation for immunosuppression was initiated. An immunosuppression panel revealed low IgA levels (46 mg/dL [reference range, 82–453 mg/dL]), low absolute CD8 level (145 cells/µL [reference range, 300–1800 cells/µL), and CD4:CD8 ratio of 4.1. Serum IgG, IgM, IgE, complement levels, and immunoelectrophoresis were within reference range. Abdominal ultrasonography was unremarkable. Taking into account the patient’s history of autoimmune hypoparathyroidism, hypothyroidism, oral candidiasis, and alopecia areata, he was diagnosed with autoimmune polyglandular syndrome.
The patient and his family were successfully treated with modified Wilkinson ointment (goudron végétal 12.5%; sulfur 12.5% in petrolatum) for 3 consecutive days. Within 1 week, the scaly plaques had disappeared and the erythematous papules had faded. The pruritus had resolved and no new papules emerged. Treatment was reapplied once more the following week and complete cure was achieved. We have been following this family for 12 months and no recurrences have occurred.
Comment
Crusted scabies is a rare and highly contagious form of scabies that is characterized by uncontrolled proliferation of mites in the skin, extensive hyperkeratotic scaling, crusted lesions, and variable pruritus.1 The stratum corneum thickens and forms warty crusts as a reaction to the high mite burden.2 The uncontrolled proliferation of mites in the skin typically develops in patients with a defective T-cell response or decreased cutaneous sensation and reduced ability to mechanically debride the mites.3 Patients should be investigated for a predisposition to crusted scabies due to an underlying condition. Crusted scabies also has been shown to develop in Australian natives with normal immunity, though the etiology of the increased susceptibility in this patient population remains unclear. Some studies have shown an association with HLA-A11.4,5 It also has been hypothesized that these patients may have a specific immunodeficiency predisposing them to hyperinfestation.6
Unlike classic scabies, crusted scabies usually does not present acutely, and it usually is insidious at onset. The eruption typically has 2 components: localized horny plaques and a more distinct erythema.3 Crusted scabies can mimic a variety of conditions such as psoriasis, eczema, seborrheic dermatitis, Darier disease, contact dermatitis, and pityriasis rubra pilaris.7 When pruritus is resistant to permethrin therapy, as in our patient, crusted scabies often is misdiagnosed as eczema or contact dermatitis. Topical and systemic corticosteroids often are prescribed, causing progression to scabies incognito.
The diagnosis of crusted scabies is confirmed by examination of scrapings and biopsies, as in classic scabies; however, treatment can be challenging due to compromised immunity, a large mite burden, and limited penetration of topical medications into the hyperkeratotic lesions. Thus treatment should include both keratolytic and scabicidal agents to remove the crusts, reduce the mite load, and enhance the scabicidal therapy.1 Our patient and his affected family members had previously been treated with topical permethrin several times without any benefit. Oral ivermectin has been proven to be effective but is not available in Turkey. Therefore, we treated the patient and his household contacts (other extended family members treated separately) with modified Wilkinson ointment (goudron végétal 12.5%; sulfur 12.5% in petrolatum) for 3 consecutive days, which is known to have both a keratolytic and scabicidal effect.8-11
Conclusion
This case highlights the importance of obtaining a complete family history, skin examination, and thorough investigation for underlying immunodeficiencies that can lead to a predisposition for crusted scabies. It is important to note that the treatment of crusted scabies can be challenging, and effective management of the condition requires a keratolytic agent in conjunction with a scabicidal agent.
1. Douri T, Shawaf AZ. Treatment of crusted scabies with albenzdazole: a case report. Dermatol Online J. 2009;15:17.
2. Burns DA. Diseases caused by arthropods and other noxious animals. In: Champion RH, Burton JL, Burns DA, et al, eds. Textbook of Dermatology. 6th ed. Oxford, England: Wiley-Blackwell; 1998:1423-1482.
3. Karthiyekan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
4. Falk ES, Thorsby E. HLA antigens in patients with scabies. Br J Dermatol. 1981;104:317-320.
5. Morsy TA, Romia SA, al-Ganayni GA, et al. Histocompatibility (HLA) antigens in Egyptians with two parasitic skin diseases (scabies and leishmaniasis). J Egypt Soc Parasitol. 1990;20:565-572.
6. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
7. Jucowics P, Ramon ME, Don PC, et al. Norwegian scabies in an infant with acquired immunodeficiency syndrome. Arch Dermatol. 1989;125:1670-1671.
8. Goldsmith WN. Wilkinson’s ointment. Br Med J. 1945;1:347-348.
9. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
10. Gupta AK, Nikol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
11. Lin AN, Moses K. Tar revisited. Int J Dermatol. 1985;24:216-219.
1. Douri T, Shawaf AZ. Treatment of crusted scabies with albenzdazole: a case report. Dermatol Online J. 2009;15:17.
2. Burns DA. Diseases caused by arthropods and other noxious animals. In: Champion RH, Burton JL, Burns DA, et al, eds. Textbook of Dermatology. 6th ed. Oxford, England: Wiley-Blackwell; 1998:1423-1482.
3. Karthiyekan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
4. Falk ES, Thorsby E. HLA antigens in patients with scabies. Br J Dermatol. 1981;104:317-320.
5. Morsy TA, Romia SA, al-Ganayni GA, et al. Histocompatibility (HLA) antigens in Egyptians with two parasitic skin diseases (scabies and leishmaniasis). J Egypt Soc Parasitol. 1990;20:565-572.
6. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
7. Jucowics P, Ramon ME, Don PC, et al. Norwegian scabies in an infant with acquired immunodeficiency syndrome. Arch Dermatol. 1989;125:1670-1671.
8. Goldsmith WN. Wilkinson’s ointment. Br Med J. 1945;1:347-348.
9. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
10. Gupta AK, Nikol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
11. Lin AN, Moses K. Tar revisited. Int J Dermatol. 1985;24:216-219.
Practice Points
- Crusted scabies can mimic a variety of conditions such as psoriasis, eczema, seborrheic dermatitis, and contact dermatitis. Therefore, suspicion is the prerequisite for disease control.
- Scabies usually is found in individuals with a compromised immune system as well as those with decreased sensory functions. Thus patients should be investigated for an underlying immunodeficiency.
- Treatment can be challenging, and effective management of the condition requires a keratolytic agent in conjunction with a scabicidal agent.
Nontender Nodules on the Lower Lip
The Diagnosis: Primary Systemic Amyloidosis
Our patient presented with multiple firm waxy nodules on the mucosal surface of the lower lip. Excision biopsy showed thickening of blood vessel walls with abundant amorphous material that was consistent with amyloid. Further staining with Congo red demonstrated brick red amorphous material within the vessel walls on routine light microscopy (Figure 1), and crystal violet stain showed metachromasia (Figure 2). Fine needle aspiration of the abdominal fat-pad showed amyloid. The final diagnosis was primary systemic amyloidosis (PSA).
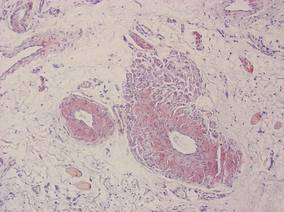 |
| Figure 1. Congo red staining showed a brick red appearance of amyloid within the vessel walls on routine light microscopy (original magnification ×200). |
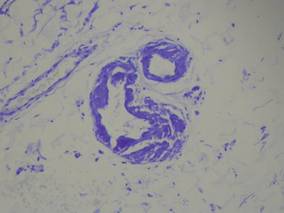 |
| Figure 2. Crystal violet staining around the blood vessels showed metachromasia (original magnification ×100). |
Amyloid is an ubiquitous fibrillar protein arranged in a cross-beta-pleated sheet that is confirmed with x-ray crystallography.1,2 More than 25 variants of amyloid have been identified.3 Pathologic deposition of amyloid-derived material results in a variable spectrum of clinical findings, collectively known as amyloidosis, with presentations ranging from nonspecific fever or fatigue to frank organ failure, depending on the organ involved. In PSA, immunoglobulin light chains are deposited throughout the body. Associated conditions include malignant or benign monoclonal gammopathy, multiple myeloma, Waldenström macroglobulinemia, malignant lymphoma, heavy chain disease, and chronic lymphocytic leukemia.2,4 The most commonly involved organ systems are the heart, lungs, liver, and kidneys. When patients present with unexplained heart failure, orthostatic hypotension, hepatomegaly, peripheral neuropathy, carpal tunnel syndrome, or renal insufficiency, amyloidosis should always be considered in the differential diagnosis.3
Cutaneous lesions of PSA tend to be vascular due to amyloid infiltration of blood vessel walls, manifesting as petechiae, purpura, ecchymoses, or nonhealing ulcers. Pinch purpura frequently are seen in the periorbital region after minor trauma and are recognized as a clinical indicator of PSA.2,4 Xerostomia from amyloid infiltrates in salivary glands is extremely common, and cases of amyloid in the eyes, bones, and thyroid gland have been reported.2 Macroglossia is seen in 12% to 40% of cases; coupled with xerostomia, it can lead to oropharyngeal dysphagia.2,5
Systemic amyloidosis can be further divided into primary (idiopathic or multiple myeloma associated) or secondary to chronic inflammatory conditions or infections; the key difference is the protein from which the abnormal amyloid is derived.1,4 The presence of cutaneous amyloidosis renders the need to rule out systemic disease because amyloidosis may be a purely localized or systemic process.4 Nodular amyloidosis is a localized form of amyloid that also has immunoglobulin light chain deposits and clinically appears exactly the same as PSA; however, the deposits are restricted to the skin.1,2,4,5
Characteristic biopsy findings in cutaneous amyloidosis include amorphous orange-red amyloid deposits on hematoxylin and eosin–stained sections. The gold standard for amyloid detection is apple green birefringence under polarized light with Congo red stain or electron microscopy.6,7 Other stains used to identify amyloid include crystal violet, methyl violet, periodic acid–Schiff, Sirius red, pagoda red, Dylon stain, and thioflavine T.1,2 Confirmation of systemic disease can be accomplished by fine needle aspiration of abdominal fat-pads or rectal mucosal biopsies.1,3,4 Biopsy of accessory salivary glands also has been reported to be very sensitive and specific.2
Treatment options remain limited; localized cutaneous disease may respond to topical corticosteroids, calcineurin inhibitors, or phototherapy. Primary systemic amyloidosis can be treated with a combination of steroids, melphalan, or colchicine often followed by autologous stem cell transplantation8; however, these regimens are not always curative and patients often have a poor prognosis.1
1. Black MM, Upjohn E, Albert S. Amyloidosis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 1. 2nd ed. Spain: Mosby Elsevier; 2008:623-631.
2. Steciuk A, Dompmartin A, Troussard X, et al. Cutaneous amyloidosis and possible association with systemic amyloidosis. Int J Dermatol. 2002;41:127-132.
3. Picken MM. Amyloidosis-where are we now and where are we heading? Arch Pathol Lab Med. 2010;134:545-551.
4. Schreml S, Szeimies RM, Vogt T, et al. Cutaneous amyloidoses and systemic amyloidoses with cutaneous involvement. Eur J Dermatol. 2010;20:152-160.
5. Breathnach SM. Amyloid and amyloidosis. J Am Acad Dermatol. 1988;18(1, pt 1):1-16.
6. Li WM. Histopathology of primary cutaneous amyloidoses and systemic amyloidosis. Clin Dermatol. 1990;8:30-35.
7. Lin CS, Wong CK. Electron microscopy of primary and secondary cutaneous amyloidoses and systemic amyloidosis. Clin Dermatol. 1990;8:36-45.
8. Dember LM. Modern treatment of amyloidosis: unresolved questions. J Am Soc Nephrol. 2009;20:469-472.
The Diagnosis: Primary Systemic Amyloidosis
Our patient presented with multiple firm waxy nodules on the mucosal surface of the lower lip. Excision biopsy showed thickening of blood vessel walls with abundant amorphous material that was consistent with amyloid. Further staining with Congo red demonstrated brick red amorphous material within the vessel walls on routine light microscopy (Figure 1), and crystal violet stain showed metachromasia (Figure 2). Fine needle aspiration of the abdominal fat-pad showed amyloid. The final diagnosis was primary systemic amyloidosis (PSA).
 |
| Figure 1. Congo red staining showed a brick red appearance of amyloid within the vessel walls on routine light microscopy (original magnification ×200). |
 |
| Figure 2. Crystal violet staining around the blood vessels showed metachromasia (original magnification ×100). |
Amyloid is an ubiquitous fibrillar protein arranged in a cross-beta-pleated sheet that is confirmed with x-ray crystallography.1,2 More than 25 variants of amyloid have been identified.3 Pathologic deposition of amyloid-derived material results in a variable spectrum of clinical findings, collectively known as amyloidosis, with presentations ranging from nonspecific fever or fatigue to frank organ failure, depending on the organ involved. In PSA, immunoglobulin light chains are deposited throughout the body. Associated conditions include malignant or benign monoclonal gammopathy, multiple myeloma, Waldenström macroglobulinemia, malignant lymphoma, heavy chain disease, and chronic lymphocytic leukemia.2,4 The most commonly involved organ systems are the heart, lungs, liver, and kidneys. When patients present with unexplained heart failure, orthostatic hypotension, hepatomegaly, peripheral neuropathy, carpal tunnel syndrome, or renal insufficiency, amyloidosis should always be considered in the differential diagnosis.3
Cutaneous lesions of PSA tend to be vascular due to amyloid infiltration of blood vessel walls, manifesting as petechiae, purpura, ecchymoses, or nonhealing ulcers. Pinch purpura frequently are seen in the periorbital region after minor trauma and are recognized as a clinical indicator of PSA.2,4 Xerostomia from amyloid infiltrates in salivary glands is extremely common, and cases of amyloid in the eyes, bones, and thyroid gland have been reported.2 Macroglossia is seen in 12% to 40% of cases; coupled with xerostomia, it can lead to oropharyngeal dysphagia.2,5
Systemic amyloidosis can be further divided into primary (idiopathic or multiple myeloma associated) or secondary to chronic inflammatory conditions or infections; the key difference is the protein from which the abnormal amyloid is derived.1,4 The presence of cutaneous amyloidosis renders the need to rule out systemic disease because amyloidosis may be a purely localized or systemic process.4 Nodular amyloidosis is a localized form of amyloid that also has immunoglobulin light chain deposits and clinically appears exactly the same as PSA; however, the deposits are restricted to the skin.1,2,4,5
Characteristic biopsy findings in cutaneous amyloidosis include amorphous orange-red amyloid deposits on hematoxylin and eosin–stained sections. The gold standard for amyloid detection is apple green birefringence under polarized light with Congo red stain or electron microscopy.6,7 Other stains used to identify amyloid include crystal violet, methyl violet, periodic acid–Schiff, Sirius red, pagoda red, Dylon stain, and thioflavine T.1,2 Confirmation of systemic disease can be accomplished by fine needle aspiration of abdominal fat-pads or rectal mucosal biopsies.1,3,4 Biopsy of accessory salivary glands also has been reported to be very sensitive and specific.2
Treatment options remain limited; localized cutaneous disease may respond to topical corticosteroids, calcineurin inhibitors, or phototherapy. Primary systemic amyloidosis can be treated with a combination of steroids, melphalan, or colchicine often followed by autologous stem cell transplantation8; however, these regimens are not always curative and patients often have a poor prognosis.1
The Diagnosis: Primary Systemic Amyloidosis
Our patient presented with multiple firm waxy nodules on the mucosal surface of the lower lip. Excision biopsy showed thickening of blood vessel walls with abundant amorphous material that was consistent with amyloid. Further staining with Congo red demonstrated brick red amorphous material within the vessel walls on routine light microscopy (Figure 1), and crystal violet stain showed metachromasia (Figure 2). Fine needle aspiration of the abdominal fat-pad showed amyloid. The final diagnosis was primary systemic amyloidosis (PSA).
 |
| Figure 1. Congo red staining showed a brick red appearance of amyloid within the vessel walls on routine light microscopy (original magnification ×200). |
 |
| Figure 2. Crystal violet staining around the blood vessels showed metachromasia (original magnification ×100). |
Amyloid is an ubiquitous fibrillar protein arranged in a cross-beta-pleated sheet that is confirmed with x-ray crystallography.1,2 More than 25 variants of amyloid have been identified.3 Pathologic deposition of amyloid-derived material results in a variable spectrum of clinical findings, collectively known as amyloidosis, with presentations ranging from nonspecific fever or fatigue to frank organ failure, depending on the organ involved. In PSA, immunoglobulin light chains are deposited throughout the body. Associated conditions include malignant or benign monoclonal gammopathy, multiple myeloma, Waldenström macroglobulinemia, malignant lymphoma, heavy chain disease, and chronic lymphocytic leukemia.2,4 The most commonly involved organ systems are the heart, lungs, liver, and kidneys. When patients present with unexplained heart failure, orthostatic hypotension, hepatomegaly, peripheral neuropathy, carpal tunnel syndrome, or renal insufficiency, amyloidosis should always be considered in the differential diagnosis.3
Cutaneous lesions of PSA tend to be vascular due to amyloid infiltration of blood vessel walls, manifesting as petechiae, purpura, ecchymoses, or nonhealing ulcers. Pinch purpura frequently are seen in the periorbital region after minor trauma and are recognized as a clinical indicator of PSA.2,4 Xerostomia from amyloid infiltrates in salivary glands is extremely common, and cases of amyloid in the eyes, bones, and thyroid gland have been reported.2 Macroglossia is seen in 12% to 40% of cases; coupled with xerostomia, it can lead to oropharyngeal dysphagia.2,5
Systemic amyloidosis can be further divided into primary (idiopathic or multiple myeloma associated) or secondary to chronic inflammatory conditions or infections; the key difference is the protein from which the abnormal amyloid is derived.1,4 The presence of cutaneous amyloidosis renders the need to rule out systemic disease because amyloidosis may be a purely localized or systemic process.4 Nodular amyloidosis is a localized form of amyloid that also has immunoglobulin light chain deposits and clinically appears exactly the same as PSA; however, the deposits are restricted to the skin.1,2,4,5
Characteristic biopsy findings in cutaneous amyloidosis include amorphous orange-red amyloid deposits on hematoxylin and eosin–stained sections. The gold standard for amyloid detection is apple green birefringence under polarized light with Congo red stain or electron microscopy.6,7 Other stains used to identify amyloid include crystal violet, methyl violet, periodic acid–Schiff, Sirius red, pagoda red, Dylon stain, and thioflavine T.1,2 Confirmation of systemic disease can be accomplished by fine needle aspiration of abdominal fat-pads or rectal mucosal biopsies.1,3,4 Biopsy of accessory salivary glands also has been reported to be very sensitive and specific.2
Treatment options remain limited; localized cutaneous disease may respond to topical corticosteroids, calcineurin inhibitors, or phototherapy. Primary systemic amyloidosis can be treated with a combination of steroids, melphalan, or colchicine often followed by autologous stem cell transplantation8; however, these regimens are not always curative and patients often have a poor prognosis.1
1. Black MM, Upjohn E, Albert S. Amyloidosis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 1. 2nd ed. Spain: Mosby Elsevier; 2008:623-631.
2. Steciuk A, Dompmartin A, Troussard X, et al. Cutaneous amyloidosis and possible association with systemic amyloidosis. Int J Dermatol. 2002;41:127-132.
3. Picken MM. Amyloidosis-where are we now and where are we heading? Arch Pathol Lab Med. 2010;134:545-551.
4. Schreml S, Szeimies RM, Vogt T, et al. Cutaneous amyloidoses and systemic amyloidoses with cutaneous involvement. Eur J Dermatol. 2010;20:152-160.
5. Breathnach SM. Amyloid and amyloidosis. J Am Acad Dermatol. 1988;18(1, pt 1):1-16.
6. Li WM. Histopathology of primary cutaneous amyloidoses and systemic amyloidosis. Clin Dermatol. 1990;8:30-35.
7. Lin CS, Wong CK. Electron microscopy of primary and secondary cutaneous amyloidoses and systemic amyloidosis. Clin Dermatol. 1990;8:36-45.
8. Dember LM. Modern treatment of amyloidosis: unresolved questions. J Am Soc Nephrol. 2009;20:469-472.
1. Black MM, Upjohn E, Albert S. Amyloidosis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 1. 2nd ed. Spain: Mosby Elsevier; 2008:623-631.
2. Steciuk A, Dompmartin A, Troussard X, et al. Cutaneous amyloidosis and possible association with systemic amyloidosis. Int J Dermatol. 2002;41:127-132.
3. Picken MM. Amyloidosis-where are we now and where are we heading? Arch Pathol Lab Med. 2010;134:545-551.
4. Schreml S, Szeimies RM, Vogt T, et al. Cutaneous amyloidoses and systemic amyloidoses with cutaneous involvement. Eur J Dermatol. 2010;20:152-160.
5. Breathnach SM. Amyloid and amyloidosis. J Am Acad Dermatol. 1988;18(1, pt 1):1-16.
6. Li WM. Histopathology of primary cutaneous amyloidoses and systemic amyloidosis. Clin Dermatol. 1990;8:30-35.
7. Lin CS, Wong CK. Electron microscopy of primary and secondary cutaneous amyloidoses and systemic amyloidosis. Clin Dermatol. 1990;8:36-45.
8. Dember LM. Modern treatment of amyloidosis: unresolved questions. J Am Soc Nephrol. 2009;20:469-472.
A 71-year-old woman presented with multiple 3×3-mm, firm, nontender nodules of 3 years’ duration on the mucosal surface of the lower lip that gradually enlarged. There was no macroglossia on presentation. The lip nodules were asymptomatic; however, they did interfere with eating and drinking, necessitating the use of a straw. Her medical history was remarkable for emphysema that required supplemental oxygen, rheumatoid arthritis, orthostatic hypotension, renal insufficiency, autonomic nervous system dysfunction, and Sjögren syndrome. She also had a recent thyroidectomy due to multiple thyroid nodules. An excision biopsy was performed of the lip nodules.
Plaques: A Rare Presentation of Acrokeratoelastoidosis
To the Editor:
Acrokeratoelastoidosis (AKE) is a rare disease first described by Costa1 in 1953. Typically it is only a cosmetic nuisance in the majority of patients and presents as asymptomatic, small, firm, flesh-colored to yellowish, round to polygonal papules with occasional keratosis or umbilication on the radial and ulnar margins of the hands and/or feet.1-3 In some cases, the lesions occur on the anterior aspects of the wrists, fingers, or lower legs.1 The lesions are always bilaterally distributed. Acrokeratoelastoidosis is a chronic skin disorder that commonly presents during childhood or adolescence, but presentation in adulthood also has been described.3 Histologically, AKE always shows hyperkeratosis, acanthosis, decrease of elastic tissue, and elastorrhexis of remaining elastic fibers. Plaque-type lesions are rare. We describe a patient who presented with plaques on the radial and ulnar margins of the hands.
A 36-year-old Chinese woman presented with asymptomatic, small, firm papules of 6 months’ duration that initially developed on the hands and gradually increased in number, coalescing into plaques. The feet were spared. She had no medical history of hyperhidrosis, chronic trauma, friction, or excessive sun exposure, and no family history of similar symptoms. No prior therapy had been attempted.
Physical examination showed nonconfluent, firm, flesh-colored to yellowish, translucent, smooth papules with wavy edges that were symmetrically distributed on the radial and ulnar margins of the hands; some papules had coalesced into plaques (Figure 1). A biopsy specimen taken from a plaque on the hypothenar eminence of the right hand revealed focal hyperkeratosis, hypergranulosis, acanthosis, and mild chronic inflammation with hematoxylin and eosin stain (Figure 2A). Aldehyde fuchsin staining showed fragmented and rarefied elastic fibers in the reticular dermis (Figure 2B). The patient was diagnosed with AKE. Oral tretinoin 10 mg twice daily was initiated and resulted in an evident response after 2 weeks of treatment. However, the patient stopped taking the medication because of pruritus and dry skin and the lesions then reappeared.
 |
| Figure 1. Nonconfluent, firm, flesh-colored to yellowish, translucent, smooth papules distributed on the radial margin of the hand; some of papules coalesced into plaques. |
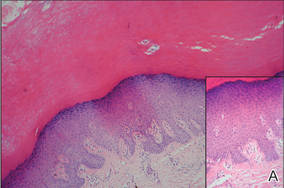 |
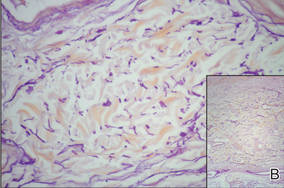 |
| Figure 2. Histopathology revealed hyperkeratosis, hypergranulosis, acanthosis, and mild chronic inflammation (A) (H&E, original magnification ×250 [inset, original magnification ×400]), as well as fragmented elastic fibers in the reticular dermis (B) (Aldehyde fuchsin, original magnification ×400 [inset, original magnification ×100]). |
Acrokeratoelastoidosis is a rare keratotic disorder. It seems to have no racial or ethnic predilection and occurs more frequently in women.4,5 It also is rare in China, with few cases reported, all women.5 The reason for the gender predilection in China remains unknown. The course is chronic, but it may rapidly progress during pregnancy.6
The pathogenesis of AKE is still unresolved.2,3 Although many cases are sporadic,5 it appears to be inherited in an autosomal-dominant fashion, most likely related to chromosome 2.7 Typically, AKE presents as papules that are discrete and bilaterally distributed in the palmoplantar margins,2,3 but some of the papules in our patient coalesced into plaques, which is unique. The histologic hallmarks indicated that the lesions were AKE.
The differential diagnosis of AKE includes hereditary papulotranslucent acrokeratoderma, focal acral hyperkeratosis, and keratoelastoidosis marginalis.8 Hereditary papulotranslucent acrokeratoderma also is inherited in an autosomal-dominant fashion and shares similar acral, translucent, keratotic papules with AKE, but there is no chronic inflammatory cell infiltrate, degeneration of collagenous fibers, or fragmentation of elastic fibers. The clinical appearance of focal acral hyperkeratosis is similar to AKE, but no changes are revealed in the elastic tissue.9 Because AKE, focal acral hyperkeratosis, and hereditary papulotranslucent acrokeratoderma have similar lesions and overlapping histologic changes, they may be considered variants of the same entity.4 Keratoelastoidosis marginalis, also called degenerative collagenous plaques of the hand, mainly affects white individuals aged 40 to 60 years with a history of prolonged sun exposure. Papules often are distributed over the junction of the dorsal and palmar skin and less often on the ulnar sides of the hands. The clinical lesions are similar to those in our patient, but histopathology of keratoelastoidosis marginalis shows amorphous, basophilic, elastotic masses and thickened, fragmented, calcified elastic fibers in the upper and mid dermis.
Therapies including liquid nitrogen, topical salicylic acid, methotrexate, dapsone, tar, cryotherapy, systemic prednisone, retinoic acid, clobetasone cream,5 and erbium:YAG laser10 have been applied. Thus far, no optimal treatment has been recommended and no tendency of spontaneous resolution has been previously reported in the literature. Our patient responded to tretinoin, but the lesions recurred after withdrawal of the medication; therefore, tretinoin may not be an optimal treatment option. Because the lesions are limited to the skin and AKE is only considered a cosmetic problem with a good prognosis, we recommend a wait-and-watch approach.
Acknowledgment—We thank Rashmi Sarkar, MD, New Delhi, India, for her assistance.
1. Costa OG. Akrokerato-elastoidosis; a hitherto undescribed skin disease. Dermatologica. 1953;107:164-168.
2. Bogle MA, Huang LY, Tschen JA. Acrokeratoelastoidosis. J Am Acad Dermatol. 2002;47:448-451.
3. Highet AS, Rook A, Anderson JR. Acrokeratoelastoidosis. Br J Dermatol. 1982;106:337-344.
4. Abulafia J, Vignale RA. Degenerative collagenous
plaques of the hands and acrokeratoelastoidosis: pathogenesis and relationship with knuckle pads. Int J Dermatol. 2000;39:424-432.
5. Luo DQ, Zhang B, Huang YB, et al. Papules on a young woman’s hands and feet. Clin Exp Dermatol. 2010;35:451-452.
6. Nelson-Adesokan P, Mallory SB, Lombardi C, et al. Acrokeratoelastoidosis of Costa [published correction appears in Int J Dermatol. 1996;35:380]. Int J Dermatol. 1995;34:431-433.
7. Greiner J, Krüger J, Palden L, et al. A linkage study of acrokeratoelastoidosis. possible mapping to chromosome 2. Hum Genet. 1983;63:222-227.
8. Hu W, Cook TF, Vicki GJ, et al. Acrokeratoelastoidosis. Pediatr Dermatol. 2002;19:320-322.
9. Dowd PM, Harman RR, Black MM. Focal acral hyperkeratosis. Br J Dermatol. 1983;109:97-103.
10. Erbil AH, Sezer E, Koç E, et al. Acrokeratoelastoidosis treated with the erbium:YAG laser [published online ahead of print November 3, 2007]. Clin Exp Dermatol. 2008;33:30-31.
To the Editor:
Acrokeratoelastoidosis (AKE) is a rare disease first described by Costa1 in 1953. Typically it is only a cosmetic nuisance in the majority of patients and presents as asymptomatic, small, firm, flesh-colored to yellowish, round to polygonal papules with occasional keratosis or umbilication on the radial and ulnar margins of the hands and/or feet.1-3 In some cases, the lesions occur on the anterior aspects of the wrists, fingers, or lower legs.1 The lesions are always bilaterally distributed. Acrokeratoelastoidosis is a chronic skin disorder that commonly presents during childhood or adolescence, but presentation in adulthood also has been described.3 Histologically, AKE always shows hyperkeratosis, acanthosis, decrease of elastic tissue, and elastorrhexis of remaining elastic fibers. Plaque-type lesions are rare. We describe a patient who presented with plaques on the radial and ulnar margins of the hands.
A 36-year-old Chinese woman presented with asymptomatic, small, firm papules of 6 months’ duration that initially developed on the hands and gradually increased in number, coalescing into plaques. The feet were spared. She had no medical history of hyperhidrosis, chronic trauma, friction, or excessive sun exposure, and no family history of similar symptoms. No prior therapy had been attempted.
Physical examination showed nonconfluent, firm, flesh-colored to yellowish, translucent, smooth papules with wavy edges that were symmetrically distributed on the radial and ulnar margins of the hands; some papules had coalesced into plaques (Figure 1). A biopsy specimen taken from a plaque on the hypothenar eminence of the right hand revealed focal hyperkeratosis, hypergranulosis, acanthosis, and mild chronic inflammation with hematoxylin and eosin stain (Figure 2A). Aldehyde fuchsin staining showed fragmented and rarefied elastic fibers in the reticular dermis (Figure 2B). The patient was diagnosed with AKE. Oral tretinoin 10 mg twice daily was initiated and resulted in an evident response after 2 weeks of treatment. However, the patient stopped taking the medication because of pruritus and dry skin and the lesions then reappeared.
 |
| Figure 1. Nonconfluent, firm, flesh-colored to yellowish, translucent, smooth papules distributed on the radial margin of the hand; some of papules coalesced into plaques. |
 |
 |
| Figure 2. Histopathology revealed hyperkeratosis, hypergranulosis, acanthosis, and mild chronic inflammation (A) (H&E, original magnification ×250 [inset, original magnification ×400]), as well as fragmented elastic fibers in the reticular dermis (B) (Aldehyde fuchsin, original magnification ×400 [inset, original magnification ×100]). |
Acrokeratoelastoidosis is a rare keratotic disorder. It seems to have no racial or ethnic predilection and occurs more frequently in women.4,5 It also is rare in China, with few cases reported, all women.5 The reason for the gender predilection in China remains unknown. The course is chronic, but it may rapidly progress during pregnancy.6
The pathogenesis of AKE is still unresolved.2,3 Although many cases are sporadic,5 it appears to be inherited in an autosomal-dominant fashion, most likely related to chromosome 2.7 Typically, AKE presents as papules that are discrete and bilaterally distributed in the palmoplantar margins,2,3 but some of the papules in our patient coalesced into plaques, which is unique. The histologic hallmarks indicated that the lesions were AKE.
The differential diagnosis of AKE includes hereditary papulotranslucent acrokeratoderma, focal acral hyperkeratosis, and keratoelastoidosis marginalis.8 Hereditary papulotranslucent acrokeratoderma also is inherited in an autosomal-dominant fashion and shares similar acral, translucent, keratotic papules with AKE, but there is no chronic inflammatory cell infiltrate, degeneration of collagenous fibers, or fragmentation of elastic fibers. The clinical appearance of focal acral hyperkeratosis is similar to AKE, but no changes are revealed in the elastic tissue.9 Because AKE, focal acral hyperkeratosis, and hereditary papulotranslucent acrokeratoderma have similar lesions and overlapping histologic changes, they may be considered variants of the same entity.4 Keratoelastoidosis marginalis, also called degenerative collagenous plaques of the hand, mainly affects white individuals aged 40 to 60 years with a history of prolonged sun exposure. Papules often are distributed over the junction of the dorsal and palmar skin and less often on the ulnar sides of the hands. The clinical lesions are similar to those in our patient, but histopathology of keratoelastoidosis marginalis shows amorphous, basophilic, elastotic masses and thickened, fragmented, calcified elastic fibers in the upper and mid dermis.
Therapies including liquid nitrogen, topical salicylic acid, methotrexate, dapsone, tar, cryotherapy, systemic prednisone, retinoic acid, clobetasone cream,5 and erbium:YAG laser10 have been applied. Thus far, no optimal treatment has been recommended and no tendency of spontaneous resolution has been previously reported in the literature. Our patient responded to tretinoin, but the lesions recurred after withdrawal of the medication; therefore, tretinoin may not be an optimal treatment option. Because the lesions are limited to the skin and AKE is only considered a cosmetic problem with a good prognosis, we recommend a wait-and-watch approach.
Acknowledgment—We thank Rashmi Sarkar, MD, New Delhi, India, for her assistance.
To the Editor:
Acrokeratoelastoidosis (AKE) is a rare disease first described by Costa1 in 1953. Typically it is only a cosmetic nuisance in the majority of patients and presents as asymptomatic, small, firm, flesh-colored to yellowish, round to polygonal papules with occasional keratosis or umbilication on the radial and ulnar margins of the hands and/or feet.1-3 In some cases, the lesions occur on the anterior aspects of the wrists, fingers, or lower legs.1 The lesions are always bilaterally distributed. Acrokeratoelastoidosis is a chronic skin disorder that commonly presents during childhood or adolescence, but presentation in adulthood also has been described.3 Histologically, AKE always shows hyperkeratosis, acanthosis, decrease of elastic tissue, and elastorrhexis of remaining elastic fibers. Plaque-type lesions are rare. We describe a patient who presented with plaques on the radial and ulnar margins of the hands.
A 36-year-old Chinese woman presented with asymptomatic, small, firm papules of 6 months’ duration that initially developed on the hands and gradually increased in number, coalescing into plaques. The feet were spared. She had no medical history of hyperhidrosis, chronic trauma, friction, or excessive sun exposure, and no family history of similar symptoms. No prior therapy had been attempted.
Physical examination showed nonconfluent, firm, flesh-colored to yellowish, translucent, smooth papules with wavy edges that were symmetrically distributed on the radial and ulnar margins of the hands; some papules had coalesced into plaques (Figure 1). A biopsy specimen taken from a plaque on the hypothenar eminence of the right hand revealed focal hyperkeratosis, hypergranulosis, acanthosis, and mild chronic inflammation with hematoxylin and eosin stain (Figure 2A). Aldehyde fuchsin staining showed fragmented and rarefied elastic fibers in the reticular dermis (Figure 2B). The patient was diagnosed with AKE. Oral tretinoin 10 mg twice daily was initiated and resulted in an evident response after 2 weeks of treatment. However, the patient stopped taking the medication because of pruritus and dry skin and the lesions then reappeared.
 |
| Figure 1. Nonconfluent, firm, flesh-colored to yellowish, translucent, smooth papules distributed on the radial margin of the hand; some of papules coalesced into plaques. |
 |
 |
| Figure 2. Histopathology revealed hyperkeratosis, hypergranulosis, acanthosis, and mild chronic inflammation (A) (H&E, original magnification ×250 [inset, original magnification ×400]), as well as fragmented elastic fibers in the reticular dermis (B) (Aldehyde fuchsin, original magnification ×400 [inset, original magnification ×100]). |
Acrokeratoelastoidosis is a rare keratotic disorder. It seems to have no racial or ethnic predilection and occurs more frequently in women.4,5 It also is rare in China, with few cases reported, all women.5 The reason for the gender predilection in China remains unknown. The course is chronic, but it may rapidly progress during pregnancy.6
The pathogenesis of AKE is still unresolved.2,3 Although many cases are sporadic,5 it appears to be inherited in an autosomal-dominant fashion, most likely related to chromosome 2.7 Typically, AKE presents as papules that are discrete and bilaterally distributed in the palmoplantar margins,2,3 but some of the papules in our patient coalesced into plaques, which is unique. The histologic hallmarks indicated that the lesions were AKE.
The differential diagnosis of AKE includes hereditary papulotranslucent acrokeratoderma, focal acral hyperkeratosis, and keratoelastoidosis marginalis.8 Hereditary papulotranslucent acrokeratoderma also is inherited in an autosomal-dominant fashion and shares similar acral, translucent, keratotic papules with AKE, but there is no chronic inflammatory cell infiltrate, degeneration of collagenous fibers, or fragmentation of elastic fibers. The clinical appearance of focal acral hyperkeratosis is similar to AKE, but no changes are revealed in the elastic tissue.9 Because AKE, focal acral hyperkeratosis, and hereditary papulotranslucent acrokeratoderma have similar lesions and overlapping histologic changes, they may be considered variants of the same entity.4 Keratoelastoidosis marginalis, also called degenerative collagenous plaques of the hand, mainly affects white individuals aged 40 to 60 years with a history of prolonged sun exposure. Papules often are distributed over the junction of the dorsal and palmar skin and less often on the ulnar sides of the hands. The clinical lesions are similar to those in our patient, but histopathology of keratoelastoidosis marginalis shows amorphous, basophilic, elastotic masses and thickened, fragmented, calcified elastic fibers in the upper and mid dermis.
Therapies including liquid nitrogen, topical salicylic acid, methotrexate, dapsone, tar, cryotherapy, systemic prednisone, retinoic acid, clobetasone cream,5 and erbium:YAG laser10 have been applied. Thus far, no optimal treatment has been recommended and no tendency of spontaneous resolution has been previously reported in the literature. Our patient responded to tretinoin, but the lesions recurred after withdrawal of the medication; therefore, tretinoin may not be an optimal treatment option. Because the lesions are limited to the skin and AKE is only considered a cosmetic problem with a good prognosis, we recommend a wait-and-watch approach.
Acknowledgment—We thank Rashmi Sarkar, MD, New Delhi, India, for her assistance.
1. Costa OG. Akrokerato-elastoidosis; a hitherto undescribed skin disease. Dermatologica. 1953;107:164-168.
2. Bogle MA, Huang LY, Tschen JA. Acrokeratoelastoidosis. J Am Acad Dermatol. 2002;47:448-451.
3. Highet AS, Rook A, Anderson JR. Acrokeratoelastoidosis. Br J Dermatol. 1982;106:337-344.
4. Abulafia J, Vignale RA. Degenerative collagenous
plaques of the hands and acrokeratoelastoidosis: pathogenesis and relationship with knuckle pads. Int J Dermatol. 2000;39:424-432.
5. Luo DQ, Zhang B, Huang YB, et al. Papules on a young woman’s hands and feet. Clin Exp Dermatol. 2010;35:451-452.
6. Nelson-Adesokan P, Mallory SB, Lombardi C, et al. Acrokeratoelastoidosis of Costa [published correction appears in Int J Dermatol. 1996;35:380]. Int J Dermatol. 1995;34:431-433.
7. Greiner J, Krüger J, Palden L, et al. A linkage study of acrokeratoelastoidosis. possible mapping to chromosome 2. Hum Genet. 1983;63:222-227.
8. Hu W, Cook TF, Vicki GJ, et al. Acrokeratoelastoidosis. Pediatr Dermatol. 2002;19:320-322.
9. Dowd PM, Harman RR, Black MM. Focal acral hyperkeratosis. Br J Dermatol. 1983;109:97-103.
10. Erbil AH, Sezer E, Koç E, et al. Acrokeratoelastoidosis treated with the erbium:YAG laser [published online ahead of print November 3, 2007]. Clin Exp Dermatol. 2008;33:30-31.
1. Costa OG. Akrokerato-elastoidosis; a hitherto undescribed skin disease. Dermatologica. 1953;107:164-168.
2. Bogle MA, Huang LY, Tschen JA. Acrokeratoelastoidosis. J Am Acad Dermatol. 2002;47:448-451.
3. Highet AS, Rook A, Anderson JR. Acrokeratoelastoidosis. Br J Dermatol. 1982;106:337-344.
4. Abulafia J, Vignale RA. Degenerative collagenous
plaques of the hands and acrokeratoelastoidosis: pathogenesis and relationship with knuckle pads. Int J Dermatol. 2000;39:424-432.
5. Luo DQ, Zhang B, Huang YB, et al. Papules on a young woman’s hands and feet. Clin Exp Dermatol. 2010;35:451-452.
6. Nelson-Adesokan P, Mallory SB, Lombardi C, et al. Acrokeratoelastoidosis of Costa [published correction appears in Int J Dermatol. 1996;35:380]. Int J Dermatol. 1995;34:431-433.
7. Greiner J, Krüger J, Palden L, et al. A linkage study of acrokeratoelastoidosis. possible mapping to chromosome 2. Hum Genet. 1983;63:222-227.
8. Hu W, Cook TF, Vicki GJ, et al. Acrokeratoelastoidosis. Pediatr Dermatol. 2002;19:320-322.
9. Dowd PM, Harman RR, Black MM. Focal acral hyperkeratosis. Br J Dermatol. 1983;109:97-103.
10. Erbil AH, Sezer E, Koç E, et al. Acrokeratoelastoidosis treated with the erbium:YAG laser [published online ahead of print November 3, 2007]. Clin Exp Dermatol. 2008;33:30-31.
Hailey-Hailey Disease
Hailey-Hailey disease (HHD), or benign familial chronic pemphigus, typically presents as suprabasal blisters with a perivascular and interstitial lymphocytic infiltrate (Figure 1).1 Villi, or elongated dermal papillae lined with a single layer of basal cells, protrude into the bullae (Figure 2). In HHD lesions, the epidermis is thickened with scale-crust, and at least the lower half of the epidermis shows acantholysis. Despite the acantholytic changes, a few intact intercellular bridges remain, giving the appearance of a dilapidated brick wall (Figure 2). There may be dyskeratotic cells among the acantholytic cells, though they are scant in many cases. These acantholytic dyskeratotic cells have eosinophilic polygonal-shaped cytoplasm. Hailey-Hailey disease typically does not show adnexal extension of the acantholysis. Direct immunofluorescence is negative in HHD.
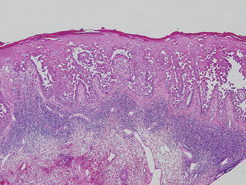
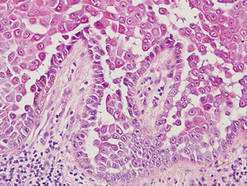
Pemphigus vulgaris is an autoimmune intraepidermal bullous disease that presents with suprabasal acantholysis (Figure 3).2 The epidermis is not thickened and acantholysis is limited to the suprabasal layer. Acantholytic cells with eosinophils and/or neutrophils are found within the bullae. Perivascular and interstitial infiltrates of lymphocytes, eosinophils, and occasionally neutrophils are seen; however, the inflammatory cell infiltrate can vary from extensive to scant. Direct immunofluorescence usually reveals IgG and/or C3 deposition on the surface of the keratinocytes throughout the epidermis.
Pemphigus foliaceus is another autoimmune intraepidermal bullous disease that is characterized by acantholysis in the granular or upper spinous layers (Figure 4).3 The epidermis is not thickened. Sometimes acantholytic cells show dyskeratotic change (Figure 4). Some biopsy specimens do not contain the roof of the bullae; therefore, only erosion is seen and the diagnosis may be missed. Moreover, when only the adnexal epithelium shows acantholysis without epidermal involvement, diagnosis can be difficult.4 Acantholysis is accompanied with a superficial perivascular and interstitial inflammatory cell infiltrate consisting of lymphocytes, eosinophils, and occasionally neutrophils. The amount of inflammatory cell infiltrate may vary. Bullous impetigo and staphylococcal scalded skin syndrome reveal a similar histopathologic pattern. Direct immunofluorescence usually discloses IgG and/or C3 deposition on cell surfaces of keratinocytes in the entire or upper epidermis.
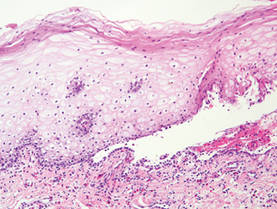
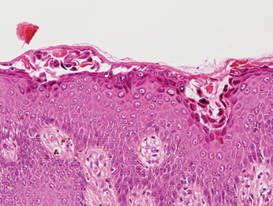
Herpesvirus infection shows ballooning (intracellular edema) of keratinocytes. Eventually acantholysis occurs and intraepidermal bullae are formed. In the bullae, virus-associated acantholytic keratinocytes, some that are multinucleated, can be easily found (Figure 5).5 These cells are larger than normal keratinocytes and have steel gray nuclei with peripheral accentuation. Some of these cells are necrotic, and the remains of necrotic multinucleated acantholytic cells are easily recognized. Adnexal epithelial cells occasionally are affected by herpesvirus infection; nuclear change is similar to the epidermis. A perivascular and interstitial infiltrate of lymphocytes and neutrophils is seen. Neutrophils accumulate within the old bullae, clinically manifesting as a pustule.
Darier disease is characterized by suprabasal clefts and acantholysis above the basal layer (Figure 6).6 Similar to HHD, villi protrude within the clefts (Figure 6). Conspicuous columns of parakeratosis above the acantholytic epidermis often are observed. Dyskeratotic cells exist among acantholytic ke-ratinocytes in the granular layer and parakeratotic column, which are known as corps ronds and crops grains, respectively. A scant to moderate lymphocytic infiltrate is found in the upper dermis.
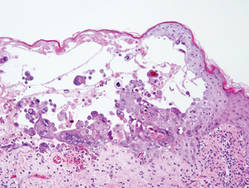
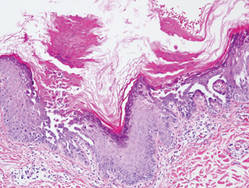
- Hernandez-Perez E. Familial benign chronic pemphigus. Cutis. 1987;39:75-77.
- Venugopal SS, Murrell DF. Diagnosis and clinical features of pemphigus vulgaris. Dermatol Clin. 2011;29:373-380, vii.
- Dasher D, Rubenstein D, Diaz LA. Pemphigus foliaceus. Curr Dir Autoimmun. 2008;10:182-194.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- King DF, King LA. Giant cells in lesions of varicella and herpes zoster. Am J Dermatopathol. 1986;8:456-458.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.
Hailey-Hailey disease (HHD), or benign familial chronic pemphigus, typically presents as suprabasal blisters with a perivascular and interstitial lymphocytic infiltrate (Figure 1).1 Villi, or elongated dermal papillae lined with a single layer of basal cells, protrude into the bullae (Figure 2). In HHD lesions, the epidermis is thickened with scale-crust, and at least the lower half of the epidermis shows acantholysis. Despite the acantholytic changes, a few intact intercellular bridges remain, giving the appearance of a dilapidated brick wall (Figure 2). There may be dyskeratotic cells among the acantholytic cells, though they are scant in many cases. These acantholytic dyskeratotic cells have eosinophilic polygonal-shaped cytoplasm. Hailey-Hailey disease typically does not show adnexal extension of the acantholysis. Direct immunofluorescence is negative in HHD.


Pemphigus vulgaris is an autoimmune intraepidermal bullous disease that presents with suprabasal acantholysis (Figure 3).2 The epidermis is not thickened and acantholysis is limited to the suprabasal layer. Acantholytic cells with eosinophils and/or neutrophils are found within the bullae. Perivascular and interstitial infiltrates of lymphocytes, eosinophils, and occasionally neutrophils are seen; however, the inflammatory cell infiltrate can vary from extensive to scant. Direct immunofluorescence usually reveals IgG and/or C3 deposition on the surface of the keratinocytes throughout the epidermis.
Pemphigus foliaceus is another autoimmune intraepidermal bullous disease that is characterized by acantholysis in the granular or upper spinous layers (Figure 4).3 The epidermis is not thickened. Sometimes acantholytic cells show dyskeratotic change (Figure 4). Some biopsy specimens do not contain the roof of the bullae; therefore, only erosion is seen and the diagnosis may be missed. Moreover, when only the adnexal epithelium shows acantholysis without epidermal involvement, diagnosis can be difficult.4 Acantholysis is accompanied with a superficial perivascular and interstitial inflammatory cell infiltrate consisting of lymphocytes, eosinophils, and occasionally neutrophils. The amount of inflammatory cell infiltrate may vary. Bullous impetigo and staphylococcal scalded skin syndrome reveal a similar histopathologic pattern. Direct immunofluorescence usually discloses IgG and/or C3 deposition on cell surfaces of keratinocytes in the entire or upper epidermis.


Herpesvirus infection shows ballooning (intracellular edema) of keratinocytes. Eventually acantholysis occurs and intraepidermal bullae are formed. In the bullae, virus-associated acantholytic keratinocytes, some that are multinucleated, can be easily found (Figure 5).5 These cells are larger than normal keratinocytes and have steel gray nuclei with peripheral accentuation. Some of these cells are necrotic, and the remains of necrotic multinucleated acantholytic cells are easily recognized. Adnexal epithelial cells occasionally are affected by herpesvirus infection; nuclear change is similar to the epidermis. A perivascular and interstitial infiltrate of lymphocytes and neutrophils is seen. Neutrophils accumulate within the old bullae, clinically manifesting as a pustule.
Darier disease is characterized by suprabasal clefts and acantholysis above the basal layer (Figure 6).6 Similar to HHD, villi protrude within the clefts (Figure 6). Conspicuous columns of parakeratosis above the acantholytic epidermis often are observed. Dyskeratotic cells exist among acantholytic ke-ratinocytes in the granular layer and parakeratotic column, which are known as corps ronds and crops grains, respectively. A scant to moderate lymphocytic infiltrate is found in the upper dermis.


Hailey-Hailey disease (HHD), or benign familial chronic pemphigus, typically presents as suprabasal blisters with a perivascular and interstitial lymphocytic infiltrate (Figure 1).1 Villi, or elongated dermal papillae lined with a single layer of basal cells, protrude into the bullae (Figure 2). In HHD lesions, the epidermis is thickened with scale-crust, and at least the lower half of the epidermis shows acantholysis. Despite the acantholytic changes, a few intact intercellular bridges remain, giving the appearance of a dilapidated brick wall (Figure 2). There may be dyskeratotic cells among the acantholytic cells, though they are scant in many cases. These acantholytic dyskeratotic cells have eosinophilic polygonal-shaped cytoplasm. Hailey-Hailey disease typically does not show adnexal extension of the acantholysis. Direct immunofluorescence is negative in HHD.


Pemphigus vulgaris is an autoimmune intraepidermal bullous disease that presents with suprabasal acantholysis (Figure 3).2 The epidermis is not thickened and acantholysis is limited to the suprabasal layer. Acantholytic cells with eosinophils and/or neutrophils are found within the bullae. Perivascular and interstitial infiltrates of lymphocytes, eosinophils, and occasionally neutrophils are seen; however, the inflammatory cell infiltrate can vary from extensive to scant. Direct immunofluorescence usually reveals IgG and/or C3 deposition on the surface of the keratinocytes throughout the epidermis.
Pemphigus foliaceus is another autoimmune intraepidermal bullous disease that is characterized by acantholysis in the granular or upper spinous layers (Figure 4).3 The epidermis is not thickened. Sometimes acantholytic cells show dyskeratotic change (Figure 4). Some biopsy specimens do not contain the roof of the bullae; therefore, only erosion is seen and the diagnosis may be missed. Moreover, when only the adnexal epithelium shows acantholysis without epidermal involvement, diagnosis can be difficult.4 Acantholysis is accompanied with a superficial perivascular and interstitial inflammatory cell infiltrate consisting of lymphocytes, eosinophils, and occasionally neutrophils. The amount of inflammatory cell infiltrate may vary. Bullous impetigo and staphylococcal scalded skin syndrome reveal a similar histopathologic pattern. Direct immunofluorescence usually discloses IgG and/or C3 deposition on cell surfaces of keratinocytes in the entire or upper epidermis.


Herpesvirus infection shows ballooning (intracellular edema) of keratinocytes. Eventually acantholysis occurs and intraepidermal bullae are formed. In the bullae, virus-associated acantholytic keratinocytes, some that are multinucleated, can be easily found (Figure 5).5 These cells are larger than normal keratinocytes and have steel gray nuclei with peripheral accentuation. Some of these cells are necrotic, and the remains of necrotic multinucleated acantholytic cells are easily recognized. Adnexal epithelial cells occasionally are affected by herpesvirus infection; nuclear change is similar to the epidermis. A perivascular and interstitial infiltrate of lymphocytes and neutrophils is seen. Neutrophils accumulate within the old bullae, clinically manifesting as a pustule.
Darier disease is characterized by suprabasal clefts and acantholysis above the basal layer (Figure 6).6 Similar to HHD, villi protrude within the clefts (Figure 6). Conspicuous columns of parakeratosis above the acantholytic epidermis often are observed. Dyskeratotic cells exist among acantholytic ke-ratinocytes in the granular layer and parakeratotic column, which are known as corps ronds and crops grains, respectively. A scant to moderate lymphocytic infiltrate is found in the upper dermis.


- Hernandez-Perez E. Familial benign chronic pemphigus. Cutis. 1987;39:75-77.
- Venugopal SS, Murrell DF. Diagnosis and clinical features of pemphigus vulgaris. Dermatol Clin. 2011;29:373-380, vii.
- Dasher D, Rubenstein D, Diaz LA. Pemphigus foliaceus. Curr Dir Autoimmun. 2008;10:182-194.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- King DF, King LA. Giant cells in lesions of varicella and herpes zoster. Am J Dermatopathol. 1986;8:456-458.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.
- Hernandez-Perez E. Familial benign chronic pemphigus. Cutis. 1987;39:75-77.
- Venugopal SS, Murrell DF. Diagnosis and clinical features of pemphigus vulgaris. Dermatol Clin. 2011;29:373-380, vii.
- Dasher D, Rubenstein D, Diaz LA. Pemphigus foliaceus. Curr Dir Autoimmun. 2008;10:182-194.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- King DF, King LA. Giant cells in lesions of varicella and herpes zoster. Am J Dermatopathol. 1986;8:456-458.
- Burge S. Management of Darier’s disease. Clin Exp Dermatol. 1999;24:53-56.