User login
Evaluating a Program Process Change to Improve Completion of Foot Exams and Amputation Risk Assessments for Veterans with Diabetes (FULL)
Individuals with diabetes mellitus (DM), peripheral vascular disease, or end-stage renal disease are at risk for a nontraumatic lower limb amputation.1 Veterans have a high number of risk factors and are especially vulnerable. More than 70% of veterans enrolled in US Department of Veterans Affairs (VA) healthcare are at increased risk for developing DM due to excess weight, poor eating habits, and physical inactivity.2 One in 4 veterans has DM, compared with 1 in 6 in the general population.2
DM can lead to long-term complications including limb amputations. Annually in the US about 73,000 nontraumatic lower limb amputations are performed and > 60% occur among persons with DM.3 Complications from diabetic wounds are the cause of 90% of lower limb amputations, and foot ulcers are the most prevalent complication.4 Diabetic ulcers are slow to heal due to vascular impairments and nerve damage.5 Peripheral vascular disease, a common comorbid condition, contributes to restricted blood flow and can lead to tissue death or gangrene requiring amputation.6
Between 2010 and 2014, VA Portland Healthcare System (VAPORHCS) had one of the highest national amputation rates in VA.7 A clinical chart review found that annual foot examinations and amputation risk assessments (ARAs) were not completed with all at-risk veterans. In 2013, a VA Office of Inspector General (OIG) national report found that more than one-third of veterans enrolled in VA with DM had no documentation of required annual foot exams.8 In 2017, VA released Directive 1410, which outlined the scope of care required to prevent and treat lower limb complications and amputations for veterans at risk for primary or secondary limb loss.1 This national initiative is a comprehensive approach that engages multiprofessional teams to perform routine foot examinations and amputation risk assessments; identify and promptly treat foot ulcers; track, monitor and educate at-risk veterans; and participate in clinical education to enhance staff skills.
To decrease the amputation rate, VAPORHCS redesigned its foot-care program to comply with the national initiative. As is typical in VA, VAPORHCS uses a team-based approach in primary care. The basic 4-member team patient-aligned care team (PACT) consists of a physician or nurse practitioner (NP) primary care provider (PCP), a registered nurse (RN) care manager, a licensed practical nurse (LPN), and a medical staff assistant (MSA) for administrative support. Each PACT cares for about 1,800 veterans. Formerly, LPNs completed the annual diabetic foot exams, and PCPs verified the exams and completed the ARA based on the LPNs’ findings. If patients were moderate risk or high risk, they were referred to podiatry. The VAPORHCS audit found that not all at-risk veterans had both the foot exam and ARA completed, or were referred to podiatry when indicated. There was a need for a process improvement project to develop a seamless program consisting of all recommended foot care components crucial for timely care.
This quality improvement project sought to evaluate the effectiveness of the process changes by examining PCPs’ adoption of, and consistency in completing annual diabetic foot exams and ARAs with veterans. The goals of the project were to evaluate changes in the: (1) Number of accurate diabetic foot exams and amputation risk assessments completed with veterans with DM; (2) Number and timeliness of appropriate referrals to podiatry for an in-depth assessment and treatment of veterans found to be at moderate-to-high risk for lower limb amputations; and (3) Number of administrative text orders entered by PCPs for nurse care managers to offer foot care education and the completion of the education with veterans found to be at normal-to-low risk for lower limb amputations. The institutional review boards of VAPORHCS and Gonzaga University approved the study.
Methods
Established by the American Diabetes Association and endorsed by the American Association of Clinical Endocrinologists, the comprehensive foot exam includes a visual exam, pedal pulse checks, and a sensory exam.9,10 The templated Computerized Patient Record System (CPRS) electronic health record note specifies normal and abnormal parameters of each section. On the same template, the provider assigns an ARA score based on the results of the completed foot exam. Risk scores range from 0 to 3 (0, normal or no risk; 1, low risk, 2; moderate risk; 3, high risk) If the veteran has normal or low risk, the PCP can encourage the veteran to remain at low risk by entering an administrative CPRS text order for the nurse care manager to offer education about daily foot care at the same visit or at a scheduled follow-up visit. This process facilitates nurse care managers to include routine foot care as integral to their usual duties coaching veterans to engage in self-care to manage chronic conditions. If the risk is assessed as moderate or high risk, PCPs are prompted to send a referral to podiatry to repeat the foot exam, verify the ARA score, and provide appropriate foot care treatment and follow-up.
On October 31, 2017, following training on the updated foot exam and ARA template with staff at the 13 VAPORHCS outpatient clinic sites, 2 sites piloted all components of the Comprehensive Foot Care program. An in-person training was completed with PCPs to review the changes of the foot care template in CPRS and to answer their questions about it. PCPs were required to complete both the 3-part foot exam and ARA at least once annually with veterans with DM.
An electronic clinical reminder was built to alert PCPs and PACTs that a veteran was either due or overdue for an exam and risk assessment. VA podiatrists agreed to complete the reminder with veterans under their care. One of the 2 sites was randomly selected for this study. Data were collected from August 1, 2017 to July 31, 2018. Patients were identified from the Diabetes Registry, a database established at VAPORHCS in 2008 to track veterans with DM to ensure quality care.11 Veterans’ personal health identifiers from the registry were used to access their health records to complete chart reviews and assess the completion, accuracy and timeliness of all foot care components.
The Diabetes Registry lists a veterans’ upcoming appointments and tracks their most recent clinic visits; laboratory tests; physical exams; and screening exams for foot, eye, and renal care. Newly diagnosed veterans are uploaded automatically into this registry by tracking all DM-related International Classification of Diseases (ICD-10) codes, hemoglobin A1c (HbA1c) levels ≥ 6.5%, or outpatient prescriptions for insulin or oral hypoglycemic agents.11
Study Design
This quality improvement project evaluated PCPs’ actions in a program process change intended to improve foot care provided with veterans at-risk for nontraumatic lower limb amputations. Audits of CPRS records and the Diabetes Registry determined the results of the practice change. Data on the total number of foot exams, amputation risk scores, appropriate podiatry referrals, administrative orders for nurse coaching, and completed foot care education were collected during the study period. Data collected for the 3-month period preceding the process change established preimplementation comparison vs the postimplementation data. Data were collected at 3, 6, and 9 months after implementation. The foot exams and ARAs were reviewed to determine whether exams and assessments were completed accurately during the pre- and post-implementation timeframes. Incomplete or clearly incorrectly completed documentation were considered inaccurate. For example, it was considered inaccurate if only the foot exam portion was completed in the assessment and the ARA was not.
Data Analysis
Data on the total number of accurately completed foot examinations and ARAs, total number of podiatry referrals, and total number of administrative text orders placed by PCPs, and education completed by nurse care managers were assessed. Statistical significance was evaluated using χ2 and Fisher exact test as appropriate. A Pearson correlation coefficient was used to determine whether there was a statistically significant increase in accurate foot examinations and ARAs as well as total number of podiatry referrals during the study period. Statistical analyses were performed using Stata 14.1 statistical software (College Station, TX).
Results
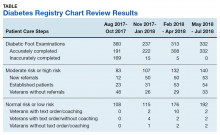
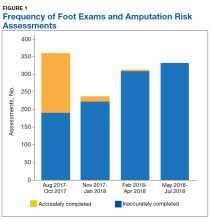
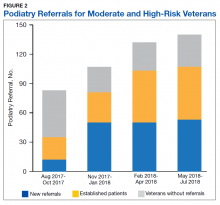
A total of 1,242 completed diabetic foot examinations were identified from August 1, 2017 to July 31, 2018 using the Diabetes Registry (Table). For the 3 months prior to the change, there were 191 appropriately completed foot examinations and ARAs. This number increased progressively over three 3-month periods (Figure 1). Within the 1-year study period, there was a statistically significant increase in the number of appropriate foot examinations (r = 0.495). PCPs placed 34 podiatry referrals during the prechange period. After the change, the number of appropriate referrals increased statistically significantly in the 3 following 3-month-periods (r = 0.222) (Figure 2).
To determine the accuracy of documentation and ratio of appropriate referrals, the 3-month prech
Notably, at the end of the first year of this evaluation, 119 veterans at the clinic did not show a recorded comprehensive foot examination since receiving a DM diagnosis and 299 veterans were due for an annual examination—a 25.2% gap of veterans without the recommended progression of foot care services. Of those that previously had a recorded foot examination, 51 (17.0%) veterans were found to be ≥ 2 years overdue.
Discussion
DM management requires a comprehensive team-based approach to help monitor for associated complications. At the VA, PACTs are veterans’ initial and primary point of contact for chronic condition management. PACTs have regular opportunities to engage veterans in initial and follow-up care and appropriate self-care. PCPs are critical in placing referrals for specialized care promptly to prevent and minimize complications such as foot ulcers, and ultimately, lower limb amputations.9,10,12
When PCPs assume responsibility for the entire foot examination, they are able to identify problems early.1 Left untreated, foot wounds and ulcers have the potential to grow into serious infections.9 Early risk identification and management can lead to increased patient satisfaction, improved life expectancy, quality of life, and ultimately, lower healthcare costs.12
Multiple studies have shown the clinical importance of foot examinations in preventative care. In one study, researchers found that completing foot examinations, among other early interventions, increased life expectancy and reduced foot complications.13 Diabetic foot management programs involving screening and categorizing patients into low- and high-risk groups had a 47.4% decrease in the incidence of amputations and 37.8% decrease in hospital admissions.14 Amputations were found to be inversely correlated with multidisciplinary foot care programs with reduction of lower limb amputations at 2 years.15 The Centers for Disease Control and Prevention found that comprehensive foot care programs that include a foot examination, ARA, appropriate referrals to specialists, and foot-care education and preventative services can reduce lower limb amputation rates by 45% to 85%.16
With one of the highest amputation rates in VA, VAPORHCS needed an integrated approach to ensure that appropriate foot care occurred regularly with veterans with DM. Prior to the process change, LPNs completed foot examinations and PCPs completed the ARA. Separating these clinical services resulted in few veterans receiving an amputation risk score. Of those with scores, the lack of a standardized program protocol resulted in discrepancies between ARAs from patient to patient as health care providers did not have clear or enough information to select the correct score and make the appropriate referrals. Thus, veterans previously identified as at moderate or high risk also lacked podiatric follow-up care.
The new quality-driven process change corrected the documentation process to nationally accepted standards. The goal was to create a consistent template in the electronic health record for all health care providers. The new template simplifies the documentation process and clarifies the amputation risk score assignment, which allows for proper foot care management. The PCP completes the process from assessment through referral, removing gaps in care and improving efficiency. Although this change was initially met with resistance from PCPs, it led to a significant increase in the number of patients with accurately documented examinations. Similarly, the number of appropriate referrals significantly rose during the study period. The standardized documentation process resulted in improved accurate examinations and ARAs over the past year. The new program also resulted in an increased number of appropriate podiatry referrals for those identified to be at moderate or high risk. This elevation of care is crucial for veterans to receive frequent follow-up visits for preventative care and/or treatment, including surgical modalities to promote limb salvage.
Barriers
This project identified several barriers to the Comprehensive Foot Care program. One major barrier was health care provider resistance to using the new process. For example, VAPORHCS podiatrists are not using the new template with established patients, which requires PCPs to complete the clinical reminder template for quality performance, an additional burden unrelated to clinical care. PCPs that do complete the foot examination/ARA templated note use the podiatrist’s visit note without personally assessing the patient.
PCPs also have been resistant to entering administrative text orders for preventative foot care in normal- or low-risk veterans (4.6% overall), which has resulted in decreased patient education (3.9% overall). Education for normal-risk and low-risk patients is designed to engage veterans in self-care and prevent risk progression, critical to prevention.
It was found that PCPs often did not ask nurses to coach normal- or low-risk veterans on preventative foot care, as suggested by the low rates at which patients were offered education. This is an area we will target with future quality improvement efforts. All patients with DM should have general education about risk factors and appropriate management of them to decrease their risk for complications.9 Preventative foot care education is a critical resource to share with patients during health coaching opportunities to clarify misunderstandings and support change talk when patients are ambivalent or resistant to change. Individual or group-based nurse visits can facilitate better coaching for patients.
At the VA, coaching begins with a conversation about what matters most to the veteran, facilitating the development of a personalized plan based on patients’ values, needs, preferences and goals.9,10,12,17 Coaching allows nurses to assess veterans’ knowledge and willingness to engage in healthy habits; and identify additional resources to help them achieve their goals.
Limitations
There are many limitations to this short quality improvement analysis. For example, only 1 of 2 clinics that piloted the program change was evaluated. In addition, there are 11 other clinics that need additional in-depth education on the program change. Although this analysis was overwhelmingly positive, it may not be as successful at other clinic sites and may be subject to the Hawthorne effect—since the 2 piloted locations knew they were being observed for the quality improvement program and may have made an extra effort to be compliant.18 Additionally, we were unable to track the records of veterans receiving care through the VA Choice Program for this analysis resulting in a lack of documentation of completed diabetic foot examinations and a lack of internal referrals to VA podiatry.
Another major limitation of this project involved calculating the number of referrals placed to podiatry. On January 1, 2018, about halfway through the program evaluation, a national VA decision enabled veterans to self-refer to podiatry, which may have limited the number of podiatry referrals placed by PCPs. Finally, patients could refuse podiatry referrals. In the 9-month postimplementation period, 57 (64.8%) veterans declined podiatry referrals, according to their CPRS records.
Although, there was an improvement in the accuracy of diabetic foot examinations, ARAs, and appropriate podiatry referrals, the ultimate goal of reducing diabetic foot ulcers and lower limb amputations was not tracked due to the limited timeframe of this analysis. Tracking these endpoints with continuous plan-do-study-act cycles are needed for this ongoing quality improvement project.
Conclusion
The goal of the VAPORHCS Comprehensive Foot Care program is to provide veterans with a program that is predictable, easy and consistent to prevent and treat foot ulcers to reduce the rate of lower limb amputations. It requires multidisciplinary team collaboration for success. Implementation of this new comprehensive program has increased the number of accurate annual foot exams, ARAs and podiatry referrals. Despite these improvements, areas of future improvement include emphasizing patient education and ongoing provider compliance with annual assessments.
Author contributions
MHG proposed the program evaluation project idea. TVQ collected and analyzed the data and wrote the manuscript. MHG oversaw the project and edited the manuscript. TVQ is the guarantor of this project and takes responsibility for the contents of this journal article.
Acknowledgments
The authors thank Tyra Haebe, VAPORHCS Prevention of Amputation in Veterans Everywhere (PAVE) Manager, and the entire VAPORHCS PAVE committee for their support in this program evaluation project.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 1410, prevention of amputation in veterans everywhere (PAVE) program. http://vaww.medical surgical.va.gov/podiatry/docs/VHADirective_1410_PAVE.pdf. Published March 31, 2017. Accessed October 11, 2019.
2. US Department of Veterans Affairs. Close to 25 percent of VA patients have diabetes http://www.va.gov/health/NewsFeatures/20111115a.asp. Accessed 14 October 2017
3. Centers for Disease Control and Prevention. National diabetes statistics report, 2017: Estimates of Diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 11, 2019.
4. Gibson LW, Abbas A: Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2012;25(1):131-134
5. Boyko EJ, Monteiro-Soares M, Wheeler SGB. “Peripheral arterial disease, foot ulcers, lower extremity amputations, and diabetes.” In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health Publication; 2017:20-21,20-34.
6. National Institute of Health, National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Updated August 13, 2019. Accessed October 11, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration, Support Services Center. Amputation cube, lower amputations 2015. http://vssc.med.va.gov/AlphaIndex. [Nonpublic source, not verified]
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: Foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed October 11, 2019.
9. American Diabetes Association. Standards of medical care in diabetes - 2017. Diabetes Care. 2017;40(suppl 1):1-142.
10. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
11. Yang J, McConnachie J, Renfro R, Schreiner S, Tallett S, Winterbottom L. The diabetes registry and future panel management tool https://docplayer.net/19062632-The-diabetes-registry-and.html. Accessed October 11, 2019.
12. National Institute of Health, Centers for Disease Control and Prevention, the National Diabetes Education Program. Working together to manage diabetes: a guide for pharmcy, podiatry, optometry, and dentistry. https://www.cdc.gov/diabetes/ndep/pdfs/ppod-guide.pdf. Accessed October 11, 2019.
13. Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care. 2004;27(4):901-907.
14. Lavery LA, Wunderlich RP, Tredwell JL. Disease management for the diabetic foot: effectiveness of a diabetic foot prevention program to reduce amputations and hospitalizations. Diabetes Res Clin Pract. 2005;70(1):31-37.
15. Paisey RB, Abbott A, Levenson R, et al; South-West Cardiovascular Strategic Clinical Network peer diabetic foot service review team. Diabetes-related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the south-west of England. Diabet Med. 2017;35(1):53-62.
16. Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed October 11, 2019.
17. US Department of Veterans Affairs. Whole health for life. https://www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Updated July 20, 2017. Accessed October 11, 2019.
18. Parsons HM. What happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974;183(4128):922–9322.
Individuals with diabetes mellitus (DM), peripheral vascular disease, or end-stage renal disease are at risk for a nontraumatic lower limb amputation.1 Veterans have a high number of risk factors and are especially vulnerable. More than 70% of veterans enrolled in US Department of Veterans Affairs (VA) healthcare are at increased risk for developing DM due to excess weight, poor eating habits, and physical inactivity.2 One in 4 veterans has DM, compared with 1 in 6 in the general population.2
DM can lead to long-term complications including limb amputations. Annually in the US about 73,000 nontraumatic lower limb amputations are performed and > 60% occur among persons with DM.3 Complications from diabetic wounds are the cause of 90% of lower limb amputations, and foot ulcers are the most prevalent complication.4 Diabetic ulcers are slow to heal due to vascular impairments and nerve damage.5 Peripheral vascular disease, a common comorbid condition, contributes to restricted blood flow and can lead to tissue death or gangrene requiring amputation.6
Between 2010 and 2014, VA Portland Healthcare System (VAPORHCS) had one of the highest national amputation rates in VA.7 A clinical chart review found that annual foot examinations and amputation risk assessments (ARAs) were not completed with all at-risk veterans. In 2013, a VA Office of Inspector General (OIG) national report found that more than one-third of veterans enrolled in VA with DM had no documentation of required annual foot exams.8 In 2017, VA released Directive 1410, which outlined the scope of care required to prevent and treat lower limb complications and amputations for veterans at risk for primary or secondary limb loss.1 This national initiative is a comprehensive approach that engages multiprofessional teams to perform routine foot examinations and amputation risk assessments; identify and promptly treat foot ulcers; track, monitor and educate at-risk veterans; and participate in clinical education to enhance staff skills.
To decrease the amputation rate, VAPORHCS redesigned its foot-care program to comply with the national initiative. As is typical in VA, VAPORHCS uses a team-based approach in primary care. The basic 4-member team patient-aligned care team (PACT) consists of a physician or nurse practitioner (NP) primary care provider (PCP), a registered nurse (RN) care manager, a licensed practical nurse (LPN), and a medical staff assistant (MSA) for administrative support. Each PACT cares for about 1,800 veterans. Formerly, LPNs completed the annual diabetic foot exams, and PCPs verified the exams and completed the ARA based on the LPNs’ findings. If patients were moderate risk or high risk, they were referred to podiatry. The VAPORHCS audit found that not all at-risk veterans had both the foot exam and ARA completed, or were referred to podiatry when indicated. There was a need for a process improvement project to develop a seamless program consisting of all recommended foot care components crucial for timely care.
This quality improvement project sought to evaluate the effectiveness of the process changes by examining PCPs’ adoption of, and consistency in completing annual diabetic foot exams and ARAs with veterans. The goals of the project were to evaluate changes in the: (1) Number of accurate diabetic foot exams and amputation risk assessments completed with veterans with DM; (2) Number and timeliness of appropriate referrals to podiatry for an in-depth assessment and treatment of veterans found to be at moderate-to-high risk for lower limb amputations; and (3) Number of administrative text orders entered by PCPs for nurse care managers to offer foot care education and the completion of the education with veterans found to be at normal-to-low risk for lower limb amputations. The institutional review boards of VAPORHCS and Gonzaga University approved the study.
Methods
Established by the American Diabetes Association and endorsed by the American Association of Clinical Endocrinologists, the comprehensive foot exam includes a visual exam, pedal pulse checks, and a sensory exam.9,10 The templated Computerized Patient Record System (CPRS) electronic health record note specifies normal and abnormal parameters of each section. On the same template, the provider assigns an ARA score based on the results of the completed foot exam. Risk scores range from 0 to 3 (0, normal or no risk; 1, low risk, 2; moderate risk; 3, high risk) If the veteran has normal or low risk, the PCP can encourage the veteran to remain at low risk by entering an administrative CPRS text order for the nurse care manager to offer education about daily foot care at the same visit or at a scheduled follow-up visit. This process facilitates nurse care managers to include routine foot care as integral to their usual duties coaching veterans to engage in self-care to manage chronic conditions. If the risk is assessed as moderate or high risk, PCPs are prompted to send a referral to podiatry to repeat the foot exam, verify the ARA score, and provide appropriate foot care treatment and follow-up.
On October 31, 2017, following training on the updated foot exam and ARA template with staff at the 13 VAPORHCS outpatient clinic sites, 2 sites piloted all components of the Comprehensive Foot Care program. An in-person training was completed with PCPs to review the changes of the foot care template in CPRS and to answer their questions about it. PCPs were required to complete both the 3-part foot exam and ARA at least once annually with veterans with DM.
An electronic clinical reminder was built to alert PCPs and PACTs that a veteran was either due or overdue for an exam and risk assessment. VA podiatrists agreed to complete the reminder with veterans under their care. One of the 2 sites was randomly selected for this study. Data were collected from August 1, 2017 to July 31, 2018. Patients were identified from the Diabetes Registry, a database established at VAPORHCS in 2008 to track veterans with DM to ensure quality care.11 Veterans’ personal health identifiers from the registry were used to access their health records to complete chart reviews and assess the completion, accuracy and timeliness of all foot care components.
The Diabetes Registry lists a veterans’ upcoming appointments and tracks their most recent clinic visits; laboratory tests; physical exams; and screening exams for foot, eye, and renal care. Newly diagnosed veterans are uploaded automatically into this registry by tracking all DM-related International Classification of Diseases (ICD-10) codes, hemoglobin A1c (HbA1c) levels ≥ 6.5%, or outpatient prescriptions for insulin or oral hypoglycemic agents.11
Study Design
This quality improvement project evaluated PCPs’ actions in a program process change intended to improve foot care provided with veterans at-risk for nontraumatic lower limb amputations. Audits of CPRS records and the Diabetes Registry determined the results of the practice change. Data on the total number of foot exams, amputation risk scores, appropriate podiatry referrals, administrative orders for nurse coaching, and completed foot care education were collected during the study period. Data collected for the 3-month period preceding the process change established preimplementation comparison vs the postimplementation data. Data were collected at 3, 6, and 9 months after implementation. The foot exams and ARAs were reviewed to determine whether exams and assessments were completed accurately during the pre- and post-implementation timeframes. Incomplete or clearly incorrectly completed documentation were considered inaccurate. For example, it was considered inaccurate if only the foot exam portion was completed in the assessment and the ARA was not.
Data Analysis
Data on the total number of accurately completed foot examinations and ARAs, total number of podiatry referrals, and total number of administrative text orders placed by PCPs, and education completed by nurse care managers were assessed. Statistical significance was evaluated using χ2 and Fisher exact test as appropriate. A Pearson correlation coefficient was used to determine whether there was a statistically significant increase in accurate foot examinations and ARAs as well as total number of podiatry referrals during the study period. Statistical analyses were performed using Stata 14.1 statistical software (College Station, TX).
Results
A total of 1,242 completed diabetic foot examinations were identified from August 1, 2017 to July 31, 2018 using the Diabetes Registry (Table). For the 3 months prior to the change, there were 191 appropriately completed foot examinations and ARAs. This number increased progressively over three 3-month periods (Figure 1). Within the 1-year study period, there was a statistically significant increase in the number of appropriate foot examinations (r = 0.495). PCPs placed 34 podiatry referrals during the prechange period. After the change, the number of appropriate referrals increased statistically significantly in the 3 following 3-month-periods (r = 0.222) (Figure 2).
To determine the accuracy of documentation and ratio of appropriate referrals, the 3-month prech
Notably, at the end of the first year of this evaluation, 119 veterans at the clinic did not show a recorded comprehensive foot examination since receiving a DM diagnosis and 299 veterans were due for an annual examination—a 25.2% gap of veterans without the recommended progression of foot care services. Of those that previously had a recorded foot examination, 51 (17.0%) veterans were found to be ≥ 2 years overdue.
Discussion
DM management requires a comprehensive team-based approach to help monitor for associated complications. At the VA, PACTs are veterans’ initial and primary point of contact for chronic condition management. PACTs have regular opportunities to engage veterans in initial and follow-up care and appropriate self-care. PCPs are critical in placing referrals for specialized care promptly to prevent and minimize complications such as foot ulcers, and ultimately, lower limb amputations.9,10,12
When PCPs assume responsibility for the entire foot examination, they are able to identify problems early.1 Left untreated, foot wounds and ulcers have the potential to grow into serious infections.9 Early risk identification and management can lead to increased patient satisfaction, improved life expectancy, quality of life, and ultimately, lower healthcare costs.12
Multiple studies have shown the clinical importance of foot examinations in preventative care. In one study, researchers found that completing foot examinations, among other early interventions, increased life expectancy and reduced foot complications.13 Diabetic foot management programs involving screening and categorizing patients into low- and high-risk groups had a 47.4% decrease in the incidence of amputations and 37.8% decrease in hospital admissions.14 Amputations were found to be inversely correlated with multidisciplinary foot care programs with reduction of lower limb amputations at 2 years.15 The Centers for Disease Control and Prevention found that comprehensive foot care programs that include a foot examination, ARA, appropriate referrals to specialists, and foot-care education and preventative services can reduce lower limb amputation rates by 45% to 85%.16
With one of the highest amputation rates in VA, VAPORHCS needed an integrated approach to ensure that appropriate foot care occurred regularly with veterans with DM. Prior to the process change, LPNs completed foot examinations and PCPs completed the ARA. Separating these clinical services resulted in few veterans receiving an amputation risk score. Of those with scores, the lack of a standardized program protocol resulted in discrepancies between ARAs from patient to patient as health care providers did not have clear or enough information to select the correct score and make the appropriate referrals. Thus, veterans previously identified as at moderate or high risk also lacked podiatric follow-up care.
The new quality-driven process change corrected the documentation process to nationally accepted standards. The goal was to create a consistent template in the electronic health record for all health care providers. The new template simplifies the documentation process and clarifies the amputation risk score assignment, which allows for proper foot care management. The PCP completes the process from assessment through referral, removing gaps in care and improving efficiency. Although this change was initially met with resistance from PCPs, it led to a significant increase in the number of patients with accurately documented examinations. Similarly, the number of appropriate referrals significantly rose during the study period. The standardized documentation process resulted in improved accurate examinations and ARAs over the past year. The new program also resulted in an increased number of appropriate podiatry referrals for those identified to be at moderate or high risk. This elevation of care is crucial for veterans to receive frequent follow-up visits for preventative care and/or treatment, including surgical modalities to promote limb salvage.
Barriers
This project identified several barriers to the Comprehensive Foot Care program. One major barrier was health care provider resistance to using the new process. For example, VAPORHCS podiatrists are not using the new template with established patients, which requires PCPs to complete the clinical reminder template for quality performance, an additional burden unrelated to clinical care. PCPs that do complete the foot examination/ARA templated note use the podiatrist’s visit note without personally assessing the patient.
PCPs also have been resistant to entering administrative text orders for preventative foot care in normal- or low-risk veterans (4.6% overall), which has resulted in decreased patient education (3.9% overall). Education for normal-risk and low-risk patients is designed to engage veterans in self-care and prevent risk progression, critical to prevention.
It was found that PCPs often did not ask nurses to coach normal- or low-risk veterans on preventative foot care, as suggested by the low rates at which patients were offered education. This is an area we will target with future quality improvement efforts. All patients with DM should have general education about risk factors and appropriate management of them to decrease their risk for complications.9 Preventative foot care education is a critical resource to share with patients during health coaching opportunities to clarify misunderstandings and support change talk when patients are ambivalent or resistant to change. Individual or group-based nurse visits can facilitate better coaching for patients.
At the VA, coaching begins with a conversation about what matters most to the veteran, facilitating the development of a personalized plan based on patients’ values, needs, preferences and goals.9,10,12,17 Coaching allows nurses to assess veterans’ knowledge and willingness to engage in healthy habits; and identify additional resources to help them achieve their goals.
Limitations
There are many limitations to this short quality improvement analysis. For example, only 1 of 2 clinics that piloted the program change was evaluated. In addition, there are 11 other clinics that need additional in-depth education on the program change. Although this analysis was overwhelmingly positive, it may not be as successful at other clinic sites and may be subject to the Hawthorne effect—since the 2 piloted locations knew they were being observed for the quality improvement program and may have made an extra effort to be compliant.18 Additionally, we were unable to track the records of veterans receiving care through the VA Choice Program for this analysis resulting in a lack of documentation of completed diabetic foot examinations and a lack of internal referrals to VA podiatry.
Another major limitation of this project involved calculating the number of referrals placed to podiatry. On January 1, 2018, about halfway through the program evaluation, a national VA decision enabled veterans to self-refer to podiatry, which may have limited the number of podiatry referrals placed by PCPs. Finally, patients could refuse podiatry referrals. In the 9-month postimplementation period, 57 (64.8%) veterans declined podiatry referrals, according to their CPRS records.
Although, there was an improvement in the accuracy of diabetic foot examinations, ARAs, and appropriate podiatry referrals, the ultimate goal of reducing diabetic foot ulcers and lower limb amputations was not tracked due to the limited timeframe of this analysis. Tracking these endpoints with continuous plan-do-study-act cycles are needed for this ongoing quality improvement project.
Conclusion
The goal of the VAPORHCS Comprehensive Foot Care program is to provide veterans with a program that is predictable, easy and consistent to prevent and treat foot ulcers to reduce the rate of lower limb amputations. It requires multidisciplinary team collaboration for success. Implementation of this new comprehensive program has increased the number of accurate annual foot exams, ARAs and podiatry referrals. Despite these improvements, areas of future improvement include emphasizing patient education and ongoing provider compliance with annual assessments.
Author contributions
MHG proposed the program evaluation project idea. TVQ collected and analyzed the data and wrote the manuscript. MHG oversaw the project and edited the manuscript. TVQ is the guarantor of this project and takes responsibility for the contents of this journal article.
Acknowledgments
The authors thank Tyra Haebe, VAPORHCS Prevention of Amputation in Veterans Everywhere (PAVE) Manager, and the entire VAPORHCS PAVE committee for their support in this program evaluation project.
Individuals with diabetes mellitus (DM), peripheral vascular disease, or end-stage renal disease are at risk for a nontraumatic lower limb amputation.1 Veterans have a high number of risk factors and are especially vulnerable. More than 70% of veterans enrolled in US Department of Veterans Affairs (VA) healthcare are at increased risk for developing DM due to excess weight, poor eating habits, and physical inactivity.2 One in 4 veterans has DM, compared with 1 in 6 in the general population.2
DM can lead to long-term complications including limb amputations. Annually in the US about 73,000 nontraumatic lower limb amputations are performed and > 60% occur among persons with DM.3 Complications from diabetic wounds are the cause of 90% of lower limb amputations, and foot ulcers are the most prevalent complication.4 Diabetic ulcers are slow to heal due to vascular impairments and nerve damage.5 Peripheral vascular disease, a common comorbid condition, contributes to restricted blood flow and can lead to tissue death or gangrene requiring amputation.6
Between 2010 and 2014, VA Portland Healthcare System (VAPORHCS) had one of the highest national amputation rates in VA.7 A clinical chart review found that annual foot examinations and amputation risk assessments (ARAs) were not completed with all at-risk veterans. In 2013, a VA Office of Inspector General (OIG) national report found that more than one-third of veterans enrolled in VA with DM had no documentation of required annual foot exams.8 In 2017, VA released Directive 1410, which outlined the scope of care required to prevent and treat lower limb complications and amputations for veterans at risk for primary or secondary limb loss.1 This national initiative is a comprehensive approach that engages multiprofessional teams to perform routine foot examinations and amputation risk assessments; identify and promptly treat foot ulcers; track, monitor and educate at-risk veterans; and participate in clinical education to enhance staff skills.
To decrease the amputation rate, VAPORHCS redesigned its foot-care program to comply with the national initiative. As is typical in VA, VAPORHCS uses a team-based approach in primary care. The basic 4-member team patient-aligned care team (PACT) consists of a physician or nurse practitioner (NP) primary care provider (PCP), a registered nurse (RN) care manager, a licensed practical nurse (LPN), and a medical staff assistant (MSA) for administrative support. Each PACT cares for about 1,800 veterans. Formerly, LPNs completed the annual diabetic foot exams, and PCPs verified the exams and completed the ARA based on the LPNs’ findings. If patients were moderate risk or high risk, they were referred to podiatry. The VAPORHCS audit found that not all at-risk veterans had both the foot exam and ARA completed, or were referred to podiatry when indicated. There was a need for a process improvement project to develop a seamless program consisting of all recommended foot care components crucial for timely care.
This quality improvement project sought to evaluate the effectiveness of the process changes by examining PCPs’ adoption of, and consistency in completing annual diabetic foot exams and ARAs with veterans. The goals of the project were to evaluate changes in the: (1) Number of accurate diabetic foot exams and amputation risk assessments completed with veterans with DM; (2) Number and timeliness of appropriate referrals to podiatry for an in-depth assessment and treatment of veterans found to be at moderate-to-high risk for lower limb amputations; and (3) Number of administrative text orders entered by PCPs for nurse care managers to offer foot care education and the completion of the education with veterans found to be at normal-to-low risk for lower limb amputations. The institutional review boards of VAPORHCS and Gonzaga University approved the study.
Methods
Established by the American Diabetes Association and endorsed by the American Association of Clinical Endocrinologists, the comprehensive foot exam includes a visual exam, pedal pulse checks, and a sensory exam.9,10 The templated Computerized Patient Record System (CPRS) electronic health record note specifies normal and abnormal parameters of each section. On the same template, the provider assigns an ARA score based on the results of the completed foot exam. Risk scores range from 0 to 3 (0, normal or no risk; 1, low risk, 2; moderate risk; 3, high risk) If the veteran has normal or low risk, the PCP can encourage the veteran to remain at low risk by entering an administrative CPRS text order for the nurse care manager to offer education about daily foot care at the same visit or at a scheduled follow-up visit. This process facilitates nurse care managers to include routine foot care as integral to their usual duties coaching veterans to engage in self-care to manage chronic conditions. If the risk is assessed as moderate or high risk, PCPs are prompted to send a referral to podiatry to repeat the foot exam, verify the ARA score, and provide appropriate foot care treatment and follow-up.
On October 31, 2017, following training on the updated foot exam and ARA template with staff at the 13 VAPORHCS outpatient clinic sites, 2 sites piloted all components of the Comprehensive Foot Care program. An in-person training was completed with PCPs to review the changes of the foot care template in CPRS and to answer their questions about it. PCPs were required to complete both the 3-part foot exam and ARA at least once annually with veterans with DM.
An electronic clinical reminder was built to alert PCPs and PACTs that a veteran was either due or overdue for an exam and risk assessment. VA podiatrists agreed to complete the reminder with veterans under their care. One of the 2 sites was randomly selected for this study. Data were collected from August 1, 2017 to July 31, 2018. Patients were identified from the Diabetes Registry, a database established at VAPORHCS in 2008 to track veterans with DM to ensure quality care.11 Veterans’ personal health identifiers from the registry were used to access their health records to complete chart reviews and assess the completion, accuracy and timeliness of all foot care components.
The Diabetes Registry lists a veterans’ upcoming appointments and tracks their most recent clinic visits; laboratory tests; physical exams; and screening exams for foot, eye, and renal care. Newly diagnosed veterans are uploaded automatically into this registry by tracking all DM-related International Classification of Diseases (ICD-10) codes, hemoglobin A1c (HbA1c) levels ≥ 6.5%, or outpatient prescriptions for insulin or oral hypoglycemic agents.11
Study Design
This quality improvement project evaluated PCPs’ actions in a program process change intended to improve foot care provided with veterans at-risk for nontraumatic lower limb amputations. Audits of CPRS records and the Diabetes Registry determined the results of the practice change. Data on the total number of foot exams, amputation risk scores, appropriate podiatry referrals, administrative orders for nurse coaching, and completed foot care education were collected during the study period. Data collected for the 3-month period preceding the process change established preimplementation comparison vs the postimplementation data. Data were collected at 3, 6, and 9 months after implementation. The foot exams and ARAs were reviewed to determine whether exams and assessments were completed accurately during the pre- and post-implementation timeframes. Incomplete or clearly incorrectly completed documentation were considered inaccurate. For example, it was considered inaccurate if only the foot exam portion was completed in the assessment and the ARA was not.
Data Analysis
Data on the total number of accurately completed foot examinations and ARAs, total number of podiatry referrals, and total number of administrative text orders placed by PCPs, and education completed by nurse care managers were assessed. Statistical significance was evaluated using χ2 and Fisher exact test as appropriate. A Pearson correlation coefficient was used to determine whether there was a statistically significant increase in accurate foot examinations and ARAs as well as total number of podiatry referrals during the study period. Statistical analyses were performed using Stata 14.1 statistical software (College Station, TX).
Results
A total of 1,242 completed diabetic foot examinations were identified from August 1, 2017 to July 31, 2018 using the Diabetes Registry (Table). For the 3 months prior to the change, there were 191 appropriately completed foot examinations and ARAs. This number increased progressively over three 3-month periods (Figure 1). Within the 1-year study period, there was a statistically significant increase in the number of appropriate foot examinations (r = 0.495). PCPs placed 34 podiatry referrals during the prechange period. After the change, the number of appropriate referrals increased statistically significantly in the 3 following 3-month-periods (r = 0.222) (Figure 2).
To determine the accuracy of documentation and ratio of appropriate referrals, the 3-month prech
Notably, at the end of the first year of this evaluation, 119 veterans at the clinic did not show a recorded comprehensive foot examination since receiving a DM diagnosis and 299 veterans were due for an annual examination—a 25.2% gap of veterans without the recommended progression of foot care services. Of those that previously had a recorded foot examination, 51 (17.0%) veterans were found to be ≥ 2 years overdue.
Discussion
DM management requires a comprehensive team-based approach to help monitor for associated complications. At the VA, PACTs are veterans’ initial and primary point of contact for chronic condition management. PACTs have regular opportunities to engage veterans in initial and follow-up care and appropriate self-care. PCPs are critical in placing referrals for specialized care promptly to prevent and minimize complications such as foot ulcers, and ultimately, lower limb amputations.9,10,12
When PCPs assume responsibility for the entire foot examination, they are able to identify problems early.1 Left untreated, foot wounds and ulcers have the potential to grow into serious infections.9 Early risk identification and management can lead to increased patient satisfaction, improved life expectancy, quality of life, and ultimately, lower healthcare costs.12
Multiple studies have shown the clinical importance of foot examinations in preventative care. In one study, researchers found that completing foot examinations, among other early interventions, increased life expectancy and reduced foot complications.13 Diabetic foot management programs involving screening and categorizing patients into low- and high-risk groups had a 47.4% decrease in the incidence of amputations and 37.8% decrease in hospital admissions.14 Amputations were found to be inversely correlated with multidisciplinary foot care programs with reduction of lower limb amputations at 2 years.15 The Centers for Disease Control and Prevention found that comprehensive foot care programs that include a foot examination, ARA, appropriate referrals to specialists, and foot-care education and preventative services can reduce lower limb amputation rates by 45% to 85%.16
With one of the highest amputation rates in VA, VAPORHCS needed an integrated approach to ensure that appropriate foot care occurred regularly with veterans with DM. Prior to the process change, LPNs completed foot examinations and PCPs completed the ARA. Separating these clinical services resulted in few veterans receiving an amputation risk score. Of those with scores, the lack of a standardized program protocol resulted in discrepancies between ARAs from patient to patient as health care providers did not have clear or enough information to select the correct score and make the appropriate referrals. Thus, veterans previously identified as at moderate or high risk also lacked podiatric follow-up care.
The new quality-driven process change corrected the documentation process to nationally accepted standards. The goal was to create a consistent template in the electronic health record for all health care providers. The new template simplifies the documentation process and clarifies the amputation risk score assignment, which allows for proper foot care management. The PCP completes the process from assessment through referral, removing gaps in care and improving efficiency. Although this change was initially met with resistance from PCPs, it led to a significant increase in the number of patients with accurately documented examinations. Similarly, the number of appropriate referrals significantly rose during the study period. The standardized documentation process resulted in improved accurate examinations and ARAs over the past year. The new program also resulted in an increased number of appropriate podiatry referrals for those identified to be at moderate or high risk. This elevation of care is crucial for veterans to receive frequent follow-up visits for preventative care and/or treatment, including surgical modalities to promote limb salvage.
Barriers
This project identified several barriers to the Comprehensive Foot Care program. One major barrier was health care provider resistance to using the new process. For example, VAPORHCS podiatrists are not using the new template with established patients, which requires PCPs to complete the clinical reminder template for quality performance, an additional burden unrelated to clinical care. PCPs that do complete the foot examination/ARA templated note use the podiatrist’s visit note without personally assessing the patient.
PCPs also have been resistant to entering administrative text orders for preventative foot care in normal- or low-risk veterans (4.6% overall), which has resulted in decreased patient education (3.9% overall). Education for normal-risk and low-risk patients is designed to engage veterans in self-care and prevent risk progression, critical to prevention.
It was found that PCPs often did not ask nurses to coach normal- or low-risk veterans on preventative foot care, as suggested by the low rates at which patients were offered education. This is an area we will target with future quality improvement efforts. All patients with DM should have general education about risk factors and appropriate management of them to decrease their risk for complications.9 Preventative foot care education is a critical resource to share with patients during health coaching opportunities to clarify misunderstandings and support change talk when patients are ambivalent or resistant to change. Individual or group-based nurse visits can facilitate better coaching for patients.
At the VA, coaching begins with a conversation about what matters most to the veteran, facilitating the development of a personalized plan based on patients’ values, needs, preferences and goals.9,10,12,17 Coaching allows nurses to assess veterans’ knowledge and willingness to engage in healthy habits; and identify additional resources to help them achieve their goals.
Limitations
There are many limitations to this short quality improvement analysis. For example, only 1 of 2 clinics that piloted the program change was evaluated. In addition, there are 11 other clinics that need additional in-depth education on the program change. Although this analysis was overwhelmingly positive, it may not be as successful at other clinic sites and may be subject to the Hawthorne effect—since the 2 piloted locations knew they were being observed for the quality improvement program and may have made an extra effort to be compliant.18 Additionally, we were unable to track the records of veterans receiving care through the VA Choice Program for this analysis resulting in a lack of documentation of completed diabetic foot examinations and a lack of internal referrals to VA podiatry.
Another major limitation of this project involved calculating the number of referrals placed to podiatry. On January 1, 2018, about halfway through the program evaluation, a national VA decision enabled veterans to self-refer to podiatry, which may have limited the number of podiatry referrals placed by PCPs. Finally, patients could refuse podiatry referrals. In the 9-month postimplementation period, 57 (64.8%) veterans declined podiatry referrals, according to their CPRS records.
Although, there was an improvement in the accuracy of diabetic foot examinations, ARAs, and appropriate podiatry referrals, the ultimate goal of reducing diabetic foot ulcers and lower limb amputations was not tracked due to the limited timeframe of this analysis. Tracking these endpoints with continuous plan-do-study-act cycles are needed for this ongoing quality improvement project.
Conclusion
The goal of the VAPORHCS Comprehensive Foot Care program is to provide veterans with a program that is predictable, easy and consistent to prevent and treat foot ulcers to reduce the rate of lower limb amputations. It requires multidisciplinary team collaboration for success. Implementation of this new comprehensive program has increased the number of accurate annual foot exams, ARAs and podiatry referrals. Despite these improvements, areas of future improvement include emphasizing patient education and ongoing provider compliance with annual assessments.
Author contributions
MHG proposed the program evaluation project idea. TVQ collected and analyzed the data and wrote the manuscript. MHG oversaw the project and edited the manuscript. TVQ is the guarantor of this project and takes responsibility for the contents of this journal article.
Acknowledgments
The authors thank Tyra Haebe, VAPORHCS Prevention of Amputation in Veterans Everywhere (PAVE) Manager, and the entire VAPORHCS PAVE committee for their support in this program evaluation project.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 1410, prevention of amputation in veterans everywhere (PAVE) program. http://vaww.medical surgical.va.gov/podiatry/docs/VHADirective_1410_PAVE.pdf. Published March 31, 2017. Accessed October 11, 2019.
2. US Department of Veterans Affairs. Close to 25 percent of VA patients have diabetes http://www.va.gov/health/NewsFeatures/20111115a.asp. Accessed 14 October 2017
3. Centers for Disease Control and Prevention. National diabetes statistics report, 2017: Estimates of Diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 11, 2019.
4. Gibson LW, Abbas A: Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2012;25(1):131-134
5. Boyko EJ, Monteiro-Soares M, Wheeler SGB. “Peripheral arterial disease, foot ulcers, lower extremity amputations, and diabetes.” In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health Publication; 2017:20-21,20-34.
6. National Institute of Health, National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Updated August 13, 2019. Accessed October 11, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration, Support Services Center. Amputation cube, lower amputations 2015. http://vssc.med.va.gov/AlphaIndex. [Nonpublic source, not verified]
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: Foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed October 11, 2019.
9. American Diabetes Association. Standards of medical care in diabetes - 2017. Diabetes Care. 2017;40(suppl 1):1-142.
10. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
11. Yang J, McConnachie J, Renfro R, Schreiner S, Tallett S, Winterbottom L. The diabetes registry and future panel management tool https://docplayer.net/19062632-The-diabetes-registry-and.html. Accessed October 11, 2019.
12. National Institute of Health, Centers for Disease Control and Prevention, the National Diabetes Education Program. Working together to manage diabetes: a guide for pharmcy, podiatry, optometry, and dentistry. https://www.cdc.gov/diabetes/ndep/pdfs/ppod-guide.pdf. Accessed October 11, 2019.
13. Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care. 2004;27(4):901-907.
14. Lavery LA, Wunderlich RP, Tredwell JL. Disease management for the diabetic foot: effectiveness of a diabetic foot prevention program to reduce amputations and hospitalizations. Diabetes Res Clin Pract. 2005;70(1):31-37.
15. Paisey RB, Abbott A, Levenson R, et al; South-West Cardiovascular Strategic Clinical Network peer diabetic foot service review team. Diabetes-related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the south-west of England. Diabet Med. 2017;35(1):53-62.
16. Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed October 11, 2019.
17. US Department of Veterans Affairs. Whole health for life. https://www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Updated July 20, 2017. Accessed October 11, 2019.
18. Parsons HM. What happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974;183(4128):922–9322.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA directive 1410, prevention of amputation in veterans everywhere (PAVE) program. http://vaww.medical surgical.va.gov/podiatry/docs/VHADirective_1410_PAVE.pdf. Published March 31, 2017. Accessed October 11, 2019.
2. US Department of Veterans Affairs. Close to 25 percent of VA patients have diabetes http://www.va.gov/health/NewsFeatures/20111115a.asp. Accessed 14 October 2017
3. Centers for Disease Control and Prevention. National diabetes statistics report, 2017: Estimates of Diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 11, 2019.
4. Gibson LW, Abbas A: Limb salvage for veterans with diabetes: to care for him who has borne the battle. Crit Care Nurs Clin North Am. 2012;25(1):131-134
5. Boyko EJ, Monteiro-Soares M, Wheeler SGB. “Peripheral arterial disease, foot ulcers, lower extremity amputations, and diabetes.” In: Cowie CC, Casagrande SS, Menke A, et al, eds. Diabetes in America. 3rd ed. Bethesda, MD: National Institutes of Health Publication; 2017:20-21,20-34.
6. National Institute of Health, National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Updated August 13, 2019. Accessed October 11, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration, Support Services Center. Amputation cube, lower amputations 2015. http://vssc.med.va.gov/AlphaIndex. [Nonpublic source, not verified]
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: Foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed October 11, 2019.
9. American Diabetes Association. Standards of medical care in diabetes - 2017. Diabetes Care. 2017;40(suppl 1):1-142.
10. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
11. Yang J, McConnachie J, Renfro R, Schreiner S, Tallett S, Winterbottom L. The diabetes registry and future panel management tool https://docplayer.net/19062632-The-diabetes-registry-and.html. Accessed October 11, 2019.
12. National Institute of Health, Centers for Disease Control and Prevention, the National Diabetes Education Program. Working together to manage diabetes: a guide for pharmcy, podiatry, optometry, and dentistry. https://www.cdc.gov/diabetes/ndep/pdfs/ppod-guide.pdf. Accessed October 11, 2019.
13. Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care. 2004;27(4):901-907.
14. Lavery LA, Wunderlich RP, Tredwell JL. Disease management for the diabetic foot: effectiveness of a diabetic foot prevention program to reduce amputations and hospitalizations. Diabetes Res Clin Pract. 2005;70(1):31-37.
15. Paisey RB, Abbott A, Levenson R, et al; South-West Cardiovascular Strategic Clinical Network peer diabetic foot service review team. Diabetes-related major lower limb amputation incidence is strongly related to diabetic foot service provision and improves with enhancement of services: peer review of the south-west of England. Diabet Med. 2017;35(1):53-62.
16. Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Published 2011. Accessed October 11, 2019.
17. US Department of Veterans Affairs. Whole health for life. https://www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Updated July 20, 2017. Accessed October 11, 2019.
18. Parsons HM. What happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974;183(4128):922–9322.
A Health Care Provider Intervention to Address Obesity in Patients with Diabetes (FULL)
Obesity is associated with a significant increase in mortality. It increases the risk of type 2 diabetes mellitus (T2DM), hypertension, hyperlipidemia, and coronary artery disease.1 T2DM is strongly associated with obesity in all ethnic groups.
Medical nutrition therapy and weight loss are very important for DM management.2 This includes providing education about diet modification, increased physical activity, daily calorie intake evaluation, and consistent carbohydrate intake. For patients with T2DM, health care providers (HCPs) should emphasize lowering caloric intake and inducing weight loss for those who are overweight (body mass index [BMI] between 25 and 29.9) and obese (BMI ≥ 30). This can improve glycemic control by decreasing insulin resistance. Initial recommendations for weight loss and physical activity are to lose between 5% and 10% of initial body weight and to accumulate at least 30 minutes of moderate physical activity over the course of most days of the week.3,4
Several formulas are available to estimate baseline caloric intake for weight maintenance. For weight loss of 1 to 2 pounds per week, lowering 500 to 1,000 calories from daily weight maintenance calories serves the goal. The American Diabetes Association (ADA) also suggests that HCPs recommend diet, physical activity, and behavioral therapy designed to achieve > 5% weight loss to overweight and obese patients with T2DM.5
Recognizing the clinical benefits of achieving weight loss in overweight or obese patients with T2DM, we aimed to increase the number of visits in the Endocrine Clinic at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock that addressed obesity, documented calorie goal for patients who are overweight or obese, and performed an intervention with further education for the patient.
Methods
The study population included veterans with either type 1 DM (T1DM) or T2DM with BMI > 25 on any DM control regimen. We performed a health record review of the eligible patients seen in the CAVHS Endocrine Clinic from June 1, 2016 to July 31, 2016 to determine the baseline percentage of visits that addressed obesity and provided weight loss advice to patients. We obtained a list of patients seen in the clinic during the study period from Strategic Management Service Services at CAVHS. We also obtained information that age, gender, medications, BMI, and last Endocrine clinic HCP assessment from the electronic health record. We reviewed the HCPs notes, including fellows and faculty who were involved in the patients’ treatment, to determine whether their notes documented a BMI > 25 and whether they discussed an intervention for overweight or obesity with the patient. The CAVHS Institutional Review Board reviewed and approved the initiative as a quality improvement study.
Intervention
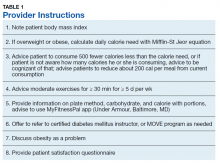
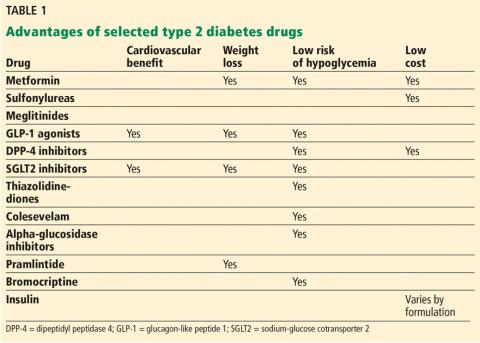
Our clinic has a defined group of HCPs that we targeted for the intervention. After getting baseline information, during August 2017 we educated these HCPs on the tools available to calculate calorie goal for the patients. We advised the HCPs to use the Mifflin St Jyor equation for estimating energy expenditure and set a goal of initial weight loss between 5% and 7% of body weight. We gave specific instructions and advice to the providers (Table 1). HCPs also received educational material to distribute to patients that provided information on the healthy plate method, discussed how to count calories, and advised them on ADA goals with carbohydrate limitation. We encouraged HCPs to recommend that patients cut between 500 and 1,000 calories daily from their current diet. HCPs also received advice to seek help from clinical dieticians and the VA MOVE! Weight Management Program when appropriate.
Study of Effect of the Intervention
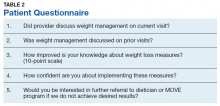
To study the effect of this intervention, we reviewed documentation by HCPs and assessed patient satisfaction. We obtained a list of patients and reviewed HCP notes on patients with BMI > 25 to assess whether providers addressed obesity in November and December 2017. We also evaluated whether HCPs offered a specific intervention to address the problem, such as providing education material to the patient or an estimate of daily calorie goal, or referring them to clinical dietician and/or the MOVE program. Patients received a 5-question survey that assessed their understanding and satisfaction at the end of the visit (Table 2).
Results
Of the 100 charts reviewed prior to intervention, HCPs discussed obesity management with only 6% of patients. After the intervention, we collected data again through chart review of the patients who were overweight or obese and seen for DM in the same clinic during a 2-month period. Of the 100 charts reviewed, we noticed that recognition and management of obesity improved to 60%.
To evaluate the impact of this intervention, patients received a questionnaire at the end of the visit. Nearly all (97%) patients mentioned that the provider discussed weight management during that visit. Most (83%) patients mentioned that weight management was discussed with them during prior visits, while 70% of patients felt their knowledge on working on weight loss had improved. Almost half (46%) were interested in further referral to a dietician or the MOVE program if they did not achieve desired results, but 78% were confident that they could implement the discussed weight management measures.
Discussion
Increased body weight is associated with worsening of DM and can result in poor glycemic control. Achieving weight loss in overweight or obese patients with DM can lead to clinical benefits; however, this is a challenge. In one study, a DM prevention program with lifestyle intervention leading to weight loss significantly reduced the rate of progression from impaired glucose tolerance to DM over a 3-year period and improved cardiovascular risk factors like elevated blood pressure and dyslipidemia.6 A randomized trial of an intensive lifestyle intervention to increase physical activity and decrease caloric intake vs standard DM education in people with T2DM showed a modest weight loss of 8.6% of initial weight at 1 year.7 This weight loss was associated with significant improvement in blood pressure, glycemic control, fasting blood glucose, high-density lipoprotein (HDL) cholesterol, and triglyceride levels and significant reductions in the use of DM, hypertension, and lipid-lowering medications.7 Obesity attributes to dyslipidemia with increased levels of cholesterol, low-density lipoprotein, very low-density lipoprotein, triglycerides, and decreased levels of HDL by about 5%.8 Obesity also is associated with hypertension, coronary heart disease, heart failure, and cardiovascular and all-cause mortality.9
Limitations
Limitations of this study include the small sample size and that multiple HCPs were involved. The nature of intervention might have differed with different HCPs or in a different setting than a VA clinic. In addition, we did not evaluate the effect on weight loss in specific patients as we only reviewed charts to check whether HCPs addressed weight loss. Nevertheless, our intervention was effective because it improved patient and provider awareness. It also gave us the opportunity to create framework for further collaborations and community building. The Endocrinology department at CAVHS is currently collaborating with the MOVE program, which is a part of the nutrition and food services. We hope to have an endocrinologist involved to provide guidance on medication management for obesity.
Conclusion
At CAVHS a simple intervention was instituted to evaluate whether HCPs were discussing weight loss in patients with DM, providing them with information to assess patients’ daily calorie goal, and prompting them for intervention to achieve weight loss. The intervention led to better management of patients with DM and obesity and greater engagement in weight loss from patients.
This project was a team effort. The clinic nurse documented patient’s BMI on the check in slip. HCPs discussed the problem and specific intervention. The clinical dieticians provided focused education for patients. The clerks collected the patient responses to questionnaire. This project also improved communication within the Endocrine Clinic team. Documentation of HCPs pertaining to addressing obesity improved by 54%. Improved patient satisfaction and insight was evident on patient responses to the questionnaire.
We believe that HCP apathy is a major contributor to the problem of obesity. Small steps like these go a long way for further management of obesity. Most VA hospitals have MOVE programs that provide dietary advice and encourage behavioral changes. However, getting patients to commit to these programs is a challenge. Primary care and endocrine clinics are important services that may help with patient awareness.
This project helped us better recognize patients with obesity and provide them with initial counseling and dietary advice. We received help from clinical dieticians and gave patients the option to join MOVE in situations where initial advice did not yield results and for more consistent follow up.
We tried to improve the care for patients with DM who were overweight or obese at CAVHS by prompting HCPs to focus on obesity as a problem and perform interventions to address this problem. The activities carried out and the data collected were used for internal quality improvement and for encouraging further interventions in the care of these patients.
1. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 suppl 2):S102-S138.
2. Evert AB, Boucher JL, Cypress M, et al; American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36(11):3821-3842.
3. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Heart, Lung, and Blood Institute; 1998.
4. US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996.
5. American Diabetes Association. 7. Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S65-S72.
6. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
7. Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374-1383.
8. Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26(5):968-976.
9. Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133(7):639-649.
Obesity is associated with a significant increase in mortality. It increases the risk of type 2 diabetes mellitus (T2DM), hypertension, hyperlipidemia, and coronary artery disease.1 T2DM is strongly associated with obesity in all ethnic groups.
Medical nutrition therapy and weight loss are very important for DM management.2 This includes providing education about diet modification, increased physical activity, daily calorie intake evaluation, and consistent carbohydrate intake. For patients with T2DM, health care providers (HCPs) should emphasize lowering caloric intake and inducing weight loss for those who are overweight (body mass index [BMI] between 25 and 29.9) and obese (BMI ≥ 30). This can improve glycemic control by decreasing insulin resistance. Initial recommendations for weight loss and physical activity are to lose between 5% and 10% of initial body weight and to accumulate at least 30 minutes of moderate physical activity over the course of most days of the week.3,4
Several formulas are available to estimate baseline caloric intake for weight maintenance. For weight loss of 1 to 2 pounds per week, lowering 500 to 1,000 calories from daily weight maintenance calories serves the goal. The American Diabetes Association (ADA) also suggests that HCPs recommend diet, physical activity, and behavioral therapy designed to achieve > 5% weight loss to overweight and obese patients with T2DM.5
Recognizing the clinical benefits of achieving weight loss in overweight or obese patients with T2DM, we aimed to increase the number of visits in the Endocrine Clinic at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock that addressed obesity, documented calorie goal for patients who are overweight or obese, and performed an intervention with further education for the patient.
Methods
The study population included veterans with either type 1 DM (T1DM) or T2DM with BMI > 25 on any DM control regimen. We performed a health record review of the eligible patients seen in the CAVHS Endocrine Clinic from June 1, 2016 to July 31, 2016 to determine the baseline percentage of visits that addressed obesity and provided weight loss advice to patients. We obtained a list of patients seen in the clinic during the study period from Strategic Management Service Services at CAVHS. We also obtained information that age, gender, medications, BMI, and last Endocrine clinic HCP assessment from the electronic health record. We reviewed the HCPs notes, including fellows and faculty who were involved in the patients’ treatment, to determine whether their notes documented a BMI > 25 and whether they discussed an intervention for overweight or obesity with the patient. The CAVHS Institutional Review Board reviewed and approved the initiative as a quality improvement study.
Intervention
Our clinic has a defined group of HCPs that we targeted for the intervention. After getting baseline information, during August 2017 we educated these HCPs on the tools available to calculate calorie goal for the patients. We advised the HCPs to use the Mifflin St Jyor equation for estimating energy expenditure and set a goal of initial weight loss between 5% and 7% of body weight. We gave specific instructions and advice to the providers (Table 1). HCPs also received educational material to distribute to patients that provided information on the healthy plate method, discussed how to count calories, and advised them on ADA goals with carbohydrate limitation. We encouraged HCPs to recommend that patients cut between 500 and 1,000 calories daily from their current diet. HCPs also received advice to seek help from clinical dieticians and the VA MOVE! Weight Management Program when appropriate.
Study of Effect of the Intervention
To study the effect of this intervention, we reviewed documentation by HCPs and assessed patient satisfaction. We obtained a list of patients and reviewed HCP notes on patients with BMI > 25 to assess whether providers addressed obesity in November and December 2017. We also evaluated whether HCPs offered a specific intervention to address the problem, such as providing education material to the patient or an estimate of daily calorie goal, or referring them to clinical dietician and/or the MOVE program. Patients received a 5-question survey that assessed their understanding and satisfaction at the end of the visit (Table 2).
Results
Of the 100 charts reviewed prior to intervention, HCPs discussed obesity management with only 6% of patients. After the intervention, we collected data again through chart review of the patients who were overweight or obese and seen for DM in the same clinic during a 2-month period. Of the 100 charts reviewed, we noticed that recognition and management of obesity improved to 60%.
To evaluate the impact of this intervention, patients received a questionnaire at the end of the visit. Nearly all (97%) patients mentioned that the provider discussed weight management during that visit. Most (83%) patients mentioned that weight management was discussed with them during prior visits, while 70% of patients felt their knowledge on working on weight loss had improved. Almost half (46%) were interested in further referral to a dietician or the MOVE program if they did not achieve desired results, but 78% were confident that they could implement the discussed weight management measures.
Discussion
Increased body weight is associated with worsening of DM and can result in poor glycemic control. Achieving weight loss in overweight or obese patients with DM can lead to clinical benefits; however, this is a challenge. In one study, a DM prevention program with lifestyle intervention leading to weight loss significantly reduced the rate of progression from impaired glucose tolerance to DM over a 3-year period and improved cardiovascular risk factors like elevated blood pressure and dyslipidemia.6 A randomized trial of an intensive lifestyle intervention to increase physical activity and decrease caloric intake vs standard DM education in people with T2DM showed a modest weight loss of 8.6% of initial weight at 1 year.7 This weight loss was associated with significant improvement in blood pressure, glycemic control, fasting blood glucose, high-density lipoprotein (HDL) cholesterol, and triglyceride levels and significant reductions in the use of DM, hypertension, and lipid-lowering medications.7 Obesity attributes to dyslipidemia with increased levels of cholesterol, low-density lipoprotein, very low-density lipoprotein, triglycerides, and decreased levels of HDL by about 5%.8 Obesity also is associated with hypertension, coronary heart disease, heart failure, and cardiovascular and all-cause mortality.9
Limitations
Limitations of this study include the small sample size and that multiple HCPs were involved. The nature of intervention might have differed with different HCPs or in a different setting than a VA clinic. In addition, we did not evaluate the effect on weight loss in specific patients as we only reviewed charts to check whether HCPs addressed weight loss. Nevertheless, our intervention was effective because it improved patient and provider awareness. It also gave us the opportunity to create framework for further collaborations and community building. The Endocrinology department at CAVHS is currently collaborating with the MOVE program, which is a part of the nutrition and food services. We hope to have an endocrinologist involved to provide guidance on medication management for obesity.
Conclusion
At CAVHS a simple intervention was instituted to evaluate whether HCPs were discussing weight loss in patients with DM, providing them with information to assess patients’ daily calorie goal, and prompting them for intervention to achieve weight loss. The intervention led to better management of patients with DM and obesity and greater engagement in weight loss from patients.
This project was a team effort. The clinic nurse documented patient’s BMI on the check in slip. HCPs discussed the problem and specific intervention. The clinical dieticians provided focused education for patients. The clerks collected the patient responses to questionnaire. This project also improved communication within the Endocrine Clinic team. Documentation of HCPs pertaining to addressing obesity improved by 54%. Improved patient satisfaction and insight was evident on patient responses to the questionnaire.
We believe that HCP apathy is a major contributor to the problem of obesity. Small steps like these go a long way for further management of obesity. Most VA hospitals have MOVE programs that provide dietary advice and encourage behavioral changes. However, getting patients to commit to these programs is a challenge. Primary care and endocrine clinics are important services that may help with patient awareness.
This project helped us better recognize patients with obesity and provide them with initial counseling and dietary advice. We received help from clinical dieticians and gave patients the option to join MOVE in situations where initial advice did not yield results and for more consistent follow up.
We tried to improve the care for patients with DM who were overweight or obese at CAVHS by prompting HCPs to focus on obesity as a problem and perform interventions to address this problem. The activities carried out and the data collected were used for internal quality improvement and for encouraging further interventions in the care of these patients.
Obesity is associated with a significant increase in mortality. It increases the risk of type 2 diabetes mellitus (T2DM), hypertension, hyperlipidemia, and coronary artery disease.1 T2DM is strongly associated with obesity in all ethnic groups.
Medical nutrition therapy and weight loss are very important for DM management.2 This includes providing education about diet modification, increased physical activity, daily calorie intake evaluation, and consistent carbohydrate intake. For patients with T2DM, health care providers (HCPs) should emphasize lowering caloric intake and inducing weight loss for those who are overweight (body mass index [BMI] between 25 and 29.9) and obese (BMI ≥ 30). This can improve glycemic control by decreasing insulin resistance. Initial recommendations for weight loss and physical activity are to lose between 5% and 10% of initial body weight and to accumulate at least 30 minutes of moderate physical activity over the course of most days of the week.3,4
Several formulas are available to estimate baseline caloric intake for weight maintenance. For weight loss of 1 to 2 pounds per week, lowering 500 to 1,000 calories from daily weight maintenance calories serves the goal. The American Diabetes Association (ADA) also suggests that HCPs recommend diet, physical activity, and behavioral therapy designed to achieve > 5% weight loss to overweight and obese patients with T2DM.5
Recognizing the clinical benefits of achieving weight loss in overweight or obese patients with T2DM, we aimed to increase the number of visits in the Endocrine Clinic at Central Arkansas Veterans Healthcare System (CAVHS) in Little Rock that addressed obesity, documented calorie goal for patients who are overweight or obese, and performed an intervention with further education for the patient.
Methods
The study population included veterans with either type 1 DM (T1DM) or T2DM with BMI > 25 on any DM control regimen. We performed a health record review of the eligible patients seen in the CAVHS Endocrine Clinic from June 1, 2016 to July 31, 2016 to determine the baseline percentage of visits that addressed obesity and provided weight loss advice to patients. We obtained a list of patients seen in the clinic during the study period from Strategic Management Service Services at CAVHS. We also obtained information that age, gender, medications, BMI, and last Endocrine clinic HCP assessment from the electronic health record. We reviewed the HCPs notes, including fellows and faculty who were involved in the patients’ treatment, to determine whether their notes documented a BMI > 25 and whether they discussed an intervention for overweight or obesity with the patient. The CAVHS Institutional Review Board reviewed and approved the initiative as a quality improvement study.
Intervention
Our clinic has a defined group of HCPs that we targeted for the intervention. After getting baseline information, during August 2017 we educated these HCPs on the tools available to calculate calorie goal for the patients. We advised the HCPs to use the Mifflin St Jyor equation for estimating energy expenditure and set a goal of initial weight loss between 5% and 7% of body weight. We gave specific instructions and advice to the providers (Table 1). HCPs also received educational material to distribute to patients that provided information on the healthy plate method, discussed how to count calories, and advised them on ADA goals with carbohydrate limitation. We encouraged HCPs to recommend that patients cut between 500 and 1,000 calories daily from their current diet. HCPs also received advice to seek help from clinical dieticians and the VA MOVE! Weight Management Program when appropriate.
Study of Effect of the Intervention
To study the effect of this intervention, we reviewed documentation by HCPs and assessed patient satisfaction. We obtained a list of patients and reviewed HCP notes on patients with BMI > 25 to assess whether providers addressed obesity in November and December 2017. We also evaluated whether HCPs offered a specific intervention to address the problem, such as providing education material to the patient or an estimate of daily calorie goal, or referring them to clinical dietician and/or the MOVE program. Patients received a 5-question survey that assessed their understanding and satisfaction at the end of the visit (Table 2).
Results
Of the 100 charts reviewed prior to intervention, HCPs discussed obesity management with only 6% of patients. After the intervention, we collected data again through chart review of the patients who were overweight or obese and seen for DM in the same clinic during a 2-month period. Of the 100 charts reviewed, we noticed that recognition and management of obesity improved to 60%.
To evaluate the impact of this intervention, patients received a questionnaire at the end of the visit. Nearly all (97%) patients mentioned that the provider discussed weight management during that visit. Most (83%) patients mentioned that weight management was discussed with them during prior visits, while 70% of patients felt their knowledge on working on weight loss had improved. Almost half (46%) were interested in further referral to a dietician or the MOVE program if they did not achieve desired results, but 78% were confident that they could implement the discussed weight management measures.
Discussion
Increased body weight is associated with worsening of DM and can result in poor glycemic control. Achieving weight loss in overweight or obese patients with DM can lead to clinical benefits; however, this is a challenge. In one study, a DM prevention program with lifestyle intervention leading to weight loss significantly reduced the rate of progression from impaired glucose tolerance to DM over a 3-year period and improved cardiovascular risk factors like elevated blood pressure and dyslipidemia.6 A randomized trial of an intensive lifestyle intervention to increase physical activity and decrease caloric intake vs standard DM education in people with T2DM showed a modest weight loss of 8.6% of initial weight at 1 year.7 This weight loss was associated with significant improvement in blood pressure, glycemic control, fasting blood glucose, high-density lipoprotein (HDL) cholesterol, and triglyceride levels and significant reductions in the use of DM, hypertension, and lipid-lowering medications.7 Obesity attributes to dyslipidemia with increased levels of cholesterol, low-density lipoprotein, very low-density lipoprotein, triglycerides, and decreased levels of HDL by about 5%.8 Obesity also is associated with hypertension, coronary heart disease, heart failure, and cardiovascular and all-cause mortality.9
Limitations
Limitations of this study include the small sample size and that multiple HCPs were involved. The nature of intervention might have differed with different HCPs or in a different setting than a VA clinic. In addition, we did not evaluate the effect on weight loss in specific patients as we only reviewed charts to check whether HCPs addressed weight loss. Nevertheless, our intervention was effective because it improved patient and provider awareness. It also gave us the opportunity to create framework for further collaborations and community building. The Endocrinology department at CAVHS is currently collaborating with the MOVE program, which is a part of the nutrition and food services. We hope to have an endocrinologist involved to provide guidance on medication management for obesity.
Conclusion
At CAVHS a simple intervention was instituted to evaluate whether HCPs were discussing weight loss in patients with DM, providing them with information to assess patients’ daily calorie goal, and prompting them for intervention to achieve weight loss. The intervention led to better management of patients with DM and obesity and greater engagement in weight loss from patients.
This project was a team effort. The clinic nurse documented patient’s BMI on the check in slip. HCPs discussed the problem and specific intervention. The clinical dieticians provided focused education for patients. The clerks collected the patient responses to questionnaire. This project also improved communication within the Endocrine Clinic team. Documentation of HCPs pertaining to addressing obesity improved by 54%. Improved patient satisfaction and insight was evident on patient responses to the questionnaire.
We believe that HCP apathy is a major contributor to the problem of obesity. Small steps like these go a long way for further management of obesity. Most VA hospitals have MOVE programs that provide dietary advice and encourage behavioral changes. However, getting patients to commit to these programs is a challenge. Primary care and endocrine clinics are important services that may help with patient awareness.
This project helped us better recognize patients with obesity and provide them with initial counseling and dietary advice. We received help from clinical dieticians and gave patients the option to join MOVE in situations where initial advice did not yield results and for more consistent follow up.
We tried to improve the care for patients with DM who were overweight or obese at CAVHS by prompting HCPs to focus on obesity as a problem and perform interventions to address this problem. The activities carried out and the data collected were used for internal quality improvement and for encouraging further interventions in the care of these patients.
1. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 suppl 2):S102-S138.
2. Evert AB, Boucher JL, Cypress M, et al; American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36(11):3821-3842.
3. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Heart, Lung, and Blood Institute; 1998.
4. US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996.
5. American Diabetes Association. 7. Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S65-S72.
6. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
7. Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374-1383.
8. Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26(5):968-976.
9. Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133(7):639-649.
1. Jensen MD, Ryan DH, Apovian CM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 suppl 2):S102-S138.
2. Evert AB, Boucher JL, Cypress M, et al; American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36(11):3821-3842.
3. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Heart, Lung, and Blood Institute; 1998.
4. US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996.
5. American Diabetes Association. 7. Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S65-S72.
6. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
7. Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374-1383.
8. Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26(5):968-976.
9. Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133(7):639-649.
Signs of adult diabetes apparent in very young children
BARCELONA – Disturbed HDL cholesterol metabolism is one of the earliest features that may predispose individuals to the development of type 2 diabetes, according to data from a genetics and metabolomics study conducted in the United Kingdom.
Changes in HDL cholesterol metabolism were seen in children as young as 8 years, decades before the clinical onset of disease, Joshua Bell, PhD, a research fellow at the University of Bristol (England), reported at the annual meeting of the European Association for the Study of Diabetes.
“We know that type 2 diabetes certainly doesn’t develop overnight,” Dr. Bell said. Indeed, data exist showing that there are changes in glucose metabolism several years before a formal diagnosis may be made in adults. “What we don’t know is what the very earliest features of diabetes look like,” he added.
“The main assumption is that type 2 diabetes is a metabolic disease, and so disease features are visible in systemic metabolism,” explained Dr. Bell. What was not clear, however, was that if any metabolic features – seen mainly in observational studies and in adults – were caused by the disease itself or perhaps were independent causes of type 2 diabetes.To investigate, Dr. Bell and associates performed a study linking genetic liability with metabolomic data collected at four time points from 4,761 offspring from participants in the Avon Longitudinal Study of Parents and Children cohort, which is also known as the Children of the 90s cohort. More than 200 metabolic traits were considered, and a genetic risk score comprising more than 162 single nucleotide polymorphisms previously linked to adult type 2 diabetes was used.
The metabolomic traits considered included lipoprotein subclass-specific cholesterol and triglyceride content, amino and fatty acids, and inflammatory glycoprotein acetyls, which had been measured in childhood at the age of 8 years, in adolescence at 16 years, in young adulthood at 18 years, and in adulthood at 25 years.
Early metabolic features of type 2 diabetes liability were grouped together and one feature that stood out was the sizes of lipid particles. In particular, it was the size of HDL cholesterol particle subtypes in children at the age of 8 years. Before other types of changes in lipid particles were being seen, there were reductions in the lipid content of HDL cholesterol particle subtypes, notably those that were very large.
By age 16 years, strong associations remained with lower lipids in HDL cholesterol particle subtypes and type 2 diabetes liability, which became stronger with preglycemic traits, such as citrate, and with glycoprotein acetyls. By age 18 years, elevations were seen in branched amino acids, and by age 25, association had strengthened for the lipid content of very low–density lipoprotein cholesterol.
“Linking genetic liability to adult disease with traits measured much earlier in life can tell you something about how the disease activity unfolds over a lifetime,” Dr. Bell said, adding that the feature that was “most consistently tracked” could be evaluated and could help reveal whether or not an individual might go on to develop type 2 diabetes.
In a press release issued by the EASD, Dr. Bell observed: “It’s remarkable that we can see signs of adult diabetes in the blood from such a young age. Knowing what early features of type 2 diabetes look like, could help us to intervene much earlier to halt progression to full-blown diabetes and its complications.”
The study was funded by Diabetes U.K., Cancer Research U.K., the Elizabeth Blackwell Institute for Health Research, the Wellcome Trust, the Medical Research Council, and the University of Bristol. Dr. Bell said he had no conflicts of interest to declare.
SOURCE: Bell J et al. bioRxiv. 2019 Sep 17. doi: 10.1101/767756.
BARCELONA – Disturbed HDL cholesterol metabolism is one of the earliest features that may predispose individuals to the development of type 2 diabetes, according to data from a genetics and metabolomics study conducted in the United Kingdom.
Changes in HDL cholesterol metabolism were seen in children as young as 8 years, decades before the clinical onset of disease, Joshua Bell, PhD, a research fellow at the University of Bristol (England), reported at the annual meeting of the European Association for the Study of Diabetes.
“We know that type 2 diabetes certainly doesn’t develop overnight,” Dr. Bell said. Indeed, data exist showing that there are changes in glucose metabolism several years before a formal diagnosis may be made in adults. “What we don’t know is what the very earliest features of diabetes look like,” he added.
“The main assumption is that type 2 diabetes is a metabolic disease, and so disease features are visible in systemic metabolism,” explained Dr. Bell. What was not clear, however, was that if any metabolic features – seen mainly in observational studies and in adults – were caused by the disease itself or perhaps were independent causes of type 2 diabetes.To investigate, Dr. Bell and associates performed a study linking genetic liability with metabolomic data collected at four time points from 4,761 offspring from participants in the Avon Longitudinal Study of Parents and Children cohort, which is also known as the Children of the 90s cohort. More than 200 metabolic traits were considered, and a genetic risk score comprising more than 162 single nucleotide polymorphisms previously linked to adult type 2 diabetes was used.
The metabolomic traits considered included lipoprotein subclass-specific cholesterol and triglyceride content, amino and fatty acids, and inflammatory glycoprotein acetyls, which had been measured in childhood at the age of 8 years, in adolescence at 16 years, in young adulthood at 18 years, and in adulthood at 25 years.
Early metabolic features of type 2 diabetes liability were grouped together and one feature that stood out was the sizes of lipid particles. In particular, it was the size of HDL cholesterol particle subtypes in children at the age of 8 years. Before other types of changes in lipid particles were being seen, there were reductions in the lipid content of HDL cholesterol particle subtypes, notably those that were very large.
By age 16 years, strong associations remained with lower lipids in HDL cholesterol particle subtypes and type 2 diabetes liability, which became stronger with preglycemic traits, such as citrate, and with glycoprotein acetyls. By age 18 years, elevations were seen in branched amino acids, and by age 25, association had strengthened for the lipid content of very low–density lipoprotein cholesterol.
“Linking genetic liability to adult disease with traits measured much earlier in life can tell you something about how the disease activity unfolds over a lifetime,” Dr. Bell said, adding that the feature that was “most consistently tracked” could be evaluated and could help reveal whether or not an individual might go on to develop type 2 diabetes.
In a press release issued by the EASD, Dr. Bell observed: “It’s remarkable that we can see signs of adult diabetes in the blood from such a young age. Knowing what early features of type 2 diabetes look like, could help us to intervene much earlier to halt progression to full-blown diabetes and its complications.”
The study was funded by Diabetes U.K., Cancer Research U.K., the Elizabeth Blackwell Institute for Health Research, the Wellcome Trust, the Medical Research Council, and the University of Bristol. Dr. Bell said he had no conflicts of interest to declare.
SOURCE: Bell J et al. bioRxiv. 2019 Sep 17. doi: 10.1101/767756.
BARCELONA – Disturbed HDL cholesterol metabolism is one of the earliest features that may predispose individuals to the development of type 2 diabetes, according to data from a genetics and metabolomics study conducted in the United Kingdom.
Changes in HDL cholesterol metabolism were seen in children as young as 8 years, decades before the clinical onset of disease, Joshua Bell, PhD, a research fellow at the University of Bristol (England), reported at the annual meeting of the European Association for the Study of Diabetes.
“We know that type 2 diabetes certainly doesn’t develop overnight,” Dr. Bell said. Indeed, data exist showing that there are changes in glucose metabolism several years before a formal diagnosis may be made in adults. “What we don’t know is what the very earliest features of diabetes look like,” he added.
“The main assumption is that type 2 diabetes is a metabolic disease, and so disease features are visible in systemic metabolism,” explained Dr. Bell. What was not clear, however, was that if any metabolic features – seen mainly in observational studies and in adults – were caused by the disease itself or perhaps were independent causes of type 2 diabetes.To investigate, Dr. Bell and associates performed a study linking genetic liability with metabolomic data collected at four time points from 4,761 offspring from participants in the Avon Longitudinal Study of Parents and Children cohort, which is also known as the Children of the 90s cohort. More than 200 metabolic traits were considered, and a genetic risk score comprising more than 162 single nucleotide polymorphisms previously linked to adult type 2 diabetes was used.
The metabolomic traits considered included lipoprotein subclass-specific cholesterol and triglyceride content, amino and fatty acids, and inflammatory glycoprotein acetyls, which had been measured in childhood at the age of 8 years, in adolescence at 16 years, in young adulthood at 18 years, and in adulthood at 25 years.
Early metabolic features of type 2 diabetes liability were grouped together and one feature that stood out was the sizes of lipid particles. In particular, it was the size of HDL cholesterol particle subtypes in children at the age of 8 years. Before other types of changes in lipid particles were being seen, there were reductions in the lipid content of HDL cholesterol particle subtypes, notably those that were very large.
By age 16 years, strong associations remained with lower lipids in HDL cholesterol particle subtypes and type 2 diabetes liability, which became stronger with preglycemic traits, such as citrate, and with glycoprotein acetyls. By age 18 years, elevations were seen in branched amino acids, and by age 25, association had strengthened for the lipid content of very low–density lipoprotein cholesterol.
“Linking genetic liability to adult disease with traits measured much earlier in life can tell you something about how the disease activity unfolds over a lifetime,” Dr. Bell said, adding that the feature that was “most consistently tracked” could be evaluated and could help reveal whether or not an individual might go on to develop type 2 diabetes.
In a press release issued by the EASD, Dr. Bell observed: “It’s remarkable that we can see signs of adult diabetes in the blood from such a young age. Knowing what early features of type 2 diabetes look like, could help us to intervene much earlier to halt progression to full-blown diabetes and its complications.”
The study was funded by Diabetes U.K., Cancer Research U.K., the Elizabeth Blackwell Institute for Health Research, the Wellcome Trust, the Medical Research Council, and the University of Bristol. Dr. Bell said he had no conflicts of interest to declare.
SOURCE: Bell J et al. bioRxiv. 2019 Sep 17. doi: 10.1101/767756.
REPORTING FROM EASD 2019
Body weight influences SGLT2-inhibitor effects in type 1 diabetes
BARCELONA – Individuals with type 1 diabetes and a high body mass index gain the most benefit with the least risk when sodium-glucose cotransporter 2 (SGLT2) inhibitors are added to insulin therapy, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
Results from new analyses of the inTandem 1 and inTandem 2 trials with sotagliflozin (Zynquista), and the DEPICT-1 and DEPICT-2 trials with dapagliflozin (Farxiga), support the recent decision of the European Medicines Agency to license the use of the drugs only in patients with a BMI of 27 kg/m2 or higher.
inTandem with sotagliflozin
Weight gain is a challenge in patients with type 1 diabetes, said Thomas Danne, MD, who presented post hoc data from the two inTandem studies. “It’s a little bit counterintuitive,” he acknowledged, “but you have to realize, particularly in patients who have hypoglycemia, that they have to take in extra carbohydrates,” which may tip them to becoming overweight or obese.
SGLT2-inhibitor therapy with sotagliflozin or dapagliflozin added to insulin therapy has been shown to reduce body weight in individuals with type 1 diabetes, but there is an increased risk for diabetic ketoacidosis (DKA). That risk, however, seems to be lower in the higher body-weight categories.
Dr. Danne, director of the department of general pediatrics, endocrinology, and diabetology, and clinical research at the Auf der Bult Hospital for Children and Adolescents, at the Hannover (Germany) Medical School, presented data looking at the outcomes of patients treated with sotagliflozin or placebo based on their BMI.
In all, 1,575 patients were included in the analysis, of whom 659 were of normal weight (BMI of less than 27 kg/m2; average mean, 24 kg/m2 at baseline), and 916 had a higher weight (BMI of 27 kg/m2 or higher; average mean, 32 kg/m2 at baseline). The mean age of patients at study entry was 42 years for those with the lower BMI, and 45 years for those with the higher BMI.
Patients in the two inTandem trials had been treated with insulin plus placebo (n = 228, BMI less than 27 kg/m2; n = 298, BMI 27 kg/m2 or higher), or insulin plus sotagliflozin at a dose of 200 mg (n = 219, BMI less than 27 kg/m2; n = 305, BMI 27 kg/m2 or higher) or 400 mg (n = 212; BMI less than 27 kg/m2; n = 313, BMI 27 kg/m2 or higher).
Glycemic control and body weight
Greater reductions in glycated hemoglobin (HbA1c) were seen with sotagliflozin versus placebo, and even more so, if the BMI was 27 kg/m2 or higher. At week 24, the least-squares mean difference in HbA1c comparing sotagliflozin 200 mg and placebo was –0.32 in patients with the lower BMI, compared with –0.39 in those with the higher BMI. Corresponding values for the 400-mg sotagliflozin group in the higher-BMI group were –0.27 and –0.45, respectively (P less than .001 for all comparisons).
In the lower-BMI group, week 24 least-squares mean differences in body weight comparing sotagliflozin and placebo were –2.06 kg for the 200-mg group and –2.55 kg for the 400-mg group, and –2.27 kg and 3.32 kg in the higher-BMI group (P less than .001 for all comparisons).
“This is why this class of drugs holds so much of a promise, [because] it’s not only one good effect regarding improvement of glycemia judged by A1c,” Dr. Danne said.
He also reported that treatment with sotagliflozin was associated with an increased time in range, compared with placebo, again, with greater effects seen in the higher- versus lower-BMI groups. In those with a BMI of 27 kg/m2 or more, there was an additional 1 hour 58 minutes time in range for the 200-mg dose, and 3 hours 37 minutes for the 200-mg dose, compared with an extra 24 minutes and 1 hour 31 minutes, respectively, in the lower-BMI category.
“We also see a trend to improved reduction in systolic blood pressure in those with the higher BMI,” Dr. Danne said.
Risk for DKA
“The big charm of these drugs is that not only do you improve A1c and all the other good things, but also you do this without increasing the risk of hypoglycemia,” said Dr. Danne. “Again, you can see a trend of a lower risk of severe hypoglycemia for both sotagliflozin doses [compared with placebo] in the group with the body mass index of greater than 27 kg/m2 [versus BMI of less than 27 kg/m2].”
The risk of DKA was higher than placebo in both BMI groups, but the number of DKA events was very small when comparing the low and high body weight categories (0 and 1 events, respectively, in the placebo groups; 7 and 9, in the sotagliflozin 200-mg group; and 9 and 11, in the 400-mg group. The absolute risk difference in the exposure adjusted incidence rate was slightly lower in the lower-BMI group, he said, but the numbers were so small that it is difficult to draw conclusions from that finding.
“There is no doubt that we have an increase for the risk of DKA with this class of drugs in general ... but it is futile to discuss whether or not, just on the basis of a body mass index or something else, we will be able to reduce it in a big fashion,” Dr. Danne suggested.
Body weight and composition
Other data on the long-term effect of sotagliflozin on body weight and composition were presented by Sangeeta Sawhney, MD, vice-president of clinical development at Lexicon Pharmaceuticals, Chapel Hill, North Carolina.
She presented data from the DEXA substudy of the inTandem phase 3 studies in which 243 patients underwent fat mass and bone density scanning.
SGLT2 inhibitors are associated with weight loss through glycuresis and net caloric loss, Dr. Sawhney reminded the audience. As sotagliflozin is a dual inhibitor of SGLT1 and SGLT2, however, it is important to estimate the contribution of changes in fat mass and lean mass to the weight loss that could be achieved with the drug.
Pooled data from the inTandem 1 and inTandem 2 studies showed that at week 24, there were reductions in body weight of –1.7 kg and –2.6 kg with sotagliflozin 200 mg and 400 mg, respectively, and at 52 weeks, reductions of –1.9 kg and –2.9 kg. However, there was an increase in body weight with placebo (+0.5 and +0.8 kg, respectively).
For the substudy, patients underwent dual-energy x-ray absorptiometry at baseline and weeks 24 and 52. Fat mass was measured at all three time points, and bone density was evaluated at the start and end of the study.
The least-square mean change in total fat mass from baseline to week 24 and week 52 were +0.6 and +0.1 kg, respectively, for placebo, –1.6 and –1.6 kg for the sotagliflozin 200-mg dose; and –1.9 and –2.1 kg for the 400-mg dose, “which really parallels the reduction in total body weight,” Dr. Sawhney observed.
The changes in total lean mass were much smaller for sotagliflozin, she added, at –0.6 kg at week 24 and 0.3 kg at week 52 for the 200-mg dose, and –0.7 kg and –0.4 kg, respectively, for the 400-mg dose, and rises in lean mass of 0.2 kg and 0.4 kg, respectively, in placebo.
Taken together, these data show that “about 80% of the body weight reduction is really from the fat mass, and a much smaller proportion of the total body weight reduction is really coming from the lean fat mass,” said Dr. Sawhney.
DEPICT with dapagliflozin
In a poster, Paresh Dandona, MD, PhD, of the State University of New York at Buffalo, and associates presented data from a pooled analysis of the DEPICT-1 and DEPICT-2 studies looking at safety and efficacy outcomes with dapagliflozin according to five BMI categories: less than or equal to 23 kg/m2; greater than 23 kg/m2 to less than or equal to 25 kg/m2; greater than 25 kg/m2 to less than or equal to 27 kg/m2; greater than 27 kg/m2 to less than or equal to 30 kg/m2; and greater than 30 kg/m2.
The pooled analysis included 548 patients treated with dapagliflozin 5 mg and 532 who received placebo. The investigators found that patients with higher BMIs who were treated with dapagliflozin had greater weight loss, showed a trend toward achieving an HbA1c reduction of 5.5 mmol/mol (greater than or equal to 0.5%) or more without the risk of severe hypoglycemia, and had fewer episodes of definite DKA, compared with those with those with lower BMIs.
The adjusted mean percentage change from baseline in body weight in the lowest BMI (less than or equal to 23 kg/m2) group at week 24 was +0.06 kg for placebo and –2.71 kg for dapagliflozin, and at week 52, +0.33 kg and –2.91 kg, respectively. Corresponding values comparing placebo and dapagliflozin at 24 and 52 weeks in the highest BMI group (greater than 30 kg/m2) were –0.30 kg and –3.03 kg, and +0.56 and –3.58 kg.
Odds ratios for achieving an HbA1c reduction of 5.5 mmol/mol (greater than or equal to 0.5%) without severe hypoglycemia at week 24 with dapagliflozin, compared with placebo, were, in increasing order of BMI groups: 1.85, 1.93, 3.87, 2.91, and 4.20.
“Generally, more events of definite DKA were observed in patients treated with dapagliflozin than in those treated with placebo,” but there were fewer events as BMI increased, Dr. Dandona and associates reported. “These data should be interpreted with caution due to the low number of events in each subgroup,” they added.
The number of adjudicated DKA events comparing dapagliflozin and placebo across the BMI groups were: 4 versus 1 (BMI less than or equal to 23 kg/m2); 6 versus 1 (BMI greater than 23 kg/m2 to less than or equal to 25 kg/m2); 7 versus 1 (BMI greater than 25 kg/m2 to less than or equal to 27 kg/m2); 3 versus 1 (BMI greater than 27 kg/m2 to less than or equal to 30 kg/m2); and 2 versus 1 (BMI greater than 30 kg/m2).
In regard to limitations, “this was a post hoc analysis,” the investigators noted, adding that the studies were not originally powered for comparison between BMI subgroups, so the results should be considered exploratory. Moreover, DKA and hypoglycemia were strictly monitored in the trials, which “may differ from real-world situations,” they said.
The inTandem studies were sponsored by Lexicon and Sanofi. Dr. Danne disclosed receiving research funding and serving as a consultant, advisory board or steering committee member, or speaker for various companies, including Sanofi. Dr. Sawhney is an employee of and holds stoke in Lexicon. The DEPICT studies were sponsored by AstraZeneca. The lead author, Dr. Dandona, disclosed employment or consultancy services for multiple companies, including AstraZeneca.
SOURCE: Danne T et al. EASD 2018, Oral Presentation 2; Dandona P et al. EASD 2019, ePoster 720; Sawhney S et al. EASD 2019, Oral Presentation 3.
BARCELONA – Individuals with type 1 diabetes and a high body mass index gain the most benefit with the least risk when sodium-glucose cotransporter 2 (SGLT2) inhibitors are added to insulin therapy, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
Results from new analyses of the inTandem 1 and inTandem 2 trials with sotagliflozin (Zynquista), and the DEPICT-1 and DEPICT-2 trials with dapagliflozin (Farxiga), support the recent decision of the European Medicines Agency to license the use of the drugs only in patients with a BMI of 27 kg/m2 or higher.
inTandem with sotagliflozin
Weight gain is a challenge in patients with type 1 diabetes, said Thomas Danne, MD, who presented post hoc data from the two inTandem studies. “It’s a little bit counterintuitive,” he acknowledged, “but you have to realize, particularly in patients who have hypoglycemia, that they have to take in extra carbohydrates,” which may tip them to becoming overweight or obese.
SGLT2-inhibitor therapy with sotagliflozin or dapagliflozin added to insulin therapy has been shown to reduce body weight in individuals with type 1 diabetes, but there is an increased risk for diabetic ketoacidosis (DKA). That risk, however, seems to be lower in the higher body-weight categories.
Dr. Danne, director of the department of general pediatrics, endocrinology, and diabetology, and clinical research at the Auf der Bult Hospital for Children and Adolescents, at the Hannover (Germany) Medical School, presented data looking at the outcomes of patients treated with sotagliflozin or placebo based on their BMI.
In all, 1,575 patients were included in the analysis, of whom 659 were of normal weight (BMI of less than 27 kg/m2; average mean, 24 kg/m2 at baseline), and 916 had a higher weight (BMI of 27 kg/m2 or higher; average mean, 32 kg/m2 at baseline). The mean age of patients at study entry was 42 years for those with the lower BMI, and 45 years for those with the higher BMI.
Patients in the two inTandem trials had been treated with insulin plus placebo (n = 228, BMI less than 27 kg/m2; n = 298, BMI 27 kg/m2 or higher), or insulin plus sotagliflozin at a dose of 200 mg (n = 219, BMI less than 27 kg/m2; n = 305, BMI 27 kg/m2 or higher) or 400 mg (n = 212; BMI less than 27 kg/m2; n = 313, BMI 27 kg/m2 or higher).
Glycemic control and body weight
Greater reductions in glycated hemoglobin (HbA1c) were seen with sotagliflozin versus placebo, and even more so, if the BMI was 27 kg/m2 or higher. At week 24, the least-squares mean difference in HbA1c comparing sotagliflozin 200 mg and placebo was –0.32 in patients with the lower BMI, compared with –0.39 in those with the higher BMI. Corresponding values for the 400-mg sotagliflozin group in the higher-BMI group were –0.27 and –0.45, respectively (P less than .001 for all comparisons).
In the lower-BMI group, week 24 least-squares mean differences in body weight comparing sotagliflozin and placebo were –2.06 kg for the 200-mg group and –2.55 kg for the 400-mg group, and –2.27 kg and 3.32 kg in the higher-BMI group (P less than .001 for all comparisons).
“This is why this class of drugs holds so much of a promise, [because] it’s not only one good effect regarding improvement of glycemia judged by A1c,” Dr. Danne said.
He also reported that treatment with sotagliflozin was associated with an increased time in range, compared with placebo, again, with greater effects seen in the higher- versus lower-BMI groups. In those with a BMI of 27 kg/m2 or more, there was an additional 1 hour 58 minutes time in range for the 200-mg dose, and 3 hours 37 minutes for the 200-mg dose, compared with an extra 24 minutes and 1 hour 31 minutes, respectively, in the lower-BMI category.
“We also see a trend to improved reduction in systolic blood pressure in those with the higher BMI,” Dr. Danne said.
Risk for DKA
“The big charm of these drugs is that not only do you improve A1c and all the other good things, but also you do this without increasing the risk of hypoglycemia,” said Dr. Danne. “Again, you can see a trend of a lower risk of severe hypoglycemia for both sotagliflozin doses [compared with placebo] in the group with the body mass index of greater than 27 kg/m2 [versus BMI of less than 27 kg/m2].”
The risk of DKA was higher than placebo in both BMI groups, but the number of DKA events was very small when comparing the low and high body weight categories (0 and 1 events, respectively, in the placebo groups; 7 and 9, in the sotagliflozin 200-mg group; and 9 and 11, in the 400-mg group. The absolute risk difference in the exposure adjusted incidence rate was slightly lower in the lower-BMI group, he said, but the numbers were so small that it is difficult to draw conclusions from that finding.
“There is no doubt that we have an increase for the risk of DKA with this class of drugs in general ... but it is futile to discuss whether or not, just on the basis of a body mass index or something else, we will be able to reduce it in a big fashion,” Dr. Danne suggested.
Body weight and composition
Other data on the long-term effect of sotagliflozin on body weight and composition were presented by Sangeeta Sawhney, MD, vice-president of clinical development at Lexicon Pharmaceuticals, Chapel Hill, North Carolina.
She presented data from the DEXA substudy of the inTandem phase 3 studies in which 243 patients underwent fat mass and bone density scanning.
SGLT2 inhibitors are associated with weight loss through glycuresis and net caloric loss, Dr. Sawhney reminded the audience. As sotagliflozin is a dual inhibitor of SGLT1 and SGLT2, however, it is important to estimate the contribution of changes in fat mass and lean mass to the weight loss that could be achieved with the drug.
Pooled data from the inTandem 1 and inTandem 2 studies showed that at week 24, there were reductions in body weight of –1.7 kg and –2.6 kg with sotagliflozin 200 mg and 400 mg, respectively, and at 52 weeks, reductions of –1.9 kg and –2.9 kg. However, there was an increase in body weight with placebo (+0.5 and +0.8 kg, respectively).
For the substudy, patients underwent dual-energy x-ray absorptiometry at baseline and weeks 24 and 52. Fat mass was measured at all three time points, and bone density was evaluated at the start and end of the study.
The least-square mean change in total fat mass from baseline to week 24 and week 52 were +0.6 and +0.1 kg, respectively, for placebo, –1.6 and –1.6 kg for the sotagliflozin 200-mg dose; and –1.9 and –2.1 kg for the 400-mg dose, “which really parallels the reduction in total body weight,” Dr. Sawhney observed.
The changes in total lean mass were much smaller for sotagliflozin, she added, at –0.6 kg at week 24 and 0.3 kg at week 52 for the 200-mg dose, and –0.7 kg and –0.4 kg, respectively, for the 400-mg dose, and rises in lean mass of 0.2 kg and 0.4 kg, respectively, in placebo.
Taken together, these data show that “about 80% of the body weight reduction is really from the fat mass, and a much smaller proportion of the total body weight reduction is really coming from the lean fat mass,” said Dr. Sawhney.
DEPICT with dapagliflozin
In a poster, Paresh Dandona, MD, PhD, of the State University of New York at Buffalo, and associates presented data from a pooled analysis of the DEPICT-1 and DEPICT-2 studies looking at safety and efficacy outcomes with dapagliflozin according to five BMI categories: less than or equal to 23 kg/m2; greater than 23 kg/m2 to less than or equal to 25 kg/m2; greater than 25 kg/m2 to less than or equal to 27 kg/m2; greater than 27 kg/m2 to less than or equal to 30 kg/m2; and greater than 30 kg/m2.
The pooled analysis included 548 patients treated with dapagliflozin 5 mg and 532 who received placebo. The investigators found that patients with higher BMIs who were treated with dapagliflozin had greater weight loss, showed a trend toward achieving an HbA1c reduction of 5.5 mmol/mol (greater than or equal to 0.5%) or more without the risk of severe hypoglycemia, and had fewer episodes of definite DKA, compared with those with those with lower BMIs.
The adjusted mean percentage change from baseline in body weight in the lowest BMI (less than or equal to 23 kg/m2) group at week 24 was +0.06 kg for placebo and –2.71 kg for dapagliflozin, and at week 52, +0.33 kg and –2.91 kg, respectively. Corresponding values comparing placebo and dapagliflozin at 24 and 52 weeks in the highest BMI group (greater than 30 kg/m2) were –0.30 kg and –3.03 kg, and +0.56 and –3.58 kg.
Odds ratios for achieving an HbA1c reduction of 5.5 mmol/mol (greater than or equal to 0.5%) without severe hypoglycemia at week 24 with dapagliflozin, compared with placebo, were, in increasing order of BMI groups: 1.85, 1.93, 3.87, 2.91, and 4.20.
“Generally, more events of definite DKA were observed in patients treated with dapagliflozin than in those treated with placebo,” but there were fewer events as BMI increased, Dr. Dandona and associates reported. “These data should be interpreted with caution due to the low number of events in each subgroup,” they added.
The number of adjudicated DKA events comparing dapagliflozin and placebo across the BMI groups were: 4 versus 1 (BMI less than or equal to 23 kg/m2); 6 versus 1 (BMI greater than 23 kg/m2 to less than or equal to 25 kg/m2); 7 versus 1 (BMI greater than 25 kg/m2 to less than or equal to 27 kg/m2); 3 versus 1 (BMI greater than 27 kg/m2 to less than or equal to 30 kg/m2); and 2 versus 1 (BMI greater than 30 kg/m2).
In regard to limitations, “this was a post hoc analysis,” the investigators noted, adding that the studies were not originally powered for comparison between BMI subgroups, so the results should be considered exploratory. Moreover, DKA and hypoglycemia were strictly monitored in the trials, which “may differ from real-world situations,” they said.
The inTandem studies were sponsored by Lexicon and Sanofi. Dr. Danne disclosed receiving research funding and serving as a consultant, advisory board or steering committee member, or speaker for various companies, including Sanofi. Dr. Sawhney is an employee of and holds stoke in Lexicon. The DEPICT studies were sponsored by AstraZeneca. The lead author, Dr. Dandona, disclosed employment or consultancy services for multiple companies, including AstraZeneca.
SOURCE: Danne T et al. EASD 2018, Oral Presentation 2; Dandona P et al. EASD 2019, ePoster 720; Sawhney S et al. EASD 2019, Oral Presentation 3.
BARCELONA – Individuals with type 1 diabetes and a high body mass index gain the most benefit with the least risk when sodium-glucose cotransporter 2 (SGLT2) inhibitors are added to insulin therapy, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
Results from new analyses of the inTandem 1 and inTandem 2 trials with sotagliflozin (Zynquista), and the DEPICT-1 and DEPICT-2 trials with dapagliflozin (Farxiga), support the recent decision of the European Medicines Agency to license the use of the drugs only in patients with a BMI of 27 kg/m2 or higher.
inTandem with sotagliflozin
Weight gain is a challenge in patients with type 1 diabetes, said Thomas Danne, MD, who presented post hoc data from the two inTandem studies. “It’s a little bit counterintuitive,” he acknowledged, “but you have to realize, particularly in patients who have hypoglycemia, that they have to take in extra carbohydrates,” which may tip them to becoming overweight or obese.
SGLT2-inhibitor therapy with sotagliflozin or dapagliflozin added to insulin therapy has been shown to reduce body weight in individuals with type 1 diabetes, but there is an increased risk for diabetic ketoacidosis (DKA). That risk, however, seems to be lower in the higher body-weight categories.
Dr. Danne, director of the department of general pediatrics, endocrinology, and diabetology, and clinical research at the Auf der Bult Hospital for Children and Adolescents, at the Hannover (Germany) Medical School, presented data looking at the outcomes of patients treated with sotagliflozin or placebo based on their BMI.
In all, 1,575 patients were included in the analysis, of whom 659 were of normal weight (BMI of less than 27 kg/m2; average mean, 24 kg/m2 at baseline), and 916 had a higher weight (BMI of 27 kg/m2 or higher; average mean, 32 kg/m2 at baseline). The mean age of patients at study entry was 42 years for those with the lower BMI, and 45 years for those with the higher BMI.
Patients in the two inTandem trials had been treated with insulin plus placebo (n = 228, BMI less than 27 kg/m2; n = 298, BMI 27 kg/m2 or higher), or insulin plus sotagliflozin at a dose of 200 mg (n = 219, BMI less than 27 kg/m2; n = 305, BMI 27 kg/m2 or higher) or 400 mg (n = 212; BMI less than 27 kg/m2; n = 313, BMI 27 kg/m2 or higher).
Glycemic control and body weight
Greater reductions in glycated hemoglobin (HbA1c) were seen with sotagliflozin versus placebo, and even more so, if the BMI was 27 kg/m2 or higher. At week 24, the least-squares mean difference in HbA1c comparing sotagliflozin 200 mg and placebo was –0.32 in patients with the lower BMI, compared with –0.39 in those with the higher BMI. Corresponding values for the 400-mg sotagliflozin group in the higher-BMI group were –0.27 and –0.45, respectively (P less than .001 for all comparisons).
In the lower-BMI group, week 24 least-squares mean differences in body weight comparing sotagliflozin and placebo were –2.06 kg for the 200-mg group and –2.55 kg for the 400-mg group, and –2.27 kg and 3.32 kg in the higher-BMI group (P less than .001 for all comparisons).
“This is why this class of drugs holds so much of a promise, [because] it’s not only one good effect regarding improvement of glycemia judged by A1c,” Dr. Danne said.
He also reported that treatment with sotagliflozin was associated with an increased time in range, compared with placebo, again, with greater effects seen in the higher- versus lower-BMI groups. In those with a BMI of 27 kg/m2 or more, there was an additional 1 hour 58 minutes time in range for the 200-mg dose, and 3 hours 37 minutes for the 200-mg dose, compared with an extra 24 minutes and 1 hour 31 minutes, respectively, in the lower-BMI category.
“We also see a trend to improved reduction in systolic blood pressure in those with the higher BMI,” Dr. Danne said.
Risk for DKA
“The big charm of these drugs is that not only do you improve A1c and all the other good things, but also you do this without increasing the risk of hypoglycemia,” said Dr. Danne. “Again, you can see a trend of a lower risk of severe hypoglycemia for both sotagliflozin doses [compared with placebo] in the group with the body mass index of greater than 27 kg/m2 [versus BMI of less than 27 kg/m2].”
The risk of DKA was higher than placebo in both BMI groups, but the number of DKA events was very small when comparing the low and high body weight categories (0 and 1 events, respectively, in the placebo groups; 7 and 9, in the sotagliflozin 200-mg group; and 9 and 11, in the 400-mg group. The absolute risk difference in the exposure adjusted incidence rate was slightly lower in the lower-BMI group, he said, but the numbers were so small that it is difficult to draw conclusions from that finding.
“There is no doubt that we have an increase for the risk of DKA with this class of drugs in general ... but it is futile to discuss whether or not, just on the basis of a body mass index or something else, we will be able to reduce it in a big fashion,” Dr. Danne suggested.
Body weight and composition
Other data on the long-term effect of sotagliflozin on body weight and composition were presented by Sangeeta Sawhney, MD, vice-president of clinical development at Lexicon Pharmaceuticals, Chapel Hill, North Carolina.
She presented data from the DEXA substudy of the inTandem phase 3 studies in which 243 patients underwent fat mass and bone density scanning.
SGLT2 inhibitors are associated with weight loss through glycuresis and net caloric loss, Dr. Sawhney reminded the audience. As sotagliflozin is a dual inhibitor of SGLT1 and SGLT2, however, it is important to estimate the contribution of changes in fat mass and lean mass to the weight loss that could be achieved with the drug.
Pooled data from the inTandem 1 and inTandem 2 studies showed that at week 24, there were reductions in body weight of –1.7 kg and –2.6 kg with sotagliflozin 200 mg and 400 mg, respectively, and at 52 weeks, reductions of –1.9 kg and –2.9 kg. However, there was an increase in body weight with placebo (+0.5 and +0.8 kg, respectively).
For the substudy, patients underwent dual-energy x-ray absorptiometry at baseline and weeks 24 and 52. Fat mass was measured at all three time points, and bone density was evaluated at the start and end of the study.
The least-square mean change in total fat mass from baseline to week 24 and week 52 were +0.6 and +0.1 kg, respectively, for placebo, –1.6 and –1.6 kg for the sotagliflozin 200-mg dose; and –1.9 and –2.1 kg for the 400-mg dose, “which really parallels the reduction in total body weight,” Dr. Sawhney observed.
The changes in total lean mass were much smaller for sotagliflozin, she added, at –0.6 kg at week 24 and 0.3 kg at week 52 for the 200-mg dose, and –0.7 kg and –0.4 kg, respectively, for the 400-mg dose, and rises in lean mass of 0.2 kg and 0.4 kg, respectively, in placebo.
Taken together, these data show that “about 80% of the body weight reduction is really from the fat mass, and a much smaller proportion of the total body weight reduction is really coming from the lean fat mass,” said Dr. Sawhney.
DEPICT with dapagliflozin
In a poster, Paresh Dandona, MD, PhD, of the State University of New York at Buffalo, and associates presented data from a pooled analysis of the DEPICT-1 and DEPICT-2 studies looking at safety and efficacy outcomes with dapagliflozin according to five BMI categories: less than or equal to 23 kg/m2; greater than 23 kg/m2 to less than or equal to 25 kg/m2; greater than 25 kg/m2 to less than or equal to 27 kg/m2; greater than 27 kg/m2 to less than or equal to 30 kg/m2; and greater than 30 kg/m2.
The pooled analysis included 548 patients treated with dapagliflozin 5 mg and 532 who received placebo. The investigators found that patients with higher BMIs who were treated with dapagliflozin had greater weight loss, showed a trend toward achieving an HbA1c reduction of 5.5 mmol/mol (greater than or equal to 0.5%) or more without the risk of severe hypoglycemia, and had fewer episodes of definite DKA, compared with those with those with lower BMIs.
The adjusted mean percentage change from baseline in body weight in the lowest BMI (less than or equal to 23 kg/m2) group at week 24 was +0.06 kg for placebo and –2.71 kg for dapagliflozin, and at week 52, +0.33 kg and –2.91 kg, respectively. Corresponding values comparing placebo and dapagliflozin at 24 and 52 weeks in the highest BMI group (greater than 30 kg/m2) were –0.30 kg and –3.03 kg, and +0.56 and –3.58 kg.
Odds ratios for achieving an HbA1c reduction of 5.5 mmol/mol (greater than or equal to 0.5%) without severe hypoglycemia at week 24 with dapagliflozin, compared with placebo, were, in increasing order of BMI groups: 1.85, 1.93, 3.87, 2.91, and 4.20.
“Generally, more events of definite DKA were observed in patients treated with dapagliflozin than in those treated with placebo,” but there were fewer events as BMI increased, Dr. Dandona and associates reported. “These data should be interpreted with caution due to the low number of events in each subgroup,” they added.
The number of adjudicated DKA events comparing dapagliflozin and placebo across the BMI groups were: 4 versus 1 (BMI less than or equal to 23 kg/m2); 6 versus 1 (BMI greater than 23 kg/m2 to less than or equal to 25 kg/m2); 7 versus 1 (BMI greater than 25 kg/m2 to less than or equal to 27 kg/m2); 3 versus 1 (BMI greater than 27 kg/m2 to less than or equal to 30 kg/m2); and 2 versus 1 (BMI greater than 30 kg/m2).
In regard to limitations, “this was a post hoc analysis,” the investigators noted, adding that the studies were not originally powered for comparison between BMI subgroups, so the results should be considered exploratory. Moreover, DKA and hypoglycemia were strictly monitored in the trials, which “may differ from real-world situations,” they said.
The inTandem studies were sponsored by Lexicon and Sanofi. Dr. Danne disclosed receiving research funding and serving as a consultant, advisory board or steering committee member, or speaker for various companies, including Sanofi. Dr. Sawhney is an employee of and holds stoke in Lexicon. The DEPICT studies were sponsored by AstraZeneca. The lead author, Dr. Dandona, disclosed employment or consultancy services for multiple companies, including AstraZeneca.
SOURCE: Danne T et al. EASD 2018, Oral Presentation 2; Dandona P et al. EASD 2019, ePoster 720; Sawhney S et al. EASD 2019, Oral Presentation 3.
REPORTING FROM EASD 2019
Correction: Diabetes management
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
Information was omitted from Table 1 on page 596 of the article, Makin V, Lansang MC. Diabetes management: beyond hemoglobin A1c (Cleve Clin J Med 2019; 86[9]:595–600, doi:10.3949/ccjm.86a.18031).
The sodium-glucose cotransporter 2 (SGLT2) inhibitors pose a low risk of hypoglyemia, and that should have been noted in the table. The corrected table appears below and online.
Open Clinical Trials for Diabetes Mellitus Harm Reduction (FULL)
Providing access to clinical trials for native American, veteran, and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the US Department of Veterans
Affairs (VA), the military, and Indian Health Service. The VA Office of Research and Development alone sponsors more than 480 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of October 24, 2018; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on preventing diabetes mellitus or improving patient care. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials. gov.
Diabetes Prevention Program Outcomes Study (DPPOS)
The Diabetes Prevention Program (DPP) was a multicenter trial examining the ability of an intensive lifestyle or metformin to prevent or delay the development of diabetes in a high risk population due to the presence of impaired glucose tolerance (IGT). The DPP has ended early demonstrating that lifestyle reduced diabetes onset by 58% and metformin reduced diabetes onset by 31%.
ID: NCT00038727
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases
Location: George Washington University, Rockville, Maryland
Efforts to Improve Diabetes Control
The primary objectives of this study are: (1) test the longterm effectiveness of a peer mentor model on improving glucose control, blood pressure, LDL levels, diabetes mellitus quality of life, and depression scores in a mixed race population of poorly controlled diabetic veterans; (2) test the effectiveness of using former peer mentees as peer mentors as a means of creating a self-sustaining program; and (3) test the effects of becoming a mentor on those who were originally mentees given a growing literature that being a mentor is good for your health. Secondary objectives include: (1) in those randomized to being a mentee, explore mentor characteristics associated with improved HbA1c.
ID: NCT01651117
Sponsor: VA Office of Research and Development
Location: Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
A Patient-Centered Strategy for Improving Diabetes Prevention in Urban American Indians
The goal of the proposed research is to identify effective patient-centered strategies to prevent diabetes in high-risk populations in real world settings. The investigators will accomplish this by conducting a randomized controlled trial comparing an enhanced Diabetes Prevention Program addressing psychosocial stressors to a standard version in a high-risk population of urban American Indian
and Alaskan Native peoples within a primary care setting.
ID: NCT02266576
Sponsor: Stanford University
Locations: Timpany Center of San Jose State University, California; Stanford University School of Medicine, California
Physical Activity and Participation
Physical activity is the cornerstone of good diabetes management, and yet effective physical activity intervention is not available. The investigators developed a lifestyle intervention based on individual’s home activity patterns. The goal of the study is to test the efficacy of this intervention among veterans with diabetes in a randomized-controlled trial. In addition to physical activity, the investigators will also assess if the intervention will improve social participation among veterans.
ID: NCT02268916
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Caring Others Increasing EngageMent in PACT (CO-IMPACT)
This trial will compare two methods of increasing engagement in care and success in diabetes management, among patients with diabetes with high-risk features, who also have family members involved in their care.
ID: NCT02328326
Sponsor: VA Office of Research and Development
Locations: VA Ann Arbor Healthcare System, Michigan;VA Pittsburgh Healthcare System, Pennsylvania
STEP UP to Avert Amputation in Diabetes (STEP UP)
This study will evaluate a comprehensive tailored behavioral intervention aimed to improve foot self-care and self-monitoring (combined with dermal thermometry) to prevent recurrent ulcers in Veterans at highest risk of amputation. This intervention may be a novel strategy for improving self-care and early detection of foot abnormalities in this at-risk population using psychological theories to target multiple health behaviors simultaneously. This could be an efficient and cost-effective approach to improve diabetes-related foot health behavior, and other risk factors in patients who are vulnerable to devastating consequences related to amputation.
ID: NCT02356848
Sponsor: VA Office of Research and Development
Location: Manhattan Campus of the VA NY Harbor Healthcare System
Physical Activity Behavior Change for Older Adults After Dysvascular Amputation (PABC)
This pilot study will use mobile-health technology to deliver an intervention designed for lasting physical activity behavior change. The study will assess the feasibility of using the Physical Activity Behavior Change (PABC) intervention for Veterans with lower limb amputation. This intervention will be delivered using wrist-worn wearable activity sensors and a home-based tablet computer to allow real-time physical activity feedback and video interface between the participants and the therapist.
ID: NCT02738086
Sponsor: VA Office of Research and Development
Location: Rocky Mountain Regional VA Medical Center, Aurora, Colorado
ForgIng New Paths to Prevent DIabeTes (FINDIT)
This study will evaluate the effects of screening for type 2 diabetes mellitus (T2DM) and brief counseling about screening test results on weight and key health behaviors among veterans with risk factors for T2DM. Study participants will be randomly assigned to 1 of 2 study groups: (1) Blood Test Group; or (2) Brochure Group. Participants in the Blood Test Group will complete a blood test called hemoglobin A1c (HbA1c) which measures average blood sugar levels. Participants will receive brief counseling about the results from their primary care provider or someone authorized to speak on their behalf. Participants randomly selected for the Brochure Group will review a handout from the VA National Center for Health Promotion and Disease Prevention (NCP) on recommended screening tests and immunizations. All participants will be asked to complete a survey prior to study group assignment, immediately after a Primary Care appointment, 3 months after enrollment, and 12 months after enrollment.
ID: NCT02747108
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Using Technology to Share Fitness Goals and Results to Improve Diabetes Outcomes
The investigators will recruit DoD beneficiaries, aged 18 years or older and diagnosed with type 2 diabetes. Patients will be randomized into one of two groups. Group 1 will use a fitness tracker but will not be able to see other participants data and group 2 will use a fitness tracker and will be able to see other members daily and weekly results. Outcome measures will be assessed at baseline, 3 months and 6 months to include hemoglobin A1c, weight, body mass index, blood pressure, and number of hours and days fitness tracker is used. The goal is to see if the group randomized into an online community will have improved activity and outcome measurements compared with those who use the pedometer alone.
ID: NCT02761018
Sponsor: Mike O’Callaghan Military Hospital
Location: Mike O’Callaghan Federal Medical Center, Nellis Air Force Base, Nevada
Healthy Living Partnerships to Prevent Diabetes in Veterans Pilot Study (HELP Vets)
Diabetes and obesity are both major public health concerns and the prevalence of diabetes is even higher in the patient population of the VA. This planning project is designed to adapt a successful weight-loss program for delivery through an existing outpatient clinic to reach local veterans at risk for developing diabetes. The information gathered as a part of this project will be used to plan a larger trial designed to improve the health of veterans by offering them a diabetes prevention program through their usual source of healthcare.
ID: NCT02835495
Sponsor: Wake Forest University Health Sciences
Location: Wake Forest School of Medicine
Mindful Stress Reduction in Diabetes Self-Management Education for Veterans (MindSTRIDE)
The purpose of this study is to see if adding Mindfulness training to diabetes education reduces feelings of stress and makes it easier to adhere to healthy behaviors that improve diabetes outcomes (such as hemoglobin A1c).
ID: NCT02928952
Sponsor: VA Office of Research and Development
Location: VA Pittsburgh Healthcare System University Drive Division, Pittsburgh, Pennsylvania
Improving Diabetes Care Through Effective Personalized Patient Portal Interactions
Patient-facing eHealth technologies are those that connect patients and the healthcare system, and include online patient portals. Although many organizations are adopting patient portals, there is limited understanding of how the different portal features help improve health outcomes. This study is designed to develop and test an intervention to improve adoption and use of patient portal features for diabetes management.
ID: NCT02953262
Sponsor: VA Office of Research and Development
Locations: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus, Massachusetts.
Home-Based Kidney Care in Native American’s of New Mexico (HBKC)
People reach end stage renal disease (ESRD) due to progressive chronic kidney disease (CKD), which is associated with increased risk for heart disease and death. The burden of chronic kidney disease is increased among minority populations compared to Caucasians. New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of ESRD by early interventions in CKD. Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered
by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Location: University of New Mexico, Albuquerque
INcreasing Veteran EngagemeNT to Prevent Diabetes (INVENT)
This study will evaluate a VA MyHealtheVet Secure Messaging intervention that uses different intervention messaging strategies designed to increase engagement in behaviors to prevent type 2 diabetes (T2DM). After completing a baseline survey, participants will be randomly assigned to receive different novel presentations of information about ways to prevent T2DM through both secure messaging and US mail. The investigators will test the 5 presentations that each: (1) represent an innovative approach from behavioral economics or health psychology with great promise to increase engagement in behaviors to prevent T2DM among patients with prediabetes; and (2) have not been tested in this setting.
ID: NCT03403231
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Self-efficacy, Beliefs and Adherence—Pilot and Feasibility Trial of a Pharmacist-led Intervention
This study uses an intervention mixed methods design. The overall purpose is to improve medication adherence and assess the clinical impact on diabetes outcomes among patients with uncontrolled diabetes. We will examine if usual care combined with a clinic-based health literacy/psychosocial support intervention improves medication adherence compared to usual care alone. A randomized controlled trial will be conducted at William S. Middleton Memorial Veterans Hospital in Madison, targeting individuals with
uncontrolled diabetes. The patient-centered health literacy intervention will focus on enhancing patients’ self-efficacy and addressing patients’ negative beliefs in medicine and illness.
ID: NCT03406923
Sponsor: University of Wisconsin, Madison
Location: William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
Practical Telemedicine to Improve Control and Engagement for Veterans With Clinic-Refractory Diabetes Mellitus (PRACTICE-DM)
Diabetes generates significant morbidity, mortality, and costs within the Veterans Health Administration (VHA). Veterans with persistently poor diabetes control despite clinic-based care are among the highest-risk diabetes patients in VHA, and contribute disproportionately to VHA’s massive burden of diabetes complications and costs. VHA critically needs effective, practical management alternatives for veterans whose diabetes does not respond to clinic-based management. The proposed study will address this need by leveraging VHA’s unique Home Telehealth capacity to deliver comprehensive telemedicine-based management for veterans with persistently poor diabetes control despite clinic-based care. Because this intensive intervention is delivered using only existing Home Telehealth workforce, infrastructure, and technical resources—which are ubiquitous at VHA centers nationwide—it could represent an effective, practical approach to improving outcomes in veterans with PPDM, potentially translating to a substantial reduction in VHA’s diabetes burden.
ID: NCT03520413
Sponsor: VA Office of Research and Development
Locations: Durham VA Medical Center, North Carolina; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Cooking for Health
Type 2 diabetes is a leading cause of morbidity and mortality among American Indians in the US. Although healthy diet is a key component of diabetes management programs, many American Indians face contextual barriers to adopting a healthy diet including: difficulty budgeting for food on low-incomes, low literacy and numeracy when purchasing food, and limited cooking skills. The proposed project will develop, implement, and evaluate a culturally-targeted healthy foods budgeting, purchasing, and cooking skills intervention aimed at improving the cardio-metabolic health of American Indians with type 2 diabetes who live in rural areas.
ID: NCT03699709
Sponsor: University of Washington
Location: Missouri Breaks Industries Research, Eagle Butte, South Dakota
Providing access to clinical trials for native American, veteran, and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the US Department of Veterans
Affairs (VA), the military, and Indian Health Service. The VA Office of Research and Development alone sponsors more than 480 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of October 24, 2018; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on preventing diabetes mellitus or improving patient care. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials. gov.
Diabetes Prevention Program Outcomes Study (DPPOS)
The Diabetes Prevention Program (DPP) was a multicenter trial examining the ability of an intensive lifestyle or metformin to prevent or delay the development of diabetes in a high risk population due to the presence of impaired glucose tolerance (IGT). The DPP has ended early demonstrating that lifestyle reduced diabetes onset by 58% and metformin reduced diabetes onset by 31%.
ID: NCT00038727
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases
Location: George Washington University, Rockville, Maryland
Efforts to Improve Diabetes Control
The primary objectives of this study are: (1) test the longterm effectiveness of a peer mentor model on improving glucose control, blood pressure, LDL levels, diabetes mellitus quality of life, and depression scores in a mixed race population of poorly controlled diabetic veterans; (2) test the effectiveness of using former peer mentees as peer mentors as a means of creating a self-sustaining program; and (3) test the effects of becoming a mentor on those who were originally mentees given a growing literature that being a mentor is good for your health. Secondary objectives include: (1) in those randomized to being a mentee, explore mentor characteristics associated with improved HbA1c.
ID: NCT01651117
Sponsor: VA Office of Research and Development
Location: Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
A Patient-Centered Strategy for Improving Diabetes Prevention in Urban American Indians
The goal of the proposed research is to identify effective patient-centered strategies to prevent diabetes in high-risk populations in real world settings. The investigators will accomplish this by conducting a randomized controlled trial comparing an enhanced Diabetes Prevention Program addressing psychosocial stressors to a standard version in a high-risk population of urban American Indian
and Alaskan Native peoples within a primary care setting.
ID: NCT02266576
Sponsor: Stanford University
Locations: Timpany Center of San Jose State University, California; Stanford University School of Medicine, California
Physical Activity and Participation
Physical activity is the cornerstone of good diabetes management, and yet effective physical activity intervention is not available. The investigators developed a lifestyle intervention based on individual’s home activity patterns. The goal of the study is to test the efficacy of this intervention among veterans with diabetes in a randomized-controlled trial. In addition to physical activity, the investigators will also assess if the intervention will improve social participation among veterans.
ID: NCT02268916
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Caring Others Increasing EngageMent in PACT (CO-IMPACT)
This trial will compare two methods of increasing engagement in care and success in diabetes management, among patients with diabetes with high-risk features, who also have family members involved in their care.
ID: NCT02328326
Sponsor: VA Office of Research and Development
Locations: VA Ann Arbor Healthcare System, Michigan;VA Pittsburgh Healthcare System, Pennsylvania
STEP UP to Avert Amputation in Diabetes (STEP UP)
This study will evaluate a comprehensive tailored behavioral intervention aimed to improve foot self-care and self-monitoring (combined with dermal thermometry) to prevent recurrent ulcers in Veterans at highest risk of amputation. This intervention may be a novel strategy for improving self-care and early detection of foot abnormalities in this at-risk population using psychological theories to target multiple health behaviors simultaneously. This could be an efficient and cost-effective approach to improve diabetes-related foot health behavior, and other risk factors in patients who are vulnerable to devastating consequences related to amputation.
ID: NCT02356848
Sponsor: VA Office of Research and Development
Location: Manhattan Campus of the VA NY Harbor Healthcare System
Physical Activity Behavior Change for Older Adults After Dysvascular Amputation (PABC)
This pilot study will use mobile-health technology to deliver an intervention designed for lasting physical activity behavior change. The study will assess the feasibility of using the Physical Activity Behavior Change (PABC) intervention for Veterans with lower limb amputation. This intervention will be delivered using wrist-worn wearable activity sensors and a home-based tablet computer to allow real-time physical activity feedback and video interface between the participants and the therapist.
ID: NCT02738086
Sponsor: VA Office of Research and Development
Location: Rocky Mountain Regional VA Medical Center, Aurora, Colorado
ForgIng New Paths to Prevent DIabeTes (FINDIT)
This study will evaluate the effects of screening for type 2 diabetes mellitus (T2DM) and brief counseling about screening test results on weight and key health behaviors among veterans with risk factors for T2DM. Study participants will be randomly assigned to 1 of 2 study groups: (1) Blood Test Group; or (2) Brochure Group. Participants in the Blood Test Group will complete a blood test called hemoglobin A1c (HbA1c) which measures average blood sugar levels. Participants will receive brief counseling about the results from their primary care provider or someone authorized to speak on their behalf. Participants randomly selected for the Brochure Group will review a handout from the VA National Center for Health Promotion and Disease Prevention (NCP) on recommended screening tests and immunizations. All participants will be asked to complete a survey prior to study group assignment, immediately after a Primary Care appointment, 3 months after enrollment, and 12 months after enrollment.
ID: NCT02747108
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Using Technology to Share Fitness Goals and Results to Improve Diabetes Outcomes
The investigators will recruit DoD beneficiaries, aged 18 years or older and diagnosed with type 2 diabetes. Patients will be randomized into one of two groups. Group 1 will use a fitness tracker but will not be able to see other participants data and group 2 will use a fitness tracker and will be able to see other members daily and weekly results. Outcome measures will be assessed at baseline, 3 months and 6 months to include hemoglobin A1c, weight, body mass index, blood pressure, and number of hours and days fitness tracker is used. The goal is to see if the group randomized into an online community will have improved activity and outcome measurements compared with those who use the pedometer alone.
ID: NCT02761018
Sponsor: Mike O’Callaghan Military Hospital
Location: Mike O’Callaghan Federal Medical Center, Nellis Air Force Base, Nevada
Healthy Living Partnerships to Prevent Diabetes in Veterans Pilot Study (HELP Vets)
Diabetes and obesity are both major public health concerns and the prevalence of diabetes is even higher in the patient population of the VA. This planning project is designed to adapt a successful weight-loss program for delivery through an existing outpatient clinic to reach local veterans at risk for developing diabetes. The information gathered as a part of this project will be used to plan a larger trial designed to improve the health of veterans by offering them a diabetes prevention program through their usual source of healthcare.
ID: NCT02835495
Sponsor: Wake Forest University Health Sciences
Location: Wake Forest School of Medicine
Mindful Stress Reduction in Diabetes Self-Management Education for Veterans (MindSTRIDE)
The purpose of this study is to see if adding Mindfulness training to diabetes education reduces feelings of stress and makes it easier to adhere to healthy behaviors that improve diabetes outcomes (such as hemoglobin A1c).
ID: NCT02928952
Sponsor: VA Office of Research and Development
Location: VA Pittsburgh Healthcare System University Drive Division, Pittsburgh, Pennsylvania
Improving Diabetes Care Through Effective Personalized Patient Portal Interactions
Patient-facing eHealth technologies are those that connect patients and the healthcare system, and include online patient portals. Although many organizations are adopting patient portals, there is limited understanding of how the different portal features help improve health outcomes. This study is designed to develop and test an intervention to improve adoption and use of patient portal features for diabetes management.
ID: NCT02953262
Sponsor: VA Office of Research and Development
Locations: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus, Massachusetts.
Home-Based Kidney Care in Native American’s of New Mexico (HBKC)
People reach end stage renal disease (ESRD) due to progressive chronic kidney disease (CKD), which is associated with increased risk for heart disease and death. The burden of chronic kidney disease is increased among minority populations compared to Caucasians. New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of ESRD by early interventions in CKD. Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered
by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Location: University of New Mexico, Albuquerque
INcreasing Veteran EngagemeNT to Prevent Diabetes (INVENT)
This study will evaluate a VA MyHealtheVet Secure Messaging intervention that uses different intervention messaging strategies designed to increase engagement in behaviors to prevent type 2 diabetes (T2DM). After completing a baseline survey, participants will be randomly assigned to receive different novel presentations of information about ways to prevent T2DM through both secure messaging and US mail. The investigators will test the 5 presentations that each: (1) represent an innovative approach from behavioral economics or health psychology with great promise to increase engagement in behaviors to prevent T2DM among patients with prediabetes; and (2) have not been tested in this setting.
ID: NCT03403231
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Self-efficacy, Beliefs and Adherence—Pilot and Feasibility Trial of a Pharmacist-led Intervention
This study uses an intervention mixed methods design. The overall purpose is to improve medication adherence and assess the clinical impact on diabetes outcomes among patients with uncontrolled diabetes. We will examine if usual care combined with a clinic-based health literacy/psychosocial support intervention improves medication adherence compared to usual care alone. A randomized controlled trial will be conducted at William S. Middleton Memorial Veterans Hospital in Madison, targeting individuals with
uncontrolled diabetes. The patient-centered health literacy intervention will focus on enhancing patients’ self-efficacy and addressing patients’ negative beliefs in medicine and illness.
ID: NCT03406923
Sponsor: University of Wisconsin, Madison
Location: William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
Practical Telemedicine to Improve Control and Engagement for Veterans With Clinic-Refractory Diabetes Mellitus (PRACTICE-DM)
Diabetes generates significant morbidity, mortality, and costs within the Veterans Health Administration (VHA). Veterans with persistently poor diabetes control despite clinic-based care are among the highest-risk diabetes patients in VHA, and contribute disproportionately to VHA’s massive burden of diabetes complications and costs. VHA critically needs effective, practical management alternatives for veterans whose diabetes does not respond to clinic-based management. The proposed study will address this need by leveraging VHA’s unique Home Telehealth capacity to deliver comprehensive telemedicine-based management for veterans with persistently poor diabetes control despite clinic-based care. Because this intensive intervention is delivered using only existing Home Telehealth workforce, infrastructure, and technical resources—which are ubiquitous at VHA centers nationwide—it could represent an effective, practical approach to improving outcomes in veterans with PPDM, potentially translating to a substantial reduction in VHA’s diabetes burden.
ID: NCT03520413
Sponsor: VA Office of Research and Development
Locations: Durham VA Medical Center, North Carolina; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Cooking for Health
Type 2 diabetes is a leading cause of morbidity and mortality among American Indians in the US. Although healthy diet is a key component of diabetes management programs, many American Indians face contextual barriers to adopting a healthy diet including: difficulty budgeting for food on low-incomes, low literacy and numeracy when purchasing food, and limited cooking skills. The proposed project will develop, implement, and evaluate a culturally-targeted healthy foods budgeting, purchasing, and cooking skills intervention aimed at improving the cardio-metabolic health of American Indians with type 2 diabetes who live in rural areas.
ID: NCT03699709
Sponsor: University of Washington
Location: Missouri Breaks Industries Research, Eagle Butte, South Dakota
Providing access to clinical trials for native American, veteran, and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the US Department of Veterans
Affairs (VA), the military, and Indian Health Service. The VA Office of Research and Development alone sponsors more than 480 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of October 24, 2018; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on preventing diabetes mellitus or improving patient care. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials. gov.
Diabetes Prevention Program Outcomes Study (DPPOS)
The Diabetes Prevention Program (DPP) was a multicenter trial examining the ability of an intensive lifestyle or metformin to prevent or delay the development of diabetes in a high risk population due to the presence of impaired glucose tolerance (IGT). The DPP has ended early demonstrating that lifestyle reduced diabetes onset by 58% and metformin reduced diabetes onset by 31%.
ID: NCT00038727
Sponsor: National Institute of Diabetes and Digestive and Kidney Diseases
Location: George Washington University, Rockville, Maryland
Efforts to Improve Diabetes Control
The primary objectives of this study are: (1) test the longterm effectiveness of a peer mentor model on improving glucose control, blood pressure, LDL levels, diabetes mellitus quality of life, and depression scores in a mixed race population of poorly controlled diabetic veterans; (2) test the effectiveness of using former peer mentees as peer mentors as a means of creating a self-sustaining program; and (3) test the effects of becoming a mentor on those who were originally mentees given a growing literature that being a mentor is good for your health. Secondary objectives include: (1) in those randomized to being a mentee, explore mentor characteristics associated with improved HbA1c.
ID: NCT01651117
Sponsor: VA Office of Research and Development
Location: Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania
A Patient-Centered Strategy for Improving Diabetes Prevention in Urban American Indians
The goal of the proposed research is to identify effective patient-centered strategies to prevent diabetes in high-risk populations in real world settings. The investigators will accomplish this by conducting a randomized controlled trial comparing an enhanced Diabetes Prevention Program addressing psychosocial stressors to a standard version in a high-risk population of urban American Indian
and Alaskan Native peoples within a primary care setting.
ID: NCT02266576
Sponsor: Stanford University
Locations: Timpany Center of San Jose State University, California; Stanford University School of Medicine, California
Physical Activity and Participation
Physical activity is the cornerstone of good diabetes management, and yet effective physical activity intervention is not available. The investigators developed a lifestyle intervention based on individual’s home activity patterns. The goal of the study is to test the efficacy of this intervention among veterans with diabetes in a randomized-controlled trial. In addition to physical activity, the investigators will also assess if the intervention will improve social participation among veterans.
ID: NCT02268916
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Caring Others Increasing EngageMent in PACT (CO-IMPACT)
This trial will compare two methods of increasing engagement in care and success in diabetes management, among patients with diabetes with high-risk features, who also have family members involved in their care.
ID: NCT02328326
Sponsor: VA Office of Research and Development
Locations: VA Ann Arbor Healthcare System, Michigan;VA Pittsburgh Healthcare System, Pennsylvania
STEP UP to Avert Amputation in Diabetes (STEP UP)
This study will evaluate a comprehensive tailored behavioral intervention aimed to improve foot self-care and self-monitoring (combined with dermal thermometry) to prevent recurrent ulcers in Veterans at highest risk of amputation. This intervention may be a novel strategy for improving self-care and early detection of foot abnormalities in this at-risk population using psychological theories to target multiple health behaviors simultaneously. This could be an efficient and cost-effective approach to improve diabetes-related foot health behavior, and other risk factors in patients who are vulnerable to devastating consequences related to amputation.
ID: NCT02356848
Sponsor: VA Office of Research and Development
Location: Manhattan Campus of the VA NY Harbor Healthcare System
Physical Activity Behavior Change for Older Adults After Dysvascular Amputation (PABC)
This pilot study will use mobile-health technology to deliver an intervention designed for lasting physical activity behavior change. The study will assess the feasibility of using the Physical Activity Behavior Change (PABC) intervention for Veterans with lower limb amputation. This intervention will be delivered using wrist-worn wearable activity sensors and a home-based tablet computer to allow real-time physical activity feedback and video interface between the participants and the therapist.
ID: NCT02738086
Sponsor: VA Office of Research and Development
Location: Rocky Mountain Regional VA Medical Center, Aurora, Colorado
ForgIng New Paths to Prevent DIabeTes (FINDIT)
This study will evaluate the effects of screening for type 2 diabetes mellitus (T2DM) and brief counseling about screening test results on weight and key health behaviors among veterans with risk factors for T2DM. Study participants will be randomly assigned to 1 of 2 study groups: (1) Blood Test Group; or (2) Brochure Group. Participants in the Blood Test Group will complete a blood test called hemoglobin A1c (HbA1c) which measures average blood sugar levels. Participants will receive brief counseling about the results from their primary care provider or someone authorized to speak on their behalf. Participants randomly selected for the Brochure Group will review a handout from the VA National Center for Health Promotion and Disease Prevention (NCP) on recommended screening tests and immunizations. All participants will be asked to complete a survey prior to study group assignment, immediately after a Primary Care appointment, 3 months after enrollment, and 12 months after enrollment.
ID: NCT02747108
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Using Technology to Share Fitness Goals and Results to Improve Diabetes Outcomes
The investigators will recruit DoD beneficiaries, aged 18 years or older and diagnosed with type 2 diabetes. Patients will be randomized into one of two groups. Group 1 will use a fitness tracker but will not be able to see other participants data and group 2 will use a fitness tracker and will be able to see other members daily and weekly results. Outcome measures will be assessed at baseline, 3 months and 6 months to include hemoglobin A1c, weight, body mass index, blood pressure, and number of hours and days fitness tracker is used. The goal is to see if the group randomized into an online community will have improved activity and outcome measurements compared with those who use the pedometer alone.
ID: NCT02761018
Sponsor: Mike O’Callaghan Military Hospital
Location: Mike O’Callaghan Federal Medical Center, Nellis Air Force Base, Nevada
Healthy Living Partnerships to Prevent Diabetes in Veterans Pilot Study (HELP Vets)
Diabetes and obesity are both major public health concerns and the prevalence of diabetes is even higher in the patient population of the VA. This planning project is designed to adapt a successful weight-loss program for delivery through an existing outpatient clinic to reach local veterans at risk for developing diabetes. The information gathered as a part of this project will be used to plan a larger trial designed to improve the health of veterans by offering them a diabetes prevention program through their usual source of healthcare.
ID: NCT02835495
Sponsor: Wake Forest University Health Sciences
Location: Wake Forest School of Medicine
Mindful Stress Reduction in Diabetes Self-Management Education for Veterans (MindSTRIDE)
The purpose of this study is to see if adding Mindfulness training to diabetes education reduces feelings of stress and makes it easier to adhere to healthy behaviors that improve diabetes outcomes (such as hemoglobin A1c).
ID: NCT02928952
Sponsor: VA Office of Research and Development
Location: VA Pittsburgh Healthcare System University Drive Division, Pittsburgh, Pennsylvania
Improving Diabetes Care Through Effective Personalized Patient Portal Interactions
Patient-facing eHealth technologies are those that connect patients and the healthcare system, and include online patient portals. Although many organizations are adopting patient portals, there is limited understanding of how the different portal features help improve health outcomes. This study is designed to develop and test an intervention to improve adoption and use of patient portal features for diabetes management.
ID: NCT02953262
Sponsor: VA Office of Research and Development
Locations: Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus, Massachusetts.
Home-Based Kidney Care in Native American’s of New Mexico (HBKC)
People reach end stage renal disease (ESRD) due to progressive chronic kidney disease (CKD), which is associated with increased risk for heart disease and death. The burden of chronic kidney disease is increased among minority populations compared to Caucasians. New Mexico American Indians are experiencing an epidemic of chronic kidney disease due primarily to the high rates of obesity and diabetes. The present study entitled Home-Based Kidney Care is designed to delay / reduce rates of ESRD by early interventions in CKD. Investigators propose to assess the safety and efficacy of conducting a full-scale study to determine if home based care delivered
by a collaborative team composed of community health workers, the Albuquerque Area Indian Health Board and University of New Mexico faculty will decrease the risk for the development and the progression of CKD.
ID: NCT03179085
Sponsor: University of New Mexico
Location: University of New Mexico, Albuquerque
INcreasing Veteran EngagemeNT to Prevent Diabetes (INVENT)
This study will evaluate a VA MyHealtheVet Secure Messaging intervention that uses different intervention messaging strategies designed to increase engagement in behaviors to prevent type 2 diabetes (T2DM). After completing a baseline survey, participants will be randomly assigned to receive different novel presentations of information about ways to prevent T2DM through both secure messaging and US mail. The investigators will test the 5 presentations that each: (1) represent an innovative approach from behavioral economics or health psychology with great promise to increase engagement in behaviors to prevent T2DM among patients with prediabetes; and (2) have not been tested in this setting.
ID: NCT03403231
Sponsor: VA Office of Research and Development
Location: VA Ann Arbor Healthcare System, Michigan
Self-efficacy, Beliefs and Adherence—Pilot and Feasibility Trial of a Pharmacist-led Intervention
This study uses an intervention mixed methods design. The overall purpose is to improve medication adherence and assess the clinical impact on diabetes outcomes among patients with uncontrolled diabetes. We will examine if usual care combined with a clinic-based health literacy/psychosocial support intervention improves medication adherence compared to usual care alone. A randomized controlled trial will be conducted at William S. Middleton Memorial Veterans Hospital in Madison, targeting individuals with
uncontrolled diabetes. The patient-centered health literacy intervention will focus on enhancing patients’ self-efficacy and addressing patients’ negative beliefs in medicine and illness.
ID: NCT03406923
Sponsor: University of Wisconsin, Madison
Location: William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
Practical Telemedicine to Improve Control and Engagement for Veterans With Clinic-Refractory Diabetes Mellitus (PRACTICE-DM)
Diabetes generates significant morbidity, mortality, and costs within the Veterans Health Administration (VHA). Veterans with persistently poor diabetes control despite clinic-based care are among the highest-risk diabetes patients in VHA, and contribute disproportionately to VHA’s massive burden of diabetes complications and costs. VHA critically needs effective, practical management alternatives for veterans whose diabetes does not respond to clinic-based management. The proposed study will address this need by leveraging VHA’s unique Home Telehealth capacity to deliver comprehensive telemedicine-based management for veterans with persistently poor diabetes control despite clinic-based care. Because this intensive intervention is delivered using only existing Home Telehealth workforce, infrastructure, and technical resources—which are ubiquitous at VHA centers nationwide—it could represent an effective, practical approach to improving outcomes in veterans with PPDM, potentially translating to a substantial reduction in VHA’s diabetes burden.
ID: NCT03520413
Sponsor: VA Office of Research and Development
Locations: Durham VA Medical Center, North Carolina; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
Cooking for Health
Type 2 diabetes is a leading cause of morbidity and mortality among American Indians in the US. Although healthy diet is a key component of diabetes management programs, many American Indians face contextual barriers to adopting a healthy diet including: difficulty budgeting for food on low-incomes, low literacy and numeracy when purchasing food, and limited cooking skills. The proposed project will develop, implement, and evaluate a culturally-targeted healthy foods budgeting, purchasing, and cooking skills intervention aimed at improving the cardio-metabolic health of American Indians with type 2 diabetes who live in rural areas.
ID: NCT03699709
Sponsor: University of Washington
Location: Missouri Breaks Industries Research, Eagle Butte, South Dakota
Synchronizing refills saves money, improves outcomes
NATIONAL HARBOR, MD. – according to research presented at the annual meeting of the Academy of Managed Care Pharmacy.
Investigators with Pharmacy Quality Alliance (PQA) used data from Truven MarketScan Research Databases to conduct a retrospective cohort study of more than 20,000 patients eligible for inclusion in PQA’s diabetes medication adherence measure. To be included, patients needed to have two or more prescriptions for diabetes medications (excluding insulin), statins, or renin-angiotensin system antagonists. About 80% of patients were commercially insured and 20% came from Medicare supplement insurance (Medigap) plans.
Commercially insured patients whose medication refills were synchronized had better medication adherence than did matched controls (67.7% vs. 57.4%) and lower median health care expenditures ($3,687 vs. $7,480).
The same was true for patients with Medicare supplemental insurance. Synchronized patients in this group also had better medication adherence than controls, at 86.5% vs. 70.4% and lower median health care expenditures ($7,353 vs. $10,592).
Based on their findings in diabetes patients, “I think we should synchronize refills,” Matthew K. Pickering, PharmD, senior director of research and quality strategies at PQA, said. “However, there are populations that were not represented in this, like COPD [chronic obstructive pulmonary disease]. That’s another high-comorbidity, high-cost population that should be studied.”
Session moderator Laura Happe, PharmD, editor in chief of the Journal of Managed Care and Specialty Pharmacy, questioned Dr. Pickering about the barriers to medication synchronization.
In previous research, “we discovered that some patients were resistant to synchronizing their medication refills because of the copays – having all of their copays at one time, rather than spreading them out over the month,” Dr. Happe said.
“Certainly, patients may not be able to afford all their copays at one time, so that can be a barrier,” Dr. Pickering said. “With medication synchronization programs, there’s a lot of variation across the board. Patients can choose which medication to synchronize in some programs. Others only synchronize the three-star medication, etc. But there are real barriers and they should be explored.”
Pharmacy Quality Alliance is a nonprofit public-private partnership that develops pharmacy quality measures in collaboration with the Centers for Medicare & Medicaid Services.
Dr. Pickering disclosed no relevant conflicts of interest.
NATIONAL HARBOR, MD. – according to research presented at the annual meeting of the Academy of Managed Care Pharmacy.
Investigators with Pharmacy Quality Alliance (PQA) used data from Truven MarketScan Research Databases to conduct a retrospective cohort study of more than 20,000 patients eligible for inclusion in PQA’s diabetes medication adherence measure. To be included, patients needed to have two or more prescriptions for diabetes medications (excluding insulin), statins, or renin-angiotensin system antagonists. About 80% of patients were commercially insured and 20% came from Medicare supplement insurance (Medigap) plans.
Commercially insured patients whose medication refills were synchronized had better medication adherence than did matched controls (67.7% vs. 57.4%) and lower median health care expenditures ($3,687 vs. $7,480).
The same was true for patients with Medicare supplemental insurance. Synchronized patients in this group also had better medication adherence than controls, at 86.5% vs. 70.4% and lower median health care expenditures ($7,353 vs. $10,592).
Based on their findings in diabetes patients, “I think we should synchronize refills,” Matthew K. Pickering, PharmD, senior director of research and quality strategies at PQA, said. “However, there are populations that were not represented in this, like COPD [chronic obstructive pulmonary disease]. That’s another high-comorbidity, high-cost population that should be studied.”
Session moderator Laura Happe, PharmD, editor in chief of the Journal of Managed Care and Specialty Pharmacy, questioned Dr. Pickering about the barriers to medication synchronization.
In previous research, “we discovered that some patients were resistant to synchronizing their medication refills because of the copays – having all of their copays at one time, rather than spreading them out over the month,” Dr. Happe said.
“Certainly, patients may not be able to afford all their copays at one time, so that can be a barrier,” Dr. Pickering said. “With medication synchronization programs, there’s a lot of variation across the board. Patients can choose which medication to synchronize in some programs. Others only synchronize the three-star medication, etc. But there are real barriers and they should be explored.”
Pharmacy Quality Alliance is a nonprofit public-private partnership that develops pharmacy quality measures in collaboration with the Centers for Medicare & Medicaid Services.
Dr. Pickering disclosed no relevant conflicts of interest.
NATIONAL HARBOR, MD. – according to research presented at the annual meeting of the Academy of Managed Care Pharmacy.
Investigators with Pharmacy Quality Alliance (PQA) used data from Truven MarketScan Research Databases to conduct a retrospective cohort study of more than 20,000 patients eligible for inclusion in PQA’s diabetes medication adherence measure. To be included, patients needed to have two or more prescriptions for diabetes medications (excluding insulin), statins, or renin-angiotensin system antagonists. About 80% of patients were commercially insured and 20% came from Medicare supplement insurance (Medigap) plans.
Commercially insured patients whose medication refills were synchronized had better medication adherence than did matched controls (67.7% vs. 57.4%) and lower median health care expenditures ($3,687 vs. $7,480).
The same was true for patients with Medicare supplemental insurance. Synchronized patients in this group also had better medication adherence than controls, at 86.5% vs. 70.4% and lower median health care expenditures ($7,353 vs. $10,592).
Based on their findings in diabetes patients, “I think we should synchronize refills,” Matthew K. Pickering, PharmD, senior director of research and quality strategies at PQA, said. “However, there are populations that were not represented in this, like COPD [chronic obstructive pulmonary disease]. That’s another high-comorbidity, high-cost population that should be studied.”
Session moderator Laura Happe, PharmD, editor in chief of the Journal of Managed Care and Specialty Pharmacy, questioned Dr. Pickering about the barriers to medication synchronization.
In previous research, “we discovered that some patients were resistant to synchronizing their medication refills because of the copays – having all of their copays at one time, rather than spreading them out over the month,” Dr. Happe said.
“Certainly, patients may not be able to afford all their copays at one time, so that can be a barrier,” Dr. Pickering said. “With medication synchronization programs, there’s a lot of variation across the board. Patients can choose which medication to synchronize in some programs. Others only synchronize the three-star medication, etc. But there are real barriers and they should be explored.”
Pharmacy Quality Alliance is a nonprofit public-private partnership that develops pharmacy quality measures in collaboration with the Centers for Medicare & Medicaid Services.
Dr. Pickering disclosed no relevant conflicts of interest.
REPORTING FROM AMCP NEXUS 2019
Net prices of drugs rising four-times faster than inflation
NATIONAL HARBOR, MD. – The net prices of drugs are increasing four times faster than the rate of inflation, despite being offset 43% from list prices.
List prices increased by 232% from 2007 to 2018 (12% per year) and net prices increased 133% during that same time period. For Medicaid, the gross-to-net discount increased from 40% in 2007 to 68% in 2018. For all other payers, the increase was 22%-50% during that same period, Inmaculada Hernandez, PharmD, and colleagues reported at annual meeting of the Academy of Managed Care Pharmacy.
The investigators also found a wide variation on discounts across therapeutic classes. For example, list price for drugs in the multiple sclerosis category increased 407% over the study period while net price increased 221%. Insulins came in second in terms of gross price increases (337%) but saw only net prices increases by 83% due to increasing discounts, according to Dr. Hernandez, assistant professor of pharmacy and therapeutics at the University of Pittsburgh.
List prices for noninsulin diabetes treatments tripled during the observation period, but net prices went up only 24%. List price increases were lowest in the antineoplastic class, averaging 135%, though there were only 34% in rebates to offset the list price, resulting in an average net price increase of 89%.
Research was based on pricing data supplied by investment firm SSR Health for branded products and U.S. sales reported by publicly traded companies. The National Heart, Lung, and Blood Institute sponsored the study.
SOURCE: Hernandez I et a. AMCP Nexus, poster U2.
NATIONAL HARBOR, MD. – The net prices of drugs are increasing four times faster than the rate of inflation, despite being offset 43% from list prices.
List prices increased by 232% from 2007 to 2018 (12% per year) and net prices increased 133% during that same time period. For Medicaid, the gross-to-net discount increased from 40% in 2007 to 68% in 2018. For all other payers, the increase was 22%-50% during that same period, Inmaculada Hernandez, PharmD, and colleagues reported at annual meeting of the Academy of Managed Care Pharmacy.
The investigators also found a wide variation on discounts across therapeutic classes. For example, list price for drugs in the multiple sclerosis category increased 407% over the study period while net price increased 221%. Insulins came in second in terms of gross price increases (337%) but saw only net prices increases by 83% due to increasing discounts, according to Dr. Hernandez, assistant professor of pharmacy and therapeutics at the University of Pittsburgh.
List prices for noninsulin diabetes treatments tripled during the observation period, but net prices went up only 24%. List price increases were lowest in the antineoplastic class, averaging 135%, though there were only 34% in rebates to offset the list price, resulting in an average net price increase of 89%.
Research was based on pricing data supplied by investment firm SSR Health for branded products and U.S. sales reported by publicly traded companies. The National Heart, Lung, and Blood Institute sponsored the study.
SOURCE: Hernandez I et a. AMCP Nexus, poster U2.
NATIONAL HARBOR, MD. – The net prices of drugs are increasing four times faster than the rate of inflation, despite being offset 43% from list prices.
List prices increased by 232% from 2007 to 2018 (12% per year) and net prices increased 133% during that same time period. For Medicaid, the gross-to-net discount increased from 40% in 2007 to 68% in 2018. For all other payers, the increase was 22%-50% during that same period, Inmaculada Hernandez, PharmD, and colleagues reported at annual meeting of the Academy of Managed Care Pharmacy.
The investigators also found a wide variation on discounts across therapeutic classes. For example, list price for drugs in the multiple sclerosis category increased 407% over the study period while net price increased 221%. Insulins came in second in terms of gross price increases (337%) but saw only net prices increases by 83% due to increasing discounts, according to Dr. Hernandez, assistant professor of pharmacy and therapeutics at the University of Pittsburgh.
List prices for noninsulin diabetes treatments tripled during the observation period, but net prices went up only 24%. List price increases were lowest in the antineoplastic class, averaging 135%, though there were only 34% in rebates to offset the list price, resulting in an average net price increase of 89%.
Research was based on pricing data supplied by investment firm SSR Health for branded products and U.S. sales reported by publicly traded companies. The National Heart, Lung, and Blood Institute sponsored the study.
SOURCE: Hernandez I et a. AMCP Nexus, poster U2.
REPORTING FROM AMCP NEXUS 2019
GABA falls short for type 1 diabetes prevention in children
BARCELONA – Gamma aminobutyric acid (GABA) alone or given in combination with glutamic acid decarboxylase (GAD) had little to no effect on the progression of type 1 diabetes in children, according to early data presented at the annual meeting of the European Association for the Study of Diabetes.
There was no difference between the two active treatment groups and placebo for the primary outcome measure, which was the effect on meal-stimulated C-peptide secretion before and after 1 year of treatment, study investigator Kenneth L. McCormick, MD, reported, nor was there any difference in glycemic control, based on hemoglobin A1c (HbA1c) and insulin dose, between the children who received GABA alone (n = 39) or combined with GAD (n = 22), and those who received placebo (n = 30).
“However, the GABA–GAD combination tended to have greater efficacy [than placebo] in terms of the daily insulin dose and the fasting C-peptide–to–glucagon ratio,” said Dr. McCormick, a pediatric endocrinologist at the University of Alabama at Birmingham.
“At 12 months, fasting glucagon was reduced [P less than .013] in the GABA–GAD group, compared with placebo,” he said. This was a “novel observation,” because stimulated glucagon was also reduced in this cohort. “This could be a potential salutatory metabolic effect in diabetes.”
The data were the first to be reported from the trial, and results of the immunologic analyses should be available by the end of the year and might reveal more positive effects of GABA and GAD, Dr. McCormick suggested. Data from a “proinsulin analysis” will also be available later.
The inspiration for the trial was a study performed in mice showing that GABA exerted a protective and regenerative effect on the islet beta cells and “reversed diabetes” (Proc Natl Acad Sci USA. 2011;108:11692-7). It took almost 4 years from the publication of that study to enroll the first patient for the current study.
“GABA was intriguing ... first of all, it is available in health food stores and in supermarkets in the United States,” said Dr. McCormick. “It has a strong safety profile, it’s tasteless, and can be given orally – what better could you ask for in a trial of children with type 1 diabetes?”
GABA is thought to have multiple effects in the pancreas, from increasing insulin secretion and suppressing glucagon secretion, to altering inflammation and T-cell populations. “That’s what’s so important to emphasize, besides its metabolic effects, this compound also has immunosuppressant action,” Dr. McCormick noted.
The study hypothesis was that treatment with oral GABA, or a combination of GABA–GAD, would hinder the progression of new-onset type 1 diabetes. The double-blind trial was designed to run for 1 year (Contemp Clin Trials. 2019;82:93-100) and recruited 97 children with newly diagnosed type 1 diabetes, aged 4-18 years, who were randomized to the three study groups. They were evaluated at baseline and months 1, 5, 8, and 12.
The trial had several limitations, however, which might explain the findings. A key limitation was that the researchers used a low dose of GABA – 1 to 1.5 g/m2 a day, given as a twice-daily oral dose, as mandated by the Food and Drug Administration. “For the GABA dose and the response, we are at the threshold. I don’t believe we are overdosing these kids,” Dr. McCormick said, noting that this is the first study done with GABA in humans.
In fact, GABA has a short half-life of around 2.5-5 hours, so the dose may need to be much higher to show an effect and perhaps administered three times a day, he said.
Another limitation was compliance with the twice-daily medication, especially because 35% of the patients were teenagers, and that it was a young population, with about a third of the patients aged younger that 8 years.
GABA and GABA–GAD should still be studied further, Dr. McCormick concluded, but “additional studies with a higher dose of GABA [given] three times a day, and not twice, are warranted.” Such studies also need to have more participants in each group.
The University of Alabama at Birmingham sponsored the study. Collaborators included Diamyd, NOW Foods, Janssen, and the Juvenile Diabetes Research Foundation. Dr. McCormick did not have any disclosures.
SOURCE: McCormick KL et al. EASD 2019, Abstract S05.1.
BARCELONA – Gamma aminobutyric acid (GABA) alone or given in combination with glutamic acid decarboxylase (GAD) had little to no effect on the progression of type 1 diabetes in children, according to early data presented at the annual meeting of the European Association for the Study of Diabetes.
There was no difference between the two active treatment groups and placebo for the primary outcome measure, which was the effect on meal-stimulated C-peptide secretion before and after 1 year of treatment, study investigator Kenneth L. McCormick, MD, reported, nor was there any difference in glycemic control, based on hemoglobin A1c (HbA1c) and insulin dose, between the children who received GABA alone (n = 39) or combined with GAD (n = 22), and those who received placebo (n = 30).
“However, the GABA–GAD combination tended to have greater efficacy [than placebo] in terms of the daily insulin dose and the fasting C-peptide–to–glucagon ratio,” said Dr. McCormick, a pediatric endocrinologist at the University of Alabama at Birmingham.
“At 12 months, fasting glucagon was reduced [P less than .013] in the GABA–GAD group, compared with placebo,” he said. This was a “novel observation,” because stimulated glucagon was also reduced in this cohort. “This could be a potential salutatory metabolic effect in diabetes.”
The data were the first to be reported from the trial, and results of the immunologic analyses should be available by the end of the year and might reveal more positive effects of GABA and GAD, Dr. McCormick suggested. Data from a “proinsulin analysis” will also be available later.
The inspiration for the trial was a study performed in mice showing that GABA exerted a protective and regenerative effect on the islet beta cells and “reversed diabetes” (Proc Natl Acad Sci USA. 2011;108:11692-7). It took almost 4 years from the publication of that study to enroll the first patient for the current study.
“GABA was intriguing ... first of all, it is available in health food stores and in supermarkets in the United States,” said Dr. McCormick. “It has a strong safety profile, it’s tasteless, and can be given orally – what better could you ask for in a trial of children with type 1 diabetes?”
GABA is thought to have multiple effects in the pancreas, from increasing insulin secretion and suppressing glucagon secretion, to altering inflammation and T-cell populations. “That’s what’s so important to emphasize, besides its metabolic effects, this compound also has immunosuppressant action,” Dr. McCormick noted.
The study hypothesis was that treatment with oral GABA, or a combination of GABA–GAD, would hinder the progression of new-onset type 1 diabetes. The double-blind trial was designed to run for 1 year (Contemp Clin Trials. 2019;82:93-100) and recruited 97 children with newly diagnosed type 1 diabetes, aged 4-18 years, who were randomized to the three study groups. They were evaluated at baseline and months 1, 5, 8, and 12.
The trial had several limitations, however, which might explain the findings. A key limitation was that the researchers used a low dose of GABA – 1 to 1.5 g/m2 a day, given as a twice-daily oral dose, as mandated by the Food and Drug Administration. “For the GABA dose and the response, we are at the threshold. I don’t believe we are overdosing these kids,” Dr. McCormick said, noting that this is the first study done with GABA in humans.
In fact, GABA has a short half-life of around 2.5-5 hours, so the dose may need to be much higher to show an effect and perhaps administered three times a day, he said.
Another limitation was compliance with the twice-daily medication, especially because 35% of the patients were teenagers, and that it was a young population, with about a third of the patients aged younger that 8 years.
GABA and GABA–GAD should still be studied further, Dr. McCormick concluded, but “additional studies with a higher dose of GABA [given] three times a day, and not twice, are warranted.” Such studies also need to have more participants in each group.
The University of Alabama at Birmingham sponsored the study. Collaborators included Diamyd, NOW Foods, Janssen, and the Juvenile Diabetes Research Foundation. Dr. McCormick did not have any disclosures.
SOURCE: McCormick KL et al. EASD 2019, Abstract S05.1.
BARCELONA – Gamma aminobutyric acid (GABA) alone or given in combination with glutamic acid decarboxylase (GAD) had little to no effect on the progression of type 1 diabetes in children, according to early data presented at the annual meeting of the European Association for the Study of Diabetes.
There was no difference between the two active treatment groups and placebo for the primary outcome measure, which was the effect on meal-stimulated C-peptide secretion before and after 1 year of treatment, study investigator Kenneth L. McCormick, MD, reported, nor was there any difference in glycemic control, based on hemoglobin A1c (HbA1c) and insulin dose, between the children who received GABA alone (n = 39) or combined with GAD (n = 22), and those who received placebo (n = 30).
“However, the GABA–GAD combination tended to have greater efficacy [than placebo] in terms of the daily insulin dose and the fasting C-peptide–to–glucagon ratio,” said Dr. McCormick, a pediatric endocrinologist at the University of Alabama at Birmingham.
“At 12 months, fasting glucagon was reduced [P less than .013] in the GABA–GAD group, compared with placebo,” he said. This was a “novel observation,” because stimulated glucagon was also reduced in this cohort. “This could be a potential salutatory metabolic effect in diabetes.”
The data were the first to be reported from the trial, and results of the immunologic analyses should be available by the end of the year and might reveal more positive effects of GABA and GAD, Dr. McCormick suggested. Data from a “proinsulin analysis” will also be available later.
The inspiration for the trial was a study performed in mice showing that GABA exerted a protective and regenerative effect on the islet beta cells and “reversed diabetes” (Proc Natl Acad Sci USA. 2011;108:11692-7). It took almost 4 years from the publication of that study to enroll the first patient for the current study.
“GABA was intriguing ... first of all, it is available in health food stores and in supermarkets in the United States,” said Dr. McCormick. “It has a strong safety profile, it’s tasteless, and can be given orally – what better could you ask for in a trial of children with type 1 diabetes?”
GABA is thought to have multiple effects in the pancreas, from increasing insulin secretion and suppressing glucagon secretion, to altering inflammation and T-cell populations. “That’s what’s so important to emphasize, besides its metabolic effects, this compound also has immunosuppressant action,” Dr. McCormick noted.
The study hypothesis was that treatment with oral GABA, or a combination of GABA–GAD, would hinder the progression of new-onset type 1 diabetes. The double-blind trial was designed to run for 1 year (Contemp Clin Trials. 2019;82:93-100) and recruited 97 children with newly diagnosed type 1 diabetes, aged 4-18 years, who were randomized to the three study groups. They were evaluated at baseline and months 1, 5, 8, and 12.
The trial had several limitations, however, which might explain the findings. A key limitation was that the researchers used a low dose of GABA – 1 to 1.5 g/m2 a day, given as a twice-daily oral dose, as mandated by the Food and Drug Administration. “For the GABA dose and the response, we are at the threshold. I don’t believe we are overdosing these kids,” Dr. McCormick said, noting that this is the first study done with GABA in humans.
In fact, GABA has a short half-life of around 2.5-5 hours, so the dose may need to be much higher to show an effect and perhaps administered three times a day, he said.
Another limitation was compliance with the twice-daily medication, especially because 35% of the patients were teenagers, and that it was a young population, with about a third of the patients aged younger that 8 years.
GABA and GABA–GAD should still be studied further, Dr. McCormick concluded, but “additional studies with a higher dose of GABA [given] three times a day, and not twice, are warranted.” Such studies also need to have more participants in each group.
The University of Alabama at Birmingham sponsored the study. Collaborators included Diamyd, NOW Foods, Janssen, and the Juvenile Diabetes Research Foundation. Dr. McCormick did not have any disclosures.
SOURCE: McCormick KL et al. EASD 2019, Abstract S05.1.
REPORTING FROM EASD 2019
SUSTAIN 10: Weight loss, glycemic control better with semaglutide than liraglutide
BARCELONA – Patients with type 2 diabetes who were treated with semaglutide achieved greater reductions in glycated hemoglobin (HbA1c) levels and body weight, compared with those receiving liraglutide, according to results presented at the annual meeting of the European Association for the Study of Diabetes.
In the phase 3b SUSTAIN 10 trial, conducted in 11 European countries, mean glycated hemoglobin at 30 weeks decreased by 1.7% with once-weekly semaglutide and 1.0% for once-daily liraglutide, from the overall baseline level of 8.2%. The estimated treatment difference (ETD) between the two treatments was –0.69 percentage points (95% confidence interval, –0.82 to –0.56; P less than .0001).
Mean body weight decreased during the same period by 5.8 kg with semaglutide and 1.9 kg with liraglutide, from a baseline of 96.9 kg. The ETD was 3.83 kg (95% CI, –4.57 to –3.09; P less than .0001).
The doses of semaglutide and liraglutide used in the study were 1.0 mg and 1.2 mg, respectively, the latter being the dose that is used most commonly in clinical practice, study investigator Matthew Capehorn, MB, CAB, explained in an interview at the meeting.
“We know that at a dose of 1.8 mg, liraglutide is more effective than 1.2 mg, but it’s about whether it is deemed more cost effective,” said Dr. Capehorn, who is clinical manager at Rotherham (England) Institute for Obesity, Clifton Medical Centre. “Certainly, in the United Kingdom, we’re encouraged to use the 1.2-mg dose” according to guidance from the National Institute for Heath and Care Excellence, and “other European countries are the same.”
Dr. Capehorn noted that studies are being done with a higher dose of semaglutide to see if it has potential as a weight loss drug in its own right in patients who do not have type 2 diabetes. “I care as much about obesity and cardiovascular disease as I do about chasing the HbA1c level and getting that reduced, so I would rather pick an agent that covers all three [components], than just looking at the HbA1c,” he said.
In SUSTAIN 10,577 adults with type 2 diabetes and an HbA1c level of between 7.0% and 11.0% who were on stable doses of one to three oral antidiabetic drugs were randomized to receive semaglutide (n = 290) or liraglutide (n = 287) for 30 weeks.
The primary endpoint was the change in HbA1c from baseline to week 30, and the secondary confirmatory endpoint was change in body weight over the same period.
In presenting the findings, which were simultaneously published in Diabetes & Metabolism, Dr. Capehorn noted that the efficacy results were consistent with those of other SUSTAIN trials that compared semaglutide with other glucagonlike peptide–1 receptor antagonists, notably SUSTAIN 3 (with exenatide extended release) and SUSTAIN 7 (with dulaglutide).
Other efficacy findings from SUSTAIN 10 were that semaglutide produced greater mean changes than did liraglutide in both fasting plasma glucose and in a 7-point, self-monitoring of blood glucose profile.
A greater percentage of people treated with semaglutide, compared with liraglutide, also achieved their glycemic targets of less than 7.0% (80% vs. 46%, respectively) and of 6.5% or less (58% vs. 25%), and their weight loss targets of 5% or more (56% vs. 18%) and 10% or more (19% vs. 4%).
In addition, more semaglutide- than liraglutide-treated patients achieved an HbA1c target of less than 7.0% without severe or blood glucose–confirmed symptomatic hypoglycemia, with or without weight gain (76% vs. 37%; P less than .0001). There were also more semaglutide patients who achieved an HbA1c reduction of 1% or more and a weight loss reduction of 10% or more (17% vs. 4% for liraglutide, P less than .0001).
The safety profiles were similar for semaglutide and liraglutide, Dr. Capehorn noted, but gastrointestinal adverse events were more prevalent in patients receiving semaglutide, compared with liraglutide (43.9% vs. 38.3%), and more patients receiving semaglutide discontinued treatment prematurely because of those adverse events (11.4% vs. 6.6% for liraglutide).
Novo Nordisk sponsored the study. Dr. Capehorn reported receiving research funding from, providing advisory board support to, and speaker fees from Novo Nordisk and from several other companies.
SOURCE: Capehorn M et al. EASD 2019, Oral Presentation 53; Capehorn M et al. Diabetes Metab. 2019 Sep 17. doi: 10.1016/j.diabet.2019.101117.
BARCELONA – Patients with type 2 diabetes who were treated with semaglutide achieved greater reductions in glycated hemoglobin (HbA1c) levels and body weight, compared with those receiving liraglutide, according to results presented at the annual meeting of the European Association for the Study of Diabetes.
In the phase 3b SUSTAIN 10 trial, conducted in 11 European countries, mean glycated hemoglobin at 30 weeks decreased by 1.7% with once-weekly semaglutide and 1.0% for once-daily liraglutide, from the overall baseline level of 8.2%. The estimated treatment difference (ETD) between the two treatments was –0.69 percentage points (95% confidence interval, –0.82 to –0.56; P less than .0001).
Mean body weight decreased during the same period by 5.8 kg with semaglutide and 1.9 kg with liraglutide, from a baseline of 96.9 kg. The ETD was 3.83 kg (95% CI, –4.57 to –3.09; P less than .0001).
The doses of semaglutide and liraglutide used in the study were 1.0 mg and 1.2 mg, respectively, the latter being the dose that is used most commonly in clinical practice, study investigator Matthew Capehorn, MB, CAB, explained in an interview at the meeting.
“We know that at a dose of 1.8 mg, liraglutide is more effective than 1.2 mg, but it’s about whether it is deemed more cost effective,” said Dr. Capehorn, who is clinical manager at Rotherham (England) Institute for Obesity, Clifton Medical Centre. “Certainly, in the United Kingdom, we’re encouraged to use the 1.2-mg dose” according to guidance from the National Institute for Heath and Care Excellence, and “other European countries are the same.”
Dr. Capehorn noted that studies are being done with a higher dose of semaglutide to see if it has potential as a weight loss drug in its own right in patients who do not have type 2 diabetes. “I care as much about obesity and cardiovascular disease as I do about chasing the HbA1c level and getting that reduced, so I would rather pick an agent that covers all three [components], than just looking at the HbA1c,” he said.
In SUSTAIN 10,577 adults with type 2 diabetes and an HbA1c level of between 7.0% and 11.0% who were on stable doses of one to three oral antidiabetic drugs were randomized to receive semaglutide (n = 290) or liraglutide (n = 287) for 30 weeks.
The primary endpoint was the change in HbA1c from baseline to week 30, and the secondary confirmatory endpoint was change in body weight over the same period.
In presenting the findings, which were simultaneously published in Diabetes & Metabolism, Dr. Capehorn noted that the efficacy results were consistent with those of other SUSTAIN trials that compared semaglutide with other glucagonlike peptide–1 receptor antagonists, notably SUSTAIN 3 (with exenatide extended release) and SUSTAIN 7 (with dulaglutide).
Other efficacy findings from SUSTAIN 10 were that semaglutide produced greater mean changes than did liraglutide in both fasting plasma glucose and in a 7-point, self-monitoring of blood glucose profile.
A greater percentage of people treated with semaglutide, compared with liraglutide, also achieved their glycemic targets of less than 7.0% (80% vs. 46%, respectively) and of 6.5% or less (58% vs. 25%), and their weight loss targets of 5% or more (56% vs. 18%) and 10% or more (19% vs. 4%).
In addition, more semaglutide- than liraglutide-treated patients achieved an HbA1c target of less than 7.0% without severe or blood glucose–confirmed symptomatic hypoglycemia, with or without weight gain (76% vs. 37%; P less than .0001). There were also more semaglutide patients who achieved an HbA1c reduction of 1% or more and a weight loss reduction of 10% or more (17% vs. 4% for liraglutide, P less than .0001).
The safety profiles were similar for semaglutide and liraglutide, Dr. Capehorn noted, but gastrointestinal adverse events were more prevalent in patients receiving semaglutide, compared with liraglutide (43.9% vs. 38.3%), and more patients receiving semaglutide discontinued treatment prematurely because of those adverse events (11.4% vs. 6.6% for liraglutide).
Novo Nordisk sponsored the study. Dr. Capehorn reported receiving research funding from, providing advisory board support to, and speaker fees from Novo Nordisk and from several other companies.
SOURCE: Capehorn M et al. EASD 2019, Oral Presentation 53; Capehorn M et al. Diabetes Metab. 2019 Sep 17. doi: 10.1016/j.diabet.2019.101117.
BARCELONA – Patients with type 2 diabetes who were treated with semaglutide achieved greater reductions in glycated hemoglobin (HbA1c) levels and body weight, compared with those receiving liraglutide, according to results presented at the annual meeting of the European Association for the Study of Diabetes.
In the phase 3b SUSTAIN 10 trial, conducted in 11 European countries, mean glycated hemoglobin at 30 weeks decreased by 1.7% with once-weekly semaglutide and 1.0% for once-daily liraglutide, from the overall baseline level of 8.2%. The estimated treatment difference (ETD) between the two treatments was –0.69 percentage points (95% confidence interval, –0.82 to –0.56; P less than .0001).
Mean body weight decreased during the same period by 5.8 kg with semaglutide and 1.9 kg with liraglutide, from a baseline of 96.9 kg. The ETD was 3.83 kg (95% CI, –4.57 to –3.09; P less than .0001).
The doses of semaglutide and liraglutide used in the study were 1.0 mg and 1.2 mg, respectively, the latter being the dose that is used most commonly in clinical practice, study investigator Matthew Capehorn, MB, CAB, explained in an interview at the meeting.
“We know that at a dose of 1.8 mg, liraglutide is more effective than 1.2 mg, but it’s about whether it is deemed more cost effective,” said Dr. Capehorn, who is clinical manager at Rotherham (England) Institute for Obesity, Clifton Medical Centre. “Certainly, in the United Kingdom, we’re encouraged to use the 1.2-mg dose” according to guidance from the National Institute for Heath and Care Excellence, and “other European countries are the same.”
Dr. Capehorn noted that studies are being done with a higher dose of semaglutide to see if it has potential as a weight loss drug in its own right in patients who do not have type 2 diabetes. “I care as much about obesity and cardiovascular disease as I do about chasing the HbA1c level and getting that reduced, so I would rather pick an agent that covers all three [components], than just looking at the HbA1c,” he said.
In SUSTAIN 10,577 adults with type 2 diabetes and an HbA1c level of between 7.0% and 11.0% who were on stable doses of one to three oral antidiabetic drugs were randomized to receive semaglutide (n = 290) or liraglutide (n = 287) for 30 weeks.
The primary endpoint was the change in HbA1c from baseline to week 30, and the secondary confirmatory endpoint was change in body weight over the same period.
In presenting the findings, which were simultaneously published in Diabetes & Metabolism, Dr. Capehorn noted that the efficacy results were consistent with those of other SUSTAIN trials that compared semaglutide with other glucagonlike peptide–1 receptor antagonists, notably SUSTAIN 3 (with exenatide extended release) and SUSTAIN 7 (with dulaglutide).
Other efficacy findings from SUSTAIN 10 were that semaglutide produced greater mean changes than did liraglutide in both fasting plasma glucose and in a 7-point, self-monitoring of blood glucose profile.
A greater percentage of people treated with semaglutide, compared with liraglutide, also achieved their glycemic targets of less than 7.0% (80% vs. 46%, respectively) and of 6.5% or less (58% vs. 25%), and their weight loss targets of 5% or more (56% vs. 18%) and 10% or more (19% vs. 4%).
In addition, more semaglutide- than liraglutide-treated patients achieved an HbA1c target of less than 7.0% without severe or blood glucose–confirmed symptomatic hypoglycemia, with or without weight gain (76% vs. 37%; P less than .0001). There were also more semaglutide patients who achieved an HbA1c reduction of 1% or more and a weight loss reduction of 10% or more (17% vs. 4% for liraglutide, P less than .0001).
The safety profiles were similar for semaglutide and liraglutide, Dr. Capehorn noted, but gastrointestinal adverse events were more prevalent in patients receiving semaglutide, compared with liraglutide (43.9% vs. 38.3%), and more patients receiving semaglutide discontinued treatment prematurely because of those adverse events (11.4% vs. 6.6% for liraglutide).
Novo Nordisk sponsored the study. Dr. Capehorn reported receiving research funding from, providing advisory board support to, and speaker fees from Novo Nordisk and from several other companies.
SOURCE: Capehorn M et al. EASD 2019, Oral Presentation 53; Capehorn M et al. Diabetes Metab. 2019 Sep 17. doi: 10.1016/j.diabet.2019.101117.
REPORTING FROM EASD 2019