User login
Overall Survival Gain With Adding Darolutamide to ADT and Docetaxel in Metastatic, Hormone-Sensitive Prostate Cancer
Study Overview
Objective: To evaluate whether the addition of the potent androgen-receptor inhibitor (ARA) darolutamide to the standard doublet androgen-deprivation therapy (ADT) and docetaxel in metastatic, hormone-sensitive prostate cancer (mHSPC) would increase survival.
Design: A randomized, double-blind, placebo-controlled, multicenter, phase 3 study. The results reported in this publication are from the prespecified interim analysis.
Intervention: Patients with mHSPC were randomly assigned to receive either darolutamide 600 mg twice daily or placebo. All patients received standard ADT with 6 cycles of docetaxel 75 mg/m2 on day 1 every 21 days along with prednisone given within 6 weeks after randomization. Patients receiving luteinizing hormone–releasing hormone (LHRH) agonists as ADT were bridged with at least 4 weeks of first-generation antiandrogen therapy, which was discontinued before randomization. Treatments were continued until symptomatic disease progression, a change in neoplastic therapy, unacceptable toxicity, patient or physician decision, death, or nonadherence.
Setting and participants: Eligible patients included those newly diagnosed with mHSPC with metastases detected on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and bone scan. Patients were excluded if they had regional lymph node–only involvement or if they had received more than 12 weeks of ADT before randomization. Between November 2016 and June 2018, 1306 patients (651 in the darolutamide group and 655 in the placebo group) were randomized in a 1:1 manner to receive darolutamide 600 mg twice daily or placebo in addition to ADT and docetaxel. Randomization was stratified based on the TNM staging system (M1a—nonregional lymph node–only metastasis, M1b—bone metastasis with or without lymph node, or M1c—bone metastases) as well as baseline alkaline phosphatase levels.
Main outcome measures: The primary end point for the study was overall survival. Other meaningful secondary end points included time to castration resistance, time to pain progression, time to first symptomatic skeletal event, symptomatic skeletal event-free survival, time to subsequent systemic antineoplastic therapy, time to worsening of disease-related physical symptoms, initiation of opioid therapy for ≥7 days, and safety.
Results: The baseline and demographic characteristics were well balanced between the 2 groups. Median age was 67 years. Nearly 80% of patients had bone metastasis, and approximately 17% had visceral metastasis. At the data cutoff date for the primary analysis, the median duration of therapy was 41 months for darolutamide compared with 16.7 months in the placebo group; 45.9% in the darolutamide group and 19.1% in the placebo group were receiving the allotted trial therapy at the time of the analysis. Six cycles of docetaxel were completed in approximately 85% of patients in both arms. Median overall survival follow-up was 43.7 months (darolutamide) and 42.4 months (placebo). A significant improvement in overall survival was observed in the darolutamide group. The risk of death was 32.5% lower in the darolutamide cohort than in the placebo cohort (hazard ratio [HR], 0.68; 95% CI, 0.57-0.80; P < .001). The overall survival at 4 years was 62.7% (95% CI, 58.7-66.7) in the darolutamide arm and 50.4% (95% CI, 46.3-54.6) in the placebo arm. The overall survival results remained favorable across most subgroups.
Darolutamide was associated with improvement in all key secondary endpoints. Time to castration-resistance was significantly longer in the darolutamide group (HR, 0.36; 95% CI, 0.30-0.42; P < .001). Time to pain progression was also significantly longer in the darolutamide group (HR, 0.79; 95% CI, 0.66-0.95; P = .01). Time to first symptomatic skeletal events (HR, 0.71; 95% CI, 0.54-0.94; P = .02) and time to initiation of subsequent systemic therapy (HR, 0.39; 95% CI, 0.33-0.46; P < .001) were also found to be longer in the darolutamide group.
Safety: The risk of grade 3 or higher adverse events was similar across the 2 groups. Most common adverse events were known toxic effects of docetaxel therapy and were highest during the initial period when both groups received this therapy. These side effects progressively decreased after the initial period. The most common grade 3 or 4 adverse event was neutropenia, and its frequency was similar between the darolutamide and placebo groups (33.7% and 34.2%, respectively). The most frequently reported adverse events were alopecia, neutropenia, fatigue, and anemia and were similar between the groups. Adverse events of special significance, including fatigue, falls, fractures, and cardiovascular events, were also similar between the 2 groups. Adverse events causing deaths in each arm were low and similar (4.1% in the darolutamide group and 4.0% in the placebo group). The rates of discontinuation of darolutamide or placebo were similar (13.5% and 10.6%, respectively).
Conclusion: Among patients with mHSPC, overall survival was significantly longer among patients who received darolutamide plus ADT and docetaxel than among those who received ADT and docetaxel alone. This was observed despite a high percentage of patients in the placebo group receiving subsequent systemic therapy at the time of progression. The survival benefit of darolutamide was maintained across most subgroups. An improvement was also observed in the darolutamide arm in terms of key secondary end points. The adverse events were similar across the groups and were consistent with known safety profiles of ADT and docetaxel, and no new safety signals were identified in this trial.
Commentary
The results of the current study add to the body of literature supporting multi-agent systemic therapy in newly diagnosed mHSPC. Prior phase 3 trials of combination therapy using androgen-receptor pathway inhibitors, ADT, and docetaxel have shown conflicting results. The results from the previously reported PEACE-1 study showed improved overall survival among patients who received abiraterone with ADT and docetaxel as compared with those who received ADT and docetaxel alone.1 However, as noted by the authors, the subgroup of patients in the ENZAMET trial who received docetaxel, enzalutamide, and ADT did not appear to have a survival advantage compared with those who received ADT and docetaxel alone.2 The results from the current ARASENS trial provide compelling evidence in a population of prospectively randomized patients that combination therapy with darolutamide, docetaxel, and ADT improves overall survival in men with mHSPC. The survival advantage was maintained across subgroups analyzed in this study. Improvements were observed in regards to several key secondary end points with use of darolutamide. This benefit was maintained despite many patients receiving subsequent therapy at the time of progression. Importantly, there did not appear to be a significant increase in toxicity with triplet therapy. However, it is important to note that this cohort of patients appeared largely asymptomatic at the time of enrollment, with 70% of patients having an Eastern Cooperative Oncology Group performance status of 0.
Additionally, the average age in this study was 67 years, with only about 15% of the population being older than 75 years. In the reported subgroup analysis, those older than 75 years appeared to derive a similar benefit in overall survival, however. Whether triplet therapy should be universally adopted in all patients remains unclear. For example, there is a subset of patients with mHSPC with favorable- risk disease (ie, those with recurrent metastatic disease, node-only disease). In this population, the risk-benefit analysis is less clear, and whether these patients should receive this combination is not certain. Nevertheless, the results of this well-designed study are compelling and certainly represent a potential new standard treatment option for men with mHSPC. One of the strengths of this study was its large sample size that allowed for vigorous statistical analysis to evaluate the efficacy of darolutamide in combination with ADT and docetaxel.
Application for Clinical Practice
The ARASENS study provides convincing evidence that in men with mHSPC, the addition of darolutamide to docetaxel and ADT improves overall survival. This combination appeared to be well tolerated, with no evidence of increased toxicity noted. Certainly, this combination represents a potential new standard treatment option in this population; however, further understanding of which subgroups of men benefit from enhanced therapy is needed to aid in proper patient selection.
—Santosh Kagathur, MD, and Daniel Isaac, DO, MS
Michigan State University, East Lansing, MI
1. Fizazi K, Carles Galceran J, Foulon S, et al. LBA5 A phase III trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate plus prednisone in PEACE-1. Ann Oncol. 2021;32:Suppl 5:S1299. doi:10.1016/j.annonc.2021.08.2099
2. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121-131. doi:10.1056/NEJMoa1903835
Study Overview
Objective: To evaluate whether the addition of the potent androgen-receptor inhibitor (ARA) darolutamide to the standard doublet androgen-deprivation therapy (ADT) and docetaxel in metastatic, hormone-sensitive prostate cancer (mHSPC) would increase survival.
Design: A randomized, double-blind, placebo-controlled, multicenter, phase 3 study. The results reported in this publication are from the prespecified interim analysis.
Intervention: Patients with mHSPC were randomly assigned to receive either darolutamide 600 mg twice daily or placebo. All patients received standard ADT with 6 cycles of docetaxel 75 mg/m2 on day 1 every 21 days along with prednisone given within 6 weeks after randomization. Patients receiving luteinizing hormone–releasing hormone (LHRH) agonists as ADT were bridged with at least 4 weeks of first-generation antiandrogen therapy, which was discontinued before randomization. Treatments were continued until symptomatic disease progression, a change in neoplastic therapy, unacceptable toxicity, patient or physician decision, death, or nonadherence.
Setting and participants: Eligible patients included those newly diagnosed with mHSPC with metastases detected on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and bone scan. Patients were excluded if they had regional lymph node–only involvement or if they had received more than 12 weeks of ADT before randomization. Between November 2016 and June 2018, 1306 patients (651 in the darolutamide group and 655 in the placebo group) were randomized in a 1:1 manner to receive darolutamide 600 mg twice daily or placebo in addition to ADT and docetaxel. Randomization was stratified based on the TNM staging system (M1a—nonregional lymph node–only metastasis, M1b—bone metastasis with or without lymph node, or M1c—bone metastases) as well as baseline alkaline phosphatase levels.
Main outcome measures: The primary end point for the study was overall survival. Other meaningful secondary end points included time to castration resistance, time to pain progression, time to first symptomatic skeletal event, symptomatic skeletal event-free survival, time to subsequent systemic antineoplastic therapy, time to worsening of disease-related physical symptoms, initiation of opioid therapy for ≥7 days, and safety.
Results: The baseline and demographic characteristics were well balanced between the 2 groups. Median age was 67 years. Nearly 80% of patients had bone metastasis, and approximately 17% had visceral metastasis. At the data cutoff date for the primary analysis, the median duration of therapy was 41 months for darolutamide compared with 16.7 months in the placebo group; 45.9% in the darolutamide group and 19.1% in the placebo group were receiving the allotted trial therapy at the time of the analysis. Six cycles of docetaxel were completed in approximately 85% of patients in both arms. Median overall survival follow-up was 43.7 months (darolutamide) and 42.4 months (placebo). A significant improvement in overall survival was observed in the darolutamide group. The risk of death was 32.5% lower in the darolutamide cohort than in the placebo cohort (hazard ratio [HR], 0.68; 95% CI, 0.57-0.80; P < .001). The overall survival at 4 years was 62.7% (95% CI, 58.7-66.7) in the darolutamide arm and 50.4% (95% CI, 46.3-54.6) in the placebo arm. The overall survival results remained favorable across most subgroups.
Darolutamide was associated with improvement in all key secondary endpoints. Time to castration-resistance was significantly longer in the darolutamide group (HR, 0.36; 95% CI, 0.30-0.42; P < .001). Time to pain progression was also significantly longer in the darolutamide group (HR, 0.79; 95% CI, 0.66-0.95; P = .01). Time to first symptomatic skeletal events (HR, 0.71; 95% CI, 0.54-0.94; P = .02) and time to initiation of subsequent systemic therapy (HR, 0.39; 95% CI, 0.33-0.46; P < .001) were also found to be longer in the darolutamide group.
Safety: The risk of grade 3 or higher adverse events was similar across the 2 groups. Most common adverse events were known toxic effects of docetaxel therapy and were highest during the initial period when both groups received this therapy. These side effects progressively decreased after the initial period. The most common grade 3 or 4 adverse event was neutropenia, and its frequency was similar between the darolutamide and placebo groups (33.7% and 34.2%, respectively). The most frequently reported adverse events were alopecia, neutropenia, fatigue, and anemia and were similar between the groups. Adverse events of special significance, including fatigue, falls, fractures, and cardiovascular events, were also similar between the 2 groups. Adverse events causing deaths in each arm were low and similar (4.1% in the darolutamide group and 4.0% in the placebo group). The rates of discontinuation of darolutamide or placebo were similar (13.5% and 10.6%, respectively).
Conclusion: Among patients with mHSPC, overall survival was significantly longer among patients who received darolutamide plus ADT and docetaxel than among those who received ADT and docetaxel alone. This was observed despite a high percentage of patients in the placebo group receiving subsequent systemic therapy at the time of progression. The survival benefit of darolutamide was maintained across most subgroups. An improvement was also observed in the darolutamide arm in terms of key secondary end points. The adverse events were similar across the groups and were consistent with known safety profiles of ADT and docetaxel, and no new safety signals were identified in this trial.
Commentary
The results of the current study add to the body of literature supporting multi-agent systemic therapy in newly diagnosed mHSPC. Prior phase 3 trials of combination therapy using androgen-receptor pathway inhibitors, ADT, and docetaxel have shown conflicting results. The results from the previously reported PEACE-1 study showed improved overall survival among patients who received abiraterone with ADT and docetaxel as compared with those who received ADT and docetaxel alone.1 However, as noted by the authors, the subgroup of patients in the ENZAMET trial who received docetaxel, enzalutamide, and ADT did not appear to have a survival advantage compared with those who received ADT and docetaxel alone.2 The results from the current ARASENS trial provide compelling evidence in a population of prospectively randomized patients that combination therapy with darolutamide, docetaxel, and ADT improves overall survival in men with mHSPC. The survival advantage was maintained across subgroups analyzed in this study. Improvements were observed in regards to several key secondary end points with use of darolutamide. This benefit was maintained despite many patients receiving subsequent therapy at the time of progression. Importantly, there did not appear to be a significant increase in toxicity with triplet therapy. However, it is important to note that this cohort of patients appeared largely asymptomatic at the time of enrollment, with 70% of patients having an Eastern Cooperative Oncology Group performance status of 0.
Additionally, the average age in this study was 67 years, with only about 15% of the population being older than 75 years. In the reported subgroup analysis, those older than 75 years appeared to derive a similar benefit in overall survival, however. Whether triplet therapy should be universally adopted in all patients remains unclear. For example, there is a subset of patients with mHSPC with favorable- risk disease (ie, those with recurrent metastatic disease, node-only disease). In this population, the risk-benefit analysis is less clear, and whether these patients should receive this combination is not certain. Nevertheless, the results of this well-designed study are compelling and certainly represent a potential new standard treatment option for men with mHSPC. One of the strengths of this study was its large sample size that allowed for vigorous statistical analysis to evaluate the efficacy of darolutamide in combination with ADT and docetaxel.
Application for Clinical Practice
The ARASENS study provides convincing evidence that in men with mHSPC, the addition of darolutamide to docetaxel and ADT improves overall survival. This combination appeared to be well tolerated, with no evidence of increased toxicity noted. Certainly, this combination represents a potential new standard treatment option in this population; however, further understanding of which subgroups of men benefit from enhanced therapy is needed to aid in proper patient selection.
—Santosh Kagathur, MD, and Daniel Isaac, DO, MS
Michigan State University, East Lansing, MI
Study Overview
Objective: To evaluate whether the addition of the potent androgen-receptor inhibitor (ARA) darolutamide to the standard doublet androgen-deprivation therapy (ADT) and docetaxel in metastatic, hormone-sensitive prostate cancer (mHSPC) would increase survival.
Design: A randomized, double-blind, placebo-controlled, multicenter, phase 3 study. The results reported in this publication are from the prespecified interim analysis.
Intervention: Patients with mHSPC were randomly assigned to receive either darolutamide 600 mg twice daily or placebo. All patients received standard ADT with 6 cycles of docetaxel 75 mg/m2 on day 1 every 21 days along with prednisone given within 6 weeks after randomization. Patients receiving luteinizing hormone–releasing hormone (LHRH) agonists as ADT were bridged with at least 4 weeks of first-generation antiandrogen therapy, which was discontinued before randomization. Treatments were continued until symptomatic disease progression, a change in neoplastic therapy, unacceptable toxicity, patient or physician decision, death, or nonadherence.
Setting and participants: Eligible patients included those newly diagnosed with mHSPC with metastases detected on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and bone scan. Patients were excluded if they had regional lymph node–only involvement or if they had received more than 12 weeks of ADT before randomization. Between November 2016 and June 2018, 1306 patients (651 in the darolutamide group and 655 in the placebo group) were randomized in a 1:1 manner to receive darolutamide 600 mg twice daily or placebo in addition to ADT and docetaxel. Randomization was stratified based on the TNM staging system (M1a—nonregional lymph node–only metastasis, M1b—bone metastasis with or without lymph node, or M1c—bone metastases) as well as baseline alkaline phosphatase levels.
Main outcome measures: The primary end point for the study was overall survival. Other meaningful secondary end points included time to castration resistance, time to pain progression, time to first symptomatic skeletal event, symptomatic skeletal event-free survival, time to subsequent systemic antineoplastic therapy, time to worsening of disease-related physical symptoms, initiation of opioid therapy for ≥7 days, and safety.
Results: The baseline and demographic characteristics were well balanced between the 2 groups. Median age was 67 years. Nearly 80% of patients had bone metastasis, and approximately 17% had visceral metastasis. At the data cutoff date for the primary analysis, the median duration of therapy was 41 months for darolutamide compared with 16.7 months in the placebo group; 45.9% in the darolutamide group and 19.1% in the placebo group were receiving the allotted trial therapy at the time of the analysis. Six cycles of docetaxel were completed in approximately 85% of patients in both arms. Median overall survival follow-up was 43.7 months (darolutamide) and 42.4 months (placebo). A significant improvement in overall survival was observed in the darolutamide group. The risk of death was 32.5% lower in the darolutamide cohort than in the placebo cohort (hazard ratio [HR], 0.68; 95% CI, 0.57-0.80; P < .001). The overall survival at 4 years was 62.7% (95% CI, 58.7-66.7) in the darolutamide arm and 50.4% (95% CI, 46.3-54.6) in the placebo arm. The overall survival results remained favorable across most subgroups.
Darolutamide was associated with improvement in all key secondary endpoints. Time to castration-resistance was significantly longer in the darolutamide group (HR, 0.36; 95% CI, 0.30-0.42; P < .001). Time to pain progression was also significantly longer in the darolutamide group (HR, 0.79; 95% CI, 0.66-0.95; P = .01). Time to first symptomatic skeletal events (HR, 0.71; 95% CI, 0.54-0.94; P = .02) and time to initiation of subsequent systemic therapy (HR, 0.39; 95% CI, 0.33-0.46; P < .001) were also found to be longer in the darolutamide group.
Safety: The risk of grade 3 or higher adverse events was similar across the 2 groups. Most common adverse events were known toxic effects of docetaxel therapy and were highest during the initial period when both groups received this therapy. These side effects progressively decreased after the initial period. The most common grade 3 or 4 adverse event was neutropenia, and its frequency was similar between the darolutamide and placebo groups (33.7% and 34.2%, respectively). The most frequently reported adverse events were alopecia, neutropenia, fatigue, and anemia and were similar between the groups. Adverse events of special significance, including fatigue, falls, fractures, and cardiovascular events, were also similar between the 2 groups. Adverse events causing deaths in each arm were low and similar (4.1% in the darolutamide group and 4.0% in the placebo group). The rates of discontinuation of darolutamide or placebo were similar (13.5% and 10.6%, respectively).
Conclusion: Among patients with mHSPC, overall survival was significantly longer among patients who received darolutamide plus ADT and docetaxel than among those who received ADT and docetaxel alone. This was observed despite a high percentage of patients in the placebo group receiving subsequent systemic therapy at the time of progression. The survival benefit of darolutamide was maintained across most subgroups. An improvement was also observed in the darolutamide arm in terms of key secondary end points. The adverse events were similar across the groups and were consistent with known safety profiles of ADT and docetaxel, and no new safety signals were identified in this trial.
Commentary
The results of the current study add to the body of literature supporting multi-agent systemic therapy in newly diagnosed mHSPC. Prior phase 3 trials of combination therapy using androgen-receptor pathway inhibitors, ADT, and docetaxel have shown conflicting results. The results from the previously reported PEACE-1 study showed improved overall survival among patients who received abiraterone with ADT and docetaxel as compared with those who received ADT and docetaxel alone.1 However, as noted by the authors, the subgroup of patients in the ENZAMET trial who received docetaxel, enzalutamide, and ADT did not appear to have a survival advantage compared with those who received ADT and docetaxel alone.2 The results from the current ARASENS trial provide compelling evidence in a population of prospectively randomized patients that combination therapy with darolutamide, docetaxel, and ADT improves overall survival in men with mHSPC. The survival advantage was maintained across subgroups analyzed in this study. Improvements were observed in regards to several key secondary end points with use of darolutamide. This benefit was maintained despite many patients receiving subsequent therapy at the time of progression. Importantly, there did not appear to be a significant increase in toxicity with triplet therapy. However, it is important to note that this cohort of patients appeared largely asymptomatic at the time of enrollment, with 70% of patients having an Eastern Cooperative Oncology Group performance status of 0.
Additionally, the average age in this study was 67 years, with only about 15% of the population being older than 75 years. In the reported subgroup analysis, those older than 75 years appeared to derive a similar benefit in overall survival, however. Whether triplet therapy should be universally adopted in all patients remains unclear. For example, there is a subset of patients with mHSPC with favorable- risk disease (ie, those with recurrent metastatic disease, node-only disease). In this population, the risk-benefit analysis is less clear, and whether these patients should receive this combination is not certain. Nevertheless, the results of this well-designed study are compelling and certainly represent a potential new standard treatment option for men with mHSPC. One of the strengths of this study was its large sample size that allowed for vigorous statistical analysis to evaluate the efficacy of darolutamide in combination with ADT and docetaxel.
Application for Clinical Practice
The ARASENS study provides convincing evidence that in men with mHSPC, the addition of darolutamide to docetaxel and ADT improves overall survival. This combination appeared to be well tolerated, with no evidence of increased toxicity noted. Certainly, this combination represents a potential new standard treatment option in this population; however, further understanding of which subgroups of men benefit from enhanced therapy is needed to aid in proper patient selection.
—Santosh Kagathur, MD, and Daniel Isaac, DO, MS
Michigan State University, East Lansing, MI
1. Fizazi K, Carles Galceran J, Foulon S, et al. LBA5 A phase III trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate plus prednisone in PEACE-1. Ann Oncol. 2021;32:Suppl 5:S1299. doi:10.1016/j.annonc.2021.08.2099
2. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121-131. doi:10.1056/NEJMoa1903835
1. Fizazi K, Carles Galceran J, Foulon S, et al. LBA5 A phase III trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate plus prednisone in PEACE-1. Ann Oncol. 2021;32:Suppl 5:S1299. doi:10.1016/j.annonc.2021.08.2099
2. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121-131. doi:10.1056/NEJMoa1903835
Evaluation of the Empower Veterans Program for Military Veterans With Chronic Pain
From Neurology/Chronic Pain Management Services, Department of Veterans Affairs (VA) Maryland Health Care System, Baltimore VA Medical Center, Baltimore, MD (Dr. Uche), and School of Nursing, Washburn University, Topeka, KS (Drs. Jamison and Waugh).
Abstract
Objective: The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the Empower Veterans Program (EVP) offered by a Veterans Administration facility in the northeastern United States.
Methods: This quality improvement project used data collected from veterans with chronic pain who completed the veterans health care facility’s EVP between August 2017 and August 2019. Pre- and post-intervention data on pain intensity, pain interference, quality of life, and pain catastrophizing were compared using paired t-tests.
Results: Although data were abstracted from 115 patients, the final sample included
Conclusion: Veterans with chronic high-impact noncancer pain who completed the EVP had reduced pain intensity, pain interference, pain catastrophizing as well as improved quality of life and satisfaction with their health.
Keywords: musculoskeletal pain, Veterans Affairs, complementary and integrative health, acceptance and commitment therapy, mind-body therapies, whole health, multidisciplinary pain management.
More than 100 million American adults suffer from chronic pain; costs associated with managing chronic pain are approximately $635 billion
One such program is the Empower Veterans Program (EVP). Originally developed at the Atlanta Veterans Affairs Health Care System, the EVP is a CIH modality based on the biopsychosocial model of pain developed by psychiatrist George Engel in 1977.3 The biopsychosocial model of pain recognizes that pain is a complex, multidimensional, biopsychosocial experience. Under this model, the mind and body work in unison as interconnected entities. Because the model acknowledges biological, psychological, and social components of pain and illness,4 treatment focuses on all aspects of a person’s health, life, and relationships.
The EVP fits into the VHA Pain Management Stepped Care Model and is an adjunctive complement for that model.5-7 The EVP complements care at the first step, where patient/family provide self-care and where care is provided by patient-aligned primary care teams, at the second step, which includes secondary consultation with multidisciplinary pain medicine specialty teams and other specialists, and at the third step, with the addition of tertiary interdisciplinary pain centers.
The VA Maryland Health Care System (VAMHCS) implemented the EVP as part of a quality improvement project for the management of chronic pain. The objectives of the program were to reduce pain intensity, pain catastrophizing, and pain interference, as well as improve functionality and quality of life among veterans with chronic high-impact noncancer pain. More than 2 years after the program was implemented, collected data had not been analyzed. The purpose of this quality improvement project was to abstract and analyze the previously collected data from veterans with high-impact chronic pain who attended an EVP offered by the VAMHCS. The results of the data analysis were used to inform decisions regarding the future of the program.
Methods
This quality improvement project used the Plan-Do-Study-Act (PDSA) process.8 The first 2 phases of the PDSA cycle (Plan and Do) were completed by a team of VA employees from the VAMHCS, who donated their time to establish and implement the program at the project site. This team consisted of psychologists, a physical therapist, a social worker, and a chaplain, and included support from medical administrative staff. This team planned and implemented the EVP at the VA facility based on the model developed at the Atlanta VA Health Care System. During the “Do” phase, the team collected data on pain intensity, pain interference, quality of life, and pain negative cognition (catastrophizing) before the intervention and post intervention. They also collected data on program outcome (patient treatment satisfaction) post intervention. Because these employees did not have time to retrieve and analyze the data, they welcomed the opportunity to have the data analyzed by the investigators during the Study phase of the PDSA cycle. Based on the results of the analysis, recommendations for program changes were made during the Act phase of the cycle.
Intervention
The EVP was developed as a 10-week (30 hours) interdisciplinary CIH approach that coached veterans with chronic pain to live fuller lives based on their individual values and what matters to them. EVP is the “What Else” management modality for the 5% of veterans with high-impact chronic pain.9 The EVP provided functional restoration through its components of whole health, mindfulness training, coaching calls, acceptance and commitment therapy, and mindful movement. It used the Wheel of Health with the 4 key components of me, self-care, professional care, and community.10,11
Veterans who had a diagnosis of chronic nonmalignant pain for 3 months or more and who agreed to participate in the EVP at this facility attended 3-hour classes every Tuesday with a cohort of 8 to 12 peers and engaged in one-on-one coaching with interdisciplinary team members. During the class sessions, veterans were coached to understand and accept their pain and commit to maintaining function despite their pain. Mindful movement by the physical therapist emphasized the pivotal place of exercise in pain management. The therapist used the mantra “Motion is Lotion.”9 The guiding principle of the EVP was that small incremental changes can have a big impact on the individual’s whole life. Emphasis was placed on increasing self-efficacy and mindful awareness for veterans with high-impact pain by giving them “Skills before Pills.”9
Outcome Measures
Outcome measures included the Numerical Pain Rating Scale (NPRS), the Multidimensional Pain Inventory (MPI), the World Health Organization Quality of Life assessment (WHOQOL-BREF), the Pain Catastrophizing Scale (PCS), and the Pain Treatment Satisfaction Scale (PTSS). Cronbach alpha coefficients were calculated to assess internal consistency reliability of these measures in the sample of veterans who completed the EVP.
NPRS. The NPRS is ubiquitous as a screening tool in many health care environments and its use is mandated by the VA health care system.12 The choice of the NPRS as the tool for pain screening in the VA health care system was based on a large body of research that supports the reliability and validity of the NPRS as a single index of pain intensity or severity. Studies suggest that the NPRS is valid for use in the assessment of acute, cancer, or chronic nonmalignant pain and in varied clinical settings.13 The NPRS has 4 items, each on a scale of 0 to 10. For the purpose of this project, only 3 items were used. The 3 items assessed the worst pain, usual pain, and the current pain (right now). The higher the score, the higher the pain intensity. Cronbach alpha coefficients on the NPRS obtained from the current sample of veterans were 0.85 on both pre- and postintervention assessments.
MPI. The MPI is an easily accessible, reliable, and valid self-report questionnaire that measures the impact of pain on an individual’s life, quality of social support, and general activity.14 This instrument is a short version of the West Haven-Yale MPI.15 The MPI contains 9 items rated on a scale from 0 to 6. The higher the score, the greater pain interference a person is experiencing. The MPI produces reliable, valid information for diagnostic purposes and for therapy outcome studies.16 The MPI had a Cronbach alpha of 0.90 on pre-intervention and 0.92 on postintervention assessments in the current sample.
WHOQOL-BREF. The WHOQOL-BREF is a measure of quality of life and is an abbreviated version of the WHOQOL-100. Quality of life is defined by the World Health Organization17 “as an individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.” The WHOQOL-BREF contains 26 items. The first 2 items were examined separately; the first item asks individuals to rate their overall quality of life and the second asks individuals how satisfied they are with their health. The remaining 24 items were used to calculate the following 4 domain scores: physical health, psychological health, social relationship, and environment.18 Each item is measured on a scale of 1 to 5. Higher scores denote higher or better quality of life. Domain scores have demonstrated good reliability and validity.19-21 Cronbach alpha coefficients for the domain subscales ranged from 0.63 to 0.84 in the current sample, with the lowest alphas for the 3-item Social Relationships Domain.
PCS. The PCS is a widely used measure of catastrophic thinking related to pain. Catastrophizing has been conceived by Sullivan and colleagues as “an exaggerated negative mental set brought to bear during actual or anticipated painful experience.”22 The PCS provides a total score and scores for the following subscales: rumination, magnification, and helplessness.23 It has been used in a variety of chronic pain populations and has demonstrated good reliability and validity in clinical as well as nonclinical samples.24-26 The PCS has 13 items rated on a scale of 0 to 4. Higher scores mean greater negative pain cognition (catastrophizing). In the current sample, the PCS total scale had a Cronbach alpha coefficient of 0.95 and 0.94 on the 2 assessments. The coefficients for the subscales ranged from 0.81 to 0.90.
PTSS. The PTSS is a 5-item tool that measures patient satisfaction with pain treatment. It includes items that address overall satisfaction, staff warmth, staff skill level, ease of scheduling appointments, and recommendation of the program to other veterans. It was derived from the post-treatment version of The Pain Outcome Questionnaire-VA and has demonstrated reliability and validity.27 The questions are scaled from 0 to 10. High scores on the PTSS denote high patient satisfaction with the EVP. The Cronbach alpha coefficient on the PTSS obtained from the current sample was 0.80.
Data Gathering and Analysis
Prior to starting the Study phase, Washburn University’s Institutional Review Board (IRB) and the VA IRB approved the project. The VA IRB, through its affiliate, gave a Not Human Research Determination and granted a waiver of informed consent and the Health Insurance Portability and Accountability Act authorization. The VA facility’s Research and Development department also approved the quality improvement project.
Once these approvals were obtained, the Study phase began with the abstraction of retrospective data obtained from veterans who participated in the VA health care facility’s EVP between
Veterans who completed the program were compared to veterans who did not complete the program on age, gender, and baseline measures. The investigators used independent samples t-tests to compare completers and noncompleters on age, pain intensity, pain interference, quality of life, and pain catastrophizing. They used the chi-square test of independence to analyze the association between gender and program completion.
Data were included in the pre- and postintervention analysis if the veteran completed the NPRS, MPI, WHOQOL-BREF, and PCS pre and post intervention. This became an important eligibility requirement as some of the tools/measures were changed towards the end of the review period in 2019. Pre- and postintervention data on pain intensity, pain interference, quality of life, pain catastrophizing, and patient satisfaction were compared using paired samples t-test at .004 level of significance based on the Bonferroni correction.28 Data on patient satisfaction with pain treatment were collected at program completion (week 8 or 10) and were analyzed using descriptive statistics.
Effect sizes (Cohen’s d) were calculated to determine the substantive significance or magnitude of the mean differences in scores. Effect sizes (expressed as absolute values of Cohen’s d) were calculated as the mean difference divided by the standard deviation. Values of 0.2 were considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size.29
Results
Data were abstracted for 115 veterans who started the EVP. Of these, 48 left the program, leaving 67 veterans (58%) who completed the program. Completers and noncompleters were similar in age, gender, and baseline measures (Table 1). Fifty-three (79%) completers and 35 (73%) noncompleters were male. A chi-square test of independence showed no significant association between gender and program completion (χ21 [N = 115] = .595, P = .440).
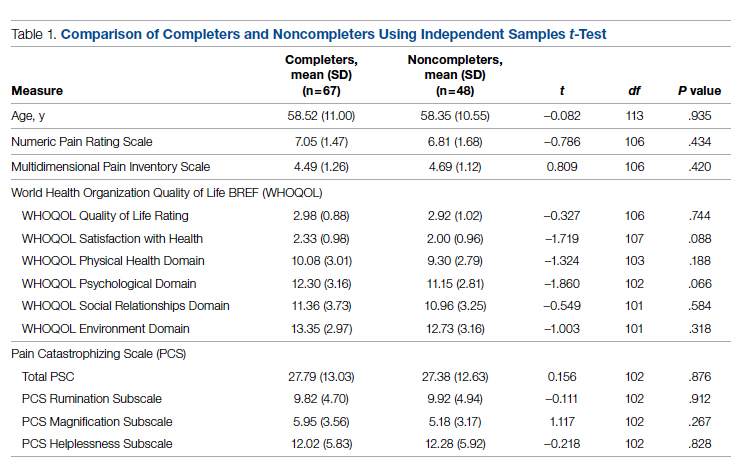
Comparison of pre-and postintervention mean scale scores resulted in statistically significant
Analysis of data obtained using the PTSS yielded high mean scores for items that focused on patient satisfaction with treatment (Table 3). Scaled statistics yielded a mean (SD) of 46.95 (4.40). These results denoted overall patient satisfaction with the EVP.

Discussion
The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the EVP. Comparison of pre-intervention and postintervention data obtained from 67 veterans who completed the program revealed improvements in pain intensity, pain interference, negative cognition (catastrophizing), and quality of life. The differences were statistically significant and clinically meaningful, with medium and large effect sizes. In addition, veterans reported high satisfaction with the EVP.
The EVP includes CIH approaches that have demonstrated effectiveness among veterans and other populations with chronic pain. A wealth of studies, for example, support the effectiveness of CIH approaches among veterans.30-34 Other studies focus on specific CIH approaches that are components of the EVP. Evidence supports, for example, the efficacy of mindfulness-based stress reduction,35-39 acceptance and commitment therapy,40-43 brief peer support intervention,44 and interdisciplinary biopsychosocial rehabilitation.45,46
While empirical evidence supports components of the EVP, only one study focused on the outcomes of the Atlanta VA EVP among veterans with chronic pain. Results of a qualitative study conducted by Penney and Haro47 described the experience of veterans with the EVP. Those veterans reported adopting new self-care or lifestyle practices for pain management and health, accepting pain, being better able to adjust and set boundaries, feeling more in control, participating in life, and changing their medication use.
The mean baseline scores from the current sample were similar to samples of patients with chronic pain in other studies (NPRS,48 MPI,48 and PCS48-51). After converting scores on the WHOQOL-BREF from those that ranged from 4 to 20 to those that ranged from 0 to 100,18 the scores from the current sample were similar to those of other studies of patients with chronic pain.48,52,53Several strengths of the project should be noted. Data were collected using well established measurement tools that had previously demonstrated reliability and validity. All the tools used in data collection demonstrated good internal consistency reliabilities in the current sample of veterans. Weaknesses of the project include the use of a convenience sample of veterans and small sample size. Data were not available on the number of veterans who were offered participation or on how many veterans declined enrollment. The sample of veterans who chose to participate in the EVP may or may not have been representative of the population of veterans with high-impact chronic pain. As a pre- and postintervention design with no comparison group, the results are subject to multiple threats to internal validity, including the Hawthorne effect, maturation in the form of healing, and attrition. Reasons for leaving the program had not been recorded, so the investigators had no way of knowing factors that may have contributed to attrition. Also, data on when veterans left the program were unavailable. Research is needed with a control group to reduce the effect of confounding variables on the outcome measures. This project used data collected at a single VA facility, which limits its generalizability.
While completers and noncompleters of the EVP were similar on age, gender, and baseline measures, there may have been unidentified characteristics that influenced program completion. The investigators noticed the presence of more missing data among noncompleters compared to completers on the pre-intervention PCS; thus, noncompleters may have scored lower than completers on this instrument simply because there were more individual items that were unanswered/missing among this group of noncompleters.
Data were analyzed using a limited number of outcome measures that had previously been collected. Other outcome measures might include whether EVP participants reduced their use of medications, clinical resources, and personnel. Future projects, for example, could determine whether the EVP is effective in reducing opioid analgesic medication use and decreasing primary care and emergency department visits. Cost-benefit analyses could be completed to determine whether EVP is associated with financial savings.
Because no follow-up assessments were made to determine whether improvements were maintained over time, the project focus was limited to an evaluation of the short-term changes in the outcome measures. Future projects could include a follow-up assessment of the veterans 1- or 2-years post completion of the EVP.
Data for the project were collected prior to the COVID-19 pandemic, when the EVP was implemented through face-to-face meetings with participants and their peers. It is not clear how changes to the delivery of the program (such as offering it through telehealth) might impact veterans’ satisfaction with the program, willingness to complete it, and other variables of interest.
The results of this project were made available to stakeholders with recommendations for program expansion both at the current location and at other VA facilities, including the recommendation to hire additional personnel that would implement the program. As the VA network of facilities expand the EVP program and adapt it for telehealth delivery, the investigators recommended a similar analysis of data be performed following telehealth delivery. If delivery through telehealth is shown to improve outcome measures, the EVP could provide pain management treatment options for patients challenged by transportation barriers, including rural veterans.
Conclusion
This quality improvement project provided evidence of improvement in measures of pain severity, pain interference, negative cognition (catastrophizing), quality of life, and patient treatment satisfaction among veterans with chronic high-impact pain. Findings have been well received by the northeastern VA as well as the Veterans Integrated Systems Network 5. The results of the analyses were used to inform decisions regarding the future of the program.
Disclaimer: This material is the result of work supported with resources and the use of facilities at the VA Maryland Health Care System, Baltimore, Maryland. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgments: The authors thank Dr. Arianna Perra, the recent past coordinator of the Empower Veterans Program (EVP), who provided initial insights and support that motivated the decision to evaluate the program. We also thank the veterans and VA EVP clinicians who contributed data for the evaluation, and Dr. Michael Saenger (Director, TelePain-EVP: EVP) and Dr. Robert Lavin for their ongoing support, care, and concern for veteran patients. We also thank Dr. Beverly Bradley and the neurology service administrative team for their guidance in the process of obtaining necessary VA approvals for this project.
Corresponding author: Jessica U. Uche, DNP, CRNP-Family; [email protected]
doi:10.12788/jcom.0089
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press (US); 2011.
2. Bastian LA, Heapy A, Becker WC, et al. Understanding pain and pain treatment for veterans: responding to the federal pain research strategy. Pain Med. 2018;19(suppl_1); S1-S4. doi:10.1093/pm/pny1433
3. Engle GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136. doi:10.1126/science.847460
4. Bevers K, Watts L, Kishino ND, et al. The biopsychosocial model of the assessment, prevention, and treatment of chronic pain. US Neurology. 2016;12(2):98-104. doi:10.17925/USN.2016.12.02.98
5. Bair MJ, Ang D, Wu J, et al. Evaluation of stepped care for chronic pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: A randomized clinical trial. JAMA Intern Med. 2015;175(5):682-689. doi:10.1001/jamainternmed.2015.97
6. Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: Department of Veterans Affairs; 2009.https://www.va.gov/painmanagement/docs/vha09paindirective.pdf
7. Moore BA, Anderson D, Dorflinger L, et al. Stepped care model for pain management and quality of pain care in long-term opioid therapy. J Rehabil Res Dev. 2016;53(1):137-146. doi:10.1682/JRRD.2014.10.0254
8. Institute for Healthcare Improvement. How to improve. Accessed March 14, 2022. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
9. Saenger M. Empower Veterans Program. APA PCSS-O Webinars. Evidence CAM LBP 2016.
10. Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: How will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
11. Veterans Health Administration. Whole health: Circle of health. Updated April 1, 2021. Accessed March 14, 2022. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
12. Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453-1458. doi:10.1007/s11606-007-0321-2
13. Veterans Health Administration. Pain as the 5th vital sign toolkit. October 2000, revised edition. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf
14. McKillop JM, Nielson WR. Improving the usefulness of the Multidimensional Pain Inventory. Pain Res Manag. 2011;16(4):239-244. doi:10.1155/2011/873424
15. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain.1985;23(4):345-356. doi:10.1016/0304-3959(85)90004-1
16. Verra ML, Angst F, Staal JB, et al. Reliability of the multidimensional pain inventory and stability of the MPI classification system in chronic back pain. BMC Musculoskelet Disord. 2012;13:155. doi:10.1186/1471-2474-13-155
17. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551-558. doi:10.1017/s0033291798006667
18. World Health Organization. Division of Mental Health. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version, December 1996. Accessed March 14, 2022. https://apps.who.int/iris/handle/10665/63529
19. Guay S, Fortin C, Fikretoglu D, et al. Validation of the WHOQOL-BREF in a sample of male treatment-seeking veterans. Mil Psychol. 2015;27(2):85-92. doi:10.1037/mil0000065
20. Skevington S, Lotfy M, O’Connell K, WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
21. Stratton KJ, Bender MC, Cameron JJ, Pickett TC. Development and evaluation of a behavioral pain management treatment program in a Veterans Affairs Medical Center. Mil Med. 2015;180(3):263-268. doi:10.7205/MILMED-D-14-00281.
22. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52-64. doi:10.1097/00002508-200103000-00008
23. Sullivan JL. The Pain Catastrophizing Scale: User manual. Accessed March 14, 2022. https://studylib.net/doc/8330191/the-pain-catastrophizing-scale---dr.-michael-sullivan
24. Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18(9):1139-1149. doi:10.1016/j.jpain.2017.05.003
25. Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589-605. doi:10.1023/a:1025570508954
26. Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assessment. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
27. Walker R, Clark M, Gironda R. Psychometric characteristics of the Pain Treatment Satisfaction Scale. J Pain. 2015;6(3Suppl.):S76.
28. Emerson RW. Bonferroni correction and type I error. J Vis Impair Blind. 2020;114(1):77-78. doi:10.1177/0145482X20901378
29. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988. doi:10.4324/9780203771587
30. Craner JR, Lake ES, Bancroft KA, George LL. Treatment outcomes and mechanisms for an ACT-based 10-week interdisciplinary chronic pain rehabilitation program. Pain Pract. 2020;20(1):44-54. doi:10.1111/papr.12824
31. Han L, Goulet JL, Skanderson M, et al. Evaluation of complementary and integrative health approaches among US veterans with musculoskeletal pain using propensity score methods. Pain Med. 2019;20(1):90-102. doi:10.1093/pm/pny027
32. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US veterans: an economic evaluation. PLoS One. 2019;14(6):e0217831. doi:10.1371/journal.pone.0217831
33. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Global Forum on Innovation in Health Professional Education; Forum on Neuroscience and Nervous System Disorders; Stroud C, Posey Norris SM, Bain L, eds. The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop. National Academies Press (US); April 12, 2019.
34. Richmond H, Hall AM, Copsey B, et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0134192. doi:10.1371/journal.pone.0134192

35. Kearney DJ, Simpson TL, Malte CA, et al. Mindfulness-based stress reduction in addition to usual care is associated with improvements in pain, fatigue, and cognitive failures among veterans with Gulf War illness. Am J Med. 2016;129(2):204-214. doi:10.1016/j.amjmed.2015.09.015
36. Khoo E, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioral therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis. Evid Based Ment Health. 2019;22(1):26-35. doi:10.1136/ebmental-2018-300062
37. Khusid MA, Vythilingam M. The emerging role of mindfulness meditation as effective self-management strategy, Part 2: clinical implications for chronic pain, substance misuse, and insomnia. Mil Med. 2016;181(9):969-975. doi:10.7205/MILMED-D-14-00678
38. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: A randomized controlled trial. Pain Med. 2015;16(4):641-652. doi:10.1111/pme.12605
39. Zou L, Zhang Y, Yang L, et al. Are mindful exercises safe and beneficial for treating chronic lower back pain? A systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2019;8(5):628. doi:10.3390/jcm8050628
40. Hughes LS, Clark J, Colclough JA, et al. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6):552-568. doi:10.1097/AJP.0000000000000425
41. Kemani MK, Olsson GL, Lekander M, et al. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: a randomized controlled trial. Clin J Pain. 2015;31(11):1004-1016. doi:10.1097/AJP.0000000000000203
42. Scott W, Daly A, Yu L, McCracken LM. Treatment of chronic pain for adults 65 and over: analyses of outcomes and changes in psychological flexibility following interdisciplinary acceptance and commitment therapy (ACT). Pain Med. 2017;18(2):252. doi:10.1093/pm/pnw073
43. Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KMG. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther. 2016;45(1):5-31. doi:10.1080/16506073.2015.1098724
44. Matthias MS, McGuire AB, Kukla M, et al. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16(1):81-87. doi:10.1111/pme.12571
45. Anamkath NS, Palyo SA, Jacobs SC, et al. An interdisciplinary pain rehabilitation program for veterans with chronic pain: description and initial evaluation of outcomes. Pain Res Manag. 2018;2018(3941682):1-9. doi:10.1155/2018/3941682
46. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;9: CD000963. doi:10.1002/14651858.CD000963.pub3
47. Penney LS, Haro E. Qualitative evaluation of an interdisciplinary chronic pain intervention: Outcomes and barriers and facilitators to ongoing pain management. J Pain Res. 2019;12:865-878. doi:10.2147/JPR.S185652
48. Murphy JL, Cordova MJ, Dedert EA. Cognitive behavioral therapy for chronic pain in veterans; Evidence for clinical effectiveness in a model program. Psychol Serv. 2022;19(1):95-102. doi:10.1037/ser0000506
49. Katz L, Patterson L, Zacharias R. Evaluation of an interdisciplinary chronic pain program and predictors of readiness for change. Can J Pain. 2019;3(1):70-78. doi:10.1080/24740527.2019.1582296
50. Majumder SMM, Ahmed S, Shazzad N, et al. Translation, cross-cultural adaptation and validation of the Pain Catastrophizing Scale (PCS) into Bengali I patients with chronic non-malignant musculoskeletal pain. Int J Rheum Dis. 2020;23:1481-1487. doi:10.1111/1756-185X.13954
51. Margiotta F, Hannigan A, Imran A, et al. Pain, perceived injustice, and pain catastrophizing in chronic pain patients in Ireland. Pain Pract. 2016;17(5):663-668. doi:10.1111/papr.12
52. Bras M, Milunovic V, Boban M, et al. Quality of live in Croatian Homeland war (1991-1995) veterans who suffer from post-traumatic stress disorder and chronic pain. Health Qual Life Out. 2011;9:56. doi:10.1186/1477-7525-9-56
53. Liu C-H, Kung Y-Y, Lin C-L, et al. Therapeutic efficacy and the impact of the “dose” effect of acupuncture to treat sciatica: A randomized controlled pilot study. J Pain Res. 2019;12:3511-3520. doi:10.2147/JPR.S210672
From Neurology/Chronic Pain Management Services, Department of Veterans Affairs (VA) Maryland Health Care System, Baltimore VA Medical Center, Baltimore, MD (Dr. Uche), and School of Nursing, Washburn University, Topeka, KS (Drs. Jamison and Waugh).
Abstract
Objective: The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the Empower Veterans Program (EVP) offered by a Veterans Administration facility in the northeastern United States.
Methods: This quality improvement project used data collected from veterans with chronic pain who completed the veterans health care facility’s EVP between August 2017 and August 2019. Pre- and post-intervention data on pain intensity, pain interference, quality of life, and pain catastrophizing were compared using paired t-tests.
Results: Although data were abstracted from 115 patients, the final sample included
Conclusion: Veterans with chronic high-impact noncancer pain who completed the EVP had reduced pain intensity, pain interference, pain catastrophizing as well as improved quality of life and satisfaction with their health.
Keywords: musculoskeletal pain, Veterans Affairs, complementary and integrative health, acceptance and commitment therapy, mind-body therapies, whole health, multidisciplinary pain management.
More than 100 million American adults suffer from chronic pain; costs associated with managing chronic pain are approximately $635 billion
One such program is the Empower Veterans Program (EVP). Originally developed at the Atlanta Veterans Affairs Health Care System, the EVP is a CIH modality based on the biopsychosocial model of pain developed by psychiatrist George Engel in 1977.3 The biopsychosocial model of pain recognizes that pain is a complex, multidimensional, biopsychosocial experience. Under this model, the mind and body work in unison as interconnected entities. Because the model acknowledges biological, psychological, and social components of pain and illness,4 treatment focuses on all aspects of a person’s health, life, and relationships.
The EVP fits into the VHA Pain Management Stepped Care Model and is an adjunctive complement for that model.5-7 The EVP complements care at the first step, where patient/family provide self-care and where care is provided by patient-aligned primary care teams, at the second step, which includes secondary consultation with multidisciplinary pain medicine specialty teams and other specialists, and at the third step, with the addition of tertiary interdisciplinary pain centers.
The VA Maryland Health Care System (VAMHCS) implemented the EVP as part of a quality improvement project for the management of chronic pain. The objectives of the program were to reduce pain intensity, pain catastrophizing, and pain interference, as well as improve functionality and quality of life among veterans with chronic high-impact noncancer pain. More than 2 years after the program was implemented, collected data had not been analyzed. The purpose of this quality improvement project was to abstract and analyze the previously collected data from veterans with high-impact chronic pain who attended an EVP offered by the VAMHCS. The results of the data analysis were used to inform decisions regarding the future of the program.
Methods
This quality improvement project used the Plan-Do-Study-Act (PDSA) process.8 The first 2 phases of the PDSA cycle (Plan and Do) were completed by a team of VA employees from the VAMHCS, who donated their time to establish and implement the program at the project site. This team consisted of psychologists, a physical therapist, a social worker, and a chaplain, and included support from medical administrative staff. This team planned and implemented the EVP at the VA facility based on the model developed at the Atlanta VA Health Care System. During the “Do” phase, the team collected data on pain intensity, pain interference, quality of life, and pain negative cognition (catastrophizing) before the intervention and post intervention. They also collected data on program outcome (patient treatment satisfaction) post intervention. Because these employees did not have time to retrieve and analyze the data, they welcomed the opportunity to have the data analyzed by the investigators during the Study phase of the PDSA cycle. Based on the results of the analysis, recommendations for program changes were made during the Act phase of the cycle.
Intervention
The EVP was developed as a 10-week (30 hours) interdisciplinary CIH approach that coached veterans with chronic pain to live fuller lives based on their individual values and what matters to them. EVP is the “What Else” management modality for the 5% of veterans with high-impact chronic pain.9 The EVP provided functional restoration through its components of whole health, mindfulness training, coaching calls, acceptance and commitment therapy, and mindful movement. It used the Wheel of Health with the 4 key components of me, self-care, professional care, and community.10,11
Veterans who had a diagnosis of chronic nonmalignant pain for 3 months or more and who agreed to participate in the EVP at this facility attended 3-hour classes every Tuesday with a cohort of 8 to 12 peers and engaged in one-on-one coaching with interdisciplinary team members. During the class sessions, veterans were coached to understand and accept their pain and commit to maintaining function despite their pain. Mindful movement by the physical therapist emphasized the pivotal place of exercise in pain management. The therapist used the mantra “Motion is Lotion.”9 The guiding principle of the EVP was that small incremental changes can have a big impact on the individual’s whole life. Emphasis was placed on increasing self-efficacy and mindful awareness for veterans with high-impact pain by giving them “Skills before Pills.”9
Outcome Measures
Outcome measures included the Numerical Pain Rating Scale (NPRS), the Multidimensional Pain Inventory (MPI), the World Health Organization Quality of Life assessment (WHOQOL-BREF), the Pain Catastrophizing Scale (PCS), and the Pain Treatment Satisfaction Scale (PTSS). Cronbach alpha coefficients were calculated to assess internal consistency reliability of these measures in the sample of veterans who completed the EVP.
NPRS. The NPRS is ubiquitous as a screening tool in many health care environments and its use is mandated by the VA health care system.12 The choice of the NPRS as the tool for pain screening in the VA health care system was based on a large body of research that supports the reliability and validity of the NPRS as a single index of pain intensity or severity. Studies suggest that the NPRS is valid for use in the assessment of acute, cancer, or chronic nonmalignant pain and in varied clinical settings.13 The NPRS has 4 items, each on a scale of 0 to 10. For the purpose of this project, only 3 items were used. The 3 items assessed the worst pain, usual pain, and the current pain (right now). The higher the score, the higher the pain intensity. Cronbach alpha coefficients on the NPRS obtained from the current sample of veterans were 0.85 on both pre- and postintervention assessments.
MPI. The MPI is an easily accessible, reliable, and valid self-report questionnaire that measures the impact of pain on an individual’s life, quality of social support, and general activity.14 This instrument is a short version of the West Haven-Yale MPI.15 The MPI contains 9 items rated on a scale from 0 to 6. The higher the score, the greater pain interference a person is experiencing. The MPI produces reliable, valid information for diagnostic purposes and for therapy outcome studies.16 The MPI had a Cronbach alpha of 0.90 on pre-intervention and 0.92 on postintervention assessments in the current sample.
WHOQOL-BREF. The WHOQOL-BREF is a measure of quality of life and is an abbreviated version of the WHOQOL-100. Quality of life is defined by the World Health Organization17 “as an individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.” The WHOQOL-BREF contains 26 items. The first 2 items were examined separately; the first item asks individuals to rate their overall quality of life and the second asks individuals how satisfied they are with their health. The remaining 24 items were used to calculate the following 4 domain scores: physical health, psychological health, social relationship, and environment.18 Each item is measured on a scale of 1 to 5. Higher scores denote higher or better quality of life. Domain scores have demonstrated good reliability and validity.19-21 Cronbach alpha coefficients for the domain subscales ranged from 0.63 to 0.84 in the current sample, with the lowest alphas for the 3-item Social Relationships Domain.
PCS. The PCS is a widely used measure of catastrophic thinking related to pain. Catastrophizing has been conceived by Sullivan and colleagues as “an exaggerated negative mental set brought to bear during actual or anticipated painful experience.”22 The PCS provides a total score and scores for the following subscales: rumination, magnification, and helplessness.23 It has been used in a variety of chronic pain populations and has demonstrated good reliability and validity in clinical as well as nonclinical samples.24-26 The PCS has 13 items rated on a scale of 0 to 4. Higher scores mean greater negative pain cognition (catastrophizing). In the current sample, the PCS total scale had a Cronbach alpha coefficient of 0.95 and 0.94 on the 2 assessments. The coefficients for the subscales ranged from 0.81 to 0.90.
PTSS. The PTSS is a 5-item tool that measures patient satisfaction with pain treatment. It includes items that address overall satisfaction, staff warmth, staff skill level, ease of scheduling appointments, and recommendation of the program to other veterans. It was derived from the post-treatment version of The Pain Outcome Questionnaire-VA and has demonstrated reliability and validity.27 The questions are scaled from 0 to 10. High scores on the PTSS denote high patient satisfaction with the EVP. The Cronbach alpha coefficient on the PTSS obtained from the current sample was 0.80.
Data Gathering and Analysis
Prior to starting the Study phase, Washburn University’s Institutional Review Board (IRB) and the VA IRB approved the project. The VA IRB, through its affiliate, gave a Not Human Research Determination and granted a waiver of informed consent and the Health Insurance Portability and Accountability Act authorization. The VA facility’s Research and Development department also approved the quality improvement project.
Once these approvals were obtained, the Study phase began with the abstraction of retrospective data obtained from veterans who participated in the VA health care facility’s EVP between
Veterans who completed the program were compared to veterans who did not complete the program on age, gender, and baseline measures. The investigators used independent samples t-tests to compare completers and noncompleters on age, pain intensity, pain interference, quality of life, and pain catastrophizing. They used the chi-square test of independence to analyze the association between gender and program completion.
Data were included in the pre- and postintervention analysis if the veteran completed the NPRS, MPI, WHOQOL-BREF, and PCS pre and post intervention. This became an important eligibility requirement as some of the tools/measures were changed towards the end of the review period in 2019. Pre- and postintervention data on pain intensity, pain interference, quality of life, pain catastrophizing, and patient satisfaction were compared using paired samples t-test at .004 level of significance based on the Bonferroni correction.28 Data on patient satisfaction with pain treatment were collected at program completion (week 8 or 10) and were analyzed using descriptive statistics.
Effect sizes (Cohen’s d) were calculated to determine the substantive significance or magnitude of the mean differences in scores. Effect sizes (expressed as absolute values of Cohen’s d) were calculated as the mean difference divided by the standard deviation. Values of 0.2 were considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size.29
Results
Data were abstracted for 115 veterans who started the EVP. Of these, 48 left the program, leaving 67 veterans (58%) who completed the program. Completers and noncompleters were similar in age, gender, and baseline measures (Table 1). Fifty-three (79%) completers and 35 (73%) noncompleters were male. A chi-square test of independence showed no significant association between gender and program completion (χ21 [N = 115] = .595, P = .440).

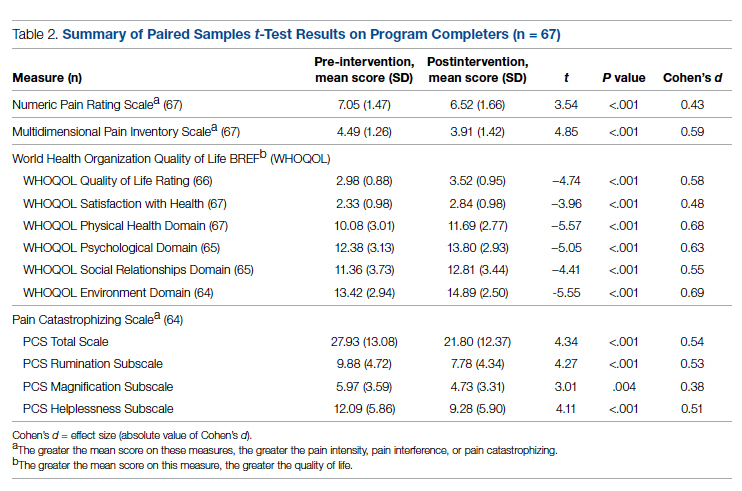
Comparison of pre-and postintervention mean scale scores resulted in statistically significant

Analysis of data obtained using the PTSS yielded high mean scores for items that focused on patient satisfaction with treatment (Table 3). Scaled statistics yielded a mean (SD) of 46.95 (4.40). These results denoted overall patient satisfaction with the EVP.

Discussion
The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the EVP. Comparison of pre-intervention and postintervention data obtained from 67 veterans who completed the program revealed improvements in pain intensity, pain interference, negative cognition (catastrophizing), and quality of life. The differences were statistically significant and clinically meaningful, with medium and large effect sizes. In addition, veterans reported high satisfaction with the EVP.
The EVP includes CIH approaches that have demonstrated effectiveness among veterans and other populations with chronic pain. A wealth of studies, for example, support the effectiveness of CIH approaches among veterans.30-34 Other studies focus on specific CIH approaches that are components of the EVP. Evidence supports, for example, the efficacy of mindfulness-based stress reduction,35-39 acceptance and commitment therapy,40-43 brief peer support intervention,44 and interdisciplinary biopsychosocial rehabilitation.45,46
While empirical evidence supports components of the EVP, only one study focused on the outcomes of the Atlanta VA EVP among veterans with chronic pain. Results of a qualitative study conducted by Penney and Haro47 described the experience of veterans with the EVP. Those veterans reported adopting new self-care or lifestyle practices for pain management and health, accepting pain, being better able to adjust and set boundaries, feeling more in control, participating in life, and changing their medication use.
The mean baseline scores from the current sample were similar to samples of patients with chronic pain in other studies (NPRS,48 MPI,48 and PCS48-51). After converting scores on the WHOQOL-BREF from those that ranged from 4 to 20 to those that ranged from 0 to 100,18 the scores from the current sample were similar to those of other studies of patients with chronic pain.48,52,53Several strengths of the project should be noted. Data were collected using well established measurement tools that had previously demonstrated reliability and validity. All the tools used in data collection demonstrated good internal consistency reliabilities in the current sample of veterans. Weaknesses of the project include the use of a convenience sample of veterans and small sample size. Data were not available on the number of veterans who were offered participation or on how many veterans declined enrollment. The sample of veterans who chose to participate in the EVP may or may not have been representative of the population of veterans with high-impact chronic pain. As a pre- and postintervention design with no comparison group, the results are subject to multiple threats to internal validity, including the Hawthorne effect, maturation in the form of healing, and attrition. Reasons for leaving the program had not been recorded, so the investigators had no way of knowing factors that may have contributed to attrition. Also, data on when veterans left the program were unavailable. Research is needed with a control group to reduce the effect of confounding variables on the outcome measures. This project used data collected at a single VA facility, which limits its generalizability.
While completers and noncompleters of the EVP were similar on age, gender, and baseline measures, there may have been unidentified characteristics that influenced program completion. The investigators noticed the presence of more missing data among noncompleters compared to completers on the pre-intervention PCS; thus, noncompleters may have scored lower than completers on this instrument simply because there were more individual items that were unanswered/missing among this group of noncompleters.
Data were analyzed using a limited number of outcome measures that had previously been collected. Other outcome measures might include whether EVP participants reduced their use of medications, clinical resources, and personnel. Future projects, for example, could determine whether the EVP is effective in reducing opioid analgesic medication use and decreasing primary care and emergency department visits. Cost-benefit analyses could be completed to determine whether EVP is associated with financial savings.
Because no follow-up assessments were made to determine whether improvements were maintained over time, the project focus was limited to an evaluation of the short-term changes in the outcome measures. Future projects could include a follow-up assessment of the veterans 1- or 2-years post completion of the EVP.
Data for the project were collected prior to the COVID-19 pandemic, when the EVP was implemented through face-to-face meetings with participants and their peers. It is not clear how changes to the delivery of the program (such as offering it through telehealth) might impact veterans’ satisfaction with the program, willingness to complete it, and other variables of interest.
The results of this project were made available to stakeholders with recommendations for program expansion both at the current location and at other VA facilities, including the recommendation to hire additional personnel that would implement the program. As the VA network of facilities expand the EVP program and adapt it for telehealth delivery, the investigators recommended a similar analysis of data be performed following telehealth delivery. If delivery through telehealth is shown to improve outcome measures, the EVP could provide pain management treatment options for patients challenged by transportation barriers, including rural veterans.
Conclusion
This quality improvement project provided evidence of improvement in measures of pain severity, pain interference, negative cognition (catastrophizing), quality of life, and patient treatment satisfaction among veterans with chronic high-impact pain. Findings have been well received by the northeastern VA as well as the Veterans Integrated Systems Network 5. The results of the analyses were used to inform decisions regarding the future of the program.
Disclaimer: This material is the result of work supported with resources and the use of facilities at the VA Maryland Health Care System, Baltimore, Maryland. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgments: The authors thank Dr. Arianna Perra, the recent past coordinator of the Empower Veterans Program (EVP), who provided initial insights and support that motivated the decision to evaluate the program. We also thank the veterans and VA EVP clinicians who contributed data for the evaluation, and Dr. Michael Saenger (Director, TelePain-EVP: EVP) and Dr. Robert Lavin for their ongoing support, care, and concern for veteran patients. We also thank Dr. Beverly Bradley and the neurology service administrative team for their guidance in the process of obtaining necessary VA approvals for this project.
Corresponding author: Jessica U. Uche, DNP, CRNP-Family; [email protected]
doi:10.12788/jcom.0089
From Neurology/Chronic Pain Management Services, Department of Veterans Affairs (VA) Maryland Health Care System, Baltimore VA Medical Center, Baltimore, MD (Dr. Uche), and School of Nursing, Washburn University, Topeka, KS (Drs. Jamison and Waugh).
Abstract
Objective: The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the Empower Veterans Program (EVP) offered by a Veterans Administration facility in the northeastern United States.
Methods: This quality improvement project used data collected from veterans with chronic pain who completed the veterans health care facility’s EVP between August 2017 and August 2019. Pre- and post-intervention data on pain intensity, pain interference, quality of life, and pain catastrophizing were compared using paired t-tests.
Results: Although data were abstracted from 115 patients, the final sample included
Conclusion: Veterans with chronic high-impact noncancer pain who completed the EVP had reduced pain intensity, pain interference, pain catastrophizing as well as improved quality of life and satisfaction with their health.
Keywords: musculoskeletal pain, Veterans Affairs, complementary and integrative health, acceptance and commitment therapy, mind-body therapies, whole health, multidisciplinary pain management.
More than 100 million American adults suffer from chronic pain; costs associated with managing chronic pain are approximately $635 billion
One such program is the Empower Veterans Program (EVP). Originally developed at the Atlanta Veterans Affairs Health Care System, the EVP is a CIH modality based on the biopsychosocial model of pain developed by psychiatrist George Engel in 1977.3 The biopsychosocial model of pain recognizes that pain is a complex, multidimensional, biopsychosocial experience. Under this model, the mind and body work in unison as interconnected entities. Because the model acknowledges biological, psychological, and social components of pain and illness,4 treatment focuses on all aspects of a person’s health, life, and relationships.
The EVP fits into the VHA Pain Management Stepped Care Model and is an adjunctive complement for that model.5-7 The EVP complements care at the first step, where patient/family provide self-care and where care is provided by patient-aligned primary care teams, at the second step, which includes secondary consultation with multidisciplinary pain medicine specialty teams and other specialists, and at the third step, with the addition of tertiary interdisciplinary pain centers.
The VA Maryland Health Care System (VAMHCS) implemented the EVP as part of a quality improvement project for the management of chronic pain. The objectives of the program were to reduce pain intensity, pain catastrophizing, and pain interference, as well as improve functionality and quality of life among veterans with chronic high-impact noncancer pain. More than 2 years after the program was implemented, collected data had not been analyzed. The purpose of this quality improvement project was to abstract and analyze the previously collected data from veterans with high-impact chronic pain who attended an EVP offered by the VAMHCS. The results of the data analysis were used to inform decisions regarding the future of the program.
Methods
This quality improvement project used the Plan-Do-Study-Act (PDSA) process.8 The first 2 phases of the PDSA cycle (Plan and Do) were completed by a team of VA employees from the VAMHCS, who donated their time to establish and implement the program at the project site. This team consisted of psychologists, a physical therapist, a social worker, and a chaplain, and included support from medical administrative staff. This team planned and implemented the EVP at the VA facility based on the model developed at the Atlanta VA Health Care System. During the “Do” phase, the team collected data on pain intensity, pain interference, quality of life, and pain negative cognition (catastrophizing) before the intervention and post intervention. They also collected data on program outcome (patient treatment satisfaction) post intervention. Because these employees did not have time to retrieve and analyze the data, they welcomed the opportunity to have the data analyzed by the investigators during the Study phase of the PDSA cycle. Based on the results of the analysis, recommendations for program changes were made during the Act phase of the cycle.
Intervention
The EVP was developed as a 10-week (30 hours) interdisciplinary CIH approach that coached veterans with chronic pain to live fuller lives based on their individual values and what matters to them. EVP is the “What Else” management modality for the 5% of veterans with high-impact chronic pain.9 The EVP provided functional restoration through its components of whole health, mindfulness training, coaching calls, acceptance and commitment therapy, and mindful movement. It used the Wheel of Health with the 4 key components of me, self-care, professional care, and community.10,11
Veterans who had a diagnosis of chronic nonmalignant pain for 3 months or more and who agreed to participate in the EVP at this facility attended 3-hour classes every Tuesday with a cohort of 8 to 12 peers and engaged in one-on-one coaching with interdisciplinary team members. During the class sessions, veterans were coached to understand and accept their pain and commit to maintaining function despite their pain. Mindful movement by the physical therapist emphasized the pivotal place of exercise in pain management. The therapist used the mantra “Motion is Lotion.”9 The guiding principle of the EVP was that small incremental changes can have a big impact on the individual’s whole life. Emphasis was placed on increasing self-efficacy and mindful awareness for veterans with high-impact pain by giving them “Skills before Pills.”9
Outcome Measures
Outcome measures included the Numerical Pain Rating Scale (NPRS), the Multidimensional Pain Inventory (MPI), the World Health Organization Quality of Life assessment (WHOQOL-BREF), the Pain Catastrophizing Scale (PCS), and the Pain Treatment Satisfaction Scale (PTSS). Cronbach alpha coefficients were calculated to assess internal consistency reliability of these measures in the sample of veterans who completed the EVP.
NPRS. The NPRS is ubiquitous as a screening tool in many health care environments and its use is mandated by the VA health care system.12 The choice of the NPRS as the tool for pain screening in the VA health care system was based on a large body of research that supports the reliability and validity of the NPRS as a single index of pain intensity or severity. Studies suggest that the NPRS is valid for use in the assessment of acute, cancer, or chronic nonmalignant pain and in varied clinical settings.13 The NPRS has 4 items, each on a scale of 0 to 10. For the purpose of this project, only 3 items were used. The 3 items assessed the worst pain, usual pain, and the current pain (right now). The higher the score, the higher the pain intensity. Cronbach alpha coefficients on the NPRS obtained from the current sample of veterans were 0.85 on both pre- and postintervention assessments.
MPI. The MPI is an easily accessible, reliable, and valid self-report questionnaire that measures the impact of pain on an individual’s life, quality of social support, and general activity.14 This instrument is a short version of the West Haven-Yale MPI.15 The MPI contains 9 items rated on a scale from 0 to 6. The higher the score, the greater pain interference a person is experiencing. The MPI produces reliable, valid information for diagnostic purposes and for therapy outcome studies.16 The MPI had a Cronbach alpha of 0.90 on pre-intervention and 0.92 on postintervention assessments in the current sample.
WHOQOL-BREF. The WHOQOL-BREF is a measure of quality of life and is an abbreviated version of the WHOQOL-100. Quality of life is defined by the World Health Organization17 “as an individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns.” The WHOQOL-BREF contains 26 items. The first 2 items were examined separately; the first item asks individuals to rate their overall quality of life and the second asks individuals how satisfied they are with their health. The remaining 24 items were used to calculate the following 4 domain scores: physical health, psychological health, social relationship, and environment.18 Each item is measured on a scale of 1 to 5. Higher scores denote higher or better quality of life. Domain scores have demonstrated good reliability and validity.19-21 Cronbach alpha coefficients for the domain subscales ranged from 0.63 to 0.84 in the current sample, with the lowest alphas for the 3-item Social Relationships Domain.
PCS. The PCS is a widely used measure of catastrophic thinking related to pain. Catastrophizing has been conceived by Sullivan and colleagues as “an exaggerated negative mental set brought to bear during actual or anticipated painful experience.”22 The PCS provides a total score and scores for the following subscales: rumination, magnification, and helplessness.23 It has been used in a variety of chronic pain populations and has demonstrated good reliability and validity in clinical as well as nonclinical samples.24-26 The PCS has 13 items rated on a scale of 0 to 4. Higher scores mean greater negative pain cognition (catastrophizing). In the current sample, the PCS total scale had a Cronbach alpha coefficient of 0.95 and 0.94 on the 2 assessments. The coefficients for the subscales ranged from 0.81 to 0.90.
PTSS. The PTSS is a 5-item tool that measures patient satisfaction with pain treatment. It includes items that address overall satisfaction, staff warmth, staff skill level, ease of scheduling appointments, and recommendation of the program to other veterans. It was derived from the post-treatment version of The Pain Outcome Questionnaire-VA and has demonstrated reliability and validity.27 The questions are scaled from 0 to 10. High scores on the PTSS denote high patient satisfaction with the EVP. The Cronbach alpha coefficient on the PTSS obtained from the current sample was 0.80.
Data Gathering and Analysis
Prior to starting the Study phase, Washburn University’s Institutional Review Board (IRB) and the VA IRB approved the project. The VA IRB, through its affiliate, gave a Not Human Research Determination and granted a waiver of informed consent and the Health Insurance Portability and Accountability Act authorization. The VA facility’s Research and Development department also approved the quality improvement project.
Once these approvals were obtained, the Study phase began with the abstraction of retrospective data obtained from veterans who participated in the VA health care facility’s EVP between
Veterans who completed the program were compared to veterans who did not complete the program on age, gender, and baseline measures. The investigators used independent samples t-tests to compare completers and noncompleters on age, pain intensity, pain interference, quality of life, and pain catastrophizing. They used the chi-square test of independence to analyze the association between gender and program completion.
Data were included in the pre- and postintervention analysis if the veteran completed the NPRS, MPI, WHOQOL-BREF, and PCS pre and post intervention. This became an important eligibility requirement as some of the tools/measures were changed towards the end of the review period in 2019. Pre- and postintervention data on pain intensity, pain interference, quality of life, pain catastrophizing, and patient satisfaction were compared using paired samples t-test at .004 level of significance based on the Bonferroni correction.28 Data on patient satisfaction with pain treatment were collected at program completion (week 8 or 10) and were analyzed using descriptive statistics.
Effect sizes (Cohen’s d) were calculated to determine the substantive significance or magnitude of the mean differences in scores. Effect sizes (expressed as absolute values of Cohen’s d) were calculated as the mean difference divided by the standard deviation. Values of 0.2 were considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size.29
Results
Data were abstracted for 115 veterans who started the EVP. Of these, 48 left the program, leaving 67 veterans (58%) who completed the program. Completers and noncompleters were similar in age, gender, and baseline measures (Table 1). Fifty-three (79%) completers and 35 (73%) noncompleters were male. A chi-square test of independence showed no significant association between gender and program completion (χ21 [N = 115] = .595, P = .440).

Comparison of pre-and postintervention mean scale scores resulted in statistically significant

Analysis of data obtained using the PTSS yielded high mean scores for items that focused on patient satisfaction with treatment (Table 3). Scaled statistics yielded a mean (SD) of 46.95 (4.40). These results denoted overall patient satisfaction with the EVP.

Discussion
The purpose of this quality improvement project was to abstract and analyze previously collected data from veterans with high-impact chronic pain who attended the EVP. Comparison of pre-intervention and postintervention data obtained from 67 veterans who completed the program revealed improvements in pain intensity, pain interference, negative cognition (catastrophizing), and quality of life. The differences were statistically significant and clinically meaningful, with medium and large effect sizes. In addition, veterans reported high satisfaction with the EVP.
The EVP includes CIH approaches that have demonstrated effectiveness among veterans and other populations with chronic pain. A wealth of studies, for example, support the effectiveness of CIH approaches among veterans.30-34 Other studies focus on specific CIH approaches that are components of the EVP. Evidence supports, for example, the efficacy of mindfulness-based stress reduction,35-39 acceptance and commitment therapy,40-43 brief peer support intervention,44 and interdisciplinary biopsychosocial rehabilitation.45,46
While empirical evidence supports components of the EVP, only one study focused on the outcomes of the Atlanta VA EVP among veterans with chronic pain. Results of a qualitative study conducted by Penney and Haro47 described the experience of veterans with the EVP. Those veterans reported adopting new self-care or lifestyle practices for pain management and health, accepting pain, being better able to adjust and set boundaries, feeling more in control, participating in life, and changing their medication use.
The mean baseline scores from the current sample were similar to samples of patients with chronic pain in other studies (NPRS,48 MPI,48 and PCS48-51). After converting scores on the WHOQOL-BREF from those that ranged from 4 to 20 to those that ranged from 0 to 100,18 the scores from the current sample were similar to those of other studies of patients with chronic pain.48,52,53Several strengths of the project should be noted. Data were collected using well established measurement tools that had previously demonstrated reliability and validity. All the tools used in data collection demonstrated good internal consistency reliabilities in the current sample of veterans. Weaknesses of the project include the use of a convenience sample of veterans and small sample size. Data were not available on the number of veterans who were offered participation or on how many veterans declined enrollment. The sample of veterans who chose to participate in the EVP may or may not have been representative of the population of veterans with high-impact chronic pain. As a pre- and postintervention design with no comparison group, the results are subject to multiple threats to internal validity, including the Hawthorne effect, maturation in the form of healing, and attrition. Reasons for leaving the program had not been recorded, so the investigators had no way of knowing factors that may have contributed to attrition. Also, data on when veterans left the program were unavailable. Research is needed with a control group to reduce the effect of confounding variables on the outcome measures. This project used data collected at a single VA facility, which limits its generalizability.
While completers and noncompleters of the EVP were similar on age, gender, and baseline measures, there may have been unidentified characteristics that influenced program completion. The investigators noticed the presence of more missing data among noncompleters compared to completers on the pre-intervention PCS; thus, noncompleters may have scored lower than completers on this instrument simply because there were more individual items that were unanswered/missing among this group of noncompleters.
Data were analyzed using a limited number of outcome measures that had previously been collected. Other outcome measures might include whether EVP participants reduced their use of medications, clinical resources, and personnel. Future projects, for example, could determine whether the EVP is effective in reducing opioid analgesic medication use and decreasing primary care and emergency department visits. Cost-benefit analyses could be completed to determine whether EVP is associated with financial savings.
Because no follow-up assessments were made to determine whether improvements were maintained over time, the project focus was limited to an evaluation of the short-term changes in the outcome measures. Future projects could include a follow-up assessment of the veterans 1- or 2-years post completion of the EVP.
Data for the project were collected prior to the COVID-19 pandemic, when the EVP was implemented through face-to-face meetings with participants and their peers. It is not clear how changes to the delivery of the program (such as offering it through telehealth) might impact veterans’ satisfaction with the program, willingness to complete it, and other variables of interest.
The results of this project were made available to stakeholders with recommendations for program expansion both at the current location and at other VA facilities, including the recommendation to hire additional personnel that would implement the program. As the VA network of facilities expand the EVP program and adapt it for telehealth delivery, the investigators recommended a similar analysis of data be performed following telehealth delivery. If delivery through telehealth is shown to improve outcome measures, the EVP could provide pain management treatment options for patients challenged by transportation barriers, including rural veterans.
Conclusion
This quality improvement project provided evidence of improvement in measures of pain severity, pain interference, negative cognition (catastrophizing), quality of life, and patient treatment satisfaction among veterans with chronic high-impact pain. Findings have been well received by the northeastern VA as well as the Veterans Integrated Systems Network 5. The results of the analyses were used to inform decisions regarding the future of the program.
Disclaimer: This material is the result of work supported with resources and the use of facilities at the VA Maryland Health Care System, Baltimore, Maryland. The views expressed are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgments: The authors thank Dr. Arianna Perra, the recent past coordinator of the Empower Veterans Program (EVP), who provided initial insights and support that motivated the decision to evaluate the program. We also thank the veterans and VA EVP clinicians who contributed data for the evaluation, and Dr. Michael Saenger (Director, TelePain-EVP: EVP) and Dr. Robert Lavin for their ongoing support, care, and concern for veteran patients. We also thank Dr. Beverly Bradley and the neurology service administrative team for their guidance in the process of obtaining necessary VA approvals for this project.
Corresponding author: Jessica U. Uche, DNP, CRNP-Family; [email protected]
doi:10.12788/jcom.0089
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press (US); 2011.
2. Bastian LA, Heapy A, Becker WC, et al. Understanding pain and pain treatment for veterans: responding to the federal pain research strategy. Pain Med. 2018;19(suppl_1); S1-S4. doi:10.1093/pm/pny1433
3. Engle GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136. doi:10.1126/science.847460
4. Bevers K, Watts L, Kishino ND, et al. The biopsychosocial model of the assessment, prevention, and treatment of chronic pain. US Neurology. 2016;12(2):98-104. doi:10.17925/USN.2016.12.02.98
5. Bair MJ, Ang D, Wu J, et al. Evaluation of stepped care for chronic pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: A randomized clinical trial. JAMA Intern Med. 2015;175(5):682-689. doi:10.1001/jamainternmed.2015.97
6. Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: Department of Veterans Affairs; 2009.https://www.va.gov/painmanagement/docs/vha09paindirective.pdf
7. Moore BA, Anderson D, Dorflinger L, et al. Stepped care model for pain management and quality of pain care in long-term opioid therapy. J Rehabil Res Dev. 2016;53(1):137-146. doi:10.1682/JRRD.2014.10.0254
8. Institute for Healthcare Improvement. How to improve. Accessed March 14, 2022. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
9. Saenger M. Empower Veterans Program. APA PCSS-O Webinars. Evidence CAM LBP 2016.
10. Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: How will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
11. Veterans Health Administration. Whole health: Circle of health. Updated April 1, 2021. Accessed March 14, 2022. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
12. Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453-1458. doi:10.1007/s11606-007-0321-2
13. Veterans Health Administration. Pain as the 5th vital sign toolkit. October 2000, revised edition. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf
14. McKillop JM, Nielson WR. Improving the usefulness of the Multidimensional Pain Inventory. Pain Res Manag. 2011;16(4):239-244. doi:10.1155/2011/873424
15. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain.1985;23(4):345-356. doi:10.1016/0304-3959(85)90004-1
16. Verra ML, Angst F, Staal JB, et al. Reliability of the multidimensional pain inventory and stability of the MPI classification system in chronic back pain. BMC Musculoskelet Disord. 2012;13:155. doi:10.1186/1471-2474-13-155
17. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551-558. doi:10.1017/s0033291798006667
18. World Health Organization. Division of Mental Health. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version, December 1996. Accessed March 14, 2022. https://apps.who.int/iris/handle/10665/63529
19. Guay S, Fortin C, Fikretoglu D, et al. Validation of the WHOQOL-BREF in a sample of male treatment-seeking veterans. Mil Psychol. 2015;27(2):85-92. doi:10.1037/mil0000065
20. Skevington S, Lotfy M, O’Connell K, WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
21. Stratton KJ, Bender MC, Cameron JJ, Pickett TC. Development and evaluation of a behavioral pain management treatment program in a Veterans Affairs Medical Center. Mil Med. 2015;180(3):263-268. doi:10.7205/MILMED-D-14-00281.
22. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52-64. doi:10.1097/00002508-200103000-00008
23. Sullivan JL. The Pain Catastrophizing Scale: User manual. Accessed March 14, 2022. https://studylib.net/doc/8330191/the-pain-catastrophizing-scale---dr.-michael-sullivan
24. Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18(9):1139-1149. doi:10.1016/j.jpain.2017.05.003
25. Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589-605. doi:10.1023/a:1025570508954
26. Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assessment. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
27. Walker R, Clark M, Gironda R. Psychometric characteristics of the Pain Treatment Satisfaction Scale. J Pain. 2015;6(3Suppl.):S76.
28. Emerson RW. Bonferroni correction and type I error. J Vis Impair Blind. 2020;114(1):77-78. doi:10.1177/0145482X20901378
29. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988. doi:10.4324/9780203771587
30. Craner JR, Lake ES, Bancroft KA, George LL. Treatment outcomes and mechanisms for an ACT-based 10-week interdisciplinary chronic pain rehabilitation program. Pain Pract. 2020;20(1):44-54. doi:10.1111/papr.12824
31. Han L, Goulet JL, Skanderson M, et al. Evaluation of complementary and integrative health approaches among US veterans with musculoskeletal pain using propensity score methods. Pain Med. 2019;20(1):90-102. doi:10.1093/pm/pny027
32. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US veterans: an economic evaluation. PLoS One. 2019;14(6):e0217831. doi:10.1371/journal.pone.0217831
33. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Global Forum on Innovation in Health Professional Education; Forum on Neuroscience and Nervous System Disorders; Stroud C, Posey Norris SM, Bain L, eds. The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop. National Academies Press (US); April 12, 2019.
34. Richmond H, Hall AM, Copsey B, et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0134192. doi:10.1371/journal.pone.0134192

35. Kearney DJ, Simpson TL, Malte CA, et al. Mindfulness-based stress reduction in addition to usual care is associated with improvements in pain, fatigue, and cognitive failures among veterans with Gulf War illness. Am J Med. 2016;129(2):204-214. doi:10.1016/j.amjmed.2015.09.015
36. Khoo E, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioral therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis. Evid Based Ment Health. 2019;22(1):26-35. doi:10.1136/ebmental-2018-300062
37. Khusid MA, Vythilingam M. The emerging role of mindfulness meditation as effective self-management strategy, Part 2: clinical implications for chronic pain, substance misuse, and insomnia. Mil Med. 2016;181(9):969-975. doi:10.7205/MILMED-D-14-00678
38. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: A randomized controlled trial. Pain Med. 2015;16(4):641-652. doi:10.1111/pme.12605
39. Zou L, Zhang Y, Yang L, et al. Are mindful exercises safe and beneficial for treating chronic lower back pain? A systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2019;8(5):628. doi:10.3390/jcm8050628
40. Hughes LS, Clark J, Colclough JA, et al. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6):552-568. doi:10.1097/AJP.0000000000000425
41. Kemani MK, Olsson GL, Lekander M, et al. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: a randomized controlled trial. Clin J Pain. 2015;31(11):1004-1016. doi:10.1097/AJP.0000000000000203
42. Scott W, Daly A, Yu L, McCracken LM. Treatment of chronic pain for adults 65 and over: analyses of outcomes and changes in psychological flexibility following interdisciplinary acceptance and commitment therapy (ACT). Pain Med. 2017;18(2):252. doi:10.1093/pm/pnw073
43. Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KMG. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther. 2016;45(1):5-31. doi:10.1080/16506073.2015.1098724
44. Matthias MS, McGuire AB, Kukla M, et al. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16(1):81-87. doi:10.1111/pme.12571
45. Anamkath NS, Palyo SA, Jacobs SC, et al. An interdisciplinary pain rehabilitation program for veterans with chronic pain: description and initial evaluation of outcomes. Pain Res Manag. 2018;2018(3941682):1-9. doi:10.1155/2018/3941682
46. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;9: CD000963. doi:10.1002/14651858.CD000963.pub3
47. Penney LS, Haro E. Qualitative evaluation of an interdisciplinary chronic pain intervention: Outcomes and barriers and facilitators to ongoing pain management. J Pain Res. 2019;12:865-878. doi:10.2147/JPR.S185652
48. Murphy JL, Cordova MJ, Dedert EA. Cognitive behavioral therapy for chronic pain in veterans; Evidence for clinical effectiveness in a model program. Psychol Serv. 2022;19(1):95-102. doi:10.1037/ser0000506
49. Katz L, Patterson L, Zacharias R. Evaluation of an interdisciplinary chronic pain program and predictors of readiness for change. Can J Pain. 2019;3(1):70-78. doi:10.1080/24740527.2019.1582296
50. Majumder SMM, Ahmed S, Shazzad N, et al. Translation, cross-cultural adaptation and validation of the Pain Catastrophizing Scale (PCS) into Bengali I patients with chronic non-malignant musculoskeletal pain. Int J Rheum Dis. 2020;23:1481-1487. doi:10.1111/1756-185X.13954
51. Margiotta F, Hannigan A, Imran A, et al. Pain, perceived injustice, and pain catastrophizing in chronic pain patients in Ireland. Pain Pract. 2016;17(5):663-668. doi:10.1111/papr.12
52. Bras M, Milunovic V, Boban M, et al. Quality of live in Croatian Homeland war (1991-1995) veterans who suffer from post-traumatic stress disorder and chronic pain. Health Qual Life Out. 2011;9:56. doi:10.1186/1477-7525-9-56
53. Liu C-H, Kung Y-Y, Lin C-L, et al. Therapeutic efficacy and the impact of the “dose” effect of acupuncture to treat sciatica: A randomized controlled pilot study. J Pain Res. 2019;12:3511-3520. doi:10.2147/JPR.S210672
1. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press (US); 2011.
2. Bastian LA, Heapy A, Becker WC, et al. Understanding pain and pain treatment for veterans: responding to the federal pain research strategy. Pain Med. 2018;19(suppl_1); S1-S4. doi:10.1093/pm/pny1433
3. Engle GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136. doi:10.1126/science.847460
4. Bevers K, Watts L, Kishino ND, et al. The biopsychosocial model of the assessment, prevention, and treatment of chronic pain. US Neurology. 2016;12(2):98-104. doi:10.17925/USN.2016.12.02.98
5. Bair MJ, Ang D, Wu J, et al. Evaluation of stepped care for chronic pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: A randomized clinical trial. JAMA Intern Med. 2015;175(5):682-689. doi:10.1001/jamainternmed.2015.97
6. Veterans Health Administration. Pain Management. VHA Directive 2009-053. Washington, DC: Department of Veterans Affairs; 2009.https://www.va.gov/painmanagement/docs/vha09paindirective.pdf
7. Moore BA, Anderson D, Dorflinger L, et al. Stepped care model for pain management and quality of pain care in long-term opioid therapy. J Rehabil Res Dev. 2016;53(1):137-146. doi:10.1682/JRRD.2014.10.0254
8. Institute for Healthcare Improvement. How to improve. Accessed March 14, 2022. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx
9. Saenger M. Empower Veterans Program. APA PCSS-O Webinars. Evidence CAM LBP 2016.
10. Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: How will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
11. Veterans Health Administration. Whole health: Circle of health. Updated April 1, 2021. Accessed March 14, 2022. https://www.va.gov/WHOLEHEALTH/circle-of-health/index.asp
12. Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453-1458. doi:10.1007/s11606-007-0321-2
13. Veterans Health Administration. Pain as the 5th vital sign toolkit. October 2000, revised edition. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf
14. McKillop JM, Nielson WR. Improving the usefulness of the Multidimensional Pain Inventory. Pain Res Manag. 2011;16(4):239-244. doi:10.1155/2011/873424
15. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain.1985;23(4):345-356. doi:10.1016/0304-3959(85)90004-1
16. Verra ML, Angst F, Staal JB, et al. Reliability of the multidimensional pain inventory and stability of the MPI classification system in chronic back pain. BMC Musculoskelet Disord. 2012;13:155. doi:10.1186/1471-2474-13-155
17. Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551-558. doi:10.1017/s0033291798006667
18. World Health Organization. Division of Mental Health. WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment: field trial version, December 1996. Accessed March 14, 2022. https://apps.who.int/iris/handle/10665/63529
19. Guay S, Fortin C, Fikretoglu D, et al. Validation of the WHOQOL-BREF in a sample of male treatment-seeking veterans. Mil Psychol. 2015;27(2):85-92. doi:10.1037/mil0000065
20. Skevington S, Lotfy M, O’Connell K, WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
21. Stratton KJ, Bender MC, Cameron JJ, Pickett TC. Development and evaluation of a behavioral pain management treatment program in a Veterans Affairs Medical Center. Mil Med. 2015;180(3):263-268. doi:10.7205/MILMED-D-14-00281.
22. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52-64. doi:10.1097/00002508-200103000-00008
23. Sullivan JL. The Pain Catastrophizing Scale: User manual. Accessed March 14, 2022. https://studylib.net/doc/8330191/the-pain-catastrophizing-scale---dr.-michael-sullivan
24. Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18(9):1139-1149. doi:10.1016/j.jpain.2017.05.003
25. Osman A, Barrios FX, Kopper BA, et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589-605. doi:10.1023/a:1025570508954
26. Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assessment. 1995;7(4):524-532. doi:10.1037/1040-3590.7.4.524
27. Walker R, Clark M, Gironda R. Psychometric characteristics of the Pain Treatment Satisfaction Scale. J Pain. 2015;6(3Suppl.):S76.
28. Emerson RW. Bonferroni correction and type I error. J Vis Impair Blind. 2020;114(1):77-78. doi:10.1177/0145482X20901378
29. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988. doi:10.4324/9780203771587
30. Craner JR, Lake ES, Bancroft KA, George LL. Treatment outcomes and mechanisms for an ACT-based 10-week interdisciplinary chronic pain rehabilitation program. Pain Pract. 2020;20(1):44-54. doi:10.1111/papr.12824
31. Han L, Goulet JL, Skanderson M, et al. Evaluation of complementary and integrative health approaches among US veterans with musculoskeletal pain using propensity score methods. Pain Med. 2019;20(1):90-102. doi:10.1093/pm/pny027
32. Herman PM, Yuan AH, Cefalu MS, et al. The use of complementary and integrative health approaches for chronic musculoskeletal pain in younger US veterans: an economic evaluation. PLoS One. 2019;14(6):e0217831. doi:10.1371/journal.pone.0217831
33. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Global Forum on Innovation in Health Professional Education; Forum on Neuroscience and Nervous System Disorders; Stroud C, Posey Norris SM, Bain L, eds. The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop. National Academies Press (US); April 12, 2019.
34. Richmond H, Hall AM, Copsey B, et al. The effectiveness of cognitive behavioural treatment for non-specific low back pain: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0134192. doi:10.1371/journal.pone.0134192

35. Kearney DJ, Simpson TL, Malte CA, et al. Mindfulness-based stress reduction in addition to usual care is associated with improvements in pain, fatigue, and cognitive failures among veterans with Gulf War illness. Am J Med. 2016;129(2):204-214. doi:10.1016/j.amjmed.2015.09.015
36. Khoo E, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioral therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis. Evid Based Ment Health. 2019;22(1):26-35. doi:10.1136/ebmental-2018-300062
37. Khusid MA, Vythilingam M. The emerging role of mindfulness meditation as effective self-management strategy, Part 2: clinical implications for chronic pain, substance misuse, and insomnia. Mil Med. 2016;181(9):969-975. doi:10.7205/MILMED-D-14-00678
38. la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: A randomized controlled trial. Pain Med. 2015;16(4):641-652. doi:10.1111/pme.12605
39. Zou L, Zhang Y, Yang L, et al. Are mindful exercises safe and beneficial for treating chronic lower back pain? A systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2019;8(5):628. doi:10.3390/jcm8050628
40. Hughes LS, Clark J, Colclough JA, et al. Acceptance and commitment therapy (ACT) for chronic pain: a systematic review and meta-analyses. Clin J Pain. 2017;33(6):552-568. doi:10.1097/AJP.0000000000000425
41. Kemani MK, Olsson GL, Lekander M, et al. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: a randomized controlled trial. Clin J Pain. 2015;31(11):1004-1016. doi:10.1097/AJP.0000000000000203
42. Scott W, Daly A, Yu L, McCracken LM. Treatment of chronic pain for adults 65 and over: analyses of outcomes and changes in psychological flexibility following interdisciplinary acceptance and commitment therapy (ACT). Pain Med. 2017;18(2):252. doi:10.1093/pm/pnw073
43. Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KMG. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther. 2016;45(1):5-31. doi:10.1080/16506073.2015.1098724
44. Matthias MS, McGuire AB, Kukla M, et al. A brief peer support intervention for veterans with chronic musculoskeletal pain: a pilot study of feasibility and effectiveness. Pain Med. 2015;16(1):81-87. doi:10.1111/pme.12571
45. Anamkath NS, Palyo SA, Jacobs SC, et al. An interdisciplinary pain rehabilitation program for veterans with chronic pain: description and initial evaluation of outcomes. Pain Res Manag. 2018;2018(3941682):1-9. doi:10.1155/2018/3941682
46. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;9: CD000963. doi:10.1002/14651858.CD000963.pub3
47. Penney LS, Haro E. Qualitative evaluation of an interdisciplinary chronic pain intervention: Outcomes and barriers and facilitators to ongoing pain management. J Pain Res. 2019;12:865-878. doi:10.2147/JPR.S185652
48. Murphy JL, Cordova MJ, Dedert EA. Cognitive behavioral therapy for chronic pain in veterans; Evidence for clinical effectiveness in a model program. Psychol Serv. 2022;19(1):95-102. doi:10.1037/ser0000506
49. Katz L, Patterson L, Zacharias R. Evaluation of an interdisciplinary chronic pain program and predictors of readiness for change. Can J Pain. 2019;3(1):70-78. doi:10.1080/24740527.2019.1582296
50. Majumder SMM, Ahmed S, Shazzad N, et al. Translation, cross-cultural adaptation and validation of the Pain Catastrophizing Scale (PCS) into Bengali I patients with chronic non-malignant musculoskeletal pain. Int J Rheum Dis. 2020;23:1481-1487. doi:10.1111/1756-185X.13954
51. Margiotta F, Hannigan A, Imran A, et al. Pain, perceived injustice, and pain catastrophizing in chronic pain patients in Ireland. Pain Pract. 2016;17(5):663-668. doi:10.1111/papr.12
52. Bras M, Milunovic V, Boban M, et al. Quality of live in Croatian Homeland war (1991-1995) veterans who suffer from post-traumatic stress disorder and chronic pain. Health Qual Life Out. 2011;9:56. doi:10.1186/1477-7525-9-56
53. Liu C-H, Kung Y-Y, Lin C-L, et al. Therapeutic efficacy and the impact of the “dose” effect of acupuncture to treat sciatica: A randomized controlled pilot study. J Pain Res. 2019;12:3511-3520. doi:10.2147/JPR.S210672
A Practical and Cost-Effective Approach to the Diagnosis of Heparin-Induced Thrombocytopenia: A Single-Center Quality Improvement Study
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.
Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.
During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.
Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; [email protected]
Disclosures: None reported.
doi: 10.12788/jcom.0087
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.

Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.

During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.

Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; [email protected]
Disclosures: None reported.
doi: 10.12788/jcom.0087
From the Veterans Affairs Ann Arbor Healthcare System Medicine Service (Dr. Cusick), University of Michigan College of Pharmacy, Clinical Pharmacy Service, Michigan Medicine (Dr. Hanigan), Department of Internal Medicine Clinical Experience and Quality, Michigan Medicine (Linda Bashaw), Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI (Dr. Heidemann), and the Operational Excellence Department, Sparrow Health System, Lansing, MI (Matthew Johnson).
Abstract
Background: Diagnosis of heparin-induced thrombocytopenia (HIT) requires completion of an enzyme-linked immunosorbent assay (ELISA)–based heparin-platelet factor 4 (PF4) antibody test. If this test is negative, HIT is excluded. If positive, a serotonin-release assay (SRA) test is indicated. The SRA is expensive and sometimes inappropriately ordered despite negative PF4 results, leading to unnecessary treatment with argatroban while awaiting SRA results.
Objectives: The primary objectives of this project were to reduce unnecessary SRA testing and argatroban utilization in patients with suspected HIT.
Methods: The authors implemented an intervention at a tertiary care academic hospital in November 2017 targeting patients hospitalized with suspected HIT. The intervention was controlled at the level of the laboratory and prevented ordering of SRA tests in the absence of a positive PF4 test. The number of SRA tests performed and argatroban bags administered were identified retrospectively via chart review before the intervention (January 2016 to November 2017) and post intervention (December 2017 to March 2020). Associated costs were calculated based on institutional SRA testing cost as well as the average wholesale price of argatroban.
Results: SRA testing decreased from an average of 3.7 SRA results per 1000 admissions before the intervention to an average of 0.6 results per 1000 admissions post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 prior to the intervention to 14.3 post intervention. Total estimated cost savings per 1000 admissions was $2361.20.
Conclusion: An evidence-based testing strategy for HIT can be effectively implemented at the level of the laboratory. This approach led to reductions in SRA testing and argatroban utilization with resultant cost savings.
Keywords: HIT, argatroban, anticoagulation, serotonin-release assay.
Thrombocytopenia is a common finding in hospitalized patients.1,2 Heparin-induced thrombocytopenia (HIT) is one of the many potential causes of thrombocytopenia in hospitalized patients and occurs when antibodies to the heparin-platelet factor 4 (PF4) complex develop after heparin exposure. This triggers a cascade of events, leading to platelet activation, platelet consumption, and thrombosis. While HIT is relatively rare, occurring in 0.3% to 0.5% of critically ill patients, many patients will be tested to rule out this potentially life-threatening cause of thrombocytopenia.3
The diagnosis of HIT utilizes a combination of both clinical suspicion and laboratory testing.4 The 4T score (Table) was developed to evaluate the clinical probability of HIT and involves assessing the degree and timing of thrombocytopenia, the presence or absence of thrombosis, and other potential causes of the thrombocytopenia.5 The 4T score is designed to be utilized to identify patients who require laboratory testing for HIT; however, it has low inter-rater agreement in patients undergoing evaluation for HIT,6 and, in our experience, completion of this scoring is time-consuming.
The enzyme-linked immunosorbent assay (ELISA) is a commonly used laboratory test to diagnose HIT that detects antibodies to the heparin-PF4 complex utilizing optical density (OD) units. When using an OD cutoff of 0.400, ELISA PF4 (PF4) tests have a sensitivity of 99.6%, but poor specificity at 69.3%.7 When the PF4 antibody test is positive with an OD ≥0.400, then a functional test is used to determine whether the antibodies detected will activate platelets. The serotonin-release assay (SRA) is a functional test that measures 14C-labeled serotonin release from donor platelets when mixed with patient serum or plasma containing HIT antibodies. In the correct clinical context, a positive ELISA PF4 antibody test along with a positive SRA is diagnostic of HIT.8
The process of diagnosing HIT in a timely and cost-effective manner is dependent on the clinician’s experience in diagnosing HIT as well as access to the laboratory testing necessary to confirm the diagnosis. PF4 antibody tests are time-consuming and not always available daily and/or are not available onsite. The SRA requires access to donor platelets and specialized radioactivity counting equipment, making it available only at particular centers.
The treatment of HIT is more straightforward and involves stopping all heparin products and starting a nonheparin anticoagulant. The direct thrombin inhibitor argatroban is one of the standard nonheparin anticoagulants used in patients with suspected HIT.4 While it is expensive, its short half-life and lack of renal clearance make it ideal for treatment of hospitalized patients with suspected HIT, many of whom need frequent procedures and/or have renal disease.
At our academic tertiary care center, we performed a retrospective analysis that showed inappropriate ordering of diagnostic HIT testing as well as unnecessary use of argatroban even when there was low suspicion for HIT based on laboratory findings. The aim of our project was to reduce unnecessary HIT testing and argatroban utilization without overburdening providers or interfering with established workflows.
Methods
Setting
The University of Michigan (UM) hospital is a 1000-bed tertiary care center in Ann Arbor, Michigan. The UM guidelines reflect evidence-based guidelines for the diagnosis and treatment of HIT.4 In 2016 the UM guidelines for laboratory testing included sending the PF4 antibody test first when there was clinical suspicion of HIT. The SRA was to be sent separately only when the PF4 returned positive (OD ≥ 0.400). Standard guidelines at UM also included switching patients with suspected HIT from heparin to a nonheparin anticoagulant and stopping all heparin products while awaiting the SRA results. The direct thrombin inhibitor argatroban is utilized at UM and monitored with anti-IIa levels. University of Michigan Hospital utilizes the Immucor PF4 IgG ELISA for detecting heparin-associated antibodies.9 In 2016, this PF4 test was performed in the UM onsite laboratory Monday through Friday. At UM the SRA is performed off site, with a turnaround time of 3 to 5 business days.
Baseline Data
We retrospectively reviewed PF4 and SRA testing as well as argatroban usage from December 2016 to May 2017. Despite the institutional guidelines, providers were sending PF4 and SRA simultaneously as soon as HIT was suspected; 62% of PF4 tests were ordered simultaneously with the SRA, but only 8% of these PF4 tests were positive with an OD ≥0.400. Of those patients with negative PF4 testing, argatroban was continued until the SRA returned negative, leading to many days of unnecessary argatroban usage. An informal survey of the anticoagulation pharmacists revealed that many recommended discontinuing argatroban when the PF4 test was negative, but providers routinely did not feel comfortable with this approach. This suggested many providers misunderstood the performance characteristics of the PF4 test.
Intervention
Our team consisted of hematology and internal medicine faculty, pharmacists, coagulation laboratory personnel, and quality improvement specialists. We designed and implemented an intervention in November 2017 focused on controlling the ordering of the SRA test. We chose to focus on this step due to the excellent sensitivity of the PF4 test with a cutoff of OD <0.400 and the significant expense of the SRA test. Under direction of the Coagulation Laboratory Director, a standard operating procedure was developed where the coagulation laboratory personnel did not send out the SRA until a positive PF4 test (OD ≥ 0.400) was reported. If the PF4 was negative, the SRA was canceled and the ordering provider received notification of the cancelled test via the electronic medical record, accompanied by education about HIT testing (Figure 1). In addition, the lab increased the availability of PF4 testing from 5 days to 7 days a week so there were no delays in tests ordered on Fridays or weekends.

Outcomes
Our primary goals were to decrease both SRA testing and argatroban use. Secondarily, we examined the cost-effectiveness of this intervention. We hypothesized that controlling the SRA testing at the laboratory level would decrease both SRA testing and argatroban use.
Data Collection
Pre- and postintervention data were collected retrospectively. Pre-intervention data were from January 2016 through November 2017, and postintervention data were from December 2017 through March 2020. The number of SRA tests performed were identified retrospectively via review of electronic ordering records. All patients who had a hospital admission after January 1, 2016, were included. These patients were filtered to include only those who had a result for an SRA test. In order to calculate cost-savings, we identified both the number of SRA tests ordered retrospectively as well as patients who had both an SRA resulted and had been administered argatroban. Cost-savings were calculated based on our institutional cost of $357 per SRA test.
At our institution, argatroban is supplied in 50-mL bags; therefore, we utilized the number of bags to identify argatroban usage. Savings were calculated using the average wholesale price (AWP) of $292.50 per 50-mL bag. The amounts billed or collected for the SRA testing or argatroban treatment were not collected. Costs were estimated using only direct costs to the institution. Safety data were not collected. As the intent of our project was a quality improvement activity, this project did not require institutional review board regulation per our institutional guidance.
Results
During the pre-intervention period, the average number of admissions (adults and children) at UM was 5863 per month. Post intervention there was an average of 5842 admissions per month. A total of 1192 PF4 tests were ordered before the intervention and 1148 were ordered post intervention. Prior to the intervention, 481 SRA tests were completed, while post intervention 105 were completed. Serotonin-release testing decreased from an average of 3.7 SRA results per 1000 admissions during the pre-intervention period to an average of 0.6 per 1000 admissions post intervention (Figure 2). Cost-savings were $1045 per 1000 admissions.

During the pre-intervention period, 2539 bags of argatroban were used, while 2337 bags were used post intervention. The number of 50-mL argatroban bags used per 1000 admissions decreased from 18.8 before the intervention to 14.3 post intervention. Cost-savings were $1316.20 per 1000 admissions. Figure 3 illustrates the monthly argatroban utilization per 1000 admissions during each quarter from January 2016 through March 2020.

Discussion
We designed and implemented an evidence-based strategy for HIT at our academic institution which led to a decrease in unnecessary SRA testing and argatroban utilization, with associated cost savings. By focusing on a single point of intervention at the laboratory level where SRA tests were held and canceled if the PF4 test was negative, we helped offload the decision-making from the provider while simultaneously providing just-in-time education to the provider. This intervention was designed with input from multiple stakeholders, including physicians, quality improvement specialists, pharmacists, and coagulation laboratory personnel.
Serotonin-release testing dramatically decreased post intervention even though a similar number of PF4 tests were performed before and after the intervention. This suggests that the decrease in SRA testing was a direct consequence of our intervention. Post intervention the number of completed SRA tests was 9% of the number of PF4 tests sent. This is consistent with our baseline pre-intervention data showing that only 8% of all PF4 tests sent were positive.
While the absolute number of argatroban bags utilized did not dramatically decrease after the intervention, the quarterly rate did, particularly after 2018. Given that argatroban data were only drawn from patients with a concurrent SRA test, this decrease is clearly from decreased usage in patients with suspected HIT. We suspect the decrease occurred because argatroban was not being continued while awaiting an SRA test in patients with a negative PF4 test. Decreasing the utilization of argatroban not only saved money but also reduced days of exposure to argatroban. While we do not have data regarding adverse events related to argatroban prior to the intervention, it is logical to conclude that reducing unnecessary exposure to argatroban reduces the risk of adverse events related to bleeding. Future studies would ideally address specific safety outcome metrics such as adverse events, bleeding risk, or missed diagnoses of HIT.
Our institutional guidelines for the diagnosis of HIT are evidence-based and helpful but are rarely followed by busy inpatient providers. Controlling the utilization of the SRA at the laboratory level had several advantages. First, removing SRA decision-making from providers who are not experts in the diagnosis of HIT guaranteed adherence to evidence-based guidelines. Second, pharmacists could safely recommend discontinuing argatroban when the PF4 test was negative as there was no SRA pending. Third, with cancellation at the laboratory level there was no need to further burden providers with yet another alert in the electronic health record. Fourth, just-in-time education was provided to the providers with justification for why the SRA test was canceled. Last, ruling out HIT within 24 hours with the PF4 test alone allowed providers to evaluate patients for other causes of thrombocytopenia much earlier than the 3 to 5 business days before the SRA results returned.
A limitation of this study is that it was conducted at a single center. Our approach is also limited by the lack of universal applicability. At our institution we are fortunate to have PF4 testing available in our coagulation laboratory 7 days a week. In addition, the coagulation laboratory controls sending the SRA to the reference laboratory. The specific intervention of controlling the SRA testing is therefore applicable only to institutions similar to ours; however, the concept of removing control of specialized testing from the provider is not unique. Inpatient thrombophilia testing has been a successful target of this approach.11-13 While electronic alerts and education of individual providers can also be effective initially, the effectiveness of these interventions has been repeatedly shown to wane over time.14-16
Conclusion
At our institution we were able to implement practical, evidence-based testing for HIT by implementing control over SRA testing at the level of the laboratory. This approach led to decreased argatroban utilization and cost savings.
Corresponding author: Alice Cusick, MD; LTC Charles S Kettles VA Medical Center, 2215 Fuller Road, Ann Arbor, MI 48105; [email protected]
Disclosures: None reported.
doi: 10.12788/jcom.0087
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
1. Fountain E, Arepally GM. Thrombocytopenia in hospitalized non-ICU patients. Blood. 2015;126(23):1060. doi:10.1182/blood.v126.23.1060.1060
2. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271-278. doi:10.1378/chest.10-2243
3. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576-585. doi:10.1097/MCC.0000000000000259
4. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi:10.1182/bloodadvances.2018024489
5. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi:10.1182/blood-2012-07-443051
6. Northam KA, Parker WF, Chen S-L, et al. Evaluation of 4Ts score inter-rater agreement in patients undergoing evaluation for heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2021;32(5):328-334. doi:10.1097/MBC.0000000000001042
7. Raschke RA, Curry SC, Warkentin TE, Gerkin RD. Improving clinical interpretation of the anti-platelet factor 4/heparin enzyme-linked immunosorbent assay for the diagnosis of heparin-induced thrombocytopenia through the use of receiver operating characteristic analysis, stratum-specific likelihood ratios, and Bayes theorem. Chest. 2013;144(4):1269-1275. doi:10.1378/chest.12-2712
8. Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol. 2015;90(6):564-572. doi:10.1002/ajh.24006
9. Use IFOR, Contents TOF. LIFECODES ® PF4 IgG assay:1-9.
10. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1-9. doi:10.1186/s12911-017-0430-8
11. O’Connor N, Carter-Johnson R. Effective screening of pathology tests controls costs: thrombophilia testing. J Clin Pathol. 2006;59(5):556. doi:10.1136/jcp.2005.030700
12. Lim MY, Greenberg CS. Inpatient thrombophilia testing: Impact of healthcare system technology and targeted clinician education on changing practice patterns. Vasc Med (United Kingdom). 2018;23(1):78-79. doi:10.1177/1358863X17742509
13. Cox JL, Shunkwiler SM, Koepsell SA. Requirement for a pathologist’s second signature limits inappropriate inpatient thrombophilia testing. Lab Med. 2017;48(4):367-371. doi:10.1093/labmed/lmx040
14. Kwang H, Mou E, Richman I, et al. Thrombophilia testing in the inpatient setting: impact of an educational intervention. BMC Med Inform Decis Mak. 2019;19(1):167. doi:10.1186/s12911-019-0889-6
15. Shah T, Patel-Teague S, Kroupa L, Meyer AND, Singh H. Impact of a national QI programme on reducing electronic health record notifications to clinicians. BMJ Qual Saf. 2019;28(1):10-14. doi:10.1136/bmjqs-2017-007447
16. Singh H, Spitzmueller C, Petersen NJ, Sawhney MK, Sittig DF. Information overload and missed test results in electronic health record-based settings. JAMA Intern Med. 2013;173(8):702-704. doi:10.1001/2013.jamainternmed.61
Differences in COVID-19 Outcomes Among Patients With Type 1 Diabetes: First vs Later Surges
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.
Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.
Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
From Hassenfeld Children’s Hospital at NYU Langone Health, New York, NY (Dr Gallagher), T1D Exchange, Boston, MA (Saketh Rompicherla; Drs Ebekozien, Noor, Odugbesan, and Mungmode; Nicole Rioles, Emma Ospelt), University of Mississippi School of Population Health, Jackson, MS (Dr. Ebekozien), Icahn School of Medicine at Mount Sinai, New York, NY (Drs. Wilkes, O’Malley, and Rapaport), Weill Cornell Medicine, New York, NY (Drs. Antal and Feuer), NYU Long Island School of Medicine, Mineola, NY (Dr. Gabriel), NYU Langone Health, New York, NY (Dr. Golden), Barbara Davis Center, Aurora, CO (Dr. Alonso), Texas Children’s Hospital/Baylor College of Medicine, Houston, TX (Dr. Lyons), Stanford University, Stanford, CA (Dr. Prahalad), Children Mercy Kansas City, MO (Dr. Clements), Indiana University School of Medicine, IN (Dr. Neyman), Rady Children’s Hospital, University of California, San Diego, CA (Dr. Demeterco-Berggren).
Background: Patient outcomes of COVID-19 have improved throughout the pandemic. However, because it is not known whether outcomes of COVID-19 in the type 1 diabetes (T1D) population improved over time, we investigated differences in COVID-19 outcomes for patients with T1D in the United States.
Methods: We analyzed data collected via a registry of patients with T1D and COVID-19 from 56 sites between April 2020 and January 2021. We grouped cases into first surge (April 9, 2020, to July 31, 2020, n = 188) and late surge (August 1, 2020, to January 31, 2021, n = 410), and then compared outcomes between both groups using descriptive statistics and logistic regression models.
Results: Adverse outcomes were more frequent during the first surge, including diabetic ketoacidosis (32% vs 15%, P < .001), severe hypoglycemia (4% vs 1%, P = .04), and hospitalization (52% vs 22%, P < .001). Patients in the first surge were older (28 [SD,18.8] years vs 18.0 [SD, 11.1] years, P < .001), had higher median hemoglobin A1c levels (9.3 [interquartile range {IQR}, 4.0] vs 8.4 (IQR, 2.8), P < .001), and were more likely to use public insurance (107 [57%] vs 154 [38%], P < .001). The odds of hospitalization for adults in the first surge were 5 times higher compared to the late surge (odds ratio, 5.01; 95% CI, 2.11-12.63).
Conclusion: Patients with T1D who presented with COVID-19 during the first surge had a higher proportion of adverse outcomes than those who presented in a later surge.
Keywords: TD1, diabetic ketoacidosis, hypoglycemia.
After the World Health Organization declared the disease caused by the novel coronavirus SARS-CoV-2, COVID-19, a pandemic on March 11, 2020, the Centers for Disease Control and Prevention identified patients with diabetes as high risk for severe illness.1-7 The case-fatality rate for COVID-19 has significantly improved over the past 2 years. Public health measures, less severe COVID-19 variants, increased access to testing, and new treatments for COVID-19 have contributed to improved outcomes.
The T1D Exchange has previously published findings on COVID-19 outcomes for patients with type 1 diabetes (T1D) using data from the T1D COVID-19 Surveillance Registry.8-12 Given improved outcomes in COVID-19 in the general population, we sought to determine if outcomes for cases of COVID-19 reported to this registry changed over time.
Methods
This study was coordinated by the T1D Exchange and approved as nonhuman subject research by the Western Institutional Review Board. All participating centers also obtained local institutional review board approval. No identifiable patient information was collected as part of this noninterventional, cross-sectional study.
The T1D Exchange Multi-center COVID-19 Surveillance Study collected data from endocrinology clinics that completed a retrospective chart review and submitted information to T1D Exchange via an online questionnaire for all patients with T1D at their sites who tested positive for COVID-19.13,14 The questionnaire was administered using the Qualtrics survey platform (www.qualtrics.com version XM) and contained 33 pre-coded and free-text response fields to collect patient and clinical attributes.
Each participating center identified 1 team member for reporting to avoid duplicate case submission. Each submitted case was reviewed for potential errors and incomplete information. The coordinating center verified the number of cases per site for data quality assurance.
Quantitative data were represented as mean (standard deviation) or median (interquartile range). Categorical data were described as the number (percentage) of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient-related and clinical characteristics. The date August 1, 2021, was selected as the end of the first surge based on a review of national COVID-19 surges.
We used the Fisher’s exact test to assess associations between hospitalization and demographics, HbA1c, diabetes duration, symptoms, and adverse outcomes. In addition, multivariate logistic regression was used to calculate odds ratios (OR). Logistic regression models were used to determine the association between time of surge and hospitalization separately for both the pediatric and adult populations. Each model was adjusted for potential sociodemographic confounders, specifically age, sex, race, insurance, and HbA1c.
All tests were 2-sided, with type 1 error set at 5%. Fisher’s exact test and logistic regression were performed using statistical software R, version 3.6.2 (R Foundation for Statistical Computing).
Results
The characteristics of COVID-19 cases in patients with T1D that were reported early in the pandemic, before August 1, 2020 (first surge), compared with those of cases reported on and after August 1, 2020 (later surges) are shown in Table 1.

Patients with T1D who presented with COVID-19 during the first surge as compared to the later surges were older (mean age 28 [SD, 18.0] years vs 18.8 [SD, 11.1] years; P < .001) and had a longer duration of diabetes (P < .001). The first-surge group also had more patients with >20 years’ diabetes duration (20% vs 9%, P < .001). Obesity, hypertension, and chronic kidney disease were also more commonly reported in first-surge cases (all P < .001).
There was a significant difference in race and ethnicity reported in the first surge vs the later surge cases, with fewer patients identifying as non-Hispanic White (39% vs, 63%, P < .001) and more patients identifying as non-Hispanic Black (29% vs 12%, P < .001). The groups also differed significantly in terms of insurance type, with more people on public insurance in the first-surge group (57% vs 38%, P < .001). In addition, median HbA1c was higher (9.3% vs 8.4%, P < .001) and continuous glucose monitor and insulin pump use were less common (P = .02 and <.001, respectively) in the early surge.
All symptoms and adverse outcomes were reported more often in the first surge, including diabetic ketoacidosis (DKA; 32% vs 15%; P < .001) and severe hypoglycemia (4% vs 1%, P = .04). Hospitalization (52% vs 13%, P < .001) and ICU admission (24% vs 9%, P < .001) were reported more often in the first-surge group.
Regression Analyses
Table 2 shows the results of logistic regression analyses for hospitalization in the pediatric (≤19 years of age) and adult (>19 years of age) groups, along with the odds of hospitalization during the first vs late surge among COVID-positive people with T1D. Adult patients who tested positive in the first surge were about 5 times more likely to be hospitalized than adults who tested positive for infection in the late surge after adjusting for age, insurance type, sex, race, and HbA1c levels. Pediatric patients also had an increased odds for hospitalization during the first surge, but this increase was not statistically significant.

Discussion
Our analysis of COVID-19 cases in patients with T1D reported by diabetes providers across the United States found that adverse outcomes were more prevalent early in the pandemic. There may be a number of reasons for this difference in outcomes between patients who presented in the first surge vs a later surge. First, because testing for COVID-19 was extremely limited and reserved for hospitalized patients early in the pandemic, the first-surge patients with confirmed COVID-19 likely represent a skewed population of higher-acuity patients. This may also explain the relative paucity of cases in younger patients reported early in the pandemic. Second, worse outcomes in the early surge may also have been associated with overwhelmed hospitals in New York City at the start of the outbreak. According to Cummings et al, the abrupt surge of critically ill patients hospitalized with severe acute respiratory distress syndrome initially outpaced their capacity to provide prone-positioning ventilation, which has been expanded since then.15 While there was very little hypertension, cardiovascular disease, or kidney disease reported in the pediatric groups, there was a higher prevalence of obesity in the pediatric group from the mid-Atlantic region. Obesity has been associated with a worse prognosis for COVID-19 illness in children.16 Finally, there were 5 deaths reported in this study, all of which were reported during the first surge. Older age and increased rates of cardiovascular disease and chronic kidney disease in the first surge cases likely contributed to worse outcomes for adults in mid-Atlantic region relative to the other regions. Minority race and the use of public insurance, risk factors for more severe outcomes in all regions, were also more common in cases reported from the mid-Atlantic region.
This study has several limitations. First, it is a cross-sectional study that relies upon voluntary provider reports. Second, availability of COVID-19 testing was limited in all regions in spring 2020. Third, different regions of the country experienced subsequent surges at different times within the reported timeframes in this analysis. Fourth, this report time period does not include the impact of the newer COVID-19 variants. Finally, trends in COVID-19 outcomes were affected by the evolution of care that developed throughout 2020.
Conclusion
Adult patients with T1D and COVID-19 who reported during the first surge had about 5 times higher hospitalization odds than those who presented in a later surge.
Corresponding author: Osagie Ebekozien, MD, MPH, 11 Avenue de Lafayette, Boston, MA 02111; [email protected]
Disclosures: Dr Ebekozien reports receiving research grants from Medtronic Diabetes, Eli Lilly, and Dexcom, and receiving honoraria from Medtronic Diabetes.
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
1. Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813-822. doi:10.1016/S2213-8587(20)30272-2
2. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J Diabetes Complications. 2020;34(12):107748. doi:10.1016/j.jdiacomp.2020.107748
3. Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823-833. doi:10.1016/S2213-8587(20)30271-0
4. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174-e177. doi:10.2337/dc20-1217
5. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44(2):526-532. doi:10.2337/dc20-2260
6. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. 2021;22(2):202-206. doi:10.1111/pedi.13158
7. Maahs DM, Alonso GT, Gallagher MP, Ebekozien O. Comment on Gregory et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care. 2021;44:526-532. Diabetes Care. 2021;44(5):e102. doi:10.2337/dc20-3119
8. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the US. Diabetes Care. 2020;43(8):e83-e85. doi:10.2337/dc20-1088
9. Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes. 2021;13(3):270-272. doi:10.1111/1753-0407
10. O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange Multicenter Surveillance study. J Clin Endocrinol Metab. 2021;106(2):e936-e942. doi:10.1210/clinem/dgaa825
11. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755-e1762. doi:10.1210/clinem/dgaa920
12. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681-687. doi:10.1111/1753-0407.13184
13. Noor N, Ebekozien O, Levin L, et al. Diabetes technology use for management of type 1 diabetes is associated with fewer adverse COVID-19 outcomes: findings from the T1D Exchange COVID-19 Surveillance Registry. Diabetes Care. 2021;44(8):e160-e162. doi:10.2337/dc21-0074
14. Demeterco-Berggren C, Ebekozien O, Rompicherla S, et al. Age and hospitalization risk in people with type 1 diabetes and COVID-19: Data from the T1D Exchange Surveillance Study. J Clin Endocrinol Metab. 2021;dgab668. doi:10.1210/clinem/dgab668
15. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2
16. Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256. doi:10.1016/j.ijid.2020.11.163
Role and Experience of a Subintensive Care Unit in Caring for Patients With COVID-19 in Italy: The CO-RESP Study
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.
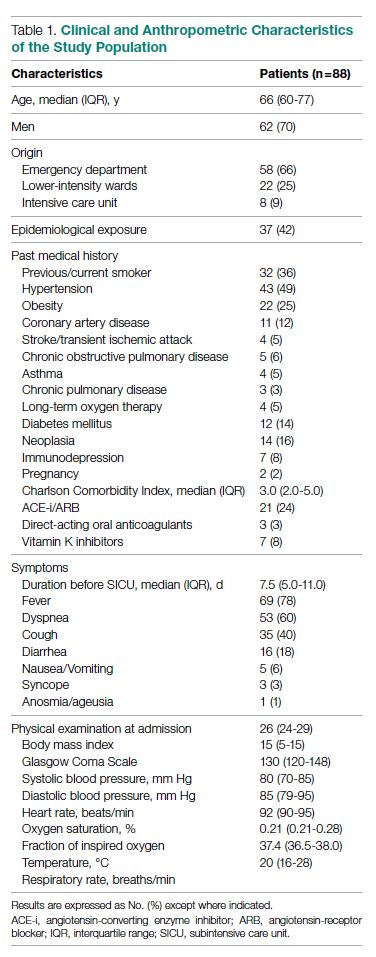
Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).
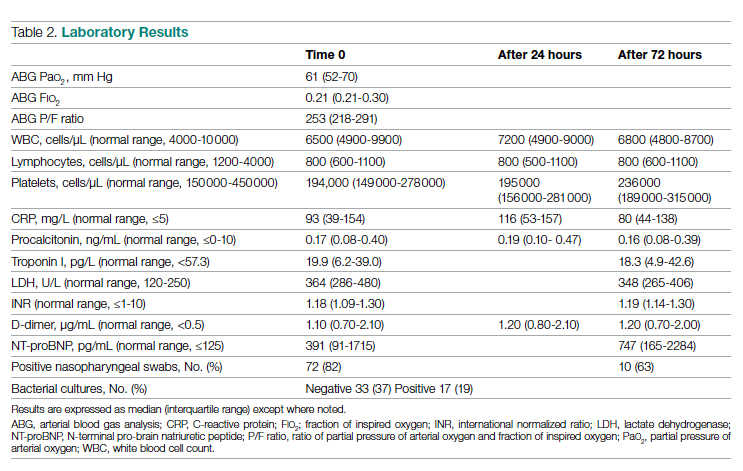
Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.
Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.
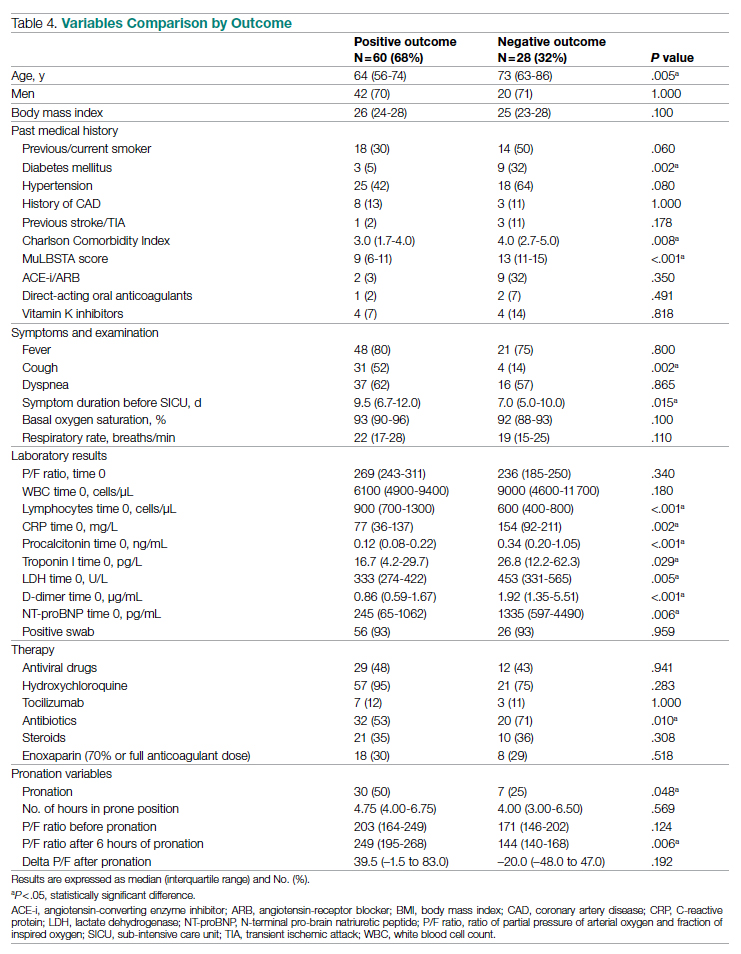
Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.

Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).

Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.

Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.

Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.

Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).

Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.

Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.

Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
A COVID-19 Clinical Management Committee to Standardize Care in a 2-Hospital System
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
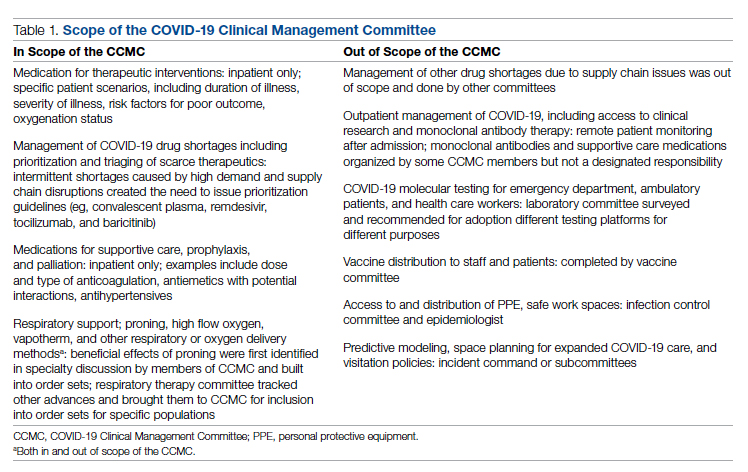
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
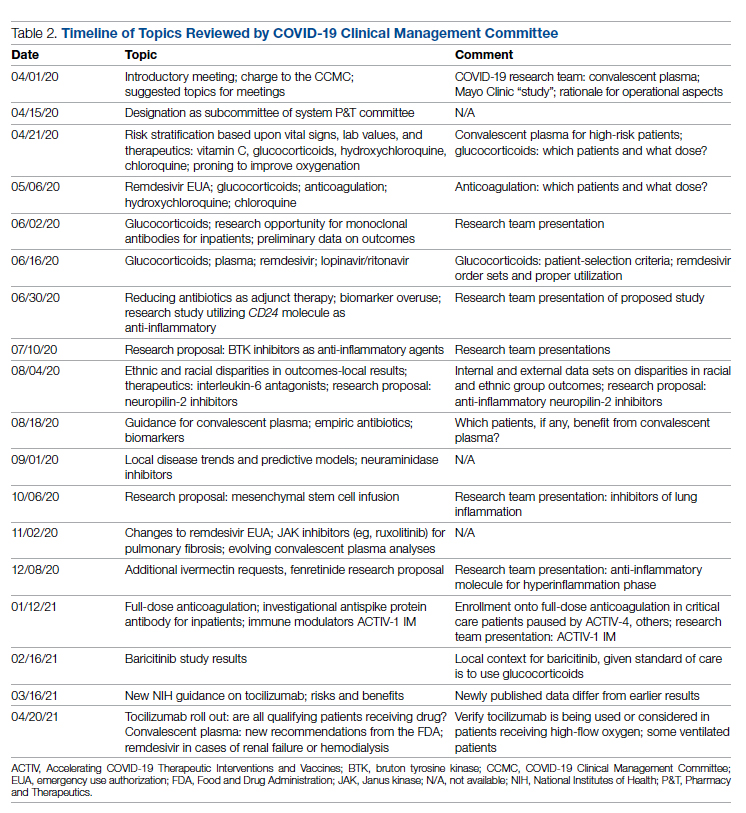
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
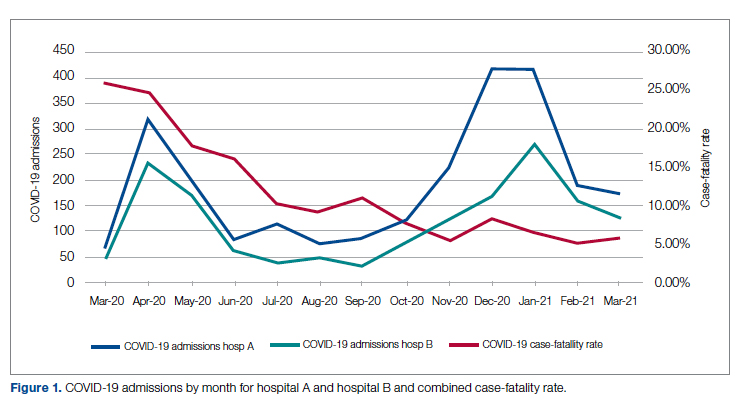
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
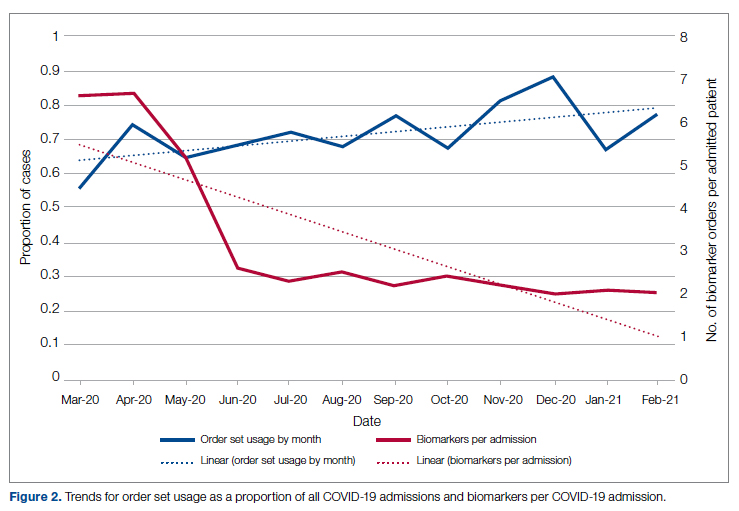
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
Assessment of Same-Day Naloxone Availability in New Mexico Pharmacies
From the Department of Medicine, University of California San Diego (Dr. Haponyuk), Department of Emergency Medicine, University of Tennessee (Dr. Dejong), the Department of Family Medicine, University of New Mexico (Dr. Gutfrucht), and the Department of Internal Medicine, University of New Mexico (Dr. Barrett)
Objective: Naloxone availability can reduce the risk of death from opioid overdoses, although prescriber, legislative, and payment barriers to accessing this life-saving medication exist. A previously underreported barrier involves same-day availability, the lack of which may force patients to travel to multiple pharmacies and having delays in access or risking not filling their prescription. This study sought to determine same-day availability of naloxone in pharmacies in the state of New Mexico.
Methods: Same-day availability of naloxone was assessed via an audit survey.
Results: Of the 183 pharamacies screened, only 84.7% had same-day availability, including only 72% in Albuquerque, the state’s most populous city/municipality.
Conclusion: These results highlight the extent of a previously underexplored challenge to patient care and barrier to patient safety, and future directions for more patient-centered care.
Keywords: naloxone; barriers to care; opioid overdose prevention.
The US is enduring an ongoing epidemic of deaths due to opioid use, which have increased in frequency since the onset of the COVID-19 pandemic.1 One strategy to reduce the risk of mortality from opioid use is to ensure the widespread availability of naloxone. Individual states have implemented harm reduction strategies to increase access to naloxone, including improving availability via a statewide standing order that it may be dispensed without a prescription.2,3 Such naloxone access laws are being widely adopted and are believed to reduce overdose deaths.4
There are many barriers to patients receiving naloxone despite their clinicians providing a prescription for it, including stigmatization, financial cost, and local availability.5-9 However, the stigma associated with naloxone extends to both patients and pharmacists. Pharmacists in West Virginia, for example, showed widespread concerns about having naloxone available for patients to purchase over the counter, for fear that increasing naloxone access may increase overdoses.6 A study in Tennessee also found pharmacists hesitant to recommend naloxone.7 Another study of rural pharmacies in Georgia found that just over half carried naloxone despite a state law that naloxone be available without a prescription.8 Challenges are not limited to rural areas, however; a study in Philadelphia found that more than one-third of pharmacies required a prescription to dispense naloxone, contrary to state law.9 Thus, in a rapidly changing regulatory environment, there are many evolving barriers to patients receiving naloxone.
New Mexico has an opioid overdose rate higher than the national average, coming in 15th out of 50 states when last ranked in 2018, with overdose rates that vary across demographic variables.10 Consequently, New Mexico state law added language requiring clinicians prescribing opioids for 5 days or longer to co-prescribe naloxone along with written information on how to administer the opioid antagonist.11 New Mexico is also a geographically large state with a relatively low overall population characterized by striking health disparities, particularly as related to access to care.
The purpose of this study is to describe the same-day availability of naloxone throughout the state of New Mexico after a change in state law requiring co-prescription was enacted, to help identify challenges to patients receiving it. Comprehensive examination of barriers to patients accessing this life-saving medication can advise strategies to both improve patient-centered care and potentially reduce deaths.
Methods
To better understand barriers to patients obtaining naloxone, in July and August of 2019 we performed an audit (“secret shopper”) study of all pharmacies in the state, posing as patients wishing to obtain naloxone. A publicly available list of every pharmacy in New Mexico was used to identify 89 pharmacies in Albuquerque (the most populous city in New Mexico) and 106 pharmacies throughout the rest of the state.12
Every pharmacy was called via a publicly available phone number during business hours (confirmed via an internet search), at least 2 hours prior to closing. One of 3 researchers telephoned pharmacies posing as a patient and inquired whether naloxone would be available for pick up the same day. If the pharmacy confirmed it was available that day, the call concluded. If naloxone was unavailable for same day pick up, researchers asked when it would be next available. Each pharmacy was called once, and neither insurance information nor cost was offered or requested. All questions were asked in English by native English speakers.
All responses were recorded in a secure spreadsheet. Once all responses were received and reviewed, they were characterized in discrete response categories: same day, within 1 to 2 days, within 3 to 4 days, within a week, or unsure/unknown. Naloxone availability was also tracked by city/municipality, and this was compared to the state’s population distribution.
No personally identifiable information was obtained. This study was Institutional Review Board exempt.
Results
Responses were recorded from 183 pharmacies. Seventeen locations were eliminated from our analysis because their phone system was inoperable or the pharmacy was permanently closed. Of the pharmacies reached, 84.7% (155/183) reported they have naloxone available for pick up on the same day (Figure 1). Of the 15.3% (28) pharmacies that did not have same-day availability, 60.7% (17 pharmacies) reported availability in 1 to 2 days, 3.6% had availability in 3 to 4 days, 3.6% had availability in 1 week, and 32.1% were unsure of next availability (Figure 2). More than one-third of the state’s patients reside in municipalities where naloxone is immediately available in at least 72% of pharmacies (Table).13
Discussion
Increased access to naloxone at the state and community level is associated with reduced risk for death from overdose, and, consequently, widespread availability is recommended.14-17 Statewide real-time pharmacy availability of naloxone—as patients would experience availability—has not been previously reported. These findings suggest unpredictable same-day availability that may affect experience and care outcomes. That other studies have found similar challenges in naloxone availability in other municipalities and regions suggests this barrier to access is widespread,6-9 and likely affects patients throughout the country.
Many patients have misgivings about naloxone, and it places an undue burden on them to travel to multiple pharmacies or take repeated trips to fill prescriptions. Additionally, patients without reliable transportation may be unable to return at a later date. Although we found most pharmacies in New Mexico without immediate availability of naloxone reported they could have it within several days, such a delay may reduce the likelihood that patients will fill their prescription at all. It is also concerning that many pharmacies are unsure of when naloxone will be available, particularly when some of these may be the only pharmacy easily accessible to patients or the one where they regularly fill their prescriptions.
Barriers to naloxone availability requires further study due to possible negative consequences for patient safety and risks for exacerbating health disparities among vulnerable populations. Further research may focus on examining the effects on patients when naloxone dispensing is delayed or impossible, why there is variability in naloxone availability between different pharmacies and municipalities, the reasons for uncertainty when naloxone will be available, and effective solutions. Expanded naloxone distribution in community locations and in clinics offers one potential patient-centered solution that should be explored, but it is likely that more widespread and systemic solutions will require policy and regulatory changes at the state and national levels.
Limitations of this study include that the findings may be relevant for solely 1 state, such as in the case of state-specific barriers to keeping naloxone in stock that we are unaware of. However, it is unclear why that would be the case, and it is more likely that similar barriers are pervasive. Additionally, repeat phone calls, which we did not follow up with, may have yielded more pharmacies with naloxone availability. However, due to the stigma associated with obtaining naloxone, it may be that patients will not make multiple calls either—highlighting how important real-time availability is.
Conclusion
Urgent solutions are needed to address the epidemic of deaths from opioid overdoses. Naloxone availability is an important tool for reducing these deaths, resulting in numerous state laws attempting to increase access. Despite this, there are persistent barriers to patients receiving naloxone, including a lack of same-day availability at pharmacies. Our results suggest that this underexplored barrier is widespread. Improving both availability and accessibility of naloxone may include legislative policy solutions as well as patient-oriented solutions, such as distribution in clinics and hospitals when opioid prescriptions are first written. Further research should be conducted to determine patient-centered, effective solutions that can improve outcomes.
Corresponding author: Eileen Barrett, MD, MPH, Department of Internal Medicine, University of New Mexico; [email protected].
Financial disclosures: None.
1. Mason M, Welch SB, Arunkumar P, et al. Notes from the field: opioid overdose deaths before, during, and after an 11-week COVID-19 stay-at-home order—Cook County, Illinois, January 1, 2018–October 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):362-363. doi:10.15585/mmwr.mm7010a3
2. Kaiser Family Foundation. Opioid overdose death rates and all drug overdose death rates per 100,000 population (age-adjusted). Accessed October 6, 2021. https://www.kff.org/other/state-indicator/opioid-overdose-death
3. Sohn M, Talbert JC, Huang Z, et al. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw Open. 2019;2(6):e196215. doi:10.1001/jamanetworkopen.2019.6215
4. Smart R, Pardo B, Davis CS. Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction. 2021;116(1):6-17. doi:10.1111/add.15163
5. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017;32(3):277-283. doi:10.1007/s11606-016-3895-8
6. Thornton JD, Lyvers E, Scott VGG, Dwibedi N. Pharmacists’ readiness to provide naloxone in community pharmacies in West Virginia. J Am Pharm Assoc (2003). 2017;57(2S):S12-S18.e4. doi:10.1016/j.japh.2016.12.070
7. Spivey C, Wilder A, Chisholm-Burns MA, et al. Evaluation of naloxone access, pricing, and barriers to dispensing in Tennessee retail community pharmacies. J Am Pharm Assoc (2003). 2020;60(5):694-701.e1. doi:10.1016/j.japh.2020.01.030
8. Nguyen JL, Gilbert LR, Beasley L, et al. Availability of naloxone at rural Georgia pharmacies, 2019. JAMA Netw Open. 2020;3(2):e1921227. doi:10.1001/jamanetworkopen.2019.21227
9. Guadamuz JS, Alexander GC, Chaudhri T, et al. Availability and cost of naloxone nasal spray at pharmacies in Philadelphia, Pennsylvania. JAMA Netw Open. 2019;2(6):e195388. doi:10.1001/jamanetworkopen.2019.5388
10. Edge K. Changes in drug overdose mortality in New Mexico. New Mexico Epidemiology. July 2020 (3). https://www.nmhealth.org/data/view/report/2402/
11. Senate Bill 221. 54th Legislature, State of New Mexico, First Session, 2019 (introduced by William P. Soules). Accessed October 6, 2021. https://nmlegis.gov/Sessions/19%20Regular/bills/senate/SB0221.pdf
12. GoodRx. Find pharmacies in New Mexico. Accessed October 6, 2021. https://www.goodrx.com/pharmacy-near-me/all/nm
13. U.S. Census Bureau. QuickFacts: New Mexico. Accessed October 6, 2021. https://www.census.gov/quickfacts/NM
14. Linas BP, Savinkina A, Madushani RWMA, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open. 2021;4(2):e2037259. doi:10.1001/jamanetworkopen.2020.37259
15. You HS, Ha J, Kang CY, et al. Regional variation in states’ naloxone accessibility laws in association with opioid overdose death rates—observational study (STROBE compliant). Medicine (Baltimore). 2020;99(22):e20033. doi:10.1097/MD.0000000000020033
16. Pew Charitable Trusts. Expanded access to naloxone can curb opioid overdose deaths. October 20, 2020. Accessed October 6, 2021. https://www.
17. Centers for Disease Control and Prevention. Still not enough naloxone where it’s most needed. August 6, 2019. Accessed October 6, 2021. https://www.cdc.gov/media/releases/2019/p0806-naloxone.html
From the Department of Medicine, University of California San Diego (Dr. Haponyuk), Department of Emergency Medicine, University of Tennessee (Dr. Dejong), the Department of Family Medicine, University of New Mexico (Dr. Gutfrucht), and the Department of Internal Medicine, University of New Mexico (Dr. Barrett)
Objective: Naloxone availability can reduce the risk of death from opioid overdoses, although prescriber, legislative, and payment barriers to accessing this life-saving medication exist. A previously underreported barrier involves same-day availability, the lack of which may force patients to travel to multiple pharmacies and having delays in access or risking not filling their prescription. This study sought to determine same-day availability of naloxone in pharmacies in the state of New Mexico.
Methods: Same-day availability of naloxone was assessed via an audit survey.
Results: Of the 183 pharamacies screened, only 84.7% had same-day availability, including only 72% in Albuquerque, the state’s most populous city/municipality.
Conclusion: These results highlight the extent of a previously underexplored challenge to patient care and barrier to patient safety, and future directions for more patient-centered care.
Keywords: naloxone; barriers to care; opioid overdose prevention.
The US is enduring an ongoing epidemic of deaths due to opioid use, which have increased in frequency since the onset of the COVID-19 pandemic.1 One strategy to reduce the risk of mortality from opioid use is to ensure the widespread availability of naloxone. Individual states have implemented harm reduction strategies to increase access to naloxone, including improving availability via a statewide standing order that it may be dispensed without a prescription.2,3 Such naloxone access laws are being widely adopted and are believed to reduce overdose deaths.4
There are many barriers to patients receiving naloxone despite their clinicians providing a prescription for it, including stigmatization, financial cost, and local availability.5-9 However, the stigma associated with naloxone extends to both patients and pharmacists. Pharmacists in West Virginia, for example, showed widespread concerns about having naloxone available for patients to purchase over the counter, for fear that increasing naloxone access may increase overdoses.6 A study in Tennessee also found pharmacists hesitant to recommend naloxone.7 Another study of rural pharmacies in Georgia found that just over half carried naloxone despite a state law that naloxone be available without a prescription.8 Challenges are not limited to rural areas, however; a study in Philadelphia found that more than one-third of pharmacies required a prescription to dispense naloxone, contrary to state law.9 Thus, in a rapidly changing regulatory environment, there are many evolving barriers to patients receiving naloxone.
New Mexico has an opioid overdose rate higher than the national average, coming in 15th out of 50 states when last ranked in 2018, with overdose rates that vary across demographic variables.10 Consequently, New Mexico state law added language requiring clinicians prescribing opioids for 5 days or longer to co-prescribe naloxone along with written information on how to administer the opioid antagonist.11 New Mexico is also a geographically large state with a relatively low overall population characterized by striking health disparities, particularly as related to access to care.
The purpose of this study is to describe the same-day availability of naloxone throughout the state of New Mexico after a change in state law requiring co-prescription was enacted, to help identify challenges to patients receiving it. Comprehensive examination of barriers to patients accessing this life-saving medication can advise strategies to both improve patient-centered care and potentially reduce deaths.
Methods
To better understand barriers to patients obtaining naloxone, in July and August of 2019 we performed an audit (“secret shopper”) study of all pharmacies in the state, posing as patients wishing to obtain naloxone. A publicly available list of every pharmacy in New Mexico was used to identify 89 pharmacies in Albuquerque (the most populous city in New Mexico) and 106 pharmacies throughout the rest of the state.12
Every pharmacy was called via a publicly available phone number during business hours (confirmed via an internet search), at least 2 hours prior to closing. One of 3 researchers telephoned pharmacies posing as a patient and inquired whether naloxone would be available for pick up the same day. If the pharmacy confirmed it was available that day, the call concluded. If naloxone was unavailable for same day pick up, researchers asked when it would be next available. Each pharmacy was called once, and neither insurance information nor cost was offered or requested. All questions were asked in English by native English speakers.
All responses were recorded in a secure spreadsheet. Once all responses were received and reviewed, they were characterized in discrete response categories: same day, within 1 to 2 days, within 3 to 4 days, within a week, or unsure/unknown. Naloxone availability was also tracked by city/municipality, and this was compared to the state’s population distribution.
No personally identifiable information was obtained. This study was Institutional Review Board exempt.

Results
Responses were recorded from 183 pharmacies. Seventeen locations were eliminated from our analysis because their phone system was inoperable or the pharmacy was permanently closed. Of the pharmacies reached, 84.7% (155/183) reported they have naloxone available for pick up on the same day (Figure 1). Of the 15.3% (28) pharmacies that did not have same-day availability, 60.7% (17 pharmacies) reported availability in 1 to 2 days, 3.6% had availability in 3 to 4 days, 3.6% had availability in 1 week, and 32.1% were unsure of next availability (Figure 2). More than one-third of the state’s patients reside in municipalities where naloxone is immediately available in at least 72% of pharmacies (Table).13

Discussion
Increased access to naloxone at the state and community level is associated with reduced risk for death from overdose, and, consequently, widespread availability is recommended.14-17 Statewide real-time pharmacy availability of naloxone—as patients would experience availability—has not been previously reported. These findings suggest unpredictable same-day availability that may affect experience and care outcomes. That other studies have found similar challenges in naloxone availability in other municipalities and regions suggests this barrier to access is widespread,6-9 and likely affects patients throughout the country.

Many patients have misgivings about naloxone, and it places an undue burden on them to travel to multiple pharmacies or take repeated trips to fill prescriptions. Additionally, patients without reliable transportation may be unable to return at a later date. Although we found most pharmacies in New Mexico without immediate availability of naloxone reported they could have it within several days, such a delay may reduce the likelihood that patients will fill their prescription at all. It is also concerning that many pharmacies are unsure of when naloxone will be available, particularly when some of these may be the only pharmacy easily accessible to patients or the one where they regularly fill their prescriptions.
Barriers to naloxone availability requires further study due to possible negative consequences for patient safety and risks for exacerbating health disparities among vulnerable populations. Further research may focus on examining the effects on patients when naloxone dispensing is delayed or impossible, why there is variability in naloxone availability between different pharmacies and municipalities, the reasons for uncertainty when naloxone will be available, and effective solutions. Expanded naloxone distribution in community locations and in clinics offers one potential patient-centered solution that should be explored, but it is likely that more widespread and systemic solutions will require policy and regulatory changes at the state and national levels.
Limitations of this study include that the findings may be relevant for solely 1 state, such as in the case of state-specific barriers to keeping naloxone in stock that we are unaware of. However, it is unclear why that would be the case, and it is more likely that similar barriers are pervasive. Additionally, repeat phone calls, which we did not follow up with, may have yielded more pharmacies with naloxone availability. However, due to the stigma associated with obtaining naloxone, it may be that patients will not make multiple calls either—highlighting how important real-time availability is.
Conclusion
Urgent solutions are needed to address the epidemic of deaths from opioid overdoses. Naloxone availability is an important tool for reducing these deaths, resulting in numerous state laws attempting to increase access. Despite this, there are persistent barriers to patients receiving naloxone, including a lack of same-day availability at pharmacies. Our results suggest that this underexplored barrier is widespread. Improving both availability and accessibility of naloxone may include legislative policy solutions as well as patient-oriented solutions, such as distribution in clinics and hospitals when opioid prescriptions are first written. Further research should be conducted to determine patient-centered, effective solutions that can improve outcomes.
Corresponding author: Eileen Barrett, MD, MPH, Department of Internal Medicine, University of New Mexico; [email protected].
Financial disclosures: None.
From the Department of Medicine, University of California San Diego (Dr. Haponyuk), Department of Emergency Medicine, University of Tennessee (Dr. Dejong), the Department of Family Medicine, University of New Mexico (Dr. Gutfrucht), and the Department of Internal Medicine, University of New Mexico (Dr. Barrett)
Objective: Naloxone availability can reduce the risk of death from opioid overdoses, although prescriber, legislative, and payment barriers to accessing this life-saving medication exist. A previously underreported barrier involves same-day availability, the lack of which may force patients to travel to multiple pharmacies and having delays in access or risking not filling their prescription. This study sought to determine same-day availability of naloxone in pharmacies in the state of New Mexico.
Methods: Same-day availability of naloxone was assessed via an audit survey.
Results: Of the 183 pharamacies screened, only 84.7% had same-day availability, including only 72% in Albuquerque, the state’s most populous city/municipality.
Conclusion: These results highlight the extent of a previously underexplored challenge to patient care and barrier to patient safety, and future directions for more patient-centered care.
Keywords: naloxone; barriers to care; opioid overdose prevention.
The US is enduring an ongoing epidemic of deaths due to opioid use, which have increased in frequency since the onset of the COVID-19 pandemic.1 One strategy to reduce the risk of mortality from opioid use is to ensure the widespread availability of naloxone. Individual states have implemented harm reduction strategies to increase access to naloxone, including improving availability via a statewide standing order that it may be dispensed without a prescription.2,3 Such naloxone access laws are being widely adopted and are believed to reduce overdose deaths.4
There are many barriers to patients receiving naloxone despite their clinicians providing a prescription for it, including stigmatization, financial cost, and local availability.5-9 However, the stigma associated with naloxone extends to both patients and pharmacists. Pharmacists in West Virginia, for example, showed widespread concerns about having naloxone available for patients to purchase over the counter, for fear that increasing naloxone access may increase overdoses.6 A study in Tennessee also found pharmacists hesitant to recommend naloxone.7 Another study of rural pharmacies in Georgia found that just over half carried naloxone despite a state law that naloxone be available without a prescription.8 Challenges are not limited to rural areas, however; a study in Philadelphia found that more than one-third of pharmacies required a prescription to dispense naloxone, contrary to state law.9 Thus, in a rapidly changing regulatory environment, there are many evolving barriers to patients receiving naloxone.
New Mexico has an opioid overdose rate higher than the national average, coming in 15th out of 50 states when last ranked in 2018, with overdose rates that vary across demographic variables.10 Consequently, New Mexico state law added language requiring clinicians prescribing opioids for 5 days or longer to co-prescribe naloxone along with written information on how to administer the opioid antagonist.11 New Mexico is also a geographically large state with a relatively low overall population characterized by striking health disparities, particularly as related to access to care.
The purpose of this study is to describe the same-day availability of naloxone throughout the state of New Mexico after a change in state law requiring co-prescription was enacted, to help identify challenges to patients receiving it. Comprehensive examination of barriers to patients accessing this life-saving medication can advise strategies to both improve patient-centered care and potentially reduce deaths.
Methods
To better understand barriers to patients obtaining naloxone, in July and August of 2019 we performed an audit (“secret shopper”) study of all pharmacies in the state, posing as patients wishing to obtain naloxone. A publicly available list of every pharmacy in New Mexico was used to identify 89 pharmacies in Albuquerque (the most populous city in New Mexico) and 106 pharmacies throughout the rest of the state.12
Every pharmacy was called via a publicly available phone number during business hours (confirmed via an internet search), at least 2 hours prior to closing. One of 3 researchers telephoned pharmacies posing as a patient and inquired whether naloxone would be available for pick up the same day. If the pharmacy confirmed it was available that day, the call concluded. If naloxone was unavailable for same day pick up, researchers asked when it would be next available. Each pharmacy was called once, and neither insurance information nor cost was offered or requested. All questions were asked in English by native English speakers.
All responses were recorded in a secure spreadsheet. Once all responses were received and reviewed, they were characterized in discrete response categories: same day, within 1 to 2 days, within 3 to 4 days, within a week, or unsure/unknown. Naloxone availability was also tracked by city/municipality, and this was compared to the state’s population distribution.
No personally identifiable information was obtained. This study was Institutional Review Board exempt.

Results
Responses were recorded from 183 pharmacies. Seventeen locations were eliminated from our analysis because their phone system was inoperable or the pharmacy was permanently closed. Of the pharmacies reached, 84.7% (155/183) reported they have naloxone available for pick up on the same day (Figure 1). Of the 15.3% (28) pharmacies that did not have same-day availability, 60.7% (17 pharmacies) reported availability in 1 to 2 days, 3.6% had availability in 3 to 4 days, 3.6% had availability in 1 week, and 32.1% were unsure of next availability (Figure 2). More than one-third of the state’s patients reside in municipalities where naloxone is immediately available in at least 72% of pharmacies (Table).13

Discussion
Increased access to naloxone at the state and community level is associated with reduced risk for death from overdose, and, consequently, widespread availability is recommended.14-17 Statewide real-time pharmacy availability of naloxone—as patients would experience availability—has not been previously reported. These findings suggest unpredictable same-day availability that may affect experience and care outcomes. That other studies have found similar challenges in naloxone availability in other municipalities and regions suggests this barrier to access is widespread,6-9 and likely affects patients throughout the country.

Many patients have misgivings about naloxone, and it places an undue burden on them to travel to multiple pharmacies or take repeated trips to fill prescriptions. Additionally, patients without reliable transportation may be unable to return at a later date. Although we found most pharmacies in New Mexico without immediate availability of naloxone reported they could have it within several days, such a delay may reduce the likelihood that patients will fill their prescription at all. It is also concerning that many pharmacies are unsure of when naloxone will be available, particularly when some of these may be the only pharmacy easily accessible to patients or the one where they regularly fill their prescriptions.
Barriers to naloxone availability requires further study due to possible negative consequences for patient safety and risks for exacerbating health disparities among vulnerable populations. Further research may focus on examining the effects on patients when naloxone dispensing is delayed or impossible, why there is variability in naloxone availability between different pharmacies and municipalities, the reasons for uncertainty when naloxone will be available, and effective solutions. Expanded naloxone distribution in community locations and in clinics offers one potential patient-centered solution that should be explored, but it is likely that more widespread and systemic solutions will require policy and regulatory changes at the state and national levels.
Limitations of this study include that the findings may be relevant for solely 1 state, such as in the case of state-specific barriers to keeping naloxone in stock that we are unaware of. However, it is unclear why that would be the case, and it is more likely that similar barriers are pervasive. Additionally, repeat phone calls, which we did not follow up with, may have yielded more pharmacies with naloxone availability. However, due to the stigma associated with obtaining naloxone, it may be that patients will not make multiple calls either—highlighting how important real-time availability is.
Conclusion
Urgent solutions are needed to address the epidemic of deaths from opioid overdoses. Naloxone availability is an important tool for reducing these deaths, resulting in numerous state laws attempting to increase access. Despite this, there are persistent barriers to patients receiving naloxone, including a lack of same-day availability at pharmacies. Our results suggest that this underexplored barrier is widespread. Improving both availability and accessibility of naloxone may include legislative policy solutions as well as patient-oriented solutions, such as distribution in clinics and hospitals when opioid prescriptions are first written. Further research should be conducted to determine patient-centered, effective solutions that can improve outcomes.
Corresponding author: Eileen Barrett, MD, MPH, Department of Internal Medicine, University of New Mexico; [email protected].
Financial disclosures: None.
1. Mason M, Welch SB, Arunkumar P, et al. Notes from the field: opioid overdose deaths before, during, and after an 11-week COVID-19 stay-at-home order—Cook County, Illinois, January 1, 2018–October 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):362-363. doi:10.15585/mmwr.mm7010a3
2. Kaiser Family Foundation. Opioid overdose death rates and all drug overdose death rates per 100,000 population (age-adjusted). Accessed October 6, 2021. https://www.kff.org/other/state-indicator/opioid-overdose-death
3. Sohn M, Talbert JC, Huang Z, et al. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw Open. 2019;2(6):e196215. doi:10.1001/jamanetworkopen.2019.6215
4. Smart R, Pardo B, Davis CS. Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction. 2021;116(1):6-17. doi:10.1111/add.15163
5. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017;32(3):277-283. doi:10.1007/s11606-016-3895-8
6. Thornton JD, Lyvers E, Scott VGG, Dwibedi N. Pharmacists’ readiness to provide naloxone in community pharmacies in West Virginia. J Am Pharm Assoc (2003). 2017;57(2S):S12-S18.e4. doi:10.1016/j.japh.2016.12.070
7. Spivey C, Wilder A, Chisholm-Burns MA, et al. Evaluation of naloxone access, pricing, and barriers to dispensing in Tennessee retail community pharmacies. J Am Pharm Assoc (2003). 2020;60(5):694-701.e1. doi:10.1016/j.japh.2020.01.030
8. Nguyen JL, Gilbert LR, Beasley L, et al. Availability of naloxone at rural Georgia pharmacies, 2019. JAMA Netw Open. 2020;3(2):e1921227. doi:10.1001/jamanetworkopen.2019.21227
9. Guadamuz JS, Alexander GC, Chaudhri T, et al. Availability and cost of naloxone nasal spray at pharmacies in Philadelphia, Pennsylvania. JAMA Netw Open. 2019;2(6):e195388. doi:10.1001/jamanetworkopen.2019.5388
10. Edge K. Changes in drug overdose mortality in New Mexico. New Mexico Epidemiology. July 2020 (3). https://www.nmhealth.org/data/view/report/2402/
11. Senate Bill 221. 54th Legislature, State of New Mexico, First Session, 2019 (introduced by William P. Soules). Accessed October 6, 2021. https://nmlegis.gov/Sessions/19%20Regular/bills/senate/SB0221.pdf
12. GoodRx. Find pharmacies in New Mexico. Accessed October 6, 2021. https://www.goodrx.com/pharmacy-near-me/all/nm
13. U.S. Census Bureau. QuickFacts: New Mexico. Accessed October 6, 2021. https://www.census.gov/quickfacts/NM
14. Linas BP, Savinkina A, Madushani RWMA, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open. 2021;4(2):e2037259. doi:10.1001/jamanetworkopen.2020.37259
15. You HS, Ha J, Kang CY, et al. Regional variation in states’ naloxone accessibility laws in association with opioid overdose death rates—observational study (STROBE compliant). Medicine (Baltimore). 2020;99(22):e20033. doi:10.1097/MD.0000000000020033
16. Pew Charitable Trusts. Expanded access to naloxone can curb opioid overdose deaths. October 20, 2020. Accessed October 6, 2021. https://www.
17. Centers for Disease Control and Prevention. Still not enough naloxone where it’s most needed. August 6, 2019. Accessed October 6, 2021. https://www.cdc.gov/media/releases/2019/p0806-naloxone.html
1. Mason M, Welch SB, Arunkumar P, et al. Notes from the field: opioid overdose deaths before, during, and after an 11-week COVID-19 stay-at-home order—Cook County, Illinois, January 1, 2018–October 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):362-363. doi:10.15585/mmwr.mm7010a3
2. Kaiser Family Foundation. Opioid overdose death rates and all drug overdose death rates per 100,000 population (age-adjusted). Accessed October 6, 2021. https://www.kff.org/other/state-indicator/opioid-overdose-death
3. Sohn M, Talbert JC, Huang Z, et al. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw Open. 2019;2(6):e196215. doi:10.1001/jamanetworkopen.2019.6215
4. Smart R, Pardo B, Davis CS. Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction. 2021;116(1):6-17. doi:10.1111/add.15163
5. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017;32(3):277-283. doi:10.1007/s11606-016-3895-8
6. Thornton JD, Lyvers E, Scott VGG, Dwibedi N. Pharmacists’ readiness to provide naloxone in community pharmacies in West Virginia. J Am Pharm Assoc (2003). 2017;57(2S):S12-S18.e4. doi:10.1016/j.japh.2016.12.070
7. Spivey C, Wilder A, Chisholm-Burns MA, et al. Evaluation of naloxone access, pricing, and barriers to dispensing in Tennessee retail community pharmacies. J Am Pharm Assoc (2003). 2020;60(5):694-701.e1. doi:10.1016/j.japh.2020.01.030
8. Nguyen JL, Gilbert LR, Beasley L, et al. Availability of naloxone at rural Georgia pharmacies, 2019. JAMA Netw Open. 2020;3(2):e1921227. doi:10.1001/jamanetworkopen.2019.21227
9. Guadamuz JS, Alexander GC, Chaudhri T, et al. Availability and cost of naloxone nasal spray at pharmacies in Philadelphia, Pennsylvania. JAMA Netw Open. 2019;2(6):e195388. doi:10.1001/jamanetworkopen.2019.5388
10. Edge K. Changes in drug overdose mortality in New Mexico. New Mexico Epidemiology. July 2020 (3). https://www.nmhealth.org/data/view/report/2402/
11. Senate Bill 221. 54th Legislature, State of New Mexico, First Session, 2019 (introduced by William P. Soules). Accessed October 6, 2021. https://nmlegis.gov/Sessions/19%20Regular/bills/senate/SB0221.pdf
12. GoodRx. Find pharmacies in New Mexico. Accessed October 6, 2021. https://www.goodrx.com/pharmacy-near-me/all/nm
13. U.S. Census Bureau. QuickFacts: New Mexico. Accessed October 6, 2021. https://www.census.gov/quickfacts/NM
14. Linas BP, Savinkina A, Madushani RWMA, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open. 2021;4(2):e2037259. doi:10.1001/jamanetworkopen.2020.37259
15. You HS, Ha J, Kang CY, et al. Regional variation in states’ naloxone accessibility laws in association with opioid overdose death rates—observational study (STROBE compliant). Medicine (Baltimore). 2020;99(22):e20033. doi:10.1097/MD.0000000000020033
16. Pew Charitable Trusts. Expanded access to naloxone can curb opioid overdose deaths. October 20, 2020. Accessed October 6, 2021. https://www.
17. Centers for Disease Control and Prevention. Still not enough naloxone where it’s most needed. August 6, 2019. Accessed October 6, 2021. https://www.cdc.gov/media/releases/2019/p0806-naloxone.html
Adjuvant Olaparib Improves Outcomes in High-Risk, HER2-Negative Early Breast Cancer Patients With Germline BRCA1 and BRCA2 Mutations
Study Overview
Objective. To assess the efficacy and safety of olaparib as an adjuvant treatment in patients with BRCA1 or BRCA2 germline mutations who are at a high-risk for relapse.
Design. A randomized, double-blind, placebo-controlled, multicenter phase III study. The published results are from the prespecified interim analysis.
Intervention. Patients were randomized in 1:1 ratio to either receive 300 mg of olaparib orally twice daily or to receive a matching placebo. Randomization was stratified by hormone receptor status (estrogen receptor and/or progesterone receptor positive/HER2-negative vs triple negative), prior neoadjuvant vs adjuvant chemotherapy, and prior platinum use for breast cancer. Treatment was continued for 52 weeks.
Setting and participants. A total of 1836 patients were randomized in a 1:1 fashion to receive olaparib or a placebo. Eligible patients had a germline BRCA1 or BRCA1 pathogenic or likely pathogenic variant. Patients had high-risk, HER2-negative primary breast cancers and all had received definitive local therapy and neoadjuvant or adjuvant chemotherapy. Patients were enrolled between 2 to 12 weeks after completion of all local therapy. Platinum chemotherapy was allowed. Patients received adjuvant endocrine therapy for hormone receptor positive disease as well as adjuvant bisphosphonates per institutional guidelines. Patients with triple negative disease who received adjuvant chemotherapy were required to be lymph node positive or have at least 2 cm invasive disease. Patients who received neoadjuvant chemotherapy were required to have residual invasive disease to be eligible. For hormone receptor positive patients receiving adjuvant chemotherapy to be eligible they had to have at least 4 pathologically confirmed lymph nodes involved. Hormone receptor positive patients who had neoadjuvant chemotherapy were required to have had residual invasive disease.
Main outcome measures. The primary endpoint for the study was invasive disease-free survival which was defined as time from randomization to date of recurrence or death from any cause. The secondary endpoints included overall survival (OS), distant disease-free survival, safety, and tolerability of olaparib.
Main results. At the time of data cutoff, 284 events had occurred with a median follow-up of 2.5 years in the intention to treat population. A total of 81% of patients had triple negative breast cancer. Most patients (94% in the olaparib group and 92% in the placebo group) received both taxane and anthracycline based chemotherapy regimens. Platinum based chemotherapy was used in 26% of patients in each group. The groups were otherwise well balanced. Germline mutations in BRCA1 were present in 72% of patients and BRCA2 in 27% of patients. These were balanced between groups.
At the time of this analysis, adjuvant olaparib reduced the risk of invasive disease-free survival by 42% compared with placebo (P < .001). At 3 years, invasive disease-free survival was 85.9% in the olaparib group and 77.1% in the placebo group (difference, 8.8 percentage points; 95% CI, 4.5-13.0; hazard ratio [HR], 0.58; 99.5% CI, 0.41-0.82; P < .001). The 3-year distant disease-free survival was 87.5% in the olaparib group and 80.4% in the placebo group (HR 0.57; 99.5% CI, 0.39-0.83; P < .001). Results also showed that olaparib was associated with fewer deaths than placebo (59 and 86, respectively) (HR, 0.68; 99% CI, 0.44-1.05; P = .02); however, there was no significant difference between treatment arms at the time of this interim analysis. Subgroup analysis showed a consistent benefit across all groups with no difference noted regarding BRCA mutation, hormone receptor status or use of neoadjuvant vs adjuvant chemotherapy.
The side effects were consistent with the safety profile of olaparib. Adverse events of grade 3 or higher more common with olaparib included anemia (8.7%), leukopenia (3%), and fatigue (1.8%). Early discontinuation of trial regimen due to adverse events of disease recurrence occurred in 25.9% in the olaparib group and 20.7% in the placebo group. Blood transfusions were required in 5.8% of patients in the olaparib group. Myelodysplasia or acute myleoid leukemia was observed in 2 patients in the olaparib group and 3 patients in the placebo group. Adverse events leading to death occurred in 1 patient in the olaparib group and 2 patients in the placebo group.
Conclusion. Among patients with high-risk, HER2-negative early breast cancer and germline BRCA1 or BRCA2 pathogenic or likely pathogenic variants, adjuvant olaparib after completion of local treatment and neoadjuvant or adjuvant chemotherapy was associated with significantly longer invasive disease-free and distant disease-free survival compared with placebo.
Commentary
The results from the current OlympiA trial provide the first evidence that adjuvant therapy with poly adenosine diphosphate-ribose polymerase (PARP) inhibitors can improve outcomes in high-risk, HER2-negative breast cancer in patients with pathogenic BRCA1 and BRCA2 mutations. The OS, while favoring olaparib, is not yet mature at the time of this analysis. Nevertheless, these results represent an important step forward in improving outcomes in this patient population. The efficacy and safety of PARP inhibitors in BRCA-mutated breast cancer has previously been shown in patients with advanced disease leading to FDA approval of both olaparib and talazoparib in this setting.1,2 With the current results, PARP inhibitors will certainly play an important role in the adjuvant setting in patients with deleterious BRCA1 or BRCA2 mutations at high risk for relapse. Importantly, the side effect profile appears acceptable with no unexpected events and a very low rate of secondary myeloid malignancies.
Subgroup analysis appears to indicate a benefit across all groups including hormone receptor–positive disease and triple negative breast cancer. Interestingly, approximately 25% of patients in both cohorts received platinum-based chemotherapy. The efficacy of adjuvant olaparib did not appear to be impacted by prior use of platinum-containing chemotherapy regimens. It is important to consider that postneoadjuvant capecitabine, per the results of the CREATE-X trial, in triple-negative patients was not permitted in the current study. Although, this has been widely adopted in clinical practice.3 The CREATE-X trial did not specify the benefit of adjuvant capecitabine in the BRCA-mutated cohort, thus, it is not clear how this subgroup fares with this approach. Thus, one cannot extrapolate the relative efficacy of olaparib compared with capecitabine, as pointed out by the authors, and whether we consider the use of capecitabine and/or olaparib in triple-negative patients with residual invasive disease after neoadjuvant chemotherapy is not clear at this time.
Nevertheless, the magnitude of benefit seen in this trial certainly provide clinically relevant and potentially practice changing results. It will be imperative to follow these results as the survival data matures and ensure no further long-term toxicity, particularly secondary myeloid malignancies, develop. These results should be discussed with each patient and informed decisions regarding the use of adjuvant olaparib should be considered for this patient population. Lastly, these results highlight the importance of germline testing for patients with breast cancer in accordance with national guideline recommendations. Moreover, these results certainly call into question whether it is time to consider expansion of our current germline testing guidelines to detect all potential patients who may benefit from this therapy.
Application for Clinical Practice
Adjuvant olaparib in high-risk patients with germline BRCA1 or BRCA2 mutations improves invasive and distant disease-free survival and should be considered in patients who meet the enrollment criteria of the current study. Furthermore, this highlights the importance of appropriate germline genetic testing in patients with breast cancer.
Financial disclosures: None.
1. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi:10.1056/NEJMoa1706450
2. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753-763. doi:10.1056/NEJMoa1802905
3. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
Study Overview
Objective. To assess the efficacy and safety of olaparib as an adjuvant treatment in patients with BRCA1 or BRCA2 germline mutations who are at a high-risk for relapse.
Design. A randomized, double-blind, placebo-controlled, multicenter phase III study. The published results are from the prespecified interim analysis.
Intervention. Patients were randomized in 1:1 ratio to either receive 300 mg of olaparib orally twice daily or to receive a matching placebo. Randomization was stratified by hormone receptor status (estrogen receptor and/or progesterone receptor positive/HER2-negative vs triple negative), prior neoadjuvant vs adjuvant chemotherapy, and prior platinum use for breast cancer. Treatment was continued for 52 weeks.
Setting and participants. A total of 1836 patients were randomized in a 1:1 fashion to receive olaparib or a placebo. Eligible patients had a germline BRCA1 or BRCA1 pathogenic or likely pathogenic variant. Patients had high-risk, HER2-negative primary breast cancers and all had received definitive local therapy and neoadjuvant or adjuvant chemotherapy. Patients were enrolled between 2 to 12 weeks after completion of all local therapy. Platinum chemotherapy was allowed. Patients received adjuvant endocrine therapy for hormone receptor positive disease as well as adjuvant bisphosphonates per institutional guidelines. Patients with triple negative disease who received adjuvant chemotherapy were required to be lymph node positive or have at least 2 cm invasive disease. Patients who received neoadjuvant chemotherapy were required to have residual invasive disease to be eligible. For hormone receptor positive patients receiving adjuvant chemotherapy to be eligible they had to have at least 4 pathologically confirmed lymph nodes involved. Hormone receptor positive patients who had neoadjuvant chemotherapy were required to have had residual invasive disease.
Main outcome measures. The primary endpoint for the study was invasive disease-free survival which was defined as time from randomization to date of recurrence or death from any cause. The secondary endpoints included overall survival (OS), distant disease-free survival, safety, and tolerability of olaparib.
Main results. At the time of data cutoff, 284 events had occurred with a median follow-up of 2.5 years in the intention to treat population. A total of 81% of patients had triple negative breast cancer. Most patients (94% in the olaparib group and 92% in the placebo group) received both taxane and anthracycline based chemotherapy regimens. Platinum based chemotherapy was used in 26% of patients in each group. The groups were otherwise well balanced. Germline mutations in BRCA1 were present in 72% of patients and BRCA2 in 27% of patients. These were balanced between groups.
At the time of this analysis, adjuvant olaparib reduced the risk of invasive disease-free survival by 42% compared with placebo (P < .001). At 3 years, invasive disease-free survival was 85.9% in the olaparib group and 77.1% in the placebo group (difference, 8.8 percentage points; 95% CI, 4.5-13.0; hazard ratio [HR], 0.58; 99.5% CI, 0.41-0.82; P < .001). The 3-year distant disease-free survival was 87.5% in the olaparib group and 80.4% in the placebo group (HR 0.57; 99.5% CI, 0.39-0.83; P < .001). Results also showed that olaparib was associated with fewer deaths than placebo (59 and 86, respectively) (HR, 0.68; 99% CI, 0.44-1.05; P = .02); however, there was no significant difference between treatment arms at the time of this interim analysis. Subgroup analysis showed a consistent benefit across all groups with no difference noted regarding BRCA mutation, hormone receptor status or use of neoadjuvant vs adjuvant chemotherapy.
The side effects were consistent with the safety profile of olaparib. Adverse events of grade 3 or higher more common with olaparib included anemia (8.7%), leukopenia (3%), and fatigue (1.8%). Early discontinuation of trial regimen due to adverse events of disease recurrence occurred in 25.9% in the olaparib group and 20.7% in the placebo group. Blood transfusions were required in 5.8% of patients in the olaparib group. Myelodysplasia or acute myleoid leukemia was observed in 2 patients in the olaparib group and 3 patients in the placebo group. Adverse events leading to death occurred in 1 patient in the olaparib group and 2 patients in the placebo group.
Conclusion. Among patients with high-risk, HER2-negative early breast cancer and germline BRCA1 or BRCA2 pathogenic or likely pathogenic variants, adjuvant olaparib after completion of local treatment and neoadjuvant or adjuvant chemotherapy was associated with significantly longer invasive disease-free and distant disease-free survival compared with placebo.
Commentary
The results from the current OlympiA trial provide the first evidence that adjuvant therapy with poly adenosine diphosphate-ribose polymerase (PARP) inhibitors can improve outcomes in high-risk, HER2-negative breast cancer in patients with pathogenic BRCA1 and BRCA2 mutations. The OS, while favoring olaparib, is not yet mature at the time of this analysis. Nevertheless, these results represent an important step forward in improving outcomes in this patient population. The efficacy and safety of PARP inhibitors in BRCA-mutated breast cancer has previously been shown in patients with advanced disease leading to FDA approval of both olaparib and talazoparib in this setting.1,2 With the current results, PARP inhibitors will certainly play an important role in the adjuvant setting in patients with deleterious BRCA1 or BRCA2 mutations at high risk for relapse. Importantly, the side effect profile appears acceptable with no unexpected events and a very low rate of secondary myeloid malignancies.
Subgroup analysis appears to indicate a benefit across all groups including hormone receptor–positive disease and triple negative breast cancer. Interestingly, approximately 25% of patients in both cohorts received platinum-based chemotherapy. The efficacy of adjuvant olaparib did not appear to be impacted by prior use of platinum-containing chemotherapy regimens. It is important to consider that postneoadjuvant capecitabine, per the results of the CREATE-X trial, in triple-negative patients was not permitted in the current study. Although, this has been widely adopted in clinical practice.3 The CREATE-X trial did not specify the benefit of adjuvant capecitabine in the BRCA-mutated cohort, thus, it is not clear how this subgroup fares with this approach. Thus, one cannot extrapolate the relative efficacy of olaparib compared with capecitabine, as pointed out by the authors, and whether we consider the use of capecitabine and/or olaparib in triple-negative patients with residual invasive disease after neoadjuvant chemotherapy is not clear at this time.
Nevertheless, the magnitude of benefit seen in this trial certainly provide clinically relevant and potentially practice changing results. It will be imperative to follow these results as the survival data matures and ensure no further long-term toxicity, particularly secondary myeloid malignancies, develop. These results should be discussed with each patient and informed decisions regarding the use of adjuvant olaparib should be considered for this patient population. Lastly, these results highlight the importance of germline testing for patients with breast cancer in accordance with national guideline recommendations. Moreover, these results certainly call into question whether it is time to consider expansion of our current germline testing guidelines to detect all potential patients who may benefit from this therapy.
Application for Clinical Practice
Adjuvant olaparib in high-risk patients with germline BRCA1 or BRCA2 mutations improves invasive and distant disease-free survival and should be considered in patients who meet the enrollment criteria of the current study. Furthermore, this highlights the importance of appropriate germline genetic testing in patients with breast cancer.
Financial disclosures: None.
Study Overview
Objective. To assess the efficacy and safety of olaparib as an adjuvant treatment in patients with BRCA1 or BRCA2 germline mutations who are at a high-risk for relapse.
Design. A randomized, double-blind, placebo-controlled, multicenter phase III study. The published results are from the prespecified interim analysis.
Intervention. Patients were randomized in 1:1 ratio to either receive 300 mg of olaparib orally twice daily or to receive a matching placebo. Randomization was stratified by hormone receptor status (estrogen receptor and/or progesterone receptor positive/HER2-negative vs triple negative), prior neoadjuvant vs adjuvant chemotherapy, and prior platinum use for breast cancer. Treatment was continued for 52 weeks.
Setting and participants. A total of 1836 patients were randomized in a 1:1 fashion to receive olaparib or a placebo. Eligible patients had a germline BRCA1 or BRCA1 pathogenic or likely pathogenic variant. Patients had high-risk, HER2-negative primary breast cancers and all had received definitive local therapy and neoadjuvant or adjuvant chemotherapy. Patients were enrolled between 2 to 12 weeks after completion of all local therapy. Platinum chemotherapy was allowed. Patients received adjuvant endocrine therapy for hormone receptor positive disease as well as adjuvant bisphosphonates per institutional guidelines. Patients with triple negative disease who received adjuvant chemotherapy were required to be lymph node positive or have at least 2 cm invasive disease. Patients who received neoadjuvant chemotherapy were required to have residual invasive disease to be eligible. For hormone receptor positive patients receiving adjuvant chemotherapy to be eligible they had to have at least 4 pathologically confirmed lymph nodes involved. Hormone receptor positive patients who had neoadjuvant chemotherapy were required to have had residual invasive disease.
Main outcome measures. The primary endpoint for the study was invasive disease-free survival which was defined as time from randomization to date of recurrence or death from any cause. The secondary endpoints included overall survival (OS), distant disease-free survival, safety, and tolerability of olaparib.
Main results. At the time of data cutoff, 284 events had occurred with a median follow-up of 2.5 years in the intention to treat population. A total of 81% of patients had triple negative breast cancer. Most patients (94% in the olaparib group and 92% in the placebo group) received both taxane and anthracycline based chemotherapy regimens. Platinum based chemotherapy was used in 26% of patients in each group. The groups were otherwise well balanced. Germline mutations in BRCA1 were present in 72% of patients and BRCA2 in 27% of patients. These were balanced between groups.
At the time of this analysis, adjuvant olaparib reduced the risk of invasive disease-free survival by 42% compared with placebo (P < .001). At 3 years, invasive disease-free survival was 85.9% in the olaparib group and 77.1% in the placebo group (difference, 8.8 percentage points; 95% CI, 4.5-13.0; hazard ratio [HR], 0.58; 99.5% CI, 0.41-0.82; P < .001). The 3-year distant disease-free survival was 87.5% in the olaparib group and 80.4% in the placebo group (HR 0.57; 99.5% CI, 0.39-0.83; P < .001). Results also showed that olaparib was associated with fewer deaths than placebo (59 and 86, respectively) (HR, 0.68; 99% CI, 0.44-1.05; P = .02); however, there was no significant difference between treatment arms at the time of this interim analysis. Subgroup analysis showed a consistent benefit across all groups with no difference noted regarding BRCA mutation, hormone receptor status or use of neoadjuvant vs adjuvant chemotherapy.
The side effects were consistent with the safety profile of olaparib. Adverse events of grade 3 or higher more common with olaparib included anemia (8.7%), leukopenia (3%), and fatigue (1.8%). Early discontinuation of trial regimen due to adverse events of disease recurrence occurred in 25.9% in the olaparib group and 20.7% in the placebo group. Blood transfusions were required in 5.8% of patients in the olaparib group. Myelodysplasia or acute myleoid leukemia was observed in 2 patients in the olaparib group and 3 patients in the placebo group. Adverse events leading to death occurred in 1 patient in the olaparib group and 2 patients in the placebo group.
Conclusion. Among patients with high-risk, HER2-negative early breast cancer and germline BRCA1 or BRCA2 pathogenic or likely pathogenic variants, adjuvant olaparib after completion of local treatment and neoadjuvant or adjuvant chemotherapy was associated with significantly longer invasive disease-free and distant disease-free survival compared with placebo.
Commentary
The results from the current OlympiA trial provide the first evidence that adjuvant therapy with poly adenosine diphosphate-ribose polymerase (PARP) inhibitors can improve outcomes in high-risk, HER2-negative breast cancer in patients with pathogenic BRCA1 and BRCA2 mutations. The OS, while favoring olaparib, is not yet mature at the time of this analysis. Nevertheless, these results represent an important step forward in improving outcomes in this patient population. The efficacy and safety of PARP inhibitors in BRCA-mutated breast cancer has previously been shown in patients with advanced disease leading to FDA approval of both olaparib and talazoparib in this setting.1,2 With the current results, PARP inhibitors will certainly play an important role in the adjuvant setting in patients with deleterious BRCA1 or BRCA2 mutations at high risk for relapse. Importantly, the side effect profile appears acceptable with no unexpected events and a very low rate of secondary myeloid malignancies.
Subgroup analysis appears to indicate a benefit across all groups including hormone receptor–positive disease and triple negative breast cancer. Interestingly, approximately 25% of patients in both cohorts received platinum-based chemotherapy. The efficacy of adjuvant olaparib did not appear to be impacted by prior use of platinum-containing chemotherapy regimens. It is important to consider that postneoadjuvant capecitabine, per the results of the CREATE-X trial, in triple-negative patients was not permitted in the current study. Although, this has been widely adopted in clinical practice.3 The CREATE-X trial did not specify the benefit of adjuvant capecitabine in the BRCA-mutated cohort, thus, it is not clear how this subgroup fares with this approach. Thus, one cannot extrapolate the relative efficacy of olaparib compared with capecitabine, as pointed out by the authors, and whether we consider the use of capecitabine and/or olaparib in triple-negative patients with residual invasive disease after neoadjuvant chemotherapy is not clear at this time.
Nevertheless, the magnitude of benefit seen in this trial certainly provide clinically relevant and potentially practice changing results. It will be imperative to follow these results as the survival data matures and ensure no further long-term toxicity, particularly secondary myeloid malignancies, develop. These results should be discussed with each patient and informed decisions regarding the use of adjuvant olaparib should be considered for this patient population. Lastly, these results highlight the importance of germline testing for patients with breast cancer in accordance with national guideline recommendations. Moreover, these results certainly call into question whether it is time to consider expansion of our current germline testing guidelines to detect all potential patients who may benefit from this therapy.
Application for Clinical Practice
Adjuvant olaparib in high-risk patients with germline BRCA1 or BRCA2 mutations improves invasive and distant disease-free survival and should be considered in patients who meet the enrollment criteria of the current study. Furthermore, this highlights the importance of appropriate germline genetic testing in patients with breast cancer.
Financial disclosures: None.
1. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi:10.1056/NEJMoa1706450
2. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753-763. doi:10.1056/NEJMoa1802905
3. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
1. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi:10.1056/NEJMoa1706450
2. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018;379(8):753-763. doi:10.1056/NEJMoa1802905
3. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
Nivolumab Plus Cabozantinib Improves Outcomes Compared With Sunitinib for Advanced Renal Cell Carcinoma
Study Overview
Objective. To evaluate the efficacy and safety of the combination of nivolumab plus cabozantinib as compared with sunitinib monotherapy in the treatment of previously untreated advanced renal cell carcinoma (RCC).
Design. Multicenter, international, open-label, randomized, phase 3 trial.
Intervention. Patients were randomized in a 1:1 fashion to 1 of 2 treatment arms:
- Arm A: Nivolumab intravenously 240 mg every 2 weeks plus cabozantinib orally 40 mg once daily.
- Arm B: Sunitinib orally 50 mg daily for 4 weeks, followed by 2 weeks off therapy (6-week cycle).
Randomization was stratified by the International Metastatic RCC Database Consortium prognostic risk score (low-, intermediate-, and high-risk). Treatment was continued until disease progression or development of unacceptable toxic side effects with a maximum of 2-year duration of Nivolumab therapy.
Settings and participants. Adults with previously untreated advanced RCC with a clear cell component were eligible for enrollment. Subjects were excluded if they had active central nervous system metastases or active autoimmune disease.
Main outcome measures. The primary outcome of this study was progression-free survival (PFS) as assessed by an independent review committee. Secondary endpoints included overall survival, objective response rate, safety, and PFS as assessed by investigators. All subgroup analyses were prespecified. Efficacy was assessed in the intention-to-treat population, including all patients who underwent randomization.
Main results. A total of 651 patients underwent randomization: 323 to the nivolumab plus cabozantinib group, and 328 to the sunitinib group. Baseline demographics were balanced. The median follow-up period for overall survival (OS) was 18.1 months. The primary reason for treatment discontinuation in any group was disease progression. PFS as indicated by an independent review committee was significantly longer in the nivolumab plus cabozantinib group compared to the sunitinib group (median 16.6 months vs 8.2 months; hazard ratio [HR] 0.51, P < .001). The median OS was not reached for any group. Overall survival was longer in the nivolumab plus cabozantinib group compared to the sunitinib group (HR 0.60, 95% CI: 0.40-0.89; P = .001). The objective response rate was 55.7% with the nivolumab plus cabozantinib group versus 27.1% with sunitinib (P < .001). The complete response rate was 8% in the nivolumab plus cabozantinib group compared to 4.6% in the sunitinib group. The median time to response was 2.8 months with nivolumab plus cabozantinib and 4.2 months in the sunitinib group, while the median duration of response was 20.2 months and 11.5 months, respectively.
Nearly all patients (about 99% in each group) had an adverse event (AE). Hypertension was the most common side effect, with grade 3 or higher seen in 12.5% in the nivolumab plus cabzantinib group and 13.1% in the sunitinib group. Other grade 3 or higher side effects occurring in at least 10% of patients in any group were hyponatremia, diarrhea, palmar-plantar erythrodysesthesia, hypothyroidism, and fatigue. AEs of any cause leading to discontinuation of the therapy occurred in 19.7% in the nivolumab plus cabzantinib group vs 16.9% of the sunitinib group. One death was considered to be treatment-related (small intestinal perforation) in the nivolumab plus cabozantinib group vs 2 treatment-related deaths with sunitinib (pneumonia and respiratory distress). In the nivolumab plus cabozantinib group, 57% of the patients had a dose reduction of cabozantinib and 52% had a reduction in sunitinib dosage.
Using the Functional Assessment of Cancer Therapy-Kidney Symptoms Index, patients in the nivolumab plus cabozantinib group reported better health-related quality of life and less disease-related symptoms compared to the sunitinib group.
Commentary
The treatment landscape for frontline therapy for patients with advanced RCC has rapidly expanded over the last several years and has revolutionized cancer care. Ushered in by the results from the CheckMate 214 study highlighting the efficacy of dual checkpoint inhibition with nivolumab and ipilimumab in intermediate and poor risk patients, several subsequent trials have demonstrated improved outcomes with combination therapy with immune checkpoint inhibitors and tyrosine-kinase inhibitors (TKI). To date, data from Keynote-426 (pembrolizumab plus axitinib vs sunitinib), Javelin Renal 101 (avelumab plus axitinib vs sunitinib) and the CLEAR trial (lenvatinib plus pembrolizumab vs levatinib plus everolimus vs sunitinib) have demonstrated superiority of immune checkpoint inhibitor/TKI combinations over sunitinb in the first-line setting.1-5
The current phase 3, CheckMate 9ER trial adds yet another dynamic option for patients with advanced clear cell RCC. While cross-trial comparisons are fraught with important caveats, the median PFS of almost 16.6 months and complete response rate of 8% the nivolumab plus cabozantinib group compares favorably with other combinations. Data from the CLEAR study with the combination of lenvatinib and pembrolizumab showed a complete response rate approaching 16%. Importantly, the current study highlights improved quality of life with the combination of cabozantinib and nivolumab compared to sunitinib alone adding to the efficacy and benefits of this combination treatment.
The selection of first line therapy for patients with advanced RCC should be always guided by individual patient characteristics, and any single immune checkpoint inhibitor/TKI combination is not “superior” to any other. Perhaps more importantly is developing an understanding of the overlapping toxicity profiles of checkpoint inhibitors and TKIs. Again, this trial results are consistent with prior studies in terms of the adverse event profile which were not trivial, and almost all patients (99%) experienced AEs. It is important for oncologists to understand the management of the toxicities with these combinations and dose reductions as appropriate. It is worth noting that 19% of patients with nivolumab plus cabozantinib received glucocorticoids for management of immune-related AEs.
While long-term follow-up data will be needed to further understand the durability of response to this combination, nivolumab-cabozantinib represents an exciting new option for patients with advanced clear cell RCC. As we continue to see improvement in outcomes in clear cell histology, further work must focus on optimization of therapy in non-clear cell RCC as this is a population that is not represented in these data sets. Furthermore, future efforts should begin to explore triplet combinations and biomarker driven patient selection for upfront therapy in ordercontinue to improve outcomes in patients with advanced RCC.
Applications for Clinical Practice
The combination of nivolumab plus cabozantinib adds to the growing list of highly active checkpoint inhibitor/TKI combinations for first-line treatment of advanced RCC. With significant higher response rates, improved outcomes, and improvement in the quality of life, this combination will add another standard treatment option for patients with previously untreated advanced RCC.
1. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14)1277-1290. doi:10.1056/NEJMoa1712126
2. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi:10.1056/NEJMoa1816714
3. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573. doi:10.1016/S1470-2045(20)30436-8
4. Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31:1030-1039. doi:10.1016/j.annonc.2020.04.010
5, Motzer R, Alekseev B, Rha SY, et al. CLEAR Trial Investigators. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
Study Overview
Objective. To evaluate the efficacy and safety of the combination of nivolumab plus cabozantinib as compared with sunitinib monotherapy in the treatment of previously untreated advanced renal cell carcinoma (RCC).
Design. Multicenter, international, open-label, randomized, phase 3 trial.
Intervention. Patients were randomized in a 1:1 fashion to 1 of 2 treatment arms:
- Arm A: Nivolumab intravenously 240 mg every 2 weeks plus cabozantinib orally 40 mg once daily.
- Arm B: Sunitinib orally 50 mg daily for 4 weeks, followed by 2 weeks off therapy (6-week cycle).
Randomization was stratified by the International Metastatic RCC Database Consortium prognostic risk score (low-, intermediate-, and high-risk). Treatment was continued until disease progression or development of unacceptable toxic side effects with a maximum of 2-year duration of Nivolumab therapy.
Settings and participants. Adults with previously untreated advanced RCC with a clear cell component were eligible for enrollment. Subjects were excluded if they had active central nervous system metastases or active autoimmune disease.
Main outcome measures. The primary outcome of this study was progression-free survival (PFS) as assessed by an independent review committee. Secondary endpoints included overall survival, objective response rate, safety, and PFS as assessed by investigators. All subgroup analyses were prespecified. Efficacy was assessed in the intention-to-treat population, including all patients who underwent randomization.
Main results. A total of 651 patients underwent randomization: 323 to the nivolumab plus cabozantinib group, and 328 to the sunitinib group. Baseline demographics were balanced. The median follow-up period for overall survival (OS) was 18.1 months. The primary reason for treatment discontinuation in any group was disease progression. PFS as indicated by an independent review committee was significantly longer in the nivolumab plus cabozantinib group compared to the sunitinib group (median 16.6 months vs 8.2 months; hazard ratio [HR] 0.51, P < .001). The median OS was not reached for any group. Overall survival was longer in the nivolumab plus cabozantinib group compared to the sunitinib group (HR 0.60, 95% CI: 0.40-0.89; P = .001). The objective response rate was 55.7% with the nivolumab plus cabozantinib group versus 27.1% with sunitinib (P < .001). The complete response rate was 8% in the nivolumab plus cabozantinib group compared to 4.6% in the sunitinib group. The median time to response was 2.8 months with nivolumab plus cabozantinib and 4.2 months in the sunitinib group, while the median duration of response was 20.2 months and 11.5 months, respectively.
Nearly all patients (about 99% in each group) had an adverse event (AE). Hypertension was the most common side effect, with grade 3 or higher seen in 12.5% in the nivolumab plus cabzantinib group and 13.1% in the sunitinib group. Other grade 3 or higher side effects occurring in at least 10% of patients in any group were hyponatremia, diarrhea, palmar-plantar erythrodysesthesia, hypothyroidism, and fatigue. AEs of any cause leading to discontinuation of the therapy occurred in 19.7% in the nivolumab plus cabzantinib group vs 16.9% of the sunitinib group. One death was considered to be treatment-related (small intestinal perforation) in the nivolumab plus cabozantinib group vs 2 treatment-related deaths with sunitinib (pneumonia and respiratory distress). In the nivolumab plus cabozantinib group, 57% of the patients had a dose reduction of cabozantinib and 52% had a reduction in sunitinib dosage.
Using the Functional Assessment of Cancer Therapy-Kidney Symptoms Index, patients in the nivolumab plus cabozantinib group reported better health-related quality of life and less disease-related symptoms compared to the sunitinib group.
Commentary
The treatment landscape for frontline therapy for patients with advanced RCC has rapidly expanded over the last several years and has revolutionized cancer care. Ushered in by the results from the CheckMate 214 study highlighting the efficacy of dual checkpoint inhibition with nivolumab and ipilimumab in intermediate and poor risk patients, several subsequent trials have demonstrated improved outcomes with combination therapy with immune checkpoint inhibitors and tyrosine-kinase inhibitors (TKI). To date, data from Keynote-426 (pembrolizumab plus axitinib vs sunitinib), Javelin Renal 101 (avelumab plus axitinib vs sunitinib) and the CLEAR trial (lenvatinib plus pembrolizumab vs levatinib plus everolimus vs sunitinib) have demonstrated superiority of immune checkpoint inhibitor/TKI combinations over sunitinb in the first-line setting.1-5
The current phase 3, CheckMate 9ER trial adds yet another dynamic option for patients with advanced clear cell RCC. While cross-trial comparisons are fraught with important caveats, the median PFS of almost 16.6 months and complete response rate of 8% the nivolumab plus cabozantinib group compares favorably with other combinations. Data from the CLEAR study with the combination of lenvatinib and pembrolizumab showed a complete response rate approaching 16%. Importantly, the current study highlights improved quality of life with the combination of cabozantinib and nivolumab compared to sunitinib alone adding to the efficacy and benefits of this combination treatment.
The selection of first line therapy for patients with advanced RCC should be always guided by individual patient characteristics, and any single immune checkpoint inhibitor/TKI combination is not “superior” to any other. Perhaps more importantly is developing an understanding of the overlapping toxicity profiles of checkpoint inhibitors and TKIs. Again, this trial results are consistent with prior studies in terms of the adverse event profile which were not trivial, and almost all patients (99%) experienced AEs. It is important for oncologists to understand the management of the toxicities with these combinations and dose reductions as appropriate. It is worth noting that 19% of patients with nivolumab plus cabozantinib received glucocorticoids for management of immune-related AEs.
While long-term follow-up data will be needed to further understand the durability of response to this combination, nivolumab-cabozantinib represents an exciting new option for patients with advanced clear cell RCC. As we continue to see improvement in outcomes in clear cell histology, further work must focus on optimization of therapy in non-clear cell RCC as this is a population that is not represented in these data sets. Furthermore, future efforts should begin to explore triplet combinations and biomarker driven patient selection for upfront therapy in ordercontinue to improve outcomes in patients with advanced RCC.
Applications for Clinical Practice
The combination of nivolumab plus cabozantinib adds to the growing list of highly active checkpoint inhibitor/TKI combinations for first-line treatment of advanced RCC. With significant higher response rates, improved outcomes, and improvement in the quality of life, this combination will add another standard treatment option for patients with previously untreated advanced RCC.
Study Overview
Objective. To evaluate the efficacy and safety of the combination of nivolumab plus cabozantinib as compared with sunitinib monotherapy in the treatment of previously untreated advanced renal cell carcinoma (RCC).
Design. Multicenter, international, open-label, randomized, phase 3 trial.
Intervention. Patients were randomized in a 1:1 fashion to 1 of 2 treatment arms:
- Arm A: Nivolumab intravenously 240 mg every 2 weeks plus cabozantinib orally 40 mg once daily.
- Arm B: Sunitinib orally 50 mg daily for 4 weeks, followed by 2 weeks off therapy (6-week cycle).
Randomization was stratified by the International Metastatic RCC Database Consortium prognostic risk score (low-, intermediate-, and high-risk). Treatment was continued until disease progression or development of unacceptable toxic side effects with a maximum of 2-year duration of Nivolumab therapy.
Settings and participants. Adults with previously untreated advanced RCC with a clear cell component were eligible for enrollment. Subjects were excluded if they had active central nervous system metastases or active autoimmune disease.
Main outcome measures. The primary outcome of this study was progression-free survival (PFS) as assessed by an independent review committee. Secondary endpoints included overall survival, objective response rate, safety, and PFS as assessed by investigators. All subgroup analyses were prespecified. Efficacy was assessed in the intention-to-treat population, including all patients who underwent randomization.
Main results. A total of 651 patients underwent randomization: 323 to the nivolumab plus cabozantinib group, and 328 to the sunitinib group. Baseline demographics were balanced. The median follow-up period for overall survival (OS) was 18.1 months. The primary reason for treatment discontinuation in any group was disease progression. PFS as indicated by an independent review committee was significantly longer in the nivolumab plus cabozantinib group compared to the sunitinib group (median 16.6 months vs 8.2 months; hazard ratio [HR] 0.51, P < .001). The median OS was not reached for any group. Overall survival was longer in the nivolumab plus cabozantinib group compared to the sunitinib group (HR 0.60, 95% CI: 0.40-0.89; P = .001). The objective response rate was 55.7% with the nivolumab plus cabozantinib group versus 27.1% with sunitinib (P < .001). The complete response rate was 8% in the nivolumab plus cabozantinib group compared to 4.6% in the sunitinib group. The median time to response was 2.8 months with nivolumab plus cabozantinib and 4.2 months in the sunitinib group, while the median duration of response was 20.2 months and 11.5 months, respectively.
Nearly all patients (about 99% in each group) had an adverse event (AE). Hypertension was the most common side effect, with grade 3 or higher seen in 12.5% in the nivolumab plus cabzantinib group and 13.1% in the sunitinib group. Other grade 3 or higher side effects occurring in at least 10% of patients in any group were hyponatremia, diarrhea, palmar-plantar erythrodysesthesia, hypothyroidism, and fatigue. AEs of any cause leading to discontinuation of the therapy occurred in 19.7% in the nivolumab plus cabzantinib group vs 16.9% of the sunitinib group. One death was considered to be treatment-related (small intestinal perforation) in the nivolumab plus cabozantinib group vs 2 treatment-related deaths with sunitinib (pneumonia and respiratory distress). In the nivolumab plus cabozantinib group, 57% of the patients had a dose reduction of cabozantinib and 52% had a reduction in sunitinib dosage.
Using the Functional Assessment of Cancer Therapy-Kidney Symptoms Index, patients in the nivolumab plus cabozantinib group reported better health-related quality of life and less disease-related symptoms compared to the sunitinib group.
Commentary
The treatment landscape for frontline therapy for patients with advanced RCC has rapidly expanded over the last several years and has revolutionized cancer care. Ushered in by the results from the CheckMate 214 study highlighting the efficacy of dual checkpoint inhibition with nivolumab and ipilimumab in intermediate and poor risk patients, several subsequent trials have demonstrated improved outcomes with combination therapy with immune checkpoint inhibitors and tyrosine-kinase inhibitors (TKI). To date, data from Keynote-426 (pembrolizumab plus axitinib vs sunitinib), Javelin Renal 101 (avelumab plus axitinib vs sunitinib) and the CLEAR trial (lenvatinib plus pembrolizumab vs levatinib plus everolimus vs sunitinib) have demonstrated superiority of immune checkpoint inhibitor/TKI combinations over sunitinb in the first-line setting.1-5
The current phase 3, CheckMate 9ER trial adds yet another dynamic option for patients with advanced clear cell RCC. While cross-trial comparisons are fraught with important caveats, the median PFS of almost 16.6 months and complete response rate of 8% the nivolumab plus cabozantinib group compares favorably with other combinations. Data from the CLEAR study with the combination of lenvatinib and pembrolizumab showed a complete response rate approaching 16%. Importantly, the current study highlights improved quality of life with the combination of cabozantinib and nivolumab compared to sunitinib alone adding to the efficacy and benefits of this combination treatment.
The selection of first line therapy for patients with advanced RCC should be always guided by individual patient characteristics, and any single immune checkpoint inhibitor/TKI combination is not “superior” to any other. Perhaps more importantly is developing an understanding of the overlapping toxicity profiles of checkpoint inhibitors and TKIs. Again, this trial results are consistent with prior studies in terms of the adverse event profile which were not trivial, and almost all patients (99%) experienced AEs. It is important for oncologists to understand the management of the toxicities with these combinations and dose reductions as appropriate. It is worth noting that 19% of patients with nivolumab plus cabozantinib received glucocorticoids for management of immune-related AEs.
While long-term follow-up data will be needed to further understand the durability of response to this combination, nivolumab-cabozantinib represents an exciting new option for patients with advanced clear cell RCC. As we continue to see improvement in outcomes in clear cell histology, further work must focus on optimization of therapy in non-clear cell RCC as this is a population that is not represented in these data sets. Furthermore, future efforts should begin to explore triplet combinations and biomarker driven patient selection for upfront therapy in ordercontinue to improve outcomes in patients with advanced RCC.
Applications for Clinical Practice
The combination of nivolumab plus cabozantinib adds to the growing list of highly active checkpoint inhibitor/TKI combinations for first-line treatment of advanced RCC. With significant higher response rates, improved outcomes, and improvement in the quality of life, this combination will add another standard treatment option for patients with previously untreated advanced RCC.
1. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14)1277-1290. doi:10.1056/NEJMoa1712126
2. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi:10.1056/NEJMoa1816714
3. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573. doi:10.1016/S1470-2045(20)30436-8
4. Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31:1030-1039. doi:10.1016/j.annonc.2020.04.010
5, Motzer R, Alekseev B, Rha SY, et al. CLEAR Trial Investigators. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
1. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14)1277-1290. doi:10.1056/NEJMoa1712126
2. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi:10.1056/NEJMoa1816714
3. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573. doi:10.1016/S1470-2045(20)30436-8
4. Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31:1030-1039. doi:10.1016/j.annonc.2020.04.010
5, Motzer R, Alekseev B, Rha SY, et al. CLEAR Trial Investigators. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
Ticagrelor or Clopidogrel in Elective Percutaneous Coronary Intervention
Study Overview
Objective: To assess whether ticagrelor was superior to clopidogrel in reducing periprocedural myocardial necrosis in stable coronary patients undergoing elective percutaneous coronary intervention (PCI).
Design: Multicenter, open-label, and prospective randomized control trial. Setting and participants: A total of 1910 patients with indication for PCI and at least 1 high risk characteristic were randomized to either ticagrelor or clopidogrel.
Main outcome measures: The primary outcome was the composite of PCI-related type 4a or 4b myocardial infarction or major myocardial injury. The primary safety outcome was major bleeding, evaluated within 48 hours of PCI.
Main results: At 48 hours, the primary outcome was observed in 334 of 941 patients (35%) in the ticagrelor group and 341 of 942 patients (36%) in the clopidogrel group (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.17; P = .75). The primary safety outcome did not differ between groups. Minor bleeding events at 30 days were more frequently observed with ticagrelor (11%) than clopidogrel (8%) (1.54; 95% CI 1.12-2.11; P = .007).
Conclusion: Among patients undergoing elective PCI, ticagrelor was not superior to clopidogrel in reducing periprocedural myocardial necrosis. Ticagrelor did not cause increase in major bleeding compared to clopidogrel but did increase the rate of minor bleeding at 30 days.
Commentary
Standard treatment after PCI includes dual antiplatelet therapy combining adenosine diphosphate (ADP) receptor antagonist and aspirin. The newer generation thienopyridine prasugrel and the reversible direct acting oral antagonist of the ADP receptor ticagrelor, provides consistent and greater antiplatelet effect compared to clopidogrel, and are superior in reducing ischemic events when compared to clopidogrel in patients presenting with acute coronary syndrome (ACS).1,2 Therefore, current guidelines recommend ticagrelor and prasugrel in preference to clopidogrel in patients presenting with ACS.3,4 However, whether these findings of improved outcomes with newer agents compared to clopidogrel extends to patients with stable ischemic heart disease presenting for elective PCI is unknown.
In this context, Silvain et al investigated this clinical question and compared ticagrelor and clopidogrel by performing a well-designed multicenter randomized control trial in patients presenting with elective PCI. At 48 hours and at 30 days the composite of PCI-related type 4 myocardial infarction or major myocardial injury defined by the third universal definition5 was similar between the ticagrelor and clopidogrel groups. Although the incidence of major bleeding was not significantly different between the 2 groups, minor bleeding at 30 days was higher in the ticagrelor group (11%) than clopidogrel (8%) (1.54; 95% CI, 1.12-2.11, P = .007).
The strengths of this current study include the randomized design and the large number of patients enrolled with adequate power to evaluate for superiority of ticagrelor compared to clopidogrel. This was a multicenter trial in Europe with 49 participating centers from France and Czech, and the interventional technique used by the operators reflects contemporary technique with 95% use of radial or ulnar access.
There are a few important points to consider in this study. First, the primary outcome was biomarker assessed myocardial necrosis and myocardial injury, and the study was not powered to assess the hard outcomes such as death and myocardial infarction. Although there have been previous reports describing the relationship between the postprocedural myocardial necrosis with worse outcomes, the definition of myocardial necrosis post-PCI and its relationship with hard outcomes remains controversial. Second, half of the patients enrolled were on chronic clopidogrel therapy which suggests that patients with inadequate platelet inhibition with clopidogrel may be under-represented in this cohort. Third, this was an open-label study and the knowledge of agent used could have affected the study results. Finally, whether the population included represents a true high-risk population is questionable. Some of the prespecified high-risk features necessary to enter the study was relatively light, such as presence of diabetes mellitus or body mass index > 30 kg/m2 compared to other criteria such as bifurcation stenting or left main stenting.
Currently, when treating patients with stable ischemic heart disease with higher risk anatomy, some operators may use ticagrelor over clopidogrel by extrapolating the study results from the ACS population. However, the results from the current study do not support the uniform use of ticagrelor in stable patients and suggests that the use of clopidogrel continues to be the standard of care. This is especially relevant considering the cost difference for the 2 agents studied. Whether there is a subgroup that benefits from ticagrelor use, such as patients with unprotected left main stenting or bifurcation stenting with 2 stent strategies, requires further investigation.
Applications for Clinical Practice
In patients presenting with stable ischemic heart disease undergoing elective PCI, ticagrelor did not lower composite of periprocedural myocardial infarction and myocardial injury at 48 hours. Clopidogrel continues to be a first line treatment after elective PCI.
1. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-15.
2. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2009;361(11):1045-57.
3. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-177.
4. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;152(5):12432-1275.
5. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581-98.
Study Overview
Objective: To assess whether ticagrelor was superior to clopidogrel in reducing periprocedural myocardial necrosis in stable coronary patients undergoing elective percutaneous coronary intervention (PCI).
Design: Multicenter, open-label, and prospective randomized control trial. Setting and participants: A total of 1910 patients with indication for PCI and at least 1 high risk characteristic were randomized to either ticagrelor or clopidogrel.
Main outcome measures: The primary outcome was the composite of PCI-related type 4a or 4b myocardial infarction or major myocardial injury. The primary safety outcome was major bleeding, evaluated within 48 hours of PCI.
Main results: At 48 hours, the primary outcome was observed in 334 of 941 patients (35%) in the ticagrelor group and 341 of 942 patients (36%) in the clopidogrel group (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.17; P = .75). The primary safety outcome did not differ between groups. Minor bleeding events at 30 days were more frequently observed with ticagrelor (11%) than clopidogrel (8%) (1.54; 95% CI 1.12-2.11; P = .007).
Conclusion: Among patients undergoing elective PCI, ticagrelor was not superior to clopidogrel in reducing periprocedural myocardial necrosis. Ticagrelor did not cause increase in major bleeding compared to clopidogrel but did increase the rate of minor bleeding at 30 days.
Commentary
Standard treatment after PCI includes dual antiplatelet therapy combining adenosine diphosphate (ADP) receptor antagonist and aspirin. The newer generation thienopyridine prasugrel and the reversible direct acting oral antagonist of the ADP receptor ticagrelor, provides consistent and greater antiplatelet effect compared to clopidogrel, and are superior in reducing ischemic events when compared to clopidogrel in patients presenting with acute coronary syndrome (ACS).1,2 Therefore, current guidelines recommend ticagrelor and prasugrel in preference to clopidogrel in patients presenting with ACS.3,4 However, whether these findings of improved outcomes with newer agents compared to clopidogrel extends to patients with stable ischemic heart disease presenting for elective PCI is unknown.
In this context, Silvain et al investigated this clinical question and compared ticagrelor and clopidogrel by performing a well-designed multicenter randomized control trial in patients presenting with elective PCI. At 48 hours and at 30 days the composite of PCI-related type 4 myocardial infarction or major myocardial injury defined by the third universal definition5 was similar between the ticagrelor and clopidogrel groups. Although the incidence of major bleeding was not significantly different between the 2 groups, minor bleeding at 30 days was higher in the ticagrelor group (11%) than clopidogrel (8%) (1.54; 95% CI, 1.12-2.11, P = .007).
The strengths of this current study include the randomized design and the large number of patients enrolled with adequate power to evaluate for superiority of ticagrelor compared to clopidogrel. This was a multicenter trial in Europe with 49 participating centers from France and Czech, and the interventional technique used by the operators reflects contemporary technique with 95% use of radial or ulnar access.
There are a few important points to consider in this study. First, the primary outcome was biomarker assessed myocardial necrosis and myocardial injury, and the study was not powered to assess the hard outcomes such as death and myocardial infarction. Although there have been previous reports describing the relationship between the postprocedural myocardial necrosis with worse outcomes, the definition of myocardial necrosis post-PCI and its relationship with hard outcomes remains controversial. Second, half of the patients enrolled were on chronic clopidogrel therapy which suggests that patients with inadequate platelet inhibition with clopidogrel may be under-represented in this cohort. Third, this was an open-label study and the knowledge of agent used could have affected the study results. Finally, whether the population included represents a true high-risk population is questionable. Some of the prespecified high-risk features necessary to enter the study was relatively light, such as presence of diabetes mellitus or body mass index > 30 kg/m2 compared to other criteria such as bifurcation stenting or left main stenting.
Currently, when treating patients with stable ischemic heart disease with higher risk anatomy, some operators may use ticagrelor over clopidogrel by extrapolating the study results from the ACS population. However, the results from the current study do not support the uniform use of ticagrelor in stable patients and suggests that the use of clopidogrel continues to be the standard of care. This is especially relevant considering the cost difference for the 2 agents studied. Whether there is a subgroup that benefits from ticagrelor use, such as patients with unprotected left main stenting or bifurcation stenting with 2 stent strategies, requires further investigation.
Applications for Clinical Practice
In patients presenting with stable ischemic heart disease undergoing elective PCI, ticagrelor did not lower composite of periprocedural myocardial infarction and myocardial injury at 48 hours. Clopidogrel continues to be a first line treatment after elective PCI.
Study Overview
Objective: To assess whether ticagrelor was superior to clopidogrel in reducing periprocedural myocardial necrosis in stable coronary patients undergoing elective percutaneous coronary intervention (PCI).
Design: Multicenter, open-label, and prospective randomized control trial. Setting and participants: A total of 1910 patients with indication for PCI and at least 1 high risk characteristic were randomized to either ticagrelor or clopidogrel.
Main outcome measures: The primary outcome was the composite of PCI-related type 4a or 4b myocardial infarction or major myocardial injury. The primary safety outcome was major bleeding, evaluated within 48 hours of PCI.
Main results: At 48 hours, the primary outcome was observed in 334 of 941 patients (35%) in the ticagrelor group and 341 of 942 patients (36%) in the clopidogrel group (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.17; P = .75). The primary safety outcome did not differ between groups. Minor bleeding events at 30 days were more frequently observed with ticagrelor (11%) than clopidogrel (8%) (1.54; 95% CI 1.12-2.11; P = .007).
Conclusion: Among patients undergoing elective PCI, ticagrelor was not superior to clopidogrel in reducing periprocedural myocardial necrosis. Ticagrelor did not cause increase in major bleeding compared to clopidogrel but did increase the rate of minor bleeding at 30 days.
Commentary
Standard treatment after PCI includes dual antiplatelet therapy combining adenosine diphosphate (ADP) receptor antagonist and aspirin. The newer generation thienopyridine prasugrel and the reversible direct acting oral antagonist of the ADP receptor ticagrelor, provides consistent and greater antiplatelet effect compared to clopidogrel, and are superior in reducing ischemic events when compared to clopidogrel in patients presenting with acute coronary syndrome (ACS).1,2 Therefore, current guidelines recommend ticagrelor and prasugrel in preference to clopidogrel in patients presenting with ACS.3,4 However, whether these findings of improved outcomes with newer agents compared to clopidogrel extends to patients with stable ischemic heart disease presenting for elective PCI is unknown.
In this context, Silvain et al investigated this clinical question and compared ticagrelor and clopidogrel by performing a well-designed multicenter randomized control trial in patients presenting with elective PCI. At 48 hours and at 30 days the composite of PCI-related type 4 myocardial infarction or major myocardial injury defined by the third universal definition5 was similar between the ticagrelor and clopidogrel groups. Although the incidence of major bleeding was not significantly different between the 2 groups, minor bleeding at 30 days was higher in the ticagrelor group (11%) than clopidogrel (8%) (1.54; 95% CI, 1.12-2.11, P = .007).
The strengths of this current study include the randomized design and the large number of patients enrolled with adequate power to evaluate for superiority of ticagrelor compared to clopidogrel. This was a multicenter trial in Europe with 49 participating centers from France and Czech, and the interventional technique used by the operators reflects contemporary technique with 95% use of radial or ulnar access.
There are a few important points to consider in this study. First, the primary outcome was biomarker assessed myocardial necrosis and myocardial injury, and the study was not powered to assess the hard outcomes such as death and myocardial infarction. Although there have been previous reports describing the relationship between the postprocedural myocardial necrosis with worse outcomes, the definition of myocardial necrosis post-PCI and its relationship with hard outcomes remains controversial. Second, half of the patients enrolled were on chronic clopidogrel therapy which suggests that patients with inadequate platelet inhibition with clopidogrel may be under-represented in this cohort. Third, this was an open-label study and the knowledge of agent used could have affected the study results. Finally, whether the population included represents a true high-risk population is questionable. Some of the prespecified high-risk features necessary to enter the study was relatively light, such as presence of diabetes mellitus or body mass index > 30 kg/m2 compared to other criteria such as bifurcation stenting or left main stenting.
Currently, when treating patients with stable ischemic heart disease with higher risk anatomy, some operators may use ticagrelor over clopidogrel by extrapolating the study results from the ACS population. However, the results from the current study do not support the uniform use of ticagrelor in stable patients and suggests that the use of clopidogrel continues to be the standard of care. This is especially relevant considering the cost difference for the 2 agents studied. Whether there is a subgroup that benefits from ticagrelor use, such as patients with unprotected left main stenting or bifurcation stenting with 2 stent strategies, requires further investigation.
Applications for Clinical Practice
In patients presenting with stable ischemic heart disease undergoing elective PCI, ticagrelor did not lower composite of periprocedural myocardial infarction and myocardial injury at 48 hours. Clopidogrel continues to be a first line treatment after elective PCI.
1. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-15.
2. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2009;361(11):1045-57.
3. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-177.
4. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;152(5):12432-1275.
5. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581-98.
1. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-15.
2. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2009;361(11):1045-57.
3. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-177.
4. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;152(5):12432-1275.
5. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581-98.