User login
Analysis supports CAC for personalizing statin use
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
In patients with intermediate risk of atherosclerotic cardiovascular disease along with risk-enhancing factors, coronary artery calcium scoring may help more precisely calculate their need for statin therapy.
Furthermore, when the need for statin treatment isn’t so clear and patients need additional risk assessment, the scoring can provide further information to personalize clinical decision making, according to a cross-sectional study of 1,688 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) published in JAMA Cardiology.
And regardless of coronary artery calcium (CAC), a low ankle brachial index (ABI) score is a marker for statin therapy, the study found.
The study looked at CAC scoring in the context of ABI and other risk-enhancing factors identified in the 2018 American Heart Association/American College of Cardiology cholesterol management guidelines: a family history of premature atherosclerotic cardiovascular disease (ASCVD), lipid and inflammatory biomarkers, chronic kidney disease, chronic inflammatory conditions, premature menopause or preeclampsia, and South Asian ancestry.
Any number of these factors can indicate the need for statins in people with borderline or intermediate risk. The guidelines also call for selective use of CAC to aid the decision-making process for statin therapy when the risk for developing atherosclerosis isn’t so clear.
“The novel risk-enhancing factors are not perfect,” said lead author Jaideep Patel, MD, director of preventive cardiology at Johns Hopkins Heart Center at Greater Baltimore Medical Center. He noted that the 2018 dyslipidemia guidelines suggested the risk for cardiovascular events rises when new risk-enhancing factors emerge, and that it was difficult to predict the extent to which each enhancer could change the 10-year risk.
Utility of CAC
“In this setting, the most significant finding that supports the utility of CAC scoring is when CAC is absent – a CAC of 0 – even in the setting of any of these enhancers, whether it be single or multiple, the 10-year risk remains extremely low – at the very least below the accepted threshold to initiate statin therapy,” Dr. Patel said.
That threshold is below the 7.5% 10-year ASCVD incidence rate. Over the 12-year mean study follow-up, the ASCVD incidence rate among patients with a CAC score of 0 for all risk-enhancing factors was 7.5 events per 1,000 person years, with one exception: ABI had an incidence rate of 10.4 events per 1,000 person years. “A low ABI score should trigger statin initiation irrespective of CAC score,” Dr. Patel said.
The study found a CAC score of 0 in 45.7% of those with one or two risk-enhancing factors versus 40.3% in those with three or more. “Across all the risk enhancers (except low ABI), the prevalence of CAC of 0 was greater than 50% in women; that is, enhancers overestimate risk,” Dr. Patel said. “The prevalence of CAC of 0 was approximately 40% across all risk enhancers; that is, enhancers overestimate risk.”
Dr. Patel said previous studies have suggested the risk of a major cardiovascular event was almost identical for statin and nonstatin users with a CAC score of 0. “If there is uncertainty about statin use after the physician-patient risk discussion,” he said, “CAC scoring may be helpful to guide the use of statin therapy.”
Senior author Mahmoud Al Rifai, MD, MPH, added: “For example, if CAC was absent, a statin could be deprescribed if there’s disutility on the part of the patient, with ongoing lifestyle and risk factor modification efforts.” Dr. Al Rifai is a cardiology fellow at Baylor College of Medicine, Houston.
Dr. Patel said: “Alternatively, if CAC was present, then it would be prudent to continue statin therapy.”
While South Asian ethnicity is a risk enhancing factor, the investigators acknowledged that MESA didn’t recruit this population group.
Study confirms guidelines
The study “supports the contention of the [AHA/ACC] guidelines that, in people who are in this intermediate risk range, there may be factors that either favor statin treatment or suggest that statin treatment could be deferred,” said Neil J. Stone, MD, of Northwestern University, Chicago, and author of the 2013 ASCVD risk calculator. “The guidelines pointed out that risk-enhancing factors may be associated with an increase in lifetime risk, not necessarily short term, and so could inform a more personalized risk discussion.”
The study findings validate the utility of CAC for guiding statin therapy, Dr. Stone said. “For those who have felt that a calcium score is not useful,” he said, “this is additional evidence to show that, in the context of making a decision in those at intermediate risk as proposed by the guidelines, a calcium score is indeed very useful.”
Dr. Stone added: “An important clinical point not mentioned by the authors is that, when the patient has a CAC score of 0 and risk factors, this may be exactly the time to be aggressive with lifestyle to prevent them from developing a positive CAC score and atherosclerosis, because once atherosclerosis is present, treatment may not restore the risk back to the original lower state.”
Dr. Patel, Dr. Al Rifai, and Dr. Stone have no relevant relationships to disclose. A number of study coauthors disclosed multiple financial relationships.
FROM JAMA CARDIOLOGY
How to proceed when it comes to vitamin D
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.
What should the family physician do?
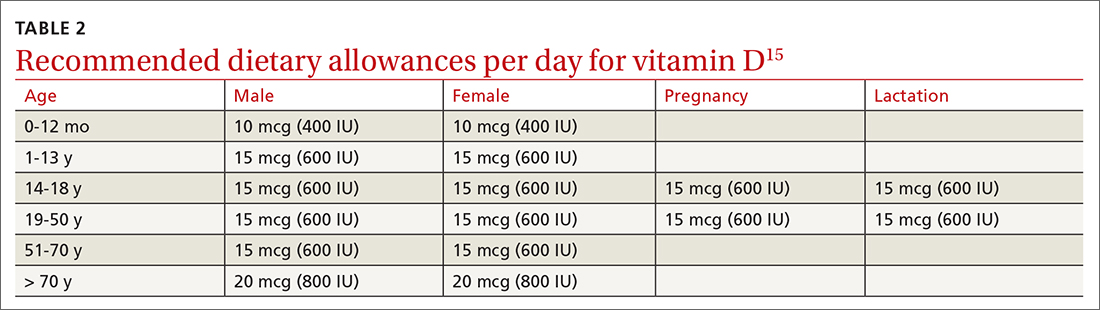
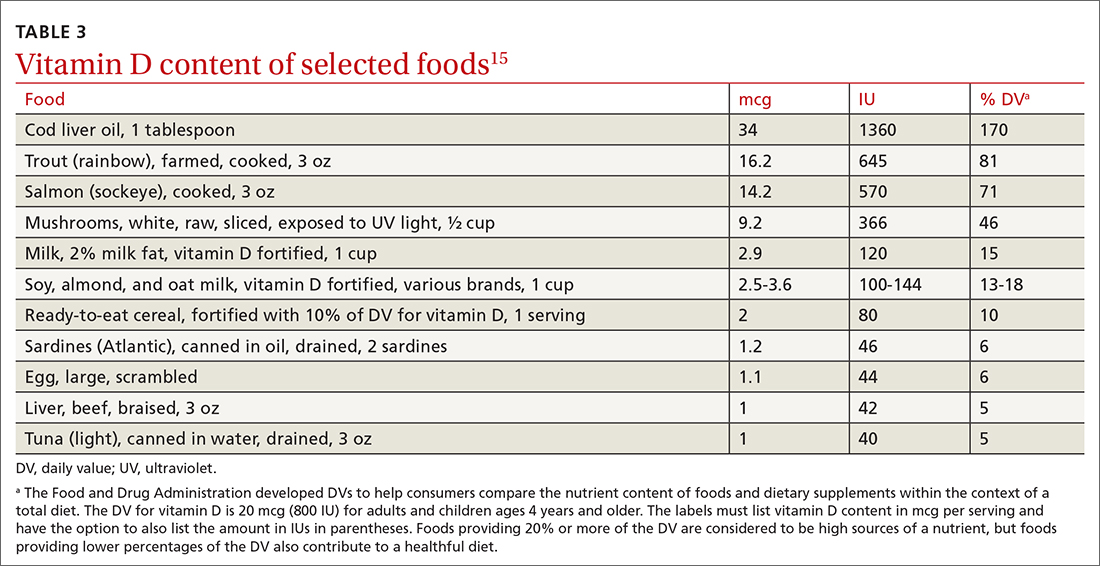
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15
Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.

What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15

Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.

What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15

Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
‘I did nothing wrong’: MDs used their own sperm for fertility patients
Martin D. Greenberg, MD, was sued in May for secretly using his own sperm to inseminate one of his infertility patients 38 years earlier. The patient’s daughter found out last year when she used a DNA test from 23andme to learn about her family history. The 77-year-old New York gynecologist is retired in Florida.
All but one of the cases took place before 1990. Most of them came to light in the past few years, when biological offspring found out from home DNA tests.
“It is a gross betrayal of the trust that a patient puts in her doctor. It is an absolute perversion of the practice of medicine,” said Dev Sethi, a plaintiff attorney who sued a Tucson, Ariz., physician who inseminated at least 10 patients with his own sperm. “The hubris of a doctor to impregnate his own patient, in some effort to either save money or populate the world with his offspring, is striking.”
Why would these physicians use their own sperm and then keep it secret? Why were there so many of them? When their offspring now try to communicate with them, do they want to have a relationship? And how do they react when they’re found out?
The doctors’ behavior mystifies Sigal Klipstein, MD, a reproductive endocrinologist in Hoffman Estates, Ill., who is chair of the ethics committee of the American Society for Reproductive Medicine.
“These doctors lived with secrets for many years. How do you live with that as a doctor?” said Dr. Klipstein, who was still in high school when most of these cases occurred. “It surprises me that anybody would do this.”
Lack of training and lots of secrecy
Were these physicians particularly selfish or egotistical? Or was expedience the prime motivation?
At the time, there was little training in the techniques and ethics of infertility care, said Jody Madeira, JD, PhD, a law professor at Indiana University, Bloomington, who has closely studied the doctors.
“Many of them were ob.gyns., but they did not take CME courses for this work,” she said. The subspecialty of reproductive endocrinology and infertility was just beginning in the early 1970s, according an ASRM spokesman.
Treatment of infertility was a rather hush-hush topic at that time, which made it easier to be deceptive. In 1955, an Illinois court held that artificial insemination constituted adultery. “The social stigma resulting from the practice forces the parents to keep secret the infant’s origin,” a law review article from 1955 stated.
“In the 1950s and 1960s and even into the 1970s and 1980s, infertility treatments were considered shameful, and patients were often advised to keep their treatment to themselves,” Dr. Madeira said. “With everything so secret, it was easy to be deceptive.”
The field has become more sophisticated since then, Dr. Klipstein said. “For known donors, there is a legal contract between the recipient and donor. And it is no longer possible to be an anonymous donor. People can find you through DNA tests.”
Owing to changes in the field as well as the growing likelihood of being caught through DNA tests, most experts believe that rampant infertility fraud ended long ago.
How they were found out
When the doctors were active, there was little risk of being exposed. In those times, paternity tests were based on broad factors such as blood type and were unreliable. More accurate DNA tests were underway, but the doctors’ offspring did not think of using them because they suspected nothing.
Most of the doctors’ deeds only came to light with the rise of a new industry – home DNA testing for people who are curious about their family background. First came 23andme in 2007, then Ancestry.com in 2015. The number of people being tested reached almost 2 million in 2016, 7 million in 2017, and 30 million in 2020.
As more people entered company databases, it became easier to pinpoint biological fathers through other relatives. This explains how doctors who had not taken a home DNA test were identified.
The home tests have been shown to be highly accurate. None of the results for doctors accused of using their own sperm have proven to be false, and courts recognize similar DNA tests as proof of paternity.
But when found out, many of the physicians disputed the results and acted as if they could still keep their secret. “I don’t deny it; I don’t admit it,” Paul Brennan Jones, MD, a Colorado doctor, said when he was accused of siring eight children through his infertility patients decades before. Asked whether he would provide a DNA sample, the 80-year-old doctor responded: “No ... because I don’t want to have any incriminating evidence against me.”
How often did it happen?
Donor Deceived, a website that monitors these cases, reports 32 cases of physicians surreptitiously providing sperm to their patients. Eleven of the doctors are linked to 1 known offspring, two are linked to more than 75 offspring, one to 15, one to 10, three to 9, three to 7, and two to 5.
“It’s unlikely that any of the doctors did it just once,” said Adam B. Wolf, a San Francisco attorney who is representing the plaintiff in the Greenberg case. “It’s happened before. When doctors get the idea to do something crazy, they do it multiple times.”
Mr. Wolf believes that, because most people haven’t taken a DNA test, there are many more biological children of infertility doctors who have yet to come forward.
Many of the doctors who were found out have negotiated settlements with patients, under which they pay undisclosed sums of money in exchange for the patient’s keeping silent. Mr. Wolf said that, of the two dozen victims of sperm-donor doctors his law firm has represented, all but three have settled.
“We give an opportunity to the doctor to resolve the claims without having to publicly out this person for using his own sperm in his patients,” Mr. Wolf said. “Most doctors jump at the opportunity to not be known as the kind of person who would do that.”
Cases about to go to trial have been withdrawn because of being settled. In May, a case against Gerald E. Mortimer, MD, in Idaho, was dismissed after 3 years of litigation. The judge had made some key decisions that made it less likely that Dr. Mortimer would win. Dr. Mortimer’s biological daughter filed the initial case. She alleged medical negligence, failure to obtain informed consent, fraud, battery, intentional infliction of emotional distress, and several other causes of action.
Dr. Madeira objects to the use of confidential settlements, because other offspring cannot be alerted. But she also believes that, as more people find out about their parentage through DNA tests, it will be harder for accused doctors to make confidential settlements with all of them, and the doctors will eventually be identified.
In settlements, offspring ask for the medical histories of these doctors. So far, offspring have linked the development of Tay-Sachs disease, cystic fibrosis, and ovarian cancer with these doctors.
Denial: Physicians’ most frequent reaction
Once identified, most of the doctors denied the charge. When Gary Phillip Wood, MD, of Arkansas, was tracked down by his biological son, Dr. Wood insisted he had had a vasectomy years before the man was born but still would not agree to a DNA test. He died in April 2021.
None of the identified sperm doctors were interested in having a relationship with their newly identified offspring. When Gary Vandenberg, MD, of California, was contacted by his biological daughter, he abruptly ended the conversation, wishing her “good luck in life,” she recalled. “When I first found out, I was very suicidal. I did not want this existence. I still have those days. My husband had to take off work and stay home quite a bit to make sure I didn’t do anything to myself.”
When Gary Don Davis, MD, of Idaho, was asked about his paternity, he replied: “Let me check on that. Goodbye.” He could not be reached after that, and he died a few months later.
The accused doctors often have no medical records of their work. Dr. Wood said that all his records had been destroyed, and Dr. Greenberg said he did not have any records on his accuser and doubted that he had ever treated her. A 1977 survey found that more than half of infertility doctors did not keep any medical records so as to preserve the donor’s anonymity.
Many of the accused doctors said they used their own sperm because they were deeply committed to helping their patients. At one physician’s trial, his defense attorney said: “If Cecil made any mistakes, it was in losing his objectivity and trying so hard to get patients pregnant.”
Was it really ethically wrong?
Many of the doctors don’t accept that they did any harm, says Julie D. Cantor, MD, JD, a former adjunct professor at the University of California, Los Angeles. “These doctors seemed to be thinking: ‘The patient wanted to get pregnant and have a baby, and that’s what happened, so no harm done.’ But the entire interaction is based on a lie.”
The doctors also had the problem of having to use fresh sperm rather than frozen sperm, as is used today. Sperm had to be used within hours of being produced. If the donor did not show up at the time of the appointment, the doctor might decide to keep the appointment with the patient anyway and provide his own sperm.
However, “these doctors didn’t have to use their own sperm,” Mr. Wolf said. “They could have rescheduled the appointment until a new donor could be found.”
Some say that the doctors seemed to have had a very high opinion of themselves and their own sperm. “Some of them had savior complexes,” Dr. Madeira said. “They seemed to be thinking: ‘I’m giving the gift of life, and I’m the only one who can really do it, because I have great genes.’ ”
When Kim McMorries, MD, of Texas, was confronted with the fact that he had donated sperm 33 years before, he insisted that it was ethical at the time. “When this occurred, it was not considered wrong,” he wrote in an email to his biological daughter.
Today, doctors are bound by the doctrine of informed consent, which holds that patients should be informed about all steps taken in their care. The term was coined by a judge in 1960, and it took some time for some in the medical world to fully accept informed consent. Still, Dr. Madeira asserts it was always unethical to secretly fertilize patients.
“Even in the more paternalistic era of the 1970s and 1980s, it was not right to lie to your patients about such an important part of their lives,” she said.
Some sperm doctors insisted that they had received informed consent when the patient agreed to use an anonymous donor. “Dr. Kiken did that which he was asked to do,” wrote the attorneys for Michael S. Kiken, MD, of Virginia. “Anonymous donor meant that the patient would not know the donor’s identity, he would be anonymous to her.”
Dr. Madeira does not accept this argument either. “The doctor may have thought it was understood that he could be the anonymous person, but the patients did not see it that way,” she said. “They were not expecting the anonymous donor would be their own doctor.”
“I think what happened is a crime,” said Dr. Klipstein. “It’s an ethical violation, a fracture in the trust between doctor and patient.”
Existing laws, however, don’t make it easy to prosecute the doctors. When lawsuits are filed against these doctors, “you have to shoehorn existing statutes to fit the facts, and that may not be a terrific fit,” Dr. Cantor said.
The doctors have been charged with battery, fraud, negligence, breach of duty, unjust enrichment, and rape. But none of them have been found guilty specifically of secretly using their own sperm. Two of the doctors were convicted, but for other offenses, such as perjury for denying their involvement.
Since 2019, five states – Arizona, Colorado, Florida, Indiana, and Texas – have passed statutes specifically outlawing infertility fraud. In addition, a 1995 California law requires identifying the sperm donor.
It may be difficult, however, to apply these new laws to offenses by aging sperm doctors that happened decades ago. “Some states have inflexible limits on the amount of time in which you can sue, even if you didn’t know about the problem until recently,” Dr. Madeira said. “Texas, for example, allows civil lawsuits only up to 10 years after commission.”
Before the fertility fraud physicians can be brought to justice, many of them might just fade away.
A version of this article first appeared on Medscape.com.
Martin D. Greenberg, MD, was sued in May for secretly using his own sperm to inseminate one of his infertility patients 38 years earlier. The patient’s daughter found out last year when she used a DNA test from 23andme to learn about her family history. The 77-year-old New York gynecologist is retired in Florida.
All but one of the cases took place before 1990. Most of them came to light in the past few years, when biological offspring found out from home DNA tests.
“It is a gross betrayal of the trust that a patient puts in her doctor. It is an absolute perversion of the practice of medicine,” said Dev Sethi, a plaintiff attorney who sued a Tucson, Ariz., physician who inseminated at least 10 patients with his own sperm. “The hubris of a doctor to impregnate his own patient, in some effort to either save money or populate the world with his offspring, is striking.”
Why would these physicians use their own sperm and then keep it secret? Why were there so many of them? When their offspring now try to communicate with them, do they want to have a relationship? And how do they react when they’re found out?
The doctors’ behavior mystifies Sigal Klipstein, MD, a reproductive endocrinologist in Hoffman Estates, Ill., who is chair of the ethics committee of the American Society for Reproductive Medicine.
“These doctors lived with secrets for many years. How do you live with that as a doctor?” said Dr. Klipstein, who was still in high school when most of these cases occurred. “It surprises me that anybody would do this.”
Lack of training and lots of secrecy
Were these physicians particularly selfish or egotistical? Or was expedience the prime motivation?
At the time, there was little training in the techniques and ethics of infertility care, said Jody Madeira, JD, PhD, a law professor at Indiana University, Bloomington, who has closely studied the doctors.
“Many of them were ob.gyns., but they did not take CME courses for this work,” she said. The subspecialty of reproductive endocrinology and infertility was just beginning in the early 1970s, according an ASRM spokesman.
Treatment of infertility was a rather hush-hush topic at that time, which made it easier to be deceptive. In 1955, an Illinois court held that artificial insemination constituted adultery. “The social stigma resulting from the practice forces the parents to keep secret the infant’s origin,” a law review article from 1955 stated.
“In the 1950s and 1960s and even into the 1970s and 1980s, infertility treatments were considered shameful, and patients were often advised to keep their treatment to themselves,” Dr. Madeira said. “With everything so secret, it was easy to be deceptive.”
The field has become more sophisticated since then, Dr. Klipstein said. “For known donors, there is a legal contract between the recipient and donor. And it is no longer possible to be an anonymous donor. People can find you through DNA tests.”
Owing to changes in the field as well as the growing likelihood of being caught through DNA tests, most experts believe that rampant infertility fraud ended long ago.
How they were found out
When the doctors were active, there was little risk of being exposed. In those times, paternity tests were based on broad factors such as blood type and were unreliable. More accurate DNA tests were underway, but the doctors’ offspring did not think of using them because they suspected nothing.
Most of the doctors’ deeds only came to light with the rise of a new industry – home DNA testing for people who are curious about their family background. First came 23andme in 2007, then Ancestry.com in 2015. The number of people being tested reached almost 2 million in 2016, 7 million in 2017, and 30 million in 2020.
As more people entered company databases, it became easier to pinpoint biological fathers through other relatives. This explains how doctors who had not taken a home DNA test were identified.
The home tests have been shown to be highly accurate. None of the results for doctors accused of using their own sperm have proven to be false, and courts recognize similar DNA tests as proof of paternity.
But when found out, many of the physicians disputed the results and acted as if they could still keep their secret. “I don’t deny it; I don’t admit it,” Paul Brennan Jones, MD, a Colorado doctor, said when he was accused of siring eight children through his infertility patients decades before. Asked whether he would provide a DNA sample, the 80-year-old doctor responded: “No ... because I don’t want to have any incriminating evidence against me.”
How often did it happen?
Donor Deceived, a website that monitors these cases, reports 32 cases of physicians surreptitiously providing sperm to their patients. Eleven of the doctors are linked to 1 known offspring, two are linked to more than 75 offspring, one to 15, one to 10, three to 9, three to 7, and two to 5.
“It’s unlikely that any of the doctors did it just once,” said Adam B. Wolf, a San Francisco attorney who is representing the plaintiff in the Greenberg case. “It’s happened before. When doctors get the idea to do something crazy, they do it multiple times.”
Mr. Wolf believes that, because most people haven’t taken a DNA test, there are many more biological children of infertility doctors who have yet to come forward.
Many of the doctors who were found out have negotiated settlements with patients, under which they pay undisclosed sums of money in exchange for the patient’s keeping silent. Mr. Wolf said that, of the two dozen victims of sperm-donor doctors his law firm has represented, all but three have settled.
“We give an opportunity to the doctor to resolve the claims without having to publicly out this person for using his own sperm in his patients,” Mr. Wolf said. “Most doctors jump at the opportunity to not be known as the kind of person who would do that.”
Cases about to go to trial have been withdrawn because of being settled. In May, a case against Gerald E. Mortimer, MD, in Idaho, was dismissed after 3 years of litigation. The judge had made some key decisions that made it less likely that Dr. Mortimer would win. Dr. Mortimer’s biological daughter filed the initial case. She alleged medical negligence, failure to obtain informed consent, fraud, battery, intentional infliction of emotional distress, and several other causes of action.
Dr. Madeira objects to the use of confidential settlements, because other offspring cannot be alerted. But she also believes that, as more people find out about their parentage through DNA tests, it will be harder for accused doctors to make confidential settlements with all of them, and the doctors will eventually be identified.
In settlements, offspring ask for the medical histories of these doctors. So far, offspring have linked the development of Tay-Sachs disease, cystic fibrosis, and ovarian cancer with these doctors.
Denial: Physicians’ most frequent reaction
Once identified, most of the doctors denied the charge. When Gary Phillip Wood, MD, of Arkansas, was tracked down by his biological son, Dr. Wood insisted he had had a vasectomy years before the man was born but still would not agree to a DNA test. He died in April 2021.
None of the identified sperm doctors were interested in having a relationship with their newly identified offspring. When Gary Vandenberg, MD, of California, was contacted by his biological daughter, he abruptly ended the conversation, wishing her “good luck in life,” she recalled. “When I first found out, I was very suicidal. I did not want this existence. I still have those days. My husband had to take off work and stay home quite a bit to make sure I didn’t do anything to myself.”
When Gary Don Davis, MD, of Idaho, was asked about his paternity, he replied: “Let me check on that. Goodbye.” He could not be reached after that, and he died a few months later.
The accused doctors often have no medical records of their work. Dr. Wood said that all his records had been destroyed, and Dr. Greenberg said he did not have any records on his accuser and doubted that he had ever treated her. A 1977 survey found that more than half of infertility doctors did not keep any medical records so as to preserve the donor’s anonymity.
Many of the accused doctors said they used their own sperm because they were deeply committed to helping their patients. At one physician’s trial, his defense attorney said: “If Cecil made any mistakes, it was in losing his objectivity and trying so hard to get patients pregnant.”
Was it really ethically wrong?
Many of the doctors don’t accept that they did any harm, says Julie D. Cantor, MD, JD, a former adjunct professor at the University of California, Los Angeles. “These doctors seemed to be thinking: ‘The patient wanted to get pregnant and have a baby, and that’s what happened, so no harm done.’ But the entire interaction is based on a lie.”
The doctors also had the problem of having to use fresh sperm rather than frozen sperm, as is used today. Sperm had to be used within hours of being produced. If the donor did not show up at the time of the appointment, the doctor might decide to keep the appointment with the patient anyway and provide his own sperm.
However, “these doctors didn’t have to use their own sperm,” Mr. Wolf said. “They could have rescheduled the appointment until a new donor could be found.”
Some say that the doctors seemed to have had a very high opinion of themselves and their own sperm. “Some of them had savior complexes,” Dr. Madeira said. “They seemed to be thinking: ‘I’m giving the gift of life, and I’m the only one who can really do it, because I have great genes.’ ”
When Kim McMorries, MD, of Texas, was confronted with the fact that he had donated sperm 33 years before, he insisted that it was ethical at the time. “When this occurred, it was not considered wrong,” he wrote in an email to his biological daughter.
Today, doctors are bound by the doctrine of informed consent, which holds that patients should be informed about all steps taken in their care. The term was coined by a judge in 1960, and it took some time for some in the medical world to fully accept informed consent. Still, Dr. Madeira asserts it was always unethical to secretly fertilize patients.
“Even in the more paternalistic era of the 1970s and 1980s, it was not right to lie to your patients about such an important part of their lives,” she said.
Some sperm doctors insisted that they had received informed consent when the patient agreed to use an anonymous donor. “Dr. Kiken did that which he was asked to do,” wrote the attorneys for Michael S. Kiken, MD, of Virginia. “Anonymous donor meant that the patient would not know the donor’s identity, he would be anonymous to her.”
Dr. Madeira does not accept this argument either. “The doctor may have thought it was understood that he could be the anonymous person, but the patients did not see it that way,” she said. “They were not expecting the anonymous donor would be their own doctor.”
“I think what happened is a crime,” said Dr. Klipstein. “It’s an ethical violation, a fracture in the trust between doctor and patient.”
Existing laws, however, don’t make it easy to prosecute the doctors. When lawsuits are filed against these doctors, “you have to shoehorn existing statutes to fit the facts, and that may not be a terrific fit,” Dr. Cantor said.
The doctors have been charged with battery, fraud, negligence, breach of duty, unjust enrichment, and rape. But none of them have been found guilty specifically of secretly using their own sperm. Two of the doctors were convicted, but for other offenses, such as perjury for denying their involvement.
Since 2019, five states – Arizona, Colorado, Florida, Indiana, and Texas – have passed statutes specifically outlawing infertility fraud. In addition, a 1995 California law requires identifying the sperm donor.
It may be difficult, however, to apply these new laws to offenses by aging sperm doctors that happened decades ago. “Some states have inflexible limits on the amount of time in which you can sue, even if you didn’t know about the problem until recently,” Dr. Madeira said. “Texas, for example, allows civil lawsuits only up to 10 years after commission.”
Before the fertility fraud physicians can be brought to justice, many of them might just fade away.
A version of this article first appeared on Medscape.com.
Martin D. Greenberg, MD, was sued in May for secretly using his own sperm to inseminate one of his infertility patients 38 years earlier. The patient’s daughter found out last year when she used a DNA test from 23andme to learn about her family history. The 77-year-old New York gynecologist is retired in Florida.
All but one of the cases took place before 1990. Most of them came to light in the past few years, when biological offspring found out from home DNA tests.
“It is a gross betrayal of the trust that a patient puts in her doctor. It is an absolute perversion of the practice of medicine,” said Dev Sethi, a plaintiff attorney who sued a Tucson, Ariz., physician who inseminated at least 10 patients with his own sperm. “The hubris of a doctor to impregnate his own patient, in some effort to either save money or populate the world with his offspring, is striking.”
Why would these physicians use their own sperm and then keep it secret? Why were there so many of them? When their offspring now try to communicate with them, do they want to have a relationship? And how do they react when they’re found out?
The doctors’ behavior mystifies Sigal Klipstein, MD, a reproductive endocrinologist in Hoffman Estates, Ill., who is chair of the ethics committee of the American Society for Reproductive Medicine.
“These doctors lived with secrets for many years. How do you live with that as a doctor?” said Dr. Klipstein, who was still in high school when most of these cases occurred. “It surprises me that anybody would do this.”
Lack of training and lots of secrecy
Were these physicians particularly selfish or egotistical? Or was expedience the prime motivation?
At the time, there was little training in the techniques and ethics of infertility care, said Jody Madeira, JD, PhD, a law professor at Indiana University, Bloomington, who has closely studied the doctors.
“Many of them were ob.gyns., but they did not take CME courses for this work,” she said. The subspecialty of reproductive endocrinology and infertility was just beginning in the early 1970s, according an ASRM spokesman.
Treatment of infertility was a rather hush-hush topic at that time, which made it easier to be deceptive. In 1955, an Illinois court held that artificial insemination constituted adultery. “The social stigma resulting from the practice forces the parents to keep secret the infant’s origin,” a law review article from 1955 stated.
“In the 1950s and 1960s and even into the 1970s and 1980s, infertility treatments were considered shameful, and patients were often advised to keep their treatment to themselves,” Dr. Madeira said. “With everything so secret, it was easy to be deceptive.”
The field has become more sophisticated since then, Dr. Klipstein said. “For known donors, there is a legal contract between the recipient and donor. And it is no longer possible to be an anonymous donor. People can find you through DNA tests.”
Owing to changes in the field as well as the growing likelihood of being caught through DNA tests, most experts believe that rampant infertility fraud ended long ago.
How they were found out
When the doctors were active, there was little risk of being exposed. In those times, paternity tests were based on broad factors such as blood type and were unreliable. More accurate DNA tests were underway, but the doctors’ offspring did not think of using them because they suspected nothing.
Most of the doctors’ deeds only came to light with the rise of a new industry – home DNA testing for people who are curious about their family background. First came 23andme in 2007, then Ancestry.com in 2015. The number of people being tested reached almost 2 million in 2016, 7 million in 2017, and 30 million in 2020.
As more people entered company databases, it became easier to pinpoint biological fathers through other relatives. This explains how doctors who had not taken a home DNA test were identified.
The home tests have been shown to be highly accurate. None of the results for doctors accused of using their own sperm have proven to be false, and courts recognize similar DNA tests as proof of paternity.
But when found out, many of the physicians disputed the results and acted as if they could still keep their secret. “I don’t deny it; I don’t admit it,” Paul Brennan Jones, MD, a Colorado doctor, said when he was accused of siring eight children through his infertility patients decades before. Asked whether he would provide a DNA sample, the 80-year-old doctor responded: “No ... because I don’t want to have any incriminating evidence against me.”
How often did it happen?
Donor Deceived, a website that monitors these cases, reports 32 cases of physicians surreptitiously providing sperm to their patients. Eleven of the doctors are linked to 1 known offspring, two are linked to more than 75 offspring, one to 15, one to 10, three to 9, three to 7, and two to 5.
“It’s unlikely that any of the doctors did it just once,” said Adam B. Wolf, a San Francisco attorney who is representing the plaintiff in the Greenberg case. “It’s happened before. When doctors get the idea to do something crazy, they do it multiple times.”
Mr. Wolf believes that, because most people haven’t taken a DNA test, there are many more biological children of infertility doctors who have yet to come forward.
Many of the doctors who were found out have negotiated settlements with patients, under which they pay undisclosed sums of money in exchange for the patient’s keeping silent. Mr. Wolf said that, of the two dozen victims of sperm-donor doctors his law firm has represented, all but three have settled.
“We give an opportunity to the doctor to resolve the claims without having to publicly out this person for using his own sperm in his patients,” Mr. Wolf said. “Most doctors jump at the opportunity to not be known as the kind of person who would do that.”
Cases about to go to trial have been withdrawn because of being settled. In May, a case against Gerald E. Mortimer, MD, in Idaho, was dismissed after 3 years of litigation. The judge had made some key decisions that made it less likely that Dr. Mortimer would win. Dr. Mortimer’s biological daughter filed the initial case. She alleged medical negligence, failure to obtain informed consent, fraud, battery, intentional infliction of emotional distress, and several other causes of action.
Dr. Madeira objects to the use of confidential settlements, because other offspring cannot be alerted. But she also believes that, as more people find out about their parentage through DNA tests, it will be harder for accused doctors to make confidential settlements with all of them, and the doctors will eventually be identified.
In settlements, offspring ask for the medical histories of these doctors. So far, offspring have linked the development of Tay-Sachs disease, cystic fibrosis, and ovarian cancer with these doctors.
Denial: Physicians’ most frequent reaction
Once identified, most of the doctors denied the charge. When Gary Phillip Wood, MD, of Arkansas, was tracked down by his biological son, Dr. Wood insisted he had had a vasectomy years before the man was born but still would not agree to a DNA test. He died in April 2021.
None of the identified sperm doctors were interested in having a relationship with their newly identified offspring. When Gary Vandenberg, MD, of California, was contacted by his biological daughter, he abruptly ended the conversation, wishing her “good luck in life,” she recalled. “When I first found out, I was very suicidal. I did not want this existence. I still have those days. My husband had to take off work and stay home quite a bit to make sure I didn’t do anything to myself.”
When Gary Don Davis, MD, of Idaho, was asked about his paternity, he replied: “Let me check on that. Goodbye.” He could not be reached after that, and he died a few months later.
The accused doctors often have no medical records of their work. Dr. Wood said that all his records had been destroyed, and Dr. Greenberg said he did not have any records on his accuser and doubted that he had ever treated her. A 1977 survey found that more than half of infertility doctors did not keep any medical records so as to preserve the donor’s anonymity.
Many of the accused doctors said they used their own sperm because they were deeply committed to helping their patients. At one physician’s trial, his defense attorney said: “If Cecil made any mistakes, it was in losing his objectivity and trying so hard to get patients pregnant.”
Was it really ethically wrong?
Many of the doctors don’t accept that they did any harm, says Julie D. Cantor, MD, JD, a former adjunct professor at the University of California, Los Angeles. “These doctors seemed to be thinking: ‘The patient wanted to get pregnant and have a baby, and that’s what happened, so no harm done.’ But the entire interaction is based on a lie.”
The doctors also had the problem of having to use fresh sperm rather than frozen sperm, as is used today. Sperm had to be used within hours of being produced. If the donor did not show up at the time of the appointment, the doctor might decide to keep the appointment with the patient anyway and provide his own sperm.
However, “these doctors didn’t have to use their own sperm,” Mr. Wolf said. “They could have rescheduled the appointment until a new donor could be found.”
Some say that the doctors seemed to have had a very high opinion of themselves and their own sperm. “Some of them had savior complexes,” Dr. Madeira said. “They seemed to be thinking: ‘I’m giving the gift of life, and I’m the only one who can really do it, because I have great genes.’ ”
When Kim McMorries, MD, of Texas, was confronted with the fact that he had donated sperm 33 years before, he insisted that it was ethical at the time. “When this occurred, it was not considered wrong,” he wrote in an email to his biological daughter.
Today, doctors are bound by the doctrine of informed consent, which holds that patients should be informed about all steps taken in their care. The term was coined by a judge in 1960, and it took some time for some in the medical world to fully accept informed consent. Still, Dr. Madeira asserts it was always unethical to secretly fertilize patients.
“Even in the more paternalistic era of the 1970s and 1980s, it was not right to lie to your patients about such an important part of their lives,” she said.
Some sperm doctors insisted that they had received informed consent when the patient agreed to use an anonymous donor. “Dr. Kiken did that which he was asked to do,” wrote the attorneys for Michael S. Kiken, MD, of Virginia. “Anonymous donor meant that the patient would not know the donor’s identity, he would be anonymous to her.”
Dr. Madeira does not accept this argument either. “The doctor may have thought it was understood that he could be the anonymous person, but the patients did not see it that way,” she said. “They were not expecting the anonymous donor would be their own doctor.”
“I think what happened is a crime,” said Dr. Klipstein. “It’s an ethical violation, a fracture in the trust between doctor and patient.”
Existing laws, however, don’t make it easy to prosecute the doctors. When lawsuits are filed against these doctors, “you have to shoehorn existing statutes to fit the facts, and that may not be a terrific fit,” Dr. Cantor said.
The doctors have been charged with battery, fraud, negligence, breach of duty, unjust enrichment, and rape. But none of them have been found guilty specifically of secretly using their own sperm. Two of the doctors were convicted, but for other offenses, such as perjury for denying their involvement.
Since 2019, five states – Arizona, Colorado, Florida, Indiana, and Texas – have passed statutes specifically outlawing infertility fraud. In addition, a 1995 California law requires identifying the sperm donor.
It may be difficult, however, to apply these new laws to offenses by aging sperm doctors that happened decades ago. “Some states have inflexible limits on the amount of time in which you can sue, even if you didn’t know about the problem until recently,” Dr. Madeira said. “Texas, for example, allows civil lawsuits only up to 10 years after commission.”
Before the fertility fraud physicians can be brought to justice, many of them might just fade away.
A version of this article first appeared on Medscape.com.
Levothyroxine overprescribing common, consistent over time
Most U.S. prescriptions for the thyroid hormone replacement drug levothyroxine are not appropriate for patients with mild subclinical hypothyroidism, a trend that has remained steady for a decade despite evidence showing no significant benefits for those patients, new research shows.
“These results suggest substantial overuse of levothyroxine during the entire duration of the study, suggesting opportunities to improve care,” wrote the authors of the study published in JAMA Internal Medicine.
“There have been previous reports of increased levothyroxine overuse in the U.S., but this is the first paper to describe the nature of the drivers of the overuse,” first author Juan P. Brito, MD, of the division of endocrinology, diabetes, metabolism and nutrition, department of internal medicine, Mayo Clinic, Rochester, Minn., said in an interview.
The findings underscore the need to improve awareness of the ongoing overuse, said the authors of an accompanying editorial.
“We hope [this study] resonates as a call to action for clinicians to stop treating patients with mild subclinical hypothyroidism,” they wrote.
Only 8% of those receiving levothyroxine had overt hypothyroidism
For the study, Dr. Brito and colleagues analyzed data of adults enrolled in Medicare Advantage who filled levothyroxine prescriptions between January 2008 and December 2018 and had thyrotropin levels measured within 3 months prior to the prescription. Patients with a history of thyroid surgery, thyroid cancer, central hypothyroidism, or who were pregnant, were excluded from the study.
In the 110,842 patients who started levothyroxine during the study period, there were no significant changes in median thyrotropin levels at the time of treatment initiation, with a median level in 2008 of 5.8 mIU/L and a level in 2018 of 5.3 mIU/L (P = .79).
In a subanalysis of 58,706 patients for whom thyrotropin as well as free thyroxine (FT4 or T4) levels were available – which allowed for the determination of the level of hypothyroidism – levothyroxine was initiated for overt hypothyroidism in only 8.4% of cases.
In as many as 61.0% of cases, patients had subclinical hypothyroidism, and in 30.5% of cases, patients had normal thyroid levels.
While the proportion of adults with overt hypothyroidism initiated on levothyroxine significantly increased over the 10 years (7.6% to 8.4%; P = .02), rates of those with subclinical hypothyroidism remained unchanged (59.3% to 65.7%; P = .36), as did the proportion with normal thyroid function (32.9% to 26.2%; P = .84).
A closer look at patients specifically with subclinical hypothyroidism showed there were also no changes in the proportion with mild subclinical hypothyroidism (thyrotropin level of 4.5 mIU/L to <10 mIU/L with normal FT4 or T4) between the beginning and end of the study period (48.2% vs. 57.9%; P = .73). Rates of moderate subclinical hypothyroidism (thyrotropin level 10-19.9 mIU/L) were also similar (8.5% to 6.4%; P = .16).
No significant benefit, but ample undesirable effects
The authors underscore that levothyroxine has been shown time and again to offer no significant benefit to patients with subclinical hypothyroidism of any type, emphasized in a 2018 meta-analysis of 21 randomized, controlled trials.
“Frequent initiation of levothyroxine in these patients is at odds with evidence demonstrating no significant association of levothyroxine replacement with measures of health-related quality of life, thyroid-related symptoms, depressive symptoms, fatigue, or cognitive function,” they explained.
In addition to showing no benefit for subclinical hypothyroidism, levothyroxine is associated with a host of unwanted side effects, noted editorialists William K. Silverstein, MD, of Sunnybrook Health Sciences Centre, department of medicine, University of Toronto, and Deborah Grady, MD, of the department of medicine, University of California, San Francisco.
Some studies have shown a link between long-term levothyroxine therapy and an increased risk of cardiovascular disease, cardiac dysrhythmias, osteoporosis, and fractures, they explained.
In addition, unnecessary treatment “increases pill burden and costs, necessitates routine physician visits and blood work, and requires modification of daily routines so that patients can take medications on an empty stomach,” the editorialists wrote.
Importantly, evidence shows that once levothyroxine treatment for subclinical hypothyroidism is started, most patients will continue the therapy for life, they added.
The fact that levothyroxine is among the most commonly prescribed drugs in the United States, with about 7% of the population estimated to have an active prescription when overt hypothyroidism affects only about 0.2%-2% of the population, underscores the extent of levothyroxine overuse, Dr. Silverstein said in an interview.
“The really notable surprise was how pervasive inappropriate use of levothyroxine was,” he said. “The fact that only 8% of patients had a biochemical indication for treatment is striking.”
Potential solutions: ‘Shift the conversation’
In terms of potential solutions to the problem, Dr. Silverstein suggested laboratories change reference ranges so that only thyrotropin values greater than 10 mIU/L are reported as abnormal.
“Studies have shown that changing the thyrotropin reference range is associated with clinicians’ prescribing patterns,” he noted.
Dr. Brito agreed, noting that “there are many guidelines with different hypothyroidism thresholds, so we need to be more consistent about the message to clinicians.
“In addition, we have to come up with different approaches to symptoms that have nothing to do with levothyroxine,” Dr. Brito said.
“I try to explain to patients that it’s very unlikely that subclinical hypothyroidism would be driving significant symptoms like fatigue, weight gain, and hair loss,” Dr. Brito said. “So, one approach is to shift the conversation from how your thyroid is causing this to ‘how are we going to treat the symptoms?’ ”
The study was supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. Silverstein has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most U.S. prescriptions for the thyroid hormone replacement drug levothyroxine are not appropriate for patients with mild subclinical hypothyroidism, a trend that has remained steady for a decade despite evidence showing no significant benefits for those patients, new research shows.
“These results suggest substantial overuse of levothyroxine during the entire duration of the study, suggesting opportunities to improve care,” wrote the authors of the study published in JAMA Internal Medicine.
“There have been previous reports of increased levothyroxine overuse in the U.S., but this is the first paper to describe the nature of the drivers of the overuse,” first author Juan P. Brito, MD, of the division of endocrinology, diabetes, metabolism and nutrition, department of internal medicine, Mayo Clinic, Rochester, Minn., said in an interview.
The findings underscore the need to improve awareness of the ongoing overuse, said the authors of an accompanying editorial.
“We hope [this study] resonates as a call to action for clinicians to stop treating patients with mild subclinical hypothyroidism,” they wrote.
Only 8% of those receiving levothyroxine had overt hypothyroidism
For the study, Dr. Brito and colleagues analyzed data of adults enrolled in Medicare Advantage who filled levothyroxine prescriptions between January 2008 and December 2018 and had thyrotropin levels measured within 3 months prior to the prescription. Patients with a history of thyroid surgery, thyroid cancer, central hypothyroidism, or who were pregnant, were excluded from the study.
In the 110,842 patients who started levothyroxine during the study period, there were no significant changes in median thyrotropin levels at the time of treatment initiation, with a median level in 2008 of 5.8 mIU/L and a level in 2018 of 5.3 mIU/L (P = .79).
In a subanalysis of 58,706 patients for whom thyrotropin as well as free thyroxine (FT4 or T4) levels were available – which allowed for the determination of the level of hypothyroidism – levothyroxine was initiated for overt hypothyroidism in only 8.4% of cases.
In as many as 61.0% of cases, patients had subclinical hypothyroidism, and in 30.5% of cases, patients had normal thyroid levels.
While the proportion of adults with overt hypothyroidism initiated on levothyroxine significantly increased over the 10 years (7.6% to 8.4%; P = .02), rates of those with subclinical hypothyroidism remained unchanged (59.3% to 65.7%; P = .36), as did the proportion with normal thyroid function (32.9% to 26.2%; P = .84).
A closer look at patients specifically with subclinical hypothyroidism showed there were also no changes in the proportion with mild subclinical hypothyroidism (thyrotropin level of 4.5 mIU/L to <10 mIU/L with normal FT4 or T4) between the beginning and end of the study period (48.2% vs. 57.9%; P = .73). Rates of moderate subclinical hypothyroidism (thyrotropin level 10-19.9 mIU/L) were also similar (8.5% to 6.4%; P = .16).
No significant benefit, but ample undesirable effects
The authors underscore that levothyroxine has been shown time and again to offer no significant benefit to patients with subclinical hypothyroidism of any type, emphasized in a 2018 meta-analysis of 21 randomized, controlled trials.
“Frequent initiation of levothyroxine in these patients is at odds with evidence demonstrating no significant association of levothyroxine replacement with measures of health-related quality of life, thyroid-related symptoms, depressive symptoms, fatigue, or cognitive function,” they explained.
In addition to showing no benefit for subclinical hypothyroidism, levothyroxine is associated with a host of unwanted side effects, noted editorialists William K. Silverstein, MD, of Sunnybrook Health Sciences Centre, department of medicine, University of Toronto, and Deborah Grady, MD, of the department of medicine, University of California, San Francisco.
Some studies have shown a link between long-term levothyroxine therapy and an increased risk of cardiovascular disease, cardiac dysrhythmias, osteoporosis, and fractures, they explained.
In addition, unnecessary treatment “increases pill burden and costs, necessitates routine physician visits and blood work, and requires modification of daily routines so that patients can take medications on an empty stomach,” the editorialists wrote.
Importantly, evidence shows that once levothyroxine treatment for subclinical hypothyroidism is started, most patients will continue the therapy for life, they added.
The fact that levothyroxine is among the most commonly prescribed drugs in the United States, with about 7% of the population estimated to have an active prescription when overt hypothyroidism affects only about 0.2%-2% of the population, underscores the extent of levothyroxine overuse, Dr. Silverstein said in an interview.
“The really notable surprise was how pervasive inappropriate use of levothyroxine was,” he said. “The fact that only 8% of patients had a biochemical indication for treatment is striking.”
Potential solutions: ‘Shift the conversation’
In terms of potential solutions to the problem, Dr. Silverstein suggested laboratories change reference ranges so that only thyrotropin values greater than 10 mIU/L are reported as abnormal.
“Studies have shown that changing the thyrotropin reference range is associated with clinicians’ prescribing patterns,” he noted.
Dr. Brito agreed, noting that “there are many guidelines with different hypothyroidism thresholds, so we need to be more consistent about the message to clinicians.
“In addition, we have to come up with different approaches to symptoms that have nothing to do with levothyroxine,” Dr. Brito said.
“I try to explain to patients that it’s very unlikely that subclinical hypothyroidism would be driving significant symptoms like fatigue, weight gain, and hair loss,” Dr. Brito said. “So, one approach is to shift the conversation from how your thyroid is causing this to ‘how are we going to treat the symptoms?’ ”
The study was supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. Silverstein has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most U.S. prescriptions for the thyroid hormone replacement drug levothyroxine are not appropriate for patients with mild subclinical hypothyroidism, a trend that has remained steady for a decade despite evidence showing no significant benefits for those patients, new research shows.
“These results suggest substantial overuse of levothyroxine during the entire duration of the study, suggesting opportunities to improve care,” wrote the authors of the study published in JAMA Internal Medicine.
“There have been previous reports of increased levothyroxine overuse in the U.S., but this is the first paper to describe the nature of the drivers of the overuse,” first author Juan P. Brito, MD, of the division of endocrinology, diabetes, metabolism and nutrition, department of internal medicine, Mayo Clinic, Rochester, Minn., said in an interview.
The findings underscore the need to improve awareness of the ongoing overuse, said the authors of an accompanying editorial.
“We hope [this study] resonates as a call to action for clinicians to stop treating patients with mild subclinical hypothyroidism,” they wrote.
Only 8% of those receiving levothyroxine had overt hypothyroidism
For the study, Dr. Brito and colleagues analyzed data of adults enrolled in Medicare Advantage who filled levothyroxine prescriptions between January 2008 and December 2018 and had thyrotropin levels measured within 3 months prior to the prescription. Patients with a history of thyroid surgery, thyroid cancer, central hypothyroidism, or who were pregnant, were excluded from the study.
In the 110,842 patients who started levothyroxine during the study period, there were no significant changes in median thyrotropin levels at the time of treatment initiation, with a median level in 2008 of 5.8 mIU/L and a level in 2018 of 5.3 mIU/L (P = .79).
In a subanalysis of 58,706 patients for whom thyrotropin as well as free thyroxine (FT4 or T4) levels were available – which allowed for the determination of the level of hypothyroidism – levothyroxine was initiated for overt hypothyroidism in only 8.4% of cases.
In as many as 61.0% of cases, patients had subclinical hypothyroidism, and in 30.5% of cases, patients had normal thyroid levels.
While the proportion of adults with overt hypothyroidism initiated on levothyroxine significantly increased over the 10 years (7.6% to 8.4%; P = .02), rates of those with subclinical hypothyroidism remained unchanged (59.3% to 65.7%; P = .36), as did the proportion with normal thyroid function (32.9% to 26.2%; P = .84).
A closer look at patients specifically with subclinical hypothyroidism showed there were also no changes in the proportion with mild subclinical hypothyroidism (thyrotropin level of 4.5 mIU/L to <10 mIU/L with normal FT4 or T4) between the beginning and end of the study period (48.2% vs. 57.9%; P = .73). Rates of moderate subclinical hypothyroidism (thyrotropin level 10-19.9 mIU/L) were also similar (8.5% to 6.4%; P = .16).
No significant benefit, but ample undesirable effects
The authors underscore that levothyroxine has been shown time and again to offer no significant benefit to patients with subclinical hypothyroidism of any type, emphasized in a 2018 meta-analysis of 21 randomized, controlled trials.
“Frequent initiation of levothyroxine in these patients is at odds with evidence demonstrating no significant association of levothyroxine replacement with measures of health-related quality of life, thyroid-related symptoms, depressive symptoms, fatigue, or cognitive function,” they explained.
In addition to showing no benefit for subclinical hypothyroidism, levothyroxine is associated with a host of unwanted side effects, noted editorialists William K. Silverstein, MD, of Sunnybrook Health Sciences Centre, department of medicine, University of Toronto, and Deborah Grady, MD, of the department of medicine, University of California, San Francisco.
Some studies have shown a link between long-term levothyroxine therapy and an increased risk of cardiovascular disease, cardiac dysrhythmias, osteoporosis, and fractures, they explained.
In addition, unnecessary treatment “increases pill burden and costs, necessitates routine physician visits and blood work, and requires modification of daily routines so that patients can take medications on an empty stomach,” the editorialists wrote.
Importantly, evidence shows that once levothyroxine treatment for subclinical hypothyroidism is started, most patients will continue the therapy for life, they added.
The fact that levothyroxine is among the most commonly prescribed drugs in the United States, with about 7% of the population estimated to have an active prescription when overt hypothyroidism affects only about 0.2%-2% of the population, underscores the extent of levothyroxine overuse, Dr. Silverstein said in an interview.
“The really notable surprise was how pervasive inappropriate use of levothyroxine was,” he said. “The fact that only 8% of patients had a biochemical indication for treatment is striking.”
Potential solutions: ‘Shift the conversation’
In terms of potential solutions to the problem, Dr. Silverstein suggested laboratories change reference ranges so that only thyrotropin values greater than 10 mIU/L are reported as abnormal.
“Studies have shown that changing the thyrotropin reference range is associated with clinicians’ prescribing patterns,” he noted.
Dr. Brito agreed, noting that “there are many guidelines with different hypothyroidism thresholds, so we need to be more consistent about the message to clinicians.
“In addition, we have to come up with different approaches to symptoms that have nothing to do with levothyroxine,” Dr. Brito said.
“I try to explain to patients that it’s very unlikely that subclinical hypothyroidism would be driving significant symptoms like fatigue, weight gain, and hair loss,” Dr. Brito said. “So, one approach is to shift the conversation from how your thyroid is causing this to ‘how are we going to treat the symptoms?’ ”
The study was supported by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. Silverstein has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADA/EASD draft guidance aims to bring adults with type 1 diabetes out of shadows
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
A new draft consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes (EASD) addresses diagnosis and management of type 1 diabetes in adults.
The impetus for the document comes from the “highly influential” EASD-ADA consensus report on the management of type 2 diabetes, which led to the realization that a comparable document was needed for adults with type 1 diabetes, said writing panel cochair Anne L. Peters, MD, professor of clinical medicine at the University of Southern California, Los Angeles.
“In recent years, there have been rapid advances in the treatment of type 1 diabetes together with a growing recognition of the psychosocial burden of living with [it],” Dr. Peters said.
She noted that although there is already some guidance available for the management of type 1 diabetes in adults, “this gets admixed into broader guidelines, and many of those are mostly derived from data in people with type 2 diabetes.”
The new draft document was coauthored by 14 content experts in type 1 diabetes, with equal numbers from the United States and Europe.
We want to be helpful to clinicians
Topics covered include diagnosis of type 1 diabetes, goals of therapy and glycemic targets, schedule of care, diabetes self-management education and additional behavioral considerations, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis, pancreas and islet cell transplantation, adjunctive therapies, special populations (including pregnant women, older adults, and inpatient management), and emergent/future perspectives, including beta-cell replacement and immunotherapy.
At the end of the document are tables of glycemic targets for adults with type 1 diabetes, schedule of care, nonglycemic factors that alter A1c levels, standardized continuous glucose meter (CGM) metrics for clinical care, examples of subcutaneous insulin regimens, and the various properties of approved and nonapproved adjunctive therapies for type 1 diabetes, including metformin, pramlintide, GLP-1 agonists, and SGLT2 inhibitors.
Several colorful flowcharts are also provided, including algorithms for diagnosing and managing type 1 diabetes in adults.
Document coauthor M. Sue Kirkman, MD, of the Diabetes Care Center’s Clinical Trials Unit at the University of North Carolina, Chapel Hill, told this news organization: “We want it to be helpful to clinicians who are diagnosing type 1 diabetes in adults or caring for adults with type 1 diabetes, whether diagnosed in childhood or adulthood.”
The authors presented an overview of the document in a symposium on June 28 at the virtual ADA scientific sessions. The final version will be presented Oct. 1 at the EASD 2021 annual meeting.
The draft document and video of the ADA meeting presentation are both available on the ADA website.
New algorithm to reduce misdiagnosis of type 1 diabetes in adults
Misdiagnosis of adult-onset type 1 diabetes is common, occurring in up to 40% of those who develop the condition after age 30 years, said J. Hans de Vries, MD, PhD, medical director, Profil Institute for Metabolic Research, Neuss, Germany.
There are multiple reasons for this, including the fact that obesity and type 2 diabetes are becoming more prevalent at younger ages, C-peptide levels may still be relatively high at the time of clinical type 1 diabetes onset, and islet autoantibodies don’t have 100% positive predictive value.
“No single feature confirms type 1 diabetes in isolation,” Dr. de Vries noted.
The document provides a detailed diagnostic algorithm specifically for adults in whom type 1 diabetes is suspected, starting with autoantibody measurement. If the diagnosis isn’t confirmed that way, the algorithm advises investigating for monogenic diabetes, including use of a maturity-onset diabetes of the young (MODY) calculator and subsequent C-peptide measurement.
Measurement of C-peptide is also recommended if the diabetes type is still uncertain more than 3 years after diabetes onset in those treated with insulin, because by that point it is likely to be <200 pmol/L in people with type 1 diabetes.
Clear statements on diabetes technology, preferred insulins
The draft document clearly states that physiologic insulin replacement using a pump or multiple daily injections, CGM, and analog rather than human insulin are standards of care for adults with type 1 diabetes. Use of hybrid closed-loop insulin delivery systems is advised when available, as they offer the “greatest benefits.”
However, the document also notes that in cases of cost barriers, subcutaneous regimens of human regular and NPH insulin may be used. It cautions, though, that these may result in higher glucose variability, higher risk of hypoglycemia, and less lifestyle flexibility.
Dr. Kirkman told this news organization: “Using human insulins such as NPH and Regular in type 1 diabetes is definitely not preferred, but sometimes due to people’s inability to afford analogs we have to use them. People need to know how to use them safely.”
As for the do-it-yourself insulin delivery systems, which many with type 1 diabetes now use with open-source software algorithms that reverse-engineer older pumps, the document advises that health care providers shouldn’t actively recommend them as they’re not approved by regulatory authorities, but should also “respect the individual’s right to make informed choices and continue to offer support,” Dr. Kirkman said when presenting the insulin therapy section.
Psychosocial aspects of type 1 diabetes ‘underappreciated’
Special emphasis is placed on psychosocial support, which may be overlooked in adults, Dr. Kirkman noted.
“Clinicians probably underappreciate what people with type 1 diabetes go through on a daily basis. A lot of the evidence out there regarding psychosocial issues is in children and families of children with type 1 diabetes, or in adults with type 2 diabetes ... Maximizing quality of life needs to be at the forefront of care, not just focusing on glycemic goals.”
Indeed, between 20% and 40% of people with type 1 diabetes experience diabetes-related emotional distress – including 15% with depression – particularly at the time of diagnosis and when complications develop, noted Frank J. Snoek, PhD, professor of medical psychology at Amsterdam University Medical Center, the Netherlands.
To address this, the draft advises that “self-management difficulties, psychological, and social problems” be screened periodically and monitored using validated screening tools.
“Health care providers should be proficient at asking questions about and discussing emotional health, psychological needs, and social challenges as part of the consultation,” Dr. Snoek said.
Dr. Peters disclosed ties with Abbott Diabetes Care, AstraZeneca, Lilly, Medscape, Novo Nordisk, Vertex, and Zealand, Omada, and Teladoc. Dr. Kirkman has received research support from Novo Nordisk and Bayer. Dr. de Vries disclosed ties with Adocia, Novo Nordisk, Zealand, Eli Lilly, and Afon Technology. Dr. Snoek reported ties with Roche Diabetes, Novo Nordisk, Sanofi, and Eli Lilly.
A version of this article first appeared on Medscape.com.
Testosterone replacement shows CV benefit in hypogonadal men
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Not so crazy: Pancreas transplants in type 2 diabetes rising
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Meta-analysis supports cardiovascular benefits of EPA
Support for a cardiovascular benefit of omega-3 fatty acids, particularly eicosapentaenoic acid (EPA), has come from a new systematic review and meta-analysis of randomized trials.
The meta-analysis of 38 randomized controlled trials found that omega-3 fatty acids improved cardiovascular outcomes, with a greater reduction in cardiovascular risk in studies of EPA alone rather than of combined eicosapentaenoic plus docosahexaenoic acid (DHA) supplements.
The paper was published online in EClinicalMedicine.
Senior author Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, was also lead investigator of the REDUCE-IT trial, which is included in the analysis and showed a 25% relative risk reduction in major cardiovascular events with a high-dose EPA product.
But the REDUCE-IT trial has been mired in controversy, with suggestions that the benefit seen might have been exaggerated because of the use of a harmful placebo. In addition, a second large trial of high-dose omega-3 fatty acids, STRENGTH (which tested a combination EPA/DHA product) showed no benefit on cardiovascular outcomes.
Dr. Bhatt said the new meta-analysis provides “a totality of evidence” that “supports a robust and consistent benefit of EPA.”
In the review, the authors concluded: “In this systematic review and meta-analysis, we noted moderate certainty of evidence favoring omega-3 fatty acids for reducing cardiovascular mortality and outcomes. ... The magnitude of relative reductions was robust in EPA trials versus those of EPA+DHA, suggesting differential effects of EPA and DHA in cardiovascular risk reduction.”
Controversy continues
But commenting on the publication for an interview, Steven Nissen, MD, Cleveland Clinic, who led the STRENGTH trial, pointed out that 85% of the EPA data in the new meta-analysis came from REDUCE-IT, so the results were a “foregone conclusion.”
“The purpose of a meta-analysis is to answer scientific questions when existing studies are too small to yield statistically robust results. That is not the case here,” Dr. Nissen stated.
He added: “There are only two major trials of EPA and both have important flaws. REDUCE-IT used a questionable placebo (mineral oil) and JELIS was an open-label trial that studied patients with baseline LDL [cholesterol] of 180 mg/dL that was not appropriately treated. A meta-analysis is only as good as the studies that it includes. The other EPA plus DHA studies were essentially neutral.”
Dr. Bhatt responded that, “to date, every randomized trial of EPA only has been positive. Some have been placebo controlled, some have been open label. This meta-analysis corroborates the results of each of those trials in a statistically robust way.”
He added: “Of course, REDUCE-IT is the most rigorous, contemporary trial of EPA. However, in our meta-analysis, even when excluding REDUCE-IT (or for that matter, JELIS), the EPA trials still significantly reduced cardiovascular events.”
Dr. Bhatt also pointed out that two randomized imaging studies, CHERRY and EVAPORATE, have shown benefits of EPA.
“Beyond the clinical trial data, there is a growing amount of evidence supporting the unique biological actions of different omega-3 fatty acids. EPA, in particular, appears to have the strongest basic science evidence supporting cardiovascular benefits. Overall, it is a remarkably consistent scientific story in support of EPA’s beneficial effects on cardiovascular health,” he stated.
38 trials included
For the current paper, Dr. Bhatt and coauthors performed a comprehensive literature search for randomized trials comparing omega-3 fatty acids with control (placebo, no supplementation, or lower dose of omega-3 fatty acids) in adults, with a follow-up of at least 12 months, and mortality and cardiovascular outcomes as endpoints.
Ultimately, 38 trials encompassing 149,051 patients were included. Of these, four trials compared EPA with control, 34 trials compared EPA+DHA with control, and 22 trials were in primary prevention. The dose of omega-3 fatty acids ranged from 0.4 g/day to 5.5 g/day.
A total of 25 trials with 143,514 individuals reported 5,550 events of cardiovascular mortality, and 24 trials with 140,983 individuals reported 10,795 events of all-cause mortality.
Omega-3 fatty acids were associated with reduced cardiovascular mortality (rate ratio, 0.93; P = .01), but not all-cause mortality (RR, 0.97; P = .27). The meta-analysis showed reduction in cardiovascular mortality with EPA monotherapy (RR, 0.82; P = .04) and EPA+DHA combination (RR, 0.94; P = .02).
A total of 20 trials with 125,611 individuals reported 2,989 nonfatal myocardial infarction events, and 29 trials with 144,384 individuals reported 9,153 coronary heart disease (CHD) events.
Omega-3 fatty acids were associated with reducing nonfatal MI (RR, 0.87; P = .0001) and CHD (RR, 0.91; P = .0002). The meta-analysis showed higher risk reductions in nonfatal MI with EPA monotherapy (RR, 0.72; P = .00002) than with EPA+DHA combination (RR, 0.92; P = .05), and also for CHD events with EPA monotherapy (RR, 0.73; P = .00004) than with EPA+DHA combination (RR, 0.94; P = .01).
A total of 17 trials (n = 135,019) reported 13,234 events of MACE, and 13 trials (n = 117,890) reported 7,416 events of revascularization.
Omega-3 fatty acids were associated with reducing MACE (RR, 0.95; P = .002) and revascularization (RR, 0.91; P = .0001). The meta-analysis showed higher risk reductions in MACE with EPA monotherapy (RR, 0.78; P = .00000001), whereas EPA+DHA combination did not reduce MACE (RR, 0.99; P = .48). This effect was consistent for revascularization.
A total of eight trials with 65,404 individuals reported 935 nonfatal strokes, and eight trials with 51,336 individuals reported 1,572 events of atrial fibrillation (AFib).
Omega-3 fatty acids did not significantly reduce nonfatal stroke (RR, 1.04; P = .55), but EPA monotherapy was associated with a reduction of nonfatal stroke, compared with control (RR: 0.71; P = .01).
Conversely, omega-3 fatty acids were associated with increased risk for AFib (RR, 1.26; P = .004), with a higher risk with EPA monotherapy than with control (RR, 1.35; P = .004).
Overall, omega-3 fatty acids did not prevent sudden cardiac death or increase gastrointestinal-related adverse events, total bleeding, or major or minor bleeding; however, the meta-analysis showed a higher risk of total bleeding with EPA monotherapy than with control (RR, 1.49; P = .006).
An influence analysis with stepwise exclusion of one trial at a time, including REDUCE-IT, did not alter the overall summary estimates. “Despite the exclusion of REDUCE-IT, EPA monotherapy reduced MACE by 23%, compared with the control,” the authors reported.
They said these new findings also have important implications for clinical practice and treatment guidelines.
“After REDUCE-IT, several national and international guidelines endorsed EPA in their therapeutic recommendations. However, the publication of two recent negative trials of EPA + DHA has created some confusion in the scientific community about the value of omega-3 FAs in preventing atherosclerotic cardiovascular disease [ASCVD],” they stated.
“This meta-analysis provides reassurance about the role of omega-3 fatty acids, specifically EPA, in the current treatment framework of ASCVD residual cardiovascular risk reduction and encourages investigators to explore further the cardiovascular effects of EPA across different clinical settings,” they added.
REDUCE-IT was sponsored by Amarin. Brigham and Women’s Hospital receives research funding from Amarin for the work Dr. Bhatt did as the trial chair and as the international principal investigator. The present analysis was unfunded.
A version of this article first appeared on Medscape.com.
Support for a cardiovascular benefit of omega-3 fatty acids, particularly eicosapentaenoic acid (EPA), has come from a new systematic review and meta-analysis of randomized trials.
The meta-analysis of 38 randomized controlled trials found that omega-3 fatty acids improved cardiovascular outcomes, with a greater reduction in cardiovascular risk in studies of EPA alone rather than of combined eicosapentaenoic plus docosahexaenoic acid (DHA) supplements.
The paper was published online in EClinicalMedicine.
Senior author Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, was also lead investigator of the REDUCE-IT trial, which is included in the analysis and showed a 25% relative risk reduction in major cardiovascular events with a high-dose EPA product.
But the REDUCE-IT trial has been mired in controversy, with suggestions that the benefit seen might have been exaggerated because of the use of a harmful placebo. In addition, a second large trial of high-dose omega-3 fatty acids, STRENGTH (which tested a combination EPA/DHA product) showed no benefit on cardiovascular outcomes.
Dr. Bhatt said the new meta-analysis provides “a totality of evidence” that “supports a robust and consistent benefit of EPA.”
In the review, the authors concluded: “In this systematic review and meta-analysis, we noted moderate certainty of evidence favoring omega-3 fatty acids for reducing cardiovascular mortality and outcomes. ... The magnitude of relative reductions was robust in EPA trials versus those of EPA+DHA, suggesting differential effects of EPA and DHA in cardiovascular risk reduction.”
Controversy continues
But commenting on the publication for an interview, Steven Nissen, MD, Cleveland Clinic, who led the STRENGTH trial, pointed out that 85% of the EPA data in the new meta-analysis came from REDUCE-IT, so the results were a “foregone conclusion.”
“The purpose of a meta-analysis is to answer scientific questions when existing studies are too small to yield statistically robust results. That is not the case here,” Dr. Nissen stated.
He added: “There are only two major trials of EPA and both have important flaws. REDUCE-IT used a questionable placebo (mineral oil) and JELIS was an open-label trial that studied patients with baseline LDL [cholesterol] of 180 mg/dL that was not appropriately treated. A meta-analysis is only as good as the studies that it includes. The other EPA plus DHA studies were essentially neutral.”
Dr. Bhatt responded that, “to date, every randomized trial of EPA only has been positive. Some have been placebo controlled, some have been open label. This meta-analysis corroborates the results of each of those trials in a statistically robust way.”
He added: “Of course, REDUCE-IT is the most rigorous, contemporary trial of EPA. However, in our meta-analysis, even when excluding REDUCE-IT (or for that matter, JELIS), the EPA trials still significantly reduced cardiovascular events.”
Dr. Bhatt also pointed out that two randomized imaging studies, CHERRY and EVAPORATE, have shown benefits of EPA.
“Beyond the clinical trial data, there is a growing amount of evidence supporting the unique biological actions of different omega-3 fatty acids. EPA, in particular, appears to have the strongest basic science evidence supporting cardiovascular benefits. Overall, it is a remarkably consistent scientific story in support of EPA’s beneficial effects on cardiovascular health,” he stated.
38 trials included
For the current paper, Dr. Bhatt and coauthors performed a comprehensive literature search for randomized trials comparing omega-3 fatty acids with control (placebo, no supplementation, or lower dose of omega-3 fatty acids) in adults, with a follow-up of at least 12 months, and mortality and cardiovascular outcomes as endpoints.
Ultimately, 38 trials encompassing 149,051 patients were included. Of these, four trials compared EPA with control, 34 trials compared EPA+DHA with control, and 22 trials were in primary prevention. The dose of omega-3 fatty acids ranged from 0.4 g/day to 5.5 g/day.
A total of 25 trials with 143,514 individuals reported 5,550 events of cardiovascular mortality, and 24 trials with 140,983 individuals reported 10,795 events of all-cause mortality.
Omega-3 fatty acids were associated with reduced cardiovascular mortality (rate ratio, 0.93; P = .01), but not all-cause mortality (RR, 0.97; P = .27). The meta-analysis showed reduction in cardiovascular mortality with EPA monotherapy (RR, 0.82; P = .04) and EPA+DHA combination (RR, 0.94; P = .02).
A total of 20 trials with 125,611 individuals reported 2,989 nonfatal myocardial infarction events, and 29 trials with 144,384 individuals reported 9,153 coronary heart disease (CHD) events.
Omega-3 fatty acids were associated with reducing nonfatal MI (RR, 0.87; P = .0001) and CHD (RR, 0.91; P = .0002). The meta-analysis showed higher risk reductions in nonfatal MI with EPA monotherapy (RR, 0.72; P = .00002) than with EPA+DHA combination (RR, 0.92; P = .05), and also for CHD events with EPA monotherapy (RR, 0.73; P = .00004) than with EPA+DHA combination (RR, 0.94; P = .01).
A total of 17 trials (n = 135,019) reported 13,234 events of MACE, and 13 trials (n = 117,890) reported 7,416 events of revascularization.
Omega-3 fatty acids were associated with reducing MACE (RR, 0.95; P = .002) and revascularization (RR, 0.91; P = .0001). The meta-analysis showed higher risk reductions in MACE with EPA monotherapy (RR, 0.78; P = .00000001), whereas EPA+DHA combination did not reduce MACE (RR, 0.99; P = .48). This effect was consistent for revascularization.
A total of eight trials with 65,404 individuals reported 935 nonfatal strokes, and eight trials with 51,336 individuals reported 1,572 events of atrial fibrillation (AFib).
Omega-3 fatty acids did not significantly reduce nonfatal stroke (RR, 1.04; P = .55), but EPA monotherapy was associated with a reduction of nonfatal stroke, compared with control (RR: 0.71; P = .01).
Conversely, omega-3 fatty acids were associated with increased risk for AFib (RR, 1.26; P = .004), with a higher risk with EPA monotherapy than with control (RR, 1.35; P = .004).
Overall, omega-3 fatty acids did not prevent sudden cardiac death or increase gastrointestinal-related adverse events, total bleeding, or major or minor bleeding; however, the meta-analysis showed a higher risk of total bleeding with EPA monotherapy than with control (RR, 1.49; P = .006).
An influence analysis with stepwise exclusion of one trial at a time, including REDUCE-IT, did not alter the overall summary estimates. “Despite the exclusion of REDUCE-IT, EPA monotherapy reduced MACE by 23%, compared with the control,” the authors reported.
They said these new findings also have important implications for clinical practice and treatment guidelines.
“After REDUCE-IT, several national and international guidelines endorsed EPA in their therapeutic recommendations. However, the publication of two recent negative trials of EPA + DHA has created some confusion in the scientific community about the value of omega-3 FAs in preventing atherosclerotic cardiovascular disease [ASCVD],” they stated.
“This meta-analysis provides reassurance about the role of omega-3 fatty acids, specifically EPA, in the current treatment framework of ASCVD residual cardiovascular risk reduction and encourages investigators to explore further the cardiovascular effects of EPA across different clinical settings,” they added.
REDUCE-IT was sponsored by Amarin. Brigham and Women’s Hospital receives research funding from Amarin for the work Dr. Bhatt did as the trial chair and as the international principal investigator. The present analysis was unfunded.
A version of this article first appeared on Medscape.com.
Support for a cardiovascular benefit of omega-3 fatty acids, particularly eicosapentaenoic acid (EPA), has come from a new systematic review and meta-analysis of randomized trials.
The meta-analysis of 38 randomized controlled trials found that omega-3 fatty acids improved cardiovascular outcomes, with a greater reduction in cardiovascular risk in studies of EPA alone rather than of combined eicosapentaenoic plus docosahexaenoic acid (DHA) supplements.
The paper was published online in EClinicalMedicine.
Senior author Deepak Bhatt, MD, Brigham and Women’s Hospital, Boston, was also lead investigator of the REDUCE-IT trial, which is included in the analysis and showed a 25% relative risk reduction in major cardiovascular events with a high-dose EPA product.
But the REDUCE-IT trial has been mired in controversy, with suggestions that the benefit seen might have been exaggerated because of the use of a harmful placebo. In addition, a second large trial of high-dose omega-3 fatty acids, STRENGTH (which tested a combination EPA/DHA product) showed no benefit on cardiovascular outcomes.
Dr. Bhatt said the new meta-analysis provides “a totality of evidence” that “supports a robust and consistent benefit of EPA.”
In the review, the authors concluded: “In this systematic review and meta-analysis, we noted moderate certainty of evidence favoring omega-3 fatty acids for reducing cardiovascular mortality and outcomes. ... The magnitude of relative reductions was robust in EPA trials versus those of EPA+DHA, suggesting differential effects of EPA and DHA in cardiovascular risk reduction.”
Controversy continues
But commenting on the publication for an interview, Steven Nissen, MD, Cleveland Clinic, who led the STRENGTH trial, pointed out that 85% of the EPA data in the new meta-analysis came from REDUCE-IT, so the results were a “foregone conclusion.”
“The purpose of a meta-analysis is to answer scientific questions when existing studies are too small to yield statistically robust results. That is not the case here,” Dr. Nissen stated.
He added: “There are only two major trials of EPA and both have important flaws. REDUCE-IT used a questionable placebo (mineral oil) and JELIS was an open-label trial that studied patients with baseline LDL [cholesterol] of 180 mg/dL that was not appropriately treated. A meta-analysis is only as good as the studies that it includes. The other EPA plus DHA studies were essentially neutral.”
Dr. Bhatt responded that, “to date, every randomized trial of EPA only has been positive. Some have been placebo controlled, some have been open label. This meta-analysis corroborates the results of each of those trials in a statistically robust way.”
He added: “Of course, REDUCE-IT is the most rigorous, contemporary trial of EPA. However, in our meta-analysis, even when excluding REDUCE-IT (or for that matter, JELIS), the EPA trials still significantly reduced cardiovascular events.”
Dr. Bhatt also pointed out that two randomized imaging studies, CHERRY and EVAPORATE, have shown benefits of EPA.
“Beyond the clinical trial data, there is a growing amount of evidence supporting the unique biological actions of different omega-3 fatty acids. EPA, in particular, appears to have the strongest basic science evidence supporting cardiovascular benefits. Overall, it is a remarkably consistent scientific story in support of EPA’s beneficial effects on cardiovascular health,” he stated.
38 trials included
For the current paper, Dr. Bhatt and coauthors performed a comprehensive literature search for randomized trials comparing omega-3 fatty acids with control (placebo, no supplementation, or lower dose of omega-3 fatty acids) in adults, with a follow-up of at least 12 months, and mortality and cardiovascular outcomes as endpoints.
Ultimately, 38 trials encompassing 149,051 patients were included. Of these, four trials compared EPA with control, 34 trials compared EPA+DHA with control, and 22 trials were in primary prevention. The dose of omega-3 fatty acids ranged from 0.4 g/day to 5.5 g/day.
A total of 25 trials with 143,514 individuals reported 5,550 events of cardiovascular mortality, and 24 trials with 140,983 individuals reported 10,795 events of all-cause mortality.
Omega-3 fatty acids were associated with reduced cardiovascular mortality (rate ratio, 0.93; P = .01), but not all-cause mortality (RR, 0.97; P = .27). The meta-analysis showed reduction in cardiovascular mortality with EPA monotherapy (RR, 0.82; P = .04) and EPA+DHA combination (RR, 0.94; P = .02).
A total of 20 trials with 125,611 individuals reported 2,989 nonfatal myocardial infarction events, and 29 trials with 144,384 individuals reported 9,153 coronary heart disease (CHD) events.
Omega-3 fatty acids were associated with reducing nonfatal MI (RR, 0.87; P = .0001) and CHD (RR, 0.91; P = .0002). The meta-analysis showed higher risk reductions in nonfatal MI with EPA monotherapy (RR, 0.72; P = .00002) than with EPA+DHA combination (RR, 0.92; P = .05), and also for CHD events with EPA monotherapy (RR, 0.73; P = .00004) than with EPA+DHA combination (RR, 0.94; P = .01).
A total of 17 trials (n = 135,019) reported 13,234 events of MACE, and 13 trials (n = 117,890) reported 7,416 events of revascularization.
Omega-3 fatty acids were associated with reducing MACE (RR, 0.95; P = .002) and revascularization (RR, 0.91; P = .0001). The meta-analysis showed higher risk reductions in MACE with EPA monotherapy (RR, 0.78; P = .00000001), whereas EPA+DHA combination did not reduce MACE (RR, 0.99; P = .48). This effect was consistent for revascularization.
A total of eight trials with 65,404 individuals reported 935 nonfatal strokes, and eight trials with 51,336 individuals reported 1,572 events of atrial fibrillation (AFib).
Omega-3 fatty acids did not significantly reduce nonfatal stroke (RR, 1.04; P = .55), but EPA monotherapy was associated with a reduction of nonfatal stroke, compared with control (RR: 0.71; P = .01).
Conversely, omega-3 fatty acids were associated with increased risk for AFib (RR, 1.26; P = .004), with a higher risk with EPA monotherapy than with control (RR, 1.35; P = .004).
Overall, omega-3 fatty acids did not prevent sudden cardiac death or increase gastrointestinal-related adverse events, total bleeding, or major or minor bleeding; however, the meta-analysis showed a higher risk of total bleeding with EPA monotherapy than with control (RR, 1.49; P = .006).
An influence analysis with stepwise exclusion of one trial at a time, including REDUCE-IT, did not alter the overall summary estimates. “Despite the exclusion of REDUCE-IT, EPA monotherapy reduced MACE by 23%, compared with the control,” the authors reported.
They said these new findings also have important implications for clinical practice and treatment guidelines.
“After REDUCE-IT, several national and international guidelines endorsed EPA in their therapeutic recommendations. However, the publication of two recent negative trials of EPA + DHA has created some confusion in the scientific community about the value of omega-3 FAs in preventing atherosclerotic cardiovascular disease [ASCVD],” they stated.
“This meta-analysis provides reassurance about the role of omega-3 fatty acids, specifically EPA, in the current treatment framework of ASCVD residual cardiovascular risk reduction and encourages investigators to explore further the cardiovascular effects of EPA across different clinical settings,” they added.
REDUCE-IT was sponsored by Amarin. Brigham and Women’s Hospital receives research funding from Amarin for the work Dr. Bhatt did as the trial chair and as the international principal investigator. The present analysis was unfunded.
A version of this article first appeared on Medscape.com.
New drug, finerenone, approved for slowing kidney disease in diabetes
The U.S. Food and Drug Administration approved finerenone (Kerendia), the first agent from a new class of nonsteroidal mineralocorticoid receptor antagonists (MRAs), on July 9 for treating patients with chronic kidney disease (CKD) associated with type 2 diabetes.
Janani Rangaswami, MD, a nephrologist not involved with finerenone’s development, hailed the action as a “welcome addition to therapies in the cardiorenal space.”
She also highlighted that until more evidence accumulates, finerenone will take a back seat to two more established renal-protective drug classes for patients with type 2 diabetes, the renin-angiotensin system inhibitors (RASIs), and the sodium-glucose cotransporter 2 (SGLT2) inhibitors.
RASIs, which include angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, remain first-line treatments for slowing the progression of CKD in patients with type 2 diabetes. The efficacy and safety of these agents are well-established. The trial that led to the FDA’s decision to approve finerenone, FIDELIO-DKD, compared it against placebo in more than 5,700 patients with type 2 diabetes who were all already taking a maximum-tolerated dose of an RASI.
Scant data on combining finerenone with an SGLT2 inhibitor
Two agents in the SGLT2 inhibitor class, approved initially for type 2 diabetes, received additional FDA approvals for slowing kidney disease: Canagliflozin (Invokana), which was approved in September 2019 on the basis of the CREDENCE trial, and dapagliflozin (Forxiga/Farxiga), which was approved in April 2021 on the basis of DAPA-CKD. Nephrologists now speak of this drug class as “practice changing.”
When FIDELIO-DKD enrolled patients from September 2015 to June 2018, it was still early days for use of SGLT2 inhibitors for patients with type 2 diabetes; hence, fewer than 5% of enrolled patients received an SGLT2 inhibitor, making it impossible to say how well finerenone works when taken along with one of these drugs.
“The big question that persists is the incremental benefit [from finerenone] on top of an SGLT2 inhibitor,” commented Dr. Rangaswami, director of the cardiorenal program at George Washington University, Washington, and chair-elect of the Council on the Kidney in Cardiovascular Disease of the American Heart Association.
“It is hard to extrapolate incremental benefit from existing finerenone trial data given the low background use of SGLT2 inhibitors [in FIDELIO-DKD],” she said in an interview.
George Bakris, MD, lead investigator for FIDELIO-DKD, agrees.
SGLT2 inhibitors are a ‘must’ for CKD
An SGLT2 inhibitor “must be used, period,” for patients with type 2 diabetes and CKD. “The evidence is very strong,” said Dr. Bakris, speaking in June 2021 during a session of the virtual annual Congress of the European Renal Association and European Dialysis and Transplant Association.
Because of inadequate evidence on how finerenone works when administered in addition to an SGLT2 inhibitor, for the time being, the combination must be considered investigational, he added.
Study results “need to show that combination therapy [with an SGLT2 inhibitor and finerenone] is better” than an SGLT2 inhibitor alone, said Dr. Bakris, professor of medicine and director of the Comprehensive Hypertension Center of the University of Chicago.
During his June talk, Dr. Bakris predicted that by 2023, enough data will exist from patients treated with both an SGLT2 inhibitor and finerenone to allow an evidence-based approach to combination treatment.
Finerenone’s approval makes it an immediate choice for patients with type 2 diabetes and CKD secondary to polycystic kidney disease, a group who are not candidates for an SGLT2 inhibitor, said Dr. Rangaswami.
But “if a patient is eligible for an SGLT2 inhibitor, I would not stop that in favor of starting finerenone” on the basis of current knowledge, she noted.
‘Not your mother’s spironolactone’
Although finerenone is classified an MRA, the class that also includes the steroidal agents spironolactone and eplerenone, the nonsteroidal structure of finerenone means “it has nothing to do with spironolactone. It’s a different molecule with different chemistry,” Dr. Bakris said in his June talk.
Although the risk for hyperkalemia has been a limiting factor and a deterrent to routine use of steroidal MRAs for preventing progression of CKD, hyperkalemia is much less of a problem with finerenone.
Main results from FIDELIO-DKD, published in late 2020, showed that the percentage of patients receiving finerenone who permanently stopped taking the drug because of hyperkalemia was 2.3%, higher than the 0.9% rate among patients in the trial who received placebo but about a third of the rate of patients treated with spironolactone in a historical cohort.
“You need to pay attention” to the potential development of hyperkalemia in patients taking finerenone, “but it is not a major issue,” Dr. Bakris said. “Finerenone is not your mother’s spironolactone,” he declared.
FIDELIO-DKD’s primary outcome, a combination of several adverse renal events, showed that treatment with finerenone cut this endpoint by a significant 18% compared with placebo. The study’s main secondary endpoint showed that finerenone cut the incidence of combined cardiovascular disease events by a significant 14% compared with placebo. Adverse events were similar in the finerenone and placebo arms.
Finerenone also shows promise for reducing CVD events
Bayer, the company that developed and will market finerenone, announced in May 2021 topline results from a companion trial, FIGARO-DKD. That trial also enrolled patients with type 2 diabetes and CKD, but a primary endpoint of that trial combined the rates of cardiovascular death and nonfatal cardiovascular disease events. The results from this trial showed a significant difference in favor of finerenone compared with placebo.
“Given the common pathways that progression of CKD and cardiovascular disease share with respect to [moderating] inflammation and [slowing development of] fibrosis, it is not surprising that a signal for benefit was seen at the different ends of the cardiorenal spectrum,” Dr. Rangaswami said.
FIDELIO-DKD and FIGARO-DKD were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Bakris has been a consultant to and has received research funding from Bayer and from numerous other companies. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved finerenone (Kerendia), the first agent from a new class of nonsteroidal mineralocorticoid receptor antagonists (MRAs), on July 9 for treating patients with chronic kidney disease (CKD) associated with type 2 diabetes.
Janani Rangaswami, MD, a nephrologist not involved with finerenone’s development, hailed the action as a “welcome addition to therapies in the cardiorenal space.”
She also highlighted that until more evidence accumulates, finerenone will take a back seat to two more established renal-protective drug classes for patients with type 2 diabetes, the renin-angiotensin system inhibitors (RASIs), and the sodium-glucose cotransporter 2 (SGLT2) inhibitors.
RASIs, which include angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, remain first-line treatments for slowing the progression of CKD in patients with type 2 diabetes. The efficacy and safety of these agents are well-established. The trial that led to the FDA’s decision to approve finerenone, FIDELIO-DKD, compared it against placebo in more than 5,700 patients with type 2 diabetes who were all already taking a maximum-tolerated dose of an RASI.
Scant data on combining finerenone with an SGLT2 inhibitor
Two agents in the SGLT2 inhibitor class, approved initially for type 2 diabetes, received additional FDA approvals for slowing kidney disease: Canagliflozin (Invokana), which was approved in September 2019 on the basis of the CREDENCE trial, and dapagliflozin (Forxiga/Farxiga), which was approved in April 2021 on the basis of DAPA-CKD. Nephrologists now speak of this drug class as “practice changing.”
When FIDELIO-DKD enrolled patients from September 2015 to June 2018, it was still early days for use of SGLT2 inhibitors for patients with type 2 diabetes; hence, fewer than 5% of enrolled patients received an SGLT2 inhibitor, making it impossible to say how well finerenone works when taken along with one of these drugs.
“The big question that persists is the incremental benefit [from finerenone] on top of an SGLT2 inhibitor,” commented Dr. Rangaswami, director of the cardiorenal program at George Washington University, Washington, and chair-elect of the Council on the Kidney in Cardiovascular Disease of the American Heart Association.
“It is hard to extrapolate incremental benefit from existing finerenone trial data given the low background use of SGLT2 inhibitors [in FIDELIO-DKD],” she said in an interview.
George Bakris, MD, lead investigator for FIDELIO-DKD, agrees.
SGLT2 inhibitors are a ‘must’ for CKD
An SGLT2 inhibitor “must be used, period,” for patients with type 2 diabetes and CKD. “The evidence is very strong,” said Dr. Bakris, speaking in June 2021 during a session of the virtual annual Congress of the European Renal Association and European Dialysis and Transplant Association.
Because of inadequate evidence on how finerenone works when administered in addition to an SGLT2 inhibitor, for the time being, the combination must be considered investigational, he added.
Study results “need to show that combination therapy [with an SGLT2 inhibitor and finerenone] is better” than an SGLT2 inhibitor alone, said Dr. Bakris, professor of medicine and director of the Comprehensive Hypertension Center of the University of Chicago.
During his June talk, Dr. Bakris predicted that by 2023, enough data will exist from patients treated with both an SGLT2 inhibitor and finerenone to allow an evidence-based approach to combination treatment.
Finerenone’s approval makes it an immediate choice for patients with type 2 diabetes and CKD secondary to polycystic kidney disease, a group who are not candidates for an SGLT2 inhibitor, said Dr. Rangaswami.
But “if a patient is eligible for an SGLT2 inhibitor, I would not stop that in favor of starting finerenone” on the basis of current knowledge, she noted.
‘Not your mother’s spironolactone’
Although finerenone is classified an MRA, the class that also includes the steroidal agents spironolactone and eplerenone, the nonsteroidal structure of finerenone means “it has nothing to do with spironolactone. It’s a different molecule with different chemistry,” Dr. Bakris said in his June talk.
Although the risk for hyperkalemia has been a limiting factor and a deterrent to routine use of steroidal MRAs for preventing progression of CKD, hyperkalemia is much less of a problem with finerenone.
Main results from FIDELIO-DKD, published in late 2020, showed that the percentage of patients receiving finerenone who permanently stopped taking the drug because of hyperkalemia was 2.3%, higher than the 0.9% rate among patients in the trial who received placebo but about a third of the rate of patients treated with spironolactone in a historical cohort.
“You need to pay attention” to the potential development of hyperkalemia in patients taking finerenone, “but it is not a major issue,” Dr. Bakris said. “Finerenone is not your mother’s spironolactone,” he declared.
FIDELIO-DKD’s primary outcome, a combination of several adverse renal events, showed that treatment with finerenone cut this endpoint by a significant 18% compared with placebo. The study’s main secondary endpoint showed that finerenone cut the incidence of combined cardiovascular disease events by a significant 14% compared with placebo. Adverse events were similar in the finerenone and placebo arms.
Finerenone also shows promise for reducing CVD events
Bayer, the company that developed and will market finerenone, announced in May 2021 topline results from a companion trial, FIGARO-DKD. That trial also enrolled patients with type 2 diabetes and CKD, but a primary endpoint of that trial combined the rates of cardiovascular death and nonfatal cardiovascular disease events. The results from this trial showed a significant difference in favor of finerenone compared with placebo.
“Given the common pathways that progression of CKD and cardiovascular disease share with respect to [moderating] inflammation and [slowing development of] fibrosis, it is not surprising that a signal for benefit was seen at the different ends of the cardiorenal spectrum,” Dr. Rangaswami said.
FIDELIO-DKD and FIGARO-DKD were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Bakris has been a consultant to and has received research funding from Bayer and from numerous other companies. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved finerenone (Kerendia), the first agent from a new class of nonsteroidal mineralocorticoid receptor antagonists (MRAs), on July 9 for treating patients with chronic kidney disease (CKD) associated with type 2 diabetes.
Janani Rangaswami, MD, a nephrologist not involved with finerenone’s development, hailed the action as a “welcome addition to therapies in the cardiorenal space.”
She also highlighted that until more evidence accumulates, finerenone will take a back seat to two more established renal-protective drug classes for patients with type 2 diabetes, the renin-angiotensin system inhibitors (RASIs), and the sodium-glucose cotransporter 2 (SGLT2) inhibitors.
RASIs, which include angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, remain first-line treatments for slowing the progression of CKD in patients with type 2 diabetes. The efficacy and safety of these agents are well-established. The trial that led to the FDA’s decision to approve finerenone, FIDELIO-DKD, compared it against placebo in more than 5,700 patients with type 2 diabetes who were all already taking a maximum-tolerated dose of an RASI.
Scant data on combining finerenone with an SGLT2 inhibitor
Two agents in the SGLT2 inhibitor class, approved initially for type 2 diabetes, received additional FDA approvals for slowing kidney disease: Canagliflozin (Invokana), which was approved in September 2019 on the basis of the CREDENCE trial, and dapagliflozin (Forxiga/Farxiga), which was approved in April 2021 on the basis of DAPA-CKD. Nephrologists now speak of this drug class as “practice changing.”
When FIDELIO-DKD enrolled patients from September 2015 to June 2018, it was still early days for use of SGLT2 inhibitors for patients with type 2 diabetes; hence, fewer than 5% of enrolled patients received an SGLT2 inhibitor, making it impossible to say how well finerenone works when taken along with one of these drugs.
“The big question that persists is the incremental benefit [from finerenone] on top of an SGLT2 inhibitor,” commented Dr. Rangaswami, director of the cardiorenal program at George Washington University, Washington, and chair-elect of the Council on the Kidney in Cardiovascular Disease of the American Heart Association.
“It is hard to extrapolate incremental benefit from existing finerenone trial data given the low background use of SGLT2 inhibitors [in FIDELIO-DKD],” she said in an interview.
George Bakris, MD, lead investigator for FIDELIO-DKD, agrees.
SGLT2 inhibitors are a ‘must’ for CKD
An SGLT2 inhibitor “must be used, period,” for patients with type 2 diabetes and CKD. “The evidence is very strong,” said Dr. Bakris, speaking in June 2021 during a session of the virtual annual Congress of the European Renal Association and European Dialysis and Transplant Association.
Because of inadequate evidence on how finerenone works when administered in addition to an SGLT2 inhibitor, for the time being, the combination must be considered investigational, he added.
Study results “need to show that combination therapy [with an SGLT2 inhibitor and finerenone] is better” than an SGLT2 inhibitor alone, said Dr. Bakris, professor of medicine and director of the Comprehensive Hypertension Center of the University of Chicago.
During his June talk, Dr. Bakris predicted that by 2023, enough data will exist from patients treated with both an SGLT2 inhibitor and finerenone to allow an evidence-based approach to combination treatment.
Finerenone’s approval makes it an immediate choice for patients with type 2 diabetes and CKD secondary to polycystic kidney disease, a group who are not candidates for an SGLT2 inhibitor, said Dr. Rangaswami.
But “if a patient is eligible for an SGLT2 inhibitor, I would not stop that in favor of starting finerenone” on the basis of current knowledge, she noted.
‘Not your mother’s spironolactone’
Although finerenone is classified an MRA, the class that also includes the steroidal agents spironolactone and eplerenone, the nonsteroidal structure of finerenone means “it has nothing to do with spironolactone. It’s a different molecule with different chemistry,” Dr. Bakris said in his June talk.
Although the risk for hyperkalemia has been a limiting factor and a deterrent to routine use of steroidal MRAs for preventing progression of CKD, hyperkalemia is much less of a problem with finerenone.
Main results from FIDELIO-DKD, published in late 2020, showed that the percentage of patients receiving finerenone who permanently stopped taking the drug because of hyperkalemia was 2.3%, higher than the 0.9% rate among patients in the trial who received placebo but about a third of the rate of patients treated with spironolactone in a historical cohort.
“You need to pay attention” to the potential development of hyperkalemia in patients taking finerenone, “but it is not a major issue,” Dr. Bakris said. “Finerenone is not your mother’s spironolactone,” he declared.
FIDELIO-DKD’s primary outcome, a combination of several adverse renal events, showed that treatment with finerenone cut this endpoint by a significant 18% compared with placebo. The study’s main secondary endpoint showed that finerenone cut the incidence of combined cardiovascular disease events by a significant 14% compared with placebo. Adverse events were similar in the finerenone and placebo arms.
Finerenone also shows promise for reducing CVD events
Bayer, the company that developed and will market finerenone, announced in May 2021 topline results from a companion trial, FIGARO-DKD. That trial also enrolled patients with type 2 diabetes and CKD, but a primary endpoint of that trial combined the rates of cardiovascular death and nonfatal cardiovascular disease events. The results from this trial showed a significant difference in favor of finerenone compared with placebo.
“Given the common pathways that progression of CKD and cardiovascular disease share with respect to [moderating] inflammation and [slowing development of] fibrosis, it is not surprising that a signal for benefit was seen at the different ends of the cardiorenal spectrum,” Dr. Rangaswami said.
FIDELIO-DKD and FIGARO-DKD were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Bakris has been a consultant to and has received research funding from Bayer and from numerous other companies. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Treat youth with gender dysphoria as individuals’
Young people with gender dysphoria should be considered as individuals rather than fall into an age-defined bracket when assessing their understanding to consent to hormone treatment, according to the Tavistock and Portman NHS Foundation Trust, as it awaits the verdict of its recent appeal in London against a High Court ruling.
The High Court ruling, made in December 2020 as reported by this news organization, stated that adolescents with gender dysphoria were unlikely to fully understand the consequences of hormone treatment for gender reassignment and was the result of a case brought by 24-year-old Keira Bell, who transitioned from female to male at the Gender Identity Development Service (GIDS), starting at the age of 16, but later “detransitioned.”
Along with changes made to rules around prescribing puberty blockers and cross-sex hormones to minors with gender dysphoria in countries such as Finland and Sweden, the English ruling signals a more cautious approach to any medical treatment for such children, as detailed in a feature published in April.
However, during the appeal, The Trust argued once more that puberty blockers give children time to “consider options” about their bodies and that the decision (the December ruling) was inconsistent with the law that “entitles children under the age of 16 to make decisions for themselves after being assessed as competent to do so by their doctor.”
Alongside other organizations, the United States–based Endocrine Society submitted written evidence in support of the Tavistock. “The High Court’s decision, if it is allowed to stand, would set a harmful precedent preventing physicians from providing transgender and gender diverse youth with high-quality medical care,” it noted in a statement.
Defending the High Court’s ruling, the lawyer for Ms. Bell said its conclusion was that puberty blockers for gender dysphoria are an “experimental” treatment with a very limited evidence base.
“The judgment of the [High Court] is entirely correct, and there is no proper basis for overturning it,” he asserted.
The 2-day appeal hearing ended on June 24, and a ruling will be made at a later date.
Do children understand the consequences of hormone treatment?
One central aspect of the overall case is the fact that Ms. Bell regrets her decision to transition at age 16, saying she only received three counseling sessions prior to endocrinology referral. And she consequently had a mastectomy at age 20, which she also bitterly regrets.
So a key concern is whether young people fully understand the consequences of taking puberty blockers and therapies that may follow, including cross-sex hormones.
Witness for the appeal Gary Butler, MD, consultant in pediatric and adolescent endocrinology at University College Hospital, London, where children are referred to from GIDS for hormone treatment, said the number of children who go on to cross-sex hormones from puberty blockers is “over 80%.”
But the actual number of children who are referred to endocrinology services (where puberty blockers are initiated) from GIDS is low, at approximately 16%, according to 2019-2020 data, said a GIDS spokesperson.
“Once at the endocrinology service, young people either participate in a group education session, or if under 15 years, an individualized session between the clinician and the patient and family members,” she added. The Trust also maintained that initiation of cross-sex hormones “is separate from the prescription of puberty blockers.”
Since the December ruling, The Trust has put in place multidisciplinary clinical reviews (MDCR) of cases, and in July, NHS England will start implementing an independent multidisciplinary professional review (MDPR) to check that the GIDS has followed due process with each case.
Slow the process down, give appropriate psychotherapy
Stella O’Malley is a psychotherapist who works with transitioners and detransitioners and is a founding member of the International Association of Therapists for Desisters and Detransitioners (IATDD).
Whatever the outcome of the appeal process, Ms. O’Malley said she would like to see the Tavistock slow down and take a broader approach to counseling children before referral to endocrinology services.
In discussing therapy prior to transition, Ms. O’Malley stated that her clients often say they did not explore their inner motivations or other possible reasons for their distress, and the therapy was focused more on when they transition, rather than being sure it was something they wanted to do.
“We need to learn from the mistakes made with people like Keira Bell. , especially when [children are] ... young and especially when they’re traumatized,” Ms. O’Malley said.
“Had they received a more conventional therapy, they might have thought about their decision from different perspectives and in the process acquired more self-awareness, which would have been more beneficial.”
“The ‘affirmative’ approach to gender therapy is too narrow; we need to look at the whole individual. Therapy in other areas would never disregard other, nongender issues such as attention deficit hyperactivity disorder or anxiety [which often co-exist with gender dysphoria] – issues bleed into each other,” Ms. O’Malley pointed out. “We need a more exploratory approach.”
“I’d also like to see other therapists all over the [U.K.] who are perfectly qualified and capable of working with gender actually start working with gender issues,” she said, noting that such an approach might also help reduce the long waiting list at the Tavistock.
The latter had been overwhelmed, and this led to a speeding up of the assessment process, which led to a number of professionals resigning from the service in recent years, saying children were being “fast-tracked” to medical transition.
Fertility and sexual function are complex issues for kids
Also asked to comment was Claire Graham, from Genspect, a group that describes itself as a voice for parents of gender-questioning kids.
She told this news organization that “parents are rightly concerned about their children’s ability to consent to treatments that may lead to infertility and issues surrounding sexual function.” She added that other countries in Europe were changing their approach. “Look to Sweden and Finland, who have both rowed back on puberty blockers and no longer recommend them.”
Ms. Graham, who has worked with children with differences in sexual development, added that it was very difficult for children and young people to understand the life-long implications of decisions made at an early age.
“How can children understand what it is to live with impaired sexual functioning if they have never had sex? Likewise, fertility is a complex issue. Most people do not want to become parents as teenagers, but we understand that this will often change as they grow,” said Ms. Graham.
“Many parents worry that their child is not being considered in the whole [and] that their child’s ability to consent to medical interventions for gender dysphoria is impacted by comorbidities, such as a diagnosis of autism or a history of mental health issues. These children are particularly vulnerable.”
“At Genspect, we hope that the decision from the ... court is upheld,” Ms. Graham concluded.
A version of this article first appeared on Medscape.com.
Young people with gender dysphoria should be considered as individuals rather than fall into an age-defined bracket when assessing their understanding to consent to hormone treatment, according to the Tavistock and Portman NHS Foundation Trust, as it awaits the verdict of its recent appeal in London against a High Court ruling.
The High Court ruling, made in December 2020 as reported by this news organization, stated that adolescents with gender dysphoria were unlikely to fully understand the consequences of hormone treatment for gender reassignment and was the result of a case brought by 24-year-old Keira Bell, who transitioned from female to male at the Gender Identity Development Service (GIDS), starting at the age of 16, but later “detransitioned.”
Along with changes made to rules around prescribing puberty blockers and cross-sex hormones to minors with gender dysphoria in countries such as Finland and Sweden, the English ruling signals a more cautious approach to any medical treatment for such children, as detailed in a feature published in April.
However, during the appeal, The Trust argued once more that puberty blockers give children time to “consider options” about their bodies and that the decision (the December ruling) was inconsistent with the law that “entitles children under the age of 16 to make decisions for themselves after being assessed as competent to do so by their doctor.”
Alongside other organizations, the United States–based Endocrine Society submitted written evidence in support of the Tavistock. “The High Court’s decision, if it is allowed to stand, would set a harmful precedent preventing physicians from providing transgender and gender diverse youth with high-quality medical care,” it noted in a statement.
Defending the High Court’s ruling, the lawyer for Ms. Bell said its conclusion was that puberty blockers for gender dysphoria are an “experimental” treatment with a very limited evidence base.
“The judgment of the [High Court] is entirely correct, and there is no proper basis for overturning it,” he asserted.
The 2-day appeal hearing ended on June 24, and a ruling will be made at a later date.
Do children understand the consequences of hormone treatment?
One central aspect of the overall case is the fact that Ms. Bell regrets her decision to transition at age 16, saying she only received three counseling sessions prior to endocrinology referral. And she consequently had a mastectomy at age 20, which she also bitterly regrets.
So a key concern is whether young people fully understand the consequences of taking puberty blockers and therapies that may follow, including cross-sex hormones.
Witness for the appeal Gary Butler, MD, consultant in pediatric and adolescent endocrinology at University College Hospital, London, where children are referred to from GIDS for hormone treatment, said the number of children who go on to cross-sex hormones from puberty blockers is “over 80%.”
But the actual number of children who are referred to endocrinology services (where puberty blockers are initiated) from GIDS is low, at approximately 16%, according to 2019-2020 data, said a GIDS spokesperson.
“Once at the endocrinology service, young people either participate in a group education session, or if under 15 years, an individualized session between the clinician and the patient and family members,” she added. The Trust also maintained that initiation of cross-sex hormones “is separate from the prescription of puberty blockers.”
Since the December ruling, The Trust has put in place multidisciplinary clinical reviews (MDCR) of cases, and in July, NHS England will start implementing an independent multidisciplinary professional review (MDPR) to check that the GIDS has followed due process with each case.
Slow the process down, give appropriate psychotherapy
Stella O’Malley is a psychotherapist who works with transitioners and detransitioners and is a founding member of the International Association of Therapists for Desisters and Detransitioners (IATDD).
Whatever the outcome of the appeal process, Ms. O’Malley said she would like to see the Tavistock slow down and take a broader approach to counseling children before referral to endocrinology services.
In discussing therapy prior to transition, Ms. O’Malley stated that her clients often say they did not explore their inner motivations or other possible reasons for their distress, and the therapy was focused more on when they transition, rather than being sure it was something they wanted to do.
“We need to learn from the mistakes made with people like Keira Bell. , especially when [children are] ... young and especially when they’re traumatized,” Ms. O’Malley said.
“Had they received a more conventional therapy, they might have thought about their decision from different perspectives and in the process acquired more self-awareness, which would have been more beneficial.”
“The ‘affirmative’ approach to gender therapy is too narrow; we need to look at the whole individual. Therapy in other areas would never disregard other, nongender issues such as attention deficit hyperactivity disorder or anxiety [which often co-exist with gender dysphoria] – issues bleed into each other,” Ms. O’Malley pointed out. “We need a more exploratory approach.”
“I’d also like to see other therapists all over the [U.K.] who are perfectly qualified and capable of working with gender actually start working with gender issues,” she said, noting that such an approach might also help reduce the long waiting list at the Tavistock.
The latter had been overwhelmed, and this led to a speeding up of the assessment process, which led to a number of professionals resigning from the service in recent years, saying children were being “fast-tracked” to medical transition.
Fertility and sexual function are complex issues for kids
Also asked to comment was Claire Graham, from Genspect, a group that describes itself as a voice for parents of gender-questioning kids.
She told this news organization that “parents are rightly concerned about their children’s ability to consent to treatments that may lead to infertility and issues surrounding sexual function.” She added that other countries in Europe were changing their approach. “Look to Sweden and Finland, who have both rowed back on puberty blockers and no longer recommend them.”
Ms. Graham, who has worked with children with differences in sexual development, added that it was very difficult for children and young people to understand the life-long implications of decisions made at an early age.
“How can children understand what it is to live with impaired sexual functioning if they have never had sex? Likewise, fertility is a complex issue. Most people do not want to become parents as teenagers, but we understand that this will often change as they grow,” said Ms. Graham.
“Many parents worry that their child is not being considered in the whole [and] that their child’s ability to consent to medical interventions for gender dysphoria is impacted by comorbidities, such as a diagnosis of autism or a history of mental health issues. These children are particularly vulnerable.”
“At Genspect, we hope that the decision from the ... court is upheld,” Ms. Graham concluded.
A version of this article first appeared on Medscape.com.
Young people with gender dysphoria should be considered as individuals rather than fall into an age-defined bracket when assessing their understanding to consent to hormone treatment, according to the Tavistock and Portman NHS Foundation Trust, as it awaits the verdict of its recent appeal in London against a High Court ruling.
The High Court ruling, made in December 2020 as reported by this news organization, stated that adolescents with gender dysphoria were unlikely to fully understand the consequences of hormone treatment for gender reassignment and was the result of a case brought by 24-year-old Keira Bell, who transitioned from female to male at the Gender Identity Development Service (GIDS), starting at the age of 16, but later “detransitioned.”
Along with changes made to rules around prescribing puberty blockers and cross-sex hormones to minors with gender dysphoria in countries such as Finland and Sweden, the English ruling signals a more cautious approach to any medical treatment for such children, as detailed in a feature published in April.
However, during the appeal, The Trust argued once more that puberty blockers give children time to “consider options” about their bodies and that the decision (the December ruling) was inconsistent with the law that “entitles children under the age of 16 to make decisions for themselves after being assessed as competent to do so by their doctor.”
Alongside other organizations, the United States–based Endocrine Society submitted written evidence in support of the Tavistock. “The High Court’s decision, if it is allowed to stand, would set a harmful precedent preventing physicians from providing transgender and gender diverse youth with high-quality medical care,” it noted in a statement.
Defending the High Court’s ruling, the lawyer for Ms. Bell said its conclusion was that puberty blockers for gender dysphoria are an “experimental” treatment with a very limited evidence base.
“The judgment of the [High Court] is entirely correct, and there is no proper basis for overturning it,” he asserted.
The 2-day appeal hearing ended on June 24, and a ruling will be made at a later date.
Do children understand the consequences of hormone treatment?
One central aspect of the overall case is the fact that Ms. Bell regrets her decision to transition at age 16, saying she only received three counseling sessions prior to endocrinology referral. And she consequently had a mastectomy at age 20, which she also bitterly regrets.
So a key concern is whether young people fully understand the consequences of taking puberty blockers and therapies that may follow, including cross-sex hormones.
Witness for the appeal Gary Butler, MD, consultant in pediatric and adolescent endocrinology at University College Hospital, London, where children are referred to from GIDS for hormone treatment, said the number of children who go on to cross-sex hormones from puberty blockers is “over 80%.”
But the actual number of children who are referred to endocrinology services (where puberty blockers are initiated) from GIDS is low, at approximately 16%, according to 2019-2020 data, said a GIDS spokesperson.
“Once at the endocrinology service, young people either participate in a group education session, or if under 15 years, an individualized session between the clinician and the patient and family members,” she added. The Trust also maintained that initiation of cross-sex hormones “is separate from the prescription of puberty blockers.”
Since the December ruling, The Trust has put in place multidisciplinary clinical reviews (MDCR) of cases, and in July, NHS England will start implementing an independent multidisciplinary professional review (MDPR) to check that the GIDS has followed due process with each case.
Slow the process down, give appropriate psychotherapy
Stella O’Malley is a psychotherapist who works with transitioners and detransitioners and is a founding member of the International Association of Therapists for Desisters and Detransitioners (IATDD).
Whatever the outcome of the appeal process, Ms. O’Malley said she would like to see the Tavistock slow down and take a broader approach to counseling children before referral to endocrinology services.
In discussing therapy prior to transition, Ms. O’Malley stated that her clients often say they did not explore their inner motivations or other possible reasons for their distress, and the therapy was focused more on when they transition, rather than being sure it was something they wanted to do.
“We need to learn from the mistakes made with people like Keira Bell. , especially when [children are] ... young and especially when they’re traumatized,” Ms. O’Malley said.
“Had they received a more conventional therapy, they might have thought about their decision from different perspectives and in the process acquired more self-awareness, which would have been more beneficial.”
“The ‘affirmative’ approach to gender therapy is too narrow; we need to look at the whole individual. Therapy in other areas would never disregard other, nongender issues such as attention deficit hyperactivity disorder or anxiety [which often co-exist with gender dysphoria] – issues bleed into each other,” Ms. O’Malley pointed out. “We need a more exploratory approach.”
“I’d also like to see other therapists all over the [U.K.] who are perfectly qualified and capable of working with gender actually start working with gender issues,” she said, noting that such an approach might also help reduce the long waiting list at the Tavistock.
The latter had been overwhelmed, and this led to a speeding up of the assessment process, which led to a number of professionals resigning from the service in recent years, saying children were being “fast-tracked” to medical transition.
Fertility and sexual function are complex issues for kids
Also asked to comment was Claire Graham, from Genspect, a group that describes itself as a voice for parents of gender-questioning kids.
She told this news organization that “parents are rightly concerned about their children’s ability to consent to treatments that may lead to infertility and issues surrounding sexual function.” She added that other countries in Europe were changing their approach. “Look to Sweden and Finland, who have both rowed back on puberty blockers and no longer recommend them.”
Ms. Graham, who has worked with children with differences in sexual development, added that it was very difficult for children and young people to understand the life-long implications of decisions made at an early age.
“How can children understand what it is to live with impaired sexual functioning if they have never had sex? Likewise, fertility is a complex issue. Most people do not want to become parents as teenagers, but we understand that this will often change as they grow,” said Ms. Graham.
“Many parents worry that their child is not being considered in the whole [and] that their child’s ability to consent to medical interventions for gender dysphoria is impacted by comorbidities, such as a diagnosis of autism or a history of mental health issues. These children are particularly vulnerable.”
“At Genspect, we hope that the decision from the ... court is upheld,” Ms. Graham concluded.
A version of this article first appeared on Medscape.com.











