User login
SGLT2 inhibitors prevent HF hospitalization regardless of baseline LVEF
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
NEW ORLEANS – based on data from a large real-world patient registry.
“The observed beneficial effects of SGLT2 inhibitors on heart failure may extend across the range of baseline ejection fractions,” Mikhail Kosiborod, MD, observed at the annual meeting of the American College of Cardiology.
This is an important new insight. The major randomized cardiovascular outcome trials that showed lower risks of heart failure hospitalization and all-cause mortality in type 2 diabetic patients on an SGLT2 inhibitor, such as EMPA-REG OUTCOME for empagliflozin (Jardiance) and CANVAS for canagliflozin (Invokana), didn’t include information on baseline LVEF. So until now it has been unclear whether the beneficial effects of the SGLT2 inhibitors preventing heart failure hospitalization vary depending upon LVEF, explained Dr. Kosiborod, a cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
He presented an analysis drawn from the patient database kept by Maccabi Healthcare Services in Israel. The study included 5,307 patients with type 2 diabetes and an LVEF measurement recorded in their chart at the time they started on either empagliflozin or dapagliflozin (Farxiga) and an equal number of propensity-matched type 2 diabetic controls who started on other glucose-lowering drugs, most commonly an oral dipeptidyl peptidase-4 inhibitor.
During roughly 16,000 person-years of follow-up, 239 deaths occurred. Compared with patients on another glucose-lowering drug, the risk of death from all causes was reduced by 47% among patients who were on an SGLT2 inhibitor and had a baseline LVEF of 50% or greater and by 62% among the 9% of subjects who had a baseline LVEF less than 50%.
Similarly, the risk of heart failure hospitalization was reduced by 29% in SGLT2 inhibitor users with a preserved LVEF and by 27% if they had a reduced LVEF.
For the composite endpoint of heart failure hospitalization or all-cause mortality, the risk reductions associated with SGLT2 inhibitor therapy were 45% with preserved and 39% with reduced LVEF.
Session comoderator Prakash C. Deedwania, MD, noted that there are ongoing major randomized trials of various SGLT2 inhibitors in patients with known heart failure, with cardiovascular death and heart failure hospitalization as primary endpoints. He asked Dr. Kosiborod whether, given that the results of these studies aren’t in yet, he thinks clinicians should be prescribing SGLT2 inhibitors to diabetic or prediabetic patients who don’t have clinical symptoms of heart failure but may have a marker of increased risk, such as an elevated B-type natriuretic peptide.
“At least in my mind, we have more than enough evidence at this point to say that SGLT2 inhibitors are effective in preventing heart failure,” Dr. Kosiborod replied.
“Obviously, if your risk for developing a condition is higher at baseline, then the absolute benefit that you’re going to get from using an agent that’s effective in preventing that event is going to be higher and the number needed to treat is going to be lower. So if you have a patient at high risk for heart failure by whatever risk predictor you’re using and the patient doesn’t yet have heart failure but does have diabetes, which is already a risk factor for heart failure, I think we have pretty solid data now that SGLT2 inhibitors will likely be effective in preventing heart failure in that kind of patient population. But I don’t think we have definitive data at this point to say that the drugs are effective in treating heart failure in people who already have a manifest clinical syndrome of heart failure, which is why we’re doing all these clinical trials now,” he continued.
Dr. Deedwania urged audience members to make the effort to become comfortable in prescribing SGLT2 inhibitors for their patients with type 2 diabetes.
“Many different surveys show that these drugs are not being utilized effectively by cardiologists,” noted Dr. Deedwania, professor of medicine at the University of California, San Francisco, and director of the heart failure program at the university’s Fresno campus.
“As cardiologists, we may not want to own diabetes, but we at least have to feel that we have the ownership of treating the diabetic patient with cardiovascular disease with appropriate drugs. We don’t need to depend on endocrinologists because if we do these patients may become lost,” he said.
Dr. Kosiborod concurred, citing evidence that diabetic patients with cardiovascular disease are much more likely to see a cardiologist than an endocrinologist in the course of usual care.
“There’s definitely a golden opportunity here to intervene to reduce risk,” he said.
Dr. Kosiborod reported serving as a consultant to roughly a dozen pharmaceutical companies.
SOURCE: Kosiborod M. ACC 19, Abstract #1024-07.
REPORTING FROM ACC 19
Fournier gangrene cases surge in patients using SGLT2 inhibitors
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
since the US Food and Drug Administration (FDA) issued a 2018 warning about this rare but serious infection, researchers say.
Health care providers prescribing SGLT2 inhibitors to patients with diabetes should have a high index of suspicion for the signs and symptoms of Fournier gangrene, given its substantial morbidity and mortality, according to Susan J. Bersoff-Matcha, MD, and her colleagues at the FDA.
“Although the risk for [Fournier gangrene] is low, serious infection should be considered and weighed against the benefits of SGLT2 inhibitor therapy,” said Dr. Bersoff-Matcha and co-authors in their recent report published in the Annals of Internal Medicine (2019 May 6. doi: 10.7326/M19-0085).
In the previous warning, FDA officials said 12 cases of Fournier gangrene in patients taking an SGLT2 inhibitor had been reported to the agency or in medical literature from March 2013, when the first such inhibitor was approved, and May 2018.
In this latest report, a total of 55 Fournier gangrene cases had been reported in patients receiving SGLT2 inhibitors from March 2, 2013 through January 31, 2019.
The influx of reports may have been prompted by growing awareness of the safety issue, investigators said, but could also reflect the increasing prevalence of diabetes combined with SGLT2 inhibitor use. The researchers also noted that diabetes is a comorbidity in 32% to 66% of cases of Fournier gangrene.
But the likliehood that diabetes mellitus alone causes Fournier gangrene seems unlikley, given that Dr. Bersoff-Matcha and co-authors only found 19 Fournier gangrene cases associated with other classes of antiglycemic agents reported to the FDA or in the literature over a 35-year time frame.
“If Fournier gangrene were associated only with diabetes mellitus and not SGLT2 inhibitors, we would expect far more cases reported with the other antiglycemic agents, considering the 35-year timeframe and the large number of agents,” they said in their report.
Cases were reported for all FDA-approved SGLT2 inhibitors besides ertugliflozin, an agent approved for use in the U.S. in December 2017. The lack of cases reported for this drug could be related to its limited time on the market, the investigators said.
Fournier gangrene, marked by rapidly progressing necrotizing infection of the genitalia, perineum, and perianal region, requires antibiotics and immediate surgery, according to Dr. Bersoff-Matcha and colleagues.
“Serious complications and death are likely if Fournier gangrene is not recognized immediately and surgical intervention is not carried out within the first few hours of diagnosis,” they said in the report.
Of the 55 cases reported in patients receiving SGLT2 inhibitors, 39 were men and 16 were women, with an average of 9 months from the start of treatment to the event, investigators said.
At least 25 patients required multiple surgeries, including one patient who had 17 trips to the operating room, they said. A total of 8 patients had a fecal diversion procedure, and 4 patients had skin grafting.
Six patients had multiple encounters with a provider before being diagnosed, suggesting that the provider may have not recognized the infection due to its nonspecific symptoms, which include fatigue, fever, and malaise.
“Pain that seems out of proportion to findings on physical examination is a strong clinical indicator of necrotizing fasciitis and may be the most important diagnostic clue,” Dr. Bersoff-Matcha and co-authors said in their report.
The incidence of Fournier gangrene in patients taking SGLT2 inhibitors can’t be established by these cases reported to the FDA, which are spontaneously provided by health care providers and patients, investigators said.
“We suspect that our numbers underestimate the true burden,” they said in their report.
Dr. Bersoff-Matcha and co-authors disclosed no conflicts of interest related to their report.
SOURCE: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6. Doi: doi:10.7326/M19-0085.
This article was updated May 9, 2019.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: The number of Fournier gangrene cases reported in patients receiving sodium-glucose cotransporter-2 (SGLT2) inhibitors has increased in the time since an FDA warning was issued about this rare but potentially serious infection.
Major finding: The previous FDA warning noted 12 reported cases from March 1, 2013 through March 1, 2018. This latest report included a total of 55 cases reported through January 31, 2019.
Study details: A review of spontaneous postmarketing cases of Fournier gangrene reported to the FDA or in the medical literature.
Disclosures: Authors disclosed no conflicts of interest related to the study.
Source: Bersoff-Matcha SJ, et al. Ann Intern Med. 2019 May 6.
Liraglutide seems safe, effective in children already on metformin
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
FROM PAS 2019
Increase in pediatric thyroid cancers: overdiagnosis or something more?
Thyroid cancer rates are on the rise in U.S. pediatric patients, a large epidemiologic study shows, and researchers say the trend can’t be explained away purely as overdiagnosis.
However, the extent of a real increase in clinically significant cancers and what might be causing that increase remains unclear, according to authors of the study and two related editorials appearing in the journal Cancer.
Cases of pediatric differentiated thyroid cancer (DTC) cases increased by 4.43% per year in the study, which was based on data from 39 U.S. cancer registries. Increases were seen in both smaller early-stage and larger late-stage tumors, leading study authors to assert that the trend was “unlikely to be explained solely by increased medical surveillance or improved detection.”
“Environmental and individual factors may also have affected rising trends,” said Meredith S. Shiels, PhD, of the National Cancer Institute and coauthors in a report on the study.
A true increase in pediatric thyroid cancer incidence is a possibility, authors of a related editorial said; however, they also expressed concern that inferences drawn from this U.S. cancer epidemiology data may be “artifactual.”
“We believe that first it is most important to closely examine the reasons for the increase that could be attributable to overdiagnosis, given this appears to be a likely explanation,” said authors of one editorial, including Amy Y. Chen, MD, MPH, of Emory University, Atlanta, and Louise Davies, MD, MS, of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H.
Overdiagnosis does occur, but the “real component” of the rise in thyroid cancer incidence cannot be ignored, according to a second editorial by David Goldenberg, MD, of Penn State University, Hershey.
“Although many authors are quick to explain the rise in thyroid cancer as an artifact of the overdiagnosis of clinically insignificant thyroid cancers, multiple groups all over the world have shown that this is not sufficient to explain the rise in thyroid cancer,” Dr. Goldenberg said in his editorial.
New data in pediatric patients
Pediatric DTCs are rare, representing just 2%-4% of pediatric malignancies, and they’re particularly rare in relation to adult cases, comprising about 2.3% of thyroid cancer diagnoses overall, according to Dr. Shiels and coauthors of the current study.
“The rarity of pediatric DTC, the lack of information on histologic features, or both have prevented prior studies from analyzing trends by tumor size or cancer stage at diagnosis,” they said.
To address this knowledge gap, Dr. Shiels and colleagues analyzed a total of 7,296 primary DTCs in children aged 0-19 years in data obtained from the North American Association of Central Cancer Registries. Ninety-one percent of these pediatric patients had papillary thyroid cancer, 83% were female, and 76% were between the ages of 15 to 19 years.
The rate of pediatric DTCs increased from 4.77 per million in 1998 to 8.82 per million in 2013, representing an increase of 4.43% per year, they found.
Both localized and more aggressive tumors increased in incidence over that time period, they also found. The annual increase was 4.06% for local stage at diagnosis, 5.68% for regional, and 8.55% for distant disease.
Similarly, increases were seen in small and large tumors alike. The annual percentage increase was 9.46% for tumors smaller than 1 cm, and 4.69% for tumors greater than 2 cm, according to the report.
Looking at age, investigators found that the increases in incidence were significant only for 10- to 19-year-olds, while significant increases were consistently observed for both sexes and for all races and ethnicities.
Overdiagnosis vs. environmental factors and lifestyle changes
If further investigations point to detection of subclinical disease as the cause of the increases in pediatric DTC incidence then initiatives may be needed to curtail use of CT scans, ultrasound, and needle biopsies, as has been done in adults, Dr. Chen and Dr. Davies said in their editorial.
“When the American Thyroid Association modified their guidelines for needle biopsy of nodules to discourage sampling of small lesions, a corresponding decrease in incidence rates was observed, suggesting that, indeed, overdiagnosis was the culprit,” they said.
Although it’s not clear what environmental factors or lifestyle changes are driving an increase, obesity has been consistently linked to increases in thyroid cancer, Dr. Goldenberg said. Conversely, smoking has been linked to reduced thyroid cancer risk, which means reduced prevalence of smoking in the community could potentially contribute to increased thyroid cancer incidence.
“It is our role as physicians to protect our patients from complacency and undertreatment,” he concluded in his editorial. “Explaining away thyroid cancers as being subclinical or clinically insignificant is reminiscent of days past when we told our patients: ‘Don’t worry, it’s good cancer.’ ”
The research by Dr. Shiels and colleagues was supported by the Intramural Research Program of the National Cancer Institute. Dr. Shiels and coauthors made no conflict of interest disclosures related to their report.
SOURCE: Shiels MO et al. Cancer. 2019 Apr 23. doi: 10.1002/cncr.32125.
Thyroid cancer rates are on the rise in U.S. pediatric patients, a large epidemiologic study shows, and researchers say the trend can’t be explained away purely as overdiagnosis.
However, the extent of a real increase in clinically significant cancers and what might be causing that increase remains unclear, according to authors of the study and two related editorials appearing in the journal Cancer.
Cases of pediatric differentiated thyroid cancer (DTC) cases increased by 4.43% per year in the study, which was based on data from 39 U.S. cancer registries. Increases were seen in both smaller early-stage and larger late-stage tumors, leading study authors to assert that the trend was “unlikely to be explained solely by increased medical surveillance or improved detection.”
“Environmental and individual factors may also have affected rising trends,” said Meredith S. Shiels, PhD, of the National Cancer Institute and coauthors in a report on the study.
A true increase in pediatric thyroid cancer incidence is a possibility, authors of a related editorial said; however, they also expressed concern that inferences drawn from this U.S. cancer epidemiology data may be “artifactual.”
“We believe that first it is most important to closely examine the reasons for the increase that could be attributable to overdiagnosis, given this appears to be a likely explanation,” said authors of one editorial, including Amy Y. Chen, MD, MPH, of Emory University, Atlanta, and Louise Davies, MD, MS, of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H.
Overdiagnosis does occur, but the “real component” of the rise in thyroid cancer incidence cannot be ignored, according to a second editorial by David Goldenberg, MD, of Penn State University, Hershey.
“Although many authors are quick to explain the rise in thyroid cancer as an artifact of the overdiagnosis of clinically insignificant thyroid cancers, multiple groups all over the world have shown that this is not sufficient to explain the rise in thyroid cancer,” Dr. Goldenberg said in his editorial.
New data in pediatric patients
Pediatric DTCs are rare, representing just 2%-4% of pediatric malignancies, and they’re particularly rare in relation to adult cases, comprising about 2.3% of thyroid cancer diagnoses overall, according to Dr. Shiels and coauthors of the current study.
“The rarity of pediatric DTC, the lack of information on histologic features, or both have prevented prior studies from analyzing trends by tumor size or cancer stage at diagnosis,” they said.
To address this knowledge gap, Dr. Shiels and colleagues analyzed a total of 7,296 primary DTCs in children aged 0-19 years in data obtained from the North American Association of Central Cancer Registries. Ninety-one percent of these pediatric patients had papillary thyroid cancer, 83% were female, and 76% were between the ages of 15 to 19 years.
The rate of pediatric DTCs increased from 4.77 per million in 1998 to 8.82 per million in 2013, representing an increase of 4.43% per year, they found.
Both localized and more aggressive tumors increased in incidence over that time period, they also found. The annual increase was 4.06% for local stage at diagnosis, 5.68% for regional, and 8.55% for distant disease.
Similarly, increases were seen in small and large tumors alike. The annual percentage increase was 9.46% for tumors smaller than 1 cm, and 4.69% for tumors greater than 2 cm, according to the report.
Looking at age, investigators found that the increases in incidence were significant only for 10- to 19-year-olds, while significant increases were consistently observed for both sexes and for all races and ethnicities.
Overdiagnosis vs. environmental factors and lifestyle changes
If further investigations point to detection of subclinical disease as the cause of the increases in pediatric DTC incidence then initiatives may be needed to curtail use of CT scans, ultrasound, and needle biopsies, as has been done in adults, Dr. Chen and Dr. Davies said in their editorial.
“When the American Thyroid Association modified their guidelines for needle biopsy of nodules to discourage sampling of small lesions, a corresponding decrease in incidence rates was observed, suggesting that, indeed, overdiagnosis was the culprit,” they said.
Although it’s not clear what environmental factors or lifestyle changes are driving an increase, obesity has been consistently linked to increases in thyroid cancer, Dr. Goldenberg said. Conversely, smoking has been linked to reduced thyroid cancer risk, which means reduced prevalence of smoking in the community could potentially contribute to increased thyroid cancer incidence.
“It is our role as physicians to protect our patients from complacency and undertreatment,” he concluded in his editorial. “Explaining away thyroid cancers as being subclinical or clinically insignificant is reminiscent of days past when we told our patients: ‘Don’t worry, it’s good cancer.’ ”
The research by Dr. Shiels and colleagues was supported by the Intramural Research Program of the National Cancer Institute. Dr. Shiels and coauthors made no conflict of interest disclosures related to their report.
SOURCE: Shiels MO et al. Cancer. 2019 Apr 23. doi: 10.1002/cncr.32125.
Thyroid cancer rates are on the rise in U.S. pediatric patients, a large epidemiologic study shows, and researchers say the trend can’t be explained away purely as overdiagnosis.
However, the extent of a real increase in clinically significant cancers and what might be causing that increase remains unclear, according to authors of the study and two related editorials appearing in the journal Cancer.
Cases of pediatric differentiated thyroid cancer (DTC) cases increased by 4.43% per year in the study, which was based on data from 39 U.S. cancer registries. Increases were seen in both smaller early-stage and larger late-stage tumors, leading study authors to assert that the trend was “unlikely to be explained solely by increased medical surveillance or improved detection.”
“Environmental and individual factors may also have affected rising trends,” said Meredith S. Shiels, PhD, of the National Cancer Institute and coauthors in a report on the study.
A true increase in pediatric thyroid cancer incidence is a possibility, authors of a related editorial said; however, they also expressed concern that inferences drawn from this U.S. cancer epidemiology data may be “artifactual.”
“We believe that first it is most important to closely examine the reasons for the increase that could be attributable to overdiagnosis, given this appears to be a likely explanation,” said authors of one editorial, including Amy Y. Chen, MD, MPH, of Emory University, Atlanta, and Louise Davies, MD, MS, of the Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, N.H.
Overdiagnosis does occur, but the “real component” of the rise in thyroid cancer incidence cannot be ignored, according to a second editorial by David Goldenberg, MD, of Penn State University, Hershey.
“Although many authors are quick to explain the rise in thyroid cancer as an artifact of the overdiagnosis of clinically insignificant thyroid cancers, multiple groups all over the world have shown that this is not sufficient to explain the rise in thyroid cancer,” Dr. Goldenberg said in his editorial.
New data in pediatric patients
Pediatric DTCs are rare, representing just 2%-4% of pediatric malignancies, and they’re particularly rare in relation to adult cases, comprising about 2.3% of thyroid cancer diagnoses overall, according to Dr. Shiels and coauthors of the current study.
“The rarity of pediatric DTC, the lack of information on histologic features, or both have prevented prior studies from analyzing trends by tumor size or cancer stage at diagnosis,” they said.
To address this knowledge gap, Dr. Shiels and colleagues analyzed a total of 7,296 primary DTCs in children aged 0-19 years in data obtained from the North American Association of Central Cancer Registries. Ninety-one percent of these pediatric patients had papillary thyroid cancer, 83% were female, and 76% were between the ages of 15 to 19 years.
The rate of pediatric DTCs increased from 4.77 per million in 1998 to 8.82 per million in 2013, representing an increase of 4.43% per year, they found.
Both localized and more aggressive tumors increased in incidence over that time period, they also found. The annual increase was 4.06% for local stage at diagnosis, 5.68% for regional, and 8.55% for distant disease.
Similarly, increases were seen in small and large tumors alike. The annual percentage increase was 9.46% for tumors smaller than 1 cm, and 4.69% for tumors greater than 2 cm, according to the report.
Looking at age, investigators found that the increases in incidence were significant only for 10- to 19-year-olds, while significant increases were consistently observed for both sexes and for all races and ethnicities.
Overdiagnosis vs. environmental factors and lifestyle changes
If further investigations point to detection of subclinical disease as the cause of the increases in pediatric DTC incidence then initiatives may be needed to curtail use of CT scans, ultrasound, and needle biopsies, as has been done in adults, Dr. Chen and Dr. Davies said in their editorial.
“When the American Thyroid Association modified their guidelines for needle biopsy of nodules to discourage sampling of small lesions, a corresponding decrease in incidence rates was observed, suggesting that, indeed, overdiagnosis was the culprit,” they said.
Although it’s not clear what environmental factors or lifestyle changes are driving an increase, obesity has been consistently linked to increases in thyroid cancer, Dr. Goldenberg said. Conversely, smoking has been linked to reduced thyroid cancer risk, which means reduced prevalence of smoking in the community could potentially contribute to increased thyroid cancer incidence.
“It is our role as physicians to protect our patients from complacency and undertreatment,” he concluded in his editorial. “Explaining away thyroid cancers as being subclinical or clinically insignificant is reminiscent of days past when we told our patients: ‘Don’t worry, it’s good cancer.’ ”
The research by Dr. Shiels and colleagues was supported by the Intramural Research Program of the National Cancer Institute. Dr. Shiels and coauthors made no conflict of interest disclosures related to their report.
SOURCE: Shiels MO et al. Cancer. 2019 Apr 23. doi: 10.1002/cncr.32125.
FROM CANCER
Knowledge gaps about long-term osteoporosis drug therapy benefits, risks remain large
Long-term use of alendronate and zoledronic acid for more than 3 years reduces the rate of vertebral fracture in treatment-naive postmenopausal women with notable, yet rare, adverse events, but too little evidence exists to make determinations on the long-term benefit/risk profile of other bisphosphonates or other osteoporosis drugs besides raloxifene and oral hormone therapy, according to a report coming out of a recent National Institutes of Health workshop.
This situation leaves a large research gap that authors of an accompanying position paper hope to bridge with recommendations for studying therapy discontinuation and drug holidays during long-term osteoporosis drug treatment.
The NIH’s Pathways to Prevention (P2P) Workshop: Appropriate Use of Drug Therapies for Osteoporotic Fracture Prevention outlined the findings of the systematic review of long-term osteoporosis drug treatment (ODT), which was commissioned by the NIH Office of Disease Prevention. The systematic review and a position paper summarizing the workshop were published April 23 in Annals of Internal Medicine.
“Clinicians and patients need increased information on benefits and risks to inform shared decision making about the use of these treatments, taking into account patients’ values and preferences,” Albert Siu, MD, of the Brookdale Department of Geriatrics and Palliative Medicine at the Icahn School of Medicine at Mount Sinai in New York, and his colleagues wrote in the position paper (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M19-0961). “The research ... is urgently needed to advance prevention of osteoporosis-related mortality and morbidity.”
In the systematic review, by a group of researchers separate from the workshop, 48 studies were identified (35 trials, 13 observational studies) that compared men and postmenopausal women 50 years or older who used treatments such as alendronate, raloxifene, zoledronic acid, and hormone therapy. The researchers found that use of alendronate for 4 years reduced the rate of clinical fractures (hazard ratio, 0.64; 95% confidence interval, 0.50-0.82) and radiographic vertebral fractures (HR, 0.50; 95% CI, 0.31-0.82) in women with osteoporosis. Raloxifene use for 4 years reduced the rate of clinical vertebral fractures (relative risk, 0.58; 95% CI, 0.43-0.79) and radiographic vertebral fractures (RR, 0.64; 95% CI, 0.53-0.76) but not nonvertebral fractures. Zoledronic acid use for 6 years was associated with a lower rate of nonvertebral fractures (HR, 0.66; 95% CI, 0.51-0.85) and clinical vertebral fractures (HR, 0.41; 95% CI, 0.22-0.75) in women with both osteoporosis and osteopenia. Estrogen-progestin use for 5.6 years and unopposed estrogen for 7 years was associated with clinical fracture reduction in women with unspecified osteoporosis and osteopenia when compared with placebo (Ann Intern Med. 2019 April 23. doi: 10.7326/M19-0533).
Controlled observational studies collectively show that long-term use of alendronate and of bisphosphonates as a class increased risk for radiologically confirmed atypical femoral fracture but by a small absolute amount, with less evidence for risks of subtrochanteric or femoral shaft fractures without radiologically confirmed atypical femoral fracture features and osteonecrosis of the jaw. However, there were no eligible observational studies with long-term use of zoledronic acid that evaluated risk for these adverse events.
Long-term raloxifene therapy was associated with a threefold increased risk for deep venous thrombosis and a three- to fourfold increased risk for pulmonary embolism, although not all results were statistically significant, the researchers said. In two long-term trials, both estrogen and estrogen-progestin compared with placebo increased risk for cardiovascular disease and cognitive impairment. Estrogen-progestin also increased risk for invasive breast cancer.
The researchers also studied abaloparatide, denosumab, ibandronate, risedronate, and teriparatide, but noted there were insufficient data to show the long-term effects of their use on fractures and other harms.
Dr. Siu and coauthors on the position paper made the following recommendations with regard to future research on long-term ODT:
• Using “innovative designs and approaches” for new research such as modeling studies, clinical trials, and observational studies of existing and potential treatments.
• Evaluating new agents or multicomponent interventions, such as fracture liaison services and oral care, that do not carry the downsides of antiresorptive therapies.
• Researching and preventing atypical femoral fracture and osteonecrosis of the jaw, particularly when associated with long-term denosumab or bisphosphonate use.
• Determining which patients are indicated for drug holidays, sequential therapies, and strategies for avoiding serious adverse events.
• Studying barriers to ODT.
“When we have information on these outcomes, such as how medication use after a fragility fracture is linked to future fractures or survival rates, we need to understand how to convey that information to patients so they can make more informed decisions about their care,” noted Dr. Siu and colleagues.
In an editorial related to both the position paper and the systematic review, Carolyn J. Crandall, MD, of the University of California, Los Angeles, agreed that clinical trial data do not answer questions about shared decision making for women with multiple comorbid conditions, the long-term effects of ODT with regard to rare fracture risk, and which patients are well-suited for drug holidays.
“The National Institutes of Health should support research to answer these high-impact clinical questions, in addition to encouraging approaches for clinicians to determine which individual patients are at greater risk for harms related to long-term bisphosphonate use,” she said. “The need to rigorously study patient preferences in the context of ODT is pressing because of the complex dosing instructions of oral bisphosphonates and the dramatic underutilization of ODT among persons who have already had a vertebral or hip fracture.”
The systematic review was funded by the National Institutes of Health and the Agency for Healthcare Research and Quality. The authors of the position paper and Dr. Crandall reported no conflicts of interest.
SOURCE: Siu A et al. Ann Intern Med. 2019 April 23. doi: 10.7326/M19-0961.
Long-term use of alendronate and zoledronic acid for more than 3 years reduces the rate of vertebral fracture in treatment-naive postmenopausal women with notable, yet rare, adverse events, but too little evidence exists to make determinations on the long-term benefit/risk profile of other bisphosphonates or other osteoporosis drugs besides raloxifene and oral hormone therapy, according to a report coming out of a recent National Institutes of Health workshop.
This situation leaves a large research gap that authors of an accompanying position paper hope to bridge with recommendations for studying therapy discontinuation and drug holidays during long-term osteoporosis drug treatment.
The NIH’s Pathways to Prevention (P2P) Workshop: Appropriate Use of Drug Therapies for Osteoporotic Fracture Prevention outlined the findings of the systematic review of long-term osteoporosis drug treatment (ODT), which was commissioned by the NIH Office of Disease Prevention. The systematic review and a position paper summarizing the workshop were published April 23 in Annals of Internal Medicine.
“Clinicians and patients need increased information on benefits and risks to inform shared decision making about the use of these treatments, taking into account patients’ values and preferences,” Albert Siu, MD, of the Brookdale Department of Geriatrics and Palliative Medicine at the Icahn School of Medicine at Mount Sinai in New York, and his colleagues wrote in the position paper (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M19-0961). “The research ... is urgently needed to advance prevention of osteoporosis-related mortality and morbidity.”
In the systematic review, by a group of researchers separate from the workshop, 48 studies were identified (35 trials, 13 observational studies) that compared men and postmenopausal women 50 years or older who used treatments such as alendronate, raloxifene, zoledronic acid, and hormone therapy. The researchers found that use of alendronate for 4 years reduced the rate of clinical fractures (hazard ratio, 0.64; 95% confidence interval, 0.50-0.82) and radiographic vertebral fractures (HR, 0.50; 95% CI, 0.31-0.82) in women with osteoporosis. Raloxifene use for 4 years reduced the rate of clinical vertebral fractures (relative risk, 0.58; 95% CI, 0.43-0.79) and radiographic vertebral fractures (RR, 0.64; 95% CI, 0.53-0.76) but not nonvertebral fractures. Zoledronic acid use for 6 years was associated with a lower rate of nonvertebral fractures (HR, 0.66; 95% CI, 0.51-0.85) and clinical vertebral fractures (HR, 0.41; 95% CI, 0.22-0.75) in women with both osteoporosis and osteopenia. Estrogen-progestin use for 5.6 years and unopposed estrogen for 7 years was associated with clinical fracture reduction in women with unspecified osteoporosis and osteopenia when compared with placebo (Ann Intern Med. 2019 April 23. doi: 10.7326/M19-0533).
Controlled observational studies collectively show that long-term use of alendronate and of bisphosphonates as a class increased risk for radiologically confirmed atypical femoral fracture but by a small absolute amount, with less evidence for risks of subtrochanteric or femoral shaft fractures without radiologically confirmed atypical femoral fracture features and osteonecrosis of the jaw. However, there were no eligible observational studies with long-term use of zoledronic acid that evaluated risk for these adverse events.
Long-term raloxifene therapy was associated with a threefold increased risk for deep venous thrombosis and a three- to fourfold increased risk for pulmonary embolism, although not all results were statistically significant, the researchers said. In two long-term trials, both estrogen and estrogen-progestin compared with placebo increased risk for cardiovascular disease and cognitive impairment. Estrogen-progestin also increased risk for invasive breast cancer.
The researchers also studied abaloparatide, denosumab, ibandronate, risedronate, and teriparatide, but noted there were insufficient data to show the long-term effects of their use on fractures and other harms.
Dr. Siu and coauthors on the position paper made the following recommendations with regard to future research on long-term ODT:
• Using “innovative designs and approaches” for new research such as modeling studies, clinical trials, and observational studies of existing and potential treatments.
• Evaluating new agents or multicomponent interventions, such as fracture liaison services and oral care, that do not carry the downsides of antiresorptive therapies.
• Researching and preventing atypical femoral fracture and osteonecrosis of the jaw, particularly when associated with long-term denosumab or bisphosphonate use.
• Determining which patients are indicated for drug holidays, sequential therapies, and strategies for avoiding serious adverse events.
• Studying barriers to ODT.
“When we have information on these outcomes, such as how medication use after a fragility fracture is linked to future fractures or survival rates, we need to understand how to convey that information to patients so they can make more informed decisions about their care,” noted Dr. Siu and colleagues.
In an editorial related to both the position paper and the systematic review, Carolyn J. Crandall, MD, of the University of California, Los Angeles, agreed that clinical trial data do not answer questions about shared decision making for women with multiple comorbid conditions, the long-term effects of ODT with regard to rare fracture risk, and which patients are well-suited for drug holidays.
“The National Institutes of Health should support research to answer these high-impact clinical questions, in addition to encouraging approaches for clinicians to determine which individual patients are at greater risk for harms related to long-term bisphosphonate use,” she said. “The need to rigorously study patient preferences in the context of ODT is pressing because of the complex dosing instructions of oral bisphosphonates and the dramatic underutilization of ODT among persons who have already had a vertebral or hip fracture.”
The systematic review was funded by the National Institutes of Health and the Agency for Healthcare Research and Quality. The authors of the position paper and Dr. Crandall reported no conflicts of interest.
SOURCE: Siu A et al. Ann Intern Med. 2019 April 23. doi: 10.7326/M19-0961.
Long-term use of alendronate and zoledronic acid for more than 3 years reduces the rate of vertebral fracture in treatment-naive postmenopausal women with notable, yet rare, adverse events, but too little evidence exists to make determinations on the long-term benefit/risk profile of other bisphosphonates or other osteoporosis drugs besides raloxifene and oral hormone therapy, according to a report coming out of a recent National Institutes of Health workshop.
This situation leaves a large research gap that authors of an accompanying position paper hope to bridge with recommendations for studying therapy discontinuation and drug holidays during long-term osteoporosis drug treatment.
The NIH’s Pathways to Prevention (P2P) Workshop: Appropriate Use of Drug Therapies for Osteoporotic Fracture Prevention outlined the findings of the systematic review of long-term osteoporosis drug treatment (ODT), which was commissioned by the NIH Office of Disease Prevention. The systematic review and a position paper summarizing the workshop were published April 23 in Annals of Internal Medicine.
“Clinicians and patients need increased information on benefits and risks to inform shared decision making about the use of these treatments, taking into account patients’ values and preferences,” Albert Siu, MD, of the Brookdale Department of Geriatrics and Palliative Medicine at the Icahn School of Medicine at Mount Sinai in New York, and his colleagues wrote in the position paper (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M19-0961). “The research ... is urgently needed to advance prevention of osteoporosis-related mortality and morbidity.”
In the systematic review, by a group of researchers separate from the workshop, 48 studies were identified (35 trials, 13 observational studies) that compared men and postmenopausal women 50 years or older who used treatments such as alendronate, raloxifene, zoledronic acid, and hormone therapy. The researchers found that use of alendronate for 4 years reduced the rate of clinical fractures (hazard ratio, 0.64; 95% confidence interval, 0.50-0.82) and radiographic vertebral fractures (HR, 0.50; 95% CI, 0.31-0.82) in women with osteoporosis. Raloxifene use for 4 years reduced the rate of clinical vertebral fractures (relative risk, 0.58; 95% CI, 0.43-0.79) and radiographic vertebral fractures (RR, 0.64; 95% CI, 0.53-0.76) but not nonvertebral fractures. Zoledronic acid use for 6 years was associated with a lower rate of nonvertebral fractures (HR, 0.66; 95% CI, 0.51-0.85) and clinical vertebral fractures (HR, 0.41; 95% CI, 0.22-0.75) in women with both osteoporosis and osteopenia. Estrogen-progestin use for 5.6 years and unopposed estrogen for 7 years was associated with clinical fracture reduction in women with unspecified osteoporosis and osteopenia when compared with placebo (Ann Intern Med. 2019 April 23. doi: 10.7326/M19-0533).
Controlled observational studies collectively show that long-term use of alendronate and of bisphosphonates as a class increased risk for radiologically confirmed atypical femoral fracture but by a small absolute amount, with less evidence for risks of subtrochanteric or femoral shaft fractures without radiologically confirmed atypical femoral fracture features and osteonecrosis of the jaw. However, there were no eligible observational studies with long-term use of zoledronic acid that evaluated risk for these adverse events.
Long-term raloxifene therapy was associated with a threefold increased risk for deep venous thrombosis and a three- to fourfold increased risk for pulmonary embolism, although not all results were statistically significant, the researchers said. In two long-term trials, both estrogen and estrogen-progestin compared with placebo increased risk for cardiovascular disease and cognitive impairment. Estrogen-progestin also increased risk for invasive breast cancer.
The researchers also studied abaloparatide, denosumab, ibandronate, risedronate, and teriparatide, but noted there were insufficient data to show the long-term effects of their use on fractures and other harms.
Dr. Siu and coauthors on the position paper made the following recommendations with regard to future research on long-term ODT:
• Using “innovative designs and approaches” for new research such as modeling studies, clinical trials, and observational studies of existing and potential treatments.
• Evaluating new agents or multicomponent interventions, such as fracture liaison services and oral care, that do not carry the downsides of antiresorptive therapies.
• Researching and preventing atypical femoral fracture and osteonecrosis of the jaw, particularly when associated with long-term denosumab or bisphosphonate use.
• Determining which patients are indicated for drug holidays, sequential therapies, and strategies for avoiding serious adverse events.
• Studying barriers to ODT.
“When we have information on these outcomes, such as how medication use after a fragility fracture is linked to future fractures or survival rates, we need to understand how to convey that information to patients so they can make more informed decisions about their care,” noted Dr. Siu and colleagues.
In an editorial related to both the position paper and the systematic review, Carolyn J. Crandall, MD, of the University of California, Los Angeles, agreed that clinical trial data do not answer questions about shared decision making for women with multiple comorbid conditions, the long-term effects of ODT with regard to rare fracture risk, and which patients are well-suited for drug holidays.
“The National Institutes of Health should support research to answer these high-impact clinical questions, in addition to encouraging approaches for clinicians to determine which individual patients are at greater risk for harms related to long-term bisphosphonate use,” she said. “The need to rigorously study patient preferences in the context of ODT is pressing because of the complex dosing instructions of oral bisphosphonates and the dramatic underutilization of ODT among persons who have already had a vertebral or hip fracture.”
The systematic review was funded by the National Institutes of Health and the Agency for Healthcare Research and Quality. The authors of the position paper and Dr. Crandall reported no conflicts of interest.
SOURCE: Siu A et al. Ann Intern Med. 2019 April 23. doi: 10.7326/M19-0961.
FROM ANNALS OF INTERNAL MEDICINE
An obese 48-year-old man with progressive fatigue and decreased libido
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
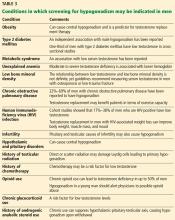
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
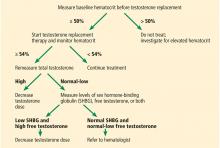
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
Click for Credit: Migraine & stroke risk; Aspirin for CV events; more
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Here are 5 articles from the May issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Subclinical hypothyroidism boosts immediate risk of heart failure
To take the posttest, go to: https://bit.ly/2IK0YiL
Expires January 24, 2020
2. Meta-analysis supports aspirin to reduce cardiovascular events
To take the posttest, go to: https://bit.ly/2GJLgSB
Expires January 24, 2020
3. Age of migraine onset may affect stroke risk
To take the posttest, go to: https://bit.ly/2ZAJ5YR
Expires January 24, 2020
4. Women with RA have reduced chance of live birth after assisted reproduction treatment
To take the posttest, go to: https://bit.ly/2VvKRLF
Expires January 27, 2020
5. New SLE disease activity measure beats SLEDAI-2K
To take the posttest, go to: https://bit.ly/2W8SVPA
Expires January 31, 2020
Tailoring the Mediterranean diet for NAFLD
Adults with nonalcoholic fatty liver disease (NAFLD) were more likely to implement the Mediterranean diet when they had greater nutritional knowledge and skills, family support, nutritional care, and positive reinforcement in the media, according to an in-depth study of 19 patients.
Barriers to adopting the diet included “an obesogenic environment, life stressors, and demand for convenience. Poor understanding of the causes and significance of NAFLD adversely affected readiness to change dietary habits,” wrote Laura Haigh of Newcastle University in Newcastle Upon Tyne, England, and associates. The study, which included both standard quantitative methods and semistructured interviews, was published in Clinical Gastroenterology and Hepatology.
The Mediterranean diet emphasizes vegetables, legumes, fish, fruits, whole grains, nuts, and olive oil in lieu of processed foods, sweets, saturated fats, and red meat. This diet has been definitively shown to improve insulin sensitivity and steatosis, even when patients do not lose weight. This has sparked interest in its use for NAFLD disease, but keys to its successful adoption in Northern Europe are not well understood.
Therefore, the researchers recruited 19 NAFLD patients from a tertiary care center in the United Kingdom for a 12-week Mediterranean diet intervention. Most were female, white, in their late 50s, obese, and had type 2 diabetes. “Participants were taught behavioral strategies through the provision of shopping lists, meal planners, and recipes. No advice was given on calorie allowances or physical activities,” the investigators noted.
By using a 14-point assessment tool, they found that dietary adherence rose significantly at 12 weeks, compared with baseline (P = .006). In all, 79% of patients lost weight (mean, 2.4 kg; P = .001 versus baseline), and 72% significantly increased their serum level of HDL cholesterol. Interviews linked successful adoption of the diet with diverse factors, such as believing that NAFLD is lifestyle associated, realizing that healthier nutrition can improve health outcomes, and having access to transportation and budget grocery stories. Patients generally saw the Mediterranean diet as flexible and affordable, but they struggled to adopt it if they worked irregular hours, experienced substantial life stress or were very busy, or tended to eat for self-reward or self-comfort.
Other cited barriers included “diet saboteurs” (including spouses), the plethora of unhealthy foods available in patients’ environments, low nutritional or medical knowledge, and cultural, social, or taste incompatibility, the researchers reported. Taken together, the findings underscore “the futility of a one-size-fits-all approach” when implementing the Mediterranean diet in this population, they concluded. Instead, their patients valued a collaborative, tailored approach – ideally one that incorporated in-person and group-based treatment, as well as online support.
Funders included the North East of England hub of the Allied Health Professions Research Network, the Elucidating Pathways of Steatohepatitis consortium, the Horizon 2020 Framework Program of the European Union, and the Newcastle NIHR Biomedical Research Centre. The researchers reported having no conflicts of interest.
SOURCE: Haigh L et al. Clin Gastroenterol Hepatol. 2018 Oct 31. doi: 10.1016/j.cgh.2018.10.044.
Adults with nonalcoholic fatty liver disease (NAFLD) were more likely to implement the Mediterranean diet when they had greater nutritional knowledge and skills, family support, nutritional care, and positive reinforcement in the media, according to an in-depth study of 19 patients.
Barriers to adopting the diet included “an obesogenic environment, life stressors, and demand for convenience. Poor understanding of the causes and significance of NAFLD adversely affected readiness to change dietary habits,” wrote Laura Haigh of Newcastle University in Newcastle Upon Tyne, England, and associates. The study, which included both standard quantitative methods and semistructured interviews, was published in Clinical Gastroenterology and Hepatology.
The Mediterranean diet emphasizes vegetables, legumes, fish, fruits, whole grains, nuts, and olive oil in lieu of processed foods, sweets, saturated fats, and red meat. This diet has been definitively shown to improve insulin sensitivity and steatosis, even when patients do not lose weight. This has sparked interest in its use for NAFLD disease, but keys to its successful adoption in Northern Europe are not well understood.
Therefore, the researchers recruited 19 NAFLD patients from a tertiary care center in the United Kingdom for a 12-week Mediterranean diet intervention. Most were female, white, in their late 50s, obese, and had type 2 diabetes. “Participants were taught behavioral strategies through the provision of shopping lists, meal planners, and recipes. No advice was given on calorie allowances or physical activities,” the investigators noted.
By using a 14-point assessment tool, they found that dietary adherence rose significantly at 12 weeks, compared with baseline (P = .006). In all, 79% of patients lost weight (mean, 2.4 kg; P = .001 versus baseline), and 72% significantly increased their serum level of HDL cholesterol. Interviews linked successful adoption of the diet with diverse factors, such as believing that NAFLD is lifestyle associated, realizing that healthier nutrition can improve health outcomes, and having access to transportation and budget grocery stories. Patients generally saw the Mediterranean diet as flexible and affordable, but they struggled to adopt it if they worked irregular hours, experienced substantial life stress or were very busy, or tended to eat for self-reward or self-comfort.
Other cited barriers included “diet saboteurs” (including spouses), the plethora of unhealthy foods available in patients’ environments, low nutritional or medical knowledge, and cultural, social, or taste incompatibility, the researchers reported. Taken together, the findings underscore “the futility of a one-size-fits-all approach” when implementing the Mediterranean diet in this population, they concluded. Instead, their patients valued a collaborative, tailored approach – ideally one that incorporated in-person and group-based treatment, as well as online support.
Funders included the North East of England hub of the Allied Health Professions Research Network, the Elucidating Pathways of Steatohepatitis consortium, the Horizon 2020 Framework Program of the European Union, and the Newcastle NIHR Biomedical Research Centre. The researchers reported having no conflicts of interest.
SOURCE: Haigh L et al. Clin Gastroenterol Hepatol. 2018 Oct 31. doi: 10.1016/j.cgh.2018.10.044.
Adults with nonalcoholic fatty liver disease (NAFLD) were more likely to implement the Mediterranean diet when they had greater nutritional knowledge and skills, family support, nutritional care, and positive reinforcement in the media, according to an in-depth study of 19 patients.
Barriers to adopting the diet included “an obesogenic environment, life stressors, and demand for convenience. Poor understanding of the causes and significance of NAFLD adversely affected readiness to change dietary habits,” wrote Laura Haigh of Newcastle University in Newcastle Upon Tyne, England, and associates. The study, which included both standard quantitative methods and semistructured interviews, was published in Clinical Gastroenterology and Hepatology.
The Mediterranean diet emphasizes vegetables, legumes, fish, fruits, whole grains, nuts, and olive oil in lieu of processed foods, sweets, saturated fats, and red meat. This diet has been definitively shown to improve insulin sensitivity and steatosis, even when patients do not lose weight. This has sparked interest in its use for NAFLD disease, but keys to its successful adoption in Northern Europe are not well understood.
Therefore, the researchers recruited 19 NAFLD patients from a tertiary care center in the United Kingdom for a 12-week Mediterranean diet intervention. Most were female, white, in their late 50s, obese, and had type 2 diabetes. “Participants were taught behavioral strategies through the provision of shopping lists, meal planners, and recipes. No advice was given on calorie allowances or physical activities,” the investigators noted.
By using a 14-point assessment tool, they found that dietary adherence rose significantly at 12 weeks, compared with baseline (P = .006). In all, 79% of patients lost weight (mean, 2.4 kg; P = .001 versus baseline), and 72% significantly increased their serum level of HDL cholesterol. Interviews linked successful adoption of the diet with diverse factors, such as believing that NAFLD is lifestyle associated, realizing that healthier nutrition can improve health outcomes, and having access to transportation and budget grocery stories. Patients generally saw the Mediterranean diet as flexible and affordable, but they struggled to adopt it if they worked irregular hours, experienced substantial life stress or were very busy, or tended to eat for self-reward or self-comfort.
Other cited barriers included “diet saboteurs” (including spouses), the plethora of unhealthy foods available in patients’ environments, low nutritional or medical knowledge, and cultural, social, or taste incompatibility, the researchers reported. Taken together, the findings underscore “the futility of a one-size-fits-all approach” when implementing the Mediterranean diet in this population, they concluded. Instead, their patients valued a collaborative, tailored approach – ideally one that incorporated in-person and group-based treatment, as well as online support.
Funders included the North East of England hub of the Allied Health Professions Research Network, the Elucidating Pathways of Steatohepatitis consortium, the Horizon 2020 Framework Program of the European Union, and the Newcastle NIHR Biomedical Research Centre. The researchers reported having no conflicts of interest.
SOURCE: Haigh L et al. Clin Gastroenterol Hepatol. 2018 Oct 31. doi: 10.1016/j.cgh.2018.10.044.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Older women with ESRD face higher mortality, compared with male counterparts
LOS ANGELES – In patients with end-stage renal disease, women older than 50 years have a significantly higher mortality, compared with their male counterparts, results from an analysis of national data showed.
“The racial and ethnic disparities in the prevalence, treatment, risks, and outcomes of [hypertension] in patients with CKD [chronic kidney disease], are well recognized,” the study’s senior author, Ricardo Correa, MD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Whites have better control of blood pressure, compared with Hispanics or African Americans with CKD, for example. On the other hand, gender differences in the outcome of blood pressure control and mortality across the different CKD stages have been very poorly studied, with conflicting results.”
The importance of gender difference has been mostly the focus in cardiovascular diseases, he continued, with compelling data revealing a higher incidence in men than in women of similar age, and a menopause-associated increase in cardiovascular disease in women.
“Whether the same can be said for hypertension, remains to be elucidated,” said Dr. Correa, an endocrinologist who directs the diabetes and metabolism fellowship at the University of Arizona in Phoenix.
In what he said is the first study of its kind, Dr. Correa and his colleagues set out to determine if gender in the U.S. population and menopausal age affect the inpatient survival rate in hypertensive patients across different stages of CKD. They drew from the 2005-2012 National Inpatient Sample to identify 2,121,750 hospitalized hypertensive patients and compared a number of factors between men and women, including crude mortality and mortality per CKD stage, menopausal age, length of stay, and total hospital charges.
Of the 2,121,750 patients, 1,092,931 (52%) were men and 1,028,819 (48%) were women; their mean age was 65 years. Among women, 32% had stage 3 CKD, 15% had stage 4 disease, 3% had stage 5 CKD, and 54% had end-stage renal disease (ESRD). Among men, 33% had stage 3 CKD, 13% had stage 4 disease, 3% had stage 5 CKD, and 51% had ESRD. The researchers observed that in-hospital crude mortality was significantly higher for men, compared with a matched group of women at CKD stages 3 and 4 (3.09% vs. 3.29% for CDK 3; P less than .0001 and 4.05% vs. 4.36% for CDK 4; P = .0004), yet was nonsignificant among those with ESRD (4.68% vs. 4.83%; P = .45).
When the researchers factored in menopausal age, they found that women with stage 3, 4, or 5 CKD who were aged 50 years or younger had a mortality rate similar to that of men with same stage disease, whereas women older than 50 years with ESRD had a significantly higher mortality, compared with their male counterparts, especially those of Asian, African American, and Hispanic descent (P less than .001, compared with those of white, non-Hispanic descent).
“One could hypothesize that cardiac remodeling in hemodialysis women may be different than that in hemodialysis men to the extent that it affects mortality,” Dr. Correa said. “However, it is unclear if the survival benefit for dialysis men is owing to the possibility of a selection bias or not. Dialysis women may not be receiving equal access to cardiovascular procedures or surgical interventions (arteriovenous fistula, for example) or women may not be offered adequate hemodialysis to the same extent as men are. Further investigations regarding sex-based differences in dialysis treatment are required.”
He acknowledged certain limitations of the study, including its observational design. “We lacked detailed information regarding the cause of death, dialysis efficiency, types of dialysis accesses, and left ventricular hypertrophy measurements. We did not account for transitions between different hemodialysis modalities [and] we do not have information about distances or traveling time to dialysis units.”
The study’s first author was Kelvin Tran, MD. The researchers reported having no financial disclosures.
LOS ANGELES – In patients with end-stage renal disease, women older than 50 years have a significantly higher mortality, compared with their male counterparts, results from an analysis of national data showed.
“The racial and ethnic disparities in the prevalence, treatment, risks, and outcomes of [hypertension] in patients with CKD [chronic kidney disease], are well recognized,” the study’s senior author, Ricardo Correa, MD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Whites have better control of blood pressure, compared with Hispanics or African Americans with CKD, for example. On the other hand, gender differences in the outcome of blood pressure control and mortality across the different CKD stages have been very poorly studied, with conflicting results.”
The importance of gender difference has been mostly the focus in cardiovascular diseases, he continued, with compelling data revealing a higher incidence in men than in women of similar age, and a menopause-associated increase in cardiovascular disease in women.
“Whether the same can be said for hypertension, remains to be elucidated,” said Dr. Correa, an endocrinologist who directs the diabetes and metabolism fellowship at the University of Arizona in Phoenix.
In what he said is the first study of its kind, Dr. Correa and his colleagues set out to determine if gender in the U.S. population and menopausal age affect the inpatient survival rate in hypertensive patients across different stages of CKD. They drew from the 2005-2012 National Inpatient Sample to identify 2,121,750 hospitalized hypertensive patients and compared a number of factors between men and women, including crude mortality and mortality per CKD stage, menopausal age, length of stay, and total hospital charges.
Of the 2,121,750 patients, 1,092,931 (52%) were men and 1,028,819 (48%) were women; their mean age was 65 years. Among women, 32% had stage 3 CKD, 15% had stage 4 disease, 3% had stage 5 CKD, and 54% had end-stage renal disease (ESRD). Among men, 33% had stage 3 CKD, 13% had stage 4 disease, 3% had stage 5 CKD, and 51% had ESRD. The researchers observed that in-hospital crude mortality was significantly higher for men, compared with a matched group of women at CKD stages 3 and 4 (3.09% vs. 3.29% for CDK 3; P less than .0001 and 4.05% vs. 4.36% for CDK 4; P = .0004), yet was nonsignificant among those with ESRD (4.68% vs. 4.83%; P = .45).
When the researchers factored in menopausal age, they found that women with stage 3, 4, or 5 CKD who were aged 50 years or younger had a mortality rate similar to that of men with same stage disease, whereas women older than 50 years with ESRD had a significantly higher mortality, compared with their male counterparts, especially those of Asian, African American, and Hispanic descent (P less than .001, compared with those of white, non-Hispanic descent).
“One could hypothesize that cardiac remodeling in hemodialysis women may be different than that in hemodialysis men to the extent that it affects mortality,” Dr. Correa said. “However, it is unclear if the survival benefit for dialysis men is owing to the possibility of a selection bias or not. Dialysis women may not be receiving equal access to cardiovascular procedures or surgical interventions (arteriovenous fistula, for example) or women may not be offered adequate hemodialysis to the same extent as men are. Further investigations regarding sex-based differences in dialysis treatment are required.”
He acknowledged certain limitations of the study, including its observational design. “We lacked detailed information regarding the cause of death, dialysis efficiency, types of dialysis accesses, and left ventricular hypertrophy measurements. We did not account for transitions between different hemodialysis modalities [and] we do not have information about distances or traveling time to dialysis units.”
The study’s first author was Kelvin Tran, MD. The researchers reported having no financial disclosures.
LOS ANGELES – In patients with end-stage renal disease, women older than 50 years have a significantly higher mortality, compared with their male counterparts, results from an analysis of national data showed.
“The racial and ethnic disparities in the prevalence, treatment, risks, and outcomes of [hypertension] in patients with CKD [chronic kidney disease], are well recognized,” the study’s senior author, Ricardo Correa, MD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Whites have better control of blood pressure, compared with Hispanics or African Americans with CKD, for example. On the other hand, gender differences in the outcome of blood pressure control and mortality across the different CKD stages have been very poorly studied, with conflicting results.”
The importance of gender difference has been mostly the focus in cardiovascular diseases, he continued, with compelling data revealing a higher incidence in men than in women of similar age, and a menopause-associated increase in cardiovascular disease in women.
“Whether the same can be said for hypertension, remains to be elucidated,” said Dr. Correa, an endocrinologist who directs the diabetes and metabolism fellowship at the University of Arizona in Phoenix.
In what he said is the first study of its kind, Dr. Correa and his colleagues set out to determine if gender in the U.S. population and menopausal age affect the inpatient survival rate in hypertensive patients across different stages of CKD. They drew from the 2005-2012 National Inpatient Sample to identify 2,121,750 hospitalized hypertensive patients and compared a number of factors between men and women, including crude mortality and mortality per CKD stage, menopausal age, length of stay, and total hospital charges.
Of the 2,121,750 patients, 1,092,931 (52%) were men and 1,028,819 (48%) were women; their mean age was 65 years. Among women, 32% had stage 3 CKD, 15% had stage 4 disease, 3% had stage 5 CKD, and 54% had end-stage renal disease (ESRD). Among men, 33% had stage 3 CKD, 13% had stage 4 disease, 3% had stage 5 CKD, and 51% had ESRD. The researchers observed that in-hospital crude mortality was significantly higher for men, compared with a matched group of women at CKD stages 3 and 4 (3.09% vs. 3.29% for CDK 3; P less than .0001 and 4.05% vs. 4.36% for CDK 4; P = .0004), yet was nonsignificant among those with ESRD (4.68% vs. 4.83%; P = .45).
When the researchers factored in menopausal age, they found that women with stage 3, 4, or 5 CKD who were aged 50 years or younger had a mortality rate similar to that of men with same stage disease, whereas women older than 50 years with ESRD had a significantly higher mortality, compared with their male counterparts, especially those of Asian, African American, and Hispanic descent (P less than .001, compared with those of white, non-Hispanic descent).
“One could hypothesize that cardiac remodeling in hemodialysis women may be different than that in hemodialysis men to the extent that it affects mortality,” Dr. Correa said. “However, it is unclear if the survival benefit for dialysis men is owing to the possibility of a selection bias or not. Dialysis women may not be receiving equal access to cardiovascular procedures or surgical interventions (arteriovenous fistula, for example) or women may not be offered adequate hemodialysis to the same extent as men are. Further investigations regarding sex-based differences in dialysis treatment are required.”
He acknowledged certain limitations of the study, including its observational design. “We lacked detailed information regarding the cause of death, dialysis efficiency, types of dialysis accesses, and left ventricular hypertrophy measurements. We did not account for transitions between different hemodialysis modalities [and] we do not have information about distances or traveling time to dialysis units.”
The study’s first author was Kelvin Tran, MD. The researchers reported having no financial disclosures.
REPORTING FROM AACE 2019
Key clinical point: .
Major finding: Women older than 50 years with end-stage renal disease had significantly higher mortality, compared with their male counterparts, especially those of Asian, African American, and Hispanic descent (P less than .001 vs. those of white, non-Hispanic descent).
Study details: An observational study of more than 2 million hypertensive patients from the Nationwide Inpatient Sample.
Disclosures: Dr. Correa reported having no financial disclosures.
Few stroke patients undergo osteoporosis screening, treatment
Although stroke is a risk factor for osteoporosis, falls, and fractures, very few people who have experienced a recent stroke are either screened for osteoporosis or treated, research suggests.
Writing in Stroke, researchers presented an analysis of Ontario registry data from 16,581 patients who were aged 65 years or older and presented with stroke between 2003 and 2013.
Overall, just 5.1% of patients underwent bone mineral density testing. Of the 1,577 patients who had experienced a prior fracture, 71 (4.7%) had bone mineral density testing, and only 2.9% of those who had not had prior bone mineral density testing were tested after their stroke. Bone mineral density testing was more likely in patients who were younger, who were female, and who experienced a low-trauma fracture in the year after their stroke.
In total, 15.5% of patients were prescribed osteoporosis drugs in the first year after their stroke. However, only 7.8% of those who had fractures before the stroke and 14.8% of those with fractures after the stroke received osteoporosis treatment after the stroke. Patients who were female, had prior osteoporosis, had experienced prior fracture, had previously undergone bone mineral density testing, or had experienced a fracture or fall after their stroke were more likely to receive osteoporosis pharmacotherapy.
The authors found that the neither the severity of stroke nor the presence of other comorbidities was associated with an increased likelihood of screening or treatment of osteoporosis after the stroke.
Stroke is associated with up to a fourfold increased risk of osteoporosis and fracture, compared with healthy controls, most probably because of reduced mobility and an increased risk of falls, wrote Eshita Kapoor of the department of medicine at the University of Toronto and her coauthors.
“Screening and treatment may be particularly low poststroke because of under-recognition of osteoporosis as a consequence of stroke, a selective focus on the management of cardiovascular risk and stroke recovery, or factors such as dysphagia precluding use of oral bisphosphonates,” the authors wrote.
While the association is noted in U.S. stroke guidelines, there are few recommendations for treatment aside from fall prevention strategies, which the authors noted was a missed opportunity for prevention.
“Use of a risk prediction score to identify those at particularly high short-term risk of fractures after stroke may help to prioritize patients for osteoporosis testing and treatment,” they suggested.
The study was funded by the Heart and Stroke Foundation of Canada and was supported by ICES (Institute for Clinical Evaluative Sciences) and the Ontario Ministry of Health and Long-Term Care. One author declared consultancies for the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Kapoor E et al. Stroke. 2019 April 25. doi: 10.1161/STROKEAHA.118.024685
Although stroke is a risk factor for osteoporosis, falls, and fractures, very few people who have experienced a recent stroke are either screened for osteoporosis or treated, research suggests.
Writing in Stroke, researchers presented an analysis of Ontario registry data from 16,581 patients who were aged 65 years or older and presented with stroke between 2003 and 2013.
Overall, just 5.1% of patients underwent bone mineral density testing. Of the 1,577 patients who had experienced a prior fracture, 71 (4.7%) had bone mineral density testing, and only 2.9% of those who had not had prior bone mineral density testing were tested after their stroke. Bone mineral density testing was more likely in patients who were younger, who were female, and who experienced a low-trauma fracture in the year after their stroke.
In total, 15.5% of patients were prescribed osteoporosis drugs in the first year after their stroke. However, only 7.8% of those who had fractures before the stroke and 14.8% of those with fractures after the stroke received osteoporosis treatment after the stroke. Patients who were female, had prior osteoporosis, had experienced prior fracture, had previously undergone bone mineral density testing, or had experienced a fracture or fall after their stroke were more likely to receive osteoporosis pharmacotherapy.
The authors found that the neither the severity of stroke nor the presence of other comorbidities was associated with an increased likelihood of screening or treatment of osteoporosis after the stroke.
Stroke is associated with up to a fourfold increased risk of osteoporosis and fracture, compared with healthy controls, most probably because of reduced mobility and an increased risk of falls, wrote Eshita Kapoor of the department of medicine at the University of Toronto and her coauthors.
“Screening and treatment may be particularly low poststroke because of under-recognition of osteoporosis as a consequence of stroke, a selective focus on the management of cardiovascular risk and stroke recovery, or factors such as dysphagia precluding use of oral bisphosphonates,” the authors wrote.
While the association is noted in U.S. stroke guidelines, there are few recommendations for treatment aside from fall prevention strategies, which the authors noted was a missed opportunity for prevention.
“Use of a risk prediction score to identify those at particularly high short-term risk of fractures after stroke may help to prioritize patients for osteoporosis testing and treatment,” they suggested.
The study was funded by the Heart and Stroke Foundation of Canada and was supported by ICES (Institute for Clinical Evaluative Sciences) and the Ontario Ministry of Health and Long-Term Care. One author declared consultancies for the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Kapoor E et al. Stroke. 2019 April 25. doi: 10.1161/STROKEAHA.118.024685
Although stroke is a risk factor for osteoporosis, falls, and fractures, very few people who have experienced a recent stroke are either screened for osteoporosis or treated, research suggests.
Writing in Stroke, researchers presented an analysis of Ontario registry data from 16,581 patients who were aged 65 years or older and presented with stroke between 2003 and 2013.
Overall, just 5.1% of patients underwent bone mineral density testing. Of the 1,577 patients who had experienced a prior fracture, 71 (4.7%) had bone mineral density testing, and only 2.9% of those who had not had prior bone mineral density testing were tested after their stroke. Bone mineral density testing was more likely in patients who were younger, who were female, and who experienced a low-trauma fracture in the year after their stroke.
In total, 15.5% of patients were prescribed osteoporosis drugs in the first year after their stroke. However, only 7.8% of those who had fractures before the stroke and 14.8% of those with fractures after the stroke received osteoporosis treatment after the stroke. Patients who were female, had prior osteoporosis, had experienced prior fracture, had previously undergone bone mineral density testing, or had experienced a fracture or fall after their stroke were more likely to receive osteoporosis pharmacotherapy.
The authors found that the neither the severity of stroke nor the presence of other comorbidities was associated with an increased likelihood of screening or treatment of osteoporosis after the stroke.
Stroke is associated with up to a fourfold increased risk of osteoporosis and fracture, compared with healthy controls, most probably because of reduced mobility and an increased risk of falls, wrote Eshita Kapoor of the department of medicine at the University of Toronto and her coauthors.
“Screening and treatment may be particularly low poststroke because of under-recognition of osteoporosis as a consequence of stroke, a selective focus on the management of cardiovascular risk and stroke recovery, or factors such as dysphagia precluding use of oral bisphosphonates,” the authors wrote.
While the association is noted in U.S. stroke guidelines, there are few recommendations for treatment aside from fall prevention strategies, which the authors noted was a missed opportunity for prevention.
“Use of a risk prediction score to identify those at particularly high short-term risk of fractures after stroke may help to prioritize patients for osteoporosis testing and treatment,” they suggested.
The study was funded by the Heart and Stroke Foundation of Canada and was supported by ICES (Institute for Clinical Evaluative Sciences) and the Ontario Ministry of Health and Long-Term Care. One author declared consultancies for the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Kapoor E et al. Stroke. 2019 April 25. doi: 10.1161/STROKEAHA.118.024685
FROM STROKE