User login
Variants in common genes linked to endometrial cancer risk
The 24 single-nucleotide polymorphisms (SNPs) were detected in genes that function in transcriptional regulation, cell survival, and estrogen metabolism.
“Understanding genetic predisposition to endometrial cancer could facilitate personalized risk assessment with a view to targeted prevention and screening interventions,” wrote Cemsel Bafligil, of the University of Manchester (England) and her coinvestigators. The group’s findings were published in the Journal of Medical Genetics.
The researchers searched major databases for primary studies that evaluated associations between endometrial cancer and SNPs. After applying the search criteria, 453 eligible records were found, and 149 of these were included in the study.
The majority of records were genome-wide association studies, case-control studies, and meta-analyses. Various data, including study type, ethnicity, and endometrial cancer type, were extracted and included in the qualitative synthesis.
After analysis, the researchers identified 24 independent genetic variants associated with a higher risk of developing endometrial cancer, and SNPs in 6 genes – CYP19A1, SOX4, HNF1B, MYC, KLF, and EIF2AK – showed a strong association.
The researchers also estimated the predictive value of the identified SNPs using a theoretical polygenic risk score model. They found that women with genome-wide significant SNPs had double the risk of developing endometrial cancer (relative risk, 2.09), and women with all 24 SNPs had a three-fold greater risk of developing the disease (RR, 3.16).
“The importance of these variants and relevance of the proximate genes in a functional or biological context is challenging to evaluate,” the researchers noted.
They also acknowledged that a key limitation of this study was the ethnic homogeneity of the cohort, with most patients being of European descent. As a result, the findings may not be fully representative of other ethnic groups.
“The multiplicative effects of these SNPs could be used in a PRS [polygenic risk score] to allow personalised risk prediction models to be developed for targeted screening and prevention interventions for women at greatest risk of endometrial cancer,” the researchers concluded.
The National Institute for Health Research Manchester Biomedical Research Centre funded the study. The authors reported having no conflicts of interest.
SOURCE: Bafligil C et al. J Med Genet. 2020 Feb 17. doi: 10.1136/jmedgenet-2019-106529.
The 24 single-nucleotide polymorphisms (SNPs) were detected in genes that function in transcriptional regulation, cell survival, and estrogen metabolism.
“Understanding genetic predisposition to endometrial cancer could facilitate personalized risk assessment with a view to targeted prevention and screening interventions,” wrote Cemsel Bafligil, of the University of Manchester (England) and her coinvestigators. The group’s findings were published in the Journal of Medical Genetics.
The researchers searched major databases for primary studies that evaluated associations between endometrial cancer and SNPs. After applying the search criteria, 453 eligible records were found, and 149 of these were included in the study.
The majority of records were genome-wide association studies, case-control studies, and meta-analyses. Various data, including study type, ethnicity, and endometrial cancer type, were extracted and included in the qualitative synthesis.
After analysis, the researchers identified 24 independent genetic variants associated with a higher risk of developing endometrial cancer, and SNPs in 6 genes – CYP19A1, SOX4, HNF1B, MYC, KLF, and EIF2AK – showed a strong association.
The researchers also estimated the predictive value of the identified SNPs using a theoretical polygenic risk score model. They found that women with genome-wide significant SNPs had double the risk of developing endometrial cancer (relative risk, 2.09), and women with all 24 SNPs had a three-fold greater risk of developing the disease (RR, 3.16).
“The importance of these variants and relevance of the proximate genes in a functional or biological context is challenging to evaluate,” the researchers noted.
They also acknowledged that a key limitation of this study was the ethnic homogeneity of the cohort, with most patients being of European descent. As a result, the findings may not be fully representative of other ethnic groups.
“The multiplicative effects of these SNPs could be used in a PRS [polygenic risk score] to allow personalised risk prediction models to be developed for targeted screening and prevention interventions for women at greatest risk of endometrial cancer,” the researchers concluded.
The National Institute for Health Research Manchester Biomedical Research Centre funded the study. The authors reported having no conflicts of interest.
SOURCE: Bafligil C et al. J Med Genet. 2020 Feb 17. doi: 10.1136/jmedgenet-2019-106529.
The 24 single-nucleotide polymorphisms (SNPs) were detected in genes that function in transcriptional regulation, cell survival, and estrogen metabolism.
“Understanding genetic predisposition to endometrial cancer could facilitate personalized risk assessment with a view to targeted prevention and screening interventions,” wrote Cemsel Bafligil, of the University of Manchester (England) and her coinvestigators. The group’s findings were published in the Journal of Medical Genetics.
The researchers searched major databases for primary studies that evaluated associations between endometrial cancer and SNPs. After applying the search criteria, 453 eligible records were found, and 149 of these were included in the study.
The majority of records were genome-wide association studies, case-control studies, and meta-analyses. Various data, including study type, ethnicity, and endometrial cancer type, were extracted and included in the qualitative synthesis.
After analysis, the researchers identified 24 independent genetic variants associated with a higher risk of developing endometrial cancer, and SNPs in 6 genes – CYP19A1, SOX4, HNF1B, MYC, KLF, and EIF2AK – showed a strong association.
The researchers also estimated the predictive value of the identified SNPs using a theoretical polygenic risk score model. They found that women with genome-wide significant SNPs had double the risk of developing endometrial cancer (relative risk, 2.09), and women with all 24 SNPs had a three-fold greater risk of developing the disease (RR, 3.16).
“The importance of these variants and relevance of the proximate genes in a functional or biological context is challenging to evaluate,” the researchers noted.
They also acknowledged that a key limitation of this study was the ethnic homogeneity of the cohort, with most patients being of European descent. As a result, the findings may not be fully representative of other ethnic groups.
“The multiplicative effects of these SNPs could be used in a PRS [polygenic risk score] to allow personalised risk prediction models to be developed for targeted screening and prevention interventions for women at greatest risk of endometrial cancer,” the researchers concluded.
The National Institute for Health Research Manchester Biomedical Research Centre funded the study. The authors reported having no conflicts of interest.
SOURCE: Bafligil C et al. J Med Genet. 2020 Feb 17. doi: 10.1136/jmedgenet-2019-106529.
FROM THE JOURNAL OF MEDICAL GENETICS
Guidelines for today and tomorrow
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Proteogenomics provides molecular insights into endometrial carcinoma
according to a proteogenomic study.
Further insights into the regulatory mechanisms of the disease were also identified, based on findings from genome-wide phosphoproteome and acetylome surveys.
“This study provides a comprehensive overview of the molecular systems of endometrial carcinoma at the genomic, transcriptomic, and proteomic levels,” wrote Yongchao Dou, PhD, of Baylor College of Medicine, Houston, and colleagues. The findings were published in Cell.
The researchers prospectively analyzed proteogenomic data from 95 endometrial carcinoma tumors, including 83 endometrioid and 12 serous samples, and 49 nonmalignant tissue samples. Whole genome and exome, DNA methylation, and total and microRNA sequencing analyses were performed for each sample.
An integrated evaluation of proteins, posttranslational modifications (phosphorylation and acetylation), DNA, and RNA were used to detect novel regulatory mechanisms and potential therapeutic targets.
The researchers confirmed previous data on the impact of gain-of-function TP53 mutations on the Aurora kinase pathway, notably the relationship between AURKA expression and negative outcomes in endometrial carcinoma.
“[These findings] provide a theoretical basis for the use of AURKA inhibitors in these tumors,” the researchers wrote.
In addition, the team found evidence of a new regulatory pathway involving ESRP2, circular RNA (circRNA), and QKI, each of which plays a key role in regulatory function.
“Through its known function in isoform regulation, ESRP2 could also directly regulate circRNA levels, and, if so, it could compete with QKI in circRNA-mediated gene regulation,” the researchers wrote.
Furthermore, they identified several gene products that could play a role in optimizing selection of patients for checkpoint blockade immunotherapy. One product in particular, CDK12, may better clinical response rates to immune checkpoint blockade.
The researchers also found evidence to suggest that measuring tumor antigen presentation defects could be more effective than measuring tumor mutation burden when selecting immunotherapy for patients with endometrial carcinoma.
“Although the results presented herein are predominantly observational, they provide the basis for multiple hypotheses of clinical relevance that can and should be further explored,” the researchers concluded.
The study was funded by the National Cancer Institute, the Cancer Prevention & Research Institutes of Texas, and the Robert and Janice McNair Foundation. The authors reported having no conflicts of interest.
SOURCE: Dou Y et al. Cell. 2020 Feb 13. doi: 10.1016/j.cell.2020.01.026.
according to a proteogenomic study.
Further insights into the regulatory mechanisms of the disease were also identified, based on findings from genome-wide phosphoproteome and acetylome surveys.
“This study provides a comprehensive overview of the molecular systems of endometrial carcinoma at the genomic, transcriptomic, and proteomic levels,” wrote Yongchao Dou, PhD, of Baylor College of Medicine, Houston, and colleagues. The findings were published in Cell.
The researchers prospectively analyzed proteogenomic data from 95 endometrial carcinoma tumors, including 83 endometrioid and 12 serous samples, and 49 nonmalignant tissue samples. Whole genome and exome, DNA methylation, and total and microRNA sequencing analyses were performed for each sample.
An integrated evaluation of proteins, posttranslational modifications (phosphorylation and acetylation), DNA, and RNA were used to detect novel regulatory mechanisms and potential therapeutic targets.
The researchers confirmed previous data on the impact of gain-of-function TP53 mutations on the Aurora kinase pathway, notably the relationship between AURKA expression and negative outcomes in endometrial carcinoma.
“[These findings] provide a theoretical basis for the use of AURKA inhibitors in these tumors,” the researchers wrote.
In addition, the team found evidence of a new regulatory pathway involving ESRP2, circular RNA (circRNA), and QKI, each of which plays a key role in regulatory function.
“Through its known function in isoform regulation, ESRP2 could also directly regulate circRNA levels, and, if so, it could compete with QKI in circRNA-mediated gene regulation,” the researchers wrote.
Furthermore, they identified several gene products that could play a role in optimizing selection of patients for checkpoint blockade immunotherapy. One product in particular, CDK12, may better clinical response rates to immune checkpoint blockade.
The researchers also found evidence to suggest that measuring tumor antigen presentation defects could be more effective than measuring tumor mutation burden when selecting immunotherapy for patients with endometrial carcinoma.
“Although the results presented herein are predominantly observational, they provide the basis for multiple hypotheses of clinical relevance that can and should be further explored,” the researchers concluded.
The study was funded by the National Cancer Institute, the Cancer Prevention & Research Institutes of Texas, and the Robert and Janice McNair Foundation. The authors reported having no conflicts of interest.
SOURCE: Dou Y et al. Cell. 2020 Feb 13. doi: 10.1016/j.cell.2020.01.026.
according to a proteogenomic study.
Further insights into the regulatory mechanisms of the disease were also identified, based on findings from genome-wide phosphoproteome and acetylome surveys.
“This study provides a comprehensive overview of the molecular systems of endometrial carcinoma at the genomic, transcriptomic, and proteomic levels,” wrote Yongchao Dou, PhD, of Baylor College of Medicine, Houston, and colleagues. The findings were published in Cell.
The researchers prospectively analyzed proteogenomic data from 95 endometrial carcinoma tumors, including 83 endometrioid and 12 serous samples, and 49 nonmalignant tissue samples. Whole genome and exome, DNA methylation, and total and microRNA sequencing analyses were performed for each sample.
An integrated evaluation of proteins, posttranslational modifications (phosphorylation and acetylation), DNA, and RNA were used to detect novel regulatory mechanisms and potential therapeutic targets.
The researchers confirmed previous data on the impact of gain-of-function TP53 mutations on the Aurora kinase pathway, notably the relationship between AURKA expression and negative outcomes in endometrial carcinoma.
“[These findings] provide a theoretical basis for the use of AURKA inhibitors in these tumors,” the researchers wrote.
In addition, the team found evidence of a new regulatory pathway involving ESRP2, circular RNA (circRNA), and QKI, each of which plays a key role in regulatory function.
“Through its known function in isoform regulation, ESRP2 could also directly regulate circRNA levels, and, if so, it could compete with QKI in circRNA-mediated gene regulation,” the researchers wrote.
Furthermore, they identified several gene products that could play a role in optimizing selection of patients for checkpoint blockade immunotherapy. One product in particular, CDK12, may better clinical response rates to immune checkpoint blockade.
The researchers also found evidence to suggest that measuring tumor antigen presentation defects could be more effective than measuring tumor mutation burden when selecting immunotherapy for patients with endometrial carcinoma.
“Although the results presented herein are predominantly observational, they provide the basis for multiple hypotheses of clinical relevance that can and should be further explored,” the researchers concluded.
The study was funded by the National Cancer Institute, the Cancer Prevention & Research Institutes of Texas, and the Robert and Janice McNair Foundation. The authors reported having no conflicts of interest.
SOURCE: Dou Y et al. Cell. 2020 Feb 13. doi: 10.1016/j.cell.2020.01.026.
FROM CELL
IFN-activated monocytes show early promise for ovarian cancer
ORLANDO –
The best response observed in the open-label, dose-escalation study was a partial response in 2 of 11 evaluable patients, with about a 30% reduction in target lesion size in both patients. An additional six patients had stable disease, and three had progressive disease.
Christopher Browning Cole, MD, PhD, of the National Cancer Institute, Bethesda, Md., reported these results at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The primary objective of this study was to determine safety and identify the maximum tolerated dose (MTD). The study enrolled 18 patients with metastatic or unresectable ovarian cancer that was platinum resistant or refractory. They had a median age of 61 years and had received a median of five prior therapies.
The patients were enrolled in four dose cohorts in which they were treated every 28 days with intraperitoneal peginterferon alfa-2b (Sylatron) at doses of 25-250 mcg and interferon gamma-1b (Actimmune) at doses of 5-50 mcg, with or without autologous monocytes (75-750 x 106 cells).
In all, 15 patients were assigned to dose levels that included monocytes (dose levels 2, 3, and 4). Two of these patients were unable to tolerate apheresis and were reallocated, after two to four cycles of therapy, to “dose level 3-b,” which included 250 mcg of peginterferon alfa-2b, 50 mcg of interferon gamma-1b, and no monocytes, Dr. Cole said.
Results
Based on overall safety and tolerability, the highest dose (250 mcg of peginterferon alfa-2b, 50 mcg of interferon gamma-1b, and 750 x 106 autologous monocytes) was the MTD. Six patients received this dose.
One of the partial responders received one cycle of the MTD, and the other received eight cycles of the lowest dose (25 mcg of peginterferon alfa-2b, 5 mcg of interferon gamma-1b, and no monocytes).
The median number of cycles patients received was 3.2, but “several patients stayed on treatment much longer than that,” Dr. Cole said. Two patients are still on study, one of whom has received 10 cycles to date.
Toxicities were “largely expected” based on prior studies, and included fatigue, nausea, and abdominal pain, Dr. Cole said. He added that monocyte collection by apheresis was “tolerated pretty well” by all but the two patients reallocated to dose level 3-b, and no grade 4 or 5 toxicities occurred.
There was one grade 3 peritoneal infection associated with a catheter used for monocyte administration. After a switch to port access for administration, no other such complications occurred, Dr. Cole noted.
Rationale and next steps
“There were three sets of studies that really provided the basis for [this trial],” Dr. Cole said, explaining that the first involves the “long-standing observation that ovarian cancer is really a peritoneal disease.”
“This has led to a lot of interest in directing therapies directly to the tumor in the peritoneum ... and many of these trials have shown some really impressive results in terms of [overall survival] advantage in long-term follow up,” he added.
The second set includes pioneering studies using interferons, which are capable of activating innate immune cells, intraperitoneally to treat ovarian cancer. Interferon-gamma and interferon-alfa showed particular promise.
The third set of studies, including extensive preclinical data, shows that autologous monocytes can be activated by interferon-gamma and interferon-alfa to become tumoricidal, Dr. Cole said.
The findings of the current study support further assessment of this approach, he said, adding that exploratory analyses are ongoing to measure plasma cytokines at baseline and after therapy, specifically looking for cytokines secreted by activated monocytes.
In addition to interferon-gamma, several cytokines were present at detectable levels in the blood, including interleukin-6 and tumor necrosis factor–alpha, Dr. Cole noted.
He and colleagues seek to understand changes in immune cell populations induced by the therapy. They have developed a comprehensive set of panels to look at ligands and markers reflective of immune system activation, and “markers which might be targets for future therapies, potentially allowing us to develop some rational combinations, such as [programmed death-1, programmed death-ligand 1, and cytotoxic T-lymphocyte-associated antigen 4],” he added.
“What we’d like to do next is enroll an expansion cohort at the MTD to learn more about efficacy and immunomodulatory effects of this regimen, particularly ... what’s happening with the immune system in the tumor microenvironment, so we’re going to perform tumor biopsies before and after therapy to learn a little more about that,” Dr. Cole said. “What we ultimately envision this regimen becoming is a platform to combine intraperitoneal cellular immune therapies in the innate immune system with other immunotherapies, such as systemic therapy targeting the adaptive immune system.”
The National Institutes of Health funded the study. Dr. Cole reported having no disclosures.
SOURCE: Cole C et al. ASCO-SITC: Abstract 1.
ORLANDO –
The best response observed in the open-label, dose-escalation study was a partial response in 2 of 11 evaluable patients, with about a 30% reduction in target lesion size in both patients. An additional six patients had stable disease, and three had progressive disease.
Christopher Browning Cole, MD, PhD, of the National Cancer Institute, Bethesda, Md., reported these results at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The primary objective of this study was to determine safety and identify the maximum tolerated dose (MTD). The study enrolled 18 patients with metastatic or unresectable ovarian cancer that was platinum resistant or refractory. They had a median age of 61 years and had received a median of five prior therapies.
The patients were enrolled in four dose cohorts in which they were treated every 28 days with intraperitoneal peginterferon alfa-2b (Sylatron) at doses of 25-250 mcg and interferon gamma-1b (Actimmune) at doses of 5-50 mcg, with or without autologous monocytes (75-750 x 106 cells).
In all, 15 patients were assigned to dose levels that included monocytes (dose levels 2, 3, and 4). Two of these patients were unable to tolerate apheresis and were reallocated, after two to four cycles of therapy, to “dose level 3-b,” which included 250 mcg of peginterferon alfa-2b, 50 mcg of interferon gamma-1b, and no monocytes, Dr. Cole said.
Results
Based on overall safety and tolerability, the highest dose (250 mcg of peginterferon alfa-2b, 50 mcg of interferon gamma-1b, and 750 x 106 autologous monocytes) was the MTD. Six patients received this dose.
One of the partial responders received one cycle of the MTD, and the other received eight cycles of the lowest dose (25 mcg of peginterferon alfa-2b, 5 mcg of interferon gamma-1b, and no monocytes).
The median number of cycles patients received was 3.2, but “several patients stayed on treatment much longer than that,” Dr. Cole said. Two patients are still on study, one of whom has received 10 cycles to date.
Toxicities were “largely expected” based on prior studies, and included fatigue, nausea, and abdominal pain, Dr. Cole said. He added that monocyte collection by apheresis was “tolerated pretty well” by all but the two patients reallocated to dose level 3-b, and no grade 4 or 5 toxicities occurred.
There was one grade 3 peritoneal infection associated with a catheter used for monocyte administration. After a switch to port access for administration, no other such complications occurred, Dr. Cole noted.
Rationale and next steps
“There were three sets of studies that really provided the basis for [this trial],” Dr. Cole said, explaining that the first involves the “long-standing observation that ovarian cancer is really a peritoneal disease.”
“This has led to a lot of interest in directing therapies directly to the tumor in the peritoneum ... and many of these trials have shown some really impressive results in terms of [overall survival] advantage in long-term follow up,” he added.
The second set includes pioneering studies using interferons, which are capable of activating innate immune cells, intraperitoneally to treat ovarian cancer. Interferon-gamma and interferon-alfa showed particular promise.
The third set of studies, including extensive preclinical data, shows that autologous monocytes can be activated by interferon-gamma and interferon-alfa to become tumoricidal, Dr. Cole said.
The findings of the current study support further assessment of this approach, he said, adding that exploratory analyses are ongoing to measure plasma cytokines at baseline and after therapy, specifically looking for cytokines secreted by activated monocytes.
In addition to interferon-gamma, several cytokines were present at detectable levels in the blood, including interleukin-6 and tumor necrosis factor–alpha, Dr. Cole noted.
He and colleagues seek to understand changes in immune cell populations induced by the therapy. They have developed a comprehensive set of panels to look at ligands and markers reflective of immune system activation, and “markers which might be targets for future therapies, potentially allowing us to develop some rational combinations, such as [programmed death-1, programmed death-ligand 1, and cytotoxic T-lymphocyte-associated antigen 4],” he added.
“What we’d like to do next is enroll an expansion cohort at the MTD to learn more about efficacy and immunomodulatory effects of this regimen, particularly ... what’s happening with the immune system in the tumor microenvironment, so we’re going to perform tumor biopsies before and after therapy to learn a little more about that,” Dr. Cole said. “What we ultimately envision this regimen becoming is a platform to combine intraperitoneal cellular immune therapies in the innate immune system with other immunotherapies, such as systemic therapy targeting the adaptive immune system.”
The National Institutes of Health funded the study. Dr. Cole reported having no disclosures.
SOURCE: Cole C et al. ASCO-SITC: Abstract 1.
ORLANDO –
The best response observed in the open-label, dose-escalation study was a partial response in 2 of 11 evaluable patients, with about a 30% reduction in target lesion size in both patients. An additional six patients had stable disease, and three had progressive disease.
Christopher Browning Cole, MD, PhD, of the National Cancer Institute, Bethesda, Md., reported these results at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The primary objective of this study was to determine safety and identify the maximum tolerated dose (MTD). The study enrolled 18 patients with metastatic or unresectable ovarian cancer that was platinum resistant or refractory. They had a median age of 61 years and had received a median of five prior therapies.
The patients were enrolled in four dose cohorts in which they were treated every 28 days with intraperitoneal peginterferon alfa-2b (Sylatron) at doses of 25-250 mcg and interferon gamma-1b (Actimmune) at doses of 5-50 mcg, with or without autologous monocytes (75-750 x 106 cells).
In all, 15 patients were assigned to dose levels that included monocytes (dose levels 2, 3, and 4). Two of these patients were unable to tolerate apheresis and were reallocated, after two to four cycles of therapy, to “dose level 3-b,” which included 250 mcg of peginterferon alfa-2b, 50 mcg of interferon gamma-1b, and no monocytes, Dr. Cole said.
Results
Based on overall safety and tolerability, the highest dose (250 mcg of peginterferon alfa-2b, 50 mcg of interferon gamma-1b, and 750 x 106 autologous monocytes) was the MTD. Six patients received this dose.
One of the partial responders received one cycle of the MTD, and the other received eight cycles of the lowest dose (25 mcg of peginterferon alfa-2b, 5 mcg of interferon gamma-1b, and no monocytes).
The median number of cycles patients received was 3.2, but “several patients stayed on treatment much longer than that,” Dr. Cole said. Two patients are still on study, one of whom has received 10 cycles to date.
Toxicities were “largely expected” based on prior studies, and included fatigue, nausea, and abdominal pain, Dr. Cole said. He added that monocyte collection by apheresis was “tolerated pretty well” by all but the two patients reallocated to dose level 3-b, and no grade 4 or 5 toxicities occurred.
There was one grade 3 peritoneal infection associated with a catheter used for monocyte administration. After a switch to port access for administration, no other such complications occurred, Dr. Cole noted.
Rationale and next steps
“There were three sets of studies that really provided the basis for [this trial],” Dr. Cole said, explaining that the first involves the “long-standing observation that ovarian cancer is really a peritoneal disease.”
“This has led to a lot of interest in directing therapies directly to the tumor in the peritoneum ... and many of these trials have shown some really impressive results in terms of [overall survival] advantage in long-term follow up,” he added.
The second set includes pioneering studies using interferons, which are capable of activating innate immune cells, intraperitoneally to treat ovarian cancer. Interferon-gamma and interferon-alfa showed particular promise.
The third set of studies, including extensive preclinical data, shows that autologous monocytes can be activated by interferon-gamma and interferon-alfa to become tumoricidal, Dr. Cole said.
The findings of the current study support further assessment of this approach, he said, adding that exploratory analyses are ongoing to measure plasma cytokines at baseline and after therapy, specifically looking for cytokines secreted by activated monocytes.
In addition to interferon-gamma, several cytokines were present at detectable levels in the blood, including interleukin-6 and tumor necrosis factor–alpha, Dr. Cole noted.
He and colleagues seek to understand changes in immune cell populations induced by the therapy. They have developed a comprehensive set of panels to look at ligands and markers reflective of immune system activation, and “markers which might be targets for future therapies, potentially allowing us to develop some rational combinations, such as [programmed death-1, programmed death-ligand 1, and cytotoxic T-lymphocyte-associated antigen 4],” he added.
“What we’d like to do next is enroll an expansion cohort at the MTD to learn more about efficacy and immunomodulatory effects of this regimen, particularly ... what’s happening with the immune system in the tumor microenvironment, so we’re going to perform tumor biopsies before and after therapy to learn a little more about that,” Dr. Cole said. “What we ultimately envision this regimen becoming is a platform to combine intraperitoneal cellular immune therapies in the innate immune system with other immunotherapies, such as systemic therapy targeting the adaptive immune system.”
The National Institutes of Health funded the study. Dr. Cole reported having no disclosures.
SOURCE: Cole C et al. ASCO-SITC: Abstract 1.
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
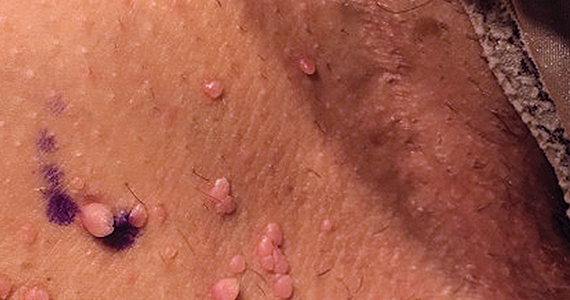
Can you identify these numerous papules in the groin area?
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2
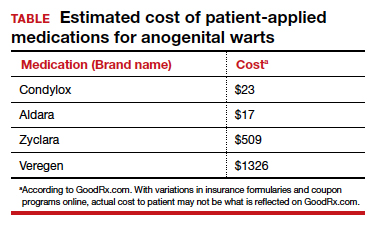
Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2

Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2

Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
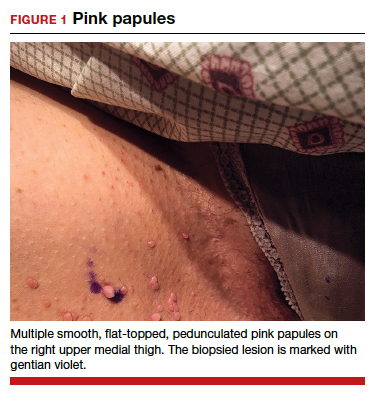
CASE Skin tags on the groin
A 47-year-old woman with no personal history of skin cancer presents to a dermatologist for annual skin surveillance examination. She notes multiple “pink skin tags” on the groin, present for 4 months. She says they are asymptomatic and have not been treated previously. She states that she is in a long-term monogamous relationship. Physical examination reveals multiple smooth, flat-topped, pedunculated pink papules on the bilateral upper inner thighs. Shave biopsy of a lesion on the right upper medial thigh is performed to aid in diagnosis (FIGURE 1).
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
Any dose of HPV vaccine is better than none
A single dose of the human papillomavirus vaccine is as effective as two or three doses for preventing cervical cancer in girls and women vaccinated at 15-19 years of age, based on data from a retrospective study of more than 100,000 girls and women.
The Centers for Disease Control and Prevention’s current recommendations include a two-dose vaccine schedule for the HPV vaccine for girls and boys younger than 15 years, and a three-dose schedule for girls and young women aged 16-26 years who had their first dose before turning 15.
However, rates of HPV vaccination in the United States fall short of those in other developed nations, and evidence supporting the protective value of a specific number of vaccine doses are mixed, wrote Ana M. Rodriguez, MD, MPH, of the University of Texas Medical Branch at Galveston, and colleagues. Fewer than three doses could have benefits, including easier logistics, lower costs, higher acceptance rates, and fewer side effects, they said. The study was published in Cancer.
The researchers reviewed data from 66,541 girls and women aged 9-26 years who had received at least one dose of HPV vaccine (4vHPV) between Jan. 1, 2006, and June 30, 2015, and 66,541 matched unvaccinated controls. The primary outcomes were histologically confirmed preinvasive cervical disease and high-grade cytology.
Overall, the adjusted hazard ratios for histologically confirmed preinvasive cervical disease among patients vaccinated at the ages of 15-19 years with one, two, and three doses were similar, at 0.64, 0.72, and 0.66, respectively, compared with unvaccinated individuals.
The risk of high-grade cytology was significantly lower for girls and women who received three doses at age 15-19 years, compared with unvaccinated individuals, but no difference was seen in high-grade cytology between unvaccinated individuals and those who received one or two doses. In addition, the unadjusted rate of preinvasive cervical disease at 5 years was 2.65% for unvaccinated teens aged 15-19 years, compared with 1.62%, 1.99%, and 1.86% in the one-, two- and three-dose groups, respectively.The findings were limited by several factors, including the use of billing codes to determine outcomes and the inability to determine potential vaccination through multiple insurance carriers, and the inclusion only of privately insured patients from the claims database, the researchers noted.
However, the results support findings from previous studies and show a similar level of association between varying vaccine doses and preinvasive cervical lesions in the 15- to 19-year-old population, they said.
“Efforts should focus on not only the need to initiate the HPV vaccine but also the need for beginning and continuing cervical cancer screening among young women who are vaccinated at older ages (18 years and older),” they said.
In an editorial accompanying the study, Julia M.L. Brotherton, PhD, MPH, and Karin Sundström, MD, PhD, of the University of Melbourne, Australia, and the Karolinska Institutet, Stockholm, respectively, wrote that the study’s strengths included the large numbers of girls and women who received a single dose of the HPV vaccine, compared with previous studies, as well as the adjustments for histories of sexually transmitted infections and pregnancy (Cancer. 2020 Feb 10. doi: 10.1002/cncr.32696). “Initial observational data from vaccination programs did not support equivalent one-dose protection against genital warts or cervical disease, but such data may have been confounded by potentially higher risk characteristics of women who only ever received one or two doses of an intended three-dose course i.e., women noncompliant with the vaccine program [amplified by the monitoring of outcomes among the initial catch-up populations of already infected women]) and by the inherent bias that prevalent infection/disease is more likely to become apparent coincidently with the earlier doses in a vaccine course,” they said. The study findings have implications for global goals to eliminate cervical cancer, the editorial authors noted.
“If one dose of an HPV vaccine were sufficient for effective protection, HPV vaccine implementation and scale-up would require less logistics (while being amenable to a periodic campaign approach), available doses could be extended further, and the overall cost would be lower,” they said.
The study was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health, and by the Cancer Prevention Research Institute of Texas. The researchers had no financial conflicts to disclose.
Dr. Brotherton disclosed serving as an investigator for Seqirus and Merck; Dr. Sundström disclosed research funding for her institution from Merck and MSD Sweden.
SOURCE: Rodriguez AM et al. Cancer. 2020 Feb 10. doi: 10.1002/cncr.32700.
A single dose of the human papillomavirus vaccine is as effective as two or three doses for preventing cervical cancer in girls and women vaccinated at 15-19 years of age, based on data from a retrospective study of more than 100,000 girls and women.
The Centers for Disease Control and Prevention’s current recommendations include a two-dose vaccine schedule for the HPV vaccine for girls and boys younger than 15 years, and a three-dose schedule for girls and young women aged 16-26 years who had their first dose before turning 15.
However, rates of HPV vaccination in the United States fall short of those in other developed nations, and evidence supporting the protective value of a specific number of vaccine doses are mixed, wrote Ana M. Rodriguez, MD, MPH, of the University of Texas Medical Branch at Galveston, and colleagues. Fewer than three doses could have benefits, including easier logistics, lower costs, higher acceptance rates, and fewer side effects, they said. The study was published in Cancer.
The researchers reviewed data from 66,541 girls and women aged 9-26 years who had received at least one dose of HPV vaccine (4vHPV) between Jan. 1, 2006, and June 30, 2015, and 66,541 matched unvaccinated controls. The primary outcomes were histologically confirmed preinvasive cervical disease and high-grade cytology.
Overall, the adjusted hazard ratios for histologically confirmed preinvasive cervical disease among patients vaccinated at the ages of 15-19 years with one, two, and three doses were similar, at 0.64, 0.72, and 0.66, respectively, compared with unvaccinated individuals.
The risk of high-grade cytology was significantly lower for girls and women who received three doses at age 15-19 years, compared with unvaccinated individuals, but no difference was seen in high-grade cytology between unvaccinated individuals and those who received one or two doses. In addition, the unadjusted rate of preinvasive cervical disease at 5 years was 2.65% for unvaccinated teens aged 15-19 years, compared with 1.62%, 1.99%, and 1.86% in the one-, two- and three-dose groups, respectively.The findings were limited by several factors, including the use of billing codes to determine outcomes and the inability to determine potential vaccination through multiple insurance carriers, and the inclusion only of privately insured patients from the claims database, the researchers noted.
However, the results support findings from previous studies and show a similar level of association between varying vaccine doses and preinvasive cervical lesions in the 15- to 19-year-old population, they said.
“Efforts should focus on not only the need to initiate the HPV vaccine but also the need for beginning and continuing cervical cancer screening among young women who are vaccinated at older ages (18 years and older),” they said.
In an editorial accompanying the study, Julia M.L. Brotherton, PhD, MPH, and Karin Sundström, MD, PhD, of the University of Melbourne, Australia, and the Karolinska Institutet, Stockholm, respectively, wrote that the study’s strengths included the large numbers of girls and women who received a single dose of the HPV vaccine, compared with previous studies, as well as the adjustments for histories of sexually transmitted infections and pregnancy (Cancer. 2020 Feb 10. doi: 10.1002/cncr.32696). “Initial observational data from vaccination programs did not support equivalent one-dose protection against genital warts or cervical disease, but such data may have been confounded by potentially higher risk characteristics of women who only ever received one or two doses of an intended three-dose course i.e., women noncompliant with the vaccine program [amplified by the monitoring of outcomes among the initial catch-up populations of already infected women]) and by the inherent bias that prevalent infection/disease is more likely to become apparent coincidently with the earlier doses in a vaccine course,” they said. The study findings have implications for global goals to eliminate cervical cancer, the editorial authors noted.
“If one dose of an HPV vaccine were sufficient for effective protection, HPV vaccine implementation and scale-up would require less logistics (while being amenable to a periodic campaign approach), available doses could be extended further, and the overall cost would be lower,” they said.
The study was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health, and by the Cancer Prevention Research Institute of Texas. The researchers had no financial conflicts to disclose.
Dr. Brotherton disclosed serving as an investigator for Seqirus and Merck; Dr. Sundström disclosed research funding for her institution from Merck and MSD Sweden.
SOURCE: Rodriguez AM et al. Cancer. 2020 Feb 10. doi: 10.1002/cncr.32700.
A single dose of the human papillomavirus vaccine is as effective as two or three doses for preventing cervical cancer in girls and women vaccinated at 15-19 years of age, based on data from a retrospective study of more than 100,000 girls and women.
The Centers for Disease Control and Prevention’s current recommendations include a two-dose vaccine schedule for the HPV vaccine for girls and boys younger than 15 years, and a three-dose schedule for girls and young women aged 16-26 years who had their first dose before turning 15.
However, rates of HPV vaccination in the United States fall short of those in other developed nations, and evidence supporting the protective value of a specific number of vaccine doses are mixed, wrote Ana M. Rodriguez, MD, MPH, of the University of Texas Medical Branch at Galveston, and colleagues. Fewer than three doses could have benefits, including easier logistics, lower costs, higher acceptance rates, and fewer side effects, they said. The study was published in Cancer.
The researchers reviewed data from 66,541 girls and women aged 9-26 years who had received at least one dose of HPV vaccine (4vHPV) between Jan. 1, 2006, and June 30, 2015, and 66,541 matched unvaccinated controls. The primary outcomes were histologically confirmed preinvasive cervical disease and high-grade cytology.
Overall, the adjusted hazard ratios for histologically confirmed preinvasive cervical disease among patients vaccinated at the ages of 15-19 years with one, two, and three doses were similar, at 0.64, 0.72, and 0.66, respectively, compared with unvaccinated individuals.
The risk of high-grade cytology was significantly lower for girls and women who received three doses at age 15-19 years, compared with unvaccinated individuals, but no difference was seen in high-grade cytology between unvaccinated individuals and those who received one or two doses. In addition, the unadjusted rate of preinvasive cervical disease at 5 years was 2.65% for unvaccinated teens aged 15-19 years, compared with 1.62%, 1.99%, and 1.86% in the one-, two- and three-dose groups, respectively.The findings were limited by several factors, including the use of billing codes to determine outcomes and the inability to determine potential vaccination through multiple insurance carriers, and the inclusion only of privately insured patients from the claims database, the researchers noted.
However, the results support findings from previous studies and show a similar level of association between varying vaccine doses and preinvasive cervical lesions in the 15- to 19-year-old population, they said.
“Efforts should focus on not only the need to initiate the HPV vaccine but also the need for beginning and continuing cervical cancer screening among young women who are vaccinated at older ages (18 years and older),” they said.
In an editorial accompanying the study, Julia M.L. Brotherton, PhD, MPH, and Karin Sundström, MD, PhD, of the University of Melbourne, Australia, and the Karolinska Institutet, Stockholm, respectively, wrote that the study’s strengths included the large numbers of girls and women who received a single dose of the HPV vaccine, compared with previous studies, as well as the adjustments for histories of sexually transmitted infections and pregnancy (Cancer. 2020 Feb 10. doi: 10.1002/cncr.32696). “Initial observational data from vaccination programs did not support equivalent one-dose protection against genital warts or cervical disease, but such data may have been confounded by potentially higher risk characteristics of women who only ever received one or two doses of an intended three-dose course i.e., women noncompliant with the vaccine program [amplified by the monitoring of outcomes among the initial catch-up populations of already infected women]) and by the inherent bias that prevalent infection/disease is more likely to become apparent coincidently with the earlier doses in a vaccine course,” they said. The study findings have implications for global goals to eliminate cervical cancer, the editorial authors noted.
“If one dose of an HPV vaccine were sufficient for effective protection, HPV vaccine implementation and scale-up would require less logistics (while being amenable to a periodic campaign approach), available doses could be extended further, and the overall cost would be lower,” they said.
The study was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health, and by the Cancer Prevention Research Institute of Texas. The researchers had no financial conflicts to disclose.
Dr. Brotherton disclosed serving as an investigator for Seqirus and Merck; Dr. Sundström disclosed research funding for her institution from Merck and MSD Sweden.
SOURCE: Rodriguez AM et al. Cancer. 2020 Feb 10. doi: 10.1002/cncr.32700.
FROM CANCER
Key clinical point: HPV vaccination was similarly effective for preventing cervical cancer in girls and women who received 1, 2, or 3 doses at age 15-19 years.
Major finding: The adjusted hazard ratios for preinvasive cervical disease for women vaccinated at age 15-19 years with 1, 2, and 3 doses of the HPV vaccine were 0.64, 0.72, and 0.66 respectively.
Study details: The data come from a retrospective matched cohort study of 133,082 women from the Optum Clinformatics DataMart Database.
Disclosures: The study was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health and by the Cancer Prevention Research Institute of Texas. The researchers disclosed no financial conflicts.
Source: Rodriguez AM et al. Cancer. 2020 Feb 10. doi: 10.1002/cncr.32700.
Lidocaine-prilocaine cream tops lidocaine injections for vulvar biopsy pain
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
FROM OBSTETRICS AND GYNECOLOGY
Vaccinating most girls could eliminate cervical cancer within a century
Cervical cancer is the second most common cancer among women in lower- and middle-income countries, but universal human papillomavirus vaccination for girls would reduce new cervical cancer cases by about 90% over the next century, according to researchers.
Adding twice-lifetime cervical screening with human papillomavirus (HPV) testing would further reduce the incidence of cervical cancer, including in countries with the highest burden, the researchers reported in The Lancet.
Marc Brisson, PhD, of Laval University, Quebec City, and colleagues conducted this study using three models identified by the World Health Organization. The models were used to project reductions in cervical cancer incidence for women in 78 low- and middle-income countries based on the following HPV vaccination and screening scenarios:
- Universal girls-only vaccination at age 9 years, assuming 90% of girls vaccinated and a vaccine that is perfectly effective
- Girls-only vaccination plus cervical screening with HPV testing at age 35 years
- Girls-only vaccination plus screening at ages 35 and 45.
All three scenarios modeled would result in the elimination of cervical cancer, Dr. Brisson and colleagues found. Elimination was defined as four or fewer new cases per 100,000 women-years.
The simplest scenario, universal girls-only vaccination, was predicted to reduce age-standardized cervical cancer incidence from 19.8 cases per 100,000 women-years to 2.1 cases per 100,000 women-years (89.4% reduction) by 2120. That amounts to about 61 million potential cases avoided, with elimination targets reached in 60% of the countries studied.
HPV vaccination plus one-time screening was predicted to reduce the incidence of cervical cancer to 1.0 case per 100,000 women-years (95.0% reduction), and HPV vaccination plus twice-lifetime screening was predicted to reduce the incidence to 0.7 cases per 100,000 women-years (96.7% reduction).
Dr. Brisson and colleagues reported that, for the countries with the highest burden of cervical cancer (more than 25 cases per 100,000 women-years), adding screening would be necessary to achieve elimination.
To meet the same targets across all 78 countries, “our models predict that scale-up of both girls-only HPV vaccination and twice-lifetime screening is necessary, with 90% HPV vaccination coverage, 90% screening uptake, and long-term protection against HPV types 16, 18, 31, 33, 45, 52, and 58,” the researchers wrote.
Dr. Brisson and colleagues claimed that a strength of this study is the modeling approach, which compared three models “that have been extensively peer reviewed and validated with postvaccination surveillance data.”
The researchers acknowledged, however, that their modeling could not account for variations in sexual behavior from country to country, and the study was not designed to anticipate behavioral or technological changes that could affect cervical cancer incidence in the decades to come.
The study was funded by the WHO, the United Nations, and the Canadian and Australian governments. The WHO contributed to the study design, data analysis and interpretation, and writing of the manuscript. Two study authors reported receiving indirect industry funding for a cervical screening trial in Australia.
SOURCE: Brisson M et al. Lancet. 2020 Jan 30. doi: 10.1016/S0140-6736(20)30068-4.
Cervical cancer is the second most common cancer among women in lower- and middle-income countries, but universal human papillomavirus vaccination for girls would reduce new cervical cancer cases by about 90% over the next century, according to researchers.
Adding twice-lifetime cervical screening with human papillomavirus (HPV) testing would further reduce the incidence of cervical cancer, including in countries with the highest burden, the researchers reported in The Lancet.
Marc Brisson, PhD, of Laval University, Quebec City, and colleagues conducted this study using three models identified by the World Health Organization. The models were used to project reductions in cervical cancer incidence for women in 78 low- and middle-income countries based on the following HPV vaccination and screening scenarios:
- Universal girls-only vaccination at age 9 years, assuming 90% of girls vaccinated and a vaccine that is perfectly effective
- Girls-only vaccination plus cervical screening with HPV testing at age 35 years
- Girls-only vaccination plus screening at ages 35 and 45.
All three scenarios modeled would result in the elimination of cervical cancer, Dr. Brisson and colleagues found. Elimination was defined as four or fewer new cases per 100,000 women-years.
The simplest scenario, universal girls-only vaccination, was predicted to reduce age-standardized cervical cancer incidence from 19.8 cases per 100,000 women-years to 2.1 cases per 100,000 women-years (89.4% reduction) by 2120. That amounts to about 61 million potential cases avoided, with elimination targets reached in 60% of the countries studied.
HPV vaccination plus one-time screening was predicted to reduce the incidence of cervical cancer to 1.0 case per 100,000 women-years (95.0% reduction), and HPV vaccination plus twice-lifetime screening was predicted to reduce the incidence to 0.7 cases per 100,000 women-years (96.7% reduction).
Dr. Brisson and colleagues reported that, for the countries with the highest burden of cervical cancer (more than 25 cases per 100,000 women-years), adding screening would be necessary to achieve elimination.
To meet the same targets across all 78 countries, “our models predict that scale-up of both girls-only HPV vaccination and twice-lifetime screening is necessary, with 90% HPV vaccination coverage, 90% screening uptake, and long-term protection against HPV types 16, 18, 31, 33, 45, 52, and 58,” the researchers wrote.
Dr. Brisson and colleagues claimed that a strength of this study is the modeling approach, which compared three models “that have been extensively peer reviewed and validated with postvaccination surveillance data.”
The researchers acknowledged, however, that their modeling could not account for variations in sexual behavior from country to country, and the study was not designed to anticipate behavioral or technological changes that could affect cervical cancer incidence in the decades to come.
The study was funded by the WHO, the United Nations, and the Canadian and Australian governments. The WHO contributed to the study design, data analysis and interpretation, and writing of the manuscript. Two study authors reported receiving indirect industry funding for a cervical screening trial in Australia.
SOURCE: Brisson M et al. Lancet. 2020 Jan 30. doi: 10.1016/S0140-6736(20)30068-4.
Cervical cancer is the second most common cancer among women in lower- and middle-income countries, but universal human papillomavirus vaccination for girls would reduce new cervical cancer cases by about 90% over the next century, according to researchers.
Adding twice-lifetime cervical screening with human papillomavirus (HPV) testing would further reduce the incidence of cervical cancer, including in countries with the highest burden, the researchers reported in The Lancet.
Marc Brisson, PhD, of Laval University, Quebec City, and colleagues conducted this study using three models identified by the World Health Organization. The models were used to project reductions in cervical cancer incidence for women in 78 low- and middle-income countries based on the following HPV vaccination and screening scenarios:
- Universal girls-only vaccination at age 9 years, assuming 90% of girls vaccinated and a vaccine that is perfectly effective
- Girls-only vaccination plus cervical screening with HPV testing at age 35 years
- Girls-only vaccination plus screening at ages 35 and 45.
All three scenarios modeled would result in the elimination of cervical cancer, Dr. Brisson and colleagues found. Elimination was defined as four or fewer new cases per 100,000 women-years.
The simplest scenario, universal girls-only vaccination, was predicted to reduce age-standardized cervical cancer incidence from 19.8 cases per 100,000 women-years to 2.1 cases per 100,000 women-years (89.4% reduction) by 2120. That amounts to about 61 million potential cases avoided, with elimination targets reached in 60% of the countries studied.
HPV vaccination plus one-time screening was predicted to reduce the incidence of cervical cancer to 1.0 case per 100,000 women-years (95.0% reduction), and HPV vaccination plus twice-lifetime screening was predicted to reduce the incidence to 0.7 cases per 100,000 women-years (96.7% reduction).
Dr. Brisson and colleagues reported that, for the countries with the highest burden of cervical cancer (more than 25 cases per 100,000 women-years), adding screening would be necessary to achieve elimination.
To meet the same targets across all 78 countries, “our models predict that scale-up of both girls-only HPV vaccination and twice-lifetime screening is necessary, with 90% HPV vaccination coverage, 90% screening uptake, and long-term protection against HPV types 16, 18, 31, 33, 45, 52, and 58,” the researchers wrote.
Dr. Brisson and colleagues claimed that a strength of this study is the modeling approach, which compared three models “that have been extensively peer reviewed and validated with postvaccination surveillance data.”
The researchers acknowledged, however, that their modeling could not account for variations in sexual behavior from country to country, and the study was not designed to anticipate behavioral or technological changes that could affect cervical cancer incidence in the decades to come.
The study was funded by the WHO, the United Nations, and the Canadian and Australian governments. The WHO contributed to the study design, data analysis and interpretation, and writing of the manuscript. Two study authors reported receiving indirect industry funding for a cervical screening trial in Australia.
SOURCE: Brisson M et al. Lancet. 2020 Jan 30. doi: 10.1016/S0140-6736(20)30068-4.
FROM THE LANCET
Global project reveals cancer’s genomic playbook
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE