User login
Cancer care and COVID-19 in Seattle, the first U.S. epicenter
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
Two months after the first patient with COVID-19 was identified in China, the first case was reported in the United States in the Seattle, Washington, metropolitan area.
Seattle rapidly became the first US epicenter for COVID-19, and local experts are now offering their expertise and advice on how to provide optimal cancer care during the pandemic in a special feature published online March 20 in the Journal of the National Comprehensive Cancer Network.
“We began implementing measures in early March, including infection control and screening of visitors, staff, and patients at the door,” said lead author Masumi Ueda, MD, who holds positions at the Seattle Cancer Care Alliance, the University of Washington, and the Fred Hutchinson Research Center.
“A lot of changes have been implemented, and it changes on a daily basis. We are responding to the growing rate of COVID-19 infection in the community,” she told Medscape Medical News.
Ueda notes that as a result of the quick implementation of new procedures, so far, very few cancer patients at their facilities have been infected by the virus. “It has not hit our cancer population hard, which is a good thing,” she said.
Create “Incident Command Structure”
In sharing their experience, the authors emphasize the importance of keeping channels of communication open between all stakeholders ― administrators and staff, patients, caregivers, and the general public. They also recommend that each facility create an “incident command structure” that can provide early coordination of institution-wide efforts and that can rapidly respond to changing information.
Ueda noted that their command structure was set up very early on, “so we could get communication set up and start building an infrastructure for response.”
Several areas of care that required new strategies were addressed, both to protect patients and to work around staff shortages caused by possible exposure and/or school closings, as well as projected shortages of supplies and hospital resources.
First and foremost was to identify patients and visitors who had respiratory symptoms and to provide them with masks. Although this is always routine practice during the respiratory virus season, screening has now been initiated at entry points throughout the system.
“We were lucky in Seattle and Washington state in that the University of Washington virology lab developed PCR [polymerase chain reaction] testing early on for COVID-19, which subsequently got FDA approval,” said Ueda. “So we were able to have local testing and didn’t have to rely on the state lab. Testing has also been rapidly scaled up.”
Initiating a comprehensive policy for testing staff, tracking results and exposures for persons under investigation, and defining when it is possible to return to work are essential elements for maintaining a stable workforce. In addition, reinforcing a strict “stay at home when ill” policy and providing access to testing for symptomatic staff have been key to limiting exposures.
“What is unique to our region is that we had testing early on, and we are turning it around in 24 hours,” she pointed out. “This is important for staff to be able to return to work.” Currently, staff, patients, and visitors are being tested only if they show the cardinal symptoms associated with COVID-19: fever, shortness of breath, and cough, although muscle aches have recently been added to their testing protocol.
“I think if we had unlimited capacity, we might consider testing people who are asymptomatic,” Ueda noted, “although if you don’t have symptoms, you may not have the viral load needed for an accurate test.”
Educational materials explaining infection control were also needed for patients and families, along with signs and a website to provide COVID-19 education. These were quickly developed.
In addition, a telephone triage line was established for patients with mild symptoms in order to minimize exposures in clinics and to lessen the number of patients presenting at emergency departments.
Outpatient Care
Because theirs is a referral center, many cancer patients come from out of town, and so there is concern about exposing nonlocal patients to COVID-19 as the virus spreads in the Seattle area. In addition, staffing shortages due to factors such as illness, exposure, and school closures are anticipated.
To address these problems, an initial priority was to establish a “multilayer” coverage system for the clinics in the event that practitioners had to be quarantined on short notice, the authors explain.
One decision was to reschedule all wellness visits for current patients or to use telemedicine. Capacity for that option expanded quickly, which was greatly helped by the recent decision by the Centers for Medicare & Medicaid Services to lift Medicare restrictions on the use of certain telemedicine services.
Another approach is to defer all consultations for second opinions for patients who were already undergoing treatment and to increase clinic hours of operations and capabilities for acute evaluations. This helps reserve emergency departments and hospital resources for patients who require higher-level care, the authors comment.
Treatment Decisions
Treatment decisions were more challenging to make, the authors note. One decision was that, despite the risk for COVID-19 for patients with solid tumors, adjuvant therapy with curative intent should proceed, they note. Similarly, patients with metastatic disease might lose the window of opportunity for treatment if it is delayed.
Treatment for aggressive hematologic malignancies is usually urgent, and stem cell transplant and cellular immunotherapies that provide curative treatments cannot be delayed in many cases.
Enrollment in clinical trials will most likely be limited to those trials that are most likely to benefit the patient.
Ueda noted that, because their patients come from all over the country, they are now conducting consultations for stem cell transplant by telephone so that nonlocal patients do not have to travel to Seattle. “If there is some way we can delay the treatment, we have taken that approach,” Ueda told Medscape Medical News. “If we can divert a patient to an area that is not as heavily affected, that’s another option we are taking.”
Although cancer surgery is not considered elective, surgical intervention needs to be prioritized, the authors comment. In the Seattle system, there is currently a 2-week ban on elective surgery in the healthcare system, owing to limited availability of personal protective equipment (PPE), staffing, and beds.
The oncology teams are currently reviewing treatment regimens to determine which treatments might lessen immunosuppression and which treatment options can be moved from the inpatient to the outpatient setting or can be delayed.
Inpatient Care
For hospitalized patients, several issues are being addressed. The priority is to prepare for an upcoming shortage of beds and resources because of the surge of patients with COVID-19 that is predicted. For both clinic and hospitalized patients, shortages of blood products have necessitated stricter adherence to thresholds for transfusion, and consideration is being given to lowering those thresholds.
Another important problem is the need to conserve PPE, which includes masks, gowns, gloves, and other products. The Seattle teams have implemented solutions such as favoring handwashing with soap and water over the use of hand gel for standard-precaution rooms, limiting the number of personnel entering patient rooms (so as to use less PPE), and reducing nursing procedures that require PPE, such as measuring urine output, unless they are necessary.
In addition, a no-visitor policy has been adopted in inpatient units to conserve PPE, with the exception of end-of-life situations.
The Future
The future trajectory of the COVID-19 pandemic is uncertain, Ueda commented. She emphasized that “we must continue to prepare for its widespread impact. The unknown is what we are looking at. We are expecting it to evolve, and the number of infections cannot go down.”
Ueda and coauthors end their article on a positive note. “To many of us, this has become the health care challenge of our generation, one that modern cancer therapy has never had to face. We will prevail, and when the pandemic ends, we will all be proud of what we did for our patients and each other in this critical moment for humanity.”
How is oncology adapting to COVID-19?
As the coronavirus pandemic escalates in the United States, Medscape Oncology reached out to a group of our contributors and asked them to provide their perspective on how their oncology departments and centers are preparing. Here are their responses to a number of issues facing oncologists in the US and around the world.
Have you shifted nonurgent follow-up visits to telemedicine, either via video or phone?
Kathy Miller, MD, Associate Director of Indiana University Simon Cancer Center: We are reviewing our clinic schedules and identifying “routine” follow-up patients who can be rescheduled. When patients are contacted to reschedule, they are asked if they have any urgent, immediate concerns that need to be addressed before the new appointment. If yes, they are offered a virtual visit.
Don Dizon, MD, Director of Women’s Cancers, Lifespan Cancer Institute; Director of Medical Oncology, Rhode Island Hospital: We have started to do this in preparation for a surge of people with COVID-19. Patients who are in long-term follow-up (no evidence of disease at 3 years or longer, being seen annually) or those in routine surveillance after curative treatment (that is, seen every 3 months) as well as those being seen for supportive care–type visits, like sexual health or survivorship, are all being contacted and visits are being moved to telehealth.
Jeffrey S. Weber, MD, PhD, Deputy Director of the Laura and Isaac Perlmutter Cancer Center at NYU Langone Medical Center: Yes. Any follow-up, nontreatment visits are done by phone or video if the patient agrees. (They all have).
Have you delayed or canceled cancer surgeries?
Ravi B. Parikh, MD, MPP, Medical oncologist at the University of Pennsylvania and the Philadelphia VA Medical Center: The University of Pennsylvania has taken this seriously. We’ve canceled all elective surgeries, have ramped up our telemedicine (video and phone) capabilities significantly, are limiting our appointments mostly to on-treatment visits, and have been asked to reconsider regular scans and reviews.
Dizon: We have not done this. There are apparently differences in interpretation in what institutions might mean as “elective surgeries.” At our institution, surgery for invasive malignancies is not elective. However, this may (or will) change if resources become an issue.
Lidia Schapira, MD, Associate Professor of Medicine and Director of Cancer Survivorship at the Stanford Comprehensive Cancer Institute: Delaying elective surgery is something that hospitals here have already implemented, and I imagine that this trend will spread. But it may be difficult to decide in situations that are not exactly “life-saving” but where an earlier intervention could preserve function or improve quality of life.
Mark A. Lewis, MD, Director of Gastrointestinal Oncology at Intermountain Healthcare in Utah: Cancer surgeries have not been deemed elective or delayed.
Have you delayed or altered the delivery of potentially immune-comprising treatments?
David Kerr, MD, Professor of Cancer Medicine at the University of Oxford in England: We are considering delaying initiation of our adjuvant colorectal cancer treatments, as we have data from our own QUASAR trials suggesting that patients who commence chemotherapy between 2 and 6 weeks do equally as well as those who begin 6-12 weeks after surgery.
Parikh: I personally haven’t delayed giving chemotherapy to avoid immune compromise, but I believe some others may have. It’s a delicate balance between wanting to ensure cancer control and making sure we are flattening the curve. As an example, though, I delayed three on-treatment visits for my clinic last Monday, and I converted 70% of my visits to telemedicine. However, I’m a genitourinary cancer specialist and the treatments I give are very different from others.
Lewis: The most difficult calculus is around adjuvant therapy. For metastatic patients, I am trying to use the least immunosuppressive regimen possible that will still control their disease. As you can imagine, it’s an assessment of competing risks.
Schapira: Patients who need essential anticancer therapy should still get it, but attempts to deintensify therapy should continue—for example, holding or postponing treatment without harm (based on evidence, not opinion). This may be possible for patients considering hormonal therapies for breast or prostate cancer.
Patients who need radiation should discuss the timing with their radiation oncologist. In some cases, it may be possible to delay treatment without affecting outcomes, but these decisions should be made carefully. Alternatively, shorter courses of radiation may be appropriate.
Have you advised your own patients differently given the high risk to cancer patients?
Kerr: We have factored potential infection with the virus into discussions where the benefits of chemotherapy are very marginal. This could tip the balance toward the patient deciding not to pursue chemotherapy.
Dizon: The data from China are not entirely crystal-clear. While they noted that people with active cancer and those who had a history of cancer are at increased risk for more severe infections and worse outcomes, the Chinese cohort was small, and compared with people without cancer, it tended to be much older and to be smokers (former or current). Having said this, we are counseling everyone about the importance of social distancing, washing hands, and not touching your face.
Lewis: If I have a complete blood count with a differential that includes lymphocytes, I can advise my lymphopenic patients (who are particularly vulnerable to viral infection) to take special precautions regarding social distancing in their own families.
Have any of your hospitalized patients been affected by policy changes to prepare beds/departments for the expected increase in COVID-19–positive patients?
Weber: Not yet.
Dizon: No, not at the moment.
Have you been asked to assist with other services or COVID-19 task forces?
Dizon: I am keenly involved in the preparations and modifications to procedures, including staffing decisions in outpatient, movement to telehealth, and work-from-home policies.
Lewis: I am engaged in system-wide COVID-19 efforts around oncology.
Kerr: Perhaps oddest of all, I am learning with some of our junior doctors to care for ventilated patients. I still consider myself enough of a general physician that I would hope to be able to contribute to the truly sick, but I accept that I do need an appropriate refresher course.
Bishal Gyawali, MD, PhD, medical oncologist at Queen’s University Cancer Research Institute: Queen’s Hospital medical students are now volunteering to help with daycare, groceries, and other tasks for staff who are working in the hospital.
Are you experiencing any shortages in personal protective equipment (PPE) at your center?
Miller: Some supplies are running short, though none are frankly out at this point. However, rationing and controls are in place to stretch the supplies as far as possible, including reusing some PPE.
Dizon: We are rationing face masks and N95 respirators, eye shields, and even surgical scrubs. We are talking about postponing elective surgery to save PPE but are not yet to that point. We’re asking that face masks be reused for at least 2 days, maybe longer. PPEs are one per day. Scrubs are kept secure.
Lewis: We are being very careful not to overuse PPE but currently have an adequate inventory. We have had to move gloves and masks to areas where they are not accessible to the general public, as otherwise they were being stolen (this started weeks ago).
Kerr: Our National Health System has an adequate supply of PPE equipment centrally, but there seems to be a problem with distribution, as some hospitals are reporting shortages.
Weber: Masks are in short supply, so they are being used for several days if not wet. We are short of plastic gowns and are using paper chemo gowns. Similar story at many places.
This article first appeared on Medscape.com.
As the coronavirus pandemic escalates in the United States, Medscape Oncology reached out to a group of our contributors and asked them to provide their perspective on how their oncology departments and centers are preparing. Here are their responses to a number of issues facing oncologists in the US and around the world.
Have you shifted nonurgent follow-up visits to telemedicine, either via video or phone?
Kathy Miller, MD, Associate Director of Indiana University Simon Cancer Center: We are reviewing our clinic schedules and identifying “routine” follow-up patients who can be rescheduled. When patients are contacted to reschedule, they are asked if they have any urgent, immediate concerns that need to be addressed before the new appointment. If yes, they are offered a virtual visit.
Don Dizon, MD, Director of Women’s Cancers, Lifespan Cancer Institute; Director of Medical Oncology, Rhode Island Hospital: We have started to do this in preparation for a surge of people with COVID-19. Patients who are in long-term follow-up (no evidence of disease at 3 years or longer, being seen annually) or those in routine surveillance after curative treatment (that is, seen every 3 months) as well as those being seen for supportive care–type visits, like sexual health or survivorship, are all being contacted and visits are being moved to telehealth.
Jeffrey S. Weber, MD, PhD, Deputy Director of the Laura and Isaac Perlmutter Cancer Center at NYU Langone Medical Center: Yes. Any follow-up, nontreatment visits are done by phone or video if the patient agrees. (They all have).
Have you delayed or canceled cancer surgeries?
Ravi B. Parikh, MD, MPP, Medical oncologist at the University of Pennsylvania and the Philadelphia VA Medical Center: The University of Pennsylvania has taken this seriously. We’ve canceled all elective surgeries, have ramped up our telemedicine (video and phone) capabilities significantly, are limiting our appointments mostly to on-treatment visits, and have been asked to reconsider regular scans and reviews.
Dizon: We have not done this. There are apparently differences in interpretation in what institutions might mean as “elective surgeries.” At our institution, surgery for invasive malignancies is not elective. However, this may (or will) change if resources become an issue.
Lidia Schapira, MD, Associate Professor of Medicine and Director of Cancer Survivorship at the Stanford Comprehensive Cancer Institute: Delaying elective surgery is something that hospitals here have already implemented, and I imagine that this trend will spread. But it may be difficult to decide in situations that are not exactly “life-saving” but where an earlier intervention could preserve function or improve quality of life.
Mark A. Lewis, MD, Director of Gastrointestinal Oncology at Intermountain Healthcare in Utah: Cancer surgeries have not been deemed elective or delayed.
Have you delayed or altered the delivery of potentially immune-comprising treatments?
David Kerr, MD, Professor of Cancer Medicine at the University of Oxford in England: We are considering delaying initiation of our adjuvant colorectal cancer treatments, as we have data from our own QUASAR trials suggesting that patients who commence chemotherapy between 2 and 6 weeks do equally as well as those who begin 6-12 weeks after surgery.
Parikh: I personally haven’t delayed giving chemotherapy to avoid immune compromise, but I believe some others may have. It’s a delicate balance between wanting to ensure cancer control and making sure we are flattening the curve. As an example, though, I delayed three on-treatment visits for my clinic last Monday, and I converted 70% of my visits to telemedicine. However, I’m a genitourinary cancer specialist and the treatments I give are very different from others.
Lewis: The most difficult calculus is around adjuvant therapy. For metastatic patients, I am trying to use the least immunosuppressive regimen possible that will still control their disease. As you can imagine, it’s an assessment of competing risks.
Schapira: Patients who need essential anticancer therapy should still get it, but attempts to deintensify therapy should continue—for example, holding or postponing treatment without harm (based on evidence, not opinion). This may be possible for patients considering hormonal therapies for breast or prostate cancer.
Patients who need radiation should discuss the timing with their radiation oncologist. In some cases, it may be possible to delay treatment without affecting outcomes, but these decisions should be made carefully. Alternatively, shorter courses of radiation may be appropriate.
Have you advised your own patients differently given the high risk to cancer patients?
Kerr: We have factored potential infection with the virus into discussions where the benefits of chemotherapy are very marginal. This could tip the balance toward the patient deciding not to pursue chemotherapy.
Dizon: The data from China are not entirely crystal-clear. While they noted that people with active cancer and those who had a history of cancer are at increased risk for more severe infections and worse outcomes, the Chinese cohort was small, and compared with people without cancer, it tended to be much older and to be smokers (former or current). Having said this, we are counseling everyone about the importance of social distancing, washing hands, and not touching your face.
Lewis: If I have a complete blood count with a differential that includes lymphocytes, I can advise my lymphopenic patients (who are particularly vulnerable to viral infection) to take special precautions regarding social distancing in their own families.
Have any of your hospitalized patients been affected by policy changes to prepare beds/departments for the expected increase in COVID-19–positive patients?
Weber: Not yet.
Dizon: No, not at the moment.
Have you been asked to assist with other services or COVID-19 task forces?
Dizon: I am keenly involved in the preparations and modifications to procedures, including staffing decisions in outpatient, movement to telehealth, and work-from-home policies.
Lewis: I am engaged in system-wide COVID-19 efforts around oncology.
Kerr: Perhaps oddest of all, I am learning with some of our junior doctors to care for ventilated patients. I still consider myself enough of a general physician that I would hope to be able to contribute to the truly sick, but I accept that I do need an appropriate refresher course.
Bishal Gyawali, MD, PhD, medical oncologist at Queen’s University Cancer Research Institute: Queen’s Hospital medical students are now volunteering to help with daycare, groceries, and other tasks for staff who are working in the hospital.
Are you experiencing any shortages in personal protective equipment (PPE) at your center?
Miller: Some supplies are running short, though none are frankly out at this point. However, rationing and controls are in place to stretch the supplies as far as possible, including reusing some PPE.
Dizon: We are rationing face masks and N95 respirators, eye shields, and even surgical scrubs. We are talking about postponing elective surgery to save PPE but are not yet to that point. We’re asking that face masks be reused for at least 2 days, maybe longer. PPEs are one per day. Scrubs are kept secure.
Lewis: We are being very careful not to overuse PPE but currently have an adequate inventory. We have had to move gloves and masks to areas where they are not accessible to the general public, as otherwise they were being stolen (this started weeks ago).
Kerr: Our National Health System has an adequate supply of PPE equipment centrally, but there seems to be a problem with distribution, as some hospitals are reporting shortages.
Weber: Masks are in short supply, so they are being used for several days if not wet. We are short of plastic gowns and are using paper chemo gowns. Similar story at many places.
This article first appeared on Medscape.com.
As the coronavirus pandemic escalates in the United States, Medscape Oncology reached out to a group of our contributors and asked them to provide their perspective on how their oncology departments and centers are preparing. Here are their responses to a number of issues facing oncologists in the US and around the world.
Have you shifted nonurgent follow-up visits to telemedicine, either via video or phone?
Kathy Miller, MD, Associate Director of Indiana University Simon Cancer Center: We are reviewing our clinic schedules and identifying “routine” follow-up patients who can be rescheduled. When patients are contacted to reschedule, they are asked if they have any urgent, immediate concerns that need to be addressed before the new appointment. If yes, they are offered a virtual visit.
Don Dizon, MD, Director of Women’s Cancers, Lifespan Cancer Institute; Director of Medical Oncology, Rhode Island Hospital: We have started to do this in preparation for a surge of people with COVID-19. Patients who are in long-term follow-up (no evidence of disease at 3 years or longer, being seen annually) or those in routine surveillance after curative treatment (that is, seen every 3 months) as well as those being seen for supportive care–type visits, like sexual health or survivorship, are all being contacted and visits are being moved to telehealth.
Jeffrey S. Weber, MD, PhD, Deputy Director of the Laura and Isaac Perlmutter Cancer Center at NYU Langone Medical Center: Yes. Any follow-up, nontreatment visits are done by phone or video if the patient agrees. (They all have).
Have you delayed or canceled cancer surgeries?
Ravi B. Parikh, MD, MPP, Medical oncologist at the University of Pennsylvania and the Philadelphia VA Medical Center: The University of Pennsylvania has taken this seriously. We’ve canceled all elective surgeries, have ramped up our telemedicine (video and phone) capabilities significantly, are limiting our appointments mostly to on-treatment visits, and have been asked to reconsider regular scans and reviews.
Dizon: We have not done this. There are apparently differences in interpretation in what institutions might mean as “elective surgeries.” At our institution, surgery for invasive malignancies is not elective. However, this may (or will) change if resources become an issue.
Lidia Schapira, MD, Associate Professor of Medicine and Director of Cancer Survivorship at the Stanford Comprehensive Cancer Institute: Delaying elective surgery is something that hospitals here have already implemented, and I imagine that this trend will spread. But it may be difficult to decide in situations that are not exactly “life-saving” but where an earlier intervention could preserve function or improve quality of life.
Mark A. Lewis, MD, Director of Gastrointestinal Oncology at Intermountain Healthcare in Utah: Cancer surgeries have not been deemed elective or delayed.
Have you delayed or altered the delivery of potentially immune-comprising treatments?
David Kerr, MD, Professor of Cancer Medicine at the University of Oxford in England: We are considering delaying initiation of our adjuvant colorectal cancer treatments, as we have data from our own QUASAR trials suggesting that patients who commence chemotherapy between 2 and 6 weeks do equally as well as those who begin 6-12 weeks after surgery.
Parikh: I personally haven’t delayed giving chemotherapy to avoid immune compromise, but I believe some others may have. It’s a delicate balance between wanting to ensure cancer control and making sure we are flattening the curve. As an example, though, I delayed three on-treatment visits for my clinic last Monday, and I converted 70% of my visits to telemedicine. However, I’m a genitourinary cancer specialist and the treatments I give are very different from others.
Lewis: The most difficult calculus is around adjuvant therapy. For metastatic patients, I am trying to use the least immunosuppressive regimen possible that will still control their disease. As you can imagine, it’s an assessment of competing risks.
Schapira: Patients who need essential anticancer therapy should still get it, but attempts to deintensify therapy should continue—for example, holding or postponing treatment without harm (based on evidence, not opinion). This may be possible for patients considering hormonal therapies for breast or prostate cancer.
Patients who need radiation should discuss the timing with their radiation oncologist. In some cases, it may be possible to delay treatment without affecting outcomes, but these decisions should be made carefully. Alternatively, shorter courses of radiation may be appropriate.
Have you advised your own patients differently given the high risk to cancer patients?
Kerr: We have factored potential infection with the virus into discussions where the benefits of chemotherapy are very marginal. This could tip the balance toward the patient deciding not to pursue chemotherapy.
Dizon: The data from China are not entirely crystal-clear. While they noted that people with active cancer and those who had a history of cancer are at increased risk for more severe infections and worse outcomes, the Chinese cohort was small, and compared with people without cancer, it tended to be much older and to be smokers (former or current). Having said this, we are counseling everyone about the importance of social distancing, washing hands, and not touching your face.
Lewis: If I have a complete blood count with a differential that includes lymphocytes, I can advise my lymphopenic patients (who are particularly vulnerable to viral infection) to take special precautions regarding social distancing in their own families.
Have any of your hospitalized patients been affected by policy changes to prepare beds/departments for the expected increase in COVID-19–positive patients?
Weber: Not yet.
Dizon: No, not at the moment.
Have you been asked to assist with other services or COVID-19 task forces?
Dizon: I am keenly involved in the preparations and modifications to procedures, including staffing decisions in outpatient, movement to telehealth, and work-from-home policies.
Lewis: I am engaged in system-wide COVID-19 efforts around oncology.
Kerr: Perhaps oddest of all, I am learning with some of our junior doctors to care for ventilated patients. I still consider myself enough of a general physician that I would hope to be able to contribute to the truly sick, but I accept that I do need an appropriate refresher course.
Bishal Gyawali, MD, PhD, medical oncologist at Queen’s University Cancer Research Institute: Queen’s Hospital medical students are now volunteering to help with daycare, groceries, and other tasks for staff who are working in the hospital.
Are you experiencing any shortages in personal protective equipment (PPE) at your center?
Miller: Some supplies are running short, though none are frankly out at this point. However, rationing and controls are in place to stretch the supplies as far as possible, including reusing some PPE.
Dizon: We are rationing face masks and N95 respirators, eye shields, and even surgical scrubs. We are talking about postponing elective surgery to save PPE but are not yet to that point. We’re asking that face masks be reused for at least 2 days, maybe longer. PPEs are one per day. Scrubs are kept secure.
Lewis: We are being very careful not to overuse PPE but currently have an adequate inventory. We have had to move gloves and masks to areas where they are not accessible to the general public, as otherwise they were being stolen (this started weeks ago).
Kerr: Our National Health System has an adequate supply of PPE equipment centrally, but there seems to be a problem with distribution, as some hospitals are reporting shortages.
Weber: Masks are in short supply, so they are being used for several days if not wet. We are short of plastic gowns and are using paper chemo gowns. Similar story at many places.
This article first appeared on Medscape.com.
Disruptions in cancer care in the era of COVID-19
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Even in the midst of the COVID-19 pandemic, cancer care must go on, but changes may need to be made in the way some care is delivered.
“We’re headed for a time when there will be significant disruptions in the care of patients with cancer,” said Len Lichtenfeld, MD, deputy chief medical officer of the American Cancer Society (ACS), in a statement. “For some it may be as straightforward as a delay in having elective surgery. For others it may be delaying preventive care or adjuvant chemotherapy that’s meant to keep cancer from returning or rescheduling appointments.”
Lichtenfeld emphasized that cancer care teams are going to do the best they can to deliver care to those most in need. However, even in those circumstances, it won’t be life as usual. “It will require patience on everyone’s part as we go through this pandemic,” he said.
“The way we treat cancer over the next few months will change enormously,” writes a British oncologist in an article published in the Guardian.
“As oncologists, we will have to find a tenuous balance between undertreating people with cancer, resulting in more deaths from the disease in the medium to long term, and increasing deaths from COVID-19 in a vulnerable patient population. Alongside our patients we will have to make difficult decisions regarding treatments, with only low-quality evidence to guide us,” writes Lucy Gossage, MD, consultant oncologist at Nottingham University Hospital, UK.
The evidence to date (from reports from China in Lancet Oncology) suggests that people with cancer have a significantly higher risk of severe illness resulting in intensive care admissions or death when infected with COVID-19, particularly if they recently had chemotherapy or surgery.
“Many of the oncology treatments we currently use, especially those given after surgery to reduce risk of cancer recurrence, have relatively small benefits,” she writes.
“In the current climate, the balance of offering these treatments may shift; a small reduction in risk of cancer recurrence over the next 5 years may be outweighed by the potential for a short-term increase in risk of death from COVID-19. In the long term, more people’s cancer will return if we aren’t able to offer these treatments,” she adds.
Postpone Routine Screening
One thing that can go on the back burner for now is routine cancer screening, which can be postponed for now in order to conserve health system resources and reduce contact with healthcare facilities, says the ACS.
“Patients seeking routine cancer screenings should delay those until further notice,” said Lichtenfeld. “While timely screening is important, the need to prevent the spread of coronavirus and to reduce the strain on the medical system is more important right now.”
But as soon as restrictions to slow the spread of COVID-19 are lifted and routine visits to health facilities are safe, regular screening tests should be rescheduled.
Guidance From ASCO
The American Society of Clinical Oncology (ASCO) has issued new guidance on caring for patients with cancer during the COVID-19 outbreak.
First and foremost, ASCO encourages providers, facilities, and anyone caring for patients with cancer to follow the existing guidelines from the Center for Disease Control and Prevention when possible.
ASCO highlights the CDC’s general recommendation for healthcare facilities that suggests “elective surgeries” at inpatient facilities be rescheduled if possible, which has also been recommended by the American College of Surgeons.
However, in many cases, cancer surgery is not elective but essential, it points out. So this is largely an individual determination that clinicians and patients will need to make, taking into account the potential harms of delaying needed cancer-related surgery.
Systemic treatments, including chemotherapy and immunotherapy, leave cancer patients vulnerable to infection, but ASCO says there is no direct evidence to support changes in regimens during the pandemic. Therefore, routinely stopping anticancer or immunosuppressive therapy is not recommended, as the balance of potential harms that may result from delaying or interrupting treatment versus the potential benefits of possibly preventing or delaying COVID-19 infection remains very unclear.
Clinical decisions must be individualized, ASCO emphasized, and suggested the following practice points be considered:
- For patients already in deep remission who are receiving maintenance therapy, stopping treatment may be an option.
- Some patients may be able to switch from IV to oral therapies, which would decrease the frequency of clinic visits.
- Decisions on modifying or withholding chemotherapy need to consider both the indication and goals of care, as well as where the patient is in the treatment regimen and tolerance to the therapy. As an example, the risk–benefit assessment for proceeding with chemotherapy in patients with untreated extensive small-cell lung cancer is quite different than proceeding with maintenance pemetrexed for metastatic non–small cell lung cancer.
- If local coronavirus transmission is an issue at a particular cancer center, reasonable options may include taking a 2-week treatment break or arranging treatment at a different facility.
- Evaluate if home infusion is medically and logistically feasible.
- In some settings, delaying or modifying adjuvant treatment presents a higher risk of compromised disease control and long-term survival than in others, but in cases where the absolute benefit of adjuvant chemotherapy may be quite small and other options are available, the risk of COVID-19 may be considered an additional factor when evaluating care.
Delay Stem Cell Transplants
For patients who are candidates for allogeneic stem cell transplantation, a delay may be reasonable if the patient is currently well controlled with conventional treatment, ASCO comments. It also directs clinicians to follow the recommendations provided by the American Society of Transplantation and Cellular Therapy and from the European Society for Blood and Marrow Transplantation regarding this issue.
Finally, there is also the question of prophylactic antiviral therapy: Should it be considered for cancer patients undergoing active therapy?
The answer to that question is currently unknown, says ASCO, but “this is an active area of research and evidence may be available at any time.”
This article first appeared on Medscape.com.
HPV vaccine-chemo combo prolongs cervical cancer survival
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: There may be an overall survival benefit of combining human papillomavirus vaccination with standard-of-care chemotherapy for cervical cancer.
Major finding: The median overall survival was 16.8 months for patients with immune responses to the vaccine that were higher than the median and 11.2 months for patients with immune responses lower than the median (hazard ratio, 0.491; P = .012).
Study details: A phase 1/2 study of 77 women with HPV16-positive advanced, metastatic, or recurrent cervical cancer.
Disclosures: The study was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
Source: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
Prospective algorithm favors vaginal hysterectomy
based on data from a prospective study of 365 patients.
“Total vaginal hysterectomy is the most cost-effective route, with a low complication rate, and, therefore, should be performed when feasible,” wrote Jennifer J. Schmitt, DO, of the Mayo Clinic, Rochester, Minn., and colleagues.
However, algorithms to support the decision to choose vaginal hysterectomy are not widely used, they said.
To assess the optimal surgical route for hysterectomy, the researchers devised a prospective algorithm and decision tree based on history of laparotomy, uterine size, and vaginal access. The results of their study were published in Obstetrics & Gynecology.
The study population included 365 women aged 18 years and older who underwent hysterectomies between Nov. 24, 2015, and Dec. 31, 2017, at a single center. A total of 202 patients (55%) met criteria for a total vaginal hysterectomy using the algorithm, and 57 (15.6%) were assigned to have an examination under anesthesia followed by total vaginal hysterectomy, for a total of 259 expected vaginal hysterectomies. Ultimately, 211 (81.5%) of the patients identified as being the best candidates for having a vaginal hysterectomy underwent the procedure. Almost all of the procedures – 99.1% – were completed successfully.
The algorithm predicted that 52 patients were expected to have an examination under anesthesia followed by a robot-assisted total laparoscopic hysterectomy and 54 were expected to have an abdominal, robotic, or laparoscopic hysterectomy. A total of 46 procedures (44 robotic, when vaginal was expected and 2 abdominal, when vaginal was expected) deviated to a more invasive route than prescribed by the algorithm, and 7 procedures deviated from the algorithm-predicted robotic or abdominal procedure to total vaginal hysterectomy.
Approximately 95% of the patients were discharged within 24 hours of surgery. These patients included 7 who had vaginal surgery when a more invasive method was predicted and did not experience intraoperative complications or Accordion grade 3 complications.
“Prospective algorithm use predicts that 55.3% of all hysterectomies were expected to have an a priori total vaginal hysterectomy, which is higher than the actual total vaginal hysterectomy rate of 11.5% reported previously,” the researchers noted, and they added that vaginal hysterectomy would be associated with cost savings of $657,524 if the total hysterectomy rate was 55% instead of 11%.
The study findings were limited by several factors including an expertise bias at the center where the study was conducted, as well as the small number of patients with algorithm deviations or poor outcomes, and the lack of a control group, the researchers noted. However, the results support the use of the algorithm “in combination with educating gynecologic surgeons about the feasibility of vaginal surgery,” they said.
“Prospective use of this algorithm nationally may increase the rate of total vaginal hysterectomy and decrease health care delivery costs,” they concluded.
“The American College of Obstetricians and Gynecologists continues to recommend vaginal hysterectomy as the approach of choice whenever feasible, and although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, the rate of vaginal hysterectomy in the United States has continued to decline,” Arnold P. Advincula, MD, of Columbia University Medical Center, New York, wrote in an accompanying editorial.
Many variables beyond clinical will determine the optimal hysterectomy route, Dr. Advincula said.
“Although historical evidence demonstrates that vaginal hysterectomy is associated with better outcomes when compared with other approaches, a multitude of studies now exist that challenge this notion. Given the financial implications and overall costs of care with surgical complications and 30-day readmissions, experienced high-volume surgeons using all available routes have shown robotics to be the best surgical approach in terms of fewer postoperative complications and lowest 30-day readmission rates,” he noted. However, “one should not split hairs and subtly pit one minimally invasive option against another, but instead should work toward the goal of minimizing laparotomy, which is still performed at a high rate,” Dr. Advincula emphasized.
The study was supported in part by the National Center for Advancing Translational Science. Dr. Schmitt had no financial conflicts to disclose. Dr. Advincula disclosed serving as a consultant for AbbVie, Baxter, ConMed, Eximis Surgical, Intuitive Surgical, and Titan Medical, and performing consultancy work and receiving royalties from Cooper Surgical.
SOURCES: Schmitt JJ et al. Obstet Gynecol. 2020;135:761-9. doi: 10.1097/AOG.0000000000003725; Advincula A. Obstet Gynecol. 2020;135:759-60. doi: doi: 10.1097/AOG.0000000000003814.
based on data from a prospective study of 365 patients.
“Total vaginal hysterectomy is the most cost-effective route, with a low complication rate, and, therefore, should be performed when feasible,” wrote Jennifer J. Schmitt, DO, of the Mayo Clinic, Rochester, Minn., and colleagues.
However, algorithms to support the decision to choose vaginal hysterectomy are not widely used, they said.
To assess the optimal surgical route for hysterectomy, the researchers devised a prospective algorithm and decision tree based on history of laparotomy, uterine size, and vaginal access. The results of their study were published in Obstetrics & Gynecology.
The study population included 365 women aged 18 years and older who underwent hysterectomies between Nov. 24, 2015, and Dec. 31, 2017, at a single center. A total of 202 patients (55%) met criteria for a total vaginal hysterectomy using the algorithm, and 57 (15.6%) were assigned to have an examination under anesthesia followed by total vaginal hysterectomy, for a total of 259 expected vaginal hysterectomies. Ultimately, 211 (81.5%) of the patients identified as being the best candidates for having a vaginal hysterectomy underwent the procedure. Almost all of the procedures – 99.1% – were completed successfully.
The algorithm predicted that 52 patients were expected to have an examination under anesthesia followed by a robot-assisted total laparoscopic hysterectomy and 54 were expected to have an abdominal, robotic, or laparoscopic hysterectomy. A total of 46 procedures (44 robotic, when vaginal was expected and 2 abdominal, when vaginal was expected) deviated to a more invasive route than prescribed by the algorithm, and 7 procedures deviated from the algorithm-predicted robotic or abdominal procedure to total vaginal hysterectomy.
Approximately 95% of the patients were discharged within 24 hours of surgery. These patients included 7 who had vaginal surgery when a more invasive method was predicted and did not experience intraoperative complications or Accordion grade 3 complications.
“Prospective algorithm use predicts that 55.3% of all hysterectomies were expected to have an a priori total vaginal hysterectomy, which is higher than the actual total vaginal hysterectomy rate of 11.5% reported previously,” the researchers noted, and they added that vaginal hysterectomy would be associated with cost savings of $657,524 if the total hysterectomy rate was 55% instead of 11%.
The study findings were limited by several factors including an expertise bias at the center where the study was conducted, as well as the small number of patients with algorithm deviations or poor outcomes, and the lack of a control group, the researchers noted. However, the results support the use of the algorithm “in combination with educating gynecologic surgeons about the feasibility of vaginal surgery,” they said.
“Prospective use of this algorithm nationally may increase the rate of total vaginal hysterectomy and decrease health care delivery costs,” they concluded.
“The American College of Obstetricians and Gynecologists continues to recommend vaginal hysterectomy as the approach of choice whenever feasible, and although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, the rate of vaginal hysterectomy in the United States has continued to decline,” Arnold P. Advincula, MD, of Columbia University Medical Center, New York, wrote in an accompanying editorial.
Many variables beyond clinical will determine the optimal hysterectomy route, Dr. Advincula said.
“Although historical evidence demonstrates that vaginal hysterectomy is associated with better outcomes when compared with other approaches, a multitude of studies now exist that challenge this notion. Given the financial implications and overall costs of care with surgical complications and 30-day readmissions, experienced high-volume surgeons using all available routes have shown robotics to be the best surgical approach in terms of fewer postoperative complications and lowest 30-day readmission rates,” he noted. However, “one should not split hairs and subtly pit one minimally invasive option against another, but instead should work toward the goal of minimizing laparotomy, which is still performed at a high rate,” Dr. Advincula emphasized.
The study was supported in part by the National Center for Advancing Translational Science. Dr. Schmitt had no financial conflicts to disclose. Dr. Advincula disclosed serving as a consultant for AbbVie, Baxter, ConMed, Eximis Surgical, Intuitive Surgical, and Titan Medical, and performing consultancy work and receiving royalties from Cooper Surgical.
SOURCES: Schmitt JJ et al. Obstet Gynecol. 2020;135:761-9. doi: 10.1097/AOG.0000000000003725; Advincula A. Obstet Gynecol. 2020;135:759-60. doi: doi: 10.1097/AOG.0000000000003814.
based on data from a prospective study of 365 patients.
“Total vaginal hysterectomy is the most cost-effective route, with a low complication rate, and, therefore, should be performed when feasible,” wrote Jennifer J. Schmitt, DO, of the Mayo Clinic, Rochester, Minn., and colleagues.
However, algorithms to support the decision to choose vaginal hysterectomy are not widely used, they said.
To assess the optimal surgical route for hysterectomy, the researchers devised a prospective algorithm and decision tree based on history of laparotomy, uterine size, and vaginal access. The results of their study were published in Obstetrics & Gynecology.
The study population included 365 women aged 18 years and older who underwent hysterectomies between Nov. 24, 2015, and Dec. 31, 2017, at a single center. A total of 202 patients (55%) met criteria for a total vaginal hysterectomy using the algorithm, and 57 (15.6%) were assigned to have an examination under anesthesia followed by total vaginal hysterectomy, for a total of 259 expected vaginal hysterectomies. Ultimately, 211 (81.5%) of the patients identified as being the best candidates for having a vaginal hysterectomy underwent the procedure. Almost all of the procedures – 99.1% – were completed successfully.
The algorithm predicted that 52 patients were expected to have an examination under anesthesia followed by a robot-assisted total laparoscopic hysterectomy and 54 were expected to have an abdominal, robotic, or laparoscopic hysterectomy. A total of 46 procedures (44 robotic, when vaginal was expected and 2 abdominal, when vaginal was expected) deviated to a more invasive route than prescribed by the algorithm, and 7 procedures deviated from the algorithm-predicted robotic or abdominal procedure to total vaginal hysterectomy.
Approximately 95% of the patients were discharged within 24 hours of surgery. These patients included 7 who had vaginal surgery when a more invasive method was predicted and did not experience intraoperative complications or Accordion grade 3 complications.
“Prospective algorithm use predicts that 55.3% of all hysterectomies were expected to have an a priori total vaginal hysterectomy, which is higher than the actual total vaginal hysterectomy rate of 11.5% reported previously,” the researchers noted, and they added that vaginal hysterectomy would be associated with cost savings of $657,524 if the total hysterectomy rate was 55% instead of 11%.
The study findings were limited by several factors including an expertise bias at the center where the study was conducted, as well as the small number of patients with algorithm deviations or poor outcomes, and the lack of a control group, the researchers noted. However, the results support the use of the algorithm “in combination with educating gynecologic surgeons about the feasibility of vaginal surgery,” they said.
“Prospective use of this algorithm nationally may increase the rate of total vaginal hysterectomy and decrease health care delivery costs,” they concluded.
“The American College of Obstetricians and Gynecologists continues to recommend vaginal hysterectomy as the approach of choice whenever feasible, and although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, the rate of vaginal hysterectomy in the United States has continued to decline,” Arnold P. Advincula, MD, of Columbia University Medical Center, New York, wrote in an accompanying editorial.
Many variables beyond clinical will determine the optimal hysterectomy route, Dr. Advincula said.
“Although historical evidence demonstrates that vaginal hysterectomy is associated with better outcomes when compared with other approaches, a multitude of studies now exist that challenge this notion. Given the financial implications and overall costs of care with surgical complications and 30-day readmissions, experienced high-volume surgeons using all available routes have shown robotics to be the best surgical approach in terms of fewer postoperative complications and lowest 30-day readmission rates,” he noted. However, “one should not split hairs and subtly pit one minimally invasive option against another, but instead should work toward the goal of minimizing laparotomy, which is still performed at a high rate,” Dr. Advincula emphasized.
The study was supported in part by the National Center for Advancing Translational Science. Dr. Schmitt had no financial conflicts to disclose. Dr. Advincula disclosed serving as a consultant for AbbVie, Baxter, ConMed, Eximis Surgical, Intuitive Surgical, and Titan Medical, and performing consultancy work and receiving royalties from Cooper Surgical.
SOURCES: Schmitt JJ et al. Obstet Gynecol. 2020;135:761-9. doi: 10.1097/AOG.0000000000003725; Advincula A. Obstet Gynecol. 2020;135:759-60. doi: doi: 10.1097/AOG.0000000000003814.
FROM OBSTETRICS & GYNECOLOGY
Largest meeting on cancer research canceled: AACR
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
2020 Update on gynecologic cancer
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
The role of hysteroscopy in diagnosing endometrial cancer
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.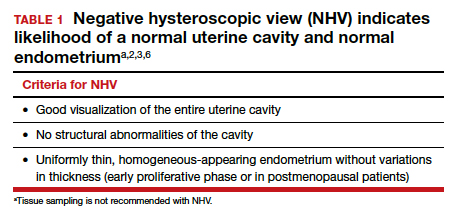
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).
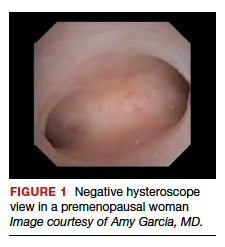
Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1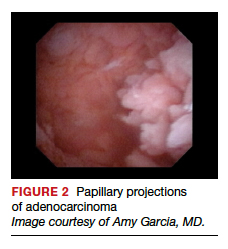
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4
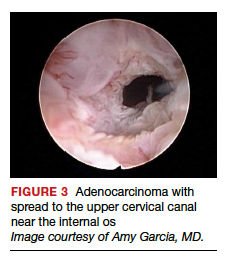
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5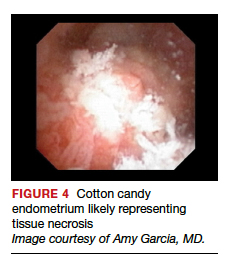
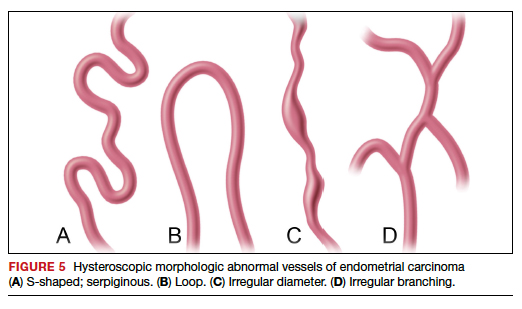
Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).
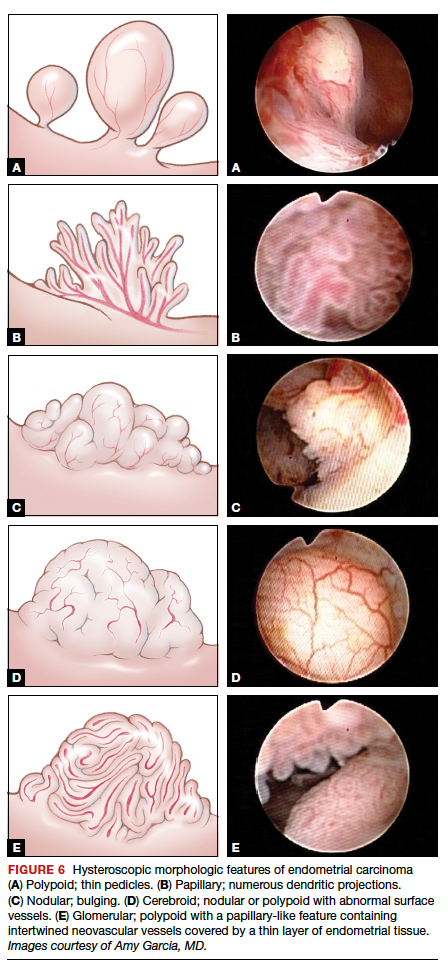
TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.
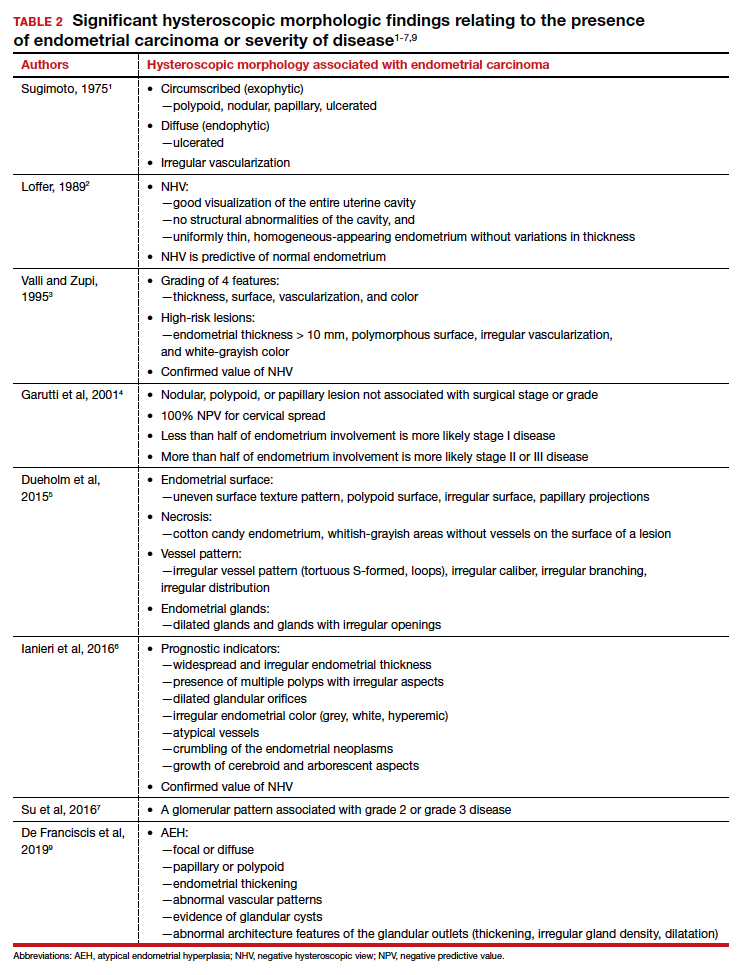
Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).

Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4

Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5

Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).

TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.

Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).

Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4

Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5

Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).

TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.

Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
Salpingectomy adds little time and no complications to cesarean delivery
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
REPORTING FROM THE PREGNANCY MEETING
SOLO3: Olaparib outperforms chemo in heavily pretreated ovarian cancer
For heavily pretreated patients with BRCA-mutated ovarian cancer, olaparib is more effective than nonplatinum chemotherapy, according to results from the SOLO3 trial.
Both objective response rate and progression-free survival were significantly better in the olaparib group, reported lead author Richard T. Penson, MD, of Massachusetts General Hospital, Boston, and colleagues. These findings were published in the Journal of Clinical Oncology.
SOLO3 provides much-needed data for an understudied but common group of patients, according to Kathleen Moore, MD, lead author of the previous SOLO1 trial and associate director of clinical research at the Stephenson Cancer Center at the University of Oklahoma in Oklahoma City.
“[Approximately] 50% of the women who participated in SOLO3 were on their fourth or more line of chemo,” Dr. Moore said in an interview. “That group of patients has not been studied in the past ... Even though we know many women receive many, many lines of chemotherapy, we really don’t have high quality clinical trial data to give women any indication of what to expect in terms of their response rate.”
SOLO3 is also notable, Dr. Moore said, because it is the first phase 3 trial to compare a PARP inhibitor with doctor’s choice chemotherapy.
SOLO3 involved 266 patients with BRCA-mutated, relapsed ovarian cancer who had received two or more lines of platinum-based chemotherapy. All patients were platinum sensitive (progression more than 12 months after last platinum-based treatment) or partially platinum sensitive (progression within 6-12 months of last platinum-based treatment).
After enrollment, participants were randomized in a 2:1 ratio to receive either olaparib (300 mg twice daily) or physician’s choice, single-agent, nonplatinum chemotherapy (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan). The primary endpoint was objective response rate, determined by blinded independent central review. Secondary endpoints included progression-free survival and overall survival.
At a median follow-up of 13.8 months in the olaparib group and 3.9 months in the chemotherapy group, olaparib showed a significant efficacy advantage. Among 223 patients with measurable disease, the objective response rate was 72.2% in the olaparib group and 51.4% in the chemotherapy group (odds ratio, 2.53; P = .002).
The superiority of olaparib was maintained in multiple subgroups of patients, including those who had received only two prior lines of therapy (OR, 3.44) and those who had three or more prior lines (OR, 2.21). Similar benefits were observed regardless of age.
Across all patients, the median progression-free survival was significantly better in the olaparib group, at 13.4 months, versus 9.2 months in the chemotherapy group (P = .013). Overall survival data were immature.
No new safety signals were encountered. The most common adverse events (AEs) in the olaparib group were nausea, fatigue/asthenia, anemia, vomiting, and diarrhea. The most common AEs in the chemotherapy group were fatigue/asthenia, palmar-plantar erythrodysesthesia, nausea, neutropenia, and anemia.
The rate of serious AEs was 23.6% in the olaparib group and 18.4% in the chemotherapy group. Three patients in the olaparib group, and none in the chemotherapy group, developed new primary malignancies. There were four patients with fatal AEs in the olaparib group (myelodysplastic syndrome, cardiopulmonary decompensation, sepsis, and acute myeloid leukemia and subarachnoid hemorrhage), and there was one fatal AE in the chemotherapy group (mesenteric vein thrombosis).
Dr. Moore pointed out that these findings are relevant to current clinical practice, but a shifting treatment landscape may soon render SOLO3 data obsolete.
“PARP [inhibitors] are likely moving into the frontline,” Dr. Moore said. “So this population of women who have not received a PARP [inhibitor] and are recurrent is still here, and they will be for several years, but there’s going to be a point when they’re not going to be here anymore, because they’ll all have received [a PARP inhibitor] front line.”
Concerning the broader research landscape for PARP inhibitors, Dr. Moore suggested that investigators are currently in a “waiting period” while the Food and Drug Administration and European Medicines Agency interpret major clinical trials, such as PAOLA-1 and PRIMA.
A variety of patient populations and clinical scenarios remain unevaluated, Dr. Moore said, including patients who don’t respond to PARP inhibitors, those who have disease recurrence while taking PARP inhibitors, and which drug combinations to use for which patients.
“There are a lot of irons in the fire right now, just getting ready to launch, but I think we need to see what the population is that’s going to be exposed to PARP [inhibitors] next, so we can design the next round of studies,” Dr. Moore said. “It’s an exciting time.”
SOLO3 was funded by AstraZeneca and Merck. The investigators reported additional relationships with Clovis Oncology, Eisai, Tesaro, and other companies. Dr. Moore disclosed relationships with Genentech, Immunogen, Mersana, and other companies.
SOURCE: Penson et al. J Clin Oncol. 2020 Feb 19. doi: 10.1200/JCO.19.02745.
For heavily pretreated patients with BRCA-mutated ovarian cancer, olaparib is more effective than nonplatinum chemotherapy, according to results from the SOLO3 trial.
Both objective response rate and progression-free survival were significantly better in the olaparib group, reported lead author Richard T. Penson, MD, of Massachusetts General Hospital, Boston, and colleagues. These findings were published in the Journal of Clinical Oncology.
SOLO3 provides much-needed data for an understudied but common group of patients, according to Kathleen Moore, MD, lead author of the previous SOLO1 trial and associate director of clinical research at the Stephenson Cancer Center at the University of Oklahoma in Oklahoma City.
“[Approximately] 50% of the women who participated in SOLO3 were on their fourth or more line of chemo,” Dr. Moore said in an interview. “That group of patients has not been studied in the past ... Even though we know many women receive many, many lines of chemotherapy, we really don’t have high quality clinical trial data to give women any indication of what to expect in terms of their response rate.”
SOLO3 is also notable, Dr. Moore said, because it is the first phase 3 trial to compare a PARP inhibitor with doctor’s choice chemotherapy.
SOLO3 involved 266 patients with BRCA-mutated, relapsed ovarian cancer who had received two or more lines of platinum-based chemotherapy. All patients were platinum sensitive (progression more than 12 months after last platinum-based treatment) or partially platinum sensitive (progression within 6-12 months of last platinum-based treatment).
After enrollment, participants were randomized in a 2:1 ratio to receive either olaparib (300 mg twice daily) or physician’s choice, single-agent, nonplatinum chemotherapy (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan). The primary endpoint was objective response rate, determined by blinded independent central review. Secondary endpoints included progression-free survival and overall survival.
At a median follow-up of 13.8 months in the olaparib group and 3.9 months in the chemotherapy group, olaparib showed a significant efficacy advantage. Among 223 patients with measurable disease, the objective response rate was 72.2% in the olaparib group and 51.4% in the chemotherapy group (odds ratio, 2.53; P = .002).
The superiority of olaparib was maintained in multiple subgroups of patients, including those who had received only two prior lines of therapy (OR, 3.44) and those who had three or more prior lines (OR, 2.21). Similar benefits were observed regardless of age.
Across all patients, the median progression-free survival was significantly better in the olaparib group, at 13.4 months, versus 9.2 months in the chemotherapy group (P = .013). Overall survival data were immature.
No new safety signals were encountered. The most common adverse events (AEs) in the olaparib group were nausea, fatigue/asthenia, anemia, vomiting, and diarrhea. The most common AEs in the chemotherapy group were fatigue/asthenia, palmar-plantar erythrodysesthesia, nausea, neutropenia, and anemia.
The rate of serious AEs was 23.6% in the olaparib group and 18.4% in the chemotherapy group. Three patients in the olaparib group, and none in the chemotherapy group, developed new primary malignancies. There were four patients with fatal AEs in the olaparib group (myelodysplastic syndrome, cardiopulmonary decompensation, sepsis, and acute myeloid leukemia and subarachnoid hemorrhage), and there was one fatal AE in the chemotherapy group (mesenteric vein thrombosis).
Dr. Moore pointed out that these findings are relevant to current clinical practice, but a shifting treatment landscape may soon render SOLO3 data obsolete.
“PARP [inhibitors] are likely moving into the frontline,” Dr. Moore said. “So this population of women who have not received a PARP [inhibitor] and are recurrent is still here, and they will be for several years, but there’s going to be a point when they’re not going to be here anymore, because they’ll all have received [a PARP inhibitor] front line.”
Concerning the broader research landscape for PARP inhibitors, Dr. Moore suggested that investigators are currently in a “waiting period” while the Food and Drug Administration and European Medicines Agency interpret major clinical trials, such as PAOLA-1 and PRIMA.
A variety of patient populations and clinical scenarios remain unevaluated, Dr. Moore said, including patients who don’t respond to PARP inhibitors, those who have disease recurrence while taking PARP inhibitors, and which drug combinations to use for which patients.
“There are a lot of irons in the fire right now, just getting ready to launch, but I think we need to see what the population is that’s going to be exposed to PARP [inhibitors] next, so we can design the next round of studies,” Dr. Moore said. “It’s an exciting time.”
SOLO3 was funded by AstraZeneca and Merck. The investigators reported additional relationships with Clovis Oncology, Eisai, Tesaro, and other companies. Dr. Moore disclosed relationships with Genentech, Immunogen, Mersana, and other companies.
SOURCE: Penson et al. J Clin Oncol. 2020 Feb 19. doi: 10.1200/JCO.19.02745.
For heavily pretreated patients with BRCA-mutated ovarian cancer, olaparib is more effective than nonplatinum chemotherapy, according to results from the SOLO3 trial.
Both objective response rate and progression-free survival were significantly better in the olaparib group, reported lead author Richard T. Penson, MD, of Massachusetts General Hospital, Boston, and colleagues. These findings were published in the Journal of Clinical Oncology.
SOLO3 provides much-needed data for an understudied but common group of patients, according to Kathleen Moore, MD, lead author of the previous SOLO1 trial and associate director of clinical research at the Stephenson Cancer Center at the University of Oklahoma in Oklahoma City.
“[Approximately] 50% of the women who participated in SOLO3 were on their fourth or more line of chemo,” Dr. Moore said in an interview. “That group of patients has not been studied in the past ... Even though we know many women receive many, many lines of chemotherapy, we really don’t have high quality clinical trial data to give women any indication of what to expect in terms of their response rate.”
SOLO3 is also notable, Dr. Moore said, because it is the first phase 3 trial to compare a PARP inhibitor with doctor’s choice chemotherapy.
SOLO3 involved 266 patients with BRCA-mutated, relapsed ovarian cancer who had received two or more lines of platinum-based chemotherapy. All patients were platinum sensitive (progression more than 12 months after last platinum-based treatment) or partially platinum sensitive (progression within 6-12 months of last platinum-based treatment).
After enrollment, participants were randomized in a 2:1 ratio to receive either olaparib (300 mg twice daily) or physician’s choice, single-agent, nonplatinum chemotherapy (pegylated liposomal doxorubicin, paclitaxel, gemcitabine, or topotecan). The primary endpoint was objective response rate, determined by blinded independent central review. Secondary endpoints included progression-free survival and overall survival.
At a median follow-up of 13.8 months in the olaparib group and 3.9 months in the chemotherapy group, olaparib showed a significant efficacy advantage. Among 223 patients with measurable disease, the objective response rate was 72.2% in the olaparib group and 51.4% in the chemotherapy group (odds ratio, 2.53; P = .002).
The superiority of olaparib was maintained in multiple subgroups of patients, including those who had received only two prior lines of therapy (OR, 3.44) and those who had three or more prior lines (OR, 2.21). Similar benefits were observed regardless of age.
Across all patients, the median progression-free survival was significantly better in the olaparib group, at 13.4 months, versus 9.2 months in the chemotherapy group (P = .013). Overall survival data were immature.
No new safety signals were encountered. The most common adverse events (AEs) in the olaparib group were nausea, fatigue/asthenia, anemia, vomiting, and diarrhea. The most common AEs in the chemotherapy group were fatigue/asthenia, palmar-plantar erythrodysesthesia, nausea, neutropenia, and anemia.
The rate of serious AEs was 23.6% in the olaparib group and 18.4% in the chemotherapy group. Three patients in the olaparib group, and none in the chemotherapy group, developed new primary malignancies. There were four patients with fatal AEs in the olaparib group (myelodysplastic syndrome, cardiopulmonary decompensation, sepsis, and acute myeloid leukemia and subarachnoid hemorrhage), and there was one fatal AE in the chemotherapy group (mesenteric vein thrombosis).
Dr. Moore pointed out that these findings are relevant to current clinical practice, but a shifting treatment landscape may soon render SOLO3 data obsolete.
“PARP [inhibitors] are likely moving into the frontline,” Dr. Moore said. “So this population of women who have not received a PARP [inhibitor] and are recurrent is still here, and they will be for several years, but there’s going to be a point when they’re not going to be here anymore, because they’ll all have received [a PARP inhibitor] front line.”
Concerning the broader research landscape for PARP inhibitors, Dr. Moore suggested that investigators are currently in a “waiting period” while the Food and Drug Administration and European Medicines Agency interpret major clinical trials, such as PAOLA-1 and PRIMA.
A variety of patient populations and clinical scenarios remain unevaluated, Dr. Moore said, including patients who don’t respond to PARP inhibitors, those who have disease recurrence while taking PARP inhibitors, and which drug combinations to use for which patients.
“There are a lot of irons in the fire right now, just getting ready to launch, but I think we need to see what the population is that’s going to be exposed to PARP [inhibitors] next, so we can design the next round of studies,” Dr. Moore said. “It’s an exciting time.”
SOLO3 was funded by AstraZeneca and Merck. The investigators reported additional relationships with Clovis Oncology, Eisai, Tesaro, and other companies. Dr. Moore disclosed relationships with Genentech, Immunogen, Mersana, and other companies.
SOURCE: Penson et al. J Clin Oncol. 2020 Feb 19. doi: 10.1200/JCO.19.02745.
FROM JOURNAL OF CLINICAL ONCOLOGY