User login
Obesity-related cancers increasing in younger adults
The incidence of obesity-related cancers such as kidney and gallbladder cancer has increased significantly in young adults over the past two decades in the United States, according to an analysis of data from 25 population-based state registries in the United States.
The incidence of 6 of the 12 obesity-related cancers increased among individuals aged 25-49 years, Hyuna Sung, PhD, of the American Cancer Society, Atlanta, and her colleagues reported Feb. 4 in the Lancet Public Health.
Among more than 14.6 million incident cases of cancer diagnosed in adults aged 25-84 years between 1995 and 2014, the greatest increase in incidence, 6.23% annually, was seen with kidney cancer among the 25- to 29-year age group. Incidence, however, also increased by at least 6.17% in those aged 30-34 years, by 5.23% in those aged 35-39 years, and by 3.88% in those aged 40-44 years.
The incidence rate for kidney cancer among individuals born around 1985 was nearly fivefold higher than in individuals born in 1950, the investigators said (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(18)30267-6).
The analysis also showed significant increases from 1995 to 2014 in the incidence of cancer of the gallbladder among younger adults: 3.71% per year among those aged 25-29 years and 2.58% per year in those aged 30-34 years.
Similarly, the incidence of uterine corpus cancer increased in the 25- to 29-year age group by 3.34% per year and by 3.22% in the 30- to 34-year age group. The incidence of colorectal cancer increased by 2.41% among those aged 25-29 years and by 2.38% in those aged 30-34 years, Dr. Sung and her associates said.
The greatest annual increase in the incidence of multiple myeloma was seen in individuals aged 30-34 years (2.21%), but significant annual increases in incidence were seen in individuals aged 30-44 years.
For pancreatic cancer, significant annual increases in incidence were seen among individuals aged 25-29 years (4.34%) and 30-34 years (2.47%).
The study also showed increases in the same obesity-related cancers – except for colorectal cancer – among adults aged 50 years and older. The incidence of colorectal cancer actually decreased annually in older adults, while the incidence of uterine corpus cancer increased among women aged 50-69 years but decreased in those over 75 years.
Dr. Sung and her coauthors suggested that these trends may be related to the rise of obesity and overweight in the United States, noting that excess body weight could be responsible for up to 60% of all endometrial cancers, 36% of gallbladder cancers, and 33% of kidney cancers in adults aged over 30 years.
“Because most epidemiological studies have primarily focused on older populations, the effect of excess bodyweight in early life or of weight change from young adulthood on cancer risk in different stages of the life course is not well characterized,” they wrote. “In concert with excess bodyweight, obesity-related health conditions and lifestyle factors can contribute to the increasing burden of obesity-related cancers in young adults, which include diabetes, gallstones, inflammatory bowel disease, and poor diet.”
The incidences of breast cancer and gastric cardia cancer were relatively stable in all age groups over the study period, and the incidence of ovarian cancer decreased in all age groups.
Researchers looked at the incidence of 30 cancers in total, including 18 cancers not related to obesity. Here they saw increases among younger adults only in the incidence of gastric noncardiac cancer – which showed a 2.16% annual increase in incidence among those aged 30-34 years – and leukemia, where there was a 1.33% annual increase in incidence in the same age group.
But the incidence of eight cancers, including those related to smoking and infection, decreased each year among younger adults.
“Our findings expose a recent change that could serve as a warning of an increased burden of obesity-related cancers to come in older adults,” study senior author Ahmedin Jemal, PhD, of the American Cancer Society, said in a statement. “Most cancers occur in older adults, which means that as the young people in our study age, the burden of obesity-related cancer cases and deaths are likely to increase even more. On the eve of World Cancer Day, it’s timely to consider what can be done to avert the impending rise.”
The future burden of these cancers could halt or even reverse the reductions in cancer mortality achieved over the past several decades, the investigators warned.
The study was funded by the American Cancer Society and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sung H et al. Lancet Public Health. 2019 Feb 4 doi: 10.1016/ S2468-2667(18)30267-6.
Cancer was long thought of as a disease of aging, but the increase in incidence of some cancers in younger age groups has driven a recent reexamination of risk factors. This study’s most striking finding is the disproportionate increase in obesity-related cancer incidence among successively younger cohorts. Coupled with the rising incidence of obesity over the same period, it provides compelling evidence of a possible causal role for obesity in the increased incidence of these cancers.
Not all obesity-related cancers, however, show this pattern of age-specific increase in incidence, which could reflect the influence of other risk factors.
The hypothesis suggested by the study’s authors is plausible but needs to be tested more directly in experimental and population-based studies.
Catherine R. Marinac, PhD, is with the department of medical oncology at the Dana-Farber Cancer Institute, Boston, and Brenda M. Birmann, ScD, is with the department of medicine at Brigham and Women’s Hospital, Boston. These comments are taken from an accompanying editorial (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(19)30017-9). No conflicts of interest were declared.
Cancer was long thought of as a disease of aging, but the increase in incidence of some cancers in younger age groups has driven a recent reexamination of risk factors. This study’s most striking finding is the disproportionate increase in obesity-related cancer incidence among successively younger cohorts. Coupled with the rising incidence of obesity over the same period, it provides compelling evidence of a possible causal role for obesity in the increased incidence of these cancers.
Not all obesity-related cancers, however, show this pattern of age-specific increase in incidence, which could reflect the influence of other risk factors.
The hypothesis suggested by the study’s authors is plausible but needs to be tested more directly in experimental and population-based studies.
Catherine R. Marinac, PhD, is with the department of medical oncology at the Dana-Farber Cancer Institute, Boston, and Brenda M. Birmann, ScD, is with the department of medicine at Brigham and Women’s Hospital, Boston. These comments are taken from an accompanying editorial (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(19)30017-9). No conflicts of interest were declared.
Cancer was long thought of as a disease of aging, but the increase in incidence of some cancers in younger age groups has driven a recent reexamination of risk factors. This study’s most striking finding is the disproportionate increase in obesity-related cancer incidence among successively younger cohorts. Coupled with the rising incidence of obesity over the same period, it provides compelling evidence of a possible causal role for obesity in the increased incidence of these cancers.
Not all obesity-related cancers, however, show this pattern of age-specific increase in incidence, which could reflect the influence of other risk factors.
The hypothesis suggested by the study’s authors is plausible but needs to be tested more directly in experimental and population-based studies.
Catherine R. Marinac, PhD, is with the department of medical oncology at the Dana-Farber Cancer Institute, Boston, and Brenda M. Birmann, ScD, is with the department of medicine at Brigham and Women’s Hospital, Boston. These comments are taken from an accompanying editorial (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(19)30017-9). No conflicts of interest were declared.
The incidence of obesity-related cancers such as kidney and gallbladder cancer has increased significantly in young adults over the past two decades in the United States, according to an analysis of data from 25 population-based state registries in the United States.
The incidence of 6 of the 12 obesity-related cancers increased among individuals aged 25-49 years, Hyuna Sung, PhD, of the American Cancer Society, Atlanta, and her colleagues reported Feb. 4 in the Lancet Public Health.
Among more than 14.6 million incident cases of cancer diagnosed in adults aged 25-84 years between 1995 and 2014, the greatest increase in incidence, 6.23% annually, was seen with kidney cancer among the 25- to 29-year age group. Incidence, however, also increased by at least 6.17% in those aged 30-34 years, by 5.23% in those aged 35-39 years, and by 3.88% in those aged 40-44 years.
The incidence rate for kidney cancer among individuals born around 1985 was nearly fivefold higher than in individuals born in 1950, the investigators said (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(18)30267-6).
The analysis also showed significant increases from 1995 to 2014 in the incidence of cancer of the gallbladder among younger adults: 3.71% per year among those aged 25-29 years and 2.58% per year in those aged 30-34 years.
Similarly, the incidence of uterine corpus cancer increased in the 25- to 29-year age group by 3.34% per year and by 3.22% in the 30- to 34-year age group. The incidence of colorectal cancer increased by 2.41% among those aged 25-29 years and by 2.38% in those aged 30-34 years, Dr. Sung and her associates said.
The greatest annual increase in the incidence of multiple myeloma was seen in individuals aged 30-34 years (2.21%), but significant annual increases in incidence were seen in individuals aged 30-44 years.
For pancreatic cancer, significant annual increases in incidence were seen among individuals aged 25-29 years (4.34%) and 30-34 years (2.47%).
The study also showed increases in the same obesity-related cancers – except for colorectal cancer – among adults aged 50 years and older. The incidence of colorectal cancer actually decreased annually in older adults, while the incidence of uterine corpus cancer increased among women aged 50-69 years but decreased in those over 75 years.
Dr. Sung and her coauthors suggested that these trends may be related to the rise of obesity and overweight in the United States, noting that excess body weight could be responsible for up to 60% of all endometrial cancers, 36% of gallbladder cancers, and 33% of kidney cancers in adults aged over 30 years.
“Because most epidemiological studies have primarily focused on older populations, the effect of excess bodyweight in early life or of weight change from young adulthood on cancer risk in different stages of the life course is not well characterized,” they wrote. “In concert with excess bodyweight, obesity-related health conditions and lifestyle factors can contribute to the increasing burden of obesity-related cancers in young adults, which include diabetes, gallstones, inflammatory bowel disease, and poor diet.”
The incidences of breast cancer and gastric cardia cancer were relatively stable in all age groups over the study period, and the incidence of ovarian cancer decreased in all age groups.
Researchers looked at the incidence of 30 cancers in total, including 18 cancers not related to obesity. Here they saw increases among younger adults only in the incidence of gastric noncardiac cancer – which showed a 2.16% annual increase in incidence among those aged 30-34 years – and leukemia, where there was a 1.33% annual increase in incidence in the same age group.
But the incidence of eight cancers, including those related to smoking and infection, decreased each year among younger adults.
“Our findings expose a recent change that could serve as a warning of an increased burden of obesity-related cancers to come in older adults,” study senior author Ahmedin Jemal, PhD, of the American Cancer Society, said in a statement. “Most cancers occur in older adults, which means that as the young people in our study age, the burden of obesity-related cancer cases and deaths are likely to increase even more. On the eve of World Cancer Day, it’s timely to consider what can be done to avert the impending rise.”
The future burden of these cancers could halt or even reverse the reductions in cancer mortality achieved over the past several decades, the investigators warned.
The study was funded by the American Cancer Society and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sung H et al. Lancet Public Health. 2019 Feb 4 doi: 10.1016/ S2468-2667(18)30267-6.
The incidence of obesity-related cancers such as kidney and gallbladder cancer has increased significantly in young adults over the past two decades in the United States, according to an analysis of data from 25 population-based state registries in the United States.
The incidence of 6 of the 12 obesity-related cancers increased among individuals aged 25-49 years, Hyuna Sung, PhD, of the American Cancer Society, Atlanta, and her colleagues reported Feb. 4 in the Lancet Public Health.
Among more than 14.6 million incident cases of cancer diagnosed in adults aged 25-84 years between 1995 and 2014, the greatest increase in incidence, 6.23% annually, was seen with kidney cancer among the 25- to 29-year age group. Incidence, however, also increased by at least 6.17% in those aged 30-34 years, by 5.23% in those aged 35-39 years, and by 3.88% in those aged 40-44 years.
The incidence rate for kidney cancer among individuals born around 1985 was nearly fivefold higher than in individuals born in 1950, the investigators said (Lancet Public Health. 2019 Feb 4. doi: 10.1016/S2468-2667(18)30267-6).
The analysis also showed significant increases from 1995 to 2014 in the incidence of cancer of the gallbladder among younger adults: 3.71% per year among those aged 25-29 years and 2.58% per year in those aged 30-34 years.
Similarly, the incidence of uterine corpus cancer increased in the 25- to 29-year age group by 3.34% per year and by 3.22% in the 30- to 34-year age group. The incidence of colorectal cancer increased by 2.41% among those aged 25-29 years and by 2.38% in those aged 30-34 years, Dr. Sung and her associates said.
The greatest annual increase in the incidence of multiple myeloma was seen in individuals aged 30-34 years (2.21%), but significant annual increases in incidence were seen in individuals aged 30-44 years.
For pancreatic cancer, significant annual increases in incidence were seen among individuals aged 25-29 years (4.34%) and 30-34 years (2.47%).
The study also showed increases in the same obesity-related cancers – except for colorectal cancer – among adults aged 50 years and older. The incidence of colorectal cancer actually decreased annually in older adults, while the incidence of uterine corpus cancer increased among women aged 50-69 years but decreased in those over 75 years.
Dr. Sung and her coauthors suggested that these trends may be related to the rise of obesity and overweight in the United States, noting that excess body weight could be responsible for up to 60% of all endometrial cancers, 36% of gallbladder cancers, and 33% of kidney cancers in adults aged over 30 years.
“Because most epidemiological studies have primarily focused on older populations, the effect of excess bodyweight in early life or of weight change from young adulthood on cancer risk in different stages of the life course is not well characterized,” they wrote. “In concert with excess bodyweight, obesity-related health conditions and lifestyle factors can contribute to the increasing burden of obesity-related cancers in young adults, which include diabetes, gallstones, inflammatory bowel disease, and poor diet.”
The incidences of breast cancer and gastric cardia cancer were relatively stable in all age groups over the study period, and the incidence of ovarian cancer decreased in all age groups.
Researchers looked at the incidence of 30 cancers in total, including 18 cancers not related to obesity. Here they saw increases among younger adults only in the incidence of gastric noncardiac cancer – which showed a 2.16% annual increase in incidence among those aged 30-34 years – and leukemia, where there was a 1.33% annual increase in incidence in the same age group.
But the incidence of eight cancers, including those related to smoking and infection, decreased each year among younger adults.
“Our findings expose a recent change that could serve as a warning of an increased burden of obesity-related cancers to come in older adults,” study senior author Ahmedin Jemal, PhD, of the American Cancer Society, said in a statement. “Most cancers occur in older adults, which means that as the young people in our study age, the burden of obesity-related cancer cases and deaths are likely to increase even more. On the eve of World Cancer Day, it’s timely to consider what can be done to avert the impending rise.”
The future burden of these cancers could halt or even reverse the reductions in cancer mortality achieved over the past several decades, the investigators warned.
The study was funded by the American Cancer Society and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sung H et al. Lancet Public Health. 2019 Feb 4 doi: 10.1016/ S2468-2667(18)30267-6.
FROM THE LANCET PUBLIC HEALTH
Key clinical point: The incidence of obesity-related cancers has increased in younger adults.
Major finding: The incidence of kidney cancer has increased by more than 6% per year in younger adults since 1995.
Study details: Analysis of data from 14,672,409 cases of cancer diagnosed between 1995 and 2014.
Disclosures: The study was funded by the American Cancer Society and the National Cancer Institute. No conflicts of interest were declared.
Source: Sung H et al. Lancet Public Health. 2019 Feb 4.
When NOT to perform a Pap test
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
Avelumab active in recurrent or refractory ovarian cancer
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
FROM JAMA ONCOLOGY
Key clinical point: Single-agent treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients.
Major finding: Treatment yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months.
Study details: Phase 1b results from the JAVELIN solid tumor trial, which included 125 women with recurrent or refractory ovarian cancer.
Disclosures: The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Study authors reported disclosures related to Pfizer, Merck, Celgene, EMD Serono, Epithany, Janssen, and Seattle Genetics, among others.
Source: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Study shows evidence of herd immunity with HPV vaccine
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
This study of the real-world effectiveness of the HPV vaccine adds to the growing body of literature, and has produced three important results.
The first is that women who had received at least one dose of the vaccine were considered vaccinated, and because of their level of sexual activity, many likely would have already been infected with some HPV subtypes. The high vaccine effectiveness seen in this study despite these factors adds weight to evidence that this HPV vaccine is highly protective.
The study also showed evidence of cross-protection, in that even women who had received only the quadrivalent vaccine still had significantly reduced rates of infection with the HPV subtypes included in the nine-valent vaccine.
It also provides significant evidence of the herd immunity effect against the subtypes included in the quadrivalent vaccine.
Amanda F. Dempsey, MD, PhD, is from the adult and child consortium for health outcomes research and delivery science at the University of Colorado, Denver. These comments are taken from an accompanying editorial (Pediatrics. 2019 Jan 22. doi: 10.1542/peds.2018-3427). Dr. Dempsey declared advisory board roles for Merck, Sanofi, and Pfizer and a consultancy for Pfizer. She received no external funding.
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
Introduction of the quadrivalent human papillomavirus (HPV) vaccine was associated with significant declines in the incidence of vaccine-type virus both in vaccinated and unvaccinated young women, according to a study published in Pediatrics.
Four surveillance studies, conducted between 2006 and 2017, examined the rate of positive tests for vaccine-type HPV among 1,580 vaccinated and unvaccinated women aged 13-26 years. The majority of participants identified as African American or multiracial.
Overall, 97% of study participants received the quadrivalent vaccine, with vaccination rates increasing from 0% to 84% over the four waves of vaccination. Vaccine effectiveness – representing the relative risk of infection in vaccinated individuals, compared with unvaccinated risk before introduction of the vaccine – increased by 72% from wave 1 to wave 2, 91% from wave 1 to wave 3, and 80% from wave 1 to wave 4.
Among women who were vaccinated, rates of the quadrivalent vaccine–type HPV decreased by 81%, from 35% to 7%. But even among women who were unvaccinated, detection of the vaccine-targeted strains of HPV decreased by 40%, from 32% to 19%.
Chelse Spinner of the University of Cincinnati and her coauthors wrote that the decline in the quadrivalent vaccine–type HPV provided evidence of direct protection and high vaccine effectiveness in this real-world setting.
“This degree of effectiveness is remarkable given the fact that vaccination was defined as having received one or more doses (i.e., was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than [were] those in the HPV vaccine clinical trials because of their reported sexual behaviors,” they wrote. “As noted in a recent review, evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.”
Twelve percent of women in the studies received the nine-valent HPV vaccine, and among these women, the rate of infection with the nine-valent vaccine-type HPV decreased from 47% in the first wave of vaccination to 14% in the last wave, representing a 71% decline.
The proportion of vaccinated women in the study who were infected with one or more of the five viral subtypes included in the nine-valent but not in the quadrivalent vaccine decreased significantly by 69%, from 23% to 7%.
However, these data also suggested a nonsignificant 58% increase among unvaccinated women in infections with one of the five subtypes covered by the nine-valent vaccine but not the quadrivalent vaccine.
Ms. Spinner and her associates noted this increase was unexpected and suggested the increase may be caused by the differences between vaccinated and unvaccinated women.
“For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV,” they wrote.
Ms. Spinner graduated from the University of Cincinnati and now is a graduate student at the University of South Florida, Tampa. The study was funded by the National Institutes of Health. Darron R. Brown declared shares of Merck, but the other coauthors declared no other relevant financial disclosures.
SOURCE: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
FROM PEDIATRICS
Key clinical point:
Major finding: Infection rates for quadrivalent vaccine-covered HPV strains declined by 81% among vaccinated women.
Study details: Surveillance studies in 1,580 vaccinated and unvaccinated young women.
Disclosures: The study was funded by the National Institutes of Health. One author declared shares of Merck, but no other conflicts of interest were declared.
Source: Spinner C et al. Pediatrics. 2019, Jan 22. doi: 10.1542/peds.2018-1902.
Nationwide implementation of MIS reduced complications and increased survival in early-stage endometrial cancer
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
CRS/HIPEC safety concerns may be outdated
, according to a retrospective study involving more than 34,000 cases.
Compared with four other surgical oncology procedures considered high risk, CRS/HIPEC had the lowest 30-day mortality rate, reported lead author Jason M. Foster, MD, of the University of Nebraska Medical Center in Omaha, and his colleagues.
“The perception of high morbidity, high mortality, and poor surgical outcomes remains a barrier to CRS/HIPEC patient referral as well as clinical trial development in the United States, despite the published noncomparative data establishing contemporary safety,” the investigators wrote in JAMA Network Open.
The study involved 34,114 patients from the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) database who underwent CRS/HIPEC (n = 1,822), trisegmental hepatectomy (n = 2,449), right lobe hepatectomy (n = 5,109), pancreaticoduodenectomy (Whipple; n = 16,793), or esophagectomy (n = 7,941) during 2005-2015. The investigators rates of overall 30-day postoperative mortality, superficial incisional infection, deep incisional infection, organ space infection, return to operating room, and length of hospital stay.
Analysis revealed that CRS/HIPEC had a 30-day mortality rate of 1.1%, which was lower than rates of 2.5%-3.9% for the comparative procedures. Similarly, organ space infection rate was lowest for CRS/HIPEC (7.2%). Superficial and deep incisional infection rates were 5.4% and 1.7%, respectively, for CRS/HIPEC, lower than all procedures except right lobe hepatectomy, with rates of 4.6% and 1.5%. Return to OR was necessary for 6.8% of CRS/HIPEC patients, a rate similar to the other procedures except esophagectomy, in which return to OR was necessary 14.4% of the time. Finally, CRS/HIPEC had a median length of stay of 8 days, which was slightly longer than right lobe or trisegmental hepatectomy (7 days), but shorter than Whipple procedure or esophagectomy (10 days.)
“This study found that CRS/HIPEC had the lowest mortality risk, almost 50%-75% lower than other advanced oncology surgical procedures,” the investigators noted. “These findings provide objective data to dispel the misperception of morbidity and mortality concerns surrounding CRS/HIPEC, and surgical risk should no longer remain a deterrent to patient referral or development of clinical trials for CRS/HIPEC.”
The study was funded by the Platon Foundation and the Hill Foundation. The authors reported no conflicts of interest.
SOURCE: Foster JM et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6847.
The recent study by Foster et al. provides insight into the national safety of reductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC); however, more detailed safety and efficacy data are needed to influence current practices, according to Margaret Smith, MD, and Hari Nathan, MD, PhD.
A closer look at the Foster et al. study reveals three key limitations: First, “cytoreductive surgery encompasses a wide range of procedures, from resection of one peritoneal nodule to multivisceral resection with peritoneal stripping, and, thus, reflects a wide range of possible morbidity,” the authors wrote in an editorial for JAMA Network Open. Therefore, the findings may not represent certain patient populations.
Second, “comparison with other procedures for different indications constructs a straw man.” In contrast with some candidates for CRS/HIPEC, “a patient with pancreatic cancer has no other curative option besides a Whipple procedure.” This imperfect comparison should be considered as such.
Third, the safety of CRS/HIPEC may not be the procedure’s primary limitation. “A more salient concern may be its oncologic effectiveness,” the authors wrote.
Although a clinical randomized trial from 2003 involving patients with colorectal peritoneal carcinomatosis showed a near doubling of overall survival with CRS/HIPEC, compared with systemic chemotherapy alone (22 vs. 12.5 months), a comprehensive understanding of safety and efficacy is lacking, particularly regarding the inclusion of HIPEC. For example, the recent phase 3 Prodige 7 trial showed that addition of HIPEC to CRS added morbidity without survival advantage in patients with colorectal peritoneal carcinomatosis; in contrast, a separate phase 3 trial in epithelial ovarian cancer showed that adding HIPEC to CRS did extend survival.
“…Others have cautioned against changing practice based on these results given concerns over small sample size, imbalances in effects seen across centers, and overall survival with CRS/HIPEC that was similar to other studies’ reported survival following interval debulking alone. Legitimate concerns regarding the efficacy of CRS/HIPEC exist, and appropriate patient selection for this aggressive treatment remains a challenge. Foster et al. demonstrates acceptable morbidity and mortality rates for CRS/HIPEC in this highly selected patient cohort. However, until the benefit for individual patients is more thoroughly understood, clinician referral and treatment practices will remain difficult to transform,” the authors wrote.
Dr. Smith and Dr. Nathan are affiliated with Michigan Medicine at the University of Michigan in Ann Arbor. These comments are adapted from the accompanying editorial (JAMA Netw Open 2019 Jan 11. doi:10.1001/jamanetworkopen.2018.6839).
The recent study by Foster et al. provides insight into the national safety of reductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC); however, more detailed safety and efficacy data are needed to influence current practices, according to Margaret Smith, MD, and Hari Nathan, MD, PhD.
A closer look at the Foster et al. study reveals three key limitations: First, “cytoreductive surgery encompasses a wide range of procedures, from resection of one peritoneal nodule to multivisceral resection with peritoneal stripping, and, thus, reflects a wide range of possible morbidity,” the authors wrote in an editorial for JAMA Network Open. Therefore, the findings may not represent certain patient populations.
Second, “comparison with other procedures for different indications constructs a straw man.” In contrast with some candidates for CRS/HIPEC, “a patient with pancreatic cancer has no other curative option besides a Whipple procedure.” This imperfect comparison should be considered as such.
Third, the safety of CRS/HIPEC may not be the procedure’s primary limitation. “A more salient concern may be its oncologic effectiveness,” the authors wrote.
Although a clinical randomized trial from 2003 involving patients with colorectal peritoneal carcinomatosis showed a near doubling of overall survival with CRS/HIPEC, compared with systemic chemotherapy alone (22 vs. 12.5 months), a comprehensive understanding of safety and efficacy is lacking, particularly regarding the inclusion of HIPEC. For example, the recent phase 3 Prodige 7 trial showed that addition of HIPEC to CRS added morbidity without survival advantage in patients with colorectal peritoneal carcinomatosis; in contrast, a separate phase 3 trial in epithelial ovarian cancer showed that adding HIPEC to CRS did extend survival.
“…Others have cautioned against changing practice based on these results given concerns over small sample size, imbalances in effects seen across centers, and overall survival with CRS/HIPEC that was similar to other studies’ reported survival following interval debulking alone. Legitimate concerns regarding the efficacy of CRS/HIPEC exist, and appropriate patient selection for this aggressive treatment remains a challenge. Foster et al. demonstrates acceptable morbidity and mortality rates for CRS/HIPEC in this highly selected patient cohort. However, until the benefit for individual patients is more thoroughly understood, clinician referral and treatment practices will remain difficult to transform,” the authors wrote.
Dr. Smith and Dr. Nathan are affiliated with Michigan Medicine at the University of Michigan in Ann Arbor. These comments are adapted from the accompanying editorial (JAMA Netw Open 2019 Jan 11. doi:10.1001/jamanetworkopen.2018.6839).
The recent study by Foster et al. provides insight into the national safety of reductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC); however, more detailed safety and efficacy data are needed to influence current practices, according to Margaret Smith, MD, and Hari Nathan, MD, PhD.
A closer look at the Foster et al. study reveals three key limitations: First, “cytoreductive surgery encompasses a wide range of procedures, from resection of one peritoneal nodule to multivisceral resection with peritoneal stripping, and, thus, reflects a wide range of possible morbidity,” the authors wrote in an editorial for JAMA Network Open. Therefore, the findings may not represent certain patient populations.
Second, “comparison with other procedures for different indications constructs a straw man.” In contrast with some candidates for CRS/HIPEC, “a patient with pancreatic cancer has no other curative option besides a Whipple procedure.” This imperfect comparison should be considered as such.
Third, the safety of CRS/HIPEC may not be the procedure’s primary limitation. “A more salient concern may be its oncologic effectiveness,” the authors wrote.
Although a clinical randomized trial from 2003 involving patients with colorectal peritoneal carcinomatosis showed a near doubling of overall survival with CRS/HIPEC, compared with systemic chemotherapy alone (22 vs. 12.5 months), a comprehensive understanding of safety and efficacy is lacking, particularly regarding the inclusion of HIPEC. For example, the recent phase 3 Prodige 7 trial showed that addition of HIPEC to CRS added morbidity without survival advantage in patients with colorectal peritoneal carcinomatosis; in contrast, a separate phase 3 trial in epithelial ovarian cancer showed that adding HIPEC to CRS did extend survival.
“…Others have cautioned against changing practice based on these results given concerns over small sample size, imbalances in effects seen across centers, and overall survival with CRS/HIPEC that was similar to other studies’ reported survival following interval debulking alone. Legitimate concerns regarding the efficacy of CRS/HIPEC exist, and appropriate patient selection for this aggressive treatment remains a challenge. Foster et al. demonstrates acceptable morbidity and mortality rates for CRS/HIPEC in this highly selected patient cohort. However, until the benefit for individual patients is more thoroughly understood, clinician referral and treatment practices will remain difficult to transform,” the authors wrote.
Dr. Smith and Dr. Nathan are affiliated with Michigan Medicine at the University of Michigan in Ann Arbor. These comments are adapted from the accompanying editorial (JAMA Netw Open 2019 Jan 11. doi:10.1001/jamanetworkopen.2018.6839).
, according to a retrospective study involving more than 34,000 cases.
Compared with four other surgical oncology procedures considered high risk, CRS/HIPEC had the lowest 30-day mortality rate, reported lead author Jason M. Foster, MD, of the University of Nebraska Medical Center in Omaha, and his colleagues.
“The perception of high morbidity, high mortality, and poor surgical outcomes remains a barrier to CRS/HIPEC patient referral as well as clinical trial development in the United States, despite the published noncomparative data establishing contemporary safety,” the investigators wrote in JAMA Network Open.
The study involved 34,114 patients from the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) database who underwent CRS/HIPEC (n = 1,822), trisegmental hepatectomy (n = 2,449), right lobe hepatectomy (n = 5,109), pancreaticoduodenectomy (Whipple; n = 16,793), or esophagectomy (n = 7,941) during 2005-2015. The investigators rates of overall 30-day postoperative mortality, superficial incisional infection, deep incisional infection, organ space infection, return to operating room, and length of hospital stay.
Analysis revealed that CRS/HIPEC had a 30-day mortality rate of 1.1%, which was lower than rates of 2.5%-3.9% for the comparative procedures. Similarly, organ space infection rate was lowest for CRS/HIPEC (7.2%). Superficial and deep incisional infection rates were 5.4% and 1.7%, respectively, for CRS/HIPEC, lower than all procedures except right lobe hepatectomy, with rates of 4.6% and 1.5%. Return to OR was necessary for 6.8% of CRS/HIPEC patients, a rate similar to the other procedures except esophagectomy, in which return to OR was necessary 14.4% of the time. Finally, CRS/HIPEC had a median length of stay of 8 days, which was slightly longer than right lobe or trisegmental hepatectomy (7 days), but shorter than Whipple procedure or esophagectomy (10 days.)
“This study found that CRS/HIPEC had the lowest mortality risk, almost 50%-75% lower than other advanced oncology surgical procedures,” the investigators noted. “These findings provide objective data to dispel the misperception of morbidity and mortality concerns surrounding CRS/HIPEC, and surgical risk should no longer remain a deterrent to patient referral or development of clinical trials for CRS/HIPEC.”
The study was funded by the Platon Foundation and the Hill Foundation. The authors reported no conflicts of interest.
SOURCE: Foster JM et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6847.
, according to a retrospective study involving more than 34,000 cases.
Compared with four other surgical oncology procedures considered high risk, CRS/HIPEC had the lowest 30-day mortality rate, reported lead author Jason M. Foster, MD, of the University of Nebraska Medical Center in Omaha, and his colleagues.
“The perception of high morbidity, high mortality, and poor surgical outcomes remains a barrier to CRS/HIPEC patient referral as well as clinical trial development in the United States, despite the published noncomparative data establishing contemporary safety,” the investigators wrote in JAMA Network Open.
The study involved 34,114 patients from the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) database who underwent CRS/HIPEC (n = 1,822), trisegmental hepatectomy (n = 2,449), right lobe hepatectomy (n = 5,109), pancreaticoduodenectomy (Whipple; n = 16,793), or esophagectomy (n = 7,941) during 2005-2015. The investigators rates of overall 30-day postoperative mortality, superficial incisional infection, deep incisional infection, organ space infection, return to operating room, and length of hospital stay.
Analysis revealed that CRS/HIPEC had a 30-day mortality rate of 1.1%, which was lower than rates of 2.5%-3.9% for the comparative procedures. Similarly, organ space infection rate was lowest for CRS/HIPEC (7.2%). Superficial and deep incisional infection rates were 5.4% and 1.7%, respectively, for CRS/HIPEC, lower than all procedures except right lobe hepatectomy, with rates of 4.6% and 1.5%. Return to OR was necessary for 6.8% of CRS/HIPEC patients, a rate similar to the other procedures except esophagectomy, in which return to OR was necessary 14.4% of the time. Finally, CRS/HIPEC had a median length of stay of 8 days, which was slightly longer than right lobe or trisegmental hepatectomy (7 days), but shorter than Whipple procedure or esophagectomy (10 days.)
“This study found that CRS/HIPEC had the lowest mortality risk, almost 50%-75% lower than other advanced oncology surgical procedures,” the investigators noted. “These findings provide objective data to dispel the misperception of morbidity and mortality concerns surrounding CRS/HIPEC, and surgical risk should no longer remain a deterrent to patient referral or development of clinical trials for CRS/HIPEC.”
The study was funded by the Platon Foundation and the Hill Foundation. The authors reported no conflicts of interest.
SOURCE: Foster JM et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6847.
FROM JAMA NETWORK OPEN
Key clinical point: Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) appears safe, and concerns about high complication rates may be outdated.
Major finding: CRS/HIPEC had a 30-day mortality rate of 1.1%, which was lower than rates of 2.5%-3.9% for comparative high-risk surgical oncology procedures.
Study details: A retrospective study of 34,114 patients from the American College of Surgeons National Surgical Quality Improvement Project (NSQIP) database who underwent CRS/HIPEC (n = 1,822), trisegmental hepatectomy (n = 2,449), right lobe hepatectomy (n = 5,109), pancreaticoduodenectomy (Whipple; n = 16,793), or esophagectomy (n = 7,941) during 2005-2015.
Disclosures: The study was funded by the Platon Foundation and the Hill Foundation. The authors reported no conflicts of interest.
Source: Foster JM et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6847.
BMI changes in adolescence linked to later cancer risk
Adiposity changes during adolescence are more strongly associated with ovarian cancer risk than changes in adiposity during adulthood, according to data from the Nurses’ Health Study.
Among the 133,526 women followed prospectively in the observational study, investigators documented 562 incident ovarian cancers in the first cohort (1980-2012) and 226 in the second cohort (1989-2013) during 32 years of follow-up. Body mass index (BMI) changes that occurred between age 10 and 18 years was strongly positively associated with ovarian cancer risk (hazard Ratio, 1.24; 95% confidence interval, 1.11-1.39; P = .0002), compared with a slight association with risk for BMI change after age 18 years (HR, 1.06; 95% CI, 0.99-1.14; P = .10), Tianyi Huang, ScD, of Harvard Medical School, Boston, and his associates reported in Annals of Oncology.
The association between adolescent BMI changes and ovarian cancer risk was stronger for premenopausal cases (HR, 2.41; 95% CI, 1.38-4.19; P = .002), compared with postmenopausal cases (HR, 1.31; 95% CI, 0.90-1.92; P = .16), and suggestively stronger for nonserous tumors versus serous ovarian tumors.
For BMI change between age 10 and 18 years, the HR for every 5 kg/m2 increase was 1.35 (1.10, 1.65) for nonserous cancer and 1.08 (0.90, 1.28) for serous cancer (P = .10).
“This study provides additional evidence to support that maintaining a healthy weight throughout the life course may have moderate benefits on ovarian cancer prevention, particularly nonserous subtypes diagnosed during premenopausal years,” the authors wrote. “Further studies are needed to understand the specific mechanisms linking peripubertal adiposity and adult ovarian cancer risk.”
SOURCE: Huang T et al. Ann Oncol. 2018 Dec 21. doi: 10.1093/annonc/mdy546.
Adiposity changes during adolescence are more strongly associated with ovarian cancer risk than changes in adiposity during adulthood, according to data from the Nurses’ Health Study.
Among the 133,526 women followed prospectively in the observational study, investigators documented 562 incident ovarian cancers in the first cohort (1980-2012) and 226 in the second cohort (1989-2013) during 32 years of follow-up. Body mass index (BMI) changes that occurred between age 10 and 18 years was strongly positively associated with ovarian cancer risk (hazard Ratio, 1.24; 95% confidence interval, 1.11-1.39; P = .0002), compared with a slight association with risk for BMI change after age 18 years (HR, 1.06; 95% CI, 0.99-1.14; P = .10), Tianyi Huang, ScD, of Harvard Medical School, Boston, and his associates reported in Annals of Oncology.
The association between adolescent BMI changes and ovarian cancer risk was stronger for premenopausal cases (HR, 2.41; 95% CI, 1.38-4.19; P = .002), compared with postmenopausal cases (HR, 1.31; 95% CI, 0.90-1.92; P = .16), and suggestively stronger for nonserous tumors versus serous ovarian tumors.
For BMI change between age 10 and 18 years, the HR for every 5 kg/m2 increase was 1.35 (1.10, 1.65) for nonserous cancer and 1.08 (0.90, 1.28) for serous cancer (P = .10).
“This study provides additional evidence to support that maintaining a healthy weight throughout the life course may have moderate benefits on ovarian cancer prevention, particularly nonserous subtypes diagnosed during premenopausal years,” the authors wrote. “Further studies are needed to understand the specific mechanisms linking peripubertal adiposity and adult ovarian cancer risk.”
SOURCE: Huang T et al. Ann Oncol. 2018 Dec 21. doi: 10.1093/annonc/mdy546.
Adiposity changes during adolescence are more strongly associated with ovarian cancer risk than changes in adiposity during adulthood, according to data from the Nurses’ Health Study.
Among the 133,526 women followed prospectively in the observational study, investigators documented 562 incident ovarian cancers in the first cohort (1980-2012) and 226 in the second cohort (1989-2013) during 32 years of follow-up. Body mass index (BMI) changes that occurred between age 10 and 18 years was strongly positively associated with ovarian cancer risk (hazard Ratio, 1.24; 95% confidence interval, 1.11-1.39; P = .0002), compared with a slight association with risk for BMI change after age 18 years (HR, 1.06; 95% CI, 0.99-1.14; P = .10), Tianyi Huang, ScD, of Harvard Medical School, Boston, and his associates reported in Annals of Oncology.
The association between adolescent BMI changes and ovarian cancer risk was stronger for premenopausal cases (HR, 2.41; 95% CI, 1.38-4.19; P = .002), compared with postmenopausal cases (HR, 1.31; 95% CI, 0.90-1.92; P = .16), and suggestively stronger for nonserous tumors versus serous ovarian tumors.
For BMI change between age 10 and 18 years, the HR for every 5 kg/m2 increase was 1.35 (1.10, 1.65) for nonserous cancer and 1.08 (0.90, 1.28) for serous cancer (P = .10).
“This study provides additional evidence to support that maintaining a healthy weight throughout the life course may have moderate benefits on ovarian cancer prevention, particularly nonserous subtypes diagnosed during premenopausal years,” the authors wrote. “Further studies are needed to understand the specific mechanisms linking peripubertal adiposity and adult ovarian cancer risk.”
SOURCE: Huang T et al. Ann Oncol. 2018 Dec 21. doi: 10.1093/annonc/mdy546.
FROM ANNALS OF ONCOLOGY
Key clinical point:
Major finding: The pooled hazard ratio associated with a body mass index increase between age 10 and 18 years was 1.24 (95% CI: 1.11-1.39; P = .0002), compared with 1.06 (95% CI: 0.99-1.14; P = .10) for BMI change after age 18 years.
Study details: A prospective observational study of 133,526 women in the Nurses’ Health Study.
Disclosures: The study was supported by a grant from the National Institute of Health. The authors reported having no conflicts of interest.
Source: Huang T et al. Ann Oncol. 2018 Dec 21. doi: 10.1093/annonc/mdy546.
Should we abandon minimally invasive surgery for cervical cancer?
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
The HPV vaccine is now recommended for adults aged 27–45: Counseling implications
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.
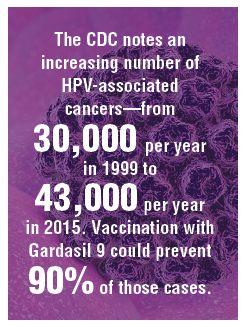
HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.
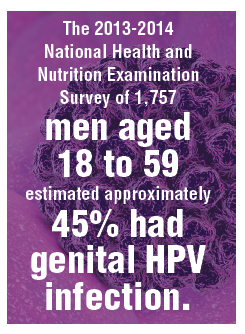
The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.

HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.

The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
The US Food and Drug Administration (FDA) recently extended the approval for Gardasil 9 (to prevent HPV-associated cancers, cancer precursors, and genital lesions) to men and women aged 27 to 45.1 In this editorial, we discuss the evolution of the HPV vaccine since its initial approval more than 10 years ago, the benefits of primary prevention with the HPV vaccine, and the case for the FDA’s recent extension of coverage to older men and women.
The evolution of the HPV vaccine
Since recognition in the 1980s and 90s that high-risk strains of HPV, notably HPV types 16 and 18, were linked to cervical cancer, there have been exciting advances in detection and prevention of high-risk HPV infection. About 70% of cervical cancers are attributable to these 2 oncogenic types.2 The first vaccine licensed, Gardasil (Merck), was approved in 2006 for girls and women aged 9 through 26 to prevent HPV-related diseases caused by types 6, 11, 16, and 18.3 The vaccine was effective for prevention of cervical cancer; genital warts; and grades 2 and 3 of cervical, vulvar, and vaginal intraepithelial neoplasia. In 2008, prevention of vulvar and vaginal cancers was added to the indication. By 2009, prevention of genital warts was added, and use in males aged 9 to 15 was approved. By 2010 sufficient data were accumulated to document prevention of anal cancer and anal intraepithelial neoplasia in men and women, and this indication was added.
In 2014 Gardasil 9 was approved to extend coverage to an additional 5 oncogenic HPV types (31, 33, 45, 52, and 58), now covering an additional 20% of cervical cancers, and in 2015 Gardasil 9 indications were expanded to include boys and men 9 to 26 years of age. Immunogenicity studies were performed to infer effectiveness of a 2-dose regimen in boys and girls aged 9 to 14 years, which was recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) in late 2016.4
Until October 2018, Gardasil 9 was indicated for prevention of genital warts, cervical, vaginal, vulvar and anal cancers and cancer precursors for males and females aged 9 to 26 years. In October the FDA extended approval of the 3-dose vaccine regimen to men and women up to age 45.

HPV vaccine uptake
HPV vaccination has been underutilized in the United States. In 2017, a disappointing 49% of adolescents were up to date on vaccination, and 66% had received at least one dose.5 In rural areas the vaccination rates are 11 points lower than in urban regions.6 The CDC notes an increasing number of HPV-associated cancers—from 30,000 per year in 1999 to 43,000 per year in 2015—due mostly to increases in oral and anal carcinomas. Vaccination with Gardasil 9 could prevent 90% of those cases.7
Non-US successes. HPV vaccine uptake in Australia provides an excellent opportunity to study the impact of universally available, school-based vaccinations. In 2007 Australia implemented a program of free HPV vaccination distributed through schools. Boys and girls aged 12 and 13 were targeted that year, with catch-up vaccinations for those aged 13 to 18 in 2007-2009 in schools and for those aged 18 to 26 reached in the community.8
Continue to: Ali and colleagues studied the...
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.

The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...
Impact of the new indication on HPV-related disease
As described above, widespread vaccination of young girls and boys is going to have major impact on HPV-related disease, including precancer and cancer. Because there is evidence that older women and men are at risk for new HPV infection,10 there likely will be some benefit from vaccination of adults. It is difficult, however, to extrapolate the degree to which adult vaccination will impact HPV-related disease. This is because we do not fully understand the rates at which new HPV infection in the cervices of older women will progress to high-grade dysplasia or cancer. Further, the pathophysiology of HPV-related cancers at other anogenital sites and new oral-pharyngeal infection is poorly understood in comparison with our knowledge of the natural history of high-risk HPV infection in younger women. That said, because of the outstanding efficacy of HPV vaccination and the low-risk profile, even if the actual impact on prevention of cancer or morbidity from dysplasia is relatively low, adult vaccination benefits outweigh the limited risks.
It may be that increased vaccination and awareness of vaccination for adults may enhance the adherence and acceptance of widespread vaccination of boys and girls. Adult vaccination could create a cultural shift toward HPV vaccination acceptance when adult parents and loved ones of vaccine-age boys and girls have been vaccinated themselves.
Current and future insurance coverage
The Affordable Care Act, otherwise known as Obamacare, mandates coverage for all immunizations recommended by the ACIP. HPV vaccination up to age 26 is fully covered, without copay or deductible. The ACIP did consider extension of the indications for HPV vaccination to men and women up to age 45 at their October 2018 meeting. They are tasked with considering not only safety and efficacy but also the cost effectiveness of implementing vaccination. They continue to study the costs and potential benefits of extending HPV vaccination to age 45. Their recommendations may be determined at the February 2019 meeting—or even later in 2019. The American College of Obstetricians and Gynecologists (ACOG) relies upon ACIP for practice guidance. Once the ACIP has made a determination, and if new guidelines are published in the Morbidity and Mortality Weekly Report, insurance coverage and ACOG guidance will be updated.
How should we react and change practice based on this new indication?
Given the information reviewed by the FDA, ObGyns will want to discuss the availability of Gardasil 9 with our patients between ages 27 and 45 who have not been previously immunized.

Especially for our patients with exposure to multiple or new sexual partners, immunization against oncogenic HPV viral types is effective in providing protection from cancer precursors and cancers of the cervix, vulva, vagina, and anus—and of course from genital warts. They should understand that, until formal recommendations are published by the ACIP, they are likely to be responsible for the cost of the vaccination series. These conversations will also remind our patients to immunize their teens against HPV. The more conversation we have regarding the benefits of vaccination against high-risk HPV types, the more likely we are to be able to achieve the impressive results seen in Australia.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- US Food and Drug Administration website. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm. Updated October 9, 2018. Accessed December 27, 2018.
- World Health Organization website. Human papillomavirus (HPV) and cervical cancer. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. February 15, 2018. Accessed December 27, 2018.
- Centers for Disease Control and Prevention website. Human papillomavirus (HPV) vaccine safety. https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Last reviewed October 27, 2015. Accessed December 27, 2018.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;65(49):1405–1408.
- AAP News website. Jenko M. CDC: 49% of teens up to date on HPV vaccine. http://www.aappublications.org/news/2018/08/23/vac cinationrates082318. August 23, 2018. Accessed December 27, 2018.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909-917.
- Montague L. Summary basis for regulatory action. October 5, 2018. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf. Accessed December 27, 2018.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645-1651.
- Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data [published correction appears in BMJ. 2013;346:F2942]. BMJ. 2013;346:F2032.
- Winer RL, Hughes JP, Feng Q, et al. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25-65 years. J Infect Dis. 2016;214:665-675.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
Don’t leave vaginal hysterectomies behind, surgeon urges
LAS VEGAS –
While “younger trainees are seeing fewer vaginal procedures being done and have less confidence to do the procedure,” research suggests that the vaginal approach can offer major benefits, compared with the alternatives, Rosanne M. Kho, MD, of the Cleveland Clinic, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Kho pointed to several studies suggesting a decline in vaginal hysterectomies as laparoscopic and robot procedures become more common. One study compared hysterectomy surgery approaches during 2007-2010 and found a sharp rise in robotic procedures (0.5% to 10%) and a big decrease in abdominal procedures (from 54% to 40%). The rate of laparoscopic procedures grew (from 24% to 30%), while vaginal procedures dipped slightly (22% to 20%) (JAMA. 2013 Feb 20;309[7]:689-98). Another study tracked hysterectomy strategies at Pittsburgh’s Magee-Womens Hospital in almost 14,000 women during 2000-2010. It found that vaginal hysterectomy rates fell from 22% to 17% while laparoscopic rates grew remarkably from 3% to 43%. Open procedures fell dramatically from 75% to 36% (Am J Obstet Gynecol. 2013 Apr. doi: 10.1016/j.ajog.2013.01.022).
These findings are “telling me that surgeons are steering away from the vaginal approach because the laparoscopic and robotic approach are much more appealing,” Dr. Koh said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Specifically, it appears that surgeons think the vaginal hysterectomy is more “challenging” and “cumbersome,” Dr. Kho said, and they lack inadequate training.
Why should vaginal hysterectomy still be considered? Dr. Kho pointed to two pieces of evidence:
- Expert opinion. A 2017 committee opinion from the American College of Obstetricians and Gynecologists examined routes of hysterectomy in benign disease and declared that, despite the decrease in its use, “evidence supports the opinion that [when feasible] vaginal hysterectomy is associated with better outcomes” than are laparoscopic or abdominal hysterectomy. Also, the decision to perform a salpingo-oophorectomy is not necessarily a contraindication to performing a vaginal hysterectomy, according to the committee opinion (Obstet Gynecol. 2017 Jun;129[6]:e155-e9).The opinion also says, “the vaginal approach is preferred among the minimally invasive approaches. Laparoscopic hysterectomy is a preferable alternative to open abdominal hysterectomy for those patients in whom a vaginal hysterectomy is not indicated or feasible. Although minimally invasive approaches to hysterectomy are the preferred route, open abdominal hysterectomy remains an important surgical option for some patients.”
- Randomized, controlled studies. A 2015 Cochrane Library systematic review examined 47 randomized, controlled trials and found that “vaginal hysterectomy should be performed whenever possible. Where vaginal hysterectomy is not possible, both a laparoscopic approach and abdominal hysterectomy have their pros and cons, and these should be incorporated in the decision-making process” (Cochrane Database Syst Rev. 2015 Aug 12. doi: 10.1002/14651858.CD003677.pub5).
What if a patient has an enlarged uterus? Dr. Kho coauthored a 2017 review that suggested that vaginal hysterectomy may be appropriate in this case. Her report found that in women with large uteri, “vaginal hysterectomy is preferred over laparoscopic and laparoscopic assistance with less operative time and hospital cost. In morbidly obese patients with large uteri, total laparoscopic hysterectomy is superior to vaginal hysterectomy with lesser odds of blood transfusion and lower length of hospital stay” (Clin Obstet Gynecol. 2017 Jun;60[2]:286-95).
What about the removal of fallopian tubes – salpingectomy – during vaginal hysterectomy? Dr. Kho highlighted a 2017 decision analysis that said these procedures are frequently performed for cancer prevention during laparoscopic and open hysterectomies “but [fallopian tubes] are not routinely removed during vaginal hysterectomy because of perceptions of increased morbidity, difficulty, or inadequate surgical training.”
The analysis, however, determined that “salpingectomy should routinely be performed with vaginal hysterectomy because it was the dominant and therefore cost-effective strategy. Complications are minimally increased, but the trade-off with cancer prevention is highly favorable.” (Am J Obstet Gynecol. 2017 Nov;217[5]:603.e1-603.e6).
Dr. Kho reported consulting for AbbVie, Olympus, and Applied Medical.
LAS VEGAS –
While “younger trainees are seeing fewer vaginal procedures being done and have less confidence to do the procedure,” research suggests that the vaginal approach can offer major benefits, compared with the alternatives, Rosanne M. Kho, MD, of the Cleveland Clinic, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Kho pointed to several studies suggesting a decline in vaginal hysterectomies as laparoscopic and robot procedures become more common. One study compared hysterectomy surgery approaches during 2007-2010 and found a sharp rise in robotic procedures (0.5% to 10%) and a big decrease in abdominal procedures (from 54% to 40%). The rate of laparoscopic procedures grew (from 24% to 30%), while vaginal procedures dipped slightly (22% to 20%) (JAMA. 2013 Feb 20;309[7]:689-98). Another study tracked hysterectomy strategies at Pittsburgh’s Magee-Womens Hospital in almost 14,000 women during 2000-2010. It found that vaginal hysterectomy rates fell from 22% to 17% while laparoscopic rates grew remarkably from 3% to 43%. Open procedures fell dramatically from 75% to 36% (Am J Obstet Gynecol. 2013 Apr. doi: 10.1016/j.ajog.2013.01.022).
These findings are “telling me that surgeons are steering away from the vaginal approach because the laparoscopic and robotic approach are much more appealing,” Dr. Koh said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Specifically, it appears that surgeons think the vaginal hysterectomy is more “challenging” and “cumbersome,” Dr. Kho said, and they lack inadequate training.
Why should vaginal hysterectomy still be considered? Dr. Kho pointed to two pieces of evidence:
- Expert opinion. A 2017 committee opinion from the American College of Obstetricians and Gynecologists examined routes of hysterectomy in benign disease and declared that, despite the decrease in its use, “evidence supports the opinion that [when feasible] vaginal hysterectomy is associated with better outcomes” than are laparoscopic or abdominal hysterectomy. Also, the decision to perform a salpingo-oophorectomy is not necessarily a contraindication to performing a vaginal hysterectomy, according to the committee opinion (Obstet Gynecol. 2017 Jun;129[6]:e155-e9).The opinion also says, “the vaginal approach is preferred among the minimally invasive approaches. Laparoscopic hysterectomy is a preferable alternative to open abdominal hysterectomy for those patients in whom a vaginal hysterectomy is not indicated or feasible. Although minimally invasive approaches to hysterectomy are the preferred route, open abdominal hysterectomy remains an important surgical option for some patients.”
- Randomized, controlled studies. A 2015 Cochrane Library systematic review examined 47 randomized, controlled trials and found that “vaginal hysterectomy should be performed whenever possible. Where vaginal hysterectomy is not possible, both a laparoscopic approach and abdominal hysterectomy have their pros and cons, and these should be incorporated in the decision-making process” (Cochrane Database Syst Rev. 2015 Aug 12. doi: 10.1002/14651858.CD003677.pub5).
What if a patient has an enlarged uterus? Dr. Kho coauthored a 2017 review that suggested that vaginal hysterectomy may be appropriate in this case. Her report found that in women with large uteri, “vaginal hysterectomy is preferred over laparoscopic and laparoscopic assistance with less operative time and hospital cost. In morbidly obese patients with large uteri, total laparoscopic hysterectomy is superior to vaginal hysterectomy with lesser odds of blood transfusion and lower length of hospital stay” (Clin Obstet Gynecol. 2017 Jun;60[2]:286-95).
What about the removal of fallopian tubes – salpingectomy – during vaginal hysterectomy? Dr. Kho highlighted a 2017 decision analysis that said these procedures are frequently performed for cancer prevention during laparoscopic and open hysterectomies “but [fallopian tubes] are not routinely removed during vaginal hysterectomy because of perceptions of increased morbidity, difficulty, or inadequate surgical training.”
The analysis, however, determined that “salpingectomy should routinely be performed with vaginal hysterectomy because it was the dominant and therefore cost-effective strategy. Complications are minimally increased, but the trade-off with cancer prevention is highly favorable.” (Am J Obstet Gynecol. 2017 Nov;217[5]:603.e1-603.e6).
Dr. Kho reported consulting for AbbVie, Olympus, and Applied Medical.
LAS VEGAS –
While “younger trainees are seeing fewer vaginal procedures being done and have less confidence to do the procedure,” research suggests that the vaginal approach can offer major benefits, compared with the alternatives, Rosanne M. Kho, MD, of the Cleveland Clinic, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Kho pointed to several studies suggesting a decline in vaginal hysterectomies as laparoscopic and robot procedures become more common. One study compared hysterectomy surgery approaches during 2007-2010 and found a sharp rise in robotic procedures (0.5% to 10%) and a big decrease in abdominal procedures (from 54% to 40%). The rate of laparoscopic procedures grew (from 24% to 30%), while vaginal procedures dipped slightly (22% to 20%) (JAMA. 2013 Feb 20;309[7]:689-98). Another study tracked hysterectomy strategies at Pittsburgh’s Magee-Womens Hospital in almost 14,000 women during 2000-2010. It found that vaginal hysterectomy rates fell from 22% to 17% while laparoscopic rates grew remarkably from 3% to 43%. Open procedures fell dramatically from 75% to 36% (Am J Obstet Gynecol. 2013 Apr. doi: 10.1016/j.ajog.2013.01.022).
These findings are “telling me that surgeons are steering away from the vaginal approach because the laparoscopic and robotic approach are much more appealing,” Dr. Koh said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Specifically, it appears that surgeons think the vaginal hysterectomy is more “challenging” and “cumbersome,” Dr. Kho said, and they lack inadequate training.
Why should vaginal hysterectomy still be considered? Dr. Kho pointed to two pieces of evidence:
- Expert opinion. A 2017 committee opinion from the American College of Obstetricians and Gynecologists examined routes of hysterectomy in benign disease and declared that, despite the decrease in its use, “evidence supports the opinion that [when feasible] vaginal hysterectomy is associated with better outcomes” than are laparoscopic or abdominal hysterectomy. Also, the decision to perform a salpingo-oophorectomy is not necessarily a contraindication to performing a vaginal hysterectomy, according to the committee opinion (Obstet Gynecol. 2017 Jun;129[6]:e155-e9).The opinion also says, “the vaginal approach is preferred among the minimally invasive approaches. Laparoscopic hysterectomy is a preferable alternative to open abdominal hysterectomy for those patients in whom a vaginal hysterectomy is not indicated or feasible. Although minimally invasive approaches to hysterectomy are the preferred route, open abdominal hysterectomy remains an important surgical option for some patients.”
- Randomized, controlled studies. A 2015 Cochrane Library systematic review examined 47 randomized, controlled trials and found that “vaginal hysterectomy should be performed whenever possible. Where vaginal hysterectomy is not possible, both a laparoscopic approach and abdominal hysterectomy have their pros and cons, and these should be incorporated in the decision-making process” (Cochrane Database Syst Rev. 2015 Aug 12. doi: 10.1002/14651858.CD003677.pub5).
What if a patient has an enlarged uterus? Dr. Kho coauthored a 2017 review that suggested that vaginal hysterectomy may be appropriate in this case. Her report found that in women with large uteri, “vaginal hysterectomy is preferred over laparoscopic and laparoscopic assistance with less operative time and hospital cost. In morbidly obese patients with large uteri, total laparoscopic hysterectomy is superior to vaginal hysterectomy with lesser odds of blood transfusion and lower length of hospital stay” (Clin Obstet Gynecol. 2017 Jun;60[2]:286-95).
What about the removal of fallopian tubes – salpingectomy – during vaginal hysterectomy? Dr. Kho highlighted a 2017 decision analysis that said these procedures are frequently performed for cancer prevention during laparoscopic and open hysterectomies “but [fallopian tubes] are not routinely removed during vaginal hysterectomy because of perceptions of increased morbidity, difficulty, or inadequate surgical training.”
The analysis, however, determined that “salpingectomy should routinely be performed with vaginal hysterectomy because it was the dominant and therefore cost-effective strategy. Complications are minimally increased, but the trade-off with cancer prevention is highly favorable.” (Am J Obstet Gynecol. 2017 Nov;217[5]:603.e1-603.e6).
Dr. Kho reported consulting for AbbVie, Olympus, and Applied Medical.
EXPERT ANALYSIS FROM PAGS










