User login
ERAS reduced opioid use, improved same-day discharge after gyn surgery
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
REPORTING FROM SGS 2018
Key clinical point: ERAS pathways improve same-day discharge rates and reduce opioid use in gynecologic surgery.
Major finding: Same-day discharge rates before and after ERAS were 25.9% and 91.7%, respectively.
Study details: A retrospective review of 258 patients; a study of 161 patients.
Disclosures: Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
Sources: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
Study: Preop EKGs have little utility for benign hysterectomy
ORLANDO – Preoperative electrocardiograms (EKGs) had no effect on management or perioperative complications in women undergoing benign hysterectomy over a 12-month period at a single medical center, according to a review of records.
Of 587 patients included in the review, 182 (31%) underwent EKG as part of their preoperative evaluation, and the majority of those were indicated according to institutional criteria (166; 28%) or National Institute for Health Care Excellence (NICE) guidelines (177; 30%), Nemi M. Shah, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“EKG was indicated in 91% of these patients according to institutional criteria, and in 97% of patients per NICE criteria,” said Dr. Shah, a third-year resident at the University of Texas Southwestern Medical Center, Dallas.
By institutional criteria, hypertension was the most common indication (68% of cases), and by NICE criteria, American Society of Anesthesia class 2 physical status was the most common indication (80% of cases), she noted, adding that obesity, which was present in 70% of patients, was the most common comorbidity classifying patients with American Society of Anesthesia class 2 or above.
Of the 182 EKGs performed, findings were abnormal in 89, but further workup was pursued in only 16 patients, and included repeat EKG, echocardiogram, and/or stress testing and cardiology consultation. Surgical delays of 1 and 4 months occurred in 2 patients as a result of the additional workup, and ultimately, all planned hysterectomies were completed by the primary surgical team without changes in management, she said.
Perioperative complications occurred in two patients, and included nonspecific postinduction EKG changes that led to surgery being aborted in one patient who had left ventricular hypertrophy on the preoperative EKG, and failed extubation in a patient with airway edema whose preoperative EKG showed a nonacute inferior infarct.
For the first, cardiology was consulted and determined the findings to be benign; the patient underwent hysterectomy at a later date without complications. The second patient was taken to the surgical intensive care unit for management, Dr. Shah said.
“Preoperative testing for benign hysterectomy is variable as there is no single standard of care,” she explained. “Though tests such as EKG are commonly ordered, there are no data linking study results to surgical outcomes.”
The current study was conducted to evaluate the rate of preoperative EKG performed in concordance with institutional and NICE guidelines, and to assess implications for management and perioperative complications.
Patients included in the review were adult women who underwent scheduled benign hysterectomy during 2016. Women who underwent emergency surgery or whose surgery was performed by gynecologic oncologists were excluded.
Subjects were primarily Hispanic, and had a mean age of 45 years, Dr. Shah noted.
Though limited by the single-center design and retrospective nature of the study, the findings suggest that preoperative EKG has little clinical utility.
“We found that practice patterns were highly concordant with institutional and NICE guidelines. However, EKG resulted in minimal impact on perioperative management, and no association between abnormal EKG and perioperative complications was found,” she said. “EKG may not accurately stratify perioperative cardiopulmonary risk, and alternative methods for preoperative evaluation should be considered.”
Dr. Shah reported having no disclosures.
SOURCE: Shah N et al. SGS 2018 Oral Poster 3.
ORLANDO – Preoperative electrocardiograms (EKGs) had no effect on management or perioperative complications in women undergoing benign hysterectomy over a 12-month period at a single medical center, according to a review of records.
Of 587 patients included in the review, 182 (31%) underwent EKG as part of their preoperative evaluation, and the majority of those were indicated according to institutional criteria (166; 28%) or National Institute for Health Care Excellence (NICE) guidelines (177; 30%), Nemi M. Shah, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“EKG was indicated in 91% of these patients according to institutional criteria, and in 97% of patients per NICE criteria,” said Dr. Shah, a third-year resident at the University of Texas Southwestern Medical Center, Dallas.
By institutional criteria, hypertension was the most common indication (68% of cases), and by NICE criteria, American Society of Anesthesia class 2 physical status was the most common indication (80% of cases), she noted, adding that obesity, which was present in 70% of patients, was the most common comorbidity classifying patients with American Society of Anesthesia class 2 or above.
Of the 182 EKGs performed, findings were abnormal in 89, but further workup was pursued in only 16 patients, and included repeat EKG, echocardiogram, and/or stress testing and cardiology consultation. Surgical delays of 1 and 4 months occurred in 2 patients as a result of the additional workup, and ultimately, all planned hysterectomies were completed by the primary surgical team without changes in management, she said.
Perioperative complications occurred in two patients, and included nonspecific postinduction EKG changes that led to surgery being aborted in one patient who had left ventricular hypertrophy on the preoperative EKG, and failed extubation in a patient with airway edema whose preoperative EKG showed a nonacute inferior infarct.
For the first, cardiology was consulted and determined the findings to be benign; the patient underwent hysterectomy at a later date without complications. The second patient was taken to the surgical intensive care unit for management, Dr. Shah said.
“Preoperative testing for benign hysterectomy is variable as there is no single standard of care,” she explained. “Though tests such as EKG are commonly ordered, there are no data linking study results to surgical outcomes.”
The current study was conducted to evaluate the rate of preoperative EKG performed in concordance with institutional and NICE guidelines, and to assess implications for management and perioperative complications.
Patients included in the review were adult women who underwent scheduled benign hysterectomy during 2016. Women who underwent emergency surgery or whose surgery was performed by gynecologic oncologists were excluded.
Subjects were primarily Hispanic, and had a mean age of 45 years, Dr. Shah noted.
Though limited by the single-center design and retrospective nature of the study, the findings suggest that preoperative EKG has little clinical utility.
“We found that practice patterns were highly concordant with institutional and NICE guidelines. However, EKG resulted in minimal impact on perioperative management, and no association between abnormal EKG and perioperative complications was found,” she said. “EKG may not accurately stratify perioperative cardiopulmonary risk, and alternative methods for preoperative evaluation should be considered.”
Dr. Shah reported having no disclosures.
SOURCE: Shah N et al. SGS 2018 Oral Poster 3.
ORLANDO – Preoperative electrocardiograms (EKGs) had no effect on management or perioperative complications in women undergoing benign hysterectomy over a 12-month period at a single medical center, according to a review of records.
Of 587 patients included in the review, 182 (31%) underwent EKG as part of their preoperative evaluation, and the majority of those were indicated according to institutional criteria (166; 28%) or National Institute for Health Care Excellence (NICE) guidelines (177; 30%), Nemi M. Shah, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
“EKG was indicated in 91% of these patients according to institutional criteria, and in 97% of patients per NICE criteria,” said Dr. Shah, a third-year resident at the University of Texas Southwestern Medical Center, Dallas.
By institutional criteria, hypertension was the most common indication (68% of cases), and by NICE criteria, American Society of Anesthesia class 2 physical status was the most common indication (80% of cases), she noted, adding that obesity, which was present in 70% of patients, was the most common comorbidity classifying patients with American Society of Anesthesia class 2 or above.
Of the 182 EKGs performed, findings were abnormal in 89, but further workup was pursued in only 16 patients, and included repeat EKG, echocardiogram, and/or stress testing and cardiology consultation. Surgical delays of 1 and 4 months occurred in 2 patients as a result of the additional workup, and ultimately, all planned hysterectomies were completed by the primary surgical team without changes in management, she said.
Perioperative complications occurred in two patients, and included nonspecific postinduction EKG changes that led to surgery being aborted in one patient who had left ventricular hypertrophy on the preoperative EKG, and failed extubation in a patient with airway edema whose preoperative EKG showed a nonacute inferior infarct.
For the first, cardiology was consulted and determined the findings to be benign; the patient underwent hysterectomy at a later date without complications. The second patient was taken to the surgical intensive care unit for management, Dr. Shah said.
“Preoperative testing for benign hysterectomy is variable as there is no single standard of care,” she explained. “Though tests such as EKG are commonly ordered, there are no data linking study results to surgical outcomes.”
The current study was conducted to evaluate the rate of preoperative EKG performed in concordance with institutional and NICE guidelines, and to assess implications for management and perioperative complications.
Patients included in the review were adult women who underwent scheduled benign hysterectomy during 2016. Women who underwent emergency surgery or whose surgery was performed by gynecologic oncologists were excluded.
Subjects were primarily Hispanic, and had a mean age of 45 years, Dr. Shah noted.
Though limited by the single-center design and retrospective nature of the study, the findings suggest that preoperative EKG has little clinical utility.
“We found that practice patterns were highly concordant with institutional and NICE guidelines. However, EKG resulted in minimal impact on perioperative management, and no association between abnormal EKG and perioperative complications was found,” she said. “EKG may not accurately stratify perioperative cardiopulmonary risk, and alternative methods for preoperative evaluation should be considered.”
Dr. Shah reported having no disclosures.
SOURCE: Shah N et al. SGS 2018 Oral Poster 3.
REPORTING FROM SGS 2018
Key clinical point: Preoperative EKG appears to have little utility in patients undergoing benign hysterectomy.
Major finding: Only 16 of 89 patients with abnormal preoperative EKG underwent further workup, and surgery was delayed in only two cases.
Study details: A retrospective review of 587 cases.
Disclosures: Dr. Shah reported having no disclosures.
Source: Shah N et al. SGS 2018 Oral Poster 3.
Residual single-site ovarian cancer surpasses multisite outcomes
NEW ORLEANS – When complete resection of advanced-stage, epithelial ovarian cancer is not possible, surgical resection that leaves a small volume of residual tumor at a single site produces significantly better outcomes than leaving minimal residual cancer at multiple sites, according to a review of 510 patients at two U.S. centers.
“In the past, we separated these patients based on whether they had a complete resection, R0 disease, or had 1 cm or less of residual disease” regardless of the number of sites with this small amount of residual tumor. The third category was patients with more than 1 cm of residual tumor at one or more sites, explained Dr. Manning-Geist in an interview. “What we did was break down the patients with 1 cm or less of residual tumor into those with one site or multiple sites. This is the first reported study to use number of sites” as a clinical characteristic for analysis in this context.
The message from the findings is that, while the goal of debulking surgery in patients with advanced epithelial ovarian cancer is complete tumor resection, if that can’t be achieved, the next goal is to leave residual tumor at just a single site, she concluded. A question that remains is whether primary debulking surgery is preferable to neoadjuvant treatment followed by interval debulking surgery. In the results Dr. Manning-Geist presented, patients who underwent primary debulking had better outcomes than those with neoadjuvant therapy followed by interval debulking, but these two subgroups also had different clinical characteristics.
The study used data from 510 patients with stage IIIC or IV epithelial ovarian cancer treated at either Brigham and Women’s or Massachusetts General Hospital during 2010-2015. The study cohort included 240 patients who underwent primary debulking surgery and 270 who first received neoadjuvant chemotherapy and then underwent interval debulking surgery. The patients who received neoadjuvant therapy were, on average, older (65 years vs. 63 years), had a higher prevalence of stage IV disease (44% vs. 16%), and had a higher prevalence of tumors with serous histology (93% vs. 77%), compared with patients who underwent primary debulking.
Complete tumor resections occurred in 39% of the primary debulking patients and in 64% of those who received neoadjuvant therapy; residual disease of 1 cm or less at one site occurred in 17% and 13%, respectively; minimal residual disease at multiple sites remained in 28% and 17% respectively; and the remaining patients had residual disease of more than 1 cm in at least one site, 16% and 6% respectively.
For this analysis, Dr. Manning-Geist and her associates considered residual disease at any of seven possible sites: diaphragm, upper abdomen, bowel mesentary, bowel serosa, abdominal peritoneum, pelvis, and nodal. Even if multiple individual metastases remained within one of these sites after surgery, it was categorized as a single site of residual disease.
Among patients who underwent primary debulking surgery, progression-free survival persisted for a median of 23 months among patients with full resection, 19 months in patients with a single site with minimal residual disease, 13 months among those with multiple sites of residual disease, and 10 months in patients with more than 1 cm of residual tumor. Median overall survival in these four subgroups was not yet reached, 64 months, 50 months, and 49 months, respectively.
Among patients who received neoadjuvant chemotherapy and then underwent interval debulking surgery, median durations of progression-free survival were 14 months, 12 months, 10 months, and 6 months, respectively. Median overall survival rates were 58 months, 37 months, 26 months, and 33 months, respectively. Within each of these four analyses, the differences in both survival and progression-free survival across the four subgroups was statistically significant, with a P less than .001 for each analysis.
In multivariate analyses, among patients who underwent primary debulking surgery, the significant linkages with worsening progression-free and overall survival were age, cancer stage, and amount and site number of residual disease. Among patients who received neoadjuvant chemotherapy followed by interval debulking residual disease diameter and site number of residual tumor was the only significant determinant for both progression-free and overall survival, Dr. Manning-Geist reported.
Dr. Manning-Geist had no disclosures.
SOURCE: Manning-Geist B et al. SGO 2018, Abstract 43.
NEW ORLEANS – When complete resection of advanced-stage, epithelial ovarian cancer is not possible, surgical resection that leaves a small volume of residual tumor at a single site produces significantly better outcomes than leaving minimal residual cancer at multiple sites, according to a review of 510 patients at two U.S. centers.
“In the past, we separated these patients based on whether they had a complete resection, R0 disease, or had 1 cm or less of residual disease” regardless of the number of sites with this small amount of residual tumor. The third category was patients with more than 1 cm of residual tumor at one or more sites, explained Dr. Manning-Geist in an interview. “What we did was break down the patients with 1 cm or less of residual tumor into those with one site or multiple sites. This is the first reported study to use number of sites” as a clinical characteristic for analysis in this context.
The message from the findings is that, while the goal of debulking surgery in patients with advanced epithelial ovarian cancer is complete tumor resection, if that can’t be achieved, the next goal is to leave residual tumor at just a single site, she concluded. A question that remains is whether primary debulking surgery is preferable to neoadjuvant treatment followed by interval debulking surgery. In the results Dr. Manning-Geist presented, patients who underwent primary debulking had better outcomes than those with neoadjuvant therapy followed by interval debulking, but these two subgroups also had different clinical characteristics.
The study used data from 510 patients with stage IIIC or IV epithelial ovarian cancer treated at either Brigham and Women’s or Massachusetts General Hospital during 2010-2015. The study cohort included 240 patients who underwent primary debulking surgery and 270 who first received neoadjuvant chemotherapy and then underwent interval debulking surgery. The patients who received neoadjuvant therapy were, on average, older (65 years vs. 63 years), had a higher prevalence of stage IV disease (44% vs. 16%), and had a higher prevalence of tumors with serous histology (93% vs. 77%), compared with patients who underwent primary debulking.
Complete tumor resections occurred in 39% of the primary debulking patients and in 64% of those who received neoadjuvant therapy; residual disease of 1 cm or less at one site occurred in 17% and 13%, respectively; minimal residual disease at multiple sites remained in 28% and 17% respectively; and the remaining patients had residual disease of more than 1 cm in at least one site, 16% and 6% respectively.
For this analysis, Dr. Manning-Geist and her associates considered residual disease at any of seven possible sites: diaphragm, upper abdomen, bowel mesentary, bowel serosa, abdominal peritoneum, pelvis, and nodal. Even if multiple individual metastases remained within one of these sites after surgery, it was categorized as a single site of residual disease.
Among patients who underwent primary debulking surgery, progression-free survival persisted for a median of 23 months among patients with full resection, 19 months in patients with a single site with minimal residual disease, 13 months among those with multiple sites of residual disease, and 10 months in patients with more than 1 cm of residual tumor. Median overall survival in these four subgroups was not yet reached, 64 months, 50 months, and 49 months, respectively.
Among patients who received neoadjuvant chemotherapy and then underwent interval debulking surgery, median durations of progression-free survival were 14 months, 12 months, 10 months, and 6 months, respectively. Median overall survival rates were 58 months, 37 months, 26 months, and 33 months, respectively. Within each of these four analyses, the differences in both survival and progression-free survival across the four subgroups was statistically significant, with a P less than .001 for each analysis.
In multivariate analyses, among patients who underwent primary debulking surgery, the significant linkages with worsening progression-free and overall survival were age, cancer stage, and amount and site number of residual disease. Among patients who received neoadjuvant chemotherapy followed by interval debulking residual disease diameter and site number of residual tumor was the only significant determinant for both progression-free and overall survival, Dr. Manning-Geist reported.
Dr. Manning-Geist had no disclosures.
SOURCE: Manning-Geist B et al. SGO 2018, Abstract 43.
NEW ORLEANS – When complete resection of advanced-stage, epithelial ovarian cancer is not possible, surgical resection that leaves a small volume of residual tumor at a single site produces significantly better outcomes than leaving minimal residual cancer at multiple sites, according to a review of 510 patients at two U.S. centers.
“In the past, we separated these patients based on whether they had a complete resection, R0 disease, or had 1 cm or less of residual disease” regardless of the number of sites with this small amount of residual tumor. The third category was patients with more than 1 cm of residual tumor at one or more sites, explained Dr. Manning-Geist in an interview. “What we did was break down the patients with 1 cm or less of residual tumor into those with one site or multiple sites. This is the first reported study to use number of sites” as a clinical characteristic for analysis in this context.
The message from the findings is that, while the goal of debulking surgery in patients with advanced epithelial ovarian cancer is complete tumor resection, if that can’t be achieved, the next goal is to leave residual tumor at just a single site, she concluded. A question that remains is whether primary debulking surgery is preferable to neoadjuvant treatment followed by interval debulking surgery. In the results Dr. Manning-Geist presented, patients who underwent primary debulking had better outcomes than those with neoadjuvant therapy followed by interval debulking, but these two subgroups also had different clinical characteristics.
The study used data from 510 patients with stage IIIC or IV epithelial ovarian cancer treated at either Brigham and Women’s or Massachusetts General Hospital during 2010-2015. The study cohort included 240 patients who underwent primary debulking surgery and 270 who first received neoadjuvant chemotherapy and then underwent interval debulking surgery. The patients who received neoadjuvant therapy were, on average, older (65 years vs. 63 years), had a higher prevalence of stage IV disease (44% vs. 16%), and had a higher prevalence of tumors with serous histology (93% vs. 77%), compared with patients who underwent primary debulking.
Complete tumor resections occurred in 39% of the primary debulking patients and in 64% of those who received neoadjuvant therapy; residual disease of 1 cm or less at one site occurred in 17% and 13%, respectively; minimal residual disease at multiple sites remained in 28% and 17% respectively; and the remaining patients had residual disease of more than 1 cm in at least one site, 16% and 6% respectively.
For this analysis, Dr. Manning-Geist and her associates considered residual disease at any of seven possible sites: diaphragm, upper abdomen, bowel mesentary, bowel serosa, abdominal peritoneum, pelvis, and nodal. Even if multiple individual metastases remained within one of these sites after surgery, it was categorized as a single site of residual disease.
Among patients who underwent primary debulking surgery, progression-free survival persisted for a median of 23 months among patients with full resection, 19 months in patients with a single site with minimal residual disease, 13 months among those with multiple sites of residual disease, and 10 months in patients with more than 1 cm of residual tumor. Median overall survival in these four subgroups was not yet reached, 64 months, 50 months, and 49 months, respectively.
Among patients who received neoadjuvant chemotherapy and then underwent interval debulking surgery, median durations of progression-free survival were 14 months, 12 months, 10 months, and 6 months, respectively. Median overall survival rates were 58 months, 37 months, 26 months, and 33 months, respectively. Within each of these four analyses, the differences in both survival and progression-free survival across the four subgroups was statistically significant, with a P less than .001 for each analysis.
In multivariate analyses, among patients who underwent primary debulking surgery, the significant linkages with worsening progression-free and overall survival were age, cancer stage, and amount and site number of residual disease. Among patients who received neoadjuvant chemotherapy followed by interval debulking residual disease diameter and site number of residual tumor was the only significant determinant for both progression-free and overall survival, Dr. Manning-Geist reported.
Dr. Manning-Geist had no disclosures.
SOURCE: Manning-Geist B et al. SGO 2018, Abstract 43.
REPORTING FROM SGO 2018
Key clinical point:
Major finding: Median overall survival after primary debulking was 64 months with single-site residual disease and 50 months with multisite disease.
Study details: Retrospective review of 510 patients from two U.S. centers.
Disclosures: Dr. Manning-Geist had no disclosures.
Source: Manning-Geist BL et al. SGO 2018, Abstract 43.
VIDEO: Interventions target opioid overprescribing after gynecologic surgery
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – U.S. clinicians prescribe opioid tablets to postsurgical patients too often and at too high a pill count, according to results from two independent studies that examined prescribing patterns and opioid use in patients following gynecologic surgery.
In addition, “setting preoperative expectations about pain management led to increased compliance at discharge,” said Dr. Mark, a gynecologic oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
Findings from the second study, of 122 women who underwent gynecologic surgery at Women and Infants Hospital in Providence, R.I., showed that 32% did not use any opioids for pain following hospital discharge, and that opioid use during hospitalization was a significant predictor of postdischarge opioid needs. This finding provided a way to devise a new prescribing guide for postsurgical patients based on their opioid use while hospitalized, said Erica Weston, MD, a gynecologic oncologist at Johns Hopkins University, Baltimore.

“No question, we are overprescribing,” Dr. Dowdy said, and described a program he and his colleagues at Mayo recently put in place that capped routine opioid pill prescriptions following various surgeries based on historic patient needs. For example, most laparotomy patients receive a prescription for 10 opioid doses on discharge. Based on the first 6 months of this program, it’s on track to cut the annual number of opioid tablets prescribed to postsurgical patients at Mayo by 35,000 for all gynecologic surgeries and by 1.5 million tablets for all Mayo surgical subspecialties, he said.
The study reported by Dr. Mark ran after the Roswell Park gynecologic oncology department implemented new guidelines for dispensing pain control medications following surgery. The guidelines called for comprehensive teaching for patients about pain expectations and pain management both before and after surgery and also established four dispensing categories:
- Patients undergoing minimally invasive or outpatient surgery and with no history of chronic pain and low opioid need while hospitalized received the default dispense of 600 mg ibuprofen every 6 hours as needed for 7 days and 500 mg acetaminophen every 6 hours as needed for 7 days.
- Patients who underwent this surgery but required 5 or more opioid tablets while hospitalized or those with a history of chronic pain and opioid use received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply with 1 tablet taken every 6 hours as needed.
- Patients who underwent laparotomy and had no chronic pain and opioid history and low opioid use while hospitalized received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply.
- Patients who underwent laparotomy and showed a higher opioid need based on their use during the 24 hours before discharge received the ibuprofen and acetaminophen regimen plus 24 opioid tablets for 3 days so they could take 2 tablets every 6 hours as needed.
Dr. Mark and his associates collected data from 337 patients managed with these guidelines during June 2017–January 2018 and compared them with 626 patients who underwent gynecologic surgery at Roswell Park during July 2016–June 2017. The data showed the average number of opioid tablets dispensed per patient for all discharges fell from 31.7 before the new guideline to 3.5, an 89% reduction. For the subgroup of patients who had undergone a laparotomy, the average pill number fell from 43.6 to 11.6, a 72% drop. Among patients treated with minimally invasive or outpatient surgery, average tablets dispensed fell from 28.1 before to 0.9 after, a 97% reduction. The reduction among opioid-naive patients was 90%, and it was 83% among patients who used opioids prior to their surgery.
Under the new program, patients requested an opioid refill 14% of the time after laparotomy and 8% of the time after minimally invasive surgery, rates that did not significantly differ from the prior era. Average postoperative pain scores were identical among patients treated under the new dispensing guidelines and those treated during the prior years, and 96% of patients said they were satisfied with the care they received during the new, restricted dispensing period, Dr. Mark reported.
The single-center experience reviewed by Dr. Weston tracked opioid use by 122 women who underwent a minimally invasive hysterectomy at Women and Infants both as inpatients and out to both 1-2 weeks and 4-6 weeks following discharge. The patients were an average age of 61 years, and included 16% who reported chronic pain and 5% with a history of chronic opioid use.
During the inpatient phase, median opioid use was three doses, with 25% of the patients using no opioids. During the first 1-2 weeks following discharge, median opioid use was nine tablets, with 37% of the patients using no opioids. By the 4- to 6-week follow-up (which collected data from 114 of the patients), median opioid use was a cumulative 11 tablets with 67% of the patients reporting no opioid use during the time between their first and second follow-up visit. During the total postdischarge period, 90% of the patients used 30 or fewer opioid tablets.
A multivariate analysis of the findings showed that opioid use while in hospital was the only significant predictor of opioid use after discharge. Age of 65 years or older showed a nonsignificant trend toward less postdischarge opioid use.
Based on these data Dr. Weston and her associates proposed a formula for estimating a patient’s opioid needs at discharge: Gynecologic surgery patients who needed no opioid medication as inpatients could receive 1-5 opioid tablets at discharge, patients who used opioids at or below the median level should receive 10-15 tablets at discharge, and those who used more than the median number of opioid tablets as inpatients should receive 25-30 tablets at discharge. For patients who undergo surgery as outpatients and have no record of pain medication needs, Dr. Weston recommended discharging them with a prescription for 25-30 tablets, possibly reducing this to 10-15 tablets for patients aged 65 years or older.
Dr. Mark, Dr. Weston, and Dr. Dowdy had no disclosures.
SOURCE: Mark J et al. SGO 2018, Abstract 7. Weston E et al. SGO 2018, Abstract 8.
The important and thought provoking reports by Dr. Mark and by Dr. Weston and their associates present innovative ways for clinicians to address the opioid crisis by prescribing fewer narcotics to postsurgical patients when they leave the hospital. Their work suggests that doing this can have little or no negative impact on patient satisfaction with their care. Their findings give us important documentation for prescribing fewer opioid tablets, while still giving patients adequate pain relief.

Their findings also provide clear documentation that, in current practice, without guidance like this opioids are often overprescribed, not out of negligence but because clinicians are simply not sure what a patient will need once they go home following gynecologic surgery. By reviewing the pain medication a patient needed in hospital we can better estimate what patients will need when they go home, and we can better avoid giving patients more opioid tablets than they will really need.
Brent Smith, MD , is a gynecologic oncologist at Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The important and thought provoking reports by Dr. Mark and by Dr. Weston and their associates present innovative ways for clinicians to address the opioid crisis by prescribing fewer narcotics to postsurgical patients when they leave the hospital. Their work suggests that doing this can have little or no negative impact on patient satisfaction with their care. Their findings give us important documentation for prescribing fewer opioid tablets, while still giving patients adequate pain relief.

Their findings also provide clear documentation that, in current practice, without guidance like this opioids are often overprescribed, not out of negligence but because clinicians are simply not sure what a patient will need once they go home following gynecologic surgery. By reviewing the pain medication a patient needed in hospital we can better estimate what patients will need when they go home, and we can better avoid giving patients more opioid tablets than they will really need.
Brent Smith, MD , is a gynecologic oncologist at Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The important and thought provoking reports by Dr. Mark and by Dr. Weston and their associates present innovative ways for clinicians to address the opioid crisis by prescribing fewer narcotics to postsurgical patients when they leave the hospital. Their work suggests that doing this can have little or no negative impact on patient satisfaction with their care. Their findings give us important documentation for prescribing fewer opioid tablets, while still giving patients adequate pain relief.

Their findings also provide clear documentation that, in current practice, without guidance like this opioids are often overprescribed, not out of negligence but because clinicians are simply not sure what a patient will need once they go home following gynecologic surgery. By reviewing the pain medication a patient needed in hospital we can better estimate what patients will need when they go home, and we can better avoid giving patients more opioid tablets than they will really need.
Brent Smith, MD , is a gynecologic oncologist at Ohio State University, Columbus. He had no disclosures. Dr. Smith made these comments in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – U.S. clinicians prescribe opioid tablets to postsurgical patients too often and at too high a pill count, according to results from two independent studies that examined prescribing patterns and opioid use in patients following gynecologic surgery.
In addition, “setting preoperative expectations about pain management led to increased compliance at discharge,” said Dr. Mark, a gynecologic oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
Findings from the second study, of 122 women who underwent gynecologic surgery at Women and Infants Hospital in Providence, R.I., showed that 32% did not use any opioids for pain following hospital discharge, and that opioid use during hospitalization was a significant predictor of postdischarge opioid needs. This finding provided a way to devise a new prescribing guide for postsurgical patients based on their opioid use while hospitalized, said Erica Weston, MD, a gynecologic oncologist at Johns Hopkins University, Baltimore.

“No question, we are overprescribing,” Dr. Dowdy said, and described a program he and his colleagues at Mayo recently put in place that capped routine opioid pill prescriptions following various surgeries based on historic patient needs. For example, most laparotomy patients receive a prescription for 10 opioid doses on discharge. Based on the first 6 months of this program, it’s on track to cut the annual number of opioid tablets prescribed to postsurgical patients at Mayo by 35,000 for all gynecologic surgeries and by 1.5 million tablets for all Mayo surgical subspecialties, he said.
The study reported by Dr. Mark ran after the Roswell Park gynecologic oncology department implemented new guidelines for dispensing pain control medications following surgery. The guidelines called for comprehensive teaching for patients about pain expectations and pain management both before and after surgery and also established four dispensing categories:
- Patients undergoing minimally invasive or outpatient surgery and with no history of chronic pain and low opioid need while hospitalized received the default dispense of 600 mg ibuprofen every 6 hours as needed for 7 days and 500 mg acetaminophen every 6 hours as needed for 7 days.
- Patients who underwent this surgery but required 5 or more opioid tablets while hospitalized or those with a history of chronic pain and opioid use received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply with 1 tablet taken every 6 hours as needed.
- Patients who underwent laparotomy and had no chronic pain and opioid history and low opioid use while hospitalized received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply.
- Patients who underwent laparotomy and showed a higher opioid need based on their use during the 24 hours before discharge received the ibuprofen and acetaminophen regimen plus 24 opioid tablets for 3 days so they could take 2 tablets every 6 hours as needed.
Dr. Mark and his associates collected data from 337 patients managed with these guidelines during June 2017–January 2018 and compared them with 626 patients who underwent gynecologic surgery at Roswell Park during July 2016–June 2017. The data showed the average number of opioid tablets dispensed per patient for all discharges fell from 31.7 before the new guideline to 3.5, an 89% reduction. For the subgroup of patients who had undergone a laparotomy, the average pill number fell from 43.6 to 11.6, a 72% drop. Among patients treated with minimally invasive or outpatient surgery, average tablets dispensed fell from 28.1 before to 0.9 after, a 97% reduction. The reduction among opioid-naive patients was 90%, and it was 83% among patients who used opioids prior to their surgery.
Under the new program, patients requested an opioid refill 14% of the time after laparotomy and 8% of the time after minimally invasive surgery, rates that did not significantly differ from the prior era. Average postoperative pain scores were identical among patients treated under the new dispensing guidelines and those treated during the prior years, and 96% of patients said they were satisfied with the care they received during the new, restricted dispensing period, Dr. Mark reported.
The single-center experience reviewed by Dr. Weston tracked opioid use by 122 women who underwent a minimally invasive hysterectomy at Women and Infants both as inpatients and out to both 1-2 weeks and 4-6 weeks following discharge. The patients were an average age of 61 years, and included 16% who reported chronic pain and 5% with a history of chronic opioid use.
During the inpatient phase, median opioid use was three doses, with 25% of the patients using no opioids. During the first 1-2 weeks following discharge, median opioid use was nine tablets, with 37% of the patients using no opioids. By the 4- to 6-week follow-up (which collected data from 114 of the patients), median opioid use was a cumulative 11 tablets with 67% of the patients reporting no opioid use during the time between their first and second follow-up visit. During the total postdischarge period, 90% of the patients used 30 or fewer opioid tablets.
A multivariate analysis of the findings showed that opioid use while in hospital was the only significant predictor of opioid use after discharge. Age of 65 years or older showed a nonsignificant trend toward less postdischarge opioid use.
Based on these data Dr. Weston and her associates proposed a formula for estimating a patient’s opioid needs at discharge: Gynecologic surgery patients who needed no opioid medication as inpatients could receive 1-5 opioid tablets at discharge, patients who used opioids at or below the median level should receive 10-15 tablets at discharge, and those who used more than the median number of opioid tablets as inpatients should receive 25-30 tablets at discharge. For patients who undergo surgery as outpatients and have no record of pain medication needs, Dr. Weston recommended discharging them with a prescription for 25-30 tablets, possibly reducing this to 10-15 tablets for patients aged 65 years or older.
Dr. Mark, Dr. Weston, and Dr. Dowdy had no disclosures.
SOURCE: Mark J et al. SGO 2018, Abstract 7. Weston E et al. SGO 2018, Abstract 8.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – U.S. clinicians prescribe opioid tablets to postsurgical patients too often and at too high a pill count, according to results from two independent studies that examined prescribing patterns and opioid use in patients following gynecologic surgery.
In addition, “setting preoperative expectations about pain management led to increased compliance at discharge,” said Dr. Mark, a gynecologic oncologist at Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
Findings from the second study, of 122 women who underwent gynecologic surgery at Women and Infants Hospital in Providence, R.I., showed that 32% did not use any opioids for pain following hospital discharge, and that opioid use during hospitalization was a significant predictor of postdischarge opioid needs. This finding provided a way to devise a new prescribing guide for postsurgical patients based on their opioid use while hospitalized, said Erica Weston, MD, a gynecologic oncologist at Johns Hopkins University, Baltimore.

“No question, we are overprescribing,” Dr. Dowdy said, and described a program he and his colleagues at Mayo recently put in place that capped routine opioid pill prescriptions following various surgeries based on historic patient needs. For example, most laparotomy patients receive a prescription for 10 opioid doses on discharge. Based on the first 6 months of this program, it’s on track to cut the annual number of opioid tablets prescribed to postsurgical patients at Mayo by 35,000 for all gynecologic surgeries and by 1.5 million tablets for all Mayo surgical subspecialties, he said.
The study reported by Dr. Mark ran after the Roswell Park gynecologic oncology department implemented new guidelines for dispensing pain control medications following surgery. The guidelines called for comprehensive teaching for patients about pain expectations and pain management both before and after surgery and also established four dispensing categories:
- Patients undergoing minimally invasive or outpatient surgery and with no history of chronic pain and low opioid need while hospitalized received the default dispense of 600 mg ibuprofen every 6 hours as needed for 7 days and 500 mg acetaminophen every 6 hours as needed for 7 days.
- Patients who underwent this surgery but required 5 or more opioid tablets while hospitalized or those with a history of chronic pain and opioid use received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply with 1 tablet taken every 6 hours as needed.
- Patients who underwent laparotomy and had no chronic pain and opioid history and low opioid use while hospitalized received the ibuprofen and acetaminophen regimen plus 12 opioid tablets, a 3-day supply.
- Patients who underwent laparotomy and showed a higher opioid need based on their use during the 24 hours before discharge received the ibuprofen and acetaminophen regimen plus 24 opioid tablets for 3 days so they could take 2 tablets every 6 hours as needed.
Dr. Mark and his associates collected data from 337 patients managed with these guidelines during June 2017–January 2018 and compared them with 626 patients who underwent gynecologic surgery at Roswell Park during July 2016–June 2017. The data showed the average number of opioid tablets dispensed per patient for all discharges fell from 31.7 before the new guideline to 3.5, an 89% reduction. For the subgroup of patients who had undergone a laparotomy, the average pill number fell from 43.6 to 11.6, a 72% drop. Among patients treated with minimally invasive or outpatient surgery, average tablets dispensed fell from 28.1 before to 0.9 after, a 97% reduction. The reduction among opioid-naive patients was 90%, and it was 83% among patients who used opioids prior to their surgery.
Under the new program, patients requested an opioid refill 14% of the time after laparotomy and 8% of the time after minimally invasive surgery, rates that did not significantly differ from the prior era. Average postoperative pain scores were identical among patients treated under the new dispensing guidelines and those treated during the prior years, and 96% of patients said they were satisfied with the care they received during the new, restricted dispensing period, Dr. Mark reported.
The single-center experience reviewed by Dr. Weston tracked opioid use by 122 women who underwent a minimally invasive hysterectomy at Women and Infants both as inpatients and out to both 1-2 weeks and 4-6 weeks following discharge. The patients were an average age of 61 years, and included 16% who reported chronic pain and 5% with a history of chronic opioid use.
During the inpatient phase, median opioid use was three doses, with 25% of the patients using no opioids. During the first 1-2 weeks following discharge, median opioid use was nine tablets, with 37% of the patients using no opioids. By the 4- to 6-week follow-up (which collected data from 114 of the patients), median opioid use was a cumulative 11 tablets with 67% of the patients reporting no opioid use during the time between their first and second follow-up visit. During the total postdischarge period, 90% of the patients used 30 or fewer opioid tablets.
A multivariate analysis of the findings showed that opioid use while in hospital was the only significant predictor of opioid use after discharge. Age of 65 years or older showed a nonsignificant trend toward less postdischarge opioid use.
Based on these data Dr. Weston and her associates proposed a formula for estimating a patient’s opioid needs at discharge: Gynecologic surgery patients who needed no opioid medication as inpatients could receive 1-5 opioid tablets at discharge, patients who used opioids at or below the median level should receive 10-15 tablets at discharge, and those who used more than the median number of opioid tablets as inpatients should receive 25-30 tablets at discharge. For patients who undergo surgery as outpatients and have no record of pain medication needs, Dr. Weston recommended discharging them with a prescription for 25-30 tablets, possibly reducing this to 10-15 tablets for patients aged 65 years or older.
Dr. Mark, Dr. Weston, and Dr. Dowdy had no disclosures.
SOURCE: Mark J et al. SGO 2018, Abstract 7. Weston E et al. SGO 2018, Abstract 8.
REPORTING FROM SGO 2018
Key clinical point: Patients who underwent gynecologic surgery received adequate pain relief when receiving fewer opioid tablets.
Major finding: A protocol that restricted opioid dispensing successfully cut the discharge allotment of opioid tablets by 89%.
Study details: A single-center review of 337 patients, and a second single-center experience with 122 patients.
Disclosures: Dr. Mark, Dr. Weston, and Dr. Dowdy had no disclosures.
Source: Mark J et al. SGO 2018, Abstract 7. Weston E et al. SGO 2018, Abstract 8.
VIDEO: Cervical cancer laparotomy outperforms minimally invasive surgery
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
REPORTING FROM SGO 2018
Key clinical point: Laparotomy produced better survival than did minimally invasive surgery for cervical cancer.
Major finding: Disease-free survival after 4.5 years was 96.5% with laparotomy and 86.0% with minimally invasive surgery.
Study details: LACC was a multicenter, randomized trial with 631 patients. The observational study included 2,221 patients from the National Cancer Database during 2010-2012.
Disclosures: Dr. Ramirez and Dr. Rauh-Hain had no disclosures.
Source: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
Same-day discharge for hysterectomy
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
There is an increased focus on reducing the costs of health care delivery, and one major driver of surgical cost is length of hospitalization. A minimally invasive surgical approach to hysterectomy is a strategy that significantly enhances recovery and shortens hospital stay, although many patients who can safely be considered for same-day discharge (SDD), including many with cancer, are still admitted to the hospital overnight. Much has been published on the predictors and pathways for successful same-day discharge after minimally invasive hysterectomy, and in this column we will review how to best predict who is a good candidate for SDD and how to optimize the success of this approach with respect to safety and patient satisfaction.
What are the benefits to SDD?
Certainly, decreased hospitalization costs are an attractive feature of SDD following hysterectomy, although surgeons should also be mindful that patient-centered outcomes, such as pain control, managing nausea, and patient satisfaction, also are considered with equal emphasis. Several studies have shown that, in appropriate candidates and when proactive pathways are used, patient satisfaction is preserved with SDD following hysterectomy.1
Choosing patient candidates
Same day discharge is most successfully accomplished in patients of good general baseline health.2 Diabetic patients, particularly those on insulin, are generally not good candidates for SDD because it is important to monitor and intervene in blood glucose changes that are influenced by a nothing-by-mouth status and surgical stress. We recommend observing patients overnight with a history of pulmonary disease who may have transient increased postoperative O2 needs. Similarly, patients with significant cardiac disease (including heart failure and coronary disease) may benefit from prolonged overnight observation.
Particular caution should be paid to patients with obstructive sleep apnea, which may be occult but anticipated in patients with very high body mass indexes (greater than 40 kg/m2). General anesthetic drugs, the trauma of intubation, and opioids all couple with the underlying airway compromise such that these patients are at risk for postoperative apnea, which, in severe cases, can result in anoxia and death. These patients should be considered for continuous pulse-ox monitoring for at least 12-24 hours postoperatively and are not good candidates for same-day discharge.
Patients who have baseline anticoagulation that has been stopped or bridged preoperatively should have prolonged observation with recheck of their postoperative hemoglobin prior to discharge.
Patients who live alone or are very elderly with baseline frailty are poor candidates for SDD and may benefit from nursing observation overnight while they metabolize their anesthesia. Patients who have chronic opioid dependency present a greater challenge to control postoperative pain; these patients are generally less good candidates for SDD.
Studies have shown that the indication for the procedure (for example, cancer with staging, fibroids, endometriosis) is less critical in determining who is a good candidate for SDD.3 However, successful SDD rates are highest in more straightforward cases with few or no prior surgeries, small uteri (less than 14 weeks), a surgical duration of less than 3 hours, and a surgical start time before 2 p.m. Longer, more complex cases are typically associated with more blood loss, higher risk for occult complications, and more time under anesthesia (and in Trendelenburg), which can exacerbate airway edema. In preparation for such cases, it might be wise to prepare patients for the possibility that they may not be good candidates for discharge on the same day. In general, most SDD pathways exclude patients with very high BMI (greater than 50 kg/m2) because of concern for airway patency and because these cases may be more complex with higher underlying risk. In addition, many of these patients have diabetes and require perioperative metabolic interventions.
Patient preparation
A key component to successful SDD is setting patient expectations. Patients should be informed at their preoperative visit that, unless there is an unexpected occurrence or response to the surgery, they will be discharged to home the same day. This allows them to prepare their home (including transportation needs) in advance. They should be provided with information about what to expect that first night after surgery (including potential residual drowsiness or nausea from anesthesia and immediate postoperative pain).
On the day of surgery, under the influence of anesthesia and pain medication, patients will have difficulty retaining complex discharge instructions. The preoperative visit is critically important because it’s the best time to provide them with this information, including postoperative activity limitations, wound and dressing care, and follow-up instructions. This is also the best time to provide prescriptions for postoperative pain, nausea, and constipation prophylaxis with detailed instructions about best use. Patients should be encouraged to fill these prescriptions preoperatively so that they have these medications on hand on the evening of their discharge.
Many programs utilize a combination of educational strategies (in person, written, video) to maximize the likelihood of retention.1 It is also important to offer an opportunity for patients to ask questions about this information after they have received it (for example, by phoning the patients prior to their procedure).
Preoperative strategies

Intraoperative strategies
Consider in-and-out catheterization rather than placement of an indwelling catheter for anticipated short cases without complex bladder dissection.5 Minimize blood loss and maximally evacuate blood and clots with suction because hemoperitoneum can induce nausea and pain.
Pain from retained gas under the diaphragm can be reduced by bathing the diaphragms with 400 cc of dilute local anesthetic made by mixing 50 mL of 0.5% bupivacaine in 1000 mL normal saline prior to removal of pneumoperitoneum and while still in Trendelenburg. Ensure there is minimal retained intraperitoneal CO2 at the completion of the surgery by asking the anesthesiologists to perform positive pressure ventilations prior to fascial closure. Consider injecting port sites (including the peritoneal and fascial layers) with a mixture of immediate and long-acting local anesthetics. Request that the anesthesia staff administer intraoperative doses of IV ketorolac, acetaminophen, and tramadol (in preference to opioids) and an aggressive perioperative cocktail of antiemetics.
Management in the recovery room
Surgeons should ensure that recovery room staff are well versed in the pathway for patients who are selected for SDD to ensure proactive implementation of analgesic and antiemetic regimens and to fast-track the various tasks and education required for discharge.5
Patients should be started on their home postoperative medication regimen in the recovery room, including an anti-inflammatory such as diclofenac, sublingual tramadol (in preference to an opioid, such as hydrocodone), docusate, and sennosides. IV opioids should be avoided because they can result in somnolence and nausea.
If placed intraoperatively, the Foley catheter should be removed early to allow adequate time to void. Backfilling the bladder prior to removal can hasten the urge to void and help objectively document completeness of evacuation. All patients should be seen by the anesthesiologist and/or surgeon prior to discharge.
For patients who are discharged same day, a follow-up phone call on postoperative day 1 is valuable to ensure that they have continued their successful postoperative transition to the home and to intervene early if there are concerns for patient satisfaction.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Fountain CR et al. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol. 2017 Sep-Oct;24(6):932-9.
2. Rivard C et al. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol. 2015 Feb;22(2):219-26.
3. Lee SJ et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014 Jun;133(3):552-5.
4. Elia N et al. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296-304.
5. Donnez O et al. Low pain score after total laparoscopic hysterectomy and same-day discharge within less than 5 hours: Results of a prospective observational study. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1293-9.
High MIH case volume may up risk for adverse events in women with large uteri
ORLANDO – High case volume for surgeons performing minimally invasive hysterectomies in women with large uteri is associated with an increased rate of perioperative adverse events – but also with a decreased rate of conversion to laparotomy – according to a review of 763 procedures.
The minimally invasive hysterectomy (MIH) procedures in the study were performed by 66 surgeons and included 416 total laparoscopic hysterectomies, 196 robotic-assisted laparoscopic hysterectomies, 90 total vaginal hysterectomies, and 61 laparoscopic-assisted vaginal hysterectomies, Carol E. Bretschneider, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
The mean monthly case volume was 16.4 and mean MIH volume was 23, said Dr. Bretschneider, a fellow at the Cleveland Clinic.
“The rate of postoperative adverse events was 17.8%, the rate of intraoperative adverse events was 4.2%, and the rate of conversion from a minimally invasive approach to a laparotomy was 5.5%,” she said, explaining that adverse events were defined as those greater than grade 2 on the Clavien-Dindo classification scale. “No differences were appreciated across routes [of MIH] in terms of perioperative adverse events or conversion to laparotomy,” she noted.
Even after investigators controlled for age, body mass index, uterine weight, history of laparotomy, and parity, as well as surgeon volume and operative time, they found that higher monthly MIH volume, estimated blood loss, and operative time remained significantly associated with both intraoperative adverse events (adjusted odds ratios, 1.9, 2.0, and 22.1, respectively) and postoperative adverse events (aOR, 1.3, 1.4, and 1.9, respectively), she said.
Higher BMI was associated with a lower incidence of intraoperative complications (aOR, 0.1).
“As for conversion to laparotomy, increasing surgeon volume was associated with a lower incidence of conversion (aOR, 0.4), but higher estimated blood loss and uterine weight were associated with a higher incidence of conversion (aOR, 2.6 and 7.1, respectively).”
Study subjects were women with a mean age of 47 years and mean BMI of 31 kg/m2 who underwent MIH during January 2014–June 2016 at a tertiary care referral center. Median uterine weight was 409 g, and indications for hysterectomy included fibroids, pelvic pain, abnormal uterine bleeding, and prolapse. Patients with malignancy were excluded.
“In the context of high-complexity cases, such as those for large uteri, many factors beyond surgical volume influence perioperative adverse events,” Dr. Bretschneider said, concluding that, to improve patient safety and outcomes, additional studies should be performed to explore the relationship between surgeon experience and patient factors on perioperative outcomes in the setting of common gynecologic procedures.
Dr. Bretschneider reported having no relevant disclosures.
SOURCE: Bretschneider C et al. SGS 2018, Oral Poster 12.
ORLANDO – High case volume for surgeons performing minimally invasive hysterectomies in women with large uteri is associated with an increased rate of perioperative adverse events – but also with a decreased rate of conversion to laparotomy – according to a review of 763 procedures.
The minimally invasive hysterectomy (MIH) procedures in the study were performed by 66 surgeons and included 416 total laparoscopic hysterectomies, 196 robotic-assisted laparoscopic hysterectomies, 90 total vaginal hysterectomies, and 61 laparoscopic-assisted vaginal hysterectomies, Carol E. Bretschneider, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
The mean monthly case volume was 16.4 and mean MIH volume was 23, said Dr. Bretschneider, a fellow at the Cleveland Clinic.
“The rate of postoperative adverse events was 17.8%, the rate of intraoperative adverse events was 4.2%, and the rate of conversion from a minimally invasive approach to a laparotomy was 5.5%,” she said, explaining that adverse events were defined as those greater than grade 2 on the Clavien-Dindo classification scale. “No differences were appreciated across routes [of MIH] in terms of perioperative adverse events or conversion to laparotomy,” she noted.
Even after investigators controlled for age, body mass index, uterine weight, history of laparotomy, and parity, as well as surgeon volume and operative time, they found that higher monthly MIH volume, estimated blood loss, and operative time remained significantly associated with both intraoperative adverse events (adjusted odds ratios, 1.9, 2.0, and 22.1, respectively) and postoperative adverse events (aOR, 1.3, 1.4, and 1.9, respectively), she said.
Higher BMI was associated with a lower incidence of intraoperative complications (aOR, 0.1).
“As for conversion to laparotomy, increasing surgeon volume was associated with a lower incidence of conversion (aOR, 0.4), but higher estimated blood loss and uterine weight were associated with a higher incidence of conversion (aOR, 2.6 and 7.1, respectively).”
Study subjects were women with a mean age of 47 years and mean BMI of 31 kg/m2 who underwent MIH during January 2014–June 2016 at a tertiary care referral center. Median uterine weight was 409 g, and indications for hysterectomy included fibroids, pelvic pain, abnormal uterine bleeding, and prolapse. Patients with malignancy were excluded.
“In the context of high-complexity cases, such as those for large uteri, many factors beyond surgical volume influence perioperative adverse events,” Dr. Bretschneider said, concluding that, to improve patient safety and outcomes, additional studies should be performed to explore the relationship between surgeon experience and patient factors on perioperative outcomes in the setting of common gynecologic procedures.
Dr. Bretschneider reported having no relevant disclosures.
SOURCE: Bretschneider C et al. SGS 2018, Oral Poster 12.
ORLANDO – High case volume for surgeons performing minimally invasive hysterectomies in women with large uteri is associated with an increased rate of perioperative adverse events – but also with a decreased rate of conversion to laparotomy – according to a review of 763 procedures.
The minimally invasive hysterectomy (MIH) procedures in the study were performed by 66 surgeons and included 416 total laparoscopic hysterectomies, 196 robotic-assisted laparoscopic hysterectomies, 90 total vaginal hysterectomies, and 61 laparoscopic-assisted vaginal hysterectomies, Carol E. Bretschneider, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
The mean monthly case volume was 16.4 and mean MIH volume was 23, said Dr. Bretschneider, a fellow at the Cleveland Clinic.
“The rate of postoperative adverse events was 17.8%, the rate of intraoperative adverse events was 4.2%, and the rate of conversion from a minimally invasive approach to a laparotomy was 5.5%,” she said, explaining that adverse events were defined as those greater than grade 2 on the Clavien-Dindo classification scale. “No differences were appreciated across routes [of MIH] in terms of perioperative adverse events or conversion to laparotomy,” she noted.
Even after investigators controlled for age, body mass index, uterine weight, history of laparotomy, and parity, as well as surgeon volume and operative time, they found that higher monthly MIH volume, estimated blood loss, and operative time remained significantly associated with both intraoperative adverse events (adjusted odds ratios, 1.9, 2.0, and 22.1, respectively) and postoperative adverse events (aOR, 1.3, 1.4, and 1.9, respectively), she said.
Higher BMI was associated with a lower incidence of intraoperative complications (aOR, 0.1).
“As for conversion to laparotomy, increasing surgeon volume was associated with a lower incidence of conversion (aOR, 0.4), but higher estimated blood loss and uterine weight were associated with a higher incidence of conversion (aOR, 2.6 and 7.1, respectively).”
Study subjects were women with a mean age of 47 years and mean BMI of 31 kg/m2 who underwent MIH during January 2014–June 2016 at a tertiary care referral center. Median uterine weight was 409 g, and indications for hysterectomy included fibroids, pelvic pain, abnormal uterine bleeding, and prolapse. Patients with malignancy were excluded.
“In the context of high-complexity cases, such as those for large uteri, many factors beyond surgical volume influence perioperative adverse events,” Dr. Bretschneider said, concluding that, to improve patient safety and outcomes, additional studies should be performed to explore the relationship between surgeon experience and patient factors on perioperative outcomes in the setting of common gynecologic procedures.
Dr. Bretschneider reported having no relevant disclosures.
SOURCE: Bretschneider C et al. SGS 2018, Oral Poster 12.
REPORTING FROM SGS 2018
Key clinical point: High case volume may increase risk for adverse events during minimally invasive hysterectomy with large uteri.
Major finding: MIH volume, estimated blood loss, and operative time were associated with intraoperative adverse events (odds ratios, 1.9, 2.0, and 22.1, respectively) and postoperative adverse events (ORs, 1.3, 1.4, and 1.9, respectively).
Study details: A retrospective cohort study of 763 women.
Disclosures: Dr. Bretschneider reported having no relevant disclosures.
Source: Bretschneider C et al. SGS 2018, Oral Poster 12.
No clear winner in Pfannenstiel vs. vertical incision for high BMI cesareans
DALLAS – though enrollment difficulties limited study numbers, with almost two-thirds of eligible women declining to participate in the surgical trial.
At 6 weeks postdelivery, 21.1% of women who had a vertical incision experienced wound complications, compared with 18.6% of those who had a Pfannenstiel incision, a nonsignificant difference. This was a smaller difference than was seen at 2 weeks postpartum, when 20% of the vertical incision group had wound complications, compared with 10.4% of those who had a Pfannenstiel, also a nonsignificant difference. Maternal and fetal outcomes didn’t differ significantly with the two surgical approaches.
Though there had been several observational studies comparing vertical with Pfannenstiel incisions for cesarean delivery in women with obesity, no randomized, controlled trials had been conducted, and observational study results were mixed, said Dr. Marrs.
Each approach comes with theoretical pros and cons: For women who have a large pannus, the incision site may lie in a moist environment with a low transverse incision, and oxygen tension may be low. However, a Pfannenstiel incision usually will have better cosmesis than will a vertical incision, and generally will result in less postoperative pain.
On the other hand, said Dr. Marrs, vertical incisions can provide improved exposure of the uterus during delivery, and the moist environment underlying the pannus is avoided. However, wound tension may be higher, and subcutaneous thickness is likely to be higher than at the Pfannenstiel incision site.
The study, conducted at two academic medical centers, enrolled women with a body mass index (BMI) of at least 40 kg/m2 at a gestational age of 24 weeks or greater who required cesarean delivery. Consenting women were then randomized to receive Pfannenstiel or vertical incisions.
Women who had clinical chorioamnionitis, whose amniotic membranes had been ruptured for 18 hours or more, or who had placenta accreta were excluded. Also excluded were women with a private physician and those desiring vaginal delivery, said Dr. Marrs, a maternal-fetal medicine fellow at the University of Texas Medical Branch, Galveston.
The study’s primary outcome measure was a composite of wound complications seen within 6 weeks of delivery, including surgical site infection, whether superficial, deep, or involving an organ or tissue space; cellulitis; seroma or hematoma; and wound separation. Other maternal outcomes tracked in the study included postoperative length of stay, transfusion requirement, sepsis, readmission, and death.
Cesarean-specific secondary outcomes included operative time and time from skin incision to delivery, estimated blood loss, and any incidence of hysterectomy through a low transverse incision. Neonatal outcomes included a 5-minute Apgar score of less than 7, umbilical cord pH of less than 7, and neonatal ICU admissions.
Dr. Mars said that the goal enrollment for the study was 300 patients, to ensure adequate statistical power. However, they found enrollment a challenge, with low consent rates during the defined time period from October 2013 to May 2017. They shifted their statistical technique to a Bayesian analysis, taking into account the estimated probability of treatment benefit.
Using this approach, they found a 59% probability that a Pfannenstiel incision would lead to a lower primary outcome rate – a better result – than would a vertical incision. This result just missed the predetermined threshold of 60%, said Dr. Marrs.
Of the 789 women who met the BMI threshold for eligibility assessment, 420 (65%) who passed the screening declined to participate. Of those who consented to participation, an additional 137 women either withdrew consent or failed further screening, leaving 50 women who were randomized to the Pfannenstiel arm and 41 who were randomized to the vertical incision arm.
Baseline characteristics were similar between groups, with a mean maternal age of 30 years in the Pfannenstiel group and 28 years in the vertical incision group. Gestational age at delivery was a mean of 37 weeks in both groups, and mean BMI was 48-50 kg/m2.
Most patients (80%-90%) had public insurance. Diabetes was more common in the Pfannenstiel group (48%) than in the vertical incision cohort (32%). Just over 40% of patients were African American.
Two women in the Pfannenstiel group and three in the vertical incision group did not receive the intended incision. After accounting for patients lost to follow-up by 6 weeks, 43 women who received Pfannenstiel and 38 women who received vertical incisions were available for full evaluation.
Dr. Marrs said that the study, the first randomized trial to address this issue, had several strengths, including its being conducted at two sites with appropriate stratification for the sites. Also, an independent data safety monitoring board and two chart reviewers helped overcome some of the limitations of a surgical study, where complete blinding is impossible.
The Bayesian analysis allowed ascertainment of the probability of treatment benefit despite the lower-than-hoped-for enrollment numbers. The primary weakness of the study, said Dr. Marrs, centered around the low consent rate, which led to a small study that was prematurely terminated.
“It’s difficult to enroll women in a trial that requires random allocation of skin incision, due to their preference to choose their own incision. A larger trial would likewise be challenging, and unlikely to yield different results,” said Dr. Marrs.
Dr. Marrs reported no conflicts of interest.
SOURCE: Marrs CC et al. Am J Obstet Gynecol. 2018 Jan;218:S29.
DALLAS – though enrollment difficulties limited study numbers, with almost two-thirds of eligible women declining to participate in the surgical trial.
At 6 weeks postdelivery, 21.1% of women who had a vertical incision experienced wound complications, compared with 18.6% of those who had a Pfannenstiel incision, a nonsignificant difference. This was a smaller difference than was seen at 2 weeks postpartum, when 20% of the vertical incision group had wound complications, compared with 10.4% of those who had a Pfannenstiel, also a nonsignificant difference. Maternal and fetal outcomes didn’t differ significantly with the two surgical approaches.
Though there had been several observational studies comparing vertical with Pfannenstiel incisions for cesarean delivery in women with obesity, no randomized, controlled trials had been conducted, and observational study results were mixed, said Dr. Marrs.
Each approach comes with theoretical pros and cons: For women who have a large pannus, the incision site may lie in a moist environment with a low transverse incision, and oxygen tension may be low. However, a Pfannenstiel incision usually will have better cosmesis than will a vertical incision, and generally will result in less postoperative pain.
On the other hand, said Dr. Marrs, vertical incisions can provide improved exposure of the uterus during delivery, and the moist environment underlying the pannus is avoided. However, wound tension may be higher, and subcutaneous thickness is likely to be higher than at the Pfannenstiel incision site.
The study, conducted at two academic medical centers, enrolled women with a body mass index (BMI) of at least 40 kg/m2 at a gestational age of 24 weeks or greater who required cesarean delivery. Consenting women were then randomized to receive Pfannenstiel or vertical incisions.
Women who had clinical chorioamnionitis, whose amniotic membranes had been ruptured for 18 hours or more, or who had placenta accreta were excluded. Also excluded were women with a private physician and those desiring vaginal delivery, said Dr. Marrs, a maternal-fetal medicine fellow at the University of Texas Medical Branch, Galveston.
The study’s primary outcome measure was a composite of wound complications seen within 6 weeks of delivery, including surgical site infection, whether superficial, deep, or involving an organ or tissue space; cellulitis; seroma or hematoma; and wound separation. Other maternal outcomes tracked in the study included postoperative length of stay, transfusion requirement, sepsis, readmission, and death.
Cesarean-specific secondary outcomes included operative time and time from skin incision to delivery, estimated blood loss, and any incidence of hysterectomy through a low transverse incision. Neonatal outcomes included a 5-minute Apgar score of less than 7, umbilical cord pH of less than 7, and neonatal ICU admissions.
Dr. Mars said that the goal enrollment for the study was 300 patients, to ensure adequate statistical power. However, they found enrollment a challenge, with low consent rates during the defined time period from October 2013 to May 2017. They shifted their statistical technique to a Bayesian analysis, taking into account the estimated probability of treatment benefit.
Using this approach, they found a 59% probability that a Pfannenstiel incision would lead to a lower primary outcome rate – a better result – than would a vertical incision. This result just missed the predetermined threshold of 60%, said Dr. Marrs.
Of the 789 women who met the BMI threshold for eligibility assessment, 420 (65%) who passed the screening declined to participate. Of those who consented to participation, an additional 137 women either withdrew consent or failed further screening, leaving 50 women who were randomized to the Pfannenstiel arm and 41 who were randomized to the vertical incision arm.
Baseline characteristics were similar between groups, with a mean maternal age of 30 years in the Pfannenstiel group and 28 years in the vertical incision group. Gestational age at delivery was a mean of 37 weeks in both groups, and mean BMI was 48-50 kg/m2.
Most patients (80%-90%) had public insurance. Diabetes was more common in the Pfannenstiel group (48%) than in the vertical incision cohort (32%). Just over 40% of patients were African American.
Two women in the Pfannenstiel group and three in the vertical incision group did not receive the intended incision. After accounting for patients lost to follow-up by 6 weeks, 43 women who received Pfannenstiel and 38 women who received vertical incisions were available for full evaluation.
Dr. Marrs said that the study, the first randomized trial to address this issue, had several strengths, including its being conducted at two sites with appropriate stratification for the sites. Also, an independent data safety monitoring board and two chart reviewers helped overcome some of the limitations of a surgical study, where complete blinding is impossible.
The Bayesian analysis allowed ascertainment of the probability of treatment benefit despite the lower-than-hoped-for enrollment numbers. The primary weakness of the study, said Dr. Marrs, centered around the low consent rate, which led to a small study that was prematurely terminated.
“It’s difficult to enroll women in a trial that requires random allocation of skin incision, due to their preference to choose their own incision. A larger trial would likewise be challenging, and unlikely to yield different results,” said Dr. Marrs.
Dr. Marrs reported no conflicts of interest.
SOURCE: Marrs CC et al. Am J Obstet Gynecol. 2018 Jan;218:S29.
DALLAS – though enrollment difficulties limited study numbers, with almost two-thirds of eligible women declining to participate in the surgical trial.
At 6 weeks postdelivery, 21.1% of women who had a vertical incision experienced wound complications, compared with 18.6% of those who had a Pfannenstiel incision, a nonsignificant difference. This was a smaller difference than was seen at 2 weeks postpartum, when 20% of the vertical incision group had wound complications, compared with 10.4% of those who had a Pfannenstiel, also a nonsignificant difference. Maternal and fetal outcomes didn’t differ significantly with the two surgical approaches.
Though there had been several observational studies comparing vertical with Pfannenstiel incisions for cesarean delivery in women with obesity, no randomized, controlled trials had been conducted, and observational study results were mixed, said Dr. Marrs.
Each approach comes with theoretical pros and cons: For women who have a large pannus, the incision site may lie in a moist environment with a low transverse incision, and oxygen tension may be low. However, a Pfannenstiel incision usually will have better cosmesis than will a vertical incision, and generally will result in less postoperative pain.
On the other hand, said Dr. Marrs, vertical incisions can provide improved exposure of the uterus during delivery, and the moist environment underlying the pannus is avoided. However, wound tension may be higher, and subcutaneous thickness is likely to be higher than at the Pfannenstiel incision site.
The study, conducted at two academic medical centers, enrolled women with a body mass index (BMI) of at least 40 kg/m2 at a gestational age of 24 weeks or greater who required cesarean delivery. Consenting women were then randomized to receive Pfannenstiel or vertical incisions.
Women who had clinical chorioamnionitis, whose amniotic membranes had been ruptured for 18 hours or more, or who had placenta accreta were excluded. Also excluded were women with a private physician and those desiring vaginal delivery, said Dr. Marrs, a maternal-fetal medicine fellow at the University of Texas Medical Branch, Galveston.
The study’s primary outcome measure was a composite of wound complications seen within 6 weeks of delivery, including surgical site infection, whether superficial, deep, or involving an organ or tissue space; cellulitis; seroma or hematoma; and wound separation. Other maternal outcomes tracked in the study included postoperative length of stay, transfusion requirement, sepsis, readmission, and death.
Cesarean-specific secondary outcomes included operative time and time from skin incision to delivery, estimated blood loss, and any incidence of hysterectomy through a low transverse incision. Neonatal outcomes included a 5-minute Apgar score of less than 7, umbilical cord pH of less than 7, and neonatal ICU admissions.
Dr. Mars said that the goal enrollment for the study was 300 patients, to ensure adequate statistical power. However, they found enrollment a challenge, with low consent rates during the defined time period from October 2013 to May 2017. They shifted their statistical technique to a Bayesian analysis, taking into account the estimated probability of treatment benefit.
Using this approach, they found a 59% probability that a Pfannenstiel incision would lead to a lower primary outcome rate – a better result – than would a vertical incision. This result just missed the predetermined threshold of 60%, said Dr. Marrs.
Of the 789 women who met the BMI threshold for eligibility assessment, 420 (65%) who passed the screening declined to participate. Of those who consented to participation, an additional 137 women either withdrew consent or failed further screening, leaving 50 women who were randomized to the Pfannenstiel arm and 41 who were randomized to the vertical incision arm.
Baseline characteristics were similar between groups, with a mean maternal age of 30 years in the Pfannenstiel group and 28 years in the vertical incision group. Gestational age at delivery was a mean of 37 weeks in both groups, and mean BMI was 48-50 kg/m2.
Most patients (80%-90%) had public insurance. Diabetes was more common in the Pfannenstiel group (48%) than in the vertical incision cohort (32%). Just over 40% of patients were African American.
Two women in the Pfannenstiel group and three in the vertical incision group did not receive the intended incision. After accounting for patients lost to follow-up by 6 weeks, 43 women who received Pfannenstiel and 38 women who received vertical incisions were available for full evaluation.
Dr. Marrs said that the study, the first randomized trial to address this issue, had several strengths, including its being conducted at two sites with appropriate stratification for the sites. Also, an independent data safety monitoring board and two chart reviewers helped overcome some of the limitations of a surgical study, where complete blinding is impossible.
The Bayesian analysis allowed ascertainment of the probability of treatment benefit despite the lower-than-hoped-for enrollment numbers. The primary weakness of the study, said Dr. Marrs, centered around the low consent rate, which led to a small study that was prematurely terminated.
“It’s difficult to enroll women in a trial that requires random allocation of skin incision, due to their preference to choose their own incision. A larger trial would likewise be challenging, and unlikely to yield different results,” said Dr. Marrs.
Dr. Marrs reported no conflicts of interest.
SOURCE: Marrs CC et al. Am J Obstet Gynecol. 2018 Jan;218:S29.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Wound complication rates were similar with Pfannenstiel and vertical incisions in women with obesity.
Major finding: At 6 weeks, 21.1% of vertical incision recipients and 18.6% of Pfannenstiel recipients had wound complications.
Study details: Randomized controlled trial of 91 women with obesity receiving cesarean section.
Disclosures: Dr. Marrs reported no conflicts of interest.
Source: Marrs CC et al. Am J Obstet Gynecol. 2018 Jan;218:S29.
Greater gynecological but not medical risks with hysteroscopic sterilization
Hysteroscopic sterilization is associated with a significantly greater risk of gynecological complications but not medical or surgical complications, compared with laparoscopic sterilization, according to data from a French nationwide cohort study.
In a report published Jan. 23 in JAMA, researchers conducted a study of 105,357 women – 71,303 (67.7%) of whom underwent hysteroscopic sterilization, and 34,054 (32.3%) of whom underwent laparoscopic sterilization – and who were followed for at least 1 year after the procedure.
Women who had the hysteroscopic procedure had a nearly threefold higher risk of tubal disorder or surgery, a sevenfold higher risk of sterilization failure, and a 25-fold higher risk of undergoing a second sterilization procedure at 1 year compared with those who had the laparoscopic procedure (P less than .001 for each). These risk increases persisted even at 3 years after the procedure (hazard ratios of 1.79, 4.66, and 16.63, respectively; 95% confidence interval for each).
“A second sterilization procedure following hysteroscopic sterilization is a well-identified risk already described in phase 2 and 3 studies, in which the risk varied between 4.0% and 4.5%,” wrote Kim Bouillon, MD, PhD, of the French National Agency for Medicines and Health Products Safety, and her coauthors.
“In the present study, this risk was 4.1% at the 1-year follow-up, comparable with that reported in previous studies conducted in real-life conditions in patients who received care in public or private hospitals, and much higher than after laparoscopic sterilization.”
However, hysteroscopic sterilization was associated with a significantly reduced risk of surgical complications, compared with laparoscopic sterilization (adjusted odds ratio, 0.18; 95% CI, 0.14-0.23). The overall rate of in-hospital surgical complications was 0.13% with the hysteroscopic procedure and 0.78% with the laparoscopic procedure. Medical complications occurred in 0.06% of hysteroscopic procedures and 0.11% of laparoscopic procedures.
Women who underwent hysteroscopic procedures also had a significantly lower risk of uterine disorders (adjusted HR, 0.85; 95% CI, 0.74-0.98), uterine bleeding, and hysterectomies at 1 year, after adjustment for known hysterectomy risk factors.
The researchers noted that in absolute terms, the differences in the risk of procedural complications were very small, compared with the differences in the risk of gynecological complications.
The risk of pregnancy was significantly lower in the hysteroscopic group, compared with the laparoscopic group at 1 year after the procedure, but by 3 years’ follow-up, the difference was no longer significant.
There were no significant differences seen in the risk of medical complications such as autoimmune disease and thyroid disorders, attempted suicide, or death between the two procedures. Women who underwent hysteroscopic sterilization had a slightly lower use of analgesics, antidepressants, and benzodiazepines at 1 year that was more pronounced by 3 years.
There was a significantly higher risk of allergic reaction seen with hysteroscopic sterilization among women with prior allergies, but the authors suggested that a null overall effect and large number of tested interactions made the finding “hypothesis-generating” only.
The study was prompted by safety concerns about hysteroscopic sterilization, with the Food and Drug Administration in 2015 receiving a large number of reports of adverse events including bleeding, pelvic pain, fallopian tube perforation, unwanted pregnancy, hysterectomies, depression, and allergic reactions.
The FDA has since ordered the device manufacturer to undertake an open-label, nonrandomized study comparing outcomes between hysteroscopic and laparoscopic sterilization, which is expected to deliver results in 2023.
“To our knowledge, this is the first study aiming at comparing medical outcomes in addition to gynecological outcomes between hysteroscopic and laparoscopic sterilization,” the authors wrote, referring to their own work. They concluded, “these findings do not support increased medical risks associated with hysteroscopic sterilization.”
One author declared personal fees from Boston Scientific but no other conflicts of interest were declared.
SOURCE: Bouillon K et al. JAMA. 2018 Jan 23;319:375-87.
In 2016, in response to safety concerns about the hysteroscopic sterilization implant Essure, the Food and Drug Administration placed a “black box” warning on the device to highlight potential risks, and a global patient advocacy movement called for a ban on the product. In this environment, there is therefore a need for strong scientific evidence to inform objective decision making.
This study provides reassuring evidence that adverse outcomes are not significantly higher after hysteroscopic sterilization compared with laparoscopic sterilization, at least up to 3 years after the procedure. However, given the powerful and very public grassroots effort to ban the hysteroscopic implant and the possibility of class action litigation, the future of hysteroscopic sterilization is uncertain.
Eve Espey, MD, MPH, and Lisa G. Hofler, MD, MPH, are in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. The comments are taken from an editorial (JAMA. 2018 Jan 23;319[4]:347-50). Dr. Hofler declared personal fees and nonfinancial support from the American College of Obstetricians and Gynecologists.
In 2016, in response to safety concerns about the hysteroscopic sterilization implant Essure, the Food and Drug Administration placed a “black box” warning on the device to highlight potential risks, and a global patient advocacy movement called for a ban on the product. In this environment, there is therefore a need for strong scientific evidence to inform objective decision making.
This study provides reassuring evidence that adverse outcomes are not significantly higher after hysteroscopic sterilization compared with laparoscopic sterilization, at least up to 3 years after the procedure. However, given the powerful and very public grassroots effort to ban the hysteroscopic implant and the possibility of class action litigation, the future of hysteroscopic sterilization is uncertain.
Eve Espey, MD, MPH, and Lisa G. Hofler, MD, MPH, are in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. The comments are taken from an editorial (JAMA. 2018 Jan 23;319[4]:347-50). Dr. Hofler declared personal fees and nonfinancial support from the American College of Obstetricians and Gynecologists.
In 2016, in response to safety concerns about the hysteroscopic sterilization implant Essure, the Food and Drug Administration placed a “black box” warning on the device to highlight potential risks, and a global patient advocacy movement called for a ban on the product. In this environment, there is therefore a need for strong scientific evidence to inform objective decision making.
This study provides reassuring evidence that adverse outcomes are not significantly higher after hysteroscopic sterilization compared with laparoscopic sterilization, at least up to 3 years after the procedure. However, given the powerful and very public grassroots effort to ban the hysteroscopic implant and the possibility of class action litigation, the future of hysteroscopic sterilization is uncertain.
Eve Espey, MD, MPH, and Lisa G. Hofler, MD, MPH, are in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. The comments are taken from an editorial (JAMA. 2018 Jan 23;319[4]:347-50). Dr. Hofler declared personal fees and nonfinancial support from the American College of Obstetricians and Gynecologists.
Hysteroscopic sterilization is associated with a significantly greater risk of gynecological complications but not medical or surgical complications, compared with laparoscopic sterilization, according to data from a French nationwide cohort study.
In a report published Jan. 23 in JAMA, researchers conducted a study of 105,357 women – 71,303 (67.7%) of whom underwent hysteroscopic sterilization, and 34,054 (32.3%) of whom underwent laparoscopic sterilization – and who were followed for at least 1 year after the procedure.
Women who had the hysteroscopic procedure had a nearly threefold higher risk of tubal disorder or surgery, a sevenfold higher risk of sterilization failure, and a 25-fold higher risk of undergoing a second sterilization procedure at 1 year compared with those who had the laparoscopic procedure (P less than .001 for each). These risk increases persisted even at 3 years after the procedure (hazard ratios of 1.79, 4.66, and 16.63, respectively; 95% confidence interval for each).
“A second sterilization procedure following hysteroscopic sterilization is a well-identified risk already described in phase 2 and 3 studies, in which the risk varied between 4.0% and 4.5%,” wrote Kim Bouillon, MD, PhD, of the French National Agency for Medicines and Health Products Safety, and her coauthors.
“In the present study, this risk was 4.1% at the 1-year follow-up, comparable with that reported in previous studies conducted in real-life conditions in patients who received care in public or private hospitals, and much higher than after laparoscopic sterilization.”
However, hysteroscopic sterilization was associated with a significantly reduced risk of surgical complications, compared with laparoscopic sterilization (adjusted odds ratio, 0.18; 95% CI, 0.14-0.23). The overall rate of in-hospital surgical complications was 0.13% with the hysteroscopic procedure and 0.78% with the laparoscopic procedure. Medical complications occurred in 0.06% of hysteroscopic procedures and 0.11% of laparoscopic procedures.
Women who underwent hysteroscopic procedures also had a significantly lower risk of uterine disorders (adjusted HR, 0.85; 95% CI, 0.74-0.98), uterine bleeding, and hysterectomies at 1 year, after adjustment for known hysterectomy risk factors.
The researchers noted that in absolute terms, the differences in the risk of procedural complications were very small, compared with the differences in the risk of gynecological complications.
The risk of pregnancy was significantly lower in the hysteroscopic group, compared with the laparoscopic group at 1 year after the procedure, but by 3 years’ follow-up, the difference was no longer significant.
There were no significant differences seen in the risk of medical complications such as autoimmune disease and thyroid disorders, attempted suicide, or death between the two procedures. Women who underwent hysteroscopic sterilization had a slightly lower use of analgesics, antidepressants, and benzodiazepines at 1 year that was more pronounced by 3 years.
There was a significantly higher risk of allergic reaction seen with hysteroscopic sterilization among women with prior allergies, but the authors suggested that a null overall effect and large number of tested interactions made the finding “hypothesis-generating” only.
The study was prompted by safety concerns about hysteroscopic sterilization, with the Food and Drug Administration in 2015 receiving a large number of reports of adverse events including bleeding, pelvic pain, fallopian tube perforation, unwanted pregnancy, hysterectomies, depression, and allergic reactions.
The FDA has since ordered the device manufacturer to undertake an open-label, nonrandomized study comparing outcomes between hysteroscopic and laparoscopic sterilization, which is expected to deliver results in 2023.
“To our knowledge, this is the first study aiming at comparing medical outcomes in addition to gynecological outcomes between hysteroscopic and laparoscopic sterilization,” the authors wrote, referring to their own work. They concluded, “these findings do not support increased medical risks associated with hysteroscopic sterilization.”
One author declared personal fees from Boston Scientific but no other conflicts of interest were declared.
SOURCE: Bouillon K et al. JAMA. 2018 Jan 23;319:375-87.
Hysteroscopic sterilization is associated with a significantly greater risk of gynecological complications but not medical or surgical complications, compared with laparoscopic sterilization, according to data from a French nationwide cohort study.
In a report published Jan. 23 in JAMA, researchers conducted a study of 105,357 women – 71,303 (67.7%) of whom underwent hysteroscopic sterilization, and 34,054 (32.3%) of whom underwent laparoscopic sterilization – and who were followed for at least 1 year after the procedure.
Women who had the hysteroscopic procedure had a nearly threefold higher risk of tubal disorder or surgery, a sevenfold higher risk of sterilization failure, and a 25-fold higher risk of undergoing a second sterilization procedure at 1 year compared with those who had the laparoscopic procedure (P less than .001 for each). These risk increases persisted even at 3 years after the procedure (hazard ratios of 1.79, 4.66, and 16.63, respectively; 95% confidence interval for each).
“A second sterilization procedure following hysteroscopic sterilization is a well-identified risk already described in phase 2 and 3 studies, in which the risk varied between 4.0% and 4.5%,” wrote Kim Bouillon, MD, PhD, of the French National Agency for Medicines and Health Products Safety, and her coauthors.
“In the present study, this risk was 4.1% at the 1-year follow-up, comparable with that reported in previous studies conducted in real-life conditions in patients who received care in public or private hospitals, and much higher than after laparoscopic sterilization.”
However, hysteroscopic sterilization was associated with a significantly reduced risk of surgical complications, compared with laparoscopic sterilization (adjusted odds ratio, 0.18; 95% CI, 0.14-0.23). The overall rate of in-hospital surgical complications was 0.13% with the hysteroscopic procedure and 0.78% with the laparoscopic procedure. Medical complications occurred in 0.06% of hysteroscopic procedures and 0.11% of laparoscopic procedures.
Women who underwent hysteroscopic procedures also had a significantly lower risk of uterine disorders (adjusted HR, 0.85; 95% CI, 0.74-0.98), uterine bleeding, and hysterectomies at 1 year, after adjustment for known hysterectomy risk factors.
The researchers noted that in absolute terms, the differences in the risk of procedural complications were very small, compared with the differences in the risk of gynecological complications.
The risk of pregnancy was significantly lower in the hysteroscopic group, compared with the laparoscopic group at 1 year after the procedure, but by 3 years’ follow-up, the difference was no longer significant.
There were no significant differences seen in the risk of medical complications such as autoimmune disease and thyroid disorders, attempted suicide, or death between the two procedures. Women who underwent hysteroscopic sterilization had a slightly lower use of analgesics, antidepressants, and benzodiazepines at 1 year that was more pronounced by 3 years.
There was a significantly higher risk of allergic reaction seen with hysteroscopic sterilization among women with prior allergies, but the authors suggested that a null overall effect and large number of tested interactions made the finding “hypothesis-generating” only.
The study was prompted by safety concerns about hysteroscopic sterilization, with the Food and Drug Administration in 2015 receiving a large number of reports of adverse events including bleeding, pelvic pain, fallopian tube perforation, unwanted pregnancy, hysterectomies, depression, and allergic reactions.
The FDA has since ordered the device manufacturer to undertake an open-label, nonrandomized study comparing outcomes between hysteroscopic and laparoscopic sterilization, which is expected to deliver results in 2023.
“To our knowledge, this is the first study aiming at comparing medical outcomes in addition to gynecological outcomes between hysteroscopic and laparoscopic sterilization,” the authors wrote, referring to their own work. They concluded, “these findings do not support increased medical risks associated with hysteroscopic sterilization.”
One author declared personal fees from Boston Scientific but no other conflicts of interest were declared.
SOURCE: Bouillon K et al. JAMA. 2018 Jan 23;319:375-87.
FROM JAMA
Key clinical point: Hysteroscopic sterilization is associated with a significantly greater risk of gynecological complications – but not medical or surgical complications – compared with laparoscopic sterilization.
Major finding: The risks of repeat sterilization procedure, sterilization failure, and tubal disorder are higher with hysteroscopic sterilization than with laparoscopic sterilization, but the surgical risks are lower and there are no significant differences in other medical risks.
Data source: Nationwide cohort study of 105,357 women.
Disclosures: One author declared personal fees from Boston Scientific but no other conflicts of interest were declared.
Source: Bouillon K et al. JAMA. 2018 Jan 23;319:375-87.
Implementing enhanced recovery protocols for gynecologic surgery
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.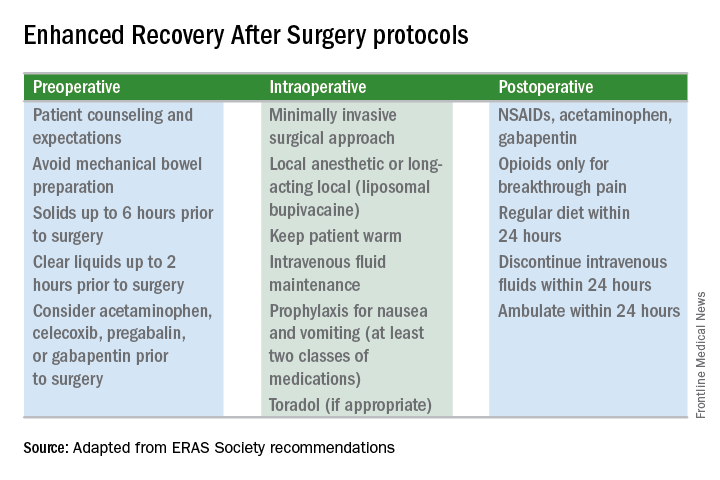
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.
“Enhanced Recovery After Surgery” (ERAS) practices and protocols have been increasingly refined and adopted for the field of gynecology, and there is hope among gynecologic surgeons – and some recent evidence – that, with the ERAS movement, we are improving patient recoveries and outcomes and minimizing the need for opioids.
This applies not only to open surgeries but also to the minimally invasive procedures that already are prized for significant reductions in morbidity and length of stay. The overarching and guiding principle of ERAS is that any surgery – whether open or minimally invasive, major or minor – places stress on the body and is associated with risks and morbidity.
Enhanced recovery protocols are multidisciplinary, perioperative approaches designed to lessen the body’s stress response to surgery. The protocols and pathways offer us a menu of small changes that, in the aggregate, can lead to large and demonstrable benefits – especially when these small changes are chosen across the preoperative, intraoperative, and postoperative arenas and then standardized in one’s practice. Among the major components of ERAS practices and protocols are limiting preoperative fasting, employing multimodal analgesia, encouraging early ambulation and early postsurgical feeding, and creating culture shift that includes greater emphasis on patient expectations.
In our practice, we are incorporating ERAS practices not only in hopes of reducing the stress of all surgeries before, during, and after, but also with the goal of achieving a postoperative opioid-free hysterectomy, myomectomy, and extensive endometriosis surgery. (All of our advanced procedures are performed laparoscopically or robotically.)
Over the past 7 or so years, we have adopted a multimodal approach to pain control that includes a bundle of preoperative analgesics – acetaminophen, pregabalin, and celecoxib (we call it “TLC” for Tylenol, Lyrica, and Celebrex) – and the use of liposomal bupivacaine in our robotic surgeries. We are now turning toward ERAS nutritional changes, most of which run counter to traditional paradigms for surgical care. And in other areas, such as dedicated preoperative counseling, we continue to refine and improve our practices.
Improved Outcomes
The ERAS mindset notably intersected gynecology with the publication in 2016 of a two-part series of guidelines for gynecology/oncology surgery from the ERAS Society. The 8-year-old society has its roots in a study group of European surgeons and others who decided to examine surgical practices and the concept of multimodal surgical care put forth in the 1990s by Henrik Kehlet, MD, PhD, then a professor at the University of Copenhagen.
The first set of recommendations addressed pre- and intraoperative care (Gynecol Oncol. 2016 Feb;140[2]:313-22), and the second set addressed postoperative care (Gynecol Oncol. 2016 Feb;140[2]:323-32). Similar evidence-based recommendations were previously written for colonic resections, rectal and pelvic surgery, and other surgical specialties.
Most of the published outcomes of enhanced recovery protocols come from colorectal surgery. As noted in the ERAS Society gynecology/oncology guidelines, the benefits include an average reduction in length of stay of 2.5 days and a decrease in complications by as much as 50%.
There is growing evidence, however, that ERAS programs are also beneficial for patients undergoing laparoscopic surgery, and outcomes from gynecology – including minimally invasive surgery – are also being reported.
For instance, a retrospective case-control study of 55 consecutive gynecologic oncology patients treated at the University of California, San Francisco, with laparoscopic or robotic surgery and an enhanced recovery pathway – and 110 historical control patients matched on the basis of age and surgery type – found significant improvements in recovery time, decreased pain despite reduced opioid use, and overall lower hospital costs (Obstet Gynecol. 2016 Jul;128[1]:138-44).
The enhanced recovery pathway included patient education, multimodal antiemetics, multimodal analgesia, and balanced fluid administration. Early catheter removal, ambulation, and feeding were also components. Analgesia included routine preoperative gabapentin, diclofenac, and acetaminophen; routine postoperative gabapentin, NSAIDs, and acetaminophen; and transversus abdominis plane blocks in 32 of the ERAS patients.
ERAS patients were significantly more likely to be discharged on day 1 (91%, compared with 60% in the control group). Opioid use decreased by 30%, and pain scores on postoperative day 1 were significantly lower.
Another study looking at the effect of enhanced recovery implementation in gynecologic surgeries at the University of Virginia, Charlottesville, (gynecologic oncology, urogynecology, and general gynecology) similarly reported benefits for vaginal and minimally invasive procedures, as well as for open procedures (Obstet Gynecol. 2016 Sep;128[3]:457-66).
In the minimally invasive group, investigators compared 324 patients before ERAS implementation with 249 patients afterward and found that the median length of stay was unchanged (1 day). However, intraoperative and postoperative opioid consumption decreased significantly and – even though actual pain scores improved only slightly – patient satisfaction scores improved markedly among post-ERAS patients. Patients gave higher marks, for instance, to questions regarding pain control (“how well your pain was controlled”) and teamwork (“staff worked together to care for you”).
Reducing Opioids
New opioid use that persists after a surgical procedure is a postsurgical complication that we all should be working to prevent. It has been estimated that 6% of surgical patients – even those who’ve had relatively minor surgical procedures – will become long-term opioid users, developing a dependence on the drugs prescribed to them for postsurgical pain.
This was shown last year in a national study of insurance claims data from between 2013 and 2014; investigators identified adults without opioid use in the year prior to surgery (including hysterectomy) and found that 5.9%-6.5% were filling opioid prescriptions 90-180 days after their surgical procedure. The incidence in a nonoperative control cohort was 0.4% (JAMA Surg. 2017 Jun 21;152[6]:e170504). Notably, this prolonged use was greatest in patients with prior pain conditions, substance abuse, and mental health disorders – a finding that may have implications for the counseling we provide prior to surgery.
It’s not clear what the optimal analgesic regimen is for minimally invasive or other gynecologic surgeries. What is clearly recommended, however, is that the approach be multifaceted. In our practice, we believe that the preoperative use of acetaminophen, pregabalin, and celecoxib plays an important role in reducing postoperative pain and opioid use. But we also have striven to create a practice-wide culture shift (throughout the operating and recovery rooms), for instance, that encourages using the least amounts of narcotics possible and using the shortest-acting formulations possible.
Transversus abdominis plane (TAP) blocks are also often part of ERAS protocols; they have been shown in at least two randomized controlled trials of abdominal hysterectomy to reduce intraoperative fentanyl requirements and to reduce immediate postoperative pain scores and postoperative morphine requirements (Anesth Analg. 2008 Dec;107[6]:2056-60; J Anaesthesiol Clin Pharmacol. 2014 Jul-Sep;30[3]:391-6).
More recently, liposomal bupivacaine, which is slowly released over several days, has gained traction as a substitute for standard bupivacaine and other agents in TAP blocks. In one recent retrospective study, abdominal incision infiltration with liposomal bupivacaine was associated with less opioid use (with no change in pain scores), compared with bupivacaine hydrochloride after laparotomy for gynecologic malignancies (Obstet Gynecol. 2016 Nov;128[5]:1009-17). It’s significantly more expensive, however, making it likely that the formulation is being used more judiciously in minimally invasive gynecologic surgery than in open surgeries.
Because of costs, we currently are restricted to using liposomal bupivacaine in our robotic surgeries only. In our practice, the single 20 mL vial (266 mg of liposomal bupivacaine) is diluted with 20 mL of normal saline, but it can be further diluted without loss of efficacy. With a 16-gauge needle, the liposomal bupivacaine is distributed across the incisions (usually 20 mL in the umbilicus with a larger incision and 10 mL in each of the two lateral incisions). Patients are counseled that they may have more discomfort after 3 days, but by this point most are mobile and feeling relatively well with a combination of NSAIDs and acetaminophen.
With growing visibility of the problem of narcotic dependence in the United States, patients seem increasingly receptive and even eager to limit or avoid the use of opioids. Patients should be counseled that minimizing or avoiding opioids may also speed recovery. Narcotics cause gut motility to slow down, which may hinder mobilization. Early mobilization (within 24 hours) is among the enhanced recovery elements that the ERAS Society guidelines say is “of particular value” for minimally invasive surgery, along with maintenance of normothermia and normovolemia with maintenance of adequate cardiac output.
Selecting Steps
Our practice is also trying to reduce preoperative bowel preparation and preoperative fasting, both of which have been found to be stressful for the body without evidence of benefit. These practices can lead to insulin resistance and hyperglycemia, which are associated with increased morbidity and length of stay.
It is now recommended that clear fluids be allowed up to 2 hours before surgery and solids up to 6 hours before. Some health systems and practices also recommend presurgical carbohydrate loading (for example, 10 ounces of apple juice 2 hours before surgery) – another small change on the ERAS menu – to further reduce postoperative insulin resistance and help the body cope with its stress response to surgery.
Along with nutritional changes are also various measures aimed at optimizing the body’s functionality before surgery (“prehabilitation”), from walking 30 minutes a day to abstaining from alcohol for patients who drink heavily.
Throughout the country, enhanced recovery protocols are taking shape in gynecologic surgery. ERAS was featured in an aptly titled panel session at the 2017 annual meeting of the American Association of Gynecologic Laparoscopists: “Outpatient Hysterectomy, ERAS, and Same-Day Discharge: The Next Big Thing in Gyn Surgery.” Others are applying ERAS to scheduled cesarean sections. And in our practice, I believe that if we continue making small changes, we will reach our goal of opioid-free recoveries and a better surgical experience for our patients.
Kirsten Sasaki, MD, is an associate of the Advanced Gynecologic Surgery Institute. She reported that she has no disclosures relevant to this Master Class.














