User login
Postmenopausal women would benefit from clinician-initiated discussion of GSM symptoms
Researchers from Kaiser Permanente Northwest and Oregon Health & Science University, both in Portland, performed a secondary analysis of a survey of postmenopausal women conducted to assess the impact of a health system intervention on genitourinary syndrome of menopause (GSM). They presented their results at the recent annual Scientific Meeting of the North American Menopause Society in Chicago, Illinois (September 25-28, 2019). The intervention included clinician education and computer support tools and was assessed in a clinic-based, cluster-randomized trial in which primary care and gynecology clinics either received the intervention or did not. Women received follow-up 2 weeks after a well-woman visit with a survey that elicited vulvovaginal, sexual, and urinary symptoms with bother.
About 45% of those responding to the survey (N = 1,533) reported 1 or more vulvovaginal atrophy (VVA) symptoms—on average described as somewhat or moderately bothersome—but less than half of those women (39%) discussed their symptom(s) at their well-woman visit. Typically it was the woman, rather than the clinician, who initiated the discussion of the VVA symptom(s) (59% vs 22%, respectively). About 16% of women reported that both parties brought up the symptom(s). Most women (83%) were satisfied with the VVA symptom discussion. Of the women not having such a discussion, 18% wished that one had occurred. A VVA symptom discussion was positively associated with clinicians providing written materials, suggesting lubricants or vaginal estrogen, and providing a referral. Therefore, there is a greater role for clinician-initiated screening for GSM, the study authors concluded.
- Clark AL, Bulkley JE, Bennett AT, et al. Discussion of vulvovaginal health at postmenopausal well woman visit—patient characteristics and visit experiences. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
Researchers from Kaiser Permanente Northwest and Oregon Health & Science University, both in Portland, performed a secondary analysis of a survey of postmenopausal women conducted to assess the impact of a health system intervention on genitourinary syndrome of menopause (GSM). They presented their results at the recent annual Scientific Meeting of the North American Menopause Society in Chicago, Illinois (September 25-28, 2019). The intervention included clinician education and computer support tools and was assessed in a clinic-based, cluster-randomized trial in which primary care and gynecology clinics either received the intervention or did not. Women received follow-up 2 weeks after a well-woman visit with a survey that elicited vulvovaginal, sexual, and urinary symptoms with bother.
About 45% of those responding to the survey (N = 1,533) reported 1 or more vulvovaginal atrophy (VVA) symptoms—on average described as somewhat or moderately bothersome—but less than half of those women (39%) discussed their symptom(s) at their well-woman visit. Typically it was the woman, rather than the clinician, who initiated the discussion of the VVA symptom(s) (59% vs 22%, respectively). About 16% of women reported that both parties brought up the symptom(s). Most women (83%) were satisfied with the VVA symptom discussion. Of the women not having such a discussion, 18% wished that one had occurred. A VVA symptom discussion was positively associated with clinicians providing written materials, suggesting lubricants or vaginal estrogen, and providing a referral. Therefore, there is a greater role for clinician-initiated screening for GSM, the study authors concluded.
Researchers from Kaiser Permanente Northwest and Oregon Health & Science University, both in Portland, performed a secondary analysis of a survey of postmenopausal women conducted to assess the impact of a health system intervention on genitourinary syndrome of menopause (GSM). They presented their results at the recent annual Scientific Meeting of the North American Menopause Society in Chicago, Illinois (September 25-28, 2019). The intervention included clinician education and computer support tools and was assessed in a clinic-based, cluster-randomized trial in which primary care and gynecology clinics either received the intervention or did not. Women received follow-up 2 weeks after a well-woman visit with a survey that elicited vulvovaginal, sexual, and urinary symptoms with bother.
About 45% of those responding to the survey (N = 1,533) reported 1 or more vulvovaginal atrophy (VVA) symptoms—on average described as somewhat or moderately bothersome—but less than half of those women (39%) discussed their symptom(s) at their well-woman visit. Typically it was the woman, rather than the clinician, who initiated the discussion of the VVA symptom(s) (59% vs 22%, respectively). About 16% of women reported that both parties brought up the symptom(s). Most women (83%) were satisfied with the VVA symptom discussion. Of the women not having such a discussion, 18% wished that one had occurred. A VVA symptom discussion was positively associated with clinicians providing written materials, suggesting lubricants or vaginal estrogen, and providing a referral. Therefore, there is a greater role for clinician-initiated screening for GSM, the study authors concluded.
- Clark AL, Bulkley JE, Bennett AT, et al. Discussion of vulvovaginal health at postmenopausal well woman visit—patient characteristics and visit experiences. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
- Clark AL, Bulkley JE, Bennett AT, et al. Discussion of vulvovaginal health at postmenopausal well woman visit—patient characteristics and visit experiences. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
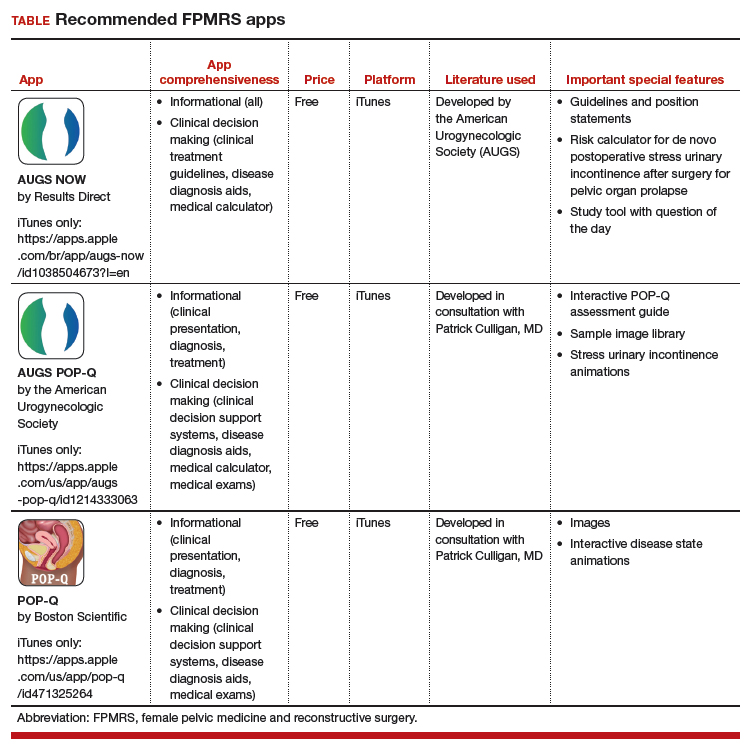
Three free apps for urogynecology providers
Thousands of medical apps are available for smart mobile devices; however, identifying accurate and high-quality apps poses a challenge to health care providers. In the field of urogynecology, also known as female pelvic medicine and reconstructive surgery (FPMRS), the authors of a recent study identified and rated a number of apps for use by urogynecologists.1
The 3 apps featured here are all free and are both informational and clinical decision-making apps.
Informational apps include one or more of the following datasets in a given condition: epidemiology, etiology/pathophysiology, histology/pathology, clinical presentation, treatment, follow-up care, prevention, and/or prognosis.
Clinical decision-making apps may have the following functionalities within the app: clinical decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, medical calculators, laboratory test ordering, laboratory test interpretation, and/or medical exams.
The TABLE details the features of these recommended apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).2 I hope urogynecologists view these apps as innovative educational resources that provide quick medical knowledge and pelvic floor patient education.
1. Wallace SL, Mehta S, Farag S, et al. In search of mobile applications for urogynecology providers. Female Pelvic Med Reconstr Surg. 2018. doi:10.1097/SPV.0000000000000580.
2. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Thousands of medical apps are available for smart mobile devices; however, identifying accurate and high-quality apps poses a challenge to health care providers. In the field of urogynecology, also known as female pelvic medicine and reconstructive surgery (FPMRS), the authors of a recent study identified and rated a number of apps for use by urogynecologists.1
The 3 apps featured here are all free and are both informational and clinical decision-making apps.
Informational apps include one or more of the following datasets in a given condition: epidemiology, etiology/pathophysiology, histology/pathology, clinical presentation, treatment, follow-up care, prevention, and/or prognosis.
Clinical decision-making apps may have the following functionalities within the app: clinical decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, medical calculators, laboratory test ordering, laboratory test interpretation, and/or medical exams.
The TABLE details the features of these recommended apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).2 I hope urogynecologists view these apps as innovative educational resources that provide quick medical knowledge and pelvic floor patient education.

Thousands of medical apps are available for smart mobile devices; however, identifying accurate and high-quality apps poses a challenge to health care providers. In the field of urogynecology, also known as female pelvic medicine and reconstructive surgery (FPMRS), the authors of a recent study identified and rated a number of apps for use by urogynecologists.1
The 3 apps featured here are all free and are both informational and clinical decision-making apps.
Informational apps include one or more of the following datasets in a given condition: epidemiology, etiology/pathophysiology, histology/pathology, clinical presentation, treatment, follow-up care, prevention, and/or prognosis.
Clinical decision-making apps may have the following functionalities within the app: clinical decision support systems, clinical treatment guidelines, disease diagnosis aids, differential diagnosis aids, medical calculators, laboratory test ordering, laboratory test interpretation, and/or medical exams.
The TABLE details the features of these recommended apps based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature used, and important special features).2 I hope urogynecologists view these apps as innovative educational resources that provide quick medical knowledge and pelvic floor patient education.

1. Wallace SL, Mehta S, Farag S, et al. In search of mobile applications for urogynecology providers. Female Pelvic Med Reconstr Surg. 2018. doi:10.1097/SPV.0000000000000580.
2. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
1. Wallace SL, Mehta S, Farag S, et al. In search of mobile applications for urogynecology providers. Female Pelvic Med Reconstr Surg. 2018. doi:10.1097/SPV.0000000000000580.
2. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
#MomsNeedToKnow mental health awareness campaign set to launch
One goal is to use social media to encourage women to let go of stigma
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
One goal is to use social media to encourage women to let go of stigma
One goal is to use social media to encourage women to let go of stigma
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
Midurethral slings have low reoperation rates for stress urinary incontinence
according to a study published in Obstetrics & Gynecology.
Alexander A. Berger, MD, MPH, of the division of female pelvic medicine and reconstructive surgery at Kaiser Permanente in San Diego, and colleagues performed a retrospective cohort study of 17,030 patients with stress urinary incontinence (SUI) who underwent midurethral sling surgery between 2005 and 2016, examining the reoperation rate at 1 year, 5 years, and 9 years after the procedure, as well as secondary outcomes of mesh revision, mesh removal, and recurrence of SUI.
Overall, the rate of reoperation at 1 year was 2.1% (95% confidence interval, 1.9%-2.4%), was 4.5% at 5 years (95% CI, 4.1%-4.8%) and 6.0% at 9 years (95% CI, 5.5%-6.5%). Compared with white patients, there was a lower rate of reoperation among Asian or Pacific Islander patients.
The rate of reoperation involving mesh removal was 0.7% at 1 year (95% CI, 0.6%-0.8%), 1.0% at 5 years (95% CI, 0.8%-1.1%) and 1.1% at 9 years (95% CI, 0.9%-1.3%).
The rate of recurrent SUI leading to operation was 1.6% at 1 year (95% CI, 1.4%-1.8%), 3.9% at 5 years (95% CI, 3.5%-4.2%) and 5.2% at 9 years (95% CI, 4.7%-5.7%), with more reoperations occurring for patients who received a single-incision sling, rather than a retropubic sling (adjusted hazard ratio, 1.5; 95% CI, 1.06-2.11; P = .03), Dr. Berger and associates wrote.
Urogynecologists, ob.gyns., and urologists who use mesh for slings and reconstructive surgery have struggled to recommend synthetic mesh slings to their patients with SUI, said Patrick J. Woodman, DO, MS, program director of obstetrics and gynecology residency at Providence Health Ascension Macomb-Oakland, Warren (Mich.) Campus, said in an interview. In 2008, the Food and Drug Administration issued a public health notification for transvaginal placement of surgical mesh in patients with pelvic organ prolapse and SUI.
“Although some of the recommendations first made by the FDA were reasoned and reasonable, such as the need for direct, premarket, patient studies instead of the mostly administrative 510(k) ‘similar-to’ process that had been used previously, physicians and patients had been eagerly awaiting the outcomes of some of these clinical studies that would help answer some of the safety and efficacy questions that had been dogging the transvaginal use of mesh material for years,” he said.
“But, to everyone’s surprise, in April [2019] they called for a recall of all vaginal mesh products, even before the study data could be analyzed, written up and released,” added Dr. Woodman. “Companies were forced to halt production, pull stocks from the shelves, and halt and reverse shipments.”
One reason the results by Berger et al. show midurethral slings have had a good safety record is because of a small incision size and low amount of mesh, noted Dr. Woodman, who was not involved with the study. “This article seems to underline and highlight the fact that reoperation is rare for midurethral slings (for all reasons), but particularly for mesh erosion or exposure. This is well within the experience of most female pelvic medicine and reconstructive surgery and urologic surgeons, and incredibly less than the 8%-24% of mesh exposures reported in the variety of mesh exposure literature on vaginal mesh procedures.”
Despite this safety record, some women may still experience adverse events with midurethral slings, admitted Dr. Woodman. “The fact remains, if a surgeon drags a large piece of synthetic fabric through a ‘clean-contaminated’ vaginal environment, and buries this mesh under the skin of the vagina, and then rests this mesh against a long incision, some women’s immune systems will not be able to handle the resultant inflammation and bacterial load, despite antibiotics, vaginal prepping, and any number of coatings or soakings of the mesh.”
The researchers noted the study’s retrospective nature is one potential limitation, and the data has not been compiled by surgeon type or skill, or considered patients with complications that did not choose reoperations.
“But, the flip side is also true,” said Dr. Woodman, an Ob.Gyn News editorial advisor. “There may have been a number of individuals who had a surgical removal who did not need or warrant it due to the societal, family, or legal ‘suggestion’ that the mesh is now ‘dangerous’ and must be removed at all costs.”
Berger et al. “hit the nail on the head” with the study, including a large amount of patients that demonstrates the safety of midurethral slings, he said. “We need a solid body of unquestioned evidence of safety and effectiveness from which to base solid, evidence-based medical decisions. If there is a way to effectively use mesh to reinforce a vaginal repair in a high-risk woman (for example, with previous failed surgeries), then we have to take the stigma away from its use: because no one wants to use it now, even if it could help.
“The best we can hope for, as physicians, is a rehabilitation of reputation for vaginal mesh,” he concluded.
The study was supported by a grant from the Regional Research Committee of Kaiser Permanente Southern California. One coauthor reported receiving royalties from UptoDate and the American Urogynecologic Society Board Member for travel for board meetings. The other authors reported no relevant conflicts of interest. Dr. Woodman said he had no relevant financial disclosures.*
SOURCE: Berger AA et al. Obstet Gynecol. 2019 Oct 10. doi:10.1097/AOG.0000000000003526.
* Updated 10/14/2019
according to a study published in Obstetrics & Gynecology.
Alexander A. Berger, MD, MPH, of the division of female pelvic medicine and reconstructive surgery at Kaiser Permanente in San Diego, and colleagues performed a retrospective cohort study of 17,030 patients with stress urinary incontinence (SUI) who underwent midurethral sling surgery between 2005 and 2016, examining the reoperation rate at 1 year, 5 years, and 9 years after the procedure, as well as secondary outcomes of mesh revision, mesh removal, and recurrence of SUI.
Overall, the rate of reoperation at 1 year was 2.1% (95% confidence interval, 1.9%-2.4%), was 4.5% at 5 years (95% CI, 4.1%-4.8%) and 6.0% at 9 years (95% CI, 5.5%-6.5%). Compared with white patients, there was a lower rate of reoperation among Asian or Pacific Islander patients.
The rate of reoperation involving mesh removal was 0.7% at 1 year (95% CI, 0.6%-0.8%), 1.0% at 5 years (95% CI, 0.8%-1.1%) and 1.1% at 9 years (95% CI, 0.9%-1.3%).
The rate of recurrent SUI leading to operation was 1.6% at 1 year (95% CI, 1.4%-1.8%), 3.9% at 5 years (95% CI, 3.5%-4.2%) and 5.2% at 9 years (95% CI, 4.7%-5.7%), with more reoperations occurring for patients who received a single-incision sling, rather than a retropubic sling (adjusted hazard ratio, 1.5; 95% CI, 1.06-2.11; P = .03), Dr. Berger and associates wrote.
Urogynecologists, ob.gyns., and urologists who use mesh for slings and reconstructive surgery have struggled to recommend synthetic mesh slings to their patients with SUI, said Patrick J. Woodman, DO, MS, program director of obstetrics and gynecology residency at Providence Health Ascension Macomb-Oakland, Warren (Mich.) Campus, said in an interview. In 2008, the Food and Drug Administration issued a public health notification for transvaginal placement of surgical mesh in patients with pelvic organ prolapse and SUI.
“Although some of the recommendations first made by the FDA were reasoned and reasonable, such as the need for direct, premarket, patient studies instead of the mostly administrative 510(k) ‘similar-to’ process that had been used previously, physicians and patients had been eagerly awaiting the outcomes of some of these clinical studies that would help answer some of the safety and efficacy questions that had been dogging the transvaginal use of mesh material for years,” he said.
“But, to everyone’s surprise, in April [2019] they called for a recall of all vaginal mesh products, even before the study data could be analyzed, written up and released,” added Dr. Woodman. “Companies were forced to halt production, pull stocks from the shelves, and halt and reverse shipments.”
One reason the results by Berger et al. show midurethral slings have had a good safety record is because of a small incision size and low amount of mesh, noted Dr. Woodman, who was not involved with the study. “This article seems to underline and highlight the fact that reoperation is rare for midurethral slings (for all reasons), but particularly for mesh erosion or exposure. This is well within the experience of most female pelvic medicine and reconstructive surgery and urologic surgeons, and incredibly less than the 8%-24% of mesh exposures reported in the variety of mesh exposure literature on vaginal mesh procedures.”
Despite this safety record, some women may still experience adverse events with midurethral slings, admitted Dr. Woodman. “The fact remains, if a surgeon drags a large piece of synthetic fabric through a ‘clean-contaminated’ vaginal environment, and buries this mesh under the skin of the vagina, and then rests this mesh against a long incision, some women’s immune systems will not be able to handle the resultant inflammation and bacterial load, despite antibiotics, vaginal prepping, and any number of coatings or soakings of the mesh.”
The researchers noted the study’s retrospective nature is one potential limitation, and the data has not been compiled by surgeon type or skill, or considered patients with complications that did not choose reoperations.
“But, the flip side is also true,” said Dr. Woodman, an Ob.Gyn News editorial advisor. “There may have been a number of individuals who had a surgical removal who did not need or warrant it due to the societal, family, or legal ‘suggestion’ that the mesh is now ‘dangerous’ and must be removed at all costs.”
Berger et al. “hit the nail on the head” with the study, including a large amount of patients that demonstrates the safety of midurethral slings, he said. “We need a solid body of unquestioned evidence of safety and effectiveness from which to base solid, evidence-based medical decisions. If there is a way to effectively use mesh to reinforce a vaginal repair in a high-risk woman (for example, with previous failed surgeries), then we have to take the stigma away from its use: because no one wants to use it now, even if it could help.
“The best we can hope for, as physicians, is a rehabilitation of reputation for vaginal mesh,” he concluded.
The study was supported by a grant from the Regional Research Committee of Kaiser Permanente Southern California. One coauthor reported receiving royalties from UptoDate and the American Urogynecologic Society Board Member for travel for board meetings. The other authors reported no relevant conflicts of interest. Dr. Woodman said he had no relevant financial disclosures.*
SOURCE: Berger AA et al. Obstet Gynecol. 2019 Oct 10. doi:10.1097/AOG.0000000000003526.
* Updated 10/14/2019
according to a study published in Obstetrics & Gynecology.
Alexander A. Berger, MD, MPH, of the division of female pelvic medicine and reconstructive surgery at Kaiser Permanente in San Diego, and colleagues performed a retrospective cohort study of 17,030 patients with stress urinary incontinence (SUI) who underwent midurethral sling surgery between 2005 and 2016, examining the reoperation rate at 1 year, 5 years, and 9 years after the procedure, as well as secondary outcomes of mesh revision, mesh removal, and recurrence of SUI.
Overall, the rate of reoperation at 1 year was 2.1% (95% confidence interval, 1.9%-2.4%), was 4.5% at 5 years (95% CI, 4.1%-4.8%) and 6.0% at 9 years (95% CI, 5.5%-6.5%). Compared with white patients, there was a lower rate of reoperation among Asian or Pacific Islander patients.
The rate of reoperation involving mesh removal was 0.7% at 1 year (95% CI, 0.6%-0.8%), 1.0% at 5 years (95% CI, 0.8%-1.1%) and 1.1% at 9 years (95% CI, 0.9%-1.3%).
The rate of recurrent SUI leading to operation was 1.6% at 1 year (95% CI, 1.4%-1.8%), 3.9% at 5 years (95% CI, 3.5%-4.2%) and 5.2% at 9 years (95% CI, 4.7%-5.7%), with more reoperations occurring for patients who received a single-incision sling, rather than a retropubic sling (adjusted hazard ratio, 1.5; 95% CI, 1.06-2.11; P = .03), Dr. Berger and associates wrote.
Urogynecologists, ob.gyns., and urologists who use mesh for slings and reconstructive surgery have struggled to recommend synthetic mesh slings to their patients with SUI, said Patrick J. Woodman, DO, MS, program director of obstetrics and gynecology residency at Providence Health Ascension Macomb-Oakland, Warren (Mich.) Campus, said in an interview. In 2008, the Food and Drug Administration issued a public health notification for transvaginal placement of surgical mesh in patients with pelvic organ prolapse and SUI.
“Although some of the recommendations first made by the FDA were reasoned and reasonable, such as the need for direct, premarket, patient studies instead of the mostly administrative 510(k) ‘similar-to’ process that had been used previously, physicians and patients had been eagerly awaiting the outcomes of some of these clinical studies that would help answer some of the safety and efficacy questions that had been dogging the transvaginal use of mesh material for years,” he said.
“But, to everyone’s surprise, in April [2019] they called for a recall of all vaginal mesh products, even before the study data could be analyzed, written up and released,” added Dr. Woodman. “Companies were forced to halt production, pull stocks from the shelves, and halt and reverse shipments.”
One reason the results by Berger et al. show midurethral slings have had a good safety record is because of a small incision size and low amount of mesh, noted Dr. Woodman, who was not involved with the study. “This article seems to underline and highlight the fact that reoperation is rare for midurethral slings (for all reasons), but particularly for mesh erosion or exposure. This is well within the experience of most female pelvic medicine and reconstructive surgery and urologic surgeons, and incredibly less than the 8%-24% of mesh exposures reported in the variety of mesh exposure literature on vaginal mesh procedures.”
Despite this safety record, some women may still experience adverse events with midurethral slings, admitted Dr. Woodman. “The fact remains, if a surgeon drags a large piece of synthetic fabric through a ‘clean-contaminated’ vaginal environment, and buries this mesh under the skin of the vagina, and then rests this mesh against a long incision, some women’s immune systems will not be able to handle the resultant inflammation and bacterial load, despite antibiotics, vaginal prepping, and any number of coatings or soakings of the mesh.”
The researchers noted the study’s retrospective nature is one potential limitation, and the data has not been compiled by surgeon type or skill, or considered patients with complications that did not choose reoperations.
“But, the flip side is also true,” said Dr. Woodman, an Ob.Gyn News editorial advisor. “There may have been a number of individuals who had a surgical removal who did not need or warrant it due to the societal, family, or legal ‘suggestion’ that the mesh is now ‘dangerous’ and must be removed at all costs.”
Berger et al. “hit the nail on the head” with the study, including a large amount of patients that demonstrates the safety of midurethral slings, he said. “We need a solid body of unquestioned evidence of safety and effectiveness from which to base solid, evidence-based medical decisions. If there is a way to effectively use mesh to reinforce a vaginal repair in a high-risk woman (for example, with previous failed surgeries), then we have to take the stigma away from its use: because no one wants to use it now, even if it could help.
“The best we can hope for, as physicians, is a rehabilitation of reputation for vaginal mesh,” he concluded.
The study was supported by a grant from the Regional Research Committee of Kaiser Permanente Southern California. One coauthor reported receiving royalties from UptoDate and the American Urogynecologic Society Board Member for travel for board meetings. The other authors reported no relevant conflicts of interest. Dr. Woodman said he had no relevant financial disclosures.*
SOURCE: Berger AA et al. Obstet Gynecol. 2019 Oct 10. doi:10.1097/AOG.0000000000003526.
* Updated 10/14/2019
FROM OBSTETRICS & GYNECOLOGY
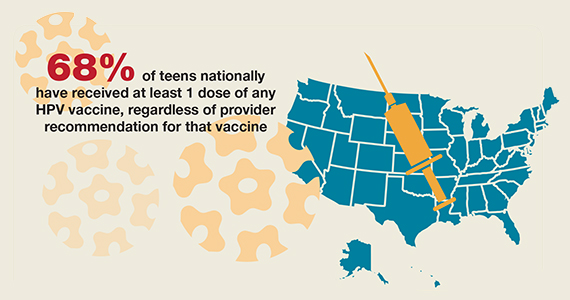
National HPV vaccination rates among teens according to provider recommendation



The electronic medical record’s role in ObGyn burnout and patient care

Physician burnout has been labeled a public health crisis by the Harvard School of Public Health and other institutions.1 A 2018 Physician’s Foundation survey found that 78% of physicians had symptoms of burnout,2 which result from chronic workplace stress and include feeling depleted of energy or exhausted, mentally distanced from or cynical about one’s job, and problems getting one’s job done successfully.3 Among ObGyns, almost half (46%) report burnout.4 One-third of ObGyns responded on a recent Medscape Burnout Report that the computerization of practice is contributing to their burnout, and 54% said too many bureaucratic tasks, including charting, were adding to their burnout.5
Inefficient electronic medical records (EMRs) have been implicated as one reason for burnout, with improvements in efficiency cited as one of several potential resolutions to the problem. About 96% of hospitals have adopted EMRs today, compared with only 9% in 2008,6 and many physicians report recognizing value in the technology. For instance, 60% of participants in Stanford Medicine’s 2018 National Physician Poll said EMRs had led to improved patient care. At the same time, however, about as many (59%) said EMRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54%) as well as from their clinical effectiveness (49%).7
With this roundtable, we explore the concerns with hours spent on the EMR with several experts, and whether it is a problem that has been contributing to burnout among staff at their institutions. In addition, are there solutions that their institutions have implemented that they can share to help to cope with the problem?
John J. Dougherty, MD, MBA: Yes, absolutely. There is not a day that goes by that I don’t hear about or experience “Epic Fails.” (We use Epic’s EMR product at our institution.) Too many clicks are needed to navigate even the simplest tasks—finding notes or results, documenting visits, and billing for services are all unnecessarily complex. In addition, we are being held accountable for achieving a long and growing list of “metrics” measures, education projects (HealthStream), and productivity goals. When do we have time to treat patients? And it is not just practicing physicians and clinicians. Our resident physicians spend an inordinate amount of time in front of the computer documenting, placing orders, and transferring patients using a system with a very inefficient user interface, to say the least.
Megan L. Evans, MD, MPH: I absolutely agree. Over the years, my institution has created a conglomerate of EMRs, requiring physicians across the hospital to be fluent in a multitude of systems. For example, you finish your clinic notes in one system, sign off on discharge summaries in another, and complete your operative notes in an entirely different system. As busy attendings, it is hard to keep ahead of all of these tasks, especially when the systems do not talk to one another. Fortunately, my hospital is changing our EMR to a single system within the next year. Until then, however, we will work in this piecemeal system.
Mark Woodland, MS, MD: EMR and computerization of medicine is the number 1 issue relating to dissatisfaction by ObGyn providers in our institution. Providers are earnest in their attempt to be compliant with EMR requirements, but the reality is that they are dealing with an automated system that does not have realistic expectations for management of results, follow-up tasks, and patient communications for a human provider. The actual charting, ordering of tests and consults, and communication between providers has been enhanced. However, the “in-basket” of tasks to be accomplished are extraordinary and much of it relies on the provider, which requires an inordinate amount of time. Additionally, while other members of the medical staff are stationary at a desk, physicians and other providers are not. They are mobile between inpatient units, labor and delivery, operating rooms, and emergency rooms. Time management does not always allow for providers to access computers from all of these areas to facilitate their managing the expectations of the EMR. This requires providers to access the EMR at off hours, extending their workload. Finally, the EMR is neither personal nor friendly. It is not designed with the clinician in mind, and it is not fun or engaging for a provider.
EMRs are not just inefficient and contributing to physician burnout, according to a joint report from Kaiser Health News (KHN) and Fortune magazine, they are inadequate and contributing to patient safety concerns.1 This was not the intended goal of the HITECH Act, signed into law in 2009 as part of the stimulus bill. HITECH was intended to promote the adoption of meaningful use of health information technology by providing financial incentives to clinicians to adopt electronic medical records (EMRs). It also intended to increase security for health care data--achieved through larger penalties for HIPAA violations.2
Ten years later, however, "America has little to show" for its $36 billion investment, according to KHN and Fortune. Yes, 96% of hospitals have one of the currently available EMRs, among thousands, but they are disconnected. And they are "glitchy." At least 2 EMR vendors have reached settlements with the federal government over egregious patient errors. At least 7 deaths have resulted from errors related to the EMR, according to the firm Quantros, reports KHN and Fortune, and the number of EMR-related safety events tops 18,000. The problem is that information, critical to a patient's well-being, may get buried in the EMR. Clinicians may not have been aware of, because they did not see, a critical medication allergy or piece of patient history.1
The problems with health information technology usability do have solutions, however, asserts Raj M. Ratwani, MD, and colleagues. In a recent article published in the Journal of the American Medical Association, the researchers propose 5 priorities for achieving progress3:
- Establishment of a national database of usability and safety issues. This database should allow sharing of safety information among EMR vendors, hospitals, and clinicians, and make the public aware of any technology risks.
- Establishment of basic design standards, which should promote innovation and be regulated by a board composed of all stakeholders: EMR vendors, researchers, clinicians, and health care organizations.
- Addressing unintended harms. Causes of harm could include "vendor design and development, vendor and health care organization implementation, and customization by the health care organization." Along with shared responsibility and collaboration comes shared liability for harms caused by inadequate usability.
- Simplification of mandated documentation requirements that affect usability. Reducing clinician's "busy work" would go a long way toward simplifying documentation requirements.
- Development of standard usability and safety measures so that progress can be tracked and the market can react. EMR vendors cannot be directly compared currently, since no standards for usability are in place.
Ratwani and colleagues cite shared responsibility and commitment among all of the parties invested in EMR usability success as keys to solving the current challenges affecting health information technology, with policy makers at the helm.3 The federal government is attempting to respond: As part of the 2016 21st Century Cures Act and with an aim toward alleviating physician time spent on the EMR, the Department of Health and Human Services is required to recommend reductions to current EMR burdens required under the HITECH Act. It plans to revise E&M codes, lessening documentation. And the Centers for Medicare and Medicaid Services aims to make meaningful use requirements more flexible, require information exchange between providers and patients, and provide incentive to clinicians to allow patient access to EMRs.4,5
References
- Fry E, Schulte F. Death by a thousand clicks. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- Burde H. The HITECH Act: an overview. AMA J Ethics. March 2011. https://journalofethics.ama-assn.org/article/hitech-act-overview/2011-03. Accessed September 9, 2019.
- Ratwani R, Reider J, Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321:743-744.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Morris G, Anthony ES. 21st Century Cures Act overview for states. Office of the National Coordinator for Health Information Technology. https://www.healthit.gov/sites/default/files/curesactlearningsession_1_v6_10818.pdf. Accessed September 11, 2019.
Continue to:
Dr. Dougherty: When our institution compared EMR offerings, EMR companies put their best collective marketing feet forward. The general notion, at least with the Epic EMR, was that “you can customize Epic to your liking.” It did not take long for a bunch of motivated Epic users to create “smart” stuff (lists, phrases, and texts) in an effort to customize workflows and create fancy-looking electronic notes. Shortly thereafter, it was obvious that, as an institution, our reporting efforts kept coming up short—our reports lacked accuracy and meaning. Everyone was documenting in different ways and in different areas. Considering that reports are currently generated using (mostly) discrete data entries (data placed in specific fields within the EMR), it became obvious that our data entry paradigm needed to change. Therefore, standardization became the leading buzzword. Our institution recently initiated a project aimed at standardizing our workflows and documentation habits. In addition, we have incorporated a third-party information exchange product into our health system data aggregation and analysis workflow. Much more needs to be done, but it is a start.
Dr. Evans: At my institution, as a group, we have created templates for routine procedures and visits that also auto populate billing codes. I know that some departments have used scribes. From the hospital side, there has been improved access to the EMR from home. Some of my colleagues like this feature; however, others, like myself, believe this contributes to some of our burnout. I like to leave work at work. Having the ability to continue working at home is not a solution in my mind.
Dr. Woodland: At our institution, we have engaged our chaperones and medical assistants to help facilitate completion of the medical records during the office visit. Providers work with their assistants to accommodate documentation of history and physical findings while also listening to the provider as they are speaking in order to document patient care plans and orders. This saves the clinicians time in reviewing and editing the record as well as making sure the appropriate care plan is instituted. Our EMR provider recently has begun experimenting with personalization of color themes as well as pictures as part of the interface. Having said this, I still ask, “Why have medical professionals allowed non–clinical agencies and information technology groups to run this show?” It is also inconceivable to me that this unfunded mandate—that has increased cost, decreased clinical efficiency, and decreased clinician satisfaction—has not been addressed by national and international medical communities.
Dr. Woodland: I feel that we need to appropriately manage expectations of the EMR and the institution with relation to EMR and providers. By this I mean that we need to make the EMR more user-friendly and appropriate for different clinicians as well as patients. We also need to manage expectations of our patients. In a digital age where immediate contact is the norm, we need to address the issue that the EMR is not social media but rather a communication tool for routine contact and information transmission. Emergencies are not typically addressed well through the EMR platform; they are better handled with a more appropriate communication interface.
Dr. Dougherty: I feel that the biggest change needed is a competent, simple, and standard user-interface. Our old charting methods were great on a number of levels. For instance, if I wanted to add an order, I flipped to the ”Orders” tab and entered an order. If I needed to document a note, I flipped to the “Notes” tab and started writing, etc. Obviously, manual charting had its downsides—like trying to decipher handwriting art! EMRs could easily adopt the stuff that worked from our old methods of documentation, while leveraging the advantages that computerized workflows can bring to practitioners, including efficient transfer of records, meaningful reporting, simple electronic ordering, and interprofessional communication portals.
Dr. Evans: Our systems need to better communicate with one another. I am in an academic practice, and I should be able to see labs, consultant notes, imaging, all in one spot to improve efficiency and ease with patient visits. Minimizing clicks would be helpful as well. I try to write as much as I can while in the room with a patient to avoid after-hours note writing, but it takes away from my interaction with each patient.
Continue to:
Dr. Evans: When I first started as a new attending, it would take me hours to finish my notes, partly because of the level of detail I would write in my history of present illness (HPI) and assessment and plan. One great piece of advice I received was to be satisfied with good notes, not perfect notes. I worked to consolidate my thoughts and use preconstructed phrases/paragraphs on common problems I saw. This saved time to focus on other aspects of my academic job.
Dr. Dougherty: We need to refocus on the patient first, and mold our systems to meet that priority. Much too often, we have our backs to the patients or spend too much time interfacing with our EMR systems, and our patients are not happy about it (as many surveys have demonstrated). More importantly, a renewed focus on patient care, not EMR care, would allow our practitioners to do what they signed up for—treating patients. In the meantime, I would suggest that practitioners stay away from EMR gimmicks and go back to old-style documentation practices (like those established by the Centers for Medicare and Medicaid Services in 1997 and 1998), and ask the IT folks to help with molding the EMR systems to meet your own standards, not the standards established by EMR companies. I am also very hopeful that the consumer will drive most of the health care-related data collection in the near future, thereby marginalizing the current generation of EMR systems.
Dr. Woodland: I would add that providers need to manage the EMR and not let the EMR manage them. Set up task reminders at point times to handle results and communications from the EMR and set up time in your schedule where you can facilitate meeting these tasks. When providers are out on vacation, make sure to have an out-of-office reminder built into their EMR so that patients and others know timing of potential responses. Try to make the EMR as enjoyable as possible and focus on the good points of the EMR, such as legibility, order verification, safety, and documentation.
1. Engage the computer in your patient encounter, says Rey Wuerth and colleagues. Share the screen, and any test results you are highlighting, with your patient by turning it toward her during your discussion. This can increase patient satisfaction.1
2. Go mobile at the point of care, suggests Tom Giannulli, MD, MS, Chief Medical Information Officer at Kareo. By using a tablet or mobile device, you can enter data while facing a patient or on the go.2
3. Use templates when documenting data, advises Wuerth and colleagues, as pre-filled templates, that are provided through the EMR or that you create within the EMR, can reduce the time required to enter patient visits, findings, and referrals.1
4. Delegate responsibility for routing documents, says Brian Anderson, MD. Hand off to staff administrative duties, such as patient forms and routine negative test results.3
5. Involve medical assistants (MAs) in the process. Make the MA feel part of the team, says R. Scott Eden, and assign them history-taking responsibilities, utilizing your EMR's templates. Assign them other tasks as well, including medication reconciliation, referrals, refills, routine screening, and patient education.4
6. Employ physical or virtual scribes who are specifically assigned to EMR duty. Although drawbacks can include patient privacy concerns and reduced practice income due to salary requirements, employing a scribe (often a pre-medical or graduate student), who trails you on patient visits, or who is connected virtually, can leave the clinician free to interact with patients.5,6
References
- Wuerth R, Campbell C, Peng MD, et al. Top 10 tips for effective use of electronic health records. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959973/. Paediatr Child Health. 2014;19:138.
- Giannulli T. 7 time-saving EHR use tips to boost physician productivity. April 28, 2016. https://ehrintelligence.com/news/7-time-saving-emr-use-tips-to-boost-physician-productivity. Accessed September 9, 2019.
- Anderson B. 5 ways to increase your EMR efficiency. October 28, 2014. https://www.kevinmd.com/blog/2014/10/5-ways-increase-emr-efficiency.html. Accessed September 9, 2019.
- Eden RS. Maximizing your medical assistant's role. Fam Pract Manag. 2016;23:5-7. https://www.aafp.org/fpm/2016/0500/p5.html.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Caliri A. The case for virtual scribes. January 2, 2019. Becker's Hospital Review. https://www.beckershospitalreview.com/hospital-physician-relationships/the-case-for-virtual-scribes.html. Accessed September 20, 2019.
Dr. Evans: Yes and no. Yes, in that it can be much easier to follow a patient’s health care history from other provider notes or prior surgeries. Information is searchable and legible. If an EMR is built correctly, it can save time for providers, through smart phrases and templates, and it can help providers with proper billing codes and documentation requirements. No, in that it can take away from important patient interaction. We are required to see more patients in less time all while using, at times, a cumbersome EMR system.
Dr. Woodland: This is a tricky question because the EMR has both positive and negative attributes. Certainly, the legibility and order verification has improved, but the ease of accessing information in the EMR has changed. Additionally, there has been a drastic increase in provider dissatisfaction that has not been addressed. Provider dissatisfaction can lead to problems in patient care. If there was a clear-cut increased value for the cost, I do not think the EMR would be such a huge focus of negative attention. Providers need to take back control of their EMR and their profession so that they can utilize the EMR as the tool it was supposed to be and not the dissatisfier that it has become.
Dr. Dougherty: I do not believe patient care has been improved by EMR systems, for all of the reasons we have discussed, and then some. But there is an enormous amount of potential, if we get the interface between humans and EMR systems right!
- A crisis in health care: a call to action on physician burnout. Massachusetts Health and Hospital Association. Massachusetts Medical Society. Harvard T.H. Chan School of Public Health. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/21/2019/01/PhysicianBurnoutReport2018FINAL.pdf. Accessed September 9, 2019.
- Physician’s Foundation. 2018 survey of America’s physicians practice patterns and perspectives. https://physiciansfoundation.org/wp-content/uploads/2018/09/physicians-survey-results-final-2018.pdf. Accessed September 9, 2019.
- Burn-out. ICD-11 for Mortality and Morbidity Statistics. Version 04/2019. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/129180281. Accessed September 11, 2019.
- Peckham C. Medscape National Physician Burnout & Depression Report 2018. January 17, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235#3. Accessed September 9, 2019.
- Kane L. Medscape National Physician Burnout, Depression & Suicide Report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#5. Accessed September 9, 2019.
- Fry E, Schulte F. Death by a thousand clicks: where electronic health records went wrong. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- How doctors feel about electronic health records: National Physician Poll by The Harris Poll. https://med.stanford.edu/content/dam/sm/ehr/documents/EHR-Poll-Presentation.pdf. Accessed September 9, 2019.

Physician burnout has been labeled a public health crisis by the Harvard School of Public Health and other institutions.1 A 2018 Physician’s Foundation survey found that 78% of physicians had symptoms of burnout,2 which result from chronic workplace stress and include feeling depleted of energy or exhausted, mentally distanced from or cynical about one’s job, and problems getting one’s job done successfully.3 Among ObGyns, almost half (46%) report burnout.4 One-third of ObGyns responded on a recent Medscape Burnout Report that the computerization of practice is contributing to their burnout, and 54% said too many bureaucratic tasks, including charting, were adding to their burnout.5
Inefficient electronic medical records (EMRs) have been implicated as one reason for burnout, with improvements in efficiency cited as one of several potential resolutions to the problem. About 96% of hospitals have adopted EMRs today, compared with only 9% in 2008,6 and many physicians report recognizing value in the technology. For instance, 60% of participants in Stanford Medicine’s 2018 National Physician Poll said EMRs had led to improved patient care. At the same time, however, about as many (59%) said EMRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54%) as well as from their clinical effectiveness (49%).7
With this roundtable, we explore the concerns with hours spent on the EMR with several experts, and whether it is a problem that has been contributing to burnout among staff at their institutions. In addition, are there solutions that their institutions have implemented that they can share to help to cope with the problem?
John J. Dougherty, MD, MBA: Yes, absolutely. There is not a day that goes by that I don’t hear about or experience “Epic Fails.” (We use Epic’s EMR product at our institution.) Too many clicks are needed to navigate even the simplest tasks—finding notes or results, documenting visits, and billing for services are all unnecessarily complex. In addition, we are being held accountable for achieving a long and growing list of “metrics” measures, education projects (HealthStream), and productivity goals. When do we have time to treat patients? And it is not just practicing physicians and clinicians. Our resident physicians spend an inordinate amount of time in front of the computer documenting, placing orders, and transferring patients using a system with a very inefficient user interface, to say the least.
Megan L. Evans, MD, MPH: I absolutely agree. Over the years, my institution has created a conglomerate of EMRs, requiring physicians across the hospital to be fluent in a multitude of systems. For example, you finish your clinic notes in one system, sign off on discharge summaries in another, and complete your operative notes in an entirely different system. As busy attendings, it is hard to keep ahead of all of these tasks, especially when the systems do not talk to one another. Fortunately, my hospital is changing our EMR to a single system within the next year. Until then, however, we will work in this piecemeal system.
Mark Woodland, MS, MD: EMR and computerization of medicine is the number 1 issue relating to dissatisfaction by ObGyn providers in our institution. Providers are earnest in their attempt to be compliant with EMR requirements, but the reality is that they are dealing with an automated system that does not have realistic expectations for management of results, follow-up tasks, and patient communications for a human provider. The actual charting, ordering of tests and consults, and communication between providers has been enhanced. However, the “in-basket” of tasks to be accomplished are extraordinary and much of it relies on the provider, which requires an inordinate amount of time. Additionally, while other members of the medical staff are stationary at a desk, physicians and other providers are not. They are mobile between inpatient units, labor and delivery, operating rooms, and emergency rooms. Time management does not always allow for providers to access computers from all of these areas to facilitate their managing the expectations of the EMR. This requires providers to access the EMR at off hours, extending their workload. Finally, the EMR is neither personal nor friendly. It is not designed with the clinician in mind, and it is not fun or engaging for a provider.
EMRs are not just inefficient and contributing to physician burnout, according to a joint report from Kaiser Health News (KHN) and Fortune magazine, they are inadequate and contributing to patient safety concerns.1 This was not the intended goal of the HITECH Act, signed into law in 2009 as part of the stimulus bill. HITECH was intended to promote the adoption of meaningful use of health information technology by providing financial incentives to clinicians to adopt electronic medical records (EMRs). It also intended to increase security for health care data--achieved through larger penalties for HIPAA violations.2
Ten years later, however, "America has little to show" for its $36 billion investment, according to KHN and Fortune. Yes, 96% of hospitals have one of the currently available EMRs, among thousands, but they are disconnected. And they are "glitchy." At least 2 EMR vendors have reached settlements with the federal government over egregious patient errors. At least 7 deaths have resulted from errors related to the EMR, according to the firm Quantros, reports KHN and Fortune, and the number of EMR-related safety events tops 18,000. The problem is that information, critical to a patient's well-being, may get buried in the EMR. Clinicians may not have been aware of, because they did not see, a critical medication allergy or piece of patient history.1
The problems with health information technology usability do have solutions, however, asserts Raj M. Ratwani, MD, and colleagues. In a recent article published in the Journal of the American Medical Association, the researchers propose 5 priorities for achieving progress3:
- Establishment of a national database of usability and safety issues. This database should allow sharing of safety information among EMR vendors, hospitals, and clinicians, and make the public aware of any technology risks.
- Establishment of basic design standards, which should promote innovation and be regulated by a board composed of all stakeholders: EMR vendors, researchers, clinicians, and health care organizations.
- Addressing unintended harms. Causes of harm could include "vendor design and development, vendor and health care organization implementation, and customization by the health care organization." Along with shared responsibility and collaboration comes shared liability for harms caused by inadequate usability.
- Simplification of mandated documentation requirements that affect usability. Reducing clinician's "busy work" would go a long way toward simplifying documentation requirements.
- Development of standard usability and safety measures so that progress can be tracked and the market can react. EMR vendors cannot be directly compared currently, since no standards for usability are in place.
Ratwani and colleagues cite shared responsibility and commitment among all of the parties invested in EMR usability success as keys to solving the current challenges affecting health information technology, with policy makers at the helm.3 The federal government is attempting to respond: As part of the 2016 21st Century Cures Act and with an aim toward alleviating physician time spent on the EMR, the Department of Health and Human Services is required to recommend reductions to current EMR burdens required under the HITECH Act. It plans to revise E&M codes, lessening documentation. And the Centers for Medicare and Medicaid Services aims to make meaningful use requirements more flexible, require information exchange between providers and patients, and provide incentive to clinicians to allow patient access to EMRs.4,5
References
- Fry E, Schulte F. Death by a thousand clicks. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- Burde H. The HITECH Act: an overview. AMA J Ethics. March 2011. https://journalofethics.ama-assn.org/article/hitech-act-overview/2011-03. Accessed September 9, 2019.
- Ratwani R, Reider J, Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321:743-744.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Morris G, Anthony ES. 21st Century Cures Act overview for states. Office of the National Coordinator for Health Information Technology. https://www.healthit.gov/sites/default/files/curesactlearningsession_1_v6_10818.pdf. Accessed September 11, 2019.
Continue to:
Dr. Dougherty: When our institution compared EMR offerings, EMR companies put their best collective marketing feet forward. The general notion, at least with the Epic EMR, was that “you can customize Epic to your liking.” It did not take long for a bunch of motivated Epic users to create “smart” stuff (lists, phrases, and texts) in an effort to customize workflows and create fancy-looking electronic notes. Shortly thereafter, it was obvious that, as an institution, our reporting efforts kept coming up short—our reports lacked accuracy and meaning. Everyone was documenting in different ways and in different areas. Considering that reports are currently generated using (mostly) discrete data entries (data placed in specific fields within the EMR), it became obvious that our data entry paradigm needed to change. Therefore, standardization became the leading buzzword. Our institution recently initiated a project aimed at standardizing our workflows and documentation habits. In addition, we have incorporated a third-party information exchange product into our health system data aggregation and analysis workflow. Much more needs to be done, but it is a start.
Dr. Evans: At my institution, as a group, we have created templates for routine procedures and visits that also auto populate billing codes. I know that some departments have used scribes. From the hospital side, there has been improved access to the EMR from home. Some of my colleagues like this feature; however, others, like myself, believe this contributes to some of our burnout. I like to leave work at work. Having the ability to continue working at home is not a solution in my mind.
Dr. Woodland: At our institution, we have engaged our chaperones and medical assistants to help facilitate completion of the medical records during the office visit. Providers work with their assistants to accommodate documentation of history and physical findings while also listening to the provider as they are speaking in order to document patient care plans and orders. This saves the clinicians time in reviewing and editing the record as well as making sure the appropriate care plan is instituted. Our EMR provider recently has begun experimenting with personalization of color themes as well as pictures as part of the interface. Having said this, I still ask, “Why have medical professionals allowed non–clinical agencies and information technology groups to run this show?” It is also inconceivable to me that this unfunded mandate—that has increased cost, decreased clinical efficiency, and decreased clinician satisfaction—has not been addressed by national and international medical communities.
Dr. Woodland: I feel that we need to appropriately manage expectations of the EMR and the institution with relation to EMR and providers. By this I mean that we need to make the EMR more user-friendly and appropriate for different clinicians as well as patients. We also need to manage expectations of our patients. In a digital age where immediate contact is the norm, we need to address the issue that the EMR is not social media but rather a communication tool for routine contact and information transmission. Emergencies are not typically addressed well through the EMR platform; they are better handled with a more appropriate communication interface.
Dr. Dougherty: I feel that the biggest change needed is a competent, simple, and standard user-interface. Our old charting methods were great on a number of levels. For instance, if I wanted to add an order, I flipped to the ”Orders” tab and entered an order. If I needed to document a note, I flipped to the “Notes” tab and started writing, etc. Obviously, manual charting had its downsides—like trying to decipher handwriting art! EMRs could easily adopt the stuff that worked from our old methods of documentation, while leveraging the advantages that computerized workflows can bring to practitioners, including efficient transfer of records, meaningful reporting, simple electronic ordering, and interprofessional communication portals.
Dr. Evans: Our systems need to better communicate with one another. I am in an academic practice, and I should be able to see labs, consultant notes, imaging, all in one spot to improve efficiency and ease with patient visits. Minimizing clicks would be helpful as well. I try to write as much as I can while in the room with a patient to avoid after-hours note writing, but it takes away from my interaction with each patient.
Continue to:
Dr. Evans: When I first started as a new attending, it would take me hours to finish my notes, partly because of the level of detail I would write in my history of present illness (HPI) and assessment and plan. One great piece of advice I received was to be satisfied with good notes, not perfect notes. I worked to consolidate my thoughts and use preconstructed phrases/paragraphs on common problems I saw. This saved time to focus on other aspects of my academic job.
Dr. Dougherty: We need to refocus on the patient first, and mold our systems to meet that priority. Much too often, we have our backs to the patients or spend too much time interfacing with our EMR systems, and our patients are not happy about it (as many surveys have demonstrated). More importantly, a renewed focus on patient care, not EMR care, would allow our practitioners to do what they signed up for—treating patients. In the meantime, I would suggest that practitioners stay away from EMR gimmicks and go back to old-style documentation practices (like those established by the Centers for Medicare and Medicaid Services in 1997 and 1998), and ask the IT folks to help with molding the EMR systems to meet your own standards, not the standards established by EMR companies. I am also very hopeful that the consumer will drive most of the health care-related data collection in the near future, thereby marginalizing the current generation of EMR systems.
Dr. Woodland: I would add that providers need to manage the EMR and not let the EMR manage them. Set up task reminders at point times to handle results and communications from the EMR and set up time in your schedule where you can facilitate meeting these tasks. When providers are out on vacation, make sure to have an out-of-office reminder built into their EMR so that patients and others know timing of potential responses. Try to make the EMR as enjoyable as possible and focus on the good points of the EMR, such as legibility, order verification, safety, and documentation.
1. Engage the computer in your patient encounter, says Rey Wuerth and colleagues. Share the screen, and any test results you are highlighting, with your patient by turning it toward her during your discussion. This can increase patient satisfaction.1
2. Go mobile at the point of care, suggests Tom Giannulli, MD, MS, Chief Medical Information Officer at Kareo. By using a tablet or mobile device, you can enter data while facing a patient or on the go.2
3. Use templates when documenting data, advises Wuerth and colleagues, as pre-filled templates, that are provided through the EMR or that you create within the EMR, can reduce the time required to enter patient visits, findings, and referrals.1
4. Delegate responsibility for routing documents, says Brian Anderson, MD. Hand off to staff administrative duties, such as patient forms and routine negative test results.3
5. Involve medical assistants (MAs) in the process. Make the MA feel part of the team, says R. Scott Eden, and assign them history-taking responsibilities, utilizing your EMR's templates. Assign them other tasks as well, including medication reconciliation, referrals, refills, routine screening, and patient education.4
6. Employ physical or virtual scribes who are specifically assigned to EMR duty. Although drawbacks can include patient privacy concerns and reduced practice income due to salary requirements, employing a scribe (often a pre-medical or graduate student), who trails you on patient visits, or who is connected virtually, can leave the clinician free to interact with patients.5,6
References
- Wuerth R, Campbell C, Peng MD, et al. Top 10 tips for effective use of electronic health records. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959973/. Paediatr Child Health. 2014;19:138.
- Giannulli T. 7 time-saving EHR use tips to boost physician productivity. April 28, 2016. https://ehrintelligence.com/news/7-time-saving-emr-use-tips-to-boost-physician-productivity. Accessed September 9, 2019.
- Anderson B. 5 ways to increase your EMR efficiency. October 28, 2014. https://www.kevinmd.com/blog/2014/10/5-ways-increase-emr-efficiency.html. Accessed September 9, 2019.
- Eden RS. Maximizing your medical assistant's role. Fam Pract Manag. 2016;23:5-7. https://www.aafp.org/fpm/2016/0500/p5.html.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Caliri A. The case for virtual scribes. January 2, 2019. Becker's Hospital Review. https://www.beckershospitalreview.com/hospital-physician-relationships/the-case-for-virtual-scribes.html. Accessed September 20, 2019.
Dr. Evans: Yes and no. Yes, in that it can be much easier to follow a patient’s health care history from other provider notes or prior surgeries. Information is searchable and legible. If an EMR is built correctly, it can save time for providers, through smart phrases and templates, and it can help providers with proper billing codes and documentation requirements. No, in that it can take away from important patient interaction. We are required to see more patients in less time all while using, at times, a cumbersome EMR system.
Dr. Woodland: This is a tricky question because the EMR has both positive and negative attributes. Certainly, the legibility and order verification has improved, but the ease of accessing information in the EMR has changed. Additionally, there has been a drastic increase in provider dissatisfaction that has not been addressed. Provider dissatisfaction can lead to problems in patient care. If there was a clear-cut increased value for the cost, I do not think the EMR would be such a huge focus of negative attention. Providers need to take back control of their EMR and their profession so that they can utilize the EMR as the tool it was supposed to be and not the dissatisfier that it has become.
Dr. Dougherty: I do not believe patient care has been improved by EMR systems, for all of the reasons we have discussed, and then some. But there is an enormous amount of potential, if we get the interface between humans and EMR systems right!

Physician burnout has been labeled a public health crisis by the Harvard School of Public Health and other institutions.1 A 2018 Physician’s Foundation survey found that 78% of physicians had symptoms of burnout,2 which result from chronic workplace stress and include feeling depleted of energy or exhausted, mentally distanced from or cynical about one’s job, and problems getting one’s job done successfully.3 Among ObGyns, almost half (46%) report burnout.4 One-third of ObGyns responded on a recent Medscape Burnout Report that the computerization of practice is contributing to their burnout, and 54% said too many bureaucratic tasks, including charting, were adding to their burnout.5
Inefficient electronic medical records (EMRs) have been implicated as one reason for burnout, with improvements in efficiency cited as one of several potential resolutions to the problem. About 96% of hospitals have adopted EMRs today, compared with only 9% in 2008,6 and many physicians report recognizing value in the technology. For instance, 60% of participants in Stanford Medicine’s 2018 National Physician Poll said EMRs had led to improved patient care. At the same time, however, about as many (59%) said EMRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54%) as well as from their clinical effectiveness (49%).7
With this roundtable, we explore the concerns with hours spent on the EMR with several experts, and whether it is a problem that has been contributing to burnout among staff at their institutions. In addition, are there solutions that their institutions have implemented that they can share to help to cope with the problem?
John J. Dougherty, MD, MBA: Yes, absolutely. There is not a day that goes by that I don’t hear about or experience “Epic Fails.” (We use Epic’s EMR product at our institution.) Too many clicks are needed to navigate even the simplest tasks—finding notes or results, documenting visits, and billing for services are all unnecessarily complex. In addition, we are being held accountable for achieving a long and growing list of “metrics” measures, education projects (HealthStream), and productivity goals. When do we have time to treat patients? And it is not just practicing physicians and clinicians. Our resident physicians spend an inordinate amount of time in front of the computer documenting, placing orders, and transferring patients using a system with a very inefficient user interface, to say the least.
Megan L. Evans, MD, MPH: I absolutely agree. Over the years, my institution has created a conglomerate of EMRs, requiring physicians across the hospital to be fluent in a multitude of systems. For example, you finish your clinic notes in one system, sign off on discharge summaries in another, and complete your operative notes in an entirely different system. As busy attendings, it is hard to keep ahead of all of these tasks, especially when the systems do not talk to one another. Fortunately, my hospital is changing our EMR to a single system within the next year. Until then, however, we will work in this piecemeal system.
Mark Woodland, MS, MD: EMR and computerization of medicine is the number 1 issue relating to dissatisfaction by ObGyn providers in our institution. Providers are earnest in their attempt to be compliant with EMR requirements, but the reality is that they are dealing with an automated system that does not have realistic expectations for management of results, follow-up tasks, and patient communications for a human provider. The actual charting, ordering of tests and consults, and communication between providers has been enhanced. However, the “in-basket” of tasks to be accomplished are extraordinary and much of it relies on the provider, which requires an inordinate amount of time. Additionally, while other members of the medical staff are stationary at a desk, physicians and other providers are not. They are mobile between inpatient units, labor and delivery, operating rooms, and emergency rooms. Time management does not always allow for providers to access computers from all of these areas to facilitate their managing the expectations of the EMR. This requires providers to access the EMR at off hours, extending their workload. Finally, the EMR is neither personal nor friendly. It is not designed with the clinician in mind, and it is not fun or engaging for a provider.
EMRs are not just inefficient and contributing to physician burnout, according to a joint report from Kaiser Health News (KHN) and Fortune magazine, they are inadequate and contributing to patient safety concerns.1 This was not the intended goal of the HITECH Act, signed into law in 2009 as part of the stimulus bill. HITECH was intended to promote the adoption of meaningful use of health information technology by providing financial incentives to clinicians to adopt electronic medical records (EMRs). It also intended to increase security for health care data--achieved through larger penalties for HIPAA violations.2
Ten years later, however, "America has little to show" for its $36 billion investment, according to KHN and Fortune. Yes, 96% of hospitals have one of the currently available EMRs, among thousands, but they are disconnected. And they are "glitchy." At least 2 EMR vendors have reached settlements with the federal government over egregious patient errors. At least 7 deaths have resulted from errors related to the EMR, according to the firm Quantros, reports KHN and Fortune, and the number of EMR-related safety events tops 18,000. The problem is that information, critical to a patient's well-being, may get buried in the EMR. Clinicians may not have been aware of, because they did not see, a critical medication allergy or piece of patient history.1
The problems with health information technology usability do have solutions, however, asserts Raj M. Ratwani, MD, and colleagues. In a recent article published in the Journal of the American Medical Association, the researchers propose 5 priorities for achieving progress3:
- Establishment of a national database of usability and safety issues. This database should allow sharing of safety information among EMR vendors, hospitals, and clinicians, and make the public aware of any technology risks.
- Establishment of basic design standards, which should promote innovation and be regulated by a board composed of all stakeholders: EMR vendors, researchers, clinicians, and health care organizations.
- Addressing unintended harms. Causes of harm could include "vendor design and development, vendor and health care organization implementation, and customization by the health care organization." Along with shared responsibility and collaboration comes shared liability for harms caused by inadequate usability.
- Simplification of mandated documentation requirements that affect usability. Reducing clinician's "busy work" would go a long way toward simplifying documentation requirements.
- Development of standard usability and safety measures so that progress can be tracked and the market can react. EMR vendors cannot be directly compared currently, since no standards for usability are in place.
Ratwani and colleagues cite shared responsibility and commitment among all of the parties invested in EMR usability success as keys to solving the current challenges affecting health information technology, with policy makers at the helm.3 The federal government is attempting to respond: As part of the 2016 21st Century Cures Act and with an aim toward alleviating physician time spent on the EMR, the Department of Health and Human Services is required to recommend reductions to current EMR burdens required under the HITECH Act. It plans to revise E&M codes, lessening documentation. And the Centers for Medicare and Medicaid Services aims to make meaningful use requirements more flexible, require information exchange between providers and patients, and provide incentive to clinicians to allow patient access to EMRs.4,5
References
- Fry E, Schulte F. Death by a thousand clicks. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- Burde H. The HITECH Act: an overview. AMA J Ethics. March 2011. https://journalofethics.ama-assn.org/article/hitech-act-overview/2011-03. Accessed September 9, 2019.
- Ratwani R, Reider J, Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321:743-744.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Morris G, Anthony ES. 21st Century Cures Act overview for states. Office of the National Coordinator for Health Information Technology. https://www.healthit.gov/sites/default/files/curesactlearningsession_1_v6_10818.pdf. Accessed September 11, 2019.
Continue to:
Dr. Dougherty: When our institution compared EMR offerings, EMR companies put their best collective marketing feet forward. The general notion, at least with the Epic EMR, was that “you can customize Epic to your liking.” It did not take long for a bunch of motivated Epic users to create “smart” stuff (lists, phrases, and texts) in an effort to customize workflows and create fancy-looking electronic notes. Shortly thereafter, it was obvious that, as an institution, our reporting efforts kept coming up short—our reports lacked accuracy and meaning. Everyone was documenting in different ways and in different areas. Considering that reports are currently generated using (mostly) discrete data entries (data placed in specific fields within the EMR), it became obvious that our data entry paradigm needed to change. Therefore, standardization became the leading buzzword. Our institution recently initiated a project aimed at standardizing our workflows and documentation habits. In addition, we have incorporated a third-party information exchange product into our health system data aggregation and analysis workflow. Much more needs to be done, but it is a start.
Dr. Evans: At my institution, as a group, we have created templates for routine procedures and visits that also auto populate billing codes. I know that some departments have used scribes. From the hospital side, there has been improved access to the EMR from home. Some of my colleagues like this feature; however, others, like myself, believe this contributes to some of our burnout. I like to leave work at work. Having the ability to continue working at home is not a solution in my mind.
Dr. Woodland: At our institution, we have engaged our chaperones and medical assistants to help facilitate completion of the medical records during the office visit. Providers work with their assistants to accommodate documentation of history and physical findings while also listening to the provider as they are speaking in order to document patient care plans and orders. This saves the clinicians time in reviewing and editing the record as well as making sure the appropriate care plan is instituted. Our EMR provider recently has begun experimenting with personalization of color themes as well as pictures as part of the interface. Having said this, I still ask, “Why have medical professionals allowed non–clinical agencies and information technology groups to run this show?” It is also inconceivable to me that this unfunded mandate—that has increased cost, decreased clinical efficiency, and decreased clinician satisfaction—has not been addressed by national and international medical communities.
Dr. Woodland: I feel that we need to appropriately manage expectations of the EMR and the institution with relation to EMR and providers. By this I mean that we need to make the EMR more user-friendly and appropriate for different clinicians as well as patients. We also need to manage expectations of our patients. In a digital age where immediate contact is the norm, we need to address the issue that the EMR is not social media but rather a communication tool for routine contact and information transmission. Emergencies are not typically addressed well through the EMR platform; they are better handled with a more appropriate communication interface.
Dr. Dougherty: I feel that the biggest change needed is a competent, simple, and standard user-interface. Our old charting methods were great on a number of levels. For instance, if I wanted to add an order, I flipped to the ”Orders” tab and entered an order. If I needed to document a note, I flipped to the “Notes” tab and started writing, etc. Obviously, manual charting had its downsides—like trying to decipher handwriting art! EMRs could easily adopt the stuff that worked from our old methods of documentation, while leveraging the advantages that computerized workflows can bring to practitioners, including efficient transfer of records, meaningful reporting, simple electronic ordering, and interprofessional communication portals.
Dr. Evans: Our systems need to better communicate with one another. I am in an academic practice, and I should be able to see labs, consultant notes, imaging, all in one spot to improve efficiency and ease with patient visits. Minimizing clicks would be helpful as well. I try to write as much as I can while in the room with a patient to avoid after-hours note writing, but it takes away from my interaction with each patient.
Continue to:
Dr. Evans: When I first started as a new attending, it would take me hours to finish my notes, partly because of the level of detail I would write in my history of present illness (HPI) and assessment and plan. One great piece of advice I received was to be satisfied with good notes, not perfect notes. I worked to consolidate my thoughts and use preconstructed phrases/paragraphs on common problems I saw. This saved time to focus on other aspects of my academic job.
Dr. Dougherty: We need to refocus on the patient first, and mold our systems to meet that priority. Much too often, we have our backs to the patients or spend too much time interfacing with our EMR systems, and our patients are not happy about it (as many surveys have demonstrated). More importantly, a renewed focus on patient care, not EMR care, would allow our practitioners to do what they signed up for—treating patients. In the meantime, I would suggest that practitioners stay away from EMR gimmicks and go back to old-style documentation practices (like those established by the Centers for Medicare and Medicaid Services in 1997 and 1998), and ask the IT folks to help with molding the EMR systems to meet your own standards, not the standards established by EMR companies. I am also very hopeful that the consumer will drive most of the health care-related data collection in the near future, thereby marginalizing the current generation of EMR systems.
Dr. Woodland: I would add that providers need to manage the EMR and not let the EMR manage them. Set up task reminders at point times to handle results and communications from the EMR and set up time in your schedule where you can facilitate meeting these tasks. When providers are out on vacation, make sure to have an out-of-office reminder built into their EMR so that patients and others know timing of potential responses. Try to make the EMR as enjoyable as possible and focus on the good points of the EMR, such as legibility, order verification, safety, and documentation.
1. Engage the computer in your patient encounter, says Rey Wuerth and colleagues. Share the screen, and any test results you are highlighting, with your patient by turning it toward her during your discussion. This can increase patient satisfaction.1
2. Go mobile at the point of care, suggests Tom Giannulli, MD, MS, Chief Medical Information Officer at Kareo. By using a tablet or mobile device, you can enter data while facing a patient or on the go.2
3. Use templates when documenting data, advises Wuerth and colleagues, as pre-filled templates, that are provided through the EMR or that you create within the EMR, can reduce the time required to enter patient visits, findings, and referrals.1
4. Delegate responsibility for routing documents, says Brian Anderson, MD. Hand off to staff administrative duties, such as patient forms and routine negative test results.3
5. Involve medical assistants (MAs) in the process. Make the MA feel part of the team, says R. Scott Eden, and assign them history-taking responsibilities, utilizing your EMR's templates. Assign them other tasks as well, including medication reconciliation, referrals, refills, routine screening, and patient education.4
6. Employ physical or virtual scribes who are specifically assigned to EMR duty. Although drawbacks can include patient privacy concerns and reduced practice income due to salary requirements, employing a scribe (often a pre-medical or graduate student), who trails you on patient visits, or who is connected virtually, can leave the clinician free to interact with patients.5,6
References
- Wuerth R, Campbell C, Peng MD, et al. Top 10 tips for effective use of electronic health records. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959973/. Paediatr Child Health. 2014;19:138.
- Giannulli T. 7 time-saving EHR use tips to boost physician productivity. April 28, 2016. https://ehrintelligence.com/news/7-time-saving-emr-use-tips-to-boost-physician-productivity. Accessed September 9, 2019.
- Anderson B. 5 ways to increase your EMR efficiency. October 28, 2014. https://www.kevinmd.com/blog/2014/10/5-ways-increase-emr-efficiency.html. Accessed September 9, 2019.
- Eden RS. Maximizing your medical assistant's role. Fam Pract Manag. 2016;23:5-7. https://www.aafp.org/fpm/2016/0500/p5.html.
- Hoffman S. Healing the healers: legal remedies for physician burnout. Case Western Reserve University School of Law. September 2018.
- Caliri A. The case for virtual scribes. January 2, 2019. Becker's Hospital Review. https://www.beckershospitalreview.com/hospital-physician-relationships/the-case-for-virtual-scribes.html. Accessed September 20, 2019.
Dr. Evans: Yes and no. Yes, in that it can be much easier to follow a patient’s health care history from other provider notes or prior surgeries. Information is searchable and legible. If an EMR is built correctly, it can save time for providers, through smart phrases and templates, and it can help providers with proper billing codes and documentation requirements. No, in that it can take away from important patient interaction. We are required to see more patients in less time all while using, at times, a cumbersome EMR system.
Dr. Woodland: This is a tricky question because the EMR has both positive and negative attributes. Certainly, the legibility and order verification has improved, but the ease of accessing information in the EMR has changed. Additionally, there has been a drastic increase in provider dissatisfaction that has not been addressed. Provider dissatisfaction can lead to problems in patient care. If there was a clear-cut increased value for the cost, I do not think the EMR would be such a huge focus of negative attention. Providers need to take back control of their EMR and their profession so that they can utilize the EMR as the tool it was supposed to be and not the dissatisfier that it has become.
Dr. Dougherty: I do not believe patient care has been improved by EMR systems, for all of the reasons we have discussed, and then some. But there is an enormous amount of potential, if we get the interface between humans and EMR systems right!
- A crisis in health care: a call to action on physician burnout. Massachusetts Health and Hospital Association. Massachusetts Medical Society. Harvard T.H. Chan School of Public Health. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/21/2019/01/PhysicianBurnoutReport2018FINAL.pdf. Accessed September 9, 2019.
- Physician’s Foundation. 2018 survey of America’s physicians practice patterns and perspectives. https://physiciansfoundation.org/wp-content/uploads/2018/09/physicians-survey-results-final-2018.pdf. Accessed September 9, 2019.
- Burn-out. ICD-11 for Mortality and Morbidity Statistics. Version 04/2019. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/129180281. Accessed September 11, 2019.
- Peckham C. Medscape National Physician Burnout & Depression Report 2018. January 17, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235#3. Accessed September 9, 2019.
- Kane L. Medscape National Physician Burnout, Depression & Suicide Report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#5. Accessed September 9, 2019.
- Fry E, Schulte F. Death by a thousand clicks: where electronic health records went wrong. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- How doctors feel about electronic health records: National Physician Poll by The Harris Poll. https://med.stanford.edu/content/dam/sm/ehr/documents/EHR-Poll-Presentation.pdf. Accessed September 9, 2019.
- A crisis in health care: a call to action on physician burnout. Massachusetts Health and Hospital Association. Massachusetts Medical Society. Harvard T.H. Chan School of Public Health. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/21/2019/01/PhysicianBurnoutReport2018FINAL.pdf. Accessed September 9, 2019.
- Physician’s Foundation. 2018 survey of America’s physicians practice patterns and perspectives. https://physiciansfoundation.org/wp-content/uploads/2018/09/physicians-survey-results-final-2018.pdf. Accessed September 9, 2019.
- Burn-out. ICD-11 for Mortality and Morbidity Statistics. Version 04/2019. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/129180281. Accessed September 11, 2019.
- Peckham C. Medscape National Physician Burnout & Depression Report 2018. January 17, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235#3. Accessed September 9, 2019.
- Kane L. Medscape National Physician Burnout, Depression & Suicide Report 2019. January 16, 2019. https://www.medscape.com/slideshow/2019-lifestyle-burnout-depression-6011056#5. Accessed September 9, 2019.
- Fry E, Schulte F. Death by a thousand clicks: where electronic health records went wrong. Fortune. March 18, 2019. http://fortune.com/longform/medical-records/. Accessed September 9, 2019.
- How doctors feel about electronic health records: National Physician Poll by The Harris Poll. https://med.stanford.edu/content/dam/sm/ehr/documents/EHR-Poll-Presentation.pdf. Accessed September 9, 2019.
When providing contraceptive counseling to women with migraine headaches, how do you identify migraine with aura?
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4
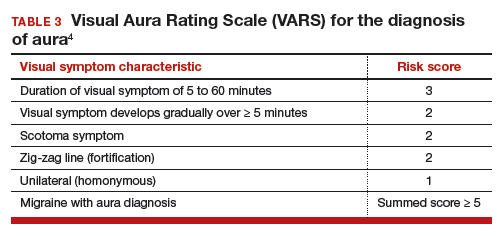
Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
Most physicians know that migraine with aura is a risk factor for ischemic stroke and that the use of an estrogen-containing contraceptive further increases this risk.1-3 Additional important and prevalent risk factors for ischemic stroke include cigarette smoking, hypertension, diabetes, and ischemic heart disease.1 The American College of Obstetricians and Gynecologists (ACOG)2 and the Centers for Disease Control and Prevention (CDC)3 recommend against the use of estrogen-containing contraceptives for women with migraine with aura because of the increased risk of ischemic stroke (Medical Eligibility Criteria [MEC] category 4—unacceptable health risk, method not to be used).
However, those who have migraine with aura can use nonhormonal and progestin-only forms of contraception, including copper- and levonorgestrel-intrauterine devices, the etonogestrel subdermal implant, depot medroxyprogesterone acetate, and progestin-only pills (MEC category 1—no restriction).2,3 ACOG and the CDC advise that estrogen-containing contraceptives can be used for those with migraine without aura who have no other risk factors for stroke (MEC category 2—advantages generally outweigh theoretical or proven risks).2,3 Given the high prevalence of migraine in reproductive-age women, accurate diagnosis of aura is of paramount importance in order to provide appropriate contraceptive counseling.
When is migraine with aura the right diagnosis?
In clinical practice, there is a high level of confusion about the migraine symptoms that warrant a diagnosis of migraine with aura. One approach to improving the accuracy of such a diagnosis is to refer every woman seeking contraceptive counseling who has migraine headaches to a neurologist for expert adjudication of the presence or absence of aura. But in the clinical context of contraceptive counseling, neurology consultation is not always readily available, and requiring consultation increases barriers to care. However, there are tools—such as the Visual Aura Rating Scale (VARS), which is discussed below—that may help non-neurologists identify migraine with aura.4 First, let us review the data that links migraine with aura with increased risk of ischemic stroke.

Migraine with aura is a risk factor for stroke
Multiple case-control studies report that migraine with aura is a risk factor for ischemic stroke.1,5,6 Studies also report that women with migraine with aura who use estrogen-containing contraceptives have an even greater risk of ischemic stroke. For example, one recent case-control study used a commercial claims database of 1,884 cases of ischemic stroke among individuals who identify as women 15 to 49 years of age matched to 7,536 controls without ischemic stroke.1 In this study, the risk of ischemic stroke was increased more than 2.5-fold by cigarette smoking (adjusted odds ratio [aOR], 2.59), hypertension (aOR, 2.73), diabetes (aOR, 2.78), migraine with aura (aOR, 2.89), and ischemic heart disease (aOR, 5.49). For those with migraine with aura who also used an estrogen-containing contraceptive, the aOR for ischemic stroke was 6.08. By contrast, the risk for stroke among those with migraine with aura who were not using an estrogen-containing contraceptive was 2.65. Furthermore, among those with migraine without aura, the risk of ischemic stroke was only 1.77 with the use of an estrogen-containing contraceptive.
Continue to: Although women with migraine...
Although women with migraine with and without aura are at increased risk for stroke, the absolute risk is still very low. For example, one review reported that the incidence of ischemic stroke per 100,000 person-years among women 20 to 44 years of age was 2.5 for those without migraine not taking estrogen-containing contraceptives, 5.9 for those with migraine with aura not taking estrogen-containing contraceptives, and 14.5 among those with migraine with aura and taking estrogen-containing contraceptives.6 Another important observation is that the incidence of thrombotic stroke dramatically increases from adolescence (3.4 per 100,000 person-years) to 45-49 years of age (64.4 per 100,000 person-years).7 Therefore, older women with migraine are at greater risk for stroke than adolescents.
Diagnostic criteria for migraine with and without aura
In contraceptive counseling, if an estrogen-containing contraceptive is being considered, it is important to identify women with migraine headache, determine migraine subtype, assess the frequency of migraines and identify other cardiovascular risk factors, such as hypertension and cigarette smoking. The International Headache Society has evolved the diagnostic criteria for migraine with and without aura, and now endorses the criteria published in the 3rd edition of the International Classification of Headache Disorders (ICHD-3; TABLES 1 and 2).8 For non-neurologists, these criteria may be difficult to remember and impractical to utilize in daily contraceptive counseling. Two simplified tools, the ID Migraine Questionnaire9 and the Visual Aura Rating Scale (TABLE 3)4 may help identify women who have migraine headaches and assess for the presence of aura.


The ID Migraine Questionnaire
In a study of 563 people seeking primary care who had headaches in the past 3 months, 3 questions were identified as being helpful in identifying women with migraine. This 3-question screening tool had reasonable sensitivity (81%), specificity (75%), and positive predictive value (93%) compared with expert diagnosis using the ICHD-3.9 The 3 questions in this screening tool, which are answered “Yes” or “No,” are:
During the last 3 months did you have the following symptoms with your headaches:
- Feel nauseated or sick to your stomach?
- Light bothered you?
- Your headaches limited your ability to work, study or do what you needed to do for at least 1 day?
If two questions are answered “Yes” the patient may have migraine headaches.
Visual Aura Rating Scale for the diagnosis of migraine with aura
More than 90% of women with migraine with aura have visual auras, leaving only a minority with non–visual aura, such as tingling or numbness in a limb, speech or language problems, or muscle weakness. Hence for non-neurologists, it is reasonable to focus on the accurate diagnosis of visual aura to identify those with migraine with aura.
In the clinical context of contraceptive counseling, the Visual Aura Rating Scale (VARS) is especially useful because it has good sensitivity and specificity, and it is easy to use in practice (TABLE 3).4 VARS assesses for 5 characteristics of a visual aura, and each characteristic is associated with a weighted risk score. The 5 symptoms assessed include:
- duration of visual symptom between 5 and 60 minutes (3 points)
- visual symptom develops gradually over 5 minutes (2 points)
- scotoma (2 points)
- zig-zag line (2 points)
- unilateral (1 point).
Continue to: Of note, visual aura is usually...
Of note, visual aura is usually slow-spreading and persists for more than 5 minutes but less than 60 minutes. If a visual symptom has a sudden onset and persists for much longer than 60 minutes, concern is heightened for a more serious neurologic diagnosis such as transient ischemic attack or stroke. A summed score of 5 or more points supports the diagnosis of migraine with aura. In one study, VARS had a sensitivity of 91% and specificity of 96% for identifying women with migraine with aura diagnosed by the ICHD-3 criteria.4

Consider using VARS to identify migraine with aura
Epidemiologic studies report that about 17% of adults have migraine, and about 5% have migraine with aura.10,11 Consequently, migraine with aura is one of the most common medical conditions encountered during contraceptive counseling. The CDC MEC recommend against the use of estrogen-containing contraceptives in women with migraine with aura (Category 4 rating). The VARS may help clinicians identify those who have migraine with aura who should not be offered estrogen-containing contraceptives. Equally important, the use of VARS could help reduce the number of women who are inappropriately diagnosed as having migraine with aura based on fleeting visual symptoms lasting far less than 5 minutes during a migraine headache.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
- Champaloux SW, Tepper NK, Monsour M, et al. Use of combined hormonal contraceptives among women with migraine and risk of ischemic stroke. Am J Obstet Gynecol. 2017;216:489.e1-e7.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Eriksen MK, Thomsen LL, Olesen J. The Visual Aura Rating Scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- Schürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914.
- Sacco S, Merki-Feld G, Aegidius KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18:108.
- Lidegaard Ø, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
- Headache Classification Committee of the International Headache Society. International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211.
- Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;12;61:375-382.
- Lipton RB, Scher AI, Kolodner K, et al. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885-894.
- Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
Can we discern optimal long-term osteoporosis treatment for women?
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
In a recent systematic review, Fink and colleagues attempted to summarize the published evidence of the efficacy and safety of long-term (> 3 years) therapy for osteoporosis.1 Unfortunately, they arrived at very limited and tentative conclusions because, as they point out, of the paucity of such evidence.
Why long-term studies stop short
Only 3 of the several tens of placebo-controlled fracture end-point studies (about 58 trials and observational studies) that Fink and colleagues reviewed evaluated treatment for more than 3 years. The nonavailability of longer-term studies is the direct consequence of a requirement by regulatory agencies for a 3-year fracture end-point study in order to register a new drug for osteoporosis. Hence, longer, placebo-controlled studies do not benefit the industry sponsor, and enrolling patients with osteoporosis or who are at high risk for fracture in any, much less long, placebo-controlled trials is now considered to be unethical.
What the authors did observe
From this limited set of information with which to evaluate, Fink and colleagues observed that long-term therapy with raloxifene reduces the risk of vertebral fractures but is associated with thromboembolic complications. In addition, treatment for more than 3 years with bisphosphonates reduces the risk of vertebral and nonvertebral fractures but may increase risk of rare adverse events (including femoral shaft fractures with atypical radiographic features).
The bisphosphonate holiday. The authors refer to the even more limited evidence about the effects of discontinuing bisphosphonate therapy. Unlike the rapid loss of bone mass density (BMD) and fracture protection upon stopping estrogen or denosumab, the offset of these treatment benefits is slower when bisphosphonates are discontinued. This, coupled with concern about increasing risk with long-term bisphosphonate therapy, led to the confusing concept of a “bisphosphonate holiday.” While recommendations to consider temporary discontinuation of bisphosphonates in patients at low risk for fracture have been made by expert panels,2 very little information exists about the benefits/risks of this strategy, how long the treatment interruption should be, or how to decide when and with what to restart therapy. Unfortunately, overall, Fink and colleagues’ observations provide little practical guidance for clinicians.
Continue to: What we can learn from longer term and recent studies of ideal treatment...
What we can learn from longer term and recent studies of ideal treatment
Since we have no “cure” for osteoporosis, and since the benefits of therapy, including protection from fractures, abate upon stopping treatment (as they do when we stop treating hypertension or diabetes), very long term if not lifelong management is required for patients with osteoporosis. Persistent or even greater reduction of fracture risk with treatment up to 10 years, compared with the rate of fracture in the placebo or treated group during the first 3 years of the study, has been observed with zoledronate and denosumab.3-5 Denosumab was not included in the systematic review by Fink and colleagues since the pivotal fracture trial with that agent was placebo-controlled for only 3 years.6
Sequential drug treatment may be best. Fink and colleagues also did not consider new evidence, which suggests that the use of osteoporosis drugs in sequence—rather than a single agent for a long time—may be the most effective management strategy.7,8
More consideration should be given to the use of estrogen and raloxifene in younger postmenopausal women at risk for vertebral but not hip fracture.
Only treat high-risk patients. Using osteoporosis therapies to only treat patients at high risk for fracture will optimize the benefit:risk ratio and cost-effectiveness of therapy.
Bisphosphonate holidays may not be as important as once thought. BMD and fracture risk reduction does not improve after 5 years of bisphosphonate therapy, and longer treatment may increase the risk of atypic
Hip BMD may serve as indicator for treatment decisions. Recent evidence indicating that the change in hip BMD with treatment or the level of hip BMD achieved on treatment correlates with fracture risk reduction may provide a useful clinical target to guide treatment decisions.9,10
Because we have a lack of pristine evidence does not mean that we shouldn’t treat osteoporosis; we have to do the best we can with the limited evidence we have. Therapy must be individualized, for we are not just treating osteoporosis, we are treating patients with osteoporosis.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
- Fink HA, MacDonald R, Forte ML, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. 2019;171:37-50.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
- Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015;30:934-944.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Ferrari S, Butler PW, Kendler DL, et al. Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab. 2019;104:3450-3461.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
- Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32:198-202.
- Hanley DA, McClung MR, Davison KS, et al; Writing Group for the Western Osteoporosis Alliance. Western Osteoporosis Alliance Clinical Practice Series: evaluating the balance of benefits and risks of long-term osteoporosis therapies. Am J Med. 2017;130:862.e1-862.e7.
- Bouxsein ML, Eastell R, Lui LY, et al; FNIH Bone Quality Project. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34:632-642.
- Ferrari S, Libanati C, Lin CJF, et al. Relationship between bone mineral density T-score and nonvertebral fracture risk over 10 years of denosumab treatment. J Bone Miner Res. 2019;34:1033-1040.
Transcervical ablation of symptomatic uterine fibroids under US guidance
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
Treating uterine fibroids
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.