User login
Treatment for Iron Deficiency Anemia Associated With Heavy Menstrual Bleeding
Iron deficiency anemia (IDA) is a serious health problem that affects millions of women globally. Heavy menstrual bleeding (HMB) is one of the most common causes of IDA in women in North America.
In this supplement to OBG Management, the authors describe the signs, symptoms, and laboratory evaluation for HMB and IDA, including a comprehensive diagnostic and treatment algorithm for the practicing physician. The authors also discuss the characteristics of iron-repletion therapies currently available in the United States to help you make the best choice for your patient.
Iron deficiency anemia (IDA) is a serious health problem that affects millions of women globally. Heavy menstrual bleeding (HMB) is one of the most common causes of IDA in women in North America.
In this supplement to OBG Management, the authors describe the signs, symptoms, and laboratory evaluation for HMB and IDA, including a comprehensive diagnostic and treatment algorithm for the practicing physician. The authors also discuss the characteristics of iron-repletion therapies currently available in the United States to help you make the best choice for your patient.
Iron deficiency anemia (IDA) is a serious health problem that affects millions of women globally. Heavy menstrual bleeding (HMB) is one of the most common causes of IDA in women in North America.
In this supplement to OBG Management, the authors describe the signs, symptoms, and laboratory evaluation for HMB and IDA, including a comprehensive diagnostic and treatment algorithm for the practicing physician. The authors also discuss the characteristics of iron-repletion therapies currently available in the United States to help you make the best choice for your patient.
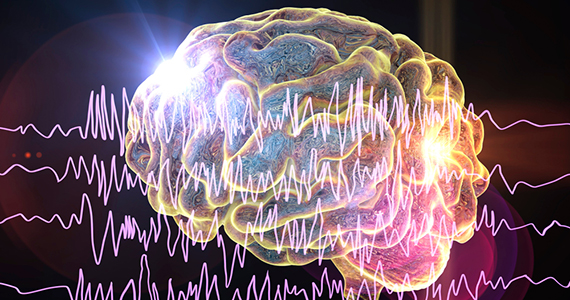
Trial of mesh vs. hysterectomy for prolapse yields inconclusive results
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
FROM JAMA
Supporting our gender-diverse patients
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
CASE Patient has adverse effects from halted estrogen pills
JR twists her hands nervously as you step into the room. “They stopped my hormones,” she sighs as you pull up her lab results.
JR recently had been admitted to an inpatient cardiology unit for several days for a heart failure exacerbation. Her ankles are still swollen beneath her floral print skirt, but she is breathing much easier now. She is back at your primary care office, hoping to get clearance to restart her estrogen pills.
JR reports having mood swings and terrible nightmares while not taking her hormones, which she has been taking for more than 3 years. She hesitates before sharing, “One of the doctors kept asking me questions about my sex life that had nothing to do with my heart condition. I don’t want to go back there.”
Providing compassionate and comprehensive care to gender-nonconforming individuals is challenging for a multitude of reasons, from clinician ignorance to systemic discrimination. About 33% of transgender patients reported being harassed, denied care, or even being assaulted when seeking health care, while 23% reported avoiding going to the doctor altogether when sick or injured out of fear of discrimination.1
Unfortunately, now, further increases to barriers to care may be put in place. In late May of this year, the Department of Health and Human Services (HHS) proposed new regulations that would reverse previous regulations granted through section 1557 of the Affordable Care Act (ACA)—the Health Care Rights Law—which affirmed the rights of gender nonbinary persons to medical care. Among the proposed changes is the elimination of protections against discrimination in health care based on gender identity.2 The proposed regulation changes come on the heels of a federal court case, which seeks to declare that hospital systems may turn away patients based on gender identity.3
Unraveling rights afforded under the ACA
The Health Care Rights Law was passed under the ACA; it prohibits discrimination based on race, color, national origin, sex, age, and disability in health programs and activities receiving federal financial assistance. Multiple lower courts have supported that the rights of transgender individuals is included within these protections against discrimination on the basis of sex.4 These court rulings not only have ensured the ability of gender-diverse individuals to access care but also have enforced insurance coverage of therapies for gender dysphoria. It was only in 2014 that Medicaid began providing coverage for gender-affirming surgeries and eliminating language that such procedures were “experimental” or “cosmetic.” The 2016 passage of the ACA mandated that private insurance companies follow suit. Unfortunately, the recent proposed regulation changes to the Health Care Rights Law may spark a reversal from insurance companies as well. Such a setback would affect gender-diverse individuals’ hormone treatments as well as their ability to access a full spectrum of care within the health care system.
Continue to: ACOG urges nondiscriminatory practices...
ACOG urges nondiscriminatory practices
The proposed regulation changes to the Health Care Rights Law are from the Conscience and Religious Freedom Division of the HHS Office for Civil Rights, which was established in 2018 and has been advocating for the rights of health care providers to refuse to treat patients based on their own religious beliefs.5 We argue, however, that providing care to persons of varying backgrounds is not an assault on our individual liberties but rather a privilege as providers. As obstetrician-gynecologists, it may be easy to only consider cis-gendered women our responsibility. But our field also emphasizes individual empowerment above all else—we fight every day for our patients’ rights to contraception, fertility, pregnancy, parenthood, and sexual freedoms. Let us continue speaking up for the rights of all those who need gynecologic care, regardless of the pronouns they use.
“The American College of Obstetricians and Gynecologists urges health care providers to foster nondiscriminatory practices and policies to increase identification and to facilitate quality health care for transgender individuals, both in assisting with the transition if desired as well as providing long-term preventive health care.”6
We urge you to take action
- Reach out to your local representatives about protecting transgender health access
- Educate yourself on the unique needs of transgender individuals
- Read personal accounts
- Share your personal story
- Find referring providers near your practice
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
- 2015 US Transgender Survey. December 2016. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF. Accessed August 30, 2019.
- Musumeci M, Kates J, Dawson J, et al. HHS’ proposed changes to non-discrimination regulations under ACA section 1557. July 1, 2019. https://www.kff.org/disparities-policy/issue-brief/hhss-proposed-changes-to-non-discrimination-regulations-under-aca-section-1557/. Accessed August 30, 2019.
- Franciscan Alliance v. Burwell. ACLU website. https://www.aclu.org/cases/franciscan-alliance-v-burwell. Accessed August 30, 2019.
- Pear R. Trump plan would cut back health care protections for transgender people. April 21, 2018. https://www.nytimes.com/2018/04/21/us/politics/trump-transgender-health-care.html. Accessed August 30, 2019.
- U.S. Department of Health and Human Services. HHS announces new conscience and religious freedom division. January 18, 2018. https://www.hhs.gov/about/news/2018/01/18/hhs-ocr-announces-new-conscience-and-religious-freedom-division.html. Accessed August 30, 2019.
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women. Committee Opinion no. 512: health care for transgender individuals. Obstet Gynecol. 2011;118:1454–1458.
Women with epilepsy: 5 clinical pearls for contraception and preconception counseling
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).
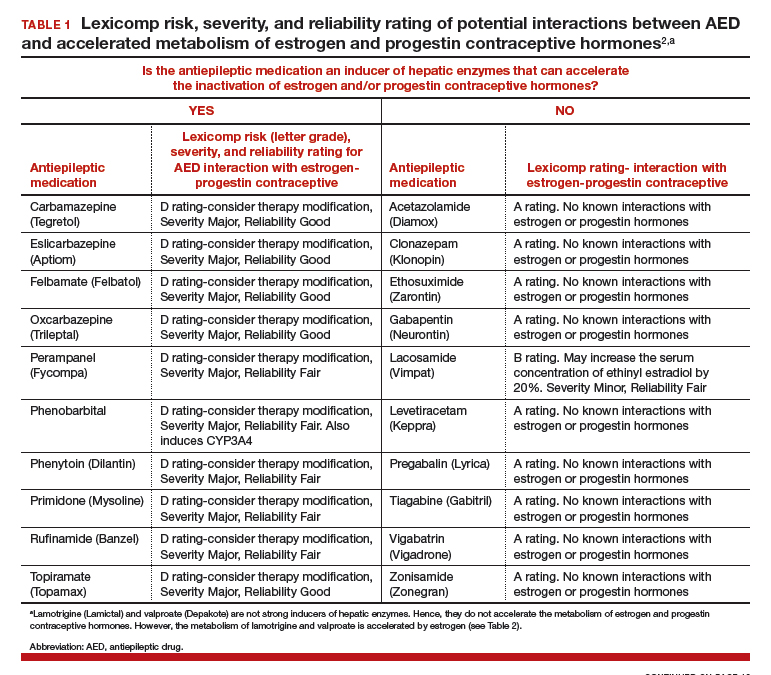

For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
Native tissue repair of POP: Surgical techniques to improve outcomes
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.
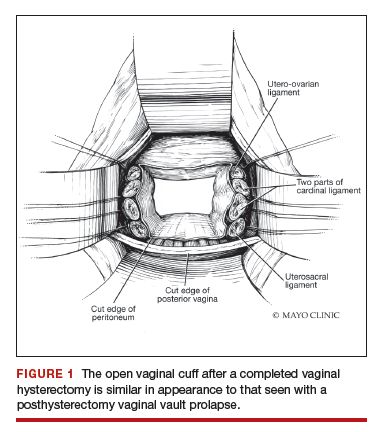
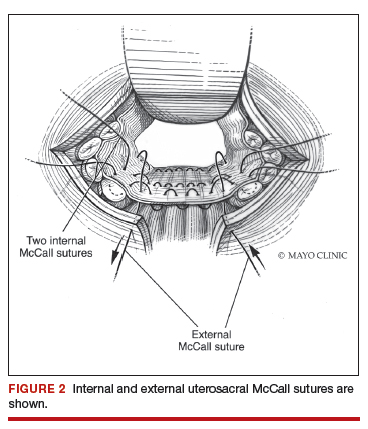
Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.
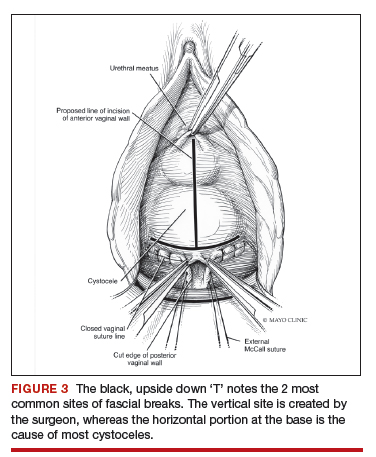
Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.
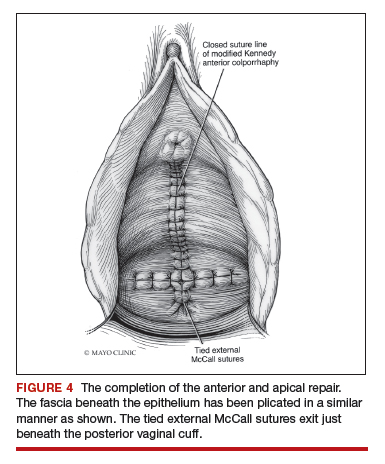
Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.
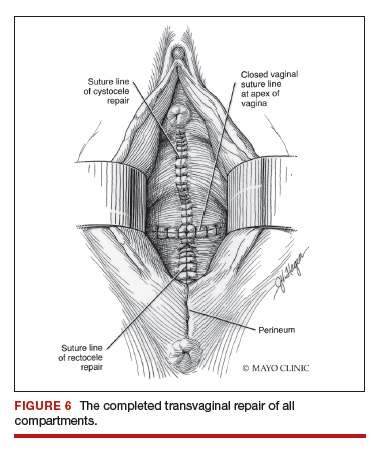
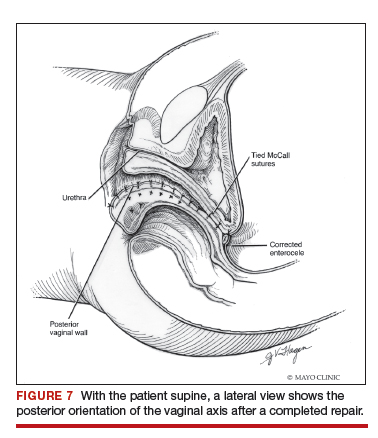
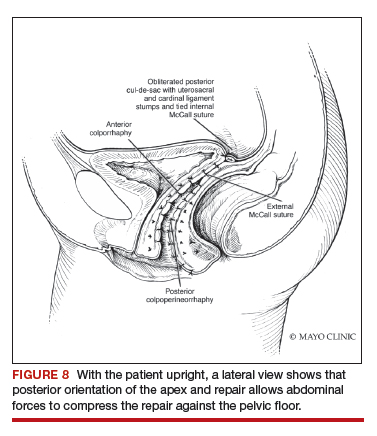
Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.

Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.

Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
Minimally invasive surgery for cervical cancer: Is surgeon volume a factor?
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
ACOG advises bleeding disorder screening for teens with heavy menstruation
Adolescent girls with heavy menstrual bleeding should be assessed for bleeding disorders, according to a Committee Opinion issued by the American College of Obstetricians and Gynecologists.
A bleeding disorder is secondary only to anovulation as a cause of heavy menstrual bleeding in adolescents.
Bleeding disorders affect 1%-2% of the general population, but are “found in approximately 20% of adolescent girls who present for evaluation of heavy menstrual bleeding and in 33% of adolescent girls hospitalized for heavy menstrual bleeding,” wrote Oluyemisi Adeyemi-Fowode, MD, and Judith Simms-Cendan, MD, and members of the ACOG Committee on Adolescent Health Care in the opinion, published in Obstetrics & Gynecology.
The committee advised that physical examination of teens with acute heavy menstrual bleeding should include assessment of hemodynamic stability with orthostatic blood pressure and pulse measurements. A speculum exam is not usually needed in teen girls with heavy menstrual bleeding. Evaluation should include screening for anemia attributable to blood loss with serum ferritin, endocrine disorders, and bleeding disorders. In suspected cases of bleeding disorders, laboratory evaluation and medical management should be done in consultation with a hematologist.
Those who are actively bleeding or hemodynamically unstable should be hospitalized for medical management, they said.
Ultrasonography is not necessary for an initial work-up of teens with heavy menstrual bleeding, but could be useful in patients who fail to respond to medical management.
Adolescent girls without contraindications to estrogen can be treated with hormone therapy in various forms including intravenous conjugated estrogen every 4-6 hours or oral 30-50 mg ethinyl estradiol every 6-8 hours until cessation of bleeding. Antifibrinolytics also can be used to stop bleeding.
Maintenance therapy after correction of acute heavy bleeding can include a combination of treatments such as hormonal contraceptives, oral and injectable progestins, and levonorgestrel-releasing intrauterine devices, the committee wrote. They also recommended oral iron replacement therapy for all women of reproductive age with anemia caused by menstrual bleeding.
If a patient fails to respond to medical therapy, nonmedical options or surgery may be considered, according to the committee. In addition, all teen girls with bleeding disorders should be advised about safe medication use, including the use of aspirin or NSAIDs only on the recommendation of a hematologist.
Patients and their families need education on menstrual issues including possible options for surgery in the future if heavy menstruation does not resolve. If a patient has a known bleeding disorder and is considering surgery, preoperative evaluation should include a consultation with a hematologist and an anesthesiologist, the committee noted.
Melissa Kottke, MD, MPH, said in an interview, “Every ob.gyn. will see a young patient with ‘heavy menstrual bleeding.’ And it becomes part of the art and challenge to work with the patient and family to collectively explore if this is, indeed, ‘heavy’ and of concern … or is it is a ‘normal’ menstrual period and simply reflects a newer life experience that would benefit from some education? And the stakes are high. Young people who have heavy menstrual cycles are much more likely to have an underlying bleeding disorder than the general population (20% vs. 1%-2%), and 75%-80% of adolescents with bleeding disorders report heavy menses as the most common clinical manifestation of their disorder.
“Fortunately, Committee Opinion 785, ‘Screening and Management of Bleeding Disorders in Adolescents with Heavy Menstrual Bleeding’ from the ACOG Committee on Adolescent Health Care is detailed and pragmatic. It outlines how to translate everyday conversations with young people about their menses into a quantifiable estimate of bleeding, including a very teen-friendly Pictorial Blood Loss Assessment Chart. It also gives ob.gyns. ever-important guidance about what to do next for evaluation and diagnosis. This committee opinion nicely outlines how to help manage heavy bleeding in an adolescent with a detailed algorithm. And very importantly, it gives clear management guidance and encourages ob.gyns. to avoid frequently unnecessary (speculum exams and ultrasounds) and excessive (early transfusion or surgical interventions) approaches to management for the young patient. I think it will be a great resource for any provider who is taking care of heavy menstrual bleeding for a young person,” said Dr. Kottke, who is director of the Jane Fonda Center for Adolescent Reproductive Health and associate professor of gynecology and obstetrics, both at Emory University, Atlanta. Dr. Kottke is not a member of the ACOG Committee on Adolescent Health and was asked to comment on the opinion.*
The complete opinion, ACOG Committee Opinion number 785, includes recommended laboratory tests, an eight-question screening tool, and a management algorithm.
The committee members had no financial conflicts to disclose. Dr. Kottke said she had no relevant financial disclosures.
SOURCE: Adeyemi-Fowode O and Simms-Cendan J. Obstet Gynecol. 2019 Sep. 134:e71-83.
*This article was updated on 9/9/2019.
Adolescent girls with heavy menstrual bleeding should be assessed for bleeding disorders, according to a Committee Opinion issued by the American College of Obstetricians and Gynecologists.
A bleeding disorder is secondary only to anovulation as a cause of heavy menstrual bleeding in adolescents.
Bleeding disorders affect 1%-2% of the general population, but are “found in approximately 20% of adolescent girls who present for evaluation of heavy menstrual bleeding and in 33% of adolescent girls hospitalized for heavy menstrual bleeding,” wrote Oluyemisi Adeyemi-Fowode, MD, and Judith Simms-Cendan, MD, and members of the ACOG Committee on Adolescent Health Care in the opinion, published in Obstetrics & Gynecology.
The committee advised that physical examination of teens with acute heavy menstrual bleeding should include assessment of hemodynamic stability with orthostatic blood pressure and pulse measurements. A speculum exam is not usually needed in teen girls with heavy menstrual bleeding. Evaluation should include screening for anemia attributable to blood loss with serum ferritin, endocrine disorders, and bleeding disorders. In suspected cases of bleeding disorders, laboratory evaluation and medical management should be done in consultation with a hematologist.
Those who are actively bleeding or hemodynamically unstable should be hospitalized for medical management, they said.
Ultrasonography is not necessary for an initial work-up of teens with heavy menstrual bleeding, but could be useful in patients who fail to respond to medical management.
Adolescent girls without contraindications to estrogen can be treated with hormone therapy in various forms including intravenous conjugated estrogen every 4-6 hours or oral 30-50 mg ethinyl estradiol every 6-8 hours until cessation of bleeding. Antifibrinolytics also can be used to stop bleeding.
Maintenance therapy after correction of acute heavy bleeding can include a combination of treatments such as hormonal contraceptives, oral and injectable progestins, and levonorgestrel-releasing intrauterine devices, the committee wrote. They also recommended oral iron replacement therapy for all women of reproductive age with anemia caused by menstrual bleeding.
If a patient fails to respond to medical therapy, nonmedical options or surgery may be considered, according to the committee. In addition, all teen girls with bleeding disorders should be advised about safe medication use, including the use of aspirin or NSAIDs only on the recommendation of a hematologist.
Patients and their families need education on menstrual issues including possible options for surgery in the future if heavy menstruation does not resolve. If a patient has a known bleeding disorder and is considering surgery, preoperative evaluation should include a consultation with a hematologist and an anesthesiologist, the committee noted.
Melissa Kottke, MD, MPH, said in an interview, “Every ob.gyn. will see a young patient with ‘heavy menstrual bleeding.’ And it becomes part of the art and challenge to work with the patient and family to collectively explore if this is, indeed, ‘heavy’ and of concern … or is it is a ‘normal’ menstrual period and simply reflects a newer life experience that would benefit from some education? And the stakes are high. Young people who have heavy menstrual cycles are much more likely to have an underlying bleeding disorder than the general population (20% vs. 1%-2%), and 75%-80% of adolescents with bleeding disorders report heavy menses as the most common clinical manifestation of their disorder.
“Fortunately, Committee Opinion 785, ‘Screening and Management of Bleeding Disorders in Adolescents with Heavy Menstrual Bleeding’ from the ACOG Committee on Adolescent Health Care is detailed and pragmatic. It outlines how to translate everyday conversations with young people about their menses into a quantifiable estimate of bleeding, including a very teen-friendly Pictorial Blood Loss Assessment Chart. It also gives ob.gyns. ever-important guidance about what to do next for evaluation and diagnosis. This committee opinion nicely outlines how to help manage heavy bleeding in an adolescent with a detailed algorithm. And very importantly, it gives clear management guidance and encourages ob.gyns. to avoid frequently unnecessary (speculum exams and ultrasounds) and excessive (early transfusion or surgical interventions) approaches to management for the young patient. I think it will be a great resource for any provider who is taking care of heavy menstrual bleeding for a young person,” said Dr. Kottke, who is director of the Jane Fonda Center for Adolescent Reproductive Health and associate professor of gynecology and obstetrics, both at Emory University, Atlanta. Dr. Kottke is not a member of the ACOG Committee on Adolescent Health and was asked to comment on the opinion.*
The complete opinion, ACOG Committee Opinion number 785, includes recommended laboratory tests, an eight-question screening tool, and a management algorithm.
The committee members had no financial conflicts to disclose. Dr. Kottke said she had no relevant financial disclosures.
SOURCE: Adeyemi-Fowode O and Simms-Cendan J. Obstet Gynecol. 2019 Sep. 134:e71-83.
*This article was updated on 9/9/2019.
Adolescent girls with heavy menstrual bleeding should be assessed for bleeding disorders, according to a Committee Opinion issued by the American College of Obstetricians and Gynecologists.
A bleeding disorder is secondary only to anovulation as a cause of heavy menstrual bleeding in adolescents.
Bleeding disorders affect 1%-2% of the general population, but are “found in approximately 20% of adolescent girls who present for evaluation of heavy menstrual bleeding and in 33% of adolescent girls hospitalized for heavy menstrual bleeding,” wrote Oluyemisi Adeyemi-Fowode, MD, and Judith Simms-Cendan, MD, and members of the ACOG Committee on Adolescent Health Care in the opinion, published in Obstetrics & Gynecology.
The committee advised that physical examination of teens with acute heavy menstrual bleeding should include assessment of hemodynamic stability with orthostatic blood pressure and pulse measurements. A speculum exam is not usually needed in teen girls with heavy menstrual bleeding. Evaluation should include screening for anemia attributable to blood loss with serum ferritin, endocrine disorders, and bleeding disorders. In suspected cases of bleeding disorders, laboratory evaluation and medical management should be done in consultation with a hematologist.
Those who are actively bleeding or hemodynamically unstable should be hospitalized for medical management, they said.
Ultrasonography is not necessary for an initial work-up of teens with heavy menstrual bleeding, but could be useful in patients who fail to respond to medical management.
Adolescent girls without contraindications to estrogen can be treated with hormone therapy in various forms including intravenous conjugated estrogen every 4-6 hours or oral 30-50 mg ethinyl estradiol every 6-8 hours until cessation of bleeding. Antifibrinolytics also can be used to stop bleeding.
Maintenance therapy after correction of acute heavy bleeding can include a combination of treatments such as hormonal contraceptives, oral and injectable progestins, and levonorgestrel-releasing intrauterine devices, the committee wrote. They also recommended oral iron replacement therapy for all women of reproductive age with anemia caused by menstrual bleeding.
If a patient fails to respond to medical therapy, nonmedical options or surgery may be considered, according to the committee. In addition, all teen girls with bleeding disorders should be advised about safe medication use, including the use of aspirin or NSAIDs only on the recommendation of a hematologist.
Patients and their families need education on menstrual issues including possible options for surgery in the future if heavy menstruation does not resolve. If a patient has a known bleeding disorder and is considering surgery, preoperative evaluation should include a consultation with a hematologist and an anesthesiologist, the committee noted.
Melissa Kottke, MD, MPH, said in an interview, “Every ob.gyn. will see a young patient with ‘heavy menstrual bleeding.’ And it becomes part of the art and challenge to work with the patient and family to collectively explore if this is, indeed, ‘heavy’ and of concern … or is it is a ‘normal’ menstrual period and simply reflects a newer life experience that would benefit from some education? And the stakes are high. Young people who have heavy menstrual cycles are much more likely to have an underlying bleeding disorder than the general population (20% vs. 1%-2%), and 75%-80% of adolescents with bleeding disorders report heavy menses as the most common clinical manifestation of their disorder.
“Fortunately, Committee Opinion 785, ‘Screening and Management of Bleeding Disorders in Adolescents with Heavy Menstrual Bleeding’ from the ACOG Committee on Adolescent Health Care is detailed and pragmatic. It outlines how to translate everyday conversations with young people about their menses into a quantifiable estimate of bleeding, including a very teen-friendly Pictorial Blood Loss Assessment Chart. It also gives ob.gyns. ever-important guidance about what to do next for evaluation and diagnosis. This committee opinion nicely outlines how to help manage heavy bleeding in an adolescent with a detailed algorithm. And very importantly, it gives clear management guidance and encourages ob.gyns. to avoid frequently unnecessary (speculum exams and ultrasounds) and excessive (early transfusion or surgical interventions) approaches to management for the young patient. I think it will be a great resource for any provider who is taking care of heavy menstrual bleeding for a young person,” said Dr. Kottke, who is director of the Jane Fonda Center for Adolescent Reproductive Health and associate professor of gynecology and obstetrics, both at Emory University, Atlanta. Dr. Kottke is not a member of the ACOG Committee on Adolescent Health and was asked to comment on the opinion.*
The complete opinion, ACOG Committee Opinion number 785, includes recommended laboratory tests, an eight-question screening tool, and a management algorithm.
The committee members had no financial conflicts to disclose. Dr. Kottke said she had no relevant financial disclosures.
SOURCE: Adeyemi-Fowode O and Simms-Cendan J. Obstet Gynecol. 2019 Sep. 134:e71-83.
*This article was updated on 9/9/2019.
FROM OBSTETRICS AND GYNECOLOGY
Addressing suicidality among Indigenous women, girls
Historical trauma and current social factors contribute to depression, PTSD, anxiety disorders
The history of abuse and genocide has its precursors in antiquity. A brief sketch of this history will provide some insights into the impact of intergenerational trauma and a rationale for the crisis of missing and murdered Indigenous women and girls in the United States and Canada, or Turtle Island, as the Indigenous People call it.
Such a review also will provide a partial explanation of why the suicide rate among non-Hispanic Native American or Alaska Native women increased by 139%1 during 1999-2017 – a time when more Indigenous women were gaining access to law and medical school, as well as positions of authority in their tribes.
Church-, state-sanctioned transgressions
The psychological impact of our past history haunts us today. Papal bulletins – decrees from the pope – gave permission to Christian explorers to take land, wealth, and slaves from any nonbeliever. This permission was labeled the Doctrine of Discovery. It was incorporated into U.S. law in 1823, and by the Supreme Court case, Johnson v. M’intosh. It also provided rationale for the Indian Removal Act, which was passed on May 28, 1830, and signed into law by U.S. President Andrew Jackson. As a result of that law, Indigenous People were forced onto reservations, often removed from their traditional and sacred homelands. Many died during forced relocation.2
From the time of “discovery” by settlers until well into the 19th century, the U.S. governmental intent was genocide. It was manifest by the outright murder of Indigenous People, displacement from land, and the disruption of families when children were taken, put into boarding schools, and were forbidden to speak their language. Indigenous medicine people were killed or jailed for practicing their traditional ceremonies. Indigenous nations had their laws, languages, and agricultural practices denied them. Even today, they must practice U.S. law, adapt colonizing forms of land ownership, and engage in the economic practices of the dominant culture. The economic system currently in place rewards rape of the land and creates a trickle-up economy that keeps rewarding the rich at the expense of the poor. The economic system even gives corporations legal status as individuals, and, in some cases, is allowed to supersede the rights of Indigenous nations.
Today, the federal government still can appropriate land for minerals, pipelines,3 and even put indigenous land and water sovereignty at risk of contamination and pollution by mines established upstream.4 Most of those practices are repugnant to Indigenous nations. The Doctrine of Discovery established prior to 1492 is still alive and well on Turtle Island.
It is this background that denies the rights of Mother Earth, and this backdrop that, in turn, generalizes the denial of the rights of Indigenous women. There are women today, who, against their will and knowledge, have been sterilized.5 There are cases in which women have been raped and beaten, and their perpetrators were never been brought to justice.6 There are jurisdictional issues in the federal law that keep non-native perpetrators from being punished for their actions on tribal sovereign land.
This history and those current practices affect Indigenous families. Historical trauma produces epigenetic changes7 that create more anxiety and depression. Families in which one or both parents were taken away have a harder time providing a loving, safe, addiction-free environment for their children. Children often have high scores on measurements of adverse childhood experiences and suffer PTSD. As psychiatrists, we have treated PTSD from residential and boarding school survivors, families with family members who were victims of being missing or murdered, and survivors of sexual abuse – both in the United States and Canada. According to the final Canadian report of the inquiry into missing and murdered Indigenous women and girls, the murder rate for Indigenous women was 12 times that of non-Indigenous women.8
We assert that this combination of historical trauma and current social factors contributes to depression, PTSD, and anxiety disorders that currently feed the rise in attempted and completed suicide. Less-than-optimal educational opportunities and unemployment, often above 10% on reservations,9 along with food insecurity, accentuate the settings in which women and girls live.
Women achieving despite challenges
Yet, Indigenous women are making great strides within their cultures and communities. For example, Indigenous women are leading language revitalization, and within their culture, are healers and carriers of knowledge. Many Indigenous women are doctors, lawyers, dentists, teachers, poets, authors, and artists.10 Voters in last year’s midterms elected two Native American women to the U.S. Congress. Often, however, those achievements within the Western culture come at a cost, and some might have difficulty balancing those roles with their traditional cultures.
Current societal pressures feed the rise of suicide. Santa Fe, N.M., is known for its affluence and reputation as a tricultural city of Anglos, Hispanics, and Native Americans, and yet, a recent health impact assessment survey of urban Indigenous families stated that food insecurity was the leading concern for those families. Unemployment on the Navajo Nation is above 50%.11 The Indian Health Service (IHS) in the United States, which provides the majority of mental services to the Indigenous population, has identified mental health issues as the No. 1 health problem. However, only 7% of the IHS budget is allocated for mental health and substance abuse services. This represents an underfudging of services to American Indian and Alaska Native communities. In fact, there were only two psychiatrists per 100,000 people served by the IHS, which is one-seventh the number of psychiatrists available to the general population in the United States.12
Best practices for psychiatrists working with Indigenous women demands that we know the history, know how that history is still being manifest in subtle ways, and understand how such antiquated papal bulletins as the Doctrine of Discovery still operate to justify the taking and misuse of indigenous land. We must realize that the dominant economic systems, laws, and policing strategies are imposed on cultures that are sophisticated in their own right. This will then allow compassionate care with a level of understanding.
13
We can advocate at all levels, considering that the role of the federal government, the state, corporations, tribes, families, and provision of quality care to individuals can continue the positive collective advancement of women, and reduce the morbidity and mortality associated with suicide attempts.
We need to be sensitive to our patients and their risks of suicide. Treat suicidal ideation as the serious threat that it is. Address the depression, anxiety, PTSD, historical trauma, substance abuse, emotional dysregulation, and loss of relationship in persons with attachment disorders as serious and valid life events than can lead to serious consequences – including completed suicide.
Indigenous women are resilient, and the approach should be to also balance knowledge of those potential barriers with validating the feminine, and supporting the traditional roles of women and men that value women and children, and revere the matriarchs. Encouraging and supporting Indigenous resurgence of cultural practices and values is significant for positive outcomes for healing and wellness. Doing so can carry a greater meaning within Indigenous and First Nations society.
References
1. Curtin SC and H Hedegaard. Suicide rates for females and males by race and ethnicity: United States, 1999 and 2017. NCHS Health E-Stat. 2019.
2. Anderson GC. Ethnic cleansing and the Indian: The crime that should haunt America. Norman, Okla.: University of Oklahoma Press, 2014.
3. Rausch N. “Standing Rock, Morton County work to mend relationships post-DAPL protests.” Billingsgazette.com. Aug 10, 2019.
4. Roy A. “5 ways the government keeps Native Americans in poverty.” Forbes.com. Mar 13, 2014.
5. Blakemore E. “The little-known history of forced sterilization of Native American women.” JSTOR.org. Aug 25, 2016.
6. Bleir G and A Zoledziowski. “Murdered and missing Native American women challenge police and courts.” Publicintegrity.org. Aug 27, 2018.
7. Brockie TN et al. A framework to examine the role of epigenetics in health disparities among Native Americans. Nurs Res Prac. 2013;2013:410395.
8. “Reclaiming power and place: The final report of the national inquiry into missing and murdered Indigenous women and girls.” Vancouver: Privy Office. Jun 3, 2019.
9. Hagan S. “Where U.S. unemployment is still sky-high: Indian reservations.” Bloomberg.com. Apr 5, 2018.
10. Morin B. “Meet 10 Indigenous women who are making the world a better place.” Indian Country Today. Jul 1, 2019.
11. Fact sheet. Discovernavajo.com.
12. Sarche M and P Spicer. Poverty and health disparities for American Indian and Alaska Native children: Current knowledge and future prospects. Ann NY Acad Sci. 2008 Jul 25;1136:126-36.
13. Lewis-Fernández R et al. Culture and psychiatric evaluation: Operationalizing cultural formulation for DSM-5. Psychiatry. 2014 Summer;77(2):130-54.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local Indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating Indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association.
Dr. Neidhardt is a board-certified psychiatrist who lives in Santa Fe and has an integrative, holistic psychiatric practice that also specializes in trauma-focused therapy. He has provided care for Indigenous People in the Southwest United States and in Canada, and has worked with Navajo medicine people to develop training for mental health professionals with his wife, Dr. Mary Hasbah Roessel. Dr. Reinhardt is a life fellow of the APA.
Historical trauma and current social factors contribute to depression, PTSD, anxiety disorders
Historical trauma and current social factors contribute to depression, PTSD, anxiety disorders
The history of abuse and genocide has its precursors in antiquity. A brief sketch of this history will provide some insights into the impact of intergenerational trauma and a rationale for the crisis of missing and murdered Indigenous women and girls in the United States and Canada, or Turtle Island, as the Indigenous People call it.
Such a review also will provide a partial explanation of why the suicide rate among non-Hispanic Native American or Alaska Native women increased by 139%1 during 1999-2017 – a time when more Indigenous women were gaining access to law and medical school, as well as positions of authority in their tribes.
Church-, state-sanctioned transgressions
The psychological impact of our past history haunts us today. Papal bulletins – decrees from the pope – gave permission to Christian explorers to take land, wealth, and slaves from any nonbeliever. This permission was labeled the Doctrine of Discovery. It was incorporated into U.S. law in 1823, and by the Supreme Court case, Johnson v. M’intosh. It also provided rationale for the Indian Removal Act, which was passed on May 28, 1830, and signed into law by U.S. President Andrew Jackson. As a result of that law, Indigenous People were forced onto reservations, often removed from their traditional and sacred homelands. Many died during forced relocation.2
From the time of “discovery” by settlers until well into the 19th century, the U.S. governmental intent was genocide. It was manifest by the outright murder of Indigenous People, displacement from land, and the disruption of families when children were taken, put into boarding schools, and were forbidden to speak their language. Indigenous medicine people were killed or jailed for practicing their traditional ceremonies. Indigenous nations had their laws, languages, and agricultural practices denied them. Even today, they must practice U.S. law, adapt colonizing forms of land ownership, and engage in the economic practices of the dominant culture. The economic system currently in place rewards rape of the land and creates a trickle-up economy that keeps rewarding the rich at the expense of the poor. The economic system even gives corporations legal status as individuals, and, in some cases, is allowed to supersede the rights of Indigenous nations.
Today, the federal government still can appropriate land for minerals, pipelines,3 and even put indigenous land and water sovereignty at risk of contamination and pollution by mines established upstream.4 Most of those practices are repugnant to Indigenous nations. The Doctrine of Discovery established prior to 1492 is still alive and well on Turtle Island.
It is this background that denies the rights of Mother Earth, and this backdrop that, in turn, generalizes the denial of the rights of Indigenous women. There are women today, who, against their will and knowledge, have been sterilized.5 There are cases in which women have been raped and beaten, and their perpetrators were never been brought to justice.6 There are jurisdictional issues in the federal law that keep non-native perpetrators from being punished for their actions on tribal sovereign land.
This history and those current practices affect Indigenous families. Historical trauma produces epigenetic changes7 that create more anxiety and depression. Families in which one or both parents were taken away have a harder time providing a loving, safe, addiction-free environment for their children. Children often have high scores on measurements of adverse childhood experiences and suffer PTSD. As psychiatrists, we have treated PTSD from residential and boarding school survivors, families with family members who were victims of being missing or murdered, and survivors of sexual abuse – both in the United States and Canada. According to the final Canadian report of the inquiry into missing and murdered Indigenous women and girls, the murder rate for Indigenous women was 12 times that of non-Indigenous women.8
We assert that this combination of historical trauma and current social factors contributes to depression, PTSD, and anxiety disorders that currently feed the rise in attempted and completed suicide. Less-than-optimal educational opportunities and unemployment, often above 10% on reservations,9 along with food insecurity, accentuate the settings in which women and girls live.
Women achieving despite challenges
Yet, Indigenous women are making great strides within their cultures and communities. For example, Indigenous women are leading language revitalization, and within their culture, are healers and carriers of knowledge. Many Indigenous women are doctors, lawyers, dentists, teachers, poets, authors, and artists.10 Voters in last year’s midterms elected two Native American women to the U.S. Congress. Often, however, those achievements within the Western culture come at a cost, and some might have difficulty balancing those roles with their traditional cultures.
Current societal pressures feed the rise of suicide. Santa Fe, N.M., is known for its affluence and reputation as a tricultural city of Anglos, Hispanics, and Native Americans, and yet, a recent health impact assessment survey of urban Indigenous families stated that food insecurity was the leading concern for those families. Unemployment on the Navajo Nation is above 50%.11 The Indian Health Service (IHS) in the United States, which provides the majority of mental services to the Indigenous population, has identified mental health issues as the No. 1 health problem. However, only 7% of the IHS budget is allocated for mental health and substance abuse services. This represents an underfudging of services to American Indian and Alaska Native communities. In fact, there were only two psychiatrists per 100,000 people served by the IHS, which is one-seventh the number of psychiatrists available to the general population in the United States.12
Best practices for psychiatrists working with Indigenous women demands that we know the history, know how that history is still being manifest in subtle ways, and understand how such antiquated papal bulletins as the Doctrine of Discovery still operate to justify the taking and misuse of indigenous land. We must realize that the dominant economic systems, laws, and policing strategies are imposed on cultures that are sophisticated in their own right. This will then allow compassionate care with a level of understanding.
13
We can advocate at all levels, considering that the role of the federal government, the state, corporations, tribes, families, and provision of quality care to individuals can continue the positive collective advancement of women, and reduce the morbidity and mortality associated with suicide attempts.
We need to be sensitive to our patients and their risks of suicide. Treat suicidal ideation as the serious threat that it is. Address the depression, anxiety, PTSD, historical trauma, substance abuse, emotional dysregulation, and loss of relationship in persons with attachment disorders as serious and valid life events than can lead to serious consequences – including completed suicide.
Indigenous women are resilient, and the approach should be to also balance knowledge of those potential barriers with validating the feminine, and supporting the traditional roles of women and men that value women and children, and revere the matriarchs. Encouraging and supporting Indigenous resurgence of cultural practices and values is significant for positive outcomes for healing and wellness. Doing so can carry a greater meaning within Indigenous and First Nations society.
References
1. Curtin SC and H Hedegaard. Suicide rates for females and males by race and ethnicity: United States, 1999 and 2017. NCHS Health E-Stat. 2019.
2. Anderson GC. Ethnic cleansing and the Indian: The crime that should haunt America. Norman, Okla.: University of Oklahoma Press, 2014.
3. Rausch N. “Standing Rock, Morton County work to mend relationships post-DAPL protests.” Billingsgazette.com. Aug 10, 2019.
4. Roy A. “5 ways the government keeps Native Americans in poverty.” Forbes.com. Mar 13, 2014.
5. Blakemore E. “The little-known history of forced sterilization of Native American women.” JSTOR.org. Aug 25, 2016.
6. Bleir G and A Zoledziowski. “Murdered and missing Native American women challenge police and courts.” Publicintegrity.org. Aug 27, 2018.
7. Brockie TN et al. A framework to examine the role of epigenetics in health disparities among Native Americans. Nurs Res Prac. 2013;2013:410395.
8. “Reclaiming power and place: The final report of the national inquiry into missing and murdered Indigenous women and girls.” Vancouver: Privy Office. Jun 3, 2019.
9. Hagan S. “Where U.S. unemployment is still sky-high: Indian reservations.” Bloomberg.com. Apr 5, 2018.
10. Morin B. “Meet 10 Indigenous women who are making the world a better place.” Indian Country Today. Jul 1, 2019.
11. Fact sheet. Discovernavajo.com.
12. Sarche M and P Spicer. Poverty and health disparities for American Indian and Alaska Native children: Current knowledge and future prospects. Ann NY Acad Sci. 2008 Jul 25;1136:126-36.
13. Lewis-Fernández R et al. Culture and psychiatric evaluation: Operationalizing cultural formulation for DSM-5. Psychiatry. 2014 Summer;77(2):130-54.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local Indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating Indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association.
Dr. Neidhardt is a board-certified psychiatrist who lives in Santa Fe and has an integrative, holistic psychiatric practice that also specializes in trauma-focused therapy. He has provided care for Indigenous People in the Southwest United States and in Canada, and has worked with Navajo medicine people to develop training for mental health professionals with his wife, Dr. Mary Hasbah Roessel. Dr. Reinhardt is a life fellow of the APA.
The history of abuse and genocide has its precursors in antiquity. A brief sketch of this history will provide some insights into the impact of intergenerational trauma and a rationale for the crisis of missing and murdered Indigenous women and girls in the United States and Canada, or Turtle Island, as the Indigenous People call it.
Such a review also will provide a partial explanation of why the suicide rate among non-Hispanic Native American or Alaska Native women increased by 139%1 during 1999-2017 – a time when more Indigenous women were gaining access to law and medical school, as well as positions of authority in their tribes.
Church-, state-sanctioned transgressions
The psychological impact of our past history haunts us today. Papal bulletins – decrees from the pope – gave permission to Christian explorers to take land, wealth, and slaves from any nonbeliever. This permission was labeled the Doctrine of Discovery. It was incorporated into U.S. law in 1823, and by the Supreme Court case, Johnson v. M’intosh. It also provided rationale for the Indian Removal Act, which was passed on May 28, 1830, and signed into law by U.S. President Andrew Jackson. As a result of that law, Indigenous People were forced onto reservations, often removed from their traditional and sacred homelands. Many died during forced relocation.2
From the time of “discovery” by settlers until well into the 19th century, the U.S. governmental intent was genocide. It was manifest by the outright murder of Indigenous People, displacement from land, and the disruption of families when children were taken, put into boarding schools, and were forbidden to speak their language. Indigenous medicine people were killed or jailed for practicing their traditional ceremonies. Indigenous nations had their laws, languages, and agricultural practices denied them. Even today, they must practice U.S. law, adapt colonizing forms of land ownership, and engage in the economic practices of the dominant culture. The economic system currently in place rewards rape of the land and creates a trickle-up economy that keeps rewarding the rich at the expense of the poor. The economic system even gives corporations legal status as individuals, and, in some cases, is allowed to supersede the rights of Indigenous nations.
Today, the federal government still can appropriate land for minerals, pipelines,3 and even put indigenous land and water sovereignty at risk of contamination and pollution by mines established upstream.4 Most of those practices are repugnant to Indigenous nations. The Doctrine of Discovery established prior to 1492 is still alive and well on Turtle Island.
It is this background that denies the rights of Mother Earth, and this backdrop that, in turn, generalizes the denial of the rights of Indigenous women. There are women today, who, against their will and knowledge, have been sterilized.5 There are cases in which women have been raped and beaten, and their perpetrators were never been brought to justice.6 There are jurisdictional issues in the federal law that keep non-native perpetrators from being punished for their actions on tribal sovereign land.
This history and those current practices affect Indigenous families. Historical trauma produces epigenetic changes7 that create more anxiety and depression. Families in which one or both parents were taken away have a harder time providing a loving, safe, addiction-free environment for their children. Children often have high scores on measurements of adverse childhood experiences and suffer PTSD. As psychiatrists, we have treated PTSD from residential and boarding school survivors, families with family members who were victims of being missing or murdered, and survivors of sexual abuse – both in the United States and Canada. According to the final Canadian report of the inquiry into missing and murdered Indigenous women and girls, the murder rate for Indigenous women was 12 times that of non-Indigenous women.8
We assert that this combination of historical trauma and current social factors contributes to depression, PTSD, and anxiety disorders that currently feed the rise in attempted and completed suicide. Less-than-optimal educational opportunities and unemployment, often above 10% on reservations,9 along with food insecurity, accentuate the settings in which women and girls live.
Women achieving despite challenges
Yet, Indigenous women are making great strides within their cultures and communities. For example, Indigenous women are leading language revitalization, and within their culture, are healers and carriers of knowledge. Many Indigenous women are doctors, lawyers, dentists, teachers, poets, authors, and artists.10 Voters in last year’s midterms elected two Native American women to the U.S. Congress. Often, however, those achievements within the Western culture come at a cost, and some might have difficulty balancing those roles with their traditional cultures.
Current societal pressures feed the rise of suicide. Santa Fe, N.M., is known for its affluence and reputation as a tricultural city of Anglos, Hispanics, and Native Americans, and yet, a recent health impact assessment survey of urban Indigenous families stated that food insecurity was the leading concern for those families. Unemployment on the Navajo Nation is above 50%.11 The Indian Health Service (IHS) in the United States, which provides the majority of mental services to the Indigenous population, has identified mental health issues as the No. 1 health problem. However, only 7% of the IHS budget is allocated for mental health and substance abuse services. This represents an underfudging of services to American Indian and Alaska Native communities. In fact, there were only two psychiatrists per 100,000 people served by the IHS, which is one-seventh the number of psychiatrists available to the general population in the United States.12
Best practices for psychiatrists working with Indigenous women demands that we know the history, know how that history is still being manifest in subtle ways, and understand how such antiquated papal bulletins as the Doctrine of Discovery still operate to justify the taking and misuse of indigenous land. We must realize that the dominant economic systems, laws, and policing strategies are imposed on cultures that are sophisticated in their own right. This will then allow compassionate care with a level of understanding.
13
We can advocate at all levels, considering that the role of the federal government, the state, corporations, tribes, families, and provision of quality care to individuals can continue the positive collective advancement of women, and reduce the morbidity and mortality associated with suicide attempts.
We need to be sensitive to our patients and their risks of suicide. Treat suicidal ideation as the serious threat that it is. Address the depression, anxiety, PTSD, historical trauma, substance abuse, emotional dysregulation, and loss of relationship in persons with attachment disorders as serious and valid life events than can lead to serious consequences – including completed suicide.
Indigenous women are resilient, and the approach should be to also balance knowledge of those potential barriers with validating the feminine, and supporting the traditional roles of women and men that value women and children, and revere the matriarchs. Encouraging and supporting Indigenous resurgence of cultural practices and values is significant for positive outcomes for healing and wellness. Doing so can carry a greater meaning within Indigenous and First Nations society.
References
1. Curtin SC and H Hedegaard. Suicide rates for females and males by race and ethnicity: United States, 1999 and 2017. NCHS Health E-Stat. 2019.
2. Anderson GC. Ethnic cleansing and the Indian: The crime that should haunt America. Norman, Okla.: University of Oklahoma Press, 2014.
3. Rausch N. “Standing Rock, Morton County work to mend relationships post-DAPL protests.” Billingsgazette.com. Aug 10, 2019.
4. Roy A. “5 ways the government keeps Native Americans in poverty.” Forbes.com. Mar 13, 2014.
5. Blakemore E. “The little-known history of forced sterilization of Native American women.” JSTOR.org. Aug 25, 2016.
6. Bleir G and A Zoledziowski. “Murdered and missing Native American women challenge police and courts.” Publicintegrity.org. Aug 27, 2018.
7. Brockie TN et al. A framework to examine the role of epigenetics in health disparities among Native Americans. Nurs Res Prac. 2013;2013:410395.
8. “Reclaiming power and place: The final report of the national inquiry into missing and murdered Indigenous women and girls.” Vancouver: Privy Office. Jun 3, 2019.
9. Hagan S. “Where U.S. unemployment is still sky-high: Indian reservations.” Bloomberg.com. Apr 5, 2018.
10. Morin B. “Meet 10 Indigenous women who are making the world a better place.” Indian Country Today. Jul 1, 2019.
11. Fact sheet. Discovernavajo.com.
12. Sarche M and P Spicer. Poverty and health disparities for American Indian and Alaska Native children: Current knowledge and future prospects. Ann NY Acad Sci. 2008 Jul 25;1136:126-36.
13. Lewis-Fernández R et al. Culture and psychiatric evaluation: Operationalizing cultural formulation for DSM-5. Psychiatry. 2014 Summer;77(2):130-54.
Dr. Roessel is a Navajo board-certified psychiatrist practicing in Santa Fe, N.M., working with the local Indigenous population. She has special expertise in cultural psychiatry; her childhood was spent growing up in the Navajo nation with her grandfather, who was a Navajo medicine man. Her psychiatric practice focuses on integrating Indigenous knowledge and principles. Dr. Roessel is a distinguished fellow of the American Psychiatric Association.
Dr. Neidhardt is a board-certified psychiatrist who lives in Santa Fe and has an integrative, holistic psychiatric practice that also specializes in trauma-focused therapy. He has provided care for Indigenous People in the Southwest United States and in Canada, and has worked with Navajo medicine people to develop training for mental health professionals with his wife, Dr. Mary Hasbah Roessel. Dr. Reinhardt is a life fellow of the APA.
Product Update: Osphena’s NDA, new hysteroscope, TempSure RF technology, Resilient stirrup covers
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
Which birth defects are associated with childhood cancer risk?