User login
Evaluation of Gender as a Clinically Relevant Outcome Variable in the Treatment of Onychomycosis With Efinaconazole Topical Solution 10%
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
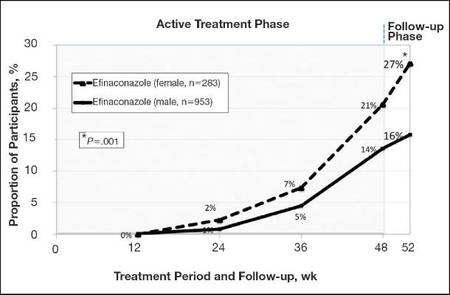
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).
|
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |
|
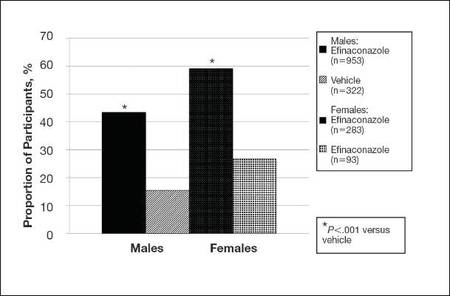
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
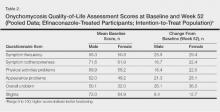
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).

|
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |

|
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).

|
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |

|
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
Practice Points
- Men, particularly as they age, are more likely to develop onychomycosis.
- Treatment adherence may be a bigger issue among male patients.
- Onychomycosis in males may be more difficult to treat for a variety of reasons.
Cosmetic Corner: Dermatologists Weigh in on OTC Dandruff Treatments
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC dandruff treatments. Consideration must be given to:
- Head & Shoulders Shampoo
Procter & Gamble
“OTC dandruff products are more for maintenance rather than active treatment, which is why many consumers and patients become frustrated with their use. I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
- Moroccanoil Treatment
Moroccanoil
“I think it’s great to actually put [this product] directly onto the scalp after shampooing to get any remaining scales off.”—Anthony M. Rossi, MD, New York, New York
- Neutrogena T/Gel Therapeutic Hair Care
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Neutrogena T/Sal Therapeutic Shampoo
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Nizoral A-D Ketoconazole Shampoo 1%
McNeil-PPC, Inc
“I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
Cutis invites readers to send us their recommendations. Eye creams, men’s shaving products, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC dandruff treatments. Consideration must be given to:
- Head & Shoulders Shampoo
Procter & Gamble
“OTC dandruff products are more for maintenance rather than active treatment, which is why many consumers and patients become frustrated with their use. I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
- Moroccanoil Treatment
Moroccanoil
“I think it’s great to actually put [this product] directly onto the scalp after shampooing to get any remaining scales off.”—Anthony M. Rossi, MD, New York, New York
- Neutrogena T/Gel Therapeutic Hair Care
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Neutrogena T/Sal Therapeutic Shampoo
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Nizoral A-D Ketoconazole Shampoo 1%
McNeil-PPC, Inc
“I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
Cutis invites readers to send us their recommendations. Eye creams, men’s shaving products, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC dandruff treatments. Consideration must be given to:
- Head & Shoulders Shampoo
Procter & Gamble
“OTC dandruff products are more for maintenance rather than active treatment, which is why many consumers and patients become frustrated with their use. I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
- Moroccanoil Treatment
Moroccanoil
“I think it’s great to actually put [this product] directly onto the scalp after shampooing to get any remaining scales off.”—Anthony M. Rossi, MD, New York, New York
- Neutrogena T/Gel Therapeutic Hair Care
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Neutrogena T/Sal Therapeutic Shampoo
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Nizoral A-D Ketoconazole Shampoo 1%
McNeil-PPC, Inc
“I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
Cutis invites readers to send us their recommendations. Eye creams, men’s shaving products, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Turn down the androgens to treat female pattern hair loss
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Onychomatricoma: An Often Misdiagnosed Tumor of the Nails
Changes in the appearance of the nail apparatus can be produced by a variety of conditions. Onychomatricoma is an unusual benign tumor with specific clinical characteristics that was first described more than 2 decades ago.1 It is often and easily misdiagnosed because the condition rarely has been described. We report a case of onychomatricoma in a 54-year-old Colombian man who presented with a deformity of the nail plate on the right index finger that corresponded with the classic description of onychomatricoma. We emphasize the importance of reporting such lesions to prevent misdiagnosis and delay of proper treatment.
Case Report
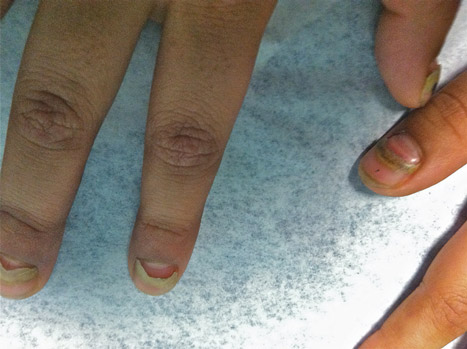
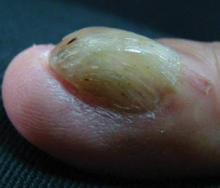
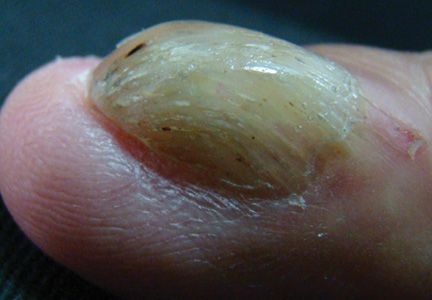
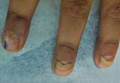
A 54-year-old Colombian man presented with nail dystrophy involving the right index finger of 2 years’ duration. He did not recall any trauma prior to the onset of the nail abnormalities. Several topical treatments had previously been ineffective. Physical examination revealed a longitudinally banded thickening of the lateral half of the nail plate on the right index finger with yellowish brown discoloration, transverse overcurvature of the nail, longitudinal white lines, and splinter hemorrhages (Figure 1). Direct microscopy and fungal culture were performed to diagnose or rule out onychomycosis.
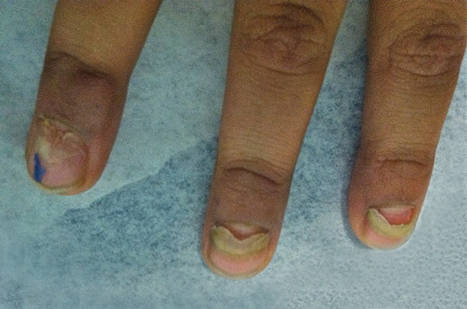
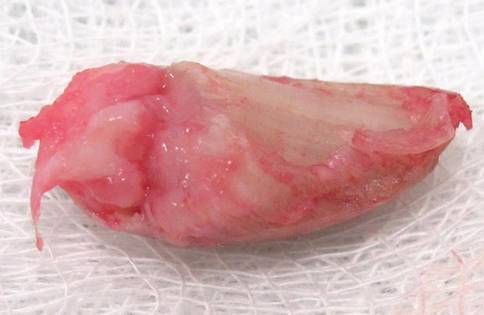
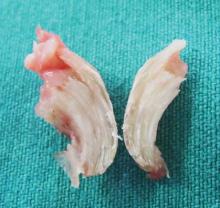
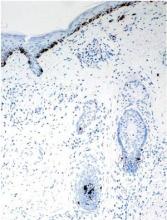
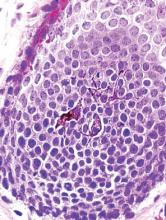
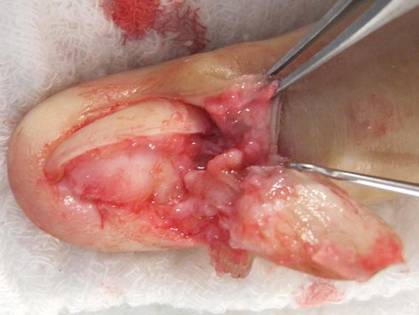
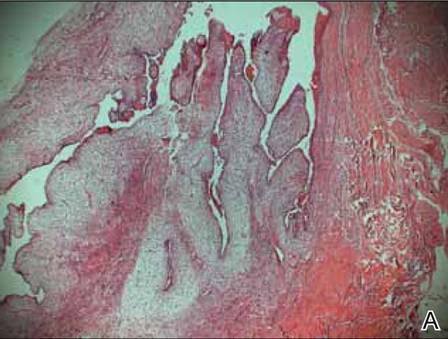
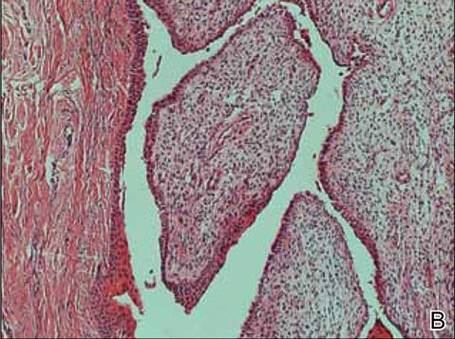
A clinical diagnosis of onychomatricoma was made, and the lesion was surgically removed and sent for histopathologic study (Figure 2). The radial half of the nail plate was avulsed, and the proximal part of the removed nail plate contained a large, firmly attached, filamentous tumor arising from the nail matrix (Figure 3) with multiple fine filiform projections (Figure 4). The nail bed was cleaned with a curette to remove any debris, the ulnar half of the nail plate and nail bed was left in place, and the radial border was reconstructed. Histology confirmed the clinical diagnosis (Figure 5). No recurrences of the tumor were seen 36 months following surgery.
| 
|
Comment
Since the original report of this tumor,1 fewer than 10 cases of onychomatricoma have been reported in Latin America,2-5 with no more than 80 cases reported worldwide.6 Clinicians and academicians are becoming interested in the topic, which will result in better recognition and more reports in the literature.
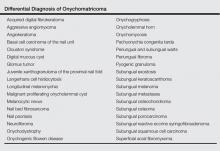
The clinical differential diagnosis of onycho-matricoma is extensive,7,8 but onychomatricoma has characteristic clinical and histopathologic features that allow its separation from other nail disorders and subungual tumors (Table).9 There are 4 cardinal clinical signs that suggest a diagnosis of onychomatricoma: (1) banded or diffuse thickening of the nail plate of variable widths; (2) yellowish discoloration of the involved nail plate, often showing fine splinter hemorrhages in the proximal nail portion; (3) transverse overcurvature of the nail; and (4) exposure of a filamentous tufted tumor emerging from the matrix in a funnel-shaped nail by avulsion.1
|
Histologic findings of onychomatricoma typically demonstrate a fibroepithelial tumor with a biphasic growth pattern mimicking normal nail matrix histology, including a proximal zone, which corresponds to the base of the fibroepithelial tumor, and a distal zone, which is composed of multiple epithelial digitations that extend into the small cavities present in the attached nail.10-12 Nevertheless, the anatomic tumor location, the often fragmented aspect of the tissue specimen, and the choice of the section planes may change the typical histologic features seen in onychomatricoma.13 Stromal prominence, cellularity, and atypia may vary in individual cases.10-12
The etiology of onychomatricoma is not yet known. Although it has been suggested that onychomatricoma could be an epithelial and connective tissue hamartoma simulating the nail matrix structure,1,10 the more recent concept of an epithelial onychogenic tumor with onychogenic mesenchyme will help to clarify its etiology because new histopathologic and immunohistochemical features suggest a neoplastic nature.14 On the other hand, predisposing factors such as trauma to the nail plate and onychomycosis may play a role,7 as the thumbs, index fingers, and great toes are more susceptible to onychomycosis and accidental trauma.
Conclusion
Our patient fulfilled the criteria of onychomatricoma.1 Onychomatricoma should be kept in mind in the differential diagnosis of subungual or periungual tumors to avoid misdiagnosis and erroneous treatments.
1. Baran R, Kint A. Onychomatrixoma: filamentous tufted tumor in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Estrada-Chavez G, Vega-Memije ME, Toussaint-Caire S, et al. Giant onychomatricoma: report of two cases with rare clinical presentation. Int J Dermatol. 2007;46: 634-636.
3. Soto R, Wortsman X, Corredoira Y. Onychomatricoma: clinical and sonographic findings. Arch Dermatol. 2009;145:1461-1462.
4. Tavares GT, Chiacchio NG, Chiacchio ND, et al. Onychomatricoma: a tumor unknown to dermatologists. An Bras Dermatol. 2015;90:265-267.
5. Fernández-Sánchez M, Saeb-Lima M, Charli-Joseph Y, et al. Onychomatricoma: an infrequent nail tumor. Indian J Dermatol Venereol Leprol. 2012;78:382-383.
6. Tavares G, Di-Chiacchio N, Di-Santis E, et al. Onycho-matricoma: epidemiological and clinical findings in a large series of 30 cases [published online ahead of print May 12, 2015]. Br J Dermatol. doi:10.1111/bjd.13900.
7. Rashid RM, Swan J. Onychomatricoma: benign sporadic nail lesion or much more? Dermatol Online J. 2006;12:4.
8. Goutos I, Furniss D, Smith GD. Onychomatricoma: an unusual case of ungual pathology. case report and review of the literature. J Plast Reconstr Aesthet Surg. 2010;63:54-57.
9. Fraga GR, Patterson JW, McHargue CA. Onychomatricoma: report of a case and its comparison with fibrokeratoma of the nailbed. Am J Dermatopathol. 2001;23:36-40.
10. Perrin C, Goettmann S, Baran R. Onychomatricoma: clinical and histopathologic findings in 12 cases. J Am Acad Dermatol. 1998;39:560-564.
11. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):S66-S69.
12. Perrin C, Baran R, Pisani A, et al. The onychomatricoma: additional histologic criteria and immunohistochemical study. Am J Dermatopathol. 2002;24:199-203.
13. Perrin C, Baran R, Balaguer T, et al. Onychomatricoma: new clinical and histological features. a review of 19 tumors. Am J Dermatopathol. 2010;32:1-8.
14. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
Changes in the appearance of the nail apparatus can be produced by a variety of conditions. Onychomatricoma is an unusual benign tumor with specific clinical characteristics that was first described more than 2 decades ago.1 It is often and easily misdiagnosed because the condition rarely has been described. We report a case of onychomatricoma in a 54-year-old Colombian man who presented with a deformity of the nail plate on the right index finger that corresponded with the classic description of onychomatricoma. We emphasize the importance of reporting such lesions to prevent misdiagnosis and delay of proper treatment.
Case Report
A 54-year-old Colombian man presented with nail dystrophy involving the right index finger of 2 years’ duration. He did not recall any trauma prior to the onset of the nail abnormalities. Several topical treatments had previously been ineffective. Physical examination revealed a longitudinally banded thickening of the lateral half of the nail plate on the right index finger with yellowish brown discoloration, transverse overcurvature of the nail, longitudinal white lines, and splinter hemorrhages (Figure 1). Direct microscopy and fungal culture were performed to diagnose or rule out onychomycosis.
A clinical diagnosis of onychomatricoma was made, and the lesion was surgically removed and sent for histopathologic study (Figure 2). The radial half of the nail plate was avulsed, and the proximal part of the removed nail plate contained a large, firmly attached, filamentous tumor arising from the nail matrix (Figure 3) with multiple fine filiform projections (Figure 4). The nail bed was cleaned with a curette to remove any debris, the ulnar half of the nail plate and nail bed was left in place, and the radial border was reconstructed. Histology confirmed the clinical diagnosis (Figure 5). No recurrences of the tumor were seen 36 months following surgery.
| 
|
Comment
Since the original report of this tumor,1 fewer than 10 cases of onychomatricoma have been reported in Latin America,2-5 with no more than 80 cases reported worldwide.6 Clinicians and academicians are becoming interested in the topic, which will result in better recognition and more reports in the literature.
The clinical differential diagnosis of onycho-matricoma is extensive,7,8 but onychomatricoma has characteristic clinical and histopathologic features that allow its separation from other nail disorders and subungual tumors (Table).9 There are 4 cardinal clinical signs that suggest a diagnosis of onychomatricoma: (1) banded or diffuse thickening of the nail plate of variable widths; (2) yellowish discoloration of the involved nail plate, often showing fine splinter hemorrhages in the proximal nail portion; (3) transverse overcurvature of the nail; and (4) exposure of a filamentous tufted tumor emerging from the matrix in a funnel-shaped nail by avulsion.1


|
Histologic findings of onychomatricoma typically demonstrate a fibroepithelial tumor with a biphasic growth pattern mimicking normal nail matrix histology, including a proximal zone, which corresponds to the base of the fibroepithelial tumor, and a distal zone, which is composed of multiple epithelial digitations that extend into the small cavities present in the attached nail.10-12 Nevertheless, the anatomic tumor location, the often fragmented aspect of the tissue specimen, and the choice of the section planes may change the typical histologic features seen in onychomatricoma.13 Stromal prominence, cellularity, and atypia may vary in individual cases.10-12
The etiology of onychomatricoma is not yet known. Although it has been suggested that onychomatricoma could be an epithelial and connective tissue hamartoma simulating the nail matrix structure,1,10 the more recent concept of an epithelial onychogenic tumor with onychogenic mesenchyme will help to clarify its etiology because new histopathologic and immunohistochemical features suggest a neoplastic nature.14 On the other hand, predisposing factors such as trauma to the nail plate and onychomycosis may play a role,7 as the thumbs, index fingers, and great toes are more susceptible to onychomycosis and accidental trauma.
Conclusion
Our patient fulfilled the criteria of onychomatricoma.1 Onychomatricoma should be kept in mind in the differential diagnosis of subungual or periungual tumors to avoid misdiagnosis and erroneous treatments.
Changes in the appearance of the nail apparatus can be produced by a variety of conditions. Onychomatricoma is an unusual benign tumor with specific clinical characteristics that was first described more than 2 decades ago.1 It is often and easily misdiagnosed because the condition rarely has been described. We report a case of onychomatricoma in a 54-year-old Colombian man who presented with a deformity of the nail plate on the right index finger that corresponded with the classic description of onychomatricoma. We emphasize the importance of reporting such lesions to prevent misdiagnosis and delay of proper treatment.
Case Report
A 54-year-old Colombian man presented with nail dystrophy involving the right index finger of 2 years’ duration. He did not recall any trauma prior to the onset of the nail abnormalities. Several topical treatments had previously been ineffective. Physical examination revealed a longitudinally banded thickening of the lateral half of the nail plate on the right index finger with yellowish brown discoloration, transverse overcurvature of the nail, longitudinal white lines, and splinter hemorrhages (Figure 1). Direct microscopy and fungal culture were performed to diagnose or rule out onychomycosis.
A clinical diagnosis of onychomatricoma was made, and the lesion was surgically removed and sent for histopathologic study (Figure 2). The radial half of the nail plate was avulsed, and the proximal part of the removed nail plate contained a large, firmly attached, filamentous tumor arising from the nail matrix (Figure 3) with multiple fine filiform projections (Figure 4). The nail bed was cleaned with a curette to remove any debris, the ulnar half of the nail plate and nail bed was left in place, and the radial border was reconstructed. Histology confirmed the clinical diagnosis (Figure 5). No recurrences of the tumor were seen 36 months following surgery.
| 
|
Comment
Since the original report of this tumor,1 fewer than 10 cases of onychomatricoma have been reported in Latin America,2-5 with no more than 80 cases reported worldwide.6 Clinicians and academicians are becoming interested in the topic, which will result in better recognition and more reports in the literature.
The clinical differential diagnosis of onycho-matricoma is extensive,7,8 but onychomatricoma has characteristic clinical and histopathologic features that allow its separation from other nail disorders and subungual tumors (Table).9 There are 4 cardinal clinical signs that suggest a diagnosis of onychomatricoma: (1) banded or diffuse thickening of the nail plate of variable widths; (2) yellowish discoloration of the involved nail plate, often showing fine splinter hemorrhages in the proximal nail portion; (3) transverse overcurvature of the nail; and (4) exposure of a filamentous tufted tumor emerging from the matrix in a funnel-shaped nail by avulsion.1


|
Histologic findings of onychomatricoma typically demonstrate a fibroepithelial tumor with a biphasic growth pattern mimicking normal nail matrix histology, including a proximal zone, which corresponds to the base of the fibroepithelial tumor, and a distal zone, which is composed of multiple epithelial digitations that extend into the small cavities present in the attached nail.10-12 Nevertheless, the anatomic tumor location, the often fragmented aspect of the tissue specimen, and the choice of the section planes may change the typical histologic features seen in onychomatricoma.13 Stromal prominence, cellularity, and atypia may vary in individual cases.10-12
The etiology of onychomatricoma is not yet known. Although it has been suggested that onychomatricoma could be an epithelial and connective tissue hamartoma simulating the nail matrix structure,1,10 the more recent concept of an epithelial onychogenic tumor with onychogenic mesenchyme will help to clarify its etiology because new histopathologic and immunohistochemical features suggest a neoplastic nature.14 On the other hand, predisposing factors such as trauma to the nail plate and onychomycosis may play a role,7 as the thumbs, index fingers, and great toes are more susceptible to onychomycosis and accidental trauma.
Conclusion
Our patient fulfilled the criteria of onychomatricoma.1 Onychomatricoma should be kept in mind in the differential diagnosis of subungual or periungual tumors to avoid misdiagnosis and erroneous treatments.
1. Baran R, Kint A. Onychomatrixoma: filamentous tufted tumor in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Estrada-Chavez G, Vega-Memije ME, Toussaint-Caire S, et al. Giant onychomatricoma: report of two cases with rare clinical presentation. Int J Dermatol. 2007;46: 634-636.
3. Soto R, Wortsman X, Corredoira Y. Onychomatricoma: clinical and sonographic findings. Arch Dermatol. 2009;145:1461-1462.
4. Tavares GT, Chiacchio NG, Chiacchio ND, et al. Onychomatricoma: a tumor unknown to dermatologists. An Bras Dermatol. 2015;90:265-267.
5. Fernández-Sánchez M, Saeb-Lima M, Charli-Joseph Y, et al. Onychomatricoma: an infrequent nail tumor. Indian J Dermatol Venereol Leprol. 2012;78:382-383.
6. Tavares G, Di-Chiacchio N, Di-Santis E, et al. Onycho-matricoma: epidemiological and clinical findings in a large series of 30 cases [published online ahead of print May 12, 2015]. Br J Dermatol. doi:10.1111/bjd.13900.
7. Rashid RM, Swan J. Onychomatricoma: benign sporadic nail lesion or much more? Dermatol Online J. 2006;12:4.
8. Goutos I, Furniss D, Smith GD. Onychomatricoma: an unusual case of ungual pathology. case report and review of the literature. J Plast Reconstr Aesthet Surg. 2010;63:54-57.
9. Fraga GR, Patterson JW, McHargue CA. Onychomatricoma: report of a case and its comparison with fibrokeratoma of the nailbed. Am J Dermatopathol. 2001;23:36-40.
10. Perrin C, Goettmann S, Baran R. Onychomatricoma: clinical and histopathologic findings in 12 cases. J Am Acad Dermatol. 1998;39:560-564.
11. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):S66-S69.
12. Perrin C, Baran R, Pisani A, et al. The onychomatricoma: additional histologic criteria and immunohistochemical study. Am J Dermatopathol. 2002;24:199-203.
13. Perrin C, Baran R, Balaguer T, et al. Onychomatricoma: new clinical and histological features. a review of 19 tumors. Am J Dermatopathol. 2010;32:1-8.
14. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
1. Baran R, Kint A. Onychomatrixoma: filamentous tufted tumor in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Estrada-Chavez G, Vega-Memije ME, Toussaint-Caire S, et al. Giant onychomatricoma: report of two cases with rare clinical presentation. Int J Dermatol. 2007;46: 634-636.
3. Soto R, Wortsman X, Corredoira Y. Onychomatricoma: clinical and sonographic findings. Arch Dermatol. 2009;145:1461-1462.
4. Tavares GT, Chiacchio NG, Chiacchio ND, et al. Onychomatricoma: a tumor unknown to dermatologists. An Bras Dermatol. 2015;90:265-267.
5. Fernández-Sánchez M, Saeb-Lima M, Charli-Joseph Y, et al. Onychomatricoma: an infrequent nail tumor. Indian J Dermatol Venereol Leprol. 2012;78:382-383.
6. Tavares G, Di-Chiacchio N, Di-Santis E, et al. Onycho-matricoma: epidemiological and clinical findings in a large series of 30 cases [published online ahead of print May 12, 2015]. Br J Dermatol. doi:10.1111/bjd.13900.
7. Rashid RM, Swan J. Onychomatricoma: benign sporadic nail lesion or much more? Dermatol Online J. 2006;12:4.
8. Goutos I, Furniss D, Smith GD. Onychomatricoma: an unusual case of ungual pathology. case report and review of the literature. J Plast Reconstr Aesthet Surg. 2010;63:54-57.
9. Fraga GR, Patterson JW, McHargue CA. Onychomatricoma: report of a case and its comparison with fibrokeratoma of the nailbed. Am J Dermatopathol. 2001;23:36-40.
10. Perrin C, Goettmann S, Baran R. Onychomatricoma: clinical and histopathologic findings in 12 cases. J Am Acad Dermatol. 1998;39:560-564.
11. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):S66-S69.
12. Perrin C, Baran R, Pisani A, et al. The onychomatricoma: additional histologic criteria and immunohistochemical study. Am J Dermatopathol. 2002;24:199-203.
13. Perrin C, Baran R, Balaguer T, et al. Onychomatricoma: new clinical and histological features. a review of 19 tumors. Am J Dermatopathol. 2010;32:1-8.
14. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
Practice Points
- Onychomatricoma has been described mostly in white individuals, but it can occur in all races and ethnic groups.
- Onychomatricoma should be kept in mind in the differential diagnosis of subungual or periungual tumors.
- Treatment of onychomatricoma is complete surgical excision.
Nail surgery: Top anesthesia tips
VANCOUVER – Achieving effective local anesthesia is the critical first step in successful nail surgery, Dr. Chris G. Adigun said at the World Congress of Dermatology.
“Always remember: Nail surgery hurts. Your patients will applaud you enthusiastically when they’re back home for your having used a long-acting anesthetic,” said Dr. Adigun, a dermatologist in group practice in Chapel Hill, N.C.
The three most widely used anesthetic agents in nail surgery are lidocaine (Xylocaine), bupivacaine (Marcaine), and ropivacaine (Naropin). Dr. Adigun said she strongly prefers ropivacaine. It combines the best features of the other two: lidocaine’s rapid onset along with a duration of action that’s even longer than bupivacaine’s, she noted. Ropivacaine’s duration of action is 8-12 hours – and it comes without bupivacaine’s potential for cardiotoxicity. Moreover, ropivacaine has a vasoconstrictive effect, which improves hemostasis and enhances visualization during the surgery.
She provided numerous additional tips on how to predictably achieve effective anesthesia for nail surgery:
• Buffer with sodium bicarbonate. The idea is to bring the anesthetic solution close to physiologic pH, which makes for a far less painful experience than injecting the acidic unbuffered solution.
• Warm it. Investigators have shown that warming anesthetic fluid reduces pain upon injection of both nonbuffered and buffered local anesthetics (Ann Emerg Med. 2011 Jul;58(1):86-98).
• Stick to a small-gauge needle. Dr. Adigan said she favors 30 gauge. It makes for a smaller, less painful puncture and limits the rate of flow of anesthetic fluid into the digital space.
• Inject in a perpendicular plane. This will disrupt fewer nerve endings than when going in at an angle.
“I think this is something that’s not frequently taught to residents in dermatology. I think we almost always go in at an angle, but if you go in at a perpendicular plane, you’re going to cause less pain,” according to Dr. Adigun.
• Inject just below the dermis. The dermis is nociceptor rich, and stretching those tissues by injecting a volume of fluid there will cause intense, continuous pain until the local anesthetic has time to take effect.
• Use distraction techniques liberally. Dr. Adigun said she likes to tell stories and jokes, which she calls “talkesthesia.” She also utilizes a battery-powered massager.
“Put the massager as close to your surgical field as you’re comfortable with. Under the gate theory of pain, you want to create as much sensory ‘noise’ as possible with your distraction techniques so that gate is filled with your sensory noise rather than pain,” the dermatologist explained.
There are three solid, time-tested completely acceptable techniques for getting the target digit numb: the wing block, the traditional digital block, and the transthecal digital block.
Dr. Adigun said she generally relies upon the wing block unless she is concerned that the associated blanching might cause her to lose her digital landmarks during surgery addressing a subtle abnormality. In that situation she turns mainly to the traditional digital block, which doesn’t interfere with digital landmarks and effectively anesthetizes both the paired digital and volar nerves.
The downside of the traditional digital block is it entails a 15- to 20-minute wait for the anesthetic to diffuse. So does the transthecal digital block, which has the additional shortcoming of achieving predictable results only when applied for surgery on the second, third, or fourth digits.
The wing block is an efficient infiltrative technique targeting the distal digit. It offers immediate anesthesia of the total nail unit. To achieve an excellent wing block, initially inject just 0.1-0.2 mL of anesthetic fluid subcutaneously into the proximal nail fold midway between the cuticle and the distal interphalangeal joint. Wait for a wheal to form; then wait an additional 45-60 seconds. At that point, inject obliquely along the lateral edge of the nail fold in the direction of the digital tip. The needle should be advanced while maintaining a gentle fluid bolus ahead of the needle tip in order to minimize the patient’s sensation of the moving needle. The process is then repeated on the opposite side of the digit.
“You want to keep that needle in the dermal plane and avoid filling the pulp with anesthetic fluid. If you do this correctly, only one prick is felt by the patient. I very rarely have to use a full cc of anesthetic fluid when I use a wing block,” Dr. Adigun said.
If any additional needle insertions are needed, make sure they’re placed into tissue that’s already been anesthetized, she added.
Dr. Adigun reported having no financial conflicts of interest.
VANCOUVER – Achieving effective local anesthesia is the critical first step in successful nail surgery, Dr. Chris G. Adigun said at the World Congress of Dermatology.
“Always remember: Nail surgery hurts. Your patients will applaud you enthusiastically when they’re back home for your having used a long-acting anesthetic,” said Dr. Adigun, a dermatologist in group practice in Chapel Hill, N.C.
The three most widely used anesthetic agents in nail surgery are lidocaine (Xylocaine), bupivacaine (Marcaine), and ropivacaine (Naropin). Dr. Adigun said she strongly prefers ropivacaine. It combines the best features of the other two: lidocaine’s rapid onset along with a duration of action that’s even longer than bupivacaine’s, she noted. Ropivacaine’s duration of action is 8-12 hours – and it comes without bupivacaine’s potential for cardiotoxicity. Moreover, ropivacaine has a vasoconstrictive effect, which improves hemostasis and enhances visualization during the surgery.
She provided numerous additional tips on how to predictably achieve effective anesthesia for nail surgery:
• Buffer with sodium bicarbonate. The idea is to bring the anesthetic solution close to physiologic pH, which makes for a far less painful experience than injecting the acidic unbuffered solution.
• Warm it. Investigators have shown that warming anesthetic fluid reduces pain upon injection of both nonbuffered and buffered local anesthetics (Ann Emerg Med. 2011 Jul;58(1):86-98).
• Stick to a small-gauge needle. Dr. Adigan said she favors 30 gauge. It makes for a smaller, less painful puncture and limits the rate of flow of anesthetic fluid into the digital space.
• Inject in a perpendicular plane. This will disrupt fewer nerve endings than when going in at an angle.
“I think this is something that’s not frequently taught to residents in dermatology. I think we almost always go in at an angle, but if you go in at a perpendicular plane, you’re going to cause less pain,” according to Dr. Adigun.
• Inject just below the dermis. The dermis is nociceptor rich, and stretching those tissues by injecting a volume of fluid there will cause intense, continuous pain until the local anesthetic has time to take effect.
• Use distraction techniques liberally. Dr. Adigun said she likes to tell stories and jokes, which she calls “talkesthesia.” She also utilizes a battery-powered massager.
“Put the massager as close to your surgical field as you’re comfortable with. Under the gate theory of pain, you want to create as much sensory ‘noise’ as possible with your distraction techniques so that gate is filled with your sensory noise rather than pain,” the dermatologist explained.
There are three solid, time-tested completely acceptable techniques for getting the target digit numb: the wing block, the traditional digital block, and the transthecal digital block.
Dr. Adigun said she generally relies upon the wing block unless she is concerned that the associated blanching might cause her to lose her digital landmarks during surgery addressing a subtle abnormality. In that situation she turns mainly to the traditional digital block, which doesn’t interfere with digital landmarks and effectively anesthetizes both the paired digital and volar nerves.
The downside of the traditional digital block is it entails a 15- to 20-minute wait for the anesthetic to diffuse. So does the transthecal digital block, which has the additional shortcoming of achieving predictable results only when applied for surgery on the second, third, or fourth digits.
The wing block is an efficient infiltrative technique targeting the distal digit. It offers immediate anesthesia of the total nail unit. To achieve an excellent wing block, initially inject just 0.1-0.2 mL of anesthetic fluid subcutaneously into the proximal nail fold midway between the cuticle and the distal interphalangeal joint. Wait for a wheal to form; then wait an additional 45-60 seconds. At that point, inject obliquely along the lateral edge of the nail fold in the direction of the digital tip. The needle should be advanced while maintaining a gentle fluid bolus ahead of the needle tip in order to minimize the patient’s sensation of the moving needle. The process is then repeated on the opposite side of the digit.
“You want to keep that needle in the dermal plane and avoid filling the pulp with anesthetic fluid. If you do this correctly, only one prick is felt by the patient. I very rarely have to use a full cc of anesthetic fluid when I use a wing block,” Dr. Adigun said.
If any additional needle insertions are needed, make sure they’re placed into tissue that’s already been anesthetized, she added.
Dr. Adigun reported having no financial conflicts of interest.
VANCOUVER – Achieving effective local anesthesia is the critical first step in successful nail surgery, Dr. Chris G. Adigun said at the World Congress of Dermatology.
“Always remember: Nail surgery hurts. Your patients will applaud you enthusiastically when they’re back home for your having used a long-acting anesthetic,” said Dr. Adigun, a dermatologist in group practice in Chapel Hill, N.C.
The three most widely used anesthetic agents in nail surgery are lidocaine (Xylocaine), bupivacaine (Marcaine), and ropivacaine (Naropin). Dr. Adigun said she strongly prefers ropivacaine. It combines the best features of the other two: lidocaine’s rapid onset along with a duration of action that’s even longer than bupivacaine’s, she noted. Ropivacaine’s duration of action is 8-12 hours – and it comes without bupivacaine’s potential for cardiotoxicity. Moreover, ropivacaine has a vasoconstrictive effect, which improves hemostasis and enhances visualization during the surgery.
She provided numerous additional tips on how to predictably achieve effective anesthesia for nail surgery:
• Buffer with sodium bicarbonate. The idea is to bring the anesthetic solution close to physiologic pH, which makes for a far less painful experience than injecting the acidic unbuffered solution.
• Warm it. Investigators have shown that warming anesthetic fluid reduces pain upon injection of both nonbuffered and buffered local anesthetics (Ann Emerg Med. 2011 Jul;58(1):86-98).
• Stick to a small-gauge needle. Dr. Adigan said she favors 30 gauge. It makes for a smaller, less painful puncture and limits the rate of flow of anesthetic fluid into the digital space.
• Inject in a perpendicular plane. This will disrupt fewer nerve endings than when going in at an angle.
“I think this is something that’s not frequently taught to residents in dermatology. I think we almost always go in at an angle, but if you go in at a perpendicular plane, you’re going to cause less pain,” according to Dr. Adigun.
• Inject just below the dermis. The dermis is nociceptor rich, and stretching those tissues by injecting a volume of fluid there will cause intense, continuous pain until the local anesthetic has time to take effect.
• Use distraction techniques liberally. Dr. Adigun said she likes to tell stories and jokes, which she calls “talkesthesia.” She also utilizes a battery-powered massager.
“Put the massager as close to your surgical field as you’re comfortable with. Under the gate theory of pain, you want to create as much sensory ‘noise’ as possible with your distraction techniques so that gate is filled with your sensory noise rather than pain,” the dermatologist explained.
There are three solid, time-tested completely acceptable techniques for getting the target digit numb: the wing block, the traditional digital block, and the transthecal digital block.
Dr. Adigun said she generally relies upon the wing block unless she is concerned that the associated blanching might cause her to lose her digital landmarks during surgery addressing a subtle abnormality. In that situation she turns mainly to the traditional digital block, which doesn’t interfere with digital landmarks and effectively anesthetizes both the paired digital and volar nerves.
The downside of the traditional digital block is it entails a 15- to 20-minute wait for the anesthetic to diffuse. So does the transthecal digital block, which has the additional shortcoming of achieving predictable results only when applied for surgery on the second, third, or fourth digits.
The wing block is an efficient infiltrative technique targeting the distal digit. It offers immediate anesthesia of the total nail unit. To achieve an excellent wing block, initially inject just 0.1-0.2 mL of anesthetic fluid subcutaneously into the proximal nail fold midway between the cuticle and the distal interphalangeal joint. Wait for a wheal to form; then wait an additional 45-60 seconds. At that point, inject obliquely along the lateral edge of the nail fold in the direction of the digital tip. The needle should be advanced while maintaining a gentle fluid bolus ahead of the needle tip in order to minimize the patient’s sensation of the moving needle. The process is then repeated on the opposite side of the digit.
“You want to keep that needle in the dermal plane and avoid filling the pulp with anesthetic fluid. If you do this correctly, only one prick is felt by the patient. I very rarely have to use a full cc of anesthetic fluid when I use a wing block,” Dr. Adigun said.
If any additional needle insertions are needed, make sure they’re placed into tissue that’s already been anesthetized, she added.
Dr. Adigun reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM WCD 2015
WCD: How to submit a proper nail specimen
VANCOUVER – Dr. Curtis T. Thompson is on a mission: to improve the often-shoddy quality of nail biopsy specimens submitted to pathologists.
No standardized protocols for nail specimens exist. The quality of pathologic diagnosis often suffers as a result, Dr. Thompson said at the World Congress of Dermatology.
“What often happens is the nail specimens get put into a bottle of formaldehyde, they float around and get torn up, and then when they come to the lab, we have no idea what’s proximal and dorsal. This is an issue. We’re all used to just putting a nail specimen in a bottle and sending it away, so all the grossing happens in the laboratory. What I submit to you is you need to be more involved in the grossing side so the specimen can be properly processed,” said Dr. Thompson, a dermatopathologist in group practice in Tigard, Ore.
He added that clear and concise guidelines for standardized specimen submission are needed, and he offered several specific suggestions regarding the orientation of the tissue and securing it for transport.
“Careful submission of tissue specimens is of great importance and allows for better diagnostics,” Dr. Thompson stressed. “There’s really nothing more terrifying than to be told you’re being sent a pigmented lesion and then not being able to find anything at all in the specimen. You really worry that it’s ended up in the trash can through leveling. This is why dermatopathologists don’t want to read nail biopsies very much.”
When a nail specimen is submitted properly, such mix-ups become “almost impossible,” according to the dermatopathologist.
Dr. Thompson borrowed one of his key ideas on efficient handling of nail specimens from opthalmologic pathology. Ophthalmologists routinely send delicate tissue segments and margins from the operating room, and they do so with consistent success because they place the segments on a cartoon of the eye so the pathologist can see exactly where the tissue was located on the patient.
Dermatologists and surgeons can do the same after printing out a sheaf of nail diagrams gratis at the Website for Dr. Thompson’s dermatopathology practice.
The rest of the necessary equipment is similarly simple and readily obtainable from any pathology laboratory, which routinely purchases small plastic cassettes by the tens of thousands for handling of tissue specimens.
“You don’t need to go out and buy them; just ask the lab you work with to send over 10 or so,” Dr. Thompson advised.
The cassette comes with a small fitted sponge to be placed over the tissue to keep it securely in place on the nail diagram rather than floating off. Ink one end of the specimen using the wooden end of a cotton-tip applicator so the lab knows which end is proximal and which is distal. The wooden tip provides more precise inking than the cotton-tip end. Then place the closed cassette in a larger bottle of formaldehyde for shipping.
One more thing: Separate the nail plate from the nail bed or matrix whenever possible, and place them in separate cassettes. Lab technicians typically devote a lot of attention to trying to get the nail plate to stick to a slide, but the diagnostic material is usually present in the nail bed or matrix, and keeping those soft tissues separate makes it less likely they’ll get lost in the shuffle.
“I recommend putting the nail plate cassette and the lesional tissue in the same bottle because then you don’t have two specimens with double the charge for the patient,” Dr. Thompson said.
He reported having no relevant financial conflicts.
VANCOUVER – Dr. Curtis T. Thompson is on a mission: to improve the often-shoddy quality of nail biopsy specimens submitted to pathologists.
No standardized protocols for nail specimens exist. The quality of pathologic diagnosis often suffers as a result, Dr. Thompson said at the World Congress of Dermatology.
“What often happens is the nail specimens get put into a bottle of formaldehyde, they float around and get torn up, and then when they come to the lab, we have no idea what’s proximal and dorsal. This is an issue. We’re all used to just putting a nail specimen in a bottle and sending it away, so all the grossing happens in the laboratory. What I submit to you is you need to be more involved in the grossing side so the specimen can be properly processed,” said Dr. Thompson, a dermatopathologist in group practice in Tigard, Ore.
He added that clear and concise guidelines for standardized specimen submission are needed, and he offered several specific suggestions regarding the orientation of the tissue and securing it for transport.
“Careful submission of tissue specimens is of great importance and allows for better diagnostics,” Dr. Thompson stressed. “There’s really nothing more terrifying than to be told you’re being sent a pigmented lesion and then not being able to find anything at all in the specimen. You really worry that it’s ended up in the trash can through leveling. This is why dermatopathologists don’t want to read nail biopsies very much.”
When a nail specimen is submitted properly, such mix-ups become “almost impossible,” according to the dermatopathologist.
Dr. Thompson borrowed one of his key ideas on efficient handling of nail specimens from opthalmologic pathology. Ophthalmologists routinely send delicate tissue segments and margins from the operating room, and they do so with consistent success because they place the segments on a cartoon of the eye so the pathologist can see exactly where the tissue was located on the patient.
Dermatologists and surgeons can do the same after printing out a sheaf of nail diagrams gratis at the Website for Dr. Thompson’s dermatopathology practice.
The rest of the necessary equipment is similarly simple and readily obtainable from any pathology laboratory, which routinely purchases small plastic cassettes by the tens of thousands for handling of tissue specimens.
“You don’t need to go out and buy them; just ask the lab you work with to send over 10 or so,” Dr. Thompson advised.
The cassette comes with a small fitted sponge to be placed over the tissue to keep it securely in place on the nail diagram rather than floating off. Ink one end of the specimen using the wooden end of a cotton-tip applicator so the lab knows which end is proximal and which is distal. The wooden tip provides more precise inking than the cotton-tip end. Then place the closed cassette in a larger bottle of formaldehyde for shipping.
One more thing: Separate the nail plate from the nail bed or matrix whenever possible, and place them in separate cassettes. Lab technicians typically devote a lot of attention to trying to get the nail plate to stick to a slide, but the diagnostic material is usually present in the nail bed or matrix, and keeping those soft tissues separate makes it less likely they’ll get lost in the shuffle.
“I recommend putting the nail plate cassette and the lesional tissue in the same bottle because then you don’t have two specimens with double the charge for the patient,” Dr. Thompson said.
He reported having no relevant financial conflicts.
VANCOUVER – Dr. Curtis T. Thompson is on a mission: to improve the often-shoddy quality of nail biopsy specimens submitted to pathologists.
No standardized protocols for nail specimens exist. The quality of pathologic diagnosis often suffers as a result, Dr. Thompson said at the World Congress of Dermatology.
“What often happens is the nail specimens get put into a bottle of formaldehyde, they float around and get torn up, and then when they come to the lab, we have no idea what’s proximal and dorsal. This is an issue. We’re all used to just putting a nail specimen in a bottle and sending it away, so all the grossing happens in the laboratory. What I submit to you is you need to be more involved in the grossing side so the specimen can be properly processed,” said Dr. Thompson, a dermatopathologist in group practice in Tigard, Ore.
He added that clear and concise guidelines for standardized specimen submission are needed, and he offered several specific suggestions regarding the orientation of the tissue and securing it for transport.
“Careful submission of tissue specimens is of great importance and allows for better diagnostics,” Dr. Thompson stressed. “There’s really nothing more terrifying than to be told you’re being sent a pigmented lesion and then not being able to find anything at all in the specimen. You really worry that it’s ended up in the trash can through leveling. This is why dermatopathologists don’t want to read nail biopsies very much.”
When a nail specimen is submitted properly, such mix-ups become “almost impossible,” according to the dermatopathologist.
Dr. Thompson borrowed one of his key ideas on efficient handling of nail specimens from opthalmologic pathology. Ophthalmologists routinely send delicate tissue segments and margins from the operating room, and they do so with consistent success because they place the segments on a cartoon of the eye so the pathologist can see exactly where the tissue was located on the patient.
Dermatologists and surgeons can do the same after printing out a sheaf of nail diagrams gratis at the Website for Dr. Thompson’s dermatopathology practice.
The rest of the necessary equipment is similarly simple and readily obtainable from any pathology laboratory, which routinely purchases small plastic cassettes by the tens of thousands for handling of tissue specimens.
“You don’t need to go out and buy them; just ask the lab you work with to send over 10 or so,” Dr. Thompson advised.
The cassette comes with a small fitted sponge to be placed over the tissue to keep it securely in place on the nail diagram rather than floating off. Ink one end of the specimen using the wooden end of a cotton-tip applicator so the lab knows which end is proximal and which is distal. The wooden tip provides more precise inking than the cotton-tip end. Then place the closed cassette in a larger bottle of formaldehyde for shipping.
One more thing: Separate the nail plate from the nail bed or matrix whenever possible, and place them in separate cassettes. Lab technicians typically devote a lot of attention to trying to get the nail plate to stick to a slide, but the diagnostic material is usually present in the nail bed or matrix, and keeping those soft tissues separate makes it less likely they’ll get lost in the shuffle.
“I recommend putting the nail plate cassette and the lesional tissue in the same bottle because then you don’t have two specimens with double the charge for the patient,” Dr. Thompson said.
He reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM WCD 2015
Repigmentation of Gray Hair in Lesions of Annular Elastolytic Giant Cell Granuloma
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.
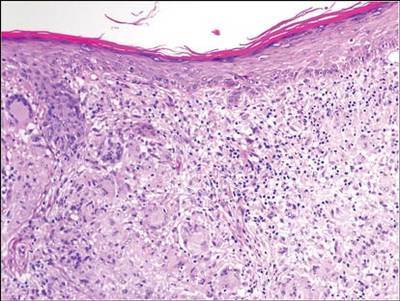
| 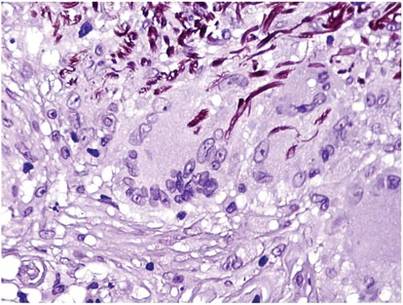
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.

| 
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
Hair pigmentation is a complex phenomenon that involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu.1 Repigmentation of gray hair has been associated with herpes zoster infection,2 use of systemic corticosteroids,3 thyroid hormone therapy,4 or treatment with interferon and ribavirin.5 We report a case of repigmentation of gray hairs in lesions of annular elastolytic giant cell granuloma (AEGCG) on the scalp of a 67-year-old man.
Case Report
A 67-year-old man presented to the dermatology department for evaluation of pruritic lesions on the face and scalp of 1 year’s duration. The patient reported that hairs in the involved areas of the scalp had turned from gray to a dark color since the appearance of the lesions. The patient had a history of hypertension and type 2 diabetes mellitus. His current medications included irbesartan, atorvastatin, metformin, acetylsalicylic acid, omeprazole, and repaglinide.
Physical examination revealed plaques on the scalp and cheeks that were 2 to 10 mm in diameter. Some of the plaques had an atrophic center and a desquamative peripheral border. The patient had androgenetic alopecia. The remaining hair was dark in the areas affected by the inflammatory plaques while it remained white-gray in the uninvolved areas (Figure 1).
A biopsy of one of the lesions was performed. Histopathology revealed a granulomatous dermatitis involving mostly the upper and mid dermis (Figure 2). Granulomas were epithelioid with many giant cells, some of which contained many nuclei. A ringed array of nuclei was noted in some histiocytes. Elastic fibers were absent in the central zone of the granulomas, a finding that was better evidenced on orcein staining (Figure 3). On the contrary, the peripheral zone of the granulomas showed an increased amount of thick elastotic material. Elastophagocytosis was observed, but no asteroid bodies, Schaumann bodies, or mucin deposits were noted. Histochemistry for microorganisms with Ziehl-Neelsen and periodic acid–Schiff staining was negative. Other findings included a mild infiltrate of melanophages in the papillary dermis as well as a mild superficial dermal inflammatory infiltrate that was rich in plasma cells. Immunostaining for Treponema pallidum was negative. The lymphocytic infiltrate was CD4+predominant. A prominent dermal elastosis also was noted. Hair follicles within the plaques were small in size, penetrating just the dermis. Immunostaining for HMB-45, melan-A, and S-100 demonstrated preserved melanocytes in the hair bulbs (Figure 4). CD68 immunostaining made the infiltrate of macrophages stand out. Based on the results of the histopathologic evaluation, a diagnosis of AEGCG was made.

| 
|
Comment
Annular elastolytic giant cell granuloma is a controversial entity that was first described by O’Brien6 in 1975 as actinic granuloma. Hanke et al7 proposed the term annular elastolytic giant cell granuloma to encompass lesions previously called actinic granuloma, atypical necrobiosis lipoidica, and Miescher granuloma. Some researchers have claimed that AEGCG is an independent entity, therefore separate and distinguishable from granuloma annulare. Histopathologic clues to distinguish AEGCG from granuloma annulare have been noted in the literature.7-9 Other investigators believe AEGCG is a type of granuloma annulare that appears on exposed skin.10 There are several variants of the classic clinical presentation of AEGCG, such as cases including presentation in unexposed areas of the skin,11 a papular variant,12 a rapidly regressive variant,13 a reticular variant,14 a variant of early childhood,15 a generalized variant,16 presentation in a necklace distribution,17 presentation as alopecia,18 a sarcoid variant,19 or presentation as reticulate erythema.20 However, no variant has been associated with hair repigmentation.
Melanin units from the proximal hair bulb are responsible for pigmentation in adult hair follicles and are integrated by the hair matrix, melanocytes, keratinocytes, and fibroblasts.21 Hair bulb melanocytes are larger and more dendritic than epidermal melanocytes (Figure 5). The hair only pigments during the anagen phase; therefore, its pigmentation is cyclic, as opposed to epidermal pigmentation, which is ongoing. Hair pigmentation is the result of a complex interaction between the epithelium, the mesenchyme, and the neuroectoderm. This complex pigmentation results from the interaction between follicular melanocytes, keratinocytes, and the fibroblasts from the hair papilla.22 Hair pigmentation involves many hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and a physicochemical milieu1,23-25 (Table), and it is regulated by autocrines, paracrines, or intracrines.21 Therefore, it is likely that many environmental factors may affect hair pigmentation, which may explain why repigmentation of the hair has been seen in the setting of herpes zoster infection,2 use of systemic corticosteroids in the treatment of bullous pemphigoid,3 thyroid hormone therapy,4 treatment with interferon and ribavirin,5 porphyria cutanea tarda,26 or lentigo maligna.27 In our patient, AEGCG might have induced some changes in the dermal environment that were responsible for the repigmentation of the patient’s gray hair. It is speculated that solar radiation and other factors can transform the antigenicity of elastic fibers and induce an immune response in AEGCG.12,15 The lymphocytic infiltrate in these lesions is predominantly CD4+, as seen in our patient, which is consistent with an autoimmune hypothesis.15 Nevertheless, it most likely is too simplistic to attribute the repigmentation to the influence of just these cells.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
1. Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-1228.
2. Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
3. Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2008;22:1018-1020.
4. Redondo P, Guzmán M, Marquina M, et al. Repigmentation of gray hair after thyroid hormone treatment [in Spanish]. Actas Dermosifiliogr. 2007;98:603-610.
5. Kavak A, Akcan Y, Korkmaz U. Hair repigmentation in a hepatitis C patient treated with interferon and ribavirin. Dermatology. 2005;211:171-172.
6. O’Brien JP. Actinic granuloma. an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
7. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
8. Al-Hoqail IA, Al-Ghamdi AM, Martinka M, et al. Actinic granuloma is a unique and distinct entity: a comparative study with granuloma annulare. Am J Dermatopathol. 2002;24:209-212.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
11. Muramatsu T, Shirai T, Yamashina Y, et al. Annular elastolytic giant cell granuloma: an unusual case with lesions arising in non-sun-exposed areas. J Dermatol. 1987;14:54-58.
12. Kelly BJ, Mrstik ME, Ramos-Caro FA, et al. Papular elastolytic giant cell granuloma responding to hydroxychloroquine and quinacrine. Int J Dermatol. 2004;43:964-966.
13. Misago N, Ohtsuka Y, Ishii K, et al. Papular and reticular elastolytic giant cell granuloma: rapid spontaneous regression. Acta Derm Venereol. 2007;87:89-90.
14. Hinrichs R, Weiss T, Peschke E, et al. A reticular variant of elastolytic giant cell granuloma. Clin Exp Dermatol. 2006;31:42-44.
15. Lee HW, Lee MW, Choi JH, et al. Annular elastolytic giant cell granuloma in an infant: improvement after treatment with oral tranilast andtopical pimecrolimus. J Am Acad Dermatol. 2005;53(5, suppl 1):S244-S246.
16. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422.
17. Meadows KP, O’Reilly MA, Harris RM, et al. Erythematous annular plaques in a necklace distribution. annular elastolytic giant cell granuloma. Arch Dermatol. 2001;137:1647-1652.
18. Delgado-Jimenez Y, Perez-Gala S, Peñas PF, et al. O’Brien actinic granuloma presenting as alopecia. J Eur Acad Dermatol Venereol. 2006;20:226-227.
19. Gambichler T, Herde M, Hoffmann K, et al. Sarcoid variant of actinic granuloma: is it annular sarcoidosis? Dermatology. 2001;203:353-354.
20. Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42:50-54.
21. Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl):90S-97S.
22. Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
23. Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24-28.
24. Slominski A, Wortsman J. Neuroendocrinology of the skin [published correction appears in Endocr Rev. 2002;23:364]. Endocr Rev. 2000;21:457-487.
25. Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979-1020.
26. Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol. 2002;146:325-329.
27. Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
Practice Points
- Hair repigmentation can be a clinical clue to a subjacent inflammatory disease.
- Hair depigmentation associated with aging may be a reversible condition under proper stimulation.
WCD: Secukinumab shows effectiveness for nail, palmoplantar psoriasis
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
VANCOUVER, B.C. – The interleukin-17A inhibitor secukinumab demonstrated the greatest improvement in nail psoriasis ever reported from a randomized, placebo-controlled trial in the phase IIIb TRANSFIGURE study, Dr. Kristian Reich reported at the World Congress of Dermatology.
At 198 patients, TRANSFIGURE is the largest-ever prospective study in patients with moderate to severe chronic plaque psoriasis and significant nail involvement. And while only the 16-week results are available thus far, when TRANSFIGURE is completed after a planned 132 weeks of treatment, it will also be the longest-ever study in the treatment of nail psoriasis, noted Dr. Reich, a dermatologist in group practice in Hamburg, Germany.
Elsewhere at WCD 2015, Dr. Alice B. Gottlieb presented the week 16 results of the phase IIIb GESTURE study, in which 205 psoriasis patients with moderate to severe psoriasis of the palms and soles were randomized to subcutaneous secukinumab (Cosentyx) at 150 or 300 mg or placebo. Dosing was weekly for the first 5 weeks and monthly thereafter.
The primary endpoint, a palmoplantar Investigator’s Global Assessment scale score of 0 or 1 – clear or almost clear – at week 16 was 33.3% with secukinumab at 300 mg, 22.1% at 150 mg, and 1.5% with placebo. The average reduction in palmoplantar PASI (Psoriasis Area Severity Index) score from baseline was 54.6% with high-dose and 35.3% with low-dose secukinumab, compared with 4.1% in placebo-treated controls, reported Dr. Gottlieb, professor and chair of dermatology at Tufts University, Boston.
Like the TRANSFIGURE trial, GESTURE will continue for 132 weeks, with the initial placebo-treated controls being randomized to secukinumab at 150 or 300 mg after week 16.
Dr. Reich reported that by 16 weeks in TRANSFIGURE, mean scores on the Nail Psoriasis Severity Index had improved by 45.3%, compared with baseline, in patients on secukinumab 300 mg, 37.9% in those on secukinumab 150 mg, and 10.8% with placebo.
The results on the skin were dramatic: a PASI 75 rate of 87.1% with secukinumab 300 mg, 77% with secukinumab 150 mg, and 5.1% with placebo. The PASI 100 response rate – meaning totally clear skin – was 31.9% with high-dose and 25.2% with lower-dose secukinumab, while there was a zero PASI 100 rate in controls.
The only adverse events more common than with placebo were nasopharyngitis and upper respiratory infections.
Dr. Reich predicted that as the ongoing TRANSFIGURE study continues well beyond 16 weeks, the nail psoriasis response rates will climb, since nails are so slow growing.
TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
AT WCD 2015
Key clinical point: Two phase IIIb trials show secukinumab at 300 mg is the most effective drug ever formally studied for nail or palmoplantar psoriasis.
Major finding: At 16 weeks, secukinumab at 300 mg improved nail psoriasis by 45.3% and palmoplantar psoriasis by 33.3%.
Data source: The phase IIIb TRANSFIGURE and GESTURE studies, ongoing randomized, prospective, initially double-blind studies in which 198 patients with significant nail psoriasis and 205 with palmoplantar psoriasis received secukinumab at 150 or 300 mg or placebo. Both studies will continue out to 132 weeks.
Disclosures: TRANSFIGURE and GESTURE are sponsored by Novartis, which markets secukinumab. Dr. Reich and Dr. Gottlieb reported having financial relationships with Novartis and numerous other pharmaceutical companies.
WCD: Topical squaric acid may help alopecia areata in kids
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
VANCOUVER, B.C. – If you haven’t tried topical 5% squaric acid dibutylester for alopecia areata in children, you probably should, according to investigators at the University of Texas in Houston.
“We have treated over 500 patients” with decent results. “A lot of children referred to us had failed topical steroids or kenalog injections. If you don’t have this in your practice and you are treating this population, this is something you really have to look into,” said investigator Dr. Marjon Vatanchi, a dermatology research fellow at the university.
She shared outcomes for 10 children, a mix of boys and girls aged 3-12 years, at the World Congress of Dermatology.
Overall, five had a good response to squaric acid, meaning regrowth of 75% or more of their hair, and three had a fair outcome, meaning regrowth of 25%-74%.
Seven had failed previous treatment with kenalog, topical steroids, minoxidil, and tacrolimus ointment. Hair regrowth was good in three and fair in two. The two poor responders – 4- and 10-year-old girls – both had areata universalis, which seems to be less responsive to treatment then areata totalis.
Two of the three children who hadn’t been treated before – a 3-year-old boy and 4-year-old girl, both with areata totalis – had a good response. The third, a 12-year-old girl with areata universalis, had a fair response.
In all, 50% of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome. It usually took 2-4 monthly treatments to see a response, but some patients needed up to 17 treatments.
The results from the University of Texas are on the high end of what’s been reported before, and that might have something to do with how squaric acid is used there.
After ruling out hormonal and other causes of hair loss and explaining the possible side effects, “the first thing we do is a patch test,” applying the solution to a small affected area to see how children respond, Dr. Vatanchi said.
Patients are then sent home with instructions to wash the area and apply a topical steroid if the irritation becomes too much. Itching, redness, and blistering can occur, but “most people don’t have a terrible reaction,” she said.
Patients come back in a month, and if there were no problems, then the whole head is treated. The solution is applied with cotton swabs to moisten the affected areas. It usually takes one to six swabs. “You don’t have to do every single centimeter, because what you are trying to do is stimulate the scalp,” she said.
Children keep the solution on overnight, and wash it off in the morning.
They return to the office the following month. “At that point, I am looking for any new hairs. If they have good regrowth, we continue with the 5% solution, one treatment once a month. If I don’t see any hair regrowth, then we go up to 10%. If they come back and say they had burning or blistering, we go down to 2.5%,” Dr. Vatanchi said. Treatment continues until it’s no longer needed.
“Many children will outgrow alopecia areata, but like [with] eczema, we tell parents we don’t know what the future holds. We treat what we can and hope children grow out of it,” she said.
Although there are several theories, it’s unknown why squaric acid, which is generally available from compounding pharmacies, helps with alopecia.
There was no funding for the project, and Dr. Vatanchi reported having no relevant financial disclosures.
AT WCD 2015
Key clinical point: Squaric acid may work when other treatments fail in kids with alopecia areata.
Major finding: Fifty percent of the children had a good outcome, and 71% who had failed previous treatments had a favorable outcome with squaric acid.
Data source: A study of 10 children with alopecia aged 3-12 years.
Disclosures: There was no outside funding for the work, and Dr. Vatanchi reported having no relevant financial disclosures.
What Is Your Diagnosis? Onychomadesis Following Hand-foot-and-mouth Disease
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.