User login
Superficial Acral Fibromyxoma and Other Slow-Growing Tumors in Acral Areas
First described by Fetsch et al1 in 2001, superficial acral fibromyxoma (SAFM) is a rare fibromyxoid mesenchymal tumor that typically affects the fingers and toes with frequent involvement of the nail unit. It is not widely recognized and remains poorly understood. We describe a series of 3 cases of SAFM encountered at our institution and provide a review of the literature on this unique tumor.
Case Reports
Patient 1
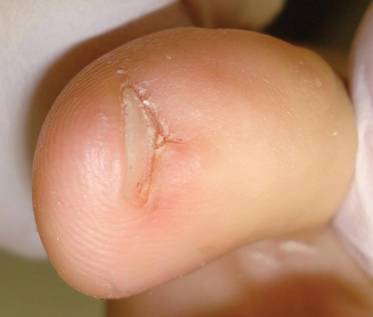
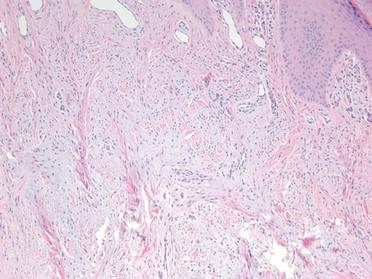
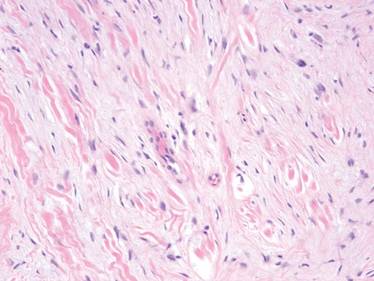
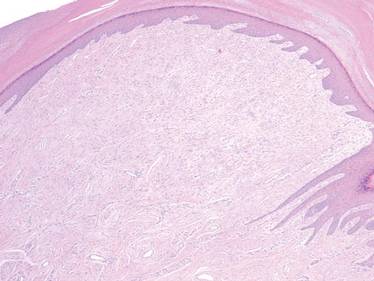
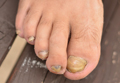
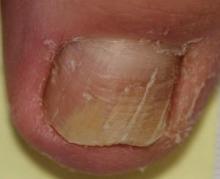
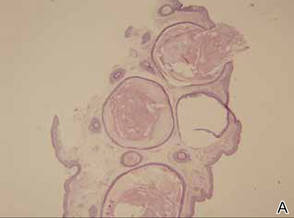
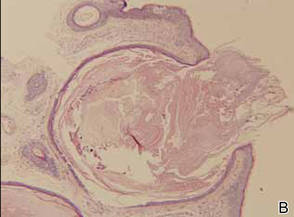
A 35-year-old man presented for treatment of a “wart” on the right fifth toe that had increased in size over the last year. He reported that the lesion was mildly painful and occasionally bled or drained clear fluid. He also noted cracking of the nail plate on the same toe. Physical examination revealed a firm, flesh-colored, 3-mm dermal papule on the proximal nail fold of the right fifth toe with subtle flattening of the underlying nail plate (Figure 1). The patient underwent biopsy of the involved proximal nail fold. Histopathology revealed a proliferation of small oval and spindle cells arranged in fascicles and bundles in the dermis (Figure 2). There was extensive mucin deposition associated with the spindle cell proliferation. Additionally, spindle cells and mucin surrounded and entrapped collagen bundles on the periphery of the lesion. Lesional cells were diffusely positive for CD34 and extended to the deep surgical margin (Figure 3). S-100 and factor XIIIa stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
|
Patient 2
A 47-year-old man presented with an asymptomatic growth on the left fourth toe that had increased in size over the last year. Physical examination revealed an 8-mm, firm, fleshy, flesh-colored, smooth and slightly pedunculated papule on the distal aspect of the left fourth toe. The nail plate and periungual region were not involved. A shave biopsy of the papule was obtained. Histopathology demonstrated dermal stellate spindle cells arranged in a loose fascicular pattern with marked mucin deposition throughout the dermis (Figure 4). Lesional cells were positive for CD34. An S-100 stain highlighted dermal dendritic cells, but lesional cells were negative. No further excision was undertaken, and there was no evidence of recurrence at 1-year follow-up. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Patient 3
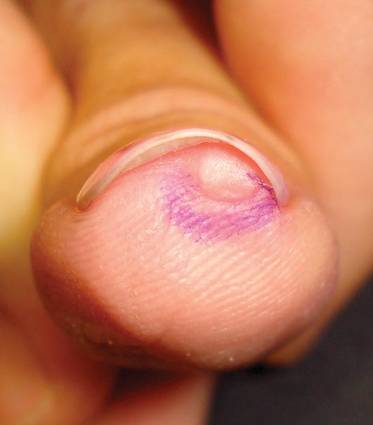
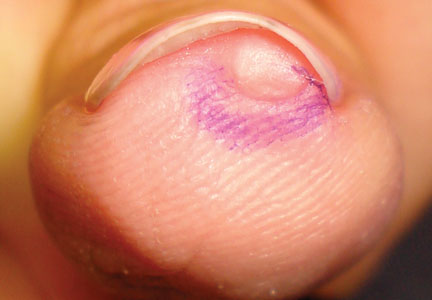
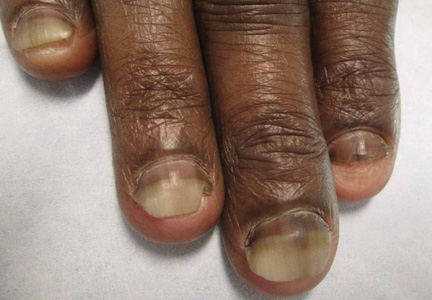
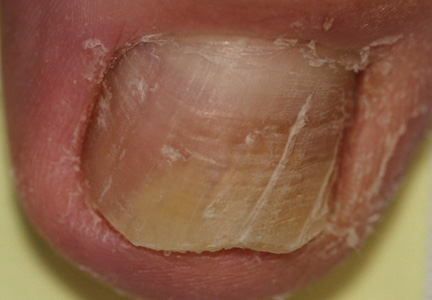
A 45-year-old woman presented with asymptomatic distal onycholysis of the right thumbnail of 1 year’s duration. She denied any history of trauma, and no bleeding or pigmentary changes were noted. Physical examination revealed a 5-mm flesh-colored papule on the hyponychium of the right thumb with focal onycholysis (Figure 5). A wedge biopsy of the lesion was performed. Histopathology showed an intradermal nodular proliferation of bland spindle cells arranged in loose fascicles and bundles and embedded in a myxoid stroma (Figure 6). CD34 staining strongly highlighted lesional cells. S-100 and neurofilament stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Comment
Clinically, SAFM typically presents as a slow-growing solitary nodule on the distal fingers or toes. The great toe is the most commonly affected digit, and the tumor may be subungual in up to two-thirds of cases.1 Unusual locations, such as the heel, also have been reported.2 Onset typically occurs in the fifth or sixth decade, and there is an approximately 2-fold higher incidence in men than women.1-3
Histopathologically, SAFM is a characteristically well-circumscribed but unencapsulated dermal tumor composed of spindle and stellate cells in a loose storiform or fascicular arrangement embedded in a myxoid, myxocollagenous, or collagenous stroma.4 The tumor often occupies the entire dermis and may extend into the subcutis or occasionally the underlying fascia and bone.4,5 Mast cells often are prominent, and microvascular accentuation also may be seen. Inflammatory infiltrates and multinucleated giant cells typically are not seen.6 Although 2 cases of atypical SAFM have been described,2 cellular atypia is not a characteristic feature of SAFM.
The immunohistochemical profile of SAFM is characterized by diffuse or focal expression of CD34, focal expression of epithelial membrane antigen (EMA), CD99 expression, and varying numbers of factor XIIIa–positive histiocytes.2,3 Positive staining for vimentin also is common. Staining typically is negative for S-100, human melanoma black 45, keratin, smooth muscle actin, and desmin.
The standard treatment of SAFM is complete local resection of the tumor, though some patients have been treated with partial excision or biopsy and partial or complete digital amputation.1 Local recurrence may occur in up to 20% of cases; however, approximately two-thirds of the reported recurrences in the literature occurred after incomplete tumor excision.1,2 It may be more appropriate to consider these cases as persistent rather than recurrent tumors. Superficial acral fibromyxoma is considered a benign tumor, with no known cases of metastases.4
|
A broad differential diagnosis exists for SAFM and it can be difficult to differentiate it from a wide variety of benign and malignant tumors that may be seen on the nail unit and distal extremities (Table). Myxoid neurofibromas typically present as solitary lesions on the hands and feet. Similar to SAFM, myxoid neurofibromas are unencapsulated dermal tumors composed of spindle-shaped cells in which mast cells often are conspicuous.2,7 However, tumor cells in myxoid neurofibromas are S-100 positive, and the lesions typically do not show vasculature accentuation.4,7
Sclerosing perineuriomas are benign fibrous tumors of the fingers and palms. Histopathologically, bland spindle cells arranged in fascicles and whorls are observed in a hyalinized collagen matrix.8 Immunohistochemically, sclerosing perineuriomas are positive for EMA and negative for S-100, but unlike SAFM, these tumors usually are CD34 negative.8
Superficial angiomyxomas typically are located on the head and neck but also may be found in other locations such as the trunk. They present as cutaneous papules or polypoid lesions. Histopathologically, superficial angiomyxomas are poorly circumscribed with a lobular pattern. Spindle-shaped fibroblasts exist in a myxoid matrix with neutrophils and thin-walled capillaries. The fibroblasts are variably positive for CD34 but also are S-100 positive.1,9
Myxoid dermatofibrosarcoma protuberans is a rare, locally aggressive, mesenchymal tumor of the skin and subcutis2 that typically presents on the trunk, proximal extremities, or head and neck; occurrence on the fingers or toes is exceedingly rare.2,10 Histopathologically, a myxoid stroma contains sheets of bland spindle-shaped cells with minimal to no atypia, sometimes arranged in a storiform pattern. The tumor characteristically invades deeply into the subcutaneous tissues. CD34 is characteristically positive and S-100 is negative.2,10
Low-grade myxofibrosarcoma is a soft tissue sarcoma easily confused with other spindle cell tumors. It is one of the most common sarcomas in adults but rarely arises in acral areas.2 It is characterized by a nodular growth pattern with marked nuclear atypia and perivascular clustering of tumor cells. CD34 staining may be positive in some cases.11
Similar to SAFM, myxoinflammatory fibroblastic sarcoma has a predilection for the extremities.4 However, it typically presents as a subcutaneous mass and has no documented tendency for nail bed involvement. Also unlike SAFM, it has a remarkable inflammatory infiltrate and characteristic virocyte or Reed-Sternberg cells.12
Acquired digital fibrokeratomas are benign neoplasms that occur on fingers and toes; the classic clinical presentation is a solitary smooth nodule or dome, often with a characteristic projecting configuration and horn shape.1 Histopathologically, these tumors are paucicellular with thick, vertically oriented, interwoven collagen bundles; cells may be positive for CD34 but are negative for EMA.1,13 Related to acquired digital fibrokeratomas are Koenen tumors, which share a similar histology but are distinguished by their clinical characteristics. For example, Koenen tumors tend to be multifocal and are strongly associated with tuberous sclerosis. These tumors also have a tendency to recur.1
Conclusion
Our report of 3 typical cases of SAFM highlights the need to keep this increasingly recognized and well-defined clinicopathological entity in the differential for slow-growing tumors in acral locations, particularly those in the periungual and subungual regions.
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
2. Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
3. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
4. André J, Theunis A, Richert B, et al. Superficial acral fibromyxoma: clinical and pathological features. Am J Dermatopathol. 2004;26:472-474.
5. Kazakov DV, Mentzel T, Burg G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
6. Meyerle JH, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
7. Graadt van Roggen JF, Hogendoorn PC, Fletcher CD. Myxoid tumours of soft tissue. Histopathology. 1999;35:291-312.
8. Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
9. Calonje E, Guerin D, McCormick D, et al. Superficial angiomyxoma: clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol. 1999;23:910-917.
10. Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans. a study of 115 cases. Cancer. 1962;15:717-725.
11. Wada T, Hasegawa T, Nagoya S, et al. Myxofibrosarcoma with an infiltrative growth pattern: a case report. Jpn J Clin Oncol. 2000;30:458-462.
12. Meis-Kindblom JM, Kindblom LG. Acral myxoinflammatory fibroblastic sarcoma: a low-grade tumor of the hands and feet. Am J Surg Pathol. 1998;22:911-924.
13. Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;97:120-129.
First described by Fetsch et al1 in 2001, superficial acral fibromyxoma (SAFM) is a rare fibromyxoid mesenchymal tumor that typically affects the fingers and toes with frequent involvement of the nail unit. It is not widely recognized and remains poorly understood. We describe a series of 3 cases of SAFM encountered at our institution and provide a review of the literature on this unique tumor.
Case Reports
Patient 1
A 35-year-old man presented for treatment of a “wart” on the right fifth toe that had increased in size over the last year. He reported that the lesion was mildly painful and occasionally bled or drained clear fluid. He also noted cracking of the nail plate on the same toe. Physical examination revealed a firm, flesh-colored, 3-mm dermal papule on the proximal nail fold of the right fifth toe with subtle flattening of the underlying nail plate (Figure 1). The patient underwent biopsy of the involved proximal nail fold. Histopathology revealed a proliferation of small oval and spindle cells arranged in fascicles and bundles in the dermis (Figure 2). There was extensive mucin deposition associated with the spindle cell proliferation. Additionally, spindle cells and mucin surrounded and entrapped collagen bundles on the periphery of the lesion. Lesional cells were diffusely positive for CD34 and extended to the deep surgical margin (Figure 3). S-100 and factor XIIIa stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
|
Patient 2
A 47-year-old man presented with an asymptomatic growth on the left fourth toe that had increased in size over the last year. Physical examination revealed an 8-mm, firm, fleshy, flesh-colored, smooth and slightly pedunculated papule on the distal aspect of the left fourth toe. The nail plate and periungual region were not involved. A shave biopsy of the papule was obtained. Histopathology demonstrated dermal stellate spindle cells arranged in a loose fascicular pattern with marked mucin deposition throughout the dermis (Figure 4). Lesional cells were positive for CD34. An S-100 stain highlighted dermal dendritic cells, but lesional cells were negative. No further excision was undertaken, and there was no evidence of recurrence at 1-year follow-up. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Patient 3
A 45-year-old woman presented with asymptomatic distal onycholysis of the right thumbnail of 1 year’s duration. She denied any history of trauma, and no bleeding or pigmentary changes were noted. Physical examination revealed a 5-mm flesh-colored papule on the hyponychium of the right thumb with focal onycholysis (Figure 5). A wedge biopsy of the lesion was performed. Histopathology showed an intradermal nodular proliferation of bland spindle cells arranged in loose fascicles and bundles and embedded in a myxoid stroma (Figure 6). CD34 staining strongly highlighted lesional cells. S-100 and neurofilament stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Comment
Clinically, SAFM typically presents as a slow-growing solitary nodule on the distal fingers or toes. The great toe is the most commonly affected digit, and the tumor may be subungual in up to two-thirds of cases.1 Unusual locations, such as the heel, also have been reported.2 Onset typically occurs in the fifth or sixth decade, and there is an approximately 2-fold higher incidence in men than women.1-3
Histopathologically, SAFM is a characteristically well-circumscribed but unencapsulated dermal tumor composed of spindle and stellate cells in a loose storiform or fascicular arrangement embedded in a myxoid, myxocollagenous, or collagenous stroma.4 The tumor often occupies the entire dermis and may extend into the subcutis or occasionally the underlying fascia and bone.4,5 Mast cells often are prominent, and microvascular accentuation also may be seen. Inflammatory infiltrates and multinucleated giant cells typically are not seen.6 Although 2 cases of atypical SAFM have been described,2 cellular atypia is not a characteristic feature of SAFM.
The immunohistochemical profile of SAFM is characterized by diffuse or focal expression of CD34, focal expression of epithelial membrane antigen (EMA), CD99 expression, and varying numbers of factor XIIIa–positive histiocytes.2,3 Positive staining for vimentin also is common. Staining typically is negative for S-100, human melanoma black 45, keratin, smooth muscle actin, and desmin.
The standard treatment of SAFM is complete local resection of the tumor, though some patients have been treated with partial excision or biopsy and partial or complete digital amputation.1 Local recurrence may occur in up to 20% of cases; however, approximately two-thirds of the reported recurrences in the literature occurred after incomplete tumor excision.1,2 It may be more appropriate to consider these cases as persistent rather than recurrent tumors. Superficial acral fibromyxoma is considered a benign tumor, with no known cases of metastases.4
|
A broad differential diagnosis exists for SAFM and it can be difficult to differentiate it from a wide variety of benign and malignant tumors that may be seen on the nail unit and distal extremities (Table). Myxoid neurofibromas typically present as solitary lesions on the hands and feet. Similar to SAFM, myxoid neurofibromas are unencapsulated dermal tumors composed of spindle-shaped cells in which mast cells often are conspicuous.2,7 However, tumor cells in myxoid neurofibromas are S-100 positive, and the lesions typically do not show vasculature accentuation.4,7
Sclerosing perineuriomas are benign fibrous tumors of the fingers and palms. Histopathologically, bland spindle cells arranged in fascicles and whorls are observed in a hyalinized collagen matrix.8 Immunohistochemically, sclerosing perineuriomas are positive for EMA and negative for S-100, but unlike SAFM, these tumors usually are CD34 negative.8
Superficial angiomyxomas typically are located on the head and neck but also may be found in other locations such as the trunk. They present as cutaneous papules or polypoid lesions. Histopathologically, superficial angiomyxomas are poorly circumscribed with a lobular pattern. Spindle-shaped fibroblasts exist in a myxoid matrix with neutrophils and thin-walled capillaries. The fibroblasts are variably positive for CD34 but also are S-100 positive.1,9
Myxoid dermatofibrosarcoma protuberans is a rare, locally aggressive, mesenchymal tumor of the skin and subcutis2 that typically presents on the trunk, proximal extremities, or head and neck; occurrence on the fingers or toes is exceedingly rare.2,10 Histopathologically, a myxoid stroma contains sheets of bland spindle-shaped cells with minimal to no atypia, sometimes arranged in a storiform pattern. The tumor characteristically invades deeply into the subcutaneous tissues. CD34 is characteristically positive and S-100 is negative.2,10
Low-grade myxofibrosarcoma is a soft tissue sarcoma easily confused with other spindle cell tumors. It is one of the most common sarcomas in adults but rarely arises in acral areas.2 It is characterized by a nodular growth pattern with marked nuclear atypia and perivascular clustering of tumor cells. CD34 staining may be positive in some cases.11
Similar to SAFM, myxoinflammatory fibroblastic sarcoma has a predilection for the extremities.4 However, it typically presents as a subcutaneous mass and has no documented tendency for nail bed involvement. Also unlike SAFM, it has a remarkable inflammatory infiltrate and characteristic virocyte or Reed-Sternberg cells.12
Acquired digital fibrokeratomas are benign neoplasms that occur on fingers and toes; the classic clinical presentation is a solitary smooth nodule or dome, often with a characteristic projecting configuration and horn shape.1 Histopathologically, these tumors are paucicellular with thick, vertically oriented, interwoven collagen bundles; cells may be positive for CD34 but are negative for EMA.1,13 Related to acquired digital fibrokeratomas are Koenen tumors, which share a similar histology but are distinguished by their clinical characteristics. For example, Koenen tumors tend to be multifocal and are strongly associated with tuberous sclerosis. These tumors also have a tendency to recur.1
Conclusion
Our report of 3 typical cases of SAFM highlights the need to keep this increasingly recognized and well-defined clinicopathological entity in the differential for slow-growing tumors in acral locations, particularly those in the periungual and subungual regions.
First described by Fetsch et al1 in 2001, superficial acral fibromyxoma (SAFM) is a rare fibromyxoid mesenchymal tumor that typically affects the fingers and toes with frequent involvement of the nail unit. It is not widely recognized and remains poorly understood. We describe a series of 3 cases of SAFM encountered at our institution and provide a review of the literature on this unique tumor.
Case Reports
Patient 1
A 35-year-old man presented for treatment of a “wart” on the right fifth toe that had increased in size over the last year. He reported that the lesion was mildly painful and occasionally bled or drained clear fluid. He also noted cracking of the nail plate on the same toe. Physical examination revealed a firm, flesh-colored, 3-mm dermal papule on the proximal nail fold of the right fifth toe with subtle flattening of the underlying nail plate (Figure 1). The patient underwent biopsy of the involved proximal nail fold. Histopathology revealed a proliferation of small oval and spindle cells arranged in fascicles and bundles in the dermis (Figure 2). There was extensive mucin deposition associated with the spindle cell proliferation. Additionally, spindle cells and mucin surrounded and entrapped collagen bundles on the periphery of the lesion. Lesional cells were diffusely positive for CD34 and extended to the deep surgical margin (Figure 3). S-100 and factor XIIIa stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
|
Patient 2
A 47-year-old man presented with an asymptomatic growth on the left fourth toe that had increased in size over the last year. Physical examination revealed an 8-mm, firm, fleshy, flesh-colored, smooth and slightly pedunculated papule on the distal aspect of the left fourth toe. The nail plate and periungual region were not involved. A shave biopsy of the papule was obtained. Histopathology demonstrated dermal stellate spindle cells arranged in a loose fascicular pattern with marked mucin deposition throughout the dermis (Figure 4). Lesional cells were positive for CD34. An S-100 stain highlighted dermal dendritic cells, but lesional cells were negative. No further excision was undertaken, and there was no evidence of recurrence at 1-year follow-up. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Patient 3
A 45-year-old woman presented with asymptomatic distal onycholysis of the right thumbnail of 1 year’s duration. She denied any history of trauma, and no bleeding or pigmentary changes were noted. Physical examination revealed a 5-mm flesh-colored papule on the hyponychium of the right thumb with focal onycholysis (Figure 5). A wedge biopsy of the lesion was performed. Histopathology showed an intradermal nodular proliferation of bland spindle cells arranged in loose fascicles and bundles and embedded in a myxoid stroma (Figure 6). CD34 staining strongly highlighted lesional cells. S-100 and neurofilament stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Comment
Clinically, SAFM typically presents as a slow-growing solitary nodule on the distal fingers or toes. The great toe is the most commonly affected digit, and the tumor may be subungual in up to two-thirds of cases.1 Unusual locations, such as the heel, also have been reported.2 Onset typically occurs in the fifth or sixth decade, and there is an approximately 2-fold higher incidence in men than women.1-3
Histopathologically, SAFM is a characteristically well-circumscribed but unencapsulated dermal tumor composed of spindle and stellate cells in a loose storiform or fascicular arrangement embedded in a myxoid, myxocollagenous, or collagenous stroma.4 The tumor often occupies the entire dermis and may extend into the subcutis or occasionally the underlying fascia and bone.4,5 Mast cells often are prominent, and microvascular accentuation also may be seen. Inflammatory infiltrates and multinucleated giant cells typically are not seen.6 Although 2 cases of atypical SAFM have been described,2 cellular atypia is not a characteristic feature of SAFM.
The immunohistochemical profile of SAFM is characterized by diffuse or focal expression of CD34, focal expression of epithelial membrane antigen (EMA), CD99 expression, and varying numbers of factor XIIIa–positive histiocytes.2,3 Positive staining for vimentin also is common. Staining typically is negative for S-100, human melanoma black 45, keratin, smooth muscle actin, and desmin.
The standard treatment of SAFM is complete local resection of the tumor, though some patients have been treated with partial excision or biopsy and partial or complete digital amputation.1 Local recurrence may occur in up to 20% of cases; however, approximately two-thirds of the reported recurrences in the literature occurred after incomplete tumor excision.1,2 It may be more appropriate to consider these cases as persistent rather than recurrent tumors. Superficial acral fibromyxoma is considered a benign tumor, with no known cases of metastases.4
|
A broad differential diagnosis exists for SAFM and it can be difficult to differentiate it from a wide variety of benign and malignant tumors that may be seen on the nail unit and distal extremities (Table). Myxoid neurofibromas typically present as solitary lesions on the hands and feet. Similar to SAFM, myxoid neurofibromas are unencapsulated dermal tumors composed of spindle-shaped cells in which mast cells often are conspicuous.2,7 However, tumor cells in myxoid neurofibromas are S-100 positive, and the lesions typically do not show vasculature accentuation.4,7
Sclerosing perineuriomas are benign fibrous tumors of the fingers and palms. Histopathologically, bland spindle cells arranged in fascicles and whorls are observed in a hyalinized collagen matrix.8 Immunohistochemically, sclerosing perineuriomas are positive for EMA and negative for S-100, but unlike SAFM, these tumors usually are CD34 negative.8
Superficial angiomyxomas typically are located on the head and neck but also may be found in other locations such as the trunk. They present as cutaneous papules or polypoid lesions. Histopathologically, superficial angiomyxomas are poorly circumscribed with a lobular pattern. Spindle-shaped fibroblasts exist in a myxoid matrix with neutrophils and thin-walled capillaries. The fibroblasts are variably positive for CD34 but also are S-100 positive.1,9
Myxoid dermatofibrosarcoma protuberans is a rare, locally aggressive, mesenchymal tumor of the skin and subcutis2 that typically presents on the trunk, proximal extremities, or head and neck; occurrence on the fingers or toes is exceedingly rare.2,10 Histopathologically, a myxoid stroma contains sheets of bland spindle-shaped cells with minimal to no atypia, sometimes arranged in a storiform pattern. The tumor characteristically invades deeply into the subcutaneous tissues. CD34 is characteristically positive and S-100 is negative.2,10
Low-grade myxofibrosarcoma is a soft tissue sarcoma easily confused with other spindle cell tumors. It is one of the most common sarcomas in adults but rarely arises in acral areas.2 It is characterized by a nodular growth pattern with marked nuclear atypia and perivascular clustering of tumor cells. CD34 staining may be positive in some cases.11
Similar to SAFM, myxoinflammatory fibroblastic sarcoma has a predilection for the extremities.4 However, it typically presents as a subcutaneous mass and has no documented tendency for nail bed involvement. Also unlike SAFM, it has a remarkable inflammatory infiltrate and characteristic virocyte or Reed-Sternberg cells.12
Acquired digital fibrokeratomas are benign neoplasms that occur on fingers and toes; the classic clinical presentation is a solitary smooth nodule or dome, often with a characteristic projecting configuration and horn shape.1 Histopathologically, these tumors are paucicellular with thick, vertically oriented, interwoven collagen bundles; cells may be positive for CD34 but are negative for EMA.1,13 Related to acquired digital fibrokeratomas are Koenen tumors, which share a similar histology but are distinguished by their clinical characteristics. For example, Koenen tumors tend to be multifocal and are strongly associated with tuberous sclerosis. These tumors also have a tendency to recur.1
Conclusion
Our report of 3 typical cases of SAFM highlights the need to keep this increasingly recognized and well-defined clinicopathological entity in the differential for slow-growing tumors in acral locations, particularly those in the periungual and subungual regions.
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
2. Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
3. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
4. André J, Theunis A, Richert B, et al. Superficial acral fibromyxoma: clinical and pathological features. Am J Dermatopathol. 2004;26:472-474.
5. Kazakov DV, Mentzel T, Burg G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
6. Meyerle JH, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
7. Graadt van Roggen JF, Hogendoorn PC, Fletcher CD. Myxoid tumours of soft tissue. Histopathology. 1999;35:291-312.
8. Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
9. Calonje E, Guerin D, McCormick D, et al. Superficial angiomyxoma: clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol. 1999;23:910-917.
10. Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans. a study of 115 cases. Cancer. 1962;15:717-725.
11. Wada T, Hasegawa T, Nagoya S, et al. Myxofibrosarcoma with an infiltrative growth pattern: a case report. Jpn J Clin Oncol. 2000;30:458-462.
12. Meis-Kindblom JM, Kindblom LG. Acral myxoinflammatory fibroblastic sarcoma: a low-grade tumor of the hands and feet. Am J Surg Pathol. 1998;22:911-924.
13. Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;97:120-129.
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
2. Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
3. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
4. André J, Theunis A, Richert B, et al. Superficial acral fibromyxoma: clinical and pathological features. Am J Dermatopathol. 2004;26:472-474.
5. Kazakov DV, Mentzel T, Burg G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
6. Meyerle JH, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
7. Graadt van Roggen JF, Hogendoorn PC, Fletcher CD. Myxoid tumours of soft tissue. Histopathology. 1999;35:291-312.
8. Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
9. Calonje E, Guerin D, McCormick D, et al. Superficial angiomyxoma: clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol. 1999;23:910-917.
10. Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans. a study of 115 cases. Cancer. 1962;15:717-725.
11. Wada T, Hasegawa T, Nagoya S, et al. Myxofibrosarcoma with an infiltrative growth pattern: a case report. Jpn J Clin Oncol. 2000;30:458-462.
12. Meis-Kindblom JM, Kindblom LG. Acral myxoinflammatory fibroblastic sarcoma: a low-grade tumor of the hands and feet. Am J Surg Pathol. 1998;22:911-924.
13. Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;97:120-129.
Practice Points
- Superficial acral fibromyxoma (SAFM) is a rare but distinct tumor that may affect the nail bed and nail plate, and it may clinically or histopathologically mimic other tumors of the distal extremities.
- Although SAFM is considered a benign tumor, it frequently persists or recurs after incomplete excision, and therefore complete local resection may be recommended, particularly for symptomatic lesions.
Subungual exostosis masquerades as nail fungus
ORLANDO – In a patient presenting with a tender, firm lesion under the nail, think subungual exostosis, Dr. Phoebe Rich advised.
Subungual exostosis is a benign bony growth that can cause discomfort by pressing up from the nail bed. It can be mistaken for onychomycosis, but keep in mind that only 50% of those presenting with nail disorders will have onychomycosis, so it is important to consider other diagnoses, Dr. Rich, director of the Nail Disorder Center at Oregon Health and Science University, Portland, said at the Orlando Dermatology Aesthetic and Clinical Conference.
She described a teenager who presented with a growth under her nail, which the patient thought was nail fungus. The lesion developed after an injury experienced during a touch football game 2 months earlier.
An X-ray will aid in the diagnosis of such lesions, Dr. Rich said.
“You want to know that’s what it is … because you don’t want to just cut into it for a biopsy if you don’t know that it’s bone,” she said.
The diagnosis can be confirmed by removing the nail to take a look at the tumor.
“They are pretty characteristic. They are a bony growth. They’re actually composed of bone with a fibro-cartilaginous cap,” she said noting that tenderness is an important clue to the diagnosis.
Subungual exostosis occurs more often in toes than fingers, more often in girls than boys, and more often in children and young adults than older individuals, Dr. Rich noted.
Surgical removal is typically successful; the recurrence rate is about 10%, and recurrence is more common in children.
Presentation varies; some lesions can be quite obvious, with the growth protruding from under the nail, while others are more subtle. Dr. Rich described one young college student who presented with slight white spotting of the nail, and slight onycholysis. The patient had tenderness of the nail area when pressed, and the tumor peeked out from under the nail.
“If you see something like this, get an X-ray,” she said, adding: “You can be a hero and make the diagnosis. Don’t just treat it as though it’s a fungus.”
“If you’re very comfortable and expert at nails, great. Otherwise, make the diagnosis and send them to someone else, but you can really help these patients a lot,” she said.
Dr. Rich has participated in clinical trials with companies with antifungal/onychomycosis drugs, including Anacor, Merz, and Valeant.
ORLANDO – In a patient presenting with a tender, firm lesion under the nail, think subungual exostosis, Dr. Phoebe Rich advised.
Subungual exostosis is a benign bony growth that can cause discomfort by pressing up from the nail bed. It can be mistaken for onychomycosis, but keep in mind that only 50% of those presenting with nail disorders will have onychomycosis, so it is important to consider other diagnoses, Dr. Rich, director of the Nail Disorder Center at Oregon Health and Science University, Portland, said at the Orlando Dermatology Aesthetic and Clinical Conference.
She described a teenager who presented with a growth under her nail, which the patient thought was nail fungus. The lesion developed after an injury experienced during a touch football game 2 months earlier.
An X-ray will aid in the diagnosis of such lesions, Dr. Rich said.
“You want to know that’s what it is … because you don’t want to just cut into it for a biopsy if you don’t know that it’s bone,” she said.
The diagnosis can be confirmed by removing the nail to take a look at the tumor.
“They are pretty characteristic. They are a bony growth. They’re actually composed of bone with a fibro-cartilaginous cap,” she said noting that tenderness is an important clue to the diagnosis.
Subungual exostosis occurs more often in toes than fingers, more often in girls than boys, and more often in children and young adults than older individuals, Dr. Rich noted.
Surgical removal is typically successful; the recurrence rate is about 10%, and recurrence is more common in children.
Presentation varies; some lesions can be quite obvious, with the growth protruding from under the nail, while others are more subtle. Dr. Rich described one young college student who presented with slight white spotting of the nail, and slight onycholysis. The patient had tenderness of the nail area when pressed, and the tumor peeked out from under the nail.
“If you see something like this, get an X-ray,” she said, adding: “You can be a hero and make the diagnosis. Don’t just treat it as though it’s a fungus.”
“If you’re very comfortable and expert at nails, great. Otherwise, make the diagnosis and send them to someone else, but you can really help these patients a lot,” she said.
Dr. Rich has participated in clinical trials with companies with antifungal/onychomycosis drugs, including Anacor, Merz, and Valeant.
ORLANDO – In a patient presenting with a tender, firm lesion under the nail, think subungual exostosis, Dr. Phoebe Rich advised.
Subungual exostosis is a benign bony growth that can cause discomfort by pressing up from the nail bed. It can be mistaken for onychomycosis, but keep in mind that only 50% of those presenting with nail disorders will have onychomycosis, so it is important to consider other diagnoses, Dr. Rich, director of the Nail Disorder Center at Oregon Health and Science University, Portland, said at the Orlando Dermatology Aesthetic and Clinical Conference.
She described a teenager who presented with a growth under her nail, which the patient thought was nail fungus. The lesion developed after an injury experienced during a touch football game 2 months earlier.
An X-ray will aid in the diagnosis of such lesions, Dr. Rich said.
“You want to know that’s what it is … because you don’t want to just cut into it for a biopsy if you don’t know that it’s bone,” she said.
The diagnosis can be confirmed by removing the nail to take a look at the tumor.
“They are pretty characteristic. They are a bony growth. They’re actually composed of bone with a fibro-cartilaginous cap,” she said noting that tenderness is an important clue to the diagnosis.
Subungual exostosis occurs more often in toes than fingers, more often in girls than boys, and more often in children and young adults than older individuals, Dr. Rich noted.
Surgical removal is typically successful; the recurrence rate is about 10%, and recurrence is more common in children.
Presentation varies; some lesions can be quite obvious, with the growth protruding from under the nail, while others are more subtle. Dr. Rich described one young college student who presented with slight white spotting of the nail, and slight onycholysis. The patient had tenderness of the nail area when pressed, and the tumor peeked out from under the nail.
“If you see something like this, get an X-ray,” she said, adding: “You can be a hero and make the diagnosis. Don’t just treat it as though it’s a fungus.”
“If you’re very comfortable and expert at nails, great. Otherwise, make the diagnosis and send them to someone else, but you can really help these patients a lot,” she said.
Dr. Rich has participated in clinical trials with companies with antifungal/onychomycosis drugs, including Anacor, Merz, and Valeant.
EXPERT ANALYSIS AT THE ODAC CONFERENCE
Antifungal treatment may cause DNA strain type switching in onychomycosis
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Antifungal treatment of onychomycosis could induce higher rates of DNA strain type switching in certain patients.
Major finding: Strain type switching occurred in 83% of the terbinafine group, 56% of the laser group, and 25% of the placebo group.
Data source: Cohort study of 23 individuals selected from 50 adults with onychomycosis who contributed samples to determine strain types.
Disclosures: The study authors did not disclose any source of funding or any relevant conflicts of interest.
Efinaconazole earns top marks for effectiveness in onychomycosis treatment
Topical treatment of toenail onychomycosis is effective with several antifungals, Dr. Aditya Gupta of the University of Toronto and his associates reported in an evidence-based review.
The researchers identified 28 relevant studies, 13 of which were of high quality. Four antifungals were an effective cure for mild to moderate toenail onychomycosis: amorolfine, ciclopirox, tavaborole, and efinaconazole. Efinaconazole was the most effective, but in all cases, outcomes were improved with longer treatment times and follow-ups.
Oral treatments are usually more effective for toenail onychomycosis, but are not always possible because of drug interactions, so topical treatments are important in certain patient populations, the investigators noted in the article abstract.
The article abstract can be found online in the American Journal of Clinical Dermatology (doi:10.1007/s40257-014-0096-2).
Topical treatment of toenail onychomycosis is effective with several antifungals, Dr. Aditya Gupta of the University of Toronto and his associates reported in an evidence-based review.
The researchers identified 28 relevant studies, 13 of which were of high quality. Four antifungals were an effective cure for mild to moderate toenail onychomycosis: amorolfine, ciclopirox, tavaborole, and efinaconazole. Efinaconazole was the most effective, but in all cases, outcomes were improved with longer treatment times and follow-ups.
Oral treatments are usually more effective for toenail onychomycosis, but are not always possible because of drug interactions, so topical treatments are important in certain patient populations, the investigators noted in the article abstract.
The article abstract can be found online in the American Journal of Clinical Dermatology (doi:10.1007/s40257-014-0096-2).
Topical treatment of toenail onychomycosis is effective with several antifungals, Dr. Aditya Gupta of the University of Toronto and his associates reported in an evidence-based review.
The researchers identified 28 relevant studies, 13 of which were of high quality. Four antifungals were an effective cure for mild to moderate toenail onychomycosis: amorolfine, ciclopirox, tavaborole, and efinaconazole. Efinaconazole was the most effective, but in all cases, outcomes were improved with longer treatment times and follow-ups.
Oral treatments are usually more effective for toenail onychomycosis, but are not always possible because of drug interactions, so topical treatments are important in certain patient populations, the investigators noted in the article abstract.
The article abstract can be found online in the American Journal of Clinical Dermatology (doi:10.1007/s40257-014-0096-2).
PACT shows promise against onychomycosis
Photodynamic antimicrobial chemotherapy is an effective option for treatment of in vitro onychomycosis, according to Dr. Tarun Mehra of Eberhard Karls University, Tübingen, Germany, and his associates.
In both a microdilution assay and a onychomycosis model, photodynamic antimicrobial chemotherapy (PACT) was effective in suppressing Trichophyton rubrum in conjunction with toluidine blue O (TBO) and LED irradiation. In another test, a patient diagnosed with distolateral onychomycosis was treated with TBO and PACT and experienced a significant improvement in nail health over the next 6 months while receiving no other treatments.
The long-term normalization of the patients’ nails suggests that PACT is a persistent cure, but patients with more than 50% of the nail affected may be more difficult to treat with PACT, the researchers said.
Read the full article online in the Journal of the European Academy of Dermatology and Venereology (doi:10:1111/jdv.12467).
Photodynamic antimicrobial chemotherapy is an effective option for treatment of in vitro onychomycosis, according to Dr. Tarun Mehra of Eberhard Karls University, Tübingen, Germany, and his associates.
In both a microdilution assay and a onychomycosis model, photodynamic antimicrobial chemotherapy (PACT) was effective in suppressing Trichophyton rubrum in conjunction with toluidine blue O (TBO) and LED irradiation. In another test, a patient diagnosed with distolateral onychomycosis was treated with TBO and PACT and experienced a significant improvement in nail health over the next 6 months while receiving no other treatments.
The long-term normalization of the patients’ nails suggests that PACT is a persistent cure, but patients with more than 50% of the nail affected may be more difficult to treat with PACT, the researchers said.
Read the full article online in the Journal of the European Academy of Dermatology and Venereology (doi:10:1111/jdv.12467).
Photodynamic antimicrobial chemotherapy is an effective option for treatment of in vitro onychomycosis, according to Dr. Tarun Mehra of Eberhard Karls University, Tübingen, Germany, and his associates.
In both a microdilution assay and a onychomycosis model, photodynamic antimicrobial chemotherapy (PACT) was effective in suppressing Trichophyton rubrum in conjunction with toluidine blue O (TBO) and LED irradiation. In another test, a patient diagnosed with distolateral onychomycosis was treated with TBO and PACT and experienced a significant improvement in nail health over the next 6 months while receiving no other treatments.
The long-term normalization of the patients’ nails suggests that PACT is a persistent cure, but patients with more than 50% of the nail affected may be more difficult to treat with PACT, the researchers said.
Read the full article online in the Journal of the European Academy of Dermatology and Venereology (doi:10:1111/jdv.12467).
Incidence and Epidemiology of Onychomycosis in Patients Visiting a Tertiary Care Hospital in India
Onychomycosis is a chronic fungal infection of the nails. Dermatophytes are the most common etiologic agents, but yeasts and nondermatophyte molds also constitute a substantial number of cases.1 An accumulation of debris under distorted, deformed, thickened, and discolored nails, particularly with ragged and furrowed edges, strongly suggests tinea unguium.2 Candidal onychomycosis (CO) lacks gross distortion and accumulated detritus and mainly affects fingernails.3 Nondermatophytic molds cause 1.5% to 6% of cases of onychomycosis, mostly seen in toenails of elderly individuals with a history of trauma.4 Onychomycosis affects 5.5% of the world population5 and represents 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.6
The incidence of onychomycosis ranges from 0.5% to 5% in the general population in India.7 The incidence is particularly high in warm humid climates such as India.8 Researchers have found certain habits of the population in the Indian subcontinent (eg, walking with bare feet, wearing ill-fitting shoes, nail-biting [eg, onychophagia], working with chemicals) to be contributing factors for onychomycosis.9 Several studies have shown that the prevalence of onychomycosis increases with age, possibly due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, prolonged exposure to pathogenic fungi, suboptimal immune function, inactivity, or inability to trim the toenails and care for the feet.10 Nail infection is a cosmetic problem with serious physical and psychological morbidity and also serves as the fungal reservoir for skin infections. Besides destruction and disfigurement of the nail plate, onychomycosis can lead to self-consciousness and impairment of daily functioning.11
Nail dystrophy occurs secondary to various systemic disorders or can be associated with other dermatologic conditions. Nail discoloration and other onychia should be differentiated from onychomycosis by classifying nail lesions as distal lateral subungual onychomycosis, proximal subungual onychomycosis (PSO), CO, white superficial onychomycosis (WSO), and total dystrophic onychomycosis.12 Laboratory investigation is necessary to accurately differentiate between fungal infections and other skin diseases before starting treatment. Our hospital-based study sought to determine the incidence and epidemiology of onychomycosis with an analysis of 134 participants with clinically suspected onychomycosis. We evaluated prevalence based on age, sex, and occupation, as well as the most common pathogens.
Materials and Methods
Study Design and Participants
The study population consisted of 134 patients with clinically suspected onychomycosis who visited the dermatology department at the Veer Chandra Singh Garhwali Government Institute of Medical Sciences and Research Institute in Uttarakhand, India (October 2010 to October 2011). A thorough history was obtained and a detailed examination of the distorted nails was conducted in the microbiology laboratory. Patient history and demographic factors such as age, sex, occupation, and related history of risk factors for onychomycosis were recorded pro forma. Some of the details such as itching, family history of fungal infection, and prior cutaneous infections were recorded. Patients who were undergoing treatment with systemic or topical antifungal agents in the 4 weeks preceding the study period were excluded to rule out false-negative cases and to avoid the influence of antifungal agents on the disease course.
Assessments
Two samples were taken from each patient on different days. Participants were divided into 4 groups based on occupation: farmer, housewife, student, and other (eg, clerk, shopkeeper, painter). Clinical presentation of discoloration, onycholysis, subungual hyperkeratosis, and nail thickening affecting the distal and/or lateral nail plate was defined as distal lateral subungual onychomycosis; discoloration and onycholysis affecting the proximal part of the nail was defined as PSO; association with paronychia and distal and lateral onycholysis was defined as CO; white opaque patches on the nail surface were defined as WSO; and end-stage nail disease was defined as total dystrophic onychomycosis.
Prior to sampling, the nails were cleaned with a 70% alcohol solution. Nail clippings were obtained using presterilized nail clippers and a blunt no. 15 scalpel blade and were placed on sterilized black paper. Each nail sample was divided into 2 parts: one for direct microscopy and one for culture. Nail clippings were subjected to microscopic examination after clearing in 20% potassium hydroxide solution. The slides were examined for fungal hyphae, arthrospores, yeasts, and pseudohyphal forms. Culture was done with Emmons modification of Sabouraud dextrose agar (incubated at 27°C for molds and 37°C for yeasts) as well as with 0.4% chloramphenicol and 5% cycloheximide (incubated at 27°C). Culture tubes were examined daily for the first week and on alternate days thereafter for 4 weeks of incubation.
Dermatophytes were identified based on the colony morphology, growth rate, texture, border, and pigmentation in the obverse and reverse of culture media and microscopic examination using lactophenol cotton blue tease mount. Yeast colonies were identified microscopically with Gram stain, and species were identified by germ tube, carbohydrate assimilation, and fermentation tests.13 Nondermatophyte molds were identified by colony morphology, microscopic examination, and slide culture. Molds were considered as pathogens in the presence of the following criteria: (1) absence of other fungal growth in the same culture tube; (2) presence of mold growth in all 3 samples; and (3) presence of filaments identified on direct examination.
Results
Of 134 clinically suspected cases of onychomycosis, 78 (58.2%) were from fingernails and 56 (41.8%) from toenails. Clinical diagnosis was confirmed in 96 (71.6%) cases by both fungal culture and direct microscopy but was confirmed by direct microscopy alone in only 76 (56.7%) cases. False-negative results were found in 23.9% (32/134) of participants with direct microscopy and 9.0% (12/134) with fungal cultures. The results of direct microscopy and fungal culture are outlined in Table 1. The study included 78 (58.2%) males and 56 (41.8%) females with a mean age of 44 years. Highest prevalence (47.8%) was seen in participants older than 40 years and lowest prevalence (11.9%) in participants younger than 20 years. In total, 32.8% of participants were farmers, 31.3% were housewives, 14.9% were students, and 20.9% performed other occupations. Disease history at the time of first presentation varied from 1 month to more than 2 years; 33.6% of participants had a 1- to 6-month history of disease, while only 3.7% had a disease history of less than 1 month at presentation. The demographic data are further outlined in Table 2.
Distal lateral subungual onychomycosis was the most prevalent clinical pattern found in 66 (49.3%) participants; fungal isolates were found in 60 of these participants. The next most prevalent clinical pattern was PSO, which was found in 34 (25.4%) participants, 12 showing fungal growth. A clinical pattern of CO was noted in 28 (20.9%) participants, 22 showing fungal growth; WSO was noted in 10 (7.5%) participants, 2 showing fungal growth.
Of 96 culture-positive cases, dermatophytes were the most common pathogens isolated in 56 (58.3%) participants, followed by Candida species in 28 (29.2%) participants. Nondermatophyte molds were isolated in 12 (12.5%) participants. The various dermatophytes, Candida species, and nondermatophyte molds that were isolated on fungal culture are outlined in Table 3. Of the 96 participants with positive fungal cultures, 30 (31.2%) were farmers working with soil, 28 (29.2%) were housewives associated with wet work, 16 (16.7%) were students associated with increased physical exercise from extracurricular activity, and 22 (22.9%) were in other occupations (Table 4).
Comment
The term onychomycosis is derived from onyx, the Greek word for nail, and mykes, the Greek word for fungus. Onychomycosis is a chronic mycotic infection of the fingernails and toenails that can have a serious impact on patients’ quality of life. The fungi known to cause onychomycosis vary among geographic areas, primarily due to differences in climate.14 The isolation rate of onychomycosis in our hospital-based study was 71.6%, which is in accordance with various studies in India and abroad, including 60% in Karnataka, India5; 82.3% in Sikkim, India6; and 86.9% in Turkey.1 However, other studies have shown lower isolation rates of 39.5% in Central Delhi, India,15 and 37.6% in Himachal Pradesh, India.16 Some patients with onychomycosis may not seek medical attention, which may explain the difference in the prevalence of onychomycosis observed worldwide.17 The prevalence of onychomycosis by age also varies. In our study, participants older than 40 years showed the highest prevalence (47.8%), which is in accordance with other studies from India18 and abroad.19,20 In contrast, some Indian studies15,21,22 have reported a higher prevalence in younger adults (ie, 21–30 years), which may be attributed to greater self-consciousness about nail discoloration and disfigurement as well as increased physical activity and different shoe-wearing habits. A higher prevalence in older adults, as observed in our study as well some other studies,19,21 may be due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, longer exposure to pathogenic fungi, suboptimal immune function, inactivity, and poor hygiene.10
In our study, suspected onychomycosis was more common in males (58.2%) than in females (41.8%). These results are in accordance with many of the studies in the worldwide literature.1,10,11,15,16,23-25 A higher isolation rate in males worldwide may be due to common use of occlusive footwear, more exposure to outdoor conditions, and increased physical activity, leading to an increased likelihood of trauma. The importance of trauma to the nails as a predisposing factor for onychomycosis is well established.24 In our study, the majority of males wore shoes regardless of occupation. Perspiration of the feet when wearing socks and/or shoes can generate a warm moist environment that promotes the growth of fungi and predisposes patients to onychomycosis. Similar observations have been reported by other investigators.21,22,25,26
The incidence of onychomycosis was almost evenly distributed among farmers, housewives, and the miscellaneous group, whereas a high isolation rate was noted among students. Of 20 students included in our study, onychomycosis was confirmed in 16, which may be related to an increased use of synthetic sports shoes and socks that retain sweat as well as vigorous physical activity frequently resulting in nail injuries among this patient population.11 Younger patients may be more conscious of their appearance and therefore may be more likely to seek treatment. Similar observations have been reported by other researchers.15,21,22
In our study, dermatophytes were the most commonly found pathogens (58.3%), which is comparable to other studies.15,18,22Trichophyton mentagrophytes was the most frequently isolated dermatophyte from cultures, which was in concordance with a study from Delhi.15 In some studies,18,20,22Trichophyton rubrum has been reported as the most prevalent dermatophyte, but we identified Trichophyton rubrum in only 18 participants, which can be attributed to variations in epidemiology based on geographic region. Nondermatophyte molds were isolated in 12.5% of participants, with Aspergillus niger being the most common isolate found in 8 cases. Other isolated species were Alternaria alternata and Fusarium solani found in 2 cases each. Aspergillus niger has been reported in worldwide studies as an important cause of onychomycosis.15,18,19,21,22
In 28 cases (29.2%) involving Candida species, Candida albicans, Candida parapsilosis, and Candida tropicalis were the most common pathogens, respectively, which is in accordance with many studies.15,20-22,25 In 28 cases of CO, females (n=16) were affected more than males (n=12). All of the females were housewives and C albicans was predominantly isolated from the fingernails. Household responsibilities involving kitchen work (eg, cutting and peeling vegetables, washing utensils, cleaning the house/laundry) may chronically expose housewives to moist environments and make them more prone to injury, thus facilitating easy entry of fungal agents.
Distal lateral subungual onychomycosis was the most prevalent clinical type found (n=66), which is comparable to other reports.20,22,25 Proximal subungual onychomycosis was the second most common type; however, a greater incidence has been reported by some researchers,23,24 while others have reported a lower incidence.20,21 Candidial onychomycosis and WSO were not common in our study, and PSO was not associated with any immunodeficiency disease, as reported by other researchers.15,20
Of 134 suspected cases of onychomycosis, 71.6% were confirmed by both direct microscopy and fungal culture, but only 56.7% were confirmed by direct microscopy alone. If we had relied on microscopy with potassium hydroxide only, we would have missed 23.9% of cases. Therefore, nail scrapings should always be subjected to fungal culture as well as direct microscopy, as both are necessary for accurate diagnosis and treatment of onychomycosis. If onychomycosis is not successfully treated, it can act as a reservoir of fungal infection affecting other parts of the body with the potential to pass infection on to others.
Conclusion
Clinical examination alone is not sufficient for diagnosing onychomycosis14,18,20; in many cases of suspected onychomycosis with nail changes, mycologic examination does not confirm fungal infection. In our study, only 71.6% of participants with nail changes proved to be of fungal etiology. Other researchers from different geographic locations have reported similar results with lower incidence (eg, 39.5%,15 37.6%,16 51.7%,18 45.3%21) of fungal etiology in such cases. Therefore, both clinical and mycologic examinations are important for establishing the diagnosis and selecting the most suitable antifungal agent, which is possible only if the underlying pathogen is correctly identified.
1. Yenişehirli G, Bulut Y, Sezer E, et al. Onychomycosis infections in the Middle Black Sea Region, Turkey. Int J Dermatol. 2009;48:956-959.
2. Kouskoukis CE, Scher RK, Ackerman AB. What histologic finding distinguishes onychomycosis and psoriasis? Am J Dermatopathol. 1983;5:501-503.
3. Rippon JW. Medical mycology. In: Wonsiewicz M, ed. The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:169-275.
4. Greer DL. Evolving role of nondermatophytes in onychomycosis. Int J Dermatol. 1995;34:521-524.
5. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
6. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
7. Sobhanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at Government General Hospital: guntur and their response to treatment with hamycin, dermostatin and dermamycin. Indian J Dermatol Venereol. 1970;36:209-214.
8. Jain S, Sehgal VN. Commentary: onychomycosis: an epidemio-etiologic perspective. Int J Dermatol. 2000;39:100-103.
9. Sehgal VN, Aggarwal AK, Srivastava G, et al. Onychomycosis: a 3 year clinicomycologic hospital-based study. Skinmed. 2007;6:11-17.
10. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for the other conditions. Arch Dermatol. 1997;133:1172-1173.
11. Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):S15.
12. Godoy-Martinez PG, Nunes FG, Tomimori-Yamashita J, et al. Onychomycosis in São Paulo, Brazil [published online ahead of print May 8, 2009]. Mycopathologia. 2009;168:111-116.
13. Larone DH. Medically Important Fungi: A Guide to Identification. 4th ed. Washington, DC: American Society for Microbiology Press; 2002.
14. Sehgal VN, Srivastava G, Dogra S, et al. Onychomycosis: an Asian perspective. Skinmed. 2010;8:37-45.
15. Sanjiv A, Shalini M, Charoo H. Etiological agents of onychomycosis from a tertiary care hospital in Central Delhi, India. Indian J Fund Appl Life Science. 2011;1:11-14.
16. Gupta M, Sharma NL, Kanga AK, et al. Onychomycosis: clinic-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007;73:389-392.
17. Eleweski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3, pt 2):S6-S9.
18. Das NK, Ghosh P, Das S, et al. A study on the etiological agent and clinico-mycological correlation of fingernail onychomycosis in eastern India. Indian J Dermatol. 2008;53:75-79.
19. Bassiri-Jahromi S, Khaksar AA. Nondermatophytic moulds as a causative agent of onychomycosis in Tehran. Indian J Dermatol. 2010;55:140-143.
20. Bokhari MA, Hussain I, Jahangir M, et al. Onychomycosis in Lahore, Pakistan. Int J Dermatol. 1999;38:591-595.
21. Jesudanam TM, Rao GR, Lakshmi DJ, et al. Onychomycosis: a significant medical problem. Indian J Dermatol Venereol Leprol. 2002;68:326-329.
22. Ahmad M, Gupta S, Gupte S. A clinico-mycological study of onychomycosis. EDOJ. 2010;6:1-9.
23. Vinod S, Grover S, Dash K, et al. A clinico-mycological evaluation of onychomycosis. Indian J Dermatol Venereol Leprol. 2000;66:238-240.
24. Veer P, Patwardhan NS, Damle AS. Study of onychomycosis: prevailing fungi and pattern of infection. Indian J Med Microbiol. 2007;25:53-56.
25. Garg A, Venkatesh V, Singh M, et al. Onychomycosis in central India: a clinicoetiologic correlation. Int J Dermatol. 2004;43:498-502.
26. Adhikari L, Das Gupta A, Pal R, et al. Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J Pathol Microbiol. 2009;52:194-197.
Onychomycosis is a chronic fungal infection of the nails. Dermatophytes are the most common etiologic agents, but yeasts and nondermatophyte molds also constitute a substantial number of cases.1 An accumulation of debris under distorted, deformed, thickened, and discolored nails, particularly with ragged and furrowed edges, strongly suggests tinea unguium.2 Candidal onychomycosis (CO) lacks gross distortion and accumulated detritus and mainly affects fingernails.3 Nondermatophytic molds cause 1.5% to 6% of cases of onychomycosis, mostly seen in toenails of elderly individuals with a history of trauma.4 Onychomycosis affects 5.5% of the world population5 and represents 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.6
The incidence of onychomycosis ranges from 0.5% to 5% in the general population in India.7 The incidence is particularly high in warm humid climates such as India.8 Researchers have found certain habits of the population in the Indian subcontinent (eg, walking with bare feet, wearing ill-fitting shoes, nail-biting [eg, onychophagia], working with chemicals) to be contributing factors for onychomycosis.9 Several studies have shown that the prevalence of onychomycosis increases with age, possibly due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, prolonged exposure to pathogenic fungi, suboptimal immune function, inactivity, or inability to trim the toenails and care for the feet.10 Nail infection is a cosmetic problem with serious physical and psychological morbidity and also serves as the fungal reservoir for skin infections. Besides destruction and disfigurement of the nail plate, onychomycosis can lead to self-consciousness and impairment of daily functioning.11
Nail dystrophy occurs secondary to various systemic disorders or can be associated with other dermatologic conditions. Nail discoloration and other onychia should be differentiated from onychomycosis by classifying nail lesions as distal lateral subungual onychomycosis, proximal subungual onychomycosis (PSO), CO, white superficial onychomycosis (WSO), and total dystrophic onychomycosis.12 Laboratory investigation is necessary to accurately differentiate between fungal infections and other skin diseases before starting treatment. Our hospital-based study sought to determine the incidence and epidemiology of onychomycosis with an analysis of 134 participants with clinically suspected onychomycosis. We evaluated prevalence based on age, sex, and occupation, as well as the most common pathogens.
Materials and Methods
Study Design and Participants
The study population consisted of 134 patients with clinically suspected onychomycosis who visited the dermatology department at the Veer Chandra Singh Garhwali Government Institute of Medical Sciences and Research Institute in Uttarakhand, India (October 2010 to October 2011). A thorough history was obtained and a detailed examination of the distorted nails was conducted in the microbiology laboratory. Patient history and demographic factors such as age, sex, occupation, and related history of risk factors for onychomycosis were recorded pro forma. Some of the details such as itching, family history of fungal infection, and prior cutaneous infections were recorded. Patients who were undergoing treatment with systemic or topical antifungal agents in the 4 weeks preceding the study period were excluded to rule out false-negative cases and to avoid the influence of antifungal agents on the disease course.
Assessments
Two samples were taken from each patient on different days. Participants were divided into 4 groups based on occupation: farmer, housewife, student, and other (eg, clerk, shopkeeper, painter). Clinical presentation of discoloration, onycholysis, subungual hyperkeratosis, and nail thickening affecting the distal and/or lateral nail plate was defined as distal lateral subungual onychomycosis; discoloration and onycholysis affecting the proximal part of the nail was defined as PSO; association with paronychia and distal and lateral onycholysis was defined as CO; white opaque patches on the nail surface were defined as WSO; and end-stage nail disease was defined as total dystrophic onychomycosis.
Prior to sampling, the nails were cleaned with a 70% alcohol solution. Nail clippings were obtained using presterilized nail clippers and a blunt no. 15 scalpel blade and were placed on sterilized black paper. Each nail sample was divided into 2 parts: one for direct microscopy and one for culture. Nail clippings were subjected to microscopic examination after clearing in 20% potassium hydroxide solution. The slides were examined for fungal hyphae, arthrospores, yeasts, and pseudohyphal forms. Culture was done with Emmons modification of Sabouraud dextrose agar (incubated at 27°C for molds and 37°C for yeasts) as well as with 0.4% chloramphenicol and 5% cycloheximide (incubated at 27°C). Culture tubes were examined daily for the first week and on alternate days thereafter for 4 weeks of incubation.
Dermatophytes were identified based on the colony morphology, growth rate, texture, border, and pigmentation in the obverse and reverse of culture media and microscopic examination using lactophenol cotton blue tease mount. Yeast colonies were identified microscopically with Gram stain, and species were identified by germ tube, carbohydrate assimilation, and fermentation tests.13 Nondermatophyte molds were identified by colony morphology, microscopic examination, and slide culture. Molds were considered as pathogens in the presence of the following criteria: (1) absence of other fungal growth in the same culture tube; (2) presence of mold growth in all 3 samples; and (3) presence of filaments identified on direct examination.
Results
Of 134 clinically suspected cases of onychomycosis, 78 (58.2%) were from fingernails and 56 (41.8%) from toenails. Clinical diagnosis was confirmed in 96 (71.6%) cases by both fungal culture and direct microscopy but was confirmed by direct microscopy alone in only 76 (56.7%) cases. False-negative results were found in 23.9% (32/134) of participants with direct microscopy and 9.0% (12/134) with fungal cultures. The results of direct microscopy and fungal culture are outlined in Table 1. The study included 78 (58.2%) males and 56 (41.8%) females with a mean age of 44 years. Highest prevalence (47.8%) was seen in participants older than 40 years and lowest prevalence (11.9%) in participants younger than 20 years. In total, 32.8% of participants were farmers, 31.3% were housewives, 14.9% were students, and 20.9% performed other occupations. Disease history at the time of first presentation varied from 1 month to more than 2 years; 33.6% of participants had a 1- to 6-month history of disease, while only 3.7% had a disease history of less than 1 month at presentation. The demographic data are further outlined in Table 2.
Distal lateral subungual onychomycosis was the most prevalent clinical pattern found in 66 (49.3%) participants; fungal isolates were found in 60 of these participants. The next most prevalent clinical pattern was PSO, which was found in 34 (25.4%) participants, 12 showing fungal growth. A clinical pattern of CO was noted in 28 (20.9%) participants, 22 showing fungal growth; WSO was noted in 10 (7.5%) participants, 2 showing fungal growth.
Of 96 culture-positive cases, dermatophytes were the most common pathogens isolated in 56 (58.3%) participants, followed by Candida species in 28 (29.2%) participants. Nondermatophyte molds were isolated in 12 (12.5%) participants. The various dermatophytes, Candida species, and nondermatophyte molds that were isolated on fungal culture are outlined in Table 3. Of the 96 participants with positive fungal cultures, 30 (31.2%) were farmers working with soil, 28 (29.2%) were housewives associated with wet work, 16 (16.7%) were students associated with increased physical exercise from extracurricular activity, and 22 (22.9%) were in other occupations (Table 4).
Comment
The term onychomycosis is derived from onyx, the Greek word for nail, and mykes, the Greek word for fungus. Onychomycosis is a chronic mycotic infection of the fingernails and toenails that can have a serious impact on patients’ quality of life. The fungi known to cause onychomycosis vary among geographic areas, primarily due to differences in climate.14 The isolation rate of onychomycosis in our hospital-based study was 71.6%, which is in accordance with various studies in India and abroad, including 60% in Karnataka, India5; 82.3% in Sikkim, India6; and 86.9% in Turkey.1 However, other studies have shown lower isolation rates of 39.5% in Central Delhi, India,15 and 37.6% in Himachal Pradesh, India.16 Some patients with onychomycosis may not seek medical attention, which may explain the difference in the prevalence of onychomycosis observed worldwide.17 The prevalence of onychomycosis by age also varies. In our study, participants older than 40 years showed the highest prevalence (47.8%), which is in accordance with other studies from India18 and abroad.19,20 In contrast, some Indian studies15,21,22 have reported a higher prevalence in younger adults (ie, 21–30 years), which may be attributed to greater self-consciousness about nail discoloration and disfigurement as well as increased physical activity and different shoe-wearing habits. A higher prevalence in older adults, as observed in our study as well some other studies,19,21 may be due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, longer exposure to pathogenic fungi, suboptimal immune function, inactivity, and poor hygiene.10
In our study, suspected onychomycosis was more common in males (58.2%) than in females (41.8%). These results are in accordance with many of the studies in the worldwide literature.1,10,11,15,16,23-25 A higher isolation rate in males worldwide may be due to common use of occlusive footwear, more exposure to outdoor conditions, and increased physical activity, leading to an increased likelihood of trauma. The importance of trauma to the nails as a predisposing factor for onychomycosis is well established.24 In our study, the majority of males wore shoes regardless of occupation. Perspiration of the feet when wearing socks and/or shoes can generate a warm moist environment that promotes the growth of fungi and predisposes patients to onychomycosis. Similar observations have been reported by other investigators.21,22,25,26
The incidence of onychomycosis was almost evenly distributed among farmers, housewives, and the miscellaneous group, whereas a high isolation rate was noted among students. Of 20 students included in our study, onychomycosis was confirmed in 16, which may be related to an increased use of synthetic sports shoes and socks that retain sweat as well as vigorous physical activity frequently resulting in nail injuries among this patient population.11 Younger patients may be more conscious of their appearance and therefore may be more likely to seek treatment. Similar observations have been reported by other researchers.15,21,22
In our study, dermatophytes were the most commonly found pathogens (58.3%), which is comparable to other studies.15,18,22Trichophyton mentagrophytes was the most frequently isolated dermatophyte from cultures, which was in concordance with a study from Delhi.15 In some studies,18,20,22Trichophyton rubrum has been reported as the most prevalent dermatophyte, but we identified Trichophyton rubrum in only 18 participants, which can be attributed to variations in epidemiology based on geographic region. Nondermatophyte molds were isolated in 12.5% of participants, with Aspergillus niger being the most common isolate found in 8 cases. Other isolated species were Alternaria alternata and Fusarium solani found in 2 cases each. Aspergillus niger has been reported in worldwide studies as an important cause of onychomycosis.15,18,19,21,22
In 28 cases (29.2%) involving Candida species, Candida albicans, Candida parapsilosis, and Candida tropicalis were the most common pathogens, respectively, which is in accordance with many studies.15,20-22,25 In 28 cases of CO, females (n=16) were affected more than males (n=12). All of the females were housewives and C albicans was predominantly isolated from the fingernails. Household responsibilities involving kitchen work (eg, cutting and peeling vegetables, washing utensils, cleaning the house/laundry) may chronically expose housewives to moist environments and make them more prone to injury, thus facilitating easy entry of fungal agents.
Distal lateral subungual onychomycosis was the most prevalent clinical type found (n=66), which is comparable to other reports.20,22,25 Proximal subungual onychomycosis was the second most common type; however, a greater incidence has been reported by some researchers,23,24 while others have reported a lower incidence.20,21 Candidial onychomycosis and WSO were not common in our study, and PSO was not associated with any immunodeficiency disease, as reported by other researchers.15,20
Of 134 suspected cases of onychomycosis, 71.6% were confirmed by both direct microscopy and fungal culture, but only 56.7% were confirmed by direct microscopy alone. If we had relied on microscopy with potassium hydroxide only, we would have missed 23.9% of cases. Therefore, nail scrapings should always be subjected to fungal culture as well as direct microscopy, as both are necessary for accurate diagnosis and treatment of onychomycosis. If onychomycosis is not successfully treated, it can act as a reservoir of fungal infection affecting other parts of the body with the potential to pass infection on to others.
Conclusion
Clinical examination alone is not sufficient for diagnosing onychomycosis14,18,20; in many cases of suspected onychomycosis with nail changes, mycologic examination does not confirm fungal infection. In our study, only 71.6% of participants with nail changes proved to be of fungal etiology. Other researchers from different geographic locations have reported similar results with lower incidence (eg, 39.5%,15 37.6%,16 51.7%,18 45.3%21) of fungal etiology in such cases. Therefore, both clinical and mycologic examinations are important for establishing the diagnosis and selecting the most suitable antifungal agent, which is possible only if the underlying pathogen is correctly identified.
Onychomycosis is a chronic fungal infection of the nails. Dermatophytes are the most common etiologic agents, but yeasts and nondermatophyte molds also constitute a substantial number of cases.1 An accumulation of debris under distorted, deformed, thickened, and discolored nails, particularly with ragged and furrowed edges, strongly suggests tinea unguium.2 Candidal onychomycosis (CO) lacks gross distortion and accumulated detritus and mainly affects fingernails.3 Nondermatophytic molds cause 1.5% to 6% of cases of onychomycosis, mostly seen in toenails of elderly individuals with a history of trauma.4 Onychomycosis affects 5.5% of the world population5 and represents 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.6
The incidence of onychomycosis ranges from 0.5% to 5% in the general population in India.7 The incidence is particularly high in warm humid climates such as India.8 Researchers have found certain habits of the population in the Indian subcontinent (eg, walking with bare feet, wearing ill-fitting shoes, nail-biting [eg, onychophagia], working with chemicals) to be contributing factors for onychomycosis.9 Several studies have shown that the prevalence of onychomycosis increases with age, possibly due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, prolonged exposure to pathogenic fungi, suboptimal immune function, inactivity, or inability to trim the toenails and care for the feet.10 Nail infection is a cosmetic problem with serious physical and psychological morbidity and also serves as the fungal reservoir for skin infections. Besides destruction and disfigurement of the nail plate, onychomycosis can lead to self-consciousness and impairment of daily functioning.11
Nail dystrophy occurs secondary to various systemic disorders or can be associated with other dermatologic conditions. Nail discoloration and other onychia should be differentiated from onychomycosis by classifying nail lesions as distal lateral subungual onychomycosis, proximal subungual onychomycosis (PSO), CO, white superficial onychomycosis (WSO), and total dystrophic onychomycosis.12 Laboratory investigation is necessary to accurately differentiate between fungal infections and other skin diseases before starting treatment. Our hospital-based study sought to determine the incidence and epidemiology of onychomycosis with an analysis of 134 participants with clinically suspected onychomycosis. We evaluated prevalence based on age, sex, and occupation, as well as the most common pathogens.
Materials and Methods
Study Design and Participants
The study population consisted of 134 patients with clinically suspected onychomycosis who visited the dermatology department at the Veer Chandra Singh Garhwali Government Institute of Medical Sciences and Research Institute in Uttarakhand, India (October 2010 to October 2011). A thorough history was obtained and a detailed examination of the distorted nails was conducted in the microbiology laboratory. Patient history and demographic factors such as age, sex, occupation, and related history of risk factors for onychomycosis were recorded pro forma. Some of the details such as itching, family history of fungal infection, and prior cutaneous infections were recorded. Patients who were undergoing treatment with systemic or topical antifungal agents in the 4 weeks preceding the study period were excluded to rule out false-negative cases and to avoid the influence of antifungal agents on the disease course.
Assessments
Two samples were taken from each patient on different days. Participants were divided into 4 groups based on occupation: farmer, housewife, student, and other (eg, clerk, shopkeeper, painter). Clinical presentation of discoloration, onycholysis, subungual hyperkeratosis, and nail thickening affecting the distal and/or lateral nail plate was defined as distal lateral subungual onychomycosis; discoloration and onycholysis affecting the proximal part of the nail was defined as PSO; association with paronychia and distal and lateral onycholysis was defined as CO; white opaque patches on the nail surface were defined as WSO; and end-stage nail disease was defined as total dystrophic onychomycosis.
Prior to sampling, the nails were cleaned with a 70% alcohol solution. Nail clippings were obtained using presterilized nail clippers and a blunt no. 15 scalpel blade and were placed on sterilized black paper. Each nail sample was divided into 2 parts: one for direct microscopy and one for culture. Nail clippings were subjected to microscopic examination after clearing in 20% potassium hydroxide solution. The slides were examined for fungal hyphae, arthrospores, yeasts, and pseudohyphal forms. Culture was done with Emmons modification of Sabouraud dextrose agar (incubated at 27°C for molds and 37°C for yeasts) as well as with 0.4% chloramphenicol and 5% cycloheximide (incubated at 27°C). Culture tubes were examined daily for the first week and on alternate days thereafter for 4 weeks of incubation.
Dermatophytes were identified based on the colony morphology, growth rate, texture, border, and pigmentation in the obverse and reverse of culture media and microscopic examination using lactophenol cotton blue tease mount. Yeast colonies were identified microscopically with Gram stain, and species were identified by germ tube, carbohydrate assimilation, and fermentation tests.13 Nondermatophyte molds were identified by colony morphology, microscopic examination, and slide culture. Molds were considered as pathogens in the presence of the following criteria: (1) absence of other fungal growth in the same culture tube; (2) presence of mold growth in all 3 samples; and (3) presence of filaments identified on direct examination.
Results
Of 134 clinically suspected cases of onychomycosis, 78 (58.2%) were from fingernails and 56 (41.8%) from toenails. Clinical diagnosis was confirmed in 96 (71.6%) cases by both fungal culture and direct microscopy but was confirmed by direct microscopy alone in only 76 (56.7%) cases. False-negative results were found in 23.9% (32/134) of participants with direct microscopy and 9.0% (12/134) with fungal cultures. The results of direct microscopy and fungal culture are outlined in Table 1. The study included 78 (58.2%) males and 56 (41.8%) females with a mean age of 44 years. Highest prevalence (47.8%) was seen in participants older than 40 years and lowest prevalence (11.9%) in participants younger than 20 years. In total, 32.8% of participants were farmers, 31.3% were housewives, 14.9% were students, and 20.9% performed other occupations. Disease history at the time of first presentation varied from 1 month to more than 2 years; 33.6% of participants had a 1- to 6-month history of disease, while only 3.7% had a disease history of less than 1 month at presentation. The demographic data are further outlined in Table 2.
Distal lateral subungual onychomycosis was the most prevalent clinical pattern found in 66 (49.3%) participants; fungal isolates were found in 60 of these participants. The next most prevalent clinical pattern was PSO, which was found in 34 (25.4%) participants, 12 showing fungal growth. A clinical pattern of CO was noted in 28 (20.9%) participants, 22 showing fungal growth; WSO was noted in 10 (7.5%) participants, 2 showing fungal growth.
Of 96 culture-positive cases, dermatophytes were the most common pathogens isolated in 56 (58.3%) participants, followed by Candida species in 28 (29.2%) participants. Nondermatophyte molds were isolated in 12 (12.5%) participants. The various dermatophytes, Candida species, and nondermatophyte molds that were isolated on fungal culture are outlined in Table 3. Of the 96 participants with positive fungal cultures, 30 (31.2%) were farmers working with soil, 28 (29.2%) were housewives associated with wet work, 16 (16.7%) were students associated with increased physical exercise from extracurricular activity, and 22 (22.9%) were in other occupations (Table 4).
Comment
The term onychomycosis is derived from onyx, the Greek word for nail, and mykes, the Greek word for fungus. Onychomycosis is a chronic mycotic infection of the fingernails and toenails that can have a serious impact on patients’ quality of life. The fungi known to cause onychomycosis vary among geographic areas, primarily due to differences in climate.14 The isolation rate of onychomycosis in our hospital-based study was 71.6%, which is in accordance with various studies in India and abroad, including 60% in Karnataka, India5; 82.3% in Sikkim, India6; and 86.9% in Turkey.1 However, other studies have shown lower isolation rates of 39.5% in Central Delhi, India,15 and 37.6% in Himachal Pradesh, India.16 Some patients with onychomycosis may not seek medical attention, which may explain the difference in the prevalence of onychomycosis observed worldwide.17 The prevalence of onychomycosis by age also varies. In our study, participants older than 40 years showed the highest prevalence (47.8%), which is in accordance with other studies from India18 and abroad.19,20 In contrast, some Indian studies15,21,22 have reported a higher prevalence in younger adults (ie, 21–30 years), which may be attributed to greater self-consciousness about nail discoloration and disfigurement as well as increased physical activity and different shoe-wearing habits. A higher prevalence in older adults, as observed in our study as well some other studies,19,21 may be due to poor peripheral circulation, diabetes mellitus, repeated nail trauma, longer exposure to pathogenic fungi, suboptimal immune function, inactivity, and poor hygiene.10
In our study, suspected onychomycosis was more common in males (58.2%) than in females (41.8%). These results are in accordance with many of the studies in the worldwide literature.1,10,11,15,16,23-25 A higher isolation rate in males worldwide may be due to common use of occlusive footwear, more exposure to outdoor conditions, and increased physical activity, leading to an increased likelihood of trauma. The importance of trauma to the nails as a predisposing factor for onychomycosis is well established.24 In our study, the majority of males wore shoes regardless of occupation. Perspiration of the feet when wearing socks and/or shoes can generate a warm moist environment that promotes the growth of fungi and predisposes patients to onychomycosis. Similar observations have been reported by other investigators.21,22,25,26
The incidence of onychomycosis was almost evenly distributed among farmers, housewives, and the miscellaneous group, whereas a high isolation rate was noted among students. Of 20 students included in our study, onychomycosis was confirmed in 16, which may be related to an increased use of synthetic sports shoes and socks that retain sweat as well as vigorous physical activity frequently resulting in nail injuries among this patient population.11 Younger patients may be more conscious of their appearance and therefore may be more likely to seek treatment. Similar observations have been reported by other researchers.15,21,22
In our study, dermatophytes were the most commonly found pathogens (58.3%), which is comparable to other studies.15,18,22Trichophyton mentagrophytes was the most frequently isolated dermatophyte from cultures, which was in concordance with a study from Delhi.15 In some studies,18,20,22Trichophyton rubrum has been reported as the most prevalent dermatophyte, but we identified Trichophyton rubrum in only 18 participants, which can be attributed to variations in epidemiology based on geographic region. Nondermatophyte molds were isolated in 12.5% of participants, with Aspergillus niger being the most common isolate found in 8 cases. Other isolated species were Alternaria alternata and Fusarium solani found in 2 cases each. Aspergillus niger has been reported in worldwide studies as an important cause of onychomycosis.15,18,19,21,22
In 28 cases (29.2%) involving Candida species, Candida albicans, Candida parapsilosis, and Candida tropicalis were the most common pathogens, respectively, which is in accordance with many studies.15,20-22,25 In 28 cases of CO, females (n=16) were affected more than males (n=12). All of the females were housewives and C albicans was predominantly isolated from the fingernails. Household responsibilities involving kitchen work (eg, cutting and peeling vegetables, washing utensils, cleaning the house/laundry) may chronically expose housewives to moist environments and make them more prone to injury, thus facilitating easy entry of fungal agents.
Distal lateral subungual onychomycosis was the most prevalent clinical type found (n=66), which is comparable to other reports.20,22,25 Proximal subungual onychomycosis was the second most common type; however, a greater incidence has been reported by some researchers,23,24 while others have reported a lower incidence.20,21 Candidial onychomycosis and WSO were not common in our study, and PSO was not associated with any immunodeficiency disease, as reported by other researchers.15,20
Of 134 suspected cases of onychomycosis, 71.6% were confirmed by both direct microscopy and fungal culture, but only 56.7% were confirmed by direct microscopy alone. If we had relied on microscopy with potassium hydroxide only, we would have missed 23.9% of cases. Therefore, nail scrapings should always be subjected to fungal culture as well as direct microscopy, as both are necessary for accurate diagnosis and treatment of onychomycosis. If onychomycosis is not successfully treated, it can act as a reservoir of fungal infection affecting other parts of the body with the potential to pass infection on to others.
Conclusion
Clinical examination alone is not sufficient for diagnosing onychomycosis14,18,20; in many cases of suspected onychomycosis with nail changes, mycologic examination does not confirm fungal infection. In our study, only 71.6% of participants with nail changes proved to be of fungal etiology. Other researchers from different geographic locations have reported similar results with lower incidence (eg, 39.5%,15 37.6%,16 51.7%,18 45.3%21) of fungal etiology in such cases. Therefore, both clinical and mycologic examinations are important for establishing the diagnosis and selecting the most suitable antifungal agent, which is possible only if the underlying pathogen is correctly identified.
1. Yenişehirli G, Bulut Y, Sezer E, et al. Onychomycosis infections in the Middle Black Sea Region, Turkey. Int J Dermatol. 2009;48:956-959.
2. Kouskoukis CE, Scher RK, Ackerman AB. What histologic finding distinguishes onychomycosis and psoriasis? Am J Dermatopathol. 1983;5:501-503.
3. Rippon JW. Medical mycology. In: Wonsiewicz M, ed. The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:169-275.
4. Greer DL. Evolving role of nondermatophytes in onychomycosis. Int J Dermatol. 1995;34:521-524.
5. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
6. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
7. Sobhanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at Government General Hospital: guntur and their response to treatment with hamycin, dermostatin and dermamycin. Indian J Dermatol Venereol. 1970;36:209-214.
8. Jain S, Sehgal VN. Commentary: onychomycosis: an epidemio-etiologic perspective. Int J Dermatol. 2000;39:100-103.
9. Sehgal VN, Aggarwal AK, Srivastava G, et al. Onychomycosis: a 3 year clinicomycologic hospital-based study. Skinmed. 2007;6:11-17.
10. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for the other conditions. Arch Dermatol. 1997;133:1172-1173.
11. Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):S15.
12. Godoy-Martinez PG, Nunes FG, Tomimori-Yamashita J, et al. Onychomycosis in São Paulo, Brazil [published online ahead of print May 8, 2009]. Mycopathologia. 2009;168:111-116.
13. Larone DH. Medically Important Fungi: A Guide to Identification. 4th ed. Washington, DC: American Society for Microbiology Press; 2002.
14. Sehgal VN, Srivastava G, Dogra S, et al. Onychomycosis: an Asian perspective. Skinmed. 2010;8:37-45.
15. Sanjiv A, Shalini M, Charoo H. Etiological agents of onychomycosis from a tertiary care hospital in Central Delhi, India. Indian J Fund Appl Life Science. 2011;1:11-14.
16. Gupta M, Sharma NL, Kanga AK, et al. Onychomycosis: clinic-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007;73:389-392.
17. Eleweski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3, pt 2):S6-S9.
18. Das NK, Ghosh P, Das S, et al. A study on the etiological agent and clinico-mycological correlation of fingernail onychomycosis in eastern India. Indian J Dermatol. 2008;53:75-79.
19. Bassiri-Jahromi S, Khaksar AA. Nondermatophytic moulds as a causative agent of onychomycosis in Tehran. Indian J Dermatol. 2010;55:140-143.
20. Bokhari MA, Hussain I, Jahangir M, et al. Onychomycosis in Lahore, Pakistan. Int J Dermatol. 1999;38:591-595.
21. Jesudanam TM, Rao GR, Lakshmi DJ, et al. Onychomycosis: a significant medical problem. Indian J Dermatol Venereol Leprol. 2002;68:326-329.
22. Ahmad M, Gupta S, Gupte S. A clinico-mycological study of onychomycosis. EDOJ. 2010;6:1-9.
23. Vinod S, Grover S, Dash K, et al. A clinico-mycological evaluation of onychomycosis. Indian J Dermatol Venereol Leprol. 2000;66:238-240.
24. Veer P, Patwardhan NS, Damle AS. Study of onychomycosis: prevailing fungi and pattern of infection. Indian J Med Microbiol. 2007;25:53-56.
25. Garg A, Venkatesh V, Singh M, et al. Onychomycosis in central India: a clinicoetiologic correlation. Int J Dermatol. 2004;43:498-502.
26. Adhikari L, Das Gupta A, Pal R, et al. Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J Pathol Microbiol. 2009;52:194-197.
1. Yenişehirli G, Bulut Y, Sezer E, et al. Onychomycosis infections in the Middle Black Sea Region, Turkey. Int J Dermatol. 2009;48:956-959.
2. Kouskoukis CE, Scher RK, Ackerman AB. What histologic finding distinguishes onychomycosis and psoriasis? Am J Dermatopathol. 1983;5:501-503.
3. Rippon JW. Medical mycology. In: Wonsiewicz M, ed. The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:169-275.
4. Greer DL. Evolving role of nondermatophytes in onychomycosis. Int J Dermatol. 1995;34:521-524.
5. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
6. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
7. Sobhanadri C, Rao DT, Babu KS. Clinical and mycological study of superficial fungal infections at Government General Hospital: guntur and their response to treatment with hamycin, dermostatin and dermamycin. Indian J Dermatol Venereol. 1970;36:209-214.
8. Jain S, Sehgal VN. Commentary: onychomycosis: an epidemio-etiologic perspective. Int J Dermatol. 2000;39:100-103.
9. Sehgal VN, Aggarwal AK, Srivastava G, et al. Onychomycosis: a 3 year clinicomycologic hospital-based study. Skinmed. 2007;6:11-17.
10. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for the other conditions. Arch Dermatol. 1997;133:1172-1173.
11. Scher RK. Onychomycosis is more than a cosmetic problem. Br J Dermatol. 1994;130(suppl 43):S15.
12. Godoy-Martinez PG, Nunes FG, Tomimori-Yamashita J, et al. Onychomycosis in São Paulo, Brazil [published online ahead of print May 8, 2009]. Mycopathologia. 2009;168:111-116.
13. Larone DH. Medically Important Fungi: A Guide to Identification. 4th ed. Washington, DC: American Society for Microbiology Press; 2002.
14. Sehgal VN, Srivastava G, Dogra S, et al. Onychomycosis: an Asian perspective. Skinmed. 2010;8:37-45.
15. Sanjiv A, Shalini M, Charoo H. Etiological agents of onychomycosis from a tertiary care hospital in Central Delhi, India. Indian J Fund Appl Life Science. 2011;1:11-14.
16. Gupta M, Sharma NL, Kanga AK, et al. Onychomycosis: clinic-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007;73:389-392.
17. Eleweski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3, pt 2):S6-S9.
18. Das NK, Ghosh P, Das S, et al. A study on the etiological agent and clinico-mycological correlation of fingernail onychomycosis in eastern India. Indian J Dermatol. 2008;53:75-79.
19. Bassiri-Jahromi S, Khaksar AA. Nondermatophytic moulds as a causative agent of onychomycosis in Tehran. Indian J Dermatol. 2010;55:140-143.
20. Bokhari MA, Hussain I, Jahangir M, et al. Onychomycosis in Lahore, Pakistan. Int J Dermatol. 1999;38:591-595.
21. Jesudanam TM, Rao GR, Lakshmi DJ, et al. Onychomycosis: a significant medical problem. Indian J Dermatol Venereol Leprol. 2002;68:326-329.
22. Ahmad M, Gupta S, Gupte S. A clinico-mycological study of onychomycosis. EDOJ. 2010;6:1-9.
23. Vinod S, Grover S, Dash K, et al. A clinico-mycological evaluation of onychomycosis. Indian J Dermatol Venereol Leprol. 2000;66:238-240.
24. Veer P, Patwardhan NS, Damle AS. Study of onychomycosis: prevailing fungi and pattern of infection. Indian J Med Microbiol. 2007;25:53-56.
25. Garg A, Venkatesh V, Singh M, et al. Onychomycosis in central India: a clinicoetiologic correlation. Int J Dermatol. 2004;43:498-502.
26. Adhikari L, Das Gupta A, Pal R, et al. Clinico-etiologic correlates of onychomycosis in Sikkim. Indian J Pathol Microbiol. 2009;52:194-197.
Practice Points
- Onychomycosis is a chronic fungal infection of the nails and represents 20% to 40% of all onycho-pathies worldwide.
- Apart from dermatophytes as etiologic agents, nondermatophyte molds and yeasts also can contribute to the disease.
- Categorization of onychomycosis clinically as well as mycologically will surely ensure better patient care.
- Avoiding certain habits (eg, walking with bare feet, wearing ill-fitting shoes, onychophagia) can decrease disease incidence.
Paclitaxel-Associated Melanonychia
To the Editor:
Taxane-based chemotherapy including paclitaxel and docetaxel is commonly used to treat solid tumor malignancies including lung, breast, ovarian, and bladder cancers.1 Taxanes work by interrupting normal microtubule function by inducing tubulin polymerization and inhibiting microtubule depolymerization, thereby leading to cell cycle arrest at the gap 2 (premitotic) and mitotic phase and the blockade of cell division.2
Cutaneous side effects have been reported with taxane-based therapies, including alopecia, skin rash and erythema, and desquamation of the hands and feet (hand-foot syndrome).3 Nail changes also have been reported to occur in 0% to 44% of treated patients,4 with one study reporting an incidence as high as 50.5%.5 Nail abnormalities that have been described primarily include onycholysis, and less frequently Beau lines, subungual hemorrhagic bullae, subungual hyperkeratosis, splinter hemorrhages, acute paronychia, and pigmentary changes such as nail bed dyschromia. Among the taxanes, nail abnormalities are more commonly seen with docetaxel; few reports address paclitaxel-induced nail changes.4 Onycholysis, diffuse fingernail orange discoloration, Beau lines, subungual distal hyperkeratosis, and brown discoloration of 3 fingernail beds sparing the lunula have been reported with paclitaxel.6-9 We report a unique case of paclitaxel-associated melanonychia.
A 54-year-old black woman with a history of multiple myeloma and breast cancer who was being treated with paclitaxel for breast cancer presented with nail changes including nail darkening since initiating paclitaxel. She was diagnosed with multiple myeloma in 2010 and received bortezomib, dexamethasone, and an autologous stem cell transplant in August 2011. She never achieved complete remission but had been on lenalidomide with stable disease. She underwent a lumpectomy in December 2012, which revealed intraductal carcinoma with ductal carcinoma in situ that was estrogen receptor and progesterone receptor negative and ERBB2 (formerly HER2) positive. She was started on weekly paclitaxel (80 mg/m2) to complete 12 cycles and trastuzumab (6 mg/kg) every 3 weeks. While on paclitaxel, she developed grade 2 neuropathy of the hands, leading to subsequent dose reduction at week 9. She denied any other changes to her medications. On clinical examination she had diffuse and well-demarcated, brown-black, longitudinal and transverse bands beginning at the proximal nail plate and progressing distally, with onycholysis involving all 20 nails (Figure, A and B). A nail clipping of the right hallux nail was sent for analysis. Pathology results showed evidence of scattered clusters of brown melanin pigment in the nail plate. Periodic acid–Schiff staining revealed numerous yeasts at the nail base but no infiltrating hyphae. Iron stain was negative for hemosiderin. The right index finger was injected with triamcinolone acetonide to treat the onycholysis. Four months after completing the paclitaxel, she began to notice lightening of the nails and improvement of the onycholysis in all nails (Figure, C and D).

| 
| |
| 
|
Initial appearance of diffuse, well-demarcated, brown-black, longitudinal and transverse bands beginning at the proximal nail plate and progressing distally, with onycholysis in the nails on the right hand (A) and left hand (B). Four months after completing paclitaxel, the patient began to notice lightening of the nails and improvement of the onycholysis in the nails on the right hand (C) and left hand (D). |
The highly proliferating cells that comprise the nail matrix epithelium mature, differentiate, and keratinize to form the nail plate and are susceptible to the antimitotic effects of systemic chemotherapy. As a result, systemic chemotherapies may lead to abnormal nail plate production and keratinization of the nail plate, causing the clinical manifestations of Beau lines, onychomadesis, and leukonychia.10
Melanonychia is the development of melanin pigmentation of the nail plate and is typically caused by matrix melanin deposition through the activation of nail matrix melanocytes. There are 3 patterns of melanonychia: longitudinal, transverse, and diffuse. A single nail plate can involve more than one pattern of melanonychia and several nails may be affected. Longitudinal melanonychia typically develops from the activation of a group of melanocytes in the nail matrix, while diffuse pigmentation arises from diffuse melanocyte activation.11 Longitudinal melanonychia is common in darker-pigmented individuals12 and can be associated with systemic diseases.10 Transverse melanonychia has been reported in association with medications including many chemotherapy agents, and each band of transverse melanonychia may correspond to a cycle of therapy.11 Drug-induced melanonychia can affect several nails and tends to resolve after completion of therapy. Melanonychia has previously been described with vincristine, doxorubicin, hydroxyurea, cyclophosphamide, 5-fluorouracil, bleomycin, dacarbazine, methotrexate, and electron beam therapy.11 Nail pigmentation changes have been reported with docetaxel; a patient developed blue discoloration on the right and left thumb lunulae that improved 3 months after discontinuation of docetaxel therapy.13 While on docetaxel, another patient developed acral erythema, onycholysis, and longitudinal melanonychia in photoexposed areas, which was thought to be secondary to possible photosensitization.14 Possible explanations for paclitaxel-induced melanonychia include a direct toxic effect on the nail bed or nail matrix, focal stimulation of nail matrix melanocytes, or photosensitization. Drug-induced melanonychia commonly appears 3 to 8 weeks after drug intake and typically resolves 6 to 8 weeks after drug discontinuation.15
Predictors of taxane-related nail changes have been studied.5 Taxane-induced nail toxicity was more prevalent in patients who were female, had a history of diabetes mellitus, had received capecitabine with docetaxel, and had a diagnosis of breast or gynecological cancer. The nail changes increased with greater number of taxane cycles administered, body mass index, and severity of treatment-related neuropathy.5 Although nail changes often are temporary and typically resolve with drug withdrawal, they may persist in some patients.16 Possible measures have been proposed to prevent taxane-induced nail toxicity including frozen gloves,17 nail cutting, and avoiding potential fingernail irritants.18
It is possible that the nails of our darker-skinned patient may have been affected by some degree of melanonychia prior to starting the therapy, which cannot be ruled out. However, according to the patient, she only noticed the change after starting paclitaxel, raising the possibility of either new, worsening, or more diffuse involvement following initiation of paclitaxel therapy. Additionally, she was receiving weekly administration of paclitaxel and experienced severe neuropathy, both predictors of nail toxicity.5 No reports of melanonychia from lenalidomide have been reported in the literature indexed for MEDLINE. Although these nail changes are not life threatening, clinicians should be aware of these side effects, as they are cosmetically distressing to many patients and can impact quality of life.19
1. Crown J, O’Leary M. The taxanes: an update. Lancet. 2000;356:507-508.
2. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277:665-667.
3. Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol. 2008;58:545-570.
4. Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;14:333-337.
5. Can G, Aydiner A, Cavdar I. Taxane-induced nail changes: predictors and efficacy of the use of frozen gloves and socks in the prevention of nail toxicity. Eur J Oncol Nurs. 2012;16:270-275.
6. Lüftner D, Flath B, Akrivakis C, et al. Dose-intensified weekly paclitaxel induces multiple nail disorders. Ann Oncol. 1998;9:1139-1141.
7. Hussain S, Anderson DN, Salvatti ME, et al. Onycholysis as a complication of systemic chemotherapy. report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer. 2000;88:2367-2371.
8. Almagro M, Del Pozo J, Garcia-Silva J, et al. Nail alterations secondary to paclitaxel therapy. Eur J Dermatol. 2000;10:146-147.
9. Flory SM, Solimando DA Jr, Webster GF, et al. Onycholysis associated with weekly administration of paclitaxel. Ann Pharmacother. 1999;33:584-586.
10. Hinds G, Thomas VD. Malignancy and cancer treatment-related hair and nail changes. Dermatol Clin. 2008;26:59-68.
11. Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Practice. 2009;15:143-55.
12. Buka R, Friedman KA, Phelps RG, et al. Childhood longitudinal melanonychia: case reports and review of the literature. Mt Sinai J Med. 2001;68:331-335.
13. Halvorson CR, Erickson CL, Gaspari AA. A rare manifestation of nail changes with docetaxel therapy. Skinmed. 2010;8:179-180.
14. Ferreira O, Baudrier T, Mota A, et al. Docetaxel-induced acral erythema and nail changes distributed to photoexposed areas. Cutan Ocul Toxicol. 2010;29:296-299.
15. Piraccini BM, Iorizzo M. Drug reactions affecting the nail unit: diagnosis and management. Dermatol Clin. 2007;25:215-221.
16. Piraccini BM, Tosti A. Drug-induced nail disorders: incidence, management and prognosis. Drug Saf. 1999;21:187-201.
17. Scotté F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23:4424-4429.
18. Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Pract. 2009;15:143-155.
19. Hackbarth M, Haas N, Fotopoulou C, et al. Chemotherapy-induced dermatological toxicity: frequencies and impact on quality of life in women’s cancers. results of a prospective study. Support Care Cancer. 2008;16:267-273.
To the Editor:
Taxane-based chemotherapy including paclitaxel and docetaxel is commonly used to treat solid tumor malignancies including lung, breast, ovarian, and bladder cancers.1 Taxanes work by interrupting normal microtubule function by inducing tubulin polymerization and inhibiting microtubule depolymerization, thereby leading to cell cycle arrest at the gap 2 (premitotic) and mitotic phase and the blockade of cell division.2
Cutaneous side effects have been reported with taxane-based therapies, including alopecia, skin rash and erythema, and desquamation of the hands and feet (hand-foot syndrome).3 Nail changes also have been reported to occur in 0% to 44% of treated patients,4 with one study reporting an incidence as high as 50.5%.5 Nail abnormalities that have been described primarily include onycholysis, and less frequently Beau lines, subungual hemorrhagic bullae, subungual hyperkeratosis, splinter hemorrhages, acute paronychia, and pigmentary changes such as nail bed dyschromia. Among the taxanes, nail abnormalities are more commonly seen with docetaxel; few reports address paclitaxel-induced nail changes.4 Onycholysis, diffuse fingernail orange discoloration, Beau lines, subungual distal hyperkeratosis, and brown discoloration of 3 fingernail beds sparing the lunula have been reported with paclitaxel.6-9 We report a unique case of paclitaxel-associated melanonychia.
A 54-year-old black woman with a history of multiple myeloma and breast cancer who was being treated with paclitaxel for breast cancer presented with nail changes including nail darkening since initiating paclitaxel. She was diagnosed with multiple myeloma in 2010 and received bortezomib, dexamethasone, and an autologous stem cell transplant in August 2011. She never achieved complete remission but had been on lenalidomide with stable disease. She underwent a lumpectomy in December 2012, which revealed intraductal carcinoma with ductal carcinoma in situ that was estrogen receptor and progesterone receptor negative and ERBB2 (formerly HER2) positive. She was started on weekly paclitaxel (80 mg/m2) to complete 12 cycles and trastuzumab (6 mg/kg) every 3 weeks. While on paclitaxel, she developed grade 2 neuropathy of the hands, leading to subsequent dose reduction at week 9. She denied any other changes to her medications. On clinical examination she had diffuse and well-demarcated, brown-black, longitudinal and transverse bands beginning at the proximal nail plate and progressing distally, with onycholysis involving all 20 nails (Figure, A and B). A nail clipping of the right hallux nail was sent for analysis. Pathology results showed evidence of scattered clusters of brown melanin pigment in the nail plate. Periodic acid–Schiff staining revealed numerous yeasts at the nail base but no infiltrating hyphae. Iron stain was negative for hemosiderin. The right index finger was injected with triamcinolone acetonide to treat the onycholysis. Four months after completing the paclitaxel, she began to notice lightening of the nails and improvement of the onycholysis in all nails (Figure, C and D).

| 
| |

| 
|
Initial appearance of diffuse, well-demarcated, brown-black, longitudinal and transverse bands beginning at the proximal nail plate and progressing distally, with onycholysis in the nails on the right hand (A) and left hand (B). Four months after completing paclitaxel, the patient began to notice lightening of the nails and improvement of the onycholysis in the nails on the right hand (C) and left hand (D). |
The highly proliferating cells that comprise the nail matrix epithelium mature, differentiate, and keratinize to form the nail plate and are susceptible to the antimitotic effects of systemic chemotherapy. As a result, systemic chemotherapies may lead to abnormal nail plate production and keratinization of the nail plate, causing the clinical manifestations of Beau lines, onychomadesis, and leukonychia.10
Melanonychia is the development of melanin pigmentation of the nail plate and is typically caused by matrix melanin deposition through the activation of nail matrix melanocytes. There are 3 patterns of melanonychia: longitudinal, transverse, and diffuse. A single nail plate can involve more than one pattern of melanonychia and several nails may be affected. Longitudinal melanonychia typically develops from the activation of a group of melanocytes in the nail matrix, while diffuse pigmentation arises from diffuse melanocyte activation.11 Longitudinal melanonychia is common in darker-pigmented individuals12 and can be associated with systemic diseases.10 Transverse melanonychia has been reported in association with medications including many chemotherapy agents, and each band of transverse melanonychia may correspond to a cycle of therapy.11 Drug-induced melanonychia can affect several nails and tends to resolve after completion of therapy. Melanonychia has previously been described with vincristine, doxorubicin, hydroxyurea, cyclophosphamide, 5-fluorouracil, bleomycin, dacarbazine, methotrexate, and electron beam therapy.11 Nail pigmentation changes have been reported with docetaxel; a patient developed blue discoloration on the right and left thumb lunulae that improved 3 months after discontinuation of docetaxel therapy.13 While on docetaxel, another patient developed acral erythema, onycholysis, and longitudinal melanonychia in photoexposed areas, which was thought to be secondary to possible photosensitization.14 Possible explanations for paclitaxel-induced melanonychia include a direct toxic effect on the nail bed or nail matrix, focal stimulation of nail matrix melanocytes, or photosensitization. Drug-induced melanonychia commonly appears 3 to 8 weeks after drug intake and typically resolves 6 to 8 weeks after drug discontinuation.15
Predictors of taxane-related nail changes have been studied.5 Taxane-induced nail toxicity was more prevalent in patients who were female, had a history of diabetes mellitus, had received capecitabine with docetaxel, and had a diagnosis of breast or gynecological cancer. The nail changes increased with greater number of taxane cycles administered, body mass index, and severity of treatment-related neuropathy.5 Although nail changes often are temporary and typically resolve with drug withdrawal, they may persist in some patients.16 Possible measures have been proposed to prevent taxane-induced nail toxicity including frozen gloves,17 nail cutting, and avoiding potential fingernail irritants.18
It is possible that the nails of our darker-skinned patient may have been affected by some degree of melanonychia prior to starting the therapy, which cannot be ruled out. However, according to the patient, she only noticed the change after starting paclitaxel, raising the possibility of either new, worsening, or more diffuse involvement following initiation of paclitaxel therapy. Additionally, she was receiving weekly administration of paclitaxel and experienced severe neuropathy, both predictors of nail toxicity.5 No reports of melanonychia from lenalidomide have been reported in the literature indexed for MEDLINE. Although these nail changes are not life threatening, clinicians should be aware of these side effects, as they are cosmetically distressing to many patients and can impact quality of life.19
To the Editor:
Taxane-based chemotherapy including paclitaxel and docetaxel is commonly used to treat solid tumor malignancies including lung, breast, ovarian, and bladder cancers.1 Taxanes work by interrupting normal microtubule function by inducing tubulin polymerization and inhibiting microtubule depolymerization, thereby leading to cell cycle arrest at the gap 2 (premitotic) and mitotic phase and the blockade of cell division.2
Cutaneous side effects have been reported with taxane-based therapies, including alopecia, skin rash and erythema, and desquamation of the hands and feet (hand-foot syndrome).3 Nail changes also have been reported to occur in 0% to 44% of treated patients,4 with one study reporting an incidence as high as 50.5%.5 Nail abnormalities that have been described primarily include onycholysis, and less frequently Beau lines, subungual hemorrhagic bullae, subungual hyperkeratosis, splinter hemorrhages, acute paronychia, and pigmentary changes such as nail bed dyschromia. Among the taxanes, nail abnormalities are more commonly seen with docetaxel; few reports address paclitaxel-induced nail changes.4 Onycholysis, diffuse fingernail orange discoloration, Beau lines, subungual distal hyperkeratosis, and brown discoloration of 3 fingernail beds sparing the lunula have been reported with paclitaxel.6-9 We report a unique case of paclitaxel-associated melanonychia.
A 54-year-old black woman with a history of multiple myeloma and breast cancer who was being treated with paclitaxel for breast cancer presented with nail changes including nail darkening since initiating paclitaxel. She was diagnosed with multiple myeloma in 2010 and received bortezomib, dexamethasone, and an autologous stem cell transplant in August 2011. She never achieved complete remission but had been on lenalidomide with stable disease. She underwent a lumpectomy in December 2012, which revealed intraductal carcinoma with ductal carcinoma in situ that was estrogen receptor and progesterone receptor negative and ERBB2 (formerly HER2) positive. She was started on weekly paclitaxel (80 mg/m2) to complete 12 cycles and trastuzumab (6 mg/kg) every 3 weeks. While on paclitaxel, she developed grade 2 neuropathy of the hands, leading to subsequent dose reduction at week 9. She denied any other changes to her medications. On clinical examination she had diffuse and well-demarcated, brown-black, longitudinal and transverse bands beginning at the proximal nail plate and progressing distally, with onycholysis involving all 20 nails (Figure, A and B). A nail clipping of the right hallux nail was sent for analysis. Pathology results showed evidence of scattered clusters of brown melanin pigment in the nail plate. Periodic acid–Schiff staining revealed numerous yeasts at the nail base but no infiltrating hyphae. Iron stain was negative for hemosiderin. The right index finger was injected with triamcinolone acetonide to treat the onycholysis. Four months after completing the paclitaxel, she began to notice lightening of the nails and improvement of the onycholysis in all nails (Figure, C and D).

| 
| |

| 
|
Initial appearance of diffuse, well-demarcated, brown-black, longitudinal and transverse bands beginning at the proximal nail plate and progressing distally, with onycholysis in the nails on the right hand (A) and left hand (B). Four months after completing paclitaxel, the patient began to notice lightening of the nails and improvement of the onycholysis in the nails on the right hand (C) and left hand (D). |
The highly proliferating cells that comprise the nail matrix epithelium mature, differentiate, and keratinize to form the nail plate and are susceptible to the antimitotic effects of systemic chemotherapy. As a result, systemic chemotherapies may lead to abnormal nail plate production and keratinization of the nail plate, causing the clinical manifestations of Beau lines, onychomadesis, and leukonychia.10
Melanonychia is the development of melanin pigmentation of the nail plate and is typically caused by matrix melanin deposition through the activation of nail matrix melanocytes. There are 3 patterns of melanonychia: longitudinal, transverse, and diffuse. A single nail plate can involve more than one pattern of melanonychia and several nails may be affected. Longitudinal melanonychia typically develops from the activation of a group of melanocytes in the nail matrix, while diffuse pigmentation arises from diffuse melanocyte activation.11 Longitudinal melanonychia is common in darker-pigmented individuals12 and can be associated with systemic diseases.10 Transverse melanonychia has been reported in association with medications including many chemotherapy agents, and each band of transverse melanonychia may correspond to a cycle of therapy.11 Drug-induced melanonychia can affect several nails and tends to resolve after completion of therapy. Melanonychia has previously been described with vincristine, doxorubicin, hydroxyurea, cyclophosphamide, 5-fluorouracil, bleomycin, dacarbazine, methotrexate, and electron beam therapy.11 Nail pigmentation changes have been reported with docetaxel; a patient developed blue discoloration on the right and left thumb lunulae that improved 3 months after discontinuation of docetaxel therapy.13 While on docetaxel, another patient developed acral erythema, onycholysis, and longitudinal melanonychia in photoexposed areas, which was thought to be secondary to possible photosensitization.14 Possible explanations for paclitaxel-induced melanonychia include a direct toxic effect on the nail bed or nail matrix, focal stimulation of nail matrix melanocytes, or photosensitization. Drug-induced melanonychia commonly appears 3 to 8 weeks after drug intake and typically resolves 6 to 8 weeks after drug discontinuation.15
Predictors of taxane-related nail changes have been studied.5 Taxane-induced nail toxicity was more prevalent in patients who were female, had a history of diabetes mellitus, had received capecitabine with docetaxel, and had a diagnosis of breast or gynecological cancer. The nail changes increased with greater number of taxane cycles administered, body mass index, and severity of treatment-related neuropathy.5 Although nail changes often are temporary and typically resolve with drug withdrawal, they may persist in some patients.16 Possible measures have been proposed to prevent taxane-induced nail toxicity including frozen gloves,17 nail cutting, and avoiding potential fingernail irritants.18
It is possible that the nails of our darker-skinned patient may have been affected by some degree of melanonychia prior to starting the therapy, which cannot be ruled out. However, according to the patient, she only noticed the change after starting paclitaxel, raising the possibility of either new, worsening, or more diffuse involvement following initiation of paclitaxel therapy. Additionally, she was receiving weekly administration of paclitaxel and experienced severe neuropathy, both predictors of nail toxicity.5 No reports of melanonychia from lenalidomide have been reported in the literature indexed for MEDLINE. Although these nail changes are not life threatening, clinicians should be aware of these side effects, as they are cosmetically distressing to many patients and can impact quality of life.19
1. Crown J, O’Leary M. The taxanes: an update. Lancet. 2000;356:507-508.
2. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277:665-667.
3. Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol. 2008;58:545-570.
4. Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;14:333-337.
5. Can G, Aydiner A, Cavdar I. Taxane-induced nail changes: predictors and efficacy of the use of frozen gloves and socks in the prevention of nail toxicity. Eur J Oncol Nurs. 2012;16:270-275.
6. Lüftner D, Flath B, Akrivakis C, et al. Dose-intensified weekly paclitaxel induces multiple nail disorders. Ann Oncol. 1998;9:1139-1141.
7. Hussain S, Anderson DN, Salvatti ME, et al. Onycholysis as a complication of systemic chemotherapy. report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer. 2000;88:2367-2371.
8. Almagro M, Del Pozo J, Garcia-Silva J, et al. Nail alterations secondary to paclitaxel therapy. Eur J Dermatol. 2000;10:146-147.
9. Flory SM, Solimando DA Jr, Webster GF, et al. Onycholysis associated with weekly administration of paclitaxel. Ann Pharmacother. 1999;33:584-586.
10. Hinds G, Thomas VD. Malignancy and cancer treatment-related hair and nail changes. Dermatol Clin. 2008;26:59-68.
11. Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Practice. 2009;15:143-55.
12. Buka R, Friedman KA, Phelps RG, et al. Childhood longitudinal melanonychia: case reports and review of the literature. Mt Sinai J Med. 2001;68:331-335.
13. Halvorson CR, Erickson CL, Gaspari AA. A rare manifestation of nail changes with docetaxel therapy. Skinmed. 2010;8:179-180.
14. Ferreira O, Baudrier T, Mota A, et al. Docetaxel-induced acral erythema and nail changes distributed to photoexposed areas. Cutan Ocul Toxicol. 2010;29:296-299.
15. Piraccini BM, Iorizzo M. Drug reactions affecting the nail unit: diagnosis and management. Dermatol Clin. 2007;25:215-221.
16. Piraccini BM, Tosti A. Drug-induced nail disorders: incidence, management and prognosis. Drug Saf. 1999;21:187-201.
17. Scotté F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23:4424-4429.
18. Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Pract. 2009;15:143-155.
19. Hackbarth M, Haas N, Fotopoulou C, et al. Chemotherapy-induced dermatological toxicity: frequencies and impact on quality of life in women’s cancers. results of a prospective study. Support Care Cancer. 2008;16:267-273.
1. Crown J, O’Leary M. The taxanes: an update. Lancet. 2000;356:507-508.
2. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by Taxol. Nature. 1979;277:665-667.
3. Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol. 2008;58:545-570.
4. Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;14:333-337.
5. Can G, Aydiner A, Cavdar I. Taxane-induced nail changes: predictors and efficacy of the use of frozen gloves and socks in the prevention of nail toxicity. Eur J Oncol Nurs. 2012;16:270-275.
6. Lüftner D, Flath B, Akrivakis C, et al. Dose-intensified weekly paclitaxel induces multiple nail disorders. Ann Oncol. 1998;9:1139-1141.
7. Hussain S, Anderson DN, Salvatti ME, et al. Onycholysis as a complication of systemic chemotherapy. report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer. 2000;88:2367-2371.
8. Almagro M, Del Pozo J, Garcia-Silva J, et al. Nail alterations secondary to paclitaxel therapy. Eur J Dermatol. 2000;10:146-147.
9. Flory SM, Solimando DA Jr, Webster GF, et al. Onycholysis associated with weekly administration of paclitaxel. Ann Pharmacother. 1999;33:584-586.
10. Hinds G, Thomas VD. Malignancy and cancer treatment-related hair and nail changes. Dermatol Clin. 2008;26:59-68.
11. Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Practice. 2009;15:143-55.
12. Buka R, Friedman KA, Phelps RG, et al. Childhood longitudinal melanonychia: case reports and review of the literature. Mt Sinai J Med. 2001;68:331-335.
13. Halvorson CR, Erickson CL, Gaspari AA. A rare manifestation of nail changes with docetaxel therapy. Skinmed. 2010;8:179-180.
14. Ferreira O, Baudrier T, Mota A, et al. Docetaxel-induced acral erythema and nail changes distributed to photoexposed areas. Cutan Ocul Toxicol. 2010;29:296-299.
15. Piraccini BM, Iorizzo M. Drug reactions affecting the nail unit: diagnosis and management. Dermatol Clin. 2007;25:215-221.
16. Piraccini BM, Tosti A. Drug-induced nail disorders: incidence, management and prognosis. Drug Saf. 1999;21:187-201.
17. Scotté F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23:4424-4429.
18. Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Pract. 2009;15:143-155.
19. Hackbarth M, Haas N, Fotopoulou C, et al. Chemotherapy-induced dermatological toxicity: frequencies and impact on quality of life in women’s cancers. results of a prospective study. Support Care Cancer. 2008;16:267-273.
Nailing Down the Data: Topicals for Onychomycosis
In summer 2014, the US Food and Drug Administration (FDA) approved 2 new topical medications for onychomycosis. In recent months, the Journal of Clinical and Aesthetic Dermatology (2014;7:10-18) and Medscape provided review materials to assist in sifting through this topic. In summary, efinaconazole, a triazole antifungal in a 10% solution recommended for daily application for 48 weeks, exhibited 17.8% complete and 55.2% mycological cure rates compared to vehicle (5.5% and 16.9%, respectively). Tavaborole, an oxaborole antifungal in a 5% solution recommended for daily application for 48 weeks, displayed 9.1% complete and 35.9% mycological cure rates versus vehicle (1.5% and 12.2%, respectively). To complete the discussion, ciclopirox nail lacquer, FDA approved in 1999 for onychomycosis for daily application for 48 weeks, heralded 8.5% complete and 36% mycological cure rates compared to vehicle (0% and 9%, respectively). Ciclopirox requires nail debridement periodically, and the newer agents do not.
What’s the issue?
Do you agree that nary a day goes by without an e-mailed article or continuing medical education opportunity tasked at “getting to know” new topical onychomycosis therapies? That being said, how often have you summarily deleted them, assuming that topicals just don’t work? I know I have, though I paused this week after thinking to myself, “How often have I written a prescription for ciclopirox nail lacquer to appease a patient who would prefer nonsystemic therapy?” And then I read on. Based on the data above, perhaps these medications, particularly efinaconazole, at least deserve perusal compared to our current meager topical and systemic options. What has your experience been with these novel topicals, their insurance coverage, and their tolerability and efficacy in your practice?
In summer 2014, the US Food and Drug Administration (FDA) approved 2 new topical medications for onychomycosis. In recent months, the Journal of Clinical and Aesthetic Dermatology (2014;7:10-18) and Medscape provided review materials to assist in sifting through this topic. In summary, efinaconazole, a triazole antifungal in a 10% solution recommended for daily application for 48 weeks, exhibited 17.8% complete and 55.2% mycological cure rates compared to vehicle (5.5% and 16.9%, respectively). Tavaborole, an oxaborole antifungal in a 5% solution recommended for daily application for 48 weeks, displayed 9.1% complete and 35.9% mycological cure rates versus vehicle (1.5% and 12.2%, respectively). To complete the discussion, ciclopirox nail lacquer, FDA approved in 1999 for onychomycosis for daily application for 48 weeks, heralded 8.5% complete and 36% mycological cure rates compared to vehicle (0% and 9%, respectively). Ciclopirox requires nail debridement periodically, and the newer agents do not.
What’s the issue?
Do you agree that nary a day goes by without an e-mailed article or continuing medical education opportunity tasked at “getting to know” new topical onychomycosis therapies? That being said, how often have you summarily deleted them, assuming that topicals just don’t work? I know I have, though I paused this week after thinking to myself, “How often have I written a prescription for ciclopirox nail lacquer to appease a patient who would prefer nonsystemic therapy?” And then I read on. Based on the data above, perhaps these medications, particularly efinaconazole, at least deserve perusal compared to our current meager topical and systemic options. What has your experience been with these novel topicals, their insurance coverage, and their tolerability and efficacy in your practice?
In summer 2014, the US Food and Drug Administration (FDA) approved 2 new topical medications for onychomycosis. In recent months, the Journal of Clinical and Aesthetic Dermatology (2014;7:10-18) and Medscape provided review materials to assist in sifting through this topic. In summary, efinaconazole, a triazole antifungal in a 10% solution recommended for daily application for 48 weeks, exhibited 17.8% complete and 55.2% mycological cure rates compared to vehicle (5.5% and 16.9%, respectively). Tavaborole, an oxaborole antifungal in a 5% solution recommended for daily application for 48 weeks, displayed 9.1% complete and 35.9% mycological cure rates versus vehicle (1.5% and 12.2%, respectively). To complete the discussion, ciclopirox nail lacquer, FDA approved in 1999 for onychomycosis for daily application for 48 weeks, heralded 8.5% complete and 36% mycological cure rates compared to vehicle (0% and 9%, respectively). Ciclopirox requires nail debridement periodically, and the newer agents do not.
What’s the issue?
Do you agree that nary a day goes by without an e-mailed article or continuing medical education opportunity tasked at “getting to know” new topical onychomycosis therapies? That being said, how often have you summarily deleted them, assuming that topicals just don’t work? I know I have, though I paused this week after thinking to myself, “How often have I written a prescription for ciclopirox nail lacquer to appease a patient who would prefer nonsystemic therapy?” And then I read on. Based on the data above, perhaps these medications, particularly efinaconazole, at least deserve perusal compared to our current meager topical and systemic options. What has your experience been with these novel topicals, their insurance coverage, and their tolerability and efficacy in your practice?
Onychomycosis: Current and Investigational Therapies
To the Editor:
Onychomycosis is a fungal infection of the nail plate by dermatophytes, yeasts, and nondermatophyte molds. It is a common problem with a prevalence of 10% to 12% in the United States.1,2 The clinical presentation of onychomycosis is shown in the Figure. Although some patients may have mild asymptomatic cases of onychomycosis and do not inquire about treatment, many will have more advanced cases, presenting with pain and discomfort, secondary infection, unattractive appearance, or problems performing everyday functions. The goal of onychomycosis treatment is to eliminate the fungus, if possible, which usually restores the nail to its normal state when it fully grows out. Patients should be counseled that it is a long process that may take 6 months or more for fingernails and 12 to 18 months for toenails. These estimates are based on a growth rate of 2 to 3 mm per month for fingernails and 1 to 2 mm per month for toenails.3 Nails grow fastest during the teenaged years and slow down with advancing age.4 It should be noted that advanced cases of onychomycosis affecting the nail matrix may cause permanent scarring; therefore, the nail unit may still appear dystrophic after the causative organism is eliminated. The US Food and Drug Administration (FDA) defines a complete cure as negative potassium hydroxide preparation and negative fungal culture plus a completely normal appearance of the nail.
Treatment of onychomycosis poses a number of challenges. First, hyperkeratosis and the fungal mass may limit the delivery of topical and systemic drugs to the source of the infection. In addition, high rates of relapse and reinfection after treatment may be due to residual hyphae or spores.5 Furthermore, the extended length of treatment limits patient adherence and many patients are unwilling to forego wearing nail cosmetics during the course of some of the treatments.
There are 4 approved classes of antifungal drugs for the treatment of onychomycosis: allylamines, azoles, morpholines, and hydroxypyridinones.6 The allylamines (eg, terbinafine) inhibit squalene epoxidase.7 Oral terbinafine (250 mg daily) taken for 6 weeks for fingernails and 12 weeks for toenails is considered the current systemic treatment preference in onychomycosis therapy8 with complete cure rates in 12-week studies of approximately 38%9 and 49%.10
The second class of drugs is the azoles, which inhibit lanosterol 14a-demethylase, a step in the ergosterol biosynthesis pathway.6 Two members of this class that are widely used in treating onychomycosis are oral itraconazole11 and off-label oral fluconazole.12 The approved dose for oral itraconazole is 200 mg daily for 3 months (or an alternative pulse regimen) with a reported complete cure rate of 14%.11 Although fluconazole is not FDA approved for the treatment of onychomycosis in the United States, it is used extensively in other countries and to some extent off label in the United States. In a study of 362 patients with onychomycosis treated with oral fluconazole, complete cure rates were 48% in patients who received 450 mg weekly, 46% in those who received 300 mg weekly, and 37% in those who received 150 mg weekly for up to 9 months.12 It should be noted that several oral triazole antifungals, namely albaconazole,13 posaconazole,14 and ravuconazole,15 have undergone phase 1 and 2 studies for the treatment of onychomycosis and have shown some efficacy.
Another class of antifungals are the morpholines including topical amorolfine, which is approved for use in Europe but not in North America.16 Amorolfine inhibits D14 reductase and D7-D8 isomerase, thus depleting ergosterol.17 In one randomized controlled study, the combination of amorolfine nail lacquer and oral terbinafine compared to oral terbinafine alone resulted in a higher clinical cure rate with the combination (59.2% vs 46%); complete cure rate was not reported.16
Finally, the hydroxypyridinone class includes topical ciclopirox, which has a poorly understood mechanism of action but may involve iron chelation or oxidative damage.18,19 Ciclopirox nail lacquer 8% was approved by the FDA in 1999 and has reported complete cure rates of 5.5% to 8.5% with monthly nail debridement.20
Based on the poor efficacy of many of the currently available treatments and time-consuming treatment courses, it is clear that there is a need for alternative and novel therapies. There has been a greater emphasis on topical agents due to their more favorable side-effect profile and lower risk for drug-drug interactions. Although there are many agents for the treatment of onychomycosis currently in development, many are in vitro studies or phase 1 and 2 studies. However, we will focus on drugs that are further along in phase 3 studies and those that were recently FDA approved.
Efinaconazole is a member of the azole class of drugs and has completed 2 phase 3 clinical trials (study 1, N=870; study 2, N=785).21 Patients in these 2 studies were randomized to receive either efinaconazole nail solution 10% or vehicle for 48 weeks followed by a 4-week washout period. Complete cure rates in the 2 studies were 17.8% and 15.2% in the treated group and 3.3% and 5.5% in the control group. The mycological cure rates were 55.2% and 53.4% in the treated group and 16.8% and 16.9% in the control group. The side-effect profile was minimal, with the most common adverse events being application-site dermatitis and vesiculation, which were not significantly higher in the treated group versus the control group.21 Efinaconazole received FDA approval for the treatment of toenail onychomycosis in June 2014.
There are some notable differences between ciclopirox and efinaconazole that may improve patient compliance with the latter. First, treatment with ciclopirox includes monthly nail debridement, which is not required with efinaconazole. Secondly, although ciclopirox lacquer must be removed weekly, efinaconazole is a solution, so no removal is necessary.
Terbinafine nail solution (TNS) is a member of the allylamine class and has completed phase 3 clinical trials.22 Three studies—2 vehicle controlled and 1 active comparator—were performed. The first compared TNS and vehicle, both applied daily for 24 weeks; the second study repeated the same for 48 weeks; and the third study compared TNS to amorolfine nail lacquer 5% daily for 48 weeks. The best results for complete cure were achieved with TNS for 48 weeks in the vehicle-controlled study with a rate of 2.2% versus 0%. The authors also concluded TNS was not more effective than amorolfine, as complete cure rates were 1.2% for TNS and 0.96% for amorolfine. The most common side effects were headache, nasopharyngitis, and influenza.22
Tavaborole is a member of the new benzoxaborole class, which inhibits protein synthesis by forming an adduct with the aminoacyl–transfer RNA synthetase.23 The topical solution was engineered to have improved penetration through the nail plate. In vitro studies showed better penetration than both ciclopirox and amorolfine.24 Two identical phase 3 randomized, double-blind, vehicle-controlled studies were completed involving 1197 patients who were treated with tavaborole topical solution 5% daily compared to vehicle for 48 weeks followed by a 4-week washout period with promising results.25 The incidence of treatment-related side effects was comparable to the vehicle. The most common adverse events were exfoliation, erythema, and dermatitis, all occurring at the application site.25 Tavaborole was approved by the FDA for the treatment of toenail onychomycosis in July 2014.
Luliconazole is a member of the azole class and a phase 2b/3 clinical trial with a 10% solution involving 334 patients was completed in June 2013.26 Results from this trial are expected in early 2015.
Lasers are a developing area for onychomycosis therapy and the appeal stems from their ability to selectively deliver energy to the target tissue, thus avoiding systemic side effects. Since 2010, the FDA has approved numerous laser devices for the temporary cosmetic improvement of onychomycosis, all of which are Nd:YAG 1064-nm lasers.27,28 It was previously thought that the mechanism of action for the fungicidal effect was achieved with heat,29 but newer in vitro studies have shown that the amount of time and level of heat required to kill Trichophyton rubrum would not be tolerable to patients.30 Although the mechanism of action is poorly understood, some clinical trials have shown success using the Nd:YAG 1064-nm laser for treatment of onychomycosis. However, in a study of 8 patients treated with the Nd:YAG 1064-nm laser for 5 treatment sessions, none had a mycological or clinical cure and there was only mild clinical improvement. In addition, most patients had pain and burning during the treatments requiring many short breaks.30 Although not yet FDA approved for the treatment of onychomycosis, other types of lasers are currently being studied, including CO2, near-infrared diode, and femtosecond-infrared laser systems.3
Plasma therapy is a developing area for the treatment of onychomycosis. Plasma was shown to be fungicidal to T rubrum in an in vitro model (MOE Medical Devices, written communication, July 2012), and a clinical trial to evaluate the safety, tolerability, and efficacy of plasma in human subjects is ongoing (registered on March 22, 2013, at www.clinicaltrials.gov with the identifier NCT01819051).
Onychomycosis is a common problem that increases in prevalence with advancing age. Oral terbinafine is considered the first-line treatment at this point in time.31 Two new topical agents, efinaconazole and tavaborole, were recently FDA approved and may be used for the treatment of toenail onychomycosis without the need for nail debridement. The Nd:YAG laser has shown some promise in earlier clinical studies but was ineffective in a more recent study.
1. Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
2. Heikkila H, Stubb S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995;133:699-703.
3. Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
4. Abdullah L, Abbas O. Common nail changes and disorders in older people: diagnosis and management. Can Fam Physician. 2011;57:173-181.
5. Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
6. Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28:151-159.
7. Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. part II. J Am Acad Dermatol. 1994;30:911-933.
8. Gupta AK, Paquet M, Simpson F, et al. Terbinafine in the treatment of dermatophyte toenail onychomycosis: a meta-analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol. 2013;27:267-272.
9. Drake LA, Shear NH, Arlette JP, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37:740-745.
10. Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. the LION Study Group. BMJ. 1999;318:1031-1035.
11. Sporanox [package insert]. Macquarie Park, Australia: Janssen-Cilag Pty Ltd; 2014.
12. Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6, pt 2):S77-S86.
13. Sigurgeirsson B, van Rossem K, Malahias S, et al. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J Am Acad Dermatol. 2013;69:416-425.
14. Elewski B, Pollak R, Ashton S, et al. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166:389-398.
15. Gupta AK, Leonardi C, Stoltz RR, et al. A phase I/II randomized, double-blind, placebo-controlled, dose-ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19:437-443.
16. Baran R, Sigurgeirsson B, de Berker D, et al. A multicentre, randomized, controlled study of the efficacy, safety and cost-effectiveness of a combination therapy with amorolfine nail lacquer and oral terbinafine compared with oral terbinafine alone for the treatment of onychomycosis with matrix involvement. Br J Dermatol. 2007;157:149-157.
17. Polak A. Preclinical data and mode of action of amorolfine. Dermatology. 1992;184(suppl 1):3-7.
18. Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350-358.
19. Lee RE, Liu TT, Barker KS, et al. Genome-wide expression profiling of the response to ciclopirox olamine in Candida albicans. J Antimicrob Chemother. 2005;55:655-662.
20. Penlac [package insert]. Bridgewater, NJ: sanofi-aventis; 2006.
21. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
22. Elewski BE, Ghannoum MA, Mayser P, et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J Eur Acad Dermatol Venereol. 2013;27:287-294.
23. Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
24. Hui X, Baker SJ, Wester RC, et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96:2622-2631.
25. Elewski BE, Rich P, Wiltz H, et al. Effectiveness and safety of tavaborole, a novel born-based molecule for the treatment of onychomycosis: results from two phase 3 studies. Poster presented at: Women’s & Pediatric Dermatology Seminar; October 4-6, 2013; Newport Beach, CA.
26. The solution study: Topica’s phase 2b/3 clinical trial. Topica Pharmaceuticals Inc Web site. http://www.
topicapharma.com/phase-2b3. Accessed December 2, 2014.
27. Gupta AK, Simpson FC. Medical devices for the treatment of onychomycosis. Dermatol Ther. 2012;25:574-581.
28. Ortiz AE, Avram MM, Wanner MA. A review of lasers and light for the treatment of onychomycosis. Lasers Surg Med. 2014;46:117-124.
29. Vural E, Winfield HL, Shingleton AW, et al. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci. 2008;23:349-353.
30. Carney C, Cantrell W, Warner J, et al. Treatment of onychomycosis using a submillisecond 1064-nm neodymium:yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69:578-582.
31. Gupta AK, Daigle D, Paquet M. Therapies for onychomycosis: a systematic review and network meta-analysis of mycological cure [published online ahead of print July 17, 2014]. J Am Podiatr Med Assoc. doi:10.7547/13-110.1.
To the Editor:
Onychomycosis is a fungal infection of the nail plate by dermatophytes, yeasts, and nondermatophyte molds. It is a common problem with a prevalence of 10% to 12% in the United States.1,2 The clinical presentation of onychomycosis is shown in the Figure. Although some patients may have mild asymptomatic cases of onychomycosis and do not inquire about treatment, many will have more advanced cases, presenting with pain and discomfort, secondary infection, unattractive appearance, or problems performing everyday functions. The goal of onychomycosis treatment is to eliminate the fungus, if possible, which usually restores the nail to its normal state when it fully grows out. Patients should be counseled that it is a long process that may take 6 months or more for fingernails and 12 to 18 months for toenails. These estimates are based on a growth rate of 2 to 3 mm per month for fingernails and 1 to 2 mm per month for toenails.3 Nails grow fastest during the teenaged years and slow down with advancing age.4 It should be noted that advanced cases of onychomycosis affecting the nail matrix may cause permanent scarring; therefore, the nail unit may still appear dystrophic after the causative organism is eliminated. The US Food and Drug Administration (FDA) defines a complete cure as negative potassium hydroxide preparation and negative fungal culture plus a completely normal appearance of the nail.
Treatment of onychomycosis poses a number of challenges. First, hyperkeratosis and the fungal mass may limit the delivery of topical and systemic drugs to the source of the infection. In addition, high rates of relapse and reinfection after treatment may be due to residual hyphae or spores.5 Furthermore, the extended length of treatment limits patient adherence and many patients are unwilling to forego wearing nail cosmetics during the course of some of the treatments.
There are 4 approved classes of antifungal drugs for the treatment of onychomycosis: allylamines, azoles, morpholines, and hydroxypyridinones.6 The allylamines (eg, terbinafine) inhibit squalene epoxidase.7 Oral terbinafine (250 mg daily) taken for 6 weeks for fingernails and 12 weeks for toenails is considered the current systemic treatment preference in onychomycosis therapy8 with complete cure rates in 12-week studies of approximately 38%9 and 49%.10
The second class of drugs is the azoles, which inhibit lanosterol 14a-demethylase, a step in the ergosterol biosynthesis pathway.6 Two members of this class that are widely used in treating onychomycosis are oral itraconazole11 and off-label oral fluconazole.12 The approved dose for oral itraconazole is 200 mg daily for 3 months (or an alternative pulse regimen) with a reported complete cure rate of 14%.11 Although fluconazole is not FDA approved for the treatment of onychomycosis in the United States, it is used extensively in other countries and to some extent off label in the United States. In a study of 362 patients with onychomycosis treated with oral fluconazole, complete cure rates were 48% in patients who received 450 mg weekly, 46% in those who received 300 mg weekly, and 37% in those who received 150 mg weekly for up to 9 months.12 It should be noted that several oral triazole antifungals, namely albaconazole,13 posaconazole,14 and ravuconazole,15 have undergone phase 1 and 2 studies for the treatment of onychomycosis and have shown some efficacy.
Another class of antifungals are the morpholines including topical amorolfine, which is approved for use in Europe but not in North America.16 Amorolfine inhibits D14 reductase and D7-D8 isomerase, thus depleting ergosterol.17 In one randomized controlled study, the combination of amorolfine nail lacquer and oral terbinafine compared to oral terbinafine alone resulted in a higher clinical cure rate with the combination (59.2% vs 46%); complete cure rate was not reported.16
Finally, the hydroxypyridinone class includes topical ciclopirox, which has a poorly understood mechanism of action but may involve iron chelation or oxidative damage.18,19 Ciclopirox nail lacquer 8% was approved by the FDA in 1999 and has reported complete cure rates of 5.5% to 8.5% with monthly nail debridement.20
Based on the poor efficacy of many of the currently available treatments and time-consuming treatment courses, it is clear that there is a need for alternative and novel therapies. There has been a greater emphasis on topical agents due to their more favorable side-effect profile and lower risk for drug-drug interactions. Although there are many agents for the treatment of onychomycosis currently in development, many are in vitro studies or phase 1 and 2 studies. However, we will focus on drugs that are further along in phase 3 studies and those that were recently FDA approved.
Efinaconazole is a member of the azole class of drugs and has completed 2 phase 3 clinical trials (study 1, N=870; study 2, N=785).21 Patients in these 2 studies were randomized to receive either efinaconazole nail solution 10% or vehicle for 48 weeks followed by a 4-week washout period. Complete cure rates in the 2 studies were 17.8% and 15.2% in the treated group and 3.3% and 5.5% in the control group. The mycological cure rates were 55.2% and 53.4% in the treated group and 16.8% and 16.9% in the control group. The side-effect profile was minimal, with the most common adverse events being application-site dermatitis and vesiculation, which were not significantly higher in the treated group versus the control group.21 Efinaconazole received FDA approval for the treatment of toenail onychomycosis in June 2014.
There are some notable differences between ciclopirox and efinaconazole that may improve patient compliance with the latter. First, treatment with ciclopirox includes monthly nail debridement, which is not required with efinaconazole. Secondly, although ciclopirox lacquer must be removed weekly, efinaconazole is a solution, so no removal is necessary.
Terbinafine nail solution (TNS) is a member of the allylamine class and has completed phase 3 clinical trials.22 Three studies—2 vehicle controlled and 1 active comparator—were performed. The first compared TNS and vehicle, both applied daily for 24 weeks; the second study repeated the same for 48 weeks; and the third study compared TNS to amorolfine nail lacquer 5% daily for 48 weeks. The best results for complete cure were achieved with TNS for 48 weeks in the vehicle-controlled study with a rate of 2.2% versus 0%. The authors also concluded TNS was not more effective than amorolfine, as complete cure rates were 1.2% for TNS and 0.96% for amorolfine. The most common side effects were headache, nasopharyngitis, and influenza.22
Tavaborole is a member of the new benzoxaborole class, which inhibits protein synthesis by forming an adduct with the aminoacyl–transfer RNA synthetase.23 The topical solution was engineered to have improved penetration through the nail plate. In vitro studies showed better penetration than both ciclopirox and amorolfine.24 Two identical phase 3 randomized, double-blind, vehicle-controlled studies were completed involving 1197 patients who were treated with tavaborole topical solution 5% daily compared to vehicle for 48 weeks followed by a 4-week washout period with promising results.25 The incidence of treatment-related side effects was comparable to the vehicle. The most common adverse events were exfoliation, erythema, and dermatitis, all occurring at the application site.25 Tavaborole was approved by the FDA for the treatment of toenail onychomycosis in July 2014.
Luliconazole is a member of the azole class and a phase 2b/3 clinical trial with a 10% solution involving 334 patients was completed in June 2013.26 Results from this trial are expected in early 2015.
Lasers are a developing area for onychomycosis therapy and the appeal stems from their ability to selectively deliver energy to the target tissue, thus avoiding systemic side effects. Since 2010, the FDA has approved numerous laser devices for the temporary cosmetic improvement of onychomycosis, all of which are Nd:YAG 1064-nm lasers.27,28 It was previously thought that the mechanism of action for the fungicidal effect was achieved with heat,29 but newer in vitro studies have shown that the amount of time and level of heat required to kill Trichophyton rubrum would not be tolerable to patients.30 Although the mechanism of action is poorly understood, some clinical trials have shown success using the Nd:YAG 1064-nm laser for treatment of onychomycosis. However, in a study of 8 patients treated with the Nd:YAG 1064-nm laser for 5 treatment sessions, none had a mycological or clinical cure and there was only mild clinical improvement. In addition, most patients had pain and burning during the treatments requiring many short breaks.30 Although not yet FDA approved for the treatment of onychomycosis, other types of lasers are currently being studied, including CO2, near-infrared diode, and femtosecond-infrared laser systems.3
Plasma therapy is a developing area for the treatment of onychomycosis. Plasma was shown to be fungicidal to T rubrum in an in vitro model (MOE Medical Devices, written communication, July 2012), and a clinical trial to evaluate the safety, tolerability, and efficacy of plasma in human subjects is ongoing (registered on March 22, 2013, at www.clinicaltrials.gov with the identifier NCT01819051).
Onychomycosis is a common problem that increases in prevalence with advancing age. Oral terbinafine is considered the first-line treatment at this point in time.31 Two new topical agents, efinaconazole and tavaborole, were recently FDA approved and may be used for the treatment of toenail onychomycosis without the need for nail debridement. The Nd:YAG laser has shown some promise in earlier clinical studies but was ineffective in a more recent study.
To the Editor:
Onychomycosis is a fungal infection of the nail plate by dermatophytes, yeasts, and nondermatophyte molds. It is a common problem with a prevalence of 10% to 12% in the United States.1,2 The clinical presentation of onychomycosis is shown in the Figure. Although some patients may have mild asymptomatic cases of onychomycosis and do not inquire about treatment, many will have more advanced cases, presenting with pain and discomfort, secondary infection, unattractive appearance, or problems performing everyday functions. The goal of onychomycosis treatment is to eliminate the fungus, if possible, which usually restores the nail to its normal state when it fully grows out. Patients should be counseled that it is a long process that may take 6 months or more for fingernails and 12 to 18 months for toenails. These estimates are based on a growth rate of 2 to 3 mm per month for fingernails and 1 to 2 mm per month for toenails.3 Nails grow fastest during the teenaged years and slow down with advancing age.4 It should be noted that advanced cases of onychomycosis affecting the nail matrix may cause permanent scarring; therefore, the nail unit may still appear dystrophic after the causative organism is eliminated. The US Food and Drug Administration (FDA) defines a complete cure as negative potassium hydroxide preparation and negative fungal culture plus a completely normal appearance of the nail.
Treatment of onychomycosis poses a number of challenges. First, hyperkeratosis and the fungal mass may limit the delivery of topical and systemic drugs to the source of the infection. In addition, high rates of relapse and reinfection after treatment may be due to residual hyphae or spores.5 Furthermore, the extended length of treatment limits patient adherence and many patients are unwilling to forego wearing nail cosmetics during the course of some of the treatments.
There are 4 approved classes of antifungal drugs for the treatment of onychomycosis: allylamines, azoles, morpholines, and hydroxypyridinones.6 The allylamines (eg, terbinafine) inhibit squalene epoxidase.7 Oral terbinafine (250 mg daily) taken for 6 weeks for fingernails and 12 weeks for toenails is considered the current systemic treatment preference in onychomycosis therapy8 with complete cure rates in 12-week studies of approximately 38%9 and 49%.10
The second class of drugs is the azoles, which inhibit lanosterol 14a-demethylase, a step in the ergosterol biosynthesis pathway.6 Two members of this class that are widely used in treating onychomycosis are oral itraconazole11 and off-label oral fluconazole.12 The approved dose for oral itraconazole is 200 mg daily for 3 months (or an alternative pulse regimen) with a reported complete cure rate of 14%.11 Although fluconazole is not FDA approved for the treatment of onychomycosis in the United States, it is used extensively in other countries and to some extent off label in the United States. In a study of 362 patients with onychomycosis treated with oral fluconazole, complete cure rates were 48% in patients who received 450 mg weekly, 46% in those who received 300 mg weekly, and 37% in those who received 150 mg weekly for up to 9 months.12 It should be noted that several oral triazole antifungals, namely albaconazole,13 posaconazole,14 and ravuconazole,15 have undergone phase 1 and 2 studies for the treatment of onychomycosis and have shown some efficacy.
Another class of antifungals are the morpholines including topical amorolfine, which is approved for use in Europe but not in North America.16 Amorolfine inhibits D14 reductase and D7-D8 isomerase, thus depleting ergosterol.17 In one randomized controlled study, the combination of amorolfine nail lacquer and oral terbinafine compared to oral terbinafine alone resulted in a higher clinical cure rate with the combination (59.2% vs 46%); complete cure rate was not reported.16
Finally, the hydroxypyridinone class includes topical ciclopirox, which has a poorly understood mechanism of action but may involve iron chelation or oxidative damage.18,19 Ciclopirox nail lacquer 8% was approved by the FDA in 1999 and has reported complete cure rates of 5.5% to 8.5% with monthly nail debridement.20
Based on the poor efficacy of many of the currently available treatments and time-consuming treatment courses, it is clear that there is a need for alternative and novel therapies. There has been a greater emphasis on topical agents due to their more favorable side-effect profile and lower risk for drug-drug interactions. Although there are many agents for the treatment of onychomycosis currently in development, many are in vitro studies or phase 1 and 2 studies. However, we will focus on drugs that are further along in phase 3 studies and those that were recently FDA approved.
Efinaconazole is a member of the azole class of drugs and has completed 2 phase 3 clinical trials (study 1, N=870; study 2, N=785).21 Patients in these 2 studies were randomized to receive either efinaconazole nail solution 10% or vehicle for 48 weeks followed by a 4-week washout period. Complete cure rates in the 2 studies were 17.8% and 15.2% in the treated group and 3.3% and 5.5% in the control group. The mycological cure rates were 55.2% and 53.4% in the treated group and 16.8% and 16.9% in the control group. The side-effect profile was minimal, with the most common adverse events being application-site dermatitis and vesiculation, which were not significantly higher in the treated group versus the control group.21 Efinaconazole received FDA approval for the treatment of toenail onychomycosis in June 2014.
There are some notable differences between ciclopirox and efinaconazole that may improve patient compliance with the latter. First, treatment with ciclopirox includes monthly nail debridement, which is not required with efinaconazole. Secondly, although ciclopirox lacquer must be removed weekly, efinaconazole is a solution, so no removal is necessary.
Terbinafine nail solution (TNS) is a member of the allylamine class and has completed phase 3 clinical trials.22 Three studies—2 vehicle controlled and 1 active comparator—were performed. The first compared TNS and vehicle, both applied daily for 24 weeks; the second study repeated the same for 48 weeks; and the third study compared TNS to amorolfine nail lacquer 5% daily for 48 weeks. The best results for complete cure were achieved with TNS for 48 weeks in the vehicle-controlled study with a rate of 2.2% versus 0%. The authors also concluded TNS was not more effective than amorolfine, as complete cure rates were 1.2% for TNS and 0.96% for amorolfine. The most common side effects were headache, nasopharyngitis, and influenza.22
Tavaborole is a member of the new benzoxaborole class, which inhibits protein synthesis by forming an adduct with the aminoacyl–transfer RNA synthetase.23 The topical solution was engineered to have improved penetration through the nail plate. In vitro studies showed better penetration than both ciclopirox and amorolfine.24 Two identical phase 3 randomized, double-blind, vehicle-controlled studies were completed involving 1197 patients who were treated with tavaborole topical solution 5% daily compared to vehicle for 48 weeks followed by a 4-week washout period with promising results.25 The incidence of treatment-related side effects was comparable to the vehicle. The most common adverse events were exfoliation, erythema, and dermatitis, all occurring at the application site.25 Tavaborole was approved by the FDA for the treatment of toenail onychomycosis in July 2014.
Luliconazole is a member of the azole class and a phase 2b/3 clinical trial with a 10% solution involving 334 patients was completed in June 2013.26 Results from this trial are expected in early 2015.
Lasers are a developing area for onychomycosis therapy and the appeal stems from their ability to selectively deliver energy to the target tissue, thus avoiding systemic side effects. Since 2010, the FDA has approved numerous laser devices for the temporary cosmetic improvement of onychomycosis, all of which are Nd:YAG 1064-nm lasers.27,28 It was previously thought that the mechanism of action for the fungicidal effect was achieved with heat,29 but newer in vitro studies have shown that the amount of time and level of heat required to kill Trichophyton rubrum would not be tolerable to patients.30 Although the mechanism of action is poorly understood, some clinical trials have shown success using the Nd:YAG 1064-nm laser for treatment of onychomycosis. However, in a study of 8 patients treated with the Nd:YAG 1064-nm laser for 5 treatment sessions, none had a mycological or clinical cure and there was only mild clinical improvement. In addition, most patients had pain and burning during the treatments requiring many short breaks.30 Although not yet FDA approved for the treatment of onychomycosis, other types of lasers are currently being studied, including CO2, near-infrared diode, and femtosecond-infrared laser systems.3
Plasma therapy is a developing area for the treatment of onychomycosis. Plasma was shown to be fungicidal to T rubrum in an in vitro model (MOE Medical Devices, written communication, July 2012), and a clinical trial to evaluate the safety, tolerability, and efficacy of plasma in human subjects is ongoing (registered on March 22, 2013, at www.clinicaltrials.gov with the identifier NCT01819051).
Onychomycosis is a common problem that increases in prevalence with advancing age. Oral terbinafine is considered the first-line treatment at this point in time.31 Two new topical agents, efinaconazole and tavaborole, were recently FDA approved and may be used for the treatment of toenail onychomycosis without the need for nail debridement. The Nd:YAG laser has shown some promise in earlier clinical studies but was ineffective in a more recent study.
1. Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
2. Heikkila H, Stubb S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995;133:699-703.
3. Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
4. Abdullah L, Abbas O. Common nail changes and disorders in older people: diagnosis and management. Can Fam Physician. 2011;57:173-181.
5. Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
6. Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28:151-159.
7. Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. part II. J Am Acad Dermatol. 1994;30:911-933.
8. Gupta AK, Paquet M, Simpson F, et al. Terbinafine in the treatment of dermatophyte toenail onychomycosis: a meta-analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol. 2013;27:267-272.
9. Drake LA, Shear NH, Arlette JP, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37:740-745.
10. Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. the LION Study Group. BMJ. 1999;318:1031-1035.
11. Sporanox [package insert]. Macquarie Park, Australia: Janssen-Cilag Pty Ltd; 2014.
12. Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6, pt 2):S77-S86.
13. Sigurgeirsson B, van Rossem K, Malahias S, et al. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J Am Acad Dermatol. 2013;69:416-425.
14. Elewski B, Pollak R, Ashton S, et al. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166:389-398.
15. Gupta AK, Leonardi C, Stoltz RR, et al. A phase I/II randomized, double-blind, placebo-controlled, dose-ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19:437-443.
16. Baran R, Sigurgeirsson B, de Berker D, et al. A multicentre, randomized, controlled study of the efficacy, safety and cost-effectiveness of a combination therapy with amorolfine nail lacquer and oral terbinafine compared with oral terbinafine alone for the treatment of onychomycosis with matrix involvement. Br J Dermatol. 2007;157:149-157.
17. Polak A. Preclinical data and mode of action of amorolfine. Dermatology. 1992;184(suppl 1):3-7.
18. Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350-358.
19. Lee RE, Liu TT, Barker KS, et al. Genome-wide expression profiling of the response to ciclopirox olamine in Candida albicans. J Antimicrob Chemother. 2005;55:655-662.
20. Penlac [package insert]. Bridgewater, NJ: sanofi-aventis; 2006.
21. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
22. Elewski BE, Ghannoum MA, Mayser P, et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J Eur Acad Dermatol Venereol. 2013;27:287-294.
23. Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
24. Hui X, Baker SJ, Wester RC, et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96:2622-2631.
25. Elewski BE, Rich P, Wiltz H, et al. Effectiveness and safety of tavaborole, a novel born-based molecule for the treatment of onychomycosis: results from two phase 3 studies. Poster presented at: Women’s & Pediatric Dermatology Seminar; October 4-6, 2013; Newport Beach, CA.
26. The solution study: Topica’s phase 2b/3 clinical trial. Topica Pharmaceuticals Inc Web site. http://www.
topicapharma.com/phase-2b3. Accessed December 2, 2014.
27. Gupta AK, Simpson FC. Medical devices for the treatment of onychomycosis. Dermatol Ther. 2012;25:574-581.
28. Ortiz AE, Avram MM, Wanner MA. A review of lasers and light for the treatment of onychomycosis. Lasers Surg Med. 2014;46:117-124.
29. Vural E, Winfield HL, Shingleton AW, et al. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci. 2008;23:349-353.
30. Carney C, Cantrell W, Warner J, et al. Treatment of onychomycosis using a submillisecond 1064-nm neodymium:yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69:578-582.
31. Gupta AK, Daigle D, Paquet M. Therapies for onychomycosis: a systematic review and network meta-analysis of mycological cure [published online ahead of print July 17, 2014]. J Am Podiatr Med Assoc. doi:10.7547/13-110.1.
1. Ghannoum MA, Hajjeh RA, Scher R, et al. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol. 2000;43:641-648.
2. Heikkila H, Stubb S. The prevalence of onychomycosis in Finland. Br J Dermatol. 1995;133:699-703.
3. Scher RK, Rich P, Pariser D, et al. The epidemiology, etiology, and pathophysiology of onychomycosis. Semin Cutan Med Surg. 2013;32(2, suppl 1):S2-S4.
4. Abdullah L, Abbas O. Common nail changes and disorders in older people: diagnosis and management. Can Fam Physician. 2011;57:173-181.
5. Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
6. Welsh O, Vera-Cabrera L, Welsh E. Onychomycosis. Clin Dermatol. 2010;28:151-159.
7. Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. part II. J Am Acad Dermatol. 1994;30:911-933.
8. Gupta AK, Paquet M, Simpson F, et al. Terbinafine in the treatment of dermatophyte toenail onychomycosis: a meta-analysis of efficacy for continuous and intermittent regimens. J Eur Acad Dermatol Venereol. 2013;27:267-272.
9. Drake LA, Shear NH, Arlette JP, et al. Oral terbinafine in the treatment of toenail onychomycosis: North American multicenter trial. J Am Acad Dermatol. 1997;37:740-745.
10. Evans EG, Sigurgeirsson B. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. the LION Study Group. BMJ. 1999;318:1031-1035.
11. Sporanox [package insert]. Macquarie Park, Australia: Janssen-Cilag Pty Ltd; 2014.
12. Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol. 1998;38(6, pt 2):S77-S86.
13. Sigurgeirsson B, van Rossem K, Malahias S, et al. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J Am Acad Dermatol. 2013;69:416-425.
14. Elewski B, Pollak R, Ashton S, et al. A randomized, placebo- and active-controlled, parallel-group, multicentre, investigator-blinded study of four treatment regimens of posaconazole in adults with toenail onychomycosis. Br J Dermatol. 2012;166:389-398.
15. Gupta AK, Leonardi C, Stoltz RR, et al. A phase I/II randomized, double-blind, placebo-controlled, dose-ranging study evaluating the efficacy, safety and pharmacokinetics of ravuconazole in the treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19:437-443.
16. Baran R, Sigurgeirsson B, de Berker D, et al. A multicentre, randomized, controlled study of the efficacy, safety and cost-effectiveness of a combination therapy with amorolfine nail lacquer and oral terbinafine compared with oral terbinafine alone for the treatment of onychomycosis with matrix involvement. Br J Dermatol. 2007;157:149-157.
17. Polak A. Preclinical data and mode of action of amorolfine. Dermatology. 1992;184(suppl 1):3-7.
18. Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350-358.
19. Lee RE, Liu TT, Barker KS, et al. Genome-wide expression profiling of the response to ciclopirox olamine in Candida albicans. J Antimicrob Chemother. 2005;55:655-662.
20. Penlac [package insert]. Bridgewater, NJ: sanofi-aventis; 2006.
21. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
22. Elewski BE, Ghannoum MA, Mayser P, et al. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J Eur Acad Dermatol Venereol. 2013;27:287-294.
23. Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
24. Hui X, Baker SJ, Wester RC, et al. In vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96:2622-2631.
25. Elewski BE, Rich P, Wiltz H, et al. Effectiveness and safety of tavaborole, a novel born-based molecule for the treatment of onychomycosis: results from two phase 3 studies. Poster presented at: Women’s & Pediatric Dermatology Seminar; October 4-6, 2013; Newport Beach, CA.
26. The solution study: Topica’s phase 2b/3 clinical trial. Topica Pharmaceuticals Inc Web site. http://www.
topicapharma.com/phase-2b3. Accessed December 2, 2014.
27. Gupta AK, Simpson FC. Medical devices for the treatment of onychomycosis. Dermatol Ther. 2012;25:574-581.
28. Ortiz AE, Avram MM, Wanner MA. A review of lasers and light for the treatment of onychomycosis. Lasers Surg Med. 2014;46:117-124.
29. Vural E, Winfield HL, Shingleton AW, et al. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci. 2008;23:349-353.
30. Carney C, Cantrell W, Warner J, et al. Treatment of onychomycosis using a submillisecond 1064-nm neodymium:yttrium-aluminum-garnet laser. J Am Acad Dermatol. 2013;69:578-582.
31. Gupta AK, Daigle D, Paquet M. Therapies for onychomycosis: a systematic review and network meta-analysis of mycological cure [published online ahead of print July 17, 2014]. J Am Podiatr Med Assoc. doi:10.7547/13-110.1.
Late-Onset Nevus Comedonicus on Both Eyelids With Hypothyroidism
To the Editor:
A 62-year-old woman was referred to the dermatology clinic for papules on both eyelids of 6 months’ duration. She underwent surgery for a thyroid gland adenoma 3 years prior and subsequently experienced hypothyroidism. Levothyroxine sodium was administered daily (100 µg initially; 50 µg over the last 1.5 years). Papules occurred on both eyelids 6 months prior to presentation and gradually increased in number. The center of each papule was filled with a black keratinous plug. The skin lesions became raised after the patient ate fatty foods. The lesions remained entirely asymptomatic and there was no family history of a similar disorder.
Physical examinations showed no systemic abnormalities. Dermatologic examination showed clustered 3- to 4-mm flesh-colored papules on both upper eyelids; the centers of the papules were filled with 1- to 2-mm black keratinous plugs (Figure 1A). Several similar skin lesions existed on the lower eyelids, nasal root, and right side of the nasal dorsum. On laboratory examination, the results of routine blood, urine, and stool tests, as well as renal and hepatic functions, electrolytes, and blood sugar levels, were within reference range. Indicators including triglyceride of 2.50 mmol/L (reference range, 0.40–1.90 mmol/L), total cholesterol of 6.31 mmol/L (reference range, 3.00–5.70 mmol/L), serum total thyroxine (T4) of 5.32 µg/dL (reference range, 6.09–12.23 µg/dL), total triiodothyronine (T3) of 64 ng/dL (reference range, 87–178 ng/dL), serum free thyroxine (FT4) of 0.41 ng/dL (reference range, 0.61–1.12 ng/dL), serum free triiodothyronine (FT3) of 182 pg/dL (reference range, 250–390 pg/dL), and thyrotropin of 33.75 µIU/mL (reference range, 0.34–5.60 µIU/mL) were not within reference range; however, thyroperoxidase antibodies, thyrotropin receptor antibodies, thyroglobulin antibodies, thyroglobulin, and calcitonin were within reference range. Color ultrasonography indicated post–subtotal resection of the bilateral thyroid glands.
|
Histopathologic analysis of the skin lesions showed that the epidermis became atrophic and thinner, and several atrophic and cyst-dilated follicular structures existed inside the dermis. Some structures opened through the epidermis; the walls were squamous epithelium and keratin filled the structures (Figure 2). The condition was diagnosed as nevus comedonicus (NC).
The patient was referred to the endocrinology department and treated with levothyroxine sodium (100 µg daily). At 5-month follow-up, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions had resolved (Figure 1B).
Nevus comedonicus is an unusual skin lesion with a predilection for the face, neck, shoulders, upper arms, and trunk. The clinical manifestations include comedonelike papules with centers that are characterized by large, black, solid keratinous plugs. When the plugs are peeled off, volcanic craterlike pits will be left. The skin lesions usually are ribbonlike and clustered on 1 side of the body. Pathologic examination often shows that the epidermis is pitted downward, and the dilated follicular ostia are plugged with keratin.1,2 Paige and Mendelson3 divided NC into 2 types: inflammatory and noninflammatory. Approximately half of NC patients experience cysts, abscesses, fistulae, and scars.4
The exact etiology of NC is unclear. Some researchers believe that it is a congenital hair follicle deformity; more specifically, that it is caused by a developmental defect in the hair follicles in the embryonic stage (ie, abnormal differentiation of epithelial stem cells that differentiate into follicles). Most incidences of NC occur at birth or before growth and development. However, few studies have reported late-onset NC.5
|
The relationship between NC and thyroid disease is unique. Clinical research has shown that hypothyroidism can result in hair loss and cracks.6 In animal experiments, hypothyroidism model mice often experienced degenerative changes of their hair follicles and hair papillae as well as changes in the telogen phase, such as thinning of the outer and inner root sheaths.7 Meanwhile, decreased cell proliferation activity in the hair follicles was observed. Therefore, it is reasonable to conclude that thyroid hormones have regulatory effects on the growth and development of hair follicles.7
A study on human hair follicles found that thyroid hormone receptor β1 is expressed in human hair follicles.8 Research on in vitro–cultured human hair follicles showed that thyroid hormones T3 and T4 upregulated the proliferation of hair matrix cells and downregulated their apoptosis. Thyroid hormones also prolonged the duration of the hair growth phase (anagen).9 Furthermore, expression of thyrotropin receptor was detected in human hair follicles. Because increased serum thyrotropin levels can lead to clinical hair loss, thyrotropin may inhibit the growth of hair follicles via thyrotropin receptor.10 In our patient, NC occurred on both eyelids when the patient experienced hypothyroidism following thyroid gland adenoma surgery. Following treatment with levothyroxine sodium, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions resolved. Therefore, the occurrence of NC may be related to hypothyroidism in this patient. The low thyroid hormone levels and elevated thyrotropin level possibly induced degenerative changes and injuries to the hair matrix cells, resulting in hair follicle obstruction and accumulation of keratin, which ultimately led to NC. However, the exact relationship between NC and thyroid diseases requires elucidation in future studies.
1. Engber PB. The nevus comedonicus syndrome: a case report with emphasis on associated internal manifestations. Int J Dermatol. 1978;17:745-749.
2. Kirtak N, Inaloz HS, Karakok M, et al. Extensive inflammatory nevus comedonicus involving half of the body. Int J Dermatol. 2004;43:434-436.
3. Paige TN, Mendelson CG. Bilateral nevus comedonicus. Arch Dermatol. 1967;96:172-175.
4. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
5. Ahn SY, Oh Y, Bak H, et al. Co-occurrence of nevus comedonicus with accessory breast tissue. Int J Dermatol. 2008;47:530-531.
6. Freinkel RK, Freinkel N. Hair growth and alopecia in hypothyroidism. Arch Dermatol. 1972;106:349-352.
7. Tsujio M, Yoshioka K, Satoh M, et al. Skin morphology of thyroidectomized rats. Vet Pathol. 2008;45:505-511.
8. Billoni N, Buan B, Gautier B, et al. Thyroid hormone receptor β1 is expressed in the human hair follicle. Br J Dermatol. 2000;142:645-652.
9. van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388.
10. Bodó E, Kromminga A, Bíró T, et al. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol. 2009;129:1126-1139.
To the Editor:
A 62-year-old woman was referred to the dermatology clinic for papules on both eyelids of 6 months’ duration. She underwent surgery for a thyroid gland adenoma 3 years prior and subsequently experienced hypothyroidism. Levothyroxine sodium was administered daily (100 µg initially; 50 µg over the last 1.5 years). Papules occurred on both eyelids 6 months prior to presentation and gradually increased in number. The center of each papule was filled with a black keratinous plug. The skin lesions became raised after the patient ate fatty foods. The lesions remained entirely asymptomatic and there was no family history of a similar disorder.
Physical examinations showed no systemic abnormalities. Dermatologic examination showed clustered 3- to 4-mm flesh-colored papules on both upper eyelids; the centers of the papules were filled with 1- to 2-mm black keratinous plugs (Figure 1A). Several similar skin lesions existed on the lower eyelids, nasal root, and right side of the nasal dorsum. On laboratory examination, the results of routine blood, urine, and stool tests, as well as renal and hepatic functions, electrolytes, and blood sugar levels, were within reference range. Indicators including triglyceride of 2.50 mmol/L (reference range, 0.40–1.90 mmol/L), total cholesterol of 6.31 mmol/L (reference range, 3.00–5.70 mmol/L), serum total thyroxine (T4) of 5.32 µg/dL (reference range, 6.09–12.23 µg/dL), total triiodothyronine (T3) of 64 ng/dL (reference range, 87–178 ng/dL), serum free thyroxine (FT4) of 0.41 ng/dL (reference range, 0.61–1.12 ng/dL), serum free triiodothyronine (FT3) of 182 pg/dL (reference range, 250–390 pg/dL), and thyrotropin of 33.75 µIU/mL (reference range, 0.34–5.60 µIU/mL) were not within reference range; however, thyroperoxidase antibodies, thyrotropin receptor antibodies, thyroglobulin antibodies, thyroglobulin, and calcitonin were within reference range. Color ultrasonography indicated post–subtotal resection of the bilateral thyroid glands.


|
Histopathologic analysis of the skin lesions showed that the epidermis became atrophic and thinner, and several atrophic and cyst-dilated follicular structures existed inside the dermis. Some structures opened through the epidermis; the walls were squamous epithelium and keratin filled the structures (Figure 2). The condition was diagnosed as nevus comedonicus (NC).
The patient was referred to the endocrinology department and treated with levothyroxine sodium (100 µg daily). At 5-month follow-up, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions had resolved (Figure 1B).
Nevus comedonicus is an unusual skin lesion with a predilection for the face, neck, shoulders, upper arms, and trunk. The clinical manifestations include comedonelike papules with centers that are characterized by large, black, solid keratinous plugs. When the plugs are peeled off, volcanic craterlike pits will be left. The skin lesions usually are ribbonlike and clustered on 1 side of the body. Pathologic examination often shows that the epidermis is pitted downward, and the dilated follicular ostia are plugged with keratin.1,2 Paige and Mendelson3 divided NC into 2 types: inflammatory and noninflammatory. Approximately half of NC patients experience cysts, abscesses, fistulae, and scars.4
The exact etiology of NC is unclear. Some researchers believe that it is a congenital hair follicle deformity; more specifically, that it is caused by a developmental defect in the hair follicles in the embryonic stage (ie, abnormal differentiation of epithelial stem cells that differentiate into follicles). Most incidences of NC occur at birth or before growth and development. However, few studies have reported late-onset NC.5
|
The relationship between NC and thyroid disease is unique. Clinical research has shown that hypothyroidism can result in hair loss and cracks.6 In animal experiments, hypothyroidism model mice often experienced degenerative changes of their hair follicles and hair papillae as well as changes in the telogen phase, such as thinning of the outer and inner root sheaths.7 Meanwhile, decreased cell proliferation activity in the hair follicles was observed. Therefore, it is reasonable to conclude that thyroid hormones have regulatory effects on the growth and development of hair follicles.7
A study on human hair follicles found that thyroid hormone receptor β1 is expressed in human hair follicles.8 Research on in vitro–cultured human hair follicles showed that thyroid hormones T3 and T4 upregulated the proliferation of hair matrix cells and downregulated their apoptosis. Thyroid hormones also prolonged the duration of the hair growth phase (anagen).9 Furthermore, expression of thyrotropin receptor was detected in human hair follicles. Because increased serum thyrotropin levels can lead to clinical hair loss, thyrotropin may inhibit the growth of hair follicles via thyrotropin receptor.10 In our patient, NC occurred on both eyelids when the patient experienced hypothyroidism following thyroid gland adenoma surgery. Following treatment with levothyroxine sodium, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions resolved. Therefore, the occurrence of NC may be related to hypothyroidism in this patient. The low thyroid hormone levels and elevated thyrotropin level possibly induced degenerative changes and injuries to the hair matrix cells, resulting in hair follicle obstruction and accumulation of keratin, which ultimately led to NC. However, the exact relationship between NC and thyroid diseases requires elucidation in future studies.
To the Editor:
A 62-year-old woman was referred to the dermatology clinic for papules on both eyelids of 6 months’ duration. She underwent surgery for a thyroid gland adenoma 3 years prior and subsequently experienced hypothyroidism. Levothyroxine sodium was administered daily (100 µg initially; 50 µg over the last 1.5 years). Papules occurred on both eyelids 6 months prior to presentation and gradually increased in number. The center of each papule was filled with a black keratinous plug. The skin lesions became raised after the patient ate fatty foods. The lesions remained entirely asymptomatic and there was no family history of a similar disorder.
Physical examinations showed no systemic abnormalities. Dermatologic examination showed clustered 3- to 4-mm flesh-colored papules on both upper eyelids; the centers of the papules were filled with 1- to 2-mm black keratinous plugs (Figure 1A). Several similar skin lesions existed on the lower eyelids, nasal root, and right side of the nasal dorsum. On laboratory examination, the results of routine blood, urine, and stool tests, as well as renal and hepatic functions, electrolytes, and blood sugar levels, were within reference range. Indicators including triglyceride of 2.50 mmol/L (reference range, 0.40–1.90 mmol/L), total cholesterol of 6.31 mmol/L (reference range, 3.00–5.70 mmol/L), serum total thyroxine (T4) of 5.32 µg/dL (reference range, 6.09–12.23 µg/dL), total triiodothyronine (T3) of 64 ng/dL (reference range, 87–178 ng/dL), serum free thyroxine (FT4) of 0.41 ng/dL (reference range, 0.61–1.12 ng/dL), serum free triiodothyronine (FT3) of 182 pg/dL (reference range, 250–390 pg/dL), and thyrotropin of 33.75 µIU/mL (reference range, 0.34–5.60 µIU/mL) were not within reference range; however, thyroperoxidase antibodies, thyrotropin receptor antibodies, thyroglobulin antibodies, thyroglobulin, and calcitonin were within reference range. Color ultrasonography indicated post–subtotal resection of the bilateral thyroid glands.


|
Histopathologic analysis of the skin lesions showed that the epidermis became atrophic and thinner, and several atrophic and cyst-dilated follicular structures existed inside the dermis. Some structures opened through the epidermis; the walls were squamous epithelium and keratin filled the structures (Figure 2). The condition was diagnosed as nevus comedonicus (NC).
The patient was referred to the endocrinology department and treated with levothyroxine sodium (100 µg daily). At 5-month follow-up, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions had resolved (Figure 1B).
Nevus comedonicus is an unusual skin lesion with a predilection for the face, neck, shoulders, upper arms, and trunk. The clinical manifestations include comedonelike papules with centers that are characterized by large, black, solid keratinous plugs. When the plugs are peeled off, volcanic craterlike pits will be left. The skin lesions usually are ribbonlike and clustered on 1 side of the body. Pathologic examination often shows that the epidermis is pitted downward, and the dilated follicular ostia are plugged with keratin.1,2 Paige and Mendelson3 divided NC into 2 types: inflammatory and noninflammatory. Approximately half of NC patients experience cysts, abscesses, fistulae, and scars.4
The exact etiology of NC is unclear. Some researchers believe that it is a congenital hair follicle deformity; more specifically, that it is caused by a developmental defect in the hair follicles in the embryonic stage (ie, abnormal differentiation of epithelial stem cells that differentiate into follicles). Most incidences of NC occur at birth or before growth and development. However, few studies have reported late-onset NC.5
|
The relationship between NC and thyroid disease is unique. Clinical research has shown that hypothyroidism can result in hair loss and cracks.6 In animal experiments, hypothyroidism model mice often experienced degenerative changes of their hair follicles and hair papillae as well as changes in the telogen phase, such as thinning of the outer and inner root sheaths.7 Meanwhile, decreased cell proliferation activity in the hair follicles was observed. Therefore, it is reasonable to conclude that thyroid hormones have regulatory effects on the growth and development of hair follicles.7
A study on human hair follicles found that thyroid hormone receptor β1 is expressed in human hair follicles.8 Research on in vitro–cultured human hair follicles showed that thyroid hormones T3 and T4 upregulated the proliferation of hair matrix cells and downregulated their apoptosis. Thyroid hormones also prolonged the duration of the hair growth phase (anagen).9 Furthermore, expression of thyrotropin receptor was detected in human hair follicles. Because increased serum thyrotropin levels can lead to clinical hair loss, thyrotropin may inhibit the growth of hair follicles via thyrotropin receptor.10 In our patient, NC occurred on both eyelids when the patient experienced hypothyroidism following thyroid gland adenoma surgery. Following treatment with levothyroxine sodium, the T4, T3, FT4, FT3, and thyrotropin levels were within reference range and most of the skin lesions resolved. Therefore, the occurrence of NC may be related to hypothyroidism in this patient. The low thyroid hormone levels and elevated thyrotropin level possibly induced degenerative changes and injuries to the hair matrix cells, resulting in hair follicle obstruction and accumulation of keratin, which ultimately led to NC. However, the exact relationship between NC and thyroid diseases requires elucidation in future studies.
1. Engber PB. The nevus comedonicus syndrome: a case report with emphasis on associated internal manifestations. Int J Dermatol. 1978;17:745-749.
2. Kirtak N, Inaloz HS, Karakok M, et al. Extensive inflammatory nevus comedonicus involving half of the body. Int J Dermatol. 2004;43:434-436.
3. Paige TN, Mendelson CG. Bilateral nevus comedonicus. Arch Dermatol. 1967;96:172-175.
4. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
5. Ahn SY, Oh Y, Bak H, et al. Co-occurrence of nevus comedonicus with accessory breast tissue. Int J Dermatol. 2008;47:530-531.
6. Freinkel RK, Freinkel N. Hair growth and alopecia in hypothyroidism. Arch Dermatol. 1972;106:349-352.
7. Tsujio M, Yoshioka K, Satoh M, et al. Skin morphology of thyroidectomized rats. Vet Pathol. 2008;45:505-511.
8. Billoni N, Buan B, Gautier B, et al. Thyroid hormone receptor β1 is expressed in the human hair follicle. Br J Dermatol. 2000;142:645-652.
9. van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388.
10. Bodó E, Kromminga A, Bíró T, et al. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol. 2009;129:1126-1139.
1. Engber PB. The nevus comedonicus syndrome: a case report with emphasis on associated internal manifestations. Int J Dermatol. 1978;17:745-749.
2. Kirtak N, Inaloz HS, Karakok M, et al. Extensive inflammatory nevus comedonicus involving half of the body. Int J Dermatol. 2004;43:434-436.
3. Paige TN, Mendelson CG. Bilateral nevus comedonicus. Arch Dermatol. 1967;96:172-175.
4. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: WB Saunders; 2006.
5. Ahn SY, Oh Y, Bak H, et al. Co-occurrence of nevus comedonicus with accessory breast tissue. Int J Dermatol. 2008;47:530-531.
6. Freinkel RK, Freinkel N. Hair growth and alopecia in hypothyroidism. Arch Dermatol. 1972;106:349-352.
7. Tsujio M, Yoshioka K, Satoh M, et al. Skin morphology of thyroidectomized rats. Vet Pathol. 2008;45:505-511.
8. Billoni N, Buan B, Gautier B, et al. Thyroid hormone receptor β1 is expressed in the human hair follicle. Br J Dermatol. 2000;142:645-652.
9. van Beek N, Bodó E, Kromminga A, et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381-4388.
10. Bodó E, Kromminga A, Bíró T, et al. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J Invest Dermatol. 2009;129:1126-1139.