User login
Digital Mindfulness Program May Reduce Anxiety in Patients With Chronic Obstructive Pulmonary Disease
TOPLINE:
An 8-week smartphone-based mindfulness program using audio-guided meditation reduced anxiety and improved emotional well-being in patients with chronic obstructive pulmonary disease (COPD), also providing relief from stress, anxiety, and dyspnea following each session.
METHODOLOGY:
- A considerable proportion of patients with COPD experience clinically significant anxiety and depressive symptoms; psychological interventions that are easy to implement as add-on treatments can alleviate these symptoms.
- In this pilot study, 30 patients (mean age, 62.68 y; 60.5% women) with COPD and subclinical symptoms of anxiety or depression were enrolled and allocated to an 8-week self-administered digital mindfulness-based intervention (n = 14) or the waitlist control (n = 16).
- Patients in the intervention group had an introductory face-to-face session, followed by daily smartphone audio-guided meditation adapted for patients with COPD. The waitlist group received the same intervention after the study period ended.
- The primary endpoints were the feasibility of the intervention and its effects on anxiety and depression symptoms at baseline, 4 weeks, and 8 weeks.
TAKEAWAY:
- Patients in the intervention group practiced mindfulness on 81.38% of the 56 intervention days.
- After 8 weeks, the intervention group showed a significant reduction in anxiety (P = .010) compared with the waitlist group; however, no significant improvement was observed for depression.
- Similarly, significant improvements were reported for emotional functioning (P = .004), but no significant reductions in perceived stress and hair cortisol levels were observed after 8 weeks.
- Significant reductions were reported for momentary subjective stress (P < .001), anxiety (P = .022), and dyspnea (P < .001) immediately after meditation sessions.
IN PRACTICE:
“The investigated self-administered digital MBI [mindfulness-based intervention], including brief 10- to 15-minute meditations, was feasible and holds potential as low-threshold add-on treatment to alleviate anxiety after 8 weeks and reduce momentary subjective stress, anxiety, and dyspnea in everyday life,” the study authors wrote.
SOURCE:
This study was led by Hannah Tschenett, Department of Clinical and Health Psychology, Faculty of Psychology, University of Vienna, Vienna, Austria, and was published online in Respiratory Research.
LIMITATIONS:
This study had several limitations including a small sample size, lack of a true control group, and potential selection bias due to recruitment from centers with patients already interested in mindfulness, which may have inflated adherence. Additionally, generalizability to all patients with COPD was limited, as many were either ineligible or declined to participate.
DISCLOSURES:
This study was funded by the Scientific Medical Fund of the City of Vienna and the Karl Landsteiner Institute (KLI) for Lung Research and Pulmonary Oncology. The KLI received funding from AstraZeneca, Boehringer Ingelheim, Chiesi, Linde plc, Menarini Pharma, Novartis, and Vivisol Austria. Three authors reported being employees of KLI or receiving lecture fees from some of these pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An 8-week smartphone-based mindfulness program using audio-guided meditation reduced anxiety and improved emotional well-being in patients with chronic obstructive pulmonary disease (COPD), also providing relief from stress, anxiety, and dyspnea following each session.
METHODOLOGY:
- A considerable proportion of patients with COPD experience clinically significant anxiety and depressive symptoms; psychological interventions that are easy to implement as add-on treatments can alleviate these symptoms.
- In this pilot study, 30 patients (mean age, 62.68 y; 60.5% women) with COPD and subclinical symptoms of anxiety or depression were enrolled and allocated to an 8-week self-administered digital mindfulness-based intervention (n = 14) or the waitlist control (n = 16).
- Patients in the intervention group had an introductory face-to-face session, followed by daily smartphone audio-guided meditation adapted for patients with COPD. The waitlist group received the same intervention after the study period ended.
- The primary endpoints were the feasibility of the intervention and its effects on anxiety and depression symptoms at baseline, 4 weeks, and 8 weeks.
TAKEAWAY:
- Patients in the intervention group practiced mindfulness on 81.38% of the 56 intervention days.
- After 8 weeks, the intervention group showed a significant reduction in anxiety (P = .010) compared with the waitlist group; however, no significant improvement was observed for depression.
- Similarly, significant improvements were reported for emotional functioning (P = .004), but no significant reductions in perceived stress and hair cortisol levels were observed after 8 weeks.
- Significant reductions were reported for momentary subjective stress (P < .001), anxiety (P = .022), and dyspnea (P < .001) immediately after meditation sessions.
IN PRACTICE:
“The investigated self-administered digital MBI [mindfulness-based intervention], including brief 10- to 15-minute meditations, was feasible and holds potential as low-threshold add-on treatment to alleviate anxiety after 8 weeks and reduce momentary subjective stress, anxiety, and dyspnea in everyday life,” the study authors wrote.
SOURCE:
This study was led by Hannah Tschenett, Department of Clinical and Health Psychology, Faculty of Psychology, University of Vienna, Vienna, Austria, and was published online in Respiratory Research.
LIMITATIONS:
This study had several limitations including a small sample size, lack of a true control group, and potential selection bias due to recruitment from centers with patients already interested in mindfulness, which may have inflated adherence. Additionally, generalizability to all patients with COPD was limited, as many were either ineligible or declined to participate.
DISCLOSURES:
This study was funded by the Scientific Medical Fund of the City of Vienna and the Karl Landsteiner Institute (KLI) for Lung Research and Pulmonary Oncology. The KLI received funding from AstraZeneca, Boehringer Ingelheim, Chiesi, Linde plc, Menarini Pharma, Novartis, and Vivisol Austria. Three authors reported being employees of KLI or receiving lecture fees from some of these pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An 8-week smartphone-based mindfulness program using audio-guided meditation reduced anxiety and improved emotional well-being in patients with chronic obstructive pulmonary disease (COPD), also providing relief from stress, anxiety, and dyspnea following each session.
METHODOLOGY:
- A considerable proportion of patients with COPD experience clinically significant anxiety and depressive symptoms; psychological interventions that are easy to implement as add-on treatments can alleviate these symptoms.
- In this pilot study, 30 patients (mean age, 62.68 y; 60.5% women) with COPD and subclinical symptoms of anxiety or depression were enrolled and allocated to an 8-week self-administered digital mindfulness-based intervention (n = 14) or the waitlist control (n = 16).
- Patients in the intervention group had an introductory face-to-face session, followed by daily smartphone audio-guided meditation adapted for patients with COPD. The waitlist group received the same intervention after the study period ended.
- The primary endpoints were the feasibility of the intervention and its effects on anxiety and depression symptoms at baseline, 4 weeks, and 8 weeks.
TAKEAWAY:
- Patients in the intervention group practiced mindfulness on 81.38% of the 56 intervention days.
- After 8 weeks, the intervention group showed a significant reduction in anxiety (P = .010) compared with the waitlist group; however, no significant improvement was observed for depression.
- Similarly, significant improvements were reported for emotional functioning (P = .004), but no significant reductions in perceived stress and hair cortisol levels were observed after 8 weeks.
- Significant reductions were reported for momentary subjective stress (P < .001), anxiety (P = .022), and dyspnea (P < .001) immediately after meditation sessions.
IN PRACTICE:
“The investigated self-administered digital MBI [mindfulness-based intervention], including brief 10- to 15-minute meditations, was feasible and holds potential as low-threshold add-on treatment to alleviate anxiety after 8 weeks and reduce momentary subjective stress, anxiety, and dyspnea in everyday life,” the study authors wrote.
SOURCE:
This study was led by Hannah Tschenett, Department of Clinical and Health Psychology, Faculty of Psychology, University of Vienna, Vienna, Austria, and was published online in Respiratory Research.
LIMITATIONS:
This study had several limitations including a small sample size, lack of a true control group, and potential selection bias due to recruitment from centers with patients already interested in mindfulness, which may have inflated adherence. Additionally, generalizability to all patients with COPD was limited, as many were either ineligible or declined to participate.
DISCLOSURES:
This study was funded by the Scientific Medical Fund of the City of Vienna and the Karl Landsteiner Institute (KLI) for Lung Research and Pulmonary Oncology. The KLI received funding from AstraZeneca, Boehringer Ingelheim, Chiesi, Linde plc, Menarini Pharma, Novartis, and Vivisol Austria. Three authors reported being employees of KLI or receiving lecture fees from some of these pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Are Your Patients With COPD Inhaling Eucalyptus Oil? Know the Risks
There’s been renewed interest in recent years for concentrated essential oils to replace or complement pharmaceutical treatments. This is especially concerning among patients with chronic obstructive pulmonary disease (COPD), who might be eager to turn to alternatives but are unaware that COPD increases sensitivity to lung irritants like essential oils.
Eucalyptus oil might be at or near the top of the essential oils list for these patients, given its storied history in both ancient and modern medicine for treating colds and respiratory illnesses. Its inclusion in the United States and European pharmacopoeias has also reinforced its legitimacy. And, today, patients are at risk of confusing the primary active ingredient in eucalyptus — the monoterpene 1,8-cineole (eucalyptol, which has been shown to reduce COPD exacerbations when used adjunctively) — with concentrated essential oils that can be purchased online and in stores here in the United States.
“The more potent active ingredient, eucalyptol (in capsule form), is approved in Germany — not the essential oil of eucalyptus, which contains other compounds. I recommend against using any sort of inhaled essential oils for patients with chronic respiratory illnesses, mainly because they are unregulated and unstandardized,” explained Ni-Chen Liang, MD, an integrative pulmonologist affiliated with Scripps Memorial Hospital Encinitas in Encinitas, California.
“The substances that come out when you create eucalyptus oil are a ‘gamash’ of all sorts of chemicals — some benign, some which taste good, and some that may be irritating or even dangerous,” said Neil Schachter, MD, pulmonologist and professor of medicine (pulmonary, critical care, and sleep medicine) at the Icahn School of Medicine at Mount Sinai, New York City.
“They can also produce volatile organic compounds (VOCs) related to their formulas, which contain fillers and other constituents,” Liang said.
Hidden Dangers
Eucalyptus oil was first used by Aboriginal Australians, who crushed the leaves for their antiseptic properties or steamed them for their expectorant activity. Today, eucalyptus oil can be found in mouthwash and soap, used topically to relieve pain or repel insects, or added to cleaning products due to its disinfectant properties.
However, inhalation via diffusers or directly from the bottle can trigger different respiratory reactions, including cough, wheezing, shortness of breath, as well as respiratory distress.
“The vapors contain oil, ie, fatty products that can be irritating in and of themselves,” said Schachter. “There are cases where people have inhaled these oils and developed lipid pneumonia, which is very hard to treat,” he said.
Anything inhaled into the lungs is a risk, said Juan Rojas, MD, assistant professor, Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at Rush University Medical Center in Chicago. Rojas compared inhaling essential oils to e-cigarettes, which, in addition to tobacco, contain a variety of chemicals and additives that cause a lung reaction in the short term and create inflammatory patterns in the medium and long term.
“Another problem is that when ingested, eucalyptus oil can be distressing to the gastrointestinal tract. In larger doses, it can actually have some neurological impact as well, including seizures,” said Kalilah L. Gates, MD, associate professor of medicine (pulmonary and critical care) and assistant dean of medical education at Northwestern Feinberg School of Medicine in Chicago.
Clinical trial data have also shown a significant association between long-term exposure to essential oils and cardiopulmonary effects such as increased heart rate and blood pressure and a decline in percentage predicted peak expiratory flow rate in healthy volunteers. In the study of 200 participants (who were homemakers), long-term exposure referred to daily hours (> 4/d) and the study period, which was 10 years.
About Eucalyptol
Eucalyptol is rapidly absorbed and quickly distributed throughout the bloodstream, which allows it to reach the bronchial system, where it is expelled by the lungs. It’s been shown in various preclinical studies to have anti-inflammatory, antioxidant, mucolytic, and bronchodilatory activity, as well as antimicrobial effects.
For the past decade, enteric-coated eucalyptol capsules containing 100 mg or 200 mg of 1,8-cineole have been available in Germany for adjunctive treatment of inflammatory respiratory disorders, including asthma and COPD. Due to its limited bioactivity, frequent administration is required.
Clinical evidence of eucalyptol’s effectiveness is somewhat limited. Findings from a 2009 double-blind, placebo-controlled, multicenter study also demonstrated that when used along with beta-agonists, anticholinergics, corticosteroids, or combinations in patients with stable COPD, severity and duration of exacerbations over 6 months were significantly decreased compared with placebo.
However, Liang was quick to point out that studies of oral eucalyptol preparations in pulmonary patients have not been robust enough.
“I haven’t been able to find anything written by a multitude of different authors, which, to me, is a red flag. We want naturally occurring substances to be well tested in multicenter studies across a variety of different patient populations outside of Germany to ensure that results are reproducible,” she said.
Rojas concurred. “Even with the data in Europe, I would say that the studies have been underpowered to support large-scale adoption or suggest that the active ingredient for patients with moderate or severe COPD could be considered an adjunctive therapy with traditional medications,” he said.
“It would be difficult for me to make a recommendation without knowing the full impact,” said Rojas.
Open Dialogue
Like many chronic diseases, it’s important to meet patients where they are, including their use of unapproved or unwise treatment strategies.
“More times than not, they’ve already figured out their triggers for worsening respiratory symptoms, what does and doesn’t work for them, and what predicts a good vs a bad day from a respiratory standpoint,” said Liang.
“There’s a lot of popularity and claims related to essential oil use, and ultimately, we need to partner to find healing modalities (which may or may not include essential oils) that are ultimately helpful and minimize harm,” she said.
Gates suggested that when it comes to eucalyptus essential oil vs eucalyptol, education of both patients and doctors is key.
“The issue is that we had a study showing that a particular component — the active ingredient of eucalyptus oil was isolated and put into the capsule form and showed benefit. And then we extrapolated and said, ‘well, let’s just take (or inhale) eucalyptus oil. It’s not the same thing,” she said.
“I feel that it’s my responsibility to make sure that patients have the information they need to make informed decisions. It’s about being willing to communicate and have open conversations about what they may be taking in addition to medications that I prescribe,” said Gates.
Liang, Schachter, Rojas, and Gates reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There’s been renewed interest in recent years for concentrated essential oils to replace or complement pharmaceutical treatments. This is especially concerning among patients with chronic obstructive pulmonary disease (COPD), who might be eager to turn to alternatives but are unaware that COPD increases sensitivity to lung irritants like essential oils.
Eucalyptus oil might be at or near the top of the essential oils list for these patients, given its storied history in both ancient and modern medicine for treating colds and respiratory illnesses. Its inclusion in the United States and European pharmacopoeias has also reinforced its legitimacy. And, today, patients are at risk of confusing the primary active ingredient in eucalyptus — the monoterpene 1,8-cineole (eucalyptol, which has been shown to reduce COPD exacerbations when used adjunctively) — with concentrated essential oils that can be purchased online and in stores here in the United States.
“The more potent active ingredient, eucalyptol (in capsule form), is approved in Germany — not the essential oil of eucalyptus, which contains other compounds. I recommend against using any sort of inhaled essential oils for patients with chronic respiratory illnesses, mainly because they are unregulated and unstandardized,” explained Ni-Chen Liang, MD, an integrative pulmonologist affiliated with Scripps Memorial Hospital Encinitas in Encinitas, California.
“The substances that come out when you create eucalyptus oil are a ‘gamash’ of all sorts of chemicals — some benign, some which taste good, and some that may be irritating or even dangerous,” said Neil Schachter, MD, pulmonologist and professor of medicine (pulmonary, critical care, and sleep medicine) at the Icahn School of Medicine at Mount Sinai, New York City.
“They can also produce volatile organic compounds (VOCs) related to their formulas, which contain fillers and other constituents,” Liang said.
Hidden Dangers
Eucalyptus oil was first used by Aboriginal Australians, who crushed the leaves for their antiseptic properties or steamed them for their expectorant activity. Today, eucalyptus oil can be found in mouthwash and soap, used topically to relieve pain or repel insects, or added to cleaning products due to its disinfectant properties.
However, inhalation via diffusers or directly from the bottle can trigger different respiratory reactions, including cough, wheezing, shortness of breath, as well as respiratory distress.
“The vapors contain oil, ie, fatty products that can be irritating in and of themselves,” said Schachter. “There are cases where people have inhaled these oils and developed lipid pneumonia, which is very hard to treat,” he said.
Anything inhaled into the lungs is a risk, said Juan Rojas, MD, assistant professor, Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at Rush University Medical Center in Chicago. Rojas compared inhaling essential oils to e-cigarettes, which, in addition to tobacco, contain a variety of chemicals and additives that cause a lung reaction in the short term and create inflammatory patterns in the medium and long term.
“Another problem is that when ingested, eucalyptus oil can be distressing to the gastrointestinal tract. In larger doses, it can actually have some neurological impact as well, including seizures,” said Kalilah L. Gates, MD, associate professor of medicine (pulmonary and critical care) and assistant dean of medical education at Northwestern Feinberg School of Medicine in Chicago.
Clinical trial data have also shown a significant association between long-term exposure to essential oils and cardiopulmonary effects such as increased heart rate and blood pressure and a decline in percentage predicted peak expiratory flow rate in healthy volunteers. In the study of 200 participants (who were homemakers), long-term exposure referred to daily hours (> 4/d) and the study period, which was 10 years.
About Eucalyptol
Eucalyptol is rapidly absorbed and quickly distributed throughout the bloodstream, which allows it to reach the bronchial system, where it is expelled by the lungs. It’s been shown in various preclinical studies to have anti-inflammatory, antioxidant, mucolytic, and bronchodilatory activity, as well as antimicrobial effects.
For the past decade, enteric-coated eucalyptol capsules containing 100 mg or 200 mg of 1,8-cineole have been available in Germany for adjunctive treatment of inflammatory respiratory disorders, including asthma and COPD. Due to its limited bioactivity, frequent administration is required.
Clinical evidence of eucalyptol’s effectiveness is somewhat limited. Findings from a 2009 double-blind, placebo-controlled, multicenter study also demonstrated that when used along with beta-agonists, anticholinergics, corticosteroids, or combinations in patients with stable COPD, severity and duration of exacerbations over 6 months were significantly decreased compared with placebo.
However, Liang was quick to point out that studies of oral eucalyptol preparations in pulmonary patients have not been robust enough.
“I haven’t been able to find anything written by a multitude of different authors, which, to me, is a red flag. We want naturally occurring substances to be well tested in multicenter studies across a variety of different patient populations outside of Germany to ensure that results are reproducible,” she said.
Rojas concurred. “Even with the data in Europe, I would say that the studies have been underpowered to support large-scale adoption or suggest that the active ingredient for patients with moderate or severe COPD could be considered an adjunctive therapy with traditional medications,” he said.
“It would be difficult for me to make a recommendation without knowing the full impact,” said Rojas.
Open Dialogue
Like many chronic diseases, it’s important to meet patients where they are, including their use of unapproved or unwise treatment strategies.
“More times than not, they’ve already figured out their triggers for worsening respiratory symptoms, what does and doesn’t work for them, and what predicts a good vs a bad day from a respiratory standpoint,” said Liang.
“There’s a lot of popularity and claims related to essential oil use, and ultimately, we need to partner to find healing modalities (which may or may not include essential oils) that are ultimately helpful and minimize harm,” she said.
Gates suggested that when it comes to eucalyptus essential oil vs eucalyptol, education of both patients and doctors is key.
“The issue is that we had a study showing that a particular component — the active ingredient of eucalyptus oil was isolated and put into the capsule form and showed benefit. And then we extrapolated and said, ‘well, let’s just take (or inhale) eucalyptus oil. It’s not the same thing,” she said.
“I feel that it’s my responsibility to make sure that patients have the information they need to make informed decisions. It’s about being willing to communicate and have open conversations about what they may be taking in addition to medications that I prescribe,” said Gates.
Liang, Schachter, Rojas, and Gates reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There’s been renewed interest in recent years for concentrated essential oils to replace or complement pharmaceutical treatments. This is especially concerning among patients with chronic obstructive pulmonary disease (COPD), who might be eager to turn to alternatives but are unaware that COPD increases sensitivity to lung irritants like essential oils.
Eucalyptus oil might be at or near the top of the essential oils list for these patients, given its storied history in both ancient and modern medicine for treating colds and respiratory illnesses. Its inclusion in the United States and European pharmacopoeias has also reinforced its legitimacy. And, today, patients are at risk of confusing the primary active ingredient in eucalyptus — the monoterpene 1,8-cineole (eucalyptol, which has been shown to reduce COPD exacerbations when used adjunctively) — with concentrated essential oils that can be purchased online and in stores here in the United States.
“The more potent active ingredient, eucalyptol (in capsule form), is approved in Germany — not the essential oil of eucalyptus, which contains other compounds. I recommend against using any sort of inhaled essential oils for patients with chronic respiratory illnesses, mainly because they are unregulated and unstandardized,” explained Ni-Chen Liang, MD, an integrative pulmonologist affiliated with Scripps Memorial Hospital Encinitas in Encinitas, California.
“The substances that come out when you create eucalyptus oil are a ‘gamash’ of all sorts of chemicals — some benign, some which taste good, and some that may be irritating or even dangerous,” said Neil Schachter, MD, pulmonologist and professor of medicine (pulmonary, critical care, and sleep medicine) at the Icahn School of Medicine at Mount Sinai, New York City.
“They can also produce volatile organic compounds (VOCs) related to their formulas, which contain fillers and other constituents,” Liang said.
Hidden Dangers
Eucalyptus oil was first used by Aboriginal Australians, who crushed the leaves for their antiseptic properties or steamed them for their expectorant activity. Today, eucalyptus oil can be found in mouthwash and soap, used topically to relieve pain or repel insects, or added to cleaning products due to its disinfectant properties.
However, inhalation via diffusers or directly from the bottle can trigger different respiratory reactions, including cough, wheezing, shortness of breath, as well as respiratory distress.
“The vapors contain oil, ie, fatty products that can be irritating in and of themselves,” said Schachter. “There are cases where people have inhaled these oils and developed lipid pneumonia, which is very hard to treat,” he said.
Anything inhaled into the lungs is a risk, said Juan Rojas, MD, assistant professor, Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine at Rush University Medical Center in Chicago. Rojas compared inhaling essential oils to e-cigarettes, which, in addition to tobacco, contain a variety of chemicals and additives that cause a lung reaction in the short term and create inflammatory patterns in the medium and long term.
“Another problem is that when ingested, eucalyptus oil can be distressing to the gastrointestinal tract. In larger doses, it can actually have some neurological impact as well, including seizures,” said Kalilah L. Gates, MD, associate professor of medicine (pulmonary and critical care) and assistant dean of medical education at Northwestern Feinberg School of Medicine in Chicago.
Clinical trial data have also shown a significant association between long-term exposure to essential oils and cardiopulmonary effects such as increased heart rate and blood pressure and a decline in percentage predicted peak expiratory flow rate in healthy volunteers. In the study of 200 participants (who were homemakers), long-term exposure referred to daily hours (> 4/d) and the study period, which was 10 years.
About Eucalyptol
Eucalyptol is rapidly absorbed and quickly distributed throughout the bloodstream, which allows it to reach the bronchial system, where it is expelled by the lungs. It’s been shown in various preclinical studies to have anti-inflammatory, antioxidant, mucolytic, and bronchodilatory activity, as well as antimicrobial effects.
For the past decade, enteric-coated eucalyptol capsules containing 100 mg or 200 mg of 1,8-cineole have been available in Germany for adjunctive treatment of inflammatory respiratory disorders, including asthma and COPD. Due to its limited bioactivity, frequent administration is required.
Clinical evidence of eucalyptol’s effectiveness is somewhat limited. Findings from a 2009 double-blind, placebo-controlled, multicenter study also demonstrated that when used along with beta-agonists, anticholinergics, corticosteroids, or combinations in patients with stable COPD, severity and duration of exacerbations over 6 months were significantly decreased compared with placebo.
However, Liang was quick to point out that studies of oral eucalyptol preparations in pulmonary patients have not been robust enough.
“I haven’t been able to find anything written by a multitude of different authors, which, to me, is a red flag. We want naturally occurring substances to be well tested in multicenter studies across a variety of different patient populations outside of Germany to ensure that results are reproducible,” she said.
Rojas concurred. “Even with the data in Europe, I would say that the studies have been underpowered to support large-scale adoption or suggest that the active ingredient for patients with moderate or severe COPD could be considered an adjunctive therapy with traditional medications,” he said.
“It would be difficult for me to make a recommendation without knowing the full impact,” said Rojas.
Open Dialogue
Like many chronic diseases, it’s important to meet patients where they are, including their use of unapproved or unwise treatment strategies.
“More times than not, they’ve already figured out their triggers for worsening respiratory symptoms, what does and doesn’t work for them, and what predicts a good vs a bad day from a respiratory standpoint,” said Liang.
“There’s a lot of popularity and claims related to essential oil use, and ultimately, we need to partner to find healing modalities (which may or may not include essential oils) that are ultimately helpful and minimize harm,” she said.
Gates suggested that when it comes to eucalyptus essential oil vs eucalyptol, education of both patients and doctors is key.
“The issue is that we had a study showing that a particular component — the active ingredient of eucalyptus oil was isolated and put into the capsule form and showed benefit. And then we extrapolated and said, ‘well, let’s just take (or inhale) eucalyptus oil. It’s not the same thing,” she said.
“I feel that it’s my responsibility to make sure that patients have the information they need to make informed decisions. It’s about being willing to communicate and have open conversations about what they may be taking in addition to medications that I prescribe,” said Gates.
Liang, Schachter, Rojas, and Gates reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Single Antiplatelet After TAVR Lowers Risk
Patients who received a single antiplatelet drug therapy — usually aspirin — after transcatheter aortic valve replacement (TAVR) had about half the risk of dying in the subsequent 6 months compared with patients who received dual antiplatelet drug therapy. The findings were similar in men and women and in patients with and without coronary artery disease.
“This is one of the first demonstrations in real-world data that single antiplatelet therapy is not only associated with a lower risk of bleeding but also lower mortality,” said lead author Francesco Pelliccia, MD, PhD, a cardiologist at Sapienza University in Rome, Italy. Mortality rates for those who received dual antiplatelet therapy increased steadily during the 6 months after the procedure, he reported at the Society for Cardiovascular Angiography and Interventions (SCAI) 2025 Scientific Sessions in Washington, DC.
Ischemic and major bleeding events were dramatically reduced in those receiving a single drug, according to a real-world study of 5514 patients undergoing TAVR at 20 centers. The centers participate in the Transfusion Requirements in Transcatheter Aortic Valve Implantation (TRITAVI) registry.
In the 6 months after the procedure, 2.4% of the 3197 patients who received a single antiplatelet drug died of any cause, as did 5.4% of 2317 patients who received two antiplatelet drugs (hazard ratio [HR], 1.65). Dual therapy was associated with a higher risk for death in both men (HR, 2.08) and women (HR, 1.53). Risk for death was also higher in patients with coronary artery disease (HR, 1.83) and without coronary artery disease (HR, 1.52). All results were statistically significant.
Balancing Risks and Benefits
The popularity of TAVR, which was introduced in 2002, has grown to the point that, in 2019, it surpassed the use of surgical aortic valve replacement. But the procedure is associated with an increased risk for both thrombosis and bleeding. Antiplatelet therapy with aspirin and clopidogrel helps prevent thrombosis but can increase the risk of bleeding. This has led to a debate about the best balance for antiplatelet therapy after TAVR with either single therapy — usually with aspirin — or dual therapy with both aspirin and clopidogrel.
A series of studies have addressed this problem. Dual therapy did not show any benefits over single therapy in terms of major adverse cardiac and cerebrovascular events in a 2011 small randomized study. A 2014 small randomized study also showed no benefit for morbidity or mortality from dual therapy. A larger 2017 randomized trial showed that single therapy reduced the risk for major or life-threatening events but did not increase the risk for myocardial infarction or stroke.
Bleeding and bleeding plus thromboembolic events were significantly lower with aspirin than with aspirin plus clopidogrel after a year’s follow-up in the 2020 POPular TAVI trial. Findings from three of these trials were pooled in a 2018 meta-analysis, which showed that dual therapy increased the risk for major adverse events after TAVR and did not prevent ischemic events any more than single therapy.
Based on this evidence, many centers changed their practice. And current European guidelines recommend a single antiplatelet drug for patients undergoing TAVR who do not have additional indications for oral anticoagulation therapy.
By the Numbers
Randomized trials are generally considered the best evidence for medical questions such as this one. “But randomized trials often do not reflect real-world reality. We have to look at what really happens,” Pelliccia said.
Retrospective data from registries can also provide large numbers of patients; in this case, TRITAVI provided data on thousands of patients rather than the hundreds examined in combined randomized trials.
“The results, for the first time, provide clinicians more information on how to treat their patients who are at high risk for bleeding and provide evidence that single antiplatelet therapy should be considered the standard of care in all patients undergoing TAVR,” Pelliccia said.
A version of this article first appeared on Medscape.com.
Patients who received a single antiplatelet drug therapy — usually aspirin — after transcatheter aortic valve replacement (TAVR) had about half the risk of dying in the subsequent 6 months compared with patients who received dual antiplatelet drug therapy. The findings were similar in men and women and in patients with and without coronary artery disease.
“This is one of the first demonstrations in real-world data that single antiplatelet therapy is not only associated with a lower risk of bleeding but also lower mortality,” said lead author Francesco Pelliccia, MD, PhD, a cardiologist at Sapienza University in Rome, Italy. Mortality rates for those who received dual antiplatelet therapy increased steadily during the 6 months after the procedure, he reported at the Society for Cardiovascular Angiography and Interventions (SCAI) 2025 Scientific Sessions in Washington, DC.
Ischemic and major bleeding events were dramatically reduced in those receiving a single drug, according to a real-world study of 5514 patients undergoing TAVR at 20 centers. The centers participate in the Transfusion Requirements in Transcatheter Aortic Valve Implantation (TRITAVI) registry.
In the 6 months after the procedure, 2.4% of the 3197 patients who received a single antiplatelet drug died of any cause, as did 5.4% of 2317 patients who received two antiplatelet drugs (hazard ratio [HR], 1.65). Dual therapy was associated with a higher risk for death in both men (HR, 2.08) and women (HR, 1.53). Risk for death was also higher in patients with coronary artery disease (HR, 1.83) and without coronary artery disease (HR, 1.52). All results were statistically significant.
Balancing Risks and Benefits
The popularity of TAVR, which was introduced in 2002, has grown to the point that, in 2019, it surpassed the use of surgical aortic valve replacement. But the procedure is associated with an increased risk for both thrombosis and bleeding. Antiplatelet therapy with aspirin and clopidogrel helps prevent thrombosis but can increase the risk of bleeding. This has led to a debate about the best balance for antiplatelet therapy after TAVR with either single therapy — usually with aspirin — or dual therapy with both aspirin and clopidogrel.
A series of studies have addressed this problem. Dual therapy did not show any benefits over single therapy in terms of major adverse cardiac and cerebrovascular events in a 2011 small randomized study. A 2014 small randomized study also showed no benefit for morbidity or mortality from dual therapy. A larger 2017 randomized trial showed that single therapy reduced the risk for major or life-threatening events but did not increase the risk for myocardial infarction or stroke.
Bleeding and bleeding plus thromboembolic events were significantly lower with aspirin than with aspirin plus clopidogrel after a year’s follow-up in the 2020 POPular TAVI trial. Findings from three of these trials were pooled in a 2018 meta-analysis, which showed that dual therapy increased the risk for major adverse events after TAVR and did not prevent ischemic events any more than single therapy.
Based on this evidence, many centers changed their practice. And current European guidelines recommend a single antiplatelet drug for patients undergoing TAVR who do not have additional indications for oral anticoagulation therapy.
By the Numbers
Randomized trials are generally considered the best evidence for medical questions such as this one. “But randomized trials often do not reflect real-world reality. We have to look at what really happens,” Pelliccia said.
Retrospective data from registries can also provide large numbers of patients; in this case, TRITAVI provided data on thousands of patients rather than the hundreds examined in combined randomized trials.
“The results, for the first time, provide clinicians more information on how to treat their patients who are at high risk for bleeding and provide evidence that single antiplatelet therapy should be considered the standard of care in all patients undergoing TAVR,” Pelliccia said.
A version of this article first appeared on Medscape.com.
Patients who received a single antiplatelet drug therapy — usually aspirin — after transcatheter aortic valve replacement (TAVR) had about half the risk of dying in the subsequent 6 months compared with patients who received dual antiplatelet drug therapy. The findings were similar in men and women and in patients with and without coronary artery disease.
“This is one of the first demonstrations in real-world data that single antiplatelet therapy is not only associated with a lower risk of bleeding but also lower mortality,” said lead author Francesco Pelliccia, MD, PhD, a cardiologist at Sapienza University in Rome, Italy. Mortality rates for those who received dual antiplatelet therapy increased steadily during the 6 months after the procedure, he reported at the Society for Cardiovascular Angiography and Interventions (SCAI) 2025 Scientific Sessions in Washington, DC.
Ischemic and major bleeding events were dramatically reduced in those receiving a single drug, according to a real-world study of 5514 patients undergoing TAVR at 20 centers. The centers participate in the Transfusion Requirements in Transcatheter Aortic Valve Implantation (TRITAVI) registry.
In the 6 months after the procedure, 2.4% of the 3197 patients who received a single antiplatelet drug died of any cause, as did 5.4% of 2317 patients who received two antiplatelet drugs (hazard ratio [HR], 1.65). Dual therapy was associated with a higher risk for death in both men (HR, 2.08) and women (HR, 1.53). Risk for death was also higher in patients with coronary artery disease (HR, 1.83) and without coronary artery disease (HR, 1.52). All results were statistically significant.
Balancing Risks and Benefits
The popularity of TAVR, which was introduced in 2002, has grown to the point that, in 2019, it surpassed the use of surgical aortic valve replacement. But the procedure is associated with an increased risk for both thrombosis and bleeding. Antiplatelet therapy with aspirin and clopidogrel helps prevent thrombosis but can increase the risk of bleeding. This has led to a debate about the best balance for antiplatelet therapy after TAVR with either single therapy — usually with aspirin — or dual therapy with both aspirin and clopidogrel.
A series of studies have addressed this problem. Dual therapy did not show any benefits over single therapy in terms of major adverse cardiac and cerebrovascular events in a 2011 small randomized study. A 2014 small randomized study also showed no benefit for morbidity or mortality from dual therapy. A larger 2017 randomized trial showed that single therapy reduced the risk for major or life-threatening events but did not increase the risk for myocardial infarction or stroke.
Bleeding and bleeding plus thromboembolic events were significantly lower with aspirin than with aspirin plus clopidogrel after a year’s follow-up in the 2020 POPular TAVI trial. Findings from three of these trials were pooled in a 2018 meta-analysis, which showed that dual therapy increased the risk for major adverse events after TAVR and did not prevent ischemic events any more than single therapy.
Based on this evidence, many centers changed their practice. And current European guidelines recommend a single antiplatelet drug for patients undergoing TAVR who do not have additional indications for oral anticoagulation therapy.
By the Numbers
Randomized trials are generally considered the best evidence for medical questions such as this one. “But randomized trials often do not reflect real-world reality. We have to look at what really happens,” Pelliccia said.
Retrospective data from registries can also provide large numbers of patients; in this case, TRITAVI provided data on thousands of patients rather than the hundreds examined in combined randomized trials.
“The results, for the first time, provide clinicians more information on how to treat their patients who are at high risk for bleeding and provide evidence that single antiplatelet therapy should be considered the standard of care in all patients undergoing TAVR,” Pelliccia said.
A version of this article first appeared on Medscape.com.
FROM SCAI 2025
Advances in Blood Cancer Care for Veterans
Advances in Blood Cancer Care for Veterans
Click to view more from Cancer Data Trends 2025.
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
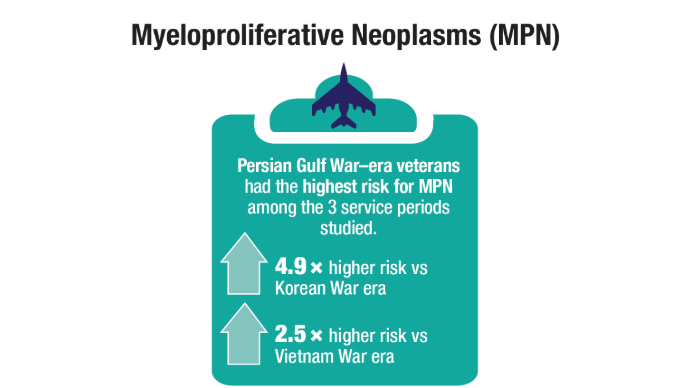
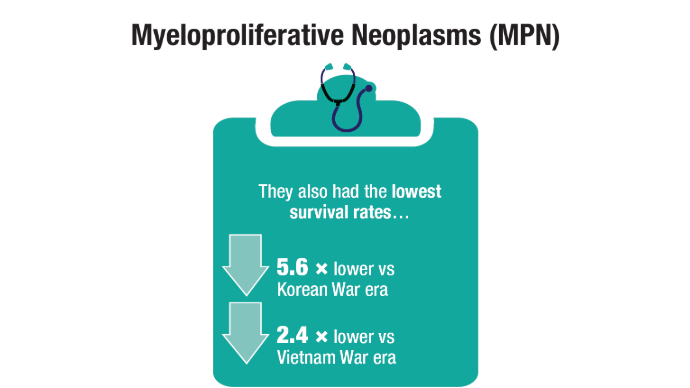
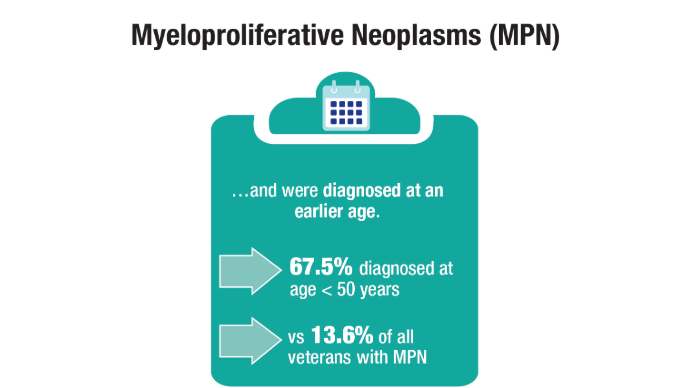
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
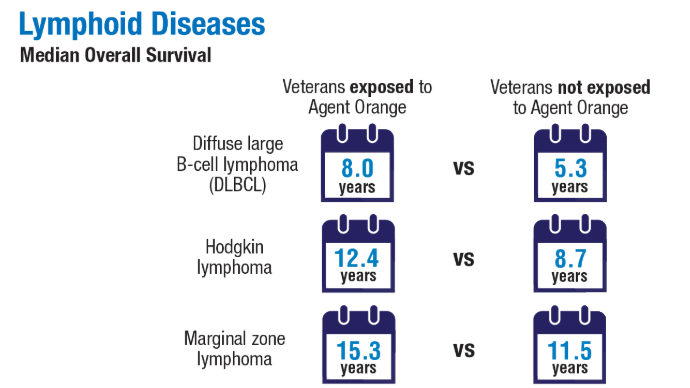
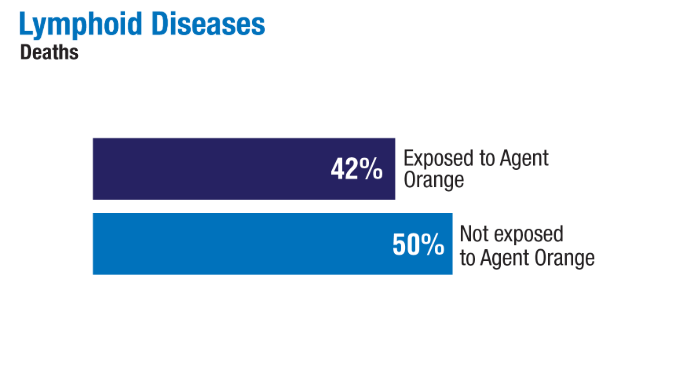
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
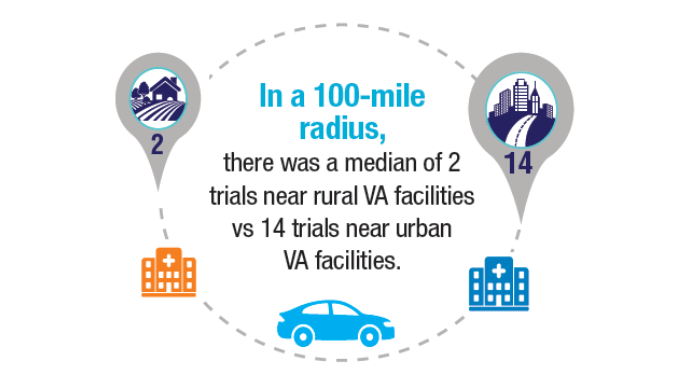
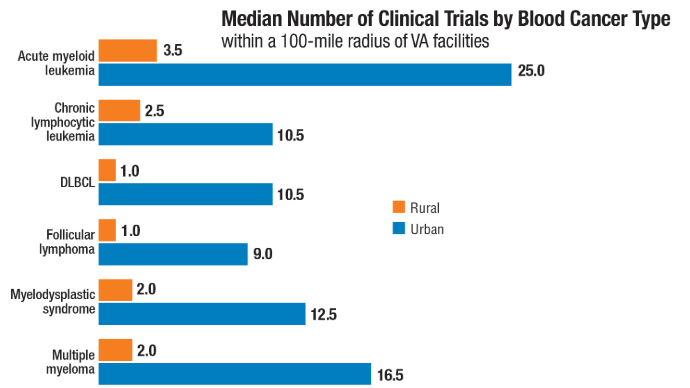
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
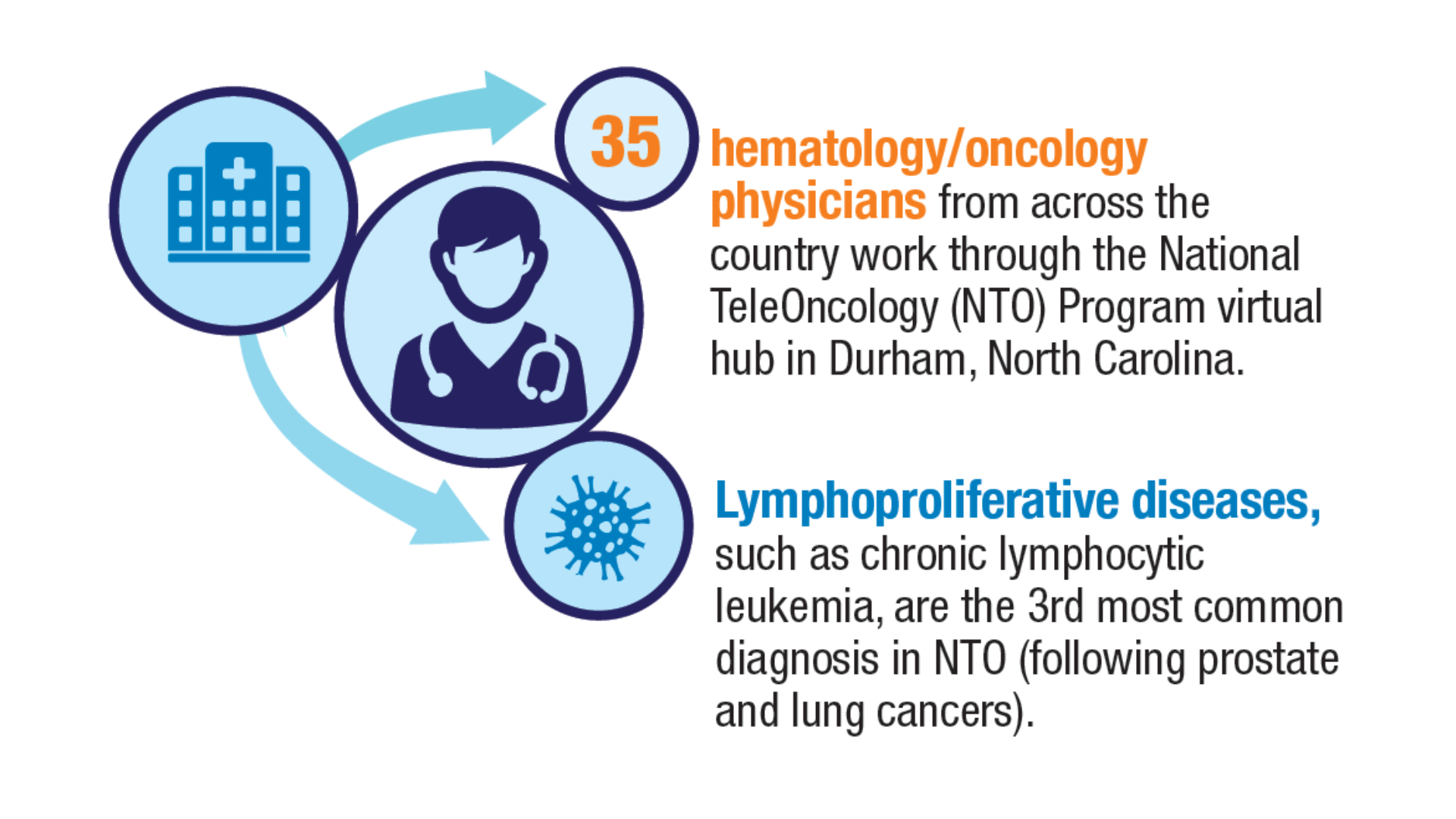
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
Click to view more from Cancer Data Trends 2025.
Click to view more from Cancer Data Trends 2025.
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
- Li W, ed. The 5th Edition of the World Health Organization Classification of
Hematolymphoid Tumors. In: Leukemia [Internet]. Brisbane (AU): Exon Publications;
October 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK586208/ - Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with
hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin
Oncol. 2020;47(1):94-101. doi:10.1053/j.seminoncol.2020.02.010. - Tiu A, McKinnell Z, Liu S, et al. Risk of myeloproliferative neoplasms among
U.S. Veterans from Korean, Vietnam, and Persian Gulf War eras. Am J Hematol.
2024;99(10):1969-1978. doi:10.1002/ajh.27438 - Ma H, Wan JY, Cortessis VK, Gupta P, Cozen W. Survival in Agent Orange
exposed and unexposed Vietnam-era veterans who were diagnosed with
lymphoid malignancies. Blood Adv. 2024;8(4):1037-1041. doi:10.1182/
bloodadvances.2023011999 - Friedman DR, Rodgers TD, Kovalick C, Yellapragada S, Szumita L, Weiss ES. Veterans
with blood cancers: Clinical trial navigation and the challenge of rurality. J Rural
Health. 2024;40(1):114-120. doi:10.1111/jrh.12773 - Parikh DA, Rodgers TD, Passero VA, et al. Teleoncology in the Veterans Health
Administration: Models of Care and the Veteran Experience. Am Soc Clin Oncol Educ
Book. 2024;44(3):e100042. doi:10.1200/EDBK_100042 - Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RVL. Technological
advancements in cancer diagnostics: Improvements and limitations. Cancer Rep
(Hoboken). 2023;6(2):e1764. doi:10.1002/cnr2.1764
Advances in Blood Cancer Care for Veterans
Advances in Blood Cancer Care for Veterans
What Hematologists Should Know About Cutaneous Porphyria and Hemochromatosis
One patient, a 39-year-old woman, went to a dermatologist seeking care for fluid-filled blisters over the backs of her hands and arms. Another patient, a 56-year-old man, sought care from his general practitioner owing to fatigue.
Their presentations were quite different, but the two patients shared one thing in common: iron overload. Both ended up in the care of hematologists who diagnosed their conditions as porphyria cutanea tarda (PCT) and hemochromatosis, respectively.
A pair of hematologists discussed the treatment of these disorders at the American Society of Hematology (ASH) 2024 Annual Meeting and in reports in Hematology: American Society of Hematology Education Program. Here’s a look at the guidance they provided.
Porphyria Cutanea Tarda: Skin Trouble
Testing revealed that the female patient had a highly elevated porphyrin levels: Her urine uroporphyrin was 3959 nmol/L (normal, < 30 nmol/L) and plasma uroporphyrin was 2.0 µg/dL (normal, < 1.0 µg/dL). Her serum ferritin level was also high, at 420 ng/mL (normal, < 200 ng/mL).
Rebecca Karp Leaf, MD, of Massachusetts General Hospital and Harvard Medical School, diagnosed her with PCT, a disorder of heme biosynthesis that often presents with skin manifestations.
As co-founder and co-director of the Boston hospital’s Porphyria Center, Karp Leaf is a leading expert in PCT, a rare disease that affects 5-10 people per 100,000. In addition to speaking at the ASH meeting in December, she described PCT in a December 2024 article in Hematology: American Society of Hematology Education Program.
PCT is caused by inhibition of an enzyme in heme biosynthesis and leads to accumulation of porphyrins in the liver and plasma, Karp Leaf said. Through a complex process, excess of iron leads to inhibition of the enzyme, which leads to a buildup of toxic porphyrins, she said. The condition causes painless, blistering lesions on sun-exposed skin, scarring, skin fragility, dark urine, and liver disease.
PCT most commonly occurs in middle age after the age of 40 and affects men more than women. “It’s the only porphyria that can occur absent a genetic variant,” she said, and 75% of cases have no genetic component.
Options for Treatment Include Antivirals and Phlebotomy
Risk factors for PCT include alcohol use, smoking, exogenous estrogen, hepatitis, and HIV mutations.
In regard to treatment, “modification of risk factors can be variably helpful: alcohol and smoking cessation, stopping exogenous estrogen, sun-protective clothing, and steroid-containing creams for lesions,” Karp Leaf said. “Most patients typically require further therapy to reduce liver porphyrins.”
Urine and plasma tests can help with diagnosis, she said. In patients with hepatitis C (HCV), “direct-acting antivirals can actually lead to resolution of PCT without any other therapy. We suspect that with effective antiviral treatment for HCV, the incidence of PCT will really go down.”
Therapeutic phlebotomy — blood removal — is another option. “It’s one of my favorite therapies because you don’t have to give somebody a drug. You can just take out iron,” Karp Leaf said. “Typically, we’ll start with venesection of 450 ccs of whole blood every 2 weeks, We target a ferritin level of 20 [ng/mL] but permit it up to 50 [ng/mL], or a little bit higher.”
The treatment leads to resolution of blisters in about 2-3 months, she said, and normalization of porphyrins by 13 months. Patients typically require about 6-8 treatments, she said.
Another option is iron chelation, iron removal via medicine, “but it’s expensive, has side effects, and is really not recommended if other treatments are available,” she said.
Hydroxychloroquine Can Be Helpful Too
Low-dose hydroxychloroquine can also be effective at 100 mg twice a week, “much lower than what we use in autoimmune disease,” Karp Leaf said. “We suspect that it’s taken up by the hepatic lysosomes and causes release of porphyrins. It causes clinical remission in about 6 months.”
However, higher doses can lead to liver injury, and the drug’s use is limited in end-stage kidney disease since porphyrins are excreted in the urine. These patients are especially difficult to treat, she said.
In the case of the 39-year-old patient, Karp Leaf recommended that the woman reduce her alcohol intake and begin using a copper intrauterine device for contraception instead of a combined oral contraceptive pill, which allowed her to undergo phlebotomy.
“She needed about eight sessions of therapeutic phlebotomy to achieve a ferritin of 30 [ng/mL], and her lesions resolved in 6 months,” Karp Leaf said. “Her plasma porphyrins resolved by 12 months. Her liver biochemistries were a bit elevated, and they subsequently normalized.”
Karp Leaf said she sees the patient about once a year.
Hemochromatosis: It’s (Probably) a Family Affair
In an adjoining presentation at ASH and in a December 2024 article in Hematology: American Society of Hematology Education Program, hematologist Domenico Girelli, MD, PhD, with the University of Verona, Italy, told colleagues about the 56-year-old male patient with fatigue. He also had a mildly enlarged liver, hyperferritinemia (890 µg/L vs normal value < 300 µg/L) and a mildly increased alanine aminotransferase level (46 U/L vs normal value < 40 U/L).
The patient was diagnosed with hemochromatosis, a genetic disorder caused by mutations that leads to increased transferrin saturation, Girelli said.
“By definition, hemochromatosis is characterized by the absence of signs of a primary red blood cell disorder — different from other disorders like transfusion iron overload or iron-loading anemias,” he said.
It’s also important to consider other possible causes of hyperferritinemia, because most cases of the symptom aren’t related to iron overload, he said. “A careful clinical history and a few laboratory parameters including transferrin saturation are generally sufficient for the differential diagnosis.”
As Girelli noted, “hemochromatosis can have a wide clinical spectrum ranging from mild to severe forms, which are strongly influenced by the co-presence of risk factors like alcohol [use], blood transfusion, and genetic factors captured by polygenic risk score.”
In Many Cases, Hemochromatosis Can Be Successfully Treated
According to Girelli, it’s important to understand the disease stage, because this information can predict the probability of advanced liver fibrosis, which can be a sign of a worse prognosis.
“The strongest clinical predictors of advanced liver fibrosis are ferritin higher than 1000 [µg/L] and the presence of arthropathy [joint disease],” he said. “If both are absent and the patient is asymptomatic, there is no need for further investigation. If both are present, further investigation — including cardiac MRI and full endocrine profile — are indicated. Liver biopsy may be indicated only in uncertain cases.”
Fortunately, “most patients are diagnosed in preclinical or early stage, and their prognosis is excellent, with a normal life expectancy,” he said
Phlebotomy remains the standard of care for hemochromatosis in uncomplicated cases. “It is safe, cheap, well-tolerated, and significantly reduces mortality and morbidity, especially when it is started before the development of cirrhosis,” he said.
Family Members Should Be Tested for Genetic Traits
It’s important to advise patients prior to phlebotomy to avoid undercooked seafood and wound contact with sea water because of the risk for sepsis due to the pathogen Vibrio vulnificus, Girelli said.
And it’s a good idea to test family members to see if they share a genetic risk for hemochromatosis, he said. The 56-year-old patient’s brother turned out to also have genetic risk, and his iron levels were very high. He had recently been diagnosed with seronegative arthritis that could be classified as secondary to hemochromatosis.
For management, Girelli said, patients should minimize or avoid alcohol consumption, eat a healthy diet, and avoid vitamin C and iron supplements even in multivitamin compounds. Patients should be encouraged to exercise and maintain an ideal weight.
The 56-year-old patient fared well, reaching a ferritin target of 50 mg/mL after multiple phlebotomy procedures that removed nearly 5 g of iron.
The patient tolerated the treatment and his fatigue resolved, Girelli said. “The maintenance treatment consisted of 3 phlebotomies per year. The patient remained asymptomatic and was eventually enrolled as a regular blood donor.”
Karp Leaf disclosed relationships with Alnylam, Recordati, and Disc Medicine. She is a member of the Porphyrias Consortium, part of the Rare Diseases Clinical Research Network, funded by the National Institutes of Health and led by the National Center for Advancing Translational Sciences (NCATS). The consortium is funded by NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases. Girelli had no disclosures.
A version of this article first appeared on Medscape.com.
One patient, a 39-year-old woman, went to a dermatologist seeking care for fluid-filled blisters over the backs of her hands and arms. Another patient, a 56-year-old man, sought care from his general practitioner owing to fatigue.
Their presentations were quite different, but the two patients shared one thing in common: iron overload. Both ended up in the care of hematologists who diagnosed their conditions as porphyria cutanea tarda (PCT) and hemochromatosis, respectively.
A pair of hematologists discussed the treatment of these disorders at the American Society of Hematology (ASH) 2024 Annual Meeting and in reports in Hematology: American Society of Hematology Education Program. Here’s a look at the guidance they provided.
Porphyria Cutanea Tarda: Skin Trouble
Testing revealed that the female patient had a highly elevated porphyrin levels: Her urine uroporphyrin was 3959 nmol/L (normal, < 30 nmol/L) and plasma uroporphyrin was 2.0 µg/dL (normal, < 1.0 µg/dL). Her serum ferritin level was also high, at 420 ng/mL (normal, < 200 ng/mL).
Rebecca Karp Leaf, MD, of Massachusetts General Hospital and Harvard Medical School, diagnosed her with PCT, a disorder of heme biosynthesis that often presents with skin manifestations.
As co-founder and co-director of the Boston hospital’s Porphyria Center, Karp Leaf is a leading expert in PCT, a rare disease that affects 5-10 people per 100,000. In addition to speaking at the ASH meeting in December, she described PCT in a December 2024 article in Hematology: American Society of Hematology Education Program.
PCT is caused by inhibition of an enzyme in heme biosynthesis and leads to accumulation of porphyrins in the liver and plasma, Karp Leaf said. Through a complex process, excess of iron leads to inhibition of the enzyme, which leads to a buildup of toxic porphyrins, she said. The condition causes painless, blistering lesions on sun-exposed skin, scarring, skin fragility, dark urine, and liver disease.
PCT most commonly occurs in middle age after the age of 40 and affects men more than women. “It’s the only porphyria that can occur absent a genetic variant,” she said, and 75% of cases have no genetic component.
Options for Treatment Include Antivirals and Phlebotomy
Risk factors for PCT include alcohol use, smoking, exogenous estrogen, hepatitis, and HIV mutations.
In regard to treatment, “modification of risk factors can be variably helpful: alcohol and smoking cessation, stopping exogenous estrogen, sun-protective clothing, and steroid-containing creams for lesions,” Karp Leaf said. “Most patients typically require further therapy to reduce liver porphyrins.”
Urine and plasma tests can help with diagnosis, she said. In patients with hepatitis C (HCV), “direct-acting antivirals can actually lead to resolution of PCT without any other therapy. We suspect that with effective antiviral treatment for HCV, the incidence of PCT will really go down.”
Therapeutic phlebotomy — blood removal — is another option. “It’s one of my favorite therapies because you don’t have to give somebody a drug. You can just take out iron,” Karp Leaf said. “Typically, we’ll start with venesection of 450 ccs of whole blood every 2 weeks, We target a ferritin level of 20 [ng/mL] but permit it up to 50 [ng/mL], or a little bit higher.”
The treatment leads to resolution of blisters in about 2-3 months, she said, and normalization of porphyrins by 13 months. Patients typically require about 6-8 treatments, she said.
Another option is iron chelation, iron removal via medicine, “but it’s expensive, has side effects, and is really not recommended if other treatments are available,” she said.
Hydroxychloroquine Can Be Helpful Too
Low-dose hydroxychloroquine can also be effective at 100 mg twice a week, “much lower than what we use in autoimmune disease,” Karp Leaf said. “We suspect that it’s taken up by the hepatic lysosomes and causes release of porphyrins. It causes clinical remission in about 6 months.”
However, higher doses can lead to liver injury, and the drug’s use is limited in end-stage kidney disease since porphyrins are excreted in the urine. These patients are especially difficult to treat, she said.
In the case of the 39-year-old patient, Karp Leaf recommended that the woman reduce her alcohol intake and begin using a copper intrauterine device for contraception instead of a combined oral contraceptive pill, which allowed her to undergo phlebotomy.
“She needed about eight sessions of therapeutic phlebotomy to achieve a ferritin of 30 [ng/mL], and her lesions resolved in 6 months,” Karp Leaf said. “Her plasma porphyrins resolved by 12 months. Her liver biochemistries were a bit elevated, and they subsequently normalized.”
Karp Leaf said she sees the patient about once a year.
Hemochromatosis: It’s (Probably) a Family Affair
In an adjoining presentation at ASH and in a December 2024 article in Hematology: American Society of Hematology Education Program, hematologist Domenico Girelli, MD, PhD, with the University of Verona, Italy, told colleagues about the 56-year-old male patient with fatigue. He also had a mildly enlarged liver, hyperferritinemia (890 µg/L vs normal value < 300 µg/L) and a mildly increased alanine aminotransferase level (46 U/L vs normal value < 40 U/L).
The patient was diagnosed with hemochromatosis, a genetic disorder caused by mutations that leads to increased transferrin saturation, Girelli said.
“By definition, hemochromatosis is characterized by the absence of signs of a primary red blood cell disorder — different from other disorders like transfusion iron overload or iron-loading anemias,” he said.
It’s also important to consider other possible causes of hyperferritinemia, because most cases of the symptom aren’t related to iron overload, he said. “A careful clinical history and a few laboratory parameters including transferrin saturation are generally sufficient for the differential diagnosis.”
As Girelli noted, “hemochromatosis can have a wide clinical spectrum ranging from mild to severe forms, which are strongly influenced by the co-presence of risk factors like alcohol [use], blood transfusion, and genetic factors captured by polygenic risk score.”
In Many Cases, Hemochromatosis Can Be Successfully Treated
According to Girelli, it’s important to understand the disease stage, because this information can predict the probability of advanced liver fibrosis, which can be a sign of a worse prognosis.
“The strongest clinical predictors of advanced liver fibrosis are ferritin higher than 1000 [µg/L] and the presence of arthropathy [joint disease],” he said. “If both are absent and the patient is asymptomatic, there is no need for further investigation. If both are present, further investigation — including cardiac MRI and full endocrine profile — are indicated. Liver biopsy may be indicated only in uncertain cases.”
Fortunately, “most patients are diagnosed in preclinical or early stage, and their prognosis is excellent, with a normal life expectancy,” he said
Phlebotomy remains the standard of care for hemochromatosis in uncomplicated cases. “It is safe, cheap, well-tolerated, and significantly reduces mortality and morbidity, especially when it is started before the development of cirrhosis,” he said.
Family Members Should Be Tested for Genetic Traits
It’s important to advise patients prior to phlebotomy to avoid undercooked seafood and wound contact with sea water because of the risk for sepsis due to the pathogen Vibrio vulnificus, Girelli said.
And it’s a good idea to test family members to see if they share a genetic risk for hemochromatosis, he said. The 56-year-old patient’s brother turned out to also have genetic risk, and his iron levels were very high. He had recently been diagnosed with seronegative arthritis that could be classified as secondary to hemochromatosis.
For management, Girelli said, patients should minimize or avoid alcohol consumption, eat a healthy diet, and avoid vitamin C and iron supplements even in multivitamin compounds. Patients should be encouraged to exercise and maintain an ideal weight.
The 56-year-old patient fared well, reaching a ferritin target of 50 mg/mL after multiple phlebotomy procedures that removed nearly 5 g of iron.
The patient tolerated the treatment and his fatigue resolved, Girelli said. “The maintenance treatment consisted of 3 phlebotomies per year. The patient remained asymptomatic and was eventually enrolled as a regular blood donor.”
Karp Leaf disclosed relationships with Alnylam, Recordati, and Disc Medicine. She is a member of the Porphyrias Consortium, part of the Rare Diseases Clinical Research Network, funded by the National Institutes of Health and led by the National Center for Advancing Translational Sciences (NCATS). The consortium is funded by NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases. Girelli had no disclosures.
A version of this article first appeared on Medscape.com.
One patient, a 39-year-old woman, went to a dermatologist seeking care for fluid-filled blisters over the backs of her hands and arms. Another patient, a 56-year-old man, sought care from his general practitioner owing to fatigue.
Their presentations were quite different, but the two patients shared one thing in common: iron overload. Both ended up in the care of hematologists who diagnosed their conditions as porphyria cutanea tarda (PCT) and hemochromatosis, respectively.
A pair of hematologists discussed the treatment of these disorders at the American Society of Hematology (ASH) 2024 Annual Meeting and in reports in Hematology: American Society of Hematology Education Program. Here’s a look at the guidance they provided.
Porphyria Cutanea Tarda: Skin Trouble
Testing revealed that the female patient had a highly elevated porphyrin levels: Her urine uroporphyrin was 3959 nmol/L (normal, < 30 nmol/L) and plasma uroporphyrin was 2.0 µg/dL (normal, < 1.0 µg/dL). Her serum ferritin level was also high, at 420 ng/mL (normal, < 200 ng/mL).
Rebecca Karp Leaf, MD, of Massachusetts General Hospital and Harvard Medical School, diagnosed her with PCT, a disorder of heme biosynthesis that often presents with skin manifestations.
As co-founder and co-director of the Boston hospital’s Porphyria Center, Karp Leaf is a leading expert in PCT, a rare disease that affects 5-10 people per 100,000. In addition to speaking at the ASH meeting in December, she described PCT in a December 2024 article in Hematology: American Society of Hematology Education Program.
PCT is caused by inhibition of an enzyme in heme biosynthesis and leads to accumulation of porphyrins in the liver and plasma, Karp Leaf said. Through a complex process, excess of iron leads to inhibition of the enzyme, which leads to a buildup of toxic porphyrins, she said. The condition causes painless, blistering lesions on sun-exposed skin, scarring, skin fragility, dark urine, and liver disease.
PCT most commonly occurs in middle age after the age of 40 and affects men more than women. “It’s the only porphyria that can occur absent a genetic variant,” she said, and 75% of cases have no genetic component.
Options for Treatment Include Antivirals and Phlebotomy
Risk factors for PCT include alcohol use, smoking, exogenous estrogen, hepatitis, and HIV mutations.
In regard to treatment, “modification of risk factors can be variably helpful: alcohol and smoking cessation, stopping exogenous estrogen, sun-protective clothing, and steroid-containing creams for lesions,” Karp Leaf said. “Most patients typically require further therapy to reduce liver porphyrins.”
Urine and plasma tests can help with diagnosis, she said. In patients with hepatitis C (HCV), “direct-acting antivirals can actually lead to resolution of PCT without any other therapy. We suspect that with effective antiviral treatment for HCV, the incidence of PCT will really go down.”
Therapeutic phlebotomy — blood removal — is another option. “It’s one of my favorite therapies because you don’t have to give somebody a drug. You can just take out iron,” Karp Leaf said. “Typically, we’ll start with venesection of 450 ccs of whole blood every 2 weeks, We target a ferritin level of 20 [ng/mL] but permit it up to 50 [ng/mL], or a little bit higher.”
The treatment leads to resolution of blisters in about 2-3 months, she said, and normalization of porphyrins by 13 months. Patients typically require about 6-8 treatments, she said.
Another option is iron chelation, iron removal via medicine, “but it’s expensive, has side effects, and is really not recommended if other treatments are available,” she said.
Hydroxychloroquine Can Be Helpful Too
Low-dose hydroxychloroquine can also be effective at 100 mg twice a week, “much lower than what we use in autoimmune disease,” Karp Leaf said. “We suspect that it’s taken up by the hepatic lysosomes and causes release of porphyrins. It causes clinical remission in about 6 months.”
However, higher doses can lead to liver injury, and the drug’s use is limited in end-stage kidney disease since porphyrins are excreted in the urine. These patients are especially difficult to treat, she said.
In the case of the 39-year-old patient, Karp Leaf recommended that the woman reduce her alcohol intake and begin using a copper intrauterine device for contraception instead of a combined oral contraceptive pill, which allowed her to undergo phlebotomy.
“She needed about eight sessions of therapeutic phlebotomy to achieve a ferritin of 30 [ng/mL], and her lesions resolved in 6 months,” Karp Leaf said. “Her plasma porphyrins resolved by 12 months. Her liver biochemistries were a bit elevated, and they subsequently normalized.”
Karp Leaf said she sees the patient about once a year.
Hemochromatosis: It’s (Probably) a Family Affair
In an adjoining presentation at ASH and in a December 2024 article in Hematology: American Society of Hematology Education Program, hematologist Domenico Girelli, MD, PhD, with the University of Verona, Italy, told colleagues about the 56-year-old male patient with fatigue. He also had a mildly enlarged liver, hyperferritinemia (890 µg/L vs normal value < 300 µg/L) and a mildly increased alanine aminotransferase level (46 U/L vs normal value < 40 U/L).
The patient was diagnosed with hemochromatosis, a genetic disorder caused by mutations that leads to increased transferrin saturation, Girelli said.
“By definition, hemochromatosis is characterized by the absence of signs of a primary red blood cell disorder — different from other disorders like transfusion iron overload or iron-loading anemias,” he said.
It’s also important to consider other possible causes of hyperferritinemia, because most cases of the symptom aren’t related to iron overload, he said. “A careful clinical history and a few laboratory parameters including transferrin saturation are generally sufficient for the differential diagnosis.”
As Girelli noted, “hemochromatosis can have a wide clinical spectrum ranging from mild to severe forms, which are strongly influenced by the co-presence of risk factors like alcohol [use], blood transfusion, and genetic factors captured by polygenic risk score.”
In Many Cases, Hemochromatosis Can Be Successfully Treated
According to Girelli, it’s important to understand the disease stage, because this information can predict the probability of advanced liver fibrosis, which can be a sign of a worse prognosis.
“The strongest clinical predictors of advanced liver fibrosis are ferritin higher than 1000 [µg/L] and the presence of arthropathy [joint disease],” he said. “If both are absent and the patient is asymptomatic, there is no need for further investigation. If both are present, further investigation — including cardiac MRI and full endocrine profile — are indicated. Liver biopsy may be indicated only in uncertain cases.”
Fortunately, “most patients are diagnosed in preclinical or early stage, and their prognosis is excellent, with a normal life expectancy,” he said
Phlebotomy remains the standard of care for hemochromatosis in uncomplicated cases. “It is safe, cheap, well-tolerated, and significantly reduces mortality and morbidity, especially when it is started before the development of cirrhosis,” he said.
Family Members Should Be Tested for Genetic Traits
It’s important to advise patients prior to phlebotomy to avoid undercooked seafood and wound contact with sea water because of the risk for sepsis due to the pathogen Vibrio vulnificus, Girelli said.
And it’s a good idea to test family members to see if they share a genetic risk for hemochromatosis, he said. The 56-year-old patient’s brother turned out to also have genetic risk, and his iron levels were very high. He had recently been diagnosed with seronegative arthritis that could be classified as secondary to hemochromatosis.
For management, Girelli said, patients should minimize or avoid alcohol consumption, eat a healthy diet, and avoid vitamin C and iron supplements even in multivitamin compounds. Patients should be encouraged to exercise and maintain an ideal weight.
The 56-year-old patient fared well, reaching a ferritin target of 50 mg/mL after multiple phlebotomy procedures that removed nearly 5 g of iron.
The patient tolerated the treatment and his fatigue resolved, Girelli said. “The maintenance treatment consisted of 3 phlebotomies per year. The patient remained asymptomatic and was eventually enrolled as a regular blood donor.”
Karp Leaf disclosed relationships with Alnylam, Recordati, and Disc Medicine. She is a member of the Porphyrias Consortium, part of the Rare Diseases Clinical Research Network, funded by the National Institutes of Health and led by the National Center for Advancing Translational Sciences (NCATS). The consortium is funded by NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases. Girelli had no disclosures.
A version of this article first appeared on Medscape.com.
Heavy Menstrual Bleeding: How Hematologists Can Help
Heavy menstrual bleeding is more than an inconvenience in adolescents: It often leads to significant medical complications, in addition to disruptions in quality of life. While measuring the true level of bleeding can be a challenge, hematologists say treatments are helpful and can be as simple — and surprising — as doses of aspirin.
About 90% of adolescents with heavy menstrual bleeding will have low ferritin, and 70% will develop anemia, said benign hematologist Juliana Perez Botero, MD, of the Mayo Clinic in Rochester, Minnesota, in a presentation at the American Society of Hematology (ASH) 2024 Annual Meeting. “This is an issue of big magnitude that has public health implications, but it’s also an issue of gender equality and social justice.”
Measuring Menstruation: What Counts as Heavy Bleeding?
According to hematologist Allison Wheeler, MD, of the University of Washington in Seattle, normal menstrual bleeding is defined as lasting for about 5 days with 30-50 mL of blood loss.
“Historically, heavy menstrual bleeding was defined as bleeding as > 7 days or > 80 mL of blood loss,” Wheeler said. “It’s pretty hard to measure those mL. So a more modern definition is increased menstrual blood loss that interferes with a female’s physical, social, emotional, or material quality of life.”
Measuring blood loss during menstruation isn’t simple. The alkaline hematin method, which measures blood in feminine hygiene products, is the “gold standard, but that’s all I’m going to say about that,” Wheeler said.
An alternative is the Pictorial Blood Loss Assessment Chart, a visual tool that patients can use to estimate blood loss, she said, although there have been questions about its accuracy. In addition, it only covers pads and tampons even though other products are now available.
Another option is the Adolescent Menstrual Bleeding Questionnaire. “It captures both how much patients are bleeding and a lot of quality-of-life aspects,” she said.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Surprisingly, Can Be Effective Treatments
It seems counterintuitive that NSAIDs can be helpful in patients with heavy bleeding. “We don’t think about them because Cox inhibition ends up leading to decreases in thromboxane A2, which is going to increase bleeding,” Wheeler said. However, she said, the drugs also decrease prostaglandin within the endometrium, which can improve menstrual bleeding.
“In a meta-analysis, when NSAIDs were compared to placebo, there was decreased menstrual bleeding,” she said. “It was pretty similar to estrogen-progesterone formulations together. And when compared to antifibrinolytics, there was a little more menstrual bleeding with the NSAID use.”
Wheeler cautioned that “it’s important to optimize NSAID dosage. You really can’t use lower doses, and you can’t use sporadic doses.”
Other Options: Tranexamic Acid, Hormone Management
Tranexamic acid is the most common antifibrinolytic treatment for heavy bleeding, Wheeler said. “This is a lysine analog that’s going to inhibit the conversion from plasminogen to plasmin,” she said. “This is going to strengthen and stabilize blood clots that are already formed.”
Multiple clinical trials have studied the treatment in heavy menstrual bleeding, she said. “There’s no increased risk of venous or arterial thrombosis among participants in these clinical trials, but patients with a history of thrombosis were excluded from the majority of them. So we do need to keep that in mind.”
As for adverse effects, they’re mild and sporadic and include headaches and stomach upset, she said.
Another treatment option is estrogen and progesterone therapy using pill, patch, or vaginal ring, Wheeler said. The treatment leads to a “stable and thin very endometrium,” and typically takes 3-6 months to fully kick in. Studies have suggested the therapy can lead to 35%-80% reduction in menstrual blood loss, she said.
Keep in mind, Wheeler noted, “that there are many contraindications associated with estrogen use. Please take note of this, especially in consideration of discussions with patients shifting over to progesterone-only hormonal therapy” — another option to treat excess bleeding.
In most patients, she added, progesterone-only therapy “is going to result in irregular bleeding when you first start taking it. That irregular bleeding can last from up to 3-6 months. It’s something that’s really important to discuss with patients.”
She also noted that this therapy can be given to patients in forms that do not prevent pregnancy.
Botero had no disclosures. Wheeler reported relationships with Novo Nordisk, Bayer, BioMarin, Bioverativ, CSL Behring, Genentech, HEMA, Octapharma, Pfizer, Sanofi-Aventis, Shire North America, Spark, and Takeda.
A version of this article first appeared on Medscape.com.
Heavy menstrual bleeding is more than an inconvenience in adolescents: It often leads to significant medical complications, in addition to disruptions in quality of life. While measuring the true level of bleeding can be a challenge, hematologists say treatments are helpful and can be as simple — and surprising — as doses of aspirin.
About 90% of adolescents with heavy menstrual bleeding will have low ferritin, and 70% will develop anemia, said benign hematologist Juliana Perez Botero, MD, of the Mayo Clinic in Rochester, Minnesota, in a presentation at the American Society of Hematology (ASH) 2024 Annual Meeting. “This is an issue of big magnitude that has public health implications, but it’s also an issue of gender equality and social justice.”
Measuring Menstruation: What Counts as Heavy Bleeding?
According to hematologist Allison Wheeler, MD, of the University of Washington in Seattle, normal menstrual bleeding is defined as lasting for about 5 days with 30-50 mL of blood loss.
“Historically, heavy menstrual bleeding was defined as bleeding as > 7 days or > 80 mL of blood loss,” Wheeler said. “It’s pretty hard to measure those mL. So a more modern definition is increased menstrual blood loss that interferes with a female’s physical, social, emotional, or material quality of life.”
Measuring blood loss during menstruation isn’t simple. The alkaline hematin method, which measures blood in feminine hygiene products, is the “gold standard, but that’s all I’m going to say about that,” Wheeler said.
An alternative is the Pictorial Blood Loss Assessment Chart, a visual tool that patients can use to estimate blood loss, she said, although there have been questions about its accuracy. In addition, it only covers pads and tampons even though other products are now available.
Another option is the Adolescent Menstrual Bleeding Questionnaire. “It captures both how much patients are bleeding and a lot of quality-of-life aspects,” she said.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Surprisingly, Can Be Effective Treatments
It seems counterintuitive that NSAIDs can be helpful in patients with heavy bleeding. “We don’t think about them because Cox inhibition ends up leading to decreases in thromboxane A2, which is going to increase bleeding,” Wheeler said. However, she said, the drugs also decrease prostaglandin within the endometrium, which can improve menstrual bleeding.
“In a meta-analysis, when NSAIDs were compared to placebo, there was decreased menstrual bleeding,” she said. “It was pretty similar to estrogen-progesterone formulations together. And when compared to antifibrinolytics, there was a little more menstrual bleeding with the NSAID use.”
Wheeler cautioned that “it’s important to optimize NSAID dosage. You really can’t use lower doses, and you can’t use sporadic doses.”
Other Options: Tranexamic Acid, Hormone Management
Tranexamic acid is the most common antifibrinolytic treatment for heavy bleeding, Wheeler said. “This is a lysine analog that’s going to inhibit the conversion from plasminogen to plasmin,” she said. “This is going to strengthen and stabilize blood clots that are already formed.”
Multiple clinical trials have studied the treatment in heavy menstrual bleeding, she said. “There’s no increased risk of venous or arterial thrombosis among participants in these clinical trials, but patients with a history of thrombosis were excluded from the majority of them. So we do need to keep that in mind.”
As for adverse effects, they’re mild and sporadic and include headaches and stomach upset, she said.
Another treatment option is estrogen and progesterone therapy using pill, patch, or vaginal ring, Wheeler said. The treatment leads to a “stable and thin very endometrium,” and typically takes 3-6 months to fully kick in. Studies have suggested the therapy can lead to 35%-80% reduction in menstrual blood loss, she said.
Keep in mind, Wheeler noted, “that there are many contraindications associated with estrogen use. Please take note of this, especially in consideration of discussions with patients shifting over to progesterone-only hormonal therapy” — another option to treat excess bleeding.
In most patients, she added, progesterone-only therapy “is going to result in irregular bleeding when you first start taking it. That irregular bleeding can last from up to 3-6 months. It’s something that’s really important to discuss with patients.”
She also noted that this therapy can be given to patients in forms that do not prevent pregnancy.
Botero had no disclosures. Wheeler reported relationships with Novo Nordisk, Bayer, BioMarin, Bioverativ, CSL Behring, Genentech, HEMA, Octapharma, Pfizer, Sanofi-Aventis, Shire North America, Spark, and Takeda.
A version of this article first appeared on Medscape.com.
Heavy menstrual bleeding is more than an inconvenience in adolescents: It often leads to significant medical complications, in addition to disruptions in quality of life. While measuring the true level of bleeding can be a challenge, hematologists say treatments are helpful and can be as simple — and surprising — as doses of aspirin.
About 90% of adolescents with heavy menstrual bleeding will have low ferritin, and 70% will develop anemia, said benign hematologist Juliana Perez Botero, MD, of the Mayo Clinic in Rochester, Minnesota, in a presentation at the American Society of Hematology (ASH) 2024 Annual Meeting. “This is an issue of big magnitude that has public health implications, but it’s also an issue of gender equality and social justice.”
Measuring Menstruation: What Counts as Heavy Bleeding?
According to hematologist Allison Wheeler, MD, of the University of Washington in Seattle, normal menstrual bleeding is defined as lasting for about 5 days with 30-50 mL of blood loss.
“Historically, heavy menstrual bleeding was defined as bleeding as > 7 days or > 80 mL of blood loss,” Wheeler said. “It’s pretty hard to measure those mL. So a more modern definition is increased menstrual blood loss that interferes with a female’s physical, social, emotional, or material quality of life.”
Measuring blood loss during menstruation isn’t simple. The alkaline hematin method, which measures blood in feminine hygiene products, is the “gold standard, but that’s all I’m going to say about that,” Wheeler said.
An alternative is the Pictorial Blood Loss Assessment Chart, a visual tool that patients can use to estimate blood loss, she said, although there have been questions about its accuracy. In addition, it only covers pads and tampons even though other products are now available.
Another option is the Adolescent Menstrual Bleeding Questionnaire. “It captures both how much patients are bleeding and a lot of quality-of-life aspects,” she said.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Surprisingly, Can Be Effective Treatments
It seems counterintuitive that NSAIDs can be helpful in patients with heavy bleeding. “We don’t think about them because Cox inhibition ends up leading to decreases in thromboxane A2, which is going to increase bleeding,” Wheeler said. However, she said, the drugs also decrease prostaglandin within the endometrium, which can improve menstrual bleeding.
“In a meta-analysis, when NSAIDs were compared to placebo, there was decreased menstrual bleeding,” she said. “It was pretty similar to estrogen-progesterone formulations together. And when compared to antifibrinolytics, there was a little more menstrual bleeding with the NSAID use.”
Wheeler cautioned that “it’s important to optimize NSAID dosage. You really can’t use lower doses, and you can’t use sporadic doses.”
Other Options: Tranexamic Acid, Hormone Management
Tranexamic acid is the most common antifibrinolytic treatment for heavy bleeding, Wheeler said. “This is a lysine analog that’s going to inhibit the conversion from plasminogen to plasmin,” she said. “This is going to strengthen and stabilize blood clots that are already formed.”
Multiple clinical trials have studied the treatment in heavy menstrual bleeding, she said. “There’s no increased risk of venous or arterial thrombosis among participants in these clinical trials, but patients with a history of thrombosis were excluded from the majority of them. So we do need to keep that in mind.”
As for adverse effects, they’re mild and sporadic and include headaches and stomach upset, she said.
Another treatment option is estrogen and progesterone therapy using pill, patch, or vaginal ring, Wheeler said. The treatment leads to a “stable and thin very endometrium,” and typically takes 3-6 months to fully kick in. Studies have suggested the therapy can lead to 35%-80% reduction in menstrual blood loss, she said.
Keep in mind, Wheeler noted, “that there are many contraindications associated with estrogen use. Please take note of this, especially in consideration of discussions with patients shifting over to progesterone-only hormonal therapy” — another option to treat excess bleeding.
In most patients, she added, progesterone-only therapy “is going to result in irregular bleeding when you first start taking it. That irregular bleeding can last from up to 3-6 months. It’s something that’s really important to discuss with patients.”
She also noted that this therapy can be given to patients in forms that do not prevent pregnancy.
Botero had no disclosures. Wheeler reported relationships with Novo Nordisk, Bayer, BioMarin, Bioverativ, CSL Behring, Genentech, HEMA, Octapharma, Pfizer, Sanofi-Aventis, Shire North America, Spark, and Takeda.
A version of this article first appeared on Medscape.com.
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.
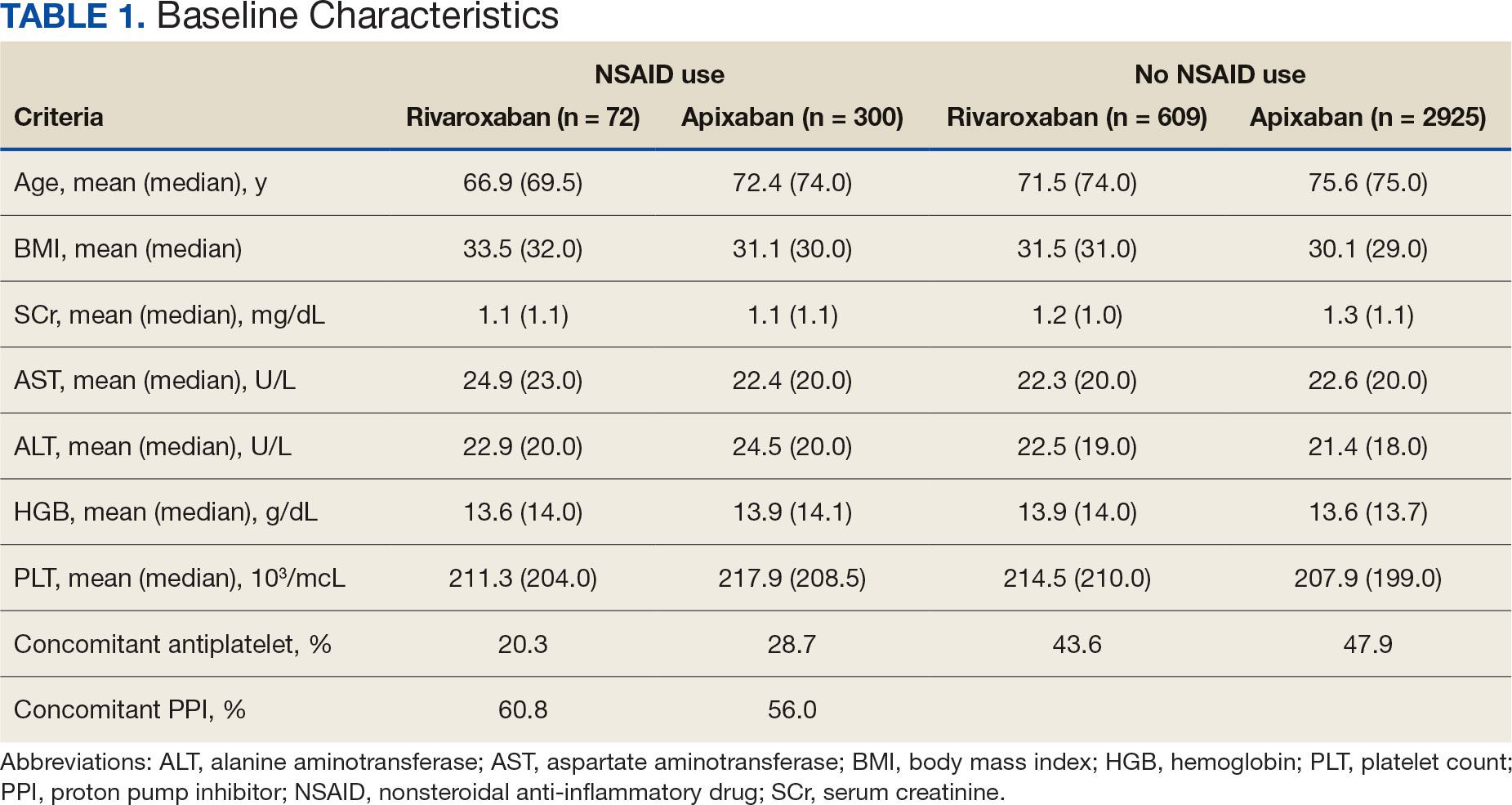
A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).
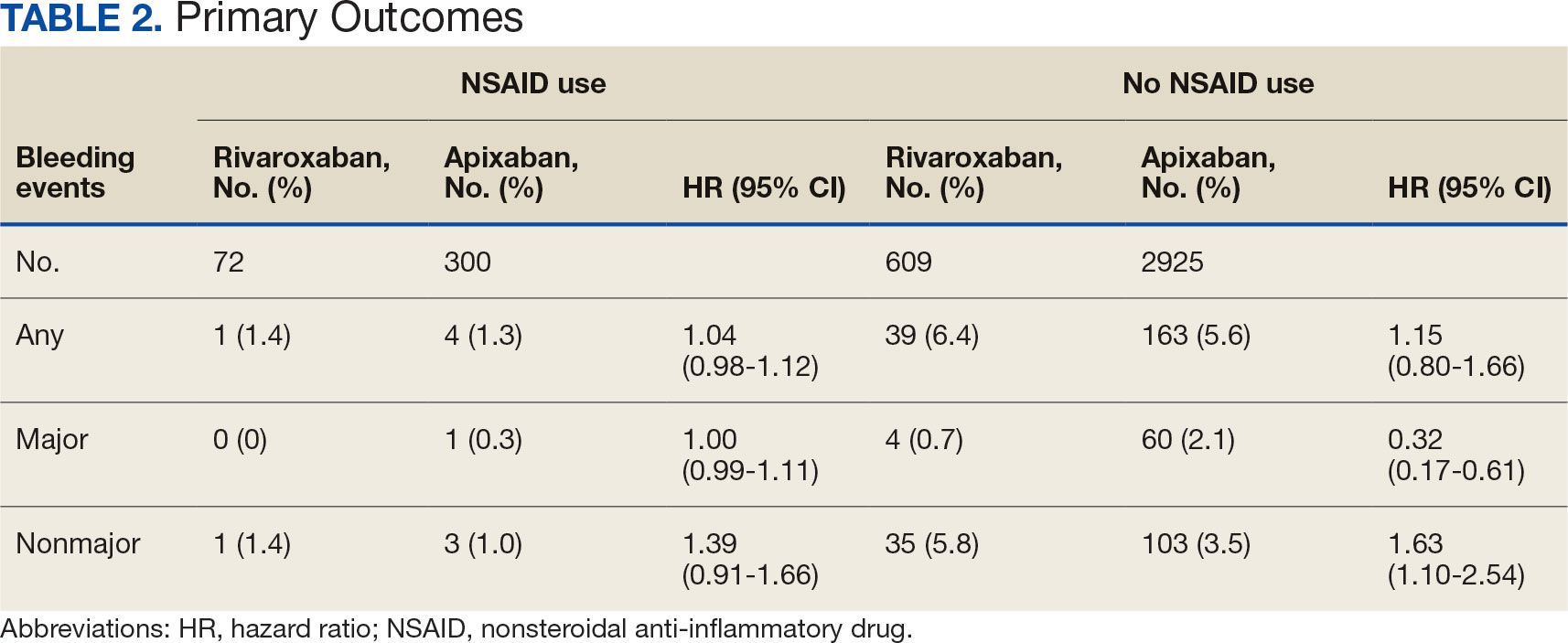
The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
Clinical practice has shifted from vitamin K antagonists to direct oral anticoagulants (DOACs) for atrial fibrillation treatment due to their more favorable risk-benefit profile and less lifestyle modification required.1,2 However, the advantage of a lower bleeding risk with DOACs could be compromised by potentially problematic pharmacokinetic interactions like those conferred by antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs).3,4 Treating a patient needing anticoagulation with a DOAC who has comorbidities may introduce unavoidable drug-drug interactions. This particularly happens with over-the-counter and prescription NSAIDs used for the management of pain and inflammatory conditions.5
NSAIDs primarily affect 2 cyclooxygenase (COX) enzyme isomers, COX-1 and COX-2.6 COX-1 helps maintain gastrointestinal (GI) mucosa integrity and platelet aggregation processes, whereas COX-2 is engaged in pain signaling and inflammation mediation. COX-1 inhibition is associated with more bleeding-related adverse events (AEs), especially in the GI tract. COX-2 inhibition is thought to provide analgesia and anti-inflammatory properties without elevating bleeding risk. This premise is responsible for the preferential use of celecoxib, a COX-2 selective NSAID, which should confer a lower bleeding risk compared to nonselective NSAIDs such as ibuprofen and naproxen.7 NSAIDs have been documented as independent risk factors for bleeding. NSAID users are about 3 times as likely to develop GI AEs compared to nonNSAID users.8
Many clinicians aim to further mitigate NSAID-associated bleeding risk by coprescribing a proton pump inhibitor (PPI). PPIs provide gastroprotection against NSAID-induced mucosal injury and sequential complication of GI bleeding. In a multicenter randomized control trial, patients who received concomitant PPI therapy while undergoing chronic NSAID therapy—including nonselective and COX-2 selective NSAIDs—had a significantly lower risk of GI ulcer development (placebo, 17.0%; 20 mg esomeprazole, 5.2%; 40 mg esomeprazole, 4.6%).9 Current clinical guidelines for preventing NSAIDassociated bleeding complications recommend using a COX-2 selective NSAID in combination with PPI therapy for patients at high risk for GI-related bleeding, including the concomitant use of anticoagulants.10
There is evidence suggesting an increased bleeding risk with NSAIDs when used in combination with vitamin K antagonists such as warfarin.11,12 A systematic review of warfarin and concomitant NSAID use found an increased risk of overall bleeding with NSAID use in combination with warfarin (odds ratio 1.58; 95% CI, 1.18-2.12), compared to warfarin alone.12
Posthoc analyses of randomized clinical trials have also demonstrated an increased bleeding risk with oral anticoagulation and concomitant NSAID use.13,14 In the RE-LY trial, NSAID users on warfarin or dabigatran had a statistically significant increased risk of major bleeding compared to non-NSAID users (hazard ratio [HR] 1.68; 95% CI, 1.40- 2.02; P < .001).13 In the ARISTOTLE trial, patients on warfarin or apixaban who were incident NSAID users were found to have an increased risk of major bleeding (HR 1.61; 95% CI, 1.11-2.33) and clinically relevant nonmajor bleeding (HR 1.70; 95% CI, 1.16- 2.48).14 These trials found a statistically significant increased bleeding risk associated with NSAID use, though the populations evaluated included patients taking warfarin and patients taking DOACs. These trials did not evaluate the bleeding risk of concomitant NSAID use among DOACs alone.
Evidence on NSAID-associated bleeding risk with DOACs is lacking in settings where the patient population, prescribing practices, and monitoring levels are variable. Within the Veterans Health Administration, clinical pharmacist practitioners (CPPs) in anticoagulation clinics oversee DOAC therapy management. CPPs monitor safety and efficacy of DOAC therapies through a population health management tool, the DOAC Dashboard.15 The DOAC Dashboard creates alerts for patients who may require an intervention based on certain clinical parameters, such as drug-drug interactions.16 Whenever a patient on a DOAC is prescribed an NSAID, an alert is generated on the DOAC Dashboard to flag the CPPs for the potential need for an intervention. If NSAID therapy remains clinically indicated, CPPs may recommend risk reduction strategies such as a COX-2 selective NSAID or coprescribing a PPI.10
The DOAC Dashboard provides an ideal setting for investigating the effects of NSAID use, NSAID selectivity, and PPI coprescribing on DOAC bleeding rates. With an increasing population of patients receiving anticoagulation therapy with a DOAC, more guidance regarding the bleeding risk of concomitant NSAID use with DOACs is needed. Studies evaluating the bleeding risk with concomitant NSAID use in patients on a DOAC alone are limited. This is the first study to date to compare bleeding risk with concomitant NSAID use between DOACs. This study provides information on bleeding risk with NSAID use among commonly prescribed DOACs, rivaroxaban and apixaban, and the potential impacts of current risk reduction strategies.
METHODS
This single-center retrospective cohort review was performed using the electronic health records (EHRs) of patients enrolled in the US Department of Veterans Affairs (VA) Mountain Home Healthcare System who received rivaroxaban or apixaban from December 2020 to December 2022. This study received approval from the East Tennessee State University/VA Institutional Review Board committee.
Patients were identified through the DOAC Dashboard, aged 21 to 100 years, and received rivaroxaban or apixaban at a therapeutic dose: rivaroxaban 10 to 20 mg daily or apixaban 2.5 to 5 mg twice daily. Patients were excluded if they were prescribed dual antiplatelet therapy, received rivaroxaban at dosing indicated for peripheral vascular disease, were undergoing dialysis, had evidence of moderate to severe hepatic impairment or any hepatic disease with coagulopathy, were undergoing chemotherapy or radiation, or had hematological conditions with predisposed bleeding risk. These patients were excluded to mitigate the potential confounding impact from nontherapeutic DOAC dosing strategies and conditions associated with an increased bleeding risk.
Eligible patients were stratified based on NSAID use. NSAID users were defined as patients prescribed an oral NSAID, including both acute and chronic courses, at any point during the study time frame while actively on a DOAC. Bleeding events were reviewed to evaluate rates between rivaroxaban and apixaban among NSAID and nonNSAID users. Identified NSAID users were further assessed for NSAID selectivity and PPI coprescribing as a subgroup analysis for the secondary assessment.
Data Collection
Baseline data were collected, including age, body mass index, anticoagulation indication, DOAC agent, DOAC dose, and DOAC total daily dose. Baseline serum creatinine levels, liver function tests, hemoglobin levels, and platelet counts were collected from the most recent data available immediately prior to the bleeding event, if applicable.
The DOAC Dashboard was reviewed for active and dismissed drug interaction alerts to identify patients taking rivaroxaban or apixaban who were prescribed an NSAID. Patients were categorized in the NSAID group if an interacting drug alert with an NSAID was reported during the study time frame. Data available through the interacting drug alerts on NSAID use were limited to the interacting drug name and date of the reported flag. Manual EHR review was required to confirm dates of NSAID therapy initiation and NSAID discontinuation, if applicable.
Data regarding concomitant antiplatelet use were obtained through review of the active and dismissed drug interaction alerts on the DOAC Dashboard. Concomitant antiplatelet use was defined as the prescribing of a single antiplatelet agent at any point while receiving DOAC therapy. Data on concomitant antiplatelets were collected regardless of NSAID status.
Data on coprescribed PPI therapy were obtained through manual EHR review of identified NSAID users. Coprescribed PPI therapy was defined as the prescribing of a PPI at any point during NSAID therapy. Data regarding PPI use among non-NSAID users were not collected because the secondary endpoint was designed to assess PPI use only among patients coprescribed a DOAC and NSAID.
Outcomes
Bleeding events were identified through an outcomes report generated by the DOAC Dashboard based on International Classification of Diseases, Tenth Revision diagnosis codes associated with a bleeding event. The outcomes report captures diagnoses from the outpatient and inpatient care settings. Reported bleeding events were limited to patients who received a DOAC at any point in the 6 months prior to the event and excluded patients with recent DOAC initiation within 7 days of the event, as these patients are not captured on the DOAC Dashboard.
All reported bleeding events were manually reviewed in the EHR and categorized as a major or clinically relevant nonmajor bleed, according to International Society of Thrombosis and Haemostasis criteria. Validated bleeding events were then crossreferenced with the interacting drug alerts report to identify events with potentially overlapping NSAID therapy at the time of the event. Overlapping NSAID therapy was defined as the prescribing of an NSAID at any point in the 6 months prior to the event. All events with potential overlapping NSAID therapies were manually reviewed for confirmation of NSAID status at the time of the event.
The primary endpoint was a composite of any bleeding event per International Society of Thrombosis and Haemostasis criteria. The secondary endpoint evaluated the potential impact of NSAID selectivity or PPI coprescribing on the bleeding rate among the NSAID user groups.
Statistical Analysis
Analyses were performed consistent with the methods used in the ARISTOTLE and RE-LY trials. It was determined that a sample size of 504 patients, with ≥ 168 patients in each group, would provide 80% power using a 2-sided a of 0.05. HRs with 95% CIs and respective P values were calculated using a SPSS-adapted online calculator.
RESULTS
The DOAC Dashboard identified 681 patients on rivaroxaban and 3225 patients on apixaban; 72 patients on rivaroxaban (10.6%) and 300 patients on apixaban (9.3%) were NSAID users. The mean age of NSAID users was 66.9 years in the rivaroxaban group and 72.4 years in the apixaban group. The mean age of non-NSAID users was 71.5 years in the rivaroxaban group and 75.6 years in the apixaban group. No appreciable differences were observed among subgroups in body mass index, renal function, hepatic function, hemoglobin, or platelet counts, and no statistically significant differences were identified (Table 1). Antiplatelet agents identified included aspirin, clopidogrel, prasugrel, and ticagrelor. Fifteen patients (20.3%) in the rivaroxaban group and 87 patients (28.7%) in the apixaban group had concomitant antiplatelet and NSAID use. Forty-five patients on rivaroxaban (60.8%) and 170 (55.9%) on apixaban were prescribed concomitant PPI and NSAID at baseline. Among non-NSAID users, there was concomitant antiplatelet use for 265 patients (43.6%) in the rivaroxaban group and 1401 patients (47.9%) in the apixaban group. Concomitant PPI use was identified among 63 patients (60.0%) taking selective NSAIDs and 182 (57.2%) taking nonselective NSAIDs.

A total of 423 courses of NSAIDs were identified: 85 NSAID courses in the rivaroxaban group and 338 NSAID courses in the apixaban group. Most NSAID courses involved a nonselective NSAID in the rivaroxaban and apixaban NSAID user groups: 75.2% (n = 318) aggregately compared to 71.8% (n = 61) and 76.0% (n = 257) in the rivaroxaban and apixaban groups, respectively. The most frequent NSAID courses identified were meloxicam (26.7%; n = 113), celecoxib (24.8%; n = 105), ibuprofen (19.1%; n = 81), and naproxen (13.5%; n = 57). Data regarding NSAID therapy initiation and discontinuation dates were not readily available. As a result, the duration of NSAID courses was not captured.
There was no statistically significant difference in bleeding rates between rivaroxaban and apixaban among NSAID users (HR 1.04; 95% CI, 0.98-1.12) or non-NSAID users (HR 1.15; 95% CI, 0.80-1.66) (Table 2). Apixaban non-NSAID users had a higher rate of major bleeds (HR 0.32; 95% CI, 0.17-0.61) while rivaroxaban non-NSAID users had a higher rate of clinically relevant nonmajor bleeds (HR 1.63; 95% CI, 1.10-2.54).

The sample size for the secondary endpoint consisted of bleeding events that were confirmed to have had an overlapping NSAID prescribed at the time of the event. For this secondary assessment, there was 1 rivaroxaban NSAID user bleeding event and 4 apixaban NSAID user bleeding events. For the rivaroxaban NSAID user bleeding event, the NSAID was nonselective and a PPI was not coprescribed. For the apixaban NSAID user bleeding events, 2 NSAIDs were nonselective and 2 were selective. All patients with apixaban and NSAID bleeding events had a coprescribed PPI. There was no clinically significant difference in the bleeding rates observed for NSAID selectivity or PPI coprescribing among the NSAID user subgroups.
DISCUSSION
This study found that there was no statistically significant difference for bleeding rates of major and nonmajor bleeding events between rivaroxaban and apixaban among NSAID users and non-NSAID users. This study did not identify a clinically significant impact on bleeding rates from NSAID selectivity or PPI coprescribing among the NSAID users.
There were notable but not statistically significant differences in baseline characteristics observed between the NSAID and non-NSAID user groups. On average, the rivaroxaban and apixaban NSAID users were younger compared with those not taking NSAIDs. NSAIDs, specifically nonselective NSAIDs, are recognized as potentially inappropriate medications for older adults given that this population is at an increased risk for GI ulcer development and/or GI bleeding.17 The non-NSAID user group likely consisted of older patients compared to the NSAID user group as clinicians may avoid prescribing NSAIDs to older adults regardless of concomitant DOAC therapy.
In addition to having an older patient population, non-NSAID users were more frequently prescribed a concomitant antiplatelet when compared with NSAID users. This prescribing pattern may be due to clinicians avoiding the use of NSAIDs in patients receiving DOAC therapy in combination with antiplatelet therapy, as these patients have been found to have an increased bleeding rate compared to DOAC therapy alone.18
Non-NSAID users had an overall higher bleeding rate for both major and nonmajor bleeding events. Based on this observation, it could be hypothesized that antiplatelet agents have a higher risk of bleeding in comparison to NSAIDs. In a subanalysis of the EXPAND study evaluating risk factors of major bleeding in patients receiving rivaroxaban, concomitant use of antiplatelet agents demonstrated a statistically significant increased risk of bleeding (HR 1.6; 95% CI, 1.2-2.3; P = .003) while concomitant use of NSAIDs did not (HR 0.8; 95% CI, 0.3-2.2; P = .67).19
In assessing PPI status at baseline, a majority of both rivaroxaban and apixaban NSAID users were coprescribed a PPI. This trend aligns with current clinical guideline recommendations for the prescribing of PPI therapy for GI protection in high-risk patients, such as those on DOAC therapy and concomitant NSAID therapy.10 Given the high proportion of NSAID users coprescribed a PPI at baseline, it may be possible that the true incidence of NSAID-associated bleeding events was higher than what this study found. This observation may reflect the impact from timely implementation of risk mitigation strategies by CPPs in the anticoagulation clinic. However, this study was not constructed to assess the efficacy of PPI use in this manner.
It is important to note the patients included in this study were followed by a pharmacist in an anticoagulation clinic using the DOAC Dashboard.15 This population management tool allows CPPs to make proactive interventions when a patient taking a DOAC receives an NSAID prescription, such as recommending the coprescribing of a PPI or use of a selective NSAID.10,16 These standards of care may have contributed to an overall reduced bleeding rate among the NSAID user group and may not be reflective of private practice.
The planned analysis of this study was modeled after the posthoc analysis of the RE-LY and ARISTOTLE trials. Both trials demonstrated an increased risk of bleeding with oral anticoagulation, including DOAC and warfarin, in combination with NSAID use. However, both trials found that NSAID use in patients treated with a DOAC was not independently associated with increased bleeding events compared with warfarin.13,14 The results of this study are comparable to the RE-LY and ARISTOTLE findings that NSAID use among patients treated with rivaroxaban or apixaban did not demonstrate a statistically significant increased bleeding risk.
Studies of NSAID use in combination with DOAC therapy have been limited to patient populations consisting of both DOAC and warfarin. Evidence from these trials outlines the increased bleeding risk associated with NSAID use in combination with oral anticoagulation; however, these patient populations include those on a DOAC and warfarin.13,14,19,20 Given the limited evidence on NSAID use among DOACs alone, it is assumed NSAID use in combination with DOACs has a similar risk of bleeding as warfarin use. This may cause clinicians to automatically exclude NSAID therapy as a treatment option for patients on a DOAC who are otherwise clinically appropriate candidates, such as those with underlying inflammatory conditions. Avoiding NSAID therapy in this patient population may lead to suboptimal pain management and increase the risk of patient harm from methods such as inappropriate opioid therapy prescribing.
DOAC therapy should not be a universal limitation to the use of NSAIDs. Although the risk of bleeding with NSAID therapy is always present, deliberate NSAID prescribing in addition to the timely implementation of risk mitigation strategies may provide an avenue for safe NSAID prescribing in patients receiving a DOAC. A population health-based approach to DOAC management, such as the DOAC Dashboard, appears to be effective at preventing patient harm when NSAIDs are prescribed in conjunction with DOACs.
Limitations
The DOAC Dashboard has been shown to be effective and efficient at monitoring DOAC therapy from a population-based approach.16 Reports generated through the DOAC Dashboard provide convenient access to patient data which allows for timely interventions; however, there are limits to its use for data collection. All the data elements necessary to properly assess bleeding risk with validated tools, such as HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly), are not available on DOAC Dashboard reports. Due to this constraint, bleeding risk assessments were not conducted at baseline and this study was unable to include risk modeling. Additionally, data elements like initiation and discontinuation dates and duration of therapies were not readily available. As a result, this study was unable to incorporate time as a data point.
This was a retrospective study that relied on manual review of chart documentation to verify bleeding events, but data obtained through the DOAC Dashboard were transferred directly from the EHR.15 Bleeding events available for evaluation were restricted to those that occurred at a VA facility. Additionally, the sample size within the rivaroxaban NSAID user group did not reach the predefined sample size required to reach power and may have been too small to detect a difference if one did exist. The secondary assessment had a low sample size of NSAID user bleeding events, making it difficult to fully assess its impact on NSAID selectivity and PPI coprescribing on bleeding rates. All courses of NSAIDs were equally valued regardless of the dose or therapy duration; however, this is consistent with how NSAID use was defined in the RE-LY and ARISTOTLE trials.
CONCLUSIONS
This retrospective cohort review found no statistically significant difference in the composite bleeding rates between rivaroxaban and apixaban among NSAID users and non-NSAID users. Moreover, there was no clinically significant impact observed for bleeding rates in regard to NSAID selectivity and PPI coprescribing among NSAID users. However, coprescribing of PPI therapy to patients on a DOAC who are clinically indicated for an NSAID may reduce the risk of bleeding. Population health management tools, such as the DOAC Dashboard, may also allow clinicians to safely prescribe NSAIDs to patients on a DOAC. Further large-scale observational studies are needed to quantify the real-world risk of bleeding with concomitant NSAID use among DOACs alone and to evaluate the impact from NSAID selectivity or PPI coprescribing.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi:10.1016/S0140-6736(13)62343-0
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-e88S. doi:10.1378/chest.11-2292
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129(11S):S33-S40. doi:10.1016/j.amjmed.2016.06.003
- Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55-61. doi:10.15420/aer.2017.50.1
- Davis JS, Lee HY, Kim J, et al. Use of non-steroidal antiinflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
- Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi:10.1002/j.1552-4604.1995.tb04050.x
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J. 2011;26(6):385-391. doi:10.5001/omj.2011.101
- Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115(10):787-796. doi:10.7326/0003-4819-115-10-787
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701-710. doi:10.1111/j.1572-0241.2006.00499.x
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi:10.1053/j.gastro.2017.01.031
- Lamberts M, Lip GYH, Hansen ML, et al. Relation of nonsteroidal anti-inflammatory drugs to serious bleeding and thromboembolism risk in patients with atrial fibrillation receiving antithrombotic therapy: a nationwide cohort study. Ann Intern Med. 2014;161(10):690-698. doi:10.7326/M13-1581
- Villa Zapata L, Hansten PD, Panic J, et al. Risk of bleeding with exposure to warfarin and nonsteroidal anti-inflammatory drugs: a systematic review and metaanalysis. Thromb Haemost. 2020;120(7):1066-1074. doi:10.1055/s-0040-1710592
- Kent AP, Brueckmann M, Fraessdorf M, et al. Concomitant oral anticoagulant and nonsteroidal anti-inflammatory drug therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72(3):255-267. doi:10.1016/j.jacc.2018.04.063
- Dalgaard F, Mulder H, Wojdyla DM, et al. Patients with atrial fibrillation taking nonsteroidal antiinflammatory drugs and oral anticoagulants in the ARISTOTLE Trial. Circulation. 2020;141(1):10-20. doi:10.1161/CIRCULATIONAHA.119.041296
- Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Asssoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
- . Valencia D, Spoutz P, Stoppi J, et al. Impact of a direct oral anticoagulant population management tool on anticoagulation therapy monitoring in clinical practice. Ann Pharmacother. 2019;53(8):806-811. doi:10.1177/1060028019835843
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Kumar S, Danik SB, Altman RK, et al. Non-vitamin K antagonist oral anticoagulants and antiplatelet therapy for stroke prevention in patients with atrial fibrillation. Cardiol Rev. 2016;24(5):218-223. doi:10.1097/CRD.0000000000000088
- Sakuma I, Uchiyama S, Atarashi H, et al. Clinical risk factors of stroke and major bleeding in patients with nonvalvular atrial fibrillation under rivaroxaban: the EXPAND study sub-analysis. Heart Vessels. 2019;34(11):1839-1851. doi:10.1007/s00380-019-01425-x
- Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med. 2014;174(6):947-953. doi:10.1001/jamainternmed.2014.946
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Impact of NSAID Use on Bleeding Rates for Patients Taking Rivaroxaban or Apixaban
Skin Cancer Risk Elevated Among Blood, Marrow Transplant Survivors
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Iron Overload: The Silent Bone Breaker
TOPLINE:
Patients with serum ferritin levels higher than 1000 μg/L show a 91% increased risk for any fracture, with a doubled risk for vertebral and humerus fractures compared with those without iron overload.
METHODOLOGY:
- Iron overload’s association with decreased bone mineral density is established, but its relationship to osteoporotic fracture risk has remained understudied and inconsistent across fracture sites.
- Researchers conducted a population-based cohort study using a UK general practice database to evaluate the fracture risk in 20,264 patients with iron overload and 192,956 matched controls without elevated ferritin (mean age, 57 years; about 40% women).
- Patients with iron overload were identified as those with laboratory-confirmed iron overload (serum ferritin levels > 1000 μg/L; n = 13,510) or a diagnosis of an iron overloading disorder, such as thalassemia major, sickle cell disease, or hemochromatosis (n = 6754).
- The primary outcome of interest was the first occurrence of an osteoporotic fracture after the diagnosis of iron overload or first record of high ferritin.
- A sensitivity analysis was conducted to check the impact of laboratory-confirmed iron overload on the risk for osteoporotic fracture compared with a diagnosis code without elevated ferritin.
TAKEAWAY:
- In the overall cohort, patients with iron overload had a 55% higher risk for any osteoporotic fracture than control individuals (adjusted hazard ratio [aHR], 1.55; 95% CI, 1.42-1.68), with the highest risk observed for vertebral fractures (aHR, 1.97; 95% CI, 1.63-2.37) and humerus fractures (aHR, 1.91; 95% CI, 1.61-2.26).
- Patients with laboratory-confirmed iron overload showed a 91% increased risk for any fracture (aHR, 1.91; 95% CI, 1.73-2.10), with a 2.5-fold higher risk observed for vertebral fractures (aHR, 2.51; 95% CI, 2.01-3.12), followed by humerus fractures (aHR, 2.41; 95% CI, 1.96-2.95).
- There was no increased risk for fracture at any site in patients with a diagnosis of an iron overloading disorder but no laboratory-confirmed iron overload.
- No sex-specific differences were identified in the association between iron overload and fracture risk.
IN PRACTICE:
“The main clinical message from our findings is that clinicians should consider iron overloading as a risk factor for fracture. Importantly, among high-risk patients presenting with serum ferritin values exceeding 1000 μg/L, osteoporosis screening and treatment strategies should be initiated in accordance with the guidelines for patients with hepatic disease,” the authors wrote.
SOURCE:
The study was led by Andrea Michelle Burden, PhD, Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zürich in Switzerland, and was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study could not assess the duration of iron overload on fracture risk, and thus, patients could enter the cohort with a single elevated serum ferritin value that may not have reflected systemic iron overload. The authors also acknowledged potential exposure misclassification among matched control individuals because only 2.9% had a serum ferritin value available at baseline. Also, researchers were unable to adjust for inflammation status due to the limited availability of C-reactive protein measurements and the lack of leukocyte count data in primary care settings.
DISCLOSURES:
This study received support through grants from the German Research Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with serum ferritin levels higher than 1000 μg/L show a 91% increased risk for any fracture, with a doubled risk for vertebral and humerus fractures compared with those without iron overload.
METHODOLOGY:
- Iron overload’s association with decreased bone mineral density is established, but its relationship to osteoporotic fracture risk has remained understudied and inconsistent across fracture sites.
- Researchers conducted a population-based cohort study using a UK general practice database to evaluate the fracture risk in 20,264 patients with iron overload and 192,956 matched controls without elevated ferritin (mean age, 57 years; about 40% women).
- Patients with iron overload were identified as those with laboratory-confirmed iron overload (serum ferritin levels > 1000 μg/L; n = 13,510) or a diagnosis of an iron overloading disorder, such as thalassemia major, sickle cell disease, or hemochromatosis (n = 6754).
- The primary outcome of interest was the first occurrence of an osteoporotic fracture after the diagnosis of iron overload or first record of high ferritin.
- A sensitivity analysis was conducted to check the impact of laboratory-confirmed iron overload on the risk for osteoporotic fracture compared with a diagnosis code without elevated ferritin.
TAKEAWAY:
- In the overall cohort, patients with iron overload had a 55% higher risk for any osteoporotic fracture than control individuals (adjusted hazard ratio [aHR], 1.55; 95% CI, 1.42-1.68), with the highest risk observed for vertebral fractures (aHR, 1.97; 95% CI, 1.63-2.37) and humerus fractures (aHR, 1.91; 95% CI, 1.61-2.26).
- Patients with laboratory-confirmed iron overload showed a 91% increased risk for any fracture (aHR, 1.91; 95% CI, 1.73-2.10), with a 2.5-fold higher risk observed for vertebral fractures (aHR, 2.51; 95% CI, 2.01-3.12), followed by humerus fractures (aHR, 2.41; 95% CI, 1.96-2.95).
- There was no increased risk for fracture at any site in patients with a diagnosis of an iron overloading disorder but no laboratory-confirmed iron overload.
- No sex-specific differences were identified in the association between iron overload and fracture risk.
IN PRACTICE:
“The main clinical message from our findings is that clinicians should consider iron overloading as a risk factor for fracture. Importantly, among high-risk patients presenting with serum ferritin values exceeding 1000 μg/L, osteoporosis screening and treatment strategies should be initiated in accordance with the guidelines for patients with hepatic disease,” the authors wrote.
SOURCE:
The study was led by Andrea Michelle Burden, PhD, Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zürich in Switzerland, and was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study could not assess the duration of iron overload on fracture risk, and thus, patients could enter the cohort with a single elevated serum ferritin value that may not have reflected systemic iron overload. The authors also acknowledged potential exposure misclassification among matched control individuals because only 2.9% had a serum ferritin value available at baseline. Also, researchers were unable to adjust for inflammation status due to the limited availability of C-reactive protein measurements and the lack of leukocyte count data in primary care settings.
DISCLOSURES:
This study received support through grants from the German Research Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Patients with serum ferritin levels higher than 1000 μg/L show a 91% increased risk for any fracture, with a doubled risk for vertebral and humerus fractures compared with those without iron overload.
METHODOLOGY:
- Iron overload’s association with decreased bone mineral density is established, but its relationship to osteoporotic fracture risk has remained understudied and inconsistent across fracture sites.
- Researchers conducted a population-based cohort study using a UK general practice database to evaluate the fracture risk in 20,264 patients with iron overload and 192,956 matched controls without elevated ferritin (mean age, 57 years; about 40% women).
- Patients with iron overload were identified as those with laboratory-confirmed iron overload (serum ferritin levels > 1000 μg/L; n = 13,510) or a diagnosis of an iron overloading disorder, such as thalassemia major, sickle cell disease, or hemochromatosis (n = 6754).
- The primary outcome of interest was the first occurrence of an osteoporotic fracture after the diagnosis of iron overload or first record of high ferritin.
- A sensitivity analysis was conducted to check the impact of laboratory-confirmed iron overload on the risk for osteoporotic fracture compared with a diagnosis code without elevated ferritin.
TAKEAWAY:
- In the overall cohort, patients with iron overload had a 55% higher risk for any osteoporotic fracture than control individuals (adjusted hazard ratio [aHR], 1.55; 95% CI, 1.42-1.68), with the highest risk observed for vertebral fractures (aHR, 1.97; 95% CI, 1.63-2.37) and humerus fractures (aHR, 1.91; 95% CI, 1.61-2.26).
- Patients with laboratory-confirmed iron overload showed a 91% increased risk for any fracture (aHR, 1.91; 95% CI, 1.73-2.10), with a 2.5-fold higher risk observed for vertebral fractures (aHR, 2.51; 95% CI, 2.01-3.12), followed by humerus fractures (aHR, 2.41; 95% CI, 1.96-2.95).
- There was no increased risk for fracture at any site in patients with a diagnosis of an iron overloading disorder but no laboratory-confirmed iron overload.
- No sex-specific differences were identified in the association between iron overload and fracture risk.
IN PRACTICE:
“The main clinical message from our findings is that clinicians should consider iron overloading as a risk factor for fracture. Importantly, among high-risk patients presenting with serum ferritin values exceeding 1000 μg/L, osteoporosis screening and treatment strategies should be initiated in accordance with the guidelines for patients with hepatic disease,” the authors wrote.
SOURCE:
The study was led by Andrea Michelle Burden, PhD, Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zürich in Switzerland, and was published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The study could not assess the duration of iron overload on fracture risk, and thus, patients could enter the cohort with a single elevated serum ferritin value that may not have reflected systemic iron overload. The authors also acknowledged potential exposure misclassification among matched control individuals because only 2.9% had a serum ferritin value available at baseline. Also, researchers were unable to adjust for inflammation status due to the limited availability of C-reactive protein measurements and the lack of leukocyte count data in primary care settings.
DISCLOSURES:
This study received support through grants from the German Research Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New Gel Stops Severe Bleeding in Seconds
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me today to discuss a novel, plant-based approach to stopping moderate to severe bleeding is Joe Landolina, CEO and cofounder of Cresilon. Welcome, Joe.
Joe Landolina, MS: Thank you so much for taking the time. It’s great to be here.
Educational Background and Inception of Cresilon
Glatter: It’s a pleasure to have you join me, and I want to congratulate you on your recent 510(k) FDA clearance for your novel product to save lives and stop bleeding. To begin with, can you explain how the idea for launching your company came about?
Landolina: The way that Cresilon came about was a little bit unorthodox, because I was 17 years old when I invented the technology behind the product that eventually became Traumagel®.
My grandfather was an ex-pharmaceutical executive, who later in life started a vineyard. I grew up on a vineyard with a winery chemistry lab across the street from my house and a grandfather who learned lab safety in the 60s. So, that meant that the day I learned how to walk, I was tossed into a lab and I fell head over heels in love with lab research.
That started experimentation and my academic pursuits. That led to discovering a blend of two plant-based polymers derived from algae that stop bleeding on contact, effectively creating a mechanical barrier and allowing anything from a gunshot wound to anything quite a bit more minor to stop in a matter of seconds.
Glatter: Your background is in biomedical engineering. How is it that you started tinkering and doing all this type of work?
Landolina: That’s correct. I did my undergrad in chemical engineering, and my graduate studies were in biomedical engineering. For me, that was supposed to be a pathway into medical school. I always wanted to be a surgeon myself, and I love the field of medicine.
As a freshman in college at NYU Engineering, I had this idea. I entered it into NYU’s business plan competition, and we won at the engineering school. That gave us just enough capital to start developing and researching Traumagel more, and Cresilon was born out of that research.
Techniques for Stopping Hemorrhage
Glatter: In terms of stopping hemorrhage, which takes so many lives in the United States and globally — certainly, uncontrolled hemorrhage — what are the techniques that you see, prior to the arrival of your product, as being effective? Can you elucidate some of these techniques?
Landolina: In emergency medicine, the primary mode of controlling hemorrhage is passive. It’s what, in Brooklyn, we like to call “pressure and a prayer”, where you have a material that’s either gauze or an impregnated gauze in most cases, where the mode of action is absorbing blood, with the adjunct of pressure by the first responder or by the clinician who’s providing aid.
These types of technologies are widespread. There are many versions of this technology carried by EMS agencies, trauma bays, US military soldiers, and soldiers across NATO countries. But these types of technologies tend to be relatively inefficient, meaning that they’re very difficult to get into wounds because of the gauze or the powder form of the devices, and it’s very hard to get them in contact with the form of bleeding.
On top of that, if the patient is clotting compromised or immunocompromised in some way, the ability to create a durable clot that will not be ripped off when you remove the product at the next level of care is also of concern. And so, this type of technology or the type of treatment of massive hemorrhage hasn’t changed in decades.
Current Applications and Potential Use
Glatter: I envision this product will be carried by paramedics, used on the battlefield at some point after your FDA clearance, and recently it went through.
Do you see any possibility that this could be an AED equivalent to Stop the Bleed? In other words, could the average lay person be trained to use your product if kits are available?
Landolina: To be very clear, Traumagel today is only approved or cleared under a “prescription-only” indication, which means that it will not initially be available OTC. However, that is our goal. Our goal is to make this product available and usable by someone with no medical training whatsoever.
The form factor of being a gel in a syringe lends itself well to that, meaning that we try to make it as easy as point and shoot to control hemorrhage, where there’s not as much technique to be learned in the application of a product like Traumagel as there is in current hemorrhage control techniques.
Mechanism of Action and Physiology
Glatter: Once you apply Traumagel, can you explain what happens to the product after it’s applied and the bleeding has stopped? Does it get reabsorbed by the body? What’s the process here?
Landolina: Under Traumagel’s indication, because it’s used in traumatic injury, it must be removed within 24 hours.
One of the big benefits of Traumagel is that when the patient produces a blood clot underneath Traumagel, it doesn’t become incorporated within the gel itself. To contrast that with the use of gauze, gauze is porous. The clot ends up wrapped around the fibers of the gauze, so if you peel the gauze away, it’s very likely that clot is coming off with it. The surgeon or the clinician at the next level of care is going to have to deal with the re-bleed.
You can remove Traumagel cleanly and entirely without disturbing the underlying clot. That’s a major benefit, not only to the patient but also to the next level of care, to the next clinician or physician that is required to remove the product.
Glatter: How is it possible to remove the substance without disturbing the clot? Can you explain in more detail?
Landolina: That’s one of the hallmarks of these plant-based polymers and the way that we design Traumagel itself. Traumagel is completely nonporous, and it has no fibrous nature to it. What that means is when the patient produces a blood clot or fibrin next to or on top of Traumagel, that fibrin ends up not incorporated within the polymers of Traumagel itself.
Over time, because Traumagel is a hydrogel, meaning that by weight it’s mostly water, you end up having less adhesion to the clot over time. When it’s time to remove Traumagel from the injury, it has lost almost all of its adhesive capabilities, meaning that when you peel it away, that clot is going to stick better to tissue than it will to the gel itself.
Glatter: Can you explain a little bit about the matrix that’s formed, the physiology, and how the polymers work to form this matrix?
Landolina: Sure. Traumagel is made of two polysaccharides that are plant derived. One polysaccharide is polyanionic, and the other is polycationic, meaning one has negative charges and the other has positive charges, which together create almost a Lego block effect, where when the material comes in contact with tissue, it adheres strongly and allows for itself to effectively create a mechanical barrier against bleeding.
Courtesy of Cresilon
Landolina: Even in the face of major arterial blood flow, Traumagel will stay where it needs to stay, and it’s not going to get washed away. This means that it is much more easily appliable to these types of surfaces and will allow the patient to produce their own endogenous fibrin clot at that location.
Like I mentioned before, when that fibrin clot is formed, because the gel itself has no pores or fibers, it doesn’t become incorporated within the fibrin clot. You can take the gel away, leaving that clot behind without the chance of a rebleed.
Testing With Major Bleeds
Glatter: In terms of bleeding itself, have you tested your product with major aortic bleeds or carotid bleeds in preclinical work?
Landolina: We have used the US military’s model for lethal hemorrhage, and the idea there is to create a model that is just that — lethal. These are the worst types of bleeds that you can possibly imagine, where the patients are clotting compromised, and where you have, in most cases, a very strong arterial component, so something like a femoral artery bleed.
We’ve also tested in carotid artery, aortic applications, as well as combinations of venous and arterial bleeds. The idea here is to show the use of the product in the absolute worst-case scenario so that when this translates into the clinic, the models that we’ve used for evaluation, hopefully, are worse than what actually rolls into the trauma bay.
Glatter: Excellent. What’s the mean time to stop an arterial vs a venous bleed? Are we talking a matter of seconds?
Landolina: In the case of a healthy patient, meaning a patient without clotting compromise, you’re in a matter of seconds. It’s less than 10 seconds.
In the case where you have clotting compromise, a deep, complicated wound geometry, we recommend holding a pressure bandage on for 3 minutes just because it increases the chance of Traumagel coming into contact with the bleed, especially when you can’t visualize the bleed in the bleed source. Because of that pressure time, that becomes the mean. But again, it’s highly dependent on the type of bleed and the style of application.
Failure Rates and Effectiveness
Glatter: As a segue to that, what is the failure rate based on your studies and internal research using Traumagel? Have there been cases where bleeding has not been able to be stopped?
Landolina: It depends on the study, but the failure rates are incredibly low with Traumagel, assuming that it’s correctly used. That’s one of the benefits to this product, where with proper technique, with overwrap with gauze, you nearly always get control of hemorrhage with a product like this.
Glatter: Is manual pressure required in that sense? From what you described earlier, manual pressure would not be required.
Landolina: It depends on the injury. What we recommend is that, if you have a very deep wound where you cannot visualize the source of bleed, you use pressure to seat Traumagel into the source of bleeding, meaning that you’re following Committee on Tactical Combat Casualty Care (Co-TCCC) regulations or requirements, where you’re over wrapping with gauze, and you’re providing a pressure wrapping to ensure that the Traumagel is in contact with the bleed while it’s doing what it’s doing.
In most cases, it doesn’t hurt to apply pressure on top of Traumagel as well. In more surface level bleeds, you don’t need pressure at all.
Applications Beyond Trauma
Glatter: Interesting. In terms of further applications (eg, nose bleeds or GYN bleeding, which are life-threatening), do you see this coming as an application for the future?
Landolina: That’s where we’re working. Traumagel is the successor to an animal health product called Vetigel. The formulations of the gel behind Vetigel and Traumagel are identical. Vetigel has a full surgical indication, and that’s everything from epistaxis to neuro and spine procedures, into cardiovascular and soft tissue surgeries, orthopedic medicine, and so on.
Cresilon’s goal is to eventually expand the indication of our technology to include surgical indications and other indications where we can help any patient that’s bleeding.
Glatter: That’s important, because we use prehospital whole blood, low titer, specifically, when patients have life-threatening hemorrhage. With your product, that would reduce the amount of blood products that would need to be administered. This could be a real game changer.
Landolina: Definitely, that’s the goal we’re working on.
Infection Risks and Biocompatibility
Glatter: In terms of any risk for infection, has that been studied as well? Does Traumagel in any way lead to increased rates of infection?
Landolina: Traumagel is biocompatible. It’s a sterile product. We’ve done the full suite of biocompatibility testing as required by FDA. On top of that, remember that Vetigel, which is the same formulation, is an implantable product. As a result, that has even extended biocompatibility testing beyond what would be necessary for an external product.
In Vetigel’s use case, which has been used now in over 60,000 patients, primarily companion animals, dogs and cats, we haven’t seen instances of infection. There’s no reason to believe that we would see that clinically with Traumagel.
Research Collaborations and Future Applications
Glatter: In terms of other research that your company’s embarked on preclinically, I understand there were some studies done at Walter Reed Army Institute of Research. I was wondering if you could expand on these, specifically, in terms of traumatic brain injury (TBI) and hemorrhage related to that. For example, with shrapnel or even a gunshot wound.
Landolina: The Walter Reed collaboration with Cresilon is something that I’m particularly excited about, because it marks Cresilon’s first project that’s outside the scope of just hemostasis. Walter Reed came to us with this proposal where there’s a big challenge in a subset of TBI called penetrating ballistic-like brain injury, where the brain has been penetrated by a bullet, shrapnel, or some other projectile, and there’s an injury that exposes the brain to the outside.
Today, there is no standard of care to treat patients with those types of injuries. In many cases, mortality is caused through swelling of the brain, or collapse of the brain. What they came to us with was the potential of using our technology, not primarily as a hemostatic agent, but to be able to stabilize that patient enough to get to the next level of care to be treated by a neurosurgeon.
That study Walter Reed did was just a pilot that was done in small animals. In that pilot, they showed that over the period of treatment, there was no negative change in vital signs, no increase in edema or in swelling, or in any of the biomarkers that were being monitored at that time.
At the very least, this is not full indication that this indication will work for Cresilon, but it shows that there’s promise. It’s something that we’re working on and hopefully we’ll be able to bring to market soon.
Glatter: Certainly, maintaining intracranial pressure and cerebral perfusion pressures are very critical. In the future, do you think this product would be able to be deployed endovascularly? Imagine this in terms of stopping bleeding from some source, whether it’s from a stroke or another intracranial source.
Landolina: That’s been an area of interest for us. We have no evidence to prove that indication works at this point, but there’s also nothing to say that it wouldn’t be possible for our technology. At this point, we’ve only looked at a cursory level at those indications.
Glatter: Does the use of Traumagel obviate the need for a more definitive repair (eg, with sutures) or something that’s more permanent?
Landolina: I always say that Traumagel — and Vetigel, for that matter — is not a replacement for good surgical technique. The surgeon always needs to make his or her best judgment when reviewing the patient. That doesn’t mean that there won’t need to be sutures or vascular repair in most of these cases, especially in major trauma.
Final Takeaways
Glatter: Do you have some bullet points or pearls you could give our audience as a takeaway?
Landolina: When Cresilon looks at Traumagel — and for us, Traumagel is the next generation of hemostatic agent, especially in trauma care and in emergency medicine — it allows for a far-simplified application of the product and much faster control of hemorrhage with better patient outcomes.
As we roll this out through EMS agencies, trauma hospitals, military agencies, and eventually to the general public through a future indication, it’s something we’re very excited about. Personally, I started this business 14 years ago, and so it’s great to see our mission of saving lives transitioning to saving human lives.
Glatter: I look forward to seeing this product in the emergency department, but also in other settings, such as in the operating room where we can really help patients who are dying from hemorrhage, certainly on the battlefield, and the lay public. If someone were to come upon a patient who’s bleeding out, this could be certainly a game changer and a lifesaver.
I want to thank you for your time. This is a really important product that’s transformed the lives of so many animals, but also people in the future.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He reported no relevant conflicts of interest. Mr. Landolina is the CEO and co-founder of Cresilon, a biotechnology company specializing in plant-based solutions for emergency bleeding control.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me today to discuss a novel, plant-based approach to stopping moderate to severe bleeding is Joe Landolina, CEO and cofounder of Cresilon. Welcome, Joe.
Joe Landolina, MS: Thank you so much for taking the time. It’s great to be here.
Educational Background and Inception of Cresilon
Glatter: It’s a pleasure to have you join me, and I want to congratulate you on your recent 510(k) FDA clearance for your novel product to save lives and stop bleeding. To begin with, can you explain how the idea for launching your company came about?
Landolina: The way that Cresilon came about was a little bit unorthodox, because I was 17 years old when I invented the technology behind the product that eventually became Traumagel®.
My grandfather was an ex-pharmaceutical executive, who later in life started a vineyard. I grew up on a vineyard with a winery chemistry lab across the street from my house and a grandfather who learned lab safety in the 60s. So, that meant that the day I learned how to walk, I was tossed into a lab and I fell head over heels in love with lab research.
That started experimentation and my academic pursuits. That led to discovering a blend of two plant-based polymers derived from algae that stop bleeding on contact, effectively creating a mechanical barrier and allowing anything from a gunshot wound to anything quite a bit more minor to stop in a matter of seconds.
Glatter: Your background is in biomedical engineering. How is it that you started tinkering and doing all this type of work?
Landolina: That’s correct. I did my undergrad in chemical engineering, and my graduate studies were in biomedical engineering. For me, that was supposed to be a pathway into medical school. I always wanted to be a surgeon myself, and I love the field of medicine.
As a freshman in college at NYU Engineering, I had this idea. I entered it into NYU’s business plan competition, and we won at the engineering school. That gave us just enough capital to start developing and researching Traumagel more, and Cresilon was born out of that research.
Techniques for Stopping Hemorrhage
Glatter: In terms of stopping hemorrhage, which takes so many lives in the United States and globally — certainly, uncontrolled hemorrhage — what are the techniques that you see, prior to the arrival of your product, as being effective? Can you elucidate some of these techniques?
Landolina: In emergency medicine, the primary mode of controlling hemorrhage is passive. It’s what, in Brooklyn, we like to call “pressure and a prayer”, where you have a material that’s either gauze or an impregnated gauze in most cases, where the mode of action is absorbing blood, with the adjunct of pressure by the first responder or by the clinician who’s providing aid.
These types of technologies are widespread. There are many versions of this technology carried by EMS agencies, trauma bays, US military soldiers, and soldiers across NATO countries. But these types of technologies tend to be relatively inefficient, meaning that they’re very difficult to get into wounds because of the gauze or the powder form of the devices, and it’s very hard to get them in contact with the form of bleeding.
On top of that, if the patient is clotting compromised or immunocompromised in some way, the ability to create a durable clot that will not be ripped off when you remove the product at the next level of care is also of concern. And so, this type of technology or the type of treatment of massive hemorrhage hasn’t changed in decades.
Current Applications and Potential Use
Glatter: I envision this product will be carried by paramedics, used on the battlefield at some point after your FDA clearance, and recently it went through.
Do you see any possibility that this could be an AED equivalent to Stop the Bleed? In other words, could the average lay person be trained to use your product if kits are available?
Landolina: To be very clear, Traumagel today is only approved or cleared under a “prescription-only” indication, which means that it will not initially be available OTC. However, that is our goal. Our goal is to make this product available and usable by someone with no medical training whatsoever.
The form factor of being a gel in a syringe lends itself well to that, meaning that we try to make it as easy as point and shoot to control hemorrhage, where there’s not as much technique to be learned in the application of a product like Traumagel as there is in current hemorrhage control techniques.
Mechanism of Action and Physiology
Glatter: Once you apply Traumagel, can you explain what happens to the product after it’s applied and the bleeding has stopped? Does it get reabsorbed by the body? What’s the process here?
Landolina: Under Traumagel’s indication, because it’s used in traumatic injury, it must be removed within 24 hours.
One of the big benefits of Traumagel is that when the patient produces a blood clot underneath Traumagel, it doesn’t become incorporated within the gel itself. To contrast that with the use of gauze, gauze is porous. The clot ends up wrapped around the fibers of the gauze, so if you peel the gauze away, it’s very likely that clot is coming off with it. The surgeon or the clinician at the next level of care is going to have to deal with the re-bleed.
You can remove Traumagel cleanly and entirely without disturbing the underlying clot. That’s a major benefit, not only to the patient but also to the next level of care, to the next clinician or physician that is required to remove the product.
Glatter: How is it possible to remove the substance without disturbing the clot? Can you explain in more detail?
Landolina: That’s one of the hallmarks of these plant-based polymers and the way that we design Traumagel itself. Traumagel is completely nonporous, and it has no fibrous nature to it. What that means is when the patient produces a blood clot or fibrin next to or on top of Traumagel, that fibrin ends up not incorporated within the polymers of Traumagel itself.
Over time, because Traumagel is a hydrogel, meaning that by weight it’s mostly water, you end up having less adhesion to the clot over time. When it’s time to remove Traumagel from the injury, it has lost almost all of its adhesive capabilities, meaning that when you peel it away, that clot is going to stick better to tissue than it will to the gel itself.
Glatter: Can you explain a little bit about the matrix that’s formed, the physiology, and how the polymers work to form this matrix?
Landolina: Sure. Traumagel is made of two polysaccharides that are plant derived. One polysaccharide is polyanionic, and the other is polycationic, meaning one has negative charges and the other has positive charges, which together create almost a Lego block effect, where when the material comes in contact with tissue, it adheres strongly and allows for itself to effectively create a mechanical barrier against bleeding.
Courtesy of Cresilon
Landolina: Even in the face of major arterial blood flow, Traumagel will stay where it needs to stay, and it’s not going to get washed away. This means that it is much more easily appliable to these types of surfaces and will allow the patient to produce their own endogenous fibrin clot at that location.
Like I mentioned before, when that fibrin clot is formed, because the gel itself has no pores or fibers, it doesn’t become incorporated within the fibrin clot. You can take the gel away, leaving that clot behind without the chance of a rebleed.
Testing With Major Bleeds
Glatter: In terms of bleeding itself, have you tested your product with major aortic bleeds or carotid bleeds in preclinical work?
Landolina: We have used the US military’s model for lethal hemorrhage, and the idea there is to create a model that is just that — lethal. These are the worst types of bleeds that you can possibly imagine, where the patients are clotting compromised, and where you have, in most cases, a very strong arterial component, so something like a femoral artery bleed.
We’ve also tested in carotid artery, aortic applications, as well as combinations of venous and arterial bleeds. The idea here is to show the use of the product in the absolute worst-case scenario so that when this translates into the clinic, the models that we’ve used for evaluation, hopefully, are worse than what actually rolls into the trauma bay.
Glatter: Excellent. What’s the mean time to stop an arterial vs a venous bleed? Are we talking a matter of seconds?
Landolina: In the case of a healthy patient, meaning a patient without clotting compromise, you’re in a matter of seconds. It’s less than 10 seconds.
In the case where you have clotting compromise, a deep, complicated wound geometry, we recommend holding a pressure bandage on for 3 minutes just because it increases the chance of Traumagel coming into contact with the bleed, especially when you can’t visualize the bleed in the bleed source. Because of that pressure time, that becomes the mean. But again, it’s highly dependent on the type of bleed and the style of application.
Failure Rates and Effectiveness
Glatter: As a segue to that, what is the failure rate based on your studies and internal research using Traumagel? Have there been cases where bleeding has not been able to be stopped?
Landolina: It depends on the study, but the failure rates are incredibly low with Traumagel, assuming that it’s correctly used. That’s one of the benefits to this product, where with proper technique, with overwrap with gauze, you nearly always get control of hemorrhage with a product like this.
Glatter: Is manual pressure required in that sense? From what you described earlier, manual pressure would not be required.
Landolina: It depends on the injury. What we recommend is that, if you have a very deep wound where you cannot visualize the source of bleed, you use pressure to seat Traumagel into the source of bleeding, meaning that you’re following Committee on Tactical Combat Casualty Care (Co-TCCC) regulations or requirements, where you’re over wrapping with gauze, and you’re providing a pressure wrapping to ensure that the Traumagel is in contact with the bleed while it’s doing what it’s doing.
In most cases, it doesn’t hurt to apply pressure on top of Traumagel as well. In more surface level bleeds, you don’t need pressure at all.
Applications Beyond Trauma
Glatter: Interesting. In terms of further applications (eg, nose bleeds or GYN bleeding, which are life-threatening), do you see this coming as an application for the future?
Landolina: That’s where we’re working. Traumagel is the successor to an animal health product called Vetigel. The formulations of the gel behind Vetigel and Traumagel are identical. Vetigel has a full surgical indication, and that’s everything from epistaxis to neuro and spine procedures, into cardiovascular and soft tissue surgeries, orthopedic medicine, and so on.
Cresilon’s goal is to eventually expand the indication of our technology to include surgical indications and other indications where we can help any patient that’s bleeding.
Glatter: That’s important, because we use prehospital whole blood, low titer, specifically, when patients have life-threatening hemorrhage. With your product, that would reduce the amount of blood products that would need to be administered. This could be a real game changer.
Landolina: Definitely, that’s the goal we’re working on.
Infection Risks and Biocompatibility
Glatter: In terms of any risk for infection, has that been studied as well? Does Traumagel in any way lead to increased rates of infection?
Landolina: Traumagel is biocompatible. It’s a sterile product. We’ve done the full suite of biocompatibility testing as required by FDA. On top of that, remember that Vetigel, which is the same formulation, is an implantable product. As a result, that has even extended biocompatibility testing beyond what would be necessary for an external product.
In Vetigel’s use case, which has been used now in over 60,000 patients, primarily companion animals, dogs and cats, we haven’t seen instances of infection. There’s no reason to believe that we would see that clinically with Traumagel.
Research Collaborations and Future Applications
Glatter: In terms of other research that your company’s embarked on preclinically, I understand there were some studies done at Walter Reed Army Institute of Research. I was wondering if you could expand on these, specifically, in terms of traumatic brain injury (TBI) and hemorrhage related to that. For example, with shrapnel or even a gunshot wound.
Landolina: The Walter Reed collaboration with Cresilon is something that I’m particularly excited about, because it marks Cresilon’s first project that’s outside the scope of just hemostasis. Walter Reed came to us with this proposal where there’s a big challenge in a subset of TBI called penetrating ballistic-like brain injury, where the brain has been penetrated by a bullet, shrapnel, or some other projectile, and there’s an injury that exposes the brain to the outside.
Today, there is no standard of care to treat patients with those types of injuries. In many cases, mortality is caused through swelling of the brain, or collapse of the brain. What they came to us with was the potential of using our technology, not primarily as a hemostatic agent, but to be able to stabilize that patient enough to get to the next level of care to be treated by a neurosurgeon.
That study Walter Reed did was just a pilot that was done in small animals. In that pilot, they showed that over the period of treatment, there was no negative change in vital signs, no increase in edema or in swelling, or in any of the biomarkers that were being monitored at that time.
At the very least, this is not full indication that this indication will work for Cresilon, but it shows that there’s promise. It’s something that we’re working on and hopefully we’ll be able to bring to market soon.
Glatter: Certainly, maintaining intracranial pressure and cerebral perfusion pressures are very critical. In the future, do you think this product would be able to be deployed endovascularly? Imagine this in terms of stopping bleeding from some source, whether it’s from a stroke or another intracranial source.
Landolina: That’s been an area of interest for us. We have no evidence to prove that indication works at this point, but there’s also nothing to say that it wouldn’t be possible for our technology. At this point, we’ve only looked at a cursory level at those indications.
Glatter: Does the use of Traumagel obviate the need for a more definitive repair (eg, with sutures) or something that’s more permanent?
Landolina: I always say that Traumagel — and Vetigel, for that matter — is not a replacement for good surgical technique. The surgeon always needs to make his or her best judgment when reviewing the patient. That doesn’t mean that there won’t need to be sutures or vascular repair in most of these cases, especially in major trauma.
Final Takeaways
Glatter: Do you have some bullet points or pearls you could give our audience as a takeaway?
Landolina: When Cresilon looks at Traumagel — and for us, Traumagel is the next generation of hemostatic agent, especially in trauma care and in emergency medicine — it allows for a far-simplified application of the product and much faster control of hemorrhage with better patient outcomes.
As we roll this out through EMS agencies, trauma hospitals, military agencies, and eventually to the general public through a future indication, it’s something we’re very excited about. Personally, I started this business 14 years ago, and so it’s great to see our mission of saving lives transitioning to saving human lives.
Glatter: I look forward to seeing this product in the emergency department, but also in other settings, such as in the operating room where we can really help patients who are dying from hemorrhage, certainly on the battlefield, and the lay public. If someone were to come upon a patient who’s bleeding out, this could be certainly a game changer and a lifesaver.
I want to thank you for your time. This is a really important product that’s transformed the lives of so many animals, but also people in the future.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He reported no relevant conflicts of interest. Mr. Landolina is the CEO and co-founder of Cresilon, a biotechnology company specializing in plant-based solutions for emergency bleeding control.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Joining me today to discuss a novel, plant-based approach to stopping moderate to severe bleeding is Joe Landolina, CEO and cofounder of Cresilon. Welcome, Joe.
Joe Landolina, MS: Thank you so much for taking the time. It’s great to be here.
Educational Background and Inception of Cresilon
Glatter: It’s a pleasure to have you join me, and I want to congratulate you on your recent 510(k) FDA clearance for your novel product to save lives and stop bleeding. To begin with, can you explain how the idea for launching your company came about?
Landolina: The way that Cresilon came about was a little bit unorthodox, because I was 17 years old when I invented the technology behind the product that eventually became Traumagel®.
My grandfather was an ex-pharmaceutical executive, who later in life started a vineyard. I grew up on a vineyard with a winery chemistry lab across the street from my house and a grandfather who learned lab safety in the 60s. So, that meant that the day I learned how to walk, I was tossed into a lab and I fell head over heels in love with lab research.
That started experimentation and my academic pursuits. That led to discovering a blend of two plant-based polymers derived from algae that stop bleeding on contact, effectively creating a mechanical barrier and allowing anything from a gunshot wound to anything quite a bit more minor to stop in a matter of seconds.
Glatter: Your background is in biomedical engineering. How is it that you started tinkering and doing all this type of work?
Landolina: That’s correct. I did my undergrad in chemical engineering, and my graduate studies were in biomedical engineering. For me, that was supposed to be a pathway into medical school. I always wanted to be a surgeon myself, and I love the field of medicine.
As a freshman in college at NYU Engineering, I had this idea. I entered it into NYU’s business plan competition, and we won at the engineering school. That gave us just enough capital to start developing and researching Traumagel more, and Cresilon was born out of that research.
Techniques for Stopping Hemorrhage
Glatter: In terms of stopping hemorrhage, which takes so many lives in the United States and globally — certainly, uncontrolled hemorrhage — what are the techniques that you see, prior to the arrival of your product, as being effective? Can you elucidate some of these techniques?
Landolina: In emergency medicine, the primary mode of controlling hemorrhage is passive. It’s what, in Brooklyn, we like to call “pressure and a prayer”, where you have a material that’s either gauze or an impregnated gauze in most cases, where the mode of action is absorbing blood, with the adjunct of pressure by the first responder or by the clinician who’s providing aid.
These types of technologies are widespread. There are many versions of this technology carried by EMS agencies, trauma bays, US military soldiers, and soldiers across NATO countries. But these types of technologies tend to be relatively inefficient, meaning that they’re very difficult to get into wounds because of the gauze or the powder form of the devices, and it’s very hard to get them in contact with the form of bleeding.
On top of that, if the patient is clotting compromised or immunocompromised in some way, the ability to create a durable clot that will not be ripped off when you remove the product at the next level of care is also of concern. And so, this type of technology or the type of treatment of massive hemorrhage hasn’t changed in decades.
Current Applications and Potential Use
Glatter: I envision this product will be carried by paramedics, used on the battlefield at some point after your FDA clearance, and recently it went through.
Do you see any possibility that this could be an AED equivalent to Stop the Bleed? In other words, could the average lay person be trained to use your product if kits are available?
Landolina: To be very clear, Traumagel today is only approved or cleared under a “prescription-only” indication, which means that it will not initially be available OTC. However, that is our goal. Our goal is to make this product available and usable by someone with no medical training whatsoever.
The form factor of being a gel in a syringe lends itself well to that, meaning that we try to make it as easy as point and shoot to control hemorrhage, where there’s not as much technique to be learned in the application of a product like Traumagel as there is in current hemorrhage control techniques.
Mechanism of Action and Physiology
Glatter: Once you apply Traumagel, can you explain what happens to the product after it’s applied and the bleeding has stopped? Does it get reabsorbed by the body? What’s the process here?
Landolina: Under Traumagel’s indication, because it’s used in traumatic injury, it must be removed within 24 hours.
One of the big benefits of Traumagel is that when the patient produces a blood clot underneath Traumagel, it doesn’t become incorporated within the gel itself. To contrast that with the use of gauze, gauze is porous. The clot ends up wrapped around the fibers of the gauze, so if you peel the gauze away, it’s very likely that clot is coming off with it. The surgeon or the clinician at the next level of care is going to have to deal with the re-bleed.
You can remove Traumagel cleanly and entirely without disturbing the underlying clot. That’s a major benefit, not only to the patient but also to the next level of care, to the next clinician or physician that is required to remove the product.
Glatter: How is it possible to remove the substance without disturbing the clot? Can you explain in more detail?
Landolina: That’s one of the hallmarks of these plant-based polymers and the way that we design Traumagel itself. Traumagel is completely nonporous, and it has no fibrous nature to it. What that means is when the patient produces a blood clot or fibrin next to or on top of Traumagel, that fibrin ends up not incorporated within the polymers of Traumagel itself.
Over time, because Traumagel is a hydrogel, meaning that by weight it’s mostly water, you end up having less adhesion to the clot over time. When it’s time to remove Traumagel from the injury, it has lost almost all of its adhesive capabilities, meaning that when you peel it away, that clot is going to stick better to tissue than it will to the gel itself.
Glatter: Can you explain a little bit about the matrix that’s formed, the physiology, and how the polymers work to form this matrix?
Landolina: Sure. Traumagel is made of two polysaccharides that are plant derived. One polysaccharide is polyanionic, and the other is polycationic, meaning one has negative charges and the other has positive charges, which together create almost a Lego block effect, where when the material comes in contact with tissue, it adheres strongly and allows for itself to effectively create a mechanical barrier against bleeding.
Courtesy of Cresilon
Landolina: Even in the face of major arterial blood flow, Traumagel will stay where it needs to stay, and it’s not going to get washed away. This means that it is much more easily appliable to these types of surfaces and will allow the patient to produce their own endogenous fibrin clot at that location.
Like I mentioned before, when that fibrin clot is formed, because the gel itself has no pores or fibers, it doesn’t become incorporated within the fibrin clot. You can take the gel away, leaving that clot behind without the chance of a rebleed.
Testing With Major Bleeds
Glatter: In terms of bleeding itself, have you tested your product with major aortic bleeds or carotid bleeds in preclinical work?
Landolina: We have used the US military’s model for lethal hemorrhage, and the idea there is to create a model that is just that — lethal. These are the worst types of bleeds that you can possibly imagine, where the patients are clotting compromised, and where you have, in most cases, a very strong arterial component, so something like a femoral artery bleed.
We’ve also tested in carotid artery, aortic applications, as well as combinations of venous and arterial bleeds. The idea here is to show the use of the product in the absolute worst-case scenario so that when this translates into the clinic, the models that we’ve used for evaluation, hopefully, are worse than what actually rolls into the trauma bay.
Glatter: Excellent. What’s the mean time to stop an arterial vs a venous bleed? Are we talking a matter of seconds?
Landolina: In the case of a healthy patient, meaning a patient without clotting compromise, you’re in a matter of seconds. It’s less than 10 seconds.
In the case where you have clotting compromise, a deep, complicated wound geometry, we recommend holding a pressure bandage on for 3 minutes just because it increases the chance of Traumagel coming into contact with the bleed, especially when you can’t visualize the bleed in the bleed source. Because of that pressure time, that becomes the mean. But again, it’s highly dependent on the type of bleed and the style of application.
Failure Rates and Effectiveness
Glatter: As a segue to that, what is the failure rate based on your studies and internal research using Traumagel? Have there been cases where bleeding has not been able to be stopped?
Landolina: It depends on the study, but the failure rates are incredibly low with Traumagel, assuming that it’s correctly used. That’s one of the benefits to this product, where with proper technique, with overwrap with gauze, you nearly always get control of hemorrhage with a product like this.
Glatter: Is manual pressure required in that sense? From what you described earlier, manual pressure would not be required.
Landolina: It depends on the injury. What we recommend is that, if you have a very deep wound where you cannot visualize the source of bleed, you use pressure to seat Traumagel into the source of bleeding, meaning that you’re following Committee on Tactical Combat Casualty Care (Co-TCCC) regulations or requirements, where you’re over wrapping with gauze, and you’re providing a pressure wrapping to ensure that the Traumagel is in contact with the bleed while it’s doing what it’s doing.
In most cases, it doesn’t hurt to apply pressure on top of Traumagel as well. In more surface level bleeds, you don’t need pressure at all.
Applications Beyond Trauma
Glatter: Interesting. In terms of further applications (eg, nose bleeds or GYN bleeding, which are life-threatening), do you see this coming as an application for the future?
Landolina: That’s where we’re working. Traumagel is the successor to an animal health product called Vetigel. The formulations of the gel behind Vetigel and Traumagel are identical. Vetigel has a full surgical indication, and that’s everything from epistaxis to neuro and spine procedures, into cardiovascular and soft tissue surgeries, orthopedic medicine, and so on.
Cresilon’s goal is to eventually expand the indication of our technology to include surgical indications and other indications where we can help any patient that’s bleeding.
Glatter: That’s important, because we use prehospital whole blood, low titer, specifically, when patients have life-threatening hemorrhage. With your product, that would reduce the amount of blood products that would need to be administered. This could be a real game changer.
Landolina: Definitely, that’s the goal we’re working on.
Infection Risks and Biocompatibility
Glatter: In terms of any risk for infection, has that been studied as well? Does Traumagel in any way lead to increased rates of infection?
Landolina: Traumagel is biocompatible. It’s a sterile product. We’ve done the full suite of biocompatibility testing as required by FDA. On top of that, remember that Vetigel, which is the same formulation, is an implantable product. As a result, that has even extended biocompatibility testing beyond what would be necessary for an external product.
In Vetigel’s use case, which has been used now in over 60,000 patients, primarily companion animals, dogs and cats, we haven’t seen instances of infection. There’s no reason to believe that we would see that clinically with Traumagel.
Research Collaborations and Future Applications
Glatter: In terms of other research that your company’s embarked on preclinically, I understand there were some studies done at Walter Reed Army Institute of Research. I was wondering if you could expand on these, specifically, in terms of traumatic brain injury (TBI) and hemorrhage related to that. For example, with shrapnel or even a gunshot wound.
Landolina: The Walter Reed collaboration with Cresilon is something that I’m particularly excited about, because it marks Cresilon’s first project that’s outside the scope of just hemostasis. Walter Reed came to us with this proposal where there’s a big challenge in a subset of TBI called penetrating ballistic-like brain injury, where the brain has been penetrated by a bullet, shrapnel, or some other projectile, and there’s an injury that exposes the brain to the outside.
Today, there is no standard of care to treat patients with those types of injuries. In many cases, mortality is caused through swelling of the brain, or collapse of the brain. What they came to us with was the potential of using our technology, not primarily as a hemostatic agent, but to be able to stabilize that patient enough to get to the next level of care to be treated by a neurosurgeon.
That study Walter Reed did was just a pilot that was done in small animals. In that pilot, they showed that over the period of treatment, there was no negative change in vital signs, no increase in edema or in swelling, or in any of the biomarkers that were being monitored at that time.
At the very least, this is not full indication that this indication will work for Cresilon, but it shows that there’s promise. It’s something that we’re working on and hopefully we’ll be able to bring to market soon.
Glatter: Certainly, maintaining intracranial pressure and cerebral perfusion pressures are very critical. In the future, do you think this product would be able to be deployed endovascularly? Imagine this in terms of stopping bleeding from some source, whether it’s from a stroke or another intracranial source.
Landolina: That’s been an area of interest for us. We have no evidence to prove that indication works at this point, but there’s also nothing to say that it wouldn’t be possible for our technology. At this point, we’ve only looked at a cursory level at those indications.
Glatter: Does the use of Traumagel obviate the need for a more definitive repair (eg, with sutures) or something that’s more permanent?
Landolina: I always say that Traumagel — and Vetigel, for that matter — is not a replacement for good surgical technique. The surgeon always needs to make his or her best judgment when reviewing the patient. That doesn’t mean that there won’t need to be sutures or vascular repair in most of these cases, especially in major trauma.
Final Takeaways
Glatter: Do you have some bullet points or pearls you could give our audience as a takeaway?
Landolina: When Cresilon looks at Traumagel — and for us, Traumagel is the next generation of hemostatic agent, especially in trauma care and in emergency medicine — it allows for a far-simplified application of the product and much faster control of hemorrhage with better patient outcomes.
As we roll this out through EMS agencies, trauma hospitals, military agencies, and eventually to the general public through a future indication, it’s something we’re very excited about. Personally, I started this business 14 years ago, and so it’s great to see our mission of saving lives transitioning to saving human lives.
Glatter: I look forward to seeing this product in the emergency department, but also in other settings, such as in the operating room where we can really help patients who are dying from hemorrhage, certainly on the battlefield, and the lay public. If someone were to come upon a patient who’s bleeding out, this could be certainly a game changer and a lifesaver.
I want to thank you for your time. This is a really important product that’s transformed the lives of so many animals, but also people in the future.
Dr. Glatter is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He reported no relevant conflicts of interest. Mr. Landolina is the CEO and co-founder of Cresilon, a biotechnology company specializing in plant-based solutions for emergency bleeding control.
A version of this article first appeared on Medscape.com.