User login
Significant HbA1c Lowering in Patients Achieving a Hepatitis C Virus Cure (FULL)
The immediate clinically significant reduction in hemoglobin A1c following HCV treatment observed in this study contrasts with the expected rise seen with normal disease progression.
According to estimates, between 2.7 and 3.9 million people are infected with hepatitis C virus (HCV) in the US, with worldwide infection estimated to be about 185 million people.1-3 The majority of patients infected with HCV develop a chronic infection, which is the leading cause of liver-related complications in the Western world, including cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.4 In addition to the direct effects HCV has on the liver, extrahepatic complications can occur, often related to the immune-mediated mechanism of cryoglobulinemia, such as vasculitis, renal disease, and palpable purpura. Additionally, > 70 studies globally have associated HCV with insulin resistance and worsening glycemic control.5,6
The prevalence of patients infected with HCV that have comorbid type 2 diabetes mellitus (T2DM) is estimated to be about 30%.7,8 The landmark cross-sectional National Health and Nutrition Examination Survey III study found the prevalence of T2DM among HCV patients in the US aged > 40 years to be about 3-fold higher than those without HCV.9 These findings were further supported by a Taiwanese prospective community-based cohort study that found a higher incidence of T2DM in HCV-positive patients compared with HCV negative patients (hazard ratio [HR], 1.7; 95% CI, 1.3-2.1).10 This relationship appears to be separate from the diabetogenic effect of cirrhosis itself as a significantly higher prevalence of DM has been observed in people with HCV when compared with people with cirrhosis due to other etiologies.11 Although the mechanism for this relationship is not fully understood and is likely multifactorial, it is believed to primarily be an effect of the HCV core protein increasing phosphorylation of insulin receptor substrate-1.6,12,13 The increased presence of the inflammatory cytokine, tumor necrosis factor-α, is also believed to play a role in the effects on insulinreceptor substrate-1 as well as mediating hepatic insulin resistance, stimulating lipolysis, down-regulating peroxisome proliferator-activated receptor-γ, and interfering with β-cell function.14-17
The relationship between HCV and T2DM has been further established by measured improvements in insulin resistance among patients undergoing HCV treatment with the pre-2011 standard of care—peginterferon and ribavirin.Kawaguchi and colleagues found sustained treatment responders to have a significant decrease in both the homeostatic model assessment-insulin resistance (HOMA-IR) score, representing insulin resistance, and the HOMA-β score, representing β-cell function.18 Improvements in the HOMA-IR score were further validated by Kim and colleagues and a nested cohort within the Hepatitis C Long-term Treatment against Cirrhosis (HALT-C) trial.19,20 Furthermore, Romero-Gómez and colleagues found that patients achieving a cure from HCV treatment defined as a sustained virologic response (SVR) had a nearly 50% reduced risk of impaired fasting glucose or T2DM over a mean posttreatment follow-up of 27 months.21
The recent development of direct-acting antivirals (DAAs) has marked significant HCV treatment advances in terms of efficacy and tolerability, leading current guidelines to emphasize that nearly all patients with HCV would benefit from treatment.22 Despite these guidelines, issues have been documented throughout the US with payors often limiting this costly treatment to only those with advanced fibrotic disease.23 Although the benefits of HCV treatment on reducing liver-related morbidity and mortality may be most appreciated in individuals with advanced fibrotic liver disease, improvements in insulin resistance would suggest potential morbidity and mortality benefits beyond the liver in many more at-risk individuals.24
Increasingly, cases are being reported of new DAA regimens having a significant impact on reducing insulin resistance as demonstrated by marked decreases in antihyperglycemic requirements, fasting blood glucose, and hemoglobin A1c (HbA1c).25-30 One striking case describes a patient being able to de-escalate his regimen from 42 daily units of insulin to a single oral dipeptidyl peptidase-4 inhibitor while maintaining goal HbA1c level over a 2-year time period.31 A database-driven study of veterans found a mean HbA1c drop of 0.37% in its overall included cohort of patients with T2DM who achieved SVR from HCV DAA treatment.32
Despite these data, the individual predictability and variable magnitude of improved insulin resistance based on baseline HbA1c remains unknown. The objective of this study was to assess the impact of HCV treatment with short course DAAs on glucose control in veteran patients with T2DM at a single center.
Methods
This retrospective cohort study was performed at the Department of Veterans Affairs (VA) Northeast Ohio Healthcare System (VANEOHS) in Cleveland. This study received approval from the VANEOHS Institutional Review Board. Retrospective patient data were collected from the Veterans Health Administration (VHA) Computerized Patient Record System (CPRS) electronic health record. Collectively, the VHA has treated > 100,000 patients with DAAs, making it the largest provider of HCV treatment in the US. VANEOHS has treated nearly 2,000 patients with DAAs, rendering it one of the largest single-institution cohorts to be able to examine the effects of HCV treatment on subpopulations, such as patients with T2DM.
Patient Population
Patients were identified using ICD-9/10 codes for T2DM and medication dispense history of hepatitis C DAAs. Patients were included if they had a diagnosis of T2DM, were initiated on a hepatitis C DAA between February 1, 2014 to September 26, 2016. To be eligible, patients were required to have both a baseline HbA1c within 6 months prior to starting HCV treatment as well as a HbA1c within 4 months posttreatment. The HCV treatment included were new short-course DAAs, including sofosbuvir, simeprevir, ombitasvir/paritaprevir/ritonavir ± dasabuvir, ledipasvir/sofosbuvir, elbasvir/grazoprevir, and sofosbuvir/velpatasvir. Patients were excluded if they were not on any antihyperglycemic medications at the start of HCV treatment or did not complete a full HCV treatment course.
Baseline Characteristics
Pertinent demographic data collected at baseline included patient age, gender, HCV genotype, and presence of advanced fibrotic liver disease (defined as a Metavir fibrosis stage 4 on liver biopsy, transient elastography > 12.5 kPa, or radiologic evidence of cirrhosis). HCV treatment initiation and completion dates were collected along with treatment response at 12 weeks posttreatment. Patients were considered to have achieved SVR12 if their hepatitis C viral load remained undetectable at posttreatment day 77 or thereafter. Treatment relapse was defined as a patient who achieved an undetectable HCV RNA by the end of treatment but subsequently had detectable HCV RNA following treatment cessation.
Outcome Measures
Baseline HbA1c was defined as the HbA1c drawn closest to the date of HCV treatment initiation, at least 6 months prior to treatment. Immediate posttreatment HbA1c was defined as HbA1c drawn up to 4 months posttreatment, and sustained HbA1c was captured up to 18 months posttreatment. Antihyperglycemic medication regimens and doses were collected at baseline, the end of treatment, and 3 months posttreatment via medication dispense history as well as provider notes documented in CPRS.
The primary endpoint was the change in HbA1c up to 4 months posttreatment in patients achieving SVR12. Secondary endpoints included the sustained change in HbA1c up to 12- and 18-months posttreatment, as well as change in antihyperglycemic medications from baseline to the end of HCV treatment and from baseline to 3 months posttreatment in patients achieving SVR12.
Statistical Analysis
The anticipated sample size after inclusion and exclusion for this study was 160 patients. As HbA1c is a continuous variable and tested prior to treatment and up to 18-months posttreatment, a paired dependent 2-sided t test was used for this study. For a paired dependent t test with an α of 0.05 and a power of 80%, a sample size of 160 would be able to detect a moderately small, but clinically relevant effect size of 0.22. Descriptive statistics were used for secondary outcomes. For categorical data, frequencies and percentages are provided.
Results
A total of 437 patients were identified as having a diagnosis of T2DM and being prescribed a HCV DAA, of which 157 patients met inclusion criteria. The 280 excluded patients included 127 who were not on antihyperglycemics at the start of HCV treatment, 147 who did not have HbA1c data within the specified time frame, 4 were excluded due to delayed treatment initiation outside of the study time period, and 2 self-discontinued HCV treatment due to adverse drug reactions.
Baseline Demographics
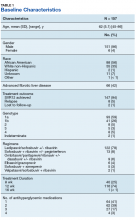
The majority of patients were male (96%), primarily African American (56%), with a mean age of 62 years (Table 1).
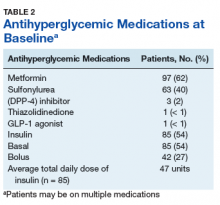
Metformin was the most commonly prescribed antihyperglycemic medication (62%), followed by insulin (54%), and sulfonylureas (40%) (Table 2).
Primary and Secondary Endpoints
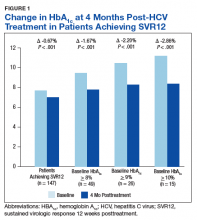
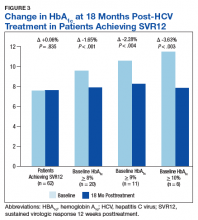
There was a significant immediate HbA1c lowering of 0.67% (from 7.67% to 7.00%; P < .001) in patients who achieved SVR12 over a mean of 2-months posttreatment (Figure 1).
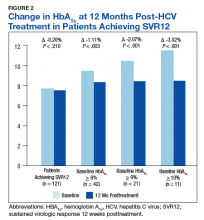
In the overall cohort of patients achieving SVR12, the HbA1c lowering was not sustained at 18 months posttreatment. However, a subanalysis demonstrated that patients with baseline HbA1c ≥ 8%, ≥ 9%, and ≥ 10% had an increasingly larger HbA1c Δ upon HCV treatment completion; the change in HbA1c for these subcohorts did remain significant at sustained time points. Patients with a baseline HbA1c ≥ 8%, ≥ 9%, and ≥ 10%, showed 18-month posttreatment HbA1c decreases of 1.65% (P < .001), 2.28% (P = .004), and 3.63% (P = .003), respectively (Figure 3).
Of the 8 patients who relapsed, there was a significant decrease in HbA1c of 0.90% from 7.54% to 6.64% (P = .024) at 4 months posttreatment. Of the relapsers who had HbA1c values up to 12 months and 18-months posttreatment, the observed change in HbA1c was 0.61% and 0.2%, respectively. However, the data are limited by its small numbers. One (13%) of the HCV treatment relapsers had an escalation of their antihyperglycemic regimen, while 1 (13%) had a de-escalation, and the remaining 6 (75%) had no change.
Discussion
The immediate reduction in HbA1c following HCV treatment observed in this study of -0.67% is clinically significant and contrasts with the expected rise in HbA1c seen with normal disease progression. The results from this study are comparable to HbA1c reductions seen with certain oral, antihyperglycemic medications, such as DPP-4 inhibitors, meglitinides, and SGLT-2 inhibitors that have an average HbA1c lowering of 0.5% to 1%. This effect was increasingly magnified in patients with a higher baseline HbA1c.
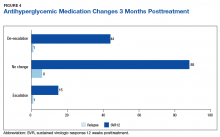
The sustained effect on HbA1c may have not been seen in the overall cohort achieving SVR12 due to the fairly well-controlled mean baseline HbA1c for this older patient cohort. In addition to improvements in HbA1c, one-third of patients achieving SVR12 required de-escalation of concomitant antihyperglycemic medications. The de-escalation of antihyperglycemics may have made the sustained HbA1c impact underappreciated in the overall cohort. There were also limited sustained HbA1c data to evaluate at the time the review was completed.
Despite the clinically significant magnitude of HbA1c change, this study suggests that this effect is not predictable for all patients with DM achieving SVR12 from HCV treatment. Nineteen percent (28/147) of these patients neither had a decrease in their HbA1c nor a de-escalation of their antihyperglycemic treatment. Patients whose T2DM onset preceded or was independent of the diabetogenic effects of HCV may be more likely to have insulin resistance unaffected by hepatitis C viral clearance. Notably, the small number of treatment relapses in this study limits this group’s ability to serve as a comparator. However, one may expect a treatment relapse to have an initial decrease in insulin resistance while the hepatitis C viral load decreases below the level of detectability, yet the effects not be sustained once the HCV relapses.
Of the 35 patients who had their HbA1c decrease to < 6% following HCV treatment, concerningly 29 (83%) had either no change or even had an escalation in their antihyperglycemic regimen. This lack of de-escalation occurred despite 45% (13/29) of these patients continuing insulin posttreatment. These patients may be at a particularly high risk for hypoglycemia. Given the mean age of patients was 62 years, extremely tight glycemic control typically is not the goal for this older patient population with numerous comorbidities and high potential for hypoglycemia unawareness.
This raises concerns that patients with T2DM undergoing HCV treatment experience a new heightened risk of hypoglycemia, particularly if neither patients or providers managing DM are aware of the high potential for decreased antihyperglycemic needs upon achieving hepatitis C virologic response. It is important that these providers are aware of the mean decreased insulin resistance achieved from hepatitis C viral clearance. Providers managing DM should advise frequent serum blood glucose monitoring with close follow-up to allow for medication adjustments to prevent hypoglycemic episodes occurring during and after HCV treatment.
Limitations
The limitations of this study included small sample sizes in subgroups, and the retrospective design prohibited the ability to quantify and describe hypoglycemic events that may have occurred as a result of HCV treatment. In addition, the documentation of medication changes in CPRS may not have fully accounted for adjustments or self-discontinuations of DM medications. An alternative definition for change in antihyperglycemic medications may have accounted for the variable HbA1c-lowering between oral antihyperglycemic medications.
Finally, hemoglobin was not collected to account for any impact ribavirin-associated anemia may have had on the immediate posttreatment HbA1c values. Phase 3 DAA trials have demonstrated that between 7% and 9% of patients on ribavirin-containing DAA regimens are expected to have a hemoglobin < 10 g/dL during the HCV treatment course.33-36 Ribavirin-containing regimens may minimally impact the immediate posttreatment HbA1c result, but not necessarily the 12- or 18-month posttreatment HbA1c levels due to the reversible nature of this adverse effect (AE) following discontinuation of ribavirin.
Future studies may be strengthened by controlling for possible confounders such as concomitant ribavirin, adherence to antihyperglycemic medications, comorbidities, years since initial DM diagnosis, and lifestyle modifications, including a decrease of alcohol consumption. A prospective study also may include data on hypoglycemic events and further determine the sustained response by including an 18- or 24-month posttreatment HbA1c in the protocol.
Conclusion
The findings of this study validate the significant HbA1c changes post-HCV treatment described in the recent veteran database study.32 However, the current study’s validated patient chart data provide a better understanding of the changes made to antihyperglycemic regimens. This also is the first study describing this phenomenon of improved insulin resistance to only be observed in approximately 80% of patients infected with HCV and comorbid T2DM. Furthermore, the variable magnitude of HbA1c impact reliant on baseline HbA1c is informative for individual patient management. In addition to the direct benefits for the liver on hepatitis C viral eradication, improvements in HbA1c and the de-escalation of antihyperglycemic regimens may be a benefit of receiving HCV treatment.
The improved DM control achieved with hepatitis C viral eradication may represent an opportunity to prevent progressive DM and cardiovascular AEs. Additionally, HCV treatment may be able to prevent the onset of T2DM in patients at risk. Arguably HCV treatment has significant benefits in terms of health outcomes, quality of life, and long-term cost avoidance to patients beyond the well-described value of decreasing liver-related morbidity and mortality. This may be an incentive for payers to improve access to HCV DAAs by expanding eligibility criteria beyond those with advanced fibrotic liver disease.
Acknowledgments
This material is the result of work supported with the resources and the use of facilities at the VA Northeast Ohio Healthcare System.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353-1363.
3. World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Published April 2014. Accessed January 24, 2019.
4. Antonelli A, Ferri C, Galeazzi C, et al. HCV infection: pathogenesis, clinical manifestations and therapy. Clin Exp Rheumatol. 2008;26(1)(suppl 48):S39-S47.
5. Jacobson IM, Cacoub P, Dal Maso L, Harrison SA, Younossi ZM. Manifestations of chronic hepatitis C virus infection beyond the liver. Clin Gastroenterol Hepatol. 2010;8(12):1017-1029.
6. Antonelli A, Ferrari SM, Giuggioli D, et al. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5(5):586-600.
7. Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes mellitus in non-cirrhotic patients with hepatitis C. Mayo Clin Proc. 2000;75(4):355-359.
8. Hammerstad SS, Grock SF, Lee HJ, Hasham A, Sundaram N, Tomer Y. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
9. Mehta SH, Brancati FI, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Interns Med. 2000;133(8):592-599.
10. Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166(2):196-203.
11. Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21(6):1135-1139.
12. Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165(5):1499-1508.
13. Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15(13):1537-1547.
14. Knobler H, Schattner A. TNF-α, chronic hepatitis C and diabetes: a novel triad. QJM. 2005;98(1):1-6.
15. Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32(suppl 3):24-34.
16. Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14(5):447-455.
17. Kralj D, Virovic´ Jukic´ L, Stojsavljevic´ S, Duvnjak M, Smolic´ M, C˘urc˘ic´ IB. Hepatitis C virus, insulin resistance, and steatosis. J Clin Transl Hepatol. 2016;4(1):66-75.
18. Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102(3):570-576.
19. Kim HJ, Park JH, Park DI, et al. Clearance of HCV by combination therapy of pegylated interferon alpha-2a and ribavirin improves insulin resistance. Gut Liver. 2009;3(2):108-115.
20. Delgado-Borrego A, Jordan SH, Negre B, et al; Halt-C Trial Group. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8(5):458-462.
21. Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, et al. Effect of sustained virologic response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48(5):721-727.
22. American Association for the Study of Liver Disease, Infectious Disease Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Updated May 24, 20187. Accessed January 24, 2019.
23. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215-223.
24. Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of clinical, economic, and quality of life benefits. BMC Infect Dis. 2015;15:19.
25. Moucari R, Forestier N, Larrey D, et al. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut. 2010;59(12):1694-1698.
26. Pedersen MR, Backstedt D, Kakati BR, et al. Sustained virologic response to direct acting antiviral therapy improves components is associated with improvements in the metabolic syndrome. Abstract 1043. Presented at: The 66th Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting, October 2015; San Francisco, CA.
27. Doyle MA, Curtis C. Successful hepatitis C antiviral therapy induces remission of type 2 diabetes: a case report. Am J Case Rep. 2015;16:745-750.
28. Pavone P, Tieghi T, d’Ettore G, et al. Rapid decline of fasting glucose in HCV diabetic patients treated with direct-acting antiviral agents. Clin Microbiol Infect. 2016;22(5):462.e1-e3.
29. Pashun RA, Shen NT, Jesudian A. Markedly improved glycemic control in poorly controlled type 2 diabetes following direct acting antiviral treatment of genotype 1 hepatitis C. Case Reports Hepatol. 2016:7807921.
30. Stine JG, Wynter JA, Niccum B, Kelly V, Caldwell SH, Shah NL. Effect of treatment with direct acting antiviral on glycemic control in patients with diabetes mellitus and chronic hepatitis C. Ann Hepatol. 2017;16(2):215-220.
31. Davis TME, Davis WA, Jeffrey G. Successful withdrawal of insulin therapy after post-treatment clearance of hepatitis C virus in a man with type 2 diabetes. Am J Case Rep. 2017;18:414-417.
32. Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40(9):1173-1180.
33. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
34. Afdhal N, Reddy R, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014:370 (16):1483-1493.
35. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
36. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
The immediate clinically significant reduction in hemoglobin A1c following HCV treatment observed in this study contrasts with the expected rise seen with normal disease progression.
The immediate clinically significant reduction in hemoglobin A1c following HCV treatment observed in this study contrasts with the expected rise seen with normal disease progression.
According to estimates, between 2.7 and 3.9 million people are infected with hepatitis C virus (HCV) in the US, with worldwide infection estimated to be about 185 million people.1-3 The majority of patients infected with HCV develop a chronic infection, which is the leading cause of liver-related complications in the Western world, including cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.4 In addition to the direct effects HCV has on the liver, extrahepatic complications can occur, often related to the immune-mediated mechanism of cryoglobulinemia, such as vasculitis, renal disease, and palpable purpura. Additionally, > 70 studies globally have associated HCV with insulin resistance and worsening glycemic control.5,6
The prevalence of patients infected with HCV that have comorbid type 2 diabetes mellitus (T2DM) is estimated to be about 30%.7,8 The landmark cross-sectional National Health and Nutrition Examination Survey III study found the prevalence of T2DM among HCV patients in the US aged > 40 years to be about 3-fold higher than those without HCV.9 These findings were further supported by a Taiwanese prospective community-based cohort study that found a higher incidence of T2DM in HCV-positive patients compared with HCV negative patients (hazard ratio [HR], 1.7; 95% CI, 1.3-2.1).10 This relationship appears to be separate from the diabetogenic effect of cirrhosis itself as a significantly higher prevalence of DM has been observed in people with HCV when compared with people with cirrhosis due to other etiologies.11 Although the mechanism for this relationship is not fully understood and is likely multifactorial, it is believed to primarily be an effect of the HCV core protein increasing phosphorylation of insulin receptor substrate-1.6,12,13 The increased presence of the inflammatory cytokine, tumor necrosis factor-α, is also believed to play a role in the effects on insulinreceptor substrate-1 as well as mediating hepatic insulin resistance, stimulating lipolysis, down-regulating peroxisome proliferator-activated receptor-γ, and interfering with β-cell function.14-17
The relationship between HCV and T2DM has been further established by measured improvements in insulin resistance among patients undergoing HCV treatment with the pre-2011 standard of care—peginterferon and ribavirin.Kawaguchi and colleagues found sustained treatment responders to have a significant decrease in both the homeostatic model assessment-insulin resistance (HOMA-IR) score, representing insulin resistance, and the HOMA-β score, representing β-cell function.18 Improvements in the HOMA-IR score were further validated by Kim and colleagues and a nested cohort within the Hepatitis C Long-term Treatment against Cirrhosis (HALT-C) trial.19,20 Furthermore, Romero-Gómez and colleagues found that patients achieving a cure from HCV treatment defined as a sustained virologic response (SVR) had a nearly 50% reduced risk of impaired fasting glucose or T2DM over a mean posttreatment follow-up of 27 months.21
The recent development of direct-acting antivirals (DAAs) has marked significant HCV treatment advances in terms of efficacy and tolerability, leading current guidelines to emphasize that nearly all patients with HCV would benefit from treatment.22 Despite these guidelines, issues have been documented throughout the US with payors often limiting this costly treatment to only those with advanced fibrotic disease.23 Although the benefits of HCV treatment on reducing liver-related morbidity and mortality may be most appreciated in individuals with advanced fibrotic liver disease, improvements in insulin resistance would suggest potential morbidity and mortality benefits beyond the liver in many more at-risk individuals.24
Increasingly, cases are being reported of new DAA regimens having a significant impact on reducing insulin resistance as demonstrated by marked decreases in antihyperglycemic requirements, fasting blood glucose, and hemoglobin A1c (HbA1c).25-30 One striking case describes a patient being able to de-escalate his regimen from 42 daily units of insulin to a single oral dipeptidyl peptidase-4 inhibitor while maintaining goal HbA1c level over a 2-year time period.31 A database-driven study of veterans found a mean HbA1c drop of 0.37% in its overall included cohort of patients with T2DM who achieved SVR from HCV DAA treatment.32
Despite these data, the individual predictability and variable magnitude of improved insulin resistance based on baseline HbA1c remains unknown. The objective of this study was to assess the impact of HCV treatment with short course DAAs on glucose control in veteran patients with T2DM at a single center.
Methods
This retrospective cohort study was performed at the Department of Veterans Affairs (VA) Northeast Ohio Healthcare System (VANEOHS) in Cleveland. This study received approval from the VANEOHS Institutional Review Board. Retrospective patient data were collected from the Veterans Health Administration (VHA) Computerized Patient Record System (CPRS) electronic health record. Collectively, the VHA has treated > 100,000 patients with DAAs, making it the largest provider of HCV treatment in the US. VANEOHS has treated nearly 2,000 patients with DAAs, rendering it one of the largest single-institution cohorts to be able to examine the effects of HCV treatment on subpopulations, such as patients with T2DM.
Patient Population
Patients were identified using ICD-9/10 codes for T2DM and medication dispense history of hepatitis C DAAs. Patients were included if they had a diagnosis of T2DM, were initiated on a hepatitis C DAA between February 1, 2014 to September 26, 2016. To be eligible, patients were required to have both a baseline HbA1c within 6 months prior to starting HCV treatment as well as a HbA1c within 4 months posttreatment. The HCV treatment included were new short-course DAAs, including sofosbuvir, simeprevir, ombitasvir/paritaprevir/ritonavir ± dasabuvir, ledipasvir/sofosbuvir, elbasvir/grazoprevir, and sofosbuvir/velpatasvir. Patients were excluded if they were not on any antihyperglycemic medications at the start of HCV treatment or did not complete a full HCV treatment course.
Baseline Characteristics
Pertinent demographic data collected at baseline included patient age, gender, HCV genotype, and presence of advanced fibrotic liver disease (defined as a Metavir fibrosis stage 4 on liver biopsy, transient elastography > 12.5 kPa, or radiologic evidence of cirrhosis). HCV treatment initiation and completion dates were collected along with treatment response at 12 weeks posttreatment. Patients were considered to have achieved SVR12 if their hepatitis C viral load remained undetectable at posttreatment day 77 or thereafter. Treatment relapse was defined as a patient who achieved an undetectable HCV RNA by the end of treatment but subsequently had detectable HCV RNA following treatment cessation.
Outcome Measures
Baseline HbA1c was defined as the HbA1c drawn closest to the date of HCV treatment initiation, at least 6 months prior to treatment. Immediate posttreatment HbA1c was defined as HbA1c drawn up to 4 months posttreatment, and sustained HbA1c was captured up to 18 months posttreatment. Antihyperglycemic medication regimens and doses were collected at baseline, the end of treatment, and 3 months posttreatment via medication dispense history as well as provider notes documented in CPRS.
The primary endpoint was the change in HbA1c up to 4 months posttreatment in patients achieving SVR12. Secondary endpoints included the sustained change in HbA1c up to 12- and 18-months posttreatment, as well as change in antihyperglycemic medications from baseline to the end of HCV treatment and from baseline to 3 months posttreatment in patients achieving SVR12.
Statistical Analysis
The anticipated sample size after inclusion and exclusion for this study was 160 patients. As HbA1c is a continuous variable and tested prior to treatment and up to 18-months posttreatment, a paired dependent 2-sided t test was used for this study. For a paired dependent t test with an α of 0.05 and a power of 80%, a sample size of 160 would be able to detect a moderately small, but clinically relevant effect size of 0.22. Descriptive statistics were used for secondary outcomes. For categorical data, frequencies and percentages are provided.
Results
A total of 437 patients were identified as having a diagnosis of T2DM and being prescribed a HCV DAA, of which 157 patients met inclusion criteria. The 280 excluded patients included 127 who were not on antihyperglycemics at the start of HCV treatment, 147 who did not have HbA1c data within the specified time frame, 4 were excluded due to delayed treatment initiation outside of the study time period, and 2 self-discontinued HCV treatment due to adverse drug reactions.
Baseline Demographics
The majority of patients were male (96%), primarily African American (56%), with a mean age of 62 years (Table 1).
Metformin was the most commonly prescribed antihyperglycemic medication (62%), followed by insulin (54%), and sulfonylureas (40%) (Table 2).
Primary and Secondary Endpoints
There was a significant immediate HbA1c lowering of 0.67% (from 7.67% to 7.00%; P < .001) in patients who achieved SVR12 over a mean of 2-months posttreatment (Figure 1).
In the overall cohort of patients achieving SVR12, the HbA1c lowering was not sustained at 18 months posttreatment. However, a subanalysis demonstrated that patients with baseline HbA1c ≥ 8%, ≥ 9%, and ≥ 10% had an increasingly larger HbA1c Δ upon HCV treatment completion; the change in HbA1c for these subcohorts did remain significant at sustained time points. Patients with a baseline HbA1c ≥ 8%, ≥ 9%, and ≥ 10%, showed 18-month posttreatment HbA1c decreases of 1.65% (P < .001), 2.28% (P = .004), and 3.63% (P = .003), respectively (Figure 3).
Of the 8 patients who relapsed, there was a significant decrease in HbA1c of 0.90% from 7.54% to 6.64% (P = .024) at 4 months posttreatment. Of the relapsers who had HbA1c values up to 12 months and 18-months posttreatment, the observed change in HbA1c was 0.61% and 0.2%, respectively. However, the data are limited by its small numbers. One (13%) of the HCV treatment relapsers had an escalation of their antihyperglycemic regimen, while 1 (13%) had a de-escalation, and the remaining 6 (75%) had no change.
Discussion
The immediate reduction in HbA1c following HCV treatment observed in this study of -0.67% is clinically significant and contrasts with the expected rise in HbA1c seen with normal disease progression. The results from this study are comparable to HbA1c reductions seen with certain oral, antihyperglycemic medications, such as DPP-4 inhibitors, meglitinides, and SGLT-2 inhibitors that have an average HbA1c lowering of 0.5% to 1%. This effect was increasingly magnified in patients with a higher baseline HbA1c.
The sustained effect on HbA1c may have not been seen in the overall cohort achieving SVR12 due to the fairly well-controlled mean baseline HbA1c for this older patient cohort. In addition to improvements in HbA1c, one-third of patients achieving SVR12 required de-escalation of concomitant antihyperglycemic medications. The de-escalation of antihyperglycemics may have made the sustained HbA1c impact underappreciated in the overall cohort. There were also limited sustained HbA1c data to evaluate at the time the review was completed.
Despite the clinically significant magnitude of HbA1c change, this study suggests that this effect is not predictable for all patients with DM achieving SVR12 from HCV treatment. Nineteen percent (28/147) of these patients neither had a decrease in their HbA1c nor a de-escalation of their antihyperglycemic treatment. Patients whose T2DM onset preceded or was independent of the diabetogenic effects of HCV may be more likely to have insulin resistance unaffected by hepatitis C viral clearance. Notably, the small number of treatment relapses in this study limits this group’s ability to serve as a comparator. However, one may expect a treatment relapse to have an initial decrease in insulin resistance while the hepatitis C viral load decreases below the level of detectability, yet the effects not be sustained once the HCV relapses.
Of the 35 patients who had their HbA1c decrease to < 6% following HCV treatment, concerningly 29 (83%) had either no change or even had an escalation in their antihyperglycemic regimen. This lack of de-escalation occurred despite 45% (13/29) of these patients continuing insulin posttreatment. These patients may be at a particularly high risk for hypoglycemia. Given the mean age of patients was 62 years, extremely tight glycemic control typically is not the goal for this older patient population with numerous comorbidities and high potential for hypoglycemia unawareness.
This raises concerns that patients with T2DM undergoing HCV treatment experience a new heightened risk of hypoglycemia, particularly if neither patients or providers managing DM are aware of the high potential for decreased antihyperglycemic needs upon achieving hepatitis C virologic response. It is important that these providers are aware of the mean decreased insulin resistance achieved from hepatitis C viral clearance. Providers managing DM should advise frequent serum blood glucose monitoring with close follow-up to allow for medication adjustments to prevent hypoglycemic episodes occurring during and after HCV treatment.
Limitations
The limitations of this study included small sample sizes in subgroups, and the retrospective design prohibited the ability to quantify and describe hypoglycemic events that may have occurred as a result of HCV treatment. In addition, the documentation of medication changes in CPRS may not have fully accounted for adjustments or self-discontinuations of DM medications. An alternative definition for change in antihyperglycemic medications may have accounted for the variable HbA1c-lowering between oral antihyperglycemic medications.
Finally, hemoglobin was not collected to account for any impact ribavirin-associated anemia may have had on the immediate posttreatment HbA1c values. Phase 3 DAA trials have demonstrated that between 7% and 9% of patients on ribavirin-containing DAA regimens are expected to have a hemoglobin < 10 g/dL during the HCV treatment course.33-36 Ribavirin-containing regimens may minimally impact the immediate posttreatment HbA1c result, but not necessarily the 12- or 18-month posttreatment HbA1c levels due to the reversible nature of this adverse effect (AE) following discontinuation of ribavirin.
Future studies may be strengthened by controlling for possible confounders such as concomitant ribavirin, adherence to antihyperglycemic medications, comorbidities, years since initial DM diagnosis, and lifestyle modifications, including a decrease of alcohol consumption. A prospective study also may include data on hypoglycemic events and further determine the sustained response by including an 18- or 24-month posttreatment HbA1c in the protocol.
Conclusion
The findings of this study validate the significant HbA1c changes post-HCV treatment described in the recent veteran database study.32 However, the current study’s validated patient chart data provide a better understanding of the changes made to antihyperglycemic regimens. This also is the first study describing this phenomenon of improved insulin resistance to only be observed in approximately 80% of patients infected with HCV and comorbid T2DM. Furthermore, the variable magnitude of HbA1c impact reliant on baseline HbA1c is informative for individual patient management. In addition to the direct benefits for the liver on hepatitis C viral eradication, improvements in HbA1c and the de-escalation of antihyperglycemic regimens may be a benefit of receiving HCV treatment.
The improved DM control achieved with hepatitis C viral eradication may represent an opportunity to prevent progressive DM and cardiovascular AEs. Additionally, HCV treatment may be able to prevent the onset of T2DM in patients at risk. Arguably HCV treatment has significant benefits in terms of health outcomes, quality of life, and long-term cost avoidance to patients beyond the well-described value of decreasing liver-related morbidity and mortality. This may be an incentive for payers to improve access to HCV DAAs by expanding eligibility criteria beyond those with advanced fibrotic liver disease.
Acknowledgments
This material is the result of work supported with the resources and the use of facilities at the VA Northeast Ohio Healthcare System.
According to estimates, between 2.7 and 3.9 million people are infected with hepatitis C virus (HCV) in the US, with worldwide infection estimated to be about 185 million people.1-3 The majority of patients infected with HCV develop a chronic infection, which is the leading cause of liver-related complications in the Western world, including cirrhosis, hepatocellular carcinoma, and the need for liver transplantation.4 In addition to the direct effects HCV has on the liver, extrahepatic complications can occur, often related to the immune-mediated mechanism of cryoglobulinemia, such as vasculitis, renal disease, and palpable purpura. Additionally, > 70 studies globally have associated HCV with insulin resistance and worsening glycemic control.5,6
The prevalence of patients infected with HCV that have comorbid type 2 diabetes mellitus (T2DM) is estimated to be about 30%.7,8 The landmark cross-sectional National Health and Nutrition Examination Survey III study found the prevalence of T2DM among HCV patients in the US aged > 40 years to be about 3-fold higher than those without HCV.9 These findings were further supported by a Taiwanese prospective community-based cohort study that found a higher incidence of T2DM in HCV-positive patients compared with HCV negative patients (hazard ratio [HR], 1.7; 95% CI, 1.3-2.1).10 This relationship appears to be separate from the diabetogenic effect of cirrhosis itself as a significantly higher prevalence of DM has been observed in people with HCV when compared with people with cirrhosis due to other etiologies.11 Although the mechanism for this relationship is not fully understood and is likely multifactorial, it is believed to primarily be an effect of the HCV core protein increasing phosphorylation of insulin receptor substrate-1.6,12,13 The increased presence of the inflammatory cytokine, tumor necrosis factor-α, is also believed to play a role in the effects on insulinreceptor substrate-1 as well as mediating hepatic insulin resistance, stimulating lipolysis, down-regulating peroxisome proliferator-activated receptor-γ, and interfering with β-cell function.14-17
The relationship between HCV and T2DM has been further established by measured improvements in insulin resistance among patients undergoing HCV treatment with the pre-2011 standard of care—peginterferon and ribavirin.Kawaguchi and colleagues found sustained treatment responders to have a significant decrease in both the homeostatic model assessment-insulin resistance (HOMA-IR) score, representing insulin resistance, and the HOMA-β score, representing β-cell function.18 Improvements in the HOMA-IR score were further validated by Kim and colleagues and a nested cohort within the Hepatitis C Long-term Treatment against Cirrhosis (HALT-C) trial.19,20 Furthermore, Romero-Gómez and colleagues found that patients achieving a cure from HCV treatment defined as a sustained virologic response (SVR) had a nearly 50% reduced risk of impaired fasting glucose or T2DM over a mean posttreatment follow-up of 27 months.21
The recent development of direct-acting antivirals (DAAs) has marked significant HCV treatment advances in terms of efficacy and tolerability, leading current guidelines to emphasize that nearly all patients with HCV would benefit from treatment.22 Despite these guidelines, issues have been documented throughout the US with payors often limiting this costly treatment to only those with advanced fibrotic disease.23 Although the benefits of HCV treatment on reducing liver-related morbidity and mortality may be most appreciated in individuals with advanced fibrotic liver disease, improvements in insulin resistance would suggest potential morbidity and mortality benefits beyond the liver in many more at-risk individuals.24
Increasingly, cases are being reported of new DAA regimens having a significant impact on reducing insulin resistance as demonstrated by marked decreases in antihyperglycemic requirements, fasting blood glucose, and hemoglobin A1c (HbA1c).25-30 One striking case describes a patient being able to de-escalate his regimen from 42 daily units of insulin to a single oral dipeptidyl peptidase-4 inhibitor while maintaining goal HbA1c level over a 2-year time period.31 A database-driven study of veterans found a mean HbA1c drop of 0.37% in its overall included cohort of patients with T2DM who achieved SVR from HCV DAA treatment.32
Despite these data, the individual predictability and variable magnitude of improved insulin resistance based on baseline HbA1c remains unknown. The objective of this study was to assess the impact of HCV treatment with short course DAAs on glucose control in veteran patients with T2DM at a single center.
Methods
This retrospective cohort study was performed at the Department of Veterans Affairs (VA) Northeast Ohio Healthcare System (VANEOHS) in Cleveland. This study received approval from the VANEOHS Institutional Review Board. Retrospective patient data were collected from the Veterans Health Administration (VHA) Computerized Patient Record System (CPRS) electronic health record. Collectively, the VHA has treated > 100,000 patients with DAAs, making it the largest provider of HCV treatment in the US. VANEOHS has treated nearly 2,000 patients with DAAs, rendering it one of the largest single-institution cohorts to be able to examine the effects of HCV treatment on subpopulations, such as patients with T2DM.
Patient Population
Patients were identified using ICD-9/10 codes for T2DM and medication dispense history of hepatitis C DAAs. Patients were included if they had a diagnosis of T2DM, were initiated on a hepatitis C DAA between February 1, 2014 to September 26, 2016. To be eligible, patients were required to have both a baseline HbA1c within 6 months prior to starting HCV treatment as well as a HbA1c within 4 months posttreatment. The HCV treatment included were new short-course DAAs, including sofosbuvir, simeprevir, ombitasvir/paritaprevir/ritonavir ± dasabuvir, ledipasvir/sofosbuvir, elbasvir/grazoprevir, and sofosbuvir/velpatasvir. Patients were excluded if they were not on any antihyperglycemic medications at the start of HCV treatment or did not complete a full HCV treatment course.
Baseline Characteristics
Pertinent demographic data collected at baseline included patient age, gender, HCV genotype, and presence of advanced fibrotic liver disease (defined as a Metavir fibrosis stage 4 on liver biopsy, transient elastography > 12.5 kPa, or radiologic evidence of cirrhosis). HCV treatment initiation and completion dates were collected along with treatment response at 12 weeks posttreatment. Patients were considered to have achieved SVR12 if their hepatitis C viral load remained undetectable at posttreatment day 77 or thereafter. Treatment relapse was defined as a patient who achieved an undetectable HCV RNA by the end of treatment but subsequently had detectable HCV RNA following treatment cessation.
Outcome Measures
Baseline HbA1c was defined as the HbA1c drawn closest to the date of HCV treatment initiation, at least 6 months prior to treatment. Immediate posttreatment HbA1c was defined as HbA1c drawn up to 4 months posttreatment, and sustained HbA1c was captured up to 18 months posttreatment. Antihyperglycemic medication regimens and doses were collected at baseline, the end of treatment, and 3 months posttreatment via medication dispense history as well as provider notes documented in CPRS.
The primary endpoint was the change in HbA1c up to 4 months posttreatment in patients achieving SVR12. Secondary endpoints included the sustained change in HbA1c up to 12- and 18-months posttreatment, as well as change in antihyperglycemic medications from baseline to the end of HCV treatment and from baseline to 3 months posttreatment in patients achieving SVR12.
Statistical Analysis
The anticipated sample size after inclusion and exclusion for this study was 160 patients. As HbA1c is a continuous variable and tested prior to treatment and up to 18-months posttreatment, a paired dependent 2-sided t test was used for this study. For a paired dependent t test with an α of 0.05 and a power of 80%, a sample size of 160 would be able to detect a moderately small, but clinically relevant effect size of 0.22. Descriptive statistics were used for secondary outcomes. For categorical data, frequencies and percentages are provided.
Results
A total of 437 patients were identified as having a diagnosis of T2DM and being prescribed a HCV DAA, of which 157 patients met inclusion criteria. The 280 excluded patients included 127 who were not on antihyperglycemics at the start of HCV treatment, 147 who did not have HbA1c data within the specified time frame, 4 were excluded due to delayed treatment initiation outside of the study time period, and 2 self-discontinued HCV treatment due to adverse drug reactions.
Baseline Demographics
The majority of patients were male (96%), primarily African American (56%), with a mean age of 62 years (Table 1).
Metformin was the most commonly prescribed antihyperglycemic medication (62%), followed by insulin (54%), and sulfonylureas (40%) (Table 2).
Primary and Secondary Endpoints
There was a significant immediate HbA1c lowering of 0.67% (from 7.67% to 7.00%; P < .001) in patients who achieved SVR12 over a mean of 2-months posttreatment (Figure 1).
In the overall cohort of patients achieving SVR12, the HbA1c lowering was not sustained at 18 months posttreatment. However, a subanalysis demonstrated that patients with baseline HbA1c ≥ 8%, ≥ 9%, and ≥ 10% had an increasingly larger HbA1c Δ upon HCV treatment completion; the change in HbA1c for these subcohorts did remain significant at sustained time points. Patients with a baseline HbA1c ≥ 8%, ≥ 9%, and ≥ 10%, showed 18-month posttreatment HbA1c decreases of 1.65% (P < .001), 2.28% (P = .004), and 3.63% (P = .003), respectively (Figure 3).
Of the 8 patients who relapsed, there was a significant decrease in HbA1c of 0.90% from 7.54% to 6.64% (P = .024) at 4 months posttreatment. Of the relapsers who had HbA1c values up to 12 months and 18-months posttreatment, the observed change in HbA1c was 0.61% and 0.2%, respectively. However, the data are limited by its small numbers. One (13%) of the HCV treatment relapsers had an escalation of their antihyperglycemic regimen, while 1 (13%) had a de-escalation, and the remaining 6 (75%) had no change.
Discussion
The immediate reduction in HbA1c following HCV treatment observed in this study of -0.67% is clinically significant and contrasts with the expected rise in HbA1c seen with normal disease progression. The results from this study are comparable to HbA1c reductions seen with certain oral, antihyperglycemic medications, such as DPP-4 inhibitors, meglitinides, and SGLT-2 inhibitors that have an average HbA1c lowering of 0.5% to 1%. This effect was increasingly magnified in patients with a higher baseline HbA1c.
The sustained effect on HbA1c may have not been seen in the overall cohort achieving SVR12 due to the fairly well-controlled mean baseline HbA1c for this older patient cohort. In addition to improvements in HbA1c, one-third of patients achieving SVR12 required de-escalation of concomitant antihyperglycemic medications. The de-escalation of antihyperglycemics may have made the sustained HbA1c impact underappreciated in the overall cohort. There were also limited sustained HbA1c data to evaluate at the time the review was completed.
Despite the clinically significant magnitude of HbA1c change, this study suggests that this effect is not predictable for all patients with DM achieving SVR12 from HCV treatment. Nineteen percent (28/147) of these patients neither had a decrease in their HbA1c nor a de-escalation of their antihyperglycemic treatment. Patients whose T2DM onset preceded or was independent of the diabetogenic effects of HCV may be more likely to have insulin resistance unaffected by hepatitis C viral clearance. Notably, the small number of treatment relapses in this study limits this group’s ability to serve as a comparator. However, one may expect a treatment relapse to have an initial decrease in insulin resistance while the hepatitis C viral load decreases below the level of detectability, yet the effects not be sustained once the HCV relapses.
Of the 35 patients who had their HbA1c decrease to < 6% following HCV treatment, concerningly 29 (83%) had either no change or even had an escalation in their antihyperglycemic regimen. This lack of de-escalation occurred despite 45% (13/29) of these patients continuing insulin posttreatment. These patients may be at a particularly high risk for hypoglycemia. Given the mean age of patients was 62 years, extremely tight glycemic control typically is not the goal for this older patient population with numerous comorbidities and high potential for hypoglycemia unawareness.
This raises concerns that patients with T2DM undergoing HCV treatment experience a new heightened risk of hypoglycemia, particularly if neither patients or providers managing DM are aware of the high potential for decreased antihyperglycemic needs upon achieving hepatitis C virologic response. It is important that these providers are aware of the mean decreased insulin resistance achieved from hepatitis C viral clearance. Providers managing DM should advise frequent serum blood glucose monitoring with close follow-up to allow for medication adjustments to prevent hypoglycemic episodes occurring during and after HCV treatment.
Limitations
The limitations of this study included small sample sizes in subgroups, and the retrospective design prohibited the ability to quantify and describe hypoglycemic events that may have occurred as a result of HCV treatment. In addition, the documentation of medication changes in CPRS may not have fully accounted for adjustments or self-discontinuations of DM medications. An alternative definition for change in antihyperglycemic medications may have accounted for the variable HbA1c-lowering between oral antihyperglycemic medications.
Finally, hemoglobin was not collected to account for any impact ribavirin-associated anemia may have had on the immediate posttreatment HbA1c values. Phase 3 DAA trials have demonstrated that between 7% and 9% of patients on ribavirin-containing DAA regimens are expected to have a hemoglobin < 10 g/dL during the HCV treatment course.33-36 Ribavirin-containing regimens may minimally impact the immediate posttreatment HbA1c result, but not necessarily the 12- or 18-month posttreatment HbA1c levels due to the reversible nature of this adverse effect (AE) following discontinuation of ribavirin.
Future studies may be strengthened by controlling for possible confounders such as concomitant ribavirin, adherence to antihyperglycemic medications, comorbidities, years since initial DM diagnosis, and lifestyle modifications, including a decrease of alcohol consumption. A prospective study also may include data on hypoglycemic events and further determine the sustained response by including an 18- or 24-month posttreatment HbA1c in the protocol.
Conclusion
The findings of this study validate the significant HbA1c changes post-HCV treatment described in the recent veteran database study.32 However, the current study’s validated patient chart data provide a better understanding of the changes made to antihyperglycemic regimens. This also is the first study describing this phenomenon of improved insulin resistance to only be observed in approximately 80% of patients infected with HCV and comorbid T2DM. Furthermore, the variable magnitude of HbA1c impact reliant on baseline HbA1c is informative for individual patient management. In addition to the direct benefits for the liver on hepatitis C viral eradication, improvements in HbA1c and the de-escalation of antihyperglycemic regimens may be a benefit of receiving HCV treatment.
The improved DM control achieved with hepatitis C viral eradication may represent an opportunity to prevent progressive DM and cardiovascular AEs. Additionally, HCV treatment may be able to prevent the onset of T2DM in patients at risk. Arguably HCV treatment has significant benefits in terms of health outcomes, quality of life, and long-term cost avoidance to patients beyond the well-described value of decreasing liver-related morbidity and mortality. This may be an incentive for payers to improve access to HCV DAAs by expanding eligibility criteria beyond those with advanced fibrotic liver disease.
Acknowledgments
This material is the result of work supported with the resources and the use of facilities at the VA Northeast Ohio Healthcare System.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353-1363.
3. World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Published April 2014. Accessed January 24, 2019.
4. Antonelli A, Ferri C, Galeazzi C, et al. HCV infection: pathogenesis, clinical manifestations and therapy. Clin Exp Rheumatol. 2008;26(1)(suppl 48):S39-S47.
5. Jacobson IM, Cacoub P, Dal Maso L, Harrison SA, Younossi ZM. Manifestations of chronic hepatitis C virus infection beyond the liver. Clin Gastroenterol Hepatol. 2010;8(12):1017-1029.
6. Antonelli A, Ferrari SM, Giuggioli D, et al. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5(5):586-600.
7. Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes mellitus in non-cirrhotic patients with hepatitis C. Mayo Clin Proc. 2000;75(4):355-359.
8. Hammerstad SS, Grock SF, Lee HJ, Hasham A, Sundaram N, Tomer Y. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
9. Mehta SH, Brancati FI, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Interns Med. 2000;133(8):592-599.
10. Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166(2):196-203.
11. Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21(6):1135-1139.
12. Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165(5):1499-1508.
13. Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15(13):1537-1547.
14. Knobler H, Schattner A. TNF-α, chronic hepatitis C and diabetes: a novel triad. QJM. 2005;98(1):1-6.
15. Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32(suppl 3):24-34.
16. Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14(5):447-455.
17. Kralj D, Virovic´ Jukic´ L, Stojsavljevic´ S, Duvnjak M, Smolic´ M, C˘urc˘ic´ IB. Hepatitis C virus, insulin resistance, and steatosis. J Clin Transl Hepatol. 2016;4(1):66-75.
18. Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102(3):570-576.
19. Kim HJ, Park JH, Park DI, et al. Clearance of HCV by combination therapy of pegylated interferon alpha-2a and ribavirin improves insulin resistance. Gut Liver. 2009;3(2):108-115.
20. Delgado-Borrego A, Jordan SH, Negre B, et al; Halt-C Trial Group. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8(5):458-462.
21. Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, et al. Effect of sustained virologic response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48(5):721-727.
22. American Association for the Study of Liver Disease, Infectious Disease Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Updated May 24, 20187. Accessed January 24, 2019.
23. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215-223.
24. Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of clinical, economic, and quality of life benefits. BMC Infect Dis. 2015;15:19.
25. Moucari R, Forestier N, Larrey D, et al. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut. 2010;59(12):1694-1698.
26. Pedersen MR, Backstedt D, Kakati BR, et al. Sustained virologic response to direct acting antiviral therapy improves components is associated with improvements in the metabolic syndrome. Abstract 1043. Presented at: The 66th Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting, October 2015; San Francisco, CA.
27. Doyle MA, Curtis C. Successful hepatitis C antiviral therapy induces remission of type 2 diabetes: a case report. Am J Case Rep. 2015;16:745-750.
28. Pavone P, Tieghi T, d’Ettore G, et al. Rapid decline of fasting glucose in HCV diabetic patients treated with direct-acting antiviral agents. Clin Microbiol Infect. 2016;22(5):462.e1-e3.
29. Pashun RA, Shen NT, Jesudian A. Markedly improved glycemic control in poorly controlled type 2 diabetes following direct acting antiviral treatment of genotype 1 hepatitis C. Case Reports Hepatol. 2016:7807921.
30. Stine JG, Wynter JA, Niccum B, Kelly V, Caldwell SH, Shah NL. Effect of treatment with direct acting antiviral on glycemic control in patients with diabetes mellitus and chronic hepatitis C. Ann Hepatol. 2017;16(2):215-220.
31. Davis TME, Davis WA, Jeffrey G. Successful withdrawal of insulin therapy after post-treatment clearance of hepatitis C virus in a man with type 2 diabetes. Am J Case Rep. 2017;18:414-417.
32. Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40(9):1173-1180.
33. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
34. Afdhal N, Reddy R, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014:370 (16):1483-1493.
35. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
36. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
1. Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549-1552.
2. Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353-1363.
3. World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Published April 2014. Accessed January 24, 2019.
4. Antonelli A, Ferri C, Galeazzi C, et al. HCV infection: pathogenesis, clinical manifestations and therapy. Clin Exp Rheumatol. 2008;26(1)(suppl 48):S39-S47.
5. Jacobson IM, Cacoub P, Dal Maso L, Harrison SA, Younossi ZM. Manifestations of chronic hepatitis C virus infection beyond the liver. Clin Gastroenterol Hepatol. 2010;8(12):1017-1029.
6. Antonelli A, Ferrari SM, Giuggioli D, et al. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5(5):586-600.
7. Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes mellitus in non-cirrhotic patients with hepatitis C. Mayo Clin Proc. 2000;75(4):355-359.
8. Hammerstad SS, Grock SF, Lee HJ, Hasham A, Sundaram N, Tomer Y. Diabetes and hepatitis C: a two-way association. Front Endocrinol (Lausanne). 2015;6:134.
9. Mehta SH, Brancati FI, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Interns Med. 2000;133(8):592-599.
10. Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166(2):196-203.
11. Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21(6):1135-1139.
12. Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165(5):1499-1508.
13. Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15(13):1537-1547.
14. Knobler H, Schattner A. TNF-α, chronic hepatitis C and diabetes: a novel triad. QJM. 2005;98(1):1-6.
15. Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32(suppl 3):24-34.
16. Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14(5):447-455.
17. Kralj D, Virovic´ Jukic´ L, Stojsavljevic´ S, Duvnjak M, Smolic´ M, C˘urc˘ic´ IB. Hepatitis C virus, insulin resistance, and steatosis. J Clin Transl Hepatol. 2016;4(1):66-75.
18. Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102(3):570-576.
19. Kim HJ, Park JH, Park DI, et al. Clearance of HCV by combination therapy of pegylated interferon alpha-2a and ribavirin improves insulin resistance. Gut Liver. 2009;3(2):108-115.
20. Delgado-Borrego A, Jordan SH, Negre B, et al; Halt-C Trial Group. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8(5):458-462.
21. Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, et al. Effect of sustained virologic response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48(5):721-727.
22. American Association for the Study of Liver Disease, Infectious Disease Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Updated May 24, 20187. Accessed January 24, 2019.
23. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215-223.
24. Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of clinical, economic, and quality of life benefits. BMC Infect Dis. 2015;15:19.
25. Moucari R, Forestier N, Larrey D, et al. Danoprevir, an HCV NS3/4A protease inhibitor, improves insulin sensitivity in patients with genotype 1 chronic hepatitis C. Gut. 2010;59(12):1694-1698.
26. Pedersen MR, Backstedt D, Kakati BR, et al. Sustained virologic response to direct acting antiviral therapy improves components is associated with improvements in the metabolic syndrome. Abstract 1043. Presented at: The 66th Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting, October 2015; San Francisco, CA.
27. Doyle MA, Curtis C. Successful hepatitis C antiviral therapy induces remission of type 2 diabetes: a case report. Am J Case Rep. 2015;16:745-750.
28. Pavone P, Tieghi T, d’Ettore G, et al. Rapid decline of fasting glucose in HCV diabetic patients treated with direct-acting antiviral agents. Clin Microbiol Infect. 2016;22(5):462.e1-e3.
29. Pashun RA, Shen NT, Jesudian A. Markedly improved glycemic control in poorly controlled type 2 diabetes following direct acting antiviral treatment of genotype 1 hepatitis C. Case Reports Hepatol. 2016:7807921.
30. Stine JG, Wynter JA, Niccum B, Kelly V, Caldwell SH, Shah NL. Effect of treatment with direct acting antiviral on glycemic control in patients with diabetes mellitus and chronic hepatitis C. Ann Hepatol. 2017;16(2):215-220.
31. Davis TME, Davis WA, Jeffrey G. Successful withdrawal of insulin therapy after post-treatment clearance of hepatitis C virus in a man with type 2 diabetes. Am J Case Rep. 2017;18:414-417.
32. Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40(9):1173-1180.
33. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
34. Afdhal N, Reddy R, Nelson DR, et al; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014:370 (16):1483-1493.
35. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
36. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
Achieving Excellence in Hepatitis B Virus Care for Veterans in the VHA (FULL)
Hepatitis B is a viral infection caused by the hepatitis B virus (HBV), which is transmitted through percutaneous (ie, puncture through the skin) or mucosal (ie, direct contact with mucous membranes) exposure to infectious blood or body fluids. Hepatitis B virus can cause chronic infection, resulting in cirrhosis of the liver, liver cancer, liver failure, and death. Persons with chronic infection also serve as the main reservoir for continued HBV transmission.1
Individuals at highest risk for infection include those born in geographic regions with a high prevalence of HBV, those with sexual partners or household contacts with chronic HBV infection, men who have sex with men (MSM), those with HIV, and individuals who inject drugs. Pregnant women also are a population of concern given the potential for perinatal transmission.2
About 850,000 to 2.2 million people in the US (about 0.3% of the civilian US population) are chronically infected with HBV.3 The prevalence of chronic HBV is much higher (10%-19%) among Asian Americans, those of Pacific Island descent, and other immigrant populations from highly endemic countries.4 In the US, HBV is responsible for 2,000 to 4,000 preventable deaths annually, primarily from cirrhosis, liver cancer, and hepatic failure.4 In the civilian US population, reported cases of acute HBV decreased 0.3% from 2011 to 2012, increased 5.4% in 2013 with an 8.5% decrease in 2014, and a 20.7% increase in 2015.4 Injection drug use is likely driving the most recent increase.5
Not all individuals exposed to HBV will develop chronic infection, and the risk of chronic HBV infection depends on an individual’s age at the time of exposure. For example, about 95% of infants exposed to HBV perinatally will develop a chronic infection compared with 5% of exposed adults.6 Of those with chronic HBV, a small proportion will develop cirrhosis and/or hepatocellular carcinoma (HCC) with increasing risk as viral DNA concentrations increase. Additional risk factors for cirrhosis include being older, male, having a persistently elevated alanine transaminase, viral superinfections, HBV reversion/reactivation, genotype, and various markers of disease severity (HCC).6 Of note, chronic HBV infection may cause HCC even in the absence of cirrhosis.7 In addition, immunosuppression (eg, from cancer chemotherapy) may allow HBV reactivation, which may result in fulminant hepatic failure. In the Veterans Health Affairs (VHA) health care system, about 17% of those with known chronic HBV also carry a diagnosis of cirrhosis.
Vaccination is the mainstay of efforts to prevent HBV infection. The first commercially available HBV vaccine was approved by the FDA in 1981, with subsequent FDA approval in 1986 of a vaccine manufactured using recombinant DNA technology.8 In 1991, the Advisory Committee on Immunization Practices (ACIP) recommended universal childhood vaccination for HBV, with subsequent recommendations for vaccination of adolescents and adults in high-risk groups in 1995, and in 1999 all remaining unvaccinated children aged ≤ 19 years.9 Military policy has been to provide hepatitis B immunization to personnel assigned to the Korean peninsula since 1986 and to all recruits since 2001.10
Following publication of an Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) report, in 2011 the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan.11 The current HHS Action Plan, along with the NASEM National Strategy for the Elimination of Hepatitis B and C: Phase Two Report, commissioned by the US Centers for Disease Control and Prevention (CDC), outlines a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.12 The VA is a critical partner in this federal collaborative effort to achieve excellence in viral hepatitis care.
In August 2016, the HIV, Hepatitis, and Related Conditions Programs in the VA Office of Specialty Care Services convened a National Hepatitis B Working Group consisting of VA subject matter experts (SMEs) and representatives from the VA Central Office stakeholder program offices, with a charge of developing a strategic plan to ensure excellence in HBV prevention, care, and management across the VHA. The task included addressing supportive processes and barriers at each level of the organization through a public health framework and using a population health management approach.
The VA National Strategic Plan for Excellence in HBV Care was focused on the following overarching aims:
- Characterizing the current state of care for veterans with HBV in VA care;
- Developing and disseminating clinical guidance on high-quality care for patients with HBV;
- Developing population data and informatics tools to streamline the identification and monitoring of patients with chronic HBV; and
- Evaluating VHA care for patients with HBV over time.
Care for Veterans With HBV at the VA
The VA health care system is America’s largest integrated health care system, providing care at 1,243 health care facilities, including 170 medical centers and 1,063 outpatient sites of care serving 9 million enrolled veterans each year.13 As of January 2018, there were 10,743 individuals with serologic evidence for chronic HBV infection in VA care, based on a definition of 2 or more detectable surface antigen (sAg) or hepatitis B DNA tests recorded at least 6 months apart.1 About 2,000 additional VA patients have a history of a single positive sAg test. These patients have unclear HBV status and require a second sAg test to determine whether they have a chronic infection.
The prevalence of HBV infection among veterans in VA care is slightly higher than that in the US civilian population at 0.4%.14 Studies of selected subpopulations of veterans have found high seropositivity of prior or chronic HBV infection among homeless veterans and veterans admitted to a psychiatric hospital.15,16 The data from 2015 suggest that homeless veterans have a chronic HBV infection rate of 1.0%.14 Of those with known chronic HBV infection, the plurality are white (40.4%) or African American (40.2%), male (92.4%), with a mean age of 59.9 (SD 12.0) years. According to National HIV, Hepatitis and Related Conditions Data and Analysis Group personal correspondence, the geographic territories with the largest chronic HBV caseload include the Southeast, Gulf Coast, and West Coast. As of January 2018, 1,210 veterans in care have HBV-related cirrhosis.
HBV Screening in VA
The current VA HBV screening guidelines follow those of the US Preventive Services Task Force (USPSTF).17 HBV screening is recommended for unvaccinated individuals in high-risk groups, such as patients with HIV or hepatitis C virus (HCV), those on hemodialysis, those with elevated alanine transaminase/aspartate transaminase of unknown etiology, those on immunosuppressive therapy, injection drug users, the MSM population, people with household contact with an HBV-infected person, people born to an HBV-infected mother, those with risk factors for HBV exposure prior to vaccination, pregnant women, and people born in highly endemic areas regardless of vaccination status.2 The VHA recommends against standardized risk assessment and laboratory screening for HBV infection in the asymptomatic general patient population. However, if risk factors become known during the course of providing usual clinical care, then laboratory screening should be considered.2
Of the 6.1 million VHA users
HBV Care and VA Antiviral Treatment
In a study of an HBV care cascade, Serper and colleagues reviewed a cohort of veterans in the VA with HBV. About 50% of the patients with known chronic HBV in the VA system from 1999 to 2013 had received infectious diseases or gastroenterology/hepatology specialty care in the previous 2 years.19 Follow-up data from the National HIV, Hepatitis and Related Conditions Data and Analysis Group indicated that this remains the case: 52.3% of patients with documented chronic HBV had received specialty care from VA sources in the prior 2 years. Serper and colleagues also reported that among veterans in VHA care with chronic HBV infection and cirrhosis from 1999 to 2013, annual imaging was < 50%, and initiation of antiviral treatment was only 39%. Antiviral therapy and liver imaging were both independently associated with lower mortality for patients with HBV and cirrhosis.19
A review of studies that evaluated the delivery of care for patients with HBV in U.S. civilian populations, including retrospective reviews of private payer claims databases and chart reviews, the Kaiser Permanente claims database, and community gastrointestinal (GI) practice chart reviews, revealed similar practice patterns with those in the VA.20 Across the US, rates of antiviral therapy and HCC surveillance for those with HBV cirrhosis were low, ranging from 14% to 50% and 19% to 60%, respectively. Several of these studies also found that being seen by an HBV specialist was associated with improved care.20
Antiviral treatment of individuals with cirrhosis and chronic HBV infection can reduce the risk of progression to decompensated cirrhosis and liver cancer. Among current VA patients with HBV cirrhosis, 62.4% received at least 1 month of HBV antiviral medication in the prior year. Additionally, biannual liver imaging is recommended in this population to screen for the development of HCC. According to National HIV, Hepatitis and Related Conditions Data and Analysis Group personal correspondence, nationally, 51.2% of individuals with HBV cirrhosis had received at least one instance of liver imaging within the past 6 months, and 71.2% received imaging within the past 12 months.
Prevention of HBV Infection and Sequelae
Vaccination rates in the US vary by age group, with higher immunization rates among those born after 1991 than the rates of those born earlier. Data from the National Health and Nutrition Examination Survey from 1988 to 2012 reported 33% immunity among veterans aged < 50 years and 6% among those aged ≥ 50 years.21 In addition to individuals who received childhood vaccination in the 1990s, all new military recruits assigned to the Korean Peninsula were vaccinated for HBV as of 1986, and those joining the military after 2002 received mandatory vaccination.
The VA follows the ACIP/CDC hepatitis B immunization guidelines.22-24 The VA currently recommends HBV immunization among previously unvaccinated adults at increased risk of contracting HBV infection and for any other adult who is seeking protection from HBV infection. The VA also offers general recommendations for prevention of transmission between veterans with known chronic HBV to their household, sexual, or drug-using partners. Transmission prevention guidelines also provide recommendations for vaccination of pregnant women with HBV risk factors and women at risk for HBV infection during pregnancy.22
HBV Care Guidance
One of the core tasks of the VA National Hepatitis B Working Group, given its broad, multidisciplinary expertise in HBV, was developing general clinical guidelines for the provision of high-quality care for patients with HBV. The group reviewed current literature and scientific evidence on care for patients with HBV. The working group relied heavily on the VA’s national guidelines for HBV screening and immunization, which are based on recommendations from the USPSTF, ACIP, CDC, and professional societies. The professional society guidelines included the American Association for the Study of Liver Disease’s Guidelines for Treatment of Chronic Hepatitis B, the America College of Gastroenterology’s Practice Guidelines: Evaluation of Abnormal Liver Chemistries, the American Gastroenterological Association Institute’s Guidelines for Prevention and Treatment of Hepatitis B Reactivation during Immunosuppressive Drug Therapy, and CDC’s Guidelines for Screening Pregnant Women for HBV.19,22-27
The working group identified areas for HBV quality improvement that were consistent with the VA and professional guidelines, specific and measurable using VA data, clinically relevant, feasible, and achievable in a defined time period. Areas for targeted improvement will include testing for HBV among high-risk patients, increasing antiviral treatment and HCC surveillance of veterans with HBV-related cirrhosis, decreasing progression of chronic HBV to cirrhosis, and expanding prevention measures, such as immunization among those at high risk for HBV and prevention of HBV reactivation.
At a national level, development of specific and measurable quality of care indicators for HBV will aid in assessing gaps in care and developing strategies to address these gaps. A broader discussion of care for patients with HBV quality with front-line health care providers (HCPs) will be paired with increased education and providing clinical support tools for those HCPs and facilities without access to specialty GI services.
Clinical pharmacists will be critical targets for the dissemination of guidance for HBV care paired with clinical informatics support tools and clinical educational opportunities. As of 2015, there were about 7,700 clinical pharmacists in the VHA and 3,200 had a scope of practice that included prescribing authority. As a result, 20% of HCV prescriptions in the VA in fiscal year 2015
Identification and Monitoring
The HBV working group and the VA Viral Hepatitis Technical Advisory Group are working with field HCPs to develop several informatics tools to promote HBV case identification and quality monitoring. These groups identified several barriers to HBV case identification and monitoring. The following informatics tools are either available or in development to reduce these barriers:
- A local clinical case registry of patients with HBV infection based on ICD codes, which allows users to create custom reports to identify, monitor, and track care;
- Because of the risk of HBV reactivation in patients with chronic HBV infection who receive anti-CD20 agents, such as rituximab, a medication order check to improve HBV screening among veterans receiving anti-CD20 medication;
- Validated patient reports based on laboratory diagnosis of HBV, drawn from all results across the VHA since 1999, made available to all facilities;
- Interactive reports summarizing quality of care for patients with HBV infection, based on facility-level indicators in development by the national HBV working group, will be distributed and enable geographic comparison;
- An HBV immunization clinical reminder that will prompt frontline HCPs to test and vaccinate; and
- An HBV clinical dashboard that will enable HCPs and facilities to identify all their HBV-positive veterans and track their care and outcomes over time.
Evaluating VA Care for Patients with HBV
As indicators of quality of HBV care are refined for VA patients and the health care delivery system, guidance will be made broadly available to frontline HCPs and administrators. The HBV quality of care recommendations will be paired with a suite of clinical informatics tools and virtual educational trainings to ensure that VA HCPs and facilities can streamline care for patients with HBV infection as much as possible. Quality improvement will be measured nationally each year, and strategies to address persistent variability and gaps in care will be developed in collaboration with the VA SME’s, facilities, and HCPs.
Conclusion
Hepatitis B virus is at least as prevalent among veterans who are cared for at VA facilities as it is in the US civilian population. Although care for patients with HBV infection in the VA is similar to care for patients with HBV infection in the community, the VA recognizes areas for improved HBV prevention, testing, care, and treatment. The VA has begun a continuous quality improvement strategic plan to enhance the level of care for patients with HBV infection in VA care. Centralized coordination and communication of VA data combined with veteran- and field-centered policies and operational planning and execution will allow clinically relevant improvements in HBV diagnosis, treatment, and prevention among veterans served by VA.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatitis B FAQs for health professionals: overview and statistics. https://www.cdc.gov/hepatitis/hbv/hbvfaq .htm#overview. Updated January 11, 2018. Accessed on February 12, 2018.
2.
3. Centers for Disease Control and Prevention. Surveillance for viral hepatitis—United States, 2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/index.htm. Updated June 19, 2017. Accessed February 12, 2018.
4. Kim WR. Epidemiology of hepatitis B in the United States. Hepatology. 2009;49(suppl 5):S28-S34.
5. Harris AM, Iqbal K, Schillie S, et al. Increases in acute hepatitis B virus infections— Kentucky, Tennessee, and West Virginia, 2006-2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):47-50.
6. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373(9663):582-592.
7. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127.
8. Weinbaum CM, Williams I, Mast EE, et al; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1-20.
9. Centers for Disease Control and Prevention. Achievements in public health: hepatitis B vaccination—United States, 1982-2002. MMWR. 2002;51(25):549-552, 563.
10. Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3-26.
11. Colvin HM, Mitchell AE, eds; Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: National Academies Press; 2010.
12. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: National Academies Press; 2017.
13. US Department of Veterans Affairs. Providing health care for veterans. https://www.va.gov/health. Updated February 20, 2018. Accessed February 22, 2018.
14. Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Prevalence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus among homeless and nonhomeless United States veterans. Clin Infect Dis. 2017;65(2):252-258.
15. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
16. Tabibian JH, Wirshing DA, Pierre JM, et al. Hepatitis B and C among veterans in a psychiatric ward. Dig Dis Sci. 2008;53(6):1693-1698
17. US Preventive Services Task Force. Final recommendation statement: screening for hepatitis B virus infection in nonpregnant adolescents and adults. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-b-virus-infection-screening-2014. Published May 2014. Updated February 2018. Accessed February 22, 2018.
18. Backus LI, Belperio PS, Loomis TP, Han SH, Mole LA. Screening for and prevalence of hepatitis B virus infection among high-risk veterans under the care of the U.S. Department of Veterans Affairs: a case report. Ann Intern Med. 2014;161(12):926-928.
19. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
20. Mellinger J, Fontana RJ. Quality of care metrics in chronic hepatitis B. Hepatology. 2016;63(6):1755-1758.
21. Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63(2):388-397.
22. US Department of Veterans Affairs. National Clinical Preventive Service Guidance Statements: Hepatitis B Immunization. http://vaww.prevention.va.gov/CPS/Hepatitis_B_Immunization.asp. Nonpublic document. Source not verified.
23. Advisory Committee on Immunization Practices (ACIP). Recommended immunization schedule for adults aged 19 years or older, United States, 2017. https://www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed February 12, 2018.
24. Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2018;67(1):1-31.
25. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283.
26. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
27. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):215-219, quiz e16-e17.
28. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
Hepatitis B is a viral infection caused by the hepatitis B virus (HBV), which is transmitted through percutaneous (ie, puncture through the skin) or mucosal (ie, direct contact with mucous membranes) exposure to infectious blood or body fluids. Hepatitis B virus can cause chronic infection, resulting in cirrhosis of the liver, liver cancer, liver failure, and death. Persons with chronic infection also serve as the main reservoir for continued HBV transmission.1
Individuals at highest risk for infection include those born in geographic regions with a high prevalence of HBV, those with sexual partners or household contacts with chronic HBV infection, men who have sex with men (MSM), those with HIV, and individuals who inject drugs. Pregnant women also are a population of concern given the potential for perinatal transmission.2
About 850,000 to 2.2 million people in the US (about 0.3% of the civilian US population) are chronically infected with HBV.3 The prevalence of chronic HBV is much higher (10%-19%) among Asian Americans, those of Pacific Island descent, and other immigrant populations from highly endemic countries.4 In the US, HBV is responsible for 2,000 to 4,000 preventable deaths annually, primarily from cirrhosis, liver cancer, and hepatic failure.4 In the civilian US population, reported cases of acute HBV decreased 0.3% from 2011 to 2012, increased 5.4% in 2013 with an 8.5% decrease in 2014, and a 20.7% increase in 2015.4 Injection drug use is likely driving the most recent increase.5
Not all individuals exposed to HBV will develop chronic infection, and the risk of chronic HBV infection depends on an individual’s age at the time of exposure. For example, about 95% of infants exposed to HBV perinatally will develop a chronic infection compared with 5% of exposed adults.6 Of those with chronic HBV, a small proportion will develop cirrhosis and/or hepatocellular carcinoma (HCC) with increasing risk as viral DNA concentrations increase. Additional risk factors for cirrhosis include being older, male, having a persistently elevated alanine transaminase, viral superinfections, HBV reversion/reactivation, genotype, and various markers of disease severity (HCC).6 Of note, chronic HBV infection may cause HCC even in the absence of cirrhosis.7 In addition, immunosuppression (eg, from cancer chemotherapy) may allow HBV reactivation, which may result in fulminant hepatic failure. In the Veterans Health Affairs (VHA) health care system, about 17% of those with known chronic HBV also carry a diagnosis of cirrhosis.
Vaccination is the mainstay of efforts to prevent HBV infection. The first commercially available HBV vaccine was approved by the FDA in 1981, with subsequent FDA approval in 1986 of a vaccine manufactured using recombinant DNA technology.8 In 1991, the Advisory Committee on Immunization Practices (ACIP) recommended universal childhood vaccination for HBV, with subsequent recommendations for vaccination of adolescents and adults in high-risk groups in 1995, and in 1999 all remaining unvaccinated children aged ≤ 19 years.9 Military policy has been to provide hepatitis B immunization to personnel assigned to the Korean peninsula since 1986 and to all recruits since 2001.10
Following publication of an Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) report, in 2011 the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan.11 The current HHS Action Plan, along with the NASEM National Strategy for the Elimination of Hepatitis B and C: Phase Two Report, commissioned by the US Centers for Disease Control and Prevention (CDC), outlines a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.12 The VA is a critical partner in this federal collaborative effort to achieve excellence in viral hepatitis care.
In August 2016, the HIV, Hepatitis, and Related Conditions Programs in the VA Office of Specialty Care Services convened a National Hepatitis B Working Group consisting of VA subject matter experts (SMEs) and representatives from the VA Central Office stakeholder program offices, with a charge of developing a strategic plan to ensure excellence in HBV prevention, care, and management across the VHA. The task included addressing supportive processes and barriers at each level of the organization through a public health framework and using a population health management approach.
The VA National Strategic Plan for Excellence in HBV Care was focused on the following overarching aims:
- Characterizing the current state of care for veterans with HBV in VA care;
- Developing and disseminating clinical guidance on high-quality care for patients with HBV;
- Developing population data and informatics tools to streamline the identification and monitoring of patients with chronic HBV; and
- Evaluating VHA care for patients with HBV over time.
Care for Veterans With HBV at the VA
The VA health care system is America’s largest integrated health care system, providing care at 1,243 health care facilities, including 170 medical centers and 1,063 outpatient sites of care serving 9 million enrolled veterans each year.13 As of January 2018, there were 10,743 individuals with serologic evidence for chronic HBV infection in VA care, based on a definition of 2 or more detectable surface antigen (sAg) or hepatitis B DNA tests recorded at least 6 months apart.1 About 2,000 additional VA patients have a history of a single positive sAg test. These patients have unclear HBV status and require a second sAg test to determine whether they have a chronic infection.
The prevalence of HBV infection among veterans in VA care is slightly higher than that in the US civilian population at 0.4%.14 Studies of selected subpopulations of veterans have found high seropositivity of prior or chronic HBV infection among homeless veterans and veterans admitted to a psychiatric hospital.15,16 The data from 2015 suggest that homeless veterans have a chronic HBV infection rate of 1.0%.14 Of those with known chronic HBV infection, the plurality are white (40.4%) or African American (40.2%), male (92.4%), with a mean age of 59.9 (SD 12.0) years. According to National HIV, Hepatitis and Related Conditions Data and Analysis Group personal correspondence, the geographic territories with the largest chronic HBV caseload include the Southeast, Gulf Coast, and West Coast. As of January 2018, 1,210 veterans in care have HBV-related cirrhosis.
HBV Screening in VA
The current VA HBV screening guidelines follow those of the US Preventive Services Task Force (USPSTF).17 HBV screening is recommended for unvaccinated individuals in high-risk groups, such as patients with HIV or hepatitis C virus (HCV), those on hemodialysis, those with elevated alanine transaminase/aspartate transaminase of unknown etiology, those on immunosuppressive therapy, injection drug users, the MSM population, people with household contact with an HBV-infected person, people born to an HBV-infected mother, those with risk factors for HBV exposure prior to vaccination, pregnant women, and people born in highly endemic areas regardless of vaccination status.2 The VHA recommends against standardized risk assessment and laboratory screening for HBV infection in the asymptomatic general patient population. However, if risk factors become known during the course of providing usual clinical care, then laboratory screening should be considered.2
Of the 6.1 million VHA users
HBV Care and VA Antiviral Treatment
In a study of an HBV care cascade, Serper and colleagues reviewed a cohort of veterans in the VA with HBV. About 50% of the patients with known chronic HBV in the VA system from 1999 to 2013 had received infectious diseases or gastroenterology/hepatology specialty care in the previous 2 years.19 Follow-up data from the National HIV, Hepatitis and Related Conditions Data and Analysis Group indicated that this remains the case: 52.3% of patients with documented chronic HBV had received specialty care from VA sources in the prior 2 years. Serper and colleagues also reported that among veterans in VHA care with chronic HBV infection and cirrhosis from 1999 to 2013, annual imaging was < 50%, and initiation of antiviral treatment was only 39%. Antiviral therapy and liver imaging were both independently associated with lower mortality for patients with HBV and cirrhosis.19
A review of studies that evaluated the delivery of care for patients with HBV in U.S. civilian populations, including retrospective reviews of private payer claims databases and chart reviews, the Kaiser Permanente claims database, and community gastrointestinal (GI) practice chart reviews, revealed similar practice patterns with those in the VA.20 Across the US, rates of antiviral therapy and HCC surveillance for those with HBV cirrhosis were low, ranging from 14% to 50% and 19% to 60%, respectively. Several of these studies also found that being seen by an HBV specialist was associated with improved care.20
Antiviral treatment of individuals with cirrhosis and chronic HBV infection can reduce the risk of progression to decompensated cirrhosis and liver cancer. Among current VA patients with HBV cirrhosis, 62.4% received at least 1 month of HBV antiviral medication in the prior year. Additionally, biannual liver imaging is recommended in this population to screen for the development of HCC. According to National HIV, Hepatitis and Related Conditions Data and Analysis Group personal correspondence, nationally, 51.2% of individuals with HBV cirrhosis had received at least one instance of liver imaging within the past 6 months, and 71.2% received imaging within the past 12 months.
Prevention of HBV Infection and Sequelae
Vaccination rates in the US vary by age group, with higher immunization rates among those born after 1991 than the rates of those born earlier. Data from the National Health and Nutrition Examination Survey from 1988 to 2012 reported 33% immunity among veterans aged < 50 years and 6% among those aged ≥ 50 years.21 In addition to individuals who received childhood vaccination in the 1990s, all new military recruits assigned to the Korean Peninsula were vaccinated for HBV as of 1986, and those joining the military after 2002 received mandatory vaccination.
The VA follows the ACIP/CDC hepatitis B immunization guidelines.22-24 The VA currently recommends HBV immunization among previously unvaccinated adults at increased risk of contracting HBV infection and for any other adult who is seeking protection from HBV infection. The VA also offers general recommendations for prevention of transmission between veterans with known chronic HBV to their household, sexual, or drug-using partners. Transmission prevention guidelines also provide recommendations for vaccination of pregnant women with HBV risk factors and women at risk for HBV infection during pregnancy.22
HBV Care Guidance
One of the core tasks of the VA National Hepatitis B Working Group, given its broad, multidisciplinary expertise in HBV, was developing general clinical guidelines for the provision of high-quality care for patients with HBV. The group reviewed current literature and scientific evidence on care for patients with HBV. The working group relied heavily on the VA’s national guidelines for HBV screening and immunization, which are based on recommendations from the USPSTF, ACIP, CDC, and professional societies. The professional society guidelines included the American Association for the Study of Liver Disease’s Guidelines for Treatment of Chronic Hepatitis B, the America College of Gastroenterology’s Practice Guidelines: Evaluation of Abnormal Liver Chemistries, the American Gastroenterological Association Institute’s Guidelines for Prevention and Treatment of Hepatitis B Reactivation during Immunosuppressive Drug Therapy, and CDC’s Guidelines for Screening Pregnant Women for HBV.19,22-27
The working group identified areas for HBV quality improvement that were consistent with the VA and professional guidelines, specific and measurable using VA data, clinically relevant, feasible, and achievable in a defined time period. Areas for targeted improvement will include testing for HBV among high-risk patients, increasing antiviral treatment and HCC surveillance of veterans with HBV-related cirrhosis, decreasing progression of chronic HBV to cirrhosis, and expanding prevention measures, such as immunization among those at high risk for HBV and prevention of HBV reactivation.
At a national level, development of specific and measurable quality of care indicators for HBV will aid in assessing gaps in care and developing strategies to address these gaps. A broader discussion of care for patients with HBV quality with front-line health care providers (HCPs) will be paired with increased education and providing clinical support tools for those HCPs and facilities without access to specialty GI services.
Clinical pharmacists will be critical targets for the dissemination of guidance for HBV care paired with clinical informatics support tools and clinical educational opportunities. As of 2015, there were about 7,700 clinical pharmacists in the VHA and 3,200 had a scope of practice that included prescribing authority. As a result, 20% of HCV prescriptions in the VA in fiscal year 2015
Identification and Monitoring
The HBV working group and the VA Viral Hepatitis Technical Advisory Group are working with field HCPs to develop several informatics tools to promote HBV case identification and quality monitoring. These groups identified several barriers to HBV case identification and monitoring. The following informatics tools are either available or in development to reduce these barriers:
- A local clinical case registry of patients with HBV infection based on ICD codes, which allows users to create custom reports to identify, monitor, and track care;
- Because of the risk of HBV reactivation in patients with chronic HBV infection who receive anti-CD20 agents, such as rituximab, a medication order check to improve HBV screening among veterans receiving anti-CD20 medication;
- Validated patient reports based on laboratory diagnosis of HBV, drawn from all results across the VHA since 1999, made available to all facilities;
- Interactive reports summarizing quality of care for patients with HBV infection, based on facility-level indicators in development by the national HBV working group, will be distributed and enable geographic comparison;
- An HBV immunization clinical reminder that will prompt frontline HCPs to test and vaccinate; and
- An HBV clinical dashboard that will enable HCPs and facilities to identify all their HBV-positive veterans and track their care and outcomes over time.
Evaluating VA Care for Patients with HBV
As indicators of quality of HBV care are refined for VA patients and the health care delivery system, guidance will be made broadly available to frontline HCPs and administrators. The HBV quality of care recommendations will be paired with a suite of clinical informatics tools and virtual educational trainings to ensure that VA HCPs and facilities can streamline care for patients with HBV infection as much as possible. Quality improvement will be measured nationally each year, and strategies to address persistent variability and gaps in care will be developed in collaboration with the VA SME’s, facilities, and HCPs.
Conclusion
Hepatitis B virus is at least as prevalent among veterans who are cared for at VA facilities as it is in the US civilian population. Although care for patients with HBV infection in the VA is similar to care for patients with HBV infection in the community, the VA recognizes areas for improved HBV prevention, testing, care, and treatment. The VA has begun a continuous quality improvement strategic plan to enhance the level of care for patients with HBV infection in VA care. Centralized coordination and communication of VA data combined with veteran- and field-centered policies and operational planning and execution will allow clinically relevant improvements in HBV diagnosis, treatment, and prevention among veterans served by VA.
Click here to read the digital edition.
Hepatitis B is a viral infection caused by the hepatitis B virus (HBV), which is transmitted through percutaneous (ie, puncture through the skin) or mucosal (ie, direct contact with mucous membranes) exposure to infectious blood or body fluids. Hepatitis B virus can cause chronic infection, resulting in cirrhosis of the liver, liver cancer, liver failure, and death. Persons with chronic infection also serve as the main reservoir for continued HBV transmission.1
Individuals at highest risk for infection include those born in geographic regions with a high prevalence of HBV, those with sexual partners or household contacts with chronic HBV infection, men who have sex with men (MSM), those with HIV, and individuals who inject drugs. Pregnant women also are a population of concern given the potential for perinatal transmission.2
About 850,000 to 2.2 million people in the US (about 0.3% of the civilian US population) are chronically infected with HBV.3 The prevalence of chronic HBV is much higher (10%-19%) among Asian Americans, those of Pacific Island descent, and other immigrant populations from highly endemic countries.4 In the US, HBV is responsible for 2,000 to 4,000 preventable deaths annually, primarily from cirrhosis, liver cancer, and hepatic failure.4 In the civilian US population, reported cases of acute HBV decreased 0.3% from 2011 to 2012, increased 5.4% in 2013 with an 8.5% decrease in 2014, and a 20.7% increase in 2015.4 Injection drug use is likely driving the most recent increase.5
Not all individuals exposed to HBV will develop chronic infection, and the risk of chronic HBV infection depends on an individual’s age at the time of exposure. For example, about 95% of infants exposed to HBV perinatally will develop a chronic infection compared with 5% of exposed adults.6 Of those with chronic HBV, a small proportion will develop cirrhosis and/or hepatocellular carcinoma (HCC) with increasing risk as viral DNA concentrations increase. Additional risk factors for cirrhosis include being older, male, having a persistently elevated alanine transaminase, viral superinfections, HBV reversion/reactivation, genotype, and various markers of disease severity (HCC).6 Of note, chronic HBV infection may cause HCC even in the absence of cirrhosis.7 In addition, immunosuppression (eg, from cancer chemotherapy) may allow HBV reactivation, which may result in fulminant hepatic failure. In the Veterans Health Affairs (VHA) health care system, about 17% of those with known chronic HBV also carry a diagnosis of cirrhosis.
Vaccination is the mainstay of efforts to prevent HBV infection. The first commercially available HBV vaccine was approved by the FDA in 1981, with subsequent FDA approval in 1986 of a vaccine manufactured using recombinant DNA technology.8 In 1991, the Advisory Committee on Immunization Practices (ACIP) recommended universal childhood vaccination for HBV, with subsequent recommendations for vaccination of adolescents and adults in high-risk groups in 1995, and in 1999 all remaining unvaccinated children aged ≤ 19 years.9 Military policy has been to provide hepatitis B immunization to personnel assigned to the Korean peninsula since 1986 and to all recruits since 2001.10
Following publication of an Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) report, in 2011 the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan.11 The current HHS Action Plan, along with the NASEM National Strategy for the Elimination of Hepatitis B and C: Phase Two Report, commissioned by the US Centers for Disease Control and Prevention (CDC), outlines a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.12 The VA is a critical partner in this federal collaborative effort to achieve excellence in viral hepatitis care.
In August 2016, the HIV, Hepatitis, and Related Conditions Programs in the VA Office of Specialty Care Services convened a National Hepatitis B Working Group consisting of VA subject matter experts (SMEs) and representatives from the VA Central Office stakeholder program offices, with a charge of developing a strategic plan to ensure excellence in HBV prevention, care, and management across the VHA. The task included addressing supportive processes and barriers at each level of the organization through a public health framework and using a population health management approach.
The VA National Strategic Plan for Excellence in HBV Care was focused on the following overarching aims:
- Characterizing the current state of care for veterans with HBV in VA care;
- Developing and disseminating clinical guidance on high-quality care for patients with HBV;
- Developing population data and informatics tools to streamline the identification and monitoring of patients with chronic HBV; and
- Evaluating VHA care for patients with HBV over time.
Care for Veterans With HBV at the VA
The VA health care system is America’s largest integrated health care system, providing care at 1,243 health care facilities, including 170 medical centers and 1,063 outpatient sites of care serving 9 million enrolled veterans each year.13 As of January 2018, there were 10,743 individuals with serologic evidence for chronic HBV infection in VA care, based on a definition of 2 or more detectable surface antigen (sAg) or hepatitis B DNA tests recorded at least 6 months apart.1 About 2,000 additional VA patients have a history of a single positive sAg test. These patients have unclear HBV status and require a second sAg test to determine whether they have a chronic infection.
The prevalence of HBV infection among veterans in VA care is slightly higher than that in the US civilian population at 0.4%.14 Studies of selected subpopulations of veterans have found high seropositivity of prior or chronic HBV infection among homeless veterans and veterans admitted to a psychiatric hospital.15,16 The data from 2015 suggest that homeless veterans have a chronic HBV infection rate of 1.0%.14 Of those with known chronic HBV infection, the plurality are white (40.4%) or African American (40.2%), male (92.4%), with a mean age of 59.9 (SD 12.0) years. According to National HIV, Hepatitis and Related Conditions Data and Analysis Group personal correspondence, the geographic territories with the largest chronic HBV caseload include the Southeast, Gulf Coast, and West Coast. As of January 2018, 1,210 veterans in care have HBV-related cirrhosis.
HBV Screening in VA
The current VA HBV screening guidelines follow those of the US Preventive Services Task Force (USPSTF).17 HBV screening is recommended for unvaccinated individuals in high-risk groups, such as patients with HIV or hepatitis C virus (HCV), those on hemodialysis, those with elevated alanine transaminase/aspartate transaminase of unknown etiology, those on immunosuppressive therapy, injection drug users, the MSM population, people with household contact with an HBV-infected person, people born to an HBV-infected mother, those with risk factors for HBV exposure prior to vaccination, pregnant women, and people born in highly endemic areas regardless of vaccination status.2 The VHA recommends against standardized risk assessment and laboratory screening for HBV infection in the asymptomatic general patient population. However, if risk factors become known during the course of providing usual clinical care, then laboratory screening should be considered.2
Of the 6.1 million VHA users
HBV Care and VA Antiviral Treatment
In a study of an HBV care cascade, Serper and colleagues reviewed a cohort of veterans in the VA with HBV. About 50% of the patients with known chronic HBV in the VA system from 1999 to 2013 had received infectious diseases or gastroenterology/hepatology specialty care in the previous 2 years.19 Follow-up data from the National HIV, Hepatitis and Related Conditions Data and Analysis Group indicated that this remains the case: 52.3% of patients with documented chronic HBV had received specialty care from VA sources in the prior 2 years. Serper and colleagues also reported that among veterans in VHA care with chronic HBV infection and cirrhosis from 1999 to 2013, annual imaging was < 50%, and initiation of antiviral treatment was only 39%. Antiviral therapy and liver imaging were both independently associated with lower mortality for patients with HBV and cirrhosis.19
A review of studies that evaluated the delivery of care for patients with HBV in U.S. civilian populations, including retrospective reviews of private payer claims databases and chart reviews, the Kaiser Permanente claims database, and community gastrointestinal (GI) practice chart reviews, revealed similar practice patterns with those in the VA.20 Across the US, rates of antiviral therapy and HCC surveillance for those with HBV cirrhosis were low, ranging from 14% to 50% and 19% to 60%, respectively. Several of these studies also found that being seen by an HBV specialist was associated with improved care.20
Antiviral treatment of individuals with cirrhosis and chronic HBV infection can reduce the risk of progression to decompensated cirrhosis and liver cancer. Among current VA patients with HBV cirrhosis, 62.4% received at least 1 month of HBV antiviral medication in the prior year. Additionally, biannual liver imaging is recommended in this population to screen for the development of HCC. According to National HIV, Hepatitis and Related Conditions Data and Analysis Group personal correspondence, nationally, 51.2% of individuals with HBV cirrhosis had received at least one instance of liver imaging within the past 6 months, and 71.2% received imaging within the past 12 months.
Prevention of HBV Infection and Sequelae
Vaccination rates in the US vary by age group, with higher immunization rates among those born after 1991 than the rates of those born earlier. Data from the National Health and Nutrition Examination Survey from 1988 to 2012 reported 33% immunity among veterans aged < 50 years and 6% among those aged ≥ 50 years.21 In addition to individuals who received childhood vaccination in the 1990s, all new military recruits assigned to the Korean Peninsula were vaccinated for HBV as of 1986, and those joining the military after 2002 received mandatory vaccination.
The VA follows the ACIP/CDC hepatitis B immunization guidelines.22-24 The VA currently recommends HBV immunization among previously unvaccinated adults at increased risk of contracting HBV infection and for any other adult who is seeking protection from HBV infection. The VA also offers general recommendations for prevention of transmission between veterans with known chronic HBV to their household, sexual, or drug-using partners. Transmission prevention guidelines also provide recommendations for vaccination of pregnant women with HBV risk factors and women at risk for HBV infection during pregnancy.22
HBV Care Guidance
One of the core tasks of the VA National Hepatitis B Working Group, given its broad, multidisciplinary expertise in HBV, was developing general clinical guidelines for the provision of high-quality care for patients with HBV. The group reviewed current literature and scientific evidence on care for patients with HBV. The working group relied heavily on the VA’s national guidelines for HBV screening and immunization, which are based on recommendations from the USPSTF, ACIP, CDC, and professional societies. The professional society guidelines included the American Association for the Study of Liver Disease’s Guidelines for Treatment of Chronic Hepatitis B, the America College of Gastroenterology’s Practice Guidelines: Evaluation of Abnormal Liver Chemistries, the American Gastroenterological Association Institute’s Guidelines for Prevention and Treatment of Hepatitis B Reactivation during Immunosuppressive Drug Therapy, and CDC’s Guidelines for Screening Pregnant Women for HBV.19,22-27
The working group identified areas for HBV quality improvement that were consistent with the VA and professional guidelines, specific and measurable using VA data, clinically relevant, feasible, and achievable in a defined time period. Areas for targeted improvement will include testing for HBV among high-risk patients, increasing antiviral treatment and HCC surveillance of veterans with HBV-related cirrhosis, decreasing progression of chronic HBV to cirrhosis, and expanding prevention measures, such as immunization among those at high risk for HBV and prevention of HBV reactivation.
At a national level, development of specific and measurable quality of care indicators for HBV will aid in assessing gaps in care and developing strategies to address these gaps. A broader discussion of care for patients with HBV quality with front-line health care providers (HCPs) will be paired with increased education and providing clinical support tools for those HCPs and facilities without access to specialty GI services.
Clinical pharmacists will be critical targets for the dissemination of guidance for HBV care paired with clinical informatics support tools and clinical educational opportunities. As of 2015, there were about 7,700 clinical pharmacists in the VHA and 3,200 had a scope of practice that included prescribing authority. As a result, 20% of HCV prescriptions in the VA in fiscal year 2015
Identification and Monitoring
The HBV working group and the VA Viral Hepatitis Technical Advisory Group are working with field HCPs to develop several informatics tools to promote HBV case identification and quality monitoring. These groups identified several barriers to HBV case identification and monitoring. The following informatics tools are either available or in development to reduce these barriers:
- A local clinical case registry of patients with HBV infection based on ICD codes, which allows users to create custom reports to identify, monitor, and track care;
- Because of the risk of HBV reactivation in patients with chronic HBV infection who receive anti-CD20 agents, such as rituximab, a medication order check to improve HBV screening among veterans receiving anti-CD20 medication;
- Validated patient reports based on laboratory diagnosis of HBV, drawn from all results across the VHA since 1999, made available to all facilities;
- Interactive reports summarizing quality of care for patients with HBV infection, based on facility-level indicators in development by the national HBV working group, will be distributed and enable geographic comparison;
- An HBV immunization clinical reminder that will prompt frontline HCPs to test and vaccinate; and
- An HBV clinical dashboard that will enable HCPs and facilities to identify all their HBV-positive veterans and track their care and outcomes over time.
Evaluating VA Care for Patients with HBV
As indicators of quality of HBV care are refined for VA patients and the health care delivery system, guidance will be made broadly available to frontline HCPs and administrators. The HBV quality of care recommendations will be paired with a suite of clinical informatics tools and virtual educational trainings to ensure that VA HCPs and facilities can streamline care for patients with HBV infection as much as possible. Quality improvement will be measured nationally each year, and strategies to address persistent variability and gaps in care will be developed in collaboration with the VA SME’s, facilities, and HCPs.
Conclusion
Hepatitis B virus is at least as prevalent among veterans who are cared for at VA facilities as it is in the US civilian population. Although care for patients with HBV infection in the VA is similar to care for patients with HBV infection in the community, the VA recognizes areas for improved HBV prevention, testing, care, and treatment. The VA has begun a continuous quality improvement strategic plan to enhance the level of care for patients with HBV infection in VA care. Centralized coordination and communication of VA data combined with veteran- and field-centered policies and operational planning and execution will allow clinically relevant improvements in HBV diagnosis, treatment, and prevention among veterans served by VA.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatitis B FAQs for health professionals: overview and statistics. https://www.cdc.gov/hepatitis/hbv/hbvfaq .htm#overview. Updated January 11, 2018. Accessed on February 12, 2018.
2.
3. Centers for Disease Control and Prevention. Surveillance for viral hepatitis—United States, 2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/index.htm. Updated June 19, 2017. Accessed February 12, 2018.
4. Kim WR. Epidemiology of hepatitis B in the United States. Hepatology. 2009;49(suppl 5):S28-S34.
5. Harris AM, Iqbal K, Schillie S, et al. Increases in acute hepatitis B virus infections— Kentucky, Tennessee, and West Virginia, 2006-2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):47-50.
6. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373(9663):582-592.
7. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127.
8. Weinbaum CM, Williams I, Mast EE, et al; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1-20.
9. Centers for Disease Control and Prevention. Achievements in public health: hepatitis B vaccination—United States, 1982-2002. MMWR. 2002;51(25):549-552, 563.
10. Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3-26.
11. Colvin HM, Mitchell AE, eds; Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: National Academies Press; 2010.
12. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: National Academies Press; 2017.
13. US Department of Veterans Affairs. Providing health care for veterans. https://www.va.gov/health. Updated February 20, 2018. Accessed February 22, 2018.
14. Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Prevalence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus among homeless and nonhomeless United States veterans. Clin Infect Dis. 2017;65(2):252-258.
15. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
16. Tabibian JH, Wirshing DA, Pierre JM, et al. Hepatitis B and C among veterans in a psychiatric ward. Dig Dis Sci. 2008;53(6):1693-1698
17. US Preventive Services Task Force. Final recommendation statement: screening for hepatitis B virus infection in nonpregnant adolescents and adults. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-b-virus-infection-screening-2014. Published May 2014. Updated February 2018. Accessed February 22, 2018.
18. Backus LI, Belperio PS, Loomis TP, Han SH, Mole LA. Screening for and prevalence of hepatitis B virus infection among high-risk veterans under the care of the U.S. Department of Veterans Affairs: a case report. Ann Intern Med. 2014;161(12):926-928.
19. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
20. Mellinger J, Fontana RJ. Quality of care metrics in chronic hepatitis B. Hepatology. 2016;63(6):1755-1758.
21. Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63(2):388-397.
22. US Department of Veterans Affairs. National Clinical Preventive Service Guidance Statements: Hepatitis B Immunization. http://vaww.prevention.va.gov/CPS/Hepatitis_B_Immunization.asp. Nonpublic document. Source not verified.
23. Advisory Committee on Immunization Practices (ACIP). Recommended immunization schedule for adults aged 19 years or older, United States, 2017. https://www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed February 12, 2018.
24. Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2018;67(1):1-31.
25. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283.
26. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
27. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):215-219, quiz e16-e17.
28. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
1. Centers for Disease Control and Prevention. Hepatitis B FAQs for health professionals: overview and statistics. https://www.cdc.gov/hepatitis/hbv/hbvfaq .htm#overview. Updated January 11, 2018. Accessed on February 12, 2018.
2.
3. Centers for Disease Control and Prevention. Surveillance for viral hepatitis—United States, 2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/index.htm. Updated June 19, 2017. Accessed February 12, 2018.
4. Kim WR. Epidemiology of hepatitis B in the United States. Hepatology. 2009;49(suppl 5):S28-S34.
5. Harris AM, Iqbal K, Schillie S, et al. Increases in acute hepatitis B virus infections— Kentucky, Tennessee, and West Virginia, 2006-2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):47-50.
6. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373(9663):582-592.
7. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127.
8. Weinbaum CM, Williams I, Mast EE, et al; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57(RR-8):1-20.
9. Centers for Disease Control and Prevention. Achievements in public health: hepatitis B vaccination—United States, 1982-2002. MMWR. 2002;51(25):549-552, 563.
10. Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3-26.
11. Colvin HM, Mitchell AE, eds; Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: National Academies Press; 2010.
12. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: National Academies Press; 2017.
13. US Department of Veterans Affairs. Providing health care for veterans. https://www.va.gov/health. Updated February 20, 2018. Accessed February 22, 2018.
14. Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Prevalence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus among homeless and nonhomeless United States veterans. Clin Infect Dis. 2017;65(2):252-258.
15. Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished US military veterans in residential care in Los Angeles. Public Health. 2001;115(4):286-291.
16. Tabibian JH, Wirshing DA, Pierre JM, et al. Hepatitis B and C among veterans in a psychiatric ward. Dig Dis Sci. 2008;53(6):1693-1698
17. US Preventive Services Task Force. Final recommendation statement: screening for hepatitis B virus infection in nonpregnant adolescents and adults. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-b-virus-infection-screening-2014. Published May 2014. Updated February 2018. Accessed February 22, 2018.
18. Backus LI, Belperio PS, Loomis TP, Han SH, Mole LA. Screening for and prevalence of hepatitis B virus infection among high-risk veterans under the care of the U.S. Department of Veterans Affairs: a case report. Ann Intern Med. 2014;161(12):926-928.
19. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
20. Mellinger J, Fontana RJ. Quality of care metrics in chronic hepatitis B. Hepatology. 2016;63(6):1755-1758.
21. Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988-2012. Hepatology. 2016;63(2):388-397.
22. US Department of Veterans Affairs. National Clinical Preventive Service Guidance Statements: Hepatitis B Immunization. http://vaww.prevention.va.gov/CPS/Hepatitis_B_Immunization.asp. Nonpublic document. Source not verified.
23. Advisory Committee on Immunization Practices (ACIP). Recommended immunization schedule for adults aged 19 years or older, United States, 2017. https://www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed February 12, 2018.
24. Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2018;67(1):1-31.
25. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283.
26. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18-35.
27. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):215-219, quiz e16-e17.
28. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
One HCV infection leads to another in HIV+ MSM
SEATTLE – Once HIV positive men who have sex with men contract the hepatitis C virus, they are more likely to get it again, according a study of 305 men in New York.
Overall, 38 men (12%) picked up another HCV infection a median of 1.9 years after clearance of their first, yielding a reinfection rate was 4.4/100 person-years, “a solid seven times higher than the primary infection rate” among HIV-positive men who have sex with men (MSM), said senior investigator Daniel Fierer, MD, an associate professor of infectious diseases at Mount Sinai Hospital, New York.
Thirty-three men cleared their second infection. Of those, six picked up a third infection at a median of 1.1 years, yielding an overall third infection incidence of 8.7/100 person-years.
The results held no matter how the men cleared HCV, whether spontaneously, as in about 10%, or by interferon before 2013, and direct-acting antivirals (DAAs) after.
Most reinfections occurred within 2 years of initial clearance, but some occurred more than a decade later.
The results suggest that there’s a particular need for HCV prevention efforts among men who have previously cleared the infection. For those patients, testing for HCV at an annual HIV checkup might not be frequent enough, Dr. Fierer said at the Conference on Retroviruses and Opportunistic Infections.
“Long-term surveillance is warranted for all HIV-infected MSM after clearance of HCV infection. Further, strategies to reduce HCV reinfections are needed to meet the goal of eliminating HCV in these men,” he said.
Also, “the large difference between primary” and secondary infection “rates suggests HCV risk is not distributed evenly between HIV-infected MSM, but concentrated among a small subpopulation. By definition, this subpopulation would have a higher prevalence” of risky behavior, such as condomless receptive anal sex and sexualized injection methamphetamine use, he said.
The high reinfection rate “tells us basically that we have not done a good job of” preventing infection and reinfection among at risk, HIV-positive men. There’s an “inadequate level of HCV treatment ... we need to eliminate restrictions on DAA” access, Dr. Fierer said.
As far as prevention goes, “I believe we just don’t know what to do. I tell all of my patients about the body fluids that have HCV in them,” which is a good start, he said.
The median age at first clearance was about 45 years, 82% of the men were white, and there was about a 50-50 split between people with private and public insurance.
The work was funded by Gilead. Dr. Fierer did not mention any disclosures.
SOURCE: Carollo JR et al. CROI 2019, Abstract 86
SEATTLE – Once HIV positive men who have sex with men contract the hepatitis C virus, they are more likely to get it again, according a study of 305 men in New York.
Overall, 38 men (12%) picked up another HCV infection a median of 1.9 years after clearance of their first, yielding a reinfection rate was 4.4/100 person-years, “a solid seven times higher than the primary infection rate” among HIV-positive men who have sex with men (MSM), said senior investigator Daniel Fierer, MD, an associate professor of infectious diseases at Mount Sinai Hospital, New York.
Thirty-three men cleared their second infection. Of those, six picked up a third infection at a median of 1.1 years, yielding an overall third infection incidence of 8.7/100 person-years.
The results held no matter how the men cleared HCV, whether spontaneously, as in about 10%, or by interferon before 2013, and direct-acting antivirals (DAAs) after.
Most reinfections occurred within 2 years of initial clearance, but some occurred more than a decade later.
The results suggest that there’s a particular need for HCV prevention efforts among men who have previously cleared the infection. For those patients, testing for HCV at an annual HIV checkup might not be frequent enough, Dr. Fierer said at the Conference on Retroviruses and Opportunistic Infections.
“Long-term surveillance is warranted for all HIV-infected MSM after clearance of HCV infection. Further, strategies to reduce HCV reinfections are needed to meet the goal of eliminating HCV in these men,” he said.
Also, “the large difference between primary” and secondary infection “rates suggests HCV risk is not distributed evenly between HIV-infected MSM, but concentrated among a small subpopulation. By definition, this subpopulation would have a higher prevalence” of risky behavior, such as condomless receptive anal sex and sexualized injection methamphetamine use, he said.
The high reinfection rate “tells us basically that we have not done a good job of” preventing infection and reinfection among at risk, HIV-positive men. There’s an “inadequate level of HCV treatment ... we need to eliminate restrictions on DAA” access, Dr. Fierer said.
As far as prevention goes, “I believe we just don’t know what to do. I tell all of my patients about the body fluids that have HCV in them,” which is a good start, he said.
The median age at first clearance was about 45 years, 82% of the men were white, and there was about a 50-50 split between people with private and public insurance.
The work was funded by Gilead. Dr. Fierer did not mention any disclosures.
SOURCE: Carollo JR et al. CROI 2019, Abstract 86
SEATTLE – Once HIV positive men who have sex with men contract the hepatitis C virus, they are more likely to get it again, according a study of 305 men in New York.
Overall, 38 men (12%) picked up another HCV infection a median of 1.9 years after clearance of their first, yielding a reinfection rate was 4.4/100 person-years, “a solid seven times higher than the primary infection rate” among HIV-positive men who have sex with men (MSM), said senior investigator Daniel Fierer, MD, an associate professor of infectious diseases at Mount Sinai Hospital, New York.
Thirty-three men cleared their second infection. Of those, six picked up a third infection at a median of 1.1 years, yielding an overall third infection incidence of 8.7/100 person-years.
The results held no matter how the men cleared HCV, whether spontaneously, as in about 10%, or by interferon before 2013, and direct-acting antivirals (DAAs) after.
Most reinfections occurred within 2 years of initial clearance, but some occurred more than a decade later.
The results suggest that there’s a particular need for HCV prevention efforts among men who have previously cleared the infection. For those patients, testing for HCV at an annual HIV checkup might not be frequent enough, Dr. Fierer said at the Conference on Retroviruses and Opportunistic Infections.
“Long-term surveillance is warranted for all HIV-infected MSM after clearance of HCV infection. Further, strategies to reduce HCV reinfections are needed to meet the goal of eliminating HCV in these men,” he said.
Also, “the large difference between primary” and secondary infection “rates suggests HCV risk is not distributed evenly between HIV-infected MSM, but concentrated among a small subpopulation. By definition, this subpopulation would have a higher prevalence” of risky behavior, such as condomless receptive anal sex and sexualized injection methamphetamine use, he said.
The high reinfection rate “tells us basically that we have not done a good job of” preventing infection and reinfection among at risk, HIV-positive men. There’s an “inadequate level of HCV treatment ... we need to eliminate restrictions on DAA” access, Dr. Fierer said.
As far as prevention goes, “I believe we just don’t know what to do. I tell all of my patients about the body fluids that have HCV in them,” which is a good start, he said.
The median age at first clearance was about 45 years, 82% of the men were white, and there was about a 50-50 split between people with private and public insurance.
The work was funded by Gilead. Dr. Fierer did not mention any disclosures.
SOURCE: Carollo JR et al. CROI 2019, Abstract 86
REPORTING FROM CROI 2019
For patients with HBV, daily aspirin may reduce risk of liver cancer
Sixteen years of data showed that daily aspirin therapy reduced the risk of HBV-related HCC by 29%, reported lead author Teng-Yu Lee, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and his colleagues. Analysis also showed that antiviral nucleos(t)ide analogue therapy and statin use were independently associated with reduced risk of HCC, whereas older age, cirrhosis, and male sex increased risk.
“Therapy with [nucleos(t)ide analogues] is associated with reductions in HCC risk, but the risk is not erased,” the investigators wrote in JAMA Internal Medicine. “Therefore, using only [nucleos(t)ide analogue] therapy may not be enough for HCC prevention. Antiviral therapy is not indicated in most HBV carriers, so another effective way of reducing HCC risk needs to be developed.”
Previous studies have shown that aspirin can reduce the risk of colorectal cancer; however, data supporting aspirin for HCC prevention are limited to a few animal models and human studies, the latter of which are statistically unreliable.
“Therefore, we conducted a nationwide cohort study to evaluate the association of daily aspirin therapy with HBV-related HCC,” the investigators wrote.
They screened 204,507 patients with HBV included in the Taiwanese National Health Insurance Research Database (NHIRD) between 1997 and 2012, first excluding any with confounding conditions, such as hepatitis C infection or alcoholic liver disease. Next, 2,123 patients were identified who had taken aspirin for 90 days or longer. Finally, these cases were randomly matched with 8,492 control patients with HBV who had never received antiplatelet therapy. The main measured outcome was diagnosis with HCC. Patients were followed until this diagnosis was made, death occurred, or the end of the study period.
Analysis showed that most patients were male (72.4%) and took aspirin for about 4 years, usually prescribed for cardiovascular disease risk factors. Almost all patients in the treatment group (98%) received an aspirin dose of 100 mg or less.
After 5 years, the cumulative incidence of HCC in the aspirin group was 5.20% versus 7.87% in the control group (P less than .001). Multivariable analysis revealed that daily aspirin was associated with a significant risk reduction of 29% (HR 0.71; P less than .001), as were nucleos(t)ide analogues and statins, which lowered risk by 46% and 38%, respectively. In contrast, risk increased with older age at the rate of 1% per year, male sex carried an additional risk of 75%, and liver cirrhosis was associated with a 2.89-fold risk increase.
“In the present study, we report that daily aspirin therapy was associated with a reduced incidence of HCC in patients with [chronic hepatitis B],” the investigators wrote. “Our findings may be of help in future efforts to further improve the chemoprevention of HBV-related HCC, and a proof-of-concept study is thus warranted.”
The investigators described several mechanisms that may have contribute to the possible risk reduction provided by aspirin. For one, aspirin inhibits platelet activation, which is associated with development of HBV-related liver disease. Additional benefit may come from induction of HCC cell apoptosis, control of tumor growth, reduced liver fibrosis, and increased liver regeneration, all of which have been associated with aspirin in rodent models.
“Hepatitis B virus–related HCC is generally a consequence of chronic inflammation due to hepatitis, fibrosis, dysplasia, and tumor growth,” the investigators wrote, suggesting that aspirin-related reductions in inflammation could also explain reduced neoplastic activity.
To assess for increased risk of peptic ulcers secondary to aspirin, the investigators performed a subanalysis of peptic ulcer bleeding. These results showed that rates of peptic ulcer bleeding, at around 5%-6%, were similar between the aspirin group and the control group. Among other variables, cirrhosis didn’t significantly affect rates of peptic ulcer bleeding, and aspirin users had similar rates of peptic ulcer bleeding regardless of HBV status. Because of the study design, however, the investigators cautioned that these analyses could underestimate ulcer risk because patients who could not tolerate aspirin for at least 90 days were excluded from the study.
Although statins stood out as another possible risk reducer, the investigators noted that “randomized clinical trials are required to confirm the chemopreventive effect of statins.”
Similarly, the investigators suggested that a prospective trial is needed before aspirin can be adopted as an HCC preventive.
The study was funded by the Ministry of Science and Technology, National Health Research Institutes, and Taichung (Taiwan) Veterans General Hospital, Taiwan. One author reported financial compensation from Gilead and Bristol-Myers Squibb.
SOURCE: Lee T-Y et al. JAMA Intern Med. 2019 Mar 18. doi:10.1001/jamainternmed.2018.8342.
Sixteen years of data showed that daily aspirin therapy reduced the risk of HBV-related HCC by 29%, reported lead author Teng-Yu Lee, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and his colleagues. Analysis also showed that antiviral nucleos(t)ide analogue therapy and statin use were independently associated with reduced risk of HCC, whereas older age, cirrhosis, and male sex increased risk.
“Therapy with [nucleos(t)ide analogues] is associated with reductions in HCC risk, but the risk is not erased,” the investigators wrote in JAMA Internal Medicine. “Therefore, using only [nucleos(t)ide analogue] therapy may not be enough for HCC prevention. Antiviral therapy is not indicated in most HBV carriers, so another effective way of reducing HCC risk needs to be developed.”
Previous studies have shown that aspirin can reduce the risk of colorectal cancer; however, data supporting aspirin for HCC prevention are limited to a few animal models and human studies, the latter of which are statistically unreliable.
“Therefore, we conducted a nationwide cohort study to evaluate the association of daily aspirin therapy with HBV-related HCC,” the investigators wrote.
They screened 204,507 patients with HBV included in the Taiwanese National Health Insurance Research Database (NHIRD) between 1997 and 2012, first excluding any with confounding conditions, such as hepatitis C infection or alcoholic liver disease. Next, 2,123 patients were identified who had taken aspirin for 90 days or longer. Finally, these cases were randomly matched with 8,492 control patients with HBV who had never received antiplatelet therapy. The main measured outcome was diagnosis with HCC. Patients were followed until this diagnosis was made, death occurred, or the end of the study period.
Analysis showed that most patients were male (72.4%) and took aspirin for about 4 years, usually prescribed for cardiovascular disease risk factors. Almost all patients in the treatment group (98%) received an aspirin dose of 100 mg or less.
After 5 years, the cumulative incidence of HCC in the aspirin group was 5.20% versus 7.87% in the control group (P less than .001). Multivariable analysis revealed that daily aspirin was associated with a significant risk reduction of 29% (HR 0.71; P less than .001), as were nucleos(t)ide analogues and statins, which lowered risk by 46% and 38%, respectively. In contrast, risk increased with older age at the rate of 1% per year, male sex carried an additional risk of 75%, and liver cirrhosis was associated with a 2.89-fold risk increase.
“In the present study, we report that daily aspirin therapy was associated with a reduced incidence of HCC in patients with [chronic hepatitis B],” the investigators wrote. “Our findings may be of help in future efforts to further improve the chemoprevention of HBV-related HCC, and a proof-of-concept study is thus warranted.”
The investigators described several mechanisms that may have contribute to the possible risk reduction provided by aspirin. For one, aspirin inhibits platelet activation, which is associated with development of HBV-related liver disease. Additional benefit may come from induction of HCC cell apoptosis, control of tumor growth, reduced liver fibrosis, and increased liver regeneration, all of which have been associated with aspirin in rodent models.
“Hepatitis B virus–related HCC is generally a consequence of chronic inflammation due to hepatitis, fibrosis, dysplasia, and tumor growth,” the investigators wrote, suggesting that aspirin-related reductions in inflammation could also explain reduced neoplastic activity.
To assess for increased risk of peptic ulcers secondary to aspirin, the investigators performed a subanalysis of peptic ulcer bleeding. These results showed that rates of peptic ulcer bleeding, at around 5%-6%, were similar between the aspirin group and the control group. Among other variables, cirrhosis didn’t significantly affect rates of peptic ulcer bleeding, and aspirin users had similar rates of peptic ulcer bleeding regardless of HBV status. Because of the study design, however, the investigators cautioned that these analyses could underestimate ulcer risk because patients who could not tolerate aspirin for at least 90 days were excluded from the study.
Although statins stood out as another possible risk reducer, the investigators noted that “randomized clinical trials are required to confirm the chemopreventive effect of statins.”
Similarly, the investigators suggested that a prospective trial is needed before aspirin can be adopted as an HCC preventive.
The study was funded by the Ministry of Science and Technology, National Health Research Institutes, and Taichung (Taiwan) Veterans General Hospital, Taiwan. One author reported financial compensation from Gilead and Bristol-Myers Squibb.
SOURCE: Lee T-Y et al. JAMA Intern Med. 2019 Mar 18. doi:10.1001/jamainternmed.2018.8342.
Sixteen years of data showed that daily aspirin therapy reduced the risk of HBV-related HCC by 29%, reported lead author Teng-Yu Lee, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and his colleagues. Analysis also showed that antiviral nucleos(t)ide analogue therapy and statin use were independently associated with reduced risk of HCC, whereas older age, cirrhosis, and male sex increased risk.
“Therapy with [nucleos(t)ide analogues] is associated with reductions in HCC risk, but the risk is not erased,” the investigators wrote in JAMA Internal Medicine. “Therefore, using only [nucleos(t)ide analogue] therapy may not be enough for HCC prevention. Antiviral therapy is not indicated in most HBV carriers, so another effective way of reducing HCC risk needs to be developed.”
Previous studies have shown that aspirin can reduce the risk of colorectal cancer; however, data supporting aspirin for HCC prevention are limited to a few animal models and human studies, the latter of which are statistically unreliable.
“Therefore, we conducted a nationwide cohort study to evaluate the association of daily aspirin therapy with HBV-related HCC,” the investigators wrote.
They screened 204,507 patients with HBV included in the Taiwanese National Health Insurance Research Database (NHIRD) between 1997 and 2012, first excluding any with confounding conditions, such as hepatitis C infection or alcoholic liver disease. Next, 2,123 patients were identified who had taken aspirin for 90 days or longer. Finally, these cases were randomly matched with 8,492 control patients with HBV who had never received antiplatelet therapy. The main measured outcome was diagnosis with HCC. Patients were followed until this diagnosis was made, death occurred, or the end of the study period.
Analysis showed that most patients were male (72.4%) and took aspirin for about 4 years, usually prescribed for cardiovascular disease risk factors. Almost all patients in the treatment group (98%) received an aspirin dose of 100 mg or less.
After 5 years, the cumulative incidence of HCC in the aspirin group was 5.20% versus 7.87% in the control group (P less than .001). Multivariable analysis revealed that daily aspirin was associated with a significant risk reduction of 29% (HR 0.71; P less than .001), as were nucleos(t)ide analogues and statins, which lowered risk by 46% and 38%, respectively. In contrast, risk increased with older age at the rate of 1% per year, male sex carried an additional risk of 75%, and liver cirrhosis was associated with a 2.89-fold risk increase.
“In the present study, we report that daily aspirin therapy was associated with a reduced incidence of HCC in patients with [chronic hepatitis B],” the investigators wrote. “Our findings may be of help in future efforts to further improve the chemoprevention of HBV-related HCC, and a proof-of-concept study is thus warranted.”
The investigators described several mechanisms that may have contribute to the possible risk reduction provided by aspirin. For one, aspirin inhibits platelet activation, which is associated with development of HBV-related liver disease. Additional benefit may come from induction of HCC cell apoptosis, control of tumor growth, reduced liver fibrosis, and increased liver regeneration, all of which have been associated with aspirin in rodent models.
“Hepatitis B virus–related HCC is generally a consequence of chronic inflammation due to hepatitis, fibrosis, dysplasia, and tumor growth,” the investigators wrote, suggesting that aspirin-related reductions in inflammation could also explain reduced neoplastic activity.
To assess for increased risk of peptic ulcers secondary to aspirin, the investigators performed a subanalysis of peptic ulcer bleeding. These results showed that rates of peptic ulcer bleeding, at around 5%-6%, were similar between the aspirin group and the control group. Among other variables, cirrhosis didn’t significantly affect rates of peptic ulcer bleeding, and aspirin users had similar rates of peptic ulcer bleeding regardless of HBV status. Because of the study design, however, the investigators cautioned that these analyses could underestimate ulcer risk because patients who could not tolerate aspirin for at least 90 days were excluded from the study.
Although statins stood out as another possible risk reducer, the investigators noted that “randomized clinical trials are required to confirm the chemopreventive effect of statins.”
Similarly, the investigators suggested that a prospective trial is needed before aspirin can be adopted as an HCC preventive.
The study was funded by the Ministry of Science and Technology, National Health Research Institutes, and Taichung (Taiwan) Veterans General Hospital, Taiwan. One author reported financial compensation from Gilead and Bristol-Myers Squibb.
SOURCE: Lee T-Y et al. JAMA Intern Med. 2019 Mar 18. doi:10.1001/jamainternmed.2018.8342.
FROM JAMA INTERNAL MEDICINE
Current State of Hepatitis C Care in the VA
VA Hepatitis C Treatment Progress
Lisa Backus, MD. For a long time the US Department of Veterans Affairs (VA) has approached hepatitis C virus (HCV) care in a comprehensive way. We have done extensive screening to look for people with HCV infection. Even before birth cohort testing was recommended by the Centers for Disease Control and Prevention (CDC), the VA had aggressive HCV screening programs.
From the VA Corporate Data Warehouse, we know that the VA has screened more than 80% of people who are in the 1945 to 1965 birth cohort in VA care. Over time, HCV prevalence has been dropping in screened veterans and by extension in those who remain to be screened. Based on internal modeling, the VA estimates that only 6,000 to 7,000 veterans in the 1945 to 1965 birth cohort remain to be found if we could somehow screen everyone in that group.
On the treatment side, the VA has provided an unparalleled amount of care. In data from the Clinical Case Registry: HCV, as of February 2018 the VA has started more than 104,000 veterans on direct-acting antiviral (DAA) treatment. When the DAAs first became available, we estimated that there were about 165,000 people who were HCV viremic and who needed to be treated. By the end of January 2018, that number was down to about 35,000 people. The VA has done an unbelievably good job of finding people, getting them into care, and treating them.
Samuel Ho, MD. I agree with Dr. Backus. The VA has done an excellent job over the past few years in treating a very significant proportion of our patients with HCV. In addition to the extensive screening efforts, I want to emphasize that going back to about the year 2000, the VA has been very active in supporting the establishment of HCV clinics within every VA medical center to identify and engage patients in treatment. At that time, of course, the treatment was with pegylated interferon and ribavirin, which was very challenging. The VA support consisted of funding 4 hepatitis C Resource Centers (HCRCs) nationwide, which were located in Minneapolis, Portland/Seattle, New Haven, and San Francisco.
The HCRCs reached out to every VA facility in the country, developed networks of health care providers (HCPs), trained them, and educated them regarding the HCV treatments and strategies to engage patients in care, especially the large numbers with comorbidities, such as psychiatric problems and substance use disorders. This highly engaged network of local HCV clinic providers was set up and running and was well poised to take advantage of the interferon-free DAAs when they became available in late 2013 and early 2014. With the continuing leadership of David Ross, MD, and many others at the national level, the VA then supported the development of HCV Innovation Teams in every VISN that continued the efforts to support local quality improvement initiatives related to HCV care.
That being said, the VA still has challenges. There are a significant number of people who have barriers to receiving treatment. For example, here at the VA San Diego Healthcare System, Dr. John Dever and our other colleagues looked at 481 patients who were high priority to get started on HCV treatment, because they were all believed to be a high risk for cirrhosis due to their Fibrosis-4 (FIB4) scores and other characteristics.1
We really worked hard on that group, and of the ones who were eligible for treatment, 30% were either unwilling or unable to engage in care over a yearlong follow-up with multiple attempts at outreach. In comparison with patients who became engaged or were engaged in care, these nonengaged patients were significantly more likely to be homeless, have other comorbidities, or active alcohol and/or drug use. Not surprisingly, they had obvious barriers to engaging in care.
Further efforts need to be made to focus on these patients, maybe with innovative ideas and strategies for outreach to get them into treatment or to bring treatment to them. I’m not sure exactly as to what the best approach would be. There is ongoing research in that regard, but it still is a challenge.
Erica Trimble, NP. Our experience at VA San Francisco Health Care System is similar. If we actively reach out to veterans already engaged in primary care, we can usually engage them in the liver clinic as well. However, there are quite a number of veterans who engage regularly with HUD-VASH (US Department of House and Urban Development-VA Supportive Housing program) and other homeless veteran services but have no primary or specialty care engagement. These veterans are very difficult to reach.
We are collaborating with HUD-VASH social workers to see if there are more creative ways to connect with these veterans. Some of the ideas include having liver providers visit veteran housing locations, having HUD-VASH social workers convey messages to difficult-to-reach veterans, and problem-solving specific transportation issues that present barriers to care.
Christina Dickson, PharmD. At the VA Maryland Health Care System Baltimore VA Medical Center, we hear from veterans in our education classes about the various myths that are still out there in the community about HCV. Some of these myths are the reason that veterans may avoid seeking treatment or even attending the HCV clinic appointments. Some veterans say they didn’t come in previously because they thought they would need a liver biopsy or because their doctor told them they had to be completely sober in order to be considered for treatment. These can be major deterrents that keep patients away despite our outreach efforts. In addition to miseducation in the community, there also is still a reluctance to talk about HCV and the risk factors. Many patients don’t want to discuss their history or are concerned about their partners finding out, so they instead choose to ignore it altogether. The negative stigma of HCV is still present even in some of our HCPs.
Just as VA San Francisco is working to engage its homeless population, we are looking to work with mental health and substance abuse programs. More and more is being written about the importance of working with such teams and even colocating the HCV clinic with their services. For example, in Baltimore, the methadone clinic is 2 floors above our clinic. Some of the remaining viremic patients will go to the methadone clinic in the morning and then leave despite having an appointment just 2 floors down. Offering HCV services at the same time, in the same area may help to engage veterans to consider their liver health.
Ms. Trimble. VA San Francisco has been fortunate to have the assistance of our opiate replacement clinic staff as well; this is particularly helpful since many veterans visit the opiate replacement clinic daily for medications and know the staff there very well. The staff facilitate communication with the liver clinic, execute warm handoffs to the liver clinic, and provide daily dispensing of hepatitis C medications for a number of veterans who have more difficulty with medication adherence. It has worked very well.
Dr. Ho. I think what you both are pointing out is very important—these patients require teamwork. A multidisciplinary group of HCPs working together in a collaborative, integrated care model has been demonstrated to significantly improve HCV engagement, care, and treatment in these highly comorbid patients.2 Whenever we can work together and build teams and recruit other HCPs in these other clinics, it will really pay off.
Dr. Backus. At VA Palo Alto Health Care System, we also run a program integrated with our 28-day and 90-day residential rehabilitation programs. We realized that those residential treatment programs were a place to reach people who we were having difficulties starting treatment. It was a perfect situation because if you were there for 28 days, we could nearly guarantee that at the very least the patient was going to get 28 days of medications. Particularly now with some of the shorter treatment courses, we only have to get a patient to take another 28 days, which is very doable. Clearly, for the people who are in 90-day programs, the full 8-week or 12-week course of treatment could be completed during the rehabilitation. In addition, we started out at a good place because the programs already screened automatically for HCV on admission to the program, so it was easy to identify people who had HCV.
Ms. Trimble. Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) also can help with outreach. Alexander Monto, MD, and Helen Yee, PharmD, conduct weekly SCAN-ECHO video telehealth conferences with outlying HCPs from other clinics. The outlying HCPs submit cases for hepatitis C treatment consideration; then they take the recommendations from their discussion with Dr. Monto and Dr. Yee but lead the treatment with their patients.
Over time, with this ongoing mentoring, the participating providers have gained a lot of expertise in hepatitis C and serve as a local resource for their clinics. One of the clinics is in Eureka, California, which is nearly 300 miles away. In contrast, the other main clinic that participates is the downtown clinic. It serves the most urban and difficult-to-reach patients. The familiarity and rapport that the downtown clinic providers have with their patients allow them to more effectively engage patients for treatment initiation and follow-up.
Dr. Dickson. Our catchment area includes West Virginia, and we do telehealth for one of the sites, which has a number of 20-year-old and 30-year-old patients. In this slightly different population it is again a challenge getting and keeping them engaged as they go through the pretreatment evaluation. Some say that there may be a benefit to getting them on treatment as quickly as possible so that they don’t have time to disengage. The age difference brings about different barriers. We have to think outside the box on how to reach out to these patients. They work, they have kids, and they don’t feel ill right now. And many are active injection drug users. Trying to get them engaged in health care in general and on HCV treatment is the next big challenge.
Health Care Provider Education
Dr. Dickson. When we reach out to viremic veterans who’ve never been to our clinic, we will sometimes find comments such as, “patient not interested” or “patient still drinking” or no comment at all in the electronic health record primary care notes. So we began to focus our HCV education not only on veterans but also the providers. Some HCPs don’t consider the benefits of referring patients to the clinic for at least the opportunity to receive education on HCV, learning if there is any scarring on their liver, and learning about their options for treatment should they choose to proceed. We are continuing to meet with HCPs in all areas to let them know what’s offered in the HCV clinics. In addition, we have found that direct contact from our HCV clinic to veterans who were not interested is very successful. We get a chance to show that the VA cares and explain what our clinic offers and find that they are more than willing to arrange an appointment with us.
Ms. Trimble. I agree. We have successfully treated many veterans who are still using alcohol or drugs, and the VA supports considering any patient for treatment regardless of substance use; however, not all providers are aware of this. One of the other main education points for patients and providers is that they need not have severe liver disease to be considered for treatment. In the past, typically only patients with moderate to advanced liver fibrosis were considered for treatment, but this approach has changed in the past couple years.
Dr. Ho. I would agree that there still is a need to educate HCPs who may have had a presentation or read something on HCV a year or 2 ago. It’s now possible to treat almost all patients with HCV. It really has been fantastic, but not everyone is aware of it right now. That means we need to continue to be active with our colleagues and get them on the team. It is very helpful to increase enthusiasm if we can publicize new data and information coming out about the success in the VA of these DAA regimens.
Dr. Backus. There was a time when the DAAs first came out and the prices were higher and there was concern about the funding. At that time, we were treating only people with more advanced liver disease. Now we are treating everyone regardless of how advanced their liver disease is, but occasionally at VA Palo Alto I’ve run into providers who say, “The patient didn’t have cirrhosis, so I didn’t refer.” Education still needs to happen. It can be a little confusing because there was a time when we were not treating everyone. Now we are, and we have to make sure to get out this message.
Dr. Dickson. For patients with unstable comorbidities, HCPs may make the choice against HCV treatment. In the Baltimore clinic, we have case managers who will work with such patients and get to know them very well. Many times we do more than just cure their HCV. We also help them with their other conditions because we see them so often, such as helping with their pill boxes and encouraging them since they can see their liver enzymes getting better. There is a lot to be said for case management, the hands-on contact, and the concern that we can show these veterans. It helps not just the HCV but also their blood pressure and cholesterol are now controlled. We hear so many thanks from the veterans that come through our program. It might have taken a lot of work to get them to treatment, but in the end, they’re better overall.
Next Steps in HCV Care
Dr. Backus. The most pressing next step is becoming really creative and integrative about how to reach the more difficult-to-treat patients with comorbidities and reach the less-engaged populations. It is probably going to take some change in the models of care. For example, we are going to have to set up a clinic that is colocated in an opioid replacement therapy clinic or in the rehabilitation program. HCV care is going to have to evolve.
I think there is another issue that Dr. Dickson pointed out. Although it is small and really only occurs in some regions, there is a young population of people with HCV. Some of the models of care that we have used may not work with this population, and we have to recognize that this will be an ongoing issue. Care for these patients will look different. For example, clinics may need to provide child care for this younger population.
Cancer is another important issue. Many of these people have cirrhosis, and even if we cure their HCV, we have to remain cognizant that they still have cirrhosis and potentially need screening for hepatocellular carcinoma. They also may need care for their cirrhosis or counseling about ongoing alcohol use, because even though their HCV was cured, continued alcohol use is not good for their cirrhosis.
Those 3 issues are still in the immediate future of HCV care in the VA. The World Health Organization has a goal for eliminating HCV. One could hope that maybe we could get there; it may be possible through screening, treatment, and prevention strategies. If we are lucky, we could put ourselves out of a job. I don’t see that happening, but it’s a hope.
Ms. Trimble. Are we seeing the same trend in new infections in young injection drug using veterans that are being seen in the nonveteran population nationally?
Dr. Backus. We have looked at this quite closely. The CDC came out with a report recently that showed a substantial increase in HCV cases in people aged 20 to 39 years. At the VA, we have not seen that uptick. The VA rates of new infections or new diagnosis of infections in peopled aged 20 to 39 years are pretty stable. The VA screening rates in people who were born after 1965 is in the high 70% range—nearly as high as in the cohort of people born between 1945 and 1965. As a result, the VA has excellent internal data about the incidence of infections in younger populations. In the VA, we are not seeing this sort of massive increase in incidence in younger populations. Definitely, there are new young injection drug users in the VA who are contracting HCV but not what the CDC is reporting in other parts of the country.4
Ms. Trimble. That’s really interesting.
Dr. Ho. Part of that has been the fact that if you’re a VA patient, you had to have been engaged at some point with the VA with access to its extensive psychiatric mental health and substance use disorder treatment infrastructure. I wonder if the availability of these services is a factor that can be protecting our patients from this recent upsurge in injection drug use.
Dr. Dickson. For our VISN, we do have smaller sites with a number of their remaining viremic veterans in this young cohort who are indeed proving to be a challenge to link to care in the HCV clinics. We continue to brainstorm ideas to determine and overcome their barriers to treatment. The VA is excellent at connecting all of us nationwide, so we look forward to hearing from other sites in a similar situation on how they are overcoming this challenge. Because when you look outside the VA, many are wondering what to do and how to engage these patients.
Dr. Backus. One of the amazing things about HCV treatment is how effective it has been. Traditionally the real-world effectiveness for medications is not nearly as good as the clinical trial efficacy. Clinical trials have extra resources, specially trained doctors and nurses, and tend to recruit engaged and cooperative patients. Often, there has been a stepdown between the clinical efficacy from the trials and what we see in the real world. A pleasant surprise about DAA treatment at the VA is that the clinical effectiveness we see in the real world almost matches the amazing results seen in clinical trials. That also has been critical to the success that we are seeing. The medications are powerful, and even outside the settings of a clinical trial, they work incredibly well.
Dr. Ho. I agree. You, Dr. Backus, along with Pam Belperio, PharmD, George Ioannou MD, MS, and other VA researchers have done excellent work in documenting the real-world effectiveness of these medications in the VA system. It was surprising but not unexpected.5-7 It is due to the VA’s excellent clinical infrastructure and that it provides an integrated system for caring for these patients. It is a measure of that success.
Dr. Dickson. The multidisciplinary teams are a major part of that. I don’t think we could care and support the veterans that we have, especially the challenging ones, the ones who are resistant, without having nursing, social work, mental health, and pharmacy involved. It’s just a huge team effort. That is what I love about caring for patients at the VA—it’s always been supportive of the multidisciplinary aspect of looking at this disease.
Click here to read the digital edition.
1. Dever JB, Ducom JH, Ma A, et al. Engagement in care of high-risk hepatitis C patients with interferon-free direct-acting antiviral therapies. Dig Dis Sci. 2017;62(6):1472-1479.
2. Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. Int J Drug Policy. 2017;47:34-46.
3. Groessl EJ, Liu L, Sklar M, Ho SB. HCV integrated care: a randomized trial to increase treatment initiation and SVR with direct acting antivirals. Int J Hepatol. 2017;2017:5834182.
4. Centers for Disease Control and Prevention. Table 4.1. Reported cases of acute hepatitis C, nationally and by state and jurisdiction—United States, 2011-2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/index.htm#tabs-6-1. Updated June 19, 2017. Accessed March 5, 2018.
5. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Comparative effectiveness of ledipasvir/sofosbuvir ± ribavirin vs. ombitasvir/paritaprevir/ritonavir + dasabuvir ± ribavirin in 6961 genotype 1 patients treated in routine medical practice. Aliment Pharmacol Ther. 2016;44(4):400-410.
6. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64(2):405-414.
7. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.e5.
VA Hepatitis C Treatment Progress
Lisa Backus, MD. For a long time the US Department of Veterans Affairs (VA) has approached hepatitis C virus (HCV) care in a comprehensive way. We have done extensive screening to look for people with HCV infection. Even before birth cohort testing was recommended by the Centers for Disease Control and Prevention (CDC), the VA had aggressive HCV screening programs.
From the VA Corporate Data Warehouse, we know that the VA has screened more than 80% of people who are in the 1945 to 1965 birth cohort in VA care. Over time, HCV prevalence has been dropping in screened veterans and by extension in those who remain to be screened. Based on internal modeling, the VA estimates that only 6,000 to 7,000 veterans in the 1945 to 1965 birth cohort remain to be found if we could somehow screen everyone in that group.
On the treatment side, the VA has provided an unparalleled amount of care. In data from the Clinical Case Registry: HCV, as of February 2018 the VA has started more than 104,000 veterans on direct-acting antiviral (DAA) treatment. When the DAAs first became available, we estimated that there were about 165,000 people who were HCV viremic and who needed to be treated. By the end of January 2018, that number was down to about 35,000 people. The VA has done an unbelievably good job of finding people, getting them into care, and treating them.
Samuel Ho, MD. I agree with Dr. Backus. The VA has done an excellent job over the past few years in treating a very significant proportion of our patients with HCV. In addition to the extensive screening efforts, I want to emphasize that going back to about the year 2000, the VA has been very active in supporting the establishment of HCV clinics within every VA medical center to identify and engage patients in treatment. At that time, of course, the treatment was with pegylated interferon and ribavirin, which was very challenging. The VA support consisted of funding 4 hepatitis C Resource Centers (HCRCs) nationwide, which were located in Minneapolis, Portland/Seattle, New Haven, and San Francisco.
The HCRCs reached out to every VA facility in the country, developed networks of health care providers (HCPs), trained them, and educated them regarding the HCV treatments and strategies to engage patients in care, especially the large numbers with comorbidities, such as psychiatric problems and substance use disorders. This highly engaged network of local HCV clinic providers was set up and running and was well poised to take advantage of the interferon-free DAAs when they became available in late 2013 and early 2014. With the continuing leadership of David Ross, MD, and many others at the national level, the VA then supported the development of HCV Innovation Teams in every VISN that continued the efforts to support local quality improvement initiatives related to HCV care.
That being said, the VA still has challenges. There are a significant number of people who have barriers to receiving treatment. For example, here at the VA San Diego Healthcare System, Dr. John Dever and our other colleagues looked at 481 patients who were high priority to get started on HCV treatment, because they were all believed to be a high risk for cirrhosis due to their Fibrosis-4 (FIB4) scores and other characteristics.1
We really worked hard on that group, and of the ones who were eligible for treatment, 30% were either unwilling or unable to engage in care over a yearlong follow-up with multiple attempts at outreach. In comparison with patients who became engaged or were engaged in care, these nonengaged patients were significantly more likely to be homeless, have other comorbidities, or active alcohol and/or drug use. Not surprisingly, they had obvious barriers to engaging in care.
Further efforts need to be made to focus on these patients, maybe with innovative ideas and strategies for outreach to get them into treatment or to bring treatment to them. I’m not sure exactly as to what the best approach would be. There is ongoing research in that regard, but it still is a challenge.
Erica Trimble, NP. Our experience at VA San Francisco Health Care System is similar. If we actively reach out to veterans already engaged in primary care, we can usually engage them in the liver clinic as well. However, there are quite a number of veterans who engage regularly with HUD-VASH (US Department of House and Urban Development-VA Supportive Housing program) and other homeless veteran services but have no primary or specialty care engagement. These veterans are very difficult to reach.
We are collaborating with HUD-VASH social workers to see if there are more creative ways to connect with these veterans. Some of the ideas include having liver providers visit veteran housing locations, having HUD-VASH social workers convey messages to difficult-to-reach veterans, and problem-solving specific transportation issues that present barriers to care.
Christina Dickson, PharmD. At the VA Maryland Health Care System Baltimore VA Medical Center, we hear from veterans in our education classes about the various myths that are still out there in the community about HCV. Some of these myths are the reason that veterans may avoid seeking treatment or even attending the HCV clinic appointments. Some veterans say they didn’t come in previously because they thought they would need a liver biopsy or because their doctor told them they had to be completely sober in order to be considered for treatment. These can be major deterrents that keep patients away despite our outreach efforts. In addition to miseducation in the community, there also is still a reluctance to talk about HCV and the risk factors. Many patients don’t want to discuss their history or are concerned about their partners finding out, so they instead choose to ignore it altogether. The negative stigma of HCV is still present even in some of our HCPs.
Just as VA San Francisco is working to engage its homeless population, we are looking to work with mental health and substance abuse programs. More and more is being written about the importance of working with such teams and even colocating the HCV clinic with their services. For example, in Baltimore, the methadone clinic is 2 floors above our clinic. Some of the remaining viremic patients will go to the methadone clinic in the morning and then leave despite having an appointment just 2 floors down. Offering HCV services at the same time, in the same area may help to engage veterans to consider their liver health.
Ms. Trimble. VA San Francisco has been fortunate to have the assistance of our opiate replacement clinic staff as well; this is particularly helpful since many veterans visit the opiate replacement clinic daily for medications and know the staff there very well. The staff facilitate communication with the liver clinic, execute warm handoffs to the liver clinic, and provide daily dispensing of hepatitis C medications for a number of veterans who have more difficulty with medication adherence. It has worked very well.
Dr. Ho. I think what you both are pointing out is very important—these patients require teamwork. A multidisciplinary group of HCPs working together in a collaborative, integrated care model has been demonstrated to significantly improve HCV engagement, care, and treatment in these highly comorbid patients.2 Whenever we can work together and build teams and recruit other HCPs in these other clinics, it will really pay off.
Dr. Backus. At VA Palo Alto Health Care System, we also run a program integrated with our 28-day and 90-day residential rehabilitation programs. We realized that those residential treatment programs were a place to reach people who we were having difficulties starting treatment. It was a perfect situation because if you were there for 28 days, we could nearly guarantee that at the very least the patient was going to get 28 days of medications. Particularly now with some of the shorter treatment courses, we only have to get a patient to take another 28 days, which is very doable. Clearly, for the people who are in 90-day programs, the full 8-week or 12-week course of treatment could be completed during the rehabilitation. In addition, we started out at a good place because the programs already screened automatically for HCV on admission to the program, so it was easy to identify people who had HCV.
Ms. Trimble. Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) also can help with outreach. Alexander Monto, MD, and Helen Yee, PharmD, conduct weekly SCAN-ECHO video telehealth conferences with outlying HCPs from other clinics. The outlying HCPs submit cases for hepatitis C treatment consideration; then they take the recommendations from their discussion with Dr. Monto and Dr. Yee but lead the treatment with their patients.
Over time, with this ongoing mentoring, the participating providers have gained a lot of expertise in hepatitis C and serve as a local resource for their clinics. One of the clinics is in Eureka, California, which is nearly 300 miles away. In contrast, the other main clinic that participates is the downtown clinic. It serves the most urban and difficult-to-reach patients. The familiarity and rapport that the downtown clinic providers have with their patients allow them to more effectively engage patients for treatment initiation and follow-up.
Dr. Dickson. Our catchment area includes West Virginia, and we do telehealth for one of the sites, which has a number of 20-year-old and 30-year-old patients. In this slightly different population it is again a challenge getting and keeping them engaged as they go through the pretreatment evaluation. Some say that there may be a benefit to getting them on treatment as quickly as possible so that they don’t have time to disengage. The age difference brings about different barriers. We have to think outside the box on how to reach out to these patients. They work, they have kids, and they don’t feel ill right now. And many are active injection drug users. Trying to get them engaged in health care in general and on HCV treatment is the next big challenge.
Health Care Provider Education
Dr. Dickson. When we reach out to viremic veterans who’ve never been to our clinic, we will sometimes find comments such as, “patient not interested” or “patient still drinking” or no comment at all in the electronic health record primary care notes. So we began to focus our HCV education not only on veterans but also the providers. Some HCPs don’t consider the benefits of referring patients to the clinic for at least the opportunity to receive education on HCV, learning if there is any scarring on their liver, and learning about their options for treatment should they choose to proceed. We are continuing to meet with HCPs in all areas to let them know what’s offered in the HCV clinics. In addition, we have found that direct contact from our HCV clinic to veterans who were not interested is very successful. We get a chance to show that the VA cares and explain what our clinic offers and find that they are more than willing to arrange an appointment with us.
Ms. Trimble. I agree. We have successfully treated many veterans who are still using alcohol or drugs, and the VA supports considering any patient for treatment regardless of substance use; however, not all providers are aware of this. One of the other main education points for patients and providers is that they need not have severe liver disease to be considered for treatment. In the past, typically only patients with moderate to advanced liver fibrosis were considered for treatment, but this approach has changed in the past couple years.
Dr. Ho. I would agree that there still is a need to educate HCPs who may have had a presentation or read something on HCV a year or 2 ago. It’s now possible to treat almost all patients with HCV. It really has been fantastic, but not everyone is aware of it right now. That means we need to continue to be active with our colleagues and get them on the team. It is very helpful to increase enthusiasm if we can publicize new data and information coming out about the success in the VA of these DAA regimens.
Dr. Backus. There was a time when the DAAs first came out and the prices were higher and there was concern about the funding. At that time, we were treating only people with more advanced liver disease. Now we are treating everyone regardless of how advanced their liver disease is, but occasionally at VA Palo Alto I’ve run into providers who say, “The patient didn’t have cirrhosis, so I didn’t refer.” Education still needs to happen. It can be a little confusing because there was a time when we were not treating everyone. Now we are, and we have to make sure to get out this message.
Dr. Dickson. For patients with unstable comorbidities, HCPs may make the choice against HCV treatment. In the Baltimore clinic, we have case managers who will work with such patients and get to know them very well. Many times we do more than just cure their HCV. We also help them with their other conditions because we see them so often, such as helping with their pill boxes and encouraging them since they can see their liver enzymes getting better. There is a lot to be said for case management, the hands-on contact, and the concern that we can show these veterans. It helps not just the HCV but also their blood pressure and cholesterol are now controlled. We hear so many thanks from the veterans that come through our program. It might have taken a lot of work to get them to treatment, but in the end, they’re better overall.
Next Steps in HCV Care
Dr. Backus. The most pressing next step is becoming really creative and integrative about how to reach the more difficult-to-treat patients with comorbidities and reach the less-engaged populations. It is probably going to take some change in the models of care. For example, we are going to have to set up a clinic that is colocated in an opioid replacement therapy clinic or in the rehabilitation program. HCV care is going to have to evolve.
I think there is another issue that Dr. Dickson pointed out. Although it is small and really only occurs in some regions, there is a young population of people with HCV. Some of the models of care that we have used may not work with this population, and we have to recognize that this will be an ongoing issue. Care for these patients will look different. For example, clinics may need to provide child care for this younger population.
Cancer is another important issue. Many of these people have cirrhosis, and even if we cure their HCV, we have to remain cognizant that they still have cirrhosis and potentially need screening for hepatocellular carcinoma. They also may need care for their cirrhosis or counseling about ongoing alcohol use, because even though their HCV was cured, continued alcohol use is not good for their cirrhosis.
Those 3 issues are still in the immediate future of HCV care in the VA. The World Health Organization has a goal for eliminating HCV. One could hope that maybe we could get there; it may be possible through screening, treatment, and prevention strategies. If we are lucky, we could put ourselves out of a job. I don’t see that happening, but it’s a hope.
Ms. Trimble. Are we seeing the same trend in new infections in young injection drug using veterans that are being seen in the nonveteran population nationally?
Dr. Backus. We have looked at this quite closely. The CDC came out with a report recently that showed a substantial increase in HCV cases in people aged 20 to 39 years. At the VA, we have not seen that uptick. The VA rates of new infections or new diagnosis of infections in peopled aged 20 to 39 years are pretty stable. The VA screening rates in people who were born after 1965 is in the high 70% range—nearly as high as in the cohort of people born between 1945 and 1965. As a result, the VA has excellent internal data about the incidence of infections in younger populations. In the VA, we are not seeing this sort of massive increase in incidence in younger populations. Definitely, there are new young injection drug users in the VA who are contracting HCV but not what the CDC is reporting in other parts of the country.4
Ms. Trimble. That’s really interesting.
Dr. Ho. Part of that has been the fact that if you’re a VA patient, you had to have been engaged at some point with the VA with access to its extensive psychiatric mental health and substance use disorder treatment infrastructure. I wonder if the availability of these services is a factor that can be protecting our patients from this recent upsurge in injection drug use.
Dr. Dickson. For our VISN, we do have smaller sites with a number of their remaining viremic veterans in this young cohort who are indeed proving to be a challenge to link to care in the HCV clinics. We continue to brainstorm ideas to determine and overcome their barriers to treatment. The VA is excellent at connecting all of us nationwide, so we look forward to hearing from other sites in a similar situation on how they are overcoming this challenge. Because when you look outside the VA, many are wondering what to do and how to engage these patients.
Dr. Backus. One of the amazing things about HCV treatment is how effective it has been. Traditionally the real-world effectiveness for medications is not nearly as good as the clinical trial efficacy. Clinical trials have extra resources, specially trained doctors and nurses, and tend to recruit engaged and cooperative patients. Often, there has been a stepdown between the clinical efficacy from the trials and what we see in the real world. A pleasant surprise about DAA treatment at the VA is that the clinical effectiveness we see in the real world almost matches the amazing results seen in clinical trials. That also has been critical to the success that we are seeing. The medications are powerful, and even outside the settings of a clinical trial, they work incredibly well.
Dr. Ho. I agree. You, Dr. Backus, along with Pam Belperio, PharmD, George Ioannou MD, MS, and other VA researchers have done excellent work in documenting the real-world effectiveness of these medications in the VA system. It was surprising but not unexpected.5-7 It is due to the VA’s excellent clinical infrastructure and that it provides an integrated system for caring for these patients. It is a measure of that success.
Dr. Dickson. The multidisciplinary teams are a major part of that. I don’t think we could care and support the veterans that we have, especially the challenging ones, the ones who are resistant, without having nursing, social work, mental health, and pharmacy involved. It’s just a huge team effort. That is what I love about caring for patients at the VA—it’s always been supportive of the multidisciplinary aspect of looking at this disease.
Click here to read the digital edition.
VA Hepatitis C Treatment Progress
Lisa Backus, MD. For a long time the US Department of Veterans Affairs (VA) has approached hepatitis C virus (HCV) care in a comprehensive way. We have done extensive screening to look for people with HCV infection. Even before birth cohort testing was recommended by the Centers for Disease Control and Prevention (CDC), the VA had aggressive HCV screening programs.
From the VA Corporate Data Warehouse, we know that the VA has screened more than 80% of people who are in the 1945 to 1965 birth cohort in VA care. Over time, HCV prevalence has been dropping in screened veterans and by extension in those who remain to be screened. Based on internal modeling, the VA estimates that only 6,000 to 7,000 veterans in the 1945 to 1965 birth cohort remain to be found if we could somehow screen everyone in that group.
On the treatment side, the VA has provided an unparalleled amount of care. In data from the Clinical Case Registry: HCV, as of February 2018 the VA has started more than 104,000 veterans on direct-acting antiviral (DAA) treatment. When the DAAs first became available, we estimated that there were about 165,000 people who were HCV viremic and who needed to be treated. By the end of January 2018, that number was down to about 35,000 people. The VA has done an unbelievably good job of finding people, getting them into care, and treating them.
Samuel Ho, MD. I agree with Dr. Backus. The VA has done an excellent job over the past few years in treating a very significant proportion of our patients with HCV. In addition to the extensive screening efforts, I want to emphasize that going back to about the year 2000, the VA has been very active in supporting the establishment of HCV clinics within every VA medical center to identify and engage patients in treatment. At that time, of course, the treatment was with pegylated interferon and ribavirin, which was very challenging. The VA support consisted of funding 4 hepatitis C Resource Centers (HCRCs) nationwide, which were located in Minneapolis, Portland/Seattle, New Haven, and San Francisco.
The HCRCs reached out to every VA facility in the country, developed networks of health care providers (HCPs), trained them, and educated them regarding the HCV treatments and strategies to engage patients in care, especially the large numbers with comorbidities, such as psychiatric problems and substance use disorders. This highly engaged network of local HCV clinic providers was set up and running and was well poised to take advantage of the interferon-free DAAs when they became available in late 2013 and early 2014. With the continuing leadership of David Ross, MD, and many others at the national level, the VA then supported the development of HCV Innovation Teams in every VISN that continued the efforts to support local quality improvement initiatives related to HCV care.
That being said, the VA still has challenges. There are a significant number of people who have barriers to receiving treatment. For example, here at the VA San Diego Healthcare System, Dr. John Dever and our other colleagues looked at 481 patients who were high priority to get started on HCV treatment, because they were all believed to be a high risk for cirrhosis due to their Fibrosis-4 (FIB4) scores and other characteristics.1
We really worked hard on that group, and of the ones who were eligible for treatment, 30% were either unwilling or unable to engage in care over a yearlong follow-up with multiple attempts at outreach. In comparison with patients who became engaged or were engaged in care, these nonengaged patients were significantly more likely to be homeless, have other comorbidities, or active alcohol and/or drug use. Not surprisingly, they had obvious barriers to engaging in care.
Further efforts need to be made to focus on these patients, maybe with innovative ideas and strategies for outreach to get them into treatment or to bring treatment to them. I’m not sure exactly as to what the best approach would be. There is ongoing research in that regard, but it still is a challenge.
Erica Trimble, NP. Our experience at VA San Francisco Health Care System is similar. If we actively reach out to veterans already engaged in primary care, we can usually engage them in the liver clinic as well. However, there are quite a number of veterans who engage regularly with HUD-VASH (US Department of House and Urban Development-VA Supportive Housing program) and other homeless veteran services but have no primary or specialty care engagement. These veterans are very difficult to reach.
We are collaborating with HUD-VASH social workers to see if there are more creative ways to connect with these veterans. Some of the ideas include having liver providers visit veteran housing locations, having HUD-VASH social workers convey messages to difficult-to-reach veterans, and problem-solving specific transportation issues that present barriers to care.
Christina Dickson, PharmD. At the VA Maryland Health Care System Baltimore VA Medical Center, we hear from veterans in our education classes about the various myths that are still out there in the community about HCV. Some of these myths are the reason that veterans may avoid seeking treatment or even attending the HCV clinic appointments. Some veterans say they didn’t come in previously because they thought they would need a liver biopsy or because their doctor told them they had to be completely sober in order to be considered for treatment. These can be major deterrents that keep patients away despite our outreach efforts. In addition to miseducation in the community, there also is still a reluctance to talk about HCV and the risk factors. Many patients don’t want to discuss their history or are concerned about their partners finding out, so they instead choose to ignore it altogether. The negative stigma of HCV is still present even in some of our HCPs.
Just as VA San Francisco is working to engage its homeless population, we are looking to work with mental health and substance abuse programs. More and more is being written about the importance of working with such teams and even colocating the HCV clinic with their services. For example, in Baltimore, the methadone clinic is 2 floors above our clinic. Some of the remaining viremic patients will go to the methadone clinic in the morning and then leave despite having an appointment just 2 floors down. Offering HCV services at the same time, in the same area may help to engage veterans to consider their liver health.
Ms. Trimble. VA San Francisco has been fortunate to have the assistance of our opiate replacement clinic staff as well; this is particularly helpful since many veterans visit the opiate replacement clinic daily for medications and know the staff there very well. The staff facilitate communication with the liver clinic, execute warm handoffs to the liver clinic, and provide daily dispensing of hepatitis C medications for a number of veterans who have more difficulty with medication adherence. It has worked very well.
Dr. Ho. I think what you both are pointing out is very important—these patients require teamwork. A multidisciplinary group of HCPs working together in a collaborative, integrated care model has been demonstrated to significantly improve HCV engagement, care, and treatment in these highly comorbid patients.2 Whenever we can work together and build teams and recruit other HCPs in these other clinics, it will really pay off.
Dr. Backus. At VA Palo Alto Health Care System, we also run a program integrated with our 28-day and 90-day residential rehabilitation programs. We realized that those residential treatment programs were a place to reach people who we were having difficulties starting treatment. It was a perfect situation because if you were there for 28 days, we could nearly guarantee that at the very least the patient was going to get 28 days of medications. Particularly now with some of the shorter treatment courses, we only have to get a patient to take another 28 days, which is very doable. Clearly, for the people who are in 90-day programs, the full 8-week or 12-week course of treatment could be completed during the rehabilitation. In addition, we started out at a good place because the programs already screened automatically for HCV on admission to the program, so it was easy to identify people who had HCV.
Ms. Trimble. Specialty Care Access Network-Extension for Community Healthcare Outcomes (SCAN-ECHO) also can help with outreach. Alexander Monto, MD, and Helen Yee, PharmD, conduct weekly SCAN-ECHO video telehealth conferences with outlying HCPs from other clinics. The outlying HCPs submit cases for hepatitis C treatment consideration; then they take the recommendations from their discussion with Dr. Monto and Dr. Yee but lead the treatment with their patients.
Over time, with this ongoing mentoring, the participating providers have gained a lot of expertise in hepatitis C and serve as a local resource for their clinics. One of the clinics is in Eureka, California, which is nearly 300 miles away. In contrast, the other main clinic that participates is the downtown clinic. It serves the most urban and difficult-to-reach patients. The familiarity and rapport that the downtown clinic providers have with their patients allow them to more effectively engage patients for treatment initiation and follow-up.
Dr. Dickson. Our catchment area includes West Virginia, and we do telehealth for one of the sites, which has a number of 20-year-old and 30-year-old patients. In this slightly different population it is again a challenge getting and keeping them engaged as they go through the pretreatment evaluation. Some say that there may be a benefit to getting them on treatment as quickly as possible so that they don’t have time to disengage. The age difference brings about different barriers. We have to think outside the box on how to reach out to these patients. They work, they have kids, and they don’t feel ill right now. And many are active injection drug users. Trying to get them engaged in health care in general and on HCV treatment is the next big challenge.
Health Care Provider Education
Dr. Dickson. When we reach out to viremic veterans who’ve never been to our clinic, we will sometimes find comments such as, “patient not interested” or “patient still drinking” or no comment at all in the electronic health record primary care notes. So we began to focus our HCV education not only on veterans but also the providers. Some HCPs don’t consider the benefits of referring patients to the clinic for at least the opportunity to receive education on HCV, learning if there is any scarring on their liver, and learning about their options for treatment should they choose to proceed. We are continuing to meet with HCPs in all areas to let them know what’s offered in the HCV clinics. In addition, we have found that direct contact from our HCV clinic to veterans who were not interested is very successful. We get a chance to show that the VA cares and explain what our clinic offers and find that they are more than willing to arrange an appointment with us.
Ms. Trimble. I agree. We have successfully treated many veterans who are still using alcohol or drugs, and the VA supports considering any patient for treatment regardless of substance use; however, not all providers are aware of this. One of the other main education points for patients and providers is that they need not have severe liver disease to be considered for treatment. In the past, typically only patients with moderate to advanced liver fibrosis were considered for treatment, but this approach has changed in the past couple years.
Dr. Ho. I would agree that there still is a need to educate HCPs who may have had a presentation or read something on HCV a year or 2 ago. It’s now possible to treat almost all patients with HCV. It really has been fantastic, but not everyone is aware of it right now. That means we need to continue to be active with our colleagues and get them on the team. It is very helpful to increase enthusiasm if we can publicize new data and information coming out about the success in the VA of these DAA regimens.
Dr. Backus. There was a time when the DAAs first came out and the prices were higher and there was concern about the funding. At that time, we were treating only people with more advanced liver disease. Now we are treating everyone regardless of how advanced their liver disease is, but occasionally at VA Palo Alto I’ve run into providers who say, “The patient didn’t have cirrhosis, so I didn’t refer.” Education still needs to happen. It can be a little confusing because there was a time when we were not treating everyone. Now we are, and we have to make sure to get out this message.
Dr. Dickson. For patients with unstable comorbidities, HCPs may make the choice against HCV treatment. In the Baltimore clinic, we have case managers who will work with such patients and get to know them very well. Many times we do more than just cure their HCV. We also help them with their other conditions because we see them so often, such as helping with their pill boxes and encouraging them since they can see their liver enzymes getting better. There is a lot to be said for case management, the hands-on contact, and the concern that we can show these veterans. It helps not just the HCV but also their blood pressure and cholesterol are now controlled. We hear so many thanks from the veterans that come through our program. It might have taken a lot of work to get them to treatment, but in the end, they’re better overall.
Next Steps in HCV Care
Dr. Backus. The most pressing next step is becoming really creative and integrative about how to reach the more difficult-to-treat patients with comorbidities and reach the less-engaged populations. It is probably going to take some change in the models of care. For example, we are going to have to set up a clinic that is colocated in an opioid replacement therapy clinic or in the rehabilitation program. HCV care is going to have to evolve.
I think there is another issue that Dr. Dickson pointed out. Although it is small and really only occurs in some regions, there is a young population of people with HCV. Some of the models of care that we have used may not work with this population, and we have to recognize that this will be an ongoing issue. Care for these patients will look different. For example, clinics may need to provide child care for this younger population.
Cancer is another important issue. Many of these people have cirrhosis, and even if we cure their HCV, we have to remain cognizant that they still have cirrhosis and potentially need screening for hepatocellular carcinoma. They also may need care for their cirrhosis or counseling about ongoing alcohol use, because even though their HCV was cured, continued alcohol use is not good for their cirrhosis.
Those 3 issues are still in the immediate future of HCV care in the VA. The World Health Organization has a goal for eliminating HCV. One could hope that maybe we could get there; it may be possible through screening, treatment, and prevention strategies. If we are lucky, we could put ourselves out of a job. I don’t see that happening, but it’s a hope.
Ms. Trimble. Are we seeing the same trend in new infections in young injection drug using veterans that are being seen in the nonveteran population nationally?
Dr. Backus. We have looked at this quite closely. The CDC came out with a report recently that showed a substantial increase in HCV cases in people aged 20 to 39 years. At the VA, we have not seen that uptick. The VA rates of new infections or new diagnosis of infections in peopled aged 20 to 39 years are pretty stable. The VA screening rates in people who were born after 1965 is in the high 70% range—nearly as high as in the cohort of people born between 1945 and 1965. As a result, the VA has excellent internal data about the incidence of infections in younger populations. In the VA, we are not seeing this sort of massive increase in incidence in younger populations. Definitely, there are new young injection drug users in the VA who are contracting HCV but not what the CDC is reporting in other parts of the country.4
Ms. Trimble. That’s really interesting.
Dr. Ho. Part of that has been the fact that if you’re a VA patient, you had to have been engaged at some point with the VA with access to its extensive psychiatric mental health and substance use disorder treatment infrastructure. I wonder if the availability of these services is a factor that can be protecting our patients from this recent upsurge in injection drug use.
Dr. Dickson. For our VISN, we do have smaller sites with a number of their remaining viremic veterans in this young cohort who are indeed proving to be a challenge to link to care in the HCV clinics. We continue to brainstorm ideas to determine and overcome their barriers to treatment. The VA is excellent at connecting all of us nationwide, so we look forward to hearing from other sites in a similar situation on how they are overcoming this challenge. Because when you look outside the VA, many are wondering what to do and how to engage these patients.
Dr. Backus. One of the amazing things about HCV treatment is how effective it has been. Traditionally the real-world effectiveness for medications is not nearly as good as the clinical trial efficacy. Clinical trials have extra resources, specially trained doctors and nurses, and tend to recruit engaged and cooperative patients. Often, there has been a stepdown between the clinical efficacy from the trials and what we see in the real world. A pleasant surprise about DAA treatment at the VA is that the clinical effectiveness we see in the real world almost matches the amazing results seen in clinical trials. That also has been critical to the success that we are seeing. The medications are powerful, and even outside the settings of a clinical trial, they work incredibly well.
Dr. Ho. I agree. You, Dr. Backus, along with Pam Belperio, PharmD, George Ioannou MD, MS, and other VA researchers have done excellent work in documenting the real-world effectiveness of these medications in the VA system. It was surprising but not unexpected.5-7 It is due to the VA’s excellent clinical infrastructure and that it provides an integrated system for caring for these patients. It is a measure of that success.
Dr. Dickson. The multidisciplinary teams are a major part of that. I don’t think we could care and support the veterans that we have, especially the challenging ones, the ones who are resistant, without having nursing, social work, mental health, and pharmacy involved. It’s just a huge team effort. That is what I love about caring for patients at the VA—it’s always been supportive of the multidisciplinary aspect of looking at this disease.
Click here to read the digital edition.
1. Dever JB, Ducom JH, Ma A, et al. Engagement in care of high-risk hepatitis C patients with interferon-free direct-acting antiviral therapies. Dig Dis Sci. 2017;62(6):1472-1479.
2. Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. Int J Drug Policy. 2017;47:34-46.
3. Groessl EJ, Liu L, Sklar M, Ho SB. HCV integrated care: a randomized trial to increase treatment initiation and SVR with direct acting antivirals. Int J Hepatol. 2017;2017:5834182.
4. Centers for Disease Control and Prevention. Table 4.1. Reported cases of acute hepatitis C, nationally and by state and jurisdiction—United States, 2011-2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/index.htm#tabs-6-1. Updated June 19, 2017. Accessed March 5, 2018.
5. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Comparative effectiveness of ledipasvir/sofosbuvir ± ribavirin vs. ombitasvir/paritaprevir/ritonavir + dasabuvir ± ribavirin in 6961 genotype 1 patients treated in routine medical practice. Aliment Pharmacol Ther. 2016;44(4):400-410.
6. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64(2):405-414.
7. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.e5.
1. Dever JB, Ducom JH, Ma A, et al. Engagement in care of high-risk hepatitis C patients with interferon-free direct-acting antiviral therapies. Dig Dis Sci. 2017;62(6):1472-1479.
2. Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: A systematic review. Int J Drug Policy. 2017;47:34-46.
3. Groessl EJ, Liu L, Sklar M, Ho SB. HCV integrated care: a randomized trial to increase treatment initiation and SVR with direct acting antivirals. Int J Hepatol. 2017;2017:5834182.
4. Centers for Disease Control and Prevention. Table 4.1. Reported cases of acute hepatitis C, nationally and by state and jurisdiction—United States, 2011-2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/index.htm#tabs-6-1. Updated June 19, 2017. Accessed March 5, 2018.
5. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Comparative effectiveness of ledipasvir/sofosbuvir ± ribavirin vs. ombitasvir/paritaprevir/ritonavir + dasabuvir ± ribavirin in 6961 genotype 1 patients treated in routine medical practice. Aliment Pharmacol Ther. 2016;44(4):400-410.
6. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64(2):405-414.
7. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.e5.
HCC with no cirrhosis is more common in HIV patients
SEATTLE – Hepatocellular carcinoma (HCC) is on the rise in HIV-positive individuals, its incidence having quadrupled since 1996, and HIV-positive individuals have about a 300% increase risk of HCC, compared with the general population. However, more than 40% of patients with HIV who develop HCC have a Fibrosis-4 score (FIB-4) suggesting a lack of cirrhosis, according to a new retrospective analysis. By contrast, only about 13% of typical HCC patients have no cirrhosis.
The study also revealed some of the risk factors associated with HCC in this population, including longer duration of HIV viremia and lower CD4 cell counts, as well as markers of metabolic syndrome. “There was some signal that perhaps other markers of metabolic syndrome, obesity and diabetes, were more prevalent in those [who developed HCC] without advanced fibrosis or cirrhosis, suggesting that there may be other underlying etiologies of liver disease that we should be wary of when evaluating somebody for their risk of HCC,” Jessie Torgersen, MD, said in an interview.
Dr. Torgersen is an instructor of medicine at the University of Pennsylvania, Philadelphia. She presented the study at the Conference on Retroviruses & Opportunistic Infections.
The results of the study are tantalizing, but not yet practice changing. “I don’t think we have enough information from this study to recommend a dramatic overhaul of the current HCC screening guidelines, but with the anticipated elimination of hepatitis C, I think the emergence of [metabolic factors and their] contributions to our HIV-positive population’s risk of HCC needs to be better understood. Hopefully this will serve as a first step in further understanding those risks,” Dr. Torgersen said.
She also hopes to get a better handle on the biological mechanisms that might drive HCC in the absence of cirrhosis. “While the mechanisms are unclear as to why HCC would develop in HIV-positive patients without cirrhosis, there are a lot of biologically plausible mechanisms that seem to make [sense],” said Dr. Torgersen. The team hopes to get a better understanding of those mechanisms in order to information evaluation and screening for HCC.
The researchers analyzed data from the Veterans Affairs Cancer Registry as well as EMRs for HIV-positive veterans across the United States. The study included 2,497 participants with a FIB-4 score greater than 3.25, and 29,836 with an FIB-4 score less than or equal to 3.25. At baseline, subjects with FIB-4 greater than 3.25 were more likely to have an alcohol-related diagnosis (47% vs. 29%), be positive for hepatitis C virus RNA (59% vs. 30%), be positive for the hepatitis B surface antigen (10% versus 5%), have HIV RNA greater than or equal to 500 copies/mL (63% vs. 56%), and to have a CD4+ cell count less than 200 cells/m3 (39% vs. 26%).
A total of 278 subjects were diagnosed with HCC; 43% had an FIB-4 less than or equal to 3.25. Among those 43%, more patients had a body mass index of 30 or higher (16% vs. 12%), had diabetes (31% vs. 25%), and tested positive for the hepatitis B surface antigen (26% vs. 17%).
Among subjects with FIB-4 less than or equal to 3.25, factors associated with greater HCC risk included higher HIV RNA level (hazard ratio, 1.24 per 1.0 log10 copies/mL), CD4+ cell count less than 200 cells/m3 (HR, 1.78), hepatitis C virus infection (HR, 6.32), and positive hepatitis B surface antigen (HR, 4.93).
Among subjects with FIB-4 greater than 3.25, increased HCC risk was associated with HCV infection (HR, 6.18) and positive hepatitis B surface antigen (HR, 2.12).
The study was funded by the National Institutes of Health. Dr. Torgersen reported no financial disclosures.
SOURCE: Torgersen J et al. CROI 2019, Abstract 90.
SEATTLE – Hepatocellular carcinoma (HCC) is on the rise in HIV-positive individuals, its incidence having quadrupled since 1996, and HIV-positive individuals have about a 300% increase risk of HCC, compared with the general population. However, more than 40% of patients with HIV who develop HCC have a Fibrosis-4 score (FIB-4) suggesting a lack of cirrhosis, according to a new retrospective analysis. By contrast, only about 13% of typical HCC patients have no cirrhosis.
The study also revealed some of the risk factors associated with HCC in this population, including longer duration of HIV viremia and lower CD4 cell counts, as well as markers of metabolic syndrome. “There was some signal that perhaps other markers of metabolic syndrome, obesity and diabetes, were more prevalent in those [who developed HCC] without advanced fibrosis or cirrhosis, suggesting that there may be other underlying etiologies of liver disease that we should be wary of when evaluating somebody for their risk of HCC,” Jessie Torgersen, MD, said in an interview.
Dr. Torgersen is an instructor of medicine at the University of Pennsylvania, Philadelphia. She presented the study at the Conference on Retroviruses & Opportunistic Infections.
The results of the study are tantalizing, but not yet practice changing. “I don’t think we have enough information from this study to recommend a dramatic overhaul of the current HCC screening guidelines, but with the anticipated elimination of hepatitis C, I think the emergence of [metabolic factors and their] contributions to our HIV-positive population’s risk of HCC needs to be better understood. Hopefully this will serve as a first step in further understanding those risks,” Dr. Torgersen said.
She also hopes to get a better handle on the biological mechanisms that might drive HCC in the absence of cirrhosis. “While the mechanisms are unclear as to why HCC would develop in HIV-positive patients without cirrhosis, there are a lot of biologically plausible mechanisms that seem to make [sense],” said Dr. Torgersen. The team hopes to get a better understanding of those mechanisms in order to information evaluation and screening for HCC.
The researchers analyzed data from the Veterans Affairs Cancer Registry as well as EMRs for HIV-positive veterans across the United States. The study included 2,497 participants with a FIB-4 score greater than 3.25, and 29,836 with an FIB-4 score less than or equal to 3.25. At baseline, subjects with FIB-4 greater than 3.25 were more likely to have an alcohol-related diagnosis (47% vs. 29%), be positive for hepatitis C virus RNA (59% vs. 30%), be positive for the hepatitis B surface antigen (10% versus 5%), have HIV RNA greater than or equal to 500 copies/mL (63% vs. 56%), and to have a CD4+ cell count less than 200 cells/m3 (39% vs. 26%).
A total of 278 subjects were diagnosed with HCC; 43% had an FIB-4 less than or equal to 3.25. Among those 43%, more patients had a body mass index of 30 or higher (16% vs. 12%), had diabetes (31% vs. 25%), and tested positive for the hepatitis B surface antigen (26% vs. 17%).
Among subjects with FIB-4 less than or equal to 3.25, factors associated with greater HCC risk included higher HIV RNA level (hazard ratio, 1.24 per 1.0 log10 copies/mL), CD4+ cell count less than 200 cells/m3 (HR, 1.78), hepatitis C virus infection (HR, 6.32), and positive hepatitis B surface antigen (HR, 4.93).
Among subjects with FIB-4 greater than 3.25, increased HCC risk was associated with HCV infection (HR, 6.18) and positive hepatitis B surface antigen (HR, 2.12).
The study was funded by the National Institutes of Health. Dr. Torgersen reported no financial disclosures.
SOURCE: Torgersen J et al. CROI 2019, Abstract 90.
SEATTLE – Hepatocellular carcinoma (HCC) is on the rise in HIV-positive individuals, its incidence having quadrupled since 1996, and HIV-positive individuals have about a 300% increase risk of HCC, compared with the general population. However, more than 40% of patients with HIV who develop HCC have a Fibrosis-4 score (FIB-4) suggesting a lack of cirrhosis, according to a new retrospective analysis. By contrast, only about 13% of typical HCC patients have no cirrhosis.
The study also revealed some of the risk factors associated with HCC in this population, including longer duration of HIV viremia and lower CD4 cell counts, as well as markers of metabolic syndrome. “There was some signal that perhaps other markers of metabolic syndrome, obesity and diabetes, were more prevalent in those [who developed HCC] without advanced fibrosis or cirrhosis, suggesting that there may be other underlying etiologies of liver disease that we should be wary of when evaluating somebody for their risk of HCC,” Jessie Torgersen, MD, said in an interview.
Dr. Torgersen is an instructor of medicine at the University of Pennsylvania, Philadelphia. She presented the study at the Conference on Retroviruses & Opportunistic Infections.
The results of the study are tantalizing, but not yet practice changing. “I don’t think we have enough information from this study to recommend a dramatic overhaul of the current HCC screening guidelines, but with the anticipated elimination of hepatitis C, I think the emergence of [metabolic factors and their] contributions to our HIV-positive population’s risk of HCC needs to be better understood. Hopefully this will serve as a first step in further understanding those risks,” Dr. Torgersen said.
She also hopes to get a better handle on the biological mechanisms that might drive HCC in the absence of cirrhosis. “While the mechanisms are unclear as to why HCC would develop in HIV-positive patients without cirrhosis, there are a lot of biologically plausible mechanisms that seem to make [sense],” said Dr. Torgersen. The team hopes to get a better understanding of those mechanisms in order to information evaluation and screening for HCC.
The researchers analyzed data from the Veterans Affairs Cancer Registry as well as EMRs for HIV-positive veterans across the United States. The study included 2,497 participants with a FIB-4 score greater than 3.25, and 29,836 with an FIB-4 score less than or equal to 3.25. At baseline, subjects with FIB-4 greater than 3.25 were more likely to have an alcohol-related diagnosis (47% vs. 29%), be positive for hepatitis C virus RNA (59% vs. 30%), be positive for the hepatitis B surface antigen (10% versus 5%), have HIV RNA greater than or equal to 500 copies/mL (63% vs. 56%), and to have a CD4+ cell count less than 200 cells/m3 (39% vs. 26%).
A total of 278 subjects were diagnosed with HCC; 43% had an FIB-4 less than or equal to 3.25. Among those 43%, more patients had a body mass index of 30 or higher (16% vs. 12%), had diabetes (31% vs. 25%), and tested positive for the hepatitis B surface antigen (26% vs. 17%).
Among subjects with FIB-4 less than or equal to 3.25, factors associated with greater HCC risk included higher HIV RNA level (hazard ratio, 1.24 per 1.0 log10 copies/mL), CD4+ cell count less than 200 cells/m3 (HR, 1.78), hepatitis C virus infection (HR, 6.32), and positive hepatitis B surface antigen (HR, 4.93).
Among subjects with FIB-4 greater than 3.25, increased HCC risk was associated with HCV infection (HR, 6.18) and positive hepatitis B surface antigen (HR, 2.12).
The study was funded by the National Institutes of Health. Dr. Torgersen reported no financial disclosures.
SOURCE: Torgersen J et al. CROI 2019, Abstract 90.
REPORTING FROM CROI 2019
Hepatitis A Virus Prevention and Vaccination Within and Outside the VHA in Light of Recent Outbreaks (FULL)
Hepatitis A virus (HAV) can result in acute infection characterized by fatigue, nausea, jaundice (yellowing of the skin) and, rarely, acute liver failure and death.1,2 In the US, HAV yearly incidence (per 100,000) has decreased from 11.7 cases in 1996 to 0.4 cases in 2015, largely due to the 2006 recommendations from the Centers for Disease Control and Prevention (CDC) that all infants receive HAV vaccination.3,4
In 2017, multiple HAV outbreaks occurred in Arizona, California, Colorado, Kentucky, Michigan, and Utah with infections concentrated among those who were homeless, used illicit drugs (both injection and noninjection), or had close contact with these groups (Table 1).5-7
In response, the CDC has recommended the administration of HAV vaccine or immune globulin (IG) as postexposure prophylaxis (PEP) to people in high-risk groups including unvaccinated individuals exposed to HAV within the prior 2 weeks.5 While the Veterans Health Administration (VHA) in the Department of Veteran’s Affairs (VA) has not noted a significant increase in the number of reported HAV infections, there have been cases of hospitalization within the VA health care system due to HAV in at least 2 of the outbreak areas. The VA facilities in outbreak areas are responding by supporting county disease-control measures that include ensuring handwashing stations and vaccinations for high-risk, in-care populations and employees in direct contact with patients at high risk for HAV.
This review provides information on HAV transmission and clinical manifestations, guidelines on the prevention of HAV infection, and baseline data on current HAV susceptibility and immunization rates in the VHA.
Transmission and Clinical Manifestations
Hepatitis A virus is primarily transmitted by ingestion of small amounts of infected stool (ie, fecal-oral route) via direct person-to-person contact or through exposure to contaminated food or water.9,10 Groups at high risk of HAV infection include those in direct contact with HAV-infected individuals, users of injection or non-injection drugs, men who have sex with men (MSM), travelers to high-risk countries, individuals with clotting disorders, and those who work with nonhuman primates.11 Individuals who are homeless are susceptible to HAV due to poor sanitary conditions, and MSM are at increased risk of HAV acquisition via exposure to infected stool during sexual activity.
Complications of acute HAV infection, including fulminant liver failure and death, are more common among patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV).12,13 While infection with HIV does not independently increase the risk of HAV acquisition, about 75% of new HIV infections in the US are among MSM or IV drug users who are at increased risk of HAV infection.14 In addition, duration of HAV viremia and resulting HAV transmissibility may be increased in HIV-infected individuals.15-17
After infection, HAV remains asymptomatic (the incubation period) for an average of 28 days with a range of 15 to 50 days.18,19 Most children younger than 6 years remain asymptomatic while older children and adults typically experience symptoms including fever, fatigue, poor appetite, abdominal pain, dark urine, clay-colored stools, and jaundice.2,20,21 Symptoms typically last less than 2 months but can persist or relapse for up to 6 months in 10% to 15% of symptomatic individuals.22,23 Those with HAV infection are capable of viral transmission from the beginning of the incubation period until about a week after jaundice appears.24 Unlike HBV and HCV, HAV does not cause chronic infection.
Fulminant liver failure, characterized by encephalopathy, jaundice, and elevated international normalized ratio (INR), occurs in < 1% of HAV infections and is more common in those with underlying liver disease and older individuals.13,25-27 In one retrospective review of fulminant liver failure from HAV infection, about half of the patients required liver transplantation or died within 3 weeks of presentation.12
Other than supportive care, there are no specific treatments for acute HAV infection. However, the CDC recommends that healthy individuals aged between 1 and 40 years with known or suspected exposure to HAV within the prior 2 weeks receive 1 dose of a single-antigen HAV vaccination. The CDC also recommends that recently exposed individuals aged < 1 year or > 40 years, or patients who are immunocompromised, have chronic liver disease (CLD), or are allergic to HAV vaccine or a vaccine component should receive a single IG injection. In addition, the CDC recommends that health care providers report all cases of acute HAV to state and local health departments.28
In patients with typical symptoms of acute viral hepatitis (eg, headache, fever, malaise, anorexia, nausea, vomiting, abdominal pain, and diarrhea) and either jaundice or elevated serum aminotransferase levels, confirmation of HAV infection is required with either a positive serologic test for immunoglobulin M (IgM) anti-HAV antibody or an epidemiologic link (eg, recent household or close contact) to a person with laboratory-confirmed HAV.5 Serum IgM anti-HAV antibodies are first detectable when symptoms begin and remain detectable for about 3 to 6 months.29,30 Serum immunoglobulin G (IgG) anti-HAV antibodies, which provide lifelong protection against reinfection, appear as symptoms improve and persist indefinitely.31,32 Therefore, the presence of anti-HAV IgG and the absence of anti-HAV IgM is indicative of immunity to HAV via past infection or vaccination.
HAV Prevention in The VHA
The mainstay of HAV prevention is vaccination with 2 doses of inactivated, single-antigen hepatitis A vaccine or 3 doses of combination (HAV and HBV) vaccine.11 Both single antigen and combination HAV vaccines are safe in immunocompromised and pregnant patients.33-39 The HAV vaccination results in 100% anti-HAV IgG seropositivity among healthy individuals, although immunogenicity might be lower for those who are immunocompromised or with CLD.31,40-47 The VHA recommends HAV immunization, unless contraindicated, for previously unvaccinated
In addition to vaccination, addressing risk factors for HAV infection and its complications could reduce the burden of disease. For instance, recent outbreaks highlight that homeless individuals and users of injection and noninjection drugs are particularly vulnerable to infections transmitted via fecal-oral contamination. Broad strategies to address homelessness and related sanitation concerns are needed to help reduce the likelihood of future HAV outbreaks.49 Specific measures to combat HAV include providing access to clean water, adequate hygiene, and clean needles for people who inject drugs.11 Hepatitis A virus can be destroyed by heating food to ≥ 185 °F for at least 1 minute, chlorinating contaminated water, or cleaning contaminated surfaces with a solution of household bleach and water.50 Moreover, it is important to identify and treat risk factors for complications of HAV infection. This includes identifying individuals with HCV and ensuring that they are immune to HAV, given data that HCV-infected individuals are at increased risk of fulminant hepatic failure from HAV.12,13
Active-duty service members have long been considered at higher risk of HAV infections due to deployments in endemic areas and exposure to contaminated food and water.51,52 Shortly after the FDA approved HAV vaccination in 1995, the Department of Defense (DoD) mandated screening and HAV immunization for all incoming active-duty service members and those deployed to areas of high endemicity.53 However, US veterans who were discharged before the adoption of universal HAV vaccination remain at increased risk for HAV infection, particularly given the high prevalence of CLD, homelessness, and substance use disord
Methods
A cross-sectional analysis of veterans in VA care from June 1, 2016 to June 1, 2017 was performed to determine national rates of HAV susceptibility among patients with HCV exposure, homelessness, SUD, or HIV infection. The definitions of homelessness, SUD (alcohol, cannabis, opioid, sedatives, hallucinogens, inhalants, stimulants, or tobacco), and HIV infection were based on the presence of appropriate ICD-9 or ICD-10 codes. History of HCV exposure was based on a positive HCV antibody test. Presence of HAV vaccination was determined based on CPT codes for administration of the single-antigen HAV vaccination or combination HAV/HBV vaccination.
While HIV infection is not independently considered an indication for HAV vaccination, the authors included this group given its high proportion of patients with other risk factors, including MSM and IV drug use. All data were obtained from the VA Corporate Data Warehouse (CDW), a comprehensive national repository of all laboratory, diagnosis, and prescription results (including vaccines) within the VHA since 1999.
Hepatitis A virus nonsusceptibility was defined as (1) documented receipt of HAV vaccination within the VHA; (2) anti-HAV IgG antibody testing within the VHA; or (3) active-duty service after October 1997. It was considered likely that patients who received HAV testing either showed evidence of HAV immunity (eg, positive anti-HAV IgG) or were anti-HAV IgG negative and subsequently immunized. Therefore, patients with anti-HAV IgG antibody testing were counted presumptively as nonsusceptible. The DoD implemented a universal HAV vaccination policy in 1995, therefore, 1997 was chosen as a time at which the military’s universal HAV vaccination campaign was likely to have achieved near 100% vaccination coverage of active-duty military.
Results
The cohort included 5,896,451 patients in VA care, including 381,628 (6.5%) who were homeless, 455,344 (7.7%) with SUD, 225,889 (3.8%) with a lifetime history of positive HCV antibody (indicating past HCV exposure), and 29,166 (0.5%) with HIV infection.
There was wide geographic variability in rates of HAV susceptibility (Figure 1).
Discussion
Widespread HAV vaccination has decreased the incidence of HAV infection in the US dramatically. Nevertheless, recent outbreaks demonstrate that substantial population susceptibility and associated risk for HAV-related morbidity and mortality remains, particularly in high-risk populations. Although the VHA has not experienced a significant increase in acute HAV infections to date, this cross-sectional analysis highlights that a large proportion of VA patients in traditionally high-risk groups remain susceptible to HAV infection.
Strengths
Strengths of this analysis include a current reflection of HAV susceptibility within the national VHA, thus informing HAV testing and vaccination strategies. This study also involves a very large cohort, which is possible because the VHA is the largest integrated healthcare system in the US. Lastly, because the VHA uses electronic medical records, there was nearly complete capture of HAV vaccinations and testing obtained through the VHA.
Limitations
This cross-sectional analysis has several potential limitations. First, findings may not be generalizable outside the VHA. In addition, determination of homelessness, substance abuse, and HIV infection were based on ICD-9 and ICD-10 codes, which have been used in previous studies but may be subject to misclassification. The authors deliberately included all patients with positive HCV antibody testing to include those with current or prior risk factors for HAV acquisition. This population does not reflect patients with HCV viremia who received HAV testing or vaccination. Lastly, misattribution of HAV susceptibility could have occurred if patients with negative HAV IgG results were not vaccinated or if patients previously received HAV vaccination outside the VHA.
Conclusion
To mitigate the risk of future HAV outbreaks, continued efforts should be made to increase vaccination among high-risk groups, improve awareness of additional prevention measures, and address risk factors for HAV acquisition, particularly in areas with active outbreaks. Further study is suggested to identify geographic areas with large caseloads of at-risk patients and to highlight best practices utilized by VHA facilities that achieved high vaccine coverage rates. Recommended approaches likely will need to include efforts to improve hygiene and reduce risks for HAV exposure associated with SUD and homelessness.
Click here to read the digital edition.
1. Kemmer NM, Miskovsky EP. Hepatitis A. Infect Dis Clin North Am. 2000;14(3):605-615.
2. Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171(suppl 1):S15-S18.
3. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis a disease in the United States. MMWR Suppl. 2016;65(1):29-41.
4. Centers for Disease Control and Prevention. Viral hepatitis surveillance, United States, 2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Published 2015. Accessed February 12, 2018.
5. Centers for Disease Control and Prevention. 2017 – Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Updated February 7, 2018. Accessed February 12, 2018.
6. Hepatitis A cases more than double in 2017; if you’re at risk, get vaccinated [press release]. https://www.colorado.gov/pacific/cdphe/news/hep-a-cases-doubled. Published August 30,2017. Accessed February 12, 2018.
7. Alltucker K. Hepatitis A outbreak spread to Maricopa County homeless from San Diego, officials say. Azcentral website. October 7, 2017. https://www.azcentral.com/story/news/local /arizona-health/2017/10/07/hepatitis-outbreak-spread-maricopa-county-homeless-san-diego-officials-say/740185001/. Accessed February 12, 2018.
8. Savage RD, Rosella LC, Brown KA, Khan K, Crowcroft NS. Underreporting of hepatitis A in non-endemic countries: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:281.
9. Purcell RH, Wong DC, Shapiro M. Relative infectivity of hepatitis A virus by the oral and intravenous routes in 2 species of nonhuman primates. J Infect Dis. 2002;185(11):1668-1671.
10. Tassopoulos NC, Papaevangelou GJ, Ticehurst JR, Purcell RH. Fecal excretion of Greek strains of hepatitis A virus in patients with hepatitis A and in experimentally infected chimpanzees. J Infect Dis. 1986;154(2):231-237.
11. Centers for Disease Control and Prevention. Hepatitis A questions and answers for health professionals. https://www.cdc.gov/hepatitis/hav/havfaq.htm. Updated November 8, 2017. Accessed February 12, 2018.
12. Taylor RM, Davern T, Munoz S, et al; US Acute Liver Failure Study Group. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology. 2006;44(6):1589-1597.
13. Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338(5):286-290.
14. Singh S, Johnson AS, McCray E, Hall HI. CDC - HIV incidence, prevalence and undiagnosed infections in men who have sex with men - HIV incidence decreased among all transmission categories except MSM. Conference on Retroviruses and Opportunistic Infections (CROI); February 13-16,2017; Seattle, WA. http://www .natap.org/2017/CROI/croi_116.htm. Accessed February 12, 2018.
15. Fonquernie L, Meynard JL, Charrois A, Delamare C, Meyohas MC, Frottier J. Occurrence of acute hepatitis A in patients infected with human immunodeficiency virus. Clin Infect Dis. 2001;32(2):297-299.
16. Ida S, Tachikawa N, Nakajima A, et al. Influence of human immunodeficiency virus type 1 infection on acute hepatitis A virus infection. Clin Infect Dis. 2002;34(3):379-385.
17. Costa-Mattioli M, Allavena C, Poirier AS, Billaudel S, Raffi F, Ferré V. Prolonged hepatitis A infection in an HIV-1 seropositive patient. J Med Virol. 2002;68(1):7-11.
18. Neefe JR, Gellis SS, Stokes J Jr. Homologous serum hepatitis and infectious (epidemic) hepatitis; studies in volunteers bearing on immunological and other characteristics of the etiological agents. Am J Med. 1946;1:3-22.
19. Krugman S, Giles JP, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200(5):365-373.
20. Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980;302(22):1222-1227.
21. Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol. 1985;122(2):226-233.
22. Gordon SC, Reddy KR, Schiff L, Schiff ER. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Intern Med. 1984;101(5):635-637.
23. Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine. 1992;10(suppl 1):S18-S20.
24. Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20(4):380-391.
25. Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998;128(2):111-114.
26. Rezende G, Roque-Afonso AM, Samuel D, et al. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003;38(3):613-618.
27. Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985;313(17):1059-1067.
28. Centers for Disease Control and Prevention. Guidelines for viral hepatitis surveillance and case management. https://www.cdc.gov/hepatitis/statistics/surveillance guidelines.htm. Updated May 31, 2015. Accessed February 8, 2018.
29. Kao HW, Ashcavai M, Redeker AG. The persistence of hepatitis A IgM antibody after acute clinical hepatitis A. Hepatology. 1984;4(5):933-936.
30. Liaw YF, Yang CY, Chu CM, Huang MJ. Appearance and persistence of hepatitis A IgM antibody in acute clinical hepatitis A observed in an outbreak. Infection. 1986;14(4):156-158.
31. Plumb ID, Bulkow LR, Bruce MG, et al. Persistence of antibody to Hepatitis A virus 20 years after receipt of Hepatitis A vaccine in Alaska. J Viral Hepat. 2017;24(7):608-612.
32. Koff RS. Clinical manifestations and diagnosis of hepatitis A virus infection. Vaccine. 1992;10 (suppl 1):S15-S17.
33. Clemens R, Safary A, Hepburn A, Roche C, Stanbury WJ, André FE. Clinical experience with an inactivated hepatitis A vaccine. J Infect Dis. 1995;171(suppl 1):S44-S49.
34. Ambrosch F, André FE, Delem A, et al. Simultaneous vaccination against hepatitis A and B: results of a controlled study. Vaccine. 1992;10(suppl 1):S142-S145.
35. Gil A, González A, Dal-Ré R, Calero JR. Interference assessment of yellow fever vaccine with the immune response to a single-dose inactivated hepatitis A vaccine (1440 EL.U.). A controlled study in adults. Vaccine. 1996;14(11):1028-1030.
36. Jong EC, Kaplan KM, Eves KA, Taddeo CA, Lakkis HD, Kuter BJ. An open randomized study of inactivated hepatitis A vaccine administered concomitantly with typhoid fever and yellow fever vaccines. J Travel Med. 2002;9(2):66-70.
37. Nolan T, Bernstein H, Blatter MM, et al. Immunogenicity and safety of an inactivated hepatitis A vaccine administered concomitantly with diphtheria-tetanus-acellular pertussis and haemophilus influenzae type B vaccines to children less than 2 years of age. Pediatrics. 2006;118(3):e602-e609.
38. Usonis V, Meriste S, Bakasenas V, et al. Immunogenicity and safety of a combined hepatitis A and B vaccine administered concomitantly with either a measles-mumps-rubella or a diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis vaccine mixed with a Haemophilus influenzae type b conjugate vaccine in infants aged 12-18 months. Vaccine. 2005;23(20):2602-2606.
39. Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014;210(6):561.e1-561.e-6.
40. André FE, D’Hondt E, Delem A, Safary A. Clinical assessment of the safety and efficacy of an inactivated hepatitis A vaccine: rationale and summary of findings. Vaccine. 1992;10(suppl 1):S160-S168.
41. Just M, Berger R. Reactogenicity and immunogenicity of inactivated hepatitis A vaccines. Vaccine. 1992;10(suppl 1):S110-S113.
42. McMahon BJ, Williams J, Bulkow L, et al. Immunogenicity of an inactivated hepatitis A vaccine in Alaska Native children and Native and non-Native adults. J Infect Dis. 1995;171(3):676-679.
43. Balcarek KB, Bagley MR, Pass RF, Schiff ER, Krause DS. Safety and immunogenicity of an inactivated hepatitis A vaccine in preschool children. J Infect Dis. 1995;171(suppl 1):S70-S72.
44. Bell BP, Negus S, Fiore AE, et al. Immunogenicity of an inactivated hepatitis A vaccine in infants and young children. Pediatr Infect Dis J. 2007;26(2):116-122.
45. Arguedas MR, Johnson A, Eloubeidi MA, Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology. 2001;34(1):28-31.
46. Overton ET, Nurutdinova D, Sungkanuparph S, Seyfried W, Groger RK, Powderly WG. Predictors of immunity after hepatitis A vaccination in HIV-infected persons. J Viral Hepat. 2007;14(3):189-193.
47. Askling HH, Rombo L, van Vollenhoven R, et al. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Travel Med Infect Dis. 2014;12(2):134-142.
48. US Department of Veterans Affairs. VHA national hepatitis A immunization guidelines. http://vaww.prevention.va.gov/CPS/Hepatitis_A_Immunization.asp. Nonpublic document. Source not verified.
49. Kushel M. Hepatitis A outbreak in California - addressing the root cause. N Engl J Med. 2018;378(3):211-213.
50. Millard J, Appleton H, Parry JV. Studies on heat inactivation of hepatitis A virus with special reference to shellfish. Part 1. Procedures for infection and recovery of virus from laboratory-maintained cockles. Epidemiol Infect. 1987;98(3):397-414.
51. Hoke CH, Jr., Binn LN, Egan JE, et al. Hepatitis A in the US Army: epidemiology and vaccine development. Vaccine. 1992;10(suppl 1):S75-S79.
52. Dooley DP. History of U.S. military contributions to the study of viral hepatitis. Mil Med. 2005;170(suppl 4):71-76.
53. Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3-26.
54. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e1475; quiz e17-e18.
55. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
56. Teeters JB, Lancaster CL, Brown DG, Back SE. Substance use disorders in military veterans: prevalence and treatment challenges. Subst Abuse Rehabil. 2017;8:69-77.
Hepatitis A virus (HAV) can result in acute infection characterized by fatigue, nausea, jaundice (yellowing of the skin) and, rarely, acute liver failure and death.1,2 In the US, HAV yearly incidence (per 100,000) has decreased from 11.7 cases in 1996 to 0.4 cases in 2015, largely due to the 2006 recommendations from the Centers for Disease Control and Prevention (CDC) that all infants receive HAV vaccination.3,4
In 2017, multiple HAV outbreaks occurred in Arizona, California, Colorado, Kentucky, Michigan, and Utah with infections concentrated among those who were homeless, used illicit drugs (both injection and noninjection), or had close contact with these groups (Table 1).5-7
In response, the CDC has recommended the administration of HAV vaccine or immune globulin (IG) as postexposure prophylaxis (PEP) to people in high-risk groups including unvaccinated individuals exposed to HAV within the prior 2 weeks.5 While the Veterans Health Administration (VHA) in the Department of Veteran’s Affairs (VA) has not noted a significant increase in the number of reported HAV infections, there have been cases of hospitalization within the VA health care system due to HAV in at least 2 of the outbreak areas. The VA facilities in outbreak areas are responding by supporting county disease-control measures that include ensuring handwashing stations and vaccinations for high-risk, in-care populations and employees in direct contact with patients at high risk for HAV.
This review provides information on HAV transmission and clinical manifestations, guidelines on the prevention of HAV infection, and baseline data on current HAV susceptibility and immunization rates in the VHA.
Transmission and Clinical Manifestations
Hepatitis A virus is primarily transmitted by ingestion of small amounts of infected stool (ie, fecal-oral route) via direct person-to-person contact or through exposure to contaminated food or water.9,10 Groups at high risk of HAV infection include those in direct contact with HAV-infected individuals, users of injection or non-injection drugs, men who have sex with men (MSM), travelers to high-risk countries, individuals with clotting disorders, and those who work with nonhuman primates.11 Individuals who are homeless are susceptible to HAV due to poor sanitary conditions, and MSM are at increased risk of HAV acquisition via exposure to infected stool during sexual activity.
Complications of acute HAV infection, including fulminant liver failure and death, are more common among patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV).12,13 While infection with HIV does not independently increase the risk of HAV acquisition, about 75% of new HIV infections in the US are among MSM or IV drug users who are at increased risk of HAV infection.14 In addition, duration of HAV viremia and resulting HAV transmissibility may be increased in HIV-infected individuals.15-17
After infection, HAV remains asymptomatic (the incubation period) for an average of 28 days with a range of 15 to 50 days.18,19 Most children younger than 6 years remain asymptomatic while older children and adults typically experience symptoms including fever, fatigue, poor appetite, abdominal pain, dark urine, clay-colored stools, and jaundice.2,20,21 Symptoms typically last less than 2 months but can persist or relapse for up to 6 months in 10% to 15% of symptomatic individuals.22,23 Those with HAV infection are capable of viral transmission from the beginning of the incubation period until about a week after jaundice appears.24 Unlike HBV and HCV, HAV does not cause chronic infection.
Fulminant liver failure, characterized by encephalopathy, jaundice, and elevated international normalized ratio (INR), occurs in < 1% of HAV infections and is more common in those with underlying liver disease and older individuals.13,25-27 In one retrospective review of fulminant liver failure from HAV infection, about half of the patients required liver transplantation or died within 3 weeks of presentation.12
Other than supportive care, there are no specific treatments for acute HAV infection. However, the CDC recommends that healthy individuals aged between 1 and 40 years with known or suspected exposure to HAV within the prior 2 weeks receive 1 dose of a single-antigen HAV vaccination. The CDC also recommends that recently exposed individuals aged < 1 year or > 40 years, or patients who are immunocompromised, have chronic liver disease (CLD), or are allergic to HAV vaccine or a vaccine component should receive a single IG injection. In addition, the CDC recommends that health care providers report all cases of acute HAV to state and local health departments.28
In patients with typical symptoms of acute viral hepatitis (eg, headache, fever, malaise, anorexia, nausea, vomiting, abdominal pain, and diarrhea) and either jaundice or elevated serum aminotransferase levels, confirmation of HAV infection is required with either a positive serologic test for immunoglobulin M (IgM) anti-HAV antibody or an epidemiologic link (eg, recent household or close contact) to a person with laboratory-confirmed HAV.5 Serum IgM anti-HAV antibodies are first detectable when symptoms begin and remain detectable for about 3 to 6 months.29,30 Serum immunoglobulin G (IgG) anti-HAV antibodies, which provide lifelong protection against reinfection, appear as symptoms improve and persist indefinitely.31,32 Therefore, the presence of anti-HAV IgG and the absence of anti-HAV IgM is indicative of immunity to HAV via past infection or vaccination.
HAV Prevention in The VHA
The mainstay of HAV prevention is vaccination with 2 doses of inactivated, single-antigen hepatitis A vaccine or 3 doses of combination (HAV and HBV) vaccine.11 Both single antigen and combination HAV vaccines are safe in immunocompromised and pregnant patients.33-39 The HAV vaccination results in 100% anti-HAV IgG seropositivity among healthy individuals, although immunogenicity might be lower for those who are immunocompromised or with CLD.31,40-47 The VHA recommends HAV immunization, unless contraindicated, for previously unvaccinated
In addition to vaccination, addressing risk factors for HAV infection and its complications could reduce the burden of disease. For instance, recent outbreaks highlight that homeless individuals and users of injection and noninjection drugs are particularly vulnerable to infections transmitted via fecal-oral contamination. Broad strategies to address homelessness and related sanitation concerns are needed to help reduce the likelihood of future HAV outbreaks.49 Specific measures to combat HAV include providing access to clean water, adequate hygiene, and clean needles for people who inject drugs.11 Hepatitis A virus can be destroyed by heating food to ≥ 185 °F for at least 1 minute, chlorinating contaminated water, or cleaning contaminated surfaces with a solution of household bleach and water.50 Moreover, it is important to identify and treat risk factors for complications of HAV infection. This includes identifying individuals with HCV and ensuring that they are immune to HAV, given data that HCV-infected individuals are at increased risk of fulminant hepatic failure from HAV.12,13
Active-duty service members have long been considered at higher risk of HAV infections due to deployments in endemic areas and exposure to contaminated food and water.51,52 Shortly after the FDA approved HAV vaccination in 1995, the Department of Defense (DoD) mandated screening and HAV immunization for all incoming active-duty service members and those deployed to areas of high endemicity.53 However, US veterans who were discharged before the adoption of universal HAV vaccination remain at increased risk for HAV infection, particularly given the high prevalence of CLD, homelessness, and substance use disord
Methods
A cross-sectional analysis of veterans in VA care from June 1, 2016 to June 1, 2017 was performed to determine national rates of HAV susceptibility among patients with HCV exposure, homelessness, SUD, or HIV infection. The definitions of homelessness, SUD (alcohol, cannabis, opioid, sedatives, hallucinogens, inhalants, stimulants, or tobacco), and HIV infection were based on the presence of appropriate ICD-9 or ICD-10 codes. History of HCV exposure was based on a positive HCV antibody test. Presence of HAV vaccination was determined based on CPT codes for administration of the single-antigen HAV vaccination or combination HAV/HBV vaccination.
While HIV infection is not independently considered an indication for HAV vaccination, the authors included this group given its high proportion of patients with other risk factors, including MSM and IV drug use. All data were obtained from the VA Corporate Data Warehouse (CDW), a comprehensive national repository of all laboratory, diagnosis, and prescription results (including vaccines) within the VHA since 1999.
Hepatitis A virus nonsusceptibility was defined as (1) documented receipt of HAV vaccination within the VHA; (2) anti-HAV IgG antibody testing within the VHA; or (3) active-duty service after October 1997. It was considered likely that patients who received HAV testing either showed evidence of HAV immunity (eg, positive anti-HAV IgG) or were anti-HAV IgG negative and subsequently immunized. Therefore, patients with anti-HAV IgG antibody testing were counted presumptively as nonsusceptible. The DoD implemented a universal HAV vaccination policy in 1995, therefore, 1997 was chosen as a time at which the military’s universal HAV vaccination campaign was likely to have achieved near 100% vaccination coverage of active-duty military.
Results
The cohort included 5,896,451 patients in VA care, including 381,628 (6.5%) who were homeless, 455,344 (7.7%) with SUD, 225,889 (3.8%) with a lifetime history of positive HCV antibody (indicating past HCV exposure), and 29,166 (0.5%) with HIV infection.
There was wide geographic variability in rates of HAV susceptibility (Figure 1).
Discussion
Widespread HAV vaccination has decreased the incidence of HAV infection in the US dramatically. Nevertheless, recent outbreaks demonstrate that substantial population susceptibility and associated risk for HAV-related morbidity and mortality remains, particularly in high-risk populations. Although the VHA has not experienced a significant increase in acute HAV infections to date, this cross-sectional analysis highlights that a large proportion of VA patients in traditionally high-risk groups remain susceptible to HAV infection.
Strengths
Strengths of this analysis include a current reflection of HAV susceptibility within the national VHA, thus informing HAV testing and vaccination strategies. This study also involves a very large cohort, which is possible because the VHA is the largest integrated healthcare system in the US. Lastly, because the VHA uses electronic medical records, there was nearly complete capture of HAV vaccinations and testing obtained through the VHA.
Limitations
This cross-sectional analysis has several potential limitations. First, findings may not be generalizable outside the VHA. In addition, determination of homelessness, substance abuse, and HIV infection were based on ICD-9 and ICD-10 codes, which have been used in previous studies but may be subject to misclassification. The authors deliberately included all patients with positive HCV antibody testing to include those with current or prior risk factors for HAV acquisition. This population does not reflect patients with HCV viremia who received HAV testing or vaccination. Lastly, misattribution of HAV susceptibility could have occurred if patients with negative HAV IgG results were not vaccinated or if patients previously received HAV vaccination outside the VHA.
Conclusion
To mitigate the risk of future HAV outbreaks, continued efforts should be made to increase vaccination among high-risk groups, improve awareness of additional prevention measures, and address risk factors for HAV acquisition, particularly in areas with active outbreaks. Further study is suggested to identify geographic areas with large caseloads of at-risk patients and to highlight best practices utilized by VHA facilities that achieved high vaccine coverage rates. Recommended approaches likely will need to include efforts to improve hygiene and reduce risks for HAV exposure associated with SUD and homelessness.
Click here to read the digital edition.
Hepatitis A virus (HAV) can result in acute infection characterized by fatigue, nausea, jaundice (yellowing of the skin) and, rarely, acute liver failure and death.1,2 In the US, HAV yearly incidence (per 100,000) has decreased from 11.7 cases in 1996 to 0.4 cases in 2015, largely due to the 2006 recommendations from the Centers for Disease Control and Prevention (CDC) that all infants receive HAV vaccination.3,4
In 2017, multiple HAV outbreaks occurred in Arizona, California, Colorado, Kentucky, Michigan, and Utah with infections concentrated among those who were homeless, used illicit drugs (both injection and noninjection), or had close contact with these groups (Table 1).5-7
In response, the CDC has recommended the administration of HAV vaccine or immune globulin (IG) as postexposure prophylaxis (PEP) to people in high-risk groups including unvaccinated individuals exposed to HAV within the prior 2 weeks.5 While the Veterans Health Administration (VHA) in the Department of Veteran’s Affairs (VA) has not noted a significant increase in the number of reported HAV infections, there have been cases of hospitalization within the VA health care system due to HAV in at least 2 of the outbreak areas. The VA facilities in outbreak areas are responding by supporting county disease-control measures that include ensuring handwashing stations and vaccinations for high-risk, in-care populations and employees in direct contact with patients at high risk for HAV.
This review provides information on HAV transmission and clinical manifestations, guidelines on the prevention of HAV infection, and baseline data on current HAV susceptibility and immunization rates in the VHA.
Transmission and Clinical Manifestations
Hepatitis A virus is primarily transmitted by ingestion of small amounts of infected stool (ie, fecal-oral route) via direct person-to-person contact or through exposure to contaminated food or water.9,10 Groups at high risk of HAV infection include those in direct contact with HAV-infected individuals, users of injection or non-injection drugs, men who have sex with men (MSM), travelers to high-risk countries, individuals with clotting disorders, and those who work with nonhuman primates.11 Individuals who are homeless are susceptible to HAV due to poor sanitary conditions, and MSM are at increased risk of HAV acquisition via exposure to infected stool during sexual activity.
Complications of acute HAV infection, including fulminant liver failure and death, are more common among patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV).12,13 While infection with HIV does not independently increase the risk of HAV acquisition, about 75% of new HIV infections in the US are among MSM or IV drug users who are at increased risk of HAV infection.14 In addition, duration of HAV viremia and resulting HAV transmissibility may be increased in HIV-infected individuals.15-17
After infection, HAV remains asymptomatic (the incubation period) for an average of 28 days with a range of 15 to 50 days.18,19 Most children younger than 6 years remain asymptomatic while older children and adults typically experience symptoms including fever, fatigue, poor appetite, abdominal pain, dark urine, clay-colored stools, and jaundice.2,20,21 Symptoms typically last less than 2 months but can persist or relapse for up to 6 months in 10% to 15% of symptomatic individuals.22,23 Those with HAV infection are capable of viral transmission from the beginning of the incubation period until about a week after jaundice appears.24 Unlike HBV and HCV, HAV does not cause chronic infection.
Fulminant liver failure, characterized by encephalopathy, jaundice, and elevated international normalized ratio (INR), occurs in < 1% of HAV infections and is more common in those with underlying liver disease and older individuals.13,25-27 In one retrospective review of fulminant liver failure from HAV infection, about half of the patients required liver transplantation or died within 3 weeks of presentation.12
Other than supportive care, there are no specific treatments for acute HAV infection. However, the CDC recommends that healthy individuals aged between 1 and 40 years with known or suspected exposure to HAV within the prior 2 weeks receive 1 dose of a single-antigen HAV vaccination. The CDC also recommends that recently exposed individuals aged < 1 year or > 40 years, or patients who are immunocompromised, have chronic liver disease (CLD), or are allergic to HAV vaccine or a vaccine component should receive a single IG injection. In addition, the CDC recommends that health care providers report all cases of acute HAV to state and local health departments.28
In patients with typical symptoms of acute viral hepatitis (eg, headache, fever, malaise, anorexia, nausea, vomiting, abdominal pain, and diarrhea) and either jaundice or elevated serum aminotransferase levels, confirmation of HAV infection is required with either a positive serologic test for immunoglobulin M (IgM) anti-HAV antibody or an epidemiologic link (eg, recent household or close contact) to a person with laboratory-confirmed HAV.5 Serum IgM anti-HAV antibodies are first detectable when symptoms begin and remain detectable for about 3 to 6 months.29,30 Serum immunoglobulin G (IgG) anti-HAV antibodies, which provide lifelong protection against reinfection, appear as symptoms improve and persist indefinitely.31,32 Therefore, the presence of anti-HAV IgG and the absence of anti-HAV IgM is indicative of immunity to HAV via past infection or vaccination.
HAV Prevention in The VHA
The mainstay of HAV prevention is vaccination with 2 doses of inactivated, single-antigen hepatitis A vaccine or 3 doses of combination (HAV and HBV) vaccine.11 Both single antigen and combination HAV vaccines are safe in immunocompromised and pregnant patients.33-39 The HAV vaccination results in 100% anti-HAV IgG seropositivity among healthy individuals, although immunogenicity might be lower for those who are immunocompromised or with CLD.31,40-47 The VHA recommends HAV immunization, unless contraindicated, for previously unvaccinated
In addition to vaccination, addressing risk factors for HAV infection and its complications could reduce the burden of disease. For instance, recent outbreaks highlight that homeless individuals and users of injection and noninjection drugs are particularly vulnerable to infections transmitted via fecal-oral contamination. Broad strategies to address homelessness and related sanitation concerns are needed to help reduce the likelihood of future HAV outbreaks.49 Specific measures to combat HAV include providing access to clean water, adequate hygiene, and clean needles for people who inject drugs.11 Hepatitis A virus can be destroyed by heating food to ≥ 185 °F for at least 1 minute, chlorinating contaminated water, or cleaning contaminated surfaces with a solution of household bleach and water.50 Moreover, it is important to identify and treat risk factors for complications of HAV infection. This includes identifying individuals with HCV and ensuring that they are immune to HAV, given data that HCV-infected individuals are at increased risk of fulminant hepatic failure from HAV.12,13
Active-duty service members have long been considered at higher risk of HAV infections due to deployments in endemic areas and exposure to contaminated food and water.51,52 Shortly after the FDA approved HAV vaccination in 1995, the Department of Defense (DoD) mandated screening and HAV immunization for all incoming active-duty service members and those deployed to areas of high endemicity.53 However, US veterans who were discharged before the adoption of universal HAV vaccination remain at increased risk for HAV infection, particularly given the high prevalence of CLD, homelessness, and substance use disord
Methods
A cross-sectional analysis of veterans in VA care from June 1, 2016 to June 1, 2017 was performed to determine national rates of HAV susceptibility among patients with HCV exposure, homelessness, SUD, or HIV infection. The definitions of homelessness, SUD (alcohol, cannabis, opioid, sedatives, hallucinogens, inhalants, stimulants, or tobacco), and HIV infection were based on the presence of appropriate ICD-9 or ICD-10 codes. History of HCV exposure was based on a positive HCV antibody test. Presence of HAV vaccination was determined based on CPT codes for administration of the single-antigen HAV vaccination or combination HAV/HBV vaccination.
While HIV infection is not independently considered an indication for HAV vaccination, the authors included this group given its high proportion of patients with other risk factors, including MSM and IV drug use. All data were obtained from the VA Corporate Data Warehouse (CDW), a comprehensive national repository of all laboratory, diagnosis, and prescription results (including vaccines) within the VHA since 1999.
Hepatitis A virus nonsusceptibility was defined as (1) documented receipt of HAV vaccination within the VHA; (2) anti-HAV IgG antibody testing within the VHA; or (3) active-duty service after October 1997. It was considered likely that patients who received HAV testing either showed evidence of HAV immunity (eg, positive anti-HAV IgG) or were anti-HAV IgG negative and subsequently immunized. Therefore, patients with anti-HAV IgG antibody testing were counted presumptively as nonsusceptible. The DoD implemented a universal HAV vaccination policy in 1995, therefore, 1997 was chosen as a time at which the military’s universal HAV vaccination campaign was likely to have achieved near 100% vaccination coverage of active-duty military.
Results
The cohort included 5,896,451 patients in VA care, including 381,628 (6.5%) who were homeless, 455,344 (7.7%) with SUD, 225,889 (3.8%) with a lifetime history of positive HCV antibody (indicating past HCV exposure), and 29,166 (0.5%) with HIV infection.
There was wide geographic variability in rates of HAV susceptibility (Figure 1).
Discussion
Widespread HAV vaccination has decreased the incidence of HAV infection in the US dramatically. Nevertheless, recent outbreaks demonstrate that substantial population susceptibility and associated risk for HAV-related morbidity and mortality remains, particularly in high-risk populations. Although the VHA has not experienced a significant increase in acute HAV infections to date, this cross-sectional analysis highlights that a large proportion of VA patients in traditionally high-risk groups remain susceptible to HAV infection.
Strengths
Strengths of this analysis include a current reflection of HAV susceptibility within the national VHA, thus informing HAV testing and vaccination strategies. This study also involves a very large cohort, which is possible because the VHA is the largest integrated healthcare system in the US. Lastly, because the VHA uses electronic medical records, there was nearly complete capture of HAV vaccinations and testing obtained through the VHA.
Limitations
This cross-sectional analysis has several potential limitations. First, findings may not be generalizable outside the VHA. In addition, determination of homelessness, substance abuse, and HIV infection were based on ICD-9 and ICD-10 codes, which have been used in previous studies but may be subject to misclassification. The authors deliberately included all patients with positive HCV antibody testing to include those with current or prior risk factors for HAV acquisition. This population does not reflect patients with HCV viremia who received HAV testing or vaccination. Lastly, misattribution of HAV susceptibility could have occurred if patients with negative HAV IgG results were not vaccinated or if patients previously received HAV vaccination outside the VHA.
Conclusion
To mitigate the risk of future HAV outbreaks, continued efforts should be made to increase vaccination among high-risk groups, improve awareness of additional prevention measures, and address risk factors for HAV acquisition, particularly in areas with active outbreaks. Further study is suggested to identify geographic areas with large caseloads of at-risk patients and to highlight best practices utilized by VHA facilities that achieved high vaccine coverage rates. Recommended approaches likely will need to include efforts to improve hygiene and reduce risks for HAV exposure associated with SUD and homelessness.
Click here to read the digital edition.
1. Kemmer NM, Miskovsky EP. Hepatitis A. Infect Dis Clin North Am. 2000;14(3):605-615.
2. Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171(suppl 1):S15-S18.
3. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis a disease in the United States. MMWR Suppl. 2016;65(1):29-41.
4. Centers for Disease Control and Prevention. Viral hepatitis surveillance, United States, 2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Published 2015. Accessed February 12, 2018.
5. Centers for Disease Control and Prevention. 2017 – Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Updated February 7, 2018. Accessed February 12, 2018.
6. Hepatitis A cases more than double in 2017; if you’re at risk, get vaccinated [press release]. https://www.colorado.gov/pacific/cdphe/news/hep-a-cases-doubled. Published August 30,2017. Accessed February 12, 2018.
7. Alltucker K. Hepatitis A outbreak spread to Maricopa County homeless from San Diego, officials say. Azcentral website. October 7, 2017. https://www.azcentral.com/story/news/local /arizona-health/2017/10/07/hepatitis-outbreak-spread-maricopa-county-homeless-san-diego-officials-say/740185001/. Accessed February 12, 2018.
8. Savage RD, Rosella LC, Brown KA, Khan K, Crowcroft NS. Underreporting of hepatitis A in non-endemic countries: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:281.
9. Purcell RH, Wong DC, Shapiro M. Relative infectivity of hepatitis A virus by the oral and intravenous routes in 2 species of nonhuman primates. J Infect Dis. 2002;185(11):1668-1671.
10. Tassopoulos NC, Papaevangelou GJ, Ticehurst JR, Purcell RH. Fecal excretion of Greek strains of hepatitis A virus in patients with hepatitis A and in experimentally infected chimpanzees. J Infect Dis. 1986;154(2):231-237.
11. Centers for Disease Control and Prevention. Hepatitis A questions and answers for health professionals. https://www.cdc.gov/hepatitis/hav/havfaq.htm. Updated November 8, 2017. Accessed February 12, 2018.
12. Taylor RM, Davern T, Munoz S, et al; US Acute Liver Failure Study Group. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology. 2006;44(6):1589-1597.
13. Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338(5):286-290.
14. Singh S, Johnson AS, McCray E, Hall HI. CDC - HIV incidence, prevalence and undiagnosed infections in men who have sex with men - HIV incidence decreased among all transmission categories except MSM. Conference on Retroviruses and Opportunistic Infections (CROI); February 13-16,2017; Seattle, WA. http://www .natap.org/2017/CROI/croi_116.htm. Accessed February 12, 2018.
15. Fonquernie L, Meynard JL, Charrois A, Delamare C, Meyohas MC, Frottier J. Occurrence of acute hepatitis A in patients infected with human immunodeficiency virus. Clin Infect Dis. 2001;32(2):297-299.
16. Ida S, Tachikawa N, Nakajima A, et al. Influence of human immunodeficiency virus type 1 infection on acute hepatitis A virus infection. Clin Infect Dis. 2002;34(3):379-385.
17. Costa-Mattioli M, Allavena C, Poirier AS, Billaudel S, Raffi F, Ferré V. Prolonged hepatitis A infection in an HIV-1 seropositive patient. J Med Virol. 2002;68(1):7-11.
18. Neefe JR, Gellis SS, Stokes J Jr. Homologous serum hepatitis and infectious (epidemic) hepatitis; studies in volunteers bearing on immunological and other characteristics of the etiological agents. Am J Med. 1946;1:3-22.
19. Krugman S, Giles JP, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200(5):365-373.
20. Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980;302(22):1222-1227.
21. Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol. 1985;122(2):226-233.
22. Gordon SC, Reddy KR, Schiff L, Schiff ER. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Intern Med. 1984;101(5):635-637.
23. Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine. 1992;10(suppl 1):S18-S20.
24. Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20(4):380-391.
25. Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998;128(2):111-114.
26. Rezende G, Roque-Afonso AM, Samuel D, et al. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003;38(3):613-618.
27. Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985;313(17):1059-1067.
28. Centers for Disease Control and Prevention. Guidelines for viral hepatitis surveillance and case management. https://www.cdc.gov/hepatitis/statistics/surveillance guidelines.htm. Updated May 31, 2015. Accessed February 8, 2018.
29. Kao HW, Ashcavai M, Redeker AG. The persistence of hepatitis A IgM antibody after acute clinical hepatitis A. Hepatology. 1984;4(5):933-936.
30. Liaw YF, Yang CY, Chu CM, Huang MJ. Appearance and persistence of hepatitis A IgM antibody in acute clinical hepatitis A observed in an outbreak. Infection. 1986;14(4):156-158.
31. Plumb ID, Bulkow LR, Bruce MG, et al. Persistence of antibody to Hepatitis A virus 20 years after receipt of Hepatitis A vaccine in Alaska. J Viral Hepat. 2017;24(7):608-612.
32. Koff RS. Clinical manifestations and diagnosis of hepatitis A virus infection. Vaccine. 1992;10 (suppl 1):S15-S17.
33. Clemens R, Safary A, Hepburn A, Roche C, Stanbury WJ, André FE. Clinical experience with an inactivated hepatitis A vaccine. J Infect Dis. 1995;171(suppl 1):S44-S49.
34. Ambrosch F, André FE, Delem A, et al. Simultaneous vaccination against hepatitis A and B: results of a controlled study. Vaccine. 1992;10(suppl 1):S142-S145.
35. Gil A, González A, Dal-Ré R, Calero JR. Interference assessment of yellow fever vaccine with the immune response to a single-dose inactivated hepatitis A vaccine (1440 EL.U.). A controlled study in adults. Vaccine. 1996;14(11):1028-1030.
36. Jong EC, Kaplan KM, Eves KA, Taddeo CA, Lakkis HD, Kuter BJ. An open randomized study of inactivated hepatitis A vaccine administered concomitantly with typhoid fever and yellow fever vaccines. J Travel Med. 2002;9(2):66-70.
37. Nolan T, Bernstein H, Blatter MM, et al. Immunogenicity and safety of an inactivated hepatitis A vaccine administered concomitantly with diphtheria-tetanus-acellular pertussis and haemophilus influenzae type B vaccines to children less than 2 years of age. Pediatrics. 2006;118(3):e602-e609.
38. Usonis V, Meriste S, Bakasenas V, et al. Immunogenicity and safety of a combined hepatitis A and B vaccine administered concomitantly with either a measles-mumps-rubella or a diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis vaccine mixed with a Haemophilus influenzae type b conjugate vaccine in infants aged 12-18 months. Vaccine. 2005;23(20):2602-2606.
39. Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014;210(6):561.e1-561.e-6.
40. André FE, D’Hondt E, Delem A, Safary A. Clinical assessment of the safety and efficacy of an inactivated hepatitis A vaccine: rationale and summary of findings. Vaccine. 1992;10(suppl 1):S160-S168.
41. Just M, Berger R. Reactogenicity and immunogenicity of inactivated hepatitis A vaccines. Vaccine. 1992;10(suppl 1):S110-S113.
42. McMahon BJ, Williams J, Bulkow L, et al. Immunogenicity of an inactivated hepatitis A vaccine in Alaska Native children and Native and non-Native adults. J Infect Dis. 1995;171(3):676-679.
43. Balcarek KB, Bagley MR, Pass RF, Schiff ER, Krause DS. Safety and immunogenicity of an inactivated hepatitis A vaccine in preschool children. J Infect Dis. 1995;171(suppl 1):S70-S72.
44. Bell BP, Negus S, Fiore AE, et al. Immunogenicity of an inactivated hepatitis A vaccine in infants and young children. Pediatr Infect Dis J. 2007;26(2):116-122.
45. Arguedas MR, Johnson A, Eloubeidi MA, Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology. 2001;34(1):28-31.
46. Overton ET, Nurutdinova D, Sungkanuparph S, Seyfried W, Groger RK, Powderly WG. Predictors of immunity after hepatitis A vaccination in HIV-infected persons. J Viral Hepat. 2007;14(3):189-193.
47. Askling HH, Rombo L, van Vollenhoven R, et al. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Travel Med Infect Dis. 2014;12(2):134-142.
48. US Department of Veterans Affairs. VHA national hepatitis A immunization guidelines. http://vaww.prevention.va.gov/CPS/Hepatitis_A_Immunization.asp. Nonpublic document. Source not verified.
49. Kushel M. Hepatitis A outbreak in California - addressing the root cause. N Engl J Med. 2018;378(3):211-213.
50. Millard J, Appleton H, Parry JV. Studies on heat inactivation of hepatitis A virus with special reference to shellfish. Part 1. Procedures for infection and recovery of virus from laboratory-maintained cockles. Epidemiol Infect. 1987;98(3):397-414.
51. Hoke CH, Jr., Binn LN, Egan JE, et al. Hepatitis A in the US Army: epidemiology and vaccine development. Vaccine. 1992;10(suppl 1):S75-S79.
52. Dooley DP. History of U.S. military contributions to the study of viral hepatitis. Mil Med. 2005;170(suppl 4):71-76.
53. Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3-26.
54. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e1475; quiz e17-e18.
55. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
56. Teeters JB, Lancaster CL, Brown DG, Back SE. Substance use disorders in military veterans: prevalence and treatment challenges. Subst Abuse Rehabil. 2017;8:69-77.
1. Kemmer NM, Miskovsky EP. Hepatitis A. Infect Dis Clin North Am. 2000;14(3):605-615.
2. Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171(suppl 1):S15-S18.
3. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis a disease in the United States. MMWR Suppl. 2016;65(1):29-41.
4. Centers for Disease Control and Prevention. Viral hepatitis surveillance, United States, 2015. https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Published 2015. Accessed February 12, 2018.
5. Centers for Disease Control and Prevention. 2017 – Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Updated February 7, 2018. Accessed February 12, 2018.
6. Hepatitis A cases more than double in 2017; if you’re at risk, get vaccinated [press release]. https://www.colorado.gov/pacific/cdphe/news/hep-a-cases-doubled. Published August 30,2017. Accessed February 12, 2018.
7. Alltucker K. Hepatitis A outbreak spread to Maricopa County homeless from San Diego, officials say. Azcentral website. October 7, 2017. https://www.azcentral.com/story/news/local /arizona-health/2017/10/07/hepatitis-outbreak-spread-maricopa-county-homeless-san-diego-officials-say/740185001/. Accessed February 12, 2018.
8. Savage RD, Rosella LC, Brown KA, Khan K, Crowcroft NS. Underreporting of hepatitis A in non-endemic countries: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:281.
9. Purcell RH, Wong DC, Shapiro M. Relative infectivity of hepatitis A virus by the oral and intravenous routes in 2 species of nonhuman primates. J Infect Dis. 2002;185(11):1668-1671.
10. Tassopoulos NC, Papaevangelou GJ, Ticehurst JR, Purcell RH. Fecal excretion of Greek strains of hepatitis A virus in patients with hepatitis A and in experimentally infected chimpanzees. J Infect Dis. 1986;154(2):231-237.
11. Centers for Disease Control and Prevention. Hepatitis A questions and answers for health professionals. https://www.cdc.gov/hepatitis/hav/havfaq.htm. Updated November 8, 2017. Accessed February 12, 2018.
12. Taylor RM, Davern T, Munoz S, et al; US Acute Liver Failure Study Group. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology. 2006;44(6):1589-1597.
13. Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338(5):286-290.
14. Singh S, Johnson AS, McCray E, Hall HI. CDC - HIV incidence, prevalence and undiagnosed infections in men who have sex with men - HIV incidence decreased among all transmission categories except MSM. Conference on Retroviruses and Opportunistic Infections (CROI); February 13-16,2017; Seattle, WA. http://www .natap.org/2017/CROI/croi_116.htm. Accessed February 12, 2018.
15. Fonquernie L, Meynard JL, Charrois A, Delamare C, Meyohas MC, Frottier J. Occurrence of acute hepatitis A in patients infected with human immunodeficiency virus. Clin Infect Dis. 2001;32(2):297-299.
16. Ida S, Tachikawa N, Nakajima A, et al. Influence of human immunodeficiency virus type 1 infection on acute hepatitis A virus infection. Clin Infect Dis. 2002;34(3):379-385.
17. Costa-Mattioli M, Allavena C, Poirier AS, Billaudel S, Raffi F, Ferré V. Prolonged hepatitis A infection in an HIV-1 seropositive patient. J Med Virol. 2002;68(1):7-11.
18. Neefe JR, Gellis SS, Stokes J Jr. Homologous serum hepatitis and infectious (epidemic) hepatitis; studies in volunteers bearing on immunological and other characteristics of the etiological agents. Am J Med. 1946;1:3-22.
19. Krugman S, Giles JP, Hammond J. Infectious hepatitis. Evidence for two distinctive clinical, epidemiological, and immunological types of infection. JAMA. 1967;200(5):365-373.
20. Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980;302(22):1222-1227.
21. Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol. 1985;122(2):226-233.
22. Gordon SC, Reddy KR, Schiff L, Schiff ER. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Intern Med. 1984;101(5):635-637.
23. Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine. 1992;10(suppl 1):S18-S20.
24. Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20(4):380-391.
25. Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998;128(2):111-114.
26. Rezende G, Roque-Afonso AM, Samuel D, et al. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003;38(3):613-618.
27. Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985;313(17):1059-1067.
28. Centers for Disease Control and Prevention. Guidelines for viral hepatitis surveillance and case management. https://www.cdc.gov/hepatitis/statistics/surveillance guidelines.htm. Updated May 31, 2015. Accessed February 8, 2018.
29. Kao HW, Ashcavai M, Redeker AG. The persistence of hepatitis A IgM antibody after acute clinical hepatitis A. Hepatology. 1984;4(5):933-936.
30. Liaw YF, Yang CY, Chu CM, Huang MJ. Appearance and persistence of hepatitis A IgM antibody in acute clinical hepatitis A observed in an outbreak. Infection. 1986;14(4):156-158.
31. Plumb ID, Bulkow LR, Bruce MG, et al. Persistence of antibody to Hepatitis A virus 20 years after receipt of Hepatitis A vaccine in Alaska. J Viral Hepat. 2017;24(7):608-612.
32. Koff RS. Clinical manifestations and diagnosis of hepatitis A virus infection. Vaccine. 1992;10 (suppl 1):S15-S17.
33. Clemens R, Safary A, Hepburn A, Roche C, Stanbury WJ, André FE. Clinical experience with an inactivated hepatitis A vaccine. J Infect Dis. 1995;171(suppl 1):S44-S49.
34. Ambrosch F, André FE, Delem A, et al. Simultaneous vaccination against hepatitis A and B: results of a controlled study. Vaccine. 1992;10(suppl 1):S142-S145.
35. Gil A, González A, Dal-Ré R, Calero JR. Interference assessment of yellow fever vaccine with the immune response to a single-dose inactivated hepatitis A vaccine (1440 EL.U.). A controlled study in adults. Vaccine. 1996;14(11):1028-1030.
36. Jong EC, Kaplan KM, Eves KA, Taddeo CA, Lakkis HD, Kuter BJ. An open randomized study of inactivated hepatitis A vaccine administered concomitantly with typhoid fever and yellow fever vaccines. J Travel Med. 2002;9(2):66-70.
37. Nolan T, Bernstein H, Blatter MM, et al. Immunogenicity and safety of an inactivated hepatitis A vaccine administered concomitantly with diphtheria-tetanus-acellular pertussis and haemophilus influenzae type B vaccines to children less than 2 years of age. Pediatrics. 2006;118(3):e602-e609.
38. Usonis V, Meriste S, Bakasenas V, et al. Immunogenicity and safety of a combined hepatitis A and B vaccine administered concomitantly with either a measles-mumps-rubella or a diphtheria-tetanus-acellular pertussis-inactivated poliomyelitis vaccine mixed with a Haemophilus influenzae type b conjugate vaccine in infants aged 12-18 months. Vaccine. 2005;23(20):2602-2606.
39. Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014;210(6):561.e1-561.e-6.
40. André FE, D’Hondt E, Delem A, Safary A. Clinical assessment of the safety and efficacy of an inactivated hepatitis A vaccine: rationale and summary of findings. Vaccine. 1992;10(suppl 1):S160-S168.
41. Just M, Berger R. Reactogenicity and immunogenicity of inactivated hepatitis A vaccines. Vaccine. 1992;10(suppl 1):S110-S113.
42. McMahon BJ, Williams J, Bulkow L, et al. Immunogenicity of an inactivated hepatitis A vaccine in Alaska Native children and Native and non-Native adults. J Infect Dis. 1995;171(3):676-679.
43. Balcarek KB, Bagley MR, Pass RF, Schiff ER, Krause DS. Safety and immunogenicity of an inactivated hepatitis A vaccine in preschool children. J Infect Dis. 1995;171(suppl 1):S70-S72.
44. Bell BP, Negus S, Fiore AE, et al. Immunogenicity of an inactivated hepatitis A vaccine in infants and young children. Pediatr Infect Dis J. 2007;26(2):116-122.
45. Arguedas MR, Johnson A, Eloubeidi MA, Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology. 2001;34(1):28-31.
46. Overton ET, Nurutdinova D, Sungkanuparph S, Seyfried W, Groger RK, Powderly WG. Predictors of immunity after hepatitis A vaccination in HIV-infected persons. J Viral Hepat. 2007;14(3):189-193.
47. Askling HH, Rombo L, van Vollenhoven R, et al. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Travel Med Infect Dis. 2014;12(2):134-142.
48. US Department of Veterans Affairs. VHA national hepatitis A immunization guidelines. http://vaww.prevention.va.gov/CPS/Hepatitis_A_Immunization.asp. Nonpublic document. Source not verified.
49. Kushel M. Hepatitis A outbreak in California - addressing the root cause. N Engl J Med. 2018;378(3):211-213.
50. Millard J, Appleton H, Parry JV. Studies on heat inactivation of hepatitis A virus with special reference to shellfish. Part 1. Procedures for infection and recovery of virus from laboratory-maintained cockles. Epidemiol Infect. 1987;98(3):397-414.
51. Hoke CH, Jr., Binn LN, Egan JE, et al. Hepatitis A in the US Army: epidemiology and vaccine development. Vaccine. 1992;10(suppl 1):S75-S79.
52. Dooley DP. History of U.S. military contributions to the study of viral hepatitis. Mil Med. 2005;170(suppl 4):71-76.
53. Grabenstein JD, Pittman PR, Greenwood JT, Engler RJ. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28:3-26.
54. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e1475; quiz e17-e18.
55. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
56. Teeters JB, Lancaster CL, Brown DG, Back SE. Substance use disorders in military veterans: prevalence and treatment challenges. Subst Abuse Rehabil. 2017;8:69-77.
Ready for universal hepatitis C testing?
Poor asthma control during pregnancy trims live birth rates. Fluorouracil beats other actinic keratosis treatments in a head-to-head trial. And vitamin C for sepsis? Experts take sides in a sharp debate.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Poor asthma control during pregnancy trims live birth rates. Fluorouracil beats other actinic keratosis treatments in a head-to-head trial. And vitamin C for sepsis? Experts take sides in a sharp debate.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Poor asthma control during pregnancy trims live birth rates. Fluorouracil beats other actinic keratosis treatments in a head-to-head trial. And vitamin C for sepsis? Experts take sides in a sharp debate.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
HCV treatment with DAA regimens linked to reduced diabetes risk
SEATTLE – Treatment of hepatitis C virus (HCV) with new direct-acting antiviral (DAA) regimens is associated with improved glucose control and reduced incidence of type 2 diabetes when compared to treatment with pegylated interferon/ribavirin (PEG/RBV ) and untreated controls, according to a new analysis of the Electronically Retrieved Cohort of HCV Infected Veterans.
“Previously, people who had diabetes were considered slightly more difficult to treat because their virologic response was a little lower, but now this is not the case, and we have the added benefit of reducing the incidence of diabetes,” said Adeel Butt, MD, professor of medicine and health care policy and research at Weill Cornell Medicine, New York and Qatar, in an interview. Dr. Butt presented the study at the Conference on Retroviruses & Opportunistic Infections.
The incidence of diabetes dropped in the overall treated cohort, compared with untreated patients, but this benefit was driven by the effect of DAAs, as there was no significant difference between PEG/RBV–treated patients and controls. “It’s another reason to argue with people who make it difficult to treat. Our biggest barriers to treating everyone with hepatitis C has to do with reimbursement and the capacity of the health care system, and this is another reason that we need to overcome those barriers. It’s an important insight that provides one more reason to try to continue to eradicate hepatitis C in our population,” said Robert Schooley, MD, professor of medicine at the University of California, San Diego, in an interview.
Patients may also need some reassurance, given concerns that have arisen over the potential for older regimens to cause diabetes. Dr. Butt cited an example of a patient who has an acute myocardial infarction, has a high body mass, and wants to know if DAAs will help or hurt them. “We see [such patients] frequently. This is pretty reassuring not only that DAAs don’t increase risk, but they actually decrease the risk of diabetes as opposed to older treatments. There is a growing body of evidence that non–liver [related conditions] significantly improve with treatment,” he said.
The results could also help prioritize patients for treatment. “It may be important to the patients who are at elevated risk of developing diabetes. They may need to be monitored more closely and offered treatment earlier, perhaps, but that requires more study,” said Dr. Butt.
The researchers excluded patients with HIV or hepatitis B virus, and those who had prevalent diabetes. The cohort included 26,043 treated patients and 26,043 propensity score–matched untreated control patients. Treated patients underwent at least 8 weeks of DAA or 24 weeks of PEG/RBV. Demographically, 54% of patients were white, 29% were black, 3% were Hispanic, and 96% of the patients were male. About one-third had a body mass index of 30 or above.
The incidence of diabetes was 20.6 per 1,000 person-years of follow-up among untreated patients, compared with 15.5 among treated patients (P less than .0001). The incidence was 19.8 in patients treated with PEG/RBV (P =.39) and 9.9 in those treated with DAAs (P less than. 001; hazard ratio, 0.48; P less than .0001). The incidence of diabetes in those with a sustained viral response (SVR) was 13.3 per 1,000 person-years, compared with 19.2 in patients with no SVR (P less than .0001). The incidence of diabetes was lower in treated patients regardless of baseline FIB-4 (Fibrosis-4, a liver fibrosis score) levels.
The study was funded by Gilead. Dr. Butt has had research grants from Gilead and Dr. Schooley is on Gilead’s scientific advisory board.
SOURCE: A Butt et al. CROI 2019. Abstract 88.
SEATTLE – Treatment of hepatitis C virus (HCV) with new direct-acting antiviral (DAA) regimens is associated with improved glucose control and reduced incidence of type 2 diabetes when compared to treatment with pegylated interferon/ribavirin (PEG/RBV ) and untreated controls, according to a new analysis of the Electronically Retrieved Cohort of HCV Infected Veterans.
“Previously, people who had diabetes were considered slightly more difficult to treat because their virologic response was a little lower, but now this is not the case, and we have the added benefit of reducing the incidence of diabetes,” said Adeel Butt, MD, professor of medicine and health care policy and research at Weill Cornell Medicine, New York and Qatar, in an interview. Dr. Butt presented the study at the Conference on Retroviruses & Opportunistic Infections.
The incidence of diabetes dropped in the overall treated cohort, compared with untreated patients, but this benefit was driven by the effect of DAAs, as there was no significant difference between PEG/RBV–treated patients and controls. “It’s another reason to argue with people who make it difficult to treat. Our biggest barriers to treating everyone with hepatitis C has to do with reimbursement and the capacity of the health care system, and this is another reason that we need to overcome those barriers. It’s an important insight that provides one more reason to try to continue to eradicate hepatitis C in our population,” said Robert Schooley, MD, professor of medicine at the University of California, San Diego, in an interview.
Patients may also need some reassurance, given concerns that have arisen over the potential for older regimens to cause diabetes. Dr. Butt cited an example of a patient who has an acute myocardial infarction, has a high body mass, and wants to know if DAAs will help or hurt them. “We see [such patients] frequently. This is pretty reassuring not only that DAAs don’t increase risk, but they actually decrease the risk of diabetes as opposed to older treatments. There is a growing body of evidence that non–liver [related conditions] significantly improve with treatment,” he said.
The results could also help prioritize patients for treatment. “It may be important to the patients who are at elevated risk of developing diabetes. They may need to be monitored more closely and offered treatment earlier, perhaps, but that requires more study,” said Dr. Butt.
The researchers excluded patients with HIV or hepatitis B virus, and those who had prevalent diabetes. The cohort included 26,043 treated patients and 26,043 propensity score–matched untreated control patients. Treated patients underwent at least 8 weeks of DAA or 24 weeks of PEG/RBV. Demographically, 54% of patients were white, 29% were black, 3% were Hispanic, and 96% of the patients were male. About one-third had a body mass index of 30 or above.
The incidence of diabetes was 20.6 per 1,000 person-years of follow-up among untreated patients, compared with 15.5 among treated patients (P less than .0001). The incidence was 19.8 in patients treated with PEG/RBV (P =.39) and 9.9 in those treated with DAAs (P less than. 001; hazard ratio, 0.48; P less than .0001). The incidence of diabetes in those with a sustained viral response (SVR) was 13.3 per 1,000 person-years, compared with 19.2 in patients with no SVR (P less than .0001). The incidence of diabetes was lower in treated patients regardless of baseline FIB-4 (Fibrosis-4, a liver fibrosis score) levels.
The study was funded by Gilead. Dr. Butt has had research grants from Gilead and Dr. Schooley is on Gilead’s scientific advisory board.
SOURCE: A Butt et al. CROI 2019. Abstract 88.
SEATTLE – Treatment of hepatitis C virus (HCV) with new direct-acting antiviral (DAA) regimens is associated with improved glucose control and reduced incidence of type 2 diabetes when compared to treatment with pegylated interferon/ribavirin (PEG/RBV ) and untreated controls, according to a new analysis of the Electronically Retrieved Cohort of HCV Infected Veterans.
“Previously, people who had diabetes were considered slightly more difficult to treat because their virologic response was a little lower, but now this is not the case, and we have the added benefit of reducing the incidence of diabetes,” said Adeel Butt, MD, professor of medicine and health care policy and research at Weill Cornell Medicine, New York and Qatar, in an interview. Dr. Butt presented the study at the Conference on Retroviruses & Opportunistic Infections.
The incidence of diabetes dropped in the overall treated cohort, compared with untreated patients, but this benefit was driven by the effect of DAAs, as there was no significant difference between PEG/RBV–treated patients and controls. “It’s another reason to argue with people who make it difficult to treat. Our biggest barriers to treating everyone with hepatitis C has to do with reimbursement and the capacity of the health care system, and this is another reason that we need to overcome those barriers. It’s an important insight that provides one more reason to try to continue to eradicate hepatitis C in our population,” said Robert Schooley, MD, professor of medicine at the University of California, San Diego, in an interview.
Patients may also need some reassurance, given concerns that have arisen over the potential for older regimens to cause diabetes. Dr. Butt cited an example of a patient who has an acute myocardial infarction, has a high body mass, and wants to know if DAAs will help or hurt them. “We see [such patients] frequently. This is pretty reassuring not only that DAAs don’t increase risk, but they actually decrease the risk of diabetes as opposed to older treatments. There is a growing body of evidence that non–liver [related conditions] significantly improve with treatment,” he said.
The results could also help prioritize patients for treatment. “It may be important to the patients who are at elevated risk of developing diabetes. They may need to be monitored more closely and offered treatment earlier, perhaps, but that requires more study,” said Dr. Butt.
The researchers excluded patients with HIV or hepatitis B virus, and those who had prevalent diabetes. The cohort included 26,043 treated patients and 26,043 propensity score–matched untreated control patients. Treated patients underwent at least 8 weeks of DAA or 24 weeks of PEG/RBV. Demographically, 54% of patients were white, 29% were black, 3% were Hispanic, and 96% of the patients were male. About one-third had a body mass index of 30 or above.
The incidence of diabetes was 20.6 per 1,000 person-years of follow-up among untreated patients, compared with 15.5 among treated patients (P less than .0001). The incidence was 19.8 in patients treated with PEG/RBV (P =.39) and 9.9 in those treated with DAAs (P less than. 001; hazard ratio, 0.48; P less than .0001). The incidence of diabetes in those with a sustained viral response (SVR) was 13.3 per 1,000 person-years, compared with 19.2 in patients with no SVR (P less than .0001). The incidence of diabetes was lower in treated patients regardless of baseline FIB-4 (Fibrosis-4, a liver fibrosis score) levels.
The study was funded by Gilead. Dr. Butt has had research grants from Gilead and Dr. Schooley is on Gilead’s scientific advisory board.
SOURCE: A Butt et al. CROI 2019. Abstract 88.
REPORTING FROM CROI 2019
One-time, universal hepatitis C testing cost effective, researchers say
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY