User login
Celebrity death finally solved – with locks of hair
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.
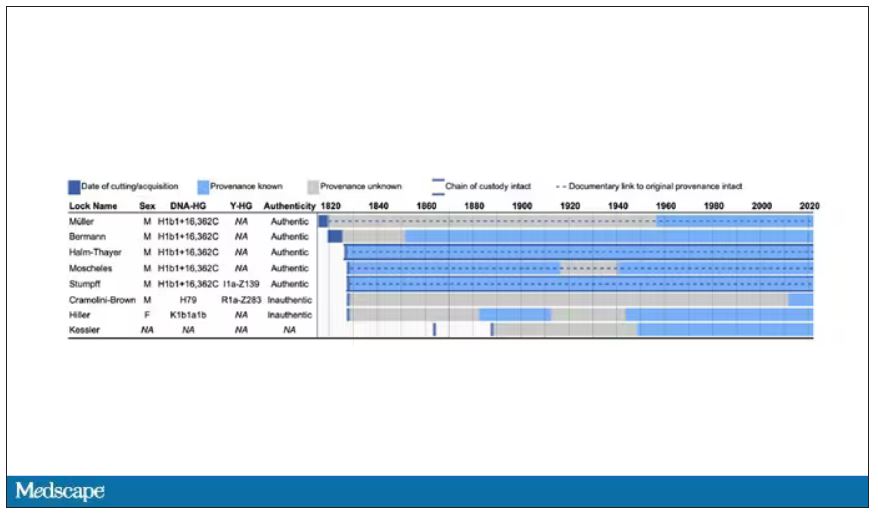
The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.
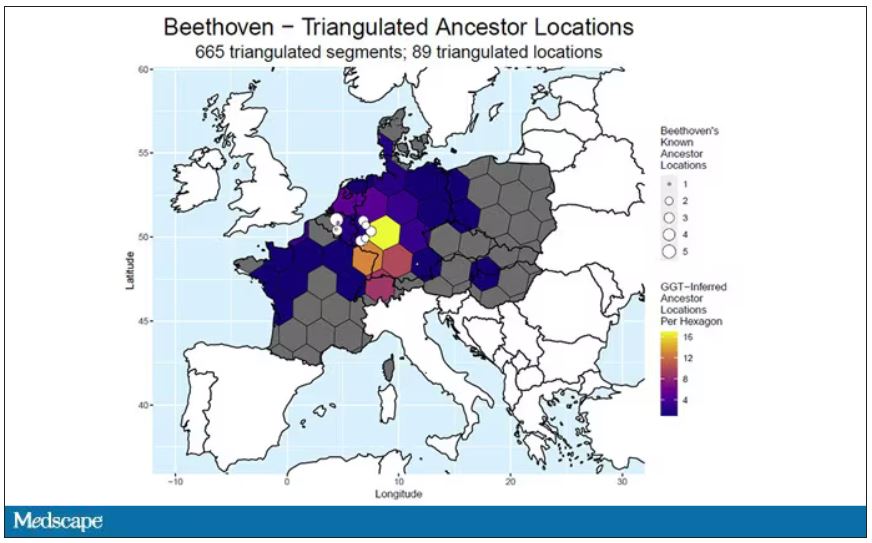
In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
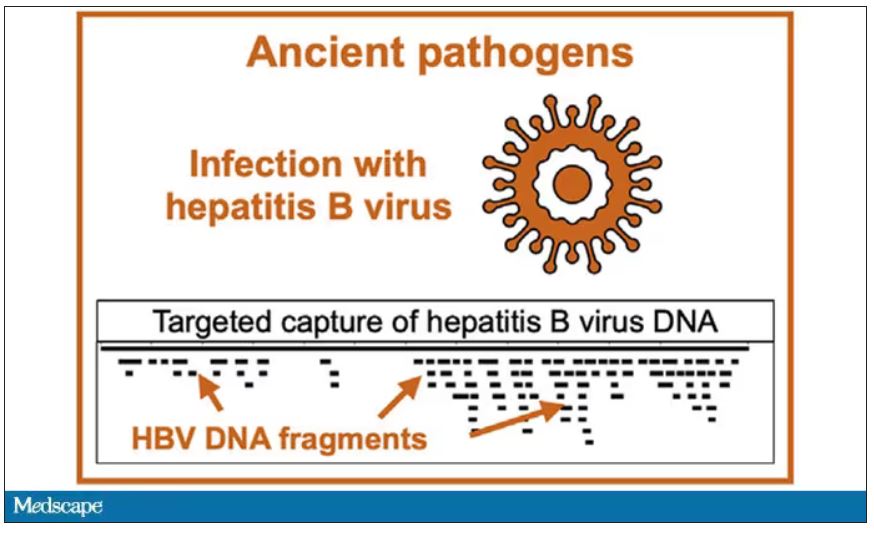
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.
It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.

The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.

In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.

It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.

The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.

In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.

It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
CDC recommends screening all adults for hepatitis B
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
NICU use up, birth weights down in babies of mothers with HCV
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
Infants born to women infected with the hepatitis C virus (HCV) faced twice the risk of stays in the neonatal ICU (NICU) and 2.7 times the risk of low birth weight, a new analysis finds, even when researchers adjusted their data to control for injectable drug use and maternal medical comorbidity.
Clinicians should be “aware that the infants of pregnant people with HCV may have a high rate of need for higher-level pediatric care,” said Brenna L. Hughes, MD, MSc, chief of maternal fetal medicine at Duke University Medical Center, Durham, N.C. She spoke in an interview about the findings, which were presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
As Dr. Hughes noted, “HCV remains a serious problem in pregnancy because it often goes undiagnosed and/or untreated prior to pregnancy. It can be passed to infants, and this can cause significant health-related outcomes for children as they age.”
For the multicenter U.S. study, researchers identified 249 pregnant mothers with HCV from a 2012-2018 cohort and matched them by gestational age to controls (n = 486). The average age was 28; 71.1% of the cases were non-Hispanic White versus 41.6% of the controls; 8.4% of cases were non-Hispanic Black versus 32.1% of controls (P < .001 for race/ethnicity analysis); and 73% of cases were smokers versus 18% of controls (P < .001). More than 19% of cases reported injectable drug use during pregnancy versus 0.2% of controls (P < .001).
The researchers adjusted their findings for maternal age, body mass index, injectable drug use, and maternal comorbidity.
An earlier analysis of the study data found that 6% of pregnant women with HCV passed it on to their infants, especially those with high levels of virus in their systems. For the new study, researchers focused on various outcomes to test the assumption that “adverse pregnancy outcomes associated with HCV are related to prematurity or to ongoing use of injection drugs,” Dr. Hughes said.
There was no increase in rates of preterm birth or adverse maternal outcomes in the HCV cases. However, infants born to women with HCV were more likely than the controls to require a stay in the NICU (45% vs. 19%; adjusted relative risk, 1.99; 95% confidence interval, 1.54-2.58). They were also more likely to have lower birth weights (small for gestational age < 5th percentile) (10.6% vs. 3.1%; ARR, 2.72; 95% CI, 1.38-5.34).
No difference in outcomes was seen when HCV cases with viremia (33%) were excluded.
“The most surprising finding was that the need for higher-level pediatric care was so high even though there wasn’t an increased risk of prematurity,” Dr. Hughes said.
She added it’s not clear why NICU stays and low birth weights were more common in infants of women with HCV. “It is possible that the higher risk of need for higher-level pediatric care was related to a need for observation or treatment due to use of opioid replacement therapies with opioid agonists.” As for lower birth weight, “there may be other unmeasured risk factors.”
Tatyana Kushner, MD, MSCE, of the division of liver diseases at Icahn School of Medicine at Mount Sinai, New York, said in an interview that the study adds to limited data about HCV in pregnancy. “These findings have been demonstrated in prior studies, and it would be important to tease apart whether [low birth weight] is related to the virus itself or more related to other confounding associated factors such as maternal substance use as well as other associated social determinants of health among women with HCV.”
As for the study’s message, Dr. Kushner said it makes it clear that “hepatitis C adversely impacts outcomes of pregnancy and it is important to identify women of childbearing age for treatment early, ideally prior to pregnancy, in order to improve their pregnancy outcomes. In addition, treatment of hepatitis C during pregnancy should be explored further to determine if treatment during pregnancy can improve outcomes.”
At the moment, she said, “there are ongoing studies to delineate the safety and efficacy of hepatitis C treatment during pregnancy. Given that we are screening for hepatitis C during pregnancy, we need clear recommendations on the use of direct-acting antivirals in people who screen positive.”
The study was funded by the National Institute of Child Health and Human Development. The authors have no disclosures. Dr. Kushner disclosed research support (Gilead) and advisory board service (Gilead, AbbVie, Bausch, GlaxoSmithKline, and Eiger).
FROM THE PREGNANCY MEETING
The five biggest changes in the 2023 adult vaccine schedules
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. I’m Dr Sandra Fryhofer. Welcome to Medicine Matters.
It’s a new year, which means a new ACIP adult immunization schedule – a valuable resource collating ACIP’s most up-to-date vaccination recommendations.
Here are this year’s five most important changes:
- COVID vaccines now front and center
- New emphasis on polio vaccination
- Inclusion of some nonvaccine products (such as monoclonal antibody products)
- Pharmacists group has approved the schedule for the first time
- New shared clinical decision-making option for pneumococcal vaccines
The schedule’s organization remains the same. It still has four sections:
- Table 1: vaccinations by age
- Table 2: vaccinations by medical condition and other indications
- The Notes section (alphabetically ordered by vaccine type)
- Appendix listing of vaccine-specific contraindications and precautions
But what’s unique this year is that some of the abbreviations have historical implications. The first change is no big surprise in light of what we’ve gone through in the past few years. COVID vaccines are listed first on the cover page by brand name for those authorized and by company name for those still under US emergency use authorization. They’re also listed first on the graphics and in the notes.
COVID and mRNA and protein-based vaccines have now been assigned official abbreviations based on vaccine platform and valency.
- 1vCOV-mRNA: Comirnaty/Pfizer-BioNTech and Spikevax Moderna COVID-19 vaccines
- 2vCOV-mRNA: Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines
- 1vCOV-aPS: Novavax COVID-19 vaccine
Also remarkable is the absence of COVID viral vector vaccines on the list. However, the viral vector COVID vaccine (which has been available but is not preferred) does have a CDC website link in the Notes section.
A sad but necessary inclusion was triggered by recent polio cases in New York. Polio was believed to be eradicated, and we thought adults no longer needed to be vaccinated against polio. In the new schedule, the polio vaccine is listed on the cover page but is not included in the tables. Current polio vaccination recommendations are now in the Notes section.
Also of historical significance and something that may set a precedent is the inclusion of nonvaccine products. The value of COVID preexposure prophylaxis with products including monoclonal antibodies (such as Evusheld) for people who are moderately or severely immunocompromised is mentioned in the Notes section.
For the first time ever, the schedule has been approved by the American Pharmacists Association, which validates pharmacists as established partners in vaccine administration.
Color-code key
One aspect of the schedule that has not changed is the color-code key:
- Yellow: Recommended if the patient meets the age requirement
- Purple: Indicated for those with additional risk factors or another indication
- Blue: Recommended based on shared clinical decision-making
- Orange: Precaution
- Red: Contraindicated or not recommended; the vaccine should not be administered. Overlays on the red more precisely clarify whether a vaccine is really contraindicated or just not recommended. An asterisk on red means vaccinate after pregnancy if indicated.
- Gray: No recommendation or not applicable
Vaccinations by age
Table 1 lists recommended vaccinations by age. There is one major change. COVID vaccines are on the first row of the graphic, with the need for both a primary series and boosters emphasized on the overlay. The notes have hyperlinks to the most up-to-date COVID vaccination recommendations.
Pneumococcal vaccination. Pneumococcal vaccination is routinely recommended starting at age 65. Current recommendations for those not previously vaccinated have not changed since last year. But on Table 1, the bottom half of the row for those 65 or older is now blue (and that’s new). This new color blue means shared clinical decision-making and applies to people who were previously considered fully vaccinated with the now extinct combination of PCV13 and PPSV23. These patients now have the option of getting a dose of PCV20 five years after completing their PCV13-PPSV23 combo series. This option is blue because the decision is up to you and your patient.
Check the notes for more pneumococcal vaccination details. For example, for those partially vaccinated using lower valency vaccines, there’s an option of substituting PCV20 for PPSV23 to broaden and increase durability of protection.
The pneumococcal vaccination recommendation options are complicated. A new pneumococcal vaccination app can help.
Hepatitis B. For adults under age 60, the color code for the hepatitis B vaccine is yellow, meaning it’s indicated for all. For older patients, the color code is purple. If a patient who is age 60 or older wants the hepatitis B vaccine, they can have it even in the absence of additional risk indications.
Vaccinations by medical condition or other indications
Other than a few minor word changes on the overlay, the only thing that’s new is the COVID vaccine row.
This table is helpful for matching vaccine recommendations with specific medical conditions, including pregnancy, immunocompromise, HIV (with specifics according to CD4 count), asplenia, complement deficiencies, heart disease, lung disease, alcoholism, chronic liver disease, diabetes, health care personnel, and men who have sex with men.
Use this table to dot the i’s and cross the t’s when it comes to vaccination recommendations. For example, take a look at the pregnancy column. Live virus vaccines, including LAIV, MMR, and varicella, are contraindicated and color-coded red. MMR and varicella also have an asterisk, meaning vaccinate after pregnancy if indicated. HPV vaccines are not live virus vaccines, but the overlay says they are not recommended during pregnancy. The asterisk indicates that you can vaccinate after pregnancy.
Vaccine notes
The notes are in alphabetical order, and their organization (routine, special situations, and shared clinical decision-making when indicated) has not changed. They are concise and succinct, but sometimes they’re not enough. That’s why vaccine-specific links to more complete recommendations are so convenient.
Notes for hepatitis B contain nuances on specific dosing for vaccinating patients on dialysis, as well as a reminder that newer hepatitis C vaccines such as Heplisav and PreHevbrio are not recommended during pregnancy due to lack of safety data.
For influenza, everyone 6 months or older still needs yearly flu vaccination with an age- and health-appropriate flu vaccine. But for those aged 65 or older, the notes specify the three vaccine versions now preferred: high-dose, recombinant, or adjuvanted versions. However, if these aren’t available, it’s better to get any flu vaccine than to go without.
Under meningococcal vaccines, the notes for MenACWY and MenB are combined. For MenB, trade names Bexsero and Trumenba are specified because the products are not interchangeable. Booster intervals for those still at risk are different for each vaccine type: every 5 years for MenACWY boosters, and every 2-3 years for boosts of MenB.
The recent polio cases in New York have put polio vaccination in the spotlight. ACIP has now reinstated its Polio Vaccine Work Group. The new schedule lists polio vaccines on the cover page. Current recommendations have been added to the notes section. Routine vaccination for adults is not necessary, at least for now. However, those at increased risk for exposure to polio fall in the special-situation category. For those at increased risk who have completed a polio vaccine series, a single lifetime IPV booster can be given. For those at increased risk who have not completed their polio vaccine series, now would be the time to finish the series.
Appendix
The final step in using the new schedule is checking the appendix and its list of vaccine-specific contraindications and precautions.
I hope this review of the new ACIP adult immunization schedule has been helpful. For Medicine Matters, I’m Dr. Sandra Fryhofer.
Dr. Fryhofer is clinical associate professor of medicine, Emory University, Atlanta. She reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
Strong support to provide DAA therapy to all patients with HCV
, a large, real-world analysis finds.
Improved outcomes were seen among patients without cirrhosis, those with compensated cirrhosis, and those with existing liver decompensation, the authors noted.
The findings highlight a “substantial need to provide DAA therapy to all patients with HCV, regardless of disease stage or financial status,” wrote Mindie Nguyen, MD, of Stanford University Medical Center, Palo Alto, Calif., and coinvestigators.
“Additional national efforts are needed to reach and treat U.S. population groups that are underinsured or not insured, incarcerated and otherwise marginalized, such as users of illicit drugs, who are also at higher risk of disease complication and reinfection,” they said.
The study was published online in JAMA Internal Medicine.
CHC and its complications are associated with high rates of illness and death. However, large-scale data on long-term liver and nonliver effects of DAA treatment are limited.
For their study, Dr. Nguyen and colleagues analyzed administrative claims data from 2010 to 2021 for 245,596 adults with CHC, of whom 40,654 had received one or more DAA therapies (without interferon) and 204,942 had not received treatment.
DAA-treated patients were slightly older than their untreated peers (mean age, 59.9 years, vs. 58.5 years) and were more likely to be male (62% vs. 58%) and White (59% vs. 57%), and to have diabetes (26% vs. 25%) and cirrhosis (44% vs. 29%).
For liver outcomes, DAA therapy was associated with a lower incidence of decompensation (28.2 vs. 40.8 per 1,000 person-years; P < .001) and hepatocellular carcinoma (HCC) in compensated cirrhosis (20.1 vs. 41.8; P < .001).
For nonliver outcomes, DAA treatment was associated with a lower incidence of diabetes (30.2 vs. 37.2 per 1,000 person-years; P < .001) and chronic kidney disease (31.1 vs. 34.1; P < .001).
The all-cause mortality rate per 1,000 person-years was 36.5 in the DAA-treated group, vs. 64.7 in the untreated group (P < .001).
In multivariable regression analysis, DAA treatment was independently associated with a significant decrease in the risk for HCC (adjusted hazard ratio [aHR], 0.73), decompensation (aHR, 0.36), diabetes (aHR, 0.74), chronic kidney disease (aHR, 0.81), cardiovascular disease (aHR, 0.90), nonliver cancer (aHR, 0.89), and mortality (aHR, 0.43).
The 57% lower mortality rate observed among DAA-treated vs. untreated patients aligns with a large French study of adults with CHC.
“Because HCV treatment with a DAA regimen is well tolerated for nearly all patients, we believe these findings provide further support for universal HCV treatment coverage for all patients affected by HCV,” Dr. Nguyen and colleagues wrote.
The strengths of this study are its large sample of DAA-treated and untreated patients from diverse racial and ethnic groups from across the United States and from diverse practice settings (not just tertiary centers).
One limitation is that the study cohort included only patients covered by private insurance; therefore, the findings may not be generalizable to individuals who are underinsured or not insured. Miscoding and misclassification are also possible with large claims databases.
Support for the study was provided by Stanford University and the Stanford Center for Population Health Sciences. Dr. Nguyen has received institutional grants and advisory board fees from Gilead Sciences outside the submitted work.
A version of this article first appeared on Medscape.com.
, a large, real-world analysis finds.
Improved outcomes were seen among patients without cirrhosis, those with compensated cirrhosis, and those with existing liver decompensation, the authors noted.
The findings highlight a “substantial need to provide DAA therapy to all patients with HCV, regardless of disease stage or financial status,” wrote Mindie Nguyen, MD, of Stanford University Medical Center, Palo Alto, Calif., and coinvestigators.
“Additional national efforts are needed to reach and treat U.S. population groups that are underinsured or not insured, incarcerated and otherwise marginalized, such as users of illicit drugs, who are also at higher risk of disease complication and reinfection,” they said.
The study was published online in JAMA Internal Medicine.
CHC and its complications are associated with high rates of illness and death. However, large-scale data on long-term liver and nonliver effects of DAA treatment are limited.
For their study, Dr. Nguyen and colleagues analyzed administrative claims data from 2010 to 2021 for 245,596 adults with CHC, of whom 40,654 had received one or more DAA therapies (without interferon) and 204,942 had not received treatment.
DAA-treated patients were slightly older than their untreated peers (mean age, 59.9 years, vs. 58.5 years) and were more likely to be male (62% vs. 58%) and White (59% vs. 57%), and to have diabetes (26% vs. 25%) and cirrhosis (44% vs. 29%).
For liver outcomes, DAA therapy was associated with a lower incidence of decompensation (28.2 vs. 40.8 per 1,000 person-years; P < .001) and hepatocellular carcinoma (HCC) in compensated cirrhosis (20.1 vs. 41.8; P < .001).
For nonliver outcomes, DAA treatment was associated with a lower incidence of diabetes (30.2 vs. 37.2 per 1,000 person-years; P < .001) and chronic kidney disease (31.1 vs. 34.1; P < .001).
The all-cause mortality rate per 1,000 person-years was 36.5 in the DAA-treated group, vs. 64.7 in the untreated group (P < .001).
In multivariable regression analysis, DAA treatment was independently associated with a significant decrease in the risk for HCC (adjusted hazard ratio [aHR], 0.73), decompensation (aHR, 0.36), diabetes (aHR, 0.74), chronic kidney disease (aHR, 0.81), cardiovascular disease (aHR, 0.90), nonliver cancer (aHR, 0.89), and mortality (aHR, 0.43).
The 57% lower mortality rate observed among DAA-treated vs. untreated patients aligns with a large French study of adults with CHC.
“Because HCV treatment with a DAA regimen is well tolerated for nearly all patients, we believe these findings provide further support for universal HCV treatment coverage for all patients affected by HCV,” Dr. Nguyen and colleagues wrote.
The strengths of this study are its large sample of DAA-treated and untreated patients from diverse racial and ethnic groups from across the United States and from diverse practice settings (not just tertiary centers).
One limitation is that the study cohort included only patients covered by private insurance; therefore, the findings may not be generalizable to individuals who are underinsured or not insured. Miscoding and misclassification are also possible with large claims databases.
Support for the study was provided by Stanford University and the Stanford Center for Population Health Sciences. Dr. Nguyen has received institutional grants and advisory board fees from Gilead Sciences outside the submitted work.
A version of this article first appeared on Medscape.com.
, a large, real-world analysis finds.
Improved outcomes were seen among patients without cirrhosis, those with compensated cirrhosis, and those with existing liver decompensation, the authors noted.
The findings highlight a “substantial need to provide DAA therapy to all patients with HCV, regardless of disease stage or financial status,” wrote Mindie Nguyen, MD, of Stanford University Medical Center, Palo Alto, Calif., and coinvestigators.
“Additional national efforts are needed to reach and treat U.S. population groups that are underinsured or not insured, incarcerated and otherwise marginalized, such as users of illicit drugs, who are also at higher risk of disease complication and reinfection,” they said.
The study was published online in JAMA Internal Medicine.
CHC and its complications are associated with high rates of illness and death. However, large-scale data on long-term liver and nonliver effects of DAA treatment are limited.
For their study, Dr. Nguyen and colleagues analyzed administrative claims data from 2010 to 2021 for 245,596 adults with CHC, of whom 40,654 had received one or more DAA therapies (without interferon) and 204,942 had not received treatment.
DAA-treated patients were slightly older than their untreated peers (mean age, 59.9 years, vs. 58.5 years) and were more likely to be male (62% vs. 58%) and White (59% vs. 57%), and to have diabetes (26% vs. 25%) and cirrhosis (44% vs. 29%).
For liver outcomes, DAA therapy was associated with a lower incidence of decompensation (28.2 vs. 40.8 per 1,000 person-years; P < .001) and hepatocellular carcinoma (HCC) in compensated cirrhosis (20.1 vs. 41.8; P < .001).
For nonliver outcomes, DAA treatment was associated with a lower incidence of diabetes (30.2 vs. 37.2 per 1,000 person-years; P < .001) and chronic kidney disease (31.1 vs. 34.1; P < .001).
The all-cause mortality rate per 1,000 person-years was 36.5 in the DAA-treated group, vs. 64.7 in the untreated group (P < .001).
In multivariable regression analysis, DAA treatment was independently associated with a significant decrease in the risk for HCC (adjusted hazard ratio [aHR], 0.73), decompensation (aHR, 0.36), diabetes (aHR, 0.74), chronic kidney disease (aHR, 0.81), cardiovascular disease (aHR, 0.90), nonliver cancer (aHR, 0.89), and mortality (aHR, 0.43).
The 57% lower mortality rate observed among DAA-treated vs. untreated patients aligns with a large French study of adults with CHC.
“Because HCV treatment with a DAA regimen is well tolerated for nearly all patients, we believe these findings provide further support for universal HCV treatment coverage for all patients affected by HCV,” Dr. Nguyen and colleagues wrote.
The strengths of this study are its large sample of DAA-treated and untreated patients from diverse racial and ethnic groups from across the United States and from diverse practice settings (not just tertiary centers).
One limitation is that the study cohort included only patients covered by private insurance; therefore, the findings may not be generalizable to individuals who are underinsured or not insured. Miscoding and misclassification are also possible with large claims databases.
Support for the study was provided by Stanford University and the Stanford Center for Population Health Sciences. Dr. Nguyen has received institutional grants and advisory board fees from Gilead Sciences outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
Direct-acting antivirals tied to better outcomes in chronic Hep C
Eiichi Ogawa, MD, PhD, with the department of general internal medicine, Kyushu University Hospital in Fukuoka, Japan, led the retrospective study of 245,596 adults with CHC. In the new research, which was published in JAMA Internal Medicine, the authors analyzed data from the Optum Clinformatics Data Mart (CDM) database, 2010-2021.
It was important to do the study because of limited and conflicting information – mostly from case reports – on safety of the DAAs when they were approved for CHC in 2014, said coauthor Mindie H. Nguyen, MD, in an interview.
‘DAA treatment is safe’
“The main message is that DAA treatment is safe,” said Dr. Nguyen, of the division of gastroenterology and hepatology at Stanford (Calif.) University Medical Center in Palo Alto. In the early days of treatment, physicians were treating the sickest patients with the DAAs, which may have introduced patient selection bias and caused lasting misperceptions about poor safety, she noted.
“I really hope to dispel this myth,” she said, adding that this study also shows improved liver and nonliver outcomes.
Of the total cohort in this study, 40,654 patients had one or more prescriptions for a DAA (without interferon) and 204,942 patients had not been treated.
All-cause mortality reduced by 57%
DAA treatment, vs. no treatment, was linked with a large and significant reduction (57%) in all-cause mortality. That finding was particularly notable, because it was seen regardless of age, sex, race and ethnicity, comorbidities, alcohol use, and presence of hepatocellular carcinoma or cirrhosis.
The authors noted that patients without cirrhosis are a population previously considered to receive less benefit from an HCV cure than patients with cirrhosis.
DAAs were associated with lower risk of hepatocellular carcinoma and decompensation as well as risk of nonliver outcomes, including diabetes, cardiovascular disease (CVD), and chronic kidney disease (CKD).
Lower risk of poor nonliver outcomes
The researchers found that when they compared DAA-treated patients with untreated patients, the incidences per 1,000 person-years of having diabetes were 30.2 vs. 37.2 (P less than .001), and of having kidney disease was 31.1 vs. 34.1 (P less than .001), respectively.
“This retrospective cohort study provides valuable information to physicians,” Noel Deep, MD, chief medical officer at Aspirus Langlade Hospital in Antigo, Wis., said, in an interview.
The study’s size helps confirm DAAs’ safety and benefit, and previously unknown added benefits, in treating CHC, he continued.
Large study confirms, introduces DAA benefits
Dr. Deep, who was not part of the study, noted that DAAs now show much promise in efficacy and tolerability in most people with chronic hepatitis C, including those with concomitant conditions such as CKD.
“Previous studies did not have such large-scale nationwide data. [The findings of the new study] greatly enhance our knowledge of DAA treatment for chronic hepatitis C patients across the spectrum from noncirrhotic to compensated cirrhotic to decompensated cirrhotic,” Dr. Deep said. “The added benefit of improved outcomes for diabetes, CVD, CKD, and nonliver cancers truly surprised me.”
Dr. Deep pointed out some limitations of the study, including that, as the authors acknowledge, only privately insured patients were included so results may not be generalizable to the underinsured/uninsured “who might have other risk factors, poorer health, and fewer resources.”
He added: “The data also may not be reflective of the outcomes in Asians who were, in my opinion, also underrepresented in this study.”
The authors cited the insurance claims database they used as a strength of the study, due to it containing information on 61 million people from across all regions of the United States.
Dr. Ogawa reports grants from Gilead Sciences outside the submitted work. Coauthor Dr. Nguyen reports institutional grants and advisory board fees from Gilead Sciences outside the submitted work. Another coauthor reports speaking/consulting fees from Gilead and Merck Sharp & Dohme outside the submitted work. No other disclosures were reported.
The Stanford Center for Population Health Sciences (PHS) supported this study by providing access to the PHS Data Core.
Dr. Deep reports no relevant financial relationships. He serves on the editorial advisory board of Internal Medicine News.
Eiichi Ogawa, MD, PhD, with the department of general internal medicine, Kyushu University Hospital in Fukuoka, Japan, led the retrospective study of 245,596 adults with CHC. In the new research, which was published in JAMA Internal Medicine, the authors analyzed data from the Optum Clinformatics Data Mart (CDM) database, 2010-2021.
It was important to do the study because of limited and conflicting information – mostly from case reports – on safety of the DAAs when they were approved for CHC in 2014, said coauthor Mindie H. Nguyen, MD, in an interview.
‘DAA treatment is safe’
“The main message is that DAA treatment is safe,” said Dr. Nguyen, of the division of gastroenterology and hepatology at Stanford (Calif.) University Medical Center in Palo Alto. In the early days of treatment, physicians were treating the sickest patients with the DAAs, which may have introduced patient selection bias and caused lasting misperceptions about poor safety, she noted.
“I really hope to dispel this myth,” she said, adding that this study also shows improved liver and nonliver outcomes.
Of the total cohort in this study, 40,654 patients had one or more prescriptions for a DAA (without interferon) and 204,942 patients had not been treated.
All-cause mortality reduced by 57%
DAA treatment, vs. no treatment, was linked with a large and significant reduction (57%) in all-cause mortality. That finding was particularly notable, because it was seen regardless of age, sex, race and ethnicity, comorbidities, alcohol use, and presence of hepatocellular carcinoma or cirrhosis.
The authors noted that patients without cirrhosis are a population previously considered to receive less benefit from an HCV cure than patients with cirrhosis.
DAAs were associated with lower risk of hepatocellular carcinoma and decompensation as well as risk of nonliver outcomes, including diabetes, cardiovascular disease (CVD), and chronic kidney disease (CKD).
Lower risk of poor nonliver outcomes
The researchers found that when they compared DAA-treated patients with untreated patients, the incidences per 1,000 person-years of having diabetes were 30.2 vs. 37.2 (P less than .001), and of having kidney disease was 31.1 vs. 34.1 (P less than .001), respectively.
“This retrospective cohort study provides valuable information to physicians,” Noel Deep, MD, chief medical officer at Aspirus Langlade Hospital in Antigo, Wis., said, in an interview.
The study’s size helps confirm DAAs’ safety and benefit, and previously unknown added benefits, in treating CHC, he continued.
Large study confirms, introduces DAA benefits
Dr. Deep, who was not part of the study, noted that DAAs now show much promise in efficacy and tolerability in most people with chronic hepatitis C, including those with concomitant conditions such as CKD.
“Previous studies did not have such large-scale nationwide data. [The findings of the new study] greatly enhance our knowledge of DAA treatment for chronic hepatitis C patients across the spectrum from noncirrhotic to compensated cirrhotic to decompensated cirrhotic,” Dr. Deep said. “The added benefit of improved outcomes for diabetes, CVD, CKD, and nonliver cancers truly surprised me.”
Dr. Deep pointed out some limitations of the study, including that, as the authors acknowledge, only privately insured patients were included so results may not be generalizable to the underinsured/uninsured “who might have other risk factors, poorer health, and fewer resources.”
He added: “The data also may not be reflective of the outcomes in Asians who were, in my opinion, also underrepresented in this study.”
The authors cited the insurance claims database they used as a strength of the study, due to it containing information on 61 million people from across all regions of the United States.
Dr. Ogawa reports grants from Gilead Sciences outside the submitted work. Coauthor Dr. Nguyen reports institutional grants and advisory board fees from Gilead Sciences outside the submitted work. Another coauthor reports speaking/consulting fees from Gilead and Merck Sharp & Dohme outside the submitted work. No other disclosures were reported.
The Stanford Center for Population Health Sciences (PHS) supported this study by providing access to the PHS Data Core.
Dr. Deep reports no relevant financial relationships. He serves on the editorial advisory board of Internal Medicine News.
Eiichi Ogawa, MD, PhD, with the department of general internal medicine, Kyushu University Hospital in Fukuoka, Japan, led the retrospective study of 245,596 adults with CHC. In the new research, which was published in JAMA Internal Medicine, the authors analyzed data from the Optum Clinformatics Data Mart (CDM) database, 2010-2021.
It was important to do the study because of limited and conflicting information – mostly from case reports – on safety of the DAAs when they were approved for CHC in 2014, said coauthor Mindie H. Nguyen, MD, in an interview.
‘DAA treatment is safe’
“The main message is that DAA treatment is safe,” said Dr. Nguyen, of the division of gastroenterology and hepatology at Stanford (Calif.) University Medical Center in Palo Alto. In the early days of treatment, physicians were treating the sickest patients with the DAAs, which may have introduced patient selection bias and caused lasting misperceptions about poor safety, she noted.
“I really hope to dispel this myth,” she said, adding that this study also shows improved liver and nonliver outcomes.
Of the total cohort in this study, 40,654 patients had one or more prescriptions for a DAA (without interferon) and 204,942 patients had not been treated.
All-cause mortality reduced by 57%
DAA treatment, vs. no treatment, was linked with a large and significant reduction (57%) in all-cause mortality. That finding was particularly notable, because it was seen regardless of age, sex, race and ethnicity, comorbidities, alcohol use, and presence of hepatocellular carcinoma or cirrhosis.
The authors noted that patients without cirrhosis are a population previously considered to receive less benefit from an HCV cure than patients with cirrhosis.
DAAs were associated with lower risk of hepatocellular carcinoma and decompensation as well as risk of nonliver outcomes, including diabetes, cardiovascular disease (CVD), and chronic kidney disease (CKD).
Lower risk of poor nonliver outcomes
The researchers found that when they compared DAA-treated patients with untreated patients, the incidences per 1,000 person-years of having diabetes were 30.2 vs. 37.2 (P less than .001), and of having kidney disease was 31.1 vs. 34.1 (P less than .001), respectively.
“This retrospective cohort study provides valuable information to physicians,” Noel Deep, MD, chief medical officer at Aspirus Langlade Hospital in Antigo, Wis., said, in an interview.
The study’s size helps confirm DAAs’ safety and benefit, and previously unknown added benefits, in treating CHC, he continued.
Large study confirms, introduces DAA benefits
Dr. Deep, who was not part of the study, noted that DAAs now show much promise in efficacy and tolerability in most people with chronic hepatitis C, including those with concomitant conditions such as CKD.
“Previous studies did not have such large-scale nationwide data. [The findings of the new study] greatly enhance our knowledge of DAA treatment for chronic hepatitis C patients across the spectrum from noncirrhotic to compensated cirrhotic to decompensated cirrhotic,” Dr. Deep said. “The added benefit of improved outcomes for diabetes, CVD, CKD, and nonliver cancers truly surprised me.”
Dr. Deep pointed out some limitations of the study, including that, as the authors acknowledge, only privately insured patients were included so results may not be generalizable to the underinsured/uninsured “who might have other risk factors, poorer health, and fewer resources.”
He added: “The data also may not be reflective of the outcomes in Asians who were, in my opinion, also underrepresented in this study.”
The authors cited the insurance claims database they used as a strength of the study, due to it containing information on 61 million people from across all regions of the United States.
Dr. Ogawa reports grants from Gilead Sciences outside the submitted work. Coauthor Dr. Nguyen reports institutional grants and advisory board fees from Gilead Sciences outside the submitted work. Another coauthor reports speaking/consulting fees from Gilead and Merck Sharp & Dohme outside the submitted work. No other disclosures were reported.
The Stanford Center for Population Health Sciences (PHS) supported this study by providing access to the PHS Data Core.
Dr. Deep reports no relevant financial relationships. He serves on the editorial advisory board of Internal Medicine News.
FROM JAMA INTERNAL MEDICINE
A bold national plan to eliminate HCV by 2050
WASHINGTON – “We don’t get to use the ‘eliminate’ word all that often with a disease that’s taking thousands or tens of thousands – or worldwide, hundreds of thousands – of lives every year, but we have that opportunity with hepatitis C.”
So said Francis S. Collins, MD, PhD, special projects advisor to the Executive Office of the President of the United States, and former director of the National Institutes of Health, speaking at a special session outlining ambitious goals for a national plan to eliminate hepatitis C virus (HCV) infections by the year 2050.
The session was held at the annual meeting of the American Association for the Study of Liver Diseases.
A public health crisis
Dr. Collins labeled HCV a public health crisis, citing statistics from the Centers for Disease Control and Prevention that show that the rate of reported acute HCV infection cases increased 400% between 2010 and 2020, with the highest rates among young adults aged 20-39 years.
In addition, an estimated 2.4 million people in the United States are living with chronic HCV infections, but as many as 40% of these people are unaware of their infection, despite broad recommendations for the screening of all adults aged 18 years and older, he said.
“Our goal is to try to do something to change this,” Dr. Collins said. He noted that for the past 8 years we have had highly effective oral agents that don’t just treat the disease but cure it – 95%-97% of the time, with only 8-12 weeks of oral therapy and relatively few side effects.
“A wonderful story, one of the most exciting stories that’s come out of biomedical research in the last couple of decades,” he said.
Yet Dr. Collins also acknowledged that the task of developing a national plan is daunting, despite that pharmaceutical triumph.
National pharmacy claims data show that the number of persons treated for HCV with direct-acting antiviral agents (DAAs) in the United States declined from a high of 164,247 in 2015 to 83,740 in 2020.
Furthermore, CDC data from 2019 and 2020 show that, of persons with a diagnosis of HCV infection, only 23% of those on Medicaid, 28% of those on Medicare, and 35% of those with private insurance were treated for their infections.
“We have a huge gap here between the ability to know you have the disease and to get treatment, and we don’t see the numbers here for the uninsured, or people in prisons, but they’re probably much worse,” he said.
Obstacles abound, as do ways to overcome them
Current barriers to treatment include the aforementioned lack of awareness of infection, a “clunky” two-step diagnosis requiring an antibody test followed by an RNA or core antigen test necessitating three visits often separated by several weeks, and the high cost of treatment (around $90,000 per patient).
In addition, insurers commonly require proof that patients remain sober for extended periods, insist that treatment monitoring be performed by specialists only, and often approve treatment only for those patients who have documented evidence of liver damage.
“Does that make sense to you?” Dr. Collins asked. “You’ve got a cure for a liver disease, and you have to wait and show that the liver’s been damaged before you receive it? That just doesn’t fit,” he said.
Dr. Collins also pointed out that we’re dealing with hard-to-reach populations (underserved, uninsured, justice-involved), and people who are in tough times. “Anything that you put in the way as a barrier is going to make this worse in terms of its ability to be implemented,” he said.
To demonstrate how a coordinated HCV-elimination program could work, Dr. Collins pointed to a Medicaid cohort study in Louisiana conducted from July 2019 through December 2021, in which 8,867 patients started on therapy, 7,763 (88%) completed therapy, and 5,882 (66%) returned for testing. Of those tested, 5,285 (90%) had sustained virologic responses.
Another model of a hepatitis C elimination program was provided by the Veterans Health Administration. They received funding for an effort for all veterans, and in the space of 7 years were able to reach out even to some of their difficult-to-reach populations and achieve high diagnosis and treatment rates in a way that could be a model for what we would want to do across the nation, Dr. Collins noted.
Doing the math
Also at the session, Jagpreet Chhatwal, PhD, director of the Massachusetts General Hospital Institute for Technology Assessment and associate professor of radiology at Harvard Medical School, Boston, described outcomes projected by a mathematical simulation model of the HCV epidemic that he and his colleagues developed.
The HEP-SIM (Hepatitis C Disease Burden Simulation) model evaluates HCV prevalence trends, the number needed to screen and treat to eliminate HCV, HCV-associated clinical outcomes, the cost of an elimination program, and the cost savings that could be realized from preventing long-term complications.
The model seeks to determine whether the upfront costs of a national HCV elimination program could be offset by savings down the road. Specifically, it assumes that within the next 5 years 1.31 million individuals would be diagnosed with HCV and projects that within that time frame 1.52 million would need to be treated to meet HCV elimination goals.
The model shows that, compared with the status quo, a concerted campaign of screening and treatment would prevent more than 10,000 HCV-related deaths by 2030, and 91,000 deaths by 2050.
A coordinated screening program is also projected to prevent 17,000 cases of hepatocellular carcinoma by 2030 and 108,000 cases by 2050, as well as avert 29,000 cases of decompensated cirrhosis by 2030 and 93,000 such cases by 2050.
The cost savings associated with an HCV elimination plan would also be substantial, Dr. Chhatwal said.
According to the model, over the next decade the cumulative costs associated with HCV would decline by $14.2 billion, compared with the status quo. Nearly 80% of those savings ($11.2 billion) would be in Medicare and Medicaid.
The total projected savings from 2024 through 2050 – in disease management, testing, treatment, and pragmatic costs – are estimated at $59.3 billion, Dr. Chhatwal said.
“This is unprecedented,” he said.
Getting it done
Rachael L. Fleurence, PhD, MSc, a health economist currently serving as a senior advisor in the Executive Office of the President, summarized efforts to build a national HCV elimination program with input from federal health care agencies, state health leaders, patients, advocacy groups, drug manufacturers, and insurers.
She noted that a large component and focus of the program will be working on diagnostic test development but also accelerating bringing tests into the United States that are currently unavailable here. “These include point-of-care RNA diagnostic tests, as well as core antigen laboratory tests,” she said.
The program will be designed to offer broad access to curative anti-HCV drugs through a national subscription model that would make DAAs available to Medicaid recipients, justice-involved populations, the uninsured, and American Indians and Alaskan Natives who receive care through the Indian Health Service.
“On the Medicare and commercial insurance fronts, we’re still exploring different approaches, including potentially a co-pay assistance for Medicare beneficiaries, as well as working with commercial insurers to reduce barriers to access,” she said.
The program would also involve screening strategies extending to more settings, especially for high-risk populations, expanding the number of providers allowed to screen and treat HCV infections through telehealth, ensuring incentives for providers, and increasing the number of community health workers and case workers to improve linkage to care.
The next steps for the program would include funding to support the NIH’s RADx diagnostics program to accelerate access to testing, planning for the subscription model for DAA purchase, and launching pilot programs with the CDC, the Health Resources and Services Administration, the Substance Abuse and Mental Health Services Administration, and the Indian Health Service.
A call to action
Dr. Collins ended this portion of the program with an exhortation to AASLD members to do their part.
“We need your help,” Dr. Collins said. “This is a bold initiative, but it’s an opportunity. It’s even a responsibility. If we can actually succeed at this kind of outreach and save lives, and at the same time save money, how can we not do that?”
Dr. Collins, Dr. Chhatwal, and Dr. Fleurence each reported having no financial conflicts.
A version of this article first appeared on Medscape.com.
WASHINGTON – “We don’t get to use the ‘eliminate’ word all that often with a disease that’s taking thousands or tens of thousands – or worldwide, hundreds of thousands – of lives every year, but we have that opportunity with hepatitis C.”
So said Francis S. Collins, MD, PhD, special projects advisor to the Executive Office of the President of the United States, and former director of the National Institutes of Health, speaking at a special session outlining ambitious goals for a national plan to eliminate hepatitis C virus (HCV) infections by the year 2050.
The session was held at the annual meeting of the American Association for the Study of Liver Diseases.
A public health crisis
Dr. Collins labeled HCV a public health crisis, citing statistics from the Centers for Disease Control and Prevention that show that the rate of reported acute HCV infection cases increased 400% between 2010 and 2020, with the highest rates among young adults aged 20-39 years.
In addition, an estimated 2.4 million people in the United States are living with chronic HCV infections, but as many as 40% of these people are unaware of their infection, despite broad recommendations for the screening of all adults aged 18 years and older, he said.
“Our goal is to try to do something to change this,” Dr. Collins said. He noted that for the past 8 years we have had highly effective oral agents that don’t just treat the disease but cure it – 95%-97% of the time, with only 8-12 weeks of oral therapy and relatively few side effects.
“A wonderful story, one of the most exciting stories that’s come out of biomedical research in the last couple of decades,” he said.
Yet Dr. Collins also acknowledged that the task of developing a national plan is daunting, despite that pharmaceutical triumph.
National pharmacy claims data show that the number of persons treated for HCV with direct-acting antiviral agents (DAAs) in the United States declined from a high of 164,247 in 2015 to 83,740 in 2020.
Furthermore, CDC data from 2019 and 2020 show that, of persons with a diagnosis of HCV infection, only 23% of those on Medicaid, 28% of those on Medicare, and 35% of those with private insurance were treated for their infections.
“We have a huge gap here between the ability to know you have the disease and to get treatment, and we don’t see the numbers here for the uninsured, or people in prisons, but they’re probably much worse,” he said.
Obstacles abound, as do ways to overcome them
Current barriers to treatment include the aforementioned lack of awareness of infection, a “clunky” two-step diagnosis requiring an antibody test followed by an RNA or core antigen test necessitating three visits often separated by several weeks, and the high cost of treatment (around $90,000 per patient).
In addition, insurers commonly require proof that patients remain sober for extended periods, insist that treatment monitoring be performed by specialists only, and often approve treatment only for those patients who have documented evidence of liver damage.
“Does that make sense to you?” Dr. Collins asked. “You’ve got a cure for a liver disease, and you have to wait and show that the liver’s been damaged before you receive it? That just doesn’t fit,” he said.
Dr. Collins also pointed out that we’re dealing with hard-to-reach populations (underserved, uninsured, justice-involved), and people who are in tough times. “Anything that you put in the way as a barrier is going to make this worse in terms of its ability to be implemented,” he said.
To demonstrate how a coordinated HCV-elimination program could work, Dr. Collins pointed to a Medicaid cohort study in Louisiana conducted from July 2019 through December 2021, in which 8,867 patients started on therapy, 7,763 (88%) completed therapy, and 5,882 (66%) returned for testing. Of those tested, 5,285 (90%) had sustained virologic responses.
Another model of a hepatitis C elimination program was provided by the Veterans Health Administration. They received funding for an effort for all veterans, and in the space of 7 years were able to reach out even to some of their difficult-to-reach populations and achieve high diagnosis and treatment rates in a way that could be a model for what we would want to do across the nation, Dr. Collins noted.
Doing the math
Also at the session, Jagpreet Chhatwal, PhD, director of the Massachusetts General Hospital Institute for Technology Assessment and associate professor of radiology at Harvard Medical School, Boston, described outcomes projected by a mathematical simulation model of the HCV epidemic that he and his colleagues developed.
The HEP-SIM (Hepatitis C Disease Burden Simulation) model evaluates HCV prevalence trends, the number needed to screen and treat to eliminate HCV, HCV-associated clinical outcomes, the cost of an elimination program, and the cost savings that could be realized from preventing long-term complications.
The model seeks to determine whether the upfront costs of a national HCV elimination program could be offset by savings down the road. Specifically, it assumes that within the next 5 years 1.31 million individuals would be diagnosed with HCV and projects that within that time frame 1.52 million would need to be treated to meet HCV elimination goals.
The model shows that, compared with the status quo, a concerted campaign of screening and treatment would prevent more than 10,000 HCV-related deaths by 2030, and 91,000 deaths by 2050.
A coordinated screening program is also projected to prevent 17,000 cases of hepatocellular carcinoma by 2030 and 108,000 cases by 2050, as well as avert 29,000 cases of decompensated cirrhosis by 2030 and 93,000 such cases by 2050.
The cost savings associated with an HCV elimination plan would also be substantial, Dr. Chhatwal said.
According to the model, over the next decade the cumulative costs associated with HCV would decline by $14.2 billion, compared with the status quo. Nearly 80% of those savings ($11.2 billion) would be in Medicare and Medicaid.
The total projected savings from 2024 through 2050 – in disease management, testing, treatment, and pragmatic costs – are estimated at $59.3 billion, Dr. Chhatwal said.
“This is unprecedented,” he said.
Getting it done
Rachael L. Fleurence, PhD, MSc, a health economist currently serving as a senior advisor in the Executive Office of the President, summarized efforts to build a national HCV elimination program with input from federal health care agencies, state health leaders, patients, advocacy groups, drug manufacturers, and insurers.
She noted that a large component and focus of the program will be working on diagnostic test development but also accelerating bringing tests into the United States that are currently unavailable here. “These include point-of-care RNA diagnostic tests, as well as core antigen laboratory tests,” she said.
The program will be designed to offer broad access to curative anti-HCV drugs through a national subscription model that would make DAAs available to Medicaid recipients, justice-involved populations, the uninsured, and American Indians and Alaskan Natives who receive care through the Indian Health Service.
“On the Medicare and commercial insurance fronts, we’re still exploring different approaches, including potentially a co-pay assistance for Medicare beneficiaries, as well as working with commercial insurers to reduce barriers to access,” she said.
The program would also involve screening strategies extending to more settings, especially for high-risk populations, expanding the number of providers allowed to screen and treat HCV infections through telehealth, ensuring incentives for providers, and increasing the number of community health workers and case workers to improve linkage to care.
The next steps for the program would include funding to support the NIH’s RADx diagnostics program to accelerate access to testing, planning for the subscription model for DAA purchase, and launching pilot programs with the CDC, the Health Resources and Services Administration, the Substance Abuse and Mental Health Services Administration, and the Indian Health Service.
A call to action
Dr. Collins ended this portion of the program with an exhortation to AASLD members to do their part.
“We need your help,” Dr. Collins said. “This is a bold initiative, but it’s an opportunity. It’s even a responsibility. If we can actually succeed at this kind of outreach and save lives, and at the same time save money, how can we not do that?”
Dr. Collins, Dr. Chhatwal, and Dr. Fleurence each reported having no financial conflicts.
A version of this article first appeared on Medscape.com.
WASHINGTON – “We don’t get to use the ‘eliminate’ word all that often with a disease that’s taking thousands or tens of thousands – or worldwide, hundreds of thousands – of lives every year, but we have that opportunity with hepatitis C.”
So said Francis S. Collins, MD, PhD, special projects advisor to the Executive Office of the President of the United States, and former director of the National Institutes of Health, speaking at a special session outlining ambitious goals for a national plan to eliminate hepatitis C virus (HCV) infections by the year 2050.
The session was held at the annual meeting of the American Association for the Study of Liver Diseases.
A public health crisis
Dr. Collins labeled HCV a public health crisis, citing statistics from the Centers for Disease Control and Prevention that show that the rate of reported acute HCV infection cases increased 400% between 2010 and 2020, with the highest rates among young adults aged 20-39 years.
In addition, an estimated 2.4 million people in the United States are living with chronic HCV infections, but as many as 40% of these people are unaware of their infection, despite broad recommendations for the screening of all adults aged 18 years and older, he said.
“Our goal is to try to do something to change this,” Dr. Collins said. He noted that for the past 8 years we have had highly effective oral agents that don’t just treat the disease but cure it – 95%-97% of the time, with only 8-12 weeks of oral therapy and relatively few side effects.
“A wonderful story, one of the most exciting stories that’s come out of biomedical research in the last couple of decades,” he said.
Yet Dr. Collins also acknowledged that the task of developing a national plan is daunting, despite that pharmaceutical triumph.
National pharmacy claims data show that the number of persons treated for HCV with direct-acting antiviral agents (DAAs) in the United States declined from a high of 164,247 in 2015 to 83,740 in 2020.
Furthermore, CDC data from 2019 and 2020 show that, of persons with a diagnosis of HCV infection, only 23% of those on Medicaid, 28% of those on Medicare, and 35% of those with private insurance were treated for their infections.
“We have a huge gap here between the ability to know you have the disease and to get treatment, and we don’t see the numbers here for the uninsured, or people in prisons, but they’re probably much worse,” he said.
Obstacles abound, as do ways to overcome them
Current barriers to treatment include the aforementioned lack of awareness of infection, a “clunky” two-step diagnosis requiring an antibody test followed by an RNA or core antigen test necessitating three visits often separated by several weeks, and the high cost of treatment (around $90,000 per patient).
In addition, insurers commonly require proof that patients remain sober for extended periods, insist that treatment monitoring be performed by specialists only, and often approve treatment only for those patients who have documented evidence of liver damage.
“Does that make sense to you?” Dr. Collins asked. “You’ve got a cure for a liver disease, and you have to wait and show that the liver’s been damaged before you receive it? That just doesn’t fit,” he said.
Dr. Collins also pointed out that we’re dealing with hard-to-reach populations (underserved, uninsured, justice-involved), and people who are in tough times. “Anything that you put in the way as a barrier is going to make this worse in terms of its ability to be implemented,” he said.
To demonstrate how a coordinated HCV-elimination program could work, Dr. Collins pointed to a Medicaid cohort study in Louisiana conducted from July 2019 through December 2021, in which 8,867 patients started on therapy, 7,763 (88%) completed therapy, and 5,882 (66%) returned for testing. Of those tested, 5,285 (90%) had sustained virologic responses.
Another model of a hepatitis C elimination program was provided by the Veterans Health Administration. They received funding for an effort for all veterans, and in the space of 7 years were able to reach out even to some of their difficult-to-reach populations and achieve high diagnosis and treatment rates in a way that could be a model for what we would want to do across the nation, Dr. Collins noted.
Doing the math
Also at the session, Jagpreet Chhatwal, PhD, director of the Massachusetts General Hospital Institute for Technology Assessment and associate professor of radiology at Harvard Medical School, Boston, described outcomes projected by a mathematical simulation model of the HCV epidemic that he and his colleagues developed.
The HEP-SIM (Hepatitis C Disease Burden Simulation) model evaluates HCV prevalence trends, the number needed to screen and treat to eliminate HCV, HCV-associated clinical outcomes, the cost of an elimination program, and the cost savings that could be realized from preventing long-term complications.
The model seeks to determine whether the upfront costs of a national HCV elimination program could be offset by savings down the road. Specifically, it assumes that within the next 5 years 1.31 million individuals would be diagnosed with HCV and projects that within that time frame 1.52 million would need to be treated to meet HCV elimination goals.
The model shows that, compared with the status quo, a concerted campaign of screening and treatment would prevent more than 10,000 HCV-related deaths by 2030, and 91,000 deaths by 2050.
A coordinated screening program is also projected to prevent 17,000 cases of hepatocellular carcinoma by 2030 and 108,000 cases by 2050, as well as avert 29,000 cases of decompensated cirrhosis by 2030 and 93,000 such cases by 2050.
The cost savings associated with an HCV elimination plan would also be substantial, Dr. Chhatwal said.
According to the model, over the next decade the cumulative costs associated with HCV would decline by $14.2 billion, compared with the status quo. Nearly 80% of those savings ($11.2 billion) would be in Medicare and Medicaid.
The total projected savings from 2024 through 2050 – in disease management, testing, treatment, and pragmatic costs – are estimated at $59.3 billion, Dr. Chhatwal said.
“This is unprecedented,” he said.
Getting it done
Rachael L. Fleurence, PhD, MSc, a health economist currently serving as a senior advisor in the Executive Office of the President, summarized efforts to build a national HCV elimination program with input from federal health care agencies, state health leaders, patients, advocacy groups, drug manufacturers, and insurers.
She noted that a large component and focus of the program will be working on diagnostic test development but also accelerating bringing tests into the United States that are currently unavailable here. “These include point-of-care RNA diagnostic tests, as well as core antigen laboratory tests,” she said.
The program will be designed to offer broad access to curative anti-HCV drugs through a national subscription model that would make DAAs available to Medicaid recipients, justice-involved populations, the uninsured, and American Indians and Alaskan Natives who receive care through the Indian Health Service.
“On the Medicare and commercial insurance fronts, we’re still exploring different approaches, including potentially a co-pay assistance for Medicare beneficiaries, as well as working with commercial insurers to reduce barriers to access,” she said.
The program would also involve screening strategies extending to more settings, especially for high-risk populations, expanding the number of providers allowed to screen and treat HCV infections through telehealth, ensuring incentives for providers, and increasing the number of community health workers and case workers to improve linkage to care.
The next steps for the program would include funding to support the NIH’s RADx diagnostics program to accelerate access to testing, planning for the subscription model for DAA purchase, and launching pilot programs with the CDC, the Health Resources and Services Administration, the Substance Abuse and Mental Health Services Administration, and the Indian Health Service.
A call to action
Dr. Collins ended this portion of the program with an exhortation to AASLD members to do their part.
“We need your help,” Dr. Collins said. “This is a bold initiative, but it’s an opportunity. It’s even a responsibility. If we can actually succeed at this kind of outreach and save lives, and at the same time save money, how can we not do that?”
Dr. Collins, Dr. Chhatwal, and Dr. Fleurence each reported having no financial conflicts.
A version of this article first appeared on Medscape.com.
AT THE LIVER MEETING
Bepirovirsen: Is a ‘functional cure’ for HBV on the horizon?
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
World falls short on HBV, HCV elimination targets
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
FDA expands tenofovir alafenamide (Vemlidy) use to adolescents with chronic HBV
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.


