User login
Bulevirtide shows real-world efficacy versus HDV
A real-world analysis of bulevirtide found a safety and efficacy profile similar to what was seen in earlier clinical trials in the treatment of hepatitis delta virus (HDV) infection.
HDV can only infect patients already carrying hepatitis B virus (HBV), but it causes the most severe form of viral hepatitis as it can progress to cirrhosis within 5 years and to hepatocellular carcinoma within 10 years.
Bulevirtide is a first-in-class medication that mimics the hepatitis B surface antigen, binding to its receptor on hepatocytes and preventing HDV viral particles from binding to it. The drug received conditional marketing approval by the European Medicines Agency in 2020 and has received a breakthrough therapy designation from the U.S. Food and Drug Administration.
The study was presented at the annual meeting of the American Association for the Study of Liver Diseases by Victor De Ledinghen, PhD, who is a professor of hepatology and head of the hepatology and liver transplantation unit at Bordeaux (France) University Hospital.
The early-access program launched after the French National Agency for Medicines and Health Products approved bulevirtide in 2019. It was made available to patients with compensated cirrhosis or severe liver fibrosis (F3) or patients with F2 fibrosis and alanine amino transferase levels more than twice the upper limit of normal for 6 months or more. Patients received bulevirtide alone (n = 77) or in combination with peg-interferon (n = 68), as determined by their physician.
The researchers defined virologic efficacy as HDV RNA levels being undetectable, or decreased by at least 2 log10 from baseline. They defined biochemical efficacy as ALT levels below 40 IU/L.
A per-protocol analysis included all patients in the bulevirtide group, but excluded 12 from the combination group who discontinued peg-interferon (n = 56). Nineteen patients in bulevirtide group had a treatment modification, and seven discontinued treatment. Five in the combination group had a treatment modification, and 14 stopped treatment. At 12 months, there was a greater decline in median log10 IU/mL in the combination group (–5.65 versus –3.64), though the study was not powered to compare the two. At 12 months, the combination group had 93.9% virologic efficacy, compared with 68.3% in the bulevirtide group.
The two groups had similar mean ALT levels at 12 months (48.91 and 48.03 IU/mL, respectively), with more patients in the bulevirtide group having normal ALT levels (<40 IU/L; 48.8% versus 36.4%). At 12 months, 39.0% of the bulevirtide group and 30.3% of the combination group had a combined response, defined as either undetectable HDV RNA or ≥2 log10 from baseline plus normal ALT levels.
Twenty-nine patients in the bulevirtide group had an adverse event, compared with 43 in the combination group. The two groups were similar in the frequency of grade 3-4 adverse events (7 versus 6), discontinuation due to adverse events (2 versus 3), deaths (0 in both), injection-site reactions (2 in both), liver-related adverse events (4 versus 2), and elevated bile acid (76 versus 68).
During the Q&A period following the presentation, Dr. De Ledinghen was asked if he has a preferred regimen for HDV patients. “I think it depends on the tolerance of peg-interferon because of all the side effects with this drug. I think we need to have predictive factors of virological response with or without interferon. At this time, I don’t have a preference, but I think at this time we need to work on predictive factors associated with virologic response,” he said.
The EMA’s conditional bulevirtide approval hinged on results from phase 2 clinical trials, while the phase 3 clinical studies are ongoing. “This was a very unusual step for the EMA to provide what is similar to emergency use approval while the phase 3 clinical trials are still ongoing,” said Anna Lok, MD, who was asked to comment on the study. Dr. Lok is a professor of internal medicine, director of clinical hepatology, and assistant dean for clinical research at the University of Michigan, Ann Arbor.
She noted that the phase 2 studies indicated that the combination with peg-interferon seems to have an additive effect on HDV suppression, while monotherapy with bulevirtide has a greater effect on normalizing ALT levels. The real-world experience confirms these findings.
But the real-world data revealed some concerns. “What really worried me is the large number of patients who required dose modifications or discontinuations, and that seems to be the case in both treatment groups. They didn’t really go into a lot of details [about] why patients needed treatment modifications, but one has to assume that this is due to side effects,” said Dr. Lok.
She also noted that the per-protocol analysis, instead of an intention-to-treat analysis, is a weakness of the study. Additionally, over time, the number of patients analyzed decreased – as many as 40% of patients didn’t have test results at month 12. “It makes you wonder what happened to those patients. Many probably didn’t respond, in which case your overall response rate will be far lower,” said Dr. Lok.
The study was funded by Gilead. Dr. De Ledinghen has financial relationships with Gilead, AbbVie, Echosens, Hologic, Intercept Pharma, Tillotts, Orphalan, Alfasigma, Bristol Myers Squibb, and Siemens Healthineers. Dr. Lok has no relevant financial disclosures.
A real-world analysis of bulevirtide found a safety and efficacy profile similar to what was seen in earlier clinical trials in the treatment of hepatitis delta virus (HDV) infection.
HDV can only infect patients already carrying hepatitis B virus (HBV), but it causes the most severe form of viral hepatitis as it can progress to cirrhosis within 5 years and to hepatocellular carcinoma within 10 years.
Bulevirtide is a first-in-class medication that mimics the hepatitis B surface antigen, binding to its receptor on hepatocytes and preventing HDV viral particles from binding to it. The drug received conditional marketing approval by the European Medicines Agency in 2020 and has received a breakthrough therapy designation from the U.S. Food and Drug Administration.
The study was presented at the annual meeting of the American Association for the Study of Liver Diseases by Victor De Ledinghen, PhD, who is a professor of hepatology and head of the hepatology and liver transplantation unit at Bordeaux (France) University Hospital.
The early-access program launched after the French National Agency for Medicines and Health Products approved bulevirtide in 2019. It was made available to patients with compensated cirrhosis or severe liver fibrosis (F3) or patients with F2 fibrosis and alanine amino transferase levels more than twice the upper limit of normal for 6 months or more. Patients received bulevirtide alone (n = 77) or in combination with peg-interferon (n = 68), as determined by their physician.
The researchers defined virologic efficacy as HDV RNA levels being undetectable, or decreased by at least 2 log10 from baseline. They defined biochemical efficacy as ALT levels below 40 IU/L.
A per-protocol analysis included all patients in the bulevirtide group, but excluded 12 from the combination group who discontinued peg-interferon (n = 56). Nineteen patients in bulevirtide group had a treatment modification, and seven discontinued treatment. Five in the combination group had a treatment modification, and 14 stopped treatment. At 12 months, there was a greater decline in median log10 IU/mL in the combination group (–5.65 versus –3.64), though the study was not powered to compare the two. At 12 months, the combination group had 93.9% virologic efficacy, compared with 68.3% in the bulevirtide group.
The two groups had similar mean ALT levels at 12 months (48.91 and 48.03 IU/mL, respectively), with more patients in the bulevirtide group having normal ALT levels (<40 IU/L; 48.8% versus 36.4%). At 12 months, 39.0% of the bulevirtide group and 30.3% of the combination group had a combined response, defined as either undetectable HDV RNA or ≥2 log10 from baseline plus normal ALT levels.
Twenty-nine patients in the bulevirtide group had an adverse event, compared with 43 in the combination group. The two groups were similar in the frequency of grade 3-4 adverse events (7 versus 6), discontinuation due to adverse events (2 versus 3), deaths (0 in both), injection-site reactions (2 in both), liver-related adverse events (4 versus 2), and elevated bile acid (76 versus 68).
During the Q&A period following the presentation, Dr. De Ledinghen was asked if he has a preferred regimen for HDV patients. “I think it depends on the tolerance of peg-interferon because of all the side effects with this drug. I think we need to have predictive factors of virological response with or without interferon. At this time, I don’t have a preference, but I think at this time we need to work on predictive factors associated with virologic response,” he said.
The EMA’s conditional bulevirtide approval hinged on results from phase 2 clinical trials, while the phase 3 clinical studies are ongoing. “This was a very unusual step for the EMA to provide what is similar to emergency use approval while the phase 3 clinical trials are still ongoing,” said Anna Lok, MD, who was asked to comment on the study. Dr. Lok is a professor of internal medicine, director of clinical hepatology, and assistant dean for clinical research at the University of Michigan, Ann Arbor.
She noted that the phase 2 studies indicated that the combination with peg-interferon seems to have an additive effect on HDV suppression, while monotherapy with bulevirtide has a greater effect on normalizing ALT levels. The real-world experience confirms these findings.
But the real-world data revealed some concerns. “What really worried me is the large number of patients who required dose modifications or discontinuations, and that seems to be the case in both treatment groups. They didn’t really go into a lot of details [about] why patients needed treatment modifications, but one has to assume that this is due to side effects,” said Dr. Lok.
She also noted that the per-protocol analysis, instead of an intention-to-treat analysis, is a weakness of the study. Additionally, over time, the number of patients analyzed decreased – as many as 40% of patients didn’t have test results at month 12. “It makes you wonder what happened to those patients. Many probably didn’t respond, in which case your overall response rate will be far lower,” said Dr. Lok.
The study was funded by Gilead. Dr. De Ledinghen has financial relationships with Gilead, AbbVie, Echosens, Hologic, Intercept Pharma, Tillotts, Orphalan, Alfasigma, Bristol Myers Squibb, and Siemens Healthineers. Dr. Lok has no relevant financial disclosures.
A real-world analysis of bulevirtide found a safety and efficacy profile similar to what was seen in earlier clinical trials in the treatment of hepatitis delta virus (HDV) infection.
HDV can only infect patients already carrying hepatitis B virus (HBV), but it causes the most severe form of viral hepatitis as it can progress to cirrhosis within 5 years and to hepatocellular carcinoma within 10 years.
Bulevirtide is a first-in-class medication that mimics the hepatitis B surface antigen, binding to its receptor on hepatocytes and preventing HDV viral particles from binding to it. The drug received conditional marketing approval by the European Medicines Agency in 2020 and has received a breakthrough therapy designation from the U.S. Food and Drug Administration.
The study was presented at the annual meeting of the American Association for the Study of Liver Diseases by Victor De Ledinghen, PhD, who is a professor of hepatology and head of the hepatology and liver transplantation unit at Bordeaux (France) University Hospital.
The early-access program launched after the French National Agency for Medicines and Health Products approved bulevirtide in 2019. It was made available to patients with compensated cirrhosis or severe liver fibrosis (F3) or patients with F2 fibrosis and alanine amino transferase levels more than twice the upper limit of normal for 6 months or more. Patients received bulevirtide alone (n = 77) or in combination with peg-interferon (n = 68), as determined by their physician.
The researchers defined virologic efficacy as HDV RNA levels being undetectable, or decreased by at least 2 log10 from baseline. They defined biochemical efficacy as ALT levels below 40 IU/L.
A per-protocol analysis included all patients in the bulevirtide group, but excluded 12 from the combination group who discontinued peg-interferon (n = 56). Nineteen patients in bulevirtide group had a treatment modification, and seven discontinued treatment. Five in the combination group had a treatment modification, and 14 stopped treatment. At 12 months, there was a greater decline in median log10 IU/mL in the combination group (–5.65 versus –3.64), though the study was not powered to compare the two. At 12 months, the combination group had 93.9% virologic efficacy, compared with 68.3% in the bulevirtide group.
The two groups had similar mean ALT levels at 12 months (48.91 and 48.03 IU/mL, respectively), with more patients in the bulevirtide group having normal ALT levels (<40 IU/L; 48.8% versus 36.4%). At 12 months, 39.0% of the bulevirtide group and 30.3% of the combination group had a combined response, defined as either undetectable HDV RNA or ≥2 log10 from baseline plus normal ALT levels.
Twenty-nine patients in the bulevirtide group had an adverse event, compared with 43 in the combination group. The two groups were similar in the frequency of grade 3-4 adverse events (7 versus 6), discontinuation due to adverse events (2 versus 3), deaths (0 in both), injection-site reactions (2 in both), liver-related adverse events (4 versus 2), and elevated bile acid (76 versus 68).
During the Q&A period following the presentation, Dr. De Ledinghen was asked if he has a preferred regimen for HDV patients. “I think it depends on the tolerance of peg-interferon because of all the side effects with this drug. I think we need to have predictive factors of virological response with or without interferon. At this time, I don’t have a preference, but I think at this time we need to work on predictive factors associated with virologic response,” he said.
The EMA’s conditional bulevirtide approval hinged on results from phase 2 clinical trials, while the phase 3 clinical studies are ongoing. “This was a very unusual step for the EMA to provide what is similar to emergency use approval while the phase 3 clinical trials are still ongoing,” said Anna Lok, MD, who was asked to comment on the study. Dr. Lok is a professor of internal medicine, director of clinical hepatology, and assistant dean for clinical research at the University of Michigan, Ann Arbor.
She noted that the phase 2 studies indicated that the combination with peg-interferon seems to have an additive effect on HDV suppression, while monotherapy with bulevirtide has a greater effect on normalizing ALT levels. The real-world experience confirms these findings.
But the real-world data revealed some concerns. “What really worried me is the large number of patients who required dose modifications or discontinuations, and that seems to be the case in both treatment groups. They didn’t really go into a lot of details [about] why patients needed treatment modifications, but one has to assume that this is due to side effects,” said Dr. Lok.
She also noted that the per-protocol analysis, instead of an intention-to-treat analysis, is a weakness of the study. Additionally, over time, the number of patients analyzed decreased – as many as 40% of patients didn’t have test results at month 12. “It makes you wonder what happened to those patients. Many probably didn’t respond, in which case your overall response rate will be far lower,” said Dr. Lok.
The study was funded by Gilead. Dr. De Ledinghen has financial relationships with Gilead, AbbVie, Echosens, Hologic, Intercept Pharma, Tillotts, Orphalan, Alfasigma, Bristol Myers Squibb, and Siemens Healthineers. Dr. Lok has no relevant financial disclosures.
FROM THE LIVER MEETING
HCV screening in pregnancy: Reducing the risk for casualties in the quest for elimination
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Because hepatitis C virus (HCV) infection is typically asymptomatic, its presence can easily be overlooked without appropriate screening efforts. For those screening efforts to be effective, they must keep pace with the changing demographic face of this increasingly prevalent but treatable disease.
Perhaps the most dramatic shift in HCV demographics in recent years has been the increase of infections among those born after 1965, a trend primarily driven by the opioid epidemic. In addition, data from the National Notifiable Diseases Surveillance System show that cases of diagnosed HCV doubled among women of childbearing age from 2006 to 2014, with new infections in younger women surpassing those in older age groups.
With such trends in mind, the Centers for Disease Control and Prevention broadened their recommendations regarding HCV in 2020 to include one-time testing in all adults aged 18 years and older and screening of all pregnant women during each pregnancy, except where the prevalence of infection is less than 0.1%, a threshold that no state has yet achieved.
The US Preventive Services Task Force (USPSTF) subsequently followed suit in their own recommendations.
The American Association for the Study of Liver Diseases/Infectious Diseases Society of America have long advocated for extensive expansion in their screening recommendations for HCV, including pregnancy.
Although the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine did not immediately adopt these recommendations, they have since endorsed them in May 2021 and June 2021, respectively.
The hepatologist perspective
As a practicing hepatologist, this seems like an uncontroversial recommendation. Obstetricians already screen for hepatitis B virus in each pregnancy. It should be easy to add HCV testing to the same lab testing.
Risk-based screening has repeatedly been demonstrated to be ineffective. It should be easier to test all women than to ask prying questions about high-risk behaviors.
Given the increase of injection drug use and resultant HCV infections in women of childbearing age, this seems like a perfect opportunity to identify chronically infected women and counsel them on transmission and cure. And pregnancy is also unique in that it is a time of near-universal health coverage.
Let’s address some of the operational issues.
The diagnostic cascade for HCV can be made very easy. HCV antibody testing is our standard screening test and, when positive, can automatically reflex to HCV polymerase chain reaction (PCR), the diagnostic test. Thus, with one blood sample, you can both screen for and diagnose infection.
Current guidelines do not recommend treating HCV during pregnancy, although therapy can be considered on an individual basis. Linkage to a knowledgeable provider who can discuss transmission and treatment, as well as assess the stage of liver injury, should decrease the burden on the ob.gyn.
The impact on pregnancy is marginal. HCV should not change either the mode of delivery or the decision to breastfeed. The AASLD/IDSA guidance outlines only four recommendations for monitoring during pregnancy:
- Obtain HCV RNA to see whether the infection is active and assess liver function at initiation of prenatal care.
- Prenatal care should be tailored to the pregnancy. There is no modification recommended to decrease mother-to-child transmission (MTCT).
- Be aware that intrahepatic is more common with HCV.
- Women with have a higher rate of adverse outcomes and should be linked to a high-risk obstetrics specialist.
But of course, what seems easy to one specialist may not be true of another. With that in mind, let’s hear the ob.gyn. perspective on these updated screening recommendations.
The ob.gyn. perspective
Recent guidelines from the CDC, ACOG, and SMFM recommend universal screening for HCV in all pregnant women. The increased availability of highly effective antiviral regimens makes universal screening a logical strategy, especially to identify candidates for this curative treatment. What is questionable, however, is the recommended timing by which this screening should take place.
HCV screening during pregnancy, as currently recommended, provides no immediate benefit for the pregnant woman or the fetus/neonate, given that antiviral treatments have not been approved during gestation, and there are no known measures that decrease MTCT or change routine perinatal care.
We also must not forget that a significant proportion of women in the United States, particularly those with limited resources, do not receive prenatal care at all. Most of them, however, will present to a hospital for delivery. Consequently, compliance with screening might be higher if performed at the time of delivery rather than antepartum.
Deferring screening until the intrapartum or immediate postpartum period, at least until antiviral treatment during pregnancy becomes a reality, was discussed. The rationale was that this approach might obviate the need to deal with the unintended consequences and burden of testing for HCV during pregnancy. Ultimately, ACOG and SMFM fell in line with the CDC recommendations.
Despite the lack of robust evidence regarding the risk for MTCT associated with commonly performed obstetric procedures (for example, genetic amniocentesis, artificial rupture of the membranes during labor, placement of an intrauterine pressure catheter), clinicians may be reluctant to perform them in HCV-infected women, resulting in potential deviations from the obstetric standard of care.
Similarly, it is likely that patients may choose to have a cesarean delivery for the sole purpose of decreasing MTCT, despite the lack of evidence for this. Such ill-advised patient-driven decisions are increasingly likely in the current environment, where social media can rapidly disseminate misinformation.
Implications for pediatric patients
One cannot isolate HCV screening in pregnancy from the consequences that may potentially occur as part of the infant’s transition to the care of a pediatrician.
Even though MTCT is estimated to occur in just 5%-15% of cases, all children born to HCV viremic mothers should be screened for HCV.
Traditionally, screening for HCV antibodies occurred after 18 months of age. In those who test positive, HCV PCR testing is recommended at 3 years. However, this algorithm is being called into question because only approximately one-third of infants are successfully screened.
HCV RNA testing in the first year after birth has been suggested. However, even proponents of this approach concur that all management decisions should be deferred until after the age of 3 years, when medications are approved for pediatric use.
In addition, HCV testing would be required again before considering therapy because children have higher rates of spontaneous clearance.
Seeking consensus beyond the controversy
Controversy remains surrounding the most recent update to the HCV screening guidelines. The current recommendation to screen during pregnancy cannot modify the risk for MTCT, has no impact on decisions regarding mode of delivery or breastfeeding, and could potentially cause harm by making obstetricians defer necessary invasive procedures even though there are no data linking them to an increase in MTCT.
Yet after extensive debate, the CDC, USPSTF, AASLD/IDSA, ACOG, and SMFM all developed their current recommendations to initiate HCV screening during pregnancy. To make this successful, screening algorithms need to be simple and consistent across all society recommendations.
HCV antibody testing should always reflex to the diagnostic test (HCV PCR) to allow confirmation in those who test positive without requiring an additional blood test. Viremic mothers (those who are HCV positive on PCR) should be linked to a provider who can discuss prognosis, transmission, and treatment. The importance of screening the infant also must be communicated to the parents and pediatrician alike.
Dr. Reau has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Gilead, Arbutus, Intercept, and Salix; received research grants from AbbVie and Gilead; and received income from AASLD. Dr. Pacheco disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HCV in pregnancy: One piece of a bigger problem
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
FROM JAMA HEALTH FORUM
Antiviral Therapy Improves Hepatocellular Cancer Survival
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
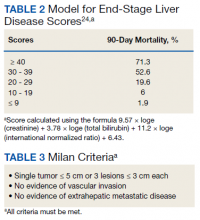
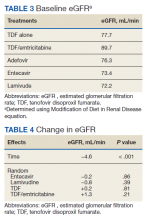
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
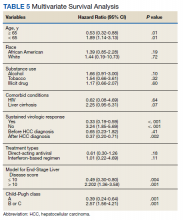
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
Comparison of Renal Function Between Tenofovir Disoproxil Fumarate and Other Nucleos(t)ide Reverse Transcriptase Inhibitors in Patients With Hepatitis B Virus Infection
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
Infection with hepatitis B virus (HBV) is associated with risk of potentially lethal, chronic infection and is a major public health problem. Infection from HBV has the potential to lead to liver failure, cirrhosis, and cancer.1,2 Chronic HBV infection exists in as many as 2.2 million Americans, and in 2015 alone, HBV was estimated to be associated with 887,000 deaths worldwide.1,3 Suppression of viral load is the basis of treatment, necessitating long-term use of medication for treatment.4 Nucleoside reverse transcriptase inhibitors (entecavir, lamivudine, telbivudine) and nucleotide reverse transcriptase inhibitors (adefovir, tenofovir), have improved the efficacy and tolerability of chronic HBV treatment compared with interferon-based agents.4-7 However, concerns remain regarding long-term risk of nephrotoxicity, in particular with tenofovir disoproxil fumarate (TDF), which could lead to a limitation of safe and effective options for certain populations.5,6,8 A newer formulation, tenofovir alafenamide fumarate (TAF), has improved the kidney risks, but expense remains a limiting factor for this agent.9
Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) have demonstrated efficacy in reducing HBV viral load and other markers of improvement in chronic HBV, but entecavir and tenofovir have tended to demonstrate greater efficacy in clinical trials.5-7 Several studies have suggested potential benefits of tenofovir-based treatment over other NRTIs, including greater viral load achievement compared with adefovir, efficacy in patients with previous failure of lamivudine or adefovir, and long-term efficacy in chronic HBV infection.10-12 A 2019 systematic review suggests TDF and TAF are more effective than other NRTIs for achieving viral load suppression.13 Other NRTIs are not without their own risks, including mitochondrial dysfunction, mostly with lamivudine and telbivudine.4
Despite these data, guidelines have varied in their treatment recommendations in the context of chronic kidney disease partly due to variations in the evidence regarding nephrotoxicity.7,14 Cohort studies and case reports have suggested association between TDF and acute kidney injury in patients with HIV infection as well as long-term reductions in kidney function.15,16 In one study, 58% of patients treated with TDF did not return to baseline kidney function after an event of acute kidney injury.17 However, little data are available on whether this association exists for chronic HBV treatment in the absence of HIV infection. One retrospective analysis comparing TDF and entecavir in chronic HBV without HIV showed greater incidence of creatinine clearance < 60 mL/min with TDF but greater incidence of serum creatinine (SCr) ≥ 2.5 mg/dL in the entacavir group, making it difficult to reach a clear conclusion on risks.18 Other studies have either suffered from small cohorts with TDF or included patients with HIV coinfection.19,20 Although a retrospective comparison of TDF and entecavir, randomly matched 1:2 to account for differences between groups, showed lower estimated glomerular filtration rate (eGFR) in the TDF group, more data are needed.21 Entecavir remains an option for many patient, but for those who have failed nucleosides, few options remain.
With the advantages available from TDF and the continued expense of TAF, more data regarding the risks of nephrotoxicity with TDF would be beneficial. The objective of this study was to compare treatment with TDF and other NRTIs in chronic HBV monoinfection to distinguish any differences in kidney function changes over time. With hopes of gathering enough data to distinguish between groups, information was gathered from across the Veterans Health Administration (VHA) system.
Methods
A nationwide, multicenter, retrospective, cohort study of veterans with HBV infection was conducted to compare the effects of various NRTIs on renal function. Patient were identified through the US Department of Veterans Affairs Corporate Data Warehouse (CDW), using data from July 1, 2005 to July 31, 2015. Patients were included who had positive HBV surface antigen (HBsAg) or newly prescribed NRTI. Multiple drug episodes could be included for each patient. That is, if a patient who had previously been included had another instance of a newly prescribed NRTI, this would be included in the analysis. Exclusion criteria were patients aged < 18 years, those with NRTI prescription for ≤ 1 month, and concurrent HIV infection. All patients with HBsAg were included for the study for increasing the sensitivity in gathering patients; however, those patients were included only if they received NRTI concurrent with the laboratory test results used for the primary endpoint (ie, SCr) to be included in the analysis.
How data are received from CDW bears some explanation. A basic way to understand the way data are received is that questions can be asked such as “for X population, at this point in time, was the patient on Y drug and what was the SCr value.” Therefore, inclusion and exclusion must first be specified to define the population, after which point certain data points can be received depending on the specifications made. For this reason, there is no way to determine, for example, whether a certain patient continued TDF use for the duration of the study, only at the defined points in time (described below) to receive the specific data.
For the patients included, information was retrieved from the first receipt of the NRTI prescription to 36 months after initiation. Baseline characteristics included age, sex, race, and ethnicity, and were defined at time of NRTI initiation. Values for SCr were compared at baseline, 3, 6, 12, 24, and 36 months after prescription of NRTI. The date of laboratory results was associated with the nearest date of comparison. Values for eGFR were determined by the modification of diet in renal disease equation. Values for eGFR are available in the CDW, whereas there is no direct means to calculate creatinine clearance with the available data, so eGFR was used for this study.
The primary endpoint was a change in eGFR in patients taking TDF after adjustment for time with the full cohort. Secondary analyses included the overall effect of time for the full cohort and change in renal function for each NRTI group. Mean and standard deviation for eGFR were determined for each NRTI group using the available data points. Analyses of the primary and secondary endpoints were completed using a linear mixed model with terms for time, to account for fixed effects, and specific NRTI used to account for random effects. A 2-sided α of .05 was used to determine statistical significance.
Results
A total of 413 drug episodes from 308 subjects met inclusion criteria for the study. Of these subjects, 229 were still living at the time of query. Most study participants were male (96%), the mean age was 62.1 years for males and 55.9 years for females; 49.5% were White and 39.7% were Black veterans (Table 1).
The NRTIs received by patients during the study period included TDF, TDF/emtricitabine, adefovir, entecavir, and lamivudine. No patients were on telbivudine. Formulations including TAF had not been approved by the US Food and Drug Administration (FDA) by the end of the study period, and as such were not found in the study.13 A plurality of participants received entecavir (94 of 223 at baseline), followed by TDF (n = 38) (Table 2). Of note, only 8 participants received TDF/emtricitabine at baseline. Differences were found between the groups in number of SCr data points available at 36 months vs baseline. The TDF group had the greatest reduction in data points available with 38 laboratory values at baseline vs 15 at 36 months (39.5% of baseline). From the available data, it is not possible to determine whether these represent medication discontinuations, missing values, lost to follow-up, or some other cause. Baseline eGFR was highest in the 2 TDF groups, with TDF alone at 77.7 mL/min (1.4-5.5 mL/min higher than the nontenofovir groups) and TDF/emtricitabine at 89.7 mL/min (13.4-17.5 mL/min higher than nontenofovir groups) (Table 3).
Table 4 contains data for the primarily and secondary analyses, examining change in eGFR. The fixed-effects analysis revealed a significant negative association between eGFR and time of −4.6 mL/min (P < .001) for all the NRTI groups combined. After accounting for this effect of time, there was no statistically significant correlation between use of TDF and change in eGFR (+0.2 mL/min, P = .81). For the TDF/emtricitabine group, a positive but statistically nonsignificant change was found (+1.3 mL/min, P = .21), but numbers were small and may have been insufficient to detect a difference. Similarly, no statistically significant change in eGFR was found after the fixed effects for either entecavir (−0.2 mL/min, P = .86) or lamivudine (−0.8 mL/min, P = .39). While included in the full analysis for fixed effects, random effects data were not received for the adefovir group due to heterogeneity and small quantity of the data, producing an unclear result.
Discussion
This study demonstrated a decline in eGFR over time in a similar fashion for all NRTIs used in patients treated for HBV monoinfection, but no greater decline in renal function was found with use of TDF vs other NRTIs. A statistically significant decline in eGFR of −4.55 mL/min over the 36-month time frame of the study was demonstrated for the full cohort, but no statistically significant change in eGFR was found for any individual NRTI after accounting for the fixed effect of time. If TDF is not associated with additional risk of nephrotoxicity compared with other NRTIs, this could have important implications for treatment when considering the evidence that tenofovir-based treatment seems to be more effective than other medications for suppressing viral load.13
This result runs contrary to data in patients given NRTIs for HIV infection as well as a more recent cohort study in chronic HBV infectioin, which showed a statistically significant difference in kidney dysfunction between TDF and entecavir (-15.73 vs -5.96 mL/min/m2, P < .001).5-7,21 Possible mechanism for differences in response between HIV and HBV patients has not been elucidated, but the inherent risk of developing chronic kidney disease from HIV disease may play a role.22 The possibility remains that all NRTIs cause a degree of kidney impairment in patients treated for chronic HBV infection as evidenced by the statistically significant fixed effect for time in the present study. The cause of this effect is unknown but may be independently related to HBV infection or may be specific to NRTI therapy. No control group of patients not receiving NRTI therapy was included in this study, so conclusions cannot be drawn regarding whether all NRTIs are associated with decline in renal function in chronic HBV infection.
Limitations
Although this study did not detect a difference in change in eGFR between TDF and other NRTI treatments, it is possible that the length of data collection was not adequate to account for possible kidney injury from TDF. A study assessing renal tubular dysfunction in patients receiving adefovir or TDF showed a mean onset of dysfunction of 49 months.15 It is possible that participants in this study would go on to develop renal dysfunction in the future. This potential also was observed in a more recent retrospective cohort study in chronic HBV infection, which showed the greatest degree of decline in kidney function between 36 and 48 months (−11.87 to −15.73 mL/min/m2 for the TDF group).21
The retrospective design created additional limitations. We attempted to account for some by using a matched cohort for the entecavir group, and there was no statistically significant difference between the groups in baseline characteristics. In HIV patients, a 10-year follow-up study continued to show decline in eGFR throughout the study, though the greatest degree of reduction occurred in the first year of the study.10 The higher baseline eGFR of the TDF recipients, 77.7 mL/min for the TDF alone group and 89.7 mL/min for the TDF/emtricitabine group vs 72.2 to 76.3 mL/min in the other NRTI groups, suggests high potential for selection bias. Some health care providers were likely to avoid TDF in patients with lower eGFR due to the data suggesting nephrotoxicity in other populations. Another limitation is that the reason for the missing laboratory values could not be determined. The TDF group had the greatest disparity in SCr data availability at baseline vs 36 months, with 39.5% concurrence with TDF alone compared with 50.0 to 63.6% in the other groups. Other treatment received outside the VHA system also could have influenced results.
Conclusions
This retrospective, multicenter, cohort study did not find a difference between TDF and other NRTIs for changes in renal function over time in patients with HBV infection without HIV. There was a fixed effect for time, ie, all NRTI groups showed some decline in renal function over time (−4.6 mL/min), but the effects were similar across groups. The results appear contrary to studies with comorbid HIV showing a decline in renal function with TDF, but present studies in HBV monotherapy have mixed results.
Further studies are needed to validate these results, as this and previous studies have several limitations. If these results are confirmed, a possible mechanism for these differences between patients with and without HIV should be examined. In addition, a study looking specifically at incidence of acute kidney injury rather than overall decline in renal function would add important data. If the results of this study are confirmed, there could be clinical implications in choice of agent with treatment of HBV monoinfection. This would add to the overall armament of medications available for chronic HBV infection and could create cost savings in certain situations if providers feel more comfortable continuing to use TDF instead of switching to the more expensive TAF.
Acknowledgments
Funding for this study was provided by the Veterans Health Administration.
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
1. Chartier M, Maier MM, Morgan TR, et al. Achieving excellence in hepatitis B virus care for veterans in the Veterans Health Administration. Fed Pract. 2018;35(suppl 2):S49-S53.
2. Chayanupatkul M, Omino R, Mittal S, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355-362. doi:10.1016/j.jhep.2016.09.013
3. World Health Organization. Global hepatitis report, 2017. Published April 19, 2017. Accessed July 15, 2021. https://www.who.int/publications/i/item/global-hepatitis-report-2017
4. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017;9(5):227-241. doi:10.4254/wjh.v9.i5.227
5. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44(1):16-34. doi:10.1111/apt.13659
6. Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39(1):35-46. doi:10.1111/apt.12538
7. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. doi:10.1002/hep.28156
8. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22(2):99-103. doi:10.1089/apc.2007.0052
9. Canadian Agency for Drugs and Technologies in Health. Pharmacoeconomic review teport: tenofovir alafenamide (Vemlidy): (Gilead Sciences Canada, Inc.): indication: treatment of chronic hepatitis B in adults with compensated liver disease. Published April 2018. Accessed July 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK532825/
10. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442-2455. doi:10.1056/NEJMoa0802878
11. van Bömmel F, de Man RA, Wedemeyer H, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51(1):73-80. doi:10.1002/hep.23246
12. Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505-513. doi:10.1002/hep.26277
13. Wong WWL, Pechivanoglou P, Wong J, et al. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8(1):207. Published 2019 Aug 19. doi:10.1186/s13643-019-1126-1
14. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and meta-analysis. Int Immunopharmacol. 2017;42:168-175. doi:10.1016/j.intimp.2016.11.022
15. Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56(4):567-575. doi:10.1093/cid/cis937
16. Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-780. doi:10.1053/j.ajkd.2011.01.022
17. Veiga TM, Prazeres AB, Silva D, et al. Tenofovir nephrotoxicity is an important cause of acute kidney injury in hiv infected inpatients. Abstract FR-PO481 presented at: American Society of Nephrology Kidney Week 2015; November 6, 2015; San Diego, CA.
18. Tan LK, Gilleece Y, Mandalia S, et al. Reduced glomerular filtration rate but sustained virologic response in HIV/hepatitis B co-infected individuals on long-term tenofovir. J Viral Hepat. 2009;16(7):471-478. doi:10.1111/j.1365-2893.2009.01084.x
19. Gish RG, Clark MD, Kane SD, Shaw RE, Mangahas MF, Baqai S. Similar risk of renal events among patients treated with tenofovir or entecavir for chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(8):941-e68. doi:10.1016/j.cgh.2012.04.008
20. Gara N, Zhao X, Collins MT, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35(11):1317-1325. doi:10.1111/j.1365-2036.2012.05093.x
21. Tsai HJ, Chuang YW, Lee SW, Wu CY, Yeh HZ, Lee TY. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47(12):1673-1681. doi:10.1111/apt.14682
22. Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145-1152. doi:10.1111/j.1523-1755.2004.00865.x
Protein expression may predict HBV DNA suppression
Stopping nucleoside analog therapy in patients with hepatitis B viral (HBV) infections results in sustained viral suppression in only a minority of patients, but a new study suggests there are immune signatures that may serve as predictive biomarkers to help clinicians determine how to improve immune responses in these patients, according to investigators.
In a study of 359 patients enrolled in clinical trials of antiviral therapy for HBV infections, there were 29 immune-related proteins that were found in significantly higher levels among patients who continued to have viral suppression 24 weeks after the end of treatment, compared with patients who did not maintain viral suppression, reported Henry L.Y. Chan, MD, from the Chinese University of Hong Kong.
“In this study, plasma proteomics shows that sustained HBV suppression following treatment discontinuation is associated with higher levels of innate and adaptive immune responses during treatment, but whether these signatures vary by specific treatment regimens remains to be determined,” he said in an oral session at the meeting sponsored by the European Association for the Study of the Liver.
The clustering of proteins differed between patients treated with nucleoside analogs and those who received pegylated interferon (PEG-IFN), Dr. Chan noted.
Is it safe?
Although current international guidelines say that clinicians may consider stopping nucleoside analogs in certain patient populations with the goal of promoting sustained off-treatment responses, pooled data from four large phase 3 studies showed that only 10% of patients had sustained HBV DNA suppression, and only 32% had persistent low-level viremia, Dr. Chan said, citing a presentation from ILC in 2019.
Dr. Chan and colleagues sought to identify immune biomarkers that at the end of treatment predict HBV off-treatment response. This is important because existing treatments do not kill the virus which – even if suppressed – can lead to hepatocellular carcinoma.
The researchers examined plasma samples from patients with chronic hepatitis B who were enrolled in two studies: a registrational study comparing tenofovir disoproxil fumarate with adefovir followed by tenofovir maintenance (GS-US-174-0102) and one comparing TDF plus PEG-IFN with either drug alone (GS-US-174-0149).
They identified a total of 359 patients who had at least two treatment-free follow-up visits, were positive for the hepatitis B S antigen (HBsAg) at the end of the treatment, including patients who had antigen loss on treatment but subsequently seroverted, and had available plasma samples collected before the end of treatment.
The study outcomes were sustained viral suppression 24 weeks after the end of treatment, defined as HBV DNA less than 29 IU/mL, and a low replicative state defined as HBV DNA below 2,000 IU/mL with ALT levels at or below the upper limit of normal.
The median patient age was 39 years. In all, 67% of the population was male, and 70% were Asian.
Immune-related proteins
The investigators performed proteomic analyses looking for expression levels in serum or plasma proteins at the end of treatment.
A total of 25 patients had HBV DNA suppression at posttreatment week 24, 111 patients had a low replicative states, and 4 had HBsAg loss.
The patients with HBV DNA suppression had significantly higher expression of 29 immune-related proteins, the majority of which were related to the host immune response.
The proteins included myeloid cell markers, leukocyte-trafficking chemokines, natural killer cell markers, and extracellular matrix and/or extracellular matrix–associated proteins.
Among patients with HBV suppression, there was evidence of enrichment for extracellular remodeling pathways, as well as pathways involved in innate immune response to viral infections and immune regulation.
Among patients with low viral replication, there was a trend toward higher CD8a expression levels at the 24-week follow-up, but there were no proteins with significantly elevated expression levels.
“Assessment of unique protein signatures associated with HBsAg loss following treatment discontinuation is ongoing,” Dr. Chan said.
Timing of expression patterns
During the question-and-answer session following his presentation, comoderator Pablo Sarobe, MD, from the Clinica Universidad de Navarra (Spain), said: “I’ve seen that you have compared the different proteins which are detected in your cell samples 24 weeks after stopping treatment. Do you think that these differences are already relevant just at the end of treatment, or that these proteins are being expressed [during] the 24 weeks between the end of treatment and your determination?”
“We only have one time-point sample, so it’s hard to say,” Dr. Chan replied, but he speculated that the delay would not have a direct impact on protein expression, “so probably this expression should last after treatment has stopped. But we only have only posttreatment 24-week data, and we believe that some of the outcome measures may change with longer follow-up. After 1 year some patients in suppression may relapse.”
Asked by an audience member whether the investigators had performed a subanalysis of patients treated with nucleoside analogs, Dr. Chan noted that such an analysis was under consideration, although the patient numbers were relatively small. He did add, however, that protein expression patterns differed among patients treated with nucleoside analogs and PEG-IFN.
The study was funded by Gilead Sciences. Dr. Chan disclosed sponsored lecture activities and consulting for Gilead and others. Dr. Sarobe reported no conflicts of interest.
Stopping nucleoside analog therapy in patients with hepatitis B viral (HBV) infections results in sustained viral suppression in only a minority of patients, but a new study suggests there are immune signatures that may serve as predictive biomarkers to help clinicians determine how to improve immune responses in these patients, according to investigators.
In a study of 359 patients enrolled in clinical trials of antiviral therapy for HBV infections, there were 29 immune-related proteins that were found in significantly higher levels among patients who continued to have viral suppression 24 weeks after the end of treatment, compared with patients who did not maintain viral suppression, reported Henry L.Y. Chan, MD, from the Chinese University of Hong Kong.
“In this study, plasma proteomics shows that sustained HBV suppression following treatment discontinuation is associated with higher levels of innate and adaptive immune responses during treatment, but whether these signatures vary by specific treatment regimens remains to be determined,” he said in an oral session at the meeting sponsored by the European Association for the Study of the Liver.
The clustering of proteins differed between patients treated with nucleoside analogs and those who received pegylated interferon (PEG-IFN), Dr. Chan noted.
Is it safe?
Although current international guidelines say that clinicians may consider stopping nucleoside analogs in certain patient populations with the goal of promoting sustained off-treatment responses, pooled data from four large phase 3 studies showed that only 10% of patients had sustained HBV DNA suppression, and only 32% had persistent low-level viremia, Dr. Chan said, citing a presentation from ILC in 2019.
Dr. Chan and colleagues sought to identify immune biomarkers that at the end of treatment predict HBV off-treatment response. This is important because existing treatments do not kill the virus which – even if suppressed – can lead to hepatocellular carcinoma.
The researchers examined plasma samples from patients with chronic hepatitis B who were enrolled in two studies: a registrational study comparing tenofovir disoproxil fumarate with adefovir followed by tenofovir maintenance (GS-US-174-0102) and one comparing TDF plus PEG-IFN with either drug alone (GS-US-174-0149).
They identified a total of 359 patients who had at least two treatment-free follow-up visits, were positive for the hepatitis B S antigen (HBsAg) at the end of the treatment, including patients who had antigen loss on treatment but subsequently seroverted, and had available plasma samples collected before the end of treatment.
The study outcomes were sustained viral suppression 24 weeks after the end of treatment, defined as HBV DNA less than 29 IU/mL, and a low replicative state defined as HBV DNA below 2,000 IU/mL with ALT levels at or below the upper limit of normal.
The median patient age was 39 years. In all, 67% of the population was male, and 70% were Asian.
Immune-related proteins
The investigators performed proteomic analyses looking for expression levels in serum or plasma proteins at the end of treatment.
A total of 25 patients had HBV DNA suppression at posttreatment week 24, 111 patients had a low replicative states, and 4 had HBsAg loss.
The patients with HBV DNA suppression had significantly higher expression of 29 immune-related proteins, the majority of which were related to the host immune response.
The proteins included myeloid cell markers, leukocyte-trafficking chemokines, natural killer cell markers, and extracellular matrix and/or extracellular matrix–associated proteins.
Among patients with HBV suppression, there was evidence of enrichment for extracellular remodeling pathways, as well as pathways involved in innate immune response to viral infections and immune regulation.
Among patients with low viral replication, there was a trend toward higher CD8a expression levels at the 24-week follow-up, but there were no proteins with significantly elevated expression levels.
“Assessment of unique protein signatures associated with HBsAg loss following treatment discontinuation is ongoing,” Dr. Chan said.
Timing of expression patterns
During the question-and-answer session following his presentation, comoderator Pablo Sarobe, MD, from the Clinica Universidad de Navarra (Spain), said: “I’ve seen that you have compared the different proteins which are detected in your cell samples 24 weeks after stopping treatment. Do you think that these differences are already relevant just at the end of treatment, or that these proteins are being expressed [during] the 24 weeks between the end of treatment and your determination?”
“We only have one time-point sample, so it’s hard to say,” Dr. Chan replied, but he speculated that the delay would not have a direct impact on protein expression, “so probably this expression should last after treatment has stopped. But we only have only posttreatment 24-week data, and we believe that some of the outcome measures may change with longer follow-up. After 1 year some patients in suppression may relapse.”
Asked by an audience member whether the investigators had performed a subanalysis of patients treated with nucleoside analogs, Dr. Chan noted that such an analysis was under consideration, although the patient numbers were relatively small. He did add, however, that protein expression patterns differed among patients treated with nucleoside analogs and PEG-IFN.
The study was funded by Gilead Sciences. Dr. Chan disclosed sponsored lecture activities and consulting for Gilead and others. Dr. Sarobe reported no conflicts of interest.
Stopping nucleoside analog therapy in patients with hepatitis B viral (HBV) infections results in sustained viral suppression in only a minority of patients, but a new study suggests there are immune signatures that may serve as predictive biomarkers to help clinicians determine how to improve immune responses in these patients, according to investigators.
In a study of 359 patients enrolled in clinical trials of antiviral therapy for HBV infections, there were 29 immune-related proteins that were found in significantly higher levels among patients who continued to have viral suppression 24 weeks after the end of treatment, compared with patients who did not maintain viral suppression, reported Henry L.Y. Chan, MD, from the Chinese University of Hong Kong.
“In this study, plasma proteomics shows that sustained HBV suppression following treatment discontinuation is associated with higher levels of innate and adaptive immune responses during treatment, but whether these signatures vary by specific treatment regimens remains to be determined,” he said in an oral session at the meeting sponsored by the European Association for the Study of the Liver.
The clustering of proteins differed between patients treated with nucleoside analogs and those who received pegylated interferon (PEG-IFN), Dr. Chan noted.
Is it safe?
Although current international guidelines say that clinicians may consider stopping nucleoside analogs in certain patient populations with the goal of promoting sustained off-treatment responses, pooled data from four large phase 3 studies showed that only 10% of patients had sustained HBV DNA suppression, and only 32% had persistent low-level viremia, Dr. Chan said, citing a presentation from ILC in 2019.
Dr. Chan and colleagues sought to identify immune biomarkers that at the end of treatment predict HBV off-treatment response. This is important because existing treatments do not kill the virus which – even if suppressed – can lead to hepatocellular carcinoma.
The researchers examined plasma samples from patients with chronic hepatitis B who were enrolled in two studies: a registrational study comparing tenofovir disoproxil fumarate with adefovir followed by tenofovir maintenance (GS-US-174-0102) and one comparing TDF plus PEG-IFN with either drug alone (GS-US-174-0149).
They identified a total of 359 patients who had at least two treatment-free follow-up visits, were positive for the hepatitis B S antigen (HBsAg) at the end of the treatment, including patients who had antigen loss on treatment but subsequently seroverted, and had available plasma samples collected before the end of treatment.
The study outcomes were sustained viral suppression 24 weeks after the end of treatment, defined as HBV DNA less than 29 IU/mL, and a low replicative state defined as HBV DNA below 2,000 IU/mL with ALT levels at or below the upper limit of normal.
The median patient age was 39 years. In all, 67% of the population was male, and 70% were Asian.
Immune-related proteins
The investigators performed proteomic analyses looking for expression levels in serum or plasma proteins at the end of treatment.
A total of 25 patients had HBV DNA suppression at posttreatment week 24, 111 patients had a low replicative states, and 4 had HBsAg loss.
The patients with HBV DNA suppression had significantly higher expression of 29 immune-related proteins, the majority of which were related to the host immune response.
The proteins included myeloid cell markers, leukocyte-trafficking chemokines, natural killer cell markers, and extracellular matrix and/or extracellular matrix–associated proteins.
Among patients with HBV suppression, there was evidence of enrichment for extracellular remodeling pathways, as well as pathways involved in innate immune response to viral infections and immune regulation.
Among patients with low viral replication, there was a trend toward higher CD8a expression levels at the 24-week follow-up, but there were no proteins with significantly elevated expression levels.
“Assessment of unique protein signatures associated with HBsAg loss following treatment discontinuation is ongoing,” Dr. Chan said.
Timing of expression patterns
During the question-and-answer session following his presentation, comoderator Pablo Sarobe, MD, from the Clinica Universidad de Navarra (Spain), said: “I’ve seen that you have compared the different proteins which are detected in your cell samples 24 weeks after stopping treatment. Do you think that these differences are already relevant just at the end of treatment, or that these proteins are being expressed [during] the 24 weeks between the end of treatment and your determination?”
“We only have one time-point sample, so it’s hard to say,” Dr. Chan replied, but he speculated that the delay would not have a direct impact on protein expression, “so probably this expression should last after treatment has stopped. But we only have only posttreatment 24-week data, and we believe that some of the outcome measures may change with longer follow-up. After 1 year some patients in suppression may relapse.”
Asked by an audience member whether the investigators had performed a subanalysis of patients treated with nucleoside analogs, Dr. Chan noted that such an analysis was under consideration, although the patient numbers were relatively small. He did add, however, that protein expression patterns differed among patients treated with nucleoside analogs and PEG-IFN.
The study was funded by Gilead Sciences. Dr. Chan disclosed sponsored lecture activities and consulting for Gilead and others. Dr. Sarobe reported no conflicts of interest.
FROM ILC 2021
Treating Hepatitis C Virus Reinfection With 8 Weeks of Ledipasvir/Sofosbuvir Achieves Sustained Virologic Response
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
Three patients reinfected with hepatitis C virus after a sustained virologic response were considered treatment naïve and treated with a short-course direct acting antiviral regimen.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
To decrease the incidence and prevalence of hepatitis C virus (HCV) in the United States, hepatology experts, public health officials, and patient advocates agree that linkage to care is essential for treatment of people who inject drugs (PWID). The most recent surveillance report from the Centers for Disease Control and Prevention (CDC) estimates that injection drug use accounts for the transmission of approximately 72% of new HCV infections.1,2
Although recent studies of direct-acting antiviral (DAA) agents have not been designed to investigate the long-term rates of reinfection in this population, various population-based studies in multiple countries have attempted to describe the rate of reinfection for this cohort.3-7 This rate varies widely based on the defined population of PWID, definition of reinfection, and the prevalence of HCV in a given PWID population. However, studies have consistently shown a relatively low historic rate of reinfection, which varies from 1 to 5 per 100 person-years in patients who have ever injected drugs, to 3 to 33 per 100 person-years in patients who continue injection drug use (IDU). Higher rates are found in those who engage in high-risk behaviors such as needle sharing.3-7 Yet, the US opioid crisis is attributable to a recent rise in both overall incidence and reinfections, highlighting the importance of determining the best treatment strategy for those who become reinfected.1
Current HCV guidelines from the American Association for the Study of Liver Diseases AASLD) and Infectious Diseases Society of America (IDSA) encourage access to retreatment for PWID who become reinfected, stating that new reinfections should follow treatment-naïve therapy recommendations.8 However, to date this recommendation has not been validated by published clinical trials or patient case reports. This is likely due in part both to the small number of reinfections among PWID requiring retreatment and barriers to payment for treatment, particularly for individuals with substance use disorders.9 While this recommendation can be found under the key population section for the “Identification and Management of HCV in People Who Inject Drugs,” health care providers (HCPs) may easily miss this statement if they alternatively refer to the “Treatment-Experienced” section that recommends escalation to either sofosbuvir/velpatasvir/voxilaprevir or glecaprevir/pibrentasvir in patients who are NS5A inhibitor DAA-experienced.8 Anecdotally, the first instinct for many HCPs when considering a treatment regimen for a reinfected patient is to refer to treatment-experienced regimen recommendations rather than appreciating the reinfected virus to be treatment naïve.
A treatment-escalation approach could have the consequence of limiting the number of times a patient could undergo treatment on successive reinfections. Additionally, these retreatment regimens often are more expensive, resulting in further cost barriers for payors approving retreatment for individuals with HCV reinfection. In contrast, demonstrating efficacy of a less costly short-course regimen would support increased access to initial and retreatment courses for PWID. The implications of enabling improved access to care is essential in the setting of the ongoing opioid epidemic in the United States.
Given the perspective that the virus should be considered treatment naïve for patients who become reinfected, we describe here 3 cases of patients previously achieving sustained virologic response (SVR) being retreated with the cost-effective 8-week regimen of ledipasvir/sofosbuvir following reinfection.
Case Reports
Case 1
A 59-year-old male presented for his third treatment course for HCV genotype 1a. The patient initially underwent 76 weeks of interferon-based HCV treatment in 2007 and 2008, from which he was determined to have achieved SVR in 24 weeks (SVR24) in April 2009. His viral load remained undetected through February 2010 but subsequently had detectable virus again in 2011 following relapsed use of alcohol, cocaine, and injection drugs. The patient elected to await approval of DAAs and eventually completed an 8-week regimen of ledipasvir/sofosbuvir from May to July 2016, achieving SVR24 in December 2016. The patient’s viral load was rechecked in October 2018 and he was again viremic following recent IDU, suggesting a second reinfection.
In preparation for his third HCV treatment, the patient was included in shared decision making to consider retreating his de novo infection as treatment naïve to provide a briefer (ie, 8 weeks) and more cost-effective treatment given his low likelihood of advanced fibrotic liver disease—his FibroScan score was 6.5 kPa, whereas scores ≥ 12.5 kPa in patients with chronic HCV suggest a higher likelihood of cirrhosis.10 At week 4, the patient’s viral load was undetected, he completed his 8-week regimen of ledipasvir/sofosbuvir as planned and achieved SVR12 (Table). He had reported excellent adherence throughout treatment with assistance of a pill box and validated by a reported pill count.
Case 2
A 32-year-old male presented with HCV genotype 1a. Like case 1, this patient had a low FibroScan score of 4.7 kPa. He was previously infected with genotype 3 and completed a 12-week course of sofosbuvir/velpatasvir in November 2016. He achieved SVR12 as evidenced by an undetected viral load in February 2017 despite questionable adherence throughout and relapsed use of heroin by the end of his regimen. He continued intermittent IDU and presented in October 2018 with a detectable viral load, now with genotype 1a. The patient similarly agreed to undergo an 8-week regimen of ledipasvir/sofosbuvir, considering his de novo infection to be treatment naïve. His viral load at treatment week 3 was quantitatively negative while qualitatively detectable at < 15 U/mL. He completed his treatment course in March 2019 and was determined to have achieved SVR24 in September 2019.
Case 3
A 51-year-old male presented with a history of HCV genotype 1a and a low FibroScan score (4.9 kPa ). The patient was previously infected with genotype 2 and had achieved SVR24 following a 12-week regimen of sofosbuvir/velpatasvir in 2017. The patient subsequently was reinfected with genotype 1a and completed an 8-week course of ledipasvir/sofosbuvir in May 2019. The patient had his SVR12 lab drawn 9 days early and was undetectable at that time. He reported 0 missed doses during treatment and achieved an undetected viral load by treatment week 4.
Discussion
We demonstrate that HCV reinfection after treatment with previous interferon and/or DAA-based regimens can be treated with less costly 8-week treatment regimens. Current guidelines include a statement allowing for reinfected patients to follow initial treatment guidelines, but this statement has previously lacked published evidence and may be overlooked by HCPs who refer to recommendations for treatment-experienced patients. Given the increasing likelihood of HCPs encountering patients who have become reinfected with HCV after achieving SVR from a DAA regimen, further delineation may be needed in the recommendations for treatment-experienced patients to highlight the important nuance of recognizing that reinfections should follow initial treatment guidance.
While all 3 of these cases met criteria for the least costly and simplest 1 pill once daily 8-week regimen of ledipasvir/sofosbuvir, patients requiring retreatment with alternative genotypes or evidence of advanced fibrotic liver disease could benefit from a similar approach of using the least expensive and/or shortest duration regimen for which they meet eligibility. With this approach, coverage could be further expanded to the PWID population to help limit HCV transmission amid the opioid crisis.1
Studies have established that PWID are able to achieve similar SVR efficacy rates similar to that of the general population when treated in the setting of an interdisciplinary treatment team that offers collaborative management of complex psychosocial comorbidities and harm reduction strategies.11,12 These integrative patient-centric strategies may include personalized behavioral health pretreatment evaluations, access to substance use treatment, harm reduction counseling, needle exchange programs, and close follow-up by a case manager.2,13 Current DAA regimens combined with 1 or more of these strategies have demonstrated SVR12 rates of 90 to 95% for initial treatment regimens.11 These high SVR12 rates were even achieved in a recent study in which 74% (76/103) of participants had self-reported IDU within 30 days of HCV treatment start and similar IDU rates throughout treatment.12 A meta-analysis, including real-world studies of DAA treatment outcomes yielded a pooled SVR of 88% (95% CI, 83‐92%) for recent PWID and 91% (95% CI, 88‐95%) for individuals using opiate substitution therapy (OST).14 Additionally, linking PWID with OST also reduces risk for reinfection.14,15
For any patient with detectable HCV after completing the initial DAA regimen, it is important to distinguish between relapse and reinfection. SVR12 is generally synonymous with a clinical cure. Patients with ongoing risk factors posttreatment should continue to have their HCV viral load monitored for evidence of reinfection. Patients without known risk factors may benefit from repeat viral load only if there is clinical concern for reinfection, for example, a rise in liver enzymes.
We have shown that patients with ongoing risk factors who are reinfected can be treated successfully with cost-effective 8-week regimens. For comparison this 8-week regimen of ledipasvir/sofosbuvir has an average wholesale price (AWP) of $28,800, while alternative regimens approved for treatment-naïve patients vary in AWP from $31,680 to $43,200, and regimens approved for retreatment of DAA failures have an AWP as high as $89,712.
An 8-week treatment regimen for both initial and reinfection regimens affords many advantages in medication adherence and both medication and provider resource cost-effectiveness. First, new HCV reinfections are disproportionally younger individuals often with complex psychosocial issues that impact retention in treatment. An 8-week course of treatment can be initiated concurrently with substance abuse treatment programs, including intensive outpatient programs and residential treatment programs that are usually at least 28 days. Many of these programs provide aftercare options that would extend the entire course of treatment. These opportunities afford individuals to receive HCV treatment in a setting that supports medication adherence, sobriety efforts, and education on harm reduction to reduce risk for reinfection.
Finally, statistical models indicate eradication of HCV will require scaling up the treatment of PWID in conjunction with harm reduction strategies such as OST and needle exchange programs.16 In contrast, there are low risks associated with retreatment given these medications are well-tolerated, treatment of PWID lowers the risk of further HCV transmission, and the understanding of these reinfections being treatment naïve disavows concerns of these patients having resistance to regimens that cleared their prior infections. The opportunity to provide retreatment without escalating regimen complexity or cost increases access to care for a vulnerable population while aiding in the eradication of HCV.
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
1. Centers for Disease Control and Prevention. Viral Hepatitis Surveillance - United States, 2018. Updated August 28, 2020. Accessed May 18, 2021. https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepC.htm 2. Grebely J, Robaeys G, Bruggmann P, et al; International Network for Hepatitis in Substance Users. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(10):1028-1038. doi:10.1016/j.drugpo.2015.07.005
3. Marco A, Esteban JI, Solé C, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45-51. doi:10.1016/j.jhep.2013.03.008
4. Midgard H, Bjøro B, Mæland A, et al. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64(5):1020-1026. doi:10.1016/j.jhep.2016.01.001
5. Currie SL, Ryan JC, Tracy D, et al. A prospective study to examine persistent HCV reinfection in injection drug users who have previously cleared the virus [published correction appears in Drug Alcohol Depend. 2008 Jul;96(1-2):192]. Drug Alcohol Depend. 2008;93(1-2):148-154. doi:10.1016/j.drugalcdep.2007.09.011
6. Grady BP, Vanhommerig JW, Schinkel J, et al. Low incidence of reinfection with the hepatitis C virus following treatment in active drug users in Amsterdam. Eur J Gastroenterol Hepatol. 2012;24(11):1302-1307. doi:10.1097/MEG.0b013e32835702a8
7. Grebely J, Pham ST, Matthews GV, et al; ATAHC Study Group. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55(4):1058-1069. doi:10.1002/hep.24754
8. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed May 26, 2021. https://www.hcvguidelines.org
9. National Viral Hepatitis Roundtable, Center for Health Law and Policy Innovation, Harvard Law School. Hepatitis C: The State of Medicaid Access. 2017 National Summary Report. Updated October 23, 2017. Accessed May 26, 2021. https://hepcstage.wpengine.com/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf
10. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544-1577. doi:10.1053/j.gastro.2017.03.016
11. Dore GJ, Altice F, Litwin AH, et al; C-EDGE CO-STAR Study Group. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165(9):625-634. doi:10.7326/M16-0816
12. Grebely J, Dalgard O, Conway B, et al; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(3):153-161. doi:10.1016/S2468-1253(17)30404-1
13. Cos TA, Bartholomew TS, Huynh, KJ. Role of behavioral health providers in treating hepatitis C. Professional Psychol Res Pract. 2019;50(4):246–254. doi:10.1037/pro0000243
14. Latham NH, Doyle JS, Palmer AY, et al. Staying hepatitis C negative: a systematic review and meta-analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39(12):2244-2260. doi:10.1111/liv.14152
15. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9(9):CD012021. Published 2017 Sep 18. doi:10.1002/14651858.CD012021.pub2
16. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402-411. doi:10.1016/j.jhep.2017.10.010
Liver transplant outcomes improving for U.S. patients with HIV/HCV
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
While liver transplant outcomes were historically poor in people coinfected with HIV and hepatitis C virus (HCV), they have improved significantly in the era of direct-acting antiviral (DAA) therapy, a recent analysis of U.S. organ transplant data showed.
The availability of highly potent DAA therapy should change how transplant specialists view patients coinfected with HIV/HCV who need a liver transplant, according to researcher Jennifer Wang, MD, chief gastroenterology fellow at the University of Chicago, who presented the results of the analysis at the annual Digestive Disease Week® (DDW). Cumulative graft survival rates since the introduction of DAAs are comparable between transplant recipients with HIV/HCV coinfection and recipients who are both HIV and HCV negative, according to the study.
“Having hepatitis C no longer confers worse patient survival in the DAA era, and this is the main takeaway from our study,” Dr. Wang said.
The study also showed that the number of liver transplants among HIV-infected patients has increased over the past 4-5 years. However, the absolute number remains low at 64 cases in 2019, or less than 1% of all liver transplants that year, and only about one-third of those HIV-positive recipients had HCV coinfection, according to Dr. Wang.
Moreover, relatively few centers are performing liver transplants for patients who are HIV/HCV coinfected, and there is significant geographic variation in where the procedures are done, she said in her presentation.
Reassuring data that should prompt referral
Taken together, these results should offer reassurance to transplant centers that patients coinfected with HIV/HCV are no longer at increased risk for poor outcomes after transplantation, said Christine M. Durand, MD, associate professor of medicine at Johns Hopkins University, Baltimore.
“The additional call for action should be beyond the transplantation community to ensure that referrals for liver transplant are where they should be,” Dr. Durand said in an interview.
“With a number of only 64 transplants a year, we’re not doing enough, and there are more patients that could benefit from liver transplants,” added Dr. Durand, who is principal investigator of HOPE in Action, a prospective, multicenter, clinical trial evaluating the safety and survival outcomes of HIV-positive deceased donor liver transplants in HIV-positive recipients.
Impact of the HOPE Act
Liver transplantation for HIV-positive patients has increased since the signing of the HIV Organ Policy Equity (HOPE) Act in 2013, according to Dr. Wang.
The HOPE act expanded the donor pool to include HIV-positive deceased donors, which not only increased the donor supply overall, but specifically helped HIV-positive individuals, who experience a higher rate of waiting-list mortality, according to a review on the topic authored by Dr. Durand and coauthors.
However, some transplant centers may be reluctant to do liver transplants in HIV-positive patients coinfected with HCV. That’s because, in previous studies that were conducted before the DAA era, outcomes after liver transplant in HIV/HCV-coinfected patients were inferior to those in patients with HIV but no HCV infection, Dr. Wang said.
Accordingly, Dr. Wang and colleagues analyzed Organ Procurement and Transplantation Network (OPTN) data on adult patients who underwent liver transplants between 2008 and 2019 to see if the introduction of DAAs had leveled the playing field for those with HCV coinfection.
Progress in a still-underserved population
The practice of liver transplant in the HIV population has been increasing since the HOPE Act, according to Dr. Wang.
Overall, out of 70,125 liver transplant recipients over the 2008-2019 period, 416 (0.6%) were HIV infected, the data show.
In 2014, 28 liver transplants (0.5%) were performed in HIV-infected individuals, which increased to 64 transplants (0.8%) in 2019, data show. Of those 64 HIV-positive liver transplant recipients in 2019, 23 (35.9%) were coinfected with HCV.
Graft survival has greatly improved, from a 3-year survival of only 58% in patients transplanted before the availability of DAAs to 82% in the DAA era, a difference that was statistically significant, Dr. Wang said.
In the DAA era, there was no significant difference in graft failure outcomes when comparing HIV/HCV-coinfected recipients with uninfected recipients, she added.
The largest proportion of liver transplantations in HIV/HCV-coinfected recipients have been done in OPTN Region 9 (New York), both in the pre- and post-DAA eras, according to Dr. Wang. Several regions have very low numbers or have performed no liver transplants in HIV/HCV-coinfected patients in either era.
“The number of transplant centers participating in liver transplant for coinfected patients is still quite low, so this is a very underserved patient population,” Dr. Wang said.
Dr. Wang provided no financial disclosures related to the research. Dr. Durand receives grants to the institution from Abbvie and GlaxoSmithKline and she receives honoraria from Gilead Sciences for serving on a grant review committee.
FROM DDW 2021
Treatment paradigm for chronic HBV in flux
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
FROM GUILD 2021
New ‘minimal monitoring’ approach to HCV treatment may simplify care
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
FROM CROI 2021