User login
Good news, bad news about HCV in kidney disease
SAN DIEGO – There’s good news and bad news about hepatitis C virus (HCV) in patients with chronic kidney disease (CKD): The new generation of drugs that cures HCV is effective in this population, but outbreaks of infection are still plaguing the nation’s dialysis clinics.
These perspectives came in a presentation about infections in CKD at Kidney Week 2018, sponsored by the American Society of Nephrology.
First, the good news about HCV. “Treatment is now feasible for all stages of chronic kidney disease,” said gastroenterologist Paul Martin, MD, of the University of Miami. “It was possible to achieve biological cure in 99% of patients, which is truly remarkable considering what a problem kidney patients were for hepatitis C until very recently.”
The key is to treat HCV with drug combinations that lower the risk of viral resistance. “These drugs are extremely well tolerated. They’re not like interferon or ribavirin,” he said, referring to a drug combo that was formerly used to treat HCV. “We can anticipate curing hepatitis C with a finite amount of therapy in virtually every patient we see, including those with kidney disease.”
In patients with CKD, all the new drugs are approved for glomerular filtration rates greater than 30 mL/min. Sofosbuvir (Sovaldi) is not approved for patients with a filtration rate under 30 mL/min, he said, but other options are available.
Ribavirin, he added, is no longer needed with current regimens.
Dr. Martin pointed to two studies that reveal the power of the new regimens against HCV in patients with CKD. One of the studies, a 2015 industry-funded report in the Lancet, found that “once-daily grazoprevir and elbasvir for 12 weeks had a low rate of adverse events and was effective in patients infected with HCV genotype 1 and stage 4-5 chronic kidney disease.” The other study, also funded by industry and published in 2017 in the New England Journal of Medicine, found that “treatment with glecaprevir and pibrentasvir for 12 weeks resulted in a high rate of sustained virologic response in patients with stage 4 or 5 chronic kidney disease and HCV infection.”
Meanwhile, there are signs that HCV treatment may boost survival in CKD patients on dialysis, Dr. Martin said.
In terms of bad news, Priti R. Patel, MD, MPH, a medical officer with the Centers for Disease Control and Prevention, warned that dialysis clinics are still seeing HCV outbreaks. “It’s a continuing problem,” she said. “What we hear about at the CDC is the tip of the iceberg.”
The CDC says it received word of 21 HCV outbreaks of two or more cases in dialysis clinics during 2008-2017. These affected 102 patients, and more than 3,000 patients were notified that they were at risk and should be screened.
One dialysis clinic in Philadelphia had 18 cases of HCV during 2008-2013; they were blamed on “multiple lapses in infection control ... including hand hygiene and glove use, vascular access care, medication preparation, cleaning, and disinfection.”
“There should be no more than one case that has to happen for a facility to detect that it has a problem and identify a solution,” Dr. Patel said.
Since acute HCV can appear without symptoms, every dialysis patients should be tested for HCV antibodies, she added. “If it’s positive, confirm it. If confirmed, they should be informed of their infection status and have an evaluation for treatment.”
Dr. Martin reported consulting for Bristol-Myers Squibb and AbbVie and receiving research funding from Gilead, Bristol-Myers Squibb, AbbVie, and Merck. Dr. Patel reported no disclosures.
SAN DIEGO – There’s good news and bad news about hepatitis C virus (HCV) in patients with chronic kidney disease (CKD): The new generation of drugs that cures HCV is effective in this population, but outbreaks of infection are still plaguing the nation’s dialysis clinics.
These perspectives came in a presentation about infections in CKD at Kidney Week 2018, sponsored by the American Society of Nephrology.
First, the good news about HCV. “Treatment is now feasible for all stages of chronic kidney disease,” said gastroenterologist Paul Martin, MD, of the University of Miami. “It was possible to achieve biological cure in 99% of patients, which is truly remarkable considering what a problem kidney patients were for hepatitis C until very recently.”
The key is to treat HCV with drug combinations that lower the risk of viral resistance. “These drugs are extremely well tolerated. They’re not like interferon or ribavirin,” he said, referring to a drug combo that was formerly used to treat HCV. “We can anticipate curing hepatitis C with a finite amount of therapy in virtually every patient we see, including those with kidney disease.”
In patients with CKD, all the new drugs are approved for glomerular filtration rates greater than 30 mL/min. Sofosbuvir (Sovaldi) is not approved for patients with a filtration rate under 30 mL/min, he said, but other options are available.
Ribavirin, he added, is no longer needed with current regimens.
Dr. Martin pointed to two studies that reveal the power of the new regimens against HCV in patients with CKD. One of the studies, a 2015 industry-funded report in the Lancet, found that “once-daily grazoprevir and elbasvir for 12 weeks had a low rate of adverse events and was effective in patients infected with HCV genotype 1 and stage 4-5 chronic kidney disease.” The other study, also funded by industry and published in 2017 in the New England Journal of Medicine, found that “treatment with glecaprevir and pibrentasvir for 12 weeks resulted in a high rate of sustained virologic response in patients with stage 4 or 5 chronic kidney disease and HCV infection.”
Meanwhile, there are signs that HCV treatment may boost survival in CKD patients on dialysis, Dr. Martin said.
In terms of bad news, Priti R. Patel, MD, MPH, a medical officer with the Centers for Disease Control and Prevention, warned that dialysis clinics are still seeing HCV outbreaks. “It’s a continuing problem,” she said. “What we hear about at the CDC is the tip of the iceberg.”
The CDC says it received word of 21 HCV outbreaks of two or more cases in dialysis clinics during 2008-2017. These affected 102 patients, and more than 3,000 patients were notified that they were at risk and should be screened.
One dialysis clinic in Philadelphia had 18 cases of HCV during 2008-2013; they were blamed on “multiple lapses in infection control ... including hand hygiene and glove use, vascular access care, medication preparation, cleaning, and disinfection.”
“There should be no more than one case that has to happen for a facility to detect that it has a problem and identify a solution,” Dr. Patel said.
Since acute HCV can appear without symptoms, every dialysis patients should be tested for HCV antibodies, she added. “If it’s positive, confirm it. If confirmed, they should be informed of their infection status and have an evaluation for treatment.”
Dr. Martin reported consulting for Bristol-Myers Squibb and AbbVie and receiving research funding from Gilead, Bristol-Myers Squibb, AbbVie, and Merck. Dr. Patel reported no disclosures.
SAN DIEGO – There’s good news and bad news about hepatitis C virus (HCV) in patients with chronic kidney disease (CKD): The new generation of drugs that cures HCV is effective in this population, but outbreaks of infection are still plaguing the nation’s dialysis clinics.
These perspectives came in a presentation about infections in CKD at Kidney Week 2018, sponsored by the American Society of Nephrology.
First, the good news about HCV. “Treatment is now feasible for all stages of chronic kidney disease,” said gastroenterologist Paul Martin, MD, of the University of Miami. “It was possible to achieve biological cure in 99% of patients, which is truly remarkable considering what a problem kidney patients were for hepatitis C until very recently.”
The key is to treat HCV with drug combinations that lower the risk of viral resistance. “These drugs are extremely well tolerated. They’re not like interferon or ribavirin,” he said, referring to a drug combo that was formerly used to treat HCV. “We can anticipate curing hepatitis C with a finite amount of therapy in virtually every patient we see, including those with kidney disease.”
In patients with CKD, all the new drugs are approved for glomerular filtration rates greater than 30 mL/min. Sofosbuvir (Sovaldi) is not approved for patients with a filtration rate under 30 mL/min, he said, but other options are available.
Ribavirin, he added, is no longer needed with current regimens.
Dr. Martin pointed to two studies that reveal the power of the new regimens against HCV in patients with CKD. One of the studies, a 2015 industry-funded report in the Lancet, found that “once-daily grazoprevir and elbasvir for 12 weeks had a low rate of adverse events and was effective in patients infected with HCV genotype 1 and stage 4-5 chronic kidney disease.” The other study, also funded by industry and published in 2017 in the New England Journal of Medicine, found that “treatment with glecaprevir and pibrentasvir for 12 weeks resulted in a high rate of sustained virologic response in patients with stage 4 or 5 chronic kidney disease and HCV infection.”
Meanwhile, there are signs that HCV treatment may boost survival in CKD patients on dialysis, Dr. Martin said.
In terms of bad news, Priti R. Patel, MD, MPH, a medical officer with the Centers for Disease Control and Prevention, warned that dialysis clinics are still seeing HCV outbreaks. “It’s a continuing problem,” she said. “What we hear about at the CDC is the tip of the iceberg.”
The CDC says it received word of 21 HCV outbreaks of two or more cases in dialysis clinics during 2008-2017. These affected 102 patients, and more than 3,000 patients were notified that they were at risk and should be screened.
One dialysis clinic in Philadelphia had 18 cases of HCV during 2008-2013; they were blamed on “multiple lapses in infection control ... including hand hygiene and glove use, vascular access care, medication preparation, cleaning, and disinfection.”
“There should be no more than one case that has to happen for a facility to detect that it has a problem and identify a solution,” Dr. Patel said.
Since acute HCV can appear without symptoms, every dialysis patients should be tested for HCV antibodies, she added. “If it’s positive, confirm it. If confirmed, they should be informed of their infection status and have an evaluation for treatment.”
Dr. Martin reported consulting for Bristol-Myers Squibb and AbbVie and receiving research funding from Gilead, Bristol-Myers Squibb, AbbVie, and Merck. Dr. Patel reported no disclosures.
EXPERT ANALYSIS FROM KIDNEY WEEK 2018
U.S. death rates from chronic liver disease continue to rise
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
AT THE LIVER MEETING 2018
Key clinical point: Nonalcoholic steatohepatitis is very common in the U.S. population.
Major finding: Between 2007 and 2016, the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population to 24.9/100,000 population.
Study details: An analysis of 838,809 chronic liver disease–related deaths from 2007 to 2016.
Disclosures: Dr. Younossi disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, Bristol-Myers Squibb, AbbVie, Viking, Term Quest Diagnostics, Echosens, and Shionogi. He has also received grant/research support from Gilead, Intercept, and Bristol-Myers Squibb.
Source: Hepatol. 2018;68[S1], Abstract 763.
Reviewing the state of HCV and HBV in children
The natural histories of hepatitis B virus (HBV) and hepatitis C virus (HCV) are very different in children, compared with their progress in adults, and depends on age at time of infection, mode of acquisition, ethnicity, and genotype, according to a review in a special pediatric issue of Clinics in Liver Disease.
Most children infected perinatally or vertically continue to be asymptomatic but are at uniquely higher risk of developing chronic viral hepatitis and progressing to liver cirrhosis and hepatocellular carcinoma (HCC), according to Krupa R. Mysore, MD, and Daniel H. Leung, MD, both of the Baylor College of Medicine, Houston. In addition, because the risk of progression to cancer along with such other liver damage is high in children, the reviewers stated that HCV and HBV can be classified as oncoviruses.
Their article assessed overall epidemiology, viral characteristics, and immune responses, as well as prevention, clinical manifestations, and current advances in the treatment of hepatitis B and C in children.
Because of the introduction of universal infant vaccination for HBV in the United States in 1991, the incidence of acute hepatitis B in U.S. children (those aged less than 19 years) has decreased from approximately 13.80/100,000 population (in children aged 10-19 years) in the 1980s to 0.34/100,000 population in 2002, Dr. Mysore and Dr. Leung wrote.
However, they added that those children who have chronic HBV remain at high risk for HCC, with a 100-fold greater incidence, compared with the HBV-negative population.
Similarly, HCV is a significant problem in children, with an estimated prevalence in the United States of 0.2% and 0.4% for children aged 6-11 years and 12-19 years, respectively. Vertical transmission from the mother is responsible for more than 60% of pediatric HCV infection and adds approximately 7,200 new cases in the United States yearly. Older children can acquire the virus through intravenous and intranasal drug use and high-risk sexual activity, they stated.
“Our understanding of the pathobiology and immunology of hepatitis B and C is unprecedented. As new antiviral therapies are being developed for the pediatric population, the differences in management and monitoring between children and adults with HBV and HCV are beginning to narrow but are still important,” the authors wrote.
They pointed out that soon-to-be-available treatments for HCV will be curative in children aged as young as 3 years. “[T]his will change the natural history of HCV and the prevalence of hepatocellular carcinoma over the next several decades for the better,” Dr. Mysore and Dr. Leung concluded.
They reported that they had no conflicts of interest to disclose.
SOURCE: Mysore KR et al. Clin Liver Dis. 2018; 22:703-22.
The natural histories of hepatitis B virus (HBV) and hepatitis C virus (HCV) are very different in children, compared with their progress in adults, and depends on age at time of infection, mode of acquisition, ethnicity, and genotype, according to a review in a special pediatric issue of Clinics in Liver Disease.
Most children infected perinatally or vertically continue to be asymptomatic but are at uniquely higher risk of developing chronic viral hepatitis and progressing to liver cirrhosis and hepatocellular carcinoma (HCC), according to Krupa R. Mysore, MD, and Daniel H. Leung, MD, both of the Baylor College of Medicine, Houston. In addition, because the risk of progression to cancer along with such other liver damage is high in children, the reviewers stated that HCV and HBV can be classified as oncoviruses.
Their article assessed overall epidemiology, viral characteristics, and immune responses, as well as prevention, clinical manifestations, and current advances in the treatment of hepatitis B and C in children.
Because of the introduction of universal infant vaccination for HBV in the United States in 1991, the incidence of acute hepatitis B in U.S. children (those aged less than 19 years) has decreased from approximately 13.80/100,000 population (in children aged 10-19 years) in the 1980s to 0.34/100,000 population in 2002, Dr. Mysore and Dr. Leung wrote.
However, they added that those children who have chronic HBV remain at high risk for HCC, with a 100-fold greater incidence, compared with the HBV-negative population.
Similarly, HCV is a significant problem in children, with an estimated prevalence in the United States of 0.2% and 0.4% for children aged 6-11 years and 12-19 years, respectively. Vertical transmission from the mother is responsible for more than 60% of pediatric HCV infection and adds approximately 7,200 new cases in the United States yearly. Older children can acquire the virus through intravenous and intranasal drug use and high-risk sexual activity, they stated.
“Our understanding of the pathobiology and immunology of hepatitis B and C is unprecedented. As new antiviral therapies are being developed for the pediatric population, the differences in management and monitoring between children and adults with HBV and HCV are beginning to narrow but are still important,” the authors wrote.
They pointed out that soon-to-be-available treatments for HCV will be curative in children aged as young as 3 years. “[T]his will change the natural history of HCV and the prevalence of hepatocellular carcinoma over the next several decades for the better,” Dr. Mysore and Dr. Leung concluded.
They reported that they had no conflicts of interest to disclose.
SOURCE: Mysore KR et al. Clin Liver Dis. 2018; 22:703-22.
The natural histories of hepatitis B virus (HBV) and hepatitis C virus (HCV) are very different in children, compared with their progress in adults, and depends on age at time of infection, mode of acquisition, ethnicity, and genotype, according to a review in a special pediatric issue of Clinics in Liver Disease.
Most children infected perinatally or vertically continue to be asymptomatic but are at uniquely higher risk of developing chronic viral hepatitis and progressing to liver cirrhosis and hepatocellular carcinoma (HCC), according to Krupa R. Mysore, MD, and Daniel H. Leung, MD, both of the Baylor College of Medicine, Houston. In addition, because the risk of progression to cancer along with such other liver damage is high in children, the reviewers stated that HCV and HBV can be classified as oncoviruses.
Their article assessed overall epidemiology, viral characteristics, and immune responses, as well as prevention, clinical manifestations, and current advances in the treatment of hepatitis B and C in children.
Because of the introduction of universal infant vaccination for HBV in the United States in 1991, the incidence of acute hepatitis B in U.S. children (those aged less than 19 years) has decreased from approximately 13.80/100,000 population (in children aged 10-19 years) in the 1980s to 0.34/100,000 population in 2002, Dr. Mysore and Dr. Leung wrote.
However, they added that those children who have chronic HBV remain at high risk for HCC, with a 100-fold greater incidence, compared with the HBV-negative population.
Similarly, HCV is a significant problem in children, with an estimated prevalence in the United States of 0.2% and 0.4% for children aged 6-11 years and 12-19 years, respectively. Vertical transmission from the mother is responsible for more than 60% of pediatric HCV infection and adds approximately 7,200 new cases in the United States yearly. Older children can acquire the virus through intravenous and intranasal drug use and high-risk sexual activity, they stated.
“Our understanding of the pathobiology and immunology of hepatitis B and C is unprecedented. As new antiviral therapies are being developed for the pediatric population, the differences in management and monitoring between children and adults with HBV and HCV are beginning to narrow but are still important,” the authors wrote.
They pointed out that soon-to-be-available treatments for HCV will be curative in children aged as young as 3 years. “[T]his will change the natural history of HCV and the prevalence of hepatocellular carcinoma over the next several decades for the better,” Dr. Mysore and Dr. Leung concluded.
They reported that they had no conflicts of interest to disclose.
SOURCE: Mysore KR et al. Clin Liver Dis. 2018; 22:703-22.
FROM CLINICS IN LIVER DISEASE
Maintaining virologic response predicted long-term survival in HBV patients with decompensated cirrhosis
according to the results of a multicenter observational study published in the December issue of Clinical Gastroenterology and Hepatology.
Survival times were “excellent” if patients survived the first 6 months of antiviral therapy and did not develop hepatocellular carcinoma, said Jeong Won Jang, MD, of the Catholic University of Korea College of Medicine in Seoul, South Korea, and his associates. Patients who developed hepatocellular carcinoma had persistent declines in survival over time, they said. Predictors of short-term mortality included a baseline Model for End-Stage Liver Disease score above 20 and multiple complications.
Chronic hepatitis B virus (HBV) infection is the most common cause of liver-related disease and death in Asia, and complications such as decompensated cirrhosis affect up to 40% of chronically infected persons. Five-year survival rates are as low as 14% if patients develop decompensated cirrhosis.
To explore whether virologic suppression with oral nucleoside or nucleotide analog therapy improves outcomes in these decompensated patients, the researchers studied 295 such individuals from the Epidemiology and Natural History of Liver Cirrhosis in Korea Study. At baseline, these patients did not have documented chronic hepatitis C virus infection, hepatocellular carcinoma, other cancers, autoimmune hepatitis, or alcohol use disorders. All patients initiated entecavir or lamivudine therapy immediately after their cirrhosis became decompensated. The primary outcome was transplant-free survival.
A total of 60.1% of patients survived 5 years and 45.7% survived 10 years without undergoing transplantation, for a median transplant-free survival time of 7.7 years. The 116 patients (39%) who consistently had undetectable HBV DNA levels (less than 20 IU/mL) throughout treatment had significantly longer transplant-free survival than did patients who did not maintain a virologic response (P less than .001). In addition, a maintained virologic response (MVR) was the strongest predictor of long-term transplant-free survival, the researchers said.
A significantly greater proportion of patients who received entecavir survived 10 years compared with patients who received lamivudine. However, there was no significant difference in long-term survival among patients with MVRs to either drug. “Importantly, it appears that improvement in patient survival is attained by antiviral response, not by the type of nucleos(t)ide analogue per se,” the researchers wrote.
Patients who achieved MVR also showed significant improvements in hepatic function, but “the preventive effects of MVR on the incidence of hepatocellular carcinoma appeared only modest,” the investigators said. “Survival of patients without hepatocellular carcinoma who survived the first 6 months after initiation of antiviral therapy was excellent, with only a 25.3% mortality rate occurring between 6 months and 10 years.”
Based on their findings, Dr. Jang and his associates recommended aiming for an HBV DNA load less than 20 IU/mL in patients with decompensated cirrhosis to significantly improve the chances of long-term survival. Survival curves were similar regardless of whether patients had HBV DNA levels less than 10 IU/mL or between and 10 and 20 IU/mL, they noted.
Funders included Korea Healthcare Technology R&D Project and the Catholic Research Coordinating Center of the Korea Health 21 R&D Project, both of the Ministry of Health and Welfare, Republic of Korea. Dr. Jang disclosed ties to Bristol-Myers Squibb, Gilead, and Merck Sharp & Dohme. Three coinvestigators also disclosed ties to Gilead, MSD, and several other pharmaceutical companies.
SOURCE: Jang JW et al. Clin Gastroenterol Hepatol. 2018 May 18. doi: 10.1016/j.cgh.2018.04.063
according to the results of a multicenter observational study published in the December issue of Clinical Gastroenterology and Hepatology.
Survival times were “excellent” if patients survived the first 6 months of antiviral therapy and did not develop hepatocellular carcinoma, said Jeong Won Jang, MD, of the Catholic University of Korea College of Medicine in Seoul, South Korea, and his associates. Patients who developed hepatocellular carcinoma had persistent declines in survival over time, they said. Predictors of short-term mortality included a baseline Model for End-Stage Liver Disease score above 20 and multiple complications.
Chronic hepatitis B virus (HBV) infection is the most common cause of liver-related disease and death in Asia, and complications such as decompensated cirrhosis affect up to 40% of chronically infected persons. Five-year survival rates are as low as 14% if patients develop decompensated cirrhosis.
To explore whether virologic suppression with oral nucleoside or nucleotide analog therapy improves outcomes in these decompensated patients, the researchers studied 295 such individuals from the Epidemiology and Natural History of Liver Cirrhosis in Korea Study. At baseline, these patients did not have documented chronic hepatitis C virus infection, hepatocellular carcinoma, other cancers, autoimmune hepatitis, or alcohol use disorders. All patients initiated entecavir or lamivudine therapy immediately after their cirrhosis became decompensated. The primary outcome was transplant-free survival.
A total of 60.1% of patients survived 5 years and 45.7% survived 10 years without undergoing transplantation, for a median transplant-free survival time of 7.7 years. The 116 patients (39%) who consistently had undetectable HBV DNA levels (less than 20 IU/mL) throughout treatment had significantly longer transplant-free survival than did patients who did not maintain a virologic response (P less than .001). In addition, a maintained virologic response (MVR) was the strongest predictor of long-term transplant-free survival, the researchers said.
A significantly greater proportion of patients who received entecavir survived 10 years compared with patients who received lamivudine. However, there was no significant difference in long-term survival among patients with MVRs to either drug. “Importantly, it appears that improvement in patient survival is attained by antiviral response, not by the type of nucleos(t)ide analogue per se,” the researchers wrote.
Patients who achieved MVR also showed significant improvements in hepatic function, but “the preventive effects of MVR on the incidence of hepatocellular carcinoma appeared only modest,” the investigators said. “Survival of patients without hepatocellular carcinoma who survived the first 6 months after initiation of antiviral therapy was excellent, with only a 25.3% mortality rate occurring between 6 months and 10 years.”
Based on their findings, Dr. Jang and his associates recommended aiming for an HBV DNA load less than 20 IU/mL in patients with decompensated cirrhosis to significantly improve the chances of long-term survival. Survival curves were similar regardless of whether patients had HBV DNA levels less than 10 IU/mL or between and 10 and 20 IU/mL, they noted.
Funders included Korea Healthcare Technology R&D Project and the Catholic Research Coordinating Center of the Korea Health 21 R&D Project, both of the Ministry of Health and Welfare, Republic of Korea. Dr. Jang disclosed ties to Bristol-Myers Squibb, Gilead, and Merck Sharp & Dohme. Three coinvestigators also disclosed ties to Gilead, MSD, and several other pharmaceutical companies.
SOURCE: Jang JW et al. Clin Gastroenterol Hepatol. 2018 May 18. doi: 10.1016/j.cgh.2018.04.063
according to the results of a multicenter observational study published in the December issue of Clinical Gastroenterology and Hepatology.
Survival times were “excellent” if patients survived the first 6 months of antiviral therapy and did not develop hepatocellular carcinoma, said Jeong Won Jang, MD, of the Catholic University of Korea College of Medicine in Seoul, South Korea, and his associates. Patients who developed hepatocellular carcinoma had persistent declines in survival over time, they said. Predictors of short-term mortality included a baseline Model for End-Stage Liver Disease score above 20 and multiple complications.
Chronic hepatitis B virus (HBV) infection is the most common cause of liver-related disease and death in Asia, and complications such as decompensated cirrhosis affect up to 40% of chronically infected persons. Five-year survival rates are as low as 14% if patients develop decompensated cirrhosis.
To explore whether virologic suppression with oral nucleoside or nucleotide analog therapy improves outcomes in these decompensated patients, the researchers studied 295 such individuals from the Epidemiology and Natural History of Liver Cirrhosis in Korea Study. At baseline, these patients did not have documented chronic hepatitis C virus infection, hepatocellular carcinoma, other cancers, autoimmune hepatitis, or alcohol use disorders. All patients initiated entecavir or lamivudine therapy immediately after their cirrhosis became decompensated. The primary outcome was transplant-free survival.
A total of 60.1% of patients survived 5 years and 45.7% survived 10 years without undergoing transplantation, for a median transplant-free survival time of 7.7 years. The 116 patients (39%) who consistently had undetectable HBV DNA levels (less than 20 IU/mL) throughout treatment had significantly longer transplant-free survival than did patients who did not maintain a virologic response (P less than .001). In addition, a maintained virologic response (MVR) was the strongest predictor of long-term transplant-free survival, the researchers said.
A significantly greater proportion of patients who received entecavir survived 10 years compared with patients who received lamivudine. However, there was no significant difference in long-term survival among patients with MVRs to either drug. “Importantly, it appears that improvement in patient survival is attained by antiviral response, not by the type of nucleos(t)ide analogue per se,” the researchers wrote.
Patients who achieved MVR also showed significant improvements in hepatic function, but “the preventive effects of MVR on the incidence of hepatocellular carcinoma appeared only modest,” the investigators said. “Survival of patients without hepatocellular carcinoma who survived the first 6 months after initiation of antiviral therapy was excellent, with only a 25.3% mortality rate occurring between 6 months and 10 years.”
Based on their findings, Dr. Jang and his associates recommended aiming for an HBV DNA load less than 20 IU/mL in patients with decompensated cirrhosis to significantly improve the chances of long-term survival. Survival curves were similar regardless of whether patients had HBV DNA levels less than 10 IU/mL or between and 10 and 20 IU/mL, they noted.
Funders included Korea Healthcare Technology R&D Project and the Catholic Research Coordinating Center of the Korea Health 21 R&D Project, both of the Ministry of Health and Welfare, Republic of Korea. Dr. Jang disclosed ties to Bristol-Myers Squibb, Gilead, and Merck Sharp & Dohme. Three coinvestigators also disclosed ties to Gilead, MSD, and several other pharmaceutical companies.
SOURCE: Jang JW et al. Clin Gastroenterol Hepatol. 2018 May 18. doi: 10.1016/j.cgh.2018.04.063
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: For patients with decompensated cirrhosis, long-term hepatitis B virus suppression was associated with significantly improved transplant-free survival.
Major finding: Lack of virologic response was associated with a more than twofold increase in hazard of long-term mortality in the multivariate analysis (HR, 2.30; 95% confidence interval, 1.60-3.29; P less than .001).
Study details: Ten-year multicenter observational study of 295 patients who began entecavir or lamivudine therapy immediately after their cirrhosis became decompensated.
Disclosures: Funders included Korea Healthcare Technology R&D Project and the Catholic Research Coordinating Center of the Korea Health 21 R&D Project, both of the Ministry of Health and Welfare, Republic of Korea. Dr. Jang disclosed ties to Bristol-Myers Squibb, Gilead, and Merck Sharp & Dohme. Three coinvestigators also disclosed ties to Gilead, MSD, and several other pharmaceutical companies.
Source: Jang JW et al. Clin Gastroenterol Hepatol. 2018 May 18. doi: 10.1016/j.cgh.2018.04.063
Norfloxacin might benefit patients with advanced cirrhosis and low ascites fluid protein levels
Six months of once-daily norfloxacin therapy did not reduce 6-month mortality among patients with Child-Pugh class C cirrhosis who had not recently received fluoroquinolone therapy.
Mortality based on the Kaplan-Meier method was 14.8% in the norfloxacin group versus 19.7% for patients receiving placebo (P = .21). “Norfloxacin, however, appear[ed] to increase survival of patients with low ascites fluid protein concentrations,” wrote Richard Moreau, MD, of Hôpital Beaujon, Paris, and his associates. The results of the multicenter, double-blind trial of 291 patients were published in the December issue of Gastroenterology.
Patients with advanced cirrhosis often develop spontaneous bacterial peritonitis and other severe bacterial infections, with potentially grave outcomes. These are often enteric gram-negative bacteria that cross the intestinal barrier, enter the systemic circulation, and travel to the site of infection.
Long-term fluoroquinolone therapy (typically with norfloxacin) might help prevent these bacterial infections, the translocation of bacterial products, systemic inflammation, and consequent end-organ dysfunction, such as acute kidney disease. However, long-term antibiotic therapy also raises the specter of multidrug resistance, which is especially concerning when it involves a crucial antibiotic class such as fluoroquinolones, the researchers noted. “[In] patients receiving prolonged fluoroquinolone therapy, the development of infections by multidrug resistant bacteria might obscure the beneficial effect of fluoroquinolones on survival,” they added.
Four previous blinded and placebo-controlled trials have investigated fluoroquinolone therapy and mortality patients with cirrhosis, but they were small, usually included mortality only as a secondary outcome, and yielded mixed results. Hence, the researchers enrolled 291 patients with advanced (Child-Pugh class C) cirrhosis from 18 clinical sites in France and randomly assigned them to receive either norfloxacin (400 mg once daily) or placebo for 6 months. Patients were evaluated monthly during treatment and then at 9 months and 12 months. The primary outcome was survival at 6 months.
In a post hoc analysis, the researchers examined cumulative death rates at 6 months after accounting for liver transplantation as a competing risk of death and including survival data for patients who developed spontaneous bacterial peritonitis. Taking this approach, the estimated cumulative rate of death at 6 months was 15.5% (95% confidence interval, 10.1-21.9) in the norfloxacin group and 24.8% (95% CI, 18.1-32.1) in the placebo group, for a hazard ratio of 0.59 (95% CI, 0.35-0.99). Among patients whose ascites fluid levels were less than 15 g/L, the hazard ratio for death at 6 months was 65% lower in the norfloxacin group than in the placebo group (HR, 0.35; 95% CI, 0.13-0.93). Norfloxacin showed no such benefit for patients with ascites fluid protein levels above 15 g/L.
Norfloxacin therapy “could reduce the incidence of death among patients with ascitic fluid protein concentrations of less than 15 g/L but not among those with ascitic fluid protein concentration of 15 g/L or more,” the researchers concluded. “Norfloxacin may prevent some infections, especially gram-negative bacterial infections, but not the development of [spontaneous bacterial peritonitis] and other noninfectious, liver-related complications.”
The study was funded by Programme Hospitalier de Recherche Clinique National 2008 of the French Ministry of Health. Dr. Moreau reported having no conflicts of interest. Two coinvestigators disclosed ties to Gore Norgine, Exalenz, and Conatus.
SOURCE: Moreau R et al. Gastroenterology. 2018 Aug 22. doi: 10.1053/j.gastro.2018.08.026.
Prolonged antimicrobial use in patients with decompensated cirrhosis is an area of unclear mortality benefit and may actually increase risk in some patients given antimicrobial resistance. This randomized double-blind, placebo-controlled trial by Moreau et al. evaluates the mortality associated with long-term fluoroquinolone therapy in patients without indications for primary or secondary prophylaxis. Although the study had limited statistical power to detect clear benefit, the authors found that 6-month mortality was not reduced in patients with Child-Pugh class C cirrhosis who received treatment with daily oral fluoroquinolone therapy for 6 months. Subgroup analysis of individuals with ascites fluid total protein levels lower than 15 g/L showed a survival benefit at 6 months.
Determining quantifiable risk for known factors associated with liver disease mortality is a pressing issue, especially in the pretransplant setting where infectious risk is compounded post transplant with changes in gut flora, addition of potent immunosuppressants, and increased metabolic demands. Biologic measurements that correlate with increased complications and mortality, like low protein ascites, are helpful in complex clinical settings.Studying patients with advanced and decompensated liver disease in a systematic, longitudinal manner with any pharmacologic intervention is a particular challenge given the unpredictable nature of decompensation events and variable outcomes from those events. However, attempts to quantify risk and benefit even in this unpredictable patient population is worthwhile to stratify patients for interventions and minimize risk of liver-related and overall mortality – as well as peritransplant complications and posttransplant survival.
Julia J. Wattacheril, MD, MPH, is a physician- scientist and director of the Nonalcoholic Fatty Liver Disease Program in the Center for Liver Disease and Transplantation at Columbia University Irving Medical Center–New York Presbyterian Hospital, New York; an assistant professor, department of medicine, division of digestive and liver diseases at the Columbia University Vagelos College of Physicians and Surgeons. She has no conflicts.
Prolonged antimicrobial use in patients with decompensated cirrhosis is an area of unclear mortality benefit and may actually increase risk in some patients given antimicrobial resistance. This randomized double-blind, placebo-controlled trial by Moreau et al. evaluates the mortality associated with long-term fluoroquinolone therapy in patients without indications for primary or secondary prophylaxis. Although the study had limited statistical power to detect clear benefit, the authors found that 6-month mortality was not reduced in patients with Child-Pugh class C cirrhosis who received treatment with daily oral fluoroquinolone therapy for 6 months. Subgroup analysis of individuals with ascites fluid total protein levels lower than 15 g/L showed a survival benefit at 6 months.
Determining quantifiable risk for known factors associated with liver disease mortality is a pressing issue, especially in the pretransplant setting where infectious risk is compounded post transplant with changes in gut flora, addition of potent immunosuppressants, and increased metabolic demands. Biologic measurements that correlate with increased complications and mortality, like low protein ascites, are helpful in complex clinical settings.Studying patients with advanced and decompensated liver disease in a systematic, longitudinal manner with any pharmacologic intervention is a particular challenge given the unpredictable nature of decompensation events and variable outcomes from those events. However, attempts to quantify risk and benefit even in this unpredictable patient population is worthwhile to stratify patients for interventions and minimize risk of liver-related and overall mortality – as well as peritransplant complications and posttransplant survival.
Julia J. Wattacheril, MD, MPH, is a physician- scientist and director of the Nonalcoholic Fatty Liver Disease Program in the Center for Liver Disease and Transplantation at Columbia University Irving Medical Center–New York Presbyterian Hospital, New York; an assistant professor, department of medicine, division of digestive and liver diseases at the Columbia University Vagelos College of Physicians and Surgeons. She has no conflicts.
Prolonged antimicrobial use in patients with decompensated cirrhosis is an area of unclear mortality benefit and may actually increase risk in some patients given antimicrobial resistance. This randomized double-blind, placebo-controlled trial by Moreau et al. evaluates the mortality associated with long-term fluoroquinolone therapy in patients without indications for primary or secondary prophylaxis. Although the study had limited statistical power to detect clear benefit, the authors found that 6-month mortality was not reduced in patients with Child-Pugh class C cirrhosis who received treatment with daily oral fluoroquinolone therapy for 6 months. Subgroup analysis of individuals with ascites fluid total protein levels lower than 15 g/L showed a survival benefit at 6 months.
Determining quantifiable risk for known factors associated with liver disease mortality is a pressing issue, especially in the pretransplant setting where infectious risk is compounded post transplant with changes in gut flora, addition of potent immunosuppressants, and increased metabolic demands. Biologic measurements that correlate with increased complications and mortality, like low protein ascites, are helpful in complex clinical settings.Studying patients with advanced and decompensated liver disease in a systematic, longitudinal manner with any pharmacologic intervention is a particular challenge given the unpredictable nature of decompensation events and variable outcomes from those events. However, attempts to quantify risk and benefit even in this unpredictable patient population is worthwhile to stratify patients for interventions and minimize risk of liver-related and overall mortality – as well as peritransplant complications and posttransplant survival.
Julia J. Wattacheril, MD, MPH, is a physician- scientist and director of the Nonalcoholic Fatty Liver Disease Program in the Center for Liver Disease and Transplantation at Columbia University Irving Medical Center–New York Presbyterian Hospital, New York; an assistant professor, department of medicine, division of digestive and liver diseases at the Columbia University Vagelos College of Physicians and Surgeons. She has no conflicts.
Six months of once-daily norfloxacin therapy did not reduce 6-month mortality among patients with Child-Pugh class C cirrhosis who had not recently received fluoroquinolone therapy.
Mortality based on the Kaplan-Meier method was 14.8% in the norfloxacin group versus 19.7% for patients receiving placebo (P = .21). “Norfloxacin, however, appear[ed] to increase survival of patients with low ascites fluid protein concentrations,” wrote Richard Moreau, MD, of Hôpital Beaujon, Paris, and his associates. The results of the multicenter, double-blind trial of 291 patients were published in the December issue of Gastroenterology.
Patients with advanced cirrhosis often develop spontaneous bacterial peritonitis and other severe bacterial infections, with potentially grave outcomes. These are often enteric gram-negative bacteria that cross the intestinal barrier, enter the systemic circulation, and travel to the site of infection.
Long-term fluoroquinolone therapy (typically with norfloxacin) might help prevent these bacterial infections, the translocation of bacterial products, systemic inflammation, and consequent end-organ dysfunction, such as acute kidney disease. However, long-term antibiotic therapy also raises the specter of multidrug resistance, which is especially concerning when it involves a crucial antibiotic class such as fluoroquinolones, the researchers noted. “[In] patients receiving prolonged fluoroquinolone therapy, the development of infections by multidrug resistant bacteria might obscure the beneficial effect of fluoroquinolones on survival,” they added.
Four previous blinded and placebo-controlled trials have investigated fluoroquinolone therapy and mortality patients with cirrhosis, but they were small, usually included mortality only as a secondary outcome, and yielded mixed results. Hence, the researchers enrolled 291 patients with advanced (Child-Pugh class C) cirrhosis from 18 clinical sites in France and randomly assigned them to receive either norfloxacin (400 mg once daily) or placebo for 6 months. Patients were evaluated monthly during treatment and then at 9 months and 12 months. The primary outcome was survival at 6 months.
In a post hoc analysis, the researchers examined cumulative death rates at 6 months after accounting for liver transplantation as a competing risk of death and including survival data for patients who developed spontaneous bacterial peritonitis. Taking this approach, the estimated cumulative rate of death at 6 months was 15.5% (95% confidence interval, 10.1-21.9) in the norfloxacin group and 24.8% (95% CI, 18.1-32.1) in the placebo group, for a hazard ratio of 0.59 (95% CI, 0.35-0.99). Among patients whose ascites fluid levels were less than 15 g/L, the hazard ratio for death at 6 months was 65% lower in the norfloxacin group than in the placebo group (HR, 0.35; 95% CI, 0.13-0.93). Norfloxacin showed no such benefit for patients with ascites fluid protein levels above 15 g/L.
Norfloxacin therapy “could reduce the incidence of death among patients with ascitic fluid protein concentrations of less than 15 g/L but not among those with ascitic fluid protein concentration of 15 g/L or more,” the researchers concluded. “Norfloxacin may prevent some infections, especially gram-negative bacterial infections, but not the development of [spontaneous bacterial peritonitis] and other noninfectious, liver-related complications.”
The study was funded by Programme Hospitalier de Recherche Clinique National 2008 of the French Ministry of Health. Dr. Moreau reported having no conflicts of interest. Two coinvestigators disclosed ties to Gore Norgine, Exalenz, and Conatus.
SOURCE: Moreau R et al. Gastroenterology. 2018 Aug 22. doi: 10.1053/j.gastro.2018.08.026.
Six months of once-daily norfloxacin therapy did not reduce 6-month mortality among patients with Child-Pugh class C cirrhosis who had not recently received fluoroquinolone therapy.
Mortality based on the Kaplan-Meier method was 14.8% in the norfloxacin group versus 19.7% for patients receiving placebo (P = .21). “Norfloxacin, however, appear[ed] to increase survival of patients with low ascites fluid protein concentrations,” wrote Richard Moreau, MD, of Hôpital Beaujon, Paris, and his associates. The results of the multicenter, double-blind trial of 291 patients were published in the December issue of Gastroenterology.
Patients with advanced cirrhosis often develop spontaneous bacterial peritonitis and other severe bacterial infections, with potentially grave outcomes. These are often enteric gram-negative bacteria that cross the intestinal barrier, enter the systemic circulation, and travel to the site of infection.
Long-term fluoroquinolone therapy (typically with norfloxacin) might help prevent these bacterial infections, the translocation of bacterial products, systemic inflammation, and consequent end-organ dysfunction, such as acute kidney disease. However, long-term antibiotic therapy also raises the specter of multidrug resistance, which is especially concerning when it involves a crucial antibiotic class such as fluoroquinolones, the researchers noted. “[In] patients receiving prolonged fluoroquinolone therapy, the development of infections by multidrug resistant bacteria might obscure the beneficial effect of fluoroquinolones on survival,” they added.
Four previous blinded and placebo-controlled trials have investigated fluoroquinolone therapy and mortality patients with cirrhosis, but they were small, usually included mortality only as a secondary outcome, and yielded mixed results. Hence, the researchers enrolled 291 patients with advanced (Child-Pugh class C) cirrhosis from 18 clinical sites in France and randomly assigned them to receive either norfloxacin (400 mg once daily) or placebo for 6 months. Patients were evaluated monthly during treatment and then at 9 months and 12 months. The primary outcome was survival at 6 months.
In a post hoc analysis, the researchers examined cumulative death rates at 6 months after accounting for liver transplantation as a competing risk of death and including survival data for patients who developed spontaneous bacterial peritonitis. Taking this approach, the estimated cumulative rate of death at 6 months was 15.5% (95% confidence interval, 10.1-21.9) in the norfloxacin group and 24.8% (95% CI, 18.1-32.1) in the placebo group, for a hazard ratio of 0.59 (95% CI, 0.35-0.99). Among patients whose ascites fluid levels were less than 15 g/L, the hazard ratio for death at 6 months was 65% lower in the norfloxacin group than in the placebo group (HR, 0.35; 95% CI, 0.13-0.93). Norfloxacin showed no such benefit for patients with ascites fluid protein levels above 15 g/L.
Norfloxacin therapy “could reduce the incidence of death among patients with ascitic fluid protein concentrations of less than 15 g/L but not among those with ascitic fluid protein concentration of 15 g/L or more,” the researchers concluded. “Norfloxacin may prevent some infections, especially gram-negative bacterial infections, but not the development of [spontaneous bacterial peritonitis] and other noninfectious, liver-related complications.”
The study was funded by Programme Hospitalier de Recherche Clinique National 2008 of the French Ministry of Health. Dr. Moreau reported having no conflicts of interest. Two coinvestigators disclosed ties to Gore Norgine, Exalenz, and Conatus.
SOURCE: Moreau R et al. Gastroenterology. 2018 Aug 22. doi: 10.1053/j.gastro.2018.08.026.
FROM GASTROENTEROLOGY
Key clinical point: Six months of once-daily norfloxacin therapy did not reduce 6-month mortality among patients with Child-Pugh class C cirrhosis who had not recently received fluoroquinolone therapy, but norfloxacin did appear to benefit a subgroup of patients with low ascites fluid protein levels.
Major finding: Mortality based on the Kaplan-Meier method was 14.8% in the norfloxacin group versus 19.7% for patients receiving placebo (P = .21). Among patients whose ascites fluid levels were less than 15 g/L, the hazard ratio for death at 6 months was 65% lower in the norfloxacin group than in the placebo group (HR, 0.35; 95% CI, 0.13-0.93).
Study details: Multicenter double-blind trial of 291 patients with Child-Pugh class C cirrhosis who had not received recent fluoroquinolone therapy.
Disclosures: The study was funded by Programme Hospitalier de Recherche Clinique National 2008 of the French Ministry of Health. Dr. Moreau reported having no conflicts of interest. Two coinvestigators disclosed ties to Gore Norgine, Exalenz, and Conatus.
Source: Moreau R et al. Gastroenterology. 2018 Aug 22. doi: 10.1053/j.gastro.2018.08.026.
ACIP votes unanimously in favor of immunization schedule update and redesign
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
Clinicians consulting the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices vaccination schedules for children, adolescents, and adults in 2019 will find a simpler design and more useful product, according to David Kim, MD, of the Immunization Services Division of the Centers for Disease Control and Prevention, Atlanta.
In a single vote to cover both adult and child/adolescent schedules, the committee voted unanimously in favor of a redesign of the schedules and several clinical updates.
In 2016, the working group for vaccination schedules conducted an ad hoc evaluation of the adult schedule to assess its usability, Dr. Kim said at a meeting of the CDC’s ACIP.
The design of the adult schedule was fully evaluated in 2018 via a three-step process – interviews with 48 health care providers, a redesign of the schedule, and a survey after the redesign. Design changes to the child/adolescent schedule were harmonized with the adult schedule, Dr. Kim explained.
The adult vaccination schedule itself includes several updates in ACIP recommendations in addition to the aesthetic design changes.
The 2019 Adult Immunization Schedule includes the option of the live attenuated influenza vaccine (LAIV) for influenza, the addition of homelessness as an indication for hepatitis A vaccination, and the use of CpG-adjuvanted hepatitis B vaccine, Dr. Kim said.
The additions to the 2019 Child and Adolescent Immunization Schedule are the optional use of the LAIV for influenza, the addition of homelessness as an indication for hepatitis A vaccination, the use of CpG-adjuvanted hepatitis B vaccine (a cytosine phosphoguanosine oligodeoxynucleotide adjuvant), and the addition of the Tdap vaccination of individuals who received Tdap at age 7-10 years.
Some of the key design changes include the use of bright purple on the child/adolescent schedule to more easily distinguish it from the adult version, said Dr. Kim.
Other changes to both schedules include shorter titles, lists of vaccines and trade names, and compartmentalized information for easier reference. Figures have been replaced by tables, and footnotes are simply “Notes” at the end of the schedule, compartmentalized for easier reading, he said. In addition, the schedules include resources for vaccination in outbreak situations and a section on how to report vaccine preventable disease outbreaks.
The ACIP committee members had no relevant financial conflicts to disclose.
AT AN ACIP MEETING
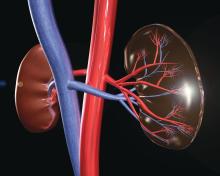
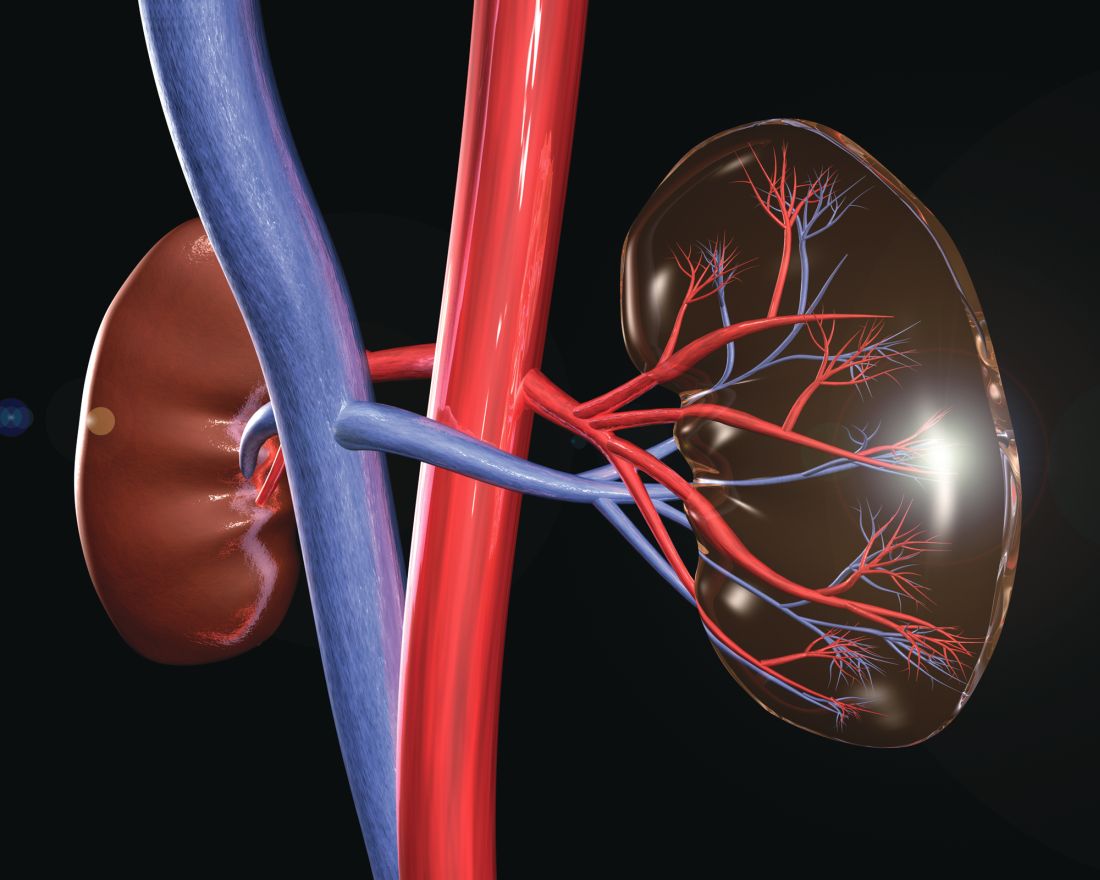
HCV, HBV, and HIV associated with autoimmune kidney diseases
Chronic viral infection can be associated with a variety of autoimmune kidney diseases, according to a review published in Rheumatic Disease Clinics.
In particular, hepatitis C virus (HCV) infection can cause several kidney disorders. These include cryoglobulinemic glomerulonephritis, membranous nephropathy, fibrillary glomerulopathy, immunotactoid glomerulopathy, and IgA nephropathy, wrote Joshua D. Long and his colleagues at Massachusetts General Hospital, Boston.
Similarly, hepatitis B virus (HBV) infection was found to be associated with both membranous nephropathy and polyarteritis nodosa, and human immunodeficiency virus (HIV) infection can cause HIV-associated nephropathy and HIV-associated immune complex diseases, which affect the kidneys.
In their detailed review, the authors discussed the various causal mechanisms and clinical presentations of each of these various autoimmune kidney diseases caused by HCV, HBV, and HIV, along with current treatment modalities.
“Control of the kidney disease relies primarily on treatment of viremia with antiviral agents; however, immunosuppression also may be needed in severe cases,” said the reviewers. However, “more clinical trials are needed to determine first-line therapies for patients who develop autoimmune kidney diseases in the context of chronic viral infections and to define when adjunctive immunosuppressive therapy is warranted,” they concluded.
One of the authors reported grant support and acting as a consultant for various pharmaceutical companies.
[email protected]
SOURCE: Long JD et al. Rheum Dis Clin N Am 2018;44:675-98.
Chronic viral infection can be associated with a variety of autoimmune kidney diseases, according to a review published in Rheumatic Disease Clinics.
In particular, hepatitis C virus (HCV) infection can cause several kidney disorders. These include cryoglobulinemic glomerulonephritis, membranous nephropathy, fibrillary glomerulopathy, immunotactoid glomerulopathy, and IgA nephropathy, wrote Joshua D. Long and his colleagues at Massachusetts General Hospital, Boston.
Similarly, hepatitis B virus (HBV) infection was found to be associated with both membranous nephropathy and polyarteritis nodosa, and human immunodeficiency virus (HIV) infection can cause HIV-associated nephropathy and HIV-associated immune complex diseases, which affect the kidneys.
In their detailed review, the authors discussed the various causal mechanisms and clinical presentations of each of these various autoimmune kidney diseases caused by HCV, HBV, and HIV, along with current treatment modalities.
“Control of the kidney disease relies primarily on treatment of viremia with antiviral agents; however, immunosuppression also may be needed in severe cases,” said the reviewers. However, “more clinical trials are needed to determine first-line therapies for patients who develop autoimmune kidney diseases in the context of chronic viral infections and to define when adjunctive immunosuppressive therapy is warranted,” they concluded.
One of the authors reported grant support and acting as a consultant for various pharmaceutical companies.
[email protected]
SOURCE: Long JD et al. Rheum Dis Clin N Am 2018;44:675-98.
Chronic viral infection can be associated with a variety of autoimmune kidney diseases, according to a review published in Rheumatic Disease Clinics.
In particular, hepatitis C virus (HCV) infection can cause several kidney disorders. These include cryoglobulinemic glomerulonephritis, membranous nephropathy, fibrillary glomerulopathy, immunotactoid glomerulopathy, and IgA nephropathy, wrote Joshua D. Long and his colleagues at Massachusetts General Hospital, Boston.
Similarly, hepatitis B virus (HBV) infection was found to be associated with both membranous nephropathy and polyarteritis nodosa, and human immunodeficiency virus (HIV) infection can cause HIV-associated nephropathy and HIV-associated immune complex diseases, which affect the kidneys.
In their detailed review, the authors discussed the various causal mechanisms and clinical presentations of each of these various autoimmune kidney diseases caused by HCV, HBV, and HIV, along with current treatment modalities.
“Control of the kidney disease relies primarily on treatment of viremia with antiviral agents; however, immunosuppression also may be needed in severe cases,” said the reviewers. However, “more clinical trials are needed to determine first-line therapies for patients who develop autoimmune kidney diseases in the context of chronic viral infections and to define when adjunctive immunosuppressive therapy is warranted,” they concluded.
One of the authors reported grant support and acting as a consultant for various pharmaceutical companies.
[email protected]
SOURCE: Long JD et al. Rheum Dis Clin N Am 2018;44:675-98.
FROM RHEUMATIC DISEASE CLINICS
HCV adapts to HIV coinfection
HCV strains evolve differently in HIV-coinfected individuals, according to the results of a database analysis of HCV genetic sequences from patients monoinfected with HCV and those who were coinfected with HIV. The study compared results from 112 coinfected persons (CIPs) and 176 monoinfected persons (MIPs), according to the report published in the journal Infection, Genetics, and Evolution.
Genetic differences between intrahost variants of the HCV hypervariable region 1 (HVR1) were sampled from CIPs and MIPs, and the nucleotide sequences of intrahost HCV HVR1 variants (n = 28,622) obtained were represented using 148 physical-chemical (PhyChem) indexes of DNA nucleotide dimers. Changes of the intrahost HCV population were detected by measuring coevolution among the HVR1 site using new PhyChem properties extracted from the next-generation sequencing of HVR1 data. Small but statistically significant variances in seven of the PhyChem indexes, measured using these intrahost HVR1 variants, was shown to be strongly associated with CIPs and MIPs (P less than .0001).
“The computational models built using these new markers provide novel opportunities for development of cybermolecular diagnostics,” wrote James Lara, PhD, and his colleagues at the Centers for Disease Control and Prevention.
All HVR1 sequences used (n = 28,622) shared only 6,782 profiles of the selected calculated dinucleotide-based auto covariances of the seven PhyChem indexes. The vast majority (98%-99%) of these profiles were found to be specific to CIPs or MIPs, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals, according to the authors.
Because of the common occurrence of HIV-coinfection in high-risk groups, “HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” Dr. Lara and his colleagues concluded.
The authors reported having no conflicts of interest.
SOURCE: Lara J et al. Infection, Genetics and Evolution. 2018. 65:216-25.
HCV strains evolve differently in HIV-coinfected individuals, according to the results of a database analysis of HCV genetic sequences from patients monoinfected with HCV and those who were coinfected with HIV. The study compared results from 112 coinfected persons (CIPs) and 176 monoinfected persons (MIPs), according to the report published in the journal Infection, Genetics, and Evolution.
Genetic differences between intrahost variants of the HCV hypervariable region 1 (HVR1) were sampled from CIPs and MIPs, and the nucleotide sequences of intrahost HCV HVR1 variants (n = 28,622) obtained were represented using 148 physical-chemical (PhyChem) indexes of DNA nucleotide dimers. Changes of the intrahost HCV population were detected by measuring coevolution among the HVR1 site using new PhyChem properties extracted from the next-generation sequencing of HVR1 data. Small but statistically significant variances in seven of the PhyChem indexes, measured using these intrahost HVR1 variants, was shown to be strongly associated with CIPs and MIPs (P less than .0001).
“The computational models built using these new markers provide novel opportunities for development of cybermolecular diagnostics,” wrote James Lara, PhD, and his colleagues at the Centers for Disease Control and Prevention.
All HVR1 sequences used (n = 28,622) shared only 6,782 profiles of the selected calculated dinucleotide-based auto covariances of the seven PhyChem indexes. The vast majority (98%-99%) of these profiles were found to be specific to CIPs or MIPs, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals, according to the authors.
Because of the common occurrence of HIV-coinfection in high-risk groups, “HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” Dr. Lara and his colleagues concluded.
The authors reported having no conflicts of interest.
SOURCE: Lara J et al. Infection, Genetics and Evolution. 2018. 65:216-25.
HCV strains evolve differently in HIV-coinfected individuals, according to the results of a database analysis of HCV genetic sequences from patients monoinfected with HCV and those who were coinfected with HIV. The study compared results from 112 coinfected persons (CIPs) and 176 monoinfected persons (MIPs), according to the report published in the journal Infection, Genetics, and Evolution.
Genetic differences between intrahost variants of the HCV hypervariable region 1 (HVR1) were sampled from CIPs and MIPs, and the nucleotide sequences of intrahost HCV HVR1 variants (n = 28,622) obtained were represented using 148 physical-chemical (PhyChem) indexes of DNA nucleotide dimers. Changes of the intrahost HCV population were detected by measuring coevolution among the HVR1 site using new PhyChem properties extracted from the next-generation sequencing of HVR1 data. Small but statistically significant variances in seven of the PhyChem indexes, measured using these intrahost HVR1 variants, was shown to be strongly associated with CIPs and MIPs (P less than .0001).
“The computational models built using these new markers provide novel opportunities for development of cybermolecular diagnostics,” wrote James Lara, PhD, and his colleagues at the Centers for Disease Control and Prevention.
All HVR1 sequences used (n = 28,622) shared only 6,782 profiles of the selected calculated dinucleotide-based auto covariances of the seven PhyChem indexes. The vast majority (98%-99%) of these profiles were found to be specific to CIPs or MIPs, indicating that coevolution among HVR1 sites reflects HCV adaptation to HIV among coinfected individuals, according to the authors.
Because of the common occurrence of HIV-coinfection in high-risk groups, “HCV strains circulating in high-risk groups need to be carefully monitored for the identification of potentially new traits of clinical and public health relevance,” Dr. Lara and his colleagues concluded.
The authors reported having no conflicts of interest.
SOURCE: Lara J et al. Infection, Genetics and Evolution. 2018. 65:216-25.
FROM INFECTION, GENETICS, AND EVOLUTION
Novel score predicts esophageal varices in patients with cirrhosis prior to EGD
PHILADELPHIA – A novel score accurately predicted the size and presence of esophageal varices in a noninvasive manner, which may help clinicians avoid unnecessary esophagogastroduodenoscopy in patients with cirrhosis, according to a recent award-winning presentation at the annual meeting of the American College of Gastroenterology.
Although there are two validated scores for predicting esophageal varices (EV), platelet count to spleen diameter ratio and Baveno VI criteria, they have drawbacks, Tien Dong, MD, from the University of California, Los Angeles said.
“The limitations to these existing scores and criteria are both of them require imaging to do, so because of that, they oftentimes are limited in national clinical use,” Dr. Dong said in his presentation of his team’s abstract, which won the ACG Governors Award for Excellence in Clinical Research. “The other thing is that, even though it’s recommended, sometimes spleen diameter on a normal ultrasound is not reported. Furthermore, elastography – even though it’s becoming more and more common – is still not yet readily available across the country.”
Dr. Dong and his colleagues sought to identify noninvasive clinical predictors of EV to create a predictive score to identify EV to overcome these drawbacks. They gathered endoscopy data from the Olive View–UCLA and West Los Angeles Veterans Affairs Hospital to create a discovery cohort (165 patients) and tested the score on patients from Ronald Reagan UCLA Medical Center in a validation cohort (73 patients).
They used a random forest classifier machine learning algorithm “to create a forest of decision trees so that it can tell us which variables it believes to be the most predictive of our outcomes of interest,” Dr. Dong said.
The algorithm identified several variables that appeared to be predictive of EV presence, such as international normalized ratio, aminotransferase, platelet mean, hemoglobin, albumin and blood urea nitrogen less than or equal to 3, which the researchers used to create an EV-endoscopy (EV-Endo) score.
In the discovery cohort, area under the curve (AUC) for the presence of EV was 0.81, compared with an AUC of 0.82 in the validation cohort, while there was an AUC of 0.77 in the discovery cohort and an AUC of 0.79 for small/absent vs. medium/large EV. Patients with Child-Pugh class A cirrhosis had an AUC of 0.81 for EV presence and an AUC of 0.77 for EV size. The researchers then created a cutoff score of 3.48 or less and 7.70 or more for EV presence, which had a sensitivity and specificity of 93.9% and 97.5%, respectively. EV-Endo score EV size cutoff scores were also 3.48 or less and 7.70 or more, with a sensitivity of 95.8% and specificity of 95.0%.
Dr. Dong reports no conflicts of interest.
SOURCE: Hauer M et al. ACG 2018. Presentation 31.
PHILADELPHIA – A novel score accurately predicted the size and presence of esophageal varices in a noninvasive manner, which may help clinicians avoid unnecessary esophagogastroduodenoscopy in patients with cirrhosis, according to a recent award-winning presentation at the annual meeting of the American College of Gastroenterology.
Although there are two validated scores for predicting esophageal varices (EV), platelet count to spleen diameter ratio and Baveno VI criteria, they have drawbacks, Tien Dong, MD, from the University of California, Los Angeles said.
“The limitations to these existing scores and criteria are both of them require imaging to do, so because of that, they oftentimes are limited in national clinical use,” Dr. Dong said in his presentation of his team’s abstract, which won the ACG Governors Award for Excellence in Clinical Research. “The other thing is that, even though it’s recommended, sometimes spleen diameter on a normal ultrasound is not reported. Furthermore, elastography – even though it’s becoming more and more common – is still not yet readily available across the country.”
Dr. Dong and his colleagues sought to identify noninvasive clinical predictors of EV to create a predictive score to identify EV to overcome these drawbacks. They gathered endoscopy data from the Olive View–UCLA and West Los Angeles Veterans Affairs Hospital to create a discovery cohort (165 patients) and tested the score on patients from Ronald Reagan UCLA Medical Center in a validation cohort (73 patients).
They used a random forest classifier machine learning algorithm “to create a forest of decision trees so that it can tell us which variables it believes to be the most predictive of our outcomes of interest,” Dr. Dong said.
The algorithm identified several variables that appeared to be predictive of EV presence, such as international normalized ratio, aminotransferase, platelet mean, hemoglobin, albumin and blood urea nitrogen less than or equal to 3, which the researchers used to create an EV-endoscopy (EV-Endo) score.
In the discovery cohort, area under the curve (AUC) for the presence of EV was 0.81, compared with an AUC of 0.82 in the validation cohort, while there was an AUC of 0.77 in the discovery cohort and an AUC of 0.79 for small/absent vs. medium/large EV. Patients with Child-Pugh class A cirrhosis had an AUC of 0.81 for EV presence and an AUC of 0.77 for EV size. The researchers then created a cutoff score of 3.48 or less and 7.70 or more for EV presence, which had a sensitivity and specificity of 93.9% and 97.5%, respectively. EV-Endo score EV size cutoff scores were also 3.48 or less and 7.70 or more, with a sensitivity of 95.8% and specificity of 95.0%.
Dr. Dong reports no conflicts of interest.
SOURCE: Hauer M et al. ACG 2018. Presentation 31.
PHILADELPHIA – A novel score accurately predicted the size and presence of esophageal varices in a noninvasive manner, which may help clinicians avoid unnecessary esophagogastroduodenoscopy in patients with cirrhosis, according to a recent award-winning presentation at the annual meeting of the American College of Gastroenterology.
Although there are two validated scores for predicting esophageal varices (EV), platelet count to spleen diameter ratio and Baveno VI criteria, they have drawbacks, Tien Dong, MD, from the University of California, Los Angeles said.
“The limitations to these existing scores and criteria are both of them require imaging to do, so because of that, they oftentimes are limited in national clinical use,” Dr. Dong said in his presentation of his team’s abstract, which won the ACG Governors Award for Excellence in Clinical Research. “The other thing is that, even though it’s recommended, sometimes spleen diameter on a normal ultrasound is not reported. Furthermore, elastography – even though it’s becoming more and more common – is still not yet readily available across the country.”
Dr. Dong and his colleagues sought to identify noninvasive clinical predictors of EV to create a predictive score to identify EV to overcome these drawbacks. They gathered endoscopy data from the Olive View–UCLA and West Los Angeles Veterans Affairs Hospital to create a discovery cohort (165 patients) and tested the score on patients from Ronald Reagan UCLA Medical Center in a validation cohort (73 patients).
They used a random forest classifier machine learning algorithm “to create a forest of decision trees so that it can tell us which variables it believes to be the most predictive of our outcomes of interest,” Dr. Dong said.
The algorithm identified several variables that appeared to be predictive of EV presence, such as international normalized ratio, aminotransferase, platelet mean, hemoglobin, albumin and blood urea nitrogen less than or equal to 3, which the researchers used to create an EV-endoscopy (EV-Endo) score.
In the discovery cohort, area under the curve (AUC) for the presence of EV was 0.81, compared with an AUC of 0.82 in the validation cohort, while there was an AUC of 0.77 in the discovery cohort and an AUC of 0.79 for small/absent vs. medium/large EV. Patients with Child-Pugh class A cirrhosis had an AUC of 0.81 for EV presence and an AUC of 0.77 for EV size. The researchers then created a cutoff score of 3.48 or less and 7.70 or more for EV presence, which had a sensitivity and specificity of 93.9% and 97.5%, respectively. EV-Endo score EV size cutoff scores were also 3.48 or less and 7.70 or more, with a sensitivity of 95.8% and specificity of 95.0%.
Dr. Dong reports no conflicts of interest.
SOURCE: Hauer M et al. ACG 2018. Presentation 31.
REPORTING FROM ACG 2018
Aspirin cuts risk of ovarian and liver cancer
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
FROM JAMA ONCOLOGY
Key clinical point: Regular aspirin use was associated with a decreased risk of ovarian cancer and hepatocellular carcinoma.
Major finding: Low-dose aspirin was associated with a 23% lower risk of ovarian cancer and a 49% reduced risk of developing hepatocellular carcinoma.
Study details: The hepatocellular carcinoma study was a population-based study of two nationwide, prospective cohorts of 87,507 men and 45,864 women; the ovarian cancer study was a cohort study using data from two prospective cohorts, with 93,664 people in one and 111,834 in the other.
Disclosures: The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The hepatocellular carcinoma study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
Sources: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.