User login
Doc accused of killing 14 patients in the ICU: Upcoming trial notes patient safety lapses
On Dec. 5, 2017, Danny Mollette, age 74, was brought to the emergency department of Mount Carmel West Medical Center in Columbus, Ohio, in critical condition. Staff inserted a breathing tube and sent him to the intensive care unit.
Mr. Mollette, who had diabetes, previously had been hospitalized for treatment of a gangrenous foot. When he arrived in the ICU, he was suffering from acute renal failure and low blood pressure, and had had two heart stoppages, according to a 2020 Ohio Board of Pharmacy report. He was placed under the care of William Husel, DO, the sole physician on duty in the ICU during the overnight shift.
Around 9:00 p.m., Dr. Husel discussed Mr. Mollette’s “grim prognosis” with family members at the patient’s bedside. He advised them that Mr. Mollette had “minutes to live” and asked, “How would you want him to take his last breath: on the ventilator or without these machines?”
In less than an hour, Mr. Mollette was dead. Some said that what happened in his case was similar to what happened with 34 other ICU patients at Mount Carmel West and Mount Carmel St. Ann’s in Westerville, Ohio, from 2014 through 2018 – all under Dr. Husel’s care.
Like Mr. Mollette, most of these gravely ill patients died minutes after receiving a single, unusually large intravenous dose of the powerful opioid fentanyl – often combined with a dose of one or more other painkillers or sedatives like hydromorphone – and being withdrawn from the ventilator. These deaths all occurred following a procedure called palliative extubation, the removal of the endotracheal tube in patients who are expected to die.
Mount Carmel fired Dr. Husel in December 2018 following an investigation that concluded that the opioid dosages he used were “significantly excessive and potentially fatal,” and “went beyond providing comfort.” His Ohio medical license was suspended. In February 2022, he is scheduled to go on trial in Columbus on 14 counts of murder.*
Hanging over the murder case against Dr. Husel is the question of how Mount Carmel, a 136-year-old Catholic hospital owned by the giant Trinity Health system, allowed this pattern of care to continue for so many patients over 4 years, and why numerous registered nurses and hospital pharmacists went along with Dr. Husel’s actions. Nearly two dozen RNs and two pharmacists involved in these cases have faced disciplinary action, mostly license suspension.
“The first time a patient died on a very high dose, someone should have flagged this,” said Lewis Nelson, MD, chair of emergency medicine at Rutgers New Jersey Medical School, Newark. “As soon as I see it the second time or 27th time, it doesn’t seem okay. There was a breakdown in oversight to allow this to continue. The hospital didn’t have guardrails in place.”
The Franklin County (Ohio) Prosecuting Attorney’s Office faces two big challenges in trying Dr. Husel for murder. The prosecutors must prove that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Federal and state agencies have cited the hospital system for faults in its patient safety systems and culture that were exposed by the Husel cases. An outside medical expert, Robert Powers, MD, a professor of emergency medicine at the University of Virginia, Charlottesville, testified in one of the dozens of wrongful death lawsuits against Mount Carmel and Dr. Husel that there was no record of anyone supervising Dr. Husel or monitoring his care.
There also are questions about why Mount Carmel administrators and physician leaders did not find out about Dr. Husel’s criminal record as a young man before hiring and credentialing him, even though the Ohio Medical Board had obtained that record. As a college freshman in West Virginia in 1994, Dr. Husel and a friend allegedly stole car stereos, and after a classmate reported their behavior, they built a pipe bomb they planned to plant under the classmate’s car, according to court records.
Dr. Husel pleaded guilty in 1996 to a federal misdemeanor for improperly storing explosive materials, and he received a 6-month sentence followed by supervision. He did not disclose that criminal conviction on his application for medical liability insurance as part of his Mount Carmel employment application, attorneys representing the families of his deceased patients say.
A Mount Carmel spokeswoman said the hospital only checks a physician applicant’s background record for the previous 10 years.
“I think [the credentialing process] should have been more careful and more comprehensive than it was,” Robert Powers testified in a September 2020 deposition. “This guy was a bomber and a thief. You don’t hire bombers and thieves to take care of patients.”
Mount Carmel and Trinity leaders say they knew nothing about Dr. Husel’s palliative extubation practices until a staffer reported Dr. Husel’s high-dose fentanyl orders in October 2018. However, three more Husel patients died under similar circumstances before he was removed from patient care in November 2018.
Mount Carmel and Trinity already have settled a number of wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. The Mount Carmel CEO, the chief clinical officer, other physician, nursing, and pharmacy leaders, as well as dozens of nurses and pharmacists have been terminated or entered into retirement.
“What happened is tragic and unacceptable,” the Mount Carmel spokeswoman said in a written statement. “We have made a number of changes designed to prevent this from ever happening again. … Our new hospital leadership team is committed to patient safety and will take immediate action whenever patient safety is at issue.”
In January 2019, Mount Carmel’s then-CEO Ed Lamb acknowledged that “processes in place were not sufficient to prevent these actions from happening.” Mr. Lamb later said Mount Carmel was investigating whether five of the ICU patients who died under Dr. Husel’s care could have been treated and survived. Mr. Lamb stepped down in June 2019.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved one gasping for breath. But most medical experts say the fentanyl doses Dr. Husel ordered – 500-2,000 mcg – were five to 20 times larger than doses normally used in palliative extubation. Such doses, they say, would quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 mcg for relieving pain, and its 2018 guidelines reduced that to 25-50 mcg.
“If I perform a painful procedure, I might give 100 or 150 micrograms of fentanyl, or 500 or 600 for open heart surgery,” said Dr. Nelson of Rutgers, who also practices medical toxicology and addiction medicine. “But you’ll be intubated and monitored carefully. Without having a tube in your airway to help you breathe, those doses will kill you.”**
Mount Carmel West hired Dr. Husel in 2013 to work the late-night shift in its ICU. It was his first job as a full-fledged physician, after completing a residency and fellowship in critical care medicine at Cleveland Clinic. A good-looking and charismatic former high school basketball star, he was a hard worker and was popular with the ICU nurses and staff, who looked to him as a teacher and mentor, according to depositions of nurses and Ohio Board of Nursing reports.
In 2014, Dr. Husel was chosen by his hospital colleagues as physician of the year. He was again nominated in 2018. Before October 2018, there were no complaints about his care, according to the deposition of Larry Swanner, MD, Mount Carmel’s former vice president of medical affairs, who was fired in 2019.
“Dr. Husel is so knowledgeable that we would try to soak up as much knowledge as we could,” said Jason Schulze, RN, in a July 2020 deposition. Mr. Schulze’s license was suspended, however, that suspension was stayed for a minimum period of two years. This was in connection with his care of one of Dr. Husel’s ICU patients, 44-year-old Troy Allison, who died 3 minutes after Mr. Schulze administered a 1,000-microgram dose of fentanyl ordered by Dr. Husel in July 2018.
Dr. Husel’s winning personality and seeming expertise in the use of pain drugs, combined with his training at the prestigious Cleveland Clinic, may have lulled other hospital staff into going along with his decisions.
“They’re thinking, the guy’s likable and he must know what he’s doing,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “But you can’t get fooled by that. You need a policy in place for what to do if pharmacists or nurses disagree with an order, and you need to have practice simulations so people know how to handle these situations.”
Dr. Husel’s criminal defense attorney, Jose Baez, said Dr. Husel’s treatment of all these palliative extubation patients, including his prescribed dosages of fentanyl and other drugs, was completely appropriate. “Dr. Husel practiced medicine with compassion, and never wanted to see any of his patients suffer, nor their family,” Mr. Baez said.
Most medical and pharmacy experts sharply disagree. “I’m a pharmacist, and I’ve never seen anything like those kinds of doses,” Mr. Cohen said. “Something strange was going on there.”
Complicating these issues, eight nurses and a pharmacist have sued Mount Carmel and Trinity for wrongful termination and defamation in connection with the Husel allegations. They strongly defend Dr. Husel’s and their care as compassionate and appropriate. Beyond that, they argue that the changes Mount Carmel and Trinity made to ICU procedures to prevent such situations from happening again are potentially harmful to patient care.
“None of the nurses ever thought that Dr. Husel did anything to harm his patients or do anything other than provide comfort care during a very difficult time,” said Robert Landy, a New York attorney who’s representing the plaintiffs in the federal wrongful termination suit. “The real harm came in January 2019, when there were substantial policy changes that were detrimental to patient care and safety.”
Many of these patient deaths occurred during a period when the Mount Carmel system and Trinity were in the process of closing the old Mount Carmel West hospital, located in the low-income, inner-city neighborhood of Columbus, and opening a new hospital in the affluent suburb of Grove City, Ohio.
“They were done with this old, worn-out, inner-city hospital and its patient base and wanted a brand-new sparkling object in the suburbs,” said Gerry Leeseberg, a Columbus attorney who is representing 17 families of patients who died under Dr. Husel’s care. “They may have directed less energy, attention, and resources to the inner-city hospital.”
The case of Danny Mollette illustrates the multiple issues with Mount Carmel’s patient safety system.
First, there was no evidence in the record that Mr. Mollette was in pain or lacked the ability to breathe on his own prior to Dr. Husel’s palliative extubation. He had received no pain medications in the hospital that day, according to the report of an Ohio Board of Nursing examiner in a licensure discipline action brought against nurse Jacob Deemer for his care of Mr. Mollette and two other ICU patients who died. Mr. Deemer said Dr. Husel told him that the patient had to be in pain given his condition.
After consulting with Mr. Mollette’s family at the bedside, Dr. Husel ordered Mr. Deemer to administer 1,000 mcg of fentanyl, followed by 2 mg of hydromorphone, and 4 mg of midzolam, a sedative. Mr. Deemer withdrew the drugs from the Pyxis dispensing cabinet, overriding the pharmacist preapproval system. He said Dr. Husel told him the pharmacist had said, “It is okay.”
Actually, according to the pharmacy board report, the pharmacist, Gregory White, wrote in the medical record system that he did not agree to the fentanyl order. But his dissent came as the drugs were being administered, the breathing tube was being removed, and the patient was about to die. Mr. White was later disciplined by the Ohio Board of Pharmacy for failing to inform his supervisors about the incident and preventing the use of those high drug dosages in the cases of Mr. Mollette and two subsequent Husel patients.
Then there are questions about whether the families of Mr. Mollette and other Husel patients were fully and accurately informed about their loved ones’ conditions before agreeing to the palliative extubation. Mr. Mollette’s son, Brian, told reporters in July 2019 that Dr. Husel “said my father’s organs were shutting down and he was brain damaged. In hindsight, we felt kind of rushed to make that decision.”
Plaintiff attorneys bringing civil wrongful death cases against Mount Carmel and Dr. Husel must overcome hurdles similar to those faced by prosecutors in the murder case against Dr. Husel. Even if the patients were likely to die from their underlying conditions, did the drugs hasten their deaths, and by how much? In the civil cases, there’s the additional question of how much a few more hours or days or weeks of life are worth in terms of monetary damages.
Another challenge in bringing both the criminal and civil cases is that physicians and other medical providers have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia, and that’s illegal in the United States.
“There is no evidence that medication played any part in the death of any of these patients,” said Mr. Landy, who’s representing the nurses and pharmacists in the wrongful termination suit. “The only evidence we have is that higher dosages of opioids following extubation extend life, not shorten it.”
Dr. Husel, as well as the nurses and pharmacists who have faced licensure actions, claim their actions were legally shielded by the double-effect principle. But the Centers for Medicare & Medicaid Services, the Ohio Board of Nursing, and Ohio Board of Pharmacy haven’t accepted that defense. Instead, they have cited Mount Carmel, Dr. Husel, and the nurses and pharmacists for numerous patient safety violations, including administering excessive dosages of fentanyl and other drugs.
Among those violations is that many of Dr. Husel’s drug orders were given verbally instead of through the standard process of entering the orders into the electronic health record. He and the nurses on duty skipped the standard nonemergency process of getting preapproval from the pharmacist on duty. Instead, they used the override function on Mount Carmel’s automated Pyxis system to withdraw the drugs from the cabinet and avoid pharmacist review. In many cases, there was no retrospective review of the appropriateness of the orders by a pharmacist after the drugs were administered, which is required.
After threatening to cut off Medicare and Medicaid payments to Mount Carmel, CMS in June 2019 accepted the hospital’s correction plan, which restricted use of verbal drug orders and prohibited Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Under the agreement with CMS, Mount Carmel physicians must receive permission from a physician executive to order painkilling drugs that exceed hospital-set dosage parameters for palliative ventilator withdrawal. In addition, pharmacists must immediately report concerns about drug-prescribing safety up the hospital pharmacy chain of command.
“We have trained staff to ensure they feel empowered to speak up when appropriate,” the Mount Carmel spokeswoman said. “Staff members have multiple avenues for elevating a complaint or concern.”
Dr. Husel’s high dosages of fentanyl and other painkillers were well-known among the ICU nurses and pharmacists, who rarely – if ever – questioned those dosages, and went along with his standard use of verbal orders and overrides of the Pyxis system, according to depositions of nurses and pharmacists in the wrongful death lawsuits.
But the Mount Carmel nurses and pharmacists had a professional responsibility to question such dosages and demand evidence from the medical literature to support their use, according to hearing examiners at the nursing and pharmacy boards, who meted out licensure actions to providers working with Dr. Husel.
Nursing board hearing examiner Jack Decker emphasized those responsibilities in his November 30, 2020, report on nurse Deemer’s actions regarding three patients who died under Dr. Husel’s care in 2017 and 2018. Mr. Deemer’s license was suspended, however, that suspension was stayed for a minimum period of three years. Mr. Decker wrote that the ICU nurses had a professional responsibility to question Dr. Husel and, if necessary, refuse to carry out the doctor’s order and report their concerns to managers.
“Challenging a physician’s order is a difficult step even under ideal circumstances,” wrote Mr. Decker, who called Mount Carmel West’s ICU a “dysfunctional” environment. “But,” he noted, “when Mr. Deemer signed on to become a nurse, he enlisted to use his own critical thinking skills to serve as a patient protector and advocate. … Clearly, Mr. Deemer trusted Dr. Husel. But Dr. Husel was not to be trusted.”
While patient safety experts say these cases reveal that Mount Carmel had a flawed system and culture that did not train and empower staff to report safety concerns up the chain of command, they acknowledged that this could have happened at many U.S. hospitals.
“Sadly, I’m not sure it’s all that uncommon,” said Dr. Nelson of Rutgers. “Nurses and pharmacists have historically been afraid to raise concerns about physicians. We’ve been trying to break down barriers, but it’s a natural human instinct to play your role in the hierarchy.”
A version of this article first appeared on Medscape.com.
Corrections 2/1/22: An earlier version of this article misstated (*) the number of murder counts and (**) Dr. Nelson's area of practice.
This article was updated 2/2/22 to reflect the fact that the license suspensions of Mr. Deemer and Mr. Schulze were stayed.
On Dec. 5, 2017, Danny Mollette, age 74, was brought to the emergency department of Mount Carmel West Medical Center in Columbus, Ohio, in critical condition. Staff inserted a breathing tube and sent him to the intensive care unit.
Mr. Mollette, who had diabetes, previously had been hospitalized for treatment of a gangrenous foot. When he arrived in the ICU, he was suffering from acute renal failure and low blood pressure, and had had two heart stoppages, according to a 2020 Ohio Board of Pharmacy report. He was placed under the care of William Husel, DO, the sole physician on duty in the ICU during the overnight shift.
Around 9:00 p.m., Dr. Husel discussed Mr. Mollette’s “grim prognosis” with family members at the patient’s bedside. He advised them that Mr. Mollette had “minutes to live” and asked, “How would you want him to take his last breath: on the ventilator or without these machines?”
In less than an hour, Mr. Mollette was dead. Some said that what happened in his case was similar to what happened with 34 other ICU patients at Mount Carmel West and Mount Carmel St. Ann’s in Westerville, Ohio, from 2014 through 2018 – all under Dr. Husel’s care.
Like Mr. Mollette, most of these gravely ill patients died minutes after receiving a single, unusually large intravenous dose of the powerful opioid fentanyl – often combined with a dose of one or more other painkillers or sedatives like hydromorphone – and being withdrawn from the ventilator. These deaths all occurred following a procedure called palliative extubation, the removal of the endotracheal tube in patients who are expected to die.
Mount Carmel fired Dr. Husel in December 2018 following an investigation that concluded that the opioid dosages he used were “significantly excessive and potentially fatal,” and “went beyond providing comfort.” His Ohio medical license was suspended. In February 2022, he is scheduled to go on trial in Columbus on 14 counts of murder.*
Hanging over the murder case against Dr. Husel is the question of how Mount Carmel, a 136-year-old Catholic hospital owned by the giant Trinity Health system, allowed this pattern of care to continue for so many patients over 4 years, and why numerous registered nurses and hospital pharmacists went along with Dr. Husel’s actions. Nearly two dozen RNs and two pharmacists involved in these cases have faced disciplinary action, mostly license suspension.
“The first time a patient died on a very high dose, someone should have flagged this,” said Lewis Nelson, MD, chair of emergency medicine at Rutgers New Jersey Medical School, Newark. “As soon as I see it the second time or 27th time, it doesn’t seem okay. There was a breakdown in oversight to allow this to continue. The hospital didn’t have guardrails in place.”
The Franklin County (Ohio) Prosecuting Attorney’s Office faces two big challenges in trying Dr. Husel for murder. The prosecutors must prove that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Federal and state agencies have cited the hospital system for faults in its patient safety systems and culture that were exposed by the Husel cases. An outside medical expert, Robert Powers, MD, a professor of emergency medicine at the University of Virginia, Charlottesville, testified in one of the dozens of wrongful death lawsuits against Mount Carmel and Dr. Husel that there was no record of anyone supervising Dr. Husel or monitoring his care.
There also are questions about why Mount Carmel administrators and physician leaders did not find out about Dr. Husel’s criminal record as a young man before hiring and credentialing him, even though the Ohio Medical Board had obtained that record. As a college freshman in West Virginia in 1994, Dr. Husel and a friend allegedly stole car stereos, and after a classmate reported their behavior, they built a pipe bomb they planned to plant under the classmate’s car, according to court records.
Dr. Husel pleaded guilty in 1996 to a federal misdemeanor for improperly storing explosive materials, and he received a 6-month sentence followed by supervision. He did not disclose that criminal conviction on his application for medical liability insurance as part of his Mount Carmel employment application, attorneys representing the families of his deceased patients say.
A Mount Carmel spokeswoman said the hospital only checks a physician applicant’s background record for the previous 10 years.
“I think [the credentialing process] should have been more careful and more comprehensive than it was,” Robert Powers testified in a September 2020 deposition. “This guy was a bomber and a thief. You don’t hire bombers and thieves to take care of patients.”
Mount Carmel and Trinity leaders say they knew nothing about Dr. Husel’s palliative extubation practices until a staffer reported Dr. Husel’s high-dose fentanyl orders in October 2018. However, three more Husel patients died under similar circumstances before he was removed from patient care in November 2018.
Mount Carmel and Trinity already have settled a number of wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. The Mount Carmel CEO, the chief clinical officer, other physician, nursing, and pharmacy leaders, as well as dozens of nurses and pharmacists have been terminated or entered into retirement.
“What happened is tragic and unacceptable,” the Mount Carmel spokeswoman said in a written statement. “We have made a number of changes designed to prevent this from ever happening again. … Our new hospital leadership team is committed to patient safety and will take immediate action whenever patient safety is at issue.”
In January 2019, Mount Carmel’s then-CEO Ed Lamb acknowledged that “processes in place were not sufficient to prevent these actions from happening.” Mr. Lamb later said Mount Carmel was investigating whether five of the ICU patients who died under Dr. Husel’s care could have been treated and survived. Mr. Lamb stepped down in June 2019.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved one gasping for breath. But most medical experts say the fentanyl doses Dr. Husel ordered – 500-2,000 mcg – were five to 20 times larger than doses normally used in palliative extubation. Such doses, they say, would quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 mcg for relieving pain, and its 2018 guidelines reduced that to 25-50 mcg.
“If I perform a painful procedure, I might give 100 or 150 micrograms of fentanyl, or 500 or 600 for open heart surgery,” said Dr. Nelson of Rutgers, who also practices medical toxicology and addiction medicine. “But you’ll be intubated and monitored carefully. Without having a tube in your airway to help you breathe, those doses will kill you.”**
Mount Carmel West hired Dr. Husel in 2013 to work the late-night shift in its ICU. It was his first job as a full-fledged physician, after completing a residency and fellowship in critical care medicine at Cleveland Clinic. A good-looking and charismatic former high school basketball star, he was a hard worker and was popular with the ICU nurses and staff, who looked to him as a teacher and mentor, according to depositions of nurses and Ohio Board of Nursing reports.
In 2014, Dr. Husel was chosen by his hospital colleagues as physician of the year. He was again nominated in 2018. Before October 2018, there were no complaints about his care, according to the deposition of Larry Swanner, MD, Mount Carmel’s former vice president of medical affairs, who was fired in 2019.
“Dr. Husel is so knowledgeable that we would try to soak up as much knowledge as we could,” said Jason Schulze, RN, in a July 2020 deposition. Mr. Schulze’s license was suspended, however, that suspension was stayed for a minimum period of two years. This was in connection with his care of one of Dr. Husel’s ICU patients, 44-year-old Troy Allison, who died 3 minutes after Mr. Schulze administered a 1,000-microgram dose of fentanyl ordered by Dr. Husel in July 2018.
Dr. Husel’s winning personality and seeming expertise in the use of pain drugs, combined with his training at the prestigious Cleveland Clinic, may have lulled other hospital staff into going along with his decisions.
“They’re thinking, the guy’s likable and he must know what he’s doing,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “But you can’t get fooled by that. You need a policy in place for what to do if pharmacists or nurses disagree with an order, and you need to have practice simulations so people know how to handle these situations.”
Dr. Husel’s criminal defense attorney, Jose Baez, said Dr. Husel’s treatment of all these palliative extubation patients, including his prescribed dosages of fentanyl and other drugs, was completely appropriate. “Dr. Husel practiced medicine with compassion, and never wanted to see any of his patients suffer, nor their family,” Mr. Baez said.
Most medical and pharmacy experts sharply disagree. “I’m a pharmacist, and I’ve never seen anything like those kinds of doses,” Mr. Cohen said. “Something strange was going on there.”
Complicating these issues, eight nurses and a pharmacist have sued Mount Carmel and Trinity for wrongful termination and defamation in connection with the Husel allegations. They strongly defend Dr. Husel’s and their care as compassionate and appropriate. Beyond that, they argue that the changes Mount Carmel and Trinity made to ICU procedures to prevent such situations from happening again are potentially harmful to patient care.
“None of the nurses ever thought that Dr. Husel did anything to harm his patients or do anything other than provide comfort care during a very difficult time,” said Robert Landy, a New York attorney who’s representing the plaintiffs in the federal wrongful termination suit. “The real harm came in January 2019, when there were substantial policy changes that were detrimental to patient care and safety.”
Many of these patient deaths occurred during a period when the Mount Carmel system and Trinity were in the process of closing the old Mount Carmel West hospital, located in the low-income, inner-city neighborhood of Columbus, and opening a new hospital in the affluent suburb of Grove City, Ohio.
“They were done with this old, worn-out, inner-city hospital and its patient base and wanted a brand-new sparkling object in the suburbs,” said Gerry Leeseberg, a Columbus attorney who is representing 17 families of patients who died under Dr. Husel’s care. “They may have directed less energy, attention, and resources to the inner-city hospital.”
The case of Danny Mollette illustrates the multiple issues with Mount Carmel’s patient safety system.
First, there was no evidence in the record that Mr. Mollette was in pain or lacked the ability to breathe on his own prior to Dr. Husel’s palliative extubation. He had received no pain medications in the hospital that day, according to the report of an Ohio Board of Nursing examiner in a licensure discipline action brought against nurse Jacob Deemer for his care of Mr. Mollette and two other ICU patients who died. Mr. Deemer said Dr. Husel told him that the patient had to be in pain given his condition.
After consulting with Mr. Mollette’s family at the bedside, Dr. Husel ordered Mr. Deemer to administer 1,000 mcg of fentanyl, followed by 2 mg of hydromorphone, and 4 mg of midzolam, a sedative. Mr. Deemer withdrew the drugs from the Pyxis dispensing cabinet, overriding the pharmacist preapproval system. He said Dr. Husel told him the pharmacist had said, “It is okay.”
Actually, according to the pharmacy board report, the pharmacist, Gregory White, wrote in the medical record system that he did not agree to the fentanyl order. But his dissent came as the drugs were being administered, the breathing tube was being removed, and the patient was about to die. Mr. White was later disciplined by the Ohio Board of Pharmacy for failing to inform his supervisors about the incident and preventing the use of those high drug dosages in the cases of Mr. Mollette and two subsequent Husel patients.
Then there are questions about whether the families of Mr. Mollette and other Husel patients were fully and accurately informed about their loved ones’ conditions before agreeing to the palliative extubation. Mr. Mollette’s son, Brian, told reporters in July 2019 that Dr. Husel “said my father’s organs were shutting down and he was brain damaged. In hindsight, we felt kind of rushed to make that decision.”
Plaintiff attorneys bringing civil wrongful death cases against Mount Carmel and Dr. Husel must overcome hurdles similar to those faced by prosecutors in the murder case against Dr. Husel. Even if the patients were likely to die from their underlying conditions, did the drugs hasten their deaths, and by how much? In the civil cases, there’s the additional question of how much a few more hours or days or weeks of life are worth in terms of monetary damages.
Another challenge in bringing both the criminal and civil cases is that physicians and other medical providers have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia, and that’s illegal in the United States.
“There is no evidence that medication played any part in the death of any of these patients,” said Mr. Landy, who’s representing the nurses and pharmacists in the wrongful termination suit. “The only evidence we have is that higher dosages of opioids following extubation extend life, not shorten it.”
Dr. Husel, as well as the nurses and pharmacists who have faced licensure actions, claim their actions were legally shielded by the double-effect principle. But the Centers for Medicare & Medicaid Services, the Ohio Board of Nursing, and Ohio Board of Pharmacy haven’t accepted that defense. Instead, they have cited Mount Carmel, Dr. Husel, and the nurses and pharmacists for numerous patient safety violations, including administering excessive dosages of fentanyl and other drugs.
Among those violations is that many of Dr. Husel’s drug orders were given verbally instead of through the standard process of entering the orders into the electronic health record. He and the nurses on duty skipped the standard nonemergency process of getting preapproval from the pharmacist on duty. Instead, they used the override function on Mount Carmel’s automated Pyxis system to withdraw the drugs from the cabinet and avoid pharmacist review. In many cases, there was no retrospective review of the appropriateness of the orders by a pharmacist after the drugs were administered, which is required.
After threatening to cut off Medicare and Medicaid payments to Mount Carmel, CMS in June 2019 accepted the hospital’s correction plan, which restricted use of verbal drug orders and prohibited Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Under the agreement with CMS, Mount Carmel physicians must receive permission from a physician executive to order painkilling drugs that exceed hospital-set dosage parameters for palliative ventilator withdrawal. In addition, pharmacists must immediately report concerns about drug-prescribing safety up the hospital pharmacy chain of command.
“We have trained staff to ensure they feel empowered to speak up when appropriate,” the Mount Carmel spokeswoman said. “Staff members have multiple avenues for elevating a complaint or concern.”
Dr. Husel’s high dosages of fentanyl and other painkillers were well-known among the ICU nurses and pharmacists, who rarely – if ever – questioned those dosages, and went along with his standard use of verbal orders and overrides of the Pyxis system, according to depositions of nurses and pharmacists in the wrongful death lawsuits.
But the Mount Carmel nurses and pharmacists had a professional responsibility to question such dosages and demand evidence from the medical literature to support their use, according to hearing examiners at the nursing and pharmacy boards, who meted out licensure actions to providers working with Dr. Husel.
Nursing board hearing examiner Jack Decker emphasized those responsibilities in his November 30, 2020, report on nurse Deemer’s actions regarding three patients who died under Dr. Husel’s care in 2017 and 2018. Mr. Deemer’s license was suspended, however, that suspension was stayed for a minimum period of three years. Mr. Decker wrote that the ICU nurses had a professional responsibility to question Dr. Husel and, if necessary, refuse to carry out the doctor’s order and report their concerns to managers.
“Challenging a physician’s order is a difficult step even under ideal circumstances,” wrote Mr. Decker, who called Mount Carmel West’s ICU a “dysfunctional” environment. “But,” he noted, “when Mr. Deemer signed on to become a nurse, he enlisted to use his own critical thinking skills to serve as a patient protector and advocate. … Clearly, Mr. Deemer trusted Dr. Husel. But Dr. Husel was not to be trusted.”
While patient safety experts say these cases reveal that Mount Carmel had a flawed system and culture that did not train and empower staff to report safety concerns up the chain of command, they acknowledged that this could have happened at many U.S. hospitals.
“Sadly, I’m not sure it’s all that uncommon,” said Dr. Nelson of Rutgers. “Nurses and pharmacists have historically been afraid to raise concerns about physicians. We’ve been trying to break down barriers, but it’s a natural human instinct to play your role in the hierarchy.”
A version of this article first appeared on Medscape.com.
Corrections 2/1/22: An earlier version of this article misstated (*) the number of murder counts and (**) Dr. Nelson's area of practice.
This article was updated 2/2/22 to reflect the fact that the license suspensions of Mr. Deemer and Mr. Schulze were stayed.
On Dec. 5, 2017, Danny Mollette, age 74, was brought to the emergency department of Mount Carmel West Medical Center in Columbus, Ohio, in critical condition. Staff inserted a breathing tube and sent him to the intensive care unit.
Mr. Mollette, who had diabetes, previously had been hospitalized for treatment of a gangrenous foot. When he arrived in the ICU, he was suffering from acute renal failure and low blood pressure, and had had two heart stoppages, according to a 2020 Ohio Board of Pharmacy report. He was placed under the care of William Husel, DO, the sole physician on duty in the ICU during the overnight shift.
Around 9:00 p.m., Dr. Husel discussed Mr. Mollette’s “grim prognosis” with family members at the patient’s bedside. He advised them that Mr. Mollette had “minutes to live” and asked, “How would you want him to take his last breath: on the ventilator or without these machines?”
In less than an hour, Mr. Mollette was dead. Some said that what happened in his case was similar to what happened with 34 other ICU patients at Mount Carmel West and Mount Carmel St. Ann’s in Westerville, Ohio, from 2014 through 2018 – all under Dr. Husel’s care.
Like Mr. Mollette, most of these gravely ill patients died minutes after receiving a single, unusually large intravenous dose of the powerful opioid fentanyl – often combined with a dose of one or more other painkillers or sedatives like hydromorphone – and being withdrawn from the ventilator. These deaths all occurred following a procedure called palliative extubation, the removal of the endotracheal tube in patients who are expected to die.
Mount Carmel fired Dr. Husel in December 2018 following an investigation that concluded that the opioid dosages he used were “significantly excessive and potentially fatal,” and “went beyond providing comfort.” His Ohio medical license was suspended. In February 2022, he is scheduled to go on trial in Columbus on 14 counts of murder.*
Hanging over the murder case against Dr. Husel is the question of how Mount Carmel, a 136-year-old Catholic hospital owned by the giant Trinity Health system, allowed this pattern of care to continue for so many patients over 4 years, and why numerous registered nurses and hospital pharmacists went along with Dr. Husel’s actions. Nearly two dozen RNs and two pharmacists involved in these cases have faced disciplinary action, mostly license suspension.
“The first time a patient died on a very high dose, someone should have flagged this,” said Lewis Nelson, MD, chair of emergency medicine at Rutgers New Jersey Medical School, Newark. “As soon as I see it the second time or 27th time, it doesn’t seem okay. There was a breakdown in oversight to allow this to continue. The hospital didn’t have guardrails in place.”
The Franklin County (Ohio) Prosecuting Attorney’s Office faces two big challenges in trying Dr. Husel for murder. The prosecutors must prove that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Federal and state agencies have cited the hospital system for faults in its patient safety systems and culture that were exposed by the Husel cases. An outside medical expert, Robert Powers, MD, a professor of emergency medicine at the University of Virginia, Charlottesville, testified in one of the dozens of wrongful death lawsuits against Mount Carmel and Dr. Husel that there was no record of anyone supervising Dr. Husel or monitoring his care.
There also are questions about why Mount Carmel administrators and physician leaders did not find out about Dr. Husel’s criminal record as a young man before hiring and credentialing him, even though the Ohio Medical Board had obtained that record. As a college freshman in West Virginia in 1994, Dr. Husel and a friend allegedly stole car stereos, and after a classmate reported their behavior, they built a pipe bomb they planned to plant under the classmate’s car, according to court records.
Dr. Husel pleaded guilty in 1996 to a federal misdemeanor for improperly storing explosive materials, and he received a 6-month sentence followed by supervision. He did not disclose that criminal conviction on his application for medical liability insurance as part of his Mount Carmel employment application, attorneys representing the families of his deceased patients say.
A Mount Carmel spokeswoman said the hospital only checks a physician applicant’s background record for the previous 10 years.
“I think [the credentialing process] should have been more careful and more comprehensive than it was,” Robert Powers testified in a September 2020 deposition. “This guy was a bomber and a thief. You don’t hire bombers and thieves to take care of patients.”
Mount Carmel and Trinity leaders say they knew nothing about Dr. Husel’s palliative extubation practices until a staffer reported Dr. Husel’s high-dose fentanyl orders in October 2018. However, three more Husel patients died under similar circumstances before he was removed from patient care in November 2018.
Mount Carmel and Trinity already have settled a number of wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. The Mount Carmel CEO, the chief clinical officer, other physician, nursing, and pharmacy leaders, as well as dozens of nurses and pharmacists have been terminated or entered into retirement.
“What happened is tragic and unacceptable,” the Mount Carmel spokeswoman said in a written statement. “We have made a number of changes designed to prevent this from ever happening again. … Our new hospital leadership team is committed to patient safety and will take immediate action whenever patient safety is at issue.”
In January 2019, Mount Carmel’s then-CEO Ed Lamb acknowledged that “processes in place were not sufficient to prevent these actions from happening.” Mr. Lamb later said Mount Carmel was investigating whether five of the ICU patients who died under Dr. Husel’s care could have been treated and survived. Mr. Lamb stepped down in June 2019.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved one gasping for breath. But most medical experts say the fentanyl doses Dr. Husel ordered – 500-2,000 mcg – were five to 20 times larger than doses normally used in palliative extubation. Such doses, they say, would quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 mcg for relieving pain, and its 2018 guidelines reduced that to 25-50 mcg.
“If I perform a painful procedure, I might give 100 or 150 micrograms of fentanyl, or 500 or 600 for open heart surgery,” said Dr. Nelson of Rutgers, who also practices medical toxicology and addiction medicine. “But you’ll be intubated and monitored carefully. Without having a tube in your airway to help you breathe, those doses will kill you.”**
Mount Carmel West hired Dr. Husel in 2013 to work the late-night shift in its ICU. It was his first job as a full-fledged physician, after completing a residency and fellowship in critical care medicine at Cleveland Clinic. A good-looking and charismatic former high school basketball star, he was a hard worker and was popular with the ICU nurses and staff, who looked to him as a teacher and mentor, according to depositions of nurses and Ohio Board of Nursing reports.
In 2014, Dr. Husel was chosen by his hospital colleagues as physician of the year. He was again nominated in 2018. Before October 2018, there were no complaints about his care, according to the deposition of Larry Swanner, MD, Mount Carmel’s former vice president of medical affairs, who was fired in 2019.
“Dr. Husel is so knowledgeable that we would try to soak up as much knowledge as we could,” said Jason Schulze, RN, in a July 2020 deposition. Mr. Schulze’s license was suspended, however, that suspension was stayed for a minimum period of two years. This was in connection with his care of one of Dr. Husel’s ICU patients, 44-year-old Troy Allison, who died 3 minutes after Mr. Schulze administered a 1,000-microgram dose of fentanyl ordered by Dr. Husel in July 2018.
Dr. Husel’s winning personality and seeming expertise in the use of pain drugs, combined with his training at the prestigious Cleveland Clinic, may have lulled other hospital staff into going along with his decisions.
“They’re thinking, the guy’s likable and he must know what he’s doing,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “But you can’t get fooled by that. You need a policy in place for what to do if pharmacists or nurses disagree with an order, and you need to have practice simulations so people know how to handle these situations.”
Dr. Husel’s criminal defense attorney, Jose Baez, said Dr. Husel’s treatment of all these palliative extubation patients, including his prescribed dosages of fentanyl and other drugs, was completely appropriate. “Dr. Husel practiced medicine with compassion, and never wanted to see any of his patients suffer, nor their family,” Mr. Baez said.
Most medical and pharmacy experts sharply disagree. “I’m a pharmacist, and I’ve never seen anything like those kinds of doses,” Mr. Cohen said. “Something strange was going on there.”
Complicating these issues, eight nurses and a pharmacist have sued Mount Carmel and Trinity for wrongful termination and defamation in connection with the Husel allegations. They strongly defend Dr. Husel’s and their care as compassionate and appropriate. Beyond that, they argue that the changes Mount Carmel and Trinity made to ICU procedures to prevent such situations from happening again are potentially harmful to patient care.
“None of the nurses ever thought that Dr. Husel did anything to harm his patients or do anything other than provide comfort care during a very difficult time,” said Robert Landy, a New York attorney who’s representing the plaintiffs in the federal wrongful termination suit. “The real harm came in January 2019, when there were substantial policy changes that were detrimental to patient care and safety.”
Many of these patient deaths occurred during a period when the Mount Carmel system and Trinity were in the process of closing the old Mount Carmel West hospital, located in the low-income, inner-city neighborhood of Columbus, and opening a new hospital in the affluent suburb of Grove City, Ohio.
“They were done with this old, worn-out, inner-city hospital and its patient base and wanted a brand-new sparkling object in the suburbs,” said Gerry Leeseberg, a Columbus attorney who is representing 17 families of patients who died under Dr. Husel’s care. “They may have directed less energy, attention, and resources to the inner-city hospital.”
The case of Danny Mollette illustrates the multiple issues with Mount Carmel’s patient safety system.
First, there was no evidence in the record that Mr. Mollette was in pain or lacked the ability to breathe on his own prior to Dr. Husel’s palliative extubation. He had received no pain medications in the hospital that day, according to the report of an Ohio Board of Nursing examiner in a licensure discipline action brought against nurse Jacob Deemer for his care of Mr. Mollette and two other ICU patients who died. Mr. Deemer said Dr. Husel told him that the patient had to be in pain given his condition.
After consulting with Mr. Mollette’s family at the bedside, Dr. Husel ordered Mr. Deemer to administer 1,000 mcg of fentanyl, followed by 2 mg of hydromorphone, and 4 mg of midzolam, a sedative. Mr. Deemer withdrew the drugs from the Pyxis dispensing cabinet, overriding the pharmacist preapproval system. He said Dr. Husel told him the pharmacist had said, “It is okay.”
Actually, according to the pharmacy board report, the pharmacist, Gregory White, wrote in the medical record system that he did not agree to the fentanyl order. But his dissent came as the drugs were being administered, the breathing tube was being removed, and the patient was about to die. Mr. White was later disciplined by the Ohio Board of Pharmacy for failing to inform his supervisors about the incident and preventing the use of those high drug dosages in the cases of Mr. Mollette and two subsequent Husel patients.
Then there are questions about whether the families of Mr. Mollette and other Husel patients were fully and accurately informed about their loved ones’ conditions before agreeing to the palliative extubation. Mr. Mollette’s son, Brian, told reporters in July 2019 that Dr. Husel “said my father’s organs were shutting down and he was brain damaged. In hindsight, we felt kind of rushed to make that decision.”
Plaintiff attorneys bringing civil wrongful death cases against Mount Carmel and Dr. Husel must overcome hurdles similar to those faced by prosecutors in the murder case against Dr. Husel. Even if the patients were likely to die from their underlying conditions, did the drugs hasten their deaths, and by how much? In the civil cases, there’s the additional question of how much a few more hours or days or weeks of life are worth in terms of monetary damages.
Another challenge in bringing both the criminal and civil cases is that physicians and other medical providers have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia, and that’s illegal in the United States.
“There is no evidence that medication played any part in the death of any of these patients,” said Mr. Landy, who’s representing the nurses and pharmacists in the wrongful termination suit. “The only evidence we have is that higher dosages of opioids following extubation extend life, not shorten it.”
Dr. Husel, as well as the nurses and pharmacists who have faced licensure actions, claim their actions were legally shielded by the double-effect principle. But the Centers for Medicare & Medicaid Services, the Ohio Board of Nursing, and Ohio Board of Pharmacy haven’t accepted that defense. Instead, they have cited Mount Carmel, Dr. Husel, and the nurses and pharmacists for numerous patient safety violations, including administering excessive dosages of fentanyl and other drugs.
Among those violations is that many of Dr. Husel’s drug orders were given verbally instead of through the standard process of entering the orders into the electronic health record. He and the nurses on duty skipped the standard nonemergency process of getting preapproval from the pharmacist on duty. Instead, they used the override function on Mount Carmel’s automated Pyxis system to withdraw the drugs from the cabinet and avoid pharmacist review. In many cases, there was no retrospective review of the appropriateness of the orders by a pharmacist after the drugs were administered, which is required.
After threatening to cut off Medicare and Medicaid payments to Mount Carmel, CMS in June 2019 accepted the hospital’s correction plan, which restricted use of verbal drug orders and prohibited Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Under the agreement with CMS, Mount Carmel physicians must receive permission from a physician executive to order painkilling drugs that exceed hospital-set dosage parameters for palliative ventilator withdrawal. In addition, pharmacists must immediately report concerns about drug-prescribing safety up the hospital pharmacy chain of command.
“We have trained staff to ensure they feel empowered to speak up when appropriate,” the Mount Carmel spokeswoman said. “Staff members have multiple avenues for elevating a complaint or concern.”
Dr. Husel’s high dosages of fentanyl and other painkillers were well-known among the ICU nurses and pharmacists, who rarely – if ever – questioned those dosages, and went along with his standard use of verbal orders and overrides of the Pyxis system, according to depositions of nurses and pharmacists in the wrongful death lawsuits.
But the Mount Carmel nurses and pharmacists had a professional responsibility to question such dosages and demand evidence from the medical literature to support their use, according to hearing examiners at the nursing and pharmacy boards, who meted out licensure actions to providers working with Dr. Husel.
Nursing board hearing examiner Jack Decker emphasized those responsibilities in his November 30, 2020, report on nurse Deemer’s actions regarding three patients who died under Dr. Husel’s care in 2017 and 2018. Mr. Deemer’s license was suspended, however, that suspension was stayed for a minimum period of three years. Mr. Decker wrote that the ICU nurses had a professional responsibility to question Dr. Husel and, if necessary, refuse to carry out the doctor’s order and report their concerns to managers.
“Challenging a physician’s order is a difficult step even under ideal circumstances,” wrote Mr. Decker, who called Mount Carmel West’s ICU a “dysfunctional” environment. “But,” he noted, “when Mr. Deemer signed on to become a nurse, he enlisted to use his own critical thinking skills to serve as a patient protector and advocate. … Clearly, Mr. Deemer trusted Dr. Husel. But Dr. Husel was not to be trusted.”
While patient safety experts say these cases reveal that Mount Carmel had a flawed system and culture that did not train and empower staff to report safety concerns up the chain of command, they acknowledged that this could have happened at many U.S. hospitals.
“Sadly, I’m not sure it’s all that uncommon,” said Dr. Nelson of Rutgers. “Nurses and pharmacists have historically been afraid to raise concerns about physicians. We’ve been trying to break down barriers, but it’s a natural human instinct to play your role in the hierarchy.”
A version of this article first appeared on Medscape.com.
Corrections 2/1/22: An earlier version of this article misstated (*) the number of murder counts and (**) Dr. Nelson's area of practice.
This article was updated 2/2/22 to reflect the fact that the license suspensions of Mr. Deemer and Mr. Schulze were stayed.
Easing dementia caregiver burden, addressing interpersonal violence
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
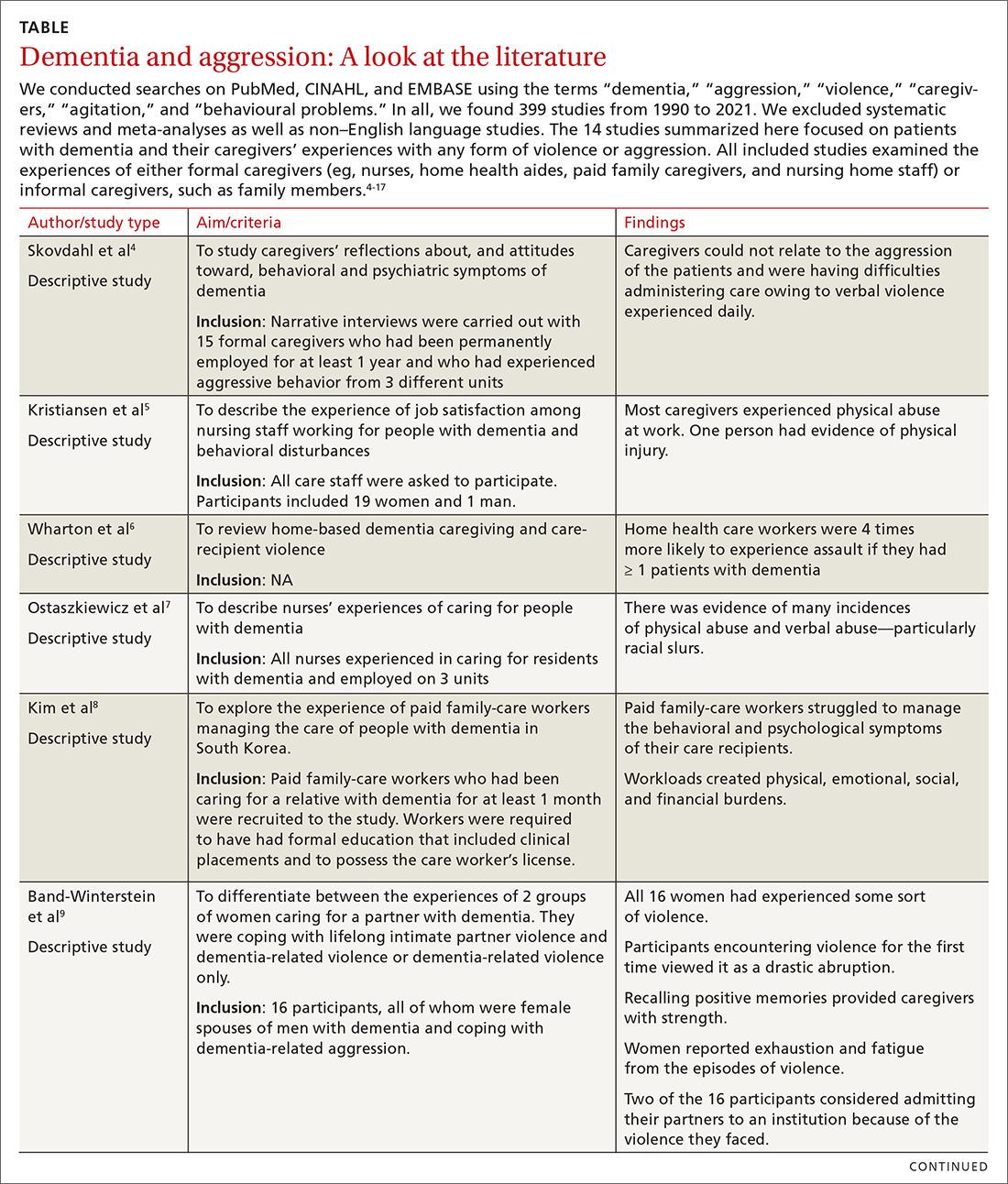
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.
CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.
A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.
However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
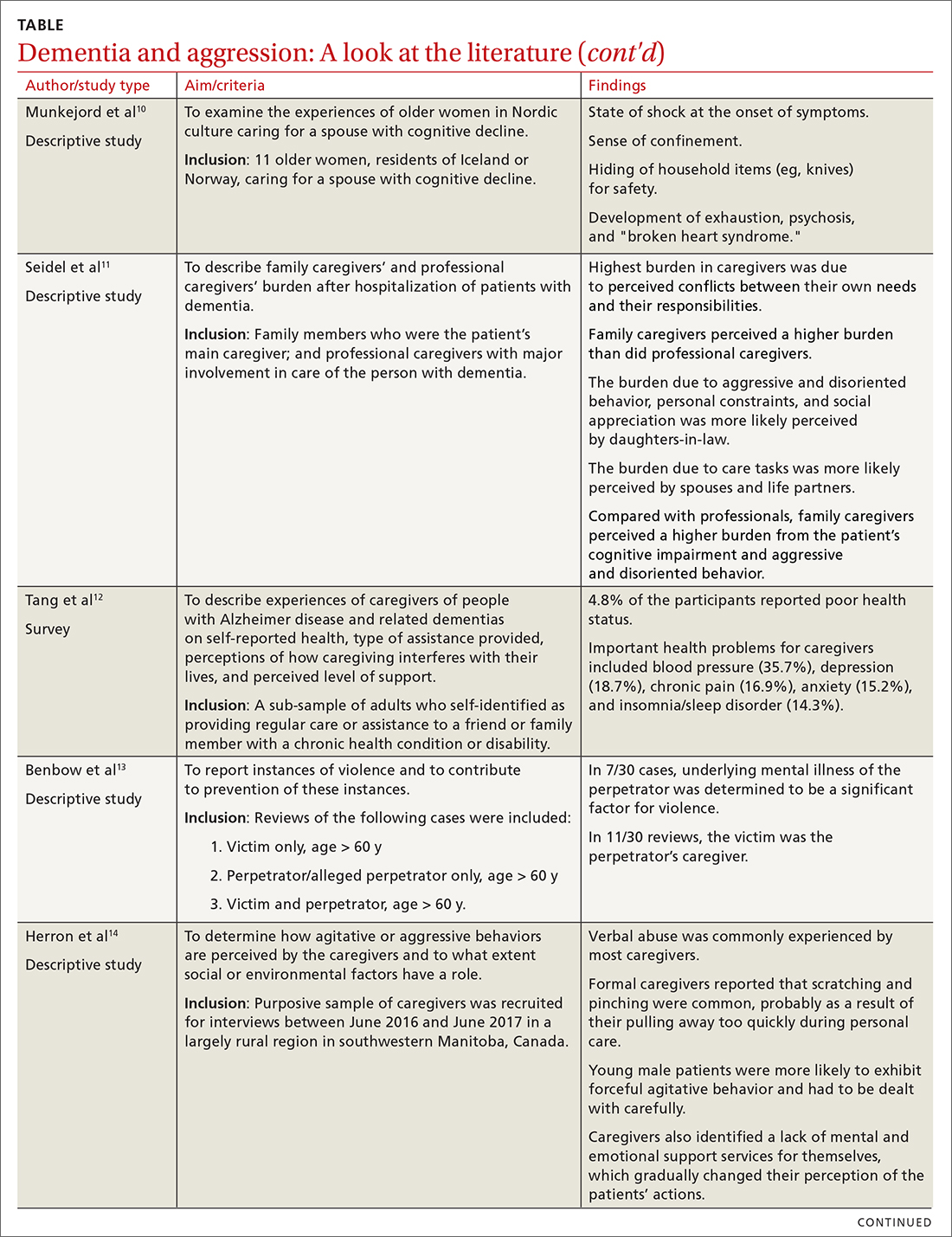
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
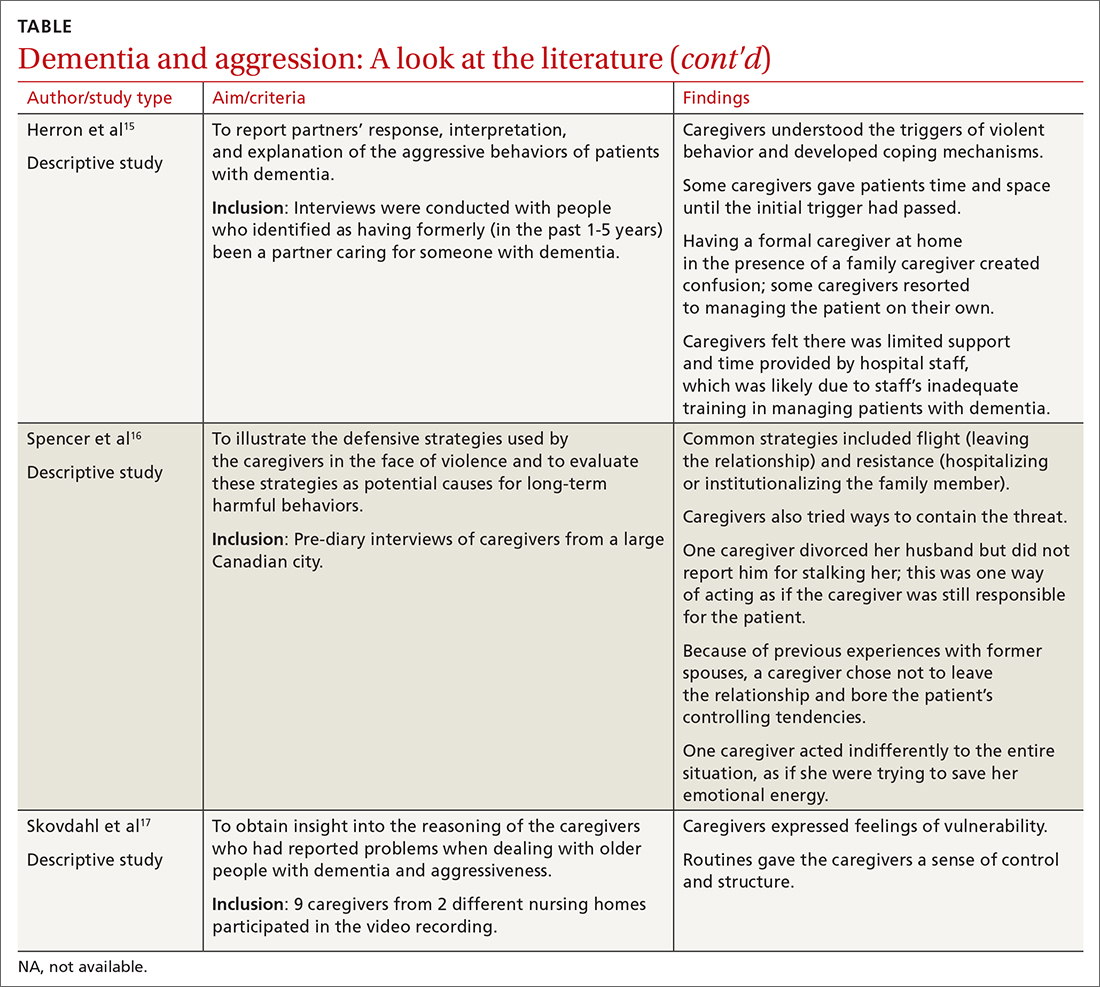
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.
CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.
A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.
However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.
CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.
A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.
However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
PRACTICE RECOMMENDATIONS
› Screen caregivers and family members of patients with dementia for signs of interpersonal violence. C
› Counsel caregivers early on that behavior changes in patients with dementia are likely and may be unpredictable. C
› Discuss safety strategies for the caregiver, including when it is appropriate to alter routines such as bedtimes and meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Palliative care specialists seek greater role in lung disease
Mrs. S.’s long-term chronic obstructive pulmonary disease (COPD) prognosis was grim, and she faced a harder time getting through each day. But neither she nor her primary care physician was willing to embrace strategies other than drugs.
“She felt guilty for continuing to smoke, but also expressed a need to smoke to help her deal with her husband’s cancer and eventual death,” recalled Georgia Narsavage, PhD, RN, ANP-BC, professor emerita of nursing at West Virginia University. “Her primary care physician was reluctant to introduce any treatment other than medications because her family was resistant to facing ‘mother dying.’ ”
But things changed when Mrs. S. was referred to a palliative-care clinical nurse specialist following a hospitalization. “The goal of palliative care is to support quality of life by relieving symptoms and decreasing suffering. She was assisted to improve functioning overall, and home support services were provided,” Dr. Narsavage said. “They allowed her to live at home relatively pain free with decreased dyspnea for 3 more years until her transition to hospice care a few months before death.
It wasn’t quite a happy ending. But it was a happier ending, and one that palliative care (PC) advocates hope will become more common in pulmonary care. They’re working to convince colleagues that PC is neither another word for hospice nor a sign that anyone is giving up on a patient.
Underutilized but beneficial
“Palliative care is underutilized in patients with chronic pulmonary disease, and it’s a missed opportunity to potentially alleviate symptoms and improve quality of life,” said Hilary DuBrock, MD, an internist and critical care pulmonologist with the Mayo Clinic in Rochester, Minn. “Chest physicians should know that it’s important to recognize your limitations in addressing all aspects of a chronic disease, and it’s OK to ask for help from a specialty multidisciplinary team of palliative care providers.”
Statistics back up Dr. DuBrock’s perspective about how PC isn’t common in pulmonary care. A 2017 study examined 181,689 U.S. adult patients who had COPD, received oxygen at home, and were hospitalized for exacerbations from 2006-2012. Just 1.7% received PC, although the number grew over the study period.
Another study published in 2017 examined 3,166 patients over the same period with end-stage idiopathic pulmonary fibrosis (IPF) who were on ventilators. The use of PC is group rose from 2.3% in 2006 to 21.6% in 2012.
More recently, a 2020 meta-analysis examined 19 studies and found that patients with lung cancer were much more likely to receive PC than were those with COPD (odds ratio (OR) = 9.59, P < .001, for hospital-based PC and OR = 8.79, P < .001, for home-based PC).
Patients with lung cancer vs. COPD were also less likely to receive invasive ventilation (OR = .26, P < .001), noninvasive ventilation (OR = .63, P = .009) or CPR (OR = .29, P < .001) or die at a nursing home/long-term care facility (OR = .32, P < .001).
Other studies support PC in COPD: Research in Europe has linked PC in COPD to fewer in-hospital deaths and lower end-of-life expenses. A Canadian study also linked PC to fewer in-hospital deaths in COPD.
Dr. DuBrock said she believes there are a couple reasons why PC isn’t more widely accepted in pulmonology. “There has been little evidence in chronic pulmonary disease regarding the role of PC, and there is a lack of standardized guidelines to help clinicians determine appropriate timing and patient selection for referral,” she said. “There is also a reluctance to refer patients to palliative care since some may think that referral implies that they are giving up on their patients.”
In fact, she said, “if appropriately explained and discussed with patients, PC does not necessarily need to imply to patients that you are giving up on them, but rather that you care enough about them to try to find novel ways to improve their quality of life and relieve their symptoms. Additionally, palliative care can be provided alongside ongoing medical care and treatment of their chronic lung disease.”
More than standard care
Another obstacle comes from pulmonologists who claim PC isn’t necessary because they’re handling patient care themselves, said University of Alabama at Birmingham critical care pulmonologist Anand S. Iyer, MD. “They’ll say: ‘I do palliative care, I palliate their breathing. I treat breathlessness and cough, that’s what I do.’ ”
But these symptoms only brush the surface of patient needs, he said. “I don’t think that the average pulmonologist goes beyond that to comprehensive symptom assessment and management of a whole host of symptoms beyond those limited to the lungs – depression, anxiety, fatigue, malnutrition.”
On that latter front, he said, pulmonologists “are really good at having end-of-life conversations at the end of life. We do that every day in the ICU.” Advocates for PC, he said, “want to push that to the clinic a year or two earlier.”
Timing and use of PC
When should pulmonologists call in a PC team? Specialists recommend early consultations, even right after a pulmonary disease is diagnosed. “When a pulmonologist diagnoses a condition as a serious illness – especially chronic pulmonary disease – a consultation with a palliative care physician or advanced practice registered nurse” can help assess the need for care and the best time to introduce palliative care to the patient and family “to provide relief and enhance quality of life,” West Virginia University’s Dr. Narsavage said. “Initial diagnosis is not too early to think about the trajectory.
Dr. Iyer agreed that early PC consultation is key. “We’re talking about comprehensive support for the physical, emotional, and spiritual needs of patients and their families. It can grow as needs of patients become more severe.”
For her part, Dr. DuBrock urged colleagues to focus on patient experiences. “The exact timing of when to refer patients with pulmonary disease is not well established,” she said. “Thus, it’s important to take cues from our patients. If they are experiencing significant symptom burden or impaired quality of life or having difficulty coping with their lung disease, then it may be helpful to call in palliative care to address these issues alongside education and discussion with the patient about the role of palliative care to address their unmet needs.”
As an example, Dr. DuBrock spoke of one of her own patients who has pulmonary hypertension (PH), connective tissue disease, and interstitial lung disease. “Her hypertension was relatively well controlled, but she was still quite symptomatic as well as depressed and having difficulty sleeping. I struggled with wanting to help her feel better but I also recognized that more PH therapy wasn’t necessarily the answer,” Dr. Dubrock said. “After some discussions, I referred her to palliative care, and they were extremely helpful with addressing her symptoms with a combination of pharmacologic and nonpharmacologic therapy and also addressing some of her underlying concerns and fears regarding her prognosis and issues related to advance-care planning. Social work was also helpful with addressing some of her financial concerns. I continue to see her on regular basis and treat her PH, but her overall quality of life, sleep, and mood have improved substantially.”
First steps
According to specialists, the first step in the PC process with patients is to make sure they understand their conditions, their prognoses, and the role of palliative care itself.
Kathleen Oare Lindell, PhD, RN, associate professor of nursing at Medical University of South Carolina, Charleston, who specializes in PC in pulmonary disease, remembers taking the histories of patients with grim prognoses and “their look on their face was like, ‘I just have a common cold.’ ” In other cases, she said, patients may fear they’ll die immediately when they have 3-5 years to live.
Dr. Lindell, who has worked at a specialty center for patients with interstitial lung disease (ILD), emphasized the importance of speaking in layperson terms that patients understand, such as referring to idiopathic pulmonary fibrosis as “unknown lung scarring.” She also said it’s crucial to be up front about their prognoses.
As for patient understanding of PC, she said, “people think it’s hospice that they’re giving. Palliative care is neither. Instead, it helps to address symptom management, I always tell patients, ‘You’ll be scared, you’ll have a cough. There are medicines and nonpharmacological therapies [that can help], and that’s what palliative care does.’ ”
Keith Swetz, MD, an internist and palliative care specialist at the University of Alabama at Birmingham, agreed that a concise discussion of prognosis is vital. “What do they know about their illness, and what do they understand about what will happen when things get worse?” he said.
“With pulmonary disease, they may be looking at months to years punctuated with a lot of ICU admissions, trips to the hospital, symptom burden, and decline in function. Some will want aggressive treatment and say they’re fine being in the hospital, while others will say being comfortable at home is more important.”
Dr. Swetz’s patients commonly have COPD, interstitial lung disease, pulmonary fibrosis, or PH, and some may have concurrent heart failure. While their prognoses may be poor, he said, discussion about their wishes probably aren’t happening outside of the PC setting.
Or if they are happening, he said, they’re lower quality, boiling down complicated care questions to “Do you want us to do everything yes, or no?
“A lot of it has to do with time,” he said. “Clinicians are busy, they might have a full ICU or pulmonary clinic with 15 minutes to see patients. Sitting down and talking about these things isn’t something that’s prioritized or fits into the work stream very well, and often it hasn’t been reimbursed.”
There typically aren’t insurance hassles regarding referrals for PC, Dr. Iyer said, although finding available specialists may be challenging. A 2019 study projected a wave of retirements of older PC physicians over the next few years, and the ratio of patients to PC specialists may not return to 2019 levels for decades. Rural areas are especially shorthanded. But telehealth may improve access, Dr. Iyer said.
What’s next? Specialists are trying to pin down guidelines for when PC consultation is appropriate in pulmonary disease.
Triggers to PC
Dr. Iyer, Dr. Lindell and others authored a 2021 report in the journal CHEST that offers guidance about triggers for PC consultation. The authors cited four “levers” or triggers that are important: worsening lung function, severe symptoms or high burden of care needs, poor prognosis, and frequent severe exacerbations.
“The overall point here is that integrating palliative care into COPD practice isn’t an on-off switch; rather, it should be based upon multiple factors and can evolve over time,” they wrote.
They noted that, “patients with COPD accept palliative care as early as moderate COPD (FEV1 < 80%), so patients may be ready sooner than clinicians think.”
They added that, “if prognosis is such a concern that a clinician is considering referral for lung transplant evaluation, then concurrent referral to specialist palliative care should be routine practice.
Finally, frequent severe exacerbations, i.e. those that require hospitalization or an emergency room visit, carry a high risk for posthospitalization mortality and are ideal inflection points in the illness trajectory of COPD.”
In the big picture, the authors contend, “palliative care should be integrated early and concurrently with COPD-directed therapies, and its intensity should increase over time as symptoms, needs, and exacerbations worsen approaching EOL [end of life].”
None of the interviewees or other authors reported having any relevant conflicts for this story.
Mrs. S.’s long-term chronic obstructive pulmonary disease (COPD) prognosis was grim, and she faced a harder time getting through each day. But neither she nor her primary care physician was willing to embrace strategies other than drugs.
“She felt guilty for continuing to smoke, but also expressed a need to smoke to help her deal with her husband’s cancer and eventual death,” recalled Georgia Narsavage, PhD, RN, ANP-BC, professor emerita of nursing at West Virginia University. “Her primary care physician was reluctant to introduce any treatment other than medications because her family was resistant to facing ‘mother dying.’ ”
But things changed when Mrs. S. was referred to a palliative-care clinical nurse specialist following a hospitalization. “The goal of palliative care is to support quality of life by relieving symptoms and decreasing suffering. She was assisted to improve functioning overall, and home support services were provided,” Dr. Narsavage said. “They allowed her to live at home relatively pain free with decreased dyspnea for 3 more years until her transition to hospice care a few months before death.
It wasn’t quite a happy ending. But it was a happier ending, and one that palliative care (PC) advocates hope will become more common in pulmonary care. They’re working to convince colleagues that PC is neither another word for hospice nor a sign that anyone is giving up on a patient.
Underutilized but beneficial
“Palliative care is underutilized in patients with chronic pulmonary disease, and it’s a missed opportunity to potentially alleviate symptoms and improve quality of life,” said Hilary DuBrock, MD, an internist and critical care pulmonologist with the Mayo Clinic in Rochester, Minn. “Chest physicians should know that it’s important to recognize your limitations in addressing all aspects of a chronic disease, and it’s OK to ask for help from a specialty multidisciplinary team of palliative care providers.”
Statistics back up Dr. DuBrock’s perspective about how PC isn’t common in pulmonary care. A 2017 study examined 181,689 U.S. adult patients who had COPD, received oxygen at home, and were hospitalized for exacerbations from 2006-2012. Just 1.7% received PC, although the number grew over the study period.
Another study published in 2017 examined 3,166 patients over the same period with end-stage idiopathic pulmonary fibrosis (IPF) who were on ventilators. The use of PC is group rose from 2.3% in 2006 to 21.6% in 2012.
More recently, a 2020 meta-analysis examined 19 studies and found that patients with lung cancer were much more likely to receive PC than were those with COPD (odds ratio (OR) = 9.59, P < .001, for hospital-based PC and OR = 8.79, P < .001, for home-based PC).
Patients with lung cancer vs. COPD were also less likely to receive invasive ventilation (OR = .26, P < .001), noninvasive ventilation (OR = .63, P = .009) or CPR (OR = .29, P < .001) or die at a nursing home/long-term care facility (OR = .32, P < .001).
Other studies support PC in COPD: Research in Europe has linked PC in COPD to fewer in-hospital deaths and lower end-of-life expenses. A Canadian study also linked PC to fewer in-hospital deaths in COPD.
Dr. DuBrock said she believes there are a couple reasons why PC isn’t more widely accepted in pulmonology. “There has been little evidence in chronic pulmonary disease regarding the role of PC, and there is a lack of standardized guidelines to help clinicians determine appropriate timing and patient selection for referral,” she said. “There is also a reluctance to refer patients to palliative care since some may think that referral implies that they are giving up on their patients.”
In fact, she said, “if appropriately explained and discussed with patients, PC does not necessarily need to imply to patients that you are giving up on them, but rather that you care enough about them to try to find novel ways to improve their quality of life and relieve their symptoms. Additionally, palliative care can be provided alongside ongoing medical care and treatment of their chronic lung disease.”
More than standard care
Another obstacle comes from pulmonologists who claim PC isn’t necessary because they’re handling patient care themselves, said University of Alabama at Birmingham critical care pulmonologist Anand S. Iyer, MD. “They’ll say: ‘I do palliative care, I palliate their breathing. I treat breathlessness and cough, that’s what I do.’ ”
But these symptoms only brush the surface of patient needs, he said. “I don’t think that the average pulmonologist goes beyond that to comprehensive symptom assessment and management of a whole host of symptoms beyond those limited to the lungs – depression, anxiety, fatigue, malnutrition.”
On that latter front, he said, pulmonologists “are really good at having end-of-life conversations at the end of life. We do that every day in the ICU.” Advocates for PC, he said, “want to push that to the clinic a year or two earlier.”
Timing and use of PC
When should pulmonologists call in a PC team? Specialists recommend early consultations, even right after a pulmonary disease is diagnosed. “When a pulmonologist diagnoses a condition as a serious illness – especially chronic pulmonary disease – a consultation with a palliative care physician or advanced practice registered nurse” can help assess the need for care and the best time to introduce palliative care to the patient and family “to provide relief and enhance quality of life,” West Virginia University’s Dr. Narsavage said. “Initial diagnosis is not too early to think about the trajectory.
Dr. Iyer agreed that early PC consultation is key. “We’re talking about comprehensive support for the physical, emotional, and spiritual needs of patients and their families. It can grow as needs of patients become more severe.”
For her part, Dr. DuBrock urged colleagues to focus on patient experiences. “The exact timing of when to refer patients with pulmonary disease is not well established,” she said. “Thus, it’s important to take cues from our patients. If they are experiencing significant symptom burden or impaired quality of life or having difficulty coping with their lung disease, then it may be helpful to call in palliative care to address these issues alongside education and discussion with the patient about the role of palliative care to address their unmet needs.”
As an example, Dr. DuBrock spoke of one of her own patients who has pulmonary hypertension (PH), connective tissue disease, and interstitial lung disease. “Her hypertension was relatively well controlled, but she was still quite symptomatic as well as depressed and having difficulty sleeping. I struggled with wanting to help her feel better but I also recognized that more PH therapy wasn’t necessarily the answer,” Dr. Dubrock said. “After some discussions, I referred her to palliative care, and they were extremely helpful with addressing her symptoms with a combination of pharmacologic and nonpharmacologic therapy and also addressing some of her underlying concerns and fears regarding her prognosis and issues related to advance-care planning. Social work was also helpful with addressing some of her financial concerns. I continue to see her on regular basis and treat her PH, but her overall quality of life, sleep, and mood have improved substantially.”
First steps
According to specialists, the first step in the PC process with patients is to make sure they understand their conditions, their prognoses, and the role of palliative care itself.
Kathleen Oare Lindell, PhD, RN, associate professor of nursing at Medical University of South Carolina, Charleston, who specializes in PC in pulmonary disease, remembers taking the histories of patients with grim prognoses and “their look on their face was like, ‘I just have a common cold.’ ” In other cases, she said, patients may fear they’ll die immediately when they have 3-5 years to live.
Dr. Lindell, who has worked at a specialty center for patients with interstitial lung disease (ILD), emphasized the importance of speaking in layperson terms that patients understand, such as referring to idiopathic pulmonary fibrosis as “unknown lung scarring.” She also said it’s crucial to be up front about their prognoses.
As for patient understanding of PC, she said, “people think it’s hospice that they’re giving. Palliative care is neither. Instead, it helps to address symptom management, I always tell patients, ‘You’ll be scared, you’ll have a cough. There are medicines and nonpharmacological therapies [that can help], and that’s what palliative care does.’ ”
Keith Swetz, MD, an internist and palliative care specialist at the University of Alabama at Birmingham, agreed that a concise discussion of prognosis is vital. “What do they know about their illness, and what do they understand about what will happen when things get worse?” he said.
“With pulmonary disease, they may be looking at months to years punctuated with a lot of ICU admissions, trips to the hospital, symptom burden, and decline in function. Some will want aggressive treatment and say they’re fine being in the hospital, while others will say being comfortable at home is more important.”
Dr. Swetz’s patients commonly have COPD, interstitial lung disease, pulmonary fibrosis, or PH, and some may have concurrent heart failure. While their prognoses may be poor, he said, discussion about their wishes probably aren’t happening outside of the PC setting.
Or if they are happening, he said, they’re lower quality, boiling down complicated care questions to “Do you want us to do everything yes, or no?
“A lot of it has to do with time,” he said. “Clinicians are busy, they might have a full ICU or pulmonary clinic with 15 minutes to see patients. Sitting down and talking about these things isn’t something that’s prioritized or fits into the work stream very well, and often it hasn’t been reimbursed.”
There typically aren’t insurance hassles regarding referrals for PC, Dr. Iyer said, although finding available specialists may be challenging. A 2019 study projected a wave of retirements of older PC physicians over the next few years, and the ratio of patients to PC specialists may not return to 2019 levels for decades. Rural areas are especially shorthanded. But telehealth may improve access, Dr. Iyer said.
What’s next? Specialists are trying to pin down guidelines for when PC consultation is appropriate in pulmonary disease.
Triggers to PC
Dr. Iyer, Dr. Lindell and others authored a 2021 report in the journal CHEST that offers guidance about triggers for PC consultation. The authors cited four “levers” or triggers that are important: worsening lung function, severe symptoms or high burden of care needs, poor prognosis, and frequent severe exacerbations.
“The overall point here is that integrating palliative care into COPD practice isn’t an on-off switch; rather, it should be based upon multiple factors and can evolve over time,” they wrote.
They noted that, “patients with COPD accept palliative care as early as moderate COPD (FEV1 < 80%), so patients may be ready sooner than clinicians think.”
They added that, “if prognosis is such a concern that a clinician is considering referral for lung transplant evaluation, then concurrent referral to specialist palliative care should be routine practice.
Finally, frequent severe exacerbations, i.e. those that require hospitalization or an emergency room visit, carry a high risk for posthospitalization mortality and are ideal inflection points in the illness trajectory of COPD.”
In the big picture, the authors contend, “palliative care should be integrated early and concurrently with COPD-directed therapies, and its intensity should increase over time as symptoms, needs, and exacerbations worsen approaching EOL [end of life].”
None of the interviewees or other authors reported having any relevant conflicts for this story.
Mrs. S.’s long-term chronic obstructive pulmonary disease (COPD) prognosis was grim, and she faced a harder time getting through each day. But neither she nor her primary care physician was willing to embrace strategies other than drugs.
“She felt guilty for continuing to smoke, but also expressed a need to smoke to help her deal with her husband’s cancer and eventual death,” recalled Georgia Narsavage, PhD, RN, ANP-BC, professor emerita of nursing at West Virginia University. “Her primary care physician was reluctant to introduce any treatment other than medications because her family was resistant to facing ‘mother dying.’ ”
But things changed when Mrs. S. was referred to a palliative-care clinical nurse specialist following a hospitalization. “The goal of palliative care is to support quality of life by relieving symptoms and decreasing suffering. She was assisted to improve functioning overall, and home support services were provided,” Dr. Narsavage said. “They allowed her to live at home relatively pain free with decreased dyspnea for 3 more years until her transition to hospice care a few months before death.
It wasn’t quite a happy ending. But it was a happier ending, and one that palliative care (PC) advocates hope will become more common in pulmonary care. They’re working to convince colleagues that PC is neither another word for hospice nor a sign that anyone is giving up on a patient.
Underutilized but beneficial
“Palliative care is underutilized in patients with chronic pulmonary disease, and it’s a missed opportunity to potentially alleviate symptoms and improve quality of life,” said Hilary DuBrock, MD, an internist and critical care pulmonologist with the Mayo Clinic in Rochester, Minn. “Chest physicians should know that it’s important to recognize your limitations in addressing all aspects of a chronic disease, and it’s OK to ask for help from a specialty multidisciplinary team of palliative care providers.”
Statistics back up Dr. DuBrock’s perspective about how PC isn’t common in pulmonary care. A 2017 study examined 181,689 U.S. adult patients who had COPD, received oxygen at home, and were hospitalized for exacerbations from 2006-2012. Just 1.7% received PC, although the number grew over the study period.
Another study published in 2017 examined 3,166 patients over the same period with end-stage idiopathic pulmonary fibrosis (IPF) who were on ventilators. The use of PC is group rose from 2.3% in 2006 to 21.6% in 2012.
More recently, a 2020 meta-analysis examined 19 studies and found that patients with lung cancer were much more likely to receive PC than were those with COPD (odds ratio (OR) = 9.59, P < .001, for hospital-based PC and OR = 8.79, P < .001, for home-based PC).
Patients with lung cancer vs. COPD were also less likely to receive invasive ventilation (OR = .26, P < .001), noninvasive ventilation (OR = .63, P = .009) or CPR (OR = .29, P < .001) or die at a nursing home/long-term care facility (OR = .32, P < .001).
Other studies support PC in COPD: Research in Europe has linked PC in COPD to fewer in-hospital deaths and lower end-of-life expenses. A Canadian study also linked PC to fewer in-hospital deaths in COPD.
Dr. DuBrock said she believes there are a couple reasons why PC isn’t more widely accepted in pulmonology. “There has been little evidence in chronic pulmonary disease regarding the role of PC, and there is a lack of standardized guidelines to help clinicians determine appropriate timing and patient selection for referral,” she said. “There is also a reluctance to refer patients to palliative care since some may think that referral implies that they are giving up on their patients.”
In fact, she said, “if appropriately explained and discussed with patients, PC does not necessarily need to imply to patients that you are giving up on them, but rather that you care enough about them to try to find novel ways to improve their quality of life and relieve their symptoms. Additionally, palliative care can be provided alongside ongoing medical care and treatment of their chronic lung disease.”
More than standard care
Another obstacle comes from pulmonologists who claim PC isn’t necessary because they’re handling patient care themselves, said University of Alabama at Birmingham critical care pulmonologist Anand S. Iyer, MD. “They’ll say: ‘I do palliative care, I palliate their breathing. I treat breathlessness and cough, that’s what I do.’ ”
But these symptoms only brush the surface of patient needs, he said. “I don’t think that the average pulmonologist goes beyond that to comprehensive symptom assessment and management of a whole host of symptoms beyond those limited to the lungs – depression, anxiety, fatigue, malnutrition.”
On that latter front, he said, pulmonologists “are really good at having end-of-life conversations at the end of life. We do that every day in the ICU.” Advocates for PC, he said, “want to push that to the clinic a year or two earlier.”
Timing and use of PC
When should pulmonologists call in a PC team? Specialists recommend early consultations, even right after a pulmonary disease is diagnosed. “When a pulmonologist diagnoses a condition as a serious illness – especially chronic pulmonary disease – a consultation with a palliative care physician or advanced practice registered nurse” can help assess the need for care and the best time to introduce palliative care to the patient and family “to provide relief and enhance quality of life,” West Virginia University’s Dr. Narsavage said. “Initial diagnosis is not too early to think about the trajectory.
Dr. Iyer agreed that early PC consultation is key. “We’re talking about comprehensive support for the physical, emotional, and spiritual needs of patients and their families. It can grow as needs of patients become more severe.”
For her part, Dr. DuBrock urged colleagues to focus on patient experiences. “The exact timing of when to refer patients with pulmonary disease is not well established,” she said. “Thus, it’s important to take cues from our patients. If they are experiencing significant symptom burden or impaired quality of life or having difficulty coping with their lung disease, then it may be helpful to call in palliative care to address these issues alongside education and discussion with the patient about the role of palliative care to address their unmet needs.”
As an example, Dr. DuBrock spoke of one of her own patients who has pulmonary hypertension (PH), connective tissue disease, and interstitial lung disease. “Her hypertension was relatively well controlled, but she was still quite symptomatic as well as depressed and having difficulty sleeping. I struggled with wanting to help her feel better but I also recognized that more PH therapy wasn’t necessarily the answer,” Dr. Dubrock said. “After some discussions, I referred her to palliative care, and they were extremely helpful with addressing her symptoms with a combination of pharmacologic and nonpharmacologic therapy and also addressing some of her underlying concerns and fears regarding her prognosis and issues related to advance-care planning. Social work was also helpful with addressing some of her financial concerns. I continue to see her on regular basis and treat her PH, but her overall quality of life, sleep, and mood have improved substantially.”
First steps
According to specialists, the first step in the PC process with patients is to make sure they understand their conditions, their prognoses, and the role of palliative care itself.
Kathleen Oare Lindell, PhD, RN, associate professor of nursing at Medical University of South Carolina, Charleston, who specializes in PC in pulmonary disease, remembers taking the histories of patients with grim prognoses and “their look on their face was like, ‘I just have a common cold.’ ” In other cases, she said, patients may fear they’ll die immediately when they have 3-5 years to live.
Dr. Lindell, who has worked at a specialty center for patients with interstitial lung disease (ILD), emphasized the importance of speaking in layperson terms that patients understand, such as referring to idiopathic pulmonary fibrosis as “unknown lung scarring.” She also said it’s crucial to be up front about their prognoses.
As for patient understanding of PC, she said, “people think it’s hospice that they’re giving. Palliative care is neither. Instead, it helps to address symptom management, I always tell patients, ‘You’ll be scared, you’ll have a cough. There are medicines and nonpharmacological therapies [that can help], and that’s what palliative care does.’ ”
Keith Swetz, MD, an internist and palliative care specialist at the University of Alabama at Birmingham, agreed that a concise discussion of prognosis is vital. “What do they know about their illness, and what do they understand about what will happen when things get worse?” he said.
“With pulmonary disease, they may be looking at months to years punctuated with a lot of ICU admissions, trips to the hospital, symptom burden, and decline in function. Some will want aggressive treatment and say they’re fine being in the hospital, while others will say being comfortable at home is more important.”
Dr. Swetz’s patients commonly have COPD, interstitial lung disease, pulmonary fibrosis, or PH, and some may have concurrent heart failure. While their prognoses may be poor, he said, discussion about their wishes probably aren’t happening outside of the PC setting.
Or if they are happening, he said, they’re lower quality, boiling down complicated care questions to “Do you want us to do everything yes, or no?
“A lot of it has to do with time,” he said. “Clinicians are busy, they might have a full ICU or pulmonary clinic with 15 minutes to see patients. Sitting down and talking about these things isn’t something that’s prioritized or fits into the work stream very well, and often it hasn’t been reimbursed.”
There typically aren’t insurance hassles regarding referrals for PC, Dr. Iyer said, although finding available specialists may be challenging. A 2019 study projected a wave of retirements of older PC physicians over the next few years, and the ratio of patients to PC specialists may not return to 2019 levels for decades. Rural areas are especially shorthanded. But telehealth may improve access, Dr. Iyer said.
What’s next? Specialists are trying to pin down guidelines for when PC consultation is appropriate in pulmonary disease.
Triggers to PC
Dr. Iyer, Dr. Lindell and others authored a 2021 report in the journal CHEST that offers guidance about triggers for PC consultation. The authors cited four “levers” or triggers that are important: worsening lung function, severe symptoms or high burden of care needs, poor prognosis, and frequent severe exacerbations.
“The overall point here is that integrating palliative care into COPD practice isn’t an on-off switch; rather, it should be based upon multiple factors and can evolve over time,” they wrote.
They noted that, “patients with COPD accept palliative care as early as moderate COPD (FEV1 < 80%), so patients may be ready sooner than clinicians think.”
They added that, “if prognosis is such a concern that a clinician is considering referral for lung transplant evaluation, then concurrent referral to specialist palliative care should be routine practice.
Finally, frequent severe exacerbations, i.e. those that require hospitalization or an emergency room visit, carry a high risk for posthospitalization mortality and are ideal inflection points in the illness trajectory of COPD.”
In the big picture, the authors contend, “palliative care should be integrated early and concurrently with COPD-directed therapies, and its intensity should increase over time as symptoms, needs, and exacerbations worsen approaching EOL [end of life].”
None of the interviewees or other authors reported having any relevant conflicts for this story.
Black-owned hospice seeks to bring greater ease in dying to Black families
This time, it didn’t take much persuading for Mary Murphy to embrace home hospice. When her mother was dying from Alzheimer’s disease in 2020, she had been reluctant until she saw what a help it was. So when her husband, Willie, neared the end of his life, she embraced hospice again.
The Murphys’ house in a leafy Nashville, Tenn., neighborhood is their happy place – full of their treasures.
“He’s good to me – buys me anything I want,” she said, as she pulled a milky glass vase out of a floor-to-ceiling cabinet with mirrored shelves.
Willie bought Mary the display case to help her to show off the trinkets she picks up at estate sales.
Down the hall, Willie was lying in their bed, now unable to speak. His heart was giving out.
“You gonna wake up for a minute?” she asked, cradling his head. She patted his back while he cleared his throat. “Cough it out.”
Heart and Soul Hospice is owned and operated by people who share the same cultural background as the patients they aim to serve.
In their application to obtain a certificate of need in Tennessee, the hospice owners made it clear they are Black and intend to serve everyone but will focus on African Americans, who are currently underserved. Tennessee data shows that in Nashville just 19% of hospice patients are Black, although they make up 27% of the capital city’s population.
Though the area already had numerous hospice agencies, regulators granted Heart and Soul permission to operate, based primarily on the value of educating an underserved group.
In Ms. Murphy’s first hospice experience, her mother had been living with dementia for decades. Still, Ms. Murphy had concerns about transitioning her mother to hospice. She felt as if she was giving up on her mom.
“My first thought was death,” she said.
National data shows that Black Medicare patients and their families are not making the move to comfort care as often as white patients are. Roughly 41% of Black Medicare beneficiaries who died in 2019 were enrolled in hospice, compared with 54% of White patients, according to data compiled annually by the National Hospice and Palliative Care Organization.
Ms. Murphy’s mother survived nearly 3 years on hospice. The benefit is meant for those in the final 6 months of life, but predicting when the end will come is difficult, especially in cases of dementia. Hospice provides palliative care for the dying and support for caregivers for a long as the process lasts.
Ms. Murphy did most of the caregiving – which can be overwhelming – but hospice helped with a few baths a week, medication in the mail and any medical equipment they needed.
And most important to Ms. Murphy was the emotional support, which came mostly from her hospice nurse.
“Wasn’t no doctor going to come here, hold my hand, stay here until the funeral home came for her,” she said about the day her mother died.
Last year, on the day after Thanksgiving, Willie Murphy died. And the same hospice nurse was at the Murphy home within minutes. She’d already stopped by that morning to check on him and returned as soon as Mary called and told her he wasn’t breathing.
“If you don’t feel like: ‘Oh my God, thank God I have hospice,’ if you can’t say that, then we’re doing something wrong,” said Keisha Mason, Heart and Soul’s director of nursing.
Ms. Mason, like Ms. Murphy, is Black and said that in her view there’s nothing fundamental keeping Black patients from using hospice except learning what the service can offer and that it’s basically free to patients – paid for by Medicare, Medicaid, and most private health plans.
“I say to them, ‘If you see a bill, then call us, because you should not,’” she said.
As Ms. Mason helped launch this new hospice agency, she began using new language, calling hospice more than a Medicare benefit. She describes it as an entitlement.
“Just as you are entitled to unemployment, as you are entitled to Social Security, you are entitled to a hospice benefit,” she said.
The investors in Heart and Soul include David Turner, owner of CNS Hospice in Detroit; Nashville pastor the Rev. Sandy McClain; and André Lee, a former hospital administrator on the campus of Meharry Medical College, a historically Black institution in Nashville.
Mr. Lee and Mr. Turner also started a Black-focused hospice agency in Michigan and have plans to replicate the model in other states.
More families need to consider home hospice as an alternative for end-of-life care, Mr. Lee said. Nursing homes are pricey. And even with Medicare, a hospital bill can be hefty.
“You’ll go in there and they’ll eat you alive,” he said. “I hate to say [something] bad about hospitals, but it’s true.”
Hospice research hasn’t come up with clear reasons to explain the gap between White and Black families’ use of the benefit. Some experts speculate it’s related to spiritual beliefs and widespread mistrust in the medical system because of decades of discrimination.
The hospice industry’s national trade group, the National Hospice and Palliative Care Organization, released a diversity and inclusion toolkit and a guide to reaching more Black patients. It recommends connecting with influential DJs, partnering with Black pastors and simply hiring more Black nurses.
Bridging the gap is not overly complicated, Mr. Lee said.
“A lot of hospices don’t employ enough Black people,” he said. “We all feel comfortable when you see someone over there that looks like you.”
Well-established hospice agencies have attempted to minimize barriers with their own diversity initiatives. Michelle Drayton of Visiting Nurse Service of New York said her large agency has met with ministers who counsel families dealing with failing health.
“Many of them did not fully understand what hospice was,” she said. “They had many of the same sort of misperceptions.”
Every hospice company, whether it’s an upstart or one of the nation’s oldest, can promote end-of-life education and ease care disparities, Ms. Drayton said. “We’re not just handing out a brochure,” she added.
This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
This time, it didn’t take much persuading for Mary Murphy to embrace home hospice. When her mother was dying from Alzheimer’s disease in 2020, she had been reluctant until she saw what a help it was. So when her husband, Willie, neared the end of his life, she embraced hospice again.
The Murphys’ house in a leafy Nashville, Tenn., neighborhood is their happy place – full of their treasures.
“He’s good to me – buys me anything I want,” she said, as she pulled a milky glass vase out of a floor-to-ceiling cabinet with mirrored shelves.
Willie bought Mary the display case to help her to show off the trinkets she picks up at estate sales.
Down the hall, Willie was lying in their bed, now unable to speak. His heart was giving out.
“You gonna wake up for a minute?” she asked, cradling his head. She patted his back while he cleared his throat. “Cough it out.”
Heart and Soul Hospice is owned and operated by people who share the same cultural background as the patients they aim to serve.
In their application to obtain a certificate of need in Tennessee, the hospice owners made it clear they are Black and intend to serve everyone but will focus on African Americans, who are currently underserved. Tennessee data shows that in Nashville just 19% of hospice patients are Black, although they make up 27% of the capital city’s population.
Though the area already had numerous hospice agencies, regulators granted Heart and Soul permission to operate, based primarily on the value of educating an underserved group.
In Ms. Murphy’s first hospice experience, her mother had been living with dementia for decades. Still, Ms. Murphy had concerns about transitioning her mother to hospice. She felt as if she was giving up on her mom.
“My first thought was death,” she said.
National data shows that Black Medicare patients and their families are not making the move to comfort care as often as white patients are. Roughly 41% of Black Medicare beneficiaries who died in 2019 were enrolled in hospice, compared with 54% of White patients, according to data compiled annually by the National Hospice and Palliative Care Organization.
Ms. Murphy’s mother survived nearly 3 years on hospice. The benefit is meant for those in the final 6 months of life, but predicting when the end will come is difficult, especially in cases of dementia. Hospice provides palliative care for the dying and support for caregivers for a long as the process lasts.
Ms. Murphy did most of the caregiving – which can be overwhelming – but hospice helped with a few baths a week, medication in the mail and any medical equipment they needed.
And most important to Ms. Murphy was the emotional support, which came mostly from her hospice nurse.
“Wasn’t no doctor going to come here, hold my hand, stay here until the funeral home came for her,” she said about the day her mother died.
Last year, on the day after Thanksgiving, Willie Murphy died. And the same hospice nurse was at the Murphy home within minutes. She’d already stopped by that morning to check on him and returned as soon as Mary called and told her he wasn’t breathing.
“If you don’t feel like: ‘Oh my God, thank God I have hospice,’ if you can’t say that, then we’re doing something wrong,” said Keisha Mason, Heart and Soul’s director of nursing.
Ms. Mason, like Ms. Murphy, is Black and said that in her view there’s nothing fundamental keeping Black patients from using hospice except learning what the service can offer and that it’s basically free to patients – paid for by Medicare, Medicaid, and most private health plans.
“I say to them, ‘If you see a bill, then call us, because you should not,’” she said.
As Ms. Mason helped launch this new hospice agency, she began using new language, calling hospice more than a Medicare benefit. She describes it as an entitlement.
“Just as you are entitled to unemployment, as you are entitled to Social Security, you are entitled to a hospice benefit,” she said.
The investors in Heart and Soul include David Turner, owner of CNS Hospice in Detroit; Nashville pastor the Rev. Sandy McClain; and André Lee, a former hospital administrator on the campus of Meharry Medical College, a historically Black institution in Nashville.
Mr. Lee and Mr. Turner also started a Black-focused hospice agency in Michigan and have plans to replicate the model in other states.
More families need to consider home hospice as an alternative for end-of-life care, Mr. Lee said. Nursing homes are pricey. And even with Medicare, a hospital bill can be hefty.
“You’ll go in there and they’ll eat you alive,” he said. “I hate to say [something] bad about hospitals, but it’s true.”
Hospice research hasn’t come up with clear reasons to explain the gap between White and Black families’ use of the benefit. Some experts speculate it’s related to spiritual beliefs and widespread mistrust in the medical system because of decades of discrimination.
The hospice industry’s national trade group, the National Hospice and Palliative Care Organization, released a diversity and inclusion toolkit and a guide to reaching more Black patients. It recommends connecting with influential DJs, partnering with Black pastors and simply hiring more Black nurses.
Bridging the gap is not overly complicated, Mr. Lee said.
“A lot of hospices don’t employ enough Black people,” he said. “We all feel comfortable when you see someone over there that looks like you.”
Well-established hospice agencies have attempted to minimize barriers with their own diversity initiatives. Michelle Drayton of Visiting Nurse Service of New York said her large agency has met with ministers who counsel families dealing with failing health.
“Many of them did not fully understand what hospice was,” she said. “They had many of the same sort of misperceptions.”
Every hospice company, whether it’s an upstart or one of the nation’s oldest, can promote end-of-life education and ease care disparities, Ms. Drayton said. “We’re not just handing out a brochure,” she added.
This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
This time, it didn’t take much persuading for Mary Murphy to embrace home hospice. When her mother was dying from Alzheimer’s disease in 2020, she had been reluctant until she saw what a help it was. So when her husband, Willie, neared the end of his life, she embraced hospice again.
The Murphys’ house in a leafy Nashville, Tenn., neighborhood is their happy place – full of their treasures.
“He’s good to me – buys me anything I want,” she said, as she pulled a milky glass vase out of a floor-to-ceiling cabinet with mirrored shelves.
Willie bought Mary the display case to help her to show off the trinkets she picks up at estate sales.
Down the hall, Willie was lying in their bed, now unable to speak. His heart was giving out.
“You gonna wake up for a minute?” she asked, cradling his head. She patted his back while he cleared his throat. “Cough it out.”
Heart and Soul Hospice is owned and operated by people who share the same cultural background as the patients they aim to serve.
In their application to obtain a certificate of need in Tennessee, the hospice owners made it clear they are Black and intend to serve everyone but will focus on African Americans, who are currently underserved. Tennessee data shows that in Nashville just 19% of hospice patients are Black, although they make up 27% of the capital city’s population.
Though the area already had numerous hospice agencies, regulators granted Heart and Soul permission to operate, based primarily on the value of educating an underserved group.
In Ms. Murphy’s first hospice experience, her mother had been living with dementia for decades. Still, Ms. Murphy had concerns about transitioning her mother to hospice. She felt as if she was giving up on her mom.
“My first thought was death,” she said.
National data shows that Black Medicare patients and their families are not making the move to comfort care as often as white patients are. Roughly 41% of Black Medicare beneficiaries who died in 2019 were enrolled in hospice, compared with 54% of White patients, according to data compiled annually by the National Hospice and Palliative Care Organization.
Ms. Murphy’s mother survived nearly 3 years on hospice. The benefit is meant for those in the final 6 months of life, but predicting when the end will come is difficult, especially in cases of dementia. Hospice provides palliative care for the dying and support for caregivers for a long as the process lasts.
Ms. Murphy did most of the caregiving – which can be overwhelming – but hospice helped with a few baths a week, medication in the mail and any medical equipment they needed.
And most important to Ms. Murphy was the emotional support, which came mostly from her hospice nurse.
“Wasn’t no doctor going to come here, hold my hand, stay here until the funeral home came for her,” she said about the day her mother died.
Last year, on the day after Thanksgiving, Willie Murphy died. And the same hospice nurse was at the Murphy home within minutes. She’d already stopped by that morning to check on him and returned as soon as Mary called and told her he wasn’t breathing.
“If you don’t feel like: ‘Oh my God, thank God I have hospice,’ if you can’t say that, then we’re doing something wrong,” said Keisha Mason, Heart and Soul’s director of nursing.
Ms. Mason, like Ms. Murphy, is Black and said that in her view there’s nothing fundamental keeping Black patients from using hospice except learning what the service can offer and that it’s basically free to patients – paid for by Medicare, Medicaid, and most private health plans.
“I say to them, ‘If you see a bill, then call us, because you should not,’” she said.
As Ms. Mason helped launch this new hospice agency, she began using new language, calling hospice more than a Medicare benefit. She describes it as an entitlement.
“Just as you are entitled to unemployment, as you are entitled to Social Security, you are entitled to a hospice benefit,” she said.
The investors in Heart and Soul include David Turner, owner of CNS Hospice in Detroit; Nashville pastor the Rev. Sandy McClain; and André Lee, a former hospital administrator on the campus of Meharry Medical College, a historically Black institution in Nashville.
Mr. Lee and Mr. Turner also started a Black-focused hospice agency in Michigan and have plans to replicate the model in other states.
More families need to consider home hospice as an alternative for end-of-life care, Mr. Lee said. Nursing homes are pricey. And even with Medicare, a hospital bill can be hefty.
“You’ll go in there and they’ll eat you alive,” he said. “I hate to say [something] bad about hospitals, but it’s true.”
Hospice research hasn’t come up with clear reasons to explain the gap between White and Black families’ use of the benefit. Some experts speculate it’s related to spiritual beliefs and widespread mistrust in the medical system because of decades of discrimination.
The hospice industry’s national trade group, the National Hospice and Palliative Care Organization, released a diversity and inclusion toolkit and a guide to reaching more Black patients. It recommends connecting with influential DJs, partnering with Black pastors and simply hiring more Black nurses.
Bridging the gap is not overly complicated, Mr. Lee said.
“A lot of hospices don’t employ enough Black people,” he said. “We all feel comfortable when you see someone over there that looks like you.”
Well-established hospice agencies have attempted to minimize barriers with their own diversity initiatives. Michelle Drayton of Visiting Nurse Service of New York said her large agency has met with ministers who counsel families dealing with failing health.
“Many of them did not fully understand what hospice was,” she said. “They had many of the same sort of misperceptions.”
Every hospice company, whether it’s an upstart or one of the nation’s oldest, can promote end-of-life education and ease care disparities, Ms. Drayton said. “We’re not just handing out a brochure,” she added.
This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
One doctor’s psychedelic journey to confront his cancer
Pradeep Bansal considered the five capsules he was about to swallow. Together they made up a 25-mg dose of a substance that, in another setting, could have landed him in federal prison.
The substance was psilocybin, the active ingredient in magic mushrooms. To be more exact, it was a synthetic form of psilocybin called COMP360, made to pharmaceutical standards by a company called COMPASS Pathways. He was taking it as part of an Food and Drug Administration–approved clinical study on mental health therapy for people with cancer.
Dr. Bansal, a New York gastroenterologist, was far more comfortable giving medical treatment than receiving it. But he was getting used to it.
He had already been through surgery and a number of other treatments to address the physical aspects of his cancer. The psilocybin was to address the mental aspects – the crushing anxiety and depression that had stuck with him after his diagnosis.
Dr. Bansal did not arrive at this moment lightly.
“I was extremely skeptical going into this process,” said Dr. Bansal, who during a long medical career had looked with distrust and even disdain at alternative therapies.
“I don’t have much patience for holistic medicine, homeopathy, acupuncture, or alternative medicines with claims of spiritual upliftment or altered states of mind.”
But Bansal had done his homework on psilocybin and was impressed.
according to studies published in 2011, 2014, and 2016.
One study from Johns Hopkins University tracked the effects of a single guided dose of psilocybin in terminal cancer patients with anxiety and depression. More than 80% had a “significant decrease” in symptoms – even 6 months after treatment – with more than 60% of the group remaining in the normal mood range.
For the study Dr. Bansal joined, there had been weeks of screening and consultation and preparation in a strictly controlled scientific trial.
And yet, even with all that he had learned, even with his psychiatrist-guide by his side, he was afraid. Afraid of what he might experience under the powerful effects of psilocybin. And afraid that this was all a misguided waste of time – that his mental angst would still be there when it was all over.
He knew that psilocybin, like other psychedelic substances, could take you on a “trip” – could remove you, at least for a time, from normal conscious experience.
Maybe he would feel “funny,” he thought. Maybe he would have some hallucinations. But how would that change the reality of his cancer? How would it lift the black dread and anxiety he felt about his future?
Stuck in a dark place
Dr. Bansal had first noticed blood in his urine – a lot of it – in September 2019.
Two months later, doctors diagnosed cancer in his right kidney. He would need surgery to remove the kidney and surrounding lymph nodes (an operation called radical nephrectomy).
It was a shock, said Dr. Bansal. But the diagnosis and the surgery happened so quickly that he hardly had time to think. And treatment results seemed good. The cancer was only in stage I and the CT scans showed no signs of cancer after surgery.
“We were so relieved. Everyone was so happy,” Dr. Bansal said. “They didn’t even give me chemotherapy after surgery because it seemed so early.”
But a routine scan in June 2020 revealed more cancer in his lung. Within a couple of months, it was in his bladder too.
“It was devastating,” Dr. Bansal said. “I went from thinking I was healthy again to stage IV cancer.”
As doctors scheduled surgery to remove part of his lung, Dr. Bansal started on painful immunotherapy (BCG therapy) for his bladder.
At this point, from a psychological standpoint, Dr. Bansal was reeling. As a doctor, he knew all too well the meaning of stage IV cancer.
With two adult children and a grandchild on the way, Dr. Bansal had been looking forward to retirement with his wife of almost 40 years. “Suddenly, I wasn’t sure I was going to last that long,” Bansal recalled. “I was in a very dark place. I was very anxious, very depressed from lack of sleep.”
He saw a therapist about his cancer diagnosis and maintained his regular meditation practice at home. He hired a personal trainer and tried to focus on any good news that he got about his treatment.
Those things helped, but not enough.
The basic facts were inescapable. His cancer might end everything. He couldn’t stop thinking about it. And then he couldn’t stop thinking about how he couldn’t stop thinking about it.
If the worst happened, he didn’t want to spend his last days in a state of such relentless existential angst. And it wasn’t just for himself. He wanted to be strong and mentally present for his family and his loved ones and his patients.
As he searched for something to ease his mental anguish, Dr. Bansal recalled some psychedelic research on end-of-life anxiety and depression that he’d read about in Michael Pollan’s book on psychedelics, “How to Change Your Mind” (New York, Penguin Press, 2018).
The studies were small and the research was new, but Dr. Bansal was impressed enough with the results to take a chance. He called a lead researcher of one of the studies, a fellow New York doctor, and eventually found himself accepted into a new study.
Starting the journey
By the time Dr. Bansal arrived at the Bill Richards Center for Healing at the Aquilino Cancer Center in Rockville, Md., he had already been through weeks of screening.
The main requirements for the study were a cancer diagnosis and a measurable level of depression. But study participants also had to be physically fit enough to handle the medication, and psychologically free from a personal or family history of psychosis or schizophrenia. (The study also required participants to slowly wean themselves from medications like SSRIs for depression or antianxiety medications under the strict supervision of a qualified doctor.)
Dr. Bansal’s week of treatment began almost immediately on arrival at Aquilino. Everything was carefully choreographed but not rushed. From Monday to Wednesday, doctors followed his physical health with exams, ECGs, and blood work. And most importantly, they began to prepare him for the “dosing session” on Thursday when he would take the psilocybin.
This is the careful crafting of “set and setting” stressed in so many psychedelic therapies. “Set” refers to your mindset going into the drug experience. “Setting” is the space and people around you when the drug sends you into an altered state of consciousness.
Dr. Bansal met several times with at least three therapists in the days leading up to his dosing. He attended 4-plus hours of therapist-led group sessions with other people who would get a dosing on the same day. Together, they talked about what to expect during the experience and what to do in the face of fear or panic.
He connected with a therapist who would be his personal guide. Dr. Bansal’s therapist was a military psychiatrist with over 30 years’ experience.
“He was there with me from day 1, and so we established a relationship,” Dr. Bansal said.
“He asked me a lot of personal background history – you know, my religious convictions, aspirations, all those things.”
“Trust and let go,” was a kind of mantra for the treatment repeated by his guide and other doctors.
For Dr. Bansal, a doctor and scientist accustomed to using hard facts rather than touchy-feely slogans to navigate the care of patients, it was an adjustment, to say the least.
But he did his best to set aside his doubts and embrace the journey he was about to take.
The day of the trip
Thursday morning finally arrived. The setting of the dosing room was warm and welcoming, more like a cozy home study than a hospital room.
This matters more than you might think. First, because it’s important that you feel safe, open, and comfortable enough to let go and enter into a therapeutic process. But also because though rare, it’s possible – especially with psilocybin – for people to lose track of where they are and what they’re doing and put themselves or others in danger.
The dose, 25 mg, had been carefully calibrated to induce a psychedelic experience sufficient for therapy. Much less than that, say 10 mg, isn’t enough for most people to enter this state. A double dose, 50 mg, though not physically unsafe, may leave you too incoherent to have the useful insights key to therapeutic value.
A doctor, the lead investigator of the study, brought the five capsules into the room in an intricately carved crucible with a small ceremonial cup that held the water with which to take it.
“It was very solemn,” Dr. Bansal said. “He sat down with me in a very calming way.”
The doctor said: “Don’t worry about it. Just trust and let go.”
And that’s just what he did.
Dr. Bansal swallowed the capsules and lay down. The doctor quietly left the room so that Dr. Bansal and his psychiatrist guide could begin their session together.
Special eye shades kept him in the pitch dark whether his eyes were open or closed. Headphones streamed a curated musical playlist – much of it Western classical like Strauss, Bach, Mozart, and Beethoven – but also modern electronica and other music from cultures around the globe.
Dr. Bansal would remain here, with his therapist-guide by his side, in largely this same position, for the next 7-and-a-half hours.
It took about 45 minutes for the medication to kick in.
The investigator
The doctor who brought the capsules into the dosing room was Manish Agrawal, MD, codirector of clinical research at the Aquilino Cancer Center and lead investigator of the study.
Dr. Agrawal trained at the National Cancer Institute and practiced for many years as an oncologist before developing an interest in psychedelic therapies. It was his work with cancer patients that drew him to psychedelics in the first place.
He had seen too many of his patients mentally wrecked by a cancer diagnosis, and he often felt helpless to comfort them.
“You take care of the physical aspects of the cancer, right? You talk about side effects and recommend another scan to look for recurrence.”
“But what about the psychological effects?”
They can be very serious and too often go ignored, said Dr. Agrawal. Your plans for the future suddenly become moot. You may be concerned about your ability to work or worried about the pain and suffering and financial strain that might be ahead for both you and your family. And to top it all off, you’re staring into the face of your own mortality.
So it’s no wonder, said Dr. Agrawal, that many people develop clinical levels of anxiety and depression after a cancer diagnosis.
Like Dr. Bansal, Dr. Agrawal had been impressed by early studies on psilocybin-assisted therapies for end-of-life anxiety and depression. He had tried other approaches – support groups, one-on-one therapy, religious counselors, psychiatrist-prescribed medication – but he was never really happy with the results.
To Dr. Agrawal, psilocybin-assisted therapy was the first thing that looked like it could really make a difference.
And so after his psychedelic certification at the California Institute of Integral Studies, Dr. Agrawal was determined to change his approach.
The result was the Bill Richards Center for Healing at Aquilino Cancer Center, built specifically to study psychedelic-assisted therapies for psychological distress in people with cancer. The mission of the center is to help develop safe, FDA-approved psychedelic therapies for the mental health of cancer patients, and, once approved, provide a state-of-the-art facility and staff to administer those treatments.
A trip into the unknown
Back in the dosing room, Dr. Bansal was starting to feel the effects of the medication. As the psilocybin kicked in, spectacular images swirled.
“It was as if a million stained glass windows had suddenly come to life and were dancing in front of my vision,” Dr. Bansal said.
There were moving landscapes and intricate swirling patterns and massive stages in the sky where he saw orchestras playing the music he was hearing.
Dr. Bansal saw himself being crushed by a huge machine and buried, dead, in the Earth. He died and returned to life several times, glided over the top of New York City with the skyscrapers just below him, and took in the vision of the entire universe.
“I saw this expanse of the sky that was limitless. And there was this prehistoric reptile creature that spanned galaxies in the sky ahead of me who was dying. I said: ‘My God, the universe is dying,’ but then after a few moments, the universe came to life again in a burst of stars exploding.”
All the while, Dr. Bansal said, he was well aware that it was simply his mind creating these images, thoughts, and ideas. He knew he was in a safe room wearing eyeshades and headphones.
And yet, he says, it felt true. “The images and feelings are so powerful that you cannot help but believe they are in some way a part of reality.”
“At one point, I saw this giant Ferris wheel coming towards me and it was full of giant crabs, clicking and clacking their pincers. And my brain told me: ‘That’s my cancer!’ ”
Dr. Bansal was terrified. But he and his therapist had arranged a system of signals before the session. “If I was feeling afraid, I would hold his hand and if I had other issues, I would raise my hand. If I was feeling good, I would give him a thumbs up.”
Dr. Bansal reached out to his therapist and grasped his hand. “I said, ‘My cancer is coming at me!’ ”
His therapist was clear about what to do: Stand firm and walk toward it.
“That’s what they tell you: If you see anything frightening, you face it. And that’s the whole point of this exercise. And so, I stood and walked forward, and it just blew off in a puff of smoke.”
A state of peace
Around 3 hours into the experience, Dr. Bansal started to feel an immense sense of peace, happiness, and even comfort.
“I felt like I was watching a movie or a multidimensional slideshow. I was also a part of the movie. I felt like I could tell my mind what I wanted to see, and it would show it to me. It’s almost like you can mold your own visions. It was mystical.”
After about 8 hours, as the effects of the drug wore off, Dr. Bansal removed his eyeshades and headphones. He was completely drained.
“Even though I was lying down on my back for 7 hours, I felt like I had been run over by a truck. I was exhausted beyond belief physically and mentally.”
This was partly because of the fact that he hadn’t eaten much during the session. But mostly, said Dr. Bansal, it was because of the searing emotional intensity of the experience.
After the journey
It’s hard to put into words, said Dr. Bansal, what this treatment has done for his life. He feels as if he has stumbled onto something very precious that had been right in front of him all along. He wrote of his change in perspective almost obsessively in his journal in the days and weeks after treatment. One passage reads:
“It seems that, as time is passing on, I’m becoming more relaxed and hopeful, more calm, and at peace. Family has become even more important to me now. Money, politics, material gains, alcohol, seem less important.”
And yet there was nothing “easy” about the experience. In fact, in some ways the experience demanded more from him. “I feel I need to be more compassionate and considerate – less irritable and angry, more understanding of others’ needs. I feel I need to be a better human being, a better patient, a better father, and a better doctor for my patients.”
The experience, he said, gave him something far more important than mere ease. It gave him a sense of meaning.
From his journal:
“I died, and I was reborn. If I survived this, then I can face anything and anybody in the cosmic scheme. I can become part of it.
“How many sorrows in the universe? My cancer is nothing. Life does not end with the end of life. What was will be again. Eternally.”
That’s not an unusual response, according to the namesake of the Bill Richards Center for Healing. Bill Richards, PhD, has worked in the world of psychedelic-assisted psychotherapy since 1963.
A psychologist with decades of experience, Dr. Richards and colleagues figure that, with few possible exceptions, he has helped treat more people with psychedelic therapies than anyone alive in Western medicine today. At Aquilino, he works directly with patients and oversees the therapy protocol that goes along with the psilocybin dosing sessions.
“It’s inspiring,” Dr. Richards said.
“You meet someone who’s very depressed and scared and isolating from family and having all kinds of physical complaints. And a few days later, you talk to the same person and they have a whole new lease on life.”
And the positive effects can extend deep into the family system, he said.
After psilocybin treatment, said Dr. Richards, the person with cancer can become a kind of social worker for the family. They’re often far better able to talk about death and loss and even money and family issues than their loved ones. It’s not uncommon after treatment to see the resolution of years-old resentments or grievances that have dogged a family for many years.
Plus, said Dr. Richards, the cancer patient often ends up as a kind model to other family members for how to approach death. “They can demonstrate how to live fully – right to the last breath – which is a real gift because those relatives and loved ones have to die someday too, you know.”
At 80 years old, Dr. Richards is still in active practice and hopes to spend the rest of his days working with people in end-of-life care.
After the experience
Psychedelic-assisted therapy does not end with the dosing session. Integration sessions, where you discuss what happened during the dosing session, are a key part of most treatments.
The goal is to help participants absorb and “integrate” their experience. It typically happens over two or more sessions of 60-90 minutes with a therapist. In some cases, the therapist may invite a significant other to join in the integration process.
Dr. Agrawal’s trial at the Bill Richards center added something new: group therapy. Not only did Dr. Bansal meet with his therapist, he also met with a group of three other people in the trial who had their dosing the same day.
The point, said Dr. Agrawal, is to try and determine the effect of the group on the therapy. After their private dosing sessions, they come back together to discuss their experiences.
“After the psilocybin, they feel like they’ve been to war together,” Dr. Agrawal said. “There is this profound openness and connection. They feel able to share things with each other that they wouldn’t with other people.”
It will take some time to figure out how the group affects the overall outcome, but Dr. Bansal thinks it was integral to the success of his treatment.
In fact, he continues to meet regularly with his therapy group, even though it’s long since past the requirements of the study.
Pradeep 2.0
Dr. Bansal still has tough days with his cancer. Recently, immunotherapy treatment for his bladder caused side effects – pain, bleeding, fever, and chills – for most of the night. He felt like he was “passing razor blades” when he peed.
“And yet it was somehow okay,” he said. “It was only pain.”
“It’s as if there is a part of me that is watching myself objectively, going through the painful process of treatments saying: ‘It’s all right. I will be with you through this journey, through this experience. Don’t worry.’”
Months after taking that one dose, Dr. Bansal still calls it as “the single most powerful experience of my life.”
The change in his mental outlook, Dr. Bansal said, was profound, particularly in regard to his cancer.
“I understood that I still had cancer and that it could kill me in a few weeks, or months, or years. But my perspective had shifted.”
Dr. Bansal was as surprised as anyone. “Had somebody told me going into this that I would come out a transformed being or a person with a completely different perspective on life, I would never have believed it.”
He even named his new outlook. “I call it Pradeep 2.0.”
A version of this article first appeared on WebMD.com.
Pradeep Bansal considered the five capsules he was about to swallow. Together they made up a 25-mg dose of a substance that, in another setting, could have landed him in federal prison.
The substance was psilocybin, the active ingredient in magic mushrooms. To be more exact, it was a synthetic form of psilocybin called COMP360, made to pharmaceutical standards by a company called COMPASS Pathways. He was taking it as part of an Food and Drug Administration–approved clinical study on mental health therapy for people with cancer.
Dr. Bansal, a New York gastroenterologist, was far more comfortable giving medical treatment than receiving it. But he was getting used to it.
He had already been through surgery and a number of other treatments to address the physical aspects of his cancer. The psilocybin was to address the mental aspects – the crushing anxiety and depression that had stuck with him after his diagnosis.
Dr. Bansal did not arrive at this moment lightly.
“I was extremely skeptical going into this process,” said Dr. Bansal, who during a long medical career had looked with distrust and even disdain at alternative therapies.
“I don’t have much patience for holistic medicine, homeopathy, acupuncture, or alternative medicines with claims of spiritual upliftment or altered states of mind.”
But Bansal had done his homework on psilocybin and was impressed.
according to studies published in 2011, 2014, and 2016.
One study from Johns Hopkins University tracked the effects of a single guided dose of psilocybin in terminal cancer patients with anxiety and depression. More than 80% had a “significant decrease” in symptoms – even 6 months after treatment – with more than 60% of the group remaining in the normal mood range.
For the study Dr. Bansal joined, there had been weeks of screening and consultation and preparation in a strictly controlled scientific trial.
And yet, even with all that he had learned, even with his psychiatrist-guide by his side, he was afraid. Afraid of what he might experience under the powerful effects of psilocybin. And afraid that this was all a misguided waste of time – that his mental angst would still be there when it was all over.
He knew that psilocybin, like other psychedelic substances, could take you on a “trip” – could remove you, at least for a time, from normal conscious experience.
Maybe he would feel “funny,” he thought. Maybe he would have some hallucinations. But how would that change the reality of his cancer? How would it lift the black dread and anxiety he felt about his future?
Stuck in a dark place
Dr. Bansal had first noticed blood in his urine – a lot of it – in September 2019.
Two months later, doctors diagnosed cancer in his right kidney. He would need surgery to remove the kidney and surrounding lymph nodes (an operation called radical nephrectomy).
It was a shock, said Dr. Bansal. But the diagnosis and the surgery happened so quickly that he hardly had time to think. And treatment results seemed good. The cancer was only in stage I and the CT scans showed no signs of cancer after surgery.
“We were so relieved. Everyone was so happy,” Dr. Bansal said. “They didn’t even give me chemotherapy after surgery because it seemed so early.”
But a routine scan in June 2020 revealed more cancer in his lung. Within a couple of months, it was in his bladder too.
“It was devastating,” Dr. Bansal said. “I went from thinking I was healthy again to stage IV cancer.”
As doctors scheduled surgery to remove part of his lung, Dr. Bansal started on painful immunotherapy (BCG therapy) for his bladder.
At this point, from a psychological standpoint, Dr. Bansal was reeling. As a doctor, he knew all too well the meaning of stage IV cancer.
With two adult children and a grandchild on the way, Dr. Bansal had been looking forward to retirement with his wife of almost 40 years. “Suddenly, I wasn’t sure I was going to last that long,” Bansal recalled. “I was in a very dark place. I was very anxious, very depressed from lack of sleep.”
He saw a therapist about his cancer diagnosis and maintained his regular meditation practice at home. He hired a personal trainer and tried to focus on any good news that he got about his treatment.
Those things helped, but not enough.
The basic facts were inescapable. His cancer might end everything. He couldn’t stop thinking about it. And then he couldn’t stop thinking about how he couldn’t stop thinking about it.
If the worst happened, he didn’t want to spend his last days in a state of such relentless existential angst. And it wasn’t just for himself. He wanted to be strong and mentally present for his family and his loved ones and his patients.
As he searched for something to ease his mental anguish, Dr. Bansal recalled some psychedelic research on end-of-life anxiety and depression that he’d read about in Michael Pollan’s book on psychedelics, “How to Change Your Mind” (New York, Penguin Press, 2018).
The studies were small and the research was new, but Dr. Bansal was impressed enough with the results to take a chance. He called a lead researcher of one of the studies, a fellow New York doctor, and eventually found himself accepted into a new study.
Starting the journey
By the time Dr. Bansal arrived at the Bill Richards Center for Healing at the Aquilino Cancer Center in Rockville, Md., he had already been through weeks of screening.
The main requirements for the study were a cancer diagnosis and a measurable level of depression. But study participants also had to be physically fit enough to handle the medication, and psychologically free from a personal or family history of psychosis or schizophrenia. (The study also required participants to slowly wean themselves from medications like SSRIs for depression or antianxiety medications under the strict supervision of a qualified doctor.)
Dr. Bansal’s week of treatment began almost immediately on arrival at Aquilino. Everything was carefully choreographed but not rushed. From Monday to Wednesday, doctors followed his physical health with exams, ECGs, and blood work. And most importantly, they began to prepare him for the “dosing session” on Thursday when he would take the psilocybin.
This is the careful crafting of “set and setting” stressed in so many psychedelic therapies. “Set” refers to your mindset going into the drug experience. “Setting” is the space and people around you when the drug sends you into an altered state of consciousness.
Dr. Bansal met several times with at least three therapists in the days leading up to his dosing. He attended 4-plus hours of therapist-led group sessions with other people who would get a dosing on the same day. Together, they talked about what to expect during the experience and what to do in the face of fear or panic.
He connected with a therapist who would be his personal guide. Dr. Bansal’s therapist was a military psychiatrist with over 30 years’ experience.
“He was there with me from day 1, and so we established a relationship,” Dr. Bansal said.
“He asked me a lot of personal background history – you know, my religious convictions, aspirations, all those things.”
“Trust and let go,” was a kind of mantra for the treatment repeated by his guide and other doctors.
For Dr. Bansal, a doctor and scientist accustomed to using hard facts rather than touchy-feely slogans to navigate the care of patients, it was an adjustment, to say the least.
But he did his best to set aside his doubts and embrace the journey he was about to take.
The day of the trip
Thursday morning finally arrived. The setting of the dosing room was warm and welcoming, more like a cozy home study than a hospital room.
This matters more than you might think. First, because it’s important that you feel safe, open, and comfortable enough to let go and enter into a therapeutic process. But also because though rare, it’s possible – especially with psilocybin – for people to lose track of where they are and what they’re doing and put themselves or others in danger.
The dose, 25 mg, had been carefully calibrated to induce a psychedelic experience sufficient for therapy. Much less than that, say 10 mg, isn’t enough for most people to enter this state. A double dose, 50 mg, though not physically unsafe, may leave you too incoherent to have the useful insights key to therapeutic value.
A doctor, the lead investigator of the study, brought the five capsules into the room in an intricately carved crucible with a small ceremonial cup that held the water with which to take it.
“It was very solemn,” Dr. Bansal said. “He sat down with me in a very calming way.”
The doctor said: “Don’t worry about it. Just trust and let go.”
And that’s just what he did.
Dr. Bansal swallowed the capsules and lay down. The doctor quietly left the room so that Dr. Bansal and his psychiatrist guide could begin their session together.
Special eye shades kept him in the pitch dark whether his eyes were open or closed. Headphones streamed a curated musical playlist – much of it Western classical like Strauss, Bach, Mozart, and Beethoven – but also modern electronica and other music from cultures around the globe.
Dr. Bansal would remain here, with his therapist-guide by his side, in largely this same position, for the next 7-and-a-half hours.
It took about 45 minutes for the medication to kick in.
The investigator
The doctor who brought the capsules into the dosing room was Manish Agrawal, MD, codirector of clinical research at the Aquilino Cancer Center and lead investigator of the study.
Dr. Agrawal trained at the National Cancer Institute and practiced for many years as an oncologist before developing an interest in psychedelic therapies. It was his work with cancer patients that drew him to psychedelics in the first place.
He had seen too many of his patients mentally wrecked by a cancer diagnosis, and he often felt helpless to comfort them.
“You take care of the physical aspects of the cancer, right? You talk about side effects and recommend another scan to look for recurrence.”
“But what about the psychological effects?”
They can be very serious and too often go ignored, said Dr. Agrawal. Your plans for the future suddenly become moot. You may be concerned about your ability to work or worried about the pain and suffering and financial strain that might be ahead for both you and your family. And to top it all off, you’re staring into the face of your own mortality.
So it’s no wonder, said Dr. Agrawal, that many people develop clinical levels of anxiety and depression after a cancer diagnosis.
Like Dr. Bansal, Dr. Agrawal had been impressed by early studies on psilocybin-assisted therapies for end-of-life anxiety and depression. He had tried other approaches – support groups, one-on-one therapy, religious counselors, psychiatrist-prescribed medication – but he was never really happy with the results.
To Dr. Agrawal, psilocybin-assisted therapy was the first thing that looked like it could really make a difference.
And so after his psychedelic certification at the California Institute of Integral Studies, Dr. Agrawal was determined to change his approach.
The result was the Bill Richards Center for Healing at Aquilino Cancer Center, built specifically to study psychedelic-assisted therapies for psychological distress in people with cancer. The mission of the center is to help develop safe, FDA-approved psychedelic therapies for the mental health of cancer patients, and, once approved, provide a state-of-the-art facility and staff to administer those treatments.
A trip into the unknown
Back in the dosing room, Dr. Bansal was starting to feel the effects of the medication. As the psilocybin kicked in, spectacular images swirled.
“It was as if a million stained glass windows had suddenly come to life and were dancing in front of my vision,” Dr. Bansal said.
There were moving landscapes and intricate swirling patterns and massive stages in the sky where he saw orchestras playing the music he was hearing.
Dr. Bansal saw himself being crushed by a huge machine and buried, dead, in the Earth. He died and returned to life several times, glided over the top of New York City with the skyscrapers just below him, and took in the vision of the entire universe.
“I saw this expanse of the sky that was limitless. And there was this prehistoric reptile creature that spanned galaxies in the sky ahead of me who was dying. I said: ‘My God, the universe is dying,’ but then after a few moments, the universe came to life again in a burst of stars exploding.”
All the while, Dr. Bansal said, he was well aware that it was simply his mind creating these images, thoughts, and ideas. He knew he was in a safe room wearing eyeshades and headphones.
And yet, he says, it felt true. “The images and feelings are so powerful that you cannot help but believe they are in some way a part of reality.”
“At one point, I saw this giant Ferris wheel coming towards me and it was full of giant crabs, clicking and clacking their pincers. And my brain told me: ‘That’s my cancer!’ ”
Dr. Bansal was terrified. But he and his therapist had arranged a system of signals before the session. “If I was feeling afraid, I would hold his hand and if I had other issues, I would raise my hand. If I was feeling good, I would give him a thumbs up.”
Dr. Bansal reached out to his therapist and grasped his hand. “I said, ‘My cancer is coming at me!’ ”
His therapist was clear about what to do: Stand firm and walk toward it.
“That’s what they tell you: If you see anything frightening, you face it. And that’s the whole point of this exercise. And so, I stood and walked forward, and it just blew off in a puff of smoke.”
A state of peace
Around 3 hours into the experience, Dr. Bansal started to feel an immense sense of peace, happiness, and even comfort.
“I felt like I was watching a movie or a multidimensional slideshow. I was also a part of the movie. I felt like I could tell my mind what I wanted to see, and it would show it to me. It’s almost like you can mold your own visions. It was mystical.”
After about 8 hours, as the effects of the drug wore off, Dr. Bansal removed his eyeshades and headphones. He was completely drained.
“Even though I was lying down on my back for 7 hours, I felt like I had been run over by a truck. I was exhausted beyond belief physically and mentally.”
This was partly because of the fact that he hadn’t eaten much during the session. But mostly, said Dr. Bansal, it was because of the searing emotional intensity of the experience.
After the journey
It’s hard to put into words, said Dr. Bansal, what this treatment has done for his life. He feels as if he has stumbled onto something very precious that had been right in front of him all along. He wrote of his change in perspective almost obsessively in his journal in the days and weeks after treatment. One passage reads:
“It seems that, as time is passing on, I’m becoming more relaxed and hopeful, more calm, and at peace. Family has become even more important to me now. Money, politics, material gains, alcohol, seem less important.”
And yet there was nothing “easy” about the experience. In fact, in some ways the experience demanded more from him. “I feel I need to be more compassionate and considerate – less irritable and angry, more understanding of others’ needs. I feel I need to be a better human being, a better patient, a better father, and a better doctor for my patients.”
The experience, he said, gave him something far more important than mere ease. It gave him a sense of meaning.
From his journal:
“I died, and I was reborn. If I survived this, then I can face anything and anybody in the cosmic scheme. I can become part of it.
“How many sorrows in the universe? My cancer is nothing. Life does not end with the end of life. What was will be again. Eternally.”
That’s not an unusual response, according to the namesake of the Bill Richards Center for Healing. Bill Richards, PhD, has worked in the world of psychedelic-assisted psychotherapy since 1963.
A psychologist with decades of experience, Dr. Richards and colleagues figure that, with few possible exceptions, he has helped treat more people with psychedelic therapies than anyone alive in Western medicine today. At Aquilino, he works directly with patients and oversees the therapy protocol that goes along with the psilocybin dosing sessions.
“It’s inspiring,” Dr. Richards said.
“You meet someone who’s very depressed and scared and isolating from family and having all kinds of physical complaints. And a few days later, you talk to the same person and they have a whole new lease on life.”
And the positive effects can extend deep into the family system, he said.
After psilocybin treatment, said Dr. Richards, the person with cancer can become a kind of social worker for the family. They’re often far better able to talk about death and loss and even money and family issues than their loved ones. It’s not uncommon after treatment to see the resolution of years-old resentments or grievances that have dogged a family for many years.
Plus, said Dr. Richards, the cancer patient often ends up as a kind model to other family members for how to approach death. “They can demonstrate how to live fully – right to the last breath – which is a real gift because those relatives and loved ones have to die someday too, you know.”
At 80 years old, Dr. Richards is still in active practice and hopes to spend the rest of his days working with people in end-of-life care.
After the experience
Psychedelic-assisted therapy does not end with the dosing session. Integration sessions, where you discuss what happened during the dosing session, are a key part of most treatments.
The goal is to help participants absorb and “integrate” their experience. It typically happens over two or more sessions of 60-90 minutes with a therapist. In some cases, the therapist may invite a significant other to join in the integration process.
Dr. Agrawal’s trial at the Bill Richards center added something new: group therapy. Not only did Dr. Bansal meet with his therapist, he also met with a group of three other people in the trial who had their dosing the same day.
The point, said Dr. Agrawal, is to try and determine the effect of the group on the therapy. After their private dosing sessions, they come back together to discuss their experiences.
“After the psilocybin, they feel like they’ve been to war together,” Dr. Agrawal said. “There is this profound openness and connection. They feel able to share things with each other that they wouldn’t with other people.”
It will take some time to figure out how the group affects the overall outcome, but Dr. Bansal thinks it was integral to the success of his treatment.
In fact, he continues to meet regularly with his therapy group, even though it’s long since past the requirements of the study.
Pradeep 2.0
Dr. Bansal still has tough days with his cancer. Recently, immunotherapy treatment for his bladder caused side effects – pain, bleeding, fever, and chills – for most of the night. He felt like he was “passing razor blades” when he peed.
“And yet it was somehow okay,” he said. “It was only pain.”
“It’s as if there is a part of me that is watching myself objectively, going through the painful process of treatments saying: ‘It’s all right. I will be with you through this journey, through this experience. Don’t worry.’”
Months after taking that one dose, Dr. Bansal still calls it as “the single most powerful experience of my life.”
The change in his mental outlook, Dr. Bansal said, was profound, particularly in regard to his cancer.
“I understood that I still had cancer and that it could kill me in a few weeks, or months, or years. But my perspective had shifted.”
Dr. Bansal was as surprised as anyone. “Had somebody told me going into this that I would come out a transformed being or a person with a completely different perspective on life, I would never have believed it.”
He even named his new outlook. “I call it Pradeep 2.0.”
A version of this article first appeared on WebMD.com.
Pradeep Bansal considered the five capsules he was about to swallow. Together they made up a 25-mg dose of a substance that, in another setting, could have landed him in federal prison.
The substance was psilocybin, the active ingredient in magic mushrooms. To be more exact, it was a synthetic form of psilocybin called COMP360, made to pharmaceutical standards by a company called COMPASS Pathways. He was taking it as part of an Food and Drug Administration–approved clinical study on mental health therapy for people with cancer.
Dr. Bansal, a New York gastroenterologist, was far more comfortable giving medical treatment than receiving it. But he was getting used to it.
He had already been through surgery and a number of other treatments to address the physical aspects of his cancer. The psilocybin was to address the mental aspects – the crushing anxiety and depression that had stuck with him after his diagnosis.
Dr. Bansal did not arrive at this moment lightly.
“I was extremely skeptical going into this process,” said Dr. Bansal, who during a long medical career had looked with distrust and even disdain at alternative therapies.
“I don’t have much patience for holistic medicine, homeopathy, acupuncture, or alternative medicines with claims of spiritual upliftment or altered states of mind.”
But Bansal had done his homework on psilocybin and was impressed.
according to studies published in 2011, 2014, and 2016.
One study from Johns Hopkins University tracked the effects of a single guided dose of psilocybin in terminal cancer patients with anxiety and depression. More than 80% had a “significant decrease” in symptoms – even 6 months after treatment – with more than 60% of the group remaining in the normal mood range.
For the study Dr. Bansal joined, there had been weeks of screening and consultation and preparation in a strictly controlled scientific trial.
And yet, even with all that he had learned, even with his psychiatrist-guide by his side, he was afraid. Afraid of what he might experience under the powerful effects of psilocybin. And afraid that this was all a misguided waste of time – that his mental angst would still be there when it was all over.
He knew that psilocybin, like other psychedelic substances, could take you on a “trip” – could remove you, at least for a time, from normal conscious experience.
Maybe he would feel “funny,” he thought. Maybe he would have some hallucinations. But how would that change the reality of his cancer? How would it lift the black dread and anxiety he felt about his future?
Stuck in a dark place
Dr. Bansal had first noticed blood in his urine – a lot of it – in September 2019.
Two months later, doctors diagnosed cancer in his right kidney. He would need surgery to remove the kidney and surrounding lymph nodes (an operation called radical nephrectomy).
It was a shock, said Dr. Bansal. But the diagnosis and the surgery happened so quickly that he hardly had time to think. And treatment results seemed good. The cancer was only in stage I and the CT scans showed no signs of cancer after surgery.
“We were so relieved. Everyone was so happy,” Dr. Bansal said. “They didn’t even give me chemotherapy after surgery because it seemed so early.”
But a routine scan in June 2020 revealed more cancer in his lung. Within a couple of months, it was in his bladder too.
“It was devastating,” Dr. Bansal said. “I went from thinking I was healthy again to stage IV cancer.”
As doctors scheduled surgery to remove part of his lung, Dr. Bansal started on painful immunotherapy (BCG therapy) for his bladder.
At this point, from a psychological standpoint, Dr. Bansal was reeling. As a doctor, he knew all too well the meaning of stage IV cancer.
With two adult children and a grandchild on the way, Dr. Bansal had been looking forward to retirement with his wife of almost 40 years. “Suddenly, I wasn’t sure I was going to last that long,” Bansal recalled. “I was in a very dark place. I was very anxious, very depressed from lack of sleep.”
He saw a therapist about his cancer diagnosis and maintained his regular meditation practice at home. He hired a personal trainer and tried to focus on any good news that he got about his treatment.
Those things helped, but not enough.
The basic facts were inescapable. His cancer might end everything. He couldn’t stop thinking about it. And then he couldn’t stop thinking about how he couldn’t stop thinking about it.
If the worst happened, he didn’t want to spend his last days in a state of such relentless existential angst. And it wasn’t just for himself. He wanted to be strong and mentally present for his family and his loved ones and his patients.
As he searched for something to ease his mental anguish, Dr. Bansal recalled some psychedelic research on end-of-life anxiety and depression that he’d read about in Michael Pollan’s book on psychedelics, “How to Change Your Mind” (New York, Penguin Press, 2018).
The studies were small and the research was new, but Dr. Bansal was impressed enough with the results to take a chance. He called a lead researcher of one of the studies, a fellow New York doctor, and eventually found himself accepted into a new study.
Starting the journey
By the time Dr. Bansal arrived at the Bill Richards Center for Healing at the Aquilino Cancer Center in Rockville, Md., he had already been through weeks of screening.
The main requirements for the study were a cancer diagnosis and a measurable level of depression. But study participants also had to be physically fit enough to handle the medication, and psychologically free from a personal or family history of psychosis or schizophrenia. (The study also required participants to slowly wean themselves from medications like SSRIs for depression or antianxiety medications under the strict supervision of a qualified doctor.)
Dr. Bansal’s week of treatment began almost immediately on arrival at Aquilino. Everything was carefully choreographed but not rushed. From Monday to Wednesday, doctors followed his physical health with exams, ECGs, and blood work. And most importantly, they began to prepare him for the “dosing session” on Thursday when he would take the psilocybin.
This is the careful crafting of “set and setting” stressed in so many psychedelic therapies. “Set” refers to your mindset going into the drug experience. “Setting” is the space and people around you when the drug sends you into an altered state of consciousness.
Dr. Bansal met several times with at least three therapists in the days leading up to his dosing. He attended 4-plus hours of therapist-led group sessions with other people who would get a dosing on the same day. Together, they talked about what to expect during the experience and what to do in the face of fear or panic.
He connected with a therapist who would be his personal guide. Dr. Bansal’s therapist was a military psychiatrist with over 30 years’ experience.
“He was there with me from day 1, and so we established a relationship,” Dr. Bansal said.
“He asked me a lot of personal background history – you know, my religious convictions, aspirations, all those things.”
“Trust and let go,” was a kind of mantra for the treatment repeated by his guide and other doctors.
For Dr. Bansal, a doctor and scientist accustomed to using hard facts rather than touchy-feely slogans to navigate the care of patients, it was an adjustment, to say the least.
But he did his best to set aside his doubts and embrace the journey he was about to take.
The day of the trip
Thursday morning finally arrived. The setting of the dosing room was warm and welcoming, more like a cozy home study than a hospital room.
This matters more than you might think. First, because it’s important that you feel safe, open, and comfortable enough to let go and enter into a therapeutic process. But also because though rare, it’s possible – especially with psilocybin – for people to lose track of where they are and what they’re doing and put themselves or others in danger.
The dose, 25 mg, had been carefully calibrated to induce a psychedelic experience sufficient for therapy. Much less than that, say 10 mg, isn’t enough for most people to enter this state. A double dose, 50 mg, though not physically unsafe, may leave you too incoherent to have the useful insights key to therapeutic value.
A doctor, the lead investigator of the study, brought the five capsules into the room in an intricately carved crucible with a small ceremonial cup that held the water with which to take it.
“It was very solemn,” Dr. Bansal said. “He sat down with me in a very calming way.”
The doctor said: “Don’t worry about it. Just trust and let go.”
And that’s just what he did.
Dr. Bansal swallowed the capsules and lay down. The doctor quietly left the room so that Dr. Bansal and his psychiatrist guide could begin their session together.
Special eye shades kept him in the pitch dark whether his eyes were open or closed. Headphones streamed a curated musical playlist – much of it Western classical like Strauss, Bach, Mozart, and Beethoven – but also modern electronica and other music from cultures around the globe.
Dr. Bansal would remain here, with his therapist-guide by his side, in largely this same position, for the next 7-and-a-half hours.
It took about 45 minutes for the medication to kick in.
The investigator
The doctor who brought the capsules into the dosing room was Manish Agrawal, MD, codirector of clinical research at the Aquilino Cancer Center and lead investigator of the study.
Dr. Agrawal trained at the National Cancer Institute and practiced for many years as an oncologist before developing an interest in psychedelic therapies. It was his work with cancer patients that drew him to psychedelics in the first place.
He had seen too many of his patients mentally wrecked by a cancer diagnosis, and he often felt helpless to comfort them.
“You take care of the physical aspects of the cancer, right? You talk about side effects and recommend another scan to look for recurrence.”
“But what about the psychological effects?”
They can be very serious and too often go ignored, said Dr. Agrawal. Your plans for the future suddenly become moot. You may be concerned about your ability to work or worried about the pain and suffering and financial strain that might be ahead for both you and your family. And to top it all off, you’re staring into the face of your own mortality.
So it’s no wonder, said Dr. Agrawal, that many people develop clinical levels of anxiety and depression after a cancer diagnosis.
Like Dr. Bansal, Dr. Agrawal had been impressed by early studies on psilocybin-assisted therapies for end-of-life anxiety and depression. He had tried other approaches – support groups, one-on-one therapy, religious counselors, psychiatrist-prescribed medication – but he was never really happy with the results.
To Dr. Agrawal, psilocybin-assisted therapy was the first thing that looked like it could really make a difference.
And so after his psychedelic certification at the California Institute of Integral Studies, Dr. Agrawal was determined to change his approach.
The result was the Bill Richards Center for Healing at Aquilino Cancer Center, built specifically to study psychedelic-assisted therapies for psychological distress in people with cancer. The mission of the center is to help develop safe, FDA-approved psychedelic therapies for the mental health of cancer patients, and, once approved, provide a state-of-the-art facility and staff to administer those treatments.
A trip into the unknown
Back in the dosing room, Dr. Bansal was starting to feel the effects of the medication. As the psilocybin kicked in, spectacular images swirled.
“It was as if a million stained glass windows had suddenly come to life and were dancing in front of my vision,” Dr. Bansal said.
There were moving landscapes and intricate swirling patterns and massive stages in the sky where he saw orchestras playing the music he was hearing.
Dr. Bansal saw himself being crushed by a huge machine and buried, dead, in the Earth. He died and returned to life several times, glided over the top of New York City with the skyscrapers just below him, and took in the vision of the entire universe.
“I saw this expanse of the sky that was limitless. And there was this prehistoric reptile creature that spanned galaxies in the sky ahead of me who was dying. I said: ‘My God, the universe is dying,’ but then after a few moments, the universe came to life again in a burst of stars exploding.”
All the while, Dr. Bansal said, he was well aware that it was simply his mind creating these images, thoughts, and ideas. He knew he was in a safe room wearing eyeshades and headphones.
And yet, he says, it felt true. “The images and feelings are so powerful that you cannot help but believe they are in some way a part of reality.”
“At one point, I saw this giant Ferris wheel coming towards me and it was full of giant crabs, clicking and clacking their pincers. And my brain told me: ‘That’s my cancer!’ ”
Dr. Bansal was terrified. But he and his therapist had arranged a system of signals before the session. “If I was feeling afraid, I would hold his hand and if I had other issues, I would raise my hand. If I was feeling good, I would give him a thumbs up.”
Dr. Bansal reached out to his therapist and grasped his hand. “I said, ‘My cancer is coming at me!’ ”
His therapist was clear about what to do: Stand firm and walk toward it.
“That’s what they tell you: If you see anything frightening, you face it. And that’s the whole point of this exercise. And so, I stood and walked forward, and it just blew off in a puff of smoke.”
A state of peace
Around 3 hours into the experience, Dr. Bansal started to feel an immense sense of peace, happiness, and even comfort.
“I felt like I was watching a movie or a multidimensional slideshow. I was also a part of the movie. I felt like I could tell my mind what I wanted to see, and it would show it to me. It’s almost like you can mold your own visions. It was mystical.”
After about 8 hours, as the effects of the drug wore off, Dr. Bansal removed his eyeshades and headphones. He was completely drained.
“Even though I was lying down on my back for 7 hours, I felt like I had been run over by a truck. I was exhausted beyond belief physically and mentally.”
This was partly because of the fact that he hadn’t eaten much during the session. But mostly, said Dr. Bansal, it was because of the searing emotional intensity of the experience.
After the journey
It’s hard to put into words, said Dr. Bansal, what this treatment has done for his life. He feels as if he has stumbled onto something very precious that had been right in front of him all along. He wrote of his change in perspective almost obsessively in his journal in the days and weeks after treatment. One passage reads:
“It seems that, as time is passing on, I’m becoming more relaxed and hopeful, more calm, and at peace. Family has become even more important to me now. Money, politics, material gains, alcohol, seem less important.”
And yet there was nothing “easy” about the experience. In fact, in some ways the experience demanded more from him. “I feel I need to be more compassionate and considerate – less irritable and angry, more understanding of others’ needs. I feel I need to be a better human being, a better patient, a better father, and a better doctor for my patients.”
The experience, he said, gave him something far more important than mere ease. It gave him a sense of meaning.
From his journal:
“I died, and I was reborn. If I survived this, then I can face anything and anybody in the cosmic scheme. I can become part of it.
“How many sorrows in the universe? My cancer is nothing. Life does not end with the end of life. What was will be again. Eternally.”
That’s not an unusual response, according to the namesake of the Bill Richards Center for Healing. Bill Richards, PhD, has worked in the world of psychedelic-assisted psychotherapy since 1963.
A psychologist with decades of experience, Dr. Richards and colleagues figure that, with few possible exceptions, he has helped treat more people with psychedelic therapies than anyone alive in Western medicine today. At Aquilino, he works directly with patients and oversees the therapy protocol that goes along with the psilocybin dosing sessions.
“It’s inspiring,” Dr. Richards said.
“You meet someone who’s very depressed and scared and isolating from family and having all kinds of physical complaints. And a few days later, you talk to the same person and they have a whole new lease on life.”
And the positive effects can extend deep into the family system, he said.
After psilocybin treatment, said Dr. Richards, the person with cancer can become a kind of social worker for the family. They’re often far better able to talk about death and loss and even money and family issues than their loved ones. It’s not uncommon after treatment to see the resolution of years-old resentments or grievances that have dogged a family for many years.
Plus, said Dr. Richards, the cancer patient often ends up as a kind model to other family members for how to approach death. “They can demonstrate how to live fully – right to the last breath – which is a real gift because those relatives and loved ones have to die someday too, you know.”
At 80 years old, Dr. Richards is still in active practice and hopes to spend the rest of his days working with people in end-of-life care.
After the experience
Psychedelic-assisted therapy does not end with the dosing session. Integration sessions, where you discuss what happened during the dosing session, are a key part of most treatments.
The goal is to help participants absorb and “integrate” their experience. It typically happens over two or more sessions of 60-90 minutes with a therapist. In some cases, the therapist may invite a significant other to join in the integration process.
Dr. Agrawal’s trial at the Bill Richards center added something new: group therapy. Not only did Dr. Bansal meet with his therapist, he also met with a group of three other people in the trial who had their dosing the same day.
The point, said Dr. Agrawal, is to try and determine the effect of the group on the therapy. After their private dosing sessions, they come back together to discuss their experiences.
“After the psilocybin, they feel like they’ve been to war together,” Dr. Agrawal said. “There is this profound openness and connection. They feel able to share things with each other that they wouldn’t with other people.”
It will take some time to figure out how the group affects the overall outcome, but Dr. Bansal thinks it was integral to the success of his treatment.
In fact, he continues to meet regularly with his therapy group, even though it’s long since past the requirements of the study.
Pradeep 2.0
Dr. Bansal still has tough days with his cancer. Recently, immunotherapy treatment for his bladder caused side effects – pain, bleeding, fever, and chills – for most of the night. He felt like he was “passing razor blades” when he peed.
“And yet it was somehow okay,” he said. “It was only pain.”
“It’s as if there is a part of me that is watching myself objectively, going through the painful process of treatments saying: ‘It’s all right. I will be with you through this journey, through this experience. Don’t worry.’”
Months after taking that one dose, Dr. Bansal still calls it as “the single most powerful experience of my life.”
The change in his mental outlook, Dr. Bansal said, was profound, particularly in regard to his cancer.
“I understood that I still had cancer and that it could kill me in a few weeks, or months, or years. But my perspective had shifted.”
Dr. Bansal was as surprised as anyone. “Had somebody told me going into this that I would come out a transformed being or a person with a completely different perspective on life, I would never have believed it.”
He even named his new outlook. “I call it Pradeep 2.0.”
A version of this article first appeared on WebMD.com.
Electrocuted by 11,000 volts, now a triple amputee ... and an MD
Bruce “BJ” Miller Jr., a 19-year-old Princeton (N.J.) University sophomore, was horsing around with friends near a train track in 1990 when they spotted a parked commuter train. They decided to climb over the train, and Mr. Miller was first up the ladder.
An explosion ripped through the air, and Mr. Miller was thrown on top of the train, his body smoking. His petrified friends called for an ambulance.
Clinging to life, Mr. Miller was airlifted to the burn unit at Saint Barnabas Medical Center in Livingston, N.J..
Physicians saved Mr. Miller’s life, but they had to amputate both of his legs below the knees and his left arm below the elbow.
“With electricity, you burn from the inside out,” said Mr. Miller, now 50. “The voltage enters your body – in my case, the wrist – and runs around internally until it finds a way out. That is often the lower extremities as the ground tends to ground the current, but not always. In my case, the current tried to come through my chest – which is also burned and required skin grafting – but not enough to spare my legs. I think I had a half-dozen or so surgeries over the first month or 2 at the hospital.”
Waking up to a new body
Mr. Miller doesn’t remember much about the accident, but he recalls waking up a few days later in the ICU and feeling the need to use the bathroom. Disoriented, Mr. Miller pulled off his ventilator, climbed out of bed, and tried to walk forward, unaware of his injuries. His feet and legs had not yet been amputated. When the catheter line ran out of slack, he collapsed.
“Eventually, a nurse came rushing in, responding to the ventilator alarm bells going off,” Mr. Miller said. “My dad wasn’t far behind. It became clear to me then that this was not a dream and [I realized] what had happened and why I was in the hospital.”
For months, Mr. Miller lived in the burn unit, undergoing countless skin grafts and surgeries. Because viable and nonviable tissue take time to be revealed after burns, surgeons take the minimum amount of tissue during each operation to give damaged tissue a chance to heal, he explained. In Mr. Miller’s case, his feet were amputated first, and later, his legs.
“In those early days from the hospital bed, my mind turned to issues related to identity,” he said. “What do I do with myself?
Mr. Miller eventually moved to the Rehabilitation Institute of Chicago (now called The Shirley Ryan AbilityLab), where he started the grueling process of rebuilding his strength and learning to walk on prosthetic legs.
“Any one day was filled with a mix of optimism and good fight and 5 minutes later, exasperation, frustration, tons of pain, and insecurity about my body,” he said. “My family and friends held the gate for me in a way, but a lot of the work was up to me. I had to believe that I deserved this love, that I wanted to be alive, and that there was still something here for me.”
Mr. Miller didn’t have to look far for inspiration. His mom had lived with polio for most of her life and acquired post-polio syndrome as she grew older, he said. When he was a child, his mom walked with crutches, and she became wheelchair-dependent by the time he was a teenager.
After the first surgery to amputate his feet, Mr. Miller and his mom shared a deep discussion about his joining the ranks of “the disabled,” and how their connection was now even stronger.
“In this way, the injuries unlocked even more experiences to share between us, and more love to feel, and therefore some early sense of gain to complement all the losses happening,” he said. “She had taught me so much about living with disability and had given me all the tools I needed to refashion my sense of self.”
From burn patient to medical student
After returning to Princeton University and finishing his undergraduate degree, Mr. Miller decided to go into medicine. He wanted to use his experience to help patients and find ways to improve weaknesses in the health care system, he said. But he made a deal with himself that he wouldn’t become a doctor for the sake of becoming one; he would enter the vocation only if he could do the work and enjoy the job.
“I wasn’t sure if I could do it,” he said. “There weren’t a lot of triple amputees to point to, to say whether this was even mechanically possible, to get through the training. The medical institutions I spoke with knew they had some obligation by law to protect me, but there’s also an obligation that I need to be able to fulfill the competencies. This was uncharted water.”
Because his greatest physical challenge was standing for long periods, instructors at the University of California, San Francisco, made accommodations to alleviate the strain. His clinical rotations for example, were organized near his home to limit the need for travel. On surgical rotations, he was allowed to sit on a stool.
Medical training progressed smoothly until Mr. Miller completed a rotation in his chosen specialty, rehabilitation medicine. He didn’t enjoy it. The passion and meaning he hoped to find was missing. Disillusioned, and with his final year in medical school coming to an end, Mr. Miller dropped out of the Match program. Around the same time, his sister, Lisa, died by suicide.
“My whole family life was in shambles,” he said. “I felt like, ‘I can’t even help my sister, how am I going to help other people?’ ”
Mr. Miller earned his MD and moved to his parents’ home in Milwaukee after his sister’s death. He was close to giving up on medicine, but his deans convinced him to do a post-doc internship. It was as an intern at the Medical College of Wisconsin, Milwaukee, that he completed an elective in palliative care.
“I fell immediately in love with it the first day,” he said. “This was a field devoted to working with things you can’t change and dealing with a lack of control, what it’s like to live with these diagnoses. This was a place where I could dig into my experience and share that with patients and families. This was a place where my life story had something to offer.”
Creating a new form of palliative care
Dr. Miller went on to complete a fellowship at Harvard Medical School, Boston, in hospice and palliative medicine. He became a palliative care physician at UCSF Health, and later directed the Zen Hospice Project, a nonprofit dedicated to teaching mindfulness-based caregiving for professionals, family members, and caregivers.
Gayle Kojimoto, a program manager who worked with Dr. Miller at UCSF’s outpatient palliative care clinic for cancer patients, said Miller was a favorite among patients because of his authenticity and his ability to make them feel understood.
“Patients love him because he is 100% present with them,” said Ms. Kojimoto. “They feel like he can understand their suffering better than other docs. He’s open to hearing about their suffering, when others may not be, and he doesn’t judge them. Many patients have said that seeing him is better than seeing a therapist.”
In 2020, Dr. Miller cofounded Mettle Health, a first-of-its-kind company that aims to reframe the way people think about their well-being as it relates to chronic and serious illness. Mettle Health’s care team provides consultations on a range of topics, including practical, emotional, and existential issues. No physician referrals are needed.
When the pandemic started, Dr. Miller said he and his colleagues felt the moment was ripe for bringing palliative care online to increase access, while decreasing caregiver and clinician burnout.
“We set up Mettle Health as an online palliative care counseling and coaching business and we pulled it out of the healthcare system so that whether you’re a patient or a caregiver you don’t need to satisfy some insurance need to get this kind of care,” he said. “We also realized there are enough people writing prescriptions. The medical piece is relatively well tended to; it’s the psychosocial and spiritual issues, and the existential issues, that are so underdeveloped. We are a social service, not a medical service, and this allows us to complement existing structures of care rather than compete with them.”
Having Dr. Miller as a leader for Mettle Health is a huge driver for why people seek out the company, said Sonya Dolan, director of operations and cofounder of Mettle Health.
“His approach to working with patients, caregivers, and clinicians is something I think sets us apart and makes us special,” she said. “His way of thinking about serious illness and death and dying is incredibly unique and he has a way of talking about and humanizing something that’s scary for a lot of us.”
‘Surprised by how much I can still do’
Since the accident, Dr. Miller has come a long way in navigating his physical limitations. In the early years, Dr. Miller said he was determined to do as many activities as he still could. He skied, biked, and pushed himself to stand for long periods on his prosthetic legs.
“For years, I would force myself to do these things just to prove I could, but not really enjoy them,” he said. “I’d get out on the dance floor or put myself out in vulnerable social situations where I might fall. It was kind of brutal and difficult. But at about year 5 or so, I became much more at ease with myself and more at peace with myself.”
Today, Dr. Miller’s prosthetics make nearly all ambulatory activities possible, but he concentrates on the activities that bring him joy.
“Probably the thing I can still do that surprises people most, including myself, is riding a motorcycle,” he said. “As for my upper body, I’m thoroughly used to living with only one hand and I continue to be surprised at how much I can still do. With enough time and experimentation, I can usually find a way to do what I need/want to do. It took me awhile to figure out how to clap! Now I just pound my chest for the same effect!”
Dr. Miller is an animal-lover and said his pets and nature are a large part of his self-care. His dog Maysie travels nearly everywhere with him and his cats, the Muffin Man and Darkness, enjoy making guest appearances on his Zoom calls. The physician frequently visits the desert in southern Utah and said he loves the arts, architecture, and design.
Dr. Miller’s advice for others who are disabled and want to go into medicine? Live out loud with your truths and be open about your disabilities. Too often, disabled individuals hide their disabilities, lie about them, or shield the world from their story, he said.
“These are rich, ripe experiences that are incredibly valuable to someone who wants to go out and be of service in the world,” he said. “We should be proud of our experiences as disabled people. The creativity we’ve had to exercise, the workarounds we’ve had to employ, these should not be points of embarrassment, but points of pride. Anyone who wants to pursue clinical training of any kind should use these experiences explicitly. These are sources of strength, not something to be forgiven or tolerated or accommodated.”
The same goes for physicians who do not have disabilities but who have lived through hardship, pain, struggle, or adversity, he emphasized.
“Find a way to learn from them, find a way to own them,” he said. “Use them as a source of strength and the rest of the world will respond to you differently.”
A version of this article first appeared on Medscape.com.
Bruce “BJ” Miller Jr., a 19-year-old Princeton (N.J.) University sophomore, was horsing around with friends near a train track in 1990 when they spotted a parked commuter train. They decided to climb over the train, and Mr. Miller was first up the ladder.

An explosion ripped through the air, and Mr. Miller was thrown on top of the train, his body smoking. His petrified friends called for an ambulance.
Clinging to life, Mr. Miller was airlifted to the burn unit at Saint Barnabas Medical Center in Livingston, N.J..
Physicians saved Mr. Miller’s life, but they had to amputate both of his legs below the knees and his left arm below the elbow.
“With electricity, you burn from the inside out,” said Mr. Miller, now 50. “The voltage enters your body – in my case, the wrist – and runs around internally until it finds a way out. That is often the lower extremities as the ground tends to ground the current, but not always. In my case, the current tried to come through my chest – which is also burned and required skin grafting – but not enough to spare my legs. I think I had a half-dozen or so surgeries over the first month or 2 at the hospital.”
Waking up to a new body
Mr. Miller doesn’t remember much about the accident, but he recalls waking up a few days later in the ICU and feeling the need to use the bathroom. Disoriented, Mr. Miller pulled off his ventilator, climbed out of bed, and tried to walk forward, unaware of his injuries. His feet and legs had not yet been amputated. When the catheter line ran out of slack, he collapsed.
“Eventually, a nurse came rushing in, responding to the ventilator alarm bells going off,” Mr. Miller said. “My dad wasn’t far behind. It became clear to me then that this was not a dream and [I realized] what had happened and why I was in the hospital.”
For months, Mr. Miller lived in the burn unit, undergoing countless skin grafts and surgeries. Because viable and nonviable tissue take time to be revealed after burns, surgeons take the minimum amount of tissue during each operation to give damaged tissue a chance to heal, he explained. In Mr. Miller’s case, his feet were amputated first, and later, his legs.
“In those early days from the hospital bed, my mind turned to issues related to identity,” he said. “What do I do with myself?
Mr. Miller eventually moved to the Rehabilitation Institute of Chicago (now called The Shirley Ryan AbilityLab), where he started the grueling process of rebuilding his strength and learning to walk on prosthetic legs.
“Any one day was filled with a mix of optimism and good fight and 5 minutes later, exasperation, frustration, tons of pain, and insecurity about my body,” he said. “My family and friends held the gate for me in a way, but a lot of the work was up to me. I had to believe that I deserved this love, that I wanted to be alive, and that there was still something here for me.”
Mr. Miller didn’t have to look far for inspiration. His mom had lived with polio for most of her life and acquired post-polio syndrome as she grew older, he said. When he was a child, his mom walked with crutches, and she became wheelchair-dependent by the time he was a teenager.
After the first surgery to amputate his feet, Mr. Miller and his mom shared a deep discussion about his joining the ranks of “the disabled,” and how their connection was now even stronger.
“In this way, the injuries unlocked even more experiences to share between us, and more love to feel, and therefore some early sense of gain to complement all the losses happening,” he said. “She had taught me so much about living with disability and had given me all the tools I needed to refashion my sense of self.”
From burn patient to medical student
After returning to Princeton University and finishing his undergraduate degree, Mr. Miller decided to go into medicine. He wanted to use his experience to help patients and find ways to improve weaknesses in the health care system, he said. But he made a deal with himself that he wouldn’t become a doctor for the sake of becoming one; he would enter the vocation only if he could do the work and enjoy the job.
“I wasn’t sure if I could do it,” he said. “There weren’t a lot of triple amputees to point to, to say whether this was even mechanically possible, to get through the training. The medical institutions I spoke with knew they had some obligation by law to protect me, but there’s also an obligation that I need to be able to fulfill the competencies. This was uncharted water.”
Because his greatest physical challenge was standing for long periods, instructors at the University of California, San Francisco, made accommodations to alleviate the strain. His clinical rotations for example, were organized near his home to limit the need for travel. On surgical rotations, he was allowed to sit on a stool.
Medical training progressed smoothly until Mr. Miller completed a rotation in his chosen specialty, rehabilitation medicine. He didn’t enjoy it. The passion and meaning he hoped to find was missing. Disillusioned, and with his final year in medical school coming to an end, Mr. Miller dropped out of the Match program. Around the same time, his sister, Lisa, died by suicide.
“My whole family life was in shambles,” he said. “I felt like, ‘I can’t even help my sister, how am I going to help other people?’ ”
Mr. Miller earned his MD and moved to his parents’ home in Milwaukee after his sister’s death. He was close to giving up on medicine, but his deans convinced him to do a post-doc internship. It was as an intern at the Medical College of Wisconsin, Milwaukee, that he completed an elective in palliative care.
“I fell immediately in love with it the first day,” he said. “This was a field devoted to working with things you can’t change and dealing with a lack of control, what it’s like to live with these diagnoses. This was a place where I could dig into my experience and share that with patients and families. This was a place where my life story had something to offer.”
Creating a new form of palliative care
Dr. Miller went on to complete a fellowship at Harvard Medical School, Boston, in hospice and palliative medicine. He became a palliative care physician at UCSF Health, and later directed the Zen Hospice Project, a nonprofit dedicated to teaching mindfulness-based caregiving for professionals, family members, and caregivers.
Gayle Kojimoto, a program manager who worked with Dr. Miller at UCSF’s outpatient palliative care clinic for cancer patients, said Miller was a favorite among patients because of his authenticity and his ability to make them feel understood.
“Patients love him because he is 100% present with them,” said Ms. Kojimoto. “They feel like he can understand their suffering better than other docs. He’s open to hearing about their suffering, when others may not be, and he doesn’t judge them. Many patients have said that seeing him is better than seeing a therapist.”
In 2020, Dr. Miller cofounded Mettle Health, a first-of-its-kind company that aims to reframe the way people think about their well-being as it relates to chronic and serious illness. Mettle Health’s care team provides consultations on a range of topics, including practical, emotional, and existential issues. No physician referrals are needed.
When the pandemic started, Dr. Miller said he and his colleagues felt the moment was ripe for bringing palliative care online to increase access, while decreasing caregiver and clinician burnout.
“We set up Mettle Health as an online palliative care counseling and coaching business and we pulled it out of the healthcare system so that whether you’re a patient or a caregiver you don’t need to satisfy some insurance need to get this kind of care,” he said. “We also realized there are enough people writing prescriptions. The medical piece is relatively well tended to; it’s the psychosocial and spiritual issues, and the existential issues, that are so underdeveloped. We are a social service, not a medical service, and this allows us to complement existing structures of care rather than compete with them.”
Having Dr. Miller as a leader for Mettle Health is a huge driver for why people seek out the company, said Sonya Dolan, director of operations and cofounder of Mettle Health.
“His approach to working with patients, caregivers, and clinicians is something I think sets us apart and makes us special,” she said. “His way of thinking about serious illness and death and dying is incredibly unique and he has a way of talking about and humanizing something that’s scary for a lot of us.”
‘Surprised by how much I can still do’
Since the accident, Dr. Miller has come a long way in navigating his physical limitations. In the early years, Dr. Miller said he was determined to do as many activities as he still could. He skied, biked, and pushed himself to stand for long periods on his prosthetic legs.
“For years, I would force myself to do these things just to prove I could, but not really enjoy them,” he said. “I’d get out on the dance floor or put myself out in vulnerable social situations where I might fall. It was kind of brutal and difficult. But at about year 5 or so, I became much more at ease with myself and more at peace with myself.”
Today, Dr. Miller’s prosthetics make nearly all ambulatory activities possible, but he concentrates on the activities that bring him joy.
“Probably the thing I can still do that surprises people most, including myself, is riding a motorcycle,” he said. “As for my upper body, I’m thoroughly used to living with only one hand and I continue to be surprised at how much I can still do. With enough time and experimentation, I can usually find a way to do what I need/want to do. It took me awhile to figure out how to clap! Now I just pound my chest for the same effect!”
Dr. Miller is an animal-lover and said his pets and nature are a large part of his self-care. His dog Maysie travels nearly everywhere with him and his cats, the Muffin Man and Darkness, enjoy making guest appearances on his Zoom calls. The physician frequently visits the desert in southern Utah and said he loves the arts, architecture, and design.
Dr. Miller’s advice for others who are disabled and want to go into medicine? Live out loud with your truths and be open about your disabilities. Too often, disabled individuals hide their disabilities, lie about them, or shield the world from their story, he said.
“These are rich, ripe experiences that are incredibly valuable to someone who wants to go out and be of service in the world,” he said. “We should be proud of our experiences as disabled people. The creativity we’ve had to exercise, the workarounds we’ve had to employ, these should not be points of embarrassment, but points of pride. Anyone who wants to pursue clinical training of any kind should use these experiences explicitly. These are sources of strength, not something to be forgiven or tolerated or accommodated.”
The same goes for physicians who do not have disabilities but who have lived through hardship, pain, struggle, or adversity, he emphasized.
“Find a way to learn from them, find a way to own them,” he said. “Use them as a source of strength and the rest of the world will respond to you differently.”
A version of this article first appeared on Medscape.com.
Bruce “BJ” Miller Jr., a 19-year-old Princeton (N.J.) University sophomore, was horsing around with friends near a train track in 1990 when they spotted a parked commuter train. They decided to climb over the train, and Mr. Miller was first up the ladder.

An explosion ripped through the air, and Mr. Miller was thrown on top of the train, his body smoking. His petrified friends called for an ambulance.
Clinging to life, Mr. Miller was airlifted to the burn unit at Saint Barnabas Medical Center in Livingston, N.J..
Physicians saved Mr. Miller’s life, but they had to amputate both of his legs below the knees and his left arm below the elbow.
“With electricity, you burn from the inside out,” said Mr. Miller, now 50. “The voltage enters your body – in my case, the wrist – and runs around internally until it finds a way out. That is often the lower extremities as the ground tends to ground the current, but not always. In my case, the current tried to come through my chest – which is also burned and required skin grafting – but not enough to spare my legs. I think I had a half-dozen or so surgeries over the first month or 2 at the hospital.”
Waking up to a new body
Mr. Miller doesn’t remember much about the accident, but he recalls waking up a few days later in the ICU and feeling the need to use the bathroom. Disoriented, Mr. Miller pulled off his ventilator, climbed out of bed, and tried to walk forward, unaware of his injuries. His feet and legs had not yet been amputated. When the catheter line ran out of slack, he collapsed.
“Eventually, a nurse came rushing in, responding to the ventilator alarm bells going off,” Mr. Miller said. “My dad wasn’t far behind. It became clear to me then that this was not a dream and [I realized] what had happened and why I was in the hospital.”
For months, Mr. Miller lived in the burn unit, undergoing countless skin grafts and surgeries. Because viable and nonviable tissue take time to be revealed after burns, surgeons take the minimum amount of tissue during each operation to give damaged tissue a chance to heal, he explained. In Mr. Miller’s case, his feet were amputated first, and later, his legs.
“In those early days from the hospital bed, my mind turned to issues related to identity,” he said. “What do I do with myself?
Mr. Miller eventually moved to the Rehabilitation Institute of Chicago (now called The Shirley Ryan AbilityLab), where he started the grueling process of rebuilding his strength and learning to walk on prosthetic legs.
“Any one day was filled with a mix of optimism and good fight and 5 minutes later, exasperation, frustration, tons of pain, and insecurity about my body,” he said. “My family and friends held the gate for me in a way, but a lot of the work was up to me. I had to believe that I deserved this love, that I wanted to be alive, and that there was still something here for me.”
Mr. Miller didn’t have to look far for inspiration. His mom had lived with polio for most of her life and acquired post-polio syndrome as she grew older, he said. When he was a child, his mom walked with crutches, and she became wheelchair-dependent by the time he was a teenager.
After the first surgery to amputate his feet, Mr. Miller and his mom shared a deep discussion about his joining the ranks of “the disabled,” and how their connection was now even stronger.
“In this way, the injuries unlocked even more experiences to share between us, and more love to feel, and therefore some early sense of gain to complement all the losses happening,” he said. “She had taught me so much about living with disability and had given me all the tools I needed to refashion my sense of self.”
From burn patient to medical student
After returning to Princeton University and finishing his undergraduate degree, Mr. Miller decided to go into medicine. He wanted to use his experience to help patients and find ways to improve weaknesses in the health care system, he said. But he made a deal with himself that he wouldn’t become a doctor for the sake of becoming one; he would enter the vocation only if he could do the work and enjoy the job.
“I wasn’t sure if I could do it,” he said. “There weren’t a lot of triple amputees to point to, to say whether this was even mechanically possible, to get through the training. The medical institutions I spoke with knew they had some obligation by law to protect me, but there’s also an obligation that I need to be able to fulfill the competencies. This was uncharted water.”
Because his greatest physical challenge was standing for long periods, instructors at the University of California, San Francisco, made accommodations to alleviate the strain. His clinical rotations for example, were organized near his home to limit the need for travel. On surgical rotations, he was allowed to sit on a stool.
Medical training progressed smoothly until Mr. Miller completed a rotation in his chosen specialty, rehabilitation medicine. He didn’t enjoy it. The passion and meaning he hoped to find was missing. Disillusioned, and with his final year in medical school coming to an end, Mr. Miller dropped out of the Match program. Around the same time, his sister, Lisa, died by suicide.
“My whole family life was in shambles,” he said. “I felt like, ‘I can’t even help my sister, how am I going to help other people?’ ”
Mr. Miller earned his MD and moved to his parents’ home in Milwaukee after his sister’s death. He was close to giving up on medicine, but his deans convinced him to do a post-doc internship. It was as an intern at the Medical College of Wisconsin, Milwaukee, that he completed an elective in palliative care.
“I fell immediately in love with it the first day,” he said. “This was a field devoted to working with things you can’t change and dealing with a lack of control, what it’s like to live with these diagnoses. This was a place where I could dig into my experience and share that with patients and families. This was a place where my life story had something to offer.”
Creating a new form of palliative care
Dr. Miller went on to complete a fellowship at Harvard Medical School, Boston, in hospice and palliative medicine. He became a palliative care physician at UCSF Health, and later directed the Zen Hospice Project, a nonprofit dedicated to teaching mindfulness-based caregiving for professionals, family members, and caregivers.
Gayle Kojimoto, a program manager who worked with Dr. Miller at UCSF’s outpatient palliative care clinic for cancer patients, said Miller was a favorite among patients because of his authenticity and his ability to make them feel understood.
“Patients love him because he is 100% present with them,” said Ms. Kojimoto. “They feel like he can understand their suffering better than other docs. He’s open to hearing about their suffering, when others may not be, and he doesn’t judge them. Many patients have said that seeing him is better than seeing a therapist.”
In 2020, Dr. Miller cofounded Mettle Health, a first-of-its-kind company that aims to reframe the way people think about their well-being as it relates to chronic and serious illness. Mettle Health’s care team provides consultations on a range of topics, including practical, emotional, and existential issues. No physician referrals are needed.
When the pandemic started, Dr. Miller said he and his colleagues felt the moment was ripe for bringing palliative care online to increase access, while decreasing caregiver and clinician burnout.
“We set up Mettle Health as an online palliative care counseling and coaching business and we pulled it out of the healthcare system so that whether you’re a patient or a caregiver you don’t need to satisfy some insurance need to get this kind of care,” he said. “We also realized there are enough people writing prescriptions. The medical piece is relatively well tended to; it’s the psychosocial and spiritual issues, and the existential issues, that are so underdeveloped. We are a social service, not a medical service, and this allows us to complement existing structures of care rather than compete with them.”
Having Dr. Miller as a leader for Mettle Health is a huge driver for why people seek out the company, said Sonya Dolan, director of operations and cofounder of Mettle Health.
“His approach to working with patients, caregivers, and clinicians is something I think sets us apart and makes us special,” she said. “His way of thinking about serious illness and death and dying is incredibly unique and he has a way of talking about and humanizing something that’s scary for a lot of us.”
‘Surprised by how much I can still do’
Since the accident, Dr. Miller has come a long way in navigating his physical limitations. In the early years, Dr. Miller said he was determined to do as many activities as he still could. He skied, biked, and pushed himself to stand for long periods on his prosthetic legs.
“For years, I would force myself to do these things just to prove I could, but not really enjoy them,” he said. “I’d get out on the dance floor or put myself out in vulnerable social situations where I might fall. It was kind of brutal and difficult. But at about year 5 or so, I became much more at ease with myself and more at peace with myself.”
Today, Dr. Miller’s prosthetics make nearly all ambulatory activities possible, but he concentrates on the activities that bring him joy.
“Probably the thing I can still do that surprises people most, including myself, is riding a motorcycle,” he said. “As for my upper body, I’m thoroughly used to living with only one hand and I continue to be surprised at how much I can still do. With enough time and experimentation, I can usually find a way to do what I need/want to do. It took me awhile to figure out how to clap! Now I just pound my chest for the same effect!”
Dr. Miller is an animal-lover and said his pets and nature are a large part of his self-care. His dog Maysie travels nearly everywhere with him and his cats, the Muffin Man and Darkness, enjoy making guest appearances on his Zoom calls. The physician frequently visits the desert in southern Utah and said he loves the arts, architecture, and design.
Dr. Miller’s advice for others who are disabled and want to go into medicine? Live out loud with your truths and be open about your disabilities. Too often, disabled individuals hide their disabilities, lie about them, or shield the world from their story, he said.
“These are rich, ripe experiences that are incredibly valuable to someone who wants to go out and be of service in the world,” he said. “We should be proud of our experiences as disabled people. The creativity we’ve had to exercise, the workarounds we’ve had to employ, these should not be points of embarrassment, but points of pride. Anyone who wants to pursue clinical training of any kind should use these experiences explicitly. These are sources of strength, not something to be forgiven or tolerated or accommodated.”
The same goes for physicians who do not have disabilities but who have lived through hardship, pain, struggle, or adversity, he emphasized.
“Find a way to learn from them, find a way to own them,” he said. “Use them as a source of strength and the rest of the world will respond to you differently.”
A version of this article first appeared on Medscape.com.
Differences in Care by Race in Older Nursing Home Residents With Dementia
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
Comprehensive and Equitable Care for Vulnerable Veterans With Integrated Palliative, Psychology, and Oncology Care
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
Sharp decrease in opioid access for dying U.S. cancer patients
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
Disconnect between POLST orders and end-of-life care
Background: In order to reduce the mismatch between patients’ desired and actual end-of-life care, the Physician Orders for Life-Sustaining Treatment (POLST) was created. POLST is a portable document delineating medical orders for emergency care treatment at the end of life including whether to attempt resuscitation and general level of medical interventions. For nursing home residents, an association between POLST creation and reduction of unwanted CPR has been substantiated. Outside of this population, the association is unknown.
Study design: Retrospective cohort study.
Setting: Two academic hospitals in Washington.
Synopsis: Patients older than age 18 years who had one of nine chronic health conditions associated with 90% of deaths among Medicare beneficiaries were identified using Washington state death certificates. Additional inclusion criteria included hospital admission in the last 6 months of life and creation of a POLST prior to this admission. This led to identification of 1,818 patients. Patients with full-treatment POLST orders were significantly more likely to be admitted to the ICU as well as receive life-sustaining treatments such as mechanical ventilation, vasoactive infusions, or CPR, compared with patients with limited interventions or comfort-only POLST orders (P < .001 for both). 38% of patients with treatment-limiting POLSTs received aggressive end-of-life care that was discordant with their previously documented wishes.
Bottom line: Completion of POLST was associated with a greater likelihood of receiving end-of-life care that was in line with patients’ previously documented wishes regarding admission to ICU and life-sustaining treatment. Washington was one of the first states to adopt POLST in 2005 and therefore these results may not be broadly applicable.
Citation: Lee RY et al. Association of physician orders for life-sustaining treatment with ICU admission among patients hospitalized hear the end of life. JAMA. 2020 Feb 16;323(10):950-60.
Dr. Dreicer is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: In order to reduce the mismatch between patients’ desired and actual end-of-life care, the Physician Orders for Life-Sustaining Treatment (POLST) was created. POLST is a portable document delineating medical orders for emergency care treatment at the end of life including whether to attempt resuscitation and general level of medical interventions. For nursing home residents, an association between POLST creation and reduction of unwanted CPR has been substantiated. Outside of this population, the association is unknown.
Study design: Retrospective cohort study.
Setting: Two academic hospitals in Washington.
Synopsis: Patients older than age 18 years who had one of nine chronic health conditions associated with 90% of deaths among Medicare beneficiaries were identified using Washington state death certificates. Additional inclusion criteria included hospital admission in the last 6 months of life and creation of a POLST prior to this admission. This led to identification of 1,818 patients. Patients with full-treatment POLST orders were significantly more likely to be admitted to the ICU as well as receive life-sustaining treatments such as mechanical ventilation, vasoactive infusions, or CPR, compared with patients with limited interventions or comfort-only POLST orders (P < .001 for both). 38% of patients with treatment-limiting POLSTs received aggressive end-of-life care that was discordant with their previously documented wishes.
Bottom line: Completion of POLST was associated with a greater likelihood of receiving end-of-life care that was in line with patients’ previously documented wishes regarding admission to ICU and life-sustaining treatment. Washington was one of the first states to adopt POLST in 2005 and therefore these results may not be broadly applicable.
Citation: Lee RY et al. Association of physician orders for life-sustaining treatment with ICU admission among patients hospitalized hear the end of life. JAMA. 2020 Feb 16;323(10):950-60.
Dr. Dreicer is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: In order to reduce the mismatch between patients’ desired and actual end-of-life care, the Physician Orders for Life-Sustaining Treatment (POLST) was created. POLST is a portable document delineating medical orders for emergency care treatment at the end of life including whether to attempt resuscitation and general level of medical interventions. For nursing home residents, an association between POLST creation and reduction of unwanted CPR has been substantiated. Outside of this population, the association is unknown.
Study design: Retrospective cohort study.
Setting: Two academic hospitals in Washington.
Synopsis: Patients older than age 18 years who had one of nine chronic health conditions associated with 90% of deaths among Medicare beneficiaries were identified using Washington state death certificates. Additional inclusion criteria included hospital admission in the last 6 months of life and creation of a POLST prior to this admission. This led to identification of 1,818 patients. Patients with full-treatment POLST orders were significantly more likely to be admitted to the ICU as well as receive life-sustaining treatments such as mechanical ventilation, vasoactive infusions, or CPR, compared with patients with limited interventions or comfort-only POLST orders (P < .001 for both). 38% of patients with treatment-limiting POLSTs received aggressive end-of-life care that was discordant with their previously documented wishes.
Bottom line: Completion of POLST was associated with a greater likelihood of receiving end-of-life care that was in line with patients’ previously documented wishes regarding admission to ICU and life-sustaining treatment. Washington was one of the first states to adopt POLST in 2005 and therefore these results may not be broadly applicable.
Citation: Lee RY et al. Association of physician orders for life-sustaining treatment with ICU admission among patients hospitalized hear the end of life. JAMA. 2020 Feb 16;323(10):950-60.
Dr. Dreicer is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.