User login
Hiccups in patients with cancer often overlooked, undertreated
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF HOSPICE AND PALLIATIVE MEDICINE
Palliative Care Disparities in Small Cell Carcinoma of the Prostate: An Analysis of the National Cancer Database
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Purpose
This study addresses a gap in knowledge regarding palliative care utilization patterns in smallcell carcinoma of the prostate.
Background
Prostate cancer is the most common cancer affecting males. One of the most aggressive malignancies of the prostate is small cell carcinoma (SCC) of the prostate. Almost 70% of patients diagnosed with SCC present with the disseminated disease with a low 5-year survival rate of less than 2%. The role of palliative care can be beneficial in metastatic prostate cancer given its largely incurable course. Despite evidence favoring palliative care for prostate cancer in several patient populations, it remains under-utilized. Palliative care utilization patterns in SCC of the prostate have not yet been studied.
Methods
This is a retrospective study of patients diagnosed with all subtypes of AJCC staged metastatic SCC of the prostate between 2004 and 2017 in the National Cancer Database (NCDB) to determine palliative care usage (n = 615). Exclusion criteria included missing data.
Data Analysis
Variables were evaluated for significance (P < .05) in relation to the receipt of palliative care using Pearson Chi-Square, ANOVA, and Kaplan- Meier tests. Multivariate analysis was performed via binary logistics regression.
Results
Among the 961 patients diagnosed with SCC of the prostate, 64% had metastatic disease (n = 615). The metastatic cohort was more likely to receive palliative care than those that did not have distant metastasis (24.2% vs 5.7%, P < .001). Palliative care use has grown between 2004 (n = 6) and 2017 (n = 20). Patients that were uninsured were more likely than insured patients to receive palliative care (50% vs 23.5%, P = .003; 95% CI, 0.051- 0.546). Non-Hispanic patients were also more likely than Hispanic patients to receive palliative care (P = .033; 95% CI, 1.154-28.140). New England locations had the highest utilization of palliative care (43.%, P = .009). Factors that impacted palliative care use included facility region, insurance status, and Hispanic status. As palliative care continues to be utilized more frequently, we hope that this study can provide a starting point in studying and preventing palliative treatment disparities.
Implementation of Clinical Triggers for Palliative Care Consultation on the Edward Hines Jr. VA Hematology/ Oncology Inpatient Service
Purpose
Hospitalized patients with advanced malignancies often have high symptom burden and poor quality of life, which are frequently under-recognized or under-treated. Accordingly, the integration of specialty palliative care (PC) in this population is imperative. Unfortunately, a sustainable referral model to capture patients for timely PC involvement is lacking. This quality improvement study evaluated the implementation of a clinical trigger-based referral process to PC for inpatients on the Hematology/Oncology (HO) service at Hines VA Hospital. Clinical outcomes studied included: Life-Sustaining Treatment (LST) note completion rates; measurement of overall survival at 3, 6, and 12 months; rate of re-hospitalization within 30 days; and venue of death and treating specialty of deceased patients.
Methods
House staff received a weekly email that included the clinical PC triggers. Admitted patients who met trigger criteria would prompt consultation to PC. Clinical triggers included: metastatic oncologic disease or relapsed hematologic disease; uncontrolled symptoms; > 2 unscheduled hospitalizations in the prior 30 days; and unscheduled hospitalizations lasting > 7 days.
Results
A total of 63 patients were admitted to the HO service between December 2020 through February 2021. Of those, 53 (84.1%) met at least 1 trigger and 36 (68%) received PC consultation. Of the patients that met trigger criteria and received a PC consult, 85.7% died with hospice compared to 44.4% in the group who did not receive a PC consult (P < .01). Nineteen (51.3%) died within 6 months of discharge compared to 7 (26.9%) who did not receive a PC consult (P = .08). Twelve (33.3%) had recurrent hospitalizations compared to 5 (29%) who did not receive a PC consult (P = .38), and 20 (55.6%) had a new or updated LST note compared to 2 (11.8%) who did not receive PC consultation (P < .01).
Conculsions
This study demonstrated the feasibility of implementing a trigger-based system for PC consultation in a veteran inpatient HO population. Notably, a large majority of HO inpatients met criteria for at least 1 PC trigger. No significant difference was found in overall survival at 6 months; however, patients who received PC consultation were more likely to receive hospice services at the end of life.
Purpose
Hospitalized patients with advanced malignancies often have high symptom burden and poor quality of life, which are frequently under-recognized or under-treated. Accordingly, the integration of specialty palliative care (PC) in this population is imperative. Unfortunately, a sustainable referral model to capture patients for timely PC involvement is lacking. This quality improvement study evaluated the implementation of a clinical trigger-based referral process to PC for inpatients on the Hematology/Oncology (HO) service at Hines VA Hospital. Clinical outcomes studied included: Life-Sustaining Treatment (LST) note completion rates; measurement of overall survival at 3, 6, and 12 months; rate of re-hospitalization within 30 days; and venue of death and treating specialty of deceased patients.
Methods
House staff received a weekly email that included the clinical PC triggers. Admitted patients who met trigger criteria would prompt consultation to PC. Clinical triggers included: metastatic oncologic disease or relapsed hematologic disease; uncontrolled symptoms; > 2 unscheduled hospitalizations in the prior 30 days; and unscheduled hospitalizations lasting > 7 days.
Results
A total of 63 patients were admitted to the HO service between December 2020 through February 2021. Of those, 53 (84.1%) met at least 1 trigger and 36 (68%) received PC consultation. Of the patients that met trigger criteria and received a PC consult, 85.7% died with hospice compared to 44.4% in the group who did not receive a PC consult (P < .01). Nineteen (51.3%) died within 6 months of discharge compared to 7 (26.9%) who did not receive a PC consult (P = .08). Twelve (33.3%) had recurrent hospitalizations compared to 5 (29%) who did not receive a PC consult (P = .38), and 20 (55.6%) had a new or updated LST note compared to 2 (11.8%) who did not receive PC consultation (P < .01).
Conculsions
This study demonstrated the feasibility of implementing a trigger-based system for PC consultation in a veteran inpatient HO population. Notably, a large majority of HO inpatients met criteria for at least 1 PC trigger. No significant difference was found in overall survival at 6 months; however, patients who received PC consultation were more likely to receive hospice services at the end of life.
Purpose
Hospitalized patients with advanced malignancies often have high symptom burden and poor quality of life, which are frequently under-recognized or under-treated. Accordingly, the integration of specialty palliative care (PC) in this population is imperative. Unfortunately, a sustainable referral model to capture patients for timely PC involvement is lacking. This quality improvement study evaluated the implementation of a clinical trigger-based referral process to PC for inpatients on the Hematology/Oncology (HO) service at Hines VA Hospital. Clinical outcomes studied included: Life-Sustaining Treatment (LST) note completion rates; measurement of overall survival at 3, 6, and 12 months; rate of re-hospitalization within 30 days; and venue of death and treating specialty of deceased patients.
Methods
House staff received a weekly email that included the clinical PC triggers. Admitted patients who met trigger criteria would prompt consultation to PC. Clinical triggers included: metastatic oncologic disease or relapsed hematologic disease; uncontrolled symptoms; > 2 unscheduled hospitalizations in the prior 30 days; and unscheduled hospitalizations lasting > 7 days.
Results
A total of 63 patients were admitted to the HO service between December 2020 through February 2021. Of those, 53 (84.1%) met at least 1 trigger and 36 (68%) received PC consultation. Of the patients that met trigger criteria and received a PC consult, 85.7% died with hospice compared to 44.4% in the group who did not receive a PC consult (P < .01). Nineteen (51.3%) died within 6 months of discharge compared to 7 (26.9%) who did not receive a PC consult (P = .08). Twelve (33.3%) had recurrent hospitalizations compared to 5 (29%) who did not receive a PC consult (P = .38), and 20 (55.6%) had a new or updated LST note compared to 2 (11.8%) who did not receive PC consultation (P < .01).
Conculsions
This study demonstrated the feasibility of implementing a trigger-based system for PC consultation in a veteran inpatient HO population. Notably, a large majority of HO inpatients met criteria for at least 1 PC trigger. No significant difference was found in overall survival at 6 months; however, patients who received PC consultation were more likely to receive hospice services at the end of life.
A Case Report of Palliative Pembrolizumab Monotherapy for a Poorly Differentiated Malignancy
Introduction
The critical role of palliative radiotherapy (RT) in the management of advanced cancer is evolving due to the advent of novel therapeuticapproaches. We report the case of a veteran with a soft tissue metastasis who had a robust response to pembrolizumab, allowing for the deferral of palliative RT.
Case Presentation
An 86-year-old male presented with a rapidly growing, painful, malodorous, fungating right inguinal soft tissue mass measuring 10×7×3 cm that had rendered the patient non-ambulatory, with subsequent imaging also demonstrating a left pleural-based lung mass. Biopsy was consistent with a poorly differentiated carcinoma, and molecular profiling revealed a KRAS G12C mutation, high tumor mutational burden (TMB 18 mutations/megabase), and high PD-L1 expression (TPS 100%). The patient’s poor functional status precluded the use of aggressive combination chemotherapy, but the molecular features were favorable for response to immune checkpoint inhibitor monotherapy, which is better tolerated. He was initiated on pembrolizumab with the goal of symptom palliation and potentially prolonging his life. However, as rapid responses to immunotherapy are uncommon, radiation oncology was consulted for palliative RT. Twenty days after starting pembrolizumab and 2 weeks after RT simulation, the inguinal mass had markedly regressed with an open tissue defect at the site. As the palliative goal had been achieved, RT was deferred to avoid the development of a non-healing wound.
Conclusions
Our case highlights palliative treatment modalities for soft tissue masses. Immunotherapy is now a component of first-line therapy in many cancer types, but rapid and robust responses to monotherapy are rare. There is the exciting potential to combine immunotherapy with RT, with small case series indicating synergy, although further research is needed. In cases with molecular characteristics favoring response to immunotherapy, an optimal sequencing approach may incorporate an initial run-in phase with immunotherapy to determine if symptom palliation can be achieved with unimodal therapy. The location of the mass in a non-radiation sensitive region allowed us to entertain the use of combination therapy for our patient, but ultimately was not needed. Palliative RT will remain an option at the time of cancer progression.
Introduction
The critical role of palliative radiotherapy (RT) in the management of advanced cancer is evolving due to the advent of novel therapeuticapproaches. We report the case of a veteran with a soft tissue metastasis who had a robust response to pembrolizumab, allowing for the deferral of palliative RT.
Case Presentation
An 86-year-old male presented with a rapidly growing, painful, malodorous, fungating right inguinal soft tissue mass measuring 10×7×3 cm that had rendered the patient non-ambulatory, with subsequent imaging also demonstrating a left pleural-based lung mass. Biopsy was consistent with a poorly differentiated carcinoma, and molecular profiling revealed a KRAS G12C mutation, high tumor mutational burden (TMB 18 mutations/megabase), and high PD-L1 expression (TPS 100%). The patient’s poor functional status precluded the use of aggressive combination chemotherapy, but the molecular features were favorable for response to immune checkpoint inhibitor monotherapy, which is better tolerated. He was initiated on pembrolizumab with the goal of symptom palliation and potentially prolonging his life. However, as rapid responses to immunotherapy are uncommon, radiation oncology was consulted for palliative RT. Twenty days after starting pembrolizumab and 2 weeks after RT simulation, the inguinal mass had markedly regressed with an open tissue defect at the site. As the palliative goal had been achieved, RT was deferred to avoid the development of a non-healing wound.
Conclusions
Our case highlights palliative treatment modalities for soft tissue masses. Immunotherapy is now a component of first-line therapy in many cancer types, but rapid and robust responses to monotherapy are rare. There is the exciting potential to combine immunotherapy with RT, with small case series indicating synergy, although further research is needed. In cases with molecular characteristics favoring response to immunotherapy, an optimal sequencing approach may incorporate an initial run-in phase with immunotherapy to determine if symptom palliation can be achieved with unimodal therapy. The location of the mass in a non-radiation sensitive region allowed us to entertain the use of combination therapy for our patient, but ultimately was not needed. Palliative RT will remain an option at the time of cancer progression.
Introduction
The critical role of palliative radiotherapy (RT) in the management of advanced cancer is evolving due to the advent of novel therapeuticapproaches. We report the case of a veteran with a soft tissue metastasis who had a robust response to pembrolizumab, allowing for the deferral of palliative RT.
Case Presentation
An 86-year-old male presented with a rapidly growing, painful, malodorous, fungating right inguinal soft tissue mass measuring 10×7×3 cm that had rendered the patient non-ambulatory, with subsequent imaging also demonstrating a left pleural-based lung mass. Biopsy was consistent with a poorly differentiated carcinoma, and molecular profiling revealed a KRAS G12C mutation, high tumor mutational burden (TMB 18 mutations/megabase), and high PD-L1 expression (TPS 100%). The patient’s poor functional status precluded the use of aggressive combination chemotherapy, but the molecular features were favorable for response to immune checkpoint inhibitor monotherapy, which is better tolerated. He was initiated on pembrolizumab with the goal of symptom palliation and potentially prolonging his life. However, as rapid responses to immunotherapy are uncommon, radiation oncology was consulted for palliative RT. Twenty days after starting pembrolizumab and 2 weeks after RT simulation, the inguinal mass had markedly regressed with an open tissue defect at the site. As the palliative goal had been achieved, RT was deferred to avoid the development of a non-healing wound.
Conclusions
Our case highlights palliative treatment modalities for soft tissue masses. Immunotherapy is now a component of first-line therapy in many cancer types, but rapid and robust responses to monotherapy are rare. There is the exciting potential to combine immunotherapy with RT, with small case series indicating synergy, although further research is needed. In cases with molecular characteristics favoring response to immunotherapy, an optimal sequencing approach may incorporate an initial run-in phase with immunotherapy to determine if symptom palliation can be achieved with unimodal therapy. The location of the mass in a non-radiation sensitive region allowed us to entertain the use of combination therapy for our patient, but ultimately was not needed. Palliative RT will remain an option at the time of cancer progression.
Life and death decisions: What keeps oncologists up at night
It was 2 a.m. And Rebecca Shatsky, MD, could not sleep.
The breast oncologist was thinking about a patient of hers with metastatic cancer.
The patient’s disease had been asymptomatic for some time. Then without warning, her cancer suddenly exploded. Her bone marrow was failing, and her liver was not far behind.
Dr. Shatsky had a treatment plan ready to go but still, she felt uneasy.
“I had to be honest with her that I didn’t know if this plan would work,” says Dr. Shatsky, a medical oncologist at University of California, San Diego (UCSD).
That night, after visiting the patient in the hospital, Dr. Shatsky lay awake going over her next move, making sure it was the right one and hoping it would help keep the disease at bay.
“It’s so much pressure when someone is depending on you to make life or death decisions,” Dr. Shatsky said.
And in the quiet hours of night, these concerns grow louder.
Dr. Shatsky is not alone.
“There’s no off button,” says Aaron Goodman, MD, a hematologist at UCSD Health who goes by “Papa Heme” on Twitter. “I’m always thinking about my patients. Constantly.”
The public rarely gets a glimpse of these private moments. On occasion, oncologists will share a personal story, but more often, insights come from broad research on the ethical, emotional, and psychological toll of practicing medicine.
Many oncologists carry this baggage home with them because they have no other option.
“There is simply no time to process the weight of the day when I’ve got seven more patients who need my full attention before lunch,” Mark Lewis, MD, director, department of gastrointestinal oncology, Intermountain Healthcare, Salt Lake City, Utah. “That is why my processing happens outside of the office, when my brain can be quiet.”
What am I missing?
Dr. Goodman recognizes the gravity of each decision he makes. He pores over every detail of a patient’s scans, lab results, history, and symptoms.
But no matter how many times he checks and rechecks, one question nags at him: What am I missing?
For Dr. Goodman, this exhaustive level of attention is worth it.
“When errors are made, it’s someone’s life,” Dr. Goodman said. “Nothing would have prepared me for this responsibility. Until it lies on you, it’s impossible to understand how much trust patients put into us.”
That trust becomes most apparent for Dr. Goodman when facing a decision about how to treat a patient with acute myeloid leukemia who’s in remission.
Give more chemotherapy to root out the leukemia cells still lurking in the body, and the patient faces a high risk of the cancer returning. Pick stem cell transplant, and the chance of being cured goes up significantly, but the patient could also die within 100 days of the transplant.
“All together, the data show I’m helping patients with a transplant, but for the individual, I could be causing harm. Someone could be living less because of a decision I made,” Dr. Goodman said.
For patients with advanced cancer, oncologists may need to think several moves ahead. Mapping out a patient’s treatment options can feel like a game of chess. Dr. Shatsky is always trying to anticipate how the tumor will behave, what is driving it, and how lifestyle factors may influence a patient’s response in the present and the future.
“It is a mind game,” she says. “Like in chess, I try to outsmart my opponent. But with advanced cancer, there are not necessarily clear-cut guidelines or one way to manage the disease, and I have to do the best I can with drugs I have.”
That’s the art of oncology: Balancing the many knowns and unknowns of a person’s cancer alongside the toxicities of treatment and a patient’s hopes and goals.
Throughout the year, Don Dizon, MD, will see a number of patients with advanced disease. In these instances, the question he often wrestles with is if the patient can’t be cured, whether more treatment will just cause greater harm.
Dr. Dizon recently faced this dilemma with an older patient with metastatic disease who had not done well with an initial treatment regimen. After outlining the risks for more chemotherapy, he explained one option would be to forgo it and simply treat her symptoms.
“It’s an impossible choice,” says Dr. Dizon, director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, Providence.
Chemotherapy can provide symptom relief, but it can also be toxic – and patients may be so frail, they can die from more therapy.
“I told my patient, if in your heart, you want to try more therapy, that’s okay. But it’s also okay if you don’t,” Dr. Dizon recalled.
Her response: “You’re supposed to give me the answer.”
However, for patients approaching the end of life, there often is no right answer.
“It’s part of the discomfort you live with as a patient and oncologist, and when I leave the clinic, that’s one thing that follows me home,” Dr. Dizon said. “At the end of the day, I need to look in the mirror and know I did the best I could.”
The difficult conversation
Every Sunday, Dr. Lewis feels the weight of the week ahead. He and his wife, a pediatrician, call it the “Sunday scaries.”
It’s when Dr. Lewis begins thinking about the delicate conversations to come, rehearsing how he’s going to share the news that a person has advanced cancer or that a cancer, once in remission, has returned.
“Before the pandemic, I had 36 people come to a visit where I delivered some very heavy news and it became a Greek chorus of sobbing,” he recalls.
For every oncologist, delivering bad news is an integral part of the job. But after spending months, sometimes years, with a patient and the family, Dr. Lewis knows how to take the temperature of the room – who will likely prefer a more blunt style and who might need a gentler touch.
“The longer you know a patient and family, the better you can gauge the best approach,” Dr. Lewis said. “And for some, you know it’ll be complete devastation no matter what.”
When Jennifer Lycette, MD, prepares for a difficult conversation, she’ll run down all the possible ways it could go. Sometimes her brain will get stuck in a loop, cycling through the different trajectories on repeat.
“For years, I didn’t know how to cope with that,” said Dr. Lycette, medical director at Providence Oncology and Hematology Care Clinic in Seaside, Ore. “I wasn’t taught the tools to cope with that in my medical training. It took midcareer professional coaching that I sought out on my own to learn to remind myself that no matter what the person says, I have the experience and skill set to handle what comes next and to simply be present in the moment with the patient.”
The question that now sits with Dr. Lycette hours after a visit is what she could have done better. She knows from experience how important it is to choose her words carefully.
Early in her career, Dr. Lycette had a patient with stage IV cancer who wanted to know more about the death process. Because most people ask about pain, she assured him that he likely wouldn’t experience too much pain with his type of cancer.
“It will probably be like falling asleep,” said Dr. Lycette, hoping she was offering comfort. “When I saw him next, he told me he hadn’t slept.”
He was afraid that if he did, he wouldn’t wake up.
In that moment, Dr. Lycette realized the power that her words carry and the importance of trying to understand the inner lives of her patients.
Life outside the clinic
Sometimes an oncologist’s late-night ruminations have little to do with cancer itself.
Manali Patel, MD, finds herself worrying if her patients will have enough to eat and whether she will be able to help.
“I was up at 3 a.m. one morning, thinking about how we’re going to fund a project for patients from low-income households who we discovered were experiencing severe food insecurity – what grants we need, what foundations we can work with,” said Dr. Patel, a medical oncologist at Stanford Hospital and Clinics and the VA Palo Alto Health Care System in California.
The past few years of the pandemic have added a new layer of worry for Dr. Patel.
“I don’t want my patients to die from a preventable virus when they’ve already been through so much suffering,” Dr. Patel said.
This thought feeds worries about how her actions outside the clinic could unintentionally harm her patients. Should she go to a big medical conference? A family gathering? The grocery store?
“There are some places you can’t avoid, but these decisions have caused a lot of strife for me,” she said. “The health and safety of our patients – that’s in our wheelhouse – but so many of the policies are outside of our control.”
The inevitable losses and the wins
For patients with metastatic disease, eventually the treatment options will run out.
Dr. Shatsky likes to be up front with patients about that reality: “There will come a day when I will tell you there’s nothing more I can do, and you need to trust that I’m being honest with you and that’s the truth.”
For Dr. Goodman, the devastation that bad news brings patients and families is glaring. He knows there will be no more normalcy in their lives.
“I see a lot of suffering, but I know the suffering happens regardless of whether I see it or not,” Dr. Goodman said.
That’s why holding on to the victories can be so important. Dr. Goodman recalled a young patient who came to him with a 20-cm tumor and is now cured. “Had I not met that individual and done what I had done, he’d be dead, but now he’s going to live his life,” Dr. Goodman said. “But I don’t wake up at 2 a.m. thinking about that.”
Dr. Shatsky gets a lot of joy from the wins – the patients who do really well, the times when she can help a friend or colleagues – and those moments go a long way to outweigh the hurt, worry, and workload.
When dealing with so much gray, “the wins are important, knowing you can make a difference is important,” Dr. Dizon said.
And there’s a delicate balance.
“I think patients want an oncologist who cares and is genuinely invested in their outcomes but not someone who is so sad all the time,” Dr. Lewis said. “When I lose a patient, I still grieve each loss, but I can’t mourn every patient’s death like it’s a family member. Otherwise, I’d break.”
What would you do if you had terminal cancer?
Dr. Dizon recalled how a friend handled the news. She went home and made dinner, he said.
Ultimately, she lived for many years. She saw her kids get married, met her first grandchild, and had time to prepare, something not everyone gets the chance to do.
That’s why it’s important to “do what you normally do as long as you can,” Dr. Dizon said. “Live your life.”
A version of this article first appeared on Medscape.com.
It was 2 a.m. And Rebecca Shatsky, MD, could not sleep.
The breast oncologist was thinking about a patient of hers with metastatic cancer.
The patient’s disease had been asymptomatic for some time. Then without warning, her cancer suddenly exploded. Her bone marrow was failing, and her liver was not far behind.
Dr. Shatsky had a treatment plan ready to go but still, she felt uneasy.
“I had to be honest with her that I didn’t know if this plan would work,” says Dr. Shatsky, a medical oncologist at University of California, San Diego (UCSD).
That night, after visiting the patient in the hospital, Dr. Shatsky lay awake going over her next move, making sure it was the right one and hoping it would help keep the disease at bay.
“It’s so much pressure when someone is depending on you to make life or death decisions,” Dr. Shatsky said.
And in the quiet hours of night, these concerns grow louder.
Dr. Shatsky is not alone.
“There’s no off button,” says Aaron Goodman, MD, a hematologist at UCSD Health who goes by “Papa Heme” on Twitter. “I’m always thinking about my patients. Constantly.”
The public rarely gets a glimpse of these private moments. On occasion, oncologists will share a personal story, but more often, insights come from broad research on the ethical, emotional, and psychological toll of practicing medicine.
Many oncologists carry this baggage home with them because they have no other option.
“There is simply no time to process the weight of the day when I’ve got seven more patients who need my full attention before lunch,” Mark Lewis, MD, director, department of gastrointestinal oncology, Intermountain Healthcare, Salt Lake City, Utah. “That is why my processing happens outside of the office, when my brain can be quiet.”
What am I missing?
Dr. Goodman recognizes the gravity of each decision he makes. He pores over every detail of a patient’s scans, lab results, history, and symptoms.
But no matter how many times he checks and rechecks, one question nags at him: What am I missing?
For Dr. Goodman, this exhaustive level of attention is worth it.
“When errors are made, it’s someone’s life,” Dr. Goodman said. “Nothing would have prepared me for this responsibility. Until it lies on you, it’s impossible to understand how much trust patients put into us.”
That trust becomes most apparent for Dr. Goodman when facing a decision about how to treat a patient with acute myeloid leukemia who’s in remission.
Give more chemotherapy to root out the leukemia cells still lurking in the body, and the patient faces a high risk of the cancer returning. Pick stem cell transplant, and the chance of being cured goes up significantly, but the patient could also die within 100 days of the transplant.
“All together, the data show I’m helping patients with a transplant, but for the individual, I could be causing harm. Someone could be living less because of a decision I made,” Dr. Goodman said.
For patients with advanced cancer, oncologists may need to think several moves ahead. Mapping out a patient’s treatment options can feel like a game of chess. Dr. Shatsky is always trying to anticipate how the tumor will behave, what is driving it, and how lifestyle factors may influence a patient’s response in the present and the future.
“It is a mind game,” she says. “Like in chess, I try to outsmart my opponent. But with advanced cancer, there are not necessarily clear-cut guidelines or one way to manage the disease, and I have to do the best I can with drugs I have.”
That’s the art of oncology: Balancing the many knowns and unknowns of a person’s cancer alongside the toxicities of treatment and a patient’s hopes and goals.
Throughout the year, Don Dizon, MD, will see a number of patients with advanced disease. In these instances, the question he often wrestles with is if the patient can’t be cured, whether more treatment will just cause greater harm.
Dr. Dizon recently faced this dilemma with an older patient with metastatic disease who had not done well with an initial treatment regimen. After outlining the risks for more chemotherapy, he explained one option would be to forgo it and simply treat her symptoms.
“It’s an impossible choice,” says Dr. Dizon, director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, Providence.
Chemotherapy can provide symptom relief, but it can also be toxic – and patients may be so frail, they can die from more therapy.
“I told my patient, if in your heart, you want to try more therapy, that’s okay. But it’s also okay if you don’t,” Dr. Dizon recalled.
Her response: “You’re supposed to give me the answer.”
However, for patients approaching the end of life, there often is no right answer.
“It’s part of the discomfort you live with as a patient and oncologist, and when I leave the clinic, that’s one thing that follows me home,” Dr. Dizon said. “At the end of the day, I need to look in the mirror and know I did the best I could.”
The difficult conversation
Every Sunday, Dr. Lewis feels the weight of the week ahead. He and his wife, a pediatrician, call it the “Sunday scaries.”
It’s when Dr. Lewis begins thinking about the delicate conversations to come, rehearsing how he’s going to share the news that a person has advanced cancer or that a cancer, once in remission, has returned.
“Before the pandemic, I had 36 people come to a visit where I delivered some very heavy news and it became a Greek chorus of sobbing,” he recalls.
For every oncologist, delivering bad news is an integral part of the job. But after spending months, sometimes years, with a patient and the family, Dr. Lewis knows how to take the temperature of the room – who will likely prefer a more blunt style and who might need a gentler touch.
“The longer you know a patient and family, the better you can gauge the best approach,” Dr. Lewis said. “And for some, you know it’ll be complete devastation no matter what.”
When Jennifer Lycette, MD, prepares for a difficult conversation, she’ll run down all the possible ways it could go. Sometimes her brain will get stuck in a loop, cycling through the different trajectories on repeat.
“For years, I didn’t know how to cope with that,” said Dr. Lycette, medical director at Providence Oncology and Hematology Care Clinic in Seaside, Ore. “I wasn’t taught the tools to cope with that in my medical training. It took midcareer professional coaching that I sought out on my own to learn to remind myself that no matter what the person says, I have the experience and skill set to handle what comes next and to simply be present in the moment with the patient.”
The question that now sits with Dr. Lycette hours after a visit is what she could have done better. She knows from experience how important it is to choose her words carefully.
Early in her career, Dr. Lycette had a patient with stage IV cancer who wanted to know more about the death process. Because most people ask about pain, she assured him that he likely wouldn’t experience too much pain with his type of cancer.
“It will probably be like falling asleep,” said Dr. Lycette, hoping she was offering comfort. “When I saw him next, he told me he hadn’t slept.”
He was afraid that if he did, he wouldn’t wake up.
In that moment, Dr. Lycette realized the power that her words carry and the importance of trying to understand the inner lives of her patients.
Life outside the clinic
Sometimes an oncologist’s late-night ruminations have little to do with cancer itself.
Manali Patel, MD, finds herself worrying if her patients will have enough to eat and whether she will be able to help.
“I was up at 3 a.m. one morning, thinking about how we’re going to fund a project for patients from low-income households who we discovered were experiencing severe food insecurity – what grants we need, what foundations we can work with,” said Dr. Patel, a medical oncologist at Stanford Hospital and Clinics and the VA Palo Alto Health Care System in California.
The past few years of the pandemic have added a new layer of worry for Dr. Patel.
“I don’t want my patients to die from a preventable virus when they’ve already been through so much suffering,” Dr. Patel said.
This thought feeds worries about how her actions outside the clinic could unintentionally harm her patients. Should she go to a big medical conference? A family gathering? The grocery store?
“There are some places you can’t avoid, but these decisions have caused a lot of strife for me,” she said. “The health and safety of our patients – that’s in our wheelhouse – but so many of the policies are outside of our control.”
The inevitable losses and the wins
For patients with metastatic disease, eventually the treatment options will run out.
Dr. Shatsky likes to be up front with patients about that reality: “There will come a day when I will tell you there’s nothing more I can do, and you need to trust that I’m being honest with you and that’s the truth.”
For Dr. Goodman, the devastation that bad news brings patients and families is glaring. He knows there will be no more normalcy in their lives.
“I see a lot of suffering, but I know the suffering happens regardless of whether I see it or not,” Dr. Goodman said.
That’s why holding on to the victories can be so important. Dr. Goodman recalled a young patient who came to him with a 20-cm tumor and is now cured. “Had I not met that individual and done what I had done, he’d be dead, but now he’s going to live his life,” Dr. Goodman said. “But I don’t wake up at 2 a.m. thinking about that.”
Dr. Shatsky gets a lot of joy from the wins – the patients who do really well, the times when she can help a friend or colleagues – and those moments go a long way to outweigh the hurt, worry, and workload.
When dealing with so much gray, “the wins are important, knowing you can make a difference is important,” Dr. Dizon said.
And there’s a delicate balance.
“I think patients want an oncologist who cares and is genuinely invested in their outcomes but not someone who is so sad all the time,” Dr. Lewis said. “When I lose a patient, I still grieve each loss, but I can’t mourn every patient’s death like it’s a family member. Otherwise, I’d break.”
What would you do if you had terminal cancer?
Dr. Dizon recalled how a friend handled the news. She went home and made dinner, he said.
Ultimately, she lived for many years. She saw her kids get married, met her first grandchild, and had time to prepare, something not everyone gets the chance to do.
That’s why it’s important to “do what you normally do as long as you can,” Dr. Dizon said. “Live your life.”
A version of this article first appeared on Medscape.com.
It was 2 a.m. And Rebecca Shatsky, MD, could not sleep.
The breast oncologist was thinking about a patient of hers with metastatic cancer.
The patient’s disease had been asymptomatic for some time. Then without warning, her cancer suddenly exploded. Her bone marrow was failing, and her liver was not far behind.
Dr. Shatsky had a treatment plan ready to go but still, she felt uneasy.
“I had to be honest with her that I didn’t know if this plan would work,” says Dr. Shatsky, a medical oncologist at University of California, San Diego (UCSD).
That night, after visiting the patient in the hospital, Dr. Shatsky lay awake going over her next move, making sure it was the right one and hoping it would help keep the disease at bay.
“It’s so much pressure when someone is depending on you to make life or death decisions,” Dr. Shatsky said.
And in the quiet hours of night, these concerns grow louder.
Dr. Shatsky is not alone.
“There’s no off button,” says Aaron Goodman, MD, a hematologist at UCSD Health who goes by “Papa Heme” on Twitter. “I’m always thinking about my patients. Constantly.”
The public rarely gets a glimpse of these private moments. On occasion, oncologists will share a personal story, but more often, insights come from broad research on the ethical, emotional, and psychological toll of practicing medicine.
Many oncologists carry this baggage home with them because they have no other option.
“There is simply no time to process the weight of the day when I’ve got seven more patients who need my full attention before lunch,” Mark Lewis, MD, director, department of gastrointestinal oncology, Intermountain Healthcare, Salt Lake City, Utah. “That is why my processing happens outside of the office, when my brain can be quiet.”
What am I missing?
Dr. Goodman recognizes the gravity of each decision he makes. He pores over every detail of a patient’s scans, lab results, history, and symptoms.
But no matter how many times he checks and rechecks, one question nags at him: What am I missing?
For Dr. Goodman, this exhaustive level of attention is worth it.
“When errors are made, it’s someone’s life,” Dr. Goodman said. “Nothing would have prepared me for this responsibility. Until it lies on you, it’s impossible to understand how much trust patients put into us.”
That trust becomes most apparent for Dr. Goodman when facing a decision about how to treat a patient with acute myeloid leukemia who’s in remission.
Give more chemotherapy to root out the leukemia cells still lurking in the body, and the patient faces a high risk of the cancer returning. Pick stem cell transplant, and the chance of being cured goes up significantly, but the patient could also die within 100 days of the transplant.
“All together, the data show I’m helping patients with a transplant, but for the individual, I could be causing harm. Someone could be living less because of a decision I made,” Dr. Goodman said.
For patients with advanced cancer, oncologists may need to think several moves ahead. Mapping out a patient’s treatment options can feel like a game of chess. Dr. Shatsky is always trying to anticipate how the tumor will behave, what is driving it, and how lifestyle factors may influence a patient’s response in the present and the future.
“It is a mind game,” she says. “Like in chess, I try to outsmart my opponent. But with advanced cancer, there are not necessarily clear-cut guidelines or one way to manage the disease, and I have to do the best I can with drugs I have.”
That’s the art of oncology: Balancing the many knowns and unknowns of a person’s cancer alongside the toxicities of treatment and a patient’s hopes and goals.
Throughout the year, Don Dizon, MD, will see a number of patients with advanced disease. In these instances, the question he often wrestles with is if the patient can’t be cured, whether more treatment will just cause greater harm.
Dr. Dizon recently faced this dilemma with an older patient with metastatic disease who had not done well with an initial treatment regimen. After outlining the risks for more chemotherapy, he explained one option would be to forgo it and simply treat her symptoms.
“It’s an impossible choice,” says Dr. Dizon, director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, Providence.
Chemotherapy can provide symptom relief, but it can also be toxic – and patients may be so frail, they can die from more therapy.
“I told my patient, if in your heart, you want to try more therapy, that’s okay. But it’s also okay if you don’t,” Dr. Dizon recalled.
Her response: “You’re supposed to give me the answer.”
However, for patients approaching the end of life, there often is no right answer.
“It’s part of the discomfort you live with as a patient and oncologist, and when I leave the clinic, that’s one thing that follows me home,” Dr. Dizon said. “At the end of the day, I need to look in the mirror and know I did the best I could.”
The difficult conversation
Every Sunday, Dr. Lewis feels the weight of the week ahead. He and his wife, a pediatrician, call it the “Sunday scaries.”
It’s when Dr. Lewis begins thinking about the delicate conversations to come, rehearsing how he’s going to share the news that a person has advanced cancer or that a cancer, once in remission, has returned.
“Before the pandemic, I had 36 people come to a visit where I delivered some very heavy news and it became a Greek chorus of sobbing,” he recalls.
For every oncologist, delivering bad news is an integral part of the job. But after spending months, sometimes years, with a patient and the family, Dr. Lewis knows how to take the temperature of the room – who will likely prefer a more blunt style and who might need a gentler touch.
“The longer you know a patient and family, the better you can gauge the best approach,” Dr. Lewis said. “And for some, you know it’ll be complete devastation no matter what.”
When Jennifer Lycette, MD, prepares for a difficult conversation, she’ll run down all the possible ways it could go. Sometimes her brain will get stuck in a loop, cycling through the different trajectories on repeat.
“For years, I didn’t know how to cope with that,” said Dr. Lycette, medical director at Providence Oncology and Hematology Care Clinic in Seaside, Ore. “I wasn’t taught the tools to cope with that in my medical training. It took midcareer professional coaching that I sought out on my own to learn to remind myself that no matter what the person says, I have the experience and skill set to handle what comes next and to simply be present in the moment with the patient.”
The question that now sits with Dr. Lycette hours after a visit is what she could have done better. She knows from experience how important it is to choose her words carefully.
Early in her career, Dr. Lycette had a patient with stage IV cancer who wanted to know more about the death process. Because most people ask about pain, she assured him that he likely wouldn’t experience too much pain with his type of cancer.
“It will probably be like falling asleep,” said Dr. Lycette, hoping she was offering comfort. “When I saw him next, he told me he hadn’t slept.”
He was afraid that if he did, he wouldn’t wake up.
In that moment, Dr. Lycette realized the power that her words carry and the importance of trying to understand the inner lives of her patients.
Life outside the clinic
Sometimes an oncologist’s late-night ruminations have little to do with cancer itself.
Manali Patel, MD, finds herself worrying if her patients will have enough to eat and whether she will be able to help.
“I was up at 3 a.m. one morning, thinking about how we’re going to fund a project for patients from low-income households who we discovered were experiencing severe food insecurity – what grants we need, what foundations we can work with,” said Dr. Patel, a medical oncologist at Stanford Hospital and Clinics and the VA Palo Alto Health Care System in California.
The past few years of the pandemic have added a new layer of worry for Dr. Patel.
“I don’t want my patients to die from a preventable virus when they’ve already been through so much suffering,” Dr. Patel said.
This thought feeds worries about how her actions outside the clinic could unintentionally harm her patients. Should she go to a big medical conference? A family gathering? The grocery store?
“There are some places you can’t avoid, but these decisions have caused a lot of strife for me,” she said. “The health and safety of our patients – that’s in our wheelhouse – but so many of the policies are outside of our control.”
The inevitable losses and the wins
For patients with metastatic disease, eventually the treatment options will run out.
Dr. Shatsky likes to be up front with patients about that reality: “There will come a day when I will tell you there’s nothing more I can do, and you need to trust that I’m being honest with you and that’s the truth.”
For Dr. Goodman, the devastation that bad news brings patients and families is glaring. He knows there will be no more normalcy in their lives.
“I see a lot of suffering, but I know the suffering happens regardless of whether I see it or not,” Dr. Goodman said.
That’s why holding on to the victories can be so important. Dr. Goodman recalled a young patient who came to him with a 20-cm tumor and is now cured. “Had I not met that individual and done what I had done, he’d be dead, but now he’s going to live his life,” Dr. Goodman said. “But I don’t wake up at 2 a.m. thinking about that.”
Dr. Shatsky gets a lot of joy from the wins – the patients who do really well, the times when she can help a friend or colleagues – and those moments go a long way to outweigh the hurt, worry, and workload.
When dealing with so much gray, “the wins are important, knowing you can make a difference is important,” Dr. Dizon said.
And there’s a delicate balance.
“I think patients want an oncologist who cares and is genuinely invested in their outcomes but not someone who is so sad all the time,” Dr. Lewis said. “When I lose a patient, I still grieve each loss, but I can’t mourn every patient’s death like it’s a family member. Otherwise, I’d break.”
What would you do if you had terminal cancer?
Dr. Dizon recalled how a friend handled the news. She went home and made dinner, he said.
Ultimately, she lived for many years. She saw her kids get married, met her first grandchild, and had time to prepare, something not everyone gets the chance to do.
That’s why it’s important to “do what you normally do as long as you can,” Dr. Dizon said. “Live your life.”
A version of this article first appeared on Medscape.com.
When too much treatment creates more harm than good
Ann Marco, 73, who was diagnosed with ovarian cancer in late 2018, credits her oncology team for saving her life. They treated her with chemotherapy, debulking surgery, and more chemotherapy. But it is her second and current care team that helped restore Ms. Marco’s quality of life, directing her toward such resources as palliative care, physical therapy and counseling for her and her husband.
“I can’t say enough about my palliative care doctor. She helped me manage pain, and the fatigue associated with chemotherapy. When she noticed that my leg was swollen she suspected a blood clot and sent me for an ultrasound,” Ms. Marco said.
The ultrasound revealed that she did indeed have a blood clot, for which she received, and continues to receive, medication. “Because with ovarian cancer, you always have blood clots. So little things like that, though they’re not that little, have really helped me in my journey with this cancer,” Ms. Marco said.
That journey has had its ups and downs. One chemotherapy regimen was so intolerable she decided to discontinue it, with full support of her oncologist. I told her, I just want to live my life, whether that’s only 6 more months or 3 years, but I don’t want to live it like this. And she said, ‘Ann, we’re going to do what you want to do.’”
Nine months later, when her cancer started growing again, Ms. Marco returned to chemotherapy. But this regimen has been much more tolerable, and it also appears to be doing its job. A recent CT scan showed that the tumors are shrinking.
“They’ll never go away. I have metastatic cancer. But they’re smaller, and I was really thrilled about that. It’s the best news I’ve had in more than 3 years,” Ms. Marco said.
End-of-life aggressive care still common
, shows a study published in JCO Oncology Practice.
“We have good evidence that the types of aggressive end-of-life care we looked at in this paper are generally related to a lower quality of life for patients, poorer bereavement outcomes for their families, and even shorter duration survivals,” said lead author Megan A. Mullins, PhD, MPH, a postdoctoral research fellow at the University of Michigan in Ann Arbor. “This suggests there’s a disconnect between what people think aggressive care might do and what it’s doing.”
In their evaluation of variation in end-of-life care, Dr. Mullins and her colleagues analyzed SEER-Medicare data on 6,288 women with ovarian cancer who died between 2016 and 2020. They found that 51% of those women received some form of aggressive cancer care. The most common forms were not being admitted to hospice (28.9%), receiving an invasive procedure (20.7%) and being admitted to an intensive care unit (18.6%).
Dr. Mullins noted that since palliative care was officially recognized as a specialty in 2006, there has been increasing guidance for earlier integration of palliative care and reducing the aggressiveness of end-of-life care; both ASCO and the National Quality Form have standards advising against aggressive end-of-life care.
“But there are a lot of complicated factors that I think make it hard to move the needle in this area,” she said. “For one thing, particularly with ovarian cancer, women tend to have recurrences. I’ve spoken with physicians who got their patients through a difficult patch; they rebounded and they did fine. You don’t know for sure if that’s going to happen again if you try something else. Prognostication is not an exact science.”
Also, end-of-life discussions can be challenging conversations. “Nobody wants to take hope away from their patients. But there’s evidence to show that these conversations don’t actually reduce patients’ hopes – that’s a misconception,” Dr. Mullins said.
“It’s challenging. In the United States, we don’t like to talk about death and dying. But I think having these conversations earlier and more often can help make them a more regular part of care,” she said.
Brittany A. Davidson, MD, a gynecologic oncologist with Duke Health in Durham, N.C., who wrote an accompanying editorial, acknowledges that end-of-life can be fraught with fear, anxiety, and a lot of emotion. But she finds helping patients and their families navigate the ups and downs of their cancer one of the most rewarding aspects of her career as a physician.
“We want to help patients and their family members make these transitions as smoothly as possible,” she said.
A proponent of communications skills training for physicians in general, Dr. Brittany said doctors can learn to identify cues that patients are ready to have conversations about their end-of-life care.
“Those cues will help us facilitate conversations sooner rather than later so we’re not waiting until the very end,” she said.
What these conversations consist of varies depending on where the patient is in her cancer trajectory. In a patient with recurrent ovarian or recurrent uterine cancer, this might start with making sure the patient understands that while their cancer is treatable, it is very unlikely to be curable.
“I have often had patients who have been treated for cancer for several years and didn’t know their cancer wasn’t curable. How many missed opportunities have we overlooked?” Dr. Davidson said.
Then the conversation can turn to the goals of treatment. What’s important to the patient? “Are there events they want to be around for? Symptoms they want to avoid? Some patients really want to know what it’s going to be like to die. I try to take the lead from the patient. Ask what kind of information is helpful to them. Is it numbers? Is it symptoms? It’s really different for everybody,” Dr. Davidson said.
Although Dr. Mullins’s research and Dr. Davidson’s editorial suggest there’s room for improvement toward achieving goal-concordant care in gynecological cancers, Dr. Davidson suspects these patients might be faring a bit better than patients with other types of cancer based on her own anecdotal observations.
“One of the unique things about gynecologic oncology is that we have an amazing longitudinal relationship with our patients – we are not only their surgeons, we’re their oncologists. In other solid tumors, care is fractionated.
“That’s one of the reasons I love gynecologic oncology. I have the opportunity to know my patients through all the stages they experience as part of their cancer. I’d like to think that allows me a better opportunity to get to know them and help them recognize the value of palliative care,” Dr. Mullins said.
Ann Marco, 73, who was diagnosed with ovarian cancer in late 2018, credits her oncology team for saving her life. They treated her with chemotherapy, debulking surgery, and more chemotherapy. But it is her second and current care team that helped restore Ms. Marco’s quality of life, directing her toward such resources as palliative care, physical therapy and counseling for her and her husband.
“I can’t say enough about my palliative care doctor. She helped me manage pain, and the fatigue associated with chemotherapy. When she noticed that my leg was swollen she suspected a blood clot and sent me for an ultrasound,” Ms. Marco said.
The ultrasound revealed that she did indeed have a blood clot, for which she received, and continues to receive, medication. “Because with ovarian cancer, you always have blood clots. So little things like that, though they’re not that little, have really helped me in my journey with this cancer,” Ms. Marco said.
That journey has had its ups and downs. One chemotherapy regimen was so intolerable she decided to discontinue it, with full support of her oncologist. I told her, I just want to live my life, whether that’s only 6 more months or 3 years, but I don’t want to live it like this. And she said, ‘Ann, we’re going to do what you want to do.’”
Nine months later, when her cancer started growing again, Ms. Marco returned to chemotherapy. But this regimen has been much more tolerable, and it also appears to be doing its job. A recent CT scan showed that the tumors are shrinking.
“They’ll never go away. I have metastatic cancer. But they’re smaller, and I was really thrilled about that. It’s the best news I’ve had in more than 3 years,” Ms. Marco said.
End-of-life aggressive care still common
, shows a study published in JCO Oncology Practice.
“We have good evidence that the types of aggressive end-of-life care we looked at in this paper are generally related to a lower quality of life for patients, poorer bereavement outcomes for their families, and even shorter duration survivals,” said lead author Megan A. Mullins, PhD, MPH, a postdoctoral research fellow at the University of Michigan in Ann Arbor. “This suggests there’s a disconnect between what people think aggressive care might do and what it’s doing.”
In their evaluation of variation in end-of-life care, Dr. Mullins and her colleagues analyzed SEER-Medicare data on 6,288 women with ovarian cancer who died between 2016 and 2020. They found that 51% of those women received some form of aggressive cancer care. The most common forms were not being admitted to hospice (28.9%), receiving an invasive procedure (20.7%) and being admitted to an intensive care unit (18.6%).
Dr. Mullins noted that since palliative care was officially recognized as a specialty in 2006, there has been increasing guidance for earlier integration of palliative care and reducing the aggressiveness of end-of-life care; both ASCO and the National Quality Form have standards advising against aggressive end-of-life care.
“But there are a lot of complicated factors that I think make it hard to move the needle in this area,” she said. “For one thing, particularly with ovarian cancer, women tend to have recurrences. I’ve spoken with physicians who got their patients through a difficult patch; they rebounded and they did fine. You don’t know for sure if that’s going to happen again if you try something else. Prognostication is not an exact science.”
Also, end-of-life discussions can be challenging conversations. “Nobody wants to take hope away from their patients. But there’s evidence to show that these conversations don’t actually reduce patients’ hopes – that’s a misconception,” Dr. Mullins said.
“It’s challenging. In the United States, we don’t like to talk about death and dying. But I think having these conversations earlier and more often can help make them a more regular part of care,” she said.
Brittany A. Davidson, MD, a gynecologic oncologist with Duke Health in Durham, N.C., who wrote an accompanying editorial, acknowledges that end-of-life can be fraught with fear, anxiety, and a lot of emotion. But she finds helping patients and their families navigate the ups and downs of their cancer one of the most rewarding aspects of her career as a physician.
“We want to help patients and their family members make these transitions as smoothly as possible,” she said.
A proponent of communications skills training for physicians in general, Dr. Brittany said doctors can learn to identify cues that patients are ready to have conversations about their end-of-life care.
“Those cues will help us facilitate conversations sooner rather than later so we’re not waiting until the very end,” she said.
What these conversations consist of varies depending on where the patient is in her cancer trajectory. In a patient with recurrent ovarian or recurrent uterine cancer, this might start with making sure the patient understands that while their cancer is treatable, it is very unlikely to be curable.
“I have often had patients who have been treated for cancer for several years and didn’t know their cancer wasn’t curable. How many missed opportunities have we overlooked?” Dr. Davidson said.
Then the conversation can turn to the goals of treatment. What’s important to the patient? “Are there events they want to be around for? Symptoms they want to avoid? Some patients really want to know what it’s going to be like to die. I try to take the lead from the patient. Ask what kind of information is helpful to them. Is it numbers? Is it symptoms? It’s really different for everybody,” Dr. Davidson said.
Although Dr. Mullins’s research and Dr. Davidson’s editorial suggest there’s room for improvement toward achieving goal-concordant care in gynecological cancers, Dr. Davidson suspects these patients might be faring a bit better than patients with other types of cancer based on her own anecdotal observations.
“One of the unique things about gynecologic oncology is that we have an amazing longitudinal relationship with our patients – we are not only their surgeons, we’re their oncologists. In other solid tumors, care is fractionated.
“That’s one of the reasons I love gynecologic oncology. I have the opportunity to know my patients through all the stages they experience as part of their cancer. I’d like to think that allows me a better opportunity to get to know them and help them recognize the value of palliative care,” Dr. Mullins said.
Ann Marco, 73, who was diagnosed with ovarian cancer in late 2018, credits her oncology team for saving her life. They treated her with chemotherapy, debulking surgery, and more chemotherapy. But it is her second and current care team that helped restore Ms. Marco’s quality of life, directing her toward such resources as palliative care, physical therapy and counseling for her and her husband.
“I can’t say enough about my palliative care doctor. She helped me manage pain, and the fatigue associated with chemotherapy. When she noticed that my leg was swollen she suspected a blood clot and sent me for an ultrasound,” Ms. Marco said.
The ultrasound revealed that she did indeed have a blood clot, for which she received, and continues to receive, medication. “Because with ovarian cancer, you always have blood clots. So little things like that, though they’re not that little, have really helped me in my journey with this cancer,” Ms. Marco said.
That journey has had its ups and downs. One chemotherapy regimen was so intolerable she decided to discontinue it, with full support of her oncologist. I told her, I just want to live my life, whether that’s only 6 more months or 3 years, but I don’t want to live it like this. And she said, ‘Ann, we’re going to do what you want to do.’”
Nine months later, when her cancer started growing again, Ms. Marco returned to chemotherapy. But this regimen has been much more tolerable, and it also appears to be doing its job. A recent CT scan showed that the tumors are shrinking.
“They’ll never go away. I have metastatic cancer. But they’re smaller, and I was really thrilled about that. It’s the best news I’ve had in more than 3 years,” Ms. Marco said.
End-of-life aggressive care still common
, shows a study published in JCO Oncology Practice.
“We have good evidence that the types of aggressive end-of-life care we looked at in this paper are generally related to a lower quality of life for patients, poorer bereavement outcomes for their families, and even shorter duration survivals,” said lead author Megan A. Mullins, PhD, MPH, a postdoctoral research fellow at the University of Michigan in Ann Arbor. “This suggests there’s a disconnect between what people think aggressive care might do and what it’s doing.”
In their evaluation of variation in end-of-life care, Dr. Mullins and her colleagues analyzed SEER-Medicare data on 6,288 women with ovarian cancer who died between 2016 and 2020. They found that 51% of those women received some form of aggressive cancer care. The most common forms were not being admitted to hospice (28.9%), receiving an invasive procedure (20.7%) and being admitted to an intensive care unit (18.6%).
Dr. Mullins noted that since palliative care was officially recognized as a specialty in 2006, there has been increasing guidance for earlier integration of palliative care and reducing the aggressiveness of end-of-life care; both ASCO and the National Quality Form have standards advising against aggressive end-of-life care.
“But there are a lot of complicated factors that I think make it hard to move the needle in this area,” she said. “For one thing, particularly with ovarian cancer, women tend to have recurrences. I’ve spoken with physicians who got their patients through a difficult patch; they rebounded and they did fine. You don’t know for sure if that’s going to happen again if you try something else. Prognostication is not an exact science.”
Also, end-of-life discussions can be challenging conversations. “Nobody wants to take hope away from their patients. But there’s evidence to show that these conversations don’t actually reduce patients’ hopes – that’s a misconception,” Dr. Mullins said.
“It’s challenging. In the United States, we don’t like to talk about death and dying. But I think having these conversations earlier and more often can help make them a more regular part of care,” she said.
Brittany A. Davidson, MD, a gynecologic oncologist with Duke Health in Durham, N.C., who wrote an accompanying editorial, acknowledges that end-of-life can be fraught with fear, anxiety, and a lot of emotion. But she finds helping patients and their families navigate the ups and downs of their cancer one of the most rewarding aspects of her career as a physician.
“We want to help patients and their family members make these transitions as smoothly as possible,” she said.
A proponent of communications skills training for physicians in general, Dr. Brittany said doctors can learn to identify cues that patients are ready to have conversations about their end-of-life care.
“Those cues will help us facilitate conversations sooner rather than later so we’re not waiting until the very end,” she said.
What these conversations consist of varies depending on where the patient is in her cancer trajectory. In a patient with recurrent ovarian or recurrent uterine cancer, this might start with making sure the patient understands that while their cancer is treatable, it is very unlikely to be curable.
“I have often had patients who have been treated for cancer for several years and didn’t know their cancer wasn’t curable. How many missed opportunities have we overlooked?” Dr. Davidson said.
Then the conversation can turn to the goals of treatment. What’s important to the patient? “Are there events they want to be around for? Symptoms they want to avoid? Some patients really want to know what it’s going to be like to die. I try to take the lead from the patient. Ask what kind of information is helpful to them. Is it numbers? Is it symptoms? It’s really different for everybody,” Dr. Davidson said.
Although Dr. Mullins’s research and Dr. Davidson’s editorial suggest there’s room for improvement toward achieving goal-concordant care in gynecological cancers, Dr. Davidson suspects these patients might be faring a bit better than patients with other types of cancer based on her own anecdotal observations.
“One of the unique things about gynecologic oncology is that we have an amazing longitudinal relationship with our patients – we are not only their surgeons, we’re their oncologists. In other solid tumors, care is fractionated.
“That’s one of the reasons I love gynecologic oncology. I have the opportunity to know my patients through all the stages they experience as part of their cancer. I’d like to think that allows me a better opportunity to get to know them and help them recognize the value of palliative care,” Dr. Mullins said.
A Quantification Method to Compare the Value of Surgery and Palliative Care in Patients With Complex Cardiac Disease: A Concept
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.
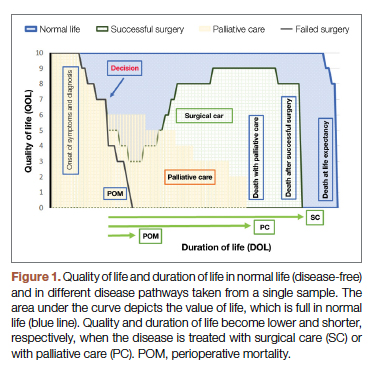
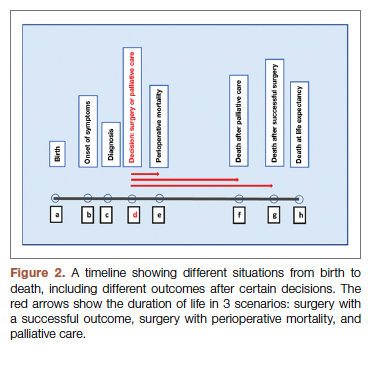
Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (
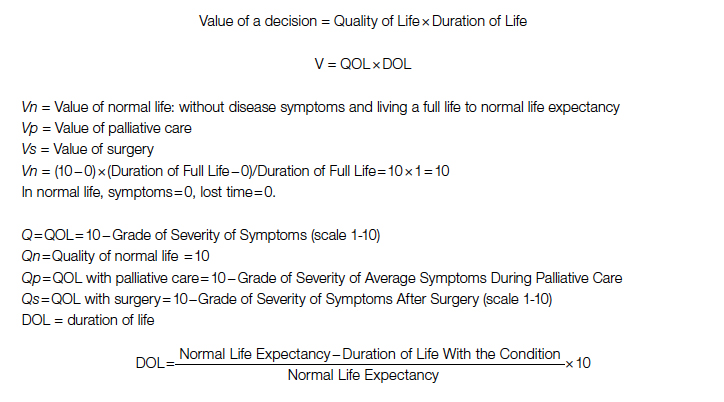
For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
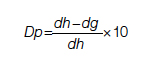
Duration for surgery:
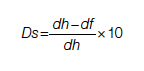
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
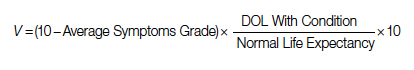
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (
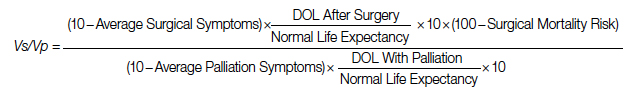
After elimination of normal life expectancy, form the numerator and denominator:
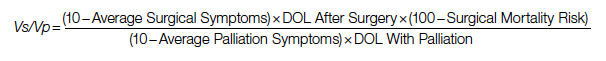
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
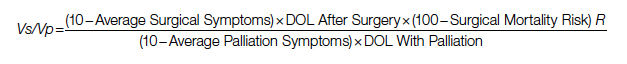
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
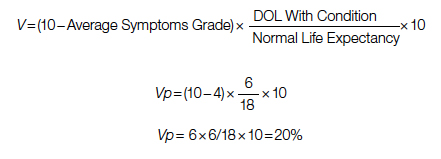
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
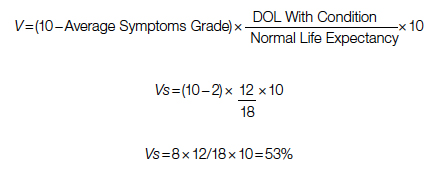
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.


Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (

For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
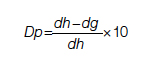
Duration for surgery:
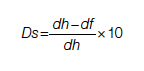
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
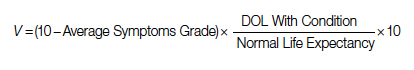
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (

After elimination of normal life expectancy, form the numerator and denominator:
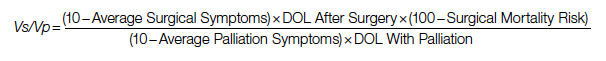
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
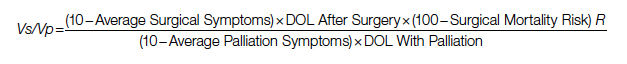
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
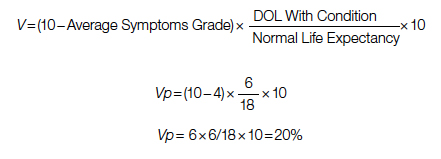
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
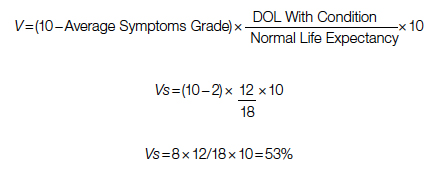
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.


Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (

For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
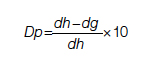
Duration for surgery:
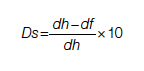
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
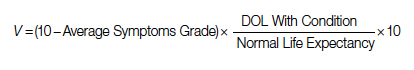
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (

After elimination of normal life expectancy, form the numerator and denominator:
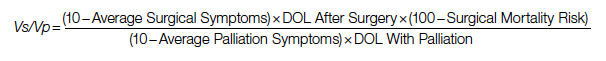
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
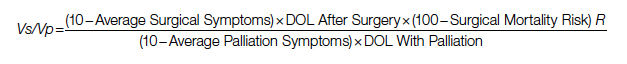
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
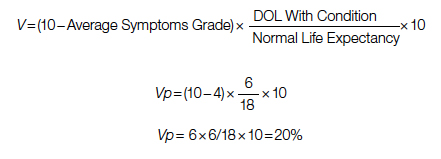
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
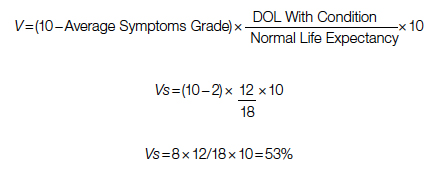
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
Doc accused of killing 14 patients found not guilty
In an unprecedented murder case about end-of-life care, a physician accused of killing 14 critically ill patients with opioid overdoses in a Columbus, Ohio, hospital ICU over a period of 4 years was found not guilty by a jury April 20.
The jury, after a 7-week trial featuring more than 50 witnesses in the Franklin County Court of Common Pleas, declared William Husel, DO, not guilty on 14 counts of murder and attempted murder.
In a news conference after the verdict was announced, lead defense attorney Jose Baez said Dr. Husel, whom he called a “great doctor,” hopes to practice medicine again in the future. “They don’t need to be looking over their shoulders worrying about whether they’ll get charged with crimes,” he said.
The prosecutors in the case declined to comment, other than to say they “accept” the verdict.
Legal experts said it’s highly unlikely that Ohio or any other state would restore Dr. Husel’s suspended medical license. “I doubt he could ever work in medicine again,” said Mark Schumacher, a Columbus medical malpractice defense attorney who retired in 2020 after practicing for 39 years. Mr. Schumacher followed the trial closely.
The trial raised the specific issue of what constitutes a medically justifiable dose of opioid painkillers during the end-of-life procedure known as palliative extubation, in which critically ill patients are withdrawn from the ventilator when they are expected to die. Under medicine’s so-called double-effect principle, physicians must weigh the benefits and risks of ordering potentially lethal doses of painkillers and sedatives to provide comfort care for critically ill patients.
To many observers, however, the case really centered on the largely hidden debate over whether it’s acceptable to hasten the deaths of dying patients who haven’t chosen that path. That’s called euthanasia, which is illegal in the United States. In contrast, 10 states plus the District of Columbia allow physicians to prescribe lethal drugs to terminally ill, mentally competent adults who can self-administer them. That’s called medical aid in dying, or physician-assisted dying or suicide.
“Maybe this is a wake-up call that people believe this is the right thing to do,” said Lewis Nelson, MD, chair of emergency medicine at New Jersey Medical School in Newark. “The medical community has a sense that we often prolong life unnecessarily. But a physician cannot unilaterally decide it’s time for someone to die. It sounds like [Dr. Husel] took that decision into his own hands.”
The case also exposed major gaps in the patient safety culture at Mount Carmel West Hospital in Columbus, which is owned by the Catholic chain Trinity Health. Experts say failures by hospital staff to question physicians’ orders and raise patient safety concerns, as happened at Mount Carmel, occur at many hospitals around the country. Experts say the Husel case offers vital lessons for health care professionals about improving safety procedures.
“This is an extreme example, that everyone should learn from, about what not to do,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “Husel was giving massive overdoses of drugs, people knew it was problematic, and someone didn’t put their foot down. You’ve got to have a process in place to address these situations where there is disagreement over the safety of a medication order.”
Dr. Husel was charged with killing the 14 patients from 2015 through 2018 by ordering single large doses of the painkiller fentanyl – from 500 to 2,000 micrograms – often in combination with other opioids and sedatives, while working as the solo physician on the overnight shift in the ICU at Mount Carmel West Hospital and at Mount Carmel St. Ann’s Hospital in Westerville, Ohio.
Dr. Husel ordered administration of the drugs while his patients were having an endotracheal tube removed as part of palliative extubation. There was conflicting testimony during the trial about whether the patients were showing signs of pain or were even capable of feeling pain.
Prosecutors argued that Dr. Husel, who did a residency and fellowship in critical care medicine at Cleveland Clinic and started working at Mount Carmel in 2013 in his first job as a full-fledged physician, intended to kill the patients or hasten their deaths. They contended that the inexperienced nurses in the ICU went along with his large drug doses because they were “in thrall” to him because of his prestigious background at the Cleveland Clinic and his willingness to take the time to teach them.
“With his training in anesthesiology, he knew what those drugs do,” assistant prosecutor David Zeyen said in closing arguments. “This isn’t negligence. This is on purpose ... Euthanizing animals with the intent to kill is fine in veterinary medicine. It’s not fine in the ICU at Mount Carmel or anywhere.”
The defense team argued that Dr. Husel was a caring and compassionate physician who ordered the drugs to relieve the patients’ pain and discomfort during the extubation process. He did not testify.
“Common sense says Dr Husel had no motive to harm patients,” defense attorney Baez said in his closing. “He dedicated his life to taking care of patients and saving lives, not taking them. ... Why would this man risk his family, career, and 17 years of trying to be a doctor to hasten someone’s death or kill them?”
There were 35 Mount Carmel patients who died in the ICU under Dr. Husel’s care after receiving large fentanyl doses during palliative extubation. The state originally charged him with murder in 25 of those cases, then reduced that to 14.
Many of Dr. Husel’s drug orders were given verbally instead of through the regular process of being entered into the electronic health record. He and the nurses on duty also skipped the standard nonemergency process of getting approval from the pharmacist on duty, instead using the override function on Mount Carmel’s automated Pyxis drug dispensing system.
Dr. Husel’s unusual dosing patterns were first reported to Mount Carmel officials by pharmacists in October 2018, spurring an investigation. The hospital system let him go in December 2018, after concluding that the opioid dosages he used were “significantly excessive and potentially fatal” and “went beyond providing comfort.”
Nearly two dozen RNs and two pharmacists involved in these cases have faced state disciplinary action, mostly license suspension. Federal and state agencies have cited the Mount Carmel system for faults in its patient safety processes and culture that were exposed by the Husel cases.
The Mount Carmel CEO; the chief clinical officer; other physician, nursing, and pharmacy leaders; and dozens of nurses and pharmacists were forced out following the Husel investigation.
In 2019, the Centers for Medicare & Medicaid Services, after threatening to cut off federal reimbursements to Mount Carmel, accepted the hospital system’s correction plan restricting the use of verbal drug orders and prohibiting Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Mount Carmel and Trinity have settled a number of civil wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. Gerry Leeseberg, a Columbus medical malpractice plaintiff attorney who is representing 17 of the families, said a number of the cases are set for trial starting in June.
During the trial, family members of many of the 14 patients whom Dr. Husel allegedly murdered testified that Dr. Husel told them their loved ones were dying. Some said they felt rushed into making a decision to extubate the person.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved ones gasping for breath. But most medical experts – including the state’s two physician expert witnesses – say the fentanyl doses Dr. Husel ordered were 5-20 times larger than doses normally used in palliative extubation. Such doses, they say, will quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 micrograms for relieving pain, and its 2018 guidelines reduced that to 25-50 micrograms.
The doses Dr. Husel ordered are lethal, even for most patients with some tolerance to opioids, said Rutgers EM chair Dr. Nelson, who practices medical toxicology and addiction medicine. “Those are doses to provide euthanasia, not to relieve pain.”
At trial, the prosecutors had to overcome two big challenges to win murder convictions against Dr. Husel: They had to prove beyond a reasonable doubt that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Late in the trial, at the state’s request, the judge ruled to allow the jury to also consider attempted murder charges, which require proof of intent but not that the defendant’s actions directly caused the deaths.
Another challenge was that physicians have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia. That’s what prosecutors accused Dr. Husel of doing.
“If you hasten a person’s death, even if their death is as sure as the sun is going to rise in the morning ... you have caused their death in the eyes of the law,” assistant prosecutor Mr. Zeyen said in his closing. “You don’t get a pass for killing a dying man ...”
Mr. Leeseberg said it was always going to be extremely hard to convince a jury to convict a physician of murder, with the potential of life in prison, in a case where the physician’s acts occurred openly over 4 years in a hospital setting where no one did anything to stop him. It would have been much easier to convince a jury to convict him of reckless homicide, a lesser offense with a shorter prison term. That would have required proving that he acted in reckless disregard for his patients’ health and safety.
In post-verdict comments to the news media, Judge Michael Holbrook said jurors told him that the procedures for the dispensing of fentanyl and other drugs at Mount Carmel weren’t properly explained to them during the trial, and that they were confused by the large number of prosecution witnesses. He also said they were confused that no one had stated a maximum dosage for fentanyl.
Mr. Schumacher, the retired malpractice defense lawyer who followed the trial, disagreed with defense attorney Baez’s takeaway about the impact of the case on pain-relief practices. In his view, the case likely will heighten rather than reduce the anxiety of physicians and nurses about administering opioids, even when the dosages are clearly needed and appropriate. He doesn’t think Dr. Husel’s dosages can be justified, however.
“Physicians have a naive overreaction to any legal development, and overgeneralize from a particular case to everyday practice,” he said.
There is only one known prior case that’s somewhat comparable of a physician tried for murder or attempted murder for giving a critically ill patient opioids for pain relief. In 1996, a Kansas jury convicted Lloyd Stanley Naramore, DO, of attempted murder in the death of a patient to whom he gave an opioid, and of second-degree murder for removing a patient from a ventilator. After Dr. Naramore served 6 months in prison, an appellate court reversed the convictions for lack of evidence.
In March, RaDonda Vaught, a nurse who worked at Vanderbilt University Medical Center in Nashville, Tenn., was convicted of criminal neglect and negligent homicide for mistakenly administering a fatal dose of the paralyzing drug vecuronium, instead of the prescribed drug Versed (midazolam), to a patient. Providers around the country were alarmed by her criminal prosecution for what was clearly an unintentional error.
But legal and medical experts said Dr. Husel’s case was sharply different from Vaught’s and Dr. Naramore’s because he deliberately and repeatedly ordered large doses of fentanyl and other drugs that he knew or should have known were potentially lethal. “You don’t need 2,000 micrograms of fentanyl plus other drugs for comfort care, and repeat that again and again for patient after patient,” said Mr. Cohen, of the Institute for Safe Medication Practices. “No one gives that to patients. You’ll knock them off.”
During the trial, prosecutors said repeatedly that no one except Dr. Husel knows what he was thinking when he ordered those huge drug dosages for his ICU patients. Judge Holbrook told the jury the state did not have to prove motive, only intent. But many observers still have wondered what his motives were.
Dr. Husel’s own view of his care in these cases soon will become public. Immediately after the April 20 verdict, Mr. Leeseberg filed a notice requesting a May 9 deposition of Dr. Husel, who will no longer be able to claim the Fifth Amendment right against self-incrimination. He predicted the deposition will last about a week, and then the transcript will be publicly available.
A version of this article first appeared on Medscape.com.
In an unprecedented murder case about end-of-life care, a physician accused of killing 14 critically ill patients with opioid overdoses in a Columbus, Ohio, hospital ICU over a period of 4 years was found not guilty by a jury April 20.
The jury, after a 7-week trial featuring more than 50 witnesses in the Franklin County Court of Common Pleas, declared William Husel, DO, not guilty on 14 counts of murder and attempted murder.
In a news conference after the verdict was announced, lead defense attorney Jose Baez said Dr. Husel, whom he called a “great doctor,” hopes to practice medicine again in the future. “They don’t need to be looking over their shoulders worrying about whether they’ll get charged with crimes,” he said.
The prosecutors in the case declined to comment, other than to say they “accept” the verdict.
Legal experts said it’s highly unlikely that Ohio or any other state would restore Dr. Husel’s suspended medical license. “I doubt he could ever work in medicine again,” said Mark Schumacher, a Columbus medical malpractice defense attorney who retired in 2020 after practicing for 39 years. Mr. Schumacher followed the trial closely.
The trial raised the specific issue of what constitutes a medically justifiable dose of opioid painkillers during the end-of-life procedure known as palliative extubation, in which critically ill patients are withdrawn from the ventilator when they are expected to die. Under medicine’s so-called double-effect principle, physicians must weigh the benefits and risks of ordering potentially lethal doses of painkillers and sedatives to provide comfort care for critically ill patients.
To many observers, however, the case really centered on the largely hidden debate over whether it’s acceptable to hasten the deaths of dying patients who haven’t chosen that path. That’s called euthanasia, which is illegal in the United States. In contrast, 10 states plus the District of Columbia allow physicians to prescribe lethal drugs to terminally ill, mentally competent adults who can self-administer them. That’s called medical aid in dying, or physician-assisted dying or suicide.
“Maybe this is a wake-up call that people believe this is the right thing to do,” said Lewis Nelson, MD, chair of emergency medicine at New Jersey Medical School in Newark. “The medical community has a sense that we often prolong life unnecessarily. But a physician cannot unilaterally decide it’s time for someone to die. It sounds like [Dr. Husel] took that decision into his own hands.”
The case also exposed major gaps in the patient safety culture at Mount Carmel West Hospital in Columbus, which is owned by the Catholic chain Trinity Health. Experts say failures by hospital staff to question physicians’ orders and raise patient safety concerns, as happened at Mount Carmel, occur at many hospitals around the country. Experts say the Husel case offers vital lessons for health care professionals about improving safety procedures.
“This is an extreme example, that everyone should learn from, about what not to do,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “Husel was giving massive overdoses of drugs, people knew it was problematic, and someone didn’t put their foot down. You’ve got to have a process in place to address these situations where there is disagreement over the safety of a medication order.”
Dr. Husel was charged with killing the 14 patients from 2015 through 2018 by ordering single large doses of the painkiller fentanyl – from 500 to 2,000 micrograms – often in combination with other opioids and sedatives, while working as the solo physician on the overnight shift in the ICU at Mount Carmel West Hospital and at Mount Carmel St. Ann’s Hospital in Westerville, Ohio.
Dr. Husel ordered administration of the drugs while his patients were having an endotracheal tube removed as part of palliative extubation. There was conflicting testimony during the trial about whether the patients were showing signs of pain or were even capable of feeling pain.
Prosecutors argued that Dr. Husel, who did a residency and fellowship in critical care medicine at Cleveland Clinic and started working at Mount Carmel in 2013 in his first job as a full-fledged physician, intended to kill the patients or hasten their deaths. They contended that the inexperienced nurses in the ICU went along with his large drug doses because they were “in thrall” to him because of his prestigious background at the Cleveland Clinic and his willingness to take the time to teach them.
“With his training in anesthesiology, he knew what those drugs do,” assistant prosecutor David Zeyen said in closing arguments. “This isn’t negligence. This is on purpose ... Euthanizing animals with the intent to kill is fine in veterinary medicine. It’s not fine in the ICU at Mount Carmel or anywhere.”
The defense team argued that Dr. Husel was a caring and compassionate physician who ordered the drugs to relieve the patients’ pain and discomfort during the extubation process. He did not testify.
“Common sense says Dr Husel had no motive to harm patients,” defense attorney Baez said in his closing. “He dedicated his life to taking care of patients and saving lives, not taking them. ... Why would this man risk his family, career, and 17 years of trying to be a doctor to hasten someone’s death or kill them?”
There were 35 Mount Carmel patients who died in the ICU under Dr. Husel’s care after receiving large fentanyl doses during palliative extubation. The state originally charged him with murder in 25 of those cases, then reduced that to 14.
Many of Dr. Husel’s drug orders were given verbally instead of through the regular process of being entered into the electronic health record. He and the nurses on duty also skipped the standard nonemergency process of getting approval from the pharmacist on duty, instead using the override function on Mount Carmel’s automated Pyxis drug dispensing system.
Dr. Husel’s unusual dosing patterns were first reported to Mount Carmel officials by pharmacists in October 2018, spurring an investigation. The hospital system let him go in December 2018, after concluding that the opioid dosages he used were “significantly excessive and potentially fatal” and “went beyond providing comfort.”
Nearly two dozen RNs and two pharmacists involved in these cases have faced state disciplinary action, mostly license suspension. Federal and state agencies have cited the Mount Carmel system for faults in its patient safety processes and culture that were exposed by the Husel cases.
The Mount Carmel CEO; the chief clinical officer; other physician, nursing, and pharmacy leaders; and dozens of nurses and pharmacists were forced out following the Husel investigation.
In 2019, the Centers for Medicare & Medicaid Services, after threatening to cut off federal reimbursements to Mount Carmel, accepted the hospital system’s correction plan restricting the use of verbal drug orders and prohibiting Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Mount Carmel and Trinity have settled a number of civil wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. Gerry Leeseberg, a Columbus medical malpractice plaintiff attorney who is representing 17 of the families, said a number of the cases are set for trial starting in June.
During the trial, family members of many of the 14 patients whom Dr. Husel allegedly murdered testified that Dr. Husel told them their loved ones were dying. Some said they felt rushed into making a decision to extubate the person.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved ones gasping for breath. But most medical experts – including the state’s two physician expert witnesses – say the fentanyl doses Dr. Husel ordered were 5-20 times larger than doses normally used in palliative extubation. Such doses, they say, will quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 micrograms for relieving pain, and its 2018 guidelines reduced that to 25-50 micrograms.
The doses Dr. Husel ordered are lethal, even for most patients with some tolerance to opioids, said Rutgers EM chair Dr. Nelson, who practices medical toxicology and addiction medicine. “Those are doses to provide euthanasia, not to relieve pain.”
At trial, the prosecutors had to overcome two big challenges to win murder convictions against Dr. Husel: They had to prove beyond a reasonable doubt that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Late in the trial, at the state’s request, the judge ruled to allow the jury to also consider attempted murder charges, which require proof of intent but not that the defendant’s actions directly caused the deaths.
Another challenge was that physicians have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia. That’s what prosecutors accused Dr. Husel of doing.
“If you hasten a person’s death, even if their death is as sure as the sun is going to rise in the morning ... you have caused their death in the eyes of the law,” assistant prosecutor Mr. Zeyen said in his closing. “You don’t get a pass for killing a dying man ...”
Mr. Leeseberg said it was always going to be extremely hard to convince a jury to convict a physician of murder, with the potential of life in prison, in a case where the physician’s acts occurred openly over 4 years in a hospital setting where no one did anything to stop him. It would have been much easier to convince a jury to convict him of reckless homicide, a lesser offense with a shorter prison term. That would have required proving that he acted in reckless disregard for his patients’ health and safety.
In post-verdict comments to the news media, Judge Michael Holbrook said jurors told him that the procedures for the dispensing of fentanyl and other drugs at Mount Carmel weren’t properly explained to them during the trial, and that they were confused by the large number of prosecution witnesses. He also said they were confused that no one had stated a maximum dosage for fentanyl.
Mr. Schumacher, the retired malpractice defense lawyer who followed the trial, disagreed with defense attorney Baez’s takeaway about the impact of the case on pain-relief practices. In his view, the case likely will heighten rather than reduce the anxiety of physicians and nurses about administering opioids, even when the dosages are clearly needed and appropriate. He doesn’t think Dr. Husel’s dosages can be justified, however.
“Physicians have a naive overreaction to any legal development, and overgeneralize from a particular case to everyday practice,” he said.
There is only one known prior case that’s somewhat comparable of a physician tried for murder or attempted murder for giving a critically ill patient opioids for pain relief. In 1996, a Kansas jury convicted Lloyd Stanley Naramore, DO, of attempted murder in the death of a patient to whom he gave an opioid, and of second-degree murder for removing a patient from a ventilator. After Dr. Naramore served 6 months in prison, an appellate court reversed the convictions for lack of evidence.
In March, RaDonda Vaught, a nurse who worked at Vanderbilt University Medical Center in Nashville, Tenn., was convicted of criminal neglect and negligent homicide for mistakenly administering a fatal dose of the paralyzing drug vecuronium, instead of the prescribed drug Versed (midazolam), to a patient. Providers around the country were alarmed by her criminal prosecution for what was clearly an unintentional error.
But legal and medical experts said Dr. Husel’s case was sharply different from Vaught’s and Dr. Naramore’s because he deliberately and repeatedly ordered large doses of fentanyl and other drugs that he knew or should have known were potentially lethal. “You don’t need 2,000 micrograms of fentanyl plus other drugs for comfort care, and repeat that again and again for patient after patient,” said Mr. Cohen, of the Institute for Safe Medication Practices. “No one gives that to patients. You’ll knock them off.”
During the trial, prosecutors said repeatedly that no one except Dr. Husel knows what he was thinking when he ordered those huge drug dosages for his ICU patients. Judge Holbrook told the jury the state did not have to prove motive, only intent. But many observers still have wondered what his motives were.
Dr. Husel’s own view of his care in these cases soon will become public. Immediately after the April 20 verdict, Mr. Leeseberg filed a notice requesting a May 9 deposition of Dr. Husel, who will no longer be able to claim the Fifth Amendment right against self-incrimination. He predicted the deposition will last about a week, and then the transcript will be publicly available.
A version of this article first appeared on Medscape.com.
In an unprecedented murder case about end-of-life care, a physician accused of killing 14 critically ill patients with opioid overdoses in a Columbus, Ohio, hospital ICU over a period of 4 years was found not guilty by a jury April 20.
The jury, after a 7-week trial featuring more than 50 witnesses in the Franklin County Court of Common Pleas, declared William Husel, DO, not guilty on 14 counts of murder and attempted murder.
In a news conference after the verdict was announced, lead defense attorney Jose Baez said Dr. Husel, whom he called a “great doctor,” hopes to practice medicine again in the future. “They don’t need to be looking over their shoulders worrying about whether they’ll get charged with crimes,” he said.
The prosecutors in the case declined to comment, other than to say they “accept” the verdict.
Legal experts said it’s highly unlikely that Ohio or any other state would restore Dr. Husel’s suspended medical license. “I doubt he could ever work in medicine again,” said Mark Schumacher, a Columbus medical malpractice defense attorney who retired in 2020 after practicing for 39 years. Mr. Schumacher followed the trial closely.
The trial raised the specific issue of what constitutes a medically justifiable dose of opioid painkillers during the end-of-life procedure known as palliative extubation, in which critically ill patients are withdrawn from the ventilator when they are expected to die. Under medicine’s so-called double-effect principle, physicians must weigh the benefits and risks of ordering potentially lethal doses of painkillers and sedatives to provide comfort care for critically ill patients.
To many observers, however, the case really centered on the largely hidden debate over whether it’s acceptable to hasten the deaths of dying patients who haven’t chosen that path. That’s called euthanasia, which is illegal in the United States. In contrast, 10 states plus the District of Columbia allow physicians to prescribe lethal drugs to terminally ill, mentally competent adults who can self-administer them. That’s called medical aid in dying, or physician-assisted dying or suicide.
“Maybe this is a wake-up call that people believe this is the right thing to do,” said Lewis Nelson, MD, chair of emergency medicine at New Jersey Medical School in Newark. “The medical community has a sense that we often prolong life unnecessarily. But a physician cannot unilaterally decide it’s time for someone to die. It sounds like [Dr. Husel] took that decision into his own hands.”
The case also exposed major gaps in the patient safety culture at Mount Carmel West Hospital in Columbus, which is owned by the Catholic chain Trinity Health. Experts say failures by hospital staff to question physicians’ orders and raise patient safety concerns, as happened at Mount Carmel, occur at many hospitals around the country. Experts say the Husel case offers vital lessons for health care professionals about improving safety procedures.
“This is an extreme example, that everyone should learn from, about what not to do,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “Husel was giving massive overdoses of drugs, people knew it was problematic, and someone didn’t put their foot down. You’ve got to have a process in place to address these situations where there is disagreement over the safety of a medication order.”
Dr. Husel was charged with killing the 14 patients from 2015 through 2018 by ordering single large doses of the painkiller fentanyl – from 500 to 2,000 micrograms – often in combination with other opioids and sedatives, while working as the solo physician on the overnight shift in the ICU at Mount Carmel West Hospital and at Mount Carmel St. Ann’s Hospital in Westerville, Ohio.
Dr. Husel ordered administration of the drugs while his patients were having an endotracheal tube removed as part of palliative extubation. There was conflicting testimony during the trial about whether the patients were showing signs of pain or were even capable of feeling pain.
Prosecutors argued that Dr. Husel, who did a residency and fellowship in critical care medicine at Cleveland Clinic and started working at Mount Carmel in 2013 in his first job as a full-fledged physician, intended to kill the patients or hasten their deaths. They contended that the inexperienced nurses in the ICU went along with his large drug doses because they were “in thrall” to him because of his prestigious background at the Cleveland Clinic and his willingness to take the time to teach them.
“With his training in anesthesiology, he knew what those drugs do,” assistant prosecutor David Zeyen said in closing arguments. “This isn’t negligence. This is on purpose ... Euthanizing animals with the intent to kill is fine in veterinary medicine. It’s not fine in the ICU at Mount Carmel or anywhere.”
The defense team argued that Dr. Husel was a caring and compassionate physician who ordered the drugs to relieve the patients’ pain and discomfort during the extubation process. He did not testify.
“Common sense says Dr Husel had no motive to harm patients,” defense attorney Baez said in his closing. “He dedicated his life to taking care of patients and saving lives, not taking them. ... Why would this man risk his family, career, and 17 years of trying to be a doctor to hasten someone’s death or kill them?”
There were 35 Mount Carmel patients who died in the ICU under Dr. Husel’s care after receiving large fentanyl doses during palliative extubation. The state originally charged him with murder in 25 of those cases, then reduced that to 14.
Many of Dr. Husel’s drug orders were given verbally instead of through the regular process of being entered into the electronic health record. He and the nurses on duty also skipped the standard nonemergency process of getting approval from the pharmacist on duty, instead using the override function on Mount Carmel’s automated Pyxis drug dispensing system.
Dr. Husel’s unusual dosing patterns were first reported to Mount Carmel officials by pharmacists in October 2018, spurring an investigation. The hospital system let him go in December 2018, after concluding that the opioid dosages he used were “significantly excessive and potentially fatal” and “went beyond providing comfort.”
Nearly two dozen RNs and two pharmacists involved in these cases have faced state disciplinary action, mostly license suspension. Federal and state agencies have cited the Mount Carmel system for faults in its patient safety processes and culture that were exposed by the Husel cases.
The Mount Carmel CEO; the chief clinical officer; other physician, nursing, and pharmacy leaders; and dozens of nurses and pharmacists were forced out following the Husel investigation.
In 2019, the Centers for Medicare & Medicaid Services, after threatening to cut off federal reimbursements to Mount Carmel, accepted the hospital system’s correction plan restricting the use of verbal drug orders and prohibiting Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Mount Carmel and Trinity have settled a number of civil wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. Gerry Leeseberg, a Columbus medical malpractice plaintiff attorney who is representing 17 of the families, said a number of the cases are set for trial starting in June.
During the trial, family members of many of the 14 patients whom Dr. Husel allegedly murdered testified that Dr. Husel told them their loved ones were dying. Some said they felt rushed into making a decision to extubate the person.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved ones gasping for breath. But most medical experts – including the state’s two physician expert witnesses – say the fentanyl doses Dr. Husel ordered were 5-20 times larger than doses normally used in palliative extubation. Such doses, they say, will quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 micrograms for relieving pain, and its 2018 guidelines reduced that to 25-50 micrograms.
The doses Dr. Husel ordered are lethal, even for most patients with some tolerance to opioids, said Rutgers EM chair Dr. Nelson, who practices medical toxicology and addiction medicine. “Those are doses to provide euthanasia, not to relieve pain.”
At trial, the prosecutors had to overcome two big challenges to win murder convictions against Dr. Husel: They had to prove beyond a reasonable doubt that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Late in the trial, at the state’s request, the judge ruled to allow the jury to also consider attempted murder charges, which require proof of intent but not that the defendant’s actions directly caused the deaths.
Another challenge was that physicians have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia. That’s what prosecutors accused Dr. Husel of doing.
“If you hasten a person’s death, even if their death is as sure as the sun is going to rise in the morning ... you have caused their death in the eyes of the law,” assistant prosecutor Mr. Zeyen said in his closing. “You don’t get a pass for killing a dying man ...”
Mr. Leeseberg said it was always going to be extremely hard to convince a jury to convict a physician of murder, with the potential of life in prison, in a case where the physician’s acts occurred openly over 4 years in a hospital setting where no one did anything to stop him. It would have been much easier to convince a jury to convict him of reckless homicide, a lesser offense with a shorter prison term. That would have required proving that he acted in reckless disregard for his patients’ health and safety.
In post-verdict comments to the news media, Judge Michael Holbrook said jurors told him that the procedures for the dispensing of fentanyl and other drugs at Mount Carmel weren’t properly explained to them during the trial, and that they were confused by the large number of prosecution witnesses. He also said they were confused that no one had stated a maximum dosage for fentanyl.
Mr. Schumacher, the retired malpractice defense lawyer who followed the trial, disagreed with defense attorney Baez’s takeaway about the impact of the case on pain-relief practices. In his view, the case likely will heighten rather than reduce the anxiety of physicians and nurses about administering opioids, even when the dosages are clearly needed and appropriate. He doesn’t think Dr. Husel’s dosages can be justified, however.
“Physicians have a naive overreaction to any legal development, and overgeneralize from a particular case to everyday practice,” he said.
There is only one known prior case that’s somewhat comparable of a physician tried for murder or attempted murder for giving a critically ill patient opioids for pain relief. In 1996, a Kansas jury convicted Lloyd Stanley Naramore, DO, of attempted murder in the death of a patient to whom he gave an opioid, and of second-degree murder for removing a patient from a ventilator. After Dr. Naramore served 6 months in prison, an appellate court reversed the convictions for lack of evidence.
In March, RaDonda Vaught, a nurse who worked at Vanderbilt University Medical Center in Nashville, Tenn., was convicted of criminal neglect and negligent homicide for mistakenly administering a fatal dose of the paralyzing drug vecuronium, instead of the prescribed drug Versed (midazolam), to a patient. Providers around the country were alarmed by her criminal prosecution for what was clearly an unintentional error.
But legal and medical experts said Dr. Husel’s case was sharply different from Vaught’s and Dr. Naramore’s because he deliberately and repeatedly ordered large doses of fentanyl and other drugs that he knew or should have known were potentially lethal. “You don’t need 2,000 micrograms of fentanyl plus other drugs for comfort care, and repeat that again and again for patient after patient,” said Mr. Cohen, of the Institute for Safe Medication Practices. “No one gives that to patients. You’ll knock them off.”
During the trial, prosecutors said repeatedly that no one except Dr. Husel knows what he was thinking when he ordered those huge drug dosages for his ICU patients. Judge Holbrook told the jury the state did not have to prove motive, only intent. But many observers still have wondered what his motives were.
Dr. Husel’s own view of his care in these cases soon will become public. Immediately after the April 20 verdict, Mr. Leeseberg filed a notice requesting a May 9 deposition of Dr. Husel, who will no longer be able to claim the Fifth Amendment right against self-incrimination. He predicted the deposition will last about a week, and then the transcript will be publicly available.
A version of this article first appeared on Medscape.com.
ILD progression, not diagnosis, triggers palliative care
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL CHEST®
Cancer Data Trends 2022
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.

