User login
How Safe is Anti–IL-6 Therapy During Pregnancy?
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
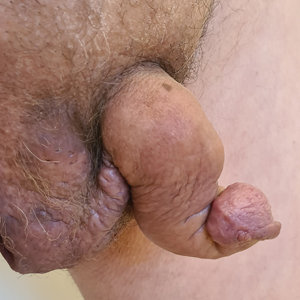
Saxophone Penis: A Forgotten Manifestation of Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a multifactorial chronic inflammatory skin disease affecting 1% to 4% of Europeans. It is characterized by recurrent inflamed nodules, abscesses, and sinus tracts in intertriginous regions.1 The genital area is affected in 11% of cases2 and usually is connected to severe forms of HS in both men and women.3 The prevalence of HS-associated genital lymphedema remains unknown.
Saxophone penis is a specific penile malformation characterized by a saxophone shape due to inflammation of the major penile lymphatic vessels that cause fibrosis of the surrounding connective tissue. Poor blood flow further causes contracture and distortion of the penile axis.4 Saxophone penis also has been associated with primary lymphedema, lymphogranuloma venereum, filariasis,5 and administration of paraffin injections.6 We describe 3 men with HS who presented with saxophone penis.
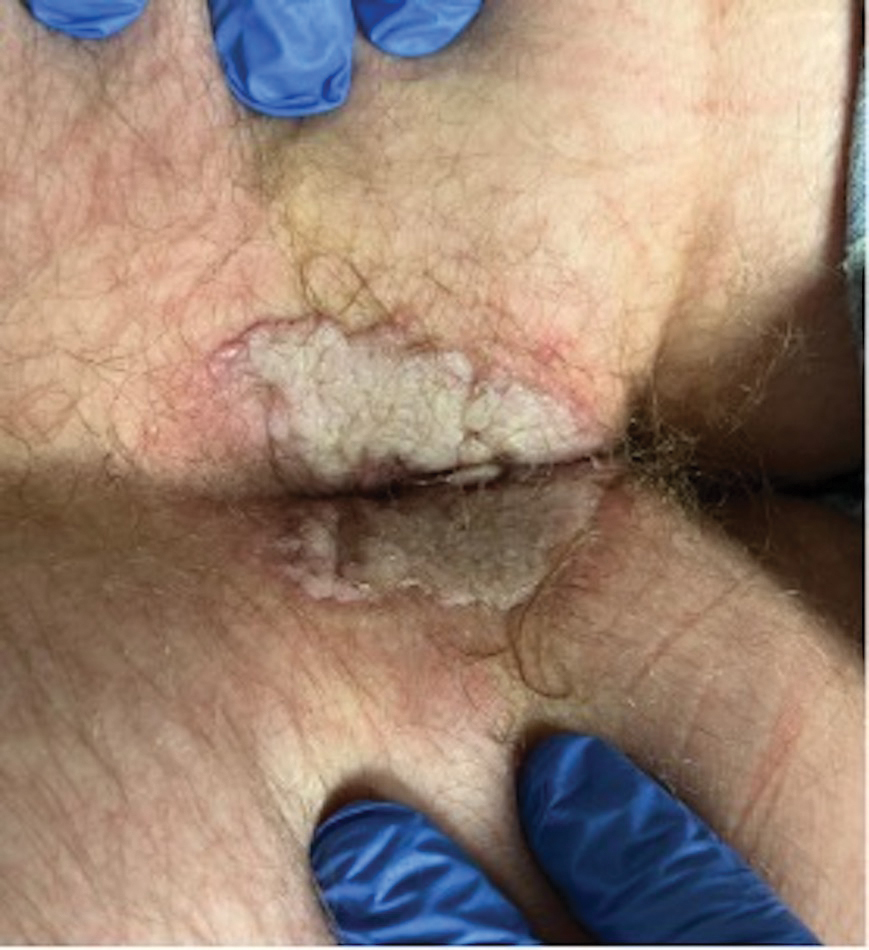
A 33-year-old man with Hurley stage III HS presented with a medical history of groin lesions and progressive penoscrotal edema of 13 years’ duration. He had a body mass index (BMI) of 37, no family history of HS or comorbidities, and a 15-year history of smoking 20 cigarettes per day. After repeated surgical drainage of the HS lesions as well as antibiotic treatment with clindamycin 600 mg/d and rifampicin 600 mg/d, the patient was kept on a maintenance therapy with adalimumab 40 mg/wk. Due to lack of response, treatment was discontinued at week 16. Clindamycin and rifampicin 300 mg were immediately reintroduced with no benefit on the genital lesions. The patient underwent genital reconstruction, including penile degloving, scrotoplasty, infrapubic fat pad removal, and perineoplasty (Figure 1). The patient currently is not undergoing any therapies.
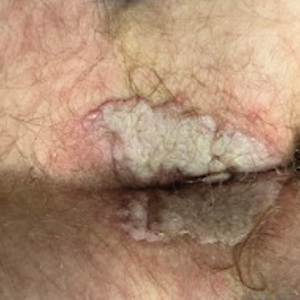
A 55-year-old man presented with Hurley stage II HS of 33 years’ duration. He had a BMI of 52; a history of hypertension, hyperuricemia, severe hip and knee osteoarthritis, and orchiopexy in childhood; a smoking history of 40 cigarettes per day; and an alcohol consumption history of 200 mL per day since 18 years of age. He had radical excision of axillary lesions 8 years prior. One year later, he was treated with concomitant clindamycin and rifampicin 300 mg twice daily for 3 months with no desirable effects. Adalimumab 40 mg/wk was initiated. After 12 weeks of treatment, he experienced 80% improvement in all areas except the genital region. He continued adalimumab for 3 years with good clinical response in all HS-affected sites except the genital region.
A 66-year-old man presented with Hurley stage III HS of 37 years’ duration. He had a smoking history of 10 cigarettes per day for 30 years, a BMI of 24.6, and a medical history of long-standing hypertension and hypothyroidism. A 3-month course of clindamycin and rifampicin 600 mg/d was ineffective; adalimumab 40 mg/wk was initiated. All affected areas improved, except for the saxophone penis. He continues his fifth year of therapy with adalimumab (Figure 2).

Hidradenitis suppurativa is associated with chronic pain, purulent malodor, and scarring with structural deformity. Repetitive inflammation causes fibrosis, scar formation, and soft-tissue destruction of lymphatic vessels, leading to lymphedema; primary lymphedema of the genitals in men has been reported to result in a saxophone penis.4
The only approved biologic treatments for moderate to severe HS are the tumor necrosis factor α inhibitor adalimumab and anti-IL-17 secukinumab.1 All 3 of our patients with HS were treated with adalimumab with reasonable success; however, the penile condition remained refractory, which we speculate may be due to adalimumab’s ability to control only active inflammatory lesions but not scars or fibrotic tissue.7 Higher adalimumab dosages were unlikely to be beneficial for their penile condition; some improvements have been reported following fluoroquinolone therapy. To our knowledge, there is no effective medical treatment for saxophone penis. However, surgery showed good results in one of our patients. Among our 3 adalimumab-treated patients, only 1 patient had corrective surgery that resulted in improvement in the penile deformity, further confirming adalimumab’s limited role in genital lymphedema.7 Extensive resection of the lymphedematous tissue, scrotoplasty, and Charles procedure are treatment options.8
Genital lymphedema has been associated with lymphangiectasia, lymphangioma circumscriptum, infections, and neoplasms such as lymphangiosarcoma and squamous cell carcinoma.9 Our patients reported discomfort, hygiene issues, and swelling. One patient reported micturition, and 2 patients reported sexual dysfunction.
Saxophone penis remains a disabling sequela of HS. Early diagnosis and treatment of HS may help prevent development of this condition.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Fertitta L, Hotz C, Wolkenstein P, et al. Efficacy and satisfaction of surgical treatment for hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34:839-845.
- Micieli R, Alavi A. Lymphedema in patients with hidradenitis suppurativa: a systematic review of published literature. Int J Dermatol. 2018;57:1471-1480.
- Maatouk I, Moutran R. Saxophone penis. JAMA Dermatol. 2013;149:802.
- Koley S, Mandal RK. Saxophone penis after unilateral inguinal bubo of lymphogranuloma venereum. Indian J Sex Transm Dis AIDS. 2013;34:149-151.
- D’Antuono A, Lambertini M, Gaspari V, et al. Visual dermatology: self-induced chronic saxophone penis due to paraffin injections. J Cutan Med Surg. 2019;23:330.
- Musumeci ML, Scilletta A, Sorci F, et al. Genital lymphedema associated with hidradenitis suppurativa unresponsive to adalimumab treatment. JAAD Case Rep. 2019;5:326-328.
- Jain V, Singh S, Garge S, et al. Saxophone penis due to primary lymphoedema. J Indian Assoc Pediatr Surg. 2009;14:230-231.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;64:1223-1224.
To the Editor:
Hidradenitis suppurativa (HS) is a multifactorial chronic inflammatory skin disease affecting 1% to 4% of Europeans. It is characterized by recurrent inflamed nodules, abscesses, and sinus tracts in intertriginous regions.1 The genital area is affected in 11% of cases2 and usually is connected to severe forms of HS in both men and women.3 The prevalence of HS-associated genital lymphedema remains unknown.
Saxophone penis is a specific penile malformation characterized by a saxophone shape due to inflammation of the major penile lymphatic vessels that cause fibrosis of the surrounding connective tissue. Poor blood flow further causes contracture and distortion of the penile axis.4 Saxophone penis also has been associated with primary lymphedema, lymphogranuloma venereum, filariasis,5 and administration of paraffin injections.6 We describe 3 men with HS who presented with saxophone penis.
A 33-year-old man with Hurley stage III HS presented with a medical history of groin lesions and progressive penoscrotal edema of 13 years’ duration. He had a body mass index (BMI) of 37, no family history of HS or comorbidities, and a 15-year history of smoking 20 cigarettes per day. After repeated surgical drainage of the HS lesions as well as antibiotic treatment with clindamycin 600 mg/d and rifampicin 600 mg/d, the patient was kept on a maintenance therapy with adalimumab 40 mg/wk. Due to lack of response, treatment was discontinued at week 16. Clindamycin and rifampicin 300 mg were immediately reintroduced with no benefit on the genital lesions. The patient underwent genital reconstruction, including penile degloving, scrotoplasty, infrapubic fat pad removal, and perineoplasty (Figure 1). The patient currently is not undergoing any therapies.
A 55-year-old man presented with Hurley stage II HS of 33 years’ duration. He had a BMI of 52; a history of hypertension, hyperuricemia, severe hip and knee osteoarthritis, and orchiopexy in childhood; a smoking history of 40 cigarettes per day; and an alcohol consumption history of 200 mL per day since 18 years of age. He had radical excision of axillary lesions 8 years prior. One year later, he was treated with concomitant clindamycin and rifampicin 300 mg twice daily for 3 months with no desirable effects. Adalimumab 40 mg/wk was initiated. After 12 weeks of treatment, he experienced 80% improvement in all areas except the genital region. He continued adalimumab for 3 years with good clinical response in all HS-affected sites except the genital region.
A 66-year-old man presented with Hurley stage III HS of 37 years’ duration. He had a smoking history of 10 cigarettes per day for 30 years, a BMI of 24.6, and a medical history of long-standing hypertension and hypothyroidism. A 3-month course of clindamycin and rifampicin 600 mg/d was ineffective; adalimumab 40 mg/wk was initiated. All affected areas improved, except for the saxophone penis. He continues his fifth year of therapy with adalimumab (Figure 2).

Hidradenitis suppurativa is associated with chronic pain, purulent malodor, and scarring with structural deformity. Repetitive inflammation causes fibrosis, scar formation, and soft-tissue destruction of lymphatic vessels, leading to lymphedema; primary lymphedema of the genitals in men has been reported to result in a saxophone penis.4
The only approved biologic treatments for moderate to severe HS are the tumor necrosis factor α inhibitor adalimumab and anti-IL-17 secukinumab.1 All 3 of our patients with HS were treated with adalimumab with reasonable success; however, the penile condition remained refractory, which we speculate may be due to adalimumab’s ability to control only active inflammatory lesions but not scars or fibrotic tissue.7 Higher adalimumab dosages were unlikely to be beneficial for their penile condition; some improvements have been reported following fluoroquinolone therapy. To our knowledge, there is no effective medical treatment for saxophone penis. However, surgery showed good results in one of our patients. Among our 3 adalimumab-treated patients, only 1 patient had corrective surgery that resulted in improvement in the penile deformity, further confirming adalimumab’s limited role in genital lymphedema.7 Extensive resection of the lymphedematous tissue, scrotoplasty, and Charles procedure are treatment options.8
Genital lymphedema has been associated with lymphangiectasia, lymphangioma circumscriptum, infections, and neoplasms such as lymphangiosarcoma and squamous cell carcinoma.9 Our patients reported discomfort, hygiene issues, and swelling. One patient reported micturition, and 2 patients reported sexual dysfunction.
Saxophone penis remains a disabling sequela of HS. Early diagnosis and treatment of HS may help prevent development of this condition.
To the Editor:
Hidradenitis suppurativa (HS) is a multifactorial chronic inflammatory skin disease affecting 1% to 4% of Europeans. It is characterized by recurrent inflamed nodules, abscesses, and sinus tracts in intertriginous regions.1 The genital area is affected in 11% of cases2 and usually is connected to severe forms of HS in both men and women.3 The prevalence of HS-associated genital lymphedema remains unknown.
Saxophone penis is a specific penile malformation characterized by a saxophone shape due to inflammation of the major penile lymphatic vessels that cause fibrosis of the surrounding connective tissue. Poor blood flow further causes contracture and distortion of the penile axis.4 Saxophone penis also has been associated with primary lymphedema, lymphogranuloma venereum, filariasis,5 and administration of paraffin injections.6 We describe 3 men with HS who presented with saxophone penis.
A 33-year-old man with Hurley stage III HS presented with a medical history of groin lesions and progressive penoscrotal edema of 13 years’ duration. He had a body mass index (BMI) of 37, no family history of HS or comorbidities, and a 15-year history of smoking 20 cigarettes per day. After repeated surgical drainage of the HS lesions as well as antibiotic treatment with clindamycin 600 mg/d and rifampicin 600 mg/d, the patient was kept on a maintenance therapy with adalimumab 40 mg/wk. Due to lack of response, treatment was discontinued at week 16. Clindamycin and rifampicin 300 mg were immediately reintroduced with no benefit on the genital lesions. The patient underwent genital reconstruction, including penile degloving, scrotoplasty, infrapubic fat pad removal, and perineoplasty (Figure 1). The patient currently is not undergoing any therapies.
A 55-year-old man presented with Hurley stage II HS of 33 years’ duration. He had a BMI of 52; a history of hypertension, hyperuricemia, severe hip and knee osteoarthritis, and orchiopexy in childhood; a smoking history of 40 cigarettes per day; and an alcohol consumption history of 200 mL per day since 18 years of age. He had radical excision of axillary lesions 8 years prior. One year later, he was treated with concomitant clindamycin and rifampicin 300 mg twice daily for 3 months with no desirable effects. Adalimumab 40 mg/wk was initiated. After 12 weeks of treatment, he experienced 80% improvement in all areas except the genital region. He continued adalimumab for 3 years with good clinical response in all HS-affected sites except the genital region.
A 66-year-old man presented with Hurley stage III HS of 37 years’ duration. He had a smoking history of 10 cigarettes per day for 30 years, a BMI of 24.6, and a medical history of long-standing hypertension and hypothyroidism. A 3-month course of clindamycin and rifampicin 600 mg/d was ineffective; adalimumab 40 mg/wk was initiated. All affected areas improved, except for the saxophone penis. He continues his fifth year of therapy with adalimumab (Figure 2).

Hidradenitis suppurativa is associated with chronic pain, purulent malodor, and scarring with structural deformity. Repetitive inflammation causes fibrosis, scar formation, and soft-tissue destruction of lymphatic vessels, leading to lymphedema; primary lymphedema of the genitals in men has been reported to result in a saxophone penis.4
The only approved biologic treatments for moderate to severe HS are the tumor necrosis factor α inhibitor adalimumab and anti-IL-17 secukinumab.1 All 3 of our patients with HS were treated with adalimumab with reasonable success; however, the penile condition remained refractory, which we speculate may be due to adalimumab’s ability to control only active inflammatory lesions but not scars or fibrotic tissue.7 Higher adalimumab dosages were unlikely to be beneficial for their penile condition; some improvements have been reported following fluoroquinolone therapy. To our knowledge, there is no effective medical treatment for saxophone penis. However, surgery showed good results in one of our patients. Among our 3 adalimumab-treated patients, only 1 patient had corrective surgery that resulted in improvement in the penile deformity, further confirming adalimumab’s limited role in genital lymphedema.7 Extensive resection of the lymphedematous tissue, scrotoplasty, and Charles procedure are treatment options.8
Genital lymphedema has been associated with lymphangiectasia, lymphangioma circumscriptum, infections, and neoplasms such as lymphangiosarcoma and squamous cell carcinoma.9 Our patients reported discomfort, hygiene issues, and swelling. One patient reported micturition, and 2 patients reported sexual dysfunction.
Saxophone penis remains a disabling sequela of HS. Early diagnosis and treatment of HS may help prevent development of this condition.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Fertitta L, Hotz C, Wolkenstein P, et al. Efficacy and satisfaction of surgical treatment for hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34:839-845.
- Micieli R, Alavi A. Lymphedema in patients with hidradenitis suppurativa: a systematic review of published literature. Int J Dermatol. 2018;57:1471-1480.
- Maatouk I, Moutran R. Saxophone penis. JAMA Dermatol. 2013;149:802.
- Koley S, Mandal RK. Saxophone penis after unilateral inguinal bubo of lymphogranuloma venereum. Indian J Sex Transm Dis AIDS. 2013;34:149-151.
- D’Antuono A, Lambertini M, Gaspari V, et al. Visual dermatology: self-induced chronic saxophone penis due to paraffin injections. J Cutan Med Surg. 2019;23:330.
- Musumeci ML, Scilletta A, Sorci F, et al. Genital lymphedema associated with hidradenitis suppurativa unresponsive to adalimumab treatment. JAAD Case Rep. 2019;5:326-328.
- Jain V, Singh S, Garge S, et al. Saxophone penis due to primary lymphoedema. J Indian Assoc Pediatr Surg. 2009;14:230-231.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;64:1223-1224.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Fertitta L, Hotz C, Wolkenstein P, et al. Efficacy and satisfaction of surgical treatment for hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34:839-845.
- Micieli R, Alavi A. Lymphedema in patients with hidradenitis suppurativa: a systematic review of published literature. Int J Dermatol. 2018;57:1471-1480.
- Maatouk I, Moutran R. Saxophone penis. JAMA Dermatol. 2013;149:802.
- Koley S, Mandal RK. Saxophone penis after unilateral inguinal bubo of lymphogranuloma venereum. Indian J Sex Transm Dis AIDS. 2013;34:149-151.
- D’Antuono A, Lambertini M, Gaspari V, et al. Visual dermatology: self-induced chronic saxophone penis due to paraffin injections. J Cutan Med Surg. 2019;23:330.
- Musumeci ML, Scilletta A, Sorci F, et al. Genital lymphedema associated with hidradenitis suppurativa unresponsive to adalimumab treatment. JAAD Case Rep. 2019;5:326-328.
- Jain V, Singh S, Garge S, et al. Saxophone penis due to primary lymphoedema. J Indian Assoc Pediatr Surg. 2009;14:230-231.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;64:1223-1224.
Practice Points
- Hidradenitis suppurativa (HS) is a multifactorial chronic inflammatory skin disease.
- Saxophone penis is a specific penile malformation characterized by a saxophone shape due to inflammation.
- Repetitive inflammation within the context of HS may cause structural deformity of the penis, resulting in a saxophone penis.
- Early diagnosis and treatment of HS may help prevent development of this condition.
Painful Anal Lesions in a Patient With HIV
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.
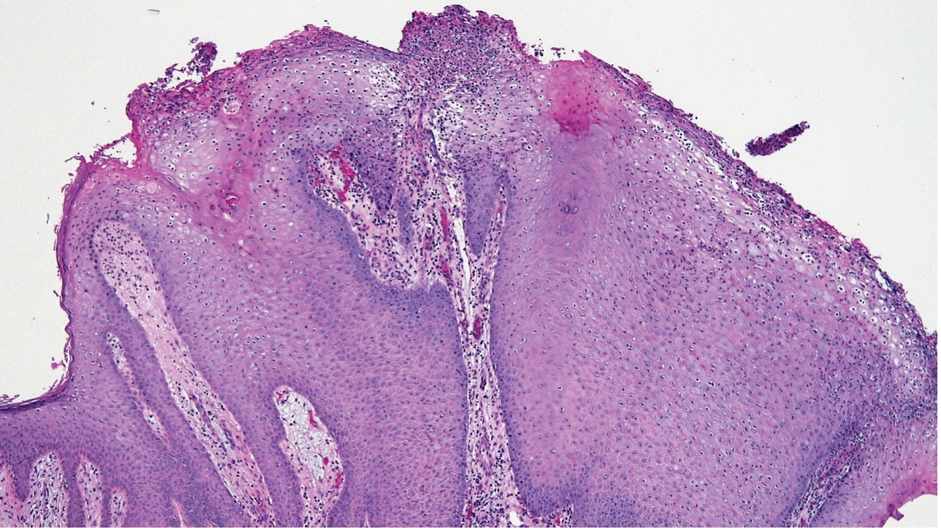
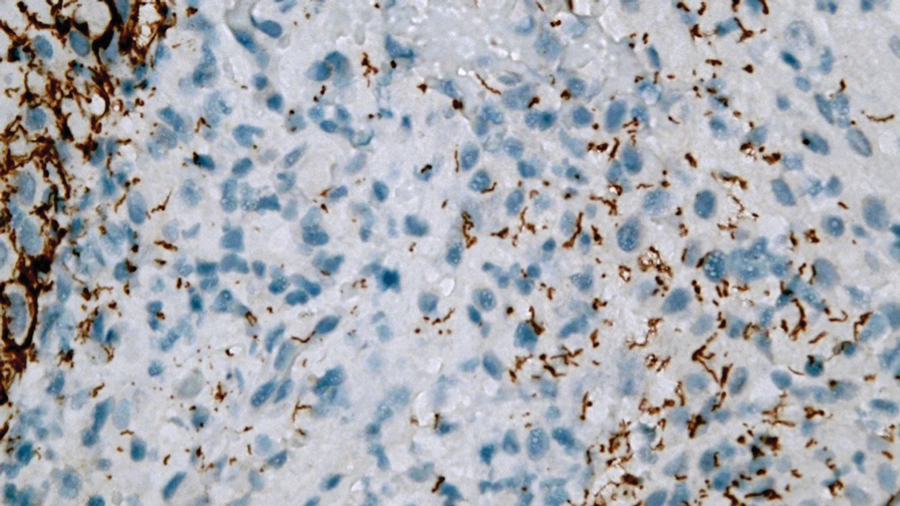
Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.


Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.


Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
A 24-year-old man presented to the emergency department with rectal pain and lesions of 3 weeks’ duration that were progressively worsening. He had a medical history of poorly controlled HIV, cerebral toxoplasmosis, and genital herpes, as well as a social history of sexual activity with other men.
He had been diagnosed with HIV 7 years prior and had been off therapy until 1 year prior to the current presentation, when he was hospitalized with encephalopathy (CD4 count, <50 cells/mm3). A diagnosis of cerebral toxoplasmosis was made, and he began a treatment regimen of sulfadiazine, pyrimethamine, and leucovorin, as well as bictegravir, emtricitabine, and tenofovir alafenamide. Since then, the patient admitted to difficulty with medication adherence.
Rapid plasma reagin, gonorrhea, and chlamydia testing were negative during a routine workup 6 months prior to the current presentation. He initially presented to an urgent care clinic for evaluation of the rectal pain and lesions and was treated empirically with topical podofilox. He presented to the emergency department 1 week later (3 weeks after symptom onset) with anal warts and apparent vesicular lesions. Empiric treatment with oral valacyclovir was prescribed.
Despite these treatments, the rectal pain became severe—especially upon sitting, defecation, and physical exertion—prompting further evaluation. Physical examination revealed soft, flat-topped, moist-appearing, gray plaques with minimal surrounding erythema at the anus. Laboratory test results demonstrated a CD4 count of 161 cells/mm3 and an HIV viral load of 137 copies/mL.
Identifying, Treating Lyme Disease in Primary Care
Geographic spread of the ticks that most often cause Lyme disease in the United States and a rise in incidence of bites, resulting in 476,000 new US cases a year, have increased the chances that physicians who have never encountered a patient with Lyme disease will see their first cases.
“It’s increasing in areas where it was not seen before,” Steven E. Schutzer, MD, with the Department of Medicine, Rutgers New Jersey Medical School, Newark, said in an interview. Dr. Schutzer coauthored a report on diagnosing and treating Lyme disease with Patricia K. Coyle, MD, Department of Neurology, Renaissance School of Medicine at Stony Brook University, Stony Brook, New York.
The report, a Curbside Consult published in New England Journal of Medicine Evidence, comes amid high season for Lyme disease. Bites from an ixodid (hard shield) tick — almost always the source of the disease in the United States — are most common from April through October.
Identifying the Bite
About 70%-90% of the time, Lyme disease will be signaled by erythema migrans (EM) or lesion expanding from the tick bite site, the authors wrote. The “classic” presentation looks like a bullseye, but most of the time the skin will show a variation of that, the authors noted.
“The presence of EM is considered the best clinical diagnostic marker for Lyme disease,” they wrote.
Other dermatologic conditions, however, can complicate diagnosis: “EM mimickers include contact dermatitis, other arthropod bites, fixed drug eruptions, granuloma annulare, cellulitis, dermatophytosis, and systemic lupus erythematosus,” they wrote.
Testing Steps
“The current recommendation is to do two-step testing almost simultaneously,” Dr. Schutzer said in an interview. The first, he said, is an ELISA (enzyme-linked immunosorbent assay)-type test and the second one, used for years, has been a pictoral view of a Western immunoblot showing which antigens of the Lyme bacteria, Borrelia burgdorferi, the antibodies are reacting to.
However, the pictoral view is subjective and some of the antigens could be cross-reactive. So the U.S. Food and Drug Administration (FDA) “has been allowing newer substitutes like a second ELISA-like assay that often uses more recombinant, less cross-reactive antigen targets,” he said. The authors advised that, “The second-tier test should not be performed alone without the first tier.”
Dr. Schutzer advised physicians to check with the lab they plan to use before sending samples.
“If you’re a practicing physician and you know you’re using a particular laboratory, you should familiarize yourself with them, talking to one of the clinical pathologists involved in advance to know what the limitations are.” Take the time to talk with the person overseeing the test and get tips on how they want the sample transported and how the cases should be reported, he said.
If the patient has neurological symptoms, he said, before treating talk with a neurologist who can advise whether, for instance, a spinal tap is in order or whether an emergency department visit is appropriate.
“If you just start proceeding you may mess up the diagnostic signs that could show up in a lab test. Don’t be hesitant to ask for extra input from colleagues,” Dr. Schutzer said.
Suspicion in Endemic Areas
On Long Island, New York, where Lyme disease is endemic, internist Ian Storch, DO, said he sees “a few cases a season.
“We have a lot of people over the summer going to the Hamptons and areas out east for the weekend and tick bites are not uncommon,” he said. “People panic.”
He said one thing it’s important to tell patients is that the tick has to be on the skin for 48-72 hours to transmit the disease. If individuals were in a wooded area and were fine before they got there and the tick was attached for less than 2 days, “they’re usually fine.”
Another issue, Dr. Storch said, is patients sometimes want to get tested for Lyme disease immediately after a tick bite. But the antibody test doesn’t turn positive for weeks, he noted, and you can get a false-negative result. “If you’re worried and you really want to test, you need to wait 6 weeks to do the blood test.”
In his region, he said that although a tick bite is a red flag, he may also suspect Lyme disease when a patient presents with otherwise unexplained joint pain, weakness, lethargy, or fever. “In our area, those are things that would make you test for Lyme.”
He also urged consideration of Lyme in this new age of long COVID. Weakness, fatigue, and lethargy are also classic symptoms of long COVID, he noted. “Keep Lyme disease in your differential because there is a lot of overlap with chronic Lyme disease,” Dr. Storch said.
Discerning Lyme from Southern Tick–Associated Rash Illness
Bonnie M. Word, MD, director of the Houston Travel Medicine Clinic in Texas, where Lyme disease is not endemic, said Lyme disease “will not and should not be on the initial differential diagnosis for those residing in nonendemic areas unless a history of travel to an endemic area is obtained.”
She noted the typical EM rash may not be as distinct or easy to discern on black and brown skin. In addition, she said, EM may have many variations in presentation, such as a crusted center or faint borders, which could lead to a delay in diagnosis and treatment. She suggested consulting the CDC guidance on Lyme disease rashes.
Another challenge in diagnosis, she said, is the patient who presents with what appears to be a classic EM lesion but does not live in a Lyme-endemic area. In Texas, Southern Tick–Associated Rash Illness (STARI) may present with a similar lesion, she said.
“It is transmitted by the Lone Star Tick, which is found in the southeast and south-central US,” Dr. Word said. “However, its habitat is moving northward and westerly,” she said.
Adding Lyme disease to the differential diagnosis is reasonable, she said, if a patient presents with neurologic symptoms “such as a facial palsy, meningitis, radiculitis, and carditis if in addition to their symptoms there is evidence of an epidemiologic link to a Lyme-endemic region.”
She noted that a detailed travel history is important as “Lyme is also endemic in Eastern Canada, Europe, states of the former Soviet Union, China, Mongolia, and Japan.”
Primary care physicians play a critical role in evaluating, diagnosing, and treating most cases of early Lyme disease, thus limiting the number of people who will develop disseminated or late Lyme disease, she said. “The two latter manifestations are most often treated by infectious disease, neurology, or rheumatology specialists.”
Treatment*
Treatment is tailored to the clinical situation, Dr. Schutzer and Dr. Coyle write. A watch-and-wait approach may be appropriate in an asymptomatic but concerned person, even in an endemic area if the person has no known tick bite and no EM lesion.
If there is high risk of an infected ixodid tick bite in a high-incidence area and the tick was attached for at least 36 hours but less than 72 hours, one dose of doxycycline has been recommended as prophylaxis.
When a diagnosis of early nondisseminated Lyme disease is made after observation of an EM lesion, oral antibiotics are typically used to treat for 10 to 14 days. Suggested oral antibiotics and doses are 100 mg of doxycycline twice a day, 500 mg of amoxicillin three times a day, or 500 mg of cefuroxime twice a day, the authors write.
Dr. Schutzer said he hopes the paper serves as a refresher for those physicians who regularly see Lyme disease cases and also helps those newly included in the disease’s spreading regions.
“The earlier you diagnose it, the earlier you can treat it and the better the chance for a favorable outcome,” he said.
Dr. Schutzer, Dr. Coyle, Dr. Storch, and Dr. Word reported no relevant financial relationships.
*This story was updated on August, 2, 2024.
Geographic spread of the ticks that most often cause Lyme disease in the United States and a rise in incidence of bites, resulting in 476,000 new US cases a year, have increased the chances that physicians who have never encountered a patient with Lyme disease will see their first cases.
“It’s increasing in areas where it was not seen before,” Steven E. Schutzer, MD, with the Department of Medicine, Rutgers New Jersey Medical School, Newark, said in an interview. Dr. Schutzer coauthored a report on diagnosing and treating Lyme disease with Patricia K. Coyle, MD, Department of Neurology, Renaissance School of Medicine at Stony Brook University, Stony Brook, New York.
The report, a Curbside Consult published in New England Journal of Medicine Evidence, comes amid high season for Lyme disease. Bites from an ixodid (hard shield) tick — almost always the source of the disease in the United States — are most common from April through October.
Identifying the Bite
About 70%-90% of the time, Lyme disease will be signaled by erythema migrans (EM) or lesion expanding from the tick bite site, the authors wrote. The “classic” presentation looks like a bullseye, but most of the time the skin will show a variation of that, the authors noted.
“The presence of EM is considered the best clinical diagnostic marker for Lyme disease,” they wrote.
Other dermatologic conditions, however, can complicate diagnosis: “EM mimickers include contact dermatitis, other arthropod bites, fixed drug eruptions, granuloma annulare, cellulitis, dermatophytosis, and systemic lupus erythematosus,” they wrote.
Testing Steps
“The current recommendation is to do two-step testing almost simultaneously,” Dr. Schutzer said in an interview. The first, he said, is an ELISA (enzyme-linked immunosorbent assay)-type test and the second one, used for years, has been a pictoral view of a Western immunoblot showing which antigens of the Lyme bacteria, Borrelia burgdorferi, the antibodies are reacting to.
However, the pictoral view is subjective and some of the antigens could be cross-reactive. So the U.S. Food and Drug Administration (FDA) “has been allowing newer substitutes like a second ELISA-like assay that often uses more recombinant, less cross-reactive antigen targets,” he said. The authors advised that, “The second-tier test should not be performed alone without the first tier.”
Dr. Schutzer advised physicians to check with the lab they plan to use before sending samples.
“If you’re a practicing physician and you know you’re using a particular laboratory, you should familiarize yourself with them, talking to one of the clinical pathologists involved in advance to know what the limitations are.” Take the time to talk with the person overseeing the test and get tips on how they want the sample transported and how the cases should be reported, he said.
If the patient has neurological symptoms, he said, before treating talk with a neurologist who can advise whether, for instance, a spinal tap is in order or whether an emergency department visit is appropriate.
“If you just start proceeding you may mess up the diagnostic signs that could show up in a lab test. Don’t be hesitant to ask for extra input from colleagues,” Dr. Schutzer said.
Suspicion in Endemic Areas
On Long Island, New York, where Lyme disease is endemic, internist Ian Storch, DO, said he sees “a few cases a season.
“We have a lot of people over the summer going to the Hamptons and areas out east for the weekend and tick bites are not uncommon,” he said. “People panic.”
He said one thing it’s important to tell patients is that the tick has to be on the skin for 48-72 hours to transmit the disease. If individuals were in a wooded area and were fine before they got there and the tick was attached for less than 2 days, “they’re usually fine.”
Another issue, Dr. Storch said, is patients sometimes want to get tested for Lyme disease immediately after a tick bite. But the antibody test doesn’t turn positive for weeks, he noted, and you can get a false-negative result. “If you’re worried and you really want to test, you need to wait 6 weeks to do the blood test.”
In his region, he said that although a tick bite is a red flag, he may also suspect Lyme disease when a patient presents with otherwise unexplained joint pain, weakness, lethargy, or fever. “In our area, those are things that would make you test for Lyme.”
He also urged consideration of Lyme in this new age of long COVID. Weakness, fatigue, and lethargy are also classic symptoms of long COVID, he noted. “Keep Lyme disease in your differential because there is a lot of overlap with chronic Lyme disease,” Dr. Storch said.
Discerning Lyme from Southern Tick–Associated Rash Illness
Bonnie M. Word, MD, director of the Houston Travel Medicine Clinic in Texas, where Lyme disease is not endemic, said Lyme disease “will not and should not be on the initial differential diagnosis for those residing in nonendemic areas unless a history of travel to an endemic area is obtained.”
She noted the typical EM rash may not be as distinct or easy to discern on black and brown skin. In addition, she said, EM may have many variations in presentation, such as a crusted center or faint borders, which could lead to a delay in diagnosis and treatment. She suggested consulting the CDC guidance on Lyme disease rashes.
Another challenge in diagnosis, she said, is the patient who presents with what appears to be a classic EM lesion but does not live in a Lyme-endemic area. In Texas, Southern Tick–Associated Rash Illness (STARI) may present with a similar lesion, she said.
“It is transmitted by the Lone Star Tick, which is found in the southeast and south-central US,” Dr. Word said. “However, its habitat is moving northward and westerly,” she said.
Adding Lyme disease to the differential diagnosis is reasonable, she said, if a patient presents with neurologic symptoms “such as a facial palsy, meningitis, radiculitis, and carditis if in addition to their symptoms there is evidence of an epidemiologic link to a Lyme-endemic region.”
She noted that a detailed travel history is important as “Lyme is also endemic in Eastern Canada, Europe, states of the former Soviet Union, China, Mongolia, and Japan.”
Primary care physicians play a critical role in evaluating, diagnosing, and treating most cases of early Lyme disease, thus limiting the number of people who will develop disseminated or late Lyme disease, she said. “The two latter manifestations are most often treated by infectious disease, neurology, or rheumatology specialists.”
Treatment*
Treatment is tailored to the clinical situation, Dr. Schutzer and Dr. Coyle write. A watch-and-wait approach may be appropriate in an asymptomatic but concerned person, even in an endemic area if the person has no known tick bite and no EM lesion.
If there is high risk of an infected ixodid tick bite in a high-incidence area and the tick was attached for at least 36 hours but less than 72 hours, one dose of doxycycline has been recommended as prophylaxis.
When a diagnosis of early nondisseminated Lyme disease is made after observation of an EM lesion, oral antibiotics are typically used to treat for 10 to 14 days. Suggested oral antibiotics and doses are 100 mg of doxycycline twice a day, 500 mg of amoxicillin three times a day, or 500 mg of cefuroxime twice a day, the authors write.
Dr. Schutzer said he hopes the paper serves as a refresher for those physicians who regularly see Lyme disease cases and also helps those newly included in the disease’s spreading regions.
“The earlier you diagnose it, the earlier you can treat it and the better the chance for a favorable outcome,” he said.
Dr. Schutzer, Dr. Coyle, Dr. Storch, and Dr. Word reported no relevant financial relationships.
*This story was updated on August, 2, 2024.
Geographic spread of the ticks that most often cause Lyme disease in the United States and a rise in incidence of bites, resulting in 476,000 new US cases a year, have increased the chances that physicians who have never encountered a patient with Lyme disease will see their first cases.
“It’s increasing in areas where it was not seen before,” Steven E. Schutzer, MD, with the Department of Medicine, Rutgers New Jersey Medical School, Newark, said in an interview. Dr. Schutzer coauthored a report on diagnosing and treating Lyme disease with Patricia K. Coyle, MD, Department of Neurology, Renaissance School of Medicine at Stony Brook University, Stony Brook, New York.
The report, a Curbside Consult published in New England Journal of Medicine Evidence, comes amid high season for Lyme disease. Bites from an ixodid (hard shield) tick — almost always the source of the disease in the United States — are most common from April through October.
Identifying the Bite
About 70%-90% of the time, Lyme disease will be signaled by erythema migrans (EM) or lesion expanding from the tick bite site, the authors wrote. The “classic” presentation looks like a bullseye, but most of the time the skin will show a variation of that, the authors noted.
“The presence of EM is considered the best clinical diagnostic marker for Lyme disease,” they wrote.
Other dermatologic conditions, however, can complicate diagnosis: “EM mimickers include contact dermatitis, other arthropod bites, fixed drug eruptions, granuloma annulare, cellulitis, dermatophytosis, and systemic lupus erythematosus,” they wrote.
Testing Steps
“The current recommendation is to do two-step testing almost simultaneously,” Dr. Schutzer said in an interview. The first, he said, is an ELISA (enzyme-linked immunosorbent assay)-type test and the second one, used for years, has been a pictoral view of a Western immunoblot showing which antigens of the Lyme bacteria, Borrelia burgdorferi, the antibodies are reacting to.
However, the pictoral view is subjective and some of the antigens could be cross-reactive. So the U.S. Food and Drug Administration (FDA) “has been allowing newer substitutes like a second ELISA-like assay that often uses more recombinant, less cross-reactive antigen targets,” he said. The authors advised that, “The second-tier test should not be performed alone without the first tier.”
Dr. Schutzer advised physicians to check with the lab they plan to use before sending samples.
“If you’re a practicing physician and you know you’re using a particular laboratory, you should familiarize yourself with them, talking to one of the clinical pathologists involved in advance to know what the limitations are.” Take the time to talk with the person overseeing the test and get tips on how they want the sample transported and how the cases should be reported, he said.
If the patient has neurological symptoms, he said, before treating talk with a neurologist who can advise whether, for instance, a spinal tap is in order or whether an emergency department visit is appropriate.
“If you just start proceeding you may mess up the diagnostic signs that could show up in a lab test. Don’t be hesitant to ask for extra input from colleagues,” Dr. Schutzer said.
Suspicion in Endemic Areas
On Long Island, New York, where Lyme disease is endemic, internist Ian Storch, DO, said he sees “a few cases a season.
“We have a lot of people over the summer going to the Hamptons and areas out east for the weekend and tick bites are not uncommon,” he said. “People panic.”
He said one thing it’s important to tell patients is that the tick has to be on the skin for 48-72 hours to transmit the disease. If individuals were in a wooded area and were fine before they got there and the tick was attached for less than 2 days, “they’re usually fine.”
Another issue, Dr. Storch said, is patients sometimes want to get tested for Lyme disease immediately after a tick bite. But the antibody test doesn’t turn positive for weeks, he noted, and you can get a false-negative result. “If you’re worried and you really want to test, you need to wait 6 weeks to do the blood test.”
In his region, he said that although a tick bite is a red flag, he may also suspect Lyme disease when a patient presents with otherwise unexplained joint pain, weakness, lethargy, or fever. “In our area, those are things that would make you test for Lyme.”
He also urged consideration of Lyme in this new age of long COVID. Weakness, fatigue, and lethargy are also classic symptoms of long COVID, he noted. “Keep Lyme disease in your differential because there is a lot of overlap with chronic Lyme disease,” Dr. Storch said.
Discerning Lyme from Southern Tick–Associated Rash Illness
Bonnie M. Word, MD, director of the Houston Travel Medicine Clinic in Texas, where Lyme disease is not endemic, said Lyme disease “will not and should not be on the initial differential diagnosis for those residing in nonendemic areas unless a history of travel to an endemic area is obtained.”
She noted the typical EM rash may not be as distinct or easy to discern on black and brown skin. In addition, she said, EM may have many variations in presentation, such as a crusted center or faint borders, which could lead to a delay in diagnosis and treatment. She suggested consulting the CDC guidance on Lyme disease rashes.
Another challenge in diagnosis, she said, is the patient who presents with what appears to be a classic EM lesion but does not live in a Lyme-endemic area. In Texas, Southern Tick–Associated Rash Illness (STARI) may present with a similar lesion, she said.
“It is transmitted by the Lone Star Tick, which is found in the southeast and south-central US,” Dr. Word said. “However, its habitat is moving northward and westerly,” she said.
Adding Lyme disease to the differential diagnosis is reasonable, she said, if a patient presents with neurologic symptoms “such as a facial palsy, meningitis, radiculitis, and carditis if in addition to their symptoms there is evidence of an epidemiologic link to a Lyme-endemic region.”
She noted that a detailed travel history is important as “Lyme is also endemic in Eastern Canada, Europe, states of the former Soviet Union, China, Mongolia, and Japan.”
Primary care physicians play a critical role in evaluating, diagnosing, and treating most cases of early Lyme disease, thus limiting the number of people who will develop disseminated or late Lyme disease, she said. “The two latter manifestations are most often treated by infectious disease, neurology, or rheumatology specialists.”
Treatment*
Treatment is tailored to the clinical situation, Dr. Schutzer and Dr. Coyle write. A watch-and-wait approach may be appropriate in an asymptomatic but concerned person, even in an endemic area if the person has no known tick bite and no EM lesion.
If there is high risk of an infected ixodid tick bite in a high-incidence area and the tick was attached for at least 36 hours but less than 72 hours, one dose of doxycycline has been recommended as prophylaxis.
When a diagnosis of early nondisseminated Lyme disease is made after observation of an EM lesion, oral antibiotics are typically used to treat for 10 to 14 days. Suggested oral antibiotics and doses are 100 mg of doxycycline twice a day, 500 mg of amoxicillin three times a day, or 500 mg of cefuroxime twice a day, the authors write.
Dr. Schutzer said he hopes the paper serves as a refresher for those physicians who regularly see Lyme disease cases and also helps those newly included in the disease’s spreading regions.
“The earlier you diagnose it, the earlier you can treat it and the better the chance for a favorable outcome,” he said.
Dr. Schutzer, Dr. Coyle, Dr. Storch, and Dr. Word reported no relevant financial relationships.
*This story was updated on August, 2, 2024.
Shortage of Blood Bottles Could Disrupt Care
Hospitals and laboratories across the United States are grappling with a shortage of Becton Dickinson BACTEC blood culture bottles that threatens to extend at least until September.
In a health advisory, the Centers for Disease Control and Prevention (CDC) warned that the critical shortage could lead to “delays in diagnosis, misdiagnosis, or other challenges” in the management of patients with infectious diseases.
Healthcare providers, laboratories, healthcare facility administrators, and state, tribal, local, and territorial health departments affected by the shortage “should immediately begin to assess their situations and develop plans and options to mitigate the potential impact,” according to the health advisory.
What to Do
To reduce the impact of the shortage, facilities are urged to:
- Determine the type of blood culture bottles they have
- Optimize the use of blood cultures at their facility
- Take steps to prevent blood culture contamination
- Ensure that the appropriate volume of blood is collected for culture
- Assess alternate options for blood cultures
- Work with a nearby facility or send samples to another laboratory
Health departments are advised to contact hospitals and laboratories in their jurisdictions to determine whether the shortage will affect them. Health departments are also encouraged to educate others on the supply shortage, optimal use of blood cultures, and mechanisms for reporting supply chain shortages or interruptions to the Food and Drug Administration (FDA), as well as to help with communication between laboratories and facilities willing to assist others in need.
To further assist affected providers, the CDC, in collaboration with the Infectious Diseases Society of America, hosted a webinar with speakers from Johns Hopkins University, Massachusetts General Hospital, and Vanderbilt University, who shared what their institutions are doing to cope with the shortage and protect patients.
Why It Happened
In June, Becton Dickinson warned its customers that they may experience “intermittent delays” in the supply of some BACTEC blood culture media over the coming months because of reduced availability of plastic bottles from its supplier.
In a July 22 update, the company said the supplier issues were “more complex” than originally communicated and it is taking steps to “resolve this challenge as quickly as possible.”
In July, the FDA published a letter to healthcare providers acknowledging the supply disruptions and recommended strategies to preserve the supply for patients at highest risk.
Becton Dickinson has promised an update by September to this “dynamic and evolving situation.”
A version of this article appeared on Medscape.com.
Hospitals and laboratories across the United States are grappling with a shortage of Becton Dickinson BACTEC blood culture bottles that threatens to extend at least until September.
In a health advisory, the Centers for Disease Control and Prevention (CDC) warned that the critical shortage could lead to “delays in diagnosis, misdiagnosis, or other challenges” in the management of patients with infectious diseases.
Healthcare providers, laboratories, healthcare facility administrators, and state, tribal, local, and territorial health departments affected by the shortage “should immediately begin to assess their situations and develop plans and options to mitigate the potential impact,” according to the health advisory.
What to Do
To reduce the impact of the shortage, facilities are urged to:
- Determine the type of blood culture bottles they have
- Optimize the use of blood cultures at their facility
- Take steps to prevent blood culture contamination
- Ensure that the appropriate volume of blood is collected for culture
- Assess alternate options for blood cultures
- Work with a nearby facility or send samples to another laboratory
Health departments are advised to contact hospitals and laboratories in their jurisdictions to determine whether the shortage will affect them. Health departments are also encouraged to educate others on the supply shortage, optimal use of blood cultures, and mechanisms for reporting supply chain shortages or interruptions to the Food and Drug Administration (FDA), as well as to help with communication between laboratories and facilities willing to assist others in need.
To further assist affected providers, the CDC, in collaboration with the Infectious Diseases Society of America, hosted a webinar with speakers from Johns Hopkins University, Massachusetts General Hospital, and Vanderbilt University, who shared what their institutions are doing to cope with the shortage and protect patients.
Why It Happened
In June, Becton Dickinson warned its customers that they may experience “intermittent delays” in the supply of some BACTEC blood culture media over the coming months because of reduced availability of plastic bottles from its supplier.
In a July 22 update, the company said the supplier issues were “more complex” than originally communicated and it is taking steps to “resolve this challenge as quickly as possible.”
In July, the FDA published a letter to healthcare providers acknowledging the supply disruptions and recommended strategies to preserve the supply for patients at highest risk.
Becton Dickinson has promised an update by September to this “dynamic and evolving situation.”
A version of this article appeared on Medscape.com.
Hospitals and laboratories across the United States are grappling with a shortage of Becton Dickinson BACTEC blood culture bottles that threatens to extend at least until September.
In a health advisory, the Centers for Disease Control and Prevention (CDC) warned that the critical shortage could lead to “delays in diagnosis, misdiagnosis, or other challenges” in the management of patients with infectious diseases.
Healthcare providers, laboratories, healthcare facility administrators, and state, tribal, local, and territorial health departments affected by the shortage “should immediately begin to assess their situations and develop plans and options to mitigate the potential impact,” according to the health advisory.
What to Do
To reduce the impact of the shortage, facilities are urged to:
- Determine the type of blood culture bottles they have
- Optimize the use of blood cultures at their facility
- Take steps to prevent blood culture contamination
- Ensure that the appropriate volume of blood is collected for culture
- Assess alternate options for blood cultures
- Work with a nearby facility or send samples to another laboratory
Health departments are advised to contact hospitals and laboratories in their jurisdictions to determine whether the shortage will affect them. Health departments are also encouraged to educate others on the supply shortage, optimal use of blood cultures, and mechanisms for reporting supply chain shortages or interruptions to the Food and Drug Administration (FDA), as well as to help with communication between laboratories and facilities willing to assist others in need.
To further assist affected providers, the CDC, in collaboration with the Infectious Diseases Society of America, hosted a webinar with speakers from Johns Hopkins University, Massachusetts General Hospital, and Vanderbilt University, who shared what their institutions are doing to cope with the shortage and protect patients.
Why It Happened
In June, Becton Dickinson warned its customers that they may experience “intermittent delays” in the supply of some BACTEC blood culture media over the coming months because of reduced availability of plastic bottles from its supplier.
In a July 22 update, the company said the supplier issues were “more complex” than originally communicated and it is taking steps to “resolve this challenge as quickly as possible.”
In July, the FDA published a letter to healthcare providers acknowledging the supply disruptions and recommended strategies to preserve the supply for patients at highest risk.
Becton Dickinson has promised an update by September to this “dynamic and evolving situation.”
A version of this article appeared on Medscape.com.
‘Doesn’t Fit Anything I Trained for’: Committee Examines Treatment for Chronic Illness After Lyme Disease
WASHINGTON — Advancing treatment for what has been variably called chronic Lyme and posttreatment Lyme disease (PTLD) is under the eyes of a National Academies of Science, Engineering, and Medicine (NASEM) committee of experts for the first time — a year after the NASEM shone a spotlight on the need to accelerate research on chronic illnesses that follow known or suspected infections.
The committee will not make recommendations on specific approaches to diagnosis and treatment when it issues a report in early 2025 but will instead present “consensus findings” on treatment for chronic illness associated with Lyme disease, including recommendations for advancing treatment.
It’s an area void of the US Food and Drug Administration–approved therapies, void of any consensus on the off-label use of medications, and without any current standard of care or proven mechanisms and pathophysiology, said John Aucott, MD, director of the Johns Hopkins Medicine Lyme Disease Clinical Research Center, Baltimore, one of the invited speakers at a public meeting held by the NASEM in Washington, DC.
“The best way to look at this illness is not from the silos of infectious disease or the silos of rheumatology; you have to look across disciplines,” Dr. Aucott, also associate professor of medicine in the Division of Rheumatology, told the committee. “The story doesn’t fit anything I trained for in my infectious disease fellowship. Even today, I’d posit that PTLD is like an island — it’s still not connected to a lot of the mainstream of medicine.”
Rhisa Parera, who wrote and directed a 2021 documentary, Your Labs Are Normal, was one of several invited speakers who amplified the patient voice. Starting around age 7, she had pain in her knees, spine, and hips and vivid nightmares. In high school, she developed gastrointestinal issues, and in college, she developed debilitating neurologic symptoms.
Depression was her eventual diagnosis after having seen “every specialist in the book,” she said. At age 29, she received a positive western blot test and a Lyme disease diagnosis, at which point “I was prescribed 4 weeks of doxycycline and left in the dark,” the 34-year-old Black patient told the committee. Her health improved only after she began working with an “LLMD,” or Lyme-literate medical doctor (a term used in the patient community), while she lived with her mother and did not work, she said.
“I don’t share my Lyme disease history with other doctors. It’s pointless when you have those who will laugh at you, say you’re fine if you were treated, or just deny the disease completely,” Ms. Parera said. “We need this to be taught in medical school. It’s a literal emergency.”
Incidence and Potential Mechanisms
Limited research has suggested that 10%-20% of patients with Lyme disease develop persistent symptoms after standard antibiotic treatment advised by the Infectious Diseases Society of America (IDSA), Dr. Aucott said. (On its web page on chronic symptoms, the Centers for Disease Control and Prevention presents a more conservative range of 5%-10%.)
His own prospective cohort study at Johns Hopkins, published in 2022, found that 13.7% of 234 patients with prior Lyme disease met symptom and functional impact criteria for PTLD, compared with 4.1% of 49 participants without a history of Lyme disease — a statistically significant difference that he said should “put to rest” the question of “is it real?”
PTLD is the research case definition proposed by the IDSA in 2006; it requires that patients have prior documented Lyme disease, no other specific comorbidities, and specific symptoms (fatigue, widespread musculoskeletal pain, and/or cognitive difficulties) causing significant functional impact at least 6 months from their initial diagnosis and treatment.
In the real world, however, where diagnostics for acute Lyme disease are often inaccurate, erythema migrans is often absent, and the symptomatology of Lyme IACI is variable (and where there is no approved laboratory test or objective biomarker for diagnosing Lyme IACI), PTLD represents only a subset of a broader, heterogeneous population with persistent symptoms.
The term “Lyme IACI,” pronounced “Lyme eye-ACK-ee” at the meeting, builds on conversations at the 2023 NASEM workshop on infection-associated chronic illnesses and “encompasses a variety of terms that are used,” including PTLD, PTLD syndrome, persistent Lyme disease, and chronic Lyme disease, according to committee documents. Symptoms are distinct from the known complications of Lyme disease, such as arthritis or carditis.
The findings from Dr. Aucott’s SLICE cohort likely represent “the best outcome,” he said. They’re “probably not generalizable to a community setting where we see lots of missed diagnoses and delayed diagnoses,” as well as other tick-borne coinfections.
One of the challenges in designing future trials, in fact, relates to enrollment criteria and whether to use strict inclusion and exclusion criteria associated with the IDSA definition or take a broader approach to trial enrollment, he and others said. “You want to enroll patients for whom there’s no controversy that they’ve had Lyme infection ... for a study people believe in,” Dr. Aucott said during a discussion period, noting that it’s typical to screen over 100 patients to find one enrollee. “But it’s a tension we’re having.”
Timothy Sellati, PhD, chief scientific officer of the Global Lyme Alliance, urged change. “It’s really important to try to figure out how to alter our thinking on identifying and diagnosing chronic Lyme patients because they need to be recruited into clinical trials,” he said during his presentation.
“We think the best way to do this is to [develop and] employ composite diagnostic testing” that looks at unique Borrelia signatures (eg, protein, DNA, RNA, or metabolites), genetic and/or epigenetic signatures, inflammation signatures, T-cell-independent antibody signatures, and other elements, Dr. Sellati said.
Researchers designing treatment trials also face unknowns, Dr. Aucott and others said, about the role of potential mechanisms of Lyme IACI, from persistent Borrelia burgdorferi (or Borrelia mayonii) infection or the persistence of bacterial remnants (eg, nucleic acids or peptidoglycans) to infection-triggered pathology such as persistent immune dysregulation, chronic inflammation, autoimmunity, microbiome alterations, and dysautonomia and other neural network alterations.
The NASEM’s spotlight on Lyme IACI follows its long COVID-driven push last year to advance a common research agenda in infection-associated chronic illnesses. Investigators see common symptoms and potential shared mechanisms between long COVID, Lyme IACI, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and other complex chronic illnesses following infections.
At the Lyme IACI meeting, invited speakers described parts of the research landscape. Avindra Nath, MD, of the National Institute of Neurological Disorders and Stroke, for instance, described a recently published deep phenotyping study of 17 patients with ME/CFS that found decreased central catecholamine synthesis, circuit dysfunction of integrative brain regions, and immune profiling differences (eg, defects in B-cell maturation or T-cell exhaustion), compared with matched controls, that suggest the persistence of microbial antigens.
And John Leong, MD, PhD, of Tufts University, Boston, described his lab’s focus on understanding the microbe-host interactions that enable bloodstream dissemination and tissue invasion of B burgdorferi to take hold, increasing the risk for persistent symptoms. Other research at Tufts, he noted during a discussion period, has demonstrated the persistence of B burgdorferi to antibiotics in microtiter dishes. “Those organisms that survive are really difficult to eradicate in vitro,” Dr. Leong said.
Other physician investigators described research on nociplastic pain — a category of pain that can be triggered by infections, causing both amplified sensory processing and augmented central nervous system pain — and on whether reactivation of the Epstein-Barr virus could potentiate autoimmunity in the context of Borrelia infection.
Researchers are ready to test therapies while pathophysiology is unraveled — provided there is funding, Dr. Aucott said. The Clinical Trials Network for Lyme and Other Tick-Borne Diseases, coordinated by Brian Fallon, MD, of Columbia University, New York City, and funded several years ago by the Steven & Alexandra Cohen Foundation, has a slate of small pilot studies underway or being planned that address potential mechanisms (eg, studies of pulse intravenous ceftriaxone, tetracycline, transauricular vagus nerve stimulation, and mast cell modulation). And should full multisite trials be designed and funded, the network is ready with an infrastructure.
Need for Patient-Centered Outcomes
Persistent symptomatology is on the NIH’s radar screen. Efforts to understand causes were part of a strategic tick-borne disease research plan developed by the NIH in 2019. And in 2023, the National Institute of Allergy and Infectious Diseases (NIAID) funded seven projects addressing persistent symptoms that will run through 2028, C. Benjamin Beard, PhD, deputy division director of the CDC’s Division of Vector-Borne Disease, said at the NASEM committee meeting.
Patient advocates maintained that too much emphasis is placed on tick biology and pathophysiology. When Wendy Adams, research grant director and advisory board member of the Bay Area Lyme Foundation, and a colleague analyzed NIAID tick-borne disease funding from 2013 to 2021, they found that 75% of the funding went toward basic research, 15% to translational research, and “only 3% went to clinical research,” Ms. Adams told the committee.
Only 3% of the basic research budget was spent on coinfections, she said, and only 1% was spent on neurologic disease associated with tick-borne infections, both of which are survey-defined patient priorities. Moreover, “12% of the overall NIAID [tick-borne diseases] budget was spent on tick biology,” she said.
Research needs to involve community physicians who are utilizing the guidelines and approaches of the International Lyme and Associated Diseases Society to treat most patients with Lyme IACI, Ms. Adams said. “They have data to be mined,” she said, as does LymeDisease.org, which maintains a patient registry, MyLymeData, with over 18,000 patients. The organization has published two treatment studies, including one on antibiotic treatment response.
Lorraine Johnson, JD, MBA, CEO of LymeDisease.org and principal investigator of MyLymeData, stressed the importance of using patient-centered outcomes that incorporate minimal clinically important differences (MCIDs). “A change in the SF-36 score [without consideration of MCIDs] is not inherently important or meaningful to patients,” she said, referring to the SF-36 survey of health-related quality of life.
“This may seem like an esoteric issue, but two of the four clinical trials done [on retreatment of] persistent Lyme disease used the SF-36 as their outcome measure, and those studies, led by [Mark] Klempner, concluded that retreatment was not effective,” Ms. Johnson said. “Patients have been and continue to be harmed by [this research] because they’re told by physicians that antibiotics don’t work.”
A 2012 biostatistical review of these four RCTs — trials that helped inform the 2006 IDSA treatment guidelines — concluded that the Klempner studies “set the bar for treatment success too high,” Ms. Johnson said. Three of the four trials were likely underpowered to detect clinically meaningful treatment effects, the review also found.
The NASEM committee will hold additional public meetings and review a wide range of literature through this year. The formation of the committee was recommended by the US Department of Health and Human Services Tick-Borne Disease Working Group that was established by Congress in 2016 and concluded its work in 2022. The committee’s work is funded by the Cohen Foundation.
A version of this article appeared on Medscape.com.
WASHINGTON — Advancing treatment for what has been variably called chronic Lyme and posttreatment Lyme disease (PTLD) is under the eyes of a National Academies of Science, Engineering, and Medicine (NASEM) committee of experts for the first time — a year after the NASEM shone a spotlight on the need to accelerate research on chronic illnesses that follow known or suspected infections.
The committee will not make recommendations on specific approaches to diagnosis and treatment when it issues a report in early 2025 but will instead present “consensus findings” on treatment for chronic illness associated with Lyme disease, including recommendations for advancing treatment.
It’s an area void of the US Food and Drug Administration–approved therapies, void of any consensus on the off-label use of medications, and without any current standard of care or proven mechanisms and pathophysiology, said John Aucott, MD, director of the Johns Hopkins Medicine Lyme Disease Clinical Research Center, Baltimore, one of the invited speakers at a public meeting held by the NASEM in Washington, DC.
“The best way to look at this illness is not from the silos of infectious disease or the silos of rheumatology; you have to look across disciplines,” Dr. Aucott, also associate professor of medicine in the Division of Rheumatology, told the committee. “The story doesn’t fit anything I trained for in my infectious disease fellowship. Even today, I’d posit that PTLD is like an island — it’s still not connected to a lot of the mainstream of medicine.”
Rhisa Parera, who wrote and directed a 2021 documentary, Your Labs Are Normal, was one of several invited speakers who amplified the patient voice. Starting around age 7, she had pain in her knees, spine, and hips and vivid nightmares. In high school, she developed gastrointestinal issues, and in college, she developed debilitating neurologic symptoms.
Depression was her eventual diagnosis after having seen “every specialist in the book,” she said. At age 29, she received a positive western blot test and a Lyme disease diagnosis, at which point “I was prescribed 4 weeks of doxycycline and left in the dark,” the 34-year-old Black patient told the committee. Her health improved only after she began working with an “LLMD,” or Lyme-literate medical doctor (a term used in the patient community), while she lived with her mother and did not work, she said.
“I don’t share my Lyme disease history with other doctors. It’s pointless when you have those who will laugh at you, say you’re fine if you were treated, or just deny the disease completely,” Ms. Parera said. “We need this to be taught in medical school. It’s a literal emergency.”
Incidence and Potential Mechanisms
Limited research has suggested that 10%-20% of patients with Lyme disease develop persistent symptoms after standard antibiotic treatment advised by the Infectious Diseases Society of America (IDSA), Dr. Aucott said. (On its web page on chronic symptoms, the Centers for Disease Control and Prevention presents a more conservative range of 5%-10%.)
His own prospective cohort study at Johns Hopkins, published in 2022, found that 13.7% of 234 patients with prior Lyme disease met symptom and functional impact criteria for PTLD, compared with 4.1% of 49 participants without a history of Lyme disease — a statistically significant difference that he said should “put to rest” the question of “is it real?”
PTLD is the research case definition proposed by the IDSA in 2006; it requires that patients have prior documented Lyme disease, no other specific comorbidities, and specific symptoms (fatigue, widespread musculoskeletal pain, and/or cognitive difficulties) causing significant functional impact at least 6 months from their initial diagnosis and treatment.
In the real world, however, where diagnostics for acute Lyme disease are often inaccurate, erythema migrans is often absent, and the symptomatology of Lyme IACI is variable (and where there is no approved laboratory test or objective biomarker for diagnosing Lyme IACI), PTLD represents only a subset of a broader, heterogeneous population with persistent symptoms.
The term “Lyme IACI,” pronounced “Lyme eye-ACK-ee” at the meeting, builds on conversations at the 2023 NASEM workshop on infection-associated chronic illnesses and “encompasses a variety of terms that are used,” including PTLD, PTLD syndrome, persistent Lyme disease, and chronic Lyme disease, according to committee documents. Symptoms are distinct from the known complications of Lyme disease, such as arthritis or carditis.
The findings from Dr. Aucott’s SLICE cohort likely represent “the best outcome,” he said. They’re “probably not generalizable to a community setting where we see lots of missed diagnoses and delayed diagnoses,” as well as other tick-borne coinfections.
One of the challenges in designing future trials, in fact, relates to enrollment criteria and whether to use strict inclusion and exclusion criteria associated with the IDSA definition or take a broader approach to trial enrollment, he and others said. “You want to enroll patients for whom there’s no controversy that they’ve had Lyme infection ... for a study people believe in,” Dr. Aucott said during a discussion period, noting that it’s typical to screen over 100 patients to find one enrollee. “But it’s a tension we’re having.”
Timothy Sellati, PhD, chief scientific officer of the Global Lyme Alliance, urged change. “It’s really important to try to figure out how to alter our thinking on identifying and diagnosing chronic Lyme patients because they need to be recruited into clinical trials,” he said during his presentation.
“We think the best way to do this is to [develop and] employ composite diagnostic testing” that looks at unique Borrelia signatures (eg, protein, DNA, RNA, or metabolites), genetic and/or epigenetic signatures, inflammation signatures, T-cell-independent antibody signatures, and other elements, Dr. Sellati said.
Researchers designing treatment trials also face unknowns, Dr. Aucott and others said, about the role of potential mechanisms of Lyme IACI, from persistent Borrelia burgdorferi (or Borrelia mayonii) infection or the persistence of bacterial remnants (eg, nucleic acids or peptidoglycans) to infection-triggered pathology such as persistent immune dysregulation, chronic inflammation, autoimmunity, microbiome alterations, and dysautonomia and other neural network alterations.
The NASEM’s spotlight on Lyme IACI follows its long COVID-driven push last year to advance a common research agenda in infection-associated chronic illnesses. Investigators see common symptoms and potential shared mechanisms between long COVID, Lyme IACI, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and other complex chronic illnesses following infections.
At the Lyme IACI meeting, invited speakers described parts of the research landscape. Avindra Nath, MD, of the National Institute of Neurological Disorders and Stroke, for instance, described a recently published deep phenotyping study of 17 patients with ME/CFS that found decreased central catecholamine synthesis, circuit dysfunction of integrative brain regions, and immune profiling differences (eg, defects in B-cell maturation or T-cell exhaustion), compared with matched controls, that suggest the persistence of microbial antigens.
And John Leong, MD, PhD, of Tufts University, Boston, described his lab’s focus on understanding the microbe-host interactions that enable bloodstream dissemination and tissue invasion of B burgdorferi to take hold, increasing the risk for persistent symptoms. Other research at Tufts, he noted during a discussion period, has demonstrated the persistence of B burgdorferi to antibiotics in microtiter dishes. “Those organisms that survive are really difficult to eradicate in vitro,” Dr. Leong said.
Other physician investigators described research on nociplastic pain — a category of pain that can be triggered by infections, causing both amplified sensory processing and augmented central nervous system pain — and on whether reactivation of the Epstein-Barr virus could potentiate autoimmunity in the context of Borrelia infection.
Researchers are ready to test therapies while pathophysiology is unraveled — provided there is funding, Dr. Aucott said. The Clinical Trials Network for Lyme and Other Tick-Borne Diseases, coordinated by Brian Fallon, MD, of Columbia University, New York City, and funded several years ago by the Steven & Alexandra Cohen Foundation, has a slate of small pilot studies underway or being planned that address potential mechanisms (eg, studies of pulse intravenous ceftriaxone, tetracycline, transauricular vagus nerve stimulation, and mast cell modulation). And should full multisite trials be designed and funded, the network is ready with an infrastructure.
Need for Patient-Centered Outcomes
Persistent symptomatology is on the NIH’s radar screen. Efforts to understand causes were part of a strategic tick-borne disease research plan developed by the NIH in 2019. And in 2023, the National Institute of Allergy and Infectious Diseases (NIAID) funded seven projects addressing persistent symptoms that will run through 2028, C. Benjamin Beard, PhD, deputy division director of the CDC’s Division of Vector-Borne Disease, said at the NASEM committee meeting.
Patient advocates maintained that too much emphasis is placed on tick biology and pathophysiology. When Wendy Adams, research grant director and advisory board member of the Bay Area Lyme Foundation, and a colleague analyzed NIAID tick-borne disease funding from 2013 to 2021, they found that 75% of the funding went toward basic research, 15% to translational research, and “only 3% went to clinical research,” Ms. Adams told the committee.
Only 3% of the basic research budget was spent on coinfections, she said, and only 1% was spent on neurologic disease associated with tick-borne infections, both of which are survey-defined patient priorities. Moreover, “12% of the overall NIAID [tick-borne diseases] budget was spent on tick biology,” she said.
Research needs to involve community physicians who are utilizing the guidelines and approaches of the International Lyme and Associated Diseases Society to treat most patients with Lyme IACI, Ms. Adams said. “They have data to be mined,” she said, as does LymeDisease.org, which maintains a patient registry, MyLymeData, with over 18,000 patients. The organization has published two treatment studies, including one on antibiotic treatment response.
Lorraine Johnson, JD, MBA, CEO of LymeDisease.org and principal investigator of MyLymeData, stressed the importance of using patient-centered outcomes that incorporate minimal clinically important differences (MCIDs). “A change in the SF-36 score [without consideration of MCIDs] is not inherently important or meaningful to patients,” she said, referring to the SF-36 survey of health-related quality of life.
“This may seem like an esoteric issue, but two of the four clinical trials done [on retreatment of] persistent Lyme disease used the SF-36 as their outcome measure, and those studies, led by [Mark] Klempner, concluded that retreatment was not effective,” Ms. Johnson said. “Patients have been and continue to be harmed by [this research] because they’re told by physicians that antibiotics don’t work.”
A 2012 biostatistical review of these four RCTs — trials that helped inform the 2006 IDSA treatment guidelines — concluded that the Klempner studies “set the bar for treatment success too high,” Ms. Johnson said. Three of the four trials were likely underpowered to detect clinically meaningful treatment effects, the review also found.
The NASEM committee will hold additional public meetings and review a wide range of literature through this year. The formation of the committee was recommended by the US Department of Health and Human Services Tick-Borne Disease Working Group that was established by Congress in 2016 and concluded its work in 2022. The committee’s work is funded by the Cohen Foundation.
A version of this article appeared on Medscape.com.
WASHINGTON — Advancing treatment for what has been variably called chronic Lyme and posttreatment Lyme disease (PTLD) is under the eyes of a National Academies of Science, Engineering, and Medicine (NASEM) committee of experts for the first time — a year after the NASEM shone a spotlight on the need to accelerate research on chronic illnesses that follow known or suspected infections.
The committee will not make recommendations on specific approaches to diagnosis and treatment when it issues a report in early 2025 but will instead present “consensus findings” on treatment for chronic illness associated with Lyme disease, including recommendations for advancing treatment.
It’s an area void of the US Food and Drug Administration–approved therapies, void of any consensus on the off-label use of medications, and without any current standard of care or proven mechanisms and pathophysiology, said John Aucott, MD, director of the Johns Hopkins Medicine Lyme Disease Clinical Research Center, Baltimore, one of the invited speakers at a public meeting held by the NASEM in Washington, DC.
“The best way to look at this illness is not from the silos of infectious disease or the silos of rheumatology; you have to look across disciplines,” Dr. Aucott, also associate professor of medicine in the Division of Rheumatology, told the committee. “The story doesn’t fit anything I trained for in my infectious disease fellowship. Even today, I’d posit that PTLD is like an island — it’s still not connected to a lot of the mainstream of medicine.”
Rhisa Parera, who wrote and directed a 2021 documentary, Your Labs Are Normal, was one of several invited speakers who amplified the patient voice. Starting around age 7, she had pain in her knees, spine, and hips and vivid nightmares. In high school, she developed gastrointestinal issues, and in college, she developed debilitating neurologic symptoms.
Depression was her eventual diagnosis after having seen “every specialist in the book,” she said. At age 29, she received a positive western blot test and a Lyme disease diagnosis, at which point “I was prescribed 4 weeks of doxycycline and left in the dark,” the 34-year-old Black patient told the committee. Her health improved only after she began working with an “LLMD,” or Lyme-literate medical doctor (a term used in the patient community), while she lived with her mother and did not work, she said.
“I don’t share my Lyme disease history with other doctors. It’s pointless when you have those who will laugh at you, say you’re fine if you were treated, or just deny the disease completely,” Ms. Parera said. “We need this to be taught in medical school. It’s a literal emergency.”
Incidence and Potential Mechanisms
Limited research has suggested that 10%-20% of patients with Lyme disease develop persistent symptoms after standard antibiotic treatment advised by the Infectious Diseases Society of America (IDSA), Dr. Aucott said. (On its web page on chronic symptoms, the Centers for Disease Control and Prevention presents a more conservative range of 5%-10%.)
His own prospective cohort study at Johns Hopkins, published in 2022, found that 13.7% of 234 patients with prior Lyme disease met symptom and functional impact criteria for PTLD, compared with 4.1% of 49 participants without a history of Lyme disease — a statistically significant difference that he said should “put to rest” the question of “is it real?”
PTLD is the research case definition proposed by the IDSA in 2006; it requires that patients have prior documented Lyme disease, no other specific comorbidities, and specific symptoms (fatigue, widespread musculoskeletal pain, and/or cognitive difficulties) causing significant functional impact at least 6 months from their initial diagnosis and treatment.
In the real world, however, where diagnostics for acute Lyme disease are often inaccurate, erythema migrans is often absent, and the symptomatology of Lyme IACI is variable (and where there is no approved laboratory test or objective biomarker for diagnosing Lyme IACI), PTLD represents only a subset of a broader, heterogeneous population with persistent symptoms.
The term “Lyme IACI,” pronounced “Lyme eye-ACK-ee” at the meeting, builds on conversations at the 2023 NASEM workshop on infection-associated chronic illnesses and “encompasses a variety of terms that are used,” including PTLD, PTLD syndrome, persistent Lyme disease, and chronic Lyme disease, according to committee documents. Symptoms are distinct from the known complications of Lyme disease, such as arthritis or carditis.
The findings from Dr. Aucott’s SLICE cohort likely represent “the best outcome,” he said. They’re “probably not generalizable to a community setting where we see lots of missed diagnoses and delayed diagnoses,” as well as other tick-borne coinfections.
One of the challenges in designing future trials, in fact, relates to enrollment criteria and whether to use strict inclusion and exclusion criteria associated with the IDSA definition or take a broader approach to trial enrollment, he and others said. “You want to enroll patients for whom there’s no controversy that they’ve had Lyme infection ... for a study people believe in,” Dr. Aucott said during a discussion period, noting that it’s typical to screen over 100 patients to find one enrollee. “But it’s a tension we’re having.”
Timothy Sellati, PhD, chief scientific officer of the Global Lyme Alliance, urged change. “It’s really important to try to figure out how to alter our thinking on identifying and diagnosing chronic Lyme patients because they need to be recruited into clinical trials,” he said during his presentation.
“We think the best way to do this is to [develop and] employ composite diagnostic testing” that looks at unique Borrelia signatures (eg, protein, DNA, RNA, or metabolites), genetic and/or epigenetic signatures, inflammation signatures, T-cell-independent antibody signatures, and other elements, Dr. Sellati said.
Researchers designing treatment trials also face unknowns, Dr. Aucott and others said, about the role of potential mechanisms of Lyme IACI, from persistent Borrelia burgdorferi (or Borrelia mayonii) infection or the persistence of bacterial remnants (eg, nucleic acids or peptidoglycans) to infection-triggered pathology such as persistent immune dysregulation, chronic inflammation, autoimmunity, microbiome alterations, and dysautonomia and other neural network alterations.
The NASEM’s spotlight on Lyme IACI follows its long COVID-driven push last year to advance a common research agenda in infection-associated chronic illnesses. Investigators see common symptoms and potential shared mechanisms between long COVID, Lyme IACI, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and other complex chronic illnesses following infections.
At the Lyme IACI meeting, invited speakers described parts of the research landscape. Avindra Nath, MD, of the National Institute of Neurological Disorders and Stroke, for instance, described a recently published deep phenotyping study of 17 patients with ME/CFS that found decreased central catecholamine synthesis, circuit dysfunction of integrative brain regions, and immune profiling differences (eg, defects in B-cell maturation or T-cell exhaustion), compared with matched controls, that suggest the persistence of microbial antigens.
And John Leong, MD, PhD, of Tufts University, Boston, described his lab’s focus on understanding the microbe-host interactions that enable bloodstream dissemination and tissue invasion of B burgdorferi to take hold, increasing the risk for persistent symptoms. Other research at Tufts, he noted during a discussion period, has demonstrated the persistence of B burgdorferi to antibiotics in microtiter dishes. “Those organisms that survive are really difficult to eradicate in vitro,” Dr. Leong said.
Other physician investigators described research on nociplastic pain — a category of pain that can be triggered by infections, causing both amplified sensory processing and augmented central nervous system pain — and on whether reactivation of the Epstein-Barr virus could potentiate autoimmunity in the context of Borrelia infection.
Researchers are ready to test therapies while pathophysiology is unraveled — provided there is funding, Dr. Aucott said. The Clinical Trials Network for Lyme and Other Tick-Borne Diseases, coordinated by Brian Fallon, MD, of Columbia University, New York City, and funded several years ago by the Steven & Alexandra Cohen Foundation, has a slate of small pilot studies underway or being planned that address potential mechanisms (eg, studies of pulse intravenous ceftriaxone, tetracycline, transauricular vagus nerve stimulation, and mast cell modulation). And should full multisite trials be designed and funded, the network is ready with an infrastructure.
Need for Patient-Centered Outcomes
Persistent symptomatology is on the NIH’s radar screen. Efforts to understand causes were part of a strategic tick-borne disease research plan developed by the NIH in 2019. And in 2023, the National Institute of Allergy and Infectious Diseases (NIAID) funded seven projects addressing persistent symptoms that will run through 2028, C. Benjamin Beard, PhD, deputy division director of the CDC’s Division of Vector-Borne Disease, said at the NASEM committee meeting.
Patient advocates maintained that too much emphasis is placed on tick biology and pathophysiology. When Wendy Adams, research grant director and advisory board member of the Bay Area Lyme Foundation, and a colleague analyzed NIAID tick-borne disease funding from 2013 to 2021, they found that 75% of the funding went toward basic research, 15% to translational research, and “only 3% went to clinical research,” Ms. Adams told the committee.
Only 3% of the basic research budget was spent on coinfections, she said, and only 1% was spent on neurologic disease associated with tick-borne infections, both of which are survey-defined patient priorities. Moreover, “12% of the overall NIAID [tick-borne diseases] budget was spent on tick biology,” she said.
Research needs to involve community physicians who are utilizing the guidelines and approaches of the International Lyme and Associated Diseases Society to treat most patients with Lyme IACI, Ms. Adams said. “They have data to be mined,” she said, as does LymeDisease.org, which maintains a patient registry, MyLymeData, with over 18,000 patients. The organization has published two treatment studies, including one on antibiotic treatment response.
Lorraine Johnson, JD, MBA, CEO of LymeDisease.org and principal investigator of MyLymeData, stressed the importance of using patient-centered outcomes that incorporate minimal clinically important differences (MCIDs). “A change in the SF-36 score [without consideration of MCIDs] is not inherently important or meaningful to patients,” she said, referring to the SF-36 survey of health-related quality of life.
“This may seem like an esoteric issue, but two of the four clinical trials done [on retreatment of] persistent Lyme disease used the SF-36 as their outcome measure, and those studies, led by [Mark] Klempner, concluded that retreatment was not effective,” Ms. Johnson said. “Patients have been and continue to be harmed by [this research] because they’re told by physicians that antibiotics don’t work.”
A 2012 biostatistical review of these four RCTs — trials that helped inform the 2006 IDSA treatment guidelines — concluded that the Klempner studies “set the bar for treatment success too high,” Ms. Johnson said. Three of the four trials were likely underpowered to detect clinically meaningful treatment effects, the review also found.
The NASEM committee will hold additional public meetings and review a wide range of literature through this year. The formation of the committee was recommended by the US Department of Health and Human Services Tick-Borne Disease Working Group that was established by Congress in 2016 and concluded its work in 2022. The committee’s work is funded by the Cohen Foundation.
A version of this article appeared on Medscape.com.
Twice-Yearly PrEP Gives ‘Huge’ 100% Protection
Twice-yearly injections are 100% effective in preventing new infections, according to the final results from the PURPOSE 1 trial of lenacapavir.
For weeks, the HIV community has been talking about this highly anticipated clinical trial and whether the strong — and to many, surprising — interim results would hold at final presentation at the International AIDS Conference 2024 in Munich, Germany.
Presenting the results, Linda-Gail Bekker, MD, director of the Desmond Tutu HIV Center at the University of Cape Town, South Africa, reported zero new infections in those who got the shots in the study of about 5000 young women. In the group given daily oral preexposure prophylaxis (PrEP), roughly 2% contracted HIV from infected partners.
“A twice-yearly PrEP choice could overcome some of the adherence and persistence challenges and contribute critically to our quest to reduce HIV infection in women around the world,” Dr. Bekker said about the results, which were published simultaneously in The New England Journal of Medicine.
PURPOSE 1 confirmed that lenacapavir is a “breakthrough” for HIV prevention, said International AIDS Society president Sharon Lewin, PhD, MBBS. It has “huge public health potential,” said Dr. Lewin, the AIDS 2024 conference cochair and director of the Peter Doherty Institute for Infection and Immunity at the University of Melbourne in Australia.
Lenacapavir is a novel, first-in-class multistage HIV-1 capsid inhibitor with a long half-life, which enables the twice-yearly dosing.
PURPOSE 1 enrolled women aged 15-25 years who were at risk for HIV in South Africa and Uganda, with a primary endpoint of HIV infection. Because of the previously announced interim results, which showed the injection was preventing infections, study sponsor Gilead Sciences discontinued the randomized phase of the trial and shifted to an open-label design for lenacapavir.
“One hundred percent efficacy is more that we could ever have hoped for a potential prevention efficacy,” said Christoph Spinner, MD, MBA, an infectious disease specialist at the University Hospital of the Technical University of Munich and AIDS 2024 conference cochair.
Dr. Spinner added that while this is the first study of lenacapavir for PrEP, it’s also the first to explore outcomes of emtricitabine-tenofovir in cisgender women.
Strong Adherence Rates
The twice-yearly injection demonstrated adherence rates above 90% in the trial for both the 6- and 12-month injection intervals.
“Adherence was 91.5% at week 26 and 92.8% at week 52,” Dr. Bekker reported.
The trial compared three PrEP options including the lenacapavir injection to once-daily oral emtricitabine 200 mg and tenofovir-alafenamide 25 mg (F/TAF) and once-daily emtricitabine 200 mg and tenofovir–disoproxil fumarate 300 mg (F/TDF).
“Most participants in both the F/TAF and F/TDF groups had low adherence, and this declined over time,” Dr. Bekker reported. At 52 weeks, the vast majority of patients on both oral therapies had low adherence with dosing, defined at less than two doses a week.
Dr. Bekker called the adherence to the oral agents in this trial “disappointing.”
Findings from the trial underscore the challenges of adherence to a daily oral medication, Rochelle Walensky, MD, and Lindsey Baden, MD, from the Harvard Kennedy School of Government and Harvard Business School in Cambridge, Massachusetts, wrote in an editorial accompanying the published results.
With almost 92% attendance for the twice-yearly lenacapavir injections, the “well-done,” large, randomized, controlled trial “exemplifies not only that women can dependably adhere to this administration schedule, but also that levels of an HIV-1 capsid inhibitor can remain high enough over a period of 6 months to reliably prevent infection,” they added.
Another key focus of the presentation was adverse events. The rate of adverse events grade 3 or more in the lenacapavir arm was 4.1%, Bekker said, which is slightly lower than the rates in the oral arms. The rates of serious adverse events were 2.8% for lenacapavir, 4% for F/TAF and 3.3% for F/TDF.
Injection Site Reactions
Injection site reactions occurred in 68% of the lenacapavir group, including 63% with subcutaneous nodules.
The injection can form “a drug depot which may be palpable as a nodule,” Dr. Bekker said. In the placebo group, 34% of patients had injection-site reactions and 16% had nodules. Nearly all injection-site reactions were grade 1 or 2, she said. “Higher grade injection-site reactions were rare and not serious and occurred in a similar percentage in lenacapavir and placebo,” she said.
Overall, more than 25,000 injections of lenacapavir have been given, Dr. Bekker said, and four patients discontinued treatment because of injection-site reactions. “Reporting of injection-site reactions, including nodules, decreased with subsequent doses,” she said.
Contraception was not a requirement for enrollment in the study, Dr. Bekker pointed out, and pregnancy outcomes across the treatment arms were similar to the general population.
First in a Series of Trials
This is the first in a series of PURPOSE trials, Bekker reported. The phase 3 PURPOSE 2 trial, enrolling 3000 gay men, transgender women, transgender men and gender nonbinary people who have sex with male partners, is the second pivotal trial now underway.
Three other smaller trials are in the clinic in the United States and Europe.
PURPOSE 1 participants will continue to access lenacapavir until the product is available in South Africa and Uganda, Dr. Bekker said. Trial sponsor Gilead Sciences is also developing a direct licensing program to expedite generic access to the drug in high-incidence, resource-limited countries, she said.
Dr. Walensky and Dr. Baden report that lenacapavir currently costs about $43,000 annually in the United States. “But the results of the PURPOSE 1 trial have now created a moral imperative to make lenacapavir broadly accessible and affordable as PrEP” to people who were enrolled, as well as all those who are similarly eligible and could benefit.
So now we have a PrEP product with high efficacy, they added. “That is great news for science but not (yet) great for women.”
Given the high pregnancy rate among participants in the PURPOSE 1 trial, Dr. Walensky and Dr. Baden point out the assessment of lenacapavir safety is a priority. They are also interested in learning more about drug resistance with this new option.
“I f approved and delivered — rapidly, affordably, and equitably — to those who need or want it, this long-acting tool could help accelerate global progress in HIV prevention,” Dr. Lewin said.
Now, she added, “we eagerly await results from PURPOSE 2.”
A version of this article first appeared on Medscape.com.
Twice-yearly injections are 100% effective in preventing new infections, according to the final results from the PURPOSE 1 trial of lenacapavir.
For weeks, the HIV community has been talking about this highly anticipated clinical trial and whether the strong — and to many, surprising — interim results would hold at final presentation at the International AIDS Conference 2024 in Munich, Germany.
Presenting the results, Linda-Gail Bekker, MD, director of the Desmond Tutu HIV Center at the University of Cape Town, South Africa, reported zero new infections in those who got the shots in the study of about 5000 young women. In the group given daily oral preexposure prophylaxis (PrEP), roughly 2% contracted HIV from infected partners.
“A twice-yearly PrEP choice could overcome some of the adherence and persistence challenges and contribute critically to our quest to reduce HIV infection in women around the world,” Dr. Bekker said about the results, which were published simultaneously in The New England Journal of Medicine.
PURPOSE 1 confirmed that lenacapavir is a “breakthrough” for HIV prevention, said International AIDS Society president Sharon Lewin, PhD, MBBS. It has “huge public health potential,” said Dr. Lewin, the AIDS 2024 conference cochair and director of the Peter Doherty Institute for Infection and Immunity at the University of Melbourne in Australia.
Lenacapavir is a novel, first-in-class multistage HIV-1 capsid inhibitor with a long half-life, which enables the twice-yearly dosing.
PURPOSE 1 enrolled women aged 15-25 years who were at risk for HIV in South Africa and Uganda, with a primary endpoint of HIV infection. Because of the previously announced interim results, which showed the injection was preventing infections, study sponsor Gilead Sciences discontinued the randomized phase of the trial and shifted to an open-label design for lenacapavir.
“One hundred percent efficacy is more that we could ever have hoped for a potential prevention efficacy,” said Christoph Spinner, MD, MBA, an infectious disease specialist at the University Hospital of the Technical University of Munich and AIDS 2024 conference cochair.
Dr. Spinner added that while this is the first study of lenacapavir for PrEP, it’s also the first to explore outcomes of emtricitabine-tenofovir in cisgender women.
Strong Adherence Rates
The twice-yearly injection demonstrated adherence rates above 90% in the trial for both the 6- and 12-month injection intervals.
“Adherence was 91.5% at week 26 and 92.8% at week 52,” Dr. Bekker reported.
The trial compared three PrEP options including the lenacapavir injection to once-daily oral emtricitabine 200 mg and tenofovir-alafenamide 25 mg (F/TAF) and once-daily emtricitabine 200 mg and tenofovir–disoproxil fumarate 300 mg (F/TDF).
“Most participants in both the F/TAF and F/TDF groups had low adherence, and this declined over time,” Dr. Bekker reported. At 52 weeks, the vast majority of patients on both oral therapies had low adherence with dosing, defined at less than two doses a week.
Dr. Bekker called the adherence to the oral agents in this trial “disappointing.”
Findings from the trial underscore the challenges of adherence to a daily oral medication, Rochelle Walensky, MD, and Lindsey Baden, MD, from the Harvard Kennedy School of Government and Harvard Business School in Cambridge, Massachusetts, wrote in an editorial accompanying the published results.
With almost 92% attendance for the twice-yearly lenacapavir injections, the “well-done,” large, randomized, controlled trial “exemplifies not only that women can dependably adhere to this administration schedule, but also that levels of an HIV-1 capsid inhibitor can remain high enough over a period of 6 months to reliably prevent infection,” they added.
Another key focus of the presentation was adverse events. The rate of adverse events grade 3 or more in the lenacapavir arm was 4.1%, Bekker said, which is slightly lower than the rates in the oral arms. The rates of serious adverse events were 2.8% for lenacapavir, 4% for F/TAF and 3.3% for F/TDF.
Injection Site Reactions
Injection site reactions occurred in 68% of the lenacapavir group, including 63% with subcutaneous nodules.
The injection can form “a drug depot which may be palpable as a nodule,” Dr. Bekker said. In the placebo group, 34% of patients had injection-site reactions and 16% had nodules. Nearly all injection-site reactions were grade 1 or 2, she said. “Higher grade injection-site reactions were rare and not serious and occurred in a similar percentage in lenacapavir and placebo,” she said.
Overall, more than 25,000 injections of lenacapavir have been given, Dr. Bekker said, and four patients discontinued treatment because of injection-site reactions. “Reporting of injection-site reactions, including nodules, decreased with subsequent doses,” she said.
Contraception was not a requirement for enrollment in the study, Dr. Bekker pointed out, and pregnancy outcomes across the treatment arms were similar to the general population.
First in a Series of Trials
This is the first in a series of PURPOSE trials, Bekker reported. The phase 3 PURPOSE 2 trial, enrolling 3000 gay men, transgender women, transgender men and gender nonbinary people who have sex with male partners, is the second pivotal trial now underway.
Three other smaller trials are in the clinic in the United States and Europe.
PURPOSE 1 participants will continue to access lenacapavir until the product is available in South Africa and Uganda, Dr. Bekker said. Trial sponsor Gilead Sciences is also developing a direct licensing program to expedite generic access to the drug in high-incidence, resource-limited countries, she said.
Dr. Walensky and Dr. Baden report that lenacapavir currently costs about $43,000 annually in the United States. “But the results of the PURPOSE 1 trial have now created a moral imperative to make lenacapavir broadly accessible and affordable as PrEP” to people who were enrolled, as well as all those who are similarly eligible and could benefit.
So now we have a PrEP product with high efficacy, they added. “That is great news for science but not (yet) great for women.”
Given the high pregnancy rate among participants in the PURPOSE 1 trial, Dr. Walensky and Dr. Baden point out the assessment of lenacapavir safety is a priority. They are also interested in learning more about drug resistance with this new option.
“I f approved and delivered — rapidly, affordably, and equitably — to those who need or want it, this long-acting tool could help accelerate global progress in HIV prevention,” Dr. Lewin said.
Now, she added, “we eagerly await results from PURPOSE 2.”
A version of this article first appeared on Medscape.com.
Twice-yearly injections are 100% effective in preventing new infections, according to the final results from the PURPOSE 1 trial of lenacapavir.
For weeks, the HIV community has been talking about this highly anticipated clinical trial and whether the strong — and to many, surprising — interim results would hold at final presentation at the International AIDS Conference 2024 in Munich, Germany.
Presenting the results, Linda-Gail Bekker, MD, director of the Desmond Tutu HIV Center at the University of Cape Town, South Africa, reported zero new infections in those who got the shots in the study of about 5000 young women. In the group given daily oral preexposure prophylaxis (PrEP), roughly 2% contracted HIV from infected partners.
“A twice-yearly PrEP choice could overcome some of the adherence and persistence challenges and contribute critically to our quest to reduce HIV infection in women around the world,” Dr. Bekker said about the results, which were published simultaneously in The New England Journal of Medicine.
PURPOSE 1 confirmed that lenacapavir is a “breakthrough” for HIV prevention, said International AIDS Society president Sharon Lewin, PhD, MBBS. It has “huge public health potential,” said Dr. Lewin, the AIDS 2024 conference cochair and director of the Peter Doherty Institute for Infection and Immunity at the University of Melbourne in Australia.
Lenacapavir is a novel, first-in-class multistage HIV-1 capsid inhibitor with a long half-life, which enables the twice-yearly dosing.
PURPOSE 1 enrolled women aged 15-25 years who were at risk for HIV in South Africa and Uganda, with a primary endpoint of HIV infection. Because of the previously announced interim results, which showed the injection was preventing infections, study sponsor Gilead Sciences discontinued the randomized phase of the trial and shifted to an open-label design for lenacapavir.
“One hundred percent efficacy is more that we could ever have hoped for a potential prevention efficacy,” said Christoph Spinner, MD, MBA, an infectious disease specialist at the University Hospital of the Technical University of Munich and AIDS 2024 conference cochair.
Dr. Spinner added that while this is the first study of lenacapavir for PrEP, it’s also the first to explore outcomes of emtricitabine-tenofovir in cisgender women.
Strong Adherence Rates
The twice-yearly injection demonstrated adherence rates above 90% in the trial for both the 6- and 12-month injection intervals.
“Adherence was 91.5% at week 26 and 92.8% at week 52,” Dr. Bekker reported.
The trial compared three PrEP options including the lenacapavir injection to once-daily oral emtricitabine 200 mg and tenofovir-alafenamide 25 mg (F/TAF) and once-daily emtricitabine 200 mg and tenofovir–disoproxil fumarate 300 mg (F/TDF).
“Most participants in both the F/TAF and F/TDF groups had low adherence, and this declined over time,” Dr. Bekker reported. At 52 weeks, the vast majority of patients on both oral therapies had low adherence with dosing, defined at less than two doses a week.
Dr. Bekker called the adherence to the oral agents in this trial “disappointing.”
Findings from the trial underscore the challenges of adherence to a daily oral medication, Rochelle Walensky, MD, and Lindsey Baden, MD, from the Harvard Kennedy School of Government and Harvard Business School in Cambridge, Massachusetts, wrote in an editorial accompanying the published results.
With almost 92% attendance for the twice-yearly lenacapavir injections, the “well-done,” large, randomized, controlled trial “exemplifies not only that women can dependably adhere to this administration schedule, but also that levels of an HIV-1 capsid inhibitor can remain high enough over a period of 6 months to reliably prevent infection,” they added.
Another key focus of the presentation was adverse events. The rate of adverse events grade 3 or more in the lenacapavir arm was 4.1%, Bekker said, which is slightly lower than the rates in the oral arms. The rates of serious adverse events were 2.8% for lenacapavir, 4% for F/TAF and 3.3% for F/TDF.
Injection Site Reactions
Injection site reactions occurred in 68% of the lenacapavir group, including 63% with subcutaneous nodules.
The injection can form “a drug depot which may be palpable as a nodule,” Dr. Bekker said. In the placebo group, 34% of patients had injection-site reactions and 16% had nodules. Nearly all injection-site reactions were grade 1 or 2, she said. “Higher grade injection-site reactions were rare and not serious and occurred in a similar percentage in lenacapavir and placebo,” she said.
Overall, more than 25,000 injections of lenacapavir have been given, Dr. Bekker said, and four patients discontinued treatment because of injection-site reactions. “Reporting of injection-site reactions, including nodules, decreased with subsequent doses,” she said.
Contraception was not a requirement for enrollment in the study, Dr. Bekker pointed out, and pregnancy outcomes across the treatment arms were similar to the general population.
First in a Series of Trials
This is the first in a series of PURPOSE trials, Bekker reported. The phase 3 PURPOSE 2 trial, enrolling 3000 gay men, transgender women, transgender men and gender nonbinary people who have sex with male partners, is the second pivotal trial now underway.
Three other smaller trials are in the clinic in the United States and Europe.
PURPOSE 1 participants will continue to access lenacapavir until the product is available in South Africa and Uganda, Dr. Bekker said. Trial sponsor Gilead Sciences is also developing a direct licensing program to expedite generic access to the drug in high-incidence, resource-limited countries, she said.
Dr. Walensky and Dr. Baden report that lenacapavir currently costs about $43,000 annually in the United States. “But the results of the PURPOSE 1 trial have now created a moral imperative to make lenacapavir broadly accessible and affordable as PrEP” to people who were enrolled, as well as all those who are similarly eligible and could benefit.
So now we have a PrEP product with high efficacy, they added. “That is great news for science but not (yet) great for women.”
Given the high pregnancy rate among participants in the PURPOSE 1 trial, Dr. Walensky and Dr. Baden point out the assessment of lenacapavir safety is a priority. They are also interested in learning more about drug resistance with this new option.
“I f approved and delivered — rapidly, affordably, and equitably — to those who need or want it, this long-acting tool could help accelerate global progress in HIV prevention,” Dr. Lewin said.
Now, she added, “we eagerly await results from PURPOSE 2.”
A version of this article first appeared on Medscape.com.
FROM AIDS 2024
Study Links Newer Shingles Vaccine to Delayed Dementia Diagnosis
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Mycobacterium interjectum Infection in an Immunocompetent Host Following Contact With Aquarium Fish
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.
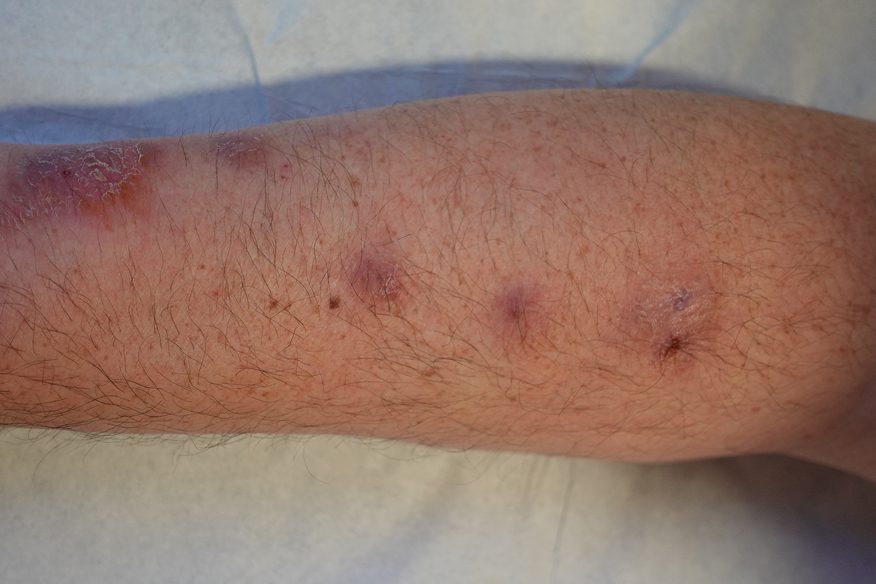
The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.

The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.

The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
Practice Points
- Mycobacterium interjectum can cause cutaneous nodules in a sporotrichoid or lymphocutaneous pattern and may affect immunocompromised and immunocompetent patients.
- This mycobacteria has been detected in water, soil, and aquarium fish. The latter could be a source of infection and should be taken into account in the anamnesis.
- There is no established therapeutic regimen for M interjectum infection. Combination therapy with rifampicin, clarithromycin, and co-trimoxazole could be an option, though it must always be adapted to an antibiogram if results are available.
Disruptive Sleep Linked to Increased Susceptibility to COVID-19
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.