User login
Low HPV Vaccination in the United States Is a Public Health ‘Failure’
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
Predicting RSV’s Role in the Upcoming Winter Respiratory Season
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
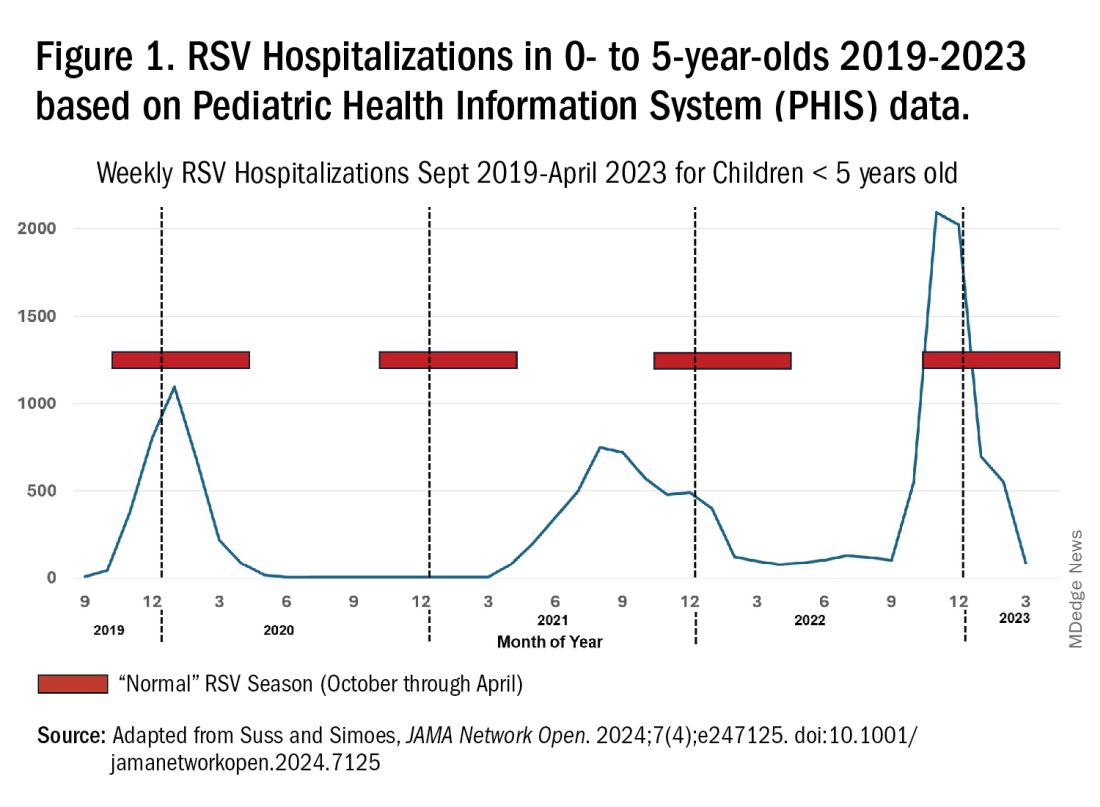
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV, influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6 
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV, influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6 
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV, influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6 
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
Misdiagnosis of Crusted Scabies: Skin Excoriations Resembling Brown Sugar Are Characteristic
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
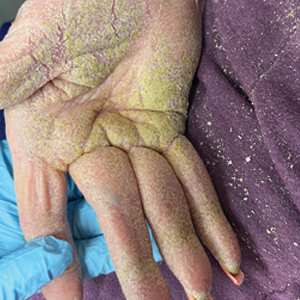
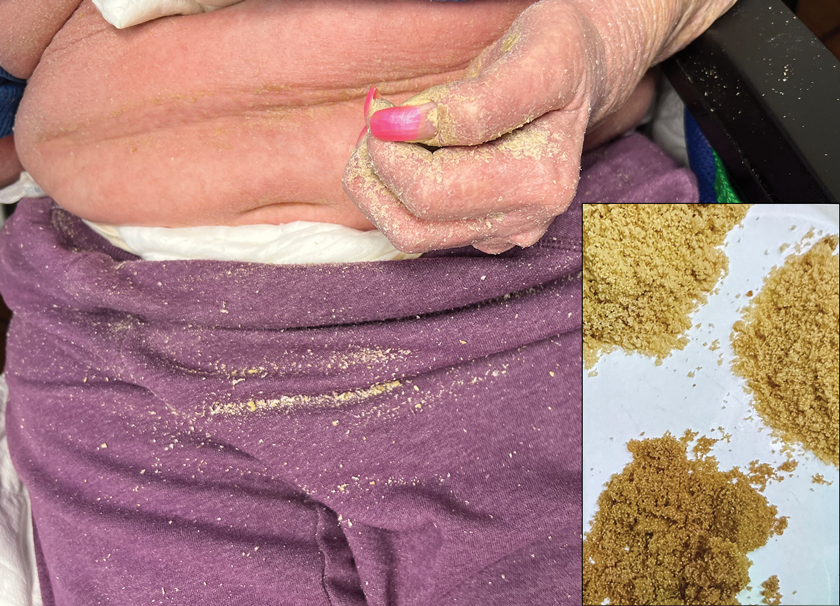
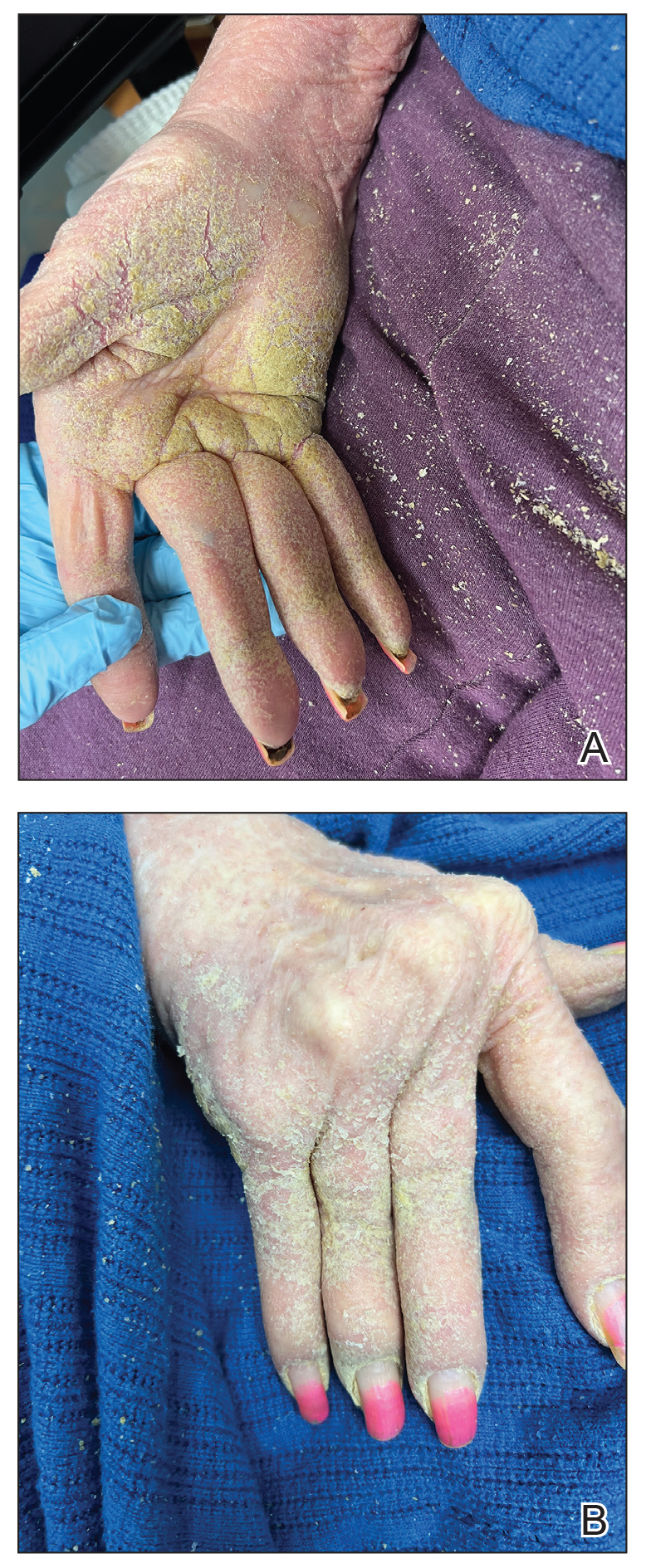
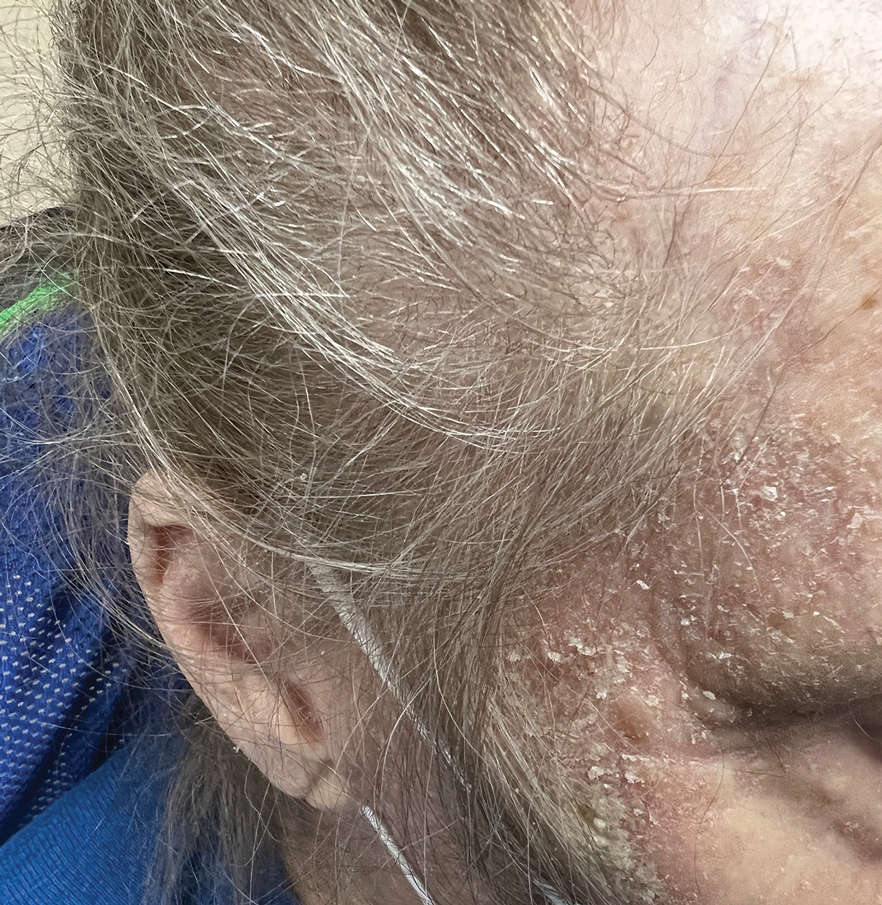
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.
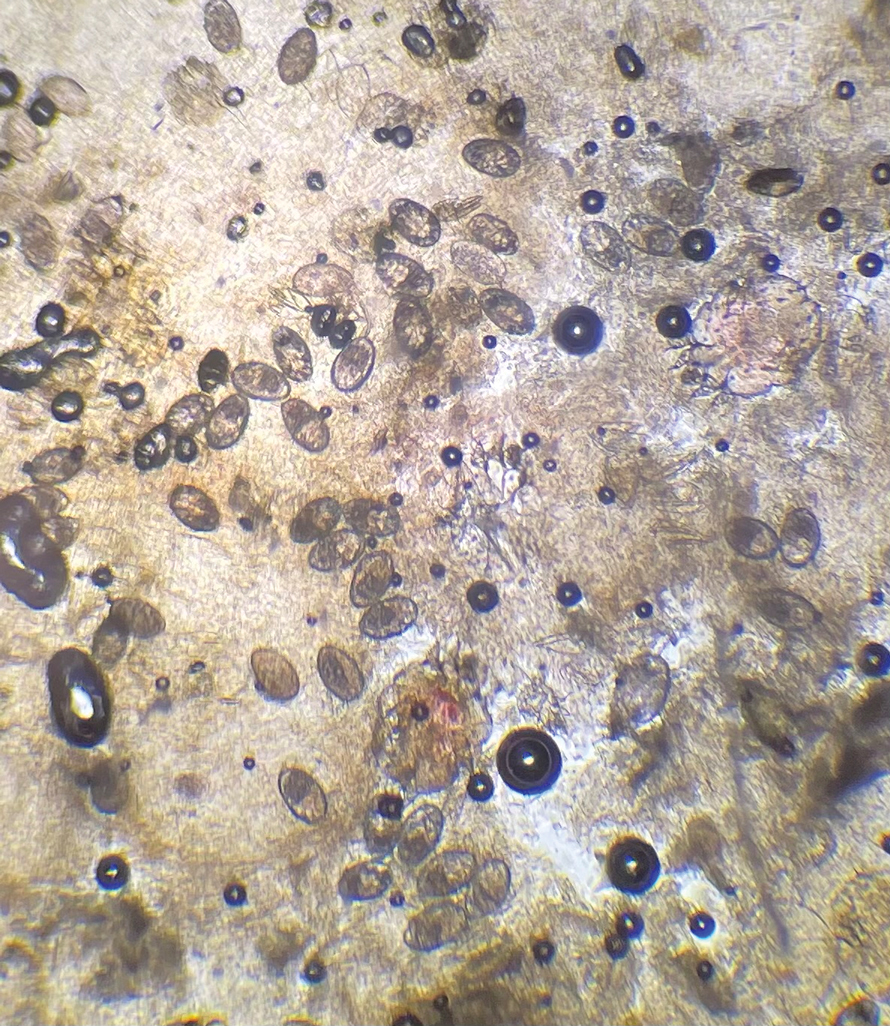
The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.
Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.



The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.

Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.



The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.

Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
PRACTICE POINTS
- Crusted scabies often is misdiagnosed because it mimics common dermatologic conditions, such as atopic dermatitis, psoriasis, drug eruption, and seborrhea. A unique feature of crusted scabies is fine or coarse scaling that resembles brown sugar.
- Immunosuppressants, such as topical corticosteroids, worsen the skin’s immune response to classic scabies infestations, which leads to parasitic overgrowth and the development of crusted scabies.
- Treatment of crusted scabies requires topical and oral scabicide; in addition, all clothing, bedding, and towels should be machine-washed and dried with hot water and hot dryer cycles to prevent environmental transmission and reinfection.
Whooping Cough Likely on Pace for a 5-Year High
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
Like many diseases, whooping cough reached record low levels during the early days of the COVID pandemic.
More than 10,000 cases of whooping cough have been reported in the United States so far this year, and weekly reports say cases have more than tripled 2023 levels as of June, according to the Centers for Disease Control and Prevention (CDC). In 2023, there were 2815 cases reported during the entire year.
“The number of reported cases this year is close to what was seen at the same time in 2019, prior to the pandemic,” the CDC reported. There were 18,617 cases of whooping cough in 2019.
There were 259 cases reported nationwide for the week ending Aug. 3, with nearly half occurring in the mid-Atlantic region. Public health officials believe the resurgence of whooping cough is likely due to declining vaccination rates, mainly due to the missed vaccines during the height of the COVID pandemic. The diphtheria, tetanus, and pertussis vaccines (DTaP) have been given together since the 1940s, typically during infancy and again during early childhood. In 1941, there were more than 220,000 cases of whooping cough.
Whooping cough is caused by the bacteria Bordetella pertussis. The bacteria attach to tiny, hair-like extensions in the upper respiratory system called cilia, and toxins released by them damage the cilia and cause airways to swell. Early symptoms are similar to the common cold, but the condition eventually leads to coughing fits and a high-pitched “whoop” sound made when inhaling after a fit subsides. Coughing fits can be so severe that people can fracture a rib.
Vaccinated people may get a less severe illness, compared to unvaccinated people, the CDC says. Babies and children are particularly at risk for severe and even potentially deadly complications. About one in three babies under age 1 who get whooping cough will need to be hospitalized, and among those hospitalized babies, 1 in 100 die from complications.
A version of this article appeared on WebMD.com.
After Rapid Weight Loss, Monitor Antiobesity Drug Dosing
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
ABIM Revokes Two Physicians’ Certifications Over Accusations of COVID Misinformation
The American Board of Internal Medicine (ABIM) has revoked certification for two physicians known for leading an organization that promotes ivermectin as a treatment for COVID-19.
Pierre Kory, MD, is no longer certified in critical care medicine, pulmonary disease, and internal medicine, according to the ABIM website. Paul Ellis Marik, MD, is no longer certified in critical care medicine or internal medicine.
Dr. Marik is the chief scientific officer and Dr. Kory is president emeritus of the Front Line COVID-19 Critical Care Alliance, a group they founded in March 2020. and also offers treatments for Lyme disease.
Ivermectin was proven to not be of use in treating COVID. Studies purporting to show a benefit were later linked to errors, and some were found to have been based on potentially fraudulent research.
The ABIM declined to comment when asked by this news organization about its action. Its website indicates that “revoked” indicates “loss of certification due to disciplinary action for which ABIM has determined that the conduct underlying the sanction does not warrant a defined pathway for restoration of certification at the time of disciplinary sanction.”
In a statement emailed to this news organization, Dr. Kory and Dr. Marik said, “we believe this decision represents a dangerous shift away from the foundation principles of medical discourse and scientific debate that have historically been the bedrock of medical education associations.”
The FLCCC said in the statement that it, along with Dr. Kory and Dr. Marik, are “evaluating options to challenge these decisions.”
Dr. Kory and Dr. Marik said they were notified in May 2022 that they were facing a potential ABIM disciplinary action. An ABIM committee recommended the revocation in July 2023, saying the two men were spreading “false or inaccurate medical information,” according to FLCCC. Dr. Kory and Dr. Marik lost an appeal.
In a 2023 statement, Dr. Kory and Dr. Marik called the ABIM action an “attack on freedom of speech.”
“This isn’t a free speech question,” said Arthur L. Caplan, PhD, the Drs. William F. and Virginia Connolly Mitty Professor of Bioethics at NYU Grossman School of Medicine’s Department of Population Health, New York City. “You do have the right to free speech, but you don’t have the right to practice outside of the standard of care boundaries,” he told this news organization.
The ABIM action “is the field standing up and saying, ‘These are the limits of what you can do,’” said Dr. Caplan. It means the profession is rejecting those “who are involved in things that harm patients or delay them getting accepted treatments,” he said. Caplan noted that a disciplinary action had been a long time in coming — 3 years since the first battles over ivermectin.
Wendy Parmet, JD, Matthews Distinguished University Professor of Law at Northeastern University School of Public Policy and Urban Affairs, Boston, said that misinformation spread by physicians is especially harmful because it comes with an air of credibility.
“We certainly want people to be able to dissent,” Ms. Parmet told this news organization. To engender trust, any sanctions by a professional board should be done in a deliberative process with a strong evidentiary base, she said.
“You want to leave sufficient room for discourse and discussion within the profession, and you don’t want the board to enforce a narrow, rigid orthodoxy,” she said. But in cases where people are “peddling information that is way outside the consensus” or are “profiting off of it, for the profession to take no action, that is, I think, detrimental also to the trust in the profession,” she said.
She was not surprised that Dr. Kory and Dr. Marik would fight to retain certification. “Board certification is an important, very worthwhile thing to have,” she said. “Losing it is not trivial.”
Dr. Kory, who is licensed in California, New York, and Wisconsin, “does not require this certification for his independent practice but is evaluating next steps with attorneys,” according to the statement from FLCCC.
Dr. Marik, whose Virginia medical license expired in 2022, “is no longer treating patients and has dedicated his time and efforts to the FLCCC Alliance,” the statement said.
Dr. Caplan served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and advisor for this news organization. Ms. Parmet reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
The American Board of Internal Medicine (ABIM) has revoked certification for two physicians known for leading an organization that promotes ivermectin as a treatment for COVID-19.
Pierre Kory, MD, is no longer certified in critical care medicine, pulmonary disease, and internal medicine, according to the ABIM website. Paul Ellis Marik, MD, is no longer certified in critical care medicine or internal medicine.
Dr. Marik is the chief scientific officer and Dr. Kory is president emeritus of the Front Line COVID-19 Critical Care Alliance, a group they founded in March 2020. and also offers treatments for Lyme disease.
Ivermectin was proven to not be of use in treating COVID. Studies purporting to show a benefit were later linked to errors, and some were found to have been based on potentially fraudulent research.
The ABIM declined to comment when asked by this news organization about its action. Its website indicates that “revoked” indicates “loss of certification due to disciplinary action for which ABIM has determined that the conduct underlying the sanction does not warrant a defined pathway for restoration of certification at the time of disciplinary sanction.”
In a statement emailed to this news organization, Dr. Kory and Dr. Marik said, “we believe this decision represents a dangerous shift away from the foundation principles of medical discourse and scientific debate that have historically been the bedrock of medical education associations.”
The FLCCC said in the statement that it, along with Dr. Kory and Dr. Marik, are “evaluating options to challenge these decisions.”
Dr. Kory and Dr. Marik said they were notified in May 2022 that they were facing a potential ABIM disciplinary action. An ABIM committee recommended the revocation in July 2023, saying the two men were spreading “false or inaccurate medical information,” according to FLCCC. Dr. Kory and Dr. Marik lost an appeal.
In a 2023 statement, Dr. Kory and Dr. Marik called the ABIM action an “attack on freedom of speech.”
“This isn’t a free speech question,” said Arthur L. Caplan, PhD, the Drs. William F. and Virginia Connolly Mitty Professor of Bioethics at NYU Grossman School of Medicine’s Department of Population Health, New York City. “You do have the right to free speech, but you don’t have the right to practice outside of the standard of care boundaries,” he told this news organization.
The ABIM action “is the field standing up and saying, ‘These are the limits of what you can do,’” said Dr. Caplan. It means the profession is rejecting those “who are involved in things that harm patients or delay them getting accepted treatments,” he said. Caplan noted that a disciplinary action had been a long time in coming — 3 years since the first battles over ivermectin.
Wendy Parmet, JD, Matthews Distinguished University Professor of Law at Northeastern University School of Public Policy and Urban Affairs, Boston, said that misinformation spread by physicians is especially harmful because it comes with an air of credibility.
“We certainly want people to be able to dissent,” Ms. Parmet told this news organization. To engender trust, any sanctions by a professional board should be done in a deliberative process with a strong evidentiary base, she said.
“You want to leave sufficient room for discourse and discussion within the profession, and you don’t want the board to enforce a narrow, rigid orthodoxy,” she said. But in cases where people are “peddling information that is way outside the consensus” or are “profiting off of it, for the profession to take no action, that is, I think, detrimental also to the trust in the profession,” she said.
She was not surprised that Dr. Kory and Dr. Marik would fight to retain certification. “Board certification is an important, very worthwhile thing to have,” she said. “Losing it is not trivial.”
Dr. Kory, who is licensed in California, New York, and Wisconsin, “does not require this certification for his independent practice but is evaluating next steps with attorneys,” according to the statement from FLCCC.
Dr. Marik, whose Virginia medical license expired in 2022, “is no longer treating patients and has dedicated his time and efforts to the FLCCC Alliance,” the statement said.
Dr. Caplan served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and advisor for this news organization. Ms. Parmet reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
The American Board of Internal Medicine (ABIM) has revoked certification for two physicians known for leading an organization that promotes ivermectin as a treatment for COVID-19.
Pierre Kory, MD, is no longer certified in critical care medicine, pulmonary disease, and internal medicine, according to the ABIM website. Paul Ellis Marik, MD, is no longer certified in critical care medicine or internal medicine.
Dr. Marik is the chief scientific officer and Dr. Kory is president emeritus of the Front Line COVID-19 Critical Care Alliance, a group they founded in March 2020. and also offers treatments for Lyme disease.
Ivermectin was proven to not be of use in treating COVID. Studies purporting to show a benefit were later linked to errors, and some were found to have been based on potentially fraudulent research.
The ABIM declined to comment when asked by this news organization about its action. Its website indicates that “revoked” indicates “loss of certification due to disciplinary action for which ABIM has determined that the conduct underlying the sanction does not warrant a defined pathway for restoration of certification at the time of disciplinary sanction.”
In a statement emailed to this news organization, Dr. Kory and Dr. Marik said, “we believe this decision represents a dangerous shift away from the foundation principles of medical discourse and scientific debate that have historically been the bedrock of medical education associations.”
The FLCCC said in the statement that it, along with Dr. Kory and Dr. Marik, are “evaluating options to challenge these decisions.”
Dr. Kory and Dr. Marik said they were notified in May 2022 that they were facing a potential ABIM disciplinary action. An ABIM committee recommended the revocation in July 2023, saying the two men were spreading “false or inaccurate medical information,” according to FLCCC. Dr. Kory and Dr. Marik lost an appeal.
In a 2023 statement, Dr. Kory and Dr. Marik called the ABIM action an “attack on freedom of speech.”
“This isn’t a free speech question,” said Arthur L. Caplan, PhD, the Drs. William F. and Virginia Connolly Mitty Professor of Bioethics at NYU Grossman School of Medicine’s Department of Population Health, New York City. “You do have the right to free speech, but you don’t have the right to practice outside of the standard of care boundaries,” he told this news organization.
The ABIM action “is the field standing up and saying, ‘These are the limits of what you can do,’” said Dr. Caplan. It means the profession is rejecting those “who are involved in things that harm patients or delay them getting accepted treatments,” he said. Caplan noted that a disciplinary action had been a long time in coming — 3 years since the first battles over ivermectin.
Wendy Parmet, JD, Matthews Distinguished University Professor of Law at Northeastern University School of Public Policy and Urban Affairs, Boston, said that misinformation spread by physicians is especially harmful because it comes with an air of credibility.
“We certainly want people to be able to dissent,” Ms. Parmet told this news organization. To engender trust, any sanctions by a professional board should be done in a deliberative process with a strong evidentiary base, she said.
“You want to leave sufficient room for discourse and discussion within the profession, and you don’t want the board to enforce a narrow, rigid orthodoxy,” she said. But in cases where people are “peddling information that is way outside the consensus” or are “profiting off of it, for the profession to take no action, that is, I think, detrimental also to the trust in the profession,” she said.
She was not surprised that Dr. Kory and Dr. Marik would fight to retain certification. “Board certification is an important, very worthwhile thing to have,” she said. “Losing it is not trivial.”
Dr. Kory, who is licensed in California, New York, and Wisconsin, “does not require this certification for his independent practice but is evaluating next steps with attorneys,” according to the statement from FLCCC.
Dr. Marik, whose Virginia medical license expired in 2022, “is no longer treating patients and has dedicated his time and efforts to the FLCCC Alliance,” the statement said.
Dr. Caplan served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and advisor for this news organization. Ms. Parmet reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
What You Need to Know About Oropouche Virus Disease
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
The European Centre for Disease Prevention and Control (ECDC) has issued a warning to travelers in areas in South and Central America and the Caribbean affected by a current outbreak of Oropouche virus (OROV) disease. The ECDC said that there had been more than 8000 cases reported in these areas since January, with 19 imported cases reported in Europe for the first time in June and July. Of these, 12 were in Spain, five were in Italy, and two were in Germany.
The ECDC’s Threat Assessment Brief of Aug. 9 said that one of those affected had traveled to Brazil and the other 18 to Cuba; however, outbreaks have also been reported this year in Bolivia, Colombia, and Peru. Though the overall risk for infection to European travelers to OROV-epidemic countries was assessed as moderate, it was higher in the more affected municipalities of the northern states of Brazil and/or the Amazon region, and/or if personal protection measures are not taken.
An editorial published Aug. 8 in The Lancet Infectious Diseases described OROV as a “mysterious threat,” which there is limited knowledge about despite some half a million cases recorded since it was first detected in Trinidad and Tobago in 1955.
OROV is transmitted primarily through bites from infected midges (Culicoides paraensis). However, some mosquitoes species can also spread the virus, which causes symptoms very similar to other arbovirus diseases from the same regions, such as dengue, chikungunya, and Zika virus infection.
Most cases are mild, but meningitis and encephalitis can occur as well as possible fetal death and deformities after infection in pregnancy. Last month, the first fatal cases were reported in two young Brazilian women who, concerningly, had no comorbidities.
This news organization asked Jan Felix Drexler, MD, of the Institute of Virology at Charité – Universitätsmedizin in Berlin, Germany, who has studied the emergence of Oropouche fever in Latin America, what clinicians should know about OROV disease.
What are the main symptoms of OROV disease for which clinicians should be alert?
The main symptoms are not different from other arboviral infections, ie, fever, maybe joint and muscle pain, maybe rash. The problem is that we do not know how often severe disease may occur because we do not know whether the severe cases that have been postulated, including death in apparently healthy people and congenital infection, are due to increased testing; an altered virus; or an altered, more intense circulation (so that many more infections simply lead to rare severe cases appearing). Be alert and ask for testing in your patients.
What is the differential diagnosis if a recent traveler to affected regions presents with symptoms? Are there any clues to suggest whether the disease is Oropouche as opposed to Zika, etc.?
The main message is: Do not assume a particular infection based on clinical symptoms. If your patient is returning from or living in an endemic area, consider OROV disease in the differential diagnosis.
What personal protective measures should clinicians advise travelers in affected areas to take? Do these differ from normal mosquito precautions?
Repellents are extremely important as usual. However, there are differences. Mosquito nets’ hole sizes need to be smaller than those used against the vectors of malaria or dengue; in other words, they need to have a higher mesh. The problem is that nets with high mesh are complicated in very hot and humid conditions because they also limit ventilation. Travelers should discuss with local suppliers about the best trade-off.
The risk for midge bites is likely highest at dawn and dusk in still and humid conditions. So on the one hand, one could recommend avoiding those areas and being outside during those times of the day. On the other hand, specific recommendations cannot be made robustly because we cannot exclude other invertebrate vectors at current knowledge. Some studies have implicated that mosquitoes may also transmit the virus. If that holds true, then we are back to reducing any bite.
Should pregnant women be advised to avoid travel to affected regions?
Not immediately, but caution must be taken. We simply do not have sufficient data to gauge the risk for potential congenital infection. Much more epidemiologic data and controlled infection experiments will be required to make evidence-based recommendations.
All the cases reported in Europe so far were imported from Cuba and Brazil. Is there any risk for local transmission, eg, via midges/mosquitoes that might hitch a ride on an aircraft, as in cases of airport malaria?
Not immediately, but it cannot be excluded. We know very little about the infection intensity in the vectors. Controlled infection experiments, including robustness of vectors against commonly used insecticides in airplanes, need to be done.
What is the risk for an animal reservoir emerging in Europe?
We do not know, but there is also no reason for ringing the alarm bells. Controlled infection experiments and surveillance will be required.
Is treatment purely supportive or are there any specific agents worth trying in case of severe symptoms/neurologic involvement?
No specific treatment can be recommended as is. However, severe dengue illustrates the relevance of supportive treatment, which is hugely effective in reducing mortality.
The Lancet paper states: “Several laboratory tests have been developed but robust commercial tests are hardly available.” How likely is it that laboratories in Europe will have the capability to test for the Oropouche organism?
European laboratory networks have already taken action, and testing is now available at least in the major and reference laboratories. If a clinician asks for OROV testing, they will probably get a robust answer in a reasonable timespan. Of course, that can be improved once we have more cases and more laboratories will be equipped for testing.
Is there anything else you think clinicians should be aware of?
The most important is to think beyond the textbooks we know from medical school. Things change rapidly in a connected world under altered climate conditions.
Dr. Drexler has no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
Viral Season 2024-2025: Try for An Ounce of Prevention
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1
They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5
Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
Study Identifies Oral Antibiotics Linked to Severe Cutaneous Reactions
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA
Comment on “Erythrodermic Pityriasis Rubra Pilaris Following COVID-19 Vaccination”
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010