User login
Mortality trends in childhood after infant bacterial meningitis
Among infants younger than 1 year of age, bacterial meningitis is associated with worse long-term mortality, even after recovery from the initial infection. Heightened mortality risk stretched out to 10 years, and was highest in the wake of infection from Streptococcus agalactiae, according to a retrospective analysis of children in the Netherlands.
“The adjusted hazard rates were high for the whole group of bacterial meningitis, especially within the first year after onset. (Staphylococcus agalactiae) meningitis has the highest mortality risk within one year of disease onset,” Linde Snoek said during her presentation of the study (abstract 913) at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Ms. Snoek is a PhD student at Amsterdam University Medical Center.
Over longer time periods, the mortality associations were different. “The adjusted hazard rates were highest for pneumococcal meningitis compared to the other pathogens. And this was the case for 1 year, 5 years, and 10 years after disease onset,” said Ms. Snoek.
The study appears to be the first to look at extended mortality following bacterial meningitis in this age group, according to Marie Rohr, MD, who comoderated the session where the research was presented.
“In a quick review of the literature I did not find any [equivalent] study concerning short- and long-term mortality after bacterial meningitis in under 1 year of age,” said Dr. Rohr, a fellow in pediatric infectious diseases at University Hospitals of Geneva. But the message to physicians is clear. “Children with history of bacterial meningitis have a higher long-term mortality than children without a history of bacterial meningitis,” said Dr. Rohr.
The study did have a key limitation: For matched controls, it relied on anonymous data from the Municipal Personal Records Database in Statistics Netherlands. “Important information like cause of death is lacking,” said Dr. Rohr.
Bacterial meningitis is associated with significant mortality and morbidity. Pathogens behind the infections vary with age group and geographic location, as well as immunization status.
To examine long-term mortality after bacterial meningitis, the researchers collected 1,646 records from an exposed cohort, with a date range of 1995 to 2018, from the Netherlands Reference Laboratory for Bacterial Meningitis. Included patients had a positive culture diagnosis of bacterial meningitis during the first year of life. Each exposed subject was compared to 10 controls matched by birth month, birth year, and sex, who had no exposure to bacterial meningitis.
Staphylococcus pneumoniae accounted for the most cases, at 32.0% (median age of onset, 180 days), followed by Neisseria meningitidis at 29.0% (median age of onset, 203 days). Other pathogens included S. agalactiae (19.7%, 10 days), Escherichia coli (8.8%, 13 days), and Haemophilus influenzae (5.4%, 231 days).
The mortality risk within 1 year of disease onset was higher for all pathogens (6.2% vs. 0.2% unexposed). The highest mortality risk was seen for S. agalactiae (8.7%), followed by E. coli (6.4%), N. meningitidis (4.9%), and H. influenzae (3.4%).
Hazard ratios (HR) for mortality were also higher, particularly in the first year after disease onset. For all pathogens, mortality rates were higher within 1 year (HR, 39.2), 5 years (HR, 28.7), and 10 years (HR, 24.1). The consistently highest mortality rates were associated with S. pneumoniae over 1-year, 5-year, and 10-year follow-up (HR, 42.8; HR, 45.6; HR, 40.6, respectively). Within 1 year, the highest mortality rate was associated with N. meningitidis (HR, 58.4).
Ms. Snoek and Dr. Rohr have no relevant financial disclosures.
Among infants younger than 1 year of age, bacterial meningitis is associated with worse long-term mortality, even after recovery from the initial infection. Heightened mortality risk stretched out to 10 years, and was highest in the wake of infection from Streptococcus agalactiae, according to a retrospective analysis of children in the Netherlands.
“The adjusted hazard rates were high for the whole group of bacterial meningitis, especially within the first year after onset. (Staphylococcus agalactiae) meningitis has the highest mortality risk within one year of disease onset,” Linde Snoek said during her presentation of the study (abstract 913) at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Ms. Snoek is a PhD student at Amsterdam University Medical Center.
Over longer time periods, the mortality associations were different. “The adjusted hazard rates were highest for pneumococcal meningitis compared to the other pathogens. And this was the case for 1 year, 5 years, and 10 years after disease onset,” said Ms. Snoek.
The study appears to be the first to look at extended mortality following bacterial meningitis in this age group, according to Marie Rohr, MD, who comoderated the session where the research was presented.
“In a quick review of the literature I did not find any [equivalent] study concerning short- and long-term mortality after bacterial meningitis in under 1 year of age,” said Dr. Rohr, a fellow in pediatric infectious diseases at University Hospitals of Geneva. But the message to physicians is clear. “Children with history of bacterial meningitis have a higher long-term mortality than children without a history of bacterial meningitis,” said Dr. Rohr.
The study did have a key limitation: For matched controls, it relied on anonymous data from the Municipal Personal Records Database in Statistics Netherlands. “Important information like cause of death is lacking,” said Dr. Rohr.
Bacterial meningitis is associated with significant mortality and morbidity. Pathogens behind the infections vary with age group and geographic location, as well as immunization status.
To examine long-term mortality after bacterial meningitis, the researchers collected 1,646 records from an exposed cohort, with a date range of 1995 to 2018, from the Netherlands Reference Laboratory for Bacterial Meningitis. Included patients had a positive culture diagnosis of bacterial meningitis during the first year of life. Each exposed subject was compared to 10 controls matched by birth month, birth year, and sex, who had no exposure to bacterial meningitis.
Staphylococcus pneumoniae accounted for the most cases, at 32.0% (median age of onset, 180 days), followed by Neisseria meningitidis at 29.0% (median age of onset, 203 days). Other pathogens included S. agalactiae (19.7%, 10 days), Escherichia coli (8.8%, 13 days), and Haemophilus influenzae (5.4%, 231 days).
The mortality risk within 1 year of disease onset was higher for all pathogens (6.2% vs. 0.2% unexposed). The highest mortality risk was seen for S. agalactiae (8.7%), followed by E. coli (6.4%), N. meningitidis (4.9%), and H. influenzae (3.4%).
Hazard ratios (HR) for mortality were also higher, particularly in the first year after disease onset. For all pathogens, mortality rates were higher within 1 year (HR, 39.2), 5 years (HR, 28.7), and 10 years (HR, 24.1). The consistently highest mortality rates were associated with S. pneumoniae over 1-year, 5-year, and 10-year follow-up (HR, 42.8; HR, 45.6; HR, 40.6, respectively). Within 1 year, the highest mortality rate was associated with N. meningitidis (HR, 58.4).
Ms. Snoek and Dr. Rohr have no relevant financial disclosures.
Among infants younger than 1 year of age, bacterial meningitis is associated with worse long-term mortality, even after recovery from the initial infection. Heightened mortality risk stretched out to 10 years, and was highest in the wake of infection from Streptococcus agalactiae, according to a retrospective analysis of children in the Netherlands.
“The adjusted hazard rates were high for the whole group of bacterial meningitis, especially within the first year after onset. (Staphylococcus agalactiae) meningitis has the highest mortality risk within one year of disease onset,” Linde Snoek said during her presentation of the study (abstract 913) at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Ms. Snoek is a PhD student at Amsterdam University Medical Center.
Over longer time periods, the mortality associations were different. “The adjusted hazard rates were highest for pneumococcal meningitis compared to the other pathogens. And this was the case for 1 year, 5 years, and 10 years after disease onset,” said Ms. Snoek.
The study appears to be the first to look at extended mortality following bacterial meningitis in this age group, according to Marie Rohr, MD, who comoderated the session where the research was presented.
“In a quick review of the literature I did not find any [equivalent] study concerning short- and long-term mortality after bacterial meningitis in under 1 year of age,” said Dr. Rohr, a fellow in pediatric infectious diseases at University Hospitals of Geneva. But the message to physicians is clear. “Children with history of bacterial meningitis have a higher long-term mortality than children without a history of bacterial meningitis,” said Dr. Rohr.
The study did have a key limitation: For matched controls, it relied on anonymous data from the Municipal Personal Records Database in Statistics Netherlands. “Important information like cause of death is lacking,” said Dr. Rohr.
Bacterial meningitis is associated with significant mortality and morbidity. Pathogens behind the infections vary with age group and geographic location, as well as immunization status.
To examine long-term mortality after bacterial meningitis, the researchers collected 1,646 records from an exposed cohort, with a date range of 1995 to 2018, from the Netherlands Reference Laboratory for Bacterial Meningitis. Included patients had a positive culture diagnosis of bacterial meningitis during the first year of life. Each exposed subject was compared to 10 controls matched by birth month, birth year, and sex, who had no exposure to bacterial meningitis.
Staphylococcus pneumoniae accounted for the most cases, at 32.0% (median age of onset, 180 days), followed by Neisseria meningitidis at 29.0% (median age of onset, 203 days). Other pathogens included S. agalactiae (19.7%, 10 days), Escherichia coli (8.8%, 13 days), and Haemophilus influenzae (5.4%, 231 days).
The mortality risk within 1 year of disease onset was higher for all pathogens (6.2% vs. 0.2% unexposed). The highest mortality risk was seen for S. agalactiae (8.7%), followed by E. coli (6.4%), N. meningitidis (4.9%), and H. influenzae (3.4%).
Hazard ratios (HR) for mortality were also higher, particularly in the first year after disease onset. For all pathogens, mortality rates were higher within 1 year (HR, 39.2), 5 years (HR, 28.7), and 10 years (HR, 24.1). The consistently highest mortality rates were associated with S. pneumoniae over 1-year, 5-year, and 10-year follow-up (HR, 42.8; HR, 45.6; HR, 40.6, respectively). Within 1 year, the highest mortality rate was associated with N. meningitidis (HR, 58.4).
Ms. Snoek and Dr. Rohr have no relevant financial disclosures.
FROM ESPID 2021
FDA approves ibrexafungerp for vaginal yeast infection
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
CDC: New botulism guidelines focus on mass casualty events
Botulinum toxin is said to be the most lethal substance known. Inhaling just 1-3 nanograms of toxin per kilogram of body mass constitutes a lethal dose.
The CDC has been working on these guidelines since 2015, initially establishing a technical development group and steering committee to prioritize topics for review and make recommendations. Since then, the agency published 15 systematic reviews in Clinical Infectious Diseases early in 2018. The reviews addressed the recognition of botulism clinically, treatment with botulinum antitoxin, and complications from that treatment. They also looked at the epidemiology of botulism outbreaks and botulism in the special populations of vulnerable pediatric and pregnant patients.
In 2016, the CDC held two extended forums and convened a workshop with 72 experts. In addition to the more standard topics of diagnosis and treatment, attention was given to crisis standards of care, caring for multiple patients at once, and ethical considerations in management.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, said in an interview that the new guidance “was really specific [and] was meant to address the gap in guidance for mass casualty settings.”
While clinicians are used to focusing on an individual patient, in times of crises, with multiple patients from a food-borne outbreak or a bioterrorism attack, the focus must shift to the population rather than the individual. The workshop explored issues of triaging, adding beds, and caring for patients when a hospital is overwhelmed with an acute influx of severely ill patients.
Such a mass casualty event is similar to the stress encountered this past year with COVID-19 patients swamping the hospitals, which had too little oxygen, too few ventilators, and too few staff members to care for the sudden influx of critically ill patients.
Diagnosis
Leslie Edwards, MHS, BSN, a CDC epidemiologist and botulism expert, said that “botulism is rare and [so] could be difficult to diagnose.” The CDC “wanted to highlight some of those key clinical factors” to speed recognition.
Hospitals and health officials are being urged to develop crisis protocols as part of emergency preparedness plans. And clinicians should be able to recognize four major syndromes: botulism from food, wounds, and inhalation, as well as iatrogenic botulism (from exposure via injection of the neurotoxin).
Botulism has a characteristic and unusual pattern of symptoms, which begin with cranial nerve palsies. Then there is typically a descending, symmetric flaccid paralysis. Symptoms might progress to respiratory failure and death. Other critical clues that implicate botulism include a lack of sensory deficits and the absence of pain.
Symptoms are most likely to be mistaken for myasthenia gravis or Guillain-Barré syndrome, but the latter has an ascending paralysis. Cranial nerve involvement can present as blurred vision, ptosis (drooping lid), diplopia (double vision), ophthalmoplegia (weak eye muscles), or difficulty with speech and swallowing. Shortness of breath and abdominal discomfort can also occur. Respiratory failure may occur from weakness or paralysis of cranial nerves. Cranial nerve signs and symptoms in the absence of fever, along with a descending paralysis, should strongly suggest the diagnosis.
With food-borne botulism, vomiting occurs in half the patients. Improperly sterilized home-canned food is the major risk factor. While the toxin is rapidly destroyed by heat, the bacterial spores are not. Wound botulism is most commonly associated with the injection of drugs, particularly black tar heroin.
Dr. Edwards stressed that “time is of the essence when it comes to botulism diagnostics and treating. Timely administration of the botulism antitoxin early in the course of illness can arrest the progression of paralysis and possibly avert the need for intubation or ventilation.”
It’s essential to note that botulism is an urgent diagnosis that has to be made on clinical grounds. Lab assays for botulinum neurotoxins take too long and are only conducted in public health laboratories. The decision to use antitoxin must not be delayed to wait for confirmation.
Clinicians should immediately contact the local or state health department’s emergency on-call team if botulism is suspected. They will arrange for expert consultation.
Treatment
Botulinum antitoxin is the only specific therapy for this infection. If given early – preferably within 24-48 hours of symptom onset – it can stop the progression of paralysis. But antitoxin will not reverse existing paralysis. If paralysis is still progressing outside of that 24- to 48-hour window, the antitoxin should still provide benefit. The antitoxin is available only through state health departments and a request to the CDC.
Botulism antitoxin is made from horse serum and therefore may cause a variety of allergic reactions. The risk for anaphylaxis is less than 2%, far lower than the mortality from untreated botulism.
While these guidelines have an important focus on triaging and treating mass casualties from botulism, it’s important to note that food-borne outbreaks and prevention issues are covered elsewhere on the CDC site.
Dr. Edwards has disclosed no relevant financial relationships. Dr. Adalja is a consultant for Emergent BioSolutions, which makes the heptavalent botulism antitoxin.
Dr. Stone is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research,” the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
Botulinum toxin is said to be the most lethal substance known. Inhaling just 1-3 nanograms of toxin per kilogram of body mass constitutes a lethal dose.
The CDC has been working on these guidelines since 2015, initially establishing a technical development group and steering committee to prioritize topics for review and make recommendations. Since then, the agency published 15 systematic reviews in Clinical Infectious Diseases early in 2018. The reviews addressed the recognition of botulism clinically, treatment with botulinum antitoxin, and complications from that treatment. They also looked at the epidemiology of botulism outbreaks and botulism in the special populations of vulnerable pediatric and pregnant patients.
In 2016, the CDC held two extended forums and convened a workshop with 72 experts. In addition to the more standard topics of diagnosis and treatment, attention was given to crisis standards of care, caring for multiple patients at once, and ethical considerations in management.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, said in an interview that the new guidance “was really specific [and] was meant to address the gap in guidance for mass casualty settings.”
While clinicians are used to focusing on an individual patient, in times of crises, with multiple patients from a food-borne outbreak or a bioterrorism attack, the focus must shift to the population rather than the individual. The workshop explored issues of triaging, adding beds, and caring for patients when a hospital is overwhelmed with an acute influx of severely ill patients.
Such a mass casualty event is similar to the stress encountered this past year with COVID-19 patients swamping the hospitals, which had too little oxygen, too few ventilators, and too few staff members to care for the sudden influx of critically ill patients.
Diagnosis
Leslie Edwards, MHS, BSN, a CDC epidemiologist and botulism expert, said that “botulism is rare and [so] could be difficult to diagnose.” The CDC “wanted to highlight some of those key clinical factors” to speed recognition.
Hospitals and health officials are being urged to develop crisis protocols as part of emergency preparedness plans. And clinicians should be able to recognize four major syndromes: botulism from food, wounds, and inhalation, as well as iatrogenic botulism (from exposure via injection of the neurotoxin).
Botulism has a characteristic and unusual pattern of symptoms, which begin with cranial nerve palsies. Then there is typically a descending, symmetric flaccid paralysis. Symptoms might progress to respiratory failure and death. Other critical clues that implicate botulism include a lack of sensory deficits and the absence of pain.
Symptoms are most likely to be mistaken for myasthenia gravis or Guillain-Barré syndrome, but the latter has an ascending paralysis. Cranial nerve involvement can present as blurred vision, ptosis (drooping lid), diplopia (double vision), ophthalmoplegia (weak eye muscles), or difficulty with speech and swallowing. Shortness of breath and abdominal discomfort can also occur. Respiratory failure may occur from weakness or paralysis of cranial nerves. Cranial nerve signs and symptoms in the absence of fever, along with a descending paralysis, should strongly suggest the diagnosis.
With food-borne botulism, vomiting occurs in half the patients. Improperly sterilized home-canned food is the major risk factor. While the toxin is rapidly destroyed by heat, the bacterial spores are not. Wound botulism is most commonly associated with the injection of drugs, particularly black tar heroin.
Dr. Edwards stressed that “time is of the essence when it comes to botulism diagnostics and treating. Timely administration of the botulism antitoxin early in the course of illness can arrest the progression of paralysis and possibly avert the need for intubation or ventilation.”
It’s essential to note that botulism is an urgent diagnosis that has to be made on clinical grounds. Lab assays for botulinum neurotoxins take too long and are only conducted in public health laboratories. The decision to use antitoxin must not be delayed to wait for confirmation.
Clinicians should immediately contact the local or state health department’s emergency on-call team if botulism is suspected. They will arrange for expert consultation.
Treatment
Botulinum antitoxin is the only specific therapy for this infection. If given early – preferably within 24-48 hours of symptom onset – it can stop the progression of paralysis. But antitoxin will not reverse existing paralysis. If paralysis is still progressing outside of that 24- to 48-hour window, the antitoxin should still provide benefit. The antitoxin is available only through state health departments and a request to the CDC.
Botulism antitoxin is made from horse serum and therefore may cause a variety of allergic reactions. The risk for anaphylaxis is less than 2%, far lower than the mortality from untreated botulism.
While these guidelines have an important focus on triaging and treating mass casualties from botulism, it’s important to note that food-borne outbreaks and prevention issues are covered elsewhere on the CDC site.
Dr. Edwards has disclosed no relevant financial relationships. Dr. Adalja is a consultant for Emergent BioSolutions, which makes the heptavalent botulism antitoxin.
Dr. Stone is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research,” the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
Botulinum toxin is said to be the most lethal substance known. Inhaling just 1-3 nanograms of toxin per kilogram of body mass constitutes a lethal dose.
The CDC has been working on these guidelines since 2015, initially establishing a technical development group and steering committee to prioritize topics for review and make recommendations. Since then, the agency published 15 systematic reviews in Clinical Infectious Diseases early in 2018. The reviews addressed the recognition of botulism clinically, treatment with botulinum antitoxin, and complications from that treatment. They also looked at the epidemiology of botulism outbreaks and botulism in the special populations of vulnerable pediatric and pregnant patients.
In 2016, the CDC held two extended forums and convened a workshop with 72 experts. In addition to the more standard topics of diagnosis and treatment, attention was given to crisis standards of care, caring for multiple patients at once, and ethical considerations in management.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, said in an interview that the new guidance “was really specific [and] was meant to address the gap in guidance for mass casualty settings.”
While clinicians are used to focusing on an individual patient, in times of crises, with multiple patients from a food-borne outbreak or a bioterrorism attack, the focus must shift to the population rather than the individual. The workshop explored issues of triaging, adding beds, and caring for patients when a hospital is overwhelmed with an acute influx of severely ill patients.
Such a mass casualty event is similar to the stress encountered this past year with COVID-19 patients swamping the hospitals, which had too little oxygen, too few ventilators, and too few staff members to care for the sudden influx of critically ill patients.
Diagnosis
Leslie Edwards, MHS, BSN, a CDC epidemiologist and botulism expert, said that “botulism is rare and [so] could be difficult to diagnose.” The CDC “wanted to highlight some of those key clinical factors” to speed recognition.
Hospitals and health officials are being urged to develop crisis protocols as part of emergency preparedness plans. And clinicians should be able to recognize four major syndromes: botulism from food, wounds, and inhalation, as well as iatrogenic botulism (from exposure via injection of the neurotoxin).
Botulism has a characteristic and unusual pattern of symptoms, which begin with cranial nerve palsies. Then there is typically a descending, symmetric flaccid paralysis. Symptoms might progress to respiratory failure and death. Other critical clues that implicate botulism include a lack of sensory deficits and the absence of pain.
Symptoms are most likely to be mistaken for myasthenia gravis or Guillain-Barré syndrome, but the latter has an ascending paralysis. Cranial nerve involvement can present as blurred vision, ptosis (drooping lid), diplopia (double vision), ophthalmoplegia (weak eye muscles), or difficulty with speech and swallowing. Shortness of breath and abdominal discomfort can also occur. Respiratory failure may occur from weakness or paralysis of cranial nerves. Cranial nerve signs and symptoms in the absence of fever, along with a descending paralysis, should strongly suggest the diagnosis.
With food-borne botulism, vomiting occurs in half the patients. Improperly sterilized home-canned food is the major risk factor. While the toxin is rapidly destroyed by heat, the bacterial spores are not. Wound botulism is most commonly associated with the injection of drugs, particularly black tar heroin.
Dr. Edwards stressed that “time is of the essence when it comes to botulism diagnostics and treating. Timely administration of the botulism antitoxin early in the course of illness can arrest the progression of paralysis and possibly avert the need for intubation or ventilation.”
It’s essential to note that botulism is an urgent diagnosis that has to be made on clinical grounds. Lab assays for botulinum neurotoxins take too long and are only conducted in public health laboratories. The decision to use antitoxin must not be delayed to wait for confirmation.
Clinicians should immediately contact the local or state health department’s emergency on-call team if botulism is suspected. They will arrange for expert consultation.
Treatment
Botulinum antitoxin is the only specific therapy for this infection. If given early – preferably within 24-48 hours of symptom onset – it can stop the progression of paralysis. But antitoxin will not reverse existing paralysis. If paralysis is still progressing outside of that 24- to 48-hour window, the antitoxin should still provide benefit. The antitoxin is available only through state health departments and a request to the CDC.
Botulism antitoxin is made from horse serum and therefore may cause a variety of allergic reactions. The risk for anaphylaxis is less than 2%, far lower than the mortality from untreated botulism.
While these guidelines have an important focus on triaging and treating mass casualties from botulism, it’s important to note that food-borne outbreaks and prevention issues are covered elsewhere on the CDC site.
Dr. Edwards has disclosed no relevant financial relationships. Dr. Adalja is a consultant for Emergent BioSolutions, which makes the heptavalent botulism antitoxin.
Dr. Stone is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research,” the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
In Zambia, PCR tracks pertussis
In the periurban slum of Lusaka, Zambia, asymptomatic pertussis infections were common among both mothers and infants, a surprising finding since asymptomatic infections are assumed to be rare in infants. The findings suggested that pertussis should be considered in cases of chronic cough, and that current standards of treating pertussis infections in low-resource settings may need to be reexamined.
The results come from testing of 1,320 infant-mother pairs who were first enrolled at a public health clinic, then followed over at least four visits. The researchers tracked pertussis infection using quantitative PCR (qPCR) on nasopharyngeal swabs. Over the course of the study, 8.9% tested positive, although only one infant developed clinical pertussis during the study.
The study was presented by Christian Gunning, a postdoctoral researcher at the University of Georgia, at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. The group also included researchers at Boston University and the University of Zambia, where PCR tests were conducted.
“That was amazing,” said session moderator Vana Spoulou, MD, PhD, professor of pediatric infectious diseases at National and Kapodistrian University of Athens, who is associated with Aghia Sofia Children’s Hospital of Athens. She noted that the study found that many physicians misdiagnosed coughs, believing them to be caused by another agent. “It was very interesting that there was so much pertussis spreading around in that community, and that nobody knew that it was around,” said Dr. Spoulou.
It’s important that physicians provide appropriate treatment, since ampicillin, which is typically prescribed for childhood upper respiratory illnesses, is believed to be ineffective against pertussis, while macrolides are effective and can prevent transmission.
Dr. Spoulou also noted that Zambia uses a whole cell vaccine, which is contraindicated in pregnant women because of potential side effects. “The good thing, despite that there was [a lot of] infection, there were no deaths, which means that maybe because the mother was infected, maybe some antibodies of the mother had passed to the child and could help the child to develop milder symptoms. So these are the pros and cons of natural infection,” said Dr. Spoulou.
The study took place in 2015, and participants were seen at the Chawama Public Health Clinic from about age 1 week to 4 months (with a target of seven clinic visits). Researchers recorded respiratory symptoms and antibiotics use at each visit, and collected a nasopharyngeal swab that was tested retrospectively using qPCR for Bordetella pertussis.
Real-time PCR analysis of the samples yields the CT value, which represents the number of amplification cycles that the PCR test must complete before Bordetella pertussis is detectable. The fewer the cycles (and the lower the CT value), the more infectious particles must have been present in the sample. For pertussis testing, a value below 35 is considered a clinically positive result. Tests that come back with higher CT values are increasingly likely to be false positives.
The researchers plotted a value called evidence for infection (EFI), which combined a range of CT values with the number of positive tests over the seven clinic visits to group patients into none, weak, or strong EFI. Among infants with no symptoms, 77% were in the no EFI category, 16% were in the weak category, and 7% were in the strong EFI group. Of infants with minimal respiratory symptoms, 18% were in the strong group, and 20% with moderate to severe symptoms were in the strong EFI group. Among mothers, 13% with no symptoms were in the strong group. 19% in the minimal symptom group were categorized as strong EFI, as were 11% in the moderate to severe symptom group.
The study used a full range of CT, not just positive test results (for pertussis, CT ≤ 35). Beyond contributing to composite measures such as EFI, CT values can serve as leading indicators of infectious disease outbreaks in a population, according to Dr. Gunning. That’s because weaker qPCR signals (CT > 35) can provide additional information within a large sample population. Higher CT values are successively more prone to false positives, but that’s less important for disease surveillance where sensitivity is of the highest importance. The false positive “noise” tends to cancel out over time. “It may be the case that you don’t make that call (correctly) 100% of the time for 100% of the people, but if you get it right in 80 out of 100 people, that’s sufficient to say we see this pathogen circulating in the population,” said Dr. Gunning.
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Gunning and Dr. Spoulou have no relevant financial disclosures.
In the periurban slum of Lusaka, Zambia, asymptomatic pertussis infections were common among both mothers and infants, a surprising finding since asymptomatic infections are assumed to be rare in infants. The findings suggested that pertussis should be considered in cases of chronic cough, and that current standards of treating pertussis infections in low-resource settings may need to be reexamined.
The results come from testing of 1,320 infant-mother pairs who were first enrolled at a public health clinic, then followed over at least four visits. The researchers tracked pertussis infection using quantitative PCR (qPCR) on nasopharyngeal swabs. Over the course of the study, 8.9% tested positive, although only one infant developed clinical pertussis during the study.
The study was presented by Christian Gunning, a postdoctoral researcher at the University of Georgia, at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. The group also included researchers at Boston University and the University of Zambia, where PCR tests were conducted.
“That was amazing,” said session moderator Vana Spoulou, MD, PhD, professor of pediatric infectious diseases at National and Kapodistrian University of Athens, who is associated with Aghia Sofia Children’s Hospital of Athens. She noted that the study found that many physicians misdiagnosed coughs, believing them to be caused by another agent. “It was very interesting that there was so much pertussis spreading around in that community, and that nobody knew that it was around,” said Dr. Spoulou.
It’s important that physicians provide appropriate treatment, since ampicillin, which is typically prescribed for childhood upper respiratory illnesses, is believed to be ineffective against pertussis, while macrolides are effective and can prevent transmission.
Dr. Spoulou also noted that Zambia uses a whole cell vaccine, which is contraindicated in pregnant women because of potential side effects. “The good thing, despite that there was [a lot of] infection, there were no deaths, which means that maybe because the mother was infected, maybe some antibodies of the mother had passed to the child and could help the child to develop milder symptoms. So these are the pros and cons of natural infection,” said Dr. Spoulou.
The study took place in 2015, and participants were seen at the Chawama Public Health Clinic from about age 1 week to 4 months (with a target of seven clinic visits). Researchers recorded respiratory symptoms and antibiotics use at each visit, and collected a nasopharyngeal swab that was tested retrospectively using qPCR for Bordetella pertussis.
Real-time PCR analysis of the samples yields the CT value, which represents the number of amplification cycles that the PCR test must complete before Bordetella pertussis is detectable. The fewer the cycles (and the lower the CT value), the more infectious particles must have been present in the sample. For pertussis testing, a value below 35 is considered a clinically positive result. Tests that come back with higher CT values are increasingly likely to be false positives.
The researchers plotted a value called evidence for infection (EFI), which combined a range of CT values with the number of positive tests over the seven clinic visits to group patients into none, weak, or strong EFI. Among infants with no symptoms, 77% were in the no EFI category, 16% were in the weak category, and 7% were in the strong EFI group. Of infants with minimal respiratory symptoms, 18% were in the strong group, and 20% with moderate to severe symptoms were in the strong EFI group. Among mothers, 13% with no symptoms were in the strong group. 19% in the minimal symptom group were categorized as strong EFI, as were 11% in the moderate to severe symptom group.
The study used a full range of CT, not just positive test results (for pertussis, CT ≤ 35). Beyond contributing to composite measures such as EFI, CT values can serve as leading indicators of infectious disease outbreaks in a population, according to Dr. Gunning. That’s because weaker qPCR signals (CT > 35) can provide additional information within a large sample population. Higher CT values are successively more prone to false positives, but that’s less important for disease surveillance where sensitivity is of the highest importance. The false positive “noise” tends to cancel out over time. “It may be the case that you don’t make that call (correctly) 100% of the time for 100% of the people, but if you get it right in 80 out of 100 people, that’s sufficient to say we see this pathogen circulating in the population,” said Dr. Gunning.
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Gunning and Dr. Spoulou have no relevant financial disclosures.
In the periurban slum of Lusaka, Zambia, asymptomatic pertussis infections were common among both mothers and infants, a surprising finding since asymptomatic infections are assumed to be rare in infants. The findings suggested that pertussis should be considered in cases of chronic cough, and that current standards of treating pertussis infections in low-resource settings may need to be reexamined.
The results come from testing of 1,320 infant-mother pairs who were first enrolled at a public health clinic, then followed over at least four visits. The researchers tracked pertussis infection using quantitative PCR (qPCR) on nasopharyngeal swabs. Over the course of the study, 8.9% tested positive, although only one infant developed clinical pertussis during the study.
The study was presented by Christian Gunning, a postdoctoral researcher at the University of Georgia, at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. The group also included researchers at Boston University and the University of Zambia, where PCR tests were conducted.
“That was amazing,” said session moderator Vana Spoulou, MD, PhD, professor of pediatric infectious diseases at National and Kapodistrian University of Athens, who is associated with Aghia Sofia Children’s Hospital of Athens. She noted that the study found that many physicians misdiagnosed coughs, believing them to be caused by another agent. “It was very interesting that there was so much pertussis spreading around in that community, and that nobody knew that it was around,” said Dr. Spoulou.
It’s important that physicians provide appropriate treatment, since ampicillin, which is typically prescribed for childhood upper respiratory illnesses, is believed to be ineffective against pertussis, while macrolides are effective and can prevent transmission.
Dr. Spoulou also noted that Zambia uses a whole cell vaccine, which is contraindicated in pregnant women because of potential side effects. “The good thing, despite that there was [a lot of] infection, there were no deaths, which means that maybe because the mother was infected, maybe some antibodies of the mother had passed to the child and could help the child to develop milder symptoms. So these are the pros and cons of natural infection,” said Dr. Spoulou.
The study took place in 2015, and participants were seen at the Chawama Public Health Clinic from about age 1 week to 4 months (with a target of seven clinic visits). Researchers recorded respiratory symptoms and antibiotics use at each visit, and collected a nasopharyngeal swab that was tested retrospectively using qPCR for Bordetella pertussis.
Real-time PCR analysis of the samples yields the CT value, which represents the number of amplification cycles that the PCR test must complete before Bordetella pertussis is detectable. The fewer the cycles (and the lower the CT value), the more infectious particles must have been present in the sample. For pertussis testing, a value below 35 is considered a clinically positive result. Tests that come back with higher CT values are increasingly likely to be false positives.
The researchers plotted a value called evidence for infection (EFI), which combined a range of CT values with the number of positive tests over the seven clinic visits to group patients into none, weak, or strong EFI. Among infants with no symptoms, 77% were in the no EFI category, 16% were in the weak category, and 7% were in the strong EFI group. Of infants with minimal respiratory symptoms, 18% were in the strong group, and 20% with moderate to severe symptoms were in the strong EFI group. Among mothers, 13% with no symptoms were in the strong group. 19% in the minimal symptom group were categorized as strong EFI, as were 11% in the moderate to severe symptom group.
The study used a full range of CT, not just positive test results (for pertussis, CT ≤ 35). Beyond contributing to composite measures such as EFI, CT values can serve as leading indicators of infectious disease outbreaks in a population, according to Dr. Gunning. That’s because weaker qPCR signals (CT > 35) can provide additional information within a large sample population. Higher CT values are successively more prone to false positives, but that’s less important for disease surveillance where sensitivity is of the highest importance. The false positive “noise” tends to cancel out over time. “It may be the case that you don’t make that call (correctly) 100% of the time for 100% of the people, but if you get it right in 80 out of 100 people, that’s sufficient to say we see this pathogen circulating in the population,” said Dr. Gunning.
The study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Gunning and Dr. Spoulou have no relevant financial disclosures.
FROM ESPID 2021
Children aged 12-15 years continue to close COVID-19 vaccination gap
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.
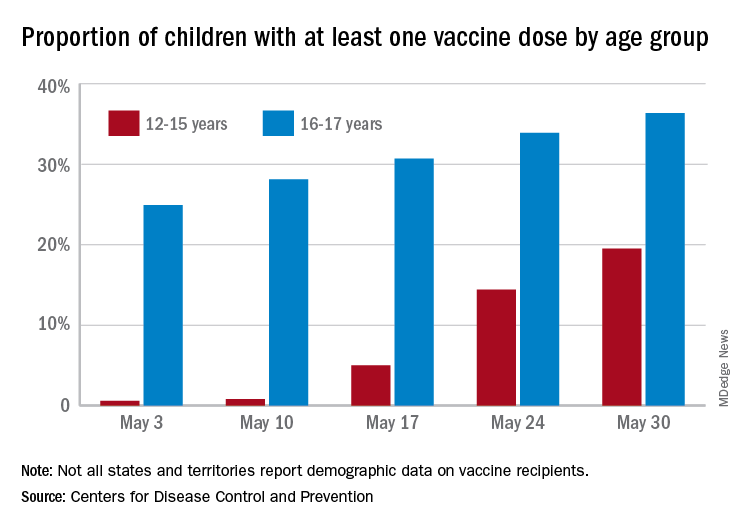
with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.

with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.

with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
Sickle cell disease: Epidemiological change in bacterial infections
Among children with sickle cell disease who have not undergone hematopoietic stem cell transplant, Salmonella is now the leading cause of invasive bacterial infection (IBI), according to a new retrospective study (BACT-SPRING) conducted in Europe. Streptococcus pneumoniae was the second most common source of infection, marking a shift from years past, when S. pneumoniae was the most common source. The epidemiology of IBI in Europe has been altered by adoption of prophylaxis and the introduction of the pneumococcal conjugated vaccine (PCV13) in 2009.
Previous studies of IBI have been single center with small sample sizes, and few have been conducted since 2016, said Jean Gaschignard, MD, PhD, during his presentation of the study at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Dr. Gaschignard is head of pediatrics at Groupe Hospitalier Nord Essonne in Longjumeau, France.
The study produced some unexpected results. “We were surprised,” said Dr. Gaschignard, by results indicating that not all children aged under 10 years were undergoing prophylaxis. Instead, the figures were closer to 80% or 90%. Among children over 10, the rate of prophylaxis varies between countries. “Our study is a clue to discuss again the indications for the age limit for prophylaxis against pneumococcus,” said Dr. Gauschignard, during the question-and-answer session following his talk.
The data give clinicians an updated picture of the epidemiology in this population following introduction of the PCV13 vaccine. “It was very important to have new data on microbiology after this implementation,” said Marie Rohr, MD, who is a fellow in pediatric infectious diseases at the University Hospitals of Geneva. Dr. Rohr moderated the session where the study was presented.
Dr. Rohr noted the shift from the dominant cause of IBI after the introduction of the PCV10/13 vaccine, from S. pneumoniae to Salmonella. The researchers also found a preponderance of bacteremia and osteoarticular infections. “The mortality and morbidity are still considerable despite infection preventive measures,” said Dr. Rohr.
The results should also prompt a second look at prevention strategies. “Even if the antibiotic prophylaxis is prescribed for a large [proportion of children with sickle cell disease] under 10 years old, the median age of invasive bacterial infection is 7 years old. This calls into question systematic antibiotic prophylaxis and case-control studies are needed to evaluate this and possibly modify antibiotic prophylaxis recommendations in the future,” said Dr. Rohr.
The BACT-SPRING study was conducted between Jan. 1, 2014, and Dec. 31, 2019, using online data. It included 217 IBI episodes from 26 centers in five European countries. Just over half were from France, while about a quarter occurred in Spain. Other countries included Belgium, Portugal, and Great Britain. Participants were younger than 18 and had an IBI confirmed by bacterial culture or PCR from normally sterile fluid.
Thirty-eight episodes occurred in children who had undergone hematopoietic stem cell transplantation (HSCT), and 179 in children who had not undergone HSCT. The presentation focused exclusively on the latter group.
Among episodes in children without HSCT, the mean age was 7. Forty-eight patients had a history of acute chest syndrome, 47 had a history of ICU admission, 29 had a history of IBI, and 27 had a history of acute splenic sequestration. Thirteen underwent a splenectomy. Almost half of children had none of these characteristics, while about one-fourth had two or more.
In the HSCT group, 141 children were on prophylaxis at the time of the infection; 74 were on hydroxyurea, and 36 were currently or previously on a transfusion program. Sixty-eight cases were primary bacteremia and 55 were osteoarticular. Other syndromes included pneumonia empyema (n = 18), and meningitis (n = 17), among others. In 44 cases, the isolated bacteria was Salmonella, followed by S. pneumoniae in 32 cases. Escherichia coli accounted for 22. Haemophilus influenza was identified in six episodes, and group A Streptococcus in three.
The study is the first large European epidemiologic study investigating IBI in children with sickle cell disease, and one of its strengths was the strict inclusion criteria. However, it was limited by its retrospective nature.
Dr. Gaschignard and Dr. Rohr have no relevant financial disclosures.
Among children with sickle cell disease who have not undergone hematopoietic stem cell transplant, Salmonella is now the leading cause of invasive bacterial infection (IBI), according to a new retrospective study (BACT-SPRING) conducted in Europe. Streptococcus pneumoniae was the second most common source of infection, marking a shift from years past, when S. pneumoniae was the most common source. The epidemiology of IBI in Europe has been altered by adoption of prophylaxis and the introduction of the pneumococcal conjugated vaccine (PCV13) in 2009.
Previous studies of IBI have been single center with small sample sizes, and few have been conducted since 2016, said Jean Gaschignard, MD, PhD, during his presentation of the study at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Dr. Gaschignard is head of pediatrics at Groupe Hospitalier Nord Essonne in Longjumeau, France.
The study produced some unexpected results. “We were surprised,” said Dr. Gaschignard, by results indicating that not all children aged under 10 years were undergoing prophylaxis. Instead, the figures were closer to 80% or 90%. Among children over 10, the rate of prophylaxis varies between countries. “Our study is a clue to discuss again the indications for the age limit for prophylaxis against pneumococcus,” said Dr. Gauschignard, during the question-and-answer session following his talk.
The data give clinicians an updated picture of the epidemiology in this population following introduction of the PCV13 vaccine. “It was very important to have new data on microbiology after this implementation,” said Marie Rohr, MD, who is a fellow in pediatric infectious diseases at the University Hospitals of Geneva. Dr. Rohr moderated the session where the study was presented.
Dr. Rohr noted the shift from the dominant cause of IBI after the introduction of the PCV10/13 vaccine, from S. pneumoniae to Salmonella. The researchers also found a preponderance of bacteremia and osteoarticular infections. “The mortality and morbidity are still considerable despite infection preventive measures,” said Dr. Rohr.
The results should also prompt a second look at prevention strategies. “Even if the antibiotic prophylaxis is prescribed for a large [proportion of children with sickle cell disease] under 10 years old, the median age of invasive bacterial infection is 7 years old. This calls into question systematic antibiotic prophylaxis and case-control studies are needed to evaluate this and possibly modify antibiotic prophylaxis recommendations in the future,” said Dr. Rohr.
The BACT-SPRING study was conducted between Jan. 1, 2014, and Dec. 31, 2019, using online data. It included 217 IBI episodes from 26 centers in five European countries. Just over half were from France, while about a quarter occurred in Spain. Other countries included Belgium, Portugal, and Great Britain. Participants were younger than 18 and had an IBI confirmed by bacterial culture or PCR from normally sterile fluid.
Thirty-eight episodes occurred in children who had undergone hematopoietic stem cell transplantation (HSCT), and 179 in children who had not undergone HSCT. The presentation focused exclusively on the latter group.
Among episodes in children without HSCT, the mean age was 7. Forty-eight patients had a history of acute chest syndrome, 47 had a history of ICU admission, 29 had a history of IBI, and 27 had a history of acute splenic sequestration. Thirteen underwent a splenectomy. Almost half of children had none of these characteristics, while about one-fourth had two or more.
In the HSCT group, 141 children were on prophylaxis at the time of the infection; 74 were on hydroxyurea, and 36 were currently or previously on a transfusion program. Sixty-eight cases were primary bacteremia and 55 were osteoarticular. Other syndromes included pneumonia empyema (n = 18), and meningitis (n = 17), among others. In 44 cases, the isolated bacteria was Salmonella, followed by S. pneumoniae in 32 cases. Escherichia coli accounted for 22. Haemophilus influenza was identified in six episodes, and group A Streptococcus in three.
The study is the first large European epidemiologic study investigating IBI in children with sickle cell disease, and one of its strengths was the strict inclusion criteria. However, it was limited by its retrospective nature.
Dr. Gaschignard and Dr. Rohr have no relevant financial disclosures.
Among children with sickle cell disease who have not undergone hematopoietic stem cell transplant, Salmonella is now the leading cause of invasive bacterial infection (IBI), according to a new retrospective study (BACT-SPRING) conducted in Europe. Streptococcus pneumoniae was the second most common source of infection, marking a shift from years past, when S. pneumoniae was the most common source. The epidemiology of IBI in Europe has been altered by adoption of prophylaxis and the introduction of the pneumococcal conjugated vaccine (PCV13) in 2009.
Previous studies of IBI have been single center with small sample sizes, and few have been conducted since 2016, said Jean Gaschignard, MD, PhD, during his presentation of the study at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Dr. Gaschignard is head of pediatrics at Groupe Hospitalier Nord Essonne in Longjumeau, France.
The study produced some unexpected results. “We were surprised,” said Dr. Gaschignard, by results indicating that not all children aged under 10 years were undergoing prophylaxis. Instead, the figures were closer to 80% or 90%. Among children over 10, the rate of prophylaxis varies between countries. “Our study is a clue to discuss again the indications for the age limit for prophylaxis against pneumococcus,” said Dr. Gauschignard, during the question-and-answer session following his talk.
The data give clinicians an updated picture of the epidemiology in this population following introduction of the PCV13 vaccine. “It was very important to have new data on microbiology after this implementation,” said Marie Rohr, MD, who is a fellow in pediatric infectious diseases at the University Hospitals of Geneva. Dr. Rohr moderated the session where the study was presented.
Dr. Rohr noted the shift from the dominant cause of IBI after the introduction of the PCV10/13 vaccine, from S. pneumoniae to Salmonella. The researchers also found a preponderance of bacteremia and osteoarticular infections. “The mortality and morbidity are still considerable despite infection preventive measures,” said Dr. Rohr.
The results should also prompt a second look at prevention strategies. “Even if the antibiotic prophylaxis is prescribed for a large [proportion of children with sickle cell disease] under 10 years old, the median age of invasive bacterial infection is 7 years old. This calls into question systematic antibiotic prophylaxis and case-control studies are needed to evaluate this and possibly modify antibiotic prophylaxis recommendations in the future,” said Dr. Rohr.
The BACT-SPRING study was conducted between Jan. 1, 2014, and Dec. 31, 2019, using online data. It included 217 IBI episodes from 26 centers in five European countries. Just over half were from France, while about a quarter occurred in Spain. Other countries included Belgium, Portugal, and Great Britain. Participants were younger than 18 and had an IBI confirmed by bacterial culture or PCR from normally sterile fluid.
Thirty-eight episodes occurred in children who had undergone hematopoietic stem cell transplantation (HSCT), and 179 in children who had not undergone HSCT. The presentation focused exclusively on the latter group.
Among episodes in children without HSCT, the mean age was 7. Forty-eight patients had a history of acute chest syndrome, 47 had a history of ICU admission, 29 had a history of IBI, and 27 had a history of acute splenic sequestration. Thirteen underwent a splenectomy. Almost half of children had none of these characteristics, while about one-fourth had two or more.
In the HSCT group, 141 children were on prophylaxis at the time of the infection; 74 were on hydroxyurea, and 36 were currently or previously on a transfusion program. Sixty-eight cases were primary bacteremia and 55 were osteoarticular. Other syndromes included pneumonia empyema (n = 18), and meningitis (n = 17), among others. In 44 cases, the isolated bacteria was Salmonella, followed by S. pneumoniae in 32 cases. Escherichia coli accounted for 22. Haemophilus influenza was identified in six episodes, and group A Streptococcus in three.
The study is the first large European epidemiologic study investigating IBI in children with sickle cell disease, and one of its strengths was the strict inclusion criteria. However, it was limited by its retrospective nature.
Dr. Gaschignard and Dr. Rohr have no relevant financial disclosures.
FROM ESPID 2021
Antiviral may improve hearing loss in congenital CMV
Infants with isolated sensorineural hearing loss as a result of congenital cytomegalovirus (cCMV) infection may benefit from treatment with valganciclovir, according to results from the CONCERT nonrandomized trial.
Subjects were found through the Newborn Hearing Screening program, using dried blood spot screening to confirm cCMV Infection. As a result of 6 weeks of therapy, more patients in the treatment group had improvements in hearing at age 20 months, and fewer had deterioration compared with untreated controls.
There is a general consensus that symptomatic cCMV should be treated with valganciclovir for 6 weeks or 6 months, but treatment of patients with only hearing loss is still under debate. The average age of participants was 8 weeks.
The study was presented by Pui Khi Chung, MD, a clinical microbiologist at the Leiden University Medical Center, the Netherlands, at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Out of 1,377 NHS-referred infants, 59 were diagnosed with cCMV (4.3%), and 35 were included in the study. Twenty-five patients received 6 weeks of valganciclovir, while 10 patients received placebo. The control group was expanded to 12 when two additional subjects were identified retrospectively and were successfully followed up at 20 months. Subjects in the treatment group were an average of 8 weeks old when treatment began. Both groups had similar neurodevelopmental outcomes at 20 months, as measured by the Bayley Scales of Infant and Toddler Development (BSID-III) and the Child Development Inventory (CDI). There were no serious adverse events associated with treatment.
To measure efficacy, the researchers used a random intercept, random slope model that accounted for repeated measurements. The differences in slopes for analyses of the best ear were significantly different between the treatment and control groups (estimated difference in slopes, –0.93; P = .0071). Further analyses of total hearing found that improvement was more common in the treatment group, and deterioration/no change was more common in the nontreatment group (P = .044). In another analysis that excluded the most profoundly impaired ears (> 70 db hearing loss), none in the control group experienced improvement and almost half deteriorated. In the treatment group, most were unchanged and a small number improved, with almost none deteriorating (P = .006).
Asked whether the treatment has any effect on the most profoundly impaired ears, Dr. Chung said she had not yet completed that analysis, but the hypothesis is that the treatment is unlikely to lead to any improvement. “When you take out the severely impaired ears, you can see a greater [treatment] effect, so it does suggest that it doesn’t do anything for those ears,” Dr. Chung said during the Q&A session following her talk.
She was also asked why the treatment period was 6 weeks, rather than 6 months – a period of treatment that has shown a better effect on long-term hearing and developmental outcomes than 6 weeks of treatment in symptomatic patients. Dr. Chung replied that she wasn’t involved in the study design, but said that at her center, the 6-month regimen is not standard.
There were two key weaknesses in the study. One was the small sample size, and the other was its nonrandomized nature, which could have led to bias in the treated versus untreated group. “Although we don’t see any baseline differences between the groups, we have to be wary in analyses. Unfortunately, an RCT proved impossible in our setting. The CONCERT Trial started as randomized but this was amended to nonrandomized, as both parents and pediatricians had a clear preference for treatment,” said Dr. Chung.
The study could provide useful information about the timing of oral antiviral medication, according to Vana Spoulou, MD, who moderated the session where the research was presented. “The earliest you can give it is best, but sometimes it’s not easy to get them diagnosed immediately after birth. What they showed us is that even giving it so late, there was some improvement,” Dr. Spoulou said in an interview.
Dr. Spoulou isn’t ready to change practice based on the results, because she noted that some other studies have shown no benefit of treatment at 3 months. “But this was a hint that maybe even in these later diagnosed cases there could be some benefit,” she said.
Dr. Chung and Dr. Spoulou have no relevant financial disclosures.
Infants with isolated sensorineural hearing loss as a result of congenital cytomegalovirus (cCMV) infection may benefit from treatment with valganciclovir, according to results from the CONCERT nonrandomized trial.
Subjects were found through the Newborn Hearing Screening program, using dried blood spot screening to confirm cCMV Infection. As a result of 6 weeks of therapy, more patients in the treatment group had improvements in hearing at age 20 months, and fewer had deterioration compared with untreated controls.
There is a general consensus that symptomatic cCMV should be treated with valganciclovir for 6 weeks or 6 months, but treatment of patients with only hearing loss is still under debate. The average age of participants was 8 weeks.
The study was presented by Pui Khi Chung, MD, a clinical microbiologist at the Leiden University Medical Center, the Netherlands, at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Out of 1,377 NHS-referred infants, 59 were diagnosed with cCMV (4.3%), and 35 were included in the study. Twenty-five patients received 6 weeks of valganciclovir, while 10 patients received placebo. The control group was expanded to 12 when two additional subjects were identified retrospectively and were successfully followed up at 20 months. Subjects in the treatment group were an average of 8 weeks old when treatment began. Both groups had similar neurodevelopmental outcomes at 20 months, as measured by the Bayley Scales of Infant and Toddler Development (BSID-III) and the Child Development Inventory (CDI). There were no serious adverse events associated with treatment.
To measure efficacy, the researchers used a random intercept, random slope model that accounted for repeated measurements. The differences in slopes for analyses of the best ear were significantly different between the treatment and control groups (estimated difference in slopes, –0.93; P = .0071). Further analyses of total hearing found that improvement was more common in the treatment group, and deterioration/no change was more common in the nontreatment group (P = .044). In another analysis that excluded the most profoundly impaired ears (> 70 db hearing loss), none in the control group experienced improvement and almost half deteriorated. In the treatment group, most were unchanged and a small number improved, with almost none deteriorating (P = .006).
Asked whether the treatment has any effect on the most profoundly impaired ears, Dr. Chung said she had not yet completed that analysis, but the hypothesis is that the treatment is unlikely to lead to any improvement. “When you take out the severely impaired ears, you can see a greater [treatment] effect, so it does suggest that it doesn’t do anything for those ears,” Dr. Chung said during the Q&A session following her talk.
She was also asked why the treatment period was 6 weeks, rather than 6 months – a period of treatment that has shown a better effect on long-term hearing and developmental outcomes than 6 weeks of treatment in symptomatic patients. Dr. Chung replied that she wasn’t involved in the study design, but said that at her center, the 6-month regimen is not standard.
There were two key weaknesses in the study. One was the small sample size, and the other was its nonrandomized nature, which could have led to bias in the treated versus untreated group. “Although we don’t see any baseline differences between the groups, we have to be wary in analyses. Unfortunately, an RCT proved impossible in our setting. The CONCERT Trial started as randomized but this was amended to nonrandomized, as both parents and pediatricians had a clear preference for treatment,” said Dr. Chung.
The study could provide useful information about the timing of oral antiviral medication, according to Vana Spoulou, MD, who moderated the session where the research was presented. “The earliest you can give it is best, but sometimes it’s not easy to get them diagnosed immediately after birth. What they showed us is that even giving it so late, there was some improvement,” Dr. Spoulou said in an interview.
Dr. Spoulou isn’t ready to change practice based on the results, because she noted that some other studies have shown no benefit of treatment at 3 months. “But this was a hint that maybe even in these later diagnosed cases there could be some benefit,” she said.
Dr. Chung and Dr. Spoulou have no relevant financial disclosures.
Infants with isolated sensorineural hearing loss as a result of congenital cytomegalovirus (cCMV) infection may benefit from treatment with valganciclovir, according to results from the CONCERT nonrandomized trial.
Subjects were found through the Newborn Hearing Screening program, using dried blood spot screening to confirm cCMV Infection. As a result of 6 weeks of therapy, more patients in the treatment group had improvements in hearing at age 20 months, and fewer had deterioration compared with untreated controls.
There is a general consensus that symptomatic cCMV should be treated with valganciclovir for 6 weeks or 6 months, but treatment of patients with only hearing loss is still under debate. The average age of participants was 8 weeks.
The study was presented by Pui Khi Chung, MD, a clinical microbiologist at the Leiden University Medical Center, the Netherlands, at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
Out of 1,377 NHS-referred infants, 59 were diagnosed with cCMV (4.3%), and 35 were included in the study. Twenty-five patients received 6 weeks of valganciclovir, while 10 patients received placebo. The control group was expanded to 12 when two additional subjects were identified retrospectively and were successfully followed up at 20 months. Subjects in the treatment group were an average of 8 weeks old when treatment began. Both groups had similar neurodevelopmental outcomes at 20 months, as measured by the Bayley Scales of Infant and Toddler Development (BSID-III) and the Child Development Inventory (CDI). There were no serious adverse events associated with treatment.
To measure efficacy, the researchers used a random intercept, random slope model that accounted for repeated measurements. The differences in slopes for analyses of the best ear were significantly different between the treatment and control groups (estimated difference in slopes, –0.93; P = .0071). Further analyses of total hearing found that improvement was more common in the treatment group, and deterioration/no change was more common in the nontreatment group (P = .044). In another analysis that excluded the most profoundly impaired ears (> 70 db hearing loss), none in the control group experienced improvement and almost half deteriorated. In the treatment group, most were unchanged and a small number improved, with almost none deteriorating (P = .006).
Asked whether the treatment has any effect on the most profoundly impaired ears, Dr. Chung said she had not yet completed that analysis, but the hypothesis is that the treatment is unlikely to lead to any improvement. “When you take out the severely impaired ears, you can see a greater [treatment] effect, so it does suggest that it doesn’t do anything for those ears,” Dr. Chung said during the Q&A session following her talk.
She was also asked why the treatment period was 6 weeks, rather than 6 months – a period of treatment that has shown a better effect on long-term hearing and developmental outcomes than 6 weeks of treatment in symptomatic patients. Dr. Chung replied that she wasn’t involved in the study design, but said that at her center, the 6-month regimen is not standard.
There were two key weaknesses in the study. One was the small sample size, and the other was its nonrandomized nature, which could have led to bias in the treated versus untreated group. “Although we don’t see any baseline differences between the groups, we have to be wary in analyses. Unfortunately, an RCT proved impossible in our setting. The CONCERT Trial started as randomized but this was amended to nonrandomized, as both parents and pediatricians had a clear preference for treatment,” said Dr. Chung.
The study could provide useful information about the timing of oral antiviral medication, according to Vana Spoulou, MD, who moderated the session where the research was presented. “The earliest you can give it is best, but sometimes it’s not easy to get them diagnosed immediately after birth. What they showed us is that even giving it so late, there was some improvement,” Dr. Spoulou said in an interview.
Dr. Spoulou isn’t ready to change practice based on the results, because she noted that some other studies have shown no benefit of treatment at 3 months. “But this was a hint that maybe even in these later diagnosed cases there could be some benefit,” she said.
Dr. Chung and Dr. Spoulou have no relevant financial disclosures.
FROM ESPID 2021
Microbiome therapeutic offers durable protection against C. difficile recurrence
SER-109, an oral microbiome therapeutic, safely protects against Clostridioides difficile recurrence for up to 24 weeks, according to a recent phase 3 trial. Three days of treatment with purified Firmicutes spores reduced risk of recurrence by 54%, suggesting a sustained, clinically meaningful response, according to a multicenter study presented at this year’s Digestive Disease Week® (DDW).
“Antibiotics targeted against C. difficile bacteria are necessary but insufficient to achieve a durable clinical response because they have no effect on C. difficile spores that germinate within a disrupted microbiome,” the investigators reported at the meeting.
“The manufacturing processes for SER-109 are designed to inactivate potential pathogens, while enriching for beneficial Firmicutes spores, which play a central role in inhibiting the cycle of C. difficile,” said Louis Y. Korman, MD, a gastroenterologist in Washington, who was lead author.
Extended data from ECOSPOR-III
The ECOSPOR-III trial involved 182 patients with at least three episodes of C. difficile infection in the previous 12 months. Patients underwent 10-21 days of antibiotic therapy with fidaxomicin or vancomycin to resolve symptoms before they were then randomized in a 1:1 ratio to receive either SER-109 (four capsules daily for 3 days) or placebo, with stratification by specific antibiotic and patient age (threshold of 65 years).
The primary objectives were safety and efficacy at 8 weeks. These results, which were previously reported at ACG 2020, showed a 68% relative risk reduction in the SER-109 group, and favorable safety data. The findings presented at DDW added to those earlier ones by providing safety and efficacy data extending to week 24. At this time point, patients treated with SER-109 had a 54% relative risk reduction in C. difficile recurrence. Recurrence rates were 21.3% and 47.3% for the treatment and placebo groups, respectively (P less than .001).
Patients 65 years and older benefited the most from SER-109 therapy, based on a relative risk reduction of 56% (P less than .001), versus a 49% relative risk reduction (lacking statistical significance) for patients younger than 65 years (P = .093). The specific antibiotic therapy patients received also appeared to impact outcomes. Patients treated with fidaxomicin had a 73% relative risk reduction (P = .009), compared with 48% for vancomycin (P = .006). Safety profiles were similar between study arms.
“By enriching for Firmicutes spores, SER-109 achieves high efficacy, while mitigating risk of transmitting infectious agents and represents a major paradigm shift in the clinical management of patients with recurrent C. difficile infection,” the investigators concluded, noting that “an open-label study for patients with recurrent C. difficile infection is currently enrolling.”
Microbiome restoration therapies
According to Sahil Khanna, MBBS, professor of medicine at Mayo Clinic, Rochester, Minn., these findings “advance the field” because they show a sustained response. “We know that microbiome restoration therapies help restore colonization resistance,” Dr. Khanna said in an interview, noting that they offer benefits comparable to fecal microbiota transplantation (FMT) without the downsides.
“The trouble with FMT is that it’s heterogenous – everybody does it differently … and also it’s an invasive procedure,” Dr. Khanna said. He noted that FMT may transmit infectious agents between donors and patients, which isn’t an issue with purified products such as SER-109.
Several other standardized microbiota restoration products are under development, Dr. Khanna said, including an enema form (RBX2660) in phase 3 testing, and two other capsules (CP101 and VE303) in phase 2 trials. “The hope would be that one or more of these products would be approved for clinical use in the near future and would probably replace the vast majority of FMT [procedures] that we do clinically,” Dr. Khanna said. “That’s where the field is headed.”
The investigators reported no conflicts of interest. Dr. Khanna disclosed research support from Finch, Rebiotix/Ferring, Vedanta, and Seres.
SER-109, an oral microbiome therapeutic, safely protects against Clostridioides difficile recurrence for up to 24 weeks, according to a recent phase 3 trial. Three days of treatment with purified Firmicutes spores reduced risk of recurrence by 54%, suggesting a sustained, clinically meaningful response, according to a multicenter study presented at this year’s Digestive Disease Week® (DDW).
“Antibiotics targeted against C. difficile bacteria are necessary but insufficient to achieve a durable clinical response because they have no effect on C. difficile spores that germinate within a disrupted microbiome,” the investigators reported at the meeting.
“The manufacturing processes for SER-109 are designed to inactivate potential pathogens, while enriching for beneficial Firmicutes spores, which play a central role in inhibiting the cycle of C. difficile,” said Louis Y. Korman, MD, a gastroenterologist in Washington, who was lead author.
Extended data from ECOSPOR-III
The ECOSPOR-III trial involved 182 patients with at least three episodes of C. difficile infection in the previous 12 months. Patients underwent 10-21 days of antibiotic therapy with fidaxomicin or vancomycin to resolve symptoms before they were then randomized in a 1:1 ratio to receive either SER-109 (four capsules daily for 3 days) or placebo, with stratification by specific antibiotic and patient age (threshold of 65 years).
The primary objectives were safety and efficacy at 8 weeks. These results, which were previously reported at ACG 2020, showed a 68% relative risk reduction in the SER-109 group, and favorable safety data. The findings presented at DDW added to those earlier ones by providing safety and efficacy data extending to week 24. At this time point, patients treated with SER-109 had a 54% relative risk reduction in C. difficile recurrence. Recurrence rates were 21.3% and 47.3% for the treatment and placebo groups, respectively (P less than .001).
Patients 65 years and older benefited the most from SER-109 therapy, based on a relative risk reduction of 56% (P less than .001), versus a 49% relative risk reduction (lacking statistical significance) for patients younger than 65 years (P = .093). The specific antibiotic therapy patients received also appeared to impact outcomes. Patients treated with fidaxomicin had a 73% relative risk reduction (P = .009), compared with 48% for vancomycin (P = .006). Safety profiles were similar between study arms.
“By enriching for Firmicutes spores, SER-109 achieves high efficacy, while mitigating risk of transmitting infectious agents and represents a major paradigm shift in the clinical management of patients with recurrent C. difficile infection,” the investigators concluded, noting that “an open-label study for patients with recurrent C. difficile infection is currently enrolling.”
Microbiome restoration therapies
According to Sahil Khanna, MBBS, professor of medicine at Mayo Clinic, Rochester, Minn., these findings “advance the field” because they show a sustained response. “We know that microbiome restoration therapies help restore colonization resistance,” Dr. Khanna said in an interview, noting that they offer benefits comparable to fecal microbiota transplantation (FMT) without the downsides.
“The trouble with FMT is that it’s heterogenous – everybody does it differently … and also it’s an invasive procedure,” Dr. Khanna said. He noted that FMT may transmit infectious agents between donors and patients, which isn’t an issue with purified products such as SER-109.
Several other standardized microbiota restoration products are under development, Dr. Khanna said, including an enema form (RBX2660) in phase 3 testing, and two other capsules (CP101 and VE303) in phase 2 trials. “The hope would be that one or more of these products would be approved for clinical use in the near future and would probably replace the vast majority of FMT [procedures] that we do clinically,” Dr. Khanna said. “That’s where the field is headed.”
The investigators reported no conflicts of interest. Dr. Khanna disclosed research support from Finch, Rebiotix/Ferring, Vedanta, and Seres.
SER-109, an oral microbiome therapeutic, safely protects against Clostridioides difficile recurrence for up to 24 weeks, according to a recent phase 3 trial. Three days of treatment with purified Firmicutes spores reduced risk of recurrence by 54%, suggesting a sustained, clinically meaningful response, according to a multicenter study presented at this year’s Digestive Disease Week® (DDW).
“Antibiotics targeted against C. difficile bacteria are necessary but insufficient to achieve a durable clinical response because they have no effect on C. difficile spores that germinate within a disrupted microbiome,” the investigators reported at the meeting.
“The manufacturing processes for SER-109 are designed to inactivate potential pathogens, while enriching for beneficial Firmicutes spores, which play a central role in inhibiting the cycle of C. difficile,” said Louis Y. Korman, MD, a gastroenterologist in Washington, who was lead author.
Extended data from ECOSPOR-III
The ECOSPOR-III trial involved 182 patients with at least three episodes of C. difficile infection in the previous 12 months. Patients underwent 10-21 days of antibiotic therapy with fidaxomicin or vancomycin to resolve symptoms before they were then randomized in a 1:1 ratio to receive either SER-109 (four capsules daily for 3 days) or placebo, with stratification by specific antibiotic and patient age (threshold of 65 years).
The primary objectives were safety and efficacy at 8 weeks. These results, which were previously reported at ACG 2020, showed a 68% relative risk reduction in the SER-109 group, and favorable safety data. The findings presented at DDW added to those earlier ones by providing safety and efficacy data extending to week 24. At this time point, patients treated with SER-109 had a 54% relative risk reduction in C. difficile recurrence. Recurrence rates were 21.3% and 47.3% for the treatment and placebo groups, respectively (P less than .001).
Patients 65 years and older benefited the most from SER-109 therapy, based on a relative risk reduction of 56% (P less than .001), versus a 49% relative risk reduction (lacking statistical significance) for patients younger than 65 years (P = .093). The specific antibiotic therapy patients received also appeared to impact outcomes. Patients treated with fidaxomicin had a 73% relative risk reduction (P = .009), compared with 48% for vancomycin (P = .006). Safety profiles were similar between study arms.
“By enriching for Firmicutes spores, SER-109 achieves high efficacy, while mitigating risk of transmitting infectious agents and represents a major paradigm shift in the clinical management of patients with recurrent C. difficile infection,” the investigators concluded, noting that “an open-label study for patients with recurrent C. difficile infection is currently enrolling.”
Microbiome restoration therapies
According to Sahil Khanna, MBBS, professor of medicine at Mayo Clinic, Rochester, Minn., these findings “advance the field” because they show a sustained response. “We know that microbiome restoration therapies help restore colonization resistance,” Dr. Khanna said in an interview, noting that they offer benefits comparable to fecal microbiota transplantation (FMT) without the downsides.
“The trouble with FMT is that it’s heterogenous – everybody does it differently … and also it’s an invasive procedure,” Dr. Khanna said. He noted that FMT may transmit infectious agents between donors and patients, which isn’t an issue with purified products such as SER-109.
Several other standardized microbiota restoration products are under development, Dr. Khanna said, including an enema form (RBX2660) in phase 3 testing, and two other capsules (CP101 and VE303) in phase 2 trials. “The hope would be that one or more of these products would be approved for clinical use in the near future and would probably replace the vast majority of FMT [procedures] that we do clinically,” Dr. Khanna said. “That’s where the field is headed.”
The investigators reported no conflicts of interest. Dr. Khanna disclosed research support from Finch, Rebiotix/Ferring, Vedanta, and Seres.
FROM DDW 2021
COVID-19: One Patient at a Time
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
I will never forget the first time I cared for a patient who tested positive for COVID-19. It was March 2020, and I was evaluating a patient in the emergency department (ED). At the time we knew very little about this virus and how it is transmitted. We had all seen the images from Wuhan, China, and had appropriate fear of the lethality of the virus, but there was not yet a clear understanding as to how best to keep health care practitioners safe as they cared for patients with COVID-19.
That evening I received a page that a middle-aged man who had tested positive for COVID-19 was in the ED with fever, cough, and hypoxia. As a hospitalist, my role is to care for these patients, those admitted to stay overnight in the hospital. Before going to see the patient, I watched a video on how to properly don personal protective equipment (PPE). I walked to the ED and suited up with a surgical mask, goggles, disposable gown, and gloves. I was very conscious of the amount of time I spent in that patient’s room, and tried to stand at the foot of the bed as much as possible so as to maximize the distance between our faces when we talked.
Upon finishing my assessment, I took off my PPE and exited the room but kept wondering if I had done so correctly. That night when I came home, I slept in the guest bedroom to minimize the risk of transmission of the virus to my wife. For the next 7 days I was terrified that I had been exposed to the virus, worried that I hadn’t worn my mask properly, or that I exposed myself to contamination when taking off my goggles and gown. I was hyperaware of my breathing and temperature, wondering if that scratch in my throat was the first sign of something worse. I never did develop any symptoms of illness but the amount of stress I felt that week was enormous.
Over the subsequent weeks I became much more comfortable with putting on and taking off PPE since the volume of COVID patients kept increasing to the point that more than 80% of the hospital patient census consisted of COVID-19 infections. Those patient interactions became less awkward once I could stop worrying about the PPE and focus on providing patient care.
Unfortunately, patient after patient entered the hospital, all with the same symptoms: cough, fever, and hypoxia. Medically there was little decision-making necessary as care was mostly supportive with supplemental oxygen to give these patients time to recover. Instead, I focused on understanding each patient’s symptoms and thinking about what could be offered to relieve bothersome symptoms. These patients were isolated in their hospital rooms – denied visitors and their interactions with hospital staff involved layers and layers of protective barrier. I sought to overcome those physical barriers through personal connection – learning about a patient’s hobbies, asking about their families, or reminiscing about one of their favorite trips.
Despite this supportive care, many patients ended up intubated in the intensive care unit. Many eventually improved, and we celebrated those individuals – a victory at a time. We even counted the COVID discharges with a running tally; first 10, then a few dozen, and eventually the number climbed into the triple digits. But not every patient was so fortunate. Hearing about a 40-something who passed away hit too close to home – what if that were me?
The hospitalists I work with rose to the occasion. We feared the virus but still showed up for work because the patients needed us and we had job obligations to honor. Everyone else was stuck at home during lockdown but we still got in our cars and drove to the hospital, suited up in our PPE, and cared for terrified patients that were struggling to breathe.
There was a satisfaction in having a job to do and being able to contribute during this time of global crisis. Staying busy gave our minds something to focus on and helped us feel a sense of purpose. Some of us stayed late to coordinate staffing. Others helped to disseminate practice guidelines and clinical knowledge. While others lent a hand wherever they could to pitch in. That sense of camaraderie served as plenty of motivation.
During the early stages of the pandemic, there was a sense that this crisis that would end after a few months and life would return to normal. By May, we experienced a dramatic decline in the number of hospitalized patients with COVID-19, which resulted in a real sense of optimism. But soon it became apparent that this pandemic was not going away anytime soon.
Cases nationwide began rising again over the summer. We saw a steady trickle of new admissions at our hospital month after month until the fall when the rate of admissions accelerated again. The hospital reactivated our surge plan, increased staffing, and confronted the new surge with growing dread. That first surge was all endorphins – but fatigue set in by the time the second wave hit. The volunteerism and sense of “we are in this together” just did not exist anymore. The stories about health care heroes in the broader community waned and the outside world seemingly had moved on from thinking about the pandemic.
Yet we remained, caring for patients with cough, fever, and low oxygen saturation. It was like living through a movie we had already seen before. We knew what we were supposed to do and we followed the script. But now it felt too much like a routine.
It has been a very long 14 months since I first cared for a patient with COVID-19. For much of this time it felt like we were just stuck on a treadmill, passing the time but not making any significant progress towards a post-COVID future state. How many times over this year did we push that date forward in our minds when “life would go back to normal”?
Now, we have reason for hope. More than 100 million Americans have been vaccinated and that number rises daily. The vaccines are remarkably effective, they are making a real difference in reducing the number of patients with COVID-19 at the hospital, and our level of daily anxiety is lower. There is still much uncertainty about the future, but at least we can feel proud of our service over the last year — proud of showing up and donning that PPE. And so, we continue one patient at a time.
Corresponding author: James A. Colbert, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA, 02462, Senior Medical Director, Blue Cross Blue Shield of Massachusetts; [email protected].
Financial disclosures: None.
Clean indoor air is vital for infection control
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.



