User login
Gene therapy is bad business, and hugging chickens is just … bad
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
The COVID-19 pandemic and changes in pediatric respiratory and nonrespiratory illnesses
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
The COVID-19 pandemic upended the U.S. health care market and disrupted much of what was thought to be consistent and necessary hospital-based care for children. Early in the pandemic, clinics closed, elective surgeries were delayed, and well visits were postponed. Mitigation strategies were launched nationwide to limit the spread of SARS-CoV-2 including mask mandates, social distancing, shelter-in-place orders, and school closures. While these measures were enacted to target COVID-19, a potential off-target effect was reductions in transmission of other respiratory illness, and potentially nonrespiratory infectious illnesses and conditions exacerbated by acute infections.1 These measures have heavily impacted the pediatric population, wherein respiratory infections are common, and also because daycares and school can be hubs for disease transmission.2
To evaluate the effect of the COVID-19 pandemic on pediatric health care utilization, we performed a multicenter, cross-sectional study of 44 children’s hospitals using the Pediatric Health Information System (PHIS) database.3 Children aged 2 months to 18 years discharged from a PHIS hospital with nonsurgical diagnoses from Jan. 1 to Sept. 30 over a 4-year period (2017-2020) were included in the study. The primary exposure was the 2020 COVID-19 pandemic, which was divided into three study periods: pre–COVID-19 (January–February 2020), early COVID-19 (March-April 2020), and COVID-19 (May-September 2020). The primary outcomes were the observed-to-expected ratio of respiratory and nonrespiratory illness encounters of the study period, compared with the 3 years prior to the pandemic. For these calculations, the expected encounters for each period was derived from the same calendar periods from prepandemic years (2017-2019).
A total of 9,051,980 pediatric encounters were included in the analyses: 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. We found a 42% reduction in overall encounters during the COVID-19 period, compared with the 3 years prior to the pandemic, with a greater reduction in respiratory, compared with nonrespiratory illnesses, which decreased 62% and 38%, respectively. These reductions were consistent across geographic and encounter type (ED vs. hospitalization). The frequency of hospital-based encounters for common pediatric respiratory illnesses was substantially reduced, with reductions in asthma exacerbations (down 76%), pneumonia (down 81%), croup (down 84%), influenza (down 87%) and bronchiolitis (down 91%). Differences in both respiratory and nonrespiratory illnesses varied by age, with larger reductions found in children aged less than 12 years. While adolescent (children aged over 12 years) encounters diminished during the early COVID period for both respiratory and nonrespiratory illnesses, their encounters returned to previous levels faster than those from younger children. For respiratory illnesses, hospital-based adolescents encounters had returned to prepandemic levels by the end of the study period (September 2020).
These findings warrant consideration as relaxation of SARS-CoV-2 mitigation are contemplated. Encounters for respiratory and nonrespiratory illnesses declined less and recovered faster in adolescents, compared with younger children. The underlying contributors to this trend are likely multifactorial. For example, respiratory illnesses such as croup and bronchiolitis are more common in younger children and adolescents may be more likely to transmit SARS-CoV-2, compared with younger age groups.4,5 However, adolescents may have had less strict adherence to social distancing measures.6 Future efforts to halt transmission of SARS-CoV-2, as well as other respiratory pathogens, should inform mitigation efforts in the adolescent population with considerations of the intensity of social mixing in different pediatric age groups.
While reductions in encounters caused by respiratory illnesses were substantial, more modest but similar age-based trends were seen in nonrespiratory illnesses. Yet, reduced transmission of infectious agents may not fully explain these findings. For example, it is possible that families sought care for mild to moderate nonrespiratory illness in clinics or via telehealth rather than the EDs.7 Provided there were no unintended negative consequences, such transition of care to non-ED settings would suggest there was overutilization of hospital resources prior to the pandemic. Additional assessments would be helpful to examine this more closely and to clarify the long-term impact of those transitions.
It is also possible that the pandemic effects on financial, social, and family stress may have led to increases in some pediatric health care encounters, such as those for mental health conditions,8 nonaccidental trauma or inability to adhere to treatment because of lack of resources.9,10 Additional study on the evolution and distribution of social and stress-related illnesses is critical to maintain and improve the health of children and adolescents.
The COVID-19 pandemic resulted in rapid and marked changes to both communicable and noncommunicable illnesses and care-seeking behaviors. Some of these findings are encouraging, such as large reductions in respiratory and nonrespiratory illnesses. However, other trends may be harbingers of negative health consequences of the pandemic, such as increases in health care utilization later in the pandemic. Further study of the evolving pandemic’s effects on disease and health care utilization is needed to benefit our children now and during the next pandemic.
Dr. Antoon is an assistant professor of pediatrics at Vanderbilt University and a pediatric hospitalist at the Monroe Carroll Jr. Children’s Hospital at Vanderbilt, both in Nashville, Tenn.
References
1. Kenyon CC et al. Initial effects of the COVID-19 pandemic on pediatric asthma emergency department utilization. J Allergy Clin Immunol Pract. 2020 Sep;8(8):2774-6.e1. doi: 10.1016/j.jaip.2020.05.045.
2. Luca G et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. doi: 10.1186/s12879-017-2934-3.
3. Antoon JW et al. The COVID-19 Pandemic and changes in healthcare utilization for pediatric respiratory and nonrespiratory illnesses in the United States. J Hosp Med. 2021 Mar 8. doi: 10.12788/jhm.3608.
4. Park YJ et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 Oct;26(10):2465-8. doi: 10.3201/eid2610.201315.
5. Davies NG et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 Aug;26(8):1205-11. doi: 10.1038/s41591-020-0962-9.
6. Andrews JL et al. Peer influence in adolescence: Public health implications for COVID-19. Trends Cogn Sci. 2020;24(8):585-7. doi: 10.1016/j.tics.2020.05.001.
7. Taquechel K et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3378-87.e11. doi: 10.1016/j.jaip.2020.07.057.
8. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. 2021;147(3):e2020029280. doi: 10.1542/peds.2020-029280.
9. Sharma S et al. COVID-19: Differences in sentinel injury and child abuse reporting during a pandemic. Child Abuse Negl. 2020 Dec;110:104709. doi: 10.1016/j.chiabu.2020.104709.
10. Lauren BN et al. Predictors of households at risk for food insecurity in the United States during the COVID-19 pandemic. Public Health Nutr. 2021 Jan 27. doi: 10.1017/S1368980021000355.
Care of post–acute COVID-19 patients requires multidisciplinary collaboration
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
FROM SHM CONVERGE 2021
Study identifies strong association between use of rotavirus vaccines, 60% reduction in infection
Two widely used rotavirus vaccines performed comparably in a meta-analysis, reducing risk of rotavirus gastroenteritis (RVGE) by more than 60% in young children. While the findings evidence a high protection level and low-risk safety profile, investigators of the study called for additional head-to-head comparisons to assess risks and benefits.
RVGE, which accounts for 28.8% of all deaths from diarrhea worldwide, is the leading cause of diarrhea in children under age 5. More than 100 countries include rotavirus vaccines in their immunization programs. Among six types of vaccines currently in use, two live-attenuated oral vaccines: the two-dose monovalent Rotarix (RV1) and three-dose pentavalent RotaTeq (RV5]) are in use worldwide.
Not much is known about their interchangeability, although a previous meta-analysis reported similarities in effectiveness of Rotarix (83%), RotaTeq (85%), and Rotarix and RotaTeq mixed series (86%) in low-mortality countries. RVGE morbidity and mortality have declined since the introduction of these vaccines, but concerns persist about their safety, Zi-Wei Sun, MSc, of Nanjing (China) Medical University and colleagues wrote in JAMA Pediatrics.
Their systematic review and meta-analysis of randomized clinical trials, case-control, and cohort studies compared benefit, risk, and immunogenicity of these vaccines and their effectiveness in reducing RVGE. Combing through databases Embase, PubMed, the Cochrane Library, and Web of Science using search terms “rotavirus” and “vaccine,” they chose 121 randomized clinical trials and cohort and case-control studies that included more than 100 children younger than 5 years. Thirty-eight of the randomized clinical trials had related data that examined the vaccines’ protection against RVGE hospitalization, study coauthor Hemant Goyal, MD, FACP, explained in an interview.
All of the studies reported on the safety and effectiveness or immunogenicity of rotavirus vaccines. The investigators used a random-effects model to calculate relative risks, odds ratios, risk differences, and 95% confidence intervals. They also stratified studies by economic development of countries, given that vaccine efficacy is often higher in middle- and high-income countries, compared with low-income countries. An adjusted indirect treatment comparison evaluated differences in vaccine protection among different subgroups, adopting P < .05 as the level of statistical significance.
Primary outcomes included RVGE, severe RVGE, and RVGE hospitalization and safety-associated outcomes such as serious adverse events, intussusception, and mortality.
Rotarix and RotaTeq reduced RVGE in children younger than 5 years by 68.4% and 63.6%, respectively. Dr. Goyal and colleagues confirmed these results in case-control studies (65.3% and 72.8%, respectively). Both vaccines significantly reduced RVGE and RVGE hospitalization risk and demonstrated higher protection against severe RVGE. In adjusted indirect comparisons, the two vaccines showed no significant differences in protection. They also found a positive correlation between immunogenicity and vaccine protection.
“RotaTeq seems to show lower protection in low-income countries, compared with Rotarix, but these estimates should be interpreted with caution as there was only one study for low-income countries and indirect comparison," said Dr. Goyal, a second-year gastroenterology fellow at the Wright Center for Graduate Medical Education, Scranton, Penn.
None of the vaccines demonstrated risk of serious adverse events. However, an Australian study in 2013 did report a small increased risk of intussusception after RV1 and RV5 vaccination. “Therefore, continuous surveillance of the benefits and adverse effects of rotavirus vaccines is required after vaccination,” the investigators noted.
Analyzing newer, less widely distributed vaccines, Rotavac, Rotasiil, and Lanzhou lamb rotavirus vaccine also showed moderate effectiveness in reducing RVGE risk.
Immunity wanes over time
Protection against rotavirus diseases seems to wane over time after vaccination. “Although our results indicated that rotavirus vaccines can provide substantial protection against RVGE during the first 2 years of life, more studies following up the vaccine efficacy for more than 2 years are required,” the investigators recommended.
Declining vaccine-induced antibodies, RVGE-acquired protection from the vaccine’s indirect effects, or exposure to unvaccinated populations may explain gradual loss of immunity.
Monitoring of rotavirus strains following vaccination should take place “to avoid population-based selection of so-called escape strains, especially fully heterotypic strains and new strains, because of the long-term pressure of vaccine immunity,” they recommended.
The findings emphasize the importance of introducing vaccines worldwide to reduce infection, summarized Dr. Goyal and colleagues. Given how challenging it is to treat the wide varieties of rotavirus, “It encouraging that RV1 and RV5 work well against heterotypic strains,” they added. Similar performance between Rotarix and RotaTeq also makes it easier for clinicians to choose a vaccine.
Increasing the availability and efficacy of these vaccines in low-income countries with high mortality rates is a high priority,
David I. Bernstein, MD, MA, wrote in a related editorial: “A clear gradient in vaccine protections was noted by country income level in the analysis presented, and much effort has been spent to understand this discrepancy.”
Overall, the study confirmed the efficacy of these two vaccines and their equivalence, noted Dr. Bernstein.
The study’s literature search process had some limitations. “Especially in stratified analyses, sparse data in some subgroups limit generalizability. ... The most accurate method, head-to-head comparisons, to evaluate the comparative efficacy of different vaccines is required in further studies,” the study investigators wrote.
Such studies would directly compare Rotarix and RotaTeq from multiple perspectives: efficacy, cost-effectiveness, strain-specific protection, the duration of protection, safety, and immunogenicity, said Dr. Goyal.
*This story was updated on May 24, 2021.
Two widely used rotavirus vaccines performed comparably in a meta-analysis, reducing risk of rotavirus gastroenteritis (RVGE) by more than 60% in young children. While the findings evidence a high protection level and low-risk safety profile, investigators of the study called for additional head-to-head comparisons to assess risks and benefits.
RVGE, which accounts for 28.8% of all deaths from diarrhea worldwide, is the leading cause of diarrhea in children under age 5. More than 100 countries include rotavirus vaccines in their immunization programs. Among six types of vaccines currently in use, two live-attenuated oral vaccines: the two-dose monovalent Rotarix (RV1) and three-dose pentavalent RotaTeq (RV5]) are in use worldwide.
Not much is known about their interchangeability, although a previous meta-analysis reported similarities in effectiveness of Rotarix (83%), RotaTeq (85%), and Rotarix and RotaTeq mixed series (86%) in low-mortality countries. RVGE morbidity and mortality have declined since the introduction of these vaccines, but concerns persist about their safety, Zi-Wei Sun, MSc, of Nanjing (China) Medical University and colleagues wrote in JAMA Pediatrics.
Their systematic review and meta-analysis of randomized clinical trials, case-control, and cohort studies compared benefit, risk, and immunogenicity of these vaccines and their effectiveness in reducing RVGE. Combing through databases Embase, PubMed, the Cochrane Library, and Web of Science using search terms “rotavirus” and “vaccine,” they chose 121 randomized clinical trials and cohort and case-control studies that included more than 100 children younger than 5 years. Thirty-eight of the randomized clinical trials had related data that examined the vaccines’ protection against RVGE hospitalization, study coauthor Hemant Goyal, MD, FACP, explained in an interview.
All of the studies reported on the safety and effectiveness or immunogenicity of rotavirus vaccines. The investigators used a random-effects model to calculate relative risks, odds ratios, risk differences, and 95% confidence intervals. They also stratified studies by economic development of countries, given that vaccine efficacy is often higher in middle- and high-income countries, compared with low-income countries. An adjusted indirect treatment comparison evaluated differences in vaccine protection among different subgroups, adopting P < .05 as the level of statistical significance.
Primary outcomes included RVGE, severe RVGE, and RVGE hospitalization and safety-associated outcomes such as serious adverse events, intussusception, and mortality.
Rotarix and RotaTeq reduced RVGE in children younger than 5 years by 68.4% and 63.6%, respectively. Dr. Goyal and colleagues confirmed these results in case-control studies (65.3% and 72.8%, respectively). Both vaccines significantly reduced RVGE and RVGE hospitalization risk and demonstrated higher protection against severe RVGE. In adjusted indirect comparisons, the two vaccines showed no significant differences in protection. They also found a positive correlation between immunogenicity and vaccine protection.
“RotaTeq seems to show lower protection in low-income countries, compared with Rotarix, but these estimates should be interpreted with caution as there was only one study for low-income countries and indirect comparison," said Dr. Goyal, a second-year gastroenterology fellow at the Wright Center for Graduate Medical Education, Scranton, Penn.
None of the vaccines demonstrated risk of serious adverse events. However, an Australian study in 2013 did report a small increased risk of intussusception after RV1 and RV5 vaccination. “Therefore, continuous surveillance of the benefits and adverse effects of rotavirus vaccines is required after vaccination,” the investigators noted.
Analyzing newer, less widely distributed vaccines, Rotavac, Rotasiil, and Lanzhou lamb rotavirus vaccine also showed moderate effectiveness in reducing RVGE risk.
Immunity wanes over time
Protection against rotavirus diseases seems to wane over time after vaccination. “Although our results indicated that rotavirus vaccines can provide substantial protection against RVGE during the first 2 years of life, more studies following up the vaccine efficacy for more than 2 years are required,” the investigators recommended.
Declining vaccine-induced antibodies, RVGE-acquired protection from the vaccine’s indirect effects, or exposure to unvaccinated populations may explain gradual loss of immunity.
Monitoring of rotavirus strains following vaccination should take place “to avoid population-based selection of so-called escape strains, especially fully heterotypic strains and new strains, because of the long-term pressure of vaccine immunity,” they recommended.
The findings emphasize the importance of introducing vaccines worldwide to reduce infection, summarized Dr. Goyal and colleagues. Given how challenging it is to treat the wide varieties of rotavirus, “It encouraging that RV1 and RV5 work well against heterotypic strains,” they added. Similar performance between Rotarix and RotaTeq also makes it easier for clinicians to choose a vaccine.
Increasing the availability and efficacy of these vaccines in low-income countries with high mortality rates is a high priority,
David I. Bernstein, MD, MA, wrote in a related editorial: “A clear gradient in vaccine protections was noted by country income level in the analysis presented, and much effort has been spent to understand this discrepancy.”
Overall, the study confirmed the efficacy of these two vaccines and their equivalence, noted Dr. Bernstein.
The study’s literature search process had some limitations. “Especially in stratified analyses, sparse data in some subgroups limit generalizability. ... The most accurate method, head-to-head comparisons, to evaluate the comparative efficacy of different vaccines is required in further studies,” the study investigators wrote.
Such studies would directly compare Rotarix and RotaTeq from multiple perspectives: efficacy, cost-effectiveness, strain-specific protection, the duration of protection, safety, and immunogenicity, said Dr. Goyal.
*This story was updated on May 24, 2021.
Two widely used rotavirus vaccines performed comparably in a meta-analysis, reducing risk of rotavirus gastroenteritis (RVGE) by more than 60% in young children. While the findings evidence a high protection level and low-risk safety profile, investigators of the study called for additional head-to-head comparisons to assess risks and benefits.
RVGE, which accounts for 28.8% of all deaths from diarrhea worldwide, is the leading cause of diarrhea in children under age 5. More than 100 countries include rotavirus vaccines in their immunization programs. Among six types of vaccines currently in use, two live-attenuated oral vaccines: the two-dose monovalent Rotarix (RV1) and three-dose pentavalent RotaTeq (RV5]) are in use worldwide.
Not much is known about their interchangeability, although a previous meta-analysis reported similarities in effectiveness of Rotarix (83%), RotaTeq (85%), and Rotarix and RotaTeq mixed series (86%) in low-mortality countries. RVGE morbidity and mortality have declined since the introduction of these vaccines, but concerns persist about their safety, Zi-Wei Sun, MSc, of Nanjing (China) Medical University and colleagues wrote in JAMA Pediatrics.
Their systematic review and meta-analysis of randomized clinical trials, case-control, and cohort studies compared benefit, risk, and immunogenicity of these vaccines and their effectiveness in reducing RVGE. Combing through databases Embase, PubMed, the Cochrane Library, and Web of Science using search terms “rotavirus” and “vaccine,” they chose 121 randomized clinical trials and cohort and case-control studies that included more than 100 children younger than 5 years. Thirty-eight of the randomized clinical trials had related data that examined the vaccines’ protection against RVGE hospitalization, study coauthor Hemant Goyal, MD, FACP, explained in an interview.
All of the studies reported on the safety and effectiveness or immunogenicity of rotavirus vaccines. The investigators used a random-effects model to calculate relative risks, odds ratios, risk differences, and 95% confidence intervals. They also stratified studies by economic development of countries, given that vaccine efficacy is often higher in middle- and high-income countries, compared with low-income countries. An adjusted indirect treatment comparison evaluated differences in vaccine protection among different subgroups, adopting P < .05 as the level of statistical significance.
Primary outcomes included RVGE, severe RVGE, and RVGE hospitalization and safety-associated outcomes such as serious adverse events, intussusception, and mortality.
Rotarix and RotaTeq reduced RVGE in children younger than 5 years by 68.4% and 63.6%, respectively. Dr. Goyal and colleagues confirmed these results in case-control studies (65.3% and 72.8%, respectively). Both vaccines significantly reduced RVGE and RVGE hospitalization risk and demonstrated higher protection against severe RVGE. In adjusted indirect comparisons, the two vaccines showed no significant differences in protection. They also found a positive correlation between immunogenicity and vaccine protection.
“RotaTeq seems to show lower protection in low-income countries, compared with Rotarix, but these estimates should be interpreted with caution as there was only one study for low-income countries and indirect comparison," said Dr. Goyal, a second-year gastroenterology fellow at the Wright Center for Graduate Medical Education, Scranton, Penn.
None of the vaccines demonstrated risk of serious adverse events. However, an Australian study in 2013 did report a small increased risk of intussusception after RV1 and RV5 vaccination. “Therefore, continuous surveillance of the benefits and adverse effects of rotavirus vaccines is required after vaccination,” the investigators noted.
Analyzing newer, less widely distributed vaccines, Rotavac, Rotasiil, and Lanzhou lamb rotavirus vaccine also showed moderate effectiveness in reducing RVGE risk.
Immunity wanes over time
Protection against rotavirus diseases seems to wane over time after vaccination. “Although our results indicated that rotavirus vaccines can provide substantial protection against RVGE during the first 2 years of life, more studies following up the vaccine efficacy for more than 2 years are required,” the investigators recommended.
Declining vaccine-induced antibodies, RVGE-acquired protection from the vaccine’s indirect effects, or exposure to unvaccinated populations may explain gradual loss of immunity.
Monitoring of rotavirus strains following vaccination should take place “to avoid population-based selection of so-called escape strains, especially fully heterotypic strains and new strains, because of the long-term pressure of vaccine immunity,” they recommended.
The findings emphasize the importance of introducing vaccines worldwide to reduce infection, summarized Dr. Goyal and colleagues. Given how challenging it is to treat the wide varieties of rotavirus, “It encouraging that RV1 and RV5 work well against heterotypic strains,” they added. Similar performance between Rotarix and RotaTeq also makes it easier for clinicians to choose a vaccine.
Increasing the availability and efficacy of these vaccines in low-income countries with high mortality rates is a high priority,
David I. Bernstein, MD, MA, wrote in a related editorial: “A clear gradient in vaccine protections was noted by country income level in the analysis presented, and much effort has been spent to understand this discrepancy.”
Overall, the study confirmed the efficacy of these two vaccines and their equivalence, noted Dr. Bernstein.
The study’s literature search process had some limitations. “Especially in stratified analyses, sparse data in some subgroups limit generalizability. ... The most accurate method, head-to-head comparisons, to evaluate the comparative efficacy of different vaccines is required in further studies,” the study investigators wrote.
Such studies would directly compare Rotarix and RotaTeq from multiple perspectives: efficacy, cost-effectiveness, strain-specific protection, the duration of protection, safety, and immunogenicity, said Dr. Goyal.
*This story was updated on May 24, 2021.
FROM JAMA PEDIATRICS
Mother-to-infant COVID-19 transmission is unlikely
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Mothers with a history of COVID-19 exposure during pregnancy are not likely to transmit the infection to their newborns, based on data from more than 2,000 women.
“Uncertainty at the onset of the COVID-19 pandemic led to varying postnatal care recommendations for newborns exposed to SARS-CoV-2 in utero,” said Margaret H. Kyle, of Columbia University, New York, and colleagues.
The Columbia University Irving Medical Center, an early epicenter of the pandemic, allowed rooming-in and encouraged direct breastfeeding between infected mothers and their newborns while adopting extensive safety measures, the researchers said.
In a study presented at the virtual meeting of the Pediatric Academic Societies (Poster 141), the researchers conducted a retrospective chart review of all newborns born at the medical center from March 22, 2020, through August 7, 2020. The study was part of Columbia University’s ongoing COVID-19 Mother Baby Outcomes (COMBO) initiative to “describe the health and well-being of mother-infant dyads with and without prenatal SARS-CoV-2 infections,” according to the researchers.
During the study period, the researchers identified newborns of 327 women who tested positive for COVID-19 at any point during pregnancy and compared them to newborns of 2,125 unexposed women. Demographics were similar between the groups.
Overall, the total test positivity was 0.7% for exposed newborns; 1.0% tested positive on an initial test, and 0% were positive on retest. During the newborn hospital stay and a 2-week follow-up, 0% of all newborns showed clinical evidence of infection.
No significant differences were noted between exposed and unexposed newborns in clinical outcomes including gestational age, mode of delivery, 5-minute Apgar score, heart rate, respiratory rate, or temperature. Although more infants of COVID-19–exposed mothers compared with unexposed mothers had an emergency department visit within the first 14 days of life (6% vs. 3%, P = .002), none of the infants was diagnosed with COVID-19 during these visits. Cough, fever, congestion, or bilirubin were more frequent reasons for emergency department visits in the exposed infants compared with unexposed infants, but these differences were not significant.
The study findings were limited by several factors, including the retrospective design and the limited follow-up period to only the first 2 weeks of life, the researchers noted. In addition, perinatal transmission rates were available only for the 202 newborns who were followed up in the hospital system, they said. However, the results suggest that the risk of mother-to-newborn vertical transmission of COVID-19 remains low, even when mothers are breastfeeding and infants are rooming in, they concluded.
Study supports safety of rooming in
The study is important because of the value of mother and infant bonding, Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview. “We know maternal and infant bonding and breastfeeding are extremely important in the first few days of life,” she said. “Initially, COVID-positive moms were separated from their babies during this important time.” Dr. Kinsella said she was not surprised by the study findings, as they reflect other research that newborns have not been getting infected with COVID-19 from their mothers.
Consequently, the take-home message is that newborns can room in with their mothers in the hospital setting, and they are at low risk for COVID-19 regardless of the mother’s exposure history, said Dr. Kinsella. Looking ahead, future areas of research could include examining SARS-CoV-2 antibodies in newborns, she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
FROM PAS 2021
New guidance for those fully vaccinated against COVID-19
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
A woman with scaling, and painful, crusted, erythematous papules and pustules on her face
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Biopsy for this patient revealed folliculitis with Demodex mites visualized on histology. Direct immunofluorescence was negative. A KOH preparation was performed and was positive for large numbers of Demodex. Bacterial cultures were negative. The patient was started on a course of submicrobial doxycycline and ivermectin and showed marked improvement 1 month following treatment.
Demodex folliculorum and Demodex brevis (collectively referred to as Demodex) are microscopic parasitic mites that commonly live on human skin.1 Typically, the mite remains asymptomatic. However, in higher numbers, the infestation may cause dermatoses, called demodicosis. Lesions often present as itchy papules, pustules, and erythematous scaling on the face, ears, and scalp. Blepharitis may be present. Demodex folliculitis is more common in immunocompromised patients.2
Demodex may have a causative role in rosacea and present similarly, with a key difference being that Demodex-type rosacea is more scaly/dry and pustular than common rosacea.1 In Demodex folliculitis, bacterial cultures are often negative. A skin scraping for KOH will reveal increased mite colonization. The Demodex mite may also be seen in histologic slides.
Treatment of Demodex folliculitis includes crotamiton cream, permethrin cream, oral tetracyclines, topical or systemic metronidazole, and topical or oral ivermectin.
This case and photos were submitted by Susannah McClain, MD, Three Rivers Dermatology, Pittsburgh.
References
1. Rather PA and Hassan I. Indian J Dermatol. 2014 Jan;59(1):60-6.
2. Bachmeyer C and Moreno-Sabater A. CMAJ. 2017 Jun 26;189(25):E865.
Procalcitonin-guided antibiotic stewardship for lower respiratory tract infection
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 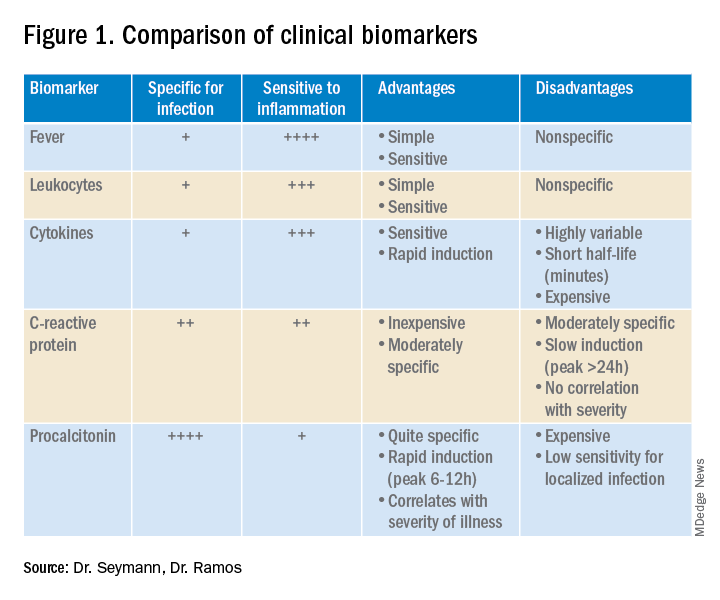
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)
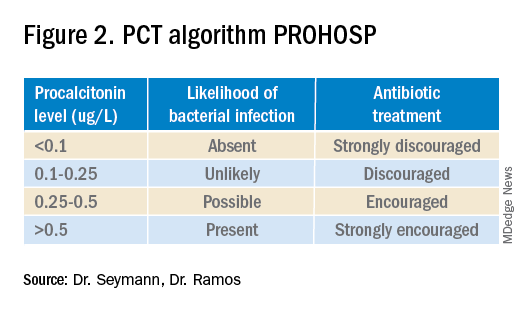
For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.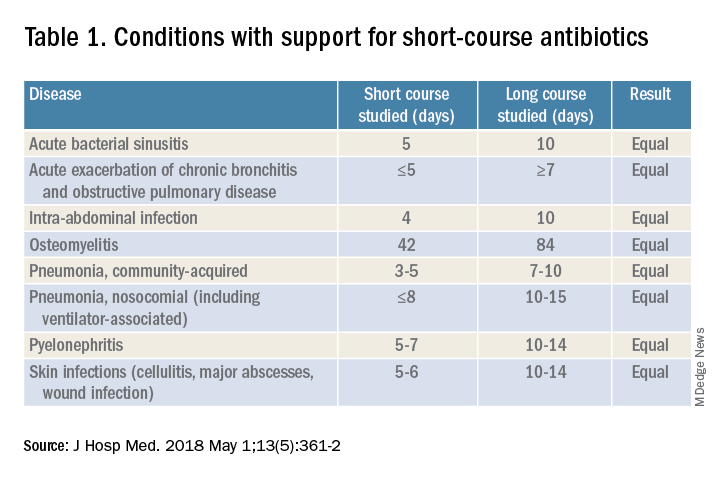
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.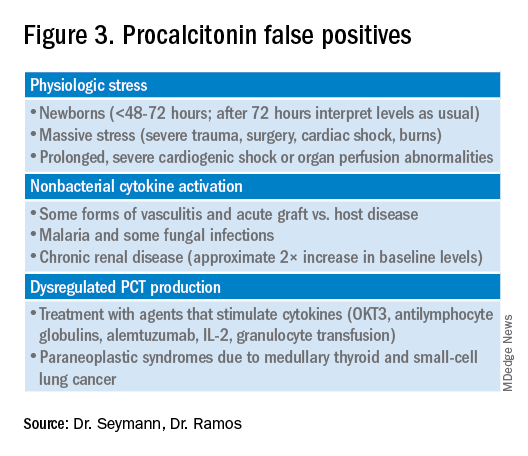
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Dynamics of the assay must be considered
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
COVID-19 in children: Weekly cases drop to 6-month low
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Infants with UTI do not have an increased risk of bacterial meningitis
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
The decision to perform a spinal tap procedure in infants to determine whether they have bacterial meningitis should not be guided by abnormal urinalysis results alone, according to new research published in JAMA Network Open.
The findings suggest febrile infants with positive urinalysis results do not have a higher risk of bacterial meningitis than those with negative urinalysis results.
Nearly 1 in 100,000 people are diagnosed with bacterial meningitis in the United States each year, according to Boston Children’s Hospital. Infants have an increased risk for bacterial meningitis, compared with those in other age groups, according to the Centers for Disease Control and Prevention. However, rates of the infectious disease have been declining in the United States since the late 1990s.
Researchers of the current study said published guidelines and quality initiatives recommend performing a lumbar puncture on febrile infants with positive urinalysis results to exclude bacterial meningitis as a cause.
“It really raises the question of should we be doing everything we’re doing?” study author Brett Burstein, MD, PhD, MPH, said in an interview. “What we conclude here is that, contrary to all the published guidelines, this invasive strategy for testing in well-appearing infants should not be guided by the urinalysis results. That’s a major departure.”
The study adds to growing research that questions whether a lumbar puncture in infants with fever and a positive urinalysis results should be routinely required.
“[Our findings] certainly goes against 30 years of clinical decisions, rules, and guidelines,” Dr. Burstein said. “We think they’re very important and they stand to change practice because approximately 500 infants will undergo these invasive procedures to not miss that needle in the haystack.”
Dr. Burstein, a clinician-scientist in pediatric emergency medicine at Montreal Children’s Hospital, led a team of researchers to perform a meta-analysis of 48 studies, including data from more than 25,000 infants.
Researchers found that the prevalence of bacterial meningitis in well-appearing febrile infants aged 29-60 days with a positive urinalysis results was 0.44%, compared with 0.50% of infants with negative urinalysis results.
Instead of relying on urinalysis results alone, Dr. Burstein suggests doctors use other stratifying biomarkers to decide whether they should perform a lumbar puncture.
“If you’ve done blood testing, for example, and your blood test results suggest serious infection, that should guide the decision to go on to invasive testing,” Dr. Burstein said. “You can use your urinary tract infection information in combination now with blood results.”
This means that, if infants have reassuring blood results, despite having a urinary tract infection, they do not need invasive testing, according to Dr. Burstein.
Some of the risks involved with invasive lumbar puncture testing include infection, bleeding, respiratory problems, as well as pain for the infant and parental anxiety.
Paul Aronson, MD, MHS, of Yale University, New Haven, Conn., who was not involved in the study, said in an interview that he has personally moved away from routine lumbar puncture in infants with a positive urinalysis, but added that many doctors have not.
Dr. Aronson said that, although there have been previous studies on this topic, what sets Dr. Burstein’s study apart is the fact that it has a “tightly defined” group of patients, which are infants aged between 29 and 60 days. He also said it is helpful that the study compared the prevalence of meningitis between infants who had positive urinalysis results with those who had negative results.
“The study compared positive urinalyses to negative analyses, which in the meta-analysis form had not been done previously,” Dr. Aronson said. “And so I think this [current study] probably provides some of the strongest evidence.”
No relevant financial relationships were reported.
FROM JAMA NETWORK OPEN