User login
Fungal cultures in bronchiectasis don’t predict outcomes
The presence of a positive fungal culture in patients with bronchiectasis does not appear to correlate with disease severity or any increased risk of an adverse outcome, according to data pulled from the Bronchiectasis and NTM Registry and presented at the 6th World Bronchiectasis & NTM Conference.
“The question we were asking is whether there is some signal that suggests we need to take care of these patients differently, and the answer is no,” reported Pamela J. McShane, MD, a pulmonologist on the faculty at the University of Texas Health Science Center at Tyler.
or more complex course than did those without a positive fungal culture.
When fungal infections are detected in an initial microbiologic evaluation of patients with bronchiectasis or other lung diseases, first-line clinicians generally assume that coverage is needed. Dr. McShane noted that many of the patients referred to her with bronchiectasis and a positive fungal culture were already on an antifungal.
These data are not supportive of treatment in the absence of fungal-related complications. Dr. McShane suggested they even raise questions about the value of culturing beyond bacterial pathogens in the absence of suspicion that fungal organisms are playing a role in symptoms. She cautioned, however, that more studies specifically studying this possibility are needed.
Study details
The data were drawn in December 2022 from the U.S.-based Bronchiectasis and NTM Registry, which at that time had 22 participating sites. Of the more than 5,000 patients enrolled, the study looked at 2,230 after several exclusions, such as a diagnosis of allergic bronchopulmonary aspergillosis (ABPA).
Of these 2,230 patients, 949 had a fungal infection at the time of diagnosis and 1,281 did not. Those with a fungal infection were further subdivided into those with an aspergillosis (331 patients) and those with a nonaspergillosis fungal infection (751 patients). The total of these two numbers is greater than the total number of fungal infections because these were not mutually exclusive.
At enrollment into the registry, there were no statistical differences between groups for age. Some statistical differences were observed among groups stratified by race, but Dr. McShane doubted that these were clinically significant with the exception of a potential disparity among Asians that might deserve further analysis.
Infection results
Of clinical features evaluated for their association with fungal infection, there was no correlation with either body mass index or history of asthma. Eosinophilia was associated significantly with positive fungal cultures.
Baseline FEV1 was slightly lower among those with a positive fungal culture even if the difference was highly significant (P = .0006). Again, Dr. McShane questioned the clinical significance of values that varied by only a few percentage points, even though she was willing to acknowledge that higher is always preferable to a lower FEV1.
In the context of other pathogens, “generally speaking, those with a positive bacterial culture were more likely to have a fungal infection,” Dr. McShane reported, although there was some variation when looking at pathogenicity of the bacteria and other variables.
“Whether this [higher rate of fungal infection] just involves the environment or our antibiotics are driving the opportunity to permit the fungi to exist, we do not have the answer,” she added.
Nontuberculosis mycobacteria (NTM) infection was similarly represented in those with or without a fungal infection, according to Dr. McShane. Noting the high use of antibiotics in an NTM population, Dr. McShane conceded that this challenges the theory that antibiotic use is driving the risk of fungal infection, but these are what the data say.
Steroid use was associated with a statistically significant risk of fungal infection, but Dr. McShane said it is unclear whether steroid use drives the risk or is an epiphenomenon.
“We looked at this a lot of different ways: oral vs. inhaled and oral vs. inhaled and oral, and it did not make much difference. Generally speaking, the fungal cultures were more likely to be positive in patients on any kind of steroid,” she said.
Finally, with the exception of the slightly lower FEV1 in patients with fungal infections, Dr. McShane said that there was no discernible relationship between the presence of a fungal infection and severity of bronchiectasis.
Because of this evidence, Dr. McShane concluded that the presence of fungus in the culture of patients with bronchiectasis does not appear to correlate with outcome or severity. Since completing the study, she said she is now using these data to reassure patients who have a positive fungal culture.
While these data do not affect the need to diagnosis fungal infections in patients who are not responding typically to therapy or otherwise have an abnormal course of bronchiectasis, raising suspicion that fungal infection is participating in the disease course, the data provide a basis for questioning whether routine cultures are needed, according to the discussion that followed Dr. McShane’s presentation.
Expert opinion
Several of the experts at the presentation provided an opinion. Some reported that they would continue to order fungal cultures on a routine basis, while others said that they now, on the basis of these data, plan to order cultures only at the first visit or when fungal infection is suspected of exacerbating the disease.
Of this latter group, which seemed to be dominant, Juzar Ali, MD, professor of medicine, Louisiana State University, New Orleans, said that he has not been ordering fungal cultures on every visit. Rather, he has been doing so selectively. Examples include those who are on steroids or those with an unusual pattern of exacerbations.
“The value of these data is that they have now provided some data to support this approach,” Dr. Ali said in an interview. Noting that this is the first large study to address this question in a systematic way, he considers this to be a valuable contribution for approaching a common clinical issue.
Dr. McShane reports no relevant financial relationships. Dr. Ali reports a financial relationship with Insmed.
A version of this article first appeared on Medscape.com.
The presence of a positive fungal culture in patients with bronchiectasis does not appear to correlate with disease severity or any increased risk of an adverse outcome, according to data pulled from the Bronchiectasis and NTM Registry and presented at the 6th World Bronchiectasis & NTM Conference.
“The question we were asking is whether there is some signal that suggests we need to take care of these patients differently, and the answer is no,” reported Pamela J. McShane, MD, a pulmonologist on the faculty at the University of Texas Health Science Center at Tyler.
or more complex course than did those without a positive fungal culture.
When fungal infections are detected in an initial microbiologic evaluation of patients with bronchiectasis or other lung diseases, first-line clinicians generally assume that coverage is needed. Dr. McShane noted that many of the patients referred to her with bronchiectasis and a positive fungal culture were already on an antifungal.
These data are not supportive of treatment in the absence of fungal-related complications. Dr. McShane suggested they even raise questions about the value of culturing beyond bacterial pathogens in the absence of suspicion that fungal organisms are playing a role in symptoms. She cautioned, however, that more studies specifically studying this possibility are needed.
Study details
The data were drawn in December 2022 from the U.S.-based Bronchiectasis and NTM Registry, which at that time had 22 participating sites. Of the more than 5,000 patients enrolled, the study looked at 2,230 after several exclusions, such as a diagnosis of allergic bronchopulmonary aspergillosis (ABPA).
Of these 2,230 patients, 949 had a fungal infection at the time of diagnosis and 1,281 did not. Those with a fungal infection were further subdivided into those with an aspergillosis (331 patients) and those with a nonaspergillosis fungal infection (751 patients). The total of these two numbers is greater than the total number of fungal infections because these were not mutually exclusive.
At enrollment into the registry, there were no statistical differences between groups for age. Some statistical differences were observed among groups stratified by race, but Dr. McShane doubted that these were clinically significant with the exception of a potential disparity among Asians that might deserve further analysis.
Infection results
Of clinical features evaluated for their association with fungal infection, there was no correlation with either body mass index or history of asthma. Eosinophilia was associated significantly with positive fungal cultures.
Baseline FEV1 was slightly lower among those with a positive fungal culture even if the difference was highly significant (P = .0006). Again, Dr. McShane questioned the clinical significance of values that varied by only a few percentage points, even though she was willing to acknowledge that higher is always preferable to a lower FEV1.
In the context of other pathogens, “generally speaking, those with a positive bacterial culture were more likely to have a fungal infection,” Dr. McShane reported, although there was some variation when looking at pathogenicity of the bacteria and other variables.
“Whether this [higher rate of fungal infection] just involves the environment or our antibiotics are driving the opportunity to permit the fungi to exist, we do not have the answer,” she added.
Nontuberculosis mycobacteria (NTM) infection was similarly represented in those with or without a fungal infection, according to Dr. McShane. Noting the high use of antibiotics in an NTM population, Dr. McShane conceded that this challenges the theory that antibiotic use is driving the risk of fungal infection, but these are what the data say.
Steroid use was associated with a statistically significant risk of fungal infection, but Dr. McShane said it is unclear whether steroid use drives the risk or is an epiphenomenon.
“We looked at this a lot of different ways: oral vs. inhaled and oral vs. inhaled and oral, and it did not make much difference. Generally speaking, the fungal cultures were more likely to be positive in patients on any kind of steroid,” she said.
Finally, with the exception of the slightly lower FEV1 in patients with fungal infections, Dr. McShane said that there was no discernible relationship between the presence of a fungal infection and severity of bronchiectasis.
Because of this evidence, Dr. McShane concluded that the presence of fungus in the culture of patients with bronchiectasis does not appear to correlate with outcome or severity. Since completing the study, she said she is now using these data to reassure patients who have a positive fungal culture.
While these data do not affect the need to diagnosis fungal infections in patients who are not responding typically to therapy or otherwise have an abnormal course of bronchiectasis, raising suspicion that fungal infection is participating in the disease course, the data provide a basis for questioning whether routine cultures are needed, according to the discussion that followed Dr. McShane’s presentation.
Expert opinion
Several of the experts at the presentation provided an opinion. Some reported that they would continue to order fungal cultures on a routine basis, while others said that they now, on the basis of these data, plan to order cultures only at the first visit or when fungal infection is suspected of exacerbating the disease.
Of this latter group, which seemed to be dominant, Juzar Ali, MD, professor of medicine, Louisiana State University, New Orleans, said that he has not been ordering fungal cultures on every visit. Rather, he has been doing so selectively. Examples include those who are on steroids or those with an unusual pattern of exacerbations.
“The value of these data is that they have now provided some data to support this approach,” Dr. Ali said in an interview. Noting that this is the first large study to address this question in a systematic way, he considers this to be a valuable contribution for approaching a common clinical issue.
Dr. McShane reports no relevant financial relationships. Dr. Ali reports a financial relationship with Insmed.
A version of this article first appeared on Medscape.com.
The presence of a positive fungal culture in patients with bronchiectasis does not appear to correlate with disease severity or any increased risk of an adverse outcome, according to data pulled from the Bronchiectasis and NTM Registry and presented at the 6th World Bronchiectasis & NTM Conference.
“The question we were asking is whether there is some signal that suggests we need to take care of these patients differently, and the answer is no,” reported Pamela J. McShane, MD, a pulmonologist on the faculty at the University of Texas Health Science Center at Tyler.
or more complex course than did those without a positive fungal culture.
When fungal infections are detected in an initial microbiologic evaluation of patients with bronchiectasis or other lung diseases, first-line clinicians generally assume that coverage is needed. Dr. McShane noted that many of the patients referred to her with bronchiectasis and a positive fungal culture were already on an antifungal.
These data are not supportive of treatment in the absence of fungal-related complications. Dr. McShane suggested they even raise questions about the value of culturing beyond bacterial pathogens in the absence of suspicion that fungal organisms are playing a role in symptoms. She cautioned, however, that more studies specifically studying this possibility are needed.
Study details
The data were drawn in December 2022 from the U.S.-based Bronchiectasis and NTM Registry, which at that time had 22 participating sites. Of the more than 5,000 patients enrolled, the study looked at 2,230 after several exclusions, such as a diagnosis of allergic bronchopulmonary aspergillosis (ABPA).
Of these 2,230 patients, 949 had a fungal infection at the time of diagnosis and 1,281 did not. Those with a fungal infection were further subdivided into those with an aspergillosis (331 patients) and those with a nonaspergillosis fungal infection (751 patients). The total of these two numbers is greater than the total number of fungal infections because these were not mutually exclusive.
At enrollment into the registry, there were no statistical differences between groups for age. Some statistical differences were observed among groups stratified by race, but Dr. McShane doubted that these were clinically significant with the exception of a potential disparity among Asians that might deserve further analysis.
Infection results
Of clinical features evaluated for their association with fungal infection, there was no correlation with either body mass index or history of asthma. Eosinophilia was associated significantly with positive fungal cultures.
Baseline FEV1 was slightly lower among those with a positive fungal culture even if the difference was highly significant (P = .0006). Again, Dr. McShane questioned the clinical significance of values that varied by only a few percentage points, even though she was willing to acknowledge that higher is always preferable to a lower FEV1.
In the context of other pathogens, “generally speaking, those with a positive bacterial culture were more likely to have a fungal infection,” Dr. McShane reported, although there was some variation when looking at pathogenicity of the bacteria and other variables.
“Whether this [higher rate of fungal infection] just involves the environment or our antibiotics are driving the opportunity to permit the fungi to exist, we do not have the answer,” she added.
Nontuberculosis mycobacteria (NTM) infection was similarly represented in those with or without a fungal infection, according to Dr. McShane. Noting the high use of antibiotics in an NTM population, Dr. McShane conceded that this challenges the theory that antibiotic use is driving the risk of fungal infection, but these are what the data say.
Steroid use was associated with a statistically significant risk of fungal infection, but Dr. McShane said it is unclear whether steroid use drives the risk or is an epiphenomenon.
“We looked at this a lot of different ways: oral vs. inhaled and oral vs. inhaled and oral, and it did not make much difference. Generally speaking, the fungal cultures were more likely to be positive in patients on any kind of steroid,” she said.
Finally, with the exception of the slightly lower FEV1 in patients with fungal infections, Dr. McShane said that there was no discernible relationship between the presence of a fungal infection and severity of bronchiectasis.
Because of this evidence, Dr. McShane concluded that the presence of fungus in the culture of patients with bronchiectasis does not appear to correlate with outcome or severity. Since completing the study, she said she is now using these data to reassure patients who have a positive fungal culture.
While these data do not affect the need to diagnosis fungal infections in patients who are not responding typically to therapy or otherwise have an abnormal course of bronchiectasis, raising suspicion that fungal infection is participating in the disease course, the data provide a basis for questioning whether routine cultures are needed, according to the discussion that followed Dr. McShane’s presentation.
Expert opinion
Several of the experts at the presentation provided an opinion. Some reported that they would continue to order fungal cultures on a routine basis, while others said that they now, on the basis of these data, plan to order cultures only at the first visit or when fungal infection is suspected of exacerbating the disease.
Of this latter group, which seemed to be dominant, Juzar Ali, MD, professor of medicine, Louisiana State University, New Orleans, said that he has not been ordering fungal cultures on every visit. Rather, he has been doing so selectively. Examples include those who are on steroids or those with an unusual pattern of exacerbations.
“The value of these data is that they have now provided some data to support this approach,” Dr. Ali said in an interview. Noting that this is the first large study to address this question in a systematic way, he considers this to be a valuable contribution for approaching a common clinical issue.
Dr. McShane reports no relevant financial relationships. Dr. Ali reports a financial relationship with Insmed.
A version of this article first appeared on Medscape.com.
Asthma severity, exacerbations increase with RV infection
TOPLINE:
Immunological and quantitative mRNA assays support a pathogenesis role for histamine-releasing factor (HRF), its interaction with HRF-reactive immunoglobulin E and rhinovirus (RV) in asthma severity and exacerbation.
METHODOLOGY:
- Clinical data for healthy controls (HCs) were compared with data from patients with asthma for three distinct cohorts recruited from programs located in Pittsburg, Boston, and Virginia.
- Cohorts differed primarily by total number of participants, median age, description of asthma severity, RV status, and longitudinal follow-up.
- Enzyme-linked immunoassay tests quantified for comparisons total IgE, IgGs, and IgG1 levels occurring in human sera samples and for HRF-reactive IgE, IgG1, and IgG2b in sera from mice inoculated with mouse .
- Anti-IgE stimulation experiments characterized bronchoalveolar lavage (BAL) cell supernatants for tryptase and PGD2 by ELISA and the mRNAs for tryptase and FCER1A
- Effect of inoculated RV infections and/or house dust mite allergen on stimulating HRF secretion from respiratory epithelial cells and in vitro–grown lung BEAS-2B cells was evaluated by Western blots.
TAKEAWAY:
- HRF-reactive IgGs and IgG1 levels in serum were lower in people with asthma than in HCs.
- People with asthma with high HRF-reactive IgE, compared with those with low levels, tended to release more tryptase prostaglandin D2 with anti-IgE stimulation of BAL cells.
- RV infection induced HFR secretions from both in vivo– and in vitro–grown respiratory epithelial cells and was associated with higher levels of HRF-IgE at the time of asthma exacerbations, compared with after resolution.
IN PRACTICE:
Inhibiting HRF and HRF-reactive IgE interactions “can be a preventative/therapeutic target” for severe and RV-induced exacerbated asthma conditions.
SOURCE:
The study led by Yu Kawakami, MD, of La Jolla Institute for Allergy & Immunology, California, and colleagues was published in the Journal of Allergy and Clinical Immunology
LIMITATIONS:
Small sample sizes, large median age differences between cohorts, and lack of data for other demographic traits and variant asthma phenotypes or endotypes in some cohorts are noted limitations that may affect result extrapolations and conclusions.
DISCLOSURES:
The authors report there are no conflicts of interest directly related to this study.
A version of this article first appeared on Medscape.com.
TOPLINE:
Immunological and quantitative mRNA assays support a pathogenesis role for histamine-releasing factor (HRF), its interaction with HRF-reactive immunoglobulin E and rhinovirus (RV) in asthma severity and exacerbation.
METHODOLOGY:
- Clinical data for healthy controls (HCs) were compared with data from patients with asthma for three distinct cohorts recruited from programs located in Pittsburg, Boston, and Virginia.
- Cohorts differed primarily by total number of participants, median age, description of asthma severity, RV status, and longitudinal follow-up.
- Enzyme-linked immunoassay tests quantified for comparisons total IgE, IgGs, and IgG1 levels occurring in human sera samples and for HRF-reactive IgE, IgG1, and IgG2b in sera from mice inoculated with mouse .
- Anti-IgE stimulation experiments characterized bronchoalveolar lavage (BAL) cell supernatants for tryptase and PGD2 by ELISA and the mRNAs for tryptase and FCER1A
- Effect of inoculated RV infections and/or house dust mite allergen on stimulating HRF secretion from respiratory epithelial cells and in vitro–grown lung BEAS-2B cells was evaluated by Western blots.
TAKEAWAY:
- HRF-reactive IgGs and IgG1 levels in serum were lower in people with asthma than in HCs.
- People with asthma with high HRF-reactive IgE, compared with those with low levels, tended to release more tryptase prostaglandin D2 with anti-IgE stimulation of BAL cells.
- RV infection induced HFR secretions from both in vivo– and in vitro–grown respiratory epithelial cells and was associated with higher levels of HRF-IgE at the time of asthma exacerbations, compared with after resolution.
IN PRACTICE:
Inhibiting HRF and HRF-reactive IgE interactions “can be a preventative/therapeutic target” for severe and RV-induced exacerbated asthma conditions.
SOURCE:
The study led by Yu Kawakami, MD, of La Jolla Institute for Allergy & Immunology, California, and colleagues was published in the Journal of Allergy and Clinical Immunology
LIMITATIONS:
Small sample sizes, large median age differences between cohorts, and lack of data for other demographic traits and variant asthma phenotypes or endotypes in some cohorts are noted limitations that may affect result extrapolations and conclusions.
DISCLOSURES:
The authors report there are no conflicts of interest directly related to this study.
A version of this article first appeared on Medscape.com.
TOPLINE:
Immunological and quantitative mRNA assays support a pathogenesis role for histamine-releasing factor (HRF), its interaction with HRF-reactive immunoglobulin E and rhinovirus (RV) in asthma severity and exacerbation.
METHODOLOGY:
- Clinical data for healthy controls (HCs) were compared with data from patients with asthma for three distinct cohorts recruited from programs located in Pittsburg, Boston, and Virginia.
- Cohorts differed primarily by total number of participants, median age, description of asthma severity, RV status, and longitudinal follow-up.
- Enzyme-linked immunoassay tests quantified for comparisons total IgE, IgGs, and IgG1 levels occurring in human sera samples and for HRF-reactive IgE, IgG1, and IgG2b in sera from mice inoculated with mouse .
- Anti-IgE stimulation experiments characterized bronchoalveolar lavage (BAL) cell supernatants for tryptase and PGD2 by ELISA and the mRNAs for tryptase and FCER1A
- Effect of inoculated RV infections and/or house dust mite allergen on stimulating HRF secretion from respiratory epithelial cells and in vitro–grown lung BEAS-2B cells was evaluated by Western blots.
TAKEAWAY:
- HRF-reactive IgGs and IgG1 levels in serum were lower in people with asthma than in HCs.
- People with asthma with high HRF-reactive IgE, compared with those with low levels, tended to release more tryptase prostaglandin D2 with anti-IgE stimulation of BAL cells.
- RV infection induced HFR secretions from both in vivo– and in vitro–grown respiratory epithelial cells and was associated with higher levels of HRF-IgE at the time of asthma exacerbations, compared with after resolution.
IN PRACTICE:
Inhibiting HRF and HRF-reactive IgE interactions “can be a preventative/therapeutic target” for severe and RV-induced exacerbated asthma conditions.
SOURCE:
The study led by Yu Kawakami, MD, of La Jolla Institute for Allergy & Immunology, California, and colleagues was published in the Journal of Allergy and Clinical Immunology
LIMITATIONS:
Small sample sizes, large median age differences between cohorts, and lack of data for other demographic traits and variant asthma phenotypes or endotypes in some cohorts are noted limitations that may affect result extrapolations and conclusions.
DISCLOSURES:
The authors report there are no conflicts of interest directly related to this study.
A version of this article first appeared on Medscape.com.
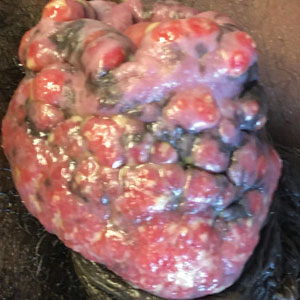
Case report describes pediatric RIME triggered by norovirus
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
Infection-related chronic illness: A new paradigm for research and treatment
Experience with long COVID has shone a spotlight on persistent Lyme disease and other often debilitating chronic illnesses that follow known or suspected infections – and on the urgent need for a common and well-funded research agenda, education of physicians, growth of multidisciplinary clinics, and financially supported clinical care.
“We critically need to understand the epidemiology and pathogenesis of chronic symptoms, and identify more effective ways to manage, treat, and potentially cure these illnesses,” Lyle Petersen, MD, MPH, director of the division of vector-borne diseases at the Centers for Disease Control and Prevention, said at the start of a 2-day National Academies of Science, Engineering, and Medicine (NASEM) workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
Thinking about infection-associated chronic illnesses as an entity – one predicated on commonalities in chronic symptoms and in leading hypotheses for causes – represents a paradigm shift that researchers and patient advocates said can avoid research redundancies and is essential to address what the NASEM calls an overlooked, growing public health problem.
An estimated 2 million people in the United States are living with what’s called posttreatment Lyme disease (PTLD) – a subset of patients with persistent or chronic Lyme disease – and an estimated 1.7-3.3 million people in the United States have diagnoses of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). More than 700,000 people are living with multiple sclerosis. And as of January 2023, 11% of people in the United States reported having long COVID symptoms; the incidence of long COVID is currently estimated at 10%-30% of nonhospitalized cases of COVID-19.
These illnesses “have come under one umbrella,” said Avindra Nath, MD, clinical director of the National Institute of Neurologic Disorders and Stroke (NINDS), Bethesda, Md.
To date, common ground in the literature has grown largely around long COVID and ME/CFS, the latter of which is often associated with a prior, often unidentified infection.
Symptoms of both have been “rigorously” studied and shown to have overlaps, and the illnesses appear to share underlying biologic abnormalities in metabolism and the gut microbiome, as well as viral reactivation and abnormalities in the immune system, central and autonomic nervous systems, and the cardiovascular and pulmonary systems, said Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School and a senior physician at Brigham & Women’s Hospital, both in Boston. (An estimated half of patients with long COVID meet the diagnostic criteria for ME/CFS.)
Although less thoroughly researched, similar symptoms are experienced by a subset of people following a variety of viral, bacterial, and protozoal infections, Dr. Komaroff said. To be determined, he said, is whether the pathophysiology believed to be shared by long COVID and ME/CFS is also shared with other postinfectious syndromes following acute illness with Ebola, West Nile, dengue, mycoplasma pneumonia, enteroviruses, and other pathogens, he said.
Persistent infection, viral reactivation
RNA viral infections can lead to persistent inflammation and dysregulated immunity, with or without viral persistence over time, Timothy J. Henrich, MD, MMSc, associate professor of medicine at the University of California, San Francisco, said in a keynote address.
Research on Ebola survivors has documented long-lasting inflammation and severe immune dysfunction 2 years after infection, for instance. And it’s well known that HIV-1 leads to aberrant immune responses, inflammation, and organ damage despite antiretroviral therapy, said Dr. Henrich, who leads a laboratory/research group that studies approaches to HIV-1 cure and PET-based imaging approaches to characterize viral reservoirs and immune sequelae.
Viral persistence, which can be difficult to measure, has also been documented in Ebola survivors. And in patients living with HIV-1, HIV-1 RNA and protein expression have been shown to persist, again despite antiretroviral therapy. The UCSF Long-Term Immunological Impact of Novel Coronavirus (LIINC) study, for which Dr. Henrich is the principal investigator, found spike RNA in colorectal tissue more than 22 months post COVID, and other research documented viral protein in gut tissue for up to 6 months, he said.
“I think we’re appreciating now, in at least the scientific and treatment community, that there’s a potential for ‘acute’ infections to exhibit some degree of persistence leading to clinical morbidity,” said Dr. Henrich, one of several speakers to describe reports of pathogen persistence. Regarding long COVID, its “etiology is likely heterogeneous,” he said, but persistence of SARS-CoV-2 “may lay behind” other described mechanisms, from clotting/microvascular dysfunction to inflammation and tissue damage to immune dysregulation.
Reactivation of existing latent viral infections in the setting of new acute microbial illness may also play an etiologic role in chronic illnesses, Dr. Henrich said. Epstein-Barr virus (EBV) reactivation has been shown in some studies, including their UCSF COVID-19 cohort, to be associated with long COVID.
“Physicians have been trained to be skeptical about the role [of latent viral infections],” Michael Peluso, MD, an infectious disease physician and assistant professor at UCSF, said during a talk on viral reactivation. This skepticism needs to be “reexamined and overcome,” he said.
Herpesviruses have frequently been associated with ME/CFS, he noted. And evidence of a strong association between EBV and multiple sclerosis came recently from a prospective study of 10 million military recruits that found a 32-fold increased risk of MS after EBV infection but no increase after infection with other viruses, Dr. Peluso and Dr. Henrich both noted.
Research needs, treatment trials
Research needs are vast: The need to learn more about the mechanisms of pathogen persistence and immune evasion, for instance, and the need for more biomarker studies, more imaging studies and tissue analyses, more study of microbiome composition and activity, and continued development and application of metagenomic next-generation sequencing.
Workshop participants also spoke of the need to better understand the molecular mimicry that can occur between pathogen-produced proteins and self-antigens, for instance, and the effects of inflammation and infection-related immune changes on neuronal and microglial function in the brain.
“We should perform similar forms of analysis [across] patients with different infection-associated chronic conditions,” said Amy Proal, PhD, president of the PolyBio Research Foundation, which funds research on infection-associated chronic infections. And within individual conditions and well-characterized study groups “we should perform many different forms of analysis … so we can define endotypes and get more solid biomarkers so that industry [will have more confidence] to run clinical trials.”
In the meantime, patients need fast-moving treatment trials for long COVID, long Lyme, and other infection-associated chronic illnesses, speakers emphasized. “We all agree that treatment trials are overdue,” said the NINDS’ Dr. Nath. “We can’t afford to wait for another decade until we understand all the mechanisms, but rather we can do clinical trials based on what we understand now and study the pathophysiology in the context of the clinical trials.”
Just as was done with HIV, said Steven G. Deeks, MD, professor of medicine at UCSF, researchers must “practice experimental medicine” and select pathways and mechanisms of interest, interrupt those pathways in a controlled manner, and assess impact. “Much of this can be done by repurposing existing drugs,” he said, like antivirals for persistent viral infection, EBV-directed therapies for EBV reactivation, anti-inflammatory drugs for inflammation, B–cell-directed therapies for autoantibodies, and antiplatelet drugs for microvascular disease.
When done correctly, he said, such “probe” studies can deepen mechanistic understandings, lead to biomarkers, and provide proof-of-concept that “will encourage massive investment in developing new therapies” for long COVID and other infection-associated chronic illnesses.
Trials of treatments for long COVID “are starting, so I’m optimistic,” said Dr. Deeks, an expert on HIV pathogenesis and treatment and a principal investigator of the Researching COVID to Enhance Recovery (RECOVER) study. Among the trials: A study of intravenous immunoglobulin (IVIG) for neurologic long COVID; a study of an anti-SARS-CoV-2 monoclonal antibody that can deplete tissue/cellular reservoirs of viral particles (replicating or not); and a study evaluating baricitinib (Olumiant), a Janus kinase inhibitor, for neurocognitive impairment and cardiopulmonary symptoms of long COVID.
Alessio Fasano, MD, professor of pediatrics at Harvard Medical School and professor of nutrition at the Harvard T.H. Chan School of Public Health, Boston, described at the workshop how he began investigating the use of larazotide acetate – an inhibitor of the protein zonulin, which increases intestinal permeability – in children with COVID-19 Multisystem Inflammatory Syndrome (MIS-C) after learning that SARS-CoV-2 viral particles persist in the gastrointestinal tract, causing dysbiosis and zonulin upregulation.
In an ongoing phase 2, double-blind, placebo-controlled trial, the agent thus far has expedited the resolution of gastrointestinal symptoms and clearance of spike protein from the circulation, he said. A phase 2 trial of the agent for pediatric patients with long COVID and SARS-CoV-2 antigenemia is underway. “What if we were to stop [chains of events] by stopping the passage of elements from the virus into circulation?’ he said.
In the realm of Lyme disease, a recently launched Clinical Trials Network for Lyme and Other Tick-Borne Diseases has awarded pilot study grants to evaluate treatments aimed at a variety of possible disease mechanisms that, notably, are similar to those of other chronic illnesses: persistence of infection or remnants of infection, immune dysregulation and autoimmune reactions, neural dysfunction, and gut microbiome changes. (Microclots and mitochondrial dysfunction have not been as well studied in Lyme.)
Current and upcoming studies include evaluations of transcutaneous auricular vagus nerve stimulation for those with persistent Lyme fatigue, transcranial direct current stimulation with cognitive retraining for Lyme brain fog, and tetracycline for PTLD, said Brian Fallon, MD, MPH, professor of clinical psychiatry at Columbia University, New York, who directs the Lyme & Tick-Borne Diseases Research Center and the coordinating center of the new network.
Moving forward, he said, it is important to loosen exclusion criteria and include patients with “probable or possible” Lyme and those with suspected infections with other tick-borne pathogens. All told, these patients comprise a large portion of those with chronic symptoms and have been neglected in an already thin research space, Dr. Fallon said, noting that “there haven’t been any clinical trials of posttreatment Lyme disease in ages – in 10-15 years.”
(PTLD refers to symptoms lasting for more than 6 months after the completion of standard Infectious Diseases Society of America–recommended antibiotic protocols. It occurs in about 15% of patients, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Research Center, Baltimore, a member of the new clinical trials network.)
Calls for a new NIH center and patient involvement
Patients and patient advocacy organizations have played a vital role in research thus far: They’ve documented post-COVID symptoms that academic researchers said they would not otherwise have known of. Leaders of the Patient-Led Research Collaborative have coauthored published reviews with leading long COVID experts. And patients with tick-borne illnesses have enrolled in the MyLymeData patient registry run by LymeDisease.org, which has documented patient-experienced efficacy of alternative treatments and described antibiotic responders and nonresponders.
At the workshop, they shared findings alongside academic experts, and researchers called for their continued involvement. “Patient engagement at every step of the research process is critical,” Dr. Nath said.
“We need to ensure that research is reflective of lived experiences … and [that we’re] accelerating clinical trials of therapeutics that are of priority to the patient community,” said Lisa McCorkell, cofounder of the long COVID-focused Patient-Led Research Collaborative.
Ms. McCorkell also called for the creation of an office for infection-associated chronic illnesses in the NIH director’s office. Others voiced their support. “I think it’s a great idea to have an NIH center for infection-associated chronic illnesses,” said Dr. Fallon. “I think it would have a profound impact.”
The other great need, of course, is funding. “We have ideas, we have drugs that can be repurposed, we have a highly informed and engaged community that will enroll in and be retained in studies, and we have outcomes we can measure,” Dr. Deeks said. “What we’re missing is industry engagement and funding. We need massive engagement from the NIH.”
Real-world treatment needs
In the meantime, patients are seeking treatment, and “clinicians need to have uncertainty tolerance” and try multiple treatments simultaneously, said David Putrino, PT, PhD, director of rehabilitation innovation for the Mount Sinai Health System and professor of rehabilitation and human performance at the Icahn School of Medicine at Mount Sinai, New York. He oversees a multidisciplinary hybrid clinical care research center that has seen over 1,500 patients with long COVID and is beginning to see patients with other infection-associated chronic illnesses.
It’s a model that should be replicated to help fill the “enormous unmet clinical need” of patients with infection-associated chronic illness, said Peter Rowe, MD, professor of pediatrics at the Johns Hopkins School of Medicine and an expert on ME/CFS. And “as we request [more research funding], we will also need [financial] support for clinical care,” he emphasized, to provide equitable access for patients and to attract treating physicians.
Moreover, said Linda Geng, MD, PhD, the culture of stigma needs to change. Right now, patients with long COVID often feel dismissed not only by friends, families, and coworkers, but by clinicians who find it find it hard “to grasp that this is real and a biological condition.”
And it’s not just conditions such as long COVID that are stigmatized, but treatments as well, she said. For instance, some clinicians view low-dose naltrexone, a treatment increasingly being used for inflammation, with suspicion because it is used for opioid use disorder and alcohol use disorder – or because the “low-dose” label summons mistrust of homeopathy. “Even with therapies, there are preconceived notions and biases,” said Dr. Geng, cofounder and codirector of the Stanford (Calif.) Long COVID program.
“What almost killed me,” said Meghan O’Rourke, who has ongoing effects from long-undiagnosed tick-borne illness, “was the invisibility of the illness.” Ms. O’Rourke teaches at Yale University and is the author of “The Invisible Kingdom: Reimagining Chronic Illness.”
Teaching young physicians about these illnesses would help, she and others said. During a question and answer session, Dr. Putrino shared that the Icahn School of Medicine has recently committed to “create a complex chronic illness medical curriculum” that will impact medical education from the first year of medical school through residencies. Dr. Putrino said his team is also working on materials to help other clinics develop care models similar to those at his Mount Sinai clinic.
The NASEM workshop did not collect or require disclosures of its participants.
Experience with long COVID has shone a spotlight on persistent Lyme disease and other often debilitating chronic illnesses that follow known or suspected infections – and on the urgent need for a common and well-funded research agenda, education of physicians, growth of multidisciplinary clinics, and financially supported clinical care.
“We critically need to understand the epidemiology and pathogenesis of chronic symptoms, and identify more effective ways to manage, treat, and potentially cure these illnesses,” Lyle Petersen, MD, MPH, director of the division of vector-borne diseases at the Centers for Disease Control and Prevention, said at the start of a 2-day National Academies of Science, Engineering, and Medicine (NASEM) workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
Thinking about infection-associated chronic illnesses as an entity – one predicated on commonalities in chronic symptoms and in leading hypotheses for causes – represents a paradigm shift that researchers and patient advocates said can avoid research redundancies and is essential to address what the NASEM calls an overlooked, growing public health problem.
An estimated 2 million people in the United States are living with what’s called posttreatment Lyme disease (PTLD) – a subset of patients with persistent or chronic Lyme disease – and an estimated 1.7-3.3 million people in the United States have diagnoses of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). More than 700,000 people are living with multiple sclerosis. And as of January 2023, 11% of people in the United States reported having long COVID symptoms; the incidence of long COVID is currently estimated at 10%-30% of nonhospitalized cases of COVID-19.
These illnesses “have come under one umbrella,” said Avindra Nath, MD, clinical director of the National Institute of Neurologic Disorders and Stroke (NINDS), Bethesda, Md.
To date, common ground in the literature has grown largely around long COVID and ME/CFS, the latter of which is often associated with a prior, often unidentified infection.
Symptoms of both have been “rigorously” studied and shown to have overlaps, and the illnesses appear to share underlying biologic abnormalities in metabolism and the gut microbiome, as well as viral reactivation and abnormalities in the immune system, central and autonomic nervous systems, and the cardiovascular and pulmonary systems, said Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School and a senior physician at Brigham & Women’s Hospital, both in Boston. (An estimated half of patients with long COVID meet the diagnostic criteria for ME/CFS.)
Although less thoroughly researched, similar symptoms are experienced by a subset of people following a variety of viral, bacterial, and protozoal infections, Dr. Komaroff said. To be determined, he said, is whether the pathophysiology believed to be shared by long COVID and ME/CFS is also shared with other postinfectious syndromes following acute illness with Ebola, West Nile, dengue, mycoplasma pneumonia, enteroviruses, and other pathogens, he said.
Persistent infection, viral reactivation
RNA viral infections can lead to persistent inflammation and dysregulated immunity, with or without viral persistence over time, Timothy J. Henrich, MD, MMSc, associate professor of medicine at the University of California, San Francisco, said in a keynote address.
Research on Ebola survivors has documented long-lasting inflammation and severe immune dysfunction 2 years after infection, for instance. And it’s well known that HIV-1 leads to aberrant immune responses, inflammation, and organ damage despite antiretroviral therapy, said Dr. Henrich, who leads a laboratory/research group that studies approaches to HIV-1 cure and PET-based imaging approaches to characterize viral reservoirs and immune sequelae.
Viral persistence, which can be difficult to measure, has also been documented in Ebola survivors. And in patients living with HIV-1, HIV-1 RNA and protein expression have been shown to persist, again despite antiretroviral therapy. The UCSF Long-Term Immunological Impact of Novel Coronavirus (LIINC) study, for which Dr. Henrich is the principal investigator, found spike RNA in colorectal tissue more than 22 months post COVID, and other research documented viral protein in gut tissue for up to 6 months, he said.
“I think we’re appreciating now, in at least the scientific and treatment community, that there’s a potential for ‘acute’ infections to exhibit some degree of persistence leading to clinical morbidity,” said Dr. Henrich, one of several speakers to describe reports of pathogen persistence. Regarding long COVID, its “etiology is likely heterogeneous,” he said, but persistence of SARS-CoV-2 “may lay behind” other described mechanisms, from clotting/microvascular dysfunction to inflammation and tissue damage to immune dysregulation.
Reactivation of existing latent viral infections in the setting of new acute microbial illness may also play an etiologic role in chronic illnesses, Dr. Henrich said. Epstein-Barr virus (EBV) reactivation has been shown in some studies, including their UCSF COVID-19 cohort, to be associated with long COVID.
“Physicians have been trained to be skeptical about the role [of latent viral infections],” Michael Peluso, MD, an infectious disease physician and assistant professor at UCSF, said during a talk on viral reactivation. This skepticism needs to be “reexamined and overcome,” he said.
Herpesviruses have frequently been associated with ME/CFS, he noted. And evidence of a strong association between EBV and multiple sclerosis came recently from a prospective study of 10 million military recruits that found a 32-fold increased risk of MS after EBV infection but no increase after infection with other viruses, Dr. Peluso and Dr. Henrich both noted.
Research needs, treatment trials
Research needs are vast: The need to learn more about the mechanisms of pathogen persistence and immune evasion, for instance, and the need for more biomarker studies, more imaging studies and tissue analyses, more study of microbiome composition and activity, and continued development and application of metagenomic next-generation sequencing.
Workshop participants also spoke of the need to better understand the molecular mimicry that can occur between pathogen-produced proteins and self-antigens, for instance, and the effects of inflammation and infection-related immune changes on neuronal and microglial function in the brain.
“We should perform similar forms of analysis [across] patients with different infection-associated chronic conditions,” said Amy Proal, PhD, president of the PolyBio Research Foundation, which funds research on infection-associated chronic infections. And within individual conditions and well-characterized study groups “we should perform many different forms of analysis … so we can define endotypes and get more solid biomarkers so that industry [will have more confidence] to run clinical trials.”
In the meantime, patients need fast-moving treatment trials for long COVID, long Lyme, and other infection-associated chronic illnesses, speakers emphasized. “We all agree that treatment trials are overdue,” said the NINDS’ Dr. Nath. “We can’t afford to wait for another decade until we understand all the mechanisms, but rather we can do clinical trials based on what we understand now and study the pathophysiology in the context of the clinical trials.”
Just as was done with HIV, said Steven G. Deeks, MD, professor of medicine at UCSF, researchers must “practice experimental medicine” and select pathways and mechanisms of interest, interrupt those pathways in a controlled manner, and assess impact. “Much of this can be done by repurposing existing drugs,” he said, like antivirals for persistent viral infection, EBV-directed therapies for EBV reactivation, anti-inflammatory drugs for inflammation, B–cell-directed therapies for autoantibodies, and antiplatelet drugs for microvascular disease.
When done correctly, he said, such “probe” studies can deepen mechanistic understandings, lead to biomarkers, and provide proof-of-concept that “will encourage massive investment in developing new therapies” for long COVID and other infection-associated chronic illnesses.
Trials of treatments for long COVID “are starting, so I’m optimistic,” said Dr. Deeks, an expert on HIV pathogenesis and treatment and a principal investigator of the Researching COVID to Enhance Recovery (RECOVER) study. Among the trials: A study of intravenous immunoglobulin (IVIG) for neurologic long COVID; a study of an anti-SARS-CoV-2 monoclonal antibody that can deplete tissue/cellular reservoirs of viral particles (replicating or not); and a study evaluating baricitinib (Olumiant), a Janus kinase inhibitor, for neurocognitive impairment and cardiopulmonary symptoms of long COVID.
Alessio Fasano, MD, professor of pediatrics at Harvard Medical School and professor of nutrition at the Harvard T.H. Chan School of Public Health, Boston, described at the workshop how he began investigating the use of larazotide acetate – an inhibitor of the protein zonulin, which increases intestinal permeability – in children with COVID-19 Multisystem Inflammatory Syndrome (MIS-C) after learning that SARS-CoV-2 viral particles persist in the gastrointestinal tract, causing dysbiosis and zonulin upregulation.
In an ongoing phase 2, double-blind, placebo-controlled trial, the agent thus far has expedited the resolution of gastrointestinal symptoms and clearance of spike protein from the circulation, he said. A phase 2 trial of the agent for pediatric patients with long COVID and SARS-CoV-2 antigenemia is underway. “What if we were to stop [chains of events] by stopping the passage of elements from the virus into circulation?’ he said.
In the realm of Lyme disease, a recently launched Clinical Trials Network for Lyme and Other Tick-Borne Diseases has awarded pilot study grants to evaluate treatments aimed at a variety of possible disease mechanisms that, notably, are similar to those of other chronic illnesses: persistence of infection or remnants of infection, immune dysregulation and autoimmune reactions, neural dysfunction, and gut microbiome changes. (Microclots and mitochondrial dysfunction have not been as well studied in Lyme.)
Current and upcoming studies include evaluations of transcutaneous auricular vagus nerve stimulation for those with persistent Lyme fatigue, transcranial direct current stimulation with cognitive retraining for Lyme brain fog, and tetracycline for PTLD, said Brian Fallon, MD, MPH, professor of clinical psychiatry at Columbia University, New York, who directs the Lyme & Tick-Borne Diseases Research Center and the coordinating center of the new network.
Moving forward, he said, it is important to loosen exclusion criteria and include patients with “probable or possible” Lyme and those with suspected infections with other tick-borne pathogens. All told, these patients comprise a large portion of those with chronic symptoms and have been neglected in an already thin research space, Dr. Fallon said, noting that “there haven’t been any clinical trials of posttreatment Lyme disease in ages – in 10-15 years.”
(PTLD refers to symptoms lasting for more than 6 months after the completion of standard Infectious Diseases Society of America–recommended antibiotic protocols. It occurs in about 15% of patients, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Research Center, Baltimore, a member of the new clinical trials network.)
Calls for a new NIH center and patient involvement
Patients and patient advocacy organizations have played a vital role in research thus far: They’ve documented post-COVID symptoms that academic researchers said they would not otherwise have known of. Leaders of the Patient-Led Research Collaborative have coauthored published reviews with leading long COVID experts. And patients with tick-borne illnesses have enrolled in the MyLymeData patient registry run by LymeDisease.org, which has documented patient-experienced efficacy of alternative treatments and described antibiotic responders and nonresponders.
At the workshop, they shared findings alongside academic experts, and researchers called for their continued involvement. “Patient engagement at every step of the research process is critical,” Dr. Nath said.
“We need to ensure that research is reflective of lived experiences … and [that we’re] accelerating clinical trials of therapeutics that are of priority to the patient community,” said Lisa McCorkell, cofounder of the long COVID-focused Patient-Led Research Collaborative.
Ms. McCorkell also called for the creation of an office for infection-associated chronic illnesses in the NIH director’s office. Others voiced their support. “I think it’s a great idea to have an NIH center for infection-associated chronic illnesses,” said Dr. Fallon. “I think it would have a profound impact.”
The other great need, of course, is funding. “We have ideas, we have drugs that can be repurposed, we have a highly informed and engaged community that will enroll in and be retained in studies, and we have outcomes we can measure,” Dr. Deeks said. “What we’re missing is industry engagement and funding. We need massive engagement from the NIH.”
Real-world treatment needs
In the meantime, patients are seeking treatment, and “clinicians need to have uncertainty tolerance” and try multiple treatments simultaneously, said David Putrino, PT, PhD, director of rehabilitation innovation for the Mount Sinai Health System and professor of rehabilitation and human performance at the Icahn School of Medicine at Mount Sinai, New York. He oversees a multidisciplinary hybrid clinical care research center that has seen over 1,500 patients with long COVID and is beginning to see patients with other infection-associated chronic illnesses.
It’s a model that should be replicated to help fill the “enormous unmet clinical need” of patients with infection-associated chronic illness, said Peter Rowe, MD, professor of pediatrics at the Johns Hopkins School of Medicine and an expert on ME/CFS. And “as we request [more research funding], we will also need [financial] support for clinical care,” he emphasized, to provide equitable access for patients and to attract treating physicians.
Moreover, said Linda Geng, MD, PhD, the culture of stigma needs to change. Right now, patients with long COVID often feel dismissed not only by friends, families, and coworkers, but by clinicians who find it find it hard “to grasp that this is real and a biological condition.”
And it’s not just conditions such as long COVID that are stigmatized, but treatments as well, she said. For instance, some clinicians view low-dose naltrexone, a treatment increasingly being used for inflammation, with suspicion because it is used for opioid use disorder and alcohol use disorder – or because the “low-dose” label summons mistrust of homeopathy. “Even with therapies, there are preconceived notions and biases,” said Dr. Geng, cofounder and codirector of the Stanford (Calif.) Long COVID program.
“What almost killed me,” said Meghan O’Rourke, who has ongoing effects from long-undiagnosed tick-borne illness, “was the invisibility of the illness.” Ms. O’Rourke teaches at Yale University and is the author of “The Invisible Kingdom: Reimagining Chronic Illness.”
Teaching young physicians about these illnesses would help, she and others said. During a question and answer session, Dr. Putrino shared that the Icahn School of Medicine has recently committed to “create a complex chronic illness medical curriculum” that will impact medical education from the first year of medical school through residencies. Dr. Putrino said his team is also working on materials to help other clinics develop care models similar to those at his Mount Sinai clinic.
The NASEM workshop did not collect or require disclosures of its participants.
Experience with long COVID has shone a spotlight on persistent Lyme disease and other often debilitating chronic illnesses that follow known or suspected infections – and on the urgent need for a common and well-funded research agenda, education of physicians, growth of multidisciplinary clinics, and financially supported clinical care.
“We critically need to understand the epidemiology and pathogenesis of chronic symptoms, and identify more effective ways to manage, treat, and potentially cure these illnesses,” Lyle Petersen, MD, MPH, director of the division of vector-borne diseases at the Centers for Disease Control and Prevention, said at the start of a 2-day National Academies of Science, Engineering, and Medicine (NASEM) workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
Thinking about infection-associated chronic illnesses as an entity – one predicated on commonalities in chronic symptoms and in leading hypotheses for causes – represents a paradigm shift that researchers and patient advocates said can avoid research redundancies and is essential to address what the NASEM calls an overlooked, growing public health problem.
An estimated 2 million people in the United States are living with what’s called posttreatment Lyme disease (PTLD) – a subset of patients with persistent or chronic Lyme disease – and an estimated 1.7-3.3 million people in the United States have diagnoses of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). More than 700,000 people are living with multiple sclerosis. And as of January 2023, 11% of people in the United States reported having long COVID symptoms; the incidence of long COVID is currently estimated at 10%-30% of nonhospitalized cases of COVID-19.
These illnesses “have come under one umbrella,” said Avindra Nath, MD, clinical director of the National Institute of Neurologic Disorders and Stroke (NINDS), Bethesda, Md.
To date, common ground in the literature has grown largely around long COVID and ME/CFS, the latter of which is often associated with a prior, often unidentified infection.
Symptoms of both have been “rigorously” studied and shown to have overlaps, and the illnesses appear to share underlying biologic abnormalities in metabolism and the gut microbiome, as well as viral reactivation and abnormalities in the immune system, central and autonomic nervous systems, and the cardiovascular and pulmonary systems, said Anthony L. Komaroff, MD, professor of medicine at Harvard Medical School and a senior physician at Brigham & Women’s Hospital, both in Boston. (An estimated half of patients with long COVID meet the diagnostic criteria for ME/CFS.)
Although less thoroughly researched, similar symptoms are experienced by a subset of people following a variety of viral, bacterial, and protozoal infections, Dr. Komaroff said. To be determined, he said, is whether the pathophysiology believed to be shared by long COVID and ME/CFS is also shared with other postinfectious syndromes following acute illness with Ebola, West Nile, dengue, mycoplasma pneumonia, enteroviruses, and other pathogens, he said.
Persistent infection, viral reactivation
RNA viral infections can lead to persistent inflammation and dysregulated immunity, with or without viral persistence over time, Timothy J. Henrich, MD, MMSc, associate professor of medicine at the University of California, San Francisco, said in a keynote address.
Research on Ebola survivors has documented long-lasting inflammation and severe immune dysfunction 2 years after infection, for instance. And it’s well known that HIV-1 leads to aberrant immune responses, inflammation, and organ damage despite antiretroviral therapy, said Dr. Henrich, who leads a laboratory/research group that studies approaches to HIV-1 cure and PET-based imaging approaches to characterize viral reservoirs and immune sequelae.
Viral persistence, which can be difficult to measure, has also been documented in Ebola survivors. And in patients living with HIV-1, HIV-1 RNA and protein expression have been shown to persist, again despite antiretroviral therapy. The UCSF Long-Term Immunological Impact of Novel Coronavirus (LIINC) study, for which Dr. Henrich is the principal investigator, found spike RNA in colorectal tissue more than 22 months post COVID, and other research documented viral protein in gut tissue for up to 6 months, he said.
“I think we’re appreciating now, in at least the scientific and treatment community, that there’s a potential for ‘acute’ infections to exhibit some degree of persistence leading to clinical morbidity,” said Dr. Henrich, one of several speakers to describe reports of pathogen persistence. Regarding long COVID, its “etiology is likely heterogeneous,” he said, but persistence of SARS-CoV-2 “may lay behind” other described mechanisms, from clotting/microvascular dysfunction to inflammation and tissue damage to immune dysregulation.
Reactivation of existing latent viral infections in the setting of new acute microbial illness may also play an etiologic role in chronic illnesses, Dr. Henrich said. Epstein-Barr virus (EBV) reactivation has been shown in some studies, including their UCSF COVID-19 cohort, to be associated with long COVID.
“Physicians have been trained to be skeptical about the role [of latent viral infections],” Michael Peluso, MD, an infectious disease physician and assistant professor at UCSF, said during a talk on viral reactivation. This skepticism needs to be “reexamined and overcome,” he said.
Herpesviruses have frequently been associated with ME/CFS, he noted. And evidence of a strong association between EBV and multiple sclerosis came recently from a prospective study of 10 million military recruits that found a 32-fold increased risk of MS after EBV infection but no increase after infection with other viruses, Dr. Peluso and Dr. Henrich both noted.
Research needs, treatment trials
Research needs are vast: The need to learn more about the mechanisms of pathogen persistence and immune evasion, for instance, and the need for more biomarker studies, more imaging studies and tissue analyses, more study of microbiome composition and activity, and continued development and application of metagenomic next-generation sequencing.
Workshop participants also spoke of the need to better understand the molecular mimicry that can occur between pathogen-produced proteins and self-antigens, for instance, and the effects of inflammation and infection-related immune changes on neuronal and microglial function in the brain.
“We should perform similar forms of analysis [across] patients with different infection-associated chronic conditions,” said Amy Proal, PhD, president of the PolyBio Research Foundation, which funds research on infection-associated chronic infections. And within individual conditions and well-characterized study groups “we should perform many different forms of analysis … so we can define endotypes and get more solid biomarkers so that industry [will have more confidence] to run clinical trials.”
In the meantime, patients need fast-moving treatment trials for long COVID, long Lyme, and other infection-associated chronic illnesses, speakers emphasized. “We all agree that treatment trials are overdue,” said the NINDS’ Dr. Nath. “We can’t afford to wait for another decade until we understand all the mechanisms, but rather we can do clinical trials based on what we understand now and study the pathophysiology in the context of the clinical trials.”
Just as was done with HIV, said Steven G. Deeks, MD, professor of medicine at UCSF, researchers must “practice experimental medicine” and select pathways and mechanisms of interest, interrupt those pathways in a controlled manner, and assess impact. “Much of this can be done by repurposing existing drugs,” he said, like antivirals for persistent viral infection, EBV-directed therapies for EBV reactivation, anti-inflammatory drugs for inflammation, B–cell-directed therapies for autoantibodies, and antiplatelet drugs for microvascular disease.
When done correctly, he said, such “probe” studies can deepen mechanistic understandings, lead to biomarkers, and provide proof-of-concept that “will encourage massive investment in developing new therapies” for long COVID and other infection-associated chronic illnesses.
Trials of treatments for long COVID “are starting, so I’m optimistic,” said Dr. Deeks, an expert on HIV pathogenesis and treatment and a principal investigator of the Researching COVID to Enhance Recovery (RECOVER) study. Among the trials: A study of intravenous immunoglobulin (IVIG) for neurologic long COVID; a study of an anti-SARS-CoV-2 monoclonal antibody that can deplete tissue/cellular reservoirs of viral particles (replicating or not); and a study evaluating baricitinib (Olumiant), a Janus kinase inhibitor, for neurocognitive impairment and cardiopulmonary symptoms of long COVID.
Alessio Fasano, MD, professor of pediatrics at Harvard Medical School and professor of nutrition at the Harvard T.H. Chan School of Public Health, Boston, described at the workshop how he began investigating the use of larazotide acetate – an inhibitor of the protein zonulin, which increases intestinal permeability – in children with COVID-19 Multisystem Inflammatory Syndrome (MIS-C) after learning that SARS-CoV-2 viral particles persist in the gastrointestinal tract, causing dysbiosis and zonulin upregulation.
In an ongoing phase 2, double-blind, placebo-controlled trial, the agent thus far has expedited the resolution of gastrointestinal symptoms and clearance of spike protein from the circulation, he said. A phase 2 trial of the agent for pediatric patients with long COVID and SARS-CoV-2 antigenemia is underway. “What if we were to stop [chains of events] by stopping the passage of elements from the virus into circulation?’ he said.
In the realm of Lyme disease, a recently launched Clinical Trials Network for Lyme and Other Tick-Borne Diseases has awarded pilot study grants to evaluate treatments aimed at a variety of possible disease mechanisms that, notably, are similar to those of other chronic illnesses: persistence of infection or remnants of infection, immune dysregulation and autoimmune reactions, neural dysfunction, and gut microbiome changes. (Microclots and mitochondrial dysfunction have not been as well studied in Lyme.)
Current and upcoming studies include evaluations of transcutaneous auricular vagus nerve stimulation for those with persistent Lyme fatigue, transcranial direct current stimulation with cognitive retraining for Lyme brain fog, and tetracycline for PTLD, said Brian Fallon, MD, MPH, professor of clinical psychiatry at Columbia University, New York, who directs the Lyme & Tick-Borne Diseases Research Center and the coordinating center of the new network.
Moving forward, he said, it is important to loosen exclusion criteria and include patients with “probable or possible” Lyme and those with suspected infections with other tick-borne pathogens. All told, these patients comprise a large portion of those with chronic symptoms and have been neglected in an already thin research space, Dr. Fallon said, noting that “there haven’t been any clinical trials of posttreatment Lyme disease in ages – in 10-15 years.”
(PTLD refers to symptoms lasting for more than 6 months after the completion of standard Infectious Diseases Society of America–recommended antibiotic protocols. It occurs in about 15% of patients, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Research Center, Baltimore, a member of the new clinical trials network.)
Calls for a new NIH center and patient involvement
Patients and patient advocacy organizations have played a vital role in research thus far: They’ve documented post-COVID symptoms that academic researchers said they would not otherwise have known of. Leaders of the Patient-Led Research Collaborative have coauthored published reviews with leading long COVID experts. And patients with tick-borne illnesses have enrolled in the MyLymeData patient registry run by LymeDisease.org, which has documented patient-experienced efficacy of alternative treatments and described antibiotic responders and nonresponders.
At the workshop, they shared findings alongside academic experts, and researchers called for their continued involvement. “Patient engagement at every step of the research process is critical,” Dr. Nath said.
“We need to ensure that research is reflective of lived experiences … and [that we’re] accelerating clinical trials of therapeutics that are of priority to the patient community,” said Lisa McCorkell, cofounder of the long COVID-focused Patient-Led Research Collaborative.
Ms. McCorkell also called for the creation of an office for infection-associated chronic illnesses in the NIH director’s office. Others voiced their support. “I think it’s a great idea to have an NIH center for infection-associated chronic illnesses,” said Dr. Fallon. “I think it would have a profound impact.”
The other great need, of course, is funding. “We have ideas, we have drugs that can be repurposed, we have a highly informed and engaged community that will enroll in and be retained in studies, and we have outcomes we can measure,” Dr. Deeks said. “What we’re missing is industry engagement and funding. We need massive engagement from the NIH.”
Real-world treatment needs
In the meantime, patients are seeking treatment, and “clinicians need to have uncertainty tolerance” and try multiple treatments simultaneously, said David Putrino, PT, PhD, director of rehabilitation innovation for the Mount Sinai Health System and professor of rehabilitation and human performance at the Icahn School of Medicine at Mount Sinai, New York. He oversees a multidisciplinary hybrid clinical care research center that has seen over 1,500 patients with long COVID and is beginning to see patients with other infection-associated chronic illnesses.
It’s a model that should be replicated to help fill the “enormous unmet clinical need” of patients with infection-associated chronic illness, said Peter Rowe, MD, professor of pediatrics at the Johns Hopkins School of Medicine and an expert on ME/CFS. And “as we request [more research funding], we will also need [financial] support for clinical care,” he emphasized, to provide equitable access for patients and to attract treating physicians.
Moreover, said Linda Geng, MD, PhD, the culture of stigma needs to change. Right now, patients with long COVID often feel dismissed not only by friends, families, and coworkers, but by clinicians who find it find it hard “to grasp that this is real and a biological condition.”
And it’s not just conditions such as long COVID that are stigmatized, but treatments as well, she said. For instance, some clinicians view low-dose naltrexone, a treatment increasingly being used for inflammation, with suspicion because it is used for opioid use disorder and alcohol use disorder – or because the “low-dose” label summons mistrust of homeopathy. “Even with therapies, there are preconceived notions and biases,” said Dr. Geng, cofounder and codirector of the Stanford (Calif.) Long COVID program.
“What almost killed me,” said Meghan O’Rourke, who has ongoing effects from long-undiagnosed tick-borne illness, “was the invisibility of the illness.” Ms. O’Rourke teaches at Yale University and is the author of “The Invisible Kingdom: Reimagining Chronic Illness.”
Teaching young physicians about these illnesses would help, she and others said. During a question and answer session, Dr. Putrino shared that the Icahn School of Medicine has recently committed to “create a complex chronic illness medical curriculum” that will impact medical education from the first year of medical school through residencies. Dr. Putrino said his team is also working on materials to help other clinics develop care models similar to those at his Mount Sinai clinic.
The NASEM workshop did not collect or require disclosures of its participants.
FROM A NATIONAL ACADEMIES OF SCIENCE, ENGINEERING, AND MEDICINE WORKSHOP
Research points toward combination therapy for Lyme and improved diagnostics
Several recent developments in Lyme disease treatment and diagnosis may pave the way forward for combating disease that persists following missed or delayed diagnoses or remains following standard treatment. These include combination therapy to address “persister” bacteria and diagnostic tests that test directly for the pathogen and/or indirectly test for host response, according to experts who presented at a 2-day National Academies of Science, Engineering and Medicine workshop on infection-associated chronic illnesses.
Research has shown that 60% of people who are infected and not treated during the early or early disseminated stages of Lyme disease go on to develop late Lyme arthritis, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center in Baltimore. And in the real world, there’s an additional category of patients: Those who are misdiagnosed and develop infection-related persistent symptoms – such as fatigue, brain fog/cognitive dysfunction, and musculoskeletal problems – that don’t match the “textbook schematic” involving late Lyme arthritis and late neurologic disease.
Moreover, of patients who are treated with protocols recommended by the Infectious Diseases Society of America (IDSA), about 15% go on to develop persistent symptoms at 6 months – again, symptoms that don’t match textbook manifestations and do match symptoms of other infection-associated chronic illnesses. As a “research construct,” this has been coined posttreatment Lyme disease (PTLD), he said at the workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
(On a practical level, it is hard to know clinically who has early disseminated disease unless they have multiple erythema migrans rashes or neurologic or cardiac involvement, he said after the meeting.)
All this points to the need for tests that are sensitive and specific for diagnosis at all stages of infection and disease, he said in a talk on diagnostics. Currently available tests – those that fit into the widely used two-tiered enzyme-linked immunosorbent assay, Western Blot serology testing – have significant limitations in sensitivity and specificity, including for acute infection when the body has not generated enough antibodies, yet treatment is most likely to succeed.
Move toward combination therapy research
Lyme disease is most commonly treated with doxycycline, and that’s problematic because the antibiotic is a microstatic whose efficacy relies on immune clearance of static bacteria, said Monica E. Embers, PhD, director of vector-borne disease at the Tulane National Primate Research Center and associate professor of immunology at Tulane University, New Orleans.
“But we know that Borrelia burgdorferi has the capability to evade the host immune response in almost every way possible. Persistence is the norm in an immunocompetent host ... [and] dormant bacteria/persisters are more tolerant of microstatic antibiotics,” she said.
Other considerations for antibiotic efficacy include the fact that B. burgdorferi survives for many months inside ticks without nutrient replenishment or replication, “so dormancy is part of their life cycle,” she said. Moreover, the bacteria can be found deep in connective tissues and joints.
The efficacy of accepted regimens of antibiotic treatment has been “a very contentious issue,” she said, noting that guidelines from the International Lyme and Associated Diseases Society “leave open the possibility for antibiotic retreatment when a chronic infection is judged to be a possible cause [of ongoing symptoms].”
The development of persister B. burgdorferi in the presence of antibiotics has been well studied in vitro, which has limitations, Dr. Embers said. But her group specializes in animal models and has shown persistence of antimicrobial-tolerant B. burgdorferi in tick-inoculated rhesus macaques 8-9 months after treatment with oral doxycycline.
“We [also] saw persistence of mild-moderate inflammation in the brain, peripheral nerves, spinal cord, joints and skeletal muscle, and in the heart,” Dr. Embers said, who coauthored a 2022 review of B. burgdorferi antimicrobial-tolerant persistence in Lyme disease and PTLD.
Her work has also shown that ceftriaxone, which is recommended by IDSA for patients with clinically evident neurological and/or cardiac involvement, does not clear infection in mice. “In general, single drugs have not been capable of clearing the infection, yet combinations show promise,” she said.
Dr. Embers has combed large drug libraries looking for combinations of antibiotics that employ different mechanisms of action in hopes of eliminating persister spirochetes. Certain combinations have shown promise in mice and have been tested in her rhesus macaque model; data analyses are underway.
Other research teams, such as that of Ying Zhang, MD, PhD, at Johns Hopkins, have similarly been screening combinations of antibiotics and other compounds, identifying candidates for further testing.
During a question and answer period, Dr. Embers said her team is also investigating the pathophysiology and long-term effects of tick-borne coinfections, including Bartonella, and is pursuing a hypothesis that infection with Borrelia allows Bartonella to cause more extensive disease and persist longer. “I think Lyme is at the core because of its ability to evade and suppress the immune response so effectively.”
Diagnostic possibilities, biomarkers for PTLD
Direct diagnostic tests for microbial nucleic acid and proteins “are promising alternatives for indirect serologic tests,” Dr. Aucott said. For instance, in addition to polymerase chain reaction tests, which “are making advances,” it may be possible to target the B. burgdorferi peptidoglycan for antigen detection.
Researchers have shown that peptidoglycan, a component of the B. burgdorferi cell envelope, is a persistent antigen in the synovial fluid of patients with Lyme arthritis who have been treated with oral and intravenous antibiotics, and that it likely contributes to inflammation.
“Maybe the infection is gone but parts of the bacteria are still there that are driving inflammation,” said Dr. Aucott, also associate professor of medicine at John Hopkins.
Researchers have also been looking at the host response to B. burgdorferi – including cytokines, chemokines, and autoantibiodies – to identify biomarkers for PTLD and to identify patients during posttreatment follow-up who are at increased risk of developing PTLD, with the hope of someday intervening. Persistently high levels of interleukin-23, CCL19, and interferon-alpha have each been associated in different studies with persistent symptoms after treatment, Dr. Aucott said.
In addition, metabolomics research is showing that patients with PTLD have metabolic fingerprints that are different from those who return to good health after treatment, and it may be possible to identify an epigenetic signature for Lyme disease. A project sponsored by the Defense Advanced Research Projects Agency called ECHO (Epigenetic Characterization and Observation) aims to identify epigenetic signatures of exposures to various threats, including B. burgdorferi.
“At the very proximal end of [indirectly testing for host response], there are modifications of the DNA that can occur in response to infectious insults ... and that changed DNA changes RNA expression and protein synthesis,” Dr. Aucott explained. DARPA’s project is “exciting because their goal [at DARPA] is to have a diagnostic test quickly as a result of this epigenetics work.”
Imaging research is also fast offering diagnostic opportunities, Dr. Aucott said. Levels of microglial activation on brain PET imaging have been found to correlate with PTLD, and a study at Johns Hopkins of multimodal neuroimaging with functional MRI and diffusion tensor imaging has shown distinct changes to white matter activation within the frontal lobe of patients with PTLD, compared with controls.
The NASEM workshop did not collect or require disclosures of its participants.
Several recent developments in Lyme disease treatment and diagnosis may pave the way forward for combating disease that persists following missed or delayed diagnoses or remains following standard treatment. These include combination therapy to address “persister” bacteria and diagnostic tests that test directly for the pathogen and/or indirectly test for host response, according to experts who presented at a 2-day National Academies of Science, Engineering and Medicine workshop on infection-associated chronic illnesses.
Research has shown that 60% of people who are infected and not treated during the early or early disseminated stages of Lyme disease go on to develop late Lyme arthritis, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center in Baltimore. And in the real world, there’s an additional category of patients: Those who are misdiagnosed and develop infection-related persistent symptoms – such as fatigue, brain fog/cognitive dysfunction, and musculoskeletal problems – that don’t match the “textbook schematic” involving late Lyme arthritis and late neurologic disease.
Moreover, of patients who are treated with protocols recommended by the Infectious Diseases Society of America (IDSA), about 15% go on to develop persistent symptoms at 6 months – again, symptoms that don’t match textbook manifestations and do match symptoms of other infection-associated chronic illnesses. As a “research construct,” this has been coined posttreatment Lyme disease (PTLD), he said at the workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
(On a practical level, it is hard to know clinically who has early disseminated disease unless they have multiple erythema migrans rashes or neurologic or cardiac involvement, he said after the meeting.)
All this points to the need for tests that are sensitive and specific for diagnosis at all stages of infection and disease, he said in a talk on diagnostics. Currently available tests – those that fit into the widely used two-tiered enzyme-linked immunosorbent assay, Western Blot serology testing – have significant limitations in sensitivity and specificity, including for acute infection when the body has not generated enough antibodies, yet treatment is most likely to succeed.
Move toward combination therapy research
Lyme disease is most commonly treated with doxycycline, and that’s problematic because the antibiotic is a microstatic whose efficacy relies on immune clearance of static bacteria, said Monica E. Embers, PhD, director of vector-borne disease at the Tulane National Primate Research Center and associate professor of immunology at Tulane University, New Orleans.
“But we know that Borrelia burgdorferi has the capability to evade the host immune response in almost every way possible. Persistence is the norm in an immunocompetent host ... [and] dormant bacteria/persisters are more tolerant of microstatic antibiotics,” she said.
Other considerations for antibiotic efficacy include the fact that B. burgdorferi survives for many months inside ticks without nutrient replenishment or replication, “so dormancy is part of their life cycle,” she said. Moreover, the bacteria can be found deep in connective tissues and joints.
The efficacy of accepted regimens of antibiotic treatment has been “a very contentious issue,” she said, noting that guidelines from the International Lyme and Associated Diseases Society “leave open the possibility for antibiotic retreatment when a chronic infection is judged to be a possible cause [of ongoing symptoms].”
The development of persister B. burgdorferi in the presence of antibiotics has been well studied in vitro, which has limitations, Dr. Embers said. But her group specializes in animal models and has shown persistence of antimicrobial-tolerant B. burgdorferi in tick-inoculated rhesus macaques 8-9 months after treatment with oral doxycycline.
“We [also] saw persistence of mild-moderate inflammation in the brain, peripheral nerves, spinal cord, joints and skeletal muscle, and in the heart,” Dr. Embers said, who coauthored a 2022 review of B. burgdorferi antimicrobial-tolerant persistence in Lyme disease and PTLD.
Her work has also shown that ceftriaxone, which is recommended by IDSA for patients with clinically evident neurological and/or cardiac involvement, does not clear infection in mice. “In general, single drugs have not been capable of clearing the infection, yet combinations show promise,” she said.
Dr. Embers has combed large drug libraries looking for combinations of antibiotics that employ different mechanisms of action in hopes of eliminating persister spirochetes. Certain combinations have shown promise in mice and have been tested in her rhesus macaque model; data analyses are underway.
Other research teams, such as that of Ying Zhang, MD, PhD, at Johns Hopkins, have similarly been screening combinations of antibiotics and other compounds, identifying candidates for further testing.
During a question and answer period, Dr. Embers said her team is also investigating the pathophysiology and long-term effects of tick-borne coinfections, including Bartonella, and is pursuing a hypothesis that infection with Borrelia allows Bartonella to cause more extensive disease and persist longer. “I think Lyme is at the core because of its ability to evade and suppress the immune response so effectively.”
Diagnostic possibilities, biomarkers for PTLD
Direct diagnostic tests for microbial nucleic acid and proteins “are promising alternatives for indirect serologic tests,” Dr. Aucott said. For instance, in addition to polymerase chain reaction tests, which “are making advances,” it may be possible to target the B. burgdorferi peptidoglycan for antigen detection.
Researchers have shown that peptidoglycan, a component of the B. burgdorferi cell envelope, is a persistent antigen in the synovial fluid of patients with Lyme arthritis who have been treated with oral and intravenous antibiotics, and that it likely contributes to inflammation.
“Maybe the infection is gone but parts of the bacteria are still there that are driving inflammation,” said Dr. Aucott, also associate professor of medicine at John Hopkins.
Researchers have also been looking at the host response to B. burgdorferi – including cytokines, chemokines, and autoantibiodies – to identify biomarkers for PTLD and to identify patients during posttreatment follow-up who are at increased risk of developing PTLD, with the hope of someday intervening. Persistently high levels of interleukin-23, CCL19, and interferon-alpha have each been associated in different studies with persistent symptoms after treatment, Dr. Aucott said.
In addition, metabolomics research is showing that patients with PTLD have metabolic fingerprints that are different from those who return to good health after treatment, and it may be possible to identify an epigenetic signature for Lyme disease. A project sponsored by the Defense Advanced Research Projects Agency called ECHO (Epigenetic Characterization and Observation) aims to identify epigenetic signatures of exposures to various threats, including B. burgdorferi.
“At the very proximal end of [indirectly testing for host response], there are modifications of the DNA that can occur in response to infectious insults ... and that changed DNA changes RNA expression and protein synthesis,” Dr. Aucott explained. DARPA’s project is “exciting because their goal [at DARPA] is to have a diagnostic test quickly as a result of this epigenetics work.”
Imaging research is also fast offering diagnostic opportunities, Dr. Aucott said. Levels of microglial activation on brain PET imaging have been found to correlate with PTLD, and a study at Johns Hopkins of multimodal neuroimaging with functional MRI and diffusion tensor imaging has shown distinct changes to white matter activation within the frontal lobe of patients with PTLD, compared with controls.
The NASEM workshop did not collect or require disclosures of its participants.
Several recent developments in Lyme disease treatment and diagnosis may pave the way forward for combating disease that persists following missed or delayed diagnoses or remains following standard treatment. These include combination therapy to address “persister” bacteria and diagnostic tests that test directly for the pathogen and/or indirectly test for host response, according to experts who presented at a 2-day National Academies of Science, Engineering and Medicine workshop on infection-associated chronic illnesses.
Research has shown that 60% of people who are infected and not treated during the early or early disseminated stages of Lyme disease go on to develop late Lyme arthritis, said John Aucott, MD, director of the Johns Hopkins Lyme Disease Clinical Research Center in Baltimore. And in the real world, there’s an additional category of patients: Those who are misdiagnosed and develop infection-related persistent symptoms – such as fatigue, brain fog/cognitive dysfunction, and musculoskeletal problems – that don’t match the “textbook schematic” involving late Lyme arthritis and late neurologic disease.
Moreover, of patients who are treated with protocols recommended by the Infectious Diseases Society of America (IDSA), about 15% go on to develop persistent symptoms at 6 months – again, symptoms that don’t match textbook manifestations and do match symptoms of other infection-associated chronic illnesses. As a “research construct,” this has been coined posttreatment Lyme disease (PTLD), he said at the workshop, “Toward a Common Research Agenda in Infection-Associated Chronic Illnesses.”
(On a practical level, it is hard to know clinically who has early disseminated disease unless they have multiple erythema migrans rashes or neurologic or cardiac involvement, he said after the meeting.)
All this points to the need for tests that are sensitive and specific for diagnosis at all stages of infection and disease, he said in a talk on diagnostics. Currently available tests – those that fit into the widely used two-tiered enzyme-linked immunosorbent assay, Western Blot serology testing – have significant limitations in sensitivity and specificity, including for acute infection when the body has not generated enough antibodies, yet treatment is most likely to succeed.
Move toward combination therapy research
Lyme disease is most commonly treated with doxycycline, and that’s problematic because the antibiotic is a microstatic whose efficacy relies on immune clearance of static bacteria, said Monica E. Embers, PhD, director of vector-borne disease at the Tulane National Primate Research Center and associate professor of immunology at Tulane University, New Orleans.
“But we know that Borrelia burgdorferi has the capability to evade the host immune response in almost every way possible. Persistence is the norm in an immunocompetent host ... [and] dormant bacteria/persisters are more tolerant of microstatic antibiotics,” she said.
Other considerations for antibiotic efficacy include the fact that B. burgdorferi survives for many months inside ticks without nutrient replenishment or replication, “so dormancy is part of their life cycle,” she said. Moreover, the bacteria can be found deep in connective tissues and joints.
The efficacy of accepted regimens of antibiotic treatment has been “a very contentious issue,” she said, noting that guidelines from the International Lyme and Associated Diseases Society “leave open the possibility for antibiotic retreatment when a chronic infection is judged to be a possible cause [of ongoing symptoms].”
The development of persister B. burgdorferi in the presence of antibiotics has been well studied in vitro, which has limitations, Dr. Embers said. But her group specializes in animal models and has shown persistence of antimicrobial-tolerant B. burgdorferi in tick-inoculated rhesus macaques 8-9 months after treatment with oral doxycycline.
“We [also] saw persistence of mild-moderate inflammation in the brain, peripheral nerves, spinal cord, joints and skeletal muscle, and in the heart,” Dr. Embers said, who coauthored a 2022 review of B. burgdorferi antimicrobial-tolerant persistence in Lyme disease and PTLD.
Her work has also shown that ceftriaxone, which is recommended by IDSA for patients with clinically evident neurological and/or cardiac involvement, does not clear infection in mice. “In general, single drugs have not been capable of clearing the infection, yet combinations show promise,” she said.
Dr. Embers has combed large drug libraries looking for combinations of antibiotics that employ different mechanisms of action in hopes of eliminating persister spirochetes. Certain combinations have shown promise in mice and have been tested in her rhesus macaque model; data analyses are underway.
Other research teams, such as that of Ying Zhang, MD, PhD, at Johns Hopkins, have similarly been screening combinations of antibiotics and other compounds, identifying candidates for further testing.
During a question and answer period, Dr. Embers said her team is also investigating the pathophysiology and long-term effects of tick-borne coinfections, including Bartonella, and is pursuing a hypothesis that infection with Borrelia allows Bartonella to cause more extensive disease and persist longer. “I think Lyme is at the core because of its ability to evade and suppress the immune response so effectively.”
Diagnostic possibilities, biomarkers for PTLD
Direct diagnostic tests for microbial nucleic acid and proteins “are promising alternatives for indirect serologic tests,” Dr. Aucott said. For instance, in addition to polymerase chain reaction tests, which “are making advances,” it may be possible to target the B. burgdorferi peptidoglycan for antigen detection.
Researchers have shown that peptidoglycan, a component of the B. burgdorferi cell envelope, is a persistent antigen in the synovial fluid of patients with Lyme arthritis who have been treated with oral and intravenous antibiotics, and that it likely contributes to inflammation.
“Maybe the infection is gone but parts of the bacteria are still there that are driving inflammation,” said Dr. Aucott, also associate professor of medicine at John Hopkins.
Researchers have also been looking at the host response to B. burgdorferi – including cytokines, chemokines, and autoantibiodies – to identify biomarkers for PTLD and to identify patients during posttreatment follow-up who are at increased risk of developing PTLD, with the hope of someday intervening. Persistently high levels of interleukin-23, CCL19, and interferon-alpha have each been associated in different studies with persistent symptoms after treatment, Dr. Aucott said.
In addition, metabolomics research is showing that patients with PTLD have metabolic fingerprints that are different from those who return to good health after treatment, and it may be possible to identify an epigenetic signature for Lyme disease. A project sponsored by the Defense Advanced Research Projects Agency called ECHO (Epigenetic Characterization and Observation) aims to identify epigenetic signatures of exposures to various threats, including B. burgdorferi.
“At the very proximal end of [indirectly testing for host response], there are modifications of the DNA that can occur in response to infectious insults ... and that changed DNA changes RNA expression and protein synthesis,” Dr. Aucott explained. DARPA’s project is “exciting because their goal [at DARPA] is to have a diagnostic test quickly as a result of this epigenetics work.”
Imaging research is also fast offering diagnostic opportunities, Dr. Aucott said. Levels of microglial activation on brain PET imaging have been found to correlate with PTLD, and a study at Johns Hopkins of multimodal neuroimaging with functional MRI and diffusion tensor imaging has shown distinct changes to white matter activation within the frontal lobe of patients with PTLD, compared with controls.
The NASEM workshop did not collect or require disclosures of its participants.
FROM A NATIONAL ACADEMIES OF SCIENCE, ENGINEERING AND MEDICINE WORKSHOP
Penile Herpes Vegetans in a Patient With Well-controlled HIV
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.
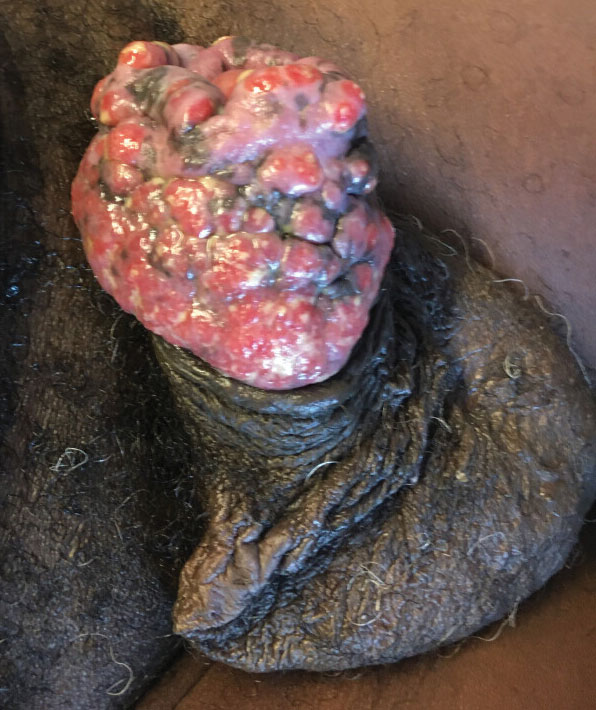
A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.
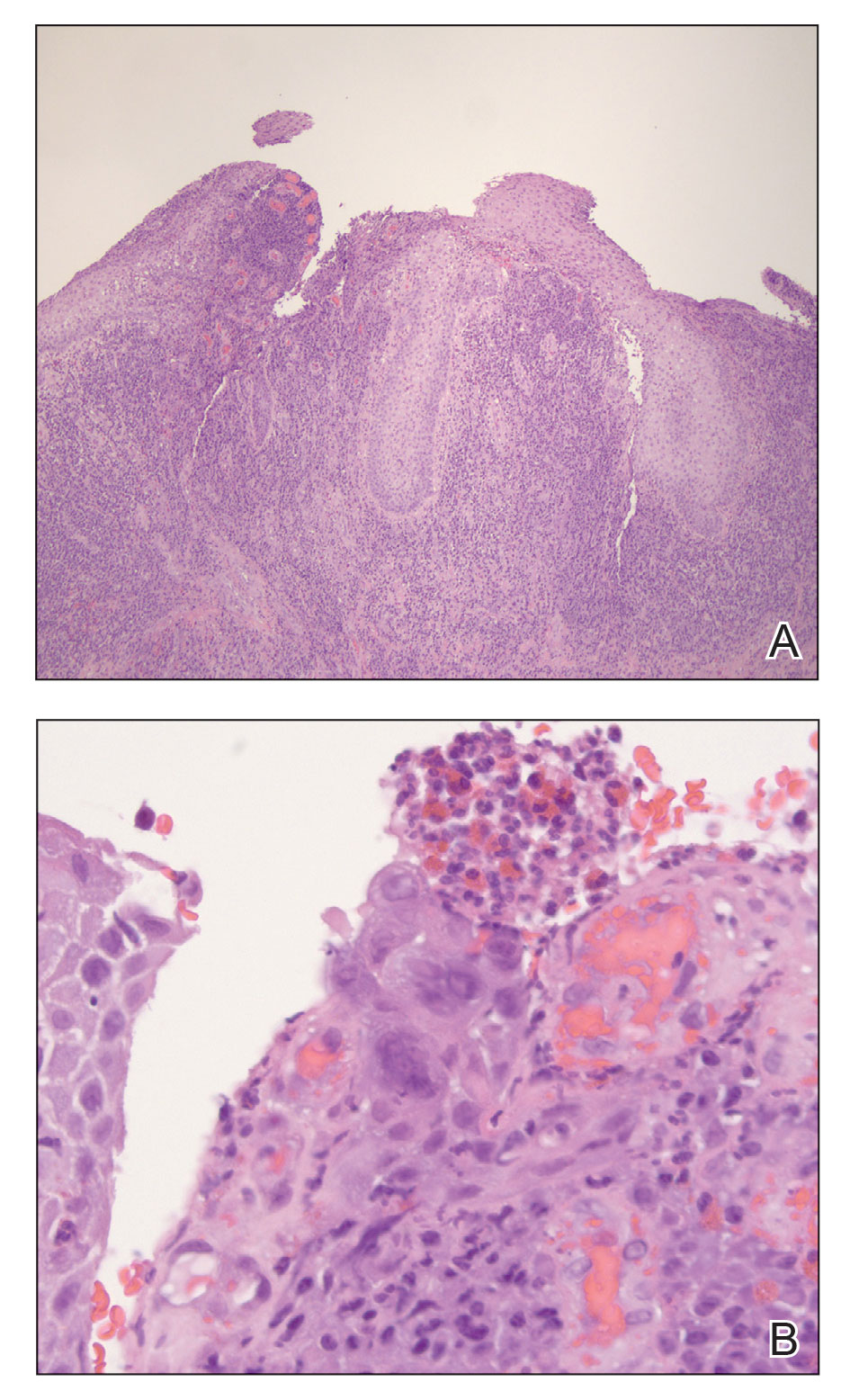
Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.
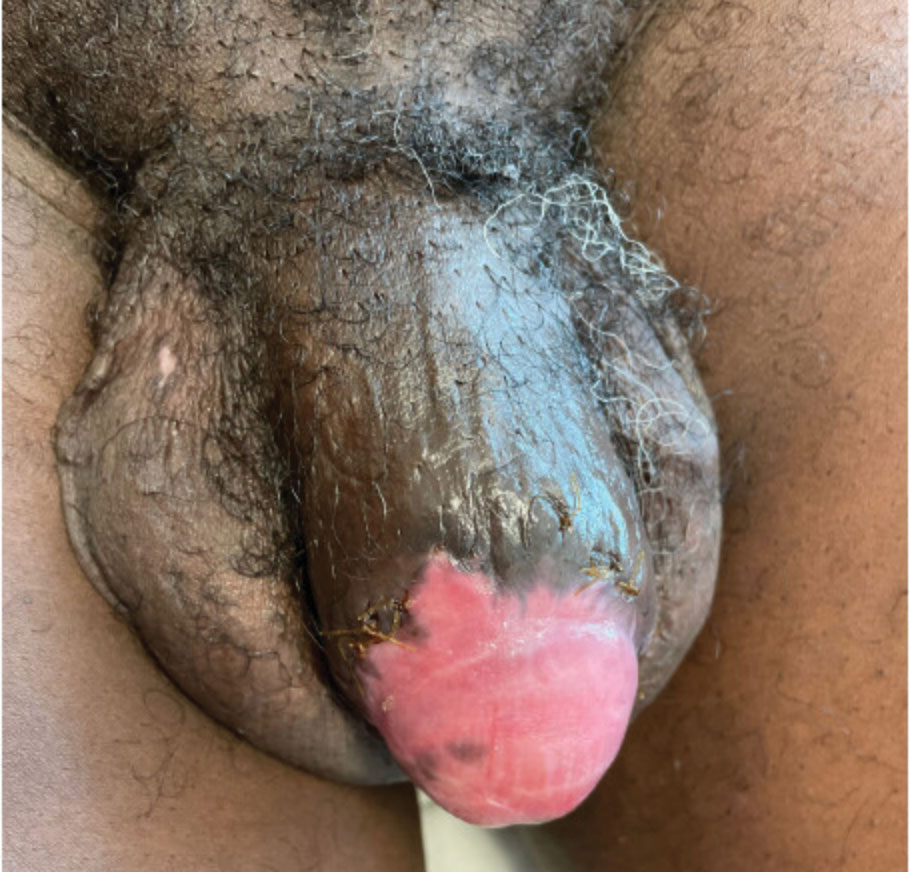
Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.

A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.

Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.

Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
To the Editor:
Herpes vegetans (HV) is an uncommon infection caused by human herpesvirus (HHV) in patients who are immunocompromised, such as those who are HIV positive.1 Unlike typical HHV infection, HV can present with exophytic exudative ulcers and papillomatous vegetations. The presentation of ulcerated genital nodules, especially in an immunocompromised patient, yields an array of disorders in the differential diagnosis, including condyloma latum, condyloma acuminatum, pyogenic granuloma (PG), and verrucous carcinoma.2,3 Histopathology of HV reveals pseudoepitheliomatous hyperplasia, plasma cell infiltration, and positivity for HHV type 1 (HHV-1) and/or HHV type 2 (HHV-2). Herpes vegetans lesions typically require a multimodal treatment approach because many cases are resistant to acyclovir. Treatment options include the nucleoside analogues foscarnet and cidofovir; immunomodulators such as topical imiquimod; and the topical antiviral trifluridine.1,4-6 We describe a case of HV in a patient with a history of well-controlled HIV infection who presented with a painful fungating penile lesion.

A 55-year-old man presented to the hospital with a painful expanding mass on the distal aspect of the penis of 3 months’ duration. He had a history of HIV infection that was well-controlled by antiretroviral therapy, prior hepatitis B virus infection and acyclovir-resistant genital HHV-2 infection. Physical examination revealed a large, firm, circumferential, exophytic, verrucous plaque with various areas of ulceration and purulent drainage on the distal shaft and glans of the penis (Figure 1). The patient’s most recent absolute CD4 count was 425 cells/mm3 (reference range, 500–1500 cells/mm3). His HIV viral load was undetectable at less than 30 copies/mL. Histopathology with hematoxylin and eosin staining of biopsy material from the penile lesion demonstrated pseudoepitheliomatous epidermal hyperplasia with focal ulceration and a mixed inflammatory infiltrate (Figure 2A). At higher magnification, clear viral cytopathic changes of HHV were noted, including multinucleation, nuclear molding, and homogenous gray nuclei (Figure 2B). Additional staining for fungi, mycobacteria, and spirochetes was negative. In-situ hybridization was negative for human papillomavirus subtypes. A bacterial culture of swabs of the purulent drainage was positive for Staphylococcus aureus and Proteus mirabilis.

Given the patient’s known history of acyclovir-resistant HHV-2 infection, he received a 28-day course of intravenous foscarnet 40 mg/kg every 12 hours. He also was given a 14-day course of intravenous ampicillin-sulbactam 3 g every 6 hours. The patient gradually improved during a 35-day hospital stay. He was discharged with cidofovir cream 1% and oral valacyclovir; the latter was subsequently discontinued by dermatology because of his known history of acyclovir resistance. Four months after discharge, the patient underwent a circumcision performed by urology to decrease the risk for recurrence and achieve the best cosmetic outcome. At the 6-month follow-up visit, dramatic clinical improvement was evident, with complete resolution of the plaque and only isolated areas of scarring (Figure 3). The patient reported that penile function was preserved.

Herpes vegetans represents a rare infection with HHV-1 or HHV-2, typically in patients who are considerably immunosuppressed, such as those with cancer, those undergoing transplantation, and those with uncontrolled HIV infection.1 Few cases of HV have been described in an immunocompetent patient.2 Our case is unique because the patient’s HIV infection was well controlled at the time HV was diagnosed, demonstrated by his modestly low CD4 count and undetectable HIV viral load.
Patients with HV can present diagnostic and therapeutic challenges. Typically, a diagnosis of cutaneous HHV infection does not require a biopsy; most cases appear as clustered vesicular lesions, making the disease easy to diagnose clinically. However, biopsies and cultures are necessary to identify the underlying cause of atypical verrucous exophytic lesions. Other conditions with clinical features similar to HV include squamous cell carcinoma, condyloma acuminatum, and deep fungal and mycobacterial infections.2,3 A tissue biopsy, histologic staining, and tissue culture should be performed to identify the causative pathogen and potential targets for treatment. Definitive diagnosis is vital to deliver proper treatment modalities, which often involve a multimodal multidisciplinary approach.
Several pathogenic mechanisms of HV have been proposed. One theory suggests that in an immunocompetent patient, HHV typically triggers a lymphocytic response, which leads to activation of interferon alpha. However, in an immunocompromised patient, such as an individual with AIDS, this interferon response is diminished, which explains why these patients typically have a chronic and resistant HHV infection. HIV has an affinity for infecting dermal dendritic cells, which signals activation of tumor necrosis factor and interleukin.6 Both cytokines contribute to an antiapoptotic environment that promotes continued proliferation of these viral cells in the epidermis. Over time, propagation of disinhibited cells can lead to the verrucous and hyperkeratotic-appearing skin that is common in patients with HV.7
Another theorized mechanism underlying hypertrophic herpetic lesions was described in the context of HHV-1 infection and subsequent PG. El Hayderi et al8 reported that histologic and immunohistochemical examination of a patient’s lesion revealed sparse epithelial cell aggregates within PG as well as HHV-1 antigens in the nuclei and cytoplasm of normal-appearing and cytopathic epithelial cells. Immunohistochemical examination also revealed vascular endothelial growth factor within HHV-1–infected epithelial cells and PG endothelial cells, suggesting that PG formation may be indirectly driven by vascular endothelial growth factor and its proangiogenic properties. The pathogenesis of PG in the setting of HHV-1 infection displays many similarities to hyperkeratotic lesions observed in atypical cutaneous manifestations of HHV-2.8
The management of patients with HV continues to be complex, often requiring a multimodal regimen. Although acyclovir has been shown to be highly effective for treating and preventing most HHV infections, acyclovir resistance frequently has been reported in immunocompromised populations.5 Acyclovir resistance can be correlated with the severity of immunodeficiency as well as the duration of acyclovir exposure. Resistance to acyclovir often results from deficient intracellular phosphorylation, which is required for activation of the drug. If patients show resistance to acyclovir and its derivatives, alternate drug classes that do not depend on thymidine kinase phosphorylation should be considered.
Our patient received a combination of intravenous foscarnet and a course of ampicillin-sulbactam while an inpatient due to his documented history of acyclovir-resistant HHV-2 infection, and he was discharged on cidofovir cream 1%. Cidofovir is US Food and Drug Administration approved for treating cytomegalovirus retinitis in patients with AIDS. Although data are limited, topical and intralesional cidofovir have been used to treat acyclovir-resistant cases of HV with documented success.1,9 In refractory HV or when the disease is slow to resolve, intralesional cidofovir has been documented to be an additional treatment option. Intralesional and topical cidofovir carry a much lower risk for adverse effects such as kidney dysfunction compared to intravenous cidofovir1 and can be considered in patients with minimal clinical improvement and those at increased risk for side effects.
Our case demonstrated how a patient with HV may require a complex and prolonged hospital course for appropriate treatment. Our patient required an array of both medical and surgical modalities to reach the desired outcome. Here, a multitude of specialties including infectious disease, dermatology, and urology worked together to reach a positive clinical and cosmetic outcome for this patient.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Bae-Harboe Y-SC, Khachemoune A. Verrucous herpetic infection of the scrotum and the groin in an immuno-competent patient: case report and review of the literature. Dermatol Online J. 2012;18. https://doi.org/10.5070/D30sv058j6
- Elosiebo RI, Koubek VA, Patel TS, et al. Vegetative sacral plaque in a patient with human immunodeficiency virus. Cutis. 2015;96:E7-E9.
- Saling C, Slim J, Szabela ME. A case of an atypical resistant granulomatous HHV-1 and HHV-2 ulceration in an AIDS patient treated with intralesional cidofovir. SAGE Open Med Case Rep. 2019;7:2050313X19847029. doi:10.1177/2050313X19847029
- Martinez V, Molina J-M, Scieux C, et al. Topical imiquimod for recurrent acyclovir-resistant HHV infection. Am J Med. 2006 May;119:E9-E11. doi:10.1016/j.amjmed.2005.06.037
- Ronkainen SD, Rothenberger M. Herpes vegetans: an unusual and acyclovir-resistant form of HHV. J Gen Intern Med. 2018;33:393. doi:10.1007/s11606-017-4256-y
- Quesada AE, Galfione S, Colome M, et al. Verrucous herpes of the scrotum presenting clinically as verrucous squamous cell carcinoma: case report and review of the literature. Ann Clin Lab Sci. 2014;44:208-212.
- El Hayderi L, Paurobally D, Fassotte MF, et al. Herpes simplex virus type-I and pyogenic granuloma: a vascular endothelial growth factor-mediated association? Case Rep Dermatol. 2013;5:236-243. doi:10.1159/000354570
- Toro JR, Sanchez S, Turiansky G, et al. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21:301-319. doi:10.1016/s0733-8635(02)00116-x
Practice Points
- Maintain a high clinical suspicion for herpes vegetans (HV) in a patient who has a history of immunosuppression and presents with exophytic genital lesions.
- A history of resistance to acyclovir requires a multimodal approach to treatment of HV lesions, including medical and surgical therapies.
Pneumococcal vaccine label adds injection-site risk
No similar safety signal has been detected for the more recently approved 15-valent and 20-valent pneumococcal conjugate vaccines, explain the investigators, led by Brendan Day, MD, MPH, from the FDA’s Center for Biologics Evaluation and Research, in their report published online in JAMA Internal Medicine.
Reports of injection-site necrosis emerged after the vaccine (Pneumovax 23, Merck) had been approved by the FDA and was administered to a large, diverse, real-world population.
Rare safety events can emerge after FDA approval, as clinical trials may not be able to detect them in a study-group population.
Therefore, “postmarketing safety surveillance is critical to further characterize the safety profile of licensed vaccines,” the investigators point out.
The FDA and the Centers for Disease Control and Prevention monitor the postmarketing safety of licensed vaccines using the Vaccine Adverse Event Reporting System (VAERS), which relies on people who get the vaccines to report adverse events.
Real-world finding
After reports indicated a safety signal in 2020, the researchers conducted a case-series review, calculated the reporting rate, and did a PubMed search for similar reports.
They found that the reporting rate for injection-site necrosis was less than 0.2 cases per 1 million vaccine doses administered. The PubMed search yielded two cases of injection-site necrosis after the vaccine.
The 23-valent vaccine helps protect people from pneumococcus bacterial infection. The manufacturer reports that it is for people at least 50 years of age and for children who are at least 2 years of age with medical conditions that put them at elevated risk for infection.
The U.S. package insert has been updated, in the Post-Marketing Experience section, to include injection-site necrosis.
Of the 104 VAERS reports identified by the researchers, 48 met the case definition. Of those cases, most were for skin necrosis (n = 43), five of which also included fat necrosis. The remaining five cases of necrosis affected fascia (n = 2); fat and fascia (n = 1); fat, fascia, and muscle (n = 1); and muscle (n = 1).
In 23 of the 48 cases (47.9%), the reactions were serious and included one death (unrelated to vaccination).
Seventeen patients (35.4%) were hospitalized and 26 (54.2%) required surgery, most commonly debridement. Eight patients (16.7%) underwent multiple surgical procedures and three (6.3%) required a skin graft.
For patients with skin necrosis (n = 43), the median age was 67 years, and most patients were female (n = 36). Twelve patients were immunocompromised.
Concomitant vaccinations were reported in 10 patients, five of whom got the shot in the same arm as the 23-valent pneumococcal vaccine. A concurrent diagnosis of cellulitis was reported in 16 patients and an abscess was reported in three patients. There were too few cases of fat, fascia, or muscle necrosis to draw conclusions, the researchers report.
Often, skin necrosis was seen after a progression of symptoms, such as redness, pain, or swelling.
“These reports are consistent with published descriptions of injection-site necrosis, which has been reported as a rare complication for many vaccines and injectable drugs,” the investigators report.
Although the researchers couldn’t conclude from the VAERS reports alone that the vaccine injection caused the necrosis, “the timing and the location of reactions at the injection site suggest a possible causal association with the vaccine,” they explain. However, they add, patient comorbidities and poor injection technique may also be contributors.
A version of this article first appeared on Medscape.com.
No similar safety signal has been detected for the more recently approved 15-valent and 20-valent pneumococcal conjugate vaccines, explain the investigators, led by Brendan Day, MD, MPH, from the FDA’s Center for Biologics Evaluation and Research, in their report published online in JAMA Internal Medicine.
Reports of injection-site necrosis emerged after the vaccine (Pneumovax 23, Merck) had been approved by the FDA and was administered to a large, diverse, real-world population.
Rare safety events can emerge after FDA approval, as clinical trials may not be able to detect them in a study-group population.
Therefore, “postmarketing safety surveillance is critical to further characterize the safety profile of licensed vaccines,” the investigators point out.
The FDA and the Centers for Disease Control and Prevention monitor the postmarketing safety of licensed vaccines using the Vaccine Adverse Event Reporting System (VAERS), which relies on people who get the vaccines to report adverse events.
Real-world finding
After reports indicated a safety signal in 2020, the researchers conducted a case-series review, calculated the reporting rate, and did a PubMed search for similar reports.
They found that the reporting rate for injection-site necrosis was less than 0.2 cases per 1 million vaccine doses administered. The PubMed search yielded two cases of injection-site necrosis after the vaccine.
The 23-valent vaccine helps protect people from pneumococcus bacterial infection. The manufacturer reports that it is for people at least 50 years of age and for children who are at least 2 years of age with medical conditions that put them at elevated risk for infection.
The U.S. package insert has been updated, in the Post-Marketing Experience section, to include injection-site necrosis.
Of the 104 VAERS reports identified by the researchers, 48 met the case definition. Of those cases, most were for skin necrosis (n = 43), five of which also included fat necrosis. The remaining five cases of necrosis affected fascia (n = 2); fat and fascia (n = 1); fat, fascia, and muscle (n = 1); and muscle (n = 1).
In 23 of the 48 cases (47.9%), the reactions were serious and included one death (unrelated to vaccination).
Seventeen patients (35.4%) were hospitalized and 26 (54.2%) required surgery, most commonly debridement. Eight patients (16.7%) underwent multiple surgical procedures and three (6.3%) required a skin graft.
For patients with skin necrosis (n = 43), the median age was 67 years, and most patients were female (n = 36). Twelve patients were immunocompromised.
Concomitant vaccinations were reported in 10 patients, five of whom got the shot in the same arm as the 23-valent pneumococcal vaccine. A concurrent diagnosis of cellulitis was reported in 16 patients and an abscess was reported in three patients. There were too few cases of fat, fascia, or muscle necrosis to draw conclusions, the researchers report.
Often, skin necrosis was seen after a progression of symptoms, such as redness, pain, or swelling.
“These reports are consistent with published descriptions of injection-site necrosis, which has been reported as a rare complication for many vaccines and injectable drugs,” the investigators report.
Although the researchers couldn’t conclude from the VAERS reports alone that the vaccine injection caused the necrosis, “the timing and the location of reactions at the injection site suggest a possible causal association with the vaccine,” they explain. However, they add, patient comorbidities and poor injection technique may also be contributors.
A version of this article first appeared on Medscape.com.
No similar safety signal has been detected for the more recently approved 15-valent and 20-valent pneumococcal conjugate vaccines, explain the investigators, led by Brendan Day, MD, MPH, from the FDA’s Center for Biologics Evaluation and Research, in their report published online in JAMA Internal Medicine.
Reports of injection-site necrosis emerged after the vaccine (Pneumovax 23, Merck) had been approved by the FDA and was administered to a large, diverse, real-world population.
Rare safety events can emerge after FDA approval, as clinical trials may not be able to detect them in a study-group population.
Therefore, “postmarketing safety surveillance is critical to further characterize the safety profile of licensed vaccines,” the investigators point out.
The FDA and the Centers for Disease Control and Prevention monitor the postmarketing safety of licensed vaccines using the Vaccine Adverse Event Reporting System (VAERS), which relies on people who get the vaccines to report adverse events.
Real-world finding
After reports indicated a safety signal in 2020, the researchers conducted a case-series review, calculated the reporting rate, and did a PubMed search for similar reports.
They found that the reporting rate for injection-site necrosis was less than 0.2 cases per 1 million vaccine doses administered. The PubMed search yielded two cases of injection-site necrosis after the vaccine.
The 23-valent vaccine helps protect people from pneumococcus bacterial infection. The manufacturer reports that it is for people at least 50 years of age and for children who are at least 2 years of age with medical conditions that put them at elevated risk for infection.
The U.S. package insert has been updated, in the Post-Marketing Experience section, to include injection-site necrosis.
Of the 104 VAERS reports identified by the researchers, 48 met the case definition. Of those cases, most were for skin necrosis (n = 43), five of which also included fat necrosis. The remaining five cases of necrosis affected fascia (n = 2); fat and fascia (n = 1); fat, fascia, and muscle (n = 1); and muscle (n = 1).
In 23 of the 48 cases (47.9%), the reactions were serious and included one death (unrelated to vaccination).
Seventeen patients (35.4%) were hospitalized and 26 (54.2%) required surgery, most commonly debridement. Eight patients (16.7%) underwent multiple surgical procedures and three (6.3%) required a skin graft.
For patients with skin necrosis (n = 43), the median age was 67 years, and most patients were female (n = 36). Twelve patients were immunocompromised.
Concomitant vaccinations were reported in 10 patients, five of whom got the shot in the same arm as the 23-valent pneumococcal vaccine. A concurrent diagnosis of cellulitis was reported in 16 patients and an abscess was reported in three patients. There were too few cases of fat, fascia, or muscle necrosis to draw conclusions, the researchers report.
Often, skin necrosis was seen after a progression of symptoms, such as redness, pain, or swelling.
“These reports are consistent with published descriptions of injection-site necrosis, which has been reported as a rare complication for many vaccines and injectable drugs,” the investigators report.
Although the researchers couldn’t conclude from the VAERS reports alone that the vaccine injection caused the necrosis, “the timing and the location of reactions at the injection site suggest a possible causal association with the vaccine,” they explain. However, they add, patient comorbidities and poor injection technique may also be contributors.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
Summer diarrhea – Time to think outside the box
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4
Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4

Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4

Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
FDA approves RSV monoclonal antibody for all infants
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
Long COVID and vaccines: Separating facts from falsehoods
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.