User login
LAMA-LABA surpasses corticosteroid combination as COPD therapy
Use of inhalers with long-acting muscarinic antagonists and long-acting beta-agonists reduced COPD exacerbations and pneumonia hospitalizations compared with inhalers with corticosteroids and long-acting beta-agonists, based on data from more than 30,000 individuals.
Current clinical guidelines for chronic obstructive pulmonary disease (COPD) patients recommend inhalers with long-acting muscarinic antagonists (LAMAs) and long-acting beta-agonists (LABAs) over those with inhaled corticosteroids (ICSs) and LABAs, but data comparing the two formulations have been inconsistent, and concerns about generalizability persist, wrote William B. Feldman, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
In a study published in JAMA Internal Medicine, the researchers reviewed data from a commercial insurance claims database of individuals diagnosed with COPD who filled a new prescription for a LAMA-LABA inhaler or ICS-LABA inhaler between Jan. 1, 2014, and Dec. 31, 2019. Patients with asthma and those younger than 40 years were excluded. The study population included 137,833 individuals with a mean age of 70.2 years; 50.4% were female. Of the 107,004 ICS-LABA users and 30,829 LAMA-LABA users, 30,216 matched pairs were included in a 1:1 propensity score matched study. The primary outcomes were effectiveness, based on the rate of first moderate or severe COPD exacerbation, and safety, based on the rate of first pneumonia hospitalization.
Use of LAMA-LABA inhalers was associated with an 8% reduction in the rate of first moderate or severe COPD exacerbation and a 20% reduction in the rate of first pneumonia hospitalization compared with use of ICS-LABA (hazard ratios 0.92 and 0.80, respectively). The absolute rate reductions with LAMA-LABA inhalers for first moderate or severe COPD exacerbations and for first pneumonia hospitalizations were was 43.0 events per 1,000 person-years and 91.8 events per person-years, respectively.
The overall rates of total moderate to severe COPD and pneumonia hospitalizations were 5% and 17% lower, respectively, among patients who used LAMA-LABA than those treated with ICS-LABA. The results were consistently robust in subgroup and sensitivity analyses, the researchers wrote in their discussion. However, the results must be interpreted cautiously in comparison to other large studies because of the significant differences in the cohorts of patients studied, notably that most patients in the current study had no received previous inhaler therapy.
The study findings were limited by several factors including the relatively short follow-up time and reliance on prescription fills as an indicator of medication use, the researchers noted. Other limitations included notable differences between the LAMA-LABA patients and ICS-LABA patients, such as more severe COPD and less access to respiratory care, they wrote.
Although the current study is not the definitive answer to conflicting results from previous trials, it is the largest know to date to compare LAMA-LABA with ICS-LABA, and the results support LAMA-LABA as the preferred therapy for COPD patients, the researchers concluded.
Findings clarify clinical practice guidelines
“This study was required to provide clarity regarding the optimal choice of treatment for COPD given conflicting data from other recent trials,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The study findings reinforce the benefits of combined LAMA-LABA in improving clinical outcomes in COPD in a real-world setting,” and the data provide further support for choosing LAMA-LABA over ICS-LABA in COPD patients, said Dr. Pal, who was not involved in the study.
However, availability and affordability of LAMA-LABA inhalers may be barriers to expanding their use in clinical practice, he noted.
“Additional research is needed to accurately define which patient populations would benefit most from the therapy and whether patients who have previously been stabilized on ICS-LABA would derive additional benefit from a change in therapy,” Dr. Pal said.
The study was supported by the National Heart, Lung, and Blood Institute and funding from the Commonwealth Fund and Arnold Ventures.
Dr. Feldman disclosed receiving personal fees from Alosa Health and Aetion, serving as an expert witness in litigation against inhaler manufacturers, and receiving an honorarium for a presentation to Blue Cross Blue Shield of Massachusetts unrelated to the current study. Dr. Pal had no financial conflicts to disclose.
Use of inhalers with long-acting muscarinic antagonists and long-acting beta-agonists reduced COPD exacerbations and pneumonia hospitalizations compared with inhalers with corticosteroids and long-acting beta-agonists, based on data from more than 30,000 individuals.
Current clinical guidelines for chronic obstructive pulmonary disease (COPD) patients recommend inhalers with long-acting muscarinic antagonists (LAMAs) and long-acting beta-agonists (LABAs) over those with inhaled corticosteroids (ICSs) and LABAs, but data comparing the two formulations have been inconsistent, and concerns about generalizability persist, wrote William B. Feldman, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
In a study published in JAMA Internal Medicine, the researchers reviewed data from a commercial insurance claims database of individuals diagnosed with COPD who filled a new prescription for a LAMA-LABA inhaler or ICS-LABA inhaler between Jan. 1, 2014, and Dec. 31, 2019. Patients with asthma and those younger than 40 years were excluded. The study population included 137,833 individuals with a mean age of 70.2 years; 50.4% were female. Of the 107,004 ICS-LABA users and 30,829 LAMA-LABA users, 30,216 matched pairs were included in a 1:1 propensity score matched study. The primary outcomes were effectiveness, based on the rate of first moderate or severe COPD exacerbation, and safety, based on the rate of first pneumonia hospitalization.
Use of LAMA-LABA inhalers was associated with an 8% reduction in the rate of first moderate or severe COPD exacerbation and a 20% reduction in the rate of first pneumonia hospitalization compared with use of ICS-LABA (hazard ratios 0.92 and 0.80, respectively). The absolute rate reductions with LAMA-LABA inhalers for first moderate or severe COPD exacerbations and for first pneumonia hospitalizations were was 43.0 events per 1,000 person-years and 91.8 events per person-years, respectively.
The overall rates of total moderate to severe COPD and pneumonia hospitalizations were 5% and 17% lower, respectively, among patients who used LAMA-LABA than those treated with ICS-LABA. The results were consistently robust in subgroup and sensitivity analyses, the researchers wrote in their discussion. However, the results must be interpreted cautiously in comparison to other large studies because of the significant differences in the cohorts of patients studied, notably that most patients in the current study had no received previous inhaler therapy.
The study findings were limited by several factors including the relatively short follow-up time and reliance on prescription fills as an indicator of medication use, the researchers noted. Other limitations included notable differences between the LAMA-LABA patients and ICS-LABA patients, such as more severe COPD and less access to respiratory care, they wrote.
Although the current study is not the definitive answer to conflicting results from previous trials, it is the largest know to date to compare LAMA-LABA with ICS-LABA, and the results support LAMA-LABA as the preferred therapy for COPD patients, the researchers concluded.
Findings clarify clinical practice guidelines
“This study was required to provide clarity regarding the optimal choice of treatment for COPD given conflicting data from other recent trials,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The study findings reinforce the benefits of combined LAMA-LABA in improving clinical outcomes in COPD in a real-world setting,” and the data provide further support for choosing LAMA-LABA over ICS-LABA in COPD patients, said Dr. Pal, who was not involved in the study.
However, availability and affordability of LAMA-LABA inhalers may be barriers to expanding their use in clinical practice, he noted.
“Additional research is needed to accurately define which patient populations would benefit most from the therapy and whether patients who have previously been stabilized on ICS-LABA would derive additional benefit from a change in therapy,” Dr. Pal said.
The study was supported by the National Heart, Lung, and Blood Institute and funding from the Commonwealth Fund and Arnold Ventures.
Dr. Feldman disclosed receiving personal fees from Alosa Health and Aetion, serving as an expert witness in litigation against inhaler manufacturers, and receiving an honorarium for a presentation to Blue Cross Blue Shield of Massachusetts unrelated to the current study. Dr. Pal had no financial conflicts to disclose.
Use of inhalers with long-acting muscarinic antagonists and long-acting beta-agonists reduced COPD exacerbations and pneumonia hospitalizations compared with inhalers with corticosteroids and long-acting beta-agonists, based on data from more than 30,000 individuals.
Current clinical guidelines for chronic obstructive pulmonary disease (COPD) patients recommend inhalers with long-acting muscarinic antagonists (LAMAs) and long-acting beta-agonists (LABAs) over those with inhaled corticosteroids (ICSs) and LABAs, but data comparing the two formulations have been inconsistent, and concerns about generalizability persist, wrote William B. Feldman, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
In a study published in JAMA Internal Medicine, the researchers reviewed data from a commercial insurance claims database of individuals diagnosed with COPD who filled a new prescription for a LAMA-LABA inhaler or ICS-LABA inhaler between Jan. 1, 2014, and Dec. 31, 2019. Patients with asthma and those younger than 40 years were excluded. The study population included 137,833 individuals with a mean age of 70.2 years; 50.4% were female. Of the 107,004 ICS-LABA users and 30,829 LAMA-LABA users, 30,216 matched pairs were included in a 1:1 propensity score matched study. The primary outcomes were effectiveness, based on the rate of first moderate or severe COPD exacerbation, and safety, based on the rate of first pneumonia hospitalization.
Use of LAMA-LABA inhalers was associated with an 8% reduction in the rate of first moderate or severe COPD exacerbation and a 20% reduction in the rate of first pneumonia hospitalization compared with use of ICS-LABA (hazard ratios 0.92 and 0.80, respectively). The absolute rate reductions with LAMA-LABA inhalers for first moderate or severe COPD exacerbations and for first pneumonia hospitalizations were was 43.0 events per 1,000 person-years and 91.8 events per person-years, respectively.
The overall rates of total moderate to severe COPD and pneumonia hospitalizations were 5% and 17% lower, respectively, among patients who used LAMA-LABA than those treated with ICS-LABA. The results were consistently robust in subgroup and sensitivity analyses, the researchers wrote in their discussion. However, the results must be interpreted cautiously in comparison to other large studies because of the significant differences in the cohorts of patients studied, notably that most patients in the current study had no received previous inhaler therapy.
The study findings were limited by several factors including the relatively short follow-up time and reliance on prescription fills as an indicator of medication use, the researchers noted. Other limitations included notable differences between the LAMA-LABA patients and ICS-LABA patients, such as more severe COPD and less access to respiratory care, they wrote.
Although the current study is not the definitive answer to conflicting results from previous trials, it is the largest know to date to compare LAMA-LABA with ICS-LABA, and the results support LAMA-LABA as the preferred therapy for COPD patients, the researchers concluded.
Findings clarify clinical practice guidelines
“This study was required to provide clarity regarding the optimal choice of treatment for COPD given conflicting data from other recent trials,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The study findings reinforce the benefits of combined LAMA-LABA in improving clinical outcomes in COPD in a real-world setting,” and the data provide further support for choosing LAMA-LABA over ICS-LABA in COPD patients, said Dr. Pal, who was not involved in the study.
However, availability and affordability of LAMA-LABA inhalers may be barriers to expanding their use in clinical practice, he noted.
“Additional research is needed to accurately define which patient populations would benefit most from the therapy and whether patients who have previously been stabilized on ICS-LABA would derive additional benefit from a change in therapy,” Dr. Pal said.
The study was supported by the National Heart, Lung, and Blood Institute and funding from the Commonwealth Fund and Arnold Ventures.
Dr. Feldman disclosed receiving personal fees from Alosa Health and Aetion, serving as an expert witness in litigation against inhaler manufacturers, and receiving an honorarium for a presentation to Blue Cross Blue Shield of Massachusetts unrelated to the current study. Dr. Pal had no financial conflicts to disclose.
FROM JAMA INTERNAL MEDICINE
A decade after first DAA, only one in three are HCV free
In the decade since safe, curative oral treatments were approved for treating hepatitis C virus (HCV) infections, only one in three U.S. patients diagnosed with the disease have been cleared of it, according to new data from the Centers for Disease Control and Prevention.
The findings indicate that current progress falls far short of the goal of the Viral Hepatitis National Strategic Plan for the United States, which calls for eliminating HCV for at least 80% of patients with the virus by 2030.
Lead author Carolyn Wester, MD, with the CDC’s Division of Viral Hepatitis, called the low numbers “stunning” and said that the researchers found that patients face barriers to being cured at every step of the way, from being diagnosed to accessing breakthrough direct-acting antiviral (DAA) agents.
The article was published online in the CDC’s Morbidity and Mortality Weekly Report.
Outcomes vary by age and insurance
Using longitudinal data from Quest Diagnostics laboratories, the researchers identified 1.7 million people who had a history of HCV infection from Jan. 1, 2013, to Dec. 31, 2022.
Of those patients, 1.5 million (88%) were categorized as having undergone viral testing.
Among those who underwent such testing, 1 million (69%) were categorized as having an initial infection. Just 356,807 patients with initial infection (34%) were cured or cleared of HCV. Of those found to be cured or cleared, 23,518 (7%) were found to have persistent infection or reinfection.
Viral clearance varied greatly by insurance. While 45% of the people covered under Medicare experienced viral clearance, only 23% of the uninsured and 31% of those on Medicaid did so.
Age also played a role in viral clearance. It was highest (42%) among those aged 60 and older. Clearance was lowest (24%) among patients in the 20-39 age group, the group most likely to be newly infected in light of the surge in HCV cases because of the opioid epidemic, Dr. Wester said. Persistent infection or reinfection was also highest in the 20-39 age group.
With respect to age and insurance type combined, the highest HCV clearance rate (49%) was for patients aged 60 and older who had commercial insurance; the lowest (16%) was for uninsured patients in the 20-39 age group.
The investigators evaluated people who had been diagnosed with HCV, Dr. Wester said. “It’s estimated about 40% of people in the U.S. are unaware of their infection.” Because of this, the numbers reported in the study may vastly underestimate the true picture, she told this news organization.
Barriers to treatment ‘insurmountable’ without major transformation
Increased access to diagnosis, treatment, and prevention services for persons with or at risk for acquiring hepatitis C needs to be addressed to prevent progression of disease and ongoing transmission and to achieve national hepatitis C elimination goals, the authors wrote.
The biggest barriers to improving HCV clearance are the high cost of treatment, widely varying insurance coverage, insurer restrictions, and challenges in diagnosing the disease, Dr. Wester added.
Overcoming these barriers requires implementation of universal HCV screening recommendations, including HCV RNA testing for all persons with reactive HCV antibody results, provision of treatment for all persons regardless of payer, and prevention services for persons at risk for acquiring new HCV infection, the authors concluded.
“The current barriers are insurmountable without a major transformation in our nation’s response,” Dr. Wester noted.
She expressed her support of the National Hepatitis C Elimination Program, offered as part of the Biden Administration’s 2024 budget proposal. She said that the initiative “is what we need to prevent the needless suffering from hepatitis C and to potentially save not only tens of thousands of lives but tens of billions of health care dollars.”
The three-part proposal includes a national subscription model to purchase DAA agents for those most underserved: Medicaid beneficiaries, incarcerated people, the uninsured, and American Indian and Alaska Native individuals treated through the Indian Health Service.
Under this model, the federal government would negotiate with manufacturers to buy as much treatment as needed for all individuals in the underserved groups.
What can physicians do?
Physicians can help improve HCV treatment and outcomes by being aware of the current testing guidelines, Dr. Wester said.
Guidelines now call for hepatitis C screening at least once in a lifetime for all adults, except in settings where the prevalence of HCV infection is less than 0.1%. They also call for screening during each pregnancy, with the same regional-prevalence exception.
Recommendations include curative treatment “for nearly everybody who is living with hepatitis C,” Dr. Wester added.
These CDC guidelines came out in April 2020, a time when the medical focus shifted to COVID-19, and that may have hurt awareness, she noted.
Physicians can also help by fighting back against non–evidence-based reasons insurance companies give for restricting coverage, Dr. Wester said.
Those restrictions include requiring specialists to prescribe DAA agents instead of allowing primary care physicians to do so, as well as requiring patients to have advanced liver disease or requiring patients to demonstrate sobriety or prove they are receiving counseling prior to their being eligible for treatment, Dr. Wester said.
Prior authorization a problem
Stacey B. Trooskin MD, PhD, MPH, assistant professor of medicine at the University of Pennsylvania in Philadelphia, told this news organization that prior authorization has been a major barrier for obtaining medications. Prior authorization requirements differ by state.
The paperwork must be submitted by already-stretched physician offices, and appeals are common. In that time, the window for keeping patients with HCV in the health care system may be lost, said Dr. Trooskin, chief medical adviser to the National Viral Hepatitis Roundtable.
“We know that about half of all Medicaid programs have removed prior authorization for most patients entirely,” she said, “but there are still half that require prior authorization.”
Action at the federal level is also needed, Dr. Trooskin said.
The countries that are successfully eliminating HCV and have successfully deployed the lifesaving medications provide governmental support for meeting patients where they are, she added.
Support can include inpatient and outpatient substance use disorder treatment programs or support in mental health settings, she noted.
“It’s not enough to want patients to come into their primary care provider and for that primary care provider to screen them,” Dr. Trooskin said. “This is about creating health care infrastructure so that we are finding patients at greatest risk for hepatitis C and integrating hepatitis C treatment into the services they are already accessing.”
Coauthor Harvey W. Kaufman, MD, is an employee of and owns stock in Quest Diagnostics. Coauthor William A. Meyer III, PhD, is a consultant to Quest Diagnostics. No other potential conflicts of interest were disclosed. Dr. Trooskin oversees C-Change, a hepatitis C elimination program, which receives funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
In the decade since safe, curative oral treatments were approved for treating hepatitis C virus (HCV) infections, only one in three U.S. patients diagnosed with the disease have been cleared of it, according to new data from the Centers for Disease Control and Prevention.
The findings indicate that current progress falls far short of the goal of the Viral Hepatitis National Strategic Plan for the United States, which calls for eliminating HCV for at least 80% of patients with the virus by 2030.
Lead author Carolyn Wester, MD, with the CDC’s Division of Viral Hepatitis, called the low numbers “stunning” and said that the researchers found that patients face barriers to being cured at every step of the way, from being diagnosed to accessing breakthrough direct-acting antiviral (DAA) agents.
The article was published online in the CDC’s Morbidity and Mortality Weekly Report.
Outcomes vary by age and insurance
Using longitudinal data from Quest Diagnostics laboratories, the researchers identified 1.7 million people who had a history of HCV infection from Jan. 1, 2013, to Dec. 31, 2022.
Of those patients, 1.5 million (88%) were categorized as having undergone viral testing.
Among those who underwent such testing, 1 million (69%) were categorized as having an initial infection. Just 356,807 patients with initial infection (34%) were cured or cleared of HCV. Of those found to be cured or cleared, 23,518 (7%) were found to have persistent infection or reinfection.
Viral clearance varied greatly by insurance. While 45% of the people covered under Medicare experienced viral clearance, only 23% of the uninsured and 31% of those on Medicaid did so.
Age also played a role in viral clearance. It was highest (42%) among those aged 60 and older. Clearance was lowest (24%) among patients in the 20-39 age group, the group most likely to be newly infected in light of the surge in HCV cases because of the opioid epidemic, Dr. Wester said. Persistent infection or reinfection was also highest in the 20-39 age group.
With respect to age and insurance type combined, the highest HCV clearance rate (49%) was for patients aged 60 and older who had commercial insurance; the lowest (16%) was for uninsured patients in the 20-39 age group.
The investigators evaluated people who had been diagnosed with HCV, Dr. Wester said. “It’s estimated about 40% of people in the U.S. are unaware of their infection.” Because of this, the numbers reported in the study may vastly underestimate the true picture, she told this news organization.
Barriers to treatment ‘insurmountable’ without major transformation
Increased access to diagnosis, treatment, and prevention services for persons with or at risk for acquiring hepatitis C needs to be addressed to prevent progression of disease and ongoing transmission and to achieve national hepatitis C elimination goals, the authors wrote.
The biggest barriers to improving HCV clearance are the high cost of treatment, widely varying insurance coverage, insurer restrictions, and challenges in diagnosing the disease, Dr. Wester added.
Overcoming these barriers requires implementation of universal HCV screening recommendations, including HCV RNA testing for all persons with reactive HCV antibody results, provision of treatment for all persons regardless of payer, and prevention services for persons at risk for acquiring new HCV infection, the authors concluded.
“The current barriers are insurmountable without a major transformation in our nation’s response,” Dr. Wester noted.
She expressed her support of the National Hepatitis C Elimination Program, offered as part of the Biden Administration’s 2024 budget proposal. She said that the initiative “is what we need to prevent the needless suffering from hepatitis C and to potentially save not only tens of thousands of lives but tens of billions of health care dollars.”
The three-part proposal includes a national subscription model to purchase DAA agents for those most underserved: Medicaid beneficiaries, incarcerated people, the uninsured, and American Indian and Alaska Native individuals treated through the Indian Health Service.
Under this model, the federal government would negotiate with manufacturers to buy as much treatment as needed for all individuals in the underserved groups.
What can physicians do?
Physicians can help improve HCV treatment and outcomes by being aware of the current testing guidelines, Dr. Wester said.
Guidelines now call for hepatitis C screening at least once in a lifetime for all adults, except in settings where the prevalence of HCV infection is less than 0.1%. They also call for screening during each pregnancy, with the same regional-prevalence exception.
Recommendations include curative treatment “for nearly everybody who is living with hepatitis C,” Dr. Wester added.
These CDC guidelines came out in April 2020, a time when the medical focus shifted to COVID-19, and that may have hurt awareness, she noted.
Physicians can also help by fighting back against non–evidence-based reasons insurance companies give for restricting coverage, Dr. Wester said.
Those restrictions include requiring specialists to prescribe DAA agents instead of allowing primary care physicians to do so, as well as requiring patients to have advanced liver disease or requiring patients to demonstrate sobriety or prove they are receiving counseling prior to their being eligible for treatment, Dr. Wester said.
Prior authorization a problem
Stacey B. Trooskin MD, PhD, MPH, assistant professor of medicine at the University of Pennsylvania in Philadelphia, told this news organization that prior authorization has been a major barrier for obtaining medications. Prior authorization requirements differ by state.
The paperwork must be submitted by already-stretched physician offices, and appeals are common. In that time, the window for keeping patients with HCV in the health care system may be lost, said Dr. Trooskin, chief medical adviser to the National Viral Hepatitis Roundtable.
“We know that about half of all Medicaid programs have removed prior authorization for most patients entirely,” she said, “but there are still half that require prior authorization.”
Action at the federal level is also needed, Dr. Trooskin said.
The countries that are successfully eliminating HCV and have successfully deployed the lifesaving medications provide governmental support for meeting patients where they are, she added.
Support can include inpatient and outpatient substance use disorder treatment programs or support in mental health settings, she noted.
“It’s not enough to want patients to come into their primary care provider and for that primary care provider to screen them,” Dr. Trooskin said. “This is about creating health care infrastructure so that we are finding patients at greatest risk for hepatitis C and integrating hepatitis C treatment into the services they are already accessing.”
Coauthor Harvey W. Kaufman, MD, is an employee of and owns stock in Quest Diagnostics. Coauthor William A. Meyer III, PhD, is a consultant to Quest Diagnostics. No other potential conflicts of interest were disclosed. Dr. Trooskin oversees C-Change, a hepatitis C elimination program, which receives funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
In the decade since safe, curative oral treatments were approved for treating hepatitis C virus (HCV) infections, only one in three U.S. patients diagnosed with the disease have been cleared of it, according to new data from the Centers for Disease Control and Prevention.
The findings indicate that current progress falls far short of the goal of the Viral Hepatitis National Strategic Plan for the United States, which calls for eliminating HCV for at least 80% of patients with the virus by 2030.
Lead author Carolyn Wester, MD, with the CDC’s Division of Viral Hepatitis, called the low numbers “stunning” and said that the researchers found that patients face barriers to being cured at every step of the way, from being diagnosed to accessing breakthrough direct-acting antiviral (DAA) agents.
The article was published online in the CDC’s Morbidity and Mortality Weekly Report.
Outcomes vary by age and insurance
Using longitudinal data from Quest Diagnostics laboratories, the researchers identified 1.7 million people who had a history of HCV infection from Jan. 1, 2013, to Dec. 31, 2022.
Of those patients, 1.5 million (88%) were categorized as having undergone viral testing.
Among those who underwent such testing, 1 million (69%) were categorized as having an initial infection. Just 356,807 patients with initial infection (34%) were cured or cleared of HCV. Of those found to be cured or cleared, 23,518 (7%) were found to have persistent infection or reinfection.
Viral clearance varied greatly by insurance. While 45% of the people covered under Medicare experienced viral clearance, only 23% of the uninsured and 31% of those on Medicaid did so.
Age also played a role in viral clearance. It was highest (42%) among those aged 60 and older. Clearance was lowest (24%) among patients in the 20-39 age group, the group most likely to be newly infected in light of the surge in HCV cases because of the opioid epidemic, Dr. Wester said. Persistent infection or reinfection was also highest in the 20-39 age group.
With respect to age and insurance type combined, the highest HCV clearance rate (49%) was for patients aged 60 and older who had commercial insurance; the lowest (16%) was for uninsured patients in the 20-39 age group.
The investigators evaluated people who had been diagnosed with HCV, Dr. Wester said. “It’s estimated about 40% of people in the U.S. are unaware of their infection.” Because of this, the numbers reported in the study may vastly underestimate the true picture, she told this news organization.
Barriers to treatment ‘insurmountable’ without major transformation
Increased access to diagnosis, treatment, and prevention services for persons with or at risk for acquiring hepatitis C needs to be addressed to prevent progression of disease and ongoing transmission and to achieve national hepatitis C elimination goals, the authors wrote.
The biggest barriers to improving HCV clearance are the high cost of treatment, widely varying insurance coverage, insurer restrictions, and challenges in diagnosing the disease, Dr. Wester added.
Overcoming these barriers requires implementation of universal HCV screening recommendations, including HCV RNA testing for all persons with reactive HCV antibody results, provision of treatment for all persons regardless of payer, and prevention services for persons at risk for acquiring new HCV infection, the authors concluded.
“The current barriers are insurmountable without a major transformation in our nation’s response,” Dr. Wester noted.
She expressed her support of the National Hepatitis C Elimination Program, offered as part of the Biden Administration’s 2024 budget proposal. She said that the initiative “is what we need to prevent the needless suffering from hepatitis C and to potentially save not only tens of thousands of lives but tens of billions of health care dollars.”
The three-part proposal includes a national subscription model to purchase DAA agents for those most underserved: Medicaid beneficiaries, incarcerated people, the uninsured, and American Indian and Alaska Native individuals treated through the Indian Health Service.
Under this model, the federal government would negotiate with manufacturers to buy as much treatment as needed for all individuals in the underserved groups.
What can physicians do?
Physicians can help improve HCV treatment and outcomes by being aware of the current testing guidelines, Dr. Wester said.
Guidelines now call for hepatitis C screening at least once in a lifetime for all adults, except in settings where the prevalence of HCV infection is less than 0.1%. They also call for screening during each pregnancy, with the same regional-prevalence exception.
Recommendations include curative treatment “for nearly everybody who is living with hepatitis C,” Dr. Wester added.
These CDC guidelines came out in April 2020, a time when the medical focus shifted to COVID-19, and that may have hurt awareness, she noted.
Physicians can also help by fighting back against non–evidence-based reasons insurance companies give for restricting coverage, Dr. Wester said.
Those restrictions include requiring specialists to prescribe DAA agents instead of allowing primary care physicians to do so, as well as requiring patients to have advanced liver disease or requiring patients to demonstrate sobriety or prove they are receiving counseling prior to their being eligible for treatment, Dr. Wester said.
Prior authorization a problem
Stacey B. Trooskin MD, PhD, MPH, assistant professor of medicine at the University of Pennsylvania in Philadelphia, told this news organization that prior authorization has been a major barrier for obtaining medications. Prior authorization requirements differ by state.
The paperwork must be submitted by already-stretched physician offices, and appeals are common. In that time, the window for keeping patients with HCV in the health care system may be lost, said Dr. Trooskin, chief medical adviser to the National Viral Hepatitis Roundtable.
“We know that about half of all Medicaid programs have removed prior authorization for most patients entirely,” she said, “but there are still half that require prior authorization.”
Action at the federal level is also needed, Dr. Trooskin said.
The countries that are successfully eliminating HCV and have successfully deployed the lifesaving medications provide governmental support for meeting patients where they are, she added.
Support can include inpatient and outpatient substance use disorder treatment programs or support in mental health settings, she noted.
“It’s not enough to want patients to come into their primary care provider and for that primary care provider to screen them,” Dr. Trooskin said. “This is about creating health care infrastructure so that we are finding patients at greatest risk for hepatitis C and integrating hepatitis C treatment into the services they are already accessing.”
Coauthor Harvey W. Kaufman, MD, is an employee of and owns stock in Quest Diagnostics. Coauthor William A. Meyer III, PhD, is a consultant to Quest Diagnostics. No other potential conflicts of interest were disclosed. Dr. Trooskin oversees C-Change, a hepatitis C elimination program, which receives funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Genital Ulcerations With Swelling
The Diagnosis: Mpox (Monkeypox)
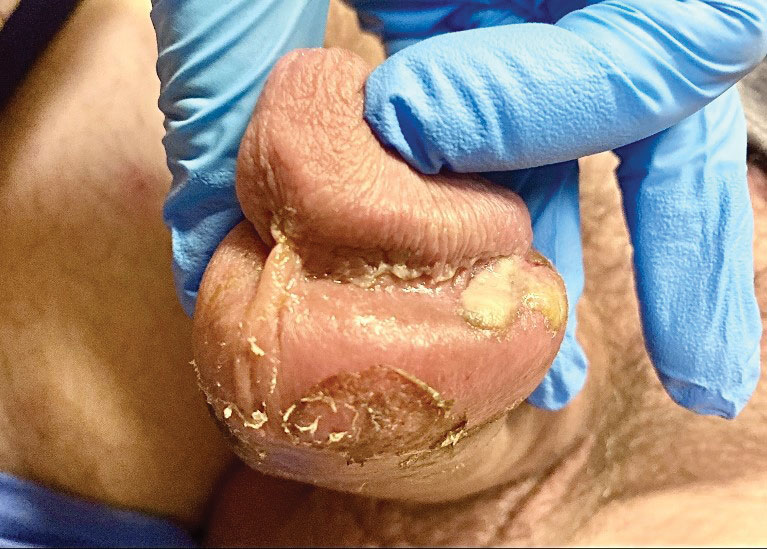
Tests for active herpes simplex virus (HHV), gonorrhea, chlamydia, HIV, and syphilis were negative. Swabs from the penile lesion demonstrated positivity for the West African clade of mpox (monkeypox) virus (MPXV) by polymerase chain reaction. The patient was treated supportively without the addition of antiviral therapy, and he experienced a complete recovery.
Mpox virus was first isolated in 1958 in a research facility and was named after the laboratory animals that were housed there. The first human documentation of the disease occurred in 1970, and it was first documented in the United States in 2003 in an infection that was traced to a shipment of small mammals from Ghana to Texas.1 The disease has always been endemic to Africa; however, the incidence has been increasing.2 A new MPXV outbreak was reported in many countries in early 2022, including the United States.1
The MPXV is a double-stranded DNA virus of the genus Orthopoxvirus, and 2 genetic clades have been identified: clade I (formerly the Central African clade) and clade II (formerly the West African clade). The virus has the capability to infect many mammals; however, its host remains unidentified.1 The exact mechanism of transmission from infected animals to humans largely is unknown; however, direct or indirect contact with infected animals likely is responsible. Human-to-human transmission can occur by many mechanisms including contact with large respiratory droplets, bodily fluids, and contaminated surfaces. The incubation period is 5 to 21 days, and the symptoms last 2 to 5 weeks.1
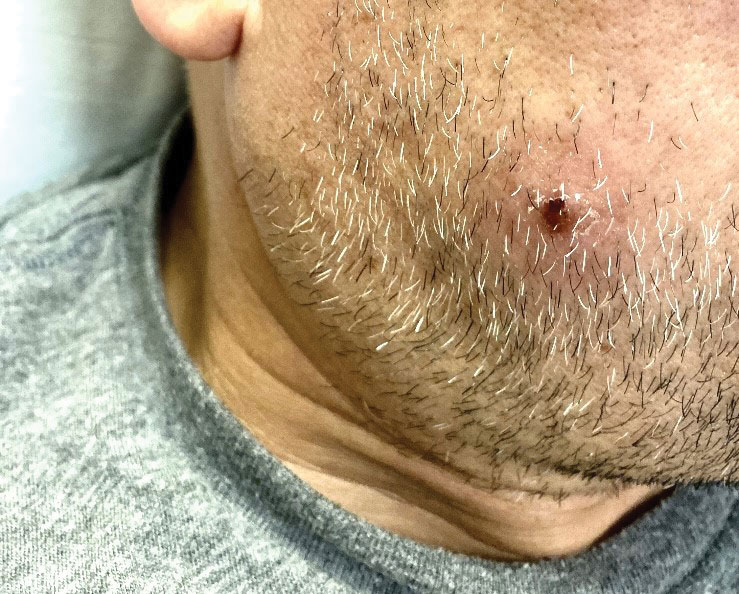
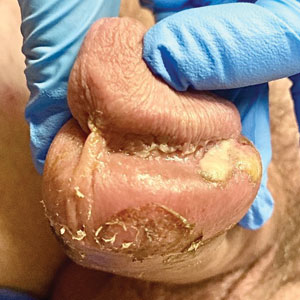
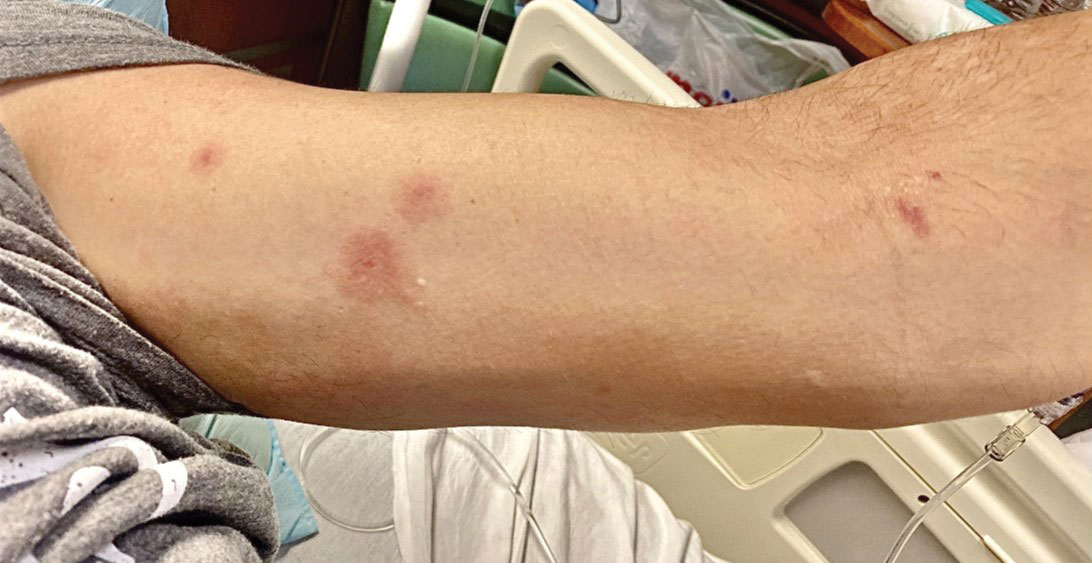
The clinical manifestations of MPXV during the most recent outbreak differ from prior outbreaks. Patients are more likely to experience minimal to no systemic symptoms, and cutaneous lesions can be few and localized to a focal area, especially on the face and in the anogenital region,3 similar to the presentation in our patient (Figure 1). Cutaneous lesions of the most recent MPXV outbreak also include painless ulcerations similar to syphilitic chancres and lesions that are in various stages of healing.3 Lesions often begin as pseudopustules, which are firm white papules with or without a necrotic center that resemble pustules; unlike true pustules, there is no identifiable purulent material within it. Bacterial superinfection of the lesions is not uncommon.4 Over time, a secondary pustular eruption resembling folliculitis also may occur,4 as noted in our patient (Figure 2).
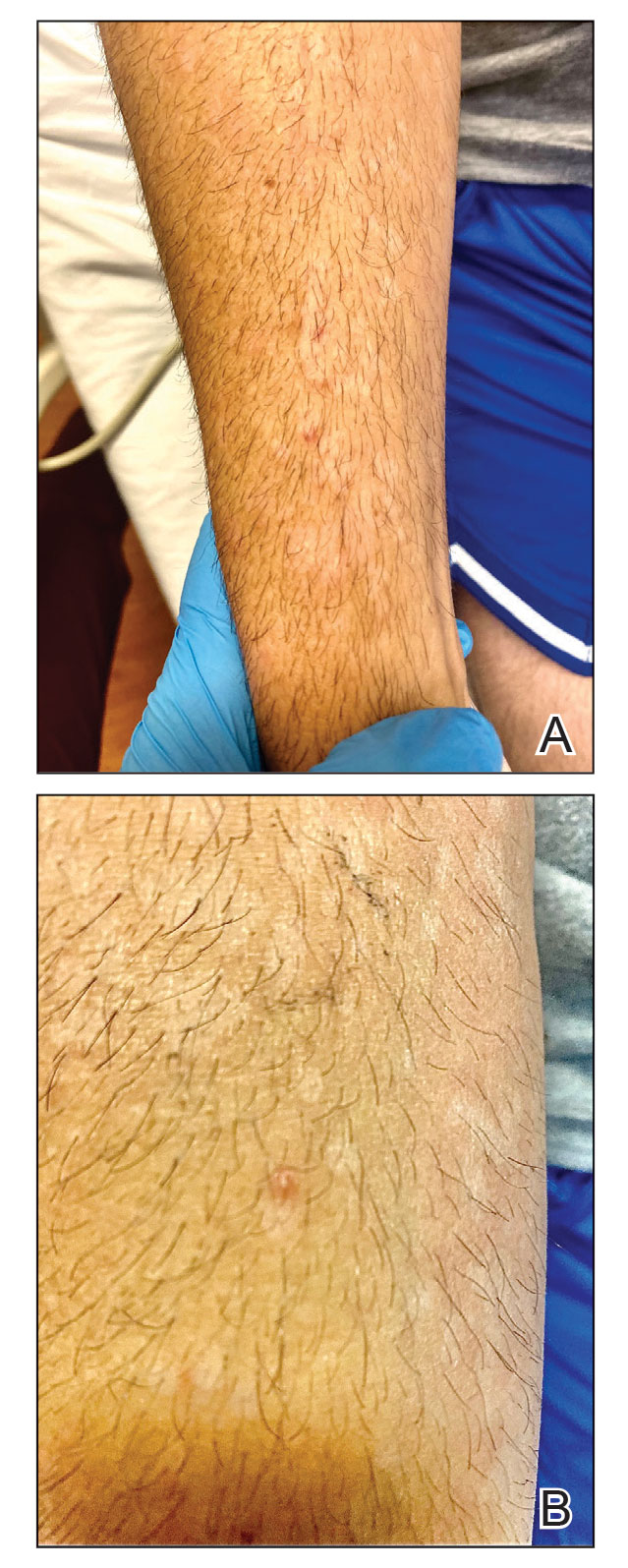
Although we did not have a biopsy to support the diagnosis of associated erythema multiforme (EM) in our patient, features supportive of this diagnosis included the classic clinical appearance of typical, well-defined, targetoid plaques with 3 distinct zones (Figure 3); the association with a known infection; the distribution on the arms with truncal sparing; and self-limited lesions. More than 90% of EM cases are associated with infection, with HHV representing the most common culprit5; therefore, the relationship with a different virus is not an unreasonable suggestion. Additionally, there have been rare reports of EM in association with MPXV.4
Histopathology of MPXV may have distinctive features. Lesions often demonstrate keratinocytic necrosis and basal layer vacuolization with an associated superficial and deep perivascular lymphohistiocytic infiltrate. When the morphology of the lesion is vesicular, histopathology reveals spongiosis and ballooning degeneration with epidermal necrosis. Viral inclusion bodies within keratinocytes may be identified.1 Death rates from MPXV has been reported from 1% to 11%, with increased mortality among high-risk populations including children and immunocompromised individuals. Treatment of the disease largely consists of supportive care and management of any associated complications including bacterial infection, pneumonia, and encephalitis.1
The differential diagnosis of MPXV includes other ulcerative lesions that can occur on the genital skin. Fixed drug eruptions often present on the penis,6 but there was no identifiable inciting drug in our patient. Herpes simplex virus infection was very high on the differential given our patient’s history of recurrent infections and association with a targetoid rash, but HHV type 1 and HHV type 2 testing of the lesion was negative. A syphilitic chancre also may present with the nontender genital ulceration7 that was seen in our patient, but serology did not support this diagnosis. Cutaneous Crohn disease also may manifest with genital ulceration even before a diagnosis of Crohn disease is made, but these lesions often present as linear knife-cut ulcerations of the anogenital region.8
Our case further supports a clinical presentation that diverges from the more traditional cases of MPXV. Additionally, associated EM may be a clue to infection, especially in cases of negative HHV and other sexually transmitted infection testing.
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? a systematic review. PLoS Negl Trop Dis. 2022;16:E0010141.
- Kumar N, Acharya A, Gendelman HE, et al. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
- Eisenstadt R, Liszewski WJ, Nguyen CV. Recognizing minimal cutaneous involvement or systemic symptoms in monkeypox. JAMA Dermatol. 2022;158:1457-1458.
- Català A, Clavo-Escribano P, Riera-Monroig J, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases [published online August 2, 2022]. Br J Dermatol. 2022;187:765-772.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Waleryie-Allanore L, Obeid G, Revuz J. Drug reactions. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:348-375.
- Stary G, Stary A. Sexually transmitted infections. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1447-1469.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1644-1663.
The Diagnosis: Mpox (Monkeypox)
Tests for active herpes simplex virus (HHV), gonorrhea, chlamydia, HIV, and syphilis were negative. Swabs from the penile lesion demonstrated positivity for the West African clade of mpox (monkeypox) virus (MPXV) by polymerase chain reaction. The patient was treated supportively without the addition of antiviral therapy, and he experienced a complete recovery.
Mpox virus was first isolated in 1958 in a research facility and was named after the laboratory animals that were housed there. The first human documentation of the disease occurred in 1970, and it was first documented in the United States in 2003 in an infection that was traced to a shipment of small mammals from Ghana to Texas.1 The disease has always been endemic to Africa; however, the incidence has been increasing.2 A new MPXV outbreak was reported in many countries in early 2022, including the United States.1
The MPXV is a double-stranded DNA virus of the genus Orthopoxvirus, and 2 genetic clades have been identified: clade I (formerly the Central African clade) and clade II (formerly the West African clade). The virus has the capability to infect many mammals; however, its host remains unidentified.1 The exact mechanism of transmission from infected animals to humans largely is unknown; however, direct or indirect contact with infected animals likely is responsible. Human-to-human transmission can occur by many mechanisms including contact with large respiratory droplets, bodily fluids, and contaminated surfaces. The incubation period is 5 to 21 days, and the symptoms last 2 to 5 weeks.1

The clinical manifestations of MPXV during the most recent outbreak differ from prior outbreaks. Patients are more likely to experience minimal to no systemic symptoms, and cutaneous lesions can be few and localized to a focal area, especially on the face and in the anogenital region,3 similar to the presentation in our patient (Figure 1). Cutaneous lesions of the most recent MPXV outbreak also include painless ulcerations similar to syphilitic chancres and lesions that are in various stages of healing.3 Lesions often begin as pseudopustules, which are firm white papules with or without a necrotic center that resemble pustules; unlike true pustules, there is no identifiable purulent material within it. Bacterial superinfection of the lesions is not uncommon.4 Over time, a secondary pustular eruption resembling folliculitis also may occur,4 as noted in our patient (Figure 2).

Although we did not have a biopsy to support the diagnosis of associated erythema multiforme (EM) in our patient, features supportive of this diagnosis included the classic clinical appearance of typical, well-defined, targetoid plaques with 3 distinct zones (Figure 3); the association with a known infection; the distribution on the arms with truncal sparing; and self-limited lesions. More than 90% of EM cases are associated with infection, with HHV representing the most common culprit5; therefore, the relationship with a different virus is not an unreasonable suggestion. Additionally, there have been rare reports of EM in association with MPXV.4

Histopathology of MPXV may have distinctive features. Lesions often demonstrate keratinocytic necrosis and basal layer vacuolization with an associated superficial and deep perivascular lymphohistiocytic infiltrate. When the morphology of the lesion is vesicular, histopathology reveals spongiosis and ballooning degeneration with epidermal necrosis. Viral inclusion bodies within keratinocytes may be identified.1 Death rates from MPXV has been reported from 1% to 11%, with increased mortality among high-risk populations including children and immunocompromised individuals. Treatment of the disease largely consists of supportive care and management of any associated complications including bacterial infection, pneumonia, and encephalitis.1
The differential diagnosis of MPXV includes other ulcerative lesions that can occur on the genital skin. Fixed drug eruptions often present on the penis,6 but there was no identifiable inciting drug in our patient. Herpes simplex virus infection was very high on the differential given our patient’s history of recurrent infections and association with a targetoid rash, but HHV type 1 and HHV type 2 testing of the lesion was negative. A syphilitic chancre also may present with the nontender genital ulceration7 that was seen in our patient, but serology did not support this diagnosis. Cutaneous Crohn disease also may manifest with genital ulceration even before a diagnosis of Crohn disease is made, but these lesions often present as linear knife-cut ulcerations of the anogenital region.8
Our case further supports a clinical presentation that diverges from the more traditional cases of MPXV. Additionally, associated EM may be a clue to infection, especially in cases of negative HHV and other sexually transmitted infection testing.
The Diagnosis: Mpox (Monkeypox)
Tests for active herpes simplex virus (HHV), gonorrhea, chlamydia, HIV, and syphilis were negative. Swabs from the penile lesion demonstrated positivity for the West African clade of mpox (monkeypox) virus (MPXV) by polymerase chain reaction. The patient was treated supportively without the addition of antiviral therapy, and he experienced a complete recovery.
Mpox virus was first isolated in 1958 in a research facility and was named after the laboratory animals that were housed there. The first human documentation of the disease occurred in 1970, and it was first documented in the United States in 2003 in an infection that was traced to a shipment of small mammals from Ghana to Texas.1 The disease has always been endemic to Africa; however, the incidence has been increasing.2 A new MPXV outbreak was reported in many countries in early 2022, including the United States.1
The MPXV is a double-stranded DNA virus of the genus Orthopoxvirus, and 2 genetic clades have been identified: clade I (formerly the Central African clade) and clade II (formerly the West African clade). The virus has the capability to infect many mammals; however, its host remains unidentified.1 The exact mechanism of transmission from infected animals to humans largely is unknown; however, direct or indirect contact with infected animals likely is responsible. Human-to-human transmission can occur by many mechanisms including contact with large respiratory droplets, bodily fluids, and contaminated surfaces. The incubation period is 5 to 21 days, and the symptoms last 2 to 5 weeks.1

The clinical manifestations of MPXV during the most recent outbreak differ from prior outbreaks. Patients are more likely to experience minimal to no systemic symptoms, and cutaneous lesions can be few and localized to a focal area, especially on the face and in the anogenital region,3 similar to the presentation in our patient (Figure 1). Cutaneous lesions of the most recent MPXV outbreak also include painless ulcerations similar to syphilitic chancres and lesions that are in various stages of healing.3 Lesions often begin as pseudopustules, which are firm white papules with or without a necrotic center that resemble pustules; unlike true pustules, there is no identifiable purulent material within it. Bacterial superinfection of the lesions is not uncommon.4 Over time, a secondary pustular eruption resembling folliculitis also may occur,4 as noted in our patient (Figure 2).

Although we did not have a biopsy to support the diagnosis of associated erythema multiforme (EM) in our patient, features supportive of this diagnosis included the classic clinical appearance of typical, well-defined, targetoid plaques with 3 distinct zones (Figure 3); the association with a known infection; the distribution on the arms with truncal sparing; and self-limited lesions. More than 90% of EM cases are associated with infection, with HHV representing the most common culprit5; therefore, the relationship with a different virus is not an unreasonable suggestion. Additionally, there have been rare reports of EM in association with MPXV.4

Histopathology of MPXV may have distinctive features. Lesions often demonstrate keratinocytic necrosis and basal layer vacuolization with an associated superficial and deep perivascular lymphohistiocytic infiltrate. When the morphology of the lesion is vesicular, histopathology reveals spongiosis and ballooning degeneration with epidermal necrosis. Viral inclusion bodies within keratinocytes may be identified.1 Death rates from MPXV has been reported from 1% to 11%, with increased mortality among high-risk populations including children and immunocompromised individuals. Treatment of the disease largely consists of supportive care and management of any associated complications including bacterial infection, pneumonia, and encephalitis.1
The differential diagnosis of MPXV includes other ulcerative lesions that can occur on the genital skin. Fixed drug eruptions often present on the penis,6 but there was no identifiable inciting drug in our patient. Herpes simplex virus infection was very high on the differential given our patient’s history of recurrent infections and association with a targetoid rash, but HHV type 1 and HHV type 2 testing of the lesion was negative. A syphilitic chancre also may present with the nontender genital ulceration7 that was seen in our patient, but serology did not support this diagnosis. Cutaneous Crohn disease also may manifest with genital ulceration even before a diagnosis of Crohn disease is made, but these lesions often present as linear knife-cut ulcerations of the anogenital region.8
Our case further supports a clinical presentation that diverges from the more traditional cases of MPXV. Additionally, associated EM may be a clue to infection, especially in cases of negative HHV and other sexually transmitted infection testing.
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? a systematic review. PLoS Negl Trop Dis. 2022;16:E0010141.
- Kumar N, Acharya A, Gendelman HE, et al. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
- Eisenstadt R, Liszewski WJ, Nguyen CV. Recognizing minimal cutaneous involvement or systemic symptoms in monkeypox. JAMA Dermatol. 2022;158:1457-1458.
- Català A, Clavo-Escribano P, Riera-Monroig J, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases [published online August 2, 2022]. Br J Dermatol. 2022;187:765-772.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Waleryie-Allanore L, Obeid G, Revuz J. Drug reactions. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:348-375.
- Stary G, Stary A. Sexually transmitted infections. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1447-1469.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1644-1663.
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? a systematic review. PLoS Negl Trop Dis. 2022;16:E0010141.
- Kumar N, Acharya A, Gendelman HE, et al. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
- Eisenstadt R, Liszewski WJ, Nguyen CV. Recognizing minimal cutaneous involvement or systemic symptoms in monkeypox. JAMA Dermatol. 2022;158:1457-1458.
- Català A, Clavo-Escribano P, Riera-Monroig J, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases [published online August 2, 2022]. Br J Dermatol. 2022;187:765-772.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Waleryie-Allanore L, Obeid G, Revuz J. Drug reactions. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:348-375.
- Stary G, Stary A. Sexually transmitted infections. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1447-1469.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1644-1663.
A 50-year-old man with a history of recurrent genital herpes simplex virus infections presented to the hospital with genital lesions and swelling of 5 days’ duration. Prior to admission, the patient was treated with a course of valacyclovir by an urgent care physician without improvement. Physical examination revealed a 3-cm, nontender, shallow, ulcerative plaque with irregular borders and a purulent yellow base distributed on the distal shaft of the penis with extension into the coronal sulcus. A few other scattered erosions were noted on the distal penile shaft. He had associated diffuse nonpitting edema of the penis and scrotum as well as tender bilateral inguinal lymphadenopathy. Three days after the genital ulcerations began, the patient developed a nontender erythematous papule with a necrotic center on the right jaw followed by an eruption of erythematous papulopustules on the arms and trunk. The patient denied dysuria, purulent penile discharge, fevers, chills, headaches, myalgia, arthralgia, nausea, vomiting, or diarrhea. The patient was sexually active exclusively with females and had more than 10 partners in the prior year. Shortly after hospital admission, the patient developed red targetoid plaques on the groin, trunk, and arms. No oral mucosal lesions were identified.
Nearly one in five in U.S. still hadn’t gotten COVID by end of 2022
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
Finding the optimal fluid strategies for sepsis
The document offers guidance on the four forms of fluid use; assessing whether intravenous fluid administration is indicated; and fluid therapy goals, timing, type, and other clinical parameters. The recommendations are based on a literature search that included 28 randomized clinical trials, 7 secondary analyses of RCTs, 20 observational studies, 5 systematic reviews or meta-analyses, 1 scoping review, 1 practice guideline, and 14 references from a reference review.
“Our review highlights that crystalloids should remain the standard of care for most critically ill patients, especially during early resuscitation,” Fernando G. Zampieri, MD, PhD, assistant adjunct professor of critical care medicine at the University of Alberta and Alberta Health Services, both in Edmonton, said in an interview. “In particular, starches should not be used in critically ill patients. Balanced solutions might be better for most patients, except for patients with traumatic brain injury, where 0.9% saline is recommended.”
The review was published online in JAMA.
Four therapeutic phases
Approximately 20%-30% of patients admitted to an intensive care unit have sepsis, and fluid therapy is a key component of their treatment. Although intravenous fluid can increase cardiac output and blood pressure, maintain or increase intravascular fluid volume, and deliver medications, too much fluid or the wrong type of fluid may cause harm.
“Deciding which type of fluid is the best for a patient [with sepsis] can be challenging,” said Dr. Zampieri.
Fluid therapy can be conceptualized as encompassing four overlapping phases from early illness through resolution of sepsis, according to the review. These phases include resuscitation (rapidly administering fluid to restore perfusion), optimization (assessing risks and benefits of additional fluids to treat shock and ensure organ perfusion), stabilization (using fluid therapy only when there is a signal of fluid responsiveness), and evacuation (eliminating excess fluid accumulated during treatment).
The review described the studies that underpin its key recommendations for management in these phases. Three RCTs included 3,723 patients with sepsis who received 1-2 L of fluid. They found that goal-directed therapy with administration of fluid boluses to attain a central venous pressure of 8-12 mm Hg, vasopressors to attain a mean arterial blood pressure of 65-90 mm Hg, and red blood cell transfusions or inotropes to attain a central venous oxygen saturation of at least 70% did not decrease mortality, compared with unstructured clinical care (24.9% vs. 25.4%, P = .68).
One RCT with 1,563 patients with sepsis and hypotension who received 1 L of fluid found that favoring vasopressor treatment did not improve mortality, compared with further fluid administration (14.0% vs. 14.9%, P = .61).
In another RCT, among 1,554 patients with septic shock who were treated in the ICU with at least 1 L of fluid, restricting fluid administration in the absence of severe hypoperfusion did not reduce mortality, compared with more liberal fluid administration (42.3% vs. 42.1%, P = .96).
An RCT of 1,000 patients with acute respiratory distress during the evacuation phase found that limiting fluid administration and giving diuretics improved the number of days alive without mechanical ventilation, compared with fluid treatment to attain higher intracardiac pressure (14.6 vs. 12.1 days, P < .001).
This study also found that hydroxyethyl starch significantly increased the incidence of kidney replacement therapy, compared with saline (7.0% vs. 5.8%, P = .04), Ringer lactate, or Ringer acetate.
Ultrasonography lacks validation
The authors summarized the key concerns about fluid therapy. Fluid therapy should be initiated for patients with evidence of sepsis-induced hypoperfusion who are likely to have increased cardiac output with fluid administration. Fluid administration should be discontinued when evidence of hypoperfusion resolves, the patient no longer responds to fluid, or the patient shows evidence of fluid overload.
Balanced solutions should be selected over 0.9% saline for fluid therapy, according to the review. Hydroxyethyl starches should not be used.
Fluid removal should be considered after the resuscitation and optimization phases and when a patient has stabilized, the authors wrote. Diuretics are first-line therapy to facilitate fluid elimination.
Kidney replacement therapy may be considered for patients with severe acute kidney injury who have complications from fluid overload and are unresponsive to diuretic therapy.
“The use of ultrasonography as a bedside tool to guide fluid resuscitation is promising but lacks validation in robust randomized controlled trials,” said Dr. Zampieri. “Point-of-care ultrasound may be useful to assess causes of shock and [helping to exclude] a life-threatening diagnosis at presentation, such as cardiac tamponade.”
Pending the emergence of further evidence, the authors suggest that clinicians prescribe fluids judiciously, preferably at aliquots followed by frequent reassessment. “Defining a resuscitation target (such as capillary refill time or lactate, among others) and performing fluid challenges to correct them while no overt signs of fluid overload (such as pulmonary edema) occur is a common practice that is also sustained by clinical research,” said Dr. Zampieri.
He added that the review’s recommendations are based on research conducted mainly in high-income settings, and that generalizability will depend on factors such as local standards of care and resource availability.
“Our review provides an overall guidance, but caution is warranted before extrapolating the suggestion to every possible clinical scenario,” he concluded.
Fluids as drugs
Commenting on the review, Hernando Gomez, MD, MPH, an associate professor of critical care medicine at the University of Pittsburgh, said: “I agree with the conclusions and commend the authors for this very practical revision of the literature.” Dr. Gomez was not involved in the review.
“I would like to stress the point, however, that although fluids can be harmful, particularly when not indicated and when used in excess, fluid resuscitation in patients with sepsis who have evidence of hypoperfusion is paramount,” he said.
“The association between fluid accumulation and poor outcomes is truly a Goldilocks problem, often described in the literature as a ‘U’ shape, where too little fluid (i.e., a very restrictive strategy) or too much fluid (i.e., use in excess and in discordance with the patient’s needs) can be harmful,” said Dr. Gomez.
Furthermore, every strategy to assess fluid responsiveness has limitations. “It is key that clinicians resist the temptation to dismiss these limitations, because decisions made on flawed data are as dangerous as not assessing fluid responsiveness in the first place,” he said.
Based on the evidence, clinicians should “think of fluids as a drug and carefully assess risks and benefits before deciding to administer fluids to their patients,” Dr. Gomez added. It is also important to separate the question “Does my patient need fluids?” from the question “Is my patient fluid responsive?”
“These are two different questions that often get conflated,” Dr. Gomez said. “If a bolus of fluid given to a patient who needs fluids and is fluid-responsive does not improve tissue perfusion, then fluids should not be given.”
No funding was reported for the review. Dr. Zampieri reported receiving fluids and logistics from Baxter Hospitalar during the conduct of the BaSICS trial, personal fees from Bactiguard for statistical consulting and from Baxter for participating in an advisory board, grants from Ionis Pharmaceuticals outside the submitted work, and serving as lead investigator of the BaSICS trial. Dr. Gomez reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The document offers guidance on the four forms of fluid use; assessing whether intravenous fluid administration is indicated; and fluid therapy goals, timing, type, and other clinical parameters. The recommendations are based on a literature search that included 28 randomized clinical trials, 7 secondary analyses of RCTs, 20 observational studies, 5 systematic reviews or meta-analyses, 1 scoping review, 1 practice guideline, and 14 references from a reference review.
“Our review highlights that crystalloids should remain the standard of care for most critically ill patients, especially during early resuscitation,” Fernando G. Zampieri, MD, PhD, assistant adjunct professor of critical care medicine at the University of Alberta and Alberta Health Services, both in Edmonton, said in an interview. “In particular, starches should not be used in critically ill patients. Balanced solutions might be better for most patients, except for patients with traumatic brain injury, where 0.9% saline is recommended.”
The review was published online in JAMA.
Four therapeutic phases
Approximately 20%-30% of patients admitted to an intensive care unit have sepsis, and fluid therapy is a key component of their treatment. Although intravenous fluid can increase cardiac output and blood pressure, maintain or increase intravascular fluid volume, and deliver medications, too much fluid or the wrong type of fluid may cause harm.
“Deciding which type of fluid is the best for a patient [with sepsis] can be challenging,” said Dr. Zampieri.
Fluid therapy can be conceptualized as encompassing four overlapping phases from early illness through resolution of sepsis, according to the review. These phases include resuscitation (rapidly administering fluid to restore perfusion), optimization (assessing risks and benefits of additional fluids to treat shock and ensure organ perfusion), stabilization (using fluid therapy only when there is a signal of fluid responsiveness), and evacuation (eliminating excess fluid accumulated during treatment).
The review described the studies that underpin its key recommendations for management in these phases. Three RCTs included 3,723 patients with sepsis who received 1-2 L of fluid. They found that goal-directed therapy with administration of fluid boluses to attain a central venous pressure of 8-12 mm Hg, vasopressors to attain a mean arterial blood pressure of 65-90 mm Hg, and red blood cell transfusions or inotropes to attain a central venous oxygen saturation of at least 70% did not decrease mortality, compared with unstructured clinical care (24.9% vs. 25.4%, P = .68).
One RCT with 1,563 patients with sepsis and hypotension who received 1 L of fluid found that favoring vasopressor treatment did not improve mortality, compared with further fluid administration (14.0% vs. 14.9%, P = .61).
In another RCT, among 1,554 patients with septic shock who were treated in the ICU with at least 1 L of fluid, restricting fluid administration in the absence of severe hypoperfusion did not reduce mortality, compared with more liberal fluid administration (42.3% vs. 42.1%, P = .96).
An RCT of 1,000 patients with acute respiratory distress during the evacuation phase found that limiting fluid administration and giving diuretics improved the number of days alive without mechanical ventilation, compared with fluid treatment to attain higher intracardiac pressure (14.6 vs. 12.1 days, P < .001).
This study also found that hydroxyethyl starch significantly increased the incidence of kidney replacement therapy, compared with saline (7.0% vs. 5.8%, P = .04), Ringer lactate, or Ringer acetate.
Ultrasonography lacks validation
The authors summarized the key concerns about fluid therapy. Fluid therapy should be initiated for patients with evidence of sepsis-induced hypoperfusion who are likely to have increased cardiac output with fluid administration. Fluid administration should be discontinued when evidence of hypoperfusion resolves, the patient no longer responds to fluid, or the patient shows evidence of fluid overload.
Balanced solutions should be selected over 0.9% saline for fluid therapy, according to the review. Hydroxyethyl starches should not be used.
Fluid removal should be considered after the resuscitation and optimization phases and when a patient has stabilized, the authors wrote. Diuretics are first-line therapy to facilitate fluid elimination.
Kidney replacement therapy may be considered for patients with severe acute kidney injury who have complications from fluid overload and are unresponsive to diuretic therapy.
“The use of ultrasonography as a bedside tool to guide fluid resuscitation is promising but lacks validation in robust randomized controlled trials,” said Dr. Zampieri. “Point-of-care ultrasound may be useful to assess causes of shock and [helping to exclude] a life-threatening diagnosis at presentation, such as cardiac tamponade.”
Pending the emergence of further evidence, the authors suggest that clinicians prescribe fluids judiciously, preferably at aliquots followed by frequent reassessment. “Defining a resuscitation target (such as capillary refill time or lactate, among others) and performing fluid challenges to correct them while no overt signs of fluid overload (such as pulmonary edema) occur is a common practice that is also sustained by clinical research,” said Dr. Zampieri.
He added that the review’s recommendations are based on research conducted mainly in high-income settings, and that generalizability will depend on factors such as local standards of care and resource availability.
“Our review provides an overall guidance, but caution is warranted before extrapolating the suggestion to every possible clinical scenario,” he concluded.
Fluids as drugs
Commenting on the review, Hernando Gomez, MD, MPH, an associate professor of critical care medicine at the University of Pittsburgh, said: “I agree with the conclusions and commend the authors for this very practical revision of the literature.” Dr. Gomez was not involved in the review.
“I would like to stress the point, however, that although fluids can be harmful, particularly when not indicated and when used in excess, fluid resuscitation in patients with sepsis who have evidence of hypoperfusion is paramount,” he said.
“The association between fluid accumulation and poor outcomes is truly a Goldilocks problem, often described in the literature as a ‘U’ shape, where too little fluid (i.e., a very restrictive strategy) or too much fluid (i.e., use in excess and in discordance with the patient’s needs) can be harmful,” said Dr. Gomez.
Furthermore, every strategy to assess fluid responsiveness has limitations. “It is key that clinicians resist the temptation to dismiss these limitations, because decisions made on flawed data are as dangerous as not assessing fluid responsiveness in the first place,” he said.
Based on the evidence, clinicians should “think of fluids as a drug and carefully assess risks and benefits before deciding to administer fluids to their patients,” Dr. Gomez added. It is also important to separate the question “Does my patient need fluids?” from the question “Is my patient fluid responsive?”
“These are two different questions that often get conflated,” Dr. Gomez said. “If a bolus of fluid given to a patient who needs fluids and is fluid-responsive does not improve tissue perfusion, then fluids should not be given.”
No funding was reported for the review. Dr. Zampieri reported receiving fluids and logistics from Baxter Hospitalar during the conduct of the BaSICS trial, personal fees from Bactiguard for statistical consulting and from Baxter for participating in an advisory board, grants from Ionis Pharmaceuticals outside the submitted work, and serving as lead investigator of the BaSICS trial. Dr. Gomez reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The document offers guidance on the four forms of fluid use; assessing whether intravenous fluid administration is indicated; and fluid therapy goals, timing, type, and other clinical parameters. The recommendations are based on a literature search that included 28 randomized clinical trials, 7 secondary analyses of RCTs, 20 observational studies, 5 systematic reviews or meta-analyses, 1 scoping review, 1 practice guideline, and 14 references from a reference review.
“Our review highlights that crystalloids should remain the standard of care for most critically ill patients, especially during early resuscitation,” Fernando G. Zampieri, MD, PhD, assistant adjunct professor of critical care medicine at the University of Alberta and Alberta Health Services, both in Edmonton, said in an interview. “In particular, starches should not be used in critically ill patients. Balanced solutions might be better for most patients, except for patients with traumatic brain injury, where 0.9% saline is recommended.”
The review was published online in JAMA.
Four therapeutic phases
Approximately 20%-30% of patients admitted to an intensive care unit have sepsis, and fluid therapy is a key component of their treatment. Although intravenous fluid can increase cardiac output and blood pressure, maintain or increase intravascular fluid volume, and deliver medications, too much fluid or the wrong type of fluid may cause harm.
“Deciding which type of fluid is the best for a patient [with sepsis] can be challenging,” said Dr. Zampieri.
Fluid therapy can be conceptualized as encompassing four overlapping phases from early illness through resolution of sepsis, according to the review. These phases include resuscitation (rapidly administering fluid to restore perfusion), optimization (assessing risks and benefits of additional fluids to treat shock and ensure organ perfusion), stabilization (using fluid therapy only when there is a signal of fluid responsiveness), and evacuation (eliminating excess fluid accumulated during treatment).
The review described the studies that underpin its key recommendations for management in these phases. Three RCTs included 3,723 patients with sepsis who received 1-2 L of fluid. They found that goal-directed therapy with administration of fluid boluses to attain a central venous pressure of 8-12 mm Hg, vasopressors to attain a mean arterial blood pressure of 65-90 mm Hg, and red blood cell transfusions or inotropes to attain a central venous oxygen saturation of at least 70% did not decrease mortality, compared with unstructured clinical care (24.9% vs. 25.4%, P = .68).
One RCT with 1,563 patients with sepsis and hypotension who received 1 L of fluid found that favoring vasopressor treatment did not improve mortality, compared with further fluid administration (14.0% vs. 14.9%, P = .61).
In another RCT, among 1,554 patients with septic shock who were treated in the ICU with at least 1 L of fluid, restricting fluid administration in the absence of severe hypoperfusion did not reduce mortality, compared with more liberal fluid administration (42.3% vs. 42.1%, P = .96).
An RCT of 1,000 patients with acute respiratory distress during the evacuation phase found that limiting fluid administration and giving diuretics improved the number of days alive without mechanical ventilation, compared with fluid treatment to attain higher intracardiac pressure (14.6 vs. 12.1 days, P < .001).
This study also found that hydroxyethyl starch significantly increased the incidence of kidney replacement therapy, compared with saline (7.0% vs. 5.8%, P = .04), Ringer lactate, or Ringer acetate.
Ultrasonography lacks validation
The authors summarized the key concerns about fluid therapy. Fluid therapy should be initiated for patients with evidence of sepsis-induced hypoperfusion who are likely to have increased cardiac output with fluid administration. Fluid administration should be discontinued when evidence of hypoperfusion resolves, the patient no longer responds to fluid, or the patient shows evidence of fluid overload.
Balanced solutions should be selected over 0.9% saline for fluid therapy, according to the review. Hydroxyethyl starches should not be used.
Fluid removal should be considered after the resuscitation and optimization phases and when a patient has stabilized, the authors wrote. Diuretics are first-line therapy to facilitate fluid elimination.
Kidney replacement therapy may be considered for patients with severe acute kidney injury who have complications from fluid overload and are unresponsive to diuretic therapy.
“The use of ultrasonography as a bedside tool to guide fluid resuscitation is promising but lacks validation in robust randomized controlled trials,” said Dr. Zampieri. “Point-of-care ultrasound may be useful to assess causes of shock and [helping to exclude] a life-threatening diagnosis at presentation, such as cardiac tamponade.”
Pending the emergence of further evidence, the authors suggest that clinicians prescribe fluids judiciously, preferably at aliquots followed by frequent reassessment. “Defining a resuscitation target (such as capillary refill time or lactate, among others) and performing fluid challenges to correct them while no overt signs of fluid overload (such as pulmonary edema) occur is a common practice that is also sustained by clinical research,” said Dr. Zampieri.
He added that the review’s recommendations are based on research conducted mainly in high-income settings, and that generalizability will depend on factors such as local standards of care and resource availability.
“Our review provides an overall guidance, but caution is warranted before extrapolating the suggestion to every possible clinical scenario,” he concluded.
Fluids as drugs
Commenting on the review, Hernando Gomez, MD, MPH, an associate professor of critical care medicine at the University of Pittsburgh, said: “I agree with the conclusions and commend the authors for this very practical revision of the literature.” Dr. Gomez was not involved in the review.
“I would like to stress the point, however, that although fluids can be harmful, particularly when not indicated and when used in excess, fluid resuscitation in patients with sepsis who have evidence of hypoperfusion is paramount,” he said.
“The association between fluid accumulation and poor outcomes is truly a Goldilocks problem, often described in the literature as a ‘U’ shape, where too little fluid (i.e., a very restrictive strategy) or too much fluid (i.e., use in excess and in discordance with the patient’s needs) can be harmful,” said Dr. Gomez.
Furthermore, every strategy to assess fluid responsiveness has limitations. “It is key that clinicians resist the temptation to dismiss these limitations, because decisions made on flawed data are as dangerous as not assessing fluid responsiveness in the first place,” he said.
Based on the evidence, clinicians should “think of fluids as a drug and carefully assess risks and benefits before deciding to administer fluids to their patients,” Dr. Gomez added. It is also important to separate the question “Does my patient need fluids?” from the question “Is my patient fluid responsive?”
“These are two different questions that often get conflated,” Dr. Gomez said. “If a bolus of fluid given to a patient who needs fluids and is fluid-responsive does not improve tissue perfusion, then fluids should not be given.”
No funding was reported for the review. Dr. Zampieri reported receiving fluids and logistics from Baxter Hospitalar during the conduct of the BaSICS trial, personal fees from Bactiguard for statistical consulting and from Baxter for participating in an advisory board, grants from Ionis Pharmaceuticals outside the submitted work, and serving as lead investigator of the BaSICS trial. Dr. Gomez reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Ticks use static electricity to latch onto hosts: Study
It turns out that some people really are tick magnets.
Researchers have discovered that ticks can defy gravity in their quest to latch onto people and animals. The key is static electricity, just like when someone rubs a balloon and things stick to it.
The study was published in the journal Current Biology. In the first phase of the research, scientists exposed ticks to furry rabbit feet and to acrylic surfaces that each had electrostatic charges.
“Ticks were readily attracted across air gaps of up to several millimeters or centimeters onto these statically charged surfaces,” the authors wrote.
In a second part of the study, the researchers created computer models simulating the electrostatic charges that exist in environments where both ticks and mammals are found. In one simulation, the researchers observed that the body parts of a cow with the most electric charge were the nose, tail, and legs, which are the body parts most likely to be encountered by a tick. They also found that the vegetation near the animal had a strong electric field that is just a few millimeters wide.
In a final phase of the study, the researchers conducted laboratory experiments in which they re-created the electric field conditions from the computer model and successfully lifted some ticks across an air gap, although some ticks did not make the full leap if they were observed to be resisting.
The authors noted that their findings could be applied to developing new tick prevention strategies, such as designing clothing that resists electrostatic charges or spraying livestock.
The study authors reported that they had no conflicts of interest.
A version of this article first appeared on WebMD.com.
It turns out that some people really are tick magnets.
Researchers have discovered that ticks can defy gravity in their quest to latch onto people and animals. The key is static electricity, just like when someone rubs a balloon and things stick to it.
The study was published in the journal Current Biology. In the first phase of the research, scientists exposed ticks to furry rabbit feet and to acrylic surfaces that each had electrostatic charges.
“Ticks were readily attracted across air gaps of up to several millimeters or centimeters onto these statically charged surfaces,” the authors wrote.
In a second part of the study, the researchers created computer models simulating the electrostatic charges that exist in environments where both ticks and mammals are found. In one simulation, the researchers observed that the body parts of a cow with the most electric charge were the nose, tail, and legs, which are the body parts most likely to be encountered by a tick. They also found that the vegetation near the animal had a strong electric field that is just a few millimeters wide.
In a final phase of the study, the researchers conducted laboratory experiments in which they re-created the electric field conditions from the computer model and successfully lifted some ticks across an air gap, although some ticks did not make the full leap if they were observed to be resisting.
The authors noted that their findings could be applied to developing new tick prevention strategies, such as designing clothing that resists electrostatic charges or spraying livestock.
The study authors reported that they had no conflicts of interest.
A version of this article first appeared on WebMD.com.
It turns out that some people really are tick magnets.
Researchers have discovered that ticks can defy gravity in their quest to latch onto people and animals. The key is static electricity, just like when someone rubs a balloon and things stick to it.
The study was published in the journal Current Biology. In the first phase of the research, scientists exposed ticks to furry rabbit feet and to acrylic surfaces that each had electrostatic charges.
“Ticks were readily attracted across air gaps of up to several millimeters or centimeters onto these statically charged surfaces,” the authors wrote.
In a second part of the study, the researchers created computer models simulating the electrostatic charges that exist in environments where both ticks and mammals are found. In one simulation, the researchers observed that the body parts of a cow with the most electric charge were the nose, tail, and legs, which are the body parts most likely to be encountered by a tick. They also found that the vegetation near the animal had a strong electric field that is just a few millimeters wide.
In a final phase of the study, the researchers conducted laboratory experiments in which they re-created the electric field conditions from the computer model and successfully lifted some ticks across an air gap, although some ticks did not make the full leap if they were observed to be resisting.
The authors noted that their findings could be applied to developing new tick prevention strategies, such as designing clothing that resists electrostatic charges or spraying livestock.
The study authors reported that they had no conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM CURRENT BIOLOGY
Malaria is spreading in the U.S. for the first time in 20 years
, the Centers for Disease Control and Prevention says.
The federal health agency recently issued a nationwide warning to health providers and officials to be on the lookout for symptoms of the potentially fatal illness. Usually, people in the U.S. who get malaria get the disease during international travel.
All five people – four in Florida and one in Texas – have received treatment and are improving, according to the CDC. The case in Texas is not related to the Florida cases, and all occurred in the past 2 months.
Malaria cannot be transmitted from person to person. It is spread by the bite of an infected female mosquito. The last cases of people being infected while in the U.S. occurred 20 years ago, when there were eight cases in Palm Beach County, Fla. The Texas Department of State Health Services said the last time malaria was locally acquired in the state was 1994.
The Florida Department of Health said it was spraying for mosquitoes in the two counties surrounding Sarasota, Fla., where the four cases occurred.
The CDC said the risk of getting malaria while in the United States “remains extremely low.” The agency advised people to protect themselves by taking precautions to prevent mosquito bites, such as wearing insect repellent and wearing long-sleeved shirts and pants. People should also do things to ensure that mosquitoes aren’t around their home, such as getting rid of standing water, which is an environment for mosquitoes to lay eggs.
More than 240 million malaria cases occur annually worldwide, the CDC said, with 95% in Africa. There are 2,000 cases diagnosed annually in the U.S. that are related to international travel. Malaria symptoms are similar to those of other illnesses and include fever, chills, a headache, and muscle aches. If not treated, malaria can be fatal.
A version of this article first appeared on WebMD.com.
, the Centers for Disease Control and Prevention says.
The federal health agency recently issued a nationwide warning to health providers and officials to be on the lookout for symptoms of the potentially fatal illness. Usually, people in the U.S. who get malaria get the disease during international travel.
All five people – four in Florida and one in Texas – have received treatment and are improving, according to the CDC. The case in Texas is not related to the Florida cases, and all occurred in the past 2 months.
Malaria cannot be transmitted from person to person. It is spread by the bite of an infected female mosquito. The last cases of people being infected while in the U.S. occurred 20 years ago, when there were eight cases in Palm Beach County, Fla. The Texas Department of State Health Services said the last time malaria was locally acquired in the state was 1994.
The Florida Department of Health said it was spraying for mosquitoes in the two counties surrounding Sarasota, Fla., where the four cases occurred.
The CDC said the risk of getting malaria while in the United States “remains extremely low.” The agency advised people to protect themselves by taking precautions to prevent mosquito bites, such as wearing insect repellent and wearing long-sleeved shirts and pants. People should also do things to ensure that mosquitoes aren’t around their home, such as getting rid of standing water, which is an environment for mosquitoes to lay eggs.
More than 240 million malaria cases occur annually worldwide, the CDC said, with 95% in Africa. There are 2,000 cases diagnosed annually in the U.S. that are related to international travel. Malaria symptoms are similar to those of other illnesses and include fever, chills, a headache, and muscle aches. If not treated, malaria can be fatal.
A version of this article first appeared on WebMD.com.
, the Centers for Disease Control and Prevention says.
The federal health agency recently issued a nationwide warning to health providers and officials to be on the lookout for symptoms of the potentially fatal illness. Usually, people in the U.S. who get malaria get the disease during international travel.
All five people – four in Florida and one in Texas – have received treatment and are improving, according to the CDC. The case in Texas is not related to the Florida cases, and all occurred in the past 2 months.
Malaria cannot be transmitted from person to person. It is spread by the bite of an infected female mosquito. The last cases of people being infected while in the U.S. occurred 20 years ago, when there were eight cases in Palm Beach County, Fla. The Texas Department of State Health Services said the last time malaria was locally acquired in the state was 1994.
The Florida Department of Health said it was spraying for mosquitoes in the two counties surrounding Sarasota, Fla., where the four cases occurred.
The CDC said the risk of getting malaria while in the United States “remains extremely low.” The agency advised people to protect themselves by taking precautions to prevent mosquito bites, such as wearing insect repellent and wearing long-sleeved shirts and pants. People should also do things to ensure that mosquitoes aren’t around their home, such as getting rid of standing water, which is an environment for mosquitoes to lay eggs.
More than 240 million malaria cases occur annually worldwide, the CDC said, with 95% in Africa. There are 2,000 cases diagnosed annually in the U.S. that are related to international travel. Malaria symptoms are similar to those of other illnesses and include fever, chills, a headache, and muscle aches. If not treated, malaria can be fatal.
A version of this article first appeared on WebMD.com.
Nebulized amphotericin B does not affect aspergillosis exacerbation-free status at 1 year
Topline
Nebulized amphotericin B does not improve exacerbation-free status at 1 year for patients with bronchopulmonary aspergillosis, though it may delay onset and incidence.
Methodology
Investigators searched PubMed and Embase databases for studies that included at least five patients with allergic bronchopulmonary aspergillosis who were managed with nebulized amphotericin B.
They included five studies, two of which were randomized controlled trials (RCTs), and three were observational studies; there was a total of 188 patients.
The primary objective of this systematic review and meta-analysis was to determine the frequency of patients remaining exacerbation free 1 year after initiating treatment with nebulized amphotericin B.
Takeaway
From the studies (one observational, two RCTs; n = 84) with exacerbation data at 1 or 2 years, the pooled proportion of patients who remained exacerbation free with nebulized amphotericin B at 1 year was 76% (I2 = 64.6%).
The pooled difference in risk with the two RCTs that assessed exacerbation-free status at 1 year was 0.33 and was not significantly different between the nebulized amphotericin B and control arms, which received nebulized saline.
Two RCTs provided the time to first exacerbation, which was significantly longer with nebulized amphotericin B than with nebulized saline (337 vs. 177 days; P = .004; I2 = 82%).
The proportion of patients who experienced two or more exacerbations was significantly lower with nebulized amphotericin B than with nebulized saline (9/33 [27.3%] vs 20/38 [52.6%]; P = .03).
In practice
Also, the proportion of subjects experiencing ≥ 2 exacerbations was also lesser with NAB than in the control,” concluded Valliappan Muthu, MD, and colleagues. However, “the ideal duration and optimal dose of LAMB for nebulization are unclear.”
Study details
“Nebulized amphotericin B for preventing exacerbations in allergic bronchopulmonary aspergillosis: A systematic review and meta-analysis” was published online in Pulmonary Pharmacology and Therapeutics.
Limitations
The current review is limited by the small number of included trials and may have a high risk of bias. Therefore, more evidence is required for the use of nebulized amphotericin B in routine care. The authors have disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Topline
Nebulized amphotericin B does not improve exacerbation-free status at 1 year for patients with bronchopulmonary aspergillosis, though it may delay onset and incidence.
Methodology
Investigators searched PubMed and Embase databases for studies that included at least five patients with allergic bronchopulmonary aspergillosis who were managed with nebulized amphotericin B.
They included five studies, two of which were randomized controlled trials (RCTs), and three were observational studies; there was a total of 188 patients.
The primary objective of this systematic review and meta-analysis was to determine the frequency of patients remaining exacerbation free 1 year after initiating treatment with nebulized amphotericin B.
Takeaway
From the studies (one observational, two RCTs; n = 84) with exacerbation data at 1 or 2 years, the pooled proportion of patients who remained exacerbation free with nebulized amphotericin B at 1 year was 76% (I2 = 64.6%).
The pooled difference in risk with the two RCTs that assessed exacerbation-free status at 1 year was 0.33 and was not significantly different between the nebulized amphotericin B and control arms, which received nebulized saline.
Two RCTs provided the time to first exacerbation, which was significantly longer with nebulized amphotericin B than with nebulized saline (337 vs. 177 days; P = .004; I2 = 82%).
The proportion of patients who experienced two or more exacerbations was significantly lower with nebulized amphotericin B than with nebulized saline (9/33 [27.3%] vs 20/38 [52.6%]; P = .03).
In practice
Also, the proportion of subjects experiencing ≥ 2 exacerbations was also lesser with NAB than in the control,” concluded Valliappan Muthu, MD, and colleagues. However, “the ideal duration and optimal dose of LAMB for nebulization are unclear.”
Study details
“Nebulized amphotericin B for preventing exacerbations in allergic bronchopulmonary aspergillosis: A systematic review and meta-analysis” was published online in Pulmonary Pharmacology and Therapeutics.
Limitations
The current review is limited by the small number of included trials and may have a high risk of bias. Therefore, more evidence is required for the use of nebulized amphotericin B in routine care. The authors have disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Topline
Nebulized amphotericin B does not improve exacerbation-free status at 1 year for patients with bronchopulmonary aspergillosis, though it may delay onset and incidence.
Methodology
Investigators searched PubMed and Embase databases for studies that included at least five patients with allergic bronchopulmonary aspergillosis who were managed with nebulized amphotericin B.
They included five studies, two of which were randomized controlled trials (RCTs), and three were observational studies; there was a total of 188 patients.
The primary objective of this systematic review and meta-analysis was to determine the frequency of patients remaining exacerbation free 1 year after initiating treatment with nebulized amphotericin B.
Takeaway
From the studies (one observational, two RCTs; n = 84) with exacerbation data at 1 or 2 years, the pooled proportion of patients who remained exacerbation free with nebulized amphotericin B at 1 year was 76% (I2 = 64.6%).
The pooled difference in risk with the two RCTs that assessed exacerbation-free status at 1 year was 0.33 and was not significantly different between the nebulized amphotericin B and control arms, which received nebulized saline.
Two RCTs provided the time to first exacerbation, which was significantly longer with nebulized amphotericin B than with nebulized saline (337 vs. 177 days; P = .004; I2 = 82%).
The proportion of patients who experienced two or more exacerbations was significantly lower with nebulized amphotericin B than with nebulized saline (9/33 [27.3%] vs 20/38 [52.6%]; P = .03).
In practice
Also, the proportion of subjects experiencing ≥ 2 exacerbations was also lesser with NAB than in the control,” concluded Valliappan Muthu, MD, and colleagues. However, “the ideal duration and optimal dose of LAMB for nebulization are unclear.”
Study details
“Nebulized amphotericin B for preventing exacerbations in allergic bronchopulmonary aspergillosis: A systematic review and meta-analysis” was published online in Pulmonary Pharmacology and Therapeutics.
Limitations
The current review is limited by the small number of included trials and may have a high risk of bias. Therefore, more evidence is required for the use of nebulized amphotericin B in routine care. The authors have disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Palliative Care: Utilization Patterns in Inpatient Dermatology
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Practice Points
- Although severe dermatologic disease negatively impacts patients’ quality of life, palliative care may be underutilized in this population.
- Palliative care should be an integral part of caring for patients who are admitted to the hospital with serious dermatologic illnesses.
Placebo effect can be found in a cup of coffee
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.