User login
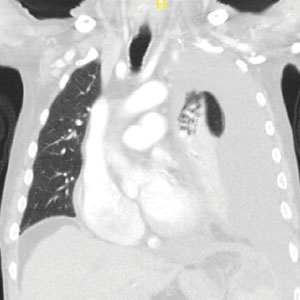
Public Health, Not Politics, Should Drive Mask Policies, Says Ethicist
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I recently saw a ban that has me very worried, concerned, and strongly in opposition.
Basically, the standard kind of medical mask would be captured, although I think their aim in doing this was to try to discourage people at political protests from being able to wear masks and hide their identity. They’re basically trying to discourage that. This is particularly triggered by, I think, protests about the invasion of Israel, the war that resulted in Gaza, and the demonstrations that have gone on around the country, with many people masked.
There may be issues about what is acceptable to wear when you go to a demonstration. I don’t claim to know about the civil rights of that.
In a time at which COVID-19 is flourishing, really on the rebound, expanding fast, and still causing 600 deaths a week; the flu season is going to be upon us soon enough; and there are also concerns about the possibility of avian flu jumping into the human population, it is absolutely the wrong time to single out those who are trying to mask for health reasons.
Basically, there are two strong reasons. One, there are people out there who wear a medical mask or mask for a medical reason because they have an underlying disease. They may have had a transplant or they may feel they’re immunocompromised for some reason. They worry that, if they don’t wear a mask, they’re going to get an infection from something like COVID-19 or flu, which could really be super-dangerous for them.
The other reason people mask is to protect their family members. They may have someone who’s immunocompromised in the family, or they’re doing it kindly and altruistically to protect the rest of us and to stop viruses from circulating.
These bans are not taking into account public health. They’re being brought forward in the midst of political heat about demonstrations and political issues. I think they should be opposed. I do not think they should be enacted.
I think the medical rights of people with disabilities and immunologic disorders, and those who want to mask to prevent getting sick at a time at which infectious diseases are still circulating and killing people, ought to take priority. Public health, in this case, should drive our policies about masks.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, NY, served on Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Six Tips on Coronavirus Testing for Doctors and Patients
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
according to the Robert Koch Institute, Germany. If a patient has a fever and cough and feels exhausted, it could be COVID-19. What significance do rapid tests have? And when should doctors advise their patients about them?
When to Test
People at a higher risk for severe COVID-19 benefit from tests. This population includes the following groups:
- Older patients
- Immunocompromised patients
- Patients with respiratory diseases
- Patients with cardiovascular diseases
- Patients with liver and kidney diseases
- Patients with neurological diseases
- Patients with obesity
If doctors detect SARS-CoV-2 infection early, they can prescribe Paxlovid, for example, to reduce morbidity and mortality risks. Conversely, people without specific risks should test themselves if they plan to visit vulnerable individuals.
Detecting New Variants
A comprehensive study from the fall of 2022 provides evidence that antigen tests targeting the nucleocapsid (N) protein of SARS-CoV-2 also detect new variants.
The researchers built a library of various versions of the SARS-CoV-2 N protein. Their collection included nearly 8000 individual amino acid substitutions, representing more than 99.5% of all statistically possible mutations of the N protein.
They then examined how these N proteins interacted with 17 antibodies used in 11 commercially available antigen rapid tests.
All antibodies were able to recognize altered N proteins. Since the researchers successfully investigated diagnostic antibodies against nearly all possible N-protein mutations, rapid tests should be able to detect future virus variants. However, sensitivity and specificity may still change.
Test Timing
Uncertainty about what time of day to test can be mitigated by performing multiple COVID-19 rapid tests over time. The Food and Drug Administration (FDA) and similar organizations make this recommendation. Studies of symptomatic individuals show that serial tests increase accuracy.
In the early stages of infection, swabs may contain too little virus material because of widespread immunity against SARS-CoV-2. That is, they may contain inadequate levels of the relevant antigen. Especially in asymptomatic individuals or patients in the incubation phase, a single test may therefore yield a false-negative result. Therefore, the FDA recommends conducting at least two additional tests 48 hours apart in case of a negative test result.
Costs of Rapid Tests
The days of free tests are long gone. In Germany, the distribution of free preventive coronavirus tests was discontinued on March 1, 2023.
Test kits are still available in pharmacies or drugstores. In packages with 5-10 tests, the individual test costs between €0.90 and €1.50, depending on the provider. If a patient still has old rapid coronavirus tests in his or her medicine cabinet, are they still suitable?
Expired Tests
Properly stored tests that have not passed their expiration dates can still be used. But microbiologist and pathologist Daniel Rhoads, MD, from the Cleveland Clinic in Ohio warns against expired rapid tests.
The chemicals may have decomposed, the solvent may have evaporated, or antibodies may have lost their effectiveness, thus making false negative results more likely. “These are proteins that can decompose over time,” said Dr. Rhoads.
Ordering PCR Tests
The polymerase chain reaction (PCR) test remains the gold standard for diagnosing COVID-19. It is still available within statutory health insurance coverage. As Germany’s National Association of Statutory Health Insurance Physicians observes, form Muster 10 is used to order the test in that country.
The fee for the swab is included in the insured patient’s basic flat rate. Laboratories bill the PCR test using fee schedule position (GOP) 32816, according to the Uniform Value Scale (EBM).
There is no possibility for billing rapid tests for SARS-CoV-2 in medical practices within the EBM. A laboratory-based SARS-CoV-2 antigen detection test (GOP 32779) can be requested via the Muster 10 form.
This story was translated from the Medscape German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
COVID Levels Start to Dip, New Variant Emerges
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
A new COVID-19 variant called XEC is on the rise, and it has experts who track variants on alert.
Each time a new variant makes a grand entrance onto tracker lists, health officials take notice because it may mean there’s an important change in behavior of SARS-CoV-2, the virus that causes COVID.
Countries reporting rising detections of XEC include Germany, the United Kingdom, and the Netherlands, Australian data scientist Mike Honey posted on the platform X this past week.
XEC’s “characteristic mutations” have been detected in at least 25 states, CBS News reported, with New Jersey, California, and Virginia labs reporting 10 or more cases each. New Jersey detections at least in part stem from the CDC’s testing program for international travelers at Newark Liberty International Airport.
Still, XEC hasn’t gained enough traction in Europe, the United States, or any other part of the world for it to be listed as a standalone variant on official watchlists maintained by the CDC, European Union, or World Health Organization.
However, Eric Topol, MD, executive vice president of Scripps Research and editor-at-large for Medscape, believes XEC is the next variant “to get legs.”
The rate at which a new variant takes the stage doesn’t always predict how severe it will be. Around this time last year, health officials sounded alarms about another Omicron variant called BA.2.86, dubbed Pirola, that ultimately didn’t make major waves.
“CDC is not aware of any specific symptoms associated with XEC or any other co-circulating SARS-CoV-2 lineage,” a CDC spokesperson said in a statement to CBS News.
Its parent lineages are KP.2 and KP.3, and all of these belong to the Omicron family. The SARS-CoV-2 virus mutates over time, and scientists use the names and labels to identify groups of viral variants based on their similarities and on which strains a mutated descendant came from.
A version of this article appeared on WebMD.com.
How Experts Predicts This COVID and Flu Season Will Unfold
What’s the outlook for COVID-19 and flu this fall and winter? It’ll probably be a lot like last year, experts say.
“We currently expect this flu season to be comparable to last year’s season,” said Adrienne Keen, PhD, of the Centers for Disease Control and Prevention’s (CDC) Center for Forecasting and Outbreak Analytics. “We expect this year’s COVID-19 season peak to be similar to last year’s or lower.” The CDC is still analyzing COVID surveillance data from the summer and will update the forecast as more is learned.
For COVID, that means it won’t be as bad as the pandemic years, and for the flu, it’s a typical pre-pandemic season. But status quo does not mean great.
Between October 2023 and April 2024, as many as 75 million people got the flu in the United States, according to CDC estimates, resulting in up to 900,000 hospitalizations and between 17,000 and 100,000 deaths. In 2023, about 900,000 Americans were hospitalized with COVID and 75,000 died.
Getting vaccinated remains crucial, public health officials stressed.
Predicting COVID
Two key predictors of how bad an upcoming COVID season will be are the cycling of new variants and the population’s immunity (protection from an infectious disease that happens when a population is immune through vaccination or previous infection).
When new variants go up and immunity goes down, “we tend to see the increase in cases,” said Michael T. Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy and a professor of public health at the University of Minnesota, Minneapolis. But if the number of variants goes down and immunity levels go up, the outlook is more favorable.
The new COVID variant called XEC has been found in at least 25 states. On September 27, the CDC added the variant to the COVID tracker. It now accounts for 6% of US cases. This was expected, as the variant has been circulating in Europe, said Amesh Adalja, MD, a senior scholar and infectious disease expert at the Center for Health Security at Johns Hopkins University, Baltimore, Maryland.
“There will always be a new variant appearing, and one falling,” he said. “So the fact that this is happening is not surprising.”
Meanwhile, the summer COVID surge has provided postinfection immunity for some people. “What’s likely is, we are going to see substantial protection of the population for several months based on previous infection and in some cases vaccination,” Dr. Osterholm said. That means protection from serious illness, hospitalizations, and deaths (but not necessarily infection). That protection could last through the year or into early 2025.
The timing of 2024’s winter surge will likely be a bit later than 2023’s, said Andrew Pekosz, PhD, a professor and vice chair of molecular microbiology and immunology at Johns Hopkins University, Baltimore, “peaking just after the Christmas/New Year holiday.”
During the 2023-2024 season, weekly COVID hospitalizations peaked the week of Dec. 30, said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill and a member of the COVID-19 Scenario Modeling Hub.
But variants are unpredictable. “There’s a chance that the XEC variant may take off and spread, or might not,” said Dr. Adalja. As of September 28, the Omicron variant KP.3.1.1 was leading, accounting for 58.7% of US cases, according to the CDC.
While Dr. Adalja agreed that 2024’s COVID season will probably be like 2023’s, “we have to be prepared for cases and hospitalizations going up,” he said, “but not to the point of a crisis.” A return to lockdowns and social distancing is unlikely.
Still, older adults and others at higher risk of getting very sick from COVID should consider masking during travel, said Rajendram Rajnarayanan, PhD, MSc, an associate professor at the New York Institute of Technology College of Osteopathic Medicine at Arkansas State University, Jonesboro.
Flu Forecasts
Predicting flu season this early is hard, said Jeffrey Shaman, PhD, a professor of environmental health sciences and professor of climate at Colombia University, New York.
“You can look at the CDC forecast and use it as a very loose guide right now,” said Dr. Shaman, who won the CDC’s first “Predict the Influenza Season Challenge” in 2014. “Until there is actually flu, it’s like trying to predict the landfall of a hurricane.” Flu activity remained low as of September 14 (the most current data available), according to the CDC.
When flu activity picks up, typically in mid-October or November, experts look at the dominant strain, exposure to similar strains in previous years, and how well-matched the current flu vaccine is to that dominant strain, Dr. Shaman said. Vaccine makers must make an educated guess months in advance regarding which strain to target, to allow time for production.
The vaccination rate plays a role, too, but that tends to remain constant, Dr. Shaman said. According to the CDC, less than half of adults age 18 and up got a flu vaccination last year.
Experts also consider flu patterns in the Southern Hemisphere, where 2024 flu activity has mostly involved two subtypes of influenza A — H1N1 and H3N2 — and some influenza B, the CDC found.
How Well Do This Year’s Vaccines and Viruses Match Up?
The FDA has authorized three updated COVID vaccines for this fall. Novavax targets the JN.1 strain of SARS-CoV-2, the virus that causes COVID-19. Both mRNA vaccines, Moderna and Pfizer, target KP.2, a descendant of JN.1. All three target current predominant variants, and any one of them is recommended by the CDC.
The vaccines are a good “though not perfect match to virtually all the circulating variants of SARS-CoV-2,” said Dr. Pekosz.
Experts said that the shots will protect against the XEC variant.
“XEC and its sublineages are expected to be the dominant fall/winter variant group,” said Dr. Rajnarayanan.
This year’s flu vaccines, all trivalent (protecting against three viruses), will target the three strains expected to circulate — H1N1, H3N2, and influenza B (Victoria), according to the CDC.
People should still get vaccinated, Dr. Adalja said, and use home tests for flu and COVID and take antivirals promptly when needed. The goal should not be status quo but rather fewer COVID and flu hospitalizations and deaths.
A version of this article first appeared on WebMD.com.
What’s the outlook for COVID-19 and flu this fall and winter? It’ll probably be a lot like last year, experts say.
“We currently expect this flu season to be comparable to last year’s season,” said Adrienne Keen, PhD, of the Centers for Disease Control and Prevention’s (CDC) Center for Forecasting and Outbreak Analytics. “We expect this year’s COVID-19 season peak to be similar to last year’s or lower.” The CDC is still analyzing COVID surveillance data from the summer and will update the forecast as more is learned.
For COVID, that means it won’t be as bad as the pandemic years, and for the flu, it’s a typical pre-pandemic season. But status quo does not mean great.
Between October 2023 and April 2024, as many as 75 million people got the flu in the United States, according to CDC estimates, resulting in up to 900,000 hospitalizations and between 17,000 and 100,000 deaths. In 2023, about 900,000 Americans were hospitalized with COVID and 75,000 died.
Getting vaccinated remains crucial, public health officials stressed.
Predicting COVID
Two key predictors of how bad an upcoming COVID season will be are the cycling of new variants and the population’s immunity (protection from an infectious disease that happens when a population is immune through vaccination or previous infection).
When new variants go up and immunity goes down, “we tend to see the increase in cases,” said Michael T. Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy and a professor of public health at the University of Minnesota, Minneapolis. But if the number of variants goes down and immunity levels go up, the outlook is more favorable.
The new COVID variant called XEC has been found in at least 25 states. On September 27, the CDC added the variant to the COVID tracker. It now accounts for 6% of US cases. This was expected, as the variant has been circulating in Europe, said Amesh Adalja, MD, a senior scholar and infectious disease expert at the Center for Health Security at Johns Hopkins University, Baltimore, Maryland.
“There will always be a new variant appearing, and one falling,” he said. “So the fact that this is happening is not surprising.”
Meanwhile, the summer COVID surge has provided postinfection immunity for some people. “What’s likely is, we are going to see substantial protection of the population for several months based on previous infection and in some cases vaccination,” Dr. Osterholm said. That means protection from serious illness, hospitalizations, and deaths (but not necessarily infection). That protection could last through the year or into early 2025.
The timing of 2024’s winter surge will likely be a bit later than 2023’s, said Andrew Pekosz, PhD, a professor and vice chair of molecular microbiology and immunology at Johns Hopkins University, Baltimore, “peaking just after the Christmas/New Year holiday.”
During the 2023-2024 season, weekly COVID hospitalizations peaked the week of Dec. 30, said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill and a member of the COVID-19 Scenario Modeling Hub.
But variants are unpredictable. “There’s a chance that the XEC variant may take off and spread, or might not,” said Dr. Adalja. As of September 28, the Omicron variant KP.3.1.1 was leading, accounting for 58.7% of US cases, according to the CDC.
While Dr. Adalja agreed that 2024’s COVID season will probably be like 2023’s, “we have to be prepared for cases and hospitalizations going up,” he said, “but not to the point of a crisis.” A return to lockdowns and social distancing is unlikely.
Still, older adults and others at higher risk of getting very sick from COVID should consider masking during travel, said Rajendram Rajnarayanan, PhD, MSc, an associate professor at the New York Institute of Technology College of Osteopathic Medicine at Arkansas State University, Jonesboro.
Flu Forecasts
Predicting flu season this early is hard, said Jeffrey Shaman, PhD, a professor of environmental health sciences and professor of climate at Colombia University, New York.
“You can look at the CDC forecast and use it as a very loose guide right now,” said Dr. Shaman, who won the CDC’s first “Predict the Influenza Season Challenge” in 2014. “Until there is actually flu, it’s like trying to predict the landfall of a hurricane.” Flu activity remained low as of September 14 (the most current data available), according to the CDC.
When flu activity picks up, typically in mid-October or November, experts look at the dominant strain, exposure to similar strains in previous years, and how well-matched the current flu vaccine is to that dominant strain, Dr. Shaman said. Vaccine makers must make an educated guess months in advance regarding which strain to target, to allow time for production.
The vaccination rate plays a role, too, but that tends to remain constant, Dr. Shaman said. According to the CDC, less than half of adults age 18 and up got a flu vaccination last year.
Experts also consider flu patterns in the Southern Hemisphere, where 2024 flu activity has mostly involved two subtypes of influenza A — H1N1 and H3N2 — and some influenza B, the CDC found.
How Well Do This Year’s Vaccines and Viruses Match Up?
The FDA has authorized three updated COVID vaccines for this fall. Novavax targets the JN.1 strain of SARS-CoV-2, the virus that causes COVID-19. Both mRNA vaccines, Moderna and Pfizer, target KP.2, a descendant of JN.1. All three target current predominant variants, and any one of them is recommended by the CDC.
The vaccines are a good “though not perfect match to virtually all the circulating variants of SARS-CoV-2,” said Dr. Pekosz.
Experts said that the shots will protect against the XEC variant.
“XEC and its sublineages are expected to be the dominant fall/winter variant group,” said Dr. Rajnarayanan.
This year’s flu vaccines, all trivalent (protecting against three viruses), will target the three strains expected to circulate — H1N1, H3N2, and influenza B (Victoria), according to the CDC.
People should still get vaccinated, Dr. Adalja said, and use home tests for flu and COVID and take antivirals promptly when needed. The goal should not be status quo but rather fewer COVID and flu hospitalizations and deaths.
A version of this article first appeared on WebMD.com.
What’s the outlook for COVID-19 and flu this fall and winter? It’ll probably be a lot like last year, experts say.
“We currently expect this flu season to be comparable to last year’s season,” said Adrienne Keen, PhD, of the Centers for Disease Control and Prevention’s (CDC) Center for Forecasting and Outbreak Analytics. “We expect this year’s COVID-19 season peak to be similar to last year’s or lower.” The CDC is still analyzing COVID surveillance data from the summer and will update the forecast as more is learned.
For COVID, that means it won’t be as bad as the pandemic years, and for the flu, it’s a typical pre-pandemic season. But status quo does not mean great.
Between October 2023 and April 2024, as many as 75 million people got the flu in the United States, according to CDC estimates, resulting in up to 900,000 hospitalizations and between 17,000 and 100,000 deaths. In 2023, about 900,000 Americans were hospitalized with COVID and 75,000 died.
Getting vaccinated remains crucial, public health officials stressed.
Predicting COVID
Two key predictors of how bad an upcoming COVID season will be are the cycling of new variants and the population’s immunity (protection from an infectious disease that happens when a population is immune through vaccination or previous infection).
When new variants go up and immunity goes down, “we tend to see the increase in cases,” said Michael T. Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy and a professor of public health at the University of Minnesota, Minneapolis. But if the number of variants goes down and immunity levels go up, the outlook is more favorable.
The new COVID variant called XEC has been found in at least 25 states. On September 27, the CDC added the variant to the COVID tracker. It now accounts for 6% of US cases. This was expected, as the variant has been circulating in Europe, said Amesh Adalja, MD, a senior scholar and infectious disease expert at the Center for Health Security at Johns Hopkins University, Baltimore, Maryland.
“There will always be a new variant appearing, and one falling,” he said. “So the fact that this is happening is not surprising.”
Meanwhile, the summer COVID surge has provided postinfection immunity for some people. “What’s likely is, we are going to see substantial protection of the population for several months based on previous infection and in some cases vaccination,” Dr. Osterholm said. That means protection from serious illness, hospitalizations, and deaths (but not necessarily infection). That protection could last through the year or into early 2025.
The timing of 2024’s winter surge will likely be a bit later than 2023’s, said Andrew Pekosz, PhD, a professor and vice chair of molecular microbiology and immunology at Johns Hopkins University, Baltimore, “peaking just after the Christmas/New Year holiday.”
During the 2023-2024 season, weekly COVID hospitalizations peaked the week of Dec. 30, said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill and a member of the COVID-19 Scenario Modeling Hub.
But variants are unpredictable. “There’s a chance that the XEC variant may take off and spread, or might not,” said Dr. Adalja. As of September 28, the Omicron variant KP.3.1.1 was leading, accounting for 58.7% of US cases, according to the CDC.
While Dr. Adalja agreed that 2024’s COVID season will probably be like 2023’s, “we have to be prepared for cases and hospitalizations going up,” he said, “but not to the point of a crisis.” A return to lockdowns and social distancing is unlikely.
Still, older adults and others at higher risk of getting very sick from COVID should consider masking during travel, said Rajendram Rajnarayanan, PhD, MSc, an associate professor at the New York Institute of Technology College of Osteopathic Medicine at Arkansas State University, Jonesboro.
Flu Forecasts
Predicting flu season this early is hard, said Jeffrey Shaman, PhD, a professor of environmental health sciences and professor of climate at Colombia University, New York.
“You can look at the CDC forecast and use it as a very loose guide right now,” said Dr. Shaman, who won the CDC’s first “Predict the Influenza Season Challenge” in 2014. “Until there is actually flu, it’s like trying to predict the landfall of a hurricane.” Flu activity remained low as of September 14 (the most current data available), according to the CDC.
When flu activity picks up, typically in mid-October or November, experts look at the dominant strain, exposure to similar strains in previous years, and how well-matched the current flu vaccine is to that dominant strain, Dr. Shaman said. Vaccine makers must make an educated guess months in advance regarding which strain to target, to allow time for production.
The vaccination rate plays a role, too, but that tends to remain constant, Dr. Shaman said. According to the CDC, less than half of adults age 18 and up got a flu vaccination last year.
Experts also consider flu patterns in the Southern Hemisphere, where 2024 flu activity has mostly involved two subtypes of influenza A — H1N1 and H3N2 — and some influenza B, the CDC found.
How Well Do This Year’s Vaccines and Viruses Match Up?
The FDA has authorized three updated COVID vaccines for this fall. Novavax targets the JN.1 strain of SARS-CoV-2, the virus that causes COVID-19. Both mRNA vaccines, Moderna and Pfizer, target KP.2, a descendant of JN.1. All three target current predominant variants, and any one of them is recommended by the CDC.
The vaccines are a good “though not perfect match to virtually all the circulating variants of SARS-CoV-2,” said Dr. Pekosz.
Experts said that the shots will protect against the XEC variant.
“XEC and its sublineages are expected to be the dominant fall/winter variant group,” said Dr. Rajnarayanan.
This year’s flu vaccines, all trivalent (protecting against three viruses), will target the three strains expected to circulate — H1N1, H3N2, and influenza B (Victoria), according to the CDC.
People should still get vaccinated, Dr. Adalja said, and use home tests for flu and COVID and take antivirals promptly when needed. The goal should not be status quo but rather fewer COVID and flu hospitalizations and deaths.
A version of this article first appeared on WebMD.com.
Guidance for Practicing Primary Care: World Health Organization’s Updated Influenza Guidelines for 2024
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
As primary care physicians, we are often the first ones patients see when they become infected with influenza. According to Centers for Disease Control and Prevention statistics, approximately 5%-20% of the US population will be infected with influenza every year. Additionally, more than 200,000 of these patients will be hospitalized because of complications related to influenza.
Earlier in September, the World Health Organization (WHO) issued its latest clinical practice guidelines for influenza for the 2024-2025 season. This is a 213-page document aimed at healthcare providers who treat patients infected with influenza. It includes treatment for those with severe and nonsevere influenza infections, those in both the outpatient and hospitalized setting, as well as medication prophylaxis for those exposed to the virus. Additionally, it defines risk estimates for those who are at risk of being hospitalized or dying. In contrast, previous updates focused on management of severe influenza or those at risk of severe influenza.
These guidelines cover recommendations regarding all the antiviral medications for treating influenza used around the world. For the purpose of this article, we will focus on those most commonly used in the United States.
A newer medication discussed was baloxavir. It is recommended to be used for patients with nonsevere influenza who are at high risk for progression to severe disease. The advice is to not use it for those with little risk of progression to severe disease. Oseltamivir is recommended for those with severe infection.
The guidelines recommend against using antibiotics for those who have a low likelihood of having a bacterial coinfection. As primary care doctors, we often prescribe medications to help with symptoms. These guidelines recommend against the use of corticosteroids and antibiotics but did advise that NSAIDs could be used for symptom relief.
One of the important parts of these guidelines is prevention in patients who have been exposed but are asymptomatic. They recommend baloxavir or oseltamivir but only for those patients who are at high risk of being hospitalized if they were to become infected. Any of the antivirals can be used for patients who are exposed to the novel influenza A, which is associated with a higher mortality rate. Caution when prescribing antivirals is recommended in immunocompromised patients because there is more drug resistance seen in these patients.
These updates also discuss the use of different influenza tests. In the outpatient setting, primary doctors don’t have time for test results that may take 2 days to come back. Only rapid tests make the sense in the primary care setting. Additionally, in the age of COVID, it is important to make an accurate diagnosis so we should be testing patients. There is resistance seen with the antivirals we prescribe for influenza so prescribing them empirically without a confirmed diagnosis of influenza may be doing more harm than good.
One gap in these recommendations is vaccination. This topic was not covered at all. It would be helpful to have a strategy in place to prevent infection in populations rather than focusing just on exposed individuals. A discussion of when and who and to vaccinate would be helpful. Research into the effectiveness of vaccines is key and more accurate development of a season’s influenza vaccine would be beneficial. Currently, there is much vaccine misinformation being spread around. Education and information regarding influenza vaccines, especially coming from WHO, is crucial.
Another failure of these recommendations is that the guidelines apply only to those who present within a few days of becoming symptomatic. As family doctors, we know many of our patients self-treat or consult Google. They often don’t come for medical care until they’ve been sick for a week or longer. There are no guidelines for these patients.
In general, these guidelines are comprehensive and do a great job discussing the current medications available. However, more is needed to increase vaccination rates. Patients need to know that if they may be sick with influenza, they need to seek medical care as soon as possible. We, as family doctors, need to do a better job of risk-stratifying our patients and prescribing prophylactic medication when suitable. Every infection we prevent aids in the health of our community and the global population at large.
Dr. Girgis practices family medicine in South River, New Jersey, and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, New Jersey. She has no relevant conflicts of interest.
Species Possibly Responsible for COVID Pandemic Identified
The origin of the COVID-19 pandemic has sparked much debate, and various hypotheses have been put forward.
“My colleagues and I have examined the issue with an open mind, taking into account all possible hypotheses. The laboratory origin hypothesis was legitimate and deserved to be investigated,” Florence Débarre, PhD, a research director at the French National Center for Scientific Research at the Institute of Ecology and Environmental Sciences in Paris, France, told this news organization. Nevertheless,
“We studied data from environmental samples taken at the Huanan market in Wuhan shortly after its closure in early 2020,” said Dr. Débarre. The data were shared by the Chinese Center for Disease Control and Prevention on open and public databases. They include the raw genetic sequences of more than 800 samples collected at the Huanan market, on cages and carts, on the floors and walls of the stalls, and in the pipes and sewers.
These data allowed researchers to highlight the co-presence at this location of genetic material from the SARS-CoV-2 virus and certain wild animals. Masked palm civets, which are wild canids similar to foxes, with a dark facial mask similar to that of raccoons, and civets, small carnivorous mammals close to mongooses, were at the site.
“These species were already involved in the emergence of the SARS epidemic in the early 2000s and considered to facilitate the transmission of the virus from animals to humans,” said Dr. Débarre.
These animals were identified based on their DNA and located in the southwest part of the market, which is also a hotspot where many samples tested positive for SARS-CoV-2.
“There is a particular stall where the virus and the animals were found,” she said.
Since the data used are based on environmental samples, it is not possible to formally demonstrate that the animals were infected, but the discovery of virus samples located in the same place as the genetic material of these animals suggests that they were.
“There were samples taken from some animals at the market, but not from others, as they had already been evacuated when the sampling services arrived,” said Dr. Débarre. These results add to a large body of evidence that all points in the same direction: an animal origin at the Wuhan market.
The team also found other zoonotic viruses, such as avian flu. “This study confirms that live animal markets pose a high health risk, especially when they are at the heart of urban centers,” said Dr. Débarre. “It can provide avenues for prevention, particularly by limiting interactions between humans and wild fauna.”
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The origin of the COVID-19 pandemic has sparked much debate, and various hypotheses have been put forward.
“My colleagues and I have examined the issue with an open mind, taking into account all possible hypotheses. The laboratory origin hypothesis was legitimate and deserved to be investigated,” Florence Débarre, PhD, a research director at the French National Center for Scientific Research at the Institute of Ecology and Environmental Sciences in Paris, France, told this news organization. Nevertheless,
“We studied data from environmental samples taken at the Huanan market in Wuhan shortly after its closure in early 2020,” said Dr. Débarre. The data were shared by the Chinese Center for Disease Control and Prevention on open and public databases. They include the raw genetic sequences of more than 800 samples collected at the Huanan market, on cages and carts, on the floors and walls of the stalls, and in the pipes and sewers.
These data allowed researchers to highlight the co-presence at this location of genetic material from the SARS-CoV-2 virus and certain wild animals. Masked palm civets, which are wild canids similar to foxes, with a dark facial mask similar to that of raccoons, and civets, small carnivorous mammals close to mongooses, were at the site.
“These species were already involved in the emergence of the SARS epidemic in the early 2000s and considered to facilitate the transmission of the virus from animals to humans,” said Dr. Débarre.
These animals were identified based on their DNA and located in the southwest part of the market, which is also a hotspot where many samples tested positive for SARS-CoV-2.
“There is a particular stall where the virus and the animals were found,” she said.
Since the data used are based on environmental samples, it is not possible to formally demonstrate that the animals were infected, but the discovery of virus samples located in the same place as the genetic material of these animals suggests that they were.
“There were samples taken from some animals at the market, but not from others, as they had already been evacuated when the sampling services arrived,” said Dr. Débarre. These results add to a large body of evidence that all points in the same direction: an animal origin at the Wuhan market.
The team also found other zoonotic viruses, such as avian flu. “This study confirms that live animal markets pose a high health risk, especially when they are at the heart of urban centers,” said Dr. Débarre. “It can provide avenues for prevention, particularly by limiting interactions between humans and wild fauna.”
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The origin of the COVID-19 pandemic has sparked much debate, and various hypotheses have been put forward.
“My colleagues and I have examined the issue with an open mind, taking into account all possible hypotheses. The laboratory origin hypothesis was legitimate and deserved to be investigated,” Florence Débarre, PhD, a research director at the French National Center for Scientific Research at the Institute of Ecology and Environmental Sciences in Paris, France, told this news organization. Nevertheless,
“We studied data from environmental samples taken at the Huanan market in Wuhan shortly after its closure in early 2020,” said Dr. Débarre. The data were shared by the Chinese Center for Disease Control and Prevention on open and public databases. They include the raw genetic sequences of more than 800 samples collected at the Huanan market, on cages and carts, on the floors and walls of the stalls, and in the pipes and sewers.
These data allowed researchers to highlight the co-presence at this location of genetic material from the SARS-CoV-2 virus and certain wild animals. Masked palm civets, which are wild canids similar to foxes, with a dark facial mask similar to that of raccoons, and civets, small carnivorous mammals close to mongooses, were at the site.
“These species were already involved in the emergence of the SARS epidemic in the early 2000s and considered to facilitate the transmission of the virus from animals to humans,” said Dr. Débarre.
These animals were identified based on their DNA and located in the southwest part of the market, which is also a hotspot where many samples tested positive for SARS-CoV-2.
“There is a particular stall where the virus and the animals were found,” she said.
Since the data used are based on environmental samples, it is not possible to formally demonstrate that the animals were infected, but the discovery of virus samples located in the same place as the genetic material of these animals suggests that they were.
“There were samples taken from some animals at the market, but not from others, as they had already been evacuated when the sampling services arrived,” said Dr. Débarre. These results add to a large body of evidence that all points in the same direction: an animal origin at the Wuhan market.
The team also found other zoonotic viruses, such as avian flu. “This study confirms that live animal markets pose a high health risk, especially when they are at the heart of urban centers,” said Dr. Débarre. “It can provide avenues for prevention, particularly by limiting interactions between humans and wild fauna.”
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Nasal Staph Aureus Carriage Linked to Surgical Infections
published in the August issue of Open Forum Infectious Diseases.
“This was a pan-European study with many hospitals, many different clinical settings, and as far as I’m aware, it hasn’t been done before. [The new study] covers a lot of European countries and a lot of surgical specialties,” said lead author Jan Kluytmans, MD. The study also captures the current state of preventive strategies in surgery, such as changes in air flow, dress, and skin preparation, he added.
The study included 5004 patients from 33 hospitals in ten European countries, of whom 67.3% were found to be SA carriers. The median age was 65 years, and 49.8% of patients were male. Open cardiac, and knee and hip prosthesis surgeries made up the largest fraction, but there were 12 types of surgery included in the study.
There were 100 SA surgical site or blood infections. The researchers found an association between surgical site or blood infection and SA carriage at any site (adjusted hazard ratio [aHR], 4.6; 95% CI, 2.1-10.0) and nasal SA carriage (aHR, 4.2; 95% CI, 2.0-8.6). Extranasal SA carriage was not associated with an increased infection risk.
Each 1-unit increase in nasal bacteria was associated with an increase in infection risk (aHR, 1.23; 95% CI, 1.05-1.43).
A strength of the study is that it is the largest prospective study yet conducted on SA carriage in surgical patients, but the researchers were unable to do a subgroup of methicillin-resistant SA (MRSA) due to small numbers of infections.
The study confirms the value of the decolonization strategy, which the World Health Organization has endorsed with the highest level of scientific evidence that is available in preventive strategies in surgery. WHO strongly recommends decolonization for cardiothoracic and orthopedic surgery using intranasal applications of mupirocin 2% ointment with or without a combination of chlorhexidine gluconate body wash. It has a conditional recommendation for a similar procedure before other types of surgery.
However, “It is not widely practiced, and although that was not a surprise to me, I think it’s really disappointing to see that proven effective strategies are not being practiced,” said Dr. Kluytmans, professor of medical microbiology at University Medical Center Utrecht, Utrecht University, the Netherlands. “If I would come into surgery being a carrier, and not be decolonized, I would really be quite angry because it puts you at risk, which is preventable. I think that’s something we owe to our patients,” he said.
He said that some may have concerns about the potential for decolonization to contribute to antibiotic resistance, but the short-term prophylaxis — typically a few days — should not foster resistance, according to Dr. Kluytmans. “If you use it short term, just before surgery, it has been shown in many studies that resistance isn’t a big problem and it can be monitored.”
The link specifically to SA nasal carriage is a mystery, according to Dr. Kluytmans. “It puzzles me still how it gets from the nares to the wound during surgery. So that’s my million-dollar question that I would like to resolve. We would like to study it, but we haven’t quite a bright idea how to do that,” he said.
The results are compelling, according to Heather Evans, MD, who was asked for comment. “On the face of it, this looks like a no-brainer. We should be decolonizing all patients that go to the operating room, and it’s not a terribly unpleasant thing for a patient to undergo to have decolonization done. Particularly for patients who are at higher risk for having a severe complication, like someone that has an operation that’s involving an implant, for example, I think it really makes a lot of sense to do this low-cost intervention for those patients,” said Dr. Evans, professor of medicine at The Medical University of South Carolina as well as the president of the Surgical Infection Society.
She noted that many facilities test for methicillin-resistant SA, but usual not SA more broadly. “This is a very interesting and compelling study that makes us rethink that, and maybe it isn’t even worth testing to see if you have staph aureus, maybe we should just be putting Betadine in everyone’s nostrils when they come to the operating room. It just seems like it would be a pretty low-cost intervention and something that could potentially have a big impact,” said Dr. Evans.
Although she was impressed by the study, Dr. Evans noted that the researchers tested for carriage at sites unrelated to the surgical site. “It really made me wonder if it would have added even more credibility to the study if there had been a sample taken after surgical prep was done to demonstrate that there is actually no staph aureus present on the skin at the time that the wound was made,” she said.
The question ties into the recent “Trojan horse” hypothesis, which suggests that endemic carriage of bacteria is responsible for most surgical site infections, rather than the long-held belief that operating room contamination is to blame. “That would sort of fly with this study, that the patient is walking around with Staph aureus and not necessarily on their skin or at their surgical site, but it’s endemic in their body,” said Dr. Evans.
Dr. Kluytmans and Dr. Evans have no relevant financial disclosures.
published in the August issue of Open Forum Infectious Diseases.
“This was a pan-European study with many hospitals, many different clinical settings, and as far as I’m aware, it hasn’t been done before. [The new study] covers a lot of European countries and a lot of surgical specialties,” said lead author Jan Kluytmans, MD. The study also captures the current state of preventive strategies in surgery, such as changes in air flow, dress, and skin preparation, he added.
The study included 5004 patients from 33 hospitals in ten European countries, of whom 67.3% were found to be SA carriers. The median age was 65 years, and 49.8% of patients were male. Open cardiac, and knee and hip prosthesis surgeries made up the largest fraction, but there were 12 types of surgery included in the study.
There were 100 SA surgical site or blood infections. The researchers found an association between surgical site or blood infection and SA carriage at any site (adjusted hazard ratio [aHR], 4.6; 95% CI, 2.1-10.0) and nasal SA carriage (aHR, 4.2; 95% CI, 2.0-8.6). Extranasal SA carriage was not associated with an increased infection risk.
Each 1-unit increase in nasal bacteria was associated with an increase in infection risk (aHR, 1.23; 95% CI, 1.05-1.43).
A strength of the study is that it is the largest prospective study yet conducted on SA carriage in surgical patients, but the researchers were unable to do a subgroup of methicillin-resistant SA (MRSA) due to small numbers of infections.
The study confirms the value of the decolonization strategy, which the World Health Organization has endorsed with the highest level of scientific evidence that is available in preventive strategies in surgery. WHO strongly recommends decolonization for cardiothoracic and orthopedic surgery using intranasal applications of mupirocin 2% ointment with or without a combination of chlorhexidine gluconate body wash. It has a conditional recommendation for a similar procedure before other types of surgery.
However, “It is not widely practiced, and although that was not a surprise to me, I think it’s really disappointing to see that proven effective strategies are not being practiced,” said Dr. Kluytmans, professor of medical microbiology at University Medical Center Utrecht, Utrecht University, the Netherlands. “If I would come into surgery being a carrier, and not be decolonized, I would really be quite angry because it puts you at risk, which is preventable. I think that’s something we owe to our patients,” he said.
He said that some may have concerns about the potential for decolonization to contribute to antibiotic resistance, but the short-term prophylaxis — typically a few days — should not foster resistance, according to Dr. Kluytmans. “If you use it short term, just before surgery, it has been shown in many studies that resistance isn’t a big problem and it can be monitored.”
The link specifically to SA nasal carriage is a mystery, according to Dr. Kluytmans. “It puzzles me still how it gets from the nares to the wound during surgery. So that’s my million-dollar question that I would like to resolve. We would like to study it, but we haven’t quite a bright idea how to do that,” he said.
The results are compelling, according to Heather Evans, MD, who was asked for comment. “On the face of it, this looks like a no-brainer. We should be decolonizing all patients that go to the operating room, and it’s not a terribly unpleasant thing for a patient to undergo to have decolonization done. Particularly for patients who are at higher risk for having a severe complication, like someone that has an operation that’s involving an implant, for example, I think it really makes a lot of sense to do this low-cost intervention for those patients,” said Dr. Evans, professor of medicine at The Medical University of South Carolina as well as the president of the Surgical Infection Society.
She noted that many facilities test for methicillin-resistant SA, but usual not SA more broadly. “This is a very interesting and compelling study that makes us rethink that, and maybe it isn’t even worth testing to see if you have staph aureus, maybe we should just be putting Betadine in everyone’s nostrils when they come to the operating room. It just seems like it would be a pretty low-cost intervention and something that could potentially have a big impact,” said Dr. Evans.
Although she was impressed by the study, Dr. Evans noted that the researchers tested for carriage at sites unrelated to the surgical site. “It really made me wonder if it would have added even more credibility to the study if there had been a sample taken after surgical prep was done to demonstrate that there is actually no staph aureus present on the skin at the time that the wound was made,” she said.
The question ties into the recent “Trojan horse” hypothesis, which suggests that endemic carriage of bacteria is responsible for most surgical site infections, rather than the long-held belief that operating room contamination is to blame. “That would sort of fly with this study, that the patient is walking around with Staph aureus and not necessarily on their skin or at their surgical site, but it’s endemic in their body,” said Dr. Evans.
Dr. Kluytmans and Dr. Evans have no relevant financial disclosures.
published in the August issue of Open Forum Infectious Diseases.
“This was a pan-European study with many hospitals, many different clinical settings, and as far as I’m aware, it hasn’t been done before. [The new study] covers a lot of European countries and a lot of surgical specialties,” said lead author Jan Kluytmans, MD. The study also captures the current state of preventive strategies in surgery, such as changes in air flow, dress, and skin preparation, he added.
The study included 5004 patients from 33 hospitals in ten European countries, of whom 67.3% were found to be SA carriers. The median age was 65 years, and 49.8% of patients were male. Open cardiac, and knee and hip prosthesis surgeries made up the largest fraction, but there were 12 types of surgery included in the study.
There were 100 SA surgical site or blood infections. The researchers found an association between surgical site or blood infection and SA carriage at any site (adjusted hazard ratio [aHR], 4.6; 95% CI, 2.1-10.0) and nasal SA carriage (aHR, 4.2; 95% CI, 2.0-8.6). Extranasal SA carriage was not associated with an increased infection risk.
Each 1-unit increase in nasal bacteria was associated with an increase in infection risk (aHR, 1.23; 95% CI, 1.05-1.43).
A strength of the study is that it is the largest prospective study yet conducted on SA carriage in surgical patients, but the researchers were unable to do a subgroup of methicillin-resistant SA (MRSA) due to small numbers of infections.
The study confirms the value of the decolonization strategy, which the World Health Organization has endorsed with the highest level of scientific evidence that is available in preventive strategies in surgery. WHO strongly recommends decolonization for cardiothoracic and orthopedic surgery using intranasal applications of mupirocin 2% ointment with or without a combination of chlorhexidine gluconate body wash. It has a conditional recommendation for a similar procedure before other types of surgery.
However, “It is not widely practiced, and although that was not a surprise to me, I think it’s really disappointing to see that proven effective strategies are not being practiced,” said Dr. Kluytmans, professor of medical microbiology at University Medical Center Utrecht, Utrecht University, the Netherlands. “If I would come into surgery being a carrier, and not be decolonized, I would really be quite angry because it puts you at risk, which is preventable. I think that’s something we owe to our patients,” he said.
He said that some may have concerns about the potential for decolonization to contribute to antibiotic resistance, but the short-term prophylaxis — typically a few days — should not foster resistance, according to Dr. Kluytmans. “If you use it short term, just before surgery, it has been shown in many studies that resistance isn’t a big problem and it can be monitored.”
The link specifically to SA nasal carriage is a mystery, according to Dr. Kluytmans. “It puzzles me still how it gets from the nares to the wound during surgery. So that’s my million-dollar question that I would like to resolve. We would like to study it, but we haven’t quite a bright idea how to do that,” he said.
The results are compelling, according to Heather Evans, MD, who was asked for comment. “On the face of it, this looks like a no-brainer. We should be decolonizing all patients that go to the operating room, and it’s not a terribly unpleasant thing for a patient to undergo to have decolonization done. Particularly for patients who are at higher risk for having a severe complication, like someone that has an operation that’s involving an implant, for example, I think it really makes a lot of sense to do this low-cost intervention for those patients,” said Dr. Evans, professor of medicine at The Medical University of South Carolina as well as the president of the Surgical Infection Society.
She noted that many facilities test for methicillin-resistant SA, but usual not SA more broadly. “This is a very interesting and compelling study that makes us rethink that, and maybe it isn’t even worth testing to see if you have staph aureus, maybe we should just be putting Betadine in everyone’s nostrils when they come to the operating room. It just seems like it would be a pretty low-cost intervention and something that could potentially have a big impact,” said Dr. Evans.
Although she was impressed by the study, Dr. Evans noted that the researchers tested for carriage at sites unrelated to the surgical site. “It really made me wonder if it would have added even more credibility to the study if there had been a sample taken after surgical prep was done to demonstrate that there is actually no staph aureus present on the skin at the time that the wound was made,” she said.
The question ties into the recent “Trojan horse” hypothesis, which suggests that endemic carriage of bacteria is responsible for most surgical site infections, rather than the long-held belief that operating room contamination is to blame. “That would sort of fly with this study, that the patient is walking around with Staph aureus and not necessarily on their skin or at their surgical site, but it’s endemic in their body,” said Dr. Evans.
Dr. Kluytmans and Dr. Evans have no relevant financial disclosures.
Pertussis Rates Up Compared With Recent Years
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
, according to data from the Centers for Disease Control and Prevention (CDC). Reports from several states illustrate this trend, thought to be due to reduced immunity across the country.
The Alaska Department of Health issued a statement on its website about the significant increase in pertussis cases in the state during the summer, with 90 cases in July and 61 in August, compared with 24 in June and a total of 26 cases in 2023.
Similarly, the Florida Department of Health reported a pertussis increase in July 2024 that was higher than the June 2024 case count and also above the previous 5-year average.
Experts in these and other states suggest that several factors are driving the nationwide increase, including the fact that fewer people are consistently wearing masks. The mass masking during the COVID-19 pandemic caused a significant drop in pertussis, but the latest data suggest a return to prepandemic levels, and waning immunity likely plays a role as well.
Pertussis, also known as whooping cough, typically begins with symptoms similar to those of the common cold, including runny nose, sneezing, mild fever, and cough, according to the CDC. However, babies with whooping cough may experience trouble breathing rather than a cough. The coughing fits often associated with pertussis may not start until 2 weeks after the onset of other symptoms, according to the CDC.
Those who have been vaccinated against pertussis can still become infected, but the risk is lower, and the illness, if it occurs, is likely to be milder. Complications such as apnea, pneumonia, and convulsions can occur in babies younger than 1 year, especially if they have not been vaccinated, according to the CDC.
Beyond Easing Pandemic Precautions
Many respiratory-based infections dipped during the COVID-19 pandemic, almost certainly from the multifactorial interventions of masking, distancing, and the general lack of comingling, said David J. Cennimo, MD, associate professor of medicine & pediatrics in the Division of Infectious Diseases at Rutgers New Jersey Medical School, Newark, New Jersey, in an interview.
The number of cases of many of these diseases returned to previous levels after COVID-19 restrictions were lifted, he said.
“However, we know pertussis immunity wanes over time. Children get DTaP at 2, 4, 6, and 15 months, and a Tdap booster at 11-12 years old gets them to adulthood,” Dr. Cennimo said. Adults should be getting a Tdap every 10 years, he added.
The latest available CDC data indicate that Tdap vaccine coverage in adults is approximately 40%, which means that there may be a large number of susceptible people who can become infected and propagate to others, said Dr. Cennimo.
Not Just the Young Ones
A recent pertussis outbreak among college students in Virginia highlighted the fact that the infection can affect all ages, and that the effectiveness of childhood vaccines may decrease over time. The majority of the recently diagnosed cases occurred in individuals who had been previously vaccinated, according to a press release from the Virginia Department of Health.
Clinical Clues
The initial stage of pertussis infection looks like a common cold with symptoms of upper respiratory infection, Dr. Cennimo told this news organization. “Unless there is reason to suspect pertussis exposure, it would almost certainly be missed,” he noted.
The characteristic barking/seal-like cough is mostly seen in children, said Dr. Cennimo. Adults and children can experience coughing fits that can lead to shortness of breath and/or vomiting, which would raise suspicion for pertussis, but is not universally present, he said. The convalescent stage of pertussis can be prolonged and is characterized by chronic coughing. “In the past, pertussis had been called the 100-day cough,” and at that point, treatment is ineffective, Dr. Cennimo said.
In clinical practice, “I advise everyone to get the Tdap vaccine every 10 years,” and remember that the “Td” is the every 10-year tetanus shot as well, Dr. Cennimo told this news organization. Reassure patients that the Tdap can be given with other vaccines, he said, and remind patients that, as with any of the respiratory illnesses, they should stay home if sick, cover a cough, consider wearing a mask in public, and wash hands frequently, he said.
Dr. Cennimo had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
A Rare Case of a Splenic Abscess as the Origin of Illness in Exudative Pleural Effusion
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6
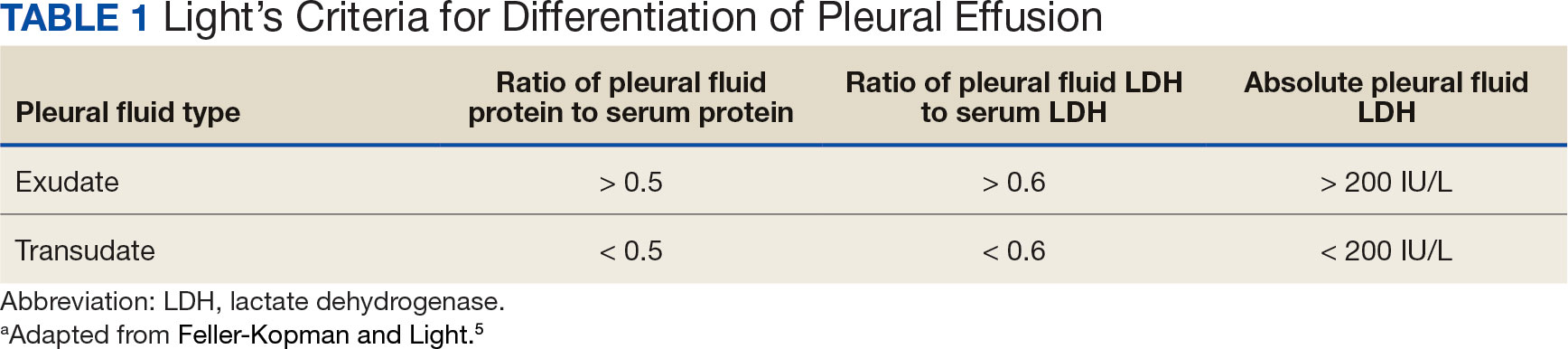
A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).
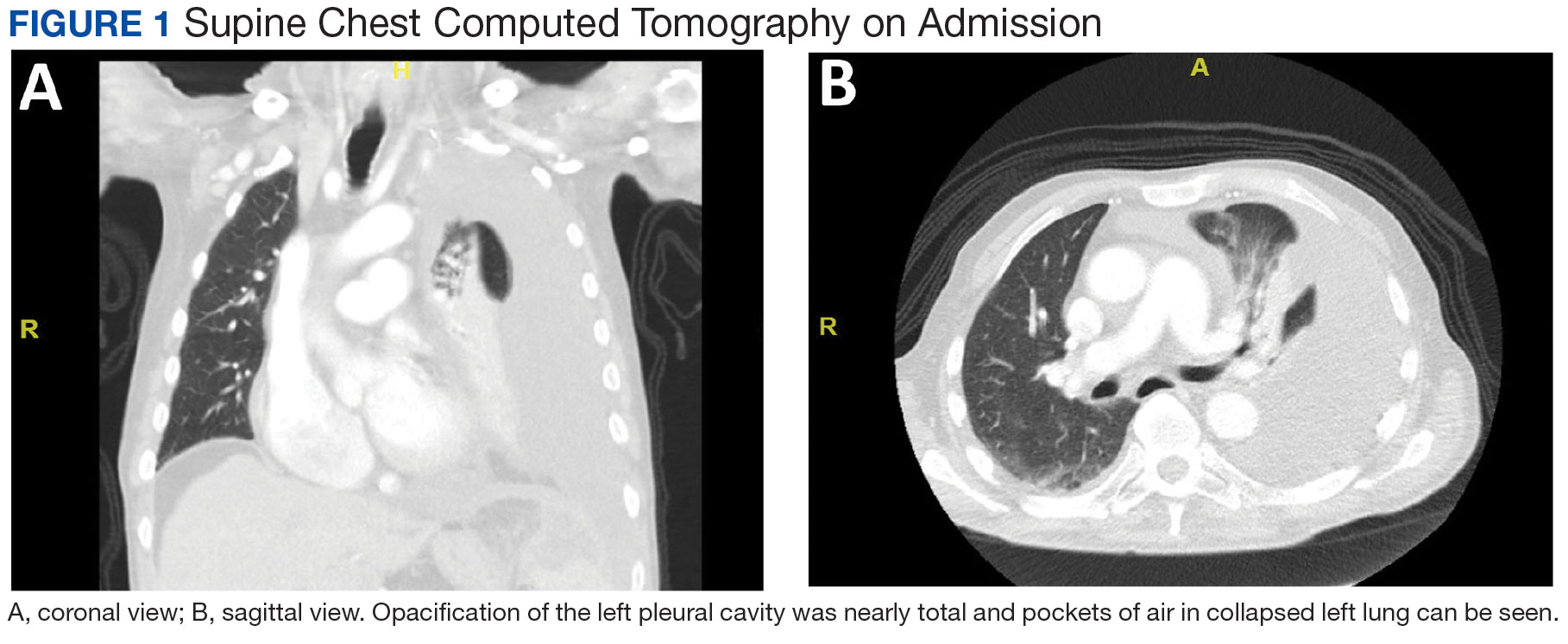
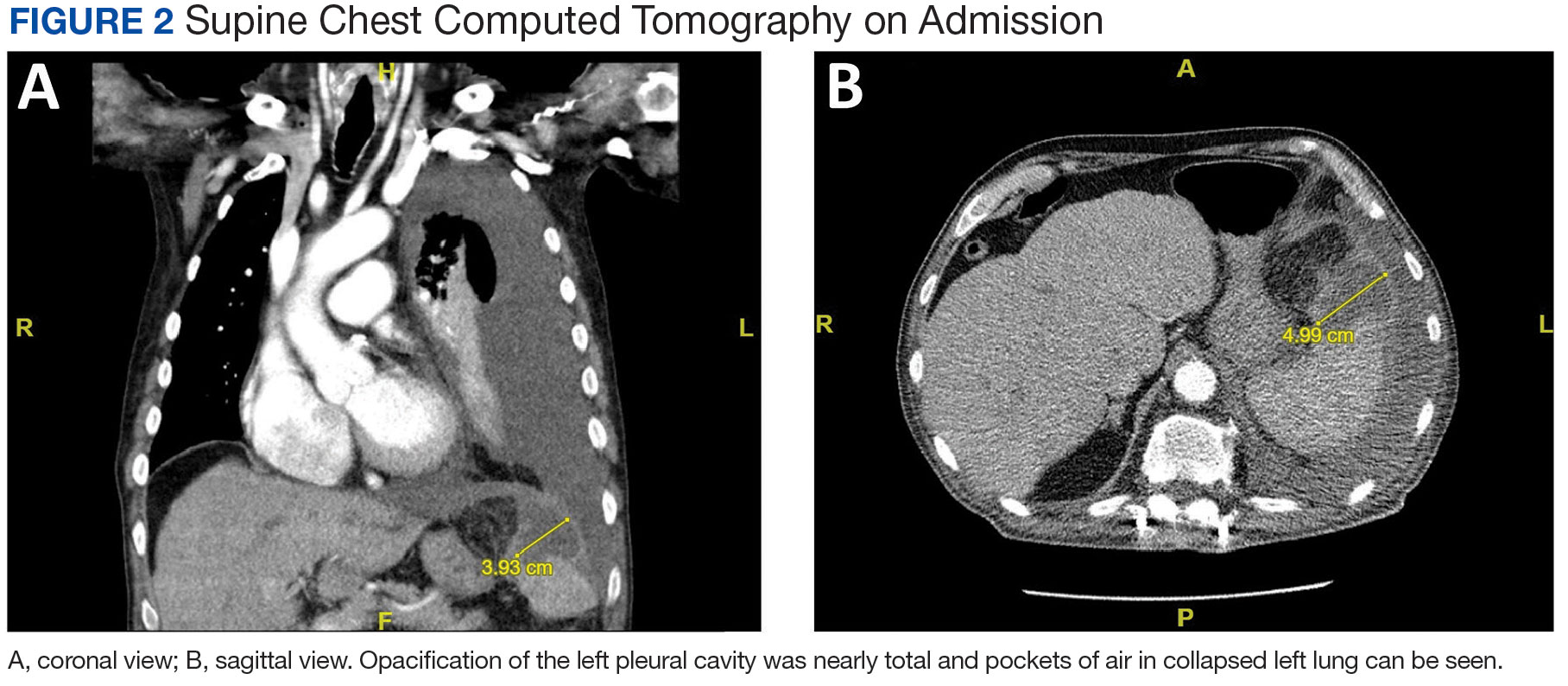
Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.
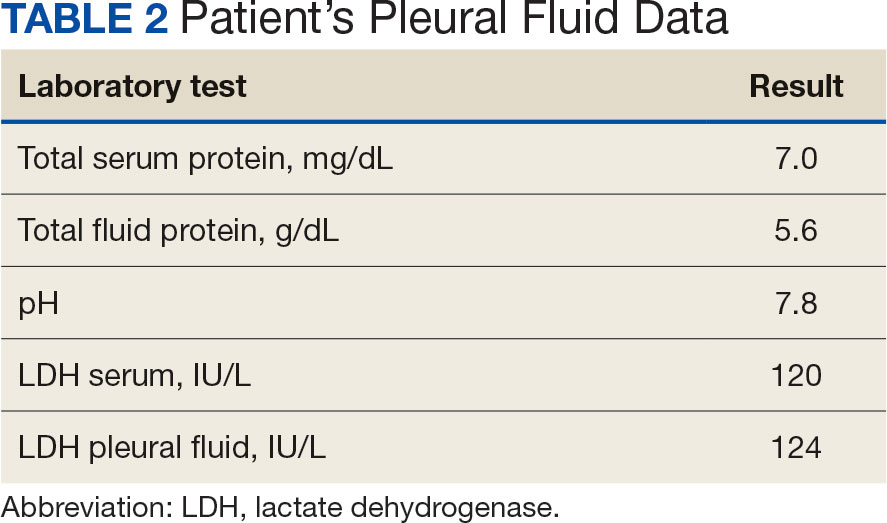
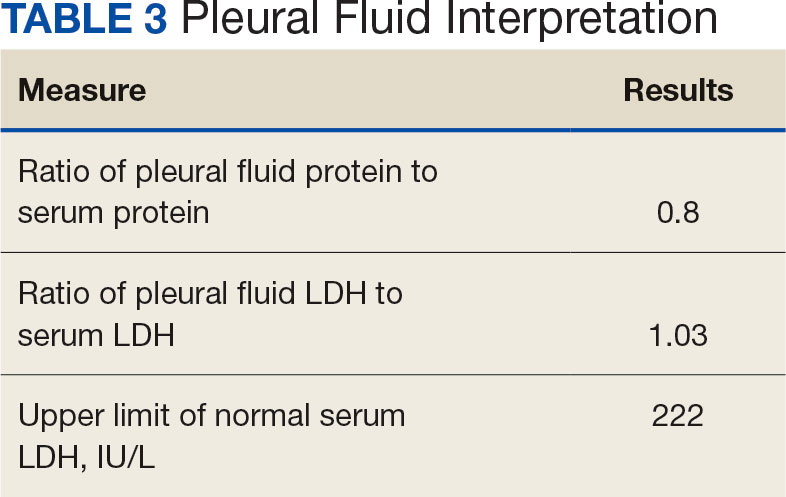
The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6

A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).


Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.


The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6

A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).


Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.


The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
Myth of the Month: Vitamin C vs the Common Cold
Case: A 38-year-old presents for acute onset runny nose, cough, and fever for the last 3 days. Her children at home have a similar presentation. She believes that she has been managing her symptoms well with Tylenol and rest. The patient is up to date on her COVID and flu shots and was wondering if there was anything else she could have done to prevent her symptoms. She saw a commercial about vitamin C supplements boosting the immune system and was wondering about their efficacy. How would you respond?
Studies of Vitamin C
Linus Pauling, FRS, did a summary of four relatively small published studies of vitamin C and concluded that vitamin C supplementation helped prevent and lessen colds.1 He mentioned a placebo-controlled study of vitamin C with viral inoculation which did not show any effect. His overall conclusion of efficacy for vitamin C led to the widespread belief that vitamin C was a proven effective therapy to prevent and treat the common cold. Since then, multiple trials and studies have examined the effect of vitamin C on the prevention and treatment of colds.
The Cochrane Review conducted a meta-analysis comparing 29 placebo-controlled trials involving 11,306 participants.2 Criteria included vitamin C supplementation of 0.2 g-1 g/day to study its efficacy in preventing the common cold. The analysis showed that supplemental vitamin C did not significantly reduce the incidence of colds. However, there was a statistically significant 8% reduction in adults and 14% in children in the duration of colds. In terms of treatment, there was no evidence of vitamin C’s efficacy.
A 2001 study conducted a small double-blind, randomized control trial to evaluate large doses of vitamin C as treatment for the common cold.3 Volunteers were divided and instructed to take varying doses ranging from 1 to 3 g of vitamin C vs a placebo at the onset of cold-like symptoms. Subjects were expected to assess the duration and severity of their cold. The data showed no significant difference in the severity or duration of cold symptoms between small or large vitamin C doses or placebo.
A more recent meta-analysis by Hemilä and Chalker looked at 10 placebo-controlled trials of vitamin C for the prevention and treatment of colds.4 The analysis showed a small 15% reduction in more severe cold symptoms.
Summary
While vitamin C is safe, there is no evidence for its ability to prevent the common cold. Although the Cochrane review and more a recent meta-analysis by Hemilä and Chalker demonstrated statistical significance in shortening the duration of symptoms, it was a minimal reduction with little clinical significance. .
Ms. Ibabao is a fourth year medical student at the University of Washington School of Medicine; Dr. Paauw is Professor of Medicine, Rathmann Family Foundation Endowed Chair Patient-centered Clinical Education, at the University of Washington School of Medicine, Seattle. They have no conflicts of interest.
References
1. Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci USA. 1971;68:2678-2671.
2. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2013;1(1).
3. Audera C et al. Mega‐dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Australia. 2001;175(7):359-362.
4. Hemilä H, Chalker E. Vitamin C reduces the severity of common colds: a meta-analysis. BMC Public Health. 2023;23:2468.
Case: A 38-year-old presents for acute onset runny nose, cough, and fever for the last 3 days. Her children at home have a similar presentation. She believes that she has been managing her symptoms well with Tylenol and rest. The patient is up to date on her COVID and flu shots and was wondering if there was anything else she could have done to prevent her symptoms. She saw a commercial about vitamin C supplements boosting the immune system and was wondering about their efficacy. How would you respond?
Studies of Vitamin C
Linus Pauling, FRS, did a summary of four relatively small published studies of vitamin C and concluded that vitamin C supplementation helped prevent and lessen colds.1 He mentioned a placebo-controlled study of vitamin C with viral inoculation which did not show any effect. His overall conclusion of efficacy for vitamin C led to the widespread belief that vitamin C was a proven effective therapy to prevent and treat the common cold. Since then, multiple trials and studies have examined the effect of vitamin C on the prevention and treatment of colds.
The Cochrane Review conducted a meta-analysis comparing 29 placebo-controlled trials involving 11,306 participants.2 Criteria included vitamin C supplementation of 0.2 g-1 g/day to study its efficacy in preventing the common cold. The analysis showed that supplemental vitamin C did not significantly reduce the incidence of colds. However, there was a statistically significant 8% reduction in adults and 14% in children in the duration of colds. In terms of treatment, there was no evidence of vitamin C’s efficacy.
A 2001 study conducted a small double-blind, randomized control trial to evaluate large doses of vitamin C as treatment for the common cold.3 Volunteers were divided and instructed to take varying doses ranging from 1 to 3 g of vitamin C vs a placebo at the onset of cold-like symptoms. Subjects were expected to assess the duration and severity of their cold. The data showed no significant difference in the severity or duration of cold symptoms between small or large vitamin C doses or placebo.
A more recent meta-analysis by Hemilä and Chalker looked at 10 placebo-controlled trials of vitamin C for the prevention and treatment of colds.4 The analysis showed a small 15% reduction in more severe cold symptoms.
Summary
While vitamin C is safe, there is no evidence for its ability to prevent the common cold. Although the Cochrane review and more a recent meta-analysis by Hemilä and Chalker demonstrated statistical significance in shortening the duration of symptoms, it was a minimal reduction with little clinical significance. .
Ms. Ibabao is a fourth year medical student at the University of Washington School of Medicine; Dr. Paauw is Professor of Medicine, Rathmann Family Foundation Endowed Chair Patient-centered Clinical Education, at the University of Washington School of Medicine, Seattle. They have no conflicts of interest.
References
1. Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci USA. 1971;68:2678-2671.
2. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2013;1(1).
3. Audera C et al. Mega‐dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Australia. 2001;175(7):359-362.
4. Hemilä H, Chalker E. Vitamin C reduces the severity of common colds: a meta-analysis. BMC Public Health. 2023;23:2468.
Case: A 38-year-old presents for acute onset runny nose, cough, and fever for the last 3 days. Her children at home have a similar presentation. She believes that she has been managing her symptoms well with Tylenol and rest. The patient is up to date on her COVID and flu shots and was wondering if there was anything else she could have done to prevent her symptoms. She saw a commercial about vitamin C supplements boosting the immune system and was wondering about their efficacy. How would you respond?
Studies of Vitamin C
Linus Pauling, FRS, did a summary of four relatively small published studies of vitamin C and concluded that vitamin C supplementation helped prevent and lessen colds.1 He mentioned a placebo-controlled study of vitamin C with viral inoculation which did not show any effect. His overall conclusion of efficacy for vitamin C led to the widespread belief that vitamin C was a proven effective therapy to prevent and treat the common cold. Since then, multiple trials and studies have examined the effect of vitamin C on the prevention and treatment of colds.
The Cochrane Review conducted a meta-analysis comparing 29 placebo-controlled trials involving 11,306 participants.2 Criteria included vitamin C supplementation of 0.2 g-1 g/day to study its efficacy in preventing the common cold. The analysis showed that supplemental vitamin C did not significantly reduce the incidence of colds. However, there was a statistically significant 8% reduction in adults and 14% in children in the duration of colds. In terms of treatment, there was no evidence of vitamin C’s efficacy.
A 2001 study conducted a small double-blind, randomized control trial to evaluate large doses of vitamin C as treatment for the common cold.3 Volunteers were divided and instructed to take varying doses ranging from 1 to 3 g of vitamin C vs a placebo at the onset of cold-like symptoms. Subjects were expected to assess the duration and severity of their cold. The data showed no significant difference in the severity or duration of cold symptoms between small or large vitamin C doses or placebo.
A more recent meta-analysis by Hemilä and Chalker looked at 10 placebo-controlled trials of vitamin C for the prevention and treatment of colds.4 The analysis showed a small 15% reduction in more severe cold symptoms.
Summary
While vitamin C is safe, there is no evidence for its ability to prevent the common cold. Although the Cochrane review and more a recent meta-analysis by Hemilä and Chalker demonstrated statistical significance in shortening the duration of symptoms, it was a minimal reduction with little clinical significance. .
Ms. Ibabao is a fourth year medical student at the University of Washington School of Medicine; Dr. Paauw is Professor of Medicine, Rathmann Family Foundation Endowed Chair Patient-centered Clinical Education, at the University of Washington School of Medicine, Seattle. They have no conflicts of interest.
References
1. Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci USA. 1971;68:2678-2671.
2. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2013;1(1).
3. Audera C et al. Mega‐dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Australia. 2001;175(7):359-362.
4. Hemilä H, Chalker E. Vitamin C reduces the severity of common colds: a meta-analysis. BMC Public Health. 2023;23:2468.