User login
Chloroquine linked to serious psychiatric side effects
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
Chloroquine may be associated with serious psychiatric side effects, even in patients with no family or personal history of psychiatric disorders, a new review suggests.
In a letter to the editor published online July 28 in The Journal of Clinical Psychiatry, the authors summarize data from several studies published as far back as 1993 and as recently as May 2020.
“In addition to previously reported side effects, chloroquine could also induce psychiatric side effects which are polymorphic and can persist even after stopping the drug,” lead author Florence Gressier, MD, PhD, CESP, Inserm, department of psychiatry, Le Kremlin Bicêtre, France, said in an interview.
“In COVID-19 patients who may still be [undergoing treatment] with chloroquine, close psychiatric assessment and monitoring should be performed,” she said.
Heated controversy
Following findings of a small French study that suggested efficacy in lowering the viral load in patients with COVID-19, President Donald Trump expressed optimism regarding the role of hydroxychloroquine in treating COVID-19, calling it a “game changer”.
Other studies, however, have called into question both the efficacy and the safety of hydroxychloroquine in treating COVID-19. On June 15, the Food and Drug Administration revoked the emergency use authorization it had given in March to chloroquine and hydroxychloroquine for the treatment of COVID-19.
Nevertheless, hydroxychloroquine continues to be prescribed for COVID-19. For example, an article that appeared in Click2Houston on June 15 quoted the chief medical officer of Houston’s United Memorial Center as saying he plans to continue prescribing hydroxychloroquine for patients with COVID-19 until he finds a better alternative.
As discussed in a Medscape expert commentary, a group of physicians who held a “white coat summit” in front of the U.S. Supreme Court building promoted the use of hydroxychloroquine for the treatment of COVID-19. The video of their summit was retweeted by President Trump and garnered millions of views before it was taken down by Twitter, Facebook, and YouTube.
Sudden onset
For the new review, “we wanted to alert the public and practitioners on the potentially psychiatric risks induced by chloroquine, as it could be taken as self-medication or potentially still prescribed,” Dr. Gressier said.
“We think the format of the letter to the editor allows information to be provided in a concise and clear manner,” she added.
According to the FDA’s Adverse Event Reporting System database, 12% of reported adverse events (520 of 4,336) following the use of chloroquine that occurred between the fourth quarter of 2012 and the fourth quarter of 2019 were neuropsychiatric. These events included amnesia, delirium, hallucinations, depression, and loss of consciousness, the authors write.
The researchers acknowledged that the incidence of psychiatric adverse effects associated with the use of chloroquine is “unclear in the absence of high-quality, randomized placebo-controlled trials of its safety.” Nevertheless, they pointed out that there have been reports of insomnia and depression when the drug was used as prophylaxis against malaria .
Moreover, some case series or case reports describe symptoms such as depression, anxiety, agitation, violent outburst, suicidal ideation, and psychosis in patients who have been treated with chloroquine for malaria, lupus erythematosus, and rheumatoid arthritis .
“In contrast to many other psychoses, chloroquine psychosis may be more affective and include prominent visual hallucinations, symptoms of derealization, and disorders of thought, with preserved insight,” the authors wrote.
They noted that the frequency of symptoms does not appear to be connected to the cumulative dose or the duration of treatment, and the onset of psychosis or other adverse effects is usually “sudden.”
In addition, they warn that the drug’s psychiatric effects may go unnoticed, especially because COVID-19 itself has been associated with neuropsychiatric symptoms, making it hard to distinguish between symptoms caused by the illness and those caused by the drug.
Although the psychiatric symptoms typically occur early after treatment initiation, some “subtle” symptoms might persist after stopping the drug, possibly owing to its “extremely long” half-life, the authors stated.
Dr. Gressier noted that practicing clinicians should look up reports about self-medication with chloroquine “and warn their patients about the risk induced by chloroquine.”
Safe but ‘not benign’
Nilanjana Bose, MD, MBA, a rheumatologist at the Rheumatology Center of Houston, said she uses hydroxychloroquine “all the time” in clinical practice to treat patients with rheumatic conditions.
“I cannot comment on whether it [hydroxychloroquine or chloroquine] is a potential prophylactic or treatment for COVID-19, but I can say that, from a safety point of view, as a rheumatologist who uses hydroxychloroquine at a dose of 400 mg/day, I do not think we need to worry about serious [psychiatric] side effects,” Dr. Bose said in an interview.
Because clinicians are trying all types of possible treatments for COVID-19, “if this medication has possible efficacy, it is a great medicine from a rheumatologic perspective and is safe,” she added.
Nevertheless, the drug is “not benign, and regular side effects will be there, and of course, higher doses will cause more side effects,” said Dr. Bose, who was not involved in authoring the letter.
She counsels patients about potential psychiatric side effects of hydroxychloroquine because some of her patients have complained about irritability, worsening anxiety and depression, and difficulty sleeping.
Be wary
James “Jimmy” Potash, MD, MPH, Henry Phipps Professor of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, said in an interview that the “take-home message of this letter is that serious psychiatric effects, psychotic illness in particular,” can occur in individuals who take chloroquine and hydroxychloroquine.
In addition, “these are potentially very concerning side effects that psychiatrists should be aware of,” noted Dr. Potash, department director and psychiatrist-in-chief at Johns Hopkins.
He said that one of his patients who had been “completely psychiatrically healthy” took chloroquine prophylactically prior to traveling overseas. After she began taking the drug, she had an episode of mania that resolved once she discontinued the medication and received treatment for the mania.
“If you add potential psychiatric side effects to the other side effects that can result from these medications, that adds up to a pretty important reason to be wary of taking them, particularly for the indication of COVID-19, where the level of evidence that it helps in any way is still quite weak,” Dr. Potash said.
In an interview, Remington Nevin, MD, MPH, DrPH, executive director at the Quinism Foundation, White River Junction, Vt., a nonprofit organization that supports and promotes education and research on disorders caused by poisoning by quinoline drugs; and faculty associate in the department of mental health at Johns Hopkins Bloomberg School of Public Health, said that the authors of the letter “are to be commended for their efforts in raising awareness of the potentially lasting and disabling psychiatric effects of chloroquine and hydroxychloroquine, which, as with similar effects from other synthetic quinoline antimalarials, have occasionally been overlooked or misattributed to other conditions.”
He added: “I have proposed that the chronic neuropsychiatric effects of this class of drug are best considered not as side effects but as signs and symptoms of a disorder known as chronic quinoline encephalopathy caused by poisoning of the central nervous system.”
Dr. Gressier and the other letter authors, Dr. Bose, and Dr. Potash have reported no relevant financial relationships. Dr. Nevin has been retained as a consultant and expert witness in legal cases involving claims of adverse effects from quinoline antimalarial drugs.
A version of this article originally appeared on Medscape.com.
Vitamin D fails to prevent late-life depression, boost mood
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
ED visits for mental health, substance use doubled in 1 decade
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).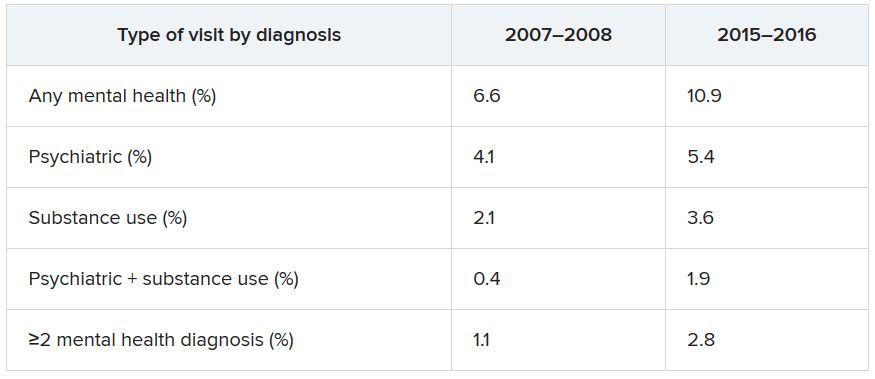
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A doctor conquers his demons
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Adam B. Hill, MD, is home on a “staycation” this week. Today, he took a 3-hour nap. I know this because I follow Dr. Hill on Twitter, where he has an active feed, a lot of posts, retweets, and more than 20,000 followers.
I also know from Twitter that he is married and has three young children, that he was once suicidal, and has been treated for major depression and alcoholism. As a palliative care doctor, Dr. Hill was required by his state medical board to blow into a breathalyzer several times a day for 5 years – something he felt quite shamed by – and while I’ve never met him, I felt just a little bit proud of this stranger when he was released from the medical board’s oversight.
Dr. Hill’s memoir, “Long Walk Out of the Woods: A Physician’s Story of Addiction, Depression, Hope, and Recovery” (Central Recovery Press, 2019) is the culmination of his efforts to use his difficulties as a way of offering hope and connection to anyone who struggled as he has, or to anyone who has struggled at all. Like his Twitter feed, it is a display of vulnerability and gratitude by someone who has been through dark times then returned to conquer his monsters.
He begins by setting the stage for us. “My name is Adam,” he announces in the preface. He tells us his various titles: human being, husband, father, physician, recovering alcoholic, and psychiatric patient. “In the midst of these struggles, working in modern medicine fractured my identity, stole my authenticity, and left me a shell of the person I wanted to be.”
We learn that he tried to buy a gun, but there was a waiting period and he could not purchase the firearm. Instead, he walked into the woods to drink himself to death, with sleeping pills as an add-on – obviously, he didn’t die, and his journey back from his failed suicidal mission is the meat of the book.
Dr. Hill was a quiet and timid child, and he was bullied at school in a way that has lingered on. He struggles with perfectionism and with a sense of never quite belonging. He was a good student, and later a competitive tennis player who was destined for a regional competition until he broke his ankle after drinking just days before the competition. He did well in college, felt more accepted, and went on to medical school after getting in from the wait list. He went on to do a pediatrics residency, and he and his wife moved from Indiana to North Carolina so he could do a hematology oncology fellowship. It was toward the end of his 2-year fellowship when he tried to purchase a gun, then walked into those woods.
His wife called, asked him to come to dinner, and he left the woods before he’d overdosed. After a meeting with his wife, parents, and sister, he returned to psychiatric care, restarted antidepressants, went to Alcoholics Anonymous, and told his employer that he had a problem. This admission started a distressing series of events, including years of being monitored by state medical boards and being labeled an “impaired physician.”
This is the only thing I didn’t like about Dr. Hill’s memoir: As open as he is about his emotional life, there were pieces missing with respect to what actually happened. At this point, I was befuddled as to why he self-reported his difficulties, and it wasn’t until he talked about starting a second fellowship in palliative care in his home state of Indiana that I could fill in some missing pieces. Dr. Hill and his wife purchased a house, and in November, he started his fellowship. The timing was off from the usual start in July, and I realized that perhaps he had gone to an inpatient setting for a number of months – his disclosure to his employer was voluntary, but his treatment likely interfered with his training and couldn’t be hidden. What else transpired he hints at: bottles hidden, driving while intoxicated, a nurse who gave him IV hydration when he came to work with a hangover.
From here, Dr. Hill’s story becomes every doctor’s nightmare. Settled into his new house and weeks into his fellowship, he is called in and fired: His application for a medical license in Indiana has been denied because of his addiction history. He met with a friend of his father’s who worked in a large pediatrics practice. The meeting went well, but the group felt he was too much of a malpractice risk.
He now needs to pay his mortgage and student loans, so he takes a position in Oklahoma with the Indian Health Service. He’s 700 miles from home, living alone in a hotel room, feeling like the work is beneath him, and the chapter is titled “Exile.” His new boss greets him with, “Listen, we all had our own stories that led us here.” Surprisingly, he likes the work and feels supported. If only it weren’t for all that loneliness, and not surprisingly, he relapses despite the mandated breathalyzer. Six beers later, and Dr. Hill is off to Chicago for a rehab program, then back to Oklahoma to finish off his stint.
What happens next is the second time I wondered about the plot of his life: Through “connections and concession,” he returns to Indiana for the palliative care fellowship, and goes on to work at Riley Children’s Hospital.
When a colleague unexpectedly dies from suicide, Dr. Hill tells others that he, too, once entertained suicidal thoughts. The story from here gets better and better: – to conquer their shame, to share their stories, to feel less alone. He and his wife become parents, he remains sober and healthy, and therapy leads him to a place of self-discovery and success.
Intertwined with telling his story, Dr. Hill takes on some of the institutional issues surrounding addiction and mental illness. He feels shamed and punished by the state medical board that mandates the terms of his medical license. Any physician who reads this book will think twice about revealing a diagnosis of depression or substance use disorder. It’s not a new idea that to protect the public, medical boards should ask about current impairments, not a past history or conditions that have been successfully treated. They should encourage treatment, not punish those who seek care.
Dr. Hill writes about how helpful it has been to allow himself to be vulnerable in the aftermath:
In my experience, the more vulnerability I show, the more opportunities I have to connect to other people. I learned the hard way that when I hide my true self from others, I spiral toward shame. Conversely, when I bury my shame, I begin to accept myself as a beautifully flawed human being, and my perspective on the world reflects that. A turn of the vulnerability dial has opened up connections to other people, while turning away pity, judgment, fear, and shame. Meanwhile, when I am to create spaces for vulnerability, permission is granted to have open and honest conversations about mental health conditions on a larger scale. But I would never have learned these lessons without having been humbled by this disease.
Perhaps the thing I liked best about Dr. Hill’s memoir is that he proposes some solutions. He talks about the importance of fighting stigma, how he finds it everywhere, and how the medical field equates mental illnesses with weakness, thereby perpetuating a self-deprecating cycle in those who have them.
In palliative care, there is an acronym – SPIKES (Set up, Perception, Invitation, Knowledge, Explore emotions, and Summary) that provides guidelines for how to deliver bad news to a family. Dr. Hill suggests using this format to discuss mental health and addictions with patients, colleagues, students. He talks about having “Compassion Rounds” to provide a safe space for his colleagues to talk about their emotional reactions to treating very ill children. And he talks about providing mental health care for trainees as an “opt out” – he schedules all his residents for a counseling session – they can cancel without repercussion, but this serves to “normalize” seeking care. I love the idea that each resident might have someone they’ve met with at least once whom they can call if the going gets rough. “As a result,” Dr. Hill writes, “once secretive conversations about attending counseling happen openly, and the physicians actually feel more comfortable going.”
Adam Hill’s memoir is short, it’s an engaging read, his openness is refreshing, and his plea to let doctors be human beings with human problems is so needed in medicine today. Thank you, Adam.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
FDA okays new indication for esketamine nasal spray
The Food and Drug Administration has approved the supplemental new drug application for esketamine nasal spray (Spravato, Janssen Pharmaceuticals) to treat depressive symptoms in adults with major depressive disorder (MDD) and acute suicidal ideation or behavior.
The FDA approved esketamine nasal spray for treatment-resistant depression in March 2019, as reported by Medscape Medical News.
– which evaluated the efficacy and safety of the nasal spray in addition to a comprehensive standard of care in adults with MDD who had active suicidal ideation with intent.
The standard of care included initial hospitalization, a newly initiated or optimized oral antidepressant, and twice-weekly treatment visits for 4 weeks. During that time, patients received esketamine nasal spray 84 mg or placebo nasal spray.
Results from the trials showed that the active treatment significantly reduced depressive symptoms within 24 hours, with some patients starting to respond as early as 4 hours after the first dose.
“Traditional oral antidepressants need weeks or more to take effect, so the availability of a medicine that can begin providing relief within a day is potentially life changing,” Theresa Nguyen, chief program officer at Mental Health America, said in a company news release.
“The clinical trials supporting this new indication provide compelling evidence that esketamine may offer clinicians a new way to provide support to patients quickly in the midst of an urgent depressive episode and help set them on the path to remission,” Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program, New Haven, Conn., and esketamine clinical trial investigator, said in the same release.
A full course of treatment for MDD with acute suicidal ideation or behavior is twice weekly for 4 weeks, “after which evidence of therapeutic benefit should be evaluated to determine need for continued treatment,” the company said.
Because of the risk for serious adverse events, including sedation and dissociation, and the potential for abuse or misuse, esketamine nasal spray is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS).
The patient self-administers esketamine nasal spray only in REMS-certified health care settings. Patients are not permitted to take the drug home.
Full prescribing information is available online.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved the supplemental new drug application for esketamine nasal spray (Spravato, Janssen Pharmaceuticals) to treat depressive symptoms in adults with major depressive disorder (MDD) and acute suicidal ideation or behavior.
The FDA approved esketamine nasal spray for treatment-resistant depression in March 2019, as reported by Medscape Medical News.
– which evaluated the efficacy and safety of the nasal spray in addition to a comprehensive standard of care in adults with MDD who had active suicidal ideation with intent.
The standard of care included initial hospitalization, a newly initiated or optimized oral antidepressant, and twice-weekly treatment visits for 4 weeks. During that time, patients received esketamine nasal spray 84 mg or placebo nasal spray.
Results from the trials showed that the active treatment significantly reduced depressive symptoms within 24 hours, with some patients starting to respond as early as 4 hours after the first dose.
“Traditional oral antidepressants need weeks or more to take effect, so the availability of a medicine that can begin providing relief within a day is potentially life changing,” Theresa Nguyen, chief program officer at Mental Health America, said in a company news release.
“The clinical trials supporting this new indication provide compelling evidence that esketamine may offer clinicians a new way to provide support to patients quickly in the midst of an urgent depressive episode and help set them on the path to remission,” Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program, New Haven, Conn., and esketamine clinical trial investigator, said in the same release.
A full course of treatment for MDD with acute suicidal ideation or behavior is twice weekly for 4 weeks, “after which evidence of therapeutic benefit should be evaluated to determine need for continued treatment,” the company said.
Because of the risk for serious adverse events, including sedation and dissociation, and the potential for abuse or misuse, esketamine nasal spray is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS).
The patient self-administers esketamine nasal spray only in REMS-certified health care settings. Patients are not permitted to take the drug home.
Full prescribing information is available online.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved the supplemental new drug application for esketamine nasal spray (Spravato, Janssen Pharmaceuticals) to treat depressive symptoms in adults with major depressive disorder (MDD) and acute suicidal ideation or behavior.
The FDA approved esketamine nasal spray for treatment-resistant depression in March 2019, as reported by Medscape Medical News.
– which evaluated the efficacy and safety of the nasal spray in addition to a comprehensive standard of care in adults with MDD who had active suicidal ideation with intent.
The standard of care included initial hospitalization, a newly initiated or optimized oral antidepressant, and twice-weekly treatment visits for 4 weeks. During that time, patients received esketamine nasal spray 84 mg or placebo nasal spray.
Results from the trials showed that the active treatment significantly reduced depressive symptoms within 24 hours, with some patients starting to respond as early as 4 hours after the first dose.
“Traditional oral antidepressants need weeks or more to take effect, so the availability of a medicine that can begin providing relief within a day is potentially life changing,” Theresa Nguyen, chief program officer at Mental Health America, said in a company news release.
“The clinical trials supporting this new indication provide compelling evidence that esketamine may offer clinicians a new way to provide support to patients quickly in the midst of an urgent depressive episode and help set them on the path to remission,” Gerard Sanacora, MD, PhD, director of the Yale Depression Research Program, New Haven, Conn., and esketamine clinical trial investigator, said in the same release.
A full course of treatment for MDD with acute suicidal ideation or behavior is twice weekly for 4 weeks, “after which evidence of therapeutic benefit should be evaluated to determine need for continued treatment,” the company said.
Because of the risk for serious adverse events, including sedation and dissociation, and the potential for abuse or misuse, esketamine nasal spray is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS).
The patient self-administers esketamine nasal spray only in REMS-certified health care settings. Patients are not permitted to take the drug home.
Full prescribing information is available online.
A version of this article originally appeared on Medscape.com.
PANS may be more prevalent than thought
Pediatric acute-onset neuropsychiatric syndrome (PANS), a rare acute onset of psychiatric symptoms, might be more common than initially thought, according to Kiki D. Chang, MD.
PANS is characterized by the National Center for Advancing Translational Sciences Genetic and Rare Diseases Information Center as a “sudden onset of obsessive-compulsive symptoms and/or severe eating restrictions, along with at least two other cognitive, behavioral, or neurological symptoms.” These symptoms can include anxiety, depression, oppositional behavior, difficulty concentrating, abnormalities in motor and sensory skills, and other somatic symptoms. The condition develops as a result of an infection that causes an autoimmune or inflammatory response in the brain, and patients tend to respond well to treatment from antibiotics, anti-inflammatory medication, and immunomodulatory therapy.
Both PANS and a subtype condition, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus infections (PANDAS), are underrecognized, Dr. Chang said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists. It is often misdiagnosed as Tourette syndrome or obsessive-compulsive disorder (OCD) because tics are present in about half of cases, he said, but more severe associated symptoms, such as psychosis, can be misdiagnosed as psychotic disorders or mood disorders. Currently, neither PANS nor PANDAS are officially recognized by the American Academy of Pediatrics or the DSM-5.
“We’re hoping that it is soon because it clearly exists,” Dr. Chang said at the meeting, presented by Global Academy for Medical Education. “If you’ve ever treated a child with PANS or PANDAS and you have seen antibiotics totally reverse OCD and tic-like behavior, if you’ve seen prednisone actually treat symptoms of mania or even psychosis and actually make those things better rather than worse, it’s really eye-opening and it makes a believer out of you.”
Anxiety is the most common psychiatric symptom in youth, and anxiety disorders are also common, said Dr. Chang. According to the National Comorbidity Survey: Adolescent Supplement, 2001-2004, 31.9% adolescents overall reported an anxiety disorder, and 8.3% said their anxiety disorder caused severe impairment. The COVID-19 pandemic has increased the level of anxiety for children and adolescents, which can lead to other disorders, such as separation anxiety disorder, panic disorder, specific phobia, social anxiety disorder, acute stress disorder, generalized anxiety disorder, OCD, or posttraumatic stress disorder. Psychiatrists should be suspicious of any sudden onset of symptoms that overlap with PANS, said Dr. Chang, who is now in private practice in Palo Alto, Calif.
“Anxiety disorders are incredibly common. Remember that you’ve got to carefully screen for other anxiety disorders, because they’re highly comorbid,” Dr. Chang said. “You’ve got to do a full workup. If there are other things going on, you’ve got to think PANS. If it’s acute onset, you’ve really got to think [PANS], and you should do that workup or refer to someone who does.”
The prevalence of PANS and PANDAS is not known, but it may be more common than psychiatrists realize, Dr. Chang said. “I’ve been doing this for about 10 years now in the PANS and PANDAS field, and it’s very clear to me that this is something that is prevalent,” he said.
Together with Jennifer Frankovich, MD, Dr. Chang founded a clinic at the Lucile Packard Children’s Hospital Stanford, and also helped to develop treatment guidelines for youth with PANS. At the clinic, patients are approximately 7.7 years old when developing the first symptoms, and are 10.7 years old when presenting for treatment. Most patients at the clinic are male (78%), and 40% are acute onset cases. Nearly all patients have symptoms of anxiety (92%), mood disorder (88%), OCD (86%), sensory/motor abnormalities (88%), irritability/aggression (82%), somatic symptoms, deterioration in school (76%), and behavioral regression (59%). More than one-third present with suicidal ideation (38%) and violence to themselves (29%), others (38%), or objects. About one-fourth have symptoms of psychosis (24%).
“These can be really sick kids,” Dr. Chang said. not able to eat because they’re afraid of things, not able to take care of their body or daily living. These were sometimes highly functional people beforehand, sometimes they weren’t, but it was still an acute change.”
Treatment for PANS
Treatment guidelines released by the PANS/PANDAS Consortium in 2017 recommend a first course of antistreptococcal treatment for new PANS cases. Psychiatrists should look for evidence of strep or other infection and use antibiotics to eradicate any underlying acute or residual infection.
“Very commonly, we’ll use things like azithromycin, or Augmentin, or amoxicillin, and you’ll see suddenly the OCD go away or at least diminish, the sleep return to normal, the mood come back down,” Dr. Chang said. “It’s pretty amazing when you see it.”
In other cases, ongoing treatment is needed for longer than the normal 5-day or 10-day course of antibiotics. “We’re not exactly sure how long: sometimes it’s 3 weeks, sometimes it’s 4 weeks, but you have to give it more than a week. Sometimes it’s the anti-inflammatory properties that are helping.” While concerns about haphazardly prescribing antibiotics are valid, “if you can cure this stuff on antibiotics, it’s low-hanging fruit,” Dr. Chang said.
There is evidence in the literature that prescribing antibiotics for PANS is beneficial. A randomized controlled trial published in 2017 showed that patients with PANS prescribed azithromycin for 4 weeks had greater reductions in severity of OCD, compared with placebo.
“We need more studies, but clearly, antibiotics do have the potential to help with certain kids. And certainly, in my practice, I see sometimes a slam-dunk response,” Dr. Chang said. “Unfortunately, sometimes you don’t see a slam-dunk response or you can’t find an infection. That’s when it might be more of an inflammation from some other reason. It could be a leftover infection, or it could be an anti-inflammatory situation.”
Immunomodulatory treatment for PANS includes use of NSAIDs, such as ibuprofen or naproxen sodium; steroids, such as prednisone or intravenous corticosteroids; intravenous immunoglobulin; or plasma exchange. Other therapies to consider are rituximab, mycophenolate mofetil, and cyclophosphamide.
Some psychiatric treatments may help patients with PANS. While there is no empirical evidence that psychotropics are effective in treating PANS, some SSRIs might help if patients are able to handle any adverse events. Psychotherapy and education of the family are also important for patients with PANS and their caregivers.
“Basically, [PANS] has as high a caregiver burden as having someone in the household with Alzheimer’s disease or cancer. It’s a huge burden, it’s very stressful, and the family needs support for this,” Dr. Chang said.
Global Academy and this news organization are owned by the same parent company. Dr. Chang reports he is a consultant for Allergan, Impel NeuroPharma, and Sunovion. He is also on the speaker’s bureau for Sunovion.
Pediatric acute-onset neuropsychiatric syndrome (PANS), a rare acute onset of psychiatric symptoms, might be more common than initially thought, according to Kiki D. Chang, MD.
PANS is characterized by the National Center for Advancing Translational Sciences Genetic and Rare Diseases Information Center as a “sudden onset of obsessive-compulsive symptoms and/or severe eating restrictions, along with at least two other cognitive, behavioral, or neurological symptoms.” These symptoms can include anxiety, depression, oppositional behavior, difficulty concentrating, abnormalities in motor and sensory skills, and other somatic symptoms. The condition develops as a result of an infection that causes an autoimmune or inflammatory response in the brain, and patients tend to respond well to treatment from antibiotics, anti-inflammatory medication, and immunomodulatory therapy.
Both PANS and a subtype condition, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus infections (PANDAS), are underrecognized, Dr. Chang said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists. It is often misdiagnosed as Tourette syndrome or obsessive-compulsive disorder (OCD) because tics are present in about half of cases, he said, but more severe associated symptoms, such as psychosis, can be misdiagnosed as psychotic disorders or mood disorders. Currently, neither PANS nor PANDAS are officially recognized by the American Academy of Pediatrics or the DSM-5.
“We’re hoping that it is soon because it clearly exists,” Dr. Chang said at the meeting, presented by Global Academy for Medical Education. “If you’ve ever treated a child with PANS or PANDAS and you have seen antibiotics totally reverse OCD and tic-like behavior, if you’ve seen prednisone actually treat symptoms of mania or even psychosis and actually make those things better rather than worse, it’s really eye-opening and it makes a believer out of you.”
Anxiety is the most common psychiatric symptom in youth, and anxiety disorders are also common, said Dr. Chang. According to the National Comorbidity Survey: Adolescent Supplement, 2001-2004, 31.9% adolescents overall reported an anxiety disorder, and 8.3% said their anxiety disorder caused severe impairment. The COVID-19 pandemic has increased the level of anxiety for children and adolescents, which can lead to other disorders, such as separation anxiety disorder, panic disorder, specific phobia, social anxiety disorder, acute stress disorder, generalized anxiety disorder, OCD, or posttraumatic stress disorder. Psychiatrists should be suspicious of any sudden onset of symptoms that overlap with PANS, said Dr. Chang, who is now in private practice in Palo Alto, Calif.
“Anxiety disorders are incredibly common. Remember that you’ve got to carefully screen for other anxiety disorders, because they’re highly comorbid,” Dr. Chang said. “You’ve got to do a full workup. If there are other things going on, you’ve got to think PANS. If it’s acute onset, you’ve really got to think [PANS], and you should do that workup or refer to someone who does.”
The prevalence of PANS and PANDAS is not known, but it may be more common than psychiatrists realize, Dr. Chang said. “I’ve been doing this for about 10 years now in the PANS and PANDAS field, and it’s very clear to me that this is something that is prevalent,” he said.
Together with Jennifer Frankovich, MD, Dr. Chang founded a clinic at the Lucile Packard Children’s Hospital Stanford, and also helped to develop treatment guidelines for youth with PANS. At the clinic, patients are approximately 7.7 years old when developing the first symptoms, and are 10.7 years old when presenting for treatment. Most patients at the clinic are male (78%), and 40% are acute onset cases. Nearly all patients have symptoms of anxiety (92%), mood disorder (88%), OCD (86%), sensory/motor abnormalities (88%), irritability/aggression (82%), somatic symptoms, deterioration in school (76%), and behavioral regression (59%). More than one-third present with suicidal ideation (38%) and violence to themselves (29%), others (38%), or objects. About one-fourth have symptoms of psychosis (24%).
“These can be really sick kids,” Dr. Chang said. not able to eat because they’re afraid of things, not able to take care of their body or daily living. These were sometimes highly functional people beforehand, sometimes they weren’t, but it was still an acute change.”
Treatment for PANS
Treatment guidelines released by the PANS/PANDAS Consortium in 2017 recommend a first course of antistreptococcal treatment for new PANS cases. Psychiatrists should look for evidence of strep or other infection and use antibiotics to eradicate any underlying acute or residual infection.
“Very commonly, we’ll use things like azithromycin, or Augmentin, or amoxicillin, and you’ll see suddenly the OCD go away or at least diminish, the sleep return to normal, the mood come back down,” Dr. Chang said. “It’s pretty amazing when you see it.”
In other cases, ongoing treatment is needed for longer than the normal 5-day or 10-day course of antibiotics. “We’re not exactly sure how long: sometimes it’s 3 weeks, sometimes it’s 4 weeks, but you have to give it more than a week. Sometimes it’s the anti-inflammatory properties that are helping.” While concerns about haphazardly prescribing antibiotics are valid, “if you can cure this stuff on antibiotics, it’s low-hanging fruit,” Dr. Chang said.
There is evidence in the literature that prescribing antibiotics for PANS is beneficial. A randomized controlled trial published in 2017 showed that patients with PANS prescribed azithromycin for 4 weeks had greater reductions in severity of OCD, compared with placebo.
“We need more studies, but clearly, antibiotics do have the potential to help with certain kids. And certainly, in my practice, I see sometimes a slam-dunk response,” Dr. Chang said. “Unfortunately, sometimes you don’t see a slam-dunk response or you can’t find an infection. That’s when it might be more of an inflammation from some other reason. It could be a leftover infection, or it could be an anti-inflammatory situation.”
Immunomodulatory treatment for PANS includes use of NSAIDs, such as ibuprofen or naproxen sodium; steroids, such as prednisone or intravenous corticosteroids; intravenous immunoglobulin; or plasma exchange. Other therapies to consider are rituximab, mycophenolate mofetil, and cyclophosphamide.
Some psychiatric treatments may help patients with PANS. While there is no empirical evidence that psychotropics are effective in treating PANS, some SSRIs might help if patients are able to handle any adverse events. Psychotherapy and education of the family are also important for patients with PANS and their caregivers.
“Basically, [PANS] has as high a caregiver burden as having someone in the household with Alzheimer’s disease or cancer. It’s a huge burden, it’s very stressful, and the family needs support for this,” Dr. Chang said.
Global Academy and this news organization are owned by the same parent company. Dr. Chang reports he is a consultant for Allergan, Impel NeuroPharma, and Sunovion. He is also on the speaker’s bureau for Sunovion.
Pediatric acute-onset neuropsychiatric syndrome (PANS), a rare acute onset of psychiatric symptoms, might be more common than initially thought, according to Kiki D. Chang, MD.
PANS is characterized by the National Center for Advancing Translational Sciences Genetic and Rare Diseases Information Center as a “sudden onset of obsessive-compulsive symptoms and/or severe eating restrictions, along with at least two other cognitive, behavioral, or neurological symptoms.” These symptoms can include anxiety, depression, oppositional behavior, difficulty concentrating, abnormalities in motor and sensory skills, and other somatic symptoms. The condition develops as a result of an infection that causes an autoimmune or inflammatory response in the brain, and patients tend to respond well to treatment from antibiotics, anti-inflammatory medication, and immunomodulatory therapy.
Both PANS and a subtype condition, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus infections (PANDAS), are underrecognized, Dr. Chang said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists. It is often misdiagnosed as Tourette syndrome or obsessive-compulsive disorder (OCD) because tics are present in about half of cases, he said, but more severe associated symptoms, such as psychosis, can be misdiagnosed as psychotic disorders or mood disorders. Currently, neither PANS nor PANDAS are officially recognized by the American Academy of Pediatrics or the DSM-5.
“We’re hoping that it is soon because it clearly exists,” Dr. Chang said at the meeting, presented by Global Academy for Medical Education. “If you’ve ever treated a child with PANS or PANDAS and you have seen antibiotics totally reverse OCD and tic-like behavior, if you’ve seen prednisone actually treat symptoms of mania or even psychosis and actually make those things better rather than worse, it’s really eye-opening and it makes a believer out of you.”
Anxiety is the most common psychiatric symptom in youth, and anxiety disorders are also common, said Dr. Chang. According to the National Comorbidity Survey: Adolescent Supplement, 2001-2004, 31.9% adolescents overall reported an anxiety disorder, and 8.3% said their anxiety disorder caused severe impairment. The COVID-19 pandemic has increased the level of anxiety for children and adolescents, which can lead to other disorders, such as separation anxiety disorder, panic disorder, specific phobia, social anxiety disorder, acute stress disorder, generalized anxiety disorder, OCD, or posttraumatic stress disorder. Psychiatrists should be suspicious of any sudden onset of symptoms that overlap with PANS, said Dr. Chang, who is now in private practice in Palo Alto, Calif.
“Anxiety disorders are incredibly common. Remember that you’ve got to carefully screen for other anxiety disorders, because they’re highly comorbid,” Dr. Chang said. “You’ve got to do a full workup. If there are other things going on, you’ve got to think PANS. If it’s acute onset, you’ve really got to think [PANS], and you should do that workup or refer to someone who does.”
The prevalence of PANS and PANDAS is not known, but it may be more common than psychiatrists realize, Dr. Chang said. “I’ve been doing this for about 10 years now in the PANS and PANDAS field, and it’s very clear to me that this is something that is prevalent,” he said.
Together with Jennifer Frankovich, MD, Dr. Chang founded a clinic at the Lucile Packard Children’s Hospital Stanford, and also helped to develop treatment guidelines for youth with PANS. At the clinic, patients are approximately 7.7 years old when developing the first symptoms, and are 10.7 years old when presenting for treatment. Most patients at the clinic are male (78%), and 40% are acute onset cases. Nearly all patients have symptoms of anxiety (92%), mood disorder (88%), OCD (86%), sensory/motor abnormalities (88%), irritability/aggression (82%), somatic symptoms, deterioration in school (76%), and behavioral regression (59%). More than one-third present with suicidal ideation (38%) and violence to themselves (29%), others (38%), or objects. About one-fourth have symptoms of psychosis (24%).
“These can be really sick kids,” Dr. Chang said. not able to eat because they’re afraid of things, not able to take care of their body or daily living. These were sometimes highly functional people beforehand, sometimes they weren’t, but it was still an acute change.”
Treatment for PANS
Treatment guidelines released by the PANS/PANDAS Consortium in 2017 recommend a first course of antistreptococcal treatment for new PANS cases. Psychiatrists should look for evidence of strep or other infection and use antibiotics to eradicate any underlying acute or residual infection.
“Very commonly, we’ll use things like azithromycin, or Augmentin, or amoxicillin, and you’ll see suddenly the OCD go away or at least diminish, the sleep return to normal, the mood come back down,” Dr. Chang said. “It’s pretty amazing when you see it.”
In other cases, ongoing treatment is needed for longer than the normal 5-day or 10-day course of antibiotics. “We’re not exactly sure how long: sometimes it’s 3 weeks, sometimes it’s 4 weeks, but you have to give it more than a week. Sometimes it’s the anti-inflammatory properties that are helping.” While concerns about haphazardly prescribing antibiotics are valid, “if you can cure this stuff on antibiotics, it’s low-hanging fruit,” Dr. Chang said.
There is evidence in the literature that prescribing antibiotics for PANS is beneficial. A randomized controlled trial published in 2017 showed that patients with PANS prescribed azithromycin for 4 weeks had greater reductions in severity of OCD, compared with placebo.
“We need more studies, but clearly, antibiotics do have the potential to help with certain kids. And certainly, in my practice, I see sometimes a slam-dunk response,” Dr. Chang said. “Unfortunately, sometimes you don’t see a slam-dunk response or you can’t find an infection. That’s when it might be more of an inflammation from some other reason. It could be a leftover infection, or it could be an anti-inflammatory situation.”
Immunomodulatory treatment for PANS includes use of NSAIDs, such as ibuprofen or naproxen sodium; steroids, such as prednisone or intravenous corticosteroids; intravenous immunoglobulin; or plasma exchange. Other therapies to consider are rituximab, mycophenolate mofetil, and cyclophosphamide.
Some psychiatric treatments may help patients with PANS. While there is no empirical evidence that psychotropics are effective in treating PANS, some SSRIs might help if patients are able to handle any adverse events. Psychotherapy and education of the family are also important for patients with PANS and their caregivers.
“Basically, [PANS] has as high a caregiver burden as having someone in the household with Alzheimer’s disease or cancer. It’s a huge burden, it’s very stressful, and the family needs support for this,” Dr. Chang said.
Global Academy and this news organization are owned by the same parent company. Dr. Chang reports he is a consultant for Allergan, Impel NeuroPharma, and Sunovion. He is also on the speaker’s bureau for Sunovion.
FROM CP/AACP PSYCHIATRY UPDATE
‘Staggering’ increase in COVID-linked depression, anxiety
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Since the start of the COVID-19 pandemic, there has been a dramatic increase in depression, anxiety, psychosis, and suicidality, new research shows.
The new data, released by Mental Health America (MHA), came from individuals who completed a voluntary online mental health screen.
As of the end of June, over 169,000 additional participants reported having moderate to severe depression or anxiety, compared with participants who completed the screen prior to the pandemic.
In June alone, 18,000 additional participants were found to be at risk for psychosis, continuing a rising pattern that began in May, when 16,000 reported psychosis risk.
“We continue to see staggering numbers that indicate increased rates in depression and anxiety because of COVID-19,” Paul Gionfriddo, president and CEO of MHA, said in a release.
“In fact, the problem is bigger than anyone imagined, making it clear how the pandemic is affecting people now and will continue to affect people who mourn loved ones and whose serious mental conditions are left untreated. So we need to take this very seriously,” Mr. Gionfriddo said in an interview.
Real-time data
MHA has been conducting online screenings for 6 years. To date, nearly 5.5 million screenings have been completed, making it the largest screening program of its kind in the United States, Mr. Gionfriddo reported.
“At the beginning of the pandemic, we were asked by a member of the media if we could offer any insight about how anxiety in particular was affecting people during the pandemic since we were the only ones with a database that could give quantitative detail,” he said.
The results of their screen could also help find that information “in real time,” he added.
More people are now undergoing mental health screenings, Mr. Gionfriddo noted.
At roughly 7,000 per day in May and June, the number of anxiety and depression screenings that were completed per day were 406% and 457% higher, respectively, than the number completed in January.
The youngest group of participants were those aged 11-17 years; the oldest age group consisted of individuals 65 years and older.
The Patient Health Questionnaire–9 was used to identify those at risk for depression, the General Anxiety Disorder–7 was used to identify those at risk for anxiety, and the Prodromal Questionnaire Brief Version was used to identify those at high risk for psychosis.
Current events
Roughly 90% screened positive for moderate to severe depression, and 80% screened positive for moderate to severe anxiety.
“Kids between the ages of 11 and 17 years have been the most stressed, but it seems to be easier to bear as you get older,” Mr. Gionfriddo said.
Loneliness and isolation were cited as contributors to depression and anxiety by the largest percentage of individuals with these conditions (74% and 65%, respectively).
In June, roughly one quarter of participants also cited grief or loss and financial concerns as contributors to anxiety (25.31% and 24.18%, respectively) and to depression (26.53% and 23.36%).
Current events were cited as an important contributor, leading to more mental health problems in June, compared with May (36.11% vs 29.41 for anxiety; 29.13% vs 21.77% for depression).
The June screen added the category of racism as a potential contributor. Close to 8% reported it as a reason for anxiety, and roughly 5% considered it a reason for depression.
“We will be releasing more data at the end of July, and it will be interesting to see how the racism category compares to data we collected at the end of June,” Mr. Gionfriddo noted.
Dramatic increase
The screen also showed a “dramatic increase” in the number of people who reported being at risk for psychosis, with 18,000 participants screening positive. This represented more than four times the baseline figures recorded through March.
“We were not surprised to see a spike in depression and anxiety, but why were we seeing a spike in psychosis in May/June?” Mr. Gionfriddo asked. He suggested that stress may play a role in driving this increased risk.
“These data, we hope, will get policymakers to pay attention, take it seriously, and intervene to prevent psychosis at an earlier stage before signs and symptoms emerge,” said Mr. Gionfriddo.
One of the most alarming findings was that in June, 25,498 participants who screened positive for depression reported thinking of suicide or self-harm on “more than half of days to nearly every day.” A total of 14,607 participants said they had these thoughts every day.
Overall, the results should reinforce the recommendations of the US Preventive Services Task Force to routinely screen for depression in any clinical setting on a regular basis, Mr. Gionfriddo said.
In addition, policymakers “need to balance reopening vs. quarantining and isolating, and we need to think about what the next 2-4 years look like in terms of balancing physical health risks and mental health risks,” he noted.
“We’ve been treating the pandemic like a sprint and now, 4 or 5 months into it, perhaps as a middle-distance run, when in fact it’s a marathon,” he added.
Advocates needed
The increase in anxiety and depression often centers on the changes and uncertainties in the college experience, such as whether classes will be held in person, online, or a hybrid of the two, said Dr. Ritchie, who was not involved with the research.
Additionally, some college students who have “left the nest” have been forced to “return to the nest,” which compounds stress, she said.
LGBTQ youngsters may be particularly affected because some have “come out of the closet” while away from home and now must negotiate going back to their home of record. They are uncertain whether or not “to go back into the closet,” added Dr. Ritchie, who is also vice chair of psychiatry at Georgetown University, Washington.
Psychiatrists and other mental health professionals should be advocates for “getting services to more people for the greatest good,” she noted.
For example, the MHA data “might be useful in advocating for keeping telehealth accessible and even promoting it,” she said.
The full report is available on MHA’s website.
Mr. Gionfriddo and Dr. Ritchie report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Microbiome research ‘opening doors’ to new Alzheimer’s disease treatments
Research into the microbiome is yielding some positive new potential treatment options for Alzheimer’s disease, according to George T. Grossberg, MD.
“I think the growing focus on the gut-brain axis is opening doors to new Alzheimer’s disease and other brain disorders, and I think the first of a possible future generation of compounds for prevention or treatment of Alzheimer’s disease may indeed be emerging,” Dr. Grossberg said at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Focus on the microbiome and microbiota is “a really hot, really new, really emerging area,” said Dr. Grossberg, professor in the department of psychiatry & behavioral neuroscience at Saint Louis University. But the microbiota, which is the microorganisms within a specific organ such as the colon, is sometimes confused with the microbiome – which is defined as all of the bacteria, viruses, fungi and other microorganisms within a habitat as well as their genomes and the environment around them. “These are often used interchangeably, but they’re not the same,” Dr. Grossberg said at the meeting, presented by Global Academy for Medical Education.
A person’s microbiome is unique to them, and nearly all of the microbiome is contained in the gut. A reduction in diversity of the microbiota in the digestive system has been linked to a wide variety of diseases, Dr. Grossberg explained. Conversely, a microbial imbalance or dysbiosis has been implicated in anxiety and/or depression, dementia, and certain cancers, he noted.
Bacteria that positively affect the microbiome come from two main genera: Lactobacillus and Bifidobacterium. Factors such as diet, medications, geography, stage of life, birthing process, infant feeding method, and stress can all affect a person’s microbiome. “We’re all beginning to understand that trying to manage or trying to diversify, trying to manipulate the microbiota may have a lot of remote effects – even effects on weight or diabetes, or other disorders,” Dr. Grossberg said.
Fecal microbiota transplantation (FMT), or the process of administering a donor’s fecal matter into a recipient’s intestinal tract, has proved beneficial in improving the health of patients suffering from recurrent Clostridioides difficile infection. A recent Harvard Health Letter, written by Jessica Allegretti, MD, MPH, observed that FMT is standard of care for patients with C. diff, and the procedure has a success rate of between 80% and 90%.
“It shows us very directly, in a very practical way, how addressing the dysbiosis – the imbalance of the gut microbiome – by infusing healthy bacteria may make a potential lifesaving difference,” Dr. Grossberg said.
Research is beginning to show that the link between gut microbiota and health extends to Alzheimer’s disease as well. Within the last few years, “we’ve started to understand that the microbial diversity in Alzheimer’s disease versus healthy age-matched controls is decreased,” Dr. Grossberg said.
In a study published by Nicholas M. Vogt and colleagues, there was decreased fecal microbial diversity among individuals with Alzheimer’s, compared with healthy individuals matched for age. Another study by Ping Liu, PhD, and colleagues found that patients with Alzheimer’s disease had decreased fecal microbial diversity, compared with individuals who had pre-onset amnestic mild cognitive impairment and normal cognition.
Dr. Grossberg noted that, while these studies do not prove that less fecal microbial diversity is responsible for mild cognitive impairment or Alzheimer’s disease, “it makes us think that, maybe, there’s a contributing factor.”
“What happens with the dysbiosis of the gut microbiome is increased permeability of the epithelial area of the gut, which can then lead to the gut-brain axis dysregulation and may in fact allow the selective entry of bacteria into the central nervous system because the blood-brain barrier comes to be dysfunctional,” he said.
Early evidence suggests that the gut-brain axis can affect cognition. In an animal model study, transferring the microbiota of a mouse with Alzheimer’s disease to one that had been bred to be germ-free resulted in cognitive decline – but there was no cognitive decline for germ-free mice that received a microbiota transplant from a mouse in a healthy control group. Results from another animal study showed that transferring healthy microbiota from a mouse model into a mouse with Alzheimer’s disease reduces amyloid and tau pathology. “The conclusions of these studies seems to be that microbiota mediated intestinal and systemic immune changes or aberrations seem to contribute to the pathogenesis of Alzheimer’s disease in these mouse models,” Dr. Grossberg said. “Consequently, restoring the gut microbial homeostasis may have beneficial effects on Alzheimer’s disease treatment.”
Periodontal disease also might be linked to Alzheimer’s disease, Dr. Grossberg said. Several studies have shown gingipains secreted from Porphyromonas gingivalis, which contribute to inflammation in the brain, have been found in cadavers of patients with Alzheimer’s disease (Sci Adv. 2019 Jan 23;5[1]:eaau3333). “There’s reason to think that the same changes may be occurring in the human brain with periodontal disease,” he said.
The relationship also might extend to the gut microbiota and the central nervous system. “There seems to be a direct communication, a direct relationship between normal gut physiology and healthy central nervous system functioning, and then, when you have abnormal gut function, it may result in a variety of abnormal central nervous system functions,” Dr. Grossberg said.
Studies that have examined a relationship between Alzheimer’s disease and gut microbiota have highlighted the potential of probiotics and prebiotics as a method of restoring the gut microbiota (Aging [Albany NY]. 2020 Mar 31; 12[6]:5539-50). Probiotics are popularly sold in health food aisles of grocery stores, and prebiotics are available in foods such as yogurts, tempeh, sauerkraut, and kimchi, as well as in drinks such as Kombucha tea. The effectiveness of probiotics and prebiotics also are being examined in randomized, controlled trials in patients with mild cognitive decline and mild Alzheimer’s disease, Dr. Grossberg said. One therapy, Sodium oligomannate, a marine algae–derived oral oligosaccharide, has shown effectiveness in remodeling gut microbiota and has been approved in China to treat patients with mild or moderate Alzheimer’s disease. Currently, no approved gut microbiota therapies are approved in the United States to treat Alzheimer’s disease; however, encouraging use of a prebiotic, a probiotic, or a Mediterranean diet is something clinicians might want to consider for their patients.
“The fact that we’re studying these things has really led to the notion that it may not be a bad idea for people to consume these healthy bacteria in later life, either as a way to prevent or delay, or to treat Alzheimer’s disease,” Dr. Grossberg said. “There’s really no downside.”
Global Academy and this news organization are owned by the same parent company. Dr. Grossberg reported that he is a consultant for Acadia, Alkahest, Avanir, Axsome, Biogen, BioXcel, Karuna, Lundbeck, Otsuka, Roche, and Takeda; receives research support from the National Institute on Aging, Janssen, and Roche; performs safety monitoring for EryDel, Merck, and Newron; and serves on data monitoring committees for Avanex and ITI Therapeutics.
Research into the microbiome is yielding some positive new potential treatment options for Alzheimer’s disease, according to George T. Grossberg, MD.
“I think the growing focus on the gut-brain axis is opening doors to new Alzheimer’s disease and other brain disorders, and I think the first of a possible future generation of compounds for prevention or treatment of Alzheimer’s disease may indeed be emerging,” Dr. Grossberg said at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Focus on the microbiome and microbiota is “a really hot, really new, really emerging area,” said Dr. Grossberg, professor in the department of psychiatry & behavioral neuroscience at Saint Louis University. But the microbiota, which is the microorganisms within a specific organ such as the colon, is sometimes confused with the microbiome – which is defined as all of the bacteria, viruses, fungi and other microorganisms within a habitat as well as their genomes and the environment around them. “These are often used interchangeably, but they’re not the same,” Dr. Grossberg said at the meeting, presented by Global Academy for Medical Education.
A person’s microbiome is unique to them, and nearly all of the microbiome is contained in the gut. A reduction in diversity of the microbiota in the digestive system has been linked to a wide variety of diseases, Dr. Grossberg explained. Conversely, a microbial imbalance or dysbiosis has been implicated in anxiety and/or depression, dementia, and certain cancers, he noted.
Bacteria that positively affect the microbiome come from two main genera: Lactobacillus and Bifidobacterium. Factors such as diet, medications, geography, stage of life, birthing process, infant feeding method, and stress can all affect a person’s microbiome. “We’re all beginning to understand that trying to manage or trying to diversify, trying to manipulate the microbiota may have a lot of remote effects – even effects on weight or diabetes, or other disorders,” Dr. Grossberg said.
Fecal microbiota transplantation (FMT), or the process of administering a donor’s fecal matter into a recipient’s intestinal tract, has proved beneficial in improving the health of patients suffering from recurrent Clostridioides difficile infection. A recent Harvard Health Letter, written by Jessica Allegretti, MD, MPH, observed that FMT is standard of care for patients with C. diff, and the procedure has a success rate of between 80% and 90%.
“It shows us very directly, in a very practical way, how addressing the dysbiosis – the imbalance of the gut microbiome – by infusing healthy bacteria may make a potential lifesaving difference,” Dr. Grossberg said.
Research is beginning to show that the link between gut microbiota and health extends to Alzheimer’s disease as well. Within the last few years, “we’ve started to understand that the microbial diversity in Alzheimer’s disease versus healthy age-matched controls is decreased,” Dr. Grossberg said.
In a study published by Nicholas M. Vogt and colleagues, there was decreased fecal microbial diversity among individuals with Alzheimer’s, compared with healthy individuals matched for age. Another study by Ping Liu, PhD, and colleagues found that patients with Alzheimer’s disease had decreased fecal microbial diversity, compared with individuals who had pre-onset amnestic mild cognitive impairment and normal cognition.
Dr. Grossberg noted that, while these studies do not prove that less fecal microbial diversity is responsible for mild cognitive impairment or Alzheimer’s disease, “it makes us think that, maybe, there’s a contributing factor.”
“What happens with the dysbiosis of the gut microbiome is increased permeability of the epithelial area of the gut, which can then lead to the gut-brain axis dysregulation and may in fact allow the selective entry of bacteria into the central nervous system because the blood-brain barrier comes to be dysfunctional,” he said.
Early evidence suggests that the gut-brain axis can affect cognition. In an animal model study, transferring the microbiota of a mouse with Alzheimer’s disease to one that had been bred to be germ-free resulted in cognitive decline – but there was no cognitive decline for germ-free mice that received a microbiota transplant from a mouse in a healthy control group. Results from another animal study showed that transferring healthy microbiota from a mouse model into a mouse with Alzheimer’s disease reduces amyloid and tau pathology. “The conclusions of these studies seems to be that microbiota mediated intestinal and systemic immune changes or aberrations seem to contribute to the pathogenesis of Alzheimer’s disease in these mouse models,” Dr. Grossberg said. “Consequently, restoring the gut microbial homeostasis may have beneficial effects on Alzheimer’s disease treatment.”
Periodontal disease also might be linked to Alzheimer’s disease, Dr. Grossberg said. Several studies have shown gingipains secreted from Porphyromonas gingivalis, which contribute to inflammation in the brain, have been found in cadavers of patients with Alzheimer’s disease (Sci Adv. 2019 Jan 23;5[1]:eaau3333). “There’s reason to think that the same changes may be occurring in the human brain with periodontal disease,” he said.
The relationship also might extend to the gut microbiota and the central nervous system. “There seems to be a direct communication, a direct relationship between normal gut physiology and healthy central nervous system functioning, and then, when you have abnormal gut function, it may result in a variety of abnormal central nervous system functions,” Dr. Grossberg said.
Studies that have examined a relationship between Alzheimer’s disease and gut microbiota have highlighted the potential of probiotics and prebiotics as a method of restoring the gut microbiota (Aging [Albany NY]. 2020 Mar 31; 12[6]:5539-50). Probiotics are popularly sold in health food aisles of grocery stores, and prebiotics are available in foods such as yogurts, tempeh, sauerkraut, and kimchi, as well as in drinks such as Kombucha tea. The effectiveness of probiotics and prebiotics also are being examined in randomized, controlled trials in patients with mild cognitive decline and mild Alzheimer’s disease, Dr. Grossberg said. One therapy, Sodium oligomannate, a marine algae–derived oral oligosaccharide, has shown effectiveness in remodeling gut microbiota and has been approved in China to treat patients with mild or moderate Alzheimer’s disease. Currently, no approved gut microbiota therapies are approved in the United States to treat Alzheimer’s disease; however, encouraging use of a prebiotic, a probiotic, or a Mediterranean diet is something clinicians might want to consider for their patients.
“The fact that we’re studying these things has really led to the notion that it may not be a bad idea for people to consume these healthy bacteria in later life, either as a way to prevent or delay, or to treat Alzheimer’s disease,” Dr. Grossberg said. “There’s really no downside.”
Global Academy and this news organization are owned by the same parent company. Dr. Grossberg reported that he is a consultant for Acadia, Alkahest, Avanir, Axsome, Biogen, BioXcel, Karuna, Lundbeck, Otsuka, Roche, and Takeda; receives research support from the National Institute on Aging, Janssen, and Roche; performs safety monitoring for EryDel, Merck, and Newron; and serves on data monitoring committees for Avanex and ITI Therapeutics.
Research into the microbiome is yielding some positive new potential treatment options for Alzheimer’s disease, according to George T. Grossberg, MD.
“I think the growing focus on the gut-brain axis is opening doors to new Alzheimer’s disease and other brain disorders, and I think the first of a possible future generation of compounds for prevention or treatment of Alzheimer’s disease may indeed be emerging,” Dr. Grossberg said at a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Focus on the microbiome and microbiota is “a really hot, really new, really emerging area,” said Dr. Grossberg, professor in the department of psychiatry & behavioral neuroscience at Saint Louis University. But the microbiota, which is the microorganisms within a specific organ such as the colon, is sometimes confused with the microbiome – which is defined as all of the bacteria, viruses, fungi and other microorganisms within a habitat as well as their genomes and the environment around them. “These are often used interchangeably, but they’re not the same,” Dr. Grossberg said at the meeting, presented by Global Academy for Medical Education.
A person’s microbiome is unique to them, and nearly all of the microbiome is contained in the gut. A reduction in diversity of the microbiota in the digestive system has been linked to a wide variety of diseases, Dr. Grossberg explained. Conversely, a microbial imbalance or dysbiosis has been implicated in anxiety and/or depression, dementia, and certain cancers, he noted.
Bacteria that positively affect the microbiome come from two main genera: Lactobacillus and Bifidobacterium. Factors such as diet, medications, geography, stage of life, birthing process, infant feeding method, and stress can all affect a person’s microbiome. “We’re all beginning to understand that trying to manage or trying to diversify, trying to manipulate the microbiota may have a lot of remote effects – even effects on weight or diabetes, or other disorders,” Dr. Grossberg said.
Fecal microbiota transplantation (FMT), or the process of administering a donor’s fecal matter into a recipient’s intestinal tract, has proved beneficial in improving the health of patients suffering from recurrent Clostridioides difficile infection. A recent Harvard Health Letter, written by Jessica Allegretti, MD, MPH, observed that FMT is standard of care for patients with C. diff, and the procedure has a success rate of between 80% and 90%.
“It shows us very directly, in a very practical way, how addressing the dysbiosis – the imbalance of the gut microbiome – by infusing healthy bacteria may make a potential lifesaving difference,” Dr. Grossberg said.
Research is beginning to show that the link between gut microbiota and health extends to Alzheimer’s disease as well. Within the last few years, “we’ve started to understand that the microbial diversity in Alzheimer’s disease versus healthy age-matched controls is decreased,” Dr. Grossberg said.
In a study published by Nicholas M. Vogt and colleagues, there was decreased fecal microbial diversity among individuals with Alzheimer’s, compared with healthy individuals matched for age. Another study by Ping Liu, PhD, and colleagues found that patients with Alzheimer’s disease had decreased fecal microbial diversity, compared with individuals who had pre-onset amnestic mild cognitive impairment and normal cognition.
Dr. Grossberg noted that, while these studies do not prove that less fecal microbial diversity is responsible for mild cognitive impairment or Alzheimer’s disease, “it makes us think that, maybe, there’s a contributing factor.”
“What happens with the dysbiosis of the gut microbiome is increased permeability of the epithelial area of the gut, which can then lead to the gut-brain axis dysregulation and may in fact allow the selective entry of bacteria into the central nervous system because the blood-brain barrier comes to be dysfunctional,” he said.
Early evidence suggests that the gut-brain axis can affect cognition. In an animal model study, transferring the microbiota of a mouse with Alzheimer’s disease to one that had been bred to be germ-free resulted in cognitive decline – but there was no cognitive decline for germ-free mice that received a microbiota transplant from a mouse in a healthy control group. Results from another animal study showed that transferring healthy microbiota from a mouse model into a mouse with Alzheimer’s disease reduces amyloid and tau pathology. “The conclusions of these studies seems to be that microbiota mediated intestinal and systemic immune changes or aberrations seem to contribute to the pathogenesis of Alzheimer’s disease in these mouse models,” Dr. Grossberg said. “Consequently, restoring the gut microbial homeostasis may have beneficial effects on Alzheimer’s disease treatment.”
Periodontal disease also might be linked to Alzheimer’s disease, Dr. Grossberg said. Several studies have shown gingipains secreted from Porphyromonas gingivalis, which contribute to inflammation in the brain, have been found in cadavers of patients with Alzheimer’s disease (Sci Adv. 2019 Jan 23;5[1]:eaau3333). “There’s reason to think that the same changes may be occurring in the human brain with periodontal disease,” he said.
The relationship also might extend to the gut microbiota and the central nervous system. “There seems to be a direct communication, a direct relationship between normal gut physiology and healthy central nervous system functioning, and then, when you have abnormal gut function, it may result in a variety of abnormal central nervous system functions,” Dr. Grossberg said.
Studies that have examined a relationship between Alzheimer’s disease and gut microbiota have highlighted the potential of probiotics and prebiotics as a method of restoring the gut microbiota (Aging [Albany NY]. 2020 Mar 31; 12[6]:5539-50). Probiotics are popularly sold in health food aisles of grocery stores, and prebiotics are available in foods such as yogurts, tempeh, sauerkraut, and kimchi, as well as in drinks such as Kombucha tea. The effectiveness of probiotics and prebiotics also are being examined in randomized, controlled trials in patients with mild cognitive decline and mild Alzheimer’s disease, Dr. Grossberg said. One therapy, Sodium oligomannate, a marine algae–derived oral oligosaccharide, has shown effectiveness in remodeling gut microbiota and has been approved in China to treat patients with mild or moderate Alzheimer’s disease. Currently, no approved gut microbiota therapies are approved in the United States to treat Alzheimer’s disease; however, encouraging use of a prebiotic, a probiotic, or a Mediterranean diet is something clinicians might want to consider for their patients.
“The fact that we’re studying these things has really led to the notion that it may not be a bad idea for people to consume these healthy bacteria in later life, either as a way to prevent or delay, or to treat Alzheimer’s disease,” Dr. Grossberg said. “There’s really no downside.”
Global Academy and this news organization are owned by the same parent company. Dr. Grossberg reported that he is a consultant for Acadia, Alkahest, Avanir, Axsome, Biogen, BioXcel, Karuna, Lundbeck, Otsuka, Roche, and Takeda; receives research support from the National Institute on Aging, Janssen, and Roche; performs safety monitoring for EryDel, Merck, and Newron; and serves on data monitoring committees for Avanex and ITI Therapeutics.
FROM CP/AACP PSYCHIATRY UPDATE
Learn to anticipate, resolve difficult interactions with patients
Every physician encounters difficult or challenging patients during their career, but learning how to anticipate and handle these interactions is not something taught in medical school or residency, according to Donald W. Black, MD, MS.
Difficult or challenging encounters with patients are not only unavoidable, they should be expected, Dr. Black said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Every doctor I know has had challenging interactions,” he said. About 15% of encounters were deemed “difficult” in a prospective study of patients by Jeffrey L. Jackson, MD, MPH, and Kurt Kroenke, MD. A depressive or anxiety disorder was present in 29% of patients, with 11% experiencing two or more disorders. Major depression was present in 8.4%, other depressive disorders in 17.4%, panic disorder in 1.4%, and other anxiety disorders in 14.2% of patients. Dr. Jackson and Dr. Kroenke found that difficult patients demonstrated disrespect and anger, made threats, and locked themselves in rooms (Arch Intern Med. 1999 May 24;159[10]:1069-75). “Rest assured, you are not the only psychiatrist to face this type of issue,” Dr. Black said at the meeting, presented by Global Academy for Medical Education.
Common scenarios can include patients who want certain tests performed after researching symptoms online, threats of legal or social media action after feeling like they are not being listened to, a demand for a second opinion after not agreeing with a physician’s diagnosis, mistrust of doctors after presenting with symptoms and not being diagnosed, patients who focus on negative outcomes, and those who do not comply with treatment. These patients can often appear angry, defensive, frightened or resistant, or manipulative; may provide vague or exaggerated symptoms; or may inappropriately rely on hospital or clinic staff for emotional and physical support.
To complicate matters, the patients’ condition also might contribute to difficult interactions, such as in patients who have conditions like chronic pain or fibromyalgia.
“These often contribute because patients never feel that their problems are being appropriately addressed,” said Dr. Black, professor of psychiatry at the University of Iowa in Iowa City. Patients with psychiatric disorders can also present unique challenges that may result in difficult encounters. Patients with anxiety might not be able to be reassured by their doctor, for example, or patients with eating disorders might refuse treatment recommendations, he said.
Difficult encounters can lead to physicians feeling angry, upset, stressed, disrespected, abused, or fearful. But “it’s not just about the patient,” Dr. Black said. Physicians can become angry or defensive because of burnout, stress, or frustration, which can lead to them snapping at patients. Physicians are also overworked, sleep-deprived, and busier than they’d like to be, he added. Personal problems can contribute, and a physician’s belief system can cause a bad interaction with a patient. If physicians “label” one of their patients, that might end up becoming a “self-fulfilling prophecy” for that patient, Dr. Black said. Poor communication, such as not conveying bad news appropriately or with empathy, seeing a patient but never making eye contact, and using medical jargon that could be confusing to the patient, also can contribute to a challenging encounter, he added.
Situational issues also might create a bad experience for the patient. For example, a patient might find it hard to make an appointment, or the clinic might be busy or have a lack of privacy or encounter administrative issues. For patients who do not speak English as their native language, not having access to an interpreter can lead to frustration on the part of both the physician and the patient. “Bad interactions are not good for patient care,” Dr. Black said.
The key to resolving these issues is to focus on the goal, Dr. Black said. “We all want the same thing. We want to help the patient get better; we want to keep patients healthy; we want to keep them happy; we want to be fulfilled; [and] we want to manage our time and make a living – and meet our professional expectations.”
Begin by recognizing the difficult situation and assessing how the patient, the environment, or you might be contributing to the problem. “You have to step back and say what’s going on with this, and what are the factors that are combining to create this situation,” Dr. Black said. It is important to be calm and professional and not argue or talk over the patient. The goal is to work with the patient to find a solution.
One technique is to verbalize the problem without blaming the patient, the physician, or the environment (“We both have very different views about how your symptoms should be investigated, and that’s causing some difficulty between us. Do you agree?”).
There also might be alternative explanations for a patient’s behavior. For example, anger could be misdirected at a physician because of anxiety surrounding an unrelated event. In this case, it is important to listen to the patient and empathize, which will help the patient feel supported and build a rapport that can aid in resolving the problem encounter, Dr. Black said. Finding common ground when patients and physicians have different ideas on treatment or diagnosis is another way to help resolve a difficult encounter.
However, setting boundaries also is important, he noted. If, after remediation or if patients demonstrate signs of threatening or abusive behavior, initiate sexual advances, refuse to follow a treatment plan, fail to pay their bills, or are potentially putting themselves in harm’s way through noncompliance, a physician might consider terminating the relationship. Terminating the patient relationship should be done after attempting to work with the patient through a case manager and team members, and clearly advising a patient about behavior that could lead to termination of the patient-provider relationship.
Global Academy and this news organization are owned by the same parent company. Dr. Black reported that he is a consultant for Otsuka and receives royalties from American Psychiatric Publishing, Oxford University Press, and UpToDate. In addition, he receives funding from Nellie Ball Trust, the National Institute on Drug Abuse, the National Institute on Aging, and the National Center for Responsible Gaming.
Every physician encounters difficult or challenging patients during their career, but learning how to anticipate and handle these interactions is not something taught in medical school or residency, according to Donald W. Black, MD, MS.
Difficult or challenging encounters with patients are not only unavoidable, they should be expected, Dr. Black said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Every doctor I know has had challenging interactions,” he said. About 15% of encounters were deemed “difficult” in a prospective study of patients by Jeffrey L. Jackson, MD, MPH, and Kurt Kroenke, MD. A depressive or anxiety disorder was present in 29% of patients, with 11% experiencing two or more disorders. Major depression was present in 8.4%, other depressive disorders in 17.4%, panic disorder in 1.4%, and other anxiety disorders in 14.2% of patients. Dr. Jackson and Dr. Kroenke found that difficult patients demonstrated disrespect and anger, made threats, and locked themselves in rooms (Arch Intern Med. 1999 May 24;159[10]:1069-75). “Rest assured, you are not the only psychiatrist to face this type of issue,” Dr. Black said at the meeting, presented by Global Academy for Medical Education.
Common scenarios can include patients who want certain tests performed after researching symptoms online, threats of legal or social media action after feeling like they are not being listened to, a demand for a second opinion after not agreeing with a physician’s diagnosis, mistrust of doctors after presenting with symptoms and not being diagnosed, patients who focus on negative outcomes, and those who do not comply with treatment. These patients can often appear angry, defensive, frightened or resistant, or manipulative; may provide vague or exaggerated symptoms; or may inappropriately rely on hospital or clinic staff for emotional and physical support.
To complicate matters, the patients’ condition also might contribute to difficult interactions, such as in patients who have conditions like chronic pain or fibromyalgia.
“These often contribute because patients never feel that their problems are being appropriately addressed,” said Dr. Black, professor of psychiatry at the University of Iowa in Iowa City. Patients with psychiatric disorders can also present unique challenges that may result in difficult encounters. Patients with anxiety might not be able to be reassured by their doctor, for example, or patients with eating disorders might refuse treatment recommendations, he said.
Difficult encounters can lead to physicians feeling angry, upset, stressed, disrespected, abused, or fearful. But “it’s not just about the patient,” Dr. Black said. Physicians can become angry or defensive because of burnout, stress, or frustration, which can lead to them snapping at patients. Physicians are also overworked, sleep-deprived, and busier than they’d like to be, he added. Personal problems can contribute, and a physician’s belief system can cause a bad interaction with a patient. If physicians “label” one of their patients, that might end up becoming a “self-fulfilling prophecy” for that patient, Dr. Black said. Poor communication, such as not conveying bad news appropriately or with empathy, seeing a patient but never making eye contact, and using medical jargon that could be confusing to the patient, also can contribute to a challenging encounter, he added.
Situational issues also might create a bad experience for the patient. For example, a patient might find it hard to make an appointment, or the clinic might be busy or have a lack of privacy or encounter administrative issues. For patients who do not speak English as their native language, not having access to an interpreter can lead to frustration on the part of both the physician and the patient. “Bad interactions are not good for patient care,” Dr. Black said.
The key to resolving these issues is to focus on the goal, Dr. Black said. “We all want the same thing. We want to help the patient get better; we want to keep patients healthy; we want to keep them happy; we want to be fulfilled; [and] we want to manage our time and make a living – and meet our professional expectations.”
Begin by recognizing the difficult situation and assessing how the patient, the environment, or you might be contributing to the problem. “You have to step back and say what’s going on with this, and what are the factors that are combining to create this situation,” Dr. Black said. It is important to be calm and professional and not argue or talk over the patient. The goal is to work with the patient to find a solution.
One technique is to verbalize the problem without blaming the patient, the physician, or the environment (“We both have very different views about how your symptoms should be investigated, and that’s causing some difficulty between us. Do you agree?”).
There also might be alternative explanations for a patient’s behavior. For example, anger could be misdirected at a physician because of anxiety surrounding an unrelated event. In this case, it is important to listen to the patient and empathize, which will help the patient feel supported and build a rapport that can aid in resolving the problem encounter, Dr. Black said. Finding common ground when patients and physicians have different ideas on treatment or diagnosis is another way to help resolve a difficult encounter.
However, setting boundaries also is important, he noted. If, after remediation or if patients demonstrate signs of threatening or abusive behavior, initiate sexual advances, refuse to follow a treatment plan, fail to pay their bills, or are potentially putting themselves in harm’s way through noncompliance, a physician might consider terminating the relationship. Terminating the patient relationship should be done after attempting to work with the patient through a case manager and team members, and clearly advising a patient about behavior that could lead to termination of the patient-provider relationship.
Global Academy and this news organization are owned by the same parent company. Dr. Black reported that he is a consultant for Otsuka and receives royalties from American Psychiatric Publishing, Oxford University Press, and UpToDate. In addition, he receives funding from Nellie Ball Trust, the National Institute on Drug Abuse, the National Institute on Aging, and the National Center for Responsible Gaming.
Every physician encounters difficult or challenging patients during their career, but learning how to anticipate and handle these interactions is not something taught in medical school or residency, according to Donald W. Black, MD, MS.
Difficult or challenging encounters with patients are not only unavoidable, they should be expected, Dr. Black said in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“Every doctor I know has had challenging interactions,” he said. About 15% of encounters were deemed “difficult” in a prospective study of patients by Jeffrey L. Jackson, MD, MPH, and Kurt Kroenke, MD. A depressive or anxiety disorder was present in 29% of patients, with 11% experiencing two or more disorders. Major depression was present in 8.4%, other depressive disorders in 17.4%, panic disorder in 1.4%, and other anxiety disorders in 14.2% of patients. Dr. Jackson and Dr. Kroenke found that difficult patients demonstrated disrespect and anger, made threats, and locked themselves in rooms (Arch Intern Med. 1999 May 24;159[10]:1069-75). “Rest assured, you are not the only psychiatrist to face this type of issue,” Dr. Black said at the meeting, presented by Global Academy for Medical Education.
Common scenarios can include patients who want certain tests performed after researching symptoms online, threats of legal or social media action after feeling like they are not being listened to, a demand for a second opinion after not agreeing with a physician’s diagnosis, mistrust of doctors after presenting with symptoms and not being diagnosed, patients who focus on negative outcomes, and those who do not comply with treatment. These patients can often appear angry, defensive, frightened or resistant, or manipulative; may provide vague or exaggerated symptoms; or may inappropriately rely on hospital or clinic staff for emotional and physical support.
To complicate matters, the patients’ condition also might contribute to difficult interactions, such as in patients who have conditions like chronic pain or fibromyalgia.
“These often contribute because patients never feel that their problems are being appropriately addressed,” said Dr. Black, professor of psychiatry at the University of Iowa in Iowa City. Patients with psychiatric disorders can also present unique challenges that may result in difficult encounters. Patients with anxiety might not be able to be reassured by their doctor, for example, or patients with eating disorders might refuse treatment recommendations, he said.
Difficult encounters can lead to physicians feeling angry, upset, stressed, disrespected, abused, or fearful. But “it’s not just about the patient,” Dr. Black said. Physicians can become angry or defensive because of burnout, stress, or frustration, which can lead to them snapping at patients. Physicians are also overworked, sleep-deprived, and busier than they’d like to be, he added. Personal problems can contribute, and a physician’s belief system can cause a bad interaction with a patient. If physicians “label” one of their patients, that might end up becoming a “self-fulfilling prophecy” for that patient, Dr. Black said. Poor communication, such as not conveying bad news appropriately or with empathy, seeing a patient but never making eye contact, and using medical jargon that could be confusing to the patient, also can contribute to a challenging encounter, he added.
Situational issues also might create a bad experience for the patient. For example, a patient might find it hard to make an appointment, or the clinic might be busy or have a lack of privacy or encounter administrative issues. For patients who do not speak English as their native language, not having access to an interpreter can lead to frustration on the part of both the physician and the patient. “Bad interactions are not good for patient care,” Dr. Black said.
The key to resolving these issues is to focus on the goal, Dr. Black said. “We all want the same thing. We want to help the patient get better; we want to keep patients healthy; we want to keep them happy; we want to be fulfilled; [and] we want to manage our time and make a living – and meet our professional expectations.”
Begin by recognizing the difficult situation and assessing how the patient, the environment, or you might be contributing to the problem. “You have to step back and say what’s going on with this, and what are the factors that are combining to create this situation,” Dr. Black said. It is important to be calm and professional and not argue or talk over the patient. The goal is to work with the patient to find a solution.
One technique is to verbalize the problem without blaming the patient, the physician, or the environment (“We both have very different views about how your symptoms should be investigated, and that’s causing some difficulty between us. Do you agree?”).
There also might be alternative explanations for a patient’s behavior. For example, anger could be misdirected at a physician because of anxiety surrounding an unrelated event. In this case, it is important to listen to the patient and empathize, which will help the patient feel supported and build a rapport that can aid in resolving the problem encounter, Dr. Black said. Finding common ground when patients and physicians have different ideas on treatment or diagnosis is another way to help resolve a difficult encounter.
However, setting boundaries also is important, he noted. If, after remediation or if patients demonstrate signs of threatening or abusive behavior, initiate sexual advances, refuse to follow a treatment plan, fail to pay their bills, or are potentially putting themselves in harm’s way through noncompliance, a physician might consider terminating the relationship. Terminating the patient relationship should be done after attempting to work with the patient through a case manager and team members, and clearly advising a patient about behavior that could lead to termination of the patient-provider relationship.
Global Academy and this news organization are owned by the same parent company. Dr. Black reported that he is a consultant for Otsuka and receives royalties from American Psychiatric Publishing, Oxford University Press, and UpToDate. In addition, he receives funding from Nellie Ball Trust, the National Institute on Drug Abuse, the National Institute on Aging, and the National Center for Responsible Gaming.
FROM CP/AACP PSYCHIATRY UPDATE
Experts call for immediate suspension of ECT, others push back
Experts are calling for the immediate suspension of electroconvulsive therapy (ECT) for major depression.
A new review by investigators led by John Read, PhD, University of East London, conclude there is no evidence to show that ECT is effective in either its target demographic or its target diagnostic group. They say its use should be suspended until more robust research proves it is safe and effective.
However, the review’s conclusions have been met with passionate opposition from expert psychiatrists who say ECT can be a lifesaving treatment for patients, many of whom have exhausted all other treatment options. Other clinicians maintain that the review itself is fraught with methodologic shortcomings that invalidate its conclusions.
“We’ve concluded there is no adequate research on which to base an answer to the question, ‘Does ECT work?,’ ” Read told Medscape Medical News. “We’re not actually saying ECT doesn’t work. We’re saying there’s no way to know whether it works or not on the basis of the current research, which, after 80 years of the treatment being used, is pretty amazing.”
On the other hand, Read said there is substantial evidence to suggest ECT causes significant adverse events. “Depending who you ask, the psychiatrists or the patients, somewhere between 12% and 55% of patients get permanent or persistent memory loss,” he said.
“So there is a very serious cost to its use, and if there’s a serious cost, you ... have to know that there’s a very strong efficacy benefit, and we just don’t know that. That’s why we’re calling for suspension until there is adequate research,” Read added.
The study was published in a recent issue of Ethical Human Psychology and Psychiatry.
Widespread use
ECT remains a popular treatment modality for resistant depression. Global data show that it is used to treat almost a million patients every year. Although ECT continues to be the subject of comparative research, the investigators say that most of these studies do not adhere to the same standards that govern clinical trials of other psychiatric medications and medical interventions.
The investigators also note that to date, only 11 placebo-controlled studies of the efficacy of ECT have been conducted. They write that the last study to compare ECT with sham or simulated ECT (SECT) – in which a general anesthetic was administered but the electricity was not – was performed in 1985. Nevertheless, this relatively small body of evidence has been the basis of many meta-analyses.
In the current review, the authors evaluated the impartiality and robustness of these previous meta-analyses and the quality of the studies that were included.
“The primary goal is not to assess whether or not ECT is effective,” they write. “The intent, instead, is to determine whether the available evidence is robust enough to answer that question.”
For Read, the decision to analyze the current state of ECT research was both personal and professional.
“As a young nursing attendant in a Bronx hospital, I had the job of sitting with people as they came around from ECT. It was my job to try to explain why they didn’t know who they were, where they were, why their head was throbbing, and why people would do something like that to them,” he said.
“On the research side, this is my sixth review, and in each one we’ve reached the same conclusion,” Read added.
Other research stands in direct opposition to the current review’s findings. Many studies have concluded that ECT is safe and effective for patients with depression.
Ongoing debate
A 2018 registry analysis showed no additional risk for cognitive impairment in patients who underwent ECT up to 40 years after therapy. A 2018 study also showed that ECT was efficacious and cost-effective for patients with treatment-resistant depression.
However, the ECT debate continues. As reported early last year, there seems to be little common ground between clinicians who believe in the utility of ECT for depression and those who vehemently do not.
For the current review, Read and colleagues performed a Medline search for meta-analyses of the efficacy of ECT for depression. Meta-analyses were only included if they comprised placebo-controlled trials that compared ECT with SECT.
Once the meta-analyses were identified, investigators assessed their component studies. This assessment was conducted by two independent reviewers who used a 24-point quality scale developed by the authors. This scale, the authors note, combines the “risk of bias” domains of the Cochrane Handbook Risk of Bias Tool with criteria related to quality of study design and reporting, as well as several criteria specific to ECT research.
The two reviewers were blinded to each other’s ratings. Interrater differences were resolved collectively.
The literature search yielded 83 potential articles; after exclusion criteria were applied, 14 remained. Three of these articles were literature reviews, one discussed different types of statistical analyses used in ECT research, one was a meta-analysis in Hungarian, one was a meta-analysis that compared ECT with SECT in a selected population of elderly people, and three focused on transcranial magnetic stimulation.
This left five meta-analyses for the review. These included from 1 to 7 of the 11 studies in question:
- Janicak et al, 1985
- Kho, van Vreewijk, Simpson, & Zwinderman, 2003
- Mutz et al, 2019
- Pagnin, de Queiroz, Pini, & Cassano, 2004
- UK ECT Review Group, 2003
The review revealed shortcomings with both the meta-analyses and the studies they included. The investigators found that the mean quality scores of the 11 studies (10.27 ± 2.45 and 11.91 ± 2.91) were not statistically different between the two raters (P = .17), whose scores were significantly correlated (P = .001).
Among the 264 total ratings, the investigators found 55 inconsistencies, which were all resolved by discussion. The mean final quality score for the 11 studies included in the review was 12.27 ± 3.20/24; eight scored 13 or less.
The results of these studies do little to support the benefits of ECT relative to SECT, the reviewers note. Indeed, only four concluded that ECT is significantly superior to SECT. Five found no significant difference, and the remaining two had mixed results.
What’s more, only two of what the investigators describe as “higher quality” studies reported follow-up data. Of these, one produced an effect size of 0.065 favoring ECT, the other showed a small benefit in favor of SECT (effect size, 0.299).
The investigators describe the five meta-analyses included in the review as “flawed,” stating that the meta-analyses “pay little or no attention to the multiple limitations of the studies they include.”
These limitations include the number of patients included in the studies (which average 37 patients); lack of a description of randomization and blinding processes; lack of patient ratings; selective reporting of findings; and the absence of assessment of patient quality of life. Furthermore, the authors note that none of the 11 studies “convincingly” demonstrate double-blinding.
Given these shortcomings, the investigators say the meta-analyses of ECT fail to prove the following:
- The short- and long-term efficacy benefits of ECT over SECT;
- Whether ECT is effective among patients who have failed other treatments for depression;
- Whether ECT prevents ;
- Whether ECT improves patients’ quality of life;
- Whether ECT is more effective in women than men;
- Whether ECT is effective in children or adolescents.
“Shoddy” research
The authors conclude that the review’s findings demonstrate the weakness of evidence that currently supports the use of ECT for depression.
“I would never have guessed how shoddy some of this research was,” said review coauthor Irving Kirsch, PhD, a lecturer on medicine at Beth Israel Deaconess Medical Center, Boston. Many of these shortcomings, Kirsch said, involve blinding and placebo effects.
“It’s not clear how you could ever run a truly blinded trial of ECT, given how pronounced the immediate side effects are,” he told Medscape Medical News. “And one of the things that’s underappreciated is the pronounced responses to placebo treatment with depression and severe depression. These can last for a very long time.”
Kirsch noted that more invasive placebo treatments, such as SECT, tend to have more pronounced effects.
“Not all placebos are created equal. We know, for example, that placebo injections are more effective than placebo pills and that placebo surgery can be extremely powerful,” he said.
The authors call for an immediate suspension of ECT until new studies address these research shortcomings.
“The doctors who perform ECT aren’t evil or stupid, they’re just ignorant of the research. What they see is very temporary benefit in some of the patients. The research suggests that about a third – half at most – get a very temporary lift in mood, which seems to be the sort of euphoria you get from mild brain injury,” Read explained.
“I have seen people who haven’t spoken or eaten for several weeks start speaking and eating, but we know that 4 weeks later, they’re going to be just as depressed as they were, or worse. And now they’re going to have brain damage as well,” said Read.
He added that physicians often don’t see long-term patient outcomes, just the immediate effect of ECT.
A “lifesaver”
No recent randomized controlled trials regarding ECT have been conducted because “no IRB [institutional review board] on planet earth will allow such a trial because of the overwhelming evidence of efficacy and the risk of anesthesia with no ECT,” noted Mark S. George, MD, the Layton McCurdy Endowed Chair in Psychiatry at the Medical University of South Carolina, Charleston.
It is a “logical fallacy” to conclude that ECT does not work because the trials were flawed, said George, who was not involved with the current review
“This is not supported by anything they have looked at,” he told Medscape Medical News. “It’s not really a scientific study when you make conclusions that aren’t based on your data, or not what you set out to do. That’s what I find egregious here.”
The way he sees it, the utility of ECT is unquestionable. “It is our most effective acute treatment for depression, and it’s our most effective treatment for suicide,” he said.
“The authors of this review don’t see the patients that I see every day, who are catatonic, who can’t eat, who are suicidal. For those people, ECT is a lifesaver,” George added.
FDA’s position
Sameer Jauhar, MBChB, PhD, a consultant psychiatrist at the South London and Maudsley NHS Foundation Trust, London, was equally unimpressed with the quality of the review.
“I try to approach everything with an open mind,” he said. “But as a doctor, if I’m reading about evidence, I expect a well-conducted meta-analysis and/or very clear systematic review with an adequate level of peer review.”
The current review, said Jauhar, doesn’t meet these criteria. He said the article reads more like a narrative review, one in which the authors dictated their own arbitrary criteria of study quality.
Most important, he said, is the fact that ECT has been the focus of a great deal of research. “The best quality synthesis of the evidence I’ve come across is the UK ECT Review Group’s 2003 meta-analysis, published in The Lancet, which asked this very question. None of the studies has changed since then.”
Jauhar also noted that in 2018, the Food and Drug Administration reclassified ECT from class III (higher risk) to class II (moderate risk). Use of ECT was also limited to treatment of “catatonia or a severe major depressive episode associated with major depressive disorder or bipolar disorder in patients age 13 years and older who are treatment-resistant or who require a rapid response treatment due to the severity of their psychiatric or medical condition.”
The FDA also noted that “[t]he safe use of ECT for treatment of these conditions has been well studied and is better understood than other uses. Therefore, sufficient information exists to establish special controls that mitigate known risks and provide a reasonable assurance of safety and effectiveness for these two uses of ECT devices.”
As part of the FDA’s 2018 reclassification, ECT manufacturers were required to file a premarket approval application for all uses that were not reclassified. The full text of the order is available in the Federal Register.
“So the FDA has been through it, The Lancet has been through it, and the findings were clear,” said Jauhar. “It’s very easy to poke holes in studies that were conducted 30 or more years ago. But the fact is, the field has moved on.”
Jauhar acknowledged there are patients who have had bad experiences with ECT, and he accepts that such events occasionally occur. Nevertheless, he said,
Jauhar also noted that the journal in which the review was published has a 2019 CiteScore of 0.3 and ranks in the 15th percentile of the Scopus Clinical Psychology category. He further noted that Kirsch is a member of the journal’s editorial board.
“I would say that the level of peer review here was negligible at best,” Jauhar said. “In addition, the ‘findings’ are driven more by ideology than evidence.”
Asked to respond to Jauhar’s comments, Kirsch noted that although he is on the journal’s advisory board, he has not been actively involved. Kirsch added that he serves on about a dozen academic advisory boards and serves as a reviewer for many top scientific and medical journals, including The Journal of the American Medical Association and the New England Journal of Medicine.
“Our article was peer reviewed,” Kirsch said, “and we revised it following the first round of reviews.” The review process did not differ from those he has gone through with more than 250 published peer-reviewed articles on which he was an author or coauthor, he noted.
A growing movement
Despite such expert opposition, the movement to suspend ECT continues. Recently, Sarah Price Hancock, MS, CRC, CPMC, who is herself a recipient of more than 100 ECT treatments, authored an international petition to standardize, regulate, and audit the modality. Hancock hopes to present the document, which currently has more than 6600 signatures, to the American Psychiatric Association and similar international societies.
A version of the petition was presented to the UK’s National Health Service on July 2, the 59th anniversary of the death by suicide of Ernest Hemingway, who had received some 20 ECT treatments himself.
“Hopefully by his 60th anniversary, America and the world will be taking his death and the thousands living with adverse reactions more seriously by auditing, regulating, and tracking patients with a history of ECT to provide much needed comprehensive brain injury rehabilitation as necessary,” Hancock told Medscape Medical News.
Among the signatories of the petition is Sue Cunliffe, MbChB, who underwent ECT for depression in 2004, with devastating effects. “I was left really badly brain damaged, and so I’ve never been allowed to work again,” she told Medscape Medical News.
Cunliffe said the immediate effects of the treatment were profound. She said her hands shook, her balance and coordination were impaired, and her memories evaporated. However, she found a neuropsychologist who she says was able to help her recover control of her life. “Now at least I’m able to plan my life so that I can live with the brain damage.”
Kirsch acknowledged that sorting through the ECT literature can be daunting. “If you’re a physician, you try to keep up with the literature, but the problem is, the literature is so old and done in ways which would not pass muster right now. The data are really poor, and I guess it’s just that people aren’t aware of it.”
George agreed that the therapy is not without its shortcomings.
“Does ECT have cognitive side effects? Unfortunately, it does. But so do lots of lifesaving therapies in medicine, like cancer chemotherapy,” he said.
“Nobody really gets well with a single ECT session. It’s usually eight to 12 over 3 or 4 weeks. So it’s not particularly durable. That’s why we often combine ECT with other forms of brain stimulation,” George added.
He noted that the recent advent of alternative forms of ECT – including right unilateral ultrabrief pulse ECT and focal electrically administered seizure therapy – are beginning to address some of these shortcomings.
“The holy grail of brain stimulation is to be able to do things less invasively, and we’re moving slowly in that direction,” he said. “But right now, we don’t have anything that is as acutely as effective as ECT. It is our lifesaver at the moment.”
The review authors as well as George, Jauhar, Cunliffe, and Hancock report no relevant financial relationships.
This article first appeared on Medscape.com.
Experts are calling for the immediate suspension of electroconvulsive therapy (ECT) for major depression.
A new review by investigators led by John Read, PhD, University of East London, conclude there is no evidence to show that ECT is effective in either its target demographic or its target diagnostic group. They say its use should be suspended until more robust research proves it is safe and effective.
However, the review’s conclusions have been met with passionate opposition from expert psychiatrists who say ECT can be a lifesaving treatment for patients, many of whom have exhausted all other treatment options. Other clinicians maintain that the review itself is fraught with methodologic shortcomings that invalidate its conclusions.
“We’ve concluded there is no adequate research on which to base an answer to the question, ‘Does ECT work?,’ ” Read told Medscape Medical News. “We’re not actually saying ECT doesn’t work. We’re saying there’s no way to know whether it works or not on the basis of the current research, which, after 80 years of the treatment being used, is pretty amazing.”
On the other hand, Read said there is substantial evidence to suggest ECT causes significant adverse events. “Depending who you ask, the psychiatrists or the patients, somewhere between 12% and 55% of patients get permanent or persistent memory loss,” he said.
“So there is a very serious cost to its use, and if there’s a serious cost, you ... have to know that there’s a very strong efficacy benefit, and we just don’t know that. That’s why we’re calling for suspension until there is adequate research,” Read added.
The study was published in a recent issue of Ethical Human Psychology and Psychiatry.
Widespread use
ECT remains a popular treatment modality for resistant depression. Global data show that it is used to treat almost a million patients every year. Although ECT continues to be the subject of comparative research, the investigators say that most of these studies do not adhere to the same standards that govern clinical trials of other psychiatric medications and medical interventions.
The investigators also note that to date, only 11 placebo-controlled studies of the efficacy of ECT have been conducted. They write that the last study to compare ECT with sham or simulated ECT (SECT) – in which a general anesthetic was administered but the electricity was not – was performed in 1985. Nevertheless, this relatively small body of evidence has been the basis of many meta-analyses.
In the current review, the authors evaluated the impartiality and robustness of these previous meta-analyses and the quality of the studies that were included.
“The primary goal is not to assess whether or not ECT is effective,” they write. “The intent, instead, is to determine whether the available evidence is robust enough to answer that question.”
For Read, the decision to analyze the current state of ECT research was both personal and professional.
“As a young nursing attendant in a Bronx hospital, I had the job of sitting with people as they came around from ECT. It was my job to try to explain why they didn’t know who they were, where they were, why their head was throbbing, and why people would do something like that to them,” he said.
“On the research side, this is my sixth review, and in each one we’ve reached the same conclusion,” Read added.
Other research stands in direct opposition to the current review’s findings. Many studies have concluded that ECT is safe and effective for patients with depression.
Ongoing debate
A 2018 registry analysis showed no additional risk for cognitive impairment in patients who underwent ECT up to 40 years after therapy. A 2018 study also showed that ECT was efficacious and cost-effective for patients with treatment-resistant depression.
However, the ECT debate continues. As reported early last year, there seems to be little common ground between clinicians who believe in the utility of ECT for depression and those who vehemently do not.
For the current review, Read and colleagues performed a Medline search for meta-analyses of the efficacy of ECT for depression. Meta-analyses were only included if they comprised placebo-controlled trials that compared ECT with SECT.
Once the meta-analyses were identified, investigators assessed their component studies. This assessment was conducted by two independent reviewers who used a 24-point quality scale developed by the authors. This scale, the authors note, combines the “risk of bias” domains of the Cochrane Handbook Risk of Bias Tool with criteria related to quality of study design and reporting, as well as several criteria specific to ECT research.
The two reviewers were blinded to each other’s ratings. Interrater differences were resolved collectively.
The literature search yielded 83 potential articles; after exclusion criteria were applied, 14 remained. Three of these articles were literature reviews, one discussed different types of statistical analyses used in ECT research, one was a meta-analysis in Hungarian, one was a meta-analysis that compared ECT with SECT in a selected population of elderly people, and three focused on transcranial magnetic stimulation.
This left five meta-analyses for the review. These included from 1 to 7 of the 11 studies in question:
- Janicak et al, 1985
- Kho, van Vreewijk, Simpson, & Zwinderman, 2003
- Mutz et al, 2019
- Pagnin, de Queiroz, Pini, & Cassano, 2004
- UK ECT Review Group, 2003
The review revealed shortcomings with both the meta-analyses and the studies they included. The investigators found that the mean quality scores of the 11 studies (10.27 ± 2.45 and 11.91 ± 2.91) were not statistically different between the two raters (P = .17), whose scores were significantly correlated (P = .001).
Among the 264 total ratings, the investigators found 55 inconsistencies, which were all resolved by discussion. The mean final quality score for the 11 studies included in the review was 12.27 ± 3.20/24; eight scored 13 or less.
The results of these studies do little to support the benefits of ECT relative to SECT, the reviewers note. Indeed, only four concluded that ECT is significantly superior to SECT. Five found no significant difference, and the remaining two had mixed results.
What’s more, only two of what the investigators describe as “higher quality” studies reported follow-up data. Of these, one produced an effect size of 0.065 favoring ECT, the other showed a small benefit in favor of SECT (effect size, 0.299).
The investigators describe the five meta-analyses included in the review as “flawed,” stating that the meta-analyses “pay little or no attention to the multiple limitations of the studies they include.”
These limitations include the number of patients included in the studies (which average 37 patients); lack of a description of randomization and blinding processes; lack of patient ratings; selective reporting of findings; and the absence of assessment of patient quality of life. Furthermore, the authors note that none of the 11 studies “convincingly” demonstrate double-blinding.
Given these shortcomings, the investigators say the meta-analyses of ECT fail to prove the following:
- The short- and long-term efficacy benefits of ECT over SECT;
- Whether ECT is effective among patients who have failed other treatments for depression;
- Whether ECT prevents ;
- Whether ECT improves patients’ quality of life;
- Whether ECT is more effective in women than men;
- Whether ECT is effective in children or adolescents.
“Shoddy” research
The authors conclude that the review’s findings demonstrate the weakness of evidence that currently supports the use of ECT for depression.
“I would never have guessed how shoddy some of this research was,” said review coauthor Irving Kirsch, PhD, a lecturer on medicine at Beth Israel Deaconess Medical Center, Boston. Many of these shortcomings, Kirsch said, involve blinding and placebo effects.
“It’s not clear how you could ever run a truly blinded trial of ECT, given how pronounced the immediate side effects are,” he told Medscape Medical News. “And one of the things that’s underappreciated is the pronounced responses to placebo treatment with depression and severe depression. These can last for a very long time.”
Kirsch noted that more invasive placebo treatments, such as SECT, tend to have more pronounced effects.
“Not all placebos are created equal. We know, for example, that placebo injections are more effective than placebo pills and that placebo surgery can be extremely powerful,” he said.
The authors call for an immediate suspension of ECT until new studies address these research shortcomings.
“The doctors who perform ECT aren’t evil or stupid, they’re just ignorant of the research. What they see is very temporary benefit in some of the patients. The research suggests that about a third – half at most – get a very temporary lift in mood, which seems to be the sort of euphoria you get from mild brain injury,” Read explained.
“I have seen people who haven’t spoken or eaten for several weeks start speaking and eating, but we know that 4 weeks later, they’re going to be just as depressed as they were, or worse. And now they’re going to have brain damage as well,” said Read.
He added that physicians often don’t see long-term patient outcomes, just the immediate effect of ECT.
A “lifesaver”
No recent randomized controlled trials regarding ECT have been conducted because “no IRB [institutional review board] on planet earth will allow such a trial because of the overwhelming evidence of efficacy and the risk of anesthesia with no ECT,” noted Mark S. George, MD, the Layton McCurdy Endowed Chair in Psychiatry at the Medical University of South Carolina, Charleston.
It is a “logical fallacy” to conclude that ECT does not work because the trials were flawed, said George, who was not involved with the current review
“This is not supported by anything they have looked at,” he told Medscape Medical News. “It’s not really a scientific study when you make conclusions that aren’t based on your data, or not what you set out to do. That’s what I find egregious here.”
The way he sees it, the utility of ECT is unquestionable. “It is our most effective acute treatment for depression, and it’s our most effective treatment for suicide,” he said.
“The authors of this review don’t see the patients that I see every day, who are catatonic, who can’t eat, who are suicidal. For those people, ECT is a lifesaver,” George added.
FDA’s position
Sameer Jauhar, MBChB, PhD, a consultant psychiatrist at the South London and Maudsley NHS Foundation Trust, London, was equally unimpressed with the quality of the review.
“I try to approach everything with an open mind,” he said. “But as a doctor, if I’m reading about evidence, I expect a well-conducted meta-analysis and/or very clear systematic review with an adequate level of peer review.”
The current review, said Jauhar, doesn’t meet these criteria. He said the article reads more like a narrative review, one in which the authors dictated their own arbitrary criteria of study quality.
Most important, he said, is the fact that ECT has been the focus of a great deal of research. “The best quality synthesis of the evidence I’ve come across is the UK ECT Review Group’s 2003 meta-analysis, published in The Lancet, which asked this very question. None of the studies has changed since then.”
Jauhar also noted that in 2018, the Food and Drug Administration reclassified ECT from class III (higher risk) to class II (moderate risk). Use of ECT was also limited to treatment of “catatonia or a severe major depressive episode associated with major depressive disorder or bipolar disorder in patients age 13 years and older who are treatment-resistant or who require a rapid response treatment due to the severity of their psychiatric or medical condition.”
The FDA also noted that “[t]he safe use of ECT for treatment of these conditions has been well studied and is better understood than other uses. Therefore, sufficient information exists to establish special controls that mitigate known risks and provide a reasonable assurance of safety and effectiveness for these two uses of ECT devices.”
As part of the FDA’s 2018 reclassification, ECT manufacturers were required to file a premarket approval application for all uses that were not reclassified. The full text of the order is available in the Federal Register.
“So the FDA has been through it, The Lancet has been through it, and the findings were clear,” said Jauhar. “It’s very easy to poke holes in studies that were conducted 30 or more years ago. But the fact is, the field has moved on.”
Jauhar acknowledged there are patients who have had bad experiences with ECT, and he accepts that such events occasionally occur. Nevertheless, he said,
Jauhar also noted that the journal in which the review was published has a 2019 CiteScore of 0.3 and ranks in the 15th percentile of the Scopus Clinical Psychology category. He further noted that Kirsch is a member of the journal’s editorial board.
“I would say that the level of peer review here was negligible at best,” Jauhar said. “In addition, the ‘findings’ are driven more by ideology than evidence.”
Asked to respond to Jauhar’s comments, Kirsch noted that although he is on the journal’s advisory board, he has not been actively involved. Kirsch added that he serves on about a dozen academic advisory boards and serves as a reviewer for many top scientific and medical journals, including The Journal of the American Medical Association and the New England Journal of Medicine.
“Our article was peer reviewed,” Kirsch said, “and we revised it following the first round of reviews.” The review process did not differ from those he has gone through with more than 250 published peer-reviewed articles on which he was an author or coauthor, he noted.
A growing movement
Despite such expert opposition, the movement to suspend ECT continues. Recently, Sarah Price Hancock, MS, CRC, CPMC, who is herself a recipient of more than 100 ECT treatments, authored an international petition to standardize, regulate, and audit the modality. Hancock hopes to present the document, which currently has more than 6600 signatures, to the American Psychiatric Association and similar international societies.
A version of the petition was presented to the UK’s National Health Service on July 2, the 59th anniversary of the death by suicide of Ernest Hemingway, who had received some 20 ECT treatments himself.
“Hopefully by his 60th anniversary, America and the world will be taking his death and the thousands living with adverse reactions more seriously by auditing, regulating, and tracking patients with a history of ECT to provide much needed comprehensive brain injury rehabilitation as necessary,” Hancock told Medscape Medical News.
Among the signatories of the petition is Sue Cunliffe, MbChB, who underwent ECT for depression in 2004, with devastating effects. “I was left really badly brain damaged, and so I’ve never been allowed to work again,” she told Medscape Medical News.
Cunliffe said the immediate effects of the treatment were profound. She said her hands shook, her balance and coordination were impaired, and her memories evaporated. However, she found a neuropsychologist who she says was able to help her recover control of her life. “Now at least I’m able to plan my life so that I can live with the brain damage.”
Kirsch acknowledged that sorting through the ECT literature can be daunting. “If you’re a physician, you try to keep up with the literature, but the problem is, the literature is so old and done in ways which would not pass muster right now. The data are really poor, and I guess it’s just that people aren’t aware of it.”
George agreed that the therapy is not without its shortcomings.
“Does ECT have cognitive side effects? Unfortunately, it does. But so do lots of lifesaving therapies in medicine, like cancer chemotherapy,” he said.
“Nobody really gets well with a single ECT session. It’s usually eight to 12 over 3 or 4 weeks. So it’s not particularly durable. That’s why we often combine ECT with other forms of brain stimulation,” George added.
He noted that the recent advent of alternative forms of ECT – including right unilateral ultrabrief pulse ECT and focal electrically administered seizure therapy – are beginning to address some of these shortcomings.
“The holy grail of brain stimulation is to be able to do things less invasively, and we’re moving slowly in that direction,” he said. “But right now, we don’t have anything that is as acutely as effective as ECT. It is our lifesaver at the moment.”
The review authors as well as George, Jauhar, Cunliffe, and Hancock report no relevant financial relationships.
This article first appeared on Medscape.com.
Experts are calling for the immediate suspension of electroconvulsive therapy (ECT) for major depression.
A new review by investigators led by John Read, PhD, University of East London, conclude there is no evidence to show that ECT is effective in either its target demographic or its target diagnostic group. They say its use should be suspended until more robust research proves it is safe and effective.
However, the review’s conclusions have been met with passionate opposition from expert psychiatrists who say ECT can be a lifesaving treatment for patients, many of whom have exhausted all other treatment options. Other clinicians maintain that the review itself is fraught with methodologic shortcomings that invalidate its conclusions.
“We’ve concluded there is no adequate research on which to base an answer to the question, ‘Does ECT work?,’ ” Read told Medscape Medical News. “We’re not actually saying ECT doesn’t work. We’re saying there’s no way to know whether it works or not on the basis of the current research, which, after 80 years of the treatment being used, is pretty amazing.”
On the other hand, Read said there is substantial evidence to suggest ECT causes significant adverse events. “Depending who you ask, the psychiatrists or the patients, somewhere between 12% and 55% of patients get permanent or persistent memory loss,” he said.
“So there is a very serious cost to its use, and if there’s a serious cost, you ... have to know that there’s a very strong efficacy benefit, and we just don’t know that. That’s why we’re calling for suspension until there is adequate research,” Read added.
The study was published in a recent issue of Ethical Human Psychology and Psychiatry.
Widespread use
ECT remains a popular treatment modality for resistant depression. Global data show that it is used to treat almost a million patients every year. Although ECT continues to be the subject of comparative research, the investigators say that most of these studies do not adhere to the same standards that govern clinical trials of other psychiatric medications and medical interventions.
The investigators also note that to date, only 11 placebo-controlled studies of the efficacy of ECT have been conducted. They write that the last study to compare ECT with sham or simulated ECT (SECT) – in which a general anesthetic was administered but the electricity was not – was performed in 1985. Nevertheless, this relatively small body of evidence has been the basis of many meta-analyses.
In the current review, the authors evaluated the impartiality and robustness of these previous meta-analyses and the quality of the studies that were included.
“The primary goal is not to assess whether or not ECT is effective,” they write. “The intent, instead, is to determine whether the available evidence is robust enough to answer that question.”
For Read, the decision to analyze the current state of ECT research was both personal and professional.
“As a young nursing attendant in a Bronx hospital, I had the job of sitting with people as they came around from ECT. It was my job to try to explain why they didn’t know who they were, where they were, why their head was throbbing, and why people would do something like that to them,” he said.
“On the research side, this is my sixth review, and in each one we’ve reached the same conclusion,” Read added.
Other research stands in direct opposition to the current review’s findings. Many studies have concluded that ECT is safe and effective for patients with depression.
Ongoing debate
A 2018 registry analysis showed no additional risk for cognitive impairment in patients who underwent ECT up to 40 years after therapy. A 2018 study also showed that ECT was efficacious and cost-effective for patients with treatment-resistant depression.
However, the ECT debate continues. As reported early last year, there seems to be little common ground between clinicians who believe in the utility of ECT for depression and those who vehemently do not.
For the current review, Read and colleagues performed a Medline search for meta-analyses of the efficacy of ECT for depression. Meta-analyses were only included if they comprised placebo-controlled trials that compared ECT with SECT.
Once the meta-analyses were identified, investigators assessed their component studies. This assessment was conducted by two independent reviewers who used a 24-point quality scale developed by the authors. This scale, the authors note, combines the “risk of bias” domains of the Cochrane Handbook Risk of Bias Tool with criteria related to quality of study design and reporting, as well as several criteria specific to ECT research.
The two reviewers were blinded to each other’s ratings. Interrater differences were resolved collectively.
The literature search yielded 83 potential articles; after exclusion criteria were applied, 14 remained. Three of these articles were literature reviews, one discussed different types of statistical analyses used in ECT research, one was a meta-analysis in Hungarian, one was a meta-analysis that compared ECT with SECT in a selected population of elderly people, and three focused on transcranial magnetic stimulation.
This left five meta-analyses for the review. These included from 1 to 7 of the 11 studies in question:
- Janicak et al, 1985
- Kho, van Vreewijk, Simpson, & Zwinderman, 2003
- Mutz et al, 2019
- Pagnin, de Queiroz, Pini, & Cassano, 2004
- UK ECT Review Group, 2003
The review revealed shortcomings with both the meta-analyses and the studies they included. The investigators found that the mean quality scores of the 11 studies (10.27 ± 2.45 and 11.91 ± 2.91) were not statistically different between the two raters (P = .17), whose scores were significantly correlated (P = .001).
Among the 264 total ratings, the investigators found 55 inconsistencies, which were all resolved by discussion. The mean final quality score for the 11 studies included in the review was 12.27 ± 3.20/24; eight scored 13 or less.
The results of these studies do little to support the benefits of ECT relative to SECT, the reviewers note. Indeed, only four concluded that ECT is significantly superior to SECT. Five found no significant difference, and the remaining two had mixed results.
What’s more, only two of what the investigators describe as “higher quality” studies reported follow-up data. Of these, one produced an effect size of 0.065 favoring ECT, the other showed a small benefit in favor of SECT (effect size, 0.299).
The investigators describe the five meta-analyses included in the review as “flawed,” stating that the meta-analyses “pay little or no attention to the multiple limitations of the studies they include.”
These limitations include the number of patients included in the studies (which average 37 patients); lack of a description of randomization and blinding processes; lack of patient ratings; selective reporting of findings; and the absence of assessment of patient quality of life. Furthermore, the authors note that none of the 11 studies “convincingly” demonstrate double-blinding.
Given these shortcomings, the investigators say the meta-analyses of ECT fail to prove the following:
- The short- and long-term efficacy benefits of ECT over SECT;
- Whether ECT is effective among patients who have failed other treatments for depression;
- Whether ECT prevents ;
- Whether ECT improves patients’ quality of life;
- Whether ECT is more effective in women than men;
- Whether ECT is effective in children or adolescents.
“Shoddy” research
The authors conclude that the review’s findings demonstrate the weakness of evidence that currently supports the use of ECT for depression.
“I would never have guessed how shoddy some of this research was,” said review coauthor Irving Kirsch, PhD, a lecturer on medicine at Beth Israel Deaconess Medical Center, Boston. Many of these shortcomings, Kirsch said, involve blinding and placebo effects.
“It’s not clear how you could ever run a truly blinded trial of ECT, given how pronounced the immediate side effects are,” he told Medscape Medical News. “And one of the things that’s underappreciated is the pronounced responses to placebo treatment with depression and severe depression. These can last for a very long time.”
Kirsch noted that more invasive placebo treatments, such as SECT, tend to have more pronounced effects.
“Not all placebos are created equal. We know, for example, that placebo injections are more effective than placebo pills and that placebo surgery can be extremely powerful,” he said.
The authors call for an immediate suspension of ECT until new studies address these research shortcomings.
“The doctors who perform ECT aren’t evil or stupid, they’re just ignorant of the research. What they see is very temporary benefit in some of the patients. The research suggests that about a third – half at most – get a very temporary lift in mood, which seems to be the sort of euphoria you get from mild brain injury,” Read explained.
“I have seen people who haven’t spoken or eaten for several weeks start speaking and eating, but we know that 4 weeks later, they’re going to be just as depressed as they were, or worse. And now they’re going to have brain damage as well,” said Read.
He added that physicians often don’t see long-term patient outcomes, just the immediate effect of ECT.
A “lifesaver”
No recent randomized controlled trials regarding ECT have been conducted because “no IRB [institutional review board] on planet earth will allow such a trial because of the overwhelming evidence of efficacy and the risk of anesthesia with no ECT,” noted Mark S. George, MD, the Layton McCurdy Endowed Chair in Psychiatry at the Medical University of South Carolina, Charleston.
It is a “logical fallacy” to conclude that ECT does not work because the trials were flawed, said George, who was not involved with the current review
“This is not supported by anything they have looked at,” he told Medscape Medical News. “It’s not really a scientific study when you make conclusions that aren’t based on your data, or not what you set out to do. That’s what I find egregious here.”
The way he sees it, the utility of ECT is unquestionable. “It is our most effective acute treatment for depression, and it’s our most effective treatment for suicide,” he said.
“The authors of this review don’t see the patients that I see every day, who are catatonic, who can’t eat, who are suicidal. For those people, ECT is a lifesaver,” George added.
FDA’s position
Sameer Jauhar, MBChB, PhD, a consultant psychiatrist at the South London and Maudsley NHS Foundation Trust, London, was equally unimpressed with the quality of the review.
“I try to approach everything with an open mind,” he said. “But as a doctor, if I’m reading about evidence, I expect a well-conducted meta-analysis and/or very clear systematic review with an adequate level of peer review.”
The current review, said Jauhar, doesn’t meet these criteria. He said the article reads more like a narrative review, one in which the authors dictated their own arbitrary criteria of study quality.
Most important, he said, is the fact that ECT has been the focus of a great deal of research. “The best quality synthesis of the evidence I’ve come across is the UK ECT Review Group’s 2003 meta-analysis, published in The Lancet, which asked this very question. None of the studies has changed since then.”
Jauhar also noted that in 2018, the Food and Drug Administration reclassified ECT from class III (higher risk) to class II (moderate risk). Use of ECT was also limited to treatment of “catatonia or a severe major depressive episode associated with major depressive disorder or bipolar disorder in patients age 13 years and older who are treatment-resistant or who require a rapid response treatment due to the severity of their psychiatric or medical condition.”
The FDA also noted that “[t]he safe use of ECT for treatment of these conditions has been well studied and is better understood than other uses. Therefore, sufficient information exists to establish special controls that mitigate known risks and provide a reasonable assurance of safety and effectiveness for these two uses of ECT devices.”
As part of the FDA’s 2018 reclassification, ECT manufacturers were required to file a premarket approval application for all uses that were not reclassified. The full text of the order is available in the Federal Register.
“So the FDA has been through it, The Lancet has been through it, and the findings were clear,” said Jauhar. “It’s very easy to poke holes in studies that were conducted 30 or more years ago. But the fact is, the field has moved on.”
Jauhar acknowledged there are patients who have had bad experiences with ECT, and he accepts that such events occasionally occur. Nevertheless, he said,
Jauhar also noted that the journal in which the review was published has a 2019 CiteScore of 0.3 and ranks in the 15th percentile of the Scopus Clinical Psychology category. He further noted that Kirsch is a member of the journal’s editorial board.
“I would say that the level of peer review here was negligible at best,” Jauhar said. “In addition, the ‘findings’ are driven more by ideology than evidence.”
Asked to respond to Jauhar’s comments, Kirsch noted that although he is on the journal’s advisory board, he has not been actively involved. Kirsch added that he serves on about a dozen academic advisory boards and serves as a reviewer for many top scientific and medical journals, including The Journal of the American Medical Association and the New England Journal of Medicine.
“Our article was peer reviewed,” Kirsch said, “and we revised it following the first round of reviews.” The review process did not differ from those he has gone through with more than 250 published peer-reviewed articles on which he was an author or coauthor, he noted.
A growing movement
Despite such expert opposition, the movement to suspend ECT continues. Recently, Sarah Price Hancock, MS, CRC, CPMC, who is herself a recipient of more than 100 ECT treatments, authored an international petition to standardize, regulate, and audit the modality. Hancock hopes to present the document, which currently has more than 6600 signatures, to the American Psychiatric Association and similar international societies.
A version of the petition was presented to the UK’s National Health Service on July 2, the 59th anniversary of the death by suicide of Ernest Hemingway, who had received some 20 ECT treatments himself.
“Hopefully by his 60th anniversary, America and the world will be taking his death and the thousands living with adverse reactions more seriously by auditing, regulating, and tracking patients with a history of ECT to provide much needed comprehensive brain injury rehabilitation as necessary,” Hancock told Medscape Medical News.
Among the signatories of the petition is Sue Cunliffe, MbChB, who underwent ECT for depression in 2004, with devastating effects. “I was left really badly brain damaged, and so I’ve never been allowed to work again,” she told Medscape Medical News.
Cunliffe said the immediate effects of the treatment were profound. She said her hands shook, her balance and coordination were impaired, and her memories evaporated. However, she found a neuropsychologist who she says was able to help her recover control of her life. “Now at least I’m able to plan my life so that I can live with the brain damage.”
Kirsch acknowledged that sorting through the ECT literature can be daunting. “If you’re a physician, you try to keep up with the literature, but the problem is, the literature is so old and done in ways which would not pass muster right now. The data are really poor, and I guess it’s just that people aren’t aware of it.”
George agreed that the therapy is not without its shortcomings.
“Does ECT have cognitive side effects? Unfortunately, it does. But so do lots of lifesaving therapies in medicine, like cancer chemotherapy,” he said.
“Nobody really gets well with a single ECT session. It’s usually eight to 12 over 3 or 4 weeks. So it’s not particularly durable. That’s why we often combine ECT with other forms of brain stimulation,” George added.
He noted that the recent advent of alternative forms of ECT – including right unilateral ultrabrief pulse ECT and focal electrically administered seizure therapy – are beginning to address some of these shortcomings.
“The holy grail of brain stimulation is to be able to do things less invasively, and we’re moving slowly in that direction,” he said. “But right now, we don’t have anything that is as acutely as effective as ECT. It is our lifesaver at the moment.”
The review authors as well as George, Jauhar, Cunliffe, and Hancock report no relevant financial relationships.
This article first appeared on Medscape.com.















