User login
Infliximab vs Adalimumab: Which Is Best for Behçet Syndrome?
TOPLINE:
Both infliximab and adalimumab are safe and effective in achieving remission in patients with severe mucocutaneous Behçet syndrome, with adalimumab demonstrating a quicker response time; both drugs also improve quality of life and disease activity scores.
METHODOLOGY:
- Researchers conducted a phase 3 prospective study to evaluate the efficacy and safety of the anti–tumor necrosis factor–alpha agents infliximab and adalimumab in patients with Behçet syndrome presenting with mucocutaneous manifestations and inadequate response to prior treatments who were recruited from four Italian tertiary referral centers specializing in Behçet syndrome.
- Patients were randomly assigned to receive either 5 mg/kg intravenous infliximab at weeks 0, 2, and 6 and then every 6-8 weeks (n = 22; mean age, 46 years; 32% women) or 40 mg subcutaneous adalimumab every 2 weeks (n = 18; mean age, 48 years; 28% women) for 24 weeks.
- Patients were followed-up for an additional 12 weeks after the treatment period, with regular assessments of disease activity, safety, and adherence to treatment.
- The primary outcome was the time to response of mucocutaneous manifestations over 6 months; the secondary outcomes included relapse rates; quality of life, assessed using the Short-Form Health Survey 36; and disease activity, assessed using the Behçet Disease Current Activity Form.
- The safety and tolerability of the drugs were evaluated as the frequency of treatment-emergent adverse events (AEs) and serious AEs, monitored every 2 weeks.
TAKEAWAY:
- The resolution of mucocutaneous manifestations was achieved significantly more quickly with adalimumab than with infliximab, with a median time to response of 42 vs 152 days (P = .001); the proportion of responders was also higher in the adalimumab group than in the infliximab group (94% vs 64%; P = .023).
- Patients in the infliximab group had a higher risk for nonresponse (adjusted hazard ratio [HR], 3.33; P = .012) and relapse (adjusted HR, 7.57; P = .036) than those in the adalimumab group.
- Both infliximab and adalimumab significantly improved the quality of life in all dimensions (P < .05 for all) and disease activity scores (P < .001 for both) from baseline to the end of the study period, with no significant differences found between the groups.
- Two AEs were reported in the adalimumab group, one of which was serious (myocardial infarction); three nonserious AEs were reported in the infliximab group.
IN PRACTICE:
“ADA [adalimumab] and IFX [infliximab] were generally well tolerated and efficacious in patients with BS [Behçet syndrome] who showed an inadequate response to prior treatments with at least AZA [azathioprine] or CyA [cyclosporine],” the authors wrote. “Although a more detailed treat-to-target profile is yet to be better defined, [the study] results are also crucial in terms of prescriptiveness (currently off label), not only in Italy but also beyond national borders, as the evidence coming from real life still needs to be confirmed by growing data from clinical trials.”
SOURCE:
The study was led by Rosaria Talarico, MD, PhD, University of Pisa in Italy, and was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The small sample size and the distinctive study design may have limited the generalizability of the findings.
DISCLOSURES:
This study was funded through a grant from the Italian Medicines Agency. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Both infliximab and adalimumab are safe and effective in achieving remission in patients with severe mucocutaneous Behçet syndrome, with adalimumab demonstrating a quicker response time; both drugs also improve quality of life and disease activity scores.
METHODOLOGY:
- Researchers conducted a phase 3 prospective study to evaluate the efficacy and safety of the anti–tumor necrosis factor–alpha agents infliximab and adalimumab in patients with Behçet syndrome presenting with mucocutaneous manifestations and inadequate response to prior treatments who were recruited from four Italian tertiary referral centers specializing in Behçet syndrome.
- Patients were randomly assigned to receive either 5 mg/kg intravenous infliximab at weeks 0, 2, and 6 and then every 6-8 weeks (n = 22; mean age, 46 years; 32% women) or 40 mg subcutaneous adalimumab every 2 weeks (n = 18; mean age, 48 years; 28% women) for 24 weeks.
- Patients were followed-up for an additional 12 weeks after the treatment period, with regular assessments of disease activity, safety, and adherence to treatment.
- The primary outcome was the time to response of mucocutaneous manifestations over 6 months; the secondary outcomes included relapse rates; quality of life, assessed using the Short-Form Health Survey 36; and disease activity, assessed using the Behçet Disease Current Activity Form.
- The safety and tolerability of the drugs were evaluated as the frequency of treatment-emergent adverse events (AEs) and serious AEs, monitored every 2 weeks.
TAKEAWAY:
- The resolution of mucocutaneous manifestations was achieved significantly more quickly with adalimumab than with infliximab, with a median time to response of 42 vs 152 days (P = .001); the proportion of responders was also higher in the adalimumab group than in the infliximab group (94% vs 64%; P = .023).
- Patients in the infliximab group had a higher risk for nonresponse (adjusted hazard ratio [HR], 3.33; P = .012) and relapse (adjusted HR, 7.57; P = .036) than those in the adalimumab group.
- Both infliximab and adalimumab significantly improved the quality of life in all dimensions (P < .05 for all) and disease activity scores (P < .001 for both) from baseline to the end of the study period, with no significant differences found between the groups.
- Two AEs were reported in the adalimumab group, one of which was serious (myocardial infarction); three nonserious AEs were reported in the infliximab group.
IN PRACTICE:
“ADA [adalimumab] and IFX [infliximab] were generally well tolerated and efficacious in patients with BS [Behçet syndrome] who showed an inadequate response to prior treatments with at least AZA [azathioprine] or CyA [cyclosporine],” the authors wrote. “Although a more detailed treat-to-target profile is yet to be better defined, [the study] results are also crucial in terms of prescriptiveness (currently off label), not only in Italy but also beyond national borders, as the evidence coming from real life still needs to be confirmed by growing data from clinical trials.”
SOURCE:
The study was led by Rosaria Talarico, MD, PhD, University of Pisa in Italy, and was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The small sample size and the distinctive study design may have limited the generalizability of the findings.
DISCLOSURES:
This study was funded through a grant from the Italian Medicines Agency. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Both infliximab and adalimumab are safe and effective in achieving remission in patients with severe mucocutaneous Behçet syndrome, with adalimumab demonstrating a quicker response time; both drugs also improve quality of life and disease activity scores.
METHODOLOGY:
- Researchers conducted a phase 3 prospective study to evaluate the efficacy and safety of the anti–tumor necrosis factor–alpha agents infliximab and adalimumab in patients with Behçet syndrome presenting with mucocutaneous manifestations and inadequate response to prior treatments who were recruited from four Italian tertiary referral centers specializing in Behçet syndrome.
- Patients were randomly assigned to receive either 5 mg/kg intravenous infliximab at weeks 0, 2, and 6 and then every 6-8 weeks (n = 22; mean age, 46 years; 32% women) or 40 mg subcutaneous adalimumab every 2 weeks (n = 18; mean age, 48 years; 28% women) for 24 weeks.
- Patients were followed-up for an additional 12 weeks after the treatment period, with regular assessments of disease activity, safety, and adherence to treatment.
- The primary outcome was the time to response of mucocutaneous manifestations over 6 months; the secondary outcomes included relapse rates; quality of life, assessed using the Short-Form Health Survey 36; and disease activity, assessed using the Behçet Disease Current Activity Form.
- The safety and tolerability of the drugs were evaluated as the frequency of treatment-emergent adverse events (AEs) and serious AEs, monitored every 2 weeks.
TAKEAWAY:
- The resolution of mucocutaneous manifestations was achieved significantly more quickly with adalimumab than with infliximab, with a median time to response of 42 vs 152 days (P = .001); the proportion of responders was also higher in the adalimumab group than in the infliximab group (94% vs 64%; P = .023).
- Patients in the infliximab group had a higher risk for nonresponse (adjusted hazard ratio [HR], 3.33; P = .012) and relapse (adjusted HR, 7.57; P = .036) than those in the adalimumab group.
- Both infliximab and adalimumab significantly improved the quality of life in all dimensions (P < .05 for all) and disease activity scores (P < .001 for both) from baseline to the end of the study period, with no significant differences found between the groups.
- Two AEs were reported in the adalimumab group, one of which was serious (myocardial infarction); three nonserious AEs were reported in the infliximab group.
IN PRACTICE:
“ADA [adalimumab] and IFX [infliximab] were generally well tolerated and efficacious in patients with BS [Behçet syndrome] who showed an inadequate response to prior treatments with at least AZA [azathioprine] or CyA [cyclosporine],” the authors wrote. “Although a more detailed treat-to-target profile is yet to be better defined, [the study] results are also crucial in terms of prescriptiveness (currently off label), not only in Italy but also beyond national borders, as the evidence coming from real life still needs to be confirmed by growing data from clinical trials.”
SOURCE:
The study was led by Rosaria Talarico, MD, PhD, University of Pisa in Italy, and was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The small sample size and the distinctive study design may have limited the generalizability of the findings.
DISCLOSURES:
This study was funded through a grant from the Italian Medicines Agency. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Study Finds Link to Increased Risk for Bulimia, Binge Eating and HS
“Clinicians should actively screen for eating disorders,” particularly bulimia nervosa and binge eating disorder, in patients with HS,” lead study author Christopher Guirguis, DMD, a student at Georgetown University School of Medicine, Washington, DC, told this news organization in advance of the annual Symposium on Hidradenitis Suppurative Advances, where the study was presented during an oral abstract session. “The significant psychological burden in these patients requires a holistic approach that integrates both dermatologic and psychosocial care. Addressing their mental health needs is essential for improving overall patient outcomes and quality of life,” he added.
In collaboration with fellow Georgetown medical student and first author Lauren Chin and Mikael Horissian, MD, a dermatologist and director of the HS Clinic at Gesinger Health System, Danville, Pennsylvania, Guirguis drew from the National Institutes of Health’s All of Us Research Program to identify 1653 individuals with a diagnosis of HS and a control group of 8265 individuals without a diagnosis of HS. They used the Observational Medical Outcomes Partnership to identify anorexia nervosa, bulimia nervosa, body dysmorphic disorder, binge eating disorder, and eating disorder, unspecified. Obsessive-compulsive disorder (OCD) was also included because of its association with bulimia. They used statistical models to compare cohorts and comorbidities. “What makes this work unique is its focus on the link between HS and eating disorders, a relationship previously underexplored,” he said.
The mean age of the overall study cohort was 46.8 years, and 78.6% were female. Univariate analysis revealed that, compared with controls, individuals in the HS cohort showed significantly increased diagnoses of bulimia, binge eating disorder, OCD, and eating disorder, unspecified, by 2.6, 5.48, 2.50, and 2.43 times, respectively (P < .05 for all associations). After adjusting for age, race, sex, and ethnicity, the researchers observed that patients with HS were 4.46 times as likely to have a diagnosis of binge eating disorder and 3.51 times as likely to have a diagnosis of bulimia as those who did not have HS (P < .05 for both associations).
Guirguis said that the absence of body dysmorphic disorder diagnoses in the HS cohort was unexpected. “Given HS’s known association with body image issues, we anticipated a higher prevalence of BDD,” he said. “This discrepancy may reflect underreporting or diagnostic overshadowing, where the physical symptoms of HS dominate clinical attention, potentially masking or complicating the identification of psychological conditions like BDD.”
He acknowledged certain limitations of the study, including the potential for variations in documentation practices in the database. “Additionally, there may be bias due to underrepresentation of certain demographic groups or underreporting of psychological comorbidities, which could influence the findings.”
Patricia M. Richey, MD, assistant professor of dermatology, at Boston University School of Medicine in Massachusetts, who was asked to comment on the study, said the results “should affect how physicians discuss lifestyle recommendations in those already at increased risk of psychiatric disease and disrupted body image.” The findings should also “prompt physicians to screen this patient population more thoroughly for eating disorders as we know they are an underrecognized and often undertreated entity,” she added.
Neither the study authors nor Richey reported having relevant disclosures.
A version of this article appeared on Medscape.com.
“Clinicians should actively screen for eating disorders,” particularly bulimia nervosa and binge eating disorder, in patients with HS,” lead study author Christopher Guirguis, DMD, a student at Georgetown University School of Medicine, Washington, DC, told this news organization in advance of the annual Symposium on Hidradenitis Suppurative Advances, where the study was presented during an oral abstract session. “The significant psychological burden in these patients requires a holistic approach that integrates both dermatologic and psychosocial care. Addressing their mental health needs is essential for improving overall patient outcomes and quality of life,” he added.
In collaboration with fellow Georgetown medical student and first author Lauren Chin and Mikael Horissian, MD, a dermatologist and director of the HS Clinic at Gesinger Health System, Danville, Pennsylvania, Guirguis drew from the National Institutes of Health’s All of Us Research Program to identify 1653 individuals with a diagnosis of HS and a control group of 8265 individuals without a diagnosis of HS. They used the Observational Medical Outcomes Partnership to identify anorexia nervosa, bulimia nervosa, body dysmorphic disorder, binge eating disorder, and eating disorder, unspecified. Obsessive-compulsive disorder (OCD) was also included because of its association with bulimia. They used statistical models to compare cohorts and comorbidities. “What makes this work unique is its focus on the link between HS and eating disorders, a relationship previously underexplored,” he said.
The mean age of the overall study cohort was 46.8 years, and 78.6% were female. Univariate analysis revealed that, compared with controls, individuals in the HS cohort showed significantly increased diagnoses of bulimia, binge eating disorder, OCD, and eating disorder, unspecified, by 2.6, 5.48, 2.50, and 2.43 times, respectively (P < .05 for all associations). After adjusting for age, race, sex, and ethnicity, the researchers observed that patients with HS were 4.46 times as likely to have a diagnosis of binge eating disorder and 3.51 times as likely to have a diagnosis of bulimia as those who did not have HS (P < .05 for both associations).
Guirguis said that the absence of body dysmorphic disorder diagnoses in the HS cohort was unexpected. “Given HS’s known association with body image issues, we anticipated a higher prevalence of BDD,” he said. “This discrepancy may reflect underreporting or diagnostic overshadowing, where the physical symptoms of HS dominate clinical attention, potentially masking or complicating the identification of psychological conditions like BDD.”
He acknowledged certain limitations of the study, including the potential for variations in documentation practices in the database. “Additionally, there may be bias due to underrepresentation of certain demographic groups or underreporting of psychological comorbidities, which could influence the findings.”
Patricia M. Richey, MD, assistant professor of dermatology, at Boston University School of Medicine in Massachusetts, who was asked to comment on the study, said the results “should affect how physicians discuss lifestyle recommendations in those already at increased risk of psychiatric disease and disrupted body image.” The findings should also “prompt physicians to screen this patient population more thoroughly for eating disorders as we know they are an underrecognized and often undertreated entity,” she added.
Neither the study authors nor Richey reported having relevant disclosures.
A version of this article appeared on Medscape.com.
“Clinicians should actively screen for eating disorders,” particularly bulimia nervosa and binge eating disorder, in patients with HS,” lead study author Christopher Guirguis, DMD, a student at Georgetown University School of Medicine, Washington, DC, told this news organization in advance of the annual Symposium on Hidradenitis Suppurative Advances, where the study was presented during an oral abstract session. “The significant psychological burden in these patients requires a holistic approach that integrates both dermatologic and psychosocial care. Addressing their mental health needs is essential for improving overall patient outcomes and quality of life,” he added.
In collaboration with fellow Georgetown medical student and first author Lauren Chin and Mikael Horissian, MD, a dermatologist and director of the HS Clinic at Gesinger Health System, Danville, Pennsylvania, Guirguis drew from the National Institutes of Health’s All of Us Research Program to identify 1653 individuals with a diagnosis of HS and a control group of 8265 individuals without a diagnosis of HS. They used the Observational Medical Outcomes Partnership to identify anorexia nervosa, bulimia nervosa, body dysmorphic disorder, binge eating disorder, and eating disorder, unspecified. Obsessive-compulsive disorder (OCD) was also included because of its association with bulimia. They used statistical models to compare cohorts and comorbidities. “What makes this work unique is its focus on the link between HS and eating disorders, a relationship previously underexplored,” he said.
The mean age of the overall study cohort was 46.8 years, and 78.6% were female. Univariate analysis revealed that, compared with controls, individuals in the HS cohort showed significantly increased diagnoses of bulimia, binge eating disorder, OCD, and eating disorder, unspecified, by 2.6, 5.48, 2.50, and 2.43 times, respectively (P < .05 for all associations). After adjusting for age, race, sex, and ethnicity, the researchers observed that patients with HS were 4.46 times as likely to have a diagnosis of binge eating disorder and 3.51 times as likely to have a diagnosis of bulimia as those who did not have HS (P < .05 for both associations).
Guirguis said that the absence of body dysmorphic disorder diagnoses in the HS cohort was unexpected. “Given HS’s known association with body image issues, we anticipated a higher prevalence of BDD,” he said. “This discrepancy may reflect underreporting or diagnostic overshadowing, where the physical symptoms of HS dominate clinical attention, potentially masking or complicating the identification of psychological conditions like BDD.”
He acknowledged certain limitations of the study, including the potential for variations in documentation practices in the database. “Additionally, there may be bias due to underrepresentation of certain demographic groups or underreporting of psychological comorbidities, which could influence the findings.”
Patricia M. Richey, MD, assistant professor of dermatology, at Boston University School of Medicine in Massachusetts, who was asked to comment on the study, said the results “should affect how physicians discuss lifestyle recommendations in those already at increased risk of psychiatric disease and disrupted body image.” The findings should also “prompt physicians to screen this patient population more thoroughly for eating disorders as we know they are an underrecognized and often undertreated entity,” she added.
Neither the study authors nor Richey reported having relevant disclosures.
A version of this article appeared on Medscape.com.
Effects of Bimekizumab Durable for HS Through One Year
AMSTERDAM — The monoclonal antibody according to new data from an open-label extension period.
“Efficacy and health-related quality-of-life outcomes were maintained through 2 years of treatment,” study presenter Christos C. Zouboulis, MD, professor of dermatology, venereology, and allergology, Brandenburg Medical School Theodor Fontane, Dessau, Germany, said at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
“No new safety signals were observed,” he added. “These data highlight the durability and consistency of bimekizumab treatment in patients with moderate to severe hidradenitis suppurativa,” Zouboulis concluded.
Efficacy Maintained
“This is the type of long-term data that clinicians hope to see in large phase 3 trials for hidradenitis suppurativa medications,” commented Jennifer L. Hsiao, MD, clinical associate professor of dermatology, University of Southern California, Los Angeles, who was not involved in the study.
She told this news organization that, beyond maintained improvement of patient-reported quality of life, the results are “raising the bar in terms of measuring treatment success,” with over three quarters of patients achieving a high level of response on the Hidradenitis Suppurativa Clinical Response (HiSCR) scale at the final 96-week follow-up.
“Clinicians and patients have struggled with maintaining treatment efficacy over time with the first [Food and Drug Administration]–approved class of biologics for hidradenitis suppurativa — TNF [tumor necrosis factor]–alpha antagonists,” Hsiao said. She emphasized that sustained treatment efficacy will reduce the need for continued treatment switching and “hopefully improve treatment adherence.”
“It was also helpful to see that, consistent with studies of bimekizumab in psoriasis, rates of oral candidiasis appear to decrease with prolonged exposure over 2 years, though as with any open-label extension study, study dropout is a limitation,” she said.
“The availability of long-term efficacy and safety data, such as those shown in this study, will help guide shared decision-making discussions with our patients.” Overall, Hsiao believes there is “much to be excited about in the field of hidradenitis suppurativa, with a robust pipeline of potential treatments.”
One-Year Extension Study
HS is a “chronic and debilitating inflammatory skin disease,” Zouboulis told the audience. He noted that interleukin (IL)–17F and IL-17A are highly expressed in lesional skin and play a role in the disease immunopathogenesis.
Bimekizumab is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits both IL-17F and IL-17A. It has previously demonstrated clinically meaningful improvements in patients with moderate to severe HS in the phase 3 BE HEARD I and BE HEARD II trials evaluating several dosing regimens.
Zouboulis said the current analysis combines data from the two phase 3 studies with the BE HEARD EXT open-label extension study, in which patients from both trials were continued on bimekizumab 320 mg every 2 weeks.
Of the 1014 patients initially enrolled in the two trials, 556 continued into the open-label extension. Their average age was 36.6 years, and 53.8% were women. The majority (80.6%) were White. Of the 556 patients enrolled in the extension, 446 completed the 1-year extension study.
The average draining tunnel count at baseline was 3.8, and 54.5% had Hurley stage II disease; the remaining 45.5% had stage III disease. The mean total Dermatology Life Quality Index (DLQI) score at baseline was 11.0, indicating the HS was having a very large impact on the patients’ lives.
After the 16-week initial treatment period and the maintenance treatment period out to 48 weeks, 64.0% of patients achieved HiSCR75, indicating at least a 75% reduction from baseline in the total abscess and inflammatory nodule count, rising to 77.1% at the end of the open-label extension, after a total follow-up of 96 weeks.
HiSCR100 scores, indicating a 100% reduction in total abscess and inflammatory nodule counts, were achieved by 30.2% of 556 patients after 48 weeks and 44.2% of 446 at the 96-week follow-up.
These findings were mirrored by substantial reductions on the International HS Severity Score System, with a 70.3% reduction over baseline at 48 weeks and a 79.8% reduction at the final follow-up.
There were also “clinically meaningful” reductions in the total draining tunnel count at 1 year that were further reduced at 2 years, Zouboulis reported, at a 57.5% reduction over baseline, increasing to 73.7% by 96 weeks. The mean draining tunnel count at the end of follow-up was 1.1.
Over the full 96 weeks, the mean DLQI score reduced from 11.0 to 4.7, with 33.9% of patients achieving a score of 0 or 1 on the scale, which he said is basically patients saying: “I don’t have disease now.”
Finally, the safety data showed that there were “no differences compared to what we knew before,” Zouboulis said, with the most common treatment-related adverse events being hidradenitis, coronavirus infection, and oral candidiasis. There were few serious and severe treatment-related adverse events, and few that led to treatment discontinuation.
The study was funded by UCB.Zouboulis declared relationships with AstraZeneca, Boehringer Ingelheim, Brandenburg Medical School Theodor Fontane, EAD, European Union, German Federal Ministry of Education and Research, GSK, InflaRx, MSD, Novartis, Relaxera, UCB, Almirall, Boehringer Ingelheim, Eli Lilly, Idorsia, Incyte, L’Oréal, NAOS-BIODERMA, Pfizer, PM, Sanofi. Hsiao is on the board of directors for the Hidradenitis Suppurativa Foundation and has declared relationships with AbbVie, Aclaris Therapeutics, Amgen, Boehringer Ingelheim, Incyte, Novartis, Sanofi-Regeneron, and UCB.
A version of this article appeared on Medscape.com.
AMSTERDAM — The monoclonal antibody according to new data from an open-label extension period.
“Efficacy and health-related quality-of-life outcomes were maintained through 2 years of treatment,” study presenter Christos C. Zouboulis, MD, professor of dermatology, venereology, and allergology, Brandenburg Medical School Theodor Fontane, Dessau, Germany, said at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
“No new safety signals were observed,” he added. “These data highlight the durability and consistency of bimekizumab treatment in patients with moderate to severe hidradenitis suppurativa,” Zouboulis concluded.
Efficacy Maintained
“This is the type of long-term data that clinicians hope to see in large phase 3 trials for hidradenitis suppurativa medications,” commented Jennifer L. Hsiao, MD, clinical associate professor of dermatology, University of Southern California, Los Angeles, who was not involved in the study.
She told this news organization that, beyond maintained improvement of patient-reported quality of life, the results are “raising the bar in terms of measuring treatment success,” with over three quarters of patients achieving a high level of response on the Hidradenitis Suppurativa Clinical Response (HiSCR) scale at the final 96-week follow-up.
“Clinicians and patients have struggled with maintaining treatment efficacy over time with the first [Food and Drug Administration]–approved class of biologics for hidradenitis suppurativa — TNF [tumor necrosis factor]–alpha antagonists,” Hsiao said. She emphasized that sustained treatment efficacy will reduce the need for continued treatment switching and “hopefully improve treatment adherence.”
“It was also helpful to see that, consistent with studies of bimekizumab in psoriasis, rates of oral candidiasis appear to decrease with prolonged exposure over 2 years, though as with any open-label extension study, study dropout is a limitation,” she said.
“The availability of long-term efficacy and safety data, such as those shown in this study, will help guide shared decision-making discussions with our patients.” Overall, Hsiao believes there is “much to be excited about in the field of hidradenitis suppurativa, with a robust pipeline of potential treatments.”
One-Year Extension Study
HS is a “chronic and debilitating inflammatory skin disease,” Zouboulis told the audience. He noted that interleukin (IL)–17F and IL-17A are highly expressed in lesional skin and play a role in the disease immunopathogenesis.
Bimekizumab is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits both IL-17F and IL-17A. It has previously demonstrated clinically meaningful improvements in patients with moderate to severe HS in the phase 3 BE HEARD I and BE HEARD II trials evaluating several dosing regimens.
Zouboulis said the current analysis combines data from the two phase 3 studies with the BE HEARD EXT open-label extension study, in which patients from both trials were continued on bimekizumab 320 mg every 2 weeks.
Of the 1014 patients initially enrolled in the two trials, 556 continued into the open-label extension. Their average age was 36.6 years, and 53.8% were women. The majority (80.6%) were White. Of the 556 patients enrolled in the extension, 446 completed the 1-year extension study.
The average draining tunnel count at baseline was 3.8, and 54.5% had Hurley stage II disease; the remaining 45.5% had stage III disease. The mean total Dermatology Life Quality Index (DLQI) score at baseline was 11.0, indicating the HS was having a very large impact on the patients’ lives.
After the 16-week initial treatment period and the maintenance treatment period out to 48 weeks, 64.0% of patients achieved HiSCR75, indicating at least a 75% reduction from baseline in the total abscess and inflammatory nodule count, rising to 77.1% at the end of the open-label extension, after a total follow-up of 96 weeks.
HiSCR100 scores, indicating a 100% reduction in total abscess and inflammatory nodule counts, were achieved by 30.2% of 556 patients after 48 weeks and 44.2% of 446 at the 96-week follow-up.
These findings were mirrored by substantial reductions on the International HS Severity Score System, with a 70.3% reduction over baseline at 48 weeks and a 79.8% reduction at the final follow-up.
There were also “clinically meaningful” reductions in the total draining tunnel count at 1 year that were further reduced at 2 years, Zouboulis reported, at a 57.5% reduction over baseline, increasing to 73.7% by 96 weeks. The mean draining tunnel count at the end of follow-up was 1.1.
Over the full 96 weeks, the mean DLQI score reduced from 11.0 to 4.7, with 33.9% of patients achieving a score of 0 or 1 on the scale, which he said is basically patients saying: “I don’t have disease now.”
Finally, the safety data showed that there were “no differences compared to what we knew before,” Zouboulis said, with the most common treatment-related adverse events being hidradenitis, coronavirus infection, and oral candidiasis. There were few serious and severe treatment-related adverse events, and few that led to treatment discontinuation.
The study was funded by UCB.Zouboulis declared relationships with AstraZeneca, Boehringer Ingelheim, Brandenburg Medical School Theodor Fontane, EAD, European Union, German Federal Ministry of Education and Research, GSK, InflaRx, MSD, Novartis, Relaxera, UCB, Almirall, Boehringer Ingelheim, Eli Lilly, Idorsia, Incyte, L’Oréal, NAOS-BIODERMA, Pfizer, PM, Sanofi. Hsiao is on the board of directors for the Hidradenitis Suppurativa Foundation and has declared relationships with AbbVie, Aclaris Therapeutics, Amgen, Boehringer Ingelheim, Incyte, Novartis, Sanofi-Regeneron, and UCB.
A version of this article appeared on Medscape.com.
AMSTERDAM — The monoclonal antibody according to new data from an open-label extension period.
“Efficacy and health-related quality-of-life outcomes were maintained through 2 years of treatment,” study presenter Christos C. Zouboulis, MD, professor of dermatology, venereology, and allergology, Brandenburg Medical School Theodor Fontane, Dessau, Germany, said at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
“No new safety signals were observed,” he added. “These data highlight the durability and consistency of bimekizumab treatment in patients with moderate to severe hidradenitis suppurativa,” Zouboulis concluded.
Efficacy Maintained
“This is the type of long-term data that clinicians hope to see in large phase 3 trials for hidradenitis suppurativa medications,” commented Jennifer L. Hsiao, MD, clinical associate professor of dermatology, University of Southern California, Los Angeles, who was not involved in the study.
She told this news organization that, beyond maintained improvement of patient-reported quality of life, the results are “raising the bar in terms of measuring treatment success,” with over three quarters of patients achieving a high level of response on the Hidradenitis Suppurativa Clinical Response (HiSCR) scale at the final 96-week follow-up.
“Clinicians and patients have struggled with maintaining treatment efficacy over time with the first [Food and Drug Administration]–approved class of biologics for hidradenitis suppurativa — TNF [tumor necrosis factor]–alpha antagonists,” Hsiao said. She emphasized that sustained treatment efficacy will reduce the need for continued treatment switching and “hopefully improve treatment adherence.”
“It was also helpful to see that, consistent with studies of bimekizumab in psoriasis, rates of oral candidiasis appear to decrease with prolonged exposure over 2 years, though as with any open-label extension study, study dropout is a limitation,” she said.
“The availability of long-term efficacy and safety data, such as those shown in this study, will help guide shared decision-making discussions with our patients.” Overall, Hsiao believes there is “much to be excited about in the field of hidradenitis suppurativa, with a robust pipeline of potential treatments.”
One-Year Extension Study
HS is a “chronic and debilitating inflammatory skin disease,” Zouboulis told the audience. He noted that interleukin (IL)–17F and IL-17A are highly expressed in lesional skin and play a role in the disease immunopathogenesis.
Bimekizumab is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits both IL-17F and IL-17A. It has previously demonstrated clinically meaningful improvements in patients with moderate to severe HS in the phase 3 BE HEARD I and BE HEARD II trials evaluating several dosing regimens.
Zouboulis said the current analysis combines data from the two phase 3 studies with the BE HEARD EXT open-label extension study, in which patients from both trials were continued on bimekizumab 320 mg every 2 weeks.
Of the 1014 patients initially enrolled in the two trials, 556 continued into the open-label extension. Their average age was 36.6 years, and 53.8% were women. The majority (80.6%) were White. Of the 556 patients enrolled in the extension, 446 completed the 1-year extension study.
The average draining tunnel count at baseline was 3.8, and 54.5% had Hurley stage II disease; the remaining 45.5% had stage III disease. The mean total Dermatology Life Quality Index (DLQI) score at baseline was 11.0, indicating the HS was having a very large impact on the patients’ lives.
After the 16-week initial treatment period and the maintenance treatment period out to 48 weeks, 64.0% of patients achieved HiSCR75, indicating at least a 75% reduction from baseline in the total abscess and inflammatory nodule count, rising to 77.1% at the end of the open-label extension, after a total follow-up of 96 weeks.
HiSCR100 scores, indicating a 100% reduction in total abscess and inflammatory nodule counts, were achieved by 30.2% of 556 patients after 48 weeks and 44.2% of 446 at the 96-week follow-up.
These findings were mirrored by substantial reductions on the International HS Severity Score System, with a 70.3% reduction over baseline at 48 weeks and a 79.8% reduction at the final follow-up.
There were also “clinically meaningful” reductions in the total draining tunnel count at 1 year that were further reduced at 2 years, Zouboulis reported, at a 57.5% reduction over baseline, increasing to 73.7% by 96 weeks. The mean draining tunnel count at the end of follow-up was 1.1.
Over the full 96 weeks, the mean DLQI score reduced from 11.0 to 4.7, with 33.9% of patients achieving a score of 0 or 1 on the scale, which he said is basically patients saying: “I don’t have disease now.”
Finally, the safety data showed that there were “no differences compared to what we knew before,” Zouboulis said, with the most common treatment-related adverse events being hidradenitis, coronavirus infection, and oral candidiasis. There were few serious and severe treatment-related adverse events, and few that led to treatment discontinuation.
The study was funded by UCB.Zouboulis declared relationships with AstraZeneca, Boehringer Ingelheim, Brandenburg Medical School Theodor Fontane, EAD, European Union, German Federal Ministry of Education and Research, GSK, InflaRx, MSD, Novartis, Relaxera, UCB, Almirall, Boehringer Ingelheim, Eli Lilly, Idorsia, Incyte, L’Oréal, NAOS-BIODERMA, Pfizer, PM, Sanofi. Hsiao is on the board of directors for the Hidradenitis Suppurativa Foundation and has declared relationships with AbbVie, Aclaris Therapeutics, Amgen, Boehringer Ingelheim, Incyte, Novartis, Sanofi-Regeneron, and UCB.
A version of this article appeared on Medscape.com.
FROM EADV 2024
Anaphylaxis Treatment Uncertainty Persists for Patients and Professionals
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Study Compares Punch Excision vs. Core Excision for Recalcitrant Keloids
according to the results of a small retrospective study.
The method “offers similar efficacy, faster healing, and fewer complications,” one of the study authors, Jinwoong Jung, MD, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the study results during an oral abstract session.
For the study, Jung, a dermatologist at Yonsei University College of Medicine, Seoul, South Korea, and colleagues retrospectively analyzed 22 patients with recalcitrant keloids treated with cryotherapy immediately following either PE or CE between May 2019 and March 2024. They used the Vancouver Scar Scale (VSS) to assess treatment efficacy.
Of the 22 patients, 16 underwent treatment with CE and 6 underwent treatment with PE. Pretreatment VSS scores showed no significant differences between the groups (P = .535). The CE group had a reduction in the VSS score from 8.13 to 4.00, while the PE group had a reduction from 7.83 to 3.67, but these declines did not differ significantly (P = .737). The PE group exhibited a shorter healing time than the CE group (a mean of 43.5 vs 63.87 days, respectively), though this difference was not statistically significant (P = .129).
“The uniqueness of this work lies in its simplified use of PE for recalcitrant keloids, which demonstrated efficacy comparable to CE, with the potential advantage of faster healing times,” Jung said. “Future studies with larger sample sizes and extended follow-up periods could help establish this approach as a standard treatment method.”
He acknowledged certain limitations of the study, including its small sample size and the lack of long-term follow-up data. The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to the results of a small retrospective study.
The method “offers similar efficacy, faster healing, and fewer complications,” one of the study authors, Jinwoong Jung, MD, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the study results during an oral abstract session.
For the study, Jung, a dermatologist at Yonsei University College of Medicine, Seoul, South Korea, and colleagues retrospectively analyzed 22 patients with recalcitrant keloids treated with cryotherapy immediately following either PE or CE between May 2019 and March 2024. They used the Vancouver Scar Scale (VSS) to assess treatment efficacy.
Of the 22 patients, 16 underwent treatment with CE and 6 underwent treatment with PE. Pretreatment VSS scores showed no significant differences between the groups (P = .535). The CE group had a reduction in the VSS score from 8.13 to 4.00, while the PE group had a reduction from 7.83 to 3.67, but these declines did not differ significantly (P = .737). The PE group exhibited a shorter healing time than the CE group (a mean of 43.5 vs 63.87 days, respectively), though this difference was not statistically significant (P = .129).
“The uniqueness of this work lies in its simplified use of PE for recalcitrant keloids, which demonstrated efficacy comparable to CE, with the potential advantage of faster healing times,” Jung said. “Future studies with larger sample sizes and extended follow-up periods could help establish this approach as a standard treatment method.”
He acknowledged certain limitations of the study, including its small sample size and the lack of long-term follow-up data. The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to the results of a small retrospective study.
The method “offers similar efficacy, faster healing, and fewer complications,” one of the study authors, Jinwoong Jung, MD, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the study results during an oral abstract session.
For the study, Jung, a dermatologist at Yonsei University College of Medicine, Seoul, South Korea, and colleagues retrospectively analyzed 22 patients with recalcitrant keloids treated with cryotherapy immediately following either PE or CE between May 2019 and March 2024. They used the Vancouver Scar Scale (VSS) to assess treatment efficacy.
Of the 22 patients, 16 underwent treatment with CE and 6 underwent treatment with PE. Pretreatment VSS scores showed no significant differences between the groups (P = .535). The CE group had a reduction in the VSS score from 8.13 to 4.00, while the PE group had a reduction from 7.83 to 3.67, but these declines did not differ significantly (P = .737). The PE group exhibited a shorter healing time than the CE group (a mean of 43.5 vs 63.87 days, respectively), though this difference was not statistically significant (P = .129).
“The uniqueness of this work lies in its simplified use of PE for recalcitrant keloids, which demonstrated efficacy comparable to CE, with the potential advantage of faster healing times,” Jung said. “Future studies with larger sample sizes and extended follow-up periods could help establish this approach as a standard treatment method.”
He acknowledged certain limitations of the study, including its small sample size and the lack of long-term follow-up data. The researchers reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ASDS 2024
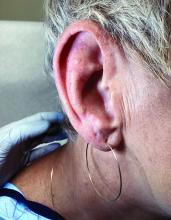
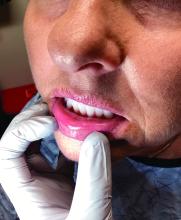
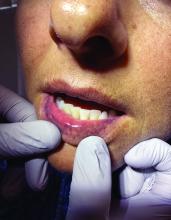
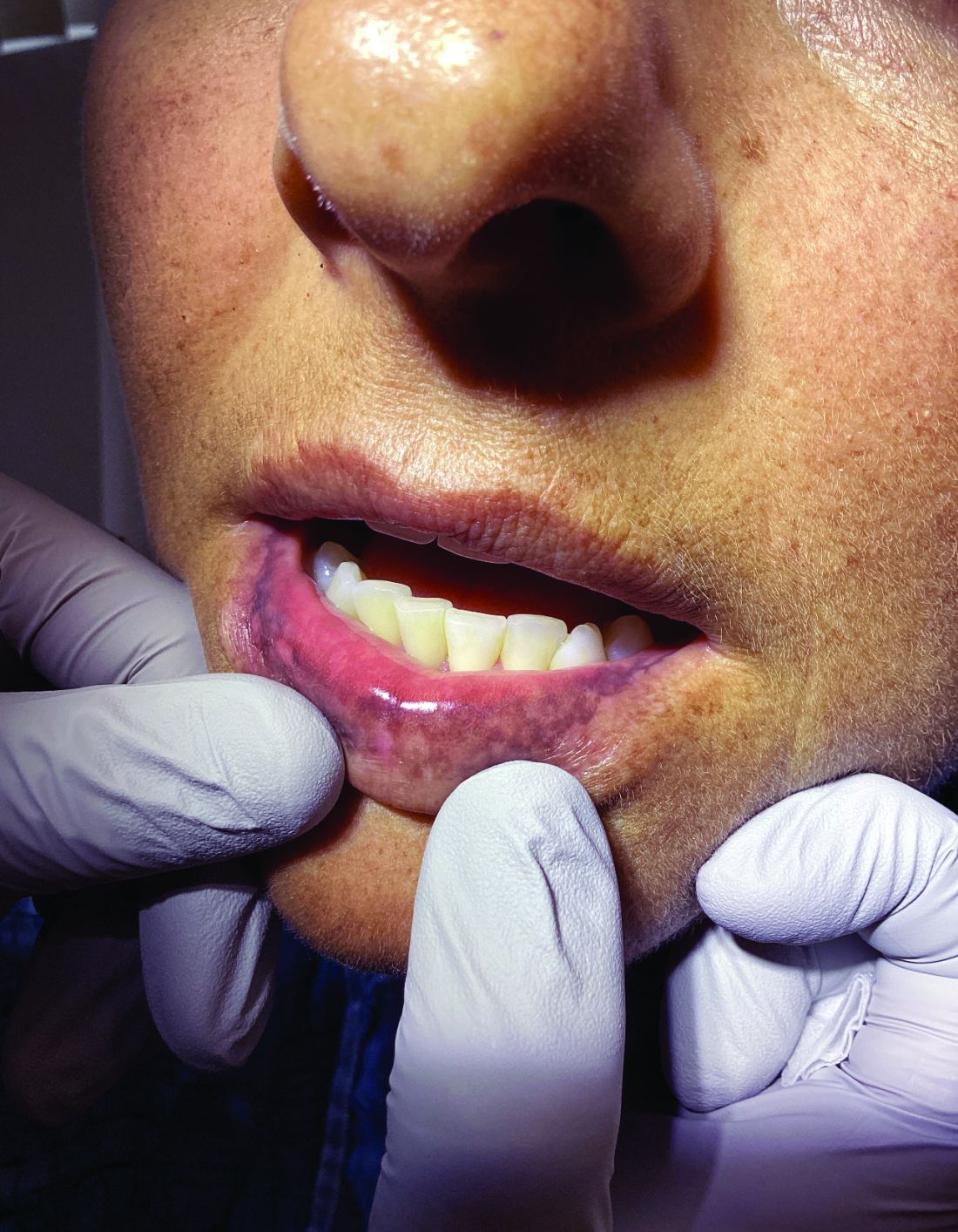
A 51-year-old woman presented for a routine full body skin exam after vacationing in Hawaii.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Primary adrenal insufficiency (Addison’s disease) results from a dysfunction of the adrenal glands, which may be secondary to autoimmune diseases, genetic conditions, infections, and vasculopathies,or may be drug-induced (e.g. checkpoint inhibitors), among others . In contrast, secondary adrenal insufficiency results from pituitary dysfunction of low adrenocorticotropic hormone (ACTH). The most common cause of primary adrenal insufficiency in developed countries is autoimmune adrenalitis, which accounts for upwards of 90% of cases. Typically, 21-hydroxylase autoantibodies are identified and account for destruction of the adrenal cortex through cell-mediated and humoral immune responses.
Palmar creases, subungual surfaces, sites of trauma, and joint spaces (including the knees, spine, elbows, and shoulders) are commonly affected. Hair depletes in the pubic area and axillary vaults. Nevi may also appear darker. In patients with autoimmune adrenalitis, vitiligo may be seen secondary to autoimmune destruction of melanocytes.
Diagnosis may be difficult in the early stages, but historical findings of fatigue and clinical findings of hyperpigmentation in classic areas may prompt appropriate lab screening workup. It is essential to determine whether adrenal insufficiency is primary or secondary. Evaluation of decreased cortisol production, determination of whether production is ACTH-dependent or -independent, and evaluation for the underlying causes of adrenal dysfunction are important. Lab screening includes morning serum cortisol, morning ACTH (cosyntropin) stimulation test, fasting CBC with differential, and CMP to evaluate for normocytic normochromic anemia, hyponatremia, hyperkalemia, hypoglycemia, plasma renin/aldosterone ratio, and 21-hydroxylase autoantibodies.
Management strategies of primary adrenal insufficiency require corticosteroid supplementation and multidisciplinary collaboration with endocrinology. If untreated, primary adrenal insufficiency can be fatal. Adrenal crisis is a critical condition following a precipitating event, such as GI infection, fever, acute stress, and/or untreated adrenal or pituitary disorders. Clinical findings include acute shock with hypotension, nausea, vomiting, abdominal pain, back or leg pain, and a change in mental status. In this scenario, increasing the dose of corticosteroid supplementation is essential for reducing mortality.
Upon examining this patient’s new skin findings of hyperpigmentation and discussing her fatigue, primary adrenal insufficiency was suspected. With further prompting, the patient reported an ICU hospitalization several months prior because of sepsis originating from a peritonsillar abscess. With these clinical and historical findings, preliminary workup was conducted by dermatology, which included morning cortisol level, ACTH, CBC with differential, CMP, plasma renin-aldosterone ratio, and 21-hydroxylase autoantibodies. Work up demonstrated a low morning cortisol level of 1.3 mcg/dL, an elevated ACTH of 2,739 pg/mL, and positive 21-hydroxylase autoantibodies. The patient was urgently referred to endocrinology and started on oral hydrocortisone. Her fatigue immediately improved, and at 1-year follow-up with dermatology, her mucocutaneous hyperpigmentation had subsided dramatically.
Dermatologists can play a major role in the early diagnosis of primary adrenal insufficiency, which is essential for reducing patient morbidity and mortality. Skin findings on full body skin exams can clue in dermatologists for ordering preliminary workup to expedite care for these patients.
The case and photos were submitted by Dr. Akhiyat, Scripps Clinic Medical Group, La Jolla, California. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
J Am Acad Dermatol. 2014 May;70(5):Supplement 1AB118. doi: 10.1016/j.jaad.2014.01.491.
Michels A, Michels N. Am Fam Physician. 2014 Apr 1;89(7):563-568.
Kauzman A et al. J Can Dent Assoc. 2004 Nov;70(10):682-683.
Phase 2 Data on New Drug Class for Prurigo Nodularis Promising
AMSTERDAM — presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress are further validated.
“We now have a pipeline of clinical studies in PN. Who would have even thought that a few years ago,” said Shawn Kwatra, MD, professor and chair, Department of Dermatology, University of Maryland School of Medicine, Baltimore. That is a remarkable turn of events for a difficult disease, he added.
Dupilumab, a monoclonal antibody that inhibits the activity of interleukin (IL)–4 and IL-13, was the first treatment approved for PN by the Food and Drug Administration 2 years ago. Approval of nemolizumab, a monoclonal antibody that targets IL-31, a cytokine strongly implicated in the itch response, followed in August 2024. Povorcitinib, which targets Janus kinase 1 (JAK1), is on track to be the third.
New data on both nemolizumab and povorcitinib were presented in late breaking news sessions at EADV.
For povorcitinib, a JAK inhibitor, Dr. Kwatra presented extended phase 2 results through 40 weeks at a late-breaker session at the EADV meeting. They follow 16-week data from a randomized study presented earlier this year.
Of the 146 patients followed in the original 16-week randomized trial, which compared 15, 45, and 75 mg of oral povorcitinib once daily against placebo, 126 entered an extension in which all patients were treated with active therapy. In this single-blind phase, those who were responders at 16 weeks received 45 mg povorcitinib, and those who were nonresponders received 75 mg povorcitinib.
At 16 weeks, all doses were superior to placebo in achieving at least a 4-point reduction on the Itch Numerical Rating Scale (NRS4) and the Investigator Global Assessment (IGA) score 0 or 1 (clear or almost clear), as well as in a composite endpoint of both. However, even though the lowest dose of povorcitinib was active, there was a “very clear dose response” demonstrated in speed of response and proportion of responders, according to Dr. Kwatra.
On the 75-mg dose, the time to improvement was a median of 19 days, while the median times to improvement were 35 days on the 45-mg dose and 58 days on the 15-mg dose.
Among povorcitinib responders, 96% had met the NRS4 response at the time they entered the extension study. During the extension study, the proportion of responders who maintained this level of itch control hovered around 90% for the duration. The proportion was 89% at week 40.
The proportion of responders at 16 weeks achieving IGA 0/1, signifying clear or almost clear, was 93%. Again, the rate hovered around 90% for the full 40 weeks. At week 40, the proportion at this outcome was also 89%. The composite outcome among responders persisted at about 80% for most of the follow-up but fell to 63% at the last follow-up.
Among nonresponders who transitioned to 75 mg povorcitinib for the extension period, the NSR4 response rates climbed within 4 weeks to approximately 60% and reached 70% at week 40. For the endpoint of IGA 0/1, rates rose incrementally among the nonresponders over time, reaching 51% at week 40. The composite endpoint was reached at 40 weeks by 41% of nonresponders switched to 75 mg during the 24-week extension.
The results at 40 weeks were highly encouraging, according to Dr. Kwatra, who reported there were no surprises in regard to safety during the extension period. He reported some transient reductions in hemoglobin and infections that resolved, but there were no cardiac events or other more serious events that have been previously associated with JAK inhibitors during the 40-week study period.
When asked if there might be an advantage for povorcitinib relative to the monoclonal antibodies in regard to speed of onset, Dr. Kwatra said that there are no comparative data. Like previous experience with dupilumab, some patients responded rapidly with povorcitinib, but others took longer to achieve benefit.
This variability in response is consistent with the growing evidence that PN is a heterogeneous disease, according to Dr. Kwatra. With multiple up-regulated cytokines implicated in the pathogenesis of PN, he suggested that more treatment options would be useful. When it comes to the multiple molecular pathways involved in the pathogenesis of PN, he said, “patients can be at a different edge of a spectrum.”
In other evidence suggesting that more options are needed, another late-breaking news study at the 2024 EADV congress underlined the fact that PN is a chronic disease. Presented by Franz J. Legat, MD, professor of dermatology at the Medical University of Graz, Graz, Austria, the data involved a withdrawal evaluation nested in a long-term extension (LTE) of the OLYMPIA pivotal trials with nemolizumab.
After 52 weeks in the LTE, 34 patients entered the OLYMPIA DURABILITY study, in which they were randomized to withdrawal or to continue on nemolizumab on an every 4-week dosing schedule.
The relapse rate over 24 weeks was 16.7% (3 of 18 patients) in the continuous nemolizumab arm and 75% (12 of 16 patients) in the withdrawal arm. The median time to relapse was 112.5 days for those in the withdrawal arm and was not reached during follow-up in the nemolizumab arm.
Praising the patients who were willing to risk PN relapse by entering this randomized trial, Dr. Legat said that the study shows a relatively high risk for relapse within months of treatment withdrawal even after good PN control over a period of 52 weeks.
“These data clearly support continuous nemolizumab beyond 52 weeks,” he said.
Dr. Kwatra reported financial relationships with AbbVie, Arcutis, Biotherapeutics, Aslan, Celldex, Galderma, Genzada, Johnson & Johnson, Novartis, Pfizer, Regeneron, Sanofi, and Incyte, which is developing povorcitinib for PN. Dr. Legat reported financial relationships with Almirall, Celgene, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Trevi, Vifor, and Galderma, which provided funding for the nemolizumab studies.
A version of this article appeared on Medscape.com.
AMSTERDAM — presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress are further validated.
“We now have a pipeline of clinical studies in PN. Who would have even thought that a few years ago,” said Shawn Kwatra, MD, professor and chair, Department of Dermatology, University of Maryland School of Medicine, Baltimore. That is a remarkable turn of events for a difficult disease, he added.
Dupilumab, a monoclonal antibody that inhibits the activity of interleukin (IL)–4 and IL-13, was the first treatment approved for PN by the Food and Drug Administration 2 years ago. Approval of nemolizumab, a monoclonal antibody that targets IL-31, a cytokine strongly implicated in the itch response, followed in August 2024. Povorcitinib, which targets Janus kinase 1 (JAK1), is on track to be the third.
New data on both nemolizumab and povorcitinib were presented in late breaking news sessions at EADV.
For povorcitinib, a JAK inhibitor, Dr. Kwatra presented extended phase 2 results through 40 weeks at a late-breaker session at the EADV meeting. They follow 16-week data from a randomized study presented earlier this year.
Of the 146 patients followed in the original 16-week randomized trial, which compared 15, 45, and 75 mg of oral povorcitinib once daily against placebo, 126 entered an extension in which all patients were treated with active therapy. In this single-blind phase, those who were responders at 16 weeks received 45 mg povorcitinib, and those who were nonresponders received 75 mg povorcitinib.
At 16 weeks, all doses were superior to placebo in achieving at least a 4-point reduction on the Itch Numerical Rating Scale (NRS4) and the Investigator Global Assessment (IGA) score 0 or 1 (clear or almost clear), as well as in a composite endpoint of both. However, even though the lowest dose of povorcitinib was active, there was a “very clear dose response” demonstrated in speed of response and proportion of responders, according to Dr. Kwatra.
On the 75-mg dose, the time to improvement was a median of 19 days, while the median times to improvement were 35 days on the 45-mg dose and 58 days on the 15-mg dose.
Among povorcitinib responders, 96% had met the NRS4 response at the time they entered the extension study. During the extension study, the proportion of responders who maintained this level of itch control hovered around 90% for the duration. The proportion was 89% at week 40.
The proportion of responders at 16 weeks achieving IGA 0/1, signifying clear or almost clear, was 93%. Again, the rate hovered around 90% for the full 40 weeks. At week 40, the proportion at this outcome was also 89%. The composite outcome among responders persisted at about 80% for most of the follow-up but fell to 63% at the last follow-up.
Among nonresponders who transitioned to 75 mg povorcitinib for the extension period, the NSR4 response rates climbed within 4 weeks to approximately 60% and reached 70% at week 40. For the endpoint of IGA 0/1, rates rose incrementally among the nonresponders over time, reaching 51% at week 40. The composite endpoint was reached at 40 weeks by 41% of nonresponders switched to 75 mg during the 24-week extension.
The results at 40 weeks were highly encouraging, according to Dr. Kwatra, who reported there were no surprises in regard to safety during the extension period. He reported some transient reductions in hemoglobin and infections that resolved, but there were no cardiac events or other more serious events that have been previously associated with JAK inhibitors during the 40-week study period.
When asked if there might be an advantage for povorcitinib relative to the monoclonal antibodies in regard to speed of onset, Dr. Kwatra said that there are no comparative data. Like previous experience with dupilumab, some patients responded rapidly with povorcitinib, but others took longer to achieve benefit.
This variability in response is consistent with the growing evidence that PN is a heterogeneous disease, according to Dr. Kwatra. With multiple up-regulated cytokines implicated in the pathogenesis of PN, he suggested that more treatment options would be useful. When it comes to the multiple molecular pathways involved in the pathogenesis of PN, he said, “patients can be at a different edge of a spectrum.”
In other evidence suggesting that more options are needed, another late-breaking news study at the 2024 EADV congress underlined the fact that PN is a chronic disease. Presented by Franz J. Legat, MD, professor of dermatology at the Medical University of Graz, Graz, Austria, the data involved a withdrawal evaluation nested in a long-term extension (LTE) of the OLYMPIA pivotal trials with nemolizumab.
After 52 weeks in the LTE, 34 patients entered the OLYMPIA DURABILITY study, in which they were randomized to withdrawal or to continue on nemolizumab on an every 4-week dosing schedule.
The relapse rate over 24 weeks was 16.7% (3 of 18 patients) in the continuous nemolizumab arm and 75% (12 of 16 patients) in the withdrawal arm. The median time to relapse was 112.5 days for those in the withdrawal arm and was not reached during follow-up in the nemolizumab arm.
Praising the patients who were willing to risk PN relapse by entering this randomized trial, Dr. Legat said that the study shows a relatively high risk for relapse within months of treatment withdrawal even after good PN control over a period of 52 weeks.
“These data clearly support continuous nemolizumab beyond 52 weeks,” he said.
Dr. Kwatra reported financial relationships with AbbVie, Arcutis, Biotherapeutics, Aslan, Celldex, Galderma, Genzada, Johnson & Johnson, Novartis, Pfizer, Regeneron, Sanofi, and Incyte, which is developing povorcitinib for PN. Dr. Legat reported financial relationships with Almirall, Celgene, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Trevi, Vifor, and Galderma, which provided funding for the nemolizumab studies.
A version of this article appeared on Medscape.com.
AMSTERDAM — presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress are further validated.
“We now have a pipeline of clinical studies in PN. Who would have even thought that a few years ago,” said Shawn Kwatra, MD, professor and chair, Department of Dermatology, University of Maryland School of Medicine, Baltimore. That is a remarkable turn of events for a difficult disease, he added.
Dupilumab, a monoclonal antibody that inhibits the activity of interleukin (IL)–4 and IL-13, was the first treatment approved for PN by the Food and Drug Administration 2 years ago. Approval of nemolizumab, a monoclonal antibody that targets IL-31, a cytokine strongly implicated in the itch response, followed in August 2024. Povorcitinib, which targets Janus kinase 1 (JAK1), is on track to be the third.
New data on both nemolizumab and povorcitinib were presented in late breaking news sessions at EADV.
For povorcitinib, a JAK inhibitor, Dr. Kwatra presented extended phase 2 results through 40 weeks at a late-breaker session at the EADV meeting. They follow 16-week data from a randomized study presented earlier this year.
Of the 146 patients followed in the original 16-week randomized trial, which compared 15, 45, and 75 mg of oral povorcitinib once daily against placebo, 126 entered an extension in which all patients were treated with active therapy. In this single-blind phase, those who were responders at 16 weeks received 45 mg povorcitinib, and those who were nonresponders received 75 mg povorcitinib.
At 16 weeks, all doses were superior to placebo in achieving at least a 4-point reduction on the Itch Numerical Rating Scale (NRS4) and the Investigator Global Assessment (IGA) score 0 or 1 (clear or almost clear), as well as in a composite endpoint of both. However, even though the lowest dose of povorcitinib was active, there was a “very clear dose response” demonstrated in speed of response and proportion of responders, according to Dr. Kwatra.
On the 75-mg dose, the time to improvement was a median of 19 days, while the median times to improvement were 35 days on the 45-mg dose and 58 days on the 15-mg dose.
Among povorcitinib responders, 96% had met the NRS4 response at the time they entered the extension study. During the extension study, the proportion of responders who maintained this level of itch control hovered around 90% for the duration. The proportion was 89% at week 40.
The proportion of responders at 16 weeks achieving IGA 0/1, signifying clear or almost clear, was 93%. Again, the rate hovered around 90% for the full 40 weeks. At week 40, the proportion at this outcome was also 89%. The composite outcome among responders persisted at about 80% for most of the follow-up but fell to 63% at the last follow-up.
Among nonresponders who transitioned to 75 mg povorcitinib for the extension period, the NSR4 response rates climbed within 4 weeks to approximately 60% and reached 70% at week 40. For the endpoint of IGA 0/1, rates rose incrementally among the nonresponders over time, reaching 51% at week 40. The composite endpoint was reached at 40 weeks by 41% of nonresponders switched to 75 mg during the 24-week extension.
The results at 40 weeks were highly encouraging, according to Dr. Kwatra, who reported there were no surprises in regard to safety during the extension period. He reported some transient reductions in hemoglobin and infections that resolved, but there were no cardiac events or other more serious events that have been previously associated with JAK inhibitors during the 40-week study period.
When asked if there might be an advantage for povorcitinib relative to the monoclonal antibodies in regard to speed of onset, Dr. Kwatra said that there are no comparative data. Like previous experience with dupilumab, some patients responded rapidly with povorcitinib, but others took longer to achieve benefit.
This variability in response is consistent with the growing evidence that PN is a heterogeneous disease, according to Dr. Kwatra. With multiple up-regulated cytokines implicated in the pathogenesis of PN, he suggested that more treatment options would be useful. When it comes to the multiple molecular pathways involved in the pathogenesis of PN, he said, “patients can be at a different edge of a spectrum.”
In other evidence suggesting that more options are needed, another late-breaking news study at the 2024 EADV congress underlined the fact that PN is a chronic disease. Presented by Franz J. Legat, MD, professor of dermatology at the Medical University of Graz, Graz, Austria, the data involved a withdrawal evaluation nested in a long-term extension (LTE) of the OLYMPIA pivotal trials with nemolizumab.
After 52 weeks in the LTE, 34 patients entered the OLYMPIA DURABILITY study, in which they were randomized to withdrawal or to continue on nemolizumab on an every 4-week dosing schedule.
The relapse rate over 24 weeks was 16.7% (3 of 18 patients) in the continuous nemolizumab arm and 75% (12 of 16 patients) in the withdrawal arm. The median time to relapse was 112.5 days for those in the withdrawal arm and was not reached during follow-up in the nemolizumab arm.
Praising the patients who were willing to risk PN relapse by entering this randomized trial, Dr. Legat said that the study shows a relatively high risk for relapse within months of treatment withdrawal even after good PN control over a period of 52 weeks.
“These data clearly support continuous nemolizumab beyond 52 weeks,” he said.
Dr. Kwatra reported financial relationships with AbbVie, Arcutis, Biotherapeutics, Aslan, Celldex, Galderma, Genzada, Johnson & Johnson, Novartis, Pfizer, Regeneron, Sanofi, and Incyte, which is developing povorcitinib for PN. Dr. Legat reported financial relationships with Almirall, Celgene, Eli Lilly, Menlo Therapeutics, Novartis, Pfizer, Trevi, Vifor, and Galderma, which provided funding for the nemolizumab studies.
A version of this article appeared on Medscape.com.
FROM EADV 2024
Hidradenitis Suppurativa: Nodules Respond to As Needed Topical JAK Inhibitor
AMSTERDAM — Following the report of results from a randomized trial in which a topically applied Janus kinase (JAK) inhibitor was highly active in
“Ruxolitinib cream may be a novel approach to address an unmet medical need in the treatment of milder HS for which there are no currently approved treatments,” reported Martina L. Porter, MD, assistant professor of dermatology, Harvard Medical School, and Beth Israel Deaconess Medical Center, both in Boston, Massachusetts.
In the earlier 16-week, double-blind, randomized period of this phase 2b study, 69 adults with mild to moderate HS were randomized to 1.5% ruxolitinib cream or vehicle, applied twice daily for 16 weeks. The new results are from the open-label extension period, where those on the vehicle were crossed over to topical ruxolitinib and treatment was continued for another 16 weeks.
Over 80% Meet Primary Endpoint at 32 Weeks
Entry criteria for the study included Hurley stage I or II HS with no draining tunnels. Hurley stage III patients were not eligible. Patients had to have an abscess or inflammatory nodule (AN) count of 3 lesions concentrated in a single anatomic area or up to 10 lesions if disseminated. The median AN count of those enrolled was 5.4.
In the randomized portion of the study and in the open-label extension, the recommendation for application was to apply the medication to nodules and a 1-cm area of surrounding skin. As-needed treatment was only recommended in the extension portion of the study and rescue medication was not allowed.
The goal of the open-label extension was to evaluate how long the improvements were sustained, according to Dr. Porter, who presented the results at the 2024 European Academy of Dermatology and Venereology (EADV) meeting.
The primary endpoints of AN50, signaling at least a 50% reduction in AN count from baseline, among those initially randomized to ruxolitinib cream climbed slightly from 79.2% at the end of 16 weeks to 81.0% at the end of 32 weeks.
This shows that the benefits recorded in the randomized phase of the trial were sustained during the open-label extension, Dr. Porter said.
For those randomized to vehicle, there was a substantial response of 56.3% for AN50 during the randomized portion of the study, but catchup in the vehicle group to those on active therapy occurred rapidly over the open-label extension. By the end of 32 weeks, the score among the crossover patients slightly exceeded that of those on continuous therapy (88.5% vs 81.0%).
AN75 responses at week 32 were 66.7% and 61.5% in the continuous arm and crossover arm, respectively. The proportion of patients reaching an AN90 or AN100 response, meaning clear or almost clear, were 19% and 38.5%, in continuous treatment and crossover arms, respectively.
One of the secondary endpoints was the HS Clinical Response 50, indicating at least a 50% reduction in the AN count with no increase in abscesses or draining fistulae. At 32 weeks, the proportions of patients who met this endpoint were 81.0% and 88.5% in the continuous treatment and crossover arms, respectively.
The mean reduction in International HS Severity Scoring System scores from baseline were 4.1 and 4.5 in the continuous treatment and crossover arms, respectively.
Patients in the Study Mostly Women, 42% Black Individuals
Most (94%) of the participants were women; about 45% and 42% were White and Black individuals, respectively. Most of the remaining patients were Asian individuals. The median age at entry was 29 years, and the mean body mass index was approximately 34 kg/m2. A substantial proportion of patients had systemic comorbidities, according to Dr. Porter, who noted that about 25% had anxiety, depression, or both.
“This phenotype — a high proportion of women with nodules but no draining tunnels and a substantial number of comorbidities — is one we often see in patients with mild HS,” Dr. Porter said.
The safety and tolerability profile of ruxolitinib cream was quite good, according to Dr. Porter, who noted that there were fewer treatment-related adverse events in the open-label extension. Overall, the number of treatment-related adverse events (3.6%), including application site reactions leading to discontinuation (1.8%) was low.
Although there is a growing list of therapies now approved for HS, Dr. Porter emphasized that all have been developed for moderate to severe disease. She suggested that there is a sizable group of patients with mild disease for whom such therapies as biologics might not be warranted even if symptom relief is needed.
Given this unmet need, she said phase 3 trials are warranted to confirm the benefits and the safety of a topical therapy that can be used as needed to control intermittent HS flares.
Asked to comment, the lead author of a recently published review article on the “evolving treatment landscape” of HS, James G. Krueger, MD, professor in clinical investigation at Rockefeller University, New York City, agreed that there is an unmet need for effective and safe therapies in milder HS.
“I agree with the premise,” said Dr. Krueger, indicating that phase 3 data will be essential to confirm the promise of this approach. Dr. Krueger, who did not hear the results presented at the EADV meeting, listed several JAK inhibitors in his review that have shown promising efficacy as oral agents and support JAK signaling as a target of HS treatment.
Topical ruxolitinib (Opzelura) is currently approved in the United States for treating nonsegmental vitiligo in patients aged ≥ 12 years and for mild to moderate atopic dermatitis in patients aged ≥ 12 years. In Europe, it is approved for treatment of nonsegmental vitiligo with facial involvement in patients aged ≥ 12 years.
Dr. Porter reported no potential conflicts of interest. Dr. Krueger reported financial relationships with more than 25 pharmaceutical companies not including Incyte, which is developing ruxolitinib cream.
A version of this article appeared on Medscape.com.
AMSTERDAM — Following the report of results from a randomized trial in which a topically applied Janus kinase (JAK) inhibitor was highly active in
“Ruxolitinib cream may be a novel approach to address an unmet medical need in the treatment of milder HS for which there are no currently approved treatments,” reported Martina L. Porter, MD, assistant professor of dermatology, Harvard Medical School, and Beth Israel Deaconess Medical Center, both in Boston, Massachusetts.
In the earlier 16-week, double-blind, randomized period of this phase 2b study, 69 adults with mild to moderate HS were randomized to 1.5% ruxolitinib cream or vehicle, applied twice daily for 16 weeks. The new results are from the open-label extension period, where those on the vehicle were crossed over to topical ruxolitinib and treatment was continued for another 16 weeks.
Over 80% Meet Primary Endpoint at 32 Weeks
Entry criteria for the study included Hurley stage I or II HS with no draining tunnels. Hurley stage III patients were not eligible. Patients had to have an abscess or inflammatory nodule (AN) count of 3 lesions concentrated in a single anatomic area or up to 10 lesions if disseminated. The median AN count of those enrolled was 5.4.
In the randomized portion of the study and in the open-label extension, the recommendation for application was to apply the medication to nodules and a 1-cm area of surrounding skin. As-needed treatment was only recommended in the extension portion of the study and rescue medication was not allowed.
The goal of the open-label extension was to evaluate how long the improvements were sustained, according to Dr. Porter, who presented the results at the 2024 European Academy of Dermatology and Venereology (EADV) meeting.
The primary endpoints of AN50, signaling at least a 50% reduction in AN count from baseline, among those initially randomized to ruxolitinib cream climbed slightly from 79.2% at the end of 16 weeks to 81.0% at the end of 32 weeks.
This shows that the benefits recorded in the randomized phase of the trial were sustained during the open-label extension, Dr. Porter said.
For those randomized to vehicle, there was a substantial response of 56.3% for AN50 during the randomized portion of the study, but catchup in the vehicle group to those on active therapy occurred rapidly over the open-label extension. By the end of 32 weeks, the score among the crossover patients slightly exceeded that of those on continuous therapy (88.5% vs 81.0%).
AN75 responses at week 32 were 66.7% and 61.5% in the continuous arm and crossover arm, respectively. The proportion of patients reaching an AN90 or AN100 response, meaning clear or almost clear, were 19% and 38.5%, in continuous treatment and crossover arms, respectively.
One of the secondary endpoints was the HS Clinical Response 50, indicating at least a 50% reduction in the AN count with no increase in abscesses or draining fistulae. At 32 weeks, the proportions of patients who met this endpoint were 81.0% and 88.5% in the continuous treatment and crossover arms, respectively.
The mean reduction in International HS Severity Scoring System scores from baseline were 4.1 and 4.5 in the continuous treatment and crossover arms, respectively.
Patients in the Study Mostly Women, 42% Black Individuals
Most (94%) of the participants were women; about 45% and 42% were White and Black individuals, respectively. Most of the remaining patients were Asian individuals. The median age at entry was 29 years, and the mean body mass index was approximately 34 kg/m2. A substantial proportion of patients had systemic comorbidities, according to Dr. Porter, who noted that about 25% had anxiety, depression, or both.
“This phenotype — a high proportion of women with nodules but no draining tunnels and a substantial number of comorbidities — is one we often see in patients with mild HS,” Dr. Porter said.
The safety and tolerability profile of ruxolitinib cream was quite good, according to Dr. Porter, who noted that there were fewer treatment-related adverse events in the open-label extension. Overall, the number of treatment-related adverse events (3.6%), including application site reactions leading to discontinuation (1.8%) was low.
Although there is a growing list of therapies now approved for HS, Dr. Porter emphasized that all have been developed for moderate to severe disease. She suggested that there is a sizable group of patients with mild disease for whom such therapies as biologics might not be warranted even if symptom relief is needed.
Given this unmet need, she said phase 3 trials are warranted to confirm the benefits and the safety of a topical therapy that can be used as needed to control intermittent HS flares.
Asked to comment, the lead author of a recently published review article on the “evolving treatment landscape” of HS, James G. Krueger, MD, professor in clinical investigation at Rockefeller University, New York City, agreed that there is an unmet need for effective and safe therapies in milder HS.
“I agree with the premise,” said Dr. Krueger, indicating that phase 3 data will be essential to confirm the promise of this approach. Dr. Krueger, who did not hear the results presented at the EADV meeting, listed several JAK inhibitors in his review that have shown promising efficacy as oral agents and support JAK signaling as a target of HS treatment.
Topical ruxolitinib (Opzelura) is currently approved in the United States for treating nonsegmental vitiligo in patients aged ≥ 12 years and for mild to moderate atopic dermatitis in patients aged ≥ 12 years. In Europe, it is approved for treatment of nonsegmental vitiligo with facial involvement in patients aged ≥ 12 years.
Dr. Porter reported no potential conflicts of interest. Dr. Krueger reported financial relationships with more than 25 pharmaceutical companies not including Incyte, which is developing ruxolitinib cream.
A version of this article appeared on Medscape.com.
AMSTERDAM — Following the report of results from a randomized trial in which a topically applied Janus kinase (JAK) inhibitor was highly active in
“Ruxolitinib cream may be a novel approach to address an unmet medical need in the treatment of milder HS for which there are no currently approved treatments,” reported Martina L. Porter, MD, assistant professor of dermatology, Harvard Medical School, and Beth Israel Deaconess Medical Center, both in Boston, Massachusetts.
In the earlier 16-week, double-blind, randomized period of this phase 2b study, 69 adults with mild to moderate HS were randomized to 1.5% ruxolitinib cream or vehicle, applied twice daily for 16 weeks. The new results are from the open-label extension period, where those on the vehicle were crossed over to topical ruxolitinib and treatment was continued for another 16 weeks.
Over 80% Meet Primary Endpoint at 32 Weeks
Entry criteria for the study included Hurley stage I or II HS with no draining tunnels. Hurley stage III patients were not eligible. Patients had to have an abscess or inflammatory nodule (AN) count of 3 lesions concentrated in a single anatomic area or up to 10 lesions if disseminated. The median AN count of those enrolled was 5.4.
In the randomized portion of the study and in the open-label extension, the recommendation for application was to apply the medication to nodules and a 1-cm area of surrounding skin. As-needed treatment was only recommended in the extension portion of the study and rescue medication was not allowed.
The goal of the open-label extension was to evaluate how long the improvements were sustained, according to Dr. Porter, who presented the results at the 2024 European Academy of Dermatology and Venereology (EADV) meeting.
The primary endpoints of AN50, signaling at least a 50% reduction in AN count from baseline, among those initially randomized to ruxolitinib cream climbed slightly from 79.2% at the end of 16 weeks to 81.0% at the end of 32 weeks.
This shows that the benefits recorded in the randomized phase of the trial were sustained during the open-label extension, Dr. Porter said.
For those randomized to vehicle, there was a substantial response of 56.3% for AN50 during the randomized portion of the study, but catchup in the vehicle group to those on active therapy occurred rapidly over the open-label extension. By the end of 32 weeks, the score among the crossover patients slightly exceeded that of those on continuous therapy (88.5% vs 81.0%).
AN75 responses at week 32 were 66.7% and 61.5% in the continuous arm and crossover arm, respectively. The proportion of patients reaching an AN90 or AN100 response, meaning clear or almost clear, were 19% and 38.5%, in continuous treatment and crossover arms, respectively.
One of the secondary endpoints was the HS Clinical Response 50, indicating at least a 50% reduction in the AN count with no increase in abscesses or draining fistulae. At 32 weeks, the proportions of patients who met this endpoint were 81.0% and 88.5% in the continuous treatment and crossover arms, respectively.
The mean reduction in International HS Severity Scoring System scores from baseline were 4.1 and 4.5 in the continuous treatment and crossover arms, respectively.
Patients in the Study Mostly Women, 42% Black Individuals
Most (94%) of the participants were women; about 45% and 42% were White and Black individuals, respectively. Most of the remaining patients were Asian individuals. The median age at entry was 29 years, and the mean body mass index was approximately 34 kg/m2. A substantial proportion of patients had systemic comorbidities, according to Dr. Porter, who noted that about 25% had anxiety, depression, or both.
“This phenotype — a high proportion of women with nodules but no draining tunnels and a substantial number of comorbidities — is one we often see in patients with mild HS,” Dr. Porter said.
The safety and tolerability profile of ruxolitinib cream was quite good, according to Dr. Porter, who noted that there were fewer treatment-related adverse events in the open-label extension. Overall, the number of treatment-related adverse events (3.6%), including application site reactions leading to discontinuation (1.8%) was low.
Although there is a growing list of therapies now approved for HS, Dr. Porter emphasized that all have been developed for moderate to severe disease. She suggested that there is a sizable group of patients with mild disease for whom such therapies as biologics might not be warranted even if symptom relief is needed.
Given this unmet need, she said phase 3 trials are warranted to confirm the benefits and the safety of a topical therapy that can be used as needed to control intermittent HS flares.
Asked to comment, the lead author of a recently published review article on the “evolving treatment landscape” of HS, James G. Krueger, MD, professor in clinical investigation at Rockefeller University, New York City, agreed that there is an unmet need for effective and safe therapies in milder HS.
“I agree with the premise,” said Dr. Krueger, indicating that phase 3 data will be essential to confirm the promise of this approach. Dr. Krueger, who did not hear the results presented at the EADV meeting, listed several JAK inhibitors in his review that have shown promising efficacy as oral agents and support JAK signaling as a target of HS treatment.
Topical ruxolitinib (Opzelura) is currently approved in the United States for treating nonsegmental vitiligo in patients aged ≥ 12 years and for mild to moderate atopic dermatitis in patients aged ≥ 12 years. In Europe, it is approved for treatment of nonsegmental vitiligo with facial involvement in patients aged ≥ 12 years.
Dr. Porter reported no potential conflicts of interest. Dr. Krueger reported financial relationships with more than 25 pharmaceutical companies not including Incyte, which is developing ruxolitinib cream.
A version of this article appeared on Medscape.com.
FROM EADV 2024
Too Few Immunocompromised Veterans Are Getting Zoster Vaccinations
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
the low rate of herpes zoster vaccination in this immunocompromised group, especially among younger individuals, is concerning.
METHODOLOGY:
- In 2021, the Food and Drug Administration authorized the use of RZV for adults aged 18 years or older on chronic immunosuppressive medications because of their high risk for herpes zoster and its related complications, followed by updated guidance from the Centers for Disease Control and Prevention and American College of Rheumatology in 2021 and 2022, respectively.
- This study aimed to assess the receipt of RZV among veterans receiving immunosuppressive medications within the Veterans Health Administration (VHA) healthcare system before and after the expanded indications in February 2022.
- It included 190,162 veterans who were prescribed one or more immunosuppressive medications for at least 90 days at 130 medical facilities between January 1, 2018, and June 30, 2023.
- A total of 23,295 veterans (12.3%) were younger than 50 years by the end of the study period.
- The outcome measured was the percentage of veterans with one or more doses of RZV documented during the study period.
TAKEAWAY:
- Among veterans aged 50 years or older, 36.2% and 49.8% received an RZV before the expanded indication and by mid-2023, respectively. Even though the rate of vaccination is higher than that observed in the 2021 National Health Interview Survey, significant room for improvement remains.
- Among veterans younger than 50 years, very few (2.8%) received an RZV before the expanded indication, and only 13.4% received it by mid-2023.
- Demographic factors associated with lower odds of vaccination included male sex, African American or unknown race, and nonurban residence (P ≤ .004 for all).
- Those who received targeted synthetic disease-modifying antirheumatic drugs (DMARDs) alone or in combination with other drugs or those who received other vaccines were more likely to receive RZV than those who received conventional synthetic DMARD monotherapy (P < .001 for both).
IN PRACTICE:
“Future work to improve RZV vaccination in patients at high risk should focus on creating informatics tools to identify individuals at high risk and standardizing vaccination guidelines across subspecialties,” the authors wrote.
SOURCE:
This study was led by Sharon Abada, MD, University of California, San Francisco. It was published online on October 11, 2024, in JAMA Network Open.
LIMITATIONS:
This study may not be generalizable to nonveteran populations or countries outside the United States. Limitations also included difficulty with capturing vaccinations not administered within the VHA system, which may have resulted in an underestimation of the percentage of patients vaccinated.
DISCLOSURES:
This work was funded by grants from the VA Quality Enhancement Research Initiative and the Agency for Healthcare Research and Quality. Some authors reported receiving grants from institutions and pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Mycosis Fungoides: Measured Approach Key to Treatment
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
FROM PDA 2024