User login
Rilzabrutinib shows positive results in phase 2b for pemphigus
accompanied by markedly reduced need for systemic corticosteroids in the phase 2b BELIEVE-PV trial, Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Moreover, in sharp contrast to the standard first-line treatments for pemphigus – rituximab (Rituxan) and high-dose corticosteroids – the treatment-emergent adverse events that arose with 6 months of rilzabrutinib in BELIEVE-PV were uniformly mild to moderate and transient, added Dr. Murrell, professor of dermatology at the University of New South Wales and head of the department of dermatology at St. George University Hospital, Sydney.
The phase 2b BELIEVE-PV trial was a small, 24-week, open-label study that included six patients with newly diagnosed pemphigus and nine others with relapsing pemphigus. The primary endpoint was control of disease activity, defined as no new lesions appearing and established lesions beginning to heal. This was achieved in 9 of 15 patients (60%) at 4 weeks and in 13 patients by week 12. Meanwhile, the mean daily dose of prednisone fell from 21 mg at baseline to 6 mg at 24 weeks.
The mean score on the Pemphigus Disease Area Index (PDAI) dropped by 79% from a baseline of 15.5. Ten of 15 subjects improved to a PDAI of 0 or 1 – clear or almost clear skin – by week 24. The complete remission rate, defined as an absence of both new and established lesions while on no or a very low dose of prednisone, was 40% at week 24.
Treatment-emergent adverse events consisted of nausea in four patients, dizziness in two, and abdominal distension in two, all of which were grade 1 or 2.
Based upon these favorable results, the pivotal phase 3, double-blind, international PEGASUS trial is underway, led by Dr. Murrell. The trial will enroll 120 pemphigus patients, randomized to rilzabrutinib at 400 mg twice daily or placebo on top of background steroid tapering.
Rilzabrutinib is also in earlier-stage clinical trials for the treatment of immune thrombocytopenia.
Dr. Murrell reported serving as a consultant to Principia Biopharma, sponsor of the BELIEVE-PV and PEGASUS trials, and has received institutional research grants from numerous pharmaceutical companies.
SOURCE: Murrell DF. AAD 2020 LBCT.
accompanied by markedly reduced need for systemic corticosteroids in the phase 2b BELIEVE-PV trial, Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Moreover, in sharp contrast to the standard first-line treatments for pemphigus – rituximab (Rituxan) and high-dose corticosteroids – the treatment-emergent adverse events that arose with 6 months of rilzabrutinib in BELIEVE-PV were uniformly mild to moderate and transient, added Dr. Murrell, professor of dermatology at the University of New South Wales and head of the department of dermatology at St. George University Hospital, Sydney.
The phase 2b BELIEVE-PV trial was a small, 24-week, open-label study that included six patients with newly diagnosed pemphigus and nine others with relapsing pemphigus. The primary endpoint was control of disease activity, defined as no new lesions appearing and established lesions beginning to heal. This was achieved in 9 of 15 patients (60%) at 4 weeks and in 13 patients by week 12. Meanwhile, the mean daily dose of prednisone fell from 21 mg at baseline to 6 mg at 24 weeks.
The mean score on the Pemphigus Disease Area Index (PDAI) dropped by 79% from a baseline of 15.5. Ten of 15 subjects improved to a PDAI of 0 or 1 – clear or almost clear skin – by week 24. The complete remission rate, defined as an absence of both new and established lesions while on no or a very low dose of prednisone, was 40% at week 24.
Treatment-emergent adverse events consisted of nausea in four patients, dizziness in two, and abdominal distension in two, all of which were grade 1 or 2.
Based upon these favorable results, the pivotal phase 3, double-blind, international PEGASUS trial is underway, led by Dr. Murrell. The trial will enroll 120 pemphigus patients, randomized to rilzabrutinib at 400 mg twice daily or placebo on top of background steroid tapering.
Rilzabrutinib is also in earlier-stage clinical trials for the treatment of immune thrombocytopenia.
Dr. Murrell reported serving as a consultant to Principia Biopharma, sponsor of the BELIEVE-PV and PEGASUS trials, and has received institutional research grants from numerous pharmaceutical companies.
SOURCE: Murrell DF. AAD 2020 LBCT.
accompanied by markedly reduced need for systemic corticosteroids in the phase 2b BELIEVE-PV trial, Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Moreover, in sharp contrast to the standard first-line treatments for pemphigus – rituximab (Rituxan) and high-dose corticosteroids – the treatment-emergent adverse events that arose with 6 months of rilzabrutinib in BELIEVE-PV were uniformly mild to moderate and transient, added Dr. Murrell, professor of dermatology at the University of New South Wales and head of the department of dermatology at St. George University Hospital, Sydney.
The phase 2b BELIEVE-PV trial was a small, 24-week, open-label study that included six patients with newly diagnosed pemphigus and nine others with relapsing pemphigus. The primary endpoint was control of disease activity, defined as no new lesions appearing and established lesions beginning to heal. This was achieved in 9 of 15 patients (60%) at 4 weeks and in 13 patients by week 12. Meanwhile, the mean daily dose of prednisone fell from 21 mg at baseline to 6 mg at 24 weeks.
The mean score on the Pemphigus Disease Area Index (PDAI) dropped by 79% from a baseline of 15.5. Ten of 15 subjects improved to a PDAI of 0 or 1 – clear or almost clear skin – by week 24. The complete remission rate, defined as an absence of both new and established lesions while on no or a very low dose of prednisone, was 40% at week 24.
Treatment-emergent adverse events consisted of nausea in four patients, dizziness in two, and abdominal distension in two, all of which were grade 1 or 2.
Based upon these favorable results, the pivotal phase 3, double-blind, international PEGASUS trial is underway, led by Dr. Murrell. The trial will enroll 120 pemphigus patients, randomized to rilzabrutinib at 400 mg twice daily or placebo on top of background steroid tapering.
Rilzabrutinib is also in earlier-stage clinical trials for the treatment of immune thrombocytopenia.
Dr. Murrell reported serving as a consultant to Principia Biopharma, sponsor of the BELIEVE-PV and PEGASUS trials, and has received institutional research grants from numerous pharmaceutical companies.
SOURCE: Murrell DF. AAD 2020 LBCT.
FROM AAD 20
COVID-19 pandemic dictates reconsideration of pemphigus therapy
The Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Together with physicians from the Mayo Clinic, Alexandria (Egypt) University, and Tehran (Iran) University, she recently published updated expert guidance for treatment of this severe, potentially fatal mucocutaneous autoimmune blistering disease, in a letter to the editor in the Journal of the American Academy of Dermatology. She presented some of the key recommendations at AAD 2020.
First off, rituximab (Rituxan), the only Food and Drug Administration–approved medication for moderate to severe pemphigus vulgaris and a biologic considered first-line therapy prepandemic, is ill-advised during the COVID-19 era. Its mechanism of benefit is through B-cell depletion. This is an irreversible effect, and reconstitution of B-cell immunity takes 6-12 months. The absence of this immunologic protection for such a long time poses potentially serious problems for pemphigus patients who become infected with SARS-CoV-2.
Also, the opportunity to administer intravenous infusions of the biologic becomes unpredictable during pandemic surges, when limitations on nonemergent medical care may be necessary, noted Dr. Murrell, professor of dermatology at the University of New South Wales and head of dermatology at St. George University Hospital, both in Sydney.
“We have taken the approach of postponing rituximab infusions temporarily, with the aim of delaying peak patient immunosuppression during peak COVID-19 incidence to reduce the risk of adverse outcomes,” Dr. Murrell and coauthors wrote in the letter (J Am Acad Dermatol. 2020 Jun;82[6]:e235-6).
The other traditional go-to therapy for pemphigus is corticosteroids. They’re effective, fast acting, and relatively inexpensive. But their nonselective immunosuppressive action boosts infection risk in general, and more specifically it increases the risk of developing severe forms of COVID-19 should a patient become infected with SARS-CoV-2.
“A basic therapeutic principle with particular importance during the pandemic is that glucocorticoids and steroid-sparing immunosuppressive agents, such as azathioprine and mycophenolate mofetil, should be tapered to the lowest effective dose. In active COVID-19 infection, immunosuppressive steroid-sparing medications should be discontinued when possible, although glucocorticoid cessation often cannot be considered due to risk for adrenal insufficiency,” the authors continued.
“Effective as adjuvant treatment in both pemphigus and COVID-19,intravenous immunoglobulin supports immunity and therefore may be useful in this setting,” they wrote. It’s not immunosuppressive, and, they noted, there’s good-quality evidence from a Japanese randomized, double-blind, controlled trial that a 5-day course of intravenous immunoglobulin is effective therapy for pemphigus (J Am Acad Dermatol. 2009 Apr;60[4]:595-603).
Moreover, intravenous immunoglobulin is also reportedly effective in severe COVID-19 (Open Forum Infect Dis. 2020 Mar 21. doi: 10.1093/ofid/ofaa102.).
Another option is to consider enrolling a patient with moderate or severe pemphigus vulgaris or foliaceus in the ongoing pivotal phase 3, international, double-blind, placebo-controlled PEGASUS trial of rilzabrutinib, a promising oral reversible Bruton tyrosine kinase inhibitor. The medication has a short half-life and a self-limited immunomodulatory effect. Moreover, the trial is set up for remote patient visits on an outpatient basis via teledermatology, so the 65-week study can continue despite the pandemic. Both newly diagnosed and relapsing patients are eligible for the trial, headed by Dr. Murrell. At AAD 2020 she reported encouraging results from a phase 2b trial of rilzabrutinib.
She is a consultant to Principia Biopharma, sponsor of the PEGASUS trial, and has received institutional research grants from numerous pharmaceutical companies.
The Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Together with physicians from the Mayo Clinic, Alexandria (Egypt) University, and Tehran (Iran) University, she recently published updated expert guidance for treatment of this severe, potentially fatal mucocutaneous autoimmune blistering disease, in a letter to the editor in the Journal of the American Academy of Dermatology. She presented some of the key recommendations at AAD 2020.
First off, rituximab (Rituxan), the only Food and Drug Administration–approved medication for moderate to severe pemphigus vulgaris and a biologic considered first-line therapy prepandemic, is ill-advised during the COVID-19 era. Its mechanism of benefit is through B-cell depletion. This is an irreversible effect, and reconstitution of B-cell immunity takes 6-12 months. The absence of this immunologic protection for such a long time poses potentially serious problems for pemphigus patients who become infected with SARS-CoV-2.
Also, the opportunity to administer intravenous infusions of the biologic becomes unpredictable during pandemic surges, when limitations on nonemergent medical care may be necessary, noted Dr. Murrell, professor of dermatology at the University of New South Wales and head of dermatology at St. George University Hospital, both in Sydney.
“We have taken the approach of postponing rituximab infusions temporarily, with the aim of delaying peak patient immunosuppression during peak COVID-19 incidence to reduce the risk of adverse outcomes,” Dr. Murrell and coauthors wrote in the letter (J Am Acad Dermatol. 2020 Jun;82[6]:e235-6).
The other traditional go-to therapy for pemphigus is corticosteroids. They’re effective, fast acting, and relatively inexpensive. But their nonselective immunosuppressive action boosts infection risk in general, and more specifically it increases the risk of developing severe forms of COVID-19 should a patient become infected with SARS-CoV-2.
“A basic therapeutic principle with particular importance during the pandemic is that glucocorticoids and steroid-sparing immunosuppressive agents, such as azathioprine and mycophenolate mofetil, should be tapered to the lowest effective dose. In active COVID-19 infection, immunosuppressive steroid-sparing medications should be discontinued when possible, although glucocorticoid cessation often cannot be considered due to risk for adrenal insufficiency,” the authors continued.
“Effective as adjuvant treatment in both pemphigus and COVID-19,intravenous immunoglobulin supports immunity and therefore may be useful in this setting,” they wrote. It’s not immunosuppressive, and, they noted, there’s good-quality evidence from a Japanese randomized, double-blind, controlled trial that a 5-day course of intravenous immunoglobulin is effective therapy for pemphigus (J Am Acad Dermatol. 2009 Apr;60[4]:595-603).
Moreover, intravenous immunoglobulin is also reportedly effective in severe COVID-19 (Open Forum Infect Dis. 2020 Mar 21. doi: 10.1093/ofid/ofaa102.).
Another option is to consider enrolling a patient with moderate or severe pemphigus vulgaris or foliaceus in the ongoing pivotal phase 3, international, double-blind, placebo-controlled PEGASUS trial of rilzabrutinib, a promising oral reversible Bruton tyrosine kinase inhibitor. The medication has a short half-life and a self-limited immunomodulatory effect. Moreover, the trial is set up for remote patient visits on an outpatient basis via teledermatology, so the 65-week study can continue despite the pandemic. Both newly diagnosed and relapsing patients are eligible for the trial, headed by Dr. Murrell. At AAD 2020 she reported encouraging results from a phase 2b trial of rilzabrutinib.
She is a consultant to Principia Biopharma, sponsor of the PEGASUS trial, and has received institutional research grants from numerous pharmaceutical companies.
The Dedee F. Murrell, MD, said at the virtual annual meeting of the American Academy of Dermatology.
Together with physicians from the Mayo Clinic, Alexandria (Egypt) University, and Tehran (Iran) University, she recently published updated expert guidance for treatment of this severe, potentially fatal mucocutaneous autoimmune blistering disease, in a letter to the editor in the Journal of the American Academy of Dermatology. She presented some of the key recommendations at AAD 2020.
First off, rituximab (Rituxan), the only Food and Drug Administration–approved medication for moderate to severe pemphigus vulgaris and a biologic considered first-line therapy prepandemic, is ill-advised during the COVID-19 era. Its mechanism of benefit is through B-cell depletion. This is an irreversible effect, and reconstitution of B-cell immunity takes 6-12 months. The absence of this immunologic protection for such a long time poses potentially serious problems for pemphigus patients who become infected with SARS-CoV-2.
Also, the opportunity to administer intravenous infusions of the biologic becomes unpredictable during pandemic surges, when limitations on nonemergent medical care may be necessary, noted Dr. Murrell, professor of dermatology at the University of New South Wales and head of dermatology at St. George University Hospital, both in Sydney.
“We have taken the approach of postponing rituximab infusions temporarily, with the aim of delaying peak patient immunosuppression during peak COVID-19 incidence to reduce the risk of adverse outcomes,” Dr. Murrell and coauthors wrote in the letter (J Am Acad Dermatol. 2020 Jun;82[6]:e235-6).
The other traditional go-to therapy for pemphigus is corticosteroids. They’re effective, fast acting, and relatively inexpensive. But their nonselective immunosuppressive action boosts infection risk in general, and more specifically it increases the risk of developing severe forms of COVID-19 should a patient become infected with SARS-CoV-2.
“A basic therapeutic principle with particular importance during the pandemic is that glucocorticoids and steroid-sparing immunosuppressive agents, such as azathioprine and mycophenolate mofetil, should be tapered to the lowest effective dose. In active COVID-19 infection, immunosuppressive steroid-sparing medications should be discontinued when possible, although glucocorticoid cessation often cannot be considered due to risk for adrenal insufficiency,” the authors continued.
“Effective as adjuvant treatment in both pemphigus and COVID-19,intravenous immunoglobulin supports immunity and therefore may be useful in this setting,” they wrote. It’s not immunosuppressive, and, they noted, there’s good-quality evidence from a Japanese randomized, double-blind, controlled trial that a 5-day course of intravenous immunoglobulin is effective therapy for pemphigus (J Am Acad Dermatol. 2009 Apr;60[4]:595-603).
Moreover, intravenous immunoglobulin is also reportedly effective in severe COVID-19 (Open Forum Infect Dis. 2020 Mar 21. doi: 10.1093/ofid/ofaa102.).
Another option is to consider enrolling a patient with moderate or severe pemphigus vulgaris or foliaceus in the ongoing pivotal phase 3, international, double-blind, placebo-controlled PEGASUS trial of rilzabrutinib, a promising oral reversible Bruton tyrosine kinase inhibitor. The medication has a short half-life and a self-limited immunomodulatory effect. Moreover, the trial is set up for remote patient visits on an outpatient basis via teledermatology, so the 65-week study can continue despite the pandemic. Both newly diagnosed and relapsing patients are eligible for the trial, headed by Dr. Murrell. At AAD 2020 she reported encouraging results from a phase 2b trial of rilzabrutinib.
She is a consultant to Principia Biopharma, sponsor of the PEGASUS trial, and has received institutional research grants from numerous pharmaceutical companies.
FROM AAD 20
Even a few days of steroids may be risky, new study suggests
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
Extended use of corticosteroids for chronic inflammatory conditions puts patients at risk for serious adverse events (AEs), including cardiovascular disease, osteoporosis, cataracts, and diabetes. Now, a growing body of evidence suggests that even short bursts of these drugs are associated with serious risks.
Most recently, a population-based study of more than 2.6 million people found that taking corticosteroids for 14 days or less was associated with a substantially greater risk for gastrointestinal (GI) bleeding, sepsis, and heart failure, particularly within the first 30 days after therapy.
In the study, Tsung-Chieh Yao, MD, PhD, a professor in the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues used a self-controlled case series to analyze data from Taiwan’s National Health Insurance Research Database of medical claims. They compared patients’ conditions in the period from 5 to 90 days before treatment to conditions from the periods from 5 to 30 days and from 31 to 90 days after therapy.
With a median duration of 3 days of treatment, the incidence rate ratios (IRRs) were 1.80 (95% confidence interval, 1.75-1.84) for GI bleeding, 1.99 (95% CI, 1.70-2.32) for sepsis, and 2.37 (95% CI, 2.13-2.63) for heart failure.
Given the findings, physicians should weigh the benefits against the risks of rare but potentially serious consequences of these anti-inflammatory drugs, according to the authors.
“After initiating patients on oral steroid bursts, physicians should be on the lookout for these severe adverse events, particularly within the first month after initiation of steroid therapy,” Dr. Yao said in an interview.
The findings were published online July 6 in Annals of Internal Medicine.
Of the 15,859,129 adult Asians in the Taiwanese database, the study included 2,623,327 adults aged 20-64 years who received single steroid bursts (14 days or less) between Jan. 1, 2013, and Dec. 31, 2015.
Almost 60% of the indications were for skin disorders, such as eczema and urticaria, and for respiratory tract infections, such as sinusitis and acute pharyngitis. Among specialties, dermatology, otolaryngology, family practice, internal medicine, and pediatrics accounted for 88% of prescriptions.
“Our findings are important for physicians and guideline developers because short-term use of oral corticosteroids is common and the real-world safety of this approach remains unclear,” the authors wrote. They acknowledged that the database did not provide information on such potential confounders as disease severity and lifestyle factors, nor did it include children and vulnerable individuals, which may limit the generalizability of the results.
The findings echo those of a 2017 cohort study conducted by researchers at the University of Michigan in Ann Arbor. That study, by Akbar K. Waljee, MD, assistant professor of gastroenterology, University of Michigan, Ann Arbor, and colleagues, included data on more than 1.5 million privately insured U.S. adults. The researchers included somewhat longer steroid bursts of up to 30 days’ duration and found that use of the drugs was associated with a greater than fivefold increased risk for sepsis, a more than threefold increased risk for venous thromboembolism, and a nearly twofold increased risk for fracture within 30 days of starting treatment.
Furthermore, the elevated risk persisted at prednisone-equivalent doses of less than 20 mg/d (IRR, 4.02 for sepsis, 3.61 for venous thromboembolism, and 1.83 for fracture; all P < .001).
The U.S. study also found that during the 3-year period from 2012 to 2014, more than 20% of patients were prescribed short-term oral corticosteroids.
“Both studies indicate that these short-term regimens are more common in the real world than was previously thought and are not risk free,” Dr. Yao said.
Recognition that corticosteroids are associated with adverse events has been building for decades, according to the authors of an editorial that accompanies the new study.
“However, we commonly use short corticosteroid ‘bursts’ for minor ailments despite a lack of evidence for meaningful benefit. We are now learning that bursts as short as 3 days may increase risk for serious AEs, even in young and healthy people,” wrote editorialists Beth I. Wallace, MD, of the Center for Clinical Management Research at the VA Ann Arbor Healthcare System and the Institute for Healthcare Policy and Innovation at Michigan Medicine, Ann Arbor, and Dr. Waljee, who led the 2017 study.
Dr. Wallace and Dr. Waljee drew parallels between corticosteroid bursts and other short-term regimens, such as of antibiotics and opiates, in which prescriber preference and sometimes patient pressure play a role. “All of these treatments have well-defined indications but can cause net harm when used. We can thus conceive of a corticosteroid stewardship model of targeted interventions that aims to reduce inappropriate prescribing,” they wrote.
In an interview, Dr. Wallace, a rheumatologist who prescribes oral steroids fairly frequently, noted that the Taiwan study is the first to investigate steroid bursts. “Up till now, these very short courses have flown under the radar. Clinicians very commonly prescribe short courses to help relieve symptoms of self-limited conditions like bronchitis, and we assume that because the exposure duration is short, the risks are low, especially for patients who are otherwise healthy.”
She warned that the data in the current study indicate that these short bursts – even at the lower end of the 1- to 2-week courses American physicians prescribe most often – carry small but real increases in risk for serious AEs. “And these increases were seen in young, healthy people, not just in people with preexisting conditions,” she said. “So, we might need to start thinking harder about how we are prescribing even these very short courses of steroids and try to use steroids only when their meaningful benefits really outweigh the risk.”
She noted that a patient with a chronic inflammatory condition such as rheumatoid arthritis may benefit substantially from short-term steroids to treat a disease flare. In that specific case, the benefits of short-term steroids may outweigh the risks, Dr. Wallace said.
But not everyone thinks a new strategy is needed. For Whitney A. High, MD, associate professor of dermatology and pathology at the University of Colorado at Denver, Aurora, the overprescribing of short-term corticosteroids is not a problem, and dermatologists are already exercising caution.
“I only prescribe these drugs short term to, at a guess, about 1 in 40 patients and only when a patient is miserable and quality of life is being seriously affected,” he said in an interview. “And that’s something that can’t be measured in a database study like the one from Taiwan but only in a risk-benefit analysis,” he said.
Furthermore, dermatologists have other drugs and technologies in their armamentarium, including topical steroids with occlusion or with wet wraps, phototherapy, phosphodiesterase inhibitors, calcipotriene, methotrexate and other immunosuppressive agents, and biologics. “In fact, many of these agents are specifically referred to as steroid-sparing,” Dr. High said.
Nor does he experience much pressure from patients to prescribe these drugs. “While occasionally I may encounter a patient who places pressure on me for oral steroids, it’s probably not nearly as frequently as providers in other fields are pressured to prescribe antibiotics or narcotics,” he said.
According to the Taiwanese researchers, the next step is to conduct more studies, including clinical trials, to determine optimal use of corticosteroids by monitoring adverse events. In the meantime, for practitioners such as Dr. Wallace and Dr. High, there is ample evidence from several recent studies of the harms of short-term corticosteroids, whereas the benefits for patients with self-limiting conditions remain uncertain. “This and other studies like it quite appropriately remind providers to avoid oral steroids when they’re not necessary and to seek alternatives where possible,” Dr. High said.
The study was supported by the National Health Research Institutes of Taiwan, the Ministry of Science and Technology of Taiwan, the Chang Gung Medical Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH). Dr. Yao has disclosed no relevant financial relationships. Dr. Wu has received grants from GlaxoSmithKline outside the submitted work. The editorialists and Dr. High have disclosed no relevant financial relationships. Dr. Wallace received an NIH grant during the writing of the editorial.
A version of this article originally appeared on Medscape.com.
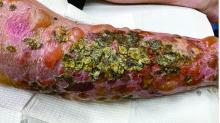
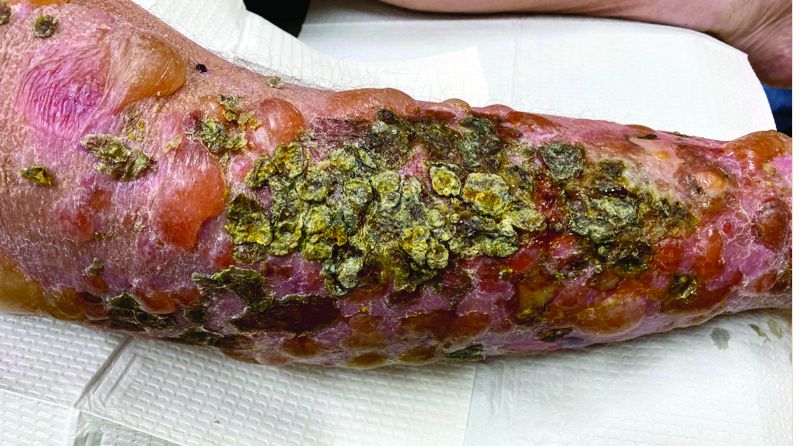
A 72-year-old with an acute, pruritic, bullous eruption involving his right pretibial extremity
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.
Localized bullous pemphigoid
with a predilection in the elderly population.1
Localized variants of bullous pemphigoid (BP) are rare and have been reported to arise at sites of mechanical trauma, prior radiation, lymphedema, surgical scars, burns, fistulas, and ostomies.1-3 Although the mechanism remains unclear, the Koebner phenomenon is thought to induce dysregulation of immunologic and vascular factors in sites of mechanical shear and trauma in susceptible individuals.3
Localized BP is an important entity for the dermatologist to be familiar with, as the diagnosis is often delayed. The localized, well-defined skin lesions frequently mimic contact dermatitis. In fact, previous reports have shown the most likely misdiagnosis of localized BP is acute allergic contact dermatitis, stasis dermatitis, and eczematous dermatitis.4,5
In this patient, histopathologic examination of a biopsy revealed a subepidermal blister with numerous eosinophils. Direct immunofluorescence study of perilesional skin showed strong linear IgG and C3 deposits at the basal membrane level. Serum level of autoantibody to BP180 antigen was elevated. Bacterial culture was positive for Staphylococcus aureus. These findings were suggestive of unilateral, localized BP with superimposed bacterial infection. Initial treatment with an extended course of doxycycline 200 mg twice daily, topical triamcinolone 0.1% ointment twice daily with compression therapy, and leg elevation led to clinical improvement with healing of previous lesions on the leg. At follow-up 3 weeks later, the patient had continued to develop new bullous lesions on the trunk and upper thighs. He was subsequently started on systemic immunosuppressive therapy for generalized bullous pemphigoid.
Importantly, localized BP generally follows a more benign disease course, although long-term follow-up is recommended for monitoring given the potential risk of developing the generalized form of BP of approximately 15%.3 Topical corticosteroids and oral antibiotics are recommended as the first-line therapy in these patients, with an escalated systemic therapy if needed for disease progression.3,5
Our case represents an important differential diagnosis to consider when evaluating an acute localized bullous eruption in an elderly patient.
Dr. Cusick and Dr. Dolohanty are with the department of dermatology, University of Rochester (N.Y.), and provided the case and photo. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kohroh K et al. J Dermatol. 2007 Jul;34(7):482-5.
2. Nguyen T et al. Dermatology 2014;229(2):88-96.
3. Sen BB et al. Indian J Dermatol Venereol Leprol. 2013;79(4):554.
4. Salomon RJ et al. Arch Dermatol. 1987 Mar;123(3):389-92.
5. Tran JT, Mutasim DF. Int J Dermatol. 2005 Nov;44(11):942-5.
Study highlights benefits of integrating dermatology into oncology centers
, according to the results of a retrospective study of 208 adults treated at the Dana-Farber Cancer Institute in Boston, or affiliated sites.
The benefits of prophylactic treatment for treatment-related skin rash in cancer patients are well established, based largely on the Skin Toxicity Evaluation Protocol With Panitumumab (STEPP) trial published in 2012, which led to the development of guidelines for preventing and managing skin toxicity associated with epidermal growth factor receptor inhibitor (EGFRi) treatment, wrote Zizi Yu of Harvard Medical School, Boston, and coauthors. However, they added, “awareness of and adherence to these guidelines among oncology clinicians are thus far poorly understood.” They pointed out that 90% of patients treated with an EGFRi develop cutaneous toxicities, which can affect quality of life, increase the risk of infection, and require dose modification, interruption, or discontinuation of treatment.
In the study, published in JAMA Dermatology, the researchers compared adherence to protocols at Dana-Farber before and after the 2014-2015 initiation of a Skin Toxicities from Anticancer Therapies (STAT) program at Dana-Farber established in 2014 by the department of dermatology.
The study population included 208 adult cancer patients with colorectal cancer, head and neck cancer, or cutaneous squamous cell cancer, treated with at least one dose of cetuximab (Erbitux); the average age of the patients was 62 years and the majority were men. Most had stage IV disease. The STAT program included the integration of 9 oncodermatologists in the head and neck, genitourinary, and cutaneous oncology clinics for 7 of 10 cancer treatment sessions per week, as well as the creation of urgent access time slots in oncodermatology clinics for 10 of 10 sessions per week.
Overall, significantly more patients were treated prophylactically for skin toxicity at the start of cetuximab treatment in 2017 vs. 2012 (47% vs. 25%, P less than .001) after the initiation of a dermatology protocol.
In addition, the preemptive use of tetracycline increased significantly from 45% to 71% (P = .02) between the two time periods, as did the use of topical corticosteroids (from 7% to 57%, P less than .001), while the use of topical antibiotics decreased from 79% to 43% (P = .02). Rates of dose changes or interruptions were significantly lower among those on prophylaxis (5% vs. 19%, P =.01), a 79% lower risk. Patients treated prophylactically were 94% less likely to need a first rescue treatment and 74% less likely to need a second rescue treatment for rash.
The study findings were limited by several factors including the retrospective design, use of data from a single institution, and incomplete documentation of some patients, the researchers noted. However, the results “highlight the value of integrating dermatologic care and education into oncology centers by increasing adherence to evidence-based prophylaxis protocols for rash and appropriate treatment agent selection, which may minimize toxicity-associated chemotherapy interruptions and improve quality of life,” they concluded.
“As novel cancer treatment options for patients continue to develop, and as patients with cancer live longer, the spectrum and prevalence of dermatologic toxic effects will continue to expand,” Bernice Y. Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, wrote in an accompanying editorial.
“Dermatologists have a critical and growing opportunity and role to engage in multidisciplinary efforts to provide expert guidance to best manage these cutaneous adverse events to achieve the best outcome for patients with cancer,” she said.
Although the prophylaxis rates at Dana-Farber improved after the establishment of the oncodermatology program, they remained relatively low, “underscoring an opportunity to improve on how to teach, execute, and improve access to oncodermatologic care for patients with cancer,” said Dr. Kwong. Knowledge gaps in the nature of skin toxicity for newer cancer drugs poses another challenge for skin toxicity management in these patients, she added.
However, “timely and consistent access to dermatologic expertise in oncology practices is critical to prevent unnecessary discontinuation of life-saving anticancer therapy, especially as multiple studies have demonstrated that anticancer therapy–associated skin toxicity may be associated with a positive response to anticancer therapy,” she emphasized.
Ms. Yu and one coauthor had no financial conflicts to disclose, the two other authors had several disclosures, outside of the submitted work. Dr. Kwong disclosed serving as a consultant for Genentech and Oncoderm and serving on the advisory board for Kyowa Kirin.
SOURCE: Yu Z et al. JAMA Dermatol. 2020 July 1. doi: 10.1001/jamadermatol.2020.1795. Kwong BY. JAMA Dermatol. 2020 Jul 1. doi: 10.1001/jamadermatol.2020.1794.
, according to the results of a retrospective study of 208 adults treated at the Dana-Farber Cancer Institute in Boston, or affiliated sites.
The benefits of prophylactic treatment for treatment-related skin rash in cancer patients are well established, based largely on the Skin Toxicity Evaluation Protocol With Panitumumab (STEPP) trial published in 2012, which led to the development of guidelines for preventing and managing skin toxicity associated with epidermal growth factor receptor inhibitor (EGFRi) treatment, wrote Zizi Yu of Harvard Medical School, Boston, and coauthors. However, they added, “awareness of and adherence to these guidelines among oncology clinicians are thus far poorly understood.” They pointed out that 90% of patients treated with an EGFRi develop cutaneous toxicities, which can affect quality of life, increase the risk of infection, and require dose modification, interruption, or discontinuation of treatment.
In the study, published in JAMA Dermatology, the researchers compared adherence to protocols at Dana-Farber before and after the 2014-2015 initiation of a Skin Toxicities from Anticancer Therapies (STAT) program at Dana-Farber established in 2014 by the department of dermatology.
The study population included 208 adult cancer patients with colorectal cancer, head and neck cancer, or cutaneous squamous cell cancer, treated with at least one dose of cetuximab (Erbitux); the average age of the patients was 62 years and the majority were men. Most had stage IV disease. The STAT program included the integration of 9 oncodermatologists in the head and neck, genitourinary, and cutaneous oncology clinics for 7 of 10 cancer treatment sessions per week, as well as the creation of urgent access time slots in oncodermatology clinics for 10 of 10 sessions per week.
Overall, significantly more patients were treated prophylactically for skin toxicity at the start of cetuximab treatment in 2017 vs. 2012 (47% vs. 25%, P less than .001) after the initiation of a dermatology protocol.
In addition, the preemptive use of tetracycline increased significantly from 45% to 71% (P = .02) between the two time periods, as did the use of topical corticosteroids (from 7% to 57%, P less than .001), while the use of topical antibiotics decreased from 79% to 43% (P = .02). Rates of dose changes or interruptions were significantly lower among those on prophylaxis (5% vs. 19%, P =.01), a 79% lower risk. Patients treated prophylactically were 94% less likely to need a first rescue treatment and 74% less likely to need a second rescue treatment for rash.
The study findings were limited by several factors including the retrospective design, use of data from a single institution, and incomplete documentation of some patients, the researchers noted. However, the results “highlight the value of integrating dermatologic care and education into oncology centers by increasing adherence to evidence-based prophylaxis protocols for rash and appropriate treatment agent selection, which may minimize toxicity-associated chemotherapy interruptions and improve quality of life,” they concluded.
“As novel cancer treatment options for patients continue to develop, and as patients with cancer live longer, the spectrum and prevalence of dermatologic toxic effects will continue to expand,” Bernice Y. Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, wrote in an accompanying editorial.
“Dermatologists have a critical and growing opportunity and role to engage in multidisciplinary efforts to provide expert guidance to best manage these cutaneous adverse events to achieve the best outcome for patients with cancer,” she said.
Although the prophylaxis rates at Dana-Farber improved after the establishment of the oncodermatology program, they remained relatively low, “underscoring an opportunity to improve on how to teach, execute, and improve access to oncodermatologic care for patients with cancer,” said Dr. Kwong. Knowledge gaps in the nature of skin toxicity for newer cancer drugs poses another challenge for skin toxicity management in these patients, she added.
However, “timely and consistent access to dermatologic expertise in oncology practices is critical to prevent unnecessary discontinuation of life-saving anticancer therapy, especially as multiple studies have demonstrated that anticancer therapy–associated skin toxicity may be associated with a positive response to anticancer therapy,” she emphasized.
Ms. Yu and one coauthor had no financial conflicts to disclose, the two other authors had several disclosures, outside of the submitted work. Dr. Kwong disclosed serving as a consultant for Genentech and Oncoderm and serving on the advisory board for Kyowa Kirin.
SOURCE: Yu Z et al. JAMA Dermatol. 2020 July 1. doi: 10.1001/jamadermatol.2020.1795. Kwong BY. JAMA Dermatol. 2020 Jul 1. doi: 10.1001/jamadermatol.2020.1794.
, according to the results of a retrospective study of 208 adults treated at the Dana-Farber Cancer Institute in Boston, or affiliated sites.
The benefits of prophylactic treatment for treatment-related skin rash in cancer patients are well established, based largely on the Skin Toxicity Evaluation Protocol With Panitumumab (STEPP) trial published in 2012, which led to the development of guidelines for preventing and managing skin toxicity associated with epidermal growth factor receptor inhibitor (EGFRi) treatment, wrote Zizi Yu of Harvard Medical School, Boston, and coauthors. However, they added, “awareness of and adherence to these guidelines among oncology clinicians are thus far poorly understood.” They pointed out that 90% of patients treated with an EGFRi develop cutaneous toxicities, which can affect quality of life, increase the risk of infection, and require dose modification, interruption, or discontinuation of treatment.
In the study, published in JAMA Dermatology, the researchers compared adherence to protocols at Dana-Farber before and after the 2014-2015 initiation of a Skin Toxicities from Anticancer Therapies (STAT) program at Dana-Farber established in 2014 by the department of dermatology.
The study population included 208 adult cancer patients with colorectal cancer, head and neck cancer, or cutaneous squamous cell cancer, treated with at least one dose of cetuximab (Erbitux); the average age of the patients was 62 years and the majority were men. Most had stage IV disease. The STAT program included the integration of 9 oncodermatologists in the head and neck, genitourinary, and cutaneous oncology clinics for 7 of 10 cancer treatment sessions per week, as well as the creation of urgent access time slots in oncodermatology clinics for 10 of 10 sessions per week.
Overall, significantly more patients were treated prophylactically for skin toxicity at the start of cetuximab treatment in 2017 vs. 2012 (47% vs. 25%, P less than .001) after the initiation of a dermatology protocol.
In addition, the preemptive use of tetracycline increased significantly from 45% to 71% (P = .02) between the two time periods, as did the use of topical corticosteroids (from 7% to 57%, P less than .001), while the use of topical antibiotics decreased from 79% to 43% (P = .02). Rates of dose changes or interruptions were significantly lower among those on prophylaxis (5% vs. 19%, P =.01), a 79% lower risk. Patients treated prophylactically were 94% less likely to need a first rescue treatment and 74% less likely to need a second rescue treatment for rash.
The study findings were limited by several factors including the retrospective design, use of data from a single institution, and incomplete documentation of some patients, the researchers noted. However, the results “highlight the value of integrating dermatologic care and education into oncology centers by increasing adherence to evidence-based prophylaxis protocols for rash and appropriate treatment agent selection, which may minimize toxicity-associated chemotherapy interruptions and improve quality of life,” they concluded.
“As novel cancer treatment options for patients continue to develop, and as patients with cancer live longer, the spectrum and prevalence of dermatologic toxic effects will continue to expand,” Bernice Y. Kwong, MD, director of the supportive dermato-oncology program at Stanford (Calif.) University, wrote in an accompanying editorial.
“Dermatologists have a critical and growing opportunity and role to engage in multidisciplinary efforts to provide expert guidance to best manage these cutaneous adverse events to achieve the best outcome for patients with cancer,” she said.
Although the prophylaxis rates at Dana-Farber improved after the establishment of the oncodermatology program, they remained relatively low, “underscoring an opportunity to improve on how to teach, execute, and improve access to oncodermatologic care for patients with cancer,” said Dr. Kwong. Knowledge gaps in the nature of skin toxicity for newer cancer drugs poses another challenge for skin toxicity management in these patients, she added.
However, “timely and consistent access to dermatologic expertise in oncology practices is critical to prevent unnecessary discontinuation of life-saving anticancer therapy, especially as multiple studies have demonstrated that anticancer therapy–associated skin toxicity may be associated with a positive response to anticancer therapy,” she emphasized.
Ms. Yu and one coauthor had no financial conflicts to disclose, the two other authors had several disclosures, outside of the submitted work. Dr. Kwong disclosed serving as a consultant for Genentech and Oncoderm and serving on the advisory board for Kyowa Kirin.
SOURCE: Yu Z et al. JAMA Dermatol. 2020 July 1. doi: 10.1001/jamadermatol.2020.1795. Kwong BY. JAMA Dermatol. 2020 Jul 1. doi: 10.1001/jamadermatol.2020.1794.
FROM JAMA DERMATOLOGY
Expert shares his approach to treating warts in children
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
In the clinical experience of Anthony J. Mancini, MD, one option for children and adolescents who present with common warts is to do nothing, since they may resolve on their own.
“Many effective treatments that we have are painful and poorly tolerated, especially in younger children,” Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “However, while they’re harmless and often self-limited, warts often form a social stigma, and parents often desire therapy.”
Even though warts may spontaneously resolve in up to 65% of patients at 2 years and 80% at 4 years, the goals of treatment are to eradicate them, minimize pain, avoid scarring, and help prevent recurrence.
One effective topical therapy he highlighted is WartPEEL cream, which is a proprietary, compounded formulation of 17% salicylic acid and 2% 5-fluorouracil. “It’s in a sustained release vehicle called Remedium, and is available from a compounding pharmacy, but not FDA approved,” said Dr. Mancini, who is also head of pediatric dermatology at Lurie Children’s Hospital of Chicago. “It’s applied nightly with plastic tape occlusion and rinsed off each morning.”
WartPEEL is available through NuCara Pharmacy at 877-268-2272. It is not covered by most insurance plans and it costs around $80. “It is very effective, tends to be totally painless, and has a much quicker response than over-the-counter salicylic acid-based treatments for warts,” he said.
Another treatment option is oral cimetidine, especially in patients who have multiple or recalcitrant warts. The recommended dosing is 30-40 mg/kg per day, divided into twice-daily dosing. “You have to give it for at least 8-12 weeks to determine whether it’s working or not,” Dr. Mancini said. “In the initial report, [investigators] described an 81% complete response rate, but subsequent randomized, controlled trials were not able to confirm that data against placebo or topical treatments. I will say, though, that cimetidine is well tolerated. It’s always worth a try but, if you do use it, always consider other medications the patient may be taking and potential drug-drug interactions.”
For flat warts, verrucous papules that commonly occur on the face, Dr. Mancini recommends off-label treatment with 5% 5-fluorouracil cream (Efudex), which is normally indicated for actinic keratoses in adults. “I have patients apply this for 3 nights per week and work their way up gradually to nightly application,” he said. “It’s really important that parents and patients understand the importance of sun protection when they’re using Efudex, and they need to know that some irritation is possible. Overall, this treatment seems to be very well tolerated.”
Other treatment options for common warts, in addition to over-the-counter products that contain salicylic acid, are home cryotherapy kits that contain a mixture of diethyl ether and propane. “These can be effective for small warts,” Dr. Mancini said. “But for larger, thicker lesions, they’re not going to quite as effective.”
Treatment options best reserved for dermatologists, he continued, include in-office liquid nitrogen cryotherapy, “if it’s tolerated,” he said. “I have a no-hold policy, so if we have to hold a child down who’s flailing and crying and screaming during treatment, we’re probably not going to use liquid nitrogen.” He also mentioned topical immunotherapy with agents like squaric acid dibutylester. “This is almost like putting poison ivy on your warts to get the immune system revved up,” he said. “It can be very effective.” Other treatment options include intralesional immune therapy, topical cidofovir, and even pulsed-dye laser.
Dr. Mancini disclosed that he is a consultant to and a member of the scientific advisory board for Verrica Pharmaceuticals.
FROM PEDIATRIC DERMATOLOGY 2020
Pulmonary function tests can’t substitute for high-resolution CT in early systemic sclerosis ILD screening
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
FROM ARTHRITIS & RHEUMATOLOGY
First validated classification criteria for discoid lupus erythematosus unveiled
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
FROM AAD 20
Pilot study shows apremilast effective for severe recurrent canker sores
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
showed.
“Canker sores [aphthous ulcers] are very common, yet are often not well managed as the diagnosis is not always correctly made,” lead study author Alison J. Bruce, MB, ChB, said in an interview following the virtual annual meeting of the American Academy of Dermatology. “They’re often mistaken for herpes infection and therefore treated with antiviral therapy. Of the available therapies, several have common side effects or require lab monitoring or are not uniformly effective.”
In their poster abstract, Dr. Bruce, of the division of dermatology at the Mayo Clinic, Jacksonville, Fla., and colleagues noted that, while no principal etiology has been established for recurrent aphthous stomatitis (RAS), immune up-regulation plays a role in the pathogenesis of the condition. “Attacks of RAS may be precipitated by local trauma, stress, food intake, drugs, hormonal changes and vitamin and trace element deficiencies,” they wrote. “Local and systemic conditions and genetic, immunological and microbial factors all may play a role in the pathogenesis.”
Apremilast, a phosphodiesterase-4 inhibitor, down-regulates inflammatory response by modulating expression of tumor necrosis factor–alpha; interferon-gamma; and interleukin-2, IL-12, IL-17, and IL-23. It is approved by the Food and Drug Administration for treating plaque psoriasis and psoriatic arthritis, and in July 2019, was approved for treating ulcers associated with Behçet’s disease, in adults.*
For the pilot study, the researchers enrolled 15 patients with RAS to receive apremilast 30 mg twice daily for 15 weeks after 1 week titration. To be eligible for the trial, patients must have had monthly oral ulcers in preceding 6 months, at least two ulcers in previous 4 weeks prior to enrollment at baseline, at least three ulcers during flares, inadequate control with topical therapy, and no evidence of systemic disease. They excluded patients on immune-modulating therapy or systemic steroids, pregnant or breastfeeding women, those with a systemic infection, those with a history of recurrent bacterial, viral, fungal, or mycobacterial infection, those with a history of depression, as well as those with a known malignancy or vitamin deficiencies. Patients were assessed monthly, evaluating number of ulcers, visual analog pain scale, physician’s global assessment and Chronic Oral Mucosal Disease Questionnaire (COMDQ).
Dr. Bruce and colleagues found that, within 4 weeks of therapy, complete clearance of RAS lesions occurred in all patients except one in whom ulcers were reported to be less severe. That patient had considerable reduction in number, size, and duration of oral ulcers. Remission in all patients was sustained during 16 weeks of treatment. COMDQ responses improved considerably from baseline to week 8, and this was continued until week 16.
“Onset of response [to apremilast] was rapid,” Dr. Bruce said. “For many other therapies, patients are counseled that [they] may take several weeks to become effective. Response was also dramatic. Almost all patients had complete remission from their ulcers, compared with other therapies where oftentimes reduction or attenuation is achieved, as opposed to complete resolution. There was a suggestion that a lower dose [of apremilast] may still be effective. This adds to our ‘toolbox’ of therapeutic options.”
The most common adverse effects were nausea/vomiting and headache, but these were mild and tolerable and generally resolved by week 4.
The researchers acknowledged certain limitations of the study, including its small sample size. “The challenge will most likely be insurance coverage,” Dr. Bruce said. “This is unfortunate, as it would be ideal to offer a safe treatment without the need for monitoring.”
The investigator-initiated study was supported by Celgene. The researchers reported having no financial disclosures.
SOURCE: Bruce AJ et al. AAD 20, Abstract 17701.
*Correction 6/23/2020: An earlier version of this story misstated the approved indications for apremilast.
FROM AAD 20
Intranasal butorphanol effectively rescues from intractable itch in retrospective study
Shawn G. Kwatra, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
Dr. Kwatra, a dermatologist at Johns Hopkins University, Baltimore, where he heads a specialized pruritus clinic, presented a retrospective study of 16 such patients treated with inhaled butorphanol. All had been responsive to a minimum of four antipruritic medications.
This is one of the largest-ever reported series of patients treated with intranasal butorphanol as acute rescue therapy for intractable itch, and it provides a strong signal of efficacy, he said in an interview.
Indeed, 11 of the 16 patients reported marked improvement in their itch after introduction of short-term treatment with butorphanol nasal spray, 1 reported no improvement, and 4 were lost to follow-up.
Itch, Dermatology Life Quality Index (DLQI), and Beck Depression Inventory scores were formally measured prior to introduction of short-term inhaled butorphanol and again at follow-up appointments at 4-6 weeks. The mean self-reported itch numeric rating scale score improved from a mean of 9.8 out of a possible 10 at baseline to 4.6 at follow-up. The reduction in itch was accompanied by major improvements in quality of life: the mean DLQI score dropped from 20.2 to 10.8, while the Beck Depression Inventory score went from 22.1 – typically interpreted as an indicator of moderate depression – to 14.2.
Three patients reported insomnia and/or lightheadedness they attributed to inhaled butorphanol.
The patients with chronic refractory itch had a wide range of associated underlying diagnoses. These included primary sclerosing cholangitis, trigeminal trophic syndrome, brachioradial pruritus, neuropathic pruritus, prurigo nodularis, chronic idiopathic urticaria, chronic aquagenic pruritus, atopic dermatitis, and itch induced by programmed death–1 immune checkpoint inhibitor therapy. It will take large randomized, controlled trials to determine which of these types of chronic pruritus benefit most from intranasal butorphanol, according to Dr. Kwatra.
Since butorphanol is a narcotic analgesic ill-suited to applications other than as short-term acute rescue therapy, there is a pressing unmet need for new therapies specifically targeting chronic itch as a symptom, he added. Several promising agents are advancing through the drug development pipeline.
Dr. Kwatra reported having no financial conflicts regarding this study, conducted free of commercial support.
Shawn G. Kwatra, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
Dr. Kwatra, a dermatologist at Johns Hopkins University, Baltimore, where he heads a specialized pruritus clinic, presented a retrospective study of 16 such patients treated with inhaled butorphanol. All had been responsive to a minimum of four antipruritic medications.
This is one of the largest-ever reported series of patients treated with intranasal butorphanol as acute rescue therapy for intractable itch, and it provides a strong signal of efficacy, he said in an interview.
Indeed, 11 of the 16 patients reported marked improvement in their itch after introduction of short-term treatment with butorphanol nasal spray, 1 reported no improvement, and 4 were lost to follow-up.
Itch, Dermatology Life Quality Index (DLQI), and Beck Depression Inventory scores were formally measured prior to introduction of short-term inhaled butorphanol and again at follow-up appointments at 4-6 weeks. The mean self-reported itch numeric rating scale score improved from a mean of 9.8 out of a possible 10 at baseline to 4.6 at follow-up. The reduction in itch was accompanied by major improvements in quality of life: the mean DLQI score dropped from 20.2 to 10.8, while the Beck Depression Inventory score went from 22.1 – typically interpreted as an indicator of moderate depression – to 14.2.
Three patients reported insomnia and/or lightheadedness they attributed to inhaled butorphanol.
The patients with chronic refractory itch had a wide range of associated underlying diagnoses. These included primary sclerosing cholangitis, trigeminal trophic syndrome, brachioradial pruritus, neuropathic pruritus, prurigo nodularis, chronic idiopathic urticaria, chronic aquagenic pruritus, atopic dermatitis, and itch induced by programmed death–1 immune checkpoint inhibitor therapy. It will take large randomized, controlled trials to determine which of these types of chronic pruritus benefit most from intranasal butorphanol, according to Dr. Kwatra.
Since butorphanol is a narcotic analgesic ill-suited to applications other than as short-term acute rescue therapy, there is a pressing unmet need for new therapies specifically targeting chronic itch as a symptom, he added. Several promising agents are advancing through the drug development pipeline.
Dr. Kwatra reported having no financial conflicts regarding this study, conducted free of commercial support.
Shawn G. Kwatra, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
Dr. Kwatra, a dermatologist at Johns Hopkins University, Baltimore, where he heads a specialized pruritus clinic, presented a retrospective study of 16 such patients treated with inhaled butorphanol. All had been responsive to a minimum of four antipruritic medications.
This is one of the largest-ever reported series of patients treated with intranasal butorphanol as acute rescue therapy for intractable itch, and it provides a strong signal of efficacy, he said in an interview.
Indeed, 11 of the 16 patients reported marked improvement in their itch after introduction of short-term treatment with butorphanol nasal spray, 1 reported no improvement, and 4 were lost to follow-up.
Itch, Dermatology Life Quality Index (DLQI), and Beck Depression Inventory scores were formally measured prior to introduction of short-term inhaled butorphanol and again at follow-up appointments at 4-6 weeks. The mean self-reported itch numeric rating scale score improved from a mean of 9.8 out of a possible 10 at baseline to 4.6 at follow-up. The reduction in itch was accompanied by major improvements in quality of life: the mean DLQI score dropped from 20.2 to 10.8, while the Beck Depression Inventory score went from 22.1 – typically interpreted as an indicator of moderate depression – to 14.2.
Three patients reported insomnia and/or lightheadedness they attributed to inhaled butorphanol.
The patients with chronic refractory itch had a wide range of associated underlying diagnoses. These included primary sclerosing cholangitis, trigeminal trophic syndrome, brachioradial pruritus, neuropathic pruritus, prurigo nodularis, chronic idiopathic urticaria, chronic aquagenic pruritus, atopic dermatitis, and itch induced by programmed death–1 immune checkpoint inhibitor therapy. It will take large randomized, controlled trials to determine which of these types of chronic pruritus benefit most from intranasal butorphanol, according to Dr. Kwatra.
Since butorphanol is a narcotic analgesic ill-suited to applications other than as short-term acute rescue therapy, there is a pressing unmet need for new therapies specifically targeting chronic itch as a symptom, he added. Several promising agents are advancing through the drug development pipeline.
Dr. Kwatra reported having no financial conflicts regarding this study, conducted free of commercial support.
FROM AAD 20
Key clinical point: Intranasal butorphanol as short-term rescue therapy is a game changer for intractable itch.
Major finding: Mean itch numeric rating scale scores improved from 9.8 to 4.6.
Study details: This was a retrospective study of 16 patients who presented to a university pruritus clinic with severe chronic itch unresponsive to at least four antipruritic therapies.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
Source: Kwatra SG. AAD 20, Abstract 17132.