User login
Treatment options for lentigo maligna far from perfect
PARK CITY, UTAH – Dr. Glen M. Bowen has been working to improve the surgical treatment of lentigo maligna ever since he joined the Huntsman Cancer Institute at the University of Utah 16 years ago. A retrospective review from Memorial Sloan-Kettering Cancer Center found that on average, 7.1-mm margins are required to remove lentigo maligna (LM) (J. Am. Acad. Dermatol. 2008;58[1]:142-8).
“If you have an LM with a 10-mm diameter to begin with, 7.1-mm margins give you a final surgical diameter of 24.2 mm,” Dr. Bowen said at the annual meeting of the Pacific Dermatologic Association. “These are very morbid surgeries in cosmetically sensitive areas for a relatively low-risk tumor.”
The risk of LM progressing to an invasive melanoma is not known, but is estimated to range between 5% and 33%. Of 2,016 patients treated for LM at the Huntsman Cancer Institute, 522 have been treated with neo-adjuvant topical imiquimod 5% cream followed by a conservative staged excision with 2-mm margins with a recurrence rate of 2.3% during median follow-up of 5 years. Of their recurrences, about 20% recurred with invasion. All recurrences to date have been less than 1 mm in depth (stage IA), which has an estimated mortality risk of 5% at 5 years. “A 5% mortality rate of the 20% that recur with invasion of the 2.3% that recur after surgery yields a mortality risk of 0.023%,” Dr. Bowen said. “Due to the very low risk of actually dying from a recurrent LM, very large and morbid surgical defects strike me as a punishment that doesn’t fit the crime in terms of the cost-benefit ratio.”
He and his associates at the Huntsman Cancer Institute have observed some deaths in patients who presented with LM melanoma (invasive melanoma) but have not observed a single death in patients who presented with LM in situ that subsequently recurred. For these reasons, Dr. Bowen favors pretreating LM with imiquimod 5% cream followed by a conservative staged excision, a process that substantially decreases the size of the surgical defects.
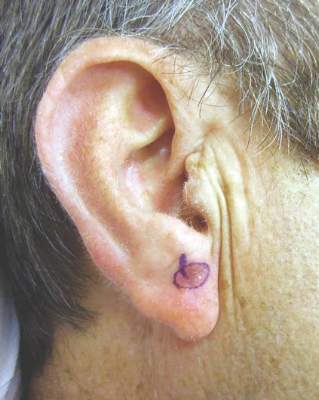
His current treatment protocol involves a five-step process that begins with removing all of the visible lentigo maligna to rule out invasion, since 16% of LMs referred to him have harbored invasion when removed and are upgraded from stage 0 to IA. “I am not going to use a topical cream on an invasive melanoma,” he said. “After an excisional biopsy with minimal margins, I usually close the defect with a purse-string suture because it avoids removing standing cones and consequently enlarging the treatment area.”
Second, he traces a template of the LM border on transparent plastic and places a tiny tattoo in the center of the biopsy site to enable pinpoint placement of the template at the time of surgery.
Third, he treats the site with imiquimod 5% cream Monday through Friday for 2-3 months and sees the patient monthly for dosage adjustments when needed. The fourth step involves enabling the site to recover for 2-6 months to allow for resolution of the inflammatory infiltrate. The final step involves re-excising around the original template with 2-mm margins for confirmation with the use of a negative control taken from an equally sun-exposed site taken some distance away from the LM. “Caucasians will have atypical junctional melanocytic hyperplasia (AJMH), which must be subtracted out as background,” he said. “Otherwise, if you hold a non–sun-exposed site as your standard for a negative margin, you will never stop cutting.”
Dr. Bowen likes to use frozen radial sections with routine staining with H and E and immunostaining with MART-1 (Melan-A) and SOX-10. Processing takes 2 hours, he continued, “so I put in relaxing sutures, which will stretch out nicely over 2 hours so I can usually close the defects primarily.”
In Dr. Bowen’s opinion, topical imiquimod as monotherapy for LM is not safe, since about 30% of patients treated with imiquimod will still harbor residual LM. “In our dataset, about 70% have no residual LM, 20% have residual LM in the center but negative perimeter margins, and 10% have LM touching a perimeter margin and require a second stage,” he said. “Taken together, 90% of patients pretreated with imiquimod will be cleared in one stage of surgery with 2 mm margins.”
Making the distinction between LM and AJMH common to chronically sun-damaged skin is no easy task. Dr. Bowen cited a concordance study between dermatopathologists interpreting staged excisional margins on permanent sections for LM where the concordance was only moderate at best. In this study, the use of a negative control improved the concordance rate on “difficult” cases from 46% to 76%; P = .001 (Arch Dermatol. 2003 May;139(5):595-604). “What we really need is a molecular marker that will tell us if a melanocyte is malignant or not,” he said. “All we have now are immunostains that tell you if it’s melanocyte but nothing more.” He went on to say that in multivariate analysis in two studies of the histologic features of LM, the only feature that consistently predicted the difference between LM and AJMH was the melanocyte density and its ratio to the negative control (Dermatol Surg. 2011;37(5):657-63 and J. Plast. Reconstr. Aesthet. Surg. 2014;67(10):1322-32). “The MART-1 immunostain is extremely sensitive, but it makes the slide somewhat muddy, so it’s hard to do an accurate cell count,” he said. “For that reason, we also use a SOX-10 immunostain which is very specific but not as sensitive. I believe that the truth lies somewhere in between those two immunostains in light of a positive control from our lab and a negative control from the patient.”
He concluded that the neoadjuvant use of imiquimod followed by a conservative staged excision “allows me to clear 90% of LM with a 2 mm margin with a recurrence rate of 2.3% in patients with a mean follow-up of 5-years or greater.”
Dr. Bowen reported having no financial disclosures.
PARK CITY, UTAH – Dr. Glen M. Bowen has been working to improve the surgical treatment of lentigo maligna ever since he joined the Huntsman Cancer Institute at the University of Utah 16 years ago. A retrospective review from Memorial Sloan-Kettering Cancer Center found that on average, 7.1-mm margins are required to remove lentigo maligna (LM) (J. Am. Acad. Dermatol. 2008;58[1]:142-8).
“If you have an LM with a 10-mm diameter to begin with, 7.1-mm margins give you a final surgical diameter of 24.2 mm,” Dr. Bowen said at the annual meeting of the Pacific Dermatologic Association. “These are very morbid surgeries in cosmetically sensitive areas for a relatively low-risk tumor.”
The risk of LM progressing to an invasive melanoma is not known, but is estimated to range between 5% and 33%. Of 2,016 patients treated for LM at the Huntsman Cancer Institute, 522 have been treated with neo-adjuvant topical imiquimod 5% cream followed by a conservative staged excision with 2-mm margins with a recurrence rate of 2.3% during median follow-up of 5 years. Of their recurrences, about 20% recurred with invasion. All recurrences to date have been less than 1 mm in depth (stage IA), which has an estimated mortality risk of 5% at 5 years. “A 5% mortality rate of the 20% that recur with invasion of the 2.3% that recur after surgery yields a mortality risk of 0.023%,” Dr. Bowen said. “Due to the very low risk of actually dying from a recurrent LM, very large and morbid surgical defects strike me as a punishment that doesn’t fit the crime in terms of the cost-benefit ratio.”
He and his associates at the Huntsman Cancer Institute have observed some deaths in patients who presented with LM melanoma (invasive melanoma) but have not observed a single death in patients who presented with LM in situ that subsequently recurred. For these reasons, Dr. Bowen favors pretreating LM with imiquimod 5% cream followed by a conservative staged excision, a process that substantially decreases the size of the surgical defects.
His current treatment protocol involves a five-step process that begins with removing all of the visible lentigo maligna to rule out invasion, since 16% of LMs referred to him have harbored invasion when removed and are upgraded from stage 0 to IA. “I am not going to use a topical cream on an invasive melanoma,” he said. “After an excisional biopsy with minimal margins, I usually close the defect with a purse-string suture because it avoids removing standing cones and consequently enlarging the treatment area.”
Second, he traces a template of the LM border on transparent plastic and places a tiny tattoo in the center of the biopsy site to enable pinpoint placement of the template at the time of surgery.
Third, he treats the site with imiquimod 5% cream Monday through Friday for 2-3 months and sees the patient monthly for dosage adjustments when needed. The fourth step involves enabling the site to recover for 2-6 months to allow for resolution of the inflammatory infiltrate. The final step involves re-excising around the original template with 2-mm margins for confirmation with the use of a negative control taken from an equally sun-exposed site taken some distance away from the LM. “Caucasians will have atypical junctional melanocytic hyperplasia (AJMH), which must be subtracted out as background,” he said. “Otherwise, if you hold a non–sun-exposed site as your standard for a negative margin, you will never stop cutting.”
Dr. Bowen likes to use frozen radial sections with routine staining with H and E and immunostaining with MART-1 (Melan-A) and SOX-10. Processing takes 2 hours, he continued, “so I put in relaxing sutures, which will stretch out nicely over 2 hours so I can usually close the defects primarily.”
In Dr. Bowen’s opinion, topical imiquimod as monotherapy for LM is not safe, since about 30% of patients treated with imiquimod will still harbor residual LM. “In our dataset, about 70% have no residual LM, 20% have residual LM in the center but negative perimeter margins, and 10% have LM touching a perimeter margin and require a second stage,” he said. “Taken together, 90% of patients pretreated with imiquimod will be cleared in one stage of surgery with 2 mm margins.”
Making the distinction between LM and AJMH common to chronically sun-damaged skin is no easy task. Dr. Bowen cited a concordance study between dermatopathologists interpreting staged excisional margins on permanent sections for LM where the concordance was only moderate at best. In this study, the use of a negative control improved the concordance rate on “difficult” cases from 46% to 76%; P = .001 (Arch Dermatol. 2003 May;139(5):595-604). “What we really need is a molecular marker that will tell us if a melanocyte is malignant or not,” he said. “All we have now are immunostains that tell you if it’s melanocyte but nothing more.” He went on to say that in multivariate analysis in two studies of the histologic features of LM, the only feature that consistently predicted the difference between LM and AJMH was the melanocyte density and its ratio to the negative control (Dermatol Surg. 2011;37(5):657-63 and J. Plast. Reconstr. Aesthet. Surg. 2014;67(10):1322-32). “The MART-1 immunostain is extremely sensitive, but it makes the slide somewhat muddy, so it’s hard to do an accurate cell count,” he said. “For that reason, we also use a SOX-10 immunostain which is very specific but not as sensitive. I believe that the truth lies somewhere in between those two immunostains in light of a positive control from our lab and a negative control from the patient.”
He concluded that the neoadjuvant use of imiquimod followed by a conservative staged excision “allows me to clear 90% of LM with a 2 mm margin with a recurrence rate of 2.3% in patients with a mean follow-up of 5-years or greater.”
Dr. Bowen reported having no financial disclosures.
PARK CITY, UTAH – Dr. Glen M. Bowen has been working to improve the surgical treatment of lentigo maligna ever since he joined the Huntsman Cancer Institute at the University of Utah 16 years ago. A retrospective review from Memorial Sloan-Kettering Cancer Center found that on average, 7.1-mm margins are required to remove lentigo maligna (LM) (J. Am. Acad. Dermatol. 2008;58[1]:142-8).
“If you have an LM with a 10-mm diameter to begin with, 7.1-mm margins give you a final surgical diameter of 24.2 mm,” Dr. Bowen said at the annual meeting of the Pacific Dermatologic Association. “These are very morbid surgeries in cosmetically sensitive areas for a relatively low-risk tumor.”
The risk of LM progressing to an invasive melanoma is not known, but is estimated to range between 5% and 33%. Of 2,016 patients treated for LM at the Huntsman Cancer Institute, 522 have been treated with neo-adjuvant topical imiquimod 5% cream followed by a conservative staged excision with 2-mm margins with a recurrence rate of 2.3% during median follow-up of 5 years. Of their recurrences, about 20% recurred with invasion. All recurrences to date have been less than 1 mm in depth (stage IA), which has an estimated mortality risk of 5% at 5 years. “A 5% mortality rate of the 20% that recur with invasion of the 2.3% that recur after surgery yields a mortality risk of 0.023%,” Dr. Bowen said. “Due to the very low risk of actually dying from a recurrent LM, very large and morbid surgical defects strike me as a punishment that doesn’t fit the crime in terms of the cost-benefit ratio.”
He and his associates at the Huntsman Cancer Institute have observed some deaths in patients who presented with LM melanoma (invasive melanoma) but have not observed a single death in patients who presented with LM in situ that subsequently recurred. For these reasons, Dr. Bowen favors pretreating LM with imiquimod 5% cream followed by a conservative staged excision, a process that substantially decreases the size of the surgical defects.
His current treatment protocol involves a five-step process that begins with removing all of the visible lentigo maligna to rule out invasion, since 16% of LMs referred to him have harbored invasion when removed and are upgraded from stage 0 to IA. “I am not going to use a topical cream on an invasive melanoma,” he said. “After an excisional biopsy with minimal margins, I usually close the defect with a purse-string suture because it avoids removing standing cones and consequently enlarging the treatment area.”
Second, he traces a template of the LM border on transparent plastic and places a tiny tattoo in the center of the biopsy site to enable pinpoint placement of the template at the time of surgery.
Third, he treats the site with imiquimod 5% cream Monday through Friday for 2-3 months and sees the patient monthly for dosage adjustments when needed. The fourth step involves enabling the site to recover for 2-6 months to allow for resolution of the inflammatory infiltrate. The final step involves re-excising around the original template with 2-mm margins for confirmation with the use of a negative control taken from an equally sun-exposed site taken some distance away from the LM. “Caucasians will have atypical junctional melanocytic hyperplasia (AJMH), which must be subtracted out as background,” he said. “Otherwise, if you hold a non–sun-exposed site as your standard for a negative margin, you will never stop cutting.”
Dr. Bowen likes to use frozen radial sections with routine staining with H and E and immunostaining with MART-1 (Melan-A) and SOX-10. Processing takes 2 hours, he continued, “so I put in relaxing sutures, which will stretch out nicely over 2 hours so I can usually close the defects primarily.”
In Dr. Bowen’s opinion, topical imiquimod as monotherapy for LM is not safe, since about 30% of patients treated with imiquimod will still harbor residual LM. “In our dataset, about 70% have no residual LM, 20% have residual LM in the center but negative perimeter margins, and 10% have LM touching a perimeter margin and require a second stage,” he said. “Taken together, 90% of patients pretreated with imiquimod will be cleared in one stage of surgery with 2 mm margins.”
Making the distinction between LM and AJMH common to chronically sun-damaged skin is no easy task. Dr. Bowen cited a concordance study between dermatopathologists interpreting staged excisional margins on permanent sections for LM where the concordance was only moderate at best. In this study, the use of a negative control improved the concordance rate on “difficult” cases from 46% to 76%; P = .001 (Arch Dermatol. 2003 May;139(5):595-604). “What we really need is a molecular marker that will tell us if a melanocyte is malignant or not,” he said. “All we have now are immunostains that tell you if it’s melanocyte but nothing more.” He went on to say that in multivariate analysis in two studies of the histologic features of LM, the only feature that consistently predicted the difference between LM and AJMH was the melanocyte density and its ratio to the negative control (Dermatol Surg. 2011;37(5):657-63 and J. Plast. Reconstr. Aesthet. Surg. 2014;67(10):1322-32). “The MART-1 immunostain is extremely sensitive, but it makes the slide somewhat muddy, so it’s hard to do an accurate cell count,” he said. “For that reason, we also use a SOX-10 immunostain which is very specific but not as sensitive. I believe that the truth lies somewhere in between those two immunostains in light of a positive control from our lab and a negative control from the patient.”
He concluded that the neoadjuvant use of imiquimod followed by a conservative staged excision “allows me to clear 90% of LM with a 2 mm margin with a recurrence rate of 2.3% in patients with a mean follow-up of 5-years or greater.”
Dr. Bowen reported having no financial disclosures.
EXPERT ANALYSIS from PDA 2015
Ingenol mebutate helped clear actinic keratoses
Cryosurgery followed by topical ingenol mebutate cleared extensive regions of actinic keratosis, which helped reveal residual squamous cell carcinomas, according to a report in the August issue of the Journal of Drugs in Dermatology.
The findings show that ingenol mebutate can clear multiple AKs and reduce the number of scarring biopsies required to identify SCCs, said Dr. Miriam S. Bettencourt, a dermatologist in group practice in Henderson, Nev. “In our dermatology clinic, many of the patients with a long history of AK who were treated with ingenol mebutate used sequentially after cryosurgery have achieved complete or partial clearance of AKs.”
Ingenol mebutate gel after cryosurgery cleared AKs more effectively than cryosurgery alone in a recent phase III trial (J Drugs Dermatol. 2014 Jun;13[6]741-7), Dr. Bettencourt noted. She described six men and one woman who each had at least 10 recurrent or hyperkeratotic AKs and previously had undergone cryosurgery. She treated all patients with cryosurgery, followed 2 weeks later by two or three once-daily applications of ingenol mebutate gel at strengths of 0.05% or 0.015%, respectively (J Drugs Dermatol. 2015 Aug;14[8];813-8). One course of ingenol mebutate gel cleared 50%-100% of AKs, Dr. Bettencourt said. She treated residual AKs with cryosurgery, and five patients also received at least one more course of ingenol mebutate to re-treat a partially cleared area or to treat a separate area. Shave biopsies of 10 residual suspicious lesions taken 3-8 months later all revealed invasive SCCs, which were treated with Mohs micrographic surgery (MMS). “These lesions may have been preexisting at the time of topical treatment but not readily recognized as suspicious in the heavily actinically damaged skin, in which suspected or small SCCs may be adjacent to or obscured by AKs,” she said. “Alternatively, these tumors may have been spontaneous new SCCs. In either case, we suggest that effective clearance of AKs from the palette of sun-damaged skin with ingenol mebutate permitted prompt recognition of these lesions as suspicious, and led to further diagnosis and treatment with MMS.”
All patients developed mild to moderate localized redness, flaking, and crusting starting on the second day of ingenol mebutate treatment and resolving within a week of finishing the course, Dr. Bettencourt said.
She reported that she had no relevant financial conflicts.
Cryosurgery followed by topical ingenol mebutate cleared extensive regions of actinic keratosis, which helped reveal residual squamous cell carcinomas, according to a report in the August issue of the Journal of Drugs in Dermatology.
The findings show that ingenol mebutate can clear multiple AKs and reduce the number of scarring biopsies required to identify SCCs, said Dr. Miriam S. Bettencourt, a dermatologist in group practice in Henderson, Nev. “In our dermatology clinic, many of the patients with a long history of AK who were treated with ingenol mebutate used sequentially after cryosurgery have achieved complete or partial clearance of AKs.”
Ingenol mebutate gel after cryosurgery cleared AKs more effectively than cryosurgery alone in a recent phase III trial (J Drugs Dermatol. 2014 Jun;13[6]741-7), Dr. Bettencourt noted. She described six men and one woman who each had at least 10 recurrent or hyperkeratotic AKs and previously had undergone cryosurgery. She treated all patients with cryosurgery, followed 2 weeks later by two or three once-daily applications of ingenol mebutate gel at strengths of 0.05% or 0.015%, respectively (J Drugs Dermatol. 2015 Aug;14[8];813-8). One course of ingenol mebutate gel cleared 50%-100% of AKs, Dr. Bettencourt said. She treated residual AKs with cryosurgery, and five patients also received at least one more course of ingenol mebutate to re-treat a partially cleared area or to treat a separate area. Shave biopsies of 10 residual suspicious lesions taken 3-8 months later all revealed invasive SCCs, which were treated with Mohs micrographic surgery (MMS). “These lesions may have been preexisting at the time of topical treatment but not readily recognized as suspicious in the heavily actinically damaged skin, in which suspected or small SCCs may be adjacent to or obscured by AKs,” she said. “Alternatively, these tumors may have been spontaneous new SCCs. In either case, we suggest that effective clearance of AKs from the palette of sun-damaged skin with ingenol mebutate permitted prompt recognition of these lesions as suspicious, and led to further diagnosis and treatment with MMS.”
All patients developed mild to moderate localized redness, flaking, and crusting starting on the second day of ingenol mebutate treatment and resolving within a week of finishing the course, Dr. Bettencourt said.
She reported that she had no relevant financial conflicts.
Cryosurgery followed by topical ingenol mebutate cleared extensive regions of actinic keratosis, which helped reveal residual squamous cell carcinomas, according to a report in the August issue of the Journal of Drugs in Dermatology.
The findings show that ingenol mebutate can clear multiple AKs and reduce the number of scarring biopsies required to identify SCCs, said Dr. Miriam S. Bettencourt, a dermatologist in group practice in Henderson, Nev. “In our dermatology clinic, many of the patients with a long history of AK who were treated with ingenol mebutate used sequentially after cryosurgery have achieved complete or partial clearance of AKs.”
Ingenol mebutate gel after cryosurgery cleared AKs more effectively than cryosurgery alone in a recent phase III trial (J Drugs Dermatol. 2014 Jun;13[6]741-7), Dr. Bettencourt noted. She described six men and one woman who each had at least 10 recurrent or hyperkeratotic AKs and previously had undergone cryosurgery. She treated all patients with cryosurgery, followed 2 weeks later by two or three once-daily applications of ingenol mebutate gel at strengths of 0.05% or 0.015%, respectively (J Drugs Dermatol. 2015 Aug;14[8];813-8). One course of ingenol mebutate gel cleared 50%-100% of AKs, Dr. Bettencourt said. She treated residual AKs with cryosurgery, and five patients also received at least one more course of ingenol mebutate to re-treat a partially cleared area or to treat a separate area. Shave biopsies of 10 residual suspicious lesions taken 3-8 months later all revealed invasive SCCs, which were treated with Mohs micrographic surgery (MMS). “These lesions may have been preexisting at the time of topical treatment but not readily recognized as suspicious in the heavily actinically damaged skin, in which suspected or small SCCs may be adjacent to or obscured by AKs,” she said. “Alternatively, these tumors may have been spontaneous new SCCs. In either case, we suggest that effective clearance of AKs from the palette of sun-damaged skin with ingenol mebutate permitted prompt recognition of these lesions as suspicious, and led to further diagnosis and treatment with MMS.”
All patients developed mild to moderate localized redness, flaking, and crusting starting on the second day of ingenol mebutate treatment and resolving within a week of finishing the course, Dr. Bettencourt said.
She reported that she had no relevant financial conflicts.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
Key clinical point: Several courses of cryosurgery and ingenol mebutate helped clear actinic keratoses, helping a clinician identify residual squamous cell carcinomas.
Major finding: Lesion counts dropped by 50%-100% after cryosurgery followed by one to three courses of ingenol mebutate gel.
Data source: A case series of seven patients who had multiple AKs and 10 SCCs.
Disclosures: Dr. Bettencourt reported that she had no relevant financial conflicts.
Immune-related patterns of response present challenges
With the race to develop cancer immunotherapies escalating and new agents appearing in the clinic, oncologists’ decision-making toolbox will need to evolve.
“As immunotherapeutics become increasingly available to patients, clinicians face a major challenge in the evaluation of these novel drugs – the accurate determination of clinical efficacy,” physician-scientists recently wrote in a commentary published online in the Journal of Clinical Oncology.
Response evaluation criteria in solid tumors (RECIST) have typically driven oncologists’ decision making. Patients undergo scans and radiographic measurements to determine the extent of change in tumor size. Scan, treat, repeat is a mantra for advanced cancer patients, so much so some patients have sought to trademark the phrase for T-shirts. And significant tumor growth has traditionally signaled treatment failure.
But although some patients have responded to immune-targeted treatment with tumor shrinkage or stable disease that would be consistent with existing RECIST criteria, distinct immune-related patterns of response are also emerging, including pseudoprogression, said Dr. Victoria L. Chiou and Dr. Mauricio Burotto, both medical oncology fellows at the National Cancer Institute, in their commentary (Jour Clin Onc. 2015 Aug 10. doi: 10.1200/JCO.2015.61.6870).
Investigators first addressed this issue by proposing immune response criteria in 2009, based on data from ipilimumab phase II trials in patients with advanced melanoma. Dr. Jedd D. Wolchok of Memorial Sloan Kettering Cancer Center, New York, and his associates expanded on RECIST by adding two additional patterns of response: response after an increase in total tumor burden and response in the presence of new lesions. All patterns of response were associated with favorable survival, Dr. Wolchok and associates said (Clin Cancer Res 2009 Dec 1. doi: 10.1158/1078-0432.CCR-09-1624).
Subsequent trials in patients with advanced melanoma have confirmed that a subset of patients who are responders using immune response criteria would have been misclassified using RECIST alone.
In a study of patients with metastatic melanoma treated with nivolumab, 10% experienced distinct immune-related responses, according to Dr. Chiou and Dr. Burotto. In another study of patients with advanced melanoma, this one evaluating anti–PD-1 monoclonal antibody pembrolizumab, 6.7% of patients of the patients experienced pseudoprogression, and overall, 12% of patients were classified as responders or as having stable disease by immune response criteria but would have been classified as having progressive disease by RECIST.
It is time to expand on those first immune response criteria, Dr. Chiou and Dr. Burotto conclude. “Five years after the introduction of the immune response criteria, it is necessary to fully characterize the patterns of immune-related phenomena, to understand these patterns across multiple solid tumor types, and to evaluate how these guidelines are used in current clinical practice,” they wrote.
The Food and Drug Administration agrees. Though guidelines for industry were published in 2011 regarding cancer vaccines, there is no specific guidance on approval criteria for other cancer immunotherapies such as checkpoint inhibitors, Dr. Gideon Blumenthal, team leader in the FDA’s Office of Hematology and Oncology Products, Center for Drug Evaluation and Research, said in an interview.
“However, the FDA is actively looking at other metrics of response beyond conventional RECIST criteria, both to help industry in early ‘go/no-go’ decision making, as well as for helping in the design of later stages of trial design and to help inform approval decisions,” he said.
The FDA is interested in investigating internal data sets of immunotherapy trials, and in collaborating with external stakeholders to determine what the optimal endpoints for cancer immunotherapies will be. Median PFS and PFS hazard ratios do not appear to capture the overall survival benefit that patients derive from immunotherapies, particularly in early lung cancer trials with which he is most familiar, Dr. Blumenthal said.
“Overall survival remains the gold standard endpoint in oncology as it is a direct measure of clinical benefit and less subject to bias, but as more effective therapies come on line and patients live longer, overall survival will become more challenging to detect,” he said.
On Twitter @NikolaidesLaura
With the race to develop cancer immunotherapies escalating and new agents appearing in the clinic, oncologists’ decision-making toolbox will need to evolve.
“As immunotherapeutics become increasingly available to patients, clinicians face a major challenge in the evaluation of these novel drugs – the accurate determination of clinical efficacy,” physician-scientists recently wrote in a commentary published online in the Journal of Clinical Oncology.
Response evaluation criteria in solid tumors (RECIST) have typically driven oncologists’ decision making. Patients undergo scans and radiographic measurements to determine the extent of change in tumor size. Scan, treat, repeat is a mantra for advanced cancer patients, so much so some patients have sought to trademark the phrase for T-shirts. And significant tumor growth has traditionally signaled treatment failure.
But although some patients have responded to immune-targeted treatment with tumor shrinkage or stable disease that would be consistent with existing RECIST criteria, distinct immune-related patterns of response are also emerging, including pseudoprogression, said Dr. Victoria L. Chiou and Dr. Mauricio Burotto, both medical oncology fellows at the National Cancer Institute, in their commentary (Jour Clin Onc. 2015 Aug 10. doi: 10.1200/JCO.2015.61.6870).
Investigators first addressed this issue by proposing immune response criteria in 2009, based on data from ipilimumab phase II trials in patients with advanced melanoma. Dr. Jedd D. Wolchok of Memorial Sloan Kettering Cancer Center, New York, and his associates expanded on RECIST by adding two additional patterns of response: response after an increase in total tumor burden and response in the presence of new lesions. All patterns of response were associated with favorable survival, Dr. Wolchok and associates said (Clin Cancer Res 2009 Dec 1. doi: 10.1158/1078-0432.CCR-09-1624).
Subsequent trials in patients with advanced melanoma have confirmed that a subset of patients who are responders using immune response criteria would have been misclassified using RECIST alone.
In a study of patients with metastatic melanoma treated with nivolumab, 10% experienced distinct immune-related responses, according to Dr. Chiou and Dr. Burotto. In another study of patients with advanced melanoma, this one evaluating anti–PD-1 monoclonal antibody pembrolizumab, 6.7% of patients of the patients experienced pseudoprogression, and overall, 12% of patients were classified as responders or as having stable disease by immune response criteria but would have been classified as having progressive disease by RECIST.
It is time to expand on those first immune response criteria, Dr. Chiou and Dr. Burotto conclude. “Five years after the introduction of the immune response criteria, it is necessary to fully characterize the patterns of immune-related phenomena, to understand these patterns across multiple solid tumor types, and to evaluate how these guidelines are used in current clinical practice,” they wrote.
The Food and Drug Administration agrees. Though guidelines for industry were published in 2011 regarding cancer vaccines, there is no specific guidance on approval criteria for other cancer immunotherapies such as checkpoint inhibitors, Dr. Gideon Blumenthal, team leader in the FDA’s Office of Hematology and Oncology Products, Center for Drug Evaluation and Research, said in an interview.
“However, the FDA is actively looking at other metrics of response beyond conventional RECIST criteria, both to help industry in early ‘go/no-go’ decision making, as well as for helping in the design of later stages of trial design and to help inform approval decisions,” he said.
The FDA is interested in investigating internal data sets of immunotherapy trials, and in collaborating with external stakeholders to determine what the optimal endpoints for cancer immunotherapies will be. Median PFS and PFS hazard ratios do not appear to capture the overall survival benefit that patients derive from immunotherapies, particularly in early lung cancer trials with which he is most familiar, Dr. Blumenthal said.
“Overall survival remains the gold standard endpoint in oncology as it is a direct measure of clinical benefit and less subject to bias, but as more effective therapies come on line and patients live longer, overall survival will become more challenging to detect,” he said.
On Twitter @NikolaidesLaura
With the race to develop cancer immunotherapies escalating and new agents appearing in the clinic, oncologists’ decision-making toolbox will need to evolve.
“As immunotherapeutics become increasingly available to patients, clinicians face a major challenge in the evaluation of these novel drugs – the accurate determination of clinical efficacy,” physician-scientists recently wrote in a commentary published online in the Journal of Clinical Oncology.
Response evaluation criteria in solid tumors (RECIST) have typically driven oncologists’ decision making. Patients undergo scans and radiographic measurements to determine the extent of change in tumor size. Scan, treat, repeat is a mantra for advanced cancer patients, so much so some patients have sought to trademark the phrase for T-shirts. And significant tumor growth has traditionally signaled treatment failure.
But although some patients have responded to immune-targeted treatment with tumor shrinkage or stable disease that would be consistent with existing RECIST criteria, distinct immune-related patterns of response are also emerging, including pseudoprogression, said Dr. Victoria L. Chiou and Dr. Mauricio Burotto, both medical oncology fellows at the National Cancer Institute, in their commentary (Jour Clin Onc. 2015 Aug 10. doi: 10.1200/JCO.2015.61.6870).
Investigators first addressed this issue by proposing immune response criteria in 2009, based on data from ipilimumab phase II trials in patients with advanced melanoma. Dr. Jedd D. Wolchok of Memorial Sloan Kettering Cancer Center, New York, and his associates expanded on RECIST by adding two additional patterns of response: response after an increase in total tumor burden and response in the presence of new lesions. All patterns of response were associated with favorable survival, Dr. Wolchok and associates said (Clin Cancer Res 2009 Dec 1. doi: 10.1158/1078-0432.CCR-09-1624).
Subsequent trials in patients with advanced melanoma have confirmed that a subset of patients who are responders using immune response criteria would have been misclassified using RECIST alone.
In a study of patients with metastatic melanoma treated with nivolumab, 10% experienced distinct immune-related responses, according to Dr. Chiou and Dr. Burotto. In another study of patients with advanced melanoma, this one evaluating anti–PD-1 monoclonal antibody pembrolizumab, 6.7% of patients of the patients experienced pseudoprogression, and overall, 12% of patients were classified as responders or as having stable disease by immune response criteria but would have been classified as having progressive disease by RECIST.
It is time to expand on those first immune response criteria, Dr. Chiou and Dr. Burotto conclude. “Five years after the introduction of the immune response criteria, it is necessary to fully characterize the patterns of immune-related phenomena, to understand these patterns across multiple solid tumor types, and to evaluate how these guidelines are used in current clinical practice,” they wrote.
The Food and Drug Administration agrees. Though guidelines for industry were published in 2011 regarding cancer vaccines, there is no specific guidance on approval criteria for other cancer immunotherapies such as checkpoint inhibitors, Dr. Gideon Blumenthal, team leader in the FDA’s Office of Hematology and Oncology Products, Center for Drug Evaluation and Research, said in an interview.
“However, the FDA is actively looking at other metrics of response beyond conventional RECIST criteria, both to help industry in early ‘go/no-go’ decision making, as well as for helping in the design of later stages of trial design and to help inform approval decisions,” he said.
The FDA is interested in investigating internal data sets of immunotherapy trials, and in collaborating with external stakeholders to determine what the optimal endpoints for cancer immunotherapies will be. Median PFS and PFS hazard ratios do not appear to capture the overall survival benefit that patients derive from immunotherapies, particularly in early lung cancer trials with which he is most familiar, Dr. Blumenthal said.
“Overall survival remains the gold standard endpoint in oncology as it is a direct measure of clinical benefit and less subject to bias, but as more effective therapies come on line and patients live longer, overall survival will become more challenging to detect,” he said.
On Twitter @NikolaidesLaura
Indoor Tanning Is More Harmful Than Americans Believe
The Surgeon General has called on partners in prevention from various sectors to address skin cancer as a major public health problem. One of the main goals outlined in The Surgeon General’s Call to Action to Prevent Skin Cancer is to reduce harm from indoor tanning, which has been linked to increased risk for skin cancer, including melanoma, basal cell carcinoma, and squamous cell carcinoma.
Based on reports from the American Cancer Society, Centers for Disease Control and Prevention, Federal Trade Commission, Mayo Clinic, and US Food and Drug Administration, the following common myths about indoor tanning should be communicated to dermatology patients.
Myth: Indoor tanning will not increase your risk for skin cancer.
Fact: As many as 90% of melanomas are caused by UV exposure. Indoor tanning exposure to UVA and UVB radiation damages the skin and may lead to cancer. Melanoma is linked to severe sunburns, especially at a young age.
Myth: Indoor tanning is safer than tanning outdoors because it is a controlled dose of UV radiation.
Fact: Both indoor tanning and tanning outside are dangerous. Tanning beds may be more dangerous than the sun because they can be used at the same high intensity every day of the year, regardless of time of day, season, or cloud cover. Furthermore, the Surgeon General and US Food and Drug Administration report that an estimated 3000 Americans each year go to emergency departments with injuries caused by indoor tanning, including burns, eye injuries, immune suppression, and allergic reactions. Indoor tanning also causes premature skin aging.
Myth: A “base tan” protects your skin from sunburn.
Fact: Although many patients believe that a few sessions of indoor tanning will prevent them from burning in the sun, a tan does little to protect the skin from future UV exposure. In fact, the Centers for Disease Control and Prevention notes that people who tan indoors are more likely to report getting sunburned. The best way to protect the skin from sunburn is by using sun protection and avoiding indoor tanning.
Myth: Indoor tanning is a safe way to increase vitamin D levels.
Fact: It is important to get enough vitamin D; however, the safest way is through what you eat. Although UVB radiation helps the body produce vitamin D, patients do not need a tan to get that benefit. Ten to 15 minutes of unprotected natural sun exposure on the face and hands 2 to 3 times a week during the summer allows for a healthy dose of vitamin D. Dietary sources, such as low-fat milk, salmon, tuna, and fortified orange juice, are the safest way to get enough vitamin D.
Myth: Indoor tanning is approved by the government.
Fact: According to the Federal Trade Commission, no US government agency recommends the use of indoor tanning equipment. Tanning bed use by minors has been banned in many states, and efforts are ongoing to protect consumers younger than 18 years on local, state, and federal levels. In July 2009, the International Agency for Research on Cancer, part of the World Health Organization, moved tanning devices that emit UV radiation into the highest cancer risk category—carcinogenic to humans—concluding that they are more dangerous than previously thought.
Studies have consistently shown that indoor tanning increases a person’s risk of getting skin cancer and indoor tanning at a young age appears to be more strongly related to lifetime skin cancer risk. Patients should be reminded that every time they tan, they increase their risk of melanoma as well as premature skin aging and other skin cancers. Dermatologists should counsel patients on using sun protection and avoiding indoor tanning.
The Surgeon General has called on partners in prevention from various sectors to address skin cancer as a major public health problem. One of the main goals outlined in The Surgeon General’s Call to Action to Prevent Skin Cancer is to reduce harm from indoor tanning, which has been linked to increased risk for skin cancer, including melanoma, basal cell carcinoma, and squamous cell carcinoma.
Based on reports from the American Cancer Society, Centers for Disease Control and Prevention, Federal Trade Commission, Mayo Clinic, and US Food and Drug Administration, the following common myths about indoor tanning should be communicated to dermatology patients.
Myth: Indoor tanning will not increase your risk for skin cancer.
Fact: As many as 90% of melanomas are caused by UV exposure. Indoor tanning exposure to UVA and UVB radiation damages the skin and may lead to cancer. Melanoma is linked to severe sunburns, especially at a young age.
Myth: Indoor tanning is safer than tanning outdoors because it is a controlled dose of UV radiation.
Fact: Both indoor tanning and tanning outside are dangerous. Tanning beds may be more dangerous than the sun because they can be used at the same high intensity every day of the year, regardless of time of day, season, or cloud cover. Furthermore, the Surgeon General and US Food and Drug Administration report that an estimated 3000 Americans each year go to emergency departments with injuries caused by indoor tanning, including burns, eye injuries, immune suppression, and allergic reactions. Indoor tanning also causes premature skin aging.
Myth: A “base tan” protects your skin from sunburn.
Fact: Although many patients believe that a few sessions of indoor tanning will prevent them from burning in the sun, a tan does little to protect the skin from future UV exposure. In fact, the Centers for Disease Control and Prevention notes that people who tan indoors are more likely to report getting sunburned. The best way to protect the skin from sunburn is by using sun protection and avoiding indoor tanning.
Myth: Indoor tanning is a safe way to increase vitamin D levels.
Fact: It is important to get enough vitamin D; however, the safest way is through what you eat. Although UVB radiation helps the body produce vitamin D, patients do not need a tan to get that benefit. Ten to 15 minutes of unprotected natural sun exposure on the face and hands 2 to 3 times a week during the summer allows for a healthy dose of vitamin D. Dietary sources, such as low-fat milk, salmon, tuna, and fortified orange juice, are the safest way to get enough vitamin D.
Myth: Indoor tanning is approved by the government.
Fact: According to the Federal Trade Commission, no US government agency recommends the use of indoor tanning equipment. Tanning bed use by minors has been banned in many states, and efforts are ongoing to protect consumers younger than 18 years on local, state, and federal levels. In July 2009, the International Agency for Research on Cancer, part of the World Health Organization, moved tanning devices that emit UV radiation into the highest cancer risk category—carcinogenic to humans—concluding that they are more dangerous than previously thought.
Studies have consistently shown that indoor tanning increases a person’s risk of getting skin cancer and indoor tanning at a young age appears to be more strongly related to lifetime skin cancer risk. Patients should be reminded that every time they tan, they increase their risk of melanoma as well as premature skin aging and other skin cancers. Dermatologists should counsel patients on using sun protection and avoiding indoor tanning.
The Surgeon General has called on partners in prevention from various sectors to address skin cancer as a major public health problem. One of the main goals outlined in The Surgeon General’s Call to Action to Prevent Skin Cancer is to reduce harm from indoor tanning, which has been linked to increased risk for skin cancer, including melanoma, basal cell carcinoma, and squamous cell carcinoma.
Based on reports from the American Cancer Society, Centers for Disease Control and Prevention, Federal Trade Commission, Mayo Clinic, and US Food and Drug Administration, the following common myths about indoor tanning should be communicated to dermatology patients.
Myth: Indoor tanning will not increase your risk for skin cancer.
Fact: As many as 90% of melanomas are caused by UV exposure. Indoor tanning exposure to UVA and UVB radiation damages the skin and may lead to cancer. Melanoma is linked to severe sunburns, especially at a young age.
Myth: Indoor tanning is safer than tanning outdoors because it is a controlled dose of UV radiation.
Fact: Both indoor tanning and tanning outside are dangerous. Tanning beds may be more dangerous than the sun because they can be used at the same high intensity every day of the year, regardless of time of day, season, or cloud cover. Furthermore, the Surgeon General and US Food and Drug Administration report that an estimated 3000 Americans each year go to emergency departments with injuries caused by indoor tanning, including burns, eye injuries, immune suppression, and allergic reactions. Indoor tanning also causes premature skin aging.
Myth: A “base tan” protects your skin from sunburn.
Fact: Although many patients believe that a few sessions of indoor tanning will prevent them from burning in the sun, a tan does little to protect the skin from future UV exposure. In fact, the Centers for Disease Control and Prevention notes that people who tan indoors are more likely to report getting sunburned. The best way to protect the skin from sunburn is by using sun protection and avoiding indoor tanning.
Myth: Indoor tanning is a safe way to increase vitamin D levels.
Fact: It is important to get enough vitamin D; however, the safest way is through what you eat. Although UVB radiation helps the body produce vitamin D, patients do not need a tan to get that benefit. Ten to 15 minutes of unprotected natural sun exposure on the face and hands 2 to 3 times a week during the summer allows for a healthy dose of vitamin D. Dietary sources, such as low-fat milk, salmon, tuna, and fortified orange juice, are the safest way to get enough vitamin D.
Myth: Indoor tanning is approved by the government.
Fact: According to the Federal Trade Commission, no US government agency recommends the use of indoor tanning equipment. Tanning bed use by minors has been banned in many states, and efforts are ongoing to protect consumers younger than 18 years on local, state, and federal levels. In July 2009, the International Agency for Research on Cancer, part of the World Health Organization, moved tanning devices that emit UV radiation into the highest cancer risk category—carcinogenic to humans—concluding that they are more dangerous than previously thought.
Studies have consistently shown that indoor tanning increases a person’s risk of getting skin cancer and indoor tanning at a young age appears to be more strongly related to lifetime skin cancer risk. Patients should be reminded that every time they tan, they increase their risk of melanoma as well as premature skin aging and other skin cancers. Dermatologists should counsel patients on using sun protection and avoiding indoor tanning.
Ipilimumab’s immune-related adverse effects greater in ‘real-world patients’
Patients treated with ipilimumab for metastatic melanoma should be prepared for immune-related adverse effects, and physicians should expect to treat them early and aggressively, according to a report published online Aug. 17 in Journal of Clinical Oncology.
Severe immune-related adverse effects such as diarrhea, hepatitis, and hypophysitis were common in a retrospective analysis of the medical records of 298 patients treated during a 27-month period at Memorial Sloan Kettering Cancer Center, New York. Corticosteroids were not adequate to control symptoms in a substantial number of cases, and additional systemic immunosuppressive therapy was required, said Dr. Troy Z. Horvat of Memorial Sloan Kettering and his associates.
From their institutional experience, the investigators suspected that the incidence of clinically significant adverse effects of ipilimumab was higher than has been previously reported and higher than would be expected just by counting the number of events qualifying as grade 3 or higher by National Cancer Institute criteria. They also suspected that the need for immunosuppressive therapy was greater than generally expected. Previous studies showed adverse event rates ranging from 6% to 19%, and did not give any information regarding corticosteroid use.
Their analysis confirmed these suspicions, showing that 31% of patients developed grade 3, 4, or 5 adverse effects and 35% required corticosteroid therapy. This is more than twice the rate of grade 3 or higher toxicity reported in clinical trials of ipilimumab. Adverse effects included diarrhea (50 patients), which led to bowel perforation in 3 patients; hepatitis (22 patients); dermatitis (21 patients); endocrinopathies (14); hypophysitis (6); uveitis (2); pneumonitis (1); seizure (1); arthritis (1); and hearing loss (1). These effects were severe enough to cause 19% of patients to discontinue ipilimumab.
About one-third of the patients who received systemic corticosteroids – 10% of the total study population – required additional immunotherapy, including infliximab, mycophenolate, or adalimumab, Dr. Horvat and his associates said (J Clin Oncol 2015 Aug 17 [doi:10.1200/JCO.2015.60.8448]).
This study’s higher rates of adverse events, of corticosteroid therapy, and of further immunosuppressive therapy are likely attributable to the treatment team’s considerable experience with ipilimumab in real-world patients. As clinicians gain such experience, they become more familiar with associated adverse events, allowing earlier identification and intervention, the investigators said.
“In our experience, if improvement in ipilimumab-related adverse effects is not evident early in the treatment with high-dose systemic corticosteroids, more prolonged treatment rarely leads to benefit, and patients usually end up requiring infliximab anyway. ... We believe the overall risk-to-benefit ratio favors the early use of infliximab rather than prolonged treatment with corticosteroids,” they noted.
The median overall survival and median time to treatment failure were not affected by either the occurrence of adverse events or the use of corticosteroids. Overall, 12% of these patients achieved long-term disease control and didn’t require further melanoma treatment. Based on their findings and those of another research group, “we believe that patients and physicians should not be concerned that ipilimumab-related adverse events requiring systemic immunosuppression will compromise the therapeutic benefit,” Dr. Horvat and his associates said.
Patients treated with ipilimumab for metastatic melanoma should be prepared for immune-related adverse effects, and physicians should expect to treat them early and aggressively, according to a report published online Aug. 17 in Journal of Clinical Oncology.
Severe immune-related adverse effects such as diarrhea, hepatitis, and hypophysitis were common in a retrospective analysis of the medical records of 298 patients treated during a 27-month period at Memorial Sloan Kettering Cancer Center, New York. Corticosteroids were not adequate to control symptoms in a substantial number of cases, and additional systemic immunosuppressive therapy was required, said Dr. Troy Z. Horvat of Memorial Sloan Kettering and his associates.
From their institutional experience, the investigators suspected that the incidence of clinically significant adverse effects of ipilimumab was higher than has been previously reported and higher than would be expected just by counting the number of events qualifying as grade 3 or higher by National Cancer Institute criteria. They also suspected that the need for immunosuppressive therapy was greater than generally expected. Previous studies showed adverse event rates ranging from 6% to 19%, and did not give any information regarding corticosteroid use.
Their analysis confirmed these suspicions, showing that 31% of patients developed grade 3, 4, or 5 adverse effects and 35% required corticosteroid therapy. This is more than twice the rate of grade 3 or higher toxicity reported in clinical trials of ipilimumab. Adverse effects included diarrhea (50 patients), which led to bowel perforation in 3 patients; hepatitis (22 patients); dermatitis (21 patients); endocrinopathies (14); hypophysitis (6); uveitis (2); pneumonitis (1); seizure (1); arthritis (1); and hearing loss (1). These effects were severe enough to cause 19% of patients to discontinue ipilimumab.
About one-third of the patients who received systemic corticosteroids – 10% of the total study population – required additional immunotherapy, including infliximab, mycophenolate, or adalimumab, Dr. Horvat and his associates said (J Clin Oncol 2015 Aug 17 [doi:10.1200/JCO.2015.60.8448]).
This study’s higher rates of adverse events, of corticosteroid therapy, and of further immunosuppressive therapy are likely attributable to the treatment team’s considerable experience with ipilimumab in real-world patients. As clinicians gain such experience, they become more familiar with associated adverse events, allowing earlier identification and intervention, the investigators said.
“In our experience, if improvement in ipilimumab-related adverse effects is not evident early in the treatment with high-dose systemic corticosteroids, more prolonged treatment rarely leads to benefit, and patients usually end up requiring infliximab anyway. ... We believe the overall risk-to-benefit ratio favors the early use of infliximab rather than prolonged treatment with corticosteroids,” they noted.
The median overall survival and median time to treatment failure were not affected by either the occurrence of adverse events or the use of corticosteroids. Overall, 12% of these patients achieved long-term disease control and didn’t require further melanoma treatment. Based on their findings and those of another research group, “we believe that patients and physicians should not be concerned that ipilimumab-related adverse events requiring systemic immunosuppression will compromise the therapeutic benefit,” Dr. Horvat and his associates said.
Patients treated with ipilimumab for metastatic melanoma should be prepared for immune-related adverse effects, and physicians should expect to treat them early and aggressively, according to a report published online Aug. 17 in Journal of Clinical Oncology.
Severe immune-related adverse effects such as diarrhea, hepatitis, and hypophysitis were common in a retrospective analysis of the medical records of 298 patients treated during a 27-month period at Memorial Sloan Kettering Cancer Center, New York. Corticosteroids were not adequate to control symptoms in a substantial number of cases, and additional systemic immunosuppressive therapy was required, said Dr. Troy Z. Horvat of Memorial Sloan Kettering and his associates.
From their institutional experience, the investigators suspected that the incidence of clinically significant adverse effects of ipilimumab was higher than has been previously reported and higher than would be expected just by counting the number of events qualifying as grade 3 or higher by National Cancer Institute criteria. They also suspected that the need for immunosuppressive therapy was greater than generally expected. Previous studies showed adverse event rates ranging from 6% to 19%, and did not give any information regarding corticosteroid use.
Their analysis confirmed these suspicions, showing that 31% of patients developed grade 3, 4, or 5 adverse effects and 35% required corticosteroid therapy. This is more than twice the rate of grade 3 or higher toxicity reported in clinical trials of ipilimumab. Adverse effects included diarrhea (50 patients), which led to bowel perforation in 3 patients; hepatitis (22 patients); dermatitis (21 patients); endocrinopathies (14); hypophysitis (6); uveitis (2); pneumonitis (1); seizure (1); arthritis (1); and hearing loss (1). These effects were severe enough to cause 19% of patients to discontinue ipilimumab.
About one-third of the patients who received systemic corticosteroids – 10% of the total study population – required additional immunotherapy, including infliximab, mycophenolate, or adalimumab, Dr. Horvat and his associates said (J Clin Oncol 2015 Aug 17 [doi:10.1200/JCO.2015.60.8448]).
This study’s higher rates of adverse events, of corticosteroid therapy, and of further immunosuppressive therapy are likely attributable to the treatment team’s considerable experience with ipilimumab in real-world patients. As clinicians gain such experience, they become more familiar with associated adverse events, allowing earlier identification and intervention, the investigators said.
“In our experience, if improvement in ipilimumab-related adverse effects is not evident early in the treatment with high-dose systemic corticosteroids, more prolonged treatment rarely leads to benefit, and patients usually end up requiring infliximab anyway. ... We believe the overall risk-to-benefit ratio favors the early use of infliximab rather than prolonged treatment with corticosteroids,” they noted.
The median overall survival and median time to treatment failure were not affected by either the occurrence of adverse events or the use of corticosteroids. Overall, 12% of these patients achieved long-term disease control and didn’t require further melanoma treatment. Based on their findings and those of another research group, “we believe that patients and physicians should not be concerned that ipilimumab-related adverse events requiring systemic immunosuppression will compromise the therapeutic benefit,” Dr. Horvat and his associates said.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Physicians and patients should be prepared for immune-related adverse effects from ipilimumab for treatment of metastatic melanoma.
Major finding: Just under one-third of patients developed grade 3, 4, or 5 adverse effects and 35% of patients required corticosteroid therapy, more than twice the rate of grade 3 or higher toxicity reported in clinical trials of ipilimumab.
Data source: A single-center retrospective analysis of adverse effects from ipilimumab therapy in 298 patients treated during a 27-month period.
Disclosures: This study was supported in part by the John K. Figge Fund. Dr. Horvat reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
The Skin Cancer Vitamin?

Martin et al recently presented a study at the 2015 American Society of Clinical Oncology Annual Meeting (J Clin Oncol. 2015;33[suppl]:9000) that reported on a phase 3 double-blind randomized trial to assess the use of oral nicotinamide to reduce actinic skin cancers, namely basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).
This study was conducted in 2 tertiary treatment centers in Sydney, Australia, from 2012 to 2014, and it included 386 immunocompetent participants with 2 or more histologically confirmed nonmelanoma skin cancers (NMSCs) in the last 5 years. Two groups were randomized (1:1 ratio) to either receive oral nicotinamide 500 mg twice daily or matched placebo for 12 months. The primary end point measured was the number of new NMSCs to 12 months. Other secondary end points included number of SCCs, BCCs, and actinic keratoses to 12 months. Dermatologists performed skin checks every 3 months on the participants.
The results of the study showed that the average NMSC rate was significantly lower for the oral nicotinamide group (1.77) compared to the placebo group (2.42). The estimated relative rate reduction (RRR) was 0.23 (95% confidence interval [CI], 0.04-0.38; P=.02) adjusting for center and NMSC history, and 0.27 (95% CI, 0.05-0.44; P=.02) with no adjustment. The effects for BCC were comparable to SCC: BCC (RRR, 0.20; 95% CI, -0.06 to 0.39; P=.1) and SCC (RRR, 0.30; 95% CI, 0-0.51; P=.05). Additionally, actinic keratosis counts were reduced by 11% at 3 months (P=.01), 14% at 6 months (P<.001), 20% at 9 months (P<.0001), and 13% at 12 months (P<.005) for the oral nicotinamide group compared to the placebo group. There was no difference in the adverse event rates between the 2 groups.
What’s the issue?
This study reported the results of a double-blind randomized study of nicotinamide (vitamin B3) to reduce actinic cancer, called the ONTRAC (Oral Nicotinamide to Reduce Actinic Cancer) study, with favorable results for the use of oral nicotinamide, an inexpensive vitamin. There was a 20% reduction in BCC and a 30% reduction in SCC in the nicotinamide group compared to the group taking a placebo with no active ingredients. This study was conducted in a heavily sun-damaged group and it is postulated that nicotinamide helps cells repair DNA damage.
The thought of using a vitamin to reduce skin cancer rates is exciting; however, this study is singular, and while it did show promising results, the number of participants is not very large. There also was no evidence that nicotinamide prevents melanoma formation. Also, there was no protective effect seen once the vitamin B3 treatment was stopped. One must be cognizant that nicotinamide is not interchangeable with other forms of vitamin B3 such as niacin.
Although this study is promising, more research is needed to determine nicotinamide’s preventative effects. Of course, strict sun protection and skin checks are the first line in the prevention of skin cancer. Will you be prescribing oral nicotinamide to your patients to prevent NMSC?

Martin et al recently presented a study at the 2015 American Society of Clinical Oncology Annual Meeting (J Clin Oncol. 2015;33[suppl]:9000) that reported on a phase 3 double-blind randomized trial to assess the use of oral nicotinamide to reduce actinic skin cancers, namely basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).
This study was conducted in 2 tertiary treatment centers in Sydney, Australia, from 2012 to 2014, and it included 386 immunocompetent participants with 2 or more histologically confirmed nonmelanoma skin cancers (NMSCs) in the last 5 years. Two groups were randomized (1:1 ratio) to either receive oral nicotinamide 500 mg twice daily or matched placebo for 12 months. The primary end point measured was the number of new NMSCs to 12 months. Other secondary end points included number of SCCs, BCCs, and actinic keratoses to 12 months. Dermatologists performed skin checks every 3 months on the participants.
The results of the study showed that the average NMSC rate was significantly lower for the oral nicotinamide group (1.77) compared to the placebo group (2.42). The estimated relative rate reduction (RRR) was 0.23 (95% confidence interval [CI], 0.04-0.38; P=.02) adjusting for center and NMSC history, and 0.27 (95% CI, 0.05-0.44; P=.02) with no adjustment. The effects for BCC were comparable to SCC: BCC (RRR, 0.20; 95% CI, -0.06 to 0.39; P=.1) and SCC (RRR, 0.30; 95% CI, 0-0.51; P=.05). Additionally, actinic keratosis counts were reduced by 11% at 3 months (P=.01), 14% at 6 months (P<.001), 20% at 9 months (P<.0001), and 13% at 12 months (P<.005) for the oral nicotinamide group compared to the placebo group. There was no difference in the adverse event rates between the 2 groups.
What’s the issue?
This study reported the results of a double-blind randomized study of nicotinamide (vitamin B3) to reduce actinic cancer, called the ONTRAC (Oral Nicotinamide to Reduce Actinic Cancer) study, with favorable results for the use of oral nicotinamide, an inexpensive vitamin. There was a 20% reduction in BCC and a 30% reduction in SCC in the nicotinamide group compared to the group taking a placebo with no active ingredients. This study was conducted in a heavily sun-damaged group and it is postulated that nicotinamide helps cells repair DNA damage.
The thought of using a vitamin to reduce skin cancer rates is exciting; however, this study is singular, and while it did show promising results, the number of participants is not very large. There also was no evidence that nicotinamide prevents melanoma formation. Also, there was no protective effect seen once the vitamin B3 treatment was stopped. One must be cognizant that nicotinamide is not interchangeable with other forms of vitamin B3 such as niacin.
Although this study is promising, more research is needed to determine nicotinamide’s preventative effects. Of course, strict sun protection and skin checks are the first line in the prevention of skin cancer. Will you be prescribing oral nicotinamide to your patients to prevent NMSC?

Martin et al recently presented a study at the 2015 American Society of Clinical Oncology Annual Meeting (J Clin Oncol. 2015;33[suppl]:9000) that reported on a phase 3 double-blind randomized trial to assess the use of oral nicotinamide to reduce actinic skin cancers, namely basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).
This study was conducted in 2 tertiary treatment centers in Sydney, Australia, from 2012 to 2014, and it included 386 immunocompetent participants with 2 or more histologically confirmed nonmelanoma skin cancers (NMSCs) in the last 5 years. Two groups were randomized (1:1 ratio) to either receive oral nicotinamide 500 mg twice daily or matched placebo for 12 months. The primary end point measured was the number of new NMSCs to 12 months. Other secondary end points included number of SCCs, BCCs, and actinic keratoses to 12 months. Dermatologists performed skin checks every 3 months on the participants.
The results of the study showed that the average NMSC rate was significantly lower for the oral nicotinamide group (1.77) compared to the placebo group (2.42). The estimated relative rate reduction (RRR) was 0.23 (95% confidence interval [CI], 0.04-0.38; P=.02) adjusting for center and NMSC history, and 0.27 (95% CI, 0.05-0.44; P=.02) with no adjustment. The effects for BCC were comparable to SCC: BCC (RRR, 0.20; 95% CI, -0.06 to 0.39; P=.1) and SCC (RRR, 0.30; 95% CI, 0-0.51; P=.05). Additionally, actinic keratosis counts were reduced by 11% at 3 months (P=.01), 14% at 6 months (P<.001), 20% at 9 months (P<.0001), and 13% at 12 months (P<.005) for the oral nicotinamide group compared to the placebo group. There was no difference in the adverse event rates between the 2 groups.
What’s the issue?
This study reported the results of a double-blind randomized study of nicotinamide (vitamin B3) to reduce actinic cancer, called the ONTRAC (Oral Nicotinamide to Reduce Actinic Cancer) study, with favorable results for the use of oral nicotinamide, an inexpensive vitamin. There was a 20% reduction in BCC and a 30% reduction in SCC in the nicotinamide group compared to the group taking a placebo with no active ingredients. This study was conducted in a heavily sun-damaged group and it is postulated that nicotinamide helps cells repair DNA damage.
The thought of using a vitamin to reduce skin cancer rates is exciting; however, this study is singular, and while it did show promising results, the number of participants is not very large. There also was no evidence that nicotinamide prevents melanoma formation. Also, there was no protective effect seen once the vitamin B3 treatment was stopped. One must be cognizant that nicotinamide is not interchangeable with other forms of vitamin B3 such as niacin.
Although this study is promising, more research is needed to determine nicotinamide’s preventative effects. Of course, strict sun protection and skin checks are the first line in the prevention of skin cancer. Will you be prescribing oral nicotinamide to your patients to prevent NMSC?
In melanoma, sentinel node results will drive targeted therapies
VANCOUVER, B.C. – Why should dermatologists care about the role of sentinel lymph node biopsy in melanoma patients? Because SNLB results will inform the use of an explosion of immunotherapies and targeted therapies emerging for melanoma and – because even after melanoma patients have been referred to a comprehensive cancer center – patients will continue to view dermatologists as the primary care providers with respect to the cancer, Dr. Timothy M. Johnson said in a plenary address at the World Congress of Dermatology.
“I guarantee you of that” scenario, predicted Dr. Johnson, professor of dermatology and clinical director of the multidisciplinary melanoma program at the University of Michigan, Ann Arbor, where more than 2,000 melanoma patients are seen each year.
New melanoma therapies are “coming in waves. They seem to be coming weekly,” he said. In June, the National Cancer Institute listed 218 therapeutic clinical trials for advanced melanoma in the United States alone. And some of these new treatments for stage IV melanoma are already starting to make their way into the adjuvant setting for stage III melanoma.
“The first round of adjuvant therapy trials has been done, and the data are being analyzed now. A new round is coming like gangbusters,” Dr. Johnson said. “This is one of the most exciting times ever in melanoma.”
Should some of these novel agents prove safe and effective as adjuvant therapy, SLNB results will determine the need for such therapy based upon individualized patient prognosis. And the SLNB results will determine the type of adjuvant therapy that’s most appropriate based upon the tumor molecular profile.
“Once we have effective adjuvant therapy, it will eradicate the need for total lymph node dissection in patients with a positive node on SLNB. Those with a positive node will likely undergo systemic therapy based on a personalized approach,” he predicted.
For the present, in Dr. Johnson’s view, much of the best guidance on when and how to employ SLNB in melanoma patients comes from the landmark National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial (MSLT-1).
The MSLT-1 results (N Engl J Med. 2014 Feb 13;370:599-609) are deemed controversial by some, Dr. Johnson noted. But they provide a strong case for widespread application of SLNB for accurate staging and regional control of the nodal basin based upon the findings in 1,270 participants with intermediate-thickness melanomas of 1.2-3.5 mm and 290 others with melanomas greater than 3.5 mm thick, he said. Both groups showed significant benefit in terms of 10-year melanoma-specific survival if randomized to SLNB with immediate total lymph node dissection if SLNB positive, as opposed to watchful waiting for an occult nodal metastasis to become clinically evident.
An important finding was that roughly 27% of patients in the observation arms who experienced a clinically evident nodal metastasis during follow-up and then underwent completion total lymph node dissection had four or more positive nodes at that time, compared to 1.6% of those with a positive SLNB who underwent immediate completion dissection.
“If an occult metastasis is present in the lymph node it’s going to grow to the point of becoming clinically evident in an average of 2-3 years. You can deal with it now or you can deal with it later, but you’re likely going to have to deal with it. Failure to detect and treat that disease early will result in increased tumor burden upon completion lymph node dissection, increased morbidity and side effects in order to remove those nodes, and a small but increased likelihood of dying from that disease. That’s a very powerful conversation to have with patients and families to help them make the best informed decision for themselves,” Dr. Johnson said.
At the University of Michigan melanoma program – the nation’s largest – patients are counseled to seriously consider SLNB if they have a clinically localized melanoma 1 mm or more in thickness provided their functional status is favorable. In contrast, there is essentially no evidence to support SLNB in patients with a melanoma less than 0.75 mm in thickness.
In patients with a melanoma thickness of 0.75-0.99 mm, however, Dr. Johnson and his colleagues may recommend SLNB in the presence of certain predictors of increased likelihood of a positive biopsy: ulceration, angiolymphatic invasion, younger age, a positive deep margin on a shave biopsy, extensive dermal regression in excess of 1 mm, and/or a mitotic rate of at least 1 mm2. Age and mitotic rate are continuous variables: The younger the patient and the higher the mitotic rate, the greater the likelihood of a positive SLNB in the setting of a thin melanoma.
At present, the decision regarding whether to recommend SLNB in a patient with a thin melanoma is based upon a general gestalt, said Dr. Johnson. He and his colleagues have received a grant to study more than 2,000 cases of thin melanoma in an effort to develop a system for weighting the individual risk factors.
“As dermatologists – you, me, we – should be one of the lead dogs with respect to melanoma advancement, knowledge, management, and guidance. To do that most effectively, you must learn from and work closely with other specialists – collegially, collaboratively, and humbly,” he concluded.
Dr. Johnson reported having no financial conflicts of interest regarding his talk.
VANCOUVER, B.C. – Why should dermatologists care about the role of sentinel lymph node biopsy in melanoma patients? Because SNLB results will inform the use of an explosion of immunotherapies and targeted therapies emerging for melanoma and – because even after melanoma patients have been referred to a comprehensive cancer center – patients will continue to view dermatologists as the primary care providers with respect to the cancer, Dr. Timothy M. Johnson said in a plenary address at the World Congress of Dermatology.
“I guarantee you of that” scenario, predicted Dr. Johnson, professor of dermatology and clinical director of the multidisciplinary melanoma program at the University of Michigan, Ann Arbor, where more than 2,000 melanoma patients are seen each year.
New melanoma therapies are “coming in waves. They seem to be coming weekly,” he said. In June, the National Cancer Institute listed 218 therapeutic clinical trials for advanced melanoma in the United States alone. And some of these new treatments for stage IV melanoma are already starting to make their way into the adjuvant setting for stage III melanoma.
“The first round of adjuvant therapy trials has been done, and the data are being analyzed now. A new round is coming like gangbusters,” Dr. Johnson said. “This is one of the most exciting times ever in melanoma.”
Should some of these novel agents prove safe and effective as adjuvant therapy, SLNB results will determine the need for such therapy based upon individualized patient prognosis. And the SLNB results will determine the type of adjuvant therapy that’s most appropriate based upon the tumor molecular profile.
“Once we have effective adjuvant therapy, it will eradicate the need for total lymph node dissection in patients with a positive node on SLNB. Those with a positive node will likely undergo systemic therapy based on a personalized approach,” he predicted.
For the present, in Dr. Johnson’s view, much of the best guidance on when and how to employ SLNB in melanoma patients comes from the landmark National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial (MSLT-1).
The MSLT-1 results (N Engl J Med. 2014 Feb 13;370:599-609) are deemed controversial by some, Dr. Johnson noted. But they provide a strong case for widespread application of SLNB for accurate staging and regional control of the nodal basin based upon the findings in 1,270 participants with intermediate-thickness melanomas of 1.2-3.5 mm and 290 others with melanomas greater than 3.5 mm thick, he said. Both groups showed significant benefit in terms of 10-year melanoma-specific survival if randomized to SLNB with immediate total lymph node dissection if SLNB positive, as opposed to watchful waiting for an occult nodal metastasis to become clinically evident.
An important finding was that roughly 27% of patients in the observation arms who experienced a clinically evident nodal metastasis during follow-up and then underwent completion total lymph node dissection had four or more positive nodes at that time, compared to 1.6% of those with a positive SLNB who underwent immediate completion dissection.
“If an occult metastasis is present in the lymph node it’s going to grow to the point of becoming clinically evident in an average of 2-3 years. You can deal with it now or you can deal with it later, but you’re likely going to have to deal with it. Failure to detect and treat that disease early will result in increased tumor burden upon completion lymph node dissection, increased morbidity and side effects in order to remove those nodes, and a small but increased likelihood of dying from that disease. That’s a very powerful conversation to have with patients and families to help them make the best informed decision for themselves,” Dr. Johnson said.
At the University of Michigan melanoma program – the nation’s largest – patients are counseled to seriously consider SLNB if they have a clinically localized melanoma 1 mm or more in thickness provided their functional status is favorable. In contrast, there is essentially no evidence to support SLNB in patients with a melanoma less than 0.75 mm in thickness.
In patients with a melanoma thickness of 0.75-0.99 mm, however, Dr. Johnson and his colleagues may recommend SLNB in the presence of certain predictors of increased likelihood of a positive biopsy: ulceration, angiolymphatic invasion, younger age, a positive deep margin on a shave biopsy, extensive dermal regression in excess of 1 mm, and/or a mitotic rate of at least 1 mm2. Age and mitotic rate are continuous variables: The younger the patient and the higher the mitotic rate, the greater the likelihood of a positive SLNB in the setting of a thin melanoma.
At present, the decision regarding whether to recommend SLNB in a patient with a thin melanoma is based upon a general gestalt, said Dr. Johnson. He and his colleagues have received a grant to study more than 2,000 cases of thin melanoma in an effort to develop a system for weighting the individual risk factors.
“As dermatologists – you, me, we – should be one of the lead dogs with respect to melanoma advancement, knowledge, management, and guidance. To do that most effectively, you must learn from and work closely with other specialists – collegially, collaboratively, and humbly,” he concluded.
Dr. Johnson reported having no financial conflicts of interest regarding his talk.
VANCOUVER, B.C. – Why should dermatologists care about the role of sentinel lymph node biopsy in melanoma patients? Because SNLB results will inform the use of an explosion of immunotherapies and targeted therapies emerging for melanoma and – because even after melanoma patients have been referred to a comprehensive cancer center – patients will continue to view dermatologists as the primary care providers with respect to the cancer, Dr. Timothy M. Johnson said in a plenary address at the World Congress of Dermatology.
“I guarantee you of that” scenario, predicted Dr. Johnson, professor of dermatology and clinical director of the multidisciplinary melanoma program at the University of Michigan, Ann Arbor, where more than 2,000 melanoma patients are seen each year.
New melanoma therapies are “coming in waves. They seem to be coming weekly,” he said. In June, the National Cancer Institute listed 218 therapeutic clinical trials for advanced melanoma in the United States alone. And some of these new treatments for stage IV melanoma are already starting to make their way into the adjuvant setting for stage III melanoma.
“The first round of adjuvant therapy trials has been done, and the data are being analyzed now. A new round is coming like gangbusters,” Dr. Johnson said. “This is one of the most exciting times ever in melanoma.”
Should some of these novel agents prove safe and effective as adjuvant therapy, SLNB results will determine the need for such therapy based upon individualized patient prognosis. And the SLNB results will determine the type of adjuvant therapy that’s most appropriate based upon the tumor molecular profile.
“Once we have effective adjuvant therapy, it will eradicate the need for total lymph node dissection in patients with a positive node on SLNB. Those with a positive node will likely undergo systemic therapy based on a personalized approach,” he predicted.
For the present, in Dr. Johnson’s view, much of the best guidance on when and how to employ SLNB in melanoma patients comes from the landmark National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial (MSLT-1).
The MSLT-1 results (N Engl J Med. 2014 Feb 13;370:599-609) are deemed controversial by some, Dr. Johnson noted. But they provide a strong case for widespread application of SLNB for accurate staging and regional control of the nodal basin based upon the findings in 1,270 participants with intermediate-thickness melanomas of 1.2-3.5 mm and 290 others with melanomas greater than 3.5 mm thick, he said. Both groups showed significant benefit in terms of 10-year melanoma-specific survival if randomized to SLNB with immediate total lymph node dissection if SLNB positive, as opposed to watchful waiting for an occult nodal metastasis to become clinically evident.
An important finding was that roughly 27% of patients in the observation arms who experienced a clinically evident nodal metastasis during follow-up and then underwent completion total lymph node dissection had four or more positive nodes at that time, compared to 1.6% of those with a positive SLNB who underwent immediate completion dissection.
“If an occult metastasis is present in the lymph node it’s going to grow to the point of becoming clinically evident in an average of 2-3 years. You can deal with it now or you can deal with it later, but you’re likely going to have to deal with it. Failure to detect and treat that disease early will result in increased tumor burden upon completion lymph node dissection, increased morbidity and side effects in order to remove those nodes, and a small but increased likelihood of dying from that disease. That’s a very powerful conversation to have with patients and families to help them make the best informed decision for themselves,” Dr. Johnson said.
At the University of Michigan melanoma program – the nation’s largest – patients are counseled to seriously consider SLNB if they have a clinically localized melanoma 1 mm or more in thickness provided their functional status is favorable. In contrast, there is essentially no evidence to support SLNB in patients with a melanoma less than 0.75 mm in thickness.
In patients with a melanoma thickness of 0.75-0.99 mm, however, Dr. Johnson and his colleagues may recommend SLNB in the presence of certain predictors of increased likelihood of a positive biopsy: ulceration, angiolymphatic invasion, younger age, a positive deep margin on a shave biopsy, extensive dermal regression in excess of 1 mm, and/or a mitotic rate of at least 1 mm2. Age and mitotic rate are continuous variables: The younger the patient and the higher the mitotic rate, the greater the likelihood of a positive SLNB in the setting of a thin melanoma.
At present, the decision regarding whether to recommend SLNB in a patient with a thin melanoma is based upon a general gestalt, said Dr. Johnson. He and his colleagues have received a grant to study more than 2,000 cases of thin melanoma in an effort to develop a system for weighting the individual risk factors.
“As dermatologists – you, me, we – should be one of the lead dogs with respect to melanoma advancement, knowledge, management, and guidance. To do that most effectively, you must learn from and work closely with other specialists – collegially, collaboratively, and humbly,” he concluded.
Dr. Johnson reported having no financial conflicts of interest regarding his talk.
EXPERT ANALYSIS FROM WCD 2015
New evidence in melanoma field may be practice changing
SEATTLE – Recent developments in the field of melanoma may help clinicians refine their approach to this disease, according to Dr. Suraj S. Venna, a dermatologist and medical director of the Inova Melanoma and Skin Cancer Center in Fairfax, Va. He discussed several key studies and guideline revisions at a joint meeting of the Global Biomarkers Consortium and World Cutaneous Malignancies Congress.
Sentinel node biopsy for thin melanomas
Guidance regarding staging of and use of sentinel lymph node (SLN) biopsy in cases of thin melanomas, those no more than 1 mm thick, is in flux, and these gray areas pose challenges to management.
In its seventh edition of the TNM Staging System published in 2010, the American Joint Committee on Cancer started incorporating mitotic rate to substage T1 melanomas, according to Dr. Venna. Those having a mitotic rate of at least 1 mitosis/mm2 are now categorized as T1b. Yet, even within this subset, there is a spectrum of 10-year survival according to mitotic rate (J Clin Oncol. 2011;29:2199-205). Also, the association of mitotic rate with regional nodal metastasis is unclear.
Therefore, “T1b is not an automatic recommendation for lymph node biopsy,” he said. Decisions about SLN in patients with such tumors should consider all available clinical and pathologic factors.
The National Comprehensive Cancer Network has also revised its guidelines in this area. In 2011, they advised that SLN could be discussed and considered in stage IA melanomas with various high-risk features and in selected stage IB melanomas based on mitotic rate and thickness.
But in their 2015 update, they gave priority to lesion depth. “What they say is other than Breslow depth, there remains little consensus regarding the significance of ulceration, mitotic rate, or lymphovascular invasion,” Dr. Venna elaborated. Now, SLN biopsy can be considered on a case-by-case basis for stage 1A melanomas that are thicker or have uncertain microstaging, and can be discussed and offered for thicker stage 1B melanomas with ulceration or an elevated mitotic rate.
“At the end of the day, when we are all in our multidisciplinary clinics trying to talk to patients about this, these are nuances that really cause a lot of angst for us and the patient in terms of making decisions,” commented Dr. Venna.
“Acknowledge the prognostic gap in the T1b cohort and that not all T1b melanomas are created equal. We all anticipate that molecular techniques may better identify who among that thin group may benefit from lymph node testing,” Dr. Venna said.
Screening strategies
Population-based screening for melanoma and other skin cancers remains controversial, and this practice is generally limited to areas of the world having high incidence, according to Dr. Venna. Germany was the first country to implement a nationwide program, starting in 2008.
“There is evidence that screening may lead to the prevention of a substantial proportion of melanoma deaths,” he maintained. However, the U.S. National Cancer Institute’s position is that current evidence is inadequate and population-based screening has potential drawbacks such as overdiagnosis.
In the meantime, many opportunities to detect melanoma during routine medical care may be missed, according to Dr. Venna; for example, auscultating a patients’ heart or lungs without pulling up their shirt may miss truncal lesions. “Use every opportunity to screen the patient – that’s opportunistic screening,” he recommended.
He also endorsed listening to patients’ concerns and balancing their requests with judicious removal of lesions, as a large share of melanomas are self-detected. In one study of note, 9 of 166 lesions removed and found to be melanoma were removed only to reassure the patient (Arch Dermatol. 2005;141:434-8).
Barriers to and imperatives for early detection
Data suggest that access to specialist care is problematic for the early detection of melanoma. In a survey of dermatologists, the median wait time for an appointment was 8 days for botulinum toxin type A (Botox) injections, compared with 26 days for evaluation of a suspicious mole (J Am Acad Dermatol. 2007;57:985-9).
“The concern of course is a delay in diagnosis and the fact that the nondermatologists will have an opportunity likely to capture melanoma before we do,” Dr. Venna commented.
Imperatives for early detection include not only better outcomes but also reduced costs, given the hefty price tag of novel targeted therapies being used to treat advanced disease, he noted.
A recent phase III trial found that progression-free survival in advanced melanoma was roughly two to four times longer with the combination of the targeted agents nivolumab (Opdivo) and ipilimumab (Yervoy) than with either agent alone (ASCO 2015, Abstract LBA1). But a discussion touching on costs noted that for the average-weight American, this combination would run about $300,000 annually (ASCO 2015, Saltz L).
Risk and protective factors
New data have implicated medications used to treat erectile dysfunction (ED) as a risk factor for melanoma, raising concern given their widespread use, Dr. Venna said.
A cohort study found that melanoma risk was 84% higher with recent use of sildenafil (Viagra) and 92% higher with ever use (JAMA Intern Med. 2014;174:964-70). But the absolute number of excess cases was fairly small.
A case-control study looking at all types of ED drugs found that receipt of a single prescription was associated with a 32% higher risk of melanoma (JAMA. 2015;313:2449-55). Use increased risk of in situ and stage I melanoma, but not more advanced disease. There was no difference between short- and long-acting agents.
“Prospective studies with clearly defined inclusions and exclusions are needed, with dosing in particular,” Dr. Venna summed up. “There appears to be a modest association, but it certainly is not enough to call for widespread discontinuation. In the patients who are on these drugs, it’s good to document [use] until we have more clarity about the biologic effects of this ultimately.”
A study using dietary surveys and having an average follow-up of more than a decade showed that coffee drinkers had a 20% lower risk of melanoma relative to nondrinkers (J Natl Cancer Inst. 2015 Jan 20;107(2)). But benefit was significant only among the subset drinking at least 4 cups a day.
“It’s premature to advise coffee intake based on this paper, although it does build upon an earlier Norwegian study from the 1990s which showed a similar trend,” Dr. Venna said. “At the end of the day, these data are intriguing, but obviously there are a lot of side effects of trying to consume 4 cups of coffee a day, so further study is certainly warranted.”
Dr. Venna disclosed that he had no relevant conflicts of interest.
SEATTLE – Recent developments in the field of melanoma may help clinicians refine their approach to this disease, according to Dr. Suraj S. Venna, a dermatologist and medical director of the Inova Melanoma and Skin Cancer Center in Fairfax, Va. He discussed several key studies and guideline revisions at a joint meeting of the Global Biomarkers Consortium and World Cutaneous Malignancies Congress.
Sentinel node biopsy for thin melanomas
Guidance regarding staging of and use of sentinel lymph node (SLN) biopsy in cases of thin melanomas, those no more than 1 mm thick, is in flux, and these gray areas pose challenges to management.
In its seventh edition of the TNM Staging System published in 2010, the American Joint Committee on Cancer started incorporating mitotic rate to substage T1 melanomas, according to Dr. Venna. Those having a mitotic rate of at least 1 mitosis/mm2 are now categorized as T1b. Yet, even within this subset, there is a spectrum of 10-year survival according to mitotic rate (J Clin Oncol. 2011;29:2199-205). Also, the association of mitotic rate with regional nodal metastasis is unclear.
Therefore, “T1b is not an automatic recommendation for lymph node biopsy,” he said. Decisions about SLN in patients with such tumors should consider all available clinical and pathologic factors.
The National Comprehensive Cancer Network has also revised its guidelines in this area. In 2011, they advised that SLN could be discussed and considered in stage IA melanomas with various high-risk features and in selected stage IB melanomas based on mitotic rate and thickness.
But in their 2015 update, they gave priority to lesion depth. “What they say is other than Breslow depth, there remains little consensus regarding the significance of ulceration, mitotic rate, or lymphovascular invasion,” Dr. Venna elaborated. Now, SLN biopsy can be considered on a case-by-case basis for stage 1A melanomas that are thicker or have uncertain microstaging, and can be discussed and offered for thicker stage 1B melanomas with ulceration or an elevated mitotic rate.
“At the end of the day, when we are all in our multidisciplinary clinics trying to talk to patients about this, these are nuances that really cause a lot of angst for us and the patient in terms of making decisions,” commented Dr. Venna.
“Acknowledge the prognostic gap in the T1b cohort and that not all T1b melanomas are created equal. We all anticipate that molecular techniques may better identify who among that thin group may benefit from lymph node testing,” Dr. Venna said.
Screening strategies
Population-based screening for melanoma and other skin cancers remains controversial, and this practice is generally limited to areas of the world having high incidence, according to Dr. Venna. Germany was the first country to implement a nationwide program, starting in 2008.
“There is evidence that screening may lead to the prevention of a substantial proportion of melanoma deaths,” he maintained. However, the U.S. National Cancer Institute’s position is that current evidence is inadequate and population-based screening has potential drawbacks such as overdiagnosis.
In the meantime, many opportunities to detect melanoma during routine medical care may be missed, according to Dr. Venna; for example, auscultating a patients’ heart or lungs without pulling up their shirt may miss truncal lesions. “Use every opportunity to screen the patient – that’s opportunistic screening,” he recommended.
He also endorsed listening to patients’ concerns and balancing their requests with judicious removal of lesions, as a large share of melanomas are self-detected. In one study of note, 9 of 166 lesions removed and found to be melanoma were removed only to reassure the patient (Arch Dermatol. 2005;141:434-8).
Barriers to and imperatives for early detection
Data suggest that access to specialist care is problematic for the early detection of melanoma. In a survey of dermatologists, the median wait time for an appointment was 8 days for botulinum toxin type A (Botox) injections, compared with 26 days for evaluation of a suspicious mole (J Am Acad Dermatol. 2007;57:985-9).
“The concern of course is a delay in diagnosis and the fact that the nondermatologists will have an opportunity likely to capture melanoma before we do,” Dr. Venna commented.
Imperatives for early detection include not only better outcomes but also reduced costs, given the hefty price tag of novel targeted therapies being used to treat advanced disease, he noted.
A recent phase III trial found that progression-free survival in advanced melanoma was roughly two to four times longer with the combination of the targeted agents nivolumab (Opdivo) and ipilimumab (Yervoy) than with either agent alone (ASCO 2015, Abstract LBA1). But a discussion touching on costs noted that for the average-weight American, this combination would run about $300,000 annually (ASCO 2015, Saltz L).
Risk and protective factors
New data have implicated medications used to treat erectile dysfunction (ED) as a risk factor for melanoma, raising concern given their widespread use, Dr. Venna said.
A cohort study found that melanoma risk was 84% higher with recent use of sildenafil (Viagra) and 92% higher with ever use (JAMA Intern Med. 2014;174:964-70). But the absolute number of excess cases was fairly small.
A case-control study looking at all types of ED drugs found that receipt of a single prescription was associated with a 32% higher risk of melanoma (JAMA. 2015;313:2449-55). Use increased risk of in situ and stage I melanoma, but not more advanced disease. There was no difference between short- and long-acting agents.
“Prospective studies with clearly defined inclusions and exclusions are needed, with dosing in particular,” Dr. Venna summed up. “There appears to be a modest association, but it certainly is not enough to call for widespread discontinuation. In the patients who are on these drugs, it’s good to document [use] until we have more clarity about the biologic effects of this ultimately.”
A study using dietary surveys and having an average follow-up of more than a decade showed that coffee drinkers had a 20% lower risk of melanoma relative to nondrinkers (J Natl Cancer Inst. 2015 Jan 20;107(2)). But benefit was significant only among the subset drinking at least 4 cups a day.
“It’s premature to advise coffee intake based on this paper, although it does build upon an earlier Norwegian study from the 1990s which showed a similar trend,” Dr. Venna said. “At the end of the day, these data are intriguing, but obviously there are a lot of side effects of trying to consume 4 cups of coffee a day, so further study is certainly warranted.”
Dr. Venna disclosed that he had no relevant conflicts of interest.
SEATTLE – Recent developments in the field of melanoma may help clinicians refine their approach to this disease, according to Dr. Suraj S. Venna, a dermatologist and medical director of the Inova Melanoma and Skin Cancer Center in Fairfax, Va. He discussed several key studies and guideline revisions at a joint meeting of the Global Biomarkers Consortium and World Cutaneous Malignancies Congress.
Sentinel node biopsy for thin melanomas
Guidance regarding staging of and use of sentinel lymph node (SLN) biopsy in cases of thin melanomas, those no more than 1 mm thick, is in flux, and these gray areas pose challenges to management.
In its seventh edition of the TNM Staging System published in 2010, the American Joint Committee on Cancer started incorporating mitotic rate to substage T1 melanomas, according to Dr. Venna. Those having a mitotic rate of at least 1 mitosis/mm2 are now categorized as T1b. Yet, even within this subset, there is a spectrum of 10-year survival according to mitotic rate (J Clin Oncol. 2011;29:2199-205). Also, the association of mitotic rate with regional nodal metastasis is unclear.
Therefore, “T1b is not an automatic recommendation for lymph node biopsy,” he said. Decisions about SLN in patients with such tumors should consider all available clinical and pathologic factors.
The National Comprehensive Cancer Network has also revised its guidelines in this area. In 2011, they advised that SLN could be discussed and considered in stage IA melanomas with various high-risk features and in selected stage IB melanomas based on mitotic rate and thickness.
But in their 2015 update, they gave priority to lesion depth. “What they say is other than Breslow depth, there remains little consensus regarding the significance of ulceration, mitotic rate, or lymphovascular invasion,” Dr. Venna elaborated. Now, SLN biopsy can be considered on a case-by-case basis for stage 1A melanomas that are thicker or have uncertain microstaging, and can be discussed and offered for thicker stage 1B melanomas with ulceration or an elevated mitotic rate.
“At the end of the day, when we are all in our multidisciplinary clinics trying to talk to patients about this, these are nuances that really cause a lot of angst for us and the patient in terms of making decisions,” commented Dr. Venna.
“Acknowledge the prognostic gap in the T1b cohort and that not all T1b melanomas are created equal. We all anticipate that molecular techniques may better identify who among that thin group may benefit from lymph node testing,” Dr. Venna said.
Screening strategies
Population-based screening for melanoma and other skin cancers remains controversial, and this practice is generally limited to areas of the world having high incidence, according to Dr. Venna. Germany was the first country to implement a nationwide program, starting in 2008.
“There is evidence that screening may lead to the prevention of a substantial proportion of melanoma deaths,” he maintained. However, the U.S. National Cancer Institute’s position is that current evidence is inadequate and population-based screening has potential drawbacks such as overdiagnosis.
In the meantime, many opportunities to detect melanoma during routine medical care may be missed, according to Dr. Venna; for example, auscultating a patients’ heart or lungs without pulling up their shirt may miss truncal lesions. “Use every opportunity to screen the patient – that’s opportunistic screening,” he recommended.
He also endorsed listening to patients’ concerns and balancing their requests with judicious removal of lesions, as a large share of melanomas are self-detected. In one study of note, 9 of 166 lesions removed and found to be melanoma were removed only to reassure the patient (Arch Dermatol. 2005;141:434-8).
Barriers to and imperatives for early detection
Data suggest that access to specialist care is problematic for the early detection of melanoma. In a survey of dermatologists, the median wait time for an appointment was 8 days for botulinum toxin type A (Botox) injections, compared with 26 days for evaluation of a suspicious mole (J Am Acad Dermatol. 2007;57:985-9).
“The concern of course is a delay in diagnosis and the fact that the nondermatologists will have an opportunity likely to capture melanoma before we do,” Dr. Venna commented.
Imperatives for early detection include not only better outcomes but also reduced costs, given the hefty price tag of novel targeted therapies being used to treat advanced disease, he noted.
A recent phase III trial found that progression-free survival in advanced melanoma was roughly two to four times longer with the combination of the targeted agents nivolumab (Opdivo) and ipilimumab (Yervoy) than with either agent alone (ASCO 2015, Abstract LBA1). But a discussion touching on costs noted that for the average-weight American, this combination would run about $300,000 annually (ASCO 2015, Saltz L).
Risk and protective factors
New data have implicated medications used to treat erectile dysfunction (ED) as a risk factor for melanoma, raising concern given their widespread use, Dr. Venna said.
A cohort study found that melanoma risk was 84% higher with recent use of sildenafil (Viagra) and 92% higher with ever use (JAMA Intern Med. 2014;174:964-70). But the absolute number of excess cases was fairly small.
A case-control study looking at all types of ED drugs found that receipt of a single prescription was associated with a 32% higher risk of melanoma (JAMA. 2015;313:2449-55). Use increased risk of in situ and stage I melanoma, but not more advanced disease. There was no difference between short- and long-acting agents.
“Prospective studies with clearly defined inclusions and exclusions are needed, with dosing in particular,” Dr. Venna summed up. “There appears to be a modest association, but it certainly is not enough to call for widespread discontinuation. In the patients who are on these drugs, it’s good to document [use] until we have more clarity about the biologic effects of this ultimately.”
A study using dietary surveys and having an average follow-up of more than a decade showed that coffee drinkers had a 20% lower risk of melanoma relative to nondrinkers (J Natl Cancer Inst. 2015 Jan 20;107(2)). But benefit was significant only among the subset drinking at least 4 cups a day.
“It’s premature to advise coffee intake based on this paper, although it does build upon an earlier Norwegian study from the 1990s which showed a similar trend,” Dr. Venna said. “At the end of the day, these data are intriguing, but obviously there are a lot of side effects of trying to consume 4 cups of coffee a day, so further study is certainly warranted.”
Dr. Venna disclosed that he had no relevant conflicts of interest.
EXPERT ANALYSIS FROM PMO LIVE
Melanoma twice as likely after CLL/SLL than other types of NHL
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma.
Major finding: A total of 202 melanomas developed during 5.5 years of follow-up, and nearly half occurred in patients with CLL/SLL rather than other types of NHL.
Data source: A large population-based study using SEER data to assess melanoma risk in 44,870 older survivors of non-Hodgkin lymphoma.
Disclosures: The National Cancer Institute supported the study. Dr. Lam and her associates reported having no relevant financial disclosures.
FDA approves hedgehog pathway inhibitor for locally advanced BCC
Sonidegib, a hedgehog pathway inhibitor, has been approved for treating patients with locally advanced basal cell carcinoma (BCC) that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy, the Food and Drug Administration announced on July 24. The drug is taken once a day, at a recommended dose of 200 mg on an empty stomach and will be marketed as Odomzo by Novartis Pharmaceuticals. Approval was based on a study that found the objective response rate to be 58% among patients treated with the 200-mg dose; an effect that lasted for about 2-19 months, according to the FDA.
“Our increasing understanding of molecular pathways involved in cancer has led to approvals of many oncology drugs in difficult-to-treat diseases for which few therapeutic options previously existed,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. “Thanks to a better understanding of the hedgehog pathway, the FDA has now approved two drugs for the treatment of basal cell carcinoma just in the last 3 years,” he added.
The first drug to treat locally advanced and metastatic basal cell carcinoma was vismodegib, marketed as Erivedge by Genentech in 2012.
The most common adverse events associated with the 200-mg dose include muscle spasms, alopecia, dysgeusia, fatigue, nausea, musculoskeletal pain, diarrhea, decreased weight, decreased appetite, myalgia, abdominal pain, headache, pain, vomiting, and pruritus (itching). It has also been associated “with rare reports” of rhabdomyolysis, muscle spasms, and myalgia, according to the FDA.
The prescribing information includes a boxed warning about the risk of embryo-fetal toxicity, and includes recommendations to verify that women of reproductive potential are not pregnant before starting treatment and to use effective contraception during treatment and for at least 20 months after the last dose. Men on this treatment should also be advised about “the potential risk of exposure through semen and to use condoms with a pregnant partner or a female partner of reproductive potential during treatment,” and for at least 8 months after stopping treatment.
More information on the approval is available on the FDA website at www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm455865.htm.
Exposed pregnancies should be reported to Novartis at 888-669-6682. Serious adverse events associated with sonidegib should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch.
Sonidegib, a hedgehog pathway inhibitor, has been approved for treating patients with locally advanced basal cell carcinoma (BCC) that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy, the Food and Drug Administration announced on July 24. The drug is taken once a day, at a recommended dose of 200 mg on an empty stomach and will be marketed as Odomzo by Novartis Pharmaceuticals. Approval was based on a study that found the objective response rate to be 58% among patients treated with the 200-mg dose; an effect that lasted for about 2-19 months, according to the FDA.
“Our increasing understanding of molecular pathways involved in cancer has led to approvals of many oncology drugs in difficult-to-treat diseases for which few therapeutic options previously existed,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. “Thanks to a better understanding of the hedgehog pathway, the FDA has now approved two drugs for the treatment of basal cell carcinoma just in the last 3 years,” he added.
The first drug to treat locally advanced and metastatic basal cell carcinoma was vismodegib, marketed as Erivedge by Genentech in 2012.
The most common adverse events associated with the 200-mg dose include muscle spasms, alopecia, dysgeusia, fatigue, nausea, musculoskeletal pain, diarrhea, decreased weight, decreased appetite, myalgia, abdominal pain, headache, pain, vomiting, and pruritus (itching). It has also been associated “with rare reports” of rhabdomyolysis, muscle spasms, and myalgia, according to the FDA.
The prescribing information includes a boxed warning about the risk of embryo-fetal toxicity, and includes recommendations to verify that women of reproductive potential are not pregnant before starting treatment and to use effective contraception during treatment and for at least 20 months after the last dose. Men on this treatment should also be advised about “the potential risk of exposure through semen and to use condoms with a pregnant partner or a female partner of reproductive potential during treatment,” and for at least 8 months after stopping treatment.
More information on the approval is available on the FDA website at www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm455865.htm.
Exposed pregnancies should be reported to Novartis at 888-669-6682. Serious adverse events associated with sonidegib should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch.
Sonidegib, a hedgehog pathway inhibitor, has been approved for treating patients with locally advanced basal cell carcinoma (BCC) that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy, the Food and Drug Administration announced on July 24. The drug is taken once a day, at a recommended dose of 200 mg on an empty stomach and will be marketed as Odomzo by Novartis Pharmaceuticals. Approval was based on a study that found the objective response rate to be 58% among patients treated with the 200-mg dose; an effect that lasted for about 2-19 months, according to the FDA.
“Our increasing understanding of molecular pathways involved in cancer has led to approvals of many oncology drugs in difficult-to-treat diseases for which few therapeutic options previously existed,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. “Thanks to a better understanding of the hedgehog pathway, the FDA has now approved two drugs for the treatment of basal cell carcinoma just in the last 3 years,” he added.
The first drug to treat locally advanced and metastatic basal cell carcinoma was vismodegib, marketed as Erivedge by Genentech in 2012.
The most common adverse events associated with the 200-mg dose include muscle spasms, alopecia, dysgeusia, fatigue, nausea, musculoskeletal pain, diarrhea, decreased weight, decreased appetite, myalgia, abdominal pain, headache, pain, vomiting, and pruritus (itching). It has also been associated “with rare reports” of rhabdomyolysis, muscle spasms, and myalgia, according to the FDA.
The prescribing information includes a boxed warning about the risk of embryo-fetal toxicity, and includes recommendations to verify that women of reproductive potential are not pregnant before starting treatment and to use effective contraception during treatment and for at least 20 months after the last dose. Men on this treatment should also be advised about “the potential risk of exposure through semen and to use condoms with a pregnant partner or a female partner of reproductive potential during treatment,” and for at least 8 months after stopping treatment.
More information on the approval is available on the FDA website at www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm455865.htm.
Exposed pregnancies should be reported to Novartis at 888-669-6682. Serious adverse events associated with sonidegib should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch.