User login
For MD-IQ use only
Remembering Why We Are In Medicine
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Dear Friends,
There have been recent policy changes that may be affecting trainees and practicing physicians, whether directly impacting our current practices or influencing the decisions that shape our careers. During these challenging times, I am trying to remind myself more often of why I am in medicine – my patients. I will continue to advocate for my patients on Hill Days to affect change in policy. I will continue to provide the best care I can and fight for resources to do so. I will continue to adapt to the changing climate and do what is best for my practice so that I can deliver the care I think my patients need. By remembering why I am in medicine, I can fight for a future of medicine and science that is still bright.
In this issue’s “In Focus” article, Dr. Yasmin G. Hernandez-Barco and Dr. Motaz Ashkar review the diagnostic and treatment approaches to exocrine pancreatic insufficiency, including common symptoms, differential diagnoses, and the different pancreatic enzyme replacement therapies.
Medications for weight loss are becoming more widely available; however, the literature on what to do with these medications in gastrointestinal endoscopy is still lacking. Dr. Sitharthan Sekar and Dr. Nikiya Asamoah summarize the current data and available guidelines in our “Short Clinical Review.”
With another new academic year upon us, this issue’s “Early Career” section features Dr. Allon Kahn’s top tips for becoming an effective gastroenterology consultant. He describes the 5 principles that would improve patient care and relationships with referring providers.
In the “Finance/Legal” section, Dr. Koushik Das dissects what happens when a physician gets sued, including the basis of malpractice suits, consequences, and anticipated timeline.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), Communications/Managing Editor of TNG.
Until next time, I leave you with a historical fun fact, because we would not be where we are now without appreciating where we were: the pancreas was first discovered by a Greek surgeon, Herophilus, in 336 BC, but its exocrine and endocrine functions were not described until the 1850s-1860s by D. Moyse in Paris and Paul Langerhans in Berlin, respectively.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Assistant Professor of Medicine
Interventional Endoscopy, Division of Gastroenterology
Washington University School of Medicine in St. Louis
Weight Loss Before Military Training May Cut Injury Risk
TOPLINE:
Army recruits who lost excess weight to enter military training experienced fewer musculoskeletal injuries (MSKIs), particularly in the lower extremities, during basic combat training than those who did not lose weight to join the service.
METHODOLOGY:
- The nation’s obesity epidemic means that fewer individuals meet the US Army’s weight and body-fat standards for entering basic combat training. Only 29% of 17- to 24-year-olds in the country would have qualified to join the military in 2018, with overweight and obesity among the leading disqualifying factors.
- Researchers analyzed data from 3168 Army trainees (mean age, 20.96 years; 62.34% men; mean maximum-ever BMI, 26.71) to examine the association between weight loss before enlistment and rates of MSKI during basic combat training.
- Trainees completed a baseline questionnaire that asked whether the person lost weight to enter the Army and included follow-up questions about the amount of weight lost, duration of weight loss, methods used, and prior physical activity.
- MSKIs were classified as any injury to the musculoskeletal system and further categorized by body region (lower extremities, upper extremities, spine/back, and other areas, including the torso and head/neck).
- Researchers identified MSKIs from medical records collected throughout basic combat training and for up to 6 weeks afterward to capture injuries that occurred during training but were documented only after its completion.
TAKEAWAY:
- Overall, 829 trainees (26.16%) reported losing weight to enter the Army, and they tended to have higher mean maximum-ever BMI, body-fat percentage, and lean mass compared with those who did not lose weight to join the service. The mean weight loss was 9.06 kg at a rate of 1.27 kg/wk among the 723 trainees with complete data.
- The most commonly reported weight-loss methods were exercising more (83.7%), changing diet (61.0%), skipping meals (39.3%), and sweating using a sauna or rubber suit (25.6%).
- Trainees who lost weight to join the service had a lower risk of any MSKI (hazard ratio [HR], 0.86) and lower extremity MSKIs (HR, 0.84) during training than those who did not lose weight to enter the Army. No difference was found between the two groups in the risk of upper extremity, spine/back, or other MSKIs.
- Among trainees who lost weight to join the Army, the amount of time it took to lose weight was not associated with the risk for any MSKI or region-specific MSKIs.
IN PRACTICE:
“The findings highlight that losing excess weight before entering military training may reduce MSKI risk for incoming recruits, enforcing the benefits of healthy weight loss programs,” the authors wrote.
SOURCE:
The study, led by Vy T. Nguyen, MS, DSc, Military Performance Division, US Army Research Institute of Environmental Medicine, Natick, Massachusetts, was published online in Obesity .
LIMITATIONS:
The study did not assess whether the association between weight loss and the rate of MSKIs persisted over long-term military service. How the two most frequently reported weight loss methods — increased exercise and dietary changes — may have influenced the observed association remains unclear. Medical records may not have captured all MSKIs if trainees did not seek medical care due to concerns about graduating on time or being placed on limited duty.
DISCLOSURES:
The study was supported by the US Army Medical Research and Development Command’s Military Operational Medicine Program. Two authors received support from the funder.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Army recruits who lost excess weight to enter military training experienced fewer musculoskeletal injuries (MSKIs), particularly in the lower extremities, during basic combat training than those who did not lose weight to join the service.
METHODOLOGY:
- The nation’s obesity epidemic means that fewer individuals meet the US Army’s weight and body-fat standards for entering basic combat training. Only 29% of 17- to 24-year-olds in the country would have qualified to join the military in 2018, with overweight and obesity among the leading disqualifying factors.
- Researchers analyzed data from 3168 Army trainees (mean age, 20.96 years; 62.34% men; mean maximum-ever BMI, 26.71) to examine the association between weight loss before enlistment and rates of MSKI during basic combat training.
- Trainees completed a baseline questionnaire that asked whether the person lost weight to enter the Army and included follow-up questions about the amount of weight lost, duration of weight loss, methods used, and prior physical activity.
- MSKIs were classified as any injury to the musculoskeletal system and further categorized by body region (lower extremities, upper extremities, spine/back, and other areas, including the torso and head/neck).
- Researchers identified MSKIs from medical records collected throughout basic combat training and for up to 6 weeks afterward to capture injuries that occurred during training but were documented only after its completion.
TAKEAWAY:
- Overall, 829 trainees (26.16%) reported losing weight to enter the Army, and they tended to have higher mean maximum-ever BMI, body-fat percentage, and lean mass compared with those who did not lose weight to join the service. The mean weight loss was 9.06 kg at a rate of 1.27 kg/wk among the 723 trainees with complete data.
- The most commonly reported weight-loss methods were exercising more (83.7%), changing diet (61.0%), skipping meals (39.3%), and sweating using a sauna or rubber suit (25.6%).
- Trainees who lost weight to join the service had a lower risk of any MSKI (hazard ratio [HR], 0.86) and lower extremity MSKIs (HR, 0.84) during training than those who did not lose weight to enter the Army. No difference was found between the two groups in the risk of upper extremity, spine/back, or other MSKIs.
- Among trainees who lost weight to join the Army, the amount of time it took to lose weight was not associated with the risk for any MSKI or region-specific MSKIs.
IN PRACTICE:
“The findings highlight that losing excess weight before entering military training may reduce MSKI risk for incoming recruits, enforcing the benefits of healthy weight loss programs,” the authors wrote.
SOURCE:
The study, led by Vy T. Nguyen, MS, DSc, Military Performance Division, US Army Research Institute of Environmental Medicine, Natick, Massachusetts, was published online in Obesity .
LIMITATIONS:
The study did not assess whether the association between weight loss and the rate of MSKIs persisted over long-term military service. How the two most frequently reported weight loss methods — increased exercise and dietary changes — may have influenced the observed association remains unclear. Medical records may not have captured all MSKIs if trainees did not seek medical care due to concerns about graduating on time or being placed on limited duty.
DISCLOSURES:
The study was supported by the US Army Medical Research and Development Command’s Military Operational Medicine Program. Two authors received support from the funder.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Army recruits who lost excess weight to enter military training experienced fewer musculoskeletal injuries (MSKIs), particularly in the lower extremities, during basic combat training than those who did not lose weight to join the service.
METHODOLOGY:
- The nation’s obesity epidemic means that fewer individuals meet the US Army’s weight and body-fat standards for entering basic combat training. Only 29% of 17- to 24-year-olds in the country would have qualified to join the military in 2018, with overweight and obesity among the leading disqualifying factors.
- Researchers analyzed data from 3168 Army trainees (mean age, 20.96 years; 62.34% men; mean maximum-ever BMI, 26.71) to examine the association between weight loss before enlistment and rates of MSKI during basic combat training.
- Trainees completed a baseline questionnaire that asked whether the person lost weight to enter the Army and included follow-up questions about the amount of weight lost, duration of weight loss, methods used, and prior physical activity.
- MSKIs were classified as any injury to the musculoskeletal system and further categorized by body region (lower extremities, upper extremities, spine/back, and other areas, including the torso and head/neck).
- Researchers identified MSKIs from medical records collected throughout basic combat training and for up to 6 weeks afterward to capture injuries that occurred during training but were documented only after its completion.
TAKEAWAY:
- Overall, 829 trainees (26.16%) reported losing weight to enter the Army, and they tended to have higher mean maximum-ever BMI, body-fat percentage, and lean mass compared with those who did not lose weight to join the service. The mean weight loss was 9.06 kg at a rate of 1.27 kg/wk among the 723 trainees with complete data.
- The most commonly reported weight-loss methods were exercising more (83.7%), changing diet (61.0%), skipping meals (39.3%), and sweating using a sauna or rubber suit (25.6%).
- Trainees who lost weight to join the service had a lower risk of any MSKI (hazard ratio [HR], 0.86) and lower extremity MSKIs (HR, 0.84) during training than those who did not lose weight to enter the Army. No difference was found between the two groups in the risk of upper extremity, spine/back, or other MSKIs.
- Among trainees who lost weight to join the Army, the amount of time it took to lose weight was not associated with the risk for any MSKI or region-specific MSKIs.
IN PRACTICE:
“The findings highlight that losing excess weight before entering military training may reduce MSKI risk for incoming recruits, enforcing the benefits of healthy weight loss programs,” the authors wrote.
SOURCE:
The study, led by Vy T. Nguyen, MS, DSc, Military Performance Division, US Army Research Institute of Environmental Medicine, Natick, Massachusetts, was published online in Obesity .
LIMITATIONS:
The study did not assess whether the association between weight loss and the rate of MSKIs persisted over long-term military service. How the two most frequently reported weight loss methods — increased exercise and dietary changes — may have influenced the observed association remains unclear. Medical records may not have captured all MSKIs if trainees did not seek medical care due to concerns about graduating on time or being placed on limited duty.
DISCLOSURES:
The study was supported by the US Army Medical Research and Development Command’s Military Operational Medicine Program. Two authors received support from the funder.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
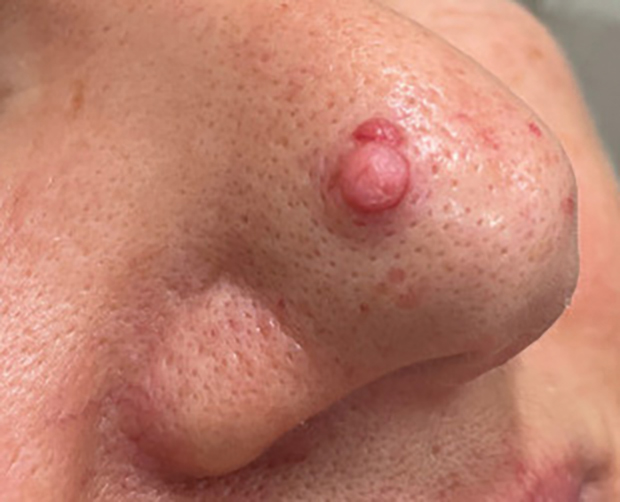
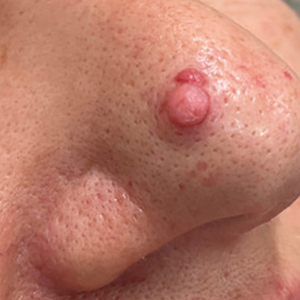
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
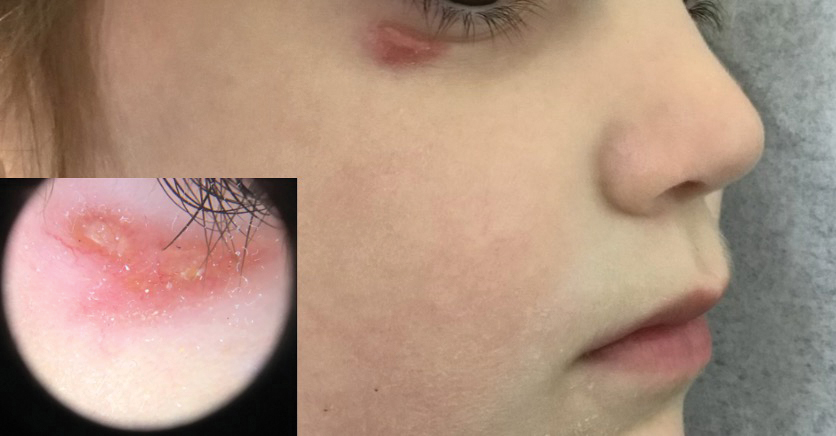
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).
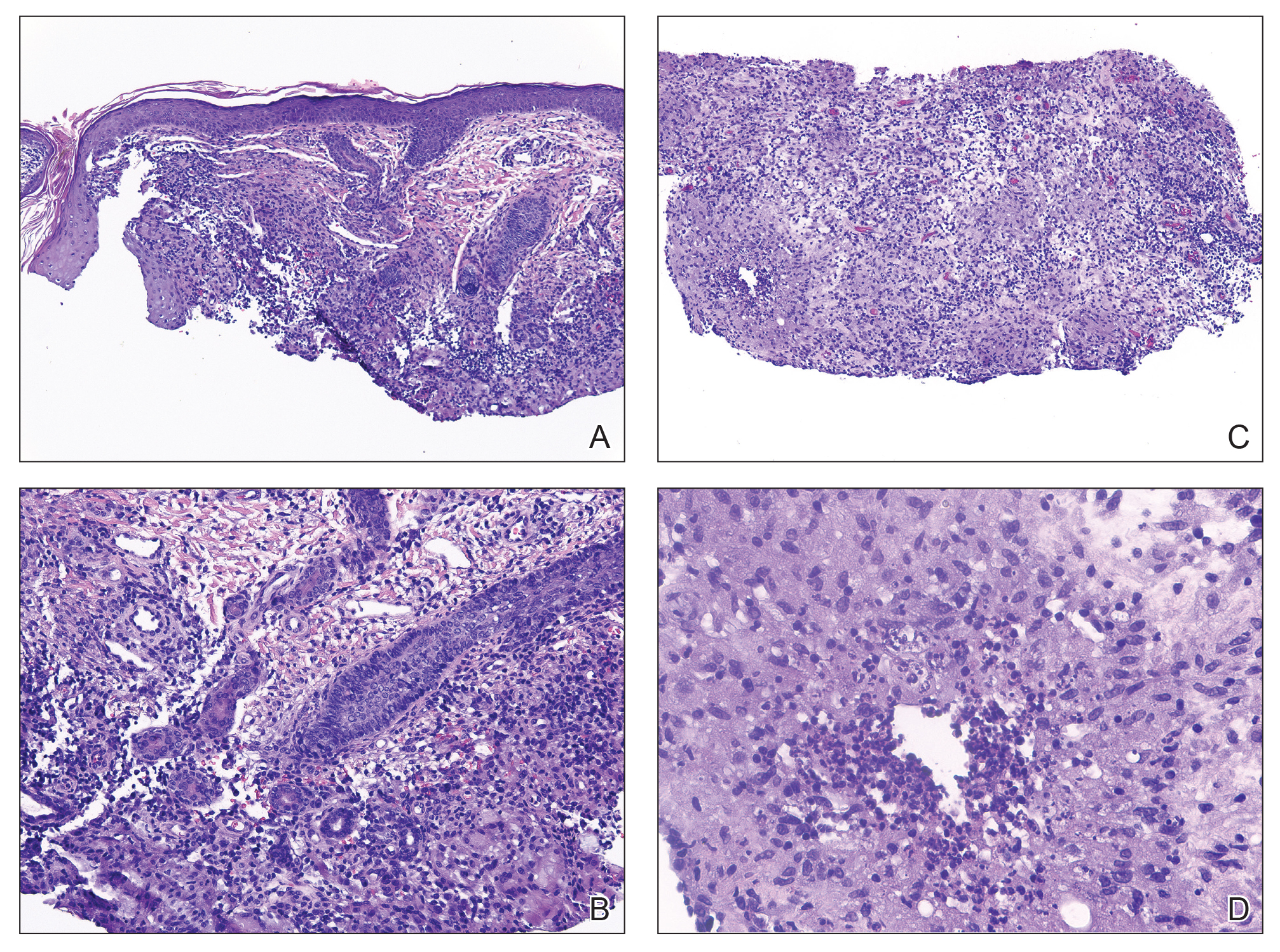
First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
THE DIAGNOSIS: Idiopathic Facial Aseptic Granuloma
Histopathology showed a ruptured follicle, perifollicular granulomatous inflammation, and admixed multinucleated giant cells in the superficial dermis. The deeper tissue exhibited edema, histiocytic/granulomatous inflammation forming ill-defined loose granulomas, and a single neutrophilic microabscess (Figure). Stains for periodic acid-Schiff with diastase and acid-fast bacillus were negative for microorganisms. The clinical examination and pathology findings supported a diagnosis of idiopathic facial aseptic granuloma (IFAG).

First reported in 1999, IFAG was described using the French term pyodermite froide du visage, which translates to “cold pyoderma of the face”; however, it was renamed to represent its granulomatous characteristics and noninfectious etiology.1 The pathogenesis of IFAG is unknown, but the leading hypothesis is that it may be a type of childhood granulomatous rosacea, given its association with relapsing chalazions, papulopustular eruptions on the face, and facial flushing.2 Other hypotheses are that IFAG is idiopathic or a granulomatous response to an insect bite, minor trauma, or embryologic remnant.3
A rare condition arising in early childhood, IFAG manifests as a single or multiple, painless, erythematous or violaceous nodule(s) on the face, most often on the cheeks or eyelids.4 A thorough history and clinical examination often suffice for diagnosis. Dermoscopy may reveal white perifollicular halos and follicular plugs on an erythematous base with linear vessels.4 If diagnostic tests are performed, there are notable characteristic findings: ultrasonography often shows a well-circumscribed, hypoechoic, ovoid dermal lesion without calcifications. Bacterial, fungal, and mycobacterial cultures commonly are negative.4 On biopsy, histopathology may reveal granulomatous inflammation in the superficial and deep dermis, multinucleated giant cells, and surrounding lymphocytic, neutrophilic, and eosinophilic infiltration with no calcium deposits.3,5,6 Histopathology findings for IFAG and rosacea lesions are similar; both may demonstrate folliculitis, perifollicular granulomas, and admixed lymphohistiocytic inflammation.7
Differentiating IFAG from other dermatologic lesions can be challenging, as the differential includes benign neoplasms (eg, dermoid cyst, chalazion, pilomatricoma, xanthoma, xanthogranuloma2) and infectious etiologies such as bacterial pyoderma and mycobacterial, fungal, and parasitic infections (eg, cutaneous leishmaniasis). Pilomatricomas, although often seen on the face or extremities in young girls, more often are well circumscribed and located in the dermis. Ultrasonography of a pilomatricoma classically shows variable foci of calcification. Xanthoma and xanthogranuloma also were considered in our case since the lesion was subtly yellowish on examination. Similar to IFAG, these conditions may manifest as single or multiple lesions. Abnormalities in the patient’s blood lipid panel or family history may be needed to diagnose xanthoma. Biopsy of a juvenile xanthogranuloma would exhibit a dense dermal nodular proliferation of histiocytic cells with a smaller number of admixed lymphocytes, neutrophils, and eosinophils, in contrast to the multiple smaller loose epithelioid granulomas seen in IFAG. Additional diagnoses in the differential for IFAG include pyogenic granuloma, Spitz nevus, nodulocystic infantile acne, granulomatous rosacea, and hemangioma.1,3,9 In particular, granulomatous rosacea is challenging to differentiate from IFAG given the overlapping clinical findings. Multiple lesions, the presence of papules and pustules, and associated rosacea symptoms such as flushing suggest a diagnosis of granulomatous rosacea over IFAG.2
The prognosis for IFAG is excellent; most lesions self-resolve without treatment or procedural intervention within 1 year without scarring or relapse.3 Topical and oral antibiotic treatments such as metronidazole 0.75% gel or cream, oral erythromycin, oral clarithromycin, and doxycycline (in patients older than 8 years) have been used to treat IFAG with variable clinic responses.2,3,6,8 Persistent IFAG has been treated with surgical excision.3 Our patient was treated with a combination of gentamicin ointment 0.3% and tacrolimus ointment 0.3% and experienced approximately 50% improvement in the first month of treatment.
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
- Roul S, Léauté-Labrèze C, Boralevi F, et al. Idiopathic aseptic facial granuloma (pyodermite froide du visage): a pediatric entity? Arch Dermatol. 2001;137:1253-1255.
- Prey S, Ezzedine K, Mazereeuw-Hautier J, et al. IFAG and childhood rosacea: a possible link? Pediatr Dermatol. 2013;30:429-432. doi:10.1111/pde.12137
- Boralevi F, Léauté-Labrèze C, Lepreux S, et al. Idiopathic facial aseptic granuloma: a multicentre prospective study of 30 cases. Br J Dermatol. 2007;156:705-708. doi:10.1111/j.1365-2133.2006.07741.x
- Lobato-Berezo A, Montoro-Romero S, Pujol RM, et al. Dermoscopic features of idiopathic facial aseptic granuloma. Pediatr Dermatol. 2018;35:E308-E309. doi:10.1111/pde.13582
- González Rodríguez AJ, Jordá Cuevas E. Idiopathic facial aseptic granuloma. Clin Exp Dermatol. 2015;40:298-300. doi:10.1111/ced.12535
- Orion C, Sfecci A, Tisseau L, et al. Idiopathic facial aseptic granuloma in a 13-year-old boy dramatically improved with oral doxycycline and topical metronidazole: evidence for a link with childhood rosacea. Case Rep Dermatol. 2016;8:197-201. doi:10.1159/000447624
- Neri I, Raone B, Dondi A, et al. Should idiopathic facial aseptic granuloma be considered granulomatous rosacea? report of three pediatric cases. Pediatr Dermatol. 2013;30:109-111. doi:10.1111 /j.1525-1470.2011.01689.x
- Miconi F, Principi N, Cassiani L, et al. A cheek nodule in a child: be aware of idiopathic facial aseptic granuloma and its differential diagnosis. Int J Environ Res Public Health. 2019;16:2471. doi:10.3390/ijerph16142471
- Baroni A, Russo T, Faccenda F, et al. Idiopathic facial aseptic granuloma in a child: a possible expression of childhood rosacea. Pediatr Dermatol. 2013;30:394-395. doi:10.1111/j.1525-1470.2012.01805.x
Painless Nodule on the Lower Eyelid
Painless Nodule on the Lower Eyelid
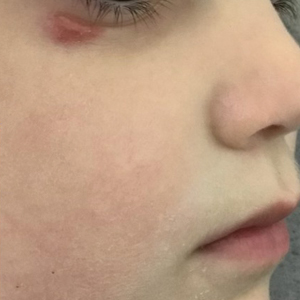
A 4-year-old girl presented to the dermatology clinic with a painless, red to golden-yellowish nodule on the right lower eyelid of 4 months’ duration. The patient had no history of skin disease and was otherwise healthy. Physical examination revealed a single 1-cm, soft, erythematous and yellowish plaque on the right lower eyelid that was subtly fluctuant on palpation. She had no associated systemic symptoms or lymphadenopathy. A punch biopsy of the lesion was performed.
Top 5 Tips for Becoming an Effective Gastroenterology Consultant
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Gastroenterology (GI) subspecialty training is carefully designed to develop expertise in digestive diseases and gastrointestinal endoscopy, while facilitating the transition from generalist to subspecialty consultant. The concept of effective consultation extends far beyond clinical expertise and has been explored repeatedly, beginning with Goldman’s “Ten Commandments” in 1983.1,2 How should these best practices be specifically applied to GI? More importantly, what kind of experience would you want if you were the referring provider or the patient themselves?
Below are
1. Be Kind
Survey studies of medical/surgical residents and attending hospitalists have demonstrated that willingness to accept consultation requests was the single factor consistently rated as most important in determining the quality of the consultation interaction.3,4 Unfortunately, nearly 65% of respondents reported encountering pushback when requesting subspecialty consultation. It is critical to recognize that when you receive a GI consult request, the requester has already decided that it is needed. Whether that request comports with our individual notion of “necessary” or “important,” this is a colleague’s request for help. There are myriad reasons why a request may be made, but they are unified in this principle.
Effective teamwork in healthcare settings enhances clinical performance and patient safety. Positive relationships with colleagues and healthcare team members also mitigate the emotional basis for physician burnout.5 Be kind and courteous to those who seek your assistance. Move beyond the notion of the “bad” or “soft” consult and seek instead to understand how you can help.
A requesting physician may phrase the consult question vaguely or may know that the patient is having a GI-related issue, but simply lack the specific knowledge to know what is needed. In these instances, it is our role to listen and help guide them to the correct thought process to ensure the best care of the patient. These important interactions establish our reputation, create our referral bases, and directly affect our sense of personal satisfaction.
2. Be Timely
GI presents an appealing breadth of pathology, but this also corresponds to a wide variety of indications for consultation and, therefore, urgency of need. In a busy clinical practice, not all requests can be urgently prioritized. However, it is the consultant’s responsibility to identify patients that require urgent evaluation and intervention to avert a potential adverse outcome.
We are well-trained in the medical triage of consultations. There are explicit guidelines for assessing urgency for GI bleeding, foreign body ingestion, choledocholithiasis, and many other indications. However, there are often special contextual circumstances that will elevate the urgency of a seemingly non-urgent consult request. Does the patient have an upcoming surgery or treatment that will depend on your input? Are they facing an imminent loss of insurance coverage? Is their non-severe GI disease leading to more severe impact on non-GI organ systems? The referring provider knows the patient better than you – seek to understand the context of the consult request.
Timeliness also applies to our communication. Communicate recommendations directly to the consulting service as soon as the patient is seen. When a colleague reaches out with a concern about a patient, make sure to take that request seriously. If you are unable to address the concern immediately, at least provide acknowledgment and an estimated timeline for response. As the maxim states, the effectiveness of a consultant is just as dependent on availability as it is on ability.
3. Be Specific
The same survey studies indicate that the second most critical aspect of successful subspecialty consultation is delivering clear recommendations. Accordingly, I always urge my trainees to challenge me when we leave a consult interaction if they feel that our plan is vague or imprecise.
Specificity in consult recommendations is an essential way to demonstrate your expertise and provide value. Clear and definitive recommendations enhance others’ perception of your skill, reduce the need for additional clarifying communication, and lead to more efficient, higher quality care. Avoid vague language, such as asking the requester to “consider” a test or intervention. When recommending medication, specify the dose, frequency, duration, and expected timeline of effect. Rather than recommending “cross-sectional imaging,” specify what modality and protocol. Instead of recommending “adequate resuscitation,” specify your target endpoints. If you engage in multidisciplinary discussion, ensure you strive for a specific group consensus plan and communicate this to all members of the team.
Specificity also applies to the quality of your documentation. Ensure that your clinical notes outline your rationale for your recommended plan, specific contingencies based on results of recommended testing, and a plan for follow-up care. When referring for open-access endoscopy, specifically outline what to look for and which specimens or endoscopic interventions are needed. Be precise in your procedure documentation – avoid vague terms such as small/medium/large and instead quantify in terms of millimeter/centimeter measurement. If you do not adopt specific classification schemes (e.g. Prague classification, Paris classification, Eosinophilic Esophagitis Endoscopic Reference Score, etc.), ensure you provide enough descriptive language to convey an adequate understanding of the findings.
4. Be Helpful
A consultant’s primary directive is to be of service to the consulting provider and the patient. As an educational leader, I am often asked what attributes separate a high-performing trainee from an average one. My feeling is that the most critical attribute is a sense of ownership over patient care.
As a consultant, when others feel we are exhibiting engagement and ownership in a patient’s care, they perceive that we are working together as an effective healthcare team. Interestingly, survey studies of inpatient care show that primary services do not necessarily value assistance with orders or care coordination – they consider these as core aspects of their daily work. What they did value was ongoing daily progress notes/communication, regardless of patient acuity or consulting specialty. This is a potent signal that our continued engagement (both inpatient and outpatient) is perceived as helpful.
Helpfulness is further aided by ensuring mutual understanding. While survey data indicate that sharing specific literature citations may not always be perceived positively, explaining the consultant’s rationale for their recommendations is highly valued. Take the time to tactfully explain your assessment of the patient and why you arrived at your specific recommendations. If your recommendations differ from what the requester expected (e.g. a procedure was expected but is not offered), ensure you explain why and answer questions they may have. This fosters mutual respect and proactively averts conflict or discontent from misunderstanding.
Multidisciplinary collaboration is another important avenue for aiding our patients and colleagues. Studies across a wide range of disease processes (including GI bleeding, IBD, etc.) and medical settings have demonstrated that multidisciplinary collaboration unequivocally improves patient outcomes.6 The success of these collaborations relies on our willingness to fully engage in these conversations, despite the fact that they may often be logistically challenging.
We all know how difficult it can be to locate and organize multiple medical specialists with complex varying clinical schedules and busy personal lives. Choosing to do so demonstrates a dedication to providing the highest level of care and elevates both patient and physician satisfaction. Having chosen to cultivate several ongoing multidisciplinary conferences/collaborations, I can attest to the notion that the outcome is well worth the effort.
5. Be Honest
While we always strive to provide the answers for our patients and colleagues, we must also acknowledge our limitations. Be honest with yourself when you encounter a scenario that pushes beyond the boundaries of your knowledge and comfort. Be willing to admit when you yourself need to consult others or seek an outside referral to provide the care a patient needs. Aspiring physicians often espouse that a devotion to lifelong learning is a key driver of their desire to pursue a career in medicine. These scenarios provide a key opportunity to expand our knowledge while doing what is right for our patients.
Be equally honest about your comfort with “curbside” consultations. Studies show that subspecialists receive on average of 3-4 such requests per week.7 The perception of these interactions is starkly discrepant between the requester and recipient. While over 80% of surveyed primary nonsurgical services felt that curbside consultations were helpful in patient care, a similar proportion of subspecialists expressed concern that insufficient clinical information was provided, even leading to a fear of litigation. While straightforward, informal conversations on narrow, well-defined questions can be helpful and efficient, the consultant should always feel comfortable seeking an opportunity for formal consultation when the details are unclear or the case/question is complex.
Closing Thoughts
Being an effective GI consultant isn’t just about what you know—it’s about how you apply it, how you communicate it, and how you make others feel in the process.
The attributes outlined above are not ancillary traits—they are essential components of high-quality consultation. When consistently applied, they enhance collaboration, improve patient outcomes, and reinforce trust within the healthcare system. By committing to them, you establish your reputation of excellence and play a role in elevating the field of gastroenterology more broadly.
Dr. Kahn is based in the Division of Gastroenterology and Hepatology at Mayo Clinic, Scottsdale, Arizona. He reports no conflicts of interest in regard to this article.
References
1. Goldman L, et al. Ten commandments for effective consultations. Arch Intern Med. 1983 Sep.
2. Salerno SM, et al. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007 Feb. doi: 10.1001/archinte.167.3.271.
3. Adams TN, et al. Hospitalist Perspective of Interactions with Medicine Subspecialty Consult Services. J Hosp Med. 2018 May. doi: 10.12788/jhm.2882.
4. Matsuo T, et al. Essential consultants’ skills and attitudes (Willing CONSULT): a cross-sectional survey. BMC Med Educ. 2021 Jul. doi: 10.1186/s12909-021-02810-9.
5. Welp A, Manser T. Integrating teamwork, clinician occupational well-being and patient safety - development of a conceptual framework based on a systematic review. BMC Health Serv Res. 2016 Jul. doi: 10.1186/s12913-016-1535-y.
6. Webster CS, et al. Interprofessional Learning in Multidisciplinary Healthcare Teams Is Associated With Reduced Patient Mortality: A Quantitative Systematic Review and Meta-analysis. J Patient Saf. 2024 Jan. doi: 10.1097/PTS.0000000000001170.
7. Lin M, et al. Curbside Consultations: The Good, the Bad, and the Ugly. Clin Gastroenterol Hepatol. 2016 Jan. doi: 10.1016/j.cgh.2015.09.026.
Military Imposters: What Drives Them and How They Damage Us All
Military Imposters: What Drives Them and How They Damage Us All
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
The better part of valor is discretion.
Henry IV, Part 1 by William Shakespeare1
This is the second part of an exploration of the phenomenon of stolen valor, where individuals claim military exploits or acts of heroism that are either fabricated or exaggerated, and/or awards and medals they did not earn.2 In June, I focused on the unsettling story of Sarah Cavanaugh, a young US Department of Veterans Affairs (VA) social worker who posed as a decorated, heroic, and seriously wounded Marine veteran for years. Cavanaugh’s manipulative masquerade allowed her to receive coveted spots in veteran recovery programs, thousands of dollars in fraudulent donations, the leadership of a local Veterans of Foreign Wars post, and eventually a federal conviction and prison sentence.3 The first column focused on the legal history of stolen valor; this editorial analyzes the clinical import and ethical impact of the behavior of military imposters. Military imposters are the culprits who steal valor.
It would be easy and perhaps reassuring to assume that stolen valor has emerged as another deplorable example of a national culture in which the betrayal of trust in human beings and loss of faith in institutions and aspirations has reached a nadir. Ironically, stolen valor is inextricably linked to the founding of the United States. When General George Washington inaugurated the American military tradition of awarding decorations to honor the bravery and sacrifices of the patriot Army, he anticipated military imposters. He tried to deter stolen valor through the threat of chastisement: “Should any who are not entitled to these honors have the insolence to assume the badges of them, they shall be severely punished,” Washington warned.4
It is plausible to think such despicable conduct occurs only as the ugly side of the beauty of our unparalleled national freedom, but this is a mistake. Cases of stolen valor have been reported in many countries around the world, with some of the most infamous found in the United Kingdom.5
While many brazen military imposters like Cavanaugh never serve, there is a small subset who honorably wore a uniform yet embellish their service record with secret missions and meritorious gallantry that purportedly earned them high rank and even higher awards. A most puzzling and disturbing example of this group is an allegation that surfaced when celebrated Navy SEAL Chris Kyle declared in American Sniper that he had won 3 additional combat awards for combat valor in addition to the Silver Star and 3 Bronze Stars actually listed in his service record.6
The fact that for centuries stolen valor has plagued multiple nations suggests, at least to this psychiatrically trained mind, that something deeper and darker in human nature than profit alone drives military imposters. Philosopher Verna Gehring has distilled these less tangible motivations into the concept of virtue imposters. According to Gehring, military phonies are a notorious exemplar: “The military phony adopts a past not her own, acts of courage she did not perform—she impersonates the heroic character and virtues she does not possess.”7 There could be no more apposite depiction of Cavanaugh, other military imposters, or a legion of other offenders of honor. 8
As with Cavanaugh, financial gain is a byproduct of the machinations of military imposters and is usually secondary to the pursuit of nonmaterial rewards such as power, influence, admiration, emulation, empathy, and charity. Gehring contends, and I agree, that virtue imposters are more pernicious and culpable than the plethora of more prosaic scammers and swindlers who use deceit primarily as a means of economic exploitation: “The virtue impostor by contrast plays on people’s better natures—their generosity, humility, and their need for heroes.”7
Military imposters cause real and lasting harm. Every veteran who exaggerates claims or scams the VA unjustly steals human and monetary resources from other deserving veterans whose integrity would not permit them to break the rules.9 Yet, even more harmful is the potential damage to therapeutic relationships: federal practitioners may become skeptical of a veteran’s history even when there is little to no grounds for suspicion. Veterans, in turn, may experience a breach of trust and betrayal not only from health care professionals and VA leaders but from their brothers and sisters in arms. On an ever-wider scale, every military impostor who is exposed may diminish the respect and honor all veterans have earned.
It is clear, then, why a small group of former service members has adopted the cause of uncovering military imposters and adroitly using the media to identify signs of stolen valor.10 Yet deception mars even these mostly well-intentioned campaigns, as some more zealous stolen valor hunters may make allegations that turn out to be false.11 Nevertheless, 500 years ago and in a very different context Shakespeare was, right on the mark: the better part of valor is discretion in describing one’s achievements, in relying on the veracity of our veteran’s narratives, and when there are sound reasons to do so verifying the truth of what our patients, friends, and even family tell us about their time in the military.1
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
- Shakespeare W. Introduction in: Henry IV, Part 1. Folger Sharespeare Library. Accessed July 24, 2025. https://www.folger.edu/explore/shakespeares-works/henry-iv-part-1/
- Geppert CM. What about stolen valor actually is illegal? Fed Pract. 2025;42(6):218-219. doi:10.12788/fp.0599
- Lehrfeld J. Woman who faked being cancer-stricken Marine gets 6 years in prison. Military Times. March 15, 2023. Accessed July 24, 2025. https://www.militarytimes.com/news/your-military/2023/03/15/woman-who-faked-being-sick-marine-purple-heart-gets-6-years-in-prison/
- Washington G. General Orders, 7 August 1782 in: Papers of George Washington. Founders Online. August 7, 1782. Accessed July 24, 2025. https://founders.archives.gov/documents/Washington/99-01-02-09056 5. Simpson LK. The men who impersonate military personnel for stolen glory. The Conversation. Updated November 17, 2016. Accessed July 24, 2025. https://theconversation.com/the-men-who-impersonate-military-personnel-for-stolen-glory-62233
- Larter DB. New questions cast doubt on ‘American Sniper‘ Chris Kyle‘s combat record. Navy Times. May 25, 2016. Accessed July 24, 2025. https://www.navytimes.com/news/your-navy/2016/05/25/new-questions-cast-doubt-on-american-sniper-chris-kyle-s-combat-record
- Gehring VV. Phonies, fakes, and frauds—and the social harms they cause. Philos Public Policy Q. 2003;23:14-20.
- Liem, E. The 6 most shocking military imposters ever. Military.com. July 7, 2015. Accessed July 29, 2025. https://www.military.com/undertheradar/2015/07/the-6-most-shocking-military-impostors-ever 9. Sisk R. Some vets with PTSD are scamming the VA: testimony. Military.com. June 8, 2017. Accessed July 24, 2025. https://www.military.com/daily-news/2017/06/08/some-vets-with-ptsd-are-scamming-va-testimony.html
- Bushatz A. How to spot a veteran. Military.com. October 3, 2022. Updated September 16, 2024. Accessed July 24, 2025. https://www.military.com/veterans-day/how-spot-veteran.html
- Monroe R. How to spot a military imposter. The New Yorker. October 19, 2020. Accessed July 24, 2025. https://www.newyorker.com/magazine/2020/10/26/how-to-spot-a-military-impostor
Military Imposters: What Drives Them and How They Damage Us All
Military Imposters: What Drives Them and How They Damage Us All
Novel Peptides Expressed in HIV Could Drive Treatment
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.
Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1
Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.
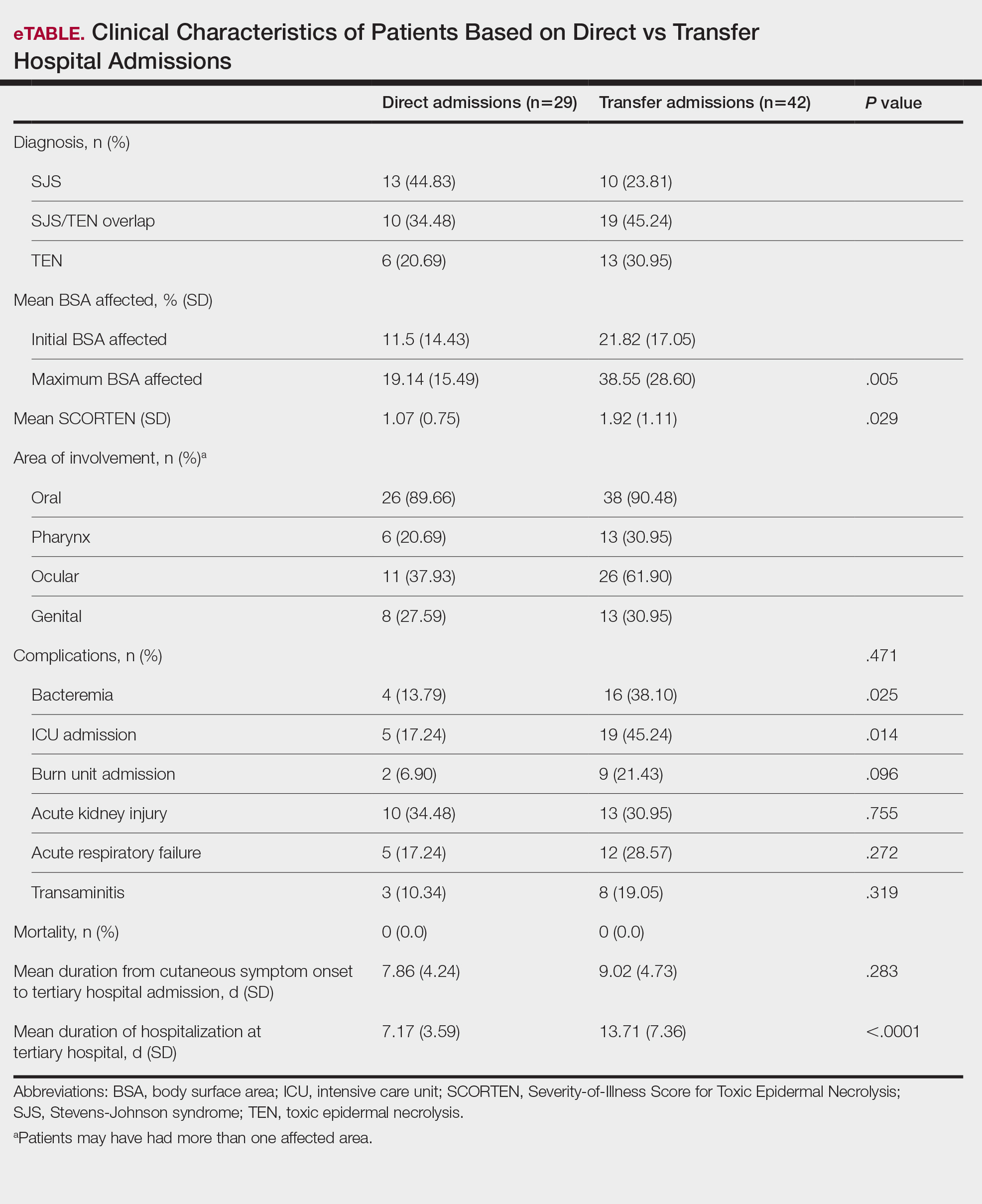
Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.
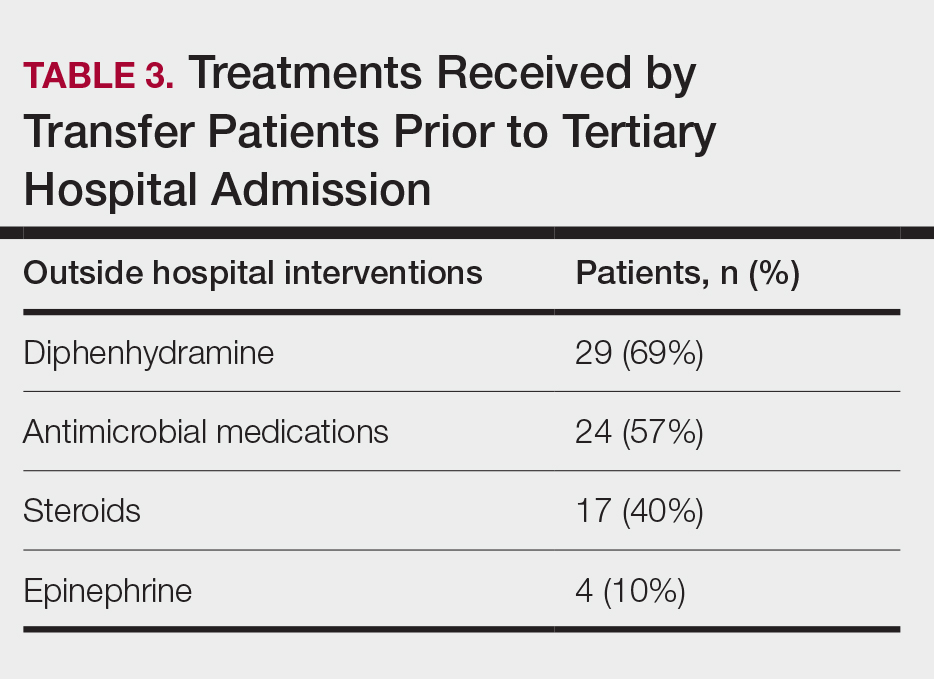
Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.

Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1

Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.

Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.

Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.

Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1

Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.

Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.

Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
PRACTICE POINTS
- Early identification and diagnosis of Stevens-Johnson syndrome and toxic epidermal necrolysis are essential to improving patient outcomes.
- Patients transferred from outside hospitals often present with more severe disease due to delays in diagnosis and initiation of appropriate treatment.
- Inpatient dermatology consultation plays a vital role in accurately diagnosing and managing life-threatening dermatologic conditions.
- Establishing timely interhospital transfer protocols may help expedite access to specialized treatment and improve patient outcomes.
Tribal Health Officials Work To Fill Vaccination Gaps as Measles Outbreak Spreads
RAPID CITY, S.D. — Cassandra Palmier had been meaning to get her son the second and final dose of the measles vaccine. But car problems made it difficult to get to the doctor.
So she pounced on the opportunity to get him vaccinated after learning that a mobile clinic would be visiting her neighborhood.
“I was definitely concerned about the epidemic and the measles,” Palmier, a member of the Oglala Sioux Tribe, said at the June event. “I wanted to do my part.”
So did her son, Makaito Cuny.
“I’m not going to be scared,” the 5-year-old announced as he walked onto the bus containing the clinic and hopped into an exam chair.
Makaito sat still as a nurse gave him the shot in his arm. “I did it!” he said while smiling at his mother.
The vaccine clinic was hosted by the Great Plains Tribal Leaders’ Health Board, which serves tribes across Iowa, Nebraska, and the Dakotas. It’s one way Native American tribes and organizations are responding to concerns about low measles vaccination rates and patients’ difficulty accessing health care as the disease spreads across the country.
Meghan O’Connell, the board’s chief public health officer, said it is also working with tribes that want to host vaccine clinics.
Elsewhere, tribal health organizations have launched social media campaigns, are making sure health providers are vaccinated, and are reaching out to the parents of unvaccinated children.
This spring, Project ECHO at the University of New Mexico hosted an online video series about measles aimed at health care professionals and organizations that serve Native American communities. The presenters outlined the basics of measles diagnosis and treatment, discussed culturally relevant communication strategies, and shared how tribes are responding to the outbreak.
Participants also strategized about ways to improve vaccination rates, said Harry Brown, a physician and an epidemiologist for the United South and Eastern Tribes, a nonprofit that works with 33 tribes in the Atlantic Coast and Southeast regions.
“It’s a pretty hot topic right now in Indian Country and I think a lot of people are being proactive,” he said.
Measles can survive for up to two hours in the air in a space where an infected person has been, sickening up to 90% of people who aren’t vaccinated, according to the Centers for Disease Control and Prevention.
The U.S. has had 1,319 confirmed cases of measles this year as of July 23, according to the CDC. It’s the largest outbreak in the U.S. since 1992. Ninety-two percent of the 2025 cases involve unvaccinated patients or people with an unknown vaccination status. Three people had died in the U.S. and 165 had been hospitalized as of July 23.
O’Connell said data on Native Americans’ vaccination rates is imperfect but that it suggests a lower percentage of them have received measles shots than the overall U.S. population.
The limited national data on measles vaccination rates for Native Americans is based on small surveys of people who self-identify as Native American. Some show that Native Americans have slightly lower measles vaccination rates, while others show significant gaps.
Data from some states, including South Dakota and Montana, shows that Native Americans are less likely than white children to be vaccinated on schedule.
The national measles vaccination rate is significantly lower for Native Americans who use the mostly rural Indian Health Service. About 76% of children 16 to 27 months old had gotten the first shot, according to data collected by the agency during recent patient visits at 156 clinics. That’s a 10-percentage-point drop from 10 years ago.
But the IHS data shows that its patients are at least as likely as other children to have received both recommended measles shots by the time they’re 17. O’Connell said it’s unclear if currently unvaccinated patients will continue the trend of eventually getting up to date on their shots or if they will remain unvaccinated.
The immunization rate is probably higher for older children since schools require students to get vaccinated unless they have an exemption, Brown said. He said it’s important that parents get their children vaccinated on time, when they’re young and more at risk of being hospitalized or dying from the disease.
Native Americans may have lower vaccination rates due to the challenges they face in accessing shots and other health care, O’Connell said. Those on rural reservations may be an hour or more from a clinic. Or, like Palmier, they may not have reliable transportation.
Another reason, O’Connell said, is that some Native Americans distrust the Indian Health Service, which is chronically underfunded and understaffed. If the only nearby health care facility is run by the agency, patients may delay or skip care.
O’Connell and Brown said vaccine skepticism and mistrust of the entire health care system are growing in Native American communities, as has occurred elsewhere nationwide.
“Prior to social media, I think our population was pretty trustful of childhood vaccination. And American Indians have a long history of being severely impacted by infectious disease,” he said.
European colonizers’ arrival in the late 1400s brought new diseases, including measles, that killed tens of millions of Indigenous people in North and South America by the early 1600s. Native Americans have also had high mortality rates in modern pandemics, including the 1918-20 Spanish flu and COVID-19.
The Great Plains Tribal Leaders’ Health Board reacted quickly when measles cases began showing up near its headquarters in South Dakota this year. Nebraska health officials announced in late May that a child had measles in a rural part of the state, close to the Pine Ridge Indian Reservation. Then, four people from the Rapid City area got sick later that month and into the middle of June.
“Our phones really rang off the hook” once that news came out, said Darren Crowe, a vice president at the board’s Oyate Health Center in Rapid City. He said parents wanted to know if their children were up to date on their measles vaccines.
Crowe said the health board ordered extra masks, created a measles command team that meets daily, and called parents when its online database showed their children needed a shot.
Brown praised that approach.
“It takes a concerted outreach effort that goes individual to individual,” he said, adding that his organization helped the Mississippi Band of Choctaw Indians and the Alabama-Coushatta Tribe of Texas with similar efforts.
Brown said reaching specific families can be a challenge in some low-income Native American communities, where many people’s phone numbers frequently change since they use temporary prepaid plans.
Once a health worker reaches a parent, Brown said, they should listen and ask questions before sharing the importance of the vaccine against measles, mumps, and rubella.
“Rather than trying to preach to somebody and beat them over the head with data or whatever to convince them that this is what they need to do, you start out by finding out where they are,” he said. “So, ‘Tell me about your experience with vaccination. Tell me what you know about vaccination.’”
Most people agree to immunize their children when presented with helpful information in a nonjudgmental way, Brown said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
RAPID CITY, S.D. — Cassandra Palmier had been meaning to get her son the second and final dose of the measles vaccine. But car problems made it difficult to get to the doctor.
So she pounced on the opportunity to get him vaccinated after learning that a mobile clinic would be visiting her neighborhood.
“I was definitely concerned about the epidemic and the measles,” Palmier, a member of the Oglala Sioux Tribe, said at the June event. “I wanted to do my part.”
So did her son, Makaito Cuny.
“I’m not going to be scared,” the 5-year-old announced as he walked onto the bus containing the clinic and hopped into an exam chair.
Makaito sat still as a nurse gave him the shot in his arm. “I did it!” he said while smiling at his mother.
The vaccine clinic was hosted by the Great Plains Tribal Leaders’ Health Board, which serves tribes across Iowa, Nebraska, and the Dakotas. It’s one way Native American tribes and organizations are responding to concerns about low measles vaccination rates and patients’ difficulty accessing health care as the disease spreads across the country.
Meghan O’Connell, the board’s chief public health officer, said it is also working with tribes that want to host vaccine clinics.
Elsewhere, tribal health organizations have launched social media campaigns, are making sure health providers are vaccinated, and are reaching out to the parents of unvaccinated children.
This spring, Project ECHO at the University of New Mexico hosted an online video series about measles aimed at health care professionals and organizations that serve Native American communities. The presenters outlined the basics of measles diagnosis and treatment, discussed culturally relevant communication strategies, and shared how tribes are responding to the outbreak.
Participants also strategized about ways to improve vaccination rates, said Harry Brown, a physician and an epidemiologist for the United South and Eastern Tribes, a nonprofit that works with 33 tribes in the Atlantic Coast and Southeast regions.
“It’s a pretty hot topic right now in Indian Country and I think a lot of people are being proactive,” he said.
Measles can survive for up to two hours in the air in a space where an infected person has been, sickening up to 90% of people who aren’t vaccinated, according to the Centers for Disease Control and Prevention.
The U.S. has had 1,319 confirmed cases of measles this year as of July 23, according to the CDC. It’s the largest outbreak in the U.S. since 1992. Ninety-two percent of the 2025 cases involve unvaccinated patients or people with an unknown vaccination status. Three people had died in the U.S. and 165 had been hospitalized as of July 23.
O’Connell said data on Native Americans’ vaccination rates is imperfect but that it suggests a lower percentage of them have received measles shots than the overall U.S. population.
The limited national data on measles vaccination rates for Native Americans is based on small surveys of people who self-identify as Native American. Some show that Native Americans have slightly lower measles vaccination rates, while others show significant gaps.
Data from some states, including South Dakota and Montana, shows that Native Americans are less likely than white children to be vaccinated on schedule.
The national measles vaccination rate is significantly lower for Native Americans who use the mostly rural Indian Health Service. About 76% of children 16 to 27 months old had gotten the first shot, according to data collected by the agency during recent patient visits at 156 clinics. That’s a 10-percentage-point drop from 10 years ago.
But the IHS data shows that its patients are at least as likely as other children to have received both recommended measles shots by the time they’re 17. O’Connell said it’s unclear if currently unvaccinated patients will continue the trend of eventually getting up to date on their shots or if they will remain unvaccinated.
The immunization rate is probably higher for older children since schools require students to get vaccinated unless they have an exemption, Brown said. He said it’s important that parents get their children vaccinated on time, when they’re young and more at risk of being hospitalized or dying from the disease.
Native Americans may have lower vaccination rates due to the challenges they face in accessing shots and other health care, O’Connell said. Those on rural reservations may be an hour or more from a clinic. Or, like Palmier, they may not have reliable transportation.
Another reason, O’Connell said, is that some Native Americans distrust the Indian Health Service, which is chronically underfunded and understaffed. If the only nearby health care facility is run by the agency, patients may delay or skip care.
O’Connell and Brown said vaccine skepticism and mistrust of the entire health care system are growing in Native American communities, as has occurred elsewhere nationwide.
“Prior to social media, I think our population was pretty trustful of childhood vaccination. And American Indians have a long history of being severely impacted by infectious disease,” he said.
European colonizers’ arrival in the late 1400s brought new diseases, including measles, that killed tens of millions of Indigenous people in North and South America by the early 1600s. Native Americans have also had high mortality rates in modern pandemics, including the 1918-20 Spanish flu and COVID-19.
The Great Plains Tribal Leaders’ Health Board reacted quickly when measles cases began showing up near its headquarters in South Dakota this year. Nebraska health officials announced in late May that a child had measles in a rural part of the state, close to the Pine Ridge Indian Reservation. Then, four people from the Rapid City area got sick later that month and into the middle of June.
“Our phones really rang off the hook” once that news came out, said Darren Crowe, a vice president at the board’s Oyate Health Center in Rapid City. He said parents wanted to know if their children were up to date on their measles vaccines.
Crowe said the health board ordered extra masks, created a measles command team that meets daily, and called parents when its online database showed their children needed a shot.
Brown praised that approach.
“It takes a concerted outreach effort that goes individual to individual,” he said, adding that his organization helped the Mississippi Band of Choctaw Indians and the Alabama-Coushatta Tribe of Texas with similar efforts.
Brown said reaching specific families can be a challenge in some low-income Native American communities, where many people’s phone numbers frequently change since they use temporary prepaid plans.
Once a health worker reaches a parent, Brown said, they should listen and ask questions before sharing the importance of the vaccine against measles, mumps, and rubella.
“Rather than trying to preach to somebody and beat them over the head with data or whatever to convince them that this is what they need to do, you start out by finding out where they are,” he said. “So, ‘Tell me about your experience with vaccination. Tell me what you know about vaccination.’”
Most people agree to immunize their children when presented with helpful information in a nonjudgmental way, Brown said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
RAPID CITY, S.D. — Cassandra Palmier had been meaning to get her son the second and final dose of the measles vaccine. But car problems made it difficult to get to the doctor.
So she pounced on the opportunity to get him vaccinated after learning that a mobile clinic would be visiting her neighborhood.
“I was definitely concerned about the epidemic and the measles,” Palmier, a member of the Oglala Sioux Tribe, said at the June event. “I wanted to do my part.”
So did her son, Makaito Cuny.
“I’m not going to be scared,” the 5-year-old announced as he walked onto the bus containing the clinic and hopped into an exam chair.
Makaito sat still as a nurse gave him the shot in his arm. “I did it!” he said while smiling at his mother.
The vaccine clinic was hosted by the Great Plains Tribal Leaders’ Health Board, which serves tribes across Iowa, Nebraska, and the Dakotas. It’s one way Native American tribes and organizations are responding to concerns about low measles vaccination rates and patients’ difficulty accessing health care as the disease spreads across the country.
Meghan O’Connell, the board’s chief public health officer, said it is also working with tribes that want to host vaccine clinics.
Elsewhere, tribal health organizations have launched social media campaigns, are making sure health providers are vaccinated, and are reaching out to the parents of unvaccinated children.
This spring, Project ECHO at the University of New Mexico hosted an online video series about measles aimed at health care professionals and organizations that serve Native American communities. The presenters outlined the basics of measles diagnosis and treatment, discussed culturally relevant communication strategies, and shared how tribes are responding to the outbreak.
Participants also strategized about ways to improve vaccination rates, said Harry Brown, a physician and an epidemiologist for the United South and Eastern Tribes, a nonprofit that works with 33 tribes in the Atlantic Coast and Southeast regions.
“It’s a pretty hot topic right now in Indian Country and I think a lot of people are being proactive,” he said.
Measles can survive for up to two hours in the air in a space where an infected person has been, sickening up to 90% of people who aren’t vaccinated, according to the Centers for Disease Control and Prevention.
The U.S. has had 1,319 confirmed cases of measles this year as of July 23, according to the CDC. It’s the largest outbreak in the U.S. since 1992. Ninety-two percent of the 2025 cases involve unvaccinated patients or people with an unknown vaccination status. Three people had died in the U.S. and 165 had been hospitalized as of July 23.
O’Connell said data on Native Americans’ vaccination rates is imperfect but that it suggests a lower percentage of them have received measles shots than the overall U.S. population.
The limited national data on measles vaccination rates for Native Americans is based on small surveys of people who self-identify as Native American. Some show that Native Americans have slightly lower measles vaccination rates, while others show significant gaps.
Data from some states, including South Dakota and Montana, shows that Native Americans are less likely than white children to be vaccinated on schedule.
The national measles vaccination rate is significantly lower for Native Americans who use the mostly rural Indian Health Service. About 76% of children 16 to 27 months old had gotten the first shot, according to data collected by the agency during recent patient visits at 156 clinics. That’s a 10-percentage-point drop from 10 years ago.
But the IHS data shows that its patients are at least as likely as other children to have received both recommended measles shots by the time they’re 17. O’Connell said it’s unclear if currently unvaccinated patients will continue the trend of eventually getting up to date on their shots or if they will remain unvaccinated.
The immunization rate is probably higher for older children since schools require students to get vaccinated unless they have an exemption, Brown said. He said it’s important that parents get their children vaccinated on time, when they’re young and more at risk of being hospitalized or dying from the disease.
Native Americans may have lower vaccination rates due to the challenges they face in accessing shots and other health care, O’Connell said. Those on rural reservations may be an hour or more from a clinic. Or, like Palmier, they may not have reliable transportation.
Another reason, O’Connell said, is that some Native Americans distrust the Indian Health Service, which is chronically underfunded and understaffed. If the only nearby health care facility is run by the agency, patients may delay or skip care.
O’Connell and Brown said vaccine skepticism and mistrust of the entire health care system are growing in Native American communities, as has occurred elsewhere nationwide.
“Prior to social media, I think our population was pretty trustful of childhood vaccination. And American Indians have a long history of being severely impacted by infectious disease,” he said.
European colonizers’ arrival in the late 1400s brought new diseases, including measles, that killed tens of millions of Indigenous people in North and South America by the early 1600s. Native Americans have also had high mortality rates in modern pandemics, including the 1918-20 Spanish flu and COVID-19.
The Great Plains Tribal Leaders’ Health Board reacted quickly when measles cases began showing up near its headquarters in South Dakota this year. Nebraska health officials announced in late May that a child had measles in a rural part of the state, close to the Pine Ridge Indian Reservation. Then, four people from the Rapid City area got sick later that month and into the middle of June.
“Our phones really rang off the hook” once that news came out, said Darren Crowe, a vice president at the board’s Oyate Health Center in Rapid City. He said parents wanted to know if their children were up to date on their measles vaccines.
Crowe said the health board ordered extra masks, created a measles command team that meets daily, and called parents when its online database showed their children needed a shot.
Brown praised that approach.
“It takes a concerted outreach effort that goes individual to individual,” he said, adding that his organization helped the Mississippi Band of Choctaw Indians and the Alabama-Coushatta Tribe of Texas with similar efforts.
Brown said reaching specific families can be a challenge in some low-income Native American communities, where many people’s phone numbers frequently change since they use temporary prepaid plans.
Once a health worker reaches a parent, Brown said, they should listen and ask questions before sharing the importance of the vaccine against measles, mumps, and rubella.
“Rather than trying to preach to somebody and beat them over the head with data or whatever to convince them that this is what they need to do, you start out by finding out where they are,” he said. “So, ‘Tell me about your experience with vaccination. Tell me what you know about vaccination.’”
Most people agree to immunize their children when presented with helpful information in a nonjudgmental way, Brown said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Federal Health Care Data Trends 2025
Federal Health Care Data Trends 2025
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends 2025
Federal Health Care Data Trends 2025
Pedunculated Pink Papule on the Nose
THE DIAGNOSIS: Pedunculated Lipofibroma
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.
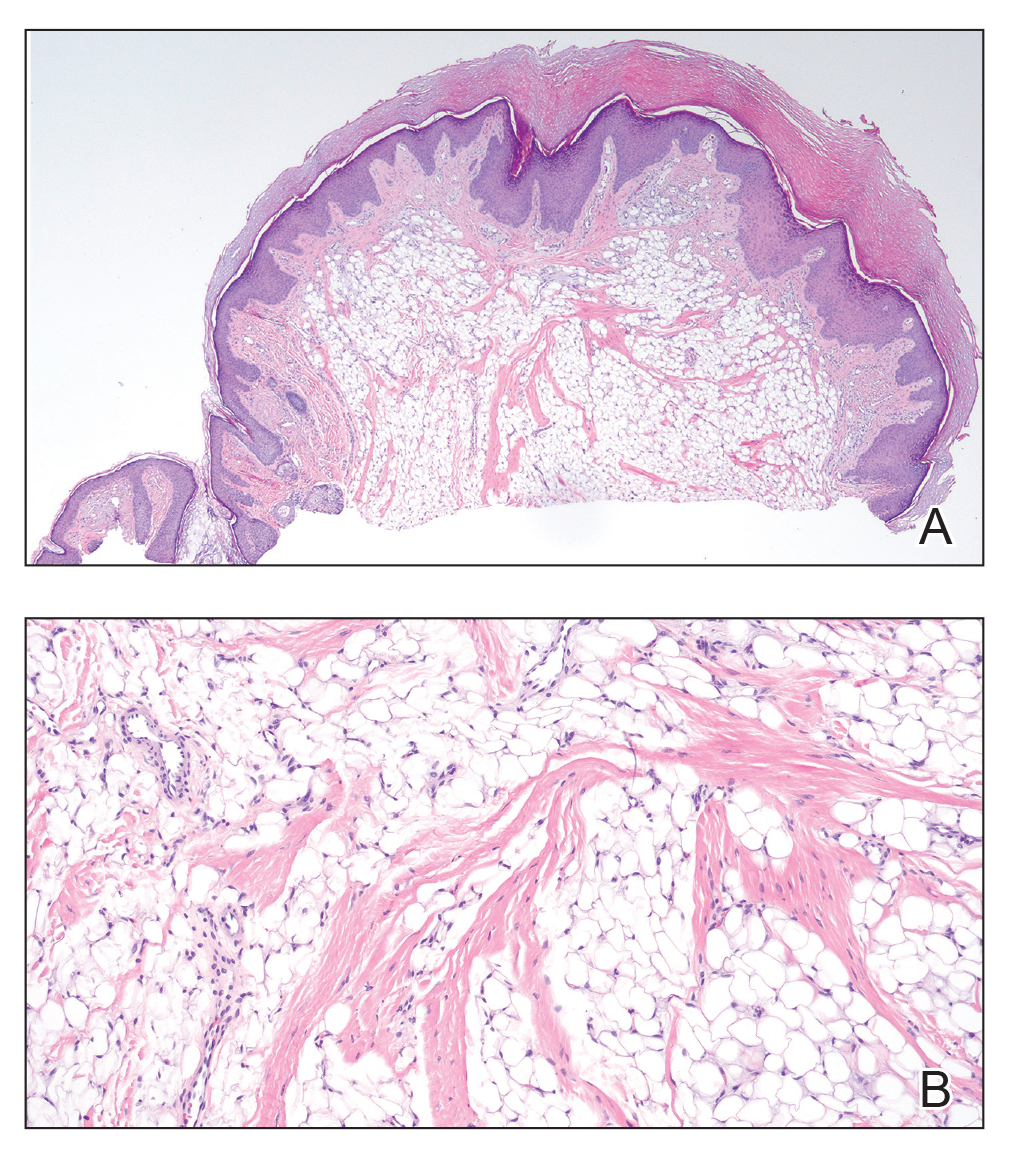
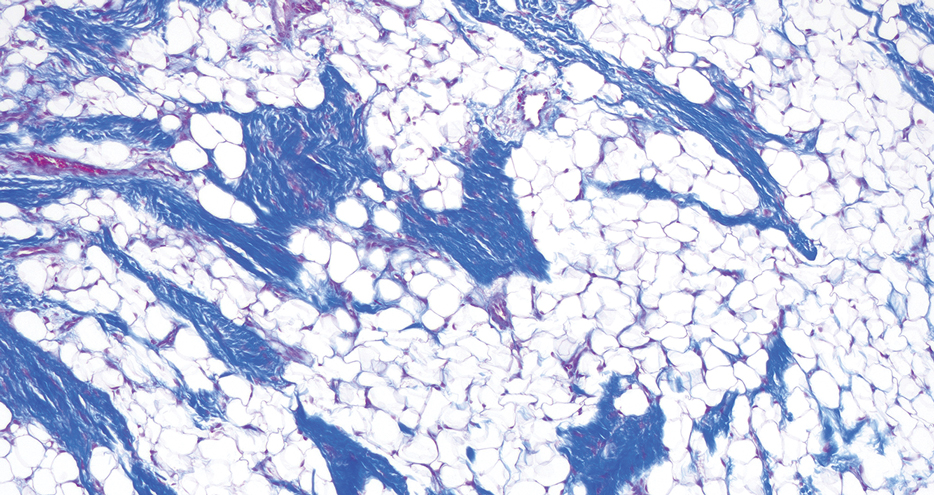
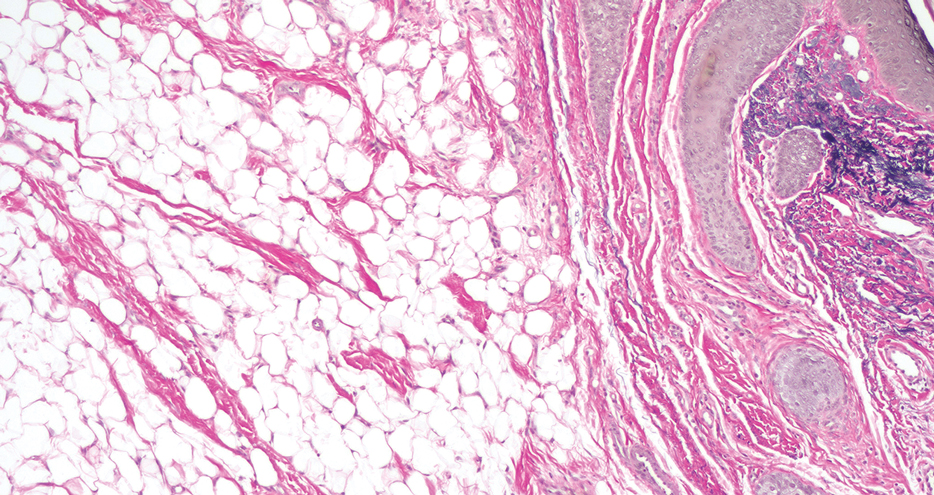
Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
THE DIAGNOSIS: Pedunculated Lipofibroma
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.



Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
THE DIAGNOSIS: Pedunculated Lipofibroma
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.



Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
A 60-year-old woman presented to the dermatology department with a 6-mm, firm, pink, nonulcerated, nonmobile papule on the right nasal side wall of 1 year’s duration. It had grown slowly and was asymptomatic with no tenderness or bleeding. No other skin lesions were noted on physical examination, and her medical history was otherwise unremarkable. A shave biopsy was performed.