User login
PCPs lag on albuminuria tests in patients with type 2 diabetes
U.S. primary care physicians are not properly checking patients with type 2 diabetes for chronic kidney disease (CKD) nearly as often as they should, meaning many of these patients miss getting a timely diagnosis.
Inadequate measurement of urinary albumin-to-creatinine ratio (uACR) is the issue.
Review of data from more than half a million U.S. primary care patients with type 2 diabetes seen at any of 1,164 practice sites run by any of 24 health care organizations during 2016-2019 showed that barely more than half, 52%, had both their uACR and estimated glomerular filtration rate (eGFR) checked annually as recommended by several U.S. medical societies, and just 73% had both values checked during a 3-year period, Nikita Stempniewicz, MSc, and associates reported in Diabetes Care.
More detailed data showed that measurement of eGFR was reasonably robust, measured at a 90% rate annually and in 97% of patients at least once every 3 years. But recording uACR values lagged, with a 53% annual rate and a 74% rate of measurement at least once every 3 years, reported Mr. Stempniewicz, director of research and analytics for the American Medical Group Association, a trade association based in Alexandria, Va. The 24 health care organizations that supplied the study’s data are all members of this association.
Prevailing recommendations from various medical societies call for annual monitoring of urinary albumin in patients with type 2 diabetes and specify the uACR, such as in the Standards of Medical Care in Diabetes from the American Diabetes Association, as well as in recommendations promoted by the National Kidney Foundation.
Missing half the CKD patients with eGFR only
“Half the patients with type 2 diabetes and chronic kidney disease have elevated albuminuria without decreased eGFR and would not be detected with eGFR testing alone,” Mr. Stempniewicz noted in an interview.
“Many patients who present for nephrology care are incompletely assessed with only low eGFR but no urine testing. Missing albuminuria testing and uACR values means patients with high levels of albuminuria but normal kidney function go undetected and thus are not able to benefit from evidenced-based interventions, including nephrology services,” said Joseph A. Vassalotti, MD, a nephrologist, chief medical officer for the National Kidney Foundation, and a coauthor of the report.
Not testing patients with type 2 diabetes regularly for their uACR “is a missed opportunity to identify the highest-risk patients and treat them,” added Josef Coresh, MD, PhD, a professor of clinical epidemiology at Johns Hopkins University, Baltimore, and senior author on the study. Measurement of albuminuria is especially important for these patients because medications from the sodium-glucose cotransporter 2 inhibitor class have been proven to slow progression of CKD in patients with type 2 diabetes, but these drugs are expensive, and in some cases have labeling that specifies the presence of albuminuria.
“I have no doubt that improving albuminuria testing is a critical step to identify patients with diabetes at highest risk who should get the best treatment possible, including SGLT2 inhibitors,” Dr. Coresh said in an interview.
The new report is not the first to document inadequate assessment of albuminuria and uACR among primary care physicians (PCPs), but it came from the largest reported U.S. study to date. “eGFR is commonly collected in a routine laboratory blood panel, but collecting urine requires additional work flow,” noted Cara B. Litvin, MD, a general internal medicine researcher at the Medical University of South Carolina, Charleston, who has tested interventions aimed at boosting CKD assessment by PCPs and was not involved in the new study.
“There have also been conflicting guidelines,” such as a “now-inactive guideline from the American College of Physicians that recommended against routine urine albumin screening in patients with diabetes and already on treatment with an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker,” she said.
New renal drugs change the stakes
The availability of newer drugs for slowing CKD progression such as the SGLT2 inhibitors will help trigger greater support for routine albuminuria testing, Dr. Litvin predicted in an interview. “Now that we have more medications that can reduce albuminuria and improve outcomes, I see screening for albuminuria increasing.” Finerenone (Kerendia) is another new agent from a new class that recently received Food and Drug Administration approval for treating CKD in patients with type 2 diabetes.
Other drivers of increased uACR testing she expects include revised clinical practice guidelines, and new quality measures of clinical care.
“Undertesting of albuminuria means that [nephrologists] have incomplete data to detect and completely risk stratify the CKD population. That in turn results in a reduced ability to match population health interventions to the severity of the condition or the risk stratification based on eGFR and uACR,” Dr. Vassalotti said in an interview.
“We are missing opportunities to prevent or delay kidney failure and reduce the risk of cardiovascular events and cardiovascular death in these patients, particularly now that we have a number of medications that offer kidney and cardiovascular protection such as SGLT2 inhibitors,” he added. “Leaders in nephrology are beginning to understand the consequences of undertesting, and are working to innovate to improve risk stratification, CKD detection, and apply interventions to give Americans living with CKD better outcomes.”
Strategies proven to boost albuminuria testing
Mr. Stempniewicz and coauthors cited in their report potential strategies for improving albuminuria testing, including benchmarking to identify best-performing sites for albumin testing within a health system and encouraging replication of identified best practices at lower-performing sites, and implementation of clinical-decision support tools in the EHR such as pop-up test reminders.
These were among the tools tested in two studies led by Dr. Litvin. One study, with results reported in 2016, involved 12 small U.S. primary care practices with a total of more than 30,000 patients and compared performance in a series of clinical quality measures at baseline with performance after 2 years of receiving various interventions designed to boost awareness for albuminuria testing.
The second study, with findings reported in 2019, involved 21 U.S. primary care practices that collectively cared for more than 100,000 patients and randomized the practices to either undergo interventions aimed at boosting testing awareness or to serve as controls.
Results from both studies showed significant and substantial increases in serial testing for albuminuria in patients with diabetes or hypertension when practices received the interventions.
“We showed that [using a] clinical-decision support tool, along with standing orders to automatically collect urine specimens, dramatically increased screening for urinary albumin in primary care practices,” Dr. Litvin said. “However, perhaps because of conflicting guidelines and clinical inertia there hasn’t been a major impetus for primary care practices in general to improve screening.” She hopes that will quickly change.
“As we have shown, adoption of EHR-based reminders along with standing orders can very quickly improve screening for albuminuria in primary care.”
Variation in testing rates among sites ‘tremendous’
One finding of the new study gives Mr. Stempniewicz hope for greater future testing: The large variance that the researchers saw in albuminuria testing rates within individual health systems.
“The paper shows that higher rates of testing are completely achievable within each system. Some clinics do very well, and the other units can learn from these local successes,” he said. At least half the organizations in the study had individual sites that fell into the top 10% for testing rates across all the greater than 1,000 sites included, and those same organizations also had at least one site that fell into the bottom 10% for testing.
“The variation is tremendous, and highlights an opportunity for improvement,” declared Mr. Stempniewicz.
“For routine testing, you need systems that help people. Clinicians shouldn’t have to think about doing routine testing. It should just happen,” said Dr. Coresh.
The study was funded in part by Janssen. Mr. Stempniewicz and Dr. Litvin had no disclosures. Dr. Coresh is an adviser to Healthy.io, a company that markets a home albuminuria testing kit to patients. Dr. Vassalotti has received personal fees from Renalytix.
U.S. primary care physicians are not properly checking patients with type 2 diabetes for chronic kidney disease (CKD) nearly as often as they should, meaning many of these patients miss getting a timely diagnosis.
Inadequate measurement of urinary albumin-to-creatinine ratio (uACR) is the issue.
Review of data from more than half a million U.S. primary care patients with type 2 diabetes seen at any of 1,164 practice sites run by any of 24 health care organizations during 2016-2019 showed that barely more than half, 52%, had both their uACR and estimated glomerular filtration rate (eGFR) checked annually as recommended by several U.S. medical societies, and just 73% had both values checked during a 3-year period, Nikita Stempniewicz, MSc, and associates reported in Diabetes Care.
More detailed data showed that measurement of eGFR was reasonably robust, measured at a 90% rate annually and in 97% of patients at least once every 3 years. But recording uACR values lagged, with a 53% annual rate and a 74% rate of measurement at least once every 3 years, reported Mr. Stempniewicz, director of research and analytics for the American Medical Group Association, a trade association based in Alexandria, Va. The 24 health care organizations that supplied the study’s data are all members of this association.
Prevailing recommendations from various medical societies call for annual monitoring of urinary albumin in patients with type 2 diabetes and specify the uACR, such as in the Standards of Medical Care in Diabetes from the American Diabetes Association, as well as in recommendations promoted by the National Kidney Foundation.
Missing half the CKD patients with eGFR only
“Half the patients with type 2 diabetes and chronic kidney disease have elevated albuminuria without decreased eGFR and would not be detected with eGFR testing alone,” Mr. Stempniewicz noted in an interview.
“Many patients who present for nephrology care are incompletely assessed with only low eGFR but no urine testing. Missing albuminuria testing and uACR values means patients with high levels of albuminuria but normal kidney function go undetected and thus are not able to benefit from evidenced-based interventions, including nephrology services,” said Joseph A. Vassalotti, MD, a nephrologist, chief medical officer for the National Kidney Foundation, and a coauthor of the report.
Not testing patients with type 2 diabetes regularly for their uACR “is a missed opportunity to identify the highest-risk patients and treat them,” added Josef Coresh, MD, PhD, a professor of clinical epidemiology at Johns Hopkins University, Baltimore, and senior author on the study. Measurement of albuminuria is especially important for these patients because medications from the sodium-glucose cotransporter 2 inhibitor class have been proven to slow progression of CKD in patients with type 2 diabetes, but these drugs are expensive, and in some cases have labeling that specifies the presence of albuminuria.
“I have no doubt that improving albuminuria testing is a critical step to identify patients with diabetes at highest risk who should get the best treatment possible, including SGLT2 inhibitors,” Dr. Coresh said in an interview.
The new report is not the first to document inadequate assessment of albuminuria and uACR among primary care physicians (PCPs), but it came from the largest reported U.S. study to date. “eGFR is commonly collected in a routine laboratory blood panel, but collecting urine requires additional work flow,” noted Cara B. Litvin, MD, a general internal medicine researcher at the Medical University of South Carolina, Charleston, who has tested interventions aimed at boosting CKD assessment by PCPs and was not involved in the new study.
“There have also been conflicting guidelines,” such as a “now-inactive guideline from the American College of Physicians that recommended against routine urine albumin screening in patients with diabetes and already on treatment with an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker,” she said.
New renal drugs change the stakes
The availability of newer drugs for slowing CKD progression such as the SGLT2 inhibitors will help trigger greater support for routine albuminuria testing, Dr. Litvin predicted in an interview. “Now that we have more medications that can reduce albuminuria and improve outcomes, I see screening for albuminuria increasing.” Finerenone (Kerendia) is another new agent from a new class that recently received Food and Drug Administration approval for treating CKD in patients with type 2 diabetes.
Other drivers of increased uACR testing she expects include revised clinical practice guidelines, and new quality measures of clinical care.
“Undertesting of albuminuria means that [nephrologists] have incomplete data to detect and completely risk stratify the CKD population. That in turn results in a reduced ability to match population health interventions to the severity of the condition or the risk stratification based on eGFR and uACR,” Dr. Vassalotti said in an interview.
“We are missing opportunities to prevent or delay kidney failure and reduce the risk of cardiovascular events and cardiovascular death in these patients, particularly now that we have a number of medications that offer kidney and cardiovascular protection such as SGLT2 inhibitors,” he added. “Leaders in nephrology are beginning to understand the consequences of undertesting, and are working to innovate to improve risk stratification, CKD detection, and apply interventions to give Americans living with CKD better outcomes.”
Strategies proven to boost albuminuria testing
Mr. Stempniewicz and coauthors cited in their report potential strategies for improving albuminuria testing, including benchmarking to identify best-performing sites for albumin testing within a health system and encouraging replication of identified best practices at lower-performing sites, and implementation of clinical-decision support tools in the EHR such as pop-up test reminders.
These were among the tools tested in two studies led by Dr. Litvin. One study, with results reported in 2016, involved 12 small U.S. primary care practices with a total of more than 30,000 patients and compared performance in a series of clinical quality measures at baseline with performance after 2 years of receiving various interventions designed to boost awareness for albuminuria testing.
The second study, with findings reported in 2019, involved 21 U.S. primary care practices that collectively cared for more than 100,000 patients and randomized the practices to either undergo interventions aimed at boosting testing awareness or to serve as controls.
Results from both studies showed significant and substantial increases in serial testing for albuminuria in patients with diabetes or hypertension when practices received the interventions.
“We showed that [using a] clinical-decision support tool, along with standing orders to automatically collect urine specimens, dramatically increased screening for urinary albumin in primary care practices,” Dr. Litvin said. “However, perhaps because of conflicting guidelines and clinical inertia there hasn’t been a major impetus for primary care practices in general to improve screening.” She hopes that will quickly change.
“As we have shown, adoption of EHR-based reminders along with standing orders can very quickly improve screening for albuminuria in primary care.”
Variation in testing rates among sites ‘tremendous’
One finding of the new study gives Mr. Stempniewicz hope for greater future testing: The large variance that the researchers saw in albuminuria testing rates within individual health systems.
“The paper shows that higher rates of testing are completely achievable within each system. Some clinics do very well, and the other units can learn from these local successes,” he said. At least half the organizations in the study had individual sites that fell into the top 10% for testing rates across all the greater than 1,000 sites included, and those same organizations also had at least one site that fell into the bottom 10% for testing.
“The variation is tremendous, and highlights an opportunity for improvement,” declared Mr. Stempniewicz.
“For routine testing, you need systems that help people. Clinicians shouldn’t have to think about doing routine testing. It should just happen,” said Dr. Coresh.
The study was funded in part by Janssen. Mr. Stempniewicz and Dr. Litvin had no disclosures. Dr. Coresh is an adviser to Healthy.io, a company that markets a home albuminuria testing kit to patients. Dr. Vassalotti has received personal fees from Renalytix.
U.S. primary care physicians are not properly checking patients with type 2 diabetes for chronic kidney disease (CKD) nearly as often as they should, meaning many of these patients miss getting a timely diagnosis.
Inadequate measurement of urinary albumin-to-creatinine ratio (uACR) is the issue.
Review of data from more than half a million U.S. primary care patients with type 2 diabetes seen at any of 1,164 practice sites run by any of 24 health care organizations during 2016-2019 showed that barely more than half, 52%, had both their uACR and estimated glomerular filtration rate (eGFR) checked annually as recommended by several U.S. medical societies, and just 73% had both values checked during a 3-year period, Nikita Stempniewicz, MSc, and associates reported in Diabetes Care.
More detailed data showed that measurement of eGFR was reasonably robust, measured at a 90% rate annually and in 97% of patients at least once every 3 years. But recording uACR values lagged, with a 53% annual rate and a 74% rate of measurement at least once every 3 years, reported Mr. Stempniewicz, director of research and analytics for the American Medical Group Association, a trade association based in Alexandria, Va. The 24 health care organizations that supplied the study’s data are all members of this association.
Prevailing recommendations from various medical societies call for annual monitoring of urinary albumin in patients with type 2 diabetes and specify the uACR, such as in the Standards of Medical Care in Diabetes from the American Diabetes Association, as well as in recommendations promoted by the National Kidney Foundation.
Missing half the CKD patients with eGFR only
“Half the patients with type 2 diabetes and chronic kidney disease have elevated albuminuria without decreased eGFR and would not be detected with eGFR testing alone,” Mr. Stempniewicz noted in an interview.
“Many patients who present for nephrology care are incompletely assessed with only low eGFR but no urine testing. Missing albuminuria testing and uACR values means patients with high levels of albuminuria but normal kidney function go undetected and thus are not able to benefit from evidenced-based interventions, including nephrology services,” said Joseph A. Vassalotti, MD, a nephrologist, chief medical officer for the National Kidney Foundation, and a coauthor of the report.
Not testing patients with type 2 diabetes regularly for their uACR “is a missed opportunity to identify the highest-risk patients and treat them,” added Josef Coresh, MD, PhD, a professor of clinical epidemiology at Johns Hopkins University, Baltimore, and senior author on the study. Measurement of albuminuria is especially important for these patients because medications from the sodium-glucose cotransporter 2 inhibitor class have been proven to slow progression of CKD in patients with type 2 diabetes, but these drugs are expensive, and in some cases have labeling that specifies the presence of albuminuria.
“I have no doubt that improving albuminuria testing is a critical step to identify patients with diabetes at highest risk who should get the best treatment possible, including SGLT2 inhibitors,” Dr. Coresh said in an interview.
The new report is not the first to document inadequate assessment of albuminuria and uACR among primary care physicians (PCPs), but it came from the largest reported U.S. study to date. “eGFR is commonly collected in a routine laboratory blood panel, but collecting urine requires additional work flow,” noted Cara B. Litvin, MD, a general internal medicine researcher at the Medical University of South Carolina, Charleston, who has tested interventions aimed at boosting CKD assessment by PCPs and was not involved in the new study.
“There have also been conflicting guidelines,” such as a “now-inactive guideline from the American College of Physicians that recommended against routine urine albumin screening in patients with diabetes and already on treatment with an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker,” she said.
New renal drugs change the stakes
The availability of newer drugs for slowing CKD progression such as the SGLT2 inhibitors will help trigger greater support for routine albuminuria testing, Dr. Litvin predicted in an interview. “Now that we have more medications that can reduce albuminuria and improve outcomes, I see screening for albuminuria increasing.” Finerenone (Kerendia) is another new agent from a new class that recently received Food and Drug Administration approval for treating CKD in patients with type 2 diabetes.
Other drivers of increased uACR testing she expects include revised clinical practice guidelines, and new quality measures of clinical care.
“Undertesting of albuminuria means that [nephrologists] have incomplete data to detect and completely risk stratify the CKD population. That in turn results in a reduced ability to match population health interventions to the severity of the condition or the risk stratification based on eGFR and uACR,” Dr. Vassalotti said in an interview.
“We are missing opportunities to prevent or delay kidney failure and reduce the risk of cardiovascular events and cardiovascular death in these patients, particularly now that we have a number of medications that offer kidney and cardiovascular protection such as SGLT2 inhibitors,” he added. “Leaders in nephrology are beginning to understand the consequences of undertesting, and are working to innovate to improve risk stratification, CKD detection, and apply interventions to give Americans living with CKD better outcomes.”
Strategies proven to boost albuminuria testing
Mr. Stempniewicz and coauthors cited in their report potential strategies for improving albuminuria testing, including benchmarking to identify best-performing sites for albumin testing within a health system and encouraging replication of identified best practices at lower-performing sites, and implementation of clinical-decision support tools in the EHR such as pop-up test reminders.
These were among the tools tested in two studies led by Dr. Litvin. One study, with results reported in 2016, involved 12 small U.S. primary care practices with a total of more than 30,000 patients and compared performance in a series of clinical quality measures at baseline with performance after 2 years of receiving various interventions designed to boost awareness for albuminuria testing.
The second study, with findings reported in 2019, involved 21 U.S. primary care practices that collectively cared for more than 100,000 patients and randomized the practices to either undergo interventions aimed at boosting testing awareness or to serve as controls.
Results from both studies showed significant and substantial increases in serial testing for albuminuria in patients with diabetes or hypertension when practices received the interventions.
“We showed that [using a] clinical-decision support tool, along with standing orders to automatically collect urine specimens, dramatically increased screening for urinary albumin in primary care practices,” Dr. Litvin said. “However, perhaps because of conflicting guidelines and clinical inertia there hasn’t been a major impetus for primary care practices in general to improve screening.” She hopes that will quickly change.
“As we have shown, adoption of EHR-based reminders along with standing orders can very quickly improve screening for albuminuria in primary care.”
Variation in testing rates among sites ‘tremendous’
One finding of the new study gives Mr. Stempniewicz hope for greater future testing: The large variance that the researchers saw in albuminuria testing rates within individual health systems.
“The paper shows that higher rates of testing are completely achievable within each system. Some clinics do very well, and the other units can learn from these local successes,” he said. At least half the organizations in the study had individual sites that fell into the top 10% for testing rates across all the greater than 1,000 sites included, and those same organizations also had at least one site that fell into the bottom 10% for testing.
“The variation is tremendous, and highlights an opportunity for improvement,” declared Mr. Stempniewicz.
“For routine testing, you need systems that help people. Clinicians shouldn’t have to think about doing routine testing. It should just happen,” said Dr. Coresh.
The study was funded in part by Janssen. Mr. Stempniewicz and Dr. Litvin had no disclosures. Dr. Coresh is an adviser to Healthy.io, a company that markets a home albuminuria testing kit to patients. Dr. Vassalotti has received personal fees from Renalytix.
FROM DIABETES CARE
No prehydration prior to contrast-enhanced CT in patients with stage 3 CKD
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Dapagliflozin safe, protective in advanced kidney disease
Patients with stage 4 chronic kidney disease (CKD) who were in the DAPA-CKD trial had cardiorenal benefits from dapagliflozin that were similar to those of patients in the overall trial, with no added safety signal.
DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) was a landmark study of more than 4,000 patients with CKD, with an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 and albuminuria with/without type 2 diabetes.
The primary results showed that patients who received the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin for a median of 2.4 years were significantly less likely to have worsening kidney disease or die from all causes than were patients who received placebo.
“This prespecified subanalysis of people with an eGFR < 30 mL/min/1.73 m2 [stage 4 CKD] in the DAPA-CKD study shows first, that in this very vulnerable population, use of the SGLT2 inhibitor is safe,” said Chantal Mathieu, MD, PhD.
Furthermore, there was no signal whatsoever of more adverse events and even a trend to fewer events, she said in an email to this news organization.
The analysis also showed that “although now in small numbers (around 300 each in the treated group vs. placebo group), there is no suggestion that the protective effect of dapagliflozin on the renal and cardiovascular front would not happen in this group” with advanced CKD. The efficacy findings just missed statistical significance, noted Dr. Mathieu, of Catholic University, Leuven, Belgium, who was not involved in the study.
Although dapagliflozin is now approved for treating patients with CKD who are at risk of kidney disease progression (on the basis of the DAPA-CKD results), guidelines have not yet been updated to reflect this, lead investigator Glenn M. Chertow, MD, MPH, of Stanford (Calif.) University, told this news organization in an email.
“For clinicians,” Dr. Mathieu said, “this is now the absolute reassurance that we do not have to stop an SGLT2 inhibitor in people with eGFR < 30 mL/min for safety reasons and that we should maintain them at these values for renal and cardiovascular protection!
“I absolutely hope labels will change soon to reflect these observations (and indeed movement on that front is happening),” she continued.
“The American Diabetes Association/European Association for the Study of Diabetes consensus on glucose-lowering therapies in type 2 diabetes already advocated keeping these agents until eGFR 30 mL/min (on the basis of evidence in 2019),” Dr. Mathieu added, “but this study will probably push the statements even further.”
“Of note,” she pointed out, “at these low eGFRs, the glucose-lowering potential of the SGLT2 inhibitor is negligible.”
Dapagliflozin risks and benefits in advanced CKD
Based on the DAPA-CKD study, published in the New England Journal of Medicine Oct. 8, 2020, the Food and Drug Administration expanded the indication for dapagliflozin (Farxiga, AstraZeneca) in April of 2021.
However, relatively little is known about the safety and efficacy of SGLT2 inhibitors in patients with advanced CKD, who are particularly vulnerable to cardiovascular events and progressive kidney failure, Dr. Chertow and colleagues wrote.
The DAPA-CKD trial randomized 4,304 patients with CKD 1:1 to dapagliflozin 10 mg/day or placebo, including 624 patients (14%) who had eGFR < 30 mL/min per 1.73 m2 and albuminuria at baseline.
Patients in the subgroup with advanced CKD had a mean age of 62 years, and 37% were female. About two-thirds had type 2 diabetes and about one-third had cardiovascular disease.
A total of 293 patients received dapagliflozin and 331 patients received placebo.
During a median follow-up of 2.4 years, patients who received dapagliflozin as opposed to placebo had a lower risk of the primary efficacy outcome – a composite of a 50% or greater sustained decline in eGFR, end-stage kidney disease, or death from cardiovascular or renal causes (hazard ratio, 0.73; 95% confidence interval, 0.53-1.02).
In secondary efficacy outcomes, patients who received dapagliflozin as opposed to placebo also had a lower risk of the following:
- A renal composite outcome – a ≥ 50% sustained decline in eGFR, end-stage kidney disease, or death from renal causes (HR, 0.71; 95% CI, 0.49-1.02).
- A cardiovascular composite outcome comprising cardiovascular death or hospitalization for heart failure (HR, 0.83; 95% CI, 0.45-1.53).
- All-cause mortality (HR, 0.68; 95% CI, 0.39 to 1.21).
The eGFR slope declined by 2.15 mL/min per 1.73 m2 per year and by 3.38 mL/min per 1.73 m2 per year in the dapagliflozin and placebo groups, respectively (P = .005).
“The trial was not powered to detect a statistically significant difference in the primary and key secondary endpoints in modest-sized subgroups,” the researchers noted.
The researchers limited their safety analysis to serious adverse events or symptoms of volume depletion, kidney-related events, major hypoglycemia, bone fractures, amputations, and potential diabetic ketoacidosis.
There was no evidence of increased risk of these adverse events in patients who received dapagliflozin.
The subanalysis of the DAPA-CKD trial was published July 16 in the Journal of the American Society of Nephrology.
The study was funded by AstraZeneca. Dr. Chertow has received fees from AstraZeneca for the DAPA-CKD trial steering committee. The disclosures of the other authors are listed in the article. Dr. Mathieu has served on the advisory panel/speakers bureau for AstraZeneca. Dr. Chertow and Dr. Mathieu also have financial relationships with many other pharmaceutical companies.
Patients with stage 4 chronic kidney disease (CKD) who were in the DAPA-CKD trial had cardiorenal benefits from dapagliflozin that were similar to those of patients in the overall trial, with no added safety signal.
DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) was a landmark study of more than 4,000 patients with CKD, with an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 and albuminuria with/without type 2 diabetes.
The primary results showed that patients who received the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin for a median of 2.4 years were significantly less likely to have worsening kidney disease or die from all causes than were patients who received placebo.
“This prespecified subanalysis of people with an eGFR < 30 mL/min/1.73 m2 [stage 4 CKD] in the DAPA-CKD study shows first, that in this very vulnerable population, use of the SGLT2 inhibitor is safe,” said Chantal Mathieu, MD, PhD.
Furthermore, there was no signal whatsoever of more adverse events and even a trend to fewer events, she said in an email to this news organization.
The analysis also showed that “although now in small numbers (around 300 each in the treated group vs. placebo group), there is no suggestion that the protective effect of dapagliflozin on the renal and cardiovascular front would not happen in this group” with advanced CKD. The efficacy findings just missed statistical significance, noted Dr. Mathieu, of Catholic University, Leuven, Belgium, who was not involved in the study.
Although dapagliflozin is now approved for treating patients with CKD who are at risk of kidney disease progression (on the basis of the DAPA-CKD results), guidelines have not yet been updated to reflect this, lead investigator Glenn M. Chertow, MD, MPH, of Stanford (Calif.) University, told this news organization in an email.
“For clinicians,” Dr. Mathieu said, “this is now the absolute reassurance that we do not have to stop an SGLT2 inhibitor in people with eGFR < 30 mL/min for safety reasons and that we should maintain them at these values for renal and cardiovascular protection!
“I absolutely hope labels will change soon to reflect these observations (and indeed movement on that front is happening),” she continued.
“The American Diabetes Association/European Association for the Study of Diabetes consensus on glucose-lowering therapies in type 2 diabetes already advocated keeping these agents until eGFR 30 mL/min (on the basis of evidence in 2019),” Dr. Mathieu added, “but this study will probably push the statements even further.”
“Of note,” she pointed out, “at these low eGFRs, the glucose-lowering potential of the SGLT2 inhibitor is negligible.”
Dapagliflozin risks and benefits in advanced CKD
Based on the DAPA-CKD study, published in the New England Journal of Medicine Oct. 8, 2020, the Food and Drug Administration expanded the indication for dapagliflozin (Farxiga, AstraZeneca) in April of 2021.
However, relatively little is known about the safety and efficacy of SGLT2 inhibitors in patients with advanced CKD, who are particularly vulnerable to cardiovascular events and progressive kidney failure, Dr. Chertow and colleagues wrote.
The DAPA-CKD trial randomized 4,304 patients with CKD 1:1 to dapagliflozin 10 mg/day or placebo, including 624 patients (14%) who had eGFR < 30 mL/min per 1.73 m2 and albuminuria at baseline.
Patients in the subgroup with advanced CKD had a mean age of 62 years, and 37% were female. About two-thirds had type 2 diabetes and about one-third had cardiovascular disease.
A total of 293 patients received dapagliflozin and 331 patients received placebo.
During a median follow-up of 2.4 years, patients who received dapagliflozin as opposed to placebo had a lower risk of the primary efficacy outcome – a composite of a 50% or greater sustained decline in eGFR, end-stage kidney disease, or death from cardiovascular or renal causes (hazard ratio, 0.73; 95% confidence interval, 0.53-1.02).
In secondary efficacy outcomes, patients who received dapagliflozin as opposed to placebo also had a lower risk of the following:
- A renal composite outcome – a ≥ 50% sustained decline in eGFR, end-stage kidney disease, or death from renal causes (HR, 0.71; 95% CI, 0.49-1.02).
- A cardiovascular composite outcome comprising cardiovascular death or hospitalization for heart failure (HR, 0.83; 95% CI, 0.45-1.53).
- All-cause mortality (HR, 0.68; 95% CI, 0.39 to 1.21).
The eGFR slope declined by 2.15 mL/min per 1.73 m2 per year and by 3.38 mL/min per 1.73 m2 per year in the dapagliflozin and placebo groups, respectively (P = .005).
“The trial was not powered to detect a statistically significant difference in the primary and key secondary endpoints in modest-sized subgroups,” the researchers noted.
The researchers limited their safety analysis to serious adverse events or symptoms of volume depletion, kidney-related events, major hypoglycemia, bone fractures, amputations, and potential diabetic ketoacidosis.
There was no evidence of increased risk of these adverse events in patients who received dapagliflozin.
The subanalysis of the DAPA-CKD trial was published July 16 in the Journal of the American Society of Nephrology.
The study was funded by AstraZeneca. Dr. Chertow has received fees from AstraZeneca for the DAPA-CKD trial steering committee. The disclosures of the other authors are listed in the article. Dr. Mathieu has served on the advisory panel/speakers bureau for AstraZeneca. Dr. Chertow and Dr. Mathieu also have financial relationships with many other pharmaceutical companies.
Patients with stage 4 chronic kidney disease (CKD) who were in the DAPA-CKD trial had cardiorenal benefits from dapagliflozin that were similar to those of patients in the overall trial, with no added safety signal.
DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) was a landmark study of more than 4,000 patients with CKD, with an estimated glomerular filtration rate (eGFR) of 25-75 mL/min per 1.73 m2 and albuminuria with/without type 2 diabetes.
The primary results showed that patients who received the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin for a median of 2.4 years were significantly less likely to have worsening kidney disease or die from all causes than were patients who received placebo.
“This prespecified subanalysis of people with an eGFR < 30 mL/min/1.73 m2 [stage 4 CKD] in the DAPA-CKD study shows first, that in this very vulnerable population, use of the SGLT2 inhibitor is safe,” said Chantal Mathieu, MD, PhD.
Furthermore, there was no signal whatsoever of more adverse events and even a trend to fewer events, she said in an email to this news organization.
The analysis also showed that “although now in small numbers (around 300 each in the treated group vs. placebo group), there is no suggestion that the protective effect of dapagliflozin on the renal and cardiovascular front would not happen in this group” with advanced CKD. The efficacy findings just missed statistical significance, noted Dr. Mathieu, of Catholic University, Leuven, Belgium, who was not involved in the study.
Although dapagliflozin is now approved for treating patients with CKD who are at risk of kidney disease progression (on the basis of the DAPA-CKD results), guidelines have not yet been updated to reflect this, lead investigator Glenn M. Chertow, MD, MPH, of Stanford (Calif.) University, told this news organization in an email.
“For clinicians,” Dr. Mathieu said, “this is now the absolute reassurance that we do not have to stop an SGLT2 inhibitor in people with eGFR < 30 mL/min for safety reasons and that we should maintain them at these values for renal and cardiovascular protection!
“I absolutely hope labels will change soon to reflect these observations (and indeed movement on that front is happening),” she continued.
“The American Diabetes Association/European Association for the Study of Diabetes consensus on glucose-lowering therapies in type 2 diabetes already advocated keeping these agents until eGFR 30 mL/min (on the basis of evidence in 2019),” Dr. Mathieu added, “but this study will probably push the statements even further.”
“Of note,” she pointed out, “at these low eGFRs, the glucose-lowering potential of the SGLT2 inhibitor is negligible.”
Dapagliflozin risks and benefits in advanced CKD
Based on the DAPA-CKD study, published in the New England Journal of Medicine Oct. 8, 2020, the Food and Drug Administration expanded the indication for dapagliflozin (Farxiga, AstraZeneca) in April of 2021.
However, relatively little is known about the safety and efficacy of SGLT2 inhibitors in patients with advanced CKD, who are particularly vulnerable to cardiovascular events and progressive kidney failure, Dr. Chertow and colleagues wrote.
The DAPA-CKD trial randomized 4,304 patients with CKD 1:1 to dapagliflozin 10 mg/day or placebo, including 624 patients (14%) who had eGFR < 30 mL/min per 1.73 m2 and albuminuria at baseline.
Patients in the subgroup with advanced CKD had a mean age of 62 years, and 37% were female. About two-thirds had type 2 diabetes and about one-third had cardiovascular disease.
A total of 293 patients received dapagliflozin and 331 patients received placebo.
During a median follow-up of 2.4 years, patients who received dapagliflozin as opposed to placebo had a lower risk of the primary efficacy outcome – a composite of a 50% or greater sustained decline in eGFR, end-stage kidney disease, or death from cardiovascular or renal causes (hazard ratio, 0.73; 95% confidence interval, 0.53-1.02).
In secondary efficacy outcomes, patients who received dapagliflozin as opposed to placebo also had a lower risk of the following:
- A renal composite outcome – a ≥ 50% sustained decline in eGFR, end-stage kidney disease, or death from renal causes (HR, 0.71; 95% CI, 0.49-1.02).
- A cardiovascular composite outcome comprising cardiovascular death or hospitalization for heart failure (HR, 0.83; 95% CI, 0.45-1.53).
- All-cause mortality (HR, 0.68; 95% CI, 0.39 to 1.21).
The eGFR slope declined by 2.15 mL/min per 1.73 m2 per year and by 3.38 mL/min per 1.73 m2 per year in the dapagliflozin and placebo groups, respectively (P = .005).
“The trial was not powered to detect a statistically significant difference in the primary and key secondary endpoints in modest-sized subgroups,” the researchers noted.
The researchers limited their safety analysis to serious adverse events or symptoms of volume depletion, kidney-related events, major hypoglycemia, bone fractures, amputations, and potential diabetic ketoacidosis.
There was no evidence of increased risk of these adverse events in patients who received dapagliflozin.
The subanalysis of the DAPA-CKD trial was published July 16 in the Journal of the American Society of Nephrology.
The study was funded by AstraZeneca. Dr. Chertow has received fees from AstraZeneca for the DAPA-CKD trial steering committee. The disclosures of the other authors are listed in the article. Dr. Mathieu has served on the advisory panel/speakers bureau for AstraZeneca. Dr. Chertow and Dr. Mathieu also have financial relationships with many other pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY
New drug, finerenone, approved for slowing kidney disease in diabetes
The U.S. Food and Drug Administration approved finerenone (Kerendia), the first agent from a new class of nonsteroidal mineralocorticoid receptor antagonists (MRAs), on July 9 for treating patients with chronic kidney disease (CKD) associated with type 2 diabetes.
Janani Rangaswami, MD, a nephrologist not involved with finerenone’s development, hailed the action as a “welcome addition to therapies in the cardiorenal space.”
She also highlighted that until more evidence accumulates, finerenone will take a back seat to two more established renal-protective drug classes for patients with type 2 diabetes, the renin-angiotensin system inhibitors (RASIs), and the sodium-glucose cotransporter 2 (SGLT2) inhibitors.
RASIs, which include angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, remain first-line treatments for slowing the progression of CKD in patients with type 2 diabetes. The efficacy and safety of these agents are well-established. The trial that led to the FDA’s decision to approve finerenone, FIDELIO-DKD, compared it against placebo in more than 5,700 patients with type 2 diabetes who were all already taking a maximum-tolerated dose of an RASI.
Scant data on combining finerenone with an SGLT2 inhibitor
Two agents in the SGLT2 inhibitor class, approved initially for type 2 diabetes, received additional FDA approvals for slowing kidney disease: Canagliflozin (Invokana), which was approved in September 2019 on the basis of the CREDENCE trial, and dapagliflozin (Forxiga/Farxiga), which was approved in April 2021 on the basis of DAPA-CKD. Nephrologists now speak of this drug class as “practice changing.”
When FIDELIO-DKD enrolled patients from September 2015 to June 2018, it was still early days for use of SGLT2 inhibitors for patients with type 2 diabetes; hence, fewer than 5% of enrolled patients received an SGLT2 inhibitor, making it impossible to say how well finerenone works when taken along with one of these drugs.
“The big question that persists is the incremental benefit [from finerenone] on top of an SGLT2 inhibitor,” commented Dr. Rangaswami, director of the cardiorenal program at George Washington University, Washington, and chair-elect of the Council on the Kidney in Cardiovascular Disease of the American Heart Association.
“It is hard to extrapolate incremental benefit from existing finerenone trial data given the low background use of SGLT2 inhibitors [in FIDELIO-DKD],” she said in an interview.
George Bakris, MD, lead investigator for FIDELIO-DKD, agrees.
SGLT2 inhibitors are a ‘must’ for CKD
An SGLT2 inhibitor “must be used, period,” for patients with type 2 diabetes and CKD. “The evidence is very strong,” said Dr. Bakris, speaking in June 2021 during a session of the virtual annual Congress of the European Renal Association and European Dialysis and Transplant Association.
Because of inadequate evidence on how finerenone works when administered in addition to an SGLT2 inhibitor, for the time being, the combination must be considered investigational, he added.
Study results “need to show that combination therapy [with an SGLT2 inhibitor and finerenone] is better” than an SGLT2 inhibitor alone, said Dr. Bakris, professor of medicine and director of the Comprehensive Hypertension Center of the University of Chicago.
During his June talk, Dr. Bakris predicted that by 2023, enough data will exist from patients treated with both an SGLT2 inhibitor and finerenone to allow an evidence-based approach to combination treatment.
Finerenone’s approval makes it an immediate choice for patients with type 2 diabetes and CKD secondary to polycystic kidney disease, a group who are not candidates for an SGLT2 inhibitor, said Dr. Rangaswami.
But “if a patient is eligible for an SGLT2 inhibitor, I would not stop that in favor of starting finerenone” on the basis of current knowledge, she noted.
‘Not your mother’s spironolactone’
Although finerenone is classified an MRA, the class that also includes the steroidal agents spironolactone and eplerenone, the nonsteroidal structure of finerenone means “it has nothing to do with spironolactone. It’s a different molecule with different chemistry,” Dr. Bakris said in his June talk.
Although the risk for hyperkalemia has been a limiting factor and a deterrent to routine use of steroidal MRAs for preventing progression of CKD, hyperkalemia is much less of a problem with finerenone.
Main results from FIDELIO-DKD, published in late 2020, showed that the percentage of patients receiving finerenone who permanently stopped taking the drug because of hyperkalemia was 2.3%, higher than the 0.9% rate among patients in the trial who received placebo but about a third of the rate of patients treated with spironolactone in a historical cohort.
“You need to pay attention” to the potential development of hyperkalemia in patients taking finerenone, “but it is not a major issue,” Dr. Bakris said. “Finerenone is not your mother’s spironolactone,” he declared.
FIDELIO-DKD’s primary outcome, a combination of several adverse renal events, showed that treatment with finerenone cut this endpoint by a significant 18% compared with placebo. The study’s main secondary endpoint showed that finerenone cut the incidence of combined cardiovascular disease events by a significant 14% compared with placebo. Adverse events were similar in the finerenone and placebo arms.
Finerenone also shows promise for reducing CVD events
Bayer, the company that developed and will market finerenone, announced in May 2021 topline results from a companion trial, FIGARO-DKD. That trial also enrolled patients with type 2 diabetes and CKD, but a primary endpoint of that trial combined the rates of cardiovascular death and nonfatal cardiovascular disease events. The results from this trial showed a significant difference in favor of finerenone compared with placebo.
“Given the common pathways that progression of CKD and cardiovascular disease share with respect to [moderating] inflammation and [slowing development of] fibrosis, it is not surprising that a signal for benefit was seen at the different ends of the cardiorenal spectrum,” Dr. Rangaswami said.
FIDELIO-DKD and FIGARO-DKD were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Bakris has been a consultant to and has received research funding from Bayer and from numerous other companies. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved finerenone (Kerendia), the first agent from a new class of nonsteroidal mineralocorticoid receptor antagonists (MRAs), on July 9 for treating patients with chronic kidney disease (CKD) associated with type 2 diabetes.
Janani Rangaswami, MD, a nephrologist not involved with finerenone’s development, hailed the action as a “welcome addition to therapies in the cardiorenal space.”
She also highlighted that until more evidence accumulates, finerenone will take a back seat to two more established renal-protective drug classes for patients with type 2 diabetes, the renin-angiotensin system inhibitors (RASIs), and the sodium-glucose cotransporter 2 (SGLT2) inhibitors.
RASIs, which include angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, remain first-line treatments for slowing the progression of CKD in patients with type 2 diabetes. The efficacy and safety of these agents are well-established. The trial that led to the FDA’s decision to approve finerenone, FIDELIO-DKD, compared it against placebo in more than 5,700 patients with type 2 diabetes who were all already taking a maximum-tolerated dose of an RASI.
Scant data on combining finerenone with an SGLT2 inhibitor
Two agents in the SGLT2 inhibitor class, approved initially for type 2 diabetes, received additional FDA approvals for slowing kidney disease: Canagliflozin (Invokana), which was approved in September 2019 on the basis of the CREDENCE trial, and dapagliflozin (Forxiga/Farxiga), which was approved in April 2021 on the basis of DAPA-CKD. Nephrologists now speak of this drug class as “practice changing.”
When FIDELIO-DKD enrolled patients from September 2015 to June 2018, it was still early days for use of SGLT2 inhibitors for patients with type 2 diabetes; hence, fewer than 5% of enrolled patients received an SGLT2 inhibitor, making it impossible to say how well finerenone works when taken along with one of these drugs.
“The big question that persists is the incremental benefit [from finerenone] on top of an SGLT2 inhibitor,” commented Dr. Rangaswami, director of the cardiorenal program at George Washington University, Washington, and chair-elect of the Council on the Kidney in Cardiovascular Disease of the American Heart Association.
“It is hard to extrapolate incremental benefit from existing finerenone trial data given the low background use of SGLT2 inhibitors [in FIDELIO-DKD],” she said in an interview.
George Bakris, MD, lead investigator for FIDELIO-DKD, agrees.
SGLT2 inhibitors are a ‘must’ for CKD
An SGLT2 inhibitor “must be used, period,” for patients with type 2 diabetes and CKD. “The evidence is very strong,” said Dr. Bakris, speaking in June 2021 during a session of the virtual annual Congress of the European Renal Association and European Dialysis and Transplant Association.
Because of inadequate evidence on how finerenone works when administered in addition to an SGLT2 inhibitor, for the time being, the combination must be considered investigational, he added.
Study results “need to show that combination therapy [with an SGLT2 inhibitor and finerenone] is better” than an SGLT2 inhibitor alone, said Dr. Bakris, professor of medicine and director of the Comprehensive Hypertension Center of the University of Chicago.
During his June talk, Dr. Bakris predicted that by 2023, enough data will exist from patients treated with both an SGLT2 inhibitor and finerenone to allow an evidence-based approach to combination treatment.
Finerenone’s approval makes it an immediate choice for patients with type 2 diabetes and CKD secondary to polycystic kidney disease, a group who are not candidates for an SGLT2 inhibitor, said Dr. Rangaswami.
But “if a patient is eligible for an SGLT2 inhibitor, I would not stop that in favor of starting finerenone” on the basis of current knowledge, she noted.
‘Not your mother’s spironolactone’
Although finerenone is classified an MRA, the class that also includes the steroidal agents spironolactone and eplerenone, the nonsteroidal structure of finerenone means “it has nothing to do with spironolactone. It’s a different molecule with different chemistry,” Dr. Bakris said in his June talk.
Although the risk for hyperkalemia has been a limiting factor and a deterrent to routine use of steroidal MRAs for preventing progression of CKD, hyperkalemia is much less of a problem with finerenone.
Main results from FIDELIO-DKD, published in late 2020, showed that the percentage of patients receiving finerenone who permanently stopped taking the drug because of hyperkalemia was 2.3%, higher than the 0.9% rate among patients in the trial who received placebo but about a third of the rate of patients treated with spironolactone in a historical cohort.
“You need to pay attention” to the potential development of hyperkalemia in patients taking finerenone, “but it is not a major issue,” Dr. Bakris said. “Finerenone is not your mother’s spironolactone,” he declared.
FIDELIO-DKD’s primary outcome, a combination of several adverse renal events, showed that treatment with finerenone cut this endpoint by a significant 18% compared with placebo. The study’s main secondary endpoint showed that finerenone cut the incidence of combined cardiovascular disease events by a significant 14% compared with placebo. Adverse events were similar in the finerenone and placebo arms.
Finerenone also shows promise for reducing CVD events
Bayer, the company that developed and will market finerenone, announced in May 2021 topline results from a companion trial, FIGARO-DKD. That trial also enrolled patients with type 2 diabetes and CKD, but a primary endpoint of that trial combined the rates of cardiovascular death and nonfatal cardiovascular disease events. The results from this trial showed a significant difference in favor of finerenone compared with placebo.
“Given the common pathways that progression of CKD and cardiovascular disease share with respect to [moderating] inflammation and [slowing development of] fibrosis, it is not surprising that a signal for benefit was seen at the different ends of the cardiorenal spectrum,” Dr. Rangaswami said.
FIDELIO-DKD and FIGARO-DKD were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Bakris has been a consultant to and has received research funding from Bayer and from numerous other companies. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved finerenone (Kerendia), the first agent from a new class of nonsteroidal mineralocorticoid receptor antagonists (MRAs), on July 9 for treating patients with chronic kidney disease (CKD) associated with type 2 diabetes.
Janani Rangaswami, MD, a nephrologist not involved with finerenone’s development, hailed the action as a “welcome addition to therapies in the cardiorenal space.”
She also highlighted that until more evidence accumulates, finerenone will take a back seat to two more established renal-protective drug classes for patients with type 2 diabetes, the renin-angiotensin system inhibitors (RASIs), and the sodium-glucose cotransporter 2 (SGLT2) inhibitors.
RASIs, which include angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, remain first-line treatments for slowing the progression of CKD in patients with type 2 diabetes. The efficacy and safety of these agents are well-established. The trial that led to the FDA’s decision to approve finerenone, FIDELIO-DKD, compared it against placebo in more than 5,700 patients with type 2 diabetes who were all already taking a maximum-tolerated dose of an RASI.
Scant data on combining finerenone with an SGLT2 inhibitor
Two agents in the SGLT2 inhibitor class, approved initially for type 2 diabetes, received additional FDA approvals for slowing kidney disease: Canagliflozin (Invokana), which was approved in September 2019 on the basis of the CREDENCE trial, and dapagliflozin (Forxiga/Farxiga), which was approved in April 2021 on the basis of DAPA-CKD. Nephrologists now speak of this drug class as “practice changing.”
When FIDELIO-DKD enrolled patients from September 2015 to June 2018, it was still early days for use of SGLT2 inhibitors for patients with type 2 diabetes; hence, fewer than 5% of enrolled patients received an SGLT2 inhibitor, making it impossible to say how well finerenone works when taken along with one of these drugs.
“The big question that persists is the incremental benefit [from finerenone] on top of an SGLT2 inhibitor,” commented Dr. Rangaswami, director of the cardiorenal program at George Washington University, Washington, and chair-elect of the Council on the Kidney in Cardiovascular Disease of the American Heart Association.
“It is hard to extrapolate incremental benefit from existing finerenone trial data given the low background use of SGLT2 inhibitors [in FIDELIO-DKD],” she said in an interview.
George Bakris, MD, lead investigator for FIDELIO-DKD, agrees.
SGLT2 inhibitors are a ‘must’ for CKD
An SGLT2 inhibitor “must be used, period,” for patients with type 2 diabetes and CKD. “The evidence is very strong,” said Dr. Bakris, speaking in June 2021 during a session of the virtual annual Congress of the European Renal Association and European Dialysis and Transplant Association.
Because of inadequate evidence on how finerenone works when administered in addition to an SGLT2 inhibitor, for the time being, the combination must be considered investigational, he added.
Study results “need to show that combination therapy [with an SGLT2 inhibitor and finerenone] is better” than an SGLT2 inhibitor alone, said Dr. Bakris, professor of medicine and director of the Comprehensive Hypertension Center of the University of Chicago.
During his June talk, Dr. Bakris predicted that by 2023, enough data will exist from patients treated with both an SGLT2 inhibitor and finerenone to allow an evidence-based approach to combination treatment.
Finerenone’s approval makes it an immediate choice for patients with type 2 diabetes and CKD secondary to polycystic kidney disease, a group who are not candidates for an SGLT2 inhibitor, said Dr. Rangaswami.
But “if a patient is eligible for an SGLT2 inhibitor, I would not stop that in favor of starting finerenone” on the basis of current knowledge, she noted.
‘Not your mother’s spironolactone’
Although finerenone is classified an MRA, the class that also includes the steroidal agents spironolactone and eplerenone, the nonsteroidal structure of finerenone means “it has nothing to do with spironolactone. It’s a different molecule with different chemistry,” Dr. Bakris said in his June talk.
Although the risk for hyperkalemia has been a limiting factor and a deterrent to routine use of steroidal MRAs for preventing progression of CKD, hyperkalemia is much less of a problem with finerenone.
Main results from FIDELIO-DKD, published in late 2020, showed that the percentage of patients receiving finerenone who permanently stopped taking the drug because of hyperkalemia was 2.3%, higher than the 0.9% rate among patients in the trial who received placebo but about a third of the rate of patients treated with spironolactone in a historical cohort.
“You need to pay attention” to the potential development of hyperkalemia in patients taking finerenone, “but it is not a major issue,” Dr. Bakris said. “Finerenone is not your mother’s spironolactone,” he declared.
FIDELIO-DKD’s primary outcome, a combination of several adverse renal events, showed that treatment with finerenone cut this endpoint by a significant 18% compared with placebo. The study’s main secondary endpoint showed that finerenone cut the incidence of combined cardiovascular disease events by a significant 14% compared with placebo. Adverse events were similar in the finerenone and placebo arms.
Finerenone also shows promise for reducing CVD events
Bayer, the company that developed and will market finerenone, announced in May 2021 topline results from a companion trial, FIGARO-DKD. That trial also enrolled patients with type 2 diabetes and CKD, but a primary endpoint of that trial combined the rates of cardiovascular death and nonfatal cardiovascular disease events. The results from this trial showed a significant difference in favor of finerenone compared with placebo.
“Given the common pathways that progression of CKD and cardiovascular disease share with respect to [moderating] inflammation and [slowing development of] fibrosis, it is not surprising that a signal for benefit was seen at the different ends of the cardiorenal spectrum,” Dr. Rangaswami said.
FIDELIO-DKD and FIGARO-DKD were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Bakris has been a consultant to and has received research funding from Bayer and from numerous other companies. Dr. Rangaswami has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lack of fever in ESRD with S. aureus bacteremia is common
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
‘Stunning’ twincretin beats semaglutide for A1c, weight reduction in T2D
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
Tirzepatide, a novel “twincretin” agent, was superior to 1-mg semaglutide treatments for reducing both hemoglobin A1c levels and body weight in patients with type 2 diabetes in a pivotal, 40-week, head-to-head trial with nearly 1,900 randomized patients, one of four positive pivotal trial results reported for tirzepatide at the annual scientific sessions of the American Diabetes Association.
“Across all four studies we see a significant and clinically meaningful decrease in A1c, and robust weight loss. The results exceeded our expectations” for both these outcomes, said Laura Fernández Landó, MD, senior medical director for tirzepatide at Lilly, the company developing the agent, and a coauthor on the semaglutide comparison study as well as on other tirzepatide reports at the meeting.
“This opens up a new avenue for results in diabetes therapy,” Jens Juul Holst, MD, remarked in a press conference.
SURPASS-2 compared three different tirzepatide doses delivered once weekly by subcutaneous injection against a 1-mg weekly, subcutaneous dose of semaglutide (Ozempic) in 1,879 adults who had been diagnosed with type 2 diabetes for an average of almost 9 years. All patients were already on metformin treatment that had proved inadequate for controlling their hyperglycemia; enrolled patients had an average A1c of 8.28%. The trial’s primary endpoint was change from baseline in A1c levels after 40 weeks.
Significant differences at each dose level
Patients on each of the three tirzepatide doses – 5 mg, 10 mg, or 15 mg once weekly – showed dose-dependent reductions in A1c that, for each dose, were significantly better than the reduction achieved with semaglutide. The highest tirzepatide dose reduced A1c levels by an average of 0.45% more than what semaglutide achieved, reported first author Juan P. Frias, MD; Dr. Landó; and their coauthors.
One key secondary endpoint was weight reduction, and each of the three tirzepatide doses again produced significant incremental loss beyond what semaglutide produced. The 5-mg weekly dose of tirzepatide produced an average 1.9-kg additional weight loss, compared with semaglutide, while the 15-mg dose resulted in an average 5.5-kg loss beyond what semaglutide achieved and a total average weight loss of 11.2 kg from baseline.
The study’s additional key secondary endpoints, the percentages of patients reaching an A1c of less than 7%, and less than 5.7%, also showed significantly better numbers with tirzepatide. The highest tirzepatide dose pushed 86% of patients below the 7% mark, compared with 79% on semaglutide, and the top tirzepatide dose resulted in 46% of patients getting their A1c below 5.7%, compared with 19% of patients on semaglutide.
The findings are “stunning, I must stay, and those results included that up to half of the patients treated with high doses of tirzepatide may reach A1c levels of less than 5.7%, which is really, really unheard of,” said Dr. Holst, professor of endocrinology and metabolism at the University of Copenhagen. Along with the “weight losses at the same time of up to 12% in that patient group, we are seeing some completely unexpected and really shocking and wonderful new advances in the therapy,” added Dr. Holst.
The safety profile of tirzepatide was roughly similar to semaglutide’s and to that other agents in the glucagonlike peptide-1 receptor agonist (GLP-1 RA) class. Concurrently with the report at the meeting, the results also appeared in an article published online in the New England Journal of Medicine.
An ‘impressive’ weight loss effect
Weight loss on tirzepatide was “impressive,” commented Katherine R. Tuttle, MD, a nephrologist affiliated with the University of Washington and executive director for research at Providence Health Care in Spokane, Wash. Another striking feature of tirzepatide’s weight-loss effect was that it did not plateau during the 40 weeks of the study, Dr. Tuttle noted in an accompanying editorial that accompanied the published report, a finding that suggests the potential for additional weight loss from continued treatment .
“The weight loss is remarkable,” commented Rodolfo J. Galindo, MD, an endocrinologist at Emory University, Atlanta. While incremental reduction of A1c on the order of less than 0.5% is helpful, incremental weight loss of more than 10 lbs on tirzepatide, compared with semaglutide “will likely be a tie-breaker” for many clinicians and patients to favor tirzepatide over semaglutide or another GLP-1 RA agent, he said in an interview. Dr. Galindo also cited other important factors that he predicted will drive decisions on using tirzepatide or a GLP-1 RA once tirzepatide reaches the U.S. market: relative cost, access, and tolerability.
The important issue of dose
But the edge that tirzepatide showed over semaglutide for weight loss did not occur on a completely level playing field. The 1 mg/week dose of semaglutide used as the comparator in SURPASS-2 was the maximum dose available at the time the study began, but in June 2021 the Food and Drug Administration approved a 2.4 mg/week dose (Wegovy) labeled specifically for weight loss. Dr. Tuttle cited the limitation this introduces in her editorial.
“The dose issue is important,” she wrote. The doses of tirzepatide and semaglutide compared in SURPASS-2 “were not comparable in terms of weight outcomes” given that prior evidence showed that the 2.4 mg/week semaglutide dose is more appropriate for weight loss.
Dr. Tuttle also cited other factors to consider when assessing tirzepatide compared with agents in the GLP-1 RA class.
Several GLP-1 RA agents, including semaglutide, have proven efficacy for reducing rates of atherosclerotic cardiovascular events and albuminuria, and they also slow decline in kidney function and progression of diabetic kidney disease. No details on the renal effects of tirzepatide appeared in the SURPASS-2 report. A press release from Lilly in May 2021 briefly mentioned results from a meta-analysis of several clinical studies of tirzepatide that showed a nonsignificant effect from tirzepatide on the incidence of major cardiovascular adverse events (death from cardiovascular or undetermined causes, MI, stroke, and hospitalization for unstable angina) relative to comparator groups. Results from a dedicated cardiovascular outcomes trial in high-risk patients treated with tirzepatide, SURPASS-CVOT, are not expected until 2024.
A further limitation of SURPASS-2 was the demographics of the enrolled population, which had a low (0.4%) enrollment rate of Black patients, and a high proportion (70%) of Hispanic patients, Dr. Tuttle observed.
Low rates of hypoglycemia
Another notable finding from SURPASS-2 was the low incidence of clinically significant hypoglycemic events (blood glucose levels less than 54 mg/dL), which occurred in 0.2%-1.7% of patients on tirzepatide, depending on their dose, and in 0.4% of patients on semaglutide. Two patients in the tirzepatide cohort had severe hypoglycemia.
These numbers are reassuring, said Dr. Galindo, and reflect the safety of tirzepatide’s dual, incretin-like mechanisms of action that make it a “twincretin.” The molecule acts as both a GLP-1 RA, and as glucose-dependent insulinotropic polypeptide, an incretin that stimulates insulin release when blood sugar is high but also increases glucagon levels when blood sugar levels are normal or low. This dual action may help explain the apparent increased potency tirzepatide showed for both A1c reduction and weight loss, compared with semaglutide, which acts only as a GLP-1 RA.
Some experts have cited the uncertainty introduced by the open-label design of SURPASS-2, a decision necessitated by the distinctly different delivery devices used for tirzepatide and semaglutide, explained Dr. Landó. But she highlighted that double blinding applied to the three different tirzepatide dosages tested in the trial. Dr. Landó said that Lilly plans to seek FDA approval for all three tested tirzepatide doses to give clinicians and patients flexibility in applying the treatment.
SURPASS-2 used a prolonged dose-escalation protocol designed to minimize gastrointestinal adverse effects that started patients on a 2.5 mg weekly dose that then increased by 2.5 mg increments every 4 weeks until patients reached their assigned target dose. This meant that patients did not begin receiving the 15-mg/week dose until halfway through the trial.
Several more tirzepatide trials
Reports from two other pivotal trials for tirzepatide also appeared as posters at the meeting. SURPASS-5 compared tirzepatide with placebo in 475 patients inadequately controlled with titrated insulin glargine (Lantus). SURPASS-3 randomized 1,444 patients to tirzepatide or titrated insulin degludec (Tresiba). In both studies treatment with tirzepatide led to significantly better reductions in A1c and in weight loss than the comparator treatments. Results from a third pivotal trial, SURPASS-1 which compared tirzepatide against placebo in 478 treatment-naive patients, will come in a report scheduled for the second day of the meeting.
The results from all the recent tirzepatide trials show a consistent benefit across the continuum of patients with type 2 diabetes regardless of whether it’s recent onset or well-established disease, said Dr. Landó.
The SURPASS studies were sponsored by Lilly, the company developing tirzepatide, and the reports include several authors who are Lilly employees. Dr. Landó is a Lilly employee and stockholder. Dr. Tuttle has been a consultant to Lilly and to Novo Nordisk, the company that markets semaglutide, as well as to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, and Janssen. She has also received travel expenses from Kyokawa Hakko Kirin, and research funding from Bayer, Goldfinch Bio, and Lilly. Dr. Galindo has been a consultant to Lilly and to Novo Nordisk, as well as to Abbott Diabetes Care, Sanofi, Valeritas, and Weight Watchers, and his institution has received grant support on his behalf from Lilly, Novo Nordisk and Dexcom. Dr. Holst had no disclosures.
FROM ADA 2021
Post–acute kidney injury proteinuria predicts subsequent kidney disease progression
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Recent studies have shown that the level of proteinuria increases after AKI. It is not yet shown if this increases risk of kidney disease progression.
Study design: Prospective matched cohort study.
Setting: North American hospitals.
Synopsis: A total of 769 hospitalized adults with AKI were matched with those without based on clinical center and preadmission chronic kidney disease (CKD) status. Study authors found that albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) 3 months after hospitalization were highly associated with kidney disease progression, with a hazard ratio of 1.53 for each doubling (95% confidence interval, 1.43-1.64).
Episodes of AKI were also associated with progression, but this is severely attenuated once adjusted for ACR, eGFR, and traditional CKD risk factors. This suggests more routine quantification of proteinuria after AKI for better risk stratification.
Bottom line: Posthospitalization ACR predicts progression of kidney disease.
Citation: Hsu CY et al. Post–acute kidney injury proteinuria and subsequent kidney disease progression. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6390.
Dr. Ho is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Fact or fiction? Intravascular contrast and acute kidney injury
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.
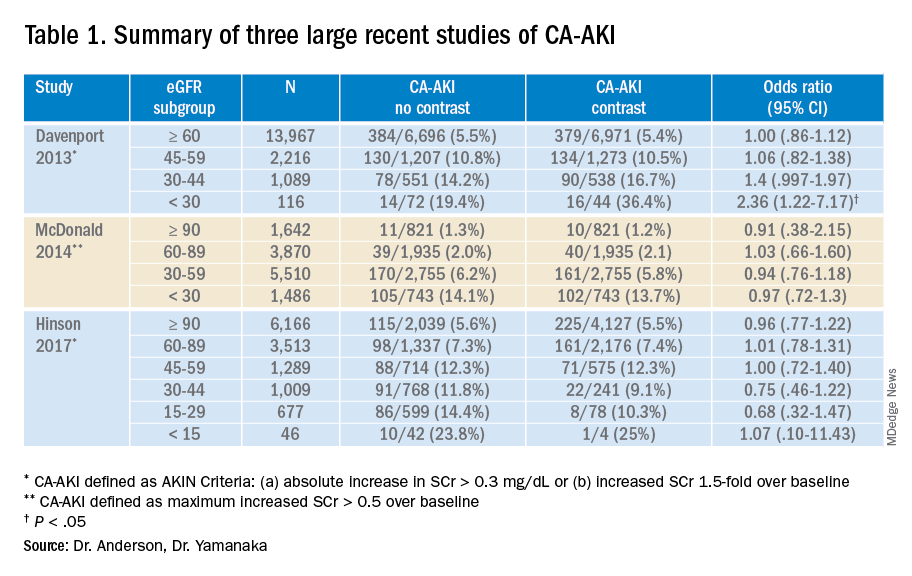
Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Withholding contrast may be the greater risk
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
The most important meal of the day, with extra zinc
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Third COVID-19 vaccine dose helped some transplant recipients
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.
All of those with low titers before the third dose had high titers after receiving the additional shot, but only about 33% of those with negative initial responses had detectable antibodies after the third dose, according to the paper, published in Annals of Internal Medicine.
Researchers at Johns Hopkins, Baltimore, who keep a COVID-19 vaccine registry, perform antibody tests on all registry subjects and inform them of their results. Registry participants were asked to inform the research team if they received a third dose, and, the research team tracked the immune responses of those who did.
The participants in this case series had low antibody levels and received a third dose of the vaccine on their own between March 20 and May 10 of 2021.
Third dose results
In this cases series – thought to be the first to look at third vaccine shots in this type of patient group – all six of those who had low antibody titers before the third dose had high-positive titers after the third dose.
Of the 24 individuals who had negative antibody titers before the third dose, just 6 had high titers after the third dose.
Two of the participants had low-positive titers, and 16 were negative.
“Several of those boosted very nicely into ranges seen, using these assays, in healthy persons,” said William Werbel, MD, a fellow in infectious disease at Johns Hopkins Medicine, Baltimore, who helped lead the study. Those with negative levels, even if they responded, tended to have lower titers, he said.
“The benefits at least from an antibody perspective were not the same for everybody and so this is obviously something that needs to be considered when thinking about selecting patients” for a COVID-19 prevention strategy, he said.
Reactions to the vaccine were low to moderate, such as some arm pain and fatigue.
“Showing that something is safe in that special, vulnerable population is important,” Dr. Werbel said. “We’re all wanting to make sure that we’re doing no harm.”
Dr. Werbel noted that there was no pattern in the small series based on the organ transplanted or in the vaccines used. As their third shot, 15 of the patients received the Johnson & Johnson vaccine; 9 received Moderna; and 6 received Pfizer-BioNTech.
Welcome news, but larger studies needed
“To think that a third dose could confer protection for a significant number of people is of course extremely welcome news,” said Christian Larsen, MD, DPhil, professor of surgery in the transplantation division at Emory University, Atlanta, who was not involved in the study. “It’s the easiest conceivable next intervention.”
He added, “We just want studies to confirm that – larger studies.”
Dr. Werbel stressed the importance of looking at third doses in these patients in a more controlled fashion in a randomized trial, to more carefully monitor safety and how patients fare when starting with one type of vaccine and switching to another, for example.
Richard Wender, MD, chair of family medicine and community health at the University of Pennsylvania, Philadelphia, said the findings are a reminder that there is still a lot that is unknown about COVID-19 and vaccination.
“We still don’t know who will or will not benefit from a third dose,” he said. “And our knowledge is evolving. For example, a recent study suggested that people with previous infection and who are vaccinated may have better and longer protection than people with vaccination alone. We’re still learning.”
He added that specialists, not primary care clinicians, should be relied upon to respond to this emerging vaccination data. Primary care doctors are very busy in other ways – such as in getting children caught up on vaccinations and helping adults return to managing their chronic diseases, Dr. Wender noted.
“Their focus needs to be on helping to overcome hesitancy, mistrust, lack of information, or antivaccination sentiment to help more people feel comfortable being vaccinated – this is a lot of work and needs constant focus. In short, primary care clinicians need to focus chiefly on the unvaccinated,” he said.
“Monitoring immunization recommendations for unique at-risk populations should be the chief responsibility of teams providing subspecialty care, [such as for] transplant patients, people with chronic kidney disease, cancer patients, and people with other chronic illnesses. This will allow primary care clinicians to tackle their many complex jobs.”
Possible solutions for those with low antibody responses
Dr. Larsen said that those with ongoing low antibody responses might still have other immune responses, such as a T-cell response. Such patients also could consider changing their vaccine type, he said.
“At the more significant intervention level, there may be circumstances where one could change the immunosuppressive drugs in a controlled way that might allow a better response,” suggested Dr. Larsen. “That’s obviously going to be something that requires a lot more thought and careful study.”
Dr. Werbel said that other options might need to be considered for those having no response following a third dose. One possibility is trying a vaccine with an adjuvant, such as the Novavax version, which might be more widely available soon.
“If you’re given a third dose of a very immunogenic vaccine – something that should work – and you just have no antibody development, it seems relatively unlikely that doing the same thing again is going to help you from that perspective, and for all we know might expose you to more risk,” Dr. Werbel noted.
Participant details
None of the 30 patients were thought to have ever had COVID-19. On average, patients had received their transplant 4.5 years before their original vaccination. In 25 patients, maintenance immunosuppression included tacrolimus or cyclosporine along with mycophenolate. Corticosteroids were also used for 24 patients, sirolimus was used for one patient, and belatacept was used for another patient.
Fifty-seven percent of patients had received the Pfizer/BioNTech vaccine originally, and 43% the Moderna vaccine. Most of the patients were kidney recipients, with two heart, three liver, one lung, one pancreas and one kidney-pancreas.
Dr. Werbel, Dr. Wender, and Dr. Larsen reported no relevant disclosures.